User login
Official Newspaper of the American College of Surgeons
True postbariatric hyperinsulinemic hypoglycemia is rare
based on a decade’s worth of experience from the Mayo Clinic, Rochester, Minn.
Of 2,386 patients who had bariatric surgery at Mayo, 60 (2.6%) had a postsurgical diagnosis code associated with hypoglycemia in their medical record. However, just five of them (0.25%) had documentation meeting the criteria for Whipple’s Triad, which consists of low blood glucose levels, symptoms associated with the low glucose levels, and symptom resolution when glucose levels are corrected, Tiffany Cortes, MD, reported in an oral presentation at Obesity Week, which is presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery .
“Postbariatric hypoglycemia is an infrequent occurrence among patients who present with suspicious symptoms,” said Dr. Cortes, an endocrinology fellow at the clinic.
Post–bariatric surgery hypoglycemia is characterized by neuroglycopenia with a documented plasma glucose of less than 54 mg/dL with symptom resolution after a rise in glucose levels; neuroglycopenia that occurs 1-3 hours after a meal; and symptom onset more than 6 months after bariatric surgery, said Dr. Cortes.
Previous work had found that the overall prevalence of post–bariatric surgery hyperinsulinemic hypoglycemia ranged from 17%-34%, with severe symptoms seen in fewer than 1% of surgery recipients.
Bariatric surgery, especially Roux-en-Y gastric bypass (RYGB), may result in wide postprandial blood glucose excursions, with a spike occurring about 30 minutes after eating. For symptomatic individuals, this postprandial glucose peak will prompt an insulin surge followed by a rapid and steep decline in serum glucose.
Looking at Mayo Clinic medical records from mid-2008 to the end of 2017, Dr. Cortes and her colleagues wanted to determine the prevalence of hyperinsulinemic hypoglycemia in the bariatric surgery population.
Additionally, the researchers wanted to see how patients who presented with symptoms suspicious for the syndrome were evaluated and to understand the efficacy of treatments.
Patients who had a diagnosis of type 1 diabetes mellitus and those who were on insulin or sulfonylureas were excluded from the retrospective chart review.
Of the 60 patients evaluated in the endocrinology clinic for symptoms suspicious for hyperinsulinemic hypoglycemia, 51 (85%) were female, and 14 had a diagnosis of diabetes before surgery. Mean patient age at surgery was 43 years.
These symptomatic patients had a mean presurgical body mass index (BMI) of 42.8 kg/m2 (range, 38.6-49.3 kg/m2). Their mean time to maximal weight loss was 1.3 years after surgery, with symptoms beginning at 1.4 years after surgery. Patients lost a mean 37.4% of their body mass to reach a mean nadir BMI of 26.2.
Overall, about two-thirds of the surgeries performed were RYGB. Of patients with hypoglycemic symptoms, 73.3% had an RYGB. Revision of gastric bypass was the next most common surgery, at 21.8% overall; these patients constituted 15% of the hypoglycemic symptom group.
Of the patients with symptoms, 80% noted symptoms only after eating, with half of patients describing symptoms coming on 1-3 hours after eating. A little over a third of the patients didn’t describe the exact timing of symptoms.
Just 20 patients had a complete hypoglycemia work up bundle documented in their medical record, said Dr. Cortes. This consisted of measures of serum glucose, insulin, and C-peptide levels. Of the 20 patients, 5 met Whipple’s Triad criteria, and 4 of these patients received a diagnosis of hyperinsulinemic hypoglycemia.
Two patients had a 72-hour fast, and neither of them met diagnostic criteria. Seventeen patients had a mixed meal tolerance test, with one individual meeting diagnostic criteria for and receiving a diagnosis of hypoinsulinemic hyperglycemia.
Of the five patients meeting diagnostic criteria (0.20% of surgical population), all had received RYGB, and two had previous weight loss procedures, said Dr. Cortes. For four of the patients, the surgical indication was weight loss; the other patient had an indication of gastroesophageal reflux disease (GERD).
“Dietary interventions are the most effective treatment” for post–bariatric surgery hyperinsulinemic hypoglycemia in the Mayo Clinic experience, said Dr. Cortes.
Turning to the investigators’ examination of treatment recommendations for the 60 patients who reported hypoglycemic symptoms, most (95%) received an initial recommendation to manage symptoms by diet changes.
Most patients (77%) had at least one follow-up visit, with over half of these patients (61%) reporting improvement in symptoms, and seven patients (16%) reporting resolution. Twelve patients (27%) either remained the same or had not had a recurrence of symptoms.
Medication was prescribed for 12 patients; of them, 8 received the alpha glucosidase inhibitor acarbose and 7 responded, according to the record review. No one reported worsening of symptoms on acarbose.
Other individual patients were prescribed octreotide alone, or octreotide, pasireotide, or diazoxide in combination with acarbose, with variable results.
Dr. Cortes reported no conflicts of interest and no external sources of funding.
SOURCE: Cortes T et al. Obesity Week 2018, Abstract T-OR-2015.
based on a decade’s worth of experience from the Mayo Clinic, Rochester, Minn.
Of 2,386 patients who had bariatric surgery at Mayo, 60 (2.6%) had a postsurgical diagnosis code associated with hypoglycemia in their medical record. However, just five of them (0.25%) had documentation meeting the criteria for Whipple’s Triad, which consists of low blood glucose levels, symptoms associated with the low glucose levels, and symptom resolution when glucose levels are corrected, Tiffany Cortes, MD, reported in an oral presentation at Obesity Week, which is presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery .
“Postbariatric hypoglycemia is an infrequent occurrence among patients who present with suspicious symptoms,” said Dr. Cortes, an endocrinology fellow at the clinic.
Post–bariatric surgery hypoglycemia is characterized by neuroglycopenia with a documented plasma glucose of less than 54 mg/dL with symptom resolution after a rise in glucose levels; neuroglycopenia that occurs 1-3 hours after a meal; and symptom onset more than 6 months after bariatric surgery, said Dr. Cortes.
Previous work had found that the overall prevalence of post–bariatric surgery hyperinsulinemic hypoglycemia ranged from 17%-34%, with severe symptoms seen in fewer than 1% of surgery recipients.
Bariatric surgery, especially Roux-en-Y gastric bypass (RYGB), may result in wide postprandial blood glucose excursions, with a spike occurring about 30 minutes after eating. For symptomatic individuals, this postprandial glucose peak will prompt an insulin surge followed by a rapid and steep decline in serum glucose.
Looking at Mayo Clinic medical records from mid-2008 to the end of 2017, Dr. Cortes and her colleagues wanted to determine the prevalence of hyperinsulinemic hypoglycemia in the bariatric surgery population.
Additionally, the researchers wanted to see how patients who presented with symptoms suspicious for the syndrome were evaluated and to understand the efficacy of treatments.
Patients who had a diagnosis of type 1 diabetes mellitus and those who were on insulin or sulfonylureas were excluded from the retrospective chart review.
Of the 60 patients evaluated in the endocrinology clinic for symptoms suspicious for hyperinsulinemic hypoglycemia, 51 (85%) were female, and 14 had a diagnosis of diabetes before surgery. Mean patient age at surgery was 43 years.
These symptomatic patients had a mean presurgical body mass index (BMI) of 42.8 kg/m2 (range, 38.6-49.3 kg/m2). Their mean time to maximal weight loss was 1.3 years after surgery, with symptoms beginning at 1.4 years after surgery. Patients lost a mean 37.4% of their body mass to reach a mean nadir BMI of 26.2.
Overall, about two-thirds of the surgeries performed were RYGB. Of patients with hypoglycemic symptoms, 73.3% had an RYGB. Revision of gastric bypass was the next most common surgery, at 21.8% overall; these patients constituted 15% of the hypoglycemic symptom group.
Of the patients with symptoms, 80% noted symptoms only after eating, with half of patients describing symptoms coming on 1-3 hours after eating. A little over a third of the patients didn’t describe the exact timing of symptoms.
Just 20 patients had a complete hypoglycemia work up bundle documented in their medical record, said Dr. Cortes. This consisted of measures of serum glucose, insulin, and C-peptide levels. Of the 20 patients, 5 met Whipple’s Triad criteria, and 4 of these patients received a diagnosis of hyperinsulinemic hypoglycemia.
Two patients had a 72-hour fast, and neither of them met diagnostic criteria. Seventeen patients had a mixed meal tolerance test, with one individual meeting diagnostic criteria for and receiving a diagnosis of hypoinsulinemic hyperglycemia.
Of the five patients meeting diagnostic criteria (0.20% of surgical population), all had received RYGB, and two had previous weight loss procedures, said Dr. Cortes. For four of the patients, the surgical indication was weight loss; the other patient had an indication of gastroesophageal reflux disease (GERD).
“Dietary interventions are the most effective treatment” for post–bariatric surgery hyperinsulinemic hypoglycemia in the Mayo Clinic experience, said Dr. Cortes.
Turning to the investigators’ examination of treatment recommendations for the 60 patients who reported hypoglycemic symptoms, most (95%) received an initial recommendation to manage symptoms by diet changes.
Most patients (77%) had at least one follow-up visit, with over half of these patients (61%) reporting improvement in symptoms, and seven patients (16%) reporting resolution. Twelve patients (27%) either remained the same or had not had a recurrence of symptoms.
Medication was prescribed for 12 patients; of them, 8 received the alpha glucosidase inhibitor acarbose and 7 responded, according to the record review. No one reported worsening of symptoms on acarbose.
Other individual patients were prescribed octreotide alone, or octreotide, pasireotide, or diazoxide in combination with acarbose, with variable results.
Dr. Cortes reported no conflicts of interest and no external sources of funding.
SOURCE: Cortes T et al. Obesity Week 2018, Abstract T-OR-2015.
based on a decade’s worth of experience from the Mayo Clinic, Rochester, Minn.
Of 2,386 patients who had bariatric surgery at Mayo, 60 (2.6%) had a postsurgical diagnosis code associated with hypoglycemia in their medical record. However, just five of them (0.25%) had documentation meeting the criteria for Whipple’s Triad, which consists of low blood glucose levels, symptoms associated with the low glucose levels, and symptom resolution when glucose levels are corrected, Tiffany Cortes, MD, reported in an oral presentation at Obesity Week, which is presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery .
“Postbariatric hypoglycemia is an infrequent occurrence among patients who present with suspicious symptoms,” said Dr. Cortes, an endocrinology fellow at the clinic.
Post–bariatric surgery hypoglycemia is characterized by neuroglycopenia with a documented plasma glucose of less than 54 mg/dL with symptom resolution after a rise in glucose levels; neuroglycopenia that occurs 1-3 hours after a meal; and symptom onset more than 6 months after bariatric surgery, said Dr. Cortes.
Previous work had found that the overall prevalence of post–bariatric surgery hyperinsulinemic hypoglycemia ranged from 17%-34%, with severe symptoms seen in fewer than 1% of surgery recipients.
Bariatric surgery, especially Roux-en-Y gastric bypass (RYGB), may result in wide postprandial blood glucose excursions, with a spike occurring about 30 minutes after eating. For symptomatic individuals, this postprandial glucose peak will prompt an insulin surge followed by a rapid and steep decline in serum glucose.
Looking at Mayo Clinic medical records from mid-2008 to the end of 2017, Dr. Cortes and her colleagues wanted to determine the prevalence of hyperinsulinemic hypoglycemia in the bariatric surgery population.
Additionally, the researchers wanted to see how patients who presented with symptoms suspicious for the syndrome were evaluated and to understand the efficacy of treatments.
Patients who had a diagnosis of type 1 diabetes mellitus and those who were on insulin or sulfonylureas were excluded from the retrospective chart review.
Of the 60 patients evaluated in the endocrinology clinic for symptoms suspicious for hyperinsulinemic hypoglycemia, 51 (85%) were female, and 14 had a diagnosis of diabetes before surgery. Mean patient age at surgery was 43 years.
These symptomatic patients had a mean presurgical body mass index (BMI) of 42.8 kg/m2 (range, 38.6-49.3 kg/m2). Their mean time to maximal weight loss was 1.3 years after surgery, with symptoms beginning at 1.4 years after surgery. Patients lost a mean 37.4% of their body mass to reach a mean nadir BMI of 26.2.
Overall, about two-thirds of the surgeries performed were RYGB. Of patients with hypoglycemic symptoms, 73.3% had an RYGB. Revision of gastric bypass was the next most common surgery, at 21.8% overall; these patients constituted 15% of the hypoglycemic symptom group.
Of the patients with symptoms, 80% noted symptoms only after eating, with half of patients describing symptoms coming on 1-3 hours after eating. A little over a third of the patients didn’t describe the exact timing of symptoms.
Just 20 patients had a complete hypoglycemia work up bundle documented in their medical record, said Dr. Cortes. This consisted of measures of serum glucose, insulin, and C-peptide levels. Of the 20 patients, 5 met Whipple’s Triad criteria, and 4 of these patients received a diagnosis of hyperinsulinemic hypoglycemia.
Two patients had a 72-hour fast, and neither of them met diagnostic criteria. Seventeen patients had a mixed meal tolerance test, with one individual meeting diagnostic criteria for and receiving a diagnosis of hypoinsulinemic hyperglycemia.
Of the five patients meeting diagnostic criteria (0.20% of surgical population), all had received RYGB, and two had previous weight loss procedures, said Dr. Cortes. For four of the patients, the surgical indication was weight loss; the other patient had an indication of gastroesophageal reflux disease (GERD).
“Dietary interventions are the most effective treatment” for post–bariatric surgery hyperinsulinemic hypoglycemia in the Mayo Clinic experience, said Dr. Cortes.
Turning to the investigators’ examination of treatment recommendations for the 60 patients who reported hypoglycemic symptoms, most (95%) received an initial recommendation to manage symptoms by diet changes.
Most patients (77%) had at least one follow-up visit, with over half of these patients (61%) reporting improvement in symptoms, and seven patients (16%) reporting resolution. Twelve patients (27%) either remained the same or had not had a recurrence of symptoms.
Medication was prescribed for 12 patients; of them, 8 received the alpha glucosidase inhibitor acarbose and 7 responded, according to the record review. No one reported worsening of symptoms on acarbose.
Other individual patients were prescribed octreotide alone, or octreotide, pasireotide, or diazoxide in combination with acarbose, with variable results.
Dr. Cortes reported no conflicts of interest and no external sources of funding.
SOURCE: Cortes T et al. Obesity Week 2018, Abstract T-OR-2015.
REPORTING FROM OBESITY WEEK 2018
Key clinical point: Less than 1% of bariatric surgery patients had hyperinsulinemic hypoglycemia.
Major finding: When strict diagnostic criteria were used, 0.20% received the diagnosis.
Study details: Single-center retrospective chart review of 2,386 patients receiving bariatric surgery.
Disclosures: Dr. Cortes reported no outside sources of funding and no conflicts of interest.
Source: Cortes T et al. Obesity Week 2018, Abstract T-OR-2015.
Cervical bupivacaine blocks pain after laparoscopic hysterectomy
LAS VEGAS – according to a small trial at the University of Tennessee, Chattanooga.
Twenty-one women were randomized to 0.5% bupivacaine, 5 mL injected into the cervix at the 3 o’clock position, and 5 mL injected into at the 9 o’clock position to a depth of 3 cm, after anesthesia induction but before insertion of the uterine manipulator. A control group of 20 women received 5 mL of 0.9% saline injected into the same positions. Surgeons were blinded to the randomization.
A stopwatch was started at extubation, and the women were asked to rate their pain on a 10-point visual analogue scale exactly at 30 and 60 minutes.
The bupivacaine group had less pain at both 30 minutes (3.2 versus 5.7 points, P = .01) and 60 minutes (2.3 versus 5.9 points, P less than .001); 71% of women in the bupivacaine group had an average score of 4 or less, indicating adequate pain control, versus just 25% in the control arm (P = .003)
“This is something we should be considering” routinely for laparoscopic hysterectomy, an audience member said after hearing the presentation at a meeting sponsored by AAGL.
Another audience member was concerned about urinary retention, but there was no increase in the treatment arm, said lead investigator Steven Radtke, MD, a former ob.gyn. surgery fellow at the university, but now at Texas Tech University, El Paso.
There have been many prior attempts to reduce pain after laparoscopic hysterectomy, such as infiltrating port sites with local anesthetic, but the results have been marginal at best, and almost all of them have focused on the abdominal wall as the source of pain.
The investigators thought that pain was more related to perimetrium dissection, colpotomy, and other parts of the operation. There also have been good studies showing that agents injected into the cervix infuse throughout the area. The team decided to try bupivacaine because it’s inexpensive and has a good duration of action, about 8 hours.
There were no significant demographic or intraoperative differences between the groups. On average, women were in their mid-40s, with a body mass index of about 31 kg/m2. The operations took about 2 hours, and were for benign indications, such as fibroids. Oophorectomy was the only concomitant procedure allowed.
The investigators are interested in repeating their investigation with liposomal bupivacaine (Exparel), which has a duration of action past 24 hours. It’s much more expensive, but the strong trial results justify the cost, Dr. Radtke said.
There was no external funding, and Dr. Radtke didn’t have any disclosures.
SOURCE: Radtke S et al. 2018 AAGL Global Congress, Abstract 130.
LAS VEGAS – according to a small trial at the University of Tennessee, Chattanooga.
Twenty-one women were randomized to 0.5% bupivacaine, 5 mL injected into the cervix at the 3 o’clock position, and 5 mL injected into at the 9 o’clock position to a depth of 3 cm, after anesthesia induction but before insertion of the uterine manipulator. A control group of 20 women received 5 mL of 0.9% saline injected into the same positions. Surgeons were blinded to the randomization.
A stopwatch was started at extubation, and the women were asked to rate their pain on a 10-point visual analogue scale exactly at 30 and 60 minutes.
The bupivacaine group had less pain at both 30 minutes (3.2 versus 5.7 points, P = .01) and 60 minutes (2.3 versus 5.9 points, P less than .001); 71% of women in the bupivacaine group had an average score of 4 or less, indicating adequate pain control, versus just 25% in the control arm (P = .003)
“This is something we should be considering” routinely for laparoscopic hysterectomy, an audience member said after hearing the presentation at a meeting sponsored by AAGL.
Another audience member was concerned about urinary retention, but there was no increase in the treatment arm, said lead investigator Steven Radtke, MD, a former ob.gyn. surgery fellow at the university, but now at Texas Tech University, El Paso.
There have been many prior attempts to reduce pain after laparoscopic hysterectomy, such as infiltrating port sites with local anesthetic, but the results have been marginal at best, and almost all of them have focused on the abdominal wall as the source of pain.
The investigators thought that pain was more related to perimetrium dissection, colpotomy, and other parts of the operation. There also have been good studies showing that agents injected into the cervix infuse throughout the area. The team decided to try bupivacaine because it’s inexpensive and has a good duration of action, about 8 hours.
There were no significant demographic or intraoperative differences between the groups. On average, women were in their mid-40s, with a body mass index of about 31 kg/m2. The operations took about 2 hours, and were for benign indications, such as fibroids. Oophorectomy was the only concomitant procedure allowed.
The investigators are interested in repeating their investigation with liposomal bupivacaine (Exparel), which has a duration of action past 24 hours. It’s much more expensive, but the strong trial results justify the cost, Dr. Radtke said.
There was no external funding, and Dr. Radtke didn’t have any disclosures.
SOURCE: Radtke S et al. 2018 AAGL Global Congress, Abstract 130.
LAS VEGAS – according to a small trial at the University of Tennessee, Chattanooga.
Twenty-one women were randomized to 0.5% bupivacaine, 5 mL injected into the cervix at the 3 o’clock position, and 5 mL injected into at the 9 o’clock position to a depth of 3 cm, after anesthesia induction but before insertion of the uterine manipulator. A control group of 20 women received 5 mL of 0.9% saline injected into the same positions. Surgeons were blinded to the randomization.
A stopwatch was started at extubation, and the women were asked to rate their pain on a 10-point visual analogue scale exactly at 30 and 60 minutes.
The bupivacaine group had less pain at both 30 minutes (3.2 versus 5.7 points, P = .01) and 60 minutes (2.3 versus 5.9 points, P less than .001); 71% of women in the bupivacaine group had an average score of 4 or less, indicating adequate pain control, versus just 25% in the control arm (P = .003)
“This is something we should be considering” routinely for laparoscopic hysterectomy, an audience member said after hearing the presentation at a meeting sponsored by AAGL.
Another audience member was concerned about urinary retention, but there was no increase in the treatment arm, said lead investigator Steven Radtke, MD, a former ob.gyn. surgery fellow at the university, but now at Texas Tech University, El Paso.
There have been many prior attempts to reduce pain after laparoscopic hysterectomy, such as infiltrating port sites with local anesthetic, but the results have been marginal at best, and almost all of them have focused on the abdominal wall as the source of pain.
The investigators thought that pain was more related to perimetrium dissection, colpotomy, and other parts of the operation. There also have been good studies showing that agents injected into the cervix infuse throughout the area. The team decided to try bupivacaine because it’s inexpensive and has a good duration of action, about 8 hours.
There were no significant demographic or intraoperative differences between the groups. On average, women were in their mid-40s, with a body mass index of about 31 kg/m2. The operations took about 2 hours, and were for benign indications, such as fibroids. Oophorectomy was the only concomitant procedure allowed.
The investigators are interested in repeating their investigation with liposomal bupivacaine (Exparel), which has a duration of action past 24 hours. It’s much more expensive, but the strong trial results justify the cost, Dr. Radtke said.
There was no external funding, and Dr. Radtke didn’t have any disclosures.
SOURCE: Radtke S et al. 2018 AAGL Global Congress, Abstract 130.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: Postoperative pain can be significantly reduced by injecting the cervix with bupivacaine prior to laparoscopic hysterectomy.
Major finding: The bupivacaine group had less pain at both 30 minutes (3.2 versus 5.7 points, P = .01) and 60 minutes (2.3 versus 5.9 points, P less than .001).
Study details: In a randomized study, 21 women received bupivacaine anesthesia and 20 control women were injected with saline.
Disclosures: There was no external funding, and Dr. Radtke didn’t have any disclosures.
Source: Radtke S et al. 2018 AAGL Global Congress, Abstract 130.
Cold packs help reduce pain after laparoscopic hysterectomy
LAS VEGAS – Patients like cold packs for pain control after laparoscopic hysterectomy, according to a small trial from Cleveland Clinic Florida (Weston).
Cold packs have been shown to reduce pain in other types of surgery, so investigators at the clinic wanted to try them out for the procedure, said study lead Pamela Frazzini Padilla, MD, an ob.gyn. at the clinic.
Twenty-eight women were randomized to get packs right after surgery, and told to use them – before turning to oxycodone tabs – every 6 hours for 72 hours – and then as needed. Twenty-eight other women were randomized to the control group. Surgery was for benign indications, most often uterine bleeding secondary to fibroids. Besides the cold packs, there were no differences between the groups in analgesia protocols.
The differences in pain control, assessed on a 10-point scale over the phone, weren’t statistically significant, but they di move in the right direction. At 24 hours, women who were cold pack users reported a median pain score of 4, versus 4.5 among controls. At 72 hours, they reported a median score of 2, versus 2.5 in the control group.
At 2 weeks postoperatively, women who were cold pack users had used a mean of 4 oxycodone pills, versus 7 among the controls, which translated into a mean of 13 IV morphine equivalents versus 24 in favor of cold packs (P = .143).
While not significantly different, overall numbers of opioid tabs consumed and morphine equivalents “demonstrated lower use in the study group,” Dr. Frazzini Padilla noted at the meeting sponsored by AAGL.
Also, 89% of women said that cold packs helped reduce their pain, and 92% said they’d use them again after an operation. “In a day when everything is driven by patient satisfaction, patients’ perception of their recovery” is important. Because cold packs are cheap, harmless, and seemed to help with patient perceptions, “we do recommend that people use” them. “It’s just another added measure that we give” at Cleveland Clinic Florida, she said.
The study team also found that 86% of the women in the trial used 10 or fewer oxycodone tabs after surgery. Across the country, women are prescribed about 25 tabs after a laparoscopic hysterectomy; the study suggests it’s overkill, as an audience member noted, especially given the current climate.
The two arms of the study were well balanced. The mean age was 46 years, and mean body mass index 30.4 kg/m2.
There was no outside funding, and the investigators didn’t have any disclosures.
aotto@mdedge.com
SOURCE: Frazzini Padilla P et al. 2018 AAGL Global Congress, Abstract 21.
LAS VEGAS – Patients like cold packs for pain control after laparoscopic hysterectomy, according to a small trial from Cleveland Clinic Florida (Weston).
Cold packs have been shown to reduce pain in other types of surgery, so investigators at the clinic wanted to try them out for the procedure, said study lead Pamela Frazzini Padilla, MD, an ob.gyn. at the clinic.
Twenty-eight women were randomized to get packs right after surgery, and told to use them – before turning to oxycodone tabs – every 6 hours for 72 hours – and then as needed. Twenty-eight other women were randomized to the control group. Surgery was for benign indications, most often uterine bleeding secondary to fibroids. Besides the cold packs, there were no differences between the groups in analgesia protocols.
The differences in pain control, assessed on a 10-point scale over the phone, weren’t statistically significant, but they di move in the right direction. At 24 hours, women who were cold pack users reported a median pain score of 4, versus 4.5 among controls. At 72 hours, they reported a median score of 2, versus 2.5 in the control group.
At 2 weeks postoperatively, women who were cold pack users had used a mean of 4 oxycodone pills, versus 7 among the controls, which translated into a mean of 13 IV morphine equivalents versus 24 in favor of cold packs (P = .143).
While not significantly different, overall numbers of opioid tabs consumed and morphine equivalents “demonstrated lower use in the study group,” Dr. Frazzini Padilla noted at the meeting sponsored by AAGL.
Also, 89% of women said that cold packs helped reduce their pain, and 92% said they’d use them again after an operation. “In a day when everything is driven by patient satisfaction, patients’ perception of their recovery” is important. Because cold packs are cheap, harmless, and seemed to help with patient perceptions, “we do recommend that people use” them. “It’s just another added measure that we give” at Cleveland Clinic Florida, she said.
The study team also found that 86% of the women in the trial used 10 or fewer oxycodone tabs after surgery. Across the country, women are prescribed about 25 tabs after a laparoscopic hysterectomy; the study suggests it’s overkill, as an audience member noted, especially given the current climate.
The two arms of the study were well balanced. The mean age was 46 years, and mean body mass index 30.4 kg/m2.
There was no outside funding, and the investigators didn’t have any disclosures.
aotto@mdedge.com
SOURCE: Frazzini Padilla P et al. 2018 AAGL Global Congress, Abstract 21.
LAS VEGAS – Patients like cold packs for pain control after laparoscopic hysterectomy, according to a small trial from Cleveland Clinic Florida (Weston).
Cold packs have been shown to reduce pain in other types of surgery, so investigators at the clinic wanted to try them out for the procedure, said study lead Pamela Frazzini Padilla, MD, an ob.gyn. at the clinic.
Twenty-eight women were randomized to get packs right after surgery, and told to use them – before turning to oxycodone tabs – every 6 hours for 72 hours – and then as needed. Twenty-eight other women were randomized to the control group. Surgery was for benign indications, most often uterine bleeding secondary to fibroids. Besides the cold packs, there were no differences between the groups in analgesia protocols.
The differences in pain control, assessed on a 10-point scale over the phone, weren’t statistically significant, but they di move in the right direction. At 24 hours, women who were cold pack users reported a median pain score of 4, versus 4.5 among controls. At 72 hours, they reported a median score of 2, versus 2.5 in the control group.
At 2 weeks postoperatively, women who were cold pack users had used a mean of 4 oxycodone pills, versus 7 among the controls, which translated into a mean of 13 IV morphine equivalents versus 24 in favor of cold packs (P = .143).
While not significantly different, overall numbers of opioid tabs consumed and morphine equivalents “demonstrated lower use in the study group,” Dr. Frazzini Padilla noted at the meeting sponsored by AAGL.
Also, 89% of women said that cold packs helped reduce their pain, and 92% said they’d use them again after an operation. “In a day when everything is driven by patient satisfaction, patients’ perception of their recovery” is important. Because cold packs are cheap, harmless, and seemed to help with patient perceptions, “we do recommend that people use” them. “It’s just another added measure that we give” at Cleveland Clinic Florida, she said.
The study team also found that 86% of the women in the trial used 10 or fewer oxycodone tabs after surgery. Across the country, women are prescribed about 25 tabs after a laparoscopic hysterectomy; the study suggests it’s overkill, as an audience member noted, especially given the current climate.
The two arms of the study were well balanced. The mean age was 46 years, and mean body mass index 30.4 kg/m2.
There was no outside funding, and the investigators didn’t have any disclosures.
aotto@mdedge.com
SOURCE: Frazzini Padilla P et al. 2018 AAGL Global Congress, Abstract 21.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point:
Major finding: Cold pack women at 2 weeks used a mean of 4 oxycodone pills, versus 7 among the controls, which translated into a mean of 13 IV morphine equivalents versus 24 in favor of cold packs (P = 0.143)
Study details: A study of 28 women using cold packs and 28 controls.
Disclosures: There was no outside funding, and the investigators didn’t have any disclosures.
Source: Frazzini Padilla P et al. 2018 AAGL Global Congress, Abstract 21.
Clinical trial: Assessment of Ventilatory Management During General Anesthesia for Robotic Surgery
The trial will assess the incidence of postoperative pulmonary complications in patients who receive mechanical ventilation while under general anesthesia during robotic surgery to characterize current ventilation practices and evaluate any association between ventilator parameters and postoperative pulmonary complications.
Patients will be included if they are at least 18 years old and had their robotic surgical procedure done under general anesthesia. Exclusion criteria include being pregnant during surgery and having their procedure done outside an operating room.
The primary outcome measure is incidence of postoperative pulmonary complications within 5 days of the procedure or hospital discharge. Secondary outcomes include intraoperative mechanical ventilation practice, mechanical ventilation practice, and postoperative pulmonary complications within 5 days of the procedure or hospital discharge, intraoperative surgical positioning and ventilation, preoperative risk for postoperative pulmonary complications, and intraoperative mechanical ventilation practice and complications.
The estimated primary completion date is March 1, 2019, and the estimated study completion date is May 1, 2019. About 500 patients are estimated to be enrolled.
Find more information on the study at Clinicaltrials.gov.
The trial will assess the incidence of postoperative pulmonary complications in patients who receive mechanical ventilation while under general anesthesia during robotic surgery to characterize current ventilation practices and evaluate any association between ventilator parameters and postoperative pulmonary complications.
Patients will be included if they are at least 18 years old and had their robotic surgical procedure done under general anesthesia. Exclusion criteria include being pregnant during surgery and having their procedure done outside an operating room.
The primary outcome measure is incidence of postoperative pulmonary complications within 5 days of the procedure or hospital discharge. Secondary outcomes include intraoperative mechanical ventilation practice, mechanical ventilation practice, and postoperative pulmonary complications within 5 days of the procedure or hospital discharge, intraoperative surgical positioning and ventilation, preoperative risk for postoperative pulmonary complications, and intraoperative mechanical ventilation practice and complications.
The estimated primary completion date is March 1, 2019, and the estimated study completion date is May 1, 2019. About 500 patients are estimated to be enrolled.
Find more information on the study at Clinicaltrials.gov.
The trial will assess the incidence of postoperative pulmonary complications in patients who receive mechanical ventilation while under general anesthesia during robotic surgery to characterize current ventilation practices and evaluate any association between ventilator parameters and postoperative pulmonary complications.
Patients will be included if they are at least 18 years old and had their robotic surgical procedure done under general anesthesia. Exclusion criteria include being pregnant during surgery and having their procedure done outside an operating room.
The primary outcome measure is incidence of postoperative pulmonary complications within 5 days of the procedure or hospital discharge. Secondary outcomes include intraoperative mechanical ventilation practice, mechanical ventilation practice, and postoperative pulmonary complications within 5 days of the procedure or hospital discharge, intraoperative surgical positioning and ventilation, preoperative risk for postoperative pulmonary complications, and intraoperative mechanical ventilation practice and complications.
The estimated primary completion date is March 1, 2019, and the estimated study completion date is May 1, 2019. About 500 patients are estimated to be enrolled.
Find more information on the study at Clinicaltrials.gov.
Clinical trial: Magnetic Resonance Imaging in Obstructive Sleep Apnea
The Magnetic Resonance Imaging in Obstructive Sleep Apnea trial is an observational cohort study recruiting adults with obstructive sleep apnea undergoing surgery.
The trial will compare drug-induced sleep endoscopy and upper airway MRI in order to determine which is the better predictor of success in patients who cannot tolerate nonsurgical solutions. Upper airway MRI is a more complete evaluation during wakefulness and is cheaper than drug-induced sleep endoscopy, but no studies have thus far utilized MRI as a surgical evaluation tool.
Patients will be included if they are at least 21 years old, have moderate to severe obstructive sleep apnea, and have a body mass index less than 40 kg/m2. Exclusion criteria include prior surgery for obstructive sleep apnea; known neurologic, cardiac, pulmonary, renal, or hepatic disorders; psychiatric problems except for treated depression or mild anxiety; a coexisting sleep disorder; or another contraindication to drug-induced sleep endoscopy or MRI, such as propofol allergy.
The primary outcome measure is surgical results after 6 months, which will be measured using sleep studies. Secondary outcomes include sleep-related quality of life after 6 months and daytime sleepiness after 6 months.
The estimated primary completion date is June 2020, and the estimated study completion date is July 2020. About 40 patients are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
The Magnetic Resonance Imaging in Obstructive Sleep Apnea trial is an observational cohort study recruiting adults with obstructive sleep apnea undergoing surgery.
The trial will compare drug-induced sleep endoscopy and upper airway MRI in order to determine which is the better predictor of success in patients who cannot tolerate nonsurgical solutions. Upper airway MRI is a more complete evaluation during wakefulness and is cheaper than drug-induced sleep endoscopy, but no studies have thus far utilized MRI as a surgical evaluation tool.
Patients will be included if they are at least 21 years old, have moderate to severe obstructive sleep apnea, and have a body mass index less than 40 kg/m2. Exclusion criteria include prior surgery for obstructive sleep apnea; known neurologic, cardiac, pulmonary, renal, or hepatic disorders; psychiatric problems except for treated depression or mild anxiety; a coexisting sleep disorder; or another contraindication to drug-induced sleep endoscopy or MRI, such as propofol allergy.
The primary outcome measure is surgical results after 6 months, which will be measured using sleep studies. Secondary outcomes include sleep-related quality of life after 6 months and daytime sleepiness after 6 months.
The estimated primary completion date is June 2020, and the estimated study completion date is July 2020. About 40 patients are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
The Magnetic Resonance Imaging in Obstructive Sleep Apnea trial is an observational cohort study recruiting adults with obstructive sleep apnea undergoing surgery.
The trial will compare drug-induced sleep endoscopy and upper airway MRI in order to determine which is the better predictor of success in patients who cannot tolerate nonsurgical solutions. Upper airway MRI is a more complete evaluation during wakefulness and is cheaper than drug-induced sleep endoscopy, but no studies have thus far utilized MRI as a surgical evaluation tool.
Patients will be included if they are at least 21 years old, have moderate to severe obstructive sleep apnea, and have a body mass index less than 40 kg/m2. Exclusion criteria include prior surgery for obstructive sleep apnea; known neurologic, cardiac, pulmonary, renal, or hepatic disorders; psychiatric problems except for treated depression or mild anxiety; a coexisting sleep disorder; or another contraindication to drug-induced sleep endoscopy or MRI, such as propofol allergy.
The primary outcome measure is surgical results after 6 months, which will be measured using sleep studies. Secondary outcomes include sleep-related quality of life after 6 months and daytime sleepiness after 6 months.
The estimated primary completion date is June 2020, and the estimated study completion date is July 2020. About 40 patients are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
Laparoscope doubles as cystoscope in robotic hysterectomy
LAS VEGAS – to check for injuries, according to a review from St. Joseph’s University Medical Center, in Paterson, N.J.
Postoperative urinary tract infections (UTIs) are the big worry with using the same scope, but they really weren’t a problem at St. Joe’s; in a study of 45 women, there was just one UTI, confirmed by culture, at the 2-week postoperative visit, yielding a rate (2.2%) that is actually better the 5%-10% reported for stand-alone cystoscopy, said lead investigator and ob.gyn. resident Nikki Amirlatifi, MD.
“This is a safe alternative to traditional cystoscopy. We had no problems with visualization, and it doesn’t increase the rate of UTIs. Of course, it’s not only cost saving but time saving, as well,” she said.
The cases all were routine, however. For tougher ones, “where we need a more in-depth look at the bladder, we would [still] do cystoscopy,” she said at the meeting sponsored by AAGL.
There’s some debate about routine cystoscopy during laparoscopic hysterectomy, but Dr. Amirlatifi noted that it’s been reported to detect up to 89% of ureter injuries and up to 95% of bladder injuries. Using the same scope for both procedures makes it easier.
After the uterus was taken out, the bladder was backfilled with 180 mL of sterile water, then the Foley catheter was pulled. The previously used 5 mm laparoscope, which had been used for abdominal entry at 0 degrees, was introduced into the bladder. Efflux from both ureteral orifices was visualized, then the catheter reinserted until the end of surgery.
The women were an average of 44 years old, with an average body mass index of 32 kg/m2. Abnormal uterine bleeding, pelvic pain, and fibroids were the main indications for surgery. No ureteral or bladder injuries were detected.
Everyone was questioned about UTI symptoms and gave a clean-catch urine sample at the first postoperative visit. Cultures were performed on the seven women who reported symptoms or had white cells in their sample, and they were treated empirically with antibiotics. Only one culture grew out despite a high prevalence of UTI risk factors, including diabetes (13%), obesity (42%), and smoking (11%).
All the women had preoperative antibiotics and phenazopyridine. Most went home on the day of surgery.
There was no outside funding for the work, and the investigators didn’t have any relevant financial disclosures.
SOURCE: J Minim. Invasive Gynecol. 2018 Nov-Dec;25[7]:S46-47.
LAS VEGAS – to check for injuries, according to a review from St. Joseph’s University Medical Center, in Paterson, N.J.
Postoperative urinary tract infections (UTIs) are the big worry with using the same scope, but they really weren’t a problem at St. Joe’s; in a study of 45 women, there was just one UTI, confirmed by culture, at the 2-week postoperative visit, yielding a rate (2.2%) that is actually better the 5%-10% reported for stand-alone cystoscopy, said lead investigator and ob.gyn. resident Nikki Amirlatifi, MD.
“This is a safe alternative to traditional cystoscopy. We had no problems with visualization, and it doesn’t increase the rate of UTIs. Of course, it’s not only cost saving but time saving, as well,” she said.
The cases all were routine, however. For tougher ones, “where we need a more in-depth look at the bladder, we would [still] do cystoscopy,” she said at the meeting sponsored by AAGL.
There’s some debate about routine cystoscopy during laparoscopic hysterectomy, but Dr. Amirlatifi noted that it’s been reported to detect up to 89% of ureter injuries and up to 95% of bladder injuries. Using the same scope for both procedures makes it easier.
After the uterus was taken out, the bladder was backfilled with 180 mL of sterile water, then the Foley catheter was pulled. The previously used 5 mm laparoscope, which had been used for abdominal entry at 0 degrees, was introduced into the bladder. Efflux from both ureteral orifices was visualized, then the catheter reinserted until the end of surgery.
The women were an average of 44 years old, with an average body mass index of 32 kg/m2. Abnormal uterine bleeding, pelvic pain, and fibroids were the main indications for surgery. No ureteral or bladder injuries were detected.
Everyone was questioned about UTI symptoms and gave a clean-catch urine sample at the first postoperative visit. Cultures were performed on the seven women who reported symptoms or had white cells in their sample, and they were treated empirically with antibiotics. Only one culture grew out despite a high prevalence of UTI risk factors, including diabetes (13%), obesity (42%), and smoking (11%).
All the women had preoperative antibiotics and phenazopyridine. Most went home on the day of surgery.
There was no outside funding for the work, and the investigators didn’t have any relevant financial disclosures.
SOURCE: J Minim. Invasive Gynecol. 2018 Nov-Dec;25[7]:S46-47.
LAS VEGAS – to check for injuries, according to a review from St. Joseph’s University Medical Center, in Paterson, N.J.
Postoperative urinary tract infections (UTIs) are the big worry with using the same scope, but they really weren’t a problem at St. Joe’s; in a study of 45 women, there was just one UTI, confirmed by culture, at the 2-week postoperative visit, yielding a rate (2.2%) that is actually better the 5%-10% reported for stand-alone cystoscopy, said lead investigator and ob.gyn. resident Nikki Amirlatifi, MD.
“This is a safe alternative to traditional cystoscopy. We had no problems with visualization, and it doesn’t increase the rate of UTIs. Of course, it’s not only cost saving but time saving, as well,” she said.
The cases all were routine, however. For tougher ones, “where we need a more in-depth look at the bladder, we would [still] do cystoscopy,” she said at the meeting sponsored by AAGL.
There’s some debate about routine cystoscopy during laparoscopic hysterectomy, but Dr. Amirlatifi noted that it’s been reported to detect up to 89% of ureter injuries and up to 95% of bladder injuries. Using the same scope for both procedures makes it easier.
After the uterus was taken out, the bladder was backfilled with 180 mL of sterile water, then the Foley catheter was pulled. The previously used 5 mm laparoscope, which had been used for abdominal entry at 0 degrees, was introduced into the bladder. Efflux from both ureteral orifices was visualized, then the catheter reinserted until the end of surgery.
The women were an average of 44 years old, with an average body mass index of 32 kg/m2. Abnormal uterine bleeding, pelvic pain, and fibroids were the main indications for surgery. No ureteral or bladder injuries were detected.
Everyone was questioned about UTI symptoms and gave a clean-catch urine sample at the first postoperative visit. Cultures were performed on the seven women who reported symptoms or had white cells in their sample, and they were treated empirically with antibiotics. Only one culture grew out despite a high prevalence of UTI risk factors, including diabetes (13%), obesity (42%), and smoking (11%).
All the women had preoperative antibiotics and phenazopyridine. Most went home on the day of surgery.
There was no outside funding for the work, and the investigators didn’t have any relevant financial disclosures.
SOURCE: J Minim. Invasive Gynecol. 2018 Nov-Dec;25[7]:S46-47.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: It is just as safe to look inside the bladder with the laparoscope after robotic hysterectomy in routine cases as to use a separate cystoscopy setup.
Major finding: Only 2.2% of women had a UTI, and there were no ureteral or bladder injuries detected using the laparoscope.
Study details: This was a prospective study of 45 women who underwent robotic hysterectomy whose bladder was inspected with the laparoscope at the end of surgery.
Disclosures: There was no outside funding for the work, and the investigators didn’t have any relevant financial disclosures.
Open enrollment: Weekly volume down again
Plan selections at HealthCare.gov fell for the second week in a row as overall volume for open enrollment 2019 continues to lag behind last year, according to the Centers for Medicare & Medicaid Services.
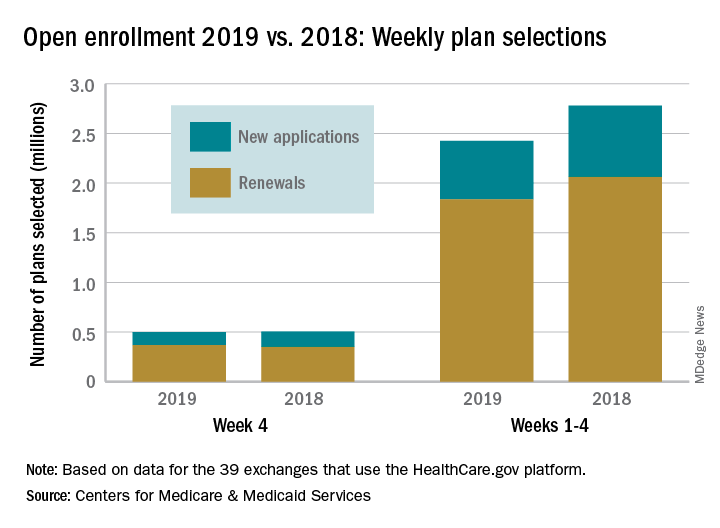
Just over 500,000 plans – 369,000 renewals and 131,000 new applications – were selected during week 4 (Nov. 18-24) for the 2019 coverage year in the 39 states that use the HealthCare.gov platform, which is down from 748,000 for week 3 and 805,000 for week 2. A similar pattern of decreases in weeks 3 and 4 was seen during last year’s open-enrollment period.
For the entire open enrollment so far this year, a little over 2.42 million plans have been selected, which is down by 12.8% from last year’s 4-week total of 2.78 million selections, the CMS data show.
Plan selections at HealthCare.gov fell for the second week in a row as overall volume for open enrollment 2019 continues to lag behind last year, according to the Centers for Medicare & Medicaid Services.

Just over 500,000 plans – 369,000 renewals and 131,000 new applications – were selected during week 4 (Nov. 18-24) for the 2019 coverage year in the 39 states that use the HealthCare.gov platform, which is down from 748,000 for week 3 and 805,000 for week 2. A similar pattern of decreases in weeks 3 and 4 was seen during last year’s open-enrollment period.
For the entire open enrollment so far this year, a little over 2.42 million plans have been selected, which is down by 12.8% from last year’s 4-week total of 2.78 million selections, the CMS data show.
Plan selections at HealthCare.gov fell for the second week in a row as overall volume for open enrollment 2019 continues to lag behind last year, according to the Centers for Medicare & Medicaid Services.

Just over 500,000 plans – 369,000 renewals and 131,000 new applications – were selected during week 4 (Nov. 18-24) for the 2019 coverage year in the 39 states that use the HealthCare.gov platform, which is down from 748,000 for week 3 and 805,000 for week 2. A similar pattern of decreases in weeks 3 and 4 was seen during last year’s open-enrollment period.
For the entire open enrollment so far this year, a little over 2.42 million plans have been selected, which is down by 12.8% from last year’s 4-week total of 2.78 million selections, the CMS data show.
Should toe amputation be delayed in diabetic patients with osteomyelitis?
Amputation: Resistance is not futile!
What’s in a toe you may ask? Why worry about saving it? Just amputate and move on ...
Not so! I implore you to resist the desire. We vascular surgeons are accustomed to cutting off toes, even feet and legs. But when it comes to diabetic feet please reconsider. Just because there is osteomyelitis, I argue that does not necessitate amputation.
We all agree that ischemic gangrene and black mummified digits are beyond salvage. That’s not what my concern is. My focus is nonhealing ulcers with underlying osteomyelitis. Whether ischemic in etiology or neuropathic (or both), give salvage a try.
Why is this so important? My opponent will try to convince you that it’s not. He’ll try to sell you on how well people walk after amputation and that functional outcomes are great. But think beyond that for a second.
Amputation changes the foot architecture and weight distribution. In a person with neuropathy, this only predisposes them to more ulcers. More ulcers will mean more infection, which will lead to more amputations. This finally culminates in a major amputation.
In one reported study,1 researchers followed more than 200,000 diabetics from 2010 until 2013. While the risk of amputation overall was relatively small (0.36% for major and 0.56% for minor amputations), prior minor amputation increased the risk of major amputation 10-fold and increased the risk of another minor (below-ankle) amputation 20-fold. Of those who had a major amputation, 57% died over the 3 years. This is not insignificant.
This does not also consider the morbidity and impact on lifestyle and quality of life for these patients. Many may not walk. Some will be relegated to nursing homes. Some will suffer from phantom limb pain. Many may never return to work. Even more will have difficulty with their daily lives, not to mention the psychological recovery also required.
The foot seems to be the only place where amputation as first-line therapy for osteomyelitis is accepted. We don’t do a hip disarticulation for ischial pressure sores with osteomyelitis. Calvarial osteomyelitis is also treated with antibiotics. I implore you: Don’t treat toes like vestigial organs.
Granted, there are subsets of patients who would benefit from amputations. A patient with painful Charcot foot may elect to have a below-knee amputation and move on with life. Another who has lost jobs or significant time due to recurrence of osteomyelitis may progress. A patient with severe sepsis and infection into a joint may need amputation.
But what other treatment options are there? I’m glad you inquired.
I primarily treat diabetic feet by treating the soft tissue envelope. Even if a patient presents with midfoot infection or necrotizing soft tissue infection, I treat it like a good old-fashioned abscess or necrotizing fasciitis:
1) Drain pus
2) Resect the dead stuff
3) Supportive care (antibiotics, fluids, aggressive wound care, etc.)
I try to leave the bones intact. When bone is exposed I take biopsies for culture and pathology. Any bone destroyed by the infection is focally debrided. I also take a specimen of the “bone margin” that I’m leaving behind and I send this to pathology looking for residual acute osteomyelitis. These steps are important as they dictate duration and choice of antibiotic therapy. This is in keeping with the consensus recommendations published in 2016.2
Even chronic wounds get a similar approach. If there is granulation, let it granulate and see if it will fill the wound. “Just because osteomyelitis is there, it doesn’t mean that for the toe we won’t care!”
There are exceptions of course. If the soft tissue is severely affected so the phalynx protrudes like something from the movie “Coco,” probably that should be amputated. Repeat offenders also may progress to amputation. But otherwise, hold off and give it a chance.
For the inpatient, aggressive irrigation of the wounds using the Veraflo system promotes granulation, even for short hospital stays of 1 week or less. Any ischemic component is worked up and addressed with percutaneous or open revascularization. We treat with prolonged antibiotics, and in questionable cases err on the side of giving long-term courses. These wounds need to be offloaded for tasks of daily living (going to the bathroom, making a sandwich, etc.) but otherwise we instruct patients to be effectively non–weight-bearing on that limb.
We also refer patients for hyperbaric therapy frequently. Now if you’re done groaning, I assure you this is not phony medicine. There is growing evidence to support not only improved rates of healing, but also significant cost savings and improved quality of life.3
In young patients or those with large defects, we also involve plastic and reconstructive surgery for secondary closure approaches (free flaps, adjacent tissue transfers, local autogenous or prosthetic grafting [Integra, Stravix, Dermacell, etc.] or other advanced techniques). This is particularly important in plantar wounds that will need to bear weight in the future, or in young patients for improved functional and cosmetic outcomes. For smaller wounds, we often use dermal/subdermal graft substitutes ourselves.
Even still, in nonambulatory or chronically debilitated and medically high-risk patients, maybe a different option is palliative wound care with or without antibiotics. A nonoperative approach to allow individuals to live the rest of their remaining days without undergoing a morbid and disfiguring amputation is not unreasonable. Many families are thankful for this option when given it. In the absence of refractory pain or overwhelming sepsis, we just let the wound do what it will do, understanding that someday the plan may change. This allows patients to continue to treat the wound without escalation to surgery or resorting to amputation.
In the end, just like we vascular surgeons tailor our “holistic” approach to the needs and desires of a single particular patient, we should approach wounds with a similar attitude. The presence of osteomyelitis in and of itself should not prompt one to bypass an entire algorithm, go straight to amputation, do not pass “Go” or “collect $200” (although the professional fee for a toe amputation is probably around $200). With a multidisciplinary and multimodal approach, and vested patients, salvage is possible in the majority of cases.
References
1. Diabetologia. 2018 Mar;61(3):626-35.
2. Diabet Foot Ankle. 2016 Jul 12. doi: 10.3402/dfa.v7.30079.
3. Int J Technol Assess Health Care. 2008 Spring;24(2):178-83.
Dr. Issam Koleilat is assistant professor and associate program director, Vascular Surgery Residency and Fellowship, Division of Vascular Surgery, Albert Einstein College of Medicine/Montefiore Medical Center, New York. He had no relevant disclosures.
Amputation: Often the best option
For many years there has been debate about the best management strategy for diabetic foot infection including osteomyelitis. The principles of appropriate antibiotics, surgical debridement, good wound care, and proper offloading will always remain. There are no randomized controlled trials of medical vs. surgical management of diabetic foot ulceration with osteomyelitis.
We now have a number of widely accepted ways to define wounds including Wagner and the SVS-adopted WIFI score. Historical papers are somewhat plagued by heterogeneity in the wounds included. This is even more apparent with any attempted meta-analyses. I think everyone would agree that the superficial toe wound with minimal cellulitis is best managed medically. The issue at hand is the profoundly neuropathic diabetic often with underlying anatomic abnormality and osteomyelitis. My esteemed colleague would suggest that we are too quick to pull a trigger and amputate a toe with underlying osteomyelitis.
I think the initial item for debate is the technique of diagnosis of osteomyelitis. We have multiple ways this is reported. Plain x-ray, bone scan, MRI, and “clinical osteomyelitis” are among the alternative ways osteomyelitis is diagnosed. The reliability of the last is the most variable because clinical osteomyelitis ranges from “probes close to bone” to exposed bone visible protruding from the wound bed. Given the variability of diagnostic techniques, the literature is an amalgam of clinical scenarios and difficult to navigate in a way to affect treatment decisions.
In addition, the medical treatment for osteomyelitis is highly variable. This commonly involves tunneled catheter insertion and 6 weeks to 3 months of IV antibiotics. In some institutions antibiotics are tailored to “wound culture.” Several of our infectious disease specialists prefer bone culture and pathology of bone demonstrating an acute destructive process. Obviously, this often requires surgical debridement to obtain a specimen. Antibiotic duration recommendations may vary from 1 week (if all infected bone is resected) to 90 days if a standalone antibiotic management is selected. Chronic osteomyelitis has a reinfection rate of up to 30%.1
Medical management is not without risk. These risks include recurring infection with resistant organisms, wound deterioration, gastrointestinal complications (Clostridium difficile), catheter-related complications, and acute kidney injury. A recent paper found over 30% of patients treated medically for osteomyelitis developed acute kidney injury. These patients had more frequent hospitalization, recurring ulceration, and infection.2 We have all experienced the patient with multiple hospitalizations and episodic AKI that culminates in ESRD requiring hemodialysis.
If the argument is with good follow-up these patients will ultimately experience preservation of the toe, I would take the stance that in our patient population of diabetics presenting with foot ulcer and osteomyelitis the average hemoglobin A1c is over 9. Although this is not only related to patient compliance, in many instances this is a large piece of the puzzle. It is hard to infer that suddenly with biopsy-proven osteomyelitis the patient will become compliant with medical management of the disease process. Certainly, in some circumstances, this is the case. There are a number of studies with a wide range of findings on HbA1c as it relates to predictive value of wound healing.
There are various studies comparing surgical to medical management for osteomyelitis. Limb salvage is contingent upon location (forefoot, midfoot, hindfoot), the extent of infection, and patient comorbidities. The conclusion of the majority of these studies is that a standalone antibiotic treatment algorithm results in greater limb loss. Patients with peripheral occlusive disease and preadmission antibiotic use have been shown to have decreased wound healing. Minor amputation has been shown to be protective from mortality, risk of major amputation, and unfavorable discharge in patients admitted with a diagnosis of osteomyelitis.3 The major limb amputation rate for antibiotics alone is 20%-30% according to two trials with duration of antibiotics of 3 months.4,5 The available randomized trials tend to exclude patients with severe infection (poorly defined), those with PAD, or those with severe comorbid conditions.
Cost of treatment is even more poorly delineated. Obviously, surgical treatment is not without cost to the health care system. Toe amputation especially when including the metatarsal head shifts pressure points and in the neuropathic patient may lead to recurrent ulceration. The average outpatient cost per patient per ulcer is often over $30,000. The goal of surgical treatment can be defined as trying to maintain the greatest degree of function with the least risk. Removing infected bone (i.e., minor amputation) limits exposure to prolonged antibiotic treatment and hopefully lessens recurring ulceration and hospitalization. This is only one piece of the puzzle, however. A multidisciplinary approach with endocrinology, infectious disease, and orthotics for offloading are keys to decrease future ulceration.
Although I do not advocate for widespread toe carnage as suggested by Dr. Koleilat, I do think liberal application of minor amputation to limit hospital stay, limit antibiotic duration and its inherent risk, and possibly affect readmission is often in the best interest of the patient and the system as a whole. Obviously, based on the variable reports in the literature there cannot be a single approach to these patients and the treatment must be individualized based on extent of infection, compliance of the patient, access to multidisciplinary care, and comorbid conditions.
References
1. World J Diabetes. 2017 Apr 15;8(4):135-42.
2. Diabetes Res Clin Pract. 2018 Jan;135:58-64.
3. Ann Surg. 2005;241(6):885-94.
4. Am J Med. 1987 Oct;83(4):653-60.
5. Am J Med.1989 Jun;86(6 Pt 2):801-8.
Dr. Mark P. Androes is division chief, vascular surgery, Greenville (S.C.) Health System. He had no relevant disclosures.
Amputation: Resistance is not futile!
What’s in a toe you may ask? Why worry about saving it? Just amputate and move on ...
Not so! I implore you to resist the desire. We vascular surgeons are accustomed to cutting off toes, even feet and legs. But when it comes to diabetic feet please reconsider. Just because there is osteomyelitis, I argue that does not necessitate amputation.
We all agree that ischemic gangrene and black mummified digits are beyond salvage. That’s not what my concern is. My focus is nonhealing ulcers with underlying osteomyelitis. Whether ischemic in etiology or neuropathic (or both), give salvage a try.
Why is this so important? My opponent will try to convince you that it’s not. He’ll try to sell you on how well people walk after amputation and that functional outcomes are great. But think beyond that for a second.
Amputation changes the foot architecture and weight distribution. In a person with neuropathy, this only predisposes them to more ulcers. More ulcers will mean more infection, which will lead to more amputations. This finally culminates in a major amputation.
In one reported study,1 researchers followed more than 200,000 diabetics from 2010 until 2013. While the risk of amputation overall was relatively small (0.36% for major and 0.56% for minor amputations), prior minor amputation increased the risk of major amputation 10-fold and increased the risk of another minor (below-ankle) amputation 20-fold. Of those who had a major amputation, 57% died over the 3 years. This is not insignificant.
This does not also consider the morbidity and impact on lifestyle and quality of life for these patients. Many may not walk. Some will be relegated to nursing homes. Some will suffer from phantom limb pain. Many may never return to work. Even more will have difficulty with their daily lives, not to mention the psychological recovery also required.
The foot seems to be the only place where amputation as first-line therapy for osteomyelitis is accepted. We don’t do a hip disarticulation for ischial pressure sores with osteomyelitis. Calvarial osteomyelitis is also treated with antibiotics. I implore you: Don’t treat toes like vestigial organs.
Granted, there are subsets of patients who would benefit from amputations. A patient with painful Charcot foot may elect to have a below-knee amputation and move on with life. Another who has lost jobs or significant time due to recurrence of osteomyelitis may progress. A patient with severe sepsis and infection into a joint may need amputation.
But what other treatment options are there? I’m glad you inquired.
I primarily treat diabetic feet by treating the soft tissue envelope. Even if a patient presents with midfoot infection or necrotizing soft tissue infection, I treat it like a good old-fashioned abscess or necrotizing fasciitis:
1) Drain pus
2) Resect the dead stuff
3) Supportive care (antibiotics, fluids, aggressive wound care, etc.)
I try to leave the bones intact. When bone is exposed I take biopsies for culture and pathology. Any bone destroyed by the infection is focally debrided. I also take a specimen of the “bone margin” that I’m leaving behind and I send this to pathology looking for residual acute osteomyelitis. These steps are important as they dictate duration and choice of antibiotic therapy. This is in keeping with the consensus recommendations published in 2016.2
Even chronic wounds get a similar approach. If there is granulation, let it granulate and see if it will fill the wound. “Just because osteomyelitis is there, it doesn’t mean that for the toe we won’t care!”
There are exceptions of course. If the soft tissue is severely affected so the phalynx protrudes like something from the movie “Coco,” probably that should be amputated. Repeat offenders also may progress to amputation. But otherwise, hold off and give it a chance.
For the inpatient, aggressive irrigation of the wounds using the Veraflo system promotes granulation, even for short hospital stays of 1 week or less. Any ischemic component is worked up and addressed with percutaneous or open revascularization. We treat with prolonged antibiotics, and in questionable cases err on the side of giving long-term courses. These wounds need to be offloaded for tasks of daily living (going to the bathroom, making a sandwich, etc.) but otherwise we instruct patients to be effectively non–weight-bearing on that limb.
We also refer patients for hyperbaric therapy frequently. Now if you’re done groaning, I assure you this is not phony medicine. There is growing evidence to support not only improved rates of healing, but also significant cost savings and improved quality of life.3
In young patients or those with large defects, we also involve plastic and reconstructive surgery for secondary closure approaches (free flaps, adjacent tissue transfers, local autogenous or prosthetic grafting [Integra, Stravix, Dermacell, etc.] or other advanced techniques). This is particularly important in plantar wounds that will need to bear weight in the future, or in young patients for improved functional and cosmetic outcomes. For smaller wounds, we often use dermal/subdermal graft substitutes ourselves.
Even still, in nonambulatory or chronically debilitated and medically high-risk patients, maybe a different option is palliative wound care with or without antibiotics. A nonoperative approach to allow individuals to live the rest of their remaining days without undergoing a morbid and disfiguring amputation is not unreasonable. Many families are thankful for this option when given it. In the absence of refractory pain or overwhelming sepsis, we just let the wound do what it will do, understanding that someday the plan may change. This allows patients to continue to treat the wound without escalation to surgery or resorting to amputation.
In the end, just like we vascular surgeons tailor our “holistic” approach to the needs and desires of a single particular patient, we should approach wounds with a similar attitude. The presence of osteomyelitis in and of itself should not prompt one to bypass an entire algorithm, go straight to amputation, do not pass “Go” or “collect $200” (although the professional fee for a toe amputation is probably around $200). With a multidisciplinary and multimodal approach, and vested patients, salvage is possible in the majority of cases.
References
1. Diabetologia. 2018 Mar;61(3):626-35.
2. Diabet Foot Ankle. 2016 Jul 12. doi: 10.3402/dfa.v7.30079.
3. Int J Technol Assess Health Care. 2008 Spring;24(2):178-83.
Dr. Issam Koleilat is assistant professor and associate program director, Vascular Surgery Residency and Fellowship, Division of Vascular Surgery, Albert Einstein College of Medicine/Montefiore Medical Center, New York. He had no relevant disclosures.
Amputation: Often the best option
For many years there has been debate about the best management strategy for diabetic foot infection including osteomyelitis. The principles of appropriate antibiotics, surgical debridement, good wound care, and proper offloading will always remain. There are no randomized controlled trials of medical vs. surgical management of diabetic foot ulceration with osteomyelitis.
We now have a number of widely accepted ways to define wounds including Wagner and the SVS-adopted WIFI score. Historical papers are somewhat plagued by heterogeneity in the wounds included. This is even more apparent with any attempted meta-analyses. I think everyone would agree that the superficial toe wound with minimal cellulitis is best managed medically. The issue at hand is the profoundly neuropathic diabetic often with underlying anatomic abnormality and osteomyelitis. My esteemed colleague would suggest that we are too quick to pull a trigger and amputate a toe with underlying osteomyelitis.
I think the initial item for debate is the technique of diagnosis of osteomyelitis. We have multiple ways this is reported. Plain x-ray, bone scan, MRI, and “clinical osteomyelitis” are among the alternative ways osteomyelitis is diagnosed. The reliability of the last is the most variable because clinical osteomyelitis ranges from “probes close to bone” to exposed bone visible protruding from the wound bed. Given the variability of diagnostic techniques, the literature is an amalgam of clinical scenarios and difficult to navigate in a way to affect treatment decisions.
In addition, the medical treatment for osteomyelitis is highly variable. This commonly involves tunneled catheter insertion and 6 weeks to 3 months of IV antibiotics. In some institutions antibiotics are tailored to “wound culture.” Several of our infectious disease specialists prefer bone culture and pathology of bone demonstrating an acute destructive process. Obviously, this often requires surgical debridement to obtain a specimen. Antibiotic duration recommendations may vary from 1 week (if all infected bone is resected) to 90 days if a standalone antibiotic management is selected. Chronic osteomyelitis has a reinfection rate of up to 30%.1
Medical management is not without risk. These risks include recurring infection with resistant organisms, wound deterioration, gastrointestinal complications (Clostridium difficile), catheter-related complications, and acute kidney injury. A recent paper found over 30% of patients treated medically for osteomyelitis developed acute kidney injury. These patients had more frequent hospitalization, recurring ulceration, and infection.2 We have all experienced the patient with multiple hospitalizations and episodic AKI that culminates in ESRD requiring hemodialysis.
If the argument is with good follow-up these patients will ultimately experience preservation of the toe, I would take the stance that in our patient population of diabetics presenting with foot ulcer and osteomyelitis the average hemoglobin A1c is over 9. Although this is not only related to patient compliance, in many instances this is a large piece of the puzzle. It is hard to infer that suddenly with biopsy-proven osteomyelitis the patient will become compliant with medical management of the disease process. Certainly, in some circumstances, this is the case. There are a number of studies with a wide range of findings on HbA1c as it relates to predictive value of wound healing.
There are various studies comparing surgical to medical management for osteomyelitis. Limb salvage is contingent upon location (forefoot, midfoot, hindfoot), the extent of infection, and patient comorbidities. The conclusion of the majority of these studies is that a standalone antibiotic treatment algorithm results in greater limb loss. Patients with peripheral occlusive disease and preadmission antibiotic use have been shown to have decreased wound healing. Minor amputation has been shown to be protective from mortality, risk of major amputation, and unfavorable discharge in patients admitted with a diagnosis of osteomyelitis.3 The major limb amputation rate for antibiotics alone is 20%-30% according to two trials with duration of antibiotics of 3 months.4,5 The available randomized trials tend to exclude patients with severe infection (poorly defined), those with PAD, or those with severe comorbid conditions.
Cost of treatment is even more poorly delineated. Obviously, surgical treatment is not without cost to the health care system. Toe amputation especially when including the metatarsal head shifts pressure points and in the neuropathic patient may lead to recurrent ulceration. The average outpatient cost per patient per ulcer is often over $30,000. The goal of surgical treatment can be defined as trying to maintain the greatest degree of function with the least risk. Removing infected bone (i.e., minor amputation) limits exposure to prolonged antibiotic treatment and hopefully lessens recurring ulceration and hospitalization. This is only one piece of the puzzle, however. A multidisciplinary approach with endocrinology, infectious disease, and orthotics for offloading are keys to decrease future ulceration.
Although I do not advocate for widespread toe carnage as suggested by Dr. Koleilat, I do think liberal application of minor amputation to limit hospital stay, limit antibiotic duration and its inherent risk, and possibly affect readmission is often in the best interest of the patient and the system as a whole. Obviously, based on the variable reports in the literature there cannot be a single approach to these patients and the treatment must be individualized based on extent of infection, compliance of the patient, access to multidisciplinary care, and comorbid conditions.
References
1. World J Diabetes. 2017 Apr 15;8(4):135-42.
2. Diabetes Res Clin Pract. 2018 Jan;135:58-64.
3. Ann Surg. 2005;241(6):885-94.
4. Am J Med. 1987 Oct;83(4):653-60.
5. Am J Med.1989 Jun;86(6 Pt 2):801-8.
Dr. Mark P. Androes is division chief, vascular surgery, Greenville (S.C.) Health System. He had no relevant disclosures.
Amputation: Resistance is not futile!
What’s in a toe you may ask? Why worry about saving it? Just amputate and move on ...
Not so! I implore you to resist the desire. We vascular surgeons are accustomed to cutting off toes, even feet and legs. But when it comes to diabetic feet please reconsider. Just because there is osteomyelitis, I argue that does not necessitate amputation.
We all agree that ischemic gangrene and black mummified digits are beyond salvage. That’s not what my concern is. My focus is nonhealing ulcers with underlying osteomyelitis. Whether ischemic in etiology or neuropathic (or both), give salvage a try.
Why is this so important? My opponent will try to convince you that it’s not. He’ll try to sell you on how well people walk after amputation and that functional outcomes are great. But think beyond that for a second.
Amputation changes the foot architecture and weight distribution. In a person with neuropathy, this only predisposes them to more ulcers. More ulcers will mean more infection, which will lead to more amputations. This finally culminates in a major amputation.
In one reported study,1 researchers followed more than 200,000 diabetics from 2010 until 2013. While the risk of amputation overall was relatively small (0.36% for major and 0.56% for minor amputations), prior minor amputation increased the risk of major amputation 10-fold and increased the risk of another minor (below-ankle) amputation 20-fold. Of those who had a major amputation, 57% died over the 3 years. This is not insignificant.
This does not also consider the morbidity and impact on lifestyle and quality of life for these patients. Many may not walk. Some will be relegated to nursing homes. Some will suffer from phantom limb pain. Many may never return to work. Even more will have difficulty with their daily lives, not to mention the psychological recovery also required.
The foot seems to be the only place where amputation as first-line therapy for osteomyelitis is accepted. We don’t do a hip disarticulation for ischial pressure sores with osteomyelitis. Calvarial osteomyelitis is also treated with antibiotics. I implore you: Don’t treat toes like vestigial organs.
Granted, there are subsets of patients who would benefit from amputations. A patient with painful Charcot foot may elect to have a below-knee amputation and move on with life. Another who has lost jobs or significant time due to recurrence of osteomyelitis may progress. A patient with severe sepsis and infection into a joint may need amputation.
But what other treatment options are there? I’m glad you inquired.
I primarily treat diabetic feet by treating the soft tissue envelope. Even if a patient presents with midfoot infection or necrotizing soft tissue infection, I treat it like a good old-fashioned abscess or necrotizing fasciitis:
1) Drain pus
2) Resect the dead stuff
3) Supportive care (antibiotics, fluids, aggressive wound care, etc.)
I try to leave the bones intact. When bone is exposed I take biopsies for culture and pathology. Any bone destroyed by the infection is focally debrided. I also take a specimen of the “bone margin” that I’m leaving behind and I send this to pathology looking for residual acute osteomyelitis. These steps are important as they dictate duration and choice of antibiotic therapy. This is in keeping with the consensus recommendations published in 2016.2
Even chronic wounds get a similar approach. If there is granulation, let it granulate and see if it will fill the wound. “Just because osteomyelitis is there, it doesn’t mean that for the toe we won’t care!”
There are exceptions of course. If the soft tissue is severely affected so the phalynx protrudes like something from the movie “Coco,” probably that should be amputated. Repeat offenders also may progress to amputation. But otherwise, hold off and give it a chance.
For the inpatient, aggressive irrigation of the wounds using the Veraflo system promotes granulation, even for short hospital stays of 1 week or less. Any ischemic component is worked up and addressed with percutaneous or open revascularization. We treat with prolonged antibiotics, and in questionable cases err on the side of giving long-term courses. These wounds need to be offloaded for tasks of daily living (going to the bathroom, making a sandwich, etc.) but otherwise we instruct patients to be effectively non–weight-bearing on that limb.
We also refer patients for hyperbaric therapy frequently. Now if you’re done groaning, I assure you this is not phony medicine. There is growing evidence to support not only improved rates of healing, but also significant cost savings and improved quality of life.3
In young patients or those with large defects, we also involve plastic and reconstructive surgery for secondary closure approaches (free flaps, adjacent tissue transfers, local autogenous or prosthetic grafting [Integra, Stravix, Dermacell, etc.] or other advanced techniques). This is particularly important in plantar wounds that will need to bear weight in the future, or in young patients for improved functional and cosmetic outcomes. For smaller wounds, we often use dermal/subdermal graft substitutes ourselves.
Even still, in nonambulatory or chronically debilitated and medically high-risk patients, maybe a different option is palliative wound care with or without antibiotics. A nonoperative approach to allow individuals to live the rest of their remaining days without undergoing a morbid and disfiguring amputation is not unreasonable. Many families are thankful for this option when given it. In the absence of refractory pain or overwhelming sepsis, we just let the wound do what it will do, understanding that someday the plan may change. This allows patients to continue to treat the wound without escalation to surgery or resorting to amputation.
In the end, just like we vascular surgeons tailor our “holistic” approach to the needs and desires of a single particular patient, we should approach wounds with a similar attitude. The presence of osteomyelitis in and of itself should not prompt one to bypass an entire algorithm, go straight to amputation, do not pass “Go” or “collect $200” (although the professional fee for a toe amputation is probably around $200). With a multidisciplinary and multimodal approach, and vested patients, salvage is possible in the majority of cases.
References
1. Diabetologia. 2018 Mar;61(3):626-35.
2. Diabet Foot Ankle. 2016 Jul 12. doi: 10.3402/dfa.v7.30079.
3. Int J Technol Assess Health Care. 2008 Spring;24(2):178-83.
Dr. Issam Koleilat is assistant professor and associate program director, Vascular Surgery Residency and Fellowship, Division of Vascular Surgery, Albert Einstein College of Medicine/Montefiore Medical Center, New York. He had no relevant disclosures.
Amputation: Often the best option
For many years there has been debate about the best management strategy for diabetic foot infection including osteomyelitis. The principles of appropriate antibiotics, surgical debridement, good wound care, and proper offloading will always remain. There are no randomized controlled trials of medical vs. surgical management of diabetic foot ulceration with osteomyelitis.
We now have a number of widely accepted ways to define wounds including Wagner and the SVS-adopted WIFI score. Historical papers are somewhat plagued by heterogeneity in the wounds included. This is even more apparent with any attempted meta-analyses. I think everyone would agree that the superficial toe wound with minimal cellulitis is best managed medically. The issue at hand is the profoundly neuropathic diabetic often with underlying anatomic abnormality and osteomyelitis. My esteemed colleague would suggest that we are too quick to pull a trigger and amputate a toe with underlying osteomyelitis.
I think the initial item for debate is the technique of diagnosis of osteomyelitis. We have multiple ways this is reported. Plain x-ray, bone scan, MRI, and “clinical osteomyelitis” are among the alternative ways osteomyelitis is diagnosed. The reliability of the last is the most variable because clinical osteomyelitis ranges from “probes close to bone” to exposed bone visible protruding from the wound bed. Given the variability of diagnostic techniques, the literature is an amalgam of clinical scenarios and difficult to navigate in a way to affect treatment decisions.
In addition, the medical treatment for osteomyelitis is highly variable. This commonly involves tunneled catheter insertion and 6 weeks to 3 months of IV antibiotics. In some institutions antibiotics are tailored to “wound culture.” Several of our infectious disease specialists prefer bone culture and pathology of bone demonstrating an acute destructive process. Obviously, this often requires surgical debridement to obtain a specimen. Antibiotic duration recommendations may vary from 1 week (if all infected bone is resected) to 90 days if a standalone antibiotic management is selected. Chronic osteomyelitis has a reinfection rate of up to 30%.1
Medical management is not without risk. These risks include recurring infection with resistant organisms, wound deterioration, gastrointestinal complications (Clostridium difficile), catheter-related complications, and acute kidney injury. A recent paper found over 30% of patients treated medically for osteomyelitis developed acute kidney injury. These patients had more frequent hospitalization, recurring ulceration, and infection.2 We have all experienced the patient with multiple hospitalizations and episodic AKI that culminates in ESRD requiring hemodialysis.
If the argument is with good follow-up these patients will ultimately experience preservation of the toe, I would take the stance that in our patient population of diabetics presenting with foot ulcer and osteomyelitis the average hemoglobin A1c is over 9. Although this is not only related to patient compliance, in many instances this is a large piece of the puzzle. It is hard to infer that suddenly with biopsy-proven osteomyelitis the patient will become compliant with medical management of the disease process. Certainly, in some circumstances, this is the case. There are a number of studies with a wide range of findings on HbA1c as it relates to predictive value of wound healing.
There are various studies comparing surgical to medical management for osteomyelitis. Limb salvage is contingent upon location (forefoot, midfoot, hindfoot), the extent of infection, and patient comorbidities. The conclusion of the majority of these studies is that a standalone antibiotic treatment algorithm results in greater limb loss. Patients with peripheral occlusive disease and preadmission antibiotic use have been shown to have decreased wound healing. Minor amputation has been shown to be protective from mortality, risk of major amputation, and unfavorable discharge in patients admitted with a diagnosis of osteomyelitis.3 The major limb amputation rate for antibiotics alone is 20%-30% according to two trials with duration of antibiotics of 3 months.4,5 The available randomized trials tend to exclude patients with severe infection (poorly defined), those with PAD, or those with severe comorbid conditions.
Cost of treatment is even more poorly delineated. Obviously, surgical treatment is not without cost to the health care system. Toe amputation especially when including the metatarsal head shifts pressure points and in the neuropathic patient may lead to recurrent ulceration. The average outpatient cost per patient per ulcer is often over $30,000. The goal of surgical treatment can be defined as trying to maintain the greatest degree of function with the least risk. Removing infected bone (i.e., minor amputation) limits exposure to prolonged antibiotic treatment and hopefully lessens recurring ulceration and hospitalization. This is only one piece of the puzzle, however. A multidisciplinary approach with endocrinology, infectious disease, and orthotics for offloading are keys to decrease future ulceration.
Although I do not advocate for widespread toe carnage as suggested by Dr. Koleilat, I do think liberal application of minor amputation to limit hospital stay, limit antibiotic duration and its inherent risk, and possibly affect readmission is often in the best interest of the patient and the system as a whole. Obviously, based on the variable reports in the literature there cannot be a single approach to these patients and the treatment must be individualized based on extent of infection, compliance of the patient, access to multidisciplinary care, and comorbid conditions.
References
1. World J Diabetes. 2017 Apr 15;8(4):135-42.
2. Diabetes Res Clin Pract. 2018 Jan;135:58-64.
3. Ann Surg. 2005;241(6):885-94.
4. Am J Med. 1987 Oct;83(4):653-60.
5. Am J Med.1989 Jun;86(6 Pt 2):801-8.
Dr. Mark P. Androes is division chief, vascular surgery, Greenville (S.C.) Health System. He had no relevant disclosures.
Under Trump, number of uninsured kids rose for first time this decade
After years of steady decline, the number of U.S. children without health insurance rose by 276,000 in 2017, according to a Georgetown University report released Nov. 22.
While not a big jump statistically – the share of uninsured kids rose to 5% in 2017 from 4.7% a year earlier – it is still striking. The uninsured rate typically remains stable or drops during times of economic growth. In September, the U.S. unemployment rate hit its lowest level since 1969.
“The nation is going backwards on insuring kids and it is likely to get worse,” said Joan Alker, coauthor of the study and executive director of Georgetown’s Center for Children and Families in Washington.
Ms. Alker and other child health advocates place the blame for this change on the Trump administration and the Republican-controlled Congress, saying their policies and actions cast a pall on enrollment.
The number of children without coverage rose to 3.9 million in 2017 from about 3.6 million a year earlier, according to Census data analyzed by Georgetown. The overall uninsured rate for people of all ages – which plummeted from 2013 to 2016 following the health law’s implementation – remained unchanged at 8.8% last year.
The share of children with employer-sponsored coverage rose modestly in 2017, but not by enough to make up for the drop in children enrolling in Medicaid or getting coverage from Obamacare insurance exchanges, Ms. Alker said.
While no states made any significant gains in lowering children’s uninsured rate, nine states experienced significant increases. The biggest occurred in South Dakota (up from 4.7% to 6.2%), Utah (up from 6% to 7.3%), and Texas (from 9.8% to 10.7%).
More than one in five uninsured children nationwide live in Texas – about 835,000 kids – by far the highest number of any state.
Florida had 325,000 uninsured children last year, as its uninsured rate for that age group rose 0.7 percentage points to 7.3%. California had 301,000 children without insurance, though its number remained virtually unchanged, relative to the previous year.
Other states with significant increases were Georgia, Massachusetts, Ohio, Tennessee, and South Carolina.
The uninsured rates for children increased at nearly triple the rates in states that did not expand Medicaid under the Affordable Care Act, according to the report. Studies have shown that children whose parents are insured are more likely to have coverage.
The uninsured rate among Hispanic children was 7.8%, compared with 4.9% among whites and 4.6% among blacks overall. (Hispanics can be of any race.)
Georgetown has been tracking these figures since 2008 when 7.6 million children – or about 10% of kids – lacked health coverage.
Because nearly all low-income children are eligible for Medicaid or the federal Children’s Health Insurance Program, known as CHIP, the challenge is making sure parents are aware of the programs, getting them enrolled, and keeping them signed up as long as they are eligible, Ms. Alker said.
Congress let the CHIP program funding lapse for several months in 2017, putting states in a position of having to warn consumers that they would soon have to freeze enrollment. Congress restored federal funding in early in 2018.
In addition, low-income families were bombarded by news reports last year of Congress threatening to repeal the health law that expanded coverage to millions. In the past 2 years, the Trump administration has slashed funding to Obamacare navigators to help people sign up for coverage.
Ms. Alker also pointed to the Trump administration’s September proposal, known as the “public charge” rule, which could make it harder for legal immigrants to get green cards if they have received certain kinds of public assistance – including Medicaid, food stamps, and housing subsidies. Green cards allow them to live and work permanently in the United States.
OLE Health, a large health provider based in Napa Valley, Calif., that serves many immigrants, said it has seen patients disenroll from Medicaid in the past year. CEO Alicia Hardy said many have dropped coverage over fears the help could jeopardize their immigration status. “They are afraid of being deported.”
All those events could have deterred families from getting their kids covered. “The welcome mat has been pulled back and as a result we see more uninsured children,” Ms. Alker said.
She said the easiest way to change the trend would be for more states to expand Medicaid under the health law. A total of 14 states have yet to do so. Though the expansion largely affects adults, as parents enroll, their children are likely to follow.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
After years of steady decline, the number of U.S. children without health insurance rose by 276,000 in 2017, according to a Georgetown University report released Nov. 22.
While not a big jump statistically – the share of uninsured kids rose to 5% in 2017 from 4.7% a year earlier – it is still striking. The uninsured rate typically remains stable or drops during times of economic growth. In September, the U.S. unemployment rate hit its lowest level since 1969.
“The nation is going backwards on insuring kids and it is likely to get worse,” said Joan Alker, coauthor of the study and executive director of Georgetown’s Center for Children and Families in Washington.
Ms. Alker and other child health advocates place the blame for this change on the Trump administration and the Republican-controlled Congress, saying their policies and actions cast a pall on enrollment.
The number of children without coverage rose to 3.9 million in 2017 from about 3.6 million a year earlier, according to Census data analyzed by Georgetown. The overall uninsured rate for people of all ages – which plummeted from 2013 to 2016 following the health law’s implementation – remained unchanged at 8.8% last year.
The share of children with employer-sponsored coverage rose modestly in 2017, but not by enough to make up for the drop in children enrolling in Medicaid or getting coverage from Obamacare insurance exchanges, Ms. Alker said.
While no states made any significant gains in lowering children’s uninsured rate, nine states experienced significant increases. The biggest occurred in South Dakota (up from 4.7% to 6.2%), Utah (up from 6% to 7.3%), and Texas (from 9.8% to 10.7%).
More than one in five uninsured children nationwide live in Texas – about 835,000 kids – by far the highest number of any state.
Florida had 325,000 uninsured children last year, as its uninsured rate for that age group rose 0.7 percentage points to 7.3%. California had 301,000 children without insurance, though its number remained virtually unchanged, relative to the previous year.
Other states with significant increases were Georgia, Massachusetts, Ohio, Tennessee, and South Carolina.
The uninsured rates for children increased at nearly triple the rates in states that did not expand Medicaid under the Affordable Care Act, according to the report. Studies have shown that children whose parents are insured are more likely to have coverage.
The uninsured rate among Hispanic children was 7.8%, compared with 4.9% among whites and 4.6% among blacks overall. (Hispanics can be of any race.)
Georgetown has been tracking these figures since 2008 when 7.6 million children – or about 10% of kids – lacked health coverage.
Because nearly all low-income children are eligible for Medicaid or the federal Children’s Health Insurance Program, known as CHIP, the challenge is making sure parents are aware of the programs, getting them enrolled, and keeping them signed up as long as they are eligible, Ms. Alker said.
Congress let the CHIP program funding lapse for several months in 2017, putting states in a position of having to warn consumers that they would soon have to freeze enrollment. Congress restored federal funding in early in 2018.
In addition, low-income families were bombarded by news reports last year of Congress threatening to repeal the health law that expanded coverage to millions. In the past 2 years, the Trump administration has slashed funding to Obamacare navigators to help people sign up for coverage.
Ms. Alker also pointed to the Trump administration’s September proposal, known as the “public charge” rule, which could make it harder for legal immigrants to get green cards if they have received certain kinds of public assistance – including Medicaid, food stamps, and housing subsidies. Green cards allow them to live and work permanently in the United States.
OLE Health, a large health provider based in Napa Valley, Calif., that serves many immigrants, said it has seen patients disenroll from Medicaid in the past year. CEO Alicia Hardy said many have dropped coverage over fears the help could jeopardize their immigration status. “They are afraid of being deported.”
All those events could have deterred families from getting their kids covered. “The welcome mat has been pulled back and as a result we see more uninsured children,” Ms. Alker said.
She said the easiest way to change the trend would be for more states to expand Medicaid under the health law. A total of 14 states have yet to do so. Though the expansion largely affects adults, as parents enroll, their children are likely to follow.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
After years of steady decline, the number of U.S. children without health insurance rose by 276,000 in 2017, according to a Georgetown University report released Nov. 22.
While not a big jump statistically – the share of uninsured kids rose to 5% in 2017 from 4.7% a year earlier – it is still striking. The uninsured rate typically remains stable or drops during times of economic growth. In September, the U.S. unemployment rate hit its lowest level since 1969.
“The nation is going backwards on insuring kids and it is likely to get worse,” said Joan Alker, coauthor of the study and executive director of Georgetown’s Center for Children and Families in Washington.
Ms. Alker and other child health advocates place the blame for this change on the Trump administration and the Republican-controlled Congress, saying their policies and actions cast a pall on enrollment.
The number of children without coverage rose to 3.9 million in 2017 from about 3.6 million a year earlier, according to Census data analyzed by Georgetown. The overall uninsured rate for people of all ages – which plummeted from 2013 to 2016 following the health law’s implementation – remained unchanged at 8.8% last year.
The share of children with employer-sponsored coverage rose modestly in 2017, but not by enough to make up for the drop in children enrolling in Medicaid or getting coverage from Obamacare insurance exchanges, Ms. Alker said.
While no states made any significant gains in lowering children’s uninsured rate, nine states experienced significant increases. The biggest occurred in South Dakota (up from 4.7% to 6.2%), Utah (up from 6% to 7.3%), and Texas (from 9.8% to 10.7%).
More than one in five uninsured children nationwide live in Texas – about 835,000 kids – by far the highest number of any state.
Florida had 325,000 uninsured children last year, as its uninsured rate for that age group rose 0.7 percentage points to 7.3%. California had 301,000 children without insurance, though its number remained virtually unchanged, relative to the previous year.
Other states with significant increases were Georgia, Massachusetts, Ohio, Tennessee, and South Carolina.
The uninsured rates for children increased at nearly triple the rates in states that did not expand Medicaid under the Affordable Care Act, according to the report. Studies have shown that children whose parents are insured are more likely to have coverage.
The uninsured rate among Hispanic children was 7.8%, compared with 4.9% among whites and 4.6% among blacks overall. (Hispanics can be of any race.)
Georgetown has been tracking these figures since 2008 when 7.6 million children – or about 10% of kids – lacked health coverage.
Because nearly all low-income children are eligible for Medicaid or the federal Children’s Health Insurance Program, known as CHIP, the challenge is making sure parents are aware of the programs, getting them enrolled, and keeping them signed up as long as they are eligible, Ms. Alker said.
Congress let the CHIP program funding lapse for several months in 2017, putting states in a position of having to warn consumers that they would soon have to freeze enrollment. Congress restored federal funding in early in 2018.
In addition, low-income families were bombarded by news reports last year of Congress threatening to repeal the health law that expanded coverage to millions. In the past 2 years, the Trump administration has slashed funding to Obamacare navigators to help people sign up for coverage.
Ms. Alker also pointed to the Trump administration’s September proposal, known as the “public charge” rule, which could make it harder for legal immigrants to get green cards if they have received certain kinds of public assistance – including Medicaid, food stamps, and housing subsidies. Green cards allow them to live and work permanently in the United States.
OLE Health, a large health provider based in Napa Valley, Calif., that serves many immigrants, said it has seen patients disenroll from Medicaid in the past year. CEO Alicia Hardy said many have dropped coverage over fears the help could jeopardize their immigration status. “They are afraid of being deported.”
All those events could have deterred families from getting their kids covered. “The welcome mat has been pulled back and as a result we see more uninsured children,” Ms. Alker said.
She said the easiest way to change the trend would be for more states to expand Medicaid under the health law. A total of 14 states have yet to do so. Though the expansion largely affects adults, as parents enroll, their children are likely to follow.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Democrats taking key leadership jobs have pocketed millions from Pharma
Three of the lawmakers who will lead the House next year as Congress focuses on skyrocketing drug costs are among the biggest recipients of campaign contributions from the pharmaceutical industry, a new KHN analysis shows.
On Nov. 28, House Democrats selected Rep. Steny Hoyer of Maryland to serve as the next majority leader and Rep. James Clyburn of South Carolina as majority whip, making them the No. 2 and No. 3 most powerful Democrats as their party regains control of the House in January.
Both lawmakers have received more than $1 million from pharmaceutical company political action committees (PACs) in the past decade. Just four members of Congress hold that distinction, including Rep. Kevin McCarthy of California, whom Republicans chose as the next House minority leader earlier this month.
Adding Rep. Nancy Pelosi, the California Democrat expected to be the next speaker, the three-person House Democratic leadership team has collected more than $2.3 million total in campaign contributions from drugmakers since the 2007-2008 election cycle, according to KHN’s database.
High drug prices surfaced as a major campaign issue in 2018. With almost half of Americans saying they were worried about prescription drug costs last summer, many Democrats told voters they’d tackle the issue in the next Congress. But the large amount of money going to key Democrats, and Republicans, raises questions about whether Congress will take on the pharmaceutical industry.
In the past decade, members of Congress from both parties have received about $81 million from 68 pharma PACs run by employees of companies that make drugs and industry trade groups.
Brendan Fischer, who directs federal reform programs at the nonpartisan Campaign Legal Center, said drugmakers, like other wealthy industries, “shower money” on congressional leaders who are mulling legislation that could affect the pharmaceutical industry.
“Both Democrats and Republicans have discussed taking action on prescription drug prices, and drug companies likely expect that big contributions will help them maintain access to, and influence over, powerful lawmakers,” he said.
Mr. McCarthy, who has close ties to President Donald Trump, has received more than $1.08 million from drugmaker PACs since 2007. According to the latest data, which runs through September, he received about $250,000 this election cycle.
The fourth lawmaker to top $1 million is Sen. Richard Burr, a North Carolina Republican who serves on both the Senate Committee on Health, Education, Labor and Pensions and the Senate Committee on Finance. North Carolina is also home to a number of research universities and drugmakers’ headquarters.
While campaign contributions may seem tantalizing as a metric for influence, industries are not necessarily buying votes with their cash. More likely, they are buying access – a sizable donation from a drugmaker’s PAC may increase the chances its lobbyists get a meeting with an influential lawmaker, for example.
Mr. Clyburn, who like Mr. Hoyer has served as a top Democratic leader since 2007, has received more from drugmaker PACs over the past decade than any other member of Congress – more than $1.09 million. During the 2018 election cycle, he received at least $170,000, despite trouncing his Republican opponent in his safely Democratic district.
A party leader and the highest-ranking African-American in Congress, Mr. Clyburn has had ties to the pharmaceutical industry over the years. In 2013, he was a featured speaker at a conference hosted by PhRMA, the industry’s leading trade group. The conference was held at the James E. Clyburn Research Center at the Medical University of South Carolina, a hub for biopharmaceutical research.
This fall, Mr. Hoyer topped the million-dollar mark in drugmaker PAC contributions over the past decade, collecting more than $1.02 million since 2007 and more than $128,000 this election cycle.
“Mr. Hoyer’s positions on legislation are based on what is in the best interest of his constituents and the American people, and he has made it clear the new Congress will tackle rising health care and prescription drug costs,” said Mariel Saez, a Hoyer spokeswoman.
The offices of Mr. Clyburn, Mr. McCarthy, and Ms. Pelosi did not respond to requests for comment.
Ms. Pelosi, in contrast to her deputies, has received nearly $193,000 total from drugmaker PACs the past decade. In the month before the midterm elections, she intensified her calls for action to control drug prices, saying on Election Day that she believed Democrats could find “common ground” with Trump on addressing the problem.
Senior committee members also tend to draw huge sums from the industries they oversee. Rep. Frank Pallone of New Jersey, the Democrat who is expected to chair the House Committee on Energy and Commerce, received nearly $169,000 this election cycle from drugmaker PACs, according to KHN’s database. Since 2007, he has collected more than $840,000.
Similarly, Rep. Greg Walden, the Oregon Republican who is finishing his term as chair of the committee, received $302,300, the most of any member this election cycle in contributions from drugmaker PACs.
By contrast, Rep. Elijah Cummings – the Maryland Democrat who is expected to head the House Committee on Oversight and Government Reform – has attracted minimal drugmaker cash, receiving just $18,500 since the 2007-2008 election cycle. He has made it clear that he intends to target pharmaceutical companies next year as he investigates climbing drug costs.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Three of the lawmakers who will lead the House next year as Congress focuses on skyrocketing drug costs are among the biggest recipients of campaign contributions from the pharmaceutical industry, a new KHN analysis shows.
On Nov. 28, House Democrats selected Rep. Steny Hoyer of Maryland to serve as the next majority leader and Rep. James Clyburn of South Carolina as majority whip, making them the No. 2 and No. 3 most powerful Democrats as their party regains control of the House in January.
Both lawmakers have received more than $1 million from pharmaceutical company political action committees (PACs) in the past decade. Just four members of Congress hold that distinction, including Rep. Kevin McCarthy of California, whom Republicans chose as the next House minority leader earlier this month.
Adding Rep. Nancy Pelosi, the California Democrat expected to be the next speaker, the three-person House Democratic leadership team has collected more than $2.3 million total in campaign contributions from drugmakers since the 2007-2008 election cycle, according to KHN’s database.
High drug prices surfaced as a major campaign issue in 2018. With almost half of Americans saying they were worried about prescription drug costs last summer, many Democrats told voters they’d tackle the issue in the next Congress. But the large amount of money going to key Democrats, and Republicans, raises questions about whether Congress will take on the pharmaceutical industry.
In the past decade, members of Congress from both parties have received about $81 million from 68 pharma PACs run by employees of companies that make drugs and industry trade groups.
Brendan Fischer, who directs federal reform programs at the nonpartisan Campaign Legal Center, said drugmakers, like other wealthy industries, “shower money” on congressional leaders who are mulling legislation that could affect the pharmaceutical industry.
“Both Democrats and Republicans have discussed taking action on prescription drug prices, and drug companies likely expect that big contributions will help them maintain access to, and influence over, powerful lawmakers,” he said.
Mr. McCarthy, who has close ties to President Donald Trump, has received more than $1.08 million from drugmaker PACs since 2007. According to the latest data, which runs through September, he received about $250,000 this election cycle.
The fourth lawmaker to top $1 million is Sen. Richard Burr, a North Carolina Republican who serves on both the Senate Committee on Health, Education, Labor and Pensions and the Senate Committee on Finance. North Carolina is also home to a number of research universities and drugmakers’ headquarters.
While campaign contributions may seem tantalizing as a metric for influence, industries are not necessarily buying votes with their cash. More likely, they are buying access – a sizable donation from a drugmaker’s PAC may increase the chances its lobbyists get a meeting with an influential lawmaker, for example.
Mr. Clyburn, who like Mr. Hoyer has served as a top Democratic leader since 2007, has received more from drugmaker PACs over the past decade than any other member of Congress – more than $1.09 million. During the 2018 election cycle, he received at least $170,000, despite trouncing his Republican opponent in his safely Democratic district.
A party leader and the highest-ranking African-American in Congress, Mr. Clyburn has had ties to the pharmaceutical industry over the years. In 2013, he was a featured speaker at a conference hosted by PhRMA, the industry’s leading trade group. The conference was held at the James E. Clyburn Research Center at the Medical University of South Carolina, a hub for biopharmaceutical research.
This fall, Mr. Hoyer topped the million-dollar mark in drugmaker PAC contributions over the past decade, collecting more than $1.02 million since 2007 and more than $128,000 this election cycle.
“Mr. Hoyer’s positions on legislation are based on what is in the best interest of his constituents and the American people, and he has made it clear the new Congress will tackle rising health care and prescription drug costs,” said Mariel Saez, a Hoyer spokeswoman.
The offices of Mr. Clyburn, Mr. McCarthy, and Ms. Pelosi did not respond to requests for comment.
Ms. Pelosi, in contrast to her deputies, has received nearly $193,000 total from drugmaker PACs the past decade. In the month before the midterm elections, she intensified her calls for action to control drug prices, saying on Election Day that she believed Democrats could find “common ground” with Trump on addressing the problem.
Senior committee members also tend to draw huge sums from the industries they oversee. Rep. Frank Pallone of New Jersey, the Democrat who is expected to chair the House Committee on Energy and Commerce, received nearly $169,000 this election cycle from drugmaker PACs, according to KHN’s database. Since 2007, he has collected more than $840,000.
Similarly, Rep. Greg Walden, the Oregon Republican who is finishing his term as chair of the committee, received $302,300, the most of any member this election cycle in contributions from drugmaker PACs.
By contrast, Rep. Elijah Cummings – the Maryland Democrat who is expected to head the House Committee on Oversight and Government Reform – has attracted minimal drugmaker cash, receiving just $18,500 since the 2007-2008 election cycle. He has made it clear that he intends to target pharmaceutical companies next year as he investigates climbing drug costs.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Three of the lawmakers who will lead the House next year as Congress focuses on skyrocketing drug costs are among the biggest recipients of campaign contributions from the pharmaceutical industry, a new KHN analysis shows.
On Nov. 28, House Democrats selected Rep. Steny Hoyer of Maryland to serve as the next majority leader and Rep. James Clyburn of South Carolina as majority whip, making them the No. 2 and No. 3 most powerful Democrats as their party regains control of the House in January.
Both lawmakers have received more than $1 million from pharmaceutical company political action committees (PACs) in the past decade. Just four members of Congress hold that distinction, including Rep. Kevin McCarthy of California, whom Republicans chose as the next House minority leader earlier this month.
Adding Rep. Nancy Pelosi, the California Democrat expected to be the next speaker, the three-person House Democratic leadership team has collected more than $2.3 million total in campaign contributions from drugmakers since the 2007-2008 election cycle, according to KHN’s database.
High drug prices surfaced as a major campaign issue in 2018. With almost half of Americans saying they were worried about prescription drug costs last summer, many Democrats told voters they’d tackle the issue in the next Congress. But the large amount of money going to key Democrats, and Republicans, raises questions about whether Congress will take on the pharmaceutical industry.
In the past decade, members of Congress from both parties have received about $81 million from 68 pharma PACs run by employees of companies that make drugs and industry trade groups.
Brendan Fischer, who directs federal reform programs at the nonpartisan Campaign Legal Center, said drugmakers, like other wealthy industries, “shower money” on congressional leaders who are mulling legislation that could affect the pharmaceutical industry.
“Both Democrats and Republicans have discussed taking action on prescription drug prices, and drug companies likely expect that big contributions will help them maintain access to, and influence over, powerful lawmakers,” he said.
Mr. McCarthy, who has close ties to President Donald Trump, has received more than $1.08 million from drugmaker PACs since 2007. According to the latest data, which runs through September, he received about $250,000 this election cycle.
The fourth lawmaker to top $1 million is Sen. Richard Burr, a North Carolina Republican who serves on both the Senate Committee on Health, Education, Labor and Pensions and the Senate Committee on Finance. North Carolina is also home to a number of research universities and drugmakers’ headquarters.
While campaign contributions may seem tantalizing as a metric for influence, industries are not necessarily buying votes with their cash. More likely, they are buying access – a sizable donation from a drugmaker’s PAC may increase the chances its lobbyists get a meeting with an influential lawmaker, for example.
Mr. Clyburn, who like Mr. Hoyer has served as a top Democratic leader since 2007, has received more from drugmaker PACs over the past decade than any other member of Congress – more than $1.09 million. During the 2018 election cycle, he received at least $170,000, despite trouncing his Republican opponent in his safely Democratic district.
A party leader and the highest-ranking African-American in Congress, Mr. Clyburn has had ties to the pharmaceutical industry over the years. In 2013, he was a featured speaker at a conference hosted by PhRMA, the industry’s leading trade group. The conference was held at the James E. Clyburn Research Center at the Medical University of South Carolina, a hub for biopharmaceutical research.
This fall, Mr. Hoyer topped the million-dollar mark in drugmaker PAC contributions over the past decade, collecting more than $1.02 million since 2007 and more than $128,000 this election cycle.
“Mr. Hoyer’s positions on legislation are based on what is in the best interest of his constituents and the American people, and he has made it clear the new Congress will tackle rising health care and prescription drug costs,” said Mariel Saez, a Hoyer spokeswoman.
The offices of Mr. Clyburn, Mr. McCarthy, and Ms. Pelosi did not respond to requests for comment.
Ms. Pelosi, in contrast to her deputies, has received nearly $193,000 total from drugmaker PACs the past decade. In the month before the midterm elections, she intensified her calls for action to control drug prices, saying on Election Day that she believed Democrats could find “common ground” with Trump on addressing the problem.
Senior committee members also tend to draw huge sums from the industries they oversee. Rep. Frank Pallone of New Jersey, the Democrat who is expected to chair the House Committee on Energy and Commerce, received nearly $169,000 this election cycle from drugmaker PACs, according to KHN’s database. Since 2007, he has collected more than $840,000.
Similarly, Rep. Greg Walden, the Oregon Republican who is finishing his term as chair of the committee, received $302,300, the most of any member this election cycle in contributions from drugmaker PACs.
By contrast, Rep. Elijah Cummings – the Maryland Democrat who is expected to head the House Committee on Oversight and Government Reform – has attracted minimal drugmaker cash, receiving just $18,500 since the 2007-2008 election cycle. He has made it clear that he intends to target pharmaceutical companies next year as he investigates climbing drug costs.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.