User login
Pamidronate Deemed Kidney-Safe in Chronic Critical Illness
HOUSTON – Intravenous pamidronate for management of bone hyperresorption in ventilator-dependent patients doesn’t adversely affect renal function, even in those with chronic kidney disease, according to a single-center retrospective study.
"This stands in contrast to prevailing concerns about bisphosphonate therapy to manage bone hyperresorption in chronically critically ill patients, many of whom have chronic kidney disease," Dr. Rifka C. Schulman reported at the annual meeting of the Endocrine Society.
"It is hoped that these results will remove barriers to more aggressive management of metabolic bone disease in chronic critical illness, which may have salutary downstream effects on morbidity and mortality," declared Dr. Schulman of the Mount Sinai School of Medicine in New York.
She presented a retrospective observational study of 315 patients admitted to the Mount Sinai Hospital respiratory care unit with ventilator-dependent chronic critical illness after surviving a bout of acute critical illness. In all, 115 received 30-90 mg of intravenous pamidronate (Aredia) infused over 4 hours, with dosing based on body weight. The other 200 patients did not receive pamidronate. All participants got calcitriol, calcium carbonate, and ergocalciferol to help protect their bones.
The study population consisted of 204 patients with either no or stage 1-2 chronic kidney disease, 41 with stage 3 CKD, 33 with stage 4 disease, and 37 with stage 5 CKD who were on hemodialysis.
The primary study end points were change in glomerular filtration rate and creatinine level following pamidronate administration. Importantly, none of the pamidronate-treated patients showed a 25% or greater reduction in GFR immediately after or at 7 or 14 days post infusion, regardless of their CKD status or dose received.
The group with no or only mild CKD showed no change in median GFR between baseline and day 7 post infusion. Those with stage 3 CKD had a median 4% drop in GFR. So did those with stage 4 disease. Patients with stage 5 CKD had a median 9% reduction in GFR on day 7. Among controls, those with stage 0-3 CKD had no change in median GFR, while those with stage 4 or stage 5 disease averaged a 6% increase in GFR during 7 days. These small fluctuations in renal function aren’t clinically meaningful, according to Dr. Schulman.
Creatinine levels rose between baseline and day 7 in lockstep with CKD status, but to the same extent in pamidronate-treated patients and controls. For example, creatinine climbed by 6.7% and 18.2%, respectively, in pamidronate-treated patients with stage 4 and stage 5 CKD, and by 8.8% and 20.8% in stage 4 and 5 controls.
She observed that metabolic bone disease involving bone hyperresorption with elevated levels of the bone turnover biomarker N-telopeptide is present in more than 90% of patients with chronic critical illness. Contributing factors include immobilization, inflammation, neuroendocrine abnormalities, low vitamin D levels and secondary hyperparathyroidism, and the use of high-dose corticosteroids and other medications with an adverse impact on bone.
Bone loss during critical illness is challenging to reverse. It predisposes to osteoporosis, fractures, and poor quality of life in patients who recover from chronic critical illness. That’s why Dr. Schulman and her coinvestigators favor turning to pamidronate when patients have a 24-hour urine N-telopeptide of 70 nmol BCE/mmol creatinine or a serum level in excess of 40 nmol BCE/L.
The study was supported by a grant from Select Medical. Dr. Schulman reported having no financial conflicts.
HOUSTON – Intravenous pamidronate for management of bone hyperresorption in ventilator-dependent patients doesn’t adversely affect renal function, even in those with chronic kidney disease, according to a single-center retrospective study.
"This stands in contrast to prevailing concerns about bisphosphonate therapy to manage bone hyperresorption in chronically critically ill patients, many of whom have chronic kidney disease," Dr. Rifka C. Schulman reported at the annual meeting of the Endocrine Society.
"It is hoped that these results will remove barriers to more aggressive management of metabolic bone disease in chronic critical illness, which may have salutary downstream effects on morbidity and mortality," declared Dr. Schulman of the Mount Sinai School of Medicine in New York.
She presented a retrospective observational study of 315 patients admitted to the Mount Sinai Hospital respiratory care unit with ventilator-dependent chronic critical illness after surviving a bout of acute critical illness. In all, 115 received 30-90 mg of intravenous pamidronate (Aredia) infused over 4 hours, with dosing based on body weight. The other 200 patients did not receive pamidronate. All participants got calcitriol, calcium carbonate, and ergocalciferol to help protect their bones.
The study population consisted of 204 patients with either no or stage 1-2 chronic kidney disease, 41 with stage 3 CKD, 33 with stage 4 disease, and 37 with stage 5 CKD who were on hemodialysis.
The primary study end points were change in glomerular filtration rate and creatinine level following pamidronate administration. Importantly, none of the pamidronate-treated patients showed a 25% or greater reduction in GFR immediately after or at 7 or 14 days post infusion, regardless of their CKD status or dose received.
The group with no or only mild CKD showed no change in median GFR between baseline and day 7 post infusion. Those with stage 3 CKD had a median 4% drop in GFR. So did those with stage 4 disease. Patients with stage 5 CKD had a median 9% reduction in GFR on day 7. Among controls, those with stage 0-3 CKD had no change in median GFR, while those with stage 4 or stage 5 disease averaged a 6% increase in GFR during 7 days. These small fluctuations in renal function aren’t clinically meaningful, according to Dr. Schulman.
Creatinine levels rose between baseline and day 7 in lockstep with CKD status, but to the same extent in pamidronate-treated patients and controls. For example, creatinine climbed by 6.7% and 18.2%, respectively, in pamidronate-treated patients with stage 4 and stage 5 CKD, and by 8.8% and 20.8% in stage 4 and 5 controls.
She observed that metabolic bone disease involving bone hyperresorption with elevated levels of the bone turnover biomarker N-telopeptide is present in more than 90% of patients with chronic critical illness. Contributing factors include immobilization, inflammation, neuroendocrine abnormalities, low vitamin D levels and secondary hyperparathyroidism, and the use of high-dose corticosteroids and other medications with an adverse impact on bone.
Bone loss during critical illness is challenging to reverse. It predisposes to osteoporosis, fractures, and poor quality of life in patients who recover from chronic critical illness. That’s why Dr. Schulman and her coinvestigators favor turning to pamidronate when patients have a 24-hour urine N-telopeptide of 70 nmol BCE/mmol creatinine or a serum level in excess of 40 nmol BCE/L.
The study was supported by a grant from Select Medical. Dr. Schulman reported having no financial conflicts.
HOUSTON – Intravenous pamidronate for management of bone hyperresorption in ventilator-dependent patients doesn’t adversely affect renal function, even in those with chronic kidney disease, according to a single-center retrospective study.
"This stands in contrast to prevailing concerns about bisphosphonate therapy to manage bone hyperresorption in chronically critically ill patients, many of whom have chronic kidney disease," Dr. Rifka C. Schulman reported at the annual meeting of the Endocrine Society.
"It is hoped that these results will remove barriers to more aggressive management of metabolic bone disease in chronic critical illness, which may have salutary downstream effects on morbidity and mortality," declared Dr. Schulman of the Mount Sinai School of Medicine in New York.
She presented a retrospective observational study of 315 patients admitted to the Mount Sinai Hospital respiratory care unit with ventilator-dependent chronic critical illness after surviving a bout of acute critical illness. In all, 115 received 30-90 mg of intravenous pamidronate (Aredia) infused over 4 hours, with dosing based on body weight. The other 200 patients did not receive pamidronate. All participants got calcitriol, calcium carbonate, and ergocalciferol to help protect their bones.
The study population consisted of 204 patients with either no or stage 1-2 chronic kidney disease, 41 with stage 3 CKD, 33 with stage 4 disease, and 37 with stage 5 CKD who were on hemodialysis.
The primary study end points were change in glomerular filtration rate and creatinine level following pamidronate administration. Importantly, none of the pamidronate-treated patients showed a 25% or greater reduction in GFR immediately after or at 7 or 14 days post infusion, regardless of their CKD status or dose received.
The group with no or only mild CKD showed no change in median GFR between baseline and day 7 post infusion. Those with stage 3 CKD had a median 4% drop in GFR. So did those with stage 4 disease. Patients with stage 5 CKD had a median 9% reduction in GFR on day 7. Among controls, those with stage 0-3 CKD had no change in median GFR, while those with stage 4 or stage 5 disease averaged a 6% increase in GFR during 7 days. These small fluctuations in renal function aren’t clinically meaningful, according to Dr. Schulman.
Creatinine levels rose between baseline and day 7 in lockstep with CKD status, but to the same extent in pamidronate-treated patients and controls. For example, creatinine climbed by 6.7% and 18.2%, respectively, in pamidronate-treated patients with stage 4 and stage 5 CKD, and by 8.8% and 20.8% in stage 4 and 5 controls.
She observed that metabolic bone disease involving bone hyperresorption with elevated levels of the bone turnover biomarker N-telopeptide is present in more than 90% of patients with chronic critical illness. Contributing factors include immobilization, inflammation, neuroendocrine abnormalities, low vitamin D levels and secondary hyperparathyroidism, and the use of high-dose corticosteroids and other medications with an adverse impact on bone.
Bone loss during critical illness is challenging to reverse. It predisposes to osteoporosis, fractures, and poor quality of life in patients who recover from chronic critical illness. That’s why Dr. Schulman and her coinvestigators favor turning to pamidronate when patients have a 24-hour urine N-telopeptide of 70 nmol BCE/mmol creatinine or a serum level in excess of 40 nmol BCE/L.
The study was supported by a grant from Select Medical. Dr. Schulman reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: In pamidronate-treated patients with chronic critical illness and stage 4 and stage 5 chronic kidney disease, creatinine climbed by 6.7% and 18.2%, respectively. In matched controls who didn’t receive pamidronate, creatinine rose by 8.8% and 20.8%.
Data Source: This was a retrospective, observational, single-center study of 315 patients with ventilator-dependent chronic critical illness, 200 of whom received intravenous pamidronate.
Disclosures: The study was supported by a grant from Select Medical. Dr. Schulman reported having no financial conflicts.
Traumatic Brain Injury Linked to GH Deficiency
HOUSTON – Growth hormone deficiency appears to be common among military veterans with mild traumatic brain injury sustained in combat, according to the first study to look at the issue.
Future studies will attempt to confirm the new finding that GH deficiency in the setting of traumatic brain injury (TBI) appears to be associated with specific neuropsychologic abnormalities, and, further, whether GH replacement therapy in affected veterans enhances TBI rehabilitation efforts, according to Dr. Adriana G. Ioachimescu of Emory University, Atlanta.
At the annual meeting of the Endocrine Society, she presented the results of a pilot study of 20 men (mean age, 34 years) with mild TBI resulting from military combat. The last injury occurred an average of 44 months earlier.
Five subjects, or 25%, were GH deficient on the basis of a peak value of less than 3 ng/mL in response to a glucagon stimulation test. One GH-deficient veteran also had an abnormally low insulin-like growth factor-1 level. But all subjects had normal thyroid status and cortisol function.
Four GH-deficient men and 12 GH-sufficient men were able to put enough effort into their neuropsychologic testing for the results to be valid. The two groups performed similarly on measures of memory, learning, and simple and complex attention.
In contrast, GH deficiency appeared to be associated with executive dysfunction, as manifest in worse performance on measures of inhibitory control and self-monitoring. Depression also was more severe in the GH-deficient men, although they did not experience greater levels of fatigue or posttraumatic stress disorder. The GH-deficient group also scored significantly lower on a validated quality-of-life measure.
This study was supported by Novo Nordisk. The presenter reported having no financial conflicts.
HOUSTON – Growth hormone deficiency appears to be common among military veterans with mild traumatic brain injury sustained in combat, according to the first study to look at the issue.
Future studies will attempt to confirm the new finding that GH deficiency in the setting of traumatic brain injury (TBI) appears to be associated with specific neuropsychologic abnormalities, and, further, whether GH replacement therapy in affected veterans enhances TBI rehabilitation efforts, according to Dr. Adriana G. Ioachimescu of Emory University, Atlanta.
At the annual meeting of the Endocrine Society, she presented the results of a pilot study of 20 men (mean age, 34 years) with mild TBI resulting from military combat. The last injury occurred an average of 44 months earlier.
Five subjects, or 25%, were GH deficient on the basis of a peak value of less than 3 ng/mL in response to a glucagon stimulation test. One GH-deficient veteran also had an abnormally low insulin-like growth factor-1 level. But all subjects had normal thyroid status and cortisol function.
Four GH-deficient men and 12 GH-sufficient men were able to put enough effort into their neuropsychologic testing for the results to be valid. The two groups performed similarly on measures of memory, learning, and simple and complex attention.
In contrast, GH deficiency appeared to be associated with executive dysfunction, as manifest in worse performance on measures of inhibitory control and self-monitoring. Depression also was more severe in the GH-deficient men, although they did not experience greater levels of fatigue or posttraumatic stress disorder. The GH-deficient group also scored significantly lower on a validated quality-of-life measure.
This study was supported by Novo Nordisk. The presenter reported having no financial conflicts.
HOUSTON – Growth hormone deficiency appears to be common among military veterans with mild traumatic brain injury sustained in combat, according to the first study to look at the issue.
Future studies will attempt to confirm the new finding that GH deficiency in the setting of traumatic brain injury (TBI) appears to be associated with specific neuropsychologic abnormalities, and, further, whether GH replacement therapy in affected veterans enhances TBI rehabilitation efforts, according to Dr. Adriana G. Ioachimescu of Emory University, Atlanta.
At the annual meeting of the Endocrine Society, she presented the results of a pilot study of 20 men (mean age, 34 years) with mild TBI resulting from military combat. The last injury occurred an average of 44 months earlier.
Five subjects, or 25%, were GH deficient on the basis of a peak value of less than 3 ng/mL in response to a glucagon stimulation test. One GH-deficient veteran also had an abnormally low insulin-like growth factor-1 level. But all subjects had normal thyroid status and cortisol function.
Four GH-deficient men and 12 GH-sufficient men were able to put enough effort into their neuropsychologic testing for the results to be valid. The two groups performed similarly on measures of memory, learning, and simple and complex attention.
In contrast, GH deficiency appeared to be associated with executive dysfunction, as manifest in worse performance on measures of inhibitory control and self-monitoring. Depression also was more severe in the GH-deficient men, although they did not experience greater levels of fatigue or posttraumatic stress disorder. The GH-deficient group also scored significantly lower on a validated quality-of-life measure.
This study was supported by Novo Nordisk. The presenter reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: Twenty-five percent of a group of military veterans with mild traumatic brain injury had growth hormone deficiency, which was associated with specific neuropsychologic abnormalities.
Data Source: This was a pilot study of 20 men with combat-induced mild traumatic brain injury.
Disclosures: The study was supported by Novo Nordisk. The presenter reported having no financial conflicts.
Oral Relaxin Improves Poststroke Recovery
HOUSTON – Oral relaxin therapy resulted in significantly improved cognitive and functional recovery in poststroke patients participating in a randomized controlled trial.
"We speculate that in the near future, relaxin hormone could represent a new therapeutic and preventative tool to afford reduction of both incidence and disability of vascular ischemic diseases, not only of the brain but also of other organs and apparatuses," Dr. Paolo Milia declared in presenting the study findings at the annual meeting of the Endocrine Society.
The trial involved 36 poststroke patients admitted to a rehabilitation unit, where they were randomized to rehabilitation therapy plus 40 mg/day of oral porcine relaxin or to rehab alone. Investigators administered validated tests of cognitive function, global impairment, and daily activity at admission and again on days 20 and 40.
Cognitive function as assessed using the Trail Making Test was significantly better in the relaxin group on day 20, when their mean score was 3.5, compared with 2.0 in controls. The difference in cognitive outcome broadened by day 40, when the average score in the relaxin group was 4.0, compared with 2.0 in controls on rehabilitation therapy only, reported Dr. Milia of the Prosperius Institute in Umbertide, Italy.
Daily activity as measured using the Functional Independence Measure improved in the relaxin group from a baseline score of 53 to 78 at day 20 and 96 on day 40. In the control group, the average score was 59 at baseline, 69 at day 20, and 75 at day 40, indicating significantly greater functional recovery in the relaxin group at the 40-day mark.
Global function as assessed using the Modified Rankin Scale was higher in the relaxin group than in controls on days 20 and 40, with the difference between groups significant at both times.
The hormone treatment was safe as well as efficacious. No side effects occurred, he said.
Relaxin, discovered in 1926, belongs to the same peptide hormone superfamily as the insulin-like peptides. Relaxin’s known function revolves around pregnancy; however, the hormone also promotes angiogenesis, inhibits collagen synthesis, enhances nitric oxide synthesis, and boosts production of matrix metalloproteinases, a spectrum of effects leaving the door open to possible broader functions not well characterized as yet. In animal models, relaxin protects against ischemic injury to the myocardium and brain (J. Chem. Neuroanat. 2011;42:262-75), Dr. Milia noted.
The porcine relaxin utilized in this study, known as Vitalaxin Plus, is marketed by Sky BioHealth. The investigators reported having no financial disclosures.
HOUSTON – Oral relaxin therapy resulted in significantly improved cognitive and functional recovery in poststroke patients participating in a randomized controlled trial.
"We speculate that in the near future, relaxin hormone could represent a new therapeutic and preventative tool to afford reduction of both incidence and disability of vascular ischemic diseases, not only of the brain but also of other organs and apparatuses," Dr. Paolo Milia declared in presenting the study findings at the annual meeting of the Endocrine Society.
The trial involved 36 poststroke patients admitted to a rehabilitation unit, where they were randomized to rehabilitation therapy plus 40 mg/day of oral porcine relaxin or to rehab alone. Investigators administered validated tests of cognitive function, global impairment, and daily activity at admission and again on days 20 and 40.
Cognitive function as assessed using the Trail Making Test was significantly better in the relaxin group on day 20, when their mean score was 3.5, compared with 2.0 in controls. The difference in cognitive outcome broadened by day 40, when the average score in the relaxin group was 4.0, compared with 2.0 in controls on rehabilitation therapy only, reported Dr. Milia of the Prosperius Institute in Umbertide, Italy.
Daily activity as measured using the Functional Independence Measure improved in the relaxin group from a baseline score of 53 to 78 at day 20 and 96 on day 40. In the control group, the average score was 59 at baseline, 69 at day 20, and 75 at day 40, indicating significantly greater functional recovery in the relaxin group at the 40-day mark.
Global function as assessed using the Modified Rankin Scale was higher in the relaxin group than in controls on days 20 and 40, with the difference between groups significant at both times.
The hormone treatment was safe as well as efficacious. No side effects occurred, he said.
Relaxin, discovered in 1926, belongs to the same peptide hormone superfamily as the insulin-like peptides. Relaxin’s known function revolves around pregnancy; however, the hormone also promotes angiogenesis, inhibits collagen synthesis, enhances nitric oxide synthesis, and boosts production of matrix metalloproteinases, a spectrum of effects leaving the door open to possible broader functions not well characterized as yet. In animal models, relaxin protects against ischemic injury to the myocardium and brain (J. Chem. Neuroanat. 2011;42:262-75), Dr. Milia noted.
The porcine relaxin utilized in this study, known as Vitalaxin Plus, is marketed by Sky BioHealth. The investigators reported having no financial disclosures.
HOUSTON – Oral relaxin therapy resulted in significantly improved cognitive and functional recovery in poststroke patients participating in a randomized controlled trial.
"We speculate that in the near future, relaxin hormone could represent a new therapeutic and preventative tool to afford reduction of both incidence and disability of vascular ischemic diseases, not only of the brain but also of other organs and apparatuses," Dr. Paolo Milia declared in presenting the study findings at the annual meeting of the Endocrine Society.
The trial involved 36 poststroke patients admitted to a rehabilitation unit, where they were randomized to rehabilitation therapy plus 40 mg/day of oral porcine relaxin or to rehab alone. Investigators administered validated tests of cognitive function, global impairment, and daily activity at admission and again on days 20 and 40.
Cognitive function as assessed using the Trail Making Test was significantly better in the relaxin group on day 20, when their mean score was 3.5, compared with 2.0 in controls. The difference in cognitive outcome broadened by day 40, when the average score in the relaxin group was 4.0, compared with 2.0 in controls on rehabilitation therapy only, reported Dr. Milia of the Prosperius Institute in Umbertide, Italy.
Daily activity as measured using the Functional Independence Measure improved in the relaxin group from a baseline score of 53 to 78 at day 20 and 96 on day 40. In the control group, the average score was 59 at baseline, 69 at day 20, and 75 at day 40, indicating significantly greater functional recovery in the relaxin group at the 40-day mark.
Global function as assessed using the Modified Rankin Scale was higher in the relaxin group than in controls on days 20 and 40, with the difference between groups significant at both times.
The hormone treatment was safe as well as efficacious. No side effects occurred, he said.
Relaxin, discovered in 1926, belongs to the same peptide hormone superfamily as the insulin-like peptides. Relaxin’s known function revolves around pregnancy; however, the hormone also promotes angiogenesis, inhibits collagen synthesis, enhances nitric oxide synthesis, and boosts production of matrix metalloproteinases, a spectrum of effects leaving the door open to possible broader functions not well characterized as yet. In animal models, relaxin protects against ischemic injury to the myocardium and brain (J. Chem. Neuroanat. 2011;42:262-75), Dr. Milia noted.
The porcine relaxin utilized in this study, known as Vitalaxin Plus, is marketed by Sky BioHealth. The investigators reported having no financial disclosures.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: Treatment with oral porcine relaxin at 40 mg/day plus rehabilitation therapy resulted in greater psychoneurologic and functional recovery than rehabilitation therapy alone in a group of poststroke patients.
Data Source: A prospective, randomized, controlled trial of 40 poststroke patients admitted to a rehabilitation center.
Disclosures: The porcine relaxin utilized in this study, known as Vitalaxin Plus, is marketed by Sky BioHealth. The investigators reported having no financial disclosures.
Tomatoes Boosted Low HDL in Small Study
HOUSTON – Consuming two midsize uncooked tomatoes daily for a month resulted in a mean 5 mg/dL gain in HDL cholesterol level in a randomized trial in patients with low HDL.
A control group assigned to eat an equal quantity of cucumber – 300 g/day – saw no change in HDL, according to Dr. Daniel Cuevas Ramos of the National Institute of Medical Sciences and Nutrition in Mexico City.
He reported on 41 women and 11 men with low HDL but normal triglyceride levels who participated in the month-long randomized trial, during which they consumed an isocaloric diet.
Over the course of 1 month of follow-up, mean HDL levels in the tomato eaters climbed from 36.5 mg/dL at baseline to 41.6 mg/dL, while the cucumber-eating controls saw no significant change over time.
A linear regression analysis adjusted for age, sex, waist-to-hip ratio, physical activity, body mass index, and intake of alcohol, simple sugars, and omega-3 fatty acids showed that tomato consumption was independently associated with the increase in HDL.
Two medium tomatoes contain about 30 mg of lycopene, the nutrient thought responsible for the HDL boost. Prior cross-sectional studies had linked lycopene intake to higher HDL, he noted.
Dr. Cuevas Ramos reported having no financial conflicts.
HOUSTON – Consuming two midsize uncooked tomatoes daily for a month resulted in a mean 5 mg/dL gain in HDL cholesterol level in a randomized trial in patients with low HDL.
A control group assigned to eat an equal quantity of cucumber – 300 g/day – saw no change in HDL, according to Dr. Daniel Cuevas Ramos of the National Institute of Medical Sciences and Nutrition in Mexico City.
He reported on 41 women and 11 men with low HDL but normal triglyceride levels who participated in the month-long randomized trial, during which they consumed an isocaloric diet.
Over the course of 1 month of follow-up, mean HDL levels in the tomato eaters climbed from 36.5 mg/dL at baseline to 41.6 mg/dL, while the cucumber-eating controls saw no significant change over time.
A linear regression analysis adjusted for age, sex, waist-to-hip ratio, physical activity, body mass index, and intake of alcohol, simple sugars, and omega-3 fatty acids showed that tomato consumption was independently associated with the increase in HDL.
Two medium tomatoes contain about 30 mg of lycopene, the nutrient thought responsible for the HDL boost. Prior cross-sectional studies had linked lycopene intake to higher HDL, he noted.
Dr. Cuevas Ramos reported having no financial conflicts.
HOUSTON – Consuming two midsize uncooked tomatoes daily for a month resulted in a mean 5 mg/dL gain in HDL cholesterol level in a randomized trial in patients with low HDL.
A control group assigned to eat an equal quantity of cucumber – 300 g/day – saw no change in HDL, according to Dr. Daniel Cuevas Ramos of the National Institute of Medical Sciences and Nutrition in Mexico City.
He reported on 41 women and 11 men with low HDL but normal triglyceride levels who participated in the month-long randomized trial, during which they consumed an isocaloric diet.
Over the course of 1 month of follow-up, mean HDL levels in the tomato eaters climbed from 36.5 mg/dL at baseline to 41.6 mg/dL, while the cucumber-eating controls saw no significant change over time.
A linear regression analysis adjusted for age, sex, waist-to-hip ratio, physical activity, body mass index, and intake of alcohol, simple sugars, and omega-3 fatty acids showed that tomato consumption was independently associated with the increase in HDL.
Two medium tomatoes contain about 30 mg of lycopene, the nutrient thought responsible for the HDL boost. Prior cross-sectional studies had linked lycopene intake to higher HDL, he noted.
Dr. Cuevas Ramos reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: Eating two midsize tomatoes per day for 1 month led to a mean 5 mg/dL increase in HDL in a randomized trial involving men and women with low HDL.
Data Source: A month-long open-label randomized trial in 52 low-HDL participants.
Disclosures: The presenter reported having no financial conflicts.
Lyme Arthritis: Highly Treatable, Slow to Resolve
VAIL, COLO. – Arthritis is far and away the most common manifestation of late Lyme disease, affecting up to 30% of children whose early-stage disease went untreated.
Lyme arthritis presents with a distinctive clinical picture. Nevertheless, this common condition is often initially mismanaged as a joint sprain or other orthopedic injury, with the correct diagnosis coming only following referral after a month or more with no improvement, according to Dr. Roberta L. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center, Washington.
"We see tons of kids with Lyme arthritis. We get two or three cases every week in our clinic," she said at the conference.
Lyme arthritis occurs months to years after an untreated exposure to Borrelia burgdorferi. Affected patients typically present with a history of recurrent, weeks- or months-long attacks of joint swelling in one or a few joints. The knee is by far the most common site, but other large joints can be involved, as can the temporomandibular joint. An involved knee may swell up to literally the size of a basketball, yet the child has no fever, erythema, or systemic complaints, and surprisingly little pain given the effusion size.
Chronic progressive arthritis that’s not preceded by brief waxing and waning attacks doesn’t fulfil the criteria for Lyme arthritis. Neither does chronic symmetric polyarthritis, Dr. DeBiasi stressed.
Patients with suspected Lyme arthritis typically undergo arthrocentesis because there are other possible explanations for reactive arthritis besides Lyme disease. The synovial fluid in a patient with Lyme arthritis shows prominent neutrophils, mild to moderate inflammation, and a median WBC of 25,000/mm3. But diagnostic confirmation of Lyme arthritis requires serologic testing, which will invariably be enzyme immunoassay-positive, IgG-positive on the Western blot test, and IgM-negative on the Western blot because arthritis is a late manifestation of infection. Dr. DeBiasi doesn’t consider synovial fluid PCR a useful adjunctive diagnostic test.
The first-line treatment recommended in the Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) is 28 days of oral therapy with doxycycline at 4 mg/kg per day divided b.i.d., to a maximum of 100 mg b.i.d.; amoxicillin at 50 mg/kg per day divided t.i.d., to a maximum of 500 mg t.i.d.; or cefuroxime axetil at 30 mg/kg per day divided b.i.d., to a maximum of 500 mg b.i.d. Unlike in late central nervous system Lyme disease, which responds swiftly to treatment, expect slow resolution of the joint inflammation.
"This is a situation in which you can avoid giving someone a PICC line. Patients do great with oral therapy. You just need to do it for quite a while. The joint may show no change after the first week of therapy and may be only slightly smaller after 2 weeks. I give anti-inflammatory drugs concurrently to speed improvement in swelling and range of motion," Dr. DeBiasi explained.
If, however, joint swelling persists at the end of 28 days of oral therapy, two options are available. Both involve a 1-month wait off treatment before considering retreatment.
The first option, which is preferred if the patient’s joint swelling is substantially improved but not completely resolved, is another 28-day course of oral antibiotic therapy.
The alternative is to initiate 2-4 weeks of intravenous ceftriaxone at 50-75 mg/kg per day in a single dose, to a maximum of 2 g. This is the favored option when a patient has shown no improvement at all or is worse after the first course of oral therapy.
"That’s happened once in the 6 years I’ve been taking care of kids with Lyme arthritis. The vast majority do well with oral therapy," she said.
Dr. DeBiasi reported having no financial conflicts.
VAIL, COLO. – Arthritis is far and away the most common manifestation of late Lyme disease, affecting up to 30% of children whose early-stage disease went untreated.
Lyme arthritis presents with a distinctive clinical picture. Nevertheless, this common condition is often initially mismanaged as a joint sprain or other orthopedic injury, with the correct diagnosis coming only following referral after a month or more with no improvement, according to Dr. Roberta L. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center, Washington.
"We see tons of kids with Lyme arthritis. We get two or three cases every week in our clinic," she said at the conference.
Lyme arthritis occurs months to years after an untreated exposure to Borrelia burgdorferi. Affected patients typically present with a history of recurrent, weeks- or months-long attacks of joint swelling in one or a few joints. The knee is by far the most common site, but other large joints can be involved, as can the temporomandibular joint. An involved knee may swell up to literally the size of a basketball, yet the child has no fever, erythema, or systemic complaints, and surprisingly little pain given the effusion size.
Chronic progressive arthritis that’s not preceded by brief waxing and waning attacks doesn’t fulfil the criteria for Lyme arthritis. Neither does chronic symmetric polyarthritis, Dr. DeBiasi stressed.
Patients with suspected Lyme arthritis typically undergo arthrocentesis because there are other possible explanations for reactive arthritis besides Lyme disease. The synovial fluid in a patient with Lyme arthritis shows prominent neutrophils, mild to moderate inflammation, and a median WBC of 25,000/mm3. But diagnostic confirmation of Lyme arthritis requires serologic testing, which will invariably be enzyme immunoassay-positive, IgG-positive on the Western blot test, and IgM-negative on the Western blot because arthritis is a late manifestation of infection. Dr. DeBiasi doesn’t consider synovial fluid PCR a useful adjunctive diagnostic test.
The first-line treatment recommended in the Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) is 28 days of oral therapy with doxycycline at 4 mg/kg per day divided b.i.d., to a maximum of 100 mg b.i.d.; amoxicillin at 50 mg/kg per day divided t.i.d., to a maximum of 500 mg t.i.d.; or cefuroxime axetil at 30 mg/kg per day divided b.i.d., to a maximum of 500 mg b.i.d. Unlike in late central nervous system Lyme disease, which responds swiftly to treatment, expect slow resolution of the joint inflammation.
"This is a situation in which you can avoid giving someone a PICC line. Patients do great with oral therapy. You just need to do it for quite a while. The joint may show no change after the first week of therapy and may be only slightly smaller after 2 weeks. I give anti-inflammatory drugs concurrently to speed improvement in swelling and range of motion," Dr. DeBiasi explained.
If, however, joint swelling persists at the end of 28 days of oral therapy, two options are available. Both involve a 1-month wait off treatment before considering retreatment.
The first option, which is preferred if the patient’s joint swelling is substantially improved but not completely resolved, is another 28-day course of oral antibiotic therapy.
The alternative is to initiate 2-4 weeks of intravenous ceftriaxone at 50-75 mg/kg per day in a single dose, to a maximum of 2 g. This is the favored option when a patient has shown no improvement at all or is worse after the first course of oral therapy.
"That’s happened once in the 6 years I’ve been taking care of kids with Lyme arthritis. The vast majority do well with oral therapy," she said.
Dr. DeBiasi reported having no financial conflicts.
VAIL, COLO. – Arthritis is far and away the most common manifestation of late Lyme disease, affecting up to 30% of children whose early-stage disease went untreated.
Lyme arthritis presents with a distinctive clinical picture. Nevertheless, this common condition is often initially mismanaged as a joint sprain or other orthopedic injury, with the correct diagnosis coming only following referral after a month or more with no improvement, according to Dr. Roberta L. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center, Washington.
"We see tons of kids with Lyme arthritis. We get two or three cases every week in our clinic," she said at the conference.
Lyme arthritis occurs months to years after an untreated exposure to Borrelia burgdorferi. Affected patients typically present with a history of recurrent, weeks- or months-long attacks of joint swelling in one or a few joints. The knee is by far the most common site, but other large joints can be involved, as can the temporomandibular joint. An involved knee may swell up to literally the size of a basketball, yet the child has no fever, erythema, or systemic complaints, and surprisingly little pain given the effusion size.
Chronic progressive arthritis that’s not preceded by brief waxing and waning attacks doesn’t fulfil the criteria for Lyme arthritis. Neither does chronic symmetric polyarthritis, Dr. DeBiasi stressed.
Patients with suspected Lyme arthritis typically undergo arthrocentesis because there are other possible explanations for reactive arthritis besides Lyme disease. The synovial fluid in a patient with Lyme arthritis shows prominent neutrophils, mild to moderate inflammation, and a median WBC of 25,000/mm3. But diagnostic confirmation of Lyme arthritis requires serologic testing, which will invariably be enzyme immunoassay-positive, IgG-positive on the Western blot test, and IgM-negative on the Western blot because arthritis is a late manifestation of infection. Dr. DeBiasi doesn’t consider synovial fluid PCR a useful adjunctive diagnostic test.
The first-line treatment recommended in the Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) is 28 days of oral therapy with doxycycline at 4 mg/kg per day divided b.i.d., to a maximum of 100 mg b.i.d.; amoxicillin at 50 mg/kg per day divided t.i.d., to a maximum of 500 mg t.i.d.; or cefuroxime axetil at 30 mg/kg per day divided b.i.d., to a maximum of 500 mg b.i.d. Unlike in late central nervous system Lyme disease, which responds swiftly to treatment, expect slow resolution of the joint inflammation.
"This is a situation in which you can avoid giving someone a PICC line. Patients do great with oral therapy. You just need to do it for quite a while. The joint may show no change after the first week of therapy and may be only slightly smaller after 2 weeks. I give anti-inflammatory drugs concurrently to speed improvement in swelling and range of motion," Dr. DeBiasi explained.
If, however, joint swelling persists at the end of 28 days of oral therapy, two options are available. Both involve a 1-month wait off treatment before considering retreatment.
The first option, which is preferred if the patient’s joint swelling is substantially improved but not completely resolved, is another 28-day course of oral antibiotic therapy.
The alternative is to initiate 2-4 weeks of intravenous ceftriaxone at 50-75 mg/kg per day in a single dose, to a maximum of 2 g. This is the favored option when a patient has shown no improvement at all or is worse after the first course of oral therapy.
"That’s happened once in the 6 years I’ve been taking care of kids with Lyme arthritis. The vast majority do well with oral therapy," she said.
Dr. DeBiasi reported having no financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL PEDIATRIC INFECTIOUS DISEASES CONFERENCE SPONSORED BY CHILDREN'S HOSPITAL COLORADO
Rheumatologic Serologies Can Be a Confounding Quagmire
ESTES PARK, COLO. – The high false-positive rates for autoimmune serologies make them unsuitable as screening tests for connective tissue diseases, according to Dr. Robert W. Janson.
Moreover, the prearranged test panels often pitched to primary care physicians are a particularly bad idea, he said. These panels might combine, for example, rheumatoid factor (RF), antinuclear antibodies, erythrocyte sedimentation rate (ESR), and C-reactive protein.
"I call them ‘rheum rally packs.’ They should be avoided. They can multiply testing errors, leading to additional unnecessary testing, needless angst, and inappropriate treatment," said Dr. Janson, chief of the rheumatology section at the Denver Veterans Affairs Medical Center.
Selectivity is the watchword when ordering autoimmune serologic tests. Their value comes when suspicion of a connective tissue disease (CTD) is already high based on history and physical examination, that is, when the pretest probability is relatively high, he explained at an update on internal medicine sponsored by the University of Colorado.
Take, for example, rheumatoid factor. The very name is a misnomer, suggesting a high test specificity that’s completely undeserved. While 80% of patients with rheumatoid arthritis are indeed RF+, the test is also positive in the majority of individuals with Sjögren’s syndrome or mixed CDT; in many patients with chronic infection, malignancy, or fibrotic pulmonary disorders; and even in 5% of healthy individuals. As a result, the test’s positive predictive value for rheumatoid arthritis is a mere 24%, although its negative predictive value is much better at 89%.
"Rheumatoid factor has little value as a screening test to diagnose or exclude CTD in patients with just plain arthralgias. It has a much higher positive predictive value if ordered selectively in patients with a higher probability of a CTD: morning stiffness, sicca symptoms, and arthralgias or synovitis in a rheumatoid arthritis distribution," the rheumatologist continued.
The combination of a positive RF and a positive test for anticyclic citrullinated peptide antibodies (ACPAs) is associated with a nearly 100% likelihood that an appropriately symptomatic patient has rheumatoid arthritis. Also, when both tests are positive, it’s an indicator of a more serious case of rheumatoid arthritis that will involve joint damage and disability.
"These patients need more aggressive therapy," Dr. Janson noted.
On the other hand, he said he’d urge that a patient with morning stiffness lasting longer than 1 hour, tenderness of the metacarpophalangeal or metatarsophalangeal joints, and suspected synovitis on physical examination be referred to a rheumatologist to see if an early inflammatory arthritis is involved, even if the RF and/or ACPA tests are negative. In this era of highly effective biologic therapy, negative serologic tests don’t preclude early and aggressive therapy, he noted.
Nearly all patients with SLE will have a positive antinuclear antibody (ANA) test. A negative test rules out SLE with 95% certainty. But the test’s positive predictive value for SLE is only 11%-30% because so many other conditions are associated with a positive ANA. These include numerous other CTDs, organ-specific autoimmune diseases such as Hashimoto’s thyroiditis, a variety of chronic infections, and lymphoproliferative disorders. In addition, low-titer positive ANA tests are not uncommon in normal women and the elderly.
In sum, ANA testing is useful to help establish a diagnosis when a patient’s clinical features suggest a CTD, as well as for excluding CTDs in patients with uncertain clinical findings, and in monitoring CTD disease activity. Rheumatologists consider ANA titers of 1:320 or higher to be more clinically meaningful, according to Dr. Janson.
He singled out a particular ANA subantibody test – that for antihistone antibodies – as one that "approaches being the perfect test." That’s because patients with drug-induced lupus don’t make antibodies to nonhistone nuclear antigens. As a result, antihistone antibody testing enjoys greater than 95% sensitivity and specificity for drug-induced lupus.
Two ANA subantibody tests are highly diagnostic for SLE. These are anti–double-stranded DNA and anti–Smith antibody tests, each with a specificity in excess of 95% for SLE.
The antineutrophil cytoplasmic antibody (ANCA) tests are the first serologies to order when a patient is suspected of having some kind of vasculitis as manifest, for example, by diffuse alveolar hemorrhage, nephritis without another explanation, or a pulmonary-renal syndrome.
ANCAs come in two types: cytoplasmic (cANCA), which are antibodies directed against proteinase 3, and perinuclear (pANCA), directed most often against myeloperoxidase.
The cANCA test has 98% specificity for active generalized granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis. However, limited granulomatosis with polyangiitis affecting, say, the sinuses but without kidney involvement may be ANCA negative in 40% of cases.
The pANCA test has 60%-90% sensitivity for microscopic polyangiitis, but poor sensitivity, as the test can be positive in rheumatoid arthritis and several other CTDs. However, the specificity for microscopic polyangiitis improves to greater than 95% in the setting of both positive pANCA and antimyeloperoxidase ELISA tests.
The acute phase reactants – C-reactive protein and ESR are useful in assessing patients with a suspected malignancy or infection, as well as in those in whom inflammatory arthritides or vasculitis is a concern. A CRP greater than 1.0 mg/dL indicates significant inflammation, and a value in excess of 10 mg/dL is due either to metastatic cancer, bacterial infection, or systemic vasculitis. And if both the ESR and CRP are normal, the probability that a patient has a vasculitis is "incredibly low," Dr. Janson said.
At least 80% of patients with polymyalgia rheumatica will have an elevated ESR, and the CRP should be elevated in those that don’t.
Dr. Janson reported having no relevant financial conflicts.
ESTES PARK, COLO. – The high false-positive rates for autoimmune serologies make them unsuitable as screening tests for connective tissue diseases, according to Dr. Robert W. Janson.
Moreover, the prearranged test panels often pitched to primary care physicians are a particularly bad idea, he said. These panels might combine, for example, rheumatoid factor (RF), antinuclear antibodies, erythrocyte sedimentation rate (ESR), and C-reactive protein.
"I call them ‘rheum rally packs.’ They should be avoided. They can multiply testing errors, leading to additional unnecessary testing, needless angst, and inappropriate treatment," said Dr. Janson, chief of the rheumatology section at the Denver Veterans Affairs Medical Center.
Selectivity is the watchword when ordering autoimmune serologic tests. Their value comes when suspicion of a connective tissue disease (CTD) is already high based on history and physical examination, that is, when the pretest probability is relatively high, he explained at an update on internal medicine sponsored by the University of Colorado.
Take, for example, rheumatoid factor. The very name is a misnomer, suggesting a high test specificity that’s completely undeserved. While 80% of patients with rheumatoid arthritis are indeed RF+, the test is also positive in the majority of individuals with Sjögren’s syndrome or mixed CDT; in many patients with chronic infection, malignancy, or fibrotic pulmonary disorders; and even in 5% of healthy individuals. As a result, the test’s positive predictive value for rheumatoid arthritis is a mere 24%, although its negative predictive value is much better at 89%.
"Rheumatoid factor has little value as a screening test to diagnose or exclude CTD in patients with just plain arthralgias. It has a much higher positive predictive value if ordered selectively in patients with a higher probability of a CTD: morning stiffness, sicca symptoms, and arthralgias or synovitis in a rheumatoid arthritis distribution," the rheumatologist continued.
The combination of a positive RF and a positive test for anticyclic citrullinated peptide antibodies (ACPAs) is associated with a nearly 100% likelihood that an appropriately symptomatic patient has rheumatoid arthritis. Also, when both tests are positive, it’s an indicator of a more serious case of rheumatoid arthritis that will involve joint damage and disability.
"These patients need more aggressive therapy," Dr. Janson noted.
On the other hand, he said he’d urge that a patient with morning stiffness lasting longer than 1 hour, tenderness of the metacarpophalangeal or metatarsophalangeal joints, and suspected synovitis on physical examination be referred to a rheumatologist to see if an early inflammatory arthritis is involved, even if the RF and/or ACPA tests are negative. In this era of highly effective biologic therapy, negative serologic tests don’t preclude early and aggressive therapy, he noted.
Nearly all patients with SLE will have a positive antinuclear antibody (ANA) test. A negative test rules out SLE with 95% certainty. But the test’s positive predictive value for SLE is only 11%-30% because so many other conditions are associated with a positive ANA. These include numerous other CTDs, organ-specific autoimmune diseases such as Hashimoto’s thyroiditis, a variety of chronic infections, and lymphoproliferative disorders. In addition, low-titer positive ANA tests are not uncommon in normal women and the elderly.
In sum, ANA testing is useful to help establish a diagnosis when a patient’s clinical features suggest a CTD, as well as for excluding CTDs in patients with uncertain clinical findings, and in monitoring CTD disease activity. Rheumatologists consider ANA titers of 1:320 or higher to be more clinically meaningful, according to Dr. Janson.
He singled out a particular ANA subantibody test – that for antihistone antibodies – as one that "approaches being the perfect test." That’s because patients with drug-induced lupus don’t make antibodies to nonhistone nuclear antigens. As a result, antihistone antibody testing enjoys greater than 95% sensitivity and specificity for drug-induced lupus.
Two ANA subantibody tests are highly diagnostic for SLE. These are anti–double-stranded DNA and anti–Smith antibody tests, each with a specificity in excess of 95% for SLE.
The antineutrophil cytoplasmic antibody (ANCA) tests are the first serologies to order when a patient is suspected of having some kind of vasculitis as manifest, for example, by diffuse alveolar hemorrhage, nephritis without another explanation, or a pulmonary-renal syndrome.
ANCAs come in two types: cytoplasmic (cANCA), which are antibodies directed against proteinase 3, and perinuclear (pANCA), directed most often against myeloperoxidase.
The cANCA test has 98% specificity for active generalized granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis. However, limited granulomatosis with polyangiitis affecting, say, the sinuses but without kidney involvement may be ANCA negative in 40% of cases.
The pANCA test has 60%-90% sensitivity for microscopic polyangiitis, but poor sensitivity, as the test can be positive in rheumatoid arthritis and several other CTDs. However, the specificity for microscopic polyangiitis improves to greater than 95% in the setting of both positive pANCA and antimyeloperoxidase ELISA tests.
The acute phase reactants – C-reactive protein and ESR are useful in assessing patients with a suspected malignancy or infection, as well as in those in whom inflammatory arthritides or vasculitis is a concern. A CRP greater than 1.0 mg/dL indicates significant inflammation, and a value in excess of 10 mg/dL is due either to metastatic cancer, bacterial infection, or systemic vasculitis. And if both the ESR and CRP are normal, the probability that a patient has a vasculitis is "incredibly low," Dr. Janson said.
At least 80% of patients with polymyalgia rheumatica will have an elevated ESR, and the CRP should be elevated in those that don’t.
Dr. Janson reported having no relevant financial conflicts.
ESTES PARK, COLO. – The high false-positive rates for autoimmune serologies make them unsuitable as screening tests for connective tissue diseases, according to Dr. Robert W. Janson.
Moreover, the prearranged test panels often pitched to primary care physicians are a particularly bad idea, he said. These panels might combine, for example, rheumatoid factor (RF), antinuclear antibodies, erythrocyte sedimentation rate (ESR), and C-reactive protein.
"I call them ‘rheum rally packs.’ They should be avoided. They can multiply testing errors, leading to additional unnecessary testing, needless angst, and inappropriate treatment," said Dr. Janson, chief of the rheumatology section at the Denver Veterans Affairs Medical Center.
Selectivity is the watchword when ordering autoimmune serologic tests. Their value comes when suspicion of a connective tissue disease (CTD) is already high based on history and physical examination, that is, when the pretest probability is relatively high, he explained at an update on internal medicine sponsored by the University of Colorado.
Take, for example, rheumatoid factor. The very name is a misnomer, suggesting a high test specificity that’s completely undeserved. While 80% of patients with rheumatoid arthritis are indeed RF+, the test is also positive in the majority of individuals with Sjögren’s syndrome or mixed CDT; in many patients with chronic infection, malignancy, or fibrotic pulmonary disorders; and even in 5% of healthy individuals. As a result, the test’s positive predictive value for rheumatoid arthritis is a mere 24%, although its negative predictive value is much better at 89%.
"Rheumatoid factor has little value as a screening test to diagnose or exclude CTD in patients with just plain arthralgias. It has a much higher positive predictive value if ordered selectively in patients with a higher probability of a CTD: morning stiffness, sicca symptoms, and arthralgias or synovitis in a rheumatoid arthritis distribution," the rheumatologist continued.
The combination of a positive RF and a positive test for anticyclic citrullinated peptide antibodies (ACPAs) is associated with a nearly 100% likelihood that an appropriately symptomatic patient has rheumatoid arthritis. Also, when both tests are positive, it’s an indicator of a more serious case of rheumatoid arthritis that will involve joint damage and disability.
"These patients need more aggressive therapy," Dr. Janson noted.
On the other hand, he said he’d urge that a patient with morning stiffness lasting longer than 1 hour, tenderness of the metacarpophalangeal or metatarsophalangeal joints, and suspected synovitis on physical examination be referred to a rheumatologist to see if an early inflammatory arthritis is involved, even if the RF and/or ACPA tests are negative. In this era of highly effective biologic therapy, negative serologic tests don’t preclude early and aggressive therapy, he noted.
Nearly all patients with SLE will have a positive antinuclear antibody (ANA) test. A negative test rules out SLE with 95% certainty. But the test’s positive predictive value for SLE is only 11%-30% because so many other conditions are associated with a positive ANA. These include numerous other CTDs, organ-specific autoimmune diseases such as Hashimoto’s thyroiditis, a variety of chronic infections, and lymphoproliferative disorders. In addition, low-titer positive ANA tests are not uncommon in normal women and the elderly.
In sum, ANA testing is useful to help establish a diagnosis when a patient’s clinical features suggest a CTD, as well as for excluding CTDs in patients with uncertain clinical findings, and in monitoring CTD disease activity. Rheumatologists consider ANA titers of 1:320 or higher to be more clinically meaningful, according to Dr. Janson.
He singled out a particular ANA subantibody test – that for antihistone antibodies – as one that "approaches being the perfect test." That’s because patients with drug-induced lupus don’t make antibodies to nonhistone nuclear antigens. As a result, antihistone antibody testing enjoys greater than 95% sensitivity and specificity for drug-induced lupus.
Two ANA subantibody tests are highly diagnostic for SLE. These are anti–double-stranded DNA and anti–Smith antibody tests, each with a specificity in excess of 95% for SLE.
The antineutrophil cytoplasmic antibody (ANCA) tests are the first serologies to order when a patient is suspected of having some kind of vasculitis as manifest, for example, by diffuse alveolar hemorrhage, nephritis without another explanation, or a pulmonary-renal syndrome.
ANCAs come in two types: cytoplasmic (cANCA), which are antibodies directed against proteinase 3, and perinuclear (pANCA), directed most often against myeloperoxidase.
The cANCA test has 98% specificity for active generalized granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis. However, limited granulomatosis with polyangiitis affecting, say, the sinuses but without kidney involvement may be ANCA negative in 40% of cases.
The pANCA test has 60%-90% sensitivity for microscopic polyangiitis, but poor sensitivity, as the test can be positive in rheumatoid arthritis and several other CTDs. However, the specificity for microscopic polyangiitis improves to greater than 95% in the setting of both positive pANCA and antimyeloperoxidase ELISA tests.
The acute phase reactants – C-reactive protein and ESR are useful in assessing patients with a suspected malignancy or infection, as well as in those in whom inflammatory arthritides or vasculitis is a concern. A CRP greater than 1.0 mg/dL indicates significant inflammation, and a value in excess of 10 mg/dL is due either to metastatic cancer, bacterial infection, or systemic vasculitis. And if both the ESR and CRP are normal, the probability that a patient has a vasculitis is "incredibly low," Dr. Janson said.
At least 80% of patients with polymyalgia rheumatica will have an elevated ESR, and the CRP should be elevated in those that don’t.
Dr. Janson reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM AN UPDATE IN INTERNAL MEDICINE SPONSORED BY THE UNIVERSITY OF COLORADO
Routine Postpneumonia X-Ray Unwarranted
ESTES PARK, COLO. – Look in the near future for widespread adoption of a more selective approach to getting chest radiographs after pneumonia.
Since 2000, the American Thoracic Society, the British Thoracic Society, and the Canadian Infectious Diseases Society along with the Canadian Thoracic Society have published guidelines for management of community-acquired pneumonia. The guidelines recommend a routine follow-up chest x-ray within a couple of months after the treatment of pneumonia. The main purpose, other than in the minority of patients with ongoing pneumonia-related symptoms, is to exclude an underlying lung cancer that may have predisposed to the pneumonia.
However, recent studies suggest that the diagnostic yield of these follow-up radiographs is too low to justify a recommendation for routine imaging. The contention is that only the subset of pneumonia patients who are at increased risk for lung cancer should be targeted for a follow-up chest x-ray. A more selective approach will save substantial health care dollars and reduce radiation exposure, according to Dr. Robert L. Keith, professor of medicine and a pulmonologist at the University of Colorado at Denver, Aurora, which sponsored this update in internal medicine.
He cited what he considers "a great study" by Canadian investigators who conducted a population-based study of 3,398 Edmonton patients who had been followed for 5 years after treatment of pneumonia. The incidence of diagnosis of new lung cancer was 1.1% at 90 days, 1.7% at 1 year, and 2.3% at 5 years, which the investigators deemed too low to provide a strong rationale for routine follow-up chest x-rays (Arch. Intern. Med. 2011;171:1193-8).
Only 40% of the patients had had a follow-up chest x-ray within 90 days, suggesting that the majority of Edmonton-area physicians were already skeptical about guideline-recommended routine postpneumonia radiography. The diagnostic yield of lung cancer achieved via the imaging studies was 2.5%.
A key study finding was that none of the 79 lung cancers that were diagnosed within 5 years following pneumonia occurred in patients younger than age 40 years at the time of their lung infection, and only five cases occurred in 40- to 49-year-olds. The investigators identified three independent risk factors associated with lung cancer within 5 years after an episode of pneumonia: age 50 years or more, with a relative risk of 19; male sex, with a 1.8-fold increased risk; and current smoking, with a relative risk of 1.7.
Dr. Keith predicted that most health care systems are going to endorse the lung cancer screening strategy that proved so successful in the landmark National Lung Screening Trial (N. Engl. J. Med. 2011;365:395-409). That study in more than 50,000 subjects showed that screening via low-dose helical chest CT reduced lung cancer mortality by 20%, compared with screening by chest x-ray in high-risk patients as defined mainly by their age and smoking history.
Based upon the results of this National Cancer Institute–sponsored study and other recent lung cancer screening studies, the National Comprehensive Cancer Network has recommended routine screening using low-dose helical CT for two high-risk groups: individuals aged 55-74 years with at least 30 pack-years of smoking who either are current smokers or who quit fewer than 15 years ago, and patients aged 50 or older with at least 20 pack-years of smoking and one additional risk factor. The additional risk factors are contact with radon, occupational exposure to asbestos or other carcinogens, a history of lung cancer in a first-degree relative, a personal history of any tobacco-related aerodigestive cancer, or chronic obstructive pulmonary disease.
Dr. Keith reported that he serves on the speakers bureaus for Boehringer Ingelheim and Pfizer.
ESTES PARK, COLO. – Look in the near future for widespread adoption of a more selective approach to getting chest radiographs after pneumonia.
Since 2000, the American Thoracic Society, the British Thoracic Society, and the Canadian Infectious Diseases Society along with the Canadian Thoracic Society have published guidelines for management of community-acquired pneumonia. The guidelines recommend a routine follow-up chest x-ray within a couple of months after the treatment of pneumonia. The main purpose, other than in the minority of patients with ongoing pneumonia-related symptoms, is to exclude an underlying lung cancer that may have predisposed to the pneumonia.
However, recent studies suggest that the diagnostic yield of these follow-up radiographs is too low to justify a recommendation for routine imaging. The contention is that only the subset of pneumonia patients who are at increased risk for lung cancer should be targeted for a follow-up chest x-ray. A more selective approach will save substantial health care dollars and reduce radiation exposure, according to Dr. Robert L. Keith, professor of medicine and a pulmonologist at the University of Colorado at Denver, Aurora, which sponsored this update in internal medicine.
He cited what he considers "a great study" by Canadian investigators who conducted a population-based study of 3,398 Edmonton patients who had been followed for 5 years after treatment of pneumonia. The incidence of diagnosis of new lung cancer was 1.1% at 90 days, 1.7% at 1 year, and 2.3% at 5 years, which the investigators deemed too low to provide a strong rationale for routine follow-up chest x-rays (Arch. Intern. Med. 2011;171:1193-8).
Only 40% of the patients had had a follow-up chest x-ray within 90 days, suggesting that the majority of Edmonton-area physicians were already skeptical about guideline-recommended routine postpneumonia radiography. The diagnostic yield of lung cancer achieved via the imaging studies was 2.5%.
A key study finding was that none of the 79 lung cancers that were diagnosed within 5 years following pneumonia occurred in patients younger than age 40 years at the time of their lung infection, and only five cases occurred in 40- to 49-year-olds. The investigators identified three independent risk factors associated with lung cancer within 5 years after an episode of pneumonia: age 50 years or more, with a relative risk of 19; male sex, with a 1.8-fold increased risk; and current smoking, with a relative risk of 1.7.
Dr. Keith predicted that most health care systems are going to endorse the lung cancer screening strategy that proved so successful in the landmark National Lung Screening Trial (N. Engl. J. Med. 2011;365:395-409). That study in more than 50,000 subjects showed that screening via low-dose helical chest CT reduced lung cancer mortality by 20%, compared with screening by chest x-ray in high-risk patients as defined mainly by their age and smoking history.
Based upon the results of this National Cancer Institute–sponsored study and other recent lung cancer screening studies, the National Comprehensive Cancer Network has recommended routine screening using low-dose helical CT for two high-risk groups: individuals aged 55-74 years with at least 30 pack-years of smoking who either are current smokers or who quit fewer than 15 years ago, and patients aged 50 or older with at least 20 pack-years of smoking and one additional risk factor. The additional risk factors are contact with radon, occupational exposure to asbestos or other carcinogens, a history of lung cancer in a first-degree relative, a personal history of any tobacco-related aerodigestive cancer, or chronic obstructive pulmonary disease.
Dr. Keith reported that he serves on the speakers bureaus for Boehringer Ingelheim and Pfizer.
ESTES PARK, COLO. – Look in the near future for widespread adoption of a more selective approach to getting chest radiographs after pneumonia.
Since 2000, the American Thoracic Society, the British Thoracic Society, and the Canadian Infectious Diseases Society along with the Canadian Thoracic Society have published guidelines for management of community-acquired pneumonia. The guidelines recommend a routine follow-up chest x-ray within a couple of months after the treatment of pneumonia. The main purpose, other than in the minority of patients with ongoing pneumonia-related symptoms, is to exclude an underlying lung cancer that may have predisposed to the pneumonia.
However, recent studies suggest that the diagnostic yield of these follow-up radiographs is too low to justify a recommendation for routine imaging. The contention is that only the subset of pneumonia patients who are at increased risk for lung cancer should be targeted for a follow-up chest x-ray. A more selective approach will save substantial health care dollars and reduce radiation exposure, according to Dr. Robert L. Keith, professor of medicine and a pulmonologist at the University of Colorado at Denver, Aurora, which sponsored this update in internal medicine.
He cited what he considers "a great study" by Canadian investigators who conducted a population-based study of 3,398 Edmonton patients who had been followed for 5 years after treatment of pneumonia. The incidence of diagnosis of new lung cancer was 1.1% at 90 days, 1.7% at 1 year, and 2.3% at 5 years, which the investigators deemed too low to provide a strong rationale for routine follow-up chest x-rays (Arch. Intern. Med. 2011;171:1193-8).
Only 40% of the patients had had a follow-up chest x-ray within 90 days, suggesting that the majority of Edmonton-area physicians were already skeptical about guideline-recommended routine postpneumonia radiography. The diagnostic yield of lung cancer achieved via the imaging studies was 2.5%.
A key study finding was that none of the 79 lung cancers that were diagnosed within 5 years following pneumonia occurred in patients younger than age 40 years at the time of their lung infection, and only five cases occurred in 40- to 49-year-olds. The investigators identified three independent risk factors associated with lung cancer within 5 years after an episode of pneumonia: age 50 years or more, with a relative risk of 19; male sex, with a 1.8-fold increased risk; and current smoking, with a relative risk of 1.7.
Dr. Keith predicted that most health care systems are going to endorse the lung cancer screening strategy that proved so successful in the landmark National Lung Screening Trial (N. Engl. J. Med. 2011;365:395-409). That study in more than 50,000 subjects showed that screening via low-dose helical chest CT reduced lung cancer mortality by 20%, compared with screening by chest x-ray in high-risk patients as defined mainly by their age and smoking history.
Based upon the results of this National Cancer Institute–sponsored study and other recent lung cancer screening studies, the National Comprehensive Cancer Network has recommended routine screening using low-dose helical CT for two high-risk groups: individuals aged 55-74 years with at least 30 pack-years of smoking who either are current smokers or who quit fewer than 15 years ago, and patients aged 50 or older with at least 20 pack-years of smoking and one additional risk factor. The additional risk factors are contact with radon, occupational exposure to asbestos or other carcinogens, a history of lung cancer in a first-degree relative, a personal history of any tobacco-related aerodigestive cancer, or chronic obstructive pulmonary disease.
Dr. Keith reported that he serves on the speakers bureaus for Boehringer Ingelheim and Pfizer.
EXPERT OPINION FROM AN UPDATE IN INTERNAL MEDICINE SPONSORED BY THE UNIVERSITY OF COLORADO
Better Atopy Outcome Measures Demanded
RALEIGH, N.C. – Atopic dermatitis is a disease that until now has gone underappreciated by regulatory authorities, the pharmaceutical industry, and the general public, according to thought leaders determined to turn the situation around.
"The first and probably foremost reason atopic dermatitis has been somewhat ignored is that it is perceived as a childhood disease, and that makes development of drugs very, very difficult. First, you have to go through studies in adults and then you have to go through children," Dr. Lisa A. Beck noted during a special plenary session devoted to atopic dermatitis at the conference.
Another stumbling block is that researchers haven’t adequately documented the negative effects atopic dermatitis can have on daily life for patients and caregivers. Clinicians who care for atopy patients are aware of it. The outside world is not, observed Dr. Beck, professor of dermatology and medicine at the University of Rochester (N.Y.). Also, better diagnostic criteria for atopic dermatitis are needed. The diagnosis isn’t always as straightforward as in psoriasis, which is "the great comparator in our range of diseases," she said.
A damaging perception that’s particularly entrenched within the regulatory agencies is that atopic dermatitis is not high priority because it lacks serious comorbidities, Dr. Beck continued.
Research Barriers
"That’s something we come up against in atopic dermatitis all the time: the idea that it’s a disease of misery rather than a disease of mortality," agreed Dr. Neil Graham of Regeneron Pharmaceuticals in Tarrytown, N.Y. "However, our view is that human misery is something we should be trying to treat therapeutically, and that this has value both economically and to society. I think we’ve seen this accomplished successfully with psoriasis, which is the template we can use in atopic dermatitis."
Biopharmaceutical companies are interested in applying the psoriasis drug development template to atopic dermatitis. Regeneron is developing a biologic agent that simultaneously blocks interleukins-4 and -13 for the treatment of atopic dermatitis and other diseases whose predominant mechanism involves Th2-driven eosinophilic inflammation, including eosinophilic asthma, chronic sinusitis with nasal polyps, and conjunctival allergic disease. The company wants an agent that can be subcutaneously injected every week or two, noted Dr. Graham.
"We hope eventually to intervene in children and potentially interrupt the atopic march. It will probably take us many years to get there," he said.
Regulatory agencies will almost certainly require that clinical trials involving any biologic agent under development for atopic dermatitis initially be restricted to patients with moderate to severe disease.
That’s a problem, according to Dr. Graham, because the Food and Drug Administration defines the clinical severity of atopic dermatitis using the Investigator’s Global Assessment (IGA) scale: a limited tool that’s not up to the task of capturing the full impact of the disease. Better means of defining the moderate to severely affected patient subset are essential. Also, a standardized, clinically relevant measure of disease severity reduction in response to treatment is needed – something akin to the Psoriasis Area Severity Index (PASI) 50 or -75 as used in psoriasis.
Dr. Eric Simpson agreed that regulatory authorities haven’t devoted sufficient attention to study design requirements or definitions of therapeutic success. For instance, the FDA requires evidence of improvement in the IGA as the bar that must be cleared in order to obtain approval of drugs for atopic dermatitis. Yet the IGA has never been adequately validated, nor has the metric’s definition been standardized. Indeed, the definition changes over time, from one phase-III clinical trial to the next.
"The inter-relater reliability of the IGA is unknown. I think it could be a major issue," said Dr. Simpson of the Oregon Health and Science University, Portland.
He proposed that participants in the SID special session on atopic dermatitis create a position paper recommending better outcome measures to the FDA. "We need to work with the agency to do what’s best for good clinical research," he said.
Identifying Biomarkers
According to Dr. Graham, atopic dermatitis is on the radar of major biotech companies that have biologic agents for psoriasis. They are working to develop biologics for atopic dermatitis, targeting a variety of pathways including interleukins-5, -4, and -13, as well as IgE.
Reliable biomarkers are needed to make drug development in atopic dermatitis go quickly and efficiently. After all, biomarker data are now routinely incorporated into early-phase development of new drugs for psoriasis, and these data are included in support of new drug applications to the FDA. But objective biomarkers have been notably lacking in atopic dermatitis, he noted.
Dr. Emma Guttman-Yassky announced help is at hand. While efforts to identify blood biomarkers have proved deeply disappointing, skin biomarkers are another story. She and her coinvestigators have identified a set of reliable skin biomarkers of therapeutic response in atopic dermatitis patients.
"We believe that these biomarkers represent the molecular fingerprints of the disease and might be key to future development of targeted treatments," said Dr. Guttman-Yassky, director of occupational and contact dermatitis at Mount Sinai Medical Center, New York.
She and her coinvestigators took lesional and nonlesional skin biopsies from 12 atopic dermatitis patients before and after undergoing narrow-band UVB phototherapy three times weekly for 12 weeks, a regimen the patients responded to with a mean 81% reduction in SCORAD index scores. Reversal of epidermal hyperplasia and abnormal keratinocyte differentiation – the histologic hallmarks of atopic dermatitis – was associated with elimination of inflammatory leukocytes and Th2-associated cytokines and chemokines (J. Allergy Clin. Immunol. 2011;128:583-93).
Among the skin biomarkers of atopic dermatitis that reversed with effective therapy were inflammatory dendritic epidermal cells, myeloid and plasmacytoid dendritic cells, CD3+ T cells, and interleukins-4 and -13.
This study, as well as another by Dr. Guttman-Yassky and her colleagues (in press), demonstrated a major role for interleukin-22 in atopic dermatitis. The cytokine was markedly overexpressed in lesional skin, with resultant upregulation of the antimicrobial protein’s S100A7, S100A8, and S100A9. After narrow-band UVB therapy, expression of IL-22 expression was downregulated, as were the IL-22-induced chemokines S100A7-9.
Expression of the three antimicrobial proteins in lesional skin also appears to be useful as a biomarker defining acute disease. Their levels jump within 3 days after onset of acute atopic dermatitis, according to Dr. Guttman-Yassky.
"Are we there yet in terms of biomarkers in atopic dermatitis? That’s the million-dollar question. Regarding biomarker availability, I think yes. We now have biomarkers that are highly informative on the reversal of disease pathology with treatment," she said. "I believe that the next step is clinical trials with specific immune antagonists that incorporate biomarkers of response. I think biomarkers are key if we want to know if new therapies really work. This will enhance early decisions and reduce the cost of developing new treatments."
An important implication of the narrow-band UVB/biomarker study in terms of atopic dermatitis pathogenesis is that the data argue against a fixed genetic phenotype, since the atopic dermatitis epidermal phenotype was shown to be reversed with broad-based immune-targeted phototherapy. Instead, the study results argue in favor of what Dr. Guttman-Yassky called "the inside-out hypothesis" of atopic dermatitis, which postulates that the epidermal abnormalities that define the disease are caused by underlying immune activation.
Her studies were supported by the National Institutes of Health.
HOME Project Seeks Standardization
During the same session, Dr. Hywel C. Williams provided an update on a major international project called Harmonising Outcome Measures for Eczema (HOME). The goal of the HOME project is to develop a consensus on a core set of outcome measures to be used in all atopic dermatitis clinical research. This should make future studies easier to compare, contrast, and synthesize in meta-analyses.
A minimum set of core outcome measures in atopic dermatitis is needed because the situation can be chaotic, noted Dr. Williams, professor of dermatology and director of the Center for Evidence-Based Dermatology at the University of Nottingham (U.K.).
"There are more than 20 named scales for atopic dermatitis, including SCORAD, POEM, SASSAD, ADASI, ADAM, EASI, and the FSSS. Some have been only partly tested. Many have not been tested at all. At international meetings I see people shouting at each other, trying to communicate what the results of their studies mean. Some people are in the SCORAD camp, some are in the SASSAD camp, others are in the EASI camp. How can we possibly communicate? This is a shameful situation which we really have to put right if we are to progress," he said.
The HOME project is modeled after Outcome Measures in Rheumatology, or OMERACT, an international consensus group that meets every 2 years with the goal of raising the quality of rheumatologic research. HOME comprises atopic dermatitis researchers, clinical experts, journal editors, patient advocates, and representatives from the European regulatory agency. The FDA was invited but didn’t attend last year’s HOME meeting in Amsterdam, where working groups were formed to identify the best evidence-based instruments for assessing atopic disease signs, symptoms, flares, quality of life, and treatment efficacy and safety. The HOME group will meet next year in San Diego on April 6-7.
Aside from Dr. Graham, a Regeneron employee, the other speakers in the special session reported receiving research grants from various sources but declared having no conflicts of interest with regard to their presentations.
RALEIGH, N.C. – Atopic dermatitis is a disease that until now has gone underappreciated by regulatory authorities, the pharmaceutical industry, and the general public, according to thought leaders determined to turn the situation around.
"The first and probably foremost reason atopic dermatitis has been somewhat ignored is that it is perceived as a childhood disease, and that makes development of drugs very, very difficult. First, you have to go through studies in adults and then you have to go through children," Dr. Lisa A. Beck noted during a special plenary session devoted to atopic dermatitis at the conference.
Another stumbling block is that researchers haven’t adequately documented the negative effects atopic dermatitis can have on daily life for patients and caregivers. Clinicians who care for atopy patients are aware of it. The outside world is not, observed Dr. Beck, professor of dermatology and medicine at the University of Rochester (N.Y.). Also, better diagnostic criteria for atopic dermatitis are needed. The diagnosis isn’t always as straightforward as in psoriasis, which is "the great comparator in our range of diseases," she said.
A damaging perception that’s particularly entrenched within the regulatory agencies is that atopic dermatitis is not high priority because it lacks serious comorbidities, Dr. Beck continued.
Research Barriers
"That’s something we come up against in atopic dermatitis all the time: the idea that it’s a disease of misery rather than a disease of mortality," agreed Dr. Neil Graham of Regeneron Pharmaceuticals in Tarrytown, N.Y. "However, our view is that human misery is something we should be trying to treat therapeutically, and that this has value both economically and to society. I think we’ve seen this accomplished successfully with psoriasis, which is the template we can use in atopic dermatitis."
Biopharmaceutical companies are interested in applying the psoriasis drug development template to atopic dermatitis. Regeneron is developing a biologic agent that simultaneously blocks interleukins-4 and -13 for the treatment of atopic dermatitis and other diseases whose predominant mechanism involves Th2-driven eosinophilic inflammation, including eosinophilic asthma, chronic sinusitis with nasal polyps, and conjunctival allergic disease. The company wants an agent that can be subcutaneously injected every week or two, noted Dr. Graham.
"We hope eventually to intervene in children and potentially interrupt the atopic march. It will probably take us many years to get there," he said.
Regulatory agencies will almost certainly require that clinical trials involving any biologic agent under development for atopic dermatitis initially be restricted to patients with moderate to severe disease.
That’s a problem, according to Dr. Graham, because the Food and Drug Administration defines the clinical severity of atopic dermatitis using the Investigator’s Global Assessment (IGA) scale: a limited tool that’s not up to the task of capturing the full impact of the disease. Better means of defining the moderate to severely affected patient subset are essential. Also, a standardized, clinically relevant measure of disease severity reduction in response to treatment is needed – something akin to the Psoriasis Area Severity Index (PASI) 50 or -75 as used in psoriasis.
Dr. Eric Simpson agreed that regulatory authorities haven’t devoted sufficient attention to study design requirements or definitions of therapeutic success. For instance, the FDA requires evidence of improvement in the IGA as the bar that must be cleared in order to obtain approval of drugs for atopic dermatitis. Yet the IGA has never been adequately validated, nor has the metric’s definition been standardized. Indeed, the definition changes over time, from one phase-III clinical trial to the next.
"The inter-relater reliability of the IGA is unknown. I think it could be a major issue," said Dr. Simpson of the Oregon Health and Science University, Portland.
He proposed that participants in the SID special session on atopic dermatitis create a position paper recommending better outcome measures to the FDA. "We need to work with the agency to do what’s best for good clinical research," he said.
Identifying Biomarkers
According to Dr. Graham, atopic dermatitis is on the radar of major biotech companies that have biologic agents for psoriasis. They are working to develop biologics for atopic dermatitis, targeting a variety of pathways including interleukins-5, -4, and -13, as well as IgE.
Reliable biomarkers are needed to make drug development in atopic dermatitis go quickly and efficiently. After all, biomarker data are now routinely incorporated into early-phase development of new drugs for psoriasis, and these data are included in support of new drug applications to the FDA. But objective biomarkers have been notably lacking in atopic dermatitis, he noted.
Dr. Emma Guttman-Yassky announced help is at hand. While efforts to identify blood biomarkers have proved deeply disappointing, skin biomarkers are another story. She and her coinvestigators have identified a set of reliable skin biomarkers of therapeutic response in atopic dermatitis patients.
"We believe that these biomarkers represent the molecular fingerprints of the disease and might be key to future development of targeted treatments," said Dr. Guttman-Yassky, director of occupational and contact dermatitis at Mount Sinai Medical Center, New York.
She and her coinvestigators took lesional and nonlesional skin biopsies from 12 atopic dermatitis patients before and after undergoing narrow-band UVB phototherapy three times weekly for 12 weeks, a regimen the patients responded to with a mean 81% reduction in SCORAD index scores. Reversal of epidermal hyperplasia and abnormal keratinocyte differentiation – the histologic hallmarks of atopic dermatitis – was associated with elimination of inflammatory leukocytes and Th2-associated cytokines and chemokines (J. Allergy Clin. Immunol. 2011;128:583-93).
Among the skin biomarkers of atopic dermatitis that reversed with effective therapy were inflammatory dendritic epidermal cells, myeloid and plasmacytoid dendritic cells, CD3+ T cells, and interleukins-4 and -13.
This study, as well as another by Dr. Guttman-Yassky and her colleagues (in press), demonstrated a major role for interleukin-22 in atopic dermatitis. The cytokine was markedly overexpressed in lesional skin, with resultant upregulation of the antimicrobial protein’s S100A7, S100A8, and S100A9. After narrow-band UVB therapy, expression of IL-22 expression was downregulated, as were the IL-22-induced chemokines S100A7-9.
Expression of the three antimicrobial proteins in lesional skin also appears to be useful as a biomarker defining acute disease. Their levels jump within 3 days after onset of acute atopic dermatitis, according to Dr. Guttman-Yassky.
"Are we there yet in terms of biomarkers in atopic dermatitis? That’s the million-dollar question. Regarding biomarker availability, I think yes. We now have biomarkers that are highly informative on the reversal of disease pathology with treatment," she said. "I believe that the next step is clinical trials with specific immune antagonists that incorporate biomarkers of response. I think biomarkers are key if we want to know if new therapies really work. This will enhance early decisions and reduce the cost of developing new treatments."
An important implication of the narrow-band UVB/biomarker study in terms of atopic dermatitis pathogenesis is that the data argue against a fixed genetic phenotype, since the atopic dermatitis epidermal phenotype was shown to be reversed with broad-based immune-targeted phototherapy. Instead, the study results argue in favor of what Dr. Guttman-Yassky called "the inside-out hypothesis" of atopic dermatitis, which postulates that the epidermal abnormalities that define the disease are caused by underlying immune activation.
Her studies were supported by the National Institutes of Health.
HOME Project Seeks Standardization
During the same session, Dr. Hywel C. Williams provided an update on a major international project called Harmonising Outcome Measures for Eczema (HOME). The goal of the HOME project is to develop a consensus on a core set of outcome measures to be used in all atopic dermatitis clinical research. This should make future studies easier to compare, contrast, and synthesize in meta-analyses.
A minimum set of core outcome measures in atopic dermatitis is needed because the situation can be chaotic, noted Dr. Williams, professor of dermatology and director of the Center for Evidence-Based Dermatology at the University of Nottingham (U.K.).
"There are more than 20 named scales for atopic dermatitis, including SCORAD, POEM, SASSAD, ADASI, ADAM, EASI, and the FSSS. Some have been only partly tested. Many have not been tested at all. At international meetings I see people shouting at each other, trying to communicate what the results of their studies mean. Some people are in the SCORAD camp, some are in the SASSAD camp, others are in the EASI camp. How can we possibly communicate? This is a shameful situation which we really have to put right if we are to progress," he said.
The HOME project is modeled after Outcome Measures in Rheumatology, or OMERACT, an international consensus group that meets every 2 years with the goal of raising the quality of rheumatologic research. HOME comprises atopic dermatitis researchers, clinical experts, journal editors, patient advocates, and representatives from the European regulatory agency. The FDA was invited but didn’t attend last year’s HOME meeting in Amsterdam, where working groups were formed to identify the best evidence-based instruments for assessing atopic disease signs, symptoms, flares, quality of life, and treatment efficacy and safety. The HOME group will meet next year in San Diego on April 6-7.
Aside from Dr. Graham, a Regeneron employee, the other speakers in the special session reported receiving research grants from various sources but declared having no conflicts of interest with regard to their presentations.
RALEIGH, N.C. – Atopic dermatitis is a disease that until now has gone underappreciated by regulatory authorities, the pharmaceutical industry, and the general public, according to thought leaders determined to turn the situation around.
"The first and probably foremost reason atopic dermatitis has been somewhat ignored is that it is perceived as a childhood disease, and that makes development of drugs very, very difficult. First, you have to go through studies in adults and then you have to go through children," Dr. Lisa A. Beck noted during a special plenary session devoted to atopic dermatitis at the conference.
Another stumbling block is that researchers haven’t adequately documented the negative effects atopic dermatitis can have on daily life for patients and caregivers. Clinicians who care for atopy patients are aware of it. The outside world is not, observed Dr. Beck, professor of dermatology and medicine at the University of Rochester (N.Y.). Also, better diagnostic criteria for atopic dermatitis are needed. The diagnosis isn’t always as straightforward as in psoriasis, which is "the great comparator in our range of diseases," she said.
A damaging perception that’s particularly entrenched within the regulatory agencies is that atopic dermatitis is not high priority because it lacks serious comorbidities, Dr. Beck continued.
Research Barriers
"That’s something we come up against in atopic dermatitis all the time: the idea that it’s a disease of misery rather than a disease of mortality," agreed Dr. Neil Graham of Regeneron Pharmaceuticals in Tarrytown, N.Y. "However, our view is that human misery is something we should be trying to treat therapeutically, and that this has value both economically and to society. I think we’ve seen this accomplished successfully with psoriasis, which is the template we can use in atopic dermatitis."
Biopharmaceutical companies are interested in applying the psoriasis drug development template to atopic dermatitis. Regeneron is developing a biologic agent that simultaneously blocks interleukins-4 and -13 for the treatment of atopic dermatitis and other diseases whose predominant mechanism involves Th2-driven eosinophilic inflammation, including eosinophilic asthma, chronic sinusitis with nasal polyps, and conjunctival allergic disease. The company wants an agent that can be subcutaneously injected every week or two, noted Dr. Graham.
"We hope eventually to intervene in children and potentially interrupt the atopic march. It will probably take us many years to get there," he said.
Regulatory agencies will almost certainly require that clinical trials involving any biologic agent under development for atopic dermatitis initially be restricted to patients with moderate to severe disease.
That’s a problem, according to Dr. Graham, because the Food and Drug Administration defines the clinical severity of atopic dermatitis using the Investigator’s Global Assessment (IGA) scale: a limited tool that’s not up to the task of capturing the full impact of the disease. Better means of defining the moderate to severely affected patient subset are essential. Also, a standardized, clinically relevant measure of disease severity reduction in response to treatment is needed – something akin to the Psoriasis Area Severity Index (PASI) 50 or -75 as used in psoriasis.
Dr. Eric Simpson agreed that regulatory authorities haven’t devoted sufficient attention to study design requirements or definitions of therapeutic success. For instance, the FDA requires evidence of improvement in the IGA as the bar that must be cleared in order to obtain approval of drugs for atopic dermatitis. Yet the IGA has never been adequately validated, nor has the metric’s definition been standardized. Indeed, the definition changes over time, from one phase-III clinical trial to the next.
"The inter-relater reliability of the IGA is unknown. I think it could be a major issue," said Dr. Simpson of the Oregon Health and Science University, Portland.
He proposed that participants in the SID special session on atopic dermatitis create a position paper recommending better outcome measures to the FDA. "We need to work with the agency to do what’s best for good clinical research," he said.
Identifying Biomarkers
According to Dr. Graham, atopic dermatitis is on the radar of major biotech companies that have biologic agents for psoriasis. They are working to develop biologics for atopic dermatitis, targeting a variety of pathways including interleukins-5, -4, and -13, as well as IgE.
Reliable biomarkers are needed to make drug development in atopic dermatitis go quickly and efficiently. After all, biomarker data are now routinely incorporated into early-phase development of new drugs for psoriasis, and these data are included in support of new drug applications to the FDA. But objective biomarkers have been notably lacking in atopic dermatitis, he noted.
Dr. Emma Guttman-Yassky announced help is at hand. While efforts to identify blood biomarkers have proved deeply disappointing, skin biomarkers are another story. She and her coinvestigators have identified a set of reliable skin biomarkers of therapeutic response in atopic dermatitis patients.
"We believe that these biomarkers represent the molecular fingerprints of the disease and might be key to future development of targeted treatments," said Dr. Guttman-Yassky, director of occupational and contact dermatitis at Mount Sinai Medical Center, New York.
She and her coinvestigators took lesional and nonlesional skin biopsies from 12 atopic dermatitis patients before and after undergoing narrow-band UVB phototherapy three times weekly for 12 weeks, a regimen the patients responded to with a mean 81% reduction in SCORAD index scores. Reversal of epidermal hyperplasia and abnormal keratinocyte differentiation – the histologic hallmarks of atopic dermatitis – was associated with elimination of inflammatory leukocytes and Th2-associated cytokines and chemokines (J. Allergy Clin. Immunol. 2011;128:583-93).
Among the skin biomarkers of atopic dermatitis that reversed with effective therapy were inflammatory dendritic epidermal cells, myeloid and plasmacytoid dendritic cells, CD3+ T cells, and interleukins-4 and -13.
This study, as well as another by Dr. Guttman-Yassky and her colleagues (in press), demonstrated a major role for interleukin-22 in atopic dermatitis. The cytokine was markedly overexpressed in lesional skin, with resultant upregulation of the antimicrobial protein’s S100A7, S100A8, and S100A9. After narrow-band UVB therapy, expression of IL-22 expression was downregulated, as were the IL-22-induced chemokines S100A7-9.
Expression of the three antimicrobial proteins in lesional skin also appears to be useful as a biomarker defining acute disease. Their levels jump within 3 days after onset of acute atopic dermatitis, according to Dr. Guttman-Yassky.
"Are we there yet in terms of biomarkers in atopic dermatitis? That’s the million-dollar question. Regarding biomarker availability, I think yes. We now have biomarkers that are highly informative on the reversal of disease pathology with treatment," she said. "I believe that the next step is clinical trials with specific immune antagonists that incorporate biomarkers of response. I think biomarkers are key if we want to know if new therapies really work. This will enhance early decisions and reduce the cost of developing new treatments."
An important implication of the narrow-band UVB/biomarker study in terms of atopic dermatitis pathogenesis is that the data argue against a fixed genetic phenotype, since the atopic dermatitis epidermal phenotype was shown to be reversed with broad-based immune-targeted phototherapy. Instead, the study results argue in favor of what Dr. Guttman-Yassky called "the inside-out hypothesis" of atopic dermatitis, which postulates that the epidermal abnormalities that define the disease are caused by underlying immune activation.
Her studies were supported by the National Institutes of Health.
HOME Project Seeks Standardization
During the same session, Dr. Hywel C. Williams provided an update on a major international project called Harmonising Outcome Measures for Eczema (HOME). The goal of the HOME project is to develop a consensus on a core set of outcome measures to be used in all atopic dermatitis clinical research. This should make future studies easier to compare, contrast, and synthesize in meta-analyses.
A minimum set of core outcome measures in atopic dermatitis is needed because the situation can be chaotic, noted Dr. Williams, professor of dermatology and director of the Center for Evidence-Based Dermatology at the University of Nottingham (U.K.).
"There are more than 20 named scales for atopic dermatitis, including SCORAD, POEM, SASSAD, ADASI, ADAM, EASI, and the FSSS. Some have been only partly tested. Many have not been tested at all. At international meetings I see people shouting at each other, trying to communicate what the results of their studies mean. Some people are in the SCORAD camp, some are in the SASSAD camp, others are in the EASI camp. How can we possibly communicate? This is a shameful situation which we really have to put right if we are to progress," he said.
The HOME project is modeled after Outcome Measures in Rheumatology, or OMERACT, an international consensus group that meets every 2 years with the goal of raising the quality of rheumatologic research. HOME comprises atopic dermatitis researchers, clinical experts, journal editors, patient advocates, and representatives from the European regulatory agency. The FDA was invited but didn’t attend last year’s HOME meeting in Amsterdam, where working groups were formed to identify the best evidence-based instruments for assessing atopic disease signs, symptoms, flares, quality of life, and treatment efficacy and safety. The HOME group will meet next year in San Diego on April 6-7.
Aside from Dr. Graham, a Regeneron employee, the other speakers in the special session reported receiving research grants from various sources but declared having no conflicts of interest with regard to their presentations.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
SAMe Is Worth Trying in Osteoarthritis, Fibromyalgia
ESTES PARK, COLO. – S-adenosyl-l-methionine doesn’t crack the annual top-10 lists of the most widely used nutritional supplements in complementary and alternative medicine surveys. But unlike other far more popular products, SAMe is supported by randomized trial evidence of efficacy for osteoarthritis, depression, and fibromyalgia.
Indeed, S-adenosyl-l-methionine (SAMe) is "buzz-worthy," Dr. Lisa Corbin declared at a conference on internal medicine sponsored by the University of Colorado.
The Natural Medicines Comprehensive Database, which she considers the best available source of physician information on supplements, rates SAMe as "likely effective" for depression and osteoarthritis and "possibly effective" for fibromyalgia, noted Dr. Corbin, medical director of the Center for Integrative Medicine and a general internist at the University of Colorado in Denver.
The Natural Medicines Comprehensive Database is subscription funded and eschews financial support from industry. The organization is famously tough in its efficacy ratings. "Likely effective" is about as good as it gets. Take, for example, hypertension. Nothing included in the natural medicines database has earned the coveted "effective" rating for treatment of hypertension, and only a single item is listed as "likely effective," namely the National Institutes of Health–backed DASH (Dietary Approaches to Stop Hypertension) diet.
SAMe is a naturally occurring homocysteine derivative present in all living human cells. Its synthesis is related to vitamin B12/folate metabolism, and SAMe serves as an essential methyl donor in cellular metabolism.
Osteoarthritis
SAMe appears to increase proteoglycan synthesis and has analgesic and anti-inflammatory effects. Numerous small studies of varying quality have demonstrated a benefit similar to that of non-cyclooxygenase-2 (non-COX2) inhibitor selective nonsteroidal anti-inflammatory drugs (NSAIDs), but with fewer side effects.
And in a University of California, Irvine, 16-week, double-blind, crossover trial of 61 patients with knee osteoarthritis, SAMe dosed at 400 mg t.i.d. and celecoxib at 200 mg/day demonstrated comparable pain reduction and improvement over time in functional health scores and isometric joint function tests.
SAMe was slower acting, taking almost a month to catch up to the COX-2 inhibitor in terms of analgesic effect. On the other hand, it appeared that the benefits of SAMe lasted after the medication was stopped.
Fibromyalgia
Danish rheumatologists randomized 43 patients with fibromyalgia to 800 mg/day of SAMe or placebo double-blind for 6 weeks. The SAMe group showed significant improvement compared with controls in fatigue, clinical disease activity, pain during the past week, mood, and morning stiffness, but not in tender point score or isokinetic muscle strength. Side effects in the SAMe group were no different from those with placebo (Scand. J. Rheumatol. 1991;20:294-302).
Another Danish double-blind randomized trial compared intravenous SAMe – a route of administration almost never used today – to placebo in 34 fibromyalgia patients. Ten days of IV SAMe at 600 mg/day showed nonsignificant trends toward better outcomes compared with placebo (Scand. J. Rheumatol. 1997; 26: 206-11).
Dr. Corbin said there have been no major safety issues with SAMe. At higher doses it has been associated with gastrointestinal side effects, headache, and loss of appetite.
"I think the financial toxicity is a potential issue," she quipped.
She urges her patients who are interested in trying dietary supplements to seek out those with "USP Verified" displayed prominently on the label. That designation signals that the product meets rigorous United States Pharmacopeia quality standards. However, SAMe is not a big seller, and no USP standards have been set for it. So she recommends that patients scan the shelves and select a SAMe product marketed by a company with USP labels on plenty of their other, more common supplements.
"That way you know that in general this manufacturer is making decent products, so that’s where I’d spend my money," Dr. Corbin said.
She reported having no financial interests relevant to her presentation.
ESTES PARK, COLO. – S-adenosyl-l-methionine doesn’t crack the annual top-10 lists of the most widely used nutritional supplements in complementary and alternative medicine surveys. But unlike other far more popular products, SAMe is supported by randomized trial evidence of efficacy for osteoarthritis, depression, and fibromyalgia.
Indeed, S-adenosyl-l-methionine (SAMe) is "buzz-worthy," Dr. Lisa Corbin declared at a conference on internal medicine sponsored by the University of Colorado.
The Natural Medicines Comprehensive Database, which she considers the best available source of physician information on supplements, rates SAMe as "likely effective" for depression and osteoarthritis and "possibly effective" for fibromyalgia, noted Dr. Corbin, medical director of the Center for Integrative Medicine and a general internist at the University of Colorado in Denver.
The Natural Medicines Comprehensive Database is subscription funded and eschews financial support from industry. The organization is famously tough in its efficacy ratings. "Likely effective" is about as good as it gets. Take, for example, hypertension. Nothing included in the natural medicines database has earned the coveted "effective" rating for treatment of hypertension, and only a single item is listed as "likely effective," namely the National Institutes of Health–backed DASH (Dietary Approaches to Stop Hypertension) diet.
SAMe is a naturally occurring homocysteine derivative present in all living human cells. Its synthesis is related to vitamin B12/folate metabolism, and SAMe serves as an essential methyl donor in cellular metabolism.
Osteoarthritis
SAMe appears to increase proteoglycan synthesis and has analgesic and anti-inflammatory effects. Numerous small studies of varying quality have demonstrated a benefit similar to that of non-cyclooxygenase-2 (non-COX2) inhibitor selective nonsteroidal anti-inflammatory drugs (NSAIDs), but with fewer side effects.
And in a University of California, Irvine, 16-week, double-blind, crossover trial of 61 patients with knee osteoarthritis, SAMe dosed at 400 mg t.i.d. and celecoxib at 200 mg/day demonstrated comparable pain reduction and improvement over time in functional health scores and isometric joint function tests.
SAMe was slower acting, taking almost a month to catch up to the COX-2 inhibitor in terms of analgesic effect. On the other hand, it appeared that the benefits of SAMe lasted after the medication was stopped.
Fibromyalgia
Danish rheumatologists randomized 43 patients with fibromyalgia to 800 mg/day of SAMe or placebo double-blind for 6 weeks. The SAMe group showed significant improvement compared with controls in fatigue, clinical disease activity, pain during the past week, mood, and morning stiffness, but not in tender point score or isokinetic muscle strength. Side effects in the SAMe group were no different from those with placebo (Scand. J. Rheumatol. 1991;20:294-302).
Another Danish double-blind randomized trial compared intravenous SAMe – a route of administration almost never used today – to placebo in 34 fibromyalgia patients. Ten days of IV SAMe at 600 mg/day showed nonsignificant trends toward better outcomes compared with placebo (Scand. J. Rheumatol. 1997; 26: 206-11).
Dr. Corbin said there have been no major safety issues with SAMe. At higher doses it has been associated with gastrointestinal side effects, headache, and loss of appetite.
"I think the financial toxicity is a potential issue," she quipped.
She urges her patients who are interested in trying dietary supplements to seek out those with "USP Verified" displayed prominently on the label. That designation signals that the product meets rigorous United States Pharmacopeia quality standards. However, SAMe is not a big seller, and no USP standards have been set for it. So she recommends that patients scan the shelves and select a SAMe product marketed by a company with USP labels on plenty of their other, more common supplements.
"That way you know that in general this manufacturer is making decent products, so that’s where I’d spend my money," Dr. Corbin said.
She reported having no financial interests relevant to her presentation.
ESTES PARK, COLO. – S-adenosyl-l-methionine doesn’t crack the annual top-10 lists of the most widely used nutritional supplements in complementary and alternative medicine surveys. But unlike other far more popular products, SAMe is supported by randomized trial evidence of efficacy for osteoarthritis, depression, and fibromyalgia.
Indeed, S-adenosyl-l-methionine (SAMe) is "buzz-worthy," Dr. Lisa Corbin declared at a conference on internal medicine sponsored by the University of Colorado.
The Natural Medicines Comprehensive Database, which she considers the best available source of physician information on supplements, rates SAMe as "likely effective" for depression and osteoarthritis and "possibly effective" for fibromyalgia, noted Dr. Corbin, medical director of the Center for Integrative Medicine and a general internist at the University of Colorado in Denver.
The Natural Medicines Comprehensive Database is subscription funded and eschews financial support from industry. The organization is famously tough in its efficacy ratings. "Likely effective" is about as good as it gets. Take, for example, hypertension. Nothing included in the natural medicines database has earned the coveted "effective" rating for treatment of hypertension, and only a single item is listed as "likely effective," namely the National Institutes of Health–backed DASH (Dietary Approaches to Stop Hypertension) diet.
SAMe is a naturally occurring homocysteine derivative present in all living human cells. Its synthesis is related to vitamin B12/folate metabolism, and SAMe serves as an essential methyl donor in cellular metabolism.
Osteoarthritis
SAMe appears to increase proteoglycan synthesis and has analgesic and anti-inflammatory effects. Numerous small studies of varying quality have demonstrated a benefit similar to that of non-cyclooxygenase-2 (non-COX2) inhibitor selective nonsteroidal anti-inflammatory drugs (NSAIDs), but with fewer side effects.
And in a University of California, Irvine, 16-week, double-blind, crossover trial of 61 patients with knee osteoarthritis, SAMe dosed at 400 mg t.i.d. and celecoxib at 200 mg/day demonstrated comparable pain reduction and improvement over time in functional health scores and isometric joint function tests.
SAMe was slower acting, taking almost a month to catch up to the COX-2 inhibitor in terms of analgesic effect. On the other hand, it appeared that the benefits of SAMe lasted after the medication was stopped.
Fibromyalgia
Danish rheumatologists randomized 43 patients with fibromyalgia to 800 mg/day of SAMe or placebo double-blind for 6 weeks. The SAMe group showed significant improvement compared with controls in fatigue, clinical disease activity, pain during the past week, mood, and morning stiffness, but not in tender point score or isokinetic muscle strength. Side effects in the SAMe group were no different from those with placebo (Scand. J. Rheumatol. 1991;20:294-302).
Another Danish double-blind randomized trial compared intravenous SAMe – a route of administration almost never used today – to placebo in 34 fibromyalgia patients. Ten days of IV SAMe at 600 mg/day showed nonsignificant trends toward better outcomes compared with placebo (Scand. J. Rheumatol. 1997; 26: 206-11).
Dr. Corbin said there have been no major safety issues with SAMe. At higher doses it has been associated with gastrointestinal side effects, headache, and loss of appetite.
"I think the financial toxicity is a potential issue," she quipped.
She urges her patients who are interested in trying dietary supplements to seek out those with "USP Verified" displayed prominently on the label. That designation signals that the product meets rigorous United States Pharmacopeia quality standards. However, SAMe is not a big seller, and no USP standards have been set for it. So she recommends that patients scan the shelves and select a SAMe product marketed by a company with USP labels on plenty of their other, more common supplements.
"That way you know that in general this manufacturer is making decent products, so that’s where I’d spend my money," Dr. Corbin said.
She reported having no financial interests relevant to her presentation.
EXPERT OPINION FROM A CONFERENCE ON INTERNAL MEDICINE SPONSORED BY THE UNIVERSITY OF COLORADO
Lyme Disease: Avoiding Inappropriate Serologic Testing
VAIL, COLO. – Misconceptions abound regarding the appropriate use of diagnostic serologic testing for Lyme disease. The end result is needless difficulties for patients, families, and their physicians, Dr. Roberta L. DeBiasi asserted at a conference on pediatric infectious diseases.
The first point to understand about serologic testing is that it isn’t even guideline recommended in patients who have early localized Lyme disease in the form of erythema migrans. Erythema migrans is a clinical diagnosis that in and of itself is sufficient to trigger the decision to treat.
"This is a major pitfall we see: A child or adult comes in with a classic rash of erythema migrans and the physician decides to do the serology. It comes back negative because the sensitivity and specificity of serologic testing at that stage is abysmal. So the patient’s Lyme disease goes untreated," she explained.
Children with untreated early-stage Lyme disease have up to a 50% risk of later developing Lyme arthritis, a 10% risk of Lyme meningitis, and a 5% risk of Lyme chronic carditis, according to Dr. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center in Washington.
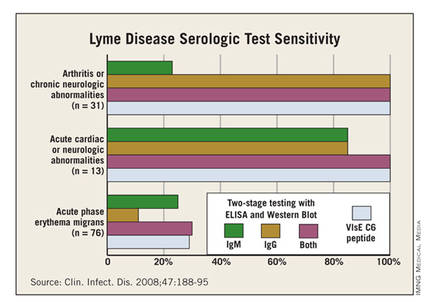
While there’s absolutely no point in doing serology in patients with early cutaneous disease manifestations only, the test sensitivity is far better in patients with extracutaneous manifestations of disease, such as meningitis, carditis, or arthritis. This was demonstrated in a classic study led by Dr. Allen C. Steere, professor of rheumatology at Harvard Medical School, Boston, who is credited with recognizing and naming the full syndrome he called Lyme disease (Clin. Infect. Dis. 2008;47:188-95).
Dr. Steere and his colleagues showed that, while the sensitivity of standard two-tiered testing was a mere 30% in the setting of acute-phase erythema migrans, it jumped to 100% in patients with arthritis, chronic neurologic abnormalities, or acute cardiac or neurologic abnormalities.
Erythema migrans appears 3-30 days and a median of 11 days after an Ixodes tick bite. It starts as a red macule or papule at the bite site and expands over days to weeks – not hours – eventually reaching a diameter of 5 cm or more, and in some cases as large as 30 cm. Circular or oval-shaped rashes are the most common. Partial clearing in the center, known as a bull’s eye, is common but not required. Up to 10% of patients with erythema migrans will have a small necrotic or vesicular/pustular are in the center of the lesion.
The most helpful features in distinguishing erythema migrans from similar-looking skin lesions are that it’s neither itchy nor painful.
"If it’s really itchy it’s usually an insect bite. If it really hurts it’s usually cellulitis," according to Dr. DeBiasi.
Differentiating erythema migrans from a tick bite hypersensitivity reaction, which is not an indicator of Borrelia burgdorferi infection, is a matter of the timing of the lesion’s onset and disappearance. Tick bite hypersensitivity reactions are erythematous lesions which appear while the tick is still attached or within 48 hours after detachment. That’s earlier than erythema migrans. And the hypersensitivity reaction typically begins to disappear 24-48 hours after its arrival; in contrast, erythema migrans grows larger during those first days after its initial appearance.
The Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) recommend treating erythema migrans without neurologic or cardiac involvement with 14 days of oral therapy. The American Academy of Pediatrics Red Book recommends 14-21 days. The first-line options are doxycycline in patients above age 8 years, amoxicillin in those under that age, and cefuroxime. Macrolide antibiotics aren’t recommended as first-line therapy because they proved less effective in clinical trials. First-generation cephalosporins are ineffective. Intravenous ceftriaxone is no more effective than oral agents.
"You might be thinking, ‘Who in the world would use IV [intravenous] ceftriaxone for erythema migrans?’ but it happens all the time. In high-incidence areas for Lyme disease, we have lots of people who call themselves Lyme specialists who set up shop and put ads in the phone book. Patients who don’t know any better go to them and can get very dangerous treatments," Dr. DeBiasi explained.
Erythema migrans resolves within the first several days of appropriate antibiotic therapy, although the oft-accompanying flu-like systemic symptoms may linger for several weeks.
Dr. DeBiasi conceded Lyme serology is confusing, even for infectious disease physicians. The key message is that it must always be a two-step process: Use an enzyme immunoassay or immunofluorescence assay as a screening test and, if it’s positive or equivocal, send the specimen for a standard IgM/IgG Western immunoblot. Antibodies aren’t detectable in the first few weeks of infection but by week 4 the screening test will pick up nearly all B. burgdorferi–infected individuals, albeit with a 5%-7% false-positive rate.
The Western immunoblot is interpreted as either positive or negative; there is no "indeterminate" with this test. IgG testing is considered positive on the Western blot if at least 5 out of 10 bands are confirmatory, while IgM requires 2 out 3 confirmatory bands.
Most patients with early disseminated or late Lyme disease have persistent antibodies for months to years.
"In no way does that correlate with the efficacy of treatment," Dr. DeBiasi stressed. "That’s a common reason people come in to the clinic. They say, ‘I want you to prove that I’m cured, and this test is negative.’ You have to explain to patients that it’s a test of antibody response, not a test for bacteria in their blood, and that just like with a vaccine, the fact that they have an antibody response is a good thing."
Many patients come in requesting the relatively new Vls C6 antibody test. It’s licensed for the diagnosis of Lyme disease, but studies indicate its sensitivity and specificity aren’t superior to the standard two-step protocol. Dr. DeBiasi reserves the Vls C6 test for travelers who may have been infected with European strains of B. burgdorferi, a situation in which it is advantageous.
She urged her colleagues not to order Lyme serologic testing for patients with fatigue, achiness, and other nonspecific symptoms in the absence of accompanying specific, objective signs of infection, such as erythema migrans or arthritis. It’s a widespread practice, but positive test results in this setting are nearly always false-positives.
Misinformation abounds regarding Lyme disease, especially on the internet. Two resources she recommends as reliable to patients are www.cdc.gov/lyme and www.idsociety.org/lyme.
Dr. DeBiasi reported having no financial conflicts.
VAIL, COLO. – Misconceptions abound regarding the appropriate use of diagnostic serologic testing for Lyme disease. The end result is needless difficulties for patients, families, and their physicians, Dr. Roberta L. DeBiasi asserted at a conference on pediatric infectious diseases.
The first point to understand about serologic testing is that it isn’t even guideline recommended in patients who have early localized Lyme disease in the form of erythema migrans. Erythema migrans is a clinical diagnosis that in and of itself is sufficient to trigger the decision to treat.
"This is a major pitfall we see: A child or adult comes in with a classic rash of erythema migrans and the physician decides to do the serology. It comes back negative because the sensitivity and specificity of serologic testing at that stage is abysmal. So the patient’s Lyme disease goes untreated," she explained.
Children with untreated early-stage Lyme disease have up to a 50% risk of later developing Lyme arthritis, a 10% risk of Lyme meningitis, and a 5% risk of Lyme chronic carditis, according to Dr. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center in Washington.

While there’s absolutely no point in doing serology in patients with early cutaneous disease manifestations only, the test sensitivity is far better in patients with extracutaneous manifestations of disease, such as meningitis, carditis, or arthritis. This was demonstrated in a classic study led by Dr. Allen C. Steere, professor of rheumatology at Harvard Medical School, Boston, who is credited with recognizing and naming the full syndrome he called Lyme disease (Clin. Infect. Dis. 2008;47:188-95).
Dr. Steere and his colleagues showed that, while the sensitivity of standard two-tiered testing was a mere 30% in the setting of acute-phase erythema migrans, it jumped to 100% in patients with arthritis, chronic neurologic abnormalities, or acute cardiac or neurologic abnormalities.
Erythema migrans appears 3-30 days and a median of 11 days after an Ixodes tick bite. It starts as a red macule or papule at the bite site and expands over days to weeks – not hours – eventually reaching a diameter of 5 cm or more, and in some cases as large as 30 cm. Circular or oval-shaped rashes are the most common. Partial clearing in the center, known as a bull’s eye, is common but not required. Up to 10% of patients with erythema migrans will have a small necrotic or vesicular/pustular are in the center of the lesion.
The most helpful features in distinguishing erythema migrans from similar-looking skin lesions are that it’s neither itchy nor painful.
"If it’s really itchy it’s usually an insect bite. If it really hurts it’s usually cellulitis," according to Dr. DeBiasi.
Differentiating erythema migrans from a tick bite hypersensitivity reaction, which is not an indicator of Borrelia burgdorferi infection, is a matter of the timing of the lesion’s onset and disappearance. Tick bite hypersensitivity reactions are erythematous lesions which appear while the tick is still attached or within 48 hours after detachment. That’s earlier than erythema migrans. And the hypersensitivity reaction typically begins to disappear 24-48 hours after its arrival; in contrast, erythema migrans grows larger during those first days after its initial appearance.
The Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) recommend treating erythema migrans without neurologic or cardiac involvement with 14 days of oral therapy. The American Academy of Pediatrics Red Book recommends 14-21 days. The first-line options are doxycycline in patients above age 8 years, amoxicillin in those under that age, and cefuroxime. Macrolide antibiotics aren’t recommended as first-line therapy because they proved less effective in clinical trials. First-generation cephalosporins are ineffective. Intravenous ceftriaxone is no more effective than oral agents.
"You might be thinking, ‘Who in the world would use IV [intravenous] ceftriaxone for erythema migrans?’ but it happens all the time. In high-incidence areas for Lyme disease, we have lots of people who call themselves Lyme specialists who set up shop and put ads in the phone book. Patients who don’t know any better go to them and can get very dangerous treatments," Dr. DeBiasi explained.
Erythema migrans resolves within the first several days of appropriate antibiotic therapy, although the oft-accompanying flu-like systemic symptoms may linger for several weeks.
Dr. DeBiasi conceded Lyme serology is confusing, even for infectious disease physicians. The key message is that it must always be a two-step process: Use an enzyme immunoassay or immunofluorescence assay as a screening test and, if it’s positive or equivocal, send the specimen for a standard IgM/IgG Western immunoblot. Antibodies aren’t detectable in the first few weeks of infection but by week 4 the screening test will pick up nearly all B. burgdorferi–infected individuals, albeit with a 5%-7% false-positive rate.
The Western immunoblot is interpreted as either positive or negative; there is no "indeterminate" with this test. IgG testing is considered positive on the Western blot if at least 5 out of 10 bands are confirmatory, while IgM requires 2 out 3 confirmatory bands.
Most patients with early disseminated or late Lyme disease have persistent antibodies for months to years.
"In no way does that correlate with the efficacy of treatment," Dr. DeBiasi stressed. "That’s a common reason people come in to the clinic. They say, ‘I want you to prove that I’m cured, and this test is negative.’ You have to explain to patients that it’s a test of antibody response, not a test for bacteria in their blood, and that just like with a vaccine, the fact that they have an antibody response is a good thing."
Many patients come in requesting the relatively new Vls C6 antibody test. It’s licensed for the diagnosis of Lyme disease, but studies indicate its sensitivity and specificity aren’t superior to the standard two-step protocol. Dr. DeBiasi reserves the Vls C6 test for travelers who may have been infected with European strains of B. burgdorferi, a situation in which it is advantageous.
She urged her colleagues not to order Lyme serologic testing for patients with fatigue, achiness, and other nonspecific symptoms in the absence of accompanying specific, objective signs of infection, such as erythema migrans or arthritis. It’s a widespread practice, but positive test results in this setting are nearly always false-positives.
Misinformation abounds regarding Lyme disease, especially on the internet. Two resources she recommends as reliable to patients are www.cdc.gov/lyme and www.idsociety.org/lyme.
Dr. DeBiasi reported having no financial conflicts.
VAIL, COLO. – Misconceptions abound regarding the appropriate use of diagnostic serologic testing for Lyme disease. The end result is needless difficulties for patients, families, and their physicians, Dr. Roberta L. DeBiasi asserted at a conference on pediatric infectious diseases.
The first point to understand about serologic testing is that it isn’t even guideline recommended in patients who have early localized Lyme disease in the form of erythema migrans. Erythema migrans is a clinical diagnosis that in and of itself is sufficient to trigger the decision to treat.
"This is a major pitfall we see: A child or adult comes in with a classic rash of erythema migrans and the physician decides to do the serology. It comes back negative because the sensitivity and specificity of serologic testing at that stage is abysmal. So the patient’s Lyme disease goes untreated," she explained.
Children with untreated early-stage Lyme disease have up to a 50% risk of later developing Lyme arthritis, a 10% risk of Lyme meningitis, and a 5% risk of Lyme chronic carditis, according to Dr. DeBiasi, acting chief of the division of pediatric infectious diseases at Children’s National Medical Center in Washington.

While there’s absolutely no point in doing serology in patients with early cutaneous disease manifestations only, the test sensitivity is far better in patients with extracutaneous manifestations of disease, such as meningitis, carditis, or arthritis. This was demonstrated in a classic study led by Dr. Allen C. Steere, professor of rheumatology at Harvard Medical School, Boston, who is credited with recognizing and naming the full syndrome he called Lyme disease (Clin. Infect. Dis. 2008;47:188-95).
Dr. Steere and his colleagues showed that, while the sensitivity of standard two-tiered testing was a mere 30% in the setting of acute-phase erythema migrans, it jumped to 100% in patients with arthritis, chronic neurologic abnormalities, or acute cardiac or neurologic abnormalities.
Erythema migrans appears 3-30 days and a median of 11 days after an Ixodes tick bite. It starts as a red macule or papule at the bite site and expands over days to weeks – not hours – eventually reaching a diameter of 5 cm or more, and in some cases as large as 30 cm. Circular or oval-shaped rashes are the most common. Partial clearing in the center, known as a bull’s eye, is common but not required. Up to 10% of patients with erythema migrans will have a small necrotic or vesicular/pustular are in the center of the lesion.
The most helpful features in distinguishing erythema migrans from similar-looking skin lesions are that it’s neither itchy nor painful.
"If it’s really itchy it’s usually an insect bite. If it really hurts it’s usually cellulitis," according to Dr. DeBiasi.
Differentiating erythema migrans from a tick bite hypersensitivity reaction, which is not an indicator of Borrelia burgdorferi infection, is a matter of the timing of the lesion’s onset and disappearance. Tick bite hypersensitivity reactions are erythematous lesions which appear while the tick is still attached or within 48 hours after detachment. That’s earlier than erythema migrans. And the hypersensitivity reaction typically begins to disappear 24-48 hours after its arrival; in contrast, erythema migrans grows larger during those first days after its initial appearance.
The Infectious Diseases Society of America guidelines (Clin. Infect. Dis. 2006;43:1089-134) recommend treating erythema migrans without neurologic or cardiac involvement with 14 days of oral therapy. The American Academy of Pediatrics Red Book recommends 14-21 days. The first-line options are doxycycline in patients above age 8 years, amoxicillin in those under that age, and cefuroxime. Macrolide antibiotics aren’t recommended as first-line therapy because they proved less effective in clinical trials. First-generation cephalosporins are ineffective. Intravenous ceftriaxone is no more effective than oral agents.
"You might be thinking, ‘Who in the world would use IV [intravenous] ceftriaxone for erythema migrans?’ but it happens all the time. In high-incidence areas for Lyme disease, we have lots of people who call themselves Lyme specialists who set up shop and put ads in the phone book. Patients who don’t know any better go to them and can get very dangerous treatments," Dr. DeBiasi explained.
Erythema migrans resolves within the first several days of appropriate antibiotic therapy, although the oft-accompanying flu-like systemic symptoms may linger for several weeks.
Dr. DeBiasi conceded Lyme serology is confusing, even for infectious disease physicians. The key message is that it must always be a two-step process: Use an enzyme immunoassay or immunofluorescence assay as a screening test and, if it’s positive or equivocal, send the specimen for a standard IgM/IgG Western immunoblot. Antibodies aren’t detectable in the first few weeks of infection but by week 4 the screening test will pick up nearly all B. burgdorferi–infected individuals, albeit with a 5%-7% false-positive rate.
The Western immunoblot is interpreted as either positive or negative; there is no "indeterminate" with this test. IgG testing is considered positive on the Western blot if at least 5 out of 10 bands are confirmatory, while IgM requires 2 out 3 confirmatory bands.
Most patients with early disseminated or late Lyme disease have persistent antibodies for months to years.
"In no way does that correlate with the efficacy of treatment," Dr. DeBiasi stressed. "That’s a common reason people come in to the clinic. They say, ‘I want you to prove that I’m cured, and this test is negative.’ You have to explain to patients that it’s a test of antibody response, not a test for bacteria in their blood, and that just like with a vaccine, the fact that they have an antibody response is a good thing."
Many patients come in requesting the relatively new Vls C6 antibody test. It’s licensed for the diagnosis of Lyme disease, but studies indicate its sensitivity and specificity aren’t superior to the standard two-step protocol. Dr. DeBiasi reserves the Vls C6 test for travelers who may have been infected with European strains of B. burgdorferi, a situation in which it is advantageous.
She urged her colleagues not to order Lyme serologic testing for patients with fatigue, achiness, and other nonspecific symptoms in the absence of accompanying specific, objective signs of infection, such as erythema migrans or arthritis. It’s a widespread practice, but positive test results in this setting are nearly always false-positives.
Misinformation abounds regarding Lyme disease, especially on the internet. Two resources she recommends as reliable to patients are www.cdc.gov/lyme and www.idsociety.org/lyme.
Dr. DeBiasi reported having no financial conflicts.
EXPERT OPINION FROM THE ANNUAL PEDIATRIC INFECTIOUS DISEASES CONFERENCE SPONSORED BY CHILDREN'S HOSPITAL COLORADO