User login
Lupus mutation may unlock targeted drugs for patient subset
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.
“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
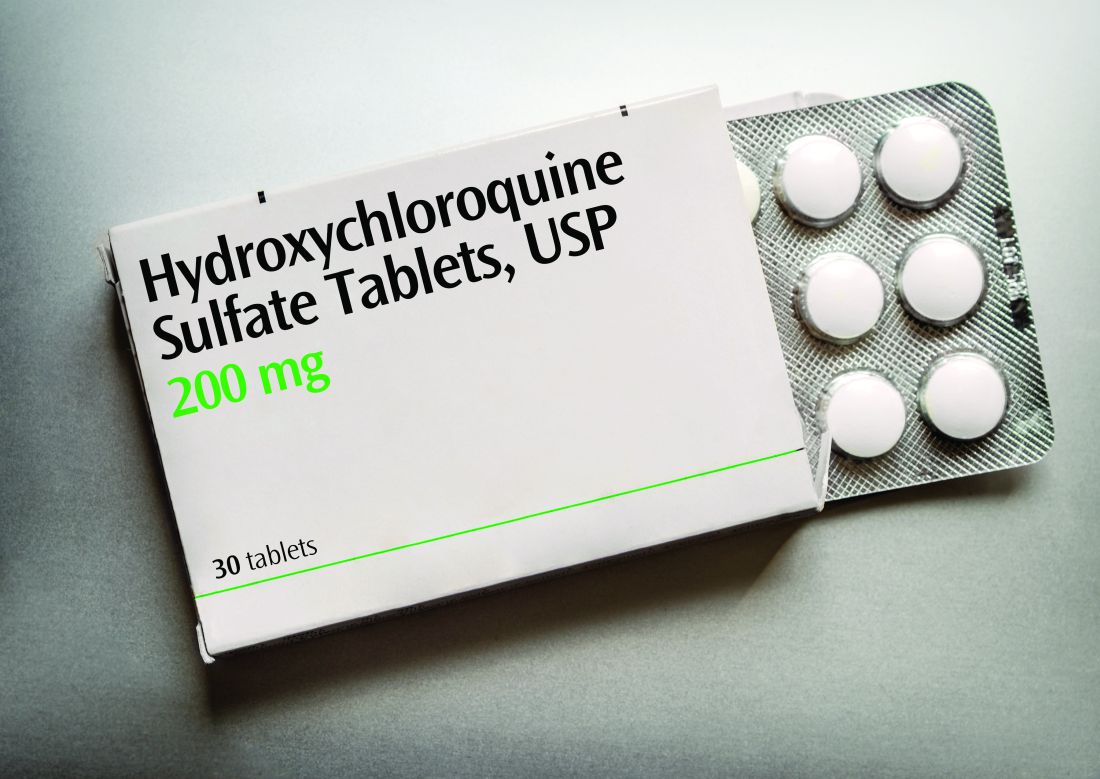
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.
“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.
“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE
Pfizer COVID vaccine performs well in youth with rheumatic diseases
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Pfizer-BioNTech mRNA vaccine (Comirnaty) showed a good safety profile with minimal short-term side effects and no negative impact on disease activity in a cohort of adolescents and young adults with rheumatic diseases, according to research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year.
Only 3% of patients experience a severe transient adverse event, according to Merav Heshin-Bekenstein, MD, of Dana-Dwek Children’s Hospital at the Tel Aviv Sourasky Medical Center in Israel. The findings were published in Rheumatology.
“We found that the mRNA Pfizer vaccine was immunogenic and induced an adequate humoral immune response in adolescent patients,” Dr. Heshin-Bekenstein told CARRA attendees. “It was definitely comparable to healthy controls and practically all patients were seropositive following the second vaccine, except for one patient with long-standing systemic sclerosis.”
The findings were not necessarily surprising but were encouraging to Melissa S. Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University, Indianapolis. Dr. Oliver wasn’t part of the study team.
“We know that the COVID vaccines in healthy adolescents have shown good efficacy with minimal side effects, and it’s good to see that this study showed that in those with rheumatic diseases on immunosuppressive therapy,” Dr. Oliver told this news organization.
Until now, the data on COVID-19 vaccines in teens with rheumatic illnesses has been limited, she said, so “many pediatric rheumatologists only have the data from adult studies to go on or personal experience with their own cohort of patients.”
But the high immunogenicity seen in the study was a pleasant surprise to Beth H. Rutstein, MD, assistant professor of clinical pediatrics in the division of rheumatology at Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania.
“I was both surprised and thrilled with Dr. Heshin-Bekenstein’s findings suggesting near-universal seroconversion for patients with rheumatic disease regardless of underlying diagnosis or immunomodulatory therapy regimen, as much of the adult data has suggested a poorer seroconversion rate” and lower antibody titers in adults with similar illnesses, Dr. Rutstein said in an interview.
The study “provides essential reassurance that vaccination against COVID-19 does not increase the risk of disease flare or worsen disease severity scores,” said Dr. Rutstein, who was not associated with the research. “Rather than speaking purely anecdotally with our patients and their families, we can refer to the science – which is always more reassuring for both our patients and ourselves.”
Study included diverse conditions and therapies
Risk factors for poor outcomes with COVID-19 in children include obesity, cardiovascular disease, chronic lung disease, diabetes, and asthma, Dr. Heshin-Bekenstein told CARRA attendees. Multisystem inflammatory syndrome in children (MIS-C) and long COVID are also potential complications of COVID-19 with less understood risk factors.
Although COVID-19 is most often mild in children, certain severe, systemic rheumatic diseases increase hospitalization risk, including systemic lupus erythematosus (SLE) and vasculitis. Evidence has also shown that COVID-19 infection increases the risk of disease flare in teens with juvenile-onset rheumatic diseases, so it’s “crucial to prevent COVID-19 disease in this population,” Dr. Heshin-Bekenstein said.
Her study therefore aimed to assess the safety and immunogenicity of the Pfizer mRNA vaccine for teens with juvenile-onset rheumatic diseases and those taking immunomodulatory medications. The international prospective multicenter study ran from April to November 2021 at three pediatric rheumatology clinics in Israel and one in Slovenia. Endpoints included short-term side effects, vaccination impact on clinical disease activity, immunogenicity at 2-9 weeks after the second dose, and, secondarily, efficacy against COVID-19 infection.
The 91 participants included adolescents aged 12-18 and young adults aged 18-21. Nearly half of the participants (46%) had juvenile idiopathic arthritis (JIA), and 14% had SLE. Other participants’ conditions included systemic vasculitis, idiopathic uveitis, inflammatory bowel disease–related arthritis, systemic or localized scleroderma, juvenile dermatomyositis, or an autoinflammatory disease. Participants’ mean disease duration was 4.8 years.
The researchers compared the patients with a control group of 40 individuals with similar demographics but without rheumatic disease. The researchers used the LIAISON quantitative assay to assess serum IgG antibody levels against the SARS-CoV-2 spike protein in both groups.
Eight in 10 participants with rheumatic disease were taking an immunomodulatory medication, including a conventional synthetic disease-modifying antirheumatic drug (csDMARD) in 40%, a biologic DMARD in 37%, tumor necrosis factor (TNF) inhibitors in 32%, hydroxychloroquine (HCQ) in 19%, glucocorticoids in 14%, and mycophenolate in 11%. A smaller proportion were on other biologics: JAK inhibitors in 6.6%, anti-CD20 drugs in 4.4%, and an IL-6 inhibitor in 1%.
Side effects similar in both groups
None of the side effects reported by participants were statistically different between those with rheumatic disease and the control group. Localized pain was the most common side effect, reported by 73%-79% of participants after each dose. About twice as many participants with rheumatic disease experienced muscle aches and joint pains, compared with the control group, but the differences were not significant. Fever occurred more often in those with rheumatic disease (6%, five cases) than without (3%, one case). One-third of those with rheumatic disease felt tiredness, compared with 20% of the control group.
None of the healthy controls were hospitalized after vaccination, but three rheumatic patients were, including two after the first dose. Both were 17 years old, had systemic vasculitis with granulomatosis with polyangiitis (GPA), and were taking rituximab (Rituxan). One patient experienced acute onset of chronic renal failure, fever, dehydration, and high C-reactive protein within hours of vaccination. The other experienced new onset of pulmonary hemorrhage a week after vaccination.
In addition, a 14-year-old female with lupus, taking only HCQ, went to the emergency department with fever, headache, vomiting, and joint pain 1 day after the second vaccine dose. She had normal inflammatory markers and no change in disease activity score, and she was discharged with low-dose steroids tapered after 2 weeks.
Immune response high in patients with rheumatic disease
Immunogenicity was similar in both groups, with 97% seropositivity in the rheumatic disease group and 100% in the control group. Average IgG titers were 242 in the rheumatic group and 388 in the control group (P < .0001). Seropositivity was 88% in those taking mycophenolate with another drug (100% with mycophenolate monotherapy), 90% with HCQ, 94% with any csDMARDs and another drug (100% with csDMARD monotherapy), and 100% for all other drugs. During 3 months’ follow-up after vaccination, there were no COVID-19 cases among the participants.
Dr. Heshin-Bekenstein noted that their results showed better immunogenicity in teens, compared with adults, for two specific drugs. Seropositivity in teens taking methotrexate (Rheumatrex, Trexall) or rituximab was 100% in this study, compared with 84% in adults taking methotrexate and 39% in adults taking rituximab in a previous study. However, only three patients in this study were taking rituximab, and only seven were taking methotrexate.
The study’s heterogenous population was both a strength and a weakness of the study. “Due to the diversity of rheumatic diseases and medications included in this cohort, it was not possible to draw significant conclusions regarding the impact of the immunomodulatory medications and type of disease” on titers, Dr. Heshin-Bekenstein told attendees.
Still, “I think as pediatric rheumatologists, we can feel reassured in recommending the COVID-19 vaccine to our patients,” Dr. Oliver said. “I will add that every patient is different, and everyone should have a conversation with their physician about receiving the COVID-19 vaccine.” Dr. Oliver said she discusses vaccination, including COVID vaccination, with every patient, and it’s been challenging to address concerns in the midst of so much misinformation circulating about the vaccine.
These findings do raise questions about whether it’s still necessary to hold immunomodulatory medications to get the vaccine,” Dr. Rutstein said.
“Many families are nervous to pause their medications before and after the vaccine as is currently recommended for many therapies by the American College of Rheumatology, and I do share that concern for some of my patients with more clinically unstable disease, so I try to work with each family to decide on best timing and have delayed or deferred the series until some patients are on a steady dose of a new immunomodulatory medication if it has been recently started,” Dr. Rutstein said. “This is one of the reasons why Dr. Heshin-Bekenstein’s study is so important – we may be holding medications that can be safely continued and even further decrease the risk of disease flare.”
None of the physicians have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CARRA 2022
Prior authorizations delay TNF inhibitors for children with JIA
Children with juvenile idiopathic arthritis (JIA) who need a tumor necrosis factor (TNF) inhibitor after failing conventional disease-modifying antirheumatic drug (DMARD) treatment often experience insurance delays before beginning the new drug because of prior authorization denials, according to research presented at the 2022 annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The findings were also published as a research letter in JAMA Network Open.
“Prompt escalation to TNF inhibitors is recommended for children with JIA refractory to DMARDs,” author Jordan Roberts, MD, a clinical fellow of the Harvard Medical School Rheumatology Program, Boston, told CARRA attendees. TNF inhibitors are increasingly used as first-line treatment in JIA since growing evidence suggests better outcomes from early treatment with biologics. “Prior authorization requirements that delay TNF inhibitor initiation among children with JIA are common in clinical practice,” Dr. Roberts said, but little evidence exists to understand the extent of this problem and its causes.
The researchers therefore conducted a retrospective cohort study using a search of electronic health records from January 2018 to December 2019 to find all children at a single center with a new diagnosis of nonsystemic JIA. Then the authors pulled the timing of prior authorization requests, approvals, denials, and first TNF inhibitor dose from the medical notes. They also sought out any children who had been recommended a TNF inhibitor but never started one.
The total population included 54 children with an average age of 10 years, about two-thirds of whom had private insurance (63%). The group was predominantly White (63%), although 13% declined to provide race, and 7% were Hispanic. Most subtypes of disease were represented: oligoarticular persistent (28%), oligoarticular extended (2%), polyarticular rheumatoid factor-negative (15%), polyarticular rheumatoid factor-positive (15%), psoriatic arthritis (26%), enthesitis-related arthritis (12%), and undifferentiated arthritis (2%).
The 44 participants with private insurance had an average of two joints with active disease, while the 10 patients with public insurance had an average of four involved joints. Nearly all the patients (91%) had previously taken or were currently taking DMARDs when the prior authorization was submitted, and 61% had received NSAIDs.
All but one of the patients’ insurance plans required a prior authorization. The first prior authorization was denied for about one-third of the public insurance patients (30%) and a quarter of the private insurance patients. About 1 in 5 patients overall (22%) required a written appeal to override the denial, and 4% required peer-to-peer review. Meanwhile, 7% of patients began another medication because of the denial.
It took a median of 3 days for prior authorizations to be approved and a median of 24 days from the time the TNF inhibitor was recommended to the patient receiving the first dose. However, 22% of patients waited at least 2 weeks before the prior authorization was approved, and more than a quarter of the requests took over 30 days before the patient could begin the medication. In the public insurance group in particular, a quarter of children waited at least 19 days for approval and at least 44 days before starting the medication.
In fact, when the researchers looked at the difference in approval time between those who did and did not receive an initial denial, the difference was stark. Median approval time was 16 days when the prior authorization was denied, compared with a median of 5 days when the first prior authorization was approved. Similarly, time to initiation of the drug after recommendation was a median of 35 days for those whose prior authorization was first denied and 17 days for those with an initial approval.
The most common reason for an initial denial was the insurance company requiring a different TNF inhibitor than the one the rheumatologist wanted to prescribe. “These were all children whose rheumatologist has recommended either infliximab or etanercept that were required to use adalimumab instead,” Dr. Roberts said.
The other reasons for initial denial were similarly familiar ones:
- Required submission to another insurer
- Additional documentation required
- Lack of medical necessity
- Prescription was for an indication not approved by the Food and Drug Administration
- Age of patient
- Nonbiologic DMARD required
- NSAID required for step therapy
Only three children who were advised to begin a TNF inhibitor did not do so, including one who was lost to follow-up, one who had injection-related anxiety, and one who had safety concerns about the medication.
“Several children were required to use alternative TNF inhibitors than the one that was recommended due to restricted formularies, which may reduce shared decisionmaking between physicians and families and may not be the optimal clinical choice for an individual child,” Dr. Roberts said in her conclusion. Most children, however, were able to get approval for the TNF inhibitor originally requested, “suggesting that utilization management strategies present barriers to timely care despite appropriate specialty medication requests,” she said. “Therefore, it’s important for us to advocate for access to medications for children with JIA.”
Findings are not surprising
“I have these same experiences at my institution – often insurance will dictate clinical practice, and step therapy is the only option, causing a delay to initiation of TNFi even if we think, as the pediatric rheumatologist, that a child needs this medicine to be initiated on presentation to our clinic,” Nayimisha Balmuri, MD, assistant professor of pediatrics in the division of allergy, immunology, and rheumatology at the Johns Hopkins School of Medicine, Baltimore, told this news organization.
Dr. Balmuri, who was not involved in the study, noted that in her clinic at Johns Hopkins, it is hit or miss if an appeal to insurance companies or to the state (if it is Medicaid coverage) will be successful. “Unfortunately, [we are] mostly unsuccessful, and we have to try another DMARD for 8 to 12 weeks first before trying to get TNFi,” she said.
Dr. Balmuri called for bringing these issues to the attention of state and federal legislators. “It’s so important for us to continue to advocate for our patients at the state and national level! We are the advocates for our patients, and we are uniquely trained to know the best medications to initiate to help patients maximize their chance to reach remission of arthritis. Insurance companies need to hear our voices!”
Dr. Roberts reported grants from CARRA, the Lupus Foundation of America, and the National Institute of Allergy and Infectious Diseases during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children with juvenile idiopathic arthritis (JIA) who need a tumor necrosis factor (TNF) inhibitor after failing conventional disease-modifying antirheumatic drug (DMARD) treatment often experience insurance delays before beginning the new drug because of prior authorization denials, according to research presented at the 2022 annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The findings were also published as a research letter in JAMA Network Open.
“Prompt escalation to TNF inhibitors is recommended for children with JIA refractory to DMARDs,” author Jordan Roberts, MD, a clinical fellow of the Harvard Medical School Rheumatology Program, Boston, told CARRA attendees. TNF inhibitors are increasingly used as first-line treatment in JIA since growing evidence suggests better outcomes from early treatment with biologics. “Prior authorization requirements that delay TNF inhibitor initiation among children with JIA are common in clinical practice,” Dr. Roberts said, but little evidence exists to understand the extent of this problem and its causes.
The researchers therefore conducted a retrospective cohort study using a search of electronic health records from January 2018 to December 2019 to find all children at a single center with a new diagnosis of nonsystemic JIA. Then the authors pulled the timing of prior authorization requests, approvals, denials, and first TNF inhibitor dose from the medical notes. They also sought out any children who had been recommended a TNF inhibitor but never started one.
The total population included 54 children with an average age of 10 years, about two-thirds of whom had private insurance (63%). The group was predominantly White (63%), although 13% declined to provide race, and 7% were Hispanic. Most subtypes of disease were represented: oligoarticular persistent (28%), oligoarticular extended (2%), polyarticular rheumatoid factor-negative (15%), polyarticular rheumatoid factor-positive (15%), psoriatic arthritis (26%), enthesitis-related arthritis (12%), and undifferentiated arthritis (2%).
The 44 participants with private insurance had an average of two joints with active disease, while the 10 patients with public insurance had an average of four involved joints. Nearly all the patients (91%) had previously taken or were currently taking DMARDs when the prior authorization was submitted, and 61% had received NSAIDs.
All but one of the patients’ insurance plans required a prior authorization. The first prior authorization was denied for about one-third of the public insurance patients (30%) and a quarter of the private insurance patients. About 1 in 5 patients overall (22%) required a written appeal to override the denial, and 4% required peer-to-peer review. Meanwhile, 7% of patients began another medication because of the denial.
It took a median of 3 days for prior authorizations to be approved and a median of 24 days from the time the TNF inhibitor was recommended to the patient receiving the first dose. However, 22% of patients waited at least 2 weeks before the prior authorization was approved, and more than a quarter of the requests took over 30 days before the patient could begin the medication. In the public insurance group in particular, a quarter of children waited at least 19 days for approval and at least 44 days before starting the medication.
In fact, when the researchers looked at the difference in approval time between those who did and did not receive an initial denial, the difference was stark. Median approval time was 16 days when the prior authorization was denied, compared with a median of 5 days when the first prior authorization was approved. Similarly, time to initiation of the drug after recommendation was a median of 35 days for those whose prior authorization was first denied and 17 days for those with an initial approval.
The most common reason for an initial denial was the insurance company requiring a different TNF inhibitor than the one the rheumatologist wanted to prescribe. “These were all children whose rheumatologist has recommended either infliximab or etanercept that were required to use adalimumab instead,” Dr. Roberts said.
The other reasons for initial denial were similarly familiar ones:
- Required submission to another insurer
- Additional documentation required
- Lack of medical necessity
- Prescription was for an indication not approved by the Food and Drug Administration
- Age of patient
- Nonbiologic DMARD required
- NSAID required for step therapy
Only three children who were advised to begin a TNF inhibitor did not do so, including one who was lost to follow-up, one who had injection-related anxiety, and one who had safety concerns about the medication.
“Several children were required to use alternative TNF inhibitors than the one that was recommended due to restricted formularies, which may reduce shared decisionmaking between physicians and families and may not be the optimal clinical choice for an individual child,” Dr. Roberts said in her conclusion. Most children, however, were able to get approval for the TNF inhibitor originally requested, “suggesting that utilization management strategies present barriers to timely care despite appropriate specialty medication requests,” she said. “Therefore, it’s important for us to advocate for access to medications for children with JIA.”
Findings are not surprising
“I have these same experiences at my institution – often insurance will dictate clinical practice, and step therapy is the only option, causing a delay to initiation of TNFi even if we think, as the pediatric rheumatologist, that a child needs this medicine to be initiated on presentation to our clinic,” Nayimisha Balmuri, MD, assistant professor of pediatrics in the division of allergy, immunology, and rheumatology at the Johns Hopkins School of Medicine, Baltimore, told this news organization.
Dr. Balmuri, who was not involved in the study, noted that in her clinic at Johns Hopkins, it is hit or miss if an appeal to insurance companies or to the state (if it is Medicaid coverage) will be successful. “Unfortunately, [we are] mostly unsuccessful, and we have to try another DMARD for 8 to 12 weeks first before trying to get TNFi,” she said.
Dr. Balmuri called for bringing these issues to the attention of state and federal legislators. “It’s so important for us to continue to advocate for our patients at the state and national level! We are the advocates for our patients, and we are uniquely trained to know the best medications to initiate to help patients maximize their chance to reach remission of arthritis. Insurance companies need to hear our voices!”
Dr. Roberts reported grants from CARRA, the Lupus Foundation of America, and the National Institute of Allergy and Infectious Diseases during the conduct of the study.
A version of this article first appeared on Medscape.com.
Children with juvenile idiopathic arthritis (JIA) who need a tumor necrosis factor (TNF) inhibitor after failing conventional disease-modifying antirheumatic drug (DMARD) treatment often experience insurance delays before beginning the new drug because of prior authorization denials, according to research presented at the 2022 annual meeting of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The findings were also published as a research letter in JAMA Network Open.
“Prompt escalation to TNF inhibitors is recommended for children with JIA refractory to DMARDs,” author Jordan Roberts, MD, a clinical fellow of the Harvard Medical School Rheumatology Program, Boston, told CARRA attendees. TNF inhibitors are increasingly used as first-line treatment in JIA since growing evidence suggests better outcomes from early treatment with biologics. “Prior authorization requirements that delay TNF inhibitor initiation among children with JIA are common in clinical practice,” Dr. Roberts said, but little evidence exists to understand the extent of this problem and its causes.
The researchers therefore conducted a retrospective cohort study using a search of electronic health records from January 2018 to December 2019 to find all children at a single center with a new diagnosis of nonsystemic JIA. Then the authors pulled the timing of prior authorization requests, approvals, denials, and first TNF inhibitor dose from the medical notes. They also sought out any children who had been recommended a TNF inhibitor but never started one.
The total population included 54 children with an average age of 10 years, about two-thirds of whom had private insurance (63%). The group was predominantly White (63%), although 13% declined to provide race, and 7% were Hispanic. Most subtypes of disease were represented: oligoarticular persistent (28%), oligoarticular extended (2%), polyarticular rheumatoid factor-negative (15%), polyarticular rheumatoid factor-positive (15%), psoriatic arthritis (26%), enthesitis-related arthritis (12%), and undifferentiated arthritis (2%).
The 44 participants with private insurance had an average of two joints with active disease, while the 10 patients with public insurance had an average of four involved joints. Nearly all the patients (91%) had previously taken or were currently taking DMARDs when the prior authorization was submitted, and 61% had received NSAIDs.
All but one of the patients’ insurance plans required a prior authorization. The first prior authorization was denied for about one-third of the public insurance patients (30%) and a quarter of the private insurance patients. About 1 in 5 patients overall (22%) required a written appeal to override the denial, and 4% required peer-to-peer review. Meanwhile, 7% of patients began another medication because of the denial.
It took a median of 3 days for prior authorizations to be approved and a median of 24 days from the time the TNF inhibitor was recommended to the patient receiving the first dose. However, 22% of patients waited at least 2 weeks before the prior authorization was approved, and more than a quarter of the requests took over 30 days before the patient could begin the medication. In the public insurance group in particular, a quarter of children waited at least 19 days for approval and at least 44 days before starting the medication.
In fact, when the researchers looked at the difference in approval time between those who did and did not receive an initial denial, the difference was stark. Median approval time was 16 days when the prior authorization was denied, compared with a median of 5 days when the first prior authorization was approved. Similarly, time to initiation of the drug after recommendation was a median of 35 days for those whose prior authorization was first denied and 17 days for those with an initial approval.
The most common reason for an initial denial was the insurance company requiring a different TNF inhibitor than the one the rheumatologist wanted to prescribe. “These were all children whose rheumatologist has recommended either infliximab or etanercept that were required to use adalimumab instead,” Dr. Roberts said.
The other reasons for initial denial were similarly familiar ones:
- Required submission to another insurer
- Additional documentation required
- Lack of medical necessity
- Prescription was for an indication not approved by the Food and Drug Administration
- Age of patient
- Nonbiologic DMARD required
- NSAID required for step therapy
Only three children who were advised to begin a TNF inhibitor did not do so, including one who was lost to follow-up, one who had injection-related anxiety, and one who had safety concerns about the medication.
“Several children were required to use alternative TNF inhibitors than the one that was recommended due to restricted formularies, which may reduce shared decisionmaking between physicians and families and may not be the optimal clinical choice for an individual child,” Dr. Roberts said in her conclusion. Most children, however, were able to get approval for the TNF inhibitor originally requested, “suggesting that utilization management strategies present barriers to timely care despite appropriate specialty medication requests,” she said. “Therefore, it’s important for us to advocate for access to medications for children with JIA.”
Findings are not surprising
“I have these same experiences at my institution – often insurance will dictate clinical practice, and step therapy is the only option, causing a delay to initiation of TNFi even if we think, as the pediatric rheumatologist, that a child needs this medicine to be initiated on presentation to our clinic,” Nayimisha Balmuri, MD, assistant professor of pediatrics in the division of allergy, immunology, and rheumatology at the Johns Hopkins School of Medicine, Baltimore, told this news organization.
Dr. Balmuri, who was not involved in the study, noted that in her clinic at Johns Hopkins, it is hit or miss if an appeal to insurance companies or to the state (if it is Medicaid coverage) will be successful. “Unfortunately, [we are] mostly unsuccessful, and we have to try another DMARD for 8 to 12 weeks first before trying to get TNFi,” she said.
Dr. Balmuri called for bringing these issues to the attention of state and federal legislators. “It’s so important for us to continue to advocate for our patients at the state and national level! We are the advocates for our patients, and we are uniquely trained to know the best medications to initiate to help patients maximize their chance to reach remission of arthritis. Insurance companies need to hear our voices!”
Dr. Roberts reported grants from CARRA, the Lupus Foundation of America, and the National Institute of Allergy and Infectious Diseases during the conduct of the study.
A version of this article first appeared on Medscape.com.
Gaps in follow-up care put kids with asthma at risk of severe recurrence
Jo Ward’s twin boys have been to the emergency department for respiratory problems about as many times as the dozen years they’ve been alive. Both have asthma and bronchopulmonary dysplasia, a form of chronic airway damage that can occur in children born premature, as the twins were. But each time Ms. Ward took them in for treatment during an acute bout of breathing distress, the staff told her to schedule a follow-up visit for the children with their physician only if they didn’t get better, not regardless of the outcome – as medical guidelines recommend.
“They asked questions, they did the exams, but they really didn’t give you a lot of information to help you at home,” Ms. Ward told this news organization. If they had, she doesn’t think she’d have needed to take them in for emergency care so often.
A new study, published in Academic Pediatrics, suggests she’s right.
Current clinical guidelines for asthma recommend that patients who visit the ED for an asthma-related problem should have a follow-up appointment within a month after the visit, independent of how well they have recovered once home, according to Naomi S. Bardach, MD, a professor of pediatrics and health policy at the University of California, San Francisco, who led the new study.
Her research found that children who have a follow-up appointment within 2 weeks of such a visit are less likely to come back again the next year. Yet the study also found that only about one in five youth had a follow-up visit within that 2-week window.
“The emergency department visit is probably a sign that they need some additional attention for their asthma,” Dr. Bardach said. “We know we can prevent emergency department visits if they get the right kind of medication or if they figure out how to avoid the things that are going to cause an asthma exacerbation or flare.”
For the study, Dr. Bardach and colleagues analyzed data from California, Vermont, and Massachusetts for all asthma-related emergency visits for patients aged 3-21 years between 2013 and 2016.
Out of the 90,267 such visits they identified, 22.6% of patients had a follow-up within 2 weeks, more often by patients who were younger, had commercial insurance, had evidence of prior asthma, or had complex chronic conditions.
Whereas 5.7% of patients who had follow-up visits returned to the ED within 60 days, 6.4% of those who didn’t came back – a 12% difference (P < .001). The gap was larger a year out, with 25% of those with follow-ups returning to the ED, compared with 28.3% of those without follow-ups returning (P < .001), according to the researchers.
Overall, Dr. Bardach’s group estimates that for every 30 children who have follow-up visits with a physician, one would avoid a return trip to the emergency department for asthma within a year.
But given the sheer number of asthma-related trips to the ED each year – 164,145 for kids age 1-17 years in the United States in 2016 alone – that translates into big numbers of kids not going back to the hospital: approximately 72,000 such trips avoided at a savings to the health care system of at least $8.6 million annually.
Missed opportunities
Had Ms. Ward’s boys been among the one in five to receive follow-up care earlier in their lives, she might have saved a significant amount of time, money, anxiety, and heartache. When the twins were 9 years old, she took them to a new pediatric pulmonologist. That changed everything. In that first visit, “they gave me way more information than I ever had in the first 9 years,” she said.
The doctor told Ms. Ward to keep steroids on hand, gave her a prescription for extra doses of the powerful medication, and explained that they needed to be used within 24 hours of the first sign of a breathing problem.
“She said if you give them the steroids right away, it keeps them out of the emergency room, and that’s actually worked,” Ms. Ward said. “She made sure we had care plans every visit and asked me each time if I still had it or we needed to rewrite it. They gave me signs to look for, for when to go to hospital visits. I think that when you go to the doctor, they should be telling you stuff like that.”
Dr. Bardach said visits with a primary care doctor or asthma specialist offer families a chance to receive information to keep the condition from becoming critical.
“Going to that follow-up visit, they can get access to education from the provider about how to avoid things that trigger asthma, and there’s medication that kids can take that keeps the lungs calm and less likely to have a big asthma reaction, so getting access to that medication can be really helpful,” she said.
That was the case for Amy Davenport, of Chapel Hill, N.C., whose 6-year-old son has been to the ED twice for his asthma.
The first time, when he was 3, he was having trouble breathing with a respiratory tract infection and received nebulizer treatment – although he received it in the ED since no beds were available in the ICU. The staff did tell Ms. Davenport to follow up with her primary care provider, but her son’s pediatrician was reluctant to diagnose him with asthma at such a young age and didn’t prescribe any maintenance medications.
A few months later, Ms. Davenport and her son found themselves back in the hospital, and an ICU bed was open this time. The critical care staff referred Davenport to a pediatric pulmonary specialist, and they haven’t been back to the hospital since. Ms. Davenport said she believes if they’d received a maintenance medication after the first visit, it likely would have prevented the second one.
“I’ve definitely seen now that, after the second admission, we got an asthma action plan and it said exactly what to do,” she said. “I felt like we had really good follow-up. We had that action plan on our refrigerator for a long time, and it helped us as parents with three small children to manage.”
Of course, follow-up care takes time – time away from work and school that not all families can spare, the researchers acknowledged. Telehealth may be an option, especially after its use expanded during the COVID-19 pandemic.
“We know that health systems have a hard time being flexible enough to actually have a kid be able to make an appointment within a short period of time, and we also know it’s hard for families sometimes to go back into a clinical setting within a certain period of time,” Dr. Bardach said. The urgency for the appointment may wane for those whose children seem to be doing better.
When the researchers adjusted their calculations for socioeconomic status, the results didn’t change much. But the study did find that patients with private insurance were about twice as likely to have follow-up visits as those on Medicaid (43.7% vs. 21.7%). And “the content and conduct” of the follow-up visit makes a difference as well.
Ms. Ward, whose boys are insured through Medicaid, recalled several visits to the ED where she had to push the staff to get the care her children needed. In one case, when one of her boys was a year old and struggling to breathe, the emergency doctor handed her a prescription and recommended she fill it at a neighborhood drugstore that would be cheaper than the hospital’s pharmacy. Then a nurse came in to begin the discharge process.
“I said no, ‘we’re not ready yet. Look at him,’” Ms. Ward said. The nurse took a pulse oximeter reading that showed the boy’s oxygen levels were at 84%, dangerously low. “If I wasn’t so knowledgeable and paid attention when they were born, since they were preemies, if it would have been somebody else, they probably would’ve went home and he’d have died.”
With the pediatric pulmonologist the boys have now, Ms. Ward said she feels more capable of managing their asthma and knowing how to reduce the likelihood that they’ll need to visit the ED.
“Part of what we’re seeing here is that having an existing and trusting relationship with a clinician can be helpful to kids with asthma,” Dr. Bardach said. “If we help establish and maintain those connections, and explain how important that connection can be, that can also help somebody with asthma overall.”
The research was funded by the Agency for Healthcare Research and Quality. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jo Ward’s twin boys have been to the emergency department for respiratory problems about as many times as the dozen years they’ve been alive. Both have asthma and bronchopulmonary dysplasia, a form of chronic airway damage that can occur in children born premature, as the twins were. But each time Ms. Ward took them in for treatment during an acute bout of breathing distress, the staff told her to schedule a follow-up visit for the children with their physician only if they didn’t get better, not regardless of the outcome – as medical guidelines recommend.
“They asked questions, they did the exams, but they really didn’t give you a lot of information to help you at home,” Ms. Ward told this news organization. If they had, she doesn’t think she’d have needed to take them in for emergency care so often.
A new study, published in Academic Pediatrics, suggests she’s right.
Current clinical guidelines for asthma recommend that patients who visit the ED for an asthma-related problem should have a follow-up appointment within a month after the visit, independent of how well they have recovered once home, according to Naomi S. Bardach, MD, a professor of pediatrics and health policy at the University of California, San Francisco, who led the new study.
Her research found that children who have a follow-up appointment within 2 weeks of such a visit are less likely to come back again the next year. Yet the study also found that only about one in five youth had a follow-up visit within that 2-week window.
“The emergency department visit is probably a sign that they need some additional attention for their asthma,” Dr. Bardach said. “We know we can prevent emergency department visits if they get the right kind of medication or if they figure out how to avoid the things that are going to cause an asthma exacerbation or flare.”
For the study, Dr. Bardach and colleagues analyzed data from California, Vermont, and Massachusetts for all asthma-related emergency visits for patients aged 3-21 years between 2013 and 2016.
Out of the 90,267 such visits they identified, 22.6% of patients had a follow-up within 2 weeks, more often by patients who were younger, had commercial insurance, had evidence of prior asthma, or had complex chronic conditions.
Whereas 5.7% of patients who had follow-up visits returned to the ED within 60 days, 6.4% of those who didn’t came back – a 12% difference (P < .001). The gap was larger a year out, with 25% of those with follow-ups returning to the ED, compared with 28.3% of those without follow-ups returning (P < .001), according to the researchers.
Overall, Dr. Bardach’s group estimates that for every 30 children who have follow-up visits with a physician, one would avoid a return trip to the emergency department for asthma within a year.
But given the sheer number of asthma-related trips to the ED each year – 164,145 for kids age 1-17 years in the United States in 2016 alone – that translates into big numbers of kids not going back to the hospital: approximately 72,000 such trips avoided at a savings to the health care system of at least $8.6 million annually.
Missed opportunities
Had Ms. Ward’s boys been among the one in five to receive follow-up care earlier in their lives, she might have saved a significant amount of time, money, anxiety, and heartache. When the twins were 9 years old, she took them to a new pediatric pulmonologist. That changed everything. In that first visit, “they gave me way more information than I ever had in the first 9 years,” she said.
The doctor told Ms. Ward to keep steroids on hand, gave her a prescription for extra doses of the powerful medication, and explained that they needed to be used within 24 hours of the first sign of a breathing problem.
“She said if you give them the steroids right away, it keeps them out of the emergency room, and that’s actually worked,” Ms. Ward said. “She made sure we had care plans every visit and asked me each time if I still had it or we needed to rewrite it. They gave me signs to look for, for when to go to hospital visits. I think that when you go to the doctor, they should be telling you stuff like that.”
Dr. Bardach said visits with a primary care doctor or asthma specialist offer families a chance to receive information to keep the condition from becoming critical.
“Going to that follow-up visit, they can get access to education from the provider about how to avoid things that trigger asthma, and there’s medication that kids can take that keeps the lungs calm and less likely to have a big asthma reaction, so getting access to that medication can be really helpful,” she said.
That was the case for Amy Davenport, of Chapel Hill, N.C., whose 6-year-old son has been to the ED twice for his asthma.
The first time, when he was 3, he was having trouble breathing with a respiratory tract infection and received nebulizer treatment – although he received it in the ED since no beds were available in the ICU. The staff did tell Ms. Davenport to follow up with her primary care provider, but her son’s pediatrician was reluctant to diagnose him with asthma at such a young age and didn’t prescribe any maintenance medications.
A few months later, Ms. Davenport and her son found themselves back in the hospital, and an ICU bed was open this time. The critical care staff referred Davenport to a pediatric pulmonary specialist, and they haven’t been back to the hospital since. Ms. Davenport said she believes if they’d received a maintenance medication after the first visit, it likely would have prevented the second one.
“I’ve definitely seen now that, after the second admission, we got an asthma action plan and it said exactly what to do,” she said. “I felt like we had really good follow-up. We had that action plan on our refrigerator for a long time, and it helped us as parents with three small children to manage.”
Of course, follow-up care takes time – time away from work and school that not all families can spare, the researchers acknowledged. Telehealth may be an option, especially after its use expanded during the COVID-19 pandemic.
“We know that health systems have a hard time being flexible enough to actually have a kid be able to make an appointment within a short period of time, and we also know it’s hard for families sometimes to go back into a clinical setting within a certain period of time,” Dr. Bardach said. The urgency for the appointment may wane for those whose children seem to be doing better.
When the researchers adjusted their calculations for socioeconomic status, the results didn’t change much. But the study did find that patients with private insurance were about twice as likely to have follow-up visits as those on Medicaid (43.7% vs. 21.7%). And “the content and conduct” of the follow-up visit makes a difference as well.
Ms. Ward, whose boys are insured through Medicaid, recalled several visits to the ED where she had to push the staff to get the care her children needed. In one case, when one of her boys was a year old and struggling to breathe, the emergency doctor handed her a prescription and recommended she fill it at a neighborhood drugstore that would be cheaper than the hospital’s pharmacy. Then a nurse came in to begin the discharge process.
“I said no, ‘we’re not ready yet. Look at him,’” Ms. Ward said. The nurse took a pulse oximeter reading that showed the boy’s oxygen levels were at 84%, dangerously low. “If I wasn’t so knowledgeable and paid attention when they were born, since they were preemies, if it would have been somebody else, they probably would’ve went home and he’d have died.”
With the pediatric pulmonologist the boys have now, Ms. Ward said she feels more capable of managing their asthma and knowing how to reduce the likelihood that they’ll need to visit the ED.
“Part of what we’re seeing here is that having an existing and trusting relationship with a clinician can be helpful to kids with asthma,” Dr. Bardach said. “If we help establish and maintain those connections, and explain how important that connection can be, that can also help somebody with asthma overall.”
The research was funded by the Agency for Healthcare Research and Quality. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jo Ward’s twin boys have been to the emergency department for respiratory problems about as many times as the dozen years they’ve been alive. Both have asthma and bronchopulmonary dysplasia, a form of chronic airway damage that can occur in children born premature, as the twins were. But each time Ms. Ward took them in for treatment during an acute bout of breathing distress, the staff told her to schedule a follow-up visit for the children with their physician only if they didn’t get better, not regardless of the outcome – as medical guidelines recommend.
“They asked questions, they did the exams, but they really didn’t give you a lot of information to help you at home,” Ms. Ward told this news organization. If they had, she doesn’t think she’d have needed to take them in for emergency care so often.
A new study, published in Academic Pediatrics, suggests she’s right.
Current clinical guidelines for asthma recommend that patients who visit the ED for an asthma-related problem should have a follow-up appointment within a month after the visit, independent of how well they have recovered once home, according to Naomi S. Bardach, MD, a professor of pediatrics and health policy at the University of California, San Francisco, who led the new study.
Her research found that children who have a follow-up appointment within 2 weeks of such a visit are less likely to come back again the next year. Yet the study also found that only about one in five youth had a follow-up visit within that 2-week window.
“The emergency department visit is probably a sign that they need some additional attention for their asthma,” Dr. Bardach said. “We know we can prevent emergency department visits if they get the right kind of medication or if they figure out how to avoid the things that are going to cause an asthma exacerbation or flare.”
For the study, Dr. Bardach and colleagues analyzed data from California, Vermont, and Massachusetts for all asthma-related emergency visits for patients aged 3-21 years between 2013 and 2016.
Out of the 90,267 such visits they identified, 22.6% of patients had a follow-up within 2 weeks, more often by patients who were younger, had commercial insurance, had evidence of prior asthma, or had complex chronic conditions.
Whereas 5.7% of patients who had follow-up visits returned to the ED within 60 days, 6.4% of those who didn’t came back – a 12% difference (P < .001). The gap was larger a year out, with 25% of those with follow-ups returning to the ED, compared with 28.3% of those without follow-ups returning (P < .001), according to the researchers.
Overall, Dr. Bardach’s group estimates that for every 30 children who have follow-up visits with a physician, one would avoid a return trip to the emergency department for asthma within a year.
But given the sheer number of asthma-related trips to the ED each year – 164,145 for kids age 1-17 years in the United States in 2016 alone – that translates into big numbers of kids not going back to the hospital: approximately 72,000 such trips avoided at a savings to the health care system of at least $8.6 million annually.
Missed opportunities
Had Ms. Ward’s boys been among the one in five to receive follow-up care earlier in their lives, she might have saved a significant amount of time, money, anxiety, and heartache. When the twins were 9 years old, she took them to a new pediatric pulmonologist. That changed everything. In that first visit, “they gave me way more information than I ever had in the first 9 years,” she said.
The doctor told Ms. Ward to keep steroids on hand, gave her a prescription for extra doses of the powerful medication, and explained that they needed to be used within 24 hours of the first sign of a breathing problem.
“She said if you give them the steroids right away, it keeps them out of the emergency room, and that’s actually worked,” Ms. Ward said. “She made sure we had care plans every visit and asked me each time if I still had it or we needed to rewrite it. They gave me signs to look for, for when to go to hospital visits. I think that when you go to the doctor, they should be telling you stuff like that.”
Dr. Bardach said visits with a primary care doctor or asthma specialist offer families a chance to receive information to keep the condition from becoming critical.
“Going to that follow-up visit, they can get access to education from the provider about how to avoid things that trigger asthma, and there’s medication that kids can take that keeps the lungs calm and less likely to have a big asthma reaction, so getting access to that medication can be really helpful,” she said.
That was the case for Amy Davenport, of Chapel Hill, N.C., whose 6-year-old son has been to the ED twice for his asthma.
The first time, when he was 3, he was having trouble breathing with a respiratory tract infection and received nebulizer treatment – although he received it in the ED since no beds were available in the ICU. The staff did tell Ms. Davenport to follow up with her primary care provider, but her son’s pediatrician was reluctant to diagnose him with asthma at such a young age and didn’t prescribe any maintenance medications.
A few months later, Ms. Davenport and her son found themselves back in the hospital, and an ICU bed was open this time. The critical care staff referred Davenport to a pediatric pulmonary specialist, and they haven’t been back to the hospital since. Ms. Davenport said she believes if they’d received a maintenance medication after the first visit, it likely would have prevented the second one.
“I’ve definitely seen now that, after the second admission, we got an asthma action plan and it said exactly what to do,” she said. “I felt like we had really good follow-up. We had that action plan on our refrigerator for a long time, and it helped us as parents with three small children to manage.”
Of course, follow-up care takes time – time away from work and school that not all families can spare, the researchers acknowledged. Telehealth may be an option, especially after its use expanded during the COVID-19 pandemic.
“We know that health systems have a hard time being flexible enough to actually have a kid be able to make an appointment within a short period of time, and we also know it’s hard for families sometimes to go back into a clinical setting within a certain period of time,” Dr. Bardach said. The urgency for the appointment may wane for those whose children seem to be doing better.
When the researchers adjusted their calculations for socioeconomic status, the results didn’t change much. But the study did find that patients with private insurance were about twice as likely to have follow-up visits as those on Medicaid (43.7% vs. 21.7%). And “the content and conduct” of the follow-up visit makes a difference as well.
Ms. Ward, whose boys are insured through Medicaid, recalled several visits to the ED where she had to push the staff to get the care her children needed. In one case, when one of her boys was a year old and struggling to breathe, the emergency doctor handed her a prescription and recommended she fill it at a neighborhood drugstore that would be cheaper than the hospital’s pharmacy. Then a nurse came in to begin the discharge process.
“I said no, ‘we’re not ready yet. Look at him,’” Ms. Ward said. The nurse took a pulse oximeter reading that showed the boy’s oxygen levels were at 84%, dangerously low. “If I wasn’t so knowledgeable and paid attention when they were born, since they were preemies, if it would have been somebody else, they probably would’ve went home and he’d have died.”
With the pediatric pulmonologist the boys have now, Ms. Ward said she feels more capable of managing their asthma and knowing how to reduce the likelihood that they’ll need to visit the ED.
“Part of what we’re seeing here is that having an existing and trusting relationship with a clinician can be helpful to kids with asthma,” Dr. Bardach said. “If we help establish and maintain those connections, and explain how important that connection can be, that can also help somebody with asthma overall.”
The research was funded by the Agency for Healthcare Research and Quality. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACADEMIC PEDIATRICS
JIA disease activity, disability linked to social factors
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRIC RHEUMATOLOGY
ADHD link to prenatal opioid exposure shifts with other substances
Children prenatally exposed to opioids alone have an increased risk of attention-deficit/hyperactivity disorder (ADHD), but interactions between opioids and both cannabis use and alcohol use were linked to varying levels of ADHD risk as well, according to findings published March 11 in JAMA Network Open.
While many prenatal exposure studies examine associations with one substance, the results of this case-control study “suggest that it is important to consider prenatal exposure to multiple substances and the interactions between these substances when counseling women regarding substance use during pregnancy,” wrote Henri M. Garrison-Desany of the Johns Hopkins University, Baltimore, and colleagues.
Using data from children in the prospective Boston Birth Cohort between 1998 and 2019, the researchers did a secondary analysis on the 3,138 children (50.4% of whom were male) with at least 2 years of follow-up, excluding children from multiple-gestation pregnancies, in vitro fertilization pregnancies, and deliveries involving major maternal trauma or major chromosomal anomalies. Mothers answered a questionnaire within 24-72 hours of delivery regarding their demographics, substance use, pregnancy history, and health status. Among the mothers, 58.6% were Black, 22.3% were Hispanic, 7.2% were White, 1.5% were Asian, and 10.4% were other races/ethnicities.
The children’s electronic medical records were used to identify those with ADHD diagnoses. The researchers did not assess prescription opioid exposure during pregnancy, but they based opioid exposure on mothers’ reports of recreationally using heroin or oxycodone, mothers’ reports of receiving methadone treatment, or a newborn diagnosis of neonatal abstinence syndrome or neonatal opioid withdrawal syndrome.
Just under a quarter of the women (24.2%) reported using at least one substance during pregnancy. After tobacco smoking (18.5%), the next most reported substances were alcohol (8.1%), cannabis (3.9%), and opioids (1.9%). With a median 12 years of follow-up, 15.5% of the children had been diagnosed with ADHD, most of whom (71.6%) were male.
Before considering interaction of different substances, children exposed to opioids had a little over twice the risk of ADHD (hazard ratio [HR], 2.19) compared to those with no prenatal substance exposure. Although neither cannabis nor alcohol was independently associated with ADHD, smoking had a 40% increased risk, and researchers found a 21% increase in risk of ADHD with each additional substance mothers used during pregnancy. The researchers had adjusted these findings for maternal age, race/ethnicity, marital status, educational level, annual household income, parity, number of perinatal visits, and general stress during pregnancy, based on a structured interview.
When the researchers considered all the substances together, opioid exposure increased risk of ADHD by 60% (HR, 1.6), opioids with cannabis increased risk by 42%, opioids with alcohol increased risk by 15%, and opioids with smoking increased risk by 17%.
”Our findings suggest opioids may interact with other substances (including cannabis), which may be particularly deleterious,” the researchers reported. “It is not clear whether this interaction is owing to biological or environmental factors, such as whether individuals with illicit polysubstance use are more likely to use more substances or whether they have other characteristics that may impact child development.”
The authors noted that cannabis exposure has been linked to other neurodevelopmental outcomes, including reduced executive and motor function in infants. ”Notably, although we did not find a significant independent association between cannabis exposure and ADHD, children exposed to both cannabis and opioids had a 23% greater risk than expected from either exposure individually,” they reported.
The researchers suggest that their findings provide data for considering harm reduction approaches that reduce use of any single substance during pregnancy. “Focusing on the most obviously harmful exposures may be a useful way to reduce the risk of ADHD,” they wrote. “Further work is needed to directly investigate this hypothesis and examine whether reduction in the use of any substance among those with polysubstance use could be acceptable compared with abstinence.”
In an invited commentary, Angela Lupattelli, PhD, and Nhung T. H. Trinh, PhD, both of the department of pharmacy at the University of Oslo, noted the methodological challenges of assessing exposures and associations from multiple different substances during pregnancy.
“First, how can we disentangle the consequences of individual and/or combined substance exposures during pregnancy from the underlying risks?” they asked. In addition to differences in baseline characteristic between those who use opioids or cannabis, Dr. Lupattelli and Dr. Trinh noted that other important unmeasured factors, such as genetics and family environment, may confound the effect size estimates for ADHD.
They also noted the need to consider intensity, dose, duration, and timing of substance use during pregnancy.
“Understanding the longer-term safety of substance use during pregnancy is paramount to inform prevention policy and shape counseling strategies. Observational studies, despite their limitations, are a necessary piece of the puzzle,” they wrote. “However, the study findings should be interpreted with caution, as the use of advanced analytical methods cannot overcome the unavailability of some important confounding factors and exposure information.”
The research was funded by the U.S. Department of Health and Human Services and the National Institutes of Health. The authors had no industry-related disclosures.
Children prenatally exposed to opioids alone have an increased risk of attention-deficit/hyperactivity disorder (ADHD), but interactions between opioids and both cannabis use and alcohol use were linked to varying levels of ADHD risk as well, according to findings published March 11 in JAMA Network Open.
While many prenatal exposure studies examine associations with one substance, the results of this case-control study “suggest that it is important to consider prenatal exposure to multiple substances and the interactions between these substances when counseling women regarding substance use during pregnancy,” wrote Henri M. Garrison-Desany of the Johns Hopkins University, Baltimore, and colleagues.
Using data from children in the prospective Boston Birth Cohort between 1998 and 2019, the researchers did a secondary analysis on the 3,138 children (50.4% of whom were male) with at least 2 years of follow-up, excluding children from multiple-gestation pregnancies, in vitro fertilization pregnancies, and deliveries involving major maternal trauma or major chromosomal anomalies. Mothers answered a questionnaire within 24-72 hours of delivery regarding their demographics, substance use, pregnancy history, and health status. Among the mothers, 58.6% were Black, 22.3% were Hispanic, 7.2% were White, 1.5% were Asian, and 10.4% were other races/ethnicities.
The children’s electronic medical records were used to identify those with ADHD diagnoses. The researchers did not assess prescription opioid exposure during pregnancy, but they based opioid exposure on mothers’ reports of recreationally using heroin or oxycodone, mothers’ reports of receiving methadone treatment, or a newborn diagnosis of neonatal abstinence syndrome or neonatal opioid withdrawal syndrome.
Just under a quarter of the women (24.2%) reported using at least one substance during pregnancy. After tobacco smoking (18.5%), the next most reported substances were alcohol (8.1%), cannabis (3.9%), and opioids (1.9%). With a median 12 years of follow-up, 15.5% of the children had been diagnosed with ADHD, most of whom (71.6%) were male.
Before considering interaction of different substances, children exposed to opioids had a little over twice the risk of ADHD (hazard ratio [HR], 2.19) compared to those with no prenatal substance exposure. Although neither cannabis nor alcohol was independently associated with ADHD, smoking had a 40% increased risk, and researchers found a 21% increase in risk of ADHD with each additional substance mothers used during pregnancy. The researchers had adjusted these findings for maternal age, race/ethnicity, marital status, educational level, annual household income, parity, number of perinatal visits, and general stress during pregnancy, based on a structured interview.
When the researchers considered all the substances together, opioid exposure increased risk of ADHD by 60% (HR, 1.6), opioids with cannabis increased risk by 42%, opioids with alcohol increased risk by 15%, and opioids with smoking increased risk by 17%.
”Our findings suggest opioids may interact with other substances (including cannabis), which may be particularly deleterious,” the researchers reported. “It is not clear whether this interaction is owing to biological or environmental factors, such as whether individuals with illicit polysubstance use are more likely to use more substances or whether they have other characteristics that may impact child development.”
The authors noted that cannabis exposure has been linked to other neurodevelopmental outcomes, including reduced executive and motor function in infants. ”Notably, although we did not find a significant independent association between cannabis exposure and ADHD, children exposed to both cannabis and opioids had a 23% greater risk than expected from either exposure individually,” they reported.
The researchers suggest that their findings provide data for considering harm reduction approaches that reduce use of any single substance during pregnancy. “Focusing on the most obviously harmful exposures may be a useful way to reduce the risk of ADHD,” they wrote. “Further work is needed to directly investigate this hypothesis and examine whether reduction in the use of any substance among those with polysubstance use could be acceptable compared with abstinence.”
In an invited commentary, Angela Lupattelli, PhD, and Nhung T. H. Trinh, PhD, both of the department of pharmacy at the University of Oslo, noted the methodological challenges of assessing exposures and associations from multiple different substances during pregnancy.
“First, how can we disentangle the consequences of individual and/or combined substance exposures during pregnancy from the underlying risks?” they asked. In addition to differences in baseline characteristic between those who use opioids or cannabis, Dr. Lupattelli and Dr. Trinh noted that other important unmeasured factors, such as genetics and family environment, may confound the effect size estimates for ADHD.
They also noted the need to consider intensity, dose, duration, and timing of substance use during pregnancy.
“Understanding the longer-term safety of substance use during pregnancy is paramount to inform prevention policy and shape counseling strategies. Observational studies, despite their limitations, are a necessary piece of the puzzle,” they wrote. “However, the study findings should be interpreted with caution, as the use of advanced analytical methods cannot overcome the unavailability of some important confounding factors and exposure information.”
The research was funded by the U.S. Department of Health and Human Services and the National Institutes of Health. The authors had no industry-related disclosures.
Children prenatally exposed to opioids alone have an increased risk of attention-deficit/hyperactivity disorder (ADHD), but interactions between opioids and both cannabis use and alcohol use were linked to varying levels of ADHD risk as well, according to findings published March 11 in JAMA Network Open.
While many prenatal exposure studies examine associations with one substance, the results of this case-control study “suggest that it is important to consider prenatal exposure to multiple substances and the interactions between these substances when counseling women regarding substance use during pregnancy,” wrote Henri M. Garrison-Desany of the Johns Hopkins University, Baltimore, and colleagues.
Using data from children in the prospective Boston Birth Cohort between 1998 and 2019, the researchers did a secondary analysis on the 3,138 children (50.4% of whom were male) with at least 2 years of follow-up, excluding children from multiple-gestation pregnancies, in vitro fertilization pregnancies, and deliveries involving major maternal trauma or major chromosomal anomalies. Mothers answered a questionnaire within 24-72 hours of delivery regarding their demographics, substance use, pregnancy history, and health status. Among the mothers, 58.6% were Black, 22.3% were Hispanic, 7.2% were White, 1.5% were Asian, and 10.4% were other races/ethnicities.
The children’s electronic medical records were used to identify those with ADHD diagnoses. The researchers did not assess prescription opioid exposure during pregnancy, but they based opioid exposure on mothers’ reports of recreationally using heroin or oxycodone, mothers’ reports of receiving methadone treatment, or a newborn diagnosis of neonatal abstinence syndrome or neonatal opioid withdrawal syndrome.
Just under a quarter of the women (24.2%) reported using at least one substance during pregnancy. After tobacco smoking (18.5%), the next most reported substances were alcohol (8.1%), cannabis (3.9%), and opioids (1.9%). With a median 12 years of follow-up, 15.5% of the children had been diagnosed with ADHD, most of whom (71.6%) were male.
Before considering interaction of different substances, children exposed to opioids had a little over twice the risk of ADHD (hazard ratio [HR], 2.19) compared to those with no prenatal substance exposure. Although neither cannabis nor alcohol was independently associated with ADHD, smoking had a 40% increased risk, and researchers found a 21% increase in risk of ADHD with each additional substance mothers used during pregnancy. The researchers had adjusted these findings for maternal age, race/ethnicity, marital status, educational level, annual household income, parity, number of perinatal visits, and general stress during pregnancy, based on a structured interview.
When the researchers considered all the substances together, opioid exposure increased risk of ADHD by 60% (HR, 1.6), opioids with cannabis increased risk by 42%, opioids with alcohol increased risk by 15%, and opioids with smoking increased risk by 17%.
”Our findings suggest opioids may interact with other substances (including cannabis), which may be particularly deleterious,” the researchers reported. “It is not clear whether this interaction is owing to biological or environmental factors, such as whether individuals with illicit polysubstance use are more likely to use more substances or whether they have other characteristics that may impact child development.”
The authors noted that cannabis exposure has been linked to other neurodevelopmental outcomes, including reduced executive and motor function in infants. ”Notably, although we did not find a significant independent association between cannabis exposure and ADHD, children exposed to both cannabis and opioids had a 23% greater risk than expected from either exposure individually,” they reported.
The researchers suggest that their findings provide data for considering harm reduction approaches that reduce use of any single substance during pregnancy. “Focusing on the most obviously harmful exposures may be a useful way to reduce the risk of ADHD,” they wrote. “Further work is needed to directly investigate this hypothesis and examine whether reduction in the use of any substance among those with polysubstance use could be acceptable compared with abstinence.”
In an invited commentary, Angela Lupattelli, PhD, and Nhung T. H. Trinh, PhD, both of the department of pharmacy at the University of Oslo, noted the methodological challenges of assessing exposures and associations from multiple different substances during pregnancy.
“First, how can we disentangle the consequences of individual and/or combined substance exposures during pregnancy from the underlying risks?” they asked. In addition to differences in baseline characteristic between those who use opioids or cannabis, Dr. Lupattelli and Dr. Trinh noted that other important unmeasured factors, such as genetics and family environment, may confound the effect size estimates for ADHD.
They also noted the need to consider intensity, dose, duration, and timing of substance use during pregnancy.
“Understanding the longer-term safety of substance use during pregnancy is paramount to inform prevention policy and shape counseling strategies. Observational studies, despite their limitations, are a necessary piece of the puzzle,” they wrote. “However, the study findings should be interpreted with caution, as the use of advanced analytical methods cannot overcome the unavailability of some important confounding factors and exposure information.”
The research was funded by the U.S. Department of Health and Human Services and the National Institutes of Health. The authors had no industry-related disclosures.
FROM JAMA NETWORK OPEN
New guidance on cannabis use for treatment-resistant epilepsy
A recent review article draws from existing clinical trials and clinical experience in New South Wales, Australia, to fill this gap with interim guidance for both pediatric and adult patients. The article was published in the British Journal of Clinical Pharmacology.
The only current U.S. guidelines are from the American Academy of Neurology’s position statement on the use of medical cannabis for neurologic disorders and the American Epilepsy Society’s position statement on cannabis as a treatment for epileptic seizures. The AAN statement “highlights the current evidence, which currently only supports [Food and Drug Administration]–approved CBD [cannabidiol] (Epidiolex) for specific epilepsy syndromes,” said Daniel Freedman, DO, an assistant professor of neurology at the University of Texas at Austin and coauthor of the AAN’s position statement.
“Rescheduling marijuana will enable researchers to study CBD, THC [tetrahydrocannabinol], and other cannabinoids in high-quality studies so that we can better understand what works and for which conditions,” said Dr. Freedman, who was not involved in the Australian guidance document. He noted that little consensus exists because little evidence exists outside the handful of trials for Epidiolex.
“There are some patients with epilepsy that can benefit from high-quality, pharmaceutical-grade CBD products,” Dr. Freedman said. “These patients need to be carefully identified by a neurologist or epileptologist and prescribed a legal, safe, quality-controlled, and FDA-regulated product.”
Appropriate patient populations
Drug-resistant epilepsy, defined as failure of two appropriate antiseizure medications, affects an estimated one third of people with epilepsy, the new guideline notes. Though many over-the-counter products are available at dispensaries in the 33 U.S. states that allow use of cannabis for medical purposes, Epidiolex (cannabidiol) is the only FDA-approved drug for epilepsy that contains a substance derived from cannabis and the only one for which evidence from randomized, controlled trials exists.
Dr. Freedman notes that hemp-derived CBD oils are classified differently in the United States than marijuana-derived CBD oil, including Epidiolex, and are loosely regulated supplements or food additives commonly seen, for example at gas station.
“The point I drive home to patients is that you wouldn’t get your antibiotics from a gas station, so please don’t get your seizure medication from there,” Dr. Freedman said. “Studies have been done on ‘over-the-counter’ CBD oils and shown that they have variable quality, sometimes no detectable CBD, and sometimes other chemicals added like THC.”
Studies of Epidiolex showed that cannabidiol more effectively reduced seizure frequency than placebo for pediatric patients with Dravet syndrome (42% reduction) and for pediatric and adult patients with Lennox-Gastaut syndrome (39% reduction) or tuberous sclerosis complex (49% reduction). Efficacy was similar across dosing from 10-50 mg/kg per day, but higher doses involved higher rates of serious adverse events.
No reliable evidence in humans exists for THC or other cannabinoids in treating epilepsy.
The Australian guidance recommends limiting cannabis treatment to patients with severe drug-resistant epilepsy; a diagnosis of Dravet syndrome, Lennox-Gastaut syndrome, or tuberous sclerosis complex; and previous treatment with four approved antiseizure medications and/or the ketogenic diet, epilepsy surgery, or neurostimulator. The authors provide specific criteria for each of these conditions and then address exceptional cases that may be considered outside that criteria, such as patients under 2 years old, severe epilepsy with extended or repeated hospitalization or ICU admission, or a dangerous seizure type. The review also includes a detailed list of exclusion criteria for CBD medicine use.
The authors advised a thorough consent process before prescribing any cannabinoids, including therapeutic goals and stopping criteria; the lack of evidence available on dosing, efficacy, and side effects; and the potential for dependence or withdrawal. Consent discussions should also note whether the products are unregistered and not covered by external payers (anything other than Epidiolex currently), any activity restrictions, and any implications for occupational drug screening.
Considerations for unapproved cannabinoids
The authors note several factors to consider if prescribing or recommending a nonapproved, nonregulated cannabis medicine, including the ”differences between registered plant-derived cannabis medicines, synthetic cannabis medicines, and unregistered hemp-derived products.” Epidiolex is plant derived while other cannabis-derived medications (Marinol, Syndos, and Cesamet) that have been approved for nonepilepsy conditions, such as nausea associated with chemotherapy, are synthetic.
The guidance document notes several reasons to use a regulated medication instead of an unregulated product:
- Manufacturing processes can differ for unregulated products, including inconsistency in batches and unknown shelf life.
- Quality control processes, including risk of impurities, are much better with regulated products, which also have a system in place for safety recalls.
- More scientific evidence is available for regulated products.
- Safety surveillance reporting is more robust and standardized for regulated products whereas adverse event reporting is less reliable for unregulated products.
- Nonregulated products are rarely covered by insurance or other reimbursement.
Legal considerations will also vary by jurisdiction. ”Right now in the U.S. we have a confused legality where state level programs are still technically illegal at the federal level and I imagine there are some quality differences amongst dispensaries and states,” Dr. Freedman said. “Whenever there is disagreement between state and federal laws, this creates tension for our patients.” He noted, for example, that a patient using a CBD product that contains THC may, even if legal in their state, be confiscated by the Transportation Security Administration at an airport since it is not FDA approved and is not legal, according to the Drug Enforcement Agency.
The authors noted that inadequate data on long-term CBD use and data on neurodevelopmental effects of THC in children, teens, and young adults means THC products should be contraindicated for these age groups. (Epidiolex has less than 2% THC.) Drug interactions should also be considered, particularly for clobazam, CYP3A4 inhibitors or inducers (including St. John’s wort), digoxin, or a mechanistic target of rapamycin inhibitor.
Dr. Freedman said that most neurologists are comfortable prescribing Epidiolex since it has FDA approval while prescribing unapproved products varies more in the field. “Now that many states have compassionate use programs for medical marijuana, some neurologists do this as well,” Dr. Freedman said. Patients often ask about unregulated CBD or CBD+THC products because they’re seen as “natural and therefore better than manufactured pharmaceuticals.”
“I think this is the naturalistic fallacy at work and try to educate my patients on that since our only high-level data to show marijuana products work for epilepsy comes from a pharmaceutical company,” Dr. Freedman said. “My reasons for hesitating on compassionate use are that there is often THC, with variable amounts of concentration, and we know that THC can harm the developing pediatric brain.”
Dosing and adverse effects
Pediatric and adult dosing differences need to be considered, and “patient response (efficacy and toxicity) to these medications varies widely,” the authors noted. They advised getting serum transaminases (ALT and AST) and total bilirubin levels before beginning treatment. All patients should begin Epidiolex at a low dose, such as 2-5 mg/kg per day of CBD in two divided doses, the authors advise, and titrate slowly while monitoring for side effects (no more than 5 mg/kg per day per week). The current dosing range for CBD is 5-20 mg/kg per day in two divided doses, with higher rates involving more risk of adverse events.
“Note that some cannabinoids auto-inhibit their own metabolism and some have active metabolites with longer half-lives,” the authors wrote. “Therefore, dose or frequency may need to be reduced over time, unless tolerance occurs.” These doses, specific to Epidiolex, “cannot necessarily be applied to other oral CBD formulations or other types of epilepsy.” This guidance also does not apply to inhaled or transdermal routes of administration.
The most common adverse events were sleepiness – which occurred in up to 60% of trial participants – as well as diarrhea, decreases in appetite and weight, and drug interactions. Risk of hepatotoxicity means there’s a need to monitor liver function and adjust dosing for patients with moderate or severe hepatic impairment. “Other short-term side effects reported only with THC-containing cannabinoid compounds include increased risk of cardiac and cerebrovascular events, anxiety and psychosis risk, dependency, and withdrawal,” the authors wrote.
Though no withdrawal syndrome has been linked to stopping CBD, the authors suggested decreasing the dose by 10% every 2 days if stopping is not urgent.
“The key points to this issue are that CBD and all marijuana products need to be safe and regulated,” Dr. Freedman said. “Any claims about them need to be backed by high-quality evidence looking at that specific product for that specific condition.”
Dr. Freedman noted the need for children to receive treatment from clinicians with expertise in their specific condition since many other evidence-based treatments exist even for patients with epilepsy syndromes that are difficult to treat, such as other medications, surgery, and specialized diets.
“We need to fix the inconsistent regulation between over-the-counter CBD products, state dispensaries, and federal laws,” Dr. Freedman added. “Any medicine being used to treat children should be held to the same FDA standard of safety and efficacy.”
Dr. Freedman and the authors had no conflicts of interest. No external funding was noted.
A recent review article draws from existing clinical trials and clinical experience in New South Wales, Australia, to fill this gap with interim guidance for both pediatric and adult patients. The article was published in the British Journal of Clinical Pharmacology.
The only current U.S. guidelines are from the American Academy of Neurology’s position statement on the use of medical cannabis for neurologic disorders and the American Epilepsy Society’s position statement on cannabis as a treatment for epileptic seizures. The AAN statement “highlights the current evidence, which currently only supports [Food and Drug Administration]–approved CBD [cannabidiol] (Epidiolex) for specific epilepsy syndromes,” said Daniel Freedman, DO, an assistant professor of neurology at the University of Texas at Austin and coauthor of the AAN’s position statement.
“Rescheduling marijuana will enable researchers to study CBD, THC [tetrahydrocannabinol], and other cannabinoids in high-quality studies so that we can better understand what works and for which conditions,” said Dr. Freedman, who was not involved in the Australian guidance document. He noted that little consensus exists because little evidence exists outside the handful of trials for Epidiolex.
“There are some patients with epilepsy that can benefit from high-quality, pharmaceutical-grade CBD products,” Dr. Freedman said. “These patients need to be carefully identified by a neurologist or epileptologist and prescribed a legal, safe, quality-controlled, and FDA-regulated product.”
Appropriate patient populations
Drug-resistant epilepsy, defined as failure of two appropriate antiseizure medications, affects an estimated one third of people with epilepsy, the new guideline notes. Though many over-the-counter products are available at dispensaries in the 33 U.S. states that allow use of cannabis for medical purposes, Epidiolex (cannabidiol) is the only FDA-approved drug for epilepsy that contains a substance derived from cannabis and the only one for which evidence from randomized, controlled trials exists.
Dr. Freedman notes that hemp-derived CBD oils are classified differently in the United States than marijuana-derived CBD oil, including Epidiolex, and are loosely regulated supplements or food additives commonly seen, for example at gas station.
“The point I drive home to patients is that you wouldn’t get your antibiotics from a gas station, so please don’t get your seizure medication from there,” Dr. Freedman said. “Studies have been done on ‘over-the-counter’ CBD oils and shown that they have variable quality, sometimes no detectable CBD, and sometimes other chemicals added like THC.”
Studies of Epidiolex showed that cannabidiol more effectively reduced seizure frequency than placebo for pediatric patients with Dravet syndrome (42% reduction) and for pediatric and adult patients with Lennox-Gastaut syndrome (39% reduction) or tuberous sclerosis complex (49% reduction). Efficacy was similar across dosing from 10-50 mg/kg per day, but higher doses involved higher rates of serious adverse events.
No reliable evidence in humans exists for THC or other cannabinoids in treating epilepsy.
The Australian guidance recommends limiting cannabis treatment to patients with severe drug-resistant epilepsy; a diagnosis of Dravet syndrome, Lennox-Gastaut syndrome, or tuberous sclerosis complex; and previous treatment with four approved antiseizure medications and/or the ketogenic diet, epilepsy surgery, or neurostimulator. The authors provide specific criteria for each of these conditions and then address exceptional cases that may be considered outside that criteria, such as patients under 2 years old, severe epilepsy with extended or repeated hospitalization or ICU admission, or a dangerous seizure type. The review also includes a detailed list of exclusion criteria for CBD medicine use.
The authors advised a thorough consent process before prescribing any cannabinoids, including therapeutic goals and stopping criteria; the lack of evidence available on dosing, efficacy, and side effects; and the potential for dependence or withdrawal. Consent discussions should also note whether the products are unregistered and not covered by external payers (anything other than Epidiolex currently), any activity restrictions, and any implications for occupational drug screening.
Considerations for unapproved cannabinoids
The authors note several factors to consider if prescribing or recommending a nonapproved, nonregulated cannabis medicine, including the ”differences between registered plant-derived cannabis medicines, synthetic cannabis medicines, and unregistered hemp-derived products.” Epidiolex is plant derived while other cannabis-derived medications (Marinol, Syndos, and Cesamet) that have been approved for nonepilepsy conditions, such as nausea associated with chemotherapy, are synthetic.
The guidance document notes several reasons to use a regulated medication instead of an unregulated product:
- Manufacturing processes can differ for unregulated products, including inconsistency in batches and unknown shelf life.
- Quality control processes, including risk of impurities, are much better with regulated products, which also have a system in place for safety recalls.
- More scientific evidence is available for regulated products.
- Safety surveillance reporting is more robust and standardized for regulated products whereas adverse event reporting is less reliable for unregulated products.
- Nonregulated products are rarely covered by insurance or other reimbursement.
Legal considerations will also vary by jurisdiction. ”Right now in the U.S. we have a confused legality where state level programs are still technically illegal at the federal level and I imagine there are some quality differences amongst dispensaries and states,” Dr. Freedman said. “Whenever there is disagreement between state and federal laws, this creates tension for our patients.” He noted, for example, that a patient using a CBD product that contains THC may, even if legal in their state, be confiscated by the Transportation Security Administration at an airport since it is not FDA approved and is not legal, according to the Drug Enforcement Agency.
The authors noted that inadequate data on long-term CBD use and data on neurodevelopmental effects of THC in children, teens, and young adults means THC products should be contraindicated for these age groups. (Epidiolex has less than 2% THC.) Drug interactions should also be considered, particularly for clobazam, CYP3A4 inhibitors or inducers (including St. John’s wort), digoxin, or a mechanistic target of rapamycin inhibitor.
Dr. Freedman said that most neurologists are comfortable prescribing Epidiolex since it has FDA approval while prescribing unapproved products varies more in the field. “Now that many states have compassionate use programs for medical marijuana, some neurologists do this as well,” Dr. Freedman said. Patients often ask about unregulated CBD or CBD+THC products because they’re seen as “natural and therefore better than manufactured pharmaceuticals.”
“I think this is the naturalistic fallacy at work and try to educate my patients on that since our only high-level data to show marijuana products work for epilepsy comes from a pharmaceutical company,” Dr. Freedman said. “My reasons for hesitating on compassionate use are that there is often THC, with variable amounts of concentration, and we know that THC can harm the developing pediatric brain.”
Dosing and adverse effects
Pediatric and adult dosing differences need to be considered, and “patient response (efficacy and toxicity) to these medications varies widely,” the authors noted. They advised getting serum transaminases (ALT and AST) and total bilirubin levels before beginning treatment. All patients should begin Epidiolex at a low dose, such as 2-5 mg/kg per day of CBD in two divided doses, the authors advise, and titrate slowly while monitoring for side effects (no more than 5 mg/kg per day per week). The current dosing range for CBD is 5-20 mg/kg per day in two divided doses, with higher rates involving more risk of adverse events.
“Note that some cannabinoids auto-inhibit their own metabolism and some have active metabolites with longer half-lives,” the authors wrote. “Therefore, dose or frequency may need to be reduced over time, unless tolerance occurs.” These doses, specific to Epidiolex, “cannot necessarily be applied to other oral CBD formulations or other types of epilepsy.” This guidance also does not apply to inhaled or transdermal routes of administration.
The most common adverse events were sleepiness – which occurred in up to 60% of trial participants – as well as diarrhea, decreases in appetite and weight, and drug interactions. Risk of hepatotoxicity means there’s a need to monitor liver function and adjust dosing for patients with moderate or severe hepatic impairment. “Other short-term side effects reported only with THC-containing cannabinoid compounds include increased risk of cardiac and cerebrovascular events, anxiety and psychosis risk, dependency, and withdrawal,” the authors wrote.
Though no withdrawal syndrome has been linked to stopping CBD, the authors suggested decreasing the dose by 10% every 2 days if stopping is not urgent.
“The key points to this issue are that CBD and all marijuana products need to be safe and regulated,” Dr. Freedman said. “Any claims about them need to be backed by high-quality evidence looking at that specific product for that specific condition.”
Dr. Freedman noted the need for children to receive treatment from clinicians with expertise in their specific condition since many other evidence-based treatments exist even for patients with epilepsy syndromes that are difficult to treat, such as other medications, surgery, and specialized diets.
“We need to fix the inconsistent regulation between over-the-counter CBD products, state dispensaries, and federal laws,” Dr. Freedman added. “Any medicine being used to treat children should be held to the same FDA standard of safety and efficacy.”
Dr. Freedman and the authors had no conflicts of interest. No external funding was noted.
A recent review article draws from existing clinical trials and clinical experience in New South Wales, Australia, to fill this gap with interim guidance for both pediatric and adult patients. The article was published in the British Journal of Clinical Pharmacology.
The only current U.S. guidelines are from the American Academy of Neurology’s position statement on the use of medical cannabis for neurologic disorders and the American Epilepsy Society’s position statement on cannabis as a treatment for epileptic seizures. The AAN statement “highlights the current evidence, which currently only supports [Food and Drug Administration]–approved CBD [cannabidiol] (Epidiolex) for specific epilepsy syndromes,” said Daniel Freedman, DO, an assistant professor of neurology at the University of Texas at Austin and coauthor of the AAN’s position statement.
“Rescheduling marijuana will enable researchers to study CBD, THC [tetrahydrocannabinol], and other cannabinoids in high-quality studies so that we can better understand what works and for which conditions,” said Dr. Freedman, who was not involved in the Australian guidance document. He noted that little consensus exists because little evidence exists outside the handful of trials for Epidiolex.
“There are some patients with epilepsy that can benefit from high-quality, pharmaceutical-grade CBD products,” Dr. Freedman said. “These patients need to be carefully identified by a neurologist or epileptologist and prescribed a legal, safe, quality-controlled, and FDA-regulated product.”
Appropriate patient populations
Drug-resistant epilepsy, defined as failure of two appropriate antiseizure medications, affects an estimated one third of people with epilepsy, the new guideline notes. Though many over-the-counter products are available at dispensaries in the 33 U.S. states that allow use of cannabis for medical purposes, Epidiolex (cannabidiol) is the only FDA-approved drug for epilepsy that contains a substance derived from cannabis and the only one for which evidence from randomized, controlled trials exists.
Dr. Freedman notes that hemp-derived CBD oils are classified differently in the United States than marijuana-derived CBD oil, including Epidiolex, and are loosely regulated supplements or food additives commonly seen, for example at gas station.
“The point I drive home to patients is that you wouldn’t get your antibiotics from a gas station, so please don’t get your seizure medication from there,” Dr. Freedman said. “Studies have been done on ‘over-the-counter’ CBD oils and shown that they have variable quality, sometimes no detectable CBD, and sometimes other chemicals added like THC.”
Studies of Epidiolex showed that cannabidiol more effectively reduced seizure frequency than placebo for pediatric patients with Dravet syndrome (42% reduction) and for pediatric and adult patients with Lennox-Gastaut syndrome (39% reduction) or tuberous sclerosis complex (49% reduction). Efficacy was similar across dosing from 10-50 mg/kg per day, but higher doses involved higher rates of serious adverse events.
No reliable evidence in humans exists for THC or other cannabinoids in treating epilepsy.
The Australian guidance recommends limiting cannabis treatment to patients with severe drug-resistant epilepsy; a diagnosis of Dravet syndrome, Lennox-Gastaut syndrome, or tuberous sclerosis complex; and previous treatment with four approved antiseizure medications and/or the ketogenic diet, epilepsy surgery, or neurostimulator. The authors provide specific criteria for each of these conditions and then address exceptional cases that may be considered outside that criteria, such as patients under 2 years old, severe epilepsy with extended or repeated hospitalization or ICU admission, or a dangerous seizure type. The review also includes a detailed list of exclusion criteria for CBD medicine use.
The authors advised a thorough consent process before prescribing any cannabinoids, including therapeutic goals and stopping criteria; the lack of evidence available on dosing, efficacy, and side effects; and the potential for dependence or withdrawal. Consent discussions should also note whether the products are unregistered and not covered by external payers (anything other than Epidiolex currently), any activity restrictions, and any implications for occupational drug screening.
Considerations for unapproved cannabinoids
The authors note several factors to consider if prescribing or recommending a nonapproved, nonregulated cannabis medicine, including the ”differences between registered plant-derived cannabis medicines, synthetic cannabis medicines, and unregistered hemp-derived products.” Epidiolex is plant derived while other cannabis-derived medications (Marinol, Syndos, and Cesamet) that have been approved for nonepilepsy conditions, such as nausea associated with chemotherapy, are synthetic.
The guidance document notes several reasons to use a regulated medication instead of an unregulated product:
- Manufacturing processes can differ for unregulated products, including inconsistency in batches and unknown shelf life.
- Quality control processes, including risk of impurities, are much better with regulated products, which also have a system in place for safety recalls.
- More scientific evidence is available for regulated products.
- Safety surveillance reporting is more robust and standardized for regulated products whereas adverse event reporting is less reliable for unregulated products.
- Nonregulated products are rarely covered by insurance or other reimbursement.
Legal considerations will also vary by jurisdiction. ”Right now in the U.S. we have a confused legality where state level programs are still technically illegal at the federal level and I imagine there are some quality differences amongst dispensaries and states,” Dr. Freedman said. “Whenever there is disagreement between state and federal laws, this creates tension for our patients.” He noted, for example, that a patient using a CBD product that contains THC may, even if legal in their state, be confiscated by the Transportation Security Administration at an airport since it is not FDA approved and is not legal, according to the Drug Enforcement Agency.
The authors noted that inadequate data on long-term CBD use and data on neurodevelopmental effects of THC in children, teens, and young adults means THC products should be contraindicated for these age groups. (Epidiolex has less than 2% THC.) Drug interactions should also be considered, particularly for clobazam, CYP3A4 inhibitors or inducers (including St. John’s wort), digoxin, or a mechanistic target of rapamycin inhibitor.
Dr. Freedman said that most neurologists are comfortable prescribing Epidiolex since it has FDA approval while prescribing unapproved products varies more in the field. “Now that many states have compassionate use programs for medical marijuana, some neurologists do this as well,” Dr. Freedman said. Patients often ask about unregulated CBD or CBD+THC products because they’re seen as “natural and therefore better than manufactured pharmaceuticals.”
“I think this is the naturalistic fallacy at work and try to educate my patients on that since our only high-level data to show marijuana products work for epilepsy comes from a pharmaceutical company,” Dr. Freedman said. “My reasons for hesitating on compassionate use are that there is often THC, with variable amounts of concentration, and we know that THC can harm the developing pediatric brain.”
Dosing and adverse effects
Pediatric and adult dosing differences need to be considered, and “patient response (efficacy and toxicity) to these medications varies widely,” the authors noted. They advised getting serum transaminases (ALT and AST) and total bilirubin levels before beginning treatment. All patients should begin Epidiolex at a low dose, such as 2-5 mg/kg per day of CBD in two divided doses, the authors advise, and titrate slowly while monitoring for side effects (no more than 5 mg/kg per day per week). The current dosing range for CBD is 5-20 mg/kg per day in two divided doses, with higher rates involving more risk of adverse events.
“Note that some cannabinoids auto-inhibit their own metabolism and some have active metabolites with longer half-lives,” the authors wrote. “Therefore, dose or frequency may need to be reduced over time, unless tolerance occurs.” These doses, specific to Epidiolex, “cannot necessarily be applied to other oral CBD formulations or other types of epilepsy.” This guidance also does not apply to inhaled or transdermal routes of administration.
The most common adverse events were sleepiness – which occurred in up to 60% of trial participants – as well as diarrhea, decreases in appetite and weight, and drug interactions. Risk of hepatotoxicity means there’s a need to monitor liver function and adjust dosing for patients with moderate or severe hepatic impairment. “Other short-term side effects reported only with THC-containing cannabinoid compounds include increased risk of cardiac and cerebrovascular events, anxiety and psychosis risk, dependency, and withdrawal,” the authors wrote.
Though no withdrawal syndrome has been linked to stopping CBD, the authors suggested decreasing the dose by 10% every 2 days if stopping is not urgent.
“The key points to this issue are that CBD and all marijuana products need to be safe and regulated,” Dr. Freedman said. “Any claims about them need to be backed by high-quality evidence looking at that specific product for that specific condition.”
Dr. Freedman noted the need for children to receive treatment from clinicians with expertise in their specific condition since many other evidence-based treatments exist even for patients with epilepsy syndromes that are difficult to treat, such as other medications, surgery, and specialized diets.
“We need to fix the inconsistent regulation between over-the-counter CBD products, state dispensaries, and federal laws,” Dr. Freedman added. “Any medicine being used to treat children should be held to the same FDA standard of safety and efficacy.”
Dr. Freedman and the authors had no conflicts of interest. No external funding was noted.
FROM THE BRITISH JOURNAL OF CLINICAL PHARMACOLOGY
Just one extra drink a day may change the brain
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
New JIA guidelines emphasize earlier DMARD use
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
New ivermectin, HCQ scripts highest in GOP-dominated counties
New prescriptions of hydroxychloroquine (HCQ) and ivermectin increased in 2020, driven particularly by rates in counties with the highest proportion of Republican votes in the 2020 U.S. presidential election, according to a cross-sectional study published in JAMA Internal Medicine.
“Our findings are consistent with the hypothesis that U.S. prescribing of hydroxychloroquine and ivermectin during the COVID-19 pandemic may have been influenced by political affiliation,” wrote Michael L. Barnett, MD, of the Harvard T.H. Chan School of Public Health in Boston and colleagues.
The researchers used data from the OptumLabs Data Warehouse to analyze commercial and Medicare Advantage medical claims from January 2019 through December 2020 for more than 18.5 million adults living in counties with at least 50 enrollees.
Using U.S. Census data and 2020 presidential election results, the researchers classified counties according to their proportion of Republican voters and then examined whether those proportions were associated with that county’s rates of new prescriptions for HCQ, ivermectin, methotrexate sodium, and albendazole. Methotrexate is prescribed for similar conditions and indications as HCQ, and albendazole is prescribed for similar reasons as ivermectin, although neither of the comparison drugs has been considered for COVID-19 treatment.
The Food and Drug Administration issued an emergency use authorization (EUA) for HCQ as a COVID-19 treatment on March 28, 2020, but the agency revoked the EUA 3 months later on June 15. Ivermectin never received an EUA for COVID treatment, but an in vitro study published April 3, 2020 claimed it had an antiviral effect.
The National Institutes of Health recommended against using ivermectin as a COVID-19 treatment on Aug. 1, 2020, but a few months later, on Nov. 13, a flawed clinical trial – later retracted – claimed ivermectin was 90% effective in treating COVID-19. Despite the lack of evidence for ivermectin’s efficacy, a Senate committee meeting on Dec. 8, 2020, included testimony from a physician who promoted its use.
In comparing ivermectin and HCQ prescription rates with counties’ political composition, the researchers adjusted their findings to account for differences in the counties’ racial composition and COVID-19 incidence as well as enrollees’ age, sex, insurance type, income, comorbidity burden, and home in a rural or urban area.
The results showed an average of 20 new HCQ prescriptions per 100,000 enrollees in 2019, but 2020 saw a sharp increase and drop in new HCQ prescriptions in March-April 2020, independent of counties’ breakdown of political affiliation.
“However, after June 2020, coinciding with the revocation of the U.S. Food and Drug Administration’s emergency use authorization for hydroxychloroquine, prescribing volume was significantly higher in the highest vs. lowest Republican vote share counties,” the authors report. The gradual increase from June through December 2020 averaged to 42 new prescriptions per 100,000, a 146% increase over 2019 rates that was driven largely by the 25% of counties with the highest proportion of Republican voters.
Similarly, rates of new ivermectin prescriptions in December 2020 were more than nine times higher in counties with the highest Republican vote share, compared with new prescriptions throughout 2019. The researchers found no differences in new prescriptions for methotrexate or albendazole in 2020 based on counties’ proportion of Republican votes.
Since the study is an ecological, observational one, it cannot show causation or shed light on what role patients, physicians, or other factors might have played in prescribing patterns. Nevertheless, the authors noted the potentially negative implications of their findings.
“Because political affiliation should not be a factor in clinical treatment decisions, our findings raise concerns for public trust in a nonpartisan health care system,” the authors write.
Coauthor Ateev Mehrotra, MD, MPH, reported personal fees from Sanofi-Aventis, and coauthor Anupam B. Jena, MD, PhD, reported personal fees from Bioverativ, Merck, Janssen, Edwards Lifesciences, Novartis, Amgen, Eisai, Otsuka, Vertex, Celgene, Sanofi-Aventis, Precision Health Economics (now PRECISIONheor), Analysis Group, and Doubleday and hosting the podcast Freakonomics, M.D. The other coauthors have disclosed no relevant financial relationships. No external funding source was noted.
A version of this article first appeared on Medscape.com.
New prescriptions of hydroxychloroquine (HCQ) and ivermectin increased in 2020, driven particularly by rates in counties with the highest proportion of Republican votes in the 2020 U.S. presidential election, according to a cross-sectional study published in JAMA Internal Medicine.
“Our findings are consistent with the hypothesis that U.S. prescribing of hydroxychloroquine and ivermectin during the COVID-19 pandemic may have been influenced by political affiliation,” wrote Michael L. Barnett, MD, of the Harvard T.H. Chan School of Public Health in Boston and colleagues.
The researchers used data from the OptumLabs Data Warehouse to analyze commercial and Medicare Advantage medical claims from January 2019 through December 2020 for more than 18.5 million adults living in counties with at least 50 enrollees.
Using U.S. Census data and 2020 presidential election results, the researchers classified counties according to their proportion of Republican voters and then examined whether those proportions were associated with that county’s rates of new prescriptions for HCQ, ivermectin, methotrexate sodium, and albendazole. Methotrexate is prescribed for similar conditions and indications as HCQ, and albendazole is prescribed for similar reasons as ivermectin, although neither of the comparison drugs has been considered for COVID-19 treatment.
The Food and Drug Administration issued an emergency use authorization (EUA) for HCQ as a COVID-19 treatment on March 28, 2020, but the agency revoked the EUA 3 months later on June 15. Ivermectin never received an EUA for COVID treatment, but an in vitro study published April 3, 2020 claimed it had an antiviral effect.
The National Institutes of Health recommended against using ivermectin as a COVID-19 treatment on Aug. 1, 2020, but a few months later, on Nov. 13, a flawed clinical trial – later retracted – claimed ivermectin was 90% effective in treating COVID-19. Despite the lack of evidence for ivermectin’s efficacy, a Senate committee meeting on Dec. 8, 2020, included testimony from a physician who promoted its use.
In comparing ivermectin and HCQ prescription rates with counties’ political composition, the researchers adjusted their findings to account for differences in the counties’ racial composition and COVID-19 incidence as well as enrollees’ age, sex, insurance type, income, comorbidity burden, and home in a rural or urban area.
The results showed an average of 20 new HCQ prescriptions per 100,000 enrollees in 2019, but 2020 saw a sharp increase and drop in new HCQ prescriptions in March-April 2020, independent of counties’ breakdown of political affiliation.
“However, after June 2020, coinciding with the revocation of the U.S. Food and Drug Administration’s emergency use authorization for hydroxychloroquine, prescribing volume was significantly higher in the highest vs. lowest Republican vote share counties,” the authors report. The gradual increase from June through December 2020 averaged to 42 new prescriptions per 100,000, a 146% increase over 2019 rates that was driven largely by the 25% of counties with the highest proportion of Republican voters.
Similarly, rates of new ivermectin prescriptions in December 2020 were more than nine times higher in counties with the highest Republican vote share, compared with new prescriptions throughout 2019. The researchers found no differences in new prescriptions for methotrexate or albendazole in 2020 based on counties’ proportion of Republican votes.
Since the study is an ecological, observational one, it cannot show causation or shed light on what role patients, physicians, or other factors might have played in prescribing patterns. Nevertheless, the authors noted the potentially negative implications of their findings.
“Because political affiliation should not be a factor in clinical treatment decisions, our findings raise concerns for public trust in a nonpartisan health care system,” the authors write.
Coauthor Ateev Mehrotra, MD, MPH, reported personal fees from Sanofi-Aventis, and coauthor Anupam B. Jena, MD, PhD, reported personal fees from Bioverativ, Merck, Janssen, Edwards Lifesciences, Novartis, Amgen, Eisai, Otsuka, Vertex, Celgene, Sanofi-Aventis, Precision Health Economics (now PRECISIONheor), Analysis Group, and Doubleday and hosting the podcast Freakonomics, M.D. The other coauthors have disclosed no relevant financial relationships. No external funding source was noted.
A version of this article first appeared on Medscape.com.
New prescriptions of hydroxychloroquine (HCQ) and ivermectin increased in 2020, driven particularly by rates in counties with the highest proportion of Republican votes in the 2020 U.S. presidential election, according to a cross-sectional study published in JAMA Internal Medicine.
“Our findings are consistent with the hypothesis that U.S. prescribing of hydroxychloroquine and ivermectin during the COVID-19 pandemic may have been influenced by political affiliation,” wrote Michael L. Barnett, MD, of the Harvard T.H. Chan School of Public Health in Boston and colleagues.
The researchers used data from the OptumLabs Data Warehouse to analyze commercial and Medicare Advantage medical claims from January 2019 through December 2020 for more than 18.5 million adults living in counties with at least 50 enrollees.
Using U.S. Census data and 2020 presidential election results, the researchers classified counties according to their proportion of Republican voters and then examined whether those proportions were associated with that county’s rates of new prescriptions for HCQ, ivermectin, methotrexate sodium, and albendazole. Methotrexate is prescribed for similar conditions and indications as HCQ, and albendazole is prescribed for similar reasons as ivermectin, although neither of the comparison drugs has been considered for COVID-19 treatment.
The Food and Drug Administration issued an emergency use authorization (EUA) for HCQ as a COVID-19 treatment on March 28, 2020, but the agency revoked the EUA 3 months later on June 15. Ivermectin never received an EUA for COVID treatment, but an in vitro study published April 3, 2020 claimed it had an antiviral effect.
The National Institutes of Health recommended against using ivermectin as a COVID-19 treatment on Aug. 1, 2020, but a few months later, on Nov. 13, a flawed clinical trial – later retracted – claimed ivermectin was 90% effective in treating COVID-19. Despite the lack of evidence for ivermectin’s efficacy, a Senate committee meeting on Dec. 8, 2020, included testimony from a physician who promoted its use.
In comparing ivermectin and HCQ prescription rates with counties’ political composition, the researchers adjusted their findings to account for differences in the counties’ racial composition and COVID-19 incidence as well as enrollees’ age, sex, insurance type, income, comorbidity burden, and home in a rural or urban area.
The results showed an average of 20 new HCQ prescriptions per 100,000 enrollees in 2019, but 2020 saw a sharp increase and drop in new HCQ prescriptions in March-April 2020, independent of counties’ breakdown of political affiliation.
“However, after June 2020, coinciding with the revocation of the U.S. Food and Drug Administration’s emergency use authorization for hydroxychloroquine, prescribing volume was significantly higher in the highest vs. lowest Republican vote share counties,” the authors report. The gradual increase from June through December 2020 averaged to 42 new prescriptions per 100,000, a 146% increase over 2019 rates that was driven largely by the 25% of counties with the highest proportion of Republican voters.
Similarly, rates of new ivermectin prescriptions in December 2020 were more than nine times higher in counties with the highest Republican vote share, compared with new prescriptions throughout 2019. The researchers found no differences in new prescriptions for methotrexate or albendazole in 2020 based on counties’ proportion of Republican votes.
Since the study is an ecological, observational one, it cannot show causation or shed light on what role patients, physicians, or other factors might have played in prescribing patterns. Nevertheless, the authors noted the potentially negative implications of their findings.
“Because political affiliation should not be a factor in clinical treatment decisions, our findings raise concerns for public trust in a nonpartisan health care system,” the authors write.
Coauthor Ateev Mehrotra, MD, MPH, reported personal fees from Sanofi-Aventis, and coauthor Anupam B. Jena, MD, PhD, reported personal fees from Bioverativ, Merck, Janssen, Edwards Lifesciences, Novartis, Amgen, Eisai, Otsuka, Vertex, Celgene, Sanofi-Aventis, Precision Health Economics (now PRECISIONheor), Analysis Group, and Doubleday and hosting the podcast Freakonomics, M.D. The other coauthors have disclosed no relevant financial relationships. No external funding source was noted.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE