User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Vaccine holdouts embrace COVID antibody treatment, mystifying doctors
Houston architect Lanson Jones is one of the nearly 80 million Americans who refuse to get a COVID-19 vaccine, arguing the shots are experimental, were rushed to market, may cause side effects, and aren’t all fully approved by federal officials.
But when he contracted COVID in September, he didn’t hesitate to seek treatment with monoclonal antibodies -- a year-old, laboratory-created therapy no less experimental than the vaccines that is not fully approved by the FDA and can also cause rare side effects.
“I haven’t done the shot because I hear a lot -- a lot -- of information about what are some of the effects of these vaccines and how it’s really not being reported, and I just felt I didn’t want to put something in me that has some question,” says Mr. Jones, 65.
“But with this monoclonal antibody treatment, I didn’t hesitate. I had no doubt in my mind -- not even one ounce of doubt about it. Not one person said, ‘Oh, well some people have had a reaction to it.’”
Mr. Jones, who was treated at Houston Methodist Hospital, is one of more than a million Americans who have received antibody IVs after getting the virus.
Those numbers are growing, with the federal government recently taking over distribution of the supplies of the drugs, which are limited in many states.
The treatment has been effective against COVID, in helping patients recover, stay out of the hospital, or die from the illness.
But what doctors and public health experts say is most surprising is that , as well.
“I think it’s irrational, quite frankly, if you have to boil it down to one word,” says Howard Huang, MD, who heads up Houston Methodist’s infusion program, which is providing up to 900 doses a week. “It really doesn’t make any sense on multiple levels.”
For one thing, he says, the FDA has just granted full approval for the COVID vaccine produced by Pfizer and BioNTech, upgrading its status from its emergency use authorization (EUA). Many experts expect the FDA to grant similar full approvals to the Moderna vaccine and possibly the Johnson and Johnson shot, which currently have EUA designations.
Many vaccine holdouts have cited the EUA status of the COVID vaccines -- one step shy of full approval -- as a reason they don’t trust the shot. But the antibody treatments have also been granted only EUA approval, which hasn’t stopped vaccine-resistant Americans from seeking them.
“So, they’re refusing an FDA-approved and tested [vaccine], and then they’re seeking something that’s still under an FDA EUA,” says Dr. Huang. “I just don’t get it. I really don’t.”
Amesh Adalja, MD, an emerging infectious diseases specialist with the Johns Hopkins University Center for Health Security, calls it “paradoxical” thinking for vaccine holdouts to refuse a shot that boosts your natural antibodies to prevent COVID, but take an antibody drug to treat it after infection.
“I don’t understand it, I can’t,” he says. “But the pandemic has been politicized and … I think consistency is not something to expect from people who are thinking about this irrationally [and] for people engaging in these conspiracies about the vaccine.
“I do think the fact that people like Joe Rogan and Gov. Abbot and Donald Trump received the monoclonal antibodies does probably play a role in some of the thinking in some of these individuals.”
Terry Scoggin, CEO of Titus Regional Medical Center in Mount Pleasant, Tex., says even the hospital’s doctors have been shocked by the demand for the new therapy among unvaccinated Texans.
“It’s mind-blowing that there’s been such resistance to the vaccine, but that demand for the monoclonal antibodies is so high,” he says, noting only 47% of adults in the region have received at least one dose of the shot. That’s far below CDC estimates that say 75.2% of American adults have received one shot, while 64.7% are fully vaccinated.
“But our doctors believe in the monoclonal antibodies, so it’s a trust factor -- they trust our community physicians,” Mr. Scoggin says. “I’ve never put the two and two together about the fear of the vaccine vs. [lack of fear] of the treatment. But it’s really interesting.”
Treatments effective, costly
Like the COVID vaccines given to nearly 214 million Americans, the antibody treatments taken by more than 1 million in the United States are highly effective and cause only rare (and usually minor) side effects.
Federal health officials say the infusions have helped keep the U.S. death toll -- now about 2,000 per day-- from soaring even higher, even as vaccine hesitancy persists, particularly in Southern states.
The FDA first authorized monoclonal antibody drugs in November 2020 -- just weeks before the vaccines were approved. But their popularity has soared as the Delta variant of the virus that causes COVID-19 has surged in recent months.
Clinical trials show that the drugs can cut COVID-related hospitalization or death in high-risk patients by as much as 70%-80%. They also can prevent infection in healthy people who have been exposed to an infected person, according to research published this month in The New England Journal of Medicine.
Monoclonal antibodies have been used for decades to treat cancer, autoimmune disorders, and other diseases, with the FDA approving nearly 100 such treatments since 1994.
The FDA has granted EUA approvals to four antibody treatments for COVID-19.
A two-antibody drug combination from Regeneron -- containing casirivimab and imdevimab -- has been shown to reduce the risk of hospitalization and death by 70% in people infected with COVID. Sotrovimab, made by GlaxoSmithKline and Vir, has had similar results.
The FDA approved a third treatment -- Eli Lilly’s combination of bamlanivimab and etesevimab -- in 2020, but the agency recommended against its use earlier this year after it proved ineffective against the Delta variant. The combination came back on the market in late August, but only in states where fewer than 5% of COVID infections are from strains, such as Delta, that are resistant to the treatment.
In June, the FDA authorized a fourth drug combination, Genentech’s tocilizumab, for people already hospitalized with COVID. But it is only moderately effective against the disease.
Lab-made monoclonal antibodies mimic the antibodies the body makes to fight viruses and illnesses. They work by targeting the spike protein on the surface of the virus. COVID vaccines work by priming the body’s immune system to recognize this very same spike protein and block it from entering your body’s cells, preventing infection.
Antibody treatments are given as an IV to treat an infection but can also be given as shots into the belly for people who have been exposed to the virus but have not yet been sickened by it, Dr. Huang says.
Timing is critical, he says, noting antibodies are most effective when given in the first few days after symptoms emerge.
Demands, concerns on the rise
Orders for monoclonal antibodies have skyrocketed in recent weeks -- to 168,000 doses per week in late August, up from 27,000 in July. The Biden administration, which has been covering the cost of the treatment for most patients, took over its distribution as well this week.
But experts foresee potential problems as patient demand increases.
Federal officials have already warned states of potential shortages ahead. Only about 2.4 million monoclonal antibody doses have been shipped nationally so far, less than half of which have been administered.
More supplies are on the way, with the federal government recently buying another 1.8 million doses for delivery in the months ahead. But for now, some hospitals are uncertain of supplies and are already struggling to meet the demand for the treatments.
Seven Southern states account for 70% of orders: Texas, Alabama, Florida, Mississippi, Tennessee, Georgia, and Louisiana. Those states have among the nation’s lowest vaccine rates and highest infection numbers.
Florida officials said the state’s latest weekly allotment left clinics 41,000 doses short of what they need. Tennessee has begun limiting treatments for unvaccinated patients to give priority to those most at risk of dying from COVID. And in Texas, elective surgeries have been postponed to make room for COVID-19 patients at some hospitals, as operating room nurses have been enlisted to give IVs.
Some strong proponents of monoclonal antibody treatments have been frustrated by Republican governors who are scrambling to push and deliver them, while opposing vaccine and mask mandates.
Raising vaccination rates, scientists say, would make the antibody treatments unnecessary in many cases.
Experts also note the drugs are far more costly than the vaccines -- with a price tag of about $2,100 for each IV, compared to $20-$40 for the shot.
“When you’re talking about just the cost to society as a whole -- turning down a [vaccine] that costs a couple dozen dollars for therapies that cost thousands of dollars -- it just doesn’t make any sense,” says Dr. Huang.
“And the tragedy is that a lot of these infections right now are preventable. It’s not like the pre-vaccine days, when we didn’t have anything better. And for these people, it’s just hard to justify that line of thinking. And so, the challenge is changing people’s minds. And that’s really been the difficult thing.”
In addition, the treatments take 90 minutes to administer, taxing health care workers in hard-hit states that have been slammed by the influx of patients.
Beyond these issues, Dr. Huang cites other public health costs of people choosing treatment over vaccination. The vaccine protects others because it limits transmission of the virus. By contrast, a single antibody IV helps only that patient and does not keep people from infecting others or becoming reinfected, requiring another IV.
“Getting the vaccine helps people beyond yourself; it helps the community, too,” he notes. “There’s just a strong argument for getting the vaccine. I obviously have a very biased opinion, but I would hope I have more of a scientific or expert opinion, but that doesn’t seem to matter these days.”
Vaccine resistance still remains for some
Seth Thurman, an IT technician from Mount Pleasant, Tex., acknowledges he was hesitant to get the vaccine at first because he felt it was fast-tracked, “experimental,” might cause unknown side effects, was developed quickly, and was being pushed by government officials.
“I shared the same sentiments as a lot of other people [as] some of the reasons why I might have been hesitant in the beginning to get the vaccine, says Mr. Thurman, 47. “A lot of people don’t trust what’s out there, maybe what the government is pushing, so I was taking a wait-and-see approach.”
In August, he relented and received the first of the two-shot Moderna vaccine. But several weeks later, he developed COVID and took his doctor’s advice to receive antibody therapy at Titus Regional Medical Center.
The results were almost immediate.
“I noticed within just a few hours of getting that infusion I was feeling better,” he says. “And by the next day, I was feeling great. No more temperature and no cough and no loss of taste and smell. And today, I’m 100%.”
Having had COVID convinced him of the importance of getting the vaccine, and he plans to get the second dose of the shot after the prescribed 90-day waiting period.
But Mr. Jones, the Houston architect, remains unconvinced, even after suffering what he describes as a “horrible” experience with COVID.
“It’s something I’m still thinking about,” he says of the vaccine. “But I can’t imagine that there wouldn’t be some sort of side effects from something that was developed so fast and had not gone through 4 or 5 years of vetting or trials. So that kind of just leaves doubt in my mind.
“And it’s just so weird that something so personal has become so public -- like people’s medical decisions now are on the front page of The New York Times. When did we think something like that would ever happen?”
The quick results of his treatment were so “remarkable” that he’d recommend it to anyone without hesitation, he says.
“If my story can help people be willing to seek out this infusion and take it early on in their COVID experience, I think it would not only save lives and keep people out of our hospitals and not overwhelm our hospital systems,” he says.
Dr. Huang agrees that the IV therapy is a great “fallback option” for people who’ve been infected, who have weakened immune systems, or can’t receive the vaccine for other health reasons. But for most people, he argues, the vaccine is the best way to go. That’s why Houston Methodist advises the shot for every patient like Mr. Jones, who’s been treated for COVID.
“Getting the vaccine is the way to go for the vast number of people,” he says.
Frederick Thurmond, MD, who oversees COVID-related care at Titus Regional Medical Center, believes it will take more than just doctors’ recommendations to move some patients to get the vaccine. The only thing that will motivate some will be contracting COVID, or knowing someone who does, he says.
“It’s clear that at least here in Texas, I swear man, you tell people they need to do something, and they just say, ‘Well, then I’m NOT going to do it,’” he says. “But once you’ve got COVID, the game becomes a whole lot more serious. And I think most people in the U.S. know someone who’s died from COVID at this point.”
Dr. Thurmond says that for some patients, stubborn resistance to legitimate medical advice persists -- on the vaccine and even treatment -- even after infection.
“We have seen more than one person avoid any medical care whatsoever after they knew they had COVID,” he says. “They languish in private and eventually come to the emergency room extremely sick and doing things with little to no medical value -- such as taking a friend’s hydroxychloroquine, random antibiotics, a horse de-worming dose of ivermectin, and gargling with Betadine and even bleach.”
But most of his patients who have the IV therapy take his advice to get the vaccine afterward.
“The only way to end the pandemic is to vaccinate everybody,” he says.
Dr. Adalja agrees.
“The monoclonal antibodies work, they are great drugs, so I think it is appropriate to praise them,” says Dr. Adalja, who’s given them to his own patients. “But it’s not appropriate to use them as an alternative to vaccination or to think, you know, don’t worry about the getting the vaccine because if you get infected and get the monoclonal antibodies to get through this -- that’s not the way to approach it.
He also worries about what he calls “dark-age mentalities” that have fueled the anti-vaccine movement, which has sought to heighten fears of modern medicine and doctors.
“The anti-vaccine movement has really capitalized on COVID-19, and it’s really a much more virulent form of the anti-vaccine movement than what we’ve seen with measles and other diseases in the past,” he notes. “And I think it’s going to be very difficult to contend with in the future, because no one thought we’d be battling the anti-vaccine movement this late in the pandemic.”
The biggest takeaway?
“When it comes to an infectious disease, prevention is always much better than treatment,” Dr. Adalja says. “If you don’t even need to get to the treatment stage because you prevent people from getting infected, that’s the goal.”
A version of this article first appeared on WebMD.com.
Houston architect Lanson Jones is one of the nearly 80 million Americans who refuse to get a COVID-19 vaccine, arguing the shots are experimental, were rushed to market, may cause side effects, and aren’t all fully approved by federal officials.
But when he contracted COVID in September, he didn’t hesitate to seek treatment with monoclonal antibodies -- a year-old, laboratory-created therapy no less experimental than the vaccines that is not fully approved by the FDA and can also cause rare side effects.
“I haven’t done the shot because I hear a lot -- a lot -- of information about what are some of the effects of these vaccines and how it’s really not being reported, and I just felt I didn’t want to put something in me that has some question,” says Mr. Jones, 65.
“But with this monoclonal antibody treatment, I didn’t hesitate. I had no doubt in my mind -- not even one ounce of doubt about it. Not one person said, ‘Oh, well some people have had a reaction to it.’”
Mr. Jones, who was treated at Houston Methodist Hospital, is one of more than a million Americans who have received antibody IVs after getting the virus.
Those numbers are growing, with the federal government recently taking over distribution of the supplies of the drugs, which are limited in many states.
The treatment has been effective against COVID, in helping patients recover, stay out of the hospital, or die from the illness.
But what doctors and public health experts say is most surprising is that , as well.
“I think it’s irrational, quite frankly, if you have to boil it down to one word,” says Howard Huang, MD, who heads up Houston Methodist’s infusion program, which is providing up to 900 doses a week. “It really doesn’t make any sense on multiple levels.”
For one thing, he says, the FDA has just granted full approval for the COVID vaccine produced by Pfizer and BioNTech, upgrading its status from its emergency use authorization (EUA). Many experts expect the FDA to grant similar full approvals to the Moderna vaccine and possibly the Johnson and Johnson shot, which currently have EUA designations.
Many vaccine holdouts have cited the EUA status of the COVID vaccines -- one step shy of full approval -- as a reason they don’t trust the shot. But the antibody treatments have also been granted only EUA approval, which hasn’t stopped vaccine-resistant Americans from seeking them.
“So, they’re refusing an FDA-approved and tested [vaccine], and then they’re seeking something that’s still under an FDA EUA,” says Dr. Huang. “I just don’t get it. I really don’t.”
Amesh Adalja, MD, an emerging infectious diseases specialist with the Johns Hopkins University Center for Health Security, calls it “paradoxical” thinking for vaccine holdouts to refuse a shot that boosts your natural antibodies to prevent COVID, but take an antibody drug to treat it after infection.
“I don’t understand it, I can’t,” he says. “But the pandemic has been politicized and … I think consistency is not something to expect from people who are thinking about this irrationally [and] for people engaging in these conspiracies about the vaccine.
“I do think the fact that people like Joe Rogan and Gov. Abbot and Donald Trump received the monoclonal antibodies does probably play a role in some of the thinking in some of these individuals.”
Terry Scoggin, CEO of Titus Regional Medical Center in Mount Pleasant, Tex., says even the hospital’s doctors have been shocked by the demand for the new therapy among unvaccinated Texans.
“It’s mind-blowing that there’s been such resistance to the vaccine, but that demand for the monoclonal antibodies is so high,” he says, noting only 47% of adults in the region have received at least one dose of the shot. That’s far below CDC estimates that say 75.2% of American adults have received one shot, while 64.7% are fully vaccinated.
“But our doctors believe in the monoclonal antibodies, so it’s a trust factor -- they trust our community physicians,” Mr. Scoggin says. “I’ve never put the two and two together about the fear of the vaccine vs. [lack of fear] of the treatment. But it’s really interesting.”
Treatments effective, costly
Like the COVID vaccines given to nearly 214 million Americans, the antibody treatments taken by more than 1 million in the United States are highly effective and cause only rare (and usually minor) side effects.
Federal health officials say the infusions have helped keep the U.S. death toll -- now about 2,000 per day-- from soaring even higher, even as vaccine hesitancy persists, particularly in Southern states.
The FDA first authorized monoclonal antibody drugs in November 2020 -- just weeks before the vaccines were approved. But their popularity has soared as the Delta variant of the virus that causes COVID-19 has surged in recent months.
Clinical trials show that the drugs can cut COVID-related hospitalization or death in high-risk patients by as much as 70%-80%. They also can prevent infection in healthy people who have been exposed to an infected person, according to research published this month in The New England Journal of Medicine.
Monoclonal antibodies have been used for decades to treat cancer, autoimmune disorders, and other diseases, with the FDA approving nearly 100 such treatments since 1994.
The FDA has granted EUA approvals to four antibody treatments for COVID-19.
A two-antibody drug combination from Regeneron -- containing casirivimab and imdevimab -- has been shown to reduce the risk of hospitalization and death by 70% in people infected with COVID. Sotrovimab, made by GlaxoSmithKline and Vir, has had similar results.
The FDA approved a third treatment -- Eli Lilly’s combination of bamlanivimab and etesevimab -- in 2020, but the agency recommended against its use earlier this year after it proved ineffective against the Delta variant. The combination came back on the market in late August, but only in states where fewer than 5% of COVID infections are from strains, such as Delta, that are resistant to the treatment.
In June, the FDA authorized a fourth drug combination, Genentech’s tocilizumab, for people already hospitalized with COVID. But it is only moderately effective against the disease.
Lab-made monoclonal antibodies mimic the antibodies the body makes to fight viruses and illnesses. They work by targeting the spike protein on the surface of the virus. COVID vaccines work by priming the body’s immune system to recognize this very same spike protein and block it from entering your body’s cells, preventing infection.
Antibody treatments are given as an IV to treat an infection but can also be given as shots into the belly for people who have been exposed to the virus but have not yet been sickened by it, Dr. Huang says.
Timing is critical, he says, noting antibodies are most effective when given in the first few days after symptoms emerge.
Demands, concerns on the rise
Orders for monoclonal antibodies have skyrocketed in recent weeks -- to 168,000 doses per week in late August, up from 27,000 in July. The Biden administration, which has been covering the cost of the treatment for most patients, took over its distribution as well this week.
But experts foresee potential problems as patient demand increases.
Federal officials have already warned states of potential shortages ahead. Only about 2.4 million monoclonal antibody doses have been shipped nationally so far, less than half of which have been administered.
More supplies are on the way, with the federal government recently buying another 1.8 million doses for delivery in the months ahead. But for now, some hospitals are uncertain of supplies and are already struggling to meet the demand for the treatments.
Seven Southern states account for 70% of orders: Texas, Alabama, Florida, Mississippi, Tennessee, Georgia, and Louisiana. Those states have among the nation’s lowest vaccine rates and highest infection numbers.
Florida officials said the state’s latest weekly allotment left clinics 41,000 doses short of what they need. Tennessee has begun limiting treatments for unvaccinated patients to give priority to those most at risk of dying from COVID. And in Texas, elective surgeries have been postponed to make room for COVID-19 patients at some hospitals, as operating room nurses have been enlisted to give IVs.
Some strong proponents of monoclonal antibody treatments have been frustrated by Republican governors who are scrambling to push and deliver them, while opposing vaccine and mask mandates.
Raising vaccination rates, scientists say, would make the antibody treatments unnecessary in many cases.
Experts also note the drugs are far more costly than the vaccines -- with a price tag of about $2,100 for each IV, compared to $20-$40 for the shot.
“When you’re talking about just the cost to society as a whole -- turning down a [vaccine] that costs a couple dozen dollars for therapies that cost thousands of dollars -- it just doesn’t make any sense,” says Dr. Huang.
“And the tragedy is that a lot of these infections right now are preventable. It’s not like the pre-vaccine days, when we didn’t have anything better. And for these people, it’s just hard to justify that line of thinking. And so, the challenge is changing people’s minds. And that’s really been the difficult thing.”
In addition, the treatments take 90 minutes to administer, taxing health care workers in hard-hit states that have been slammed by the influx of patients.
Beyond these issues, Dr. Huang cites other public health costs of people choosing treatment over vaccination. The vaccine protects others because it limits transmission of the virus. By contrast, a single antibody IV helps only that patient and does not keep people from infecting others or becoming reinfected, requiring another IV.
“Getting the vaccine helps people beyond yourself; it helps the community, too,” he notes. “There’s just a strong argument for getting the vaccine. I obviously have a very biased opinion, but I would hope I have more of a scientific or expert opinion, but that doesn’t seem to matter these days.”
Vaccine resistance still remains for some
Seth Thurman, an IT technician from Mount Pleasant, Tex., acknowledges he was hesitant to get the vaccine at first because he felt it was fast-tracked, “experimental,” might cause unknown side effects, was developed quickly, and was being pushed by government officials.
“I shared the same sentiments as a lot of other people [as] some of the reasons why I might have been hesitant in the beginning to get the vaccine, says Mr. Thurman, 47. “A lot of people don’t trust what’s out there, maybe what the government is pushing, so I was taking a wait-and-see approach.”
In August, he relented and received the first of the two-shot Moderna vaccine. But several weeks later, he developed COVID and took his doctor’s advice to receive antibody therapy at Titus Regional Medical Center.
The results were almost immediate.
“I noticed within just a few hours of getting that infusion I was feeling better,” he says. “And by the next day, I was feeling great. No more temperature and no cough and no loss of taste and smell. And today, I’m 100%.”
Having had COVID convinced him of the importance of getting the vaccine, and he plans to get the second dose of the shot after the prescribed 90-day waiting period.
But Mr. Jones, the Houston architect, remains unconvinced, even after suffering what he describes as a “horrible” experience with COVID.
“It’s something I’m still thinking about,” he says of the vaccine. “But I can’t imagine that there wouldn’t be some sort of side effects from something that was developed so fast and had not gone through 4 or 5 years of vetting or trials. So that kind of just leaves doubt in my mind.
“And it’s just so weird that something so personal has become so public -- like people’s medical decisions now are on the front page of The New York Times. When did we think something like that would ever happen?”
The quick results of his treatment were so “remarkable” that he’d recommend it to anyone without hesitation, he says.
“If my story can help people be willing to seek out this infusion and take it early on in their COVID experience, I think it would not only save lives and keep people out of our hospitals and not overwhelm our hospital systems,” he says.
Dr. Huang agrees that the IV therapy is a great “fallback option” for people who’ve been infected, who have weakened immune systems, or can’t receive the vaccine for other health reasons. But for most people, he argues, the vaccine is the best way to go. That’s why Houston Methodist advises the shot for every patient like Mr. Jones, who’s been treated for COVID.
“Getting the vaccine is the way to go for the vast number of people,” he says.
Frederick Thurmond, MD, who oversees COVID-related care at Titus Regional Medical Center, believes it will take more than just doctors’ recommendations to move some patients to get the vaccine. The only thing that will motivate some will be contracting COVID, or knowing someone who does, he says.
“It’s clear that at least here in Texas, I swear man, you tell people they need to do something, and they just say, ‘Well, then I’m NOT going to do it,’” he says. “But once you’ve got COVID, the game becomes a whole lot more serious. And I think most people in the U.S. know someone who’s died from COVID at this point.”
Dr. Thurmond says that for some patients, stubborn resistance to legitimate medical advice persists -- on the vaccine and even treatment -- even after infection.
“We have seen more than one person avoid any medical care whatsoever after they knew they had COVID,” he says. “They languish in private and eventually come to the emergency room extremely sick and doing things with little to no medical value -- such as taking a friend’s hydroxychloroquine, random antibiotics, a horse de-worming dose of ivermectin, and gargling with Betadine and even bleach.”
But most of his patients who have the IV therapy take his advice to get the vaccine afterward.
“The only way to end the pandemic is to vaccinate everybody,” he says.
Dr. Adalja agrees.
“The monoclonal antibodies work, they are great drugs, so I think it is appropriate to praise them,” says Dr. Adalja, who’s given them to his own patients. “But it’s not appropriate to use them as an alternative to vaccination or to think, you know, don’t worry about the getting the vaccine because if you get infected and get the monoclonal antibodies to get through this -- that’s not the way to approach it.
He also worries about what he calls “dark-age mentalities” that have fueled the anti-vaccine movement, which has sought to heighten fears of modern medicine and doctors.
“The anti-vaccine movement has really capitalized on COVID-19, and it’s really a much more virulent form of the anti-vaccine movement than what we’ve seen with measles and other diseases in the past,” he notes. “And I think it’s going to be very difficult to contend with in the future, because no one thought we’d be battling the anti-vaccine movement this late in the pandemic.”
The biggest takeaway?
“When it comes to an infectious disease, prevention is always much better than treatment,” Dr. Adalja says. “If you don’t even need to get to the treatment stage because you prevent people from getting infected, that’s the goal.”
A version of this article first appeared on WebMD.com.
Houston architect Lanson Jones is one of the nearly 80 million Americans who refuse to get a COVID-19 vaccine, arguing the shots are experimental, were rushed to market, may cause side effects, and aren’t all fully approved by federal officials.
But when he contracted COVID in September, he didn’t hesitate to seek treatment with monoclonal antibodies -- a year-old, laboratory-created therapy no less experimental than the vaccines that is not fully approved by the FDA and can also cause rare side effects.
“I haven’t done the shot because I hear a lot -- a lot -- of information about what are some of the effects of these vaccines and how it’s really not being reported, and I just felt I didn’t want to put something in me that has some question,” says Mr. Jones, 65.
“But with this monoclonal antibody treatment, I didn’t hesitate. I had no doubt in my mind -- not even one ounce of doubt about it. Not one person said, ‘Oh, well some people have had a reaction to it.’”
Mr. Jones, who was treated at Houston Methodist Hospital, is one of more than a million Americans who have received antibody IVs after getting the virus.
Those numbers are growing, with the federal government recently taking over distribution of the supplies of the drugs, which are limited in many states.
The treatment has been effective against COVID, in helping patients recover, stay out of the hospital, or die from the illness.
But what doctors and public health experts say is most surprising is that , as well.
“I think it’s irrational, quite frankly, if you have to boil it down to one word,” says Howard Huang, MD, who heads up Houston Methodist’s infusion program, which is providing up to 900 doses a week. “It really doesn’t make any sense on multiple levels.”
For one thing, he says, the FDA has just granted full approval for the COVID vaccine produced by Pfizer and BioNTech, upgrading its status from its emergency use authorization (EUA). Many experts expect the FDA to grant similar full approvals to the Moderna vaccine and possibly the Johnson and Johnson shot, which currently have EUA designations.
Many vaccine holdouts have cited the EUA status of the COVID vaccines -- one step shy of full approval -- as a reason they don’t trust the shot. But the antibody treatments have also been granted only EUA approval, which hasn’t stopped vaccine-resistant Americans from seeking them.
“So, they’re refusing an FDA-approved and tested [vaccine], and then they’re seeking something that’s still under an FDA EUA,” says Dr. Huang. “I just don’t get it. I really don’t.”
Amesh Adalja, MD, an emerging infectious diseases specialist with the Johns Hopkins University Center for Health Security, calls it “paradoxical” thinking for vaccine holdouts to refuse a shot that boosts your natural antibodies to prevent COVID, but take an antibody drug to treat it after infection.
“I don’t understand it, I can’t,” he says. “But the pandemic has been politicized and … I think consistency is not something to expect from people who are thinking about this irrationally [and] for people engaging in these conspiracies about the vaccine.
“I do think the fact that people like Joe Rogan and Gov. Abbot and Donald Trump received the monoclonal antibodies does probably play a role in some of the thinking in some of these individuals.”
Terry Scoggin, CEO of Titus Regional Medical Center in Mount Pleasant, Tex., says even the hospital’s doctors have been shocked by the demand for the new therapy among unvaccinated Texans.
“It’s mind-blowing that there’s been such resistance to the vaccine, but that demand for the monoclonal antibodies is so high,” he says, noting only 47% of adults in the region have received at least one dose of the shot. That’s far below CDC estimates that say 75.2% of American adults have received one shot, while 64.7% are fully vaccinated.
“But our doctors believe in the monoclonal antibodies, so it’s a trust factor -- they trust our community physicians,” Mr. Scoggin says. “I’ve never put the two and two together about the fear of the vaccine vs. [lack of fear] of the treatment. But it’s really interesting.”
Treatments effective, costly
Like the COVID vaccines given to nearly 214 million Americans, the antibody treatments taken by more than 1 million in the United States are highly effective and cause only rare (and usually minor) side effects.
Federal health officials say the infusions have helped keep the U.S. death toll -- now about 2,000 per day-- from soaring even higher, even as vaccine hesitancy persists, particularly in Southern states.
The FDA first authorized monoclonal antibody drugs in November 2020 -- just weeks before the vaccines were approved. But their popularity has soared as the Delta variant of the virus that causes COVID-19 has surged in recent months.
Clinical trials show that the drugs can cut COVID-related hospitalization or death in high-risk patients by as much as 70%-80%. They also can prevent infection in healthy people who have been exposed to an infected person, according to research published this month in The New England Journal of Medicine.
Monoclonal antibodies have been used for decades to treat cancer, autoimmune disorders, and other diseases, with the FDA approving nearly 100 such treatments since 1994.
The FDA has granted EUA approvals to four antibody treatments for COVID-19.
A two-antibody drug combination from Regeneron -- containing casirivimab and imdevimab -- has been shown to reduce the risk of hospitalization and death by 70% in people infected with COVID. Sotrovimab, made by GlaxoSmithKline and Vir, has had similar results.
The FDA approved a third treatment -- Eli Lilly’s combination of bamlanivimab and etesevimab -- in 2020, but the agency recommended against its use earlier this year after it proved ineffective against the Delta variant. The combination came back on the market in late August, but only in states where fewer than 5% of COVID infections are from strains, such as Delta, that are resistant to the treatment.
In June, the FDA authorized a fourth drug combination, Genentech’s tocilizumab, for people already hospitalized with COVID. But it is only moderately effective against the disease.
Lab-made monoclonal antibodies mimic the antibodies the body makes to fight viruses and illnesses. They work by targeting the spike protein on the surface of the virus. COVID vaccines work by priming the body’s immune system to recognize this very same spike protein and block it from entering your body’s cells, preventing infection.
Antibody treatments are given as an IV to treat an infection but can also be given as shots into the belly for people who have been exposed to the virus but have not yet been sickened by it, Dr. Huang says.
Timing is critical, he says, noting antibodies are most effective when given in the first few days after symptoms emerge.
Demands, concerns on the rise
Orders for monoclonal antibodies have skyrocketed in recent weeks -- to 168,000 doses per week in late August, up from 27,000 in July. The Biden administration, which has been covering the cost of the treatment for most patients, took over its distribution as well this week.
But experts foresee potential problems as patient demand increases.
Federal officials have already warned states of potential shortages ahead. Only about 2.4 million monoclonal antibody doses have been shipped nationally so far, less than half of which have been administered.
More supplies are on the way, with the federal government recently buying another 1.8 million doses for delivery in the months ahead. But for now, some hospitals are uncertain of supplies and are already struggling to meet the demand for the treatments.
Seven Southern states account for 70% of orders: Texas, Alabama, Florida, Mississippi, Tennessee, Georgia, and Louisiana. Those states have among the nation’s lowest vaccine rates and highest infection numbers.
Florida officials said the state’s latest weekly allotment left clinics 41,000 doses short of what they need. Tennessee has begun limiting treatments for unvaccinated patients to give priority to those most at risk of dying from COVID. And in Texas, elective surgeries have been postponed to make room for COVID-19 patients at some hospitals, as operating room nurses have been enlisted to give IVs.
Some strong proponents of monoclonal antibody treatments have been frustrated by Republican governors who are scrambling to push and deliver them, while opposing vaccine and mask mandates.
Raising vaccination rates, scientists say, would make the antibody treatments unnecessary in many cases.
Experts also note the drugs are far more costly than the vaccines -- with a price tag of about $2,100 for each IV, compared to $20-$40 for the shot.
“When you’re talking about just the cost to society as a whole -- turning down a [vaccine] that costs a couple dozen dollars for therapies that cost thousands of dollars -- it just doesn’t make any sense,” says Dr. Huang.
“And the tragedy is that a lot of these infections right now are preventable. It’s not like the pre-vaccine days, when we didn’t have anything better. And for these people, it’s just hard to justify that line of thinking. And so, the challenge is changing people’s minds. And that’s really been the difficult thing.”
In addition, the treatments take 90 minutes to administer, taxing health care workers in hard-hit states that have been slammed by the influx of patients.
Beyond these issues, Dr. Huang cites other public health costs of people choosing treatment over vaccination. The vaccine protects others because it limits transmission of the virus. By contrast, a single antibody IV helps only that patient and does not keep people from infecting others or becoming reinfected, requiring another IV.
“Getting the vaccine helps people beyond yourself; it helps the community, too,” he notes. “There’s just a strong argument for getting the vaccine. I obviously have a very biased opinion, but I would hope I have more of a scientific or expert opinion, but that doesn’t seem to matter these days.”
Vaccine resistance still remains for some
Seth Thurman, an IT technician from Mount Pleasant, Tex., acknowledges he was hesitant to get the vaccine at first because he felt it was fast-tracked, “experimental,” might cause unknown side effects, was developed quickly, and was being pushed by government officials.
“I shared the same sentiments as a lot of other people [as] some of the reasons why I might have been hesitant in the beginning to get the vaccine, says Mr. Thurman, 47. “A lot of people don’t trust what’s out there, maybe what the government is pushing, so I was taking a wait-and-see approach.”
In August, he relented and received the first of the two-shot Moderna vaccine. But several weeks later, he developed COVID and took his doctor’s advice to receive antibody therapy at Titus Regional Medical Center.
The results were almost immediate.
“I noticed within just a few hours of getting that infusion I was feeling better,” he says. “And by the next day, I was feeling great. No more temperature and no cough and no loss of taste and smell. And today, I’m 100%.”
Having had COVID convinced him of the importance of getting the vaccine, and he plans to get the second dose of the shot after the prescribed 90-day waiting period.
But Mr. Jones, the Houston architect, remains unconvinced, even after suffering what he describes as a “horrible” experience with COVID.
“It’s something I’m still thinking about,” he says of the vaccine. “But I can’t imagine that there wouldn’t be some sort of side effects from something that was developed so fast and had not gone through 4 or 5 years of vetting or trials. So that kind of just leaves doubt in my mind.
“And it’s just so weird that something so personal has become so public -- like people’s medical decisions now are on the front page of The New York Times. When did we think something like that would ever happen?”
The quick results of his treatment were so “remarkable” that he’d recommend it to anyone without hesitation, he says.
“If my story can help people be willing to seek out this infusion and take it early on in their COVID experience, I think it would not only save lives and keep people out of our hospitals and not overwhelm our hospital systems,” he says.
Dr. Huang agrees that the IV therapy is a great “fallback option” for people who’ve been infected, who have weakened immune systems, or can’t receive the vaccine for other health reasons. But for most people, he argues, the vaccine is the best way to go. That’s why Houston Methodist advises the shot for every patient like Mr. Jones, who’s been treated for COVID.
“Getting the vaccine is the way to go for the vast number of people,” he says.
Frederick Thurmond, MD, who oversees COVID-related care at Titus Regional Medical Center, believes it will take more than just doctors’ recommendations to move some patients to get the vaccine. The only thing that will motivate some will be contracting COVID, or knowing someone who does, he says.
“It’s clear that at least here in Texas, I swear man, you tell people they need to do something, and they just say, ‘Well, then I’m NOT going to do it,’” he says. “But once you’ve got COVID, the game becomes a whole lot more serious. And I think most people in the U.S. know someone who’s died from COVID at this point.”
Dr. Thurmond says that for some patients, stubborn resistance to legitimate medical advice persists -- on the vaccine and even treatment -- even after infection.
“We have seen more than one person avoid any medical care whatsoever after they knew they had COVID,” he says. “They languish in private and eventually come to the emergency room extremely sick and doing things with little to no medical value -- such as taking a friend’s hydroxychloroquine, random antibiotics, a horse de-worming dose of ivermectin, and gargling with Betadine and even bleach.”
But most of his patients who have the IV therapy take his advice to get the vaccine afterward.
“The only way to end the pandemic is to vaccinate everybody,” he says.
Dr. Adalja agrees.
“The monoclonal antibodies work, they are great drugs, so I think it is appropriate to praise them,” says Dr. Adalja, who’s given them to his own patients. “But it’s not appropriate to use them as an alternative to vaccination or to think, you know, don’t worry about the getting the vaccine because if you get infected and get the monoclonal antibodies to get through this -- that’s not the way to approach it.
He also worries about what he calls “dark-age mentalities” that have fueled the anti-vaccine movement, which has sought to heighten fears of modern medicine and doctors.
“The anti-vaccine movement has really capitalized on COVID-19, and it’s really a much more virulent form of the anti-vaccine movement than what we’ve seen with measles and other diseases in the past,” he notes. “And I think it’s going to be very difficult to contend with in the future, because no one thought we’d be battling the anti-vaccine movement this late in the pandemic.”
The biggest takeaway?
“When it comes to an infectious disease, prevention is always much better than treatment,” Dr. Adalja says. “If you don’t even need to get to the treatment stage because you prevent people from getting infected, that’s the goal.”
A version of this article first appeared on WebMD.com.
Pfizer COVID vaccine antibodies may disappear in 7 months, study says
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
Opioid prescribing mapped: Alabama highest, New York lowest
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.
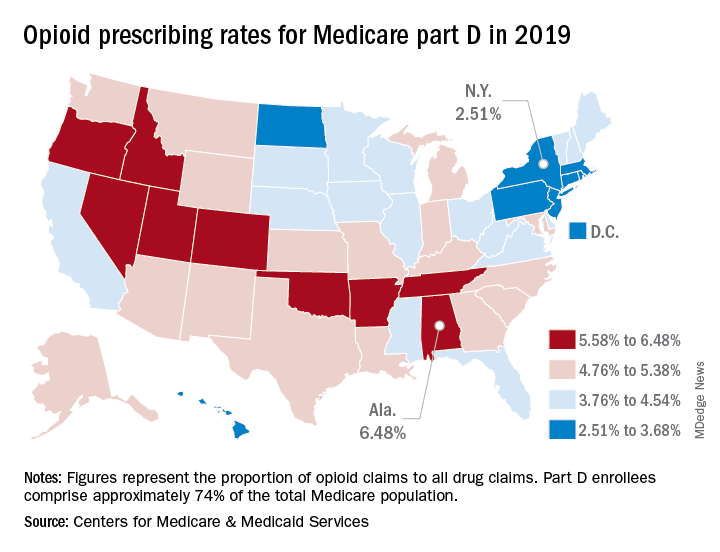
That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Should clinicians recommend vitamin D for psychiatric patients during COVID-19?
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
New data illustrate pandemic pivot to telehealth by patients, physicians
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Predicted pandemic retirement of many physicians hasn’t happened
The number of physicians who have chosen early retirement or have left medicine because of the COVID-19 pandemic may be considerably lower than previously thought, results of a new study suggest.
The research letter in the Journal of the American Medical Association, based on Medicare claims data, stated that “practice interruption rates were similar before and during the COVID-19 pandemic, except for a spike in April 2020.”
By contrast, in a Physicians Foundation Survey conducted in August 2020, 8% of physicians said they had closed their practices as a result of COVID, and 4% of the respondents said they planned to leave their practices within the next 12 months.
Similarly, a Jackson Physician Search survey in the fourth quarter of 2020 found that 54% of physicians surveyed had changed their employment plans. Of those doctors, 21% said they might hang up their white coat for early retirement. That works out to about 11% of the respondents.
The JAMA study’s authors analyzed the Medicare claims data from Jan. 1, 2019, to Dec. 30, 2020, to see how many physicians with Medicare patients had stopped filing claims for a period during those 2 years.
If a doctor had ceased submitting claims and then resumed filing them within 6 months after the last billing month, the lapse in filing was defined as “interruption with return.” If a physician stopped filing claims to Medicare and did not resume within 6 months, the gap in filing was called “interruption without return.”
In April 2020, 6.9% of physicians billing Medicare had a practice interruption, compared to 1.4% in 2019. But only 1.1% of physicians stopped practice in April 2020 and did not return, compared with 0.33% in 2019.
Physicians aged 55 or older had higher rates of interruption both with and without return than younger doctors did. The change in interruption rates for older doctors was 7.2% vs. 3.9% for younger physicians. The change in older physicians’ interruption-without-return rate was 1.3% vs. 0.34% for younger colleagues.
“Female physicians, specialists, physicians in smaller practices, those not in a health professional shortage area, and those practicing in a metropolitan area experienced greater increases in practice interruption rates in April 2020 vs. April 2019,” the study states. “But those groups typically had higher rates of return, so the overall changes in practice interruptions without return were similar across characteristics other than age.”
Significance for retirement rate
Discussing these results, the authors stressed that practice interruptions without return can’t necessarily be attributed to retirement, and that practice interruptions with return don’t necessarily signify that doctors had been furloughed from their practices.
Also, they said, “this measure of practice interruption likely misses meaningful interruptions that lasted for less than a month or did not involve complete cessation in treating Medicare patients.”
Nevertheless, “the study does capture a signal of some doctors probably retiring,” Jonathan Weiner, DPH, professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health, said in an interview.
But he added, “Some of those people who interrupted their practices and didn’t return may still come back. And there are probably a lot of other doctors who are leaving or changing practices that they didn’t capture.” For example, it’s possible that some doctors who went to work for other health care organizations stopped billing under their own names.
In Dr. Weiner’s view, the true percentage of physicians who have retired since the start of the pandemic is probably somewhere between the portion of doctors who interrupted their practice without return, according to the JAMA study, and the percentage of physicians who said they had closed their practices in the Physicians Foundation survey.
No mass exodus seen
Michael Belkin, JD, divisional vice president of recruiting for Merritt Hawkins, a physician search firm, said in an interview that the real number may be closer to the interruption-without-return figure in the JAMA study.
While many physician practices were disrupted in spring of 2020, he said, “it really didn’t result in a mass exodus [from health care]. We’re not talking to a lot of candidates who retired or walked away from their practices. We are talking to candidates who slowed down last year and then realized that they wanted to get back into medicine. And now they’re actively looking.”
One change in job candidates’ attitude, Mr. Belkin said, is that, because of COVID-19–related burnout, their quality of life is more important to them.
“They want to know, ‘What’s the culture of the employer like? What did they do last year during COVID? How did they handle it? Have they put together any protocols for the next pandemic?’ “
Demand for doctors has returned
In the summer of 2020, there was a major drop in physician recruitment by hospitals and health systems, partly because of fewer patient visits and procedures. But demand for doctors has bounced back over the past year, Mr. Belkin noted. One reason is the pent-up need for care among patients who avoided health care providers in 2020.
Another reason is that some employed doctors – particularly older physicians – have slowed down. Many doctors prefer to work remotely 1 or 2 days a week, providing telehealth visits to patients. That has led to a loss of productivity in many health care organizations and, consequently, a need to hire additional physicians.
Nevertheless, not many doctors are heading for the exit earlier than physicians did before COVID-19.
“They may work reduced hours,” Mr. Belkin said. “But the sense from a physician’s perspective is that this is all they know. For them to walk away from their life in medicine, from who they are, is problematic. So they’re continuing to practice, but at a reduced capacity.”
A version of this article first appeared on Medscape.com.
The number of physicians who have chosen early retirement or have left medicine because of the COVID-19 pandemic may be considerably lower than previously thought, results of a new study suggest.
The research letter in the Journal of the American Medical Association, based on Medicare claims data, stated that “practice interruption rates were similar before and during the COVID-19 pandemic, except for a spike in April 2020.”
By contrast, in a Physicians Foundation Survey conducted in August 2020, 8% of physicians said they had closed their practices as a result of COVID, and 4% of the respondents said they planned to leave their practices within the next 12 months.
Similarly, a Jackson Physician Search survey in the fourth quarter of 2020 found that 54% of physicians surveyed had changed their employment plans. Of those doctors, 21% said they might hang up their white coat for early retirement. That works out to about 11% of the respondents.
The JAMA study’s authors analyzed the Medicare claims data from Jan. 1, 2019, to Dec. 30, 2020, to see how many physicians with Medicare patients had stopped filing claims for a period during those 2 years.
If a doctor had ceased submitting claims and then resumed filing them within 6 months after the last billing month, the lapse in filing was defined as “interruption with return.” If a physician stopped filing claims to Medicare and did not resume within 6 months, the gap in filing was called “interruption without return.”
In April 2020, 6.9% of physicians billing Medicare had a practice interruption, compared to 1.4% in 2019. But only 1.1% of physicians stopped practice in April 2020 and did not return, compared with 0.33% in 2019.
Physicians aged 55 or older had higher rates of interruption both with and without return than younger doctors did. The change in interruption rates for older doctors was 7.2% vs. 3.9% for younger physicians. The change in older physicians’ interruption-without-return rate was 1.3% vs. 0.34% for younger colleagues.
“Female physicians, specialists, physicians in smaller practices, those not in a health professional shortage area, and those practicing in a metropolitan area experienced greater increases in practice interruption rates in April 2020 vs. April 2019,” the study states. “But those groups typically had higher rates of return, so the overall changes in practice interruptions without return were similar across characteristics other than age.”
Significance for retirement rate
Discussing these results, the authors stressed that practice interruptions without return can’t necessarily be attributed to retirement, and that practice interruptions with return don’t necessarily signify that doctors had been furloughed from their practices.
Also, they said, “this measure of practice interruption likely misses meaningful interruptions that lasted for less than a month or did not involve complete cessation in treating Medicare patients.”
Nevertheless, “the study does capture a signal of some doctors probably retiring,” Jonathan Weiner, DPH, professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health, said in an interview.
But he added, “Some of those people who interrupted their practices and didn’t return may still come back. And there are probably a lot of other doctors who are leaving or changing practices that they didn’t capture.” For example, it’s possible that some doctors who went to work for other health care organizations stopped billing under their own names.
In Dr. Weiner’s view, the true percentage of physicians who have retired since the start of the pandemic is probably somewhere between the portion of doctors who interrupted their practice without return, according to the JAMA study, and the percentage of physicians who said they had closed their practices in the Physicians Foundation survey.
No mass exodus seen
Michael Belkin, JD, divisional vice president of recruiting for Merritt Hawkins, a physician search firm, said in an interview that the real number may be closer to the interruption-without-return figure in the JAMA study.
While many physician practices were disrupted in spring of 2020, he said, “it really didn’t result in a mass exodus [from health care]. We’re not talking to a lot of candidates who retired or walked away from their practices. We are talking to candidates who slowed down last year and then realized that they wanted to get back into medicine. And now they’re actively looking.”
One change in job candidates’ attitude, Mr. Belkin said, is that, because of COVID-19–related burnout, their quality of life is more important to them.
“They want to know, ‘What’s the culture of the employer like? What did they do last year during COVID? How did they handle it? Have they put together any protocols for the next pandemic?’ “
Demand for doctors has returned
In the summer of 2020, there was a major drop in physician recruitment by hospitals and health systems, partly because of fewer patient visits and procedures. But demand for doctors has bounced back over the past year, Mr. Belkin noted. One reason is the pent-up need for care among patients who avoided health care providers in 2020.
Another reason is that some employed doctors – particularly older physicians – have slowed down. Many doctors prefer to work remotely 1 or 2 days a week, providing telehealth visits to patients. That has led to a loss of productivity in many health care organizations and, consequently, a need to hire additional physicians.
Nevertheless, not many doctors are heading for the exit earlier than physicians did before COVID-19.
“They may work reduced hours,” Mr. Belkin said. “But the sense from a physician’s perspective is that this is all they know. For them to walk away from their life in medicine, from who they are, is problematic. So they’re continuing to practice, but at a reduced capacity.”
A version of this article first appeared on Medscape.com.
The number of physicians who have chosen early retirement or have left medicine because of the COVID-19 pandemic may be considerably lower than previously thought, results of a new study suggest.
The research letter in the Journal of the American Medical Association, based on Medicare claims data, stated that “practice interruption rates were similar before and during the COVID-19 pandemic, except for a spike in April 2020.”
By contrast, in a Physicians Foundation Survey conducted in August 2020, 8% of physicians said they had closed their practices as a result of COVID, and 4% of the respondents said they planned to leave their practices within the next 12 months.
Similarly, a Jackson Physician Search survey in the fourth quarter of 2020 found that 54% of physicians surveyed had changed their employment plans. Of those doctors, 21% said they might hang up their white coat for early retirement. That works out to about 11% of the respondents.
The JAMA study’s authors analyzed the Medicare claims data from Jan. 1, 2019, to Dec. 30, 2020, to see how many physicians with Medicare patients had stopped filing claims for a period during those 2 years.
If a doctor had ceased submitting claims and then resumed filing them within 6 months after the last billing month, the lapse in filing was defined as “interruption with return.” If a physician stopped filing claims to Medicare and did not resume within 6 months, the gap in filing was called “interruption without return.”
In April 2020, 6.9% of physicians billing Medicare had a practice interruption, compared to 1.4% in 2019. But only 1.1% of physicians stopped practice in April 2020 and did not return, compared with 0.33% in 2019.
Physicians aged 55 or older had higher rates of interruption both with and without return than younger doctors did. The change in interruption rates for older doctors was 7.2% vs. 3.9% for younger physicians. The change in older physicians’ interruption-without-return rate was 1.3% vs. 0.34% for younger colleagues.
“Female physicians, specialists, physicians in smaller practices, those not in a health professional shortage area, and those practicing in a metropolitan area experienced greater increases in practice interruption rates in April 2020 vs. April 2019,” the study states. “But those groups typically had higher rates of return, so the overall changes in practice interruptions without return were similar across characteristics other than age.”
Significance for retirement rate
Discussing these results, the authors stressed that practice interruptions without return can’t necessarily be attributed to retirement, and that practice interruptions with return don’t necessarily signify that doctors had been furloughed from their practices.
Also, they said, “this measure of practice interruption likely misses meaningful interruptions that lasted for less than a month or did not involve complete cessation in treating Medicare patients.”
Nevertheless, “the study does capture a signal of some doctors probably retiring,” Jonathan Weiner, DPH, professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health, said in an interview.
But he added, “Some of those people who interrupted their practices and didn’t return may still come back. And there are probably a lot of other doctors who are leaving or changing practices that they didn’t capture.” For example, it’s possible that some doctors who went to work for other health care organizations stopped billing under their own names.
In Dr. Weiner’s view, the true percentage of physicians who have retired since the start of the pandemic is probably somewhere between the portion of doctors who interrupted their practice without return, according to the JAMA study, and the percentage of physicians who said they had closed their practices in the Physicians Foundation survey.
No mass exodus seen
Michael Belkin, JD, divisional vice president of recruiting for Merritt Hawkins, a physician search firm, said in an interview that the real number may be closer to the interruption-without-return figure in the JAMA study.
While many physician practices were disrupted in spring of 2020, he said, “it really didn’t result in a mass exodus [from health care]. We’re not talking to a lot of candidates who retired or walked away from their practices. We are talking to candidates who slowed down last year and then realized that they wanted to get back into medicine. And now they’re actively looking.”
One change in job candidates’ attitude, Mr. Belkin said, is that, because of COVID-19–related burnout, their quality of life is more important to them.
“They want to know, ‘What’s the culture of the employer like? What did they do last year during COVID? How did they handle it? Have they put together any protocols for the next pandemic?’ “
Demand for doctors has returned
In the summer of 2020, there was a major drop in physician recruitment by hospitals and health systems, partly because of fewer patient visits and procedures. But demand for doctors has bounced back over the past year, Mr. Belkin noted. One reason is the pent-up need for care among patients who avoided health care providers in 2020.
Another reason is that some employed doctors – particularly older physicians – have slowed down. Many doctors prefer to work remotely 1 or 2 days a week, providing telehealth visits to patients. That has led to a loss of productivity in many health care organizations and, consequently, a need to hire additional physicians.
Nevertheless, not many doctors are heading for the exit earlier than physicians did before COVID-19.
“They may work reduced hours,” Mr. Belkin said. “But the sense from a physician’s perspective is that this is all they know. For them to walk away from their life in medicine, from who they are, is problematic. So they’re continuing to practice, but at a reduced capacity.”
A version of this article first appeared on Medscape.com.
MIND diet preserves cognition, new data show
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study finds paying people to participate in clinical trials is not unethical
Paying people to participate in clinical trials remains controversial. But to date, most reservations are based on hypothetical scenarios or expert opinion with few real-world data to support them.
Research released this week could change that.
Investigators offered nearly 1,300 participants in two clinical trials either no payment or incentives up to $500 to partake in a smoking cessation study or an analysis of a behavioral intervention to increase ambulation in hospitalized patients.
More cash was associated with greater agreement to participate in the smoking cessation study but not the ambulation trial.
But the bigger news may be that offering payment did not appear to get people to accept more risks or skew participation to lower-income individuals, as some ethicists have warned.
“With the publication of our study, investigators finally have data that they can cite to put to rest any lingering concerns about offering moderate incentives in low-risk trials,” lead author Scott D. Halpern, MD, PhD, the John M. Eisenberg Professor of Medicine, Epidemiology, and Medical Ethics & Health Policy at the University of Pennsylvania, Philadelphia, told this news organization.
This initial real-world data centers on low-risk interventions and more research is needed to analyze the ethics and effectiveness of paying people to join clinical trials with more inherent risk, the researchers note.
The study was published online Sept. 20 in JAMA Internal Medicine.
A good first step?
“Payments to research participants are notoriously controversial. Many people oppose payments altogether or insist on minimal payments out of concern that people might be unduly influenced to participate,” Ana S. Iltis, PhD, told this news organization when asked for comment. “Others worry that incentives will disproportionately motivate the less well-off to participate.”
“This is an important study that begins to assess whether these concerns are justified in a real-world context,” added Dr. Iltis, director of the Center for Bioethics, Health and Society and professor of philosophy at Wake Forest University in Winston-Salem, N.C.
In an accompanying invited commentary, Sang Ngo, Anthony S. Kim, MD, and Winston Chiong, MD, PhD, write: “This work is welcome, as it presents experimental data to a bioethical debate that so far has been largely driven by conjecture and competing suppositions.”
The commentary authors, however, question the conclusiveness of the findings. “Interpreting the authors’ findings is complex and illustrates some of the challenges inherent to applying empirical data to ethical problems,” they write.
Recruitment realities
When asked his advice for researchers considering financial incentives, Dr. Halpern said: “All researchers would happily include incentives in their trial budgets if not for concerns that the sponsor or institutional review board might not approve of them.”
“By far the biggest threat to a trial’s success is the inability to enroll enough participants,” he added.
Dr. Iltis agreed, framing the need to boost enrollment in ethical terms. “There is another important ethical issue that often gets ignored, and that is the issue of studies that fail to enroll enough participants and are never completed or are underpowered,” she said.
“These studies end up exposing people to research risks and burdens without a compensating social benefit.”
“If incentives help to increase enrollment and do not necessarily result in undue influence or unfair participant selection, then there might be ethical reasons to offer incentives,” Dr. Iltis added.
Building on previous work assessing financial incentives in hypothetical clinical trials, Dr. Halpern and colleagues studied 654 participants with major depressive disorder in a smoking cessation trial. They also studied another 642 participants in a study that compared a gamification strategy to usual care for encouraging hospitalized patients to get out of bed and walk.
Dr. Halpern and colleagues randomly assigned people in the smoking cessation study to receive no financial compensation, $200, or $500. In the ambulation trial, participants were randomly allocated to receive no compensation, $100, or $300.
Key findings
A total of 22% of those offered no incentive enrolled in the smoking cessation study. In contrast, 36% offered $200 agreed, as did 47% of those offered $500, which the investigators say supports offering cash incentives to boost enrollment. The differences were significant (P < .001).
In contrast, the amount offered did not significantly incentivize more people to participate in the ambulation trial (P = .62). Rates were 45% with no compensation, 48% with $100 payment, and 43% with $300 payment.
In an analysis that adjusted for demographic differences, financial well-being, and Research Attitudes Questionnaire (RAQ-7) scores, each increase in cash incentive increased the odds of enrollment in the smoking cessation trial by 70% (adjusted odds ratio, 1.70; 95% confidence interval, 1.34-2.17).
The same effect was not seen in the ambulation trial, where each higher cash incentive did not make a significant difference (aOR, 0.88; 95% CI, 0.64-1.22).
“The ambulation trial was a lower-risk trial in which patients’ willingness to participate was higher in general. So there were likely fewer people whose participation decisions could be influenced by offers of money,” Dr. Halpern said.
Inducement vs. coercion
The incentives in the study “did not function as unjust inducements, as they were not preferentially motivating across groups with different income levels or financial well-being in either trial,” the researchers note.
Dr. Halpern and colleagues also checked for any perceptions of coercion. More than 70% of participants in each smoking cessation trial group perceived no coercion, as did more than 93% of participants in each ambulation trial group, according to scores on a modified Perceived Coercion Scale of the MacArthur Admission Experience Survey.
Furthermore, perception of risks did not significantly alter the association between cash incentives and enrollment in either trial.
After collecting the findings, Dr. Halpern and colleagues informed participants about their participation in RETAIN and explained the rationale for using different cash incentives. They also let all participants know they would ultimately receive the maximum incentive – either $500 or $300, depending on the trial.
Research implications
A study limitation was reliance on participant risk perception, as was an inability to measure perceived coercion among people who chose not to participant in the trials. Another potential limitation is that “neither of these parent trials posed particularly high risks. Future tests of incentives of different sizes, and in the context of higher-risk parent trials, including trials that test treatments of serious illnesses, are warranted,” the researchers note.
“While there are many more questions to ask and contexts in which to study the effects of incentives, this study calls on opponents of incentivizing research participants with money to be more humble,” Dr. Iltis said. “Incentives might not have the effects they assume they have and which they have long held make such incentives unethical.”
“I encourage researchers who are offering incentives to consider working with people doing ethics research to assess the effects of incentives in their studies,” Dr. Halpern said. “Real-world, as opposed to hypothetical studies that can improve our understanding of the impact of incentives can improve the ethical conduct of research over time.”
Responding to criticism
The authors of the invited commentary questioned the definitions Dr. Halpern and colleagues used for undue or unjust inducement. “Among bioethicists, there is no consensus about what counts as undue inducement or an unjust distribution of research burdens. In this article, the authors have operationalized these constructs based on their own interpretations of undue and unjust inducement, which may not capture all the concerns that scholars have raised about inducement.”
Asked to respond to this and other criticisms raised in the commentary, Dr. Halpern said: “Did our study answer all possible questions about incentives? Absolutely not. But when it comes to incentives for research participation, an ounce of data is worth a pound of conjecture.”
There was agreement, however, that the findings could now put the onus on opponents of financial incentives for trial participants.
“I agree with the commentary’s authors that our study essentially shifts the burden of proof, such that, as they say, ‘those who would limit [incentives’] application may owe us an applicable criterion,’ ” Dr. Halpern said.
The authors of the invited commentary also criticized use of the study’s noninferiority design to rule out undue or unjust inducement. They note this design “may be unfamiliar to many bioethicists and can place substantial evaluative demands on readers.”
“As for the authors’ claim that noninferiority designs are difficult to interpret and unfamiliar to most clinicians and ethicists, I certainly agree,” Dr. Halpern said. “But that is hardly a reason to not employ the most rigorous methods possible to answer important questions.”
The study was supported by funding from the National Cancer Institute.
A version of this article first appeared on Medscape.com.
Paying people to participate in clinical trials remains controversial. But to date, most reservations are based on hypothetical scenarios or expert opinion with few real-world data to support them.
Research released this week could change that.
Investigators offered nearly 1,300 participants in two clinical trials either no payment or incentives up to $500 to partake in a smoking cessation study or an analysis of a behavioral intervention to increase ambulation in hospitalized patients.
More cash was associated with greater agreement to participate in the smoking cessation study but not the ambulation trial.
But the bigger news may be that offering payment did not appear to get people to accept more risks or skew participation to lower-income individuals, as some ethicists have warned.
“With the publication of our study, investigators finally have data that they can cite to put to rest any lingering concerns about offering moderate incentives in low-risk trials,” lead author Scott D. Halpern, MD, PhD, the John M. Eisenberg Professor of Medicine, Epidemiology, and Medical Ethics & Health Policy at the University of Pennsylvania, Philadelphia, told this news organization.
This initial real-world data centers on low-risk interventions and more research is needed to analyze the ethics and effectiveness of paying people to join clinical trials with more inherent risk, the researchers note.
The study was published online Sept. 20 in JAMA Internal Medicine.
A good first step?
“Payments to research participants are notoriously controversial. Many people oppose payments altogether or insist on minimal payments out of concern that people might be unduly influenced to participate,” Ana S. Iltis, PhD, told this news organization when asked for comment. “Others worry that incentives will disproportionately motivate the less well-off to participate.”
“This is an important study that begins to assess whether these concerns are justified in a real-world context,” added Dr. Iltis, director of the Center for Bioethics, Health and Society and professor of philosophy at Wake Forest University in Winston-Salem, N.C.
In an accompanying invited commentary, Sang Ngo, Anthony S. Kim, MD, and Winston Chiong, MD, PhD, write: “This work is welcome, as it presents experimental data to a bioethical debate that so far has been largely driven by conjecture and competing suppositions.”
The commentary authors, however, question the conclusiveness of the findings. “Interpreting the authors’ findings is complex and illustrates some of the challenges inherent to applying empirical data to ethical problems,” they write.
Recruitment realities
When asked his advice for researchers considering financial incentives, Dr. Halpern said: “All researchers would happily include incentives in their trial budgets if not for concerns that the sponsor or institutional review board might not approve of them.”
“By far the biggest threat to a trial’s success is the inability to enroll enough participants,” he added.
Dr. Iltis agreed, framing the need to boost enrollment in ethical terms. “There is another important ethical issue that often gets ignored, and that is the issue of studies that fail to enroll enough participants and are never completed or are underpowered,” she said.
“These studies end up exposing people to research risks and burdens without a compensating social benefit.”
“If incentives help to increase enrollment and do not necessarily result in undue influence or unfair participant selection, then there might be ethical reasons to offer incentives,” Dr. Iltis added.
Building on previous work assessing financial incentives in hypothetical clinical trials, Dr. Halpern and colleagues studied 654 participants with major depressive disorder in a smoking cessation trial. They also studied another 642 participants in a study that compared a gamification strategy to usual care for encouraging hospitalized patients to get out of bed and walk.
Dr. Halpern and colleagues randomly assigned people in the smoking cessation study to receive no financial compensation, $200, or $500. In the ambulation trial, participants were randomly allocated to receive no compensation, $100, or $300.
Key findings
A total of 22% of those offered no incentive enrolled in the smoking cessation study. In contrast, 36% offered $200 agreed, as did 47% of those offered $500, which the investigators say supports offering cash incentives to boost enrollment. The differences were significant (P < .001).
In contrast, the amount offered did not significantly incentivize more people to participate in the ambulation trial (P = .62). Rates were 45% with no compensation, 48% with $100 payment, and 43% with $300 payment.
In an analysis that adjusted for demographic differences, financial well-being, and Research Attitudes Questionnaire (RAQ-7) scores, each increase in cash incentive increased the odds of enrollment in the smoking cessation trial by 70% (adjusted odds ratio, 1.70; 95% confidence interval, 1.34-2.17).
The same effect was not seen in the ambulation trial, where each higher cash incentive did not make a significant difference (aOR, 0.88; 95% CI, 0.64-1.22).
“The ambulation trial was a lower-risk trial in which patients’ willingness to participate was higher in general. So there were likely fewer people whose participation decisions could be influenced by offers of money,” Dr. Halpern said.
Inducement vs. coercion
The incentives in the study “did not function as unjust inducements, as they were not preferentially motivating across groups with different income levels or financial well-being in either trial,” the researchers note.
Dr. Halpern and colleagues also checked for any perceptions of coercion. More than 70% of participants in each smoking cessation trial group perceived no coercion, as did more than 93% of participants in each ambulation trial group, according to scores on a modified Perceived Coercion Scale of the MacArthur Admission Experience Survey.
Furthermore, perception of risks did not significantly alter the association between cash incentives and enrollment in either trial.
After collecting the findings, Dr. Halpern and colleagues informed participants about their participation in RETAIN and explained the rationale for using different cash incentives. They also let all participants know they would ultimately receive the maximum incentive – either $500 or $300, depending on the trial.
Research implications
A study limitation was reliance on participant risk perception, as was an inability to measure perceived coercion among people who chose not to participant in the trials. Another potential limitation is that “neither of these parent trials posed particularly high risks. Future tests of incentives of different sizes, and in the context of higher-risk parent trials, including trials that test treatments of serious illnesses, are warranted,” the researchers note.
“While there are many more questions to ask and contexts in which to study the effects of incentives, this study calls on opponents of incentivizing research participants with money to be more humble,” Dr. Iltis said. “Incentives might not have the effects they assume they have and which they have long held make such incentives unethical.”
“I encourage researchers who are offering incentives to consider working with people doing ethics research to assess the effects of incentives in their studies,” Dr. Halpern said. “Real-world, as opposed to hypothetical studies that can improve our understanding of the impact of incentives can improve the ethical conduct of research over time.”
Responding to criticism
The authors of the invited commentary questioned the definitions Dr. Halpern and colleagues used for undue or unjust inducement. “Among bioethicists, there is no consensus about what counts as undue inducement or an unjust distribution of research burdens. In this article, the authors have operationalized these constructs based on their own interpretations of undue and unjust inducement, which may not capture all the concerns that scholars have raised about inducement.”
Asked to respond to this and other criticisms raised in the commentary, Dr. Halpern said: “Did our study answer all possible questions about incentives? Absolutely not. But when it comes to incentives for research participation, an ounce of data is worth a pound of conjecture.”
There was agreement, however, that the findings could now put the onus on opponents of financial incentives for trial participants.
“I agree with the commentary’s authors that our study essentially shifts the burden of proof, such that, as they say, ‘those who would limit [incentives’] application may owe us an applicable criterion,’ ” Dr. Halpern said.
The authors of the invited commentary also criticized use of the study’s noninferiority design to rule out undue or unjust inducement. They note this design “may be unfamiliar to many bioethicists and can place substantial evaluative demands on readers.”
“As for the authors’ claim that noninferiority designs are difficult to interpret and unfamiliar to most clinicians and ethicists, I certainly agree,” Dr. Halpern said. “But that is hardly a reason to not employ the most rigorous methods possible to answer important questions.”
The study was supported by funding from the National Cancer Institute.
A version of this article first appeared on Medscape.com.
Paying people to participate in clinical trials remains controversial. But to date, most reservations are based on hypothetical scenarios or expert opinion with few real-world data to support them.
Research released this week could change that.
Investigators offered nearly 1,300 participants in two clinical trials either no payment or incentives up to $500 to partake in a smoking cessation study or an analysis of a behavioral intervention to increase ambulation in hospitalized patients.
More cash was associated with greater agreement to participate in the smoking cessation study but not the ambulation trial.
But the bigger news may be that offering payment did not appear to get people to accept more risks or skew participation to lower-income individuals, as some ethicists have warned.
“With the publication of our study, investigators finally have data that they can cite to put to rest any lingering concerns about offering moderate incentives in low-risk trials,” lead author Scott D. Halpern, MD, PhD, the John M. Eisenberg Professor of Medicine, Epidemiology, and Medical Ethics & Health Policy at the University of Pennsylvania, Philadelphia, told this news organization.
This initial real-world data centers on low-risk interventions and more research is needed to analyze the ethics and effectiveness of paying people to join clinical trials with more inherent risk, the researchers note.
The study was published online Sept. 20 in JAMA Internal Medicine.
A good first step?
“Payments to research participants are notoriously controversial. Many people oppose payments altogether or insist on minimal payments out of concern that people might be unduly influenced to participate,” Ana S. Iltis, PhD, told this news organization when asked for comment. “Others worry that incentives will disproportionately motivate the less well-off to participate.”
“This is an important study that begins to assess whether these concerns are justified in a real-world context,” added Dr. Iltis, director of the Center for Bioethics, Health and Society and professor of philosophy at Wake Forest University in Winston-Salem, N.C.
In an accompanying invited commentary, Sang Ngo, Anthony S. Kim, MD, and Winston Chiong, MD, PhD, write: “This work is welcome, as it presents experimental data to a bioethical debate that so far has been largely driven by conjecture and competing suppositions.”
The commentary authors, however, question the conclusiveness of the findings. “Interpreting the authors’ findings is complex and illustrates some of the challenges inherent to applying empirical data to ethical problems,” they write.
Recruitment realities
When asked his advice for researchers considering financial incentives, Dr. Halpern said: “All researchers would happily include incentives in their trial budgets if not for concerns that the sponsor or institutional review board might not approve of them.”
“By far the biggest threat to a trial’s success is the inability to enroll enough participants,” he added.
Dr. Iltis agreed, framing the need to boost enrollment in ethical terms. “There is another important ethical issue that often gets ignored, and that is the issue of studies that fail to enroll enough participants and are never completed or are underpowered,” she said.
“These studies end up exposing people to research risks and burdens without a compensating social benefit.”
“If incentives help to increase enrollment and do not necessarily result in undue influence or unfair participant selection, then there might be ethical reasons to offer incentives,” Dr. Iltis added.
Building on previous work assessing financial incentives in hypothetical clinical trials, Dr. Halpern and colleagues studied 654 participants with major depressive disorder in a smoking cessation trial. They also studied another 642 participants in a study that compared a gamification strategy to usual care for encouraging hospitalized patients to get out of bed and walk.
Dr. Halpern and colleagues randomly assigned people in the smoking cessation study to receive no financial compensation, $200, or $500. In the ambulation trial, participants were randomly allocated to receive no compensation, $100, or $300.
Key findings
A total of 22% of those offered no incentive enrolled in the smoking cessation study. In contrast, 36% offered $200 agreed, as did 47% of those offered $500, which the investigators say supports offering cash incentives to boost enrollment. The differences were significant (P < .001).
In contrast, the amount offered did not significantly incentivize more people to participate in the ambulation trial (P = .62). Rates were 45% with no compensation, 48% with $100 payment, and 43% with $300 payment.
In an analysis that adjusted for demographic differences, financial well-being, and Research Attitudes Questionnaire (RAQ-7) scores, each increase in cash incentive increased the odds of enrollment in the smoking cessation trial by 70% (adjusted odds ratio, 1.70; 95% confidence interval, 1.34-2.17).
The same effect was not seen in the ambulation trial, where each higher cash incentive did not make a significant difference (aOR, 0.88; 95% CI, 0.64-1.22).
“The ambulation trial was a lower-risk trial in which patients’ willingness to participate was higher in general. So there were likely fewer people whose participation decisions could be influenced by offers of money,” Dr. Halpern said.
Inducement vs. coercion
The incentives in the study “did not function as unjust inducements, as they were not preferentially motivating across groups with different income levels or financial well-being in either trial,” the researchers note.
Dr. Halpern and colleagues also checked for any perceptions of coercion. More than 70% of participants in each smoking cessation trial group perceived no coercion, as did more than 93% of participants in each ambulation trial group, according to scores on a modified Perceived Coercion Scale of the MacArthur Admission Experience Survey.
Furthermore, perception of risks did not significantly alter the association between cash incentives and enrollment in either trial.
After collecting the findings, Dr. Halpern and colleagues informed participants about their participation in RETAIN and explained the rationale for using different cash incentives. They also let all participants know they would ultimately receive the maximum incentive – either $500 or $300, depending on the trial.
Research implications
A study limitation was reliance on participant risk perception, as was an inability to measure perceived coercion among people who chose not to participant in the trials. Another potential limitation is that “neither of these parent trials posed particularly high risks. Future tests of incentives of different sizes, and in the context of higher-risk parent trials, including trials that test treatments of serious illnesses, are warranted,” the researchers note.
“While there are many more questions to ask and contexts in which to study the effects of incentives, this study calls on opponents of incentivizing research participants with money to be more humble,” Dr. Iltis said. “Incentives might not have the effects they assume they have and which they have long held make such incentives unethical.”
“I encourage researchers who are offering incentives to consider working with people doing ethics research to assess the effects of incentives in their studies,” Dr. Halpern said. “Real-world, as opposed to hypothetical studies that can improve our understanding of the impact of incentives can improve the ethical conduct of research over time.”
Responding to criticism
The authors of the invited commentary questioned the definitions Dr. Halpern and colleagues used for undue or unjust inducement. “Among bioethicists, there is no consensus about what counts as undue inducement or an unjust distribution of research burdens. In this article, the authors have operationalized these constructs based on their own interpretations of undue and unjust inducement, which may not capture all the concerns that scholars have raised about inducement.”
Asked to respond to this and other criticisms raised in the commentary, Dr. Halpern said: “Did our study answer all possible questions about incentives? Absolutely not. But when it comes to incentives for research participation, an ounce of data is worth a pound of conjecture.”
There was agreement, however, that the findings could now put the onus on opponents of financial incentives for trial participants.
“I agree with the commentary’s authors that our study essentially shifts the burden of proof, such that, as they say, ‘those who would limit [incentives’] application may owe us an applicable criterion,’ ” Dr. Halpern said.
The authors of the invited commentary also criticized use of the study’s noninferiority design to rule out undue or unjust inducement. They note this design “may be unfamiliar to many bioethicists and can place substantial evaluative demands on readers.”
“As for the authors’ claim that noninferiority designs are difficult to interpret and unfamiliar to most clinicians and ethicists, I certainly agree,” Dr. Halpern said. “But that is hardly a reason to not employ the most rigorous methods possible to answer important questions.”
The study was supported by funding from the National Cancer Institute.
A version of this article first appeared on Medscape.com.
Dr. Judy C. Washington shows URM physicians how to lead
For URM physicians, she also imparts a shared experience of being a minority in the field and helps prepare them for the challenges of facing racism or feeling marginalized or not equitably supported in academic life – and for making change.
While family medicine’s demographics have become more diverse over time, and more so than other specialties, they are not yet representative of the U.S. population. Within academia, male physicians who are Black or African American, or Hispanic or Latino, comprised about 4% and 5% of family medicine faculty, respectively, at the end of 2019, according to data from the Association of American Medical Colleges. For women, these numbers were about 9% and 4%, respectively. (Only those with an MD degree exclusively were included in the report.)
“When you have the privilege to serve in leadership, you have the responsibility to reach back and identify and help others who would not otherwise have the opportunity to be recognized,” Dr. Washington said.
Her mentorship work stems in large part from her long-time involvement and leadership roles in the Society of Teachers of Family Medicine (STFM) – roles she considers a pillar of her professional life. She currently serves as president of the STFM Foundation and is associate chief medical officer of the Atlantic Medical Group, a large multisite physician-led organization. She is also coordinator of women’s health for the Overlook Family Medicine Residency Program, which is affiliated with Atlantic Medical Group.
In Dr. Washington’s role as associate chief medical officer of Atlantic Medical Group in Summit, N.J., she focuses on physician engagement, satisfaction, and diversity. She also assists in areas such as population health. For the Overlook Family Medicine Residency Program also in Summit, she precepts residents in the obstetrics clinic and in the family medicine outpatient clinic.
Diana N. Carvajal, MD, MPH, one of Dr. Washington’s mentees, called her an “inspirational leader” for young academic faculty and said she is a familiar speaker at STFM meetings on topics of workforce diversity, equity, and leadership. She is “passionate” about mentorship, Dr. Carvajal said, and has understood “that URMs and women of color were not always getting [the mentorship they need to be successful].”
Guiding future leaders
Ivonne McLean, MD, assistant professor of family and community medicine at Icahn School of Medicine at Mount Sinai, New York, and an attending at a community health center in the Bronx, called Dr. Washington for advice a couple of years ago when she was considering her next career move.
“She took a genuine interest in me. She never said, this is what you should do. But the questions she asked and the examples she gave from her own life were incredibly helpful to me [in deciding to pursue a research fellowship] ... it was a pivotal conversation,” said Dr. McLean, associate director of a reproductive health fellowship and a research fellow in a New York State–funded program.
“From a lived experience angle, she also told me, here are some of the challenges you’ll have as a woman of color, and here are some of the ways you can approach that,” she said.
Dr. Carvajal, also a URM family physician, credits Dr. Washington’s mentorship with the development of a day-long workshop – held before the annual Society of Teachers of Family Medicine (STFM) meeting – on the low and declining rates of Black males in medicine. “We’d planned it as a presentation, and [she heard of it and] helped us expand it,” she said, calling Dr. Washington “warm, welcoming, and encouraging.
“That work and collaboration with her and the others she brought [into the process] have resulted in publications and more presentations and strategy building for diversifying the workforce,” said Dr. Carvajal, assistant professor, director of reproductive health education in family medicine, and codirector of the research section, all in the department of family and community medicine at the University of Maryland, Baltimore.
STFM involvement
Dr. Washington, who says that all or almost all of her mentees are now leaders in their academic institutions and communities, has been instrumental in developing STFM’s mentoring programming and in facilitating the organization’s multifaceted URM Initiative.
She has been active in STFM since the start of her academic career, and in 2009, while serving as assistant program director for the residency program in which she’d trained, she joined two other African American women, Monique Y. Davis-Smith, MD, and Joedrecka Brown-Speights, MD, in cochairing the society’s Group on Minority and Multicultural Health.
It was in this space, that Dr. Washington said she “heard people’s stories of being in major academic institutions and not feeling supported, not being given roadmaps to success, not getting assistance with publishing, or just kind of feeling like an outsider ... of not being pulled in.” Hispanic and African American females, in particular, “were feeling marginalized,” she said.
In 2018, having co-led development of the STFM Quality Mentoring Program for URM faculty, Dr. Washington was asked to join the STFM Foundation and subsequently led the STFM Foundation’s fundraising campaign for a new URM Initiative. She exceeded her goal, increasing support for URM participation in meetings and activities, and then participated in an STFM steering committee to create broader and longer-lasting support for URM faculty, community teachers, and medical students and residents going into academic family medicine.
Increasing the percentage of URM family medicine faculty in leadership positions – and raising awareness of structural barriers to achievement – is one of the current pillars of the URM Initiative.
Navigating the ‘minority tax’
As part of her mentoring, Dr. Washington helps URM physicians navigate the minority tax – a term referring to the uncompensated citizenship tasks that are more often assigned to Black and other URM physicians than to White physicians, and that take time away from scholarship, further perpetuating inequities.
“Some of our young faculty members find themselves thrust into being the diversity and inclusion leaders in their institutions at a level at which they feel little power and little buy-in from [leadership],” she noted.
A commentary written by Dr. Washington and several colleagues on the minority tax as it impacts women – and the need to build a “tax shelter” to make academic medicine a more just environment for URM women – was published earlier this year in the Journal of Women’s Health.
She also answers e-mails and fields phone calls from young URM faculty who are mulling career moves and facing other familiar challenges.
Physicians who are URM, and African American physicians in particular, tend to “get pulled into the [often underserved] communities, into the patient care and community service areas,” Dr. Washington explained. “But unless you convert these projects into scholarship and publications, and unless you serve on a national committee outside of your institution, you’re not going to be promoted.”
Dr. Washington helps junior faculty envision themselves 5-plus years down the road, find what she calls scholarly “passion projects,” and prepare themselves for their next steps.
She helps her mentees navigate other parts of the continuum of unconscious bias and racism as well, from microaggressions from colleagues to overt discrimination from patients.
“I spend countless minutes fielding texts and phone calls from those who need support,” she wrote in a blog post. “They are a constant reminder that I must continue to speak up when I get the opportunity to do so.”
A journey through family medicine, and through bias and racism
Dr. Washington’s early days in medicine included graduating from Meharry Medical College in 1983 and the Mountainside Family Practice Residency Program in 1990. Following 6 years of working in a private practice in rural Maryland, she moved to academia, spending 6 years at East Tennessee State University and 4 years at the UMDNJ–New Jersey Medical School in Newark as an assistant professor of family medicine.
As had happened in rural Maryland, bias and racism have too often lurked during her career as a physician.
“I grew up in Alabama so I was pretty much ready to deal with racism in the South,” Dr. Washington said. “What I was not ready for was coming to the Northeast and seeing that you’re marginalized because you’re not invited into the room. Or if you do go into spaces when you’re the only one, you often don’t feel as welcomed as you thought you might be.”
Her ideas and contributions were too often dismissed, she wrote in a 2020 blog entry posted on her LinkedIn page. And during contract negotiations, “I was not aware of all the information that my White colleagues had. They had the advantage of inside information.”
Dr. Washington says that “it took a village” to make her who she is today: teachers in her segregated schools in Alabama, one of her college professors, her best friend in medical school – and STFM, “where the list [of her own mentors] is long.”
For URM physicians, she also imparts a shared experience of being a minority in the field and helps prepare them for the challenges of facing racism or feeling marginalized or not equitably supported in academic life – and for making change.
While family medicine’s demographics have become more diverse over time, and more so than other specialties, they are not yet representative of the U.S. population. Within academia, male physicians who are Black or African American, or Hispanic or Latino, comprised about 4% and 5% of family medicine faculty, respectively, at the end of 2019, according to data from the Association of American Medical Colleges. For women, these numbers were about 9% and 4%, respectively. (Only those with an MD degree exclusively were included in the report.)
“When you have the privilege to serve in leadership, you have the responsibility to reach back and identify and help others who would not otherwise have the opportunity to be recognized,” Dr. Washington said.
Her mentorship work stems in large part from her long-time involvement and leadership roles in the Society of Teachers of Family Medicine (STFM) – roles she considers a pillar of her professional life. She currently serves as president of the STFM Foundation and is associate chief medical officer of the Atlantic Medical Group, a large multisite physician-led organization. She is also coordinator of women’s health for the Overlook Family Medicine Residency Program, which is affiliated with Atlantic Medical Group.
In Dr. Washington’s role as associate chief medical officer of Atlantic Medical Group in Summit, N.J., she focuses on physician engagement, satisfaction, and diversity. She also assists in areas such as population health. For the Overlook Family Medicine Residency Program also in Summit, she precepts residents in the obstetrics clinic and in the family medicine outpatient clinic.
Diana N. Carvajal, MD, MPH, one of Dr. Washington’s mentees, called her an “inspirational leader” for young academic faculty and said she is a familiar speaker at STFM meetings on topics of workforce diversity, equity, and leadership. She is “passionate” about mentorship, Dr. Carvajal said, and has understood “that URMs and women of color were not always getting [the mentorship they need to be successful].”
Guiding future leaders
Ivonne McLean, MD, assistant professor of family and community medicine at Icahn School of Medicine at Mount Sinai, New York, and an attending at a community health center in the Bronx, called Dr. Washington for advice a couple of years ago when she was considering her next career move.
“She took a genuine interest in me. She never said, this is what you should do. But the questions she asked and the examples she gave from her own life were incredibly helpful to me [in deciding to pursue a research fellowship] ... it was a pivotal conversation,” said Dr. McLean, associate director of a reproductive health fellowship and a research fellow in a New York State–funded program.
“From a lived experience angle, she also told me, here are some of the challenges you’ll have as a woman of color, and here are some of the ways you can approach that,” she said.
Dr. Carvajal, also a URM family physician, credits Dr. Washington’s mentorship with the development of a day-long workshop – held before the annual Society of Teachers of Family Medicine (STFM) meeting – on the low and declining rates of Black males in medicine. “We’d planned it as a presentation, and [she heard of it and] helped us expand it,” she said, calling Dr. Washington “warm, welcoming, and encouraging.
“That work and collaboration with her and the others she brought [into the process] have resulted in publications and more presentations and strategy building for diversifying the workforce,” said Dr. Carvajal, assistant professor, director of reproductive health education in family medicine, and codirector of the research section, all in the department of family and community medicine at the University of Maryland, Baltimore.
STFM involvement
Dr. Washington, who says that all or almost all of her mentees are now leaders in their academic institutions and communities, has been instrumental in developing STFM’s mentoring programming and in facilitating the organization’s multifaceted URM Initiative.
She has been active in STFM since the start of her academic career, and in 2009, while serving as assistant program director for the residency program in which she’d trained, she joined two other African American women, Monique Y. Davis-Smith, MD, and Joedrecka Brown-Speights, MD, in cochairing the society’s Group on Minority and Multicultural Health.
It was in this space, that Dr. Washington said she “heard people’s stories of being in major academic institutions and not feeling supported, not being given roadmaps to success, not getting assistance with publishing, or just kind of feeling like an outsider ... of not being pulled in.” Hispanic and African American females, in particular, “were feeling marginalized,” she said.
In 2018, having co-led development of the STFM Quality Mentoring Program for URM faculty, Dr. Washington was asked to join the STFM Foundation and subsequently led the STFM Foundation’s fundraising campaign for a new URM Initiative. She exceeded her goal, increasing support for URM participation in meetings and activities, and then participated in an STFM steering committee to create broader and longer-lasting support for URM faculty, community teachers, and medical students and residents going into academic family medicine.
Increasing the percentage of URM family medicine faculty in leadership positions – and raising awareness of structural barriers to achievement – is one of the current pillars of the URM Initiative.
Navigating the ‘minority tax’
As part of her mentoring, Dr. Washington helps URM physicians navigate the minority tax – a term referring to the uncompensated citizenship tasks that are more often assigned to Black and other URM physicians than to White physicians, and that take time away from scholarship, further perpetuating inequities.
“Some of our young faculty members find themselves thrust into being the diversity and inclusion leaders in their institutions at a level at which they feel little power and little buy-in from [leadership],” she noted.
A commentary written by Dr. Washington and several colleagues on the minority tax as it impacts women – and the need to build a “tax shelter” to make academic medicine a more just environment for URM women – was published earlier this year in the Journal of Women’s Health.
She also answers e-mails and fields phone calls from young URM faculty who are mulling career moves and facing other familiar challenges.
Physicians who are URM, and African American physicians in particular, tend to “get pulled into the [often underserved] communities, into the patient care and community service areas,” Dr. Washington explained. “But unless you convert these projects into scholarship and publications, and unless you serve on a national committee outside of your institution, you’re not going to be promoted.”
Dr. Washington helps junior faculty envision themselves 5-plus years down the road, find what she calls scholarly “passion projects,” and prepare themselves for their next steps.
She helps her mentees navigate other parts of the continuum of unconscious bias and racism as well, from microaggressions from colleagues to overt discrimination from patients.
“I spend countless minutes fielding texts and phone calls from those who need support,” she wrote in a blog post. “They are a constant reminder that I must continue to speak up when I get the opportunity to do so.”
A journey through family medicine, and through bias and racism
Dr. Washington’s early days in medicine included graduating from Meharry Medical College in 1983 and the Mountainside Family Practice Residency Program in 1990. Following 6 years of working in a private practice in rural Maryland, she moved to academia, spending 6 years at East Tennessee State University and 4 years at the UMDNJ–New Jersey Medical School in Newark as an assistant professor of family medicine.
As had happened in rural Maryland, bias and racism have too often lurked during her career as a physician.
“I grew up in Alabama so I was pretty much ready to deal with racism in the South,” Dr. Washington said. “What I was not ready for was coming to the Northeast and seeing that you’re marginalized because you’re not invited into the room. Or if you do go into spaces when you’re the only one, you often don’t feel as welcomed as you thought you might be.”
Her ideas and contributions were too often dismissed, she wrote in a 2020 blog entry posted on her LinkedIn page. And during contract negotiations, “I was not aware of all the information that my White colleagues had. They had the advantage of inside information.”
Dr. Washington says that “it took a village” to make her who she is today: teachers in her segregated schools in Alabama, one of her college professors, her best friend in medical school – and STFM, “where the list [of her own mentors] is long.”
For URM physicians, she also imparts a shared experience of being a minority in the field and helps prepare them for the challenges of facing racism or feeling marginalized or not equitably supported in academic life – and for making change.
While family medicine’s demographics have become more diverse over time, and more so than other specialties, they are not yet representative of the U.S. population. Within academia, male physicians who are Black or African American, or Hispanic or Latino, comprised about 4% and 5% of family medicine faculty, respectively, at the end of 2019, according to data from the Association of American Medical Colleges. For women, these numbers were about 9% and 4%, respectively. (Only those with an MD degree exclusively were included in the report.)
“When you have the privilege to serve in leadership, you have the responsibility to reach back and identify and help others who would not otherwise have the opportunity to be recognized,” Dr. Washington said.
Her mentorship work stems in large part from her long-time involvement and leadership roles in the Society of Teachers of Family Medicine (STFM) – roles she considers a pillar of her professional life. She currently serves as president of the STFM Foundation and is associate chief medical officer of the Atlantic Medical Group, a large multisite physician-led organization. She is also coordinator of women’s health for the Overlook Family Medicine Residency Program, which is affiliated with Atlantic Medical Group.
In Dr. Washington’s role as associate chief medical officer of Atlantic Medical Group in Summit, N.J., she focuses on physician engagement, satisfaction, and diversity. She also assists in areas such as population health. For the Overlook Family Medicine Residency Program also in Summit, she precepts residents in the obstetrics clinic and in the family medicine outpatient clinic.
Diana N. Carvajal, MD, MPH, one of Dr. Washington’s mentees, called her an “inspirational leader” for young academic faculty and said she is a familiar speaker at STFM meetings on topics of workforce diversity, equity, and leadership. She is “passionate” about mentorship, Dr. Carvajal said, and has understood “that URMs and women of color were not always getting [the mentorship they need to be successful].”
Guiding future leaders
Ivonne McLean, MD, assistant professor of family and community medicine at Icahn School of Medicine at Mount Sinai, New York, and an attending at a community health center in the Bronx, called Dr. Washington for advice a couple of years ago when she was considering her next career move.
“She took a genuine interest in me. She never said, this is what you should do. But the questions she asked and the examples she gave from her own life were incredibly helpful to me [in deciding to pursue a research fellowship] ... it was a pivotal conversation,” said Dr. McLean, associate director of a reproductive health fellowship and a research fellow in a New York State–funded program.
“From a lived experience angle, she also told me, here are some of the challenges you’ll have as a woman of color, and here are some of the ways you can approach that,” she said.
Dr. Carvajal, also a URM family physician, credits Dr. Washington’s mentorship with the development of a day-long workshop – held before the annual Society of Teachers of Family Medicine (STFM) meeting – on the low and declining rates of Black males in medicine. “We’d planned it as a presentation, and [she heard of it and] helped us expand it,” she said, calling Dr. Washington “warm, welcoming, and encouraging.
“That work and collaboration with her and the others she brought [into the process] have resulted in publications and more presentations and strategy building for diversifying the workforce,” said Dr. Carvajal, assistant professor, director of reproductive health education in family medicine, and codirector of the research section, all in the department of family and community medicine at the University of Maryland, Baltimore.
STFM involvement
Dr. Washington, who says that all or almost all of her mentees are now leaders in their academic institutions and communities, has been instrumental in developing STFM’s mentoring programming and in facilitating the organization’s multifaceted URM Initiative.
She has been active in STFM since the start of her academic career, and in 2009, while serving as assistant program director for the residency program in which she’d trained, she joined two other African American women, Monique Y. Davis-Smith, MD, and Joedrecka Brown-Speights, MD, in cochairing the society’s Group on Minority and Multicultural Health.
It was in this space, that Dr. Washington said she “heard people’s stories of being in major academic institutions and not feeling supported, not being given roadmaps to success, not getting assistance with publishing, or just kind of feeling like an outsider ... of not being pulled in.” Hispanic and African American females, in particular, “were feeling marginalized,” she said.
In 2018, having co-led development of the STFM Quality Mentoring Program for URM faculty, Dr. Washington was asked to join the STFM Foundation and subsequently led the STFM Foundation’s fundraising campaign for a new URM Initiative. She exceeded her goal, increasing support for URM participation in meetings and activities, and then participated in an STFM steering committee to create broader and longer-lasting support for URM faculty, community teachers, and medical students and residents going into academic family medicine.
Increasing the percentage of URM family medicine faculty in leadership positions – and raising awareness of structural barriers to achievement – is one of the current pillars of the URM Initiative.
Navigating the ‘minority tax’
As part of her mentoring, Dr. Washington helps URM physicians navigate the minority tax – a term referring to the uncompensated citizenship tasks that are more often assigned to Black and other URM physicians than to White physicians, and that take time away from scholarship, further perpetuating inequities.
“Some of our young faculty members find themselves thrust into being the diversity and inclusion leaders in their institutions at a level at which they feel little power and little buy-in from [leadership],” she noted.
A commentary written by Dr. Washington and several colleagues on the minority tax as it impacts women – and the need to build a “tax shelter” to make academic medicine a more just environment for URM women – was published earlier this year in the Journal of Women’s Health.
She also answers e-mails and fields phone calls from young URM faculty who are mulling career moves and facing other familiar challenges.
Physicians who are URM, and African American physicians in particular, tend to “get pulled into the [often underserved] communities, into the patient care and community service areas,” Dr. Washington explained. “But unless you convert these projects into scholarship and publications, and unless you serve on a national committee outside of your institution, you’re not going to be promoted.”
Dr. Washington helps junior faculty envision themselves 5-plus years down the road, find what she calls scholarly “passion projects,” and prepare themselves for their next steps.
She helps her mentees navigate other parts of the continuum of unconscious bias and racism as well, from microaggressions from colleagues to overt discrimination from patients.
“I spend countless minutes fielding texts and phone calls from those who need support,” she wrote in a blog post. “They are a constant reminder that I must continue to speak up when I get the opportunity to do so.”
A journey through family medicine, and through bias and racism
Dr. Washington’s early days in medicine included graduating from Meharry Medical College in 1983 and the Mountainside Family Practice Residency Program in 1990. Following 6 years of working in a private practice in rural Maryland, she moved to academia, spending 6 years at East Tennessee State University and 4 years at the UMDNJ–New Jersey Medical School in Newark as an assistant professor of family medicine.
As had happened in rural Maryland, bias and racism have too often lurked during her career as a physician.
“I grew up in Alabama so I was pretty much ready to deal with racism in the South,” Dr. Washington said. “What I was not ready for was coming to the Northeast and seeing that you’re marginalized because you’re not invited into the room. Or if you do go into spaces when you’re the only one, you often don’t feel as welcomed as you thought you might be.”
Her ideas and contributions were too often dismissed, she wrote in a 2020 blog entry posted on her LinkedIn page. And during contract negotiations, “I was not aware of all the information that my White colleagues had. They had the advantage of inside information.”
Dr. Washington says that “it took a village” to make her who she is today: teachers in her segregated schools in Alabama, one of her college professors, her best friend in medical school – and STFM, “where the list [of her own mentors] is long.”
Military sexual trauma tied to risk for hypertension
a new study suggests.
“Understanding a patient’s trauma history is invaluable for treating the whole person,” Allison E. Gaffey, PhD, Yale University, New Haven, Conn., and the Veterans Affairs Connecticut Healthcare System, told this news organization.
“Assessing men and women’s history of trauma, including sexual trauma, is critical for recognizing nontraditional factors that contribute to their cardiovascular risk and to gain a more comprehensive understanding of their mental and physical health,” Dr. Gaffey added.
She presented her research at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Lasting impact on physical health
Dr. Gaffey and colleagues analyzed data from the VA for roughly 1.2 million veterans (mean age, 30.2 years; 12% female) who were discharged from the military after Sept. 30, 2001, and who received health care services at VA medical centers from 2001 to 2017.
All were screened for sexual harassment and assault, known as military sexual trauma (MST), when they first began receiving VA care.
During 16 years of follow-up, 33,881 veterans screened positive for MST (65% women), and 307,332 developed hypertension (15% women).
Overall, MST was associated with a 30% increase in risk for incident hypertension in unadjusted models (hazard ratio, 1.30; 95% confidence interval, 1.28-1.33; P < .001).
After adjustment for demographic characteristics, lifestyle factors, cardiovascular comorbidities, PTSD, anxiety, and depression, MST remained significantly associated with hypertension (adjusted HR, 1.10; 95% CI, 1.08-1.12; P < .001).
When women and men were examined separately, the link between MST and risk for hypertension remained for both groups, but was slightly stronger among women.
“Sexual trauma has been associated with autonomic dysfunction, inflammation, and dysregulation in the hypothalamic pituitary adrenal axis and renin-angiotensin-aldosterone system, which could lead to elevations in BP over time,” Dr. Gaffey told this news organization.
“These findings show that even many years after being discharged from military service, exposure to military sexual trauma can continue to significantly influence veterans’ physical health,” she added.
Dr. Gaffey said it will be important to determine if early treatment of MST improves hypertension risk, particularly among those showing elevated blood pressure or stage 1 hypertension.
Social determinants of health
Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee, said the findings in this study are “in line with what we know about the impact of social determinants of health on high blood pressure.”
“There are studies that suggest that things that we historically don’t look at as risk factors for hypertension – lifelong racism, crime, mental health status – do in fact predict your risk of developing hypertension,” Dr. Lawrence, from Spectrum Health in Benton Harbor, Mich., told this news organization.
“It’s not just your genetics that will determine your health, and there are a lot of things that will affect your blood pressure. Your blood pressure is really just a barometer of everything that’s going on in your life and some of the things that have gone on in your life in the past,” added Dr. Lawrence, who wasn’t involved in the study.
Funding for the study was provided by the Department of Veterans Affairs. Dr. Gaffey and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
a new study suggests.
“Understanding a patient’s trauma history is invaluable for treating the whole person,” Allison E. Gaffey, PhD, Yale University, New Haven, Conn., and the Veterans Affairs Connecticut Healthcare System, told this news organization.
“Assessing men and women’s history of trauma, including sexual trauma, is critical for recognizing nontraditional factors that contribute to their cardiovascular risk and to gain a more comprehensive understanding of their mental and physical health,” Dr. Gaffey added.
She presented her research at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Lasting impact on physical health
Dr. Gaffey and colleagues analyzed data from the VA for roughly 1.2 million veterans (mean age, 30.2 years; 12% female) who were discharged from the military after Sept. 30, 2001, and who received health care services at VA medical centers from 2001 to 2017.
All were screened for sexual harassment and assault, known as military sexual trauma (MST), when they first began receiving VA care.
During 16 years of follow-up, 33,881 veterans screened positive for MST (65% women), and 307,332 developed hypertension (15% women).
Overall, MST was associated with a 30% increase in risk for incident hypertension in unadjusted models (hazard ratio, 1.30; 95% confidence interval, 1.28-1.33; P < .001).
After adjustment for demographic characteristics, lifestyle factors, cardiovascular comorbidities, PTSD, anxiety, and depression, MST remained significantly associated with hypertension (adjusted HR, 1.10; 95% CI, 1.08-1.12; P < .001).
When women and men were examined separately, the link between MST and risk for hypertension remained for both groups, but was slightly stronger among women.
“Sexual trauma has been associated with autonomic dysfunction, inflammation, and dysregulation in the hypothalamic pituitary adrenal axis and renin-angiotensin-aldosterone system, which could lead to elevations in BP over time,” Dr. Gaffey told this news organization.
“These findings show that even many years after being discharged from military service, exposure to military sexual trauma can continue to significantly influence veterans’ physical health,” she added.
Dr. Gaffey said it will be important to determine if early treatment of MST improves hypertension risk, particularly among those showing elevated blood pressure or stage 1 hypertension.
Social determinants of health
Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee, said the findings in this study are “in line with what we know about the impact of social determinants of health on high blood pressure.”
“There are studies that suggest that things that we historically don’t look at as risk factors for hypertension – lifelong racism, crime, mental health status – do in fact predict your risk of developing hypertension,” Dr. Lawrence, from Spectrum Health in Benton Harbor, Mich., told this news organization.
“It’s not just your genetics that will determine your health, and there are a lot of things that will affect your blood pressure. Your blood pressure is really just a barometer of everything that’s going on in your life and some of the things that have gone on in your life in the past,” added Dr. Lawrence, who wasn’t involved in the study.
Funding for the study was provided by the Department of Veterans Affairs. Dr. Gaffey and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
a new study suggests.
“Understanding a patient’s trauma history is invaluable for treating the whole person,” Allison E. Gaffey, PhD, Yale University, New Haven, Conn., and the Veterans Affairs Connecticut Healthcare System, told this news organization.
“Assessing men and women’s history of trauma, including sexual trauma, is critical for recognizing nontraditional factors that contribute to their cardiovascular risk and to gain a more comprehensive understanding of their mental and physical health,” Dr. Gaffey added.
She presented her research at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Lasting impact on physical health
Dr. Gaffey and colleagues analyzed data from the VA for roughly 1.2 million veterans (mean age, 30.2 years; 12% female) who were discharged from the military after Sept. 30, 2001, and who received health care services at VA medical centers from 2001 to 2017.
All were screened for sexual harassment and assault, known as military sexual trauma (MST), when they first began receiving VA care.
During 16 years of follow-up, 33,881 veterans screened positive for MST (65% women), and 307,332 developed hypertension (15% women).
Overall, MST was associated with a 30% increase in risk for incident hypertension in unadjusted models (hazard ratio, 1.30; 95% confidence interval, 1.28-1.33; P < .001).
After adjustment for demographic characteristics, lifestyle factors, cardiovascular comorbidities, PTSD, anxiety, and depression, MST remained significantly associated with hypertension (adjusted HR, 1.10; 95% CI, 1.08-1.12; P < .001).
When women and men were examined separately, the link between MST and risk for hypertension remained for both groups, but was slightly stronger among women.
“Sexual trauma has been associated with autonomic dysfunction, inflammation, and dysregulation in the hypothalamic pituitary adrenal axis and renin-angiotensin-aldosterone system, which could lead to elevations in BP over time,” Dr. Gaffey told this news organization.
“These findings show that even many years after being discharged from military service, exposure to military sexual trauma can continue to significantly influence veterans’ physical health,” she added.
Dr. Gaffey said it will be important to determine if early treatment of MST improves hypertension risk, particularly among those showing elevated blood pressure or stage 1 hypertension.
Social determinants of health
Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee, said the findings in this study are “in line with what we know about the impact of social determinants of health on high blood pressure.”
“There are studies that suggest that things that we historically don’t look at as risk factors for hypertension – lifelong racism, crime, mental health status – do in fact predict your risk of developing hypertension,” Dr. Lawrence, from Spectrum Health in Benton Harbor, Mich., told this news organization.
“It’s not just your genetics that will determine your health, and there are a lot of things that will affect your blood pressure. Your blood pressure is really just a barometer of everything that’s going on in your life and some of the things that have gone on in your life in the past,” added Dr. Lawrence, who wasn’t involved in the study.
Funding for the study was provided by the Department of Veterans Affairs. Dr. Gaffey and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
















