User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Check for neuromyelitis optica spectrum disorder in suspect HIV patients
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
FROM MULTIPLE SCLEROSIS AND RELATED DISORDERS
Open enrollment 2019: Busiest week so far at HealthCare.gov
but the weekly and cumulative totals for plans selected continued to run below last year’s levels, according to the Centers for Medicare & Medicaid Services.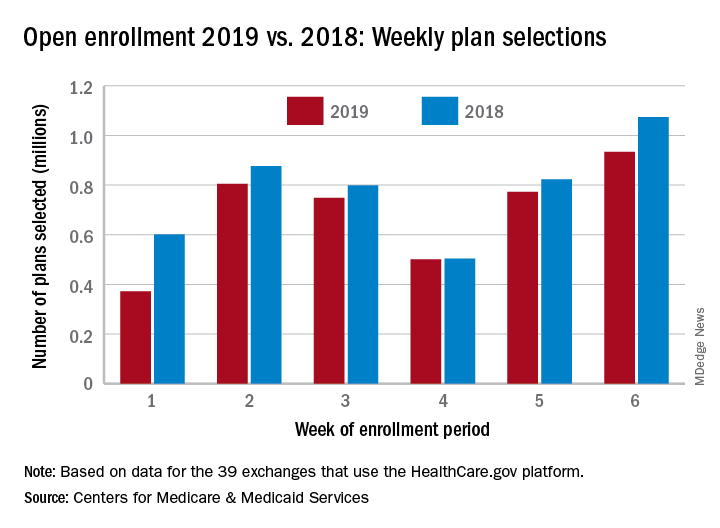
Over 934,000 plans were selected from Dec. 2 to Dec. 8, which puts the total at 4.13 million plans for the 2019 coverage year in the 39 states that use the HealthCare.gov platform, the CMS reported. Consumers renewing their coverage make up the majority of plans selected during week 6 (640,000) and cumulatively for the season (3.03 million), with new applications running at 295,000 for week 6 and 1.1 million overall.
Those numbers are down from last year, when 1.07 million plans (685,000 renewals and 389,000 new applications) were selected during week 6 of open enrollment for the 2018 coverage year, which brought the total for the season at the time to 4.68 million (3.30 million/1.38 million), CMS data show.
The deadline to enroll in a plan for 2019 is Dec. 15.
but the weekly and cumulative totals for plans selected continued to run below last year’s levels, according to the Centers for Medicare & Medicaid Services.
Over 934,000 plans were selected from Dec. 2 to Dec. 8, which puts the total at 4.13 million plans for the 2019 coverage year in the 39 states that use the HealthCare.gov platform, the CMS reported. Consumers renewing their coverage make up the majority of plans selected during week 6 (640,000) and cumulatively for the season (3.03 million), with new applications running at 295,000 for week 6 and 1.1 million overall.
Those numbers are down from last year, when 1.07 million plans (685,000 renewals and 389,000 new applications) were selected during week 6 of open enrollment for the 2018 coverage year, which brought the total for the season at the time to 4.68 million (3.30 million/1.38 million), CMS data show.
The deadline to enroll in a plan for 2019 is Dec. 15.
but the weekly and cumulative totals for plans selected continued to run below last year’s levels, according to the Centers for Medicare & Medicaid Services.
Over 934,000 plans were selected from Dec. 2 to Dec. 8, which puts the total at 4.13 million plans for the 2019 coverage year in the 39 states that use the HealthCare.gov platform, the CMS reported. Consumers renewing their coverage make up the majority of plans selected during week 6 (640,000) and cumulatively for the season (3.03 million), with new applications running at 295,000 for week 6 and 1.1 million overall.
Those numbers are down from last year, when 1.07 million plans (685,000 renewals and 389,000 new applications) were selected during week 6 of open enrollment for the 2018 coverage year, which brought the total for the season at the time to 4.68 million (3.30 million/1.38 million), CMS data show.
The deadline to enroll in a plan for 2019 is Dec. 15.
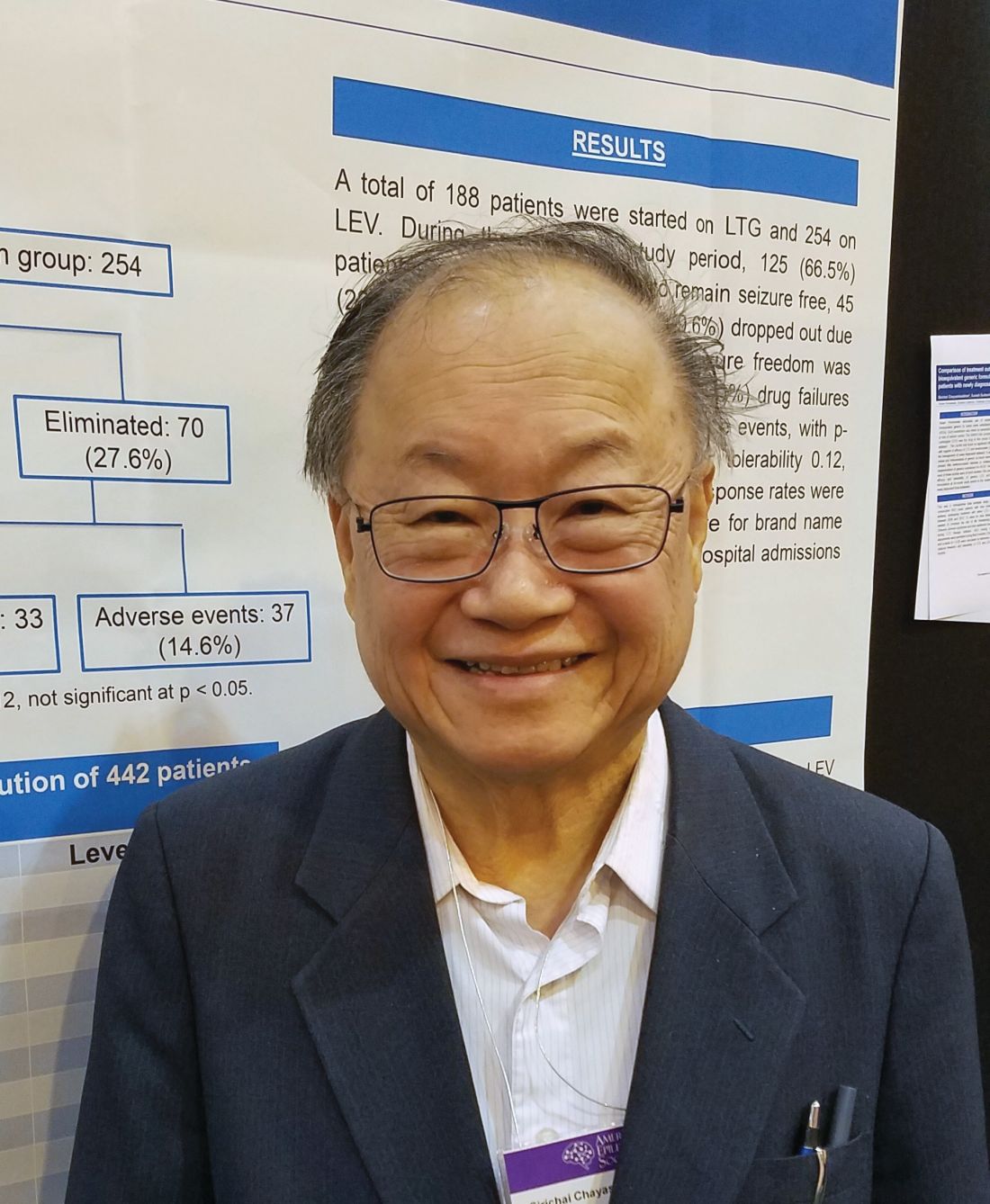
Bioequivalents lamotrigine, levetiracetam control new-onset focal seizures equally well
NEW ORLEANS – Bioequivalent generic formulations of levetiracetam and lamotrigine reduced seizures by a similar extent over 2 years in a retrospective study of patients with newly diagnosed focal epilepsy.
Each drug had a specific adverse event profile, with lamotrigine associated with rash and levetiracetam with mood disorders, Sirichai Chayasirisobhon, MD, said at the annual meeting of the American Epilepsy Society. This finding can play into the initial therapeutic decision, said Dr. Chayasirisobhon of Kaiser Permanente Southern California. “If someone comes in with depression or mood disorder, I will start on lamotrigine, not levetiracetam. And we can decrease the chance of rash with a very slow titration, as we did here, starting with just 5 mg/kg and working up over 6 months.”
Although the drugs have a somewhat similar teratogenic profile, Dr. Chayasirisobhon added that he favors lamotrigine for women of childbearing years. “It’s a little bit better choice for them I think.”
His retrospective analysis followed 442 patients from first seizure and medical therapy for 2 years. The generic medications came from Kaiser Permanente’s central pharmacy. They were single-source, with a proven 95% bioequivalence. The main outcome was the percentage of patients who became seizure free and remained so. Any seizure, whether febrile, breakthroughs, or from titration, was considered a failure. These patients were dropped from the study. Any patient who developed a drug-related rash was dropped from the study and started on another medication.
More women than men took lamotrigine (113 vs. 75), whereas more men took levetiracetam (148 vs. 106). Those taking lamotrigine were younger than were those taking levetiracetam (30 vs. 40 years).
At the end of 2 years, there was no statistically significant difference in the primary outcome of being free from seizures (66.5% with lamotrigine vs. 72.4% with levetiracetam). In the lamotrigine group, 33.5% were eliminated from the study, 24% because they had a seizure, and the rest due to an adverse event. In the levetiracetam group, 27.6% were eliminated, 13% because they had a seizure and the rest because of an adverse event.
Adverse events in the lamotrigine group included rash (12), dizziness (3), lethargy (1), and mood changes (2). Among the levetiracetam group, adverse events included dizziness (3), lethargy (7), mood changes (20), slowed thinking (4), depression (2) and headache (1).
“Rash was the main event we saw in this group, and this was even when we did a very slow titration of 5 mg/kg per week,” Dr. Chayasirisobhon said. “Any sign of rash or itching at all, we told them to stop immediately and call us. Fortunately, we had no cases of Steven-Johnson syndrome and all our cases of rash were transient. But in the levetiracetam group, the mood changes are the major thing. Some of the patients became very agitated and aggressive. Whenever we see a patient for the first time, we always ask about mood changes, and we instruct the family to call and report any changes in mood immediately.”
Aside from reproductive age, however, Dr. Chayasirisobhon generally prefers to start new patients on levetiracetam. Its safety profile is remarkable, he said, recounting a case report he published in 2010 (Acta Neurol Taiwan. 2010;19:292-5).
The paper describes a male patient who decided to commit suicide after an argument with his wife. He took his levetiracetam and walked to his father’s grave, swallowing pills the entire time. When he arrived at the grave, he had taken around 65 grams of the medication. “The amazing thing was, he’s still walking, just a little unsteady. Then he decided he’s not ready to die,” Dr. Chayasirisobhon said. “He was able to call 911, so he’s still talking fine. When they checked his level it was so high, but he remained unimpaired except for the unsteady gait and some nystagmus.”
The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
NEW ORLEANS – Bioequivalent generic formulations of levetiracetam and lamotrigine reduced seizures by a similar extent over 2 years in a retrospective study of patients with newly diagnosed focal epilepsy.
Each drug had a specific adverse event profile, with lamotrigine associated with rash and levetiracetam with mood disorders, Sirichai Chayasirisobhon, MD, said at the annual meeting of the American Epilepsy Society. This finding can play into the initial therapeutic decision, said Dr. Chayasirisobhon of Kaiser Permanente Southern California. “If someone comes in with depression or mood disorder, I will start on lamotrigine, not levetiracetam. And we can decrease the chance of rash with a very slow titration, as we did here, starting with just 5 mg/kg and working up over 6 months.”
Although the drugs have a somewhat similar teratogenic profile, Dr. Chayasirisobhon added that he favors lamotrigine for women of childbearing years. “It’s a little bit better choice for them I think.”
His retrospective analysis followed 442 patients from first seizure and medical therapy for 2 years. The generic medications came from Kaiser Permanente’s central pharmacy. They were single-source, with a proven 95% bioequivalence. The main outcome was the percentage of patients who became seizure free and remained so. Any seizure, whether febrile, breakthroughs, or from titration, was considered a failure. These patients were dropped from the study. Any patient who developed a drug-related rash was dropped from the study and started on another medication.
More women than men took lamotrigine (113 vs. 75), whereas more men took levetiracetam (148 vs. 106). Those taking lamotrigine were younger than were those taking levetiracetam (30 vs. 40 years).
At the end of 2 years, there was no statistically significant difference in the primary outcome of being free from seizures (66.5% with lamotrigine vs. 72.4% with levetiracetam). In the lamotrigine group, 33.5% were eliminated from the study, 24% because they had a seizure, and the rest due to an adverse event. In the levetiracetam group, 27.6% were eliminated, 13% because they had a seizure and the rest because of an adverse event.
Adverse events in the lamotrigine group included rash (12), dizziness (3), lethargy (1), and mood changes (2). Among the levetiracetam group, adverse events included dizziness (3), lethargy (7), mood changes (20), slowed thinking (4), depression (2) and headache (1).
“Rash was the main event we saw in this group, and this was even when we did a very slow titration of 5 mg/kg per week,” Dr. Chayasirisobhon said. “Any sign of rash or itching at all, we told them to stop immediately and call us. Fortunately, we had no cases of Steven-Johnson syndrome and all our cases of rash were transient. But in the levetiracetam group, the mood changes are the major thing. Some of the patients became very agitated and aggressive. Whenever we see a patient for the first time, we always ask about mood changes, and we instruct the family to call and report any changes in mood immediately.”
Aside from reproductive age, however, Dr. Chayasirisobhon generally prefers to start new patients on levetiracetam. Its safety profile is remarkable, he said, recounting a case report he published in 2010 (Acta Neurol Taiwan. 2010;19:292-5).
The paper describes a male patient who decided to commit suicide after an argument with his wife. He took his levetiracetam and walked to his father’s grave, swallowing pills the entire time. When he arrived at the grave, he had taken around 65 grams of the medication. “The amazing thing was, he’s still walking, just a little unsteady. Then he decided he’s not ready to die,” Dr. Chayasirisobhon said. “He was able to call 911, so he’s still talking fine. When they checked his level it was so high, but he remained unimpaired except for the unsteady gait and some nystagmus.”
The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
NEW ORLEANS – Bioequivalent generic formulations of levetiracetam and lamotrigine reduced seizures by a similar extent over 2 years in a retrospective study of patients with newly diagnosed focal epilepsy.
Each drug had a specific adverse event profile, with lamotrigine associated with rash and levetiracetam with mood disorders, Sirichai Chayasirisobhon, MD, said at the annual meeting of the American Epilepsy Society. This finding can play into the initial therapeutic decision, said Dr. Chayasirisobhon of Kaiser Permanente Southern California. “If someone comes in with depression or mood disorder, I will start on lamotrigine, not levetiracetam. And we can decrease the chance of rash with a very slow titration, as we did here, starting with just 5 mg/kg and working up over 6 months.”
Although the drugs have a somewhat similar teratogenic profile, Dr. Chayasirisobhon added that he favors lamotrigine for women of childbearing years. “It’s a little bit better choice for them I think.”
His retrospective analysis followed 442 patients from first seizure and medical therapy for 2 years. The generic medications came from Kaiser Permanente’s central pharmacy. They were single-source, with a proven 95% bioequivalence. The main outcome was the percentage of patients who became seizure free and remained so. Any seizure, whether febrile, breakthroughs, or from titration, was considered a failure. These patients were dropped from the study. Any patient who developed a drug-related rash was dropped from the study and started on another medication.
More women than men took lamotrigine (113 vs. 75), whereas more men took levetiracetam (148 vs. 106). Those taking lamotrigine were younger than were those taking levetiracetam (30 vs. 40 years).
At the end of 2 years, there was no statistically significant difference in the primary outcome of being free from seizures (66.5% with lamotrigine vs. 72.4% with levetiracetam). In the lamotrigine group, 33.5% were eliminated from the study, 24% because they had a seizure, and the rest due to an adverse event. In the levetiracetam group, 27.6% were eliminated, 13% because they had a seizure and the rest because of an adverse event.
Adverse events in the lamotrigine group included rash (12), dizziness (3), lethargy (1), and mood changes (2). Among the levetiracetam group, adverse events included dizziness (3), lethargy (7), mood changes (20), slowed thinking (4), depression (2) and headache (1).
“Rash was the main event we saw in this group, and this was even when we did a very slow titration of 5 mg/kg per week,” Dr. Chayasirisobhon said. “Any sign of rash or itching at all, we told them to stop immediately and call us. Fortunately, we had no cases of Steven-Johnson syndrome and all our cases of rash were transient. But in the levetiracetam group, the mood changes are the major thing. Some of the patients became very agitated and aggressive. Whenever we see a patient for the first time, we always ask about mood changes, and we instruct the family to call and report any changes in mood immediately.”
Aside from reproductive age, however, Dr. Chayasirisobhon generally prefers to start new patients on levetiracetam. Its safety profile is remarkable, he said, recounting a case report he published in 2010 (Acta Neurol Taiwan. 2010;19:292-5).
The paper describes a male patient who decided to commit suicide after an argument with his wife. He took his levetiracetam and walked to his father’s grave, swallowing pills the entire time. When he arrived at the grave, he had taken around 65 grams of the medication. “The amazing thing was, he’s still walking, just a little unsteady. Then he decided he’s not ready to die,” Dr. Chayasirisobhon said. “He was able to call 911, so he’s still talking fine. When they checked his level it was so high, but he remained unimpaired except for the unsteady gait and some nystagmus.”
The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
REPORTING FROM AES 2018
Key clinical point:
Major finding: At 2 years, 66.5% of the lamotrigine group and 72.4% of the levetiracetam group were seizure free.
Study details: The retrospective study comprised 442 patients.
Disclosures: The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
Source: Chayasirisobhon S et al. AES 2018, Abstract 2.147.
Rural teleprescribing for opioid use disorder shows success
BONITA SPRINGS, FLA. – Clinician shortages and alarming opioid overdose rates are prompting rural health care centers to turn to telemedicine for delivering treatments for opioid use disorder (OUD). Several studies suggest that these treatments are being delivered effectively, experts said at the annual meeting of the American Academy of Addiction Psychiatry.
In both Maryland and West Virginia, for example, success using a telehealth approach has been reported recently, said David Moore, MD, PhD, assistant professor of psychiatry at Yale University, New Haven, Conn.
Specifically, in rural Maryland, physicians used telemedicine to provide buprenorphine treatment for OUD at a treatment center in August 2015. Researchers at the University of Maryland, Baltimore, looked at the first 177 of the patients treated with the approach. They found that retention in treatment was 91% at 1 month and 57% at 3 months. Of patients still in treatment at 3 months, 86% had urine that was opioid negative, researchers said (Am J Addict. 2018 Dec;27[8]:612-17).
And in West Virginia, researchers reviewed the records of 100 patients receiving buprenorphine treatment to compare outcomes of those treated with telemedicine and those treated face-to-face. They found no significant differences between the groups in additional substance use, time to achieve 30 days and 90 days of abstinence, or retention rates at 3 months and 1 year (J Addict Med. 2017 Mar-Apr;11[2]:138-44).
In addition, Dr. Moore said, he has had success with home inductions in the northern reaches of Maine. His first home induction involved a 55-year-old veteran with a history of oxycodone and hydrocodone use who used illicit buprenorphine when he could. Dr. Moore said the man was referred to him on a Monday. He called in a prescription for the drug the next day and gave the patient a handout on how to do a home induction. “Then he had a phone check-in, and we followed up on Thursday. It actually worked really well,” Dr. Moore said.
The dearth of buprenorphine providers in northern Maine makes those kinds of arrangements attractive, he said. In Maine’s Piscataquis County, he said, there is one buprenorphine provider for every 2,000 square miles. “We have about 1 in every 5 square miles in New Haven,” he said. “Thinking about the distance you have to travel, it gets to be pretty daunting.”
The ability of clinicians to provide buprenorphine with telemedicine varies by state. Among the resources needed to provide telemedicine services are reliable Internet access and an ability for a patient to consent to the treatment.
Nationwide, 56.3% of rural counties have no buprenorphine provider, according to a recent study, said Lewei (Allison) Lin, MD, assistant professor of psychiatry at the University of Michigan, Ann Arbor. A survey of 1,100 rural providers, results of which were included in that study, found that 48% of them said concerns about substance diversion or misuse were a barrier to providing buprenorphine, and 44% cited a lack of mental health or psychosocial support services.
“Although this country still has a major issue with access to treatment, we see that the access problem is about double in rural areas,” she said. “If you add on the distance issue and time, this becomes an even greater challenge.”
Nurse practitioners and physician assistants are now able to obtain a Drug Enforcement Administration waiver that will allow them to prescribe OUD, thanks to the Comprehensive Addiction and Recovery Act of 2016.
BONITA SPRINGS, FLA. – Clinician shortages and alarming opioid overdose rates are prompting rural health care centers to turn to telemedicine for delivering treatments for opioid use disorder (OUD). Several studies suggest that these treatments are being delivered effectively, experts said at the annual meeting of the American Academy of Addiction Psychiatry.
In both Maryland and West Virginia, for example, success using a telehealth approach has been reported recently, said David Moore, MD, PhD, assistant professor of psychiatry at Yale University, New Haven, Conn.
Specifically, in rural Maryland, physicians used telemedicine to provide buprenorphine treatment for OUD at a treatment center in August 2015. Researchers at the University of Maryland, Baltimore, looked at the first 177 of the patients treated with the approach. They found that retention in treatment was 91% at 1 month and 57% at 3 months. Of patients still in treatment at 3 months, 86% had urine that was opioid negative, researchers said (Am J Addict. 2018 Dec;27[8]:612-17).
And in West Virginia, researchers reviewed the records of 100 patients receiving buprenorphine treatment to compare outcomes of those treated with telemedicine and those treated face-to-face. They found no significant differences between the groups in additional substance use, time to achieve 30 days and 90 days of abstinence, or retention rates at 3 months and 1 year (J Addict Med. 2017 Mar-Apr;11[2]:138-44).
In addition, Dr. Moore said, he has had success with home inductions in the northern reaches of Maine. His first home induction involved a 55-year-old veteran with a history of oxycodone and hydrocodone use who used illicit buprenorphine when he could. Dr. Moore said the man was referred to him on a Monday. He called in a prescription for the drug the next day and gave the patient a handout on how to do a home induction. “Then he had a phone check-in, and we followed up on Thursday. It actually worked really well,” Dr. Moore said.
The dearth of buprenorphine providers in northern Maine makes those kinds of arrangements attractive, he said. In Maine’s Piscataquis County, he said, there is one buprenorphine provider for every 2,000 square miles. “We have about 1 in every 5 square miles in New Haven,” he said. “Thinking about the distance you have to travel, it gets to be pretty daunting.”
The ability of clinicians to provide buprenorphine with telemedicine varies by state. Among the resources needed to provide telemedicine services are reliable Internet access and an ability for a patient to consent to the treatment.
Nationwide, 56.3% of rural counties have no buprenorphine provider, according to a recent study, said Lewei (Allison) Lin, MD, assistant professor of psychiatry at the University of Michigan, Ann Arbor. A survey of 1,100 rural providers, results of which were included in that study, found that 48% of them said concerns about substance diversion or misuse were a barrier to providing buprenorphine, and 44% cited a lack of mental health or psychosocial support services.
“Although this country still has a major issue with access to treatment, we see that the access problem is about double in rural areas,” she said. “If you add on the distance issue and time, this becomes an even greater challenge.”
Nurse practitioners and physician assistants are now able to obtain a Drug Enforcement Administration waiver that will allow them to prescribe OUD, thanks to the Comprehensive Addiction and Recovery Act of 2016.
BONITA SPRINGS, FLA. – Clinician shortages and alarming opioid overdose rates are prompting rural health care centers to turn to telemedicine for delivering treatments for opioid use disorder (OUD). Several studies suggest that these treatments are being delivered effectively, experts said at the annual meeting of the American Academy of Addiction Psychiatry.
In both Maryland and West Virginia, for example, success using a telehealth approach has been reported recently, said David Moore, MD, PhD, assistant professor of psychiatry at Yale University, New Haven, Conn.
Specifically, in rural Maryland, physicians used telemedicine to provide buprenorphine treatment for OUD at a treatment center in August 2015. Researchers at the University of Maryland, Baltimore, looked at the first 177 of the patients treated with the approach. They found that retention in treatment was 91% at 1 month and 57% at 3 months. Of patients still in treatment at 3 months, 86% had urine that was opioid negative, researchers said (Am J Addict. 2018 Dec;27[8]:612-17).
And in West Virginia, researchers reviewed the records of 100 patients receiving buprenorphine treatment to compare outcomes of those treated with telemedicine and those treated face-to-face. They found no significant differences between the groups in additional substance use, time to achieve 30 days and 90 days of abstinence, or retention rates at 3 months and 1 year (J Addict Med. 2017 Mar-Apr;11[2]:138-44).
In addition, Dr. Moore said, he has had success with home inductions in the northern reaches of Maine. His first home induction involved a 55-year-old veteran with a history of oxycodone and hydrocodone use who used illicit buprenorphine when he could. Dr. Moore said the man was referred to him on a Monday. He called in a prescription for the drug the next day and gave the patient a handout on how to do a home induction. “Then he had a phone check-in, and we followed up on Thursday. It actually worked really well,” Dr. Moore said.
The dearth of buprenorphine providers in northern Maine makes those kinds of arrangements attractive, he said. In Maine’s Piscataquis County, he said, there is one buprenorphine provider for every 2,000 square miles. “We have about 1 in every 5 square miles in New Haven,” he said. “Thinking about the distance you have to travel, it gets to be pretty daunting.”
The ability of clinicians to provide buprenorphine with telemedicine varies by state. Among the resources needed to provide telemedicine services are reliable Internet access and an ability for a patient to consent to the treatment.
Nationwide, 56.3% of rural counties have no buprenorphine provider, according to a recent study, said Lewei (Allison) Lin, MD, assistant professor of psychiatry at the University of Michigan, Ann Arbor. A survey of 1,100 rural providers, results of which were included in that study, found that 48% of them said concerns about substance diversion or misuse were a barrier to providing buprenorphine, and 44% cited a lack of mental health or psychosocial support services.
“Although this country still has a major issue with access to treatment, we see that the access problem is about double in rural areas,” she said. “If you add on the distance issue and time, this becomes an even greater challenge.”
Nurse practitioners and physician assistants are now able to obtain a Drug Enforcement Administration waiver that will allow them to prescribe OUD, thanks to the Comprehensive Addiction and Recovery Act of 2016.
REPORTING FROM AAAP 2018
Real-world data reveal long-lasting effects achieved with RRMS treatments
BERLIN – Real-world data from six postmarketing surveillance studies suggest that currently available disease-modifying treatments (DMTs) for relapsing-remitting multiple sclerosis (RRMS) have long-lasting effects that are matched by reasonable tolerability.
Long-term efficacy and safety data on natalizumab (Tysabri), fingolimod (Gilenya), alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), and teriflunomide (Aubagio) from four Swedish studies, one French study, and one international study were reported during a poster session on long-term treatment monitoring at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The IMSE 1 study with natalizumab
The Immunomodulation and Multiple Sclerosis Epidemiology (IMSE) studies are Swedish postmarketing surveillance studies that were started with the launch of various DMTs in Sweden: natalizumab since 2006 (IMSE 1), fingolimod in 2015 (IMSE 2), alemtuzumab in 2014 (IMSE 3), and dimethyl fumarate in 2014 (IMSE 5).
“Postmarketing surveillance is important for determination of long-term safety and effectiveness in a real-world setting,” Stina Kågström and her associates observed in their poster reporting some findings of the IMSE 1 study with natalizumab (Mult Scler. 2018;24[S2]:699-700, Abstract P1232).
Ms. Kågström of the department of clinical neuroscience at the Karolinska Institute in Stockholm and her colleagues reported that data on 3,108 patients who were seen at 54 Swedish clinics had been collated via the nationwide Swedish Quality Registry for Neurological Care (NEUROreg). NEUROreg started out as an MS register but has since widened its remit to include other neurologic diagnoses.
For the IMSE 1 study, prospectively recorded data regarding natalizumab treatment, adverse events, JC-virus (JCV) status and clinical effectiveness measures were obtained from NEUROreg for 2,225 women and 883 men. Just over one-third (37%, n = 1,150) were still receiving natalizumab at the time of the analysis.
The mean age at which natalizumab was started was 39 years, with treatment primarily given for RRMS (81% of patients) and less often for secondary progressive multiple sclerosis (SPMS, 15%) and rarely for other types of progressive MS. The mean treatment duration was just under 4 years (47.6 months).
JCV testing was introduced in 2011 in Sweden, and this “has led to fewer treated JCV-positive patients,” the IMSE 1 study investigators reported. “This likely explains a reduced incidence of PML [progressive multifocal leukoencephalopathy],” they suggested. There were nine PML cases diagnosed in Sweden from 2008 to the data cut-off point in 2018, one of which was fatal.
JCV status from 2011 onward was available for 1,269 patients, of whom 39% were JCV positive and 61% were JCV negative. The overall drug survival rate was 72% for JCV-negative and 14% for JCV-positive patients. Improved health status was seen, as measured by the Expanded Disability Status Scale (EDSS), the Multiple Sclerosis Severity Score (MSSS), and the physical and psychological Multiple Sclerosis Impact Scale–29 (MSIS-29) components.
A total of 644 of 1,269 patients discontinued treatment with natalizumab at some point, of whom 67% discontinued because of being JCV positive. The main reason for discontinuation in JCV-negative patients was pregnancy or planning a pregnancy (38%), with lack of effect (10%) and adverse events (11%) as other key reasons for stopping natalizumab.
Ms. Kågström and her associates concluded that natalizumab was “generally well tolerated with sustained effectiveness.”
The IMSE 2 study with fingolimod
Data on the long-term safety and efficacy of fingolimod were reported from the IMSE 2 study (Mult Scler. 2018;24[S2]:696-7, Abstract P1228). Lead author Anna Fält, also of the Karolinska Institute, and her associates analyzed data for 1,634 patients who had been treated with fingolimod from June 2015 to September 2018.
Most patients were older than 30 years (79%), and those aged 30 and older were predominantly female (69%), had an RRMS diagnosis (88%), and been treated for a mean of about 3 years (37 months). A total of 829 were being treated with fingolimod at the time of the analysis, with 844 having discontinued treatment at some point. The main reason for discontinuing treatment with fingolimod was a lack of effect (42% of cases) or an adverse effect (34%). The IMSE 2 study authors reported in their abstract that most patients were switched to rituximab after discontinuing fingolimod.
The number of relapses per 1,000 patient-years was reduced by fingolimod treatment from 280 to 82, comparing before and during treatment for all age groups studied. Relapse rate dropped from 694 per 1,000 patient-years before treatment to 138 during treatment in patients aged 20 years or younger, from 454 to 122 in those aged 21-30 years, and from 257 to 72 in those older than 31 years.
After 1 year of treatment, improvements were seen in the health status of patients as measured by various scales, including the EDSS, MSSS, MSIS-29 Physical, and MSIS-29 Psychological. When the researchers analyzed data by age groups, significant improvements were seen in patients aged 21-30 years and older than 30 years.
Ninety nonserious and 62 serious adverse events were reported in fingolimod-treated patients during the time of analysis. Of the latter, 13 serious adverse events involved cardiac disorders, 12 neoplasms, and 10 infections and infestations.
Overall, the IMSE 2 study investigators said that fingolimod was generally tolerable and reduced disease activity in MS.
French experience with fingolimod: The VIRGILE study
Real-world data on the long-term safety and efficacy of fingolimod in France from the VIRGILE study were reported by Christine Lebrun-Frenay, MD, PhD, and her associates (Mult Scler. 2018;24[S2]:698-9, Abstract P1231).
Dr. Lebrun-Frenay of Pasteur 2 Hospital in Nice and her coauthors noted that VIRGILE study included patients starting treatment with fingolimod between January 2014 and February 2016. A total of 1,047 patients were included, and another 330 patients treated with natalizumab were included at the behest of the French health authorities.
The annualized relapse rate after 2 years of follow-up was 0.30 in the fingolimod group. Dr. Lebrun-Frenay and her colleagues noted: “The 3-year data from this interim analysis provide evidence for sustained efficacy of fingolimod.” Indeed, they report that almost 60% of patients did not relapse and 64% had no worsening of disease. On average, EDSS was stable during the 3-year follow-up period.
“Safety and tolerability profiles of fingolimod were in line with previous clinical experience, with lymphopenia being the most frequent AE [adverse event] reported,” they added.
The IMSE 3 study with alemtuzumab
Long-term experience with alemtuzumab as a treatment for RRMS in the real-world setting is more limited as it only became available for use for this indication in 2014, but some insight is provided by the results of the IMSE 3 study (Mult Scler. 2018;24[S2]:706-7, Abstract P1240).
In total, there were 113 patients treated with alemtuzumab; the vast majority (94%) had RRMS and were aged a mean of 34 years at the start of treatment. Treatment was for more than 12 months in 101 patients, more than 24 months in 86 patients, and more than 36 months in 36 patients.
“In patients treated for at least 12 months, significant improvements were seen in several clinical parameters,” Dr. Fält and her associates observed in their poster at ECTRIMS. The mean baseline and 12-month values for the EDSS were 2.0 and 1.6, and for the MSSS they were 3.46 and 2.61. The mean baseline and 12-month values for the MSIS-29 Psychological subscale were 35.1 and 30.8, respectively, and for MSIS-29 Physical they were 22.7 and 17.7.
Overall, there were 14 nonserious and 11 serious adverse events, the most common of which were infections and infestations, metabolism and nutrition disorders, and immune system disorders.
“A longer follow-up period is needed to assess the real-world effectiveness and safety of alemtuzumab,” the IMSE 3 study authors noted.
The IMSE 5 study with dimethyl fumarate
Similarly, the authors of the IMSE 5 study (Mult Scler. 2018;24[S2]:701-2, Abstract 1234) concluded that a longer follow-up period is need to assess the real-world effectiveness of dimethyl fumarate. Selin Safer Demirbüker, also of the Karolinska Institute, and her associates looked at data on 2,108 patients treated with dimethyl fumarate between March 2014 and April 2018, of whom 1,150 were still receiving treatment at the time of their assessment.
The mean age of patients at the start of treatment was 41 years, 91% had RRMS, and 73% were female. The mean treatment duration was 22.3 months. The majority of patients (n = 867) had been previously treated with interferon and glatiramer acetate (Copaxone) prior to dimethyl fumarate, with 538 being naive to treatment.
“Dimethyl fumarate seems to have a positive effect for patients remaining on treatment,” wrote Ms. Safer Demirbüker and her colleagues. The overall 1-year drug survival reported in their abstract was 74%. Their poster showed a lower 2-year drug survival rate of 63.5% for men and 56.4% for women.
“Swedish patients show cognitive, psychological, and physical benefits after 2 or more years of treatment,” the IMSE 5 study authors further noted. Mean EDSS, MSSS, and MSIS-29 Psychological values all fell from baseline to 2 years.
Overall, 958 (47%) of patients discontinued treatment with dimethyl fumarate at some point, primarily (in 52% of cases) because of adverse events or lack of effect (29% of cases). Most patients (39%) switched to rituximab (15% had no new treatment registered), but 35% of patients continued treatment for 3 or more years.
Twelve-year follow-up of teriflunomide shows continued efficacy, safety
Mark Freedman, MD, of the University of Ottawa and the Ottawa Hospital Research Institute and his associates reported long-term follow-up data on the efficacy and safety of teriflunomide (Aubagio) in relapsing forms of MS (Mult Scler. 2018;24[S2]:700-1, Abstract P1233). After up to 12 years’ follow up, teriflunomide 14 mg was associated with an overall annualized relapse rate of 0.228.
Yearly annualized relapse rates were “low and stable,” Dr. Freedman and his coauthors from the United States, Spain, Italy, France, Germany, England, the Republic of Korea, and Australia noted in their poster.
“As of August 2018, over 93,000 patients were being treated with teriflunomide,” the authors stated. This represented a real-world exposure of approximately 186,000 patient-years up to December 2017, they added.
For the analysis, data from one phase 2 study and three phase 3 studies (TEMSO, TOWER, and TENERE) and their long-term extension studies were pooled. In all, there were 1,696 patients treated with 14 mg of teriflunomide in these studies.
Annualized relapse rates ranged from 0.321 in the first year of follow-up in the studies to 0.080 by the 12th year. The proportions of patients remaining relapse free “were high and stable (ranging from 0.75 in year 1 to 0.93 in years 8 and 9).” EDSS scores were 2.57 at baseline and 2.27 at year 12.
Importantly, no new safety signals were reported, Dr. Freedman and his colleagues wrote, adding that most adverse events were mild to moderate in severity.
Taken together, “these data demonstrate the long-term efficacy and safety of teriflunomide,” they concluded.
Study and author disclosures
The teriflunomide analysis was supported by Sanofi. Dr. Freedman disclosed receiving research or educational grant support from Bayer and Genzyme; honoraria/consulting fees from Bayer, Biogen, EMD Canada, Novartis, Sanofi, and Teva; and membership on company advisory boards/boards of directors/other similar groups for Bayer, Biogen, Chugai, Merck Serono, Novartis, Opexa Therapeutics, Sanofi, and Teva.
The IMSE 1 and 5 studies were supported by Biogen and the IMSE 2 and 3 studies by Novartis. The lead study authors for the IMSE studies – Dr. Kågström, Dr. Fält, and Dr. Safer Demirbüker – had nothing personal to disclose. Other authors included employees of the sponsoring companies or those who had received research funding or honoraria for consultancy work from the companies.
The VIRGILE study was supported by Novartis Pharma AG, Switzerland. Dr. Lebrun-Frenay disclosed receiving consultancy fees from Merck, Novartis, Biogen, MedDay, Roche, Teva, and Genzyme. Coauthors included Novartis employees.
BERLIN – Real-world data from six postmarketing surveillance studies suggest that currently available disease-modifying treatments (DMTs) for relapsing-remitting multiple sclerosis (RRMS) have long-lasting effects that are matched by reasonable tolerability.
Long-term efficacy and safety data on natalizumab (Tysabri), fingolimod (Gilenya), alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), and teriflunomide (Aubagio) from four Swedish studies, one French study, and one international study were reported during a poster session on long-term treatment monitoring at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The IMSE 1 study with natalizumab
The Immunomodulation and Multiple Sclerosis Epidemiology (IMSE) studies are Swedish postmarketing surveillance studies that were started with the launch of various DMTs in Sweden: natalizumab since 2006 (IMSE 1), fingolimod in 2015 (IMSE 2), alemtuzumab in 2014 (IMSE 3), and dimethyl fumarate in 2014 (IMSE 5).
“Postmarketing surveillance is important for determination of long-term safety and effectiveness in a real-world setting,” Stina Kågström and her associates observed in their poster reporting some findings of the IMSE 1 study with natalizumab (Mult Scler. 2018;24[S2]:699-700, Abstract P1232).
Ms. Kågström of the department of clinical neuroscience at the Karolinska Institute in Stockholm and her colleagues reported that data on 3,108 patients who were seen at 54 Swedish clinics had been collated via the nationwide Swedish Quality Registry for Neurological Care (NEUROreg). NEUROreg started out as an MS register but has since widened its remit to include other neurologic diagnoses.
For the IMSE 1 study, prospectively recorded data regarding natalizumab treatment, adverse events, JC-virus (JCV) status and clinical effectiveness measures were obtained from NEUROreg for 2,225 women and 883 men. Just over one-third (37%, n = 1,150) were still receiving natalizumab at the time of the analysis.
The mean age at which natalizumab was started was 39 years, with treatment primarily given for RRMS (81% of patients) and less often for secondary progressive multiple sclerosis (SPMS, 15%) and rarely for other types of progressive MS. The mean treatment duration was just under 4 years (47.6 months).
JCV testing was introduced in 2011 in Sweden, and this “has led to fewer treated JCV-positive patients,” the IMSE 1 study investigators reported. “This likely explains a reduced incidence of PML [progressive multifocal leukoencephalopathy],” they suggested. There were nine PML cases diagnosed in Sweden from 2008 to the data cut-off point in 2018, one of which was fatal.
JCV status from 2011 onward was available for 1,269 patients, of whom 39% were JCV positive and 61% were JCV negative. The overall drug survival rate was 72% for JCV-negative and 14% for JCV-positive patients. Improved health status was seen, as measured by the Expanded Disability Status Scale (EDSS), the Multiple Sclerosis Severity Score (MSSS), and the physical and psychological Multiple Sclerosis Impact Scale–29 (MSIS-29) components.
A total of 644 of 1,269 patients discontinued treatment with natalizumab at some point, of whom 67% discontinued because of being JCV positive. The main reason for discontinuation in JCV-negative patients was pregnancy or planning a pregnancy (38%), with lack of effect (10%) and adverse events (11%) as other key reasons for stopping natalizumab.
Ms. Kågström and her associates concluded that natalizumab was “generally well tolerated with sustained effectiveness.”
The IMSE 2 study with fingolimod
Data on the long-term safety and efficacy of fingolimod were reported from the IMSE 2 study (Mult Scler. 2018;24[S2]:696-7, Abstract P1228). Lead author Anna Fält, also of the Karolinska Institute, and her associates analyzed data for 1,634 patients who had been treated with fingolimod from June 2015 to September 2018.
Most patients were older than 30 years (79%), and those aged 30 and older were predominantly female (69%), had an RRMS diagnosis (88%), and been treated for a mean of about 3 years (37 months). A total of 829 were being treated with fingolimod at the time of the analysis, with 844 having discontinued treatment at some point. The main reason for discontinuing treatment with fingolimod was a lack of effect (42% of cases) or an adverse effect (34%). The IMSE 2 study authors reported in their abstract that most patients were switched to rituximab after discontinuing fingolimod.
The number of relapses per 1,000 patient-years was reduced by fingolimod treatment from 280 to 82, comparing before and during treatment for all age groups studied. Relapse rate dropped from 694 per 1,000 patient-years before treatment to 138 during treatment in patients aged 20 years or younger, from 454 to 122 in those aged 21-30 years, and from 257 to 72 in those older than 31 years.
After 1 year of treatment, improvements were seen in the health status of patients as measured by various scales, including the EDSS, MSSS, MSIS-29 Physical, and MSIS-29 Psychological. When the researchers analyzed data by age groups, significant improvements were seen in patients aged 21-30 years and older than 30 years.
Ninety nonserious and 62 serious adverse events were reported in fingolimod-treated patients during the time of analysis. Of the latter, 13 serious adverse events involved cardiac disorders, 12 neoplasms, and 10 infections and infestations.
Overall, the IMSE 2 study investigators said that fingolimod was generally tolerable and reduced disease activity in MS.
French experience with fingolimod: The VIRGILE study
Real-world data on the long-term safety and efficacy of fingolimod in France from the VIRGILE study were reported by Christine Lebrun-Frenay, MD, PhD, and her associates (Mult Scler. 2018;24[S2]:698-9, Abstract P1231).
Dr. Lebrun-Frenay of Pasteur 2 Hospital in Nice and her coauthors noted that VIRGILE study included patients starting treatment with fingolimod between January 2014 and February 2016. A total of 1,047 patients were included, and another 330 patients treated with natalizumab were included at the behest of the French health authorities.
The annualized relapse rate after 2 years of follow-up was 0.30 in the fingolimod group. Dr. Lebrun-Frenay and her colleagues noted: “The 3-year data from this interim analysis provide evidence for sustained efficacy of fingolimod.” Indeed, they report that almost 60% of patients did not relapse and 64% had no worsening of disease. On average, EDSS was stable during the 3-year follow-up period.
“Safety and tolerability profiles of fingolimod were in line with previous clinical experience, with lymphopenia being the most frequent AE [adverse event] reported,” they added.
The IMSE 3 study with alemtuzumab
Long-term experience with alemtuzumab as a treatment for RRMS in the real-world setting is more limited as it only became available for use for this indication in 2014, but some insight is provided by the results of the IMSE 3 study (Mult Scler. 2018;24[S2]:706-7, Abstract P1240).
In total, there were 113 patients treated with alemtuzumab; the vast majority (94%) had RRMS and were aged a mean of 34 years at the start of treatment. Treatment was for more than 12 months in 101 patients, more than 24 months in 86 patients, and more than 36 months in 36 patients.
“In patients treated for at least 12 months, significant improvements were seen in several clinical parameters,” Dr. Fält and her associates observed in their poster at ECTRIMS. The mean baseline and 12-month values for the EDSS were 2.0 and 1.6, and for the MSSS they were 3.46 and 2.61. The mean baseline and 12-month values for the MSIS-29 Psychological subscale were 35.1 and 30.8, respectively, and for MSIS-29 Physical they were 22.7 and 17.7.
Overall, there were 14 nonserious and 11 serious adverse events, the most common of which were infections and infestations, metabolism and nutrition disorders, and immune system disorders.
“A longer follow-up period is needed to assess the real-world effectiveness and safety of alemtuzumab,” the IMSE 3 study authors noted.
The IMSE 5 study with dimethyl fumarate
Similarly, the authors of the IMSE 5 study (Mult Scler. 2018;24[S2]:701-2, Abstract 1234) concluded that a longer follow-up period is need to assess the real-world effectiveness of dimethyl fumarate. Selin Safer Demirbüker, also of the Karolinska Institute, and her associates looked at data on 2,108 patients treated with dimethyl fumarate between March 2014 and April 2018, of whom 1,150 were still receiving treatment at the time of their assessment.
The mean age of patients at the start of treatment was 41 years, 91% had RRMS, and 73% were female. The mean treatment duration was 22.3 months. The majority of patients (n = 867) had been previously treated with interferon and glatiramer acetate (Copaxone) prior to dimethyl fumarate, with 538 being naive to treatment.
“Dimethyl fumarate seems to have a positive effect for patients remaining on treatment,” wrote Ms. Safer Demirbüker and her colleagues. The overall 1-year drug survival reported in their abstract was 74%. Their poster showed a lower 2-year drug survival rate of 63.5% for men and 56.4% for women.
“Swedish patients show cognitive, psychological, and physical benefits after 2 or more years of treatment,” the IMSE 5 study authors further noted. Mean EDSS, MSSS, and MSIS-29 Psychological values all fell from baseline to 2 years.
Overall, 958 (47%) of patients discontinued treatment with dimethyl fumarate at some point, primarily (in 52% of cases) because of adverse events or lack of effect (29% of cases). Most patients (39%) switched to rituximab (15% had no new treatment registered), but 35% of patients continued treatment for 3 or more years.
Twelve-year follow-up of teriflunomide shows continued efficacy, safety
Mark Freedman, MD, of the University of Ottawa and the Ottawa Hospital Research Institute and his associates reported long-term follow-up data on the efficacy and safety of teriflunomide (Aubagio) in relapsing forms of MS (Mult Scler. 2018;24[S2]:700-1, Abstract P1233). After up to 12 years’ follow up, teriflunomide 14 mg was associated with an overall annualized relapse rate of 0.228.
Yearly annualized relapse rates were “low and stable,” Dr. Freedman and his coauthors from the United States, Spain, Italy, France, Germany, England, the Republic of Korea, and Australia noted in their poster.
“As of August 2018, over 93,000 patients were being treated with teriflunomide,” the authors stated. This represented a real-world exposure of approximately 186,000 patient-years up to December 2017, they added.
For the analysis, data from one phase 2 study and three phase 3 studies (TEMSO, TOWER, and TENERE) and their long-term extension studies were pooled. In all, there were 1,696 patients treated with 14 mg of teriflunomide in these studies.
Annualized relapse rates ranged from 0.321 in the first year of follow-up in the studies to 0.080 by the 12th year. The proportions of patients remaining relapse free “were high and stable (ranging from 0.75 in year 1 to 0.93 in years 8 and 9).” EDSS scores were 2.57 at baseline and 2.27 at year 12.
Importantly, no new safety signals were reported, Dr. Freedman and his colleagues wrote, adding that most adverse events were mild to moderate in severity.
Taken together, “these data demonstrate the long-term efficacy and safety of teriflunomide,” they concluded.
Study and author disclosures
The teriflunomide analysis was supported by Sanofi. Dr. Freedman disclosed receiving research or educational grant support from Bayer and Genzyme; honoraria/consulting fees from Bayer, Biogen, EMD Canada, Novartis, Sanofi, and Teva; and membership on company advisory boards/boards of directors/other similar groups for Bayer, Biogen, Chugai, Merck Serono, Novartis, Opexa Therapeutics, Sanofi, and Teva.
The IMSE 1 and 5 studies were supported by Biogen and the IMSE 2 and 3 studies by Novartis. The lead study authors for the IMSE studies – Dr. Kågström, Dr. Fält, and Dr. Safer Demirbüker – had nothing personal to disclose. Other authors included employees of the sponsoring companies or those who had received research funding or honoraria for consultancy work from the companies.
The VIRGILE study was supported by Novartis Pharma AG, Switzerland. Dr. Lebrun-Frenay disclosed receiving consultancy fees from Merck, Novartis, Biogen, MedDay, Roche, Teva, and Genzyme. Coauthors included Novartis employees.
BERLIN – Real-world data from six postmarketing surveillance studies suggest that currently available disease-modifying treatments (DMTs) for relapsing-remitting multiple sclerosis (RRMS) have long-lasting effects that are matched by reasonable tolerability.
Long-term efficacy and safety data on natalizumab (Tysabri), fingolimod (Gilenya), alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), and teriflunomide (Aubagio) from four Swedish studies, one French study, and one international study were reported during a poster session on long-term treatment monitoring at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
The IMSE 1 study with natalizumab
The Immunomodulation and Multiple Sclerosis Epidemiology (IMSE) studies are Swedish postmarketing surveillance studies that were started with the launch of various DMTs in Sweden: natalizumab since 2006 (IMSE 1), fingolimod in 2015 (IMSE 2), alemtuzumab in 2014 (IMSE 3), and dimethyl fumarate in 2014 (IMSE 5).
“Postmarketing surveillance is important for determination of long-term safety and effectiveness in a real-world setting,” Stina Kågström and her associates observed in their poster reporting some findings of the IMSE 1 study with natalizumab (Mult Scler. 2018;24[S2]:699-700, Abstract P1232).
Ms. Kågström of the department of clinical neuroscience at the Karolinska Institute in Stockholm and her colleagues reported that data on 3,108 patients who were seen at 54 Swedish clinics had been collated via the nationwide Swedish Quality Registry for Neurological Care (NEUROreg). NEUROreg started out as an MS register but has since widened its remit to include other neurologic diagnoses.
For the IMSE 1 study, prospectively recorded data regarding natalizumab treatment, adverse events, JC-virus (JCV) status and clinical effectiveness measures were obtained from NEUROreg for 2,225 women and 883 men. Just over one-third (37%, n = 1,150) were still receiving natalizumab at the time of the analysis.
The mean age at which natalizumab was started was 39 years, with treatment primarily given for RRMS (81% of patients) and less often for secondary progressive multiple sclerosis (SPMS, 15%) and rarely for other types of progressive MS. The mean treatment duration was just under 4 years (47.6 months).
JCV testing was introduced in 2011 in Sweden, and this “has led to fewer treated JCV-positive patients,” the IMSE 1 study investigators reported. “This likely explains a reduced incidence of PML [progressive multifocal leukoencephalopathy],” they suggested. There were nine PML cases diagnosed in Sweden from 2008 to the data cut-off point in 2018, one of which was fatal.
JCV status from 2011 onward was available for 1,269 patients, of whom 39% were JCV positive and 61% were JCV negative. The overall drug survival rate was 72% for JCV-negative and 14% for JCV-positive patients. Improved health status was seen, as measured by the Expanded Disability Status Scale (EDSS), the Multiple Sclerosis Severity Score (MSSS), and the physical and psychological Multiple Sclerosis Impact Scale–29 (MSIS-29) components.
A total of 644 of 1,269 patients discontinued treatment with natalizumab at some point, of whom 67% discontinued because of being JCV positive. The main reason for discontinuation in JCV-negative patients was pregnancy or planning a pregnancy (38%), with lack of effect (10%) and adverse events (11%) as other key reasons for stopping natalizumab.
Ms. Kågström and her associates concluded that natalizumab was “generally well tolerated with sustained effectiveness.”
The IMSE 2 study with fingolimod
Data on the long-term safety and efficacy of fingolimod were reported from the IMSE 2 study (Mult Scler. 2018;24[S2]:696-7, Abstract P1228). Lead author Anna Fält, also of the Karolinska Institute, and her associates analyzed data for 1,634 patients who had been treated with fingolimod from June 2015 to September 2018.
Most patients were older than 30 years (79%), and those aged 30 and older were predominantly female (69%), had an RRMS diagnosis (88%), and been treated for a mean of about 3 years (37 months). A total of 829 were being treated with fingolimod at the time of the analysis, with 844 having discontinued treatment at some point. The main reason for discontinuing treatment with fingolimod was a lack of effect (42% of cases) or an adverse effect (34%). The IMSE 2 study authors reported in their abstract that most patients were switched to rituximab after discontinuing fingolimod.
The number of relapses per 1,000 patient-years was reduced by fingolimod treatment from 280 to 82, comparing before and during treatment for all age groups studied. Relapse rate dropped from 694 per 1,000 patient-years before treatment to 138 during treatment in patients aged 20 years or younger, from 454 to 122 in those aged 21-30 years, and from 257 to 72 in those older than 31 years.
After 1 year of treatment, improvements were seen in the health status of patients as measured by various scales, including the EDSS, MSSS, MSIS-29 Physical, and MSIS-29 Psychological. When the researchers analyzed data by age groups, significant improvements were seen in patients aged 21-30 years and older than 30 years.
Ninety nonserious and 62 serious adverse events were reported in fingolimod-treated patients during the time of analysis. Of the latter, 13 serious adverse events involved cardiac disorders, 12 neoplasms, and 10 infections and infestations.
Overall, the IMSE 2 study investigators said that fingolimod was generally tolerable and reduced disease activity in MS.
French experience with fingolimod: The VIRGILE study
Real-world data on the long-term safety and efficacy of fingolimod in France from the VIRGILE study were reported by Christine Lebrun-Frenay, MD, PhD, and her associates (Mult Scler. 2018;24[S2]:698-9, Abstract P1231).
Dr. Lebrun-Frenay of Pasteur 2 Hospital in Nice and her coauthors noted that VIRGILE study included patients starting treatment with fingolimod between January 2014 and February 2016. A total of 1,047 patients were included, and another 330 patients treated with natalizumab were included at the behest of the French health authorities.
The annualized relapse rate after 2 years of follow-up was 0.30 in the fingolimod group. Dr. Lebrun-Frenay and her colleagues noted: “The 3-year data from this interim analysis provide evidence for sustained efficacy of fingolimod.” Indeed, they report that almost 60% of patients did not relapse and 64% had no worsening of disease. On average, EDSS was stable during the 3-year follow-up period.
“Safety and tolerability profiles of fingolimod were in line with previous clinical experience, with lymphopenia being the most frequent AE [adverse event] reported,” they added.
The IMSE 3 study with alemtuzumab
Long-term experience with alemtuzumab as a treatment for RRMS in the real-world setting is more limited as it only became available for use for this indication in 2014, but some insight is provided by the results of the IMSE 3 study (Mult Scler. 2018;24[S2]:706-7, Abstract P1240).
In total, there were 113 patients treated with alemtuzumab; the vast majority (94%) had RRMS and were aged a mean of 34 years at the start of treatment. Treatment was for more than 12 months in 101 patients, more than 24 months in 86 patients, and more than 36 months in 36 patients.
“In patients treated for at least 12 months, significant improvements were seen in several clinical parameters,” Dr. Fält and her associates observed in their poster at ECTRIMS. The mean baseline and 12-month values for the EDSS were 2.0 and 1.6, and for the MSSS they were 3.46 and 2.61. The mean baseline and 12-month values for the MSIS-29 Psychological subscale were 35.1 and 30.8, respectively, and for MSIS-29 Physical they were 22.7 and 17.7.
Overall, there were 14 nonserious and 11 serious adverse events, the most common of which were infections and infestations, metabolism and nutrition disorders, and immune system disorders.
“A longer follow-up period is needed to assess the real-world effectiveness and safety of alemtuzumab,” the IMSE 3 study authors noted.
The IMSE 5 study with dimethyl fumarate
Similarly, the authors of the IMSE 5 study (Mult Scler. 2018;24[S2]:701-2, Abstract 1234) concluded that a longer follow-up period is need to assess the real-world effectiveness of dimethyl fumarate. Selin Safer Demirbüker, also of the Karolinska Institute, and her associates looked at data on 2,108 patients treated with dimethyl fumarate between March 2014 and April 2018, of whom 1,150 were still receiving treatment at the time of their assessment.
The mean age of patients at the start of treatment was 41 years, 91% had RRMS, and 73% were female. The mean treatment duration was 22.3 months. The majority of patients (n = 867) had been previously treated with interferon and glatiramer acetate (Copaxone) prior to dimethyl fumarate, with 538 being naive to treatment.
“Dimethyl fumarate seems to have a positive effect for patients remaining on treatment,” wrote Ms. Safer Demirbüker and her colleagues. The overall 1-year drug survival reported in their abstract was 74%. Their poster showed a lower 2-year drug survival rate of 63.5% for men and 56.4% for women.
“Swedish patients show cognitive, psychological, and physical benefits after 2 or more years of treatment,” the IMSE 5 study authors further noted. Mean EDSS, MSSS, and MSIS-29 Psychological values all fell from baseline to 2 years.
Overall, 958 (47%) of patients discontinued treatment with dimethyl fumarate at some point, primarily (in 52% of cases) because of adverse events or lack of effect (29% of cases). Most patients (39%) switched to rituximab (15% had no new treatment registered), but 35% of patients continued treatment for 3 or more years.
Twelve-year follow-up of teriflunomide shows continued efficacy, safety
Mark Freedman, MD, of the University of Ottawa and the Ottawa Hospital Research Institute and his associates reported long-term follow-up data on the efficacy and safety of teriflunomide (Aubagio) in relapsing forms of MS (Mult Scler. 2018;24[S2]:700-1, Abstract P1233). After up to 12 years’ follow up, teriflunomide 14 mg was associated with an overall annualized relapse rate of 0.228.
Yearly annualized relapse rates were “low and stable,” Dr. Freedman and his coauthors from the United States, Spain, Italy, France, Germany, England, the Republic of Korea, and Australia noted in their poster.
“As of August 2018, over 93,000 patients were being treated with teriflunomide,” the authors stated. This represented a real-world exposure of approximately 186,000 patient-years up to December 2017, they added.
For the analysis, data from one phase 2 study and three phase 3 studies (TEMSO, TOWER, and TENERE) and their long-term extension studies were pooled. In all, there were 1,696 patients treated with 14 mg of teriflunomide in these studies.
Annualized relapse rates ranged from 0.321 in the first year of follow-up in the studies to 0.080 by the 12th year. The proportions of patients remaining relapse free “were high and stable (ranging from 0.75 in year 1 to 0.93 in years 8 and 9).” EDSS scores were 2.57 at baseline and 2.27 at year 12.
Importantly, no new safety signals were reported, Dr. Freedman and his colleagues wrote, adding that most adverse events were mild to moderate in severity.
Taken together, “these data demonstrate the long-term efficacy and safety of teriflunomide,” they concluded.
Study and author disclosures
The teriflunomide analysis was supported by Sanofi. Dr. Freedman disclosed receiving research or educational grant support from Bayer and Genzyme; honoraria/consulting fees from Bayer, Biogen, EMD Canada, Novartis, Sanofi, and Teva; and membership on company advisory boards/boards of directors/other similar groups for Bayer, Biogen, Chugai, Merck Serono, Novartis, Opexa Therapeutics, Sanofi, and Teva.
The IMSE 1 and 5 studies were supported by Biogen and the IMSE 2 and 3 studies by Novartis. The lead study authors for the IMSE studies – Dr. Kågström, Dr. Fält, and Dr. Safer Demirbüker – had nothing personal to disclose. Other authors included employees of the sponsoring companies or those who had received research funding or honoraria for consultancy work from the companies.
The VIRGILE study was supported by Novartis Pharma AG, Switzerland. Dr. Lebrun-Frenay disclosed receiving consultancy fees from Merck, Novartis, Biogen, MedDay, Roche, Teva, and Genzyme. Coauthors included Novartis employees.
REPORTING FROM ECTRIMS 2018
Parental leave for residents
Also today, exercise is important for patients with sickle cell, COPD patients are experiencing a risk in non-TB mycobacteria infections, and how to be an influencer on social media.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, exercise is important for patients with sickle cell, COPD patients are experiencing a risk in non-TB mycobacteria infections, and how to be an influencer on social media.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, exercise is important for patients with sickle cell, COPD patients are experiencing a risk in non-TB mycobacteria infections, and how to be an influencer on social media.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Brain injury in sickle cell merits more attention
BETHESDA, MD. – The risk of brain damage from sickle cell disease (SCD) merits more attention, even with progress made in recent decades to prevent strokes, according to Lori Jordan, MD, PhD, of Vanderbilt University, Nashville, Tenn.
“Whether we can see it or not, the same injury we’ve been talking about in the kidney and the liver and other places is occurring in the brain” with sickle cell disease, Dr. Jordan said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
The concern about long-term brain injury reflects major shifts in SCD treatment. Improved medical care has transformed SCD from a disease that often resulted in an early death to more of a chronic condition, Dr. Jordan said, citing research that shows a survival rate of roughly 99% to age 18 years (Br J Haematol. 2016 Jun;173[6]:927-37).
One of the major success stories in SCD treatment also has been using primary prevention steps to cut the risk of overt stroke at least 10-fold, Dr. Jordan said. Primary prevention includes annual scans with transcranial Doppler ultrasound to identify children with SCD at high risk of stroke.
“What’s not changing is that there is silent injury that accumulates” and can cause lifelong harm, she said. “We want to protect our patients long term so that they can have a successful adult life, not just a successful childhood.”
Research done by one of Dr. Jordan’s colleagues at Vanderbilt, Michael R. DeBaun, MD, showed that regular blood-transfusion therapy significantly reduced the incidence of the recurrence of brain infarct in children with sickle cell anemia. (N Engl J Med. 2014 Aug 21;371[8]:699-710).
But the lessons from the work of Dr. DeBaun and his colleagues with their Silent Cerebral Infarct Multi-Center Clinical (SIT) Trial have not yet been fully adopted, Dr. Jordan said. That’s partly due to the inconvenience and cost of routinely administered blood transfusions to prevent silent cerebral infarcts, which, when used long term, cause side effects, she said.
Dr. Jordan said there’s growing interest in identifying patients at high risk for stroke and moving them toward stem cell transplant, though studies are ongoing. She urged greater attention to the high lifetime costs of strokes and other cerebrovascular complications, particularly in children and young adults.
While some of the brain infarcts are small and don’t result in focal weakness of the body, these “silent infarcts” do produce cognitive effects that reduce function, school performance, employment, and quality of life, she said.
“The injury to the brain is present, whether we can see it or not,” Dr. Jordan said. “In these precious patients, slow cognitive decline isn’t acceptable, frankly.”
Dr. Jordan reported having received funding from the American Heart Association and the National Institutes of Health for stroke prevention studies in SCD.
BETHESDA, MD. – The risk of brain damage from sickle cell disease (SCD) merits more attention, even with progress made in recent decades to prevent strokes, according to Lori Jordan, MD, PhD, of Vanderbilt University, Nashville, Tenn.
“Whether we can see it or not, the same injury we’ve been talking about in the kidney and the liver and other places is occurring in the brain” with sickle cell disease, Dr. Jordan said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
The concern about long-term brain injury reflects major shifts in SCD treatment. Improved medical care has transformed SCD from a disease that often resulted in an early death to more of a chronic condition, Dr. Jordan said, citing research that shows a survival rate of roughly 99% to age 18 years (Br J Haematol. 2016 Jun;173[6]:927-37).
One of the major success stories in SCD treatment also has been using primary prevention steps to cut the risk of overt stroke at least 10-fold, Dr. Jordan said. Primary prevention includes annual scans with transcranial Doppler ultrasound to identify children with SCD at high risk of stroke.
“What’s not changing is that there is silent injury that accumulates” and can cause lifelong harm, she said. “We want to protect our patients long term so that they can have a successful adult life, not just a successful childhood.”
Research done by one of Dr. Jordan’s colleagues at Vanderbilt, Michael R. DeBaun, MD, showed that regular blood-transfusion therapy significantly reduced the incidence of the recurrence of brain infarct in children with sickle cell anemia. (N Engl J Med. 2014 Aug 21;371[8]:699-710).
But the lessons from the work of Dr. DeBaun and his colleagues with their Silent Cerebral Infarct Multi-Center Clinical (SIT) Trial have not yet been fully adopted, Dr. Jordan said. That’s partly due to the inconvenience and cost of routinely administered blood transfusions to prevent silent cerebral infarcts, which, when used long term, cause side effects, she said.
Dr. Jordan said there’s growing interest in identifying patients at high risk for stroke and moving them toward stem cell transplant, though studies are ongoing. She urged greater attention to the high lifetime costs of strokes and other cerebrovascular complications, particularly in children and young adults.
While some of the brain infarcts are small and don’t result in focal weakness of the body, these “silent infarcts” do produce cognitive effects that reduce function, school performance, employment, and quality of life, she said.
“The injury to the brain is present, whether we can see it or not,” Dr. Jordan said. “In these precious patients, slow cognitive decline isn’t acceptable, frankly.”
Dr. Jordan reported having received funding from the American Heart Association and the National Institutes of Health for stroke prevention studies in SCD.
BETHESDA, MD. – The risk of brain damage from sickle cell disease (SCD) merits more attention, even with progress made in recent decades to prevent strokes, according to Lori Jordan, MD, PhD, of Vanderbilt University, Nashville, Tenn.
“Whether we can see it or not, the same injury we’ve been talking about in the kidney and the liver and other places is occurring in the brain” with sickle cell disease, Dr. Jordan said at Sickle Cell in Focus, a conference held by the National Institutes of Health.
The concern about long-term brain injury reflects major shifts in SCD treatment. Improved medical care has transformed SCD from a disease that often resulted in an early death to more of a chronic condition, Dr. Jordan said, citing research that shows a survival rate of roughly 99% to age 18 years (Br J Haematol. 2016 Jun;173[6]:927-37).
One of the major success stories in SCD treatment also has been using primary prevention steps to cut the risk of overt stroke at least 10-fold, Dr. Jordan said. Primary prevention includes annual scans with transcranial Doppler ultrasound to identify children with SCD at high risk of stroke.
“What’s not changing is that there is silent injury that accumulates” and can cause lifelong harm, she said. “We want to protect our patients long term so that they can have a successful adult life, not just a successful childhood.”
Research done by one of Dr. Jordan’s colleagues at Vanderbilt, Michael R. DeBaun, MD, showed that regular blood-transfusion therapy significantly reduced the incidence of the recurrence of brain infarct in children with sickle cell anemia. (N Engl J Med. 2014 Aug 21;371[8]:699-710).
But the lessons from the work of Dr. DeBaun and his colleagues with their Silent Cerebral Infarct Multi-Center Clinical (SIT) Trial have not yet been fully adopted, Dr. Jordan said. That’s partly due to the inconvenience and cost of routinely administered blood transfusions to prevent silent cerebral infarcts, which, when used long term, cause side effects, she said.
Dr. Jordan said there’s growing interest in identifying patients at high risk for stroke and moving them toward stem cell transplant, though studies are ongoing. She urged greater attention to the high lifetime costs of strokes and other cerebrovascular complications, particularly in children and young adults.
While some of the brain infarcts are small and don’t result in focal weakness of the body, these “silent infarcts” do produce cognitive effects that reduce function, school performance, employment, and quality of life, she said.
“The injury to the brain is present, whether we can see it or not,” Dr. Jordan said. “In these precious patients, slow cognitive decline isn’t acceptable, frankly.”
Dr. Jordan reported having received funding from the American Heart Association and the National Institutes of Health for stroke prevention studies in SCD.
EXPERT ANALYSIS FROM SICKLE CELL IN FOCUS
No matter the valve, protective device cuts post-TAVR stroke risk
SAN DIEGO – A retrospective study and combined meta-analysis of patients undergoing transcatheter aortic valve replacement (TAVR) confirms the protective effect of the Sentinel cerebral embolic protection (CEP) device, regardless of the valve type used, on periprocedural stroke and mortality.
“The only significant predictor for being stroke free was use of the protective device. If you look at different valve types, you have an effect with use of the protection device with each of them,” Julia Seeger, MD, said in an interview at the Transcatheter Cardiovascular Therapeutics annual meeting.The finding just reinforces a decision that the institution made several years ago, to uniformly use embolic protection in TAVR procedures. Asked if she was convinced by the latest data on the utility of the device, she replied “Yes, definitely.”
Use of the device adds only a couple of minutes to the procedure time, and there haven’t been any adverse events associated with it, and no additional imaging agent was required, said Dr. Seeger, an interventional cardiologist at the University of Ulm (Germany).
The studies included patients being treated with the Medtronic CoreValve/Evolut, the mechanically implantable Boston Scientific Lotus, and the balloon-expandable Edwards Sapien. Subanalyses for all three valve types showed strong trends for reduction of strokes and mortality. Sentinel is the only Food and Drug Administration–approved device for reduction of strokes during TAVR procedures.
The Sentinel and Clean-TAVI trials showed the efficacy of the Sentinel device in reducing the number and volume of periprocedural cerebral lesions, but there were insufficient randomized data to draw conclusions about its relative efficacy among valve types. Dr. Seeger’s team analyzed data from 984 consecutive TAVR patients. The Sentinel device was used in 548, and not used in 436 consecutive patients. Self-expandable valves were used significantly more often in patients who underwent the procedure with CEP (22% vs. 6.0%). In the study population, 590 balloon-expandable valves, 246 mechanically implantable valves, and 148 self-expandable valves were used.
In the 72 hours after the procedure, mortality or stroke was lower in the CEP group (1.5% versus 4.4%, P less than .01), as was disabling stroke (0.6% versus 3.2%, P less than .01). When results were analyzed by valve types, the researchers found a relative risk reduction for all stroke of 76% with the use of CEP with balloon-expandable devices, 68% with mechanically expandable devices, and 57% with self-expandable devices.
The researchers also conducted a patient-level meta-analysis, incorporating data on 1,306 subjects with symptomatic severe aortic stenosis, including 363 from the Sentinel trial (243 with CEP), 100 patients from the CLEAN-TAVI trial (1:1 randomization to CEP), and 843 patients from the Sentinel-Ulm study (423 with CEP).
They matched patients for valve type, Society of Thoracic Surgeons’ risk score, atrial fibrillation, diabetes, sex, coronary artery disease, and peripheral vascular disease. The all-procedural stroke rate was 5.4% in patients who did not receive CEP, and 1.9% in those who did, for a risk reduction of 65%. Similarly, 72-hour mortality stroke risk was reduced by 66% with the CEP device. It occurred in 6.0% of non-CEP patients, compared to 2.1% of the CEP patients.
The meeting was sponsored by the Cardiovascular Research Foundation.
SAN DIEGO – A retrospective study and combined meta-analysis of patients undergoing transcatheter aortic valve replacement (TAVR) confirms the protective effect of the Sentinel cerebral embolic protection (CEP) device, regardless of the valve type used, on periprocedural stroke and mortality.
“The only significant predictor for being stroke free was use of the protective device. If you look at different valve types, you have an effect with use of the protection device with each of them,” Julia Seeger, MD, said in an interview at the Transcatheter Cardiovascular Therapeutics annual meeting.The finding just reinforces a decision that the institution made several years ago, to uniformly use embolic protection in TAVR procedures. Asked if she was convinced by the latest data on the utility of the device, she replied “Yes, definitely.”
Use of the device adds only a couple of minutes to the procedure time, and there haven’t been any adverse events associated with it, and no additional imaging agent was required, said Dr. Seeger, an interventional cardiologist at the University of Ulm (Germany).
The studies included patients being treated with the Medtronic CoreValve/Evolut, the mechanically implantable Boston Scientific Lotus, and the balloon-expandable Edwards Sapien. Subanalyses for all three valve types showed strong trends for reduction of strokes and mortality. Sentinel is the only Food and Drug Administration–approved device for reduction of strokes during TAVR procedures.
The Sentinel and Clean-TAVI trials showed the efficacy of the Sentinel device in reducing the number and volume of periprocedural cerebral lesions, but there were insufficient randomized data to draw conclusions about its relative efficacy among valve types. Dr. Seeger’s team analyzed data from 984 consecutive TAVR patients. The Sentinel device was used in 548, and not used in 436 consecutive patients. Self-expandable valves were used significantly more often in patients who underwent the procedure with CEP (22% vs. 6.0%). In the study population, 590 balloon-expandable valves, 246 mechanically implantable valves, and 148 self-expandable valves were used.
In the 72 hours after the procedure, mortality or stroke was lower in the CEP group (1.5% versus 4.4%, P less than .01), as was disabling stroke (0.6% versus 3.2%, P less than .01). When results were analyzed by valve types, the researchers found a relative risk reduction for all stroke of 76% with the use of CEP with balloon-expandable devices, 68% with mechanically expandable devices, and 57% with self-expandable devices.
The researchers also conducted a patient-level meta-analysis, incorporating data on 1,306 subjects with symptomatic severe aortic stenosis, including 363 from the Sentinel trial (243 with CEP), 100 patients from the CLEAN-TAVI trial (1:1 randomization to CEP), and 843 patients from the Sentinel-Ulm study (423 with CEP).
They matched patients for valve type, Society of Thoracic Surgeons’ risk score, atrial fibrillation, diabetes, sex, coronary artery disease, and peripheral vascular disease. The all-procedural stroke rate was 5.4% in patients who did not receive CEP, and 1.9% in those who did, for a risk reduction of 65%. Similarly, 72-hour mortality stroke risk was reduced by 66% with the CEP device. It occurred in 6.0% of non-CEP patients, compared to 2.1% of the CEP patients.
The meeting was sponsored by the Cardiovascular Research Foundation.
SAN DIEGO – A retrospective study and combined meta-analysis of patients undergoing transcatheter aortic valve replacement (TAVR) confirms the protective effect of the Sentinel cerebral embolic protection (CEP) device, regardless of the valve type used, on periprocedural stroke and mortality.
“The only significant predictor for being stroke free was use of the protective device. If you look at different valve types, you have an effect with use of the protection device with each of them,” Julia Seeger, MD, said in an interview at the Transcatheter Cardiovascular Therapeutics annual meeting.The finding just reinforces a decision that the institution made several years ago, to uniformly use embolic protection in TAVR procedures. Asked if she was convinced by the latest data on the utility of the device, she replied “Yes, definitely.”
Use of the device adds only a couple of minutes to the procedure time, and there haven’t been any adverse events associated with it, and no additional imaging agent was required, said Dr. Seeger, an interventional cardiologist at the University of Ulm (Germany).
The studies included patients being treated with the Medtronic CoreValve/Evolut, the mechanically implantable Boston Scientific Lotus, and the balloon-expandable Edwards Sapien. Subanalyses for all three valve types showed strong trends for reduction of strokes and mortality. Sentinel is the only Food and Drug Administration–approved device for reduction of strokes during TAVR procedures.
The Sentinel and Clean-TAVI trials showed the efficacy of the Sentinel device in reducing the number and volume of periprocedural cerebral lesions, but there were insufficient randomized data to draw conclusions about its relative efficacy among valve types. Dr. Seeger’s team analyzed data from 984 consecutive TAVR patients. The Sentinel device was used in 548, and not used in 436 consecutive patients. Self-expandable valves were used significantly more often in patients who underwent the procedure with CEP (22% vs. 6.0%). In the study population, 590 balloon-expandable valves, 246 mechanically implantable valves, and 148 self-expandable valves were used.
In the 72 hours after the procedure, mortality or stroke was lower in the CEP group (1.5% versus 4.4%, P less than .01), as was disabling stroke (0.6% versus 3.2%, P less than .01). When results were analyzed by valve types, the researchers found a relative risk reduction for all stroke of 76% with the use of CEP with balloon-expandable devices, 68% with mechanically expandable devices, and 57% with self-expandable devices.
The researchers also conducted a patient-level meta-analysis, incorporating data on 1,306 subjects with symptomatic severe aortic stenosis, including 363 from the Sentinel trial (243 with CEP), 100 patients from the CLEAN-TAVI trial (1:1 randomization to CEP), and 843 patients from the Sentinel-Ulm study (423 with CEP).
They matched patients for valve type, Society of Thoracic Surgeons’ risk score, atrial fibrillation, diabetes, sex, coronary artery disease, and peripheral vascular disease. The all-procedural stroke rate was 5.4% in patients who did not receive CEP, and 1.9% in those who did, for a risk reduction of 65%. Similarly, 72-hour mortality stroke risk was reduced by 66% with the CEP device. It occurred in 6.0% of non-CEP patients, compared to 2.1% of the CEP patients.
The meeting was sponsored by the Cardiovascular Research Foundation.
REPORTING FROM TCT 2018
More data link severe sleep apnea and aggressive melanoma
in a study of 443 adults.
Sleep-disordered breathing (SDB) has been associated with increased cancer risk and mortality, but no large studies have examined the association in specific cancers, wrote Miguel Angel Martinez-Garcia, MD, of La Fe University and Polytechnic Hospital, Valencia, Spain, and his colleagues.
The researchers conducted a sleep study of 443 adults with melanoma within 6 months of their diagnoses. The findings were published in the journal CHEST®. Overall, patients with more severe sleep apnea were nearly twice as likely to have aggressive type melanoma, compared with those with less severe sleep apnea.
Sleep apnea was defined via the Apnea Hypopnea Index (AHI) and the desaturation indices (DI). Aggressive melanoma was defined as a Breslow index greater than 1 mm, compared with 1 mm or less.
Patients with AHI greater than 15.6 events per hour or in the DI4% tertile (more than 9.3 desaturations per hour) were approximately twice as likely (1.94 and 1.93 times, respectively) to have a more aggressive melanoma as were those with less severe sleep apnea, after adjustment for age, gender, body mass index, and melanoma location.
The average age of the patients was 60 years, 51% were male, and the average time between the melanoma diagnosis and the sleep study was 82 days. Sleep symptoms were not significantly different between the patients with aggressive or less aggressive melanoma. However, in addition to more severe sleep apnea, those with aggressive melanoma were significantly more likely to be older, male, and have a higher BMI than were those with less aggressive disease.
“Among the most salient findings were the dependence of the association between SDB and the markers of melanoma aggressiveness on both age and the actual indicators of tumor aggressiveness,” the researchers noted. The association was significant in patients younger than 55 years only if their Breslow index was greater than 2 mm, the researchers said.
The study findings were limited by the absence of full overnight polysomnography to assess SDB because not all participating centers had access to that option, the researchers noted. However, the results support an independent link between sleep apnea and common measures of aggressive melanoma, especially in younger patients, they said. “Future prospective studies are needed to confirm whether the presence and treatment of SDB and its evolution over time are also associated with poor melanoma outcomes, including death, the pathophysiological mechanisms underlying this association,” they added.
The study was supported in part by Fondo de Investigation Sanitaria, SEPAR, Red Respira, and Sociedad Valenciana de Neumología. The researchers had no financial conflicts to disclose
SOURCE: Martinez-Garcia M et al. CHEST. 2018; doi: 10.1016/j.chest.2018.07.015.
in a study of 443 adults.
Sleep-disordered breathing (SDB) has been associated with increased cancer risk and mortality, but no large studies have examined the association in specific cancers, wrote Miguel Angel Martinez-Garcia, MD, of La Fe University and Polytechnic Hospital, Valencia, Spain, and his colleagues.
The researchers conducted a sleep study of 443 adults with melanoma within 6 months of their diagnoses. The findings were published in the journal CHEST®. Overall, patients with more severe sleep apnea were nearly twice as likely to have aggressive type melanoma, compared with those with less severe sleep apnea.
Sleep apnea was defined via the Apnea Hypopnea Index (AHI) and the desaturation indices (DI). Aggressive melanoma was defined as a Breslow index greater than 1 mm, compared with 1 mm or less.
Patients with AHI greater than 15.6 events per hour or in the DI4% tertile (more than 9.3 desaturations per hour) were approximately twice as likely (1.94 and 1.93 times, respectively) to have a more aggressive melanoma as were those with less severe sleep apnea, after adjustment for age, gender, body mass index, and melanoma location.
The average age of the patients was 60 years, 51% were male, and the average time between the melanoma diagnosis and the sleep study was 82 days. Sleep symptoms were not significantly different between the patients with aggressive or less aggressive melanoma. However, in addition to more severe sleep apnea, those with aggressive melanoma were significantly more likely to be older, male, and have a higher BMI than were those with less aggressive disease.
“Among the most salient findings were the dependence of the association between SDB and the markers of melanoma aggressiveness on both age and the actual indicators of tumor aggressiveness,” the researchers noted. The association was significant in patients younger than 55 years only if their Breslow index was greater than 2 mm, the researchers said.
The study findings were limited by the absence of full overnight polysomnography to assess SDB because not all participating centers had access to that option, the researchers noted. However, the results support an independent link between sleep apnea and common measures of aggressive melanoma, especially in younger patients, they said. “Future prospective studies are needed to confirm whether the presence and treatment of SDB and its evolution over time are also associated with poor melanoma outcomes, including death, the pathophysiological mechanisms underlying this association,” they added.
The study was supported in part by Fondo de Investigation Sanitaria, SEPAR, Red Respira, and Sociedad Valenciana de Neumología. The researchers had no financial conflicts to disclose
SOURCE: Martinez-Garcia M et al. CHEST. 2018; doi: 10.1016/j.chest.2018.07.015.
in a study of 443 adults.
Sleep-disordered breathing (SDB) has been associated with increased cancer risk and mortality, but no large studies have examined the association in specific cancers, wrote Miguel Angel Martinez-Garcia, MD, of La Fe University and Polytechnic Hospital, Valencia, Spain, and his colleagues.
The researchers conducted a sleep study of 443 adults with melanoma within 6 months of their diagnoses. The findings were published in the journal CHEST®. Overall, patients with more severe sleep apnea were nearly twice as likely to have aggressive type melanoma, compared with those with less severe sleep apnea.
Sleep apnea was defined via the Apnea Hypopnea Index (AHI) and the desaturation indices (DI). Aggressive melanoma was defined as a Breslow index greater than 1 mm, compared with 1 mm or less.
Patients with AHI greater than 15.6 events per hour or in the DI4% tertile (more than 9.3 desaturations per hour) were approximately twice as likely (1.94 and 1.93 times, respectively) to have a more aggressive melanoma as were those with less severe sleep apnea, after adjustment for age, gender, body mass index, and melanoma location.
The average age of the patients was 60 years, 51% were male, and the average time between the melanoma diagnosis and the sleep study was 82 days. Sleep symptoms were not significantly different between the patients with aggressive or less aggressive melanoma. However, in addition to more severe sleep apnea, those with aggressive melanoma were significantly more likely to be older, male, and have a higher BMI than were those with less aggressive disease.
“Among the most salient findings were the dependence of the association between SDB and the markers of melanoma aggressiveness on both age and the actual indicators of tumor aggressiveness,” the researchers noted. The association was significant in patients younger than 55 years only if their Breslow index was greater than 2 mm, the researchers said.
The study findings were limited by the absence of full overnight polysomnography to assess SDB because not all participating centers had access to that option, the researchers noted. However, the results support an independent link between sleep apnea and common measures of aggressive melanoma, especially in younger patients, they said. “Future prospective studies are needed to confirm whether the presence and treatment of SDB and its evolution over time are also associated with poor melanoma outcomes, including death, the pathophysiological mechanisms underlying this association,” they added.
The study was supported in part by Fondo de Investigation Sanitaria, SEPAR, Red Respira, and Sociedad Valenciana de Neumología. The researchers had no financial conflicts to disclose
SOURCE: Martinez-Garcia M et al. CHEST. 2018; doi: 10.1016/j.chest.2018.07.015.
FROM CHEST®
Key clinical point: Severe sleep disordered breathing is independently associated with more severe melanoma.
Major finding: Patients in the upper tertiles of AHI or DI4% were 1.94 and 1.93 times more likely, respectively, to have aggressive melanoma compared with patients with less severe sleep apnea.
Study details: The data come from an observational study of 443 adults diagnosed with melanoma.
Disclosures: The study was supported in part by Fondo de Investigation Sanitaria, SEPAR, Red Respira, and Sociedad Valenciana de Neumología. The researchers had no financial conflicts to disclose.
Source: Martinez-Garcia M et al. Chest. 2018. doi: 10.1016/j.chest.2018.07.015.
‘Error neuron’ EEG findings could open up future clinical applications
, and this activity can be tracked through a scalp EEG pattern called error-related negativity, according to findings from experiments carried out during intracranial EEG recordings of candidates for surgical treatment of epilepsy.
“Our results suggest that coordinated neural activity can serve as a substrate for information routing that enables the performance-monitoring system to communicate the need for behavioral control to other brain regions, including those that maintain flexible goal information, such as the lateral prefrontal cortex and the frontal polar cortex,” first author Zhongzheng Fu, a PhD student at the California Institute of Technology in Pasadena, Calif., and Cedars-Sinai Medical Center, Los Angeles, and his colleagues reported in Neuron.
The findings offer insights that could lead to treatments for conditions in which the important executive function task of error self-monitoring is unbalanced, such as obsessive-compulsive disorder and schizophrenia, the authors noted in a press release.
“We discovered that the activity of error neurons correlates with the size of the ERN [error-related negativity],” Mr. Fu said. “This identifies the brain area that causes the ERN and helps explain what it signifies. This new insight might allow doctors to use the ERN as a standard tool to diagnose mental diseases and monitor responses to treatment.”
Error neuron firing and intracranial ERN occurred first in pre-supplementary motor area (pre-SMA), then in the dorsal anterior cingulate cortex (dACC) about 50 ms later, with significant correlations between firing and intracranial ERN in both locations. In dACC, this activity, with error-integrating neuron responses, correlated with magnitude of post-error slowing (PES).
Previous research suggested a link between “the detection of self-generated errors, as reflected in the ERN, with changes in cognitive control, as exhibited behaviorally in PES,” the investigators wrote. “However, several electroencephalogram (EEG) studies have failed to find a significant relationship between PES and ERN.”
The present study involved intracranial EEG of 29 candidates for surgical treatment of epilepsy and scalp EEG of 12 control participants, with each modality measuring activity in the frontal cortex. Both cohorts performed a rapid version of the color-word Stroop task, in which the words “red,” “green,” or “blue” were printed either in corresponding or noncorresponding colors of red, green, or blue. Subjects were presented various color-word combinations while being asked to click one of three buttons indicating the color of the word as quickly as possible. The investigators monitored neuronal activity throughout, discarding responses that were too slow.
As found in previous trials, the subjects demonstrated the “Stroop effect,” which refers to a slower response when word and color are incongruent (224.9 ms difference; P less than .001). As anticipated, correct responses following correct responses were faster than were correct responses following erroneous responses, which defines PES.
In the intracranial EEG group, the investigators isolated 1,171 neurons, of which 618 were located in dACC and 553 in pre-SMA. Using a Poisson regression model and correlations with erroneous responses, the investigators identified 99 “type I” error neurons in dACC and 118 in pre-SMA, based on higher frequency of firing during erroneous responses than during correct responses. At a single-cell level, error neuron mean spike rates were highest when intracranial ERN amplitude was greatest, such that error neuron firing in dACC and pre-SMA had maximal likelihood ratios of 7.9 (P = .01) and 15.1 (P less than .001), respectively. The strength of correlation between intracranial ERN and error neuron firing rate was directly related to PES magnitude exclusively in the dACC (maximum likelihood ratio of 13.9; P = .015). In post-error trials, faster error-integrating neuron firing rates in dACC predicted greater PES (maximal likelihood ratio of 18.3; P less than .001).
The study was funded by the National Institutes of Health, the McKnight Endowment for Neuroscience, and the National Science Foundation. The authors declared no conflicts of interest.
SOURCE: Fu Z et al. Neuron. 2018 Dec 4. doi: 10.1016/j.neuron.2018.11.016
, and this activity can be tracked through a scalp EEG pattern called error-related negativity, according to findings from experiments carried out during intracranial EEG recordings of candidates for surgical treatment of epilepsy.
“Our results suggest that coordinated neural activity can serve as a substrate for information routing that enables the performance-monitoring system to communicate the need for behavioral control to other brain regions, including those that maintain flexible goal information, such as the lateral prefrontal cortex and the frontal polar cortex,” first author Zhongzheng Fu, a PhD student at the California Institute of Technology in Pasadena, Calif., and Cedars-Sinai Medical Center, Los Angeles, and his colleagues reported in Neuron.
The findings offer insights that could lead to treatments for conditions in which the important executive function task of error self-monitoring is unbalanced, such as obsessive-compulsive disorder and schizophrenia, the authors noted in a press release.
“We discovered that the activity of error neurons correlates with the size of the ERN [error-related negativity],” Mr. Fu said. “This identifies the brain area that causes the ERN and helps explain what it signifies. This new insight might allow doctors to use the ERN as a standard tool to diagnose mental diseases and monitor responses to treatment.”
Error neuron firing and intracranial ERN occurred first in pre-supplementary motor area (pre-SMA), then in the dorsal anterior cingulate cortex (dACC) about 50 ms later, with significant correlations between firing and intracranial ERN in both locations. In dACC, this activity, with error-integrating neuron responses, correlated with magnitude of post-error slowing (PES).
Previous research suggested a link between “the detection of self-generated errors, as reflected in the ERN, with changes in cognitive control, as exhibited behaviorally in PES,” the investigators wrote. “However, several electroencephalogram (EEG) studies have failed to find a significant relationship between PES and ERN.”
The present study involved intracranial EEG of 29 candidates for surgical treatment of epilepsy and scalp EEG of 12 control participants, with each modality measuring activity in the frontal cortex. Both cohorts performed a rapid version of the color-word Stroop task, in which the words “red,” “green,” or “blue” were printed either in corresponding or noncorresponding colors of red, green, or blue. Subjects were presented various color-word combinations while being asked to click one of three buttons indicating the color of the word as quickly as possible. The investigators monitored neuronal activity throughout, discarding responses that were too slow.
As found in previous trials, the subjects demonstrated the “Stroop effect,” which refers to a slower response when word and color are incongruent (224.9 ms difference; P less than .001). As anticipated, correct responses following correct responses were faster than were correct responses following erroneous responses, which defines PES.
In the intracranial EEG group, the investigators isolated 1,171 neurons, of which 618 were located in dACC and 553 in pre-SMA. Using a Poisson regression model and correlations with erroneous responses, the investigators identified 99 “type I” error neurons in dACC and 118 in pre-SMA, based on higher frequency of firing during erroneous responses than during correct responses. At a single-cell level, error neuron mean spike rates were highest when intracranial ERN amplitude was greatest, such that error neuron firing in dACC and pre-SMA had maximal likelihood ratios of 7.9 (P = .01) and 15.1 (P less than .001), respectively. The strength of correlation between intracranial ERN and error neuron firing rate was directly related to PES magnitude exclusively in the dACC (maximum likelihood ratio of 13.9; P = .015). In post-error trials, faster error-integrating neuron firing rates in dACC predicted greater PES (maximal likelihood ratio of 18.3; P less than .001).
The study was funded by the National Institutes of Health, the McKnight Endowment for Neuroscience, and the National Science Foundation. The authors declared no conflicts of interest.
SOURCE: Fu Z et al. Neuron. 2018 Dec 4. doi: 10.1016/j.neuron.2018.11.016
, and this activity can be tracked through a scalp EEG pattern called error-related negativity, according to findings from experiments carried out during intracranial EEG recordings of candidates for surgical treatment of epilepsy.
“Our results suggest that coordinated neural activity can serve as a substrate for information routing that enables the performance-monitoring system to communicate the need for behavioral control to other brain regions, including those that maintain flexible goal information, such as the lateral prefrontal cortex and the frontal polar cortex,” first author Zhongzheng Fu, a PhD student at the California Institute of Technology in Pasadena, Calif., and Cedars-Sinai Medical Center, Los Angeles, and his colleagues reported in Neuron.
The findings offer insights that could lead to treatments for conditions in which the important executive function task of error self-monitoring is unbalanced, such as obsessive-compulsive disorder and schizophrenia, the authors noted in a press release.
“We discovered that the activity of error neurons correlates with the size of the ERN [error-related negativity],” Mr. Fu said. “This identifies the brain area that causes the ERN and helps explain what it signifies. This new insight might allow doctors to use the ERN as a standard tool to diagnose mental diseases and monitor responses to treatment.”
Error neuron firing and intracranial ERN occurred first in pre-supplementary motor area (pre-SMA), then in the dorsal anterior cingulate cortex (dACC) about 50 ms later, with significant correlations between firing and intracranial ERN in both locations. In dACC, this activity, with error-integrating neuron responses, correlated with magnitude of post-error slowing (PES).
Previous research suggested a link between “the detection of self-generated errors, as reflected in the ERN, with changes in cognitive control, as exhibited behaviorally in PES,” the investigators wrote. “However, several electroencephalogram (EEG) studies have failed to find a significant relationship between PES and ERN.”
The present study involved intracranial EEG of 29 candidates for surgical treatment of epilepsy and scalp EEG of 12 control participants, with each modality measuring activity in the frontal cortex. Both cohorts performed a rapid version of the color-word Stroop task, in which the words “red,” “green,” or “blue” were printed either in corresponding or noncorresponding colors of red, green, or blue. Subjects were presented various color-word combinations while being asked to click one of three buttons indicating the color of the word as quickly as possible. The investigators monitored neuronal activity throughout, discarding responses that were too slow.
As found in previous trials, the subjects demonstrated the “Stroop effect,” which refers to a slower response when word and color are incongruent (224.9 ms difference; P less than .001). As anticipated, correct responses following correct responses were faster than were correct responses following erroneous responses, which defines PES.
In the intracranial EEG group, the investigators isolated 1,171 neurons, of which 618 were located in dACC and 553 in pre-SMA. Using a Poisson regression model and correlations with erroneous responses, the investigators identified 99 “type I” error neurons in dACC and 118 in pre-SMA, based on higher frequency of firing during erroneous responses than during correct responses. At a single-cell level, error neuron mean spike rates were highest when intracranial ERN amplitude was greatest, such that error neuron firing in dACC and pre-SMA had maximal likelihood ratios of 7.9 (P = .01) and 15.1 (P less than .001), respectively. The strength of correlation between intracranial ERN and error neuron firing rate was directly related to PES magnitude exclusively in the dACC (maximum likelihood ratio of 13.9; P = .015). In post-error trials, faster error-integrating neuron firing rates in dACC predicted greater PES (maximal likelihood ratio of 18.3; P less than .001).
The study was funded by the National Institutes of Health, the McKnight Endowment for Neuroscience, and the National Science Foundation. The authors declared no conflicts of interest.
SOURCE: Fu Z et al. Neuron. 2018 Dec 4. doi: 10.1016/j.neuron.2018.11.016
FROM NEURON