User login
Can a novel, rapid-acting oral treatment effectively manage PPD?
Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp.20220785.
EXPERT COMMENTARY
Postpartum depression affects approximately 17.2% of patients in the peripartum period.1 Typical pharmacologic treatment of PPD includes selective serotonin reuptake inhibitors (SSRIs), which may take up to 12 weeks to take effect. Postpartum depression is thought to be secondary to maladaptation to hormonal fluctuations in the peripartum period, including allopregnanolone, a positive allosteric modulator of GABAA (γ-aminobutyric acid type A)receptors and a metabolite of progesterone, levels of which increase in pregnancy and abruptly decrease following delivery.1 In 2019, the GABAA receptor modulator brexanalone was approved by the US Food and Drug Administration (FDA) to treat PPD through continuous intravenous infusion over 60 hours in the hospital setting.
Zuranolone, an allosteric modulator of GABAA receptors, also has been studied as an investigational medication for rapid treatment of PPD. Prior studies demonstrated the efficacy of oral zuranolone 30 mg daily for the treatment of PPD2 and 50 mg for the treatment of major depression in nonpregnant patients.3 Deligiannidis and colleagues conducted a trial to investigate the 50-mg dose of zuranolone for the treatment of PPD. (Notably, in August 2023, the FDA approved oral zuranolone once daily for 14 days for the treatment of PPD.) Following the FDA approval, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory recommending consideration of zuranolone for PPD that takes into account balancing the benefits and risks, including known sedative effects, potential need for decreasing the dose due to adverse effects, lack of safety data in lactation, and unknown long-term efficacy.4
Details of the study
This randomized, double-blind, placebo-controlled study included 196 patients with an episode of major depression, characterized as a baseline score of 26 or greater on the Hamilton Depression Rating Scale (HAM-D) beginning in the third trimester or within the first 4 weeks postpartum. Patients were randomly assigned in a 1:1 ratio to receive zuranolone 50 mg daily or placebo, with stratification by stable concurrent antidepressant use. Treatment duration was for 14 days, with follow-up through day 45.
The study’s primary outcome was a change in the baseline HAM-D score at day 15. Changes in HAM-D score also were recorded at days 3, 28, and 45.
The 2 study groups were well balanced by demographic and baseline characteristics. In both groups, the majority of patients experienced the onset of their major depressive episodes within the first 4 weeks postpartum. Completion rates of the 14-day treatment course and 45-day follow-up were high and similar in both groups; 170 patients completed the study. The rate of concurrent psychiatric medications taken, most of which were SSRIs, was similar between the 2 groups at approximately 15% of patients.
Results. A statistically significant improvement in the primary outcome (the change in HAM-D score) at day 15 occurred in patients who received zuranolone versus placebo (P = .001). Additionally, there were statistically significant improvements in the secondary outcomes HAM-D scores at days 3, 28, and 45. Initial response, as measured by changes in HAM-D scores, occurred at a median duration of 9 days in the zuranolone group and 43 days in the placebo group. More patients in the zuranolone group achieved a reduction in HAM-D score at 15 days (57.0% vs 38.9%; P = .02). Zuranolone was associated with a higher rate of HAM-D remission at day 45 (44.0% vs 29.4%; P = .02).
With regard to safety, 16.3% of patients (17) in the zuranolone group (vs 1% in the placebo group) experienced an adverse event, most commonly somnolence, dizziness, and sedation, which led to a dose reduction. However, 15 of these 17 patients still completed the study, and there were no serious adverse events.
Study strengths and limitations
This study’s strengths include the double-blinded design that was continued throughout the duration of the follow-up. Additionally, the study population was heterogeneous andreflective of patients from diverse racial and ethnic backgrounds. Lastly, only minor and moderate adverse events were reported and, despite this, nearly all patients who experienced adverse events completed the study.
Limitations of the study include the lack of generalizability, as patients with bipolar disorder and mild or moderate PPD were excluded. Additionally, the majority of patients had depressive episodes within the first 4 weeks postpartum, thereby excluding patients with depressive episodes at other time points in the peripartum period. Further, as breastfeeding was prohibited, safety in lactating patients using zuranolone is unknown. Lastly, the study follow-up period was 45 days; therefore, the long-term efficacy of zuranolone treatment is unclear. ●
Zuranolone, a GABAA allosteric modulator, shows promise as an alternative to existing pharmacologic treatments for severe PPD that is orally administered and rapidly acting. While it is reasonable to consider its use in the specific patient population that benefited in this study, further studies are needed to determine its efficacy in other populations, the lowest effective dose for clinical improvement, and its interaction with other medications and breastfeeding. Additionally, the long-term remission rates of depressive symptoms in patients treated with zuranolone are unknown and warrant further study.
JAIMEY M. PAULI, MD; KENDALL CUNNINGHAM, MD
- Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp .20220785
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78:951-959. doi:10.1001/jamapsychiatry.2021.1559
- Clayton AH, Lasser R, Parikh SV, et al. Zuranolone for the treatment of adults with major depressive disorder: a randomized, placebo-controlled phase 3 trial. Am J Psychiatry. 2023;180:676-684. doi:10.1176/appi.ajp.20220459
- Zuranolone for the treatment of postpartum depression. Practice Advisory. American College of Obstetricians and Gynecologists. August 2023. Accessed September 18, 2023. https://www.acog.org/clinical/clinical-guidance/practice -advisory/articles/2023/08/zuranolone-for-the-treatment-of -postpartum-depression
Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp.20220785.
EXPERT COMMENTARY
Postpartum depression affects approximately 17.2% of patients in the peripartum period.1 Typical pharmacologic treatment of PPD includes selective serotonin reuptake inhibitors (SSRIs), which may take up to 12 weeks to take effect. Postpartum depression is thought to be secondary to maladaptation to hormonal fluctuations in the peripartum period, including allopregnanolone, a positive allosteric modulator of GABAA (γ-aminobutyric acid type A)receptors and a metabolite of progesterone, levels of which increase in pregnancy and abruptly decrease following delivery.1 In 2019, the GABAA receptor modulator brexanalone was approved by the US Food and Drug Administration (FDA) to treat PPD through continuous intravenous infusion over 60 hours in the hospital setting.
Zuranolone, an allosteric modulator of GABAA receptors, also has been studied as an investigational medication for rapid treatment of PPD. Prior studies demonstrated the efficacy of oral zuranolone 30 mg daily for the treatment of PPD2 and 50 mg for the treatment of major depression in nonpregnant patients.3 Deligiannidis and colleagues conducted a trial to investigate the 50-mg dose of zuranolone for the treatment of PPD. (Notably, in August 2023, the FDA approved oral zuranolone once daily for 14 days for the treatment of PPD.) Following the FDA approval, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory recommending consideration of zuranolone for PPD that takes into account balancing the benefits and risks, including known sedative effects, potential need for decreasing the dose due to adverse effects, lack of safety data in lactation, and unknown long-term efficacy.4
Details of the study
This randomized, double-blind, placebo-controlled study included 196 patients with an episode of major depression, characterized as a baseline score of 26 or greater on the Hamilton Depression Rating Scale (HAM-D) beginning in the third trimester or within the first 4 weeks postpartum. Patients were randomly assigned in a 1:1 ratio to receive zuranolone 50 mg daily or placebo, with stratification by stable concurrent antidepressant use. Treatment duration was for 14 days, with follow-up through day 45.
The study’s primary outcome was a change in the baseline HAM-D score at day 15. Changes in HAM-D score also were recorded at days 3, 28, and 45.
The 2 study groups were well balanced by demographic and baseline characteristics. In both groups, the majority of patients experienced the onset of their major depressive episodes within the first 4 weeks postpartum. Completion rates of the 14-day treatment course and 45-day follow-up were high and similar in both groups; 170 patients completed the study. The rate of concurrent psychiatric medications taken, most of which were SSRIs, was similar between the 2 groups at approximately 15% of patients.
Results. A statistically significant improvement in the primary outcome (the change in HAM-D score) at day 15 occurred in patients who received zuranolone versus placebo (P = .001). Additionally, there were statistically significant improvements in the secondary outcomes HAM-D scores at days 3, 28, and 45. Initial response, as measured by changes in HAM-D scores, occurred at a median duration of 9 days in the zuranolone group and 43 days in the placebo group. More patients in the zuranolone group achieved a reduction in HAM-D score at 15 days (57.0% vs 38.9%; P = .02). Zuranolone was associated with a higher rate of HAM-D remission at day 45 (44.0% vs 29.4%; P = .02).
With regard to safety, 16.3% of patients (17) in the zuranolone group (vs 1% in the placebo group) experienced an adverse event, most commonly somnolence, dizziness, and sedation, which led to a dose reduction. However, 15 of these 17 patients still completed the study, and there were no serious adverse events.
Study strengths and limitations
This study’s strengths include the double-blinded design that was continued throughout the duration of the follow-up. Additionally, the study population was heterogeneous andreflective of patients from diverse racial and ethnic backgrounds. Lastly, only minor and moderate adverse events were reported and, despite this, nearly all patients who experienced adverse events completed the study.
Limitations of the study include the lack of generalizability, as patients with bipolar disorder and mild or moderate PPD were excluded. Additionally, the majority of patients had depressive episodes within the first 4 weeks postpartum, thereby excluding patients with depressive episodes at other time points in the peripartum period. Further, as breastfeeding was prohibited, safety in lactating patients using zuranolone is unknown. Lastly, the study follow-up period was 45 days; therefore, the long-term efficacy of zuranolone treatment is unclear. ●
Zuranolone, a GABAA allosteric modulator, shows promise as an alternative to existing pharmacologic treatments for severe PPD that is orally administered and rapidly acting. While it is reasonable to consider its use in the specific patient population that benefited in this study, further studies are needed to determine its efficacy in other populations, the lowest effective dose for clinical improvement, and its interaction with other medications and breastfeeding. Additionally, the long-term remission rates of depressive symptoms in patients treated with zuranolone are unknown and warrant further study.
JAIMEY M. PAULI, MD; KENDALL CUNNINGHAM, MD
Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp.20220785.
EXPERT COMMENTARY
Postpartum depression affects approximately 17.2% of patients in the peripartum period.1 Typical pharmacologic treatment of PPD includes selective serotonin reuptake inhibitors (SSRIs), which may take up to 12 weeks to take effect. Postpartum depression is thought to be secondary to maladaptation to hormonal fluctuations in the peripartum period, including allopregnanolone, a positive allosteric modulator of GABAA (γ-aminobutyric acid type A)receptors and a metabolite of progesterone, levels of which increase in pregnancy and abruptly decrease following delivery.1 In 2019, the GABAA receptor modulator brexanalone was approved by the US Food and Drug Administration (FDA) to treat PPD through continuous intravenous infusion over 60 hours in the hospital setting.
Zuranolone, an allosteric modulator of GABAA receptors, also has been studied as an investigational medication for rapid treatment of PPD. Prior studies demonstrated the efficacy of oral zuranolone 30 mg daily for the treatment of PPD2 and 50 mg for the treatment of major depression in nonpregnant patients.3 Deligiannidis and colleagues conducted a trial to investigate the 50-mg dose of zuranolone for the treatment of PPD. (Notably, in August 2023, the FDA approved oral zuranolone once daily for 14 days for the treatment of PPD.) Following the FDA approval, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory recommending consideration of zuranolone for PPD that takes into account balancing the benefits and risks, including known sedative effects, potential need for decreasing the dose due to adverse effects, lack of safety data in lactation, and unknown long-term efficacy.4
Details of the study
This randomized, double-blind, placebo-controlled study included 196 patients with an episode of major depression, characterized as a baseline score of 26 or greater on the Hamilton Depression Rating Scale (HAM-D) beginning in the third trimester or within the first 4 weeks postpartum. Patients were randomly assigned in a 1:1 ratio to receive zuranolone 50 mg daily or placebo, with stratification by stable concurrent antidepressant use. Treatment duration was for 14 days, with follow-up through day 45.
The study’s primary outcome was a change in the baseline HAM-D score at day 15. Changes in HAM-D score also were recorded at days 3, 28, and 45.
The 2 study groups were well balanced by demographic and baseline characteristics. In both groups, the majority of patients experienced the onset of their major depressive episodes within the first 4 weeks postpartum. Completion rates of the 14-day treatment course and 45-day follow-up were high and similar in both groups; 170 patients completed the study. The rate of concurrent psychiatric medications taken, most of which were SSRIs, was similar between the 2 groups at approximately 15% of patients.
Results. A statistically significant improvement in the primary outcome (the change in HAM-D score) at day 15 occurred in patients who received zuranolone versus placebo (P = .001). Additionally, there were statistically significant improvements in the secondary outcomes HAM-D scores at days 3, 28, and 45. Initial response, as measured by changes in HAM-D scores, occurred at a median duration of 9 days in the zuranolone group and 43 days in the placebo group. More patients in the zuranolone group achieved a reduction in HAM-D score at 15 days (57.0% vs 38.9%; P = .02). Zuranolone was associated with a higher rate of HAM-D remission at day 45 (44.0% vs 29.4%; P = .02).
With regard to safety, 16.3% of patients (17) in the zuranolone group (vs 1% in the placebo group) experienced an adverse event, most commonly somnolence, dizziness, and sedation, which led to a dose reduction. However, 15 of these 17 patients still completed the study, and there were no serious adverse events.
Study strengths and limitations
This study’s strengths include the double-blinded design that was continued throughout the duration of the follow-up. Additionally, the study population was heterogeneous andreflective of patients from diverse racial and ethnic backgrounds. Lastly, only minor and moderate adverse events were reported and, despite this, nearly all patients who experienced adverse events completed the study.
Limitations of the study include the lack of generalizability, as patients with bipolar disorder and mild or moderate PPD were excluded. Additionally, the majority of patients had depressive episodes within the first 4 weeks postpartum, thereby excluding patients with depressive episodes at other time points in the peripartum period. Further, as breastfeeding was prohibited, safety in lactating patients using zuranolone is unknown. Lastly, the study follow-up period was 45 days; therefore, the long-term efficacy of zuranolone treatment is unclear. ●
Zuranolone, a GABAA allosteric modulator, shows promise as an alternative to existing pharmacologic treatments for severe PPD that is orally administered and rapidly acting. While it is reasonable to consider its use in the specific patient population that benefited in this study, further studies are needed to determine its efficacy in other populations, the lowest effective dose for clinical improvement, and its interaction with other medications and breastfeeding. Additionally, the long-term remission rates of depressive symptoms in patients treated with zuranolone are unknown and warrant further study.
JAIMEY M. PAULI, MD; KENDALL CUNNINGHAM, MD
- Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp .20220785
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78:951-959. doi:10.1001/jamapsychiatry.2021.1559
- Clayton AH, Lasser R, Parikh SV, et al. Zuranolone for the treatment of adults with major depressive disorder: a randomized, placebo-controlled phase 3 trial. Am J Psychiatry. 2023;180:676-684. doi:10.1176/appi.ajp.20220459
- Zuranolone for the treatment of postpartum depression. Practice Advisory. American College of Obstetricians and Gynecologists. August 2023. Accessed September 18, 2023. https://www.acog.org/clinical/clinical-guidance/practice -advisory/articles/2023/08/zuranolone-for-the-treatment-of -postpartum-depression
- Deligiannidis KM, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of postpartum depression. Am J Psychiatry. 2023;180:668-675. doi:10.1176/appi.ajp .20220785
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78:951-959. doi:10.1001/jamapsychiatry.2021.1559
- Clayton AH, Lasser R, Parikh SV, et al. Zuranolone for the treatment of adults with major depressive disorder: a randomized, placebo-controlled phase 3 trial. Am J Psychiatry. 2023;180:676-684. doi:10.1176/appi.ajp.20220459
- Zuranolone for the treatment of postpartum depression. Practice Advisory. American College of Obstetricians and Gynecologists. August 2023. Accessed September 18, 2023. https://www.acog.org/clinical/clinical-guidance/practice -advisory/articles/2023/08/zuranolone-for-the-treatment-of -postpartum-depression
Can these salt substitutes prevent complications of hypertension?
ILLUSTRATIVE CASE
A 47-year-old man in generally good health presents to a family medicine clinic for a well visit. He does not use tobacco products and had a benign colonoscopy last year. He reports walking for 30 minutes 3 to 4 times per week for exercise, althoug h he has gained 3 lbs over the past 2 years. He has no family history of early coronary artery disease, but his father and older brother have hypertension. His mother has a history of diabetes and hyperlipidemia.
The patient’s physical exam is unremarkable except for an elevated BP reading of 151/82 mm Hg. A review of his chart indicates he has had multiple elevated readings in the past that have ranged from 132/72 mm Hg to 139/89 mm Hg. The patient is interested in antihypertensive treatment but wants to know if modifying his diet and replacing his regular table salt with a salt substitute will lower his high BP. What can you recommend?
Hypertension is a leading cause of CV morbidity and mortality worldwide and is linked to increased dietary sodium intake. An estimated 1.28 billion people worldwide have hypertension; however, more than half of cases are undiagnosed.2The US Preventive Services Task Force recommends screening for hypertension in adults older than 18 years and confirming elevated measurements conducted in a nonclinical setting before starting medication (grade “A”).3
Cut-points for the diagnosis of hypertension vary. The American Academy of Family Physicians, 4 the Eighth Joint National Committee (JNC 8), 5 the International Society of Hypertension, 6 and the European Society of Cardiology 7 use ≥ 140 mm Hg systolic BP (SBP) or ≥ 90 mm Hg diastolic BP (DBP) to define hypertension. The American College of Cardiology/American Heart Association guidelines use ≥ 130/80 mm Hg. 8
When treating patients with hypertension, primary care physicians often recommend lifestyle modifications such as the
Systematic reviews have shown a measurable improvement in BP with sodium reduction and potassium substitution. 10-12 More importantly, high-quality evidence demonstrates a decreased risk for CV disease, kidney disease, and all-cause mortality with lower dietary sodium intake. 13 Previous studies have shown that potassium-enriched salt substitutes lower BP, but their impact on CV morbidity and mortality is not well defined. Although lowering BP is associated with improved clinical impact, there is a lack of patient-oriented evidence that demonstrates improvement in CV disease and mortality.
The Salt Substitute and Stroke Study (SSaSS), published in 2021, demonstrated the protective effect of salt substitution against stroke, other CV events, and death. 14 Furthermore, this 5-year, cluster-randomized controlled trial of 20,995 participants across 600 villages in China demonstrated reduced CV mortality and BP reduction similar to standard pharmacologic treatment. Prior to SSaSS, 17 randomized controlled trials demonstrated a BP-lowering effect of salt substitutes but did not directly study the impact on clinical outcomes. 13
Continue to: In this 2022 systematic review...
In this 2022 systematic review and meta-analysis, 1 Yin et al evaluated 21 trials, including SSaSS, for the effect of salt substitutes on BP and other clinical outcomes, and the generalizability of the study results to diverse populations. The systematic review included parallel-group, step-wedge, and cluster-randomized controlled trials reporting the effect of salt substitutes on BP or clinical outcomes.
STUDY SUMMARY
Salt substitutes reduced BP across diverse populations
This systematic review and meta-analysis reviewed existing literature for randomized controlled trials investigating the effects of potassium-enriched salt substitutes on clinical outcomes for patients without kidney disease. The most commonly used salt substitute was potassium chloride, at 25% to 65% potassium.
The systematic review identified 21 trials comprising 31,949 study participants from 15 different countries with 1 to 60 months’ duration. Meta-analyses were performed using 19 trials for BP outcomes and 5 trials for vascular outcomes. Eleven trials were rated as having low risk for bias, 8 were deemed to have some concern, and 2 were rated as high risk for bias. Comparisons of data excluding studies with high risk for bias yielded results similar to comparisons of all studies.
The meta-analysis of 19 trials demonstrated reduced SBP (–4.6 mm Hg; 95% CI, –6.1 to –3.1) and DBP (–1.6 mm Hg; 95% CI, –2.4 to –0.8) in participants using potassium-enriched salt substitutes. However, the authors noted substantial heterogeneity among the studies (I 2 > 70%) for both SBP and DBP outcomes. Although there were no subgroup differences for age, sex, hypertension history, or other biomarkers, outcome differences were associated with trial duration, baseline potassium intake, and composition of the salt substitute.
Potassium-enriched salt substitutes were associated with reduced total mortality (risk ratio [RR] = 0.89; 95% CI, 0.85-0.94), CV mortality (RR = 0.87; 95% CI, 0.81-0.94), and CV events (RR = 0.89; 95% CI, 0.85-0.94). In a meta-regression, each 10% reduction in the sodium content of the salt substitute was associated with a 1.5–mm Hg greater reduction in SBP (95% CI, –3.0 to –0.03) and a 1.0–mm Hg greater reduction in DBP (95% CI, –1.8 to –0.1). However, the authors suggest interpreting meta-regression results with caution.
Continue to: Only 2 of the studes...
Only 2 of the studies in the systematic review explicitly reported the adverse effect of hyperkalemia, and there was no statistical difference in events between randomized groups. Eight other studies reported no serious adverse events related to hyperkalemia , and 11 studies did not report on the risk for hyperkalemia.
WHAT’S NEW
High-quality data demonstrate beneficial outcomes
Previous observational and interventional studies demonstrated a BP-lowering effect of salt substitutes, but limited data with poor-quality evidence existed for the impact of salt substitutes on clinical outcomes such as mortality and CV events. This systematic review and meta-analysis suggests that potassium-supplemented salt may reduce BP and secondarily reduce the risk for CV events, CV mortality, and total mortality, without clear harmful effects reported.
CAVEATS
Some patient populations, comorbidities excluded from study
The study did not include patients with kidney disease or those taking potassium-sparing diuretics. Furthermore, the available data do not include primary prevention participants.
Subgroup analyses should be interpreted with caution due to the small number of trials available for individual subgroups. In addition, funnel plot asymmetry for studies reporting DBP suggests at least some effect of publication bias for that outcome.
Although BP reduction due to salt substitutes may be small at an individual level, these levels of reduction may be important at a population level.
CHALLENGES TO IMPLEMENTATION
For appropriate patients, no challenges anticipated
There are no significant challenges to implementing conclusions from this study in the primary care setting. Family physicians should be able to recommend potassium-enriched salt substitutes to patients with hypertension who are not at risk for hyperkalemia, including those with kidney disease, on potassium-sparing diuretics, or with a history of hyperkalemia/hyperkalemic conditions. Salt substitutes, including potassium-enriched salts, are readily available in stores.
1. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957-980. doi: 10.1016/S0140-6736(21)01330-1
3. USPSTF. Hypertension in adults: screening. Final recommendation statement. Published April 27, 2021. Accessed September 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
4. Coles S, Fisher L, Lin KW, et al. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Published November 4, 2022. Accessed September 18, 2023. www.aafp.org/dam/AAFP/documents/journals/afp/AAFPHypertensionGuideline.pdf
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. doi: 10.1001/jama. 2013.284427
6. Unger T, Borgi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
7. Mancia G, Kreutz R, Brunstrom M, et al; the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. 2023 ESH Guidelines for the management of arterial hypertension. Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; Jun 21. doi: 10.1097/HJH.0000000000003480
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. 10.1161/HYP.0000000000000065
9. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2014 state and national summary tables. Accessed June 27, 2023. www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
10. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315
11. Filippini T, Violi F, D’Amico R, et al. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127-135. doi: 10.1016/j.ijcard.2016.12.048
12. Brand A, Visser ME, Schoonees A, et al. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8:CD015207. doi: 10.1002/14651858.CD015207
13. He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632-647. doi: 10.1016/j.jacc.2019.11.055
14. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067-1077. doi: 10.1056/NEJMoa2105675
ILLUSTRATIVE CASE
A 47-year-old man in generally good health presents to a family medicine clinic for a well visit. He does not use tobacco products and had a benign colonoscopy last year. He reports walking for 30 minutes 3 to 4 times per week for exercise, althoug h he has gained 3 lbs over the past 2 years. He has no family history of early coronary artery disease, but his father and older brother have hypertension. His mother has a history of diabetes and hyperlipidemia.
The patient’s physical exam is unremarkable except for an elevated BP reading of 151/82 mm Hg. A review of his chart indicates he has had multiple elevated readings in the past that have ranged from 132/72 mm Hg to 139/89 mm Hg. The patient is interested in antihypertensive treatment but wants to know if modifying his diet and replacing his regular table salt with a salt substitute will lower his high BP. What can you recommend?
Hypertension is a leading cause of CV morbidity and mortality worldwide and is linked to increased dietary sodium intake. An estimated 1.28 billion people worldwide have hypertension; however, more than half of cases are undiagnosed.2The US Preventive Services Task Force recommends screening for hypertension in adults older than 18 years and confirming elevated measurements conducted in a nonclinical setting before starting medication (grade “A”).3
Cut-points for the diagnosis of hypertension vary. The American Academy of Family Physicians, 4 the Eighth Joint National Committee (JNC 8), 5 the International Society of Hypertension, 6 and the European Society of Cardiology 7 use ≥ 140 mm Hg systolic BP (SBP) or ≥ 90 mm Hg diastolic BP (DBP) to define hypertension. The American College of Cardiology/American Heart Association guidelines use ≥ 130/80 mm Hg. 8
When treating patients with hypertension, primary care physicians often recommend lifestyle modifications such as the
Systematic reviews have shown a measurable improvement in BP with sodium reduction and potassium substitution. 10-12 More importantly, high-quality evidence demonstrates a decreased risk for CV disease, kidney disease, and all-cause mortality with lower dietary sodium intake. 13 Previous studies have shown that potassium-enriched salt substitutes lower BP, but their impact on CV morbidity and mortality is not well defined. Although lowering BP is associated with improved clinical impact, there is a lack of patient-oriented evidence that demonstrates improvement in CV disease and mortality.
The Salt Substitute and Stroke Study (SSaSS), published in 2021, demonstrated the protective effect of salt substitution against stroke, other CV events, and death. 14 Furthermore, this 5-year, cluster-randomized controlled trial of 20,995 participants across 600 villages in China demonstrated reduced CV mortality and BP reduction similar to standard pharmacologic treatment. Prior to SSaSS, 17 randomized controlled trials demonstrated a BP-lowering effect of salt substitutes but did not directly study the impact on clinical outcomes. 13
Continue to: In this 2022 systematic review...
In this 2022 systematic review and meta-analysis, 1 Yin et al evaluated 21 trials, including SSaSS, for the effect of salt substitutes on BP and other clinical outcomes, and the generalizability of the study results to diverse populations. The systematic review included parallel-group, step-wedge, and cluster-randomized controlled trials reporting the effect of salt substitutes on BP or clinical outcomes.
STUDY SUMMARY
Salt substitutes reduced BP across diverse populations
This systematic review and meta-analysis reviewed existing literature for randomized controlled trials investigating the effects of potassium-enriched salt substitutes on clinical outcomes for patients without kidney disease. The most commonly used salt substitute was potassium chloride, at 25% to 65% potassium.
The systematic review identified 21 trials comprising 31,949 study participants from 15 different countries with 1 to 60 months’ duration. Meta-analyses were performed using 19 trials for BP outcomes and 5 trials for vascular outcomes. Eleven trials were rated as having low risk for bias, 8 were deemed to have some concern, and 2 were rated as high risk for bias. Comparisons of data excluding studies with high risk for bias yielded results similar to comparisons of all studies.
The meta-analysis of 19 trials demonstrated reduced SBP (–4.6 mm Hg; 95% CI, –6.1 to –3.1) and DBP (–1.6 mm Hg; 95% CI, –2.4 to –0.8) in participants using potassium-enriched salt substitutes. However, the authors noted substantial heterogeneity among the studies (I 2 > 70%) for both SBP and DBP outcomes. Although there were no subgroup differences for age, sex, hypertension history, or other biomarkers, outcome differences were associated with trial duration, baseline potassium intake, and composition of the salt substitute.
Potassium-enriched salt substitutes were associated with reduced total mortality (risk ratio [RR] = 0.89; 95% CI, 0.85-0.94), CV mortality (RR = 0.87; 95% CI, 0.81-0.94), and CV events (RR = 0.89; 95% CI, 0.85-0.94). In a meta-regression, each 10% reduction in the sodium content of the salt substitute was associated with a 1.5–mm Hg greater reduction in SBP (95% CI, –3.0 to –0.03) and a 1.0–mm Hg greater reduction in DBP (95% CI, –1.8 to –0.1). However, the authors suggest interpreting meta-regression results with caution.
Continue to: Only 2 of the studes...
Only 2 of the studies in the systematic review explicitly reported the adverse effect of hyperkalemia, and there was no statistical difference in events between randomized groups. Eight other studies reported no serious adverse events related to hyperkalemia , and 11 studies did not report on the risk for hyperkalemia.
WHAT’S NEW
High-quality data demonstrate beneficial outcomes
Previous observational and interventional studies demonstrated a BP-lowering effect of salt substitutes, but limited data with poor-quality evidence existed for the impact of salt substitutes on clinical outcomes such as mortality and CV events. This systematic review and meta-analysis suggests that potassium-supplemented salt may reduce BP and secondarily reduce the risk for CV events, CV mortality, and total mortality, without clear harmful effects reported.
CAVEATS
Some patient populations, comorbidities excluded from study
The study did not include patients with kidney disease or those taking potassium-sparing diuretics. Furthermore, the available data do not include primary prevention participants.
Subgroup analyses should be interpreted with caution due to the small number of trials available for individual subgroups. In addition, funnel plot asymmetry for studies reporting DBP suggests at least some effect of publication bias for that outcome.
Although BP reduction due to salt substitutes may be small at an individual level, these levels of reduction may be important at a population level.
CHALLENGES TO IMPLEMENTATION
For appropriate patients, no challenges anticipated
There are no significant challenges to implementing conclusions from this study in the primary care setting. Family physicians should be able to recommend potassium-enriched salt substitutes to patients with hypertension who are not at risk for hyperkalemia, including those with kidney disease, on potassium-sparing diuretics, or with a history of hyperkalemia/hyperkalemic conditions. Salt substitutes, including potassium-enriched salts, are readily available in stores.
ILLUSTRATIVE CASE
A 47-year-old man in generally good health presents to a family medicine clinic for a well visit. He does not use tobacco products and had a benign colonoscopy last year. He reports walking for 30 minutes 3 to 4 times per week for exercise, althoug h he has gained 3 lbs over the past 2 years. He has no family history of early coronary artery disease, but his father and older brother have hypertension. His mother has a history of diabetes and hyperlipidemia.
The patient’s physical exam is unremarkable except for an elevated BP reading of 151/82 mm Hg. A review of his chart indicates he has had multiple elevated readings in the past that have ranged from 132/72 mm Hg to 139/89 mm Hg. The patient is interested in antihypertensive treatment but wants to know if modifying his diet and replacing his regular table salt with a salt substitute will lower his high BP. What can you recommend?
Hypertension is a leading cause of CV morbidity and mortality worldwide and is linked to increased dietary sodium intake. An estimated 1.28 billion people worldwide have hypertension; however, more than half of cases are undiagnosed.2The US Preventive Services Task Force recommends screening for hypertension in adults older than 18 years and confirming elevated measurements conducted in a nonclinical setting before starting medication (grade “A”).3
Cut-points for the diagnosis of hypertension vary. The American Academy of Family Physicians, 4 the Eighth Joint National Committee (JNC 8), 5 the International Society of Hypertension, 6 and the European Society of Cardiology 7 use ≥ 140 mm Hg systolic BP (SBP) or ≥ 90 mm Hg diastolic BP (DBP) to define hypertension. The American College of Cardiology/American Heart Association guidelines use ≥ 130/80 mm Hg. 8
When treating patients with hypertension, primary care physicians often recommend lifestyle modifications such as the
Systematic reviews have shown a measurable improvement in BP with sodium reduction and potassium substitution. 10-12 More importantly, high-quality evidence demonstrates a decreased risk for CV disease, kidney disease, and all-cause mortality with lower dietary sodium intake. 13 Previous studies have shown that potassium-enriched salt substitutes lower BP, but their impact on CV morbidity and mortality is not well defined. Although lowering BP is associated with improved clinical impact, there is a lack of patient-oriented evidence that demonstrates improvement in CV disease and mortality.
The Salt Substitute and Stroke Study (SSaSS), published in 2021, demonstrated the protective effect of salt substitution against stroke, other CV events, and death. 14 Furthermore, this 5-year, cluster-randomized controlled trial of 20,995 participants across 600 villages in China demonstrated reduced CV mortality and BP reduction similar to standard pharmacologic treatment. Prior to SSaSS, 17 randomized controlled trials demonstrated a BP-lowering effect of salt substitutes but did not directly study the impact on clinical outcomes. 13
Continue to: In this 2022 systematic review...
In this 2022 systematic review and meta-analysis, 1 Yin et al evaluated 21 trials, including SSaSS, for the effect of salt substitutes on BP and other clinical outcomes, and the generalizability of the study results to diverse populations. The systematic review included parallel-group, step-wedge, and cluster-randomized controlled trials reporting the effect of salt substitutes on BP or clinical outcomes.
STUDY SUMMARY
Salt substitutes reduced BP across diverse populations
This systematic review and meta-analysis reviewed existing literature for randomized controlled trials investigating the effects of potassium-enriched salt substitutes on clinical outcomes for patients without kidney disease. The most commonly used salt substitute was potassium chloride, at 25% to 65% potassium.
The systematic review identified 21 trials comprising 31,949 study participants from 15 different countries with 1 to 60 months’ duration. Meta-analyses were performed using 19 trials for BP outcomes and 5 trials for vascular outcomes. Eleven trials were rated as having low risk for bias, 8 were deemed to have some concern, and 2 were rated as high risk for bias. Comparisons of data excluding studies with high risk for bias yielded results similar to comparisons of all studies.
The meta-analysis of 19 trials demonstrated reduced SBP (–4.6 mm Hg; 95% CI, –6.1 to –3.1) and DBP (–1.6 mm Hg; 95% CI, –2.4 to –0.8) in participants using potassium-enriched salt substitutes. However, the authors noted substantial heterogeneity among the studies (I 2 > 70%) for both SBP and DBP outcomes. Although there were no subgroup differences for age, sex, hypertension history, or other biomarkers, outcome differences were associated with trial duration, baseline potassium intake, and composition of the salt substitute.
Potassium-enriched salt substitutes were associated with reduced total mortality (risk ratio [RR] = 0.89; 95% CI, 0.85-0.94), CV mortality (RR = 0.87; 95% CI, 0.81-0.94), and CV events (RR = 0.89; 95% CI, 0.85-0.94). In a meta-regression, each 10% reduction in the sodium content of the salt substitute was associated with a 1.5–mm Hg greater reduction in SBP (95% CI, –3.0 to –0.03) and a 1.0–mm Hg greater reduction in DBP (95% CI, –1.8 to –0.1). However, the authors suggest interpreting meta-regression results with caution.
Continue to: Only 2 of the studes...
Only 2 of the studies in the systematic review explicitly reported the adverse effect of hyperkalemia, and there was no statistical difference in events between randomized groups. Eight other studies reported no serious adverse events related to hyperkalemia , and 11 studies did not report on the risk for hyperkalemia.
WHAT’S NEW
High-quality data demonstrate beneficial outcomes
Previous observational and interventional studies demonstrated a BP-lowering effect of salt substitutes, but limited data with poor-quality evidence existed for the impact of salt substitutes on clinical outcomes such as mortality and CV events. This systematic review and meta-analysis suggests that potassium-supplemented salt may reduce BP and secondarily reduce the risk for CV events, CV mortality, and total mortality, without clear harmful effects reported.
CAVEATS
Some patient populations, comorbidities excluded from study
The study did not include patients with kidney disease or those taking potassium-sparing diuretics. Furthermore, the available data do not include primary prevention participants.
Subgroup analyses should be interpreted with caution due to the small number of trials available for individual subgroups. In addition, funnel plot asymmetry for studies reporting DBP suggests at least some effect of publication bias for that outcome.
Although BP reduction due to salt substitutes may be small at an individual level, these levels of reduction may be important at a population level.
CHALLENGES TO IMPLEMENTATION
For appropriate patients, no challenges anticipated
There are no significant challenges to implementing conclusions from this study in the primary care setting. Family physicians should be able to recommend potassium-enriched salt substitutes to patients with hypertension who are not at risk for hyperkalemia, including those with kidney disease, on potassium-sparing diuretics, or with a history of hyperkalemia/hyperkalemic conditions. Salt substitutes, including potassium-enriched salts, are readily available in stores.
1. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957-980. doi: 10.1016/S0140-6736(21)01330-1
3. USPSTF. Hypertension in adults: screening. Final recommendation statement. Published April 27, 2021. Accessed September 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
4. Coles S, Fisher L, Lin KW, et al. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Published November 4, 2022. Accessed September 18, 2023. www.aafp.org/dam/AAFP/documents/journals/afp/AAFPHypertensionGuideline.pdf
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. doi: 10.1001/jama. 2013.284427
6. Unger T, Borgi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
7. Mancia G, Kreutz R, Brunstrom M, et al; the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. 2023 ESH Guidelines for the management of arterial hypertension. Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; Jun 21. doi: 10.1097/HJH.0000000000003480
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. 10.1161/HYP.0000000000000065
9. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2014 state and national summary tables. Accessed June 27, 2023. www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
10. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315
11. Filippini T, Violi F, D’Amico R, et al. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127-135. doi: 10.1016/j.ijcard.2016.12.048
12. Brand A, Visser ME, Schoonees A, et al. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8:CD015207. doi: 10.1002/14651858.CD015207
13. He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632-647. doi: 10.1016/j.jacc.2019.11.055
14. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067-1077. doi: 10.1056/NEJMoa2105675
1. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957-980. doi: 10.1016/S0140-6736(21)01330-1
3. USPSTF. Hypertension in adults: screening. Final recommendation statement. Published April 27, 2021. Accessed September 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
4. Coles S, Fisher L, Lin KW, et al. Blood pressure targets in adults with hypertension: a clinical practice guideline from the AAFP. Published November 4, 2022. Accessed September 18, 2023. www.aafp.org/dam/AAFP/documents/journals/afp/AAFPHypertensionGuideline.pdf
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. doi: 10.1001/jama. 2013.284427
6. Unger T, Borgi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
7. Mancia G, Kreutz R, Brunstrom M, et al; the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension. 2023 ESH Guidelines for the management of arterial hypertension. Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; Jun 21. doi: 10.1097/HJH.0000000000003480
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. 10.1161/HYP.0000000000000065
9. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2014 state and national summary tables. Accessed June 27, 2023. www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf
10. Huang L, Trieu K, Yoshimura S, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315
11. Filippini T, Violi F, D’Amico R, et al. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127-135. doi: 10.1016/j.ijcard.2016.12.048
12. Brand A, Visser ME, Schoonees A, et al. Replacing salt with low-sodium salt substitutes (LSSS) for cardiovascular health in adults, children and pregnant women. Cochrane Database Syst Rev. 2022;8:CD015207. doi: 10.1002/14651858.CD015207
13. He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:632-647. doi: 10.1016/j.jacc.2019.11.055
14. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067-1077. doi: 10.1056/NEJMoa2105675
PRACTICE CHANGER
Consider recommending potassium-enriched salt substitutes for appropriate patients with hypertension to reduce blood pressure (BP) and risk for related cardiovascular (CV) events or mortality.
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of controlled trials. 1
Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart . 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
Feeling salty about our sodium intake
The World Health Organization (WHO) recently released its inaugural report on the devastating global effects of hypertension, including recommendations for combatting this “silent killer.”1 Notable in the 276-page report is the emphasis on improving access to antihypertensive medications, in part through team-based care and simple evidence-based protocols. This strategy is not surprising given that in clinical medicine we focus on the “high-risk” strategy for prevention—ie, identify people at increased risk for an adverse health outcome (in this case, cardiovascular disease events) and offer them medication to reduce that risk.2
As part of the high-risk strategy, we also counsel at the individual level about lifestyle modifications—but unfortunately, we tend not to get very far. Given the substantial evidence demonstrating its benefits, a low-sodium DASH (Dietary Approaches to Stop Hypertension) eating plan is one of the lifestyle recommendations we make for our patients with hypertension.3,4 The DASH part of the diet involves getting our patients to eat more fruits, vegetables, and whole grains and limit sugar and saturated fats. To achieve the low-sodium part, we might counsel against added table salt, but mostly we discourage consumption of canned and other foods that are commercially processed, packaged, and prepared, because that’s the source of more than 70% of our sodium intake.5 It’s not difficult to understand why real-world uptake of the low-sodium DASH eating plan is low.6
This issue of The Journal of Family Practice features a PURL that supports a much more prominent role for salt substitutes in our counseling recommendations.7 Potassium-enriched salt substitutes not only lower blood pressure (BP) but also reduce the risk for cardiovascular events and death.8 They are widely available, and while more expensive per ounce than regular salt (sodium chloride), are still affordable.
Still, encouraging salt substitution with one patient at a time is relying on the high-risk strategy, with its inherently limited potential.2 An alternative is the population strategy. For hypertension, that would mean doing something for the entire population that would lead to a downward shift in the distribution of BP.2 The shift does not have to be large. We’ve known for more than 3 decades that just a 2–mm Hg reduction in the population’s average systolic BP would reduce stroke mortality by about 6%, coronary heart disease mortality by 4%, and total mortality by 3%.9 A 5–mm Hg reduction more than doubles those benefits. We are talking about tens of thousands fewer patients with heart disease and stroke each year and billions of dollars in health care cost savings.
Reducing our nation’s sodium intake, a quintessential population approach, has proven difficult. Our average daily sodium intake is about 3600 mg.10 Guidance on sodium reduction from the US Food and Drug Administration (targeted to industry) has aimed to reduce Americans’ average sodium intake to 3000 mg/d over the short term, fully acknowledging that the recommended sodium limit is 2300 mg/d.11 We’ve got a long way to go.
Might salt substitution at the population level be a way to simultaneously reduce our sodium intake and increase our potassium intake?12 The closest I found to a populationwide substitution study was a cluster randomized trial conducted in 6 villages in Peru.13 In a stepped-wedge design, households had 25% of their regular salt replaced with potassium salt. Small shops, bakeries, community kitchens, and food vendors also had salt replacement. The intention-to-treat analysis showed a small reduction in systolic BP (1.3 mm Hg) among those with hypertension at baseline (n = 428) and a 51% reduced incidence of developing hypertension among the other 1891 participants over the 4673 person-years of follow-up.
I found this study interesting and its results compelling, leading me to wonder: In the United States, where most of our sodium comes from the food industry, should we replace even a small amount of the sodium in processed foods with potassium? We’re not getting there with DASH alone.
1. World Health Organization. Global report on hypertension: the race against a silent killer. Published September 19, 2023. Accessed September 29, 2023. www.who.int/publications/i/item/9789240081062
2. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432. doi: 10.1093/ije/30.3.427
3. Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338
4. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253-1261. doi: 10.1016/j.numecd.2014.06.008
5. Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775-1783. doi: 10.1161/CIRCULATIONAHA.116.024446
6. Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308-314. doi: 10.1001/archinternmed.2007.119
7. Chang ET, Powell R, Reese T. Can potassium-enriched salt substitutes prevent complications of hypertension? J Fam Pract. 2023;72:342-344. doi: 10.12788/jfp.0667
8. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
9. Whelton PK, He J, Appel LJ, et al; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882-1888. doi: 10.1001/jama.288.15.1882
10. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209-1220. doi: 1001/jama.2018.1156
11. FDA. Guidance for industry: voluntary sodium reduction goals. Published October 2021. Accessed September 28, 2023. www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals
12. Nissaisorakarn V, Ormseth G, Earle W, et al. Less sodium, more potassium, or both: population-wide strategies to prevent hypertension. Am J Physiol Renal Physiol. 2023;325:F99-F104. doi: 10.1152/ajprenal.00007.202
13. Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374-378. doi: 10.1038/s41591-020-0754-2
The World Health Organization (WHO) recently released its inaugural report on the devastating global effects of hypertension, including recommendations for combatting this “silent killer.”1 Notable in the 276-page report is the emphasis on improving access to antihypertensive medications, in part through team-based care and simple evidence-based protocols. This strategy is not surprising given that in clinical medicine we focus on the “high-risk” strategy for prevention—ie, identify people at increased risk for an adverse health outcome (in this case, cardiovascular disease events) and offer them medication to reduce that risk.2
As part of the high-risk strategy, we also counsel at the individual level about lifestyle modifications—but unfortunately, we tend not to get very far. Given the substantial evidence demonstrating its benefits, a low-sodium DASH (Dietary Approaches to Stop Hypertension) eating plan is one of the lifestyle recommendations we make for our patients with hypertension.3,4 The DASH part of the diet involves getting our patients to eat more fruits, vegetables, and whole grains and limit sugar and saturated fats. To achieve the low-sodium part, we might counsel against added table salt, but mostly we discourage consumption of canned and other foods that are commercially processed, packaged, and prepared, because that’s the source of more than 70% of our sodium intake.5 It’s not difficult to understand why real-world uptake of the low-sodium DASH eating plan is low.6
This issue of The Journal of Family Practice features a PURL that supports a much more prominent role for salt substitutes in our counseling recommendations.7 Potassium-enriched salt substitutes not only lower blood pressure (BP) but also reduce the risk for cardiovascular events and death.8 They are widely available, and while more expensive per ounce than regular salt (sodium chloride), are still affordable.
Still, encouraging salt substitution with one patient at a time is relying on the high-risk strategy, with its inherently limited potential.2 An alternative is the population strategy. For hypertension, that would mean doing something for the entire population that would lead to a downward shift in the distribution of BP.2 The shift does not have to be large. We’ve known for more than 3 decades that just a 2–mm Hg reduction in the population’s average systolic BP would reduce stroke mortality by about 6%, coronary heart disease mortality by 4%, and total mortality by 3%.9 A 5–mm Hg reduction more than doubles those benefits. We are talking about tens of thousands fewer patients with heart disease and stroke each year and billions of dollars in health care cost savings.
Reducing our nation’s sodium intake, a quintessential population approach, has proven difficult. Our average daily sodium intake is about 3600 mg.10 Guidance on sodium reduction from the US Food and Drug Administration (targeted to industry) has aimed to reduce Americans’ average sodium intake to 3000 mg/d over the short term, fully acknowledging that the recommended sodium limit is 2300 mg/d.11 We’ve got a long way to go.
Might salt substitution at the population level be a way to simultaneously reduce our sodium intake and increase our potassium intake?12 The closest I found to a populationwide substitution study was a cluster randomized trial conducted in 6 villages in Peru.13 In a stepped-wedge design, households had 25% of their regular salt replaced with potassium salt. Small shops, bakeries, community kitchens, and food vendors also had salt replacement. The intention-to-treat analysis showed a small reduction in systolic BP (1.3 mm Hg) among those with hypertension at baseline (n = 428) and a 51% reduced incidence of developing hypertension among the other 1891 participants over the 4673 person-years of follow-up.
I found this study interesting and its results compelling, leading me to wonder: In the United States, where most of our sodium comes from the food industry, should we replace even a small amount of the sodium in processed foods with potassium? We’re not getting there with DASH alone.
The World Health Organization (WHO) recently released its inaugural report on the devastating global effects of hypertension, including recommendations for combatting this “silent killer.”1 Notable in the 276-page report is the emphasis on improving access to antihypertensive medications, in part through team-based care and simple evidence-based protocols. This strategy is not surprising given that in clinical medicine we focus on the “high-risk” strategy for prevention—ie, identify people at increased risk for an adverse health outcome (in this case, cardiovascular disease events) and offer them medication to reduce that risk.2
As part of the high-risk strategy, we also counsel at the individual level about lifestyle modifications—but unfortunately, we tend not to get very far. Given the substantial evidence demonstrating its benefits, a low-sodium DASH (Dietary Approaches to Stop Hypertension) eating plan is one of the lifestyle recommendations we make for our patients with hypertension.3,4 The DASH part of the diet involves getting our patients to eat more fruits, vegetables, and whole grains and limit sugar and saturated fats. To achieve the low-sodium part, we might counsel against added table salt, but mostly we discourage consumption of canned and other foods that are commercially processed, packaged, and prepared, because that’s the source of more than 70% of our sodium intake.5 It’s not difficult to understand why real-world uptake of the low-sodium DASH eating plan is low.6
This issue of The Journal of Family Practice features a PURL that supports a much more prominent role for salt substitutes in our counseling recommendations.7 Potassium-enriched salt substitutes not only lower blood pressure (BP) but also reduce the risk for cardiovascular events and death.8 They are widely available, and while more expensive per ounce than regular salt (sodium chloride), are still affordable.
Still, encouraging salt substitution with one patient at a time is relying on the high-risk strategy, with its inherently limited potential.2 An alternative is the population strategy. For hypertension, that would mean doing something for the entire population that would lead to a downward shift in the distribution of BP.2 The shift does not have to be large. We’ve known for more than 3 decades that just a 2–mm Hg reduction in the population’s average systolic BP would reduce stroke mortality by about 6%, coronary heart disease mortality by 4%, and total mortality by 3%.9 A 5–mm Hg reduction more than doubles those benefits. We are talking about tens of thousands fewer patients with heart disease and stroke each year and billions of dollars in health care cost savings.
Reducing our nation’s sodium intake, a quintessential population approach, has proven difficult. Our average daily sodium intake is about 3600 mg.10 Guidance on sodium reduction from the US Food and Drug Administration (targeted to industry) has aimed to reduce Americans’ average sodium intake to 3000 mg/d over the short term, fully acknowledging that the recommended sodium limit is 2300 mg/d.11 We’ve got a long way to go.
Might salt substitution at the population level be a way to simultaneously reduce our sodium intake and increase our potassium intake?12 The closest I found to a populationwide substitution study was a cluster randomized trial conducted in 6 villages in Peru.13 In a stepped-wedge design, households had 25% of their regular salt replaced with potassium salt. Small shops, bakeries, community kitchens, and food vendors also had salt replacement. The intention-to-treat analysis showed a small reduction in systolic BP (1.3 mm Hg) among those with hypertension at baseline (n = 428) and a 51% reduced incidence of developing hypertension among the other 1891 participants over the 4673 person-years of follow-up.
I found this study interesting and its results compelling, leading me to wonder: In the United States, where most of our sodium comes from the food industry, should we replace even a small amount of the sodium in processed foods with potassium? We’re not getting there with DASH alone.
1. World Health Organization. Global report on hypertension: the race against a silent killer. Published September 19, 2023. Accessed September 29, 2023. www.who.int/publications/i/item/9789240081062
2. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432. doi: 10.1093/ije/30.3.427
3. Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338
4. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253-1261. doi: 10.1016/j.numecd.2014.06.008
5. Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775-1783. doi: 10.1161/CIRCULATIONAHA.116.024446
6. Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308-314. doi: 10.1001/archinternmed.2007.119
7. Chang ET, Powell R, Reese T. Can potassium-enriched salt substitutes prevent complications of hypertension? J Fam Pract. 2023;72:342-344. doi: 10.12788/jfp.0667
8. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
9. Whelton PK, He J, Appel LJ, et al; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882-1888. doi: 10.1001/jama.288.15.1882
10. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209-1220. doi: 1001/jama.2018.1156
11. FDA. Guidance for industry: voluntary sodium reduction goals. Published October 2021. Accessed September 28, 2023. www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals
12. Nissaisorakarn V, Ormseth G, Earle W, et al. Less sodium, more potassium, or both: population-wide strategies to prevent hypertension. Am J Physiol Renal Physiol. 2023;325:F99-F104. doi: 10.1152/ajprenal.00007.202
13. Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374-378. doi: 10.1038/s41591-020-0754-2
1. World Health Organization. Global report on hypertension: the race against a silent killer. Published September 19, 2023. Accessed September 29, 2023. www.who.int/publications/i/item/9789240081062
2. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432. doi: 10.1093/ije/30.3.427
3. Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338
4. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, et al. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253-1261. doi: 10.1016/j.numecd.2014.06.008
5. Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775-1783. doi: 10.1161/CIRCULATIONAHA.116.024446
6. Mellen PB, Gao SK, Vitolins MZ, et al. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308-314. doi: 10.1001/archinternmed.2007.119
7. Chang ET, Powell R, Reese T. Can potassium-enriched salt substitutes prevent complications of hypertension? J Fam Pract. 2023;72:342-344. doi: 10.12788/jfp.0667
8. Yin X, Rodgers A, Perkovic A, et al. Effects of salt substitutes on clinical outcomes: a systematic review and meta-analysis. Heart. 2022;108:1608-1615. doi: 10.1136/heartjnl-2022-321332
9. Whelton PK, He J, Appel LJ, et al; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882-1888. doi: 10.1001/jama.288.15.1882
10. Cogswell ME, Loria CM, Terry AL, et al. Estimated 24-Hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209-1220. doi: 1001/jama.2018.1156
11. FDA. Guidance for industry: voluntary sodium reduction goals. Published October 2021. Accessed September 28, 2023. www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-voluntary-sodium-reduction-goals
12. Nissaisorakarn V, Ormseth G, Earle W, et al. Less sodium, more potassium, or both: population-wide strategies to prevent hypertension. Am J Physiol Renal Physiol. 2023;325:F99-F104. doi: 10.1152/ajprenal.00007.202
13. Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, et al. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med. 2020;26:374-378. doi: 10.1038/s41591-020-0754-2
52-year-old man • intermittent fevers • recently received second dose of COVID-19 vaccine • tremors in all 4 extremities • Dx?
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; Leara@ccf.org
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; Leara@ccf.org
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; Leara@ccf.org
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
► Intermittent fevers
► Recently received second dose of COVID-19 vaccine
► Tremors in all 4 extremities
ACIP updates recommendations for influenza vaccination
When the Advisory Committee on Immunization Practices (ACIP) met in June and adopted recommendations for influenza vaccines for the 2023-2024 season, the major discussions focused on the timing of vaccine administration, the composition of the vaccine, and what (if any) special precautions are needed when administering an egg-based vaccine to a person with a history of egg allergy. Here are the takeaways.
When should flu vaccine be administered?
Influenza activity usually peaks between December and the end of March; only twice between 1982 and 2022 did it peak before December. Thus, most people should receive the vaccine in September or October, a recommendation that has not changed from last year. This is early enough to provide adequate protection in most influenza seasons, but late enough to allow protection to persist through the entire season. Vaccination should continue to be offered to those who are unvaccinated throughout the influenza season, as long as influenza viruses are circulating.
Earlier administration is not recommended for most people and is recommended against for those ages 65 years and older (because their immunity from the vaccine may wane faster) and for pregnant people in their first or second trimester (because the vaccine is more effective in preventing influenza in newborns if administered in the third trimester). Evidence regarding waning immunity is inconsistent; however, some studies have shown greater loss of immunity in the elderly compared to younger age groups, as time from vaccination increases.1
What’s in this year’s vaccines?
The composition of the vaccines used in North America was determined by the World Health Organization in February, based on the most commonly circulating strains. All vaccines approved for use in the 2023-2024 season are quadrivalent and contain 1 influenza A (H1N1) strain, 1 influenza A (H3N2) strain, and 2 influenza B strains. The specifics of each strain are listed in TABLE 1.2 The 2 influenza A strains are slightly different for the egg-based and non-egg-based vaccines.2 There is no known effectiveness advantage of one antigen strain vs the other.

Should you take special precautions with egg allergy?
There is new wording to the recommendations on the use of egg-based influenza vaccines for those with a history of egg allergy (TABLE 22). Previously, the ACIP had recommended that if an egg-based vaccine is given to a person with a history of egg allergy, it should be administered in an inpatient or outpatient medical setting (eg, hospital, clinic, health department, physician office) and should be supervised by a health care provider who is able to recognize and manage severe allergic reactions. These added precautions were out of step with other organizations, including the American Academy of Pediatrics and allergy-related specialty societies, all of whom recommend no special procedures or precautions when administering any influenza vaccine to those with a history of egg allergy.3

Why the change? Several factors contributed to ACIP’s decision to reword its recommendation. One is that the ovalbumin content of all current influenza vaccines (TABLE 33) is considered too low to trigger an allergic reaction.
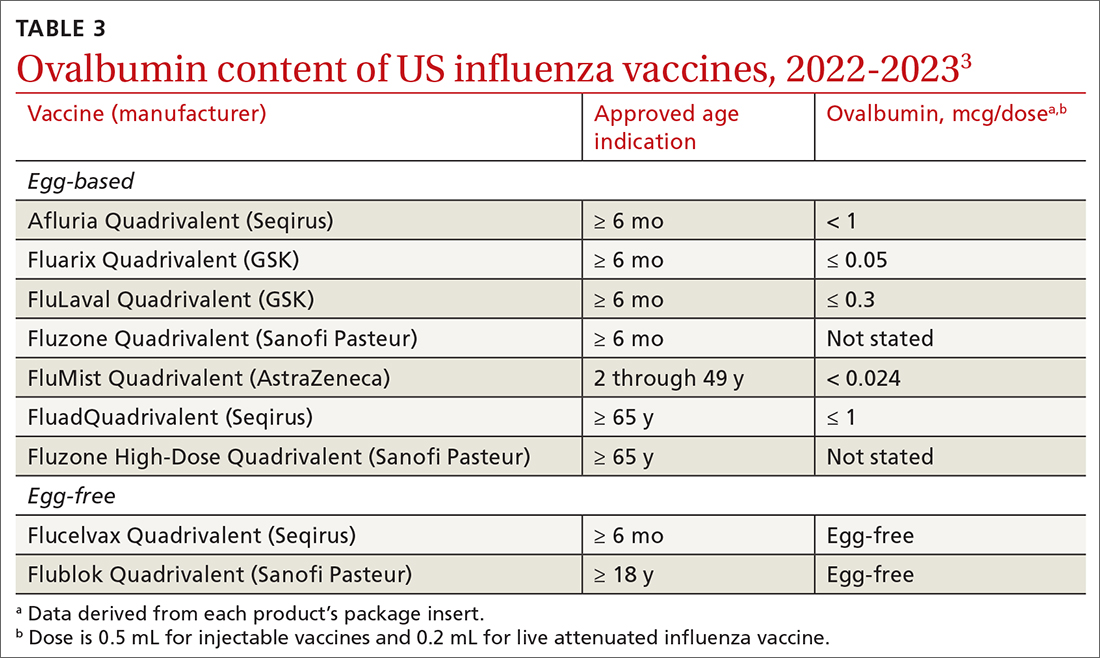
Another is the paucity of evidence that egg-based vaccines convey increased risk beyond that for any other vaccine. Although 1% to 3% of children are reported to have an egg allergy, there is no evidence that they are at increased risk for a serious allergic reaction if administered an egg-based vaccine.3 A systematic review of 31 studies (mostly low-quality observational studies and case series) conducted by the ACIP Influenza Work Group found no risk for severe anaphylaxis, hospitalization, or death, even in those with a history of an anaphylactic reaction to eggs.2 A review of Vaccine Adverse Events Reporting System (VAERS) data identified 18 cases of reported anaphylaxis after receipt of an inactivated influenza vaccine over a 5-year period, but clinical review confirmed only 7.2
Continue to: And finally, appropriate precautions already...
And finally, appropriate precautions already are recommended for administration of any vaccine. The CDC guidance for best practices for administering vaccines states: “Although allergic reactions are a common concern for vaccine providers, these reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines. Epinephrine and equipment for managing an airway should be available for immediate use.”4
What does this mean in practice? Family physicians who administer influenza vaccines do not need to use special precautions for any influenza vaccine, or use non-egg-based vaccines, for those who have a history of egg allergy. However, they should be prepared to respond to a severe allergic reaction just as they would for any other vaccine. Any vestigial practices pertaining to egg allergy and influenza vaccines—such as vaccine skin testing prior to vaccination (with dilution of vaccine if positive), vaccination deferral or administration via alternative dosing protocols, and split dosing of vaccine—are unnecessary and should be abandoned.
1. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71:1-28. doi: 10.15585/mmwr.rr7101a1
2. Grohskopf LA. Influenza vaccine safety update and proposed recommendations for the 2023-24 influenza season. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-influenza-grohskopf-508.pdf
3. Blanton LH, Grohskopf LA. Influenza vaccination of person with egg allergy: evidence to recommendations discussion and work group considerations. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-influenza-grohskopf-508.pdf
4. Kroger AT, Bahta L, Long S, et al. General best practice guidelines for immunization. Updated August 1, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
When the Advisory Committee on Immunization Practices (ACIP) met in June and adopted recommendations for influenza vaccines for the 2023-2024 season, the major discussions focused on the timing of vaccine administration, the composition of the vaccine, and what (if any) special precautions are needed when administering an egg-based vaccine to a person with a history of egg allergy. Here are the takeaways.
When should flu vaccine be administered?
Influenza activity usually peaks between December and the end of March; only twice between 1982 and 2022 did it peak before December. Thus, most people should receive the vaccine in September or October, a recommendation that has not changed from last year. This is early enough to provide adequate protection in most influenza seasons, but late enough to allow protection to persist through the entire season. Vaccination should continue to be offered to those who are unvaccinated throughout the influenza season, as long as influenza viruses are circulating.
Earlier administration is not recommended for most people and is recommended against for those ages 65 years and older (because their immunity from the vaccine may wane faster) and for pregnant people in their first or second trimester (because the vaccine is more effective in preventing influenza in newborns if administered in the third trimester). Evidence regarding waning immunity is inconsistent; however, some studies have shown greater loss of immunity in the elderly compared to younger age groups, as time from vaccination increases.1
What’s in this year’s vaccines?
The composition of the vaccines used in North America was determined by the World Health Organization in February, based on the most commonly circulating strains. All vaccines approved for use in the 2023-2024 season are quadrivalent and contain 1 influenza A (H1N1) strain, 1 influenza A (H3N2) strain, and 2 influenza B strains. The specifics of each strain are listed in TABLE 1.2 The 2 influenza A strains are slightly different for the egg-based and non-egg-based vaccines.2 There is no known effectiveness advantage of one antigen strain vs the other.

Should you take special precautions with egg allergy?
There is new wording to the recommendations on the use of egg-based influenza vaccines for those with a history of egg allergy (TABLE 22). Previously, the ACIP had recommended that if an egg-based vaccine is given to a person with a history of egg allergy, it should be administered in an inpatient or outpatient medical setting (eg, hospital, clinic, health department, physician office) and should be supervised by a health care provider who is able to recognize and manage severe allergic reactions. These added precautions were out of step with other organizations, including the American Academy of Pediatrics and allergy-related specialty societies, all of whom recommend no special procedures or precautions when administering any influenza vaccine to those with a history of egg allergy.3

Why the change? Several factors contributed to ACIP’s decision to reword its recommendation. One is that the ovalbumin content of all current influenza vaccines (TABLE 33) is considered too low to trigger an allergic reaction.

Another is the paucity of evidence that egg-based vaccines convey increased risk beyond that for any other vaccine. Although 1% to 3% of children are reported to have an egg allergy, there is no evidence that they are at increased risk for a serious allergic reaction if administered an egg-based vaccine.3 A systematic review of 31 studies (mostly low-quality observational studies and case series) conducted by the ACIP Influenza Work Group found no risk for severe anaphylaxis, hospitalization, or death, even in those with a history of an anaphylactic reaction to eggs.2 A review of Vaccine Adverse Events Reporting System (VAERS) data identified 18 cases of reported anaphylaxis after receipt of an inactivated influenza vaccine over a 5-year period, but clinical review confirmed only 7.2
Continue to: And finally, appropriate precautions already...
And finally, appropriate precautions already are recommended for administration of any vaccine. The CDC guidance for best practices for administering vaccines states: “Although allergic reactions are a common concern for vaccine providers, these reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines. Epinephrine and equipment for managing an airway should be available for immediate use.”4
What does this mean in practice? Family physicians who administer influenza vaccines do not need to use special precautions for any influenza vaccine, or use non-egg-based vaccines, for those who have a history of egg allergy. However, they should be prepared to respond to a severe allergic reaction just as they would for any other vaccine. Any vestigial practices pertaining to egg allergy and influenza vaccines—such as vaccine skin testing prior to vaccination (with dilution of vaccine if positive), vaccination deferral or administration via alternative dosing protocols, and split dosing of vaccine—are unnecessary and should be abandoned.
When the Advisory Committee on Immunization Practices (ACIP) met in June and adopted recommendations for influenza vaccines for the 2023-2024 season, the major discussions focused on the timing of vaccine administration, the composition of the vaccine, and what (if any) special precautions are needed when administering an egg-based vaccine to a person with a history of egg allergy. Here are the takeaways.
When should flu vaccine be administered?
Influenza activity usually peaks between December and the end of March; only twice between 1982 and 2022 did it peak before December. Thus, most people should receive the vaccine in September or October, a recommendation that has not changed from last year. This is early enough to provide adequate protection in most influenza seasons, but late enough to allow protection to persist through the entire season. Vaccination should continue to be offered to those who are unvaccinated throughout the influenza season, as long as influenza viruses are circulating.
Earlier administration is not recommended for most people and is recommended against for those ages 65 years and older (because their immunity from the vaccine may wane faster) and for pregnant people in their first or second trimester (because the vaccine is more effective in preventing influenza in newborns if administered in the third trimester). Evidence regarding waning immunity is inconsistent; however, some studies have shown greater loss of immunity in the elderly compared to younger age groups, as time from vaccination increases.1
What’s in this year’s vaccines?
The composition of the vaccines used in North America was determined by the World Health Organization in February, based on the most commonly circulating strains. All vaccines approved for use in the 2023-2024 season are quadrivalent and contain 1 influenza A (H1N1) strain, 1 influenza A (H3N2) strain, and 2 influenza B strains. The specifics of each strain are listed in TABLE 1.2 The 2 influenza A strains are slightly different for the egg-based and non-egg-based vaccines.2 There is no known effectiveness advantage of one antigen strain vs the other.

Should you take special precautions with egg allergy?
There is new wording to the recommendations on the use of egg-based influenza vaccines for those with a history of egg allergy (TABLE 22). Previously, the ACIP had recommended that if an egg-based vaccine is given to a person with a history of egg allergy, it should be administered in an inpatient or outpatient medical setting (eg, hospital, clinic, health department, physician office) and should be supervised by a health care provider who is able to recognize and manage severe allergic reactions. These added precautions were out of step with other organizations, including the American Academy of Pediatrics and allergy-related specialty societies, all of whom recommend no special procedures or precautions when administering any influenza vaccine to those with a history of egg allergy.3

Why the change? Several factors contributed to ACIP’s decision to reword its recommendation. One is that the ovalbumin content of all current influenza vaccines (TABLE 33) is considered too low to trigger an allergic reaction.

Another is the paucity of evidence that egg-based vaccines convey increased risk beyond that for any other vaccine. Although 1% to 3% of children are reported to have an egg allergy, there is no evidence that they are at increased risk for a serious allergic reaction if administered an egg-based vaccine.3 A systematic review of 31 studies (mostly low-quality observational studies and case series) conducted by the ACIP Influenza Work Group found no risk for severe anaphylaxis, hospitalization, or death, even in those with a history of an anaphylactic reaction to eggs.2 A review of Vaccine Adverse Events Reporting System (VAERS) data identified 18 cases of reported anaphylaxis after receipt of an inactivated influenza vaccine over a 5-year period, but clinical review confirmed only 7.2
Continue to: And finally, appropriate precautions already...
And finally, appropriate precautions already are recommended for administration of any vaccine. The CDC guidance for best practices for administering vaccines states: “Although allergic reactions are a common concern for vaccine providers, these reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines. Epinephrine and equipment for managing an airway should be available for immediate use.”4
What does this mean in practice? Family physicians who administer influenza vaccines do not need to use special precautions for any influenza vaccine, or use non-egg-based vaccines, for those who have a history of egg allergy. However, they should be prepared to respond to a severe allergic reaction just as they would for any other vaccine. Any vestigial practices pertaining to egg allergy and influenza vaccines—such as vaccine skin testing prior to vaccination (with dilution of vaccine if positive), vaccination deferral or administration via alternative dosing protocols, and split dosing of vaccine—are unnecessary and should be abandoned.
1. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71:1-28. doi: 10.15585/mmwr.rr7101a1
2. Grohskopf LA. Influenza vaccine safety update and proposed recommendations for the 2023-24 influenza season. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-influenza-grohskopf-508.pdf
3. Blanton LH, Grohskopf LA. Influenza vaccination of person with egg allergy: evidence to recommendations discussion and work group considerations. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-influenza-grohskopf-508.pdf
4. Kroger AT, Bahta L, Long S, et al. General best practice guidelines for immunization. Updated August 1, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
1. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71:1-28. doi: 10.15585/mmwr.rr7101a1
2. Grohskopf LA. Influenza vaccine safety update and proposed recommendations for the 2023-24 influenza season. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/03-influenza-grohskopf-508.pdf
3. Blanton LH, Grohskopf LA. Influenza vaccination of person with egg allergy: evidence to recommendations discussion and work group considerations. Presented to the ACIP on June 21, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-influenza-grohskopf-508.pdf
4. Kroger AT, Bahta L, Long S, et al. General best practice guidelines for immunization. Updated August 1, 2023. Accessed September 20, 2023. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
Inadequate sleep & obesity: Breaking the vicious cycle
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45
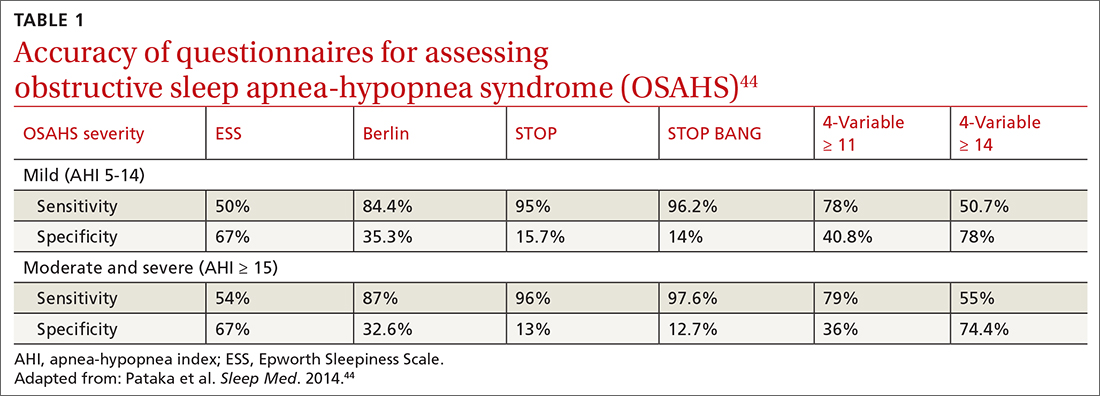
Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene(TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52
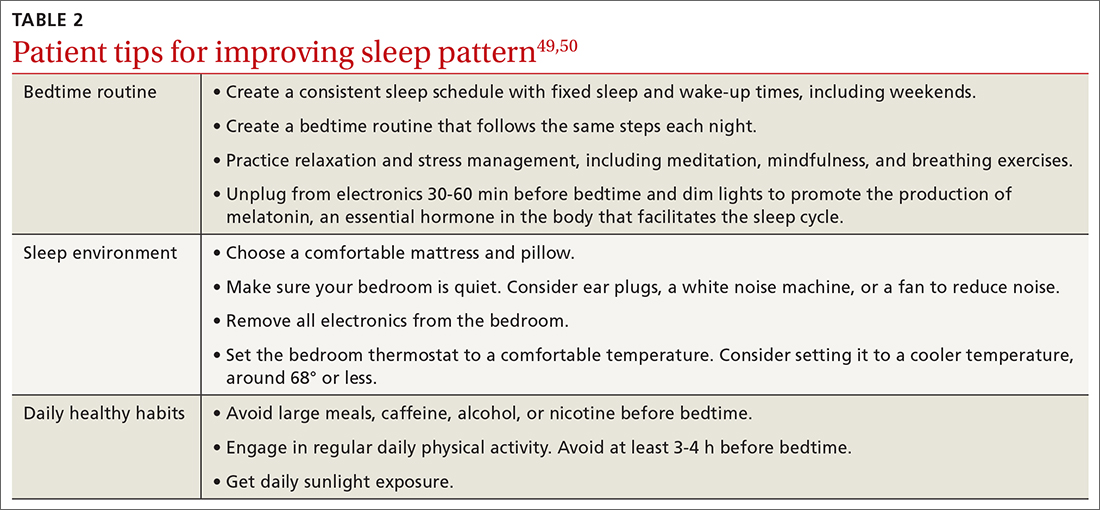
Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; ejaqua@llu.edu
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene(TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; ejaqua@llu.edu
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene(TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; ejaqua@llu.edu
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
PRACTICE RECOMMENDATIONS
› Consider cognitive behaviorial therapy for insomnia (CBT-I) first-line treatment for insomnia. A
› Carefully review patients’ medication lists, as many pharmaceuticals can affect weight and sleep. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Nonhealing Ulcer in a Patient With Crohn Disease
The Diagnosis: Mycobacterium abscessus Infection
Upon further testing, cultures were positive for Mycobacterium abscessus. Our patient was referred to infectious disease for co-management, and his treatment plan consisted of intravenous amikacin 885 mg 3 times weekly, intravenous imipenem 1 g twice daily, azithromycin 500 mg/d, and omadacycline 150 mg/d for at least 3 months. Magnetic resonance imaging findings were consistent with a combination of cellulitis and osteomyelitis, and our patient was referred to plastic surgery for debridement. He subsequently was lost to follow-up.
Mycobacterium abscessus is classified as both a nontuberculous and rapidly growing mycobacterium. Mycobacterium abscessus recently has emerged as a pathogen of increasing public health concern, especially due to its high rate of antibiotic resistance.1-5 It is highly prevalent in the environment, and infection has been reported from a wide variety of environmental sources.6-8 Immunocompromised individuals, such as our patient, undergoing anti–tumor necrosis factor therapy are at increased risk for infection from all Mycobacterium species.9-11 Recognizing these infections quickly is a priority for patient care, as M abscessus can lead to disseminated infection and high mortality rates.1
Histopathology of M abscessus consists of granulomatous inflammation with mixed granulomas12; however, these findings are not always appreciable, and staining does not always reveal visible organisms. In our patient, histopathology revealed patchy plasmalymphocytic infiltrates of the dermis and subcutaneous tissue, which are signs of generalized inflammation (Figure). Therefore, cultures positive for M abscessus are the gold standard for diagnosis and established the diagnosis in this case.

The differential diagnoses for our patient’s ulceration included squamous cell carcinoma, pyoderma gangrenosum, aseptic abscess ulcer, and pyodermatitispyostomatitis vegetans. Immunosuppressive therapy is a risk factor for squamous cell carcinoma13,14; however, ulcerated squamous cell carcinoma typically presents with prominent everted edges with a necrotic tumor base.15 Biopsy reveals cells with abundant eosinophilic cytoplasm, large nuclei, and variable keratin pearls.16 Pyoderma gangrenosum is an inflammatory skin condition associated with Crohn disease and often is a diagnosis of exclusion characterized by neutrophilic infiltrates on biopsy.17-19 Aseptic abscess ulcers are characterized by neutrophil-filled lesions that respond to corticosteroids but not antibiotics.20 Pyodermatitis-pyostomatitis vegetans is a rare skin manifestation of inflammatory bowel disease associated with a pustular eruption of the skin and/or mouth. Histopathology reveals pustules within or below the epidermis with many eosinophils or neutrophils. Granulomas do not occur as in M abscessus.21
Treatment of M abscessus infection requires the coadministration of several antibiotics across multiple classes to ensure complete disease resolution. High rates of antibiotic resistance are characterized by at least partial resistance to almost every antibiotic; clarithromycin has near-complete efficacy, but resistant strains have started to emerge. Amikacin and cefoxitin are other antibiotics that have reported a resistance rate of less than 50%, but they are only effective 90% and 70% of the time, respectively.1,22 The antibiotic omadacycline, which is approved by the US Food and Drug Administration to treat acute bacterial skin and soft-tissue infections, also may have utility in treating M abscessus infections.23,24 Finally, phage therapy may offer a potential mode of treatment for this bacterium and was used to treat pulmonary infection in a patient with cystic fibrosis.25 Despite these newer innovations, the current standard of care involves clarithromycin or azithromycin in combination with a parenteral antibiotic such as cefoxitin, amikacin, or imipenem for at least 4 months.1
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Strnad L, Winthrop KL. Treatment of Mycobacterium abscessus complex. Semin Respir Crit Care Med. 2018;39:362-376.
- Cardenas DD, Yasmin T, Ahmed S. A rare insidious case of skin and soft tissue infection due to Mycobacterium abscessus: a case report. Cureus. 2022;14:E25725.
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-577.
- Dickison P, Howard V, O’Kane G, et al. Mycobacterium abscessus infection following penetrations through wetsuits. Australas J Dermatol. 2019;60:57-59.
- Choi H, Kim YI, Na CH, et al. Mycobacterium abscessus skin infection associated with shaving activity in a 75-year-old man. Ann Geriatr Med Res. 2018;22:204.
- Costa-Silva M, Cesar A, Gomes NP, et al. Mycobacterium abscessus infection in a spa worker. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:159-161.
- Besada E. Rapid growing mycobacteria and TNF-α blockers: case report of a fatal lung infection with Mycobacterium abscessus. Clin Exp Rheumatol. 2011;29:705-707.
- Mufti AH, Toye BW, Mckendry RR, et al. Mycobacterium abscessus infection after use of tumor necrosis factor α inhibitor therapy: case report and review of infectious complications associated with tumor necrosis factor α inhibitor use. Diagn Microbiol Infect Dis. 2005;53:233-238.
- Lee SK, Kim SY, Kim EY, et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung. 2013;191:565-571.
- Rodríguez G, Ortegón M, Camargo D, et al. Iatrogenic Mycobacterium abscessus infection: histopathology of 71 patients. Br J Dermatol. 1997;137:214-218.
- Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161-168.
- Walker HS, Hardwicke J. Non-melanoma skin cancer. Surgery (Oxford). 2022;40:39-45.
- Browse NL. The skin. In: Browse NL, ed. An Introduction to the Symptoms and Signs of Surgical Disease. 3rd ed. London Arnold Publications; 2001:66-69.
- Weedon D. Squamous cell carcinoma. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010;691-700.
- Powell F, Schroeter A, Su W, et al. Pyoderma gangrenosum: a review of 86 patients. QJM Int J Med. 1985;55:173-186.
- Brunsting LA, Goeckerman WH, O’Leary PA. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occurring in adults. Arch Dermatol. 1982;118:743-768.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86:145. doi:10.1097/md.0b013e18064f9f3
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36:67-78.
- Duah M, Beshay M. Omadacycline in first-line combination therapy for pulmonary Mycobacterium abscessus infection: a case series. Int J Infect Dis. 2022;122:953-956.
- Minhas R, Sharma S, Kundu S. Utilizing the promise of omadacycline in a resistant, non-tubercular mycobacterial pulmonary infection. Cureus. 2019;11:E5112.
- Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25:730-733.
The Diagnosis: Mycobacterium abscessus Infection
Upon further testing, cultures were positive for Mycobacterium abscessus. Our patient was referred to infectious disease for co-management, and his treatment plan consisted of intravenous amikacin 885 mg 3 times weekly, intravenous imipenem 1 g twice daily, azithromycin 500 mg/d, and omadacycline 150 mg/d for at least 3 months. Magnetic resonance imaging findings were consistent with a combination of cellulitis and osteomyelitis, and our patient was referred to plastic surgery for debridement. He subsequently was lost to follow-up.
Mycobacterium abscessus is classified as both a nontuberculous and rapidly growing mycobacterium. Mycobacterium abscessus recently has emerged as a pathogen of increasing public health concern, especially due to its high rate of antibiotic resistance.1-5 It is highly prevalent in the environment, and infection has been reported from a wide variety of environmental sources.6-8 Immunocompromised individuals, such as our patient, undergoing anti–tumor necrosis factor therapy are at increased risk for infection from all Mycobacterium species.9-11 Recognizing these infections quickly is a priority for patient care, as M abscessus can lead to disseminated infection and high mortality rates.1
Histopathology of M abscessus consists of granulomatous inflammation with mixed granulomas12; however, these findings are not always appreciable, and staining does not always reveal visible organisms. In our patient, histopathology revealed patchy plasmalymphocytic infiltrates of the dermis and subcutaneous tissue, which are signs of generalized inflammation (Figure). Therefore, cultures positive for M abscessus are the gold standard for diagnosis and established the diagnosis in this case.

The differential diagnoses for our patient’s ulceration included squamous cell carcinoma, pyoderma gangrenosum, aseptic abscess ulcer, and pyodermatitispyostomatitis vegetans. Immunosuppressive therapy is a risk factor for squamous cell carcinoma13,14; however, ulcerated squamous cell carcinoma typically presents with prominent everted edges with a necrotic tumor base.15 Biopsy reveals cells with abundant eosinophilic cytoplasm, large nuclei, and variable keratin pearls.16 Pyoderma gangrenosum is an inflammatory skin condition associated with Crohn disease and often is a diagnosis of exclusion characterized by neutrophilic infiltrates on biopsy.17-19 Aseptic abscess ulcers are characterized by neutrophil-filled lesions that respond to corticosteroids but not antibiotics.20 Pyodermatitis-pyostomatitis vegetans is a rare skin manifestation of inflammatory bowel disease associated with a pustular eruption of the skin and/or mouth. Histopathology reveals pustules within or below the epidermis with many eosinophils or neutrophils. Granulomas do not occur as in M abscessus.21
Treatment of M abscessus infection requires the coadministration of several antibiotics across multiple classes to ensure complete disease resolution. High rates of antibiotic resistance are characterized by at least partial resistance to almost every antibiotic; clarithromycin has near-complete efficacy, but resistant strains have started to emerge. Amikacin and cefoxitin are other antibiotics that have reported a resistance rate of less than 50%, but they are only effective 90% and 70% of the time, respectively.1,22 The antibiotic omadacycline, which is approved by the US Food and Drug Administration to treat acute bacterial skin and soft-tissue infections, also may have utility in treating M abscessus infections.23,24 Finally, phage therapy may offer a potential mode of treatment for this bacterium and was used to treat pulmonary infection in a patient with cystic fibrosis.25 Despite these newer innovations, the current standard of care involves clarithromycin or azithromycin in combination with a parenteral antibiotic such as cefoxitin, amikacin, or imipenem for at least 4 months.1
The Diagnosis: Mycobacterium abscessus Infection
Upon further testing, cultures were positive for Mycobacterium abscessus. Our patient was referred to infectious disease for co-management, and his treatment plan consisted of intravenous amikacin 885 mg 3 times weekly, intravenous imipenem 1 g twice daily, azithromycin 500 mg/d, and omadacycline 150 mg/d for at least 3 months. Magnetic resonance imaging findings were consistent with a combination of cellulitis and osteomyelitis, and our patient was referred to plastic surgery for debridement. He subsequently was lost to follow-up.
Mycobacterium abscessus is classified as both a nontuberculous and rapidly growing mycobacterium. Mycobacterium abscessus recently has emerged as a pathogen of increasing public health concern, especially due to its high rate of antibiotic resistance.1-5 It is highly prevalent in the environment, and infection has been reported from a wide variety of environmental sources.6-8 Immunocompromised individuals, such as our patient, undergoing anti–tumor necrosis factor therapy are at increased risk for infection from all Mycobacterium species.9-11 Recognizing these infections quickly is a priority for patient care, as M abscessus can lead to disseminated infection and high mortality rates.1
Histopathology of M abscessus consists of granulomatous inflammation with mixed granulomas12; however, these findings are not always appreciable, and staining does not always reveal visible organisms. In our patient, histopathology revealed patchy plasmalymphocytic infiltrates of the dermis and subcutaneous tissue, which are signs of generalized inflammation (Figure). Therefore, cultures positive for M abscessus are the gold standard for diagnosis and established the diagnosis in this case.

The differential diagnoses for our patient’s ulceration included squamous cell carcinoma, pyoderma gangrenosum, aseptic abscess ulcer, and pyodermatitispyostomatitis vegetans. Immunosuppressive therapy is a risk factor for squamous cell carcinoma13,14; however, ulcerated squamous cell carcinoma typically presents with prominent everted edges with a necrotic tumor base.15 Biopsy reveals cells with abundant eosinophilic cytoplasm, large nuclei, and variable keratin pearls.16 Pyoderma gangrenosum is an inflammatory skin condition associated with Crohn disease and often is a diagnosis of exclusion characterized by neutrophilic infiltrates on biopsy.17-19 Aseptic abscess ulcers are characterized by neutrophil-filled lesions that respond to corticosteroids but not antibiotics.20 Pyodermatitis-pyostomatitis vegetans is a rare skin manifestation of inflammatory bowel disease associated with a pustular eruption of the skin and/or mouth. Histopathology reveals pustules within or below the epidermis with many eosinophils or neutrophils. Granulomas do not occur as in M abscessus.21
Treatment of M abscessus infection requires the coadministration of several antibiotics across multiple classes to ensure complete disease resolution. High rates of antibiotic resistance are characterized by at least partial resistance to almost every antibiotic; clarithromycin has near-complete efficacy, but resistant strains have started to emerge. Amikacin and cefoxitin are other antibiotics that have reported a resistance rate of less than 50%, but they are only effective 90% and 70% of the time, respectively.1,22 The antibiotic omadacycline, which is approved by the US Food and Drug Administration to treat acute bacterial skin and soft-tissue infections, also may have utility in treating M abscessus infections.23,24 Finally, phage therapy may offer a potential mode of treatment for this bacterium and was used to treat pulmonary infection in a patient with cystic fibrosis.25 Despite these newer innovations, the current standard of care involves clarithromycin or azithromycin in combination with a parenteral antibiotic such as cefoxitin, amikacin, or imipenem for at least 4 months.1
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Strnad L, Winthrop KL. Treatment of Mycobacterium abscessus complex. Semin Respir Crit Care Med. 2018;39:362-376.
- Cardenas DD, Yasmin T, Ahmed S. A rare insidious case of skin and soft tissue infection due to Mycobacterium abscessus: a case report. Cureus. 2022;14:E25725.
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-577.
- Dickison P, Howard V, O’Kane G, et al. Mycobacterium abscessus infection following penetrations through wetsuits. Australas J Dermatol. 2019;60:57-59.
- Choi H, Kim YI, Na CH, et al. Mycobacterium abscessus skin infection associated with shaving activity in a 75-year-old man. Ann Geriatr Med Res. 2018;22:204.
- Costa-Silva M, Cesar A, Gomes NP, et al. Mycobacterium abscessus infection in a spa worker. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:159-161.
- Besada E. Rapid growing mycobacteria and TNF-α blockers: case report of a fatal lung infection with Mycobacterium abscessus. Clin Exp Rheumatol. 2011;29:705-707.
- Mufti AH, Toye BW, Mckendry RR, et al. Mycobacterium abscessus infection after use of tumor necrosis factor α inhibitor therapy: case report and review of infectious complications associated with tumor necrosis factor α inhibitor use. Diagn Microbiol Infect Dis. 2005;53:233-238.
- Lee SK, Kim SY, Kim EY, et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung. 2013;191:565-571.
- Rodríguez G, Ortegón M, Camargo D, et al. Iatrogenic Mycobacterium abscessus infection: histopathology of 71 patients. Br J Dermatol. 1997;137:214-218.
- Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161-168.
- Walker HS, Hardwicke J. Non-melanoma skin cancer. Surgery (Oxford). 2022;40:39-45.
- Browse NL. The skin. In: Browse NL, ed. An Introduction to the Symptoms and Signs of Surgical Disease. 3rd ed. London Arnold Publications; 2001:66-69.
- Weedon D. Squamous cell carcinoma. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010;691-700.
- Powell F, Schroeter A, Su W, et al. Pyoderma gangrenosum: a review of 86 patients. QJM Int J Med. 1985;55:173-186.
- Brunsting LA, Goeckerman WH, O’Leary PA. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occurring in adults. Arch Dermatol. 1982;118:743-768.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86:145. doi:10.1097/md.0b013e18064f9f3
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36:67-78.
- Duah M, Beshay M. Omadacycline in first-line combination therapy for pulmonary Mycobacterium abscessus infection: a case series. Int J Infect Dis. 2022;122:953-956.
- Minhas R, Sharma S, Kundu S. Utilizing the promise of omadacycline in a resistant, non-tubercular mycobacterial pulmonary infection. Cureus. 2019;11:E5112.
- Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25:730-733.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Strnad L, Winthrop KL. Treatment of Mycobacterium abscessus complex. Semin Respir Crit Care Med. 2018;39:362-376.
- Cardenas DD, Yasmin T, Ahmed S. A rare insidious case of skin and soft tissue infection due to Mycobacterium abscessus: a case report. Cureus. 2022;14:E25725.
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-577.
- Dickison P, Howard V, O’Kane G, et al. Mycobacterium abscessus infection following penetrations through wetsuits. Australas J Dermatol. 2019;60:57-59.
- Choi H, Kim YI, Na CH, et al. Mycobacterium abscessus skin infection associated with shaving activity in a 75-year-old man. Ann Geriatr Med Res. 2018;22:204.
- Costa-Silva M, Cesar A, Gomes NP, et al. Mycobacterium abscessus infection in a spa worker. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:159-161.
- Besada E. Rapid growing mycobacteria and TNF-α blockers: case report of a fatal lung infection with Mycobacterium abscessus. Clin Exp Rheumatol. 2011;29:705-707.
- Mufti AH, Toye BW, Mckendry RR, et al. Mycobacterium abscessus infection after use of tumor necrosis factor α inhibitor therapy: case report and review of infectious complications associated with tumor necrosis factor α inhibitor use. Diagn Microbiol Infect Dis. 2005;53:233-238.
- Lee SK, Kim SY, Kim EY, et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung. 2013;191:565-571.
- Rodríguez G, Ortegón M, Camargo D, et al. Iatrogenic Mycobacterium abscessus infection: histopathology of 71 patients. Br J Dermatol. 1997;137:214-218.
- Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161-168.
- Walker HS, Hardwicke J. Non-melanoma skin cancer. Surgery (Oxford). 2022;40:39-45.
- Browse NL. The skin. In: Browse NL, ed. An Introduction to the Symptoms and Signs of Surgical Disease. 3rd ed. London Arnold Publications; 2001:66-69.
- Weedon D. Squamous cell carcinoma. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010;691-700.
- Powell F, Schroeter A, Su W, et al. Pyoderma gangrenosum: a review of 86 patients. QJM Int J Med. 1985;55:173-186.
- Brunsting LA, Goeckerman WH, O’Leary PA. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occurring in adults. Arch Dermatol. 1982;118:743-768.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86:145. doi:10.1097/md.0b013e18064f9f3
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36:67-78.
- Duah M, Beshay M. Omadacycline in first-line combination therapy for pulmonary Mycobacterium abscessus infection: a case series. Int J Infect Dis. 2022;122:953-956.
- Minhas R, Sharma S, Kundu S. Utilizing the promise of omadacycline in a resistant, non-tubercular mycobacterial pulmonary infection. Cureus. 2019;11:E5112.
- Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25:730-733.
A 24-year-old man presented to our dermatology clinic with a painful lesion on the right buccal cheek of 4 months’ duration that had not changed in size or appearance. He had a history of Crohn disease that was being treated with 6-mercaptopurine and infliximab. He underwent jaw surgery 7 years prior for correction of an underbite, followed by subsequent surgery to remove the hardware 1 year after the initial procedure. He experienced recurring skin abscesses following the initial jaw surgery roughly once a year that were treated with bedside incision and drainage procedures in the emergency department followed by trimethoprim-sulfamethoxazole with complete resolution; however, treatment with mupirocin ointment 2%, trimethoprim-sulfamethoxazole, and azithromycin did not provide symptomatic relief or resolution for the current lesion. Physical examination revealed a 4-cm ulceration with actively draining serosanguineous discharge. Two punch biopsies were performed; 48-hour bacterial and fungal cultures, as well as Giemsa, acid-fast bacilli, and periodic acid–Schiff staining were negative.
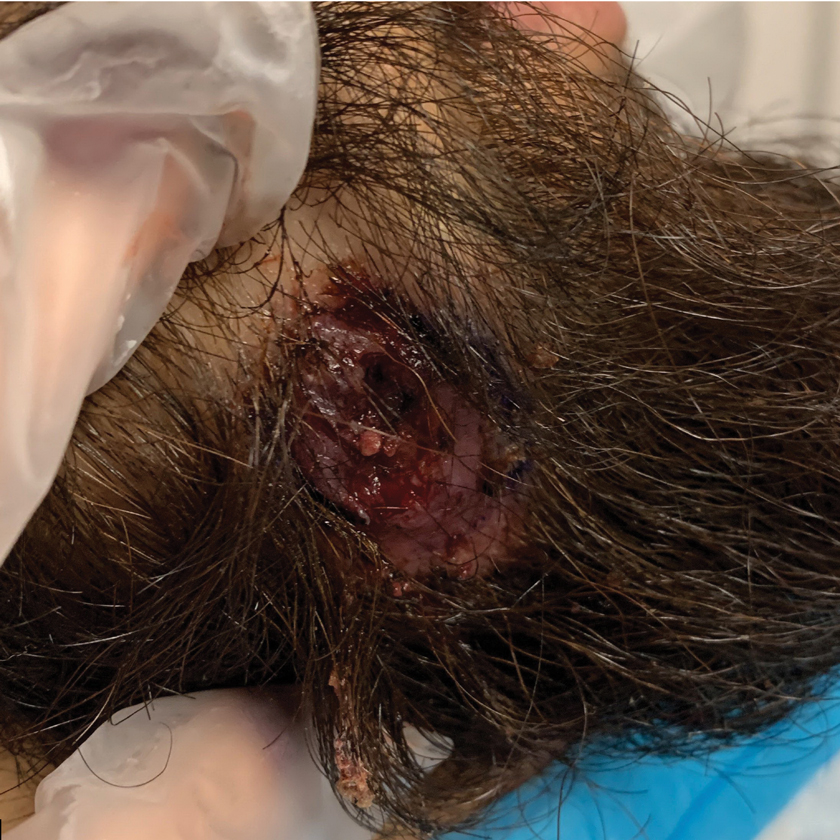
Management of Metastatic Triple-Negative Breast Cancer
Enlarging lesion on temple
A shave biopsy revealed acanthosis, papillomatosis, hyperkeratosis, hypergranulosis, parakeratosis, and cytoplasmic viral-like inclusions without atypia, consistent with a diagnosis of a common wart. The biopsy ruled out other possible diagnoses, which included keratoacanthoma, seborrheic keratosis, and squamous cell carcinoma.
Cutaneous warts can manifest as common warts (verruca vulgaris), plantar warts (verruca plantaris), or plane warts (verruca plana). These benign skin lesions are caused by human papillomavirus and can manifest in areas of skin trauma; this is known as the Koebner phenomenon. Most warts can be diagnosed through clinical history and examination. Dermoscopy, if performed, may reveal thrombosed capillaries as dotted structures, but there is an increased risk of cross-contamination.1 That said, some dermatoscopes have disposable covers or can be cleaned with antiviral, antibacterial wipes. If the diagnosis is unclear or the exam is clinically suspicious, a biopsy may be required.
Cases with progressive enlargement and extensive involvement of the skin (as was seen here) are generally associated with certain predisposing conditions, such as atopic dermatitis and immunosuppression.2 Our patient screened negative for HIV infection, and further evaluation did not reveal any concerns for immunosuppression.
Treatment for a common wart depends on patient characteristics, preferences, cost, and possible adverse effects. Standard treatment options are topical salicylic acid and cryotherapy with liquid nitrogen. Depending on the location and type of the wart, multiple treatments may be required, and recurrences are common. Intralesional injection with bleomycin, 5‐fluorouracil, or cidofovir is often used for recurrent and refractory warts.
Patients unable to tolerate cryotherapy or local injections may benefit from thermotherapy by heating the wart with a pulsed dye laser.3 Observation is also a reasonable course of action for new warts, as they may spontaneously resolve within a year.
In this case, the patient opted for over-the-counter salicylic acid 17% to be applied nightly until resolution. Cryosurgery would be a next step for him if the lesion does not resolve after 3 months of treatment.
Image courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013;3:33-34. doi: 10.5826/dpc.0304a07
2. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. doi: 10.1016/j.jaci.2012.07.049
3. Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15:284-301. doi: 10.1111/jebm.12494

A shave biopsy revealed acanthosis, papillomatosis, hyperkeratosis, hypergranulosis, parakeratosis, and cytoplasmic viral-like inclusions without atypia, consistent with a diagnosis of a common wart. The biopsy ruled out other possible diagnoses, which included keratoacanthoma, seborrheic keratosis, and squamous cell carcinoma.
Cutaneous warts can manifest as common warts (verruca vulgaris), plantar warts (verruca plantaris), or plane warts (verruca plana). These benign skin lesions are caused by human papillomavirus and can manifest in areas of skin trauma; this is known as the Koebner phenomenon. Most warts can be diagnosed through clinical history and examination. Dermoscopy, if performed, may reveal thrombosed capillaries as dotted structures, but there is an increased risk of cross-contamination.1 That said, some dermatoscopes have disposable covers or can be cleaned with antiviral, antibacterial wipes. If the diagnosis is unclear or the exam is clinically suspicious, a biopsy may be required.
Cases with progressive enlargement and extensive involvement of the skin (as was seen here) are generally associated with certain predisposing conditions, such as atopic dermatitis and immunosuppression.2 Our patient screened negative for HIV infection, and further evaluation did not reveal any concerns for immunosuppression.
Treatment for a common wart depends on patient characteristics, preferences, cost, and possible adverse effects. Standard treatment options are topical salicylic acid and cryotherapy with liquid nitrogen. Depending on the location and type of the wart, multiple treatments may be required, and recurrences are common. Intralesional injection with bleomycin, 5‐fluorouracil, or cidofovir is often used for recurrent and refractory warts.
Patients unable to tolerate cryotherapy or local injections may benefit from thermotherapy by heating the wart with a pulsed dye laser.3 Observation is also a reasonable course of action for new warts, as they may spontaneously resolve within a year.
In this case, the patient opted for over-the-counter salicylic acid 17% to be applied nightly until resolution. Cryosurgery would be a next step for him if the lesion does not resolve after 3 months of treatment.
Image courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

A shave biopsy revealed acanthosis, papillomatosis, hyperkeratosis, hypergranulosis, parakeratosis, and cytoplasmic viral-like inclusions without atypia, consistent with a diagnosis of a common wart. The biopsy ruled out other possible diagnoses, which included keratoacanthoma, seborrheic keratosis, and squamous cell carcinoma.
Cutaneous warts can manifest as common warts (verruca vulgaris), plantar warts (verruca plantaris), or plane warts (verruca plana). These benign skin lesions are caused by human papillomavirus and can manifest in areas of skin trauma; this is known as the Koebner phenomenon. Most warts can be diagnosed through clinical history and examination. Dermoscopy, if performed, may reveal thrombosed capillaries as dotted structures, but there is an increased risk of cross-contamination.1 That said, some dermatoscopes have disposable covers or can be cleaned with antiviral, antibacterial wipes. If the diagnosis is unclear or the exam is clinically suspicious, a biopsy may be required.
Cases with progressive enlargement and extensive involvement of the skin (as was seen here) are generally associated with certain predisposing conditions, such as atopic dermatitis and immunosuppression.2 Our patient screened negative for HIV infection, and further evaluation did not reveal any concerns for immunosuppression.
Treatment for a common wart depends on patient characteristics, preferences, cost, and possible adverse effects. Standard treatment options are topical salicylic acid and cryotherapy with liquid nitrogen. Depending on the location and type of the wart, multiple treatments may be required, and recurrences are common. Intralesional injection with bleomycin, 5‐fluorouracil, or cidofovir is often used for recurrent and refractory warts.
Patients unable to tolerate cryotherapy or local injections may benefit from thermotherapy by heating the wart with a pulsed dye laser.3 Observation is also a reasonable course of action for new warts, as they may spontaneously resolve within a year.
In this case, the patient opted for over-the-counter salicylic acid 17% to be applied nightly until resolution. Cryosurgery would be a next step for him if the lesion does not resolve after 3 months of treatment.
Image courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013;3:33-34. doi: 10.5826/dpc.0304a07
2. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. doi: 10.1016/j.jaci.2012.07.049
3. Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15:284-301. doi: 10.1111/jebm.12494
1. Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013;3:33-34. doi: 10.5826/dpc.0304a07
2. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. doi: 10.1016/j.jaci.2012.07.049
3. Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15:284-301. doi: 10.1111/jebm.12494
Commentary: "Migraine Plus" Symptoms, October 2023
This month we will discuss "migraine plus" conditions: menstrual migraine as well as migraine-associated symptoms, including allodynia, photophobia, and nausea.
Migraine is one of the most common and disabling conditions worldwide, and it is three times more likely to be found in women than men. This is even more so during reproductive years, where many women experience hormonally triggered migraine attacks. Although some women will experience migraine exclusively perimenstrually, most women who experience menstrual migraine attacks also will have migraine attacks that are not hormonally triggered. It is often challenging to find the correct acute treatment for specific kinds of migraine attacks, and many women will describe specific acute medications as more effective for their nonmenstrual or "regular" migraine attacks compared with their perimenstrual attacks. The study by MacGregor and colleagues investigated the use of ubrogepant and compared its effect between these two subtypes of attacks.
This trial was an extension of the initial phase 3 trial of ubrogepant, called ACHIEVE II. Initial investigators enrolled over 700 patients into an open-label extension, and the participants were randomly assigned 1:1:1 to their "usual care," 50 mg ubrogepant, or 100 mg ubrogepant. Participants were blinded to the dose of ubrogepant even though they knew that they were taking ubrogepant or their standard acute medication. The purpose of the "usual care" arm was not to collect efficacy results; rather, it was for safety, specifically to evaluate the long-term hepatic safety with ubrogepant.
Participants were allowed to treat up to eight migraine attacks per 4-week interval. The duration of the trial was 52 weeks, and a second dose of medication was allowed, identical to the initial dose. Women in this trial recorded their menstrual start date and whether they treated menstrually related attacks. An attack was considered menstrually related if the headache was within a 5-day window of the onset of menstruation. Of the 734 women enrolled in the intention-to-treat population, 354 reported at least one menstrual cycle start date with a headache day. Efficacy outcome measures included pain freedom at 2 hours post-dose, pain relief at 2 hours post-dose, absence of photophobia, phonophobia, and nausea at 2 hours post-dose, normal function at 2 hours post-dose, and use of rescue medication within 24 hours of the initial dose. All information was collected via an electronic diary.
There was no statistically significant difference between 2-hour pain freedom outcomes of menstrual and nonmenstrual migraine attacks, although there was a numerically higher mean percentage of menstrual attacks that was not statistically significant. This was noted for both doses of ubrogepant. This was also the case for 2-hour pain relief; the migraine-associated symptoms of photophobia, phonophobia, and nausea; for functional disability; and the use of a rescue medication. Among all outcomes it appears that both doses of ubrogepant are equally effective for both menstrual and nonmenstrual migraine attacks. On the basis of this evidence, clinicians may be able to consolidate different acute medications for different migraine subtypes and consider the use of this calcitonin gene-related peptide (CGRP) antagonist for all the patient's attacks.
Allodynia is a condition whereby a nonpainful stimulus is perceived as painful. In the context of migraine, this often will occur in the head and neck region and as a result of the chronification of migraine — headache frequency increasing to > 15 days per month. One significant risk factor for the development of chronic migraine is medication overuse, when an acute medication for migraine is used more often than its recommended use. Pijpers and colleagues sought to determine whether the presence of allodynia was predictive for the prognosis of chronic migraine complicated by medication overuse.
This study was a subset of the Chronification And Reversibility of Migraine (CHARM) study, a randomized, double-blind, placebo-controlled trial that aimed to investigate whether treatment with botulinum toxin A was of added value in addition to withdrawal therapy in chronic migraine patients with medication overuse headache. Diagnoses were made in consultation with headache experts and confirmed by a headache diary. Exclusion criteria were: (1) other primary headache or neurologic disorders; (2) other chronic pain disorders with medium to high pain intensity or requiring pain medication; (3) major psychiatric disorders other than depression; (4) major cognitive, behavioral, or oncologic disorders; (5) contraindications for treatment or inability to adhere to the study protocol; (6) (planned) pregnancy or breastfeeding; (7) use of ergots, opioids, or barbiturates; or (8) abuse of drugs in the past 12 months. Allodynia was determined by the Allodynia Symptom Checklist (ASC) .
The primary outcome was reversion from chronic to episodic migraine; secondary outcomes were > 50% reduction in monthly migraine days and reduction in number of monthly headache days. A total of 173 participants in the CHARM trial provided baseline allodynia data and were included in this current study. Participants with cutaneous allodynia were mainly women and did not differ significantly in age, number of monthly migraine or headache days, age of onset, use of acute or prophylactic treatment, or being treated with botulinum toxin.
The absence of cutaneous allodynia was predictive for good outcome after 12 weeks. For the primary endpoint, the odds for reversion from chronic migraine to episodic migraine were 2.5 times higher for participants without allodynia vs with allodynia. In all, 75.0% of participants without allodynia vs 57.4% of participants with allodynia reverted to episodic migraine. These helpful data will allow us to better predict accurately the disease process and better set expectations for our patients with chronic migraine.
In the earlier days of headache treatment, the focus for both acute and preventive medications was a decrease in the severity or frequency of pain. As time has progressed and our understanding of migraine has broadened, we now consider pain one of the many features of migraine, albeit usually the most prominent feature. The CGRP antagonist class of migraine medications has revolutionized how migraine is treated, both acutely and preventively; however, the initial studies all focused on pain-related outcomes. Alpuente and colleagues sought to better determine the effect of CGRP monoclonal antibody medications on other migraine-associated symptoms, specifically photophobia, photophobia, nausea, dizziness, and aura.
All injectable CGRP antibody medications were studied. Responses were recorded in an electronic diary. Patients were followed at 3 and 6 months and were excluded if their diary was < 80% complete; a total of 158 patients were included in this study. At 3 months, groups of patients were further divided between those who had > 50% decrease in monthly headache days and those that had < 50% reduction.
The > 50% group showed statistically significant reductions in the ratios of photophobia, phonophobia, and aura after 6 months of treatment, and, of note, these symptoms decreased at a higher rate than the reduction in headache days per month after 6 months. Rates of nausea and dizziness only reduced proportionally to the monthly headache days. For the < 50% group, there was a rebound of dizziness in between months 3 and 6, but all other outcomes decreased in proportion to the monthly headache days.
Our patients all experience symptoms other than headache pain as part of their migraine attacks. When we discuss the risks and benefits of a new treatment, we can now more accurately address many of the other associated symptoms and explain what our patients are likely to expect when starting a new medication. Similar studies have described these findings with the oral CGRP antagonists as well, and most acute migraine studies now use "most bothersome symptom" rather than pain severity as their primary outcome.
This month we will discuss "migraine plus" conditions: menstrual migraine as well as migraine-associated symptoms, including allodynia, photophobia, and nausea.
Migraine is one of the most common and disabling conditions worldwide, and it is three times more likely to be found in women than men. This is even more so during reproductive years, where many women experience hormonally triggered migraine attacks. Although some women will experience migraine exclusively perimenstrually, most women who experience menstrual migraine attacks also will have migraine attacks that are not hormonally triggered. It is often challenging to find the correct acute treatment for specific kinds of migraine attacks, and many women will describe specific acute medications as more effective for their nonmenstrual or "regular" migraine attacks compared with their perimenstrual attacks. The study by MacGregor and colleagues investigated the use of ubrogepant and compared its effect between these two subtypes of attacks.
This trial was an extension of the initial phase 3 trial of ubrogepant, called ACHIEVE II. Initial investigators enrolled over 700 patients into an open-label extension, and the participants were randomly assigned 1:1:1 to their "usual care," 50 mg ubrogepant, or 100 mg ubrogepant. Participants were blinded to the dose of ubrogepant even though they knew that they were taking ubrogepant or their standard acute medication. The purpose of the "usual care" arm was not to collect efficacy results; rather, it was for safety, specifically to evaluate the long-term hepatic safety with ubrogepant.
Participants were allowed to treat up to eight migraine attacks per 4-week interval. The duration of the trial was 52 weeks, and a second dose of medication was allowed, identical to the initial dose. Women in this trial recorded their menstrual start date and whether they treated menstrually related attacks. An attack was considered menstrually related if the headache was within a 5-day window of the onset of menstruation. Of the 734 women enrolled in the intention-to-treat population, 354 reported at least one menstrual cycle start date with a headache day. Efficacy outcome measures included pain freedom at 2 hours post-dose, pain relief at 2 hours post-dose, absence of photophobia, phonophobia, and nausea at 2 hours post-dose, normal function at 2 hours post-dose, and use of rescue medication within 24 hours of the initial dose. All information was collected via an electronic diary.
There was no statistically significant difference between 2-hour pain freedom outcomes of menstrual and nonmenstrual migraine attacks, although there was a numerically higher mean percentage of menstrual attacks that was not statistically significant. This was noted for both doses of ubrogepant. This was also the case for 2-hour pain relief; the migraine-associated symptoms of photophobia, phonophobia, and nausea; for functional disability; and the use of a rescue medication. Among all outcomes it appears that both doses of ubrogepant are equally effective for both menstrual and nonmenstrual migraine attacks. On the basis of this evidence, clinicians may be able to consolidate different acute medications for different migraine subtypes and consider the use of this calcitonin gene-related peptide (CGRP) antagonist for all the patient's attacks.
Allodynia is a condition whereby a nonpainful stimulus is perceived as painful. In the context of migraine, this often will occur in the head and neck region and as a result of the chronification of migraine — headache frequency increasing to > 15 days per month. One significant risk factor for the development of chronic migraine is medication overuse, when an acute medication for migraine is used more often than its recommended use. Pijpers and colleagues sought to determine whether the presence of allodynia was predictive for the prognosis of chronic migraine complicated by medication overuse.
This study was a subset of the Chronification And Reversibility of Migraine (CHARM) study, a randomized, double-blind, placebo-controlled trial that aimed to investigate whether treatment with botulinum toxin A was of added value in addition to withdrawal therapy in chronic migraine patients with medication overuse headache. Diagnoses were made in consultation with headache experts and confirmed by a headache diary. Exclusion criteria were: (1) other primary headache or neurologic disorders; (2) other chronic pain disorders with medium to high pain intensity or requiring pain medication; (3) major psychiatric disorders other than depression; (4) major cognitive, behavioral, or oncologic disorders; (5) contraindications for treatment or inability to adhere to the study protocol; (6) (planned) pregnancy or breastfeeding; (7) use of ergots, opioids, or barbiturates; or (8) abuse of drugs in the past 12 months. Allodynia was determined by the Allodynia Symptom Checklist (ASC) .
The primary outcome was reversion from chronic to episodic migraine; secondary outcomes were > 50% reduction in monthly migraine days and reduction in number of monthly headache days. A total of 173 participants in the CHARM trial provided baseline allodynia data and were included in this current study. Participants with cutaneous allodynia were mainly women and did not differ significantly in age, number of monthly migraine or headache days, age of onset, use of acute or prophylactic treatment, or being treated with botulinum toxin.
The absence of cutaneous allodynia was predictive for good outcome after 12 weeks. For the primary endpoint, the odds for reversion from chronic migraine to episodic migraine were 2.5 times higher for participants without allodynia vs with allodynia. In all, 75.0% of participants without allodynia vs 57.4% of participants with allodynia reverted to episodic migraine. These helpful data will allow us to better predict accurately the disease process and better set expectations for our patients with chronic migraine.
In the earlier days of headache treatment, the focus for both acute and preventive medications was a decrease in the severity or frequency of pain. As time has progressed and our understanding of migraine has broadened, we now consider pain one of the many features of migraine, albeit usually the most prominent feature. The CGRP antagonist class of migraine medications has revolutionized how migraine is treated, both acutely and preventively; however, the initial studies all focused on pain-related outcomes. Alpuente and colleagues sought to better determine the effect of CGRP monoclonal antibody medications on other migraine-associated symptoms, specifically photophobia, photophobia, nausea, dizziness, and aura.
All injectable CGRP antibody medications were studied. Responses were recorded in an electronic diary. Patients were followed at 3 and 6 months and were excluded if their diary was < 80% complete; a total of 158 patients were included in this study. At 3 months, groups of patients were further divided between those who had > 50% decrease in monthly headache days and those that had < 50% reduction.
The > 50% group showed statistically significant reductions in the ratios of photophobia, phonophobia, and aura after 6 months of treatment, and, of note, these symptoms decreased at a higher rate than the reduction in headache days per month after 6 months. Rates of nausea and dizziness only reduced proportionally to the monthly headache days. For the < 50% group, there was a rebound of dizziness in between months 3 and 6, but all other outcomes decreased in proportion to the monthly headache days.
Our patients all experience symptoms other than headache pain as part of their migraine attacks. When we discuss the risks and benefits of a new treatment, we can now more accurately address many of the other associated symptoms and explain what our patients are likely to expect when starting a new medication. Similar studies have described these findings with the oral CGRP antagonists as well, and most acute migraine studies now use "most bothersome symptom" rather than pain severity as their primary outcome.
This month we will discuss "migraine plus" conditions: menstrual migraine as well as migraine-associated symptoms, including allodynia, photophobia, and nausea.
Migraine is one of the most common and disabling conditions worldwide, and it is three times more likely to be found in women than men. This is even more so during reproductive years, where many women experience hormonally triggered migraine attacks. Although some women will experience migraine exclusively perimenstrually, most women who experience menstrual migraine attacks also will have migraine attacks that are not hormonally triggered. It is often challenging to find the correct acute treatment for specific kinds of migraine attacks, and many women will describe specific acute medications as more effective for their nonmenstrual or "regular" migraine attacks compared with their perimenstrual attacks. The study by MacGregor and colleagues investigated the use of ubrogepant and compared its effect between these two subtypes of attacks.
This trial was an extension of the initial phase 3 trial of ubrogepant, called ACHIEVE II. Initial investigators enrolled over 700 patients into an open-label extension, and the participants were randomly assigned 1:1:1 to their "usual care," 50 mg ubrogepant, or 100 mg ubrogepant. Participants were blinded to the dose of ubrogepant even though they knew that they were taking ubrogepant or their standard acute medication. The purpose of the "usual care" arm was not to collect efficacy results; rather, it was for safety, specifically to evaluate the long-term hepatic safety with ubrogepant.
Participants were allowed to treat up to eight migraine attacks per 4-week interval. The duration of the trial was 52 weeks, and a second dose of medication was allowed, identical to the initial dose. Women in this trial recorded their menstrual start date and whether they treated menstrually related attacks. An attack was considered menstrually related if the headache was within a 5-day window of the onset of menstruation. Of the 734 women enrolled in the intention-to-treat population, 354 reported at least one menstrual cycle start date with a headache day. Efficacy outcome measures included pain freedom at 2 hours post-dose, pain relief at 2 hours post-dose, absence of photophobia, phonophobia, and nausea at 2 hours post-dose, normal function at 2 hours post-dose, and use of rescue medication within 24 hours of the initial dose. All information was collected via an electronic diary.
There was no statistically significant difference between 2-hour pain freedom outcomes of menstrual and nonmenstrual migraine attacks, although there was a numerically higher mean percentage of menstrual attacks that was not statistically significant. This was noted for both doses of ubrogepant. This was also the case for 2-hour pain relief; the migraine-associated symptoms of photophobia, phonophobia, and nausea; for functional disability; and the use of a rescue medication. Among all outcomes it appears that both doses of ubrogepant are equally effective for both menstrual and nonmenstrual migraine attacks. On the basis of this evidence, clinicians may be able to consolidate different acute medications for different migraine subtypes and consider the use of this calcitonin gene-related peptide (CGRP) antagonist for all the patient's attacks.
Allodynia is a condition whereby a nonpainful stimulus is perceived as painful. In the context of migraine, this often will occur in the head and neck region and as a result of the chronification of migraine — headache frequency increasing to > 15 days per month. One significant risk factor for the development of chronic migraine is medication overuse, when an acute medication for migraine is used more often than its recommended use. Pijpers and colleagues sought to determine whether the presence of allodynia was predictive for the prognosis of chronic migraine complicated by medication overuse.
This study was a subset of the Chronification And Reversibility of Migraine (CHARM) study, a randomized, double-blind, placebo-controlled trial that aimed to investigate whether treatment with botulinum toxin A was of added value in addition to withdrawal therapy in chronic migraine patients with medication overuse headache. Diagnoses were made in consultation with headache experts and confirmed by a headache diary. Exclusion criteria were: (1) other primary headache or neurologic disorders; (2) other chronic pain disorders with medium to high pain intensity or requiring pain medication; (3) major psychiatric disorders other than depression; (4) major cognitive, behavioral, or oncologic disorders; (5) contraindications for treatment or inability to adhere to the study protocol; (6) (planned) pregnancy or breastfeeding; (7) use of ergots, opioids, or barbiturates; or (8) abuse of drugs in the past 12 months. Allodynia was determined by the Allodynia Symptom Checklist (ASC) .
The primary outcome was reversion from chronic to episodic migraine; secondary outcomes were > 50% reduction in monthly migraine days and reduction in number of monthly headache days. A total of 173 participants in the CHARM trial provided baseline allodynia data and were included in this current study. Participants with cutaneous allodynia were mainly women and did not differ significantly in age, number of monthly migraine or headache days, age of onset, use of acute or prophylactic treatment, or being treated with botulinum toxin.
The absence of cutaneous allodynia was predictive for good outcome after 12 weeks. For the primary endpoint, the odds for reversion from chronic migraine to episodic migraine were 2.5 times higher for participants without allodynia vs with allodynia. In all, 75.0% of participants without allodynia vs 57.4% of participants with allodynia reverted to episodic migraine. These helpful data will allow us to better predict accurately the disease process and better set expectations for our patients with chronic migraine.
In the earlier days of headache treatment, the focus for both acute and preventive medications was a decrease in the severity or frequency of pain. As time has progressed and our understanding of migraine has broadened, we now consider pain one of the many features of migraine, albeit usually the most prominent feature. The CGRP antagonist class of migraine medications has revolutionized how migraine is treated, both acutely and preventively; however, the initial studies all focused on pain-related outcomes. Alpuente and colleagues sought to better determine the effect of CGRP monoclonal antibody medications on other migraine-associated symptoms, specifically photophobia, photophobia, nausea, dizziness, and aura.
All injectable CGRP antibody medications were studied. Responses were recorded in an electronic diary. Patients were followed at 3 and 6 months and were excluded if their diary was < 80% complete; a total of 158 patients were included in this study. At 3 months, groups of patients were further divided between those who had > 50% decrease in monthly headache days and those that had < 50% reduction.
The > 50% group showed statistically significant reductions in the ratios of photophobia, phonophobia, and aura after 6 months of treatment, and, of note, these symptoms decreased at a higher rate than the reduction in headache days per month after 6 months. Rates of nausea and dizziness only reduced proportionally to the monthly headache days. For the < 50% group, there was a rebound of dizziness in between months 3 and 6, but all other outcomes decreased in proportion to the monthly headache days.
Our patients all experience symptoms other than headache pain as part of their migraine attacks. When we discuss the risks and benefits of a new treatment, we can now more accurately address many of the other associated symptoms and explain what our patients are likely to expect when starting a new medication. Similar studies have described these findings with the oral CGRP antagonists as well, and most acute migraine studies now use "most bothersome symptom" rather than pain severity as their primary outcome.