User login
FDA approves drug to treat low sexual desire in women
The .
“There are women who, for no known reason, have reduced sexual desire that causes marked distress, and who can benefit from safe and effective pharmacologic treatment,” Hylton V. Joffe, MD, director of the Center for Drug Evaluation and Research’s Division of Bone, Reproductive, and Urologic Products, stated in a press release. “Today’s approval provides women with another treatment option for this condition.”
HSDD is characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not caused by a medical or psychiatric condition. Acquired HSDD develops in a patient who previously experienced no problems with sexual desire, and generalized HSDD is a lack of desire that occurs regardless of the type of sexual activity, situation, or partner.
Vyleesi was studied in two 24-week, randomized, double-blind, placebo-controlled trials in 1,247 premenopausal women with acquired, generalized HSDD. The women used Vyleesi two or three times per month and no more than once a week. About one-quarter of patients treated with Vyleesi had an increase of 1.2 or more in their sexual desire score (scored on a range of 1.2 to 6.0, with higher scores indicating greater sexual desire), compared with about 17% of those who took placebo. About 35% of the patients treated with Vyleesi had a decrease of one or more in their distress score (scored on a range of 0-4, with higher scores indicating greater distress from low sexual desire) compared with about 31% of those who took placebo.
The drug is injected under the skin of the abdomen or thigh at least 45 minutes before anticipated sexual activity. Patients may decide the optimal time to use Vyleesi based on the duration of benefit and any side effects, such as nausea. Patients should not take more than one dose of Vyleesi within 24 hours, or more than eight doses per month. Patients should discontinue treatment after 8 weeks if they do not report an improvement in sexual desire and associated distress.
Vyleesi works by activating melanocortin receptors but the exact mechanism for improving sexual desire is unknown. Some side effects were reported. “The most common side effects of Vyleesi are nausea and vomiting, flushing, injection site reactions, and headache. About 40% of patients in the clinical trials experienced nausea, most commonly with the first Vyleesi injection, and 13% needed medications for the treatment of nausea. About 1% of patients treated with Vyleesi in the clinical trials reported darkening of the gums and parts of the skin, including the face and breasts, which did not go away in about half the patients after stopping treatment. Patients with dark skin were more likely to develop this side effect,” according to the press release.
A temporary increase in blood pressure in patients after dosing with Vyleesi was observed during the clinical trials and therefore the drug is not recommended in patients at high risk for cardiovascular disease. In addition, patients who take a naltrexone-containing medication by mouth to treat alcohol or opioid dependence should not use Vyleesi because it may significantly decrease the levels of naltrexone in the blood and could lead to naltrexone treatment failure.
The full press release can be found on the FDA website.
The .
“There are women who, for no known reason, have reduced sexual desire that causes marked distress, and who can benefit from safe and effective pharmacologic treatment,” Hylton V. Joffe, MD, director of the Center for Drug Evaluation and Research’s Division of Bone, Reproductive, and Urologic Products, stated in a press release. “Today’s approval provides women with another treatment option for this condition.”
HSDD is characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not caused by a medical or psychiatric condition. Acquired HSDD develops in a patient who previously experienced no problems with sexual desire, and generalized HSDD is a lack of desire that occurs regardless of the type of sexual activity, situation, or partner.
Vyleesi was studied in two 24-week, randomized, double-blind, placebo-controlled trials in 1,247 premenopausal women with acquired, generalized HSDD. The women used Vyleesi two or three times per month and no more than once a week. About one-quarter of patients treated with Vyleesi had an increase of 1.2 or more in their sexual desire score (scored on a range of 1.2 to 6.0, with higher scores indicating greater sexual desire), compared with about 17% of those who took placebo. About 35% of the patients treated with Vyleesi had a decrease of one or more in their distress score (scored on a range of 0-4, with higher scores indicating greater distress from low sexual desire) compared with about 31% of those who took placebo.
The drug is injected under the skin of the abdomen or thigh at least 45 minutes before anticipated sexual activity. Patients may decide the optimal time to use Vyleesi based on the duration of benefit and any side effects, such as nausea. Patients should not take more than one dose of Vyleesi within 24 hours, or more than eight doses per month. Patients should discontinue treatment after 8 weeks if they do not report an improvement in sexual desire and associated distress.
Vyleesi works by activating melanocortin receptors but the exact mechanism for improving sexual desire is unknown. Some side effects were reported. “The most common side effects of Vyleesi are nausea and vomiting, flushing, injection site reactions, and headache. About 40% of patients in the clinical trials experienced nausea, most commonly with the first Vyleesi injection, and 13% needed medications for the treatment of nausea. About 1% of patients treated with Vyleesi in the clinical trials reported darkening of the gums and parts of the skin, including the face and breasts, which did not go away in about half the patients after stopping treatment. Patients with dark skin were more likely to develop this side effect,” according to the press release.
A temporary increase in blood pressure in patients after dosing with Vyleesi was observed during the clinical trials and therefore the drug is not recommended in patients at high risk for cardiovascular disease. In addition, patients who take a naltrexone-containing medication by mouth to treat alcohol or opioid dependence should not use Vyleesi because it may significantly decrease the levels of naltrexone in the blood and could lead to naltrexone treatment failure.
The full press release can be found on the FDA website.
The .
“There are women who, for no known reason, have reduced sexual desire that causes marked distress, and who can benefit from safe and effective pharmacologic treatment,” Hylton V. Joffe, MD, director of the Center for Drug Evaluation and Research’s Division of Bone, Reproductive, and Urologic Products, stated in a press release. “Today’s approval provides women with another treatment option for this condition.”
HSDD is characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not caused by a medical or psychiatric condition. Acquired HSDD develops in a patient who previously experienced no problems with sexual desire, and generalized HSDD is a lack of desire that occurs regardless of the type of sexual activity, situation, or partner.
Vyleesi was studied in two 24-week, randomized, double-blind, placebo-controlled trials in 1,247 premenopausal women with acquired, generalized HSDD. The women used Vyleesi two or three times per month and no more than once a week. About one-quarter of patients treated with Vyleesi had an increase of 1.2 or more in their sexual desire score (scored on a range of 1.2 to 6.0, with higher scores indicating greater sexual desire), compared with about 17% of those who took placebo. About 35% of the patients treated with Vyleesi had a decrease of one or more in their distress score (scored on a range of 0-4, with higher scores indicating greater distress from low sexual desire) compared with about 31% of those who took placebo.
The drug is injected under the skin of the abdomen or thigh at least 45 minutes before anticipated sexual activity. Patients may decide the optimal time to use Vyleesi based on the duration of benefit and any side effects, such as nausea. Patients should not take more than one dose of Vyleesi within 24 hours, or more than eight doses per month. Patients should discontinue treatment after 8 weeks if they do not report an improvement in sexual desire and associated distress.
Vyleesi works by activating melanocortin receptors but the exact mechanism for improving sexual desire is unknown. Some side effects were reported. “The most common side effects of Vyleesi are nausea and vomiting, flushing, injection site reactions, and headache. About 40% of patients in the clinical trials experienced nausea, most commonly with the first Vyleesi injection, and 13% needed medications for the treatment of nausea. About 1% of patients treated with Vyleesi in the clinical trials reported darkening of the gums and parts of the skin, including the face and breasts, which did not go away in about half the patients after stopping treatment. Patients with dark skin were more likely to develop this side effect,” according to the press release.
A temporary increase in blood pressure in patients after dosing with Vyleesi was observed during the clinical trials and therefore the drug is not recommended in patients at high risk for cardiovascular disease. In addition, patients who take a naltrexone-containing medication by mouth to treat alcohol or opioid dependence should not use Vyleesi because it may significantly decrease the levels of naltrexone in the blood and could lead to naltrexone treatment failure.
The full press release can be found on the FDA website.
Appeals court allows Title X restrictions to take effect
The panel ruled that the Trump administration’s funding restrictions were a reasonable interpretation of the federal Title X statute and that the administration is likely to prevail in its argument that lower courts erroneously halted the rules from taking effect. The ruling means the restrictions can take effect in every state except for Maryland, which passed a 2019 measure approving the use of state funds to replace federal Title X money if the new rule is enacted.
Alex Azar, secretary of the Department of Health & Human Services, said agency officials were pleased the 9th Circuit recognized there was no need to hold up the new family planning rules that simply enforce laws already on the books.
“We are also pleased that the [9th] Circuit agreed that the three preliminary injunctions against the new rules, including two nationwide injunctions, were inappropriate,” Mr. Azar said in the statement. “This decision is a major step toward the Trump administration being able to ensure that all Title X projects comply with the Title X statute and do not support abortion as a method of family planning.”
Leana Wen, MD, president for the Planned Parenthood Federation of America called the court ruling “devastating” for the millions of patients who rely on Title X health centers for cancer screenings, HIV tests, affordable birth control, and other critical primary and preventive care.
“We will be immediately seeking emergency relief from the [U.S.] Court of Appeals,” Dr. Wen said in a statement. “Planned Parenthood will not let the government censor our doctors and nurses from informing patients where and how they can access health care. We will continue to fight the Trump administration in the courts and alongside champions in Congress to protect everyone’s fundamental right to health care.”
The changes to the Title X program – originally scheduled to take effect May 3 – make health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. The 9th Circuit ruling overturns these injunctions.
The American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, and other groups have voiced their opposition to the Title X restrictions.
In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
The panel ruled that the Trump administration’s funding restrictions were a reasonable interpretation of the federal Title X statute and that the administration is likely to prevail in its argument that lower courts erroneously halted the rules from taking effect. The ruling means the restrictions can take effect in every state except for Maryland, which passed a 2019 measure approving the use of state funds to replace federal Title X money if the new rule is enacted.
Alex Azar, secretary of the Department of Health & Human Services, said agency officials were pleased the 9th Circuit recognized there was no need to hold up the new family planning rules that simply enforce laws already on the books.
“We are also pleased that the [9th] Circuit agreed that the three preliminary injunctions against the new rules, including two nationwide injunctions, were inappropriate,” Mr. Azar said in the statement. “This decision is a major step toward the Trump administration being able to ensure that all Title X projects comply with the Title X statute and do not support abortion as a method of family planning.”
Leana Wen, MD, president for the Planned Parenthood Federation of America called the court ruling “devastating” for the millions of patients who rely on Title X health centers for cancer screenings, HIV tests, affordable birth control, and other critical primary and preventive care.
“We will be immediately seeking emergency relief from the [U.S.] Court of Appeals,” Dr. Wen said in a statement. “Planned Parenthood will not let the government censor our doctors and nurses from informing patients where and how they can access health care. We will continue to fight the Trump administration in the courts and alongside champions in Congress to protect everyone’s fundamental right to health care.”
The changes to the Title X program – originally scheduled to take effect May 3 – make health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. The 9th Circuit ruling overturns these injunctions.
The American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, and other groups have voiced their opposition to the Title X restrictions.
In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
The panel ruled that the Trump administration’s funding restrictions were a reasonable interpretation of the federal Title X statute and that the administration is likely to prevail in its argument that lower courts erroneously halted the rules from taking effect. The ruling means the restrictions can take effect in every state except for Maryland, which passed a 2019 measure approving the use of state funds to replace federal Title X money if the new rule is enacted.
Alex Azar, secretary of the Department of Health & Human Services, said agency officials were pleased the 9th Circuit recognized there was no need to hold up the new family planning rules that simply enforce laws already on the books.
“We are also pleased that the [9th] Circuit agreed that the three preliminary injunctions against the new rules, including two nationwide injunctions, were inappropriate,” Mr. Azar said in the statement. “This decision is a major step toward the Trump administration being able to ensure that all Title X projects comply with the Title X statute and do not support abortion as a method of family planning.”
Leana Wen, MD, president for the Planned Parenthood Federation of America called the court ruling “devastating” for the millions of patients who rely on Title X health centers for cancer screenings, HIV tests, affordable birth control, and other critical primary and preventive care.
“We will be immediately seeking emergency relief from the [U.S.] Court of Appeals,” Dr. Wen said in a statement. “Planned Parenthood will not let the government censor our doctors and nurses from informing patients where and how they can access health care. We will continue to fight the Trump administration in the courts and alongside champions in Congress to protect everyone’s fundamental right to health care.”
The changes to the Title X program – originally scheduled to take effect May 3 – make health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. The 9th Circuit ruling overturns these injunctions.
The American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, and other groups have voiced their opposition to the Title X restrictions.
In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Illinois law expands abortion rights for women
A new Illinois law makes abortion a fundamental right and requires insurers to pay for the procedure as they would any other medical procedure.
The Illinois Reproductive Health Act repeals the Illinois Abortion Law of 1975 and the Partial-Birth Abortion Ban Act, two restrictive laws that have been largely blocked from enforcement for years by the courts. The replacement law removes criminal penalties for physicians who perform abortions, eliminates waiting periods before women can receive an abortion, and lifts a requirement that married women receive spousal consent before obtaining the procedure. Illinois Gov. JB Pritzker (D) signed the law on June 12.
“In a time when too many states across the nation are taking a step backward, Illinois is taking a giant step forward for women’s health,” Gov. Pritzker said in a statement. “Illinois is demonstrating what it means to affirm the rights of individuals to make the most personal and fundamental decisions of their lives, no matter your income level, race, ethnicity, or religion. When it comes to contraception, abortion, and reproductive care, this law puts the decision making where it belongs: in the hands of women and their doctors.”
As part of the law, private health insurance plans in Illinois are required to cover abortion. Previously, the plans were mandated to cover only contraception, infertility treatments, and maternity care. The law also states that a fertilized egg, embryo, or fetus does not have independent rights under Illinois law.
The law comes as states across the country are enacting more restrictive abortion measures. Recent laws in six states – Louisiana, Georgia, Kentucky, Mississippi, Missouri, and Ohio – bar abortions after a heartbeat is detected. A measure in Alabama meanwhile, prohibits abortion at every pregnancy stage and penalizes physicians with a Class A felony for performing an abortion and a Class C felony for attempting to perform an abortion. Analysts say those laws will likely lead to a review of Roe v. Wade by the Supreme Court later this year.
Also in June, the Department Health & Human Services said scientists are no longer allowed to use fetal tissue from abortions in research. In a statement, the agency said the decision comes amid a comprehensive review of all HHS research involving human fetal tissue from elective abortions to ensure consistency with statutes and regulations governing such research. The ban on fetal tissue research led to the cancellation of an existing HIV research contract between the federal government and the University of California, San Francisco, according to HHS.
“Promoting the dignity of human life from conception to natural death is one of the very top priorities of President Trump’s administration,” according to an HHS statement. “The audit and review helped inform the policy process that led to the administration’s decision to let the contract with UCSF expire and to discontinue intramural research – research conducted within the National Institutes of Health (NIH) – involving the use of human fetal tissue from elective abortion. Intramural research that requires new acquisition of fetal tissue from elective abortions will not be conducted.”
A new Illinois law makes abortion a fundamental right and requires insurers to pay for the procedure as they would any other medical procedure.
The Illinois Reproductive Health Act repeals the Illinois Abortion Law of 1975 and the Partial-Birth Abortion Ban Act, two restrictive laws that have been largely blocked from enforcement for years by the courts. The replacement law removes criminal penalties for physicians who perform abortions, eliminates waiting periods before women can receive an abortion, and lifts a requirement that married women receive spousal consent before obtaining the procedure. Illinois Gov. JB Pritzker (D) signed the law on June 12.
“In a time when too many states across the nation are taking a step backward, Illinois is taking a giant step forward for women’s health,” Gov. Pritzker said in a statement. “Illinois is demonstrating what it means to affirm the rights of individuals to make the most personal and fundamental decisions of their lives, no matter your income level, race, ethnicity, or religion. When it comes to contraception, abortion, and reproductive care, this law puts the decision making where it belongs: in the hands of women and their doctors.”
As part of the law, private health insurance plans in Illinois are required to cover abortion. Previously, the plans were mandated to cover only contraception, infertility treatments, and maternity care. The law also states that a fertilized egg, embryo, or fetus does not have independent rights under Illinois law.
The law comes as states across the country are enacting more restrictive abortion measures. Recent laws in six states – Louisiana, Georgia, Kentucky, Mississippi, Missouri, and Ohio – bar abortions after a heartbeat is detected. A measure in Alabama meanwhile, prohibits abortion at every pregnancy stage and penalizes physicians with a Class A felony for performing an abortion and a Class C felony for attempting to perform an abortion. Analysts say those laws will likely lead to a review of Roe v. Wade by the Supreme Court later this year.
Also in June, the Department Health & Human Services said scientists are no longer allowed to use fetal tissue from abortions in research. In a statement, the agency said the decision comes amid a comprehensive review of all HHS research involving human fetal tissue from elective abortions to ensure consistency with statutes and regulations governing such research. The ban on fetal tissue research led to the cancellation of an existing HIV research contract between the federal government and the University of California, San Francisco, according to HHS.
“Promoting the dignity of human life from conception to natural death is one of the very top priorities of President Trump’s administration,” according to an HHS statement. “The audit and review helped inform the policy process that led to the administration’s decision to let the contract with UCSF expire and to discontinue intramural research – research conducted within the National Institutes of Health (NIH) – involving the use of human fetal tissue from elective abortion. Intramural research that requires new acquisition of fetal tissue from elective abortions will not be conducted.”
A new Illinois law makes abortion a fundamental right and requires insurers to pay for the procedure as they would any other medical procedure.
The Illinois Reproductive Health Act repeals the Illinois Abortion Law of 1975 and the Partial-Birth Abortion Ban Act, two restrictive laws that have been largely blocked from enforcement for years by the courts. The replacement law removes criminal penalties for physicians who perform abortions, eliminates waiting periods before women can receive an abortion, and lifts a requirement that married women receive spousal consent before obtaining the procedure. Illinois Gov. JB Pritzker (D) signed the law on June 12.
“In a time when too many states across the nation are taking a step backward, Illinois is taking a giant step forward for women’s health,” Gov. Pritzker said in a statement. “Illinois is demonstrating what it means to affirm the rights of individuals to make the most personal and fundamental decisions of their lives, no matter your income level, race, ethnicity, or religion. When it comes to contraception, abortion, and reproductive care, this law puts the decision making where it belongs: in the hands of women and their doctors.”
As part of the law, private health insurance plans in Illinois are required to cover abortion. Previously, the plans were mandated to cover only contraception, infertility treatments, and maternity care. The law also states that a fertilized egg, embryo, or fetus does not have independent rights under Illinois law.
The law comes as states across the country are enacting more restrictive abortion measures. Recent laws in six states – Louisiana, Georgia, Kentucky, Mississippi, Missouri, and Ohio – bar abortions after a heartbeat is detected. A measure in Alabama meanwhile, prohibits abortion at every pregnancy stage and penalizes physicians with a Class A felony for performing an abortion and a Class C felony for attempting to perform an abortion. Analysts say those laws will likely lead to a review of Roe v. Wade by the Supreme Court later this year.
Also in June, the Department Health & Human Services said scientists are no longer allowed to use fetal tissue from abortions in research. In a statement, the agency said the decision comes amid a comprehensive review of all HHS research involving human fetal tissue from elective abortions to ensure consistency with statutes and regulations governing such research. The ban on fetal tissue research led to the cancellation of an existing HIV research contract between the federal government and the University of California, San Francisco, according to HHS.
“Promoting the dignity of human life from conception to natural death is one of the very top priorities of President Trump’s administration,” according to an HHS statement. “The audit and review helped inform the policy process that led to the administration’s decision to let the contract with UCSF expire and to discontinue intramural research – research conducted within the National Institutes of Health (NIH) – involving the use of human fetal tissue from elective abortion. Intramural research that requires new acquisition of fetal tissue from elective abortions will not be conducted.”
Consider drug treatment in late-life women with osteoporosis
and therefore have more to gain from osteoporosis treatment, regardless of the presence of any comorbidities, according to a new study.
To determine how and when to treat older women for osteoporosis, Kristine E. Ensrud, MD, of the University of Minnesota, Minneapolis, and coauthors studied active surviving participants in the Study of Osteoporotic Fractures. The cohort comprised 1,528 women who met criteria for either osteoporosis (n = 761) or without osteoporosis but at high fracture risk (n = 767). Mean age at the time of examination was 84 years and mean femoral neck bone mineral density (BMD) T-score was −2.24.
During an average follow-up period of 4.4 years after initial examination, 125 women (9%) experienced a hip fracture and 287 (19%) died without experiencing that outcome. The 5-year absolute probability of mortality was 25% (95% confidence interval, 21.8%-28.1%) in women with osteoporosis and 19% (95% CI, 16.6%-22.3%) in women without osteoporosis but at high fracture risk. Although both groups saw mortality probability increase with more comorbidities and poorer prognosis, 5-year hip fracture probability was 13% (95% CI, 10.7%-15.5%) among women with osteoporosis and 4% (95% CI, 2.8%-5.6%) among women without osteoporosis but at high fracture risk.
This probability of the women with osteoporosis experiencing a hip fracture, “even after considering their competing mortality risk” suggests that “initiation of drug treatment in late-life women with osteoporosis may still be effective in the prevention of subsequent hip fracture,” the researchers wrote in JAMA Internal Medicine.
Dr. Ensrud and associates acknowledged their study’s limitations, including the cohort being made up of community-dwelling white women and thus the results not being generalizable to men or women of other racial or ethnic groups. But the researchers noted that the mean femoral neck BMD of women in the study “was essentially identical to that of a nationally representative sample of community-dwelling women 80 years and older enrolled in the 2005 to 2008 NHANES [National Health and Nutrition Examination Survey].”
Dr. Cynthia M. Boyd reported receiving royalties from UpToDate and a grant from the National Institutes of Aging Dr. Katie L. Stone reported receiving grant support from Merck, and Dr. Lisa Langsetmo reported receiving grants from the National Institutes of Health and Merck. No other authors reported any relevant financial disclosures. The Study of Osteoporotic Fractures was supported by NIH and grants from NIA.
SOURCE: Ensrud KE et al. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0682.
Older patients with osteoporosis with multimorbidities are the most at risk for hip fractures, which should place an emphasis on research into their treatment, Sarah D. Berry, MD, MPH; Sandra Shi, MD; and Douglas P. Kiel, MD, MPH, of Harvard Medical School, Boston, wrote in an invited commentary.
The coauthors noted that the study by Ensrud et al. is of “great clinical importance, given the ongoing recognition that clinical guidelines should consider multimorbidity.” Currently, the guidelines for treating osteoporosis do not consider age, comorbidities, or frailty, but this study indicates that older women can see benefits from treatment.
They also acknowledged the value of patient preference, referencing a study where 80% of older women “would prefer death as opposed to a hip fracture leading to institutionalization.” All in all, the work of Ensrud et al. is a reminder of “the dangers in ignoring the problem” and the need for future guidelines in osteoporosis treatment to address osteoporosis treatment for older patients with multimorbidity.
These comments are adapted from an invited commentary accompanying the article by Ensrud et al. (JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0688 ). Dr. Berry reported receiving royalties from UpToDate outside the submitted work. Dr. Kiel reported receiving royalties from UpToDate, along with grants from the Dairy Council and Radius Health, and personal fees from Springer outside the submitted work. Dr. Shi reported no relevant financial disclosures. No funding for this editorial was reported.
Older patients with osteoporosis with multimorbidities are the most at risk for hip fractures, which should place an emphasis on research into their treatment, Sarah D. Berry, MD, MPH; Sandra Shi, MD; and Douglas P. Kiel, MD, MPH, of Harvard Medical School, Boston, wrote in an invited commentary.
The coauthors noted that the study by Ensrud et al. is of “great clinical importance, given the ongoing recognition that clinical guidelines should consider multimorbidity.” Currently, the guidelines for treating osteoporosis do not consider age, comorbidities, or frailty, but this study indicates that older women can see benefits from treatment.
They also acknowledged the value of patient preference, referencing a study where 80% of older women “would prefer death as opposed to a hip fracture leading to institutionalization.” All in all, the work of Ensrud et al. is a reminder of “the dangers in ignoring the problem” and the need for future guidelines in osteoporosis treatment to address osteoporosis treatment for older patients with multimorbidity.
These comments are adapted from an invited commentary accompanying the article by Ensrud et al. (JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0688 ). Dr. Berry reported receiving royalties from UpToDate outside the submitted work. Dr. Kiel reported receiving royalties from UpToDate, along with grants from the Dairy Council and Radius Health, and personal fees from Springer outside the submitted work. Dr. Shi reported no relevant financial disclosures. No funding for this editorial was reported.
Older patients with osteoporosis with multimorbidities are the most at risk for hip fractures, which should place an emphasis on research into their treatment, Sarah D. Berry, MD, MPH; Sandra Shi, MD; and Douglas P. Kiel, MD, MPH, of Harvard Medical School, Boston, wrote in an invited commentary.
The coauthors noted that the study by Ensrud et al. is of “great clinical importance, given the ongoing recognition that clinical guidelines should consider multimorbidity.” Currently, the guidelines for treating osteoporosis do not consider age, comorbidities, or frailty, but this study indicates that older women can see benefits from treatment.
They also acknowledged the value of patient preference, referencing a study where 80% of older women “would prefer death as opposed to a hip fracture leading to institutionalization.” All in all, the work of Ensrud et al. is a reminder of “the dangers in ignoring the problem” and the need for future guidelines in osteoporosis treatment to address osteoporosis treatment for older patients with multimorbidity.
These comments are adapted from an invited commentary accompanying the article by Ensrud et al. (JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0688 ). Dr. Berry reported receiving royalties from UpToDate outside the submitted work. Dr. Kiel reported receiving royalties from UpToDate, along with grants from the Dairy Council and Radius Health, and personal fees from Springer outside the submitted work. Dr. Shi reported no relevant financial disclosures. No funding for this editorial was reported.
and therefore have more to gain from osteoporosis treatment, regardless of the presence of any comorbidities, according to a new study.
To determine how and when to treat older women for osteoporosis, Kristine E. Ensrud, MD, of the University of Minnesota, Minneapolis, and coauthors studied active surviving participants in the Study of Osteoporotic Fractures. The cohort comprised 1,528 women who met criteria for either osteoporosis (n = 761) or without osteoporosis but at high fracture risk (n = 767). Mean age at the time of examination was 84 years and mean femoral neck bone mineral density (BMD) T-score was −2.24.
During an average follow-up period of 4.4 years after initial examination, 125 women (9%) experienced a hip fracture and 287 (19%) died without experiencing that outcome. The 5-year absolute probability of mortality was 25% (95% confidence interval, 21.8%-28.1%) in women with osteoporosis and 19% (95% CI, 16.6%-22.3%) in women without osteoporosis but at high fracture risk. Although both groups saw mortality probability increase with more comorbidities and poorer prognosis, 5-year hip fracture probability was 13% (95% CI, 10.7%-15.5%) among women with osteoporosis and 4% (95% CI, 2.8%-5.6%) among women without osteoporosis but at high fracture risk.
This probability of the women with osteoporosis experiencing a hip fracture, “even after considering their competing mortality risk” suggests that “initiation of drug treatment in late-life women with osteoporosis may still be effective in the prevention of subsequent hip fracture,” the researchers wrote in JAMA Internal Medicine.
Dr. Ensrud and associates acknowledged their study’s limitations, including the cohort being made up of community-dwelling white women and thus the results not being generalizable to men or women of other racial or ethnic groups. But the researchers noted that the mean femoral neck BMD of women in the study “was essentially identical to that of a nationally representative sample of community-dwelling women 80 years and older enrolled in the 2005 to 2008 NHANES [National Health and Nutrition Examination Survey].”
Dr. Cynthia M. Boyd reported receiving royalties from UpToDate and a grant from the National Institutes of Aging Dr. Katie L. Stone reported receiving grant support from Merck, and Dr. Lisa Langsetmo reported receiving grants from the National Institutes of Health and Merck. No other authors reported any relevant financial disclosures. The Study of Osteoporotic Fractures was supported by NIH and grants from NIA.
SOURCE: Ensrud KE et al. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0682.
and therefore have more to gain from osteoporosis treatment, regardless of the presence of any comorbidities, according to a new study.
To determine how and when to treat older women for osteoporosis, Kristine E. Ensrud, MD, of the University of Minnesota, Minneapolis, and coauthors studied active surviving participants in the Study of Osteoporotic Fractures. The cohort comprised 1,528 women who met criteria for either osteoporosis (n = 761) or without osteoporosis but at high fracture risk (n = 767). Mean age at the time of examination was 84 years and mean femoral neck bone mineral density (BMD) T-score was −2.24.
During an average follow-up period of 4.4 years after initial examination, 125 women (9%) experienced a hip fracture and 287 (19%) died without experiencing that outcome. The 5-year absolute probability of mortality was 25% (95% confidence interval, 21.8%-28.1%) in women with osteoporosis and 19% (95% CI, 16.6%-22.3%) in women without osteoporosis but at high fracture risk. Although both groups saw mortality probability increase with more comorbidities and poorer prognosis, 5-year hip fracture probability was 13% (95% CI, 10.7%-15.5%) among women with osteoporosis and 4% (95% CI, 2.8%-5.6%) among women without osteoporosis but at high fracture risk.
This probability of the women with osteoporosis experiencing a hip fracture, “even after considering their competing mortality risk” suggests that “initiation of drug treatment in late-life women with osteoporosis may still be effective in the prevention of subsequent hip fracture,” the researchers wrote in JAMA Internal Medicine.
Dr. Ensrud and associates acknowledged their study’s limitations, including the cohort being made up of community-dwelling white women and thus the results not being generalizable to men or women of other racial or ethnic groups. But the researchers noted that the mean femoral neck BMD of women in the study “was essentially identical to that of a nationally representative sample of community-dwelling women 80 years and older enrolled in the 2005 to 2008 NHANES [National Health and Nutrition Examination Survey].”
Dr. Cynthia M. Boyd reported receiving royalties from UpToDate and a grant from the National Institutes of Aging Dr. Katie L. Stone reported receiving grant support from Merck, and Dr. Lisa Langsetmo reported receiving grants from the National Institutes of Health and Merck. No other authors reported any relevant financial disclosures. The Study of Osteoporotic Fractures was supported by NIH and grants from NIA.
SOURCE: Ensrud KE et al. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0682.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Medical treatment to prevent osteoporotic hip fracture in women over age 80 likely is worthwhile.
Major finding: Five-year hip fracture probability was 13% among women with osteoporosis and 4% among women without osteoporosis but at high fracture risk.
Study details: A prospective cohort study of 1,528 women 80 years and older who were potential candidates for osteoporosis drug treatment.
Disclosures: Dr. Cynthia M. Boyd reported receiving royalties from UpToDate and a grant from the National Institutes of Aging Dr. Katie L. Stone reported receiving grant support from Merck, and Dr. Lisa Langsetmo reported receiving grants from the National Institutes of Health and Merck. No other authors reported any relevant financial disclosures. The Study of Osteoporotic Fractures was supported by NIH and grants from NIA.
Source: Ensrud KE et al. JAMA Intern Med. 2019 Jun 17. doi: 10.1001/jamainternmed.2019.0682.
U.S. travelers to Europe need up to date measles immunization
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
FROM PEDIATRICS
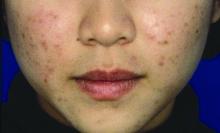
Ovarian reserve markers fall on isotretinoin, but rebound after stopping treatment
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
REPORTING FROM WCD2019
In MS, the challenges for women are unique
SEATTLE – Mitzi Joi Williams, MD.
About three in four people with MS are female – about 750,000 in the United States. And the risk and incidence may be highest in African American women.
In a presentation about the unique needs of women with MS, Dr. Williams, an assistant professor of internal medicine at the Morehouse School of Medicine in Atlanta, offered these tips at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pay attention to sexual dysfunction
Patients with MS often are ashamed to talk about sexual dysfunction, Dr. Williams said, but it is on many minds. “If I have a program on intimacy in MS, people are out the door.”
She urged colleagues to understand that MS can affect sexuality through three routes: primary, secondary, and tertiary dysfunction.
In primary sexual dysfunction, brain and spinal lesions directly related to MS can cause problems such as lack of sensation or abnormal sensations, decreased libido, vaginal dryness, and difficult orgasm.
Secondary sexual dysfunction refers to problems caused by symptoms of MS such as fatigue, which can worsen as the day progresses and affect nighttime intimacy, she said. Bladder dysfunction is another sensitive area in sexuality, with patients – especially women – “concerned that they will lose control of their bladder or they have already lost control.”
Cognitive dysfunction also can disrupt sexual function. “It is important to focus, and certain things cannot happen if you do not. If you are not able to focus and concentrate, it can affect interest,” Dr. Williams said.
Additionally, medications can improve some symptoms while making others worse. For example, a drug may relieve spasticity but boost fatigue. “We have to walk this tightrope,” she said. “But if we are not asking our patients, they may not volunteer this information.”
Finally, she said, MS can spark tertiary sexual dysfunction – poor body image, depression, anxiety, and disruptive changes in familial roles. For example, one partner may become a caregiver, and “it is hard to go from caregiving to sexy time.”
“It is something we have to acknowledge and find ways to deal with,” Dr. Williams said.
To address these issues, she pointed to strategies for symptomatic relief and disease-modifying therapy (DMT) and pinpointed several treatment options.
- Fatigue – stimulants, diet, exercise.
- Spasticity – muscle relaxants, exercise.
- Bladder dysfunction – fluid restriction, medication.
- Paresthesia – antidepressants, anticonvulsants.
- Numbness – vibrators, devices to increase stimulation.
Sexual therapy, couples therapy, and pelvic floor physical therapy also can be helpful.
Be aware of special needs during prepregnancy and pregnancy
“MS itself does not have a lot of effects on fertility, pregnancy, or pregnancy outcomes,” Dr. Williams said. However, “medications cause concern about how we manage pregnancy and fertility.”
In vitro fertilization may increase the risk of relapse, she added, and patients on dimethyl fumarate who experience vomiting or diarrhea may not be able to properly absorb oral contraceptives.
Women with MS may not need to go off DMT when they are trying to conceive, she said. “If patients have very aggressive disease, they may need to be on DMT through conception, through the first trimester, and even the entire pregnancy to prevent long-term disability.”
What about pregnancy itself? “An MS diagnosis alone does not mean that a pregnancy is high risk,” she said. “There are not necessarily additional tests and ultrasounds that are recommended for our patients based on MS diagnosis alone.”
Treatment discontinuation may be warranted during pregnancy, when MS generally improves. However, some MS symptoms – fatigue, bladder dysfunction, and balance – may increase. Corticosteroids can be appropriate if relapses occur during pregnancy.
Menopause and MS symptoms may overlap
Symptoms such as hot flashes, mood changes, sleep disturbance, bladder dysfunction, and decreased energy may be signs of MS, or they could indicate menopause, Dr. Williams said. “Sometimes patients come in and they are getting worse, and we look into it and discover they are premenopausal.”
A decline in estrogen during menopause may worsen MS symptoms, she added, and hormone therapy may be appropriate. A phase 2 study found a benefit in menopausal patients with MS for estriol in conjunction with a DMT, but more studies are needed.
Dr. Williams reported no relevant financial disclosures.
SEATTLE – Mitzi Joi Williams, MD.
About three in four people with MS are female – about 750,000 in the United States. And the risk and incidence may be highest in African American women.
In a presentation about the unique needs of women with MS, Dr. Williams, an assistant professor of internal medicine at the Morehouse School of Medicine in Atlanta, offered these tips at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pay attention to sexual dysfunction
Patients with MS often are ashamed to talk about sexual dysfunction, Dr. Williams said, but it is on many minds. “If I have a program on intimacy in MS, people are out the door.”
She urged colleagues to understand that MS can affect sexuality through three routes: primary, secondary, and tertiary dysfunction.
In primary sexual dysfunction, brain and spinal lesions directly related to MS can cause problems such as lack of sensation or abnormal sensations, decreased libido, vaginal dryness, and difficult orgasm.
Secondary sexual dysfunction refers to problems caused by symptoms of MS such as fatigue, which can worsen as the day progresses and affect nighttime intimacy, she said. Bladder dysfunction is another sensitive area in sexuality, with patients – especially women – “concerned that they will lose control of their bladder or they have already lost control.”
Cognitive dysfunction also can disrupt sexual function. “It is important to focus, and certain things cannot happen if you do not. If you are not able to focus and concentrate, it can affect interest,” Dr. Williams said.
Additionally, medications can improve some symptoms while making others worse. For example, a drug may relieve spasticity but boost fatigue. “We have to walk this tightrope,” she said. “But if we are not asking our patients, they may not volunteer this information.”
Finally, she said, MS can spark tertiary sexual dysfunction – poor body image, depression, anxiety, and disruptive changes in familial roles. For example, one partner may become a caregiver, and “it is hard to go from caregiving to sexy time.”
“It is something we have to acknowledge and find ways to deal with,” Dr. Williams said.
To address these issues, she pointed to strategies for symptomatic relief and disease-modifying therapy (DMT) and pinpointed several treatment options.
- Fatigue – stimulants, diet, exercise.
- Spasticity – muscle relaxants, exercise.
- Bladder dysfunction – fluid restriction, medication.
- Paresthesia – antidepressants, anticonvulsants.
- Numbness – vibrators, devices to increase stimulation.
Sexual therapy, couples therapy, and pelvic floor physical therapy also can be helpful.
Be aware of special needs during prepregnancy and pregnancy
“MS itself does not have a lot of effects on fertility, pregnancy, or pregnancy outcomes,” Dr. Williams said. However, “medications cause concern about how we manage pregnancy and fertility.”
In vitro fertilization may increase the risk of relapse, she added, and patients on dimethyl fumarate who experience vomiting or diarrhea may not be able to properly absorb oral contraceptives.
Women with MS may not need to go off DMT when they are trying to conceive, she said. “If patients have very aggressive disease, they may need to be on DMT through conception, through the first trimester, and even the entire pregnancy to prevent long-term disability.”
What about pregnancy itself? “An MS diagnosis alone does not mean that a pregnancy is high risk,” she said. “There are not necessarily additional tests and ultrasounds that are recommended for our patients based on MS diagnosis alone.”
Treatment discontinuation may be warranted during pregnancy, when MS generally improves. However, some MS symptoms – fatigue, bladder dysfunction, and balance – may increase. Corticosteroids can be appropriate if relapses occur during pregnancy.
Menopause and MS symptoms may overlap
Symptoms such as hot flashes, mood changes, sleep disturbance, bladder dysfunction, and decreased energy may be signs of MS, or they could indicate menopause, Dr. Williams said. “Sometimes patients come in and they are getting worse, and we look into it and discover they are premenopausal.”
A decline in estrogen during menopause may worsen MS symptoms, she added, and hormone therapy may be appropriate. A phase 2 study found a benefit in menopausal patients with MS for estriol in conjunction with a DMT, but more studies are needed.
Dr. Williams reported no relevant financial disclosures.
SEATTLE – Mitzi Joi Williams, MD.
About three in four people with MS are female – about 750,000 in the United States. And the risk and incidence may be highest in African American women.
In a presentation about the unique needs of women with MS, Dr. Williams, an assistant professor of internal medicine at the Morehouse School of Medicine in Atlanta, offered these tips at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pay attention to sexual dysfunction
Patients with MS often are ashamed to talk about sexual dysfunction, Dr. Williams said, but it is on many minds. “If I have a program on intimacy in MS, people are out the door.”
She urged colleagues to understand that MS can affect sexuality through three routes: primary, secondary, and tertiary dysfunction.
In primary sexual dysfunction, brain and spinal lesions directly related to MS can cause problems such as lack of sensation or abnormal sensations, decreased libido, vaginal dryness, and difficult orgasm.
Secondary sexual dysfunction refers to problems caused by symptoms of MS such as fatigue, which can worsen as the day progresses and affect nighttime intimacy, she said. Bladder dysfunction is another sensitive area in sexuality, with patients – especially women – “concerned that they will lose control of their bladder or they have already lost control.”
Cognitive dysfunction also can disrupt sexual function. “It is important to focus, and certain things cannot happen if you do not. If you are not able to focus and concentrate, it can affect interest,” Dr. Williams said.
Additionally, medications can improve some symptoms while making others worse. For example, a drug may relieve spasticity but boost fatigue. “We have to walk this tightrope,” she said. “But if we are not asking our patients, they may not volunteer this information.”
Finally, she said, MS can spark tertiary sexual dysfunction – poor body image, depression, anxiety, and disruptive changes in familial roles. For example, one partner may become a caregiver, and “it is hard to go from caregiving to sexy time.”
“It is something we have to acknowledge and find ways to deal with,” Dr. Williams said.
To address these issues, she pointed to strategies for symptomatic relief and disease-modifying therapy (DMT) and pinpointed several treatment options.
- Fatigue – stimulants, diet, exercise.
- Spasticity – muscle relaxants, exercise.
- Bladder dysfunction – fluid restriction, medication.
- Paresthesia – antidepressants, anticonvulsants.
- Numbness – vibrators, devices to increase stimulation.
Sexual therapy, couples therapy, and pelvic floor physical therapy also can be helpful.
Be aware of special needs during prepregnancy and pregnancy
“MS itself does not have a lot of effects on fertility, pregnancy, or pregnancy outcomes,” Dr. Williams said. However, “medications cause concern about how we manage pregnancy and fertility.”
In vitro fertilization may increase the risk of relapse, she added, and patients on dimethyl fumarate who experience vomiting or diarrhea may not be able to properly absorb oral contraceptives.
Women with MS may not need to go off DMT when they are trying to conceive, she said. “If patients have very aggressive disease, they may need to be on DMT through conception, through the first trimester, and even the entire pregnancy to prevent long-term disability.”
What about pregnancy itself? “An MS diagnosis alone does not mean that a pregnancy is high risk,” she said. “There are not necessarily additional tests and ultrasounds that are recommended for our patients based on MS diagnosis alone.”
Treatment discontinuation may be warranted during pregnancy, when MS generally improves. However, some MS symptoms – fatigue, bladder dysfunction, and balance – may increase. Corticosteroids can be appropriate if relapses occur during pregnancy.
Menopause and MS symptoms may overlap
Symptoms such as hot flashes, mood changes, sleep disturbance, bladder dysfunction, and decreased energy may be signs of MS, or they could indicate menopause, Dr. Williams said. “Sometimes patients come in and they are getting worse, and we look into it and discover they are premenopausal.”
A decline in estrogen during menopause may worsen MS symptoms, she added, and hormone therapy may be appropriate. A phase 2 study found a benefit in menopausal patients with MS for estriol in conjunction with a DMT, but more studies are needed.
Dr. Williams reported no relevant financial disclosures.
EXPERT ANALYSIS FROM CMSC 2019
Judge bars contraceptive mandate from being enforced
In a June 5, 2019, opinion, U.S. District Judge Reed O’Connor granted a permanent injunction on the contraceptive mandate, ruling that both the mandate and the accommodation process violate the Religious Freedom Restoration Act. The injunction applies to all individuals and employers – regardless of size or nonprofit status – that oppose contraceptive coverage based on religious beliefs.
In his ruling, Judge O’Connor said the contraceptive mandate substantially burdens the plaintiffs’ religious exercise.
“The point of the contraceptive mandate is to ensure all ACA-compliant insurance plans include cost-free coverage of all FDA [Food and Drug Administration]-approved contraceptive methods [and] the point of the individual mandate is to ensure individuals purchase ACA-compliant insurance plans,” Judge O’Conner wrote. “The result? The individual plaintiffs are forced out of either the health insurance market or their religious exercise. And by choosing to adhere to their religious beliefs, not only are the individual plaintiffs excluded from the insurance market, they are forced to violate federal law. That the contraceptive mandate systematically discriminates against the individual class by blocking members’ entrance into the marketplace – due to religious exercise – is a substantial burden of the highest order.”
The case, DeOtte v. Azar, started with an October 2018 legal challenge by several Texas residents and a business over having to comply with the Affordable Care Act mandate. The plaintiffs argued the requirement violates their religious freedom, and that the court should strike it down as unconstitutional. The current Justice Department has largely chosen not to defend the case, agreeing that forcing people and employers with religious objections to comply with the contraceptive mandate violates the Religious Freedom Restoration Act. In 2018, the department issued new rules expanding exemptions to the ACA’s contraceptive mandate on moral or religious grounds.
Legal challenges against the expanded exemptions continue through the courts. Judges in California and Pennsylvania have temporarily banned the rules from taking effect. Analysts say the final answer on the contraceptive mandate could come from the U.S. Supreme Court.
In a June 5, 2019, opinion, U.S. District Judge Reed O’Connor granted a permanent injunction on the contraceptive mandate, ruling that both the mandate and the accommodation process violate the Religious Freedom Restoration Act. The injunction applies to all individuals and employers – regardless of size or nonprofit status – that oppose contraceptive coverage based on religious beliefs.
In his ruling, Judge O’Connor said the contraceptive mandate substantially burdens the plaintiffs’ religious exercise.
“The point of the contraceptive mandate is to ensure all ACA-compliant insurance plans include cost-free coverage of all FDA [Food and Drug Administration]-approved contraceptive methods [and] the point of the individual mandate is to ensure individuals purchase ACA-compliant insurance plans,” Judge O’Conner wrote. “The result? The individual plaintiffs are forced out of either the health insurance market or their religious exercise. And by choosing to adhere to their religious beliefs, not only are the individual plaintiffs excluded from the insurance market, they are forced to violate federal law. That the contraceptive mandate systematically discriminates against the individual class by blocking members’ entrance into the marketplace – due to religious exercise – is a substantial burden of the highest order.”
The case, DeOtte v. Azar, started with an October 2018 legal challenge by several Texas residents and a business over having to comply with the Affordable Care Act mandate. The plaintiffs argued the requirement violates their religious freedom, and that the court should strike it down as unconstitutional. The current Justice Department has largely chosen not to defend the case, agreeing that forcing people and employers with religious objections to comply with the contraceptive mandate violates the Religious Freedom Restoration Act. In 2018, the department issued new rules expanding exemptions to the ACA’s contraceptive mandate on moral or religious grounds.
Legal challenges against the expanded exemptions continue through the courts. Judges in California and Pennsylvania have temporarily banned the rules from taking effect. Analysts say the final answer on the contraceptive mandate could come from the U.S. Supreme Court.
In a June 5, 2019, opinion, U.S. District Judge Reed O’Connor granted a permanent injunction on the contraceptive mandate, ruling that both the mandate and the accommodation process violate the Religious Freedom Restoration Act. The injunction applies to all individuals and employers – regardless of size or nonprofit status – that oppose contraceptive coverage based on religious beliefs.
In his ruling, Judge O’Connor said the contraceptive mandate substantially burdens the plaintiffs’ religious exercise.
“The point of the contraceptive mandate is to ensure all ACA-compliant insurance plans include cost-free coverage of all FDA [Food and Drug Administration]-approved contraceptive methods [and] the point of the individual mandate is to ensure individuals purchase ACA-compliant insurance plans,” Judge O’Conner wrote. “The result? The individual plaintiffs are forced out of either the health insurance market or their religious exercise. And by choosing to adhere to their religious beliefs, not only are the individual plaintiffs excluded from the insurance market, they are forced to violate federal law. That the contraceptive mandate systematically discriminates against the individual class by blocking members’ entrance into the marketplace – due to religious exercise – is a substantial burden of the highest order.”
The case, DeOtte v. Azar, started with an October 2018 legal challenge by several Texas residents and a business over having to comply with the Affordable Care Act mandate. The plaintiffs argued the requirement violates their religious freedom, and that the court should strike it down as unconstitutional. The current Justice Department has largely chosen not to defend the case, agreeing that forcing people and employers with religious objections to comply with the contraceptive mandate violates the Religious Freedom Restoration Act. In 2018, the department issued new rules expanding exemptions to the ACA’s contraceptive mandate on moral or religious grounds.
Legal challenges against the expanded exemptions continue through the courts. Judges in California and Pennsylvania have temporarily banned the rules from taking effect. Analysts say the final answer on the contraceptive mandate could come from the U.S. Supreme Court.
Postpartum LARC uptake increased with separate payment
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
FROM JAMA
Diverse vaginal microbiome may signal risk for preterm birth
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
FROM NATURE MEDICINE