User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Continuous treatment reduces risk of confirmed disability progression in MS
STOCKHOLM – (CDP), according to an investigation presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Using several confirmation points for Expanded Disability Status Scale (EDSS) progression (e.g., 12 months and 24 months), researchers detected a clear gradient of treatment effect. Identification of the most reliable outcome definitions will require further investigations, they said.
“The ultimate goal of MS treatment is the prevention of long-term disability accumulation,” said Giuseppe Lucisano, a biostatistician at the Center for Outcomes Research and Clinical Epidemiology in Pescara, Italy. “Continuous DMT exposure can impact long-term disability accumulation in MS, but it has not been definitively demonstrated yet.”
Registries and clinical databases provide the opportunity to collect longitudinal data for treated and untreated patients as a means of investigating questions such as this one, the researchers said. The Danish, Italian, and Swedish national MS registries, MSBase, and the Observatoire of MS (OFSEP) merged their data in the Big Multiple Sclerosis Data (BMSD) Network, which includes approximately 150,000 patients and more than 470,000 EDSS evaluations. The result is a large dataset suitable for long-term longitudinal studies.
Mr. Lucisano and colleagues sought to examine the long-term effect of DMTs on CDP and irreversible disability milestones (i.e., EDSS scores of 4 and 6) in relapsing-remitting MS. The researchers used marginal structural proportional models, a novel technique that enables them to correct modeling for confounders that vary with time in longitudinal observational studies. Such confounders include treatment switches, on-treatment relapses, and treatment gaps.
The investigators selected patients with 10 or more years’ follow-up and one or more EDSS score evaluations per year from the BMSD pooled cohort. Using marginal structural proportional models, the investigators evaluated cumulative hazards of 3-, 12- and 24-month CDP (i.e., CDP3, CDP12, CDP24) events in 6-month periods. They created stabilized inverse probability of treatment weights (IPTWs) at each 6-month period using survival models according to treatment status (i.e., treated versus untreated). Treatment status was assigned for each patient according to the percentage of time that he or she spent receiving DMT in each 6-month period. A patient who received treatment for 70% or more of the period studied was considered treated; patients who did not meet this threshold were considered untreated. The weights were calculated on the basis of sex, age, occurrence of relapse, EDSS score, and registry source. Finally, the researchers used Cox regression models estimating the effect of DMTs on the risk of reaching CDP3, CDP12, and CDP24, adjusted by the IPTWs, to compare cohorts that remained treated or untreated throughout follow-up.
The investigators identified a cohort of 15,602 patients with relapsing-remitting MS, and this group had 312,040 EDSS score evaluations. Approximately 28% of patients were male. Median age at disease onset was 28.3 years, and median disease duration was 18.7 years. Median follow-up duration was 13.8 years.
During follow-up, 43.3% of patients had CDP3, 27.7% had CDP12, and 14.4% had CDP24 events. In addition, 23.6% of patients reached an EDSS score of 4, and 11.2% reached an EDSS score of 6.
Cox models adjusted by IPTW demonstrated increasing positive evidence of the effect of cumulative treatment exposure, compared with cumulative untreated epochs, according to the length of confirmation time used for defining the CDP. The investigators did not observe an effect of treatment on the probability of reaching CDP3 (hazard ratio [HR], 1.02), but treatment had a protective effect on CDP12 (HR, 0.90) and CDP24 (HR, 0.65) endpoints. During treated epochs, the HR of EDSS 4 was 0.89, and the HR of EDSS 6 was 0.86. Sensitivity analyses largely confirmed the results of the main analysis.
Two of the researchers are employees of Biogen International, which supported the research. Several investigators received compensation or funding from various pharmaceutical companies.
STOCKHOLM – (CDP), according to an investigation presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Using several confirmation points for Expanded Disability Status Scale (EDSS) progression (e.g., 12 months and 24 months), researchers detected a clear gradient of treatment effect. Identification of the most reliable outcome definitions will require further investigations, they said.
“The ultimate goal of MS treatment is the prevention of long-term disability accumulation,” said Giuseppe Lucisano, a biostatistician at the Center for Outcomes Research and Clinical Epidemiology in Pescara, Italy. “Continuous DMT exposure can impact long-term disability accumulation in MS, but it has not been definitively demonstrated yet.”
Registries and clinical databases provide the opportunity to collect longitudinal data for treated and untreated patients as a means of investigating questions such as this one, the researchers said. The Danish, Italian, and Swedish national MS registries, MSBase, and the Observatoire of MS (OFSEP) merged their data in the Big Multiple Sclerosis Data (BMSD) Network, which includes approximately 150,000 patients and more than 470,000 EDSS evaluations. The result is a large dataset suitable for long-term longitudinal studies.
Mr. Lucisano and colleagues sought to examine the long-term effect of DMTs on CDP and irreversible disability milestones (i.e., EDSS scores of 4 and 6) in relapsing-remitting MS. The researchers used marginal structural proportional models, a novel technique that enables them to correct modeling for confounders that vary with time in longitudinal observational studies. Such confounders include treatment switches, on-treatment relapses, and treatment gaps.
The investigators selected patients with 10 or more years’ follow-up and one or more EDSS score evaluations per year from the BMSD pooled cohort. Using marginal structural proportional models, the investigators evaluated cumulative hazards of 3-, 12- and 24-month CDP (i.e., CDP3, CDP12, CDP24) events in 6-month periods. They created stabilized inverse probability of treatment weights (IPTWs) at each 6-month period using survival models according to treatment status (i.e., treated versus untreated). Treatment status was assigned for each patient according to the percentage of time that he or she spent receiving DMT in each 6-month period. A patient who received treatment for 70% or more of the period studied was considered treated; patients who did not meet this threshold were considered untreated. The weights were calculated on the basis of sex, age, occurrence of relapse, EDSS score, and registry source. Finally, the researchers used Cox regression models estimating the effect of DMTs on the risk of reaching CDP3, CDP12, and CDP24, adjusted by the IPTWs, to compare cohorts that remained treated or untreated throughout follow-up.
The investigators identified a cohort of 15,602 patients with relapsing-remitting MS, and this group had 312,040 EDSS score evaluations. Approximately 28% of patients were male. Median age at disease onset was 28.3 years, and median disease duration was 18.7 years. Median follow-up duration was 13.8 years.
During follow-up, 43.3% of patients had CDP3, 27.7% had CDP12, and 14.4% had CDP24 events. In addition, 23.6% of patients reached an EDSS score of 4, and 11.2% reached an EDSS score of 6.
Cox models adjusted by IPTW demonstrated increasing positive evidence of the effect of cumulative treatment exposure, compared with cumulative untreated epochs, according to the length of confirmation time used for defining the CDP. The investigators did not observe an effect of treatment on the probability of reaching CDP3 (hazard ratio [HR], 1.02), but treatment had a protective effect on CDP12 (HR, 0.90) and CDP24 (HR, 0.65) endpoints. During treated epochs, the HR of EDSS 4 was 0.89, and the HR of EDSS 6 was 0.86. Sensitivity analyses largely confirmed the results of the main analysis.
Two of the researchers are employees of Biogen International, which supported the research. Several investigators received compensation or funding from various pharmaceutical companies.
STOCKHOLM – (CDP), according to an investigation presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Using several confirmation points for Expanded Disability Status Scale (EDSS) progression (e.g., 12 months and 24 months), researchers detected a clear gradient of treatment effect. Identification of the most reliable outcome definitions will require further investigations, they said.
“The ultimate goal of MS treatment is the prevention of long-term disability accumulation,” said Giuseppe Lucisano, a biostatistician at the Center for Outcomes Research and Clinical Epidemiology in Pescara, Italy. “Continuous DMT exposure can impact long-term disability accumulation in MS, but it has not been definitively demonstrated yet.”
Registries and clinical databases provide the opportunity to collect longitudinal data for treated and untreated patients as a means of investigating questions such as this one, the researchers said. The Danish, Italian, and Swedish national MS registries, MSBase, and the Observatoire of MS (OFSEP) merged their data in the Big Multiple Sclerosis Data (BMSD) Network, which includes approximately 150,000 patients and more than 470,000 EDSS evaluations. The result is a large dataset suitable for long-term longitudinal studies.
Mr. Lucisano and colleagues sought to examine the long-term effect of DMTs on CDP and irreversible disability milestones (i.e., EDSS scores of 4 and 6) in relapsing-remitting MS. The researchers used marginal structural proportional models, a novel technique that enables them to correct modeling for confounders that vary with time in longitudinal observational studies. Such confounders include treatment switches, on-treatment relapses, and treatment gaps.
The investigators selected patients with 10 or more years’ follow-up and one or more EDSS score evaluations per year from the BMSD pooled cohort. Using marginal structural proportional models, the investigators evaluated cumulative hazards of 3-, 12- and 24-month CDP (i.e., CDP3, CDP12, CDP24) events in 6-month periods. They created stabilized inverse probability of treatment weights (IPTWs) at each 6-month period using survival models according to treatment status (i.e., treated versus untreated). Treatment status was assigned for each patient according to the percentage of time that he or she spent receiving DMT in each 6-month period. A patient who received treatment for 70% or more of the period studied was considered treated; patients who did not meet this threshold were considered untreated. The weights were calculated on the basis of sex, age, occurrence of relapse, EDSS score, and registry source. Finally, the researchers used Cox regression models estimating the effect of DMTs on the risk of reaching CDP3, CDP12, and CDP24, adjusted by the IPTWs, to compare cohorts that remained treated or untreated throughout follow-up.
The investigators identified a cohort of 15,602 patients with relapsing-remitting MS, and this group had 312,040 EDSS score evaluations. Approximately 28% of patients were male. Median age at disease onset was 28.3 years, and median disease duration was 18.7 years. Median follow-up duration was 13.8 years.
During follow-up, 43.3% of patients had CDP3, 27.7% had CDP12, and 14.4% had CDP24 events. In addition, 23.6% of patients reached an EDSS score of 4, and 11.2% reached an EDSS score of 6.
Cox models adjusted by IPTW demonstrated increasing positive evidence of the effect of cumulative treatment exposure, compared with cumulative untreated epochs, according to the length of confirmation time used for defining the CDP. The investigators did not observe an effect of treatment on the probability of reaching CDP3 (hazard ratio [HR], 1.02), but treatment had a protective effect on CDP12 (HR, 0.90) and CDP24 (HR, 0.65) endpoints. During treated epochs, the HR of EDSS 4 was 0.89, and the HR of EDSS 6 was 0.86. Sensitivity analyses largely confirmed the results of the main analysis.
Two of the researchers are employees of Biogen International, which supported the research. Several investigators received compensation or funding from various pharmaceutical companies.
REPORTING FROM ECTRIMS 2019
Neurologists need not discourage breastfeeding in women with MS
STOCKHOLM – Most neurologists are overly conservative when it comes to advising women with multiple sclerosis (MS) about breastfeeding, discouraging this broadly beneficial practice in favor of early resumption of treatment post pregnancy, Kerstin Hellwig, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We should change our behavior, and I predict we will change it so that more women are breastfeeding while under MS medication within the next couple years. ,” said Dr. Hellwig, senior consultant and MS specialist in the department of neurology at St. Josef Hospital/Ruhr University in Bochum, Germany.
She was a coauthor of a groundbreaking 2012 meta-analysis that concluded that breastfeeding by MS patients is not harmful (J Neurol. 2012 Oct;259[10]:2246-8), a finding since confirmed in multiple additional studies.
“Women with MS who want to breastfeed should be supported in doing so,” Dr. Hellwig said.
In this regard, many neurologists are out of step with their colleagues in rheumatology and gastroenterology, who commonly endorse breastfeeding by their patients while on monoclonal antibodies for other autoimmune diseases, according to Dr. Hellwig.
It is important to recognize that most women of reproductive age with MS have milder forms of the disease, she said. They can safely breastfeed without being on any MS medications at all for the duration.
For women who want to breastfeed and have more-active disease where early treatment resumption is warranted, the key is to select a breastfeeding-compatible medication. The main determinant of whether a drug will enter the mother’s breast milk is the size of the drug molecule, with large molecules being unlikely to make their way into breast milk in anything approaching clinically meaningful amounts. The injectable first-line disease-modifying drugs are good options: For example, interferon-beta is a very large molecule which has been detected in breast milk at 0.0006% of the relative infant dose. That’s reassuring, Dr. Hellwig said, since anything less than a relative infant dose of 10% is generally considered to be safe for a baby. And while glatiramer acetate, another injectable, has not been tested, it is metabolized so rapidly that it is unlikely to be detectable in breast milk, according to Dr. Hellwig.
Monoclonal antibodies are also compatible with breastfeeding. Rituximab has been detected in breast milk at 1/240th of the maternal serum level, and natalizumab at less than 1/200th. These are large molecules with a low likelihood of infant absorption, since they are probably destroyed in the child’s gastrointestinal tract. Ocrelizumab has not been studied in breast milk, but it is an IgG1 monoclonal antibody, as is rituximab, and so should likewise pose “exceedingly low risk,” Dr. Hellwig said.
At last year’s ECTRIMS conference, she presented reassuring 1-year follow-up data on a cohort of infants breastfed by mothers with MS while on interferon-beta. “We do not see any growth disturbances, any severe infections, hospitalizations, excess antibiotic use, or postponed reaching of developmental milestones in babies being breastfed under the injectables,” she said.
Dr. Hellwig has served on scientific advisory board for Bayer, Biogen, Genzyme Sanofi, Teva, Roche, Novartis, and Merck. She has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, SanofiGenzyme, and Teva, and has received support for congress participation from Bayer, Biogen, Genzyme, Teva, Roche, and Merck.
STOCKHOLM – Most neurologists are overly conservative when it comes to advising women with multiple sclerosis (MS) about breastfeeding, discouraging this broadly beneficial practice in favor of early resumption of treatment post pregnancy, Kerstin Hellwig, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We should change our behavior, and I predict we will change it so that more women are breastfeeding while under MS medication within the next couple years. ,” said Dr. Hellwig, senior consultant and MS specialist in the department of neurology at St. Josef Hospital/Ruhr University in Bochum, Germany.
She was a coauthor of a groundbreaking 2012 meta-analysis that concluded that breastfeeding by MS patients is not harmful (J Neurol. 2012 Oct;259[10]:2246-8), a finding since confirmed in multiple additional studies.
“Women with MS who want to breastfeed should be supported in doing so,” Dr. Hellwig said.
In this regard, many neurologists are out of step with their colleagues in rheumatology and gastroenterology, who commonly endorse breastfeeding by their patients while on monoclonal antibodies for other autoimmune diseases, according to Dr. Hellwig.
It is important to recognize that most women of reproductive age with MS have milder forms of the disease, she said. They can safely breastfeed without being on any MS medications at all for the duration.
For women who want to breastfeed and have more-active disease where early treatment resumption is warranted, the key is to select a breastfeeding-compatible medication. The main determinant of whether a drug will enter the mother’s breast milk is the size of the drug molecule, with large molecules being unlikely to make their way into breast milk in anything approaching clinically meaningful amounts. The injectable first-line disease-modifying drugs are good options: For example, interferon-beta is a very large molecule which has been detected in breast milk at 0.0006% of the relative infant dose. That’s reassuring, Dr. Hellwig said, since anything less than a relative infant dose of 10% is generally considered to be safe for a baby. And while glatiramer acetate, another injectable, has not been tested, it is metabolized so rapidly that it is unlikely to be detectable in breast milk, according to Dr. Hellwig.
Monoclonal antibodies are also compatible with breastfeeding. Rituximab has been detected in breast milk at 1/240th of the maternal serum level, and natalizumab at less than 1/200th. These are large molecules with a low likelihood of infant absorption, since they are probably destroyed in the child’s gastrointestinal tract. Ocrelizumab has not been studied in breast milk, but it is an IgG1 monoclonal antibody, as is rituximab, and so should likewise pose “exceedingly low risk,” Dr. Hellwig said.
At last year’s ECTRIMS conference, she presented reassuring 1-year follow-up data on a cohort of infants breastfed by mothers with MS while on interferon-beta. “We do not see any growth disturbances, any severe infections, hospitalizations, excess antibiotic use, or postponed reaching of developmental milestones in babies being breastfed under the injectables,” she said.
Dr. Hellwig has served on scientific advisory board for Bayer, Biogen, Genzyme Sanofi, Teva, Roche, Novartis, and Merck. She has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, SanofiGenzyme, and Teva, and has received support for congress participation from Bayer, Biogen, Genzyme, Teva, Roche, and Merck.
STOCKHOLM – Most neurologists are overly conservative when it comes to advising women with multiple sclerosis (MS) about breastfeeding, discouraging this broadly beneficial practice in favor of early resumption of treatment post pregnancy, Kerstin Hellwig, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We should change our behavior, and I predict we will change it so that more women are breastfeeding while under MS medication within the next couple years. ,” said Dr. Hellwig, senior consultant and MS specialist in the department of neurology at St. Josef Hospital/Ruhr University in Bochum, Germany.
She was a coauthor of a groundbreaking 2012 meta-analysis that concluded that breastfeeding by MS patients is not harmful (J Neurol. 2012 Oct;259[10]:2246-8), a finding since confirmed in multiple additional studies.
“Women with MS who want to breastfeed should be supported in doing so,” Dr. Hellwig said.
In this regard, many neurologists are out of step with their colleagues in rheumatology and gastroenterology, who commonly endorse breastfeeding by their patients while on monoclonal antibodies for other autoimmune diseases, according to Dr. Hellwig.
It is important to recognize that most women of reproductive age with MS have milder forms of the disease, she said. They can safely breastfeed without being on any MS medications at all for the duration.
For women who want to breastfeed and have more-active disease where early treatment resumption is warranted, the key is to select a breastfeeding-compatible medication. The main determinant of whether a drug will enter the mother’s breast milk is the size of the drug molecule, with large molecules being unlikely to make their way into breast milk in anything approaching clinically meaningful amounts. The injectable first-line disease-modifying drugs are good options: For example, interferon-beta is a very large molecule which has been detected in breast milk at 0.0006% of the relative infant dose. That’s reassuring, Dr. Hellwig said, since anything less than a relative infant dose of 10% is generally considered to be safe for a baby. And while glatiramer acetate, another injectable, has not been tested, it is metabolized so rapidly that it is unlikely to be detectable in breast milk, according to Dr. Hellwig.
Monoclonal antibodies are also compatible with breastfeeding. Rituximab has been detected in breast milk at 1/240th of the maternal serum level, and natalizumab at less than 1/200th. These are large molecules with a low likelihood of infant absorption, since they are probably destroyed in the child’s gastrointestinal tract. Ocrelizumab has not been studied in breast milk, but it is an IgG1 monoclonal antibody, as is rituximab, and so should likewise pose “exceedingly low risk,” Dr. Hellwig said.
At last year’s ECTRIMS conference, she presented reassuring 1-year follow-up data on a cohort of infants breastfed by mothers with MS while on interferon-beta. “We do not see any growth disturbances, any severe infections, hospitalizations, excess antibiotic use, or postponed reaching of developmental milestones in babies being breastfed under the injectables,” she said.
Dr. Hellwig has served on scientific advisory board for Bayer, Biogen, Genzyme Sanofi, Teva, Roche, Novartis, and Merck. She has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, SanofiGenzyme, and Teva, and has received support for congress participation from Bayer, Biogen, Genzyme, Teva, Roche, and Merck.
EXPERT ANALYSIS FROM ECTRIMS 2019
Can a novel steroidal anti-inflammatory drug benefit patients with Duchenne muscular dystrophy?
Daily treatment with vamorolone at doses of 2.0 mg/kg per day and 6.0 mg/kg per day suggested possible efficacy in a 24-week study, researchers said. The exploratory study included 48 boys who had completed a phase 2a trial.
The treatment was safe and well tolerated, and patients who received 2.0 mg/kg per day had significantly improved muscle function, as assessed by time to stand, compared with natural history controls, according to the results, which were published in Neurology.
In addition, the novel drug may reduce “safety concerns typically seen with traditional glucocorticoids,” wrote Eric P. Hoffman, PhD, and coauthors. Dr. Hoffman is president and CEO of ReveraGen BioPharma in Rockville, Md., which is developing the drug, and associate dean for research in the school of pharmacy and pharmaceutical sciences at Binghamton (N.Y.) University.
In preclinical studies, vamorolone retained anti-inflammatory efficacy while reducing adverse effects, compared with prednisolone, in a manner that is “consistent with vamorolone blocking [nuclear factor-kappa beta]–associated proinflammatory signals as a ligand/receptor monomeric state instead of the traditional molecular models of ligand/receptor dimeric complexes,” the authors said.
Phase 1 and phase 2a studies suggest that the drug may have an improved safety profile. To assess possible efficacy and define optimal doses, the investigators conducted the 24-week extension study. Participants were boys aged 4 years to younger than 7 years who had never been treated with glucocorticoids. They received 0.25, 0.75, 2.0, or 6.0 mg/kg per day vamorolone in an oral suspension formulation. Twelve boys received each dose level.
“Vamorolone was well tolerated ... with no adverse events leading to reduction of drug dosing or withdrawal from the trial,” they said. “The [timed stand from supine] primary outcome measure in vamorolone-treated patients with DMD supports efficacy of the 2.0-mg/kg/d dose ... at 24 weeks,” they said. A secondary outcome measure, the 6-minute walk test, supports efficacy at this dose at 12 and 24 weeks of treatment.
Furthermore, the data indicate that the 2.0-mg/kg per day dose may be associated with less weight gain and improved bone turnover and insulin resistance biomarkers, relative to prednisone therapy. “There was evidence of adrenal suppression in a subset of boys with DMD treated with 2.0 mg/kg/d vamorolone, with 18% of patients showing reduced morning cortisol levels,” the authors said. “Future studies of vamorolone will include adrenocorticotropic hormone–challenge tests to further explore adrenal function.”
A double-blind, placebo-controlled trial of vamorolone is underway. Investigators are testing two doses of vamorolone (2.0 and 6.0 mg/kg per day) versus placebo and prednisone (0.75 mg/kg per day). Researchers plan to enroll 120 patients, with 30 patients in each arm.
ReveraGen BioPharma received funds for the present study from Actelion Pharmaceuticals, U.S. and European government agencies, and nonprofit foundations. Dr. Hoffman and some of his collaborators are cofounders of ReveraGen. Other coauthors received support from the company.
SOURCE: Hoffman EP et al. Neurology. 2019 Aug 26. doi: 10.1212/WNL.0000000000008168.
Daily treatment with vamorolone at doses of 2.0 mg/kg per day and 6.0 mg/kg per day suggested possible efficacy in a 24-week study, researchers said. The exploratory study included 48 boys who had completed a phase 2a trial.
The treatment was safe and well tolerated, and patients who received 2.0 mg/kg per day had significantly improved muscle function, as assessed by time to stand, compared with natural history controls, according to the results, which were published in Neurology.
In addition, the novel drug may reduce “safety concerns typically seen with traditional glucocorticoids,” wrote Eric P. Hoffman, PhD, and coauthors. Dr. Hoffman is president and CEO of ReveraGen BioPharma in Rockville, Md., which is developing the drug, and associate dean for research in the school of pharmacy and pharmaceutical sciences at Binghamton (N.Y.) University.
In preclinical studies, vamorolone retained anti-inflammatory efficacy while reducing adverse effects, compared with prednisolone, in a manner that is “consistent with vamorolone blocking [nuclear factor-kappa beta]–associated proinflammatory signals as a ligand/receptor monomeric state instead of the traditional molecular models of ligand/receptor dimeric complexes,” the authors said.
Phase 1 and phase 2a studies suggest that the drug may have an improved safety profile. To assess possible efficacy and define optimal doses, the investigators conducted the 24-week extension study. Participants were boys aged 4 years to younger than 7 years who had never been treated with glucocorticoids. They received 0.25, 0.75, 2.0, or 6.0 mg/kg per day vamorolone in an oral suspension formulation. Twelve boys received each dose level.
“Vamorolone was well tolerated ... with no adverse events leading to reduction of drug dosing or withdrawal from the trial,” they said. “The [timed stand from supine] primary outcome measure in vamorolone-treated patients with DMD supports efficacy of the 2.0-mg/kg/d dose ... at 24 weeks,” they said. A secondary outcome measure, the 6-minute walk test, supports efficacy at this dose at 12 and 24 weeks of treatment.
Furthermore, the data indicate that the 2.0-mg/kg per day dose may be associated with less weight gain and improved bone turnover and insulin resistance biomarkers, relative to prednisone therapy. “There was evidence of adrenal suppression in a subset of boys with DMD treated with 2.0 mg/kg/d vamorolone, with 18% of patients showing reduced morning cortisol levels,” the authors said. “Future studies of vamorolone will include adrenocorticotropic hormone–challenge tests to further explore adrenal function.”
A double-blind, placebo-controlled trial of vamorolone is underway. Investigators are testing two doses of vamorolone (2.0 and 6.0 mg/kg per day) versus placebo and prednisone (0.75 mg/kg per day). Researchers plan to enroll 120 patients, with 30 patients in each arm.
ReveraGen BioPharma received funds for the present study from Actelion Pharmaceuticals, U.S. and European government agencies, and nonprofit foundations. Dr. Hoffman and some of his collaborators are cofounders of ReveraGen. Other coauthors received support from the company.
SOURCE: Hoffman EP et al. Neurology. 2019 Aug 26. doi: 10.1212/WNL.0000000000008168.
Daily treatment with vamorolone at doses of 2.0 mg/kg per day and 6.0 mg/kg per day suggested possible efficacy in a 24-week study, researchers said. The exploratory study included 48 boys who had completed a phase 2a trial.
The treatment was safe and well tolerated, and patients who received 2.0 mg/kg per day had significantly improved muscle function, as assessed by time to stand, compared with natural history controls, according to the results, which were published in Neurology.
In addition, the novel drug may reduce “safety concerns typically seen with traditional glucocorticoids,” wrote Eric P. Hoffman, PhD, and coauthors. Dr. Hoffman is president and CEO of ReveraGen BioPharma in Rockville, Md., which is developing the drug, and associate dean for research in the school of pharmacy and pharmaceutical sciences at Binghamton (N.Y.) University.
In preclinical studies, vamorolone retained anti-inflammatory efficacy while reducing adverse effects, compared with prednisolone, in a manner that is “consistent with vamorolone blocking [nuclear factor-kappa beta]–associated proinflammatory signals as a ligand/receptor monomeric state instead of the traditional molecular models of ligand/receptor dimeric complexes,” the authors said.
Phase 1 and phase 2a studies suggest that the drug may have an improved safety profile. To assess possible efficacy and define optimal doses, the investigators conducted the 24-week extension study. Participants were boys aged 4 years to younger than 7 years who had never been treated with glucocorticoids. They received 0.25, 0.75, 2.0, or 6.0 mg/kg per day vamorolone in an oral suspension formulation. Twelve boys received each dose level.
“Vamorolone was well tolerated ... with no adverse events leading to reduction of drug dosing or withdrawal from the trial,” they said. “The [timed stand from supine] primary outcome measure in vamorolone-treated patients with DMD supports efficacy of the 2.0-mg/kg/d dose ... at 24 weeks,” they said. A secondary outcome measure, the 6-minute walk test, supports efficacy at this dose at 12 and 24 weeks of treatment.
Furthermore, the data indicate that the 2.0-mg/kg per day dose may be associated with less weight gain and improved bone turnover and insulin resistance biomarkers, relative to prednisone therapy. “There was evidence of adrenal suppression in a subset of boys with DMD treated with 2.0 mg/kg/d vamorolone, with 18% of patients showing reduced morning cortisol levels,” the authors said. “Future studies of vamorolone will include adrenocorticotropic hormone–challenge tests to further explore adrenal function.”
A double-blind, placebo-controlled trial of vamorolone is underway. Investigators are testing two doses of vamorolone (2.0 and 6.0 mg/kg per day) versus placebo and prednisone (0.75 mg/kg per day). Researchers plan to enroll 120 patients, with 30 patients in each arm.
ReveraGen BioPharma received funds for the present study from Actelion Pharmaceuticals, U.S. and European government agencies, and nonprofit foundations. Dr. Hoffman and some of his collaborators are cofounders of ReveraGen. Other coauthors received support from the company.
SOURCE: Hoffman EP et al. Neurology. 2019 Aug 26. doi: 10.1212/WNL.0000000000008168.
FROM NEUROLOGY
Insurers to pay record number of rebates to patients
Health insurance companies are getting ready to disburse a record $1.3 billion in medical loss ratio (MLR) rebates, according to an analysis by the Kaiser Family Foundation.
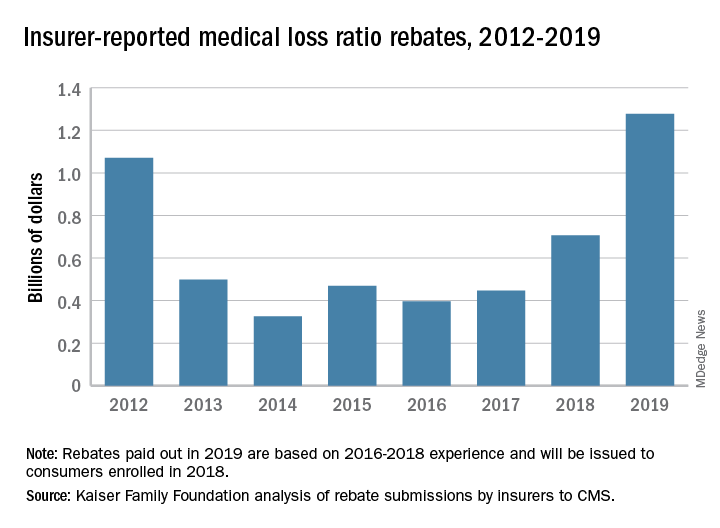
The $1.3 billion surpasses the previous rebate record of $1.1 billion, issued in 2012.
The increase is driven largely by individual market insurers who will pay $743 million in rebates this year, according to the report, which analyzed insurer data submitted to the Centers for Medicare & Medicaid Services. Rebates in the small-group and large-group insurance markets are similar to previous years, with expected paybacks of $250 million from small- and $284 million from large-group markets, according to the Kaiser report. Insurance companies have until September 30, 2019, to start issuing rebates.
The rebates stem from the MLR requirement imposed by the Affordable Care Act (ACA), which limits the amount of premium dollars that can be used for administration, marketing, and profit. Under the health law, companies are required to publicly report how much they spend on health care, quality improvement, and other activities using premium funds. Individual and small-group market insurers must spend at least 80% on health care claims and quality improvement,while large-group plans must spend at least 85%. Rebates are based on a 3-year average of financial data by each insurer.
Patients in the individual insurance market can expect their rebate in either a premium credit or a check. In the large and small group markets, rebates may be split between employee and employer depending on the plan contract.
The volume of rebates differed greatly across the states, with some states paying zero rebates and others paying millions. Virginia insurers for example, will pay the highest number of total rebates ($150 million), followed by Pennsylvania ($130 million) and Florida ($107 million), according to the report. Payments by insurers in the individual market alone ranged from zero dollars in 13 states to $111 million in Virginia. Individual market insurers in Arizona will pay $92 million in rebates to patients, while individual plans in Texas will pay $80 million. Florida insurers will pay the highest in rebates in both the small-group and large-group market at $44 million and $42 million respectively.
The largest rebates within the individual market will come from Centene, HCSC, Cigna, and Highmark. Authors of the report noted that these insurers tend to have higher enrollment and are active in multiple states.
Individual marketplace insurers will likely pay high rebates against next year, based on an individual market that remains strong and profitable, despite the recent elimination of the individual mandate penalty, according to the authors.
Health insurance companies are getting ready to disburse a record $1.3 billion in medical loss ratio (MLR) rebates, according to an analysis by the Kaiser Family Foundation.

The $1.3 billion surpasses the previous rebate record of $1.1 billion, issued in 2012.
The increase is driven largely by individual market insurers who will pay $743 million in rebates this year, according to the report, which analyzed insurer data submitted to the Centers for Medicare & Medicaid Services. Rebates in the small-group and large-group insurance markets are similar to previous years, with expected paybacks of $250 million from small- and $284 million from large-group markets, according to the Kaiser report. Insurance companies have until September 30, 2019, to start issuing rebates.
The rebates stem from the MLR requirement imposed by the Affordable Care Act (ACA), which limits the amount of premium dollars that can be used for administration, marketing, and profit. Under the health law, companies are required to publicly report how much they spend on health care, quality improvement, and other activities using premium funds. Individual and small-group market insurers must spend at least 80% on health care claims and quality improvement,while large-group plans must spend at least 85%. Rebates are based on a 3-year average of financial data by each insurer.
Patients in the individual insurance market can expect their rebate in either a premium credit or a check. In the large and small group markets, rebates may be split between employee and employer depending on the plan contract.
The volume of rebates differed greatly across the states, with some states paying zero rebates and others paying millions. Virginia insurers for example, will pay the highest number of total rebates ($150 million), followed by Pennsylvania ($130 million) and Florida ($107 million), according to the report. Payments by insurers in the individual market alone ranged from zero dollars in 13 states to $111 million in Virginia. Individual market insurers in Arizona will pay $92 million in rebates to patients, while individual plans in Texas will pay $80 million. Florida insurers will pay the highest in rebates in both the small-group and large-group market at $44 million and $42 million respectively.
The largest rebates within the individual market will come from Centene, HCSC, Cigna, and Highmark. Authors of the report noted that these insurers tend to have higher enrollment and are active in multiple states.
Individual marketplace insurers will likely pay high rebates against next year, based on an individual market that remains strong and profitable, despite the recent elimination of the individual mandate penalty, according to the authors.
Health insurance companies are getting ready to disburse a record $1.3 billion in medical loss ratio (MLR) rebates, according to an analysis by the Kaiser Family Foundation.

The $1.3 billion surpasses the previous rebate record of $1.1 billion, issued in 2012.
The increase is driven largely by individual market insurers who will pay $743 million in rebates this year, according to the report, which analyzed insurer data submitted to the Centers for Medicare & Medicaid Services. Rebates in the small-group and large-group insurance markets are similar to previous years, with expected paybacks of $250 million from small- and $284 million from large-group markets, according to the Kaiser report. Insurance companies have until September 30, 2019, to start issuing rebates.
The rebates stem from the MLR requirement imposed by the Affordable Care Act (ACA), which limits the amount of premium dollars that can be used for administration, marketing, and profit. Under the health law, companies are required to publicly report how much they spend on health care, quality improvement, and other activities using premium funds. Individual and small-group market insurers must spend at least 80% on health care claims and quality improvement,while large-group plans must spend at least 85%. Rebates are based on a 3-year average of financial data by each insurer.
Patients in the individual insurance market can expect their rebate in either a premium credit or a check. In the large and small group markets, rebates may be split between employee and employer depending on the plan contract.
The volume of rebates differed greatly across the states, with some states paying zero rebates and others paying millions. Virginia insurers for example, will pay the highest number of total rebates ($150 million), followed by Pennsylvania ($130 million) and Florida ($107 million), according to the report. Payments by insurers in the individual market alone ranged from zero dollars in 13 states to $111 million in Virginia. Individual market insurers in Arizona will pay $92 million in rebates to patients, while individual plans in Texas will pay $80 million. Florida insurers will pay the highest in rebates in both the small-group and large-group market at $44 million and $42 million respectively.
The largest rebates within the individual market will come from Centene, HCSC, Cigna, and Highmark. Authors of the report noted that these insurers tend to have higher enrollment and are active in multiple states.
Individual marketplace insurers will likely pay high rebates against next year, based on an individual market that remains strong and profitable, despite the recent elimination of the individual mandate penalty, according to the authors.
Review your insurance
Insurance, so goes the hoary cliché, is the one product you buy hoping never to use. While no one enjoys foreseeing unforeseeable calamities, if you haven’t reviewed your insurance coverage recently, there is no time like the present.
, but the cost has become prohibitive in many areas, when insurers are willing to write them at all. “Claims made” policies are cheaper and provide the same protection, but only while coverage is in effect. You will need “tail” coverage against belated claims after your policy lapses, but many companies provide free tail coverage if you are retiring. If you are simply switching workplaces (or policies), ask your new insurer about “nose” coverage, for claims involving acts that occurred before the new policy takes effect.
Other alternatives are gaining popularity as the demand for reasonably priced insurance increases. The most common, known as reciprocal exchanges, are very similar to traditional insurers, but require policyholders to make capital contributions in addition to payment of premiums, at least in their early stages. You get your investment back, with interest, when (if) the exchange becomes solvent.
Another option, called a captive, is a company formed by a consortium of medical practices to write their own insurance policies. All participants are shareholders, and all premiums (less administrative expenses) go toward building the security of the captive. Most captives purchase reinsurance to protect against catastrophic losses. If all goes well, individual owners sell their shares at retirement for a profit, which has grown tax-free in the interim.
Those willing to shoulder more risk might consider a risk retention group (RRG), a sort of combination of an exchange and a captive. Again, the owners are the insureds themselves, but all responsibility for management and adequate funding falls on their shoulders, and reinsurance is not usually an option. Most medical malpractice RRGs are licensed in Vermont or South Carolina, because of favorable laws in those states, but can be based in any state that allows them (36 at this writing). RRGs provide profit opportunities not available with traditional insurance, but there is risk: A few large claims could eat up all the profits, or even put owners in a financial hole.
Malpractice insurance requirements will remain fairly static throughout your career, but other insurance needs evolve over time. A good example is life insurance: As retirement savings increase, the need for life insurance decreases – especially expensive “whole life” coverage, which can often be eliminated or converted to cheaper “term” insurance.
Health insurance premiums continue to soar, but the Affordable Care Act might offer a favorable alternative for your office policy. If you are considering that, the Centers for Medicare & Medicaid Services maintains a website summarizing the various options for employers.
Worker compensation insurance is mandatory in most states and heavily regulated, so there is little wiggle room. However, some states do not require you, as the employer, to cover yourself, so eliminating that coverage could save you a substantial amount. This is only worth considering, of course, if you’re in excellent health and have very good personal health and disability coverage.
Disability insurance is not something to skimp on, but if you are approaching retirement age and have no major health issues, you may be able to decrease your coverage, or even eliminate it entirely if your retirement plan is far enough along.
Liability insurance is likewise no place to pinch pennies, but you might be able to add an “umbrella” policy providing comprehensive catastrophic coverage, which may allow you to decrease your regular coverage, or raise your deductible limits.
Two additional policies to consider are office overhead insurance, to cover the costs of keeping your office open should you be temporarily incapacitated, and employee practices liability insurance (EPLI), which protects you from lawsuits brought by militant or disgruntled employees. I covered EPLI in detail several months ago.
If you are over 50, I strongly recommend long-term-care insurance as well. It’s relatively inexpensive if you buy it while you’re still healthy, and it could save you and your heirs a load of money and aggravation on the other end. If you have shouldered the expense of caring for a chronically ill parent or grandparent, you know what I’m talking about. More about that next month.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Insurance, so goes the hoary cliché, is the one product you buy hoping never to use. While no one enjoys foreseeing unforeseeable calamities, if you haven’t reviewed your insurance coverage recently, there is no time like the present.
, but the cost has become prohibitive in many areas, when insurers are willing to write them at all. “Claims made” policies are cheaper and provide the same protection, but only while coverage is in effect. You will need “tail” coverage against belated claims after your policy lapses, but many companies provide free tail coverage if you are retiring. If you are simply switching workplaces (or policies), ask your new insurer about “nose” coverage, for claims involving acts that occurred before the new policy takes effect.
Other alternatives are gaining popularity as the demand for reasonably priced insurance increases. The most common, known as reciprocal exchanges, are very similar to traditional insurers, but require policyholders to make capital contributions in addition to payment of premiums, at least in their early stages. You get your investment back, with interest, when (if) the exchange becomes solvent.
Another option, called a captive, is a company formed by a consortium of medical practices to write their own insurance policies. All participants are shareholders, and all premiums (less administrative expenses) go toward building the security of the captive. Most captives purchase reinsurance to protect against catastrophic losses. If all goes well, individual owners sell their shares at retirement for a profit, which has grown tax-free in the interim.
Those willing to shoulder more risk might consider a risk retention group (RRG), a sort of combination of an exchange and a captive. Again, the owners are the insureds themselves, but all responsibility for management and adequate funding falls on their shoulders, and reinsurance is not usually an option. Most medical malpractice RRGs are licensed in Vermont or South Carolina, because of favorable laws in those states, but can be based in any state that allows them (36 at this writing). RRGs provide profit opportunities not available with traditional insurance, but there is risk: A few large claims could eat up all the profits, or even put owners in a financial hole.
Malpractice insurance requirements will remain fairly static throughout your career, but other insurance needs evolve over time. A good example is life insurance: As retirement savings increase, the need for life insurance decreases – especially expensive “whole life” coverage, which can often be eliminated or converted to cheaper “term” insurance.
Health insurance premiums continue to soar, but the Affordable Care Act might offer a favorable alternative for your office policy. If you are considering that, the Centers for Medicare & Medicaid Services maintains a website summarizing the various options for employers.
Worker compensation insurance is mandatory in most states and heavily regulated, so there is little wiggle room. However, some states do not require you, as the employer, to cover yourself, so eliminating that coverage could save you a substantial amount. This is only worth considering, of course, if you’re in excellent health and have very good personal health and disability coverage.
Disability insurance is not something to skimp on, but if you are approaching retirement age and have no major health issues, you may be able to decrease your coverage, or even eliminate it entirely if your retirement plan is far enough along.
Liability insurance is likewise no place to pinch pennies, but you might be able to add an “umbrella” policy providing comprehensive catastrophic coverage, which may allow you to decrease your regular coverage, or raise your deductible limits.
Two additional policies to consider are office overhead insurance, to cover the costs of keeping your office open should you be temporarily incapacitated, and employee practices liability insurance (EPLI), which protects you from lawsuits brought by militant or disgruntled employees. I covered EPLI in detail several months ago.
If you are over 50, I strongly recommend long-term-care insurance as well. It’s relatively inexpensive if you buy it while you’re still healthy, and it could save you and your heirs a load of money and aggravation on the other end. If you have shouldered the expense of caring for a chronically ill parent or grandparent, you know what I’m talking about. More about that next month.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Insurance, so goes the hoary cliché, is the one product you buy hoping never to use. While no one enjoys foreseeing unforeseeable calamities, if you haven’t reviewed your insurance coverage recently, there is no time like the present.
, but the cost has become prohibitive in many areas, when insurers are willing to write them at all. “Claims made” policies are cheaper and provide the same protection, but only while coverage is in effect. You will need “tail” coverage against belated claims after your policy lapses, but many companies provide free tail coverage if you are retiring. If you are simply switching workplaces (or policies), ask your new insurer about “nose” coverage, for claims involving acts that occurred before the new policy takes effect.
Other alternatives are gaining popularity as the demand for reasonably priced insurance increases. The most common, known as reciprocal exchanges, are very similar to traditional insurers, but require policyholders to make capital contributions in addition to payment of premiums, at least in their early stages. You get your investment back, with interest, when (if) the exchange becomes solvent.
Another option, called a captive, is a company formed by a consortium of medical practices to write their own insurance policies. All participants are shareholders, and all premiums (less administrative expenses) go toward building the security of the captive. Most captives purchase reinsurance to protect against catastrophic losses. If all goes well, individual owners sell their shares at retirement for a profit, which has grown tax-free in the interim.
Those willing to shoulder more risk might consider a risk retention group (RRG), a sort of combination of an exchange and a captive. Again, the owners are the insureds themselves, but all responsibility for management and adequate funding falls on their shoulders, and reinsurance is not usually an option. Most medical malpractice RRGs are licensed in Vermont or South Carolina, because of favorable laws in those states, but can be based in any state that allows them (36 at this writing). RRGs provide profit opportunities not available with traditional insurance, but there is risk: A few large claims could eat up all the profits, or even put owners in a financial hole.
Malpractice insurance requirements will remain fairly static throughout your career, but other insurance needs evolve over time. A good example is life insurance: As retirement savings increase, the need for life insurance decreases – especially expensive “whole life” coverage, which can often be eliminated or converted to cheaper “term” insurance.
Health insurance premiums continue to soar, but the Affordable Care Act might offer a favorable alternative for your office policy. If you are considering that, the Centers for Medicare & Medicaid Services maintains a website summarizing the various options for employers.
Worker compensation insurance is mandatory in most states and heavily regulated, so there is little wiggle room. However, some states do not require you, as the employer, to cover yourself, so eliminating that coverage could save you a substantial amount. This is only worth considering, of course, if you’re in excellent health and have very good personal health and disability coverage.
Disability insurance is not something to skimp on, but if you are approaching retirement age and have no major health issues, you may be able to decrease your coverage, or even eliminate it entirely if your retirement plan is far enough along.
Liability insurance is likewise no place to pinch pennies, but you might be able to add an “umbrella” policy providing comprehensive catastrophic coverage, which may allow you to decrease your regular coverage, or raise your deductible limits.
Two additional policies to consider are office overhead insurance, to cover the costs of keeping your office open should you be temporarily incapacitated, and employee practices liability insurance (EPLI), which protects you from lawsuits brought by militant or disgruntled employees. I covered EPLI in detail several months ago.
If you are over 50, I strongly recommend long-term-care insurance as well. It’s relatively inexpensive if you buy it while you’re still healthy, and it could save you and your heirs a load of money and aggravation on the other end. If you have shouldered the expense of caring for a chronically ill parent or grandparent, you know what I’m talking about. More about that next month.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
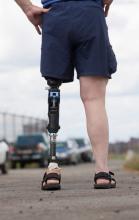
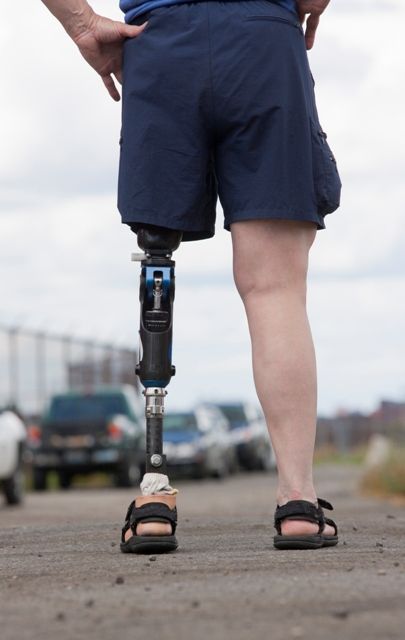
Sensory feedback may smooth walking with a prosthetic leg
A prosthetic leg that elicits the sensation of knee motion and the feeling of the sole of the foot touching the ground may improve walking performance and reduce phantom limb pain, according to a proof-of-concept study with two patients.
With the bionic leg system, the patients performed better during clinically important tests indoors and outdoors, study author Stanisa Raspopovic, PhD, explained during a press briefing about the research. The findings were published in Nature Medicine.
The results indicate that the use of sensory feedback “could be common practice” in prosthetic devices in the future, he said. Dr. Raspopovic is a researcher at Swiss Federal Institute of Technology Zürich and a founder of SensArs Neuroprosthetics, which is based in Lausanne, Switzerland.
Neural prosthetics allow the nervous system and external devices to interact. These brain-machine interfaces may improve quality of life for patients with brain or spinal cord injuries, degenerative disease, or loss of limbs.
“Conventional leg prostheses do not convey sensory information about motion or interaction with the ground to above-knee amputees, thereby reducing confidence and walking speed in the users,” the study authors wrote. Users may also have high levels of mental and physical fatigue, and the lack of physiologic feedback from the extremity to the brain may contribute to the generation of phantom limb pain.
To evaluate whether neural sensory feedback restoration could address these issues, investigators conducted a study with two patients who had undergone transfemoral amputations as a result of traumatic events. The patients were implanted with four intraneural stimulation electrodes in the remaining tibial nerve. The prosthetic leg device included sensors to represent foot touch and pressure and knee joint angle. The sensors transmitted sensory signals to the nervous system through the stimulation electrodes in the tibial nerve.
When the patients walked outdoors over a path traced in the sand, “participants’ speeds were significantly higher when sensory feedback was provided,” the authors wrote. One participant walked 3.56 m/min faster, and the other walked 5.68 m/min faster.
The participants also rated their confidence in the prosthesis on a scale from 0 to 10. For patient 1, self-rated confidence improved from 4.85 to 7.71 with the device. Patient 2 reported a confidence level that climbed from 2.7 to 5.55.
When tested indoors, both patients reached a 0.5 km/hour higher speed on the treadmill when stimulation was provided and both had a lower mean rate of oxygen uptake during the sensory feedback trials, the study authors reported.
Levels of phantom limb pain also decreased significantly after 10-minute stimulation sessions, but not during control sessions.
Longer studies with more patients are required, and fully implantable devices without transcutaneous cables need to be developed, the authors wrote.
Grants from the European Research Council, European Commission, and Swiss National Science Foundation funded the research. Dr. Raspopovic and two coauthors hold shares of SensArs Neuroprosthetics, a start-up company dealing with the commercialization of neurocontrolled artificial limbs.
SOURCE: Petrini FM et al. Nat Med. 2019 Sep 9. doi: 10.1038/s41591-019-0567-3.
A prosthetic leg that elicits the sensation of knee motion and the feeling of the sole of the foot touching the ground may improve walking performance and reduce phantom limb pain, according to a proof-of-concept study with two patients.
With the bionic leg system, the patients performed better during clinically important tests indoors and outdoors, study author Stanisa Raspopovic, PhD, explained during a press briefing about the research. The findings were published in Nature Medicine.
The results indicate that the use of sensory feedback “could be common practice” in prosthetic devices in the future, he said. Dr. Raspopovic is a researcher at Swiss Federal Institute of Technology Zürich and a founder of SensArs Neuroprosthetics, which is based in Lausanne, Switzerland.
Neural prosthetics allow the nervous system and external devices to interact. These brain-machine interfaces may improve quality of life for patients with brain or spinal cord injuries, degenerative disease, or loss of limbs.
“Conventional leg prostheses do not convey sensory information about motion or interaction with the ground to above-knee amputees, thereby reducing confidence and walking speed in the users,” the study authors wrote. Users may also have high levels of mental and physical fatigue, and the lack of physiologic feedback from the extremity to the brain may contribute to the generation of phantom limb pain.
To evaluate whether neural sensory feedback restoration could address these issues, investigators conducted a study with two patients who had undergone transfemoral amputations as a result of traumatic events. The patients were implanted with four intraneural stimulation electrodes in the remaining tibial nerve. The prosthetic leg device included sensors to represent foot touch and pressure and knee joint angle. The sensors transmitted sensory signals to the nervous system through the stimulation electrodes in the tibial nerve.
When the patients walked outdoors over a path traced in the sand, “participants’ speeds were significantly higher when sensory feedback was provided,” the authors wrote. One participant walked 3.56 m/min faster, and the other walked 5.68 m/min faster.
The participants also rated their confidence in the prosthesis on a scale from 0 to 10. For patient 1, self-rated confidence improved from 4.85 to 7.71 with the device. Patient 2 reported a confidence level that climbed from 2.7 to 5.55.
When tested indoors, both patients reached a 0.5 km/hour higher speed on the treadmill when stimulation was provided and both had a lower mean rate of oxygen uptake during the sensory feedback trials, the study authors reported.
Levels of phantom limb pain also decreased significantly after 10-minute stimulation sessions, but not during control sessions.
Longer studies with more patients are required, and fully implantable devices without transcutaneous cables need to be developed, the authors wrote.
Grants from the European Research Council, European Commission, and Swiss National Science Foundation funded the research. Dr. Raspopovic and two coauthors hold shares of SensArs Neuroprosthetics, a start-up company dealing with the commercialization of neurocontrolled artificial limbs.
SOURCE: Petrini FM et al. Nat Med. 2019 Sep 9. doi: 10.1038/s41591-019-0567-3.
A prosthetic leg that elicits the sensation of knee motion and the feeling of the sole of the foot touching the ground may improve walking performance and reduce phantom limb pain, according to a proof-of-concept study with two patients.
With the bionic leg system, the patients performed better during clinically important tests indoors and outdoors, study author Stanisa Raspopovic, PhD, explained during a press briefing about the research. The findings were published in Nature Medicine.
The results indicate that the use of sensory feedback “could be common practice” in prosthetic devices in the future, he said. Dr. Raspopovic is a researcher at Swiss Federal Institute of Technology Zürich and a founder of SensArs Neuroprosthetics, which is based in Lausanne, Switzerland.
Neural prosthetics allow the nervous system and external devices to interact. These brain-machine interfaces may improve quality of life for patients with brain or spinal cord injuries, degenerative disease, or loss of limbs.
“Conventional leg prostheses do not convey sensory information about motion or interaction with the ground to above-knee amputees, thereby reducing confidence and walking speed in the users,” the study authors wrote. Users may also have high levels of mental and physical fatigue, and the lack of physiologic feedback from the extremity to the brain may contribute to the generation of phantom limb pain.
To evaluate whether neural sensory feedback restoration could address these issues, investigators conducted a study with two patients who had undergone transfemoral amputations as a result of traumatic events. The patients were implanted with four intraneural stimulation electrodes in the remaining tibial nerve. The prosthetic leg device included sensors to represent foot touch and pressure and knee joint angle. The sensors transmitted sensory signals to the nervous system through the stimulation electrodes in the tibial nerve.
When the patients walked outdoors over a path traced in the sand, “participants’ speeds were significantly higher when sensory feedback was provided,” the authors wrote. One participant walked 3.56 m/min faster, and the other walked 5.68 m/min faster.
The participants also rated their confidence in the prosthesis on a scale from 0 to 10. For patient 1, self-rated confidence improved from 4.85 to 7.71 with the device. Patient 2 reported a confidence level that climbed from 2.7 to 5.55.
When tested indoors, both patients reached a 0.5 km/hour higher speed on the treadmill when stimulation was provided and both had a lower mean rate of oxygen uptake during the sensory feedback trials, the study authors reported.
Levels of phantom limb pain also decreased significantly after 10-minute stimulation sessions, but not during control sessions.
Longer studies with more patients are required, and fully implantable devices without transcutaneous cables need to be developed, the authors wrote.
Grants from the European Research Council, European Commission, and Swiss National Science Foundation funded the research. Dr. Raspopovic and two coauthors hold shares of SensArs Neuroprosthetics, a start-up company dealing with the commercialization of neurocontrolled artificial limbs.
SOURCE: Petrini FM et al. Nat Med. 2019 Sep 9. doi: 10.1038/s41591-019-0567-3.
FROM NATURE MEDICINE
CVS-Aetna merger approval gets poor review from physicians
Physician groups are criticizing a judge’s decision to approve the merger of pharmacy chain CVS Health and health insurer Aetna, saying the deal will raise prices and lower quality.
Judge Richard J. Leon of the U.S. District Court for the District of Columbia allowed the merger to move forward on Sept. 4, ruling that the acquisition was legal under antitrust law. The merger was approved by the Department of Justice in October 2018 on the condition that Aetna sell its Medicare prescription drug plan (PDP) business to independently owned competitor, WellCare Health Plans. Judge Leon has been examining the government’s plan since late 2018.
In his decision, Judge Leon acknowledged that the merger will have widespread effects for millions of patients and noted the many industry stakeholders, consumer groups, and state regulatory bodies have raised concerns about the merger.
“Although [the opposition] raised substantial concerns that warranted serious consideration, CVS’s and the government’s witnesses, when combined with the existing record, persuasively support why the markets at issue are not only very competitive today, but are likely to remain so post merger,” Judge Leon wrote. “Consequently, the harms to the public interest the [opposition] raised were not sufficiently established to undermine the government’s conclusion to the contrary. As such, for all of the above reasons, I have concluded that the proposed settlement is well ‘within the reaches’ of the public interest and the government’s motion ... should therefore be granted.”
Patrice A. Harris, MD, president for the American Medical Association, said the judge’s decision fails patients and will likely raise prices, lower quality, reduce choice, and stifle innovation.
“The American people and our health system will not be served well by allowing a merger that combines health insurance giant Aetna Inc. with CVS Health Corporation,” Dr. Harris said in a statement. “For patients and employers struggling with recurrent increases to health insurance premiums, out-of-pocket costs, and prescription drug prices, it’s hard to find any upside to a merger that leaves them with fewer choices. Nothing in the deal guarantees reductions on insurance premiums or prescription drug costs. As for promised efficiency savings, that money will likely go straight to CVS’s bottom line.”
Angus Worthing, MD, government affairs committee chair for the American College of Rheumatology, said his group is concerned that the merger will hinder progress that has been made toward creating cost transparency and will make it easier for costs savings to remain secret.
“We hope that regulators will now actively watch the conduct of the merged company to ensure patients are protected,” Dr. Worthing said in a statement.
In a statement, CVS noted that CVS Health and Aetna have been one company since November 2018, stating that the court’s action “makes that 100 percent clear.”
“We remain focused on transforming the consumer health care experience in America,” they said.
CVS Health announced it would buy Aetna for $69 billion in 2017 and finalized the acquisition in 2018. CVS Health President and CEO Larry J. Merlo said the combined company would connect consumers with the powerful health resources of CVS Health in communities across the country and Aetna’s network of providers to help remove barriers to high quality care and build lasting relationships with patients.
Assistant Attorney General Makan Delrahim of the Justice Department’s antitrust division said the agency was pleased with the court’s decision to approve the government’s plan and finalize the merger.
“The divestiture of Aetna’s individual PDP business provides a comprehensive remedy to the harms the Justice Department identified,” Mr. Delrahim said in a statement. “The entry of the final judgment protects seniors and other vulnerable customers of individual PDPs from the anticompetitive effects that would have occurred if CVS and Aetna had merged their individual PDP businesses.”
Physician groups are criticizing a judge’s decision to approve the merger of pharmacy chain CVS Health and health insurer Aetna, saying the deal will raise prices and lower quality.
Judge Richard J. Leon of the U.S. District Court for the District of Columbia allowed the merger to move forward on Sept. 4, ruling that the acquisition was legal under antitrust law. The merger was approved by the Department of Justice in October 2018 on the condition that Aetna sell its Medicare prescription drug plan (PDP) business to independently owned competitor, WellCare Health Plans. Judge Leon has been examining the government’s plan since late 2018.
In his decision, Judge Leon acknowledged that the merger will have widespread effects for millions of patients and noted the many industry stakeholders, consumer groups, and state regulatory bodies have raised concerns about the merger.
“Although [the opposition] raised substantial concerns that warranted serious consideration, CVS’s and the government’s witnesses, when combined with the existing record, persuasively support why the markets at issue are not only very competitive today, but are likely to remain so post merger,” Judge Leon wrote. “Consequently, the harms to the public interest the [opposition] raised were not sufficiently established to undermine the government’s conclusion to the contrary. As such, for all of the above reasons, I have concluded that the proposed settlement is well ‘within the reaches’ of the public interest and the government’s motion ... should therefore be granted.”
Patrice A. Harris, MD, president for the American Medical Association, said the judge’s decision fails patients and will likely raise prices, lower quality, reduce choice, and stifle innovation.
“The American people and our health system will not be served well by allowing a merger that combines health insurance giant Aetna Inc. with CVS Health Corporation,” Dr. Harris said in a statement. “For patients and employers struggling with recurrent increases to health insurance premiums, out-of-pocket costs, and prescription drug prices, it’s hard to find any upside to a merger that leaves them with fewer choices. Nothing in the deal guarantees reductions on insurance premiums or prescription drug costs. As for promised efficiency savings, that money will likely go straight to CVS’s bottom line.”
Angus Worthing, MD, government affairs committee chair for the American College of Rheumatology, said his group is concerned that the merger will hinder progress that has been made toward creating cost transparency and will make it easier for costs savings to remain secret.
“We hope that regulators will now actively watch the conduct of the merged company to ensure patients are protected,” Dr. Worthing said in a statement.
In a statement, CVS noted that CVS Health and Aetna have been one company since November 2018, stating that the court’s action “makes that 100 percent clear.”
“We remain focused on transforming the consumer health care experience in America,” they said.
CVS Health announced it would buy Aetna for $69 billion in 2017 and finalized the acquisition in 2018. CVS Health President and CEO Larry J. Merlo said the combined company would connect consumers with the powerful health resources of CVS Health in communities across the country and Aetna’s network of providers to help remove barriers to high quality care and build lasting relationships with patients.
Assistant Attorney General Makan Delrahim of the Justice Department’s antitrust division said the agency was pleased with the court’s decision to approve the government’s plan and finalize the merger.
“The divestiture of Aetna’s individual PDP business provides a comprehensive remedy to the harms the Justice Department identified,” Mr. Delrahim said in a statement. “The entry of the final judgment protects seniors and other vulnerable customers of individual PDPs from the anticompetitive effects that would have occurred if CVS and Aetna had merged their individual PDP businesses.”
Physician groups are criticizing a judge’s decision to approve the merger of pharmacy chain CVS Health and health insurer Aetna, saying the deal will raise prices and lower quality.
Judge Richard J. Leon of the U.S. District Court for the District of Columbia allowed the merger to move forward on Sept. 4, ruling that the acquisition was legal under antitrust law. The merger was approved by the Department of Justice in October 2018 on the condition that Aetna sell its Medicare prescription drug plan (PDP) business to independently owned competitor, WellCare Health Plans. Judge Leon has been examining the government’s plan since late 2018.
In his decision, Judge Leon acknowledged that the merger will have widespread effects for millions of patients and noted the many industry stakeholders, consumer groups, and state regulatory bodies have raised concerns about the merger.
“Although [the opposition] raised substantial concerns that warranted serious consideration, CVS’s and the government’s witnesses, when combined with the existing record, persuasively support why the markets at issue are not only very competitive today, but are likely to remain so post merger,” Judge Leon wrote. “Consequently, the harms to the public interest the [opposition] raised were not sufficiently established to undermine the government’s conclusion to the contrary. As such, for all of the above reasons, I have concluded that the proposed settlement is well ‘within the reaches’ of the public interest and the government’s motion ... should therefore be granted.”
Patrice A. Harris, MD, president for the American Medical Association, said the judge’s decision fails patients and will likely raise prices, lower quality, reduce choice, and stifle innovation.
“The American people and our health system will not be served well by allowing a merger that combines health insurance giant Aetna Inc. with CVS Health Corporation,” Dr. Harris said in a statement. “For patients and employers struggling with recurrent increases to health insurance premiums, out-of-pocket costs, and prescription drug prices, it’s hard to find any upside to a merger that leaves them with fewer choices. Nothing in the deal guarantees reductions on insurance premiums or prescription drug costs. As for promised efficiency savings, that money will likely go straight to CVS’s bottom line.”
Angus Worthing, MD, government affairs committee chair for the American College of Rheumatology, said his group is concerned that the merger will hinder progress that has been made toward creating cost transparency and will make it easier for costs savings to remain secret.
“We hope that regulators will now actively watch the conduct of the merged company to ensure patients are protected,” Dr. Worthing said in a statement.
In a statement, CVS noted that CVS Health and Aetna have been one company since November 2018, stating that the court’s action “makes that 100 percent clear.”
“We remain focused on transforming the consumer health care experience in America,” they said.
CVS Health announced it would buy Aetna for $69 billion in 2017 and finalized the acquisition in 2018. CVS Health President and CEO Larry J. Merlo said the combined company would connect consumers with the powerful health resources of CVS Health in communities across the country and Aetna’s network of providers to help remove barriers to high quality care and build lasting relationships with patients.
Assistant Attorney General Makan Delrahim of the Justice Department’s antitrust division said the agency was pleased with the court’s decision to approve the government’s plan and finalize the merger.
“The divestiture of Aetna’s individual PDP business provides a comprehensive remedy to the harms the Justice Department identified,” Mr. Delrahim said in a statement. “The entry of the final judgment protects seniors and other vulnerable customers of individual PDPs from the anticompetitive effects that would have occurred if CVS and Aetna had merged their individual PDP businesses.”
Guideline: Blood CO2 can be used to screen for OHS
A blood test for elevated carbon dioxide may be used in screening adults for obesity hypoventilation syndrome, according to new guidelines.
Obese adults with sleep-disordered breathing and increased blood carbon dioxide levels during the day are likely to have obesity hypoventilation syndrome (OHS), a result of shallow or slow breathing that can lead to respiratory failure, heart failure, pulmonary hypertension, and death. Pulmonologists and sleep specialists may be the first to see and diagnose patients with OHS in the outpatient setting, while other cases are diagnosed in the hospital when patients present with hypercapnic respiratory failure.
Screening for OHS usually involves measuring arterial blood gases, which is not standard practice in outpatient clinics. Patients often remain undiagnosed and untreated until late in the course of the disease, according to the American Thoracic Society, which in August published a new diagnosis and management guideline aiming to boost early diagnosis and reduce variability in treatment (Am J Respir Crit Care Med. 2019;200:3,e6–e24).
The guideline authors, led by Babak Mokhlesi, MD, of the University of Chicago, recommend a simpler screening method – measuring serum bicarbonate only – to rule out OHS in obese patients with nighttime breathing problems.
Serum bicarbonate should be measured in obese patients with sleep-disordered breathing and a low likelihood of OHS, Dr. Mokhlesi and colleagues recommend in the guideline. If serum bicarbonate is below 27 mmol/L, it is not necessary to conduct further testing as the patient is unlikely to have OHS.
In patients whose serum bicarbonate is higher than 27 mmol/L, or who are strongly suspected of having OHS at presentation because of severe obesity or other symptoms, arterial blood gases should be measured and a sleep study conducted. The guideline authors said that there is insufficient evidence to recommend that pulse oximetry be used in the diagnostic pathway for OHS.
First-line treatment for stable, ambulatory patients with OHS should be positive airway pressure during sleep, rather than noninvasive ventilation, Dr. Mokhlesi and colleagues concluded. For patients with comorbid obstructive sleep apnea – as many as 70% of OHS patients also have OSA – the first-line treatment should be continuous positive airway pressure (CPAP) at night, the guideline states.
Patients hospitalized with respiratory failure and suspected of having OHS should be discharged with noninvasive ventilation until diagnostic procedures can be performed, along with PAP titration in a sleep lab.
In patients initially treated with CPAP who remain symptomatic or whose blood carbon dioxide does not improve, noninvasive ventilation can be tried, the guidelines say. Finally, patients diagnosed with OHS should be guided to weight loss interventions with the aim of reducing body weight by 25%-30%. This can include referral for bariatric surgery in patients without contraindications.
Dr. Mokhlesi and colleagues acknowledged that all of the recommendations contained in the guideline are classed as “conditional,” based on the quality of evidence assessed.
The American Thoracic Society funded the study. Dr. Mokhlesi and 7 coauthors disclosed financial conflicts of interest, while an additional 13 coauthors had none. Disclosures can be found on the AJRCCM website.
SOURCE: Mokhlesi B et al. Am J Respir Crit Care Med. 2019;200:3,e6-e24
A blood test for elevated carbon dioxide may be used in screening adults for obesity hypoventilation syndrome, according to new guidelines.
Obese adults with sleep-disordered breathing and increased blood carbon dioxide levels during the day are likely to have obesity hypoventilation syndrome (OHS), a result of shallow or slow breathing that can lead to respiratory failure, heart failure, pulmonary hypertension, and death. Pulmonologists and sleep specialists may be the first to see and diagnose patients with OHS in the outpatient setting, while other cases are diagnosed in the hospital when patients present with hypercapnic respiratory failure.
Screening for OHS usually involves measuring arterial blood gases, which is not standard practice in outpatient clinics. Patients often remain undiagnosed and untreated until late in the course of the disease, according to the American Thoracic Society, which in August published a new diagnosis and management guideline aiming to boost early diagnosis and reduce variability in treatment (Am J Respir Crit Care Med. 2019;200:3,e6–e24).
The guideline authors, led by Babak Mokhlesi, MD, of the University of Chicago, recommend a simpler screening method – measuring serum bicarbonate only – to rule out OHS in obese patients with nighttime breathing problems.
Serum bicarbonate should be measured in obese patients with sleep-disordered breathing and a low likelihood of OHS, Dr. Mokhlesi and colleagues recommend in the guideline. If serum bicarbonate is below 27 mmol/L, it is not necessary to conduct further testing as the patient is unlikely to have OHS.
In patients whose serum bicarbonate is higher than 27 mmol/L, or who are strongly suspected of having OHS at presentation because of severe obesity or other symptoms, arterial blood gases should be measured and a sleep study conducted. The guideline authors said that there is insufficient evidence to recommend that pulse oximetry be used in the diagnostic pathway for OHS.
First-line treatment for stable, ambulatory patients with OHS should be positive airway pressure during sleep, rather than noninvasive ventilation, Dr. Mokhlesi and colleagues concluded. For patients with comorbid obstructive sleep apnea – as many as 70% of OHS patients also have OSA – the first-line treatment should be continuous positive airway pressure (CPAP) at night, the guideline states.
Patients hospitalized with respiratory failure and suspected of having OHS should be discharged with noninvasive ventilation until diagnostic procedures can be performed, along with PAP titration in a sleep lab.
In patients initially treated with CPAP who remain symptomatic or whose blood carbon dioxide does not improve, noninvasive ventilation can be tried, the guidelines say. Finally, patients diagnosed with OHS should be guided to weight loss interventions with the aim of reducing body weight by 25%-30%. This can include referral for bariatric surgery in patients without contraindications.
Dr. Mokhlesi and colleagues acknowledged that all of the recommendations contained in the guideline are classed as “conditional,” based on the quality of evidence assessed.
The American Thoracic Society funded the study. Dr. Mokhlesi and 7 coauthors disclosed financial conflicts of interest, while an additional 13 coauthors had none. Disclosures can be found on the AJRCCM website.
SOURCE: Mokhlesi B et al. Am J Respir Crit Care Med. 2019;200:3,e6-e24
A blood test for elevated carbon dioxide may be used in screening adults for obesity hypoventilation syndrome, according to new guidelines.
Obese adults with sleep-disordered breathing and increased blood carbon dioxide levels during the day are likely to have obesity hypoventilation syndrome (OHS), a result of shallow or slow breathing that can lead to respiratory failure, heart failure, pulmonary hypertension, and death. Pulmonologists and sleep specialists may be the first to see and diagnose patients with OHS in the outpatient setting, while other cases are diagnosed in the hospital when patients present with hypercapnic respiratory failure.
Screening for OHS usually involves measuring arterial blood gases, which is not standard practice in outpatient clinics. Patients often remain undiagnosed and untreated until late in the course of the disease, according to the American Thoracic Society, which in August published a new diagnosis and management guideline aiming to boost early diagnosis and reduce variability in treatment (Am J Respir Crit Care Med. 2019;200:3,e6–e24).
The guideline authors, led by Babak Mokhlesi, MD, of the University of Chicago, recommend a simpler screening method – measuring serum bicarbonate only – to rule out OHS in obese patients with nighttime breathing problems.
Serum bicarbonate should be measured in obese patients with sleep-disordered breathing and a low likelihood of OHS, Dr. Mokhlesi and colleagues recommend in the guideline. If serum bicarbonate is below 27 mmol/L, it is not necessary to conduct further testing as the patient is unlikely to have OHS.
In patients whose serum bicarbonate is higher than 27 mmol/L, or who are strongly suspected of having OHS at presentation because of severe obesity or other symptoms, arterial blood gases should be measured and a sleep study conducted. The guideline authors said that there is insufficient evidence to recommend that pulse oximetry be used in the diagnostic pathway for OHS.
First-line treatment for stable, ambulatory patients with OHS should be positive airway pressure during sleep, rather than noninvasive ventilation, Dr. Mokhlesi and colleagues concluded. For patients with comorbid obstructive sleep apnea – as many as 70% of OHS patients also have OSA – the first-line treatment should be continuous positive airway pressure (CPAP) at night, the guideline states.
Patients hospitalized with respiratory failure and suspected of having OHS should be discharged with noninvasive ventilation until diagnostic procedures can be performed, along with PAP titration in a sleep lab.
In patients initially treated with CPAP who remain symptomatic or whose blood carbon dioxide does not improve, noninvasive ventilation can be tried, the guidelines say. Finally, patients diagnosed with OHS should be guided to weight loss interventions with the aim of reducing body weight by 25%-30%. This can include referral for bariatric surgery in patients without contraindications.
Dr. Mokhlesi and colleagues acknowledged that all of the recommendations contained in the guideline are classed as “conditional,” based on the quality of evidence assessed.
The American Thoracic Society funded the study. Dr. Mokhlesi and 7 coauthors disclosed financial conflicts of interest, while an additional 13 coauthors had none. Disclosures can be found on the AJRCCM website.
SOURCE: Mokhlesi B et al. Am J Respir Crit Care Med. 2019;200:3,e6-e24
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Migraines linked to higher risk of dementia
, according to research published online Sept. 4 in the International Journal of Geriatric Psychiatry.
In the Manitoba Study of Health and Aging, a population-based, prospective cohort study, 679 community-dwelling adults with a mean age of 75.9 years were followed for 5 years. Participants screened as cognitively intact at baseline had complete data on migraine history and all covariates at baseline and were assessed for cognitive outcomes 5 years later.
The study showed that a history of migraines was associated with a 2.97-fold greater likelihood of dementia, after adjustment for age, education, and a history of stroke, compared with individuals without a history of migraine. Individuals with Alzheimer’s disease were more than four times more likely to have a history of migraines (odds ratio 4.22).
However, researchers found no significant association between vascular dementia and a history of migraines, either before or after adjusting for confounders but particularly after incorporating a history of stroke into the model.
Lead investigator Suzanne L. Tyas, PhD, associate professor in the School of Public Health and Health Systems at the University of Waterloo, Ont., and coauthors suggested that the association between migraine and dementia was largely driven by the strong association between migraines and Alzheimer’s disease.
“This interpretation is supported by the weaker association for dementia than for Alzheimer’s disease, reflecting a dilution of the association with migraines across all types of dementia including vascular dementia, where a significant association was not found,” the researchers wrote.
The study population was 61.9% female, and no men reporting a history of migraine were diagnosed with dementia. While the study reflected a strong association between migraine and dementia in women, the researchers said they were unable to assess potential gender differences in this association.
Commenting on possible mechanisms behind the association, the authors wrote that there were overlaps underlying the biological mechanisms of migraine and dementia. Vascular risk factors such as diabetes, hypertension, heart attack, and stroke are associated with the development of dementia, and a relationship of these risk factors and migraine also has been seen.
“Many of the mechanisms involved in migraine neurophysiology, such as inflammation and reduced cerebral blood flow, are also underlying causes of dementia,” they wrote. “Repeated activation of these pathways in chronic migraineurs has been shown to cause permanent neurological and vascular damage.”
They also observed that the association could be influenced by genetic factors, as individuals with presenilin-1 mutations, which predispose them to Alzheimer’s disease, are more likely to experience migraines or recurrent headaches.
They suggested their findings could inform preventive strategies and treatments for Alzheimer’s disease, as well as interventions such as earlier screening for cognitive decline in individuals who experience migraines.
The study was funded by Manitoba Health and the National Health Research and Development Program of Health Canada. No conflicts of interest were declared.
SOURCE: Morton R et al. Int J Geriatr Psychiatry, 2019 Sep 4. doi: 10.1002/gps.5180.
, according to research published online Sept. 4 in the International Journal of Geriatric Psychiatry.
In the Manitoba Study of Health and Aging, a population-based, prospective cohort study, 679 community-dwelling adults with a mean age of 75.9 years were followed for 5 years. Participants screened as cognitively intact at baseline had complete data on migraine history and all covariates at baseline and were assessed for cognitive outcomes 5 years later.
The study showed that a history of migraines was associated with a 2.97-fold greater likelihood of dementia, after adjustment for age, education, and a history of stroke, compared with individuals without a history of migraine. Individuals with Alzheimer’s disease were more than four times more likely to have a history of migraines (odds ratio 4.22).
However, researchers found no significant association between vascular dementia and a history of migraines, either before or after adjusting for confounders but particularly after incorporating a history of stroke into the model.
Lead investigator Suzanne L. Tyas, PhD, associate professor in the School of Public Health and Health Systems at the University of Waterloo, Ont., and coauthors suggested that the association between migraine and dementia was largely driven by the strong association between migraines and Alzheimer’s disease.
“This interpretation is supported by the weaker association for dementia than for Alzheimer’s disease, reflecting a dilution of the association with migraines across all types of dementia including vascular dementia, where a significant association was not found,” the researchers wrote.
The study population was 61.9% female, and no men reporting a history of migraine were diagnosed with dementia. While the study reflected a strong association between migraine and dementia in women, the researchers said they were unable to assess potential gender differences in this association.
Commenting on possible mechanisms behind the association, the authors wrote that there were overlaps underlying the biological mechanisms of migraine and dementia. Vascular risk factors such as diabetes, hypertension, heart attack, and stroke are associated with the development of dementia, and a relationship of these risk factors and migraine also has been seen.
“Many of the mechanisms involved in migraine neurophysiology, such as inflammation and reduced cerebral blood flow, are also underlying causes of dementia,” they wrote. “Repeated activation of these pathways in chronic migraineurs has been shown to cause permanent neurological and vascular damage.”
They also observed that the association could be influenced by genetic factors, as individuals with presenilin-1 mutations, which predispose them to Alzheimer’s disease, are more likely to experience migraines or recurrent headaches.
They suggested their findings could inform preventive strategies and treatments for Alzheimer’s disease, as well as interventions such as earlier screening for cognitive decline in individuals who experience migraines.
The study was funded by Manitoba Health and the National Health Research and Development Program of Health Canada. No conflicts of interest were declared.
SOURCE: Morton R et al. Int J Geriatr Psychiatry, 2019 Sep 4. doi: 10.1002/gps.5180.
, according to research published online Sept. 4 in the International Journal of Geriatric Psychiatry.
In the Manitoba Study of Health and Aging, a population-based, prospective cohort study, 679 community-dwelling adults with a mean age of 75.9 years were followed for 5 years. Participants screened as cognitively intact at baseline had complete data on migraine history and all covariates at baseline and were assessed for cognitive outcomes 5 years later.
The study showed that a history of migraines was associated with a 2.97-fold greater likelihood of dementia, after adjustment for age, education, and a history of stroke, compared with individuals without a history of migraine. Individuals with Alzheimer’s disease were more than four times more likely to have a history of migraines (odds ratio 4.22).
However, researchers found no significant association between vascular dementia and a history of migraines, either before or after adjusting for confounders but particularly after incorporating a history of stroke into the model.
Lead investigator Suzanne L. Tyas, PhD, associate professor in the School of Public Health and Health Systems at the University of Waterloo, Ont., and coauthors suggested that the association between migraine and dementia was largely driven by the strong association between migraines and Alzheimer’s disease.
“This interpretation is supported by the weaker association for dementia than for Alzheimer’s disease, reflecting a dilution of the association with migraines across all types of dementia including vascular dementia, where a significant association was not found,” the researchers wrote.
The study population was 61.9% female, and no men reporting a history of migraine were diagnosed with dementia. While the study reflected a strong association between migraine and dementia in women, the researchers said they were unable to assess potential gender differences in this association.
Commenting on possible mechanisms behind the association, the authors wrote that there were overlaps underlying the biological mechanisms of migraine and dementia. Vascular risk factors such as diabetes, hypertension, heart attack, and stroke are associated with the development of dementia, and a relationship of these risk factors and migraine also has been seen.
“Many of the mechanisms involved in migraine neurophysiology, such as inflammation and reduced cerebral blood flow, are also underlying causes of dementia,” they wrote. “Repeated activation of these pathways in chronic migraineurs has been shown to cause permanent neurological and vascular damage.”
They also observed that the association could be influenced by genetic factors, as individuals with presenilin-1 mutations, which predispose them to Alzheimer’s disease, are more likely to experience migraines or recurrent headaches.
They suggested their findings could inform preventive strategies and treatments for Alzheimer’s disease, as well as interventions such as earlier screening for cognitive decline in individuals who experience migraines.
The study was funded by Manitoba Health and the National Health Research and Development Program of Health Canada. No conflicts of interest were declared.
SOURCE: Morton R et al. Int J Geriatr Psychiatry, 2019 Sep 4. doi: 10.1002/gps.5180.
FROM THE INTERNATIONAL JOURNAL OF GERIATRIC PSYCHIATRY
It’s board recertification time!
Kernohan’s notch false localizing sign. PPRF. The 7th nerve fascicle wraps around the 6th nerve nucleus. (Or is it the other way around?)
Yes, I’m studying for my 10-year boards.
It’s funny how many of these details you forget over time. I used to be able to rattle off names, syndromes, and pathways at the dreaded Thursday morning differential conference in residency. To not know them would get you a dreaded glare from the chairman. Now ... not as much.
Granted, the names of such things become less important over time. What’s important is the instinctive understanding of them that comes with experience. Remembering the specific name of a neural pathway becomes less relevant compared to recognizing where the problem is when you see that patient, and translating that into appropriate testing and treatment.
But, every 10 years, I have to go back to the books. Relearn the faded details of enzyme pathways, miscellaneous receptor actions, and courses of nerve tracts.
A lot of it is done on my iPad, a gadget I never imagined back in medical school, but it’s still the same routine I knew so well back then: Reading a page, staring blankly off to commit some point to memory, taking a practice test, and reviewing the answers. Occasionally, wandering off to get a can of soda or make tea.
Of course, today I have to work that around my family and job, concerns I didn’t have to split my time with in medical school. I had classmates who were married and had kids, and this always gives me a new respect for how they managed it.
Does knowing these details again make me a better doctor? I have no idea. I understand the idea that we need some way of showing we’re still on top of things after 20 years in the field. I’m not sure the current maintenance of certification practices are the best way to do that, but admittedly I don’t have any better ideas.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Kernohan’s notch false localizing sign. PPRF. The 7th nerve fascicle wraps around the 6th nerve nucleus. (Or is it the other way around?)
Yes, I’m studying for my 10-year boards.
It’s funny how many of these details you forget over time. I used to be able to rattle off names, syndromes, and pathways at the dreaded Thursday morning differential conference in residency. To not know them would get you a dreaded glare from the chairman. Now ... not as much.
Granted, the names of such things become less important over time. What’s important is the instinctive understanding of them that comes with experience. Remembering the specific name of a neural pathway becomes less relevant compared to recognizing where the problem is when you see that patient, and translating that into appropriate testing and treatment.
But, every 10 years, I have to go back to the books. Relearn the faded details of enzyme pathways, miscellaneous receptor actions, and courses of nerve tracts.
A lot of it is done on my iPad, a gadget I never imagined back in medical school, but it’s still the same routine I knew so well back then: Reading a page, staring blankly off to commit some point to memory, taking a practice test, and reviewing the answers. Occasionally, wandering off to get a can of soda or make tea.
Of course, today I have to work that around my family and job, concerns I didn’t have to split my time with in medical school. I had classmates who were married and had kids, and this always gives me a new respect for how they managed it.
Does knowing these details again make me a better doctor? I have no idea. I understand the idea that we need some way of showing we’re still on top of things after 20 years in the field. I’m not sure the current maintenance of certification practices are the best way to do that, but admittedly I don’t have any better ideas.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Kernohan’s notch false localizing sign. PPRF. The 7th nerve fascicle wraps around the 6th nerve nucleus. (Or is it the other way around?)
Yes, I’m studying for my 10-year boards.
It’s funny how many of these details you forget over time. I used to be able to rattle off names, syndromes, and pathways at the dreaded Thursday morning differential conference in residency. To not know them would get you a dreaded glare from the chairman. Now ... not as much.
Granted, the names of such things become less important over time. What’s important is the instinctive understanding of them that comes with experience. Remembering the specific name of a neural pathway becomes less relevant compared to recognizing where the problem is when you see that patient, and translating that into appropriate testing and treatment.
But, every 10 years, I have to go back to the books. Relearn the faded details of enzyme pathways, miscellaneous receptor actions, and courses of nerve tracts.
A lot of it is done on my iPad, a gadget I never imagined back in medical school, but it’s still the same routine I knew so well back then: Reading a page, staring blankly off to commit some point to memory, taking a practice test, and reviewing the answers. Occasionally, wandering off to get a can of soda or make tea.
Of course, today I have to work that around my family and job, concerns I didn’t have to split my time with in medical school. I had classmates who were married and had kids, and this always gives me a new respect for how they managed it.
Does knowing these details again make me a better doctor? I have no idea. I understand the idea that we need some way of showing we’re still on top of things after 20 years in the field. I’m not sure the current maintenance of certification practices are the best way to do that, but admittedly I don’t have any better ideas.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.