User login
Updates to CDC’s STI guidelines relevant to midlife women too
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
FROM NAMS 2021
COVID-19 disease may actually cause preeclampsia, suggests study
New evidence strongly suggests that COVID-19 disease causes an increased risk of preeclampsia and preterm birth in those who have an infection while pregnant, according to a retrospective observational study published in the American Journal of Obstetrics and Gynecology. Though the study was observational, its primary finding was a dose-response relationship between the severity of COVID-19 disease and the likelihood of preeclampsia or preterm birth, fulfilling a key criterion for establishing causality in an association.
“The fact that 43% (13/30) of the cases of preeclampsia diagnosed after SARS-Cov-2 infection were preterm preeclampsia (< 37 weeks) suggests that COVID-19 may be a cause for medically indicated preterm birth that contributes to the excess preterm birth delivery rate previously reported,” wrote Jonathan Lai, MD, of the Fetal Medicine Research Institute of King’s College Hospital, London, and colleagues. The study also found an increased likelihood of COVID-19 disease in those who had preeclampsia before their infection. “Whether preeclampsia can predispose COVID-19 some cases, or that the two conditions may co-occur because they share similar risk factors requires further investigation,” the authors wrote.
It’s also unclear whether the increased risk of pre-eclampsia is contributing to the higher preterm birth risk, according to Linda Eckert, MD, a professor of Ob.Gyn. at The University of Washington who specializes in maternal immunization.
“COVID is linked to preeclampsia in this study, and COVID is linked to preterm birth,” Dr. Eckert said in an interview. “The question of whether preeclampsia leading to preterm birth is also linked to infection is not possible to tease out in this study as all the factors are likely interrelated. There is a relationship between COVID and preterm birth absent preeclampsia.”
The researchers retrospectively examined data from 1,223 pregnant women who tested positive for SARS-CoV-2 between February 2020 and March 2021 at any of 14 National Health Service maternity hospitals in the United Kingdom. The researchers compared the severity of disease among the women with their risk of preeclampsia as a primary outcome, followed by the outcomes of preterm birth and gestational age at delivery.
COVID-19 infections were classified as asymptomatic, mild illness (lacking shortness of breath, dyspnea, or abnormal chest imaging), moderate illness (evidence of lower respiratory disease but an oxygen saturation of at least 94%), and severe illness (requiring “high dependency or intensive care secondary to respiratory impairment/failure or multiorgan dysfunction”).
The researchers adjusted their analysis of preeclampsia to account for prior risk of preeclampsia based on maternal characteristics and medical history. Analysis of preterm birth risk included adjustment for maternal age, weight, height, race, method of conception, chronic hypertension, smoking, and diabetes.
Preeclampsia occurred in 4.2% of the women, and 17.6% of the women had a preterm birth. In addition, 1.3% of the cohort had a miscarriage, and there were 10 (0.81%) fetal deaths. Since 21 cases of preeclampsia occurred before the women tested positive, the researchers removed those cases from the analysis. Among the remaining 30 cases, 13 women had preterm preeclampsia and 17 had term preeclampsia.
When the researchers compared the study population’s risk of preeclampsia with that of a separate population with similar risk factors, they found a dose-response increased risk in those with COVID-19 infections. While 1.9% of asymptomatic patients had preeclampsia, incidence was 2.2% in patients with mild disease, 5.7% in those with moderate disease, and 11.1% in those with severe disease. Women with severe COVID-19 tended to be older and to have a higher body mass index.
After adjustments, women were nearly five times more likely to develop preeclampsia if they had severe COVID-19 compared to women with asymptomatic infection (adjusted relative risk [aRR] = 4.9). Those with moderate or severe disease had triple the risk of preeclampsia compared to those with mild or asymptomatic infection (aRR = 3.3).
To investigate whether having preeclampsia predisposes women to develop COVID-19 disease, the researchers compared the women who had preeclampsia before their infection with women in the study who never developed preeclampsia. Although they found a trend toward higher risk of moderate or severe COVID-19 following preeclampsia, the association was not significant before or after adjustment.
The researchers also found a dose-response relationship in risk of preterm birth. While 11.7% of asymptomatic patients had preterm birth, the incidence was 12.8% in those with mild COVID-19, 29.9% in those with moderate disease, and 69.4% in those with severe disease. Women with severe disease were more than five times more likely to have a preterm birth than were women with an asymptomatic infection (aRR = 5.64), and the risk of preterm birth was 2.5 times greater in women with moderate disease (aRR = 2.47).
“Moreover, there was a dose-response relationship between gestational age at delivery and the severity of SARS-CoV-2 infection,” the authors reported. Mean gestational age at delivery was 38.7 weeks in asymptomatic women compared to 37.5 weeks for those with moderate disease and 33 weeks in those with severe disease (P < .001).
”The more severe the infection with SARS-CoV-2, the greater the risk of preeclampsia and preterm birth,” the authors wrote. “SARS-CoV-2 infection can lead to endothelial dysfunction, intravascular inflammation, proteinuria, activation of thrombin, and hypertension, which are all features of preeclampsia. Therefore, a causal relationship must be considered.”
A dose-response association is only one criterion for causality, however, so it’s still premature to say definitively that a causal relationship exists, Dr. Eckert said.
“More investigation in different populations across different ethnicities is needed before causality can be confidently assured,” she said.
Anthony Sciscione, DO, director of maternal-fetal medicine and the ob.gyn. residency at ChristianaCare in Delaware, agreed that the precise relationship between the two remains unresolved.
”We don’t know what causes preeclampsia,” but “we strongly suspect it has to do with a placental dysfunction, or endothelial dysfunction, and it’s really clear that women who get COVID have a much higher risk of preeclampsia,” Dr. Sciscione said in an interview. It’s possible that no real relationship exists between the two (or that greater surveillance of women with COVID-19 is picking up the relationship) but it’s more likely that one of two other situations is happening, Dr. Sciscione said. Either COVID-19 involves a syndrome that looks like preeclampsia in pregnant women, or the disease “leads to the cascade that causes preeclampsia,” he said.
One clear clinical implication of these findings is that “women who have severe COVID early in pregnancy may need to be watched more closely for signs of developing preeclampsia” and that “women with severe COVID are more likely to have preterm births,” Dr. Eckert said. “This absolutely lends support to the need for pregnant individuals to receive a COVID vaccine.”
Dr. Sciscione said his experience counseling pregnant patients about the vaccine has made it clear that patients generally want to do what’s safest for their babies and may feel uneasiness about the safety of the vaccine. “The truth is, now there’s mounting evidence that there are fetal effects, not just maternal effects” from COVID-19 disease. He added that preterm birth is associated with a variety of long-term adverse outcomes, such as cerebral palsy and learning disabilities.
“At this time it’s critically important that women be offered and get the vaccine because we know that people that are vaccinated don’t get as sick,” Dr. Sciscione said.
The research was funded by the Fetal Medicine Foundation and the National Institutes of Health. The authors and Dr. Eckert have no disclosures. Dr. Sciscione is the associate editor of the American Journal of Obstetrics and Gynecology, where the study appeared.
New evidence strongly suggests that COVID-19 disease causes an increased risk of preeclampsia and preterm birth in those who have an infection while pregnant, according to a retrospective observational study published in the American Journal of Obstetrics and Gynecology. Though the study was observational, its primary finding was a dose-response relationship between the severity of COVID-19 disease and the likelihood of preeclampsia or preterm birth, fulfilling a key criterion for establishing causality in an association.
“The fact that 43% (13/30) of the cases of preeclampsia diagnosed after SARS-Cov-2 infection were preterm preeclampsia (< 37 weeks) suggests that COVID-19 may be a cause for medically indicated preterm birth that contributes to the excess preterm birth delivery rate previously reported,” wrote Jonathan Lai, MD, of the Fetal Medicine Research Institute of King’s College Hospital, London, and colleagues. The study also found an increased likelihood of COVID-19 disease in those who had preeclampsia before their infection. “Whether preeclampsia can predispose COVID-19 some cases, or that the two conditions may co-occur because they share similar risk factors requires further investigation,” the authors wrote.
It’s also unclear whether the increased risk of pre-eclampsia is contributing to the higher preterm birth risk, according to Linda Eckert, MD, a professor of Ob.Gyn. at The University of Washington who specializes in maternal immunization.
“COVID is linked to preeclampsia in this study, and COVID is linked to preterm birth,” Dr. Eckert said in an interview. “The question of whether preeclampsia leading to preterm birth is also linked to infection is not possible to tease out in this study as all the factors are likely interrelated. There is a relationship between COVID and preterm birth absent preeclampsia.”
The researchers retrospectively examined data from 1,223 pregnant women who tested positive for SARS-CoV-2 between February 2020 and March 2021 at any of 14 National Health Service maternity hospitals in the United Kingdom. The researchers compared the severity of disease among the women with their risk of preeclampsia as a primary outcome, followed by the outcomes of preterm birth and gestational age at delivery.
COVID-19 infections were classified as asymptomatic, mild illness (lacking shortness of breath, dyspnea, or abnormal chest imaging), moderate illness (evidence of lower respiratory disease but an oxygen saturation of at least 94%), and severe illness (requiring “high dependency or intensive care secondary to respiratory impairment/failure or multiorgan dysfunction”).
The researchers adjusted their analysis of preeclampsia to account for prior risk of preeclampsia based on maternal characteristics and medical history. Analysis of preterm birth risk included adjustment for maternal age, weight, height, race, method of conception, chronic hypertension, smoking, and diabetes.
Preeclampsia occurred in 4.2% of the women, and 17.6% of the women had a preterm birth. In addition, 1.3% of the cohort had a miscarriage, and there were 10 (0.81%) fetal deaths. Since 21 cases of preeclampsia occurred before the women tested positive, the researchers removed those cases from the analysis. Among the remaining 30 cases, 13 women had preterm preeclampsia and 17 had term preeclampsia.
When the researchers compared the study population’s risk of preeclampsia with that of a separate population with similar risk factors, they found a dose-response increased risk in those with COVID-19 infections. While 1.9% of asymptomatic patients had preeclampsia, incidence was 2.2% in patients with mild disease, 5.7% in those with moderate disease, and 11.1% in those with severe disease. Women with severe COVID-19 tended to be older and to have a higher body mass index.
After adjustments, women were nearly five times more likely to develop preeclampsia if they had severe COVID-19 compared to women with asymptomatic infection (adjusted relative risk [aRR] = 4.9). Those with moderate or severe disease had triple the risk of preeclampsia compared to those with mild or asymptomatic infection (aRR = 3.3).
To investigate whether having preeclampsia predisposes women to develop COVID-19 disease, the researchers compared the women who had preeclampsia before their infection with women in the study who never developed preeclampsia. Although they found a trend toward higher risk of moderate or severe COVID-19 following preeclampsia, the association was not significant before or after adjustment.
The researchers also found a dose-response relationship in risk of preterm birth. While 11.7% of asymptomatic patients had preterm birth, the incidence was 12.8% in those with mild COVID-19, 29.9% in those with moderate disease, and 69.4% in those with severe disease. Women with severe disease were more than five times more likely to have a preterm birth than were women with an asymptomatic infection (aRR = 5.64), and the risk of preterm birth was 2.5 times greater in women with moderate disease (aRR = 2.47).
“Moreover, there was a dose-response relationship between gestational age at delivery and the severity of SARS-CoV-2 infection,” the authors reported. Mean gestational age at delivery was 38.7 weeks in asymptomatic women compared to 37.5 weeks for those with moderate disease and 33 weeks in those with severe disease (P < .001).
”The more severe the infection with SARS-CoV-2, the greater the risk of preeclampsia and preterm birth,” the authors wrote. “SARS-CoV-2 infection can lead to endothelial dysfunction, intravascular inflammation, proteinuria, activation of thrombin, and hypertension, which are all features of preeclampsia. Therefore, a causal relationship must be considered.”
A dose-response association is only one criterion for causality, however, so it’s still premature to say definitively that a causal relationship exists, Dr. Eckert said.
“More investigation in different populations across different ethnicities is needed before causality can be confidently assured,” she said.
Anthony Sciscione, DO, director of maternal-fetal medicine and the ob.gyn. residency at ChristianaCare in Delaware, agreed that the precise relationship between the two remains unresolved.
”We don’t know what causes preeclampsia,” but “we strongly suspect it has to do with a placental dysfunction, or endothelial dysfunction, and it’s really clear that women who get COVID have a much higher risk of preeclampsia,” Dr. Sciscione said in an interview. It’s possible that no real relationship exists between the two (or that greater surveillance of women with COVID-19 is picking up the relationship) but it’s more likely that one of two other situations is happening, Dr. Sciscione said. Either COVID-19 involves a syndrome that looks like preeclampsia in pregnant women, or the disease “leads to the cascade that causes preeclampsia,” he said.
One clear clinical implication of these findings is that “women who have severe COVID early in pregnancy may need to be watched more closely for signs of developing preeclampsia” and that “women with severe COVID are more likely to have preterm births,” Dr. Eckert said. “This absolutely lends support to the need for pregnant individuals to receive a COVID vaccine.”
Dr. Sciscione said his experience counseling pregnant patients about the vaccine has made it clear that patients generally want to do what’s safest for their babies and may feel uneasiness about the safety of the vaccine. “The truth is, now there’s mounting evidence that there are fetal effects, not just maternal effects” from COVID-19 disease. He added that preterm birth is associated with a variety of long-term adverse outcomes, such as cerebral palsy and learning disabilities.
“At this time it’s critically important that women be offered and get the vaccine because we know that people that are vaccinated don’t get as sick,” Dr. Sciscione said.
The research was funded by the Fetal Medicine Foundation and the National Institutes of Health. The authors and Dr. Eckert have no disclosures. Dr. Sciscione is the associate editor of the American Journal of Obstetrics and Gynecology, where the study appeared.
New evidence strongly suggests that COVID-19 disease causes an increased risk of preeclampsia and preterm birth in those who have an infection while pregnant, according to a retrospective observational study published in the American Journal of Obstetrics and Gynecology. Though the study was observational, its primary finding was a dose-response relationship between the severity of COVID-19 disease and the likelihood of preeclampsia or preterm birth, fulfilling a key criterion for establishing causality in an association.
“The fact that 43% (13/30) of the cases of preeclampsia diagnosed after SARS-Cov-2 infection were preterm preeclampsia (< 37 weeks) suggests that COVID-19 may be a cause for medically indicated preterm birth that contributes to the excess preterm birth delivery rate previously reported,” wrote Jonathan Lai, MD, of the Fetal Medicine Research Institute of King’s College Hospital, London, and colleagues. The study also found an increased likelihood of COVID-19 disease in those who had preeclampsia before their infection. “Whether preeclampsia can predispose COVID-19 some cases, or that the two conditions may co-occur because they share similar risk factors requires further investigation,” the authors wrote.
It’s also unclear whether the increased risk of pre-eclampsia is contributing to the higher preterm birth risk, according to Linda Eckert, MD, a professor of Ob.Gyn. at The University of Washington who specializes in maternal immunization.
“COVID is linked to preeclampsia in this study, and COVID is linked to preterm birth,” Dr. Eckert said in an interview. “The question of whether preeclampsia leading to preterm birth is also linked to infection is not possible to tease out in this study as all the factors are likely interrelated. There is a relationship between COVID and preterm birth absent preeclampsia.”
The researchers retrospectively examined data from 1,223 pregnant women who tested positive for SARS-CoV-2 between February 2020 and March 2021 at any of 14 National Health Service maternity hospitals in the United Kingdom. The researchers compared the severity of disease among the women with their risk of preeclampsia as a primary outcome, followed by the outcomes of preterm birth and gestational age at delivery.
COVID-19 infections were classified as asymptomatic, mild illness (lacking shortness of breath, dyspnea, or abnormal chest imaging), moderate illness (evidence of lower respiratory disease but an oxygen saturation of at least 94%), and severe illness (requiring “high dependency or intensive care secondary to respiratory impairment/failure or multiorgan dysfunction”).
The researchers adjusted their analysis of preeclampsia to account for prior risk of preeclampsia based on maternal characteristics and medical history. Analysis of preterm birth risk included adjustment for maternal age, weight, height, race, method of conception, chronic hypertension, smoking, and diabetes.
Preeclampsia occurred in 4.2% of the women, and 17.6% of the women had a preterm birth. In addition, 1.3% of the cohort had a miscarriage, and there were 10 (0.81%) fetal deaths. Since 21 cases of preeclampsia occurred before the women tested positive, the researchers removed those cases from the analysis. Among the remaining 30 cases, 13 women had preterm preeclampsia and 17 had term preeclampsia.
When the researchers compared the study population’s risk of preeclampsia with that of a separate population with similar risk factors, they found a dose-response increased risk in those with COVID-19 infections. While 1.9% of asymptomatic patients had preeclampsia, incidence was 2.2% in patients with mild disease, 5.7% in those with moderate disease, and 11.1% in those with severe disease. Women with severe COVID-19 tended to be older and to have a higher body mass index.
After adjustments, women were nearly five times more likely to develop preeclampsia if they had severe COVID-19 compared to women with asymptomatic infection (adjusted relative risk [aRR] = 4.9). Those with moderate or severe disease had triple the risk of preeclampsia compared to those with mild or asymptomatic infection (aRR = 3.3).
To investigate whether having preeclampsia predisposes women to develop COVID-19 disease, the researchers compared the women who had preeclampsia before their infection with women in the study who never developed preeclampsia. Although they found a trend toward higher risk of moderate or severe COVID-19 following preeclampsia, the association was not significant before or after adjustment.
The researchers also found a dose-response relationship in risk of preterm birth. While 11.7% of asymptomatic patients had preterm birth, the incidence was 12.8% in those with mild COVID-19, 29.9% in those with moderate disease, and 69.4% in those with severe disease. Women with severe disease were more than five times more likely to have a preterm birth than were women with an asymptomatic infection (aRR = 5.64), and the risk of preterm birth was 2.5 times greater in women with moderate disease (aRR = 2.47).
“Moreover, there was a dose-response relationship between gestational age at delivery and the severity of SARS-CoV-2 infection,” the authors reported. Mean gestational age at delivery was 38.7 weeks in asymptomatic women compared to 37.5 weeks for those with moderate disease and 33 weeks in those with severe disease (P < .001).
”The more severe the infection with SARS-CoV-2, the greater the risk of preeclampsia and preterm birth,” the authors wrote. “SARS-CoV-2 infection can lead to endothelial dysfunction, intravascular inflammation, proteinuria, activation of thrombin, and hypertension, which are all features of preeclampsia. Therefore, a causal relationship must be considered.”
A dose-response association is only one criterion for causality, however, so it’s still premature to say definitively that a causal relationship exists, Dr. Eckert said.
“More investigation in different populations across different ethnicities is needed before causality can be confidently assured,” she said.
Anthony Sciscione, DO, director of maternal-fetal medicine and the ob.gyn. residency at ChristianaCare in Delaware, agreed that the precise relationship between the two remains unresolved.
”We don’t know what causes preeclampsia,” but “we strongly suspect it has to do with a placental dysfunction, or endothelial dysfunction, and it’s really clear that women who get COVID have a much higher risk of preeclampsia,” Dr. Sciscione said in an interview. It’s possible that no real relationship exists between the two (or that greater surveillance of women with COVID-19 is picking up the relationship) but it’s more likely that one of two other situations is happening, Dr. Sciscione said. Either COVID-19 involves a syndrome that looks like preeclampsia in pregnant women, or the disease “leads to the cascade that causes preeclampsia,” he said.
One clear clinical implication of these findings is that “women who have severe COVID early in pregnancy may need to be watched more closely for signs of developing preeclampsia” and that “women with severe COVID are more likely to have preterm births,” Dr. Eckert said. “This absolutely lends support to the need for pregnant individuals to receive a COVID vaccine.”
Dr. Sciscione said his experience counseling pregnant patients about the vaccine has made it clear that patients generally want to do what’s safest for their babies and may feel uneasiness about the safety of the vaccine. “The truth is, now there’s mounting evidence that there are fetal effects, not just maternal effects” from COVID-19 disease. He added that preterm birth is associated with a variety of long-term adverse outcomes, such as cerebral palsy and learning disabilities.
“At this time it’s critically important that women be offered and get the vaccine because we know that people that are vaccinated don’t get as sick,” Dr. Sciscione said.
The research was funded by the Fetal Medicine Foundation and the National Institutes of Health. The authors and Dr. Eckert have no disclosures. Dr. Sciscione is the associate editor of the American Journal of Obstetrics and Gynecology, where the study appeared.
FROM THE JOURNAL OF OBSTETRICS AND GYNECOLOGY
Bystander rescue breathing CPR in kids tied to better survival
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prevalence of high-risk HPV types dwindled since vaccine approval
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 mitigation measures led to shifts in typical annual respiratory virus patterns
Nonpharmaceutical interventions, such as masking, staying home, limiting travel, and social distancing, have been doing more than reducing the risk for COVID-19. They’re also having an impact on infection rates and the timing of seasonal surges of other common respiratory diseases, according to an article published July 23 in Morbidity and Mortality Weekly Report.
Typically, respiratory pathogens such as respiratory syncytial virus (RSV), common cold coronaviruses, parainfluenza viruses, and respiratory adenoviruses increase in the fall and remain high throughout winter, following the same basic patterns as influenza. Although the historically low rates of influenza remained low into spring 2021, that’s not the case for several other common respiratory viruses.
“Clinicians should be aware of increases in some respiratory virus activity and remain vigilant for off-season increases,” wrote Sonja J. Olsen, PhD, and her colleagues at the Centers for Disease Control and Prevention. She told this news organization that clinicians should use multipathogen testing to help guide treatment.
The authors also underscore the importance of fall influenza vaccination campaigns for anyone aged 6 months or older.
Timothy Brewer, MD, MPH, a professor of medicine in the Division of Infectious Diseases at the University of California, Los Angeles (UCLA), and of epidemiology at the UCLA Fielding School of Public Health, agreed that it’s important for health care professionals to consider off-season illnesses in their patients.
“Practitioners should be aware that if they see a sick child in the summer, outside of what normally might be influenza season, but they look like they have influenza, consider potentially influenza and test for it, because it might be possible that we may have disrupted that natural pattern,” Dr. Brewer told this news organization. Dr. Brewer, who was not involved in the CDC research, said it’s also “critically important” to encourage influenza vaccination as the season approaches.
The CDC researchers used the U.S. World Health Organization Collaborating Laboratories System and the CDC’s National Respiratory and Enteric Virus Surveillance System to analyze virologic data from Oct. 3, 2020, to May 22, 2021, for influenza and Jan. 4, 2020, to May 22, 2021, for other respiratory viruses. The authors compared virus circulation during these periods to circulation during the same dates from four previous years.
Data to calculate influenza and RSV hospitalization rates came from the Influenza Hospitalization Surveillance Network and RSV Hospitalization Surveillance Network.
The authors report that flu activity dropped dramatically in March 2020 to its lowest levels since 1997, the earliest season for which data are available. Only 0.2% of more than 1 million specimens tested positive for influenza; the rate of hospitalizations for lab-confirmed flu was 0.8 per 100,000 people. Flu levels remained low through the summer, fall, and on to May 2021.
A potential drawback to this low activity, however, is a more prevalent and severe upcoming flu season, the authors write. The repeated exposure to flu viruses every year often “does not lead to illness, but it does serve to boost our immune response to influenza viruses,” Dr. Olsen said in an interview. “The absence of influenza viruses in the community over the last year means that we are not getting these regular boosts to our immune system. When we finally get exposed, our body may mount a weak response, and this could mean we develop a more clinically severe illness.”
Children are most susceptible to that phenomenon because they haven’t had a lifetime of exposure to flu viruses, Dr. Olsen said.
“An immunologically naive child may be more likely to develop a severe illness than someone who has lived through several influenza seasons,” she said. “This is why it is especially important for everyone 6 months and older to get vaccinated against influenza this season.”
Rhinovirus and enterovirus infections rebounded fairly quickly after their decline in March 2020 and started increasing in May 2020 until they reached “near prepandemic seasonal levels,” the authors write.
RSV infections dropped from 15.3% of weekly positive results in January 2020 to 1.4% by April and then stayed below 1% through the end of 2020. In past years, weekly positive results climbed to 3% in October and peaked at 12.5% to 16.7% in late December. Instead, RSV weekly positive results began increasing in April 2021, rising from 1.1% to 2.8% in May.
The “unusually timed” late spring increase in RSV “is probably associated with various nonpharmaceutical measures that have been in place but are now relaxing,” Dr. Olsen stated.
The RSV hospitalization rate was 0.3 per 100,000 people from October 2020 to April 2021, compared to 27.1 and 33.4 per 100,000 people in the previous 2 years. Of all RSV hospitalizations in the past year, 76.5% occurred in April-May 2021.
Rates of illness caused by the four common human coronaviruses (OC43, NL63, 229E, and HKU1) dropped from 7.5% of weekly positive results in January 2020 to 1.3% in April 2020 and stayed below 1% through February 2021. Then they climbed to 6.6% by May 2021. Infection rates of parainfluenza viruses types 1-4 similarly dropped from 2.6% in January 2020 to 1% in March 2020 and stayed below 1% until April 2021. Since then, rates of the common coronaviruses increased to 6.6% and parainfluenza viruses to 10.9% in May 2021.
Normally, parainfluenza viruses peak in October-November and May-June, so “the current increase could represent a return to prepandemic seasonality,” the authors write.
Human pneumoviruses’ weekly positive results initially increased from 4.2% in January 2020 to 7% in March and then fell to 1.9% the second week of April and remained below 1% through May 2021. In typical years, these viruses peak from 6.2% to 7.7% in March-April. Respiratory adenovirus activity similarly dropped to historically low levels in April 2021 and then began increasing to reach 3% by May 2021, the usual level for that month.
“The different circulation patterns observed across respiratory viruses probably also reflect differences in the virus transmission routes and how effective various nonpharmaceutical measures are at stopping transmission,” Dr. Olsen said in an interview. “As pandemic mitigation measures continue to be adjusted, we expect to see more changes in the circulation of these viruses, including a return to prepandemic circulation, as seen for rhinoviruses and enteroviruses.”
Rhinovirus and enterovirus rates dropped from 14.9% in March 2020 to 3.2% in May – lower than typical – and then climbed to a peak in October 2020. The peak (21.7% weekly positive results) was, however, still lower than the usual median of 32.8%. After dropping to 9.9% in January 2021, it then rose 19.1% in May, potentially reflecting “the usual spring peak that has occurred in previous years,” the authors write.
The authors note that it’s not yet clear how the COVID-19 pandemic and related mitigation measures will continue to affect respiratory virus circulation.
The authors hypothesize that the reasons for a seeming return to seasonal activity of respiratory adenoviruses, rhinoviruses, and enteroviruses could involve “different transmission mechanisms, the role of asymptomatic transmission, and prolonged survival of these nonenveloped viruses on surfaces, all of which might make these viruses less susceptible to nonpharmaceutical interventions.”
Dr. Brewer, of UCLA, agreed.
All the viruses basically “flatline except for adenoviruses and enteroviruses, and they behave a little differently in terms of how they spread,” he said. “Enteroviruses are much more likely to be fecal-oral spread than the other viruses [in the study].”
The delayed circulation of parainfluenza and human coronaviruses may have resulted from suspension of in-person classes through late winter 2020, they write, but that doesn’t explain the relative absence of pneumovirus activity, which usually affects the same young pediatric populations as RSV.
Dr. Brewer said California is seeing a surge of RSV right now, as are many states, especially throughout in the South. He’s not surprised by RSV’s deferred season, because those most affected – children younger than 2 years – are less likely to wear masks now and were “not going to daycare, not being out in public” in 2020. “As people are doing more activities, that’s probably why RSV has been starting to go up since April,” he said.
Despite the fact that, unlike many East Asian cultures, the United States has not traditionally been a mask-wearing culture, Dr. Brewer wouldn’t be surprised if more Americans begin wearing masks during flu season. “Hopefully another thing that will come out of this is better hand hygiene, with people just getting used to washing their hands more, particularly after they come home from being out,” he added.
Dr. Brewer similarly emphasized the importance of flu vaccination for the upcoming season, especially for younger children who may have poorer natural immunity to influenza, owing to its low circulation rates in 2020-2021.
The study was funded by the CDC. Dr. Brewer and Dr. Olsen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonpharmaceutical interventions, such as masking, staying home, limiting travel, and social distancing, have been doing more than reducing the risk for COVID-19. They’re also having an impact on infection rates and the timing of seasonal surges of other common respiratory diseases, according to an article published July 23 in Morbidity and Mortality Weekly Report.
Typically, respiratory pathogens such as respiratory syncytial virus (RSV), common cold coronaviruses, parainfluenza viruses, and respiratory adenoviruses increase in the fall and remain high throughout winter, following the same basic patterns as influenza. Although the historically low rates of influenza remained low into spring 2021, that’s not the case for several other common respiratory viruses.
“Clinicians should be aware of increases in some respiratory virus activity and remain vigilant for off-season increases,” wrote Sonja J. Olsen, PhD, and her colleagues at the Centers for Disease Control and Prevention. She told this news organization that clinicians should use multipathogen testing to help guide treatment.
The authors also underscore the importance of fall influenza vaccination campaigns for anyone aged 6 months or older.
Timothy Brewer, MD, MPH, a professor of medicine in the Division of Infectious Diseases at the University of California, Los Angeles (UCLA), and of epidemiology at the UCLA Fielding School of Public Health, agreed that it’s important for health care professionals to consider off-season illnesses in their patients.
“Practitioners should be aware that if they see a sick child in the summer, outside of what normally might be influenza season, but they look like they have influenza, consider potentially influenza and test for it, because it might be possible that we may have disrupted that natural pattern,” Dr. Brewer told this news organization. Dr. Brewer, who was not involved in the CDC research, said it’s also “critically important” to encourage influenza vaccination as the season approaches.
The CDC researchers used the U.S. World Health Organization Collaborating Laboratories System and the CDC’s National Respiratory and Enteric Virus Surveillance System to analyze virologic data from Oct. 3, 2020, to May 22, 2021, for influenza and Jan. 4, 2020, to May 22, 2021, for other respiratory viruses. The authors compared virus circulation during these periods to circulation during the same dates from four previous years.
Data to calculate influenza and RSV hospitalization rates came from the Influenza Hospitalization Surveillance Network and RSV Hospitalization Surveillance Network.
The authors report that flu activity dropped dramatically in March 2020 to its lowest levels since 1997, the earliest season for which data are available. Only 0.2% of more than 1 million specimens tested positive for influenza; the rate of hospitalizations for lab-confirmed flu was 0.8 per 100,000 people. Flu levels remained low through the summer, fall, and on to May 2021.
A potential drawback to this low activity, however, is a more prevalent and severe upcoming flu season, the authors write. The repeated exposure to flu viruses every year often “does not lead to illness, but it does serve to boost our immune response to influenza viruses,” Dr. Olsen said in an interview. “The absence of influenza viruses in the community over the last year means that we are not getting these regular boosts to our immune system. When we finally get exposed, our body may mount a weak response, and this could mean we develop a more clinically severe illness.”
Children are most susceptible to that phenomenon because they haven’t had a lifetime of exposure to flu viruses, Dr. Olsen said.
“An immunologically naive child may be more likely to develop a severe illness than someone who has lived through several influenza seasons,” she said. “This is why it is especially important for everyone 6 months and older to get vaccinated against influenza this season.”
Rhinovirus and enterovirus infections rebounded fairly quickly after their decline in March 2020 and started increasing in May 2020 until they reached “near prepandemic seasonal levels,” the authors write.
RSV infections dropped from 15.3% of weekly positive results in January 2020 to 1.4% by April and then stayed below 1% through the end of 2020. In past years, weekly positive results climbed to 3% in October and peaked at 12.5% to 16.7% in late December. Instead, RSV weekly positive results began increasing in April 2021, rising from 1.1% to 2.8% in May.
The “unusually timed” late spring increase in RSV “is probably associated with various nonpharmaceutical measures that have been in place but are now relaxing,” Dr. Olsen stated.
The RSV hospitalization rate was 0.3 per 100,000 people from October 2020 to April 2021, compared to 27.1 and 33.4 per 100,000 people in the previous 2 years. Of all RSV hospitalizations in the past year, 76.5% occurred in April-May 2021.
Rates of illness caused by the four common human coronaviruses (OC43, NL63, 229E, and HKU1) dropped from 7.5% of weekly positive results in January 2020 to 1.3% in April 2020 and stayed below 1% through February 2021. Then they climbed to 6.6% by May 2021. Infection rates of parainfluenza viruses types 1-4 similarly dropped from 2.6% in January 2020 to 1% in March 2020 and stayed below 1% until April 2021. Since then, rates of the common coronaviruses increased to 6.6% and parainfluenza viruses to 10.9% in May 2021.
Normally, parainfluenza viruses peak in October-November and May-June, so “the current increase could represent a return to prepandemic seasonality,” the authors write.
Human pneumoviruses’ weekly positive results initially increased from 4.2% in January 2020 to 7% in March and then fell to 1.9% the second week of April and remained below 1% through May 2021. In typical years, these viruses peak from 6.2% to 7.7% in March-April. Respiratory adenovirus activity similarly dropped to historically low levels in April 2021 and then began increasing to reach 3% by May 2021, the usual level for that month.
“The different circulation patterns observed across respiratory viruses probably also reflect differences in the virus transmission routes and how effective various nonpharmaceutical measures are at stopping transmission,” Dr. Olsen said in an interview. “As pandemic mitigation measures continue to be adjusted, we expect to see more changes in the circulation of these viruses, including a return to prepandemic circulation, as seen for rhinoviruses and enteroviruses.”
Rhinovirus and enterovirus rates dropped from 14.9% in March 2020 to 3.2% in May – lower than typical – and then climbed to a peak in October 2020. The peak (21.7% weekly positive results) was, however, still lower than the usual median of 32.8%. After dropping to 9.9% in January 2021, it then rose 19.1% in May, potentially reflecting “the usual spring peak that has occurred in previous years,” the authors write.
The authors note that it’s not yet clear how the COVID-19 pandemic and related mitigation measures will continue to affect respiratory virus circulation.
The authors hypothesize that the reasons for a seeming return to seasonal activity of respiratory adenoviruses, rhinoviruses, and enteroviruses could involve “different transmission mechanisms, the role of asymptomatic transmission, and prolonged survival of these nonenveloped viruses on surfaces, all of which might make these viruses less susceptible to nonpharmaceutical interventions.”
Dr. Brewer, of UCLA, agreed.
All the viruses basically “flatline except for adenoviruses and enteroviruses, and they behave a little differently in terms of how they spread,” he said. “Enteroviruses are much more likely to be fecal-oral spread than the other viruses [in the study].”
The delayed circulation of parainfluenza and human coronaviruses may have resulted from suspension of in-person classes through late winter 2020, they write, but that doesn’t explain the relative absence of pneumovirus activity, which usually affects the same young pediatric populations as RSV.
Dr. Brewer said California is seeing a surge of RSV right now, as are many states, especially throughout in the South. He’s not surprised by RSV’s deferred season, because those most affected – children younger than 2 years – are less likely to wear masks now and were “not going to daycare, not being out in public” in 2020. “As people are doing more activities, that’s probably why RSV has been starting to go up since April,” he said.
Despite the fact that, unlike many East Asian cultures, the United States has not traditionally been a mask-wearing culture, Dr. Brewer wouldn’t be surprised if more Americans begin wearing masks during flu season. “Hopefully another thing that will come out of this is better hand hygiene, with people just getting used to washing their hands more, particularly after they come home from being out,” he added.
Dr. Brewer similarly emphasized the importance of flu vaccination for the upcoming season, especially for younger children who may have poorer natural immunity to influenza, owing to its low circulation rates in 2020-2021.
The study was funded by the CDC. Dr. Brewer and Dr. Olsen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonpharmaceutical interventions, such as masking, staying home, limiting travel, and social distancing, have been doing more than reducing the risk for COVID-19. They’re also having an impact on infection rates and the timing of seasonal surges of other common respiratory diseases, according to an article published July 23 in Morbidity and Mortality Weekly Report.
Typically, respiratory pathogens such as respiratory syncytial virus (RSV), common cold coronaviruses, parainfluenza viruses, and respiratory adenoviruses increase in the fall and remain high throughout winter, following the same basic patterns as influenza. Although the historically low rates of influenza remained low into spring 2021, that’s not the case for several other common respiratory viruses.
“Clinicians should be aware of increases in some respiratory virus activity and remain vigilant for off-season increases,” wrote Sonja J. Olsen, PhD, and her colleagues at the Centers for Disease Control and Prevention. She told this news organization that clinicians should use multipathogen testing to help guide treatment.
The authors also underscore the importance of fall influenza vaccination campaigns for anyone aged 6 months or older.
Timothy Brewer, MD, MPH, a professor of medicine in the Division of Infectious Diseases at the University of California, Los Angeles (UCLA), and of epidemiology at the UCLA Fielding School of Public Health, agreed that it’s important for health care professionals to consider off-season illnesses in their patients.
“Practitioners should be aware that if they see a sick child in the summer, outside of what normally might be influenza season, but they look like they have influenza, consider potentially influenza and test for it, because it might be possible that we may have disrupted that natural pattern,” Dr. Brewer told this news organization. Dr. Brewer, who was not involved in the CDC research, said it’s also “critically important” to encourage influenza vaccination as the season approaches.
The CDC researchers used the U.S. World Health Organization Collaborating Laboratories System and the CDC’s National Respiratory and Enteric Virus Surveillance System to analyze virologic data from Oct. 3, 2020, to May 22, 2021, for influenza and Jan. 4, 2020, to May 22, 2021, for other respiratory viruses. The authors compared virus circulation during these periods to circulation during the same dates from four previous years.
Data to calculate influenza and RSV hospitalization rates came from the Influenza Hospitalization Surveillance Network and RSV Hospitalization Surveillance Network.
The authors report that flu activity dropped dramatically in March 2020 to its lowest levels since 1997, the earliest season for which data are available. Only 0.2% of more than 1 million specimens tested positive for influenza; the rate of hospitalizations for lab-confirmed flu was 0.8 per 100,000 people. Flu levels remained low through the summer, fall, and on to May 2021.
A potential drawback to this low activity, however, is a more prevalent and severe upcoming flu season, the authors write. The repeated exposure to flu viruses every year often “does not lead to illness, but it does serve to boost our immune response to influenza viruses,” Dr. Olsen said in an interview. “The absence of influenza viruses in the community over the last year means that we are not getting these regular boosts to our immune system. When we finally get exposed, our body may mount a weak response, and this could mean we develop a more clinically severe illness.”
Children are most susceptible to that phenomenon because they haven’t had a lifetime of exposure to flu viruses, Dr. Olsen said.
“An immunologically naive child may be more likely to develop a severe illness than someone who has lived through several influenza seasons,” she said. “This is why it is especially important for everyone 6 months and older to get vaccinated against influenza this season.”
Rhinovirus and enterovirus infections rebounded fairly quickly after their decline in March 2020 and started increasing in May 2020 until they reached “near prepandemic seasonal levels,” the authors write.
RSV infections dropped from 15.3% of weekly positive results in January 2020 to 1.4% by April and then stayed below 1% through the end of 2020. In past years, weekly positive results climbed to 3% in October and peaked at 12.5% to 16.7% in late December. Instead, RSV weekly positive results began increasing in April 2021, rising from 1.1% to 2.8% in May.
The “unusually timed” late spring increase in RSV “is probably associated with various nonpharmaceutical measures that have been in place but are now relaxing,” Dr. Olsen stated.
The RSV hospitalization rate was 0.3 per 100,000 people from October 2020 to April 2021, compared to 27.1 and 33.4 per 100,000 people in the previous 2 years. Of all RSV hospitalizations in the past year, 76.5% occurred in April-May 2021.
Rates of illness caused by the four common human coronaviruses (OC43, NL63, 229E, and HKU1) dropped from 7.5% of weekly positive results in January 2020 to 1.3% in April 2020 and stayed below 1% through February 2021. Then they climbed to 6.6% by May 2021. Infection rates of parainfluenza viruses types 1-4 similarly dropped from 2.6% in January 2020 to 1% in March 2020 and stayed below 1% until April 2021. Since then, rates of the common coronaviruses increased to 6.6% and parainfluenza viruses to 10.9% in May 2021.
Normally, parainfluenza viruses peak in October-November and May-June, so “the current increase could represent a return to prepandemic seasonality,” the authors write.
Human pneumoviruses’ weekly positive results initially increased from 4.2% in January 2020 to 7% in March and then fell to 1.9% the second week of April and remained below 1% through May 2021. In typical years, these viruses peak from 6.2% to 7.7% in March-April. Respiratory adenovirus activity similarly dropped to historically low levels in April 2021 and then began increasing to reach 3% by May 2021, the usual level for that month.
“The different circulation patterns observed across respiratory viruses probably also reflect differences in the virus transmission routes and how effective various nonpharmaceutical measures are at stopping transmission,” Dr. Olsen said in an interview. “As pandemic mitigation measures continue to be adjusted, we expect to see more changes in the circulation of these viruses, including a return to prepandemic circulation, as seen for rhinoviruses and enteroviruses.”
Rhinovirus and enterovirus rates dropped from 14.9% in March 2020 to 3.2% in May – lower than typical – and then climbed to a peak in October 2020. The peak (21.7% weekly positive results) was, however, still lower than the usual median of 32.8%. After dropping to 9.9% in January 2021, it then rose 19.1% in May, potentially reflecting “the usual spring peak that has occurred in previous years,” the authors write.
The authors note that it’s not yet clear how the COVID-19 pandemic and related mitigation measures will continue to affect respiratory virus circulation.
The authors hypothesize that the reasons for a seeming return to seasonal activity of respiratory adenoviruses, rhinoviruses, and enteroviruses could involve “different transmission mechanisms, the role of asymptomatic transmission, and prolonged survival of these nonenveloped viruses on surfaces, all of which might make these viruses less susceptible to nonpharmaceutical interventions.”
Dr. Brewer, of UCLA, agreed.
All the viruses basically “flatline except for adenoviruses and enteroviruses, and they behave a little differently in terms of how they spread,” he said. “Enteroviruses are much more likely to be fecal-oral spread than the other viruses [in the study].”
The delayed circulation of parainfluenza and human coronaviruses may have resulted from suspension of in-person classes through late winter 2020, they write, but that doesn’t explain the relative absence of pneumovirus activity, which usually affects the same young pediatric populations as RSV.
Dr. Brewer said California is seeing a surge of RSV right now, as are many states, especially throughout in the South. He’s not surprised by RSV’s deferred season, because those most affected – children younger than 2 years – are less likely to wear masks now and were “not going to daycare, not being out in public” in 2020. “As people are doing more activities, that’s probably why RSV has been starting to go up since April,” he said.
Despite the fact that, unlike many East Asian cultures, the United States has not traditionally been a mask-wearing culture, Dr. Brewer wouldn’t be surprised if more Americans begin wearing masks during flu season. “Hopefully another thing that will come out of this is better hand hygiene, with people just getting used to washing their hands more, particularly after they come home from being out,” he added.
Dr. Brewer similarly emphasized the importance of flu vaccination for the upcoming season, especially for younger children who may have poorer natural immunity to influenza, owing to its low circulation rates in 2020-2021.
The study was funded by the CDC. Dr. Brewer and Dr. Olsen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Achieving a ‘new sexual-health paradigm’ means expanding STI care
A vital aspect of expanding access and care for sexually transmitted infections (STIs) in the United States is broadening responsibility for this care across the health care system and other community resources, according to an article published online July 6 in Clinical Infectious Diseases. This expansion and decentralization of care are central to adopting the “new sexual health paradigm” recommended by a National Academies report that was published in March.
“STIs represent a sizable, longstanding, and growing public health challenge,” write Vincent Guilamo-Ramos, PhD, MPH, dean and professor at the Duke University School of Nursing and director of the Center for Latino Adolescent and Family Health (CLAFH) at Duke University, both in Durham, N.C., and his colleagues. Yet the limitations on the current STI workforce and limited federal funding and support for STI prevention and care mean it will take clinicians of all types from across the health care spectrum to meet the challenge, they explain.
“For too long, STI prevention and treatment has been perceived as the sole responsibility of a narrow workforce of specialized STI and HIV service providers,” Dr. Guilamo-Ramos and his coauthor, Marco Thimm-Kaiser, MPH, associate in research at Duke University and epidemiologist at CLAFH, wrote in an email.
“However, the resources allocated to this STI specialty workforce have diminished over time, along with decreasing investments in the broader U.S. public health infrastructure,” they continued. “At the same time – and in part due to this underinvestment – STI rates have soared, reaching a record high for the sixth year in a row in 2019.”
Those factors led to the National Academies report, which recommends moving “away from the traditional, disease-focused perspective on STIs in favor of a holistic perspective of sexual health as an integral component of overall health and well-being,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote to this news organization.
In their article, the authors review the limitations in the STI workforce, the implications of those limitations for the broader health care industry, and what it will take for STI and HIV specialists as well as regulators to ensure it’s possible to achieve the paradigm shift recommended by the National Academies.
Currently, the biggest limitation is access to care, said Laura Mercer, MD, MBA, of the department of obstetrics and gynecology and the ob.gyn. clerkship director at the University of Arizona, Phoenix. Dr. Mercer, who was not involved with the National Academies report or the analysis of it, said in an interview that it’s essential to emphasize “sexual health as a core element of routine primary and preventative care” to ensure it becomes more accessible to patients without the need to seek out specialty care.
Dr. Guilamo-Ramos and his colleagues drive home the importance of such a shift by noting that more than 200 million Americans live in counties with no practicing infectious disease physicians. The disparities are greatest in Southern states, which account for 40% of all reported STIs. The workforce shortage has continued to worsen alongside the deterioration of the clinical infrastructure supporting STI specialty services, the authors write.
Hence the need to expand accountability for care not only to primary-care physicians but also to nurses, pharmacists, physician assistants, nurse practitioners, and behavioral health practitioners. Doing so also requires normalizing sexual health services across health care professions.
“Prevention is a crucial first step” to this, Dr. Mercer said. “This is particularly important as we recall that almost half of new sexually transmitted infections occur in teenagers. Destigmatizing sexual health and sexual health education will also help encourage patients of all ages to request and accept testing.”
Further, with primary care practitioners managing most STI testing and treatment, subspecialists can focus primarily on complex or refractory cases, she added. Ways to help broaden care include developing point-of-care testing for STIs and improving the accuracy of existing testing, she said.
“The goal is to make routine sexual health services accessible in a wide range of settings, such as in primary care, at pharmacies, and in community-based settings, and to draw on a broader workforce for delivery of sexual health services,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview.
Kevin Ault, MD, professor of obstetrics and gynecology and director of clinical and translational research at the University of Kansas Medical Center in Kansas City, said that many medical organizations, such as the American College of Obstetricians and Gynecologists, have long advocated incorporating sexual health into routine preventive care. He also noted that pharmacists have already become proactive in preventing STIs and could continue to do so.
“Vaccines for hepatitis and human papillomavirus are commonly available at pharmacies,” Dr. Ault said. He was not involved in the article by Dr. Guilamo-Ramos and colleagues or the original report. “Pharmacists could also fill a gap by administering injectable medications such as penicillin. States would have to approve changes in policy, but many states have already done this for expedited partner therapy.”
Dr. Guilamo-Ramos and Mr. Thimm-Kaiser noted similar barriers that must be removed to broaden delivery of STI services.
“Unfortunately, too many highly trained health care providers who are well-positioned for the delivery of sexual health services face regulatory or administrative barriers to practice to the full scope of their training,” they wrote. “These barriers can have a particularly negative impact in medically underserved communities, where physician shortages are common and where novel, decentralized health care service delivery models that draw on nonphysician providers may hold the greatest promise.”
As more diverse health care practitioners take on these roles, ID and HIV specialists can provide their expertise in developing training and technical assistance to support generalists, Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They can also aid in aligning “clinical training curricula, licensing criteria, and practice guidelines with routine delivery of sexual health services.”
Dr. Guilamo-Ramos and his coauthors offer specific recommendations for professional training, licensing, and practice guidelines to help overcome the “insufficient knowledge, inadequate training, and absence of explicit protocols” that currently impede delivery of STI services in general practice settings.
Although the paradigm shift recommended by the National Academies is ambitious, it’s also necessary, and “none of the recommendations are out of reach,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview. They pointed out how the COVID-19 pandemic has highlighted how underresourced the health care workforce and infrastructure are and how great health care disparities are.
“There is momentum toward rebuilding the nation’s health and public health system in a more effective and efficient way,” they said, and many of the STI report’s recommendations “overlap with priorities for the broader health and public health system moving forward.”
Dr. Mercer also believes the recommendations are realistic, “but only the beginning,” she told this news organization. “Comprehensive sexual education to expand knowledge about STI prevention and public health campaigns to help destigmatize sexual health care in general will remain crucial,” she said.
Sexual education, expanded access, and destigmatizing sexual care are particularly important for reaching the populations most in need of care, such as adolescents and young adults, as well as ethnic, racial, sexual, and gender-minority youth.
“It cannot be overstated how important of a priority population adolescents and young adults are,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They noted that those aged 15-24 account for half of all STIs each year but represent only a quarter of the sexually active population. “Targeted efforts for STI prevention and treatment among adolescents and young adults are therefore essential for an overall successful strategy to address STIs and sexual health in the United States.”
The National Academies report was supported by the Centers for Disease Control and Prevention and the National Association of County and City Health Officials. Dr. Mercer, Dr. Ault, and Mr. Thimm-Kaiser have disclosed no relevant financial relationships. Dr. Guilamo-Ramos has received grants and personal fees from ViiV Health care.
A version of this article first appeared on Medscape.com.
A vital aspect of expanding access and care for sexually transmitted infections (STIs) in the United States is broadening responsibility for this care across the health care system and other community resources, according to an article published online July 6 in Clinical Infectious Diseases. This expansion and decentralization of care are central to adopting the “new sexual health paradigm” recommended by a National Academies report that was published in March.
“STIs represent a sizable, longstanding, and growing public health challenge,” write Vincent Guilamo-Ramos, PhD, MPH, dean and professor at the Duke University School of Nursing and director of the Center for Latino Adolescent and Family Health (CLAFH) at Duke University, both in Durham, N.C., and his colleagues. Yet the limitations on the current STI workforce and limited federal funding and support for STI prevention and care mean it will take clinicians of all types from across the health care spectrum to meet the challenge, they explain.
“For too long, STI prevention and treatment has been perceived as the sole responsibility of a narrow workforce of specialized STI and HIV service providers,” Dr. Guilamo-Ramos and his coauthor, Marco Thimm-Kaiser, MPH, associate in research at Duke University and epidemiologist at CLAFH, wrote in an email.
“However, the resources allocated to this STI specialty workforce have diminished over time, along with decreasing investments in the broader U.S. public health infrastructure,” they continued. “At the same time – and in part due to this underinvestment – STI rates have soared, reaching a record high for the sixth year in a row in 2019.”
Those factors led to the National Academies report, which recommends moving “away from the traditional, disease-focused perspective on STIs in favor of a holistic perspective of sexual health as an integral component of overall health and well-being,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote to this news organization.
In their article, the authors review the limitations in the STI workforce, the implications of those limitations for the broader health care industry, and what it will take for STI and HIV specialists as well as regulators to ensure it’s possible to achieve the paradigm shift recommended by the National Academies.
Currently, the biggest limitation is access to care, said Laura Mercer, MD, MBA, of the department of obstetrics and gynecology and the ob.gyn. clerkship director at the University of Arizona, Phoenix. Dr. Mercer, who was not involved with the National Academies report or the analysis of it, said in an interview that it’s essential to emphasize “sexual health as a core element of routine primary and preventative care” to ensure it becomes more accessible to patients without the need to seek out specialty care.
Dr. Guilamo-Ramos and his colleagues drive home the importance of such a shift by noting that more than 200 million Americans live in counties with no practicing infectious disease physicians. The disparities are greatest in Southern states, which account for 40% of all reported STIs. The workforce shortage has continued to worsen alongside the deterioration of the clinical infrastructure supporting STI specialty services, the authors write.
Hence the need to expand accountability for care not only to primary-care physicians but also to nurses, pharmacists, physician assistants, nurse practitioners, and behavioral health practitioners. Doing so also requires normalizing sexual health services across health care professions.
“Prevention is a crucial first step” to this, Dr. Mercer said. “This is particularly important as we recall that almost half of new sexually transmitted infections occur in teenagers. Destigmatizing sexual health and sexual health education will also help encourage patients of all ages to request and accept testing.”
Further, with primary care practitioners managing most STI testing and treatment, subspecialists can focus primarily on complex or refractory cases, she added. Ways to help broaden care include developing point-of-care testing for STIs and improving the accuracy of existing testing, she said.
“The goal is to make routine sexual health services accessible in a wide range of settings, such as in primary care, at pharmacies, and in community-based settings, and to draw on a broader workforce for delivery of sexual health services,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview.
Kevin Ault, MD, professor of obstetrics and gynecology and director of clinical and translational research at the University of Kansas Medical Center in Kansas City, said that many medical organizations, such as the American College of Obstetricians and Gynecologists, have long advocated incorporating sexual health into routine preventive care. He also noted that pharmacists have already become proactive in preventing STIs and could continue to do so.
“Vaccines for hepatitis and human papillomavirus are commonly available at pharmacies,” Dr. Ault said. He was not involved in the article by Dr. Guilamo-Ramos and colleagues or the original report. “Pharmacists could also fill a gap by administering injectable medications such as penicillin. States would have to approve changes in policy, but many states have already done this for expedited partner therapy.”
Dr. Guilamo-Ramos and Mr. Thimm-Kaiser noted similar barriers that must be removed to broaden delivery of STI services.
“Unfortunately, too many highly trained health care providers who are well-positioned for the delivery of sexual health services face regulatory or administrative barriers to practice to the full scope of their training,” they wrote. “These barriers can have a particularly negative impact in medically underserved communities, where physician shortages are common and where novel, decentralized health care service delivery models that draw on nonphysician providers may hold the greatest promise.”
As more diverse health care practitioners take on these roles, ID and HIV specialists can provide their expertise in developing training and technical assistance to support generalists, Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They can also aid in aligning “clinical training curricula, licensing criteria, and practice guidelines with routine delivery of sexual health services.”
Dr. Guilamo-Ramos and his coauthors offer specific recommendations for professional training, licensing, and practice guidelines to help overcome the “insufficient knowledge, inadequate training, and absence of explicit protocols” that currently impede delivery of STI services in general practice settings.
Although the paradigm shift recommended by the National Academies is ambitious, it’s also necessary, and “none of the recommendations are out of reach,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview. They pointed out how the COVID-19 pandemic has highlighted how underresourced the health care workforce and infrastructure are and how great health care disparities are.
“There is momentum toward rebuilding the nation’s health and public health system in a more effective and efficient way,” they said, and many of the STI report’s recommendations “overlap with priorities for the broader health and public health system moving forward.”
Dr. Mercer also believes the recommendations are realistic, “but only the beginning,” she told this news organization. “Comprehensive sexual education to expand knowledge about STI prevention and public health campaigns to help destigmatize sexual health care in general will remain crucial,” she said.
Sexual education, expanded access, and destigmatizing sexual care are particularly important for reaching the populations most in need of care, such as adolescents and young adults, as well as ethnic, racial, sexual, and gender-minority youth.
“It cannot be overstated how important of a priority population adolescents and young adults are,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They noted that those aged 15-24 account for half of all STIs each year but represent only a quarter of the sexually active population. “Targeted efforts for STI prevention and treatment among adolescents and young adults are therefore essential for an overall successful strategy to address STIs and sexual health in the United States.”
The National Academies report was supported by the Centers for Disease Control and Prevention and the National Association of County and City Health Officials. Dr. Mercer, Dr. Ault, and Mr. Thimm-Kaiser have disclosed no relevant financial relationships. Dr. Guilamo-Ramos has received grants and personal fees from ViiV Health care.
A version of this article first appeared on Medscape.com.
A vital aspect of expanding access and care for sexually transmitted infections (STIs) in the United States is broadening responsibility for this care across the health care system and other community resources, according to an article published online July 6 in Clinical Infectious Diseases. This expansion and decentralization of care are central to adopting the “new sexual health paradigm” recommended by a National Academies report that was published in March.
“STIs represent a sizable, longstanding, and growing public health challenge,” write Vincent Guilamo-Ramos, PhD, MPH, dean and professor at the Duke University School of Nursing and director of the Center for Latino Adolescent and Family Health (CLAFH) at Duke University, both in Durham, N.C., and his colleagues. Yet the limitations on the current STI workforce and limited federal funding and support for STI prevention and care mean it will take clinicians of all types from across the health care spectrum to meet the challenge, they explain.
“For too long, STI prevention and treatment has been perceived as the sole responsibility of a narrow workforce of specialized STI and HIV service providers,” Dr. Guilamo-Ramos and his coauthor, Marco Thimm-Kaiser, MPH, associate in research at Duke University and epidemiologist at CLAFH, wrote in an email.
“However, the resources allocated to this STI specialty workforce have diminished over time, along with decreasing investments in the broader U.S. public health infrastructure,” they continued. “At the same time – and in part due to this underinvestment – STI rates have soared, reaching a record high for the sixth year in a row in 2019.”
Those factors led to the National Academies report, which recommends moving “away from the traditional, disease-focused perspective on STIs in favor of a holistic perspective of sexual health as an integral component of overall health and well-being,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote to this news organization.
In their article, the authors review the limitations in the STI workforce, the implications of those limitations for the broader health care industry, and what it will take for STI and HIV specialists as well as regulators to ensure it’s possible to achieve the paradigm shift recommended by the National Academies.
Currently, the biggest limitation is access to care, said Laura Mercer, MD, MBA, of the department of obstetrics and gynecology and the ob.gyn. clerkship director at the University of Arizona, Phoenix. Dr. Mercer, who was not involved with the National Academies report or the analysis of it, said in an interview that it’s essential to emphasize “sexual health as a core element of routine primary and preventative care” to ensure it becomes more accessible to patients without the need to seek out specialty care.
Dr. Guilamo-Ramos and his colleagues drive home the importance of such a shift by noting that more than 200 million Americans live in counties with no practicing infectious disease physicians. The disparities are greatest in Southern states, which account for 40% of all reported STIs. The workforce shortage has continued to worsen alongside the deterioration of the clinical infrastructure supporting STI specialty services, the authors write.
Hence the need to expand accountability for care not only to primary-care physicians but also to nurses, pharmacists, physician assistants, nurse practitioners, and behavioral health practitioners. Doing so also requires normalizing sexual health services across health care professions.
“Prevention is a crucial first step” to this, Dr. Mercer said. “This is particularly important as we recall that almost half of new sexually transmitted infections occur in teenagers. Destigmatizing sexual health and sexual health education will also help encourage patients of all ages to request and accept testing.”
Further, with primary care practitioners managing most STI testing and treatment, subspecialists can focus primarily on complex or refractory cases, she added. Ways to help broaden care include developing point-of-care testing for STIs and improving the accuracy of existing testing, she said.
“The goal is to make routine sexual health services accessible in a wide range of settings, such as in primary care, at pharmacies, and in community-based settings, and to draw on a broader workforce for delivery of sexual health services,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview.
Kevin Ault, MD, professor of obstetrics and gynecology and director of clinical and translational research at the University of Kansas Medical Center in Kansas City, said that many medical organizations, such as the American College of Obstetricians and Gynecologists, have long advocated incorporating sexual health into routine preventive care. He also noted that pharmacists have already become proactive in preventing STIs and could continue to do so.
“Vaccines for hepatitis and human papillomavirus are commonly available at pharmacies,” Dr. Ault said. He was not involved in the article by Dr. Guilamo-Ramos and colleagues or the original report. “Pharmacists could also fill a gap by administering injectable medications such as penicillin. States would have to approve changes in policy, but many states have already done this for expedited partner therapy.”
Dr. Guilamo-Ramos and Mr. Thimm-Kaiser noted similar barriers that must be removed to broaden delivery of STI services.
“Unfortunately, too many highly trained health care providers who are well-positioned for the delivery of sexual health services face regulatory or administrative barriers to practice to the full scope of their training,” they wrote. “These barriers can have a particularly negative impact in medically underserved communities, where physician shortages are common and where novel, decentralized health care service delivery models that draw on nonphysician providers may hold the greatest promise.”
As more diverse health care practitioners take on these roles, ID and HIV specialists can provide their expertise in developing training and technical assistance to support generalists, Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They can also aid in aligning “clinical training curricula, licensing criteria, and practice guidelines with routine delivery of sexual health services.”
Dr. Guilamo-Ramos and his coauthors offer specific recommendations for professional training, licensing, and practice guidelines to help overcome the “insufficient knowledge, inadequate training, and absence of explicit protocols” that currently impede delivery of STI services in general practice settings.
Although the paradigm shift recommended by the National Academies is ambitious, it’s also necessary, and “none of the recommendations are out of reach,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview. They pointed out how the COVID-19 pandemic has highlighted how underresourced the health care workforce and infrastructure are and how great health care disparities are.
“There is momentum toward rebuilding the nation’s health and public health system in a more effective and efficient way,” they said, and many of the STI report’s recommendations “overlap with priorities for the broader health and public health system moving forward.”
Dr. Mercer also believes the recommendations are realistic, “but only the beginning,” she told this news organization. “Comprehensive sexual education to expand knowledge about STI prevention and public health campaigns to help destigmatize sexual health care in general will remain crucial,” she said.
Sexual education, expanded access, and destigmatizing sexual care are particularly important for reaching the populations most in need of care, such as adolescents and young adults, as well as ethnic, racial, sexual, and gender-minority youth.
“It cannot be overstated how important of a priority population adolescents and young adults are,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They noted that those aged 15-24 account for half of all STIs each year but represent only a quarter of the sexually active population. “Targeted efforts for STI prevention and treatment among adolescents and young adults are therefore essential for an overall successful strategy to address STIs and sexual health in the United States.”
The National Academies report was supported by the Centers for Disease Control and Prevention and the National Association of County and City Health Officials. Dr. Mercer, Dr. Ault, and Mr. Thimm-Kaiser have disclosed no relevant financial relationships. Dr. Guilamo-Ramos has received grants and personal fees from ViiV Health care.
A version of this article first appeared on Medscape.com.
C. diff eradication not necessary for clinical cure of recurrent infections with fecal transplant
It’s not necessary to completely eradicate all Clostridioides difficile to successfully treat recurrent C. difficile infections with fecal microbiota transplant (FMT), according to a study presented online July 12 at the European Congress of Clinical Microbiology & Infectious Diseases.
C. difficile colonization persisted for 3 weeks after FMT in about one-quarter of patients, but it’s not clear whether this is a persistent infection, a newly acquired infection, or partial persistence of a mixed infection, said Elisabeth Terveer, MD, a medical microbiologist at Leiden (the Netherlands) University Medical Center. In addition, “82% of patients with detectable C. diff do not relapse, so it’s absolutely not necessary for a cure,” she said.
Several mechanisms explain why FMT is a highly effective therapy for recurrent C. difficile infections, including restoration of bacterial metabolism in the gut, immune modulation, and direct competition between bacteria, Dr. Terveer said, but it’s less clear whether eradication of C. difficile spores is among these mechanisms.
Between May 2016 and April 2020, the researchers analyzed fecal samples from 84 patients who took vancomycin for at least 4 days before undergoing FMT. The researchers took fecal samples from patients before FMT and 3 weeks after FMT to culture them and the donor samples for presence of C. difficile, and they assessed clinical outcomes at 3 weeks and 6 months after FMT.
After antibiotic treatment but prior to FMT, 19% of patients (n = 16) still had a toxigenic C. difficile culture while the other 81% had a negative culture. None of the donor samples had a positive C. difficile culture. After FMT treatment, five patients who had a positive pre-FMT culture remained positive, and the other 11 were negative. Among the 81% of patients (n = 68) who had a negative culture just before FMT, 22 had a positive culture and 46 had a negative culture after FMT. Overall, 26% of patients post FMT had a positive C. difficile culture, a finding that was 10-fold higher than another study that assessed C. difficile with PCR testing, Dr. Terveer said.
The clinical cure rate after FMT was 94%, and five patients had relapses within 2 months of their FMT. These relapses were more prevalent in patients with a positive C. difficile culture prior to FMT (odds ratio [OR], 7.6; P = .045) and a positive C. difficile culture after FMT (OR, 13.6; P = .016). Still, 82% of patients who had a positive C. difficile culture post FMT remained clinically cured 2 months later.
It’s unclear why 19% of patients had a positive culture after their antibiotic pretreatment prior to FMT, Dr. Terveer said, but it may be because the pretreatment was of such a short duration.
“I think the advice should be: Give a full anti–C. diff antibiotic course to treat the C. diff infection, and then give FMT afterward to restore the microbiota and prevent further relapses,” Dr. Terveer told attendees.
Dimitri Drekonja, MD, chief of the Minneapolis VA Infectious Disease Section, said the findings were not necessarily surprising, but it would have been interesting for the researchers to have conducted DNA sequencing of the patients’ fecal samples post FMT to see what the biological diversity looked like.
“One school of thought has been that you have to repopulate the normal diverse microbiota of the colon” with FMT, and the other “is that you need to get rid of the C. diff that›s there,” Dr. Drekonja, who was not involved in the study, said in an interview. “I think more people think it’s the diverse microbiota because if it’s just getting rid of C. diff, we can get do that with antibiotics – but that gets rid of the other organisms.”
As long as you have a diverse microbiota post FMT, Dr. Drekonja said, then “having a few residual organisms, even if they get magnified in the culture process, is probably not that big a deal.”
But there’s a third school of thought that Dr. Drekonja said he himself falls into: “I don’t really care how it works, just that in well-done trials, it does work.” As long as large, robust, well-blinded trials show that FMT works, “I’m open to all sorts of ideas of what the mechanism is,” he said. “The main thing is that it does or doesn’t work.”
These findings basically reinforce current guidance not to test patients’ stools if they are asymptomatic, Dr. Drekonja said. In the past, clinicians sometimes tested patients’ stool after therapy to ensure the C. difficile was eradicated, regardless of whether the patient had symptoms of infection, he said.
“We’ve since become much more attuned that there are lots of people who have detectable C. diff in their stool without any symptoms,” whether detectable by culture or PCR, Dr. Drekonja said. “Generally, if you’re doing well and you’re not having diarrhea, don’t test, and if someone does test and finds it, pretend you didn’t see the test,” he advised. “This is a big part of diagnostic stewardship, which is: You don’t go testing people who are doing well.”
The Netherlands Donor Feces Bank used in the research is funded by a grant from Vedanta Biosciences. Dr. Drekonja had no disclosures.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
It’s not necessary to completely eradicate all Clostridioides difficile to successfully treat recurrent C. difficile infections with fecal microbiota transplant (FMT), according to a study presented online July 12 at the European Congress of Clinical Microbiology & Infectious Diseases.
C. difficile colonization persisted for 3 weeks after FMT in about one-quarter of patients, but it’s not clear whether this is a persistent infection, a newly acquired infection, or partial persistence of a mixed infection, said Elisabeth Terveer, MD, a medical microbiologist at Leiden (the Netherlands) University Medical Center. In addition, “82% of patients with detectable C. diff do not relapse, so it’s absolutely not necessary for a cure,” she said.
Several mechanisms explain why FMT is a highly effective therapy for recurrent C. difficile infections, including restoration of bacterial metabolism in the gut, immune modulation, and direct competition between bacteria, Dr. Terveer said, but it’s less clear whether eradication of C. difficile spores is among these mechanisms.
Between May 2016 and April 2020, the researchers analyzed fecal samples from 84 patients who took vancomycin for at least 4 days before undergoing FMT. The researchers took fecal samples from patients before FMT and 3 weeks after FMT to culture them and the donor samples for presence of C. difficile, and they assessed clinical outcomes at 3 weeks and 6 months after FMT.
After antibiotic treatment but prior to FMT, 19% of patients (n = 16) still had a toxigenic C. difficile culture while the other 81% had a negative culture. None of the donor samples had a positive C. difficile culture. After FMT treatment, five patients who had a positive pre-FMT culture remained positive, and the other 11 were negative. Among the 81% of patients (n = 68) who had a negative culture just before FMT, 22 had a positive culture and 46 had a negative culture after FMT. Overall, 26% of patients post FMT had a positive C. difficile culture, a finding that was 10-fold higher than another study that assessed C. difficile with PCR testing, Dr. Terveer said.
The clinical cure rate after FMT was 94%, and five patients had relapses within 2 months of their FMT. These relapses were more prevalent in patients with a positive C. difficile culture prior to FMT (odds ratio [OR], 7.6; P = .045) and a positive C. difficile culture after FMT (OR, 13.6; P = .016). Still, 82% of patients who had a positive C. difficile culture post FMT remained clinically cured 2 months later.
It’s unclear why 19% of patients had a positive culture after their antibiotic pretreatment prior to FMT, Dr. Terveer said, but it may be because the pretreatment was of such a short duration.
“I think the advice should be: Give a full anti–C. diff antibiotic course to treat the C. diff infection, and then give FMT afterward to restore the microbiota and prevent further relapses,” Dr. Terveer told attendees.
Dimitri Drekonja, MD, chief of the Minneapolis VA Infectious Disease Section, said the findings were not necessarily surprising, but it would have been interesting for the researchers to have conducted DNA sequencing of the patients’ fecal samples post FMT to see what the biological diversity looked like.
“One school of thought has been that you have to repopulate the normal diverse microbiota of the colon” with FMT, and the other “is that you need to get rid of the C. diff that›s there,” Dr. Drekonja, who was not involved in the study, said in an interview. “I think more people think it’s the diverse microbiota because if it’s just getting rid of C. diff, we can get do that with antibiotics – but that gets rid of the other organisms.”
As long as you have a diverse microbiota post FMT, Dr. Drekonja said, then “having a few residual organisms, even if they get magnified in the culture process, is probably not that big a deal.”
But there’s a third school of thought that Dr. Drekonja said he himself falls into: “I don’t really care how it works, just that in well-done trials, it does work.” As long as large, robust, well-blinded trials show that FMT works, “I’m open to all sorts of ideas of what the mechanism is,” he said. “The main thing is that it does or doesn’t work.”
These findings basically reinforce current guidance not to test patients’ stools if they are asymptomatic, Dr. Drekonja said. In the past, clinicians sometimes tested patients’ stool after therapy to ensure the C. difficile was eradicated, regardless of whether the patient had symptoms of infection, he said.
“We’ve since become much more attuned that there are lots of people who have detectable C. diff in their stool without any symptoms,” whether detectable by culture or PCR, Dr. Drekonja said. “Generally, if you’re doing well and you’re not having diarrhea, don’t test, and if someone does test and finds it, pretend you didn’t see the test,” he advised. “This is a big part of diagnostic stewardship, which is: You don’t go testing people who are doing well.”
The Netherlands Donor Feces Bank used in the research is funded by a grant from Vedanta Biosciences. Dr. Drekonja had no disclosures.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
It’s not necessary to completely eradicate all Clostridioides difficile to successfully treat recurrent C. difficile infections with fecal microbiota transplant (FMT), according to a study presented online July 12 at the European Congress of Clinical Microbiology & Infectious Diseases.
C. difficile colonization persisted for 3 weeks after FMT in about one-quarter of patients, but it’s not clear whether this is a persistent infection, a newly acquired infection, or partial persistence of a mixed infection, said Elisabeth Terveer, MD, a medical microbiologist at Leiden (the Netherlands) University Medical Center. In addition, “82% of patients with detectable C. diff do not relapse, so it’s absolutely not necessary for a cure,” she said.
Several mechanisms explain why FMT is a highly effective therapy for recurrent C. difficile infections, including restoration of bacterial metabolism in the gut, immune modulation, and direct competition between bacteria, Dr. Terveer said, but it’s less clear whether eradication of C. difficile spores is among these mechanisms.
Between May 2016 and April 2020, the researchers analyzed fecal samples from 84 patients who took vancomycin for at least 4 days before undergoing FMT. The researchers took fecal samples from patients before FMT and 3 weeks after FMT to culture them and the donor samples for presence of C. difficile, and they assessed clinical outcomes at 3 weeks and 6 months after FMT.
After antibiotic treatment but prior to FMT, 19% of patients (n = 16) still had a toxigenic C. difficile culture while the other 81% had a negative culture. None of the donor samples had a positive C. difficile culture. After FMT treatment, five patients who had a positive pre-FMT culture remained positive, and the other 11 were negative. Among the 81% of patients (n = 68) who had a negative culture just before FMT, 22 had a positive culture and 46 had a negative culture after FMT. Overall, 26% of patients post FMT had a positive C. difficile culture, a finding that was 10-fold higher than another study that assessed C. difficile with PCR testing, Dr. Terveer said.
The clinical cure rate after FMT was 94%, and five patients had relapses within 2 months of their FMT. These relapses were more prevalent in patients with a positive C. difficile culture prior to FMT (odds ratio [OR], 7.6; P = .045) and a positive C. difficile culture after FMT (OR, 13.6; P = .016). Still, 82% of patients who had a positive C. difficile culture post FMT remained clinically cured 2 months later.
It’s unclear why 19% of patients had a positive culture after their antibiotic pretreatment prior to FMT, Dr. Terveer said, but it may be because the pretreatment was of such a short duration.
“I think the advice should be: Give a full anti–C. diff antibiotic course to treat the C. diff infection, and then give FMT afterward to restore the microbiota and prevent further relapses,” Dr. Terveer told attendees.
Dimitri Drekonja, MD, chief of the Minneapolis VA Infectious Disease Section, said the findings were not necessarily surprising, but it would have been interesting for the researchers to have conducted DNA sequencing of the patients’ fecal samples post FMT to see what the biological diversity looked like.
“One school of thought has been that you have to repopulate the normal diverse microbiota of the colon” with FMT, and the other “is that you need to get rid of the C. diff that›s there,” Dr. Drekonja, who was not involved in the study, said in an interview. “I think more people think it’s the diverse microbiota because if it’s just getting rid of C. diff, we can get do that with antibiotics – but that gets rid of the other organisms.”
As long as you have a diverse microbiota post FMT, Dr. Drekonja said, then “having a few residual organisms, even if they get magnified in the culture process, is probably not that big a deal.”
But there’s a third school of thought that Dr. Drekonja said he himself falls into: “I don’t really care how it works, just that in well-done trials, it does work.” As long as large, robust, well-blinded trials show that FMT works, “I’m open to all sorts of ideas of what the mechanism is,” he said. “The main thing is that it does or doesn’t work.”
These findings basically reinforce current guidance not to test patients’ stools if they are asymptomatic, Dr. Drekonja said. In the past, clinicians sometimes tested patients’ stool after therapy to ensure the C. difficile was eradicated, regardless of whether the patient had symptoms of infection, he said.
“We’ve since become much more attuned that there are lots of people who have detectable C. diff in their stool without any symptoms,” whether detectable by culture or PCR, Dr. Drekonja said. “Generally, if you’re doing well and you’re not having diarrhea, don’t test, and if someone does test and finds it, pretend you didn’t see the test,” he advised. “This is a big part of diagnostic stewardship, which is: You don’t go testing people who are doing well.”
The Netherlands Donor Feces Bank used in the research is funded by a grant from Vedanta Biosciences. Dr. Drekonja had no disclosures.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
Hyperimmune globulin fails to prevent congenital CMV infection
Administering hyperimmune globulin to pregnant women who tested positive for cytomegalovirus did not reduce CMV infections or deaths among their fetuses or newborns, according to a randomized controlled trial published online July 28 in the New England Journal of Medicine.
Up to 40,000 infants a year have congenital CMV infections, which can lead to stillbirth, neonatal death, deafness, and cognitive and motor delay. An estimated 35%-40% of fetuses of women with a primary CMV infection will develop an infection, write Brenna Hughes, MD, an associate professor of ob/gyn and chief of the division of maternal fetal medicine at Duke University, Durham, N.C., and colleagues.
Previous trials and observational studies have shown mixed results with hyperimmune globulin for the prevention of congenital CMV infection.
“It was surprising to us that none of the outcomes in this trial were in the direction of potential benefit,” Dr. Hughes told this news organization. “However, this is why it is important to do large trials in a diverse population.”
The study cohort comprised 206,082 pregnant women who were screened for CMV infection before 23 weeks’ gestation. Of those women, 712 (0.35%) tested positive for CMV. The researchers enrolled 399 women who had tested positive and randomly assigned them to receive either a monthly infusion of CMV hyperimmune globulin (100 mg/kg) or placebo until delivery. The researchers used a composite of CMV infection or, if no testing occurred, fetal/neonatal death as the primary endpoint.
The trial was stopped early for futility when data from 394 participants revealed that 22.7% of offspring in the hyperimmune globulin group and 19.4% of those in the placebo group had had a CMV infection or had died (relative risk = 1.17; P = .42).
When individual endpoints were examined, trends were detected in favor of the placebo, but they did not reach statistical significance. The incidence of death was higher in the hyperimmune globulin group (4.9%) than in the placebo group (2.6%). The rate of preterm birth was also higher in the intervention group (12.2%) than in the group that received placebo (8.3%). The incidence of birth weight below the fifth percentile was 10.3% in the intervention group and 5.4% in the placebo group.
One woman who received hyperimmune globulin experienced a severe allergic reaction to the first infusion. Additionally, more women in the hyperimmune globulin group experienced headaches and shaking chills during infusions than did those who received placebo. There were no differences in maternal outcomes between the groups. There were no thromboembolic or ischemic events in either group.
“These findings suggest CMV hyperimmune globulin should not be used for the prevention of congenital CMV in pregnant patients with primary CMV during pregnancy,” Dr. Hughes said in an interview.
“A CMV vaccine is likely to be the most effective public health measure that we can offer, and that should be at the forefront of research investments,” she said. “But some of the other medications that work against CMV should be tested on a large scale as well,” she said. For example, a small trial in Israel showed that high-dose valacyclovir in early pregnancy decreased congenital CMV, and thus the drug merits study in a larger trial, she said.
Other experts agree that developing a vaccine should be the priority.
“The ultimate goal for preventing the brain damage and birth defects caused by congenital CMV infection is a vaccine that is as effective as the rubella vaccine has been for eliminating congenital rubella syndrome and that can be given well before pregnancy,” said Sallie Permar, MD, PhD, chair of pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York–Presbyterian/Weill Cornell Medical Center and the New York–Presbyterian Komansky Children’s Hospital in New York.
“While trials of vaccines are ongoing, there is a need to have a therapeutic option, especially for the high-risk setting of a mother acquiring the virus for the first time during pregnancy,” Dr. Permar said in an interview.
Dr. Permar was not involved in this study but is involved in follow-up studies of this cohort and is conducting research on CMV maternal vaccines. She noted the need for safe, effective antiviral treatments and for research into newer immunoglobulin products, such as monoclonal antibodies.
Both Dr. Permar and Dr. Hughes highlighted the challenge of raising awareness about the danger of CMV infections during pregnancy.
“Pregnant women, and especially those who have or work with young children, who are frequently carriers of the infection, should be informed of this risk,” Dr. Permar said. She hopes universal testing of newborns will be implemented and that it enables people to recognize the frequency and burden of these infections. She remains optimistic about a vaccine.
“After 60 years of research into a CMV vaccine, I believe we are currently in a ‘golden age’ of CMV vaccine development,” she said. She noted that Moderna is about to launch a phase 3 mRNA vaccine trial for CMV. “Moreover, immune correlates of protection against CMV have been identified from previous partially effective vaccines, and animal models have improved for preclinical studies. Therefore, I believe we will have an effective and safe vaccine against this most common congenital infection in the coming years.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Dr. Hughes has served on Merck’s scientific advisory board. Various coauthors have received personal fees from Medela and nonfinancial support from Hologic; personal fees from Moderna and VBI vaccines, and grants from Novavax. Dr. Permar consults for Pfizer, Moderna, Merck, Sanofi, and Dynavax on their CMV vaccine programs, and she has a sponsored research program with Merck and Moderna on CMV vaccines.
A version of this article first appeared on Medscape.com.
Administering hyperimmune globulin to pregnant women who tested positive for cytomegalovirus did not reduce CMV infections or deaths among their fetuses or newborns, according to a randomized controlled trial published online July 28 in the New England Journal of Medicine.
Up to 40,000 infants a year have congenital CMV infections, which can lead to stillbirth, neonatal death, deafness, and cognitive and motor delay. An estimated 35%-40% of fetuses of women with a primary CMV infection will develop an infection, write Brenna Hughes, MD, an associate professor of ob/gyn and chief of the division of maternal fetal medicine at Duke University, Durham, N.C., and colleagues.
Previous trials and observational studies have shown mixed results with hyperimmune globulin for the prevention of congenital CMV infection.
“It was surprising to us that none of the outcomes in this trial were in the direction of potential benefit,” Dr. Hughes told this news organization. “However, this is why it is important to do large trials in a diverse population.”
The study cohort comprised 206,082 pregnant women who were screened for CMV infection before 23 weeks’ gestation. Of those women, 712 (0.35%) tested positive for CMV. The researchers enrolled 399 women who had tested positive and randomly assigned them to receive either a monthly infusion of CMV hyperimmune globulin (100 mg/kg) or placebo until delivery. The researchers used a composite of CMV infection or, if no testing occurred, fetal/neonatal death as the primary endpoint.
The trial was stopped early for futility when data from 394 participants revealed that 22.7% of offspring in the hyperimmune globulin group and 19.4% of those in the placebo group had had a CMV infection or had died (relative risk = 1.17; P = .42).
When individual endpoints were examined, trends were detected in favor of the placebo, but they did not reach statistical significance. The incidence of death was higher in the hyperimmune globulin group (4.9%) than in the placebo group (2.6%). The rate of preterm birth was also higher in the intervention group (12.2%) than in the group that received placebo (8.3%). The incidence of birth weight below the fifth percentile was 10.3% in the intervention group and 5.4% in the placebo group.
One woman who received hyperimmune globulin experienced a severe allergic reaction to the first infusion. Additionally, more women in the hyperimmune globulin group experienced headaches and shaking chills during infusions than did those who received placebo. There were no differences in maternal outcomes between the groups. There were no thromboembolic or ischemic events in either group.
“These findings suggest CMV hyperimmune globulin should not be used for the prevention of congenital CMV in pregnant patients with primary CMV during pregnancy,” Dr. Hughes said in an interview.
“A CMV vaccine is likely to be the most effective public health measure that we can offer, and that should be at the forefront of research investments,” she said. “But some of the other medications that work against CMV should be tested on a large scale as well,” she said. For example, a small trial in Israel showed that high-dose valacyclovir in early pregnancy decreased congenital CMV, and thus the drug merits study in a larger trial, she said.
Other experts agree that developing a vaccine should be the priority.
“The ultimate goal for preventing the brain damage and birth defects caused by congenital CMV infection is a vaccine that is as effective as the rubella vaccine has been for eliminating congenital rubella syndrome and that can be given well before pregnancy,” said Sallie Permar, MD, PhD, chair of pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York–Presbyterian/Weill Cornell Medical Center and the New York–Presbyterian Komansky Children’s Hospital in New York.
“While trials of vaccines are ongoing, there is a need to have a therapeutic option, especially for the high-risk setting of a mother acquiring the virus for the first time during pregnancy,” Dr. Permar said in an interview.
Dr. Permar was not involved in this study but is involved in follow-up studies of this cohort and is conducting research on CMV maternal vaccines. She noted the need for safe, effective antiviral treatments and for research into newer immunoglobulin products, such as monoclonal antibodies.
Both Dr. Permar and Dr. Hughes highlighted the challenge of raising awareness about the danger of CMV infections during pregnancy.
“Pregnant women, and especially those who have or work with young children, who are frequently carriers of the infection, should be informed of this risk,” Dr. Permar said. She hopes universal testing of newborns will be implemented and that it enables people to recognize the frequency and burden of these infections. She remains optimistic about a vaccine.
“After 60 years of research into a CMV vaccine, I believe we are currently in a ‘golden age’ of CMV vaccine development,” she said. She noted that Moderna is about to launch a phase 3 mRNA vaccine trial for CMV. “Moreover, immune correlates of protection against CMV have been identified from previous partially effective vaccines, and animal models have improved for preclinical studies. Therefore, I believe we will have an effective and safe vaccine against this most common congenital infection in the coming years.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Dr. Hughes has served on Merck’s scientific advisory board. Various coauthors have received personal fees from Medela and nonfinancial support from Hologic; personal fees from Moderna and VBI vaccines, and grants from Novavax. Dr. Permar consults for Pfizer, Moderna, Merck, Sanofi, and Dynavax on their CMV vaccine programs, and she has a sponsored research program with Merck and Moderna on CMV vaccines.
A version of this article first appeared on Medscape.com.
Administering hyperimmune globulin to pregnant women who tested positive for cytomegalovirus did not reduce CMV infections or deaths among their fetuses or newborns, according to a randomized controlled trial published online July 28 in the New England Journal of Medicine.
Up to 40,000 infants a year have congenital CMV infections, which can lead to stillbirth, neonatal death, deafness, and cognitive and motor delay. An estimated 35%-40% of fetuses of women with a primary CMV infection will develop an infection, write Brenna Hughes, MD, an associate professor of ob/gyn and chief of the division of maternal fetal medicine at Duke University, Durham, N.C., and colleagues.
Previous trials and observational studies have shown mixed results with hyperimmune globulin for the prevention of congenital CMV infection.
“It was surprising to us that none of the outcomes in this trial were in the direction of potential benefit,” Dr. Hughes told this news organization. “However, this is why it is important to do large trials in a diverse population.”
The study cohort comprised 206,082 pregnant women who were screened for CMV infection before 23 weeks’ gestation. Of those women, 712 (0.35%) tested positive for CMV. The researchers enrolled 399 women who had tested positive and randomly assigned them to receive either a monthly infusion of CMV hyperimmune globulin (100 mg/kg) or placebo until delivery. The researchers used a composite of CMV infection or, if no testing occurred, fetal/neonatal death as the primary endpoint.
The trial was stopped early for futility when data from 394 participants revealed that 22.7% of offspring in the hyperimmune globulin group and 19.4% of those in the placebo group had had a CMV infection or had died (relative risk = 1.17; P = .42).
When individual endpoints were examined, trends were detected in favor of the placebo, but they did not reach statistical significance. The incidence of death was higher in the hyperimmune globulin group (4.9%) than in the placebo group (2.6%). The rate of preterm birth was also higher in the intervention group (12.2%) than in the group that received placebo (8.3%). The incidence of birth weight below the fifth percentile was 10.3% in the intervention group and 5.4% in the placebo group.
One woman who received hyperimmune globulin experienced a severe allergic reaction to the first infusion. Additionally, more women in the hyperimmune globulin group experienced headaches and shaking chills during infusions than did those who received placebo. There were no differences in maternal outcomes between the groups. There were no thromboembolic or ischemic events in either group.
“These findings suggest CMV hyperimmune globulin should not be used for the prevention of congenital CMV in pregnant patients with primary CMV during pregnancy,” Dr. Hughes said in an interview.
“A CMV vaccine is likely to be the most effective public health measure that we can offer, and that should be at the forefront of research investments,” she said. “But some of the other medications that work against CMV should be tested on a large scale as well,” she said. For example, a small trial in Israel showed that high-dose valacyclovir in early pregnancy decreased congenital CMV, and thus the drug merits study in a larger trial, she said.
Other experts agree that developing a vaccine should be the priority.
“The ultimate goal for preventing the brain damage and birth defects caused by congenital CMV infection is a vaccine that is as effective as the rubella vaccine has been for eliminating congenital rubella syndrome and that can be given well before pregnancy,” said Sallie Permar, MD, PhD, chair of pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York–Presbyterian/Weill Cornell Medical Center and the New York–Presbyterian Komansky Children’s Hospital in New York.
“While trials of vaccines are ongoing, there is a need to have a therapeutic option, especially for the high-risk setting of a mother acquiring the virus for the first time during pregnancy,” Dr. Permar said in an interview.
Dr. Permar was not involved in this study but is involved in follow-up studies of this cohort and is conducting research on CMV maternal vaccines. She noted the need for safe, effective antiviral treatments and for research into newer immunoglobulin products, such as monoclonal antibodies.
Both Dr. Permar and Dr. Hughes highlighted the challenge of raising awareness about the danger of CMV infections during pregnancy.
“Pregnant women, and especially those who have or work with young children, who are frequently carriers of the infection, should be informed of this risk,” Dr. Permar said. She hopes universal testing of newborns will be implemented and that it enables people to recognize the frequency and burden of these infections. She remains optimistic about a vaccine.
“After 60 years of research into a CMV vaccine, I believe we are currently in a ‘golden age’ of CMV vaccine development,” she said. She noted that Moderna is about to launch a phase 3 mRNA vaccine trial for CMV. “Moreover, immune correlates of protection against CMV have been identified from previous partially effective vaccines, and animal models have improved for preclinical studies. Therefore, I believe we will have an effective and safe vaccine against this most common congenital infection in the coming years.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Dr. Hughes has served on Merck’s scientific advisory board. Various coauthors have received personal fees from Medela and nonfinancial support from Hologic; personal fees from Moderna and VBI vaccines, and grants from Novavax. Dr. Permar consults for Pfizer, Moderna, Merck, Sanofi, and Dynavax on their CMV vaccine programs, and she has a sponsored research program with Merck and Moderna on CMV vaccines.
A version of this article first appeared on Medscape.com.
C. Diff eradication not necessary for clinical cure of recurrent infections with fecal transplant
It’s not necessary to completely eradicate all Clostridioides difficile to successfully treat recurrent C. difficile infections with fecal microbiota transplant (FMT), according to a study presented online July 12 at the European Congress of Clinical Microbiology & Infectious Diseases.
C. difficile colonization persisted for 3 weeks after FMT in about one-quarter of patients, but it’s not clear whether this is a persistent infection, a newly acquired infection, or partial persistence of a mixed infection, said Elisabeth Terveer, MD, a medical microbiologist at Leiden (the Netherlands) University Medical Center. In addition, “82% of patients with detectable C. diff do not relapse, so it’s absolutely not necessary for a cure,” she said.
Several mechanisms explain why FMT is a highly effective therapy for recurrent C. difficile infections, including restoration of bacterial metabolism in the gut, immune modulation, and direct competition between bacteria, Dr. Terveer said, but it’s less clear whether eradication of C. difficile spores is among these mechanisms.
Between May 2016 and April 2020, the researchers analyzed fecal samples from 84 patients who took vancomycin for at least 4 days before undergoing FMT. The researchers took fecal samples from patients before FMT and 3 weeks after FMT to culture them and the donor samples for presence of C. difficile, and they assessed clinical outcomes at 3 weeks and 6 months after FMT.
After antibiotic treatment but prior to FMT, 19% of patients (n = 16) still had a toxigenic C. difficile culture while the other 81% had a negative culture. None of the donor samples had a positive C. difficile culture. After FMT treatment, five patients who had a positive pre-FMT culture remained positive, and the other 11 were negative. Among the 81% of patients (n = 68) who had a negative culture just before FMT, 22 had a positive culture and 46 had a negative culture after FMT. Overall, 26% of patients post FMT had a positive C. difficile culture, a finding that was 10-fold higher than another study that assessed C. difficile with PCR testing, Dr. Terveer said.
The clinical cure rate after FMT was 94%, and five patients had relapses within 2 months of their FMT. These relapses were more prevalent in patients with a positive C. difficile culture prior to FMT (odds ratio [OR], 7.6; P = .045) and a positive C. difficile culture after FMT (OR, 13.6; P = .016). Still, 82% of patients who had a positive C. difficile culture post FMT remained clinically cured 2 months later.
It’s unclear why 19% of patients had a positive culture after their antibiotic pretreatment prior to FMT, Dr. Terveer said, but it may be because the pretreatment was of such a short duration.
“I think the advice should be: Give a full anti–C. diff antibiotic course to treat the C. diff infection, and then give FMT afterward to restore the microbiota and prevent further relapses,” Dr. Terveer told attendees.
Dimitri Drekonja, MD, chief of the Minneapolis VA Infectious Disease Section, said the findings were not necessarily surprising, but it would have been interesting for the researchers to have conducted DNA sequencing of the patients’ fecal samples post FMT to see what the biological diversity looked like.
“One school of thought has been that you have to repopulate the normal diverse microbiota of the colon” with FMT, and the other “is that you need to get rid of the C. diff that›s there,” Dr. Drekonja, who was not involved in the study, said in an interview. “I think more people think it’s the diverse microbiota because if it’s just getting rid of C. diff, we can get do that with antibiotics – but that gets rid of the other organisms.”
As long as you have a diverse microbiota post FMT, Dr. Drekonja said, then “having a few residual organisms, even if they get magnified in the culture process, is probably not that big a deal.”
But there’s a third school of thought that Dr. Drekonja said he himself falls into: “I don’t really care how it works, just that in well-done trials, it does work.” As long as large, robust, well-blinded trials show that FMT works, “I’m open to all sorts of ideas of what the mechanism is,” he said. “The main thing is that it does or doesn’t work.”
These findings basically reinforce current guidance not to test patients’ stools if they are asymptomatic, Dr. Drekonja said. In the past, clinicians sometimes tested patients’ stool after therapy to ensure the C. difficile was eradicated, regardless of whether the patient had symptoms of infection, he said.
“We’ve since become much more attuned that there are lots of people who have detectable C. diff in their stool without any symptoms,” whether detectable by culture or PCR, Dr. Drekonja said. “Generally, if you’re doing well and you’re not having diarrhea, don’t test, and if someone does test and finds it, pretend you didn’t see the test,” he advised. “This is a big part of diagnostic stewardship, which is: You don’t go testing people who are doing well.”
The Netherlands Donor Feces Bank used in the research is funded by a grant from Vedanta Biosciences. Dr. Drekonja had no disclosures.
A version of this article first appeared on Medscape.com.
It’s not necessary to completely eradicate all Clostridioides difficile to successfully treat recurrent C. difficile infections with fecal microbiota transplant (FMT), according to a study presented online July 12 at the European Congress of Clinical Microbiology & Infectious Diseases.
C. difficile colonization persisted for 3 weeks after FMT in about one-quarter of patients, but it’s not clear whether this is a persistent infection, a newly acquired infection, or partial persistence of a mixed infection, said Elisabeth Terveer, MD, a medical microbiologist at Leiden (the Netherlands) University Medical Center. In addition, “82% of patients with detectable C. diff do not relapse, so it’s absolutely not necessary for a cure,” she said.
Several mechanisms explain why FMT is a highly effective therapy for recurrent C. difficile infections, including restoration of bacterial metabolism in the gut, immune modulation, and direct competition between bacteria, Dr. Terveer said, but it’s less clear whether eradication of C. difficile spores is among these mechanisms.
Between May 2016 and April 2020, the researchers analyzed fecal samples from 84 patients who took vancomycin for at least 4 days before undergoing FMT. The researchers took fecal samples from patients before FMT and 3 weeks after FMT to culture them and the donor samples for presence of C. difficile, and they assessed clinical outcomes at 3 weeks and 6 months after FMT.
After antibiotic treatment but prior to FMT, 19% of patients (n = 16) still had a toxigenic C. difficile culture while the other 81% had a negative culture. None of the donor samples had a positive C. difficile culture. After FMT treatment, five patients who had a positive pre-FMT culture remained positive, and the other 11 were negative. Among the 81% of patients (n = 68) who had a negative culture just before FMT, 22 had a positive culture and 46 had a negative culture after FMT. Overall, 26% of patients post FMT had a positive C. difficile culture, a finding that was 10-fold higher than another study that assessed C. difficile with PCR testing, Dr. Terveer said.
The clinical cure rate after FMT was 94%, and five patients had relapses within 2 months of their FMT. These relapses were more prevalent in patients with a positive C. difficile culture prior to FMT (odds ratio [OR], 7.6; P = .045) and a positive C. difficile culture after FMT (OR, 13.6; P = .016). Still, 82% of patients who had a positive C. difficile culture post FMT remained clinically cured 2 months later.
It’s unclear why 19% of patients had a positive culture after their antibiotic pretreatment prior to FMT, Dr. Terveer said, but it may be because the pretreatment was of such a short duration.
“I think the advice should be: Give a full anti–C. diff antibiotic course to treat the C. diff infection, and then give FMT afterward to restore the microbiota and prevent further relapses,” Dr. Terveer told attendees.
Dimitri Drekonja, MD, chief of the Minneapolis VA Infectious Disease Section, said the findings were not necessarily surprising, but it would have been interesting for the researchers to have conducted DNA sequencing of the patients’ fecal samples post FMT to see what the biological diversity looked like.
“One school of thought has been that you have to repopulate the normal diverse microbiota of the colon” with FMT, and the other “is that you need to get rid of the C. diff that›s there,” Dr. Drekonja, who was not involved in the study, said in an interview. “I think more people think it’s the diverse microbiota because if it’s just getting rid of C. diff, we can get do that with antibiotics – but that gets rid of the other organisms.”
As long as you have a diverse microbiota post FMT, Dr. Drekonja said, then “having a few residual organisms, even if they get magnified in the culture process, is probably not that big a deal.”
But there’s a third school of thought that Dr. Drekonja said he himself falls into: “I don’t really care how it works, just that in well-done trials, it does work.” As long as large, robust, well-blinded trials show that FMT works, “I’m open to all sorts of ideas of what the mechanism is,” he said. “The main thing is that it does or doesn’t work.”
These findings basically reinforce current guidance not to test patients’ stools if they are asymptomatic, Dr. Drekonja said. In the past, clinicians sometimes tested patients’ stool after therapy to ensure the C. difficile was eradicated, regardless of whether the patient had symptoms of infection, he said.
“We’ve since become much more attuned that there are lots of people who have detectable C. diff in their stool without any symptoms,” whether detectable by culture or PCR, Dr. Drekonja said. “Generally, if you’re doing well and you’re not having diarrhea, don’t test, and if someone does test and finds it, pretend you didn’t see the test,” he advised. “This is a big part of diagnostic stewardship, which is: You don’t go testing people who are doing well.”
The Netherlands Donor Feces Bank used in the research is funded by a grant from Vedanta Biosciences. Dr. Drekonja had no disclosures.
A version of this article first appeared on Medscape.com.
It’s not necessary to completely eradicate all Clostridioides difficile to successfully treat recurrent C. difficile infections with fecal microbiota transplant (FMT), according to a study presented online July 12 at the European Congress of Clinical Microbiology & Infectious Diseases.
C. difficile colonization persisted for 3 weeks after FMT in about one-quarter of patients, but it’s not clear whether this is a persistent infection, a newly acquired infection, or partial persistence of a mixed infection, said Elisabeth Terveer, MD, a medical microbiologist at Leiden (the Netherlands) University Medical Center. In addition, “82% of patients with detectable C. diff do not relapse, so it’s absolutely not necessary for a cure,” she said.
Several mechanisms explain why FMT is a highly effective therapy for recurrent C. difficile infections, including restoration of bacterial metabolism in the gut, immune modulation, and direct competition between bacteria, Dr. Terveer said, but it’s less clear whether eradication of C. difficile spores is among these mechanisms.
Between May 2016 and April 2020, the researchers analyzed fecal samples from 84 patients who took vancomycin for at least 4 days before undergoing FMT. The researchers took fecal samples from patients before FMT and 3 weeks after FMT to culture them and the donor samples for presence of C. difficile, and they assessed clinical outcomes at 3 weeks and 6 months after FMT.
After antibiotic treatment but prior to FMT, 19% of patients (n = 16) still had a toxigenic C. difficile culture while the other 81% had a negative culture. None of the donor samples had a positive C. difficile culture. After FMT treatment, five patients who had a positive pre-FMT culture remained positive, and the other 11 were negative. Among the 81% of patients (n = 68) who had a negative culture just before FMT, 22 had a positive culture and 46 had a negative culture after FMT. Overall, 26% of patients post FMT had a positive C. difficile culture, a finding that was 10-fold higher than another study that assessed C. difficile with PCR testing, Dr. Terveer said.
The clinical cure rate after FMT was 94%, and five patients had relapses within 2 months of their FMT. These relapses were more prevalent in patients with a positive C. difficile culture prior to FMT (odds ratio [OR], 7.6; P = .045) and a positive C. difficile culture after FMT (OR, 13.6; P = .016). Still, 82% of patients who had a positive C. difficile culture post FMT remained clinically cured 2 months later.
It’s unclear why 19% of patients had a positive culture after their antibiotic pretreatment prior to FMT, Dr. Terveer said, but it may be because the pretreatment was of such a short duration.
“I think the advice should be: Give a full anti–C. diff antibiotic course to treat the C. diff infection, and then give FMT afterward to restore the microbiota and prevent further relapses,” Dr. Terveer told attendees.
Dimitri Drekonja, MD, chief of the Minneapolis VA Infectious Disease Section, said the findings were not necessarily surprising, but it would have been interesting for the researchers to have conducted DNA sequencing of the patients’ fecal samples post FMT to see what the biological diversity looked like.
“One school of thought has been that you have to repopulate the normal diverse microbiota of the colon” with FMT, and the other “is that you need to get rid of the C. diff that›s there,” Dr. Drekonja, who was not involved in the study, said in an interview. “I think more people think it’s the diverse microbiota because if it’s just getting rid of C. diff, we can get do that with antibiotics – but that gets rid of the other organisms.”
As long as you have a diverse microbiota post FMT, Dr. Drekonja said, then “having a few residual organisms, even if they get magnified in the culture process, is probably not that big a deal.”
But there’s a third school of thought that Dr. Drekonja said he himself falls into: “I don’t really care how it works, just that in well-done trials, it does work.” As long as large, robust, well-blinded trials show that FMT works, “I’m open to all sorts of ideas of what the mechanism is,” he said. “The main thing is that it does or doesn’t work.”
These findings basically reinforce current guidance not to test patients’ stools if they are asymptomatic, Dr. Drekonja said. In the past, clinicians sometimes tested patients’ stool after therapy to ensure the C. difficile was eradicated, regardless of whether the patient had symptoms of infection, he said.
“We’ve since become much more attuned that there are lots of people who have detectable C. diff in their stool without any symptoms,” whether detectable by culture or PCR, Dr. Drekonja said. “Generally, if you’re doing well and you’re not having diarrhea, don’t test, and if someone does test and finds it, pretend you didn’t see the test,” he advised. “This is a big part of diagnostic stewardship, which is: You don’t go testing people who are doing well.”
The Netherlands Donor Feces Bank used in the research is funded by a grant from Vedanta Biosciences. Dr. Drekonja had no disclosures.
A version of this article first appeared on Medscape.com.
Long COVID seen in patients with severe and mild disease
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.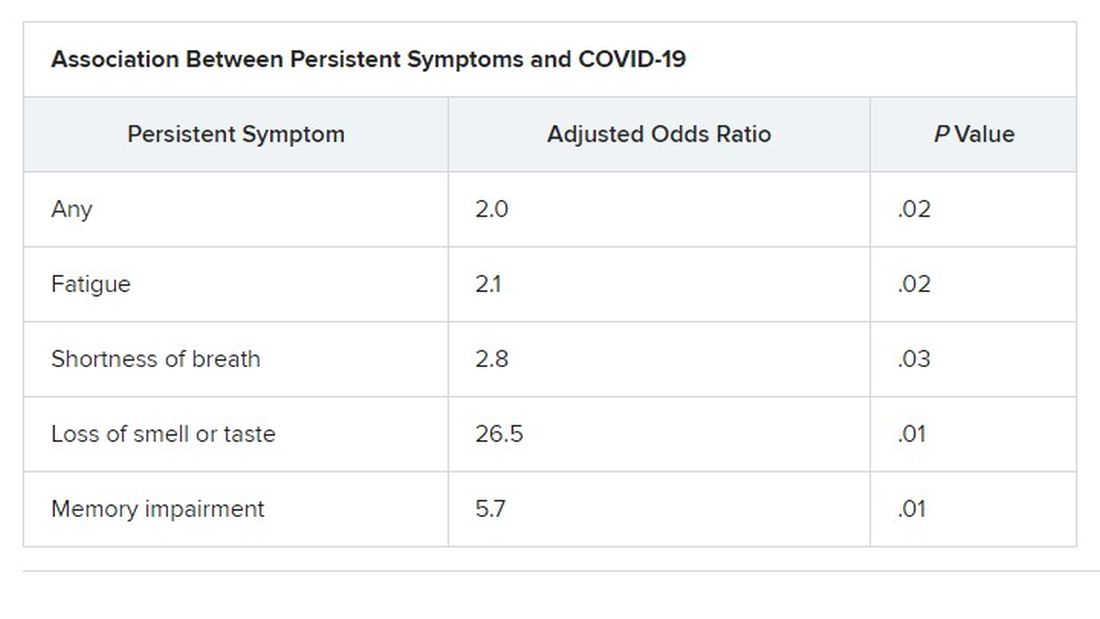
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN CONGRESS OF CLINICAL MICROBIOLOGY & INFECTIOUS DISEASES