User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
CDC, SAMHSA commit $1.8 billion to combat opioid crisis
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
Is serum serotonin level associated with risk of seizure-related breathing dysfunction?
, according to research published online Sept. 4 in Neurology. The change in serotonin level may reflect physiologic changes that protect against harmful processes that promote sudden unexpected death in epilepsy (SUDEP), the authors wrote.
“Our results give new insight into a possible link between serotonin levels and breathing during and after seizure,” Samden D. Lhatoo, MD, professor of neurology at McGovern Medical School at the University of Texas Health Science Center in Houston, said in a press release. “This may give hope that perhaps someday new therapies could be developed that may help prevent SUDEP. However, our study was small, and much more research is needed to confirm our findings in larger groups before any treatment decisions can be made. It is also important to note that excess serotonin can be harmful, so we strongly recommend against anyone trying to find ways to increase their serotonin levels in response to our study findings.”
Animal and human studies have indicated that breathing dysfunction related to SUDEP may involve serotonergic pathways. Compared with controls, patients with SUDEP have fewer midline serotonergic neurons. Furthermore, a 2018 study suggested an association between severe seizures and decreased serotonergic tone in the postictal state.
Dr. Lhatoo and colleagues examined a prospective cohort of patients with intractable epilepsy to understand the relationship between serum serotonin levels, ictal central apnea (ICA), and postconvulsive central apnea (PCCA). Patients were aged 18 years or older, were admitted to the epilepsy monitoring unit from January 2015 to April 2018, and agreed to take part in an investigation of SUDEP biomarkers. Dr. Lhatoo and colleagues evaluated video EEG, plethysmography, capillary oxygen saturation, and ECG for 49 patients. After a patient had a clinical seizure, the researchers collected postictal and interictal venous blood samples from him or her to measure serum serotonin levels. They classified seizures using the International League Against Epilepsy 2017 seizure classification. Dr. Lhatoo and colleagues analyzed 49 seizures with and without ICA and 27 generalized convulsive seizures with and without PCCA.
Of the 49 patients, 29 were female. Participants’ mean age was 42 years, mean age at epilepsy onset was 25.2 years, and mean epilepsy duration was 16.8 years. The population’s mean body mass index was 28.9. Dr. Lhatoo and colleagues observed ICA in 17 of 49 (34.7%) seizures and PCCA in 8 of 27 (29.6%) seizures.
Postictal serum serotonin levels were significantly higher than interictal levels for seizures without ICA, but not for seizures with ICA. Among patients with generalized convulsive seizures without PCCA, serum serotonin levels were significantly increased postictally, compared with interictal levels, but not among patients with seizures with PCCA. The change in postictal and interictal serotonin levels also differed significantly between participants with and without PCCA. In patients without PCCA, an increase in serotonin was associated with an increase in heart rate, but not in patients with PCCA.
“Large postictal increases in serum serotonin may play a role in modulation of respiration in these patients,” wrote Dr. Lhatoo and colleagues. “Alternatively, the increase in serum serotonin that we measured may be a surrogate for an increase in brain serotonin levels that may depend on similar physiologic mechanisms, rather than serum serotonin directly stimulating breathing.” Low levels of postictal serum serotonin are associated with potentially harmful breathing phenomena that should be investigated in larger studies, the investigators concluded.
The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
SOURCE: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
, according to research published online Sept. 4 in Neurology. The change in serotonin level may reflect physiologic changes that protect against harmful processes that promote sudden unexpected death in epilepsy (SUDEP), the authors wrote.
“Our results give new insight into a possible link between serotonin levels and breathing during and after seizure,” Samden D. Lhatoo, MD, professor of neurology at McGovern Medical School at the University of Texas Health Science Center in Houston, said in a press release. “This may give hope that perhaps someday new therapies could be developed that may help prevent SUDEP. However, our study was small, and much more research is needed to confirm our findings in larger groups before any treatment decisions can be made. It is also important to note that excess serotonin can be harmful, so we strongly recommend against anyone trying to find ways to increase their serotonin levels in response to our study findings.”
Animal and human studies have indicated that breathing dysfunction related to SUDEP may involve serotonergic pathways. Compared with controls, patients with SUDEP have fewer midline serotonergic neurons. Furthermore, a 2018 study suggested an association between severe seizures and decreased serotonergic tone in the postictal state.
Dr. Lhatoo and colleagues examined a prospective cohort of patients with intractable epilepsy to understand the relationship between serum serotonin levels, ictal central apnea (ICA), and postconvulsive central apnea (PCCA). Patients were aged 18 years or older, were admitted to the epilepsy monitoring unit from January 2015 to April 2018, and agreed to take part in an investigation of SUDEP biomarkers. Dr. Lhatoo and colleagues evaluated video EEG, plethysmography, capillary oxygen saturation, and ECG for 49 patients. After a patient had a clinical seizure, the researchers collected postictal and interictal venous blood samples from him or her to measure serum serotonin levels. They classified seizures using the International League Against Epilepsy 2017 seizure classification. Dr. Lhatoo and colleagues analyzed 49 seizures with and without ICA and 27 generalized convulsive seizures with and without PCCA.
Of the 49 patients, 29 were female. Participants’ mean age was 42 years, mean age at epilepsy onset was 25.2 years, and mean epilepsy duration was 16.8 years. The population’s mean body mass index was 28.9. Dr. Lhatoo and colleagues observed ICA in 17 of 49 (34.7%) seizures and PCCA in 8 of 27 (29.6%) seizures.
Postictal serum serotonin levels were significantly higher than interictal levels for seizures without ICA, but not for seizures with ICA. Among patients with generalized convulsive seizures without PCCA, serum serotonin levels were significantly increased postictally, compared with interictal levels, but not among patients with seizures with PCCA. The change in postictal and interictal serotonin levels also differed significantly between participants with and without PCCA. In patients without PCCA, an increase in serotonin was associated with an increase in heart rate, but not in patients with PCCA.
“Large postictal increases in serum serotonin may play a role in modulation of respiration in these patients,” wrote Dr. Lhatoo and colleagues. “Alternatively, the increase in serum serotonin that we measured may be a surrogate for an increase in brain serotonin levels that may depend on similar physiologic mechanisms, rather than serum serotonin directly stimulating breathing.” Low levels of postictal serum serotonin are associated with potentially harmful breathing phenomena that should be investigated in larger studies, the investigators concluded.
The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
SOURCE: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
, according to research published online Sept. 4 in Neurology. The change in serotonin level may reflect physiologic changes that protect against harmful processes that promote sudden unexpected death in epilepsy (SUDEP), the authors wrote.
“Our results give new insight into a possible link between serotonin levels and breathing during and after seizure,” Samden D. Lhatoo, MD, professor of neurology at McGovern Medical School at the University of Texas Health Science Center in Houston, said in a press release. “This may give hope that perhaps someday new therapies could be developed that may help prevent SUDEP. However, our study was small, and much more research is needed to confirm our findings in larger groups before any treatment decisions can be made. It is also important to note that excess serotonin can be harmful, so we strongly recommend against anyone trying to find ways to increase their serotonin levels in response to our study findings.”
Animal and human studies have indicated that breathing dysfunction related to SUDEP may involve serotonergic pathways. Compared with controls, patients with SUDEP have fewer midline serotonergic neurons. Furthermore, a 2018 study suggested an association between severe seizures and decreased serotonergic tone in the postictal state.
Dr. Lhatoo and colleagues examined a prospective cohort of patients with intractable epilepsy to understand the relationship between serum serotonin levels, ictal central apnea (ICA), and postconvulsive central apnea (PCCA). Patients were aged 18 years or older, were admitted to the epilepsy monitoring unit from January 2015 to April 2018, and agreed to take part in an investigation of SUDEP biomarkers. Dr. Lhatoo and colleagues evaluated video EEG, plethysmography, capillary oxygen saturation, and ECG for 49 patients. After a patient had a clinical seizure, the researchers collected postictal and interictal venous blood samples from him or her to measure serum serotonin levels. They classified seizures using the International League Against Epilepsy 2017 seizure classification. Dr. Lhatoo and colleagues analyzed 49 seizures with and without ICA and 27 generalized convulsive seizures with and without PCCA.
Of the 49 patients, 29 were female. Participants’ mean age was 42 years, mean age at epilepsy onset was 25.2 years, and mean epilepsy duration was 16.8 years. The population’s mean body mass index was 28.9. Dr. Lhatoo and colleagues observed ICA in 17 of 49 (34.7%) seizures and PCCA in 8 of 27 (29.6%) seizures.
Postictal serum serotonin levels were significantly higher than interictal levels for seizures without ICA, but not for seizures with ICA. Among patients with generalized convulsive seizures without PCCA, serum serotonin levels were significantly increased postictally, compared with interictal levels, but not among patients with seizures with PCCA. The change in postictal and interictal serotonin levels also differed significantly between participants with and without PCCA. In patients without PCCA, an increase in serotonin was associated with an increase in heart rate, but not in patients with PCCA.
“Large postictal increases in serum serotonin may play a role in modulation of respiration in these patients,” wrote Dr. Lhatoo and colleagues. “Alternatively, the increase in serum serotonin that we measured may be a surrogate for an increase in brain serotonin levels that may depend on similar physiologic mechanisms, rather than serum serotonin directly stimulating breathing.” Low levels of postictal serum serotonin are associated with potentially harmful breathing phenomena that should be investigated in larger studies, the investigators concluded.
The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
SOURCE: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
FROM NEUROLOGY
Key clinical point: Significant increases in serum serotonin after a seizure are associated with lower risk of seizure-related breathing dysfunction.
Major finding: In patients without ictal central apnea, mean interictal serotonin level was 109.1 ng/mL, and postictal levels were 139.8 ng/mL.
Study details: A prospective cohort study of 49 patients with intractable epilepsy.
Disclosures: The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
Source: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
Hyperphosphorylated tau visible in TBI survivors decades after brain injury
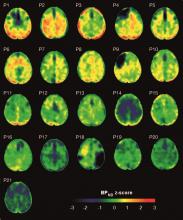
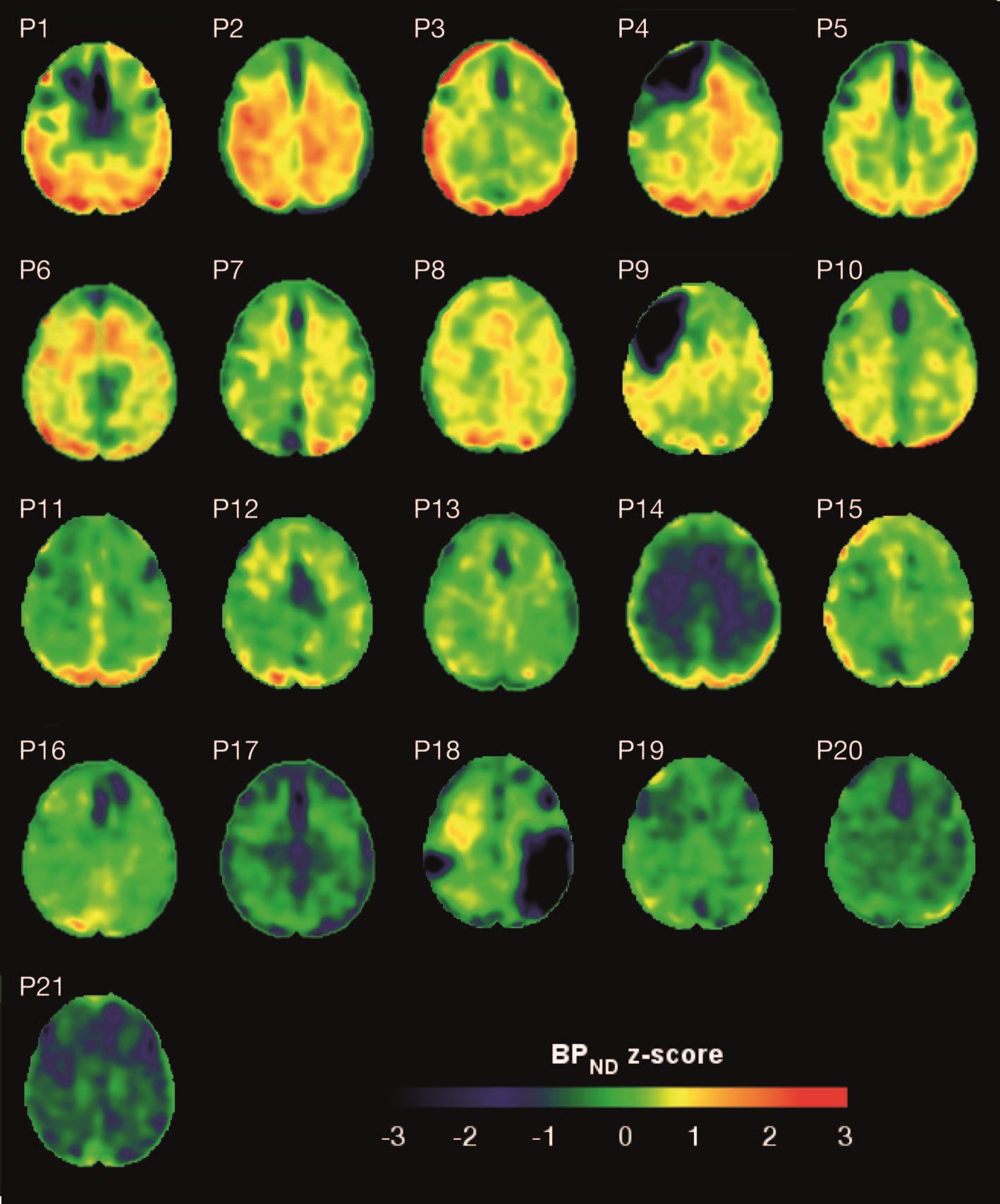
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
FROM SCIENCE TRANSLATIONAL MEDICINE
Our EHRs have a drug problem
The “opioid epidemic” has become, perhaps, the most talked-about health crisis of the 21st century. It is a pervasive topic of discussion in the health literature and beyond, written about on the front pages of national newspapers and even mentioned in presidential state-of-the-union addresses.
As practicing physicians, we are all too familiar with the ills of chronic opioid use and have dealt with the implications of the crisis long before the issue attracted the public’s attention. In many ways, we have felt alone in bearing the burdens of caring for patients on chronic controlled substances. Until this point it has been our sacred duty to determine which patients are truly in need of those medications, and which are merely dependent on or – even worse – abusing them.
Health care providers have been largely blamed for the creation of this crisis, but we are not alone. Responsibility must also be shared by the pharmaceutical industry, health insurers, and even the government. Marketing practices, inadequate coverage of pain-relieving procedures and rehabilitation, and poorly-conceived drug policies have created an environment where it has been far too difficult to provide appropriate care for patients with chronic pain. As a result, patients who may have had an alternative to opioids were still started on these medications, and we – their physicians – have been left alone to manage the outcome.
Recently, however, health policy and public awareness have signaled a dramatic shift in the management of long-term pain medication. Significant legislation has been enacted on national, state, and local levels, and parties who are perceived to be responsible for the crisis are being held to task. For example, in August a landmark legal case was decided in an Oklahoma district court. Johnson & Johnson Pharmaceuticals was found guilty of promoting drug addiction through false and misleading marketing and was thus ordered to pay $572 million to the state to fund drug rehabilitation programs. This is likely a harbinger of many more such decisions to come, and the industry as a whole is bracing for the worst.
Physician prescribing practices are also being carefully scrutinized by the DEA, and a significant number of new “checks and balances” have been put in place to address dependence and addiction concerns. Unfortunately, as with all sweeping reform programs, there are good – and not-so-good – aspects to these changes. In many ways, the new tools at our disposal are a powerful way of mitigating drug dependence and diversion while protecting the sanctity of our “prescription pads.” Yet, as with so many other government mandates, we are burdened with the onus of complying with the new mandates for each and every opioid prescription, while our EHRs provide little help. This means more “clicks” for us, which can feel quite burdensome. It doesn’t need to be this way. Below are two straightforward things that can and should occur in order for providers to feel unburdened and to fully embrace the changes.
PDMP integration
One of the major ways of controlling prescription opioid abuse is through effective monitoring. Forty-nine of the 50 U.S. states have developed Prescription Drug Monitoring Programs (PDMPs), with Missouri being the only holdout (due to the politics of individual privacy concerns and conflation with gun control legislation). Most – though not all – of the states with a PDMP also mandate that physicians query a database prior to prescribing controlled substances. While noble and helpful in principle, querying a PDMP can be cumbersome, and the process is rarely integrated into the EHR workflow. Instead, physicians typically need to login to a separate website and manually transpose patient data to search the database. While most states have offered to subsidize PDMP integration with electronic records, EHR vendors have been very slow to develop the capability, leaving most physicians with no choice but to continue the aforementioned workflow. That is, if they comply at all; many well-meaning physicians have told us that they find themselves too harried to use the PDMP consistently. This reduces the value of these databases and places the physicians at significant risk. In some states, failure to query the database can lead to loss of a doctor’s medical license. It is high time that EHR vendors step up and integrate with every state’s prescription drug database.
Electronic prescribing of controlled substances
The other major milestone in prescription opioid management is the electronic prescribing of controlled substances (EPCS). This received national priority when the SUPPORT for Patients and Communities Act was signed into federal law in October of 2018. Included in this act is a requirement that, by January of 2021, all controlled substance prescriptions covered under Medicare Part D be sent electronically. Taking this as inspiration, many states and private companies have adopted more aggressive policies, choosing to implement electronic prescription requirements prior to the 2021 deadline. In Pennsylvania, where we practice, an EPCS requirement goes into effect in October of this year (2019). National pharmacy chains have also taken a more proactive approach. Walmart, for example, has decided that it will require EPCS nationwide in all of its stores beginning in January of 2020.
Essentially physicians have no choice – if they plan to continue to prescribe controlled substances, they will need to begin doing so electronically. Unfortunately, this may not be a straightforward process. While most EHRs offer some sort of EPCS solution, it is typically far from user friendly. Setting up EPCS can be costly and incredibly time consuming, and the procedure of actually submitting controlled prescriptions can be onerous and add many extra clicks. If vendors are serious about assisting in solving the opioid crisis, they need to make streamlining the steps of EPCS a high priority.
A prescription for success
As with so many other topics we’ve written about, we face an ever-increasing burden to provide quality patient care while complying with cumbersome and often unfunded external mandates. In the case of the opioid crisis, we believe we can do better. Our prescription for success? Streamlined workflow, smarter EHRs, and fewer clicks. There is no question that physicians and patients will benefit from effective implementation of the new tools at our disposal, but we need EHR vendors to step up and help carry the load.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
The “opioid epidemic” has become, perhaps, the most talked-about health crisis of the 21st century. It is a pervasive topic of discussion in the health literature and beyond, written about on the front pages of national newspapers and even mentioned in presidential state-of-the-union addresses.
As practicing physicians, we are all too familiar with the ills of chronic opioid use and have dealt with the implications of the crisis long before the issue attracted the public’s attention. In many ways, we have felt alone in bearing the burdens of caring for patients on chronic controlled substances. Until this point it has been our sacred duty to determine which patients are truly in need of those medications, and which are merely dependent on or – even worse – abusing them.
Health care providers have been largely blamed for the creation of this crisis, but we are not alone. Responsibility must also be shared by the pharmaceutical industry, health insurers, and even the government. Marketing practices, inadequate coverage of pain-relieving procedures and rehabilitation, and poorly-conceived drug policies have created an environment where it has been far too difficult to provide appropriate care for patients with chronic pain. As a result, patients who may have had an alternative to opioids were still started on these medications, and we – their physicians – have been left alone to manage the outcome.
Recently, however, health policy and public awareness have signaled a dramatic shift in the management of long-term pain medication. Significant legislation has been enacted on national, state, and local levels, and parties who are perceived to be responsible for the crisis are being held to task. For example, in August a landmark legal case was decided in an Oklahoma district court. Johnson & Johnson Pharmaceuticals was found guilty of promoting drug addiction through false and misleading marketing and was thus ordered to pay $572 million to the state to fund drug rehabilitation programs. This is likely a harbinger of many more such decisions to come, and the industry as a whole is bracing for the worst.
Physician prescribing practices are also being carefully scrutinized by the DEA, and a significant number of new “checks and balances” have been put in place to address dependence and addiction concerns. Unfortunately, as with all sweeping reform programs, there are good – and not-so-good – aspects to these changes. In many ways, the new tools at our disposal are a powerful way of mitigating drug dependence and diversion while protecting the sanctity of our “prescription pads.” Yet, as with so many other government mandates, we are burdened with the onus of complying with the new mandates for each and every opioid prescription, while our EHRs provide little help. This means more “clicks” for us, which can feel quite burdensome. It doesn’t need to be this way. Below are two straightforward things that can and should occur in order for providers to feel unburdened and to fully embrace the changes.
PDMP integration
One of the major ways of controlling prescription opioid abuse is through effective monitoring. Forty-nine of the 50 U.S. states have developed Prescription Drug Monitoring Programs (PDMPs), with Missouri being the only holdout (due to the politics of individual privacy concerns and conflation with gun control legislation). Most – though not all – of the states with a PDMP also mandate that physicians query a database prior to prescribing controlled substances. While noble and helpful in principle, querying a PDMP can be cumbersome, and the process is rarely integrated into the EHR workflow. Instead, physicians typically need to login to a separate website and manually transpose patient data to search the database. While most states have offered to subsidize PDMP integration with electronic records, EHR vendors have been very slow to develop the capability, leaving most physicians with no choice but to continue the aforementioned workflow. That is, if they comply at all; many well-meaning physicians have told us that they find themselves too harried to use the PDMP consistently. This reduces the value of these databases and places the physicians at significant risk. In some states, failure to query the database can lead to loss of a doctor’s medical license. It is high time that EHR vendors step up and integrate with every state’s prescription drug database.
Electronic prescribing of controlled substances
The other major milestone in prescription opioid management is the electronic prescribing of controlled substances (EPCS). This received national priority when the SUPPORT for Patients and Communities Act was signed into federal law in October of 2018. Included in this act is a requirement that, by January of 2021, all controlled substance prescriptions covered under Medicare Part D be sent electronically. Taking this as inspiration, many states and private companies have adopted more aggressive policies, choosing to implement electronic prescription requirements prior to the 2021 deadline. In Pennsylvania, where we practice, an EPCS requirement goes into effect in October of this year (2019). National pharmacy chains have also taken a more proactive approach. Walmart, for example, has decided that it will require EPCS nationwide in all of its stores beginning in January of 2020.
Essentially physicians have no choice – if they plan to continue to prescribe controlled substances, they will need to begin doing so electronically. Unfortunately, this may not be a straightforward process. While most EHRs offer some sort of EPCS solution, it is typically far from user friendly. Setting up EPCS can be costly and incredibly time consuming, and the procedure of actually submitting controlled prescriptions can be onerous and add many extra clicks. If vendors are serious about assisting in solving the opioid crisis, they need to make streamlining the steps of EPCS a high priority.
A prescription for success
As with so many other topics we’ve written about, we face an ever-increasing burden to provide quality patient care while complying with cumbersome and often unfunded external mandates. In the case of the opioid crisis, we believe we can do better. Our prescription for success? Streamlined workflow, smarter EHRs, and fewer clicks. There is no question that physicians and patients will benefit from effective implementation of the new tools at our disposal, but we need EHR vendors to step up and help carry the load.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
The “opioid epidemic” has become, perhaps, the most talked-about health crisis of the 21st century. It is a pervasive topic of discussion in the health literature and beyond, written about on the front pages of national newspapers and even mentioned in presidential state-of-the-union addresses.
As practicing physicians, we are all too familiar with the ills of chronic opioid use and have dealt with the implications of the crisis long before the issue attracted the public’s attention. In many ways, we have felt alone in bearing the burdens of caring for patients on chronic controlled substances. Until this point it has been our sacred duty to determine which patients are truly in need of those medications, and which are merely dependent on or – even worse – abusing them.
Health care providers have been largely blamed for the creation of this crisis, but we are not alone. Responsibility must also be shared by the pharmaceutical industry, health insurers, and even the government. Marketing practices, inadequate coverage of pain-relieving procedures and rehabilitation, and poorly-conceived drug policies have created an environment where it has been far too difficult to provide appropriate care for patients with chronic pain. As a result, patients who may have had an alternative to opioids were still started on these medications, and we – their physicians – have been left alone to manage the outcome.
Recently, however, health policy and public awareness have signaled a dramatic shift in the management of long-term pain medication. Significant legislation has been enacted on national, state, and local levels, and parties who are perceived to be responsible for the crisis are being held to task. For example, in August a landmark legal case was decided in an Oklahoma district court. Johnson & Johnson Pharmaceuticals was found guilty of promoting drug addiction through false and misleading marketing and was thus ordered to pay $572 million to the state to fund drug rehabilitation programs. This is likely a harbinger of many more such decisions to come, and the industry as a whole is bracing for the worst.
Physician prescribing practices are also being carefully scrutinized by the DEA, and a significant number of new “checks and balances” have been put in place to address dependence and addiction concerns. Unfortunately, as with all sweeping reform programs, there are good – and not-so-good – aspects to these changes. In many ways, the new tools at our disposal are a powerful way of mitigating drug dependence and diversion while protecting the sanctity of our “prescription pads.” Yet, as with so many other government mandates, we are burdened with the onus of complying with the new mandates for each and every opioid prescription, while our EHRs provide little help. This means more “clicks” for us, which can feel quite burdensome. It doesn’t need to be this way. Below are two straightforward things that can and should occur in order for providers to feel unburdened and to fully embrace the changes.
PDMP integration
One of the major ways of controlling prescription opioid abuse is through effective monitoring. Forty-nine of the 50 U.S. states have developed Prescription Drug Monitoring Programs (PDMPs), with Missouri being the only holdout (due to the politics of individual privacy concerns and conflation with gun control legislation). Most – though not all – of the states with a PDMP also mandate that physicians query a database prior to prescribing controlled substances. While noble and helpful in principle, querying a PDMP can be cumbersome, and the process is rarely integrated into the EHR workflow. Instead, physicians typically need to login to a separate website and manually transpose patient data to search the database. While most states have offered to subsidize PDMP integration with electronic records, EHR vendors have been very slow to develop the capability, leaving most physicians with no choice but to continue the aforementioned workflow. That is, if they comply at all; many well-meaning physicians have told us that they find themselves too harried to use the PDMP consistently. This reduces the value of these databases and places the physicians at significant risk. In some states, failure to query the database can lead to loss of a doctor’s medical license. It is high time that EHR vendors step up and integrate with every state’s prescription drug database.
Electronic prescribing of controlled substances
The other major milestone in prescription opioid management is the electronic prescribing of controlled substances (EPCS). This received national priority when the SUPPORT for Patients and Communities Act was signed into federal law in October of 2018. Included in this act is a requirement that, by January of 2021, all controlled substance prescriptions covered under Medicare Part D be sent electronically. Taking this as inspiration, many states and private companies have adopted more aggressive policies, choosing to implement electronic prescription requirements prior to the 2021 deadline. In Pennsylvania, where we practice, an EPCS requirement goes into effect in October of this year (2019). National pharmacy chains have also taken a more proactive approach. Walmart, for example, has decided that it will require EPCS nationwide in all of its stores beginning in January of 2020.
Essentially physicians have no choice – if they plan to continue to prescribe controlled substances, they will need to begin doing so electronically. Unfortunately, this may not be a straightforward process. While most EHRs offer some sort of EPCS solution, it is typically far from user friendly. Setting up EPCS can be costly and incredibly time consuming, and the procedure of actually submitting controlled prescriptions can be onerous and add many extra clicks. If vendors are serious about assisting in solving the opioid crisis, they need to make streamlining the steps of EPCS a high priority.
A prescription for success
As with so many other topics we’ve written about, we face an ever-increasing burden to provide quality patient care while complying with cumbersome and often unfunded external mandates. In the case of the opioid crisis, we believe we can do better. Our prescription for success? Streamlined workflow, smarter EHRs, and fewer clicks. There is no question that physicians and patients will benefit from effective implementation of the new tools at our disposal, but we need EHR vendors to step up and help carry the load.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Cerliponase alfa continues to impress for CLN2 disease
BANGKOK – Biweekly cerliponase alfa continued to show durable and clinically important therapeutic benefit in children with neuronal ceroid lipofuscinosis type 2 (CLN2) disease at the 3-year mark in an ongoing international study, Marina Trivisano, MD, reported at the International Epilepsy Congress.
Cerliponase alfa, approved under the trade name Brineura by the Food and Drug Administration and European Commission, is a recombinant human tripeptidyl peptidase 1 designed as enzyme replacement therapy delivered by a surgically implanted intraventricular infusion device in children with this rare lysosomal storage disease, a form of Batten disease, she explained at the congress sponsored by the International League Against Epilepsy.
When both healthy parents carry one defective gene, each of their children has a one in four chance of inheriting this devastating disease that causes rapidly progressive dementia. CLN2 disease typically reveals itself when a child reaches about 3 years of age, with seizures, language delay, or loss of acquired language being the most common first indications.
Of 23 patients enrolled in the open-label study, 21 remained participants at 3 years of follow-up. The two dropouts weren’t caused by treatment-related adverse events, but rather by the formidable logistic challenges posed because the treatment – 300 mg of cerliponase alfa delivered by intraventricular infusion over a 4-hour period every 2 weeks – was available only at five medical centers located in Rome; London; New York; Hamburg, Germany; and Columbus, Ohio.
At 3 years of follow-up, 83% of patients met the primary study endpoint, defined as the absence of a 2-point or greater decline in the motor-language score on the 0-6 CLN2 Clinical Rating Scale. This was a success rate 12 times greater than in 42 historical controls. Indeed, at 3 years the cerliponase alfa–treated patients had an average CLN2 Clinical Rating Scale motor-language score 3.8 points better than the historical controls, reported Dr. Trivisano, a pediatric neurologist at Bambino Gesu Children’s Hospital in Rome.
Side effects included several cases of device failure, infection, and hypersensitivity reactions.
In an earlier report based upon 96 weeks of follow-up, the mean rate of decline in the motor-language score was 0.27 points per 48 weeks in treated patients, compared with 2.12 points in the historical controls (N Engl J Med. 2018 May 17;378[20]:1898-1907).
The study was funded by BioMarin Pharmaceutical, which markets Brineura. Dr. Trivisano was a subinvestigator in the trial.
SOURCE: Trivisano M et al. IEC 2019, Abstract P333.
BANGKOK – Biweekly cerliponase alfa continued to show durable and clinically important therapeutic benefit in children with neuronal ceroid lipofuscinosis type 2 (CLN2) disease at the 3-year mark in an ongoing international study, Marina Trivisano, MD, reported at the International Epilepsy Congress.
Cerliponase alfa, approved under the trade name Brineura by the Food and Drug Administration and European Commission, is a recombinant human tripeptidyl peptidase 1 designed as enzyme replacement therapy delivered by a surgically implanted intraventricular infusion device in children with this rare lysosomal storage disease, a form of Batten disease, she explained at the congress sponsored by the International League Against Epilepsy.
When both healthy parents carry one defective gene, each of their children has a one in four chance of inheriting this devastating disease that causes rapidly progressive dementia. CLN2 disease typically reveals itself when a child reaches about 3 years of age, with seizures, language delay, or loss of acquired language being the most common first indications.
Of 23 patients enrolled in the open-label study, 21 remained participants at 3 years of follow-up. The two dropouts weren’t caused by treatment-related adverse events, but rather by the formidable logistic challenges posed because the treatment – 300 mg of cerliponase alfa delivered by intraventricular infusion over a 4-hour period every 2 weeks – was available only at five medical centers located in Rome; London; New York; Hamburg, Germany; and Columbus, Ohio.
At 3 years of follow-up, 83% of patients met the primary study endpoint, defined as the absence of a 2-point or greater decline in the motor-language score on the 0-6 CLN2 Clinical Rating Scale. This was a success rate 12 times greater than in 42 historical controls. Indeed, at 3 years the cerliponase alfa–treated patients had an average CLN2 Clinical Rating Scale motor-language score 3.8 points better than the historical controls, reported Dr. Trivisano, a pediatric neurologist at Bambino Gesu Children’s Hospital in Rome.
Side effects included several cases of device failure, infection, and hypersensitivity reactions.
In an earlier report based upon 96 weeks of follow-up, the mean rate of decline in the motor-language score was 0.27 points per 48 weeks in treated patients, compared with 2.12 points in the historical controls (N Engl J Med. 2018 May 17;378[20]:1898-1907).
The study was funded by BioMarin Pharmaceutical, which markets Brineura. Dr. Trivisano was a subinvestigator in the trial.
SOURCE: Trivisano M et al. IEC 2019, Abstract P333.
BANGKOK – Biweekly cerliponase alfa continued to show durable and clinically important therapeutic benefit in children with neuronal ceroid lipofuscinosis type 2 (CLN2) disease at the 3-year mark in an ongoing international study, Marina Trivisano, MD, reported at the International Epilepsy Congress.
Cerliponase alfa, approved under the trade name Brineura by the Food and Drug Administration and European Commission, is a recombinant human tripeptidyl peptidase 1 designed as enzyme replacement therapy delivered by a surgically implanted intraventricular infusion device in children with this rare lysosomal storage disease, a form of Batten disease, she explained at the congress sponsored by the International League Against Epilepsy.
When both healthy parents carry one defective gene, each of their children has a one in four chance of inheriting this devastating disease that causes rapidly progressive dementia. CLN2 disease typically reveals itself when a child reaches about 3 years of age, with seizures, language delay, or loss of acquired language being the most common first indications.
Of 23 patients enrolled in the open-label study, 21 remained participants at 3 years of follow-up. The two dropouts weren’t caused by treatment-related adverse events, but rather by the formidable logistic challenges posed because the treatment – 300 mg of cerliponase alfa delivered by intraventricular infusion over a 4-hour period every 2 weeks – was available only at five medical centers located in Rome; London; New York; Hamburg, Germany; and Columbus, Ohio.
At 3 years of follow-up, 83% of patients met the primary study endpoint, defined as the absence of a 2-point or greater decline in the motor-language score on the 0-6 CLN2 Clinical Rating Scale. This was a success rate 12 times greater than in 42 historical controls. Indeed, at 3 years the cerliponase alfa–treated patients had an average CLN2 Clinical Rating Scale motor-language score 3.8 points better than the historical controls, reported Dr. Trivisano, a pediatric neurologist at Bambino Gesu Children’s Hospital in Rome.
Side effects included several cases of device failure, infection, and hypersensitivity reactions.
In an earlier report based upon 96 weeks of follow-up, the mean rate of decline in the motor-language score was 0.27 points per 48 weeks in treated patients, compared with 2.12 points in the historical controls (N Engl J Med. 2018 May 17;378[20]:1898-1907).
The study was funded by BioMarin Pharmaceutical, which markets Brineura. Dr. Trivisano was a subinvestigator in the trial.
SOURCE: Trivisano M et al. IEC 2019, Abstract P333.
REPORTING FROM IEC 2019
Combo therapy outcomes for West syndrome prove no better than monotherapy
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM IEC 2019
Tandem transplants provide EFS edge in pediatric neuroblastoma
For young patients with high-risk neuroblastoma, an intensive consolidation regimen with tandem autologous stem cell transplants was associated with significantly better event-free survival, compared with single-transplant consolidation, results of a randomized trial show.
Among 355 patients with high-risk neuroblastoma, the 3-year event-free survival (EFS) rate was 61.6% for patients randomized to tandem (sequential) autologous stem cell transplants, compared with 48.4% for patients randomized to a single transplant (P = .006), reported Julie R. Park, MD from Seattle Children’s Hospital in Washington, and coinvestigators in the Children’s Oncology Group’s ANBL0532 trial.
“Results of the current study are consistent with earlier trials demonstrating that induction chemotherapy followed by consolidation with autologous transplant improved EFS, compared with less intensive consolidation, and that further intensification of consolidation benefits some patients,” they wrote in JAMA.
But of the 652 patients enrolled in the study, only 355 were actually randomized. Although the randomization rate was slightly higher than anticipated, the authors acknowledged that the results may not apply to all patients with high-risk neuroblastoma.
Patients eligible for the trial included those with International Neuroblastoma Staging System (INSS) stage 4 neuroblastoma aged older than 18 months; INSS stage 3 neuroblastoma aged older than 18 months with International Neuroblastoma Pathology Classification of unfavorable histology; INSS stage 2, 3, 4, or 4S neuroblastoma with MYCN amplification; and INSS stage 4 neuroblastoma diagnosed from age 12-18 months whose tumors showed any unfavorable features. Patients initially diagnosed with non–high-risk neuroblastoma (including stage 1) who had not received chemotherapy and whose disease had progressed to high-risk neuroblastoma were also eligible.
Following induction with two cycles of topotecan and cyclophosphamide, patients underwent peripheral blood stem cell collection, followed by four alternating cycles of cisplatin and etoposide and doxorubicin and cyclophosphamide, and vincristine.
For those patients who did not have primary tumors resected at diagnosis, resection was performed after the fourth or fifth cycle.
Those patients who after induction had no disease progression, no uncontrolled infection, sufficient stem cell levels, and adequate organ function were then eligible for randomization. One patient did not receive any therapy, 27 were nonrandomly assigned to single transplant, 62 were not eligible for randomization, and 207 were not randomized because of physician or family preference.
Of the remaining patients (median age at diagnosis, 36.1 months) 176 were randomized to receive tandem transplant with thiotepa and cyclophosphamide followed by dose-reduced carboplatin, etoposide, and melphalan conditioning, and 179 were randomized to single transplant with standard-dose carboplatin, etoposide, and melphalan.
A total of 17 patients died on study from toxicity; 7 during induction and 10 during consolidation. Significant transplant-related toxicities included mucositis in 11.7% of tandem-transplant patients and 15.4% of single-transplant patients, and infections in 17.8% versus 18.3%, respectively.
As noted before, 3-year EFS from the time of randomization, the primary endpoint, was higher for patients in the tandem-transplant arm (61.6% vs. 48.4%, P = .006).
The median duration of follow-up after randomization for patients without relapse, disease progression, second malignancy, or death was 5.6 years.
A post hoc analysis of the randomized patients showed a 3-year overall survival rate of 71.6%, which did not differ significantly between the study arms (74.1% for the tandem-transplant group vs. 68.1% for the single-transplant group). The analysis also showed that 3-year EFS and overall survival was higher in the tandem- versus single-transplant groups among 250 patients who also received immunotherapy with isotretinoin plus an anti-GD2 chimeric antibody and cytokines.
The trial was supported by grants from the National Institutes of Health, National Cancer Institute, National Clinical Trials Network Operations Center, and St. Baldrick’s Foundation. Dr. Park reported no relevant disclosures. Multiple coauthors disclosed grants or personal feeds outside the submitted work.
SOURCE: Park JR et al. JAMA. 2019;322(8):746-55.
The ANBL0532 trial also does not address the important question as to whether tandem high-dose chemotherapy with autologous stem cell transplant results in benefit for all-comers with high-risk neuroblastoma, because just over half of the eligible patients underwent randomization. Although characteristics of the entire cohort and the randomized cohort were similar with respect to age, stage, tumor histology, and MYCN status, there may be differences unrelated to widely accepted neuroblastoma risk variables. A separate but important challenge in interpretation of these results, as with any clinical trial results, is to understand the generalizability of findings to patient populations who may not be enrolling in trials. Previous work in pediatric oncology showed that trial enrollment correlated with race, age, and zip code, and it is difficult to know whether the results of the ANBL0532 trial are applicable to patient groups who may not be well represented.
An additional challenge is that even though a difference in event-free survival was detected between groups assigned to receive single versus tandem transplant, and a difference in overall survival was detected in a post hoc analysis of patients who received immunotherapy, no difference in overall survival was detected in the overall randomized cohort. Overall survival was evaluated as a secondary outcome but the trial was not powered to detect a difference in overall survival. Moreover, as noted by the authors, overall survival can be influenced by therapies delivered after relapse. This is particularly relevant in an era in which relapse therapies have been shown to induce responses, including periods of remission.
Remarks from Rochelle Bagatell, MD, from the University of Pennsylvania, Philadelphia, and Meredith S. Irwin, MD, from the Hospital for Sick Children in Toronto, are adapted and condensed from an editorial accompanying the study by Park et al. Dr. Bagatell is the vice chair of the Children’s Oncology Group Neuroblastoma Disease Committee. Dr. Irwin reported receiving personal fees from Bayer Canada outside the submitted work and is the vice chair of the Children’s Oncology Group Neuroblastoma Biology Committee. Neither Dr. Bagatell nor Dr. Irwin was involved in the design of the ANBL0532 trial or in the analysis of the results.
The ANBL0532 trial also does not address the important question as to whether tandem high-dose chemotherapy with autologous stem cell transplant results in benefit for all-comers with high-risk neuroblastoma, because just over half of the eligible patients underwent randomization. Although characteristics of the entire cohort and the randomized cohort were similar with respect to age, stage, tumor histology, and MYCN status, there may be differences unrelated to widely accepted neuroblastoma risk variables. A separate but important challenge in interpretation of these results, as with any clinical trial results, is to understand the generalizability of findings to patient populations who may not be enrolling in trials. Previous work in pediatric oncology showed that trial enrollment correlated with race, age, and zip code, and it is difficult to know whether the results of the ANBL0532 trial are applicable to patient groups who may not be well represented.
An additional challenge is that even though a difference in event-free survival was detected between groups assigned to receive single versus tandem transplant, and a difference in overall survival was detected in a post hoc analysis of patients who received immunotherapy, no difference in overall survival was detected in the overall randomized cohort. Overall survival was evaluated as a secondary outcome but the trial was not powered to detect a difference in overall survival. Moreover, as noted by the authors, overall survival can be influenced by therapies delivered after relapse. This is particularly relevant in an era in which relapse therapies have been shown to induce responses, including periods of remission.
Remarks from Rochelle Bagatell, MD, from the University of Pennsylvania, Philadelphia, and Meredith S. Irwin, MD, from the Hospital for Sick Children in Toronto, are adapted and condensed from an editorial accompanying the study by Park et al. Dr. Bagatell is the vice chair of the Children’s Oncology Group Neuroblastoma Disease Committee. Dr. Irwin reported receiving personal fees from Bayer Canada outside the submitted work and is the vice chair of the Children’s Oncology Group Neuroblastoma Biology Committee. Neither Dr. Bagatell nor Dr. Irwin was involved in the design of the ANBL0532 trial or in the analysis of the results.
The ANBL0532 trial also does not address the important question as to whether tandem high-dose chemotherapy with autologous stem cell transplant results in benefit for all-comers with high-risk neuroblastoma, because just over half of the eligible patients underwent randomization. Although characteristics of the entire cohort and the randomized cohort were similar with respect to age, stage, tumor histology, and MYCN status, there may be differences unrelated to widely accepted neuroblastoma risk variables. A separate but important challenge in interpretation of these results, as with any clinical trial results, is to understand the generalizability of findings to patient populations who may not be enrolling in trials. Previous work in pediatric oncology showed that trial enrollment correlated with race, age, and zip code, and it is difficult to know whether the results of the ANBL0532 trial are applicable to patient groups who may not be well represented.
An additional challenge is that even though a difference in event-free survival was detected between groups assigned to receive single versus tandem transplant, and a difference in overall survival was detected in a post hoc analysis of patients who received immunotherapy, no difference in overall survival was detected in the overall randomized cohort. Overall survival was evaluated as a secondary outcome but the trial was not powered to detect a difference in overall survival. Moreover, as noted by the authors, overall survival can be influenced by therapies delivered after relapse. This is particularly relevant in an era in which relapse therapies have been shown to induce responses, including periods of remission.
Remarks from Rochelle Bagatell, MD, from the University of Pennsylvania, Philadelphia, and Meredith S. Irwin, MD, from the Hospital for Sick Children in Toronto, are adapted and condensed from an editorial accompanying the study by Park et al. Dr. Bagatell is the vice chair of the Children’s Oncology Group Neuroblastoma Disease Committee. Dr. Irwin reported receiving personal fees from Bayer Canada outside the submitted work and is the vice chair of the Children’s Oncology Group Neuroblastoma Biology Committee. Neither Dr. Bagatell nor Dr. Irwin was involved in the design of the ANBL0532 trial or in the analysis of the results.
For young patients with high-risk neuroblastoma, an intensive consolidation regimen with tandem autologous stem cell transplants was associated with significantly better event-free survival, compared with single-transplant consolidation, results of a randomized trial show.
Among 355 patients with high-risk neuroblastoma, the 3-year event-free survival (EFS) rate was 61.6% for patients randomized to tandem (sequential) autologous stem cell transplants, compared with 48.4% for patients randomized to a single transplant (P = .006), reported Julie R. Park, MD from Seattle Children’s Hospital in Washington, and coinvestigators in the Children’s Oncology Group’s ANBL0532 trial.
“Results of the current study are consistent with earlier trials demonstrating that induction chemotherapy followed by consolidation with autologous transplant improved EFS, compared with less intensive consolidation, and that further intensification of consolidation benefits some patients,” they wrote in JAMA.
But of the 652 patients enrolled in the study, only 355 were actually randomized. Although the randomization rate was slightly higher than anticipated, the authors acknowledged that the results may not apply to all patients with high-risk neuroblastoma.
Patients eligible for the trial included those with International Neuroblastoma Staging System (INSS) stage 4 neuroblastoma aged older than 18 months; INSS stage 3 neuroblastoma aged older than 18 months with International Neuroblastoma Pathology Classification of unfavorable histology; INSS stage 2, 3, 4, or 4S neuroblastoma with MYCN amplification; and INSS stage 4 neuroblastoma diagnosed from age 12-18 months whose tumors showed any unfavorable features. Patients initially diagnosed with non–high-risk neuroblastoma (including stage 1) who had not received chemotherapy and whose disease had progressed to high-risk neuroblastoma were also eligible.
Following induction with two cycles of topotecan and cyclophosphamide, patients underwent peripheral blood stem cell collection, followed by four alternating cycles of cisplatin and etoposide and doxorubicin and cyclophosphamide, and vincristine.
For those patients who did not have primary tumors resected at diagnosis, resection was performed after the fourth or fifth cycle.
Those patients who after induction had no disease progression, no uncontrolled infection, sufficient stem cell levels, and adequate organ function were then eligible for randomization. One patient did not receive any therapy, 27 were nonrandomly assigned to single transplant, 62 were not eligible for randomization, and 207 were not randomized because of physician or family preference.
Of the remaining patients (median age at diagnosis, 36.1 months) 176 were randomized to receive tandem transplant with thiotepa and cyclophosphamide followed by dose-reduced carboplatin, etoposide, and melphalan conditioning, and 179 were randomized to single transplant with standard-dose carboplatin, etoposide, and melphalan.
A total of 17 patients died on study from toxicity; 7 during induction and 10 during consolidation. Significant transplant-related toxicities included mucositis in 11.7% of tandem-transplant patients and 15.4% of single-transplant patients, and infections in 17.8% versus 18.3%, respectively.
As noted before, 3-year EFS from the time of randomization, the primary endpoint, was higher for patients in the tandem-transplant arm (61.6% vs. 48.4%, P = .006).
The median duration of follow-up after randomization for patients without relapse, disease progression, second malignancy, or death was 5.6 years.
A post hoc analysis of the randomized patients showed a 3-year overall survival rate of 71.6%, which did not differ significantly between the study arms (74.1% for the tandem-transplant group vs. 68.1% for the single-transplant group). The analysis also showed that 3-year EFS and overall survival was higher in the tandem- versus single-transplant groups among 250 patients who also received immunotherapy with isotretinoin plus an anti-GD2 chimeric antibody and cytokines.
The trial was supported by grants from the National Institutes of Health, National Cancer Institute, National Clinical Trials Network Operations Center, and St. Baldrick’s Foundation. Dr. Park reported no relevant disclosures. Multiple coauthors disclosed grants or personal feeds outside the submitted work.
SOURCE: Park JR et al. JAMA. 2019;322(8):746-55.
For young patients with high-risk neuroblastoma, an intensive consolidation regimen with tandem autologous stem cell transplants was associated with significantly better event-free survival, compared with single-transplant consolidation, results of a randomized trial show.
Among 355 patients with high-risk neuroblastoma, the 3-year event-free survival (EFS) rate was 61.6% for patients randomized to tandem (sequential) autologous stem cell transplants, compared with 48.4% for patients randomized to a single transplant (P = .006), reported Julie R. Park, MD from Seattle Children’s Hospital in Washington, and coinvestigators in the Children’s Oncology Group’s ANBL0532 trial.
“Results of the current study are consistent with earlier trials demonstrating that induction chemotherapy followed by consolidation with autologous transplant improved EFS, compared with less intensive consolidation, and that further intensification of consolidation benefits some patients,” they wrote in JAMA.
But of the 652 patients enrolled in the study, only 355 were actually randomized. Although the randomization rate was slightly higher than anticipated, the authors acknowledged that the results may not apply to all patients with high-risk neuroblastoma.
Patients eligible for the trial included those with International Neuroblastoma Staging System (INSS) stage 4 neuroblastoma aged older than 18 months; INSS stage 3 neuroblastoma aged older than 18 months with International Neuroblastoma Pathology Classification of unfavorable histology; INSS stage 2, 3, 4, or 4S neuroblastoma with MYCN amplification; and INSS stage 4 neuroblastoma diagnosed from age 12-18 months whose tumors showed any unfavorable features. Patients initially diagnosed with non–high-risk neuroblastoma (including stage 1) who had not received chemotherapy and whose disease had progressed to high-risk neuroblastoma were also eligible.
Following induction with two cycles of topotecan and cyclophosphamide, patients underwent peripheral blood stem cell collection, followed by four alternating cycles of cisplatin and etoposide and doxorubicin and cyclophosphamide, and vincristine.
For those patients who did not have primary tumors resected at diagnosis, resection was performed after the fourth or fifth cycle.
Those patients who after induction had no disease progression, no uncontrolled infection, sufficient stem cell levels, and adequate organ function were then eligible for randomization. One patient did not receive any therapy, 27 were nonrandomly assigned to single transplant, 62 were not eligible for randomization, and 207 were not randomized because of physician or family preference.
Of the remaining patients (median age at diagnosis, 36.1 months) 176 were randomized to receive tandem transplant with thiotepa and cyclophosphamide followed by dose-reduced carboplatin, etoposide, and melphalan conditioning, and 179 were randomized to single transplant with standard-dose carboplatin, etoposide, and melphalan.
A total of 17 patients died on study from toxicity; 7 during induction and 10 during consolidation. Significant transplant-related toxicities included mucositis in 11.7% of tandem-transplant patients and 15.4% of single-transplant patients, and infections in 17.8% versus 18.3%, respectively.
As noted before, 3-year EFS from the time of randomization, the primary endpoint, was higher for patients in the tandem-transplant arm (61.6% vs. 48.4%, P = .006).
The median duration of follow-up after randomization for patients without relapse, disease progression, second malignancy, or death was 5.6 years.
A post hoc analysis of the randomized patients showed a 3-year overall survival rate of 71.6%, which did not differ significantly between the study arms (74.1% for the tandem-transplant group vs. 68.1% for the single-transplant group). The analysis also showed that 3-year EFS and overall survival was higher in the tandem- versus single-transplant groups among 250 patients who also received immunotherapy with isotretinoin plus an anti-GD2 chimeric antibody and cytokines.
The trial was supported by grants from the National Institutes of Health, National Cancer Institute, National Clinical Trials Network Operations Center, and St. Baldrick’s Foundation. Dr. Park reported no relevant disclosures. Multiple coauthors disclosed grants or personal feeds outside the submitted work.
SOURCE: Park JR et al. JAMA. 2019;322(8):746-55.
FROM JAMA
Health spending nears $23,000 per family

That average cost represents the employer’s contribution to the insurance premium ($15,159), along with the employee’s premium ($4,706) and the family’s out-of-pocket spending ($3,020), according to a KFF analysis of IBM MarketScan data and the 2018 KFF Employer Health Benefits Survey.
“Buying a new car every year would be a very impractical expense. It would also be cheaper than a year’s worth of health care for a family,” KFF President and CEO Drew Altman, PhD, wrote in his Axios column.
A little searching on the Kelley Blue Book Car Finder shows that the average family could have purchased a pretty nice new vehicle for the $22,885 that was spent on their health care in 2018:
- Mazda6 sedan: $22,845.
- Mini 2-door hatchback: $22,450.
- Jeep Renegade SUV: $21,040.
- Nissan Frontier king cab pickup: $20,035.
“The cost-shifting and complexity of health insurance can hide its high cost, which crowds out families’ other needs and depresses workers’ wages,” Dr. Altman said.

That average cost represents the employer’s contribution to the insurance premium ($15,159), along with the employee’s premium ($4,706) and the family’s out-of-pocket spending ($3,020), according to a KFF analysis of IBM MarketScan data and the 2018 KFF Employer Health Benefits Survey.
“Buying a new car every year would be a very impractical expense. It would also be cheaper than a year’s worth of health care for a family,” KFF President and CEO Drew Altman, PhD, wrote in his Axios column.
A little searching on the Kelley Blue Book Car Finder shows that the average family could have purchased a pretty nice new vehicle for the $22,885 that was spent on their health care in 2018:
- Mazda6 sedan: $22,845.
- Mini 2-door hatchback: $22,450.
- Jeep Renegade SUV: $21,040.
- Nissan Frontier king cab pickup: $20,035.
“The cost-shifting and complexity of health insurance can hide its high cost, which crowds out families’ other needs and depresses workers’ wages,” Dr. Altman said.

That average cost represents the employer’s contribution to the insurance premium ($15,159), along with the employee’s premium ($4,706) and the family’s out-of-pocket spending ($3,020), according to a KFF analysis of IBM MarketScan data and the 2018 KFF Employer Health Benefits Survey.
“Buying a new car every year would be a very impractical expense. It would also be cheaper than a year’s worth of health care for a family,” KFF President and CEO Drew Altman, PhD, wrote in his Axios column.
A little searching on the Kelley Blue Book Car Finder shows that the average family could have purchased a pretty nice new vehicle for the $22,885 that was spent on their health care in 2018:
- Mazda6 sedan: $22,845.
- Mini 2-door hatchback: $22,450.
- Jeep Renegade SUV: $21,040.
- Nissan Frontier king cab pickup: $20,035.
“The cost-shifting and complexity of health insurance can hide its high cost, which crowds out families’ other needs and depresses workers’ wages,” Dr. Altman said.
MenB vaccination coverage higher in those receiving MenB-4C
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
lfranki@mdedge.com
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
lfranki@mdedge.com
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
lfranki@mdedge.com
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
FROM VACCINE
FDA approves istradefylline for Parkinson’s disease
The Food and Drug Administration on Aug. 27 approved Nourianz (istradefylline) tablets as an add-on treatment to levodopa/carbidopa in adult patients with Parkinson’s disease experiencing off episodes. During off episodes, patients’ medications do not work well, and symptoms such as tremor and difficulty walking increase.
The effectiveness of Nourianz for this indication was shown in four 12-week placebo-controlled clinical studies that included 1,143 participants. In all four studies, patients treated with Nourianz experienced a statistically significant decrease from baseline in daily off time, compared with patients who received placebo.
The most common adverse reactions to istradefylline with an incidence of 5% or greater and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%, for Nourianz 20 mg, 40 mg, and placebo, respectively), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%). In clinical trials, 1% of patients treated with Nourianz 20 mg or 40 mg discontinued treatment because of dyskinesia, compared with no patients who received placebo.
In addition,one patient treated with Nourianz 40 mg experienced impulse control disorder, compared with no patients who received Nourianz 20 mg or placebo.
If hallucinations, psychotic behavior, or impulsive or compulsive behavior occurs, a dosage reduction or stoppage should be considered, according to the FDA. Use of Nourianz during pregnancy is not recommended, and women of childbearing potential should be advised to use contraception during treatment.
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily, and clinicians should avoid use of Nourianz with strong CYP3A4 inducers.
Istradefylline is the first adenosine A2A receptor antagonist for use in Parkinson’s disease in the United States, and the drug provides patients with a novel nondopaminergic daily oral treatment option, according to a news release from Kyowa Kirin, the company that markets the drug.
Since 2013, istradefylline has been marketed at Nouriast in Japan, where it is indicated for the wearing-off phenomenon in patients with Parkinson’s disease who take preparations containing levodopa.
The Food and Drug Administration on Aug. 27 approved Nourianz (istradefylline) tablets as an add-on treatment to levodopa/carbidopa in adult patients with Parkinson’s disease experiencing off episodes. During off episodes, patients’ medications do not work well, and symptoms such as tremor and difficulty walking increase.
The effectiveness of Nourianz for this indication was shown in four 12-week placebo-controlled clinical studies that included 1,143 participants. In all four studies, patients treated with Nourianz experienced a statistically significant decrease from baseline in daily off time, compared with patients who received placebo.
The most common adverse reactions to istradefylline with an incidence of 5% or greater and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%, for Nourianz 20 mg, 40 mg, and placebo, respectively), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%). In clinical trials, 1% of patients treated with Nourianz 20 mg or 40 mg discontinued treatment because of dyskinesia, compared with no patients who received placebo.
In addition,one patient treated with Nourianz 40 mg experienced impulse control disorder, compared with no patients who received Nourianz 20 mg or placebo.
If hallucinations, psychotic behavior, or impulsive or compulsive behavior occurs, a dosage reduction or stoppage should be considered, according to the FDA. Use of Nourianz during pregnancy is not recommended, and women of childbearing potential should be advised to use contraception during treatment.
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily, and clinicians should avoid use of Nourianz with strong CYP3A4 inducers.
Istradefylline is the first adenosine A2A receptor antagonist for use in Parkinson’s disease in the United States, and the drug provides patients with a novel nondopaminergic daily oral treatment option, according to a news release from Kyowa Kirin, the company that markets the drug.
Since 2013, istradefylline has been marketed at Nouriast in Japan, where it is indicated for the wearing-off phenomenon in patients with Parkinson’s disease who take preparations containing levodopa.
The Food and Drug Administration on Aug. 27 approved Nourianz (istradefylline) tablets as an add-on treatment to levodopa/carbidopa in adult patients with Parkinson’s disease experiencing off episodes. During off episodes, patients’ medications do not work well, and symptoms such as tremor and difficulty walking increase.
The effectiveness of Nourianz for this indication was shown in four 12-week placebo-controlled clinical studies that included 1,143 participants. In all four studies, patients treated with Nourianz experienced a statistically significant decrease from baseline in daily off time, compared with patients who received placebo.
The most common adverse reactions to istradefylline with an incidence of 5% or greater and occurring more frequently than with placebo were dyskinesia (15%, 17%, and 8%, for Nourianz 20 mg, 40 mg, and placebo, respectively), dizziness (3%, 6%, and 4%), constipation (5%, 6%, and 3%), nausea (4%, 6%, and 5%), hallucination (2%, 6%, and 3%), and insomnia (1%, 6%, and 4%). In clinical trials, 1% of patients treated with Nourianz 20 mg or 40 mg discontinued treatment because of dyskinesia, compared with no patients who received placebo.
In addition,one patient treated with Nourianz 40 mg experienced impulse control disorder, compared with no patients who received Nourianz 20 mg or placebo.
If hallucinations, psychotic behavior, or impulsive or compulsive behavior occurs, a dosage reduction or stoppage should be considered, according to the FDA. Use of Nourianz during pregnancy is not recommended, and women of childbearing potential should be advised to use contraception during treatment.
The maximum recommended dosage in patients taking strong CYP3A4 inhibitors is 20 mg once daily, and clinicians should avoid use of Nourianz with strong CYP3A4 inducers.
Istradefylline is the first adenosine A2A receptor antagonist for use in Parkinson’s disease in the United States, and the drug provides patients with a novel nondopaminergic daily oral treatment option, according to a news release from Kyowa Kirin, the company that markets the drug.
Since 2013, istradefylline has been marketed at Nouriast in Japan, where it is indicated for the wearing-off phenomenon in patients with Parkinson’s disease who take preparations containing levodopa.