User login
OMV meningococcal vaccine also protected against gonorrhea
A group B meningococcal outer-membrane-vesicle (OMV) vaccine used during a meningitis outbreak in New Zealand also protected against gonorrhea, according to a report published online July 10 in the Lancet.
Even though Neisseria meningitidis and Neisseria gonorrhoeae cause distinctly different diseases, the bacteria are closely related and are genetically and antigenically very similar. Most of the virulence factors present in one pathogen have an equivalent in the other, “providing at least one biologically plausible mechanism for cross-protection,” said Helen Petousis-Harris, PhD, of the department of general practice and primary health care, University of Auckland (New Zealand), and her associates.
Approximately 1 million people – 81% of the New Zealand population younger than 20 years – received almost 3 million doses of the OMV meningococcal B vaccine (MeNZB) in a 2-year mass immunization program during the outbreak, allowing the investigators to compare the rate of gonorrhea between vaccinated and unvaccinated people. They performed a retrospective case-control study involving 14,730 participants, using information from a national health care database, a national immunization registry, and 11 sexual health clinics covering diverse geographic regions. This included 1,241 cases of gonorrhea (cases), 12,487 cases of chlamydia (controls), and 1,002 cases of gonorrhea plus chlamydia coinfection (categorized as controls or cases in separate analyses).
“The adjusted estimate for vaccine effectiveness of the MeNZB against confirmed cases of gonorrhea” was 31% (95% confidence interval, 21-39; P less than .0001), a finding that remained robust across several sensitivity analyses, Dr. Petousis-Harris and her associates said (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31449-6).
“To our knowledge, ours is the first study to show an association between a vaccine and a reduction in the risk of gonorrhea,” they noted. “The potential ability of an OMV group B meningococcal vaccine to provide even modest protection against gonorrhea would have substantial public health benefits in view of the prevalence of gonorrhea. Modeling suggests that a vaccine with 30% efficacy could decrease the prevalence of gonorrhea by more than 30% within 15 years, if immunity is maintained.”
These findings also are important in view of the organism’s increasing resistance to existing antibiotics. Moreover, if further study confirms that the MeNZB vaccine offers some degree of cross-protection against gonorrhea, these data can inform the development of a gonorrhea vaccine, the investigators added.
This study was funded by GlaxoSmithKline Vaccines and Auckland UniServices. Dr. Petousis-Harris reported serving as a consultant for GSK, Merck, and Pfizer, and one of her associates reported ties to Novartis Vaccines, GSK, Protein Sciences, and Merck.
Over decades of research, all of the attempts to create a vaccine against gonorrhea have failed, largely because of the variable nature of Neisseria gonorrhoeae antigens and the failure of the bacteria to induce a protective immune response, so the findings of Dr. Petousis-Harris and her associates are “a step in the right direction” and should reinvigorate interest and investment in this endeavor.
Although MeNZB is no longer available, another meningococcal vaccine (4CMenB, Bexsero) contains the same outer-membrane-vesicle antigen and three of the same recombinant proteins. As the authors pointed out, immunizing adolescents with this vaccine could reduce the rate of gonorrhea substantially, even if it has only moderate efficacy and duration of effect. In particular, reducing the pool of asymptomatic carriers would decrease both transmission and the severe sequelae that develop when the infection goes undetected.
Kate L. Seib, PhD, is a microbiologist at the Institute for Glycomics at Griffith University in Southport, Australia. She reported support by a career development fellowship from the Australian National Health and Medical Research Council. Dr. Seib made these remarks in an accompanying editorial comment (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31605-7).
Over decades of research, all of the attempts to create a vaccine against gonorrhea have failed, largely because of the variable nature of Neisseria gonorrhoeae antigens and the failure of the bacteria to induce a protective immune response, so the findings of Dr. Petousis-Harris and her associates are “a step in the right direction” and should reinvigorate interest and investment in this endeavor.
Although MeNZB is no longer available, another meningococcal vaccine (4CMenB, Bexsero) contains the same outer-membrane-vesicle antigen and three of the same recombinant proteins. As the authors pointed out, immunizing adolescents with this vaccine could reduce the rate of gonorrhea substantially, even if it has only moderate efficacy and duration of effect. In particular, reducing the pool of asymptomatic carriers would decrease both transmission and the severe sequelae that develop when the infection goes undetected.
Kate L. Seib, PhD, is a microbiologist at the Institute for Glycomics at Griffith University in Southport, Australia. She reported support by a career development fellowship from the Australian National Health and Medical Research Council. Dr. Seib made these remarks in an accompanying editorial comment (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31605-7).
Over decades of research, all of the attempts to create a vaccine against gonorrhea have failed, largely because of the variable nature of Neisseria gonorrhoeae antigens and the failure of the bacteria to induce a protective immune response, so the findings of Dr. Petousis-Harris and her associates are “a step in the right direction” and should reinvigorate interest and investment in this endeavor.
Although MeNZB is no longer available, another meningococcal vaccine (4CMenB, Bexsero) contains the same outer-membrane-vesicle antigen and three of the same recombinant proteins. As the authors pointed out, immunizing adolescents with this vaccine could reduce the rate of gonorrhea substantially, even if it has only moderate efficacy and duration of effect. In particular, reducing the pool of asymptomatic carriers would decrease both transmission and the severe sequelae that develop when the infection goes undetected.
Kate L. Seib, PhD, is a microbiologist at the Institute for Glycomics at Griffith University in Southport, Australia. She reported support by a career development fellowship from the Australian National Health and Medical Research Council. Dr. Seib made these remarks in an accompanying editorial comment (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31605-7).
A group B meningococcal outer-membrane-vesicle (OMV) vaccine used during a meningitis outbreak in New Zealand also protected against gonorrhea, according to a report published online July 10 in the Lancet.
Even though Neisseria meningitidis and Neisseria gonorrhoeae cause distinctly different diseases, the bacteria are closely related and are genetically and antigenically very similar. Most of the virulence factors present in one pathogen have an equivalent in the other, “providing at least one biologically plausible mechanism for cross-protection,” said Helen Petousis-Harris, PhD, of the department of general practice and primary health care, University of Auckland (New Zealand), and her associates.
Approximately 1 million people – 81% of the New Zealand population younger than 20 years – received almost 3 million doses of the OMV meningococcal B vaccine (MeNZB) in a 2-year mass immunization program during the outbreak, allowing the investigators to compare the rate of gonorrhea between vaccinated and unvaccinated people. They performed a retrospective case-control study involving 14,730 participants, using information from a national health care database, a national immunization registry, and 11 sexual health clinics covering diverse geographic regions. This included 1,241 cases of gonorrhea (cases), 12,487 cases of chlamydia (controls), and 1,002 cases of gonorrhea plus chlamydia coinfection (categorized as controls or cases in separate analyses).
“The adjusted estimate for vaccine effectiveness of the MeNZB against confirmed cases of gonorrhea” was 31% (95% confidence interval, 21-39; P less than .0001), a finding that remained robust across several sensitivity analyses, Dr. Petousis-Harris and her associates said (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31449-6).
“To our knowledge, ours is the first study to show an association between a vaccine and a reduction in the risk of gonorrhea,” they noted. “The potential ability of an OMV group B meningococcal vaccine to provide even modest protection against gonorrhea would have substantial public health benefits in view of the prevalence of gonorrhea. Modeling suggests that a vaccine with 30% efficacy could decrease the prevalence of gonorrhea by more than 30% within 15 years, if immunity is maintained.”
These findings also are important in view of the organism’s increasing resistance to existing antibiotics. Moreover, if further study confirms that the MeNZB vaccine offers some degree of cross-protection against gonorrhea, these data can inform the development of a gonorrhea vaccine, the investigators added.
This study was funded by GlaxoSmithKline Vaccines and Auckland UniServices. Dr. Petousis-Harris reported serving as a consultant for GSK, Merck, and Pfizer, and one of her associates reported ties to Novartis Vaccines, GSK, Protein Sciences, and Merck.
A group B meningococcal outer-membrane-vesicle (OMV) vaccine used during a meningitis outbreak in New Zealand also protected against gonorrhea, according to a report published online July 10 in the Lancet.
Even though Neisseria meningitidis and Neisseria gonorrhoeae cause distinctly different diseases, the bacteria are closely related and are genetically and antigenically very similar. Most of the virulence factors present in one pathogen have an equivalent in the other, “providing at least one biologically plausible mechanism for cross-protection,” said Helen Petousis-Harris, PhD, of the department of general practice and primary health care, University of Auckland (New Zealand), and her associates.
Approximately 1 million people – 81% of the New Zealand population younger than 20 years – received almost 3 million doses of the OMV meningococcal B vaccine (MeNZB) in a 2-year mass immunization program during the outbreak, allowing the investigators to compare the rate of gonorrhea between vaccinated and unvaccinated people. They performed a retrospective case-control study involving 14,730 participants, using information from a national health care database, a national immunization registry, and 11 sexual health clinics covering diverse geographic regions. This included 1,241 cases of gonorrhea (cases), 12,487 cases of chlamydia (controls), and 1,002 cases of gonorrhea plus chlamydia coinfection (categorized as controls or cases in separate analyses).
“The adjusted estimate for vaccine effectiveness of the MeNZB against confirmed cases of gonorrhea” was 31% (95% confidence interval, 21-39; P less than .0001), a finding that remained robust across several sensitivity analyses, Dr. Petousis-Harris and her associates said (Lancet. 2017 July 10. doi: 10.1016/S0140-6736(17)31449-6).
“To our knowledge, ours is the first study to show an association between a vaccine and a reduction in the risk of gonorrhea,” they noted. “The potential ability of an OMV group B meningococcal vaccine to provide even modest protection against gonorrhea would have substantial public health benefits in view of the prevalence of gonorrhea. Modeling suggests that a vaccine with 30% efficacy could decrease the prevalence of gonorrhea by more than 30% within 15 years, if immunity is maintained.”
These findings also are important in view of the organism’s increasing resistance to existing antibiotics. Moreover, if further study confirms that the MeNZB vaccine offers some degree of cross-protection against gonorrhea, these data can inform the development of a gonorrhea vaccine, the investigators added.
This study was funded by GlaxoSmithKline Vaccines and Auckland UniServices. Dr. Petousis-Harris reported serving as a consultant for GSK, Merck, and Pfizer, and one of her associates reported ties to Novartis Vaccines, GSK, Protein Sciences, and Merck.
FROM THE LANCET
Key clinical point:
Major finding: The adjusted estimate for the effectiveness of the MeNZB vaccine against cases of gonorrhea was 31%.
Data source: A retrospective case-control study involving 14,730 patients at 11 sexual health clinics across New Zealand.
Disclosures: This study was funded by GlaxoSmithKline Vaccines and Auckland UniServices. Dr. Petousis-Harris reported serving as a consultant for GSK, Merck, and Pfizer, and one of her associates reported ties to Novartis Vaccines, GSK, Protein Sciences, and Merck.
Vaccination does not eliminate risk for meningococcal disease in eculizumab recipients
Patients taking eculizumab are at a significant risk for meningococcal disease even if they have received the quadrivalent meningococcal conjugate (MenACWY) and serogroup B (MenB) meningococcal vaccines, according to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report, released July 7.
Between 2008 and 2016, 16 cases of meningococcal disease were reported in eculizumab users in 10 jurisdictions within the United States. Of those infected, 14 had received MenACWY and MenB vaccines as recommended by the Advisory Committee on Immunization Practices, according to the CDC report.
Required vaccination plus antimicrobial prophylaxis for the duration of eculizumab treatment might reduce the risk for meningococcal disease in these patients, but the addition of antibiotic prophylaxis is no guarantee that all cases of meningococcal disease would be prevented, wrote Lucy A. McNamara, PhD, of the division of bacterial diseases, National Center for Immunization and Respiratory Diseases, CDC, and her colleagues.
They advised physician and patient vigilance regarding meningococcal disease symptoms and urged that patients be advised to seek immediate care and be rapidly treated, regardless of meningococcal vaccination or antimicrobial prophylaxis status.
Health organizations in Europe, including France and the United Kingdom, are recommending eculizumab users receive penicillin during eculizumab treatment. A recent study of invasive meningococcal isolates in the United States found most were susceptible to penicillin, according to the report.
In the 16 U.S. cases reported, nongroupable Neisseria meningitidis caused meningococcal disease in 11 of the patients, serogroup Y was the cause in 4 patients, and the cause was not identified in 1 patient.
Ten patients had meningococcemia without meningitis, the researchers noted. “Initial symptoms of meningococcemia are often relatively mild and nonspecific and might include fever, chills, fatigue, vomiting, diarrhea, and aches or pains in the muscles, joints, chest, or abdomen; however, these symptoms can progress to severe illness and death within hours.”
Eculizumab (Soliris, Alexion Pharmaceuticals) is licensed in the United States for treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome, two diseases that are rare and can be fatal.
Eculizumab is associated with a 1,000-fold to 2,000-fold increased incidence of meningococcal disease among persons receiving the drug. The Food and Drug Administration–approved prescribing information includes a boxed warning regarding increased risk for meningococcal disease.
The CDC is collecting reports from state health departments for further analysis of the risk among eculizumab recipients.
The researchers reported having no conflicts of interest.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
Patients taking eculizumab are at a significant risk for meningococcal disease even if they have received the quadrivalent meningococcal conjugate (MenACWY) and serogroup B (MenB) meningococcal vaccines, according to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report, released July 7.
Between 2008 and 2016, 16 cases of meningococcal disease were reported in eculizumab users in 10 jurisdictions within the United States. Of those infected, 14 had received MenACWY and MenB vaccines as recommended by the Advisory Committee on Immunization Practices, according to the CDC report.
Required vaccination plus antimicrobial prophylaxis for the duration of eculizumab treatment might reduce the risk for meningococcal disease in these patients, but the addition of antibiotic prophylaxis is no guarantee that all cases of meningococcal disease would be prevented, wrote Lucy A. McNamara, PhD, of the division of bacterial diseases, National Center for Immunization and Respiratory Diseases, CDC, and her colleagues.
They advised physician and patient vigilance regarding meningococcal disease symptoms and urged that patients be advised to seek immediate care and be rapidly treated, regardless of meningococcal vaccination or antimicrobial prophylaxis status.
Health organizations in Europe, including France and the United Kingdom, are recommending eculizumab users receive penicillin during eculizumab treatment. A recent study of invasive meningococcal isolates in the United States found most were susceptible to penicillin, according to the report.
In the 16 U.S. cases reported, nongroupable Neisseria meningitidis caused meningococcal disease in 11 of the patients, serogroup Y was the cause in 4 patients, and the cause was not identified in 1 patient.
Ten patients had meningococcemia without meningitis, the researchers noted. “Initial symptoms of meningococcemia are often relatively mild and nonspecific and might include fever, chills, fatigue, vomiting, diarrhea, and aches or pains in the muscles, joints, chest, or abdomen; however, these symptoms can progress to severe illness and death within hours.”
Eculizumab (Soliris, Alexion Pharmaceuticals) is licensed in the United States for treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome, two diseases that are rare and can be fatal.
Eculizumab is associated with a 1,000-fold to 2,000-fold increased incidence of meningococcal disease among persons receiving the drug. The Food and Drug Administration–approved prescribing information includes a boxed warning regarding increased risk for meningococcal disease.
The CDC is collecting reports from state health departments for further analysis of the risk among eculizumab recipients.
The researchers reported having no conflicts of interest.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
Patients taking eculizumab are at a significant risk for meningococcal disease even if they have received the quadrivalent meningococcal conjugate (MenACWY) and serogroup B (MenB) meningococcal vaccines, according to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report, released July 7.
Between 2008 and 2016, 16 cases of meningococcal disease were reported in eculizumab users in 10 jurisdictions within the United States. Of those infected, 14 had received MenACWY and MenB vaccines as recommended by the Advisory Committee on Immunization Practices, according to the CDC report.
Required vaccination plus antimicrobial prophylaxis for the duration of eculizumab treatment might reduce the risk for meningococcal disease in these patients, but the addition of antibiotic prophylaxis is no guarantee that all cases of meningococcal disease would be prevented, wrote Lucy A. McNamara, PhD, of the division of bacterial diseases, National Center for Immunization and Respiratory Diseases, CDC, and her colleagues.
They advised physician and patient vigilance regarding meningococcal disease symptoms and urged that patients be advised to seek immediate care and be rapidly treated, regardless of meningococcal vaccination or antimicrobial prophylaxis status.
Health organizations in Europe, including France and the United Kingdom, are recommending eculizumab users receive penicillin during eculizumab treatment. A recent study of invasive meningococcal isolates in the United States found most were susceptible to penicillin, according to the report.
In the 16 U.S. cases reported, nongroupable Neisseria meningitidis caused meningococcal disease in 11 of the patients, serogroup Y was the cause in 4 patients, and the cause was not identified in 1 patient.
Ten patients had meningococcemia without meningitis, the researchers noted. “Initial symptoms of meningococcemia are often relatively mild and nonspecific and might include fever, chills, fatigue, vomiting, diarrhea, and aches or pains in the muscles, joints, chest, or abdomen; however, these symptoms can progress to severe illness and death within hours.”
Eculizumab (Soliris, Alexion Pharmaceuticals) is licensed in the United States for treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome, two diseases that are rare and can be fatal.
Eculizumab is associated with a 1,000-fold to 2,000-fold increased incidence of meningococcal disease among persons receiving the drug. The Food and Drug Administration–approved prescribing information includes a boxed warning regarding increased risk for meningococcal disease.
The CDC is collecting reports from state health departments for further analysis of the risk among eculizumab recipients.
The researchers reported having no conflicts of interest.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
FROM MMWR
How to raise HPV vaccine rates: Work together
Despite widespread availability of the human papillomavirus vaccine over the last 11 years, vaccination rates continue to lag behind national targets and are far behind other vaccines routinely administered in adolescence, such as the meningococcal and tetanus vaccines.
Better collaboration among pediatricians and obstetrician-gynecologists to promote the HPV vaccine may be one answer to turning the tide, said David W. Kimberlin, MD, codirector of the division of pediatric infectious diseases at the University of Alabama at Birmingham and president of the Pediatric Infectious Diseases Society.
As of 2015, just 63% of eligible U.S. girls completed the first dose of the HPV vaccination, 52% completed two doses, and 42% finished the three-dose series, according to a recent “Call to Action” paper in the American Journal of Obstetrics and Gynecology (doi: 10.1016/j.ajog.2017.02.026). Although the HPV vaccine has been recommended for boys since 2011, just half of eligible boys completed the first dose, 39% completed two doses, and 28% finished the full series. By contrast, 86% of adolescents received the tetanus, diphtheria, and acellular pertussis vaccine, and 81% received the first dose of the meningococcal vaccine. The federal government’s Office of Disease Prevention and Health Promotion aims for an 80% HPV vaccination completion rate for girls and boys aged 13-15 years by 2020.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/how-to-raise-hpv-vaccine-rates?iframe=1"}]The CDC now recommends that 11- to 12-year-olds get two doses of the HPV vaccine, rather than three, with the second dose given 6-12 months after the first (MMWR. 2016;65:1405-8).
The common ways in which the HPV vaccine is introduced to parents likely contributes to the low vaccination rates, said Beth Auslander, PhD, a clinical psychologist and associate professor in the department of pediatrics at the University of Texas Medical Branch in Galveston. Some pediatricians may tell parents about school-mandated vaccines first and then as a side note, mention the HPV vaccine.
“The way it’s presented at times is being separate from the other vaccines,” Dr. Auslander said. “Sometimes it sounds optional.”
Parents often are uncertain about the safety and efficacy of the HPV vaccine, she added, and some wrongly assume the vaccine will lead to sexual activity among their children.
“Sometimes it can take a little longer to talk about,” Dr. Yoost said in an interview. “A lot of times, parents will bring up questions or concerns about the HPV vaccine. If physicians aren’t comfortable talking about those topics, they may not give the best recommendation. Pediatricians are not dealing with cervical cancer, so they may have a harder time recommending a vaccine based on outcomes they don’t deal with.”
Ob.gyns. are in a unique position to reach out to their pediatric counterparts and discuss strategies for catching more patients eligible for the HPV vaccine, said Sarah Dilley, MD, a gynecologic oncology fellow at the University of Alabama at Birmingham and the lead author of the recent Call to Action paper.
“We offer a unique perspective in that we are treating the conditions that the HPV vaccine is preventing, so we have more of a sense of urgency and an understanding of why that is so important,” Dr. Dilley said in an interview. “Obviously, pediatricians understand this as well, but it’s not something they see every day in their practice. We, as ob.gyns., have the opportunity to talk to our pediatric colleagues about the importance and really how devastating these conditions can be and how important it is to prevent them.”
In the recent paper, Dr. Dilley and her colleagues recommend that ob.gyns. speak to pediatricians and primary care physicians in their community to promote the vaccine and encourage them to view the Centers for Disease Control and Prevention’s You Are the Key presentation. The CDC resources include tips for how to discuss the burden of HPV-related diseases and effective communication with parents, an update on state vaccination rates, and the latest HPV vaccination recommendations.
Dr. Dilley encourages ob.gyns. and pediatricians to find different opportunities and venues to discuss the HPV vaccine. Ask about the pediatrician’s current approach to the vaccine, the doctor’s communication with parents, and how such practices could be improved, she said.
“People like to hear from their colleagues,” Dr. Dilley said. “Hearing from ob.gyns. [about] their experiences could be really helpful, whether it’s doing lunch and learns, formal education, grand rounds, or even more informal talks at the hospital.”
Ob.gyns. and pediatricians also need to better coordinate their messaging so that there is more consistent emphasis during each patient encounter about the need of the HPV vaccination, Dr. Kimberlin said. There needs to be a renewed focus on the vaccine as a cancer vaccination, he said.
“The nuances of HPV and the way that HPV is acquired, namely sexually transmitted, has taken too much of a front row consideration in the conversations that parents sometimes want to have with their child’s health care providers,” Dr. Kimberlin said. “We have to stress this is a cancer vaccine. This is a vaccine that prevents the deaths of thousands of women and men. We simply need to get that message out more forcefully.”
In addition, there’s a need for joint action to debunk myths about the vaccine and work toward eliminating the stigma surrounding it, Dr. Dilley said.
“I talk to a lot parents about the HPV vaccine and there’s so much misinformation online,” she said. “But a lot of patients do look at websites of their ob.gyn. or their pediatrician, [and] if they see something reputable coming from one of those sites, they might listen. We have a lot of patients who are mothers or grandmothers of kids; that’s also an opportunity for us to say, ‘Hey while we’re screening you for cervical cancer, let’s talk about the HPV vaccine.’ That’s a really good opportunity to help our [pediatric] colleagues out.”
5 steps to increase HPV vaccination
Melissa Kottke, MD, director of the Jane Fonda Center for Adolescent Reproductive Health at Emory University offered her practice steps for increased HPV vaccination rates.
1. Be clear about your recommendation. For example, “I recommend the HPV vaccine. It can help prevent cancer.”
3. Educate the entire clinical team (front desk staff, nursing, medical assistants, etc.) about the HPV vaccine so there is consistent messaging and delivery.
4. Establish streamlined systems. The vaccine recommendation, order, and follow-up should be streamlined and automated, if possible. Systems should also ensure documentation of vaccine receipt.
5. Make time for conversations with patients who are mothers and grandmothers. Recommend the HPV vaccine for males and females aged 9-26 years old. Encourage parents/grandparents to follow-up with the child’s doctor or offer to provide the vaccine in your office.
*This story was updated 8/22/2017.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Despite widespread availability of the human papillomavirus vaccine over the last 11 years, vaccination rates continue to lag behind national targets and are far behind other vaccines routinely administered in adolescence, such as the meningococcal and tetanus vaccines.
Better collaboration among pediatricians and obstetrician-gynecologists to promote the HPV vaccine may be one answer to turning the tide, said David W. Kimberlin, MD, codirector of the division of pediatric infectious diseases at the University of Alabama at Birmingham and president of the Pediatric Infectious Diseases Society.
As of 2015, just 63% of eligible U.S. girls completed the first dose of the HPV vaccination, 52% completed two doses, and 42% finished the three-dose series, according to a recent “Call to Action” paper in the American Journal of Obstetrics and Gynecology (doi: 10.1016/j.ajog.2017.02.026). Although the HPV vaccine has been recommended for boys since 2011, just half of eligible boys completed the first dose, 39% completed two doses, and 28% finished the full series. By contrast, 86% of adolescents received the tetanus, diphtheria, and acellular pertussis vaccine, and 81% received the first dose of the meningococcal vaccine. The federal government’s Office of Disease Prevention and Health Promotion aims for an 80% HPV vaccination completion rate for girls and boys aged 13-15 years by 2020.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/how-to-raise-hpv-vaccine-rates?iframe=1"}]The CDC now recommends that 11- to 12-year-olds get two doses of the HPV vaccine, rather than three, with the second dose given 6-12 months after the first (MMWR. 2016;65:1405-8).
The common ways in which the HPV vaccine is introduced to parents likely contributes to the low vaccination rates, said Beth Auslander, PhD, a clinical psychologist and associate professor in the department of pediatrics at the University of Texas Medical Branch in Galveston. Some pediatricians may tell parents about school-mandated vaccines first and then as a side note, mention the HPV vaccine.
“The way it’s presented at times is being separate from the other vaccines,” Dr. Auslander said. “Sometimes it sounds optional.”
Parents often are uncertain about the safety and efficacy of the HPV vaccine, she added, and some wrongly assume the vaccine will lead to sexual activity among their children.
“Sometimes it can take a little longer to talk about,” Dr. Yoost said in an interview. “A lot of times, parents will bring up questions or concerns about the HPV vaccine. If physicians aren’t comfortable talking about those topics, they may not give the best recommendation. Pediatricians are not dealing with cervical cancer, so they may have a harder time recommending a vaccine based on outcomes they don’t deal with.”
Ob.gyns. are in a unique position to reach out to their pediatric counterparts and discuss strategies for catching more patients eligible for the HPV vaccine, said Sarah Dilley, MD, a gynecologic oncology fellow at the University of Alabama at Birmingham and the lead author of the recent Call to Action paper.
“We offer a unique perspective in that we are treating the conditions that the HPV vaccine is preventing, so we have more of a sense of urgency and an understanding of why that is so important,” Dr. Dilley said in an interview. “Obviously, pediatricians understand this as well, but it’s not something they see every day in their practice. We, as ob.gyns., have the opportunity to talk to our pediatric colleagues about the importance and really how devastating these conditions can be and how important it is to prevent them.”
In the recent paper, Dr. Dilley and her colleagues recommend that ob.gyns. speak to pediatricians and primary care physicians in their community to promote the vaccine and encourage them to view the Centers for Disease Control and Prevention’s You Are the Key presentation. The CDC resources include tips for how to discuss the burden of HPV-related diseases and effective communication with parents, an update on state vaccination rates, and the latest HPV vaccination recommendations.
Dr. Dilley encourages ob.gyns. and pediatricians to find different opportunities and venues to discuss the HPV vaccine. Ask about the pediatrician’s current approach to the vaccine, the doctor’s communication with parents, and how such practices could be improved, she said.
“People like to hear from their colleagues,” Dr. Dilley said. “Hearing from ob.gyns. [about] their experiences could be really helpful, whether it’s doing lunch and learns, formal education, grand rounds, or even more informal talks at the hospital.”
Ob.gyns. and pediatricians also need to better coordinate their messaging so that there is more consistent emphasis during each patient encounter about the need of the HPV vaccination, Dr. Kimberlin said. There needs to be a renewed focus on the vaccine as a cancer vaccination, he said.
“The nuances of HPV and the way that HPV is acquired, namely sexually transmitted, has taken too much of a front row consideration in the conversations that parents sometimes want to have with their child’s health care providers,” Dr. Kimberlin said. “We have to stress this is a cancer vaccine. This is a vaccine that prevents the deaths of thousands of women and men. We simply need to get that message out more forcefully.”
In addition, there’s a need for joint action to debunk myths about the vaccine and work toward eliminating the stigma surrounding it, Dr. Dilley said.
“I talk to a lot parents about the HPV vaccine and there’s so much misinformation online,” she said. “But a lot of patients do look at websites of their ob.gyn. or their pediatrician, [and] if they see something reputable coming from one of those sites, they might listen. We have a lot of patients who are mothers or grandmothers of kids; that’s also an opportunity for us to say, ‘Hey while we’re screening you for cervical cancer, let’s talk about the HPV vaccine.’ That’s a really good opportunity to help our [pediatric] colleagues out.”
5 steps to increase HPV vaccination
Melissa Kottke, MD, director of the Jane Fonda Center for Adolescent Reproductive Health at Emory University offered her practice steps for increased HPV vaccination rates.
1. Be clear about your recommendation. For example, “I recommend the HPV vaccine. It can help prevent cancer.”
3. Educate the entire clinical team (front desk staff, nursing, medical assistants, etc.) about the HPV vaccine so there is consistent messaging and delivery.
4. Establish streamlined systems. The vaccine recommendation, order, and follow-up should be streamlined and automated, if possible. Systems should also ensure documentation of vaccine receipt.
5. Make time for conversations with patients who are mothers and grandmothers. Recommend the HPV vaccine for males and females aged 9-26 years old. Encourage parents/grandparents to follow-up with the child’s doctor or offer to provide the vaccine in your office.
*This story was updated 8/22/2017.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Despite widespread availability of the human papillomavirus vaccine over the last 11 years, vaccination rates continue to lag behind national targets and are far behind other vaccines routinely administered in adolescence, such as the meningococcal and tetanus vaccines.
Better collaboration among pediatricians and obstetrician-gynecologists to promote the HPV vaccine may be one answer to turning the tide, said David W. Kimberlin, MD, codirector of the division of pediatric infectious diseases at the University of Alabama at Birmingham and president of the Pediatric Infectious Diseases Society.
As of 2015, just 63% of eligible U.S. girls completed the first dose of the HPV vaccination, 52% completed two doses, and 42% finished the three-dose series, according to a recent “Call to Action” paper in the American Journal of Obstetrics and Gynecology (doi: 10.1016/j.ajog.2017.02.026). Although the HPV vaccine has been recommended for boys since 2011, just half of eligible boys completed the first dose, 39% completed two doses, and 28% finished the full series. By contrast, 86% of adolescents received the tetanus, diphtheria, and acellular pertussis vaccine, and 81% received the first dose of the meningococcal vaccine. The federal government’s Office of Disease Prevention and Health Promotion aims for an 80% HPV vaccination completion rate for girls and boys aged 13-15 years by 2020.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/how-to-raise-hpv-vaccine-rates?iframe=1"}]The CDC now recommends that 11- to 12-year-olds get two doses of the HPV vaccine, rather than three, with the second dose given 6-12 months after the first (MMWR. 2016;65:1405-8).
The common ways in which the HPV vaccine is introduced to parents likely contributes to the low vaccination rates, said Beth Auslander, PhD, a clinical psychologist and associate professor in the department of pediatrics at the University of Texas Medical Branch in Galveston. Some pediatricians may tell parents about school-mandated vaccines first and then as a side note, mention the HPV vaccine.
“The way it’s presented at times is being separate from the other vaccines,” Dr. Auslander said. “Sometimes it sounds optional.”
Parents often are uncertain about the safety and efficacy of the HPV vaccine, she added, and some wrongly assume the vaccine will lead to sexual activity among their children.
“Sometimes it can take a little longer to talk about,” Dr. Yoost said in an interview. “A lot of times, parents will bring up questions or concerns about the HPV vaccine. If physicians aren’t comfortable talking about those topics, they may not give the best recommendation. Pediatricians are not dealing with cervical cancer, so they may have a harder time recommending a vaccine based on outcomes they don’t deal with.”
Ob.gyns. are in a unique position to reach out to their pediatric counterparts and discuss strategies for catching more patients eligible for the HPV vaccine, said Sarah Dilley, MD, a gynecologic oncology fellow at the University of Alabama at Birmingham and the lead author of the recent Call to Action paper.
“We offer a unique perspective in that we are treating the conditions that the HPV vaccine is preventing, so we have more of a sense of urgency and an understanding of why that is so important,” Dr. Dilley said in an interview. “Obviously, pediatricians understand this as well, but it’s not something they see every day in their practice. We, as ob.gyns., have the opportunity to talk to our pediatric colleagues about the importance and really how devastating these conditions can be and how important it is to prevent them.”
In the recent paper, Dr. Dilley and her colleagues recommend that ob.gyns. speak to pediatricians and primary care physicians in their community to promote the vaccine and encourage them to view the Centers for Disease Control and Prevention’s You Are the Key presentation. The CDC resources include tips for how to discuss the burden of HPV-related diseases and effective communication with parents, an update on state vaccination rates, and the latest HPV vaccination recommendations.
Dr. Dilley encourages ob.gyns. and pediatricians to find different opportunities and venues to discuss the HPV vaccine. Ask about the pediatrician’s current approach to the vaccine, the doctor’s communication with parents, and how such practices could be improved, she said.
“People like to hear from their colleagues,” Dr. Dilley said. “Hearing from ob.gyns. [about] their experiences could be really helpful, whether it’s doing lunch and learns, formal education, grand rounds, or even more informal talks at the hospital.”
Ob.gyns. and pediatricians also need to better coordinate their messaging so that there is more consistent emphasis during each patient encounter about the need of the HPV vaccination, Dr. Kimberlin said. There needs to be a renewed focus on the vaccine as a cancer vaccination, he said.
“The nuances of HPV and the way that HPV is acquired, namely sexually transmitted, has taken too much of a front row consideration in the conversations that parents sometimes want to have with their child’s health care providers,” Dr. Kimberlin said. “We have to stress this is a cancer vaccine. This is a vaccine that prevents the deaths of thousands of women and men. We simply need to get that message out more forcefully.”
In addition, there’s a need for joint action to debunk myths about the vaccine and work toward eliminating the stigma surrounding it, Dr. Dilley said.
“I talk to a lot parents about the HPV vaccine and there’s so much misinformation online,” she said. “But a lot of patients do look at websites of their ob.gyn. or their pediatrician, [and] if they see something reputable coming from one of those sites, they might listen. We have a lot of patients who are mothers or grandmothers of kids; that’s also an opportunity for us to say, ‘Hey while we’re screening you for cervical cancer, let’s talk about the HPV vaccine.’ That’s a really good opportunity to help our [pediatric] colleagues out.”
5 steps to increase HPV vaccination
Melissa Kottke, MD, director of the Jane Fonda Center for Adolescent Reproductive Health at Emory University offered her practice steps for increased HPV vaccination rates.
1. Be clear about your recommendation. For example, “I recommend the HPV vaccine. It can help prevent cancer.”
3. Educate the entire clinical team (front desk staff, nursing, medical assistants, etc.) about the HPV vaccine so there is consistent messaging and delivery.
4. Establish streamlined systems. The vaccine recommendation, order, and follow-up should be streamlined and automated, if possible. Systems should also ensure documentation of vaccine receipt.
5. Make time for conversations with patients who are mothers and grandmothers. Recommend the HPV vaccine for males and females aged 9-26 years old. Encourage parents/grandparents to follow-up with the child’s doctor or offer to provide the vaccine in your office.
*This story was updated 8/22/2017.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Measles: Why it’s still a threat
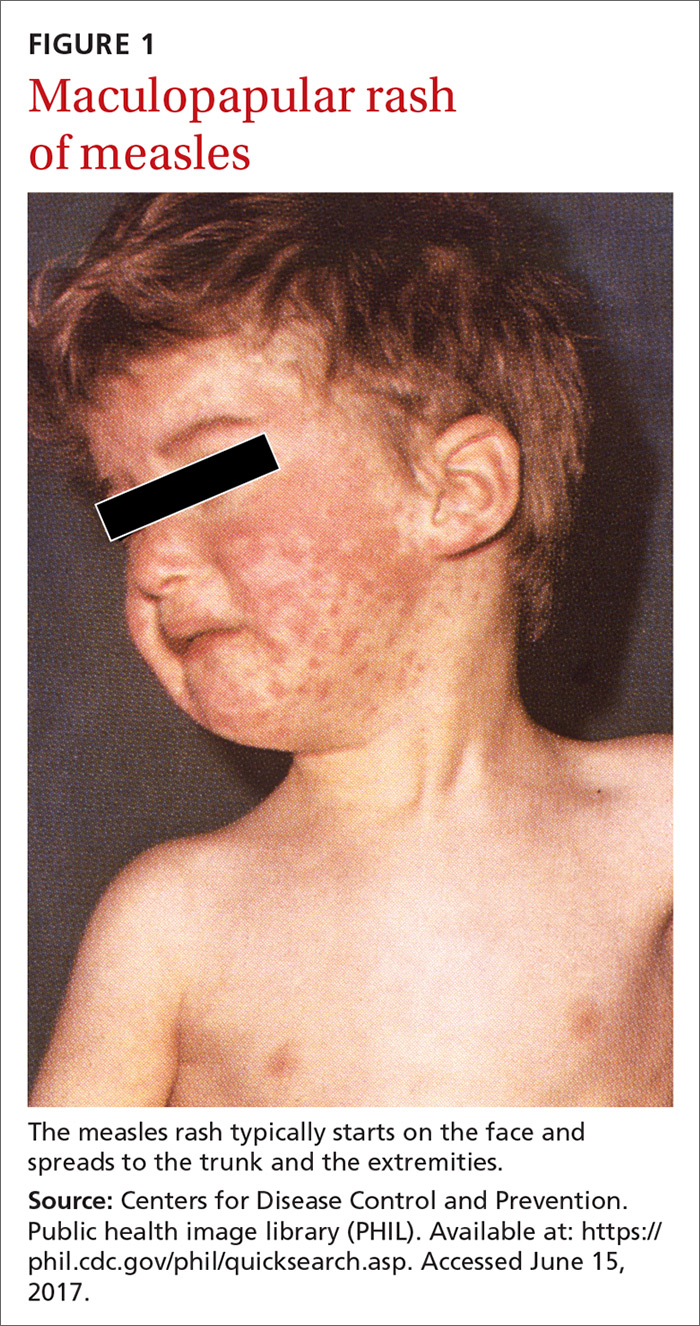
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
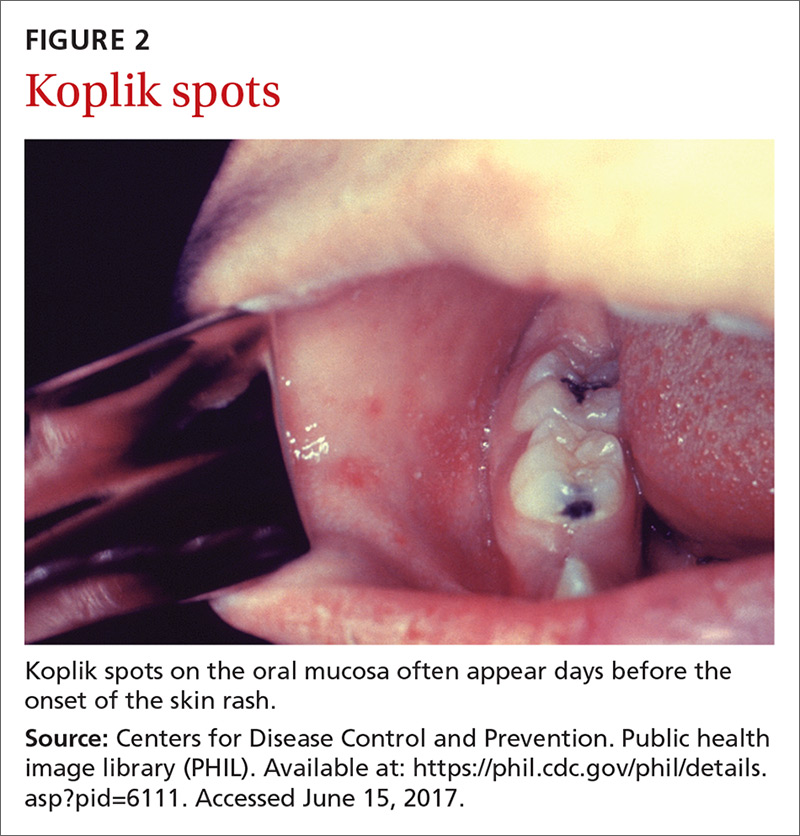
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3
Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3
Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3
Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
Understanding the human papillomavirus
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
Childhood vaccine trauma decreases adolescent HPV immunization uptake
The more vaccines children get at any one office visit between the ages of 4 and 6 years, the more fearful they are of needles later on and the less likely they are to start human papillomavirus vaccine (HPV) as adolescents, according to an investigation of 120 children.
Shot-stacking between ages 4 and 6 years is not uncommon, especially if children might not be back in the office any time soon. Although it’s convenient and often better reimbursed to give all the recommended 4- to 6-year-old shots – MMR, DTaP, varicella, IPV, and maybe flu and hepatitis B vaccines – in one visit, the investigators found a hidden cost.
“We don’t need to change the vaccine schedule,” but we do have to be careful because 4- to 6-year-olds are especially vulnerable to phobias. “I think the best thing would be to give one vaccine at age 4, two at age 5, and two at age 6,” years, said lead investigator and pediatric emergency physician Amy Baxter, MD, a clinical associate professor at the Medical College of Georgia, Augusta.
“I’ve dealt with needle phobia in the ED. These kids come in and they are already freaked out about needles. If they get diagnosed with diabetes or leukemia, parents don’t think they are going to be able to handle it,” she said in an interview.
Expanded school vaccination programs might help by making it more convenient for parents to space out shots. The development of patch, microneedle, or effective sublingual or intranasal options also would help.
Dr. Baxter and her colleagues asked 120 children aged 10 to 12 years old to rate their fear of needles on a visual analogue scale (VAS), from 0 points meaning no fear to 100. The investigators matched the scores against the children’s immunization records (Vaccine. 2017 Jun 20. doi: 10.1016/j.vaccine.2017.06.029).
There was no significant relationship between needle fear and the total number of injections. However, fear did correlate with the number of shots children received in 1 day from ages 4 to 6 years old. Six children (50%) who had four same-day injections during that period scored in the upper quartile of anxiety as adolescents (VAS greater than 83), versus 22 (27%) who had three, and 2 (10%) who had two. None of the children who had just one shot per office visit as preschoolers were very worried about needles (P = .0387).
The investigators found that the likelihood of being in the upper quartile of fear as adolescents increased with each additional same-day injection between the ages of 4 and 6 years (odds ratio, 3.108; 95% confidence interval, 1.311-7.367; P = .01).
The team checked back with the children a few years later to see who had started HPV immunizations by age 14 years. Just 27% of children in the upper quartile of needle fear had done so, versus 48% in the least anxious quartile (VAS less than 32). The study wasn’t powered to detect a statistically significant difference in HPV vaccine uptake, but decreased uptake with higher needle fear came close (OR, 2.57; 95% CI, 0.864-7.621; P = .0889).
“It’s the stacking that causes fear. There was no difference in the uptake of vaccines when they were spread out, but more shots” at one time “causes more distress, so there was a difference in fear 5 years later,” Dr. Baxter said.
The investigators also assessed parental concerns about immunizations. “What was interesting was that whether the parents were in the upper or lower quartile of anxiety didn’t impact HPV initiation at all. The children’s anxiety 2 years earlier seemed much more relevant,” Dr. Baxter said.
In a literature review of 15 studies from 1958-2016 that included over 8,000 subjects, the investigators also found that needle fear tracked neatly with the sixfold increase in scheduled vaccines since the 1970s, with more than 60% of people now endorsing some degree of injection anxiety – more than ever before. “The curve of increasing needle fear correlated strongly with increasing vaccine number,” they found (Kendall’s tau b = 0.747; 95% CI, 0.513-0.982; P = .0003).
As two-thirds of the literature review sample were adults, “these results imply that fear acquired in childhood persists,” they concluded.
The majority of children in the study were white. Their mothers were a mean of 44 years, with a mean education level of 18 years. Demographic differences didn’t affect needle fear.
Dr. Baxter is a pediatric pain researcher and the inventor of Buzzy, a bumblebee shaped vibrator to distract children and relieve pain during shots. When asked if readers could trust her study findings given her interest in selling the device, she noted that, in a previous study, “Buzzy for 15 seconds did not work well for 4 to 6 year olds” getting multiple injections at one office visit. “Buzzy is not the solution for giving four shots at once.”
The more vaccines children get at any one office visit between the ages of 4 and 6 years, the more fearful they are of needles later on and the less likely they are to start human papillomavirus vaccine (HPV) as adolescents, according to an investigation of 120 children.
Shot-stacking between ages 4 and 6 years is not uncommon, especially if children might not be back in the office any time soon. Although it’s convenient and often better reimbursed to give all the recommended 4- to 6-year-old shots – MMR, DTaP, varicella, IPV, and maybe flu and hepatitis B vaccines – in one visit, the investigators found a hidden cost.
“We don’t need to change the vaccine schedule,” but we do have to be careful because 4- to 6-year-olds are especially vulnerable to phobias. “I think the best thing would be to give one vaccine at age 4, two at age 5, and two at age 6,” years, said lead investigator and pediatric emergency physician Amy Baxter, MD, a clinical associate professor at the Medical College of Georgia, Augusta.
“I’ve dealt with needle phobia in the ED. These kids come in and they are already freaked out about needles. If they get diagnosed with diabetes or leukemia, parents don’t think they are going to be able to handle it,” she said in an interview.
Expanded school vaccination programs might help by making it more convenient for parents to space out shots. The development of patch, microneedle, or effective sublingual or intranasal options also would help.
Dr. Baxter and her colleagues asked 120 children aged 10 to 12 years old to rate their fear of needles on a visual analogue scale (VAS), from 0 points meaning no fear to 100. The investigators matched the scores against the children’s immunization records (Vaccine. 2017 Jun 20. doi: 10.1016/j.vaccine.2017.06.029).
There was no significant relationship between needle fear and the total number of injections. However, fear did correlate with the number of shots children received in 1 day from ages 4 to 6 years old. Six children (50%) who had four same-day injections during that period scored in the upper quartile of anxiety as adolescents (VAS greater than 83), versus 22 (27%) who had three, and 2 (10%) who had two. None of the children who had just one shot per office visit as preschoolers were very worried about needles (P = .0387).
The investigators found that the likelihood of being in the upper quartile of fear as adolescents increased with each additional same-day injection between the ages of 4 and 6 years (odds ratio, 3.108; 95% confidence interval, 1.311-7.367; P = .01).
The team checked back with the children a few years later to see who had started HPV immunizations by age 14 years. Just 27% of children in the upper quartile of needle fear had done so, versus 48% in the least anxious quartile (VAS less than 32). The study wasn’t powered to detect a statistically significant difference in HPV vaccine uptake, but decreased uptake with higher needle fear came close (OR, 2.57; 95% CI, 0.864-7.621; P = .0889).
“It’s the stacking that causes fear. There was no difference in the uptake of vaccines when they were spread out, but more shots” at one time “causes more distress, so there was a difference in fear 5 years later,” Dr. Baxter said.
The investigators also assessed parental concerns about immunizations. “What was interesting was that whether the parents were in the upper or lower quartile of anxiety didn’t impact HPV initiation at all. The children’s anxiety 2 years earlier seemed much more relevant,” Dr. Baxter said.
In a literature review of 15 studies from 1958-2016 that included over 8,000 subjects, the investigators also found that needle fear tracked neatly with the sixfold increase in scheduled vaccines since the 1970s, with more than 60% of people now endorsing some degree of injection anxiety – more than ever before. “The curve of increasing needle fear correlated strongly with increasing vaccine number,” they found (Kendall’s tau b = 0.747; 95% CI, 0.513-0.982; P = .0003).
As two-thirds of the literature review sample were adults, “these results imply that fear acquired in childhood persists,” they concluded.
The majority of children in the study were white. Their mothers were a mean of 44 years, with a mean education level of 18 years. Demographic differences didn’t affect needle fear.
Dr. Baxter is a pediatric pain researcher and the inventor of Buzzy, a bumblebee shaped vibrator to distract children and relieve pain during shots. When asked if readers could trust her study findings given her interest in selling the device, she noted that, in a previous study, “Buzzy for 15 seconds did not work well for 4 to 6 year olds” getting multiple injections at one office visit. “Buzzy is not the solution for giving four shots at once.”
The more vaccines children get at any one office visit between the ages of 4 and 6 years, the more fearful they are of needles later on and the less likely they are to start human papillomavirus vaccine (HPV) as adolescents, according to an investigation of 120 children.
Shot-stacking between ages 4 and 6 years is not uncommon, especially if children might not be back in the office any time soon. Although it’s convenient and often better reimbursed to give all the recommended 4- to 6-year-old shots – MMR, DTaP, varicella, IPV, and maybe flu and hepatitis B vaccines – in one visit, the investigators found a hidden cost.
“We don’t need to change the vaccine schedule,” but we do have to be careful because 4- to 6-year-olds are especially vulnerable to phobias. “I think the best thing would be to give one vaccine at age 4, two at age 5, and two at age 6,” years, said lead investigator and pediatric emergency physician Amy Baxter, MD, a clinical associate professor at the Medical College of Georgia, Augusta.
“I’ve dealt with needle phobia in the ED. These kids come in and they are already freaked out about needles. If they get diagnosed with diabetes or leukemia, parents don’t think they are going to be able to handle it,” she said in an interview.
Expanded school vaccination programs might help by making it more convenient for parents to space out shots. The development of patch, microneedle, or effective sublingual or intranasal options also would help.
Dr. Baxter and her colleagues asked 120 children aged 10 to 12 years old to rate their fear of needles on a visual analogue scale (VAS), from 0 points meaning no fear to 100. The investigators matched the scores against the children’s immunization records (Vaccine. 2017 Jun 20. doi: 10.1016/j.vaccine.2017.06.029).
There was no significant relationship between needle fear and the total number of injections. However, fear did correlate with the number of shots children received in 1 day from ages 4 to 6 years old. Six children (50%) who had four same-day injections during that period scored in the upper quartile of anxiety as adolescents (VAS greater than 83), versus 22 (27%) who had three, and 2 (10%) who had two. None of the children who had just one shot per office visit as preschoolers were very worried about needles (P = .0387).
The investigators found that the likelihood of being in the upper quartile of fear as adolescents increased with each additional same-day injection between the ages of 4 and 6 years (odds ratio, 3.108; 95% confidence interval, 1.311-7.367; P = .01).
The team checked back with the children a few years later to see who had started HPV immunizations by age 14 years. Just 27% of children in the upper quartile of needle fear had done so, versus 48% in the least anxious quartile (VAS less than 32). The study wasn’t powered to detect a statistically significant difference in HPV vaccine uptake, but decreased uptake with higher needle fear came close (OR, 2.57; 95% CI, 0.864-7.621; P = .0889).
“It’s the stacking that causes fear. There was no difference in the uptake of vaccines when they were spread out, but more shots” at one time “causes more distress, so there was a difference in fear 5 years later,” Dr. Baxter said.
The investigators also assessed parental concerns about immunizations. “What was interesting was that whether the parents were in the upper or lower quartile of anxiety didn’t impact HPV initiation at all. The children’s anxiety 2 years earlier seemed much more relevant,” Dr. Baxter said.
In a literature review of 15 studies from 1958-2016 that included over 8,000 subjects, the investigators also found that needle fear tracked neatly with the sixfold increase in scheduled vaccines since the 1970s, with more than 60% of people now endorsing some degree of injection anxiety – more than ever before. “The curve of increasing needle fear correlated strongly with increasing vaccine number,” they found (Kendall’s tau b = 0.747; 95% CI, 0.513-0.982; P = .0003).
As two-thirds of the literature review sample were adults, “these results imply that fear acquired in childhood persists,” they concluded.
The majority of children in the study were white. Their mothers were a mean of 44 years, with a mean education level of 18 years. Demographic differences didn’t affect needle fear.
Dr. Baxter is a pediatric pain researcher and the inventor of Buzzy, a bumblebee shaped vibrator to distract children and relieve pain during shots. When asked if readers could trust her study findings given her interest in selling the device, she noted that, in a previous study, “Buzzy for 15 seconds did not work well for 4 to 6 year olds” getting multiple injections at one office visit. “Buzzy is not the solution for giving four shots at once.”
FROM VACCINE
Key clinical point:
Major finding: Six children (50%) who had four same-day injections during that period scored in the upper quartile of anxiety as adolescents (VAS greater than 83), versus 22 (27%) who had three, and 2 (10%) who had two same-day injections. Just 27% of children in the upper quartile of needle fear had started HPV immunization at age 14 years, versus 48% in the least anxious quartile.
Data source: Survey and follow-up of 120 children and their parents.
Disclosures: The lead investigator is a pediatric pain researcher and the inventor of Buzzy, a bumblebee shaped vibrator to distract children and relieve pain during shots. When asked if readers could trust her findings given her interest in selling the device, she noted that, in a previous study, “Buzzy for 15 seconds did not work well for 4-6 year olds” getting multiple injections in one office visit. “Buzzy is not the solution for giving four shots at once.”
Nurses help more rheumatic disease patients get vaccinated
MADRID – A nurse-led program successfully increased the uptake of pneumococcal vaccination among patients with chronic inflammatory rheumatic diseases in a single-center study.
From the start to the end of a 4-month evaluation period, the rate of vaccination of at-risk patients increased from 17.1% (13/76) to 77.6% (59/76; P less than .001).
It is well known that patients with inflammatory rheumatic diseases, such as systemic lupus erythematous (SLE) and systemic vasculitis, are at high risk for contracting pneumococcal disease, reported Tiphaine Goulenok, MD, of Bichat Hospital, Paris, at the European Congress of Rheumatology. This is particularly the case if they are receiving immunosuppressive treatments.
Although French national guidelines were introduced in 2011 that recommend that such patients routinely receive pneumococcal vaccination, the vaccination rate is often lower than is desirable, Dr. Goulenok observed at a press briefing. Her prior research suggests that only 16.2% of patients with an indication for the PCV13 vaccine actually received it.
In the current study, 126 patients with inflammatory rheumatic diseases were consecutively recruited and seen at the day unit of Bichat Hospital. Of these patients, 76 were candidates for pneumococcal vaccination because they were receiving steroids or other immunosuppressive drugs. Of these patients, 13 were already vaccinated, and, of the 63 who were not, nurses correctly identified 56 (88.9%) who needed to be vaccinated, of whom 46 agreed and 10 refused.
“We found a low rate of pneumococcal vaccination among patients,” said Dr. Goulenok, “but, thanks to high screening by the nurses, vaccination coverage was increased and the nurse-led vaccination program was very efficient”.
Robert Landewé, MD, who chaired the press briefing, observed that, despite being in an “era of guidelines,” ways of successfully implementing them in practice remained a challenge. The nurse-led program appeared to be one way to increase pneumococcal vaccination uptake, but perhaps other ways need to be sought, especially as there may be substantial resistance to vaccination among patients, he said.
“Patients are sometimes more afraid of the consequences of vaccination than [of] the disease that is prevented by vaccination,” said Dr. Landewé, who is professor of rheumatology at the Academic Medical Center in Amsterdam (the Netherlands).
Dr. Goulenok and Dr. Landewé reported no disclosures.
MADRID – A nurse-led program successfully increased the uptake of pneumococcal vaccination among patients with chronic inflammatory rheumatic diseases in a single-center study.
From the start to the end of a 4-month evaluation period, the rate of vaccination of at-risk patients increased from 17.1% (13/76) to 77.6% (59/76; P less than .001).
It is well known that patients with inflammatory rheumatic diseases, such as systemic lupus erythematous (SLE) and systemic vasculitis, are at high risk for contracting pneumococcal disease, reported Tiphaine Goulenok, MD, of Bichat Hospital, Paris, at the European Congress of Rheumatology. This is particularly the case if they are receiving immunosuppressive treatments.
Although French national guidelines were introduced in 2011 that recommend that such patients routinely receive pneumococcal vaccination, the vaccination rate is often lower than is desirable, Dr. Goulenok observed at a press briefing. Her prior research suggests that only 16.2% of patients with an indication for the PCV13 vaccine actually received it.
In the current study, 126 patients with inflammatory rheumatic diseases were consecutively recruited and seen at the day unit of Bichat Hospital. Of these patients, 76 were candidates for pneumococcal vaccination because they were receiving steroids or other immunosuppressive drugs. Of these patients, 13 were already vaccinated, and, of the 63 who were not, nurses correctly identified 56 (88.9%) who needed to be vaccinated, of whom 46 agreed and 10 refused.
“We found a low rate of pneumococcal vaccination among patients,” said Dr. Goulenok, “but, thanks to high screening by the nurses, vaccination coverage was increased and the nurse-led vaccination program was very efficient”.
Robert Landewé, MD, who chaired the press briefing, observed that, despite being in an “era of guidelines,” ways of successfully implementing them in practice remained a challenge. The nurse-led program appeared to be one way to increase pneumococcal vaccination uptake, but perhaps other ways need to be sought, especially as there may be substantial resistance to vaccination among patients, he said.
“Patients are sometimes more afraid of the consequences of vaccination than [of] the disease that is prevented by vaccination,” said Dr. Landewé, who is professor of rheumatology at the Academic Medical Center in Amsterdam (the Netherlands).
Dr. Goulenok and Dr. Landewé reported no disclosures.
MADRID – A nurse-led program successfully increased the uptake of pneumococcal vaccination among patients with chronic inflammatory rheumatic diseases in a single-center study.
From the start to the end of a 4-month evaluation period, the rate of vaccination of at-risk patients increased from 17.1% (13/76) to 77.6% (59/76; P less than .001).
It is well known that patients with inflammatory rheumatic diseases, such as systemic lupus erythematous (SLE) and systemic vasculitis, are at high risk for contracting pneumococcal disease, reported Tiphaine Goulenok, MD, of Bichat Hospital, Paris, at the European Congress of Rheumatology. This is particularly the case if they are receiving immunosuppressive treatments.
Although French national guidelines were introduced in 2011 that recommend that such patients routinely receive pneumococcal vaccination, the vaccination rate is often lower than is desirable, Dr. Goulenok observed at a press briefing. Her prior research suggests that only 16.2% of patients with an indication for the PCV13 vaccine actually received it.
In the current study, 126 patients with inflammatory rheumatic diseases were consecutively recruited and seen at the day unit of Bichat Hospital. Of these patients, 76 were candidates for pneumococcal vaccination because they were receiving steroids or other immunosuppressive drugs. Of these patients, 13 were already vaccinated, and, of the 63 who were not, nurses correctly identified 56 (88.9%) who needed to be vaccinated, of whom 46 agreed and 10 refused.
“We found a low rate of pneumococcal vaccination among patients,” said Dr. Goulenok, “but, thanks to high screening by the nurses, vaccination coverage was increased and the nurse-led vaccination program was very efficient”.
Robert Landewé, MD, who chaired the press briefing, observed that, despite being in an “era of guidelines,” ways of successfully implementing them in practice remained a challenge. The nurse-led program appeared to be one way to increase pneumococcal vaccination uptake, but perhaps other ways need to be sought, especially as there may be substantial resistance to vaccination among patients, he said.
“Patients are sometimes more afraid of the consequences of vaccination than [of] the disease that is prevented by vaccination,” said Dr. Landewé, who is professor of rheumatology at the Academic Medical Center in Amsterdam (the Netherlands).
Dr. Goulenok and Dr. Landewé reported no disclosures.
AT THE EULAR 2017 CONGRESS
Key clinical point: A nurse-led program increased the uptake of a guideline-recommended vaccination in patients with chronic inflammatory rheumatic diseases.
Major finding: The pre- and postintervention pneumococcal vaccination rates were 17.1% (13/76) and 77.6% (59/76) of at-risk patients (P less than .001).
Data source: A 4-month, prospective pilot study of 126 consecutively recruited patients with chronic inflammatory rheumatic diseases.
Disclosures: The presenter and commentator had no disclosures to report.
English infant quadravalent group B meningococcal vaccine pays off
MADRID – Cases of invasive meningococcal disease in English 1 year olds dropped fourfold in the first year after the multicomponent group B meningococcal (MenB) vaccine (4CMenB, Bexsero) was added to the national infant immunization list, Shamez Ladhani, MD, Ph.D, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In 1 year olds, we have seen a significant decrease in the number of cases, compared with the number of cases predicted. I suspect the vaccine effectiveness is close to 94% against meningococcal group B,” said Dr. Ladhani of Public Health England, London.
Meningococcal meningitis and septicemia remain the leading cause of death for children under 5 in the UK. Public awareness of this threat is high, so 4CMenB has been well accepted. Of eligible infants, 96% have received the first dose and 89% the second. The first cohort became available for the 12-month booster dose in May 2016.
Between May and December of 2016, the Public Health England intensive surveillance program confirmed six cases of invasive meningococcal disease. During the same time frame in 2015, there were 24 cases, whereas, during the 4 years prior to introduction of 4CMenB, the average was 18 cases per year.
Three of the six 1-year-olds had meningitis, and three had septicemia. One was admitted to an ICU. None died. None had comorbid conditions placing them at increased risk. Five of the six children with invasive meningococcal disease had received two doses of the vaccine, and one child became ill 3 months after receiving the third dose.
The vaccine is licensed to be given as a four-dose series: three primary doses plus one booster dose. UK health officials deemed that excessive and not cost effective. Based on data from the vaccine trials, they determined that three doses are sufficient. This cut the cost of the national program by 25% while maintaining protection (Lancet. 2016 Dec 3;388(10061):2775-82).
Pediatricians from other countries were extremely curious about this innovative immunization program. What about vaccine side effects in infants? they asked.
Dr. Ladhani replied that studies in Australia, Northern Ireland, and Scotland have all shown a small uptick in mild fever and irritability in the first 3 days after the first dose of 4CMenB. It’s less of an issue with the second dose.
“One dose is not protective. You need two. Parents understand that, while these side effects are annoying, the risks associated with meningitis are far greater. They are very, very accepting of the vaccine,” according to Dr. Ladhani.
What about using 4CMenB in teenagers? physicians asked.
Dr. Ladhani predicted that the infant immunization program will have zero effect in adolescents. Teens are the main carriers of meningococcal bacteria. So, in theory, vaccinating them could not only protect the adolescents themselves but could benefit the whole population through herd immunity.
“The problem is we don’t have solid evidence that Bexsero protects against carriage. A massive carriage study is underway in Australia. It should provide data in 2 or 3 years. If we do see a carriage effect, then vaccinating teenagers becomes a very attractive option because you protect them and others around them,” he explained.
Dr. Ladhani reported having no financial conflicts regarding his study, funded by Public Health England.
MADRID – Cases of invasive meningococcal disease in English 1 year olds dropped fourfold in the first year after the multicomponent group B meningococcal (MenB) vaccine (4CMenB, Bexsero) was added to the national infant immunization list, Shamez Ladhani, MD, Ph.D, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In 1 year olds, we have seen a significant decrease in the number of cases, compared with the number of cases predicted. I suspect the vaccine effectiveness is close to 94% against meningococcal group B,” said Dr. Ladhani of Public Health England, London.
Meningococcal meningitis and septicemia remain the leading cause of death for children under 5 in the UK. Public awareness of this threat is high, so 4CMenB has been well accepted. Of eligible infants, 96% have received the first dose and 89% the second. The first cohort became available for the 12-month booster dose in May 2016.
Between May and December of 2016, the Public Health England intensive surveillance program confirmed six cases of invasive meningococcal disease. During the same time frame in 2015, there were 24 cases, whereas, during the 4 years prior to introduction of 4CMenB, the average was 18 cases per year.
Three of the six 1-year-olds had meningitis, and three had septicemia. One was admitted to an ICU. None died. None had comorbid conditions placing them at increased risk. Five of the six children with invasive meningococcal disease had received two doses of the vaccine, and one child became ill 3 months after receiving the third dose.
The vaccine is licensed to be given as a four-dose series: three primary doses plus one booster dose. UK health officials deemed that excessive and not cost effective. Based on data from the vaccine trials, they determined that three doses are sufficient. This cut the cost of the national program by 25% while maintaining protection (Lancet. 2016 Dec 3;388(10061):2775-82).
Pediatricians from other countries were extremely curious about this innovative immunization program. What about vaccine side effects in infants? they asked.
Dr. Ladhani replied that studies in Australia, Northern Ireland, and Scotland have all shown a small uptick in mild fever and irritability in the first 3 days after the first dose of 4CMenB. It’s less of an issue with the second dose.
“One dose is not protective. You need two. Parents understand that, while these side effects are annoying, the risks associated with meningitis are far greater. They are very, very accepting of the vaccine,” according to Dr. Ladhani.
What about using 4CMenB in teenagers? physicians asked.
Dr. Ladhani predicted that the infant immunization program will have zero effect in adolescents. Teens are the main carriers of meningococcal bacteria. So, in theory, vaccinating them could not only protect the adolescents themselves but could benefit the whole population through herd immunity.
“The problem is we don’t have solid evidence that Bexsero protects against carriage. A massive carriage study is underway in Australia. It should provide data in 2 or 3 years. If we do see a carriage effect, then vaccinating teenagers becomes a very attractive option because you protect them and others around them,” he explained.
Dr. Ladhani reported having no financial conflicts regarding his study, funded by Public Health England.
MADRID – Cases of invasive meningococcal disease in English 1 year olds dropped fourfold in the first year after the multicomponent group B meningococcal (MenB) vaccine (4CMenB, Bexsero) was added to the national infant immunization list, Shamez Ladhani, MD, Ph.D, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In 1 year olds, we have seen a significant decrease in the number of cases, compared with the number of cases predicted. I suspect the vaccine effectiveness is close to 94% against meningococcal group B,” said Dr. Ladhani of Public Health England, London.
Meningococcal meningitis and septicemia remain the leading cause of death for children under 5 in the UK. Public awareness of this threat is high, so 4CMenB has been well accepted. Of eligible infants, 96% have received the first dose and 89% the second. The first cohort became available for the 12-month booster dose in May 2016.
Between May and December of 2016, the Public Health England intensive surveillance program confirmed six cases of invasive meningococcal disease. During the same time frame in 2015, there were 24 cases, whereas, during the 4 years prior to introduction of 4CMenB, the average was 18 cases per year.
Three of the six 1-year-olds had meningitis, and three had septicemia. One was admitted to an ICU. None died. None had comorbid conditions placing them at increased risk. Five of the six children with invasive meningococcal disease had received two doses of the vaccine, and one child became ill 3 months after receiving the third dose.
The vaccine is licensed to be given as a four-dose series: three primary doses plus one booster dose. UK health officials deemed that excessive and not cost effective. Based on data from the vaccine trials, they determined that three doses are sufficient. This cut the cost of the national program by 25% while maintaining protection (Lancet. 2016 Dec 3;388(10061):2775-82).
Pediatricians from other countries were extremely curious about this innovative immunization program. What about vaccine side effects in infants? they asked.
Dr. Ladhani replied that studies in Australia, Northern Ireland, and Scotland have all shown a small uptick in mild fever and irritability in the first 3 days after the first dose of 4CMenB. It’s less of an issue with the second dose.
“One dose is not protective. You need two. Parents understand that, while these side effects are annoying, the risks associated with meningitis are far greater. They are very, very accepting of the vaccine,” according to Dr. Ladhani.
What about using 4CMenB in teenagers? physicians asked.
Dr. Ladhani predicted that the infant immunization program will have zero effect in adolescents. Teens are the main carriers of meningococcal bacteria. So, in theory, vaccinating them could not only protect the adolescents themselves but could benefit the whole population through herd immunity.
“The problem is we don’t have solid evidence that Bexsero protects against carriage. A massive carriage study is underway in Australia. It should provide data in 2 or 3 years. If we do see a carriage effect, then vaccinating teenagers becomes a very attractive option because you protect them and others around them,” he explained.
Dr. Ladhani reported having no financial conflicts regarding his study, funded by Public Health England.
AT ESPID 2017
Key clinical point:
Major finding: The number of confirmed cases of invasive meningococcal disease in English 1-year-olds dropped to six the year after introduction of the 4CMenB vaccine in the infant immunization schedule, down from 24 cases the previous year.
Data source: An enhanced surveillance study utilizing a combination of laboratory, public health, and clinical reporting to track and confirm all cases of invasive meningococcal disease in English 1-year-olds.
Disclosures: The study was funded by Public Health England. The presenter, who is employed by the agency, reported having no financial conflicts.
Small study: Patients prefer microneedle flu vaccine
Influenza vaccinations given through a microneedle patch (MNP) received higher patient approval compared with traditional inoculation methods, according to a small study funded by the National Institutes of Health.
In a phase I, randomized, placebo-controlled study, 100 patients between the ages of 18 and 49 years were split into four groups: one given the patch by a health care worker, one instructed to apply the patch at home, one given a vaccine through a traditional intramuscular injection, and one given a placebo.
Of those who took the patch, 70% (33 of 47) preferred the patch to intramuscular injection (The Lancet. 2017 Jun 27. doi: 10.1016/S0140-6736[17]30575-5).
All nonplacebo groups were given Fluvirin, the 2014-2015 licensed trivalent inactivated influenza vaccine, according to the researchers.
Protection against the virus 6 months after vaccination was similar across all groups other than the placebo group: 20-24 (83%-100%) of 24 participants given the patch by a health care worker, 18-24 (75%-100%) of 24 in the group of patients who gave themselves the patch, and 20-25 (80%-100%) of 25 in the injection group having achieved seroprotection against the three influenza strains 6 months after vaccination.
When measuring reactogenicity, the investigators did find more patients (41 of 50) reported cases of pruritis in the microneedle group than in the injection group (4 of 25).
However, these cases were mostly mild, while the injection group reported more grade 2 and grade 3 reactions, with grade 4 being the most severe.
Given the storage temperature of 40° C and ease of use without a health care provider, the investigators discussed the financial and clinical prospects of the patch.
“Increased acceptability could enable increased rates of influenza vaccination, which are currently less than 50% in adult populations,” they noted. “Moreover, because participants were able to self-vaccinate and 70% or more preferred it, significant cost savings could be enabled by microneedle patches due to a reduction in health-care worker time devoted to vaccination.”
Along with storage life and simple application process, the fact that the microneedles dissolve safely into the skin creates the potential for use in patients’ homes and offices, the researchers noted.
There may also be potential for use among pediatric patients, who may be resistant to vaccinations because of the injection method, they added.
“Microneedle patches have the potential to become ideal candidates for vaccination programmes, not only in poorly resourced settings, but also for individuals who currently prefer not to get vaccinated, potentially even being an attractive vaccine for the paediatric population,” Katja Höschler, PhD, and Maria C. Zambon, PhD, of Public Health England, London, said in a comment published with the study (The Lancet. 2017 Jun 27. doi: 10.1016/S0140-6736[17]31364-8). “The delivery advantages could also be exploited for non-influenza vaccines.”
Further studies must be conducted to test the efficacy of this vaccination system, because this study was limited by its size, the researchers noted.
The population was less inclined to receive an influenza injection because of the method itself, which may have affected the levels of preference for the patch, they added.
Some of the researchers are employees of Micron Biomedical, a company that manufactures microneedle products, and are listed as inventors on the licensed patents of these products. The investigators reported no other relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter@eaztweets
Influenza vaccinations given through a microneedle patch (MNP) received higher patient approval compared with traditional inoculation methods, according to a small study funded by the National Institutes of Health.
In a phase I, randomized, placebo-controlled study, 100 patients between the ages of 18 and 49 years were split into four groups: one given the patch by a health care worker, one instructed to apply the patch at home, one given a vaccine through a traditional intramuscular injection, and one given a placebo.
Of those who took the patch, 70% (33 of 47) preferred the patch to intramuscular injection (The Lancet. 2017 Jun 27. doi: 10.1016/S0140-6736[17]30575-5).
All nonplacebo groups were given Fluvirin, the 2014-2015 licensed trivalent inactivated influenza vaccine, according to the researchers.
Protection against the virus 6 months after vaccination was similar across all groups other than the placebo group: 20-24 (83%-100%) of 24 participants given the patch by a health care worker, 18-24 (75%-100%) of 24 in the group of patients who gave themselves the patch, and 20-25 (80%-100%) of 25 in the injection group having achieved seroprotection against the three influenza strains 6 months after vaccination.
When measuring reactogenicity, the investigators did find more patients (41 of 50) reported cases of pruritis in the microneedle group than in the injection group (4 of 25).
However, these cases were mostly mild, while the injection group reported more grade 2 and grade 3 reactions, with grade 4 being the most severe.
Given the storage temperature of 40° C and ease of use without a health care provider, the investigators discussed the financial and clinical prospects of the patch.
“Increased acceptability could enable increased rates of influenza vaccination, which are currently less than 50% in adult populations,” they noted. “Moreover, because participants were able to self-vaccinate and 70% or more preferred it, significant cost savings could be enabled by microneedle patches due to a reduction in health-care worker time devoted to vaccination.”
Along with storage life and simple application process, the fact that the microneedles dissolve safely into the skin creates the potential for use in patients’ homes and offices, the researchers noted.
There may also be potential for use among pediatric patients, who may be resistant to vaccinations because of the injection method, they added.
“Microneedle patches have the potential to become ideal candidates for vaccination programmes, not only in poorly resourced settings, but also for individuals who currently prefer not to get vaccinated, potentially even being an attractive vaccine for the paediatric population,” Katja Höschler, PhD, and Maria C. Zambon, PhD, of Public Health England, London, said in a comment published with the study (The Lancet. 2017 Jun 27. doi: 10.1016/S0140-6736[17]31364-8). “The delivery advantages could also be exploited for non-influenza vaccines.”
Further studies must be conducted to test the efficacy of this vaccination system, because this study was limited by its size, the researchers noted.
The population was less inclined to receive an influenza injection because of the method itself, which may have affected the levels of preference for the patch, they added.
Some of the researchers are employees of Micron Biomedical, a company that manufactures microneedle products, and are listed as inventors on the licensed patents of these products. The investigators reported no other relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter@eaztweets
Influenza vaccinations given through a microneedle patch (MNP) received higher patient approval compared with traditional inoculation methods, according to a small study funded by the National Institutes of Health.
In a phase I, randomized, placebo-controlled study, 100 patients between the ages of 18 and 49 years were split into four groups: one given the patch by a health care worker, one instructed to apply the patch at home, one given a vaccine through a traditional intramuscular injection, and one given a placebo.
Of those who took the patch, 70% (33 of 47) preferred the patch to intramuscular injection (The Lancet. 2017 Jun 27. doi: 10.1016/S0140-6736[17]30575-5).
All nonplacebo groups were given Fluvirin, the 2014-2015 licensed trivalent inactivated influenza vaccine, according to the researchers.
Protection against the virus 6 months after vaccination was similar across all groups other than the placebo group: 20-24 (83%-100%) of 24 participants given the patch by a health care worker, 18-24 (75%-100%) of 24 in the group of patients who gave themselves the patch, and 20-25 (80%-100%) of 25 in the injection group having achieved seroprotection against the three influenza strains 6 months after vaccination.
When measuring reactogenicity, the investigators did find more patients (41 of 50) reported cases of pruritis in the microneedle group than in the injection group (4 of 25).
However, these cases were mostly mild, while the injection group reported more grade 2 and grade 3 reactions, with grade 4 being the most severe.
Given the storage temperature of 40° C and ease of use without a health care provider, the investigators discussed the financial and clinical prospects of the patch.
“Increased acceptability could enable increased rates of influenza vaccination, which are currently less than 50% in adult populations,” they noted. “Moreover, because participants were able to self-vaccinate and 70% or more preferred it, significant cost savings could be enabled by microneedle patches due to a reduction in health-care worker time devoted to vaccination.”
Along with storage life and simple application process, the fact that the microneedles dissolve safely into the skin creates the potential for use in patients’ homes and offices, the researchers noted.
There may also be potential for use among pediatric patients, who may be resistant to vaccinations because of the injection method, they added.
“Microneedle patches have the potential to become ideal candidates for vaccination programmes, not only in poorly resourced settings, but also for individuals who currently prefer not to get vaccinated, potentially even being an attractive vaccine for the paediatric population,” Katja Höschler, PhD, and Maria C. Zambon, PhD, of Public Health England, London, said in a comment published with the study (The Lancet. 2017 Jun 27. doi: 10.1016/S0140-6736[17]31364-8). “The delivery advantages could also be exploited for non-influenza vaccines.”
Further studies must be conducted to test the efficacy of this vaccination system, because this study was limited by its size, the researchers noted.
The population was less inclined to receive an influenza injection because of the method itself, which may have affected the levels of preference for the patch, they added.
Some of the researchers are employees of Micron Biomedical, a company that manufactures microneedle products, and are listed as inventors on the licensed patents of these products. The investigators reported no other relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter@eaztweets
FROM THE LANCET
Key clinical point:
Major finding: Twenty-eight days after vaccination, 33 of 47 of patients (70%) who received the microneedle patch vaccine preferred that method to traditional inoculation.
Data source: A phase I, randomized, placebo-controlled study of 100 patients between the ages of 18 and 49 years.
Disclosures: Three of the researchers are employees of Micron Biomedical, a company that manufactures microneedle products, and are listed as inventors on the licensed patents of these products. The investigators reported no other relevant financial disclosures.
ACIP approves new influenza vaccine recommendations
FROM AN ACIP MEETING
by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) after a lengthy debate over specifics regarding recommendations for pregnant women.
The proposed recommendation that sparked the debate would change the wording of the previous recommendation for pregnant women to receive a seasonal inactivated vaccine (IIV) to “any licensed, recommended, and age-appropriate, trivalent or quadrivalent IIV or RIV [recombinant influenza vaccine] may be used.”
“I think there’s a subtle, but important difference here between making what would appear to be an affirmative statement that RIV is safe in pregnant women, versus just staying silent on it, and saying ‘we’re not saying you shouldn’t use it, but we don’t have enough data to affirmatively say it is safe,’ ” said Cindy Pellegrini, senior vice president of Public Policy and Government Affairs at the March of Dimes Foundation.
In response, members of the committee pointed out that the responsibility of determining safety lies with the Food and Drug Administration, which has already licensed the Flublok trivalent vaccine with expectations that the quadrivalent vaccine soon will follow.
While Lisa Grohskopf, MD, MPH, medical officer of the influenza division of the CDC, did acknowledge that there were more data on the safety of inactivated influenza vaccines, she asserted to the committee that “the general overall safety profile of Flublok in comparison to inactivated vaccines is reassuring.”
“For example, one concern that arises is reactogenicity and inflammation. [In terms of] overall reactogenicity in the studies where Flublok and inactivated vaccines have been compared, rates of the adverse and systemic reactions were similar,” Dr. Grohskopf said.
A motion was made to change the wording of the recommendation; however, the motion was not passed, and the eventual vote on the approval was conducted.
The ACIP also voted unanimously to change the safe age limit noted in influenza guidelines for use of Afluria (IIV3) from 9 years and older to 5 years and older. A footnote saying that the ACIP recommends Afluria for children 9 years and older will be removed.
This change, which mirrors the licensing Afluria has with the FDA, was based on research conducted by Seqirus that showed fever levels were the same for Afluria trivalent and quadrivalent vaccines in children 5 to 9 years old, both of which were less than historical vaccine rates.
The approved recommendations will be sent to the director of the CDC and the U.S. Department of Health and Human Services. Once reviewed and approved, the final recommendations will be published in the CDC’s Morbidity and Mortality Weekly Report. The committee members had no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
FROM AN ACIP MEETING
by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) after a lengthy debate over specifics regarding recommendations for pregnant women.
The proposed recommendation that sparked the debate would change the wording of the previous recommendation for pregnant women to receive a seasonal inactivated vaccine (IIV) to “any licensed, recommended, and age-appropriate, trivalent or quadrivalent IIV or RIV [recombinant influenza vaccine] may be used.”
“I think there’s a subtle, but important difference here between making what would appear to be an affirmative statement that RIV is safe in pregnant women, versus just staying silent on it, and saying ‘we’re not saying you shouldn’t use it, but we don’t have enough data to affirmatively say it is safe,’ ” said Cindy Pellegrini, senior vice president of Public Policy and Government Affairs at the March of Dimes Foundation.
In response, members of the committee pointed out that the responsibility of determining safety lies with the Food and Drug Administration, which has already licensed the Flublok trivalent vaccine with expectations that the quadrivalent vaccine soon will follow.
While Lisa Grohskopf, MD, MPH, medical officer of the influenza division of the CDC, did acknowledge that there were more data on the safety of inactivated influenza vaccines, she asserted to the committee that “the general overall safety profile of Flublok in comparison to inactivated vaccines is reassuring.”
“For example, one concern that arises is reactogenicity and inflammation. [In terms of] overall reactogenicity in the studies where Flublok and inactivated vaccines have been compared, rates of the adverse and systemic reactions were similar,” Dr. Grohskopf said.
A motion was made to change the wording of the recommendation; however, the motion was not passed, and the eventual vote on the approval was conducted.
The ACIP also voted unanimously to change the safe age limit noted in influenza guidelines for use of Afluria (IIV3) from 9 years and older to 5 years and older. A footnote saying that the ACIP recommends Afluria for children 9 years and older will be removed.
This change, which mirrors the licensing Afluria has with the FDA, was based on research conducted by Seqirus that showed fever levels were the same for Afluria trivalent and quadrivalent vaccines in children 5 to 9 years old, both of which were less than historical vaccine rates.
The approved recommendations will be sent to the director of the CDC and the U.S. Department of Health and Human Services. Once reviewed and approved, the final recommendations will be published in the CDC’s Morbidity and Mortality Weekly Report. The committee members had no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
FROM AN ACIP MEETING
by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) after a lengthy debate over specifics regarding recommendations for pregnant women.
The proposed recommendation that sparked the debate would change the wording of the previous recommendation for pregnant women to receive a seasonal inactivated vaccine (IIV) to “any licensed, recommended, and age-appropriate, trivalent or quadrivalent IIV or RIV [recombinant influenza vaccine] may be used.”
“I think there’s a subtle, but important difference here between making what would appear to be an affirmative statement that RIV is safe in pregnant women, versus just staying silent on it, and saying ‘we’re not saying you shouldn’t use it, but we don’t have enough data to affirmatively say it is safe,’ ” said Cindy Pellegrini, senior vice president of Public Policy and Government Affairs at the March of Dimes Foundation.
In response, members of the committee pointed out that the responsibility of determining safety lies with the Food and Drug Administration, which has already licensed the Flublok trivalent vaccine with expectations that the quadrivalent vaccine soon will follow.
While Lisa Grohskopf, MD, MPH, medical officer of the influenza division of the CDC, did acknowledge that there were more data on the safety of inactivated influenza vaccines, she asserted to the committee that “the general overall safety profile of Flublok in comparison to inactivated vaccines is reassuring.”
“For example, one concern that arises is reactogenicity and inflammation. [In terms of] overall reactogenicity in the studies where Flublok and inactivated vaccines have been compared, rates of the adverse and systemic reactions were similar,” Dr. Grohskopf said.
A motion was made to change the wording of the recommendation; however, the motion was not passed, and the eventual vote on the approval was conducted.
The ACIP also voted unanimously to change the safe age limit noted in influenza guidelines for use of Afluria (IIV3) from 9 years and older to 5 years and older. A footnote saying that the ACIP recommends Afluria for children 9 years and older will be removed.
This change, which mirrors the licensing Afluria has with the FDA, was based on research conducted by Seqirus that showed fever levels were the same for Afluria trivalent and quadrivalent vaccines in children 5 to 9 years old, both of which were less than historical vaccine rates.
The approved recommendations will be sent to the director of the CDC and the U.S. Department of Health and Human Services. Once reviewed and approved, the final recommendations will be published in the CDC’s Morbidity and Mortality Weekly Report. The committee members had no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets