User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Noninvasive laser therapy tied to improved short-term memory
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Terminally ill cancer patients struggle to access psilocybin
In March 2020, when the world was struck by the news of the COVID-19 pandemic, Erinn Baldeschwiler received her own gut punch. She was diagnosed with stage IV metastatic breast cancer and was given about 2 years to live.
Then 48, the mother of two teenagers had just started a new chapter in her life. She’d gotten divorced, moved to a new home, and left a small business she had spent 18 years cultivating. The prospect that her life story might soon be ending, that she wouldn’t see her children grow up, was a twist of fate almost too devastating to bear.
“Are you kidding me that this is happening?” she thought.
But she also wanted to keep learning and growing in her remaining years, to devote them to creating meaningful memories, contemplating her mortality, and trying to find inner peace.
“The last 2 years have kind of been this dance with Lady Death,” she said.
They have also been a dance with Lady Justice.
In March 2021, Ms. Baldeschwiler, along with Michal Bloom, who also has terminal cancer, and their palliative care physician, Sunil Aggarwal, MD, PhD, decided to sue the Drug Enforcement Administration (DEA) for the right to access psilocybin, the psychoactive ingredient in “magic” mushrooms.
Psilocybin-assisted therapy has been shown to help terminally ill people overcome their fear, anxiety, and despair about death and to experience the kind of peace Ms. Baldeschwiler is seeking.
Psilocybin is illegal in the United States, but the plaintiffs argue they should be able to take the substance through the Right to Try Act. The 2018 federal law says that people with life-threatening conditions who have exhausted all approved treatment options can access drugs that have not yet been approved by the Food and Drug Administration but have passed phase 1 clinical trials.
This case marks the first time patients have fought to use a Schedule I drug under the Right to Try Act.
The push to expand access to psilocybin is picking up steam in the United States. In 2023, facilitated use of psilocybin will become legal in Oregon and Colorado. Recent proposals from the Biden administration and members of Congress could make psilocybin more widely accessible in the next few years.
It is also gaining momentum outside the United States. In Canada, patients are suing the government to help patients obtain psilocybin-assisted therapy for medical purposes.
“I think what we have here is a confluence of events that are driving toward the mandatory opening of a path to access psilocybin for therapeutic use sooner rather than later,” said Kathryn Tucker, lead counsel in the case against the DEA.
Reverberations of Right to Try
The story of Right to Try began with Abigail Burroughs, who was diagnosed with head and neck cancer at age 19.
After conventional therapies failed, Ms. Burroughs’ oncologist recommended cetuximab, a drug targeting EGFR that was experimental at the time. Because the drug was available only through colon cancer trials, she was denied access.
She died in 2001 at age 21.
Ms. Burroughs’ father, Frank Burroughs, formed an organization that in 2003 sued the FDA to provide terminally ill patients access to unapproved drugs. In 2005, they lost, and subsequent attempts to appeal the decision failed.
Still, the case sparked a Right to Try movement.
“Right to Try laws swept the U.S. in a firestorm,” Ms. Tucker said.
Along with the federal law, which passed in 2018, 41 states have enacted Right to Try laws.
The movement intrigued Dr. Aggarwal, codirector of the Advanced Integrative Medical Science (AIMS) Institute in Seattle. Dr. Aggarwal had been treating patients with cannabis, and after taking psilocybin himself and finding it therapeutic, he thought Ms. Baldeschwiler could benefit.
“I always knew that the powerful medicines within Schedule I had a significant role to play in healing,” he said. “That was baked into my decision to become a doctor, to research, and to innovate.”
He applied for the right to cultivate psilocybin mushrooms, but the fungus doesn’t meet Right to Try requirements. He then found a manufacturer willing to supply synthesized psilocybin, but because it’s a Schedule I drug, the manufacturer needed an okay from the DEA.
Dr. Aggarwal joined forces with Ms. Tucker, who has spent 35 years protecting the rights of terminally ill patients. In January 2021, Ms. Tucker contacted the DEA about allowing dying patients, including Ms. Baldeschwiler and Mr. Bloom, to access psilocybin-assisted therapy.
The response, she said, was predictable.
“The DEA’s knee always jerks in the direction of no access,” Ms. Tucker said. “So it said ‘no access.’ “
The reason: In a letter dated February 2021, the DEA said it “has no authority to waive” any requirements of the Controlled Substances Act under Right to Try laws.
Suing the DEA
Dr. Aggarwal and Ms. Tucker did not accept the DEA’s “no access” answer.
They decided to sue.
Dr. Aggarwal and Ms. Tucker took the matter to the Ninth Circuit Court in March 2021. In January 2022, the court dismissed the case after the DEA claimed its initial denial was not final.
The following month, the plaintiffs petitioned the DEA to deliver a concrete answer.
In May, while waiting for a response, demonstrators gathered at the DEA’s headquarters to call for legal access to psilocybin. One of the protesters was Ms. Baldeschwiler, who choked back tears as she told the crowd she was likely missing her last Mother’s Day with her children to attend the event. She was arrested, along with 16 other people.
In late June, the DEA provided its final answer: No access.
In a letter addressed to Ms. Tucker, Thomas W. Prevoznik, the DEA’s deputy assistant administrator, said it “finds no basis” to reconsider its initial denial in February 2021 “because the legal and factual considerations remain unchanged.”
In an appeal, Ms. Tucker wrote: “In denying Petitioners’ requested accommodation in the Final Agency Action, DEA hides behind a smokescreen, neglecting its duty to implement the federal [Right to Try Act] and violating the state [Right to Try law].”
The government’s response is due in January 2023.
Ms. Tucker and her legal team also petitioned the DEA on behalf of Dr. Aggarwal to reschedule psilocybin from Schedule I to Schedule II.
The DEA defines Schedule I substances as “drugs with no currently accepted medical use and a high potential for abuse.” But the FDA has designated psilocybin as a breakthrough therapy for depression, which, Ms. Tucker noted, “reflects that there is a currently accepted medical use.”
Nevertheless, in September, the DEA denied Ms. Tucker’s petition to reschedule psilocybin, and her team is now petitioning the Ninth Circuit Court for a review of that decision.
Despite the setbacks, actions from the Biden administration and members of Congress could help improve access.
In July, Senators Cory Booker and Rand Paul introduced the Right to Try Clarification Act to clarify that the federal law includes Schedule I substances. If passed, Ms. Tucker said, it would negate the DEA’s “no access” argument.
And earlier this year, the Biden administration announced plans to establish a federal task force to address the “myriad of complex issues” associated with the anticipated FDA approval of psilocybin to treat depression. The task force will explore “the potential of psychedelic-assisted therapies” to tackle the mental health crisis as well as any “risks to public health” that “may require harm reduction, risk mitigation, and safety monitoring.”
The fight north of the border
In 2016, Canadian resident Thomas Hartle, then 48, awoke from surgery for a bowel obstruction to learn he had stage IV colon cancer.
After another surgery, his doctors believed the tumors were gone. But in 2019, the cancer came back, along with extreme anxiety and distress over his impending death and how his two special-needs children would cope.
Mr. Hartle wanted to try magic mushroom–assisted psychotherapy. The Saskatoon resident sought help from TheraPsil, a Canadian nonprofit organization that advocates for therapeutic psilocybin. They applied for access under Section 56, which allows health officials to exempt patients from certain provisions of drug law.
In 2020, Hartle became the first Canadian to legally obtain psilocybin-assisted therapy.
“It has been nothing short of life changing for me,” Mr. Hartle said at a palliative care conference in Saskatoon this past June. “I am now no longer actively dying. I feel like I am genuinely actively living.”
TheraPsil has obtained Section 56 exemptions for around 60 patients to access psilocybin-assisted therapy as well as 19 health care professionals who are training to become psilocybin-assisted therapists.
But then an election ushered in new health ministers, and in early 2022, the exemptions evaporated. Thousands of patients and health care practitioners on TheraPsil’s waiting list were left in limbo.
Health Canada told CBC News that the rule change came about because “while psilocybin has shown promise in clinical trials for the treatment of some indications, further research is still needed to determine its safety and efficacy.”
As an alternative, TheraPsil began applying for access under Canada’s Special Access Program, which is similar to Right to Try laws in the United States. But Canada’s program doesn’t apply to therapists in training, and the petition process is so slow that many patients die before requests can be approved.
“People like to pretend that the Special Access Program is not political, but it is very political,” said TheraPsil’s CEO, Spencer Hawkswell. “It means a patient and a doctor are asking a politician for access to their medicine, which is absolutely unacceptable.”
Now, TheraPsil is helping patients take the Canadian government to court. In July, Mr. Hartle and seven others with conditions ranging from cancer to chronic pain filed a lawsuit against Canada’s health ministry that challenges the limited legal pathways to the use of psilocybin. The lawsuit argues that patients have a “constitutional right to access psilocybin for medicinal purposes,” and it advocates for access to regulated psilocybin products from licensed dealers, much like Canada’s medical marijuana program already does.
In the filing, TheraPsil said that as of February 2022, it has a wait-list of more than 800 patients who are requesting help in obtaining psilocybin-assisted psychotherapy.
An uncertain future
Despite the groundswell of support, many unknowns remain about the safety of expanding access to psilocybin-assisted therapy.
When Oregon and Colorado launch their psilocybin programs in 2023, the licensed centers will provide testing grounds for the safety and efficacy of broader access to psilocybin for people with depression or terminal cancer as well as those looking to grow spiritually.
Although in clinical trials psilocybin has been found to ease symptoms of depression and end-of-life demoralization for people with life-threatening conditions, it has not been adequately tested in people with a range of mental health problems, traumas, and racial backgrounds.
That uncertainty has given some people pause. In recent months, some researchers and journalists have pushed back against the frenzy over the promise of psychedelics.
In September, David Yaden, PhD, a psychedelics researcher at Johns Hopkins, spoke at the Interdisciplinary Conference on Psychedelic Research in the Netherlands. He encouraged people to pay more attention to potential adverse effects of psychedelics, which could include anything from headaches to lingering dysphoria.
“Oftentimes, we hear only the positive anecdotes,” Dr. Yaden said. “We don’t hear ... neutral or negative ones. So, I think all of those anecdotes need to be part of the picture.”
A recent piece in Wired noted that mentioning the potential harms of psychedelics amid its renaissance has been “taboo,” but the authors cautioned that as clinical trials involving psychedelics grow larger and the drugs become commercialized, “more negative outcomes are likely to transpire.”
But Ms. Baldeschwiler remains steadfast in her pursuit. While it’s important to approach broader access to psychedelics with caution, “end-of-life patients don’t have time to wait,” she said.
A version of this article first appeared on Medscape.com.
In March 2020, when the world was struck by the news of the COVID-19 pandemic, Erinn Baldeschwiler received her own gut punch. She was diagnosed with stage IV metastatic breast cancer and was given about 2 years to live.
Then 48, the mother of two teenagers had just started a new chapter in her life. She’d gotten divorced, moved to a new home, and left a small business she had spent 18 years cultivating. The prospect that her life story might soon be ending, that she wouldn’t see her children grow up, was a twist of fate almost too devastating to bear.
“Are you kidding me that this is happening?” she thought.
But she also wanted to keep learning and growing in her remaining years, to devote them to creating meaningful memories, contemplating her mortality, and trying to find inner peace.
“The last 2 years have kind of been this dance with Lady Death,” she said.
They have also been a dance with Lady Justice.
In March 2021, Ms. Baldeschwiler, along with Michal Bloom, who also has terminal cancer, and their palliative care physician, Sunil Aggarwal, MD, PhD, decided to sue the Drug Enforcement Administration (DEA) for the right to access psilocybin, the psychoactive ingredient in “magic” mushrooms.
Psilocybin-assisted therapy has been shown to help terminally ill people overcome their fear, anxiety, and despair about death and to experience the kind of peace Ms. Baldeschwiler is seeking.
Psilocybin is illegal in the United States, but the plaintiffs argue they should be able to take the substance through the Right to Try Act. The 2018 federal law says that people with life-threatening conditions who have exhausted all approved treatment options can access drugs that have not yet been approved by the Food and Drug Administration but have passed phase 1 clinical trials.
This case marks the first time patients have fought to use a Schedule I drug under the Right to Try Act.
The push to expand access to psilocybin is picking up steam in the United States. In 2023, facilitated use of psilocybin will become legal in Oregon and Colorado. Recent proposals from the Biden administration and members of Congress could make psilocybin more widely accessible in the next few years.
It is also gaining momentum outside the United States. In Canada, patients are suing the government to help patients obtain psilocybin-assisted therapy for medical purposes.
“I think what we have here is a confluence of events that are driving toward the mandatory opening of a path to access psilocybin for therapeutic use sooner rather than later,” said Kathryn Tucker, lead counsel in the case against the DEA.
Reverberations of Right to Try
The story of Right to Try began with Abigail Burroughs, who was diagnosed with head and neck cancer at age 19.
After conventional therapies failed, Ms. Burroughs’ oncologist recommended cetuximab, a drug targeting EGFR that was experimental at the time. Because the drug was available only through colon cancer trials, she was denied access.
She died in 2001 at age 21.
Ms. Burroughs’ father, Frank Burroughs, formed an organization that in 2003 sued the FDA to provide terminally ill patients access to unapproved drugs. In 2005, they lost, and subsequent attempts to appeal the decision failed.
Still, the case sparked a Right to Try movement.
“Right to Try laws swept the U.S. in a firestorm,” Ms. Tucker said.
Along with the federal law, which passed in 2018, 41 states have enacted Right to Try laws.
The movement intrigued Dr. Aggarwal, codirector of the Advanced Integrative Medical Science (AIMS) Institute in Seattle. Dr. Aggarwal had been treating patients with cannabis, and after taking psilocybin himself and finding it therapeutic, he thought Ms. Baldeschwiler could benefit.
“I always knew that the powerful medicines within Schedule I had a significant role to play in healing,” he said. “That was baked into my decision to become a doctor, to research, and to innovate.”
He applied for the right to cultivate psilocybin mushrooms, but the fungus doesn’t meet Right to Try requirements. He then found a manufacturer willing to supply synthesized psilocybin, but because it’s a Schedule I drug, the manufacturer needed an okay from the DEA.
Dr. Aggarwal joined forces with Ms. Tucker, who has spent 35 years protecting the rights of terminally ill patients. In January 2021, Ms. Tucker contacted the DEA about allowing dying patients, including Ms. Baldeschwiler and Mr. Bloom, to access psilocybin-assisted therapy.
The response, she said, was predictable.
“The DEA’s knee always jerks in the direction of no access,” Ms. Tucker said. “So it said ‘no access.’ “
The reason: In a letter dated February 2021, the DEA said it “has no authority to waive” any requirements of the Controlled Substances Act under Right to Try laws.
Suing the DEA
Dr. Aggarwal and Ms. Tucker did not accept the DEA’s “no access” answer.
They decided to sue.
Dr. Aggarwal and Ms. Tucker took the matter to the Ninth Circuit Court in March 2021. In January 2022, the court dismissed the case after the DEA claimed its initial denial was not final.
The following month, the plaintiffs petitioned the DEA to deliver a concrete answer.
In May, while waiting for a response, demonstrators gathered at the DEA’s headquarters to call for legal access to psilocybin. One of the protesters was Ms. Baldeschwiler, who choked back tears as she told the crowd she was likely missing her last Mother’s Day with her children to attend the event. She was arrested, along with 16 other people.
In late June, the DEA provided its final answer: No access.
In a letter addressed to Ms. Tucker, Thomas W. Prevoznik, the DEA’s deputy assistant administrator, said it “finds no basis” to reconsider its initial denial in February 2021 “because the legal and factual considerations remain unchanged.”
In an appeal, Ms. Tucker wrote: “In denying Petitioners’ requested accommodation in the Final Agency Action, DEA hides behind a smokescreen, neglecting its duty to implement the federal [Right to Try Act] and violating the state [Right to Try law].”
The government’s response is due in January 2023.
Ms. Tucker and her legal team also petitioned the DEA on behalf of Dr. Aggarwal to reschedule psilocybin from Schedule I to Schedule II.
The DEA defines Schedule I substances as “drugs with no currently accepted medical use and a high potential for abuse.” But the FDA has designated psilocybin as a breakthrough therapy for depression, which, Ms. Tucker noted, “reflects that there is a currently accepted medical use.”
Nevertheless, in September, the DEA denied Ms. Tucker’s petition to reschedule psilocybin, and her team is now petitioning the Ninth Circuit Court for a review of that decision.
Despite the setbacks, actions from the Biden administration and members of Congress could help improve access.
In July, Senators Cory Booker and Rand Paul introduced the Right to Try Clarification Act to clarify that the federal law includes Schedule I substances. If passed, Ms. Tucker said, it would negate the DEA’s “no access” argument.
And earlier this year, the Biden administration announced plans to establish a federal task force to address the “myriad of complex issues” associated with the anticipated FDA approval of psilocybin to treat depression. The task force will explore “the potential of psychedelic-assisted therapies” to tackle the mental health crisis as well as any “risks to public health” that “may require harm reduction, risk mitigation, and safety monitoring.”
The fight north of the border
In 2016, Canadian resident Thomas Hartle, then 48, awoke from surgery for a bowel obstruction to learn he had stage IV colon cancer.
After another surgery, his doctors believed the tumors were gone. But in 2019, the cancer came back, along with extreme anxiety and distress over his impending death and how his two special-needs children would cope.
Mr. Hartle wanted to try magic mushroom–assisted psychotherapy. The Saskatoon resident sought help from TheraPsil, a Canadian nonprofit organization that advocates for therapeutic psilocybin. They applied for access under Section 56, which allows health officials to exempt patients from certain provisions of drug law.
In 2020, Hartle became the first Canadian to legally obtain psilocybin-assisted therapy.
“It has been nothing short of life changing for me,” Mr. Hartle said at a palliative care conference in Saskatoon this past June. “I am now no longer actively dying. I feel like I am genuinely actively living.”
TheraPsil has obtained Section 56 exemptions for around 60 patients to access psilocybin-assisted therapy as well as 19 health care professionals who are training to become psilocybin-assisted therapists.
But then an election ushered in new health ministers, and in early 2022, the exemptions evaporated. Thousands of patients and health care practitioners on TheraPsil’s waiting list were left in limbo.
Health Canada told CBC News that the rule change came about because “while psilocybin has shown promise in clinical trials for the treatment of some indications, further research is still needed to determine its safety and efficacy.”
As an alternative, TheraPsil began applying for access under Canada’s Special Access Program, which is similar to Right to Try laws in the United States. But Canada’s program doesn’t apply to therapists in training, and the petition process is so slow that many patients die before requests can be approved.
“People like to pretend that the Special Access Program is not political, but it is very political,” said TheraPsil’s CEO, Spencer Hawkswell. “It means a patient and a doctor are asking a politician for access to their medicine, which is absolutely unacceptable.”
Now, TheraPsil is helping patients take the Canadian government to court. In July, Mr. Hartle and seven others with conditions ranging from cancer to chronic pain filed a lawsuit against Canada’s health ministry that challenges the limited legal pathways to the use of psilocybin. The lawsuit argues that patients have a “constitutional right to access psilocybin for medicinal purposes,” and it advocates for access to regulated psilocybin products from licensed dealers, much like Canada’s medical marijuana program already does.
In the filing, TheraPsil said that as of February 2022, it has a wait-list of more than 800 patients who are requesting help in obtaining psilocybin-assisted psychotherapy.
An uncertain future
Despite the groundswell of support, many unknowns remain about the safety of expanding access to psilocybin-assisted therapy.
When Oregon and Colorado launch their psilocybin programs in 2023, the licensed centers will provide testing grounds for the safety and efficacy of broader access to psilocybin for people with depression or terminal cancer as well as those looking to grow spiritually.
Although in clinical trials psilocybin has been found to ease symptoms of depression and end-of-life demoralization for people with life-threatening conditions, it has not been adequately tested in people with a range of mental health problems, traumas, and racial backgrounds.
That uncertainty has given some people pause. In recent months, some researchers and journalists have pushed back against the frenzy over the promise of psychedelics.
In September, David Yaden, PhD, a psychedelics researcher at Johns Hopkins, spoke at the Interdisciplinary Conference on Psychedelic Research in the Netherlands. He encouraged people to pay more attention to potential adverse effects of psychedelics, which could include anything from headaches to lingering dysphoria.
“Oftentimes, we hear only the positive anecdotes,” Dr. Yaden said. “We don’t hear ... neutral or negative ones. So, I think all of those anecdotes need to be part of the picture.”
A recent piece in Wired noted that mentioning the potential harms of psychedelics amid its renaissance has been “taboo,” but the authors cautioned that as clinical trials involving psychedelics grow larger and the drugs become commercialized, “more negative outcomes are likely to transpire.”
But Ms. Baldeschwiler remains steadfast in her pursuit. While it’s important to approach broader access to psychedelics with caution, “end-of-life patients don’t have time to wait,” she said.
A version of this article first appeared on Medscape.com.
In March 2020, when the world was struck by the news of the COVID-19 pandemic, Erinn Baldeschwiler received her own gut punch. She was diagnosed with stage IV metastatic breast cancer and was given about 2 years to live.
Then 48, the mother of two teenagers had just started a new chapter in her life. She’d gotten divorced, moved to a new home, and left a small business she had spent 18 years cultivating. The prospect that her life story might soon be ending, that she wouldn’t see her children grow up, was a twist of fate almost too devastating to bear.
“Are you kidding me that this is happening?” she thought.
But she also wanted to keep learning and growing in her remaining years, to devote them to creating meaningful memories, contemplating her mortality, and trying to find inner peace.
“The last 2 years have kind of been this dance with Lady Death,” she said.
They have also been a dance with Lady Justice.
In March 2021, Ms. Baldeschwiler, along with Michal Bloom, who also has terminal cancer, and their palliative care physician, Sunil Aggarwal, MD, PhD, decided to sue the Drug Enforcement Administration (DEA) for the right to access psilocybin, the psychoactive ingredient in “magic” mushrooms.
Psilocybin-assisted therapy has been shown to help terminally ill people overcome their fear, anxiety, and despair about death and to experience the kind of peace Ms. Baldeschwiler is seeking.
Psilocybin is illegal in the United States, but the plaintiffs argue they should be able to take the substance through the Right to Try Act. The 2018 federal law says that people with life-threatening conditions who have exhausted all approved treatment options can access drugs that have not yet been approved by the Food and Drug Administration but have passed phase 1 clinical trials.
This case marks the first time patients have fought to use a Schedule I drug under the Right to Try Act.
The push to expand access to psilocybin is picking up steam in the United States. In 2023, facilitated use of psilocybin will become legal in Oregon and Colorado. Recent proposals from the Biden administration and members of Congress could make psilocybin more widely accessible in the next few years.
It is also gaining momentum outside the United States. In Canada, patients are suing the government to help patients obtain psilocybin-assisted therapy for medical purposes.
“I think what we have here is a confluence of events that are driving toward the mandatory opening of a path to access psilocybin for therapeutic use sooner rather than later,” said Kathryn Tucker, lead counsel in the case against the DEA.
Reverberations of Right to Try
The story of Right to Try began with Abigail Burroughs, who was diagnosed with head and neck cancer at age 19.
After conventional therapies failed, Ms. Burroughs’ oncologist recommended cetuximab, a drug targeting EGFR that was experimental at the time. Because the drug was available only through colon cancer trials, she was denied access.
She died in 2001 at age 21.
Ms. Burroughs’ father, Frank Burroughs, formed an organization that in 2003 sued the FDA to provide terminally ill patients access to unapproved drugs. In 2005, they lost, and subsequent attempts to appeal the decision failed.
Still, the case sparked a Right to Try movement.
“Right to Try laws swept the U.S. in a firestorm,” Ms. Tucker said.
Along with the federal law, which passed in 2018, 41 states have enacted Right to Try laws.
The movement intrigued Dr. Aggarwal, codirector of the Advanced Integrative Medical Science (AIMS) Institute in Seattle. Dr. Aggarwal had been treating patients with cannabis, and after taking psilocybin himself and finding it therapeutic, he thought Ms. Baldeschwiler could benefit.
“I always knew that the powerful medicines within Schedule I had a significant role to play in healing,” he said. “That was baked into my decision to become a doctor, to research, and to innovate.”
He applied for the right to cultivate psilocybin mushrooms, but the fungus doesn’t meet Right to Try requirements. He then found a manufacturer willing to supply synthesized psilocybin, but because it’s a Schedule I drug, the manufacturer needed an okay from the DEA.
Dr. Aggarwal joined forces with Ms. Tucker, who has spent 35 years protecting the rights of terminally ill patients. In January 2021, Ms. Tucker contacted the DEA about allowing dying patients, including Ms. Baldeschwiler and Mr. Bloom, to access psilocybin-assisted therapy.
The response, she said, was predictable.
“The DEA’s knee always jerks in the direction of no access,” Ms. Tucker said. “So it said ‘no access.’ “
The reason: In a letter dated February 2021, the DEA said it “has no authority to waive” any requirements of the Controlled Substances Act under Right to Try laws.
Suing the DEA
Dr. Aggarwal and Ms. Tucker did not accept the DEA’s “no access” answer.
They decided to sue.
Dr. Aggarwal and Ms. Tucker took the matter to the Ninth Circuit Court in March 2021. In January 2022, the court dismissed the case after the DEA claimed its initial denial was not final.
The following month, the plaintiffs petitioned the DEA to deliver a concrete answer.
In May, while waiting for a response, demonstrators gathered at the DEA’s headquarters to call for legal access to psilocybin. One of the protesters was Ms. Baldeschwiler, who choked back tears as she told the crowd she was likely missing her last Mother’s Day with her children to attend the event. She was arrested, along with 16 other people.
In late June, the DEA provided its final answer: No access.
In a letter addressed to Ms. Tucker, Thomas W. Prevoznik, the DEA’s deputy assistant administrator, said it “finds no basis” to reconsider its initial denial in February 2021 “because the legal and factual considerations remain unchanged.”
In an appeal, Ms. Tucker wrote: “In denying Petitioners’ requested accommodation in the Final Agency Action, DEA hides behind a smokescreen, neglecting its duty to implement the federal [Right to Try Act] and violating the state [Right to Try law].”
The government’s response is due in January 2023.
Ms. Tucker and her legal team also petitioned the DEA on behalf of Dr. Aggarwal to reschedule psilocybin from Schedule I to Schedule II.
The DEA defines Schedule I substances as “drugs with no currently accepted medical use and a high potential for abuse.” But the FDA has designated psilocybin as a breakthrough therapy for depression, which, Ms. Tucker noted, “reflects that there is a currently accepted medical use.”
Nevertheless, in September, the DEA denied Ms. Tucker’s petition to reschedule psilocybin, and her team is now petitioning the Ninth Circuit Court for a review of that decision.
Despite the setbacks, actions from the Biden administration and members of Congress could help improve access.
In July, Senators Cory Booker and Rand Paul introduced the Right to Try Clarification Act to clarify that the federal law includes Schedule I substances. If passed, Ms. Tucker said, it would negate the DEA’s “no access” argument.
And earlier this year, the Biden administration announced plans to establish a federal task force to address the “myriad of complex issues” associated with the anticipated FDA approval of psilocybin to treat depression. The task force will explore “the potential of psychedelic-assisted therapies” to tackle the mental health crisis as well as any “risks to public health” that “may require harm reduction, risk mitigation, and safety monitoring.”
The fight north of the border
In 2016, Canadian resident Thomas Hartle, then 48, awoke from surgery for a bowel obstruction to learn he had stage IV colon cancer.
After another surgery, his doctors believed the tumors were gone. But in 2019, the cancer came back, along with extreme anxiety and distress over his impending death and how his two special-needs children would cope.
Mr. Hartle wanted to try magic mushroom–assisted psychotherapy. The Saskatoon resident sought help from TheraPsil, a Canadian nonprofit organization that advocates for therapeutic psilocybin. They applied for access under Section 56, which allows health officials to exempt patients from certain provisions of drug law.
In 2020, Hartle became the first Canadian to legally obtain psilocybin-assisted therapy.
“It has been nothing short of life changing for me,” Mr. Hartle said at a palliative care conference in Saskatoon this past June. “I am now no longer actively dying. I feel like I am genuinely actively living.”
TheraPsil has obtained Section 56 exemptions for around 60 patients to access psilocybin-assisted therapy as well as 19 health care professionals who are training to become psilocybin-assisted therapists.
But then an election ushered in new health ministers, and in early 2022, the exemptions evaporated. Thousands of patients and health care practitioners on TheraPsil’s waiting list were left in limbo.
Health Canada told CBC News that the rule change came about because “while psilocybin has shown promise in clinical trials for the treatment of some indications, further research is still needed to determine its safety and efficacy.”
As an alternative, TheraPsil began applying for access under Canada’s Special Access Program, which is similar to Right to Try laws in the United States. But Canada’s program doesn’t apply to therapists in training, and the petition process is so slow that many patients die before requests can be approved.
“People like to pretend that the Special Access Program is not political, but it is very political,” said TheraPsil’s CEO, Spencer Hawkswell. “It means a patient and a doctor are asking a politician for access to their medicine, which is absolutely unacceptable.”
Now, TheraPsil is helping patients take the Canadian government to court. In July, Mr. Hartle and seven others with conditions ranging from cancer to chronic pain filed a lawsuit against Canada’s health ministry that challenges the limited legal pathways to the use of psilocybin. The lawsuit argues that patients have a “constitutional right to access psilocybin for medicinal purposes,” and it advocates for access to regulated psilocybin products from licensed dealers, much like Canada’s medical marijuana program already does.
In the filing, TheraPsil said that as of February 2022, it has a wait-list of more than 800 patients who are requesting help in obtaining psilocybin-assisted psychotherapy.
An uncertain future
Despite the groundswell of support, many unknowns remain about the safety of expanding access to psilocybin-assisted therapy.
When Oregon and Colorado launch their psilocybin programs in 2023, the licensed centers will provide testing grounds for the safety and efficacy of broader access to psilocybin for people with depression or terminal cancer as well as those looking to grow spiritually.
Although in clinical trials psilocybin has been found to ease symptoms of depression and end-of-life demoralization for people with life-threatening conditions, it has not been adequately tested in people with a range of mental health problems, traumas, and racial backgrounds.
That uncertainty has given some people pause. In recent months, some researchers and journalists have pushed back against the frenzy over the promise of psychedelics.
In September, David Yaden, PhD, a psychedelics researcher at Johns Hopkins, spoke at the Interdisciplinary Conference on Psychedelic Research in the Netherlands. He encouraged people to pay more attention to potential adverse effects of psychedelics, which could include anything from headaches to lingering dysphoria.
“Oftentimes, we hear only the positive anecdotes,” Dr. Yaden said. “We don’t hear ... neutral or negative ones. So, I think all of those anecdotes need to be part of the picture.”
A recent piece in Wired noted that mentioning the potential harms of psychedelics amid its renaissance has been “taboo,” but the authors cautioned that as clinical trials involving psychedelics grow larger and the drugs become commercialized, “more negative outcomes are likely to transpire.”
But Ms. Baldeschwiler remains steadfast in her pursuit. While it’s important to approach broader access to psychedelics with caution, “end-of-life patients don’t have time to wait,” she said.
A version of this article first appeared on Medscape.com.
Should you quit employment to open a practice? These docs share how they did it
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
The kids may not be alright, but psychiatry can help
When I was growing up, I can remember experiencing “duck and cover” drills at school. If a flash appeared in our peripheral vision, we were told we should not look at it but crawl under our desks. My classmates and I were being taught how to protect ourselves in case of a nuclear attack.
Clearly, had there been such an attack, ducking under our desks would not have saved us. Thankfully, such a conflict never occurred – and hopefully never will. Still, the warning did penetrate our psyches. In those days, families and children in schools were worried, and some were scared.
The situation is quite different today. Our children and grandchildren are being taught to protect themselves not from actions overseas – that never happened – but from what someone living in their community might do that has been occurring in real time. According to my daughter-in-law, her young children are taught during “lockdowns” to hide in their classrooms’ closets. During these drills, some children are directed to line up against a wall that would be out of sight of a shooter, and to stay as still as possible.
Since 2017, the number of intentional shootings in U.S. kindergarten through grade 12 schools increased precipitously (Prev Med. 2022 Dec. doi: 10.1016/j.ypmed.2022.107280). Imagine the psychological impact that the vigilance required to deal with such impending threats must be having on our children, as they learn to fear injury and possible death every day they go to school. I’ve talked with numerous parents about this, including my own adult children, and this is clearly a new dimension of life that is on everyone’s minds. Schools, once bastions of safety, are no longer that safe.
For many years, I’ve written about the need to destigmatize mental illness so that it is treated on a par with physical illness. As we look at the challenges faced by young people, reframing mental illness is more important now than ever. This means finding ways to increase the funding of studies that help us understand young people with mental health issues. It also means encouraging patients to pursue treatment from psychiatrists, psychologists, or mental health counselors who specialize in short-term therapy.
The emphasis here on short-term therapy is not to discourage longer-term care when needed, but clearly short-term care strategies, such as cognitive-behavioral therapies, not only work for problem resolution, they also help in the destigmatization of mental health care – as the circumscribed treatment with a clear beginning, middle, and end is consistent with CBT and consistent with much of medical care for physical disorders.
Furthermore, as we aim to destigmatize mental health care, it’s important to equate it with physical care. For example, taking a day or two from school or work for a sprained ankle, seeing a dentist, or an eye exam, plus a myriad of physical issues is quite acceptable. Why is it not also acceptable for a mental health issue and evaluation, such as for anxiety or PTSD, plus being able to talk about it without stigma? Seeing the “shrink” needs to be removed as a negative but viewed as a very positive move toward care for oneself.
In addition, children and adolescents are battling countless other health challenges that could have implications for mental health professionals, for example:
- During the height of the coronavirus pandemic, pediatric endocrinologists reportedly saw a surge of referrals for girls experiencing early puberty. Puberty should never be medicalized, but early maturation has been linked to numerous psychiatric disorders such as depression, anxiety, and eating disorders (J Pediatr Adolec Gynecol. 2022 Oct. doi: 10.1016/j.jpag.2022.05.005).
- A global epidemiologic study of children estimates that nearly 8 million youth lost a parent or caregiver because of a pandemic-related cause between Jan. 1, 2020, and May 1, 2022. An additional 2.5 million children were affected by the loss of secondary caregivers such as grandparents (JAMA Pediatr. 2022 Sept. doi: 10.1001/jamapediatrics.2022.3157).
- The inpatient and outpatient volume of adolescents and young adults receiving care for eating disorders skyrocketed before and after the pandemic, according to the results of case study series (JAMA Pediatrics. 2022 Nov 7. doi: 10.1001/jamapediatrics.2022.4346).
- Children and adolescents who developed COVID-19 suffered tremendously during the height of the pandemic. A nationwide analysis shows that COVID-19 nearly tripled children’s risks of developing new mental health illnesses, such as attention-deficit/hyperactivity disorder, anxiety, trauma, or stress disorder (Psychiatric Services. 2022 Jun 2. doi: 10.1176/appi.ps.202100646).
In addition to those challenges, young children are facing an increase in respiratory syncytial virus (RSV) infection. We were told the “flu” would be quite bad this year and to beware of monkeypox. However, very little mention is made of the equally distressing “epidemic” of mental health issues, PTSD, anxiety, and depression as we are still in the midst of the COVID pandemic in the United States with almost 400 deaths a day – a very unacceptable number.
Interestingly, we seem to have abandoned the use of masks as protection against COVID and other respiratory diseases, despite their effectiveness. A study in Boston that looked at children in two school districts that did not lift mask mandates demonstrated that mask wearing does indeed lead to significant reductions in the number of pediatric COVID cases. In addition to societal violence and school shootings – which certainly exacerbate anxiety – the fear of dying or the death of a loved one, tied to COVID, may lead to epidemic proportions of PTSD in children. As an article in WebMD noted, “pediatricians are imploring the federal government to declare a national emergency as cases of pediatric respiratory illnesses continue to soar.”
In light of the acknowledged mental health crisis in children, which appears epidemic, I would hope the psychiatric and psychological associations would publicly sound an alarm so that resources could be brought to bear to address this critical issue. I believe doing so would also aid in destigmatizing mental disorders, and increase education and treatment.
Layered on top of those issues are natural disasters, such as the fallout from Tropical Storm Nicole when it recently caused devastation across western Florida. The mental health trauma caused by recent tropical storms seems all but forgotten – except for those who are still suffering. All of this adds up to a society-wide mental health crisis, which seems far more expansive than monkeypox, for example. Yet monkeypox, which did lead to thousands of cases and approximately 29 deaths in the United States, was declared a national public health emergency.
Additionally, RSV killed 100-500 U.S. children under age 5 each year before the pandemic, according to the Centers for Disease Control and Prevention, and currently it appears even worse. Yet despite the seriousness of RSV, it nowhere matches the emotional toll COVID has taken on children globally.
Let’s make it standard practice for children – and of course, adults – to be taught that anxiety is a normal response at times. We should teach that, in some cases, feeling “down” or in despair and even experiencing symptoms of PTSD based on what’s going on personally and within our environment (i.e., COVID, school shootings, etc.) are triggers and responses that can be addressed and often quickly treated by talking with a mental health professional.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
When I was growing up, I can remember experiencing “duck and cover” drills at school. If a flash appeared in our peripheral vision, we were told we should not look at it but crawl under our desks. My classmates and I were being taught how to protect ourselves in case of a nuclear attack.
Clearly, had there been such an attack, ducking under our desks would not have saved us. Thankfully, such a conflict never occurred – and hopefully never will. Still, the warning did penetrate our psyches. In those days, families and children in schools were worried, and some were scared.
The situation is quite different today. Our children and grandchildren are being taught to protect themselves not from actions overseas – that never happened – but from what someone living in their community might do that has been occurring in real time. According to my daughter-in-law, her young children are taught during “lockdowns” to hide in their classrooms’ closets. During these drills, some children are directed to line up against a wall that would be out of sight of a shooter, and to stay as still as possible.
Since 2017, the number of intentional shootings in U.S. kindergarten through grade 12 schools increased precipitously (Prev Med. 2022 Dec. doi: 10.1016/j.ypmed.2022.107280). Imagine the psychological impact that the vigilance required to deal with such impending threats must be having on our children, as they learn to fear injury and possible death every day they go to school. I’ve talked with numerous parents about this, including my own adult children, and this is clearly a new dimension of life that is on everyone’s minds. Schools, once bastions of safety, are no longer that safe.
For many years, I’ve written about the need to destigmatize mental illness so that it is treated on a par with physical illness. As we look at the challenges faced by young people, reframing mental illness is more important now than ever. This means finding ways to increase the funding of studies that help us understand young people with mental health issues. It also means encouraging patients to pursue treatment from psychiatrists, psychologists, or mental health counselors who specialize in short-term therapy.
The emphasis here on short-term therapy is not to discourage longer-term care when needed, but clearly short-term care strategies, such as cognitive-behavioral therapies, not only work for problem resolution, they also help in the destigmatization of mental health care – as the circumscribed treatment with a clear beginning, middle, and end is consistent with CBT and consistent with much of medical care for physical disorders.
Furthermore, as we aim to destigmatize mental health care, it’s important to equate it with physical care. For example, taking a day or two from school or work for a sprained ankle, seeing a dentist, or an eye exam, plus a myriad of physical issues is quite acceptable. Why is it not also acceptable for a mental health issue and evaluation, such as for anxiety or PTSD, plus being able to talk about it without stigma? Seeing the “shrink” needs to be removed as a negative but viewed as a very positive move toward care for oneself.
In addition, children and adolescents are battling countless other health challenges that could have implications for mental health professionals, for example:
- During the height of the coronavirus pandemic, pediatric endocrinologists reportedly saw a surge of referrals for girls experiencing early puberty. Puberty should never be medicalized, but early maturation has been linked to numerous psychiatric disorders such as depression, anxiety, and eating disorders (J Pediatr Adolec Gynecol. 2022 Oct. doi: 10.1016/j.jpag.2022.05.005).
- A global epidemiologic study of children estimates that nearly 8 million youth lost a parent or caregiver because of a pandemic-related cause between Jan. 1, 2020, and May 1, 2022. An additional 2.5 million children were affected by the loss of secondary caregivers such as grandparents (JAMA Pediatr. 2022 Sept. doi: 10.1001/jamapediatrics.2022.3157).
- The inpatient and outpatient volume of adolescents and young adults receiving care for eating disorders skyrocketed before and after the pandemic, according to the results of case study series (JAMA Pediatrics. 2022 Nov 7. doi: 10.1001/jamapediatrics.2022.4346).
- Children and adolescents who developed COVID-19 suffered tremendously during the height of the pandemic. A nationwide analysis shows that COVID-19 nearly tripled children’s risks of developing new mental health illnesses, such as attention-deficit/hyperactivity disorder, anxiety, trauma, or stress disorder (Psychiatric Services. 2022 Jun 2. doi: 10.1176/appi.ps.202100646).
In addition to those challenges, young children are facing an increase in respiratory syncytial virus (RSV) infection. We were told the “flu” would be quite bad this year and to beware of monkeypox. However, very little mention is made of the equally distressing “epidemic” of mental health issues, PTSD, anxiety, and depression as we are still in the midst of the COVID pandemic in the United States with almost 400 deaths a day – a very unacceptable number.
Interestingly, we seem to have abandoned the use of masks as protection against COVID and other respiratory diseases, despite their effectiveness. A study in Boston that looked at children in two school districts that did not lift mask mandates demonstrated that mask wearing does indeed lead to significant reductions in the number of pediatric COVID cases. In addition to societal violence and school shootings – which certainly exacerbate anxiety – the fear of dying or the death of a loved one, tied to COVID, may lead to epidemic proportions of PTSD in children. As an article in WebMD noted, “pediatricians are imploring the federal government to declare a national emergency as cases of pediatric respiratory illnesses continue to soar.”
In light of the acknowledged mental health crisis in children, which appears epidemic, I would hope the psychiatric and psychological associations would publicly sound an alarm so that resources could be brought to bear to address this critical issue. I believe doing so would also aid in destigmatizing mental disorders, and increase education and treatment.
Layered on top of those issues are natural disasters, such as the fallout from Tropical Storm Nicole when it recently caused devastation across western Florida. The mental health trauma caused by recent tropical storms seems all but forgotten – except for those who are still suffering. All of this adds up to a society-wide mental health crisis, which seems far more expansive than monkeypox, for example. Yet monkeypox, which did lead to thousands of cases and approximately 29 deaths in the United States, was declared a national public health emergency.
Additionally, RSV killed 100-500 U.S. children under age 5 each year before the pandemic, according to the Centers for Disease Control and Prevention, and currently it appears even worse. Yet despite the seriousness of RSV, it nowhere matches the emotional toll COVID has taken on children globally.
Let’s make it standard practice for children – and of course, adults – to be taught that anxiety is a normal response at times. We should teach that, in some cases, feeling “down” or in despair and even experiencing symptoms of PTSD based on what’s going on personally and within our environment (i.e., COVID, school shootings, etc.) are triggers and responses that can be addressed and often quickly treated by talking with a mental health professional.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
When I was growing up, I can remember experiencing “duck and cover” drills at school. If a flash appeared in our peripheral vision, we were told we should not look at it but crawl under our desks. My classmates and I were being taught how to protect ourselves in case of a nuclear attack.
Clearly, had there been such an attack, ducking under our desks would not have saved us. Thankfully, such a conflict never occurred – and hopefully never will. Still, the warning did penetrate our psyches. In those days, families and children in schools were worried, and some were scared.
The situation is quite different today. Our children and grandchildren are being taught to protect themselves not from actions overseas – that never happened – but from what someone living in their community might do that has been occurring in real time. According to my daughter-in-law, her young children are taught during “lockdowns” to hide in their classrooms’ closets. During these drills, some children are directed to line up against a wall that would be out of sight of a shooter, and to stay as still as possible.
Since 2017, the number of intentional shootings in U.S. kindergarten through grade 12 schools increased precipitously (Prev Med. 2022 Dec. doi: 10.1016/j.ypmed.2022.107280). Imagine the psychological impact that the vigilance required to deal with such impending threats must be having on our children, as they learn to fear injury and possible death every day they go to school. I’ve talked with numerous parents about this, including my own adult children, and this is clearly a new dimension of life that is on everyone’s minds. Schools, once bastions of safety, are no longer that safe.
For many years, I’ve written about the need to destigmatize mental illness so that it is treated on a par with physical illness. As we look at the challenges faced by young people, reframing mental illness is more important now than ever. This means finding ways to increase the funding of studies that help us understand young people with mental health issues. It also means encouraging patients to pursue treatment from psychiatrists, psychologists, or mental health counselors who specialize in short-term therapy.
The emphasis here on short-term therapy is not to discourage longer-term care when needed, but clearly short-term care strategies, such as cognitive-behavioral therapies, not only work for problem resolution, they also help in the destigmatization of mental health care – as the circumscribed treatment with a clear beginning, middle, and end is consistent with CBT and consistent with much of medical care for physical disorders.
Furthermore, as we aim to destigmatize mental health care, it’s important to equate it with physical care. For example, taking a day or two from school or work for a sprained ankle, seeing a dentist, or an eye exam, plus a myriad of physical issues is quite acceptable. Why is it not also acceptable for a mental health issue and evaluation, such as for anxiety or PTSD, plus being able to talk about it without stigma? Seeing the “shrink” needs to be removed as a negative but viewed as a very positive move toward care for oneself.
In addition, children and adolescents are battling countless other health challenges that could have implications for mental health professionals, for example:
- During the height of the coronavirus pandemic, pediatric endocrinologists reportedly saw a surge of referrals for girls experiencing early puberty. Puberty should never be medicalized, but early maturation has been linked to numerous psychiatric disorders such as depression, anxiety, and eating disorders (J Pediatr Adolec Gynecol. 2022 Oct. doi: 10.1016/j.jpag.2022.05.005).
- A global epidemiologic study of children estimates that nearly 8 million youth lost a parent or caregiver because of a pandemic-related cause between Jan. 1, 2020, and May 1, 2022. An additional 2.5 million children were affected by the loss of secondary caregivers such as grandparents (JAMA Pediatr. 2022 Sept. doi: 10.1001/jamapediatrics.2022.3157).
- The inpatient and outpatient volume of adolescents and young adults receiving care for eating disorders skyrocketed before and after the pandemic, according to the results of case study series (JAMA Pediatrics. 2022 Nov 7. doi: 10.1001/jamapediatrics.2022.4346).
- Children and adolescents who developed COVID-19 suffered tremendously during the height of the pandemic. A nationwide analysis shows that COVID-19 nearly tripled children’s risks of developing new mental health illnesses, such as attention-deficit/hyperactivity disorder, anxiety, trauma, or stress disorder (Psychiatric Services. 2022 Jun 2. doi: 10.1176/appi.ps.202100646).
In addition to those challenges, young children are facing an increase in respiratory syncytial virus (RSV) infection. We were told the “flu” would be quite bad this year and to beware of monkeypox. However, very little mention is made of the equally distressing “epidemic” of mental health issues, PTSD, anxiety, and depression as we are still in the midst of the COVID pandemic in the United States with almost 400 deaths a day – a very unacceptable number.
Interestingly, we seem to have abandoned the use of masks as protection against COVID and other respiratory diseases, despite their effectiveness. A study in Boston that looked at children in two school districts that did not lift mask mandates demonstrated that mask wearing does indeed lead to significant reductions in the number of pediatric COVID cases. In addition to societal violence and school shootings – which certainly exacerbate anxiety – the fear of dying or the death of a loved one, tied to COVID, may lead to epidemic proportions of PTSD in children. As an article in WebMD noted, “pediatricians are imploring the federal government to declare a national emergency as cases of pediatric respiratory illnesses continue to soar.”
In light of the acknowledged mental health crisis in children, which appears epidemic, I would hope the psychiatric and psychological associations would publicly sound an alarm so that resources could be brought to bear to address this critical issue. I believe doing so would also aid in destigmatizing mental disorders, and increase education and treatment.
Layered on top of those issues are natural disasters, such as the fallout from Tropical Storm Nicole when it recently caused devastation across western Florida. The mental health trauma caused by recent tropical storms seems all but forgotten – except for those who are still suffering. All of this adds up to a society-wide mental health crisis, which seems far more expansive than monkeypox, for example. Yet monkeypox, which did lead to thousands of cases and approximately 29 deaths in the United States, was declared a national public health emergency.
Additionally, RSV killed 100-500 U.S. children under age 5 each year before the pandemic, according to the Centers for Disease Control and Prevention, and currently it appears even worse. Yet despite the seriousness of RSV, it nowhere matches the emotional toll COVID has taken on children globally.
Let’s make it standard practice for children – and of course, adults – to be taught that anxiety is a normal response at times. We should teach that, in some cases, feeling “down” or in despair and even experiencing symptoms of PTSD based on what’s going on personally and within our environment (i.e., COVID, school shootings, etc.) are triggers and responses that can be addressed and often quickly treated by talking with a mental health professional.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Seizures in dementia hasten decline and death
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
AT AES 2022
Immune dysregulation may drive long-term postpartum depression
Postpartum depression, anxiety, and posttraumatic stress disorder that persist 2-3 years after birth are associated with a dysregulated immune system that is characterized by increased inflammatory signaling, according to investigators.
These findings suggest that mental health screening for women who have given birth should continue beyond the first year post partum, reported lead author Jennifer M. Nicoloro-SantaBarbara, PhD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
“Delayed postpartum depression, also known as late-onset postpartum depression, can affect women up to 18 months after delivery,” the investigators wrote in the American Journal of Reproductive Immunology. “It can appear even later in some women, depending on the hormonal changes that occur after having a baby (for example, timing of weaning). However, the majority of research on maternal mental health focuses on the first year post birth, leaving a gap in research beyond 12 months post partum.”
To address this gap, the investigators enrolled 33 women who were 2-3 years post partum. Participants completed self-guided questionnaires on PTSD, depression, and anxiety, and provided blood samples for gene expression analysis.
Sixteen of the 33 women had clinically significant mood disturbances. and significantly reduced activation of genes associated with viral response.
“The results provide preliminary evidence of a mechanism (e.g., immune dysregulation) that might be contributing to mood disorders and bring us closer to the goal of identifying targetable biomarkers for mood disorders,” Dr. Nicoloro-SantaBarbara said in a written comment. “This work highlights the need for standardized and continual depression and anxiety screening in ob.gyn. and primary care settings that extends beyond the 6-week maternal visit and possibly beyond the first postpartum year.”
Findings draw skepticism
“The authors argue that mothers need to be screened for depression/anxiety longer than the first year post partum, and this is true, but it has nothing to do with their findings,” said Jennifer L. Payne, MD, an expert in reproductive psychiatry at the University of Virginia, Charlottesville.
In a written comment, she explained that the cross-sectional design makes it impossible to know whether the mood disturbances were linked with delivery at all.
“It is unclear if the depression/anxiety symptoms began after delivery or not,” Dr. Payne said. “In addition, it is unclear if the findings are causative or a result of depression/anxiety symptoms (the authors admit this in the limitations section). It is likely that the findings are not specific or even related to having delivered a child, but rather reflect a more general process related to depression/anxiety outside of the postpartum time period.”
Only prospective studies can answer these questions, she said.
Dr. Nicoloro-SantaBarbara agreed that further research is needed.
“Our findings are exciting, but still need to be replicated in larger samples with diverse women in order to make sure they generalize,” she said. “More work is needed to understand why inflammation plays a role in postpartum mental illness for some women and not others.”
The study was supported by a Cedars-Sinai Precision Health Grant, the Cousins Center for Psychoneuroimmunology, University of California, Los Angeles, and the National Institute of Mental Health. The investigators and Dr. Payne disclosed no relevant conflicts of interest.
Postpartum depression, anxiety, and posttraumatic stress disorder that persist 2-3 years after birth are associated with a dysregulated immune system that is characterized by increased inflammatory signaling, according to investigators.
These findings suggest that mental health screening for women who have given birth should continue beyond the first year post partum, reported lead author Jennifer M. Nicoloro-SantaBarbara, PhD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
“Delayed postpartum depression, also known as late-onset postpartum depression, can affect women up to 18 months after delivery,” the investigators wrote in the American Journal of Reproductive Immunology. “It can appear even later in some women, depending on the hormonal changes that occur after having a baby (for example, timing of weaning). However, the majority of research on maternal mental health focuses on the first year post birth, leaving a gap in research beyond 12 months post partum.”
To address this gap, the investigators enrolled 33 women who were 2-3 years post partum. Participants completed self-guided questionnaires on PTSD, depression, and anxiety, and provided blood samples for gene expression analysis.
Sixteen of the 33 women had clinically significant mood disturbances. and significantly reduced activation of genes associated with viral response.
“The results provide preliminary evidence of a mechanism (e.g., immune dysregulation) that might be contributing to mood disorders and bring us closer to the goal of identifying targetable biomarkers for mood disorders,” Dr. Nicoloro-SantaBarbara said in a written comment. “This work highlights the need for standardized and continual depression and anxiety screening in ob.gyn. and primary care settings that extends beyond the 6-week maternal visit and possibly beyond the first postpartum year.”
Findings draw skepticism
“The authors argue that mothers need to be screened for depression/anxiety longer than the first year post partum, and this is true, but it has nothing to do with their findings,” said Jennifer L. Payne, MD, an expert in reproductive psychiatry at the University of Virginia, Charlottesville.
In a written comment, she explained that the cross-sectional design makes it impossible to know whether the mood disturbances were linked with delivery at all.
“It is unclear if the depression/anxiety symptoms began after delivery or not,” Dr. Payne said. “In addition, it is unclear if the findings are causative or a result of depression/anxiety symptoms (the authors admit this in the limitations section). It is likely that the findings are not specific or even related to having delivered a child, but rather reflect a more general process related to depression/anxiety outside of the postpartum time period.”
Only prospective studies can answer these questions, she said.
Dr. Nicoloro-SantaBarbara agreed that further research is needed.
“Our findings are exciting, but still need to be replicated in larger samples with diverse women in order to make sure they generalize,” she said. “More work is needed to understand why inflammation plays a role in postpartum mental illness for some women and not others.”
The study was supported by a Cedars-Sinai Precision Health Grant, the Cousins Center for Psychoneuroimmunology, University of California, Los Angeles, and the National Institute of Mental Health. The investigators and Dr. Payne disclosed no relevant conflicts of interest.
Postpartum depression, anxiety, and posttraumatic stress disorder that persist 2-3 years after birth are associated with a dysregulated immune system that is characterized by increased inflammatory signaling, according to investigators.
These findings suggest that mental health screening for women who have given birth should continue beyond the first year post partum, reported lead author Jennifer M. Nicoloro-SantaBarbara, PhD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
“Delayed postpartum depression, also known as late-onset postpartum depression, can affect women up to 18 months after delivery,” the investigators wrote in the American Journal of Reproductive Immunology. “It can appear even later in some women, depending on the hormonal changes that occur after having a baby (for example, timing of weaning). However, the majority of research on maternal mental health focuses on the first year post birth, leaving a gap in research beyond 12 months post partum.”
To address this gap, the investigators enrolled 33 women who were 2-3 years post partum. Participants completed self-guided questionnaires on PTSD, depression, and anxiety, and provided blood samples for gene expression analysis.
Sixteen of the 33 women had clinically significant mood disturbances. and significantly reduced activation of genes associated with viral response.
“The results provide preliminary evidence of a mechanism (e.g., immune dysregulation) that might be contributing to mood disorders and bring us closer to the goal of identifying targetable biomarkers for mood disorders,” Dr. Nicoloro-SantaBarbara said in a written comment. “This work highlights the need for standardized and continual depression and anxiety screening in ob.gyn. and primary care settings that extends beyond the 6-week maternal visit and possibly beyond the first postpartum year.”
Findings draw skepticism
“The authors argue that mothers need to be screened for depression/anxiety longer than the first year post partum, and this is true, but it has nothing to do with their findings,” said Jennifer L. Payne, MD, an expert in reproductive psychiatry at the University of Virginia, Charlottesville.
In a written comment, she explained that the cross-sectional design makes it impossible to know whether the mood disturbances were linked with delivery at all.
“It is unclear if the depression/anxiety symptoms began after delivery or not,” Dr. Payne said. “In addition, it is unclear if the findings are causative or a result of depression/anxiety symptoms (the authors admit this in the limitations section). It is likely that the findings are not specific or even related to having delivered a child, but rather reflect a more general process related to depression/anxiety outside of the postpartum time period.”
Only prospective studies can answer these questions, she said.
Dr. Nicoloro-SantaBarbara agreed that further research is needed.
“Our findings are exciting, but still need to be replicated in larger samples with diverse women in order to make sure they generalize,” she said. “More work is needed to understand why inflammation plays a role in postpartum mental illness for some women and not others.”
The study was supported by a Cedars-Sinai Precision Health Grant, the Cousins Center for Psychoneuroimmunology, University of California, Los Angeles, and the National Institute of Mental Health. The investigators and Dr. Payne disclosed no relevant conflicts of interest.
FROM THE AMERICAN JOURNAL OF REPRODUCTIVE IMMUNOLOGY
SSRI tied to improved cognition in comorbid depression, dementia
The results of the 12-week open-label, single-group study are positive, study investigator Michael Cronquist Christensen, MPA, DrPH, a director with the Lundbeck pharmaceutical company, told this news organization before presenting the results in a poster at the 15th Clinical Trials on Alzheimer’s Disease conference.
“The study confirms earlier findings of improvement in both depressive symptoms and cognitive performance with vortioxetine in patients with depression and dementia and adds to this research that these clinical effects also extend to improvement in health-related quality of life and patients’ daily functioning,” Dr. Christensen said.
“It also demonstrates that patients with depression and comorbid dementia can be safely treated with 20 mg vortioxetine – starting dose of 5 mg for the first week and up-titration to 10 mg at day 8,” he added.
However, he reported that Lundbeck doesn’t plan to seek approval from the U.S. Food and Drug Administration for a new indication. Vortioxetine received FDA approval in 2013 to treat MDD, but 3 years later the agency rejected an expansion of its indication to include cognitive dysfunction.
“Vortioxetine is approved for MDD, but the product can be used in patients with MDD who have other diseases, including other mental illnesses,” Dr. Christensen said.
Potential neurotransmission modulator
Vortioxetine is a selective serotonin reuptake inhibitor and serotonin receptor modulator. According to Dr. Christensen, evidence suggests the drug’s receptor targets “have the potential to modulate neurotransmitter systems that are essential for regulation of cognitive function.”
The researchers recruited 83 individuals aged 55-85 with recurrent MDD that had started before the age of 55. All had MDD episodes within the previous 6 months and comorbid dementia for at least 6 months.
Of the participants, 65.9% were female. In addition, 42.7% had Alzheimer’s disease, 26.8% had mixed-type dementia, and the rest had other types of dementia.
The daily oral dose of vortioxetine started at 5 mg for up to week 1 and then was increased to 10 mg. It was then increased to 20 mg or decreased to 5 mg “based on investigator judgment and patient response.” The average daily dose was 12.3 mg.
In regard to the primary outcome, at week 12 (n = 70), scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) fell by a mean of –12.4 (.78, P < .0001), which researchers deemed to be a significant reduction in severe symptoms.
“A significant and clinically meaningful effect was observed from week 1,” the researchers reported.
“As a basis for comparison, we typically see an improvement around 13-14 points during 8 weeks of antidepressant treatment in adults with MDD who do not have dementia,” Dr. Christensen added.
More than a third of patients (35.7%) saw a reduction in MADRS score by more than 50% at week 12, and 17.2% were considered to have reached MDD depression remission, defined as a MADRS score at or under 10.
For secondary outcomes, the total Digit Symbol Substitution test score grew by 0.65 (standardized effect size) by week 12, showing significant improvement (P < .0001). In addition, participants improved on some other cognitive measures, and Dr. Christensen noted that “significant improvement was also observed in the patients’ health-related quality of life and daily functioning.”
A third of patients had drug-related treatment-emergent adverse events.
Vortioxetine is one of the most expensive antidepressants: It has a list price of $444 a month, and no generic version is currently available.
Small trial, open-label design
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, said the study “reflects a valuable aspect of treatment research because of the close connection between depression and dementia. Depression is a known risk factor for dementia, including Alzheimer’s disease, and those who have dementia may experience depression.”
She cautioned, however, that the trial was small and had an open-label design instead of the “gold standard” of a double-blinded trial with a control group.
The study was funded by Lundbeck, where Dr. Christensen is an employee. Another author is a Lundbeck employee, and a third author reported various disclosures. Dr. Sexton reported no disclosures.
A version of this article first appeared on Medscape.com.
The results of the 12-week open-label, single-group study are positive, study investigator Michael Cronquist Christensen, MPA, DrPH, a director with the Lundbeck pharmaceutical company, told this news organization before presenting the results in a poster at the 15th Clinical Trials on Alzheimer’s Disease conference.
“The study confirms earlier findings of improvement in both depressive symptoms and cognitive performance with vortioxetine in patients with depression and dementia and adds to this research that these clinical effects also extend to improvement in health-related quality of life and patients’ daily functioning,” Dr. Christensen said.
“It also demonstrates that patients with depression and comorbid dementia can be safely treated with 20 mg vortioxetine – starting dose of 5 mg for the first week and up-titration to 10 mg at day 8,” he added.
However, he reported that Lundbeck doesn’t plan to seek approval from the U.S. Food and Drug Administration for a new indication. Vortioxetine received FDA approval in 2013 to treat MDD, but 3 years later the agency rejected an expansion of its indication to include cognitive dysfunction.
“Vortioxetine is approved for MDD, but the product can be used in patients with MDD who have other diseases, including other mental illnesses,” Dr. Christensen said.
Potential neurotransmission modulator
Vortioxetine is a selective serotonin reuptake inhibitor and serotonin receptor modulator. According to Dr. Christensen, evidence suggests the drug’s receptor targets “have the potential to modulate neurotransmitter systems that are essential for regulation of cognitive function.”
The researchers recruited 83 individuals aged 55-85 with recurrent MDD that had started before the age of 55. All had MDD episodes within the previous 6 months and comorbid dementia for at least 6 months.
Of the participants, 65.9% were female. In addition, 42.7% had Alzheimer’s disease, 26.8% had mixed-type dementia, and the rest had other types of dementia.
The daily oral dose of vortioxetine started at 5 mg for up to week 1 and then was increased to 10 mg. It was then increased to 20 mg or decreased to 5 mg “based on investigator judgment and patient response.” The average daily dose was 12.3 mg.
In regard to the primary outcome, at week 12 (n = 70), scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) fell by a mean of –12.4 (.78, P < .0001), which researchers deemed to be a significant reduction in severe symptoms.
“A significant and clinically meaningful effect was observed from week 1,” the researchers reported.
“As a basis for comparison, we typically see an improvement around 13-14 points during 8 weeks of antidepressant treatment in adults with MDD who do not have dementia,” Dr. Christensen added.
More than a third of patients (35.7%) saw a reduction in MADRS score by more than 50% at week 12, and 17.2% were considered to have reached MDD depression remission, defined as a MADRS score at or under 10.
For secondary outcomes, the total Digit Symbol Substitution test score grew by 0.65 (standardized effect size) by week 12, showing significant improvement (P < .0001). In addition, participants improved on some other cognitive measures, and Dr. Christensen noted that “significant improvement was also observed in the patients’ health-related quality of life and daily functioning.”
A third of patients had drug-related treatment-emergent adverse events.
Vortioxetine is one of the most expensive antidepressants: It has a list price of $444 a month, and no generic version is currently available.
Small trial, open-label design
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, said the study “reflects a valuable aspect of treatment research because of the close connection between depression and dementia. Depression is a known risk factor for dementia, including Alzheimer’s disease, and those who have dementia may experience depression.”
She cautioned, however, that the trial was small and had an open-label design instead of the “gold standard” of a double-blinded trial with a control group.
The study was funded by Lundbeck, where Dr. Christensen is an employee. Another author is a Lundbeck employee, and a third author reported various disclosures. Dr. Sexton reported no disclosures.
A version of this article first appeared on Medscape.com.
The results of the 12-week open-label, single-group study are positive, study investigator Michael Cronquist Christensen, MPA, DrPH, a director with the Lundbeck pharmaceutical company, told this news organization before presenting the results in a poster at the 15th Clinical Trials on Alzheimer’s Disease conference.
“The study confirms earlier findings of improvement in both depressive symptoms and cognitive performance with vortioxetine in patients with depression and dementia and adds to this research that these clinical effects also extend to improvement in health-related quality of life and patients’ daily functioning,” Dr. Christensen said.
“It also demonstrates that patients with depression and comorbid dementia can be safely treated with 20 mg vortioxetine – starting dose of 5 mg for the first week and up-titration to 10 mg at day 8,” he added.
However, he reported that Lundbeck doesn’t plan to seek approval from the U.S. Food and Drug Administration for a new indication. Vortioxetine received FDA approval in 2013 to treat MDD, but 3 years later the agency rejected an expansion of its indication to include cognitive dysfunction.
“Vortioxetine is approved for MDD, but the product can be used in patients with MDD who have other diseases, including other mental illnesses,” Dr. Christensen said.
Potential neurotransmission modulator
Vortioxetine is a selective serotonin reuptake inhibitor and serotonin receptor modulator. According to Dr. Christensen, evidence suggests the drug’s receptor targets “have the potential to modulate neurotransmitter systems that are essential for regulation of cognitive function.”
The researchers recruited 83 individuals aged 55-85 with recurrent MDD that had started before the age of 55. All had MDD episodes within the previous 6 months and comorbid dementia for at least 6 months.
Of the participants, 65.9% were female. In addition, 42.7% had Alzheimer’s disease, 26.8% had mixed-type dementia, and the rest had other types of dementia.
The daily oral dose of vortioxetine started at 5 mg for up to week 1 and then was increased to 10 mg. It was then increased to 20 mg or decreased to 5 mg “based on investigator judgment and patient response.” The average daily dose was 12.3 mg.
In regard to the primary outcome, at week 12 (n = 70), scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) fell by a mean of –12.4 (.78, P < .0001), which researchers deemed to be a significant reduction in severe symptoms.
“A significant and clinically meaningful effect was observed from week 1,” the researchers reported.
“As a basis for comparison, we typically see an improvement around 13-14 points during 8 weeks of antidepressant treatment in adults with MDD who do not have dementia,” Dr. Christensen added.
More than a third of patients (35.7%) saw a reduction in MADRS score by more than 50% at week 12, and 17.2% were considered to have reached MDD depression remission, defined as a MADRS score at or under 10.
For secondary outcomes, the total Digit Symbol Substitution test score grew by 0.65 (standardized effect size) by week 12, showing significant improvement (P < .0001). In addition, participants improved on some other cognitive measures, and Dr. Christensen noted that “significant improvement was also observed in the patients’ health-related quality of life and daily functioning.”
A third of patients had drug-related treatment-emergent adverse events.
Vortioxetine is one of the most expensive antidepressants: It has a list price of $444 a month, and no generic version is currently available.
Small trial, open-label design
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, said the study “reflects a valuable aspect of treatment research because of the close connection between depression and dementia. Depression is a known risk factor for dementia, including Alzheimer’s disease, and those who have dementia may experience depression.”
She cautioned, however, that the trial was small and had an open-label design instead of the “gold standard” of a double-blinded trial with a control group.
The study was funded by Lundbeck, where Dr. Christensen is an employee. Another author is a Lundbeck employee, and a third author reported various disclosures. Dr. Sexton reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM CTAD 2022
Let people take illegal drugs under medical supervision?
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m the director of the division of medical ethics at New York University.
One is up in Washington Heights in Manhattan; the other, I believe, is over in Harlem.
These two centers will supervise people taking drugs. They have available all of the anti-overdose medications, such as Narcan. If you overdose, they will help you and try to counsel you to get off drugs, but they don’t insist that you do so. You can go there, even if you’re an addict, and continue to take drugs under supervision. This is called a risk-reduction strategy.
Some people note that there are over 100 centers like this worldwide. They’re in Canada, Switzerland, and many other countries, and they seem to work. “Working” means more people seem to come off drugs slowly – not huge numbers, but some – than if you don’t do something like this, and death rates from overdose go way down.
By the way, having these centers in place has other benefits. They save money because when someone overdoses out in the community, you have to pay all the costs of the ambulances and emergency rooms, and there are risks to the first responders due to fentanyl or other things. There are fewer syringes littering parks and public places where people shoot up. You have everything controlled when they come into a center, so that’s less burden on the community.
It turns out that you have less crime because people just aren’t out there harming or robbing other people to get money to get their next fix. The drugs are provided for them. Crime rates in neighborhoods around the world where these centers operate seem to dip. There are many positives.
There are also some negatives. People say it shouldn’t be the job of the state to keep people addicted. It’s just not the right role. Everything should be aimed at getting people off drugs, maybe including criminal penalties if that’s what it takes to get them to stop using.
My own view is that hasn’t worked. Implementing tough prison sentences in trying to fight the war on drugs just doesn’t seem to work. We had 100,000 deaths last year from drug overdoses. That number has been climbing. We all know that we’ve got a terrible epidemic of deaths due to drug overdose.
It seems to me that these centers that are involved in risk reduction are a better option for now, until we figure out some interventions that can cut the desire or the drive to use drugs, or antidotes that are effective for months or years, to prevent people from getting high no matter what drugs they take.
I’m going to come out and say that I think the New York experiment has worked. I think it has saved upward of 600 lives, they estimate, in the past year that would have been overdoses. I think costwise, it’s effective. [Reductions in] related damages and injuries from syringes being scattered around, and robbery, and so forth, are all to the good. There are even a few people coming off drugs due to counseling, which is a better outcome than we get when they’re just out in the streets.
I think other cities want to try this. I know Philadelphia does. I know New York wants to expand its program. The federal government isn’t sure, but I think the time has come to try an expansion. I think we’ve got something that – although far from perfect and I wish we had other tools – may be the best we’ve got. In the war on drugs, little victories ought to be reinforced.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m the director of the division of medical ethics at New York University.
One is up in Washington Heights in Manhattan; the other, I believe, is over in Harlem.
These two centers will supervise people taking drugs. They have available all of the anti-overdose medications, such as Narcan. If you overdose, they will help you and try to counsel you to get off drugs, but they don’t insist that you do so. You can go there, even if you’re an addict, and continue to take drugs under supervision. This is called a risk-reduction strategy.
Some people note that there are over 100 centers like this worldwide. They’re in Canada, Switzerland, and many other countries, and they seem to work. “Working” means more people seem to come off drugs slowly – not huge numbers, but some – than if you don’t do something like this, and death rates from overdose go way down.
By the way, having these centers in place has other benefits. They save money because when someone overdoses out in the community, you have to pay all the costs of the ambulances and emergency rooms, and there are risks to the first responders due to fentanyl or other things. There are fewer syringes littering parks and public places where people shoot up. You have everything controlled when they come into a center, so that’s less burden on the community.
It turns out that you have less crime because people just aren’t out there harming or robbing other people to get money to get their next fix. The drugs are provided for them. Crime rates in neighborhoods around the world where these centers operate seem to dip. There are many positives.
There are also some negatives. People say it shouldn’t be the job of the state to keep people addicted. It’s just not the right role. Everything should be aimed at getting people off drugs, maybe including criminal penalties if that’s what it takes to get them to stop using.
My own view is that hasn’t worked. Implementing tough prison sentences in trying to fight the war on drugs just doesn’t seem to work. We had 100,000 deaths last year from drug overdoses. That number has been climbing. We all know that we’ve got a terrible epidemic of deaths due to drug overdose.
It seems to me that these centers that are involved in risk reduction are a better option for now, until we figure out some interventions that can cut the desire or the drive to use drugs, or antidotes that are effective for months or years, to prevent people from getting high no matter what drugs they take.
I’m going to come out and say that I think the New York experiment has worked. I think it has saved upward of 600 lives, they estimate, in the past year that would have been overdoses. I think costwise, it’s effective. [Reductions in] related damages and injuries from syringes being scattered around, and robbery, and so forth, are all to the good. There are even a few people coming off drugs due to counseling, which is a better outcome than we get when they’re just out in the streets.
I think other cities want to try this. I know Philadelphia does. I know New York wants to expand its program. The federal government isn’t sure, but I think the time has come to try an expansion. I think we’ve got something that – although far from perfect and I wish we had other tools – may be the best we’ve got. In the war on drugs, little victories ought to be reinforced.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m the director of the division of medical ethics at New York University.
One is up in Washington Heights in Manhattan; the other, I believe, is over in Harlem.
These two centers will supervise people taking drugs. They have available all of the anti-overdose medications, such as Narcan. If you overdose, they will help you and try to counsel you to get off drugs, but they don’t insist that you do so. You can go there, even if you’re an addict, and continue to take drugs under supervision. This is called a risk-reduction strategy.
Some people note that there are over 100 centers like this worldwide. They’re in Canada, Switzerland, and many other countries, and they seem to work. “Working” means more people seem to come off drugs slowly – not huge numbers, but some – than if you don’t do something like this, and death rates from overdose go way down.
By the way, having these centers in place has other benefits. They save money because when someone overdoses out in the community, you have to pay all the costs of the ambulances and emergency rooms, and there are risks to the first responders due to fentanyl or other things. There are fewer syringes littering parks and public places where people shoot up. You have everything controlled when they come into a center, so that’s less burden on the community.
It turns out that you have less crime because people just aren’t out there harming or robbing other people to get money to get their next fix. The drugs are provided for them. Crime rates in neighborhoods around the world where these centers operate seem to dip. There are many positives.
There are also some negatives. People say it shouldn’t be the job of the state to keep people addicted. It’s just not the right role. Everything should be aimed at getting people off drugs, maybe including criminal penalties if that’s what it takes to get them to stop using.
My own view is that hasn’t worked. Implementing tough prison sentences in trying to fight the war on drugs just doesn’t seem to work. We had 100,000 deaths last year from drug overdoses. That number has been climbing. We all know that we’ve got a terrible epidemic of deaths due to drug overdose.
It seems to me that these centers that are involved in risk reduction are a better option for now, until we figure out some interventions that can cut the desire or the drive to use drugs, or antidotes that are effective for months or years, to prevent people from getting high no matter what drugs they take.
I’m going to come out and say that I think the New York experiment has worked. I think it has saved upward of 600 lives, they estimate, in the past year that would have been overdoses. I think costwise, it’s effective. [Reductions in] related damages and injuries from syringes being scattered around, and robbery, and so forth, are all to the good. There are even a few people coming off drugs due to counseling, which is a better outcome than we get when they’re just out in the streets.
I think other cities want to try this. I know Philadelphia does. I know New York wants to expand its program. The federal government isn’t sure, but I think the time has come to try an expansion. I think we’ve got something that – although far from perfect and I wish we had other tools – may be the best we’ve got. In the war on drugs, little victories ought to be reinforced.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
Why doctors are losing trust in patients; what should be done?
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
Mood stabilizers, particularly lithium, potential lifesavers in bipolar disorder
Investigators led by Pao-Huan Chen, MD, of the department of psychiatry, Taipei Medical University Hospital, Taiwan, evaluated the association between the use of mood stabilizers and the risks for all-cause mortality, suicide, and natural mortality in more than 25,000 patients with BD and found that those with BD had higher mortality.
However, they also found that patients with BD had a significantly decreased adjusted 5-year risk of dying from any cause, suicide, and natural causes. Lithium was associated with the largest risk reduction compared with the other mood stabilizers.
“The present findings highlight the potential role of mood stabilizers, particularly lithium, in reducing mortality among patients with bipolar disorder,” the authors write.
“The findings of this study could inform future clinical and mechanistic research evaluating the multifaceted effects of mood stabilizers, particularly lithium, on the psychological and physiological statuses of patients with bipolar disorder,” they add.
The study was published online in Acta Psychiatrica Scandinavica.
Research gap
Patients with BD have an elevated risk for multiple comorbidities in addition to mood symptoms and neurocognitive dysfunction, with previous research suggesting a mortality rate due to suicide and natural causes that is at least twice as high as that of the general population, the authors write.
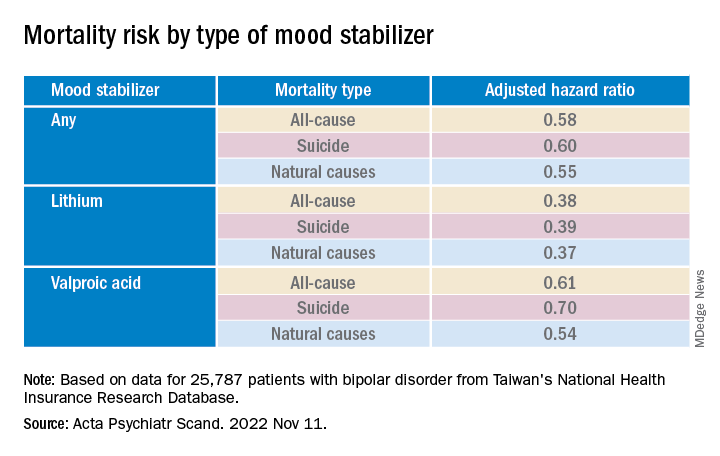
Lithium, in particular, has been associated with decreased risk for all-cause mortality and suicide in patients with BD, but findings regarding anticonvulsant mood stabilizers have been “inconsistent.”
To fill this research gap, the researchers evaluated 16 years of data from Taiwan’s National Health Insurance Research Database, which includes information about more than 23 million residents of Taiwan. The current study, which encompassed 25,787 patients with BD, looked at data from the 5-year period after index hospitalization.
The researchers hypothesized that mood stabilizers “would decrease the risk of mortality” among patients with BD and that “different mood stabilizers would exhibit different associations with mortality, owing to their varying effects on mood symptoms and physiological function.”
Covariates included sex, age, employment status, comorbidities, and concomitant drugs.
Of the patients with BD, 4,000 died within the 5-year period. Suicide and natural causes accounted for 19.0% and 73.7% of these deaths, respectively.
Cardioprotective effects?
The standardized mortality ratios (SMRs) – the ratios of observed mortality in the BD cohort to the number of expected deaths in the general population – were 5.26 for all causes (95% confidence interval, 5.10-5.43), 26.02 for suicide (95% CI, 24.20-27.93), and 4.68 for natural causes (95% CI, 4.51-4.85).
The cumulative mortality rate was higher among men vs. women, a difference that was even larger among patients who had died from any cause or natural causes (crude hazard ratios, .60 and .52, respectively; both Ps < .001).
The suicide risk peaked between ages 45 and 65 years, whereas the risks for all-cause and natural mortality increased with age and were highest in those older than 65 years.
Patients who had died from any cause or from natural causes had a higher risk for physical and psychiatric comorbidities, whereas those who had died by suicide had a higher risk for primarily psychiatric comorbidities.
Mood stabilizers were associated with decreased risks for all-cause mortality and natural mortality, with lithium and valproic acid tied to the lowest risk for all three mortality types (all Ps < .001).
Lamotrigine and carbamazepine were “not significantly associated with any type of mortality,” the authors report.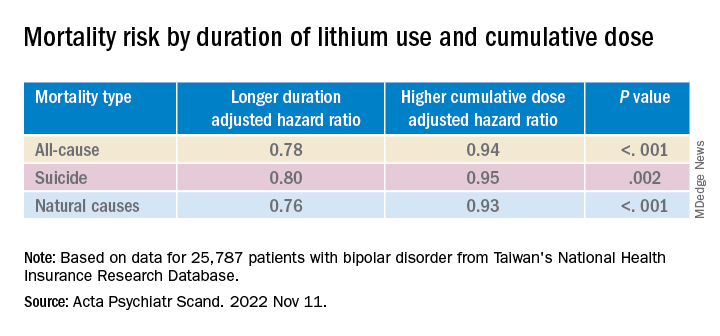
Longer duration of lithium use and a higher cumulative dose of lithium were both associated with lower risks for all three types of mortality (all Ps < .001).
Valproic acid was associated with dose-dependent decreases in all-cause and natural mortality risks.
The findings suggest that mood stabilizers “may improve not only psychosocial outcomes but also the physical health of patients with BD,” the investigators note.
The association between mood stabilizer use and reduced natural mortality risk “may be attributable to the potential benefits of psychiatric care” but may also “have resulted from the direct effects of mood stabilizers on physiological functions,” they add.
Some research suggests lithium treatment may reduce the risk for cardiovascular disease in patients with BD. Mechanistic studies have also pointed to potential cardioprotective effects from valproic acid.
The authors note several study limitations. Focusing on hospitalized patients “may have led to selection bias and overestimated mortality risk.” Moreover, the analyses were “based on the prescription, not the consumption, of mood stabilizers” and information regarding adherence was unavailable.
The absence of a protective mechanism of lamotrigine and carbamazepine may be attributable to “bias toward the relatively poor treatment responses” of these agents, neither of which is used as a first-line medication to treat BD in Taiwan. Patients taking these agents “may not receive medical care at a level equal to those taking lithium, who tend to receive closer surveillance, owing to the narrow therapeutic index.”
First-line treatment
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the data “add to a growing confluence of data from observational studies indicating that lithium especially is capable of reducing all-cause mortality, suicide mortality, and natural mortality.”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, agreed with the authors that lamotrigine is “not a very popular drug in Taiwan, therefore we may not have sufficient assay sensitivity to document the effect.”
But lamotrigine “does have recurrence prevention effects in BD, especially bipolar depression, and it would be expected that it would reduce suicide potentially especially in such a large sample.”
The study’s take-home message “is that the extant evidence now indicates that lithium should be a first-line treatment in persons who live with BD who are experiencing suicidal ideation and/or behavior and these data should inform algorithms of treatment selection and sequencing in clinical practice guidelines,” said Dr. McIntyre.
This research was supported by grants from the Ministry of Science and Technology in Taiwan and Taipei City Hospital. The authors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China, and the Milken Institute; and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe Pharma, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators led by Pao-Huan Chen, MD, of the department of psychiatry, Taipei Medical University Hospital, Taiwan, evaluated the association between the use of mood stabilizers and the risks for all-cause mortality, suicide, and natural mortality in more than 25,000 patients with BD and found that those with BD had higher mortality.
However, they also found that patients with BD had a significantly decreased adjusted 5-year risk of dying from any cause, suicide, and natural causes. Lithium was associated with the largest risk reduction compared with the other mood stabilizers.
“The present findings highlight the potential role of mood stabilizers, particularly lithium, in reducing mortality among patients with bipolar disorder,” the authors write.
“The findings of this study could inform future clinical and mechanistic research evaluating the multifaceted effects of mood stabilizers, particularly lithium, on the psychological and physiological statuses of patients with bipolar disorder,” they add.
The study was published online in Acta Psychiatrica Scandinavica.
Research gap
Patients with BD have an elevated risk for multiple comorbidities in addition to mood symptoms and neurocognitive dysfunction, with previous research suggesting a mortality rate due to suicide and natural causes that is at least twice as high as that of the general population, the authors write.

Lithium, in particular, has been associated with decreased risk for all-cause mortality and suicide in patients with BD, but findings regarding anticonvulsant mood stabilizers have been “inconsistent.”
To fill this research gap, the researchers evaluated 16 years of data from Taiwan’s National Health Insurance Research Database, which includes information about more than 23 million residents of Taiwan. The current study, which encompassed 25,787 patients with BD, looked at data from the 5-year period after index hospitalization.
The researchers hypothesized that mood stabilizers “would decrease the risk of mortality” among patients with BD and that “different mood stabilizers would exhibit different associations with mortality, owing to their varying effects on mood symptoms and physiological function.”
Covariates included sex, age, employment status, comorbidities, and concomitant drugs.
Of the patients with BD, 4,000 died within the 5-year period. Suicide and natural causes accounted for 19.0% and 73.7% of these deaths, respectively.
Cardioprotective effects?
The standardized mortality ratios (SMRs) – the ratios of observed mortality in the BD cohort to the number of expected deaths in the general population – were 5.26 for all causes (95% confidence interval, 5.10-5.43), 26.02 for suicide (95% CI, 24.20-27.93), and 4.68 for natural causes (95% CI, 4.51-4.85).
The cumulative mortality rate was higher among men vs. women, a difference that was even larger among patients who had died from any cause or natural causes (crude hazard ratios, .60 and .52, respectively; both Ps < .001).
The suicide risk peaked between ages 45 and 65 years, whereas the risks for all-cause and natural mortality increased with age and were highest in those older than 65 years.
Patients who had died from any cause or from natural causes had a higher risk for physical and psychiatric comorbidities, whereas those who had died by suicide had a higher risk for primarily psychiatric comorbidities.
Mood stabilizers were associated with decreased risks for all-cause mortality and natural mortality, with lithium and valproic acid tied to the lowest risk for all three mortality types (all Ps < .001).
Lamotrigine and carbamazepine were “not significantly associated with any type of mortality,” the authors report.
Longer duration of lithium use and a higher cumulative dose of lithium were both associated with lower risks for all three types of mortality (all Ps < .001).
Valproic acid was associated with dose-dependent decreases in all-cause and natural mortality risks.
The findings suggest that mood stabilizers “may improve not only psychosocial outcomes but also the physical health of patients with BD,” the investigators note.
The association between mood stabilizer use and reduced natural mortality risk “may be attributable to the potential benefits of psychiatric care” but may also “have resulted from the direct effects of mood stabilizers on physiological functions,” they add.
Some research suggests lithium treatment may reduce the risk for cardiovascular disease in patients with BD. Mechanistic studies have also pointed to potential cardioprotective effects from valproic acid.
The authors note several study limitations. Focusing on hospitalized patients “may have led to selection bias and overestimated mortality risk.” Moreover, the analyses were “based on the prescription, not the consumption, of mood stabilizers” and information regarding adherence was unavailable.
The absence of a protective mechanism of lamotrigine and carbamazepine may be attributable to “bias toward the relatively poor treatment responses” of these agents, neither of which is used as a first-line medication to treat BD in Taiwan. Patients taking these agents “may not receive medical care at a level equal to those taking lithium, who tend to receive closer surveillance, owing to the narrow therapeutic index.”
First-line treatment
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the data “add to a growing confluence of data from observational studies indicating that lithium especially is capable of reducing all-cause mortality, suicide mortality, and natural mortality.”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, agreed with the authors that lamotrigine is “not a very popular drug in Taiwan, therefore we may not have sufficient assay sensitivity to document the effect.”
But lamotrigine “does have recurrence prevention effects in BD, especially bipolar depression, and it would be expected that it would reduce suicide potentially especially in such a large sample.”
The study’s take-home message “is that the extant evidence now indicates that lithium should be a first-line treatment in persons who live with BD who are experiencing suicidal ideation and/or behavior and these data should inform algorithms of treatment selection and sequencing in clinical practice guidelines,” said Dr. McIntyre.
This research was supported by grants from the Ministry of Science and Technology in Taiwan and Taipei City Hospital. The authors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China, and the Milken Institute; and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe Pharma, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators led by Pao-Huan Chen, MD, of the department of psychiatry, Taipei Medical University Hospital, Taiwan, evaluated the association between the use of mood stabilizers and the risks for all-cause mortality, suicide, and natural mortality in more than 25,000 patients with BD and found that those with BD had higher mortality.
However, they also found that patients with BD had a significantly decreased adjusted 5-year risk of dying from any cause, suicide, and natural causes. Lithium was associated with the largest risk reduction compared with the other mood stabilizers.
“The present findings highlight the potential role of mood stabilizers, particularly lithium, in reducing mortality among patients with bipolar disorder,” the authors write.
“The findings of this study could inform future clinical and mechanistic research evaluating the multifaceted effects of mood stabilizers, particularly lithium, on the psychological and physiological statuses of patients with bipolar disorder,” they add.
The study was published online in Acta Psychiatrica Scandinavica.
Research gap
Patients with BD have an elevated risk for multiple comorbidities in addition to mood symptoms and neurocognitive dysfunction, with previous research suggesting a mortality rate due to suicide and natural causes that is at least twice as high as that of the general population, the authors write.

Lithium, in particular, has been associated with decreased risk for all-cause mortality and suicide in patients with BD, but findings regarding anticonvulsant mood stabilizers have been “inconsistent.”
To fill this research gap, the researchers evaluated 16 years of data from Taiwan’s National Health Insurance Research Database, which includes information about more than 23 million residents of Taiwan. The current study, which encompassed 25,787 patients with BD, looked at data from the 5-year period after index hospitalization.
The researchers hypothesized that mood stabilizers “would decrease the risk of mortality” among patients with BD and that “different mood stabilizers would exhibit different associations with mortality, owing to their varying effects on mood symptoms and physiological function.”
Covariates included sex, age, employment status, comorbidities, and concomitant drugs.
Of the patients with BD, 4,000 died within the 5-year period. Suicide and natural causes accounted for 19.0% and 73.7% of these deaths, respectively.
Cardioprotective effects?
The standardized mortality ratios (SMRs) – the ratios of observed mortality in the BD cohort to the number of expected deaths in the general population – were 5.26 for all causes (95% confidence interval, 5.10-5.43), 26.02 for suicide (95% CI, 24.20-27.93), and 4.68 for natural causes (95% CI, 4.51-4.85).
The cumulative mortality rate was higher among men vs. women, a difference that was even larger among patients who had died from any cause or natural causes (crude hazard ratios, .60 and .52, respectively; both Ps < .001).
The suicide risk peaked between ages 45 and 65 years, whereas the risks for all-cause and natural mortality increased with age and were highest in those older than 65 years.
Patients who had died from any cause or from natural causes had a higher risk for physical and psychiatric comorbidities, whereas those who had died by suicide had a higher risk for primarily psychiatric comorbidities.
Mood stabilizers were associated with decreased risks for all-cause mortality and natural mortality, with lithium and valproic acid tied to the lowest risk for all three mortality types (all Ps < .001).
Lamotrigine and carbamazepine were “not significantly associated with any type of mortality,” the authors report.
Longer duration of lithium use and a higher cumulative dose of lithium were both associated with lower risks for all three types of mortality (all Ps < .001).
Valproic acid was associated with dose-dependent decreases in all-cause and natural mortality risks.
The findings suggest that mood stabilizers “may improve not only psychosocial outcomes but also the physical health of patients with BD,” the investigators note.
The association between mood stabilizer use and reduced natural mortality risk “may be attributable to the potential benefits of psychiatric care” but may also “have resulted from the direct effects of mood stabilizers on physiological functions,” they add.
Some research suggests lithium treatment may reduce the risk for cardiovascular disease in patients with BD. Mechanistic studies have also pointed to potential cardioprotective effects from valproic acid.
The authors note several study limitations. Focusing on hospitalized patients “may have led to selection bias and overestimated mortality risk.” Moreover, the analyses were “based on the prescription, not the consumption, of mood stabilizers” and information regarding adherence was unavailable.
The absence of a protective mechanism of lamotrigine and carbamazepine may be attributable to “bias toward the relatively poor treatment responses” of these agents, neither of which is used as a first-line medication to treat BD in Taiwan. Patients taking these agents “may not receive medical care at a level equal to those taking lithium, who tend to receive closer surveillance, owing to the narrow therapeutic index.”
First-line treatment
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the data “add to a growing confluence of data from observational studies indicating that lithium especially is capable of reducing all-cause mortality, suicide mortality, and natural mortality.”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, agreed with the authors that lamotrigine is “not a very popular drug in Taiwan, therefore we may not have sufficient assay sensitivity to document the effect.”
But lamotrigine “does have recurrence prevention effects in BD, especially bipolar depression, and it would be expected that it would reduce suicide potentially especially in such a large sample.”
The study’s take-home message “is that the extant evidence now indicates that lithium should be a first-line treatment in persons who live with BD who are experiencing suicidal ideation and/or behavior and these data should inform algorithms of treatment selection and sequencing in clinical practice guidelines,” said Dr. McIntyre.
This research was supported by grants from the Ministry of Science and Technology in Taiwan and Taipei City Hospital. The authors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China, and the Milken Institute; and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe Pharma, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
FROM ACTA PSYCHIATRICA SCANDINAVICA







