User login
Pregnancy studies on psoriasis, PsA medications pick up
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
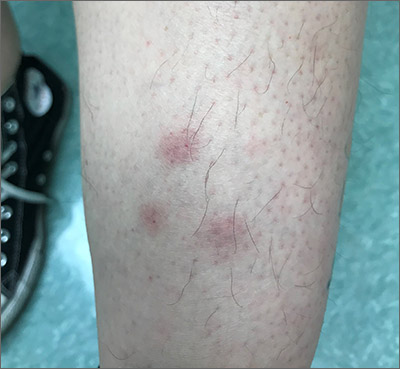
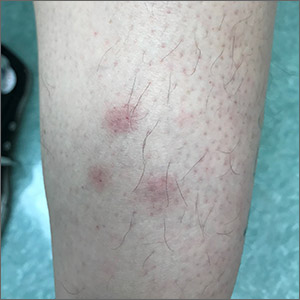
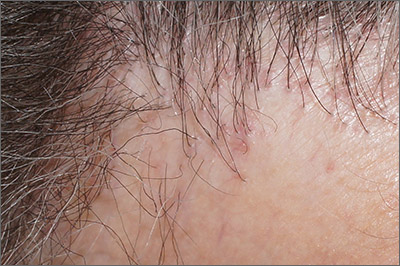
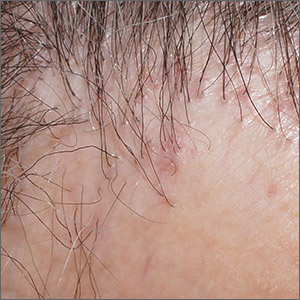
Recurrent leg lesions
Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Nearly half of brachial plexus injury cases occur without shoulder dystocia
according to research published in Obstetrics & Gynecology.
Grace J. Johnson, MD, and colleagues at Baylor College of Medicine in Houston performed a medical review of 41,525 deliveries at Texas Children’s Hospital between March 2012 and July 2019, identifying cases of brachial plexus injury, with and without shoulder dystocia, occurring and persisting. The researchers also evaluated whether clinical experience (5 years or fewer, 6-15 years, or more than 15 years since training) and education impacted the risk of children developing shoulder dystocia or brachial plexus injury.
There were 547 cases of shoulder dystocia in 26,163 vaginal births (2.1%) and 9 cases in 15,362 cesarean births (0.06%), while 33 cases of brachial plexus injury occurred overall. Nearly all brachial plexus injuries were in vaginal deliveries (30 cases; 0.1%), while 3 cases occurred in cesarean deliveries (0.02%). Of these, 14 cases (42%) of brachial plexus injury did not co-occur with shoulder dystocia. Brachial plexus injury that persisted to discharge was similar for children with shoulder dystocia (17 of 19 cases; 89%) and without shoulder dystocia (10 of 14 cases; 71%). In the 27 children with persistent brachial plexus injury, 2 of 23 children who received follow-up care continued to experience persistent brachial plexus injury at 9 months (1 case with shoulder dystocia) and 12 months (1 case without shoulder dystocia).
“The frequent co-occurrence of shoulder dystocia and brachial plexus injury coupled with the equally frequent occurrence of isolated brachial plexus injury suggests that both brachial plexus injury and shoulder dystocia often reflect two causally unrelated complications of uterine forces driving a fetus through the birth canal in the presence of disproportion between the passage and the shoulder girdle of the passenger,” Dr. Johnson and colleagues wrote.
Results unchanged by clinician experience
Factors that impacted the risk of brachial plexus injury in children without shoulder dystocia were lack of maternal diabetes (0 women vs. 6 women; P = .03) and second-stage labor length (mean 103 minutes vs. 53 minutes; P = .08). Dr. Johnson and colleagues found no significant between-group differences regarding operative delivery, maternal age, or gestational age.
The researchers also examined the experience of the clinician who delivered children with brachial plexus injuries, and discovered there were no significant differences in children who had transient as opposed to persistent brachial plexus injury based on the number of years a clinician had been in practice (P = .97). There also were no significant changes in the “ratios of brachial plexus injury per total deliveries, brachial plexus injury per vaginal deliveries, and brachial plexus injury per shoulder dystocia” despite the presence of education and training for shoulder dystocia.
Questions require further study
Torri Metz, MD, MS, a maternal-fetal medicine subspecialist and associate professor of obstetrics and gynecology at University of Utah Health in Salt Lake City, said in an interview that the review by Johnson and colleagues was able to address limitations in previous studies by looking at the medical records of shoulder dystocia cases at a single tertiary care center.
“Brachial plexus injury occurs both with and without a diagnosis of shoulder dystocia. The finding that the non–shoulder dystocia brachial plexus injuries were associated with a longer second stage of labor suggests that these injuries can occur even prior to delivery of the fetal head and are often not related to maneuvers employed by an obstetrician during delivery,” Dr. Metz said.
The findings that brachial plexus injury severity was unrelated to clinician experience suggests “the occurrence, severity, and persistence of brachial plexus injury may be unrelated to maneuvers by the practitioner at the time of delivery,” she said.
Although Johnson et al. found education and training initiatives did not significantly impact the ratio of brachial plexus injury cases, “importantly, there are likely many other benefits to shoulder dystocia simulation including team communication and comfort of the practitioner in an obstetrical emergency. Thus, the conclusion should not be that simulation training should be abandoned,” Dr. Metz explained.
The results of the study should be confirmed in future research, she noted. “Despite looking at all cases of shoulder dystocia at a tertiary center over a 7-year period, the incidence of brachial plexus injury is low enough that only 33 cases were evaluated. As such, many questions about obstetrical management and the risk of brachial plexus injury still require further study,” said Dr. Metz, who was asked to comment on the study.
The authors reported no relevant financial disclosures. Dr. Metz is an editorial board member for Obstetrics and Gynecology. She was not involved in the review of this manuscript or the decision to publish it.
SOURCE: Johnson GJ et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004013.
according to research published in Obstetrics & Gynecology.
Grace J. Johnson, MD, and colleagues at Baylor College of Medicine in Houston performed a medical review of 41,525 deliveries at Texas Children’s Hospital between March 2012 and July 2019, identifying cases of brachial plexus injury, with and without shoulder dystocia, occurring and persisting. The researchers also evaluated whether clinical experience (5 years or fewer, 6-15 years, or more than 15 years since training) and education impacted the risk of children developing shoulder dystocia or brachial plexus injury.
There were 547 cases of shoulder dystocia in 26,163 vaginal births (2.1%) and 9 cases in 15,362 cesarean births (0.06%), while 33 cases of brachial plexus injury occurred overall. Nearly all brachial plexus injuries were in vaginal deliveries (30 cases; 0.1%), while 3 cases occurred in cesarean deliveries (0.02%). Of these, 14 cases (42%) of brachial plexus injury did not co-occur with shoulder dystocia. Brachial plexus injury that persisted to discharge was similar for children with shoulder dystocia (17 of 19 cases; 89%) and without shoulder dystocia (10 of 14 cases; 71%). In the 27 children with persistent brachial plexus injury, 2 of 23 children who received follow-up care continued to experience persistent brachial plexus injury at 9 months (1 case with shoulder dystocia) and 12 months (1 case without shoulder dystocia).
“The frequent co-occurrence of shoulder dystocia and brachial plexus injury coupled with the equally frequent occurrence of isolated brachial plexus injury suggests that both brachial plexus injury and shoulder dystocia often reflect two causally unrelated complications of uterine forces driving a fetus through the birth canal in the presence of disproportion between the passage and the shoulder girdle of the passenger,” Dr. Johnson and colleagues wrote.
Results unchanged by clinician experience
Factors that impacted the risk of brachial plexus injury in children without shoulder dystocia were lack of maternal diabetes (0 women vs. 6 women; P = .03) and second-stage labor length (mean 103 minutes vs. 53 minutes; P = .08). Dr. Johnson and colleagues found no significant between-group differences regarding operative delivery, maternal age, or gestational age.
The researchers also examined the experience of the clinician who delivered children with brachial plexus injuries, and discovered there were no significant differences in children who had transient as opposed to persistent brachial plexus injury based on the number of years a clinician had been in practice (P = .97). There also were no significant changes in the “ratios of brachial plexus injury per total deliveries, brachial plexus injury per vaginal deliveries, and brachial plexus injury per shoulder dystocia” despite the presence of education and training for shoulder dystocia.
Questions require further study
Torri Metz, MD, MS, a maternal-fetal medicine subspecialist and associate professor of obstetrics and gynecology at University of Utah Health in Salt Lake City, said in an interview that the review by Johnson and colleagues was able to address limitations in previous studies by looking at the medical records of shoulder dystocia cases at a single tertiary care center.
“Brachial plexus injury occurs both with and without a diagnosis of shoulder dystocia. The finding that the non–shoulder dystocia brachial plexus injuries were associated with a longer second stage of labor suggests that these injuries can occur even prior to delivery of the fetal head and are often not related to maneuvers employed by an obstetrician during delivery,” Dr. Metz said.
The findings that brachial plexus injury severity was unrelated to clinician experience suggests “the occurrence, severity, and persistence of brachial plexus injury may be unrelated to maneuvers by the practitioner at the time of delivery,” she said.
Although Johnson et al. found education and training initiatives did not significantly impact the ratio of brachial plexus injury cases, “importantly, there are likely many other benefits to shoulder dystocia simulation including team communication and comfort of the practitioner in an obstetrical emergency. Thus, the conclusion should not be that simulation training should be abandoned,” Dr. Metz explained.
The results of the study should be confirmed in future research, she noted. “Despite looking at all cases of shoulder dystocia at a tertiary center over a 7-year period, the incidence of brachial plexus injury is low enough that only 33 cases were evaluated. As such, many questions about obstetrical management and the risk of brachial plexus injury still require further study,” said Dr. Metz, who was asked to comment on the study.
The authors reported no relevant financial disclosures. Dr. Metz is an editorial board member for Obstetrics and Gynecology. She was not involved in the review of this manuscript or the decision to publish it.
SOURCE: Johnson GJ et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004013.
according to research published in Obstetrics & Gynecology.
Grace J. Johnson, MD, and colleagues at Baylor College of Medicine in Houston performed a medical review of 41,525 deliveries at Texas Children’s Hospital between March 2012 and July 2019, identifying cases of brachial plexus injury, with and without shoulder dystocia, occurring and persisting. The researchers also evaluated whether clinical experience (5 years or fewer, 6-15 years, or more than 15 years since training) and education impacted the risk of children developing shoulder dystocia or brachial plexus injury.
There were 547 cases of shoulder dystocia in 26,163 vaginal births (2.1%) and 9 cases in 15,362 cesarean births (0.06%), while 33 cases of brachial plexus injury occurred overall. Nearly all brachial plexus injuries were in vaginal deliveries (30 cases; 0.1%), while 3 cases occurred in cesarean deliveries (0.02%). Of these, 14 cases (42%) of brachial plexus injury did not co-occur with shoulder dystocia. Brachial plexus injury that persisted to discharge was similar for children with shoulder dystocia (17 of 19 cases; 89%) and without shoulder dystocia (10 of 14 cases; 71%). In the 27 children with persistent brachial plexus injury, 2 of 23 children who received follow-up care continued to experience persistent brachial plexus injury at 9 months (1 case with shoulder dystocia) and 12 months (1 case without shoulder dystocia).
“The frequent co-occurrence of shoulder dystocia and brachial plexus injury coupled with the equally frequent occurrence of isolated brachial plexus injury suggests that both brachial plexus injury and shoulder dystocia often reflect two causally unrelated complications of uterine forces driving a fetus through the birth canal in the presence of disproportion between the passage and the shoulder girdle of the passenger,” Dr. Johnson and colleagues wrote.
Results unchanged by clinician experience
Factors that impacted the risk of brachial plexus injury in children without shoulder dystocia were lack of maternal diabetes (0 women vs. 6 women; P = .03) and second-stage labor length (mean 103 minutes vs. 53 minutes; P = .08). Dr. Johnson and colleagues found no significant between-group differences regarding operative delivery, maternal age, or gestational age.
The researchers also examined the experience of the clinician who delivered children with brachial plexus injuries, and discovered there were no significant differences in children who had transient as opposed to persistent brachial plexus injury based on the number of years a clinician had been in practice (P = .97). There also were no significant changes in the “ratios of brachial plexus injury per total deliveries, brachial plexus injury per vaginal deliveries, and brachial plexus injury per shoulder dystocia” despite the presence of education and training for shoulder dystocia.
Questions require further study
Torri Metz, MD, MS, a maternal-fetal medicine subspecialist and associate professor of obstetrics and gynecology at University of Utah Health in Salt Lake City, said in an interview that the review by Johnson and colleagues was able to address limitations in previous studies by looking at the medical records of shoulder dystocia cases at a single tertiary care center.
“Brachial plexus injury occurs both with and without a diagnosis of shoulder dystocia. The finding that the non–shoulder dystocia brachial plexus injuries were associated with a longer second stage of labor suggests that these injuries can occur even prior to delivery of the fetal head and are often not related to maneuvers employed by an obstetrician during delivery,” Dr. Metz said.
The findings that brachial plexus injury severity was unrelated to clinician experience suggests “the occurrence, severity, and persistence of brachial plexus injury may be unrelated to maneuvers by the practitioner at the time of delivery,” she said.
Although Johnson et al. found education and training initiatives did not significantly impact the ratio of brachial plexus injury cases, “importantly, there are likely many other benefits to shoulder dystocia simulation including team communication and comfort of the practitioner in an obstetrical emergency. Thus, the conclusion should not be that simulation training should be abandoned,” Dr. Metz explained.
The results of the study should be confirmed in future research, she noted. “Despite looking at all cases of shoulder dystocia at a tertiary center over a 7-year period, the incidence of brachial plexus injury is low enough that only 33 cases were evaluated. As such, many questions about obstetrical management and the risk of brachial plexus injury still require further study,” said Dr. Metz, who was asked to comment on the study.
The authors reported no relevant financial disclosures. Dr. Metz is an editorial board member for Obstetrics and Gynecology. She was not involved in the review of this manuscript or the decision to publish it.
SOURCE: Johnson GJ et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004013.
FROM OBSTETRICS & GYNECOLOGY
Pandemic drives demand for self-managed abortions
Requests for self-managed abortion via a telemedicine service increased by 27% from March 20, 2020, to April 11, 2020, in the United States in the wake of widespread lockdowns and shelter-in-place directives because of the COVID-19 pandemic, based on data from a provider of such services.
Access to abortion care is challenging in many areas under ordinary circumstances, but the disruption of the COVID-19 pandemic led to many states suspending or limiting in-clinic services, wrote Abigail R.A. Aiken, MD, PhD, of the University of Texas at Austin and colleagues.
“As a result, people may increasingly be seeking self-managed abortion outside the formal health care system,” they said.
In a research letter published in Obstetrics & Gynecology, the investigators reviewed request data from Aid Access, a telemedicine service that provides medication for abortion at up to 10 weeks’ gestation for users who complete an online consultation form. They also collected data on the implementation and scope of COVID-19–related abortion restrictions by state.
The analysis included all 49,935 requests made between January 1, 2019, and April 11, 2020.
Overall, the rate of requests for self-managed medical abortions increased significantly, by 27%, during the period from March 20, 2020, to April 11, 2020, which reflected the average period after clinic restrictions or closures at the state level. A total of 11 states showed individually significant increases in requests for self-managed medical abortions, with the highest of 94% in Texas and the lowest of 22% in Ohio. In these 11 states, the median time spent at home was 5% higher than in states without significant increases in requests for self-managed medical abortions during the same period. These states also had “particularly high COVID-19 rates or more severe COVID-19–related restrictions on in-clinic abortion access,” the researchers noted.
Patients want alternatives to in-person care
“Our results may reflect two distinct phenomena,” Dr. Aiken and associates wrote. “First, more people may be seeking abortion through all channels, whether due to COVID-19 risks during pregnancy, reduced access to prenatal care, or the pandemic-related economic downturn. Second, there may be shift in demand from in-clinic to self-managed abortion during the pandemic, possibly owing to fear of infection during in-person care or inability to get to a clinic because of childcare and transit disruptions,” they explained.
The study findings were limited by the inability to measure all options for women to achieve self-managed abortions and a lack of power to detect changes in states with low request numbers or where restrictions were implemented at later dates, the researchers noted. However, the results suggest that telemedicine services for medication abortion should be a policy priority because patients may continue to seek alternatives while in-clinic services remain restricted, they said.
In fact, “the World Health Organization recommends telemedicine and self-management abortion-care models during the pandemic, and the United Kingdom has temporarily implemented fully remote provision of abortion medications,” the researchers wrote. However, similar strategies in the United States “would depend on sustained changes to the U.S. Food and Drug Administration’s Risk Evaluation and Mitigation Strategy, which requires patients to collect mifepristone at a hospital or medical facility, as well as changes to state-specific laws that prohibit remote provider consultation,” Dr. Aiken and associates concluded.
Lift barriers to protect patients
Eve Espey, MD, of the University of New Mexico, Albuquerque, said in an interview.
“As background, state abortion restrictions have increased exponentially over the last decade, while research over the same time period has demonstrated the safety of telemedicine abortion – a form of self-managed abortion – with no in-person visit for appropriate candidates,” she said.
“Enter the coronavirus pandemic with safety concerns related to in-person medical visits and certain states leveraging the opportunity to enact even more stringent abortion restrictions. Unsurprisingly, the result, as documented in this excellent research report, is a significant increase in requests for telemedicine abortion in many states, particularly the most restrictive, from the single online service in the United States, Aid Access,” said Dr. Espey.
“Barriers to self-managed abortion include the [FDA] Risk Evaluation and Mitigation Strategy for mifepristone, a set of unnecessary restrictions requiring that providers meet certain qualifications and dispense the medication only in a clinic, office, or hospital,” she said. “The REMS precludes the use of telemedicine abortion; Aid Access and the FDA are in legal proceedings,” she noted.
“Most recently, the [American Civil Liberties Union] sued the FDA on behalf of a coalition of medical experts led by [American College of Obstetricians and Gynecologists] to suspend the REMS for mifepristone during the COVID public health emergency, to allow patients to receive the medications for early abortion without a visit to a health care provider,” Dr. Espey said. “Fortunately, a federal district court required the temporary suspension of the in-person dispensing restriction. Although this is a great step to improve abortion access during the pandemic, a permanent removal of the REMS would pave the way for ongoing safe, effective, and patient-centered early abortion care,” noted Dr. Espey, who was asked to comment on the research letter.
Dr. Aiken disclosed serving as a consultant for Agile Therapeutics, and a coauthor is the founder and director of Aid Access. Dr. Espey had no financial conflicts to disclose. She is a member of the Ob.Gyn. News Editorial Advisory Board.
SOURCE: Aiken ARA et al. Obstet Gynecol. 2020 Jul 21. doi: 10.1097/AOG.0000000000004081.
Requests for self-managed abortion via a telemedicine service increased by 27% from March 20, 2020, to April 11, 2020, in the United States in the wake of widespread lockdowns and shelter-in-place directives because of the COVID-19 pandemic, based on data from a provider of such services.
Access to abortion care is challenging in many areas under ordinary circumstances, but the disruption of the COVID-19 pandemic led to many states suspending or limiting in-clinic services, wrote Abigail R.A. Aiken, MD, PhD, of the University of Texas at Austin and colleagues.
“As a result, people may increasingly be seeking self-managed abortion outside the formal health care system,” they said.
In a research letter published in Obstetrics & Gynecology, the investigators reviewed request data from Aid Access, a telemedicine service that provides medication for abortion at up to 10 weeks’ gestation for users who complete an online consultation form. They also collected data on the implementation and scope of COVID-19–related abortion restrictions by state.
The analysis included all 49,935 requests made between January 1, 2019, and April 11, 2020.
Overall, the rate of requests for self-managed medical abortions increased significantly, by 27%, during the period from March 20, 2020, to April 11, 2020, which reflected the average period after clinic restrictions or closures at the state level. A total of 11 states showed individually significant increases in requests for self-managed medical abortions, with the highest of 94% in Texas and the lowest of 22% in Ohio. In these 11 states, the median time spent at home was 5% higher than in states without significant increases in requests for self-managed medical abortions during the same period. These states also had “particularly high COVID-19 rates or more severe COVID-19–related restrictions on in-clinic abortion access,” the researchers noted.
Patients want alternatives to in-person care
“Our results may reflect two distinct phenomena,” Dr. Aiken and associates wrote. “First, more people may be seeking abortion through all channels, whether due to COVID-19 risks during pregnancy, reduced access to prenatal care, or the pandemic-related economic downturn. Second, there may be shift in demand from in-clinic to self-managed abortion during the pandemic, possibly owing to fear of infection during in-person care or inability to get to a clinic because of childcare and transit disruptions,” they explained.
The study findings were limited by the inability to measure all options for women to achieve self-managed abortions and a lack of power to detect changes in states with low request numbers or where restrictions were implemented at later dates, the researchers noted. However, the results suggest that telemedicine services for medication abortion should be a policy priority because patients may continue to seek alternatives while in-clinic services remain restricted, they said.
In fact, “the World Health Organization recommends telemedicine and self-management abortion-care models during the pandemic, and the United Kingdom has temporarily implemented fully remote provision of abortion medications,” the researchers wrote. However, similar strategies in the United States “would depend on sustained changes to the U.S. Food and Drug Administration’s Risk Evaluation and Mitigation Strategy, which requires patients to collect mifepristone at a hospital or medical facility, as well as changes to state-specific laws that prohibit remote provider consultation,” Dr. Aiken and associates concluded.
Lift barriers to protect patients
Eve Espey, MD, of the University of New Mexico, Albuquerque, said in an interview.
“As background, state abortion restrictions have increased exponentially over the last decade, while research over the same time period has demonstrated the safety of telemedicine abortion – a form of self-managed abortion – with no in-person visit for appropriate candidates,” she said.
“Enter the coronavirus pandemic with safety concerns related to in-person medical visits and certain states leveraging the opportunity to enact even more stringent abortion restrictions. Unsurprisingly, the result, as documented in this excellent research report, is a significant increase in requests for telemedicine abortion in many states, particularly the most restrictive, from the single online service in the United States, Aid Access,” said Dr. Espey.
“Barriers to self-managed abortion include the [FDA] Risk Evaluation and Mitigation Strategy for mifepristone, a set of unnecessary restrictions requiring that providers meet certain qualifications and dispense the medication only in a clinic, office, or hospital,” she said. “The REMS precludes the use of telemedicine abortion; Aid Access and the FDA are in legal proceedings,” she noted.
“Most recently, the [American Civil Liberties Union] sued the FDA on behalf of a coalition of medical experts led by [American College of Obstetricians and Gynecologists] to suspend the REMS for mifepristone during the COVID public health emergency, to allow patients to receive the medications for early abortion without a visit to a health care provider,” Dr. Espey said. “Fortunately, a federal district court required the temporary suspension of the in-person dispensing restriction. Although this is a great step to improve abortion access during the pandemic, a permanent removal of the REMS would pave the way for ongoing safe, effective, and patient-centered early abortion care,” noted Dr. Espey, who was asked to comment on the research letter.
Dr. Aiken disclosed serving as a consultant for Agile Therapeutics, and a coauthor is the founder and director of Aid Access. Dr. Espey had no financial conflicts to disclose. She is a member of the Ob.Gyn. News Editorial Advisory Board.
SOURCE: Aiken ARA et al. Obstet Gynecol. 2020 Jul 21. doi: 10.1097/AOG.0000000000004081.
Requests for self-managed abortion via a telemedicine service increased by 27% from March 20, 2020, to April 11, 2020, in the United States in the wake of widespread lockdowns and shelter-in-place directives because of the COVID-19 pandemic, based on data from a provider of such services.
Access to abortion care is challenging in many areas under ordinary circumstances, but the disruption of the COVID-19 pandemic led to many states suspending or limiting in-clinic services, wrote Abigail R.A. Aiken, MD, PhD, of the University of Texas at Austin and colleagues.
“As a result, people may increasingly be seeking self-managed abortion outside the formal health care system,” they said.
In a research letter published in Obstetrics & Gynecology, the investigators reviewed request data from Aid Access, a telemedicine service that provides medication for abortion at up to 10 weeks’ gestation for users who complete an online consultation form. They also collected data on the implementation and scope of COVID-19–related abortion restrictions by state.
The analysis included all 49,935 requests made between January 1, 2019, and April 11, 2020.
Overall, the rate of requests for self-managed medical abortions increased significantly, by 27%, during the period from March 20, 2020, to April 11, 2020, which reflected the average period after clinic restrictions or closures at the state level. A total of 11 states showed individually significant increases in requests for self-managed medical abortions, with the highest of 94% in Texas and the lowest of 22% in Ohio. In these 11 states, the median time spent at home was 5% higher than in states without significant increases in requests for self-managed medical abortions during the same period. These states also had “particularly high COVID-19 rates or more severe COVID-19–related restrictions on in-clinic abortion access,” the researchers noted.
Patients want alternatives to in-person care
“Our results may reflect two distinct phenomena,” Dr. Aiken and associates wrote. “First, more people may be seeking abortion through all channels, whether due to COVID-19 risks during pregnancy, reduced access to prenatal care, or the pandemic-related economic downturn. Second, there may be shift in demand from in-clinic to self-managed abortion during the pandemic, possibly owing to fear of infection during in-person care or inability to get to a clinic because of childcare and transit disruptions,” they explained.
The study findings were limited by the inability to measure all options for women to achieve self-managed abortions and a lack of power to detect changes in states with low request numbers or where restrictions were implemented at later dates, the researchers noted. However, the results suggest that telemedicine services for medication abortion should be a policy priority because patients may continue to seek alternatives while in-clinic services remain restricted, they said.
In fact, “the World Health Organization recommends telemedicine and self-management abortion-care models during the pandemic, and the United Kingdom has temporarily implemented fully remote provision of abortion medications,” the researchers wrote. However, similar strategies in the United States “would depend on sustained changes to the U.S. Food and Drug Administration’s Risk Evaluation and Mitigation Strategy, which requires patients to collect mifepristone at a hospital or medical facility, as well as changes to state-specific laws that prohibit remote provider consultation,” Dr. Aiken and associates concluded.
Lift barriers to protect patients
Eve Espey, MD, of the University of New Mexico, Albuquerque, said in an interview.
“As background, state abortion restrictions have increased exponentially over the last decade, while research over the same time period has demonstrated the safety of telemedicine abortion – a form of self-managed abortion – with no in-person visit for appropriate candidates,” she said.
“Enter the coronavirus pandemic with safety concerns related to in-person medical visits and certain states leveraging the opportunity to enact even more stringent abortion restrictions. Unsurprisingly, the result, as documented in this excellent research report, is a significant increase in requests for telemedicine abortion in many states, particularly the most restrictive, from the single online service in the United States, Aid Access,” said Dr. Espey.
“Barriers to self-managed abortion include the [FDA] Risk Evaluation and Mitigation Strategy for mifepristone, a set of unnecessary restrictions requiring that providers meet certain qualifications and dispense the medication only in a clinic, office, or hospital,” she said. “The REMS precludes the use of telemedicine abortion; Aid Access and the FDA are in legal proceedings,” she noted.
“Most recently, the [American Civil Liberties Union] sued the FDA on behalf of a coalition of medical experts led by [American College of Obstetricians and Gynecologists] to suspend the REMS for mifepristone during the COVID public health emergency, to allow patients to receive the medications for early abortion without a visit to a health care provider,” Dr. Espey said. “Fortunately, a federal district court required the temporary suspension of the in-person dispensing restriction. Although this is a great step to improve abortion access during the pandemic, a permanent removal of the REMS would pave the way for ongoing safe, effective, and patient-centered early abortion care,” noted Dr. Espey, who was asked to comment on the research letter.
Dr. Aiken disclosed serving as a consultant for Agile Therapeutics, and a coauthor is the founder and director of Aid Access. Dr. Espey had no financial conflicts to disclose. She is a member of the Ob.Gyn. News Editorial Advisory Board.
SOURCE: Aiken ARA et al. Obstet Gynecol. 2020 Jul 21. doi: 10.1097/AOG.0000000000004081.
FROM OBSTETRICS & GYNECOLOGY
Combination beats misoprostol monotherapy on cost effectiveness
A combination of mifepristone followed by misoprostol was significantly more cost effective for the medical management of miscarriage than misoprostol alone, based on a decision-tree model and simulations using a range of patient income levels, cost variables, and practice patterns.
Although the American College of Obstetricians and Gynecologists recommends a combination of mifepristone and misoprostol for the medical management of miscarriage, some physicians may hesitate because of the high cost of mifepristone, wrote Holly H. Berkley, MD, of the Naval Medical Center, San Diego, and colleagues.
Previous research has supported the cost effectiveness of combination therapy, but the data came from a secondary analysis that limited the generalizability of the findings, they wrote. In a study published in Obstetrics & Gynecology, the researchers created a decision-tree model using two standard practice patterns.
In the first, patients received mifepristone and one dose of misoprostol (combination therapy) or one dose of misoprostol alone (monotherapy) at their initial visit with follow-up within 3 days. Combination therapy was defined as 200 mg of oral mifepristone followed by one or two doses of 800 micrograms of vaginal misoprostol; monotherapy was defined as one or two doses of 800 micrograms of vaginal misoprostol.
“If miscarriage is not completed, a second dose of misoprostol is given, and the patient will have a second follow-up visit 8 days after initiation of treatment. If miscarriage is not complete at the second follow-up visit, surgical management is prescribed,” Dr. Berkley and associates reported.
In the second pattern, patients receive two doses of misoprostol at the first visit and an initial follow-up visit 8 days later.
Patient hourly income was based on the wages of three employment levels of the military patient population, estimated at $7.25/hour, $15.90/hour, and $35.10 per hour. “For clinicians outside of the military health system, these wage categories may also serve as an estimate of earnings for low-income, low-middle income, and middle-income patients across the United States,” Dr. Berkley and colleagues noted.
The researchers also considered costs for time of work, transportation, and the costs of the medical visits. Costs also were computed for surgical management with in–operating room dilation and curettage or in-office manual vacuum aspiration, if needed.
The greatest difference in favor of combination therapy resulted in a savings of $190.20 per patient, compared with monotherapy, in the first practice pattern and the lowest wage group (19.5%).
“In every scenario, and for every wage level, the average cost of combination therapy is less than that of monotherapy,” Dr. Berkley and associates noted. In addition, the differences in cost between combination therapy and monotherapy increased with patients’ wages, “reflecting wage differences as well as the net savings owing to increased completion rates.”
Completion rates are key to cost effectiveness
“The higher completion rate of combination therapy leads to decreased time spent on treatment and therefore decreased time off work, decreased office visits, and a decreased need for surgical management for persistent pregnancy, which significantly reduces cost,” they noted.
The model shows that the cost of mifepristone, which some clinicians may see as a barrier, contributes little to the overall treatment costs, Dr. Berkley and colleagues emphasized.
The study findings were limited by several factors including the large ranges in costs for office visits and procedures and the inability to replicate all clinical settings and variables, the researchers noted. However, the results were strengthened by the use of current practice patterns and costs, and they support the mifepristone/misoprostol combination as being the most cost effective for medical management of miscarriage, they said.
The findings of the current study, combined with higher effectiveness reported in recent randomized controlled trials and the endorsement of the American College of Obstetricians and Gynecologists “make a strong case for mifepristone followed by misoprostol to become the standard, first-line treatment regimen for the medical management of miscarriage,” Dr. Berkley and associates concluded.
Research is clear, policy needs to catch up
“There is clear research showing that using mifepristone with misoprostol to medically manage early pregnancy loss is significantly more effective than misoprostol alone,” Sarah Prager, MD, of the University of Washington, Seattle, said in an interview. “The combination protocol does include an expensive medication, so it’s important to evaluate if the cost of this more effective method is ‘worth it,’ ” she said. “ including fewer projected days off work and fewer patients needing procedures to complete their miscarriage.”
Dr. Prager said she was not surprised by the study findings because similar results have been found in other studies evaluating treatment of abortion with misoprostol alone versus mifepristone and misoprostol. “When something is significantly more effective, it usually will also come with a cost benefit.”
Potential barriers to the use of combination therapy are related to policy rather than drug safety or effectiveness, according to Dr. Prager.
“The primary barrier is that mifepristone use is regulated by a REMS [Risk Evaluation and Mitigation Strategy] restriction which requires that providers dispense the medication directly to patients, rather than being able to prescribe it and have patients then pick it up at a pharmacy,” she said. “This restriction is typically used for medications that are dangerous and need to be closely controlled. In the case of mifepristone, the restriction does not serve a safety purpose; it simply limits access to the medication which is still primarily used to medically treat abortion.
“The secondary barrier is stigma against using a medication that is seen as an abortion medication. I fear many providers or practices may avoid putting it on formulary due to this stigma,” Dr. Prager noted.
“There is already sufficient evidence that the combination therapy is superior to monotherapy, and there is also evidence that mifepristone can be safely prescribed [not dispensed] and does not need the REMS requirement,” Dr. Prager said. “I don’t believe more research is needed; just policy change.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Berkley HH et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004063.
A combination of mifepristone followed by misoprostol was significantly more cost effective for the medical management of miscarriage than misoprostol alone, based on a decision-tree model and simulations using a range of patient income levels, cost variables, and practice patterns.
Although the American College of Obstetricians and Gynecologists recommends a combination of mifepristone and misoprostol for the medical management of miscarriage, some physicians may hesitate because of the high cost of mifepristone, wrote Holly H. Berkley, MD, of the Naval Medical Center, San Diego, and colleagues.
Previous research has supported the cost effectiveness of combination therapy, but the data came from a secondary analysis that limited the generalizability of the findings, they wrote. In a study published in Obstetrics & Gynecology, the researchers created a decision-tree model using two standard practice patterns.
In the first, patients received mifepristone and one dose of misoprostol (combination therapy) or one dose of misoprostol alone (monotherapy) at their initial visit with follow-up within 3 days. Combination therapy was defined as 200 mg of oral mifepristone followed by one or two doses of 800 micrograms of vaginal misoprostol; monotherapy was defined as one or two doses of 800 micrograms of vaginal misoprostol.
“If miscarriage is not completed, a second dose of misoprostol is given, and the patient will have a second follow-up visit 8 days after initiation of treatment. If miscarriage is not complete at the second follow-up visit, surgical management is prescribed,” Dr. Berkley and associates reported.
In the second pattern, patients receive two doses of misoprostol at the first visit and an initial follow-up visit 8 days later.
Patient hourly income was based on the wages of three employment levels of the military patient population, estimated at $7.25/hour, $15.90/hour, and $35.10 per hour. “For clinicians outside of the military health system, these wage categories may also serve as an estimate of earnings for low-income, low-middle income, and middle-income patients across the United States,” Dr. Berkley and colleagues noted.
The researchers also considered costs for time of work, transportation, and the costs of the medical visits. Costs also were computed for surgical management with in–operating room dilation and curettage or in-office manual vacuum aspiration, if needed.
The greatest difference in favor of combination therapy resulted in a savings of $190.20 per patient, compared with monotherapy, in the first practice pattern and the lowest wage group (19.5%).
“In every scenario, and for every wage level, the average cost of combination therapy is less than that of monotherapy,” Dr. Berkley and associates noted. In addition, the differences in cost between combination therapy and monotherapy increased with patients’ wages, “reflecting wage differences as well as the net savings owing to increased completion rates.”
Completion rates are key to cost effectiveness
“The higher completion rate of combination therapy leads to decreased time spent on treatment and therefore decreased time off work, decreased office visits, and a decreased need for surgical management for persistent pregnancy, which significantly reduces cost,” they noted.
The model shows that the cost of mifepristone, which some clinicians may see as a barrier, contributes little to the overall treatment costs, Dr. Berkley and colleagues emphasized.
The study findings were limited by several factors including the large ranges in costs for office visits and procedures and the inability to replicate all clinical settings and variables, the researchers noted. However, the results were strengthened by the use of current practice patterns and costs, and they support the mifepristone/misoprostol combination as being the most cost effective for medical management of miscarriage, they said.
The findings of the current study, combined with higher effectiveness reported in recent randomized controlled trials and the endorsement of the American College of Obstetricians and Gynecologists “make a strong case for mifepristone followed by misoprostol to become the standard, first-line treatment regimen for the medical management of miscarriage,” Dr. Berkley and associates concluded.
Research is clear, policy needs to catch up
“There is clear research showing that using mifepristone with misoprostol to medically manage early pregnancy loss is significantly more effective than misoprostol alone,” Sarah Prager, MD, of the University of Washington, Seattle, said in an interview. “The combination protocol does include an expensive medication, so it’s important to evaluate if the cost of this more effective method is ‘worth it,’ ” she said. “ including fewer projected days off work and fewer patients needing procedures to complete their miscarriage.”
Dr. Prager said she was not surprised by the study findings because similar results have been found in other studies evaluating treatment of abortion with misoprostol alone versus mifepristone and misoprostol. “When something is significantly more effective, it usually will also come with a cost benefit.”
Potential barriers to the use of combination therapy are related to policy rather than drug safety or effectiveness, according to Dr. Prager.
“The primary barrier is that mifepristone use is regulated by a REMS [Risk Evaluation and Mitigation Strategy] restriction which requires that providers dispense the medication directly to patients, rather than being able to prescribe it and have patients then pick it up at a pharmacy,” she said. “This restriction is typically used for medications that are dangerous and need to be closely controlled. In the case of mifepristone, the restriction does not serve a safety purpose; it simply limits access to the medication which is still primarily used to medically treat abortion.
“The secondary barrier is stigma against using a medication that is seen as an abortion medication. I fear many providers or practices may avoid putting it on formulary due to this stigma,” Dr. Prager noted.
“There is already sufficient evidence that the combination therapy is superior to monotherapy, and there is also evidence that mifepristone can be safely prescribed [not dispensed] and does not need the REMS requirement,” Dr. Prager said. “I don’t believe more research is needed; just policy change.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Berkley HH et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004063.
A combination of mifepristone followed by misoprostol was significantly more cost effective for the medical management of miscarriage than misoprostol alone, based on a decision-tree model and simulations using a range of patient income levels, cost variables, and practice patterns.
Although the American College of Obstetricians and Gynecologists recommends a combination of mifepristone and misoprostol for the medical management of miscarriage, some physicians may hesitate because of the high cost of mifepristone, wrote Holly H. Berkley, MD, of the Naval Medical Center, San Diego, and colleagues.
Previous research has supported the cost effectiveness of combination therapy, but the data came from a secondary analysis that limited the generalizability of the findings, they wrote. In a study published in Obstetrics & Gynecology, the researchers created a decision-tree model using two standard practice patterns.
In the first, patients received mifepristone and one dose of misoprostol (combination therapy) or one dose of misoprostol alone (monotherapy) at their initial visit with follow-up within 3 days. Combination therapy was defined as 200 mg of oral mifepristone followed by one or two doses of 800 micrograms of vaginal misoprostol; monotherapy was defined as one or two doses of 800 micrograms of vaginal misoprostol.
“If miscarriage is not completed, a second dose of misoprostol is given, and the patient will have a second follow-up visit 8 days after initiation of treatment. If miscarriage is not complete at the second follow-up visit, surgical management is prescribed,” Dr. Berkley and associates reported.
In the second pattern, patients receive two doses of misoprostol at the first visit and an initial follow-up visit 8 days later.
Patient hourly income was based on the wages of three employment levels of the military patient population, estimated at $7.25/hour, $15.90/hour, and $35.10 per hour. “For clinicians outside of the military health system, these wage categories may also serve as an estimate of earnings for low-income, low-middle income, and middle-income patients across the United States,” Dr. Berkley and colleagues noted.
The researchers also considered costs for time of work, transportation, and the costs of the medical visits. Costs also were computed for surgical management with in–operating room dilation and curettage or in-office manual vacuum aspiration, if needed.
The greatest difference in favor of combination therapy resulted in a savings of $190.20 per patient, compared with monotherapy, in the first practice pattern and the lowest wage group (19.5%).
“In every scenario, and for every wage level, the average cost of combination therapy is less than that of monotherapy,” Dr. Berkley and associates noted. In addition, the differences in cost between combination therapy and monotherapy increased with patients’ wages, “reflecting wage differences as well as the net savings owing to increased completion rates.”
Completion rates are key to cost effectiveness
“The higher completion rate of combination therapy leads to decreased time spent on treatment and therefore decreased time off work, decreased office visits, and a decreased need for surgical management for persistent pregnancy, which significantly reduces cost,” they noted.
The model shows that the cost of mifepristone, which some clinicians may see as a barrier, contributes little to the overall treatment costs, Dr. Berkley and colleagues emphasized.
The study findings were limited by several factors including the large ranges in costs for office visits and procedures and the inability to replicate all clinical settings and variables, the researchers noted. However, the results were strengthened by the use of current practice patterns and costs, and they support the mifepristone/misoprostol combination as being the most cost effective for medical management of miscarriage, they said.
The findings of the current study, combined with higher effectiveness reported in recent randomized controlled trials and the endorsement of the American College of Obstetricians and Gynecologists “make a strong case for mifepristone followed by misoprostol to become the standard, first-line treatment regimen for the medical management of miscarriage,” Dr. Berkley and associates concluded.
Research is clear, policy needs to catch up
“There is clear research showing that using mifepristone with misoprostol to medically manage early pregnancy loss is significantly more effective than misoprostol alone,” Sarah Prager, MD, of the University of Washington, Seattle, said in an interview. “The combination protocol does include an expensive medication, so it’s important to evaluate if the cost of this more effective method is ‘worth it,’ ” she said. “ including fewer projected days off work and fewer patients needing procedures to complete their miscarriage.”
Dr. Prager said she was not surprised by the study findings because similar results have been found in other studies evaluating treatment of abortion with misoprostol alone versus mifepristone and misoprostol. “When something is significantly more effective, it usually will also come with a cost benefit.”
Potential barriers to the use of combination therapy are related to policy rather than drug safety or effectiveness, according to Dr. Prager.
“The primary barrier is that mifepristone use is regulated by a REMS [Risk Evaluation and Mitigation Strategy] restriction which requires that providers dispense the medication directly to patients, rather than being able to prescribe it and have patients then pick it up at a pharmacy,” she said. “This restriction is typically used for medications that are dangerous and need to be closely controlled. In the case of mifepristone, the restriction does not serve a safety purpose; it simply limits access to the medication which is still primarily used to medically treat abortion.
“The secondary barrier is stigma against using a medication that is seen as an abortion medication. I fear many providers or practices may avoid putting it on formulary due to this stigma,” Dr. Prager noted.
“There is already sufficient evidence that the combination therapy is superior to monotherapy, and there is also evidence that mifepristone can be safely prescribed [not dispensed] and does not need the REMS requirement,” Dr. Prager said. “I don’t believe more research is needed; just policy change.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Berkley HH et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004063.
FROM OBSTETRICS & GYNECOLOGY
Consider switching up treatment regimens for recurrent bacterial vaginosis
Limited data are available to guide treatment of recurrent bacterial vaginosis, but behavioral changes and switching between approved medication regimens may help, according to a presenter at the virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
Investigational treatments – such as a live biotherapeutic product delivered vaginally or vaginal microbiome transplantation – could someday be additional options if they prove safe and effective. said Debra L. Birenbaum, MD, assistant professor of obstetrics and gynecology at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
As for home remedies, Dr. Birenbaum and another presenter at the conference, Cynthia Rasmussen, MD, urged caution during a panel discussion.
“I think the vagina knows its business, and the more you mess with it, the more you invite trouble,” said Dr. Rasmussen, director emerita of vulvovaginal services at Atrius Health in Burlington, Mass. For instance, tea tree oil, often cited as a home remedy, can be an allergen and very irritating.
“I want to know what women are using, but I try and dissuade them,” said Dr. Birenbaum. “I have to be careful what I say, because you’ll antagonize patients” if you come out strongly against home treatments. “I try to encourage them not to go by things they read on the Internet, because I think that’s where many people are finding their home remedies.”
When counseling patients, an analogy shared during the meeting – the vagina is a self-cleaning oven – may help get the point across. “I love the comment,” Dr. Birenbaum said. “I’ve never used that before. I’m going to start saying that.”
Possible causes and risk factors
Bacterial vaginosis, also known as vaginal dysbiosis, is the most common cause of discharge in women of reproductive age worldwide. Growth of a biofilm may cause the condition, which is characterized by a shift in vaginal flora from a Lactobacilli-dominant environment to one of other bacterial types.
Risk factors include douching, smoking, sex with an uncircumcised partner, and having multiple sexual partners. Bacterial vaginosis may be associated with various complications and infections, including increased risk of preterm delivery, postpartum endometritis, postabortal infection, Trichomonas, chlamydia, and HIV.
Unlike recurrent yeast, which is characterized by four or more episodes per year, recurrent bacterial vaginosis has no official criteria, Dr. Birenbaum said. However, recurrence of bacterial vaginosis “is extremely common,” she said. “Up to 30% of women with [bacterial vaginosis] may recur within 3 months, and up to 50% after 12 months.”
Lifestyle changes and treatments
Recommendations to use condoms, stop smoking, and not douche are important.
In addition, 11 treatment regimens for four drugs – metronidazole, clindamycin, tinidazole, or secnidazole – are available for the treatment of bacterial vaginosis. For recurrent cases, adjusting and switching between the drugs and modes of delivery may help. If a patient started with vaginal gel, they can try an oral medication, or vice versa.
“There’s very little data to guide the optimal therapy for this,” Dr. Birenbaum said. “All of this is worth a try to see if you can beat this before this becomes an ongoing issue.”
As an example of one possible regimen for recurrent bacterial vaginosis, Dr. Birenbaum suggested that a patient could complete a 2- to 4-week course of oral metronidazole instead of the usual 1-week course. The regimen could incorporate boric acid vaginal suppositories 600 mg nightly for 21 days, followed by metronidazole gel 0.75% (one applicator twice per week for 6 months).
New therapies may be on the horizon
In a randomized, double-blind, phase 2b trial published in the New England Journal of Medicine that included more than 220 participants, patients who received an investigational product containing Lactobacillus crispatus CTV-05 (Lactin-V) were less likely to have recurrent bacterial vaginosis at 12 weeks, compared with those who received placebo (30% vs. 45%).
A product in development known as TOL-463, a boric acid–based vaginal anti-infective enhanced with ethylenediaminetetraacetic acid, may be safe and effective, a phase 2 study published in Clinical Infectious Diseases suggests.
Investigators in the United Kingdom designed a trial to compare lactic acid gel and metronidazole, and the findings published in the Trials journal may clarify inconsistent results from prior studies.
Furthermore, preclinical research in Pathogens and Disease has identified cationic amphiphiles that might help fight the biofilm that is formed with Gardnerella vaginalis in patients with bacterial vaginosis, Dr. Birenbaum said.
Finally, an exploratory study in Israel published in Nature Medicine evaluated vaginal microbiome transplants in five patients, three of whom required repeat transplantation. Four patients had long-term remission, and one had a reduction in symptoms
Dr. Birenbaum is a reviewer for UpToDate. Dr. Rasmussen had no relevant disclosures.
Limited data are available to guide treatment of recurrent bacterial vaginosis, but behavioral changes and switching between approved medication regimens may help, according to a presenter at the virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
Investigational treatments – such as a live biotherapeutic product delivered vaginally or vaginal microbiome transplantation – could someday be additional options if they prove safe and effective. said Debra L. Birenbaum, MD, assistant professor of obstetrics and gynecology at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
As for home remedies, Dr. Birenbaum and another presenter at the conference, Cynthia Rasmussen, MD, urged caution during a panel discussion.
“I think the vagina knows its business, and the more you mess with it, the more you invite trouble,” said Dr. Rasmussen, director emerita of vulvovaginal services at Atrius Health in Burlington, Mass. For instance, tea tree oil, often cited as a home remedy, can be an allergen and very irritating.
“I want to know what women are using, but I try and dissuade them,” said Dr. Birenbaum. “I have to be careful what I say, because you’ll antagonize patients” if you come out strongly against home treatments. “I try to encourage them not to go by things they read on the Internet, because I think that’s where many people are finding their home remedies.”
When counseling patients, an analogy shared during the meeting – the vagina is a self-cleaning oven – may help get the point across. “I love the comment,” Dr. Birenbaum said. “I’ve never used that before. I’m going to start saying that.”
Possible causes and risk factors
Bacterial vaginosis, also known as vaginal dysbiosis, is the most common cause of discharge in women of reproductive age worldwide. Growth of a biofilm may cause the condition, which is characterized by a shift in vaginal flora from a Lactobacilli-dominant environment to one of other bacterial types.
Risk factors include douching, smoking, sex with an uncircumcised partner, and having multiple sexual partners. Bacterial vaginosis may be associated with various complications and infections, including increased risk of preterm delivery, postpartum endometritis, postabortal infection, Trichomonas, chlamydia, and HIV.
Unlike recurrent yeast, which is characterized by four or more episodes per year, recurrent bacterial vaginosis has no official criteria, Dr. Birenbaum said. However, recurrence of bacterial vaginosis “is extremely common,” she said. “Up to 30% of women with [bacterial vaginosis] may recur within 3 months, and up to 50% after 12 months.”
Lifestyle changes and treatments
Recommendations to use condoms, stop smoking, and not douche are important.
In addition, 11 treatment regimens for four drugs – metronidazole, clindamycin, tinidazole, or secnidazole – are available for the treatment of bacterial vaginosis. For recurrent cases, adjusting and switching between the drugs and modes of delivery may help. If a patient started with vaginal gel, they can try an oral medication, or vice versa.
“There’s very little data to guide the optimal therapy for this,” Dr. Birenbaum said. “All of this is worth a try to see if you can beat this before this becomes an ongoing issue.”
As an example of one possible regimen for recurrent bacterial vaginosis, Dr. Birenbaum suggested that a patient could complete a 2- to 4-week course of oral metronidazole instead of the usual 1-week course. The regimen could incorporate boric acid vaginal suppositories 600 mg nightly for 21 days, followed by metronidazole gel 0.75% (one applicator twice per week for 6 months).
New therapies may be on the horizon
In a randomized, double-blind, phase 2b trial published in the New England Journal of Medicine that included more than 220 participants, patients who received an investigational product containing Lactobacillus crispatus CTV-05 (Lactin-V) were less likely to have recurrent bacterial vaginosis at 12 weeks, compared with those who received placebo (30% vs. 45%).
A product in development known as TOL-463, a boric acid–based vaginal anti-infective enhanced with ethylenediaminetetraacetic acid, may be safe and effective, a phase 2 study published in Clinical Infectious Diseases suggests.
Investigators in the United Kingdom designed a trial to compare lactic acid gel and metronidazole, and the findings published in the Trials journal may clarify inconsistent results from prior studies.
Furthermore, preclinical research in Pathogens and Disease has identified cationic amphiphiles that might help fight the biofilm that is formed with Gardnerella vaginalis in patients with bacterial vaginosis, Dr. Birenbaum said.
Finally, an exploratory study in Israel published in Nature Medicine evaluated vaginal microbiome transplants in five patients, three of whom required repeat transplantation. Four patients had long-term remission, and one had a reduction in symptoms
Dr. Birenbaum is a reviewer for UpToDate. Dr. Rasmussen had no relevant disclosures.
Limited data are available to guide treatment of recurrent bacterial vaginosis, but behavioral changes and switching between approved medication regimens may help, according to a presenter at the virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
Investigational treatments – such as a live biotherapeutic product delivered vaginally or vaginal microbiome transplantation – could someday be additional options if they prove safe and effective. said Debra L. Birenbaum, MD, assistant professor of obstetrics and gynecology at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
As for home remedies, Dr. Birenbaum and another presenter at the conference, Cynthia Rasmussen, MD, urged caution during a panel discussion.
“I think the vagina knows its business, and the more you mess with it, the more you invite trouble,” said Dr. Rasmussen, director emerita of vulvovaginal services at Atrius Health in Burlington, Mass. For instance, tea tree oil, often cited as a home remedy, can be an allergen and very irritating.
“I want to know what women are using, but I try and dissuade them,” said Dr. Birenbaum. “I have to be careful what I say, because you’ll antagonize patients” if you come out strongly against home treatments. “I try to encourage them not to go by things they read on the Internet, because I think that’s where many people are finding their home remedies.”
When counseling patients, an analogy shared during the meeting – the vagina is a self-cleaning oven – may help get the point across. “I love the comment,” Dr. Birenbaum said. “I’ve never used that before. I’m going to start saying that.”
Possible causes and risk factors
Bacterial vaginosis, also known as vaginal dysbiosis, is the most common cause of discharge in women of reproductive age worldwide. Growth of a biofilm may cause the condition, which is characterized by a shift in vaginal flora from a Lactobacilli-dominant environment to one of other bacterial types.
Risk factors include douching, smoking, sex with an uncircumcised partner, and having multiple sexual partners. Bacterial vaginosis may be associated with various complications and infections, including increased risk of preterm delivery, postpartum endometritis, postabortal infection, Trichomonas, chlamydia, and HIV.
Unlike recurrent yeast, which is characterized by four or more episodes per year, recurrent bacterial vaginosis has no official criteria, Dr. Birenbaum said. However, recurrence of bacterial vaginosis “is extremely common,” she said. “Up to 30% of women with [bacterial vaginosis] may recur within 3 months, and up to 50% after 12 months.”
Lifestyle changes and treatments
Recommendations to use condoms, stop smoking, and not douche are important.
In addition, 11 treatment regimens for four drugs – metronidazole, clindamycin, tinidazole, or secnidazole – are available for the treatment of bacterial vaginosis. For recurrent cases, adjusting and switching between the drugs and modes of delivery may help. If a patient started with vaginal gel, they can try an oral medication, or vice versa.
“There’s very little data to guide the optimal therapy for this,” Dr. Birenbaum said. “All of this is worth a try to see if you can beat this before this becomes an ongoing issue.”
As an example of one possible regimen for recurrent bacterial vaginosis, Dr. Birenbaum suggested that a patient could complete a 2- to 4-week course of oral metronidazole instead of the usual 1-week course. The regimen could incorporate boric acid vaginal suppositories 600 mg nightly for 21 days, followed by metronidazole gel 0.75% (one applicator twice per week for 6 months).
New therapies may be on the horizon
In a randomized, double-blind, phase 2b trial published in the New England Journal of Medicine that included more than 220 participants, patients who received an investigational product containing Lactobacillus crispatus CTV-05 (Lactin-V) were less likely to have recurrent bacterial vaginosis at 12 weeks, compared with those who received placebo (30% vs. 45%).
A product in development known as TOL-463, a boric acid–based vaginal anti-infective enhanced with ethylenediaminetetraacetic acid, may be safe and effective, a phase 2 study published in Clinical Infectious Diseases suggests.
Investigators in the United Kingdom designed a trial to compare lactic acid gel and metronidazole, and the findings published in the Trials journal may clarify inconsistent results from prior studies.
Furthermore, preclinical research in Pathogens and Disease has identified cationic amphiphiles that might help fight the biofilm that is formed with Gardnerella vaginalis in patients with bacterial vaginosis, Dr. Birenbaum said.
Finally, an exploratory study in Israel published in Nature Medicine evaluated vaginal microbiome transplants in five patients, three of whom required repeat transplantation. Four patients had long-term remission, and one had a reduction in symptoms
Dr. Birenbaum is a reviewer for UpToDate. Dr. Rasmussen had no relevant disclosures.
FROM THE ISSVD BIENNIAL CONFERENCE
PRGLAC recommendations
1. Include and integrate pregnant women and lactating women in the clinical research agenda.
2. Increase the quantity, quality, and timeliness of research on safety and efficacy of therapeutic products used by pregnant women and lactating women.
3. Expand the workforce of clinicians and research investigators with expertise in obstetric and lactation pharmacology and therapeutics.
4. Remove regulatory barriers to research in pregnant women.
5. Create a public awareness campaign to engage the public and health care providers in research on pregnant women and lactating women.
6. Develop and implement evidence-based communication strategies with health care providers on information relevant to research on pregnant women and lactating women.
7. Develop separate programs to study therapeutic products used off patent in pregnant women and lactating women using the National Institute of Health Best Pharmaceuticals for Children Act (BPCA) as a model.
8. Reduce liability to facilitate an evidence base for new therapeutic products that may be used by women who are or may become pregnant and by lactating women.
9. Implement a proactive approach to protocol development and study design to include pregnant women and lactating women in clinical research.
10. Develop programs to drive discovery and development of therapeutics and new therapeutic products for conditions specific to pregnant women and lactating women.
11. Utilize and improve existing resources for data to inform the evidence and provide a foundation for research on pregnant women and lactating women.
12. Leverage established and support new infrastructures/collaborations to perform research in pregnant women and lactating women.
13. Optimize registries for pregnancy and lactation.
14. The Department of Health & Human Services Secretary should consider exercising the authority provided in law to extend the PRGLAC Task Force when its charter expires in March 2019.
15. Establish an Advisory Committee to monitor and report on implementation of recommendations, updating regulations, and guidance, as applicable, regarding the inclusion of pregnant women and lactating women in clinical research.
Source: Task Force on Research Specific to Pregnant Women and Lactating Women; Report to Secretary, Health and Human Services, Congress, September 2018
1. Include and integrate pregnant women and lactating women in the clinical research agenda.
2. Increase the quantity, quality, and timeliness of research on safety and efficacy of therapeutic products used by pregnant women and lactating women.
3. Expand the workforce of clinicians and research investigators with expertise in obstetric and lactation pharmacology and therapeutics.
4. Remove regulatory barriers to research in pregnant women.
5. Create a public awareness campaign to engage the public and health care providers in research on pregnant women and lactating women.
6. Develop and implement evidence-based communication strategies with health care providers on information relevant to research on pregnant women and lactating women.
7. Develop separate programs to study therapeutic products used off patent in pregnant women and lactating women using the National Institute of Health Best Pharmaceuticals for Children Act (BPCA) as a model.
8. Reduce liability to facilitate an evidence base for new therapeutic products that may be used by women who are or may become pregnant and by lactating women.
9. Implement a proactive approach to protocol development and study design to include pregnant women and lactating women in clinical research.
10. Develop programs to drive discovery and development of therapeutics and new therapeutic products for conditions specific to pregnant women and lactating women.
11. Utilize and improve existing resources for data to inform the evidence and provide a foundation for research on pregnant women and lactating women.
12. Leverage established and support new infrastructures/collaborations to perform research in pregnant women and lactating women.
13. Optimize registries for pregnancy and lactation.
14. The Department of Health & Human Services Secretary should consider exercising the authority provided in law to extend the PRGLAC Task Force when its charter expires in March 2019.
15. Establish an Advisory Committee to monitor and report on implementation of recommendations, updating regulations, and guidance, as applicable, regarding the inclusion of pregnant women and lactating women in clinical research.
Source: Task Force on Research Specific to Pregnant Women and Lactating Women; Report to Secretary, Health and Human Services, Congress, September 2018
1. Include and integrate pregnant women and lactating women in the clinical research agenda.
2. Increase the quantity, quality, and timeliness of research on safety and efficacy of therapeutic products used by pregnant women and lactating women.
3. Expand the workforce of clinicians and research investigators with expertise in obstetric and lactation pharmacology and therapeutics.
4. Remove regulatory barriers to research in pregnant women.
5. Create a public awareness campaign to engage the public and health care providers in research on pregnant women and lactating women.
6. Develop and implement evidence-based communication strategies with health care providers on information relevant to research on pregnant women and lactating women.
7. Develop separate programs to study therapeutic products used off patent in pregnant women and lactating women using the National Institute of Health Best Pharmaceuticals for Children Act (BPCA) as a model.
8. Reduce liability to facilitate an evidence base for new therapeutic products that may be used by women who are or may become pregnant and by lactating women.
9. Implement a proactive approach to protocol development and study design to include pregnant women and lactating women in clinical research.
10. Develop programs to drive discovery and development of therapeutics and new therapeutic products for conditions specific to pregnant women and lactating women.
11. Utilize and improve existing resources for data to inform the evidence and provide a foundation for research on pregnant women and lactating women.
12. Leverage established and support new infrastructures/collaborations to perform research in pregnant women and lactating women.
13. Optimize registries for pregnancy and lactation.
14. The Department of Health & Human Services Secretary should consider exercising the authority provided in law to extend the PRGLAC Task Force when its charter expires in March 2019.
15. Establish an Advisory Committee to monitor and report on implementation of recommendations, updating regulations, and guidance, as applicable, regarding the inclusion of pregnant women and lactating women in clinical research.
Source: Task Force on Research Specific to Pregnant Women and Lactating Women; Report to Secretary, Health and Human Services, Congress, September 2018
Safe, effective therapies: Establishing a path forward
I have had friends and colleagues visibly shrink away when I say that my work involves the study of medication safety in pregnancy. “Yikes! I would never let my daughter participate in a clinical study if she was pregnant!” I hear. It’s an interesting response. Understandably protective of a loved one, except that the loved one is an adult woman who presumably can make her own choices. And the response reveals an assumption that medications are tested in all populations before approval for market. Sadly, the response is ill-informed given that pregnant women are still excluded from most if not all clinical research. My work, by the way, is focused on postapproval studies.
Translating the above response to a larger picture, health care providers and pharmaceutical manufacturers also have their concerns about pregnant women and lactating women participating in clinical research. Along with the patient and her loved ones, all parties’ concerns are valid. However, there is a harsh reality: According to a study in the American Journal of Obstetrics & Gynecology, an estimated 50% of U.S. women take one or more prescription medications during pregnancy. Once marketed, therapies are prescribed to pregnant women, knowingly and unknowingly, and without evidence-based knowledge of their safety. If postapproval safety studies are undertaken, decades may pass as data accrue and before results become available. In general, even less is known about the safety of medications in breastmilk.
Without a path forward that includes pregnant women and lactating women in clinical research, we will remain without timely knowledge of medication safety. Further, our understanding of efficacy will be based on clinical studies of nonpregnant women. Recognizing the need for this information, the Task Force on Research Specific to Pregnant Women and Lactating Women (PRGLAC) was convened in 2017 and tasked with determining this path forward.
The PRGLAC was established by the 21st Century Cures Act, a law designed to help speed up medical product development. Managed by the National Institutes of Health, the PRGLAC is made up of representatives of all federal agencies with responsibilities for women’s health and research, as well as clinicians, industry experts, and other experts. The PRGLAC’s work has been conducted in two phases.
In Phase I, PRGLAC was charged with identifying gaps in knowledge and research regarding safe and effective therapies for pregnant women and lactating women. The Task Force conducted four public meetings in 2017 and 2018, and submitted their conclusions to Congress and the Secretary of Health & Human Services in a publicly available report. The report provides 15 specific recommendations, several of which are directly relevant to obstetricians: No. 3 recommends expanding the workforce of clinicians and research investigators with expertise in obstetric and lactation pharmacology, No. 6 recommends the development and implementation of evidence-based communication strategies with health care providers, and No. 13 recommends optimization of registries for pregnancy and lactation. Obstetricians can make a positive contribution to accruing medication safety data by being aware of pregnancy registries and indicating their availability to eligible patients.
In the spring of 2019, the PRGLAC reconvened with a 2-year mandate and a new charge for Phase II: to develop plans for implementing the recommendations laid out in the Phase I report. Four working groups (WGs) were identified to address the recommendations of the report: WG1 Research and Training, WG2 Regulatory, WG3 Communication and Registries, and WG4 Discovery. The four groups have deliberated, and a new report is being finalized. The PRGLAC’s efforts provide a fresh conversation to address long-standing issues to provide evidence-based information for the treatment of pregnant and lactating women. Once available, the final report will be posted on the PRGLAC website.
The recommendations in this report, when implemented, are directly relevant to patient care and clinician training and will provide a path forward for the inclusion of pregnant and lactating women in clinical research or a firm justification for their exclusion.
Dr. Hardy is a consultant on global maternal-child health and pharmacoepidemiology. She also represents the Society for Birth Defects Research and Prevention and the Organization of Teratology Information Specialists at PRGLAC meetings. Dr. Hardy disclosed she has worked with multiple pharmaceutical manufacturers regarding medication safety studies in pregnancy, most recently Biohaven. Email her at obnews@mdedge.com.
I have had friends and colleagues visibly shrink away when I say that my work involves the study of medication safety in pregnancy. “Yikes! I would never let my daughter participate in a clinical study if she was pregnant!” I hear. It’s an interesting response. Understandably protective of a loved one, except that the loved one is an adult woman who presumably can make her own choices. And the response reveals an assumption that medications are tested in all populations before approval for market. Sadly, the response is ill-informed given that pregnant women are still excluded from most if not all clinical research. My work, by the way, is focused on postapproval studies.
Translating the above response to a larger picture, health care providers and pharmaceutical manufacturers also have their concerns about pregnant women and lactating women participating in clinical research. Along with the patient and her loved ones, all parties’ concerns are valid. However, there is a harsh reality: According to a study in the American Journal of Obstetrics & Gynecology, an estimated 50% of U.S. women take one or more prescription medications during pregnancy. Once marketed, therapies are prescribed to pregnant women, knowingly and unknowingly, and without evidence-based knowledge of their safety. If postapproval safety studies are undertaken, decades may pass as data accrue and before results become available. In general, even less is known about the safety of medications in breastmilk.
Without a path forward that includes pregnant women and lactating women in clinical research, we will remain without timely knowledge of medication safety. Further, our understanding of efficacy will be based on clinical studies of nonpregnant women. Recognizing the need for this information, the Task Force on Research Specific to Pregnant Women and Lactating Women (PRGLAC) was convened in 2017 and tasked with determining this path forward.
The PRGLAC was established by the 21st Century Cures Act, a law designed to help speed up medical product development. Managed by the National Institutes of Health, the PRGLAC is made up of representatives of all federal agencies with responsibilities for women’s health and research, as well as clinicians, industry experts, and other experts. The PRGLAC’s work has been conducted in two phases.
In Phase I, PRGLAC was charged with identifying gaps in knowledge and research regarding safe and effective therapies for pregnant women and lactating women. The Task Force conducted four public meetings in 2017 and 2018, and submitted their conclusions to Congress and the Secretary of Health & Human Services in a publicly available report. The report provides 15 specific recommendations, several of which are directly relevant to obstetricians: No. 3 recommends expanding the workforce of clinicians and research investigators with expertise in obstetric and lactation pharmacology, No. 6 recommends the development and implementation of evidence-based communication strategies with health care providers, and No. 13 recommends optimization of registries for pregnancy and lactation. Obstetricians can make a positive contribution to accruing medication safety data by being aware of pregnancy registries and indicating their availability to eligible patients.
In the spring of 2019, the PRGLAC reconvened with a 2-year mandate and a new charge for Phase II: to develop plans for implementing the recommendations laid out in the Phase I report. Four working groups (WGs) were identified to address the recommendations of the report: WG1 Research and Training, WG2 Regulatory, WG3 Communication and Registries, and WG4 Discovery. The four groups have deliberated, and a new report is being finalized. The PRGLAC’s efforts provide a fresh conversation to address long-standing issues to provide evidence-based information for the treatment of pregnant and lactating women. Once available, the final report will be posted on the PRGLAC website.
The recommendations in this report, when implemented, are directly relevant to patient care and clinician training and will provide a path forward for the inclusion of pregnant and lactating women in clinical research or a firm justification for their exclusion.
Dr. Hardy is a consultant on global maternal-child health and pharmacoepidemiology. She also represents the Society for Birth Defects Research and Prevention and the Organization of Teratology Information Specialists at PRGLAC meetings. Dr. Hardy disclosed she has worked with multiple pharmaceutical manufacturers regarding medication safety studies in pregnancy, most recently Biohaven. Email her at obnews@mdedge.com.
I have had friends and colleagues visibly shrink away when I say that my work involves the study of medication safety in pregnancy. “Yikes! I would never let my daughter participate in a clinical study if she was pregnant!” I hear. It’s an interesting response. Understandably protective of a loved one, except that the loved one is an adult woman who presumably can make her own choices. And the response reveals an assumption that medications are tested in all populations before approval for market. Sadly, the response is ill-informed given that pregnant women are still excluded from most if not all clinical research. My work, by the way, is focused on postapproval studies.
Translating the above response to a larger picture, health care providers and pharmaceutical manufacturers also have their concerns about pregnant women and lactating women participating in clinical research. Along with the patient and her loved ones, all parties’ concerns are valid. However, there is a harsh reality: According to a study in the American Journal of Obstetrics & Gynecology, an estimated 50% of U.S. women take one or more prescription medications during pregnancy. Once marketed, therapies are prescribed to pregnant women, knowingly and unknowingly, and without evidence-based knowledge of their safety. If postapproval safety studies are undertaken, decades may pass as data accrue and before results become available. In general, even less is known about the safety of medications in breastmilk.
Without a path forward that includes pregnant women and lactating women in clinical research, we will remain without timely knowledge of medication safety. Further, our understanding of efficacy will be based on clinical studies of nonpregnant women. Recognizing the need for this information, the Task Force on Research Specific to Pregnant Women and Lactating Women (PRGLAC) was convened in 2017 and tasked with determining this path forward.
The PRGLAC was established by the 21st Century Cures Act, a law designed to help speed up medical product development. Managed by the National Institutes of Health, the PRGLAC is made up of representatives of all federal agencies with responsibilities for women’s health and research, as well as clinicians, industry experts, and other experts. The PRGLAC’s work has been conducted in two phases.
In Phase I, PRGLAC was charged with identifying gaps in knowledge and research regarding safe and effective therapies for pregnant women and lactating women. The Task Force conducted four public meetings in 2017 and 2018, and submitted their conclusions to Congress and the Secretary of Health & Human Services in a publicly available report. The report provides 15 specific recommendations, several of which are directly relevant to obstetricians: No. 3 recommends expanding the workforce of clinicians and research investigators with expertise in obstetric and lactation pharmacology, No. 6 recommends the development and implementation of evidence-based communication strategies with health care providers, and No. 13 recommends optimization of registries for pregnancy and lactation. Obstetricians can make a positive contribution to accruing medication safety data by being aware of pregnancy registries and indicating their availability to eligible patients.
In the spring of 2019, the PRGLAC reconvened with a 2-year mandate and a new charge for Phase II: to develop plans for implementing the recommendations laid out in the Phase I report. Four working groups (WGs) were identified to address the recommendations of the report: WG1 Research and Training, WG2 Regulatory, WG3 Communication and Registries, and WG4 Discovery. The four groups have deliberated, and a new report is being finalized. The PRGLAC’s efforts provide a fresh conversation to address long-standing issues to provide evidence-based information for the treatment of pregnant and lactating women. Once available, the final report will be posted on the PRGLAC website.
The recommendations in this report, when implemented, are directly relevant to patient care and clinician training and will provide a path forward for the inclusion of pregnant and lactating women in clinical research or a firm justification for their exclusion.
Dr. Hardy is a consultant on global maternal-child health and pharmacoepidemiology. She also represents the Society for Birth Defects Research and Prevention and the Organization of Teratology Information Specialists at PRGLAC meetings. Dr. Hardy disclosed she has worked with multiple pharmaceutical manufacturers regarding medication safety studies in pregnancy, most recently Biohaven. Email her at obnews@mdedge.com.
Hair loss and scalp papules
The punch biopsies were consistent with lichen planopilaris, an idiopathic, immune-mediated scarring alopecia that largely affects women between the ages of 40 and 70 years. In this variant of lichen planus, T cells target hair bulbs and cause destruction with scarring and permanent hair loss. Distribution may be patchy or may be more concentrated on the crown or involve the frontal scalp—a subtype called frontal fibrosing alopecia. Early recognition and intervention may save hair follicles and minimize disease severity.
The differential diagnosis includes traction alopecia, discoid lupus erythematosus, alopecia areata, centrifugal cicatricial alopecia, and folliculitis decalvans. The diagnosis may be confirmed with a scalp biopsy of actively inflamed follicles. Biopsy of scarred areas is likely to be nonspecific and unhelpful.
Treatment is targeted at slowing progression and symptom management. First-line therapy often includes potent corticosteroids (intralesional, topical, or systemic). Longer courses of steroid-sparing agents may be considered, including hydroxychloroquine, tacrolimus, ciclosporin, methotrexate, or acitretin. Hair styling and coloring, as well as hairpieces, often are used to conceal patches of hair loss. Hair transplantation is expensive but can be used to increase hair density in scarred areas once disease is controlled.
In this case, the patient was started on clobetasol solution 0.05% to be applied nightly to affected areas of the scalp. This treatment helped with the itching, but the inflammation and hair loss continued to worsen after 2 months. At that point, hydroxychloroquine 200 mg bid was added to the regimen, and hair loss and associated symptoms stopped. The patient remained on this therapy for 16 months. The hydroxychloroquine was then stopped, and the patient was advised to use the topical clobetasol, as needed.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Errichetti E, Figini M, Croatto M, et al. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. 2018;11:91-102.
The punch biopsies were consistent with lichen planopilaris, an idiopathic, immune-mediated scarring alopecia that largely affects women between the ages of 40 and 70 years. In this variant of lichen planus, T cells target hair bulbs and cause destruction with scarring and permanent hair loss. Distribution may be patchy or may be more concentrated on the crown or involve the frontal scalp—a subtype called frontal fibrosing alopecia. Early recognition and intervention may save hair follicles and minimize disease severity.
The differential diagnosis includes traction alopecia, discoid lupus erythematosus, alopecia areata, centrifugal cicatricial alopecia, and folliculitis decalvans. The diagnosis may be confirmed with a scalp biopsy of actively inflamed follicles. Biopsy of scarred areas is likely to be nonspecific and unhelpful.
Treatment is targeted at slowing progression and symptom management. First-line therapy often includes potent corticosteroids (intralesional, topical, or systemic). Longer courses of steroid-sparing agents may be considered, including hydroxychloroquine, tacrolimus, ciclosporin, methotrexate, or acitretin. Hair styling and coloring, as well as hairpieces, often are used to conceal patches of hair loss. Hair transplantation is expensive but can be used to increase hair density in scarred areas once disease is controlled.
In this case, the patient was started on clobetasol solution 0.05% to be applied nightly to affected areas of the scalp. This treatment helped with the itching, but the inflammation and hair loss continued to worsen after 2 months. At that point, hydroxychloroquine 200 mg bid was added to the regimen, and hair loss and associated symptoms stopped. The patient remained on this therapy for 16 months. The hydroxychloroquine was then stopped, and the patient was advised to use the topical clobetasol, as needed.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
The punch biopsies were consistent with lichen planopilaris, an idiopathic, immune-mediated scarring alopecia that largely affects women between the ages of 40 and 70 years. In this variant of lichen planus, T cells target hair bulbs and cause destruction with scarring and permanent hair loss. Distribution may be patchy or may be more concentrated on the crown or involve the frontal scalp—a subtype called frontal fibrosing alopecia. Early recognition and intervention may save hair follicles and minimize disease severity.
The differential diagnosis includes traction alopecia, discoid lupus erythematosus, alopecia areata, centrifugal cicatricial alopecia, and folliculitis decalvans. The diagnosis may be confirmed with a scalp biopsy of actively inflamed follicles. Biopsy of scarred areas is likely to be nonspecific and unhelpful.
Treatment is targeted at slowing progression and symptom management. First-line therapy often includes potent corticosteroids (intralesional, topical, or systemic). Longer courses of steroid-sparing agents may be considered, including hydroxychloroquine, tacrolimus, ciclosporin, methotrexate, or acitretin. Hair styling and coloring, as well as hairpieces, often are used to conceal patches of hair loss. Hair transplantation is expensive but can be used to increase hair density in scarred areas once disease is controlled.
In this case, the patient was started on clobetasol solution 0.05% to be applied nightly to affected areas of the scalp. This treatment helped with the itching, but the inflammation and hair loss continued to worsen after 2 months. At that point, hydroxychloroquine 200 mg bid was added to the regimen, and hair loss and associated symptoms stopped. The patient remained on this therapy for 16 months. The hydroxychloroquine was then stopped, and the patient was advised to use the topical clobetasol, as needed.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Errichetti E, Figini M, Croatto M, et al. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. 2018;11:91-102.
Errichetti E, Figini M, Croatto M, et al. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. 2018;11:91-102.
Study offers reassurance to postmenopausal women taking hormone therapy
References
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;32:369-380.
- The North American Menopause Society. Menopause Guidebook. 8th ed. www.menopause.org/publications/consumer-publications/-em-menopause-guidebook-em-8th-edition. Accessed September 25, 2020.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;318:2224-2233.
References
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;32:369-380.
- The North American Menopause Society. Menopause Guidebook. 8th ed. www.menopause.org/publications/consumer-publications/-em-menopause-guidebook-em-8th-edition. Accessed September 25, 2020.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;318:2224-2233.
References
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;32:369-380.
- The North American Menopause Society. Menopause Guidebook. 8th ed. www.menopause.org/publications/consumer-publications/-em-menopause-guidebook-em-8th-edition. Accessed September 25, 2020.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;318:2224-2233.