User login
Three-step approach may help relieve one of the itchiest vulvar conditions
A three-step approach may help relieve itch in patients with lichen simplex chronicus, “one of the itchiest conditions that we ever see on the vulva,” an expert advised at the virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
For some patients, such as those with excessive sweating or underlying psoriasis, seeing a dermatologist may be beneficial, physicians at the meeting suggested.
Treatment should aim to optimize epithelial barrier function, reduce inflammation, and stop scratching, Lynette Margesson, MD, said in a lecture at the biennial meeting, which is held by the International Society for the Study of Vulvovaginal Disease (ISSVD). “With this condition, please look always for more than one problem.”
said Dr. Margesson, an obstetrician and gynecologist at Geisel School of Medicine at Dartmouth in Hanover, N.H. “It is because of chronic rubbing and scratching on top of something else.”
It may develop on top of atopic dermatitis, psoriasis, or contact dermatitis, as well as infection, lichen sclerosus, lichen planus, or neoplasia.
Lichen simplex chronicus is characterized by years of relentless itching, and patients may wake up at night scratching. The skin looks and feels leathery, and the condition can be localized or around the entire vulva. Heat, humidity, stress, and irritants may exacerbate the condition.
Patients often try to wash the rash away with scrubbers and cleansers, which only makes it worse, Dr. Margesson said.
To get patients better, improve barrier function, such as by controlling infections, reducing sweating, avoiding irritants, and stopping excessive hygiene. Immediate therapy may include soaks, cool compresses, and ointments.
A superpotent steroid taper (e.g., clobetasol 0.05% ointment), a prednisone taper, or intramuscular triamcinolone may reduce inflammation. Dr. Margesson usually uses clobetasol, although this treatment or halobetasol can burn if patients have open skin. In such cases, she uses prednisone or intramuscular triamcinolone.
Sedating medications may help patients stop scratching, especially at night. Hydroxyzine, doxepin, or amitriptyline 2-3 hours before bedtime can help. Scratching can be a form of obsessive-compulsive disorder, and a small dose of citalopram may help during the day. Patients with significant psychological factors can be difficult to manage and tend to relapse easily, Dr. Margesson said.
If lichen simplex chronicus recurs, test for infections and allergies. “Maybe they need a mild corticosteroid all the time, like 2.5% hydrocortisone to alternate with your superpotent steroid so you can use it longer without thinning the skin,” she suggested.
Although Dr. Margesson does not often treat hyperhidrosis, addressing excessive sweating can make a big difference for patients, she said.
If a gynecologist identifies a patient who may benefit from treatment of hyperhidrosis but has limited experience with medications for this condition, it might make sense to work with a dermatologist, Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., suggested during a panel discussion. Most dermatologists treat hyperhidrosis regularly, she said.
Dermatologists also may help treat patients with psoriasis who need systemic medication, Dr. Margesson said.
“In terms of ... doing the lab monitoring and knowing what side effects to look out for, your colleagues who use these medicines more are going to be more comfortable with that,” Dr. Venkatesan said. They also may have more experience navigating insurance denials to obtain a therapy. “Don’t think you are passing the buck to someone else. Sometimes that is the right thing to do, to get that help from someone else.”
Dr. Margesson is an author for UpToDate. Dr. Venkatesan had no conflicts of interest.
A three-step approach may help relieve itch in patients with lichen simplex chronicus, “one of the itchiest conditions that we ever see on the vulva,” an expert advised at the virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
For some patients, such as those with excessive sweating or underlying psoriasis, seeing a dermatologist may be beneficial, physicians at the meeting suggested.
Treatment should aim to optimize epithelial barrier function, reduce inflammation, and stop scratching, Lynette Margesson, MD, said in a lecture at the biennial meeting, which is held by the International Society for the Study of Vulvovaginal Disease (ISSVD). “With this condition, please look always for more than one problem.”
said Dr. Margesson, an obstetrician and gynecologist at Geisel School of Medicine at Dartmouth in Hanover, N.H. “It is because of chronic rubbing and scratching on top of something else.”
It may develop on top of atopic dermatitis, psoriasis, or contact dermatitis, as well as infection, lichen sclerosus, lichen planus, or neoplasia.
Lichen simplex chronicus is characterized by years of relentless itching, and patients may wake up at night scratching. The skin looks and feels leathery, and the condition can be localized or around the entire vulva. Heat, humidity, stress, and irritants may exacerbate the condition.
Patients often try to wash the rash away with scrubbers and cleansers, which only makes it worse, Dr. Margesson said.
To get patients better, improve barrier function, such as by controlling infections, reducing sweating, avoiding irritants, and stopping excessive hygiene. Immediate therapy may include soaks, cool compresses, and ointments.
A superpotent steroid taper (e.g., clobetasol 0.05% ointment), a prednisone taper, or intramuscular triamcinolone may reduce inflammation. Dr. Margesson usually uses clobetasol, although this treatment or halobetasol can burn if patients have open skin. In such cases, she uses prednisone or intramuscular triamcinolone.
Sedating medications may help patients stop scratching, especially at night. Hydroxyzine, doxepin, or amitriptyline 2-3 hours before bedtime can help. Scratching can be a form of obsessive-compulsive disorder, and a small dose of citalopram may help during the day. Patients with significant psychological factors can be difficult to manage and tend to relapse easily, Dr. Margesson said.
If lichen simplex chronicus recurs, test for infections and allergies. “Maybe they need a mild corticosteroid all the time, like 2.5% hydrocortisone to alternate with your superpotent steroid so you can use it longer without thinning the skin,” she suggested.
Although Dr. Margesson does not often treat hyperhidrosis, addressing excessive sweating can make a big difference for patients, she said.
If a gynecologist identifies a patient who may benefit from treatment of hyperhidrosis but has limited experience with medications for this condition, it might make sense to work with a dermatologist, Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., suggested during a panel discussion. Most dermatologists treat hyperhidrosis regularly, she said.
Dermatologists also may help treat patients with psoriasis who need systemic medication, Dr. Margesson said.
“In terms of ... doing the lab monitoring and knowing what side effects to look out for, your colleagues who use these medicines more are going to be more comfortable with that,” Dr. Venkatesan said. They also may have more experience navigating insurance denials to obtain a therapy. “Don’t think you are passing the buck to someone else. Sometimes that is the right thing to do, to get that help from someone else.”
Dr. Margesson is an author for UpToDate. Dr. Venkatesan had no conflicts of interest.
A three-step approach may help relieve itch in patients with lichen simplex chronicus, “one of the itchiest conditions that we ever see on the vulva,” an expert advised at the virtual conference on diseases of the vulva and vagina, hosted by the International Society for the Study of Vulvovaginal Disease.
For some patients, such as those with excessive sweating or underlying psoriasis, seeing a dermatologist may be beneficial, physicians at the meeting suggested.
Treatment should aim to optimize epithelial barrier function, reduce inflammation, and stop scratching, Lynette Margesson, MD, said in a lecture at the biennial meeting, which is held by the International Society for the Study of Vulvovaginal Disease (ISSVD). “With this condition, please look always for more than one problem.”
said Dr. Margesson, an obstetrician and gynecologist at Geisel School of Medicine at Dartmouth in Hanover, N.H. “It is because of chronic rubbing and scratching on top of something else.”
It may develop on top of atopic dermatitis, psoriasis, or contact dermatitis, as well as infection, lichen sclerosus, lichen planus, or neoplasia.
Lichen simplex chronicus is characterized by years of relentless itching, and patients may wake up at night scratching. The skin looks and feels leathery, and the condition can be localized or around the entire vulva. Heat, humidity, stress, and irritants may exacerbate the condition.
Patients often try to wash the rash away with scrubbers and cleansers, which only makes it worse, Dr. Margesson said.
To get patients better, improve barrier function, such as by controlling infections, reducing sweating, avoiding irritants, and stopping excessive hygiene. Immediate therapy may include soaks, cool compresses, and ointments.
A superpotent steroid taper (e.g., clobetasol 0.05% ointment), a prednisone taper, or intramuscular triamcinolone may reduce inflammation. Dr. Margesson usually uses clobetasol, although this treatment or halobetasol can burn if patients have open skin. In such cases, she uses prednisone or intramuscular triamcinolone.
Sedating medications may help patients stop scratching, especially at night. Hydroxyzine, doxepin, or amitriptyline 2-3 hours before bedtime can help. Scratching can be a form of obsessive-compulsive disorder, and a small dose of citalopram may help during the day. Patients with significant psychological factors can be difficult to manage and tend to relapse easily, Dr. Margesson said.
If lichen simplex chronicus recurs, test for infections and allergies. “Maybe they need a mild corticosteroid all the time, like 2.5% hydrocortisone to alternate with your superpotent steroid so you can use it longer without thinning the skin,” she suggested.
Although Dr. Margesson does not often treat hyperhidrosis, addressing excessive sweating can make a big difference for patients, she said.
If a gynecologist identifies a patient who may benefit from treatment of hyperhidrosis but has limited experience with medications for this condition, it might make sense to work with a dermatologist, Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., suggested during a panel discussion. Most dermatologists treat hyperhidrosis regularly, she said.
Dermatologists also may help treat patients with psoriasis who need systemic medication, Dr. Margesson said.
“In terms of ... doing the lab monitoring and knowing what side effects to look out for, your colleagues who use these medicines more are going to be more comfortable with that,” Dr. Venkatesan said. They also may have more experience navigating insurance denials to obtain a therapy. “Don’t think you are passing the buck to someone else. Sometimes that is the right thing to do, to get that help from someone else.”
Dr. Margesson is an author for UpToDate. Dr. Venkatesan had no conflicts of interest.
FROM ISSVD BIENNIAL CONFERENCE
Without Ginsburg, judicial threats to the ACA, reproductive rights heighten
On Feb. 27, 2018, I got an email from the Heritage Foundation that alerted me to a news conference that afternoon held by Republican attorneys general of Texas and other states. It was referred to only as a “discussion about the Affordable Care Act lawsuit.”
I sent the following note to my editor: “I’m off to the Hill anyway. I could stop by this. You never know what it might morph into.”
Few people took that case very seriously – barely a handful of reporters attended the news conference. But it has now “morphed into” the latest existential threat to the Affordable Care Act, scheduled for oral arguments at the Supreme Court a week after the general election in November. And with the death of Justice Ruth Bader Ginsburg on Friday, that case could well morph into the threat that brings down the law in its entirety.
Democrats are raising alarms about the future of the law without Ms. Ginsburg. House Speaker Nancy Pelosi, speaking on ABC’s “This Week” Sunday morning, said that part of the strategy by President Trump and Senate Republicans to quickly fill her seat was to help undermine the ACA.
“The president is rushing to make some kind of a decision because … Nov. 10 is when the arguments begin on the Affordable Care Act,” she said. “He doesn’t want to crush the virus. He wants to crush the Affordable Care Act.”
Ms. Ginsburg’s death could throw an already chaotic general election campaign during a pandemic into even more turmoil.
Let’s take them one at a time.
The ACA under fire – again
The GOP attorneys general argued in February 2018 that the Republican-sponsored tax cut bill Congress passed two months earlier had rendered the ACA unconstitutional by reducing to zero the ACA’s penalty for not having insurance. They based their argument on Chief Justice John Roberts’ 2012 conclusion that the ACA was valid, interpreting that penalty as a constitutionally appropriate tax.
Most legal scholars, including several who challenged the law before the Supreme Court in 2012 and again in 2015, find the argument that the entire law should fall to be unconvincing. “If courts invalidate an entire law merely because Congress eliminates or revises one part, as happened here, that may well inhibit necessary reform of federal legislation in the future by turning it into an ‘all or nothing’ proposition,” wrote a group of conservative and liberal law professors in a brief filed in the case.
Still, in December 2018, U.S. District Judge Reed O’Connor in Texas accepted the GOP argument and declared the law unconstitutional. In December 2019, a three-judge 5th Circuit appeals court panel in New Orleans agreed that without the penalty the requirement to buy insurance is unconstitutional. But it sent the case back to Mr. O’Connor to suggest that perhaps the entire law need not fall.
Not wanting to wait the months or years that reconsideration would take, Democratic attorneys general defending the ACA asked the Supreme Court to hear the case this year. (Democrats are defending the law in court because the Trump administration decided to support the GOP attorneys general’s case.) The court agreed to take the case but scheduled arguments for the week after the November election.
While the fate of the ACA was and is a live political issue, few legal observers were terribly worried about the legal outcome of the case, now known as Texas v. California, if only because the case seemed much weaker than the 2012 and 2015 cases in which Mr. Roberts joined the court’s four liberals. In the 2015 case, which challenged the validity of federal tax subsidies helping millions of Americans buy health insurance on the ACA’s marketplaces, both Mr. Roberts and now-retired Justice Anthony Kennedy voted to uphold the law.
But without Ms. Ginsburg, the case could wind up in a 4-4 tie, even if Mr. Roberts supports the law’s constitutionality. That could let the lower-court ruling stand, although it would not be binding on other courts outside of the 5th Circuit. The court could also put off the arguments or, if the Republican Senate replaces Ms. Ginsburg with another conservative justice before arguments are heard, Republicans could secure a 5-4 ruling against the law. Some court observers argue that Justice Brett Kavanaugh has not favored invalidating an entire statute if only part of it is flawed and might not approve overturning the ACA. Still, what started out as an effort to energize Republican voters for the 2018 midterms after Congress failed to “repeal and replace” the health law in 2017 could end up throwing the nation’s entire health system into chaos.
At least 20 million Americans – and likely many more who sought coverage since the start of the coronavirus pandemic — who buy insurance through the ACA marketplaces or have Medicaid through the law’s expansion could lose coverage right away. Many millions more would lose the law’s popular protections guaranteeing coverage for people with preexisting health conditions, including those who have had COVID-19.
Adult children under age 26 years would no longer be guaranteed the right to remain on their parents’ health plans, and Medicare patients would lose enhanced prescription drug coverage. Women would lose guaranteed access to birth control at no out-of-pocket cost.
But a sudden elimination would affect more than just health care consumers. Insurance companies, drug companies, hospitals, and doctors have all changed the way they do business because of incentives and penalties in the health law. If it’s struck down, many of the “rules of the road” would literally be wiped away, including billing and payment mechanisms.
A new Democratic president could not drop the lawsuit because the Trump administration is not the plaintiff (the GOP attorneys general are). But a Democratic Congress and president could in theory make the entire issue go away by reinstating the penalty for failure to have insurance, even at a minimal amount. However, as far as the health law goes, for now, nothing is a sure thing.
As Nicholas Bagley, a law professor at the University of Michigan, Ann Arbor, who specializes in health issues, tweeted: “Among other things, the Affordable Care Act now dangles from a thread.”
Reproductive rights
A woman’s right to abortion – and even to birth control – also has been hanging by a thread at the high court for more than a decade. This past term, Mr. Roberts joined the liberals to invalidate a Louisiana law that would have closed most of the state’s abortion clinics, but he made it clear it was not a vote for abortion rights. The Louisiana law was too similar to a Texas law the court (without his vote) struck down in 2016, Mr. Roberts argued.
Ms. Ginsburg had been a stalwart supporter of reproductive freedom for women. In her nearly 3 decades on the court, she always voted with backers of abortion rights and birth control and led the dissenters in 2007 when the court upheld a federal ban on a specific abortion procedure.
Adding a justice opposed to abortion to the bench – which is what Trump has promised his supporters – would almost certainly tilt the court in favor of far more dramatic restrictions on the procedure and possibly an overturn of the landmark 1973 ruling Roe v. Wade.
But not only is abortion on the line: The court in recent years has repeatedly ruled that employers with religious objections can refuse to provide contraception.
And waiting in the lower-court pipeline are cases involving federal funding of Planned Parenthood in both the Medicaid and federal family planning programs, and the ability of individual health workers to decline to participate in abortion and other procedures.
For Ms. Ginsburg, those issues came down to a clear question of a woman’s guarantee of equal status under the law.
“Women, it is now acknowledged, have the talent, capacity, and right ‘to participate equally in the economic and social life of the Nation,’ ” she wrote in her dissent in that 2007 abortion case. “Their ability to realize their full potential, the Court recognized, is intimately connected to ‘their ability to control their reproductive lives.’ ”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
On Feb. 27, 2018, I got an email from the Heritage Foundation that alerted me to a news conference that afternoon held by Republican attorneys general of Texas and other states. It was referred to only as a “discussion about the Affordable Care Act lawsuit.”
I sent the following note to my editor: “I’m off to the Hill anyway. I could stop by this. You never know what it might morph into.”
Few people took that case very seriously – barely a handful of reporters attended the news conference. But it has now “morphed into” the latest existential threat to the Affordable Care Act, scheduled for oral arguments at the Supreme Court a week after the general election in November. And with the death of Justice Ruth Bader Ginsburg on Friday, that case could well morph into the threat that brings down the law in its entirety.
Democrats are raising alarms about the future of the law without Ms. Ginsburg. House Speaker Nancy Pelosi, speaking on ABC’s “This Week” Sunday morning, said that part of the strategy by President Trump and Senate Republicans to quickly fill her seat was to help undermine the ACA.
“The president is rushing to make some kind of a decision because … Nov. 10 is when the arguments begin on the Affordable Care Act,” she said. “He doesn’t want to crush the virus. He wants to crush the Affordable Care Act.”
Ms. Ginsburg’s death could throw an already chaotic general election campaign during a pandemic into even more turmoil.
Let’s take them one at a time.
The ACA under fire – again
The GOP attorneys general argued in February 2018 that the Republican-sponsored tax cut bill Congress passed two months earlier had rendered the ACA unconstitutional by reducing to zero the ACA’s penalty for not having insurance. They based their argument on Chief Justice John Roberts’ 2012 conclusion that the ACA was valid, interpreting that penalty as a constitutionally appropriate tax.
Most legal scholars, including several who challenged the law before the Supreme Court in 2012 and again in 2015, find the argument that the entire law should fall to be unconvincing. “If courts invalidate an entire law merely because Congress eliminates or revises one part, as happened here, that may well inhibit necessary reform of federal legislation in the future by turning it into an ‘all or nothing’ proposition,” wrote a group of conservative and liberal law professors in a brief filed in the case.
Still, in December 2018, U.S. District Judge Reed O’Connor in Texas accepted the GOP argument and declared the law unconstitutional. In December 2019, a three-judge 5th Circuit appeals court panel in New Orleans agreed that without the penalty the requirement to buy insurance is unconstitutional. But it sent the case back to Mr. O’Connor to suggest that perhaps the entire law need not fall.
Not wanting to wait the months or years that reconsideration would take, Democratic attorneys general defending the ACA asked the Supreme Court to hear the case this year. (Democrats are defending the law in court because the Trump administration decided to support the GOP attorneys general’s case.) The court agreed to take the case but scheduled arguments for the week after the November election.
While the fate of the ACA was and is a live political issue, few legal observers were terribly worried about the legal outcome of the case, now known as Texas v. California, if only because the case seemed much weaker than the 2012 and 2015 cases in which Mr. Roberts joined the court’s four liberals. In the 2015 case, which challenged the validity of federal tax subsidies helping millions of Americans buy health insurance on the ACA’s marketplaces, both Mr. Roberts and now-retired Justice Anthony Kennedy voted to uphold the law.
But without Ms. Ginsburg, the case could wind up in a 4-4 tie, even if Mr. Roberts supports the law’s constitutionality. That could let the lower-court ruling stand, although it would not be binding on other courts outside of the 5th Circuit. The court could also put off the arguments or, if the Republican Senate replaces Ms. Ginsburg with another conservative justice before arguments are heard, Republicans could secure a 5-4 ruling against the law. Some court observers argue that Justice Brett Kavanaugh has not favored invalidating an entire statute if only part of it is flawed and might not approve overturning the ACA. Still, what started out as an effort to energize Republican voters for the 2018 midterms after Congress failed to “repeal and replace” the health law in 2017 could end up throwing the nation’s entire health system into chaos.
At least 20 million Americans – and likely many more who sought coverage since the start of the coronavirus pandemic — who buy insurance through the ACA marketplaces or have Medicaid through the law’s expansion could lose coverage right away. Many millions more would lose the law’s popular protections guaranteeing coverage for people with preexisting health conditions, including those who have had COVID-19.
Adult children under age 26 years would no longer be guaranteed the right to remain on their parents’ health plans, and Medicare patients would lose enhanced prescription drug coverage. Women would lose guaranteed access to birth control at no out-of-pocket cost.
But a sudden elimination would affect more than just health care consumers. Insurance companies, drug companies, hospitals, and doctors have all changed the way they do business because of incentives and penalties in the health law. If it’s struck down, many of the “rules of the road” would literally be wiped away, including billing and payment mechanisms.
A new Democratic president could not drop the lawsuit because the Trump administration is not the plaintiff (the GOP attorneys general are). But a Democratic Congress and president could in theory make the entire issue go away by reinstating the penalty for failure to have insurance, even at a minimal amount. However, as far as the health law goes, for now, nothing is a sure thing.
As Nicholas Bagley, a law professor at the University of Michigan, Ann Arbor, who specializes in health issues, tweeted: “Among other things, the Affordable Care Act now dangles from a thread.”
Reproductive rights
A woman’s right to abortion – and even to birth control – also has been hanging by a thread at the high court for more than a decade. This past term, Mr. Roberts joined the liberals to invalidate a Louisiana law that would have closed most of the state’s abortion clinics, but he made it clear it was not a vote for abortion rights. The Louisiana law was too similar to a Texas law the court (without his vote) struck down in 2016, Mr. Roberts argued.
Ms. Ginsburg had been a stalwart supporter of reproductive freedom for women. In her nearly 3 decades on the court, she always voted with backers of abortion rights and birth control and led the dissenters in 2007 when the court upheld a federal ban on a specific abortion procedure.
Adding a justice opposed to abortion to the bench – which is what Trump has promised his supporters – would almost certainly tilt the court in favor of far more dramatic restrictions on the procedure and possibly an overturn of the landmark 1973 ruling Roe v. Wade.
But not only is abortion on the line: The court in recent years has repeatedly ruled that employers with religious objections can refuse to provide contraception.
And waiting in the lower-court pipeline are cases involving federal funding of Planned Parenthood in both the Medicaid and federal family planning programs, and the ability of individual health workers to decline to participate in abortion and other procedures.
For Ms. Ginsburg, those issues came down to a clear question of a woman’s guarantee of equal status under the law.
“Women, it is now acknowledged, have the talent, capacity, and right ‘to participate equally in the economic and social life of the Nation,’ ” she wrote in her dissent in that 2007 abortion case. “Their ability to realize their full potential, the Court recognized, is intimately connected to ‘their ability to control their reproductive lives.’ ”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
On Feb. 27, 2018, I got an email from the Heritage Foundation that alerted me to a news conference that afternoon held by Republican attorneys general of Texas and other states. It was referred to only as a “discussion about the Affordable Care Act lawsuit.”
I sent the following note to my editor: “I’m off to the Hill anyway. I could stop by this. You never know what it might morph into.”
Few people took that case very seriously – barely a handful of reporters attended the news conference. But it has now “morphed into” the latest existential threat to the Affordable Care Act, scheduled for oral arguments at the Supreme Court a week after the general election in November. And with the death of Justice Ruth Bader Ginsburg on Friday, that case could well morph into the threat that brings down the law in its entirety.
Democrats are raising alarms about the future of the law without Ms. Ginsburg. House Speaker Nancy Pelosi, speaking on ABC’s “This Week” Sunday morning, said that part of the strategy by President Trump and Senate Republicans to quickly fill her seat was to help undermine the ACA.
“The president is rushing to make some kind of a decision because … Nov. 10 is when the arguments begin on the Affordable Care Act,” she said. “He doesn’t want to crush the virus. He wants to crush the Affordable Care Act.”
Ms. Ginsburg’s death could throw an already chaotic general election campaign during a pandemic into even more turmoil.
Let’s take them one at a time.
The ACA under fire – again
The GOP attorneys general argued in February 2018 that the Republican-sponsored tax cut bill Congress passed two months earlier had rendered the ACA unconstitutional by reducing to zero the ACA’s penalty for not having insurance. They based their argument on Chief Justice John Roberts’ 2012 conclusion that the ACA was valid, interpreting that penalty as a constitutionally appropriate tax.
Most legal scholars, including several who challenged the law before the Supreme Court in 2012 and again in 2015, find the argument that the entire law should fall to be unconvincing. “If courts invalidate an entire law merely because Congress eliminates or revises one part, as happened here, that may well inhibit necessary reform of federal legislation in the future by turning it into an ‘all or nothing’ proposition,” wrote a group of conservative and liberal law professors in a brief filed in the case.
Still, in December 2018, U.S. District Judge Reed O’Connor in Texas accepted the GOP argument and declared the law unconstitutional. In December 2019, a three-judge 5th Circuit appeals court panel in New Orleans agreed that without the penalty the requirement to buy insurance is unconstitutional. But it sent the case back to Mr. O’Connor to suggest that perhaps the entire law need not fall.
Not wanting to wait the months or years that reconsideration would take, Democratic attorneys general defending the ACA asked the Supreme Court to hear the case this year. (Democrats are defending the law in court because the Trump administration decided to support the GOP attorneys general’s case.) The court agreed to take the case but scheduled arguments for the week after the November election.
While the fate of the ACA was and is a live political issue, few legal observers were terribly worried about the legal outcome of the case, now known as Texas v. California, if only because the case seemed much weaker than the 2012 and 2015 cases in which Mr. Roberts joined the court’s four liberals. In the 2015 case, which challenged the validity of federal tax subsidies helping millions of Americans buy health insurance on the ACA’s marketplaces, both Mr. Roberts and now-retired Justice Anthony Kennedy voted to uphold the law.
But without Ms. Ginsburg, the case could wind up in a 4-4 tie, even if Mr. Roberts supports the law’s constitutionality. That could let the lower-court ruling stand, although it would not be binding on other courts outside of the 5th Circuit. The court could also put off the arguments or, if the Republican Senate replaces Ms. Ginsburg with another conservative justice before arguments are heard, Republicans could secure a 5-4 ruling against the law. Some court observers argue that Justice Brett Kavanaugh has not favored invalidating an entire statute if only part of it is flawed and might not approve overturning the ACA. Still, what started out as an effort to energize Republican voters for the 2018 midterms after Congress failed to “repeal and replace” the health law in 2017 could end up throwing the nation’s entire health system into chaos.
At least 20 million Americans – and likely many more who sought coverage since the start of the coronavirus pandemic — who buy insurance through the ACA marketplaces or have Medicaid through the law’s expansion could lose coverage right away. Many millions more would lose the law’s popular protections guaranteeing coverage for people with preexisting health conditions, including those who have had COVID-19.
Adult children under age 26 years would no longer be guaranteed the right to remain on their parents’ health plans, and Medicare patients would lose enhanced prescription drug coverage. Women would lose guaranteed access to birth control at no out-of-pocket cost.
But a sudden elimination would affect more than just health care consumers. Insurance companies, drug companies, hospitals, and doctors have all changed the way they do business because of incentives and penalties in the health law. If it’s struck down, many of the “rules of the road” would literally be wiped away, including billing and payment mechanisms.
A new Democratic president could not drop the lawsuit because the Trump administration is not the plaintiff (the GOP attorneys general are). But a Democratic Congress and president could in theory make the entire issue go away by reinstating the penalty for failure to have insurance, even at a minimal amount. However, as far as the health law goes, for now, nothing is a sure thing.
As Nicholas Bagley, a law professor at the University of Michigan, Ann Arbor, who specializes in health issues, tweeted: “Among other things, the Affordable Care Act now dangles from a thread.”
Reproductive rights
A woman’s right to abortion – and even to birth control – also has been hanging by a thread at the high court for more than a decade. This past term, Mr. Roberts joined the liberals to invalidate a Louisiana law that would have closed most of the state’s abortion clinics, but he made it clear it was not a vote for abortion rights. The Louisiana law was too similar to a Texas law the court (without his vote) struck down in 2016, Mr. Roberts argued.
Ms. Ginsburg had been a stalwart supporter of reproductive freedom for women. In her nearly 3 decades on the court, she always voted with backers of abortion rights and birth control and led the dissenters in 2007 when the court upheld a federal ban on a specific abortion procedure.
Adding a justice opposed to abortion to the bench – which is what Trump has promised his supporters – would almost certainly tilt the court in favor of far more dramatic restrictions on the procedure and possibly an overturn of the landmark 1973 ruling Roe v. Wade.
But not only is abortion on the line: The court in recent years has repeatedly ruled that employers with religious objections can refuse to provide contraception.
And waiting in the lower-court pipeline are cases involving federal funding of Planned Parenthood in both the Medicaid and federal family planning programs, and the ability of individual health workers to decline to participate in abortion and other procedures.
For Ms. Ginsburg, those issues came down to a clear question of a woman’s guarantee of equal status under the law.
“Women, it is now acknowledged, have the talent, capacity, and right ‘to participate equally in the economic and social life of the Nation,’ ” she wrote in her dissent in that 2007 abortion case. “Their ability to realize their full potential, the Court recognized, is intimately connected to ‘their ability to control their reproductive lives.’ ”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
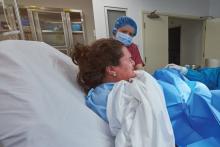
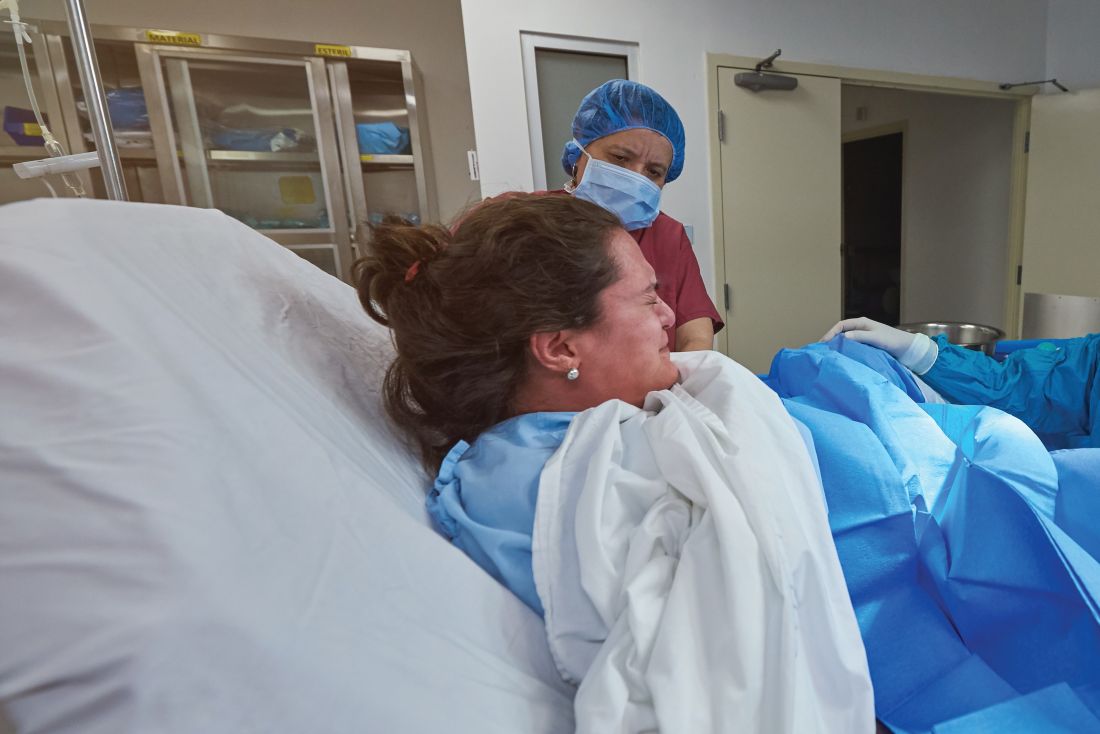
Vacuum device quickly stops postpartum hemorrhage
A novel intrauterine device that uses suction to compress the uterus to control postpartum hemorrhage has received high marks for effectiveness and ease of use, researchers say.
Calling this approach “a stroke of brilliance,” and is less risky as well.
“This device can be placed in the uterus within a minute or so and does not need any initial anesthesia and would not be associated with the delay needed for a surgical approach,” Dr. Byrne explained. Dr. Byrne, who was not involved in the study, is chair of the department of obstetrics and gynecology at Santa Clara Valley Medical Center in San Jose, Calif.
To test the efficacy and safety of the device (Jada System, Alydia Health), Mary E. D’Alton, MD, and colleagues conducted a prospective, observational treatment study in 12 U.S. medical centers. They reported their findings in an article published online Sept. 9 in Obstetrics and Gynecology.
“The Jada System (novel intrauterine vacuum-induced hemorrhage-control device) was specifically designed to offer rapid treatment by applying low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis,” Dr. D’Alton, from New York–Presbyterian/Columbia University Irving Medical Center in New York, and colleagues wrote.
“The device had a low rate of adverse events during this study, all of which were expected risks and resolved with treatment without serious clinical sequelae. Investigators, all first-time users of the device, found the system easy to use, which suggests that, after device education and with availability of a quick reference guide outlining steps, there is a minimal learning curve for use,” they added.
Alydia Health, the company that developed the device, funded this study and supported the research staff, who recruited participants and gathered follow-up data on them. On Sept. 9, the U.S. Food and Drug Administration granted 510(k) clearance for the device, according to a company news release.
Effective, safe
The multicenter study included 107 patients (mean age, 29.7 years) with postpartum hemorrhage or abnormal postpartum uterine bleeding, 106 of whom received any study treatment with the device attached to vacuum. More than half (57%) of the participants were White, and just fewer than one-quarter (24%) were Black.
Treatment was successful in 94% (100/106) of participants, with “definitive control of abnormal bleeding” occurring in a median of 3 minutes after attachment to vacuum.
Eight adverse events were judged to have been possibly related to the device or procedure: four cases of endometritis, and one case each of presumed endometritis, bacterial vaginosis, vaginal candidiasis, and disruption of a vaginal laceration repair. The eight adverse events were identified as potential risks, and all resolved without serious clinical consequences.
Thirty-five patients required transfusions of 1-3 U of red blood cells, and five patients required at least 4 U of red blood cells.
Uterine atony most frequent culprit
As many as 80% of postpartum hemorrhages are caused by uterine atony, according to the authors.
Dr. Byrne explained that the uterus is a muscular organ that contains many “spiral arteries” that are “squeezed” by the uterus as it tightens down after childbirth, which prevents them from bleeding excessively.
“With uterine atony, the uterus muscle doesn’t squeeze effectively, and therefore it’s not one or two arteries, it’s hundreds and hundreds of small arteries and capillaries [and] arterioles all bleeding; it’s a wide area of uterus,” he continued.
When medications alone are ineffective at controlling bleeding, tamponade is often added to put outward pressure on the inner wall of the uterus for 12-24 hours. Although tamponade is effective in approximately 87% of atony-related cases of postpartum hemorrhage, the use of outward pressure on the uterine walls “is counterintuitive if the ultimate goal is uterine contraction,” the authors wrote.
Dr. Byrne said he and his colleagues saw this device several years ago, and they felt at the time that it appeared to be “more intuitive to use vacuum to compress the uterus inward compared to the nonetheless valuable and effective Bakri balloon and other techniques that expand the uterus outward.”
The fact that there is no need for prophylactic antibiotics also sets the vacuum device apart from the Bakri balloon, use of which routinely involves administration of prophylactic antibiotics, Dr. Byrne said.
In the current study, 64% of participants were obese, which makes management of postpartum hemorrhage “really challenging” because it’s difficult to effectively massage the uterus through adipose tissue, Dr. Byrne explained. Patients with obesity “also have different hemodynamics for how effectively [injected medications will] be delivered to the uterus,” he added.
“A device like this that could be placed and works so efficiently – even with an obese patient – that’s actually very powerful,” Dr. Byrne said.
Quick placement, almost immediate improvement
The discomfort experienced during placement of the device is similar to that experienced during sweeping of the uterus, Dr. Byrne explained. “You’d want a patient comfortable, ideally with an epidural already active, but if it’s an emergency, you wouldn’t have to wait for that; you could sweep the uterus quickly, place this, initiate suction, and it would all be so quick you could usually talk a patient through it and get it done,” Dr. Byrne continued.
Almost all of the investigators (98%) said the device was easy to use, and 97% said they would recommend it.
The vacuum device is made of medical-grade silicone and consists of an oval-shaped intrauterine loop at one end and a vacuum connector at the other end that can be attached to a standard suction cannister. On the inner side of the intrauterine loop are 20 vacuum pores covered by a shield that protects uterine tissue and prevents the vacuum pores from clogging with tissue or clotted blood.
Before insertion of the vacuum device, the clinician manually sweeps the uterus to identify retained placental fragments and to assess the uterine cavity. The distal end of the device is inserted into the uterus, and a cervical seal, positioned just outside the cervical os, is filled with 60 to 120 cc of sterile fluid. The proximal end is attached to low-level vacuum at a pressure of 80 ± 10 mm Hg. The device is left in place with continued suction for at least 1 hour after bleeding is controlled, at which time the suction is disconnected and the cervical seal is emptied. The device remains in place for at least 30 minutes, during which the patient is observed closely.
“It looks like 75%-80% of cases stop bleeding within 5 minutes. ... Then you stop the pressure after an hour [and] wait at least 30 minutes. You could actually have this out of the patient’s body within 2 hours,” Dr. Byrne said.
Dr. Byrne has disclosed no such financial relationships.
A version of this article originally appeared on Medscape.com.
A novel intrauterine device that uses suction to compress the uterus to control postpartum hemorrhage has received high marks for effectiveness and ease of use, researchers say.
Calling this approach “a stroke of brilliance,” and is less risky as well.
“This device can be placed in the uterus within a minute or so and does not need any initial anesthesia and would not be associated with the delay needed for a surgical approach,” Dr. Byrne explained. Dr. Byrne, who was not involved in the study, is chair of the department of obstetrics and gynecology at Santa Clara Valley Medical Center in San Jose, Calif.
To test the efficacy and safety of the device (Jada System, Alydia Health), Mary E. D’Alton, MD, and colleagues conducted a prospective, observational treatment study in 12 U.S. medical centers. They reported their findings in an article published online Sept. 9 in Obstetrics and Gynecology.
“The Jada System (novel intrauterine vacuum-induced hemorrhage-control device) was specifically designed to offer rapid treatment by applying low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis,” Dr. D’Alton, from New York–Presbyterian/Columbia University Irving Medical Center in New York, and colleagues wrote.
“The device had a low rate of adverse events during this study, all of which were expected risks and resolved with treatment without serious clinical sequelae. Investigators, all first-time users of the device, found the system easy to use, which suggests that, after device education and with availability of a quick reference guide outlining steps, there is a minimal learning curve for use,” they added.
Alydia Health, the company that developed the device, funded this study and supported the research staff, who recruited participants and gathered follow-up data on them. On Sept. 9, the U.S. Food and Drug Administration granted 510(k) clearance for the device, according to a company news release.
Effective, safe
The multicenter study included 107 patients (mean age, 29.7 years) with postpartum hemorrhage or abnormal postpartum uterine bleeding, 106 of whom received any study treatment with the device attached to vacuum. More than half (57%) of the participants were White, and just fewer than one-quarter (24%) were Black.
Treatment was successful in 94% (100/106) of participants, with “definitive control of abnormal bleeding” occurring in a median of 3 minutes after attachment to vacuum.
Eight adverse events were judged to have been possibly related to the device or procedure: four cases of endometritis, and one case each of presumed endometritis, bacterial vaginosis, vaginal candidiasis, and disruption of a vaginal laceration repair. The eight adverse events were identified as potential risks, and all resolved without serious clinical consequences.
Thirty-five patients required transfusions of 1-3 U of red blood cells, and five patients required at least 4 U of red blood cells.
Uterine atony most frequent culprit
As many as 80% of postpartum hemorrhages are caused by uterine atony, according to the authors.
Dr. Byrne explained that the uterus is a muscular organ that contains many “spiral arteries” that are “squeezed” by the uterus as it tightens down after childbirth, which prevents them from bleeding excessively.
“With uterine atony, the uterus muscle doesn’t squeeze effectively, and therefore it’s not one or two arteries, it’s hundreds and hundreds of small arteries and capillaries [and] arterioles all bleeding; it’s a wide area of uterus,” he continued.
When medications alone are ineffective at controlling bleeding, tamponade is often added to put outward pressure on the inner wall of the uterus for 12-24 hours. Although tamponade is effective in approximately 87% of atony-related cases of postpartum hemorrhage, the use of outward pressure on the uterine walls “is counterintuitive if the ultimate goal is uterine contraction,” the authors wrote.
Dr. Byrne said he and his colleagues saw this device several years ago, and they felt at the time that it appeared to be “more intuitive to use vacuum to compress the uterus inward compared to the nonetheless valuable and effective Bakri balloon and other techniques that expand the uterus outward.”
The fact that there is no need for prophylactic antibiotics also sets the vacuum device apart from the Bakri balloon, use of which routinely involves administration of prophylactic antibiotics, Dr. Byrne said.
In the current study, 64% of participants were obese, which makes management of postpartum hemorrhage “really challenging” because it’s difficult to effectively massage the uterus through adipose tissue, Dr. Byrne explained. Patients with obesity “also have different hemodynamics for how effectively [injected medications will] be delivered to the uterus,” he added.
“A device like this that could be placed and works so efficiently – even with an obese patient – that’s actually very powerful,” Dr. Byrne said.
Quick placement, almost immediate improvement
The discomfort experienced during placement of the device is similar to that experienced during sweeping of the uterus, Dr. Byrne explained. “You’d want a patient comfortable, ideally with an epidural already active, but if it’s an emergency, you wouldn’t have to wait for that; you could sweep the uterus quickly, place this, initiate suction, and it would all be so quick you could usually talk a patient through it and get it done,” Dr. Byrne continued.
Almost all of the investigators (98%) said the device was easy to use, and 97% said they would recommend it.
The vacuum device is made of medical-grade silicone and consists of an oval-shaped intrauterine loop at one end and a vacuum connector at the other end that can be attached to a standard suction cannister. On the inner side of the intrauterine loop are 20 vacuum pores covered by a shield that protects uterine tissue and prevents the vacuum pores from clogging with tissue or clotted blood.
Before insertion of the vacuum device, the clinician manually sweeps the uterus to identify retained placental fragments and to assess the uterine cavity. The distal end of the device is inserted into the uterus, and a cervical seal, positioned just outside the cervical os, is filled with 60 to 120 cc of sterile fluid. The proximal end is attached to low-level vacuum at a pressure of 80 ± 10 mm Hg. The device is left in place with continued suction for at least 1 hour after bleeding is controlled, at which time the suction is disconnected and the cervical seal is emptied. The device remains in place for at least 30 minutes, during which the patient is observed closely.
“It looks like 75%-80% of cases stop bleeding within 5 minutes. ... Then you stop the pressure after an hour [and] wait at least 30 minutes. You could actually have this out of the patient’s body within 2 hours,” Dr. Byrne said.
Dr. Byrne has disclosed no such financial relationships.
A version of this article originally appeared on Medscape.com.
A novel intrauterine device that uses suction to compress the uterus to control postpartum hemorrhage has received high marks for effectiveness and ease of use, researchers say.
Calling this approach “a stroke of brilliance,” and is less risky as well.
“This device can be placed in the uterus within a minute or so and does not need any initial anesthesia and would not be associated with the delay needed for a surgical approach,” Dr. Byrne explained. Dr. Byrne, who was not involved in the study, is chair of the department of obstetrics and gynecology at Santa Clara Valley Medical Center in San Jose, Calif.
To test the efficacy and safety of the device (Jada System, Alydia Health), Mary E. D’Alton, MD, and colleagues conducted a prospective, observational treatment study in 12 U.S. medical centers. They reported their findings in an article published online Sept. 9 in Obstetrics and Gynecology.
“The Jada System (novel intrauterine vacuum-induced hemorrhage-control device) was specifically designed to offer rapid treatment by applying low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis,” Dr. D’Alton, from New York–Presbyterian/Columbia University Irving Medical Center in New York, and colleagues wrote.
“The device had a low rate of adverse events during this study, all of which were expected risks and resolved with treatment without serious clinical sequelae. Investigators, all first-time users of the device, found the system easy to use, which suggests that, after device education and with availability of a quick reference guide outlining steps, there is a minimal learning curve for use,” they added.
Alydia Health, the company that developed the device, funded this study and supported the research staff, who recruited participants and gathered follow-up data on them. On Sept. 9, the U.S. Food and Drug Administration granted 510(k) clearance for the device, according to a company news release.
Effective, safe
The multicenter study included 107 patients (mean age, 29.7 years) with postpartum hemorrhage or abnormal postpartum uterine bleeding, 106 of whom received any study treatment with the device attached to vacuum. More than half (57%) of the participants were White, and just fewer than one-quarter (24%) were Black.
Treatment was successful in 94% (100/106) of participants, with “definitive control of abnormal bleeding” occurring in a median of 3 minutes after attachment to vacuum.
Eight adverse events were judged to have been possibly related to the device or procedure: four cases of endometritis, and one case each of presumed endometritis, bacterial vaginosis, vaginal candidiasis, and disruption of a vaginal laceration repair. The eight adverse events were identified as potential risks, and all resolved without serious clinical consequences.
Thirty-five patients required transfusions of 1-3 U of red blood cells, and five patients required at least 4 U of red blood cells.
Uterine atony most frequent culprit
As many as 80% of postpartum hemorrhages are caused by uterine atony, according to the authors.
Dr. Byrne explained that the uterus is a muscular organ that contains many “spiral arteries” that are “squeezed” by the uterus as it tightens down after childbirth, which prevents them from bleeding excessively.
“With uterine atony, the uterus muscle doesn’t squeeze effectively, and therefore it’s not one or two arteries, it’s hundreds and hundreds of small arteries and capillaries [and] arterioles all bleeding; it’s a wide area of uterus,” he continued.
When medications alone are ineffective at controlling bleeding, tamponade is often added to put outward pressure on the inner wall of the uterus for 12-24 hours. Although tamponade is effective in approximately 87% of atony-related cases of postpartum hemorrhage, the use of outward pressure on the uterine walls “is counterintuitive if the ultimate goal is uterine contraction,” the authors wrote.
Dr. Byrne said he and his colleagues saw this device several years ago, and they felt at the time that it appeared to be “more intuitive to use vacuum to compress the uterus inward compared to the nonetheless valuable and effective Bakri balloon and other techniques that expand the uterus outward.”
The fact that there is no need for prophylactic antibiotics also sets the vacuum device apart from the Bakri balloon, use of which routinely involves administration of prophylactic antibiotics, Dr. Byrne said.
In the current study, 64% of participants were obese, which makes management of postpartum hemorrhage “really challenging” because it’s difficult to effectively massage the uterus through adipose tissue, Dr. Byrne explained. Patients with obesity “also have different hemodynamics for how effectively [injected medications will] be delivered to the uterus,” he added.
“A device like this that could be placed and works so efficiently – even with an obese patient – that’s actually very powerful,” Dr. Byrne said.
Quick placement, almost immediate improvement
The discomfort experienced during placement of the device is similar to that experienced during sweeping of the uterus, Dr. Byrne explained. “You’d want a patient comfortable, ideally with an epidural already active, but if it’s an emergency, you wouldn’t have to wait for that; you could sweep the uterus quickly, place this, initiate suction, and it would all be so quick you could usually talk a patient through it and get it done,” Dr. Byrne continued.
Almost all of the investigators (98%) said the device was easy to use, and 97% said they would recommend it.
The vacuum device is made of medical-grade silicone and consists of an oval-shaped intrauterine loop at one end and a vacuum connector at the other end that can be attached to a standard suction cannister. On the inner side of the intrauterine loop are 20 vacuum pores covered by a shield that protects uterine tissue and prevents the vacuum pores from clogging with tissue or clotted blood.
Before insertion of the vacuum device, the clinician manually sweeps the uterus to identify retained placental fragments and to assess the uterine cavity. The distal end of the device is inserted into the uterus, and a cervical seal, positioned just outside the cervical os, is filled with 60 to 120 cc of sterile fluid. The proximal end is attached to low-level vacuum at a pressure of 80 ± 10 mm Hg. The device is left in place with continued suction for at least 1 hour after bleeding is controlled, at which time the suction is disconnected and the cervical seal is emptied. The device remains in place for at least 30 minutes, during which the patient is observed closely.
“It looks like 75%-80% of cases stop bleeding within 5 minutes. ... Then you stop the pressure after an hour [and] wait at least 30 minutes. You could actually have this out of the patient’s body within 2 hours,” Dr. Byrne said.
Dr. Byrne has disclosed no such financial relationships.
A version of this article originally appeared on Medscape.com.
Counterintuitive findings for domestic violence during COVID-19
Intimate partner violence (IPV) has not increased during the COVID-19 pandemic, at least during the early stages of the pandemic, new research suggests.
In April 2020, investigators surveyed over 1,750 individuals in intimate partner relationships. The survey was drawn from social media and email distribution lists. The researchers found that, of the roughly one-fifth who screened positive for IPV, half stated that the degree of victimization had remained the same since the COVID-19 outbreak; 17% reported that it had worsened; and one third reported that it had gotten better.
Those who reported worsening victimization said that sexual and physical violence, in particular, were exacerbated early in the pandemic’s course.
“I was surprised by this finding, and we certainly were not expecting it – in fact, I expected that the vast majority of victims would report that victimization got worse during stay-at-home policies, but that wasn’t the case,” lead author Katelyn Jetelina, PhD, MPH, assistant professor in the department of epidemiology, human genetics, and environmental sciences, University of Texas Health Science Center, Dallas, said in an interview.
“I think the biggest take-home message is that some victims got better, but the vast majority stayed the same. These victims, men and women, were isolated with their perpetrator during COVID-19, so she added.
The study was published online Sept. 1 in Injury Prevention.
‘Shadow pandemic?’
The World Health Organization called upon health care organizations to be prepared to curb a potential IPV “shadow pandemic” during the COVID-19 pandemic.
However, no study has specifically evaluated whether self-reported victimization, particularly with regard to the severity and type of abuse, changed during the early period after COVID-19 social distancing polices were mandated.
“We scrambled right away when the pandemic hit because it was a unique opportunity to examine how behaviors change due to early stay-at-home policies; and, as a violence and injury epidemiologist, I am always curious about IPV, and this was a small subanalysis of that larger question,” Dr. Jetelina said.
The researchers recruited participants through their university and private social media accounts as well as professional distribution lists. Of those who completed the survey, 1,759 (mean age, 42 years) reported that they currently had an intimate partner. These participants were included in the study.
IPV was determined using the five-item Extended Hurt, Insulted, Threatened, and Scream (E-HITS) construct. Respondents were asked how often their partner physically hurt them, insulted them, threatened them with harm, screamed or cursed at them, or forced them to engage in sexual activities.
Each item was answered using a 5-point Likert scale. Scores ranged from 1, indicating never, to 5, indicating frequently. Participants who scored ≥7 were considered IPV positive.
Participants were also asked whether IPV severity had gotten much/somewhat better, had remained the same, or had gotten somewhat/much worse.
First peek
Of the total sample, 18% screened positive for IPV. Of these, 54% reported that the victimization had remained the same, 17% reported that it had worsened, and 30% said it had improved.
The majority of IPV victims experienced being insulted (97%) or being screamed at (86%).
Among those who reported worsening of IPV, the risk for physical violence was 4.38 times higher than the risk for nonphysical victimization. The risk for sexual victimization was 2.31 times higher than the risk for nonsexual victimization.
Among those who reported that IPV had gotten better, the improvement was 3.47 times higher with regard to physical victimization, compared with nonphysical victimization. Dr. Jetelina acknowledged that the findings cannot be generalized to the broader population.
“This was a convenience sample, but it is the first peek into what is happening behind closed doors and a first step to hearing collecting data from the victims themselves to better understand this ‘shadow pandemic’ and inform creative efforts to create better services for them while they are in isolation,” she said.
Lethality indicators
Commenting on the study, Peter Cronholm, MD, MSCE, associate professor of family medicine and community health at the Hospital of the University of Pennsylvania, Philadelphia, questioned the use of a score of 7 on the E-HITS screen to determine the presence of IPV.
“I think there are other thresholds that might be important, and even low levels of sexual violence may be different than higher levels of emotional violence,” said Dr. Cronholm, who was not involved with the study.
“Someone may have been sexually assaulted frequently but not cross the threshold, so I think it would have been helpful for the researchers to look at different types of violence,” he said.
Also commenting on the study, Jessica Palardy, LSW, program supervisor at STOP Intimate Partner Violence, Philadelphia, said, the findings “solidify a trend we sensed was happening but couldn’t confirm.”
She said her agency’s clients “have had a wide variety of experiences, in terms of increases or decreases in victimization.”
Some clients were able to use the quarantine as an excuse to stay with family or friends and so could avoid seeing their partners. “Others indicated that because their partners were distracted by figuring out a new method of work, the tension shifted away from the victim,” said Ms. Palardy, who was not involved in the research.
“For those who saw an increase in victimization, we noticed that this increase also came with an increase in lethality indicators, such as strangulation, physical violence, use of weapons and substances, etc,” she said.
She emphasized that it is critical to screen people for IPV to ensure their safety.
“The goal is to connect people with resources before they are in a more lethal situation so that they can increase their safety and know their options,” Ms. Palardy said.
The National Domestic Violence Hotline and the Crisis Text Line are two sources of support for IPV victims.
Dr. Jetelina and coauthors, Dr. Cronholm, and Ms. Palardy reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intimate partner violence (IPV) has not increased during the COVID-19 pandemic, at least during the early stages of the pandemic, new research suggests.
In April 2020, investigators surveyed over 1,750 individuals in intimate partner relationships. The survey was drawn from social media and email distribution lists. The researchers found that, of the roughly one-fifth who screened positive for IPV, half stated that the degree of victimization had remained the same since the COVID-19 outbreak; 17% reported that it had worsened; and one third reported that it had gotten better.
Those who reported worsening victimization said that sexual and physical violence, in particular, were exacerbated early in the pandemic’s course.
“I was surprised by this finding, and we certainly were not expecting it – in fact, I expected that the vast majority of victims would report that victimization got worse during stay-at-home policies, but that wasn’t the case,” lead author Katelyn Jetelina, PhD, MPH, assistant professor in the department of epidemiology, human genetics, and environmental sciences, University of Texas Health Science Center, Dallas, said in an interview.
“I think the biggest take-home message is that some victims got better, but the vast majority stayed the same. These victims, men and women, were isolated with their perpetrator during COVID-19, so she added.
The study was published online Sept. 1 in Injury Prevention.
‘Shadow pandemic?’
The World Health Organization called upon health care organizations to be prepared to curb a potential IPV “shadow pandemic” during the COVID-19 pandemic.
However, no study has specifically evaluated whether self-reported victimization, particularly with regard to the severity and type of abuse, changed during the early period after COVID-19 social distancing polices were mandated.
“We scrambled right away when the pandemic hit because it was a unique opportunity to examine how behaviors change due to early stay-at-home policies; and, as a violence and injury epidemiologist, I am always curious about IPV, and this was a small subanalysis of that larger question,” Dr. Jetelina said.
The researchers recruited participants through their university and private social media accounts as well as professional distribution lists. Of those who completed the survey, 1,759 (mean age, 42 years) reported that they currently had an intimate partner. These participants were included in the study.
IPV was determined using the five-item Extended Hurt, Insulted, Threatened, and Scream (E-HITS) construct. Respondents were asked how often their partner physically hurt them, insulted them, threatened them with harm, screamed or cursed at them, or forced them to engage in sexual activities.
Each item was answered using a 5-point Likert scale. Scores ranged from 1, indicating never, to 5, indicating frequently. Participants who scored ≥7 were considered IPV positive.
Participants were also asked whether IPV severity had gotten much/somewhat better, had remained the same, or had gotten somewhat/much worse.
First peek
Of the total sample, 18% screened positive for IPV. Of these, 54% reported that the victimization had remained the same, 17% reported that it had worsened, and 30% said it had improved.
The majority of IPV victims experienced being insulted (97%) or being screamed at (86%).
Among those who reported worsening of IPV, the risk for physical violence was 4.38 times higher than the risk for nonphysical victimization. The risk for sexual victimization was 2.31 times higher than the risk for nonsexual victimization.
Among those who reported that IPV had gotten better, the improvement was 3.47 times higher with regard to physical victimization, compared with nonphysical victimization. Dr. Jetelina acknowledged that the findings cannot be generalized to the broader population.
“This was a convenience sample, but it is the first peek into what is happening behind closed doors and a first step to hearing collecting data from the victims themselves to better understand this ‘shadow pandemic’ and inform creative efforts to create better services for them while they are in isolation,” she said.
Lethality indicators
Commenting on the study, Peter Cronholm, MD, MSCE, associate professor of family medicine and community health at the Hospital of the University of Pennsylvania, Philadelphia, questioned the use of a score of 7 on the E-HITS screen to determine the presence of IPV.
“I think there are other thresholds that might be important, and even low levels of sexual violence may be different than higher levels of emotional violence,” said Dr. Cronholm, who was not involved with the study.
“Someone may have been sexually assaulted frequently but not cross the threshold, so I think it would have been helpful for the researchers to look at different types of violence,” he said.
Also commenting on the study, Jessica Palardy, LSW, program supervisor at STOP Intimate Partner Violence, Philadelphia, said, the findings “solidify a trend we sensed was happening but couldn’t confirm.”
She said her agency’s clients “have had a wide variety of experiences, in terms of increases or decreases in victimization.”
Some clients were able to use the quarantine as an excuse to stay with family or friends and so could avoid seeing their partners. “Others indicated that because their partners were distracted by figuring out a new method of work, the tension shifted away from the victim,” said Ms. Palardy, who was not involved in the research.
“For those who saw an increase in victimization, we noticed that this increase also came with an increase in lethality indicators, such as strangulation, physical violence, use of weapons and substances, etc,” she said.
She emphasized that it is critical to screen people for IPV to ensure their safety.
“The goal is to connect people with resources before they are in a more lethal situation so that they can increase their safety and know their options,” Ms. Palardy said.
The National Domestic Violence Hotline and the Crisis Text Line are two sources of support for IPV victims.
Dr. Jetelina and coauthors, Dr. Cronholm, and Ms. Palardy reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intimate partner violence (IPV) has not increased during the COVID-19 pandemic, at least during the early stages of the pandemic, new research suggests.
In April 2020, investigators surveyed over 1,750 individuals in intimate partner relationships. The survey was drawn from social media and email distribution lists. The researchers found that, of the roughly one-fifth who screened positive for IPV, half stated that the degree of victimization had remained the same since the COVID-19 outbreak; 17% reported that it had worsened; and one third reported that it had gotten better.
Those who reported worsening victimization said that sexual and physical violence, in particular, were exacerbated early in the pandemic’s course.
“I was surprised by this finding, and we certainly were not expecting it – in fact, I expected that the vast majority of victims would report that victimization got worse during stay-at-home policies, but that wasn’t the case,” lead author Katelyn Jetelina, PhD, MPH, assistant professor in the department of epidemiology, human genetics, and environmental sciences, University of Texas Health Science Center, Dallas, said in an interview.
“I think the biggest take-home message is that some victims got better, but the vast majority stayed the same. These victims, men and women, were isolated with their perpetrator during COVID-19, so she added.
The study was published online Sept. 1 in Injury Prevention.
‘Shadow pandemic?’
The World Health Organization called upon health care organizations to be prepared to curb a potential IPV “shadow pandemic” during the COVID-19 pandemic.
However, no study has specifically evaluated whether self-reported victimization, particularly with regard to the severity and type of abuse, changed during the early period after COVID-19 social distancing polices were mandated.
“We scrambled right away when the pandemic hit because it was a unique opportunity to examine how behaviors change due to early stay-at-home policies; and, as a violence and injury epidemiologist, I am always curious about IPV, and this was a small subanalysis of that larger question,” Dr. Jetelina said.
The researchers recruited participants through their university and private social media accounts as well as professional distribution lists. Of those who completed the survey, 1,759 (mean age, 42 years) reported that they currently had an intimate partner. These participants were included in the study.
IPV was determined using the five-item Extended Hurt, Insulted, Threatened, and Scream (E-HITS) construct. Respondents were asked how often their partner physically hurt them, insulted them, threatened them with harm, screamed or cursed at them, or forced them to engage in sexual activities.
Each item was answered using a 5-point Likert scale. Scores ranged from 1, indicating never, to 5, indicating frequently. Participants who scored ≥7 were considered IPV positive.
Participants were also asked whether IPV severity had gotten much/somewhat better, had remained the same, or had gotten somewhat/much worse.
First peek
Of the total sample, 18% screened positive for IPV. Of these, 54% reported that the victimization had remained the same, 17% reported that it had worsened, and 30% said it had improved.
The majority of IPV victims experienced being insulted (97%) or being screamed at (86%).
Among those who reported worsening of IPV, the risk for physical violence was 4.38 times higher than the risk for nonphysical victimization. The risk for sexual victimization was 2.31 times higher than the risk for nonsexual victimization.
Among those who reported that IPV had gotten better, the improvement was 3.47 times higher with regard to physical victimization, compared with nonphysical victimization. Dr. Jetelina acknowledged that the findings cannot be generalized to the broader population.
“This was a convenience sample, but it is the first peek into what is happening behind closed doors and a first step to hearing collecting data from the victims themselves to better understand this ‘shadow pandemic’ and inform creative efforts to create better services for them while they are in isolation,” she said.
Lethality indicators
Commenting on the study, Peter Cronholm, MD, MSCE, associate professor of family medicine and community health at the Hospital of the University of Pennsylvania, Philadelphia, questioned the use of a score of 7 on the E-HITS screen to determine the presence of IPV.
“I think there are other thresholds that might be important, and even low levels of sexual violence may be different than higher levels of emotional violence,” said Dr. Cronholm, who was not involved with the study.
“Someone may have been sexually assaulted frequently but not cross the threshold, so I think it would have been helpful for the researchers to look at different types of violence,” he said.
Also commenting on the study, Jessica Palardy, LSW, program supervisor at STOP Intimate Partner Violence, Philadelphia, said, the findings “solidify a trend we sensed was happening but couldn’t confirm.”
She said her agency’s clients “have had a wide variety of experiences, in terms of increases or decreases in victimization.”
Some clients were able to use the quarantine as an excuse to stay with family or friends and so could avoid seeing their partners. “Others indicated that because their partners were distracted by figuring out a new method of work, the tension shifted away from the victim,” said Ms. Palardy, who was not involved in the research.
“For those who saw an increase in victimization, we noticed that this increase also came with an increase in lethality indicators, such as strangulation, physical violence, use of weapons and substances, etc,” she said.
She emphasized that it is critical to screen people for IPV to ensure their safety.
“The goal is to connect people with resources before they are in a more lethal situation so that they can increase their safety and know their options,” Ms. Palardy said.
The National Domestic Violence Hotline and the Crisis Text Line are two sources of support for IPV victims.
Dr. Jetelina and coauthors, Dr. Cronholm, and Ms. Palardy reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Mega vitamin D harms bone in women, not men, without osteoporosis
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.
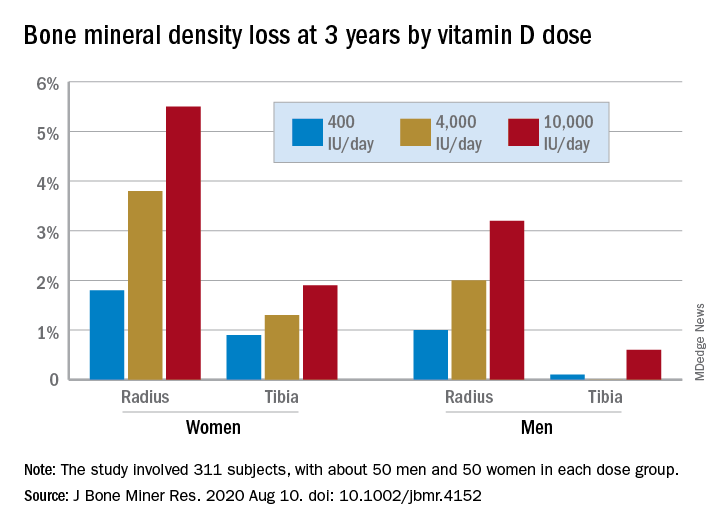
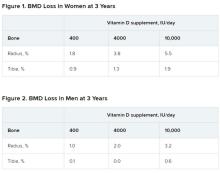
In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.

In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.

In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
Rural areas with local obstetrical care have better perinatal outcomes
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
FROM ANNALS OF FAMILY MEDICINE
Key clinical point: The absence of labor and delivery (L&D) services in rural counties predicts adverse outcomes, including higher child mortality.
Major finding: In the absence of L&D units, the risk of perinatal mortality per 1,000 live births is 19% higher (5.67 vs. 4.74; P = .0034).
Data Source: Retrospective cohort study.
Disclosures: Potential conflicts of interest involving this topic were not reported.
Source: Waits JB et al. Ann Fam Med. 2020;18:446-51.
Which medications work best for menorrhagia?
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE-BASED ANSWER:
Four medications have been shown to reduce menstrual blood loss (MBL) significantly in placebo-controlled randomized controlled trials (RCTs): the levonorgestrel-releasing intrauterine system (LNG-IUS), tranexamic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), and danazol, a synthetic steroid (strength of recommendation: A, meta-analyses of RCTs).
A single trial showed that the LNG-IUS reduced MBL by about 100 mL, compared with placebo. In a meta-analysis of 4 placebo-controlled RCTs, tranexamic acid reduced MBL by about 53 mL, roughly a 40% to 50% decrease. The 8 NSAID trials (5 mefenamic acid, 2 naproxen, 1 ibuprofen) demonstrated effectiveness, but the effect size is difficult to quantify. The single danazol RCT used a subjective scoring system without reporting MBL.
No studies compared all effective medical therapies against one another. In head-to-head comparisons, women were more likely to experience improvement with the LNG-IUS than with tranexamic acid (number needed to treat [NNT] = 2 to 6). Both treatments are superior to NSAIDs. Danazol is also more efficacious than NSAIDs, but its use is limited by its adverse effects, including teratogenicity.
No placebo-controlled trials have studied oral contraceptive pills (OCPs) or oral progesterone to treat menorrhagia. However, multiple comparative RCTs have demonstrated that these commonly prescribed medications significantly decrease MBL. Trials have shown the reduction to be inferior to LNG-IUS and danazol and equivalent to NSAIDs.
Study: 10% of pregnant women test positive for COVID-19, with most asymptomatic
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
FROM BMJ
Antidepressant use shows gender, racial disparities
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.
Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.

Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.

Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Psoriasis, PsA, and pregnancy: Tailoring treatment with increasing data
With an average age of diagnosis of 28 years, and one of two incidence peaks occurring at 15-30 years, psoriasis affects many women in the midst of their reproductive years. The prospect of pregnancy – or the reality of a surprise pregnancy – drives questions about heritability of the disease in offspring, the impact of the disease on pregnancy outcomes and breastfeeding, and how to best balance risks of treatments with risks of uncontrolled psoriasis and/or psoriatic arthritis (PsA).
While answers to these questions are not always clear, discussions about pregnancy and psoriasis management “shouldn’t be scary,” said Jenny E. Murase, MD, a dermatologist who speaks and writes widely about her research and experience with psoriasis and pregnancy. “We have access to information and data and educational resources to [work with] and reassure our patients – we just need to use it. Right now, there’s unnecessary suffering [with some patients unnecessarily stopping all treatment].”
Much has been learned in the past 2 decades about the course of psoriasis in pregnancy, and pregnancy outcomes data on the safety of biologics during pregnancy are increasingly emerging – particularly for tumor necrosis factor (TNF)–alpha inhibitors.
Ideally, since half of all pregnancies are unplanned, the implications of therapeutic options should be discussed with all women with psoriasis who are of reproductive age, whether they are sexually active or not. “The onus is on us to make sure that we’re considering the possibility [that our patient] could become pregnant without consulting us first,” said Dr. Murase, associate professor of dermatology at the University of California, San Francisco, and director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif.
Lisa R. Sammaritano, MD, associate professor of clinical medicine at Weill Cornell Medicine and a rheumatologist at the Hospital for Special Surgery, both in New York, urges similar attention for PsA. “Pregnancy is best planned while patients have quiescent disease on pregnancy-compatible medications,” she said. “We encourage [more] rheumatologists to be actively involved in pregnancy planning [in order] to guide therapy.”
The impact of estrogen
Dr. Murase was inspired to study psoriasis and pregnancy in part by a patient she met as a medical student. “She had severe psoriasis covering her body, and she said that the only times her psoriasis cleared was during her three pregnancies,” Dr. Murase recalled. “I wondered: What about the pregnancies resulted in such a substantial reduction of her psoriasis?”
She subsequently led a study, published in 2005, of 47 pregnant and 27 nonpregnant patients with psoriasis. More than half of the patients – 55% – reported improvements in their psoriasis during pregnancy, 21% reported no change, and 23% reported worsening. Among the 16 patients who had 10% or greater psoriatic body surface area (BSA) involvement and reported improvements, lesions decreased by 84%.
In the postpartum period, only 9% reported improvement, 26% reported no change, and 65% reported worsening. The increased BSA values observed 6 weeks postpartum did not exceed those of the first trimester, suggesting a return to the patients’ baseline status.
Earlier and smaller retrospective studies had also shown that approximately half of patients improve during pregnancy, and it was believed that progesterone was most likely responsible for this improvement. Dr. Murase’s study moved the needle in that it examined BSA in pregnancy and the postpartum period. It also turned the spotlight on estrogen: Patients who had higher levels of improvement also had higher levels of estradiol, estrone, and the ratio of estrogen to progesterone. However, there was no correlation between psoriatic change and levels of progesterone.
To promote fetal survival, pregnancy triggers a shift from Th1 cell–mediated immunity – and Th17 immunity – to Th2 immunity. While there’s no proof of a causative effect, increased estrogen appears to play a role in this shift and in the reduced production of Th1 and Th17 cytokines. Psoriasis is believed to be primarily a Th17-mediated disease, with some Th1 involvement, so this down-regulation can result in improved disease status, Dr. Murase said. (A host of other autoimmune diseases categorized as Th1 mediated similarly tend to improve during pregnancy, she added.)
Information on the effect of pregnancy on PsA is “conflicting,” Dr. Sammaritano said. “Some [of a limited number of studies] suggest a beneficial effect as is generally seen for rheumatoid arthritis. Others, however, have found an increased risk of disease activity during pregnancy ... It may be that psoriatic arthritis can be quite variable from patient to patient in its clinical presentation.”
At least one study, Dr. Sammaritano added, “has shown that the arthritis in pregnancy patients with PsA did not improve, compared to control nonpregnant patients, while the psoriasis rash did improve.”
The mixed findings don’t surprise Dr. Murase. “It harder to quantify joint disease in general,” she said. “And during pregnancy, physiologic changes relating to the pregnancy itself can cause discomfort – your joints ache. The numbers [of improved] cases aren’t as high with PsA, but it’s a more complex question.”
In the postpartum period, however, research findings “all suggest an increased risk of flare” of PsA, Dr. Sammaritano said, just as with psoriasis.
Assessing risk of treatment
Understanding the immunologic effects of pregnancy on psoriasis and PsA – and appreciating the concept of a hormonal component – is an important part of treatment decision making. So is understanding pregnancy outcomes data.
Researchers have looked at a host of pregnancy outcomes – including congenital malformations, preterm birth, spontaneous abortion, low birth weight, macrosomia, and gestational diabetes and hypertension – in women with psoriasis or psoriasis/PsA, compared with control groups. Some studies have suggested a link between disease activity and pregnancy complications or adverse pregnancy outcomes, “just as a result of having moderate to severe disease,” while others have found no evidence of increased risk, Dr. Murase said.
“It’s a bit unclear and a difficult question to answer; it depends on what study you look at and what data you believe. It would be nice to have some clarity, but basically the jury is still out,” said Dr. Murase, who, with coauthors Alice B. Gottlieb, MD, PhD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and Caitriona Ryan, MD, of the Blackrock Clinic and Charles Institute of Dermatology, University College Dublin, discussed the pregnancy outcomes data in a recently published review of psoriasis in women.
“In my opinion, because we have therapies that are so low risk and well tolerated, it’s better to make sure that the inflammatory cascade and inflammation created by psoriasis is under control,” she said. “So whether or not the pregnancy itself causes the patient to go into remission, or whether you have to use therapy to help the patient stay in remission, it’s important to control the inflammation.”
Contraindicated in pregnancy are oral psoralen, methotrexate, and acitretin, the latter of which should be avoided for several years before pregnancy and “therefore shouldn’t be used in a woman of childbearing age,” said Dr. Murase. Methotrexate, said Dr. Sammaritano, should generally be stopped 1-3 months prior to conception.
For psoriasis, the therapy that’s “classically considered the safest in pregnancy is UVB light therapy, specifically the 300-nm wavelength of light, which works really well as an anti-inflammatory,” Dr. Murase said. Because of the potential for maternal folate degradation with phototherapy and the long-known association of folate deficiency with neural tube defects, women of childbearing age who are receiving light therapy should take daily folic acid supplementation. (She prescribes a daily prenatal vitamin containing at least 1 mg of folic acid for women who are utilizing light therapy.)
Many topical agents can be used during pregnancy, Dr. Murase said. Topical corticosteroids, she noted, have the most safety-affirming data of any topical medication.
Regarding oral therapies, Dr. Murase recommends against the use of apremilast (Otezla) for her patients. “It’s not contraindicated, but the animal studies don’t look promising, so I don’t use that one in women of childbearing age just in case. There’s just very little data to support the safety of this medication [in pregnancy].”
There are no therapeutic guidelines in the United States for guiding the management of psoriasis in women who are considering pregnancy. In 2012, the medical board of the National Psoriasis Foundation published a review of treatment options for psoriasis in pregnant or lactating women, the “closest thing to guidelines that we’ve had,” said Dr. Murase. (Now almost a decade old, the review addresses TNF inhibitors but does not cover the anti-interleukin agents more recently approved for moderate to severe psoriasis and PsA.)
For treating PsA, rheumatologists now have the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases to reference. The 2020 guideline does not address PsA specifically, but its section on pregnancy and lactation includes recommendations on biologic and other therapies used to treat the disease.
Guidelines aside, physician-patient discussions over drug safety have the potential to be much more meaningful now that drug labels offer clinical summaries, data, and risk summaries regarding potential use in pregnancy. The labels have “more of a narrative, which is a more useful way to counsel patients and make risk-benefit decisions” than the former system of five-letter categories, said Dr. Murase. (The changes were made per the Pregnancy and Lactation Labeling Rule of 2015.)
MothertoBaby, a service of the nonprofit Organization of Teratology Information Specialists, also provides good evidence-based information to physicians and mothers, Dr. Sammaritano noted.
The use of biologic therapies
In a 2017 review of biologic safety for patients with psoriasis during pregnancy, Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School, Boston; Martina L. Porter, MD, currently with the department of dermatology at Beth Israel Deaconess Medical Center, Boston; and Stephen J. Lockwood, MD, MPH, of the department of dermatology at Harvard Medical School, concluded that an increasing body of literature suggests that biologic agents can be used during pregnancy and breastfeeding. Anti-TNF agents “should be considered over IL-12/23 and IL-17 inhibitors due to the increased availability of long-term data,” they wrote.
“In general,” said Dr. Murase, “there’s more and more data coming out from gastroenterology and rheumatology to reassure patients and prescribing physicians that the TNF-blocker class is likely safe to use in pregnancy,” particularly during the first trimester and early second trimester, when the transport of maternal antibodies across the placenta is “essentially nonexistent.” In the third trimester, the active transport of IgG antibodies increases rapidly.
If possible, said Dr. Sammaritano, who served as lead author of the ACR’s reproductive health guideline, TNF inhibitors “will be stopped prior to the third trimester to avoid [the possibility of] high drug levels in the infant at birth, which raises concern for immunosuppression in the newborn. If disease is very active, however, they can be continued throughout the pregnancy.”
The TNF inhibitor certolizumab pegol (Cimzia) has the advantage of being transported only minimally across the placenta, if at all, she and Dr. Murase both explained. “To be actively carried across, antibodies need what’s called an Fc region for the placenta to grab onto,” Dr. Murase said. Certolizumab – a pegylated anti–binding fragment antibody – lacks this Fc region.
Two recent studies – CRIB and a UCB Pharma safety database analysis – showed “essentially no medication crossing – there were barely detectable levels,” Dr. Murase said. Certolizumab’s label contains this information and other clinical trial data as well as findings from safety database analyses/surveillance registries.
“Before we had much data for the biologics, I’d advise transitioning patients to light therapy from their biologics and a lot of times their psoriasis would improve, but it was more of a dance,” she said. “Now we tend to look at [certolizumab] when they’re of childbearing age and keep them on the treatment. I know that the baby is not being immunosuppressed.”
Consideration of the use of certolizumab when treatment with biologic agents is required throughout the pregnancy is a recommendation included in Dr. Kimball’s 2017 review.
As newer anti-interleukin agents – the IL-12/23 and IL-17 inhibitors – play a growing role in the treatment of psoriasis and PsA, questions loom about their safety profile. Dr. Murase and Dr. Sammaritano are waiting for more data. “In general,” Dr. Sammaritano said, “we recommend stopping them at the time pregnancy is detected, based on a lack of data at this time.”
Small-molecule drugs are also less well studied, she noted. “Because of their low molecular weight, we anticipate they will easily cross the placenta, so we recommend avoiding use during pregnancy until more information is available.”
Postpartum care
The good news, both experts say, is that the vast majority of medications, including biologics, are safe to use during breastfeeding. Methotrexate should be avoided, Dr. Sammaritano pointed out, and the impact of novel small-molecule therapies on breast milk has not been studied.
In her 2019 review of psoriasis in women, Dr. Murase and coauthors wrote that too many dermatologists believe that breastfeeding women should either not be on biologics or are uncertain about biologic use during breastfeeding. However, “biologics are considered compatible for use while breastfeeding due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract,” they added.
Counseling and support for breastfeeding is especially important for women with psoriasis, Dr. Murase emphasized. “Breastfeeding is very traumatizing to the skin, and psoriasis can form in skin that’s injured. I have my patients set up an office visit very soon after the pregnancy to make sure they’re doing alright with their breastfeeding and that they’re coating their nipple area with some type of moisturizer and keeping the health of their nipples in good shape.”
Timely reviews of therapy and adjustments are also a priority, she said. “We need to prepare for 6 weeks post partum” when psoriasis will often flare without treatment.
Dr. Murase disclosed that she is a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron. She is also coeditor in chief of the International Journal of Women’s Dermatology. Dr. Sammaritano reported that she has no disclosures relating to the treatment of PsA.
With an average age of diagnosis of 28 years, and one of two incidence peaks occurring at 15-30 years, psoriasis affects many women in the midst of their reproductive years. The prospect of pregnancy – or the reality of a surprise pregnancy – drives questions about heritability of the disease in offspring, the impact of the disease on pregnancy outcomes and breastfeeding, and how to best balance risks of treatments with risks of uncontrolled psoriasis and/or psoriatic arthritis (PsA).
While answers to these questions are not always clear, discussions about pregnancy and psoriasis management “shouldn’t be scary,” said Jenny E. Murase, MD, a dermatologist who speaks and writes widely about her research and experience with psoriasis and pregnancy. “We have access to information and data and educational resources to [work with] and reassure our patients – we just need to use it. Right now, there’s unnecessary suffering [with some patients unnecessarily stopping all treatment].”
Much has been learned in the past 2 decades about the course of psoriasis in pregnancy, and pregnancy outcomes data on the safety of biologics during pregnancy are increasingly emerging – particularly for tumor necrosis factor (TNF)–alpha inhibitors.
Ideally, since half of all pregnancies are unplanned, the implications of therapeutic options should be discussed with all women with psoriasis who are of reproductive age, whether they are sexually active or not. “The onus is on us to make sure that we’re considering the possibility [that our patient] could become pregnant without consulting us first,” said Dr. Murase, associate professor of dermatology at the University of California, San Francisco, and director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif.
Lisa R. Sammaritano, MD, associate professor of clinical medicine at Weill Cornell Medicine and a rheumatologist at the Hospital for Special Surgery, both in New York, urges similar attention for PsA. “Pregnancy is best planned while patients have quiescent disease on pregnancy-compatible medications,” she said. “We encourage [more] rheumatologists to be actively involved in pregnancy planning [in order] to guide therapy.”
The impact of estrogen
Dr. Murase was inspired to study psoriasis and pregnancy in part by a patient she met as a medical student. “She had severe psoriasis covering her body, and she said that the only times her psoriasis cleared was during her three pregnancies,” Dr. Murase recalled. “I wondered: What about the pregnancies resulted in such a substantial reduction of her psoriasis?”
She subsequently led a study, published in 2005, of 47 pregnant and 27 nonpregnant patients with psoriasis. More than half of the patients – 55% – reported improvements in their psoriasis during pregnancy, 21% reported no change, and 23% reported worsening. Among the 16 patients who had 10% or greater psoriatic body surface area (BSA) involvement and reported improvements, lesions decreased by 84%.
In the postpartum period, only 9% reported improvement, 26% reported no change, and 65% reported worsening. The increased BSA values observed 6 weeks postpartum did not exceed those of the first trimester, suggesting a return to the patients’ baseline status.
Earlier and smaller retrospective studies had also shown that approximately half of patients improve during pregnancy, and it was believed that progesterone was most likely responsible for this improvement. Dr. Murase’s study moved the needle in that it examined BSA in pregnancy and the postpartum period. It also turned the spotlight on estrogen: Patients who had higher levels of improvement also had higher levels of estradiol, estrone, and the ratio of estrogen to progesterone. However, there was no correlation between psoriatic change and levels of progesterone.
To promote fetal survival, pregnancy triggers a shift from Th1 cell–mediated immunity – and Th17 immunity – to Th2 immunity. While there’s no proof of a causative effect, increased estrogen appears to play a role in this shift and in the reduced production of Th1 and Th17 cytokines. Psoriasis is believed to be primarily a Th17-mediated disease, with some Th1 involvement, so this down-regulation can result in improved disease status, Dr. Murase said. (A host of other autoimmune diseases categorized as Th1 mediated similarly tend to improve during pregnancy, she added.)
Information on the effect of pregnancy on PsA is “conflicting,” Dr. Sammaritano said. “Some [of a limited number of studies] suggest a beneficial effect as is generally seen for rheumatoid arthritis. Others, however, have found an increased risk of disease activity during pregnancy ... It may be that psoriatic arthritis can be quite variable from patient to patient in its clinical presentation.”
At least one study, Dr. Sammaritano added, “has shown that the arthritis in pregnancy patients with PsA did not improve, compared to control nonpregnant patients, while the psoriasis rash did improve.”
The mixed findings don’t surprise Dr. Murase. “It harder to quantify joint disease in general,” she said. “And during pregnancy, physiologic changes relating to the pregnancy itself can cause discomfort – your joints ache. The numbers [of improved] cases aren’t as high with PsA, but it’s a more complex question.”
In the postpartum period, however, research findings “all suggest an increased risk of flare” of PsA, Dr. Sammaritano said, just as with psoriasis.
Assessing risk of treatment
Understanding the immunologic effects of pregnancy on psoriasis and PsA – and appreciating the concept of a hormonal component – is an important part of treatment decision making. So is understanding pregnancy outcomes data.
Researchers have looked at a host of pregnancy outcomes – including congenital malformations, preterm birth, spontaneous abortion, low birth weight, macrosomia, and gestational diabetes and hypertension – in women with psoriasis or psoriasis/PsA, compared with control groups. Some studies have suggested a link between disease activity and pregnancy complications or adverse pregnancy outcomes, “just as a result of having moderate to severe disease,” while others have found no evidence of increased risk, Dr. Murase said.
“It’s a bit unclear and a difficult question to answer; it depends on what study you look at and what data you believe. It would be nice to have some clarity, but basically the jury is still out,” said Dr. Murase, who, with coauthors Alice B. Gottlieb, MD, PhD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and Caitriona Ryan, MD, of the Blackrock Clinic and Charles Institute of Dermatology, University College Dublin, discussed the pregnancy outcomes data in a recently published review of psoriasis in women.
“In my opinion, because we have therapies that are so low risk and well tolerated, it’s better to make sure that the inflammatory cascade and inflammation created by psoriasis is under control,” she said. “So whether or not the pregnancy itself causes the patient to go into remission, or whether you have to use therapy to help the patient stay in remission, it’s important to control the inflammation.”
Contraindicated in pregnancy are oral psoralen, methotrexate, and acitretin, the latter of which should be avoided for several years before pregnancy and “therefore shouldn’t be used in a woman of childbearing age,” said Dr. Murase. Methotrexate, said Dr. Sammaritano, should generally be stopped 1-3 months prior to conception.
For psoriasis, the therapy that’s “classically considered the safest in pregnancy is UVB light therapy, specifically the 300-nm wavelength of light, which works really well as an anti-inflammatory,” Dr. Murase said. Because of the potential for maternal folate degradation with phototherapy and the long-known association of folate deficiency with neural tube defects, women of childbearing age who are receiving light therapy should take daily folic acid supplementation. (She prescribes a daily prenatal vitamin containing at least 1 mg of folic acid for women who are utilizing light therapy.)
Many topical agents can be used during pregnancy, Dr. Murase said. Topical corticosteroids, she noted, have the most safety-affirming data of any topical medication.
Regarding oral therapies, Dr. Murase recommends against the use of apremilast (Otezla) for her patients. “It’s not contraindicated, but the animal studies don’t look promising, so I don’t use that one in women of childbearing age just in case. There’s just very little data to support the safety of this medication [in pregnancy].”
There are no therapeutic guidelines in the United States for guiding the management of psoriasis in women who are considering pregnancy. In 2012, the medical board of the National Psoriasis Foundation published a review of treatment options for psoriasis in pregnant or lactating women, the “closest thing to guidelines that we’ve had,” said Dr. Murase. (Now almost a decade old, the review addresses TNF inhibitors but does not cover the anti-interleukin agents more recently approved for moderate to severe psoriasis and PsA.)
For treating PsA, rheumatologists now have the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases to reference. The 2020 guideline does not address PsA specifically, but its section on pregnancy and lactation includes recommendations on biologic and other therapies used to treat the disease.
Guidelines aside, physician-patient discussions over drug safety have the potential to be much more meaningful now that drug labels offer clinical summaries, data, and risk summaries regarding potential use in pregnancy. The labels have “more of a narrative, which is a more useful way to counsel patients and make risk-benefit decisions” than the former system of five-letter categories, said Dr. Murase. (The changes were made per the Pregnancy and Lactation Labeling Rule of 2015.)
MothertoBaby, a service of the nonprofit Organization of Teratology Information Specialists, also provides good evidence-based information to physicians and mothers, Dr. Sammaritano noted.
The use of biologic therapies
In a 2017 review of biologic safety for patients with psoriasis during pregnancy, Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School, Boston; Martina L. Porter, MD, currently with the department of dermatology at Beth Israel Deaconess Medical Center, Boston; and Stephen J. Lockwood, MD, MPH, of the department of dermatology at Harvard Medical School, concluded that an increasing body of literature suggests that biologic agents can be used during pregnancy and breastfeeding. Anti-TNF agents “should be considered over IL-12/23 and IL-17 inhibitors due to the increased availability of long-term data,” they wrote.
“In general,” said Dr. Murase, “there’s more and more data coming out from gastroenterology and rheumatology to reassure patients and prescribing physicians that the TNF-blocker class is likely safe to use in pregnancy,” particularly during the first trimester and early second trimester, when the transport of maternal antibodies across the placenta is “essentially nonexistent.” In the third trimester, the active transport of IgG antibodies increases rapidly.
If possible, said Dr. Sammaritano, who served as lead author of the ACR’s reproductive health guideline, TNF inhibitors “will be stopped prior to the third trimester to avoid [the possibility of] high drug levels in the infant at birth, which raises concern for immunosuppression in the newborn. If disease is very active, however, they can be continued throughout the pregnancy.”
The TNF inhibitor certolizumab pegol (Cimzia) has the advantage of being transported only minimally across the placenta, if at all, she and Dr. Murase both explained. “To be actively carried across, antibodies need what’s called an Fc region for the placenta to grab onto,” Dr. Murase said. Certolizumab – a pegylated anti–binding fragment antibody – lacks this Fc region.
Two recent studies – CRIB and a UCB Pharma safety database analysis – showed “essentially no medication crossing – there were barely detectable levels,” Dr. Murase said. Certolizumab’s label contains this information and other clinical trial data as well as findings from safety database analyses/surveillance registries.
“Before we had much data for the biologics, I’d advise transitioning patients to light therapy from their biologics and a lot of times their psoriasis would improve, but it was more of a dance,” she said. “Now we tend to look at [certolizumab] when they’re of childbearing age and keep them on the treatment. I know that the baby is not being immunosuppressed.”
Consideration of the use of certolizumab when treatment with biologic agents is required throughout the pregnancy is a recommendation included in Dr. Kimball’s 2017 review.
As newer anti-interleukin agents – the IL-12/23 and IL-17 inhibitors – play a growing role in the treatment of psoriasis and PsA, questions loom about their safety profile. Dr. Murase and Dr. Sammaritano are waiting for more data. “In general,” Dr. Sammaritano said, “we recommend stopping them at the time pregnancy is detected, based on a lack of data at this time.”
Small-molecule drugs are also less well studied, she noted. “Because of their low molecular weight, we anticipate they will easily cross the placenta, so we recommend avoiding use during pregnancy until more information is available.”
Postpartum care
The good news, both experts say, is that the vast majority of medications, including biologics, are safe to use during breastfeeding. Methotrexate should be avoided, Dr. Sammaritano pointed out, and the impact of novel small-molecule therapies on breast milk has not been studied.
In her 2019 review of psoriasis in women, Dr. Murase and coauthors wrote that too many dermatologists believe that breastfeeding women should either not be on biologics or are uncertain about biologic use during breastfeeding. However, “biologics are considered compatible for use while breastfeeding due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract,” they added.
Counseling and support for breastfeeding is especially important for women with psoriasis, Dr. Murase emphasized. “Breastfeeding is very traumatizing to the skin, and psoriasis can form in skin that’s injured. I have my patients set up an office visit very soon after the pregnancy to make sure they’re doing alright with their breastfeeding and that they’re coating their nipple area with some type of moisturizer and keeping the health of their nipples in good shape.”
Timely reviews of therapy and adjustments are also a priority, she said. “We need to prepare for 6 weeks post partum” when psoriasis will often flare without treatment.
Dr. Murase disclosed that she is a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron. She is also coeditor in chief of the International Journal of Women’s Dermatology. Dr. Sammaritano reported that she has no disclosures relating to the treatment of PsA.
With an average age of diagnosis of 28 years, and one of two incidence peaks occurring at 15-30 years, psoriasis affects many women in the midst of their reproductive years. The prospect of pregnancy – or the reality of a surprise pregnancy – drives questions about heritability of the disease in offspring, the impact of the disease on pregnancy outcomes and breastfeeding, and how to best balance risks of treatments with risks of uncontrolled psoriasis and/or psoriatic arthritis (PsA).
While answers to these questions are not always clear, discussions about pregnancy and psoriasis management “shouldn’t be scary,” said Jenny E. Murase, MD, a dermatologist who speaks and writes widely about her research and experience with psoriasis and pregnancy. “We have access to information and data and educational resources to [work with] and reassure our patients – we just need to use it. Right now, there’s unnecessary suffering [with some patients unnecessarily stopping all treatment].”
Much has been learned in the past 2 decades about the course of psoriasis in pregnancy, and pregnancy outcomes data on the safety of biologics during pregnancy are increasingly emerging – particularly for tumor necrosis factor (TNF)–alpha inhibitors.
Ideally, since half of all pregnancies are unplanned, the implications of therapeutic options should be discussed with all women with psoriasis who are of reproductive age, whether they are sexually active or not. “The onus is on us to make sure that we’re considering the possibility [that our patient] could become pregnant without consulting us first,” said Dr. Murase, associate professor of dermatology at the University of California, San Francisco, and director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif.
Lisa R. Sammaritano, MD, associate professor of clinical medicine at Weill Cornell Medicine and a rheumatologist at the Hospital for Special Surgery, both in New York, urges similar attention for PsA. “Pregnancy is best planned while patients have quiescent disease on pregnancy-compatible medications,” she said. “We encourage [more] rheumatologists to be actively involved in pregnancy planning [in order] to guide therapy.”
The impact of estrogen
Dr. Murase was inspired to study psoriasis and pregnancy in part by a patient she met as a medical student. “She had severe psoriasis covering her body, and she said that the only times her psoriasis cleared was during her three pregnancies,” Dr. Murase recalled. “I wondered: What about the pregnancies resulted in such a substantial reduction of her psoriasis?”
She subsequently led a study, published in 2005, of 47 pregnant and 27 nonpregnant patients with psoriasis. More than half of the patients – 55% – reported improvements in their psoriasis during pregnancy, 21% reported no change, and 23% reported worsening. Among the 16 patients who had 10% or greater psoriatic body surface area (BSA) involvement and reported improvements, lesions decreased by 84%.
In the postpartum period, only 9% reported improvement, 26% reported no change, and 65% reported worsening. The increased BSA values observed 6 weeks postpartum did not exceed those of the first trimester, suggesting a return to the patients’ baseline status.
Earlier and smaller retrospective studies had also shown that approximately half of patients improve during pregnancy, and it was believed that progesterone was most likely responsible for this improvement. Dr. Murase’s study moved the needle in that it examined BSA in pregnancy and the postpartum period. It also turned the spotlight on estrogen: Patients who had higher levels of improvement also had higher levels of estradiol, estrone, and the ratio of estrogen to progesterone. However, there was no correlation between psoriatic change and levels of progesterone.
To promote fetal survival, pregnancy triggers a shift from Th1 cell–mediated immunity – and Th17 immunity – to Th2 immunity. While there’s no proof of a causative effect, increased estrogen appears to play a role in this shift and in the reduced production of Th1 and Th17 cytokines. Psoriasis is believed to be primarily a Th17-mediated disease, with some Th1 involvement, so this down-regulation can result in improved disease status, Dr. Murase said. (A host of other autoimmune diseases categorized as Th1 mediated similarly tend to improve during pregnancy, she added.)
Information on the effect of pregnancy on PsA is “conflicting,” Dr. Sammaritano said. “Some [of a limited number of studies] suggest a beneficial effect as is generally seen for rheumatoid arthritis. Others, however, have found an increased risk of disease activity during pregnancy ... It may be that psoriatic arthritis can be quite variable from patient to patient in its clinical presentation.”
At least one study, Dr. Sammaritano added, “has shown that the arthritis in pregnancy patients with PsA did not improve, compared to control nonpregnant patients, while the psoriasis rash did improve.”
The mixed findings don’t surprise Dr. Murase. “It harder to quantify joint disease in general,” she said. “And during pregnancy, physiologic changes relating to the pregnancy itself can cause discomfort – your joints ache. The numbers [of improved] cases aren’t as high with PsA, but it’s a more complex question.”
In the postpartum period, however, research findings “all suggest an increased risk of flare” of PsA, Dr. Sammaritano said, just as with psoriasis.
Assessing risk of treatment
Understanding the immunologic effects of pregnancy on psoriasis and PsA – and appreciating the concept of a hormonal component – is an important part of treatment decision making. So is understanding pregnancy outcomes data.
Researchers have looked at a host of pregnancy outcomes – including congenital malformations, preterm birth, spontaneous abortion, low birth weight, macrosomia, and gestational diabetes and hypertension – in women with psoriasis or psoriasis/PsA, compared with control groups. Some studies have suggested a link between disease activity and pregnancy complications or adverse pregnancy outcomes, “just as a result of having moderate to severe disease,” while others have found no evidence of increased risk, Dr. Murase said.
“It’s a bit unclear and a difficult question to answer; it depends on what study you look at and what data you believe. It would be nice to have some clarity, but basically the jury is still out,” said Dr. Murase, who, with coauthors Alice B. Gottlieb, MD, PhD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and Caitriona Ryan, MD, of the Blackrock Clinic and Charles Institute of Dermatology, University College Dublin, discussed the pregnancy outcomes data in a recently published review of psoriasis in women.
“In my opinion, because we have therapies that are so low risk and well tolerated, it’s better to make sure that the inflammatory cascade and inflammation created by psoriasis is under control,” she said. “So whether or not the pregnancy itself causes the patient to go into remission, or whether you have to use therapy to help the patient stay in remission, it’s important to control the inflammation.”
Contraindicated in pregnancy are oral psoralen, methotrexate, and acitretin, the latter of which should be avoided for several years before pregnancy and “therefore shouldn’t be used in a woman of childbearing age,” said Dr. Murase. Methotrexate, said Dr. Sammaritano, should generally be stopped 1-3 months prior to conception.
For psoriasis, the therapy that’s “classically considered the safest in pregnancy is UVB light therapy, specifically the 300-nm wavelength of light, which works really well as an anti-inflammatory,” Dr. Murase said. Because of the potential for maternal folate degradation with phototherapy and the long-known association of folate deficiency with neural tube defects, women of childbearing age who are receiving light therapy should take daily folic acid supplementation. (She prescribes a daily prenatal vitamin containing at least 1 mg of folic acid for women who are utilizing light therapy.)
Many topical agents can be used during pregnancy, Dr. Murase said. Topical corticosteroids, she noted, have the most safety-affirming data of any topical medication.
Regarding oral therapies, Dr. Murase recommends against the use of apremilast (Otezla) for her patients. “It’s not contraindicated, but the animal studies don’t look promising, so I don’t use that one in women of childbearing age just in case. There’s just very little data to support the safety of this medication [in pregnancy].”
There are no therapeutic guidelines in the United States for guiding the management of psoriasis in women who are considering pregnancy. In 2012, the medical board of the National Psoriasis Foundation published a review of treatment options for psoriasis in pregnant or lactating women, the “closest thing to guidelines that we’ve had,” said Dr. Murase. (Now almost a decade old, the review addresses TNF inhibitors but does not cover the anti-interleukin agents more recently approved for moderate to severe psoriasis and PsA.)
For treating PsA, rheumatologists now have the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases to reference. The 2020 guideline does not address PsA specifically, but its section on pregnancy and lactation includes recommendations on biologic and other therapies used to treat the disease.
Guidelines aside, physician-patient discussions over drug safety have the potential to be much more meaningful now that drug labels offer clinical summaries, data, and risk summaries regarding potential use in pregnancy. The labels have “more of a narrative, which is a more useful way to counsel patients and make risk-benefit decisions” than the former system of five-letter categories, said Dr. Murase. (The changes were made per the Pregnancy and Lactation Labeling Rule of 2015.)
MothertoBaby, a service of the nonprofit Organization of Teratology Information Specialists, also provides good evidence-based information to physicians and mothers, Dr. Sammaritano noted.
The use of biologic therapies
In a 2017 review of biologic safety for patients with psoriasis during pregnancy, Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School, Boston; Martina L. Porter, MD, currently with the department of dermatology at Beth Israel Deaconess Medical Center, Boston; and Stephen J. Lockwood, MD, MPH, of the department of dermatology at Harvard Medical School, concluded that an increasing body of literature suggests that biologic agents can be used during pregnancy and breastfeeding. Anti-TNF agents “should be considered over IL-12/23 and IL-17 inhibitors due to the increased availability of long-term data,” they wrote.
“In general,” said Dr. Murase, “there’s more and more data coming out from gastroenterology and rheumatology to reassure patients and prescribing physicians that the TNF-blocker class is likely safe to use in pregnancy,” particularly during the first trimester and early second trimester, when the transport of maternal antibodies across the placenta is “essentially nonexistent.” In the third trimester, the active transport of IgG antibodies increases rapidly.
If possible, said Dr. Sammaritano, who served as lead author of the ACR’s reproductive health guideline, TNF inhibitors “will be stopped prior to the third trimester to avoid [the possibility of] high drug levels in the infant at birth, which raises concern for immunosuppression in the newborn. If disease is very active, however, they can be continued throughout the pregnancy.”
The TNF inhibitor certolizumab pegol (Cimzia) has the advantage of being transported only minimally across the placenta, if at all, she and Dr. Murase both explained. “To be actively carried across, antibodies need what’s called an Fc region for the placenta to grab onto,” Dr. Murase said. Certolizumab – a pegylated anti–binding fragment antibody – lacks this Fc region.
Two recent studies – CRIB and a UCB Pharma safety database analysis – showed “essentially no medication crossing – there were barely detectable levels,” Dr. Murase said. Certolizumab’s label contains this information and other clinical trial data as well as findings from safety database analyses/surveillance registries.
“Before we had much data for the biologics, I’d advise transitioning patients to light therapy from their biologics and a lot of times their psoriasis would improve, but it was more of a dance,” she said. “Now we tend to look at [certolizumab] when they’re of childbearing age and keep them on the treatment. I know that the baby is not being immunosuppressed.”
Consideration of the use of certolizumab when treatment with biologic agents is required throughout the pregnancy is a recommendation included in Dr. Kimball’s 2017 review.
As newer anti-interleukin agents – the IL-12/23 and IL-17 inhibitors – play a growing role in the treatment of psoriasis and PsA, questions loom about their safety profile. Dr. Murase and Dr. Sammaritano are waiting for more data. “In general,” Dr. Sammaritano said, “we recommend stopping them at the time pregnancy is detected, based on a lack of data at this time.”
Small-molecule drugs are also less well studied, she noted. “Because of their low molecular weight, we anticipate they will easily cross the placenta, so we recommend avoiding use during pregnancy until more information is available.”
Postpartum care
The good news, both experts say, is that the vast majority of medications, including biologics, are safe to use during breastfeeding. Methotrexate should be avoided, Dr. Sammaritano pointed out, and the impact of novel small-molecule therapies on breast milk has not been studied.
In her 2019 review of psoriasis in women, Dr. Murase and coauthors wrote that too many dermatologists believe that breastfeeding women should either not be on biologics or are uncertain about biologic use during breastfeeding. However, “biologics are considered compatible for use while breastfeeding due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract,” they added.
Counseling and support for breastfeeding is especially important for women with psoriasis, Dr. Murase emphasized. “Breastfeeding is very traumatizing to the skin, and psoriasis can form in skin that’s injured. I have my patients set up an office visit very soon after the pregnancy to make sure they’re doing alright with their breastfeeding and that they’re coating their nipple area with some type of moisturizer and keeping the health of their nipples in good shape.”
Timely reviews of therapy and adjustments are also a priority, she said. “We need to prepare for 6 weeks post partum” when psoriasis will often flare without treatment.
Dr. Murase disclosed that she is a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron. She is also coeditor in chief of the International Journal of Women’s Dermatology. Dr. Sammaritano reported that she has no disclosures relating to the treatment of PsA.