User login
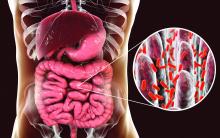
RA treatment responders show unique differences in gut microbiome
The gut microbiome, previously shown to have an association with rheumatoid arthritis, may also provide signals of a patient’s disease prognosis, researchers at the Mayo Clinic have reported.
“We found that the gut microbiome is linked to whether patients with RA improve in their clinical symptoms or not,” cosenior author Jaeyun Sung, PhD, said in an interview. “We found features of the gut microbiome that linked to improvement, and we also put those features in a machine-learning model that can actually predict improvement at a follow-up visit.” Dr. Sung is a computational biologist with Mayo Clinic’s Center for Individualized Medicine in Rochester, Minn.
The retrospective, observational cohort study included 32 patients diagnosed with RA between 1988 and 2014. The researchers performed meta-genome shotgun sequencing on 64 stool samples kept in a biobank and collected at two separate visits 6-12 months apart. Dr. Sung and colleagues observed significantly different microbiome traits between patients who eventually showed minimally clinically important improvement and those who didn’t.
The study also provided a proof of concept for using machine-learning technology to analyze the gut microbiome to predict the course of the disease, Dr. Sung said.
Cosenior author John M. Davis III, MD, a clinical rheumatologist and rheumatology research chair of the Mayo Clinic, noted that their own previous study had confirmed dysbiosis in people with RA when compared with controls. “We had some preliminary insight that it may be linked to some extent to the disease state and maybe treatments,” Dr. Davis said. “So that led us to hypothesize that there may be an association between the gut microbiome and response to treatment or disease activity over time.”
The study found that age was the dominant factor in determining variations in the gut microbiome composition, but the next prevailing factor was minimum clinically important improvement status, which 12 of the 32 study participants achieved at their follow-up visits. At baseline, all patients were on some type of treatment – either biologic or conventional disease-modifying antirheumatic drugs (DMARDs, 46.9% and 87.5%, respectively), or prednisone (46.9%).
Gut microbiome composition
The patients who achieved minimum clinically important improvement had an average decline in Clinical Disease Activity Index of 16.7 units (standard deviation, 12.8) versus a gain of 5.7 (SD, 8.9) in the remaining patients. The study found higher species-level alpha diversity and richness and higher beta diversity in the group that achieved minimum clinically important improvement, compared with those who did not.
They identified six microbial taxa as higher in abundance in the improved patients: Negativicutes (class); Selenomonadales (order); Prevotellaceae (family); Coprococcus (genus); Bacteroides sp. 3_1_19 (species); and Bilophila sp. 4_1_30 (species). In the patients who showed no improvement, Eubacterium sp. 3_1_31 (species) was found to be higher (P < .05). They also found 15 metabolic pathways that were differently abundant between the two groups at baseline.
Two things make this study different from other studies of the gut microbiome in RA, Dr. Sung said: It didn’t have a control group, only RA patients, and it didn’t evaluate a specific drug in RA patients.
“We’re thinking beyond just drug or treatment, independent of prior treatment, independent of prior clinical measurements, independent of age, sex, and other factors, can we predict RA response just using the gut microbiome alone?” Dr. Sung said. “Is there an association between clinical improvement and the gut microbiome?”
The study also showed that the microbiome may be a modifiable target for RA, Dr. Davis said.
“This research is attractive because it may complement medical treatment for RA if we can identify dietary modifications,” he said. “Still, there’s the question if probiotics or prebiotics can influence the gut. Can we modify the gut microbiome to further ameliorate the disease state? That’s something I think is an open question that’s specifically called out in our paper.”
This study included patients with long-term disease, but the group’s ongoing research is focusing on patients with earlier-stage RA, Dr. Davis said. “The next steps have to be in validating [the findings] in additional and external populations and looking at patients with very early disease where a lot of the decision-making is very active and happening in real time.”
James T. Rosenbaum, MD, an ophthalmologist and rheumatologist at Oregon Health & Science University, Portland, acknowledged that this is the first study in RA to find an effect on the gut microbiome using the minimum clinically important improvement endpoint.
“It also raises a ‘chicken-egg’ dilemma,” Dr. Rosenbaum said in an interview. “Did the patients improve and then their microbiome changed, or was the microbiome the first change that led to the clinical improvement? If the latter is correct, we potentially could alter the microbiome, for example, by diet, to treat rheumatic disease.”
He noted that studies with fecal transplants for ulcerative colitis support the therapeutic potential of microbiome modification. “But,” he added, “we are still a long way from putting this in practice.”
“The outcome is promising,” Claudia Mauri, PhD, a professor of immunology at University College London, said of the study. “Obviously, if this can be repeated in a very large cohort of patients, it would give us the possibility to be able to, based on the composition of the microbiota, to predict who is going to respond to treatment or not.”
She noted that, while RA has a broader array of available treatments than other autoimmune diseases, some RA patients don’t respond their first biologic treatment. “If from the outset we would be able to see who may not respond based on the microbiota, we may prepare physicians to better target these patients by, for example, offering them an alternative second biologic agent,” Dr. Mauri said.
Dr. Davis reported receiving research grants from Pfizer. Dr. Sung and other study coauthors have no financial relationships to disclose. Dr. Rosenbaum reported that the National Institutes of Health supports his research. Dr. Mauri has no financial relationships to disclose.
The gut microbiome, previously shown to have an association with rheumatoid arthritis, may also provide signals of a patient’s disease prognosis, researchers at the Mayo Clinic have reported.
“We found that the gut microbiome is linked to whether patients with RA improve in their clinical symptoms or not,” cosenior author Jaeyun Sung, PhD, said in an interview. “We found features of the gut microbiome that linked to improvement, and we also put those features in a machine-learning model that can actually predict improvement at a follow-up visit.” Dr. Sung is a computational biologist with Mayo Clinic’s Center for Individualized Medicine in Rochester, Minn.
The retrospective, observational cohort study included 32 patients diagnosed with RA between 1988 and 2014. The researchers performed meta-genome shotgun sequencing on 64 stool samples kept in a biobank and collected at two separate visits 6-12 months apart. Dr. Sung and colleagues observed significantly different microbiome traits between patients who eventually showed minimally clinically important improvement and those who didn’t.
The study also provided a proof of concept for using machine-learning technology to analyze the gut microbiome to predict the course of the disease, Dr. Sung said.
Cosenior author John M. Davis III, MD, a clinical rheumatologist and rheumatology research chair of the Mayo Clinic, noted that their own previous study had confirmed dysbiosis in people with RA when compared with controls. “We had some preliminary insight that it may be linked to some extent to the disease state and maybe treatments,” Dr. Davis said. “So that led us to hypothesize that there may be an association between the gut microbiome and response to treatment or disease activity over time.”
The study found that age was the dominant factor in determining variations in the gut microbiome composition, but the next prevailing factor was minimum clinically important improvement status, which 12 of the 32 study participants achieved at their follow-up visits. At baseline, all patients were on some type of treatment – either biologic or conventional disease-modifying antirheumatic drugs (DMARDs, 46.9% and 87.5%, respectively), or prednisone (46.9%).
Gut microbiome composition
The patients who achieved minimum clinically important improvement had an average decline in Clinical Disease Activity Index of 16.7 units (standard deviation, 12.8) versus a gain of 5.7 (SD, 8.9) in the remaining patients. The study found higher species-level alpha diversity and richness and higher beta diversity in the group that achieved minimum clinically important improvement, compared with those who did not.
They identified six microbial taxa as higher in abundance in the improved patients: Negativicutes (class); Selenomonadales (order); Prevotellaceae (family); Coprococcus (genus); Bacteroides sp. 3_1_19 (species); and Bilophila sp. 4_1_30 (species). In the patients who showed no improvement, Eubacterium sp. 3_1_31 (species) was found to be higher (P < .05). They also found 15 metabolic pathways that were differently abundant between the two groups at baseline.
Two things make this study different from other studies of the gut microbiome in RA, Dr. Sung said: It didn’t have a control group, only RA patients, and it didn’t evaluate a specific drug in RA patients.
“We’re thinking beyond just drug or treatment, independent of prior treatment, independent of prior clinical measurements, independent of age, sex, and other factors, can we predict RA response just using the gut microbiome alone?” Dr. Sung said. “Is there an association between clinical improvement and the gut microbiome?”
The study also showed that the microbiome may be a modifiable target for RA, Dr. Davis said.
“This research is attractive because it may complement medical treatment for RA if we can identify dietary modifications,” he said. “Still, there’s the question if probiotics or prebiotics can influence the gut. Can we modify the gut microbiome to further ameliorate the disease state? That’s something I think is an open question that’s specifically called out in our paper.”
This study included patients with long-term disease, but the group’s ongoing research is focusing on patients with earlier-stage RA, Dr. Davis said. “The next steps have to be in validating [the findings] in additional and external populations and looking at patients with very early disease where a lot of the decision-making is very active and happening in real time.”
James T. Rosenbaum, MD, an ophthalmologist and rheumatologist at Oregon Health & Science University, Portland, acknowledged that this is the first study in RA to find an effect on the gut microbiome using the minimum clinically important improvement endpoint.
“It also raises a ‘chicken-egg’ dilemma,” Dr. Rosenbaum said in an interview. “Did the patients improve and then their microbiome changed, or was the microbiome the first change that led to the clinical improvement? If the latter is correct, we potentially could alter the microbiome, for example, by diet, to treat rheumatic disease.”
He noted that studies with fecal transplants for ulcerative colitis support the therapeutic potential of microbiome modification. “But,” he added, “we are still a long way from putting this in practice.”
“The outcome is promising,” Claudia Mauri, PhD, a professor of immunology at University College London, said of the study. “Obviously, if this can be repeated in a very large cohort of patients, it would give us the possibility to be able to, based on the composition of the microbiota, to predict who is going to respond to treatment or not.”
She noted that, while RA has a broader array of available treatments than other autoimmune diseases, some RA patients don’t respond their first biologic treatment. “If from the outset we would be able to see who may not respond based on the microbiota, we may prepare physicians to better target these patients by, for example, offering them an alternative second biologic agent,” Dr. Mauri said.
Dr. Davis reported receiving research grants from Pfizer. Dr. Sung and other study coauthors have no financial relationships to disclose. Dr. Rosenbaum reported that the National Institutes of Health supports his research. Dr. Mauri has no financial relationships to disclose.
The gut microbiome, previously shown to have an association with rheumatoid arthritis, may also provide signals of a patient’s disease prognosis, researchers at the Mayo Clinic have reported.
“We found that the gut microbiome is linked to whether patients with RA improve in their clinical symptoms or not,” cosenior author Jaeyun Sung, PhD, said in an interview. “We found features of the gut microbiome that linked to improvement, and we also put those features in a machine-learning model that can actually predict improvement at a follow-up visit.” Dr. Sung is a computational biologist with Mayo Clinic’s Center for Individualized Medicine in Rochester, Minn.
The retrospective, observational cohort study included 32 patients diagnosed with RA between 1988 and 2014. The researchers performed meta-genome shotgun sequencing on 64 stool samples kept in a biobank and collected at two separate visits 6-12 months apart. Dr. Sung and colleagues observed significantly different microbiome traits between patients who eventually showed minimally clinically important improvement and those who didn’t.
The study also provided a proof of concept for using machine-learning technology to analyze the gut microbiome to predict the course of the disease, Dr. Sung said.
Cosenior author John M. Davis III, MD, a clinical rheumatologist and rheumatology research chair of the Mayo Clinic, noted that their own previous study had confirmed dysbiosis in people with RA when compared with controls. “We had some preliminary insight that it may be linked to some extent to the disease state and maybe treatments,” Dr. Davis said. “So that led us to hypothesize that there may be an association between the gut microbiome and response to treatment or disease activity over time.”
The study found that age was the dominant factor in determining variations in the gut microbiome composition, but the next prevailing factor was minimum clinically important improvement status, which 12 of the 32 study participants achieved at their follow-up visits. At baseline, all patients were on some type of treatment – either biologic or conventional disease-modifying antirheumatic drugs (DMARDs, 46.9% and 87.5%, respectively), or prednisone (46.9%).
Gut microbiome composition
The patients who achieved minimum clinically important improvement had an average decline in Clinical Disease Activity Index of 16.7 units (standard deviation, 12.8) versus a gain of 5.7 (SD, 8.9) in the remaining patients. The study found higher species-level alpha diversity and richness and higher beta diversity in the group that achieved minimum clinically important improvement, compared with those who did not.
They identified six microbial taxa as higher in abundance in the improved patients: Negativicutes (class); Selenomonadales (order); Prevotellaceae (family); Coprococcus (genus); Bacteroides sp. 3_1_19 (species); and Bilophila sp. 4_1_30 (species). In the patients who showed no improvement, Eubacterium sp. 3_1_31 (species) was found to be higher (P < .05). They also found 15 metabolic pathways that were differently abundant between the two groups at baseline.
Two things make this study different from other studies of the gut microbiome in RA, Dr. Sung said: It didn’t have a control group, only RA patients, and it didn’t evaluate a specific drug in RA patients.
“We’re thinking beyond just drug or treatment, independent of prior treatment, independent of prior clinical measurements, independent of age, sex, and other factors, can we predict RA response just using the gut microbiome alone?” Dr. Sung said. “Is there an association between clinical improvement and the gut microbiome?”
The study also showed that the microbiome may be a modifiable target for RA, Dr. Davis said.
“This research is attractive because it may complement medical treatment for RA if we can identify dietary modifications,” he said. “Still, there’s the question if probiotics or prebiotics can influence the gut. Can we modify the gut microbiome to further ameliorate the disease state? That’s something I think is an open question that’s specifically called out in our paper.”
This study included patients with long-term disease, but the group’s ongoing research is focusing on patients with earlier-stage RA, Dr. Davis said. “The next steps have to be in validating [the findings] in additional and external populations and looking at patients with very early disease where a lot of the decision-making is very active and happening in real time.”
James T. Rosenbaum, MD, an ophthalmologist and rheumatologist at Oregon Health & Science University, Portland, acknowledged that this is the first study in RA to find an effect on the gut microbiome using the minimum clinically important improvement endpoint.
“It also raises a ‘chicken-egg’ dilemma,” Dr. Rosenbaum said in an interview. “Did the patients improve and then their microbiome changed, or was the microbiome the first change that led to the clinical improvement? If the latter is correct, we potentially could alter the microbiome, for example, by diet, to treat rheumatic disease.”
He noted that studies with fecal transplants for ulcerative colitis support the therapeutic potential of microbiome modification. “But,” he added, “we are still a long way from putting this in practice.”
“The outcome is promising,” Claudia Mauri, PhD, a professor of immunology at University College London, said of the study. “Obviously, if this can be repeated in a very large cohort of patients, it would give us the possibility to be able to, based on the composition of the microbiota, to predict who is going to respond to treatment or not.”
She noted that, while RA has a broader array of available treatments than other autoimmune diseases, some RA patients don’t respond their first biologic treatment. “If from the outset we would be able to see who may not respond based on the microbiota, we may prepare physicians to better target these patients by, for example, offering them an alternative second biologic agent,” Dr. Mauri said.
Dr. Davis reported receiving research grants from Pfizer. Dr. Sung and other study coauthors have no financial relationships to disclose. Dr. Rosenbaum reported that the National Institutes of Health supports his research. Dr. Mauri has no financial relationships to disclose.
FROM GENOME MEDICINE
Friedreich’s ataxia treatment shows extended benefit
according to results of a clinical trial presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders.
The study, labeled the Delayed-Start Study, is an extension study of the two-part MOXIE phase 2 trial of omaveloxolone.
“This study shows two things,” said David Lynch, MD, PhD, of Children’s Hospital of Philadelphia. “It doesn’t matter when you started omaveloxolone for you to see a benefit; and that the benefit that the active group saw in the first part of the study was maintained as they went into the delayed-start part. So in fact omaveloxolone does modify the long-term behavior of the disease.”
Friedreich’s ataxia only affects about 22,000 people worldwide, and children typically present between the ages of 5 and 15, Dr. Lynch said.
The extension study included 73 patients who completed either of the first two parts of the MOXIe trial. The MOXIe trial randomized patients on a 3:1 basis to either omaveloxolone 2.5-300 mg or placebo for 12 weeks in the first part. The second part was a double-blind trial of 103 patients randomized on a 1:1 basis to 150 mg omaveloxolone or placebo for 48 weeks. Participants had a baseline modified Friedreich’s ataxia scale (mFARS) of 20-80 and were aged 16-40 years.
Patients in the extension study did not have severe pes cavus. The extension study was a 72-week evaluation of patients who were in either the treatment or placebo groups in the first two parts. There was a 4-week off-treatment period between the end of MOXIe part 2 and the beginning of the extension study, in which all patients received omaveloxolone.
At the end of the placebo-controlled study, patients taking omaveloxolone showed a –2.18-point (±0.96) difference in improvement in mFARS score (P = .027), compared with the placebo group, which was preserved at the end of the delayed-start period, with a –2.92-point (±2.13) improvement (P = .179), Dr. Lynch said.
In the extension study, former placebo patients who went on omaveloxolone had annualized mFARS slopes similar to the previously treated patients – 0.29 (±0.68) and 0.17 (±0.61), respectively (P = .85) – from weeks 48 to 144, Dr. Lynch said.
“This study showed that, when analyzed in a delayed-start fashion, it does not matter when you start omaveloxolone to see a benefit: Each cohort benefited almost equally once they started the drug,” Dr. Lynch said in an interview. “Also, in both groups, once they started omaveloxolone, they changed slower than people in natural history studies.”
A clinically meaningful difference?
Reached for comment, Massimo Pandolfo, MD, a neurologist at McGill University, Montreal, noted that the Delayed-Start Study included only patients without pes cavus, an indication that the patients had less severe disease. “It would be important to see how overall patients with Friedreich’s ataxia would have responded to the medication without this kind of selection,” Dr. Pandolfo said in an interview.
He also noted that the seemingly modest improvement in mFARS score could be an issue. “It’s a very difficult question: What is a clinically meaningful difference in this kind of rating scale? I would argue that probably 2 points is not a huge difference by itself, but it may be meaningful and one indicator of that is that if it was accompanied by also a significant difference in activities of daily living scale.”
In any event, Dr. Pandolfo said this is the first medication for Friedreich’s ataxia that has “survived” a randomized clinical trial.
Dr. Lynch said the study sponsor, Reata, may prepare a new drug application for omaveloxolone in patients ages 16 and older. “That would leave a need for investigation in younger FA patients.”
Dr. Lynch disclosed that his institution receives a grant from trial sponsor Reata to conduct the MOXIe trial. Dr. Pandolfo reports financial relationships with Design Therapeutics, Exicure and Voyager Therapeutics.
according to results of a clinical trial presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders.
The study, labeled the Delayed-Start Study, is an extension study of the two-part MOXIE phase 2 trial of omaveloxolone.
“This study shows two things,” said David Lynch, MD, PhD, of Children’s Hospital of Philadelphia. “It doesn’t matter when you started omaveloxolone for you to see a benefit; and that the benefit that the active group saw in the first part of the study was maintained as they went into the delayed-start part. So in fact omaveloxolone does modify the long-term behavior of the disease.”
Friedreich’s ataxia only affects about 22,000 people worldwide, and children typically present between the ages of 5 and 15, Dr. Lynch said.
The extension study included 73 patients who completed either of the first two parts of the MOXIe trial. The MOXIe trial randomized patients on a 3:1 basis to either omaveloxolone 2.5-300 mg or placebo for 12 weeks in the first part. The second part was a double-blind trial of 103 patients randomized on a 1:1 basis to 150 mg omaveloxolone or placebo for 48 weeks. Participants had a baseline modified Friedreich’s ataxia scale (mFARS) of 20-80 and were aged 16-40 years.
Patients in the extension study did not have severe pes cavus. The extension study was a 72-week evaluation of patients who were in either the treatment or placebo groups in the first two parts. There was a 4-week off-treatment period between the end of MOXIe part 2 and the beginning of the extension study, in which all patients received omaveloxolone.
At the end of the placebo-controlled study, patients taking omaveloxolone showed a –2.18-point (±0.96) difference in improvement in mFARS score (P = .027), compared with the placebo group, which was preserved at the end of the delayed-start period, with a –2.92-point (±2.13) improvement (P = .179), Dr. Lynch said.
In the extension study, former placebo patients who went on omaveloxolone had annualized mFARS slopes similar to the previously treated patients – 0.29 (±0.68) and 0.17 (±0.61), respectively (P = .85) – from weeks 48 to 144, Dr. Lynch said.
“This study showed that, when analyzed in a delayed-start fashion, it does not matter when you start omaveloxolone to see a benefit: Each cohort benefited almost equally once they started the drug,” Dr. Lynch said in an interview. “Also, in both groups, once they started omaveloxolone, they changed slower than people in natural history studies.”
A clinically meaningful difference?
Reached for comment, Massimo Pandolfo, MD, a neurologist at McGill University, Montreal, noted that the Delayed-Start Study included only patients without pes cavus, an indication that the patients had less severe disease. “It would be important to see how overall patients with Friedreich’s ataxia would have responded to the medication without this kind of selection,” Dr. Pandolfo said in an interview.
He also noted that the seemingly modest improvement in mFARS score could be an issue. “It’s a very difficult question: What is a clinically meaningful difference in this kind of rating scale? I would argue that probably 2 points is not a huge difference by itself, but it may be meaningful and one indicator of that is that if it was accompanied by also a significant difference in activities of daily living scale.”
In any event, Dr. Pandolfo said this is the first medication for Friedreich’s ataxia that has “survived” a randomized clinical trial.
Dr. Lynch said the study sponsor, Reata, may prepare a new drug application for omaveloxolone in patients ages 16 and older. “That would leave a need for investigation in younger FA patients.”
Dr. Lynch disclosed that his institution receives a grant from trial sponsor Reata to conduct the MOXIe trial. Dr. Pandolfo reports financial relationships with Design Therapeutics, Exicure and Voyager Therapeutics.
according to results of a clinical trial presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders.
The study, labeled the Delayed-Start Study, is an extension study of the two-part MOXIE phase 2 trial of omaveloxolone.
“This study shows two things,” said David Lynch, MD, PhD, of Children’s Hospital of Philadelphia. “It doesn’t matter when you started omaveloxolone for you to see a benefit; and that the benefit that the active group saw in the first part of the study was maintained as they went into the delayed-start part. So in fact omaveloxolone does modify the long-term behavior of the disease.”
Friedreich’s ataxia only affects about 22,000 people worldwide, and children typically present between the ages of 5 and 15, Dr. Lynch said.
The extension study included 73 patients who completed either of the first two parts of the MOXIe trial. The MOXIe trial randomized patients on a 3:1 basis to either omaveloxolone 2.5-300 mg or placebo for 12 weeks in the first part. The second part was a double-blind trial of 103 patients randomized on a 1:1 basis to 150 mg omaveloxolone or placebo for 48 weeks. Participants had a baseline modified Friedreich’s ataxia scale (mFARS) of 20-80 and were aged 16-40 years.
Patients in the extension study did not have severe pes cavus. The extension study was a 72-week evaluation of patients who were in either the treatment or placebo groups in the first two parts. There was a 4-week off-treatment period between the end of MOXIe part 2 and the beginning of the extension study, in which all patients received omaveloxolone.
At the end of the placebo-controlled study, patients taking omaveloxolone showed a –2.18-point (±0.96) difference in improvement in mFARS score (P = .027), compared with the placebo group, which was preserved at the end of the delayed-start period, with a –2.92-point (±2.13) improvement (P = .179), Dr. Lynch said.
In the extension study, former placebo patients who went on omaveloxolone had annualized mFARS slopes similar to the previously treated patients – 0.29 (±0.68) and 0.17 (±0.61), respectively (P = .85) – from weeks 48 to 144, Dr. Lynch said.
“This study showed that, when analyzed in a delayed-start fashion, it does not matter when you start omaveloxolone to see a benefit: Each cohort benefited almost equally once they started the drug,” Dr. Lynch said in an interview. “Also, in both groups, once they started omaveloxolone, they changed slower than people in natural history studies.”
A clinically meaningful difference?
Reached for comment, Massimo Pandolfo, MD, a neurologist at McGill University, Montreal, noted that the Delayed-Start Study included only patients without pes cavus, an indication that the patients had less severe disease. “It would be important to see how overall patients with Friedreich’s ataxia would have responded to the medication without this kind of selection,” Dr. Pandolfo said in an interview.
He also noted that the seemingly modest improvement in mFARS score could be an issue. “It’s a very difficult question: What is a clinically meaningful difference in this kind of rating scale? I would argue that probably 2 points is not a huge difference by itself, but it may be meaningful and one indicator of that is that if it was accompanied by also a significant difference in activities of daily living scale.”
In any event, Dr. Pandolfo said this is the first medication for Friedreich’s ataxia that has “survived” a randomized clinical trial.
Dr. Lynch said the study sponsor, Reata, may prepare a new drug application for omaveloxolone in patients ages 16 and older. “That would leave a need for investigation in younger FA patients.”
Dr. Lynch disclosed that his institution receives a grant from trial sponsor Reata to conduct the MOXIe trial. Dr. Pandolfo reports financial relationships with Design Therapeutics, Exicure and Voyager Therapeutics.
FROM MDS VIRTUAL CONGRESS 2021
Survey identifies clinicians’ unease with genetic testing
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
FROM MDS VIRTUAL CONGRESS 2021
Premature menopause a ‘warning sign’ for greater ASCVD risk
Premature menopause is well known to be linked to cardiovascular disease in women, but it may not carry as much weight as more traditional cardiovascular risk factors in determining a patient’s 10-year risk of having a heart attack or stroke in this population, a cohort study that evaluated the veracity of premature menopause found.
Premature menopause can serve as a “marker or warning sign” that cardiologists should pay closer attention to traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, lead study author Sadiya S. Khan, MD, MS, said in an interview. “When we looked at the addition of premature menopause into the risk-prediction equation, we did not see that it meaningfully improved the ability of the risk predictions of pooled cohort equations [PCEs] to identify who developed cardiovascular disease,” said Dr. Khan, a cardiologist at Northwestern University, Chicago.
The cohort study included 5,466 Black women and 10,584 White women from seven U.S. population-based cohorts, including the Women’s Health Initiative, of whom 951 and 1,039, respectively, self-reported early menopause. The cohort study researchers noted that the 2019 American College of Cardiology/American Heart Association guideline for prevention of CVD acknowledged premature menopause as risk-enhancing factor in the CVD assessment in women younger than 40.
The cohort study found that Black women had almost twice the rate of premature menopause than White women, 17.4% and 9.8%, respectively. And it found that premature menopause was significantly linked with ASCVD in both populations independent of traditional risk factors – a 24% greater risk for Black women and 28% greater risk for White women.
‘Surprising’ finding
However, when premature menopause was added to the pooled cohort equations per the 2013 ACC/AHA guideline, the researchers found no incremental benefit, a finding Dr. Khan called “really surprising to us.”
She added, “If we look at the differences in the characteristics of women who have premature menopause, compared with those who didn’t, there were slight differences in terms of higher blood pressure, higher body mass index, and slightly higher glucose. So maybe what we’re seeing – and this is more speculative – is that risk factors are developing after early menopause, and the focus should be earlier in the patient’s life course to try to prevent hypertension, diabetes, and obesity.”
Dr. Khan emphasized that the findings don’t obviate the value of premature menopause in assessing ASCVD risk in women. “We still know that this is an important marker for women and their risk for heart disease, and it should be a warning sign to pay close attention to those other risk factors and what other preventive measures can be taken,” she said.
Christie Ballantyne, MD, said it’s important to note that the study did not dismiss the relevance of premature menopause in shared decision-making for postmenopausal women. “It certainly doesn’t mean that premature menopause is not a risk,” Dr. Ballantyne said in an interview. “Premature menopause may cause a worsening of traditional CVD risk factors, so that’s one possible explanation for it. The other possible explanation is that women with worse ASCVD risk factors – who are more overweight, have higher blood pressure, and have more diabetes and insulin resistance – are more likely to have earlier menopause.” Dr. Ballantyne is chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston.
“You should still look very carefully at the patient’s risk factors, calculate the pooled cohort equations, and make sure you get a recommendation,” he said. “If their risks are up, give recommendations on how to improve diet and exercise. Consider if you need to treat lipids or treat blood pressure with more than diet and exercise because there’s nothing magical about 7.5%”, the threshold for lipid-lowering therapy in the ASCVD risk calculator.
Dr. Khan and coauthors disclosed receiving grants from the National Institutes of Health and the American Heart Association. One coauthor reported a financial relationship with HGM Biopharmaceuticals. Dr. Ballantyne is a lead investigator of the Atherosclerosis Risk in Communities study, one of the population-based cohorts used in the cohort study. He has no other relevant relationships to disclose.
Premature menopause is well known to be linked to cardiovascular disease in women, but it may not carry as much weight as more traditional cardiovascular risk factors in determining a patient’s 10-year risk of having a heart attack or stroke in this population, a cohort study that evaluated the veracity of premature menopause found.
Premature menopause can serve as a “marker or warning sign” that cardiologists should pay closer attention to traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, lead study author Sadiya S. Khan, MD, MS, said in an interview. “When we looked at the addition of premature menopause into the risk-prediction equation, we did not see that it meaningfully improved the ability of the risk predictions of pooled cohort equations [PCEs] to identify who developed cardiovascular disease,” said Dr. Khan, a cardiologist at Northwestern University, Chicago.
The cohort study included 5,466 Black women and 10,584 White women from seven U.S. population-based cohorts, including the Women’s Health Initiative, of whom 951 and 1,039, respectively, self-reported early menopause. The cohort study researchers noted that the 2019 American College of Cardiology/American Heart Association guideline for prevention of CVD acknowledged premature menopause as risk-enhancing factor in the CVD assessment in women younger than 40.
The cohort study found that Black women had almost twice the rate of premature menopause than White women, 17.4% and 9.8%, respectively. And it found that premature menopause was significantly linked with ASCVD in both populations independent of traditional risk factors – a 24% greater risk for Black women and 28% greater risk for White women.
‘Surprising’ finding
However, when premature menopause was added to the pooled cohort equations per the 2013 ACC/AHA guideline, the researchers found no incremental benefit, a finding Dr. Khan called “really surprising to us.”
She added, “If we look at the differences in the characteristics of women who have premature menopause, compared with those who didn’t, there were slight differences in terms of higher blood pressure, higher body mass index, and slightly higher glucose. So maybe what we’re seeing – and this is more speculative – is that risk factors are developing after early menopause, and the focus should be earlier in the patient’s life course to try to prevent hypertension, diabetes, and obesity.”
Dr. Khan emphasized that the findings don’t obviate the value of premature menopause in assessing ASCVD risk in women. “We still know that this is an important marker for women and their risk for heart disease, and it should be a warning sign to pay close attention to those other risk factors and what other preventive measures can be taken,” she said.
Christie Ballantyne, MD, said it’s important to note that the study did not dismiss the relevance of premature menopause in shared decision-making for postmenopausal women. “It certainly doesn’t mean that premature menopause is not a risk,” Dr. Ballantyne said in an interview. “Premature menopause may cause a worsening of traditional CVD risk factors, so that’s one possible explanation for it. The other possible explanation is that women with worse ASCVD risk factors – who are more overweight, have higher blood pressure, and have more diabetes and insulin resistance – are more likely to have earlier menopause.” Dr. Ballantyne is chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston.
“You should still look very carefully at the patient’s risk factors, calculate the pooled cohort equations, and make sure you get a recommendation,” he said. “If their risks are up, give recommendations on how to improve diet and exercise. Consider if you need to treat lipids or treat blood pressure with more than diet and exercise because there’s nothing magical about 7.5%”, the threshold for lipid-lowering therapy in the ASCVD risk calculator.
Dr. Khan and coauthors disclosed receiving grants from the National Institutes of Health and the American Heart Association. One coauthor reported a financial relationship with HGM Biopharmaceuticals. Dr. Ballantyne is a lead investigator of the Atherosclerosis Risk in Communities study, one of the population-based cohorts used in the cohort study. He has no other relevant relationships to disclose.
Premature menopause is well known to be linked to cardiovascular disease in women, but it may not carry as much weight as more traditional cardiovascular risk factors in determining a patient’s 10-year risk of having a heart attack or stroke in this population, a cohort study that evaluated the veracity of premature menopause found.
Premature menopause can serve as a “marker or warning sign” that cardiologists should pay closer attention to traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, lead study author Sadiya S. Khan, MD, MS, said in an interview. “When we looked at the addition of premature menopause into the risk-prediction equation, we did not see that it meaningfully improved the ability of the risk predictions of pooled cohort equations [PCEs] to identify who developed cardiovascular disease,” said Dr. Khan, a cardiologist at Northwestern University, Chicago.
The cohort study included 5,466 Black women and 10,584 White women from seven U.S. population-based cohorts, including the Women’s Health Initiative, of whom 951 and 1,039, respectively, self-reported early menopause. The cohort study researchers noted that the 2019 American College of Cardiology/American Heart Association guideline for prevention of CVD acknowledged premature menopause as risk-enhancing factor in the CVD assessment in women younger than 40.
The cohort study found that Black women had almost twice the rate of premature menopause than White women, 17.4% and 9.8%, respectively. And it found that premature menopause was significantly linked with ASCVD in both populations independent of traditional risk factors – a 24% greater risk for Black women and 28% greater risk for White women.
‘Surprising’ finding
However, when premature menopause was added to the pooled cohort equations per the 2013 ACC/AHA guideline, the researchers found no incremental benefit, a finding Dr. Khan called “really surprising to us.”
She added, “If we look at the differences in the characteristics of women who have premature menopause, compared with those who didn’t, there were slight differences in terms of higher blood pressure, higher body mass index, and slightly higher glucose. So maybe what we’re seeing – and this is more speculative – is that risk factors are developing after early menopause, and the focus should be earlier in the patient’s life course to try to prevent hypertension, diabetes, and obesity.”
Dr. Khan emphasized that the findings don’t obviate the value of premature menopause in assessing ASCVD risk in women. “We still know that this is an important marker for women and their risk for heart disease, and it should be a warning sign to pay close attention to those other risk factors and what other preventive measures can be taken,” she said.
Christie Ballantyne, MD, said it’s important to note that the study did not dismiss the relevance of premature menopause in shared decision-making for postmenopausal women. “It certainly doesn’t mean that premature menopause is not a risk,” Dr. Ballantyne said in an interview. “Premature menopause may cause a worsening of traditional CVD risk factors, so that’s one possible explanation for it. The other possible explanation is that women with worse ASCVD risk factors – who are more overweight, have higher blood pressure, and have more diabetes and insulin resistance – are more likely to have earlier menopause.” Dr. Ballantyne is chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston.
“You should still look very carefully at the patient’s risk factors, calculate the pooled cohort equations, and make sure you get a recommendation,” he said. “If their risks are up, give recommendations on how to improve diet and exercise. Consider if you need to treat lipids or treat blood pressure with more than diet and exercise because there’s nothing magical about 7.5%”, the threshold for lipid-lowering therapy in the ASCVD risk calculator.
Dr. Khan and coauthors disclosed receiving grants from the National Institutes of Health and the American Heart Association. One coauthor reported a financial relationship with HGM Biopharmaceuticals. Dr. Ballantyne is a lead investigator of the Atherosclerosis Risk in Communities study, one of the population-based cohorts used in the cohort study. He has no other relevant relationships to disclose.
FROM JAMA CARDIOLOGY
Walnuts lowered LDL cholesterol in healthy seniors: WAHA study
Benefit sustained over 2 years
Healthy elderly people who ate a walnut-supplemented diet for 2 years showed significant reductions in LDL cholesterol, according to a randomized study that used imaging to evaluate lipid changes.
“Regularly eating walnuts will lower your LDL cholesterol and improve the quality of LDL particles, rendering them less prone to enter the arterial wall and build up atherosclerosis, and this will occur without unwanted weight gain in spite of the high-fat – healthy vegetable fat, though – content of walnuts,” Emilio Ros, MD, PhD, senior author of the Walnuts and Healthy Aging (WAHA) study, said in an interview.
WAHA is a parallel-group, randomized, controlled trial that followed 636 patients over 2 years at centers in Loma Linda, Calif., and Barcelona. They were randomly assigned to either a walnut-free or walnut-supplemented diet, and every 2 months they were underwent nuclear magnetic resonance spectroscopy and recorded their compliance, toleration, medication changes, and body weight.
The researchers reported “significantly decreased” total cholesterol, LDL cholesterol and intermediate-density lipoprotein cholesterol, along with reductions in total LDL cholesterol particles and small LDL cholesterol particle number in patients on a walnut-supplemented diet, compared with controls. However, triglycerides and HDL cholesterol were unaffected.
Study results
The study reported mean reductions in the following lipid categories among what the researchers called the “walnut group”:
- Total cholesterol, –8.5 mg/dL (95% confidence interval, –11.2 to –5.4), a 4.4% mean reduction.
- LDL-C, –4.3 mg/dL (95% CI, –6.6 to –1.6), a 3.6% reduction.
- Intermediate-density lipoprotein cholesterol, –1.3 mg/dL (95% CI, –1.5 to –1.0] for a 16.8% reduction.
- Total LDL cholesterol particles, a reduction of 4.3%.
- Small LDL cholesterol particle number, a 6.1% decrease.
“WAHA is the largest and longest randomized nut trial to date, which overcomes power limitations of former trials, smaller and of shorter duration,” said Dr. Ros, of the lipid clinic, endocrinology, nutrition service at the Hospital Clinic Villarroel at the University of Barcelona. He noted that studies he has participated in have already shown that the walnut-supplemented diet had beneficial effects on blood pressure, systemic inflammation and endothelial function.
Other strengths of the study, he said, were that it recruited patients from two distinct locations and that it retained 90% of participants over 2 years (the study started out with 708 participants).
Christie Ballantyne, MD, concurred that the size and duration of the study are worth noting. “People always have questions about what they should eat,” said Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston, said in an interview. “It’s very difficult to do nutritional studies. Most studies are small and short term, so it’s unusual to have study that’s large with 2 years of follow-up. This is a larger-than-usual study.”
Potential study limitations
Dr. Ros did acknowledge some limitations of the study. Participants weren’t blinded, the feeding setting wasn’t controlled, and participants were generally healthy and had average normal lipid profiles because they were taking statins, which may explain the modest lipid improvements in the study. “LDL cholesterol lowering by nuts is related to baseline levels, hence the reduction observed in our study was modest,” he said.
Additionally, because the study group was elderly, the results don’t apply to younger people. “Yet,” Dr. Ros added, “we know from many prior studies that nuts in general and walnuts in particular will lower blood cholesterol regardless of age.”
Dr. Ballantyne noted that the use of nuclear magnetic resonance spectroscopy to evaluate lipid levels involves a methodology that isn’t as systematic or as standardized as the typical lipid profile, and that different systems use proprietary software to interpret results. “That’s a little bit of an issue,” he said. “What exactly do the numbers mean?”
Overall, though, Dr. Ballantyne said the study is a significant addition to the literature. “The study is large, well done, and it confirms the benefits of something we’ve been telling patients is a good choice. It’s useful because there’s so much noise. It is important because there’s tremendous confusion and misinformation about what’s really healthy to eat.”
The study was supported by a grant from the California Walnut Commission (CWC), from which Dr. Ros and two coauthors received research funding through their institutions. Dr. Ros also reported receiving compensation from CWC and serves on a CWC advisory council. The other authors have no relationships to disclose. The WAHA study was supported by a grant from the CWC, from which Dr. Ros and some coinvestigators have received research funding through their institutions. Dr. Ballantyne has no relevant relationships to disclose.
Benefit sustained over 2 years
Benefit sustained over 2 years
Healthy elderly people who ate a walnut-supplemented diet for 2 years showed significant reductions in LDL cholesterol, according to a randomized study that used imaging to evaluate lipid changes.
“Regularly eating walnuts will lower your LDL cholesterol and improve the quality of LDL particles, rendering them less prone to enter the arterial wall and build up atherosclerosis, and this will occur without unwanted weight gain in spite of the high-fat – healthy vegetable fat, though – content of walnuts,” Emilio Ros, MD, PhD, senior author of the Walnuts and Healthy Aging (WAHA) study, said in an interview.
WAHA is a parallel-group, randomized, controlled trial that followed 636 patients over 2 years at centers in Loma Linda, Calif., and Barcelona. They were randomly assigned to either a walnut-free or walnut-supplemented diet, and every 2 months they were underwent nuclear magnetic resonance spectroscopy and recorded their compliance, toleration, medication changes, and body weight.
The researchers reported “significantly decreased” total cholesterol, LDL cholesterol and intermediate-density lipoprotein cholesterol, along with reductions in total LDL cholesterol particles and small LDL cholesterol particle number in patients on a walnut-supplemented diet, compared with controls. However, triglycerides and HDL cholesterol were unaffected.
Study results
The study reported mean reductions in the following lipid categories among what the researchers called the “walnut group”:
- Total cholesterol, –8.5 mg/dL (95% confidence interval, –11.2 to –5.4), a 4.4% mean reduction.
- LDL-C, –4.3 mg/dL (95% CI, –6.6 to –1.6), a 3.6% reduction.
- Intermediate-density lipoprotein cholesterol, –1.3 mg/dL (95% CI, –1.5 to –1.0] for a 16.8% reduction.
- Total LDL cholesterol particles, a reduction of 4.3%.
- Small LDL cholesterol particle number, a 6.1% decrease.
“WAHA is the largest and longest randomized nut trial to date, which overcomes power limitations of former trials, smaller and of shorter duration,” said Dr. Ros, of the lipid clinic, endocrinology, nutrition service at the Hospital Clinic Villarroel at the University of Barcelona. He noted that studies he has participated in have already shown that the walnut-supplemented diet had beneficial effects on blood pressure, systemic inflammation and endothelial function.
Other strengths of the study, he said, were that it recruited patients from two distinct locations and that it retained 90% of participants over 2 years (the study started out with 708 participants).
Christie Ballantyne, MD, concurred that the size and duration of the study are worth noting. “People always have questions about what they should eat,” said Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston, said in an interview. “It’s very difficult to do nutritional studies. Most studies are small and short term, so it’s unusual to have study that’s large with 2 years of follow-up. This is a larger-than-usual study.”
Potential study limitations
Dr. Ros did acknowledge some limitations of the study. Participants weren’t blinded, the feeding setting wasn’t controlled, and participants were generally healthy and had average normal lipid profiles because they were taking statins, which may explain the modest lipid improvements in the study. “LDL cholesterol lowering by nuts is related to baseline levels, hence the reduction observed in our study was modest,” he said.
Additionally, because the study group was elderly, the results don’t apply to younger people. “Yet,” Dr. Ros added, “we know from many prior studies that nuts in general and walnuts in particular will lower blood cholesterol regardless of age.”
Dr. Ballantyne noted that the use of nuclear magnetic resonance spectroscopy to evaluate lipid levels involves a methodology that isn’t as systematic or as standardized as the typical lipid profile, and that different systems use proprietary software to interpret results. “That’s a little bit of an issue,” he said. “What exactly do the numbers mean?”
Overall, though, Dr. Ballantyne said the study is a significant addition to the literature. “The study is large, well done, and it confirms the benefits of something we’ve been telling patients is a good choice. It’s useful because there’s so much noise. It is important because there’s tremendous confusion and misinformation about what’s really healthy to eat.”
The study was supported by a grant from the California Walnut Commission (CWC), from which Dr. Ros and two coauthors received research funding through their institutions. Dr. Ros also reported receiving compensation from CWC and serves on a CWC advisory council. The other authors have no relationships to disclose. The WAHA study was supported by a grant from the CWC, from which Dr. Ros and some coinvestigators have received research funding through their institutions. Dr. Ballantyne has no relevant relationships to disclose.
Healthy elderly people who ate a walnut-supplemented diet for 2 years showed significant reductions in LDL cholesterol, according to a randomized study that used imaging to evaluate lipid changes.
“Regularly eating walnuts will lower your LDL cholesterol and improve the quality of LDL particles, rendering them less prone to enter the arterial wall and build up atherosclerosis, and this will occur without unwanted weight gain in spite of the high-fat – healthy vegetable fat, though – content of walnuts,” Emilio Ros, MD, PhD, senior author of the Walnuts and Healthy Aging (WAHA) study, said in an interview.
WAHA is a parallel-group, randomized, controlled trial that followed 636 patients over 2 years at centers in Loma Linda, Calif., and Barcelona. They were randomly assigned to either a walnut-free or walnut-supplemented diet, and every 2 months they were underwent nuclear magnetic resonance spectroscopy and recorded their compliance, toleration, medication changes, and body weight.
The researchers reported “significantly decreased” total cholesterol, LDL cholesterol and intermediate-density lipoprotein cholesterol, along with reductions in total LDL cholesterol particles and small LDL cholesterol particle number in patients on a walnut-supplemented diet, compared with controls. However, triglycerides and HDL cholesterol were unaffected.
Study results
The study reported mean reductions in the following lipid categories among what the researchers called the “walnut group”:
- Total cholesterol, –8.5 mg/dL (95% confidence interval, –11.2 to –5.4), a 4.4% mean reduction.
- LDL-C, –4.3 mg/dL (95% CI, –6.6 to –1.6), a 3.6% reduction.
- Intermediate-density lipoprotein cholesterol, –1.3 mg/dL (95% CI, –1.5 to –1.0] for a 16.8% reduction.
- Total LDL cholesterol particles, a reduction of 4.3%.
- Small LDL cholesterol particle number, a 6.1% decrease.
“WAHA is the largest and longest randomized nut trial to date, which overcomes power limitations of former trials, smaller and of shorter duration,” said Dr. Ros, of the lipid clinic, endocrinology, nutrition service at the Hospital Clinic Villarroel at the University of Barcelona. He noted that studies he has participated in have already shown that the walnut-supplemented diet had beneficial effects on blood pressure, systemic inflammation and endothelial function.
Other strengths of the study, he said, were that it recruited patients from two distinct locations and that it retained 90% of participants over 2 years (the study started out with 708 participants).
Christie Ballantyne, MD, concurred that the size and duration of the study are worth noting. “People always have questions about what they should eat,” said Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston, said in an interview. “It’s very difficult to do nutritional studies. Most studies are small and short term, so it’s unusual to have study that’s large with 2 years of follow-up. This is a larger-than-usual study.”
Potential study limitations
Dr. Ros did acknowledge some limitations of the study. Participants weren’t blinded, the feeding setting wasn’t controlled, and participants were generally healthy and had average normal lipid profiles because they were taking statins, which may explain the modest lipid improvements in the study. “LDL cholesterol lowering by nuts is related to baseline levels, hence the reduction observed in our study was modest,” he said.
Additionally, because the study group was elderly, the results don’t apply to younger people. “Yet,” Dr. Ros added, “we know from many prior studies that nuts in general and walnuts in particular will lower blood cholesterol regardless of age.”
Dr. Ballantyne noted that the use of nuclear magnetic resonance spectroscopy to evaluate lipid levels involves a methodology that isn’t as systematic or as standardized as the typical lipid profile, and that different systems use proprietary software to interpret results. “That’s a little bit of an issue,” he said. “What exactly do the numbers mean?”
Overall, though, Dr. Ballantyne said the study is a significant addition to the literature. “The study is large, well done, and it confirms the benefits of something we’ve been telling patients is a good choice. It’s useful because there’s so much noise. It is important because there’s tremendous confusion and misinformation about what’s really healthy to eat.”
The study was supported by a grant from the California Walnut Commission (CWC), from which Dr. Ros and two coauthors received research funding through their institutions. Dr. Ros also reported receiving compensation from CWC and serves on a CWC advisory council. The other authors have no relationships to disclose. The WAHA study was supported by a grant from the CWC, from which Dr. Ros and some coinvestigators have received research funding through their institutions. Dr. Ballantyne has no relevant relationships to disclose.
FROM CIRCULATION
AHA targets rising prevalence of obstructive sleep apnea in children
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Tocilizumab shortage continues as pandemic wears on
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at COVID@rheumatology.org.
A version of this article first appeared on Medscape.com.
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at COVID@rheumatology.org.
A version of this article first appeared on Medscape.com.
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at COVID@rheumatology.org.
A version of this article first appeared on Medscape.com.
Heparin’s COVID-19 benefit greatest in moderately ill patients
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Critically ill derive no benefit
Critically ill derive no benefit
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
Therapeutic levels of heparin can have widely varying effects on COVID-19 patients depending on the severity of their disease, according to a multiplatform clinical trial that analyzed patient data from three international trials.
COVID-19 patients in the ICU, or at least receiving ICU-level care, derived no benefit from anticoagulation with heparin, while non–critically ill COVID-19 patients – those who were hospitalized but not receiving ICU-level care – on the same anticoagulation were less likely to progress to need respiratory or cardiovascular organ support despite a slightly heightened risk of bleeding events.
Reporting in two articles published online in the New England Journal of Medicine, authors of three international trials combined their data into one multiplatform trial that makes a strong case for prescribing therapeutic levels of heparin in hospitalized patients not receiving ICU-level care were non–critically ill and critically ill.
“I think this is going to be a game changer,” said Jeffrey S. Berger, MD, ACTIV-4a co–principal investigator and co–first author of the study of non–critically ill patients. “I think that using therapeutic-dose anticoagulation should improve outcomes in the tens of thousands of patients worldwide. I hope our data can have a global impact.”
Outcomes based on disease severity
The multiplatform trial analyzed data from the Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC); A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19 (ACTIV-4a); and Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
The trial evaluated 2,219 non–critically ill hospitalized patients, 1,181 of whom were randomized to therapeutic-dose anticoagulation; and 1,098 critically ill patients, 534 of whom were prescribed therapeutic levels of heparin.
In the critically ill patients, those on heparin were no more likely to get discharged or spend fewer days on respiratory or CV organ support – oxygen, mechanical ventilation, life support, vasopressors or inotropes – than were those on usual-care thromboprophylaxis. The investigators stopped the trial in both patient populations: in critically ill patients when it became obvious therapeutic-dose anticoagulation was having no impact; and in moderately ill patients when the trial met the prespecified criteria for the superiority of therapeutic-dose anticoagulation.
ICU patients on therapeutic-level heparin spent an average of 1 day free of organ support vs. 4 for patients on usual-care prophylactic antithrombotic drugs. The percentage of patients who survived to hospital discharge was similar in the therapeutic-level and usual-care critically ill patients: 62.7% and 64.5%, respectively. Major bleeding occurred in 3.8% and 2.8%, respectively. Demographic and clinical characteristics were similar between both patient groups.
However, in non–critically ill patients, therapeutic levels of heparin resulted in a marked improvement in outcomes. The researchers estimated that, for every 1,000 hospitalized patients with what they labeled moderate disease, an initial treatment with therapeutic-dose heparin resulted in 40 additional patients surviving compared to usual-care thromboprophylaxis.
The percentages of patients not needing organ support before hospital discharge was 80.2% on therapeutic-dose heparin and 76.4% on usual-care therapy. In terms of adjusted odds ratio, the anticoagulation group had a 27% improved chance of not needing daily organ support.
Those improvements came with an additional seven major bleeding events per 1,000 patients. That broke down to a rate of 1.9% in the therapeutic-dose and 0.9% in the usual-care patients.
As the Delta variant of COVID-19 spreads, Patrick R. Lawler, MD, MPH, principal investigator of the ATTACC trial, said there’s no reason these findings shouldn’t apply for all variants of the disease.
Dr. Lawler, a physician-scientist at Peter Munk Cardiac Centre at Toronto General Hospital, noted that the multiplatform study did not account for disease variant. “Ongoing clinical trials are tracking the variant patients have or the variants that are most prevalent in an area at that time,” he said. “It may be easier in future trials to look at that question.”
Explaining heparin’s varying effects
The study did not specifically sort out why moderately ill patients fared better on heparin than their critically ill counterparts, but Dr. Lawler speculated on possible reasons. “One might be that the extent of illness severity is too extreme in the ICU-level population for heparin to have a beneficial extent,” he said.
He acknowledged that higher rates of macrovascular thrombosis, such as venous thromboembolism, in ICU patients would suggest that heparin would have a greater beneficial effect, but, he added, “it may also suggest how advanced that process is, and perhaps heparin is not adequate to reverse the course at that point given relatively extensive thrombosis and associate organ failure.”
As clinicians have gained experience dealing with COVID-19, they’ve learned that infected patients carry a high burden of macro- and microthrombosis, Dr. Berger said, which may explain why critically ill patients didn’t respond as well to therapeutic levels of heparin. “I think the cat is out of the bag; patients who are severe are too ill to benefit,” he said. “I would think there’s too much microthrombosis that is already in their bodies.”
However, this doesn’t completely rule out therapeutic levels of heparin in critically ill COVID-19 patients. There are some scenarios where it’s needed, said Dr. Berger, associate professor of medicine and surgery and director of the Center for the Prevention of Cardiovascular Disease at New York University Langone Health. “Anyone who has a known clot already, like a known macrothrombosis in their leg or lung, needs to be on full-dose heparin,” he said.
That rationale can help reconcile the different outcomes in the critically and non–critically ill COVID-19 patients, wrote Hugo ten Cate, MD, PhD, of Maastricht University in the Netherlands, wrote in an accompanying editorial. But differences in the study populations may also explain the divergent outcomes, Dr. ten Cate noted.
The studies suggest that critically ill patients may need hon-heparin antithrombotic approaches “or even profibrinolytic strategies,” Dr. Cate wrote, and that the safety and effectiveness of thromboprophylaxis “remains an important question.” Nonetheless, he added, treating physicians must deal with the bleeding risk when using heparin or low-molecular-weight heparin in moderately ill COVID-19 patients.
Deepak L. Bhatt MD, MPH, of Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that reconciling the two studies was “a bit challenging,” because effective therapies tend to have a greater impact in sicker patients.
“Of course, with antithrombotic therapies, bleeding side effects can sometimes overwhelm benefits in patients who are at high risk of both bleeding and ischemic complications, though that does not seem to be the explanation here,” Dr. Bhatt said. “I do think we need more data to clarify exactly which COVID patients benefit from various antithrombotic regimens, and fortunately, there are other ongoing studies, some of which will report relatively soon.”
He concurred with Dr. Berger that patients who need anticoagulation should receive it “apart from their COVID status,” Dr. Bhatt said. “Sick, hospitalized patients with or without COVID should receive appropriate prophylactic doses of anticoagulation.” However, he added, “Whether we should routinely go beyond that in COVID-positive inpatients, I think we need more data.”
The ATTACC platform received grants from the Canadian Institutes of Health Research and several other research foundations. The ACTIV-4a platform received funding from the National Heart, Lung, and Blood Institute. REMAP-CAP received funding from the European Union and several international research foundations, as well as Amgen and Eisai.
Dr. Lawler had no relationships to disclose. Dr. Berger disclosed receiving grants from the NHLBI, and financial relationships with AstraZeneca, Janssen, and Amgen outside the submitted work. Dr. ten Cate reported relationships with Alveron, Coagulation Profile, Portola/Alexion, Bayer, Pfizer, Stago, Leo Pharma, Daiichi, and Gilead/Galapagos. Dr. Bhatt is chair of the data safety and monitoring board of the FREEDOM COVID anticoagulation clinical trial.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Can a blood-based test predict TNFi nonresponse in RA?
A blood test that uses a patient’s unique genetic signature has shown some ability to predict nonresponse to tumor necrosis factor inhibitors as treatment for rheumatoid arthritis, an observational clinical study has found, but the test’s predictive accuracy was well below 100%.
The test is the blood-based molecular signature response classifier (MSRC) that uses RNA sequencing data based on 23 different biomarkers: 19 RNA transcripts and 4 clinical features. The clinical features are body mass index, gender, patient global assessment, and anticyclic citrullinated protein (anti-CCP) status.
The NETWORK-004 study, published in Rheumatology and Therapy, was able to stratify patients who were likely to respond inadequately to TNFi therapy and could provide patient-specific information to guide therapy choice in RA patients regardless of whether they’ve already been on TNFi therapy. The study evaluated the MSRC test in 504 patients, 391 of whom were treatment naive.
Avoiding ‘fail first’ approach
The idea behind the test is to circumvent the “fail first” approach in finding the right therapy for RA in an individual patient. While the test costs $4,995, Alif Saleh, chief executive officer of Scipher Medicine, which markets the test under the name PrismRA, said in a press release that it has the potential to reduce costs by $19,000 or more per patient per year by avoiding treatments that don’t work. A previous study, which Scipher funded, reported that the test resulted in savings of $7,379 in per-patient costs of ineffective therapy. The same study reported a 25% decrease in costs for ineffective treatments for Medicare-eligible patients.
The price of RA drugs, particularly anti-TNF agents, is hefty and rising. GoodRx has reported that the price of RA drugs increased 92% from 2014 to 2019, and the prices for anti-TNF agents such as etanercept and adalimumab more than doubled in that period. Adalimumab can cost upwards of $84,000 per year while etanercept has a list price of around $72,000 a year. The pharmacy benefit manager WellDyne started covering the test MSRC in February.
Nehad Soloman, MD, a rheumatologist and internist at Midwestern University Arizona College of Osteopathic Medicine in Glendale and a compensated NETWORK-004 investigator, said the MSRC test would be indicated for confirmed RA patients for whom rheumatologists are considering biologic agents, particularly TNFi drugs. “You wouldn’t do it on an RA patient who’s been on several different medications because it doesn’t serve a purpose at that point,” he said.
The potential cost savings may not be the only reason to use the test, Dr. Soloman said. “You don’t want to be dabbling with the wrong drug if there’s another path you can try and save society some money as well as the time and energy it takes to monitor the patients – as well as the patient’s pain,” he said.
How the MSRC test works
The MSRC test detects a signal that’s associated with a high or very high likelihood of inadequate response to TNFi therapies and indicates that the patient is unlikely to achieve low disease activity or remission with TNFi therapies. Response is defined as achieving ACR50 – meaning 50% improvement in American College of Rheumatology response criteria – at 6 months.
Test results are reported on a continuous 1-25 scale, explained Slava Akmaev, PhD, chief technology officer and head of therapeutics at Scipher. “The higher the score, the more likely the patient will have an inadequate response to TNFi therapies and be unable to reach low disease activity; the lower the score, the less likely the patient will have an inadequate response to TNFi therapies,” he said. However, Dr. Akmaev noted that a low score does not ensure a positive response to TNFi therapies.
The MSRC test differs from the multibiomarker disease activity blood test (MBDA; marketed as Vectra by Myriad Genetics) in the number of biomarkers it measures: 19 RNA transcripts vs. 12 serum protein biomarkers in MBDA. The MBDA test is also intended to provide a quantitative, objective measurement of RA disease activity rather than to predict nonresponse to TNFi or other biologics. A number of studies have validated the MBDA test for predicting disease control in RA patients, but not necessarily response to TNFi therapy.
The “high” category threshold of the MSRC test corresponds to an approximate 90% chance of inadequate response to TNFi therapy, or a 10% chance of responding. The “very high” category threshold corresponds to an approximate 95% chance of inadequate response to TNFi therapy, Dr. Akmaev said.
NETWORK-004 used area under the curve (AUC) to measure the accuracy of the MSRC test. An AUC of 1 represents 100% accuracy. Overall, the MSRC had an AUC of 0.64, or 64% accuracy of predicting patients unlikely to respond to TNFi therapy and to achieve ACR50 at 6 months, with an odds ratio of 4.1 (95% confidence interval, 2.0-8.3; P = .0001).
The predictive accuracy went up to 74% with ORs of 3.4-8.8 for additional endpoints at 3 and 6 months (P < .01). Among patients who had already been on TNFi therapy, the predictive accuracy was 83% and associated with ORs of 3.3-26.6 based on ACR, 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP), and Clinical Disease Activity Index (CDAI) metrics.
The investigators also performed an in-cohort cross-validation of the MSRC using baseline blood samples of 245 treatment-naive patients from the CERTAIN study, which yielded a 66% predictive accuracy for the ACR50 outcome at 6 months. Using the 19 RNA transcripts from the test, but not the clinical factors, the predictive accuracy was 62.5%. Using ACR70, CDAI, and DAS28 as measures for 6-month response, the cross-validation analysis of all 23 MSRC features yielded predictive accuracy of 64%-67%.
The study found significant differences in model scores between patients who did and did not have the molecular signal of nonresponse, and the proportion of patients who achieved low disease activity or remission at 6 months based on CDAI and DAS28-CRP measures was greater among those who lacked a molecular signature of nonresponse.
“Those who lack this signature can proceed with TNFi therapy and possibly achieve an increased response rate relative to the unstratified population,” wrote lead study author Stanley B. Cohen, MD, and colleagues.
Daniel E. Furst, MD, emeritus professor at the University of California, Los Angeles, described the design of the NETWORK-004 study as “excellent,” but said that it didn’t overcome potential issues with the MSRC test itself. “The results unfortunately are great for group data but not for individuals, with a predictive area under the curve of 60% to 80%, it really is not that useful,” he said. “Let’s say you’re a patient who’s not doing well, and I do a test and it’s positive; that still means that 20% of the time you will respond.”
He also noted that he coauthored a paper that used decreases in DAS28 to predict nonresponse to certolizumab pegol plus methotrexate with 95% probability in the first 12 weeks of treatment. “That’s closer to what we need,” Dr. Furst said.
However, the MSRC test is a promising sign of where testing for predicting RA therapy is headed, he said. “We are steadily working toward genetic signatures that really are predictive on an individual basis,” Dr. Furst said. “It’s coming; it’s just not here yet.”
Dr. Furst had no relevant financial relationships to disclose. Dr. Soloman is a paid investigator and consultant to Scipher Medicine.
A blood test that uses a patient’s unique genetic signature has shown some ability to predict nonresponse to tumor necrosis factor inhibitors as treatment for rheumatoid arthritis, an observational clinical study has found, but the test’s predictive accuracy was well below 100%.
The test is the blood-based molecular signature response classifier (MSRC) that uses RNA sequencing data based on 23 different biomarkers: 19 RNA transcripts and 4 clinical features. The clinical features are body mass index, gender, patient global assessment, and anticyclic citrullinated protein (anti-CCP) status.
The NETWORK-004 study, published in Rheumatology and Therapy, was able to stratify patients who were likely to respond inadequately to TNFi therapy and could provide patient-specific information to guide therapy choice in RA patients regardless of whether they’ve already been on TNFi therapy. The study evaluated the MSRC test in 504 patients, 391 of whom were treatment naive.
Avoiding ‘fail first’ approach
The idea behind the test is to circumvent the “fail first” approach in finding the right therapy for RA in an individual patient. While the test costs $4,995, Alif Saleh, chief executive officer of Scipher Medicine, which markets the test under the name PrismRA, said in a press release that it has the potential to reduce costs by $19,000 or more per patient per year by avoiding treatments that don’t work. A previous study, which Scipher funded, reported that the test resulted in savings of $7,379 in per-patient costs of ineffective therapy. The same study reported a 25% decrease in costs for ineffective treatments for Medicare-eligible patients.
The price of RA drugs, particularly anti-TNF agents, is hefty and rising. GoodRx has reported that the price of RA drugs increased 92% from 2014 to 2019, and the prices for anti-TNF agents such as etanercept and adalimumab more than doubled in that period. Adalimumab can cost upwards of $84,000 per year while etanercept has a list price of around $72,000 a year. The pharmacy benefit manager WellDyne started covering the test MSRC in February.
Nehad Soloman, MD, a rheumatologist and internist at Midwestern University Arizona College of Osteopathic Medicine in Glendale and a compensated NETWORK-004 investigator, said the MSRC test would be indicated for confirmed RA patients for whom rheumatologists are considering biologic agents, particularly TNFi drugs. “You wouldn’t do it on an RA patient who’s been on several different medications because it doesn’t serve a purpose at that point,” he said.
The potential cost savings may not be the only reason to use the test, Dr. Soloman said. “You don’t want to be dabbling with the wrong drug if there’s another path you can try and save society some money as well as the time and energy it takes to monitor the patients – as well as the patient’s pain,” he said.
How the MSRC test works
The MSRC test detects a signal that’s associated with a high or very high likelihood of inadequate response to TNFi therapies and indicates that the patient is unlikely to achieve low disease activity or remission with TNFi therapies. Response is defined as achieving ACR50 – meaning 50% improvement in American College of Rheumatology response criteria – at 6 months.
Test results are reported on a continuous 1-25 scale, explained Slava Akmaev, PhD, chief technology officer and head of therapeutics at Scipher. “The higher the score, the more likely the patient will have an inadequate response to TNFi therapies and be unable to reach low disease activity; the lower the score, the less likely the patient will have an inadequate response to TNFi therapies,” he said. However, Dr. Akmaev noted that a low score does not ensure a positive response to TNFi therapies.
The MSRC test differs from the multibiomarker disease activity blood test (MBDA; marketed as Vectra by Myriad Genetics) in the number of biomarkers it measures: 19 RNA transcripts vs. 12 serum protein biomarkers in MBDA. The MBDA test is also intended to provide a quantitative, objective measurement of RA disease activity rather than to predict nonresponse to TNFi or other biologics. A number of studies have validated the MBDA test for predicting disease control in RA patients, but not necessarily response to TNFi therapy.
The “high” category threshold of the MSRC test corresponds to an approximate 90% chance of inadequate response to TNFi therapy, or a 10% chance of responding. The “very high” category threshold corresponds to an approximate 95% chance of inadequate response to TNFi therapy, Dr. Akmaev said.
NETWORK-004 used area under the curve (AUC) to measure the accuracy of the MSRC test. An AUC of 1 represents 100% accuracy. Overall, the MSRC had an AUC of 0.64, or 64% accuracy of predicting patients unlikely to respond to TNFi therapy and to achieve ACR50 at 6 months, with an odds ratio of 4.1 (95% confidence interval, 2.0-8.3; P = .0001).
The predictive accuracy went up to 74% with ORs of 3.4-8.8 for additional endpoints at 3 and 6 months (P < .01). Among patients who had already been on TNFi therapy, the predictive accuracy was 83% and associated with ORs of 3.3-26.6 based on ACR, 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP), and Clinical Disease Activity Index (CDAI) metrics.
The investigators also performed an in-cohort cross-validation of the MSRC using baseline blood samples of 245 treatment-naive patients from the CERTAIN study, which yielded a 66% predictive accuracy for the ACR50 outcome at 6 months. Using the 19 RNA transcripts from the test, but not the clinical factors, the predictive accuracy was 62.5%. Using ACR70, CDAI, and DAS28 as measures for 6-month response, the cross-validation analysis of all 23 MSRC features yielded predictive accuracy of 64%-67%.
The study found significant differences in model scores between patients who did and did not have the molecular signal of nonresponse, and the proportion of patients who achieved low disease activity or remission at 6 months based on CDAI and DAS28-CRP measures was greater among those who lacked a molecular signature of nonresponse.
“Those who lack this signature can proceed with TNFi therapy and possibly achieve an increased response rate relative to the unstratified population,” wrote lead study author Stanley B. Cohen, MD, and colleagues.
Daniel E. Furst, MD, emeritus professor at the University of California, Los Angeles, described the design of the NETWORK-004 study as “excellent,” but said that it didn’t overcome potential issues with the MSRC test itself. “The results unfortunately are great for group data but not for individuals, with a predictive area under the curve of 60% to 80%, it really is not that useful,” he said. “Let’s say you’re a patient who’s not doing well, and I do a test and it’s positive; that still means that 20% of the time you will respond.”
He also noted that he coauthored a paper that used decreases in DAS28 to predict nonresponse to certolizumab pegol plus methotrexate with 95% probability in the first 12 weeks of treatment. “That’s closer to what we need,” Dr. Furst said.
However, the MSRC test is a promising sign of where testing for predicting RA therapy is headed, he said. “We are steadily working toward genetic signatures that really are predictive on an individual basis,” Dr. Furst said. “It’s coming; it’s just not here yet.”
Dr. Furst had no relevant financial relationships to disclose. Dr. Soloman is a paid investigator and consultant to Scipher Medicine.
A blood test that uses a patient’s unique genetic signature has shown some ability to predict nonresponse to tumor necrosis factor inhibitors as treatment for rheumatoid arthritis, an observational clinical study has found, but the test’s predictive accuracy was well below 100%.
The test is the blood-based molecular signature response classifier (MSRC) that uses RNA sequencing data based on 23 different biomarkers: 19 RNA transcripts and 4 clinical features. The clinical features are body mass index, gender, patient global assessment, and anticyclic citrullinated protein (anti-CCP) status.
The NETWORK-004 study, published in Rheumatology and Therapy, was able to stratify patients who were likely to respond inadequately to TNFi therapy and could provide patient-specific information to guide therapy choice in RA patients regardless of whether they’ve already been on TNFi therapy. The study evaluated the MSRC test in 504 patients, 391 of whom were treatment naive.
Avoiding ‘fail first’ approach
The idea behind the test is to circumvent the “fail first” approach in finding the right therapy for RA in an individual patient. While the test costs $4,995, Alif Saleh, chief executive officer of Scipher Medicine, which markets the test under the name PrismRA, said in a press release that it has the potential to reduce costs by $19,000 or more per patient per year by avoiding treatments that don’t work. A previous study, which Scipher funded, reported that the test resulted in savings of $7,379 in per-patient costs of ineffective therapy. The same study reported a 25% decrease in costs for ineffective treatments for Medicare-eligible patients.
The price of RA drugs, particularly anti-TNF agents, is hefty and rising. GoodRx has reported that the price of RA drugs increased 92% from 2014 to 2019, and the prices for anti-TNF agents such as etanercept and adalimumab more than doubled in that period. Adalimumab can cost upwards of $84,000 per year while etanercept has a list price of around $72,000 a year. The pharmacy benefit manager WellDyne started covering the test MSRC in February.
Nehad Soloman, MD, a rheumatologist and internist at Midwestern University Arizona College of Osteopathic Medicine in Glendale and a compensated NETWORK-004 investigator, said the MSRC test would be indicated for confirmed RA patients for whom rheumatologists are considering biologic agents, particularly TNFi drugs. “You wouldn’t do it on an RA patient who’s been on several different medications because it doesn’t serve a purpose at that point,” he said.
The potential cost savings may not be the only reason to use the test, Dr. Soloman said. “You don’t want to be dabbling with the wrong drug if there’s another path you can try and save society some money as well as the time and energy it takes to monitor the patients – as well as the patient’s pain,” he said.
How the MSRC test works
The MSRC test detects a signal that’s associated with a high or very high likelihood of inadequate response to TNFi therapies and indicates that the patient is unlikely to achieve low disease activity or remission with TNFi therapies. Response is defined as achieving ACR50 – meaning 50% improvement in American College of Rheumatology response criteria – at 6 months.
Test results are reported on a continuous 1-25 scale, explained Slava Akmaev, PhD, chief technology officer and head of therapeutics at Scipher. “The higher the score, the more likely the patient will have an inadequate response to TNFi therapies and be unable to reach low disease activity; the lower the score, the less likely the patient will have an inadequate response to TNFi therapies,” he said. However, Dr. Akmaev noted that a low score does not ensure a positive response to TNFi therapies.
The MSRC test differs from the multibiomarker disease activity blood test (MBDA; marketed as Vectra by Myriad Genetics) in the number of biomarkers it measures: 19 RNA transcripts vs. 12 serum protein biomarkers in MBDA. The MBDA test is also intended to provide a quantitative, objective measurement of RA disease activity rather than to predict nonresponse to TNFi or other biologics. A number of studies have validated the MBDA test for predicting disease control in RA patients, but not necessarily response to TNFi therapy.
The “high” category threshold of the MSRC test corresponds to an approximate 90% chance of inadequate response to TNFi therapy, or a 10% chance of responding. The “very high” category threshold corresponds to an approximate 95% chance of inadequate response to TNFi therapy, Dr. Akmaev said.
NETWORK-004 used area under the curve (AUC) to measure the accuracy of the MSRC test. An AUC of 1 represents 100% accuracy. Overall, the MSRC had an AUC of 0.64, or 64% accuracy of predicting patients unlikely to respond to TNFi therapy and to achieve ACR50 at 6 months, with an odds ratio of 4.1 (95% confidence interval, 2.0-8.3; P = .0001).
The predictive accuracy went up to 74% with ORs of 3.4-8.8 for additional endpoints at 3 and 6 months (P < .01). Among patients who had already been on TNFi therapy, the predictive accuracy was 83% and associated with ORs of 3.3-26.6 based on ACR, 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP), and Clinical Disease Activity Index (CDAI) metrics.
The investigators also performed an in-cohort cross-validation of the MSRC using baseline blood samples of 245 treatment-naive patients from the CERTAIN study, which yielded a 66% predictive accuracy for the ACR50 outcome at 6 months. Using the 19 RNA transcripts from the test, but not the clinical factors, the predictive accuracy was 62.5%. Using ACR70, CDAI, and DAS28 as measures for 6-month response, the cross-validation analysis of all 23 MSRC features yielded predictive accuracy of 64%-67%.
The study found significant differences in model scores between patients who did and did not have the molecular signal of nonresponse, and the proportion of patients who achieved low disease activity or remission at 6 months based on CDAI and DAS28-CRP measures was greater among those who lacked a molecular signature of nonresponse.
“Those who lack this signature can proceed with TNFi therapy and possibly achieve an increased response rate relative to the unstratified population,” wrote lead study author Stanley B. Cohen, MD, and colleagues.
Daniel E. Furst, MD, emeritus professor at the University of California, Los Angeles, described the design of the NETWORK-004 study as “excellent,” but said that it didn’t overcome potential issues with the MSRC test itself. “The results unfortunately are great for group data but not for individuals, with a predictive area under the curve of 60% to 80%, it really is not that useful,” he said. “Let’s say you’re a patient who’s not doing well, and I do a test and it’s positive; that still means that 20% of the time you will respond.”
He also noted that he coauthored a paper that used decreases in DAS28 to predict nonresponse to certolizumab pegol plus methotrexate with 95% probability in the first 12 weeks of treatment. “That’s closer to what we need,” Dr. Furst said.
However, the MSRC test is a promising sign of where testing for predicting RA therapy is headed, he said. “We are steadily working toward genetic signatures that really are predictive on an individual basis,” Dr. Furst said. “It’s coming; it’s just not here yet.”
Dr. Furst had no relevant financial relationships to disclose. Dr. Soloman is a paid investigator and consultant to Scipher Medicine.
FROM RHEUMATOLOGY AND THERAPY
How forgone heart failure care drives up costs
Elderly carry disproportionate cost burden
About one in six patients with heart failure in the United States are skipping doctors’ appointments, not taking their medications as directed, or forgoing some other type of care, and their recalcitrance actually ends up costing the health care system 20%-30% more per patient.
That’s according to a cross-sectional study using data from a large public database reported in JACC: Heart Failure on Aug. 11.
The investigators pooled data on 2,050 patients with HF enrolled in the Medical Expenditure Panel Survey, a public database sponsored by the Agency for Healthcare Research and Quality, from 2004 to 2015. Some 339 of those HF patients were classified in the forgone or delayed (F/D) care group. This is the first study to describe the cost impact of missed care in heart failure, lead author Alexander Thomas, MD, and colleagues wrote.
“I think we make a pretty strong case linking forgone care to higher cost,” Dr. Thomas said in an interview. “Obviously this is a prospective study, so causality is not made based on this study, but there’s a strong association, it appears.” He acknowledged that prospective studies would need to confirm these findings, but added, “I think even this is enough to give us cause to look at the somewhat granular reasons as to why patients are forgoing care.”
Average annual total costs for adults who skipped care were $8,027 higher than for those heart failure patients who kept up with their regimen (P = .02). However, for people 65 and older, the cost differential was even higher: $10,581 (P = .02), which translates into a 50% premium over average costs for compliant elderly patients, noted Dr. Thomas, a cardiovascular medicine fellow at Yale University, New Haven, Conn.
In the nonelderly F/D group, average per-patient costs were $27,000 annually; in the elderly, it was a little over $30,000.
However, nonelderly patients were more likely to forgo care. While 16% of all patients in the study were considered F/D, 27% of nonelderly patients were classified as such vs. 10% of the elderly.
Elderly forgoing care drives cost
But, Dr. Thomas said, elderly patients forgoing care drive higher costs because they’re more fragile, have more comorbidities and more advanced heart failure, and tend to decompensate more quickly. “For them, missing one appointment or missing some medications is not nearly as well tolerated as it is in the younger population,” he said. In these elderly patients, inpatient costs accounted for 75% of the increased expense, Dr. Thomas added.
Reasons for forgoing care range from the obvious, such as cost or insurance issues – 191 individuals cited the former, 69 the latter – to more granular reasons, such as transportation issues or lack of time.
The study also found a number of demographic features that were associated with forgone or delayed care: non-Hispanic Black race; lack of insurance; low-income; and worse cardiovascular risk factor profiles.
The study’s call to action is for further research and policy changes aimed at better understanding barriers to care and improving access. “It’s a really great driver for policy interventions to attack this problem and to have the financial backing also to put money and resources into fixing this problem – which is probably not only going to decrease costs and allow more cost-effective care, but lead to better outcomes in this population as well,” Dr. Thomas said.
The sentiment for policy changes was echoed in an accompanying editorial by Khadijah Breathett, MD, MS, a heart failure/transplant cardiologist at the Sarver Heart Center, University of Arizona, Tucson. But she also noted the study exposed insufficiencies with Medicare. In an interview, she expanded on that.
“Our system is failing this population,” she said of the elderly with heart failure who have forgone care. “I think that’s because of the complexity of our system: One person’s Medicare is different from another’s. There are multiple parts to Medicare that provide different forms of care – hospital care, prescription care, and so forth – and everyone does not necessarily have the same parts.” Also, many Medicare beneficiaries are still working, and “they’re still trying to figure out how to make ends meet,” Dr. Breathett added.
The study also showed that the pre-Medicare population requires the attention of policymakers. “How do we help the younger population so that they don’t develop the comorbidities that worsen their quality of life and contribute to rising hospital costs?” Dr. Breathett said.
Dr. Thomas had no financial relationships to disclose. Dr. Breathett has received grants from the National Heart, Lung, and Blood Institute.
Elderly carry disproportionate cost burden
Elderly carry disproportionate cost burden
About one in six patients with heart failure in the United States are skipping doctors’ appointments, not taking their medications as directed, or forgoing some other type of care, and their recalcitrance actually ends up costing the health care system 20%-30% more per patient.
That’s according to a cross-sectional study using data from a large public database reported in JACC: Heart Failure on Aug. 11.
The investigators pooled data on 2,050 patients with HF enrolled in the Medical Expenditure Panel Survey, a public database sponsored by the Agency for Healthcare Research and Quality, from 2004 to 2015. Some 339 of those HF patients were classified in the forgone or delayed (F/D) care group. This is the first study to describe the cost impact of missed care in heart failure, lead author Alexander Thomas, MD, and colleagues wrote.
“I think we make a pretty strong case linking forgone care to higher cost,” Dr. Thomas said in an interview. “Obviously this is a prospective study, so causality is not made based on this study, but there’s a strong association, it appears.” He acknowledged that prospective studies would need to confirm these findings, but added, “I think even this is enough to give us cause to look at the somewhat granular reasons as to why patients are forgoing care.”
Average annual total costs for adults who skipped care were $8,027 higher than for those heart failure patients who kept up with their regimen (P = .02). However, for people 65 and older, the cost differential was even higher: $10,581 (P = .02), which translates into a 50% premium over average costs for compliant elderly patients, noted Dr. Thomas, a cardiovascular medicine fellow at Yale University, New Haven, Conn.
In the nonelderly F/D group, average per-patient costs were $27,000 annually; in the elderly, it was a little over $30,000.
However, nonelderly patients were more likely to forgo care. While 16% of all patients in the study were considered F/D, 27% of nonelderly patients were classified as such vs. 10% of the elderly.
Elderly forgoing care drives cost
But, Dr. Thomas said, elderly patients forgoing care drive higher costs because they’re more fragile, have more comorbidities and more advanced heart failure, and tend to decompensate more quickly. “For them, missing one appointment or missing some medications is not nearly as well tolerated as it is in the younger population,” he said. In these elderly patients, inpatient costs accounted for 75% of the increased expense, Dr. Thomas added.
Reasons for forgoing care range from the obvious, such as cost or insurance issues – 191 individuals cited the former, 69 the latter – to more granular reasons, such as transportation issues or lack of time.
The study also found a number of demographic features that were associated with forgone or delayed care: non-Hispanic Black race; lack of insurance; low-income; and worse cardiovascular risk factor profiles.
The study’s call to action is for further research and policy changes aimed at better understanding barriers to care and improving access. “It’s a really great driver for policy interventions to attack this problem and to have the financial backing also to put money and resources into fixing this problem – which is probably not only going to decrease costs and allow more cost-effective care, but lead to better outcomes in this population as well,” Dr. Thomas said.
The sentiment for policy changes was echoed in an accompanying editorial by Khadijah Breathett, MD, MS, a heart failure/transplant cardiologist at the Sarver Heart Center, University of Arizona, Tucson. But she also noted the study exposed insufficiencies with Medicare. In an interview, she expanded on that.
“Our system is failing this population,” she said of the elderly with heart failure who have forgone care. “I think that’s because of the complexity of our system: One person’s Medicare is different from another’s. There are multiple parts to Medicare that provide different forms of care – hospital care, prescription care, and so forth – and everyone does not necessarily have the same parts.” Also, many Medicare beneficiaries are still working, and “they’re still trying to figure out how to make ends meet,” Dr. Breathett added.
The study also showed that the pre-Medicare population requires the attention of policymakers. “How do we help the younger population so that they don’t develop the comorbidities that worsen their quality of life and contribute to rising hospital costs?” Dr. Breathett said.
Dr. Thomas had no financial relationships to disclose. Dr. Breathett has received grants from the National Heart, Lung, and Blood Institute.
About one in six patients with heart failure in the United States are skipping doctors’ appointments, not taking their medications as directed, or forgoing some other type of care, and their recalcitrance actually ends up costing the health care system 20%-30% more per patient.
That’s according to a cross-sectional study using data from a large public database reported in JACC: Heart Failure on Aug. 11.
The investigators pooled data on 2,050 patients with HF enrolled in the Medical Expenditure Panel Survey, a public database sponsored by the Agency for Healthcare Research and Quality, from 2004 to 2015. Some 339 of those HF patients were classified in the forgone or delayed (F/D) care group. This is the first study to describe the cost impact of missed care in heart failure, lead author Alexander Thomas, MD, and colleagues wrote.
“I think we make a pretty strong case linking forgone care to higher cost,” Dr. Thomas said in an interview. “Obviously this is a prospective study, so causality is not made based on this study, but there’s a strong association, it appears.” He acknowledged that prospective studies would need to confirm these findings, but added, “I think even this is enough to give us cause to look at the somewhat granular reasons as to why patients are forgoing care.”
Average annual total costs for adults who skipped care were $8,027 higher than for those heart failure patients who kept up with their regimen (P = .02). However, for people 65 and older, the cost differential was even higher: $10,581 (P = .02), which translates into a 50% premium over average costs for compliant elderly patients, noted Dr. Thomas, a cardiovascular medicine fellow at Yale University, New Haven, Conn.
In the nonelderly F/D group, average per-patient costs were $27,000 annually; in the elderly, it was a little over $30,000.
However, nonelderly patients were more likely to forgo care. While 16% of all patients in the study were considered F/D, 27% of nonelderly patients were classified as such vs. 10% of the elderly.
Elderly forgoing care drives cost
But, Dr. Thomas said, elderly patients forgoing care drive higher costs because they’re more fragile, have more comorbidities and more advanced heart failure, and tend to decompensate more quickly. “For them, missing one appointment or missing some medications is not nearly as well tolerated as it is in the younger population,” he said. In these elderly patients, inpatient costs accounted for 75% of the increased expense, Dr. Thomas added.
Reasons for forgoing care range from the obvious, such as cost or insurance issues – 191 individuals cited the former, 69 the latter – to more granular reasons, such as transportation issues or lack of time.
The study also found a number of demographic features that were associated with forgone or delayed care: non-Hispanic Black race; lack of insurance; low-income; and worse cardiovascular risk factor profiles.
The study’s call to action is for further research and policy changes aimed at better understanding barriers to care and improving access. “It’s a really great driver for policy interventions to attack this problem and to have the financial backing also to put money and resources into fixing this problem – which is probably not only going to decrease costs and allow more cost-effective care, but lead to better outcomes in this population as well,” Dr. Thomas said.
The sentiment for policy changes was echoed in an accompanying editorial by Khadijah Breathett, MD, MS, a heart failure/transplant cardiologist at the Sarver Heart Center, University of Arizona, Tucson. But she also noted the study exposed insufficiencies with Medicare. In an interview, she expanded on that.
“Our system is failing this population,” she said of the elderly with heart failure who have forgone care. “I think that’s because of the complexity of our system: One person’s Medicare is different from another’s. There are multiple parts to Medicare that provide different forms of care – hospital care, prescription care, and so forth – and everyone does not necessarily have the same parts.” Also, many Medicare beneficiaries are still working, and “they’re still trying to figure out how to make ends meet,” Dr. Breathett added.
The study also showed that the pre-Medicare population requires the attention of policymakers. “How do we help the younger population so that they don’t develop the comorbidities that worsen their quality of life and contribute to rising hospital costs?” Dr. Breathett said.
Dr. Thomas had no financial relationships to disclose. Dr. Breathett has received grants from the National Heart, Lung, and Blood Institute.
FROM JACC: HEART FAILURE