User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Are You Using the Correct Medication or a Look-Alike?
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Controlling Six Risk Factors Can Combat CKD in Obesity
TOPLINE:
Optimal management of blood pressure, A1c levels, low-density lipoprotein cholesterol (LDL-C), albuminuria, smoking, and physical activity may reduce the excess risk for chronic kidney disease (CKD) typically linked to obesity. The protective effect is more pronounced in men, in those with lower healthy food scores, and in users of diabetes medication.
METHODOLOGY:
- Obesity is a significant risk factor for CKD, but it is unknown if managing multiple other obesity-related CKD risk factors can mitigate the excess CKD risk.
- Researchers assessed CKD risk factor control in 97,538 participants with obesity from the UK Biobank and compared them with an equal number of age- and sex-matched control participants with normal body weight and no CKD at baseline.
- Participants with obesity were assessed for six modifiable risk factors: Blood pressure, A1c levels, LDL-C, albuminuria, smoking, and physical activity.
- Overall, 2487, 12,720, 32,388, 36,988, and 15,381 participants with obesity had at most two, three, four, five, and six risk factors under combined control, respectively, with the two or fewer group serving as the reference.
- The primary outcome was incident CKD and the degree of combined risk factor control in persons. The CKD risk and risk factor control in participants with obesity were also compared with CKD incidence in matched normal weight participants.
TAKEAWAY:
- During a median follow-up period of 10.8 years, 3954 cases of incident CKD were reported in participants with obesity and 1498 cases in matched persons of normal body mass index (BMI).
- In a stepwise pattern, optimal control of each additional risk factor was associated with 11% (adjusted hazard ratio [aHR], 0.89; 95% CI, 0.86-0.91) reduction in the incidence of CKD events, down to a 49% reduction in CKD incidence (aHR, 0.51; 95% CI, 0.43-0.61) for combined control of all six risk factors in participants with obesity.
- The protective effect of combined control of risk factors was more pronounced in men vs women, in those with lower vs higher healthy diet scores, and in users vs nonusers of diabetes medication.
- A similar stepwise pattern emerged between the number of risk factors controlled and CKD risk in participants with obesity compared with matched individuals of normal BMI, with the excess CKD risk eliminated in participants with obesity with six risk factors under control.
IN PRACTICE:
“Comprehensive control of risk factors might effectively neutralize the excessive CKD risk associated with obesity, emphasizing the potential of a joint management approach in the prevention of CKD in this population,” the authors wrote.
SOURCE:
The study was led by Rui Tang, MS, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, Louisiana. It was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The evaluated risk factors for CKD were arbitrarily selected, which may not represent the ideal group. The study did not consider the time-varying effect of joint risk factor control owing to the lack of some variables such as A1c. The generalizability of the findings was limited because over 90% of the UK Biobank cohort is composed of White people and individuals with healthier behaviors compared with the overall UK population.
DISCLOSURES:
The study was supported by grants from the US National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Optimal management of blood pressure, A1c levels, low-density lipoprotein cholesterol (LDL-C), albuminuria, smoking, and physical activity may reduce the excess risk for chronic kidney disease (CKD) typically linked to obesity. The protective effect is more pronounced in men, in those with lower healthy food scores, and in users of diabetes medication.
METHODOLOGY:
- Obesity is a significant risk factor for CKD, but it is unknown if managing multiple other obesity-related CKD risk factors can mitigate the excess CKD risk.
- Researchers assessed CKD risk factor control in 97,538 participants with obesity from the UK Biobank and compared them with an equal number of age- and sex-matched control participants with normal body weight and no CKD at baseline.
- Participants with obesity were assessed for six modifiable risk factors: Blood pressure, A1c levels, LDL-C, albuminuria, smoking, and physical activity.
- Overall, 2487, 12,720, 32,388, 36,988, and 15,381 participants with obesity had at most two, three, four, five, and six risk factors under combined control, respectively, with the two or fewer group serving as the reference.
- The primary outcome was incident CKD and the degree of combined risk factor control in persons. The CKD risk and risk factor control in participants with obesity were also compared with CKD incidence in matched normal weight participants.
TAKEAWAY:
- During a median follow-up period of 10.8 years, 3954 cases of incident CKD were reported in participants with obesity and 1498 cases in matched persons of normal body mass index (BMI).
- In a stepwise pattern, optimal control of each additional risk factor was associated with 11% (adjusted hazard ratio [aHR], 0.89; 95% CI, 0.86-0.91) reduction in the incidence of CKD events, down to a 49% reduction in CKD incidence (aHR, 0.51; 95% CI, 0.43-0.61) for combined control of all six risk factors in participants with obesity.
- The protective effect of combined control of risk factors was more pronounced in men vs women, in those with lower vs higher healthy diet scores, and in users vs nonusers of diabetes medication.
- A similar stepwise pattern emerged between the number of risk factors controlled and CKD risk in participants with obesity compared with matched individuals of normal BMI, with the excess CKD risk eliminated in participants with obesity with six risk factors under control.
IN PRACTICE:
“Comprehensive control of risk factors might effectively neutralize the excessive CKD risk associated with obesity, emphasizing the potential of a joint management approach in the prevention of CKD in this population,” the authors wrote.
SOURCE:
The study was led by Rui Tang, MS, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, Louisiana. It was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The evaluated risk factors for CKD were arbitrarily selected, which may not represent the ideal group. The study did not consider the time-varying effect of joint risk factor control owing to the lack of some variables such as A1c. The generalizability of the findings was limited because over 90% of the UK Biobank cohort is composed of White people and individuals with healthier behaviors compared with the overall UK population.
DISCLOSURES:
The study was supported by grants from the US National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Optimal management of blood pressure, A1c levels, low-density lipoprotein cholesterol (LDL-C), albuminuria, smoking, and physical activity may reduce the excess risk for chronic kidney disease (CKD) typically linked to obesity. The protective effect is more pronounced in men, in those with lower healthy food scores, and in users of diabetes medication.
METHODOLOGY:
- Obesity is a significant risk factor for CKD, but it is unknown if managing multiple other obesity-related CKD risk factors can mitigate the excess CKD risk.
- Researchers assessed CKD risk factor control in 97,538 participants with obesity from the UK Biobank and compared them with an equal number of age- and sex-matched control participants with normal body weight and no CKD at baseline.
- Participants with obesity were assessed for six modifiable risk factors: Blood pressure, A1c levels, LDL-C, albuminuria, smoking, and physical activity.
- Overall, 2487, 12,720, 32,388, 36,988, and 15,381 participants with obesity had at most two, three, four, five, and six risk factors under combined control, respectively, with the two or fewer group serving as the reference.
- The primary outcome was incident CKD and the degree of combined risk factor control in persons. The CKD risk and risk factor control in participants with obesity were also compared with CKD incidence in matched normal weight participants.
TAKEAWAY:
- During a median follow-up period of 10.8 years, 3954 cases of incident CKD were reported in participants with obesity and 1498 cases in matched persons of normal body mass index (BMI).
- In a stepwise pattern, optimal control of each additional risk factor was associated with 11% (adjusted hazard ratio [aHR], 0.89; 95% CI, 0.86-0.91) reduction in the incidence of CKD events, down to a 49% reduction in CKD incidence (aHR, 0.51; 95% CI, 0.43-0.61) for combined control of all six risk factors in participants with obesity.
- The protective effect of combined control of risk factors was more pronounced in men vs women, in those with lower vs higher healthy diet scores, and in users vs nonusers of diabetes medication.
- A similar stepwise pattern emerged between the number of risk factors controlled and CKD risk in participants with obesity compared with matched individuals of normal BMI, with the excess CKD risk eliminated in participants with obesity with six risk factors under control.
IN PRACTICE:
“Comprehensive control of risk factors might effectively neutralize the excessive CKD risk associated with obesity, emphasizing the potential of a joint management approach in the prevention of CKD in this population,” the authors wrote.
SOURCE:
The study was led by Rui Tang, MS, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, Louisiana. It was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The evaluated risk factors for CKD were arbitrarily selected, which may not represent the ideal group. The study did not consider the time-varying effect of joint risk factor control owing to the lack of some variables such as A1c. The generalizability of the findings was limited because over 90% of the UK Biobank cohort is composed of White people and individuals with healthier behaviors compared with the overall UK population.
DISCLOSURES:
The study was supported by grants from the US National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Should There Be a Mandatory Retirement Age for Physicians?
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
When You and Your Malpractice Insurer Disagree on Your Case
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
Coffee’s ‘Sweet Spot’: Daily Consumption and Cardiometabolic Risk
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
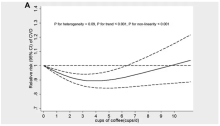
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
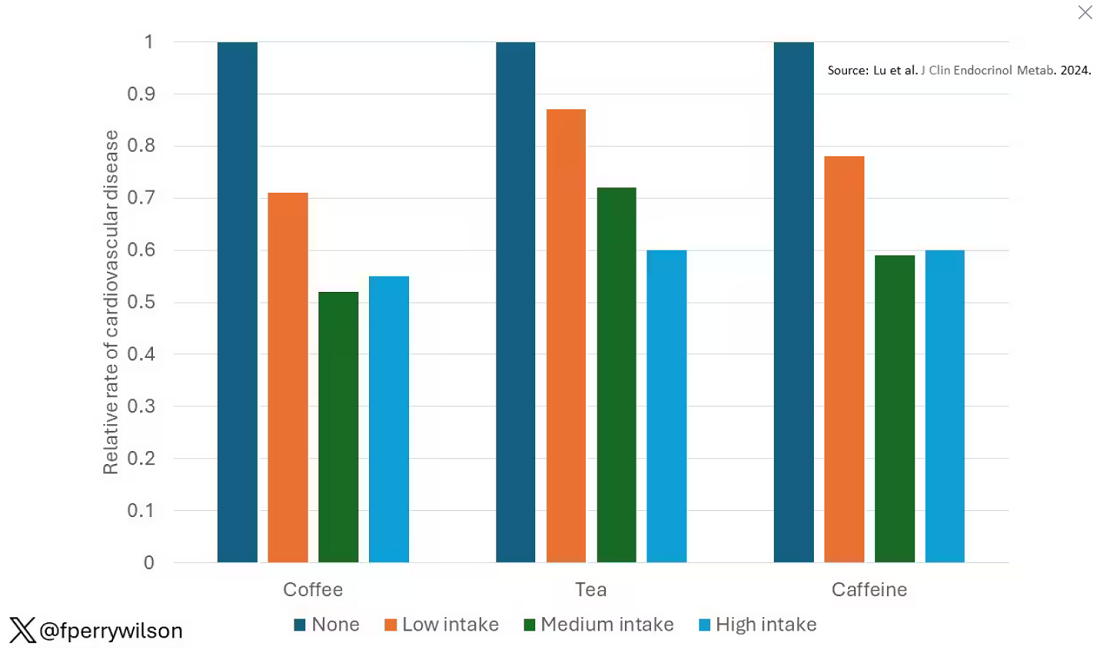
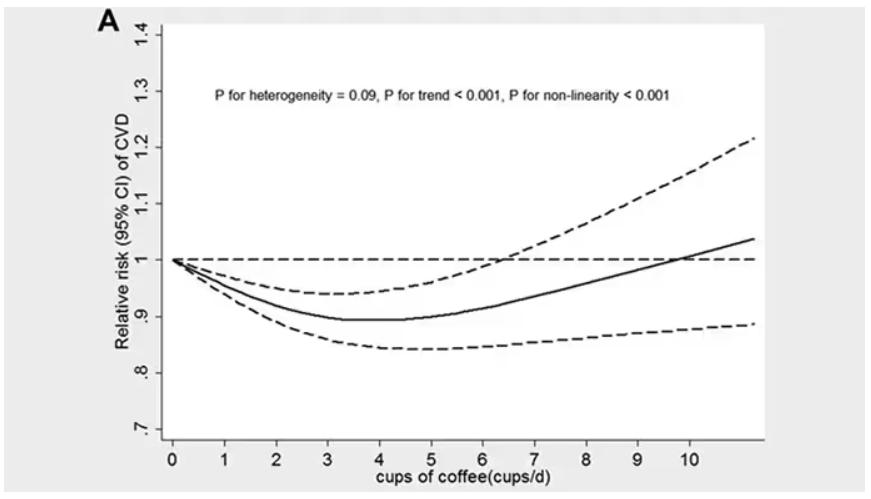
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
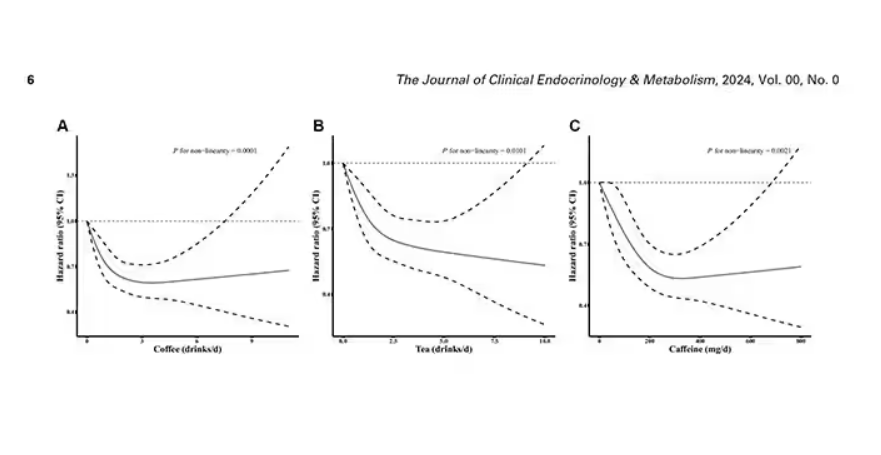
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine.
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
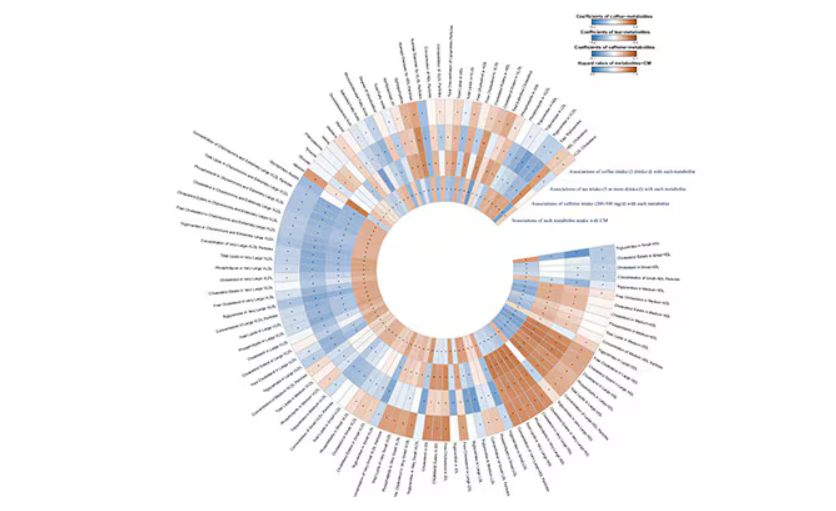
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
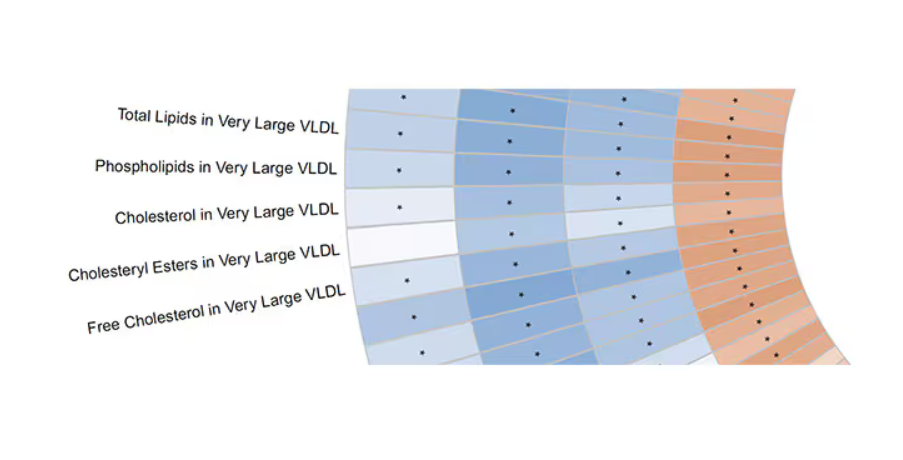
This figure summarizes the findings, and yes, this is way too complicated.
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
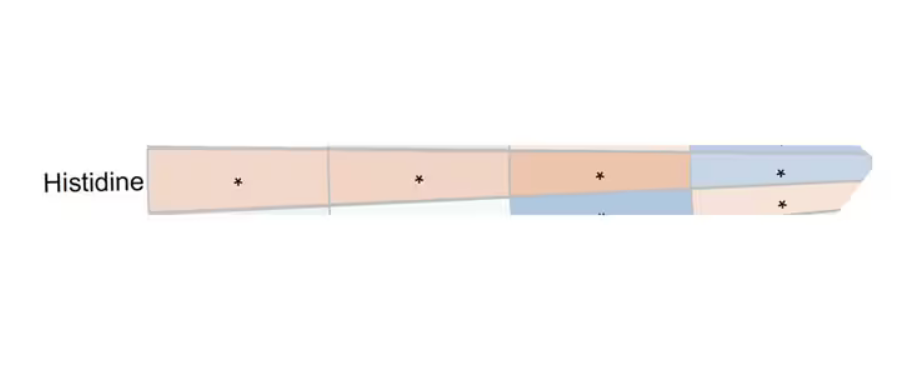
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine.
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated.
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine.
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated.
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Hot Flashes: Do They Predict CVD and Dementia?
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
‘Reform School’ for Pharmacy Benefit Managers: How Might Legislation Help Patients?
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
AI-Powered Clinical Documentation Tool Reduces EHR Time for Clinicians
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
‘Remarkable’ Weight Loss Seen With Safe, Tolerable Novel Oral Combination
MADRID — Amycretin, a dual-pathway, oral weight loss drug, led to up to 13% body weight loss in participants with overweight or obesity according to phase 1, first-in-human study data presented at the European Association for the Study of Diabetes (EASD) 2024 annual meeting.
Body weight loss was “remarkable for an orally delivered biologic,” said Agnes Gasiorek, PhD, senior clinical pharmacology specialist at Novo Nordisk, Måløv, Denmark, who presented the results. And “there was no plateauing of weight loss in the treatment period.”
With respect to the primary endpoint, stepwise dose escalation demonstrated that all tested dose levels up to and including 2 × 50 mg over a 12-week escalation period were safe and tolerable, Dr. Gasiorek reported.
The adverse events were in line with what was expected from targeting these receptors, and no new safety signals appeared during the study, she added.
Dual Pathways
Amycretin is a novel protein-based unimolecular amylin combined with a glucagon-like peptide 1 receptor agonist (GLP-1 RA) and is the first oral formulation of this combination under development.
The two components are both known to reduce appetite and energy intake and increase satiety, said Dr. Gasiorek, but amylin is considered to potentially increase leptin sensitivity and GLP-1 RAs are known to increase insulin secretion and biosynthesis. Together, the two components improve insulin sensitivity, decrease glucagon secretion, and lead to acute delay in gastric emptying.
The single-center, placebo-controlled, double-blind phase 1 study enrolled men and women aged 18-55 years (mean, 38-42 years across groups) with a body mass index of 25.0-39.9, without diabetes, and considered otherwise healthy.
Participants were randomly assigned to receive to receive oral amycretin (n = 95) or placebo (n = 29) once a day for up to 12 weeks. Study arms comprised single-ascending dosing (increasing from 1 mg/d to 25 mg), and multiple-ascending dosing. The latter consisted of multiple ascending doses (from 3 to 12 mg) over 10 days and multiple ascending doses (stepwise dose escalation, from 3 mg up to a final dose of 2 × 50 mg) over 12 weeks.
In her presentation at the EASD meeting, Dr. Gasiorek focused on results of the 12-week multiple ascending dose schedule with amycretin 50 mg (n = 16), amycretin 2 × 50 mg (n = 16), and placebo (n = 12).
The primary endpoint of the study was the number of treatment-emergent adverse events, while the area under the amycretin plasma concentration time curve and the maximum plasma concentration of amycretin were secondary endpoints.
The researchers also added percentage change in body weight after 12 weeks of treatment as an exploratory endpoint.
Safety Findings of Multiple Dosing
A total of 242 treatment-emergent adverse events were reported in the combined active and placebo groups and were of mild to moderate severity.
Treatment-emergent adverse events were found in 75% of the amycretin 50 mg group, 93.8% of the amycretin 2 × 50 mg group, and 33.3% of placebo recipients.
“Most adverse events reported were mild to moderate in severity and related to gastrointestinal discomfort (nausea and vomiting) and occurred in a dose-proportional manner,” reported Dr. Gasiorek.
Gastrointestinal events were experienced by 50%, 87.5%, and 16.7% of participants receiving amycretin 50 mg, amycretin 2 × 50 mg, and placebo, respectively (112 in total).
Decreased appetite was also found in 56.3%, 81.3%, and 16.7% of the amycretin 50 mg, amycretin 2 × 50 mg, and placebo groups, respectively.
Two serious adverse events occurred, one of which was acute cholecystitis and the other diabetic ketoacidosis; “however, the [latter] participant was found to have autoantibodies for beta cells before treatment and was later diagnosed with type 1 diabetes,” Dr. Gasiorek said.
Body Weight Reduction
Participants on 50 mg amycretin lost an average of 10.4% of their body weight (estimated treatment difference vs placebo, –9.2; 95% CI, –12.0 to –6.5), whereas those on 2 × 50 mg amycretin lost 13.1% of their body weight (estimated treatment difference vs placebo, –11.8; 95% CI, –14.6 to –9.0). Placebo group participants lost 1.2% of their body weight over the 12 weeks.
Although no plateauing of weight loss was seen, said Dr. Gasiorek, it is important to consider the relatively short treatment duration and the limited time on the final dose, which could potentially introduce bias.
To date, weight loss medications based on GLP-1 RA technology are injectables. A combination of the injectable amylin analogue cagrilintide and the GLP-1 RA semaglutide is also being explored as a subcutaneous treatment solution.
In a comment, Martin Holst Lange, MD, PhD, executive vice president of development at Novo Nordisk, said that “amycretin is the first treatment to harness the two distinct biological pathways stimulated by amylin and GLP-1 in a single molecule.”
The safety and tolerability profiles and the magnitude of weight loss support further development of amycretin in patients with overweight or obesity, said Dr. Lange, who noted that the company was awaiting data from the ongoing phase 1 trial with subcutaneous amycretin, expected in 2025.
Having heard the presentation, co-moderator Timo Müller, PhD, professor at Ludwig Maximilian University of Munich, Germany, gave a considered response. “The drug was relatively well tolerated, with the typical GLP-1–induced GI [gastrointestinal] adverse effects being the most frequently reported.”
But he pointed out that questions remain. “We still need to know whether, at the given dose, the drug outperforms best-in-class drugs like semaglutide or tirzepatide at the highest approved doses. Furthermore, it warrants clarification if and to what extent the activation of the amylin receptor contributes to the shown effect and if and to what extent the glycemic benefits result from activation of the glucagon receptor (amylin improved glycemia by decreasing the secretion of glucagon). In any way, the current data remain friendly and support phase 2 development.”
Oral Meds Could Bring Down Cost
Commenting on the data, Nerys Astbury, PhD, associate professor of diet and obesity at Nuffield Department of Primary Health Care Sciences, University of Oxford, England, said, “It is important to note that whilst the participants in this trial did lose weight over the 12-week study — and this was statistically more weight than in the placebo group — this study was not designed or powered to detect differences in body weight over longer periods of time.”
If the results are confirmed in future studies, amycretin might widen the treatment options and introduce competition, probably bringing down the costs in the longer-term, said Dr. Astbury, who welcomes the prospect.
“It is possible that some people might find the oral medications more acceptable than the injectable GLP-1 agonists currently available,” she said. And the current options are expensive, “which raises challenges to a taxpayer-funded health system like the NHS [National Health Service].”
“Furthermore, if the growing number of oral obesity medications prove safe, tolerable, and effective ... they are likely to significantly reduce the risks of developing many complications of obesity.”
Naveed Sattar, MD, professor of cardiometabolic medicine and honorary consultant, University of Glasgow, Scotland, agreed. “The more medicines coming forward to treat obesity, the better,” he said. In particular, oral medications would be more easily available, and cheaper, “for the many millions around the world struggling with obesity and its complications.”
Dr. Gasiorek declares she is an employee of and a shareholder in Novo Nordisk. Dr. Astbury declares no financial disclosures. Dr. Sattar declares having consulted for several companies that make diabetes medicines but also contributed to several lifestyle trials. For Novo Nordisk, he has consulted for the company on advisory boards, but not on any of their weight loss drug trial committees, and he is on the steering committee for the ZEUS trial, which is not a weight loss trial product but an anti-inflammatory. He does not have any shares for any product in health etc. He declares consulting fees and/or speaker honoraria from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi, and grant support paid to his university from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics. Dr. Müller received financial support or an honorarium from Novo Nordisk, Merck, Eli Lilly, Boehringer Ingelheim, and Mercodia; he further holds stocks at Novo Nordisk and Eli Lilly and is cofounder of Bluewater Biosciences.
A version of this article first appeared on Medscape.com.
MADRID — Amycretin, a dual-pathway, oral weight loss drug, led to up to 13% body weight loss in participants with overweight or obesity according to phase 1, first-in-human study data presented at the European Association for the Study of Diabetes (EASD) 2024 annual meeting.
Body weight loss was “remarkable for an orally delivered biologic,” said Agnes Gasiorek, PhD, senior clinical pharmacology specialist at Novo Nordisk, Måløv, Denmark, who presented the results. And “there was no plateauing of weight loss in the treatment period.”
With respect to the primary endpoint, stepwise dose escalation demonstrated that all tested dose levels up to and including 2 × 50 mg over a 12-week escalation period were safe and tolerable, Dr. Gasiorek reported.
The adverse events were in line with what was expected from targeting these receptors, and no new safety signals appeared during the study, she added.
Dual Pathways
Amycretin is a novel protein-based unimolecular amylin combined with a glucagon-like peptide 1 receptor agonist (GLP-1 RA) and is the first oral formulation of this combination under development.
The two components are both known to reduce appetite and energy intake and increase satiety, said Dr. Gasiorek, but amylin is considered to potentially increase leptin sensitivity and GLP-1 RAs are known to increase insulin secretion and biosynthesis. Together, the two components improve insulin sensitivity, decrease glucagon secretion, and lead to acute delay in gastric emptying.
The single-center, placebo-controlled, double-blind phase 1 study enrolled men and women aged 18-55 years (mean, 38-42 years across groups) with a body mass index of 25.0-39.9, without diabetes, and considered otherwise healthy.
Participants were randomly assigned to receive to receive oral amycretin (n = 95) or placebo (n = 29) once a day for up to 12 weeks. Study arms comprised single-ascending dosing (increasing from 1 mg/d to 25 mg), and multiple-ascending dosing. The latter consisted of multiple ascending doses (from 3 to 12 mg) over 10 days and multiple ascending doses (stepwise dose escalation, from 3 mg up to a final dose of 2 × 50 mg) over 12 weeks.
In her presentation at the EASD meeting, Dr. Gasiorek focused on results of the 12-week multiple ascending dose schedule with amycretin 50 mg (n = 16), amycretin 2 × 50 mg (n = 16), and placebo (n = 12).
The primary endpoint of the study was the number of treatment-emergent adverse events, while the area under the amycretin plasma concentration time curve and the maximum plasma concentration of amycretin were secondary endpoints.
The researchers also added percentage change in body weight after 12 weeks of treatment as an exploratory endpoint.
Safety Findings of Multiple Dosing
A total of 242 treatment-emergent adverse events were reported in the combined active and placebo groups and were of mild to moderate severity.
Treatment-emergent adverse events were found in 75% of the amycretin 50 mg group, 93.8% of the amycretin 2 × 50 mg group, and 33.3% of placebo recipients.
“Most adverse events reported were mild to moderate in severity and related to gastrointestinal discomfort (nausea and vomiting) and occurred in a dose-proportional manner,” reported Dr. Gasiorek.
Gastrointestinal events were experienced by 50%, 87.5%, and 16.7% of participants receiving amycretin 50 mg, amycretin 2 × 50 mg, and placebo, respectively (112 in total).
Decreased appetite was also found in 56.3%, 81.3%, and 16.7% of the amycretin 50 mg, amycretin 2 × 50 mg, and placebo groups, respectively.
Two serious adverse events occurred, one of which was acute cholecystitis and the other diabetic ketoacidosis; “however, the [latter] participant was found to have autoantibodies for beta cells before treatment and was later diagnosed with type 1 diabetes,” Dr. Gasiorek said.
Body Weight Reduction
Participants on 50 mg amycretin lost an average of 10.4% of their body weight (estimated treatment difference vs placebo, –9.2; 95% CI, –12.0 to –6.5), whereas those on 2 × 50 mg amycretin lost 13.1% of their body weight (estimated treatment difference vs placebo, –11.8; 95% CI, –14.6 to –9.0). Placebo group participants lost 1.2% of their body weight over the 12 weeks.
Although no plateauing of weight loss was seen, said Dr. Gasiorek, it is important to consider the relatively short treatment duration and the limited time on the final dose, which could potentially introduce bias.
To date, weight loss medications based on GLP-1 RA technology are injectables. A combination of the injectable amylin analogue cagrilintide and the GLP-1 RA semaglutide is also being explored as a subcutaneous treatment solution.
In a comment, Martin Holst Lange, MD, PhD, executive vice president of development at Novo Nordisk, said that “amycretin is the first treatment to harness the two distinct biological pathways stimulated by amylin and GLP-1 in a single molecule.”
The safety and tolerability profiles and the magnitude of weight loss support further development of amycretin in patients with overweight or obesity, said Dr. Lange, who noted that the company was awaiting data from the ongoing phase 1 trial with subcutaneous amycretin, expected in 2025.
Having heard the presentation, co-moderator Timo Müller, PhD, professor at Ludwig Maximilian University of Munich, Germany, gave a considered response. “The drug was relatively well tolerated, with the typical GLP-1–induced GI [gastrointestinal] adverse effects being the most frequently reported.”
But he pointed out that questions remain. “We still need to know whether, at the given dose, the drug outperforms best-in-class drugs like semaglutide or tirzepatide at the highest approved doses. Furthermore, it warrants clarification if and to what extent the activation of the amylin receptor contributes to the shown effect and if and to what extent the glycemic benefits result from activation of the glucagon receptor (amylin improved glycemia by decreasing the secretion of glucagon). In any way, the current data remain friendly and support phase 2 development.”
Oral Meds Could Bring Down Cost
Commenting on the data, Nerys Astbury, PhD, associate professor of diet and obesity at Nuffield Department of Primary Health Care Sciences, University of Oxford, England, said, “It is important to note that whilst the participants in this trial did lose weight over the 12-week study — and this was statistically more weight than in the placebo group — this study was not designed or powered to detect differences in body weight over longer periods of time.”
If the results are confirmed in future studies, amycretin might widen the treatment options and introduce competition, probably bringing down the costs in the longer-term, said Dr. Astbury, who welcomes the prospect.
“It is possible that some people might find the oral medications more acceptable than the injectable GLP-1 agonists currently available,” she said. And the current options are expensive, “which raises challenges to a taxpayer-funded health system like the NHS [National Health Service].”
“Furthermore, if the growing number of oral obesity medications prove safe, tolerable, and effective ... they are likely to significantly reduce the risks of developing many complications of obesity.”
Naveed Sattar, MD, professor of cardiometabolic medicine and honorary consultant, University of Glasgow, Scotland, agreed. “The more medicines coming forward to treat obesity, the better,” he said. In particular, oral medications would be more easily available, and cheaper, “for the many millions around the world struggling with obesity and its complications.”
Dr. Gasiorek declares she is an employee of and a shareholder in Novo Nordisk. Dr. Astbury declares no financial disclosures. Dr. Sattar declares having consulted for several companies that make diabetes medicines but also contributed to several lifestyle trials. For Novo Nordisk, he has consulted for the company on advisory boards, but not on any of their weight loss drug trial committees, and he is on the steering committee for the ZEUS trial, which is not a weight loss trial product but an anti-inflammatory. He does not have any shares for any product in health etc. He declares consulting fees and/or speaker honoraria from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi, and grant support paid to his university from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics. Dr. Müller received financial support or an honorarium from Novo Nordisk, Merck, Eli Lilly, Boehringer Ingelheim, and Mercodia; he further holds stocks at Novo Nordisk and Eli Lilly and is cofounder of Bluewater Biosciences.
A version of this article first appeared on Medscape.com.
MADRID — Amycretin, a dual-pathway, oral weight loss drug, led to up to 13% body weight loss in participants with overweight or obesity according to phase 1, first-in-human study data presented at the European Association for the Study of Diabetes (EASD) 2024 annual meeting.
Body weight loss was “remarkable for an orally delivered biologic,” said Agnes Gasiorek, PhD, senior clinical pharmacology specialist at Novo Nordisk, Måløv, Denmark, who presented the results. And “there was no plateauing of weight loss in the treatment period.”
With respect to the primary endpoint, stepwise dose escalation demonstrated that all tested dose levels up to and including 2 × 50 mg over a 12-week escalation period were safe and tolerable, Dr. Gasiorek reported.
The adverse events were in line with what was expected from targeting these receptors, and no new safety signals appeared during the study, she added.
Dual Pathways
Amycretin is a novel protein-based unimolecular amylin combined with a glucagon-like peptide 1 receptor agonist (GLP-1 RA) and is the first oral formulation of this combination under development.
The two components are both known to reduce appetite and energy intake and increase satiety, said Dr. Gasiorek, but amylin is considered to potentially increase leptin sensitivity and GLP-1 RAs are known to increase insulin secretion and biosynthesis. Together, the two components improve insulin sensitivity, decrease glucagon secretion, and lead to acute delay in gastric emptying.
The single-center, placebo-controlled, double-blind phase 1 study enrolled men and women aged 18-55 years (mean, 38-42 years across groups) with a body mass index of 25.0-39.9, without diabetes, and considered otherwise healthy.
Participants were randomly assigned to receive to receive oral amycretin (n = 95) or placebo (n = 29) once a day for up to 12 weeks. Study arms comprised single-ascending dosing (increasing from 1 mg/d to 25 mg), and multiple-ascending dosing. The latter consisted of multiple ascending doses (from 3 to 12 mg) over 10 days and multiple ascending doses (stepwise dose escalation, from 3 mg up to a final dose of 2 × 50 mg) over 12 weeks.
In her presentation at the EASD meeting, Dr. Gasiorek focused on results of the 12-week multiple ascending dose schedule with amycretin 50 mg (n = 16), amycretin 2 × 50 mg (n = 16), and placebo (n = 12).
The primary endpoint of the study was the number of treatment-emergent adverse events, while the area under the amycretin plasma concentration time curve and the maximum plasma concentration of amycretin were secondary endpoints.
The researchers also added percentage change in body weight after 12 weeks of treatment as an exploratory endpoint.
Safety Findings of Multiple Dosing
A total of 242 treatment-emergent adverse events were reported in the combined active and placebo groups and were of mild to moderate severity.
Treatment-emergent adverse events were found in 75% of the amycretin 50 mg group, 93.8% of the amycretin 2 × 50 mg group, and 33.3% of placebo recipients.
“Most adverse events reported were mild to moderate in severity and related to gastrointestinal discomfort (nausea and vomiting) and occurred in a dose-proportional manner,” reported Dr. Gasiorek.
Gastrointestinal events were experienced by 50%, 87.5%, and 16.7% of participants receiving amycretin 50 mg, amycretin 2 × 50 mg, and placebo, respectively (112 in total).
Decreased appetite was also found in 56.3%, 81.3%, and 16.7% of the amycretin 50 mg, amycretin 2 × 50 mg, and placebo groups, respectively.
Two serious adverse events occurred, one of which was acute cholecystitis and the other diabetic ketoacidosis; “however, the [latter] participant was found to have autoantibodies for beta cells before treatment and was later diagnosed with type 1 diabetes,” Dr. Gasiorek said.
Body Weight Reduction
Participants on 50 mg amycretin lost an average of 10.4% of their body weight (estimated treatment difference vs placebo, –9.2; 95% CI, –12.0 to –6.5), whereas those on 2 × 50 mg amycretin lost 13.1% of their body weight (estimated treatment difference vs placebo, –11.8; 95% CI, –14.6 to –9.0). Placebo group participants lost 1.2% of their body weight over the 12 weeks.
Although no plateauing of weight loss was seen, said Dr. Gasiorek, it is important to consider the relatively short treatment duration and the limited time on the final dose, which could potentially introduce bias.
To date, weight loss medications based on GLP-1 RA technology are injectables. A combination of the injectable amylin analogue cagrilintide and the GLP-1 RA semaglutide is also being explored as a subcutaneous treatment solution.
In a comment, Martin Holst Lange, MD, PhD, executive vice president of development at Novo Nordisk, said that “amycretin is the first treatment to harness the two distinct biological pathways stimulated by amylin and GLP-1 in a single molecule.”
The safety and tolerability profiles and the magnitude of weight loss support further development of amycretin in patients with overweight or obesity, said Dr. Lange, who noted that the company was awaiting data from the ongoing phase 1 trial with subcutaneous amycretin, expected in 2025.
Having heard the presentation, co-moderator Timo Müller, PhD, professor at Ludwig Maximilian University of Munich, Germany, gave a considered response. “The drug was relatively well tolerated, with the typical GLP-1–induced GI [gastrointestinal] adverse effects being the most frequently reported.”
But he pointed out that questions remain. “We still need to know whether, at the given dose, the drug outperforms best-in-class drugs like semaglutide or tirzepatide at the highest approved doses. Furthermore, it warrants clarification if and to what extent the activation of the amylin receptor contributes to the shown effect and if and to what extent the glycemic benefits result from activation of the glucagon receptor (amylin improved glycemia by decreasing the secretion of glucagon). In any way, the current data remain friendly and support phase 2 development.”
Oral Meds Could Bring Down Cost
Commenting on the data, Nerys Astbury, PhD, associate professor of diet and obesity at Nuffield Department of Primary Health Care Sciences, University of Oxford, England, said, “It is important to note that whilst the participants in this trial did lose weight over the 12-week study — and this was statistically more weight than in the placebo group — this study was not designed or powered to detect differences in body weight over longer periods of time.”
If the results are confirmed in future studies, amycretin might widen the treatment options and introduce competition, probably bringing down the costs in the longer-term, said Dr. Astbury, who welcomes the prospect.
“It is possible that some people might find the oral medications more acceptable than the injectable GLP-1 agonists currently available,” she said. And the current options are expensive, “which raises challenges to a taxpayer-funded health system like the NHS [National Health Service].”
“Furthermore, if the growing number of oral obesity medications prove safe, tolerable, and effective ... they are likely to significantly reduce the risks of developing many complications of obesity.”
Naveed Sattar, MD, professor of cardiometabolic medicine and honorary consultant, University of Glasgow, Scotland, agreed. “The more medicines coming forward to treat obesity, the better,” he said. In particular, oral medications would be more easily available, and cheaper, “for the many millions around the world struggling with obesity and its complications.”
Dr. Gasiorek declares she is an employee of and a shareholder in Novo Nordisk. Dr. Astbury declares no financial disclosures. Dr. Sattar declares having consulted for several companies that make diabetes medicines but also contributed to several lifestyle trials. For Novo Nordisk, he has consulted for the company on advisory boards, but not on any of their weight loss drug trial committees, and he is on the steering committee for the ZEUS trial, which is not a weight loss trial product but an anti-inflammatory. He does not have any shares for any product in health etc. He declares consulting fees and/or speaker honoraria from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi, and grant support paid to his university from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics. Dr. Müller received financial support or an honorarium from Novo Nordisk, Merck, Eli Lilly, Boehringer Ingelheim, and Mercodia; he further holds stocks at Novo Nordisk and Eli Lilly and is cofounder of Bluewater Biosciences.
A version of this article first appeared on Medscape.com.
FROM EASD 2024
Genitourinary Symptoms in Men: Canaries in the Coal Mine for Underlying Chronic Disease
At age 57, a senior scientific researcher in Santa Barbara, California, complained of chronic erectile dysfunction (ED) in what had been a sexually active marriage. “I just couldn’t get an erection, let alone sustain one. Apart from that, I maybe felt a bit tired but generally okay,” he said. Though seemingly well otherwise, 18 months later he was dead of a hereditary right-sided colon cancer.
While not all cases of ED are associated with a dire outcome, the genitourinary signals of ED and lower urinary tract symptoms (LUTS), especially nocturia, serve as sentinel indicators of the presence of, or risk factors for, serious chronic conditions. These commonly include cardiovascular disease (CVD), diabetes, and metabolic syndrome and are associated with obesity, depression, and obstructive sleep apnea.
Sometimes these serious conditions may stay under the radar until men seek help for ED or LUTS.
“We know that among men who had a heart attack, 50% had some degree of ED within 3 years of their cardiac event,” Sam Tafari, MBBS, of the Endocrine and Metabolic Unit at Royal Adelaide Hospital in Adelaide, South Australia, said in an interview.
That’s the bad news. The good news is that these two problems may specifically incentivize men to seek timely care for serious conditions they might otherwise not get, according to Dr. Tafari. And primary care doctors are ideally positioned to get men early multifaceted care. He recently coauthored a call to action on this issue in a review appearing in the Journal of Men’s Health.
In Dr. Tafari’s experience, most patients seeking urological care are unaware of the multiple conditions linked to ED and LUTS. “Many consider these to be due to issues like low testosterone, which actually make up a very small proportion of cases of ED,” he said. Aging, obesity, inactivity, smoking, alcohol abuse, and prescription and street drugs can also contribute to the development of ED.
In most affected men, ED is of vascular etiology, with endothelial dysfunction of the inner lining of blood vessels and smooth muscle the common denominator.
This dysfunction causes inadequate blood supply to both the coronary and the penile arteries, so ED and CVD are considered different manifestations of the same systemic disorder. Because the tumescence-controlling cavernosal vessels of the penis are considerably smaller, the same level of arteriopathy causes a more severe reduction in blood in the erectile tissue. As a result, ED often precedes CVD and presents an early opportunity to screen men for CVD.
As to the mechanisms behind LUTS, Peter N. Tsambarlis, MD, a urologist at Northwestern Medicine in Chicago, subscribes to the inflammation theory. “Suboptimal health issues such as high [blood] pressure, blood lipids, and blood glucose lead to chronic widespread inflammation, which makes the bladder less flexible as a storage vessel,” he explained. “It’s not able to stretch adequately overnight to hold the urine until morning.”
Ask Early, Ask Often
Jeffrey P. Weiss, MD, PhD, chair of the Department of Urology at SUNY Downstate Health Sciences University in Brooklyn, New York, has done research that uncovered a relationship between structural cardiac disease and nocturia. “So if you had to ask a patient a single question that would point to a global health issue, it would be ‘Do you have frequent nighttime urination,’ ” he said.
It’s never too soon to ask men about these symptoms, said Dr. Tsambarlis. The best time to raise issues of ED and LUTS is when a man enters primary care — regardless of age or absence of symptoms. “That way you have a baseline and can watch for changes and do early intervention as needed. Men don’t usually want to bring up sexual dysfunction or urinary health, but asking doesn’t need to dominate the visit,” he said.
Dr. Tafari recommends that primary care physicians adopt a targeted approach using ED and nocturia as entry points for engaging men in their healthcare. While acknowledging that primary care physicians have an ever-growing checklist of questions to ask patients and hardly need one more thing to screen for, he suggests asking two quick, and easy “before you go” genitourinary queries:
- Are you having trouble with erections or having sex?
- Are you getting up at night to pass urine more than once?
“The men really appreciate being asked,” he said. “But what worries me is all the men we don’t see who have these symptoms but don’t know they’re important, and no one is asking about them.”
Gideon Richards, MD, a urologist at the Northwell Health Physician Partners Smith Institute for Urology at Garden City, and director of Men’s Health, Central Region, for Northwell Health in New Hyde Park, both in New York, said erectile problems should not wait for specialty care. By the time men with ED are referred to urology, they may already have failed treatment with first-line phosphodiesterase 5 inhibitor therapy, he said. “A significant proportion will have arteriogenic erectile dysfunction, a measurable decrease in the amount of blood flow into the erectile bodies.”
Addressing the Issue
Addressing genitourinary-signaled issues has the double benefit of easing ED and LUTS and improving men’s health and longevity and may help narrow the worldwide gender gap in life expectancy. As a recent global analysis found, there’s a 5-year longevity disparity favoring women over men. Biology aside, men do not access healthcare as often as women, who consult their general practitioners regularly throughout their lifespan for multiple reasons, including reproductive care, and more screening programs are aimed at women.
Added Dr. Tsambarlis, “Men should know that losing weight and switching to a healthy lifestyle can improve sexual function about half as much as phosphodiesterase 5 inhibitors such as sildenafil [Viagra] or tadalafil [Cialis].”
“Many, however, would prefer just to take drugs rather than change their lifestyle and lose weight. There are certainly effective options available, but these are not uniformly effective,” said Dr. Weiss.
Dr. Tafari’s group is designing a short, simple, culturally acceptable screening tool for use in primary care practice and will monitor its impact on physician prescribing habits and overall men’s health outcomes.
Dr. Tafari received funding from the Hospital Research Foundation and Freemasons Centre for Male Health and Wellbeing in Adelaide, South Australia. Dr. Tafari, Dr. Tsambarlis, Dr. Weiss, and Dr. Richards had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
At age 57, a senior scientific researcher in Santa Barbara, California, complained of chronic erectile dysfunction (ED) in what had been a sexually active marriage. “I just couldn’t get an erection, let alone sustain one. Apart from that, I maybe felt a bit tired but generally okay,” he said. Though seemingly well otherwise, 18 months later he was dead of a hereditary right-sided colon cancer.
While not all cases of ED are associated with a dire outcome, the genitourinary signals of ED and lower urinary tract symptoms (LUTS), especially nocturia, serve as sentinel indicators of the presence of, or risk factors for, serious chronic conditions. These commonly include cardiovascular disease (CVD), diabetes, and metabolic syndrome and are associated with obesity, depression, and obstructive sleep apnea.
Sometimes these serious conditions may stay under the radar until men seek help for ED or LUTS.
“We know that among men who had a heart attack, 50% had some degree of ED within 3 years of their cardiac event,” Sam Tafari, MBBS, of the Endocrine and Metabolic Unit at Royal Adelaide Hospital in Adelaide, South Australia, said in an interview.
That’s the bad news. The good news is that these two problems may specifically incentivize men to seek timely care for serious conditions they might otherwise not get, according to Dr. Tafari. And primary care doctors are ideally positioned to get men early multifaceted care. He recently coauthored a call to action on this issue in a review appearing in the Journal of Men’s Health.
In Dr. Tafari’s experience, most patients seeking urological care are unaware of the multiple conditions linked to ED and LUTS. “Many consider these to be due to issues like low testosterone, which actually make up a very small proportion of cases of ED,” he said. Aging, obesity, inactivity, smoking, alcohol abuse, and prescription and street drugs can also contribute to the development of ED.
In most affected men, ED is of vascular etiology, with endothelial dysfunction of the inner lining of blood vessels and smooth muscle the common denominator.
This dysfunction causes inadequate blood supply to both the coronary and the penile arteries, so ED and CVD are considered different manifestations of the same systemic disorder. Because the tumescence-controlling cavernosal vessels of the penis are considerably smaller, the same level of arteriopathy causes a more severe reduction in blood in the erectile tissue. As a result, ED often precedes CVD and presents an early opportunity to screen men for CVD.
As to the mechanisms behind LUTS, Peter N. Tsambarlis, MD, a urologist at Northwestern Medicine in Chicago, subscribes to the inflammation theory. “Suboptimal health issues such as high [blood] pressure, blood lipids, and blood glucose lead to chronic widespread inflammation, which makes the bladder less flexible as a storage vessel,” he explained. “It’s not able to stretch adequately overnight to hold the urine until morning.”
Ask Early, Ask Often
Jeffrey P. Weiss, MD, PhD, chair of the Department of Urology at SUNY Downstate Health Sciences University in Brooklyn, New York, has done research that uncovered a relationship between structural cardiac disease and nocturia. “So if you had to ask a patient a single question that would point to a global health issue, it would be ‘Do you have frequent nighttime urination,’ ” he said.
It’s never too soon to ask men about these symptoms, said Dr. Tsambarlis. The best time to raise issues of ED and LUTS is when a man enters primary care — regardless of age or absence of symptoms. “That way you have a baseline and can watch for changes and do early intervention as needed. Men don’t usually want to bring up sexual dysfunction or urinary health, but asking doesn’t need to dominate the visit,” he said.
Dr. Tafari recommends that primary care physicians adopt a targeted approach using ED and nocturia as entry points for engaging men in their healthcare. While acknowledging that primary care physicians have an ever-growing checklist of questions to ask patients and hardly need one more thing to screen for, he suggests asking two quick, and easy “before you go” genitourinary queries:
- Are you having trouble with erections or having sex?
- Are you getting up at night to pass urine more than once?
“The men really appreciate being asked,” he said. “But what worries me is all the men we don’t see who have these symptoms but don’t know they’re important, and no one is asking about them.”
Gideon Richards, MD, a urologist at the Northwell Health Physician Partners Smith Institute for Urology at Garden City, and director of Men’s Health, Central Region, for Northwell Health in New Hyde Park, both in New York, said erectile problems should not wait for specialty care. By the time men with ED are referred to urology, they may already have failed treatment with first-line phosphodiesterase 5 inhibitor therapy, he said. “A significant proportion will have arteriogenic erectile dysfunction, a measurable decrease in the amount of blood flow into the erectile bodies.”
Addressing the Issue
Addressing genitourinary-signaled issues has the double benefit of easing ED and LUTS and improving men’s health and longevity and may help narrow the worldwide gender gap in life expectancy. As a recent global analysis found, there’s a 5-year longevity disparity favoring women over men. Biology aside, men do not access healthcare as often as women, who consult their general practitioners regularly throughout their lifespan for multiple reasons, including reproductive care, and more screening programs are aimed at women.
Added Dr. Tsambarlis, “Men should know that losing weight and switching to a healthy lifestyle can improve sexual function about half as much as phosphodiesterase 5 inhibitors such as sildenafil [Viagra] or tadalafil [Cialis].”
“Many, however, would prefer just to take drugs rather than change their lifestyle and lose weight. There are certainly effective options available, but these are not uniformly effective,” said Dr. Weiss.
Dr. Tafari’s group is designing a short, simple, culturally acceptable screening tool for use in primary care practice and will monitor its impact on physician prescribing habits and overall men’s health outcomes.
Dr. Tafari received funding from the Hospital Research Foundation and Freemasons Centre for Male Health and Wellbeing in Adelaide, South Australia. Dr. Tafari, Dr. Tsambarlis, Dr. Weiss, and Dr. Richards had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
At age 57, a senior scientific researcher in Santa Barbara, California, complained of chronic erectile dysfunction (ED) in what had been a sexually active marriage. “I just couldn’t get an erection, let alone sustain one. Apart from that, I maybe felt a bit tired but generally okay,” he said. Though seemingly well otherwise, 18 months later he was dead of a hereditary right-sided colon cancer.
While not all cases of ED are associated with a dire outcome, the genitourinary signals of ED and lower urinary tract symptoms (LUTS), especially nocturia, serve as sentinel indicators of the presence of, or risk factors for, serious chronic conditions. These commonly include cardiovascular disease (CVD), diabetes, and metabolic syndrome and are associated with obesity, depression, and obstructive sleep apnea.
Sometimes these serious conditions may stay under the radar until men seek help for ED or LUTS.
“We know that among men who had a heart attack, 50% had some degree of ED within 3 years of their cardiac event,” Sam Tafari, MBBS, of the Endocrine and Metabolic Unit at Royal Adelaide Hospital in Adelaide, South Australia, said in an interview.
That’s the bad news. The good news is that these two problems may specifically incentivize men to seek timely care for serious conditions they might otherwise not get, according to Dr. Tafari. And primary care doctors are ideally positioned to get men early multifaceted care. He recently coauthored a call to action on this issue in a review appearing in the Journal of Men’s Health.
In Dr. Tafari’s experience, most patients seeking urological care are unaware of the multiple conditions linked to ED and LUTS. “Many consider these to be due to issues like low testosterone, which actually make up a very small proportion of cases of ED,” he said. Aging, obesity, inactivity, smoking, alcohol abuse, and prescription and street drugs can also contribute to the development of ED.
In most affected men, ED is of vascular etiology, with endothelial dysfunction of the inner lining of blood vessels and smooth muscle the common denominator.
This dysfunction causes inadequate blood supply to both the coronary and the penile arteries, so ED and CVD are considered different manifestations of the same systemic disorder. Because the tumescence-controlling cavernosal vessels of the penis are considerably smaller, the same level of arteriopathy causes a more severe reduction in blood in the erectile tissue. As a result, ED often precedes CVD and presents an early opportunity to screen men for CVD.
As to the mechanisms behind LUTS, Peter N. Tsambarlis, MD, a urologist at Northwestern Medicine in Chicago, subscribes to the inflammation theory. “Suboptimal health issues such as high [blood] pressure, blood lipids, and blood glucose lead to chronic widespread inflammation, which makes the bladder less flexible as a storage vessel,” he explained. “It’s not able to stretch adequately overnight to hold the urine until morning.”
Ask Early, Ask Often
Jeffrey P. Weiss, MD, PhD, chair of the Department of Urology at SUNY Downstate Health Sciences University in Brooklyn, New York, has done research that uncovered a relationship between structural cardiac disease and nocturia. “So if you had to ask a patient a single question that would point to a global health issue, it would be ‘Do you have frequent nighttime urination,’ ” he said.
It’s never too soon to ask men about these symptoms, said Dr. Tsambarlis. The best time to raise issues of ED and LUTS is when a man enters primary care — regardless of age or absence of symptoms. “That way you have a baseline and can watch for changes and do early intervention as needed. Men don’t usually want to bring up sexual dysfunction or urinary health, but asking doesn’t need to dominate the visit,” he said.
Dr. Tafari recommends that primary care physicians adopt a targeted approach using ED and nocturia as entry points for engaging men in their healthcare. While acknowledging that primary care physicians have an ever-growing checklist of questions to ask patients and hardly need one more thing to screen for, he suggests asking two quick, and easy “before you go” genitourinary queries:
- Are you having trouble with erections or having sex?
- Are you getting up at night to pass urine more than once?
“The men really appreciate being asked,” he said. “But what worries me is all the men we don’t see who have these symptoms but don’t know they’re important, and no one is asking about them.”
Gideon Richards, MD, a urologist at the Northwell Health Physician Partners Smith Institute for Urology at Garden City, and director of Men’s Health, Central Region, for Northwell Health in New Hyde Park, both in New York, said erectile problems should not wait for specialty care. By the time men with ED are referred to urology, they may already have failed treatment with first-line phosphodiesterase 5 inhibitor therapy, he said. “A significant proportion will have arteriogenic erectile dysfunction, a measurable decrease in the amount of blood flow into the erectile bodies.”
Addressing the Issue
Addressing genitourinary-signaled issues has the double benefit of easing ED and LUTS and improving men’s health and longevity and may help narrow the worldwide gender gap in life expectancy. As a recent global analysis found, there’s a 5-year longevity disparity favoring women over men. Biology aside, men do not access healthcare as often as women, who consult their general practitioners regularly throughout their lifespan for multiple reasons, including reproductive care, and more screening programs are aimed at women.
Added Dr. Tsambarlis, “Men should know that losing weight and switching to a healthy lifestyle can improve sexual function about half as much as phosphodiesterase 5 inhibitors such as sildenafil [Viagra] or tadalafil [Cialis].”
“Many, however, would prefer just to take drugs rather than change their lifestyle and lose weight. There are certainly effective options available, but these are not uniformly effective,” said Dr. Weiss.
Dr. Tafari’s group is designing a short, simple, culturally acceptable screening tool for use in primary care practice and will monitor its impact on physician prescribing habits and overall men’s health outcomes.
Dr. Tafari received funding from the Hospital Research Foundation and Freemasons Centre for Male Health and Wellbeing in Adelaide, South Australia. Dr. Tafari, Dr. Tsambarlis, Dr. Weiss, and Dr. Richards had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.