User login
Smoking increases heart failure risk in blacks
Cigarette smoking is an important risk factor for heart failure in blacks, according to results of an investigation of patients in the Jackson Heart Study.
Current smoking among blacks was associated with higher mean left ventricular (LV) mass and lower mean LV systolic function, even after adjustment for confounding factors, authors of the analysis reported in the journal Circulation.
While blacks are known to have a higher incidence of heart failure than do whites, Hispanics, and Asians, this is believed to be the first prospective study of a large black cohort demonstrating a dose-response relationship between smoking and incident heart failure.
“Smoking cessation may be a potential strategy to attenuate the higher rate of heart failure in blacks,” wrote Dr. Kamimura and coauthors.
The published analysis included data on 4,129 participants in the Jackson Heart Study, a large, prospective, community-based observational study investigating cardiovascular risk factors in blacks.
That group, which was 63% female, included 503 current smokers, 742 former smokers, and 2,884 individuals who had never smoked.
At baseline, no patients had a history of heart failure or coronary heart disease, and over a median follow-up of 8.0 years, there were 147 hospitalizations for heart failure in the cohort, the investigators reported.
Current smoking, compared with never smoking, was significantly associated with incident heart failure hospitalization after adjusting for risk factors and coronary heart disease (hazard ratio, 2.82; 95% confidence interval, 1.71-4.64).
Likewise, smoking intensity of at least 20 cigarettes a day (HR, 3.48; 95% CI, 1.65-7.32) and smoking burden of at least 15 pack-years (HR, 2.06; 95% CI, 1.29-3.33) both were significantly associated with incident heart failure hospitalization .
Compared with never smoking, current smoking was significantly associated with higher mean LV mass index and lower mean LV circumferential strain, even after adjusting for confounding variables (P less than 0.05 for both comparisons).
Smoking status also was associated with higher mean levels of brain natriuretic peptide, as were smoking intensity and burden (P less than 0.05 for all three comparisons), data show.
While cigarette smoking is a well-known risk factor for cardiovascular disease, Dr. Kamimura and coauthors said the influences on cardiac structure and function may not be fully appreciated because of the strong association with coronary heart disease, a major cause of heart failure.
“These relationships were significant after adjustment for coronary heart disease, suggesting mechanisms beyond atherosclerosis probably contribute to myocardial dysfunction and increased risk of heart failure in smokers,” they wrote in a discussion of the results.
Authors reported that they had no conflicts of interest related to the study. The Jackson Heart Study is supported by Jackson (Miss.) State University, Tougaloo College, and the University of Mississippi Medical Center, all in Jackson, contracts from the National Heart, Lung, and Blood Institute and the National Institute for Minority Health and Health Disparities. This study was supported by the NHLBI. One author has also received support from the National Institute of Diabetes and Digestive and Kidney Diseases and The National Institute of General Medical Sciences.
SOURCE: Kamimura D et al. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.117.031912.
Cigarette smoking is an important risk factor for heart failure in blacks, according to results of an investigation of patients in the Jackson Heart Study.
Current smoking among blacks was associated with higher mean left ventricular (LV) mass and lower mean LV systolic function, even after adjustment for confounding factors, authors of the analysis reported in the journal Circulation.
While blacks are known to have a higher incidence of heart failure than do whites, Hispanics, and Asians, this is believed to be the first prospective study of a large black cohort demonstrating a dose-response relationship between smoking and incident heart failure.
“Smoking cessation may be a potential strategy to attenuate the higher rate of heart failure in blacks,” wrote Dr. Kamimura and coauthors.
The published analysis included data on 4,129 participants in the Jackson Heart Study, a large, prospective, community-based observational study investigating cardiovascular risk factors in blacks.
That group, which was 63% female, included 503 current smokers, 742 former smokers, and 2,884 individuals who had never smoked.
At baseline, no patients had a history of heart failure or coronary heart disease, and over a median follow-up of 8.0 years, there were 147 hospitalizations for heart failure in the cohort, the investigators reported.
Current smoking, compared with never smoking, was significantly associated with incident heart failure hospitalization after adjusting for risk factors and coronary heart disease (hazard ratio, 2.82; 95% confidence interval, 1.71-4.64).
Likewise, smoking intensity of at least 20 cigarettes a day (HR, 3.48; 95% CI, 1.65-7.32) and smoking burden of at least 15 pack-years (HR, 2.06; 95% CI, 1.29-3.33) both were significantly associated with incident heart failure hospitalization .
Compared with never smoking, current smoking was significantly associated with higher mean LV mass index and lower mean LV circumferential strain, even after adjusting for confounding variables (P less than 0.05 for both comparisons).
Smoking status also was associated with higher mean levels of brain natriuretic peptide, as were smoking intensity and burden (P less than 0.05 for all three comparisons), data show.
While cigarette smoking is a well-known risk factor for cardiovascular disease, Dr. Kamimura and coauthors said the influences on cardiac structure and function may not be fully appreciated because of the strong association with coronary heart disease, a major cause of heart failure.
“These relationships were significant after adjustment for coronary heart disease, suggesting mechanisms beyond atherosclerosis probably contribute to myocardial dysfunction and increased risk of heart failure in smokers,” they wrote in a discussion of the results.
Authors reported that they had no conflicts of interest related to the study. The Jackson Heart Study is supported by Jackson (Miss.) State University, Tougaloo College, and the University of Mississippi Medical Center, all in Jackson, contracts from the National Heart, Lung, and Blood Institute and the National Institute for Minority Health and Health Disparities. This study was supported by the NHLBI. One author has also received support from the National Institute of Diabetes and Digestive and Kidney Diseases and The National Institute of General Medical Sciences.
SOURCE: Kamimura D et al. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.117.031912.
Cigarette smoking is an important risk factor for heart failure in blacks, according to results of an investigation of patients in the Jackson Heart Study.
Current smoking among blacks was associated with higher mean left ventricular (LV) mass and lower mean LV systolic function, even after adjustment for confounding factors, authors of the analysis reported in the journal Circulation.
While blacks are known to have a higher incidence of heart failure than do whites, Hispanics, and Asians, this is believed to be the first prospective study of a large black cohort demonstrating a dose-response relationship between smoking and incident heart failure.
“Smoking cessation may be a potential strategy to attenuate the higher rate of heart failure in blacks,” wrote Dr. Kamimura and coauthors.
The published analysis included data on 4,129 participants in the Jackson Heart Study, a large, prospective, community-based observational study investigating cardiovascular risk factors in blacks.
That group, which was 63% female, included 503 current smokers, 742 former smokers, and 2,884 individuals who had never smoked.
At baseline, no patients had a history of heart failure or coronary heart disease, and over a median follow-up of 8.0 years, there were 147 hospitalizations for heart failure in the cohort, the investigators reported.
Current smoking, compared with never smoking, was significantly associated with incident heart failure hospitalization after adjusting for risk factors and coronary heart disease (hazard ratio, 2.82; 95% confidence interval, 1.71-4.64).
Likewise, smoking intensity of at least 20 cigarettes a day (HR, 3.48; 95% CI, 1.65-7.32) and smoking burden of at least 15 pack-years (HR, 2.06; 95% CI, 1.29-3.33) both were significantly associated with incident heart failure hospitalization .
Compared with never smoking, current smoking was significantly associated with higher mean LV mass index and lower mean LV circumferential strain, even after adjusting for confounding variables (P less than 0.05 for both comparisons).
Smoking status also was associated with higher mean levels of brain natriuretic peptide, as were smoking intensity and burden (P less than 0.05 for all three comparisons), data show.
While cigarette smoking is a well-known risk factor for cardiovascular disease, Dr. Kamimura and coauthors said the influences on cardiac structure and function may not be fully appreciated because of the strong association with coronary heart disease, a major cause of heart failure.
“These relationships were significant after adjustment for coronary heart disease, suggesting mechanisms beyond atherosclerosis probably contribute to myocardial dysfunction and increased risk of heart failure in smokers,” they wrote in a discussion of the results.
Authors reported that they had no conflicts of interest related to the study. The Jackson Heart Study is supported by Jackson (Miss.) State University, Tougaloo College, and the University of Mississippi Medical Center, all in Jackson, contracts from the National Heart, Lung, and Blood Institute and the National Institute for Minority Health and Health Disparities. This study was supported by the NHLBI. One author has also received support from the National Institute of Diabetes and Digestive and Kidney Diseases and The National Institute of General Medical Sciences.
SOURCE: Kamimura D et al. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.117.031912.
FROM CIRCULATION
Key clinical point: Major finding: Current smoking, cigarettes per day, and smoking burden in pack-years were all independently associated with incident heart failure hospitalization (hazard ratio 2.82, 3.48, and 2.06, respectively) even after adjusting for risk factors and coronary heart disease.
Study details: Analysis of 4,129 participants in the Jackson Heart Study, a large, prospective, community-based observational study investigating cardiovascular risk factors in blacks.
Disclosures: Authors reported that they had no conflicts of interest related to the study. The Jackson Heart Study is supported by Jackson (Miss.) State University; Tougaloo College, and the University of Mississippi Medical Center, all in Jackson, contracts from the National Heart, Lung, and Blood Institute and the National Institute for Minority Health and Health Disparities. This study was supported by the NHLBI. One author has received support from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of General Medical Sciences.
Source: Kamimura D et al. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.117.031912.
SLE: Specialized lupus clinics may offer superior quality of care
Patients with systemic lupus erythematosus (SLE) more often received care consistent with quality measures when they were seen in a specialized lupus clinic than they did in a general rheumatology clinic, according to results of a recent single-center, retrospective study.
Compared with a general rheumatology clinic, the lupus clinic had superior quality measure performance overall and for specific measures related to testing, treatment, and counseling, study authors reported in Arthritis Care & Research.
“Providing quality care in SLE is challenging as patients require ongoing, time consuming, multidisciplinary care,” wrote Shilpa Arora, MD, of John H. Stroger Hospital in Chicago, and her coauthors.
Lupus clinics and other disease-focused clinics at academic centers may provide better patient care by leveraging subspecialists’ current knowledge, experience, care processes, and multidisciplinary networks, according to Dr. Arora and her colleagues.
To assess whether a lupus clinic makes any difference in quality of care, Dr. Arora and her coinvestigators conducted a cross-sectional, retrospective chart review including 150 consecutive patients who received care at Rush University in Chicago.
Of that group of patients, 77 had received care in a subspecialty lupus clinic, and 73 received care at the general rheumatology clinic, according to the report.
Looking at validated quality measures for SLE testing, treatment, and counseling, Dr. Arora and her colleagues found performance was significantly greater overall for the lupus clinic than it was for the general rheumatology clinic (85.8% vs. 70.2%; P = 0.001).
In particular, patients treated at the lupus clinic were significantly more likely to get antiphospholipid antibody testing and bone mineral density testing, they said.
Lupus clinic patients were also more likely to be offered preventive measures, such as sunscreen counseling, cardiovascular disease risk assessment, and prescription of an angiotensin converting enzyme inhibitor when appropriate, they added.
To see whether there was any relationship between patient volume and quality measures, the researchers also looked at the number of SLE patients seen by each rheumatologist.
Dr. Arora and her associates did find a moderate correlation between the number of patients seen per rheumatologist and quality measure performance (rho, 0.48; P less than 0.001).
While this small study suggests subspecialty clinics provide high quality care, the authors said multicenter, prospective studies are needed to evaluate whether they also improve patient outcomes.
“It would be pertinent to see if these efficiencies translate over time to measurable gains in health status and health resource utilizations,” Dr. Arora and her colleagues concluded in their report.
Dr. Arora and her associates said there were no relevant financial disclosures or funding.
SOURCE: Arora S et al. Arthritis Care Res (Hoboken). 2018 Apr 2. doi: 10.1002/acr.23569.
Patients with systemic lupus erythematosus (SLE) more often received care consistent with quality measures when they were seen in a specialized lupus clinic than they did in a general rheumatology clinic, according to results of a recent single-center, retrospective study.
Compared with a general rheumatology clinic, the lupus clinic had superior quality measure performance overall and for specific measures related to testing, treatment, and counseling, study authors reported in Arthritis Care & Research.
“Providing quality care in SLE is challenging as patients require ongoing, time consuming, multidisciplinary care,” wrote Shilpa Arora, MD, of John H. Stroger Hospital in Chicago, and her coauthors.
Lupus clinics and other disease-focused clinics at academic centers may provide better patient care by leveraging subspecialists’ current knowledge, experience, care processes, and multidisciplinary networks, according to Dr. Arora and her colleagues.
To assess whether a lupus clinic makes any difference in quality of care, Dr. Arora and her coinvestigators conducted a cross-sectional, retrospective chart review including 150 consecutive patients who received care at Rush University in Chicago.
Of that group of patients, 77 had received care in a subspecialty lupus clinic, and 73 received care at the general rheumatology clinic, according to the report.
Looking at validated quality measures for SLE testing, treatment, and counseling, Dr. Arora and her colleagues found performance was significantly greater overall for the lupus clinic than it was for the general rheumatology clinic (85.8% vs. 70.2%; P = 0.001).
In particular, patients treated at the lupus clinic were significantly more likely to get antiphospholipid antibody testing and bone mineral density testing, they said.
Lupus clinic patients were also more likely to be offered preventive measures, such as sunscreen counseling, cardiovascular disease risk assessment, and prescription of an angiotensin converting enzyme inhibitor when appropriate, they added.
To see whether there was any relationship between patient volume and quality measures, the researchers also looked at the number of SLE patients seen by each rheumatologist.
Dr. Arora and her associates did find a moderate correlation between the number of patients seen per rheumatologist and quality measure performance (rho, 0.48; P less than 0.001).
While this small study suggests subspecialty clinics provide high quality care, the authors said multicenter, prospective studies are needed to evaluate whether they also improve patient outcomes.
“It would be pertinent to see if these efficiencies translate over time to measurable gains in health status and health resource utilizations,” Dr. Arora and her colleagues concluded in their report.
Dr. Arora and her associates said there were no relevant financial disclosures or funding.
SOURCE: Arora S et al. Arthritis Care Res (Hoboken). 2018 Apr 2. doi: 10.1002/acr.23569.
Patients with systemic lupus erythematosus (SLE) more often received care consistent with quality measures when they were seen in a specialized lupus clinic than they did in a general rheumatology clinic, according to results of a recent single-center, retrospective study.
Compared with a general rheumatology clinic, the lupus clinic had superior quality measure performance overall and for specific measures related to testing, treatment, and counseling, study authors reported in Arthritis Care & Research.
“Providing quality care in SLE is challenging as patients require ongoing, time consuming, multidisciplinary care,” wrote Shilpa Arora, MD, of John H. Stroger Hospital in Chicago, and her coauthors.
Lupus clinics and other disease-focused clinics at academic centers may provide better patient care by leveraging subspecialists’ current knowledge, experience, care processes, and multidisciplinary networks, according to Dr. Arora and her colleagues.
To assess whether a lupus clinic makes any difference in quality of care, Dr. Arora and her coinvestigators conducted a cross-sectional, retrospective chart review including 150 consecutive patients who received care at Rush University in Chicago.
Of that group of patients, 77 had received care in a subspecialty lupus clinic, and 73 received care at the general rheumatology clinic, according to the report.
Looking at validated quality measures for SLE testing, treatment, and counseling, Dr. Arora and her colleagues found performance was significantly greater overall for the lupus clinic than it was for the general rheumatology clinic (85.8% vs. 70.2%; P = 0.001).
In particular, patients treated at the lupus clinic were significantly more likely to get antiphospholipid antibody testing and bone mineral density testing, they said.
Lupus clinic patients were also more likely to be offered preventive measures, such as sunscreen counseling, cardiovascular disease risk assessment, and prescription of an angiotensin converting enzyme inhibitor when appropriate, they added.
To see whether there was any relationship between patient volume and quality measures, the researchers also looked at the number of SLE patients seen by each rheumatologist.
Dr. Arora and her associates did find a moderate correlation between the number of patients seen per rheumatologist and quality measure performance (rho, 0.48; P less than 0.001).
While this small study suggests subspecialty clinics provide high quality care, the authors said multicenter, prospective studies are needed to evaluate whether they also improve patient outcomes.
“It would be pertinent to see if these efficiencies translate over time to measurable gains in health status and health resource utilizations,” Dr. Arora and her colleagues concluded in their report.
Dr. Arora and her associates said there were no relevant financial disclosures or funding.
SOURCE: Arora S et al. Arthritis Care Res (Hoboken). 2018 Apr 2. doi: 10.1002/acr.23569.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Quality measure performance in the lupus clinic was superior to that in a general rheumatology clinic (85.8% vs. 70.2%; P = 0.001).
Study details: A cross-sectional, retrospective chart review including 150 consecutive patients who received care at Rush University in Chicago in either the subspecialty lupus clinic or the general rheumatology clinic.
Disclosures: Dr. Arora and her associates said there were no relevant disclosures or funding.
Source: Arora S et al. Arthritis Care Res (Hoboken). 2018 Apr 2. doi: 10.1002/acr.23569.
Blinatumomab triggers complete MRD response in ALL
After treatment with blinatumomab, most patients with minimal residual disease–positive acute lymphoblastic leukemia (ALL) achieved complete MRD response, according to results of a single-arm phase 2 study.
Achieving complete MRD response was associated with significantly longer relapse-free and overall survival in the patients, who were already in hematologic complete remission, researchers reported in the journal Blood.
“Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies,” Nicola Gökbuget, MD, University Hospital, Frankfurt, Germany, and her coauthors wrote.
This is the first international multicenter study to specifically enroll MRD-positive ALL patients and evaluate them for an MRD-based primary outcome in a cohort of MRD-positive ALL patients, according to the authors.
Preemptively treating low but measurable disease in ALL in remission, instead of waiting for overt relapse, is a strategy that may prolong overall survival, Dr. Gökbuget and her colleagues said in describing the rationale for their study. While there is no standard therapy yet for ALL patients with detectable MRD after intensive chemotherapy, hematopoietic stem cell transplantation (HSCT) is recommended, based on data that it may improve outcomes in patients with persistent MRD. However, other studies suggest detectable MRD before HSCT is associated with higher relapse rates, and many patients relapse while waiting for HSCT, the researchers noted.
To test an MRD-directed treatment strategy, Dr. Gökbuget and colleagues at 46 centers in Europe and Russia conducted an open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission. Patients in the study received up to four cycles of blinatumomab, a bispecific, T cell–engager antibody construct that enables T cells to recognize and eliminate CD19-positive cells.
Of 113 evaluable patients, 88 (78%) achieved complete MRD response after one cycle, the primary end point of the study. Relapse-free survival at 18 months was estimated at 54% and median overall survival was 36.5 months in the subset of 110 patients with Philadelphia chromosome–negative ALL in hematologic remission.
Complete MRD responders had improved relapse-free survival versus MRD nonresponders (23.6 vs. 5.7 months; P = .002), they reported. Likewise, overall survival was improved for MRD responders (38.9 vs. 12.5 months; P = .002).
Adverse events were consistent with what was previously reported for blinatumomab and included grade 3 and 4 neurologic events in 12 patients (10%) and 3 patients (3%), respectively. Cytokine-release syndrome was seen in four patients, with grade 1 and grade 3 cases.
The study was not designed to assess the impact of HSCT, which most patients (n = 76) underwent. However, a number of patients with complete MRD response but no HSCT remained in long-term remission, confirming results of an earlier blinatumomab pilot study, according to the researchers.
“This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients,” Dr. Gökbuget and her coauthors wrote.
Additional studies are needed to clarify the role and indications for HSCT in this setting, they added.
The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
SOURCE: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
The study by Dr. Gökbuget and her colleagues provides “strong evidence” that blinatumomab immunotherapy eliminates residual B-cell acute lymphoblastic leukemia (ALL) cells, thereby preventing relapse and improving survival, according to Patrick Brown, MD.
“This addresses the most important unsolved clinical problem in adults with B-ALL: the development of chemotherapy-resistant relapsed disease,” Dr. Brown wrote in an editorial.
Persistence of minimal residual disease (MRD) is the strongest independent predictor of outcomes in B-cell ALL, and is seen in up to 50% of adult patients after chemotherapy, according to Dr. Brown.
The “well-designed and well-executed” multicenter phase 2 study demonstrated an MRD clearance rate of 78% after one cycle of blinatumomab with modest adverse effects, according to Dr. Brown. Moreover, the results show a doubling of overall survival and tripling of relapse-free survival in MRD responders versus nonresponders, he said.
“An important caveat, however, is that, although the MRD clearance rate was no lower in the 35% of patients who had already relapsed once before enrolling, these patients had a substantially inferior RFS [relapse-free survival] and OS [overall survival], compared with those treated in first remission,” he added. “The clear lesson is that the impact of immunotherapeutic clearance of MRD on survival is greatest when applied early in the disease course.
The “most pressing question” not answered by this study is the impact of hematopoietic stem cell transplantation after complete MRD response, since the study allowed optional HSCT.
Patrick A. Brown, MD, is with Johns Hopkins University, Baltimore. These comments are adapted from his editorial in Blood (2018;131:1497-8). Dr. Brown reported having no competing financial interests related to his editorial.
The study by Dr. Gökbuget and her colleagues provides “strong evidence” that blinatumomab immunotherapy eliminates residual B-cell acute lymphoblastic leukemia (ALL) cells, thereby preventing relapse and improving survival, according to Patrick Brown, MD.
“This addresses the most important unsolved clinical problem in adults with B-ALL: the development of chemotherapy-resistant relapsed disease,” Dr. Brown wrote in an editorial.
Persistence of minimal residual disease (MRD) is the strongest independent predictor of outcomes in B-cell ALL, and is seen in up to 50% of adult patients after chemotherapy, according to Dr. Brown.
The “well-designed and well-executed” multicenter phase 2 study demonstrated an MRD clearance rate of 78% after one cycle of blinatumomab with modest adverse effects, according to Dr. Brown. Moreover, the results show a doubling of overall survival and tripling of relapse-free survival in MRD responders versus nonresponders, he said.
“An important caveat, however, is that, although the MRD clearance rate was no lower in the 35% of patients who had already relapsed once before enrolling, these patients had a substantially inferior RFS [relapse-free survival] and OS [overall survival], compared with those treated in first remission,” he added. “The clear lesson is that the impact of immunotherapeutic clearance of MRD on survival is greatest when applied early in the disease course.
The “most pressing question” not answered by this study is the impact of hematopoietic stem cell transplantation after complete MRD response, since the study allowed optional HSCT.
Patrick A. Brown, MD, is with Johns Hopkins University, Baltimore. These comments are adapted from his editorial in Blood (2018;131:1497-8). Dr. Brown reported having no competing financial interests related to his editorial.
The study by Dr. Gökbuget and her colleagues provides “strong evidence” that blinatumomab immunotherapy eliminates residual B-cell acute lymphoblastic leukemia (ALL) cells, thereby preventing relapse and improving survival, according to Patrick Brown, MD.
“This addresses the most important unsolved clinical problem in adults with B-ALL: the development of chemotherapy-resistant relapsed disease,” Dr. Brown wrote in an editorial.
Persistence of minimal residual disease (MRD) is the strongest independent predictor of outcomes in B-cell ALL, and is seen in up to 50% of adult patients after chemotherapy, according to Dr. Brown.
The “well-designed and well-executed” multicenter phase 2 study demonstrated an MRD clearance rate of 78% after one cycle of blinatumomab with modest adverse effects, according to Dr. Brown. Moreover, the results show a doubling of overall survival and tripling of relapse-free survival in MRD responders versus nonresponders, he said.
“An important caveat, however, is that, although the MRD clearance rate was no lower in the 35% of patients who had already relapsed once before enrolling, these patients had a substantially inferior RFS [relapse-free survival] and OS [overall survival], compared with those treated in first remission,” he added. “The clear lesson is that the impact of immunotherapeutic clearance of MRD on survival is greatest when applied early in the disease course.
The “most pressing question” not answered by this study is the impact of hematopoietic stem cell transplantation after complete MRD response, since the study allowed optional HSCT.
Patrick A. Brown, MD, is with Johns Hopkins University, Baltimore. These comments are adapted from his editorial in Blood (2018;131:1497-8). Dr. Brown reported having no competing financial interests related to his editorial.
After treatment with blinatumomab, most patients with minimal residual disease–positive acute lymphoblastic leukemia (ALL) achieved complete MRD response, according to results of a single-arm phase 2 study.
Achieving complete MRD response was associated with significantly longer relapse-free and overall survival in the patients, who were already in hematologic complete remission, researchers reported in the journal Blood.
“Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies,” Nicola Gökbuget, MD, University Hospital, Frankfurt, Germany, and her coauthors wrote.
This is the first international multicenter study to specifically enroll MRD-positive ALL patients and evaluate them for an MRD-based primary outcome in a cohort of MRD-positive ALL patients, according to the authors.
Preemptively treating low but measurable disease in ALL in remission, instead of waiting for overt relapse, is a strategy that may prolong overall survival, Dr. Gökbuget and her colleagues said in describing the rationale for their study. While there is no standard therapy yet for ALL patients with detectable MRD after intensive chemotherapy, hematopoietic stem cell transplantation (HSCT) is recommended, based on data that it may improve outcomes in patients with persistent MRD. However, other studies suggest detectable MRD before HSCT is associated with higher relapse rates, and many patients relapse while waiting for HSCT, the researchers noted.
To test an MRD-directed treatment strategy, Dr. Gökbuget and colleagues at 46 centers in Europe and Russia conducted an open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission. Patients in the study received up to four cycles of blinatumomab, a bispecific, T cell–engager antibody construct that enables T cells to recognize and eliminate CD19-positive cells.
Of 113 evaluable patients, 88 (78%) achieved complete MRD response after one cycle, the primary end point of the study. Relapse-free survival at 18 months was estimated at 54% and median overall survival was 36.5 months in the subset of 110 patients with Philadelphia chromosome–negative ALL in hematologic remission.
Complete MRD responders had improved relapse-free survival versus MRD nonresponders (23.6 vs. 5.7 months; P = .002), they reported. Likewise, overall survival was improved for MRD responders (38.9 vs. 12.5 months; P = .002).
Adverse events were consistent with what was previously reported for blinatumomab and included grade 3 and 4 neurologic events in 12 patients (10%) and 3 patients (3%), respectively. Cytokine-release syndrome was seen in four patients, with grade 1 and grade 3 cases.
The study was not designed to assess the impact of HSCT, which most patients (n = 76) underwent. However, a number of patients with complete MRD response but no HSCT remained in long-term remission, confirming results of an earlier blinatumomab pilot study, according to the researchers.
“This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients,” Dr. Gökbuget and her coauthors wrote.
Additional studies are needed to clarify the role and indications for HSCT in this setting, they added.
The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
SOURCE: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
After treatment with blinatumomab, most patients with minimal residual disease–positive acute lymphoblastic leukemia (ALL) achieved complete MRD response, according to results of a single-arm phase 2 study.
Achieving complete MRD response was associated with significantly longer relapse-free and overall survival in the patients, who were already in hematologic complete remission, researchers reported in the journal Blood.
“Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies,” Nicola Gökbuget, MD, University Hospital, Frankfurt, Germany, and her coauthors wrote.
This is the first international multicenter study to specifically enroll MRD-positive ALL patients and evaluate them for an MRD-based primary outcome in a cohort of MRD-positive ALL patients, according to the authors.
Preemptively treating low but measurable disease in ALL in remission, instead of waiting for overt relapse, is a strategy that may prolong overall survival, Dr. Gökbuget and her colleagues said in describing the rationale for their study. While there is no standard therapy yet for ALL patients with detectable MRD after intensive chemotherapy, hematopoietic stem cell transplantation (HSCT) is recommended, based on data that it may improve outcomes in patients with persistent MRD. However, other studies suggest detectable MRD before HSCT is associated with higher relapse rates, and many patients relapse while waiting for HSCT, the researchers noted.
To test an MRD-directed treatment strategy, Dr. Gökbuget and colleagues at 46 centers in Europe and Russia conducted an open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission. Patients in the study received up to four cycles of blinatumomab, a bispecific, T cell–engager antibody construct that enables T cells to recognize and eliminate CD19-positive cells.
Of 113 evaluable patients, 88 (78%) achieved complete MRD response after one cycle, the primary end point of the study. Relapse-free survival at 18 months was estimated at 54% and median overall survival was 36.5 months in the subset of 110 patients with Philadelphia chromosome–negative ALL in hematologic remission.
Complete MRD responders had improved relapse-free survival versus MRD nonresponders (23.6 vs. 5.7 months; P = .002), they reported. Likewise, overall survival was improved for MRD responders (38.9 vs. 12.5 months; P = .002).
Adverse events were consistent with what was previously reported for blinatumomab and included grade 3 and 4 neurologic events in 12 patients (10%) and 3 patients (3%), respectively. Cytokine-release syndrome was seen in four patients, with grade 1 and grade 3 cases.
The study was not designed to assess the impact of HSCT, which most patients (n = 76) underwent. However, a number of patients with complete MRD response but no HSCT remained in long-term remission, confirming results of an earlier blinatumomab pilot study, according to the researchers.
“This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients,” Dr. Gökbuget and her coauthors wrote.
Additional studies are needed to clarify the role and indications for HSCT in this setting, they added.
The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
SOURCE: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
FROM BLOOD
Key clinical point:
Major finding: Complete MRD response, seen in 78% of blinatumomab-treated patients, was associated with improved relapse-free and overall survival.
Study details: An open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission, conducted at 46 centers in Europe and Russia.
Disclosures: The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
Source: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
Zika virus: Sexual contact risk may be limited to short window
Shedding of infectious Zika virus in the semen of symptomatic infected men appears to be uncommon and limited to the first few weeks after onset of illness, according to results of a recent prospective study.
Out of all semen samples with detectable Zika virus RNA, the only ones with infectious virus were those that had been obtained within 30 days of illness onset, study authors reported in the New England Journal of Medicine.
Sexual transmission of Zika virus, first documented in 2011, has now been reported in at least 13 countries, Dr. Mead and his colleagues wrote.
Usually, the cases have involved transmission from a symptomatic man to a woman, they added.
Previously, some investigators had proposed that sexual transmission of Zika virus could pose a greater risk of fetal infection than could mosquito-borne transmission, Dr. Mead and colleagues noted in their report. “If so, the interruption of sexual transmission could play a critical role in preventing the serious complications that have been associated with fetal infection,” they wrote.
To investigate further, Dr. Mead and his colleagues conducted a prospective study of men with symptomatic Zika virus infection. They collected 1,327 semen samples from 184 men and 1,038 urine samples from 183 men, according to the report.
They obtained specimens twice monthly for 6 months. Samples were tested for Zika RNA using real-time reverse transcriptase polymerase chain reaction assay and for infectious Zika virus using Vero cell culture and plaque assay.
Investigators detected Zika virus RNA in the semen of 60 men (33%), including semen samples from 22 of the 36 men (61%) tested within 30 days after illness onset, investigators said in the report.
While Zika virus RNA shedding decreased considerably in the 3 months after illness onset, it did continue for 281 days in one man, they noted.
Men who were older and those who ejaculated less frequently were more likely to have prolonged RNA shedding in semen, results of multivariable analysis showed.
Infectious Zika virus was isolated from just 3 out of the 78 semen samples with detectable Zika virus RNA that were tested by culture, investigators said. Notably, all 3 of the cases were among the 19 of those samples obtained within 30 days of illness onset, they reported.
Detection of Zika virus RNA in urine was rare, occurring in only 7 men (4%), possibly because of the timing of the first specimen collection, according to investigators. They said previous studies suggest a rapid decline in Zika virus shedding in urine during the first few weeks after onset of illness.
Important questions remain regarding sexual transmission of Zika virus, such as whether maternal infection through sex poses similar risks to the fetus as compared with maternal infection via mosquito bite, Dr. Mead and his coauthors said in the report.
“A better understanding of these issues is needed to guide the development of effective prevention strategies,” they wrote.
The study was supported by the Centers for Disease Control and Prevention. Dr. Mead and his coauthors reported they had no disclosures related to the study.
SOURCE: Mead PS et al. N Engl J Med. 2018;378(15):1377-85.
This study illustrates the apparent shortcomings of current virus-detection standards in terms of their relevance to public health, according to Heinz Feldmann, MD.
Approximately 4% of Zika virus RNA-positive semen samples were infectious, according to the report, and of those infectious samples, all were obtained within 30 days of the onset of illness. “This finding suggests that there is a short period during which Zika virus–infected men might transmit this virus through sexual contact,” Dr. Feldmann wrote in an editorial.
Current practice in some areas is to test semen samples sequentially until two or more consecutive negative results are obtained; however, that approach is controversial, according to Dr. Feldmann, because the person could be shedding the virus intermittently because of the potential for virus latency and reactivation.
“This also raises the question of whether modern molecular approaches are properly positioned to detect virus latency rather than persistence,” he said in his editorial. The goal, he added, should be to determine infectivity, which is probably best assessed by means of viral isolation – which is believed to be less sensitive than molecular detection.
“Thus, the diagnostic situation is far more complicated than it seems,” he noted.
However, he added, these diagnostic scenarios may be less applicable for public health entities, which have “quickly” disseminated recommendations for safer sex to prevent Zika virus spread and the potentially devastating consequences of fetal infection.
“These recommendations leverage the best data available and have been implemented, but ought to be updated as new data emerge,” Dr. Feldmann wrote.
Dr. Feldmann is with the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and Rocky Mountain Laboratories, Hamilton, Mont. These comments are derived from his editorial N Engl J Med 2018;378:1377-85 . Dr. Feldmann reported that he had nothing to disclose related to the editorial.
This study illustrates the apparent shortcomings of current virus-detection standards in terms of their relevance to public health, according to Heinz Feldmann, MD.
Approximately 4% of Zika virus RNA-positive semen samples were infectious, according to the report, and of those infectious samples, all were obtained within 30 days of the onset of illness. “This finding suggests that there is a short period during which Zika virus–infected men might transmit this virus through sexual contact,” Dr. Feldmann wrote in an editorial.
Current practice in some areas is to test semen samples sequentially until two or more consecutive negative results are obtained; however, that approach is controversial, according to Dr. Feldmann, because the person could be shedding the virus intermittently because of the potential for virus latency and reactivation.
“This also raises the question of whether modern molecular approaches are properly positioned to detect virus latency rather than persistence,” he said in his editorial. The goal, he added, should be to determine infectivity, which is probably best assessed by means of viral isolation – which is believed to be less sensitive than molecular detection.
“Thus, the diagnostic situation is far more complicated than it seems,” he noted.
However, he added, these diagnostic scenarios may be less applicable for public health entities, which have “quickly” disseminated recommendations for safer sex to prevent Zika virus spread and the potentially devastating consequences of fetal infection.
“These recommendations leverage the best data available and have been implemented, but ought to be updated as new data emerge,” Dr. Feldmann wrote.
Dr. Feldmann is with the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and Rocky Mountain Laboratories, Hamilton, Mont. These comments are derived from his editorial N Engl J Med 2018;378:1377-85 . Dr. Feldmann reported that he had nothing to disclose related to the editorial.
This study illustrates the apparent shortcomings of current virus-detection standards in terms of their relevance to public health, according to Heinz Feldmann, MD.
Approximately 4% of Zika virus RNA-positive semen samples were infectious, according to the report, and of those infectious samples, all were obtained within 30 days of the onset of illness. “This finding suggests that there is a short period during which Zika virus–infected men might transmit this virus through sexual contact,” Dr. Feldmann wrote in an editorial.
Current practice in some areas is to test semen samples sequentially until two or more consecutive negative results are obtained; however, that approach is controversial, according to Dr. Feldmann, because the person could be shedding the virus intermittently because of the potential for virus latency and reactivation.
“This also raises the question of whether modern molecular approaches are properly positioned to detect virus latency rather than persistence,” he said in his editorial. The goal, he added, should be to determine infectivity, which is probably best assessed by means of viral isolation – which is believed to be less sensitive than molecular detection.
“Thus, the diagnostic situation is far more complicated than it seems,” he noted.
However, he added, these diagnostic scenarios may be less applicable for public health entities, which have “quickly” disseminated recommendations for safer sex to prevent Zika virus spread and the potentially devastating consequences of fetal infection.
“These recommendations leverage the best data available and have been implemented, but ought to be updated as new data emerge,” Dr. Feldmann wrote.
Dr. Feldmann is with the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and Rocky Mountain Laboratories, Hamilton, Mont. These comments are derived from his editorial N Engl J Med 2018;378:1377-85 . Dr. Feldmann reported that he had nothing to disclose related to the editorial.
Shedding of infectious Zika virus in the semen of symptomatic infected men appears to be uncommon and limited to the first few weeks after onset of illness, according to results of a recent prospective study.
Out of all semen samples with detectable Zika virus RNA, the only ones with infectious virus were those that had been obtained within 30 days of illness onset, study authors reported in the New England Journal of Medicine.
Sexual transmission of Zika virus, first documented in 2011, has now been reported in at least 13 countries, Dr. Mead and his colleagues wrote.
Usually, the cases have involved transmission from a symptomatic man to a woman, they added.
Previously, some investigators had proposed that sexual transmission of Zika virus could pose a greater risk of fetal infection than could mosquito-borne transmission, Dr. Mead and colleagues noted in their report. “If so, the interruption of sexual transmission could play a critical role in preventing the serious complications that have been associated with fetal infection,” they wrote.
To investigate further, Dr. Mead and his colleagues conducted a prospective study of men with symptomatic Zika virus infection. They collected 1,327 semen samples from 184 men and 1,038 urine samples from 183 men, according to the report.
They obtained specimens twice monthly for 6 months. Samples were tested for Zika RNA using real-time reverse transcriptase polymerase chain reaction assay and for infectious Zika virus using Vero cell culture and plaque assay.
Investigators detected Zika virus RNA in the semen of 60 men (33%), including semen samples from 22 of the 36 men (61%) tested within 30 days after illness onset, investigators said in the report.
While Zika virus RNA shedding decreased considerably in the 3 months after illness onset, it did continue for 281 days in one man, they noted.
Men who were older and those who ejaculated less frequently were more likely to have prolonged RNA shedding in semen, results of multivariable analysis showed.
Infectious Zika virus was isolated from just 3 out of the 78 semen samples with detectable Zika virus RNA that were tested by culture, investigators said. Notably, all 3 of the cases were among the 19 of those samples obtained within 30 days of illness onset, they reported.
Detection of Zika virus RNA in urine was rare, occurring in only 7 men (4%), possibly because of the timing of the first specimen collection, according to investigators. They said previous studies suggest a rapid decline in Zika virus shedding in urine during the first few weeks after onset of illness.
Important questions remain regarding sexual transmission of Zika virus, such as whether maternal infection through sex poses similar risks to the fetus as compared with maternal infection via mosquito bite, Dr. Mead and his coauthors said in the report.
“A better understanding of these issues is needed to guide the development of effective prevention strategies,” they wrote.
The study was supported by the Centers for Disease Control and Prevention. Dr. Mead and his coauthors reported they had no disclosures related to the study.
SOURCE: Mead PS et al. N Engl J Med. 2018;378(15):1377-85.
Shedding of infectious Zika virus in the semen of symptomatic infected men appears to be uncommon and limited to the first few weeks after onset of illness, according to results of a recent prospective study.
Out of all semen samples with detectable Zika virus RNA, the only ones with infectious virus were those that had been obtained within 30 days of illness onset, study authors reported in the New England Journal of Medicine.
Sexual transmission of Zika virus, first documented in 2011, has now been reported in at least 13 countries, Dr. Mead and his colleagues wrote.
Usually, the cases have involved transmission from a symptomatic man to a woman, they added.
Previously, some investigators had proposed that sexual transmission of Zika virus could pose a greater risk of fetal infection than could mosquito-borne transmission, Dr. Mead and colleagues noted in their report. “If so, the interruption of sexual transmission could play a critical role in preventing the serious complications that have been associated with fetal infection,” they wrote.
To investigate further, Dr. Mead and his colleagues conducted a prospective study of men with symptomatic Zika virus infection. They collected 1,327 semen samples from 184 men and 1,038 urine samples from 183 men, according to the report.
They obtained specimens twice monthly for 6 months. Samples were tested for Zika RNA using real-time reverse transcriptase polymerase chain reaction assay and for infectious Zika virus using Vero cell culture and plaque assay.
Investigators detected Zika virus RNA in the semen of 60 men (33%), including semen samples from 22 of the 36 men (61%) tested within 30 days after illness onset, investigators said in the report.
While Zika virus RNA shedding decreased considerably in the 3 months after illness onset, it did continue for 281 days in one man, they noted.
Men who were older and those who ejaculated less frequently were more likely to have prolonged RNA shedding in semen, results of multivariable analysis showed.
Infectious Zika virus was isolated from just 3 out of the 78 semen samples with detectable Zika virus RNA that were tested by culture, investigators said. Notably, all 3 of the cases were among the 19 of those samples obtained within 30 days of illness onset, they reported.
Detection of Zika virus RNA in urine was rare, occurring in only 7 men (4%), possibly because of the timing of the first specimen collection, according to investigators. They said previous studies suggest a rapid decline in Zika virus shedding in urine during the first few weeks after onset of illness.
Important questions remain regarding sexual transmission of Zika virus, such as whether maternal infection through sex poses similar risks to the fetus as compared with maternal infection via mosquito bite, Dr. Mead and his coauthors said in the report.
“A better understanding of these issues is needed to guide the development of effective prevention strategies,” they wrote.
The study was supported by the Centers for Disease Control and Prevention. Dr. Mead and his coauthors reported they had no disclosures related to the study.
SOURCE: Mead PS et al. N Engl J Med. 2018;378(15):1377-85.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: While Zika virus RNA is common and may persist for months in the semen of symptomatic infected men, shedding of infectious virus appears to be much less common and limited to the first few weeks after onset of illness.
Major finding: Out of 78 semen samples with detectable Zika virus RNA, 3 had infectious virus, and all 3 were obtained within 30 days of illness onset.
Study details: A prospective study of 1,327 semen samples from 184 men with symptomatic Zika virus infection.
Disclosures: The study was supported by the Centers for Disease Control and Prevention. Study authors reported they had nothing to disclose relative to the study.
Source: Mead PS et al. N Engl J Med. 2018 Apr 12;378(15):1377-85.
Life and health are not even across the U.S.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.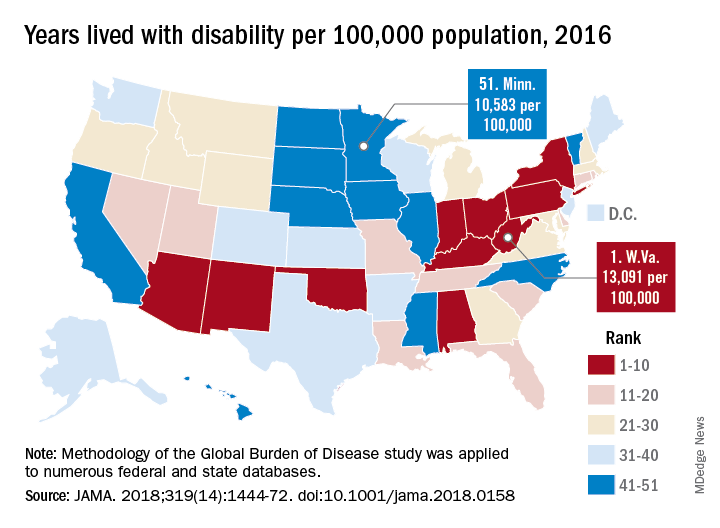
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
FROM JAMA
Key clinical point: While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors.
Major finding: Life expectancy ranged from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, and previously decreasing death rates for adults have reversed in 19 states.
Study details: A U.S. state-level analysis of results from the Global Burden of Disease (GBD) study illustrating trends in diseases, injuries, risk factors, and deaths from 1990 to 2016.
Disclosures: The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Study authors reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
Source: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-1472.
Early reading aloud, play reduced hyperactivity at school entry
and had sustained behavioral effects after the program was completed, according to results of a randomized clinical trial.
The Video Interaction Project (VIP), in which parents review and reflect upon recordings of themselves interacting with their children, is a low-cost, scalable intervention that has a “high potential” for enhancing social and emotional development by reducing disruptive behaviors, the study authors reported in Pediatrics.
The study included 675 parent-child dyads enrolled post partum at an urban public hospital serving low-income families. Of that group, 450 families were randomized to the VIP program from 0 to 3 years of age, a control group, or a third group that included a different intervention called Building Blocks that incorporates parenting education newsletters, learning materials, and parent questionnaires.
In the VIP intervention, parent-child dyads participated in up to 15 one-on-one sessions from 2 weeks of age to 3 years. In each 30-minute session, the parent and child were video recorded for 5 minutes of play or shared reading; immediately afterward, the parent would review the video with a bilingual facilitator to identify positive interactions and reflect on them.
As previously reported, the VIP intervention had enhanced children’s social and emotional development. Compared with controls, children in the VIP group had higher scores in imitation/play and attention at the end of the program and lower scores in separation distress, hyperactivity, and externalizing problems, according to investigators.
Now, investigators are reporting results that include a second phase of random assignment to VIP from 3-5 years or a control group. The second-phase VIP intervention included nine 30- to 45-minute sessions enhanced with new strategies designed to support the rapidly emerging developmental capacities of preschoolers, Dr. Mendelsohn and associates said. Ultimately, 252 families completed the 4.5 year assessment.
Those new strategies included building sessions around themes (such as birthday party), incorporation of writing into play (such as party invitations), focusing on story characters’ feelings, and video recording both reading and play, with the story serving as the basis for the play.
The initial VIP 0-3 year intervention and the VIP 3-5 year intervention were both independently associated with improved T-scores at 4.5 years on Behavior Assessment System for Children, Second Edition, rating scales, with Cohen’s d effect sizes ranging from approximately –0.25 to –0.30, according to investigators.
Participating in both VIP interventions was associated with a significant reduction in hyperactivity (effect size, –0.63; P = 0.001), Dr. Mendelsohn and his associates also reported.
Moreover, participation in the first VIP session was associated with a reduction in clinically significant hyperactivity (relative risk reduction, 69%; P = .03), they added.
The cost of the VIP program for 0-3 years is approximately $175-$200 per child per year, including staff, equipment, rent, and other expenses, according to the report, which notes that one interventionist can provide services for 400-500 families.
Taken together, these findings suggest the VIP intervention is a low-cost intervention that may prevent poverty-related disparities, investigators said.
“In this study, we provide strong support for the use of pediatric primary care to promote positive parenting activities such as reading aloud and play and the potential for such programs to promote social-emotional development as reflected through reductions in disruptive behaviors,” they wrote.
Dr. Mendelsohn and his coauthors reported no relevant financial disclosures. The study was supported by grants from the National Institutes of Health and the National Institute of Child Health and Human Development; the Tiger Foundation; the Marks Family Foundation; Children of Bellevue; KiDS of New York University Foundation; and Rhodebeck Charitable Trust. Several of the investigators were supported in part by awards or grants.
SOURCE: Mendelsohn AL et al. Pediatrics. 2018;141(5):e20173393.
and had sustained behavioral effects after the program was completed, according to results of a randomized clinical trial.
The Video Interaction Project (VIP), in which parents review and reflect upon recordings of themselves interacting with their children, is a low-cost, scalable intervention that has a “high potential” for enhancing social and emotional development by reducing disruptive behaviors, the study authors reported in Pediatrics.
The study included 675 parent-child dyads enrolled post partum at an urban public hospital serving low-income families. Of that group, 450 families were randomized to the VIP program from 0 to 3 years of age, a control group, or a third group that included a different intervention called Building Blocks that incorporates parenting education newsletters, learning materials, and parent questionnaires.
In the VIP intervention, parent-child dyads participated in up to 15 one-on-one sessions from 2 weeks of age to 3 years. In each 30-minute session, the parent and child were video recorded for 5 minutes of play or shared reading; immediately afterward, the parent would review the video with a bilingual facilitator to identify positive interactions and reflect on them.
As previously reported, the VIP intervention had enhanced children’s social and emotional development. Compared with controls, children in the VIP group had higher scores in imitation/play and attention at the end of the program and lower scores in separation distress, hyperactivity, and externalizing problems, according to investigators.
Now, investigators are reporting results that include a second phase of random assignment to VIP from 3-5 years or a control group. The second-phase VIP intervention included nine 30- to 45-minute sessions enhanced with new strategies designed to support the rapidly emerging developmental capacities of preschoolers, Dr. Mendelsohn and associates said. Ultimately, 252 families completed the 4.5 year assessment.
Those new strategies included building sessions around themes (such as birthday party), incorporation of writing into play (such as party invitations), focusing on story characters’ feelings, and video recording both reading and play, with the story serving as the basis for the play.
The initial VIP 0-3 year intervention and the VIP 3-5 year intervention were both independently associated with improved T-scores at 4.5 years on Behavior Assessment System for Children, Second Edition, rating scales, with Cohen’s d effect sizes ranging from approximately –0.25 to –0.30, according to investigators.
Participating in both VIP interventions was associated with a significant reduction in hyperactivity (effect size, –0.63; P = 0.001), Dr. Mendelsohn and his associates also reported.
Moreover, participation in the first VIP session was associated with a reduction in clinically significant hyperactivity (relative risk reduction, 69%; P = .03), they added.
The cost of the VIP program for 0-3 years is approximately $175-$200 per child per year, including staff, equipment, rent, and other expenses, according to the report, which notes that one interventionist can provide services for 400-500 families.
Taken together, these findings suggest the VIP intervention is a low-cost intervention that may prevent poverty-related disparities, investigators said.
“In this study, we provide strong support for the use of pediatric primary care to promote positive parenting activities such as reading aloud and play and the potential for such programs to promote social-emotional development as reflected through reductions in disruptive behaviors,” they wrote.
Dr. Mendelsohn and his coauthors reported no relevant financial disclosures. The study was supported by grants from the National Institutes of Health and the National Institute of Child Health and Human Development; the Tiger Foundation; the Marks Family Foundation; Children of Bellevue; KiDS of New York University Foundation; and Rhodebeck Charitable Trust. Several of the investigators were supported in part by awards or grants.
SOURCE: Mendelsohn AL et al. Pediatrics. 2018;141(5):e20173393.
and had sustained behavioral effects after the program was completed, according to results of a randomized clinical trial.
The Video Interaction Project (VIP), in which parents review and reflect upon recordings of themselves interacting with their children, is a low-cost, scalable intervention that has a “high potential” for enhancing social and emotional development by reducing disruptive behaviors, the study authors reported in Pediatrics.
The study included 675 parent-child dyads enrolled post partum at an urban public hospital serving low-income families. Of that group, 450 families were randomized to the VIP program from 0 to 3 years of age, a control group, or a third group that included a different intervention called Building Blocks that incorporates parenting education newsletters, learning materials, and parent questionnaires.
In the VIP intervention, parent-child dyads participated in up to 15 one-on-one sessions from 2 weeks of age to 3 years. In each 30-minute session, the parent and child were video recorded for 5 minutes of play or shared reading; immediately afterward, the parent would review the video with a bilingual facilitator to identify positive interactions and reflect on them.
As previously reported, the VIP intervention had enhanced children’s social and emotional development. Compared with controls, children in the VIP group had higher scores in imitation/play and attention at the end of the program and lower scores in separation distress, hyperactivity, and externalizing problems, according to investigators.
Now, investigators are reporting results that include a second phase of random assignment to VIP from 3-5 years or a control group. The second-phase VIP intervention included nine 30- to 45-minute sessions enhanced with new strategies designed to support the rapidly emerging developmental capacities of preschoolers, Dr. Mendelsohn and associates said. Ultimately, 252 families completed the 4.5 year assessment.
Those new strategies included building sessions around themes (such as birthday party), incorporation of writing into play (such as party invitations), focusing on story characters’ feelings, and video recording both reading and play, with the story serving as the basis for the play.
The initial VIP 0-3 year intervention and the VIP 3-5 year intervention were both independently associated with improved T-scores at 4.5 years on Behavior Assessment System for Children, Second Edition, rating scales, with Cohen’s d effect sizes ranging from approximately –0.25 to –0.30, according to investigators.
Participating in both VIP interventions was associated with a significant reduction in hyperactivity (effect size, –0.63; P = 0.001), Dr. Mendelsohn and his associates also reported.
Moreover, participation in the first VIP session was associated with a reduction in clinically significant hyperactivity (relative risk reduction, 69%; P = .03), they added.
The cost of the VIP program for 0-3 years is approximately $175-$200 per child per year, including staff, equipment, rent, and other expenses, according to the report, which notes that one interventionist can provide services for 400-500 families.
Taken together, these findings suggest the VIP intervention is a low-cost intervention that may prevent poverty-related disparities, investigators said.
“In this study, we provide strong support for the use of pediatric primary care to promote positive parenting activities such as reading aloud and play and the potential for such programs to promote social-emotional development as reflected through reductions in disruptive behaviors,” they wrote.
Dr. Mendelsohn and his coauthors reported no relevant financial disclosures. The study was supported by grants from the National Institutes of Health and the National Institute of Child Health and Human Development; the Tiger Foundation; the Marks Family Foundation; Children of Bellevue; KiDS of New York University Foundation; and Rhodebeck Charitable Trust. Several of the investigators were supported in part by awards or grants.
SOURCE: Mendelsohn AL et al. Pediatrics. 2018;141(5):e20173393.
FROM PEDIATRICS
Key clinical point: A video-based intervention designed to promote parent-child reading aloud and play reduced hyperactivity at school entry and had sustained behavioral effects over time.
Major finding: Parent-child participation in the Video Interaction Project (VIP) was independently associated with improved T-scores at 4.5 years on Behavior Assessment System for Children, Second Edition, rating scales, with effect sizes ranging from approximately –0.25 to –0.30.
Study details: A randomized controlled trial including 450 families enrolled at an urban public hospital that serves low-income families.
Disclosures: The researchers reported no relevant financial disclosures. The study was supported by grants from the National Institutes of Health and the National Institute of Child Health and Human Development; the Tiger Foundation; the Marks Family Foundation; Children of Bellevue; KiDS of New York University Foundation; and Rhodebeck Charitable Trust. Several of the investigators were supported in part by awards or grants.
Source: Mendelsohn AL et al. Pediatrics. 2018;141(5):e20173393.
Do industry payments increase prescribing for some targeted therapies?
Physicians receiving general payments from the company marketing a targeted cancer therapy were more likely to prescribe it in three out of six drugs evaluated, researchers reported.
Prescribing of sunitinib, dasatinib, and nilotinib was increased for physicians receiving such payments versus not receiving them, while prescribing of imatinib, sorafenib, and pazopanib were not, according to the analysis by Aaron P. Mitchell, MD, of the Lineberger Comprehensive Cancer Center, UNC School of Medicine, University of North Carolina at Chapel Hill, and his coauthors.
In previous studies, pharmaceutical industry payments to physicians have been associated with “higher-cost, brand-name pharmaceutical prescribing,” Dr. Mitchell and his colleagues wrote. The report was published in JAMA Internal Medicine.
“Whether industry payments are associated with physician treatment choice in oncology is uncertain,” they said.
To evaluate the association between payments to oncologists and drug selection, Dr. Mitchell and his colleagues linked Open Payments data from the Centers for Medicare & Medicaid Services to data from Medicare Part D Prescriber Public Use File for the years 2013-2014.
The primary variable in the study was payments received during 2013, according to investigators, and the primary outcome of the analysis was prescriptions filled during 2014.
Open Payments reported in 2013 had a total dollar value of $4.08 billion, including $1.20 billion paid to physicians, according to CMS data.
The researchers focused on targeted therapies for two therapeutic areas: metastatic renal cell carcinoma (RCC), including sorafenib, sunitinib, and pazopanib; and chronic myeloid leukemia (CML), including imatinib, dasatinib, and nilotinib.
They limited their analysis to physicians listed as oncologists who filled at least 20 prescriptions for each of the three drugs in metastatic RCC (n = 354) or in CML (n = 2,225).
Receiving payments categorized as “general,” such as gifts, speaker fees, meals, and travel, increased the odds of prescribing drugs for both metastatic RCC (odds ratio, 2.05; 95% confidence interval, 1.34-3.14; P = .001) and for CML (odds ratio, 1.29; 95% CI, 1.13-1.47; P less than .001).
By contrast, research payments did not increase the odds of prescribing those drugs, the investigators reported.
Looking at specific drugs, they found that receipt of general payments from a drug’s manufacturer was associated with increased prescribing of sunitinib (50.5% versus 34.4%, P = .01), dasatinib (13.8% versus 11.4%, P = .02), and nilotinib (15.4% vs 12.5%, P = .01).
However, no such association was found for sorafenib or pazopanib.
For imatinib, by contrast, investigators said industry payments were associated with a prescribing decrease.
“This may reflect a strategy by the manufacturer of imatinib, which also produces nilotinib, to promote switching to nilotinib before the patent expiration of imatinib in 2015,” the researchers wrote.
Dr. Mitchell and his coauthors reported no conflict of interest disclosures related to the study.
SOURCE: Mitchell AP, et al. JAMA Intern Med. 2018 Apr 9. doi: 0.1001/jamainternmed.2018.0776.
Physicians receiving general payments from the company marketing a targeted cancer therapy were more likely to prescribe it in three out of six drugs evaluated, researchers reported.
Prescribing of sunitinib, dasatinib, and nilotinib was increased for physicians receiving such payments versus not receiving them, while prescribing of imatinib, sorafenib, and pazopanib were not, according to the analysis by Aaron P. Mitchell, MD, of the Lineberger Comprehensive Cancer Center, UNC School of Medicine, University of North Carolina at Chapel Hill, and his coauthors.
In previous studies, pharmaceutical industry payments to physicians have been associated with “higher-cost, brand-name pharmaceutical prescribing,” Dr. Mitchell and his colleagues wrote. The report was published in JAMA Internal Medicine.
“Whether industry payments are associated with physician treatment choice in oncology is uncertain,” they said.
To evaluate the association between payments to oncologists and drug selection, Dr. Mitchell and his colleagues linked Open Payments data from the Centers for Medicare & Medicaid Services to data from Medicare Part D Prescriber Public Use File for the years 2013-2014.
The primary variable in the study was payments received during 2013, according to investigators, and the primary outcome of the analysis was prescriptions filled during 2014.
Open Payments reported in 2013 had a total dollar value of $4.08 billion, including $1.20 billion paid to physicians, according to CMS data.
The researchers focused on targeted therapies for two therapeutic areas: metastatic renal cell carcinoma (RCC), including sorafenib, sunitinib, and pazopanib; and chronic myeloid leukemia (CML), including imatinib, dasatinib, and nilotinib.
They limited their analysis to physicians listed as oncologists who filled at least 20 prescriptions for each of the three drugs in metastatic RCC (n = 354) or in CML (n = 2,225).
Receiving payments categorized as “general,” such as gifts, speaker fees, meals, and travel, increased the odds of prescribing drugs for both metastatic RCC (odds ratio, 2.05; 95% confidence interval, 1.34-3.14; P = .001) and for CML (odds ratio, 1.29; 95% CI, 1.13-1.47; P less than .001).
By contrast, research payments did not increase the odds of prescribing those drugs, the investigators reported.
Looking at specific drugs, they found that receipt of general payments from a drug’s manufacturer was associated with increased prescribing of sunitinib (50.5% versus 34.4%, P = .01), dasatinib (13.8% versus 11.4%, P = .02), and nilotinib (15.4% vs 12.5%, P = .01).
However, no such association was found for sorafenib or pazopanib.
For imatinib, by contrast, investigators said industry payments were associated with a prescribing decrease.
“This may reflect a strategy by the manufacturer of imatinib, which also produces nilotinib, to promote switching to nilotinib before the patent expiration of imatinib in 2015,” the researchers wrote.
Dr. Mitchell and his coauthors reported no conflict of interest disclosures related to the study.
SOURCE: Mitchell AP, et al. JAMA Intern Med. 2018 Apr 9. doi: 0.1001/jamainternmed.2018.0776.
Physicians receiving general payments from the company marketing a targeted cancer therapy were more likely to prescribe it in three out of six drugs evaluated, researchers reported.
Prescribing of sunitinib, dasatinib, and nilotinib was increased for physicians receiving such payments versus not receiving them, while prescribing of imatinib, sorafenib, and pazopanib were not, according to the analysis by Aaron P. Mitchell, MD, of the Lineberger Comprehensive Cancer Center, UNC School of Medicine, University of North Carolina at Chapel Hill, and his coauthors.
In previous studies, pharmaceutical industry payments to physicians have been associated with “higher-cost, brand-name pharmaceutical prescribing,” Dr. Mitchell and his colleagues wrote. The report was published in JAMA Internal Medicine.
“Whether industry payments are associated with physician treatment choice in oncology is uncertain,” they said.
To evaluate the association between payments to oncologists and drug selection, Dr. Mitchell and his colleagues linked Open Payments data from the Centers for Medicare & Medicaid Services to data from Medicare Part D Prescriber Public Use File for the years 2013-2014.
The primary variable in the study was payments received during 2013, according to investigators, and the primary outcome of the analysis was prescriptions filled during 2014.
Open Payments reported in 2013 had a total dollar value of $4.08 billion, including $1.20 billion paid to physicians, according to CMS data.
The researchers focused on targeted therapies for two therapeutic areas: metastatic renal cell carcinoma (RCC), including sorafenib, sunitinib, and pazopanib; and chronic myeloid leukemia (CML), including imatinib, dasatinib, and nilotinib.
They limited their analysis to physicians listed as oncologists who filled at least 20 prescriptions for each of the three drugs in metastatic RCC (n = 354) or in CML (n = 2,225).
Receiving payments categorized as “general,” such as gifts, speaker fees, meals, and travel, increased the odds of prescribing drugs for both metastatic RCC (odds ratio, 2.05; 95% confidence interval, 1.34-3.14; P = .001) and for CML (odds ratio, 1.29; 95% CI, 1.13-1.47; P less than .001).
By contrast, research payments did not increase the odds of prescribing those drugs, the investigators reported.
Looking at specific drugs, they found that receipt of general payments from a drug’s manufacturer was associated with increased prescribing of sunitinib (50.5% versus 34.4%, P = .01), dasatinib (13.8% versus 11.4%, P = .02), and nilotinib (15.4% vs 12.5%, P = .01).
However, no such association was found for sorafenib or pazopanib.
For imatinib, by contrast, investigators said industry payments were associated with a prescribing decrease.
“This may reflect a strategy by the manufacturer of imatinib, which also produces nilotinib, to promote switching to nilotinib before the patent expiration of imatinib in 2015,” the researchers wrote.
Dr. Mitchell and his coauthors reported no conflict of interest disclosures related to the study.
SOURCE: Mitchell AP, et al. JAMA Intern Med. 2018 Apr 9. doi: 0.1001/jamainternmed.2018.0776.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Oncologists receiving general payments from the company marketing a cancer drug were more likely to prescribe it in three out of six drugs evaluated.
Major finding: Prescribing was significantly increased for sunitinib (50.5% versus 34.4%, P = .01), dasatinib (13.8% versus 11.4%, P = .02), and nilotinib (15.4% vs. 12.5%, P = .01), but not for imatinib, sorafenib, or pazopanib.
Study details: An analysis of Centers for Medicare & Medicaid Services Open Payments data and Medicare Part D Prescriber Public Use File for the years 2013 to 2014.
Disclosures: The authors reported no conflict of interest disclosures related to the study.
Source: Mitchell AP et al. JAMA Intern Med. 2018 Apr 9. doi: 0.1001/jamainternmed.2018.0776.
SSRI exposure in utero may change brain structure and connectivity
Prenatal exposure to selective serotonin reuptake inhibitors (SSRIs) was associated with fetal brain development in brain regions important in emotional processing, results of an imaging study show.
Compared to controls, SSRI-exposed infants had significant gray matter volume expansion and increased white matter structural connectivity in the amygdala and insula, according to results published in JAMA Pediatrics.
“Our findings suggest a potential association between prenatal SSRI exposure, likely via aberrant serotonin signaling, and the development of the amygdala-insula circuit in the fetal brain,” wrote Claudia Lugo-Candelas, PhD, of Columbia University Medical Center, New York, and her coauthors.
An increasing number of pregnant women are taking SSRIs, in part due to increased awareness of the negative effects of untreated prenatal maternal depression (PMD), the investigators said in their report.
“Because untreated PMD poses risks to both the infant and mother, the decision to initiate, continue, or suspend SSRI treatment remains a clinical dilemma,” they wrote.
Animal studies suggest atypical serotonergic signaling from prenatal SSRI exposure could change fetal brain development and affect function later in life, they explained.
Studies in humans have produced mixed results, but in a recent national registry study including more than 15,000 individuals exposed to SSRIs prenatally, exposure was linked to increased rates of depression.
To evaluate the impact of prenatal SSRI exposure on brain development, Dr. Lugo-Candelas and colleagues used structural and diffusion magnetic resonance imaging (MRI) to evaluate the brains of 98 infants.
They included 16 infants with in utero exposure to SSRIs, 21 born to mothers with untreated maternal depression, and 61 healthy control subjects, all evaluated between 2011 and 2016.
Infants exposed to SSRIs in utero had significant (P less than .05) gray matter volume expansion versus controls in both the right amygdala (Cohen’s d, 0.65; 95 %CI, 0.06-1.23) and the right insula (Cohen’s d = 0.86; 95% CI, 0.26-1.14).
The SSRI-exposed infants also had a significant (P less than .05) increase in connectivity between the right amygdala and the right insula versus controls (Cohen’s d, 0.99; 95% CI, 0.40-1.57).
Whether these neurodevelopmental changes translate into long-term behavioral or psychological outcomes should be evaluated in subsequent studies, Dr. Lugo-Candelas and colleagues said.
Abnormalities in amygdala-insula circuitry may lead to anxiety or depression, they wrote.
“The structurally primed circuit in the infant brains could lead to maladaptive fear processing in their later life, such as generalization of conditioned fear or negative attention bias,” they added.
Dr. Lugo-Candelas reported no conflicts of interest related to the study. One study coauthor reported research support from Shire Pharmaceuticals and Aevi Genomics.
SOURCE: Lugo-Candelas C, et al. JAMA Pediatr. 2018 Apr 9. doi: 10.1001/jamapediatrics.2017.5227.
Prenatal exposure to selective serotonin reuptake inhibitors (SSRIs) was associated with fetal brain development in brain regions important in emotional processing, results of an imaging study show.
Compared to controls, SSRI-exposed infants had significant gray matter volume expansion and increased white matter structural connectivity in the amygdala and insula, according to results published in JAMA Pediatrics.
“Our findings suggest a potential association between prenatal SSRI exposure, likely via aberrant serotonin signaling, and the development of the amygdala-insula circuit in the fetal brain,” wrote Claudia Lugo-Candelas, PhD, of Columbia University Medical Center, New York, and her coauthors.
An increasing number of pregnant women are taking SSRIs, in part due to increased awareness of the negative effects of untreated prenatal maternal depression (PMD), the investigators said in their report.
“Because untreated PMD poses risks to both the infant and mother, the decision to initiate, continue, or suspend SSRI treatment remains a clinical dilemma,” they wrote.
Animal studies suggest atypical serotonergic signaling from prenatal SSRI exposure could change fetal brain development and affect function later in life, they explained.
Studies in humans have produced mixed results, but in a recent national registry study including more than 15,000 individuals exposed to SSRIs prenatally, exposure was linked to increased rates of depression.
To evaluate the impact of prenatal SSRI exposure on brain development, Dr. Lugo-Candelas and colleagues used structural and diffusion magnetic resonance imaging (MRI) to evaluate the brains of 98 infants.
They included 16 infants with in utero exposure to SSRIs, 21 born to mothers with untreated maternal depression, and 61 healthy control subjects, all evaluated between 2011 and 2016.
Infants exposed to SSRIs in utero had significant (P less than .05) gray matter volume expansion versus controls in both the right amygdala (Cohen’s d, 0.65; 95 %CI, 0.06-1.23) and the right insula (Cohen’s d = 0.86; 95% CI, 0.26-1.14).
The SSRI-exposed infants also had a significant (P less than .05) increase in connectivity between the right amygdala and the right insula versus controls (Cohen’s d, 0.99; 95% CI, 0.40-1.57).
Whether these neurodevelopmental changes translate into long-term behavioral or psychological outcomes should be evaluated in subsequent studies, Dr. Lugo-Candelas and colleagues said.
Abnormalities in amygdala-insula circuitry may lead to anxiety or depression, they wrote.
“The structurally primed circuit in the infant brains could lead to maladaptive fear processing in their later life, such as generalization of conditioned fear or negative attention bias,” they added.
Dr. Lugo-Candelas reported no conflicts of interest related to the study. One study coauthor reported research support from Shire Pharmaceuticals and Aevi Genomics.
SOURCE: Lugo-Candelas C, et al. JAMA Pediatr. 2018 Apr 9. doi: 10.1001/jamapediatrics.2017.5227.
Prenatal exposure to selective serotonin reuptake inhibitors (SSRIs) was associated with fetal brain development in brain regions important in emotional processing, results of an imaging study show.
Compared to controls, SSRI-exposed infants had significant gray matter volume expansion and increased white matter structural connectivity in the amygdala and insula, according to results published in JAMA Pediatrics.
“Our findings suggest a potential association between prenatal SSRI exposure, likely via aberrant serotonin signaling, and the development of the amygdala-insula circuit in the fetal brain,” wrote Claudia Lugo-Candelas, PhD, of Columbia University Medical Center, New York, and her coauthors.
An increasing number of pregnant women are taking SSRIs, in part due to increased awareness of the negative effects of untreated prenatal maternal depression (PMD), the investigators said in their report.
“Because untreated PMD poses risks to both the infant and mother, the decision to initiate, continue, or suspend SSRI treatment remains a clinical dilemma,” they wrote.
Animal studies suggest atypical serotonergic signaling from prenatal SSRI exposure could change fetal brain development and affect function later in life, they explained.
Studies in humans have produced mixed results, but in a recent national registry study including more than 15,000 individuals exposed to SSRIs prenatally, exposure was linked to increased rates of depression.
To evaluate the impact of prenatal SSRI exposure on brain development, Dr. Lugo-Candelas and colleagues used structural and diffusion magnetic resonance imaging (MRI) to evaluate the brains of 98 infants.
They included 16 infants with in utero exposure to SSRIs, 21 born to mothers with untreated maternal depression, and 61 healthy control subjects, all evaluated between 2011 and 2016.
Infants exposed to SSRIs in utero had significant (P less than .05) gray matter volume expansion versus controls in both the right amygdala (Cohen’s d, 0.65; 95 %CI, 0.06-1.23) and the right insula (Cohen’s d = 0.86; 95% CI, 0.26-1.14).
The SSRI-exposed infants also had a significant (P less than .05) increase in connectivity between the right amygdala and the right insula versus controls (Cohen’s d, 0.99; 95% CI, 0.40-1.57).
Whether these neurodevelopmental changes translate into long-term behavioral or psychological outcomes should be evaluated in subsequent studies, Dr. Lugo-Candelas and colleagues said.
Abnormalities in amygdala-insula circuitry may lead to anxiety or depression, they wrote.
“The structurally primed circuit in the infant brains could lead to maladaptive fear processing in their later life, such as generalization of conditioned fear or negative attention bias,” they added.
Dr. Lugo-Candelas reported no conflicts of interest related to the study. One study coauthor reported research support from Shire Pharmaceuticals and Aevi Genomics.
SOURCE: Lugo-Candelas C, et al. JAMA Pediatr. 2018 Apr 9. doi: 10.1001/jamapediatrics.2017.5227.
FROM JAMA PEDIATRICS
Key clinical point: Prenatal selective serotonin reuptake inhibitor (SSRI) exposure was associated with fetal brain development, especially in regions of the brain important to emotional processing.
Major finding: Compared to controls, SSRI-exposed infants had significant gray matter volume expansion and increased white matter structural connectivity in the amygdala and insula.
Study details: A two-center cohort study of data collected between 2011 and 2016 for 98 infants, including 16 with in utero exposure to SSRIs.
Disclosures: One study author reported research support from Shire Pharmaceuticals and Aevi Genomics.
Source: Lugo-Candelas C et al. JAMA Pediatr. 2018 Apr 9. doi: 10.1001/jamapediatrics.2017.5227.
Tivozanib after sorafenib promising in patients with advanced RCC
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
FROM THE EUROPEAN JOURNAL OF CANCER
Key clinical point: Tivozanib has potent antitumor activity in patients with advanced renal cell carcinoma (RCC) who previously progressed on sorafenib.
Major finding: Median progression-free survival was 11.0 months, and median overall survival was 21.6 months for patients receiving tivozanib.
Study details: A single-arm, phase 2 crossover study of patients previously randomized to the sorafenib arm of the phase 3 TIVO-1 study.
Disclosures: AVEO Oncology and Astellas Pharma US, Inc. funded the study. Investigators reported potential conflict of interests related to AVEO, Novartis, Eisai, Pfizer, and others.
Source: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
Age and race affect access to myeloma treatment
Older patients, African Americans, and individuals of low socioeconomic status may be less likely to receive systemic treatment for newly diagnosed multiple myeloma, results of a recent study suggest.
Comorbidities and poor performance indicators also reduced the likelihood of receiving first-line treatment, according to results of the retrospective cohort study published in Clinical Lymphoma, Myeloma & Leukemia.
The findings highlight the need for a “multifaceted approach” to address outcome disparities in multiple myeloma, according to researcher Bita Fakhri, MD, MPH, of the division of oncology at Washington University, St. Louis, and her coinvestigators.
“Particular attention to aging-related issues is essential to ensure older patients will benefit from the advances achieved in the field, similar to young patients,” the investigators wrote.
Racial and socioeconomic barriers should also be addressed, they added.
The retrospective cohort analysis included data on 3,814 patients with active multiple myeloma in the Surveillance, Epidemiology, and End Results–Medicare database from 2007 to 2011. Investigators found that overall, 1,445 patients (38%) had no insurance claims confirming that they had received systemic treatment.
Older age increased the odds of not receiving treatment, with the likelihood increasing by 7% for each year of advancing age (adjusted odds ratio, 1.07; 95% confidence interval, 1.06-1.08). Likewise, African American patients were 26% more likely to have had no treatment (aOR, 1.26; 95% CI, 1.03-1.54), and patients who were enrolled in both Medicaid and Medicare – a proxy for lower income – had a 21% increased odds of no treatment (aOR, 1.21; 95% CI, 1.02-1.42).
Similarly increased odds of no treatment were reported for patients with comorbidities and poor performance status indicators.
“In a subset of older and frail patients, the risks of treatments approved for [multiple myeloma] might outweigh the benefits or might not be in line with the individual’s goals of care,” the investigators wrote.
The study did not track supportive-care treatments that patients may have received instead of active disease treatment, such as bisphosphonates for skeletal lesions or plasmapheresis for hyperviscosity syndrome.
Lack of treatment was associated with poorer survival in the study. Median overall survival was just 9.6 months for individuals with no record of treatment, compared with 32.3 months for patients who had received treatment.
Dr. Fakhri and coauthors reported having no financial disclosures related to the study, which was supported by the National Cancer Institute.
SOURCE: Fakhri B et al. Clin Lymphoma Myeloma Leuk. 2018 Mar;18(3):219-24.
Older patients, African Americans, and individuals of low socioeconomic status may be less likely to receive systemic treatment for newly diagnosed multiple myeloma, results of a recent study suggest.
Comorbidities and poor performance indicators also reduced the likelihood of receiving first-line treatment, according to results of the retrospective cohort study published in Clinical Lymphoma, Myeloma & Leukemia.
The findings highlight the need for a “multifaceted approach” to address outcome disparities in multiple myeloma, according to researcher Bita Fakhri, MD, MPH, of the division of oncology at Washington University, St. Louis, and her coinvestigators.
“Particular attention to aging-related issues is essential to ensure older patients will benefit from the advances achieved in the field, similar to young patients,” the investigators wrote.
Racial and socioeconomic barriers should also be addressed, they added.
The retrospective cohort analysis included data on 3,814 patients with active multiple myeloma in the Surveillance, Epidemiology, and End Results–Medicare database from 2007 to 2011. Investigators found that overall, 1,445 patients (38%) had no insurance claims confirming that they had received systemic treatment.
Older age increased the odds of not receiving treatment, with the likelihood increasing by 7% for each year of advancing age (adjusted odds ratio, 1.07; 95% confidence interval, 1.06-1.08). Likewise, African American patients were 26% more likely to have had no treatment (aOR, 1.26; 95% CI, 1.03-1.54), and patients who were enrolled in both Medicaid and Medicare – a proxy for lower income – had a 21% increased odds of no treatment (aOR, 1.21; 95% CI, 1.02-1.42).
Similarly increased odds of no treatment were reported for patients with comorbidities and poor performance status indicators.
“In a subset of older and frail patients, the risks of treatments approved for [multiple myeloma] might outweigh the benefits or might not be in line with the individual’s goals of care,” the investigators wrote.
The study did not track supportive-care treatments that patients may have received instead of active disease treatment, such as bisphosphonates for skeletal lesions or plasmapheresis for hyperviscosity syndrome.
Lack of treatment was associated with poorer survival in the study. Median overall survival was just 9.6 months for individuals with no record of treatment, compared with 32.3 months for patients who had received treatment.
Dr. Fakhri and coauthors reported having no financial disclosures related to the study, which was supported by the National Cancer Institute.
SOURCE: Fakhri B et al. Clin Lymphoma Myeloma Leuk. 2018 Mar;18(3):219-24.
Older patients, African Americans, and individuals of low socioeconomic status may be less likely to receive systemic treatment for newly diagnosed multiple myeloma, results of a recent study suggest.
Comorbidities and poor performance indicators also reduced the likelihood of receiving first-line treatment, according to results of the retrospective cohort study published in Clinical Lymphoma, Myeloma & Leukemia.
The findings highlight the need for a “multifaceted approach” to address outcome disparities in multiple myeloma, according to researcher Bita Fakhri, MD, MPH, of the division of oncology at Washington University, St. Louis, and her coinvestigators.
“Particular attention to aging-related issues is essential to ensure older patients will benefit from the advances achieved in the field, similar to young patients,” the investigators wrote.
Racial and socioeconomic barriers should also be addressed, they added.
The retrospective cohort analysis included data on 3,814 patients with active multiple myeloma in the Surveillance, Epidemiology, and End Results–Medicare database from 2007 to 2011. Investigators found that overall, 1,445 patients (38%) had no insurance claims confirming that they had received systemic treatment.
Older age increased the odds of not receiving treatment, with the likelihood increasing by 7% for each year of advancing age (adjusted odds ratio, 1.07; 95% confidence interval, 1.06-1.08). Likewise, African American patients were 26% more likely to have had no treatment (aOR, 1.26; 95% CI, 1.03-1.54), and patients who were enrolled in both Medicaid and Medicare – a proxy for lower income – had a 21% increased odds of no treatment (aOR, 1.21; 95% CI, 1.02-1.42).
Similarly increased odds of no treatment were reported for patients with comorbidities and poor performance status indicators.
“In a subset of older and frail patients, the risks of treatments approved for [multiple myeloma] might outweigh the benefits or might not be in line with the individual’s goals of care,” the investigators wrote.
The study did not track supportive-care treatments that patients may have received instead of active disease treatment, such as bisphosphonates for skeletal lesions or plasmapheresis for hyperviscosity syndrome.
Lack of treatment was associated with poorer survival in the study. Median overall survival was just 9.6 months for individuals with no record of treatment, compared with 32.3 months for patients who had received treatment.
Dr. Fakhri and coauthors reported having no financial disclosures related to the study, which was supported by the National Cancer Institute.
SOURCE: Fakhri B et al. Clin Lymphoma Myeloma Leuk. 2018 Mar;18(3):219-24.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Factors significantly associated with no systemic treatment included older age (adjusted odds ratio, 1.07 per year), African American descent (aOR, 1.26), and dual Medicare-Medicaid enrollment (aOR, 1.21).
Study details: A retrospective cohort analysis including data on 3,814 patients with active multiple myeloma in the Surveillance, Epidemiology, and End Results–Medicare database from 2007 to 2011.
Disclosures: The research was supported by the National Cancer Institute. The investigators reported having no financial disclosures.
Source: Fakhri B et al. Clin Lymphoma Myeloma Leuk. Mar 2018;18(3):219-24.