User login
Outpatient talc administration improves malignant effusion outcomes
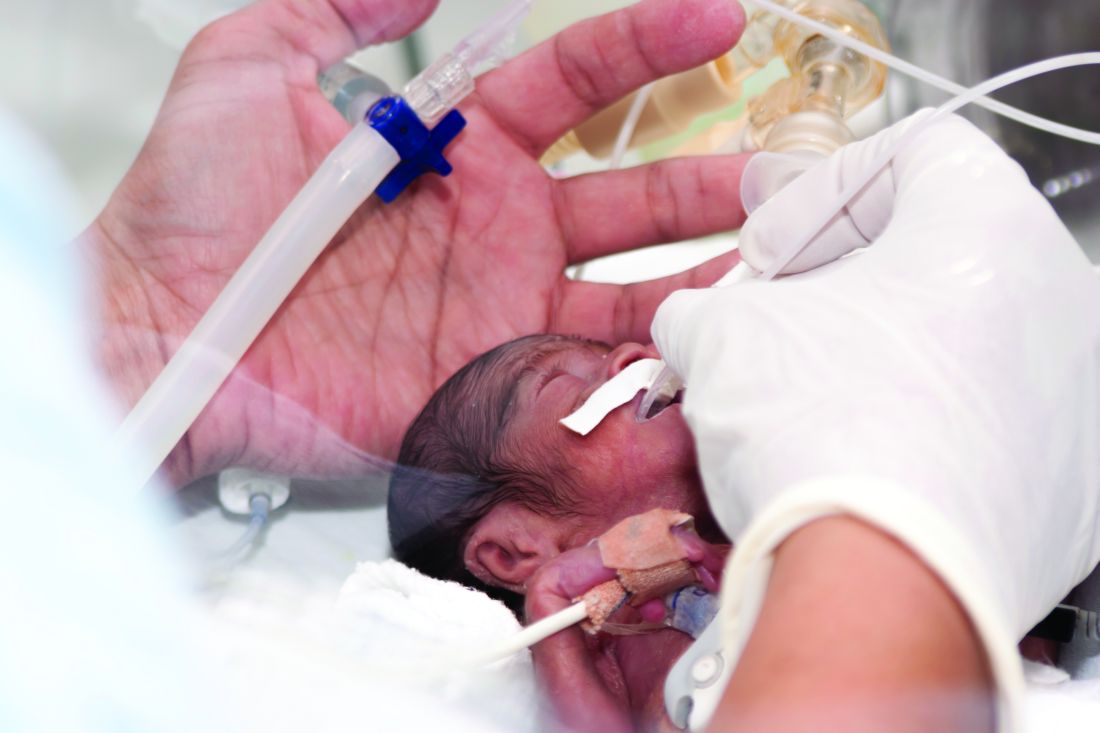
Patients with malignant pleural effusion treated with an indwelling pleural catheter have an improved chance of a positive outcome when talc administration is part of their procedure, suggest the results of a randomized, placebo-controlled study.
Malignant pleural effusion, which is usually caused by the spread of metastatic cancer, is typically treated by inducement of pleurodesis. Talc is probably the most effective agent for achieving this result, but there are drawbacks to using talc to induce pleurodesis. Patients who receive this treatment often need to stay in the hospital for 4-7 days, according to Rahul Bhatnagar, PhD, and the coauthors of a study published in the New England Journal of Medicine). Indwelling pleural catheters provide an “ambulatory alternative” for fluid management, they noted. In a noncomparative series of 22 patients, administering talc through such a catheter produced high rates of pleurodesis, they added.
In the new study, Dr. Bhatnagar of the Academic Respiratory Unit, University of Bristol, England, and his coauthors evaluated the use of an indwelling catheter, with or without talc, in patients with malignant pleural effusion recruited at 18 centers in the United Kingdom over 4 years.
“Our primary-outcome results, which were backed up by robust sensitivity analyses, strongly suggest that the administration of talc through an indwelling pleural catheter was significantly more efficacious than the use of an indwelling pleural catheter alone among patients without substantial lung entrapment,” the authors wrote.
A total of 154 patients underwent randomization to the talc or placebo group, and 139 had sufficient data to evaluate the primary outcome of successful pleurodesis at 35 days after randomization. The researchers excluded patients with evidence of lung entrapment, or nonexpandable lung, according to the study report.
In the talc group, pleurodesis was successful at day 35 in 30 of 69 patients (43%) versus 16 of 70 patients (23%) in the placebo group (P = .008).
At day 70, the success rate was 51% for the talc group vs. 27% for the placebo group, respectively.
The rate of pleurodesis was significantly higher when talc was administered through an indwelling pleural catheter, Dr. Bhatnagar and his colleagues noted.
“Success rates at day 70 suggested that pleurodesis was maintained to a point that is clinically relevant for patients with short median survival,” they added.
No excess of side effects or catheter blockages were associated with talc vs. placebo administration through a catheter. Additionally, no differences were seen between the talc and placebo groups in the number of adverse events, number of inpatient days, mortality, or other outcomes tracked by the researchers.
Dr. Bhatnagar reported he had no disclosures related to the study. Study coauthors reported disclosures related to Becton Dickinson – CareFusion, Rosetrees Trust, GE Medical, and Rocket Medical. Becton Dickinson supported the trial with an unrestricted research grant and supplied catheters and drainage bottles for the study’s participants.
SOURCE: Bhatnagar R et al. N Engl J Med. 2018;378:1313-22.
Patients with malignant pleural effusion treated with an indwelling pleural catheter have an improved chance of a positive outcome when talc administration is part of their procedure, suggest the results of a randomized, placebo-controlled study.
Malignant pleural effusion, which is usually caused by the spread of metastatic cancer, is typically treated by inducement of pleurodesis. Talc is probably the most effective agent for achieving this result, but there are drawbacks to using talc to induce pleurodesis. Patients who receive this treatment often need to stay in the hospital for 4-7 days, according to Rahul Bhatnagar, PhD, and the coauthors of a study published in the New England Journal of Medicine). Indwelling pleural catheters provide an “ambulatory alternative” for fluid management, they noted. In a noncomparative series of 22 patients, administering talc through such a catheter produced high rates of pleurodesis, they added.
In the new study, Dr. Bhatnagar of the Academic Respiratory Unit, University of Bristol, England, and his coauthors evaluated the use of an indwelling catheter, with or without talc, in patients with malignant pleural effusion recruited at 18 centers in the United Kingdom over 4 years.
“Our primary-outcome results, which were backed up by robust sensitivity analyses, strongly suggest that the administration of talc through an indwelling pleural catheter was significantly more efficacious than the use of an indwelling pleural catheter alone among patients without substantial lung entrapment,” the authors wrote.
A total of 154 patients underwent randomization to the talc or placebo group, and 139 had sufficient data to evaluate the primary outcome of successful pleurodesis at 35 days after randomization. The researchers excluded patients with evidence of lung entrapment, or nonexpandable lung, according to the study report.
In the talc group, pleurodesis was successful at day 35 in 30 of 69 patients (43%) versus 16 of 70 patients (23%) in the placebo group (P = .008).
At day 70, the success rate was 51% for the talc group vs. 27% for the placebo group, respectively.
The rate of pleurodesis was significantly higher when talc was administered through an indwelling pleural catheter, Dr. Bhatnagar and his colleagues noted.
“Success rates at day 70 suggested that pleurodesis was maintained to a point that is clinically relevant for patients with short median survival,” they added.
No excess of side effects or catheter blockages were associated with talc vs. placebo administration through a catheter. Additionally, no differences were seen between the talc and placebo groups in the number of adverse events, number of inpatient days, mortality, or other outcomes tracked by the researchers.
Dr. Bhatnagar reported he had no disclosures related to the study. Study coauthors reported disclosures related to Becton Dickinson – CareFusion, Rosetrees Trust, GE Medical, and Rocket Medical. Becton Dickinson supported the trial with an unrestricted research grant and supplied catheters and drainage bottles for the study’s participants.
SOURCE: Bhatnagar R et al. N Engl J Med. 2018;378:1313-22.
Patients with malignant pleural effusion treated with an indwelling pleural catheter have an improved chance of a positive outcome when talc administration is part of their procedure, suggest the results of a randomized, placebo-controlled study.
Malignant pleural effusion, which is usually caused by the spread of metastatic cancer, is typically treated by inducement of pleurodesis. Talc is probably the most effective agent for achieving this result, but there are drawbacks to using talc to induce pleurodesis. Patients who receive this treatment often need to stay in the hospital for 4-7 days, according to Rahul Bhatnagar, PhD, and the coauthors of a study published in the New England Journal of Medicine). Indwelling pleural catheters provide an “ambulatory alternative” for fluid management, they noted. In a noncomparative series of 22 patients, administering talc through such a catheter produced high rates of pleurodesis, they added.
In the new study, Dr. Bhatnagar of the Academic Respiratory Unit, University of Bristol, England, and his coauthors evaluated the use of an indwelling catheter, with or without talc, in patients with malignant pleural effusion recruited at 18 centers in the United Kingdom over 4 years.
“Our primary-outcome results, which were backed up by robust sensitivity analyses, strongly suggest that the administration of talc through an indwelling pleural catheter was significantly more efficacious than the use of an indwelling pleural catheter alone among patients without substantial lung entrapment,” the authors wrote.
A total of 154 patients underwent randomization to the talc or placebo group, and 139 had sufficient data to evaluate the primary outcome of successful pleurodesis at 35 days after randomization. The researchers excluded patients with evidence of lung entrapment, or nonexpandable lung, according to the study report.
In the talc group, pleurodesis was successful at day 35 in 30 of 69 patients (43%) versus 16 of 70 patients (23%) in the placebo group (P = .008).
At day 70, the success rate was 51% for the talc group vs. 27% for the placebo group, respectively.
The rate of pleurodesis was significantly higher when talc was administered through an indwelling pleural catheter, Dr. Bhatnagar and his colleagues noted.
“Success rates at day 70 suggested that pleurodesis was maintained to a point that is clinically relevant for patients with short median survival,” they added.
No excess of side effects or catheter blockages were associated with talc vs. placebo administration through a catheter. Additionally, no differences were seen between the talc and placebo groups in the number of adverse events, number of inpatient days, mortality, or other outcomes tracked by the researchers.
Dr. Bhatnagar reported he had no disclosures related to the study. Study coauthors reported disclosures related to Becton Dickinson – CareFusion, Rosetrees Trust, GE Medical, and Rocket Medical. Becton Dickinson supported the trial with an unrestricted research grant and supplied catheters and drainage bottles for the study’s participants.
SOURCE: Bhatnagar R et al. N Engl J Med. 2018;378:1313-22.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: with no deleterious effects.
Major finding: At 35 days post randomization, pleurodesis was successful in 30 of 69 patients (43%) in the talc group versus 16 of 70 patients (23%) in the placebo group (P = .008).
Study details: A randomized, placebo-controlled, single-blind, parallel-group trial including 154 patients with malignant pleural effusion recruited at 18 U.K. centers over a period of 4 years.
Disclosures: Becton Dickinson supported the trial with an unrestricted research grant and supplied catheters and drainage bottles for participants. Study authors reported disclosures related to Becton Dickinson – CareFusion, Rosetrees Trust, GE Medical, and Rocket Medical.
Source: Bhatnagar R et al. N Engl J Med. 2018;378:1313-22.
Lenalidomide yields responses in a rare cutaneous lymphoma
The oral immunomodulatory drug lenalidomide is active and may provide prolonged responses in certain patients with a rare and aggressive subtype of primary cutaneous lymphoma, according to results of a phase 2 study.
In the study, which comprised 19 patients with primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), 5 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months. The findings were reported in the Journal of Investigative Dermatology.
In an exploratory analysis, reducing the dose of lenalidomide was associated with prolonged response and improved survival, noted lead author Marie Beylot-Barry, MD, of the dermatology department, Hôpital Saint-André, CHU Bordeaux, France, and her colleagues.
“Lenalidomide at reduced doses may allow prolonged responses in few patients, and represents a therapeutic option in relapsing/refractory PCDLBCL, LT,” the researchers wrote.
Found mostly on the lower limbs of elderly patients, PCDLBCL, LT exhibits aggressive behavior and is associated with a high rate of skin recurrences. First-line therapy for the cutaneous lymphoma is typically rituximab and chemotherapy, regardless of clinical stage or patient age, the researchers wrote, though primary resistance or recurrence after treatment occurs in about half of patients. “In such relapsing or refractory cases, no treatment has demonstrated a sustained benefit thus far,” they noted.
Lenalidomide has already demonstrated efficacy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and it induces inhibition of cell signaling, engaging NF-kappaB signaling. PCDLBCL, LT is marked by genetic alterations leading to the NF-kappaB pathway, which represents a therapeutic target.
Dr. Beylot-Barry and her colleagues initiated a multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT. Median progression-free survival in the trial was 4.9 months. The 6-month overall response rate – the primary endpoint of the trial – was 26.3%, which was not significantly superior to a prespecified 20% minimal response rate, according to the researchers.
“However, it was a stringent goal, and other secondary evaluations have to be considered in this context, such as a 6-month disease control rate at 42%,” they wrote.
Reduced doses were associated with improved outcomes, they added. Comparing the nine patients who had lenalidomide dose reductions to those who did not, there was a higher likelihood of 6- to 11-month overall response rate (44.4% vs. 10.0%; P = .11) and lower risk of disease progression or death (hazard ratio, 0.54; 95% confidence interval, 0.19-1.59; P = .27).
Grade 3 adverse events were primarily hematologic, and two deaths occurred (pulmonary embolism and sepsis).
Taken together, the encouraging results at reduced doses, the advanced age of the patients, and the high rate of adverse events suggests a role for lenalidomide as a part of combination treatment for PCDLBCL, LT in future trials, the researchers concluded.
The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
SOURCE: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
The oral immunomodulatory drug lenalidomide is active and may provide prolonged responses in certain patients with a rare and aggressive subtype of primary cutaneous lymphoma, according to results of a phase 2 study.
In the study, which comprised 19 patients with primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), 5 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months. The findings were reported in the Journal of Investigative Dermatology.
In an exploratory analysis, reducing the dose of lenalidomide was associated with prolonged response and improved survival, noted lead author Marie Beylot-Barry, MD, of the dermatology department, Hôpital Saint-André, CHU Bordeaux, France, and her colleagues.
“Lenalidomide at reduced doses may allow prolonged responses in few patients, and represents a therapeutic option in relapsing/refractory PCDLBCL, LT,” the researchers wrote.
Found mostly on the lower limbs of elderly patients, PCDLBCL, LT exhibits aggressive behavior and is associated with a high rate of skin recurrences. First-line therapy for the cutaneous lymphoma is typically rituximab and chemotherapy, regardless of clinical stage or patient age, the researchers wrote, though primary resistance or recurrence after treatment occurs in about half of patients. “In such relapsing or refractory cases, no treatment has demonstrated a sustained benefit thus far,” they noted.
Lenalidomide has already demonstrated efficacy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and it induces inhibition of cell signaling, engaging NF-kappaB signaling. PCDLBCL, LT is marked by genetic alterations leading to the NF-kappaB pathway, which represents a therapeutic target.
Dr. Beylot-Barry and her colleagues initiated a multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT. Median progression-free survival in the trial was 4.9 months. The 6-month overall response rate – the primary endpoint of the trial – was 26.3%, which was not significantly superior to a prespecified 20% minimal response rate, according to the researchers.
“However, it was a stringent goal, and other secondary evaluations have to be considered in this context, such as a 6-month disease control rate at 42%,” they wrote.
Reduced doses were associated with improved outcomes, they added. Comparing the nine patients who had lenalidomide dose reductions to those who did not, there was a higher likelihood of 6- to 11-month overall response rate (44.4% vs. 10.0%; P = .11) and lower risk of disease progression or death (hazard ratio, 0.54; 95% confidence interval, 0.19-1.59; P = .27).
Grade 3 adverse events were primarily hematologic, and two deaths occurred (pulmonary embolism and sepsis).
Taken together, the encouraging results at reduced doses, the advanced age of the patients, and the high rate of adverse events suggests a role for lenalidomide as a part of combination treatment for PCDLBCL, LT in future trials, the researchers concluded.
The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
SOURCE: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
The oral immunomodulatory drug lenalidomide is active and may provide prolonged responses in certain patients with a rare and aggressive subtype of primary cutaneous lymphoma, according to results of a phase 2 study.
In the study, which comprised 19 patients with primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), 5 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months. The findings were reported in the Journal of Investigative Dermatology.
In an exploratory analysis, reducing the dose of lenalidomide was associated with prolonged response and improved survival, noted lead author Marie Beylot-Barry, MD, of the dermatology department, Hôpital Saint-André, CHU Bordeaux, France, and her colleagues.
“Lenalidomide at reduced doses may allow prolonged responses in few patients, and represents a therapeutic option in relapsing/refractory PCDLBCL, LT,” the researchers wrote.
Found mostly on the lower limbs of elderly patients, PCDLBCL, LT exhibits aggressive behavior and is associated with a high rate of skin recurrences. First-line therapy for the cutaneous lymphoma is typically rituximab and chemotherapy, regardless of clinical stage or patient age, the researchers wrote, though primary resistance or recurrence after treatment occurs in about half of patients. “In such relapsing or refractory cases, no treatment has demonstrated a sustained benefit thus far,” they noted.
Lenalidomide has already demonstrated efficacy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and it induces inhibition of cell signaling, engaging NF-kappaB signaling. PCDLBCL, LT is marked by genetic alterations leading to the NF-kappaB pathway, which represents a therapeutic target.
Dr. Beylot-Barry and her colleagues initiated a multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT. Median progression-free survival in the trial was 4.9 months. The 6-month overall response rate – the primary endpoint of the trial – was 26.3%, which was not significantly superior to a prespecified 20% minimal response rate, according to the researchers.
“However, it was a stringent goal, and other secondary evaluations have to be considered in this context, such as a 6-month disease control rate at 42%,” they wrote.
Reduced doses were associated with improved outcomes, they added. Comparing the nine patients who had lenalidomide dose reductions to those who did not, there was a higher likelihood of 6- to 11-month overall response rate (44.4% vs. 10.0%; P = .11) and lower risk of disease progression or death (hazard ratio, 0.54; 95% confidence interval, 0.19-1.59; P = .27).
Grade 3 adverse events were primarily hematologic, and two deaths occurred (pulmonary embolism and sepsis).
Taken together, the encouraging results at reduced doses, the advanced age of the patients, and the high rate of adverse events suggests a role for lenalidomide as a part of combination treatment for PCDLBCL, LT in future trials, the researchers concluded.
The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
SOURCE: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point:
Major finding: Five of 19 patients (26.3%) had a response at 6 months, and there were still 3 patients in response at 12 months.
Study details: A multicenter, single-arm, phase 2 trial of 19 patients refractory/relapsing PCDLBCL, LT.
Disclosures: The study was supported by grants from the French Ministry of Health and Celgene. The researchers reported having no financial disclosures.
Source: Beylot-Barry M et al. J Invest Dermatol. 2018 Mar 26. doi: 10.1016/j.jid.2018.03.1516.
Distinguish neurogenic from nonneurogenic orthostatic hypotension
In order to may be superior to looking at heart rate alone.
A cHR/cSBP ratio of 0.492 beats per minute (bpm)/mm Hg had the best sensitivity and specificity to distinguish neurogenic from nonneurogenic causes, according to results of a prospective study published in Annals of Neurology.
“If you just look at the heart rate increase alone, of course it is blunted, but it doesn’t really give you the bigger picture that you get when you look at the heart rate in relation to the blood pressure fall,” Dr. Norcliffe-Kaufmann said in an interview.
Neurogenic orthostatic hypotension, which indicates an underlying pathology affecting autonomic neurons, has a much worse prognosis than does nonneurogenic orthostatic hypotension, according to Dr. Norcliffe-Kaufmann and her colleagues, who published the results on behalf of the Autonomic Disorders Consortium.
One key difference between the two groups, they added, is that patients with neurogenic orthostatic hypotension typically have little or no heart rate (HR) increase in the upright position, while patients with nonneurogenic orthostatic hypotension may have marked tachycardia.
Despite the importance of orthostatic HR changes in differential diagnosis, however, there has been no systematic evaluation of HR ranges that may be diagnostic, and proposed ranges have been based on expert clinical experience rather than clinical data, they said.
Accordingly, Dr. Norcliffe-Kaufmann and her colleagues conducted a study of consecutive adult patients referred for autonomic evaluation at sites of the U.S. Autonomic Consortium.
The analysis was based on 402 patients with orthostatic hypotension who had normal sinus rhythm at the time of evaluation. Of that group, 378 had neurogenic orthostatic hypotension and were diagnosed with Parkinson disease, dementia with Lewy bodies, pure autonomic failure, or multiple system atrophy.
Patients with neurogenic orthostatic hypotension had twice the fall in SBP versus those with nonneurogenic causes (–43 vs. –21 mm Hg; P less than .0001), yet only about a third of the HR increase (8 vs. 25 bpm; P less than .0001), researchers reported.
They found the cHR/cSBP ratio of 0.492 bpm/mm Hg had the best sensitivity (91.3%) and specificity (88.4%) to distinguish between patients with neurogenic and nonneurogenic orthostatic hypertension.
By contrast, orthostatic HR increase by itself was a poor discriminator, according to the researchers, who reported that an HR increase of less than 17 bpm had just moderate sensitivity (79%) and specificity (87%).
“Using this simple bedside test of how much the blood pressure falls and heart rate increases can help in screening these patients,” Dr. Norcliffe-Kaufmann said of the results. “Then they can be sent to an autonomic clinic to really confirm the diagnosis with a sophisticated autonomic function test.”
The researchers also sought to determine whether the differences in heart rate could distinguish between central and peripheral causes of neurogenic orthostatic hypotension. They found that heart rate increased more in patients with multiple system atrophy, but noted “considerable overlap” with patients with Lewy body disorders, according to the findings.
“It didn’t really pan out as a way to distinguish the two forms from one another with enough sensitivity or specificity,” Dr. Norcliffe-Kaufmann said.
The findings do suggest, however, that looking at the cHR/cSBP ratio could help identify neurogenic orthostatic hypotension earlier, reducing delays in treatment and decreasing the need for expensive testing, the researchers said.
“I think there will be a place for genuine, solid autonomic function tests, but many patients cannot get referred to these services, or they don’t have these specialist medical centers on their doorstep, particularly in rural communities,” Dr. Norcliffe-Kaufmann said in the interview.
The study was supported by the National institutes of Health Rare Disease Clinical Research Network. Dr. Norcliffe-Kaufmann and her coauthors reported no potential conflicts of interest.
SOURCE: Norcliffe-Kaufmann L et al. Ann Neurol. 2018 Mar;83(3):522-31.
In order to may be superior to looking at heart rate alone.
A cHR/cSBP ratio of 0.492 beats per minute (bpm)/mm Hg had the best sensitivity and specificity to distinguish neurogenic from nonneurogenic causes, according to results of a prospective study published in Annals of Neurology.
“If you just look at the heart rate increase alone, of course it is blunted, but it doesn’t really give you the bigger picture that you get when you look at the heart rate in relation to the blood pressure fall,” Dr. Norcliffe-Kaufmann said in an interview.
Neurogenic orthostatic hypotension, which indicates an underlying pathology affecting autonomic neurons, has a much worse prognosis than does nonneurogenic orthostatic hypotension, according to Dr. Norcliffe-Kaufmann and her colleagues, who published the results on behalf of the Autonomic Disorders Consortium.
One key difference between the two groups, they added, is that patients with neurogenic orthostatic hypotension typically have little or no heart rate (HR) increase in the upright position, while patients with nonneurogenic orthostatic hypotension may have marked tachycardia.
Despite the importance of orthostatic HR changes in differential diagnosis, however, there has been no systematic evaluation of HR ranges that may be diagnostic, and proposed ranges have been based on expert clinical experience rather than clinical data, they said.
Accordingly, Dr. Norcliffe-Kaufmann and her colleagues conducted a study of consecutive adult patients referred for autonomic evaluation at sites of the U.S. Autonomic Consortium.
The analysis was based on 402 patients with orthostatic hypotension who had normal sinus rhythm at the time of evaluation. Of that group, 378 had neurogenic orthostatic hypotension and were diagnosed with Parkinson disease, dementia with Lewy bodies, pure autonomic failure, or multiple system atrophy.
Patients with neurogenic orthostatic hypotension had twice the fall in SBP versus those with nonneurogenic causes (–43 vs. –21 mm Hg; P less than .0001), yet only about a third of the HR increase (8 vs. 25 bpm; P less than .0001), researchers reported.
They found the cHR/cSBP ratio of 0.492 bpm/mm Hg had the best sensitivity (91.3%) and specificity (88.4%) to distinguish between patients with neurogenic and nonneurogenic orthostatic hypertension.
By contrast, orthostatic HR increase by itself was a poor discriminator, according to the researchers, who reported that an HR increase of less than 17 bpm had just moderate sensitivity (79%) and specificity (87%).
“Using this simple bedside test of how much the blood pressure falls and heart rate increases can help in screening these patients,” Dr. Norcliffe-Kaufmann said of the results. “Then they can be sent to an autonomic clinic to really confirm the diagnosis with a sophisticated autonomic function test.”
The researchers also sought to determine whether the differences in heart rate could distinguish between central and peripheral causes of neurogenic orthostatic hypotension. They found that heart rate increased more in patients with multiple system atrophy, but noted “considerable overlap” with patients with Lewy body disorders, according to the findings.
“It didn’t really pan out as a way to distinguish the two forms from one another with enough sensitivity or specificity,” Dr. Norcliffe-Kaufmann said.
The findings do suggest, however, that looking at the cHR/cSBP ratio could help identify neurogenic orthostatic hypotension earlier, reducing delays in treatment and decreasing the need for expensive testing, the researchers said.
“I think there will be a place for genuine, solid autonomic function tests, but many patients cannot get referred to these services, or they don’t have these specialist medical centers on their doorstep, particularly in rural communities,” Dr. Norcliffe-Kaufmann said in the interview.
The study was supported by the National institutes of Health Rare Disease Clinical Research Network. Dr. Norcliffe-Kaufmann and her coauthors reported no potential conflicts of interest.
SOURCE: Norcliffe-Kaufmann L et al. Ann Neurol. 2018 Mar;83(3):522-31.
In order to may be superior to looking at heart rate alone.
A cHR/cSBP ratio of 0.492 beats per minute (bpm)/mm Hg had the best sensitivity and specificity to distinguish neurogenic from nonneurogenic causes, according to results of a prospective study published in Annals of Neurology.
“If you just look at the heart rate increase alone, of course it is blunted, but it doesn’t really give you the bigger picture that you get when you look at the heart rate in relation to the blood pressure fall,” Dr. Norcliffe-Kaufmann said in an interview.
Neurogenic orthostatic hypotension, which indicates an underlying pathology affecting autonomic neurons, has a much worse prognosis than does nonneurogenic orthostatic hypotension, according to Dr. Norcliffe-Kaufmann and her colleagues, who published the results on behalf of the Autonomic Disorders Consortium.
One key difference between the two groups, they added, is that patients with neurogenic orthostatic hypotension typically have little or no heart rate (HR) increase in the upright position, while patients with nonneurogenic orthostatic hypotension may have marked tachycardia.
Despite the importance of orthostatic HR changes in differential diagnosis, however, there has been no systematic evaluation of HR ranges that may be diagnostic, and proposed ranges have been based on expert clinical experience rather than clinical data, they said.
Accordingly, Dr. Norcliffe-Kaufmann and her colleagues conducted a study of consecutive adult patients referred for autonomic evaluation at sites of the U.S. Autonomic Consortium.
The analysis was based on 402 patients with orthostatic hypotension who had normal sinus rhythm at the time of evaluation. Of that group, 378 had neurogenic orthostatic hypotension and were diagnosed with Parkinson disease, dementia with Lewy bodies, pure autonomic failure, or multiple system atrophy.
Patients with neurogenic orthostatic hypotension had twice the fall in SBP versus those with nonneurogenic causes (–43 vs. –21 mm Hg; P less than .0001), yet only about a third of the HR increase (8 vs. 25 bpm; P less than .0001), researchers reported.
They found the cHR/cSBP ratio of 0.492 bpm/mm Hg had the best sensitivity (91.3%) and specificity (88.4%) to distinguish between patients with neurogenic and nonneurogenic orthostatic hypertension.
By contrast, orthostatic HR increase by itself was a poor discriminator, according to the researchers, who reported that an HR increase of less than 17 bpm had just moderate sensitivity (79%) and specificity (87%).
“Using this simple bedside test of how much the blood pressure falls and heart rate increases can help in screening these patients,” Dr. Norcliffe-Kaufmann said of the results. “Then they can be sent to an autonomic clinic to really confirm the diagnosis with a sophisticated autonomic function test.”
The researchers also sought to determine whether the differences in heart rate could distinguish between central and peripheral causes of neurogenic orthostatic hypotension. They found that heart rate increased more in patients with multiple system atrophy, but noted “considerable overlap” with patients with Lewy body disorders, according to the findings.
“It didn’t really pan out as a way to distinguish the two forms from one another with enough sensitivity or specificity,” Dr. Norcliffe-Kaufmann said.
The findings do suggest, however, that looking at the cHR/cSBP ratio could help identify neurogenic orthostatic hypotension earlier, reducing delays in treatment and decreasing the need for expensive testing, the researchers said.
“I think there will be a place for genuine, solid autonomic function tests, but many patients cannot get referred to these services, or they don’t have these specialist medical centers on their doorstep, particularly in rural communities,” Dr. Norcliffe-Kaufmann said in the interview.
The study was supported by the National institutes of Health Rare Disease Clinical Research Network. Dr. Norcliffe-Kaufmann and her coauthors reported no potential conflicts of interest.
SOURCE: Norcliffe-Kaufmann L et al. Ann Neurol. 2018 Mar;83(3):522-31.
FROM ANNALS OF NEUROLOGY
Key clinical point: The ratio of change in heart rate (cHR) to change in systolic blood pressure (cSBP) was better than HR increase alone in distinguishing between neurogenic and nonneurogenic causes of orthostatic hypotension.
Major finding: A cHR/cSBP ratio of 0.492 bpm/mm Hg had the best sensitivity (91.3%) and specificity (88.4%) to distinguish neurogenic from nonneurogenic causes.
Study details: A prospective study including 444 adult patients with OH referred for autonomic evaluation to sites in the U.S. Autonomic Disorders Consortium.
Disclosures: The study authors reported no potential conflicts of interest.
Source: Norcliffe-Kaufmann L et al. Ann Neurol. 2018 Mar;83(3):522-31.
Most PsA patients discontinue initial biologic within 12 months
Most adult patients with psoriatic arthritis who newly initiate biologic therapy with a tumor necrosis factor (TNF) inhibitor or anti–interleukin-12/23 inhibitor discontinued the treatment before a year is up, according to a recent analysis of a U.S. claims database.
Over a 12-month follow-up period, 27% of psoriatic arthritis (PsA) patients discontinued the index biologic, 23% switched to a different biologic, and 6% discontinued the index biologic but later restarted it, according to the results, which were published in the Journal of Managed Care & Specialty Pharmacy.
“In this population of patients with PsA, additional options for concomitant therapies or alternatives to TNF inhibitors and anti–IL-12/23 inhibitors may be important,” the authors wrote.
The retrospective, observational study included administrative claims data from the Optum research database representing 1,235 adults with PsA who newly initiated a biologic therapy between Jan. 1, 2013, and Jan. 31, 2015. The patients (53% female; mean age, 50.3 years) had received biologic therapies approved for treatment of PsA at the time. These patients had commercial health coverage or Medicare Advantage, and nearly half were from the South. About half (48%) received etanercept, 24% received adalimumab, 10% received infliximab, and the rest received golimumab, ustekinumab, or certolizumab pegol.
The mean duration of persistence with a newly initiated biologic was just 246 days, the investigators reported. Infliximab had the highest 12-month persistence in this study, investigators said, with a mean of 293 days, while certolizumab pegol had the shortest, at a mean of 207 days.
Among patients who stayed on the index biologic for at least 90 days, nearly half started an adjunctive treatment, which was usually corticosteroids (22%), opioids (17%), or an NSAID (13%), Dr. Walsh and her coauthors said.
Dose escalation of the index biologic occurred in 9.6% of patients over the 12-month follow-up, they added.
“High rates of discontinuation and switching of biologic therapies, along with high rates of dose escalation, suggest a high frequency of suboptimal biologic experience in patients with PsA,” they wrote. Although the study did not address why patients discontinued or switched, previous studies suggest adverse effects and lack of efficacy are the most commonly reported reasons.
“Insufficient control of symptoms may lead patients to discontinue biologic therapy, which can contribute to disease progression,” the authors said in their conclusion.
Novartis sponsored the study. Some study authors reported disclosures related to Novartis, including consultancy and employment, and to Optum, which was commissioned to conduct the study.
SOURCE: Walsh JA et al. J Manag Care Spec Pharm. 2018 Mar 20. doi: 10.18553/jmcp.2018.17388.
Most adult patients with psoriatic arthritis who newly initiate biologic therapy with a tumor necrosis factor (TNF) inhibitor or anti–interleukin-12/23 inhibitor discontinued the treatment before a year is up, according to a recent analysis of a U.S. claims database.
Over a 12-month follow-up period, 27% of psoriatic arthritis (PsA) patients discontinued the index biologic, 23% switched to a different biologic, and 6% discontinued the index biologic but later restarted it, according to the results, which were published in the Journal of Managed Care & Specialty Pharmacy.
“In this population of patients with PsA, additional options for concomitant therapies or alternatives to TNF inhibitors and anti–IL-12/23 inhibitors may be important,” the authors wrote.
The retrospective, observational study included administrative claims data from the Optum research database representing 1,235 adults with PsA who newly initiated a biologic therapy between Jan. 1, 2013, and Jan. 31, 2015. The patients (53% female; mean age, 50.3 years) had received biologic therapies approved for treatment of PsA at the time. These patients had commercial health coverage or Medicare Advantage, and nearly half were from the South. About half (48%) received etanercept, 24% received adalimumab, 10% received infliximab, and the rest received golimumab, ustekinumab, or certolizumab pegol.
The mean duration of persistence with a newly initiated biologic was just 246 days, the investigators reported. Infliximab had the highest 12-month persistence in this study, investigators said, with a mean of 293 days, while certolizumab pegol had the shortest, at a mean of 207 days.
Among patients who stayed on the index biologic for at least 90 days, nearly half started an adjunctive treatment, which was usually corticosteroids (22%), opioids (17%), or an NSAID (13%), Dr. Walsh and her coauthors said.
Dose escalation of the index biologic occurred in 9.6% of patients over the 12-month follow-up, they added.
“High rates of discontinuation and switching of biologic therapies, along with high rates of dose escalation, suggest a high frequency of suboptimal biologic experience in patients with PsA,” they wrote. Although the study did not address why patients discontinued or switched, previous studies suggest adverse effects and lack of efficacy are the most commonly reported reasons.
“Insufficient control of symptoms may lead patients to discontinue biologic therapy, which can contribute to disease progression,” the authors said in their conclusion.
Novartis sponsored the study. Some study authors reported disclosures related to Novartis, including consultancy and employment, and to Optum, which was commissioned to conduct the study.
SOURCE: Walsh JA et al. J Manag Care Spec Pharm. 2018 Mar 20. doi: 10.18553/jmcp.2018.17388.
Most adult patients with psoriatic arthritis who newly initiate biologic therapy with a tumor necrosis factor (TNF) inhibitor or anti–interleukin-12/23 inhibitor discontinued the treatment before a year is up, according to a recent analysis of a U.S. claims database.
Over a 12-month follow-up period, 27% of psoriatic arthritis (PsA) patients discontinued the index biologic, 23% switched to a different biologic, and 6% discontinued the index biologic but later restarted it, according to the results, which were published in the Journal of Managed Care & Specialty Pharmacy.
“In this population of patients with PsA, additional options for concomitant therapies or alternatives to TNF inhibitors and anti–IL-12/23 inhibitors may be important,” the authors wrote.
The retrospective, observational study included administrative claims data from the Optum research database representing 1,235 adults with PsA who newly initiated a biologic therapy between Jan. 1, 2013, and Jan. 31, 2015. The patients (53% female; mean age, 50.3 years) had received biologic therapies approved for treatment of PsA at the time. These patients had commercial health coverage or Medicare Advantage, and nearly half were from the South. About half (48%) received etanercept, 24% received adalimumab, 10% received infliximab, and the rest received golimumab, ustekinumab, or certolizumab pegol.
The mean duration of persistence with a newly initiated biologic was just 246 days, the investigators reported. Infliximab had the highest 12-month persistence in this study, investigators said, with a mean of 293 days, while certolizumab pegol had the shortest, at a mean of 207 days.
Among patients who stayed on the index biologic for at least 90 days, nearly half started an adjunctive treatment, which was usually corticosteroids (22%), opioids (17%), or an NSAID (13%), Dr. Walsh and her coauthors said.
Dose escalation of the index biologic occurred in 9.6% of patients over the 12-month follow-up, they added.
“High rates of discontinuation and switching of biologic therapies, along with high rates of dose escalation, suggest a high frequency of suboptimal biologic experience in patients with PsA,” they wrote. Although the study did not address why patients discontinued or switched, previous studies suggest adverse effects and lack of efficacy are the most commonly reported reasons.
“Insufficient control of symptoms may lead patients to discontinue biologic therapy, which can contribute to disease progression,” the authors said in their conclusion.
Novartis sponsored the study. Some study authors reported disclosures related to Novartis, including consultancy and employment, and to Optum, which was commissioned to conduct the study.
SOURCE: Walsh JA et al. J Manag Care Spec Pharm. 2018 Mar 20. doi: 10.18553/jmcp.2018.17388.
FROM THE JOURNAL OF MANAGED CARE & SPECIALTY PHARMACY
Key clinical point: While treatment persistence is important to achieve optimal outcomes,
Major finding: Over a 12-month follow-up period, 27% of patients discontinued the index biologic, 23% switched to a different biologic, and 6% discontinued the index biologic but later restarted it.
Study details: A retrospective, observational study of U.S. administrative claims data representing 1,235 adults with PsA who newly initiated a biologic therapy between Jan. 1, 2013, and Jan. 31, 2015.
Disclosures: Novartis sponsored the study. Some study authors reported disclosures related to Novartis, including consultancy and employment, and to Optum, which was commissioned to conduct the study.
Source: Walsh JA et al. J Manag Care Spec Pharm. 2018 Mar 20. doi: 10.18553/jmcp.2018.17388.
Certolizumab pegol: Has serious infection risk been overstated?
Contrary to prior reports, certolizumab pegol may not be associated with an increased risk of serious infections versus other biologics used in the treatment of rheumatoid arthritis, according to results of a recent U.K. registry study.
Certolizumab pegol (Cimzia) actually had a lower risk of serious infections, compared with etanercept in the primary analysis, according to a report on the study recently published in Annals of the Rheumatic Diseases.
In sensitivity analyses, though, the result in favor of certolizumab pegol was no longer significant, according to lead author Andrew I. Rutherford, MBBS, of King’s College London, and his coauthors.
“From these results, it would be wrong to conclude that certolizumab pegol has a lower rate of [serious infection] than other biologics,” Dr. Rutherford and his colleagues said in their report. “However, the risk does not appear to be significantly higher as has previously been suggested.”
The prospective, observational cohort study, based on data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA), represented 19,282 patients with 46,771 years of follow-up, according to the authors.
For the entire cohort, the incidence of serious infections was 5.51 cases per 100 patient-years (95% confidence interval, 5.29-5.71), and the 30-day mortality after serious infection was 10.4% (95% CI, 9.2%-11.6%), the report said. Compared with etanercept, the risk of serious infections was lower for certolizumab pegol (adjusted hazard ratio, 0.75; 95% CI, 0.58-0.97). Etanercept was used as the reference arm for comparison since it was the most widely prescribed drug in the registry.
That finding is in “direct contradiction” to a 2011 Cochrane review finding that certolizumab pegol was associated with an infection rate three to four times higher than other anti–tumor necrosis factor (anti-TNF) drugs (Cochrane Database Syst Rev. 2011;2:Cd008794), the authors noted.
“It would seem unusual for drugs acting on the same pathway with similar efficacy to have such drastically different infection risks,” Dr. Rutherford and his coauthors observed in their report.
However, investigators noticed that in the certolizumab pegol cohort, a large number of patients had not previously been on a biologic. When those biologic-naive patients were excluded from analysis, there was no longer a difference in infection rate favoring certolizumab pegol. “This suggests that unmeasured confounders may be responsible for the difference that was observed in the primary analysis,” the investigators said in their discussion of the results.
The Cochrane review showing an increased risk of serious infections with certolizumab pegol was a large network meta-analysis, which they said relies on indirect comparisons between drugs and could be prone to error if there are differences in study design.
“In contrast, national registers use the same methodology for detecting and reporting of adverse events for each drug,” they added.
Dr. Rutherford reported no disclosures. Study coauthors reported disclosures related to Pfizer, AbbVie, Bristol-Myers Squibb, UCB, and Celgene. The British Society for Rheumatology, which commissioned the study, receives income from AbbVie, Celltrion, Hospira, Pfizer, UCB, and Roche related to a different contract.
SOURCE: Rutherford AI et al. Ann Rheum Dis. 2018 Mar 28. doi: 10.1136/annrheumdis-2017-212825.
Contrary to prior reports, certolizumab pegol may not be associated with an increased risk of serious infections versus other biologics used in the treatment of rheumatoid arthritis, according to results of a recent U.K. registry study.
Certolizumab pegol (Cimzia) actually had a lower risk of serious infections, compared with etanercept in the primary analysis, according to a report on the study recently published in Annals of the Rheumatic Diseases.
In sensitivity analyses, though, the result in favor of certolizumab pegol was no longer significant, according to lead author Andrew I. Rutherford, MBBS, of King’s College London, and his coauthors.
“From these results, it would be wrong to conclude that certolizumab pegol has a lower rate of [serious infection] than other biologics,” Dr. Rutherford and his colleagues said in their report. “However, the risk does not appear to be significantly higher as has previously been suggested.”
The prospective, observational cohort study, based on data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA), represented 19,282 patients with 46,771 years of follow-up, according to the authors.
For the entire cohort, the incidence of serious infections was 5.51 cases per 100 patient-years (95% confidence interval, 5.29-5.71), and the 30-day mortality after serious infection was 10.4% (95% CI, 9.2%-11.6%), the report said. Compared with etanercept, the risk of serious infections was lower for certolizumab pegol (adjusted hazard ratio, 0.75; 95% CI, 0.58-0.97). Etanercept was used as the reference arm for comparison since it was the most widely prescribed drug in the registry.
That finding is in “direct contradiction” to a 2011 Cochrane review finding that certolizumab pegol was associated with an infection rate three to four times higher than other anti–tumor necrosis factor (anti-TNF) drugs (Cochrane Database Syst Rev. 2011;2:Cd008794), the authors noted.
“It would seem unusual for drugs acting on the same pathway with similar efficacy to have such drastically different infection risks,” Dr. Rutherford and his coauthors observed in their report.
However, investigators noticed that in the certolizumab pegol cohort, a large number of patients had not previously been on a biologic. When those biologic-naive patients were excluded from analysis, there was no longer a difference in infection rate favoring certolizumab pegol. “This suggests that unmeasured confounders may be responsible for the difference that was observed in the primary analysis,” the investigators said in their discussion of the results.
The Cochrane review showing an increased risk of serious infections with certolizumab pegol was a large network meta-analysis, which they said relies on indirect comparisons between drugs and could be prone to error if there are differences in study design.
“In contrast, national registers use the same methodology for detecting and reporting of adverse events for each drug,” they added.
Dr. Rutherford reported no disclosures. Study coauthors reported disclosures related to Pfizer, AbbVie, Bristol-Myers Squibb, UCB, and Celgene. The British Society for Rheumatology, which commissioned the study, receives income from AbbVie, Celltrion, Hospira, Pfizer, UCB, and Roche related to a different contract.
SOURCE: Rutherford AI et al. Ann Rheum Dis. 2018 Mar 28. doi: 10.1136/annrheumdis-2017-212825.
Contrary to prior reports, certolizumab pegol may not be associated with an increased risk of serious infections versus other biologics used in the treatment of rheumatoid arthritis, according to results of a recent U.K. registry study.
Certolizumab pegol (Cimzia) actually had a lower risk of serious infections, compared with etanercept in the primary analysis, according to a report on the study recently published in Annals of the Rheumatic Diseases.
In sensitivity analyses, though, the result in favor of certolizumab pegol was no longer significant, according to lead author Andrew I. Rutherford, MBBS, of King’s College London, and his coauthors.
“From these results, it would be wrong to conclude that certolizumab pegol has a lower rate of [serious infection] than other biologics,” Dr. Rutherford and his colleagues said in their report. “However, the risk does not appear to be significantly higher as has previously been suggested.”
The prospective, observational cohort study, based on data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA), represented 19,282 patients with 46,771 years of follow-up, according to the authors.
For the entire cohort, the incidence of serious infections was 5.51 cases per 100 patient-years (95% confidence interval, 5.29-5.71), and the 30-day mortality after serious infection was 10.4% (95% CI, 9.2%-11.6%), the report said. Compared with etanercept, the risk of serious infections was lower for certolizumab pegol (adjusted hazard ratio, 0.75; 95% CI, 0.58-0.97). Etanercept was used as the reference arm for comparison since it was the most widely prescribed drug in the registry.
That finding is in “direct contradiction” to a 2011 Cochrane review finding that certolizumab pegol was associated with an infection rate three to four times higher than other anti–tumor necrosis factor (anti-TNF) drugs (Cochrane Database Syst Rev. 2011;2:Cd008794), the authors noted.
“It would seem unusual for drugs acting on the same pathway with similar efficacy to have such drastically different infection risks,” Dr. Rutherford and his coauthors observed in their report.
However, investigators noticed that in the certolizumab pegol cohort, a large number of patients had not previously been on a biologic. When those biologic-naive patients were excluded from analysis, there was no longer a difference in infection rate favoring certolizumab pegol. “This suggests that unmeasured confounders may be responsible for the difference that was observed in the primary analysis,” the investigators said in their discussion of the results.
The Cochrane review showing an increased risk of serious infections with certolizumab pegol was a large network meta-analysis, which they said relies on indirect comparisons between drugs and could be prone to error if there are differences in study design.
“In contrast, national registers use the same methodology for detecting and reporting of adverse events for each drug,” they added.
Dr. Rutherford reported no disclosures. Study coauthors reported disclosures related to Pfizer, AbbVie, Bristol-Myers Squibb, UCB, and Celgene. The British Society for Rheumatology, which commissioned the study, receives income from AbbVie, Celltrion, Hospira, Pfizer, UCB, and Roche related to a different contract.
SOURCE: Rutherford AI et al. Ann Rheum Dis. 2018 Mar 28. doi: 10.1136/annrheumdis-2017-212825.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Certolizumab pegol had a lower risk of serious infections compared to etanercept (HR, 0.75; 95% CI, 0.58-0.97), though in sensitivity analyses, the difference was no longer significant.
Study details: A prospective observational cohort study of data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) representing 19,282 patients with 46,771 years of follow-up.
Disclosures: Study authors reported disclosures related to Pfizer, AbbVie, Bristol-Myers Squibb, UCB, and Celgene. The British Society for Rheumatology, which commissioned the study, receives income from AbbVie, Celltrion, Hospira, Pfizer, UCB, and Roche related to a different contract.
Source: Rutherford AI et al. Ann Rheum Dis. 2018 Mar 28. doi: 10.1136/annrheumdis-2017-212825.
Pot legalization tied to drop in opioid prescribing rates
Laws covering medical or recreational use of marijuana are associated with reduced rates of opioid prescribing among federal health care program enrollees, results of two recently published investigations show.
In one study, researchers investigated whether medical cannabis access affected opioid prescribing in Medicare Part D, the federal program that subsidizes cost of prescription drugs and drug insurance premiums.
“Medical cannabis policies may be one mechanism that can encourage lower prescription opioid use and serve as a harm abatement tool in the opioid crisis,” Ms. Bradford and her coauthors wrote in JAMA Internal Medicine.
Medical marijuana laws were associated with a decrease of 2.11 million daily opioid doses yearly from an average of 23.08 million doses yearly in the Medicare Part D population, according to results of the longitudinal analysis daily opioids doses filled in Medicare Part D from 2010 through 2015.
In a second study, medical marijuana laws were associated with lower opioid prescribing rates among Medicaid enrollees.
That finding was consistent with earlier studies looking more broadly at pain prescriptions covered by Medicaid that also showed a reduction, researchers Hefei Wen, PhD, and Jason M. Hockenberry, PhD, wrote in their JAMA Internal Medicine article.
However, adult-use marijuana laws were associated with “even-lower” opioid prescribing rates, something that had not been investigated previously, according to Dr. Wen, who is with the University of Kentucky, Lexington, and Dr. Hockenberry of Emory University, Atlanta.
“Medical and adult-use marijuana laws have the potential to lower opioid prescribing for Medicaid enrollees, a high-risk population for chronic pain, opioid use disorder, and opioid overdose,” Dr. Wen and Dr. Hockenberry wrote in their report on the study, a cross-sectional analysis including all Medicaid fee-for-service and managed care enrollees during 2011-2016.
The rate of opioid prescribing in the study was –5.88% lower (95% confidence interval, –11.55% to approximately –0.21%) in association with medical marijuana laws, and –6.38% lower (95% CI, –12.20% to approximately –0.56%) for adult-use laws, they reported.
Based on those findings, policy discussions about the opioid epidemic should include the potential for liberalization of marijuana policies to reduce prescription opioid use and consequences in Medicaid enrollees, Dr. Wen and Dr. Hockenberry concluded.
However, legal marijuana alone won’t solve the opioid epidemic, they cautioned.
“As with other policies evaluated in the previous literature, marijuana liberalization is but one potential aspect of a comprehensive package to tackle the epidemic,” they said in the article.
None of the study authors reported conflicts of interest.
SOURCES: Bradford AC et al. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0266; Wen H, Hockenberry JM. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.1007.
Results of these two investigations suggest that the legalization of marijuana may help combat the opioid crisis, but more rigorous investigations are needed, according to Kevin P. Hill, MD, and Andrew J. Saxon, MD.
The new studies show an association between state marijuana laws and fewer opioid prescriptions in Medicare and Medicaid populations.
Those findings do support previous investigations of administrative data sets suggesting that cannabis legalization policies are associated with reductions in opioid use and mortality, Dr. Hill and Dr. Saxon said in an editorial.
However, not all studies suggest that cannabis replaces opioid use, according to the authors, who cited a study suggesting an association between illicit cannabis use and subsequent cannabis use.
“The association between illicit cannabis use and opioid use may be different than the association of legalized cannabis use and opioids,” the editorial authors wrote. “Nevertheless, the findings demonstrating that cannabis use is associated with initiation of or increase in opioid use underscores the fact that rigorous scientific studies are needed.”
Those studies should focus not only analysis of policies on legal medical and recreational cannabis but also on clinical trials of cannabis and cannabinoids for chronic pain and other conditions where opioids are used, they added.
One limitation of the studies is that , according to the authors.
Nevertheless, the authors wrote, a decrease tied to legal marijuana availability would “dovetail” with preclinical evidence that cannabinoid and opioid receptor systems mediate signaling pathways involved in tolerance, dependence, and addiction.
“These concepts support anecdotal evidence from patients who describe a decreased need for opioids to treat chronic pain after initiation of medical cannabis pharmacotherapy,” the authors wrote.
Kevin P. Hill, MD, is with the division of addiction psychiatry at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston. Andrew J. Saxon, MD, is with the Center of Excellence in Substance Abuse Treatment and Education at the VA Puget Sound Healthcare System, and the department of psychiatry and behavioral sciences at University of Washington, both in Seattle. These comments are derived from their editorial (JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0254). The authors reported no conflicts of interest.
Results of these two investigations suggest that the legalization of marijuana may help combat the opioid crisis, but more rigorous investigations are needed, according to Kevin P. Hill, MD, and Andrew J. Saxon, MD.
The new studies show an association between state marijuana laws and fewer opioid prescriptions in Medicare and Medicaid populations.
Those findings do support previous investigations of administrative data sets suggesting that cannabis legalization policies are associated with reductions in opioid use and mortality, Dr. Hill and Dr. Saxon said in an editorial.
However, not all studies suggest that cannabis replaces opioid use, according to the authors, who cited a study suggesting an association between illicit cannabis use and subsequent cannabis use.
“The association between illicit cannabis use and opioid use may be different than the association of legalized cannabis use and opioids,” the editorial authors wrote. “Nevertheless, the findings demonstrating that cannabis use is associated with initiation of or increase in opioid use underscores the fact that rigorous scientific studies are needed.”
Those studies should focus not only analysis of policies on legal medical and recreational cannabis but also on clinical trials of cannabis and cannabinoids for chronic pain and other conditions where opioids are used, they added.
One limitation of the studies is that , according to the authors.
Nevertheless, the authors wrote, a decrease tied to legal marijuana availability would “dovetail” with preclinical evidence that cannabinoid and opioid receptor systems mediate signaling pathways involved in tolerance, dependence, and addiction.
“These concepts support anecdotal evidence from patients who describe a decreased need for opioids to treat chronic pain after initiation of medical cannabis pharmacotherapy,” the authors wrote.
Kevin P. Hill, MD, is with the division of addiction psychiatry at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston. Andrew J. Saxon, MD, is with the Center of Excellence in Substance Abuse Treatment and Education at the VA Puget Sound Healthcare System, and the department of psychiatry and behavioral sciences at University of Washington, both in Seattle. These comments are derived from their editorial (JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0254). The authors reported no conflicts of interest.
Results of these two investigations suggest that the legalization of marijuana may help combat the opioid crisis, but more rigorous investigations are needed, according to Kevin P. Hill, MD, and Andrew J. Saxon, MD.
The new studies show an association between state marijuana laws and fewer opioid prescriptions in Medicare and Medicaid populations.
Those findings do support previous investigations of administrative data sets suggesting that cannabis legalization policies are associated with reductions in opioid use and mortality, Dr. Hill and Dr. Saxon said in an editorial.
However, not all studies suggest that cannabis replaces opioid use, according to the authors, who cited a study suggesting an association between illicit cannabis use and subsequent cannabis use.
“The association between illicit cannabis use and opioid use may be different than the association of legalized cannabis use and opioids,” the editorial authors wrote. “Nevertheless, the findings demonstrating that cannabis use is associated with initiation of or increase in opioid use underscores the fact that rigorous scientific studies are needed.”
Those studies should focus not only analysis of policies on legal medical and recreational cannabis but also on clinical trials of cannabis and cannabinoids for chronic pain and other conditions where opioids are used, they added.
One limitation of the studies is that , according to the authors.
Nevertheless, the authors wrote, a decrease tied to legal marijuana availability would “dovetail” with preclinical evidence that cannabinoid and opioid receptor systems mediate signaling pathways involved in tolerance, dependence, and addiction.
“These concepts support anecdotal evidence from patients who describe a decreased need for opioids to treat chronic pain after initiation of medical cannabis pharmacotherapy,” the authors wrote.
Kevin P. Hill, MD, is with the division of addiction psychiatry at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston. Andrew J. Saxon, MD, is with the Center of Excellence in Substance Abuse Treatment and Education at the VA Puget Sound Healthcare System, and the department of psychiatry and behavioral sciences at University of Washington, both in Seattle. These comments are derived from their editorial (JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0254). The authors reported no conflicts of interest.
Laws covering medical or recreational use of marijuana are associated with reduced rates of opioid prescribing among federal health care program enrollees, results of two recently published investigations show.
In one study, researchers investigated whether medical cannabis access affected opioid prescribing in Medicare Part D, the federal program that subsidizes cost of prescription drugs and drug insurance premiums.
“Medical cannabis policies may be one mechanism that can encourage lower prescription opioid use and serve as a harm abatement tool in the opioid crisis,” Ms. Bradford and her coauthors wrote in JAMA Internal Medicine.
Medical marijuana laws were associated with a decrease of 2.11 million daily opioid doses yearly from an average of 23.08 million doses yearly in the Medicare Part D population, according to results of the longitudinal analysis daily opioids doses filled in Medicare Part D from 2010 through 2015.
In a second study, medical marijuana laws were associated with lower opioid prescribing rates among Medicaid enrollees.
That finding was consistent with earlier studies looking more broadly at pain prescriptions covered by Medicaid that also showed a reduction, researchers Hefei Wen, PhD, and Jason M. Hockenberry, PhD, wrote in their JAMA Internal Medicine article.
However, adult-use marijuana laws were associated with “even-lower” opioid prescribing rates, something that had not been investigated previously, according to Dr. Wen, who is with the University of Kentucky, Lexington, and Dr. Hockenberry of Emory University, Atlanta.
“Medical and adult-use marijuana laws have the potential to lower opioid prescribing for Medicaid enrollees, a high-risk population for chronic pain, opioid use disorder, and opioid overdose,” Dr. Wen and Dr. Hockenberry wrote in their report on the study, a cross-sectional analysis including all Medicaid fee-for-service and managed care enrollees during 2011-2016.
The rate of opioid prescribing in the study was –5.88% lower (95% confidence interval, –11.55% to approximately –0.21%) in association with medical marijuana laws, and –6.38% lower (95% CI, –12.20% to approximately –0.56%) for adult-use laws, they reported.
Based on those findings, policy discussions about the opioid epidemic should include the potential for liberalization of marijuana policies to reduce prescription opioid use and consequences in Medicaid enrollees, Dr. Wen and Dr. Hockenberry concluded.
However, legal marijuana alone won’t solve the opioid epidemic, they cautioned.
“As with other policies evaluated in the previous literature, marijuana liberalization is but one potential aspect of a comprehensive package to tackle the epidemic,” they said in the article.
None of the study authors reported conflicts of interest.
SOURCES: Bradford AC et al. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0266; Wen H, Hockenberry JM. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.1007.
Laws covering medical or recreational use of marijuana are associated with reduced rates of opioid prescribing among federal health care program enrollees, results of two recently published investigations show.
In one study, researchers investigated whether medical cannabis access affected opioid prescribing in Medicare Part D, the federal program that subsidizes cost of prescription drugs and drug insurance premiums.
“Medical cannabis policies may be one mechanism that can encourage lower prescription opioid use and serve as a harm abatement tool in the opioid crisis,” Ms. Bradford and her coauthors wrote in JAMA Internal Medicine.
Medical marijuana laws were associated with a decrease of 2.11 million daily opioid doses yearly from an average of 23.08 million doses yearly in the Medicare Part D population, according to results of the longitudinal analysis daily opioids doses filled in Medicare Part D from 2010 through 2015.
In a second study, medical marijuana laws were associated with lower opioid prescribing rates among Medicaid enrollees.
That finding was consistent with earlier studies looking more broadly at pain prescriptions covered by Medicaid that also showed a reduction, researchers Hefei Wen, PhD, and Jason M. Hockenberry, PhD, wrote in their JAMA Internal Medicine article.
However, adult-use marijuana laws were associated with “even-lower” opioid prescribing rates, something that had not been investigated previously, according to Dr. Wen, who is with the University of Kentucky, Lexington, and Dr. Hockenberry of Emory University, Atlanta.
“Medical and adult-use marijuana laws have the potential to lower opioid prescribing for Medicaid enrollees, a high-risk population for chronic pain, opioid use disorder, and opioid overdose,” Dr. Wen and Dr. Hockenberry wrote in their report on the study, a cross-sectional analysis including all Medicaid fee-for-service and managed care enrollees during 2011-2016.
The rate of opioid prescribing in the study was –5.88% lower (95% confidence interval, –11.55% to approximately –0.21%) in association with medical marijuana laws, and –6.38% lower (95% CI, –12.20% to approximately –0.56%) for adult-use laws, they reported.
Based on those findings, policy discussions about the opioid epidemic should include the potential for liberalization of marijuana policies to reduce prescription opioid use and consequences in Medicaid enrollees, Dr. Wen and Dr. Hockenberry concluded.
However, legal marijuana alone won’t solve the opioid epidemic, they cautioned.
“As with other policies evaluated in the previous literature, marijuana liberalization is but one potential aspect of a comprehensive package to tackle the epidemic,” they said in the article.
None of the study authors reported conflicts of interest.
SOURCES: Bradford AC et al. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0266; Wen H, Hockenberry JM. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.1007.
FROM JAMA INTERNAL MEDICINE
Key clinical point: State laws governing medical and adult use of marijuana may lower prescription opioid use in Medicaid enrollees – a population at high risk for chronic pain and opioid overdose – and in the Medicare Part D population.
Major finding: Medical and adult-use marijuana laws were associated with lower opioid prescribing rates in Medicaid prescription data (–5.88% and –6.38%, respectively). Medical marijuana laws were associated with a decrease of 2.11 million daily doses yearly from an average of 23.08 million doses yearly in the Medicare Part D population.
Study details: A cross-sectional, quasiexperimental study of opioid prescribing trends between 2011 and 2016 for all Medicaid fee-for-service and managed care enrollees, and a longitudinal analysis daily opioids doses filled in Medicare Part D from 2010 through 2015.
Disclosures: None of the study authors reported conflicts of interest.
Sources: Bradford AC et al. JAMA Intern Med. 2018 Apr 2. doi:10.1001/jamainternmed.2018.0266; Wen H, Hockenberry JM. JAMA Intern Med. 2018 Apr 2. doi:10.1001/jamainternmed.2018.1007.
Oncologist-led BRCA mutation testing and counseling may reduce wait times for women with ovarian cancer
For women with ovarian cancer, an oncologist-led BRCA1/2 (BRCAm) counseling process is associated with favorable waiting times for test results and high levels of satisfaction, according to results of a prospective observational study.
The median turnaround time from initial counseling to receiving a test result was 9.1 weeks, investigators reported in the Journal of Clinical Oncology.
“Following a pathway similar to the one used in this study could allow faster treatment decisions and better use of resources in the management of patients with ovarian cancer,” said lead author Nicoletta Colombo, MD, of European Institute of Oncology, University of Milan-Bicocca, Italy, and her associates.
Establishing an ovarian cancer patient’s BRCAm status provides useful prognostic information and helps identify patients most likely to benefit from therapy with poly(ADP-ribose) polymerase (PARP) inhibitors, Dr. Colombo and her colleagues wrote.
However, despite guideline recommendations, many patients with an ovarian cancer diagnosis are currently not receiving BRCAm testing, they added.
“Given the high volume of BRCAm tests now being ordered, a new, more streamlined testing approach is needed to shorten testing turnaround times and to ease the pressure on genetic counselors,” the authors said.
In a pilot study from the United Kingdom, a streamlined, oncologist-led BRCAm testing model reduced a 20-week average turnaround time by fourfold, Dr. Colombo and her colleagues said.
Accordingly, the prospective, observational ENGAGE study sought to evaluate a streamlined oncologist-led BRCAm testing pathway in 700 patients with ovarian cancer at 26 sites in the United States, Spain, and Italy.
Oncologists and oncology nurses involved in the study received training on pretest genetic counseling techniques and on how to discuss the role of BRCAm testing with patients, according to the study description. Patients with a positive test were recommended for an appointment with a geneticist or genetic counselor.
The median time from initial counseling to receiving a test result was 9.1 weeks, the investigators reported. For patients in the United States, that median turnaround time was 4.1 weeks, while turnaround times in Spain and Italy were 12.0 and 20.4 weeks, respectively.
“BRCAm testing usually occurred shortly after the initial oncology team counseling, whereas the average time from patient consent to BRCAm testing was expected to be more than 1 month in approximately 25% of patients using standard procedures,” the investigators said in their report.
More than 99% of patients expressed satisfaction with the oncologist-led testing pathway, Dr. Colombo and her associates said. In addition, more than 80% of oncologists said the testing worked well and that counseling was an efficient use of their time.
Geneticists and genetic counselors showed less enthusiasm for the oncologist-led approach, according to investigators.
Less than half of surveyed geneticists or genetic counselors felt that patients received accurate information about the BRCAm test in the pretest counseling session, according to the report.
“It should be noted that the purpose of the oncologist-led pretest counseling was to provide enough information on why the patient should have the test, rather than full genetic counseling, which is appropriate once the test result is known,” investigators said in the report.
The study was supported by AstraZeneca. Dr. Colombo and her associates reported potential conflicts of interest related to AstraZeneca, Genentech, PharmaMar, Amgen, Clovis Oncology, Pfizer, MSD, Tesaro, and others.
SOURCE: Colombo N et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.76.278.
For women with ovarian cancer, an oncologist-led BRCA1/2 (BRCAm) counseling process is associated with favorable waiting times for test results and high levels of satisfaction, according to results of a prospective observational study.
The median turnaround time from initial counseling to receiving a test result was 9.1 weeks, investigators reported in the Journal of Clinical Oncology.
“Following a pathway similar to the one used in this study could allow faster treatment decisions and better use of resources in the management of patients with ovarian cancer,” said lead author Nicoletta Colombo, MD, of European Institute of Oncology, University of Milan-Bicocca, Italy, and her associates.
Establishing an ovarian cancer patient’s BRCAm status provides useful prognostic information and helps identify patients most likely to benefit from therapy with poly(ADP-ribose) polymerase (PARP) inhibitors, Dr. Colombo and her colleagues wrote.
However, despite guideline recommendations, many patients with an ovarian cancer diagnosis are currently not receiving BRCAm testing, they added.
“Given the high volume of BRCAm tests now being ordered, a new, more streamlined testing approach is needed to shorten testing turnaround times and to ease the pressure on genetic counselors,” the authors said.
In a pilot study from the United Kingdom, a streamlined, oncologist-led BRCAm testing model reduced a 20-week average turnaround time by fourfold, Dr. Colombo and her colleagues said.
Accordingly, the prospective, observational ENGAGE study sought to evaluate a streamlined oncologist-led BRCAm testing pathway in 700 patients with ovarian cancer at 26 sites in the United States, Spain, and Italy.
Oncologists and oncology nurses involved in the study received training on pretest genetic counseling techniques and on how to discuss the role of BRCAm testing with patients, according to the study description. Patients with a positive test were recommended for an appointment with a geneticist or genetic counselor.
The median time from initial counseling to receiving a test result was 9.1 weeks, the investigators reported. For patients in the United States, that median turnaround time was 4.1 weeks, while turnaround times in Spain and Italy were 12.0 and 20.4 weeks, respectively.
“BRCAm testing usually occurred shortly after the initial oncology team counseling, whereas the average time from patient consent to BRCAm testing was expected to be more than 1 month in approximately 25% of patients using standard procedures,” the investigators said in their report.
More than 99% of patients expressed satisfaction with the oncologist-led testing pathway, Dr. Colombo and her associates said. In addition, more than 80% of oncologists said the testing worked well and that counseling was an efficient use of their time.
Geneticists and genetic counselors showed less enthusiasm for the oncologist-led approach, according to investigators.
Less than half of surveyed geneticists or genetic counselors felt that patients received accurate information about the BRCAm test in the pretest counseling session, according to the report.
“It should be noted that the purpose of the oncologist-led pretest counseling was to provide enough information on why the patient should have the test, rather than full genetic counseling, which is appropriate once the test result is known,” investigators said in the report.
The study was supported by AstraZeneca. Dr. Colombo and her associates reported potential conflicts of interest related to AstraZeneca, Genentech, PharmaMar, Amgen, Clovis Oncology, Pfizer, MSD, Tesaro, and others.
SOURCE: Colombo N et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.76.278.
For women with ovarian cancer, an oncologist-led BRCA1/2 (BRCAm) counseling process is associated with favorable waiting times for test results and high levels of satisfaction, according to results of a prospective observational study.
The median turnaround time from initial counseling to receiving a test result was 9.1 weeks, investigators reported in the Journal of Clinical Oncology.
“Following a pathway similar to the one used in this study could allow faster treatment decisions and better use of resources in the management of patients with ovarian cancer,” said lead author Nicoletta Colombo, MD, of European Institute of Oncology, University of Milan-Bicocca, Italy, and her associates.
Establishing an ovarian cancer patient’s BRCAm status provides useful prognostic information and helps identify patients most likely to benefit from therapy with poly(ADP-ribose) polymerase (PARP) inhibitors, Dr. Colombo and her colleagues wrote.
However, despite guideline recommendations, many patients with an ovarian cancer diagnosis are currently not receiving BRCAm testing, they added.
“Given the high volume of BRCAm tests now being ordered, a new, more streamlined testing approach is needed to shorten testing turnaround times and to ease the pressure on genetic counselors,” the authors said.
In a pilot study from the United Kingdom, a streamlined, oncologist-led BRCAm testing model reduced a 20-week average turnaround time by fourfold, Dr. Colombo and her colleagues said.
Accordingly, the prospective, observational ENGAGE study sought to evaluate a streamlined oncologist-led BRCAm testing pathway in 700 patients with ovarian cancer at 26 sites in the United States, Spain, and Italy.
Oncologists and oncology nurses involved in the study received training on pretest genetic counseling techniques and on how to discuss the role of BRCAm testing with patients, according to the study description. Patients with a positive test were recommended for an appointment with a geneticist or genetic counselor.
The median time from initial counseling to receiving a test result was 9.1 weeks, the investigators reported. For patients in the United States, that median turnaround time was 4.1 weeks, while turnaround times in Spain and Italy were 12.0 and 20.4 weeks, respectively.
“BRCAm testing usually occurred shortly after the initial oncology team counseling, whereas the average time from patient consent to BRCAm testing was expected to be more than 1 month in approximately 25% of patients using standard procedures,” the investigators said in their report.
More than 99% of patients expressed satisfaction with the oncologist-led testing pathway, Dr. Colombo and her associates said. In addition, more than 80% of oncologists said the testing worked well and that counseling was an efficient use of their time.
Geneticists and genetic counselors showed less enthusiasm for the oncologist-led approach, according to investigators.
Less than half of surveyed geneticists or genetic counselors felt that patients received accurate information about the BRCAm test in the pretest counseling session, according to the report.
“It should be noted that the purpose of the oncologist-led pretest counseling was to provide enough information on why the patient should have the test, rather than full genetic counseling, which is appropriate once the test result is known,” investigators said in the report.
The study was supported by AstraZeneca. Dr. Colombo and her associates reported potential conflicts of interest related to AstraZeneca, Genentech, PharmaMar, Amgen, Clovis Oncology, Pfizer, MSD, Tesaro, and others.
SOURCE: Colombo N et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.76.278.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: An oncologist-led BRCA1/2 (BRCAm) counseling process is associated with favorable waiting times for test results and high levels of satisfaction among women with ovarian cancer.
Major finding: The median turnaround time from initial counseling to receiving a test result was 9.1 weeks.
Study details: The prospective, observational ENGAGE study evaluating a streamlined oncologist-led BRCAm testing pathway in 700 patients with ovarian cancer at 26 sites in the United States, Spain, and Italy.
Disclosures: The study was supported by AstraZeneca. Study authors reported potential conflicts of interest related to AstraZeneca, Genentech, PharmaMar, Amgen, Clovis Oncology, Pfizer, MSD, Tesaro, and others.
Source: Colombo N et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.76.278.
Ibrutinib plus venetoclax is active in mantle cell lymphoma
In mantle cell lymphoma (MCL), ibrutinib plus venetoclax significantly improved the complete response rate, compared with what has been previously reported for ibrutinib alone, according to results of a phase 2 study.
Clinical outcomes with the combination seem superior to previously reported results for either treatment alone, said lead investigator Constantine S. Tam, MBBS, MD, of the Peter MacCallum Cancer Centre, Melbourne, and his coinvestigators.
“The results of our study, which used a historical cohort as a control, are consistent with the notion that the combination of ibrutinib and venetoclax is highly effective in mantle-cell lymphoma,” the investigators wrote in the New England Journal of Medicine.
The BTK inhibitor ibrutinib and the BCL2 inhibitor venetoclax are two of the most active agents for this B-cell cancer, investigators reported. The rationale for combining the agents is “compelling” because they affect different critical pathways in the malignant B cell.
Both agents have demonstrated complete response rates of 21% in previous studies of relapsed or refractory MCL, and preclinical studies suggest the combination of ibrutinib and venetoclax would be synergistic.
In the present single-group, phase 2 study, 24 patients with MCL (23 relapsed or refractory, 1 previously untreated) started ibrutinib 560 mg daily; at 4 weeks, venetoclax was started at a low dose and increased to 400 mg daily.
The study primary end point – complete response rate at week 16 assessed by CT – was 42%, compared with 9% for ibrutinib monotherapy in the phase 2 PCYC-1104-CA study (P less than .001).
Computed tomography assessment was used for the primary end point to allow comparison to the ibrutinib monotherapy study, which did not use positron emission tomography for restaging. “Our study was designed to have 80% power to reject a complete response rate of 9% (at a one-sided alpha level of 0.05) if the rate of complete response was at least 30%,” the investigators noted.
Complete response rate assessed by positron emission tomography at week 16 was 62%, and was 71% overall.
In all, 67% of patients had absence of minimal residual disease by flow cytometry. At 15 months, 78% of the responses were ongoing, and at 18 months, 57% of patients were alive and progression free.
“Such outcomes appear to be substantially better than those that have been reported for ibrutinib or venetoclax monotherapy,” the investigators wrote.
The combination had side effects that are “acceptable to both patients and physicians,” investigators wrote. Side effects, usually low grade, included diarrhea in 83% of patients, fatigue in 75%, and nausea or vomiting in 71%. Tumor lysis syndrome was seen in two patients.
Whether ibrutinib plus venetoclax is superior to ibrutinib alone is being formally evaluated in an ongoing phase 3 study.
Janssen and AbbVie partially funded the current phase 2 study. Dr. Tam reported financial ties to Janssen, AbbVie, and Pharmacyclics. Other study authors reported financial ties to various pharmaceutical companies.
SOURCE: Tam C et al. N Engl J Med. 2018;378:1211-23.
In mantle cell lymphoma (MCL), ibrutinib plus venetoclax significantly improved the complete response rate, compared with what has been previously reported for ibrutinib alone, according to results of a phase 2 study.
Clinical outcomes with the combination seem superior to previously reported results for either treatment alone, said lead investigator Constantine S. Tam, MBBS, MD, of the Peter MacCallum Cancer Centre, Melbourne, and his coinvestigators.
“The results of our study, which used a historical cohort as a control, are consistent with the notion that the combination of ibrutinib and venetoclax is highly effective in mantle-cell lymphoma,” the investigators wrote in the New England Journal of Medicine.
The BTK inhibitor ibrutinib and the BCL2 inhibitor venetoclax are two of the most active agents for this B-cell cancer, investigators reported. The rationale for combining the agents is “compelling” because they affect different critical pathways in the malignant B cell.
Both agents have demonstrated complete response rates of 21% in previous studies of relapsed or refractory MCL, and preclinical studies suggest the combination of ibrutinib and venetoclax would be synergistic.
In the present single-group, phase 2 study, 24 patients with MCL (23 relapsed or refractory, 1 previously untreated) started ibrutinib 560 mg daily; at 4 weeks, venetoclax was started at a low dose and increased to 400 mg daily.
The study primary end point – complete response rate at week 16 assessed by CT – was 42%, compared with 9% for ibrutinib monotherapy in the phase 2 PCYC-1104-CA study (P less than .001).
Computed tomography assessment was used for the primary end point to allow comparison to the ibrutinib monotherapy study, which did not use positron emission tomography for restaging. “Our study was designed to have 80% power to reject a complete response rate of 9% (at a one-sided alpha level of 0.05) if the rate of complete response was at least 30%,” the investigators noted.
Complete response rate assessed by positron emission tomography at week 16 was 62%, and was 71% overall.
In all, 67% of patients had absence of minimal residual disease by flow cytometry. At 15 months, 78% of the responses were ongoing, and at 18 months, 57% of patients were alive and progression free.
“Such outcomes appear to be substantially better than those that have been reported for ibrutinib or venetoclax monotherapy,” the investigators wrote.
The combination had side effects that are “acceptable to both patients and physicians,” investigators wrote. Side effects, usually low grade, included diarrhea in 83% of patients, fatigue in 75%, and nausea or vomiting in 71%. Tumor lysis syndrome was seen in two patients.
Whether ibrutinib plus venetoclax is superior to ibrutinib alone is being formally evaluated in an ongoing phase 3 study.
Janssen and AbbVie partially funded the current phase 2 study. Dr. Tam reported financial ties to Janssen, AbbVie, and Pharmacyclics. Other study authors reported financial ties to various pharmaceutical companies.
SOURCE: Tam C et al. N Engl J Med. 2018;378:1211-23.
In mantle cell lymphoma (MCL), ibrutinib plus venetoclax significantly improved the complete response rate, compared with what has been previously reported for ibrutinib alone, according to results of a phase 2 study.
Clinical outcomes with the combination seem superior to previously reported results for either treatment alone, said lead investigator Constantine S. Tam, MBBS, MD, of the Peter MacCallum Cancer Centre, Melbourne, and his coinvestigators.
“The results of our study, which used a historical cohort as a control, are consistent with the notion that the combination of ibrutinib and venetoclax is highly effective in mantle-cell lymphoma,” the investigators wrote in the New England Journal of Medicine.
The BTK inhibitor ibrutinib and the BCL2 inhibitor venetoclax are two of the most active agents for this B-cell cancer, investigators reported. The rationale for combining the agents is “compelling” because they affect different critical pathways in the malignant B cell.
Both agents have demonstrated complete response rates of 21% in previous studies of relapsed or refractory MCL, and preclinical studies suggest the combination of ibrutinib and venetoclax would be synergistic.
In the present single-group, phase 2 study, 24 patients with MCL (23 relapsed or refractory, 1 previously untreated) started ibrutinib 560 mg daily; at 4 weeks, venetoclax was started at a low dose and increased to 400 mg daily.
The study primary end point – complete response rate at week 16 assessed by CT – was 42%, compared with 9% for ibrutinib monotherapy in the phase 2 PCYC-1104-CA study (P less than .001).
Computed tomography assessment was used for the primary end point to allow comparison to the ibrutinib monotherapy study, which did not use positron emission tomography for restaging. “Our study was designed to have 80% power to reject a complete response rate of 9% (at a one-sided alpha level of 0.05) if the rate of complete response was at least 30%,” the investigators noted.
Complete response rate assessed by positron emission tomography at week 16 was 62%, and was 71% overall.
In all, 67% of patients had absence of minimal residual disease by flow cytometry. At 15 months, 78% of the responses were ongoing, and at 18 months, 57% of patients were alive and progression free.
“Such outcomes appear to be substantially better than those that have been reported for ibrutinib or venetoclax monotherapy,” the investigators wrote.
The combination had side effects that are “acceptable to both patients and physicians,” investigators wrote. Side effects, usually low grade, included diarrhea in 83% of patients, fatigue in 75%, and nausea or vomiting in 71%. Tumor lysis syndrome was seen in two patients.
Whether ibrutinib plus venetoclax is superior to ibrutinib alone is being formally evaluated in an ongoing phase 3 study.
Janssen and AbbVie partially funded the current phase 2 study. Dr. Tam reported financial ties to Janssen, AbbVie, and Pharmacyclics. Other study authors reported financial ties to various pharmaceutical companies.
SOURCE: Tam C et al. N Engl J Med. 2018;378:1211-23.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Dual targeting of BTK and BCL2 with ibrutinib and venetoclax may improve complete response rate versus ibrutinib alone in patients with mantle cell lymphoma.
Major finding: Complete response rate at week 16 as assessed by CT was 42%, compared with 9% with ibrutinib monotherapy in a previous study (P less than .001).
Study details: A single-group phase 2 study of daily oral ibrutinib and venetoclax in 24 patients with mantle cell lymphoma (23 relapsed or refractory, 1 previously untreated), as compared with historical controls.
Disclosures: Janssen and AbbVie partially funded the study. Dr. Tam reported financial ties to Janssen, Abbvie, and Pharmacyclics. Other study authors reported financial ties to various pharmaceutical companies.
Source: Tam C et al. N Engl J Med. 2018;378:1211-23.
Frankincense extract may reduce disease activity in relapsing-remitting MS
A standardized frankincense extract was safe, well tolerated, and potentially efficacious as an oral treatment in patients with relapsing-remitting multiple sclerosis (RRMS), according to results of a small, nonrandomized study.
Patients receiving the herbal treatment had significantly fewer contrast-enhancing lesions on MRI versus baseline in the study, which was published in the Journal of Neurology, Neurosurgery & Psychiatry.
“Despite our encouraging results, it is difficult to forecast the efficacy of a standardized frankincense extract in RRMS,” Dr. Stürner and her colleagues wrote in their report on the study.
Boswellic acids, believed to be the active compound in frankincense, has been used as an anti-inflammatory substance for thousands of years in Eastern medicine, according to the study authors.
Frankincense extracts were safe and well tolerated in several small, randomized trials including patients with inflammatory or autoimmune diseases, they noted.
Dr. Stürner and her colleagues tested a standardized frankincense extract in the SABA phase 2a trial, an investigator-initiated, open-label, pilot study including 38 patients with RRMS.
Patients underwent observation for 4 months, then were treated with the extract for up to 8 months plus an optional extension phase of up to 36 months. A total of 28 patients completed the initial treatment period, and 18 participated in the extension period, according to the study results.
The median number of monthly contrast-enhancing lesions was significantly reduced from 1.00 at baseline to 0.50 during the initial treatment period (P less than .0001). In addition, significantly less brain atrophy was noted after the treatment phase as compared with the baseline observation phase (P = .0081).
Adverse events, mainly infections or gastrointestinal symptoms, were mild (57.7%) or moderate (38.6%), investigators added.
Treatment significantly increased regulatory CD4-positive T cell markers and decreased interleukin-17A–producing CD8-positive T cells, according to results of mechanistic studies that were also reported.
Other anti-inflammatory drugs that have been licensed act broadly on the immune system, according to investigators, and so those agents require careful monitoring for side effects.
“The right balance between efficacy and safety profile becomes increasingly more important for a young patient population, who require long-term treatment,” Dr. Stürner and her coauthors wrote. “The results of the SABA trial are promising from this perspective.”
The study was funded in part by Alpinia Laudanum Institute, which supplied the standardized frankincense extract at no charge. Individual authors reported competing interests with Alpinia Laudanum, Biogen, Sanofi Genzyme, Novartis, Merck Serono, and others.
SOURCE: Stürner KH et al. J Neurol Neurosurg Psychiatry. 2018 Apr;89(4):330-8.
Results of the SABA phase 2a trial suggest that frankincense could be a new therapeutic agent for mildly disabled young patients with RRMS who require long-term treatment, according to Dr. Francesco Patti.
In the study, administration of standardized oral frankincense extract significantly reduced the median number and volume of contrast-enhancing lesions and the number of new T2 lesions. The treatment also increased brain parenchymal volume and reduced the annualized relapse rate. While disability scores remained unchanged, measures of function and quality of life improved.
The treatment effects also appeared to be durable, based on the extension phase results.
Blood and immunologic findings suggested that the treatment was not toxic and that it exerted immunomodulatory activity by reduction of IL-17–producing CD8-positive T cells, as well as anti-inflammatory properties through inhibitory effects on 5-lipo-oxygenase, microsomial prostaglandin E2 synthase-1, LL-37, and nuclear factor-kB activities.
“This mechanism of action, attributing a role of these enzymes in neuroinflammation, might offer a new therapeutic approach,” he concluded in his editorial.
Francesco Patti, MD , is with the Multiple Sclerosis Hub Center, University of Catania (Italy). These comments are derived from his editorial ( J Neurol Neurosurg Psychiatry. 2018;89:327 ). Dr. Patti declared no competing interests related to the editorial.
Results of the SABA phase 2a trial suggest that frankincense could be a new therapeutic agent for mildly disabled young patients with RRMS who require long-term treatment, according to Dr. Francesco Patti.
In the study, administration of standardized oral frankincense extract significantly reduced the median number and volume of contrast-enhancing lesions and the number of new T2 lesions. The treatment also increased brain parenchymal volume and reduced the annualized relapse rate. While disability scores remained unchanged, measures of function and quality of life improved.
The treatment effects also appeared to be durable, based on the extension phase results.
Blood and immunologic findings suggested that the treatment was not toxic and that it exerted immunomodulatory activity by reduction of IL-17–producing CD8-positive T cells, as well as anti-inflammatory properties through inhibitory effects on 5-lipo-oxygenase, microsomial prostaglandin E2 synthase-1, LL-37, and nuclear factor-kB activities.
“This mechanism of action, attributing a role of these enzymes in neuroinflammation, might offer a new therapeutic approach,” he concluded in his editorial.
Francesco Patti, MD , is with the Multiple Sclerosis Hub Center, University of Catania (Italy). These comments are derived from his editorial ( J Neurol Neurosurg Psychiatry. 2018;89:327 ). Dr. Patti declared no competing interests related to the editorial.
Results of the SABA phase 2a trial suggest that frankincense could be a new therapeutic agent for mildly disabled young patients with RRMS who require long-term treatment, according to Dr. Francesco Patti.
In the study, administration of standardized oral frankincense extract significantly reduced the median number and volume of contrast-enhancing lesions and the number of new T2 lesions. The treatment also increased brain parenchymal volume and reduced the annualized relapse rate. While disability scores remained unchanged, measures of function and quality of life improved.
The treatment effects also appeared to be durable, based on the extension phase results.
Blood and immunologic findings suggested that the treatment was not toxic and that it exerted immunomodulatory activity by reduction of IL-17–producing CD8-positive T cells, as well as anti-inflammatory properties through inhibitory effects on 5-lipo-oxygenase, microsomial prostaglandin E2 synthase-1, LL-37, and nuclear factor-kB activities.
“This mechanism of action, attributing a role of these enzymes in neuroinflammation, might offer a new therapeutic approach,” he concluded in his editorial.
Francesco Patti, MD , is with the Multiple Sclerosis Hub Center, University of Catania (Italy). These comments are derived from his editorial ( J Neurol Neurosurg Psychiatry. 2018;89:327 ). Dr. Patti declared no competing interests related to the editorial.
A standardized frankincense extract was safe, well tolerated, and potentially efficacious as an oral treatment in patients with relapsing-remitting multiple sclerosis (RRMS), according to results of a small, nonrandomized study.
Patients receiving the herbal treatment had significantly fewer contrast-enhancing lesions on MRI versus baseline in the study, which was published in the Journal of Neurology, Neurosurgery & Psychiatry.
“Despite our encouraging results, it is difficult to forecast the efficacy of a standardized frankincense extract in RRMS,” Dr. Stürner and her colleagues wrote in their report on the study.
Boswellic acids, believed to be the active compound in frankincense, has been used as an anti-inflammatory substance for thousands of years in Eastern medicine, according to the study authors.
Frankincense extracts were safe and well tolerated in several small, randomized trials including patients with inflammatory or autoimmune diseases, they noted.
Dr. Stürner and her colleagues tested a standardized frankincense extract in the SABA phase 2a trial, an investigator-initiated, open-label, pilot study including 38 patients with RRMS.
Patients underwent observation for 4 months, then were treated with the extract for up to 8 months plus an optional extension phase of up to 36 months. A total of 28 patients completed the initial treatment period, and 18 participated in the extension period, according to the study results.
The median number of monthly contrast-enhancing lesions was significantly reduced from 1.00 at baseline to 0.50 during the initial treatment period (P less than .0001). In addition, significantly less brain atrophy was noted after the treatment phase as compared with the baseline observation phase (P = .0081).
Adverse events, mainly infections or gastrointestinal symptoms, were mild (57.7%) or moderate (38.6%), investigators added.
Treatment significantly increased regulatory CD4-positive T cell markers and decreased interleukin-17A–producing CD8-positive T cells, according to results of mechanistic studies that were also reported.
Other anti-inflammatory drugs that have been licensed act broadly on the immune system, according to investigators, and so those agents require careful monitoring for side effects.
“The right balance between efficacy and safety profile becomes increasingly more important for a young patient population, who require long-term treatment,” Dr. Stürner and her coauthors wrote. “The results of the SABA trial are promising from this perspective.”
The study was funded in part by Alpinia Laudanum Institute, which supplied the standardized frankincense extract at no charge. Individual authors reported competing interests with Alpinia Laudanum, Biogen, Sanofi Genzyme, Novartis, Merck Serono, and others.
SOURCE: Stürner KH et al. J Neurol Neurosurg Psychiatry. 2018 Apr;89(4):330-8.
A standardized frankincense extract was safe, well tolerated, and potentially efficacious as an oral treatment in patients with relapsing-remitting multiple sclerosis (RRMS), according to results of a small, nonrandomized study.
Patients receiving the herbal treatment had significantly fewer contrast-enhancing lesions on MRI versus baseline in the study, which was published in the Journal of Neurology, Neurosurgery & Psychiatry.
“Despite our encouraging results, it is difficult to forecast the efficacy of a standardized frankincense extract in RRMS,” Dr. Stürner and her colleagues wrote in their report on the study.
Boswellic acids, believed to be the active compound in frankincense, has been used as an anti-inflammatory substance for thousands of years in Eastern medicine, according to the study authors.
Frankincense extracts were safe and well tolerated in several small, randomized trials including patients with inflammatory or autoimmune diseases, they noted.
Dr. Stürner and her colleagues tested a standardized frankincense extract in the SABA phase 2a trial, an investigator-initiated, open-label, pilot study including 38 patients with RRMS.
Patients underwent observation for 4 months, then were treated with the extract for up to 8 months plus an optional extension phase of up to 36 months. A total of 28 patients completed the initial treatment period, and 18 participated in the extension period, according to the study results.
The median number of monthly contrast-enhancing lesions was significantly reduced from 1.00 at baseline to 0.50 during the initial treatment period (P less than .0001). In addition, significantly less brain atrophy was noted after the treatment phase as compared with the baseline observation phase (P = .0081).
Adverse events, mainly infections or gastrointestinal symptoms, were mild (57.7%) or moderate (38.6%), investigators added.
Treatment significantly increased regulatory CD4-positive T cell markers and decreased interleukin-17A–producing CD8-positive T cells, according to results of mechanistic studies that were also reported.
Other anti-inflammatory drugs that have been licensed act broadly on the immune system, according to investigators, and so those agents require careful monitoring for side effects.
“The right balance between efficacy and safety profile becomes increasingly more important for a young patient population, who require long-term treatment,” Dr. Stürner and her coauthors wrote. “The results of the SABA trial are promising from this perspective.”
The study was funded in part by Alpinia Laudanum Institute, which supplied the standardized frankincense extract at no charge. Individual authors reported competing interests with Alpinia Laudanum, Biogen, Sanofi Genzyme, Novartis, Merck Serono, and others.
SOURCE: Stürner KH et al. J Neurol Neurosurg Psychiatry. 2018 Apr;89(4):330-8.
FROM Journal of Neurology, Neurosurgery & Psychiatry
Key clinical point:
Major finding: The median number of monthly contrast-enhancing lesions was significantly reduced from 1.00 at baseline to 0.50 on treatment (P less than .0001).
Study details: The SABA phase 2a trial, an investigator-initiated, open-label, pilot study including 38 patients with RRMS.
Disclosures: The study was funded in part by Alpinia Laudanum Institute, which supplied the standardized frankincense extract at no charge. Individual authors reported competing interests with Alpinia Laudanum, Biogen, Sanofi Genzyme, Novartis, Merck Serono, and others.
Source: Stürner KH et al. J Neurol Neurosurg Psychiatry. 2018;89:330-8.
High-dose oral ibuprofen most effective for PDA closure
compared with other pharmacological treatments, according to results of a recent systematic review and meta-analysis.
However, using placebo or no treatment at all did not increase the odds of mortality, necrotizing enterocolitis, or intraventricular hemorrhage in the study, published in JAMA.
PDA is a common cardiovascular issue among prematurely born infants. According to Dr. Mitra and his coauthors, it’s thought that a large proportion of PDAs spontaneously close in a few days and have minimal effect on clinical outcomes.
As a result, treatment is often targeted to PDAs deemed hemodynamically significant based on clinical and echocardiographic parameters, the authors wrote, although there is little guidance on what, if any, treatment to use in this situation.
“The dilemma is whether to use pharmacotherapy at all, and if a decision is made to treat the PDA medically, what should be the ideal choice of pharmacotherapy,” they wrote.
Dr. Mitra and colleagues conducted a systematic review and meta-analysis of 68 randomized clinical trials including 4,802 infants with clinically or echocardiographically diagnosed, hemodynamically significant PDA.
They found that closure of hemodynamically significant PDA was significantly more likely with high-dose oral ibuprofen, compared with the two of the most widely used treatments, namely standard-dose intravenous ibuprofen (odds ratio, 3.59) and standard-dose intravenous indomethacin (odds ratio, 2.35).
Despite that finding, there was no significant difference in the odds of mortality, necrotizing enterocolitis, or intraventricular hemorrhage for use of placebo or no treatment, compared with any of the treatment modalities, the investigators added.
“With increasing emphasis on conservative management of PDA, these results may encourage researchers to revisit placebo-controlled trials against newer pharmacotherapeutic options,” they said.
Study authors reported no relevant potential conflicts of interest.
SOURCE: Mitra S et al. JAMA. 2018;319(12):1221-38.
compared with other pharmacological treatments, according to results of a recent systematic review and meta-analysis.
However, using placebo or no treatment at all did not increase the odds of mortality, necrotizing enterocolitis, or intraventricular hemorrhage in the study, published in JAMA.
PDA is a common cardiovascular issue among prematurely born infants. According to Dr. Mitra and his coauthors, it’s thought that a large proportion of PDAs spontaneously close in a few days and have minimal effect on clinical outcomes.
As a result, treatment is often targeted to PDAs deemed hemodynamically significant based on clinical and echocardiographic parameters, the authors wrote, although there is little guidance on what, if any, treatment to use in this situation.
“The dilemma is whether to use pharmacotherapy at all, and if a decision is made to treat the PDA medically, what should be the ideal choice of pharmacotherapy,” they wrote.
Dr. Mitra and colleagues conducted a systematic review and meta-analysis of 68 randomized clinical trials including 4,802 infants with clinically or echocardiographically diagnosed, hemodynamically significant PDA.
They found that closure of hemodynamically significant PDA was significantly more likely with high-dose oral ibuprofen, compared with the two of the most widely used treatments, namely standard-dose intravenous ibuprofen (odds ratio, 3.59) and standard-dose intravenous indomethacin (odds ratio, 2.35).
Despite that finding, there was no significant difference in the odds of mortality, necrotizing enterocolitis, or intraventricular hemorrhage for use of placebo or no treatment, compared with any of the treatment modalities, the investigators added.
“With increasing emphasis on conservative management of PDA, these results may encourage researchers to revisit placebo-controlled trials against newer pharmacotherapeutic options,” they said.
Study authors reported no relevant potential conflicts of interest.
SOURCE: Mitra S et al. JAMA. 2018;319(12):1221-38.
compared with other pharmacological treatments, according to results of a recent systematic review and meta-analysis.
However, using placebo or no treatment at all did not increase the odds of mortality, necrotizing enterocolitis, or intraventricular hemorrhage in the study, published in JAMA.
PDA is a common cardiovascular issue among prematurely born infants. According to Dr. Mitra and his coauthors, it’s thought that a large proportion of PDAs spontaneously close in a few days and have minimal effect on clinical outcomes.
As a result, treatment is often targeted to PDAs deemed hemodynamically significant based on clinical and echocardiographic parameters, the authors wrote, although there is little guidance on what, if any, treatment to use in this situation.
“The dilemma is whether to use pharmacotherapy at all, and if a decision is made to treat the PDA medically, what should be the ideal choice of pharmacotherapy,” they wrote.
Dr. Mitra and colleagues conducted a systematic review and meta-analysis of 68 randomized clinical trials including 4,802 infants with clinically or echocardiographically diagnosed, hemodynamically significant PDA.
They found that closure of hemodynamically significant PDA was significantly more likely with high-dose oral ibuprofen, compared with the two of the most widely used treatments, namely standard-dose intravenous ibuprofen (odds ratio, 3.59) and standard-dose intravenous indomethacin (odds ratio, 2.35).
Despite that finding, there was no significant difference in the odds of mortality, necrotizing enterocolitis, or intraventricular hemorrhage for use of placebo or no treatment, compared with any of the treatment modalities, the investigators added.
“With increasing emphasis on conservative management of PDA, these results may encourage researchers to revisit placebo-controlled trials against newer pharmacotherapeutic options,” they said.
Study authors reported no relevant potential conflicts of interest.
SOURCE: Mitra S et al. JAMA. 2018;319(12):1221-38.
FROM JAMA
Key clinical point: Compared with other pharmacological treatments, high-dose oral ibuprofen may be most likely to result in closure of hemodynamically significant PDA in preterm infants.
Major finding: Closure of hemodynamically significant PDA was significantly more likely with high-dose oral ibuprofen, compared with standard-dose intravenous ibuprofen (odds ratio, 3.59) and intravenous indomethacin (odds ratio, 2.35).
Study details: A systematic review and meta-analysis of 68 randomized clinical trials including 4,802 infants with clinically or echocardiographically diagnosed, hemodynamically significant PDA.
Disclosures: Study authors reported no relevant potential conflicts of interest.
Source: Mitra S et al. JAMA. 2018;319(12):1221-38.