User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Digital treatment may help relieve PTSD, panic disorder
The 28-day home-based treatment, known as the capnometry guided respiratory intervention (CGRI), uses an app-based feedback protocol to normalize respiration and increase patients’ ability to cope with symptoms of stress, anxiety, and panic by providing real time breath-to-breath feedback of respiratory rate and carbon dioxide (CO2) levels via a nasal cannula.
Results from the large real-world study showed that over 65% of patients with PD and over 72% of those with PTSD responded to the treatment. In addition, almost 75% of participants adhered to the study protocol, with low dropout rates.
“The brief duration of treatment, high adherence rates, and clinical benefit suggests that CGRI provides an important addition to treatment options for PD and PTSD,” the investigators write.
The study was published online in Frontiers in Digital Health.
‘New kid on the block’
The “respiratory dysregulation hypothesis” links CO2 sensitivity to panic attacks and PD, and similar reactivity has been identified in PTSD, but a “common limitation of psychotherapeutic and pharmacologic approaches to PD and PTSD is that neither address the role of respiratory physiology and breathing style,” the investigators note.
The most widely studied treatment for PTSD is trauma-focused psychotherapy, in which the patient reviews and revisits the trauma, but it has a high dropout rate, study investigator Michael Telch, PhD, director of the Laboratory for the Study of Anxiety Disorders, University of Texas, Austin, told this news organization.
He described CGRI for PTSD as a “relatively new kid on the block, so to speak.” The intervention was cleared by the U.S. Food and Drug Administration for treatment of PD and PTSD in 2013 and 2018, respectively, and is currently available through the Veterans Administration for veterans with PTSD. It is also covered by some commercial insurance plans.
“The underlying assumption [of CGRI] is that a person can learn to develop skills for controlling some of their physiological reactions that are triggered as a result of trauma,” said Dr. Telch.
The device uses a biofeedback approach to give patients “greater control over their physiological reactions, such as hyperventilation and increased respiration rate, and the focus is on providing a sense of mastery,” he said.
Participants with PTSD were assigned to a health coach. The device was delivered to the patient’s home, and patients met with the trained coach weekly and could check in between visits via text or e-mail. Twice-daily sessions were recommended.
“The coach gets feedback about what’s happening with the patient’s respiration and end-tidal CO2 levels [etCO2] and instructs participants how to keep their respiration rate and etCO2 at a more normal level,” said Dr. Telch.
The CGRI “teaches a specific breathing style via a system providing real-time feedback of respiratory rate (RR) and exhaled carbon dioxide levels facilitated by data capture,” the authors note.
Sense of mastery
Of the 1,569 participants, 1,395 had PD and 174 had PTSD (mean age, 39.2 [standard deviation, 13.9] years and 40.9 [SD, 14.9] years, respectively; 76% and 73% female, respectively). Those with PD completed the Panic Disorder Severity Scale (PDSS) and those with PTSD completed the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), before and after the intervention.
The treatment response rate for PD was defined as a 40% or greater reduction in PDSS total scores, whereas treatment response rate for PTSD was defined as a 10-point or greater reduction in PCL-5 scores.
At baseline, patients were classified either as normocapnic or hypocapnic (etCO2 ≥ 37 or < 37, respectively), with 65% classified as normocapnic and 35% classified as hypocapnic.
Among patients with PD, there was a 50.2% mean pre- to posttreatment reduction in total PDSS scores (P < .001; d = 1.31), with a treatment response rate of 65.3% of patients.
Among patients with PTSD, there was a 41.1% pre- to posttreatment reduction in total PCL-5 scores (P < .001; d = 1.16), with a treatment response rate of 72.4%.
When investigators analyzed the response at the individual level, they found that 55.7% of patients with PD and 53.5% of those with PTSD were classified as treatment responders. This determination was based on a two-pronged approach that first calculated the Reliable Change Index (RCI) for each participant, and, in participants showing statistically reliable improvement, whether the posttreatment score was closer to the distribution of scores for patients without or with the given disorder.
“Patients with both normal and below-normal baseline exhaled CO2 levels experienced comparable benefit,” the authors report.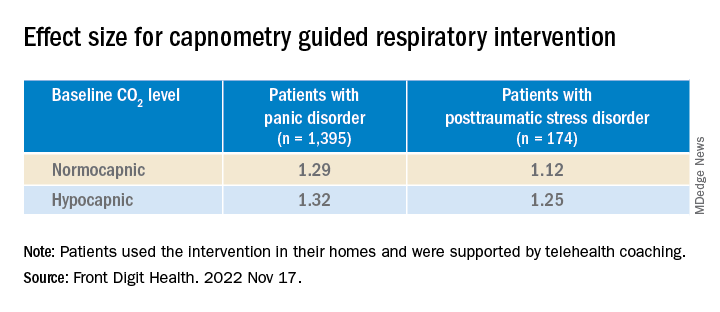
There were high levels of adherence across the full treatment period in both the PD and the PTSD groups (74.8% and 74.9%, respectively), with low dropout rates (10% and 11%, respectively).
“Not every single patient who undergoes any treatment has a perfect response, but the response rates to this treatment have, surprisingly, been quite positive and there have been no negative side effects,” Dr. Telch remarked.
He noted that one of the effects of PTSD is that the “patient has negative beliefs about their ability to control the world. ‘I can’t control my reactions. At any time, I could have a flashback.’ Helping the patient to develop any sense of mastery over some of their reactions can spill over and give them a greater sense of mastery and control, which can have a positive effect in reducing PTSD symptoms.”
‘A viable alternative’
Commenting on the research, Charles Marmar, MD, chair and Peter H. Schub Professor of Psychiatry, department of psychiatry, New York University, said that the study has some limitations, probably the most significant of which is that most participants had normal baseline CO2 levels.
“The treatment is fundamentally designed for people who hyperventilate and blow off too much CO2 so they can breathe in a more calm, relaxed way, but most people in the trial had normal CO2 to begin with,” said Dr. Marmar, who was not involved with the study.
“It’s likely that the major benefits were the relaxation from doing the breathing exercises rather than the change in CO2 levels,” he speculated.
The treatment is “probably a good thing for those patients who actually have abnormal CO2 levels. This treatment could be used in precision medicine, where you tailor treatments to those who actually need them rather than giving the same treatment to everyone,” he said.
“For patients who don’t respond to trauma-focused therapy or it’s too aversive for them to undergo, this new intervention provides a viable alternative,” Dr. Telch added.
The study was internally funded by Freespira. Dr. Telch is a scientific advisor at Freespira and receives compensation by way of stock options. The other authors’ disclosures are listed on the original paper. Dr. Marmar has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 28-day home-based treatment, known as the capnometry guided respiratory intervention (CGRI), uses an app-based feedback protocol to normalize respiration and increase patients’ ability to cope with symptoms of stress, anxiety, and panic by providing real time breath-to-breath feedback of respiratory rate and carbon dioxide (CO2) levels via a nasal cannula.
Results from the large real-world study showed that over 65% of patients with PD and over 72% of those with PTSD responded to the treatment. In addition, almost 75% of participants adhered to the study protocol, with low dropout rates.
“The brief duration of treatment, high adherence rates, and clinical benefit suggests that CGRI provides an important addition to treatment options for PD and PTSD,” the investigators write.
The study was published online in Frontiers in Digital Health.
‘New kid on the block’
The “respiratory dysregulation hypothesis” links CO2 sensitivity to panic attacks and PD, and similar reactivity has been identified in PTSD, but a “common limitation of psychotherapeutic and pharmacologic approaches to PD and PTSD is that neither address the role of respiratory physiology and breathing style,” the investigators note.
The most widely studied treatment for PTSD is trauma-focused psychotherapy, in which the patient reviews and revisits the trauma, but it has a high dropout rate, study investigator Michael Telch, PhD, director of the Laboratory for the Study of Anxiety Disorders, University of Texas, Austin, told this news organization.
He described CGRI for PTSD as a “relatively new kid on the block, so to speak.” The intervention was cleared by the U.S. Food and Drug Administration for treatment of PD and PTSD in 2013 and 2018, respectively, and is currently available through the Veterans Administration for veterans with PTSD. It is also covered by some commercial insurance plans.
“The underlying assumption [of CGRI] is that a person can learn to develop skills for controlling some of their physiological reactions that are triggered as a result of trauma,” said Dr. Telch.
The device uses a biofeedback approach to give patients “greater control over their physiological reactions, such as hyperventilation and increased respiration rate, and the focus is on providing a sense of mastery,” he said.
Participants with PTSD were assigned to a health coach. The device was delivered to the patient’s home, and patients met with the trained coach weekly and could check in between visits via text or e-mail. Twice-daily sessions were recommended.
“The coach gets feedback about what’s happening with the patient’s respiration and end-tidal CO2 levels [etCO2] and instructs participants how to keep their respiration rate and etCO2 at a more normal level,” said Dr. Telch.
The CGRI “teaches a specific breathing style via a system providing real-time feedback of respiratory rate (RR) and exhaled carbon dioxide levels facilitated by data capture,” the authors note.
Sense of mastery
Of the 1,569 participants, 1,395 had PD and 174 had PTSD (mean age, 39.2 [standard deviation, 13.9] years and 40.9 [SD, 14.9] years, respectively; 76% and 73% female, respectively). Those with PD completed the Panic Disorder Severity Scale (PDSS) and those with PTSD completed the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), before and after the intervention.
The treatment response rate for PD was defined as a 40% or greater reduction in PDSS total scores, whereas treatment response rate for PTSD was defined as a 10-point or greater reduction in PCL-5 scores.
At baseline, patients were classified either as normocapnic or hypocapnic (etCO2 ≥ 37 or < 37, respectively), with 65% classified as normocapnic and 35% classified as hypocapnic.
Among patients with PD, there was a 50.2% mean pre- to posttreatment reduction in total PDSS scores (P < .001; d = 1.31), with a treatment response rate of 65.3% of patients.
Among patients with PTSD, there was a 41.1% pre- to posttreatment reduction in total PCL-5 scores (P < .001; d = 1.16), with a treatment response rate of 72.4%.
When investigators analyzed the response at the individual level, they found that 55.7% of patients with PD and 53.5% of those with PTSD were classified as treatment responders. This determination was based on a two-pronged approach that first calculated the Reliable Change Index (RCI) for each participant, and, in participants showing statistically reliable improvement, whether the posttreatment score was closer to the distribution of scores for patients without or with the given disorder.
“Patients with both normal and below-normal baseline exhaled CO2 levels experienced comparable benefit,” the authors report.
There were high levels of adherence across the full treatment period in both the PD and the PTSD groups (74.8% and 74.9%, respectively), with low dropout rates (10% and 11%, respectively).
“Not every single patient who undergoes any treatment has a perfect response, but the response rates to this treatment have, surprisingly, been quite positive and there have been no negative side effects,” Dr. Telch remarked.
He noted that one of the effects of PTSD is that the “patient has negative beliefs about their ability to control the world. ‘I can’t control my reactions. At any time, I could have a flashback.’ Helping the patient to develop any sense of mastery over some of their reactions can spill over and give them a greater sense of mastery and control, which can have a positive effect in reducing PTSD symptoms.”
‘A viable alternative’
Commenting on the research, Charles Marmar, MD, chair and Peter H. Schub Professor of Psychiatry, department of psychiatry, New York University, said that the study has some limitations, probably the most significant of which is that most participants had normal baseline CO2 levels.
“The treatment is fundamentally designed for people who hyperventilate and blow off too much CO2 so they can breathe in a more calm, relaxed way, but most people in the trial had normal CO2 to begin with,” said Dr. Marmar, who was not involved with the study.
“It’s likely that the major benefits were the relaxation from doing the breathing exercises rather than the change in CO2 levels,” he speculated.
The treatment is “probably a good thing for those patients who actually have abnormal CO2 levels. This treatment could be used in precision medicine, where you tailor treatments to those who actually need them rather than giving the same treatment to everyone,” he said.
“For patients who don’t respond to trauma-focused therapy or it’s too aversive for them to undergo, this new intervention provides a viable alternative,” Dr. Telch added.
The study was internally funded by Freespira. Dr. Telch is a scientific advisor at Freespira and receives compensation by way of stock options. The other authors’ disclosures are listed on the original paper. Dr. Marmar has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 28-day home-based treatment, known as the capnometry guided respiratory intervention (CGRI), uses an app-based feedback protocol to normalize respiration and increase patients’ ability to cope with symptoms of stress, anxiety, and panic by providing real time breath-to-breath feedback of respiratory rate and carbon dioxide (CO2) levels via a nasal cannula.
Results from the large real-world study showed that over 65% of patients with PD and over 72% of those with PTSD responded to the treatment. In addition, almost 75% of participants adhered to the study protocol, with low dropout rates.
“The brief duration of treatment, high adherence rates, and clinical benefit suggests that CGRI provides an important addition to treatment options for PD and PTSD,” the investigators write.
The study was published online in Frontiers in Digital Health.
‘New kid on the block’
The “respiratory dysregulation hypothesis” links CO2 sensitivity to panic attacks and PD, and similar reactivity has been identified in PTSD, but a “common limitation of psychotherapeutic and pharmacologic approaches to PD and PTSD is that neither address the role of respiratory physiology and breathing style,” the investigators note.
The most widely studied treatment for PTSD is trauma-focused psychotherapy, in which the patient reviews and revisits the trauma, but it has a high dropout rate, study investigator Michael Telch, PhD, director of the Laboratory for the Study of Anxiety Disorders, University of Texas, Austin, told this news organization.
He described CGRI for PTSD as a “relatively new kid on the block, so to speak.” The intervention was cleared by the U.S. Food and Drug Administration for treatment of PD and PTSD in 2013 and 2018, respectively, and is currently available through the Veterans Administration for veterans with PTSD. It is also covered by some commercial insurance plans.
“The underlying assumption [of CGRI] is that a person can learn to develop skills for controlling some of their physiological reactions that are triggered as a result of trauma,” said Dr. Telch.
The device uses a biofeedback approach to give patients “greater control over their physiological reactions, such as hyperventilation and increased respiration rate, and the focus is on providing a sense of mastery,” he said.
Participants with PTSD were assigned to a health coach. The device was delivered to the patient’s home, and patients met with the trained coach weekly and could check in between visits via text or e-mail. Twice-daily sessions were recommended.
“The coach gets feedback about what’s happening with the patient’s respiration and end-tidal CO2 levels [etCO2] and instructs participants how to keep their respiration rate and etCO2 at a more normal level,” said Dr. Telch.
The CGRI “teaches a specific breathing style via a system providing real-time feedback of respiratory rate (RR) and exhaled carbon dioxide levels facilitated by data capture,” the authors note.
Sense of mastery
Of the 1,569 participants, 1,395 had PD and 174 had PTSD (mean age, 39.2 [standard deviation, 13.9] years and 40.9 [SD, 14.9] years, respectively; 76% and 73% female, respectively). Those with PD completed the Panic Disorder Severity Scale (PDSS) and those with PTSD completed the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), before and after the intervention.
The treatment response rate for PD was defined as a 40% or greater reduction in PDSS total scores, whereas treatment response rate for PTSD was defined as a 10-point or greater reduction in PCL-5 scores.
At baseline, patients were classified either as normocapnic or hypocapnic (etCO2 ≥ 37 or < 37, respectively), with 65% classified as normocapnic and 35% classified as hypocapnic.
Among patients with PD, there was a 50.2% mean pre- to posttreatment reduction in total PDSS scores (P < .001; d = 1.31), with a treatment response rate of 65.3% of patients.
Among patients with PTSD, there was a 41.1% pre- to posttreatment reduction in total PCL-5 scores (P < .001; d = 1.16), with a treatment response rate of 72.4%.
When investigators analyzed the response at the individual level, they found that 55.7% of patients with PD and 53.5% of those with PTSD were classified as treatment responders. This determination was based on a two-pronged approach that first calculated the Reliable Change Index (RCI) for each participant, and, in participants showing statistically reliable improvement, whether the posttreatment score was closer to the distribution of scores for patients without or with the given disorder.
“Patients with both normal and below-normal baseline exhaled CO2 levels experienced comparable benefit,” the authors report.
There were high levels of adherence across the full treatment period in both the PD and the PTSD groups (74.8% and 74.9%, respectively), with low dropout rates (10% and 11%, respectively).
“Not every single patient who undergoes any treatment has a perfect response, but the response rates to this treatment have, surprisingly, been quite positive and there have been no negative side effects,” Dr. Telch remarked.
He noted that one of the effects of PTSD is that the “patient has negative beliefs about their ability to control the world. ‘I can’t control my reactions. At any time, I could have a flashback.’ Helping the patient to develop any sense of mastery over some of their reactions can spill over and give them a greater sense of mastery and control, which can have a positive effect in reducing PTSD symptoms.”
‘A viable alternative’
Commenting on the research, Charles Marmar, MD, chair and Peter H. Schub Professor of Psychiatry, department of psychiatry, New York University, said that the study has some limitations, probably the most significant of which is that most participants had normal baseline CO2 levels.
“The treatment is fundamentally designed for people who hyperventilate and blow off too much CO2 so they can breathe in a more calm, relaxed way, but most people in the trial had normal CO2 to begin with,” said Dr. Marmar, who was not involved with the study.
“It’s likely that the major benefits were the relaxation from doing the breathing exercises rather than the change in CO2 levels,” he speculated.
The treatment is “probably a good thing for those patients who actually have abnormal CO2 levels. This treatment could be used in precision medicine, where you tailor treatments to those who actually need them rather than giving the same treatment to everyone,” he said.
“For patients who don’t respond to trauma-focused therapy or it’s too aversive for them to undergo, this new intervention provides a viable alternative,” Dr. Telch added.
The study was internally funded by Freespira. Dr. Telch is a scientific advisor at Freespira and receives compensation by way of stock options. The other authors’ disclosures are listed on the original paper. Dr. Marmar has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN DIGITAL HEALTH
How your voice could reveal hidden disease
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
No, you can’t see a different doctor: We need zero tolerance of patient bias
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
States cracking down harder on docs who sexually abuse patients
It’s the latest example of states taking doctor sexual misconduct more seriously after longstanding criticism that medical boards have been too lenient.
The law, which takes effect in January 2023, requires the state’s medical board to permanently revoke these doctors’ licenses instead of allowing them to petition the board for reinstatement after 3 years.
“Physician licenses should not be reinstated after egregious sexual misconduct with patients. The doctor-patient relationship has to remain sacrosanct and trusted,” said Peter Yellowlees, MD, a professor of psychiatry at the University of California, Davis.
Although the vast majority of the nation’s estimated 1 million doctors don’t sexually abuse patients, the problem is a national one.
The Federation of State Medical Boards defines sexual misconduct as the exploitation of the physician-patient relationship in a sexual way. The exploitation may be verbal or physical and can occur in person or virtually.
The FSMB conducted a 2-year review of how medical boards handled cases of sexual misconduct, issuing a report in 2020 that contained 38 recommended actions.
Four states in addition to California have enacted laws that incorporate some FSMB recommendations. These include revoking doctors’ licenses after a single egregious act of sexual misconduct (including sexual assault), regardless of whether the physician was charged or convicted; increased reporting by hospitals and doctors of sexual misconduct; and training of physicians to recognize and report sexual misconduct.
The four state laws are:
- Georgia’s HB 458. It was signed into law in May 2021, and it authorizes the medical board to revoke or suspend a license if a physician is found guilty of sexually assaulting a patient in a criminal case. Doctors are required to report other doctors who have sexually abused patients and to take continuing medical education (CME) units on sexual misconduct.
- Florida’s SB 1934. This legislation was signed into law in June 2021, and it bars physicians charged with serious crimes such as sexual assault, sexual misconduct against patients, or possession of child pornography from seeing patients until those charges are resolved by the legal system.
- West Virginia’s SB 603. Signed into law in March 2022 it prohibits the medical board from issuing a license to a physician who engaged in sexual activity or misconduct with a patient whose license was revoked in another state or was involved in other violations.
- Tennessee HB 1045. It was signed into law in May 2021, and authorizes the medical board, upon learning of an indictment against a physician for a controlled substance violation or sexual offense, to immediately suspend the doctor’s ability to prescribe controlled substances until the doctor’s case is resolved.
A published study identified a total of 1,721 reports of physician sexual misconduct that were submitted to the National Practitioner Data Bank between 2000 and 2019. The annual incidence of sexual misconduct reports averaged 10.8 per 100,000 U.S. physician licensees, said the researchers.
In a groundbreaking 2016 investigation, the Atlanta Journal-Constitution reviewed thousands of documents and found more than 2,400 doctors whose sexual misconduct cases clearly involved patients since 1999.
Physician sexual misconduct is likely underreported
The actual incidence of physician-patient sexual misconduct is likely higher as a result of underreporting, according to the researchers.
Because a substantial power differential exists between patients and their physicians, the researchers noted, it follows that patient victims, like other sexual assault victims, may be unwilling or unable to report the incident in question.
Many violations involving physician sexual misconduct of patients never came to the attention of state regulators, according to the Journal-Constitution investigation. Reporting showed that hospitals, clinics, and fellow doctors fail to report sexual misconduct to regulators, despite laws in most states requiring them to do so.
Media investigations highlight medical board shortcomings
Public pressure on the California Medical Board increased after the Los Angeles Times investigated what happened to doctors who surrendered or had their licenses revoked after being reported for sexual abuse with patients. The Times revealed in 2021 that the board reinstated 10 of 17 doctors who petitioned for reinstatement.
They include Esmail Nadjmabadi, MD, of Bakersfield, Calif., who had sexually abused six female patients, including one in her mid-teens. The Times reported that, in 2009, he pleaded no contest to a criminal charge that he sexually exploited two or more women and surrendered his medical license the following year.
Five years later, Dr. Nadjmabadi petitioned the medical board to be reinstated and the board approved his request.
The California board has also reinstated several doctors who underwent sex offender rehabilitation. Board members rely heavily on a doctor’s evidence of rehabilitation, usually with the testimony of therapists hired by the doctor, and no input from the patients who were harmed, according to the Times’ investigation.
High-profile sexual misconduct or abuse cases involving Larry Nassar, MD, and Robert Anderson, MD, in Michigan; Richard Strauss, MD, in Ohio; and Ricardo Cruciani, MD, in New York, added to the mounting criticism that medical boards were too lenient in their handling of complaints of sexual misconduct.
Another state tackles sexual misconduct
Ohio’s medical board created an administrative rule stating that licensed physicians have a legal and ethical duty to report colleagues for sexual misconduct with patients and to complete a 1-hour CME training. Failure to report sexual misconduct complaints can lead to a doctor being permanently stripped of his license.
This happened to Robert S. Geiger, MD, in 2016 after not reporting his colleague James Bressi, MD, to the medical board after receiving complaints that Dr. Bressi was sexually abusing female patients at their pain clinic.
Dr. Bressi was convicted of sexual misconduct with a patient, stripped of his medical license, and sentenced to 59 days in prison.
“I think all of these reforms are a step in the right direction and will help to deter doctors from committing sexual misconduct to some extent,” said California activist Marian Hollingsworth, cofounder of the Patient Safety League.
But there’s room for improvement, she said, since “most states fall short in not requiring medical boards to notify law enforcement when they get a complaint of doctor sexual misconduct so the public can be aware of it.”
A version of this article first appeared on Medscape.com.
It’s the latest example of states taking doctor sexual misconduct more seriously after longstanding criticism that medical boards have been too lenient.
The law, which takes effect in January 2023, requires the state’s medical board to permanently revoke these doctors’ licenses instead of allowing them to petition the board for reinstatement after 3 years.
“Physician licenses should not be reinstated after egregious sexual misconduct with patients. The doctor-patient relationship has to remain sacrosanct and trusted,” said Peter Yellowlees, MD, a professor of psychiatry at the University of California, Davis.
Although the vast majority of the nation’s estimated 1 million doctors don’t sexually abuse patients, the problem is a national one.
The Federation of State Medical Boards defines sexual misconduct as the exploitation of the physician-patient relationship in a sexual way. The exploitation may be verbal or physical and can occur in person or virtually.
The FSMB conducted a 2-year review of how medical boards handled cases of sexual misconduct, issuing a report in 2020 that contained 38 recommended actions.
Four states in addition to California have enacted laws that incorporate some FSMB recommendations. These include revoking doctors’ licenses after a single egregious act of sexual misconduct (including sexual assault), regardless of whether the physician was charged or convicted; increased reporting by hospitals and doctors of sexual misconduct; and training of physicians to recognize and report sexual misconduct.
The four state laws are:
- Georgia’s HB 458. It was signed into law in May 2021, and it authorizes the medical board to revoke or suspend a license if a physician is found guilty of sexually assaulting a patient in a criminal case. Doctors are required to report other doctors who have sexually abused patients and to take continuing medical education (CME) units on sexual misconduct.
- Florida’s SB 1934. This legislation was signed into law in June 2021, and it bars physicians charged with serious crimes such as sexual assault, sexual misconduct against patients, or possession of child pornography from seeing patients until those charges are resolved by the legal system.
- West Virginia’s SB 603. Signed into law in March 2022 it prohibits the medical board from issuing a license to a physician who engaged in sexual activity or misconduct with a patient whose license was revoked in another state or was involved in other violations.
- Tennessee HB 1045. It was signed into law in May 2021, and authorizes the medical board, upon learning of an indictment against a physician for a controlled substance violation or sexual offense, to immediately suspend the doctor’s ability to prescribe controlled substances until the doctor’s case is resolved.
A published study identified a total of 1,721 reports of physician sexual misconduct that were submitted to the National Practitioner Data Bank between 2000 and 2019. The annual incidence of sexual misconduct reports averaged 10.8 per 100,000 U.S. physician licensees, said the researchers.
In a groundbreaking 2016 investigation, the Atlanta Journal-Constitution reviewed thousands of documents and found more than 2,400 doctors whose sexual misconduct cases clearly involved patients since 1999.
Physician sexual misconduct is likely underreported
The actual incidence of physician-patient sexual misconduct is likely higher as a result of underreporting, according to the researchers.
Because a substantial power differential exists between patients and their physicians, the researchers noted, it follows that patient victims, like other sexual assault victims, may be unwilling or unable to report the incident in question.
Many violations involving physician sexual misconduct of patients never came to the attention of state regulators, according to the Journal-Constitution investigation. Reporting showed that hospitals, clinics, and fellow doctors fail to report sexual misconduct to regulators, despite laws in most states requiring them to do so.
Media investigations highlight medical board shortcomings
Public pressure on the California Medical Board increased after the Los Angeles Times investigated what happened to doctors who surrendered or had their licenses revoked after being reported for sexual abuse with patients. The Times revealed in 2021 that the board reinstated 10 of 17 doctors who petitioned for reinstatement.
They include Esmail Nadjmabadi, MD, of Bakersfield, Calif., who had sexually abused six female patients, including one in her mid-teens. The Times reported that, in 2009, he pleaded no contest to a criminal charge that he sexually exploited two or more women and surrendered his medical license the following year.
Five years later, Dr. Nadjmabadi petitioned the medical board to be reinstated and the board approved his request.
The California board has also reinstated several doctors who underwent sex offender rehabilitation. Board members rely heavily on a doctor’s evidence of rehabilitation, usually with the testimony of therapists hired by the doctor, and no input from the patients who were harmed, according to the Times’ investigation.
High-profile sexual misconduct or abuse cases involving Larry Nassar, MD, and Robert Anderson, MD, in Michigan; Richard Strauss, MD, in Ohio; and Ricardo Cruciani, MD, in New York, added to the mounting criticism that medical boards were too lenient in their handling of complaints of sexual misconduct.
Another state tackles sexual misconduct
Ohio’s medical board created an administrative rule stating that licensed physicians have a legal and ethical duty to report colleagues for sexual misconduct with patients and to complete a 1-hour CME training. Failure to report sexual misconduct complaints can lead to a doctor being permanently stripped of his license.
This happened to Robert S. Geiger, MD, in 2016 after not reporting his colleague James Bressi, MD, to the medical board after receiving complaints that Dr. Bressi was sexually abusing female patients at their pain clinic.
Dr. Bressi was convicted of sexual misconduct with a patient, stripped of his medical license, and sentenced to 59 days in prison.
“I think all of these reforms are a step in the right direction and will help to deter doctors from committing sexual misconduct to some extent,” said California activist Marian Hollingsworth, cofounder of the Patient Safety League.
But there’s room for improvement, she said, since “most states fall short in not requiring medical boards to notify law enforcement when they get a complaint of doctor sexual misconduct so the public can be aware of it.”
A version of this article first appeared on Medscape.com.
It’s the latest example of states taking doctor sexual misconduct more seriously after longstanding criticism that medical boards have been too lenient.
The law, which takes effect in January 2023, requires the state’s medical board to permanently revoke these doctors’ licenses instead of allowing them to petition the board for reinstatement after 3 years.
“Physician licenses should not be reinstated after egregious sexual misconduct with patients. The doctor-patient relationship has to remain sacrosanct and trusted,” said Peter Yellowlees, MD, a professor of psychiatry at the University of California, Davis.
Although the vast majority of the nation’s estimated 1 million doctors don’t sexually abuse patients, the problem is a national one.
The Federation of State Medical Boards defines sexual misconduct as the exploitation of the physician-patient relationship in a sexual way. The exploitation may be verbal or physical and can occur in person or virtually.
The FSMB conducted a 2-year review of how medical boards handled cases of sexual misconduct, issuing a report in 2020 that contained 38 recommended actions.
Four states in addition to California have enacted laws that incorporate some FSMB recommendations. These include revoking doctors’ licenses after a single egregious act of sexual misconduct (including sexual assault), regardless of whether the physician was charged or convicted; increased reporting by hospitals and doctors of sexual misconduct; and training of physicians to recognize and report sexual misconduct.
The four state laws are:
- Georgia’s HB 458. It was signed into law in May 2021, and it authorizes the medical board to revoke or suspend a license if a physician is found guilty of sexually assaulting a patient in a criminal case. Doctors are required to report other doctors who have sexually abused patients and to take continuing medical education (CME) units on sexual misconduct.
- Florida’s SB 1934. This legislation was signed into law in June 2021, and it bars physicians charged with serious crimes such as sexual assault, sexual misconduct against patients, or possession of child pornography from seeing patients until those charges are resolved by the legal system.
- West Virginia’s SB 603. Signed into law in March 2022 it prohibits the medical board from issuing a license to a physician who engaged in sexual activity or misconduct with a patient whose license was revoked in another state or was involved in other violations.
- Tennessee HB 1045. It was signed into law in May 2021, and authorizes the medical board, upon learning of an indictment against a physician for a controlled substance violation or sexual offense, to immediately suspend the doctor’s ability to prescribe controlled substances until the doctor’s case is resolved.
A published study identified a total of 1,721 reports of physician sexual misconduct that were submitted to the National Practitioner Data Bank between 2000 and 2019. The annual incidence of sexual misconduct reports averaged 10.8 per 100,000 U.S. physician licensees, said the researchers.
In a groundbreaking 2016 investigation, the Atlanta Journal-Constitution reviewed thousands of documents and found more than 2,400 doctors whose sexual misconduct cases clearly involved patients since 1999.
Physician sexual misconduct is likely underreported
The actual incidence of physician-patient sexual misconduct is likely higher as a result of underreporting, according to the researchers.
Because a substantial power differential exists between patients and their physicians, the researchers noted, it follows that patient victims, like other sexual assault victims, may be unwilling or unable to report the incident in question.
Many violations involving physician sexual misconduct of patients never came to the attention of state regulators, according to the Journal-Constitution investigation. Reporting showed that hospitals, clinics, and fellow doctors fail to report sexual misconduct to regulators, despite laws in most states requiring them to do so.
Media investigations highlight medical board shortcomings
Public pressure on the California Medical Board increased after the Los Angeles Times investigated what happened to doctors who surrendered or had their licenses revoked after being reported for sexual abuse with patients. The Times revealed in 2021 that the board reinstated 10 of 17 doctors who petitioned for reinstatement.
They include Esmail Nadjmabadi, MD, of Bakersfield, Calif., who had sexually abused six female patients, including one in her mid-teens. The Times reported that, in 2009, he pleaded no contest to a criminal charge that he sexually exploited two or more women and surrendered his medical license the following year.
Five years later, Dr. Nadjmabadi petitioned the medical board to be reinstated and the board approved his request.
The California board has also reinstated several doctors who underwent sex offender rehabilitation. Board members rely heavily on a doctor’s evidence of rehabilitation, usually with the testimony of therapists hired by the doctor, and no input from the patients who were harmed, according to the Times’ investigation.
High-profile sexual misconduct or abuse cases involving Larry Nassar, MD, and Robert Anderson, MD, in Michigan; Richard Strauss, MD, in Ohio; and Ricardo Cruciani, MD, in New York, added to the mounting criticism that medical boards were too lenient in their handling of complaints of sexual misconduct.
Another state tackles sexual misconduct
Ohio’s medical board created an administrative rule stating that licensed physicians have a legal and ethical duty to report colleagues for sexual misconduct with patients and to complete a 1-hour CME training. Failure to report sexual misconduct complaints can lead to a doctor being permanently stripped of his license.
This happened to Robert S. Geiger, MD, in 2016 after not reporting his colleague James Bressi, MD, to the medical board after receiving complaints that Dr. Bressi was sexually abusing female patients at their pain clinic.
Dr. Bressi was convicted of sexual misconduct with a patient, stripped of his medical license, and sentenced to 59 days in prison.
“I think all of these reforms are a step in the right direction and will help to deter doctors from committing sexual misconduct to some extent,” said California activist Marian Hollingsworth, cofounder of the Patient Safety League.
But there’s room for improvement, she said, since “most states fall short in not requiring medical boards to notify law enforcement when they get a complaint of doctor sexual misconduct so the public can be aware of it.”
A version of this article first appeared on Medscape.com.
Poison centers fielding more calls about teen cannabis use
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Teens’ undisclosed dieting may precede anorexia nervosa diagnosis
Adolescents later diagnosed with anorexia nervosa (AN) likely embark on the trajectory to AN with undisclosed dieting for weight loss at about age 14, a study of teens and parents found.
In the interview-based study, both adolescents and their parents described a similar prediagnosis sequence of behavioral changes occurring over roughly 1 year to 18 months, but parents lagged some 6 months behind in noticing their children’s disordered eating.
The findings suggest that even teens of normal weight should be asked about their eating habits and monitored more closely for contact with those who endorse these potentially harmful eating behaviors, according to Lisa M. Ranzenhofer, PhD, assistant professor of clinical psychology in psychiatry at Columbia University Medical Center in New York, and colleagues. Their report is in the Journal of Adolescent Health.
“We know that adolescents often have eating disorder behaviors long before they’re diagnosed, so we developed this interview as a tool to figure out how long a maladaptive behavior has been present,” Dr. Ranzenhofer said in an interview. “Most studies that report illness duration do so based on diagnosis, so this interview provides a more fine-grained assessment of the duration of problematic behavior, which may help improve understanding of the impact of duration on outcome, and hopefully facilitate better methods for early detection.” Since healthy adolescents are often seen once per year at an annual pediatrician visit, she added, teens engaging in significant dieting might benefit from more frequent monitoring since this behavior can evolve into an eating disorder over a relatively short time frame.
AN is associated with significant medical and psychiatric comorbidity and has a mortality rate among the highest of any psychiatric illness, the authors noted.
The study
The study cohort consisted of 71 girls ages 12-18 years participating in research from 2017 to 2021 at the Eating Disorders Research Unit of New York (N.Y.) State Psychiatric Institute. Patients had either the restricting or binge-eating/purging subtype of AN as diagnosed by the Eating Disorder Assessment–5 questionnaire. A semistructured 15-minute interview with the girls and their parents explored food restriction, dieting, loss of control/binge eating, purging, excessive/compulsive exercise, weight history, and amenorrhea.
Both parents and children were asked whether and when the children had been underweight or overweight, and whether and when primary amenorrhea (no menarche) or secondary amenorrhea (periods missed for 3 months) became evident. Dieting was defined as “deliberately changing eating patterns in any way to influence your shape or weight,” and restriction as “deliberately cutting down on the amount of food that you are eating, in order to change your shape or weight.” Loss-of-control eating was defined as “feeling unable to stop eating or control what or how much you are eating.”
In other characterizations, purging was defined as making yourself vomit on purpose, taking diuretics, or feeling driven to engage in these behaviors. Questions on exercise explored whether children might feel anxious when they do not exercise or inclined to exercise even if sick or injured, with excessive exercise defined as “Feeling like you must exercise, might continue exercising, sometimes in secret, if parents or doctors have told you to stop.”
Other questions focused on use of diuretics or laxatives and other strategies to compensate for calories consumed.
Responses revealed that restriction, underweight, dieting, and excessive exercise were present in most of the sample, while purging, loss-of-control eating, and overweight were reported by fewer than a third. With dieting typically emerging first around age 14, the other behaviors tended to manifest from age 14 to 14 and a half. The average age of formal diagnosis was just over 15 years. Parent-child dyads showed good agreement on the presence and timing of all behaviors except for dieting, for which children reported onset about 6 months earlier or longer duration compared with parents.
Although older age at the time of interview was associated with a lower body mass index percentile and higher eating disorder score, neither age of onset nor duration of disordered eating was associated with severity when researchers controlled for current age.
Telltale signs for parents
“For teens starting at a healthy weight, significant and intentional weight loss of more than 5-10 pounds can be a cause for concern,” Dr. Ranzenhofer said. Missed periods, refusing meals, skipping meals, fighting or arguing about eating, and withdrawal from normal activities and relationships are other signs of disordered eating. For overweight or obese teens, rapid weight loss and weight loss above and beyond that recommended are also concerning.
As for compulsive exercise, she said, “Altered exercise behavior might look like exercise that interferes with other activities, for example, being late to school or not doing homework in order to exercise.” Other red flags would be physical activity that varies considerably from that of peers, for instance, going running after a 2-hour sports practice and an inflexible routine that precludes being able to skip a day.
“All adolescents, male and female, should be screened regardless of weight trends – underweight, overweight, obese, or normal weight – regarding their body image and thoughts of dieting,” said Margaret E. Thew, DNP, FNP-BC, of the Medical College of Wisconsin, and medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee, commenting on the study but not involved in it. “Most adolescents make decisions to lose weight after trying to ‘eat healthy’ but may take an aggressive approach when they don’t see the weight loss they hope to see.”
According to Ms. Thew, the study findings support the benefit of giving medical caregivers and parents training on the red flags regarding eating disorders to foster early detection. “These include starting a new fad diet, eliminating foods, ‘healthy eating,’ over-exercising, skipping meals, or no longer eating foods they previously loved.”
She added that times of transition are key junctures to watch: The transition from grade school to middle school, middle to high school, and high school to college. “These tend to provoke eating disorder onset or relapse of eating disorder thoughts and behaviors after diagnosis,” Ms. Thew said. “It would benefit the patient to screen for concerns about disordered eating and provide resources, including consultation with a dietitian, as appropriate.”
This study was supported by grants from the National Institute of Mental Health and the Hilda and Preston Davis Foundation. Coauthor Joanna E. Steinglass, MD, disclosed receiving royalties from UpToDate. Ms. Thew disclosed no competing interests with regard to her comments.
Adolescents later diagnosed with anorexia nervosa (AN) likely embark on the trajectory to AN with undisclosed dieting for weight loss at about age 14, a study of teens and parents found.
In the interview-based study, both adolescents and their parents described a similar prediagnosis sequence of behavioral changes occurring over roughly 1 year to 18 months, but parents lagged some 6 months behind in noticing their children’s disordered eating.
The findings suggest that even teens of normal weight should be asked about their eating habits and monitored more closely for contact with those who endorse these potentially harmful eating behaviors, according to Lisa M. Ranzenhofer, PhD, assistant professor of clinical psychology in psychiatry at Columbia University Medical Center in New York, and colleagues. Their report is in the Journal of Adolescent Health.
“We know that adolescents often have eating disorder behaviors long before they’re diagnosed, so we developed this interview as a tool to figure out how long a maladaptive behavior has been present,” Dr. Ranzenhofer said in an interview. “Most studies that report illness duration do so based on diagnosis, so this interview provides a more fine-grained assessment of the duration of problematic behavior, which may help improve understanding of the impact of duration on outcome, and hopefully facilitate better methods for early detection.” Since healthy adolescents are often seen once per year at an annual pediatrician visit, she added, teens engaging in significant dieting might benefit from more frequent monitoring since this behavior can evolve into an eating disorder over a relatively short time frame.
AN is associated with significant medical and psychiatric comorbidity and has a mortality rate among the highest of any psychiatric illness, the authors noted.
The study
The study cohort consisted of 71 girls ages 12-18 years participating in research from 2017 to 2021 at the Eating Disorders Research Unit of New York (N.Y.) State Psychiatric Institute. Patients had either the restricting or binge-eating/purging subtype of AN as diagnosed by the Eating Disorder Assessment–5 questionnaire. A semistructured 15-minute interview with the girls and their parents explored food restriction, dieting, loss of control/binge eating, purging, excessive/compulsive exercise, weight history, and amenorrhea.
Both parents and children were asked whether and when the children had been underweight or overweight, and whether and when primary amenorrhea (no menarche) or secondary amenorrhea (periods missed for 3 months) became evident. Dieting was defined as “deliberately changing eating patterns in any way to influence your shape or weight,” and restriction as “deliberately cutting down on the amount of food that you are eating, in order to change your shape or weight.” Loss-of-control eating was defined as “feeling unable to stop eating or control what or how much you are eating.”
In other characterizations, purging was defined as making yourself vomit on purpose, taking diuretics, or feeling driven to engage in these behaviors. Questions on exercise explored whether children might feel anxious when they do not exercise or inclined to exercise even if sick or injured, with excessive exercise defined as “Feeling like you must exercise, might continue exercising, sometimes in secret, if parents or doctors have told you to stop.”
Other questions focused on use of diuretics or laxatives and other strategies to compensate for calories consumed.
Responses revealed that restriction, underweight, dieting, and excessive exercise were present in most of the sample, while purging, loss-of-control eating, and overweight were reported by fewer than a third. With dieting typically emerging first around age 14, the other behaviors tended to manifest from age 14 to 14 and a half. The average age of formal diagnosis was just over 15 years. Parent-child dyads showed good agreement on the presence and timing of all behaviors except for dieting, for which children reported onset about 6 months earlier or longer duration compared with parents.
Although older age at the time of interview was associated with a lower body mass index percentile and higher eating disorder score, neither age of onset nor duration of disordered eating was associated with severity when researchers controlled for current age.
Telltale signs for parents
“For teens starting at a healthy weight, significant and intentional weight loss of more than 5-10 pounds can be a cause for concern,” Dr. Ranzenhofer said. Missed periods, refusing meals, skipping meals, fighting or arguing about eating, and withdrawal from normal activities and relationships are other signs of disordered eating. For overweight or obese teens, rapid weight loss and weight loss above and beyond that recommended are also concerning.
As for compulsive exercise, she said, “Altered exercise behavior might look like exercise that interferes with other activities, for example, being late to school or not doing homework in order to exercise.” Other red flags would be physical activity that varies considerably from that of peers, for instance, going running after a 2-hour sports practice and an inflexible routine that precludes being able to skip a day.
“All adolescents, male and female, should be screened regardless of weight trends – underweight, overweight, obese, or normal weight – regarding their body image and thoughts of dieting,” said Margaret E. Thew, DNP, FNP-BC, of the Medical College of Wisconsin, and medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee, commenting on the study but not involved in it. “Most adolescents make decisions to lose weight after trying to ‘eat healthy’ but may take an aggressive approach when they don’t see the weight loss they hope to see.”
According to Ms. Thew, the study findings support the benefit of giving medical caregivers and parents training on the red flags regarding eating disorders to foster early detection. “These include starting a new fad diet, eliminating foods, ‘healthy eating,’ over-exercising, skipping meals, or no longer eating foods they previously loved.”
She added that times of transition are key junctures to watch: The transition from grade school to middle school, middle to high school, and high school to college. “These tend to provoke eating disorder onset or relapse of eating disorder thoughts and behaviors after diagnosis,” Ms. Thew said. “It would benefit the patient to screen for concerns about disordered eating and provide resources, including consultation with a dietitian, as appropriate.”
This study was supported by grants from the National Institute of Mental Health and the Hilda and Preston Davis Foundation. Coauthor Joanna E. Steinglass, MD, disclosed receiving royalties from UpToDate. Ms. Thew disclosed no competing interests with regard to her comments.
Adolescents later diagnosed with anorexia nervosa (AN) likely embark on the trajectory to AN with undisclosed dieting for weight loss at about age 14, a study of teens and parents found.
In the interview-based study, both adolescents and their parents described a similar prediagnosis sequence of behavioral changes occurring over roughly 1 year to 18 months, but parents lagged some 6 months behind in noticing their children’s disordered eating.
The findings suggest that even teens of normal weight should be asked about their eating habits and monitored more closely for contact with those who endorse these potentially harmful eating behaviors, according to Lisa M. Ranzenhofer, PhD, assistant professor of clinical psychology in psychiatry at Columbia University Medical Center in New York, and colleagues. Their report is in the Journal of Adolescent Health.
“We know that adolescents often have eating disorder behaviors long before they’re diagnosed, so we developed this interview as a tool to figure out how long a maladaptive behavior has been present,” Dr. Ranzenhofer said in an interview. “Most studies that report illness duration do so based on diagnosis, so this interview provides a more fine-grained assessment of the duration of problematic behavior, which may help improve understanding of the impact of duration on outcome, and hopefully facilitate better methods for early detection.” Since healthy adolescents are often seen once per year at an annual pediatrician visit, she added, teens engaging in significant dieting might benefit from more frequent monitoring since this behavior can evolve into an eating disorder over a relatively short time frame.
AN is associated with significant medical and psychiatric comorbidity and has a mortality rate among the highest of any psychiatric illness, the authors noted.
The study
The study cohort consisted of 71 girls ages 12-18 years participating in research from 2017 to 2021 at the Eating Disorders Research Unit of New York (N.Y.) State Psychiatric Institute. Patients had either the restricting or binge-eating/purging subtype of AN as diagnosed by the Eating Disorder Assessment–5 questionnaire. A semistructured 15-minute interview with the girls and their parents explored food restriction, dieting, loss of control/binge eating, purging, excessive/compulsive exercise, weight history, and amenorrhea.
Both parents and children were asked whether and when the children had been underweight or overweight, and whether and when primary amenorrhea (no menarche) or secondary amenorrhea (periods missed for 3 months) became evident. Dieting was defined as “deliberately changing eating patterns in any way to influence your shape or weight,” and restriction as “deliberately cutting down on the amount of food that you are eating, in order to change your shape or weight.” Loss-of-control eating was defined as “feeling unable to stop eating or control what or how much you are eating.”
In other characterizations, purging was defined as making yourself vomit on purpose, taking diuretics, or feeling driven to engage in these behaviors. Questions on exercise explored whether children might feel anxious when they do not exercise or inclined to exercise even if sick or injured, with excessive exercise defined as “Feeling like you must exercise, might continue exercising, sometimes in secret, if parents or doctors have told you to stop.”
Other questions focused on use of diuretics or laxatives and other strategies to compensate for calories consumed.
Responses revealed that restriction, underweight, dieting, and excessive exercise were present in most of the sample, while purging, loss-of-control eating, and overweight were reported by fewer than a third. With dieting typically emerging first around age 14, the other behaviors tended to manifest from age 14 to 14 and a half. The average age of formal diagnosis was just over 15 years. Parent-child dyads showed good agreement on the presence and timing of all behaviors except for dieting, for which children reported onset about 6 months earlier or longer duration compared with parents.
Although older age at the time of interview was associated with a lower body mass index percentile and higher eating disorder score, neither age of onset nor duration of disordered eating was associated with severity when researchers controlled for current age.
Telltale signs for parents
“For teens starting at a healthy weight, significant and intentional weight loss of more than 5-10 pounds can be a cause for concern,” Dr. Ranzenhofer said. Missed periods, refusing meals, skipping meals, fighting or arguing about eating, and withdrawal from normal activities and relationships are other signs of disordered eating. For overweight or obese teens, rapid weight loss and weight loss above and beyond that recommended are also concerning.
As for compulsive exercise, she said, “Altered exercise behavior might look like exercise that interferes with other activities, for example, being late to school or not doing homework in order to exercise.” Other red flags would be physical activity that varies considerably from that of peers, for instance, going running after a 2-hour sports practice and an inflexible routine that precludes being able to skip a day.
“All adolescents, male and female, should be screened regardless of weight trends – underweight, overweight, obese, or normal weight – regarding their body image and thoughts of dieting,” said Margaret E. Thew, DNP, FNP-BC, of the Medical College of Wisconsin, and medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee, commenting on the study but not involved in it. “Most adolescents make decisions to lose weight after trying to ‘eat healthy’ but may take an aggressive approach when they don’t see the weight loss they hope to see.”
According to Ms. Thew, the study findings support the benefit of giving medical caregivers and parents training on the red flags regarding eating disorders to foster early detection. “These include starting a new fad diet, eliminating foods, ‘healthy eating,’ over-exercising, skipping meals, or no longer eating foods they previously loved.”
She added that times of transition are key junctures to watch: The transition from grade school to middle school, middle to high school, and high school to college. “These tend to provoke eating disorder onset or relapse of eating disorder thoughts and behaviors after diagnosis,” Ms. Thew said. “It would benefit the patient to screen for concerns about disordered eating and provide resources, including consultation with a dietitian, as appropriate.”
This study was supported by grants from the National Institute of Mental Health and the Hilda and Preston Davis Foundation. Coauthor Joanna E. Steinglass, MD, disclosed receiving royalties from UpToDate. Ms. Thew disclosed no competing interests with regard to her comments.
FROM JOURNAL OF ADOLESCENT HEALTH
Does dopamine dysregulation cause schizophrenia?
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
Everyone wins when losers get paid
Bribery really is the solution to all of life’s problems
Breaking news: The United States has a bit of an obesity epidemic. Okay, maybe not so breaking news. But it’s a problem we’ve been struggling with for a very long time. Part of the issue is that there really is no secret to weight loss. Pretty much anything can work if you’re committed. The millions of diets floating around are testament to this idea.
The problem of losing weight is amplified if you don’t rake in the big bucks. Lower-income individuals often can’t afford healthy superfoods, and they’re often too busy to spend time at classes, exercising, or following programs. A group of researchers at New York University has offered up an alternate solution to encourage weight loss in low-income people: Pay them.
Specifically, pay them for losing weight. A reward, if you will. The researchers recruited several hundred lower-income people and split them into three groups. All participants received a free 1-year membership to a gym and weight-loss program, as well as food journals and fitness devices, but one group received payment (on average, about $300 overall) for attending meetings, exercising a certain amount every week, or weighing themselves twice a week. About 40% of people in this group lost 5% of their body weight after 6 months, twice as many as in the group that did not receive payment for performing these tasks.
The big winners, however, were those in the third group. They also received the free stuff, but the researchers offered them a more simple and direct bribe: Lose 5% of your weight over 6 months and we’ll pay you. The reward? About $450 on average, and it worked very well, with half this group losing the weight after 6 months. That said, after a year something like a fifth of this group put the weight back on, bringing them in line with the group that was paid to perform tasks. Still, both groups outperformed the control group, which received no money.
The takeaway from this research is pretty obvious. Pay people a fair price to do something, and they’ll do it. This is a lesson that has absolutely no relevance in the modern world. Nope, none whatsoever. We all receive completely fair wages. We all have plenty of money to pay for things. Everything is fine.
More green space, less medicine
Have you heard of the 3-30-300 rule? Proposed by urban forester Cecil Konijnendijk, it’s become the rule of thumb for urban planners and other foresters into getting more green space in populated areas. A recent study has found that people who lived within this 3-30-300 rule had better mental health and less medication use.
If you’re not an urban forester, however, you may not know what the 3-30-300 rule is. But it’s pretty simple, people should be able to see at least three trees from their home, have 30% tree canopy in their neighborhood, and have 300 Spartans to defend against the Persian army.
We may have made that last one up. It’s actually have a green space or park within 300 meters of your home.
In the new study, only 4.7% of people surveyed lived in an area that followed all three rules. About 62% of the surveyed lived with a green space at least 300 meters away, 43% had at least three trees within 15 meters from their home, and a rather pitiful 9% had adequate tree canopy coverage in their neighborhood.
Greater adherence to the 3-30-300 rule was associated with fewer visits to the psychologist, with 8.3% of the participants reporting a psychologist visit in the last year. The data come from a sample of a little over 3,000 Barcelona residents aged 15-97 who were randomly selected to participate in the Barcelona Public Health Agency Survey.
“There is an urgent need to provide citizens with more green space,” said Mark Nieuwenhuijsen, lead author of the study. “We may need to tear out asphalt and plant more trees, which would not only improve health, but also reduce heat island effects and contribute to carbon capture.”
The main goal and message is that more green space is good for everyone. So if you’re feeling a little overwhelmed, take a breather and sit somewhere green. Or call those 300 Spartans and get them to start knocking some buildings down.
Said the toilet to the engineer: Do you hear what I hear?
A mythical hero’s journey took Dorothy along the yellow brick road to find the Wizard of Oz. Huckleberry Finn used a raft to float down the Mississippi River. Luke Skywalker did most of his traveling between planets. For the rest of us, the journey may be just a bit shorter.
Also a bit less heroic. Unless, of course, you’re prepping for a colonoscopy. Yup, we’re headed to the toilet, but not just any toilet. This toilet was the subject of a presentation at the annual meeting of the Acoustical Society of America, titled “The feces thesis: Using machine learning to detect diarrhea,” and that presentation was the hero’s journey of Maia Gatlin, PhD, a research engineer at the Georgia Institute of Technology.
She and her team attached a noninvasive microphone sensor to a toilet, and now they can identify bowel diseases without collecting any identifiable information.
The audio sample of an excretion event is “transformed into a spectrogram, which essentially captures the sound in an image. Different events produce different features in the audio and the spectrogram. For example, urination creates a consistent tone, while defecation may have a singular tone. In contrast, diarrhea is more random,” they explained in the written statement.
They used a machine learning algorithm to classify each spectrogram based on its features. “The algorithm’s performance was tested against data with and without background noises to make sure it was learning the right sound features, regardless of the sensor’s environment,” Dr. Gatlin and associates wrote.
Their goal is to use the toilet sensor in areas where cholera is common to prevent the spread of disease. After that, who knows? “Perhaps someday, our algorithm can be used with existing in-home smart devices to monitor one’s own bowel movements and health!” she suggested.
That would be a heroic toilet indeed.
Bribery really is the solution to all of life’s problems
Breaking news: The United States has a bit of an obesity epidemic. Okay, maybe not so breaking news. But it’s a problem we’ve been struggling with for a very long time. Part of the issue is that there really is no secret to weight loss. Pretty much anything can work if you’re committed. The millions of diets floating around are testament to this idea.
The problem of losing weight is amplified if you don’t rake in the big bucks. Lower-income individuals often can’t afford healthy superfoods, and they’re often too busy to spend time at classes, exercising, or following programs. A group of researchers at New York University has offered up an alternate solution to encourage weight loss in low-income people: Pay them.
Specifically, pay them for losing weight. A reward, if you will. The researchers recruited several hundred lower-income people and split them into three groups. All participants received a free 1-year membership to a gym and weight-loss program, as well as food journals and fitness devices, but one group received payment (on average, about $300 overall) for attending meetings, exercising a certain amount every week, or weighing themselves twice a week. About 40% of people in this group lost 5% of their body weight after 6 months, twice as many as in the group that did not receive payment for performing these tasks.
The big winners, however, were those in the third group. They also received the free stuff, but the researchers offered them a more simple and direct bribe: Lose 5% of your weight over 6 months and we’ll pay you. The reward? About $450 on average, and it worked very well, with half this group losing the weight after 6 months. That said, after a year something like a fifth of this group put the weight back on, bringing them in line with the group that was paid to perform tasks. Still, both groups outperformed the control group, which received no money.
The takeaway from this research is pretty obvious. Pay people a fair price to do something, and they’ll do it. This is a lesson that has absolutely no relevance in the modern world. Nope, none whatsoever. We all receive completely fair wages. We all have plenty of money to pay for things. Everything is fine.
More green space, less medicine
Have you heard of the 3-30-300 rule? Proposed by urban forester Cecil Konijnendijk, it’s become the rule of thumb for urban planners and other foresters into getting more green space in populated areas. A recent study has found that people who lived within this 3-30-300 rule had better mental health and less medication use.
If you’re not an urban forester, however, you may not know what the 3-30-300 rule is. But it’s pretty simple, people should be able to see at least three trees from their home, have 30% tree canopy in their neighborhood, and have 300 Spartans to defend against the Persian army.
We may have made that last one up. It’s actually have a green space or park within 300 meters of your home.
In the new study, only 4.7% of people surveyed lived in an area that followed all three rules. About 62% of the surveyed lived with a green space at least 300 meters away, 43% had at least three trees within 15 meters from their home, and a rather pitiful 9% had adequate tree canopy coverage in their neighborhood.
Greater adherence to the 3-30-300 rule was associated with fewer visits to the psychologist, with 8.3% of the participants reporting a psychologist visit in the last year. The data come from a sample of a little over 3,000 Barcelona residents aged 15-97 who were randomly selected to participate in the Barcelona Public Health Agency Survey.
“There is an urgent need to provide citizens with more green space,” said Mark Nieuwenhuijsen, lead author of the study. “We may need to tear out asphalt and plant more trees, which would not only improve health, but also reduce heat island effects and contribute to carbon capture.”
The main goal and message is that more green space is good for everyone. So if you’re feeling a little overwhelmed, take a breather and sit somewhere green. Or call those 300 Spartans and get them to start knocking some buildings down.
Said the toilet to the engineer: Do you hear what I hear?
A mythical hero’s journey took Dorothy along the yellow brick road to find the Wizard of Oz. Huckleberry Finn used a raft to float down the Mississippi River. Luke Skywalker did most of his traveling between planets. For the rest of us, the journey may be just a bit shorter.
Also a bit less heroic. Unless, of course, you’re prepping for a colonoscopy. Yup, we’re headed to the toilet, but not just any toilet. This toilet was the subject of a presentation at the annual meeting of the Acoustical Society of America, titled “The feces thesis: Using machine learning to detect diarrhea,” and that presentation was the hero’s journey of Maia Gatlin, PhD, a research engineer at the Georgia Institute of Technology.
She and her team attached a noninvasive microphone sensor to a toilet, and now they can identify bowel diseases without collecting any identifiable information.
The audio sample of an excretion event is “transformed into a spectrogram, which essentially captures the sound in an image. Different events produce different features in the audio and the spectrogram. For example, urination creates a consistent tone, while defecation may have a singular tone. In contrast, diarrhea is more random,” they explained in the written statement.
They used a machine learning algorithm to classify each spectrogram based on its features. “The algorithm’s performance was tested against data with and without background noises to make sure it was learning the right sound features, regardless of the sensor’s environment,” Dr. Gatlin and associates wrote.
Their goal is to use the toilet sensor in areas where cholera is common to prevent the spread of disease. After that, who knows? “Perhaps someday, our algorithm can be used with existing in-home smart devices to monitor one’s own bowel movements and health!” she suggested.
That would be a heroic toilet indeed.
Bribery really is the solution to all of life’s problems
Breaking news: The United States has a bit of an obesity epidemic. Okay, maybe not so breaking news. But it’s a problem we’ve been struggling with for a very long time. Part of the issue is that there really is no secret to weight loss. Pretty much anything can work if you’re committed. The millions of diets floating around are testament to this idea.
The problem of losing weight is amplified if you don’t rake in the big bucks. Lower-income individuals often can’t afford healthy superfoods, and they’re often too busy to spend time at classes, exercising, or following programs. A group of researchers at New York University has offered up an alternate solution to encourage weight loss in low-income people: Pay them.
Specifically, pay them for losing weight. A reward, if you will. The researchers recruited several hundred lower-income people and split them into three groups. All participants received a free 1-year membership to a gym and weight-loss program, as well as food journals and fitness devices, but one group received payment (on average, about $300 overall) for attending meetings, exercising a certain amount every week, or weighing themselves twice a week. About 40% of people in this group lost 5% of their body weight after 6 months, twice as many as in the group that did not receive payment for performing these tasks.
The big winners, however, were those in the third group. They also received the free stuff, but the researchers offered them a more simple and direct bribe: Lose 5% of your weight over 6 months and we’ll pay you. The reward? About $450 on average, and it worked very well, with half this group losing the weight after 6 months. That said, after a year something like a fifth of this group put the weight back on, bringing them in line with the group that was paid to perform tasks. Still, both groups outperformed the control group, which received no money.
The takeaway from this research is pretty obvious. Pay people a fair price to do something, and they’ll do it. This is a lesson that has absolutely no relevance in the modern world. Nope, none whatsoever. We all receive completely fair wages. We all have plenty of money to pay for things. Everything is fine.
More green space, less medicine
Have you heard of the 3-30-300 rule? Proposed by urban forester Cecil Konijnendijk, it’s become the rule of thumb for urban planners and other foresters into getting more green space in populated areas. A recent study has found that people who lived within this 3-30-300 rule had better mental health and less medication use.
If you’re not an urban forester, however, you may not know what the 3-30-300 rule is. But it’s pretty simple, people should be able to see at least three trees from their home, have 30% tree canopy in their neighborhood, and have 300 Spartans to defend against the Persian army.
We may have made that last one up. It’s actually have a green space or park within 300 meters of your home.
In the new study, only 4.7% of people surveyed lived in an area that followed all three rules. About 62% of the surveyed lived with a green space at least 300 meters away, 43% had at least three trees within 15 meters from their home, and a rather pitiful 9% had adequate tree canopy coverage in their neighborhood.
Greater adherence to the 3-30-300 rule was associated with fewer visits to the psychologist, with 8.3% of the participants reporting a psychologist visit in the last year. The data come from a sample of a little over 3,000 Barcelona residents aged 15-97 who were randomly selected to participate in the Barcelona Public Health Agency Survey.
“There is an urgent need to provide citizens with more green space,” said Mark Nieuwenhuijsen, lead author of the study. “We may need to tear out asphalt and plant more trees, which would not only improve health, but also reduce heat island effects and contribute to carbon capture.”
The main goal and message is that more green space is good for everyone. So if you’re feeling a little overwhelmed, take a breather and sit somewhere green. Or call those 300 Spartans and get them to start knocking some buildings down.
Said the toilet to the engineer: Do you hear what I hear?
A mythical hero’s journey took Dorothy along the yellow brick road to find the Wizard of Oz. Huckleberry Finn used a raft to float down the Mississippi River. Luke Skywalker did most of his traveling between planets. For the rest of us, the journey may be just a bit shorter.
Also a bit less heroic. Unless, of course, you’re prepping for a colonoscopy. Yup, we’re headed to the toilet, but not just any toilet. This toilet was the subject of a presentation at the annual meeting of the Acoustical Society of America, titled “The feces thesis: Using machine learning to detect diarrhea,” and that presentation was the hero’s journey of Maia Gatlin, PhD, a research engineer at the Georgia Institute of Technology.
She and her team attached a noninvasive microphone sensor to a toilet, and now they can identify bowel diseases without collecting any identifiable information.
The audio sample of an excretion event is “transformed into a spectrogram, which essentially captures the sound in an image. Different events produce different features in the audio and the spectrogram. For example, urination creates a consistent tone, while defecation may have a singular tone. In contrast, diarrhea is more random,” they explained in the written statement.
They used a machine learning algorithm to classify each spectrogram based on its features. “The algorithm’s performance was tested against data with and without background noises to make sure it was learning the right sound features, regardless of the sensor’s environment,” Dr. Gatlin and associates wrote.
Their goal is to use the toilet sensor in areas where cholera is common to prevent the spread of disease. After that, who knows? “Perhaps someday, our algorithm can be used with existing in-home smart devices to monitor one’s own bowel movements and health!” she suggested.
That would be a heroic toilet indeed.
‘Meth’ heart failure on the rise, often more severe
a literature review indicates.
MethHF is associated with increased severity for HF, longer inpatient stay, and more readmissions, compared with non-MethHF, the data show.
Clinicians “need to consider methamphetamine as a potential etiology for heart failure and include a substance use history when evaluating patients. Treating methamphetamine use disorder improves heart failure outcomes,” first author Veena Manja, MD, PhD, with Stanford (Calif.) University, said in an interview.
The study was published online in the journal Heart.
Poor outcomes, ‘staggering’ costs
This “thoughtful” review is “important and necessary,” Jonathan Davis, MD, director of the heart failure program, Zuckerberg San Francisco General Hospital, wrote in an editorial in the journal.
Dr. Davis noted that patients with Meth HF are at increased risk for poor outcomes and death and the health care costs related to MethHF are “staggering.”
As an example, inpatient data for California show annual charges related to MethHF rose by 840% from 2008 to 2018, from $41.5 million to $390.2 million, compared with 82% for all HF, which rose from $3.5 billion to $6.8 billion.
Illicit use of methamphetamine – also known as “crystal meth,” “ice,” and “speed” – has been linked to hypertension, MI, stroke, aortic dissection, and sudden death. But until now, there was no comprehensive systematic review of published studies on MethHF.
“Our goal was to compile current knowledge on the topic, increase awareness of this condition and identify areas for future research,” Dr. Manja said.
The researchers reviewed 21 observational studies, mostly from the United States (14 from California), between 1997 and 2020. The mean age of adults with MethHF ranged in age from 35 to 60 and more than half were male (57%).
Illicit methamphetamine was inhaled, injected, swallowed, smoked, and snorted. The reported frequency ranged from daily to every other week, and the total monthly dose ranged from 0.35 g to 24.5 g.
The average duration of meth use before HF diagnosis was 5 years. However, 18% of users developed HF within 1 year of starting to use illicit methamphetamine. In some cases, HF was diagnosed after a single use.
The researchers also note that MethHF with preserved left ventricular ejection fraction, seen in up to 44% of cases, is a distinct entity that may progress to reduced LVEF with continued use.
MethHF is also associated with a greater likelihood of other substance abuse, PTSD, depression, and other heart and kidney disease.
Factors associated with improved MethHF outcomes include female sex, meth abstinence, and adherence to guideline-directed HF therapy.
Improvement in MethHF outcomes is possible even if abstinence is not consistent, a finding that lends support to harm reduction principles of “meeting patients where they are instead of insisting on complete abstinence,” the researchers said.
Large gaps in knowledge
They were unable to combine the results into a meta-analysis because of heterogeneity in study design, population, comparator, and outcome assessment. Also, the overall risk of bias is moderate because of the presence of confounders, selection bias and poor matching, and the overall certainty in the evidence is very low,.
No study evaluated the incidence or prevalence of HF among methamphetamine users and inconsistent history taking and testing in patients with HF impeded accurate MethHF prevalence assessment.
Several studies, however, document an increasing incidence of MethHF, particularly over the past decade.
One study from California reported a 585% increase in MethHF hospital admissions between 2008 and 2018. An analysis of the National Inpatient Survey found a 12-fold increase in annual MethHF hospitalizations between 2002 and 2014.
“The results of this systematic review highlight large gaps in our knowledge” of MethHF, Dr. Manja said in an interview.
“We need to understand the epidemiology, prevalence, factors that confer susceptibility to cardiovascular outcomes, and need research into treatment targeted toward this disease,” Dr. Manja added. “We should consider options to integrate substance use treatment in HF/cardiology/primary care clinics and design a multidisciplinary patient-centered approach.”
Dr. Davis agreed. This work “highlights that the standard of care academically and clinically must be a broad team across the care spectrum to simultaneously address methamphetamine use, heart failure, and social determinants of health.”
This research had no specific funding. Dr. Manja and Dr. Davis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
a literature review indicates.
MethHF is associated with increased severity for HF, longer inpatient stay, and more readmissions, compared with non-MethHF, the data show.
Clinicians “need to consider methamphetamine as a potential etiology for heart failure and include a substance use history when evaluating patients. Treating methamphetamine use disorder improves heart failure outcomes,” first author Veena Manja, MD, PhD, with Stanford (Calif.) University, said in an interview.
The study was published online in the journal Heart.
Poor outcomes, ‘staggering’ costs
This “thoughtful” review is “important and necessary,” Jonathan Davis, MD, director of the heart failure program, Zuckerberg San Francisco General Hospital, wrote in an editorial in the journal.
Dr. Davis noted that patients with Meth HF are at increased risk for poor outcomes and death and the health care costs related to MethHF are “staggering.”
As an example, inpatient data for California show annual charges related to MethHF rose by 840% from 2008 to 2018, from $41.5 million to $390.2 million, compared with 82% for all HF, which rose from $3.5 billion to $6.8 billion.
Illicit use of methamphetamine – also known as “crystal meth,” “ice,” and “speed” – has been linked to hypertension, MI, stroke, aortic dissection, and sudden death. But until now, there was no comprehensive systematic review of published studies on MethHF.
“Our goal was to compile current knowledge on the topic, increase awareness of this condition and identify areas for future research,” Dr. Manja said.
The researchers reviewed 21 observational studies, mostly from the United States (14 from California), between 1997 and 2020. The mean age of adults with MethHF ranged in age from 35 to 60 and more than half were male (57%).
Illicit methamphetamine was inhaled, injected, swallowed, smoked, and snorted. The reported frequency ranged from daily to every other week, and the total monthly dose ranged from 0.35 g to 24.5 g.
The average duration of meth use before HF diagnosis was 5 years. However, 18% of users developed HF within 1 year of starting to use illicit methamphetamine. In some cases, HF was diagnosed after a single use.
The researchers also note that MethHF with preserved left ventricular ejection fraction, seen in up to 44% of cases, is a distinct entity that may progress to reduced LVEF with continued use.
MethHF is also associated with a greater likelihood of other substance abuse, PTSD, depression, and other heart and kidney disease.
Factors associated with improved MethHF outcomes include female sex, meth abstinence, and adherence to guideline-directed HF therapy.
Improvement in MethHF outcomes is possible even if abstinence is not consistent, a finding that lends support to harm reduction principles of “meeting patients where they are instead of insisting on complete abstinence,” the researchers said.
Large gaps in knowledge
They were unable to combine the results into a meta-analysis because of heterogeneity in study design, population, comparator, and outcome assessment. Also, the overall risk of bias is moderate because of the presence of confounders, selection bias and poor matching, and the overall certainty in the evidence is very low,.
No study evaluated the incidence or prevalence of HF among methamphetamine users and inconsistent history taking and testing in patients with HF impeded accurate MethHF prevalence assessment.
Several studies, however, document an increasing incidence of MethHF, particularly over the past decade.
One study from California reported a 585% increase in MethHF hospital admissions between 2008 and 2018. An analysis of the National Inpatient Survey found a 12-fold increase in annual MethHF hospitalizations between 2002 and 2014.
“The results of this systematic review highlight large gaps in our knowledge” of MethHF, Dr. Manja said in an interview.
“We need to understand the epidemiology, prevalence, factors that confer susceptibility to cardiovascular outcomes, and need research into treatment targeted toward this disease,” Dr. Manja added. “We should consider options to integrate substance use treatment in HF/cardiology/primary care clinics and design a multidisciplinary patient-centered approach.”
Dr. Davis agreed. This work “highlights that the standard of care academically and clinically must be a broad team across the care spectrum to simultaneously address methamphetamine use, heart failure, and social determinants of health.”
This research had no specific funding. Dr. Manja and Dr. Davis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
a literature review indicates.
MethHF is associated with increased severity for HF, longer inpatient stay, and more readmissions, compared with non-MethHF, the data show.
Clinicians “need to consider methamphetamine as a potential etiology for heart failure and include a substance use history when evaluating patients. Treating methamphetamine use disorder improves heart failure outcomes,” first author Veena Manja, MD, PhD, with Stanford (Calif.) University, said in an interview.
The study was published online in the journal Heart.
Poor outcomes, ‘staggering’ costs
This “thoughtful” review is “important and necessary,” Jonathan Davis, MD, director of the heart failure program, Zuckerberg San Francisco General Hospital, wrote in an editorial in the journal.
Dr. Davis noted that patients with Meth HF are at increased risk for poor outcomes and death and the health care costs related to MethHF are “staggering.”
As an example, inpatient data for California show annual charges related to MethHF rose by 840% from 2008 to 2018, from $41.5 million to $390.2 million, compared with 82% for all HF, which rose from $3.5 billion to $6.8 billion.
Illicit use of methamphetamine – also known as “crystal meth,” “ice,” and “speed” – has been linked to hypertension, MI, stroke, aortic dissection, and sudden death. But until now, there was no comprehensive systematic review of published studies on MethHF.
“Our goal was to compile current knowledge on the topic, increase awareness of this condition and identify areas for future research,” Dr. Manja said.
The researchers reviewed 21 observational studies, mostly from the United States (14 from California), between 1997 and 2020. The mean age of adults with MethHF ranged in age from 35 to 60 and more than half were male (57%).
Illicit methamphetamine was inhaled, injected, swallowed, smoked, and snorted. The reported frequency ranged from daily to every other week, and the total monthly dose ranged from 0.35 g to 24.5 g.
The average duration of meth use before HF diagnosis was 5 years. However, 18% of users developed HF within 1 year of starting to use illicit methamphetamine. In some cases, HF was diagnosed after a single use.
The researchers also note that MethHF with preserved left ventricular ejection fraction, seen in up to 44% of cases, is a distinct entity that may progress to reduced LVEF with continued use.
MethHF is also associated with a greater likelihood of other substance abuse, PTSD, depression, and other heart and kidney disease.
Factors associated with improved MethHF outcomes include female sex, meth abstinence, and adherence to guideline-directed HF therapy.
Improvement in MethHF outcomes is possible even if abstinence is not consistent, a finding that lends support to harm reduction principles of “meeting patients where they are instead of insisting on complete abstinence,” the researchers said.
Large gaps in knowledge
They were unable to combine the results into a meta-analysis because of heterogeneity in study design, population, comparator, and outcome assessment. Also, the overall risk of bias is moderate because of the presence of confounders, selection bias and poor matching, and the overall certainty in the evidence is very low,.
No study evaluated the incidence or prevalence of HF among methamphetamine users and inconsistent history taking and testing in patients with HF impeded accurate MethHF prevalence assessment.
Several studies, however, document an increasing incidence of MethHF, particularly over the past decade.
One study from California reported a 585% increase in MethHF hospital admissions between 2008 and 2018. An analysis of the National Inpatient Survey found a 12-fold increase in annual MethHF hospitalizations between 2002 and 2014.
“The results of this systematic review highlight large gaps in our knowledge” of MethHF, Dr. Manja said in an interview.
“We need to understand the epidemiology, prevalence, factors that confer susceptibility to cardiovascular outcomes, and need research into treatment targeted toward this disease,” Dr. Manja added. “We should consider options to integrate substance use treatment in HF/cardiology/primary care clinics and design a multidisciplinary patient-centered approach.”
Dr. Davis agreed. This work “highlights that the standard of care academically and clinically must be a broad team across the care spectrum to simultaneously address methamphetamine use, heart failure, and social determinants of health.”
This research had no specific funding. Dr. Manja and Dr. Davis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HEART
Clinical factors drive hospitalization after self-harm
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRIC RESEARCH











