User login
Safety first: Regulations
The word “regulations” gets a mixed response.
This is common in all industries, and certainly pharmaceuticals. On any given day there are stories on industry news sites about disputes between companies and regulatory agencies.
I’d agree that some regulation is needed. The history of pharmacy has had both remarkable successes – and failures.
Let’s look at migraines, since that’s in my field. The calcitonin gene-related peptide (CGRP) drugs have been a remarkable breakthrough, certainly the biggest one since the triptans in 1992. There are currently seven on the market for both prevention and abortive use. They’re effective and (to date) pretty safe.
But it wasn’t always that way. Look back just 14 years ago to 2009, when the first promising CGRP agent (MK-3207) had its development halted because of hepatic abnormalities. It’s cousin telcagepant (MK-0974) came to a similar end 2 years later.
Without regulations in place (and the potential for lawsuits) these might have made it to market, bringing migraine relief to some and potentially serious liver damage to others. So Merck made the right decision to axe them. Researchers learned from the experience, went back to the drawing board, and developed the current generation of far-safer drugs.
This came into sharp focus in another industry recently, when the eyes of the world were on the north Atlantic. A small tourist submarine imploded and killed five people. During the inevitable media coverage it came out that the submarine hadn’t been certified for safety by any of the agencies that handle such things, falling into a gray area in international waters where inspections aren’t required.
This isn’t to say it wasn’t safe – it had made several dives before – but obviously not safe enough. While I didn’t know the late Stockton Rush (the owner/designer) it sounds like he viewed regulations as stifling innovation, and in one interview said “at some point, safety is just pure waste.” He ignored warnings from several sides about the submersible’s ability to handle deep ocean pressure and the inevitable wear and tear repeated dives will have on the hull.
I understand there’s a margin of luck, too. Bad things can happen to any of us – or any company. Some things can’t be clearly foreseen. Some drugs don’t start to show problems until they’re on the market and reach a certain number of prescriptions.
But there’s a reason we have regulations. Pretty much every government has, going back to the Roman Empire, covering numerous things. In a perfect world we wouldn’t need them.
But people are far from perfect. And the consequences can be terrible.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The word “regulations” gets a mixed response.
This is common in all industries, and certainly pharmaceuticals. On any given day there are stories on industry news sites about disputes between companies and regulatory agencies.
I’d agree that some regulation is needed. The history of pharmacy has had both remarkable successes – and failures.
Let’s look at migraines, since that’s in my field. The calcitonin gene-related peptide (CGRP) drugs have been a remarkable breakthrough, certainly the biggest one since the triptans in 1992. There are currently seven on the market for both prevention and abortive use. They’re effective and (to date) pretty safe.
But it wasn’t always that way. Look back just 14 years ago to 2009, when the first promising CGRP agent (MK-3207) had its development halted because of hepatic abnormalities. It’s cousin telcagepant (MK-0974) came to a similar end 2 years later.
Without regulations in place (and the potential for lawsuits) these might have made it to market, bringing migraine relief to some and potentially serious liver damage to others. So Merck made the right decision to axe them. Researchers learned from the experience, went back to the drawing board, and developed the current generation of far-safer drugs.
This came into sharp focus in another industry recently, when the eyes of the world were on the north Atlantic. A small tourist submarine imploded and killed five people. During the inevitable media coverage it came out that the submarine hadn’t been certified for safety by any of the agencies that handle such things, falling into a gray area in international waters where inspections aren’t required.
This isn’t to say it wasn’t safe – it had made several dives before – but obviously not safe enough. While I didn’t know the late Stockton Rush (the owner/designer) it sounds like he viewed regulations as stifling innovation, and in one interview said “at some point, safety is just pure waste.” He ignored warnings from several sides about the submersible’s ability to handle deep ocean pressure and the inevitable wear and tear repeated dives will have on the hull.
I understand there’s a margin of luck, too. Bad things can happen to any of us – or any company. Some things can’t be clearly foreseen. Some drugs don’t start to show problems until they’re on the market and reach a certain number of prescriptions.
But there’s a reason we have regulations. Pretty much every government has, going back to the Roman Empire, covering numerous things. In a perfect world we wouldn’t need them.
But people are far from perfect. And the consequences can be terrible.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The word “regulations” gets a mixed response.
This is common in all industries, and certainly pharmaceuticals. On any given day there are stories on industry news sites about disputes between companies and regulatory agencies.
I’d agree that some regulation is needed. The history of pharmacy has had both remarkable successes – and failures.
Let’s look at migraines, since that’s in my field. The calcitonin gene-related peptide (CGRP) drugs have been a remarkable breakthrough, certainly the biggest one since the triptans in 1992. There are currently seven on the market for both prevention and abortive use. They’re effective and (to date) pretty safe.
But it wasn’t always that way. Look back just 14 years ago to 2009, when the first promising CGRP agent (MK-3207) had its development halted because of hepatic abnormalities. It’s cousin telcagepant (MK-0974) came to a similar end 2 years later.
Without regulations in place (and the potential for lawsuits) these might have made it to market, bringing migraine relief to some and potentially serious liver damage to others. So Merck made the right decision to axe them. Researchers learned from the experience, went back to the drawing board, and developed the current generation of far-safer drugs.
This came into sharp focus in another industry recently, when the eyes of the world were on the north Atlantic. A small tourist submarine imploded and killed five people. During the inevitable media coverage it came out that the submarine hadn’t been certified for safety by any of the agencies that handle such things, falling into a gray area in international waters where inspections aren’t required.
This isn’t to say it wasn’t safe – it had made several dives before – but obviously not safe enough. While I didn’t know the late Stockton Rush (the owner/designer) it sounds like he viewed regulations as stifling innovation, and in one interview said “at some point, safety is just pure waste.” He ignored warnings from several sides about the submersible’s ability to handle deep ocean pressure and the inevitable wear and tear repeated dives will have on the hull.
I understand there’s a margin of luck, too. Bad things can happen to any of us – or any company. Some things can’t be clearly foreseen. Some drugs don’t start to show problems until they’re on the market and reach a certain number of prescriptions.
But there’s a reason we have regulations. Pretty much every government has, going back to the Roman Empire, covering numerous things. In a perfect world we wouldn’t need them.
But people are far from perfect. And the consequences can be terrible.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Tirzepatide: Therapeutic titan or costly cure?
As a general practitioner with a specialist interest in diabetes, I am increasingly diagnosing younger people living with type 2 diabetes and obesity. Sadly, my youngest patient living with type 2 diabetes and obesity is only in her early 20s.
In fact, in England, there are now more people under the age of 40 years living with type 2 diabetes than type 1 diabetes. These younger individuals tend to present with very high hemoglobin A1c levels; I am routinely seeing double-digit A1c percentage levels in my practice. Indeed, the patient mentioned above presented with an A1c of more than 13%.
The lifetime cardiometabolic risk of individuals like her is considerable and very worrying: Younger adults with type 2 diabetes often have adverse cardiometabolic risk profiles at diagnosis, with higher body mass indices, marked dyslipidemia, hypertension, and abnormal liver profiles suggesting nonalcoholic fatty liver disease. The cumulative impact of this risk profile is a significant impact on quality and quantity of life. Evidence tells us that a younger age of diagnosis with type 2 diabetes is associated with an increased risk for premature death, especially from cardiovascular disease.
Early treatment intensification is warranted in younger individuals living with type 2 diabetes and obesity. My patient above is now on triple therapy with metformin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, and a glucagonlike peptide–1 (GLP-1) receptor agonist. I gave her an urgent referral to my local weight management service for weight, nutritional, and psychological support. I have also issued her a real-time continuous glucose monitoring (rt-CGM) device: Whilst she does not meet any current U.K. criteria for using rt-CGM, I feel that the role of CGM as an educational tool for her is invaluable and equally important to her pharmacologic therapies. We are in desperate need of effective pharmacologic and lifestyle interventions to tackle this epidemic of cardiometabolic disease in the young.
I attended the recent ADA 2023 congress in San Diego, including the presentation of the SURMOUNT-2 trial data. SURMOUNT-2 explored the efficacy and safety of the dual GLP-GIP agonist tirzepatide for weight management in patients with obesity and type 2 diabetes. Tirzepatide was associated with significant reductions in weight (average weight loss, 14-16 kg after 72 weeks) and glycemia (2.1% reduction in A1c after 72 weeks), as well as reductions in clinically meaningful cardiometabolic risk factors, including systolic blood pressure, liver enzymes, and fasting non–HDL cholesterol levels. The overall safety profile of tirzepatide was also reassuring and consistent with the GLP-1 class. Most adverse effects were gastrointestinal and of mild to moderate severity. These adverse effects decreased over time.
These results perfectly position tirzepatide for my younger patients like the young woman mentioned above. The significant improvements in weight, glycemia, and cardiometabolic risk factors will not only help mitigate her future cardiometabolic risk but also help the sustainability of the U.K.’s National Health System. The cost of diabetes to the NHS in the United Kingdom is more than 10% of the entire NHS budget for England and Wales. More than 80% of this cost, however, is related not to the medications and devices we prescribe for diabetes but to the downstream complications of diabetes, such as hospital admissions for cardiovascular events and amputations, as well as regular hospital attendance for dialysis for end-stage kidney disease.
There is no doubt, however, that modern obesity medications such as semaglutide and tirzepatide are expensive, and demand has been astronomical. This demand has been driven by private weight-management services and celebrity influencers, and has resulted in major U.K.-wide GLP-1 shortages.
This situation is tragically widening health inequalities, as many of my patients who have been on GLP-1 receptor agonists for many years are unable to obtain them. I am having to consider switching therapies, often to less efficacious options without the compelling cardiorenal benefits. Furthermore, the GLP-1 shortages have prevented GLP-1 initiation for my other high-risk younger patients, potentially increasing future cardiometabolic risk.
There remain unanswered questions for tirzepatide: What is the durability of effect of tirzepatide after 72 weeks (that is, the trial duration of SURMOUNT-2)? Crucially, what is the effect of withdrawal of tirzepatide on weight loss maintenance? Previous evidence has suggested weight regain after discontinuation of a GLP-1 receptor agonist for obesity. This, of course, has further financial and sustainability implications for health care systems such as the NHS.
Finally, we are increasingly seeing younger women of childbearing age with or at risk for cardiometabolic disease. Again, my patient above is one example. Many of the therapies we use for cardiometabolic disease management, including GLP-1 receptor agonists and tirzepatide, have not been studied, and hence have not been licensed in pregnant women. Therefore, frank discussions are required with patients about future family plans and the importance of contraception. Often, the significant weight loss seen with GLP-1 receptor agonists can improve hormonal profiles and fertility in women and result in unexpected pregnancies if robust contraception is not in place.
Tirzepatide has yet to be made commercially available in the United Kingdom, and its price has also yet to be set. But I already envision a clear role for tirzepatide in my treatment armamentarium. I will be positioning tirzepatide as my first injectable of choice after oral treatment escalation with metformin and an SGLT2 inhibitor in all my patients who require treatment intensification – not just my younger, higher-risk individuals. This may remain an aspirational goal until supply chains and cost are defined. There is no doubt, however, that the compelling weight and glycemic benefits of tirzepatide alongside individualized lifestyle interventions can help improve the quality and quantity of life of my patients living with type 2 diabetes and obesity.
Dr. Fernando is a general practitioner near Edinburgh. He reported receiving speaker fees from Eli Lilly and Novo Nordisk..
A version of this article first appeared on Medscape.com.
As a general practitioner with a specialist interest in diabetes, I am increasingly diagnosing younger people living with type 2 diabetes and obesity. Sadly, my youngest patient living with type 2 diabetes and obesity is only in her early 20s.
In fact, in England, there are now more people under the age of 40 years living with type 2 diabetes than type 1 diabetes. These younger individuals tend to present with very high hemoglobin A1c levels; I am routinely seeing double-digit A1c percentage levels in my practice. Indeed, the patient mentioned above presented with an A1c of more than 13%.
The lifetime cardiometabolic risk of individuals like her is considerable and very worrying: Younger adults with type 2 diabetes often have adverse cardiometabolic risk profiles at diagnosis, with higher body mass indices, marked dyslipidemia, hypertension, and abnormal liver profiles suggesting nonalcoholic fatty liver disease. The cumulative impact of this risk profile is a significant impact on quality and quantity of life. Evidence tells us that a younger age of diagnosis with type 2 diabetes is associated with an increased risk for premature death, especially from cardiovascular disease.
Early treatment intensification is warranted in younger individuals living with type 2 diabetes and obesity. My patient above is now on triple therapy with metformin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, and a glucagonlike peptide–1 (GLP-1) receptor agonist. I gave her an urgent referral to my local weight management service for weight, nutritional, and psychological support. I have also issued her a real-time continuous glucose monitoring (rt-CGM) device: Whilst she does not meet any current U.K. criteria for using rt-CGM, I feel that the role of CGM as an educational tool for her is invaluable and equally important to her pharmacologic therapies. We are in desperate need of effective pharmacologic and lifestyle interventions to tackle this epidemic of cardiometabolic disease in the young.
I attended the recent ADA 2023 congress in San Diego, including the presentation of the SURMOUNT-2 trial data. SURMOUNT-2 explored the efficacy and safety of the dual GLP-GIP agonist tirzepatide for weight management in patients with obesity and type 2 diabetes. Tirzepatide was associated with significant reductions in weight (average weight loss, 14-16 kg after 72 weeks) and glycemia (2.1% reduction in A1c after 72 weeks), as well as reductions in clinically meaningful cardiometabolic risk factors, including systolic blood pressure, liver enzymes, and fasting non–HDL cholesterol levels. The overall safety profile of tirzepatide was also reassuring and consistent with the GLP-1 class. Most adverse effects were gastrointestinal and of mild to moderate severity. These adverse effects decreased over time.
These results perfectly position tirzepatide for my younger patients like the young woman mentioned above. The significant improvements in weight, glycemia, and cardiometabolic risk factors will not only help mitigate her future cardiometabolic risk but also help the sustainability of the U.K.’s National Health System. The cost of diabetes to the NHS in the United Kingdom is more than 10% of the entire NHS budget for England and Wales. More than 80% of this cost, however, is related not to the medications and devices we prescribe for diabetes but to the downstream complications of diabetes, such as hospital admissions for cardiovascular events and amputations, as well as regular hospital attendance for dialysis for end-stage kidney disease.
There is no doubt, however, that modern obesity medications such as semaglutide and tirzepatide are expensive, and demand has been astronomical. This demand has been driven by private weight-management services and celebrity influencers, and has resulted in major U.K.-wide GLP-1 shortages.
This situation is tragically widening health inequalities, as many of my patients who have been on GLP-1 receptor agonists for many years are unable to obtain them. I am having to consider switching therapies, often to less efficacious options without the compelling cardiorenal benefits. Furthermore, the GLP-1 shortages have prevented GLP-1 initiation for my other high-risk younger patients, potentially increasing future cardiometabolic risk.
There remain unanswered questions for tirzepatide: What is the durability of effect of tirzepatide after 72 weeks (that is, the trial duration of SURMOUNT-2)? Crucially, what is the effect of withdrawal of tirzepatide on weight loss maintenance? Previous evidence has suggested weight regain after discontinuation of a GLP-1 receptor agonist for obesity. This, of course, has further financial and sustainability implications for health care systems such as the NHS.
Finally, we are increasingly seeing younger women of childbearing age with or at risk for cardiometabolic disease. Again, my patient above is one example. Many of the therapies we use for cardiometabolic disease management, including GLP-1 receptor agonists and tirzepatide, have not been studied, and hence have not been licensed in pregnant women. Therefore, frank discussions are required with patients about future family plans and the importance of contraception. Often, the significant weight loss seen with GLP-1 receptor agonists can improve hormonal profiles and fertility in women and result in unexpected pregnancies if robust contraception is not in place.
Tirzepatide has yet to be made commercially available in the United Kingdom, and its price has also yet to be set. But I already envision a clear role for tirzepatide in my treatment armamentarium. I will be positioning tirzepatide as my first injectable of choice after oral treatment escalation with metformin and an SGLT2 inhibitor in all my patients who require treatment intensification – not just my younger, higher-risk individuals. This may remain an aspirational goal until supply chains and cost are defined. There is no doubt, however, that the compelling weight and glycemic benefits of tirzepatide alongside individualized lifestyle interventions can help improve the quality and quantity of life of my patients living with type 2 diabetes and obesity.
Dr. Fernando is a general practitioner near Edinburgh. He reported receiving speaker fees from Eli Lilly and Novo Nordisk..
A version of this article first appeared on Medscape.com.
As a general practitioner with a specialist interest in diabetes, I am increasingly diagnosing younger people living with type 2 diabetes and obesity. Sadly, my youngest patient living with type 2 diabetes and obesity is only in her early 20s.
In fact, in England, there are now more people under the age of 40 years living with type 2 diabetes than type 1 diabetes. These younger individuals tend to present with very high hemoglobin A1c levels; I am routinely seeing double-digit A1c percentage levels in my practice. Indeed, the patient mentioned above presented with an A1c of more than 13%.
The lifetime cardiometabolic risk of individuals like her is considerable and very worrying: Younger adults with type 2 diabetes often have adverse cardiometabolic risk profiles at diagnosis, with higher body mass indices, marked dyslipidemia, hypertension, and abnormal liver profiles suggesting nonalcoholic fatty liver disease. The cumulative impact of this risk profile is a significant impact on quality and quantity of life. Evidence tells us that a younger age of diagnosis with type 2 diabetes is associated with an increased risk for premature death, especially from cardiovascular disease.
Early treatment intensification is warranted in younger individuals living with type 2 diabetes and obesity. My patient above is now on triple therapy with metformin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, and a glucagonlike peptide–1 (GLP-1) receptor agonist. I gave her an urgent referral to my local weight management service for weight, nutritional, and psychological support. I have also issued her a real-time continuous glucose monitoring (rt-CGM) device: Whilst she does not meet any current U.K. criteria for using rt-CGM, I feel that the role of CGM as an educational tool for her is invaluable and equally important to her pharmacologic therapies. We are in desperate need of effective pharmacologic and lifestyle interventions to tackle this epidemic of cardiometabolic disease in the young.
I attended the recent ADA 2023 congress in San Diego, including the presentation of the SURMOUNT-2 trial data. SURMOUNT-2 explored the efficacy and safety of the dual GLP-GIP agonist tirzepatide for weight management in patients with obesity and type 2 diabetes. Tirzepatide was associated with significant reductions in weight (average weight loss, 14-16 kg after 72 weeks) and glycemia (2.1% reduction in A1c after 72 weeks), as well as reductions in clinically meaningful cardiometabolic risk factors, including systolic blood pressure, liver enzymes, and fasting non–HDL cholesterol levels. The overall safety profile of tirzepatide was also reassuring and consistent with the GLP-1 class. Most adverse effects were gastrointestinal and of mild to moderate severity. These adverse effects decreased over time.
These results perfectly position tirzepatide for my younger patients like the young woman mentioned above. The significant improvements in weight, glycemia, and cardiometabolic risk factors will not only help mitigate her future cardiometabolic risk but also help the sustainability of the U.K.’s National Health System. The cost of diabetes to the NHS in the United Kingdom is more than 10% of the entire NHS budget for England and Wales. More than 80% of this cost, however, is related not to the medications and devices we prescribe for diabetes but to the downstream complications of diabetes, such as hospital admissions for cardiovascular events and amputations, as well as regular hospital attendance for dialysis for end-stage kidney disease.
There is no doubt, however, that modern obesity medications such as semaglutide and tirzepatide are expensive, and demand has been astronomical. This demand has been driven by private weight-management services and celebrity influencers, and has resulted in major U.K.-wide GLP-1 shortages.
This situation is tragically widening health inequalities, as many of my patients who have been on GLP-1 receptor agonists for many years are unable to obtain them. I am having to consider switching therapies, often to less efficacious options without the compelling cardiorenal benefits. Furthermore, the GLP-1 shortages have prevented GLP-1 initiation for my other high-risk younger patients, potentially increasing future cardiometabolic risk.
There remain unanswered questions for tirzepatide: What is the durability of effect of tirzepatide after 72 weeks (that is, the trial duration of SURMOUNT-2)? Crucially, what is the effect of withdrawal of tirzepatide on weight loss maintenance? Previous evidence has suggested weight regain after discontinuation of a GLP-1 receptor agonist for obesity. This, of course, has further financial and sustainability implications for health care systems such as the NHS.
Finally, we are increasingly seeing younger women of childbearing age with or at risk for cardiometabolic disease. Again, my patient above is one example. Many of the therapies we use for cardiometabolic disease management, including GLP-1 receptor agonists and tirzepatide, have not been studied, and hence have not been licensed in pregnant women. Therefore, frank discussions are required with patients about future family plans and the importance of contraception. Often, the significant weight loss seen with GLP-1 receptor agonists can improve hormonal profiles and fertility in women and result in unexpected pregnancies if robust contraception is not in place.
Tirzepatide has yet to be made commercially available in the United Kingdom, and its price has also yet to be set. But I already envision a clear role for tirzepatide in my treatment armamentarium. I will be positioning tirzepatide as my first injectable of choice after oral treatment escalation with metformin and an SGLT2 inhibitor in all my patients who require treatment intensification – not just my younger, higher-risk individuals. This may remain an aspirational goal until supply chains and cost are defined. There is no doubt, however, that the compelling weight and glycemic benefits of tirzepatide alongside individualized lifestyle interventions can help improve the quality and quantity of life of my patients living with type 2 diabetes and obesity.
Dr. Fernando is a general practitioner near Edinburgh. He reported receiving speaker fees from Eli Lilly and Novo Nordisk..
A version of this article first appeared on Medscape.com.
Beta cells from stem cells: Nearing a cure for type 1 diabetes?
This transcript has been edited for clarity.
Those of us in the field of diabetes have long wanted to cure type 1 diabetes, and there are little steps making me feel like this might be a possibility. One of those steps is that a company named Vertex – I’m actually on the steering committee for Vertex in terms of this project – has made beta cells from stem cells. Now, instead of waiting for a cadaveric donor, we can make little beta cells. They started giving them to people in human trials. The Food and Drug Administration has been cautious because it’s new, and I get that.
In the first part of these trials, we could only give half a dose of these beta cells. The doses were determined based on what we know from giving beta-cell transplants from cadaveric donors. We gave half a dose of these stem cell–derived beta cells to two people who were having episodes of severe hypoglycemia.
In patient 1, these beta cells worked incredibly well. He became insulin independent, and now after over a year, he’s basically free of his type 1 diabetes. Patient 2 received half a dose, and she did get some activity of the beta cells, but not enough to achieve insulin independence, so she got a second dose. Shortly after the second dose, she decided she didn’t want to participate in the trial anymore and she was lost to follow-up.
Patient 2 didn’t get the same response as patient 1, but then we moved on to four more patients who got a full dose to start with. Now, there’s a total of six patients. Of those additional four patients, one of them has now been followed for a year. Just like patient 1, he’s off insulin. It’s as though his body has normal beta cells and he’s doing great. For the next three patients, we don’t have enough follow-up data to tell you what’s going to happen to them at a year.
I can tell you that, in all six patients, the beta cells worked. They basically were producing insulin, they had positive C-peptide levels, and it showed that these beta cells work when given to human beings. Now the trial is going to start giving more patients these stem cell–derived beta cells.
One of the things that’s important to realize is that this is a very small sample size, at just six individuals. Even within those six individuals, there was variation in terms of the response to the treatment. Probably, just like with all things in medicine, there will be different doses, different ways in which people do respond, people who get off of insulin completely, and people who may require some ongoing insulin therapy. I have no idea what this is going to look like as we test this in more people.
Everybody did start making C-peptide, they were having an effect of these beta cells, and it was working. We’ll have to see how well it works, how well it works in whom, and how we’re going to be able to use these types of therapies in the future.
In terms of side effects, they were really related to immunosuppression. There were no real surprises, but again, this is a very small sample size.
In summary, I think this is really hopeful. I don’t like to give false hope, but each step of this development process has shown that these beta cells derived from stem cells do seem to work in human beings as native beta cells might. Hopefully, this portends a future of newer therapies in the treatment of people with type 1 diabetes. Thank you.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Those of us in the field of diabetes have long wanted to cure type 1 diabetes, and there are little steps making me feel like this might be a possibility. One of those steps is that a company named Vertex – I’m actually on the steering committee for Vertex in terms of this project – has made beta cells from stem cells. Now, instead of waiting for a cadaveric donor, we can make little beta cells. They started giving them to people in human trials. The Food and Drug Administration has been cautious because it’s new, and I get that.
In the first part of these trials, we could only give half a dose of these beta cells. The doses were determined based on what we know from giving beta-cell transplants from cadaveric donors. We gave half a dose of these stem cell–derived beta cells to two people who were having episodes of severe hypoglycemia.
In patient 1, these beta cells worked incredibly well. He became insulin independent, and now after over a year, he’s basically free of his type 1 diabetes. Patient 2 received half a dose, and she did get some activity of the beta cells, but not enough to achieve insulin independence, so she got a second dose. Shortly after the second dose, she decided she didn’t want to participate in the trial anymore and she was lost to follow-up.
Patient 2 didn’t get the same response as patient 1, but then we moved on to four more patients who got a full dose to start with. Now, there’s a total of six patients. Of those additional four patients, one of them has now been followed for a year. Just like patient 1, he’s off insulin. It’s as though his body has normal beta cells and he’s doing great. For the next three patients, we don’t have enough follow-up data to tell you what’s going to happen to them at a year.
I can tell you that, in all six patients, the beta cells worked. They basically were producing insulin, they had positive C-peptide levels, and it showed that these beta cells work when given to human beings. Now the trial is going to start giving more patients these stem cell–derived beta cells.
One of the things that’s important to realize is that this is a very small sample size, at just six individuals. Even within those six individuals, there was variation in terms of the response to the treatment. Probably, just like with all things in medicine, there will be different doses, different ways in which people do respond, people who get off of insulin completely, and people who may require some ongoing insulin therapy. I have no idea what this is going to look like as we test this in more people.
Everybody did start making C-peptide, they were having an effect of these beta cells, and it was working. We’ll have to see how well it works, how well it works in whom, and how we’re going to be able to use these types of therapies in the future.
In terms of side effects, they were really related to immunosuppression. There were no real surprises, but again, this is a very small sample size.
In summary, I think this is really hopeful. I don’t like to give false hope, but each step of this development process has shown that these beta cells derived from stem cells do seem to work in human beings as native beta cells might. Hopefully, this portends a future of newer therapies in the treatment of people with type 1 diabetes. Thank you.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Those of us in the field of diabetes have long wanted to cure type 1 diabetes, and there are little steps making me feel like this might be a possibility. One of those steps is that a company named Vertex – I’m actually on the steering committee for Vertex in terms of this project – has made beta cells from stem cells. Now, instead of waiting for a cadaveric donor, we can make little beta cells. They started giving them to people in human trials. The Food and Drug Administration has been cautious because it’s new, and I get that.
In the first part of these trials, we could only give half a dose of these beta cells. The doses were determined based on what we know from giving beta-cell transplants from cadaveric donors. We gave half a dose of these stem cell–derived beta cells to two people who were having episodes of severe hypoglycemia.
In patient 1, these beta cells worked incredibly well. He became insulin independent, and now after over a year, he’s basically free of his type 1 diabetes. Patient 2 received half a dose, and she did get some activity of the beta cells, but not enough to achieve insulin independence, so she got a second dose. Shortly after the second dose, she decided she didn’t want to participate in the trial anymore and she was lost to follow-up.
Patient 2 didn’t get the same response as patient 1, but then we moved on to four more patients who got a full dose to start with. Now, there’s a total of six patients. Of those additional four patients, one of them has now been followed for a year. Just like patient 1, he’s off insulin. It’s as though his body has normal beta cells and he’s doing great. For the next three patients, we don’t have enough follow-up data to tell you what’s going to happen to them at a year.
I can tell you that, in all six patients, the beta cells worked. They basically were producing insulin, they had positive C-peptide levels, and it showed that these beta cells work when given to human beings. Now the trial is going to start giving more patients these stem cell–derived beta cells.
One of the things that’s important to realize is that this is a very small sample size, at just six individuals. Even within those six individuals, there was variation in terms of the response to the treatment. Probably, just like with all things in medicine, there will be different doses, different ways in which people do respond, people who get off of insulin completely, and people who may require some ongoing insulin therapy. I have no idea what this is going to look like as we test this in more people.
Everybody did start making C-peptide, they were having an effect of these beta cells, and it was working. We’ll have to see how well it works, how well it works in whom, and how we’re going to be able to use these types of therapies in the future.
In terms of side effects, they were really related to immunosuppression. There were no real surprises, but again, this is a very small sample size.
In summary, I think this is really hopeful. I don’t like to give false hope, but each step of this development process has shown that these beta cells derived from stem cells do seem to work in human beings as native beta cells might. Hopefully, this portends a future of newer therapies in the treatment of people with type 1 diabetes. Thank you.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen.
A version of this article originally appeared on Medscape.com.
New DEA CME mandate affects 2 million prescribers
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.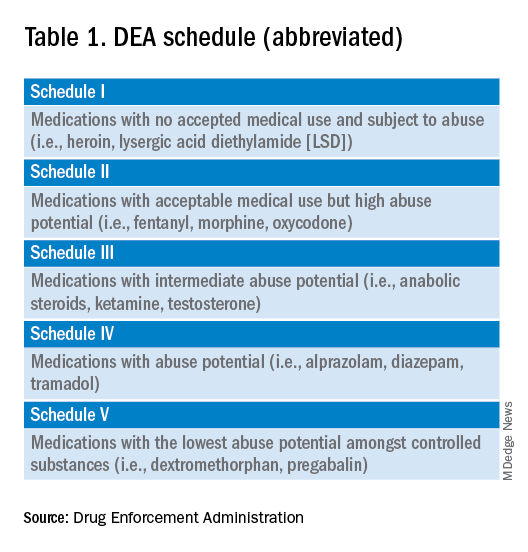
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 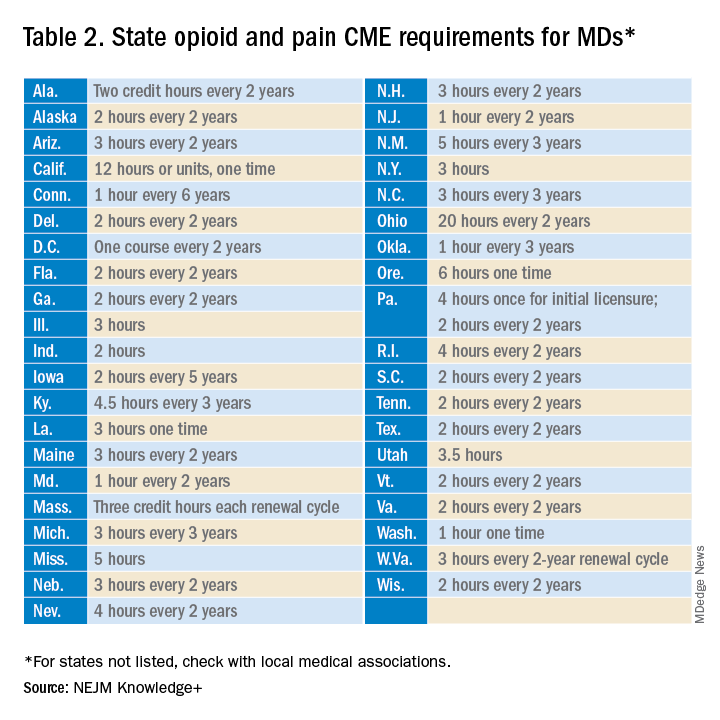
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
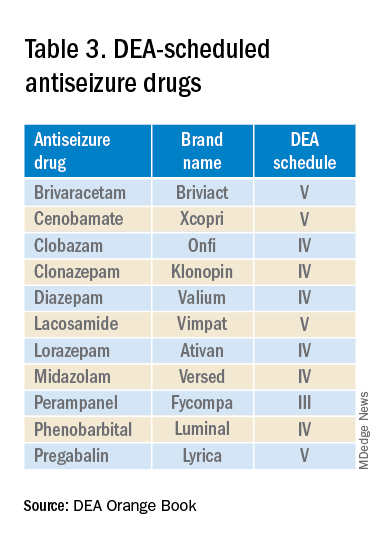
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).
Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The most important question in medicine
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Today I am going to tell you the single best question you can ask any doctor, the one that has saved my butt countless times throughout my career, the one that every attending physician should be asking every intern and resident when they present a new case. That question: “What else could this be?”
I know, I know – “When you hear hoofbeats, think horses, not zebras.” I get it. But sometimes we get so good at our jobs, so good at recognizing horses, that we stop asking ourselves about zebras at all. You see this in a phenomenon known as “anchoring bias” where physicians, when presented with a diagnosis, tend to latch on to that diagnosis based on the first piece of information given, paying attention to data that support it and ignoring data that point in other directions.
That special question: “What else could this be?”, breaks through that barrier. It forces you, the medical team, everyone, to go through the exercise of real, old-fashioned differential diagnosis. And I promise that if you do this enough, at some point it will save someone’s life.
Though the concept of anchoring bias in medicine is broadly understood, it hasn’t been broadly studied until now, with this study appearing in JAMA Internal Medicine.
Here’s the setup.
The authors hypothesized that there would be substantial anchoring bias when patients with heart failure presented to the emergency department with shortness of breath if the triage “visit reason” section mentioned HF. We’re talking about the subtle difference between the following:
- Visit reason: Shortness of breath
- Visit reason: Shortness of breath/HF
People with HF can be short of breath for lots of reasons. HF exacerbation comes immediately to mind and it should. But there are obviously lots of answers to that “What else could this be?” question: pneumonia, pneumothorax, heart attack, COPD, and, of course, pulmonary embolism (PE).
The authors leveraged the nationwide VA database, allowing them to examine data from over 100,000 patients presenting to various VA EDs with shortness of breath. They then looked for particular tests – D-dimer, CT chest with contrast, V/Q scan, lower-extremity Doppler — that would suggest that the doctor was thinking about PE. The question, then, is whether mentioning HF in that little “visit reason” section would influence the likelihood of testing for PE.
I know what you’re thinking: Not everyone who is short of breath needs an evaluation for PE. And the authors did a nice job accounting for a variety of factors that might predict a PE workup: malignancy, recent surgery, elevated heart rate, low oxygen saturation, etc. Of course, some of those same factors might predict whether that triage nurse will write HF in the visit reason section. All of these things need to be accounted for statistically, and were, but – the unofficial Impact Factor motto reminds us that “there are always more confounders.”
But let’s dig into the results. I’m going to give you the raw numbers first. There were 4,392 people with HF whose visit reason section, in addition to noting shortness of breath, explicitly mentioned HF. Of those, 360 had PE testing and two had a PE diagnosed during that ED visit. So that’s around an 8% testing rate and a 0.5% hit rate for testing. But 43 people, presumably not tested in the ED, had a PE diagnosed within the next 30 days. Assuming that those PEs were present at the ED visit, that means the ED missed 95% of the PEs in the group with that HF label attached to them.
Let’s do the same thing for those whose visit reason just said “shortness of breath.”
Of the 103,627 people in that category, 13,886 were tested for PE and 231 of those tested positive. So that is an overall testing rate of around 13% and a hit rate of 1.7%. And 1,081 of these people had a PE diagnosed within 30 days. Assuming that those PEs were actually present at the ED visit, the docs missed 79% of them.
There’s one other thing to notice from the data: The overall PE rate (diagnosed by 30 days) was basically the same in both groups. That HF label does not really flag a group at lower risk for PE.
Yes, there are a lot of assumptions here, including that all PEs that were actually there in the ED got caught within 30 days, but the numbers do paint a picture. In this unadjusted analysis, it seems that the HF label leads to less testing and more missed PEs. Classic anchoring bias.
The adjusted analysis, accounting for all those PE risk factors, really didn’t change these results. You get nearly the same numbers and thus nearly the same conclusions.
Now, the main missing piece of this puzzle is in the mind of the clinician. We don’t know whether they didn’t consider PE or whether they considered PE but thought it unlikely. And in the end, it’s clear that the vast majority of people in this study did not have PE (though I suspect not all had a simple HF exacerbation). But this type of analysis is useful not only for the empiric evidence of the clinical impact of anchoring bias but because of the fact that it reminds us all to ask that all-important question: What else could this be?
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Today I am going to tell you the single best question you can ask any doctor, the one that has saved my butt countless times throughout my career, the one that every attending physician should be asking every intern and resident when they present a new case. That question: “What else could this be?”
I know, I know – “When you hear hoofbeats, think horses, not zebras.” I get it. But sometimes we get so good at our jobs, so good at recognizing horses, that we stop asking ourselves about zebras at all. You see this in a phenomenon known as “anchoring bias” where physicians, when presented with a diagnosis, tend to latch on to that diagnosis based on the first piece of information given, paying attention to data that support it and ignoring data that point in other directions.
That special question: “What else could this be?”, breaks through that barrier. It forces you, the medical team, everyone, to go through the exercise of real, old-fashioned differential diagnosis. And I promise that if you do this enough, at some point it will save someone’s life.
Though the concept of anchoring bias in medicine is broadly understood, it hasn’t been broadly studied until now, with this study appearing in JAMA Internal Medicine.
Here’s the setup.
The authors hypothesized that there would be substantial anchoring bias when patients with heart failure presented to the emergency department with shortness of breath if the triage “visit reason” section mentioned HF. We’re talking about the subtle difference between the following:
- Visit reason: Shortness of breath
- Visit reason: Shortness of breath/HF
People with HF can be short of breath for lots of reasons. HF exacerbation comes immediately to mind and it should. But there are obviously lots of answers to that “What else could this be?” question: pneumonia, pneumothorax, heart attack, COPD, and, of course, pulmonary embolism (PE).
The authors leveraged the nationwide VA database, allowing them to examine data from over 100,000 patients presenting to various VA EDs with shortness of breath. They then looked for particular tests – D-dimer, CT chest with contrast, V/Q scan, lower-extremity Doppler — that would suggest that the doctor was thinking about PE. The question, then, is whether mentioning HF in that little “visit reason” section would influence the likelihood of testing for PE.
I know what you’re thinking: Not everyone who is short of breath needs an evaluation for PE. And the authors did a nice job accounting for a variety of factors that might predict a PE workup: malignancy, recent surgery, elevated heart rate, low oxygen saturation, etc. Of course, some of those same factors might predict whether that triage nurse will write HF in the visit reason section. All of these things need to be accounted for statistically, and were, but – the unofficial Impact Factor motto reminds us that “there are always more confounders.”
But let’s dig into the results. I’m going to give you the raw numbers first. There were 4,392 people with HF whose visit reason section, in addition to noting shortness of breath, explicitly mentioned HF. Of those, 360 had PE testing and two had a PE diagnosed during that ED visit. So that’s around an 8% testing rate and a 0.5% hit rate for testing. But 43 people, presumably not tested in the ED, had a PE diagnosed within the next 30 days. Assuming that those PEs were present at the ED visit, that means the ED missed 95% of the PEs in the group with that HF label attached to them.
Let’s do the same thing for those whose visit reason just said “shortness of breath.”
Of the 103,627 people in that category, 13,886 were tested for PE and 231 of those tested positive. So that is an overall testing rate of around 13% and a hit rate of 1.7%. And 1,081 of these people had a PE diagnosed within 30 days. Assuming that those PEs were actually present at the ED visit, the docs missed 79% of them.
There’s one other thing to notice from the data: The overall PE rate (diagnosed by 30 days) was basically the same in both groups. That HF label does not really flag a group at lower risk for PE.
Yes, there are a lot of assumptions here, including that all PEs that were actually there in the ED got caught within 30 days, but the numbers do paint a picture. In this unadjusted analysis, it seems that the HF label leads to less testing and more missed PEs. Classic anchoring bias.
The adjusted analysis, accounting for all those PE risk factors, really didn’t change these results. You get nearly the same numbers and thus nearly the same conclusions.
Now, the main missing piece of this puzzle is in the mind of the clinician. We don’t know whether they didn’t consider PE or whether they considered PE but thought it unlikely. And in the end, it’s clear that the vast majority of people in this study did not have PE (though I suspect not all had a simple HF exacerbation). But this type of analysis is useful not only for the empiric evidence of the clinical impact of anchoring bias but because of the fact that it reminds us all to ask that all-important question: What else could this be?
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Today I am going to tell you the single best question you can ask any doctor, the one that has saved my butt countless times throughout my career, the one that every attending physician should be asking every intern and resident when they present a new case. That question: “What else could this be?”
I know, I know – “When you hear hoofbeats, think horses, not zebras.” I get it. But sometimes we get so good at our jobs, so good at recognizing horses, that we stop asking ourselves about zebras at all. You see this in a phenomenon known as “anchoring bias” where physicians, when presented with a diagnosis, tend to latch on to that diagnosis based on the first piece of information given, paying attention to data that support it and ignoring data that point in other directions.
That special question: “What else could this be?”, breaks through that barrier. It forces you, the medical team, everyone, to go through the exercise of real, old-fashioned differential diagnosis. And I promise that if you do this enough, at some point it will save someone’s life.
Though the concept of anchoring bias in medicine is broadly understood, it hasn’t been broadly studied until now, with this study appearing in JAMA Internal Medicine.
Here’s the setup.
The authors hypothesized that there would be substantial anchoring bias when patients with heart failure presented to the emergency department with shortness of breath if the triage “visit reason” section mentioned HF. We’re talking about the subtle difference between the following:
- Visit reason: Shortness of breath
- Visit reason: Shortness of breath/HF
People with HF can be short of breath for lots of reasons. HF exacerbation comes immediately to mind and it should. But there are obviously lots of answers to that “What else could this be?” question: pneumonia, pneumothorax, heart attack, COPD, and, of course, pulmonary embolism (PE).
The authors leveraged the nationwide VA database, allowing them to examine data from over 100,000 patients presenting to various VA EDs with shortness of breath. They then looked for particular tests – D-dimer, CT chest with contrast, V/Q scan, lower-extremity Doppler — that would suggest that the doctor was thinking about PE. The question, then, is whether mentioning HF in that little “visit reason” section would influence the likelihood of testing for PE.
I know what you’re thinking: Not everyone who is short of breath needs an evaluation for PE. And the authors did a nice job accounting for a variety of factors that might predict a PE workup: malignancy, recent surgery, elevated heart rate, low oxygen saturation, etc. Of course, some of those same factors might predict whether that triage nurse will write HF in the visit reason section. All of these things need to be accounted for statistically, and were, but – the unofficial Impact Factor motto reminds us that “there are always more confounders.”
But let’s dig into the results. I’m going to give you the raw numbers first. There were 4,392 people with HF whose visit reason section, in addition to noting shortness of breath, explicitly mentioned HF. Of those, 360 had PE testing and two had a PE diagnosed during that ED visit. So that’s around an 8% testing rate and a 0.5% hit rate for testing. But 43 people, presumably not tested in the ED, had a PE diagnosed within the next 30 days. Assuming that those PEs were present at the ED visit, that means the ED missed 95% of the PEs in the group with that HF label attached to them.
Let’s do the same thing for those whose visit reason just said “shortness of breath.”
Of the 103,627 people in that category, 13,886 were tested for PE and 231 of those tested positive. So that is an overall testing rate of around 13% and a hit rate of 1.7%. And 1,081 of these people had a PE diagnosed within 30 days. Assuming that those PEs were actually present at the ED visit, the docs missed 79% of them.
There’s one other thing to notice from the data: The overall PE rate (diagnosed by 30 days) was basically the same in both groups. That HF label does not really flag a group at lower risk for PE.
Yes, there are a lot of assumptions here, including that all PEs that were actually there in the ED got caught within 30 days, but the numbers do paint a picture. In this unadjusted analysis, it seems that the HF label leads to less testing and more missed PEs. Classic anchoring bias.
The adjusted analysis, accounting for all those PE risk factors, really didn’t change these results. You get nearly the same numbers and thus nearly the same conclusions.
Now, the main missing piece of this puzzle is in the mind of the clinician. We don’t know whether they didn’t consider PE or whether they considered PE but thought it unlikely. And in the end, it’s clear that the vast majority of people in this study did not have PE (though I suspect not all had a simple HF exacerbation). But this type of analysis is useful not only for the empiric evidence of the clinical impact of anchoring bias but because of the fact that it reminds us all to ask that all-important question: What else could this be?
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
In defense of artificial sweeteners
More than 140 million Americans use artificial sweeteners, a habit driven by the irrefutable fact that excess sugar is harmful. But I’m continually amazed by alarmist headlines on the topic.
In May, the World Health Organization (WHO) released a report to support its “conditional recommendation” against the use of non-sugar sweeteners (NSS) for weight control. Despite the WHO’s goal “to provide evidence-informed guidance,” the report includes the disclaimer that “The recommendation is based on evidence of low certainty.”
Low certainty is an accurate descriptor for the findings of many of the 280-plus studies in the report. That the guidance does not apply to patients with diabetes was easily lost in the repeated mentions of the perceived dangers of these sugar alternatives.
The review included various table-top and beverage sweeteners, including acesulfame K, aspartame, saccharin, sucralose, stevia, and stevia derivatives. Low-calorie sugars and sugar alcohols such as erythritol were excluded.
The WHO looked at long- and short-term trials, randomized controlled trials (RCTs), prospective studies, and case-control studies measuring a wide range of endpoints, from dental caries to cancer. The report highlighted that some findings cannot be attributed directly to NSS use but may simply be due to their substitution for sugar. Differences in outcomes due to sex, ethnicity, and body weight status could not be assessed either. And the WHO conceded the possibility of reverse causation in observational studies wherein higher-risk individuals may consume more NSS.
Nonnutritive sweeteners are given little credit for weight loss. “A significant difference in body weight and BMI was only observed in trials that reported a reduction in energy intake ... rather than primarily by an inherent property of NSS that can modulate body weight (independently of energy intake),” the report reads. But isn’t the desired effect of using an artificial sweetener instead of table sugar that you lower your calorie intake?
The WHO noted that weight loss was not sustained – a finding in nearly every weight loss trial in history and something more attributable to human nature than the sweetener one chooses.
The document outlines that meta-analyses of prospective cohort studies show that higher intakes of NSS were associated with an increased risk for type 2 diabetes and elevated fasting glucose, while meta-analyses of randomized trials suggest no significant effect on “biomarkers used in the assessment and diagnosis of diabetes and insulin resistance, including fasting glucose, fasting insulin and hemoglobin A1c.”
Similar disparities are noted with cardiovascular risk. Prospective trials suggest an increased risk for CVD, including stroke and its precursor, hypertension; but again, the RCT data found no evidence to suggest a significant effect “on biomarkers used in the assessment and diagnosis of CVDs, including blood pressure, low-density lipoprotein cholesterol and other blood lipids.”
Splenda and stevia under fire
Predictably, some in the nonnutritive sweetener industry are incensed.
Ted Gelov, CEO of Heartland Food Products Group, maker of Splenda, responded in a press release, “Every few years now it seems I have to come to you and clarify misleading headlines ... Suggesting that sweeteners like Splenda cannot have long-term benefits is a disservice to healthcare providers, their patients, and all consumers.”
Splenda has been on the U.S. market since 1999, and Mr. Gelov reportedly uses three to eight packets daily in his coffee and tea.
I reached out to Heartland and they sent me an eight-page document consisting of over 50 statements, summaries, and clinical trials supporting the safety of artificial sweeteners, including sucralose, an ingredient in Splenda. In 2016, Mr. Gelov rebutted claims that sucralose was linked to cancer in Swiss male mice. These “dramatized headlines are based on one flawed study by an isolated Italian research laboratory, the Ramazzini Institute,” Mr. Gelov wrote.
Another recent headline was about the DNA-damaging effects of sucralose-6-acetate (S6A) seen in an in vitro study published in the Journal of Toxicology and Environmental Health. According to the authors, commercial sucralose samples contain up to 0.67% S6A, a manufacturing impurity.
Despite many reports linking this study to Splenda, Heartland wrote that “Splenda and its ingredients were never studied or tested in this research. We, and our suppliers, rigorously and routinely test and monitor for any impurities in our products. We can confirm that S6A is not present in Splenda Brand sucralose down to the lowest detection limit possible, which is .001% sensitivity level.”
F. Perry Wilson, MD, director of Clinical and Translational Research Accelerator at Yale and a regular contributor to this news organization, took to Twitter to put this study in context: “The human exposure equivalent to sucralose would be 60 packets per day,” he pointed out. And the blood levels of S6A with normal consumption would not “come close to the DNA damage threshold noted in the article.”
Perhaps the most concerning scientific data suggesting a link between artificial sweetener use and ill health is a Cleveland Clinic study showing an association between higher blood levels of erythritol and adverse cardiovascular outcomes such as heart attack, stroke, or death. The researchers also found that erythritol, which is found in stevia and some keto food products, made platelet activation and clot formation easier.
When I asked about these findings, Heartland stated, “The study was primarily conducted on patients who were at an elevated risk of cardiovascular events due to their advanced age, elevated body mass and presence of pre-existing health conditions ... the stated findings were only an association and cannot imply causation.”
The main conclusion I’ve drawn on the topic of artificial sweeteners is that a lot of resources were wasted in performing underpowered, poorly designed trials on compounds that are already generally regarded as safe (GRAS) by the FDA. The WHO “conditional guideline” is, by its own description, based on a plethora of “low certainty” to “very low certainty” evidence.
The monies to produce the WHO report and many of these trials would have been better spent educating the public on the difference between simple and complex carbohydrates; the inflammatory and disease-producing effects of excess sugars; and how to prevent, diagnose, and treat diabetes.
If more trials on artificial sweeteners are planned, they should be performed on people doing human things – which does not include ingesting 60 packets of any sweetener in a single day.
In my personal N-of-1 trial, consuming sugar makes me crave more, feel sluggish, and gain weight. I don’t believe that NSS alone will control my weight. But I’ll continue to drink two cups of stevia-laced coffee every morning, take walks, avoid alcohol, eat my vegetables, and hope for the best.
Dr. Walton-Shirley is a clinical cardiologist in Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
More than 140 million Americans use artificial sweeteners, a habit driven by the irrefutable fact that excess sugar is harmful. But I’m continually amazed by alarmist headlines on the topic.
In May, the World Health Organization (WHO) released a report to support its “conditional recommendation” against the use of non-sugar sweeteners (NSS) for weight control. Despite the WHO’s goal “to provide evidence-informed guidance,” the report includes the disclaimer that “The recommendation is based on evidence of low certainty.”
Low certainty is an accurate descriptor for the findings of many of the 280-plus studies in the report. That the guidance does not apply to patients with diabetes was easily lost in the repeated mentions of the perceived dangers of these sugar alternatives.
The review included various table-top and beverage sweeteners, including acesulfame K, aspartame, saccharin, sucralose, stevia, and stevia derivatives. Low-calorie sugars and sugar alcohols such as erythritol were excluded.
The WHO looked at long- and short-term trials, randomized controlled trials (RCTs), prospective studies, and case-control studies measuring a wide range of endpoints, from dental caries to cancer. The report highlighted that some findings cannot be attributed directly to NSS use but may simply be due to their substitution for sugar. Differences in outcomes due to sex, ethnicity, and body weight status could not be assessed either. And the WHO conceded the possibility of reverse causation in observational studies wherein higher-risk individuals may consume more NSS.
Nonnutritive sweeteners are given little credit for weight loss. “A significant difference in body weight and BMI was only observed in trials that reported a reduction in energy intake ... rather than primarily by an inherent property of NSS that can modulate body weight (independently of energy intake),” the report reads. But isn’t the desired effect of using an artificial sweetener instead of table sugar that you lower your calorie intake?
The WHO noted that weight loss was not sustained – a finding in nearly every weight loss trial in history and something more attributable to human nature than the sweetener one chooses.
The document outlines that meta-analyses of prospective cohort studies show that higher intakes of NSS were associated with an increased risk for type 2 diabetes and elevated fasting glucose, while meta-analyses of randomized trials suggest no significant effect on “biomarkers used in the assessment and diagnosis of diabetes and insulin resistance, including fasting glucose, fasting insulin and hemoglobin A1c.”
Similar disparities are noted with cardiovascular risk. Prospective trials suggest an increased risk for CVD, including stroke and its precursor, hypertension; but again, the RCT data found no evidence to suggest a significant effect “on biomarkers used in the assessment and diagnosis of CVDs, including blood pressure, low-density lipoprotein cholesterol and other blood lipids.”
Splenda and stevia under fire
Predictably, some in the nonnutritive sweetener industry are incensed.
Ted Gelov, CEO of Heartland Food Products Group, maker of Splenda, responded in a press release, “Every few years now it seems I have to come to you and clarify misleading headlines ... Suggesting that sweeteners like Splenda cannot have long-term benefits is a disservice to healthcare providers, their patients, and all consumers.”
Splenda has been on the U.S. market since 1999, and Mr. Gelov reportedly uses three to eight packets daily in his coffee and tea.
I reached out to Heartland and they sent me an eight-page document consisting of over 50 statements, summaries, and clinical trials supporting the safety of artificial sweeteners, including sucralose, an ingredient in Splenda. In 2016, Mr. Gelov rebutted claims that sucralose was linked to cancer in Swiss male mice. These “dramatized headlines are based on one flawed study by an isolated Italian research laboratory, the Ramazzini Institute,” Mr. Gelov wrote.
Another recent headline was about the DNA-damaging effects of sucralose-6-acetate (S6A) seen in an in vitro study published in the Journal of Toxicology and Environmental Health. According to the authors, commercial sucralose samples contain up to 0.67% S6A, a manufacturing impurity.
Despite many reports linking this study to Splenda, Heartland wrote that “Splenda and its ingredients were never studied or tested in this research. We, and our suppliers, rigorously and routinely test and monitor for any impurities in our products. We can confirm that S6A is not present in Splenda Brand sucralose down to the lowest detection limit possible, which is .001% sensitivity level.”
F. Perry Wilson, MD, director of Clinical and Translational Research Accelerator at Yale and a regular contributor to this news organization, took to Twitter to put this study in context: “The human exposure equivalent to sucralose would be 60 packets per day,” he pointed out. And the blood levels of S6A with normal consumption would not “come close to the DNA damage threshold noted in the article.”
Perhaps the most concerning scientific data suggesting a link between artificial sweetener use and ill health is a Cleveland Clinic study showing an association between higher blood levels of erythritol and adverse cardiovascular outcomes such as heart attack, stroke, or death. The researchers also found that erythritol, which is found in stevia and some keto food products, made platelet activation and clot formation easier.
When I asked about these findings, Heartland stated, “The study was primarily conducted on patients who were at an elevated risk of cardiovascular events due to their advanced age, elevated body mass and presence of pre-existing health conditions ... the stated findings were only an association and cannot imply causation.”
The main conclusion I’ve drawn on the topic of artificial sweeteners is that a lot of resources were wasted in performing underpowered, poorly designed trials on compounds that are already generally regarded as safe (GRAS) by the FDA. The WHO “conditional guideline” is, by its own description, based on a plethora of “low certainty” to “very low certainty” evidence.
The monies to produce the WHO report and many of these trials would have been better spent educating the public on the difference between simple and complex carbohydrates; the inflammatory and disease-producing effects of excess sugars; and how to prevent, diagnose, and treat diabetes.
If more trials on artificial sweeteners are planned, they should be performed on people doing human things – which does not include ingesting 60 packets of any sweetener in a single day.
In my personal N-of-1 trial, consuming sugar makes me crave more, feel sluggish, and gain weight. I don’t believe that NSS alone will control my weight. But I’ll continue to drink two cups of stevia-laced coffee every morning, take walks, avoid alcohol, eat my vegetables, and hope for the best.
Dr. Walton-Shirley is a clinical cardiologist in Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
More than 140 million Americans use artificial sweeteners, a habit driven by the irrefutable fact that excess sugar is harmful. But I’m continually amazed by alarmist headlines on the topic.
In May, the World Health Organization (WHO) released a report to support its “conditional recommendation” against the use of non-sugar sweeteners (NSS) for weight control. Despite the WHO’s goal “to provide evidence-informed guidance,” the report includes the disclaimer that “The recommendation is based on evidence of low certainty.”
Low certainty is an accurate descriptor for the findings of many of the 280-plus studies in the report. That the guidance does not apply to patients with diabetes was easily lost in the repeated mentions of the perceived dangers of these sugar alternatives.
The review included various table-top and beverage sweeteners, including acesulfame K, aspartame, saccharin, sucralose, stevia, and stevia derivatives. Low-calorie sugars and sugar alcohols such as erythritol were excluded.
The WHO looked at long- and short-term trials, randomized controlled trials (RCTs), prospective studies, and case-control studies measuring a wide range of endpoints, from dental caries to cancer. The report highlighted that some findings cannot be attributed directly to NSS use but may simply be due to their substitution for sugar. Differences in outcomes due to sex, ethnicity, and body weight status could not be assessed either. And the WHO conceded the possibility of reverse causation in observational studies wherein higher-risk individuals may consume more NSS.
Nonnutritive sweeteners are given little credit for weight loss. “A significant difference in body weight and BMI was only observed in trials that reported a reduction in energy intake ... rather than primarily by an inherent property of NSS that can modulate body weight (independently of energy intake),” the report reads. But isn’t the desired effect of using an artificial sweetener instead of table sugar that you lower your calorie intake?
The WHO noted that weight loss was not sustained – a finding in nearly every weight loss trial in history and something more attributable to human nature than the sweetener one chooses.
The document outlines that meta-analyses of prospective cohort studies show that higher intakes of NSS were associated with an increased risk for type 2 diabetes and elevated fasting glucose, while meta-analyses of randomized trials suggest no significant effect on “biomarkers used in the assessment and diagnosis of diabetes and insulin resistance, including fasting glucose, fasting insulin and hemoglobin A1c.”
Similar disparities are noted with cardiovascular risk. Prospective trials suggest an increased risk for CVD, including stroke and its precursor, hypertension; but again, the RCT data found no evidence to suggest a significant effect “on biomarkers used in the assessment and diagnosis of CVDs, including blood pressure, low-density lipoprotein cholesterol and other blood lipids.”
Splenda and stevia under fire
Predictably, some in the nonnutritive sweetener industry are incensed.
Ted Gelov, CEO of Heartland Food Products Group, maker of Splenda, responded in a press release, “Every few years now it seems I have to come to you and clarify misleading headlines ... Suggesting that sweeteners like Splenda cannot have long-term benefits is a disservice to healthcare providers, their patients, and all consumers.”
Splenda has been on the U.S. market since 1999, and Mr. Gelov reportedly uses three to eight packets daily in his coffee and tea.
I reached out to Heartland and they sent me an eight-page document consisting of over 50 statements, summaries, and clinical trials supporting the safety of artificial sweeteners, including sucralose, an ingredient in Splenda. In 2016, Mr. Gelov rebutted claims that sucralose was linked to cancer in Swiss male mice. These “dramatized headlines are based on one flawed study by an isolated Italian research laboratory, the Ramazzini Institute,” Mr. Gelov wrote.
Another recent headline was about the DNA-damaging effects of sucralose-6-acetate (S6A) seen in an in vitro study published in the Journal of Toxicology and Environmental Health. According to the authors, commercial sucralose samples contain up to 0.67% S6A, a manufacturing impurity.
Despite many reports linking this study to Splenda, Heartland wrote that “Splenda and its ingredients were never studied or tested in this research. We, and our suppliers, rigorously and routinely test and monitor for any impurities in our products. We can confirm that S6A is not present in Splenda Brand sucralose down to the lowest detection limit possible, which is .001% sensitivity level.”
F. Perry Wilson, MD, director of Clinical and Translational Research Accelerator at Yale and a regular contributor to this news organization, took to Twitter to put this study in context: “The human exposure equivalent to sucralose would be 60 packets per day,” he pointed out. And the blood levels of S6A with normal consumption would not “come close to the DNA damage threshold noted in the article.”
Perhaps the most concerning scientific data suggesting a link between artificial sweetener use and ill health is a Cleveland Clinic study showing an association between higher blood levels of erythritol and adverse cardiovascular outcomes such as heart attack, stroke, or death. The researchers also found that erythritol, which is found in stevia and some keto food products, made platelet activation and clot formation easier.
When I asked about these findings, Heartland stated, “The study was primarily conducted on patients who were at an elevated risk of cardiovascular events due to their advanced age, elevated body mass and presence of pre-existing health conditions ... the stated findings were only an association and cannot imply causation.”
The main conclusion I’ve drawn on the topic of artificial sweeteners is that a lot of resources were wasted in performing underpowered, poorly designed trials on compounds that are already generally regarded as safe (GRAS) by the FDA. The WHO “conditional guideline” is, by its own description, based on a plethora of “low certainty” to “very low certainty” evidence.
The monies to produce the WHO report and many of these trials would have been better spent educating the public on the difference between simple and complex carbohydrates; the inflammatory and disease-producing effects of excess sugars; and how to prevent, diagnose, and treat diabetes.
If more trials on artificial sweeteners are planned, they should be performed on people doing human things – which does not include ingesting 60 packets of any sweetener in a single day.
In my personal N-of-1 trial, consuming sugar makes me crave more, feel sluggish, and gain weight. I don’t believe that NSS alone will control my weight. But I’ll continue to drink two cups of stevia-laced coffee every morning, take walks, avoid alcohol, eat my vegetables, and hope for the best.
Dr. Walton-Shirley is a clinical cardiologist in Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Updates on pregnancy outcomes in transgender men
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologists and midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately,
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
For patients seeking to become pregnant, testosterone must be discontinued. Testosterone is teratogenic; it can cause abnormal urogenital development in the female fetus and should be avoided even prior to conception.1,2 The timing of testosterone discontinuation is debatable. There are no well-established guidelines dictating how early pregnancy can be attempted after cessation of testosterone, but typically if menses has resumed, the teratogenic effects of testosterone are less likely.
For amenorrheic patients on testosterone, menses will occur, on average, 3-6 months after testosterone is stopped. Of note, the longer that testosterone has been suspended, the greater the likelihood of achieving pregnancy.3 In a study by Light et al., 72% of patients conceived within 6 months of attempting pregnancy, 80% resumed menses within 6 months of stopping testosterone, and 20% of individuals conceived while they were amenorrheic from testosterone.4
Psychosocial support is an essential part of pregnancy care in transgender men. For some patients, pregnancy can worsen gender dysphoria, whereas others are empowered by the experience. Insurance companies may also deny obstetric care services to transgender males who have already changed their gender marker from female to male on insurance policies.
Whether transmasculine individuals are at higher risk for pregnancy complications is largely unknown, although emerging research in this field has yielded interesting results. While testosterone can cause vaginal atrophy, it does not seem to increase a patient’s risk of vaginal lacerations or their ability to have a successful vaginal delivery. For transgender men with significant discomfort around their genitalia, an elective cesarean section may be appropriate.5
More recently, Stroumsa et al. conducted an analysis of all deliveries at a Michigan institution from 2014 to 2018. Patients with male gender at the time of delivery or with the diagnostic code of gender dysphoria were identified as transgender.6 The primary outcome of this study was severe parental morbidity (such as amniotic fluid embolism, acute myocardial infarction, eclampsia, etc.), with secondary outcomes investigating rates of cesarean delivery and preterm birth.
During this time period, the researchers identified 256 transgender patients and 1.3 million cisgender patients in their Medicaid database and 1,651 transgender patients and 1.5 million cisgender patients in the commercial database who had experienced a delivery.6 Compared with cisgender patients, transgender patients in the Medicaid database were younger, less likely to be white, and more likely to have a chronic condition.6 Compared with cisgender patients in the commercial database, transgender patients experienced higher rates of anxiety and depression.6 Both transgender and cisgender patients had similar rates of severe parental morbidity. Ironically, rates of cesarean delivery were lower, compared with cisgender patients, in both the Medicaid and commercial databases, with no differences observed between rates of preterm birth.6
While more research is needed on pregnancy in transgender men, this analysis is not only one of the largest to date, but it also challenges many misconceptions providers have regarding pregnancy outcomes. Even though transmasculine patients may require additional medical interventions to achieve pregnancy, such as assisted reproductive technology, or increased psychosocial support during the process, these initial studies are reassuring. Based on current evidence, these patients are not at greater risk for perinatal complications than their cisgender counterparts.
Despite these encouraging findings, there are still several challenges faced by transgender men when it comes to getting pregnant. For instance, they may have difficulty accessing fertility services because of financial constraints or experience a lack of awareness or prejudice from providers; they might also be subject to discrimination or stigma within health care settings. As front-line providers for obstetrical care, we must lead the way towards improving the care for pregnant transmasculine individuals.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Light A et al. Family planning and contraception use in transgender men. Contraception. 2018 Oct. doi: 10.1016/j.contraception.2018.06.006.
2. Krempasky C et al. Contraception across the transmasculine spectrum. Am J Obstet Gynecol. 2020 Feb. doi: 10.1016/j.ajog.2019.07.043.
3. Obedin-Maliver J, De Haan G. “Gynecologic care for transgender patients” in Ferrando C, ed., Comprehensive Care of the Transgender Patient. Philadelphia: Elsevier, 2019. 131-51.
4. Light AD et al. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014 Dec. doi: 10.1097/AOG.0000000000000540.
5. Brandt JS et al. Transgender men, pregnancy, and the “new” advanced paternal age: A review of the literature. Maturitas. 2019 Oct. doi: 10.1016/j.maturitas.2019.07.004.
6. Stroumsa D et al. Pregnancy outcomes in a U.S. cohort of transgender people. JAMA. 2023 Jun 6. doi: 10.1001/jama.2023.7688.
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologists and midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately,
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
For patients seeking to become pregnant, testosterone must be discontinued. Testosterone is teratogenic; it can cause abnormal urogenital development in the female fetus and should be avoided even prior to conception.1,2 The timing of testosterone discontinuation is debatable. There are no well-established guidelines dictating how early pregnancy can be attempted after cessation of testosterone, but typically if menses has resumed, the teratogenic effects of testosterone are less likely.
For amenorrheic patients on testosterone, menses will occur, on average, 3-6 months after testosterone is stopped. Of note, the longer that testosterone has been suspended, the greater the likelihood of achieving pregnancy.3 In a study by Light et al., 72% of patients conceived within 6 months of attempting pregnancy, 80% resumed menses within 6 months of stopping testosterone, and 20% of individuals conceived while they were amenorrheic from testosterone.4
Psychosocial support is an essential part of pregnancy care in transgender men. For some patients, pregnancy can worsen gender dysphoria, whereas others are empowered by the experience. Insurance companies may also deny obstetric care services to transgender males who have already changed their gender marker from female to male on insurance policies.
Whether transmasculine individuals are at higher risk for pregnancy complications is largely unknown, although emerging research in this field has yielded interesting results. While testosterone can cause vaginal atrophy, it does not seem to increase a patient’s risk of vaginal lacerations or their ability to have a successful vaginal delivery. For transgender men with significant discomfort around their genitalia, an elective cesarean section may be appropriate.5
More recently, Stroumsa et al. conducted an analysis of all deliveries at a Michigan institution from 2014 to 2018. Patients with male gender at the time of delivery or with the diagnostic code of gender dysphoria were identified as transgender.6 The primary outcome of this study was severe parental morbidity (such as amniotic fluid embolism, acute myocardial infarction, eclampsia, etc.), with secondary outcomes investigating rates of cesarean delivery and preterm birth.
During this time period, the researchers identified 256 transgender patients and 1.3 million cisgender patients in their Medicaid database and 1,651 transgender patients and 1.5 million cisgender patients in the commercial database who had experienced a delivery.6 Compared with cisgender patients, transgender patients in the Medicaid database were younger, less likely to be white, and more likely to have a chronic condition.6 Compared with cisgender patients in the commercial database, transgender patients experienced higher rates of anxiety and depression.6 Both transgender and cisgender patients had similar rates of severe parental morbidity. Ironically, rates of cesarean delivery were lower, compared with cisgender patients, in both the Medicaid and commercial databases, with no differences observed between rates of preterm birth.6
While more research is needed on pregnancy in transgender men, this analysis is not only one of the largest to date, but it also challenges many misconceptions providers have regarding pregnancy outcomes. Even though transmasculine patients may require additional medical interventions to achieve pregnancy, such as assisted reproductive technology, or increased psychosocial support during the process, these initial studies are reassuring. Based on current evidence, these patients are not at greater risk for perinatal complications than their cisgender counterparts.
Despite these encouraging findings, there are still several challenges faced by transgender men when it comes to getting pregnant. For instance, they may have difficulty accessing fertility services because of financial constraints or experience a lack of awareness or prejudice from providers; they might also be subject to discrimination or stigma within health care settings. As front-line providers for obstetrical care, we must lead the way towards improving the care for pregnant transmasculine individuals.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Light A et al. Family planning and contraception use in transgender men. Contraception. 2018 Oct. doi: 10.1016/j.contraception.2018.06.006.
2. Krempasky C et al. Contraception across the transmasculine spectrum. Am J Obstet Gynecol. 2020 Feb. doi: 10.1016/j.ajog.2019.07.043.
3. Obedin-Maliver J, De Haan G. “Gynecologic care for transgender patients” in Ferrando C, ed., Comprehensive Care of the Transgender Patient. Philadelphia: Elsevier, 2019. 131-51.
4. Light AD et al. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014 Dec. doi: 10.1097/AOG.0000000000000540.
5. Brandt JS et al. Transgender men, pregnancy, and the “new” advanced paternal age: A review of the literature. Maturitas. 2019 Oct. doi: 10.1016/j.maturitas.2019.07.004.
6. Stroumsa D et al. Pregnancy outcomes in a U.S. cohort of transgender people. JAMA. 2023 Jun 6. doi: 10.1001/jama.2023.7688.
Despite increased societal gains, transgender individuals are still a medically and socially underserved group. The historic rise of antitransgender legislation and the overturning of Roe v. Wade, further compound existing health care disparities, particularly in the realm of contraception and pregnancy. Obstetrician-gynecologists and midwives are typically first-line providers when discussing family planning and fertility options for all patients assigned female at birth. Unfortunately,
Only individuals who are assigned female at birth and have a uterus are capable of pregnancy. This can include both cisgender women and nonbinary/transgender men. However, societal and medical institutions are struggling with this shift in perspective from a traditionally gendered role to a more inclusive one. Obstetrician-gynecologists and midwives can serve to bridge this gap between these patients and societal misconceptions surrounding transgender men who desire and experience pregnancy.
Providers need to remember that many transmasculine individuals will still retain their uterus and are therefore capable of getting pregnant. While testosterone causes amenorrhea, if patients are engaging in penile-vaginal intercourse, conception is still possible. If a patient does not desire pregnancy, all contraceptive options available for cisgender women, which also include combined oral contraceptives, should be offered.
For patients seeking to become pregnant, testosterone must be discontinued. Testosterone is teratogenic; it can cause abnormal urogenital development in the female fetus and should be avoided even prior to conception.1,2 The timing of testosterone discontinuation is debatable. There are no well-established guidelines dictating how early pregnancy can be attempted after cessation of testosterone, but typically if menses has resumed, the teratogenic effects of testosterone are less likely.
For amenorrheic patients on testosterone, menses will occur, on average, 3-6 months after testosterone is stopped. Of note, the longer that testosterone has been suspended, the greater the likelihood of achieving pregnancy.3 In a study by Light et al., 72% of patients conceived within 6 months of attempting pregnancy, 80% resumed menses within 6 months of stopping testosterone, and 20% of individuals conceived while they were amenorrheic from testosterone.4
Psychosocial support is an essential part of pregnancy care in transgender men. For some patients, pregnancy can worsen gender dysphoria, whereas others are empowered by the experience. Insurance companies may also deny obstetric care services to transgender males who have already changed their gender marker from female to male on insurance policies.
Whether transmasculine individuals are at higher risk for pregnancy complications is largely unknown, although emerging research in this field has yielded interesting results. While testosterone can cause vaginal atrophy, it does not seem to increase a patient’s risk of vaginal lacerations or their ability to have a successful vaginal delivery. For transgender men with significant discomfort around their genitalia, an elective cesarean section may be appropriate.5
More recently, Stroumsa et al. conducted an analysis of all deliveries at a Michigan institution from 2014 to 2018. Patients with male gender at the time of delivery or with the diagnostic code of gender dysphoria were identified as transgender.6 The primary outcome of this study was severe parental morbidity (such as amniotic fluid embolism, acute myocardial infarction, eclampsia, etc.), with secondary outcomes investigating rates of cesarean delivery and preterm birth.
During this time period, the researchers identified 256 transgender patients and 1.3 million cisgender patients in their Medicaid database and 1,651 transgender patients and 1.5 million cisgender patients in the commercial database who had experienced a delivery.6 Compared with cisgender patients, transgender patients in the Medicaid database were younger, less likely to be white, and more likely to have a chronic condition.6 Compared with cisgender patients in the commercial database, transgender patients experienced higher rates of anxiety and depression.6 Both transgender and cisgender patients had similar rates of severe parental morbidity. Ironically, rates of cesarean delivery were lower, compared with cisgender patients, in both the Medicaid and commercial databases, with no differences observed between rates of preterm birth.6
While more research is needed on pregnancy in transgender men, this analysis is not only one of the largest to date, but it also challenges many misconceptions providers have regarding pregnancy outcomes. Even though transmasculine patients may require additional medical interventions to achieve pregnancy, such as assisted reproductive technology, or increased psychosocial support during the process, these initial studies are reassuring. Based on current evidence, these patients are not at greater risk for perinatal complications than their cisgender counterparts.
Despite these encouraging findings, there are still several challenges faced by transgender men when it comes to getting pregnant. For instance, they may have difficulty accessing fertility services because of financial constraints or experience a lack of awareness or prejudice from providers; they might also be subject to discrimination or stigma within health care settings. As front-line providers for obstetrical care, we must lead the way towards improving the care for pregnant transmasculine individuals.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Light A et al. Family planning and contraception use in transgender men. Contraception. 2018 Oct. doi: 10.1016/j.contraception.2018.06.006.
2. Krempasky C et al. Contraception across the transmasculine spectrum. Am J Obstet Gynecol. 2020 Feb. doi: 10.1016/j.ajog.2019.07.043.
3. Obedin-Maliver J, De Haan G. “Gynecologic care for transgender patients” in Ferrando C, ed., Comprehensive Care of the Transgender Patient. Philadelphia: Elsevier, 2019. 131-51.
4. Light AD et al. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014 Dec. doi: 10.1097/AOG.0000000000000540.
5. Brandt JS et al. Transgender men, pregnancy, and the “new” advanced paternal age: A review of the literature. Maturitas. 2019 Oct. doi: 10.1016/j.maturitas.2019.07.004.
6. Stroumsa D et al. Pregnancy outcomes in a U.S. cohort of transgender people. JAMA. 2023 Jun 6. doi: 10.1001/jama.2023.7688.
Does ‘skeletal age’ describe fracture impact on mortality?
Thach Tran, MD, and colleagues introduced the concept of “skeletal age” in a recently published paper that aims to incorporate the impact of fragility, or low trauma, fractures – which can occur in patients with osteoporosis – on mortality risk.
They defined “skeletal age” as the age of the skeleton following a fragility fracture. This is calculated as the chronological age of the individual plus the number of years of “life lost” as a consequence of the specific fracture.
The risk for premature death following fragility fractures is concerning, with 22%-58% of patients with hip fracture dying within a year (Brauer et al.; Rapp et al.). Thus, it’s important to treat osteoporosis in a timely fashion to reduce the risk for such fractures and the excess mortality risk associated with them.
Implementation and uptake of such treatment, however, either before or after a fragility fracture, is far from optimal (Solomon et al). This may be because patients don’t fully understand the consequence of such a fracture, and outcomes measures currently in use (such as relative risk or hazard of mortality) are difficult to communicate to patients.
In the recent paper by Dr. Tran and colleagues, the authors examined the association between fractures and mortality based on sex, age, associated comorbidities, and fracture site. They pooled this information to create a “skeletal age” for each fracture site, using data from the Danish National Hospital Discharge Registry, which documents fractures and related mortality for all Danish people.
They examined mortality over a period of at least 2 years following a fragility fracture in individuals aged 50 or older, and reported that occurrence of any fragility fracture is associated with a 30%-45% increased risk for death, with the highest risk noted for hip and femur fractures (twofold increase). Fractures of the pelvis, vertebrae, humerus, ribs, clavicle, and lower leg were also associated with increased mortality risk, but no increase was seen with fractures of the forearm, knee, ankle, hand, or foot.
The number of years of life lost at any age depending on the fracture site is represented as a linear graph of skeletal age for any chronological age, for specific fracture sites, separated by sex.
For example, the skeletal age of a 50-year-old man who has a hip fracture is 57 years (7 years of life lost as a consequence of the fracture), while that for a 70-year-old man with the same fracture is 75 years (5 years of life lost because of the fracture). Similarly, the skeletal age of a 50-year-old man with a fracture of the pelvis, femur, vertebrae, and humerus is 55 years (5 years of life lost). Fractures of the lower leg, humerus, and clavicle lead to fewer lost years of life.
The authors are to be commended for creating a simple strategy to quantify mortality risk following low-impact or fragility fractures in older individuals; this could enable providers to communicate the importance of osteoporosis treatment more effectively to patients on the basis of their skeletal age, and for patients to better understand this information.
The study design appears reasonably robust as the authors considered many factors that might affect mortality risk, such as sex, age, and comorbidities, and the results are based on information from a very large number of people – 1.6 million.
However, there’s a major issue with the concept of “skeletal age” as proposed by Dr. Tran and colleagues. The term is already in use and defines the maturity of bones in children and adolescents, also called “bone age” (Greulich and Pyle 1959; Skeletal Age, Radiology Key). This is a real oversight and could cause confusion in interpreting “skeletal age.”
Skeletal age as currently defined in children and adolescents is influenced by chronological age, exposure to certain hormones, nutritional deficiencies, and systemic diseases, and is a predictor of adult height based on the skeletal age and current height. This concept is completely different from that being proposed by the authors in this paper. Dr. Tran and colleagues (and the reviewers of this paper) are probably not familiar with the use of the terminology in youth, which is a major oversight; they should consider changing the terminology given this overlap.
Further, fragility fractures can occur from osteoporosis at any age, and this study doesn’t provide information regarding years of life lost from occurrence of fragility fractures at younger ages, or the age at which mortality risk starts to increase (as the study was performed only in those aged 50 or older).
While the study takes into account general comorbidities in developing the model to define years of life lost, it doesn’t account for other factors that can influence fracture risk, such as lifestyle factors, activity level, and genetic risk (family history of osteoporosis, for example). Of note, the impact of additional fractures isn’t considered either and should be factored into future investigations.
Overall, the study is robust and important and provides valuable information regarding mortality risk from a fragility fracture in older people. However, there are some flaws that need to be considered and addressed, the most serious of which is that the term “skeletal age” has been in existence for decades, applied to a much younger age group, and its implications are completely different from those being proposed by the authors here.
A version of this article first appeared on Medscape.com.
Thach Tran, MD, and colleagues introduced the concept of “skeletal age” in a recently published paper that aims to incorporate the impact of fragility, or low trauma, fractures – which can occur in patients with osteoporosis – on mortality risk.
They defined “skeletal age” as the age of the skeleton following a fragility fracture. This is calculated as the chronological age of the individual plus the number of years of “life lost” as a consequence of the specific fracture.
The risk for premature death following fragility fractures is concerning, with 22%-58% of patients with hip fracture dying within a year (Brauer et al.; Rapp et al.). Thus, it’s important to treat osteoporosis in a timely fashion to reduce the risk for such fractures and the excess mortality risk associated with them.
Implementation and uptake of such treatment, however, either before or after a fragility fracture, is far from optimal (Solomon et al). This may be because patients don’t fully understand the consequence of such a fracture, and outcomes measures currently in use (such as relative risk or hazard of mortality) are difficult to communicate to patients.
In the recent paper by Dr. Tran and colleagues, the authors examined the association between fractures and mortality based on sex, age, associated comorbidities, and fracture site. They pooled this information to create a “skeletal age” for each fracture site, using data from the Danish National Hospital Discharge Registry, which documents fractures and related mortality for all Danish people.
They examined mortality over a period of at least 2 years following a fragility fracture in individuals aged 50 or older, and reported that occurrence of any fragility fracture is associated with a 30%-45% increased risk for death, with the highest risk noted for hip and femur fractures (twofold increase). Fractures of the pelvis, vertebrae, humerus, ribs, clavicle, and lower leg were also associated with increased mortality risk, but no increase was seen with fractures of the forearm, knee, ankle, hand, or foot.
The number of years of life lost at any age depending on the fracture site is represented as a linear graph of skeletal age for any chronological age, for specific fracture sites, separated by sex.
For example, the skeletal age of a 50-year-old man who has a hip fracture is 57 years (7 years of life lost as a consequence of the fracture), while that for a 70-year-old man with the same fracture is 75 years (5 years of life lost because of the fracture). Similarly, the skeletal age of a 50-year-old man with a fracture of the pelvis, femur, vertebrae, and humerus is 55 years (5 years of life lost). Fractures of the lower leg, humerus, and clavicle lead to fewer lost years of life.
The authors are to be commended for creating a simple strategy to quantify mortality risk following low-impact or fragility fractures in older individuals; this could enable providers to communicate the importance of osteoporosis treatment more effectively to patients on the basis of their skeletal age, and for patients to better understand this information.
The study design appears reasonably robust as the authors considered many factors that might affect mortality risk, such as sex, age, and comorbidities, and the results are based on information from a very large number of people – 1.6 million.
However, there’s a major issue with the concept of “skeletal age” as proposed by Dr. Tran and colleagues. The term is already in use and defines the maturity of bones in children and adolescents, also called “bone age” (Greulich and Pyle 1959; Skeletal Age, Radiology Key). This is a real oversight and could cause confusion in interpreting “skeletal age.”
Skeletal age as currently defined in children and adolescents is influenced by chronological age, exposure to certain hormones, nutritional deficiencies, and systemic diseases, and is a predictor of adult height based on the skeletal age and current height. This concept is completely different from that being proposed by the authors in this paper. Dr. Tran and colleagues (and the reviewers of this paper) are probably not familiar with the use of the terminology in youth, which is a major oversight; they should consider changing the terminology given this overlap.
Further, fragility fractures can occur from osteoporosis at any age, and this study doesn’t provide information regarding years of life lost from occurrence of fragility fractures at younger ages, or the age at which mortality risk starts to increase (as the study was performed only in those aged 50 or older).
While the study takes into account general comorbidities in developing the model to define years of life lost, it doesn’t account for other factors that can influence fracture risk, such as lifestyle factors, activity level, and genetic risk (family history of osteoporosis, for example). Of note, the impact of additional fractures isn’t considered either and should be factored into future investigations.
Overall, the study is robust and important and provides valuable information regarding mortality risk from a fragility fracture in older people. However, there are some flaws that need to be considered and addressed, the most serious of which is that the term “skeletal age” has been in existence for decades, applied to a much younger age group, and its implications are completely different from those being proposed by the authors here.
A version of this article first appeared on Medscape.com.
Thach Tran, MD, and colleagues introduced the concept of “skeletal age” in a recently published paper that aims to incorporate the impact of fragility, or low trauma, fractures – which can occur in patients with osteoporosis – on mortality risk.
They defined “skeletal age” as the age of the skeleton following a fragility fracture. This is calculated as the chronological age of the individual plus the number of years of “life lost” as a consequence of the specific fracture.
The risk for premature death following fragility fractures is concerning, with 22%-58% of patients with hip fracture dying within a year (Brauer et al.; Rapp et al.). Thus, it’s important to treat osteoporosis in a timely fashion to reduce the risk for such fractures and the excess mortality risk associated with them.
Implementation and uptake of such treatment, however, either before or after a fragility fracture, is far from optimal (Solomon et al). This may be because patients don’t fully understand the consequence of such a fracture, and outcomes measures currently in use (such as relative risk or hazard of mortality) are difficult to communicate to patients.
In the recent paper by Dr. Tran and colleagues, the authors examined the association between fractures and mortality based on sex, age, associated comorbidities, and fracture site. They pooled this information to create a “skeletal age” for each fracture site, using data from the Danish National Hospital Discharge Registry, which documents fractures and related mortality for all Danish people.
They examined mortality over a period of at least 2 years following a fragility fracture in individuals aged 50 or older, and reported that occurrence of any fragility fracture is associated with a 30%-45% increased risk for death, with the highest risk noted for hip and femur fractures (twofold increase). Fractures of the pelvis, vertebrae, humerus, ribs, clavicle, and lower leg were also associated with increased mortality risk, but no increase was seen with fractures of the forearm, knee, ankle, hand, or foot.
The number of years of life lost at any age depending on the fracture site is represented as a linear graph of skeletal age for any chronological age, for specific fracture sites, separated by sex.
For example, the skeletal age of a 50-year-old man who has a hip fracture is 57 years (7 years of life lost as a consequence of the fracture), while that for a 70-year-old man with the same fracture is 75 years (5 years of life lost because of the fracture). Similarly, the skeletal age of a 50-year-old man with a fracture of the pelvis, femur, vertebrae, and humerus is 55 years (5 years of life lost). Fractures of the lower leg, humerus, and clavicle lead to fewer lost years of life.
The authors are to be commended for creating a simple strategy to quantify mortality risk following low-impact or fragility fractures in older individuals; this could enable providers to communicate the importance of osteoporosis treatment more effectively to patients on the basis of their skeletal age, and for patients to better understand this information.
The study design appears reasonably robust as the authors considered many factors that might affect mortality risk, such as sex, age, and comorbidities, and the results are based on information from a very large number of people – 1.6 million.
However, there’s a major issue with the concept of “skeletal age” as proposed by Dr. Tran and colleagues. The term is already in use and defines the maturity of bones in children and adolescents, also called “bone age” (Greulich and Pyle 1959; Skeletal Age, Radiology Key). This is a real oversight and could cause confusion in interpreting “skeletal age.”
Skeletal age as currently defined in children and adolescents is influenced by chronological age, exposure to certain hormones, nutritional deficiencies, and systemic diseases, and is a predictor of adult height based on the skeletal age and current height. This concept is completely different from that being proposed by the authors in this paper. Dr. Tran and colleagues (and the reviewers of this paper) are probably not familiar with the use of the terminology in youth, which is a major oversight; they should consider changing the terminology given this overlap.
Further, fragility fractures can occur from osteoporosis at any age, and this study doesn’t provide information regarding years of life lost from occurrence of fragility fractures at younger ages, or the age at which mortality risk starts to increase (as the study was performed only in those aged 50 or older).
While the study takes into account general comorbidities in developing the model to define years of life lost, it doesn’t account for other factors that can influence fracture risk, such as lifestyle factors, activity level, and genetic risk (family history of osteoporosis, for example). Of note, the impact of additional fractures isn’t considered either and should be factored into future investigations.
Overall, the study is robust and important and provides valuable information regarding mortality risk from a fragility fracture in older people. However, there are some flaws that need to be considered and addressed, the most serious of which is that the term “skeletal age” has been in existence for decades, applied to a much younger age group, and its implications are completely different from those being proposed by the authors here.
A version of this article first appeared on Medscape.com.
DDW 2023: Common GI conditions in primary care
This transcript has been edited for clarity.
Hello and welcome. I am Vivek Kaul, MD, from the University of Rochester (N.Y.) Medical Center. It gives me great pleasure, once again, to collaborate with WebMD and Medscape to present this video capsule.
This time, we have picked the best GI and GI surgery papers from the recently concluded Digestive Disease Week 2023 international meeting in Chicago. For this edition of the Medscape GI/Primary Care video capsule, I thought it would be nice to present a couple of GI surgical papers that have major impact on common GI conditions seen in primary care.
This first paper in the GI surgery realm is “Diabetes Mellitus Remission in Patients with BMI > 50 kg/m2 after Bariatric Surgeries: A Real-World Multi-Centered Study.” This comes to us from the Mayo Clinic in Rochester, Minn.
This was a retrospective study of 329 patients who had type 2 diabetes, who underwent either Roux-en-Y gastric bypass (two-thirds of them) or a surgical sleeve gastrectomy (about one third of them). The mean follow-up was about 6 years. Type 2 diabetes remission was seen in about half of them in this long-term follow-up.
There were significant improvements in hemoglobin A1c, fasting blood glucose, diabetes, and, of course, weight loss – and all were statistically significant. The type of surgery did not seem to make any difference. Both groups really benefited from this.
The take-home point of this study is that patients who have a high BMI and metabolic syndrome, whether they undergo Roux-en-Y gastric bypass or sleeve gastrectomy, should expect to see long-term, durable, positive impact on their diabetes, as well as weight loss, of course, and all the metrics for blood glucose and hemodynamics.
This is an important study and helps us in counseling patients appropriately when they present for selection for these types of surgeries in the appropriate clinical context.
The next paper in the surgical realm is from the University of Padova (Italy), which is “Antireflux Surgery’s Lifespan: 20 Years After Laparoscopic Fundoplication.” This is a prospective study of 137 patients who underwent laparoscopic fundoplication for the management of gastroesophageal reflux disease (n = 107) or a large hiatal hernia (n = 30).
The median follow-up in this study was 22 years, which is among the longest I’ve seen in recent years for any study. A very small percentage of patients underwent revision surgery, which speaks to the skill set and careful selection of the patient cohort.
Only nine patients of 137 had repeat surgeries. Positive outcomes, on the other hand, were seen in the vast majority of patients with reflux, at 84%, and still, two thirds of patients with hiatal hernia had positive outcomes over the long term.
Failure-free survival was much higher for the gastroesophageal reflux cohort, as expected, compared with the hiatal hernia cohort. Patient satisfaction after two decades was almost 90% both for reflux and for hiatal hernia, which is quite a significant milestone.
The take-home point from this study is that laparoscopic fundoplication is quite durable, with success rates approaching 90%, even 2 decades after the surgical intervention. This is quite an important data point for us to discuss with patients when they present with a question around surgery for these indications. Although, as we know well in the era of PPI therapy, surgical indications are definitely reduced, compared with 20 years ago.
The next paper in this group is related to another very important clinical entity, which is acute pancreatitis and the question of pancreatic cancer in those patients who present with acute pancreatitis.
Our previous work in this realm, published a couple of years ago, suggested that about 3% of patients who come to the hospital with acute pancreatitis may harbor pancreatic malignancy, which can be picked up if we do a diligent follow-up with cross-sectional imaging and endoscopic ultrasound.
This paper talks about acute pancreatitis and the modeling of risk in terms of prediction for early cancer. This study was conducted at the University of Pennsylvania and basically speaks to a clinical prediction model to assess the risk for pancreatic cancer after acute pancreatitis diagnosis.
As we know, the risk for pancreatic cancer is highest within 2 years of the initial presentation of acute pancreatitis, especially if the etiology of that pancreatitis was unclear. In this clinical prediction model, as shown on this slide, a variety of demographic and clinical criteria were used, all of which are easily accessible to us in GI and in primary care to calculate the modeling risk.
A large number of patients were used to apply this model: 51,613 patients. The mean age was 62%, the overwhelming majority were male, and half were Caucasian. Using this clinical prediction model, a 2-year incidence of pancreatic ductal adenocarcinoma was calculated to be 1.6% for an absolute number of 800 cases.
Nearly 50% were discovered after 3 months. There was a positive association with pancreatic cystic disease in these patients, which makes sense in terms of their preneoplastic potential.
The take-home point from this study is that readily available demographic and clinical criteria may be useful in helping us predict diagnosis of early pancreatic cancer in a subset of patients who present with acute pancreatitis, and in my opinion, particularly those who present with pancreatitis for which we cannot explain the etiology.
The next paper we have refers to a very common problem, which is Helicobacter pylori infection. This is almost an endemic problem in the Far East and in the third world, but also an increasing problem in the United States.
This particular study comes to us from Taiwan and is looking at different treatment strategies for H. pylori infection. In Taiwan and the Far East, it has been proposed that high acid content in the food might be interfering with efficacy rates. The consideration for antibiotic resistance may be an issue as well. That’s in the background of this paper.
This paper is titled “Enhanced Efficacy of Combined Bismuth and High-Dose Dual Therapy versus High-Dose Dual Therapy Alone or Quadruple Therapy for First-Line H pylori Eradication.” This was an interim report from a large multicenter, randomized controlled trial and included 436 patients with H. pylori infection.
Each patient had biopsies with H. pylori cultures, and they were randomized to one of three regimens as listed here. They received high-dose dual therapy or bismuth along with high-dose dual therapy – so, triple therapy – or they received the amoxicillin-based bismuth and quadruple-therapy regimen. Breath tests were used to check for eradication at the end of the treatment period.
In terms of the results from this study, it’s very clear that the bismuth-based regimen, which is listed here in the middle column, had the highest treatment efficacy rates and moderate adverse event rates when compared with the other two arms.
Reduced eradication in the dual-therapy arm was seen probably due to increased acid food intake. Reduced eradication in the quadruple-therapy arm was likely associated with antibiotic resistance and also with poor compliance, which is understandable when you have more medications to take compared with the group that has fewer medications to take.
The take-home point from this study was that the addition of bismuth might be the silver bullet when you add that to the dual-therapy regimen in difficult cases of H pylori infection. More to come on this, and certainly of relevance to us here as we tackle more and more cases of H. pylori infection stateside.
Last but not least, GI and primary care discussions can never be complete without a discussion on colorectal cancer screening. As the NordICC trial has shown us, it’s not the issue with the screening modality but more about patients’ acceptance of screening, particularly invasive screening.
This paper looked at different strategies and tried to figure out which strategy would be most attractive to bring more people in for screening. This is a large, randomized controlled trial with good distribution of patient populations with approximately half female, half White, and a mean age around 48 years, with more than 20,000 patients.
One of four screening strategies was used. Patients were invited for a fecal immunochemical test, a colonoscopy, or they were invited to choose between a FIT test and a colonoscopy. The final group was mailed a FIT test kit as an outreach mechanism.
Invitations were sent via electronic patient portals, which are very common nowadays, and via the United States Postal Service. Text-message reminders were sent a couple of weeks later. The primary outcome of this study was to look at any colorectal cancer completion rate at 26 weeks.
The results of the study were quite interesting. Screening completion rates were relatively low at 18%. It was interesting to note that the highest screening rates were seen with those who had the FIT test mailed to them, whereas each of the other three groups that had only invitations sent to them had relatively lower screening compliance rates.
The lowest participation was in the colonoscopy invitation group, I suspect due to the invasive nature of the procedure and the patients’ perception of that. When patients were offered a choice, they were more likely to be compliant with screening. In the end, more patients chose colonoscopy as their screening test of choice compared with FIT testing.
The take-home point from this study is that directly sending test kits as an outreach might be more effective than simply inviting subjects to be screened. From those who do respond to be screened, more patients would choose colonoscopy, compared with the FIT test.
With that, I come to the end of this video capsule summary for the Best of GI for Primary Care from DDW 2023. We covered a variety of topics, ranging from pancreatitis to H pylori to colon cancer screening and a couple of GI surgical interventions with long-term outcomes that are very favorable.
Dr. Kaul disclosed conflicts of interest with AMBU, Cook Medical, CDS, CDX,Steris, and Motus GI.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello and welcome. I am Vivek Kaul, MD, from the University of Rochester (N.Y.) Medical Center. It gives me great pleasure, once again, to collaborate with WebMD and Medscape to present this video capsule.
This time, we have picked the best GI and GI surgery papers from the recently concluded Digestive Disease Week 2023 international meeting in Chicago. For this edition of the Medscape GI/Primary Care video capsule, I thought it would be nice to present a couple of GI surgical papers that have major impact on common GI conditions seen in primary care.
This first paper in the GI surgery realm is “Diabetes Mellitus Remission in Patients with BMI > 50 kg/m2 after Bariatric Surgeries: A Real-World Multi-Centered Study.” This comes to us from the Mayo Clinic in Rochester, Minn.
This was a retrospective study of 329 patients who had type 2 diabetes, who underwent either Roux-en-Y gastric bypass (two-thirds of them) or a surgical sleeve gastrectomy (about one third of them). The mean follow-up was about 6 years. Type 2 diabetes remission was seen in about half of them in this long-term follow-up.
There were significant improvements in hemoglobin A1c, fasting blood glucose, diabetes, and, of course, weight loss – and all were statistically significant. The type of surgery did not seem to make any difference. Both groups really benefited from this.
The take-home point of this study is that patients who have a high BMI and metabolic syndrome, whether they undergo Roux-en-Y gastric bypass or sleeve gastrectomy, should expect to see long-term, durable, positive impact on their diabetes, as well as weight loss, of course, and all the metrics for blood glucose and hemodynamics.
This is an important study and helps us in counseling patients appropriately when they present for selection for these types of surgeries in the appropriate clinical context.
The next paper in the surgical realm is from the University of Padova (Italy), which is “Antireflux Surgery’s Lifespan: 20 Years After Laparoscopic Fundoplication.” This is a prospective study of 137 patients who underwent laparoscopic fundoplication for the management of gastroesophageal reflux disease (n = 107) or a large hiatal hernia (n = 30).
The median follow-up in this study was 22 years, which is among the longest I’ve seen in recent years for any study. A very small percentage of patients underwent revision surgery, which speaks to the skill set and careful selection of the patient cohort.
Only nine patients of 137 had repeat surgeries. Positive outcomes, on the other hand, were seen in the vast majority of patients with reflux, at 84%, and still, two thirds of patients with hiatal hernia had positive outcomes over the long term.
Failure-free survival was much higher for the gastroesophageal reflux cohort, as expected, compared with the hiatal hernia cohort. Patient satisfaction after two decades was almost 90% both for reflux and for hiatal hernia, which is quite a significant milestone.
The take-home point from this study is that laparoscopic fundoplication is quite durable, with success rates approaching 90%, even 2 decades after the surgical intervention. This is quite an important data point for us to discuss with patients when they present with a question around surgery for these indications. Although, as we know well in the era of PPI therapy, surgical indications are definitely reduced, compared with 20 years ago.
The next paper in this group is related to another very important clinical entity, which is acute pancreatitis and the question of pancreatic cancer in those patients who present with acute pancreatitis.
Our previous work in this realm, published a couple of years ago, suggested that about 3% of patients who come to the hospital with acute pancreatitis may harbor pancreatic malignancy, which can be picked up if we do a diligent follow-up with cross-sectional imaging and endoscopic ultrasound.
This paper talks about acute pancreatitis and the modeling of risk in terms of prediction for early cancer. This study was conducted at the University of Pennsylvania and basically speaks to a clinical prediction model to assess the risk for pancreatic cancer after acute pancreatitis diagnosis.
As we know, the risk for pancreatic cancer is highest within 2 years of the initial presentation of acute pancreatitis, especially if the etiology of that pancreatitis was unclear. In this clinical prediction model, as shown on this slide, a variety of demographic and clinical criteria were used, all of which are easily accessible to us in GI and in primary care to calculate the modeling risk.
A large number of patients were used to apply this model: 51,613 patients. The mean age was 62%, the overwhelming majority were male, and half were Caucasian. Using this clinical prediction model, a 2-year incidence of pancreatic ductal adenocarcinoma was calculated to be 1.6% for an absolute number of 800 cases.
Nearly 50% were discovered after 3 months. There was a positive association with pancreatic cystic disease in these patients, which makes sense in terms of their preneoplastic potential.
The take-home point from this study is that readily available demographic and clinical criteria may be useful in helping us predict diagnosis of early pancreatic cancer in a subset of patients who present with acute pancreatitis, and in my opinion, particularly those who present with pancreatitis for which we cannot explain the etiology.
The next paper we have refers to a very common problem, which is Helicobacter pylori infection. This is almost an endemic problem in the Far East and in the third world, but also an increasing problem in the United States.
This particular study comes to us from Taiwan and is looking at different treatment strategies for H. pylori infection. In Taiwan and the Far East, it has been proposed that high acid content in the food might be interfering with efficacy rates. The consideration for antibiotic resistance may be an issue as well. That’s in the background of this paper.
This paper is titled “Enhanced Efficacy of Combined Bismuth and High-Dose Dual Therapy versus High-Dose Dual Therapy Alone or Quadruple Therapy for First-Line H pylori Eradication.” This was an interim report from a large multicenter, randomized controlled trial and included 436 patients with H. pylori infection.
Each patient had biopsies with H. pylori cultures, and they were randomized to one of three regimens as listed here. They received high-dose dual therapy or bismuth along with high-dose dual therapy – so, triple therapy – or they received the amoxicillin-based bismuth and quadruple-therapy regimen. Breath tests were used to check for eradication at the end of the treatment period.
In terms of the results from this study, it’s very clear that the bismuth-based regimen, which is listed here in the middle column, had the highest treatment efficacy rates and moderate adverse event rates when compared with the other two arms.
Reduced eradication in the dual-therapy arm was seen probably due to increased acid food intake. Reduced eradication in the quadruple-therapy arm was likely associated with antibiotic resistance and also with poor compliance, which is understandable when you have more medications to take compared with the group that has fewer medications to take.
The take-home point from this study was that the addition of bismuth might be the silver bullet when you add that to the dual-therapy regimen in difficult cases of H pylori infection. More to come on this, and certainly of relevance to us here as we tackle more and more cases of H. pylori infection stateside.
Last but not least, GI and primary care discussions can never be complete without a discussion on colorectal cancer screening. As the NordICC trial has shown us, it’s not the issue with the screening modality but more about patients’ acceptance of screening, particularly invasive screening.
This paper looked at different strategies and tried to figure out which strategy would be most attractive to bring more people in for screening. This is a large, randomized controlled trial with good distribution of patient populations with approximately half female, half White, and a mean age around 48 years, with more than 20,000 patients.
One of four screening strategies was used. Patients were invited for a fecal immunochemical test, a colonoscopy, or they were invited to choose between a FIT test and a colonoscopy. The final group was mailed a FIT test kit as an outreach mechanism.
Invitations were sent via electronic patient portals, which are very common nowadays, and via the United States Postal Service. Text-message reminders were sent a couple of weeks later. The primary outcome of this study was to look at any colorectal cancer completion rate at 26 weeks.
The results of the study were quite interesting. Screening completion rates were relatively low at 18%. It was interesting to note that the highest screening rates were seen with those who had the FIT test mailed to them, whereas each of the other three groups that had only invitations sent to them had relatively lower screening compliance rates.
The lowest participation was in the colonoscopy invitation group, I suspect due to the invasive nature of the procedure and the patients’ perception of that. When patients were offered a choice, they were more likely to be compliant with screening. In the end, more patients chose colonoscopy as their screening test of choice compared with FIT testing.
The take-home point from this study is that directly sending test kits as an outreach might be more effective than simply inviting subjects to be screened. From those who do respond to be screened, more patients would choose colonoscopy, compared with the FIT test.
With that, I come to the end of this video capsule summary for the Best of GI for Primary Care from DDW 2023. We covered a variety of topics, ranging from pancreatitis to H pylori to colon cancer screening and a couple of GI surgical interventions with long-term outcomes that are very favorable.
Dr. Kaul disclosed conflicts of interest with AMBU, Cook Medical, CDS, CDX,Steris, and Motus GI.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello and welcome. I am Vivek Kaul, MD, from the University of Rochester (N.Y.) Medical Center. It gives me great pleasure, once again, to collaborate with WebMD and Medscape to present this video capsule.
This time, we have picked the best GI and GI surgery papers from the recently concluded Digestive Disease Week 2023 international meeting in Chicago. For this edition of the Medscape GI/Primary Care video capsule, I thought it would be nice to present a couple of GI surgical papers that have major impact on common GI conditions seen in primary care.
This first paper in the GI surgery realm is “Diabetes Mellitus Remission in Patients with BMI > 50 kg/m2 after Bariatric Surgeries: A Real-World Multi-Centered Study.” This comes to us from the Mayo Clinic in Rochester, Minn.
This was a retrospective study of 329 patients who had type 2 diabetes, who underwent either Roux-en-Y gastric bypass (two-thirds of them) or a surgical sleeve gastrectomy (about one third of them). The mean follow-up was about 6 years. Type 2 diabetes remission was seen in about half of them in this long-term follow-up.
There were significant improvements in hemoglobin A1c, fasting blood glucose, diabetes, and, of course, weight loss – and all were statistically significant. The type of surgery did not seem to make any difference. Both groups really benefited from this.
The take-home point of this study is that patients who have a high BMI and metabolic syndrome, whether they undergo Roux-en-Y gastric bypass or sleeve gastrectomy, should expect to see long-term, durable, positive impact on their diabetes, as well as weight loss, of course, and all the metrics for blood glucose and hemodynamics.
This is an important study and helps us in counseling patients appropriately when they present for selection for these types of surgeries in the appropriate clinical context.
The next paper in the surgical realm is from the University of Padova (Italy), which is “Antireflux Surgery’s Lifespan: 20 Years After Laparoscopic Fundoplication.” This is a prospective study of 137 patients who underwent laparoscopic fundoplication for the management of gastroesophageal reflux disease (n = 107) or a large hiatal hernia (n = 30).
The median follow-up in this study was 22 years, which is among the longest I’ve seen in recent years for any study. A very small percentage of patients underwent revision surgery, which speaks to the skill set and careful selection of the patient cohort.
Only nine patients of 137 had repeat surgeries. Positive outcomes, on the other hand, were seen in the vast majority of patients with reflux, at 84%, and still, two thirds of patients with hiatal hernia had positive outcomes over the long term.
Failure-free survival was much higher for the gastroesophageal reflux cohort, as expected, compared with the hiatal hernia cohort. Patient satisfaction after two decades was almost 90% both for reflux and for hiatal hernia, which is quite a significant milestone.
The take-home point from this study is that laparoscopic fundoplication is quite durable, with success rates approaching 90%, even 2 decades after the surgical intervention. This is quite an important data point for us to discuss with patients when they present with a question around surgery for these indications. Although, as we know well in the era of PPI therapy, surgical indications are definitely reduced, compared with 20 years ago.
The next paper in this group is related to another very important clinical entity, which is acute pancreatitis and the question of pancreatic cancer in those patients who present with acute pancreatitis.
Our previous work in this realm, published a couple of years ago, suggested that about 3% of patients who come to the hospital with acute pancreatitis may harbor pancreatic malignancy, which can be picked up if we do a diligent follow-up with cross-sectional imaging and endoscopic ultrasound.
This paper talks about acute pancreatitis and the modeling of risk in terms of prediction for early cancer. This study was conducted at the University of Pennsylvania and basically speaks to a clinical prediction model to assess the risk for pancreatic cancer after acute pancreatitis diagnosis.
As we know, the risk for pancreatic cancer is highest within 2 years of the initial presentation of acute pancreatitis, especially if the etiology of that pancreatitis was unclear. In this clinical prediction model, as shown on this slide, a variety of demographic and clinical criteria were used, all of which are easily accessible to us in GI and in primary care to calculate the modeling risk.
A large number of patients were used to apply this model: 51,613 patients. The mean age was 62%, the overwhelming majority were male, and half were Caucasian. Using this clinical prediction model, a 2-year incidence of pancreatic ductal adenocarcinoma was calculated to be 1.6% for an absolute number of 800 cases.
Nearly 50% were discovered after 3 months. There was a positive association with pancreatic cystic disease in these patients, which makes sense in terms of their preneoplastic potential.
The take-home point from this study is that readily available demographic and clinical criteria may be useful in helping us predict diagnosis of early pancreatic cancer in a subset of patients who present with acute pancreatitis, and in my opinion, particularly those who present with pancreatitis for which we cannot explain the etiology.
The next paper we have refers to a very common problem, which is Helicobacter pylori infection. This is almost an endemic problem in the Far East and in the third world, but also an increasing problem in the United States.
This particular study comes to us from Taiwan and is looking at different treatment strategies for H. pylori infection. In Taiwan and the Far East, it has been proposed that high acid content in the food might be interfering with efficacy rates. The consideration for antibiotic resistance may be an issue as well. That’s in the background of this paper.
This paper is titled “Enhanced Efficacy of Combined Bismuth and High-Dose Dual Therapy versus High-Dose Dual Therapy Alone or Quadruple Therapy for First-Line H pylori Eradication.” This was an interim report from a large multicenter, randomized controlled trial and included 436 patients with H. pylori infection.
Each patient had biopsies with H. pylori cultures, and they were randomized to one of three regimens as listed here. They received high-dose dual therapy or bismuth along with high-dose dual therapy – so, triple therapy – or they received the amoxicillin-based bismuth and quadruple-therapy regimen. Breath tests were used to check for eradication at the end of the treatment period.
In terms of the results from this study, it’s very clear that the bismuth-based regimen, which is listed here in the middle column, had the highest treatment efficacy rates and moderate adverse event rates when compared with the other two arms.
Reduced eradication in the dual-therapy arm was seen probably due to increased acid food intake. Reduced eradication in the quadruple-therapy arm was likely associated with antibiotic resistance and also with poor compliance, which is understandable when you have more medications to take compared with the group that has fewer medications to take.
The take-home point from this study was that the addition of bismuth might be the silver bullet when you add that to the dual-therapy regimen in difficult cases of H pylori infection. More to come on this, and certainly of relevance to us here as we tackle more and more cases of H. pylori infection stateside.
Last but not least, GI and primary care discussions can never be complete without a discussion on colorectal cancer screening. As the NordICC trial has shown us, it’s not the issue with the screening modality but more about patients’ acceptance of screening, particularly invasive screening.
This paper looked at different strategies and tried to figure out which strategy would be most attractive to bring more people in for screening. This is a large, randomized controlled trial with good distribution of patient populations with approximately half female, half White, and a mean age around 48 years, with more than 20,000 patients.
One of four screening strategies was used. Patients were invited for a fecal immunochemical test, a colonoscopy, or they were invited to choose between a FIT test and a colonoscopy. The final group was mailed a FIT test kit as an outreach mechanism.
Invitations were sent via electronic patient portals, which are very common nowadays, and via the United States Postal Service. Text-message reminders were sent a couple of weeks later. The primary outcome of this study was to look at any colorectal cancer completion rate at 26 weeks.
The results of the study were quite interesting. Screening completion rates were relatively low at 18%. It was interesting to note that the highest screening rates were seen with those who had the FIT test mailed to them, whereas each of the other three groups that had only invitations sent to them had relatively lower screening compliance rates.
The lowest participation was in the colonoscopy invitation group, I suspect due to the invasive nature of the procedure and the patients’ perception of that. When patients were offered a choice, they were more likely to be compliant with screening. In the end, more patients chose colonoscopy as their screening test of choice compared with FIT testing.
The take-home point from this study is that directly sending test kits as an outreach might be more effective than simply inviting subjects to be screened. From those who do respond to be screened, more patients would choose colonoscopy, compared with the FIT test.
With that, I come to the end of this video capsule summary for the Best of GI for Primary Care from DDW 2023. We covered a variety of topics, ranging from pancreatitis to H pylori to colon cancer screening and a couple of GI surgical interventions with long-term outcomes that are very favorable.
Dr. Kaul disclosed conflicts of interest with AMBU, Cook Medical, CDS, CDX,Steris, and Motus GI.
A version of this article first appeared on Medscape.com.
Ruptured aneurysm turns MD couple into doctor-patient
Dr. Taylor Delgado: It was Saturday night, and we had just gone to bed. Suddenly, Ali sat up, and screamed, “My head!” She then became nonresponsive and had a seizure. I was in disbelief, but I also knew exactly what was happening. I called 911: “My wife is having a head bleed. I need an ambulance.” It was a bad connection, and they could barely understand me.
As I tried to carry Ali downstairs, she vomited. She still had rubber bands in her mouth from the jaw fracture that was a result of her accident just a month ago. I knew she needed an airway.
I grabbed a tracheostomy tube, but the opening over her trachea put in for the accident had since closed. I tried to push the tube through her neck, but it hurt her; her eyes opened.
I thought to myself: Maybe she doesn’t need it. This can wait until she gets to the hospital. I can’t do this to her. But she vomited again, and I knew what I had to do.
We were at the top of our stairs. I didn’t have a blade or any other equipment, just the tracheostomy tube with the dilator. I pushed hard, and she started fighting me. I had to hold her hands away with one arm. The tube popped in and she stared back at me in pain and fear.
I finally got her downstairs and called medical control at University Hospital of Cincinnati. I was able to speak with one of the attendings: “Ali’s aneurysm ruptured, and she just had a seizure. She has a GCS of 11 or 12. I replaced her tracheostomy tube. We’ll be there shortly.”
When I heard sirens come down our street, I carried Ali outside, but the sirens were from a firetruck. They likely assumed someone had fallen and had a head laceration. It was beyond deflating. I yelled incredulously: “We need an ambulance here now!”
When the ambulance finally arrived, they tried to tell me that I could not ride with them. Or if I did, I would have to sit up front. After arguing back and forth for a few seconds, I finally demanded: “This is medical control. This is MD-88, and this is my patient. I’m sitting in back with you. She needs four Zofran and two midazolam IV now.”
One month earlier ...
Dr. Alison Delgado: Taylor and I were both 4 months into our second year of residency, and we had been married for 5 months. I was a pediatric resident at Cincinnati Children’s Hospital. She was an emergency medicine resident at the University Hospital. I was having my first day off in a couple weeks, and she was working a shift in the emergency department. She was also a part of the flight crew that day. Second-year residents would go out to the scenes of accidents or to other hospitals to transport the patient back to their Level I trauma center via helicopter. The resident was the physician and considered the leader on these flights.
That afternoon, I went for a bicycle ride. About three-quarters of the way through my ride, I was struck by a car.
The EMS crew got to me fairly quickly. They intubated me at the scene and got me to the closest hospital. Immediately, the hospital realized my case was outside the scope of their care. They contacted University Hospital requesting that their flight crew come to transport me.
Dr. Taylor Delgado: At around 5:30 p.m. the day of my shift, the tones went out on the radio: “AirCare 1 and Pod Doc, you are requested for interhospital transfer, 27-year-old Jane Doe, GCS 5.” That was the only information given.
When we landed at the hospital, I walked in with my nurse. I was listening to the doctor’s report and doing my once over. The patient was a little bit bradycardic, heart rate in the 40s or 50s. Blood pressure was normal if not a little bit elevated. There was obvious facial trauma. The endotracheal tube in place.
She was covered with a blanket, but some of her clothing was visible. Suddenly, I recognized it. It was our cycling team’s kit. I thought, please don’t let it be Ali.
My flight nurse went out and called back to dispatch. “This is my doc’s wife. Dispatch the second helicopter!” She had to repeat herself a few times before they understood what was happening.
As Ali’s spouse, I couldn’t be the flight doctor. I didn’t care. I called medical control myself and told them: “This is Ali. We have to fly her. She has a head injury.” They said: “You can’t fly her.” I said: “We can’t delay her care. I have to fly her.” They said: “No, you can’t fly her.” I broke down. Devastated.
I went back into the room and looked at Ali. Her heart rate was dropping. My flight nurse was in the trauma bay with the emergency physician. We realized definitive care was being delayed because of my presence, which was an awful feeling to have. I think at that point we realized, you do nothing, or you act. So, we acted.
I told my flight nurse: “Let’s give her atropine to increase her heart rate.” I asked about sedation, and she hadn’t had anything. I spurted off some doses: “a hundred of fentanyl and five of midazolam.” My flight nurse actually administered smaller doses. She thought it was a bit aggressive, and she was correct. I was trying to maintain composure, but it was hard.
The emergency medicine physician volunteered to fly with her, so I called back medical control in desperation: “This doctor’s willing to fly. Let him take her.”
They told me apologetically, knowing my agony, that he was not trained to fly and therefore could not do so. I sat down in the ambulance bay crying, waiting for the second helicopter to arrive.
When we got Ali onto AirCare 2, my nurse then told me I couldn’t fly with her. I said, “I’m flying with her.” She said, “no, it’s not safe.” I said, “I’m not leaving her. I’ll sit in the front. What do you think I’m going to do? Jump out of the helicopter?” I think they realized there was no other option that I would agree to. I rode up front.
It was the fastest flight to the trauma center that I had ever experienced. They did a hot offload, meaning they didn’t even shut down the blades. We got her to the trauma center. And then it was a whole other layer of chaos.
Dr. Alison Delgado: Taylor’s presence may have delayed my transfer, but the University emergency department was prepped and waiting for me. Radiology was on hold, surgery and neurosurgery were there waiting. Everyone was in the trauma bay.
Dr. Taylor Delgado: My younger sister was a social worker in that emergency department, and she was on shift. She and my residency director went to CT with Ali. As the images from Ali’s CT scan showed up on the screens, everyone in the room gasped. She had a nonsurvivable head injury.
The AirCare 2 doctor collapsed into our director’s arms and cried: “She’s going to die tonight.” He responded: “I know. But we’ve got work to do.” Then he asked my sister how close she was with me. She told him we were extremely close. “Good, because we have to break the news that she’s going to die tonight.”
But the doctor never told me. I was in the consultation room. He came in and told me that she had a lot of bleeding around the brain, but he couldn’t find the words to tell me the true severity. He didn’t have to.
Dr. Alison Delgado: I was in a coma for 5 days. Shift by shift, they were amazed that I was still there. I had a broken jaw, broken vertebrae in my spine, a broken clavicle and sternum and contusions to my heart and lungs. I was later found to have a dissection of my carotid artery as well as an aneurysm to the carotid artery. These were both caused by the accident.
My jaw was wired shut and a tracheostomy was placed. They coiled the aneurysm and put a stent in the dissection. I was placed on dual antiplatelet therapy to prevent stent thrombosis.
When I initially woke from the coma during my hospital stay, I could not speak, but I remember being told why I was there. My first two thoughts were: Was it my fault? and I need to get back to work.
Two and a half weeks later, I was stable enough to go to an in-patient rehab facility.
I was very motivated. I made a lot of good progress, because Taylor was there with me. We looked through pictures, trying to jog my memory and help with my vocabulary. I’d look at a bird and know this is a flying animal but couldn’t think of the word bird. I couldn’t remember my mom’s name.
Dr. Taylor Delgado: She was becoming more fluent with her speech each day. Her right arm was working more normally. We started going on walks outside. Within 14 days she was discharged home.
When we left the rehab facility, I took a couple extra tracheostomy tubes and supplies, because I didn’t know how long Ali would have her trach. The emergency medicine person in me just thought, always have these things on hand.
A few days later, her ENT doctor decannulated her tracheostomy tube. In our minds, we were done.
The next night, she had the intracranial hemorrhage.
Return to the hospital ...
Dr. Taylor Delgado: The aneurysm they had coiled had ruptured. Ali had a recurrent subarachnoid hemorrhage and an intracranial hemorrhage, and she was still bleeding. So, they took her to IR to try to embolize it and accomplished as much as they possibly could.
She had hydrocephalus, the ventricles in her brain were enlarged. Normally, they would put in a drain, but they couldn’t because she was on aspirin and Plavix (clopidogrel). That would risk her having a bleed around that insertion site, which would cause a brain hemorrhage.
Dr. Alison Delgado: I was like a ticking time bomb. We knew I would have to have surgery as soon as possible to open my skull and clip the aneurysm. But I had to be on the Plavix and aspirin for at least 6 weeks before it would be considered safe to discontinue them. It was another 3 weeks before they could proceed with the surgery.
The second hospitalization was scarier than the first, because I was much more aware. I knew that I might not be able to return to my residency and do the thing I had dreamed of doing. There were risks of me becoming blind or paralyzed during the surgery. I might not even leave the hospital.
Dr. Taylor Delgado: It was mid-December by then, and my dad asked her, “Ali, what do you want for Christmas?” She looked at him deadpan and said, “normal brain.”
Dr. Alison Delgado: The surgery was successful. I went home a few days later. But I’d lost everything I had gained in rehabilitation. My speech was back to square one.
None of the doctors really expected me to go back to work. But from my standpoint, I thought, I could have died the day I was hit. I could have died when the aneurysm ruptured, or at any point along the way. But I’m here and I’m going back to work.
Dr. Taylor Delgado: In January, I went back to work and I had to fly on the helicopter. They were worried about how I would react. My flight director flew with me on my first shift. Our first flight was an inter-facility STEMI transfer. No big deal. The second one was a car accident outside of Batesville, Ind. We were in the back of the ambulance, and I looked at this woman. She was 27 years old, thin, with long hair. She looked exactly like Ali.
Ali flashed into my mind, and I was like, nope. Ali’s at home. She’s fine. This person is right here, right now. Do what you do. I intubated her in the helicopter. We gave her hypertonic saline. I started a blood transfusion. Afterward, my flight director came up to me and said: “You’re released back to full duty. That was the hardest test you could possibly have on your first day back flying, and you nailed it.”
Dr. Alison Delgado: I finished my residency in December of 2012 and passed my pediatric board exam on the first try, almost exactly 3 years after my accident.
The spring before I started medical school in 2005, I had won the Cincinnati Flying Pig marathon. In 2011, a few months after my accident, they invited us to be the starters of the race. When we stood at the starting line, I decided right then I was going to run this marathon again the next year. In spring 2012, I returned and finished in fourth place, beating my previous winning time by two minutes.
I have a different level of empathy for my patients now. I know what it’s like to be scared. I know what it’s like to not know if you’re going to leave the hospital. I’ve lived that. The process of writing my book was also cathartic for me. I told my story to try to give people hope.
Dr. Taylor Delgado: I have a tattoo on my wrist showing the date of Ali’s accident. The idea was to remind myself of what we’ve come through and everyone who went above and beyond. To show gratitude to them and remember everything that they did for us. It’s also to remember that every patient I see is somebody else’s Alison.
A version of this article first appeared on Medscape.com.
Dr. Taylor Delgado: It was Saturday night, and we had just gone to bed. Suddenly, Ali sat up, and screamed, “My head!” She then became nonresponsive and had a seizure. I was in disbelief, but I also knew exactly what was happening. I called 911: “My wife is having a head bleed. I need an ambulance.” It was a bad connection, and they could barely understand me.
As I tried to carry Ali downstairs, she vomited. She still had rubber bands in her mouth from the jaw fracture that was a result of her accident just a month ago. I knew she needed an airway.
I grabbed a tracheostomy tube, but the opening over her trachea put in for the accident had since closed. I tried to push the tube through her neck, but it hurt her; her eyes opened.
I thought to myself: Maybe she doesn’t need it. This can wait until she gets to the hospital. I can’t do this to her. But she vomited again, and I knew what I had to do.
We were at the top of our stairs. I didn’t have a blade or any other equipment, just the tracheostomy tube with the dilator. I pushed hard, and she started fighting me. I had to hold her hands away with one arm. The tube popped in and she stared back at me in pain and fear.
I finally got her downstairs and called medical control at University Hospital of Cincinnati. I was able to speak with one of the attendings: “Ali’s aneurysm ruptured, and she just had a seizure. She has a GCS of 11 or 12. I replaced her tracheostomy tube. We’ll be there shortly.”
When I heard sirens come down our street, I carried Ali outside, but the sirens were from a firetruck. They likely assumed someone had fallen and had a head laceration. It was beyond deflating. I yelled incredulously: “We need an ambulance here now!”
When the ambulance finally arrived, they tried to tell me that I could not ride with them. Or if I did, I would have to sit up front. After arguing back and forth for a few seconds, I finally demanded: “This is medical control. This is MD-88, and this is my patient. I’m sitting in back with you. She needs four Zofran and two midazolam IV now.”
One month earlier ...
Dr. Alison Delgado: Taylor and I were both 4 months into our second year of residency, and we had been married for 5 months. I was a pediatric resident at Cincinnati Children’s Hospital. She was an emergency medicine resident at the University Hospital. I was having my first day off in a couple weeks, and she was working a shift in the emergency department. She was also a part of the flight crew that day. Second-year residents would go out to the scenes of accidents or to other hospitals to transport the patient back to their Level I trauma center via helicopter. The resident was the physician and considered the leader on these flights.
That afternoon, I went for a bicycle ride. About three-quarters of the way through my ride, I was struck by a car.
The EMS crew got to me fairly quickly. They intubated me at the scene and got me to the closest hospital. Immediately, the hospital realized my case was outside the scope of their care. They contacted University Hospital requesting that their flight crew come to transport me.
Dr. Taylor Delgado: At around 5:30 p.m. the day of my shift, the tones went out on the radio: “AirCare 1 and Pod Doc, you are requested for interhospital transfer, 27-year-old Jane Doe, GCS 5.” That was the only information given.
When we landed at the hospital, I walked in with my nurse. I was listening to the doctor’s report and doing my once over. The patient was a little bit bradycardic, heart rate in the 40s or 50s. Blood pressure was normal if not a little bit elevated. There was obvious facial trauma. The endotracheal tube in place.
She was covered with a blanket, but some of her clothing was visible. Suddenly, I recognized it. It was our cycling team’s kit. I thought, please don’t let it be Ali.
My flight nurse went out and called back to dispatch. “This is my doc’s wife. Dispatch the second helicopter!” She had to repeat herself a few times before they understood what was happening.
As Ali’s spouse, I couldn’t be the flight doctor. I didn’t care. I called medical control myself and told them: “This is Ali. We have to fly her. She has a head injury.” They said: “You can’t fly her.” I said: “We can’t delay her care. I have to fly her.” They said: “No, you can’t fly her.” I broke down. Devastated.
I went back into the room and looked at Ali. Her heart rate was dropping. My flight nurse was in the trauma bay with the emergency physician. We realized definitive care was being delayed because of my presence, which was an awful feeling to have. I think at that point we realized, you do nothing, or you act. So, we acted.
I told my flight nurse: “Let’s give her atropine to increase her heart rate.” I asked about sedation, and she hadn’t had anything. I spurted off some doses: “a hundred of fentanyl and five of midazolam.” My flight nurse actually administered smaller doses. She thought it was a bit aggressive, and she was correct. I was trying to maintain composure, but it was hard.
The emergency medicine physician volunteered to fly with her, so I called back medical control in desperation: “This doctor’s willing to fly. Let him take her.”
They told me apologetically, knowing my agony, that he was not trained to fly and therefore could not do so. I sat down in the ambulance bay crying, waiting for the second helicopter to arrive.
When we got Ali onto AirCare 2, my nurse then told me I couldn’t fly with her. I said, “I’m flying with her.” She said, “no, it’s not safe.” I said, “I’m not leaving her. I’ll sit in the front. What do you think I’m going to do? Jump out of the helicopter?” I think they realized there was no other option that I would agree to. I rode up front.
It was the fastest flight to the trauma center that I had ever experienced. They did a hot offload, meaning they didn’t even shut down the blades. We got her to the trauma center. And then it was a whole other layer of chaos.
Dr. Alison Delgado: Taylor’s presence may have delayed my transfer, but the University emergency department was prepped and waiting for me. Radiology was on hold, surgery and neurosurgery were there waiting. Everyone was in the trauma bay.
Dr. Taylor Delgado: My younger sister was a social worker in that emergency department, and she was on shift. She and my residency director went to CT with Ali. As the images from Ali’s CT scan showed up on the screens, everyone in the room gasped. She had a nonsurvivable head injury.
The AirCare 2 doctor collapsed into our director’s arms and cried: “She’s going to die tonight.” He responded: “I know. But we’ve got work to do.” Then he asked my sister how close she was with me. She told him we were extremely close. “Good, because we have to break the news that she’s going to die tonight.”
But the doctor never told me. I was in the consultation room. He came in and told me that she had a lot of bleeding around the brain, but he couldn’t find the words to tell me the true severity. He didn’t have to.
Dr. Alison Delgado: I was in a coma for 5 days. Shift by shift, they were amazed that I was still there. I had a broken jaw, broken vertebrae in my spine, a broken clavicle and sternum and contusions to my heart and lungs. I was later found to have a dissection of my carotid artery as well as an aneurysm to the carotid artery. These were both caused by the accident.
My jaw was wired shut and a tracheostomy was placed. They coiled the aneurysm and put a stent in the dissection. I was placed on dual antiplatelet therapy to prevent stent thrombosis.
When I initially woke from the coma during my hospital stay, I could not speak, but I remember being told why I was there. My first two thoughts were: Was it my fault? and I need to get back to work.
Two and a half weeks later, I was stable enough to go to an in-patient rehab facility.
I was very motivated. I made a lot of good progress, because Taylor was there with me. We looked through pictures, trying to jog my memory and help with my vocabulary. I’d look at a bird and know this is a flying animal but couldn’t think of the word bird. I couldn’t remember my mom’s name.
Dr. Taylor Delgado: She was becoming more fluent with her speech each day. Her right arm was working more normally. We started going on walks outside. Within 14 days she was discharged home.
When we left the rehab facility, I took a couple extra tracheostomy tubes and supplies, because I didn’t know how long Ali would have her trach. The emergency medicine person in me just thought, always have these things on hand.
A few days later, her ENT doctor decannulated her tracheostomy tube. In our minds, we were done.
The next night, she had the intracranial hemorrhage.
Return to the hospital ...
Dr. Taylor Delgado: The aneurysm they had coiled had ruptured. Ali had a recurrent subarachnoid hemorrhage and an intracranial hemorrhage, and she was still bleeding. So, they took her to IR to try to embolize it and accomplished as much as they possibly could.
She had hydrocephalus, the ventricles in her brain were enlarged. Normally, they would put in a drain, but they couldn’t because she was on aspirin and Plavix (clopidogrel). That would risk her having a bleed around that insertion site, which would cause a brain hemorrhage.
Dr. Alison Delgado: I was like a ticking time bomb. We knew I would have to have surgery as soon as possible to open my skull and clip the aneurysm. But I had to be on the Plavix and aspirin for at least 6 weeks before it would be considered safe to discontinue them. It was another 3 weeks before they could proceed with the surgery.
The second hospitalization was scarier than the first, because I was much more aware. I knew that I might not be able to return to my residency and do the thing I had dreamed of doing. There were risks of me becoming blind or paralyzed during the surgery. I might not even leave the hospital.
Dr. Taylor Delgado: It was mid-December by then, and my dad asked her, “Ali, what do you want for Christmas?” She looked at him deadpan and said, “normal brain.”
Dr. Alison Delgado: The surgery was successful. I went home a few days later. But I’d lost everything I had gained in rehabilitation. My speech was back to square one.
None of the doctors really expected me to go back to work. But from my standpoint, I thought, I could have died the day I was hit. I could have died when the aneurysm ruptured, or at any point along the way. But I’m here and I’m going back to work.
Dr. Taylor Delgado: In January, I went back to work and I had to fly on the helicopter. They were worried about how I would react. My flight director flew with me on my first shift. Our first flight was an inter-facility STEMI transfer. No big deal. The second one was a car accident outside of Batesville, Ind. We were in the back of the ambulance, and I looked at this woman. She was 27 years old, thin, with long hair. She looked exactly like Ali.
Ali flashed into my mind, and I was like, nope. Ali’s at home. She’s fine. This person is right here, right now. Do what you do. I intubated her in the helicopter. We gave her hypertonic saline. I started a blood transfusion. Afterward, my flight director came up to me and said: “You’re released back to full duty. That was the hardest test you could possibly have on your first day back flying, and you nailed it.”
Dr. Alison Delgado: I finished my residency in December of 2012 and passed my pediatric board exam on the first try, almost exactly 3 years after my accident.
The spring before I started medical school in 2005, I had won the Cincinnati Flying Pig marathon. In 2011, a few months after my accident, they invited us to be the starters of the race. When we stood at the starting line, I decided right then I was going to run this marathon again the next year. In spring 2012, I returned and finished in fourth place, beating my previous winning time by two minutes.
I have a different level of empathy for my patients now. I know what it’s like to be scared. I know what it’s like to not know if you’re going to leave the hospital. I’ve lived that. The process of writing my book was also cathartic for me. I told my story to try to give people hope.
Dr. Taylor Delgado: I have a tattoo on my wrist showing the date of Ali’s accident. The idea was to remind myself of what we’ve come through and everyone who went above and beyond. To show gratitude to them and remember everything that they did for us. It’s also to remember that every patient I see is somebody else’s Alison.
A version of this article first appeared on Medscape.com.
Dr. Taylor Delgado: It was Saturday night, and we had just gone to bed. Suddenly, Ali sat up, and screamed, “My head!” She then became nonresponsive and had a seizure. I was in disbelief, but I also knew exactly what was happening. I called 911: “My wife is having a head bleed. I need an ambulance.” It was a bad connection, and they could barely understand me.
As I tried to carry Ali downstairs, she vomited. She still had rubber bands in her mouth from the jaw fracture that was a result of her accident just a month ago. I knew she needed an airway.
I grabbed a tracheostomy tube, but the opening over her trachea put in for the accident had since closed. I tried to push the tube through her neck, but it hurt her; her eyes opened.
I thought to myself: Maybe she doesn’t need it. This can wait until she gets to the hospital. I can’t do this to her. But she vomited again, and I knew what I had to do.
We were at the top of our stairs. I didn’t have a blade or any other equipment, just the tracheostomy tube with the dilator. I pushed hard, and she started fighting me. I had to hold her hands away with one arm. The tube popped in and she stared back at me in pain and fear.
I finally got her downstairs and called medical control at University Hospital of Cincinnati. I was able to speak with one of the attendings: “Ali’s aneurysm ruptured, and she just had a seizure. She has a GCS of 11 or 12. I replaced her tracheostomy tube. We’ll be there shortly.”
When I heard sirens come down our street, I carried Ali outside, but the sirens were from a firetruck. They likely assumed someone had fallen and had a head laceration. It was beyond deflating. I yelled incredulously: “We need an ambulance here now!”
When the ambulance finally arrived, they tried to tell me that I could not ride with them. Or if I did, I would have to sit up front. After arguing back and forth for a few seconds, I finally demanded: “This is medical control. This is MD-88, and this is my patient. I’m sitting in back with you. She needs four Zofran and two midazolam IV now.”
One month earlier ...
Dr. Alison Delgado: Taylor and I were both 4 months into our second year of residency, and we had been married for 5 months. I was a pediatric resident at Cincinnati Children’s Hospital. She was an emergency medicine resident at the University Hospital. I was having my first day off in a couple weeks, and she was working a shift in the emergency department. She was also a part of the flight crew that day. Second-year residents would go out to the scenes of accidents or to other hospitals to transport the patient back to their Level I trauma center via helicopter. The resident was the physician and considered the leader on these flights.
That afternoon, I went for a bicycle ride. About three-quarters of the way through my ride, I was struck by a car.
The EMS crew got to me fairly quickly. They intubated me at the scene and got me to the closest hospital. Immediately, the hospital realized my case was outside the scope of their care. They contacted University Hospital requesting that their flight crew come to transport me.
Dr. Taylor Delgado: At around 5:30 p.m. the day of my shift, the tones went out on the radio: “AirCare 1 and Pod Doc, you are requested for interhospital transfer, 27-year-old Jane Doe, GCS 5.” That was the only information given.
When we landed at the hospital, I walked in with my nurse. I was listening to the doctor’s report and doing my once over. The patient was a little bit bradycardic, heart rate in the 40s or 50s. Blood pressure was normal if not a little bit elevated. There was obvious facial trauma. The endotracheal tube in place.
She was covered with a blanket, but some of her clothing was visible. Suddenly, I recognized it. It was our cycling team’s kit. I thought, please don’t let it be Ali.
My flight nurse went out and called back to dispatch. “This is my doc’s wife. Dispatch the second helicopter!” She had to repeat herself a few times before they understood what was happening.
As Ali’s spouse, I couldn’t be the flight doctor. I didn’t care. I called medical control myself and told them: “This is Ali. We have to fly her. She has a head injury.” They said: “You can’t fly her.” I said: “We can’t delay her care. I have to fly her.” They said: “No, you can’t fly her.” I broke down. Devastated.
I went back into the room and looked at Ali. Her heart rate was dropping. My flight nurse was in the trauma bay with the emergency physician. We realized definitive care was being delayed because of my presence, which was an awful feeling to have. I think at that point we realized, you do nothing, or you act. So, we acted.
I told my flight nurse: “Let’s give her atropine to increase her heart rate.” I asked about sedation, and she hadn’t had anything. I spurted off some doses: “a hundred of fentanyl and five of midazolam.” My flight nurse actually administered smaller doses. She thought it was a bit aggressive, and she was correct. I was trying to maintain composure, but it was hard.
The emergency medicine physician volunteered to fly with her, so I called back medical control in desperation: “This doctor’s willing to fly. Let him take her.”
They told me apologetically, knowing my agony, that he was not trained to fly and therefore could not do so. I sat down in the ambulance bay crying, waiting for the second helicopter to arrive.
When we got Ali onto AirCare 2, my nurse then told me I couldn’t fly with her. I said, “I’m flying with her.” She said, “no, it’s not safe.” I said, “I’m not leaving her. I’ll sit in the front. What do you think I’m going to do? Jump out of the helicopter?” I think they realized there was no other option that I would agree to. I rode up front.
It was the fastest flight to the trauma center that I had ever experienced. They did a hot offload, meaning they didn’t even shut down the blades. We got her to the trauma center. And then it was a whole other layer of chaos.
Dr. Alison Delgado: Taylor’s presence may have delayed my transfer, but the University emergency department was prepped and waiting for me. Radiology was on hold, surgery and neurosurgery were there waiting. Everyone was in the trauma bay.
Dr. Taylor Delgado: My younger sister was a social worker in that emergency department, and she was on shift. She and my residency director went to CT with Ali. As the images from Ali’s CT scan showed up on the screens, everyone in the room gasped. She had a nonsurvivable head injury.
The AirCare 2 doctor collapsed into our director’s arms and cried: “She’s going to die tonight.” He responded: “I know. But we’ve got work to do.” Then he asked my sister how close she was with me. She told him we were extremely close. “Good, because we have to break the news that she’s going to die tonight.”
But the doctor never told me. I was in the consultation room. He came in and told me that she had a lot of bleeding around the brain, but he couldn’t find the words to tell me the true severity. He didn’t have to.
Dr. Alison Delgado: I was in a coma for 5 days. Shift by shift, they were amazed that I was still there. I had a broken jaw, broken vertebrae in my spine, a broken clavicle and sternum and contusions to my heart and lungs. I was later found to have a dissection of my carotid artery as well as an aneurysm to the carotid artery. These were both caused by the accident.
My jaw was wired shut and a tracheostomy was placed. They coiled the aneurysm and put a stent in the dissection. I was placed on dual antiplatelet therapy to prevent stent thrombosis.
When I initially woke from the coma during my hospital stay, I could not speak, but I remember being told why I was there. My first two thoughts were: Was it my fault? and I need to get back to work.
Two and a half weeks later, I was stable enough to go to an in-patient rehab facility.
I was very motivated. I made a lot of good progress, because Taylor was there with me. We looked through pictures, trying to jog my memory and help with my vocabulary. I’d look at a bird and know this is a flying animal but couldn’t think of the word bird. I couldn’t remember my mom’s name.
Dr. Taylor Delgado: She was becoming more fluent with her speech each day. Her right arm was working more normally. We started going on walks outside. Within 14 days she was discharged home.
When we left the rehab facility, I took a couple extra tracheostomy tubes and supplies, because I didn’t know how long Ali would have her trach. The emergency medicine person in me just thought, always have these things on hand.
A few days later, her ENT doctor decannulated her tracheostomy tube. In our minds, we were done.
The next night, she had the intracranial hemorrhage.
Return to the hospital ...
Dr. Taylor Delgado: The aneurysm they had coiled had ruptured. Ali had a recurrent subarachnoid hemorrhage and an intracranial hemorrhage, and she was still bleeding. So, they took her to IR to try to embolize it and accomplished as much as they possibly could.
She had hydrocephalus, the ventricles in her brain were enlarged. Normally, they would put in a drain, but they couldn’t because she was on aspirin and Plavix (clopidogrel). That would risk her having a bleed around that insertion site, which would cause a brain hemorrhage.
Dr. Alison Delgado: I was like a ticking time bomb. We knew I would have to have surgery as soon as possible to open my skull and clip the aneurysm. But I had to be on the Plavix and aspirin for at least 6 weeks before it would be considered safe to discontinue them. It was another 3 weeks before they could proceed with the surgery.
The second hospitalization was scarier than the first, because I was much more aware. I knew that I might not be able to return to my residency and do the thing I had dreamed of doing. There were risks of me becoming blind or paralyzed during the surgery. I might not even leave the hospital.
Dr. Taylor Delgado: It was mid-December by then, and my dad asked her, “Ali, what do you want for Christmas?” She looked at him deadpan and said, “normal brain.”
Dr. Alison Delgado: The surgery was successful. I went home a few days later. But I’d lost everything I had gained in rehabilitation. My speech was back to square one.
None of the doctors really expected me to go back to work. But from my standpoint, I thought, I could have died the day I was hit. I could have died when the aneurysm ruptured, or at any point along the way. But I’m here and I’m going back to work.
Dr. Taylor Delgado: In January, I went back to work and I had to fly on the helicopter. They were worried about how I would react. My flight director flew with me on my first shift. Our first flight was an inter-facility STEMI transfer. No big deal. The second one was a car accident outside of Batesville, Ind. We were in the back of the ambulance, and I looked at this woman. She was 27 years old, thin, with long hair. She looked exactly like Ali.
Ali flashed into my mind, and I was like, nope. Ali’s at home. She’s fine. This person is right here, right now. Do what you do. I intubated her in the helicopter. We gave her hypertonic saline. I started a blood transfusion. Afterward, my flight director came up to me and said: “You’re released back to full duty. That was the hardest test you could possibly have on your first day back flying, and you nailed it.”
Dr. Alison Delgado: I finished my residency in December of 2012 and passed my pediatric board exam on the first try, almost exactly 3 years after my accident.
The spring before I started medical school in 2005, I had won the Cincinnati Flying Pig marathon. In 2011, a few months after my accident, they invited us to be the starters of the race. When we stood at the starting line, I decided right then I was going to run this marathon again the next year. In spring 2012, I returned and finished in fourth place, beating my previous winning time by two minutes.
I have a different level of empathy for my patients now. I know what it’s like to be scared. I know what it’s like to not know if you’re going to leave the hospital. I’ve lived that. The process of writing my book was also cathartic for me. I told my story to try to give people hope.
Dr. Taylor Delgado: I have a tattoo on my wrist showing the date of Ali’s accident. The idea was to remind myself of what we’ve come through and everyone who went above and beyond. To show gratitude to them and remember everything that they did for us. It’s also to remember that every patient I see is somebody else’s Alison.
A version of this article first appeared on Medscape.com.



