User login
Status Epilepticus in Pregnancy
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
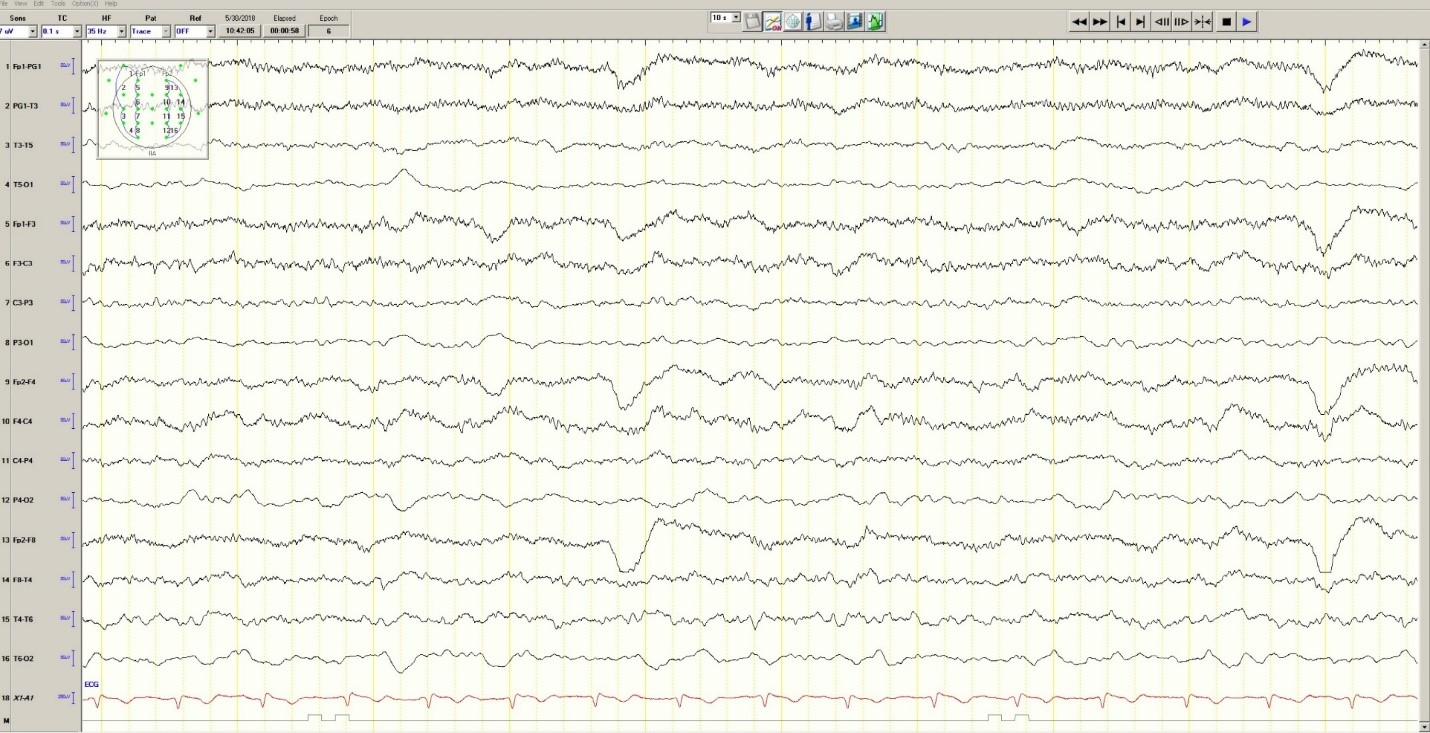
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing
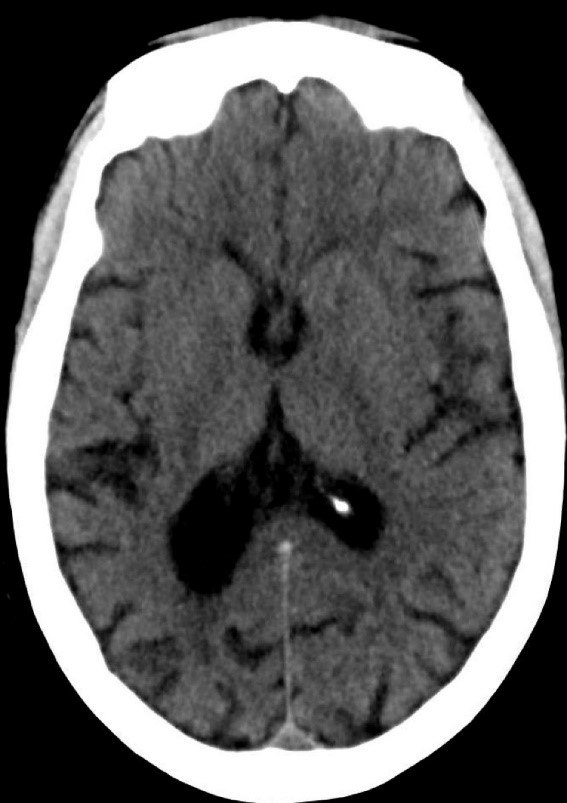
During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region
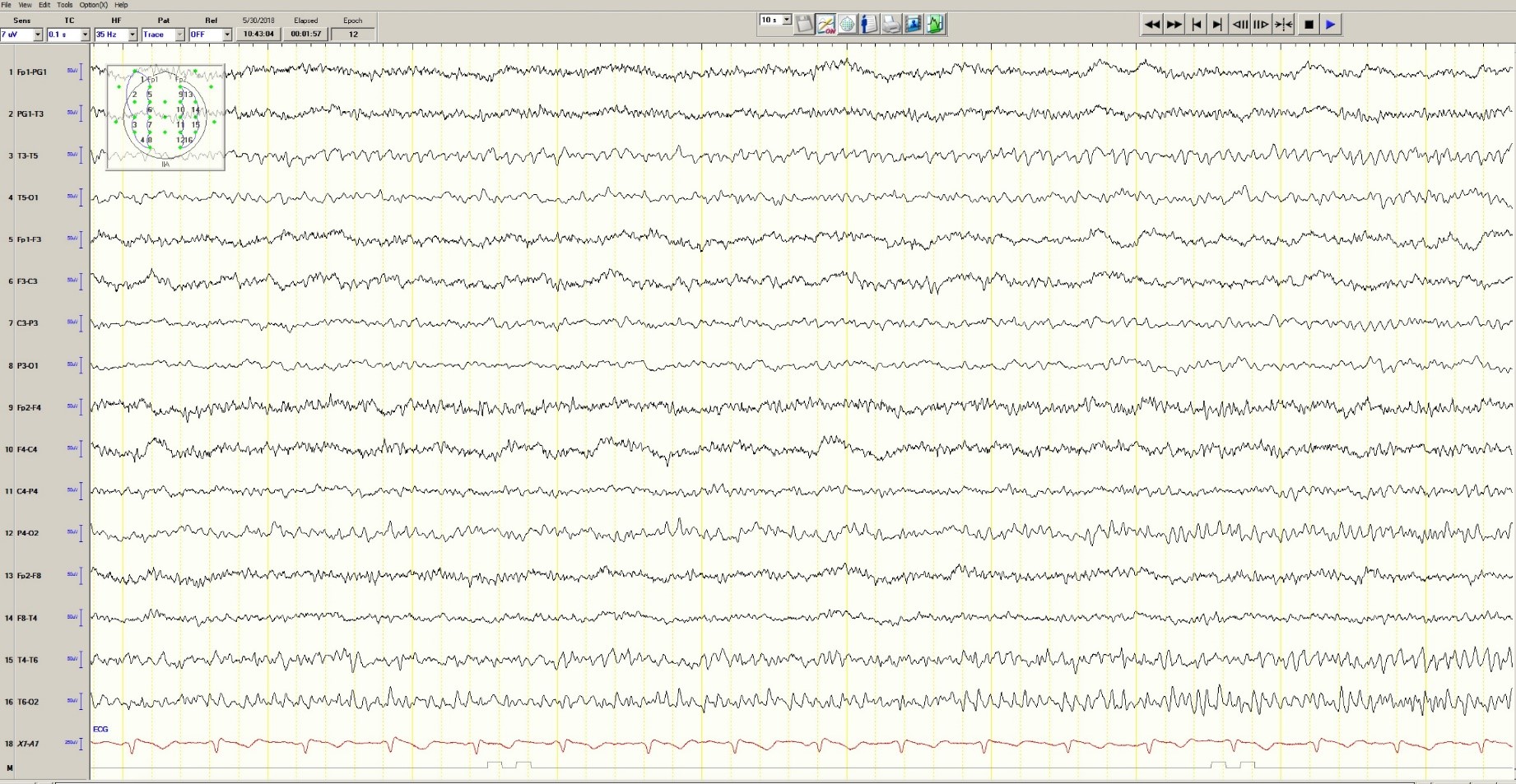
Figure 3. Mild generalized arophy, greater in right hemisphere
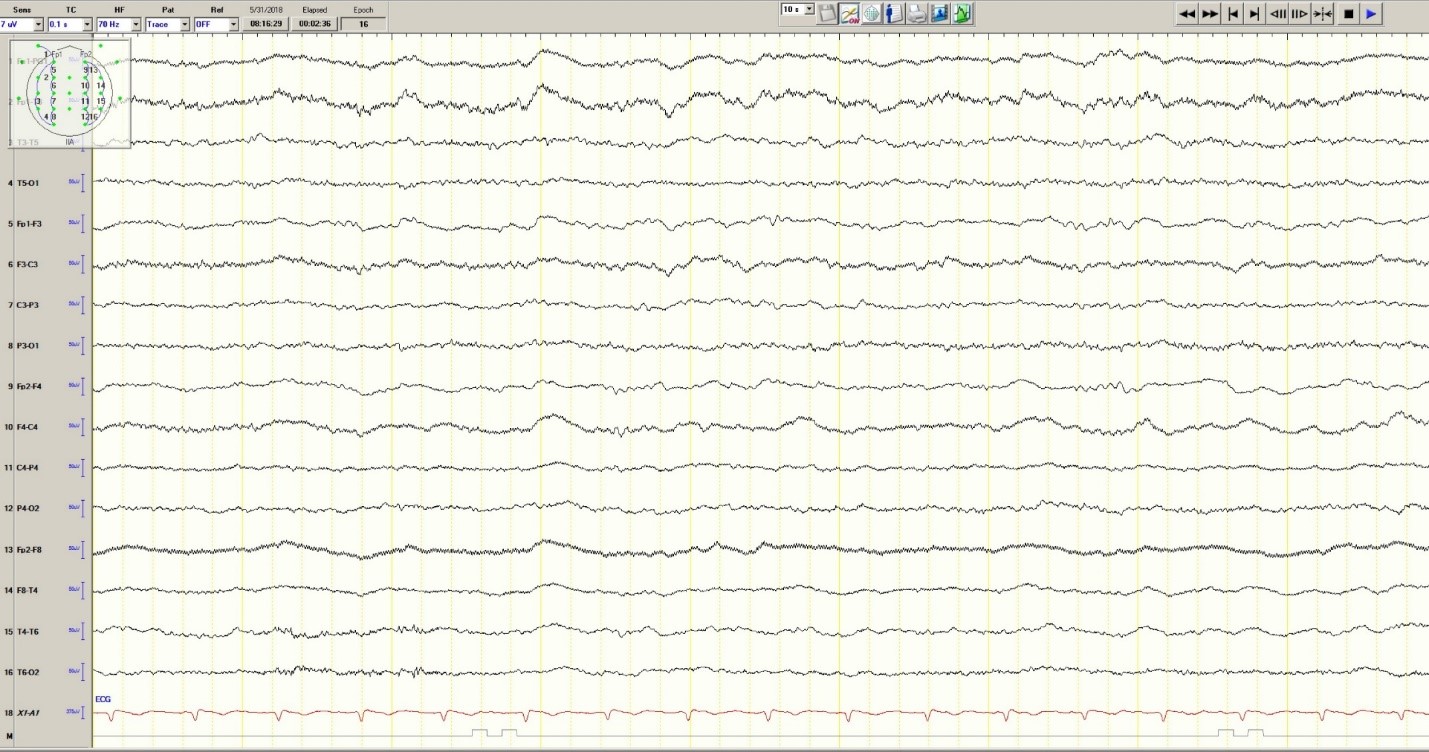
The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing
Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing

During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region

Figure 3. Mild generalized arophy, greater in right hemisphere

The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing

Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing

During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region

Figure 3. Mild generalized arophy, greater in right hemisphere

The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing

Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
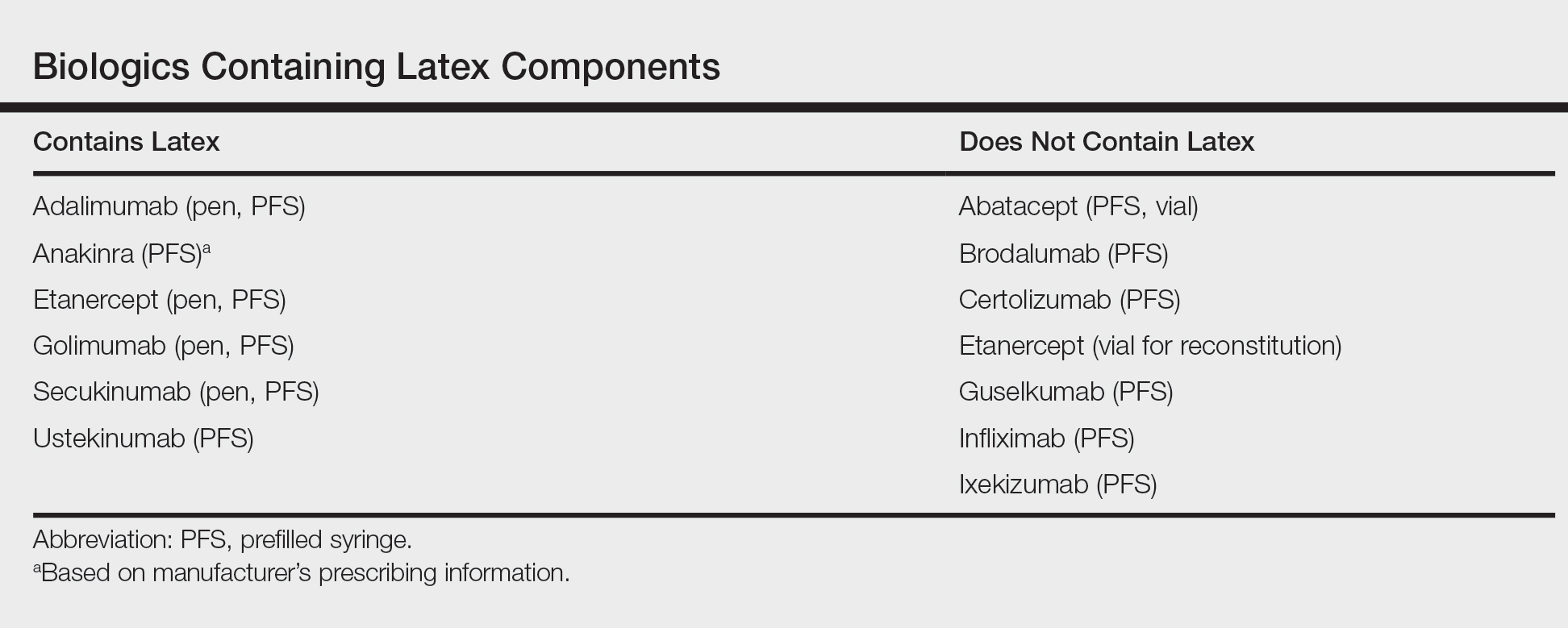
Latex Hypersensitivity to Injection Devices for Biologic Therapies in Psoriasis Patients
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
Inflammatory Linear Verrucous Epidermal Nevus Responsive to 308-nm Excimer Laser Treatment
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
Do Erythropoiesis-Stimulating Agents Have a Risk Evaluation and Mitigation Strategy? (FULL)
Epoetin alfa and darbepoetin alfa are erythropoiesis-stimulating agents (ESAs), approved for the treatment of anemia (low red blood cells [RBCs]) resulting from chronic kidney disease, chemotherapy, and certain treatments for HIV. These ESAs also are used to reduce the number of blood transfusions during and after certain major surgeries. Erythropoiesis-stimulating agents work like the human protein erythropoietin, which stimulates bone marrow to make RBCs. Epoetin alfa (marketed as Procrit and Epogen) and darbepoetin alfa (marketed as Aranesp) are manufactured by Amgen, Inc. (Thousand Oaks, CA).
In 1989 epoetin alfa was approved for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 1993 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy. Epoetin alfa also is indicated for anemia due to zidovudine in patients with HIV and reduction of RBC transfusions during certain surgeries.
Darbepoetin alfa was approved in 2001 for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 2006 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy.
Risk Evaluation and Mitigation Strategies
Both epoetin alfa and darbepoetin alfa increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access and tumor progression or recurrence. Epoetin alfa also can lead to an increase in adverse cardiovascular events, hypertension, seizures, and severe anemia.
In 2008, the FDA determined that Risk Evaluation and Mitigation Strategies (REMS) were necessary for ESAs (darbopoetin alfa and epoetin alfa), to ensure that the benefits for use as treatment for anemia associated with myelosuppressive chemotherapy outweigh the risk of shortened overall survival (OS) and/or the increased risk of tumor progression or recurrence in patients with cancer. The REMS was approved in 2010.
Under the ESA REMS program, referred to as the ESA APPRISE Oncology Program, health care providers (HCPs) that prescribed and/or dispensed darbopoetin alfa to patients with cancer and hospitals that dispensed darbopoetin alfa to patients with cancer were required to enroll and become certified in the ESA REMS. The ESA REMS also required the completion of a Patient and Healthcare Provider Acknowledgement Form for each patient with cancer before the new ESA treatment course to ensure patients were counseled about the benefits and risks of these products.
In April 2017, the FDA determined that the ESA REMS that was limited to the use of epoetin alfa and darbopoetin alfa to treat patients with anemia due to associated myelosuppressive chemotherapy was no longer necessary; the benefits of ESAs outweighed the risks of shortened OS and/or increased risk of tumor progression or recurrence in patients with cancer. 1 The FDA recognized the burden that some REMS can place on HCPs and patients. The agency has authority to modify or remove the REMS to minimize the burden on the health care delivery system of complying with the strategy.
Data
The FDA discontinued the REMS based on an evaluation of the results of the REMS Assessments submitted by Amgen and additional FDA analyses to understand the impact of the various regulatory and other actions on the use of ESAs. The REMS Assessment showed the following:
- The results from surveyed prescribers demonstrated acceptable knowledge of the product risks of decreased survival and/or the increased risk of tumor progression or recurrence and the need to counsel patients about these risks; and
- The drug utilization data indicated appropriate prescribing of ESAs consistent with the intended use as a treatment alternative to RBC transfusion for anemia associated with myelosuppressive chemotherapy.
The FDA also conducted an evaluation of the impact of multiple actions, including the ESA REMS, on the use of the ESAs using sponsor-submitted data from outpatient oncology practices between 2006 and 2014. During 2004 to 2009, the FDA took multiple regulatory actions, including labeling changes. In 2007, the Center for Medicare and Medicaid Services (CMS) made a National Coverage Determination (NCD) to limit coverage of ESAs for nonrenal disease indications. These actions coincided with the following:
- A decrease in the proportion of patients receiving chemotherapy using ESAs;
- An increase in the proportion of patients receiving chemotherapy who initiate ESAs at a hemoglobin level < 10 g/dL; and
- An increase in the proportion of patients who initiate ESAs at a dosage consistent with product prescribing information.
Full implementation of the ESA REMS in 2011 had minimal impact on trends in these 3 ESA utilization metrics beyond the changes observed after the CMS coverage determination and multiple other FDA regulatory actions.
This information led the FDA to conclude that it was no longer necessary to require the certification of prescribers and hospitals that prescribe and/or dispense ESAs to patients with cancer in order to ensure that the benefits outweigh the risks.
The FDA has released the REMS requirements for the epoetin alfa and darbopoetin alfa ESA products, and the risks can be communicated by the current product prescribing information. The appropriate use of ESAs is supported by the CMS NCD, the American Society of Clinical Oncology, and American Society of Hematology clinical guidelines, which are evidence-based guidelines intended to provide a basis for the standard of care in clinical oncology.
Education
While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened OS and/or increased risk of tumor progression or recurrence associated with these drugs remain. The boxed warning language remains as follows: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE. Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Click here to read the digital edition.
1. U.S. Food & Drug Administration. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Updated April 13, 2017. Accessed July 13, 2017.
Epoetin alfa and darbepoetin alfa are erythropoiesis-stimulating agents (ESAs), approved for the treatment of anemia (low red blood cells [RBCs]) resulting from chronic kidney disease, chemotherapy, and certain treatments for HIV. These ESAs also are used to reduce the number of blood transfusions during and after certain major surgeries. Erythropoiesis-stimulating agents work like the human protein erythropoietin, which stimulates bone marrow to make RBCs. Epoetin alfa (marketed as Procrit and Epogen) and darbepoetin alfa (marketed as Aranesp) are manufactured by Amgen, Inc. (Thousand Oaks, CA).
In 1989 epoetin alfa was approved for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 1993 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy. Epoetin alfa also is indicated for anemia due to zidovudine in patients with HIV and reduction of RBC transfusions during certain surgeries.
Darbepoetin alfa was approved in 2001 for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 2006 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy.
Risk Evaluation and Mitigation Strategies
Both epoetin alfa and darbepoetin alfa increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access and tumor progression or recurrence. Epoetin alfa also can lead to an increase in adverse cardiovascular events, hypertension, seizures, and severe anemia.
In 2008, the FDA determined that Risk Evaluation and Mitigation Strategies (REMS) were necessary for ESAs (darbopoetin alfa and epoetin alfa), to ensure that the benefits for use as treatment for anemia associated with myelosuppressive chemotherapy outweigh the risk of shortened overall survival (OS) and/or the increased risk of tumor progression or recurrence in patients with cancer. The REMS was approved in 2010.
Under the ESA REMS program, referred to as the ESA APPRISE Oncology Program, health care providers (HCPs) that prescribed and/or dispensed darbopoetin alfa to patients with cancer and hospitals that dispensed darbopoetin alfa to patients with cancer were required to enroll and become certified in the ESA REMS. The ESA REMS also required the completion of a Patient and Healthcare Provider Acknowledgement Form for each patient with cancer before the new ESA treatment course to ensure patients were counseled about the benefits and risks of these products.
In April 2017, the FDA determined that the ESA REMS that was limited to the use of epoetin alfa and darbopoetin alfa to treat patients with anemia due to associated myelosuppressive chemotherapy was no longer necessary; the benefits of ESAs outweighed the risks of shortened OS and/or increased risk of tumor progression or recurrence in patients with cancer. 1 The FDA recognized the burden that some REMS can place on HCPs and patients. The agency has authority to modify or remove the REMS to minimize the burden on the health care delivery system of complying with the strategy.
Data
The FDA discontinued the REMS based on an evaluation of the results of the REMS Assessments submitted by Amgen and additional FDA analyses to understand the impact of the various regulatory and other actions on the use of ESAs. The REMS Assessment showed the following:
- The results from surveyed prescribers demonstrated acceptable knowledge of the product risks of decreased survival and/or the increased risk of tumor progression or recurrence and the need to counsel patients about these risks; and
- The drug utilization data indicated appropriate prescribing of ESAs consistent with the intended use as a treatment alternative to RBC transfusion for anemia associated with myelosuppressive chemotherapy.
The FDA also conducted an evaluation of the impact of multiple actions, including the ESA REMS, on the use of the ESAs using sponsor-submitted data from outpatient oncology practices between 2006 and 2014. During 2004 to 2009, the FDA took multiple regulatory actions, including labeling changes. In 2007, the Center for Medicare and Medicaid Services (CMS) made a National Coverage Determination (NCD) to limit coverage of ESAs for nonrenal disease indications. These actions coincided with the following:
- A decrease in the proportion of patients receiving chemotherapy using ESAs;
- An increase in the proportion of patients receiving chemotherapy who initiate ESAs at a hemoglobin level < 10 g/dL; and
- An increase in the proportion of patients who initiate ESAs at a dosage consistent with product prescribing information.
Full implementation of the ESA REMS in 2011 had minimal impact on trends in these 3 ESA utilization metrics beyond the changes observed after the CMS coverage determination and multiple other FDA regulatory actions.
This information led the FDA to conclude that it was no longer necessary to require the certification of prescribers and hospitals that prescribe and/or dispense ESAs to patients with cancer in order to ensure that the benefits outweigh the risks.
The FDA has released the REMS requirements for the epoetin alfa and darbopoetin alfa ESA products, and the risks can be communicated by the current product prescribing information. The appropriate use of ESAs is supported by the CMS NCD, the American Society of Clinical Oncology, and American Society of Hematology clinical guidelines, which are evidence-based guidelines intended to provide a basis for the standard of care in clinical oncology.
Education
While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened OS and/or increased risk of tumor progression or recurrence associated with these drugs remain. The boxed warning language remains as follows: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE. Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Click here to read the digital edition.
Epoetin alfa and darbepoetin alfa are erythropoiesis-stimulating agents (ESAs), approved for the treatment of anemia (low red blood cells [RBCs]) resulting from chronic kidney disease, chemotherapy, and certain treatments for HIV. These ESAs also are used to reduce the number of blood transfusions during and after certain major surgeries. Erythropoiesis-stimulating agents work like the human protein erythropoietin, which stimulates bone marrow to make RBCs. Epoetin alfa (marketed as Procrit and Epogen) and darbepoetin alfa (marketed as Aranesp) are manufactured by Amgen, Inc. (Thousand Oaks, CA).
In 1989 epoetin alfa was approved for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 1993 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy. Epoetin alfa also is indicated for anemia due to zidovudine in patients with HIV and reduction of RBC transfusions during certain surgeries.
Darbepoetin alfa was approved in 2001 for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 2006 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy.
Risk Evaluation and Mitigation Strategies
Both epoetin alfa and darbepoetin alfa increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access and tumor progression or recurrence. Epoetin alfa also can lead to an increase in adverse cardiovascular events, hypertension, seizures, and severe anemia.
In 2008, the FDA determined that Risk Evaluation and Mitigation Strategies (REMS) were necessary for ESAs (darbopoetin alfa and epoetin alfa), to ensure that the benefits for use as treatment for anemia associated with myelosuppressive chemotherapy outweigh the risk of shortened overall survival (OS) and/or the increased risk of tumor progression or recurrence in patients with cancer. The REMS was approved in 2010.
Under the ESA REMS program, referred to as the ESA APPRISE Oncology Program, health care providers (HCPs) that prescribed and/or dispensed darbopoetin alfa to patients with cancer and hospitals that dispensed darbopoetin alfa to patients with cancer were required to enroll and become certified in the ESA REMS. The ESA REMS also required the completion of a Patient and Healthcare Provider Acknowledgement Form for each patient with cancer before the new ESA treatment course to ensure patients were counseled about the benefits and risks of these products.
In April 2017, the FDA determined that the ESA REMS that was limited to the use of epoetin alfa and darbopoetin alfa to treat patients with anemia due to associated myelosuppressive chemotherapy was no longer necessary; the benefits of ESAs outweighed the risks of shortened OS and/or increased risk of tumor progression or recurrence in patients with cancer. 1 The FDA recognized the burden that some REMS can place on HCPs and patients. The agency has authority to modify or remove the REMS to minimize the burden on the health care delivery system of complying with the strategy.
Data
The FDA discontinued the REMS based on an evaluation of the results of the REMS Assessments submitted by Amgen and additional FDA analyses to understand the impact of the various regulatory and other actions on the use of ESAs. The REMS Assessment showed the following:
- The results from surveyed prescribers demonstrated acceptable knowledge of the product risks of decreased survival and/or the increased risk of tumor progression or recurrence and the need to counsel patients about these risks; and
- The drug utilization data indicated appropriate prescribing of ESAs consistent with the intended use as a treatment alternative to RBC transfusion for anemia associated with myelosuppressive chemotherapy.
The FDA also conducted an evaluation of the impact of multiple actions, including the ESA REMS, on the use of the ESAs using sponsor-submitted data from outpatient oncology practices between 2006 and 2014. During 2004 to 2009, the FDA took multiple regulatory actions, including labeling changes. In 2007, the Center for Medicare and Medicaid Services (CMS) made a National Coverage Determination (NCD) to limit coverage of ESAs for nonrenal disease indications. These actions coincided with the following:
- A decrease in the proportion of patients receiving chemotherapy using ESAs;
- An increase in the proportion of patients receiving chemotherapy who initiate ESAs at a hemoglobin level < 10 g/dL; and
- An increase in the proportion of patients who initiate ESAs at a dosage consistent with product prescribing information.
Full implementation of the ESA REMS in 2011 had minimal impact on trends in these 3 ESA utilization metrics beyond the changes observed after the CMS coverage determination and multiple other FDA regulatory actions.
This information led the FDA to conclude that it was no longer necessary to require the certification of prescribers and hospitals that prescribe and/or dispense ESAs to patients with cancer in order to ensure that the benefits outweigh the risks.
The FDA has released the REMS requirements for the epoetin alfa and darbopoetin alfa ESA products, and the risks can be communicated by the current product prescribing information. The appropriate use of ESAs is supported by the CMS NCD, the American Society of Clinical Oncology, and American Society of Hematology clinical guidelines, which are evidence-based guidelines intended to provide a basis for the standard of care in clinical oncology.
Education
While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened OS and/or increased risk of tumor progression or recurrence associated with these drugs remain. The boxed warning language remains as follows: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE. Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Click here to read the digital edition.
1. U.S. Food & Drug Administration. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Updated April 13, 2017. Accessed July 13, 2017.
1. U.S. Food & Drug Administration. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Updated April 13, 2017. Accessed July 13, 2017.
Breast Implant Rupture After Radiation
The rupture rate for breast implants is about 10% at 10 years after insertion. That means women aged ≥ 70 years have a greater risk of rupture. For women who had breast augmentation or reconstruction before the advent of fifth-generation implants, there are no specific recommendations regarding follow-up and very little guidance in the literature about management for those who have had implants after radiation, say clinicians from Mayo Clinic.
They report on a 74-year-old patient who was treated for breast cancer in 1987 and 1988. She underwent lumpectomy, adjuvant unilateral radiation, a right simple mastectomy, left modified radical mastectomy, and implant-based reconstruction. Nearly 30 years later, she felt an asymmetry in 1 breast. Magnetic resonance imaging and ultrasound revealed that both implants had ruptured.
It is well known, the clinicians say, that complications of postmastectomy radiotherapy include capsular contracture, infection, and loss of prosthesis in implant-based reconstruction. Studies have shown that fibrosis, a hallmark of chronic radiation therapy, can show up even several years after radiotherapy—underscoring the importance of long-term follow-up for these patients. Moreover, the fact that the consequences of silicone on irradiated mastectomy flaps is unknown posed a further challenge.
While the cause of their patient’s implant rupture is unknown, the clinicians say it is “very likely” that delayed-onset fibrosis and capsular contracture secondary to radiation played a role. Such complications, though rare, should be kept in mind, the clinicians advise, when evaluating patients who had radiation and implants.
Source:
Molinar VE, Sabbagh MD, Manrique OJ. BMJ Case Rep. 2018; pii: bcr-2018-224578.
doi: 10.1136/bcr-2018-224578.
The rupture rate for breast implants is about 10% at 10 years after insertion. That means women aged ≥ 70 years have a greater risk of rupture. For women who had breast augmentation or reconstruction before the advent of fifth-generation implants, there are no specific recommendations regarding follow-up and very little guidance in the literature about management for those who have had implants after radiation, say clinicians from Mayo Clinic.
They report on a 74-year-old patient who was treated for breast cancer in 1987 and 1988. She underwent lumpectomy, adjuvant unilateral radiation, a right simple mastectomy, left modified radical mastectomy, and implant-based reconstruction. Nearly 30 years later, she felt an asymmetry in 1 breast. Magnetic resonance imaging and ultrasound revealed that both implants had ruptured.
It is well known, the clinicians say, that complications of postmastectomy radiotherapy include capsular contracture, infection, and loss of prosthesis in implant-based reconstruction. Studies have shown that fibrosis, a hallmark of chronic radiation therapy, can show up even several years after radiotherapy—underscoring the importance of long-term follow-up for these patients. Moreover, the fact that the consequences of silicone on irradiated mastectomy flaps is unknown posed a further challenge.
While the cause of their patient’s implant rupture is unknown, the clinicians say it is “very likely” that delayed-onset fibrosis and capsular contracture secondary to radiation played a role. Such complications, though rare, should be kept in mind, the clinicians advise, when evaluating patients who had radiation and implants.
Source:
Molinar VE, Sabbagh MD, Manrique OJ. BMJ Case Rep. 2018; pii: bcr-2018-224578.
doi: 10.1136/bcr-2018-224578.
The rupture rate for breast implants is about 10% at 10 years after insertion. That means women aged ≥ 70 years have a greater risk of rupture. For women who had breast augmentation or reconstruction before the advent of fifth-generation implants, there are no specific recommendations regarding follow-up and very little guidance in the literature about management for those who have had implants after radiation, say clinicians from Mayo Clinic.
They report on a 74-year-old patient who was treated for breast cancer in 1987 and 1988. She underwent lumpectomy, adjuvant unilateral radiation, a right simple mastectomy, left modified radical mastectomy, and implant-based reconstruction. Nearly 30 years later, she felt an asymmetry in 1 breast. Magnetic resonance imaging and ultrasound revealed that both implants had ruptured.
It is well known, the clinicians say, that complications of postmastectomy radiotherapy include capsular contracture, infection, and loss of prosthesis in implant-based reconstruction. Studies have shown that fibrosis, a hallmark of chronic radiation therapy, can show up even several years after radiotherapy—underscoring the importance of long-term follow-up for these patients. Moreover, the fact that the consequences of silicone on irradiated mastectomy flaps is unknown posed a further challenge.
While the cause of their patient’s implant rupture is unknown, the clinicians say it is “very likely” that delayed-onset fibrosis and capsular contracture secondary to radiation played a role. Such complications, though rare, should be kept in mind, the clinicians advise, when evaluating patients who had radiation and implants.
Source:
Molinar VE, Sabbagh MD, Manrique OJ. BMJ Case Rep. 2018; pii: bcr-2018-224578.
doi: 10.1136/bcr-2018-224578.
Investigators Describe the MS Prodrome
Patients who later develop MS are more likely than others to consult physicians for nervous system and genitourinary symptoms.
The prodrome of multiple sclerosis (MS) may include an increased risk of nervous system, sensory, and musculoskeletal disorders, according to research published online ahead of print July 1 in Multiple Sclerosis Journal. Patients who later develop MS also may be more likely to have genitourinary and psychiatric symptoms in the five years before diagnosis.
“The existence of such warning signs is well-accepted for Alzheimer’s disease and Parkinson’s disease, but there has been little investigation into a similar pattern for MS,” said Helen Tremlett, PhD, Professor in the Division of Neurology at the University of British Columbia in Canada. “We now need to delve deeper into this phenomenon, perhaps using data-mining techniques. We want to see if there are discernible patterns related to sex, age, or the type of MS they eventually develop.”
Clinical and Administrative Matched Cohorts
Dr. Tremlett and colleagues analyzed data from a matched-cohort record-linkage study to examine the MS prodrome. The investigators used population-based health administrative data and clinical data from the Canadian provinces of British Columbia, Saskatchewan, Manitoba, and Nova Scotia. The information included demographics, hospital visits, physician encounters, and prescriptions filled. Clinical data were for patients diagnosed by a neurologist at an MS clinic and included first clinical visit (or date of diagnosis) and date of symptom onset. Data were collected from April 1984 to April 2014.
Using the data, Dr. Tremlett and colleagues created a health-administrative cohort and a clinical cohort. The clinical cohort did not include data from Saskatchewan. To create the cohorts, the investigators identified patients with MS and matched them by sex, year of birth, and postal code with as many as five controls. The index date was the earliest recorded claim for a demyelinating disease for the health-administrative cohort and the date of MS symptom onset for the clinical cohort. Study outcomes were the number of physician and hospital encounters per ICD-10 chapter, the number of physician encounters per physician specialty, and the percentage of people with one or more prescriptions per drug class in the five years before the index date.
Clinical Cohort Results May Be More Accurate
The administrative cohort included 13,951 cases and 66,940 controls. The clinical cohort included 3,202 cases and 16,006 controls. Compared with controls, people with MS had more physician and hospital encounters for the nervous (rate ratio [RR], 2.31 to 4.75), sensory (RR, 1.40 to 2.28), musculoskeletal (RR, 1.19 to 1.70), and genitourinary systems (RR, 1.17 to 1.59) in the five years before the first demyelinating claim or symptom onset. Cases had more visits with psychiatrists and urologists (RR, 1.48 to 1.80) and higher proportions of musculoskeletal, genitourinary, or hormonal-related prescriptions (1.1–1.5 times higher), compared with controls. People with MS had fewer pregnancy-related encounters than controls, however (RR, 0.78 to 0.88).
The “more conservative” results for the clinical cohort are more likely to reflect the MS prodrome accurately because they are “unlikely to be influenced by a physician’s suspicion or consideration of MS,” said Dr. Tremlett and colleagues. “Although not all individuals with MS attend an MS specialty clinic, the clinical cohort represents a subgroup of the population that may differ with respect to demographic and clinical characteristics from nonclinic attendees (eg, have fewer comorbidities),” they added. NR
—Erik Greb
Suggested Reading
Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: Phenotyping the prodrome. Mult Scler. 2018 Jul 1 [Epub ahead of print].
Patients who later develop MS are more likely than others to consult physicians for nervous system and genitourinary symptoms.
Patients who later develop MS are more likely than others to consult physicians for nervous system and genitourinary symptoms.
The prodrome of multiple sclerosis (MS) may include an increased risk of nervous system, sensory, and musculoskeletal disorders, according to research published online ahead of print July 1 in Multiple Sclerosis Journal. Patients who later develop MS also may be more likely to have genitourinary and psychiatric symptoms in the five years before diagnosis.
“The existence of such warning signs is well-accepted for Alzheimer’s disease and Parkinson’s disease, but there has been little investigation into a similar pattern for MS,” said Helen Tremlett, PhD, Professor in the Division of Neurology at the University of British Columbia in Canada. “We now need to delve deeper into this phenomenon, perhaps using data-mining techniques. We want to see if there are discernible patterns related to sex, age, or the type of MS they eventually develop.”
Clinical and Administrative Matched Cohorts
Dr. Tremlett and colleagues analyzed data from a matched-cohort record-linkage study to examine the MS prodrome. The investigators used population-based health administrative data and clinical data from the Canadian provinces of British Columbia, Saskatchewan, Manitoba, and Nova Scotia. The information included demographics, hospital visits, physician encounters, and prescriptions filled. Clinical data were for patients diagnosed by a neurologist at an MS clinic and included first clinical visit (or date of diagnosis) and date of symptom onset. Data were collected from April 1984 to April 2014.
Using the data, Dr. Tremlett and colleagues created a health-administrative cohort and a clinical cohort. The clinical cohort did not include data from Saskatchewan. To create the cohorts, the investigators identified patients with MS and matched them by sex, year of birth, and postal code with as many as five controls. The index date was the earliest recorded claim for a demyelinating disease for the health-administrative cohort and the date of MS symptom onset for the clinical cohort. Study outcomes were the number of physician and hospital encounters per ICD-10 chapter, the number of physician encounters per physician specialty, and the percentage of people with one or more prescriptions per drug class in the five years before the index date.
Clinical Cohort Results May Be More Accurate
The administrative cohort included 13,951 cases and 66,940 controls. The clinical cohort included 3,202 cases and 16,006 controls. Compared with controls, people with MS had more physician and hospital encounters for the nervous (rate ratio [RR], 2.31 to 4.75), sensory (RR, 1.40 to 2.28), musculoskeletal (RR, 1.19 to 1.70), and genitourinary systems (RR, 1.17 to 1.59) in the five years before the first demyelinating claim or symptom onset. Cases had more visits with psychiatrists and urologists (RR, 1.48 to 1.80) and higher proportions of musculoskeletal, genitourinary, or hormonal-related prescriptions (1.1–1.5 times higher), compared with controls. People with MS had fewer pregnancy-related encounters than controls, however (RR, 0.78 to 0.88).
The “more conservative” results for the clinical cohort are more likely to reflect the MS prodrome accurately because they are “unlikely to be influenced by a physician’s suspicion or consideration of MS,” said Dr. Tremlett and colleagues. “Although not all individuals with MS attend an MS specialty clinic, the clinical cohort represents a subgroup of the population that may differ with respect to demographic and clinical characteristics from nonclinic attendees (eg, have fewer comorbidities),” they added. NR
—Erik Greb
Suggested Reading
Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: Phenotyping the prodrome. Mult Scler. 2018 Jul 1 [Epub ahead of print].
The prodrome of multiple sclerosis (MS) may include an increased risk of nervous system, sensory, and musculoskeletal disorders, according to research published online ahead of print July 1 in Multiple Sclerosis Journal. Patients who later develop MS also may be more likely to have genitourinary and psychiatric symptoms in the five years before diagnosis.
“The existence of such warning signs is well-accepted for Alzheimer’s disease and Parkinson’s disease, but there has been little investigation into a similar pattern for MS,” said Helen Tremlett, PhD, Professor in the Division of Neurology at the University of British Columbia in Canada. “We now need to delve deeper into this phenomenon, perhaps using data-mining techniques. We want to see if there are discernible patterns related to sex, age, or the type of MS they eventually develop.”
Clinical and Administrative Matched Cohorts
Dr. Tremlett and colleagues analyzed data from a matched-cohort record-linkage study to examine the MS prodrome. The investigators used population-based health administrative data and clinical data from the Canadian provinces of British Columbia, Saskatchewan, Manitoba, and Nova Scotia. The information included demographics, hospital visits, physician encounters, and prescriptions filled. Clinical data were for patients diagnosed by a neurologist at an MS clinic and included first clinical visit (or date of diagnosis) and date of symptom onset. Data were collected from April 1984 to April 2014.
Using the data, Dr. Tremlett and colleagues created a health-administrative cohort and a clinical cohort. The clinical cohort did not include data from Saskatchewan. To create the cohorts, the investigators identified patients with MS and matched them by sex, year of birth, and postal code with as many as five controls. The index date was the earliest recorded claim for a demyelinating disease for the health-administrative cohort and the date of MS symptom onset for the clinical cohort. Study outcomes were the number of physician and hospital encounters per ICD-10 chapter, the number of physician encounters per physician specialty, and the percentage of people with one or more prescriptions per drug class in the five years before the index date.
Clinical Cohort Results May Be More Accurate
The administrative cohort included 13,951 cases and 66,940 controls. The clinical cohort included 3,202 cases and 16,006 controls. Compared with controls, people with MS had more physician and hospital encounters for the nervous (rate ratio [RR], 2.31 to 4.75), sensory (RR, 1.40 to 2.28), musculoskeletal (RR, 1.19 to 1.70), and genitourinary systems (RR, 1.17 to 1.59) in the five years before the first demyelinating claim or symptom onset. Cases had more visits with psychiatrists and urologists (RR, 1.48 to 1.80) and higher proportions of musculoskeletal, genitourinary, or hormonal-related prescriptions (1.1–1.5 times higher), compared with controls. People with MS had fewer pregnancy-related encounters than controls, however (RR, 0.78 to 0.88).
The “more conservative” results for the clinical cohort are more likely to reflect the MS prodrome accurately because they are “unlikely to be influenced by a physician’s suspicion or consideration of MS,” said Dr. Tremlett and colleagues. “Although not all individuals with MS attend an MS specialty clinic, the clinical cohort represents a subgroup of the population that may differ with respect to demographic and clinical characteristics from nonclinic attendees (eg, have fewer comorbidities),” they added. NR
—Erik Greb
Suggested Reading
Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: Phenotyping the prodrome. Mult Scler. 2018 Jul 1 [Epub ahead of print].
Ketamine Plus Memantine-Based Multimodality Treatment of Chronic Refractory Migraine
Dr. Charles is Clinical Associate Professor Neurology, Rutgers–New Jersey Medical School, Newark, NJ; Neurology Attending, Holy Name Medical Center, Teaneck, NJ (jacharlesmd@gmail.com).
Dr. Gallo is Interventional Radiology Attending, Holy Name Medical Center, Teaneck, NJ (Vgallo83@gmail.com).
DISCLOSURES
The authors have no financial relationships to disclose relevant to the manuscript. There was no sponsorship of, or funding for, the study.
Dr. Charles designed and conceptualized the study; analyzed study data and performed the statistical analysis; and drafted the manuscript for intellectual content. Dr. Gallo had a major role in the acquisition of interventional sphenopalatine ganglion data.
ABSTRACT
Objective
Chronic refractory migraine patients who failed repetitive dihydroergotamine/dopamine infusion protocols and conventional preventives were treated with repeated low-dose ketamine-based parenteral protocols, followed by memantine-based preventive therapy, and observed for immediate reduction in pain intensity and headache frequency.
Methods
Ten patients were treated at an outpatient infusion center for 2 to 5 sequential days with AM and PM courses of intravenous diphenhydramine, prochlorperazine, and dihydroergotamine. A daily sphenopalatine ganglion block and low-dose intramuscular ketamine were given midday between treatments, with dexamethasone given on the last infusion day. The Numeric Pain Rating Scale was measured after infusion. Carryover effect was assessed 1 month and 2 months after infusion by headache frequency while being treated with memantine and various other preventive and abortive therapies.
Results
Reduction in headache pain of 71% was achieved at the end of the infusion period. Sedation was the only adverse effect. Decreased headache frequency persisted beyond the infusion period, with an 88.6% reduction in headache days per month at 1 month and a 79.4% reduction in headache days per month at 2 months, without adverse effects.
Conclusions
Data indicate that 1) repetitive low-dose, ketamine-based parenteral therapy, followed by memantine-based preventive therapy, reduced refractory headache pain and 2) the decremental effect on headache frequency persisted beyond the infusion period. Our results support the hypothesis that multimechanistic therapies might be better than single-modality treatment. More studies, with a larger patient population, are needed to confirm whether these multimodality ketamine/memantine therapies should become the preferred approach for these extremely disabled patients.
Chronic refractory migraine (CRM) degrades function and quality of life despite elimination of triggers and adequate trials of acute and preventive medicines that have established efficacy. This definition requires that patients with chronic migraine fail adequate trials of preventive drugs, alone or in combination, in at least 2 of 4 drug classes, including beta blockers, anticonvulsants, tricyclic antidepressants, onabotulinumtoxin A, and calcium-channel blockers. Patients must also fail adequate trials of abortive medicines, including both a triptan and dihydroergotamine (DHE), intranasal or injectable formulation, and either a nonsteroidal anti-inflammatory drug or a combination analgesic, unless contraindicated.1-4
In 1986, Raskin published a nonrandomized, nonblinded study of 2 treatments for intractable migraine in which repetitive inpatient intravenous (IV) DHE, administered in the hospital, was statistically more effective than IV diazepam in terminating cycles of intractable migraine.5 Most headache specialists have adopted the so-called Raskin protocol, as originally described or in any of several variations, as cornerstone therapy for CRM, chronic migraine, and prolonged status migrainosus.6 However, DHE-based infusion protocols do not always effectively reset the brain’s pain modulatory pathways in chronic migraine immediately posttreatment and might not induce a meaningful carryover effect.
We present 10 patients with CRM who met criteria for refractory migraine, including failure to terminate their headache with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols, with or without sporadic administration of a sphenopalatine ganglion block. We treated these patients multimechanistically with repetitive IV DHE, a dopamine antagonist, an antihistamine, sphenopalatine ganglion (SPG) block, and low-dose ketamine, plus last-infusion-day dexamethasone, followed by outpatient oral memantine. Subsequently, we observed them for 2 months.
Ketamine is a phencyclidine derivative introduced the early 1960s as an IV anesthetic. Low-dose ketamine has been used successfully in the treatment of chronic pain. Today, increased interest in the application of low-dose ketamine includes cancer pain; treatment and prevention of acute and chronic pain, with and without neuropathic analgesia; fibromyalgia; complex regional pain; and migraine.7,8 The effectiveness of ketamine in different pain disorders may arise through different pathways and/or by way of activity at various receptor systems. Effects arise predominantly by noncompetitive antagonism of the glutamate N-methyl-D-aspartate (NMDA ) receptor.7,8
Memantine also is an NMDA receptor antagonist that is used effectively as an oral agent in CRM.9
METHODS
Patients enrolled in this prospective study had CRM for periods ranging from 1 to 2 years. All had daily headache that could not be terminated with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols with or without sporadic administration of an SPG block. Age ranged from 18 and 68 years; all patients were female. Patients were excluded if they had known coronary artery disease, uncontrolled hypertension, or peripheral arterial disease; a history of stroke, transient ischemic attack, or pregnancy; impaired liver or renal function; smoked a tobacco product; or were taking a protease inhibitor or macrolide antibiotic.
Approval by the institutional review board was unnecessary because all drugs and procedures are FDA-approved and have published evidence-based efficacy for migraine and other diseases.
The Numeric Pain Rating Scale (NPRS; a scale of 0 to 10) was utilized to rate the intensity of pain from the beginning of the infusion to the end of the multiday infusion protocol, when the catheter was removed. All patients but 1 were treated for 5 days; for the 1 exception, treatment was terminated after 48 hours because of a scheduling conflict. The observational follow‐up periods for assessment of outcomes were 1 month and 2 months post-infusion.
Patients started the study with a baseline NPRS of 9 or 10. They were treated at the institution’s headache outpatient infusion center. In the morning, patients received, by sequential IV infusion, diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 1 mg. They then received a midday SPG block under fluoroscopic guidance and ketamine, 0.45 mg/kg intramuscularly (IM), given in the post-anesthesia care unit. In the late afternoon, the patients received diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 0.5 mg, in the Headache Outpatient Infusion Center. Patients were discharged to home by 6 PM. They received IV dexamethasone, 20 mg, on the last day of therapy.
Oral preventive agents were continued and abortives were temporarily discontinued during infusion therapy. Oral memantine was used immediately before, during, and, in all cases, after infusion, at a daily dosage that ranged from 10 mg BID to 28 mg, once-daily extended release.
RESULTS
Therapies were well-tolerated by all patients. On the last day of treatment, the entire cohort (N = 10) demonstrated an average of 71% (mean standard deviation [SD], 10.1%) reduction in pain intensity. The average reduction in headache days per month at 1 month was 88.6% (mean SD, 6.24%) and at 2 months was 79.4% (mean SD, 17.13%) (Table). Adverse effects were mild temporary sedation from ketamine. Pulse oximetry revealed no abnormal decrease in O2 saturation. All patients reported marked overall reduction in headache disability at the end of the infusion protocol. Self-administered abortive therapies posttreatment were more efficacious than they were pretreatment. All patients indicated less headache disability overall by the end of the 2-month observation period.
Table. Chronic Refractory Migraine Baseline Data and Treatment Resultsa
Name | Age (y) | Sex | Treatment Duration (days) | Baseline NPRS | Post-treatment NPRS | One Month Follow-upb | Two Month Follow-upb |
SL | 45 | F | 5 | 10 | 2 | 3 | 3 |
RR | 44 | F | 5 | 9 | 1 | 1 | 3 |
MP | 41 | F | 5 | 10 | 4 | 3 | 6 |
AP | 35 | F | 5 | 10 | 3 | 8 | 15 |
SW | 27 | F | 5 | 10 | 2 | 6 | 12 |
HC | 47 | F | 5 | 10 | 4 | 4 | 6 |
KK | 56 | F | 5 | 10 | 3 | 3 | 8 |
MG | 53 | F | 5 | 9 | 4 | 2 | 3 |
DM | 68 | F | 2 | 9 | 2 | 2 | 4 |
AO | 18 | F | 5 | 9 | 3 | 2 | 2 |
aAll patients had daily headache at initiation of treatment.
bHeadache days/month.
NPRS, Numeric Pain Rating Scale.
DISCUSSION
In our study of 10 patients with CRM who had daily headache treated repetitively in an outpatient infusion center with multimodality therapies, including sub-anesthetic doses of ketamine, all patients experienced marked reduction in headache pain intensity, with a whole-group average reduction of 71% by the end of infusion treatment. During post-infusion observation, all patients continued various preventive therapies, including memantine. At 1 month, the average reduction in headache frequency was 88.6%. Two months post-infusion, the average reduction in headache frequency was 79.4%. Adverse effects were minimal. Overall, the treatment was found to be safe and efficacious. All patients felt less headache disability after 2 months.
Because the protocol was administered comfortably in the Headache Outpatient Infusion Center, the inconvenience and higher cost of inpatient parenteral treatment were avoided. Ketamine, 0.45 mg/kg IM is a sub-anesthetic dose with proven efficacy in treating migraine without adverse effects in an outpatient setting.8 Low-dose ketamine obviated the need for anesthesia personnel and precautions. Temporary sedation was the only adverse effect. Ketamine was administered by a nurse in the post-anesthesia care unit while patients were under observation with conventional measurement of vital signs and pulse oximetry. Memantine, also an NMDA receptor antagonist, is postulated to prolong the NMDA antagonism of ketamine.
Inpatient and outpatient continuous IV DHE and repetitive IV DHE, often combined with dopamine antagonists in controlled and comparator studies, have demonstrated equal effectiveness for the treatment of chronic migraine.5,10,11 Our patients failed these therapies. This raises the question: Should our combined multimodality, ketamine-based approach be standard parenteral therapy for CRM?
In a recent study of continuous inpatient single-modality IV ketamine, a less-impressive carryover effect was obtained, with 23% to 50% 1-month sustained responders.12 Multimechanistic treatment superiority over monotherapy is legendary in the treatment of cancer and human immunodeficiency infection. Sumatriptan plus naproxen sodium as a single tablet for acute treatment of migraine resulted in more favorable clinical benefit compared with either monotherapy, with an acceptable, well-tolerated adverse effect profile. Because multiple pathogenic mechanisms putatively are involved in generation of the migraine symptom complex, multimechanism-targeted therapy may confer advantages over individual monotherapy. Drugs in 2 classes of migraine pharmacotherapy—triptans and nonsteroidal anti-inflammatory drugs —target distinct aspects of the vascular and inflammatory processes hypothesized to underlie migraine.13
Although combination therapy for CRM has not been systematically studied in randomized trials, clinical experience suggests that a rational approach to CRM treatment, utilizing a combination of treatments, may be effective when monotherapy has failed.14 During the infusion protocol, we re-set the trigeminovascular pain pathways 1) by repetitively blocking NMDA receptors (with ketamine), dopamine receptors (with prochlorperazine), and histamine receptors (with diphenhydramine); 2) by lidocaine anesthetic block of the sphenopalatine ganglia; and, on the last day of the protocol, 3) administering 1 large dose of IV dexamethasone to help prevent recurrence.15 NMDA blockade continued with oral outpatient memantine.
Virtually all patients were taking other preventives during the pretreatment period and 2-month observation period, including topiramate, venlafaxine, beta blockers, candesartan, zonisamide, onabotulinumtoxin A, neuromodulation (Cefaly Technology), and transcranial magnetic stimulation (springTMS®). Self-administered abortives were more effective in the 2-month observational period; these included IM/IV DHE; oral, spray, and subcutaneous triptans; IM ketorolac; diclofenac buffered solution; and transcranial magnetic stimulation (springTMS®). The cornerstone strategy of our treatment group that was a constant was the use of low-dose IM sub-anesthetic ketamine at a dosage of 0.45 mg/kg/d and the use of oral memantine during the follow-up observation period, at dosages ranging from 10 mg BID to 28 mg, once-daily extended release.
Limitations of this study design are:
- lack of a control group
- lack of subject randomization for comparative outcomes
- patients remaining on a variety of prophylactic regimens
- patients permitted to take any rescue therapy.
The effect of repetitive SPG block cannot be teased out of the efficacy data, but many of our patients had a poor or temporary response to infrequent sporadic SPG blocks prior to participating in our protocol.
Many migraineurs who seek care in a headache clinic are refractory to treatment, despite advances in headache therapy; refractory migraine was found in 5.1% of these patients.16 In this small series of patients, we demonstrated immediate relief and a significant 2-month carryover effect with our multimodality parenteral protocol. Larger, controlled studies are needed to further explore this protocol with repetitive DHE, diphenhydramine, prochlorperazine, SPG block, and low-dose IM ketamine, followed by outpatient memantine. Such studies would determine whether our protocol should be utilized as a primary treatment, instead of the conventional DHE-based Raskin and modified Raskin protocols.
Although this is a small series of patients, lack of adverse effects and impressive results should give credence to utilizing our protocol as treatment for this extremely debilitated, often desperate subset of headache patients. Data indicate that, whereas ketamine combined with other therapies immediately reduced refractory headache pain, the ameliorating effect of ketamine on CRM headache frequency and pain in our protocol persisted beyond the infusion period. This phenomenon indicates a disease-modulating role for ketamine in refractory migraine pain, possibly by means of desensitization of NMDA receptors in the trigeminal nucleus caudalis—desensitization that continued with the NMDA receptor antagonist memantine and/or restoration of inhibitory sensory control in the brain.
CONCLUSION
Our results support the hypothesis that multimechanistic therapies, including low-dose IM ketamine and memantine, might be better than single-modality treatment in this debilitated, refractory population. Future studies, with larger patient populations, are needed to confirm whether these multimodality ketamine/memantine-inclusive therapies should become the preferred approach for these extremely disabled patients.
REFERENCES
1. Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick DW. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168-1170.
2. Schulman EA, Lipton R, Peterlin BL, Levin M, Grosberg BM. Commentary from the Refractory Headache Special Interest Section on defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(10):1637-1639.
3. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
4. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921-936.
5. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995‐997.
6. Charles JA, von Dohln P. Outpatient home-based continuous intravenous dihydroergotamine therapy for intractable migraine. Headache. 2010;50(5):852-860.
7. Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in complex regional pain syndrome type 1 patients. Eur J Pain. 2010;14(3):302-307.
8. Krusz J, Cagle J, Hall S. Intramuscular (IM) ketamine for treating headache and pain flare-ups in the clinic. J Pain. 2008;9(4):30.
9. Bigal M Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337-1342.
10. Ford RG, Ford KT. Continuous intravenous dihydroergotamine for treatment of intractable headache. Headache. 1997;37(3):129‐136.
11. Boudreau G, Aghai E, Marchand L, Langlois M. Outpatient intravenous dihydroergotamine for probable medication overuse headache. Headache Care. 2006;3(1):45‐49.
12. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
13. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
14. Peterlin BL, Calhoun AH, Siegel S, Mathew NT. Rational combination therapy in refractory migraine. Headache. 2008;48(6):805-819.
15. Innes G, Macphail I, Dillon EC, Metcalfe C, Gao M. Dexamethasone prevents relapse after emergency department treatment of acute migraine: a randomized clinical trial. CJEM. 2015;1(1):26-33.
16. Irimia P, Palma JA, Fernandez-Torron R, Martinez-Vila E. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.
Dr. Charles is Clinical Associate Professor Neurology, Rutgers–New Jersey Medical School, Newark, NJ; Neurology Attending, Holy Name Medical Center, Teaneck, NJ (jacharlesmd@gmail.com).
Dr. Gallo is Interventional Radiology Attending, Holy Name Medical Center, Teaneck, NJ (Vgallo83@gmail.com).
DISCLOSURES
The authors have no financial relationships to disclose relevant to the manuscript. There was no sponsorship of, or funding for, the study.
Dr. Charles designed and conceptualized the study; analyzed study data and performed the statistical analysis; and drafted the manuscript for intellectual content. Dr. Gallo had a major role in the acquisition of interventional sphenopalatine ganglion data.
ABSTRACT
Objective
Chronic refractory migraine patients who failed repetitive dihydroergotamine/dopamine infusion protocols and conventional preventives were treated with repeated low-dose ketamine-based parenteral protocols, followed by memantine-based preventive therapy, and observed for immediate reduction in pain intensity and headache frequency.
Methods
Ten patients were treated at an outpatient infusion center for 2 to 5 sequential days with AM and PM courses of intravenous diphenhydramine, prochlorperazine, and dihydroergotamine. A daily sphenopalatine ganglion block and low-dose intramuscular ketamine were given midday between treatments, with dexamethasone given on the last infusion day. The Numeric Pain Rating Scale was measured after infusion. Carryover effect was assessed 1 month and 2 months after infusion by headache frequency while being treated with memantine and various other preventive and abortive therapies.
Results
Reduction in headache pain of 71% was achieved at the end of the infusion period. Sedation was the only adverse effect. Decreased headache frequency persisted beyond the infusion period, with an 88.6% reduction in headache days per month at 1 month and a 79.4% reduction in headache days per month at 2 months, without adverse effects.
Conclusions
Data indicate that 1) repetitive low-dose, ketamine-based parenteral therapy, followed by memantine-based preventive therapy, reduced refractory headache pain and 2) the decremental effect on headache frequency persisted beyond the infusion period. Our results support the hypothesis that multimechanistic therapies might be better than single-modality treatment. More studies, with a larger patient population, are needed to confirm whether these multimodality ketamine/memantine therapies should become the preferred approach for these extremely disabled patients.
Chronic refractory migraine (CRM) degrades function and quality of life despite elimination of triggers and adequate trials of acute and preventive medicines that have established efficacy. This definition requires that patients with chronic migraine fail adequate trials of preventive drugs, alone or in combination, in at least 2 of 4 drug classes, including beta blockers, anticonvulsants, tricyclic antidepressants, onabotulinumtoxin A, and calcium-channel blockers. Patients must also fail adequate trials of abortive medicines, including both a triptan and dihydroergotamine (DHE), intranasal or injectable formulation, and either a nonsteroidal anti-inflammatory drug or a combination analgesic, unless contraindicated.1-4
In 1986, Raskin published a nonrandomized, nonblinded study of 2 treatments for intractable migraine in which repetitive inpatient intravenous (IV) DHE, administered in the hospital, was statistically more effective than IV diazepam in terminating cycles of intractable migraine.5 Most headache specialists have adopted the so-called Raskin protocol, as originally described or in any of several variations, as cornerstone therapy for CRM, chronic migraine, and prolonged status migrainosus.6 However, DHE-based infusion protocols do not always effectively reset the brain’s pain modulatory pathways in chronic migraine immediately posttreatment and might not induce a meaningful carryover effect.
We present 10 patients with CRM who met criteria for refractory migraine, including failure to terminate their headache with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols, with or without sporadic administration of a sphenopalatine ganglion block. We treated these patients multimechanistically with repetitive IV DHE, a dopamine antagonist, an antihistamine, sphenopalatine ganglion (SPG) block, and low-dose ketamine, plus last-infusion-day dexamethasone, followed by outpatient oral memantine. Subsequently, we observed them for 2 months.
Ketamine is a phencyclidine derivative introduced the early 1960s as an IV anesthetic. Low-dose ketamine has been used successfully in the treatment of chronic pain. Today, increased interest in the application of low-dose ketamine includes cancer pain; treatment and prevention of acute and chronic pain, with and without neuropathic analgesia; fibromyalgia; complex regional pain; and migraine.7,8 The effectiveness of ketamine in different pain disorders may arise through different pathways and/or by way of activity at various receptor systems. Effects arise predominantly by noncompetitive antagonism of the glutamate N-methyl-D-aspartate (NMDA ) receptor.7,8
Memantine also is an NMDA receptor antagonist that is used effectively as an oral agent in CRM.9
METHODS
Patients enrolled in this prospective study had CRM for periods ranging from 1 to 2 years. All had daily headache that could not be terminated with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols with or without sporadic administration of an SPG block. Age ranged from 18 and 68 years; all patients were female. Patients were excluded if they had known coronary artery disease, uncontrolled hypertension, or peripheral arterial disease; a history of stroke, transient ischemic attack, or pregnancy; impaired liver or renal function; smoked a tobacco product; or were taking a protease inhibitor or macrolide antibiotic.
Approval by the institutional review board was unnecessary because all drugs and procedures are FDA-approved and have published evidence-based efficacy for migraine and other diseases.
The Numeric Pain Rating Scale (NPRS; a scale of 0 to 10) was utilized to rate the intensity of pain from the beginning of the infusion to the end of the multiday infusion protocol, when the catheter was removed. All patients but 1 were treated for 5 days; for the 1 exception, treatment was terminated after 48 hours because of a scheduling conflict. The observational follow‐up periods for assessment of outcomes were 1 month and 2 months post-infusion.
Patients started the study with a baseline NPRS of 9 or 10. They were treated at the institution’s headache outpatient infusion center. In the morning, patients received, by sequential IV infusion, diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 1 mg. They then received a midday SPG block under fluoroscopic guidance and ketamine, 0.45 mg/kg intramuscularly (IM), given in the post-anesthesia care unit. In the late afternoon, the patients received diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 0.5 mg, in the Headache Outpatient Infusion Center. Patients were discharged to home by 6 PM. They received IV dexamethasone, 20 mg, on the last day of therapy.
Oral preventive agents were continued and abortives were temporarily discontinued during infusion therapy. Oral memantine was used immediately before, during, and, in all cases, after infusion, at a daily dosage that ranged from 10 mg BID to 28 mg, once-daily extended release.
RESULTS
Therapies were well-tolerated by all patients. On the last day of treatment, the entire cohort (N = 10) demonstrated an average of 71% (mean standard deviation [SD], 10.1%) reduction in pain intensity. The average reduction in headache days per month at 1 month was 88.6% (mean SD, 6.24%) and at 2 months was 79.4% (mean SD, 17.13%) (Table). Adverse effects were mild temporary sedation from ketamine. Pulse oximetry revealed no abnormal decrease in O2 saturation. All patients reported marked overall reduction in headache disability at the end of the infusion protocol. Self-administered abortive therapies posttreatment were more efficacious than they were pretreatment. All patients indicated less headache disability overall by the end of the 2-month observation period.
Table. Chronic Refractory Migraine Baseline Data and Treatment Resultsa
Name | Age (y) | Sex | Treatment Duration (days) | Baseline NPRS | Post-treatment NPRS | One Month Follow-upb | Two Month Follow-upb |
SL | 45 | F | 5 | 10 | 2 | 3 | 3 |
RR | 44 | F | 5 | 9 | 1 | 1 | 3 |
MP | 41 | F | 5 | 10 | 4 | 3 | 6 |
AP | 35 | F | 5 | 10 | 3 | 8 | 15 |
SW | 27 | F | 5 | 10 | 2 | 6 | 12 |
HC | 47 | F | 5 | 10 | 4 | 4 | 6 |
KK | 56 | F | 5 | 10 | 3 | 3 | 8 |
MG | 53 | F | 5 | 9 | 4 | 2 | 3 |
DM | 68 | F | 2 | 9 | 2 | 2 | 4 |
AO | 18 | F | 5 | 9 | 3 | 2 | 2 |
aAll patients had daily headache at initiation of treatment.
bHeadache days/month.
NPRS, Numeric Pain Rating Scale.
DISCUSSION
In our study of 10 patients with CRM who had daily headache treated repetitively in an outpatient infusion center with multimodality therapies, including sub-anesthetic doses of ketamine, all patients experienced marked reduction in headache pain intensity, with a whole-group average reduction of 71% by the end of infusion treatment. During post-infusion observation, all patients continued various preventive therapies, including memantine. At 1 month, the average reduction in headache frequency was 88.6%. Two months post-infusion, the average reduction in headache frequency was 79.4%. Adverse effects were minimal. Overall, the treatment was found to be safe and efficacious. All patients felt less headache disability after 2 months.
Because the protocol was administered comfortably in the Headache Outpatient Infusion Center, the inconvenience and higher cost of inpatient parenteral treatment were avoided. Ketamine, 0.45 mg/kg IM is a sub-anesthetic dose with proven efficacy in treating migraine without adverse effects in an outpatient setting.8 Low-dose ketamine obviated the need for anesthesia personnel and precautions. Temporary sedation was the only adverse effect. Ketamine was administered by a nurse in the post-anesthesia care unit while patients were under observation with conventional measurement of vital signs and pulse oximetry. Memantine, also an NMDA receptor antagonist, is postulated to prolong the NMDA antagonism of ketamine.
Inpatient and outpatient continuous IV DHE and repetitive IV DHE, often combined with dopamine antagonists in controlled and comparator studies, have demonstrated equal effectiveness for the treatment of chronic migraine.5,10,11 Our patients failed these therapies. This raises the question: Should our combined multimodality, ketamine-based approach be standard parenteral therapy for CRM?
In a recent study of continuous inpatient single-modality IV ketamine, a less-impressive carryover effect was obtained, with 23% to 50% 1-month sustained responders.12 Multimechanistic treatment superiority over monotherapy is legendary in the treatment of cancer and human immunodeficiency infection. Sumatriptan plus naproxen sodium as a single tablet for acute treatment of migraine resulted in more favorable clinical benefit compared with either monotherapy, with an acceptable, well-tolerated adverse effect profile. Because multiple pathogenic mechanisms putatively are involved in generation of the migraine symptom complex, multimechanism-targeted therapy may confer advantages over individual monotherapy. Drugs in 2 classes of migraine pharmacotherapy—triptans and nonsteroidal anti-inflammatory drugs —target distinct aspects of the vascular and inflammatory processes hypothesized to underlie migraine.13
Although combination therapy for CRM has not been systematically studied in randomized trials, clinical experience suggests that a rational approach to CRM treatment, utilizing a combination of treatments, may be effective when monotherapy has failed.14 During the infusion protocol, we re-set the trigeminovascular pain pathways 1) by repetitively blocking NMDA receptors (with ketamine), dopamine receptors (with prochlorperazine), and histamine receptors (with diphenhydramine); 2) by lidocaine anesthetic block of the sphenopalatine ganglia; and, on the last day of the protocol, 3) administering 1 large dose of IV dexamethasone to help prevent recurrence.15 NMDA blockade continued with oral outpatient memantine.
Virtually all patients were taking other preventives during the pretreatment period and 2-month observation period, including topiramate, venlafaxine, beta blockers, candesartan, zonisamide, onabotulinumtoxin A, neuromodulation (Cefaly Technology), and transcranial magnetic stimulation (springTMS®). Self-administered abortives were more effective in the 2-month observational period; these included IM/IV DHE; oral, spray, and subcutaneous triptans; IM ketorolac; diclofenac buffered solution; and transcranial magnetic stimulation (springTMS®). The cornerstone strategy of our treatment group that was a constant was the use of low-dose IM sub-anesthetic ketamine at a dosage of 0.45 mg/kg/d and the use of oral memantine during the follow-up observation period, at dosages ranging from 10 mg BID to 28 mg, once-daily extended release.
Limitations of this study design are:
- lack of a control group
- lack of subject randomization for comparative outcomes
- patients remaining on a variety of prophylactic regimens
- patients permitted to take any rescue therapy.
The effect of repetitive SPG block cannot be teased out of the efficacy data, but many of our patients had a poor or temporary response to infrequent sporadic SPG blocks prior to participating in our protocol.
Many migraineurs who seek care in a headache clinic are refractory to treatment, despite advances in headache therapy; refractory migraine was found in 5.1% of these patients.16 In this small series of patients, we demonstrated immediate relief and a significant 2-month carryover effect with our multimodality parenteral protocol. Larger, controlled studies are needed to further explore this protocol with repetitive DHE, diphenhydramine, prochlorperazine, SPG block, and low-dose IM ketamine, followed by outpatient memantine. Such studies would determine whether our protocol should be utilized as a primary treatment, instead of the conventional DHE-based Raskin and modified Raskin protocols.
Although this is a small series of patients, lack of adverse effects and impressive results should give credence to utilizing our protocol as treatment for this extremely debilitated, often desperate subset of headache patients. Data indicate that, whereas ketamine combined with other therapies immediately reduced refractory headache pain, the ameliorating effect of ketamine on CRM headache frequency and pain in our protocol persisted beyond the infusion period. This phenomenon indicates a disease-modulating role for ketamine in refractory migraine pain, possibly by means of desensitization of NMDA receptors in the trigeminal nucleus caudalis—desensitization that continued with the NMDA receptor antagonist memantine and/or restoration of inhibitory sensory control in the brain.
CONCLUSION
Our results support the hypothesis that multimechanistic therapies, including low-dose IM ketamine and memantine, might be better than single-modality treatment in this debilitated, refractory population. Future studies, with larger patient populations, are needed to confirm whether these multimodality ketamine/memantine-inclusive therapies should become the preferred approach for these extremely disabled patients.
REFERENCES
1. Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick DW. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168-1170.
2. Schulman EA, Lipton R, Peterlin BL, Levin M, Grosberg BM. Commentary from the Refractory Headache Special Interest Section on defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(10):1637-1639.
3. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
4. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921-936.
5. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995‐997.
6. Charles JA, von Dohln P. Outpatient home-based continuous intravenous dihydroergotamine therapy for intractable migraine. Headache. 2010;50(5):852-860.
7. Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in complex regional pain syndrome type 1 patients. Eur J Pain. 2010;14(3):302-307.
8. Krusz J, Cagle J, Hall S. Intramuscular (IM) ketamine for treating headache and pain flare-ups in the clinic. J Pain. 2008;9(4):30.
9. Bigal M Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337-1342.
10. Ford RG, Ford KT. Continuous intravenous dihydroergotamine for treatment of intractable headache. Headache. 1997;37(3):129‐136.
11. Boudreau G, Aghai E, Marchand L, Langlois M. Outpatient intravenous dihydroergotamine for probable medication overuse headache. Headache Care. 2006;3(1):45‐49.
12. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
13. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
14. Peterlin BL, Calhoun AH, Siegel S, Mathew NT. Rational combination therapy in refractory migraine. Headache. 2008;48(6):805-819.
15. Innes G, Macphail I, Dillon EC, Metcalfe C, Gao M. Dexamethasone prevents relapse after emergency department treatment of acute migraine: a randomized clinical trial. CJEM. 2015;1(1):26-33.
16. Irimia P, Palma JA, Fernandez-Torron R, Martinez-Vila E. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.
Dr. Charles is Clinical Associate Professor Neurology, Rutgers–New Jersey Medical School, Newark, NJ; Neurology Attending, Holy Name Medical Center, Teaneck, NJ (jacharlesmd@gmail.com).
Dr. Gallo is Interventional Radiology Attending, Holy Name Medical Center, Teaneck, NJ (Vgallo83@gmail.com).
DISCLOSURES
The authors have no financial relationships to disclose relevant to the manuscript. There was no sponsorship of, or funding for, the study.
Dr. Charles designed and conceptualized the study; analyzed study data and performed the statistical analysis; and drafted the manuscript for intellectual content. Dr. Gallo had a major role in the acquisition of interventional sphenopalatine ganglion data.
ABSTRACT
Objective
Chronic refractory migraine patients who failed repetitive dihydroergotamine/dopamine infusion protocols and conventional preventives were treated with repeated low-dose ketamine-based parenteral protocols, followed by memantine-based preventive therapy, and observed for immediate reduction in pain intensity and headache frequency.
Methods
Ten patients were treated at an outpatient infusion center for 2 to 5 sequential days with AM and PM courses of intravenous diphenhydramine, prochlorperazine, and dihydroergotamine. A daily sphenopalatine ganglion block and low-dose intramuscular ketamine were given midday between treatments, with dexamethasone given on the last infusion day. The Numeric Pain Rating Scale was measured after infusion. Carryover effect was assessed 1 month and 2 months after infusion by headache frequency while being treated with memantine and various other preventive and abortive therapies.
Results
Reduction in headache pain of 71% was achieved at the end of the infusion period. Sedation was the only adverse effect. Decreased headache frequency persisted beyond the infusion period, with an 88.6% reduction in headache days per month at 1 month and a 79.4% reduction in headache days per month at 2 months, without adverse effects.
Conclusions
Data indicate that 1) repetitive low-dose, ketamine-based parenteral therapy, followed by memantine-based preventive therapy, reduced refractory headache pain and 2) the decremental effect on headache frequency persisted beyond the infusion period. Our results support the hypothesis that multimechanistic therapies might be better than single-modality treatment. More studies, with a larger patient population, are needed to confirm whether these multimodality ketamine/memantine therapies should become the preferred approach for these extremely disabled patients.
Chronic refractory migraine (CRM) degrades function and quality of life despite elimination of triggers and adequate trials of acute and preventive medicines that have established efficacy. This definition requires that patients with chronic migraine fail adequate trials of preventive drugs, alone or in combination, in at least 2 of 4 drug classes, including beta blockers, anticonvulsants, tricyclic antidepressants, onabotulinumtoxin A, and calcium-channel blockers. Patients must also fail adequate trials of abortive medicines, including both a triptan and dihydroergotamine (DHE), intranasal or injectable formulation, and either a nonsteroidal anti-inflammatory drug or a combination analgesic, unless contraindicated.1-4
In 1986, Raskin published a nonrandomized, nonblinded study of 2 treatments for intractable migraine in which repetitive inpatient intravenous (IV) DHE, administered in the hospital, was statistically more effective than IV diazepam in terminating cycles of intractable migraine.5 Most headache specialists have adopted the so-called Raskin protocol, as originally described or in any of several variations, as cornerstone therapy for CRM, chronic migraine, and prolonged status migrainosus.6 However, DHE-based infusion protocols do not always effectively reset the brain’s pain modulatory pathways in chronic migraine immediately posttreatment and might not induce a meaningful carryover effect.
We present 10 patients with CRM who met criteria for refractory migraine, including failure to terminate their headache with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols, with or without sporadic administration of a sphenopalatine ganglion block. We treated these patients multimechanistically with repetitive IV DHE, a dopamine antagonist, an antihistamine, sphenopalatine ganglion (SPG) block, and low-dose ketamine, plus last-infusion-day dexamethasone, followed by outpatient oral memantine. Subsequently, we observed them for 2 months.
Ketamine is a phencyclidine derivative introduced the early 1960s as an IV anesthetic. Low-dose ketamine has been used successfully in the treatment of chronic pain. Today, increased interest in the application of low-dose ketamine includes cancer pain; treatment and prevention of acute and chronic pain, with and without neuropathic analgesia; fibromyalgia; complex regional pain; and migraine.7,8 The effectiveness of ketamine in different pain disorders may arise through different pathways and/or by way of activity at various receptor systems. Effects arise predominantly by noncompetitive antagonism of the glutamate N-methyl-D-aspartate (NMDA ) receptor.7,8
Memantine also is an NMDA receptor antagonist that is used effectively as an oral agent in CRM.9
METHODS
Patients enrolled in this prospective study had CRM for periods ranging from 1 to 2 years. All had daily headache that could not be terminated with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols with or without sporadic administration of an SPG block. Age ranged from 18 and 68 years; all patients were female. Patients were excluded if they had known coronary artery disease, uncontrolled hypertension, or peripheral arterial disease; a history of stroke, transient ischemic attack, or pregnancy; impaired liver or renal function; smoked a tobacco product; or were taking a protease inhibitor or macrolide antibiotic.
Approval by the institutional review board was unnecessary because all drugs and procedures are FDA-approved and have published evidence-based efficacy for migraine and other diseases.
The Numeric Pain Rating Scale (NPRS; a scale of 0 to 10) was utilized to rate the intensity of pain from the beginning of the infusion to the end of the multiday infusion protocol, when the catheter was removed. All patients but 1 were treated for 5 days; for the 1 exception, treatment was terminated after 48 hours because of a scheduling conflict. The observational follow‐up periods for assessment of outcomes were 1 month and 2 months post-infusion.
Patients started the study with a baseline NPRS of 9 or 10. They were treated at the institution’s headache outpatient infusion center. In the morning, patients received, by sequential IV infusion, diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 1 mg. They then received a midday SPG block under fluoroscopic guidance and ketamine, 0.45 mg/kg intramuscularly (IM), given in the post-anesthesia care unit. In the late afternoon, the patients received diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 0.5 mg, in the Headache Outpatient Infusion Center. Patients were discharged to home by 6 PM. They received IV dexamethasone, 20 mg, on the last day of therapy.
Oral preventive agents were continued and abortives were temporarily discontinued during infusion therapy. Oral memantine was used immediately before, during, and, in all cases, after infusion, at a daily dosage that ranged from 10 mg BID to 28 mg, once-daily extended release.
RESULTS
Therapies were well-tolerated by all patients. On the last day of treatment, the entire cohort (N = 10) demonstrated an average of 71% (mean standard deviation [SD], 10.1%) reduction in pain intensity. The average reduction in headache days per month at 1 month was 88.6% (mean SD, 6.24%) and at 2 months was 79.4% (mean SD, 17.13%) (Table). Adverse effects were mild temporary sedation from ketamine. Pulse oximetry revealed no abnormal decrease in O2 saturation. All patients reported marked overall reduction in headache disability at the end of the infusion protocol. Self-administered abortive therapies posttreatment were more efficacious than they were pretreatment. All patients indicated less headache disability overall by the end of the 2-month observation period.
Table. Chronic Refractory Migraine Baseline Data and Treatment Resultsa
Name | Age (y) | Sex | Treatment Duration (days) | Baseline NPRS | Post-treatment NPRS | One Month Follow-upb | Two Month Follow-upb |
SL | 45 | F | 5 | 10 | 2 | 3 | 3 |
RR | 44 | F | 5 | 9 | 1 | 1 | 3 |
MP | 41 | F | 5 | 10 | 4 | 3 | 6 |
AP | 35 | F | 5 | 10 | 3 | 8 | 15 |
SW | 27 | F | 5 | 10 | 2 | 6 | 12 |
HC | 47 | F | 5 | 10 | 4 | 4 | 6 |
KK | 56 | F | 5 | 10 | 3 | 3 | 8 |
MG | 53 | F | 5 | 9 | 4 | 2 | 3 |
DM | 68 | F | 2 | 9 | 2 | 2 | 4 |
AO | 18 | F | 5 | 9 | 3 | 2 | 2 |
aAll patients had daily headache at initiation of treatment.
bHeadache days/month.
NPRS, Numeric Pain Rating Scale.
DISCUSSION
In our study of 10 patients with CRM who had daily headache treated repetitively in an outpatient infusion center with multimodality therapies, including sub-anesthetic doses of ketamine, all patients experienced marked reduction in headache pain intensity, with a whole-group average reduction of 71% by the end of infusion treatment. During post-infusion observation, all patients continued various preventive therapies, including memantine. At 1 month, the average reduction in headache frequency was 88.6%. Two months post-infusion, the average reduction in headache frequency was 79.4%. Adverse effects were minimal. Overall, the treatment was found to be safe and efficacious. All patients felt less headache disability after 2 months.
Because the protocol was administered comfortably in the Headache Outpatient Infusion Center, the inconvenience and higher cost of inpatient parenteral treatment were avoided. Ketamine, 0.45 mg/kg IM is a sub-anesthetic dose with proven efficacy in treating migraine without adverse effects in an outpatient setting.8 Low-dose ketamine obviated the need for anesthesia personnel and precautions. Temporary sedation was the only adverse effect. Ketamine was administered by a nurse in the post-anesthesia care unit while patients were under observation with conventional measurement of vital signs and pulse oximetry. Memantine, also an NMDA receptor antagonist, is postulated to prolong the NMDA antagonism of ketamine.
Inpatient and outpatient continuous IV DHE and repetitive IV DHE, often combined with dopamine antagonists in controlled and comparator studies, have demonstrated equal effectiveness for the treatment of chronic migraine.5,10,11 Our patients failed these therapies. This raises the question: Should our combined multimodality, ketamine-based approach be standard parenteral therapy for CRM?
In a recent study of continuous inpatient single-modality IV ketamine, a less-impressive carryover effect was obtained, with 23% to 50% 1-month sustained responders.12 Multimechanistic treatment superiority over monotherapy is legendary in the treatment of cancer and human immunodeficiency infection. Sumatriptan plus naproxen sodium as a single tablet for acute treatment of migraine resulted in more favorable clinical benefit compared with either monotherapy, with an acceptable, well-tolerated adverse effect profile. Because multiple pathogenic mechanisms putatively are involved in generation of the migraine symptom complex, multimechanism-targeted therapy may confer advantages over individual monotherapy. Drugs in 2 classes of migraine pharmacotherapy—triptans and nonsteroidal anti-inflammatory drugs —target distinct aspects of the vascular and inflammatory processes hypothesized to underlie migraine.13
Although combination therapy for CRM has not been systematically studied in randomized trials, clinical experience suggests that a rational approach to CRM treatment, utilizing a combination of treatments, may be effective when monotherapy has failed.14 During the infusion protocol, we re-set the trigeminovascular pain pathways 1) by repetitively blocking NMDA receptors (with ketamine), dopamine receptors (with prochlorperazine), and histamine receptors (with diphenhydramine); 2) by lidocaine anesthetic block of the sphenopalatine ganglia; and, on the last day of the protocol, 3) administering 1 large dose of IV dexamethasone to help prevent recurrence.15 NMDA blockade continued with oral outpatient memantine.
Virtually all patients were taking other preventives during the pretreatment period and 2-month observation period, including topiramate, venlafaxine, beta blockers, candesartan, zonisamide, onabotulinumtoxin A, neuromodulation (Cefaly Technology), and transcranial magnetic stimulation (springTMS®). Self-administered abortives were more effective in the 2-month observational period; these included IM/IV DHE; oral, spray, and subcutaneous triptans; IM ketorolac; diclofenac buffered solution; and transcranial magnetic stimulation (springTMS®). The cornerstone strategy of our treatment group that was a constant was the use of low-dose IM sub-anesthetic ketamine at a dosage of 0.45 mg/kg/d and the use of oral memantine during the follow-up observation period, at dosages ranging from 10 mg BID to 28 mg, once-daily extended release.
Limitations of this study design are:
- lack of a control group
- lack of subject randomization for comparative outcomes
- patients remaining on a variety of prophylactic regimens
- patients permitted to take any rescue therapy.
The effect of repetitive SPG block cannot be teased out of the efficacy data, but many of our patients had a poor or temporary response to infrequent sporadic SPG blocks prior to participating in our protocol.
Many migraineurs who seek care in a headache clinic are refractory to treatment, despite advances in headache therapy; refractory migraine was found in 5.1% of these patients.16 In this small series of patients, we demonstrated immediate relief and a significant 2-month carryover effect with our multimodality parenteral protocol. Larger, controlled studies are needed to further explore this protocol with repetitive DHE, diphenhydramine, prochlorperazine, SPG block, and low-dose IM ketamine, followed by outpatient memantine. Such studies would determine whether our protocol should be utilized as a primary treatment, instead of the conventional DHE-based Raskin and modified Raskin protocols.
Although this is a small series of patients, lack of adverse effects and impressive results should give credence to utilizing our protocol as treatment for this extremely debilitated, often desperate subset of headache patients. Data indicate that, whereas ketamine combined with other therapies immediately reduced refractory headache pain, the ameliorating effect of ketamine on CRM headache frequency and pain in our protocol persisted beyond the infusion period. This phenomenon indicates a disease-modulating role for ketamine in refractory migraine pain, possibly by means of desensitization of NMDA receptors in the trigeminal nucleus caudalis—desensitization that continued with the NMDA receptor antagonist memantine and/or restoration of inhibitory sensory control in the brain.
CONCLUSION
Our results support the hypothesis that multimechanistic therapies, including low-dose IM ketamine and memantine, might be better than single-modality treatment in this debilitated, refractory population. Future studies, with larger patient populations, are needed to confirm whether these multimodality ketamine/memantine-inclusive therapies should become the preferred approach for these extremely disabled patients.
REFERENCES
1. Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick DW. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168-1170.
2. Schulman EA, Lipton R, Peterlin BL, Levin M, Grosberg BM. Commentary from the Refractory Headache Special Interest Section on defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(10):1637-1639.
3. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
4. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921-936.
5. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995‐997.
6. Charles JA, von Dohln P. Outpatient home-based continuous intravenous dihydroergotamine therapy for intractable migraine. Headache. 2010;50(5):852-860.
7. Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in complex regional pain syndrome type 1 patients. Eur J Pain. 2010;14(3):302-307.
8. Krusz J, Cagle J, Hall S. Intramuscular (IM) ketamine for treating headache and pain flare-ups in the clinic. J Pain. 2008;9(4):30.
9. Bigal M Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337-1342.
10. Ford RG, Ford KT. Continuous intravenous dihydroergotamine for treatment of intractable headache. Headache. 1997;37(3):129‐136.
11. Boudreau G, Aghai E, Marchand L, Langlois M. Outpatient intravenous dihydroergotamine for probable medication overuse headache. Headache Care. 2006;3(1):45‐49.
12. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
13. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
14. Peterlin BL, Calhoun AH, Siegel S, Mathew NT. Rational combination therapy in refractory migraine. Headache. 2008;48(6):805-819.
15. Innes G, Macphail I, Dillon EC, Metcalfe C, Gao M. Dexamethasone prevents relapse after emergency department treatment of acute migraine: a randomized clinical trial. CJEM. 2015;1(1):26-33.
16. Irimia P, Palma JA, Fernandez-Torron R, Martinez-Vila E. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.
CTCs linked to late recurrence in HER2–, HR+ breast cancer
Circulating tumor cells could be used to stratify patients with hormone receptor (HR)–positive, HER2-negative breast cancer for late recurrence risk, results of a secondary analysis of a randomized clinical trial suggest.
Risk of late clinical recurrence was about 13-fold higher among HR-positive patients with a positive circulating tumor cell (CTC) assay result, according to results of the study, published in JAMA Oncology.
“This prospectively conducted study offers a high level of evidence supporting the association between a positive CTC assay result and risk of clinical recurrence,” said Joseph A. Sparano, MD, of Albert Einstein College of Medicine, New York, and his coauthors.
The present study is the first to show that this CTC assay may play a role in determining late clinical recurrence after local and systemic adjuvant therapy, according to the investigators.
The study is a secondary analysis of E5103, a phase 3 trial of adjuvant doxorubicin and cyclophosphamide followed by paclitaxel with bevacizumab in patients with HER2-negative stage II-III breast cancer. Investigators included a total of 547 patients who had no clinical evidence of recurrence between 4.5 and 7.5 years of registration in that trial.
Positive CTC assay results occurred in 26 of those patients (4.8%), they found.
At a median follow-up of 2.6 years, 24 patients had a clinical recurrence, including 23 HR-positive patients and just 1 HR-negative patient. Accordingly, the investigators focused most of their further analysis on the HR-positive subset.
A total of 7 of 23 patients with HR-positive disease (30.4%) had a positive CTC assay result.
A positive CTC result in HR-positive patients was associated with a 13.1-fold increased risk of recurrence, multivariate analysis showed.
Higher CTC burden appeared to be associated with a numerically higher recurrence risk in HR-positive patients, the investigators found. They saw recurrences in 16 of 335 patients with a CTC count of 0 cells per 7.5 mL blood (4.8%), compared with 2 of 12 patients with 1 cell per 7.5 mL blood (16.7%), and 5 of 6 patients with 2 or more cells per 7.5 mL (83.3%).
Taken together, these results provided proof of concept to support additional investigations of the CTC assay and other blood-based biomarker tests in the setting of late clinical recurrence in HR-positive patients, the researchers said.
They acknowledged several limitations of this study: It was small, it had relatively short follow-up, and it did not evaluate the CTC assay in the context of other assays.
“Notwithstanding proof of concept, further evaluation is required to confirm the clinical validity and determine the clinical utility of performing the CTC assay in this context,” Dr. Sparano and his coauthors wrote.
Late recurrences, or those that occur more than 5 years after diagnosis, account for about half of all recurrences among HR-positive receptive breast cancers, Dr. Sparano and his colleagues said.
The researchers had no conflicts of interest to report. The study was supported by grants from the National Cancer Institute, National Institutes of Health, Breast Cancer Research Foundation, and Susan G. Komen Foundation.
SOURCE: Sparano J et al. JAMA Oncol. 2018 Jul 26. doi: 10.1001/jamaoncol.2018.2574.
Circulating tumor cells could be used to stratify patients with hormone receptor (HR)–positive, HER2-negative breast cancer for late recurrence risk, results of a secondary analysis of a randomized clinical trial suggest.
Risk of late clinical recurrence was about 13-fold higher among HR-positive patients with a positive circulating tumor cell (CTC) assay result, according to results of the study, published in JAMA Oncology.
“This prospectively conducted study offers a high level of evidence supporting the association between a positive CTC assay result and risk of clinical recurrence,” said Joseph A. Sparano, MD, of Albert Einstein College of Medicine, New York, and his coauthors.
The present study is the first to show that this CTC assay may play a role in determining late clinical recurrence after local and systemic adjuvant therapy, according to the investigators.
The study is a secondary analysis of E5103, a phase 3 trial of adjuvant doxorubicin and cyclophosphamide followed by paclitaxel with bevacizumab in patients with HER2-negative stage II-III breast cancer. Investigators included a total of 547 patients who had no clinical evidence of recurrence between 4.5 and 7.5 years of registration in that trial.
Positive CTC assay results occurred in 26 of those patients (4.8%), they found.
At a median follow-up of 2.6 years, 24 patients had a clinical recurrence, including 23 HR-positive patients and just 1 HR-negative patient. Accordingly, the investigators focused most of their further analysis on the HR-positive subset.
A total of 7 of 23 patients with HR-positive disease (30.4%) had a positive CTC assay result.
A positive CTC result in HR-positive patients was associated with a 13.1-fold increased risk of recurrence, multivariate analysis showed.
Higher CTC burden appeared to be associated with a numerically higher recurrence risk in HR-positive patients, the investigators found. They saw recurrences in 16 of 335 patients with a CTC count of 0 cells per 7.5 mL blood (4.8%), compared with 2 of 12 patients with 1 cell per 7.5 mL blood (16.7%), and 5 of 6 patients with 2 or more cells per 7.5 mL (83.3%).
Taken together, these results provided proof of concept to support additional investigations of the CTC assay and other blood-based biomarker tests in the setting of late clinical recurrence in HR-positive patients, the researchers said.
They acknowledged several limitations of this study: It was small, it had relatively short follow-up, and it did not evaluate the CTC assay in the context of other assays.
“Notwithstanding proof of concept, further evaluation is required to confirm the clinical validity and determine the clinical utility of performing the CTC assay in this context,” Dr. Sparano and his coauthors wrote.
Late recurrences, or those that occur more than 5 years after diagnosis, account for about half of all recurrences among HR-positive receptive breast cancers, Dr. Sparano and his colleagues said.
The researchers had no conflicts of interest to report. The study was supported by grants from the National Cancer Institute, National Institutes of Health, Breast Cancer Research Foundation, and Susan G. Komen Foundation.
SOURCE: Sparano J et al. JAMA Oncol. 2018 Jul 26. doi: 10.1001/jamaoncol.2018.2574.
Circulating tumor cells could be used to stratify patients with hormone receptor (HR)–positive, HER2-negative breast cancer for late recurrence risk, results of a secondary analysis of a randomized clinical trial suggest.
Risk of late clinical recurrence was about 13-fold higher among HR-positive patients with a positive circulating tumor cell (CTC) assay result, according to results of the study, published in JAMA Oncology.
“This prospectively conducted study offers a high level of evidence supporting the association between a positive CTC assay result and risk of clinical recurrence,” said Joseph A. Sparano, MD, of Albert Einstein College of Medicine, New York, and his coauthors.
The present study is the first to show that this CTC assay may play a role in determining late clinical recurrence after local and systemic adjuvant therapy, according to the investigators.
The study is a secondary analysis of E5103, a phase 3 trial of adjuvant doxorubicin and cyclophosphamide followed by paclitaxel with bevacizumab in patients with HER2-negative stage II-III breast cancer. Investigators included a total of 547 patients who had no clinical evidence of recurrence between 4.5 and 7.5 years of registration in that trial.
Positive CTC assay results occurred in 26 of those patients (4.8%), they found.
At a median follow-up of 2.6 years, 24 patients had a clinical recurrence, including 23 HR-positive patients and just 1 HR-negative patient. Accordingly, the investigators focused most of their further analysis on the HR-positive subset.
A total of 7 of 23 patients with HR-positive disease (30.4%) had a positive CTC assay result.
A positive CTC result in HR-positive patients was associated with a 13.1-fold increased risk of recurrence, multivariate analysis showed.
Higher CTC burden appeared to be associated with a numerically higher recurrence risk in HR-positive patients, the investigators found. They saw recurrences in 16 of 335 patients with a CTC count of 0 cells per 7.5 mL blood (4.8%), compared with 2 of 12 patients with 1 cell per 7.5 mL blood (16.7%), and 5 of 6 patients with 2 or more cells per 7.5 mL (83.3%).
Taken together, these results provided proof of concept to support additional investigations of the CTC assay and other blood-based biomarker tests in the setting of late clinical recurrence in HR-positive patients, the researchers said.
They acknowledged several limitations of this study: It was small, it had relatively short follow-up, and it did not evaluate the CTC assay in the context of other assays.
“Notwithstanding proof of concept, further evaluation is required to confirm the clinical validity and determine the clinical utility of performing the CTC assay in this context,” Dr. Sparano and his coauthors wrote.
Late recurrences, or those that occur more than 5 years after diagnosis, account for about half of all recurrences among HR-positive receptive breast cancers, Dr. Sparano and his colleagues said.
The researchers had no conflicts of interest to report. The study was supported by grants from the National Cancer Institute, National Institutes of Health, Breast Cancer Research Foundation, and Susan G. Komen Foundation.
SOURCE: Sparano J et al. JAMA Oncol. 2018 Jul 26. doi: 10.1001/jamaoncol.2018.2574.
FROM JAMA ONCOLOGY
Key clinical point: Circulating tumor cells (CTC) may help to evaluate late recurrence risk in patients with HER2-negative breast cancer.
Major finding: A positive CTC result was associated with a 13.1-fold increased risk of recurrence in hormone receptor–positive patients.
Study details: Secondary analysis of a randomized clinical trial including 547 patients with HER2-negative stage II-III breast cancer.
Disclosures: The study was supported by grants from the National Cancer Institute, National Institutes of Health, Breast Cancer Research Foundation, and Susan G. Komen Foundation. The authors reported no conflicts of interest.
Source: Sparano J et al. JAMA Oncol. 2018 Jul 26. doi: 10.1001/jamaoncol.2018.2574.
Lower CTC count IDs indolent MBC disease subset
CHICAGO – A circulating tumor cell (CTC) count less than 5 per 7.5 mL of blood in patients with metastatic breast cancer indicates an indolent disease subset, according to a pooled analysis of individual patient data from two large cohorts.
The findings, which were independent of molecular subtype, disease location, or line of treatment, have important implications for CTC-based staging of metastatic breast cancer (MBC), which in turn could guide treatment decision making and drug development, Andrew A. Davis, MD, of Northwestern University, Chicago, and his colleagues reported in a poster at the annual meeting of the American Society of Clinical Oncology.
In 1,944 patients from the European Pooled Analysis Consortium (EPAC) and 492 from MD Anderson Cancer Center, CTC counts of 5 per 7.5mL or greater were associated with worse outcomes overall (hazard ratio, 2.43), the investigators said.
Median overall survival (OS) among all patients with CTC counts less than 5, who were considered to have stage IV indolent disease (stage IVindolent), was 36.3 months, and OS among those with de novo disease and CDC counts less than 5 was greater than 5.5 years, they said, noting that the survival benefit persisted across all disease subtypes.
For example, median OS in patients with stage IVindolent vs. stage IVaggressive (those with CTC counts of 5 or greater ) was 44.0 vs. 17.3 months in patients with hormone receptor–positive disease, 23.8 vs. 9.0 months in triple negative breast cancer patients, and 36.7 vs. 20.4 months in patients with HER2+ disease, respectively, they explained.They also noted that stage IVindolent disease could discriminate a less aggressive cohort both for patients with and without prior treatment; the hazard ratios were 0.40 and 0.42 favoring indolent disease for both first-line treatment and treatment beyond the first line, respectively.
In early-stage breast cancer, diagnostic tools have been incorporated into practice to help identify patients who will benefit from conservative vs. aggressive therapy, and the current findings suggest that CTC counts could be used in that manner for staging MBC.
“We propose a CTC-based staging system for MBC based on indolent and aggressive disease to incorporate into the American Joint Committee on Cancer [tumor node metastasis] staging classification,” they wrote, adding that prospective studies of single-agent, cost-effective treatments for stage IVindolent disease in the first-line setting are needed.
This study was supported by the Lynn Sage Breast Cancer Research OncoSET Program at Robert H. Lurie Cancer Center. Dr. Davis reported having no disclosures.
SOURCE: Davis A et al., ASCO 2018 Poster 1019.
CHICAGO – A circulating tumor cell (CTC) count less than 5 per 7.5 mL of blood in patients with metastatic breast cancer indicates an indolent disease subset, according to a pooled analysis of individual patient data from two large cohorts.
The findings, which were independent of molecular subtype, disease location, or line of treatment, have important implications for CTC-based staging of metastatic breast cancer (MBC), which in turn could guide treatment decision making and drug development, Andrew A. Davis, MD, of Northwestern University, Chicago, and his colleagues reported in a poster at the annual meeting of the American Society of Clinical Oncology.
In 1,944 patients from the European Pooled Analysis Consortium (EPAC) and 492 from MD Anderson Cancer Center, CTC counts of 5 per 7.5mL or greater were associated with worse outcomes overall (hazard ratio, 2.43), the investigators said.
Median overall survival (OS) among all patients with CTC counts less than 5, who were considered to have stage IV indolent disease (stage IVindolent), was 36.3 months, and OS among those with de novo disease and CDC counts less than 5 was greater than 5.5 years, they said, noting that the survival benefit persisted across all disease subtypes.
For example, median OS in patients with stage IVindolent vs. stage IVaggressive (those with CTC counts of 5 or greater ) was 44.0 vs. 17.3 months in patients with hormone receptor–positive disease, 23.8 vs. 9.0 months in triple negative breast cancer patients, and 36.7 vs. 20.4 months in patients with HER2+ disease, respectively, they explained.They also noted that stage IVindolent disease could discriminate a less aggressive cohort both for patients with and without prior treatment; the hazard ratios were 0.40 and 0.42 favoring indolent disease for both first-line treatment and treatment beyond the first line, respectively.
In early-stage breast cancer, diagnostic tools have been incorporated into practice to help identify patients who will benefit from conservative vs. aggressive therapy, and the current findings suggest that CTC counts could be used in that manner for staging MBC.
“We propose a CTC-based staging system for MBC based on indolent and aggressive disease to incorporate into the American Joint Committee on Cancer [tumor node metastasis] staging classification,” they wrote, adding that prospective studies of single-agent, cost-effective treatments for stage IVindolent disease in the first-line setting are needed.
This study was supported by the Lynn Sage Breast Cancer Research OncoSET Program at Robert H. Lurie Cancer Center. Dr. Davis reported having no disclosures.
SOURCE: Davis A et al., ASCO 2018 Poster 1019.
CHICAGO – A circulating tumor cell (CTC) count less than 5 per 7.5 mL of blood in patients with metastatic breast cancer indicates an indolent disease subset, according to a pooled analysis of individual patient data from two large cohorts.
The findings, which were independent of molecular subtype, disease location, or line of treatment, have important implications for CTC-based staging of metastatic breast cancer (MBC), which in turn could guide treatment decision making and drug development, Andrew A. Davis, MD, of Northwestern University, Chicago, and his colleagues reported in a poster at the annual meeting of the American Society of Clinical Oncology.
In 1,944 patients from the European Pooled Analysis Consortium (EPAC) and 492 from MD Anderson Cancer Center, CTC counts of 5 per 7.5mL or greater were associated with worse outcomes overall (hazard ratio, 2.43), the investigators said.
Median overall survival (OS) among all patients with CTC counts less than 5, who were considered to have stage IV indolent disease (stage IVindolent), was 36.3 months, and OS among those with de novo disease and CDC counts less than 5 was greater than 5.5 years, they said, noting that the survival benefit persisted across all disease subtypes.
For example, median OS in patients with stage IVindolent vs. stage IVaggressive (those with CTC counts of 5 or greater ) was 44.0 vs. 17.3 months in patients with hormone receptor–positive disease, 23.8 vs. 9.0 months in triple negative breast cancer patients, and 36.7 vs. 20.4 months in patients with HER2+ disease, respectively, they explained.They also noted that stage IVindolent disease could discriminate a less aggressive cohort both for patients with and without prior treatment; the hazard ratios were 0.40 and 0.42 favoring indolent disease for both first-line treatment and treatment beyond the first line, respectively.
In early-stage breast cancer, diagnostic tools have been incorporated into practice to help identify patients who will benefit from conservative vs. aggressive therapy, and the current findings suggest that CTC counts could be used in that manner for staging MBC.
“We propose a CTC-based staging system for MBC based on indolent and aggressive disease to incorporate into the American Joint Committee on Cancer [tumor node metastasis] staging classification,” they wrote, adding that prospective studies of single-agent, cost-effective treatments for stage IVindolent disease in the first-line setting are needed.
This study was supported by the Lynn Sage Breast Cancer Research OncoSET Program at Robert H. Lurie Cancer Center. Dr. Davis reported having no disclosures.
SOURCE: Davis A et al., ASCO 2018 Poster 1019.
REPORTING FROM ASCO 2018
Key clinical point: A CTC count less than 5 per 7.5 mL of blood in patients with MBC indicates an indolent disease subset.
Major finding: Median OS for stage IVindolent vs. stage IVaggressive disease was 4.0 vs. 17.3 months in HER2-negative patients, 23.8 vs. 9.0 months in TNBC patients, and 36.7 vs. 20.4 months in HER2-positive disease.
Study details: A pooled analysis of data from two cohort studies including 2,436 patients.
Disclosures: This study was supported by the Lynn Sage Breast Cancer Research OncoSET Program at Robert H. Lurie Cancer Center. Dr. Davis reported having no disclosures.
Source: Davis A et al. ASCO 2018 Poster 1019.
Breast cancer patients don’t get the financial counseling they want from their clinicians
About half of medical oncologists – and even fewer surgeons and radiation oncologists – have someone in their practice to discuss the financial implications of treating breast cancer, a physician-patient survey has found.
Patients are feeling that lack of service, too; 73% of women in the survey said their providers didn’t offer much, or even any, help in tackling the potentially devastating financial impact of their cancer. Women reported a variety of these issues, including increased debt, lost time at work, skimping on their food budget, and even losing their homes as the medical bills added up.
“The privations observed in the current study are sobering and consistent with studies published before the widespread awareness of the potential for financial toxicity after the diagnosis and treatment of cancer,” wrote Reshma Jagsi, MD, and coauthors. The report was published in Cancer.
“… Unfortunately, unmet needs for discussion persist, as does unresolved worry. The percentage of patients who perceive meaningful clinician engagement is low, with far fewer than one-quarter of respondents reporting more than a little discussion of these issues, which is strikingly lower than the percentage of providers who perceive routinely making services available,” they wrote.
Dr. Jagsi, of the University of Michigan, Ann Arbor, and her colleagues used the Surveillance, Epidemiology, and End Results (SEER) database to identify 2,502 women in Georgia and Los Angeles County who were diagnosed with early-stage breast cancer from 2013 to 2015. They contacted these women, who were at least 1 year out from diagnosis, and their oncology providers with a survey designed to determine how both groups communicated about financial issues, and how those issues affected patients’ day-to-day lives.
Most of the clinicians were surgeons (370); the rest were medical oncologists (306) or radiation oncologists (169). About a quarter of each group was in a teaching practice.
Among the medical oncologists, 50.9% reported that someone in their practice often or always discussed financial burden with patients, as did 15.6% of surgeons and 43.2% of radiation oncologists. Medical oncologists were also more likely to respond that they were very aware of out-of-pocket costs for patients, as did 27.3% of surgeons and 34.3% of radiation oncologists.
About 57% of medical oncologists thought it was quite or extremely important to save their patients money; 35.3% of surgeons and 55.8% of radiation oncologists also responded so.
Many women reported at least some measure of financial toxicity related to their cancer and its treatment, and this varied widely by ethnicity and race. Debt was common, noted by 58.9% of black patients, 33.5% of Latina patients, and about 28% of both white and Asian patients.
“Many patients also had substantial lost income and out-of-pocket expenses that they attributed to breast cancer,” the authors wrote. “Overall, 14% of patients reported lost income that was [at least] 10% of their household income, 17% of patients reported spending [at least] 10% of household income on out-of-pocket medical expenses, and 7% of patients reported spending [at least] 10% of household income on out-of-pocket nonmedical expenses.
Housing loss attributed to breast cancer was most common among blacks (6%) and Latinas (4.7%), and less so among whites and Asians (about 1% each).
Blacks and Latinas also were more likely to report a utility disconnection due to unpaid bills (5.9% and 3.2%, respectively) compared with whites and Asians (1.7% and 0.5%).
One way women financially coped, the survey found, was to cut the food budget. “One in five whites [21.5%] and Asians [22.5%] cut down spending on food, as did nearly one-half of black individuals [45.2%] and greater than one-third of Latinas [35.8%].”
Worry about finances was most common among blacks and Latinas (about 50%), but about a third of white and Asian women also reported worry. Survey results suggested that clinicians were not addressing these issues.
Women – especially nonwhite women – wanted to have these talks, with 15.2% of whites, 31.1% of blacks, 30.3% of Latinas, and 25.4% of Asians reporting this desire.
“Unmet patient needs for engagement with physicians regarding financial concerns were common. Of the 945 women who expressed worrying at least somewhat, 679 (72.8%) indicated that cancer physicians and their staff did not help at least somewhat,” the authors said.
More than half of the 523 women who expressed a desire to talk to health care providers regarding the impact of breast cancer on employment or finances (55.4%) reported that this discussion never took place, either with the oncologist, primary care provider, social worker, or any other professional involved in their care.
A multivariate analysis examined patient characteristics associated with the desire to discuss financial toxicity with a health care provider. Younger age, nonwhite race, lower income, being employed, receiving chemotherapy, and living in Georgia all showed significant, independent interaction.
“Given these findings, it is clear that thoughtfully designed, prospective interventions are necessary to address the remarkably common experiences of financial burden that patients report even in the modern era,” the investigators wrote. “These interventions might include training for physicians and their staff regarding how to have effective conversations in this context, in ways that are sensitive to cultural differences and needs. Other promising approaches might include the use of advanced technology to engage patients in interactive exercises that elicit their financial concerns and experiences and alert providers to their needs.”
The study was largely funded by a grant from the National Cancer Institute and the University of Michigan. Dr. Jagsi disclosed that she has been a consultant for Amgen but not relative to this study.
SOURCE: Jagsi R et al. Cancer 2018 Jul 23. doi: 10.1002/cncr.31532.
About half of medical oncologists – and even fewer surgeons and radiation oncologists – have someone in their practice to discuss the financial implications of treating breast cancer, a physician-patient survey has found.
Patients are feeling that lack of service, too; 73% of women in the survey said their providers didn’t offer much, or even any, help in tackling the potentially devastating financial impact of their cancer. Women reported a variety of these issues, including increased debt, lost time at work, skimping on their food budget, and even losing their homes as the medical bills added up.
“The privations observed in the current study are sobering and consistent with studies published before the widespread awareness of the potential for financial toxicity after the diagnosis and treatment of cancer,” wrote Reshma Jagsi, MD, and coauthors. The report was published in Cancer.
“… Unfortunately, unmet needs for discussion persist, as does unresolved worry. The percentage of patients who perceive meaningful clinician engagement is low, with far fewer than one-quarter of respondents reporting more than a little discussion of these issues, which is strikingly lower than the percentage of providers who perceive routinely making services available,” they wrote.
Dr. Jagsi, of the University of Michigan, Ann Arbor, and her colleagues used the Surveillance, Epidemiology, and End Results (SEER) database to identify 2,502 women in Georgia and Los Angeles County who were diagnosed with early-stage breast cancer from 2013 to 2015. They contacted these women, who were at least 1 year out from diagnosis, and their oncology providers with a survey designed to determine how both groups communicated about financial issues, and how those issues affected patients’ day-to-day lives.
Most of the clinicians were surgeons (370); the rest were medical oncologists (306) or radiation oncologists (169). About a quarter of each group was in a teaching practice.
Among the medical oncologists, 50.9% reported that someone in their practice often or always discussed financial burden with patients, as did 15.6% of surgeons and 43.2% of radiation oncologists. Medical oncologists were also more likely to respond that they were very aware of out-of-pocket costs for patients, as did 27.3% of surgeons and 34.3% of radiation oncologists.
About 57% of medical oncologists thought it was quite or extremely important to save their patients money; 35.3% of surgeons and 55.8% of radiation oncologists also responded so.
Many women reported at least some measure of financial toxicity related to their cancer and its treatment, and this varied widely by ethnicity and race. Debt was common, noted by 58.9% of black patients, 33.5% of Latina patients, and about 28% of both white and Asian patients.
“Many patients also had substantial lost income and out-of-pocket expenses that they attributed to breast cancer,” the authors wrote. “Overall, 14% of patients reported lost income that was [at least] 10% of their household income, 17% of patients reported spending [at least] 10% of household income on out-of-pocket medical expenses, and 7% of patients reported spending [at least] 10% of household income on out-of-pocket nonmedical expenses.
Housing loss attributed to breast cancer was most common among blacks (6%) and Latinas (4.7%), and less so among whites and Asians (about 1% each).
Blacks and Latinas also were more likely to report a utility disconnection due to unpaid bills (5.9% and 3.2%, respectively) compared with whites and Asians (1.7% and 0.5%).
One way women financially coped, the survey found, was to cut the food budget. “One in five whites [21.5%] and Asians [22.5%] cut down spending on food, as did nearly one-half of black individuals [45.2%] and greater than one-third of Latinas [35.8%].”
Worry about finances was most common among blacks and Latinas (about 50%), but about a third of white and Asian women also reported worry. Survey results suggested that clinicians were not addressing these issues.
Women – especially nonwhite women – wanted to have these talks, with 15.2% of whites, 31.1% of blacks, 30.3% of Latinas, and 25.4% of Asians reporting this desire.
“Unmet patient needs for engagement with physicians regarding financial concerns were common. Of the 945 women who expressed worrying at least somewhat, 679 (72.8%) indicated that cancer physicians and their staff did not help at least somewhat,” the authors said.
More than half of the 523 women who expressed a desire to talk to health care providers regarding the impact of breast cancer on employment or finances (55.4%) reported that this discussion never took place, either with the oncologist, primary care provider, social worker, or any other professional involved in their care.
A multivariate analysis examined patient characteristics associated with the desire to discuss financial toxicity with a health care provider. Younger age, nonwhite race, lower income, being employed, receiving chemotherapy, and living in Georgia all showed significant, independent interaction.
“Given these findings, it is clear that thoughtfully designed, prospective interventions are necessary to address the remarkably common experiences of financial burden that patients report even in the modern era,” the investigators wrote. “These interventions might include training for physicians and their staff regarding how to have effective conversations in this context, in ways that are sensitive to cultural differences and needs. Other promising approaches might include the use of advanced technology to engage patients in interactive exercises that elicit their financial concerns and experiences and alert providers to their needs.”
The study was largely funded by a grant from the National Cancer Institute and the University of Michigan. Dr. Jagsi disclosed that she has been a consultant for Amgen but not relative to this study.
SOURCE: Jagsi R et al. Cancer 2018 Jul 23. doi: 10.1002/cncr.31532.
About half of medical oncologists – and even fewer surgeons and radiation oncologists – have someone in their practice to discuss the financial implications of treating breast cancer, a physician-patient survey has found.
Patients are feeling that lack of service, too; 73% of women in the survey said their providers didn’t offer much, or even any, help in tackling the potentially devastating financial impact of their cancer. Women reported a variety of these issues, including increased debt, lost time at work, skimping on their food budget, and even losing their homes as the medical bills added up.
“The privations observed in the current study are sobering and consistent with studies published before the widespread awareness of the potential for financial toxicity after the diagnosis and treatment of cancer,” wrote Reshma Jagsi, MD, and coauthors. The report was published in Cancer.
“… Unfortunately, unmet needs for discussion persist, as does unresolved worry. The percentage of patients who perceive meaningful clinician engagement is low, with far fewer than one-quarter of respondents reporting more than a little discussion of these issues, which is strikingly lower than the percentage of providers who perceive routinely making services available,” they wrote.
Dr. Jagsi, of the University of Michigan, Ann Arbor, and her colleagues used the Surveillance, Epidemiology, and End Results (SEER) database to identify 2,502 women in Georgia and Los Angeles County who were diagnosed with early-stage breast cancer from 2013 to 2015. They contacted these women, who were at least 1 year out from diagnosis, and their oncology providers with a survey designed to determine how both groups communicated about financial issues, and how those issues affected patients’ day-to-day lives.
Most of the clinicians were surgeons (370); the rest were medical oncologists (306) or radiation oncologists (169). About a quarter of each group was in a teaching practice.
Among the medical oncologists, 50.9% reported that someone in their practice often or always discussed financial burden with patients, as did 15.6% of surgeons and 43.2% of radiation oncologists. Medical oncologists were also more likely to respond that they were very aware of out-of-pocket costs for patients, as did 27.3% of surgeons and 34.3% of radiation oncologists.
About 57% of medical oncologists thought it was quite or extremely important to save their patients money; 35.3% of surgeons and 55.8% of radiation oncologists also responded so.
Many women reported at least some measure of financial toxicity related to their cancer and its treatment, and this varied widely by ethnicity and race. Debt was common, noted by 58.9% of black patients, 33.5% of Latina patients, and about 28% of both white and Asian patients.
“Many patients also had substantial lost income and out-of-pocket expenses that they attributed to breast cancer,” the authors wrote. “Overall, 14% of patients reported lost income that was [at least] 10% of their household income, 17% of patients reported spending [at least] 10% of household income on out-of-pocket medical expenses, and 7% of patients reported spending [at least] 10% of household income on out-of-pocket nonmedical expenses.
Housing loss attributed to breast cancer was most common among blacks (6%) and Latinas (4.7%), and less so among whites and Asians (about 1% each).
Blacks and Latinas also were more likely to report a utility disconnection due to unpaid bills (5.9% and 3.2%, respectively) compared with whites and Asians (1.7% and 0.5%).
One way women financially coped, the survey found, was to cut the food budget. “One in five whites [21.5%] and Asians [22.5%] cut down spending on food, as did nearly one-half of black individuals [45.2%] and greater than one-third of Latinas [35.8%].”
Worry about finances was most common among blacks and Latinas (about 50%), but about a third of white and Asian women also reported worry. Survey results suggested that clinicians were not addressing these issues.
Women – especially nonwhite women – wanted to have these talks, with 15.2% of whites, 31.1% of blacks, 30.3% of Latinas, and 25.4% of Asians reporting this desire.
“Unmet patient needs for engagement with physicians regarding financial concerns were common. Of the 945 women who expressed worrying at least somewhat, 679 (72.8%) indicated that cancer physicians and their staff did not help at least somewhat,” the authors said.
More than half of the 523 women who expressed a desire to talk to health care providers regarding the impact of breast cancer on employment or finances (55.4%) reported that this discussion never took place, either with the oncologist, primary care provider, social worker, or any other professional involved in their care.
A multivariate analysis examined patient characteristics associated with the desire to discuss financial toxicity with a health care provider. Younger age, nonwhite race, lower income, being employed, receiving chemotherapy, and living in Georgia all showed significant, independent interaction.
“Given these findings, it is clear that thoughtfully designed, prospective interventions are necessary to address the remarkably common experiences of financial burden that patients report even in the modern era,” the investigators wrote. “These interventions might include training for physicians and their staff regarding how to have effective conversations in this context, in ways that are sensitive to cultural differences and needs. Other promising approaches might include the use of advanced technology to engage patients in interactive exercises that elicit their financial concerns and experiences and alert providers to their needs.”
The study was largely funded by a grant from the National Cancer Institute and the University of Michigan. Dr. Jagsi disclosed that she has been a consultant for Amgen but not relative to this study.
SOURCE: Jagsi R et al. Cancer 2018 Jul 23. doi: 10.1002/cncr.31532.
FROM CANCER
Key clinical point: Oncology care providers aren’t providing adequate financial counseling for patients with breast cancer.
Major finding: Half of medical oncologists say they don’t have a staff member routinely discuss the financial impact of breast cancer, and 73% of patients say they’ve never had this discussion with their doctor.
Study details: The survey comprised 2,502 patients and 845 physicians.
Disclosures: The study was largely funded by a grant from the National Cancer Institute and the University of Michigan. Dr. Jagsi disclosed that she has been a consultant for Amgen but not relative to this study.
Source: Jagsi R et al. Cancer 2018 Jul 23. doi: 10.1002/cncr.31532.