User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
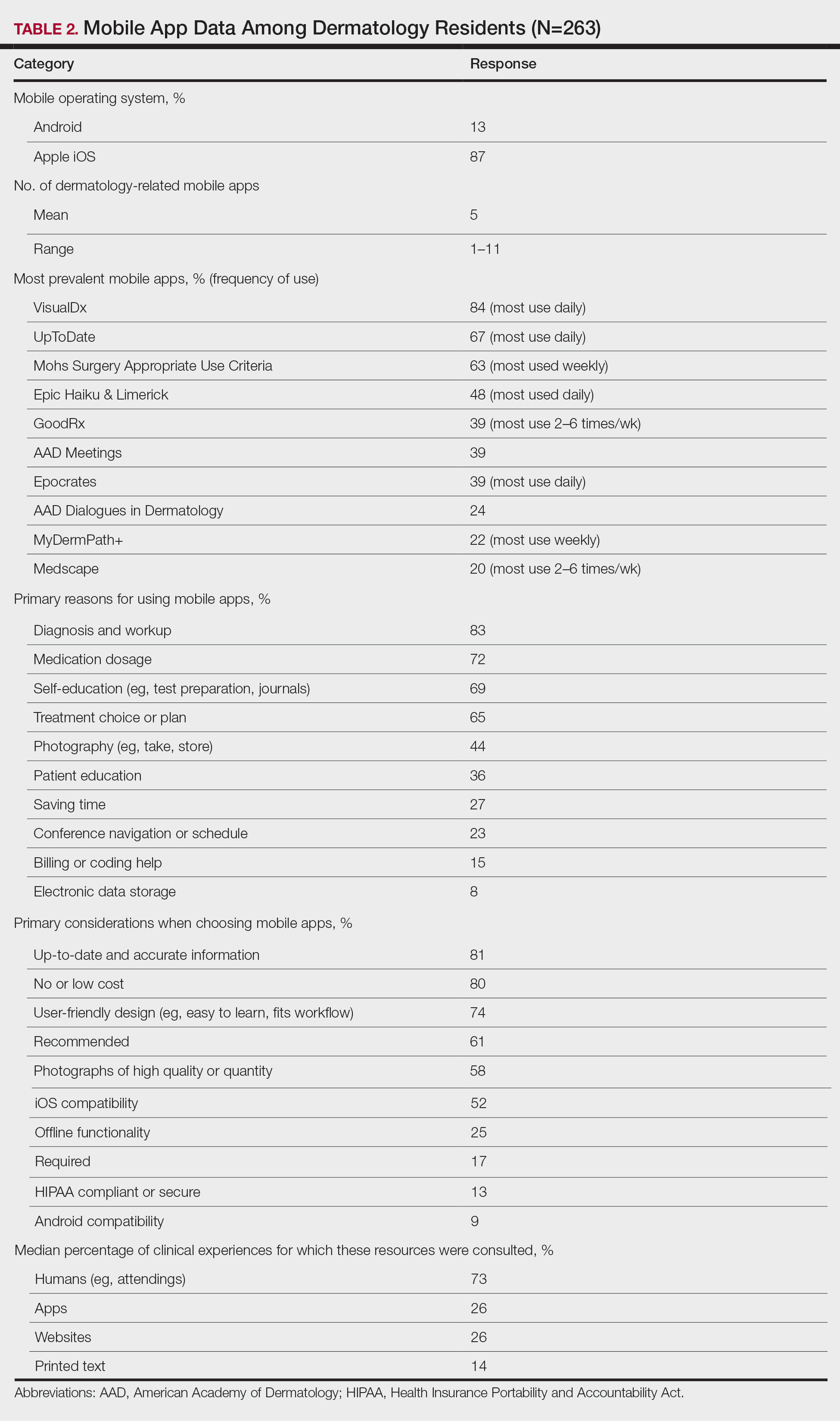
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Anecdote Increases Patient Willingness to Take a Biologic Medication for Psoriasis
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Practice Points
- Patients often are apprehensive to start biologic medications for their psoriasis.
- Clinical trial evidence of a biologic medication’s efficacy and safety as well as anecdotes of patient experiences appear to be important factors for patients when considering taking a medication.
- The use of an anecdote—alone or in combination with clinical trial evidence—to help patients overcome fears of starting a biologic medication for their psoriasis may be an effective way to improve patients’ willingness to take treatment.
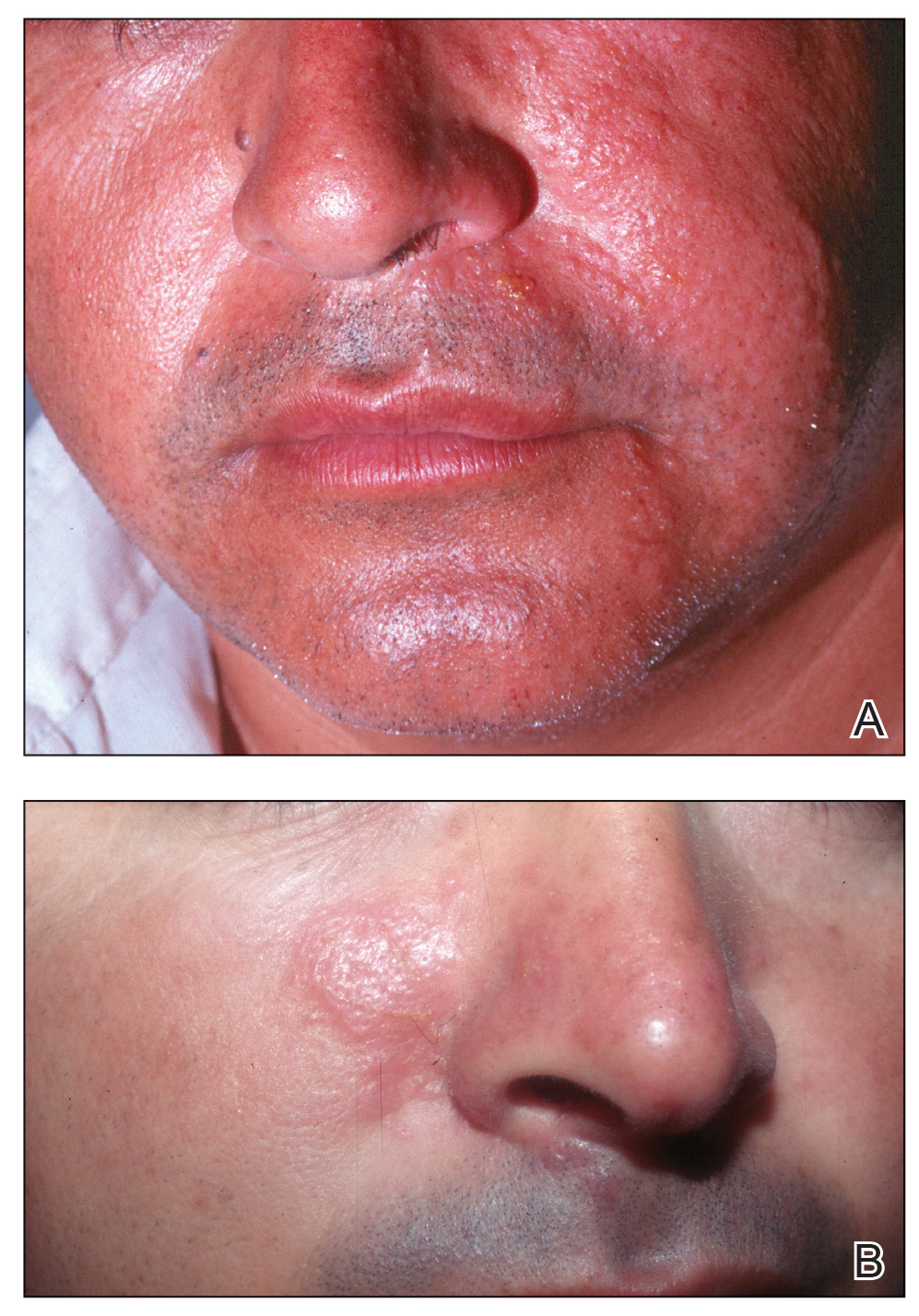
Ulcerated and Verrucous Plaque on the Chest
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
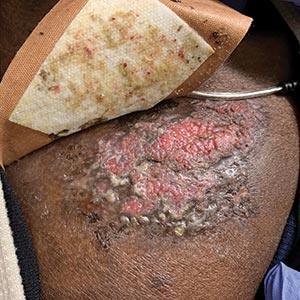
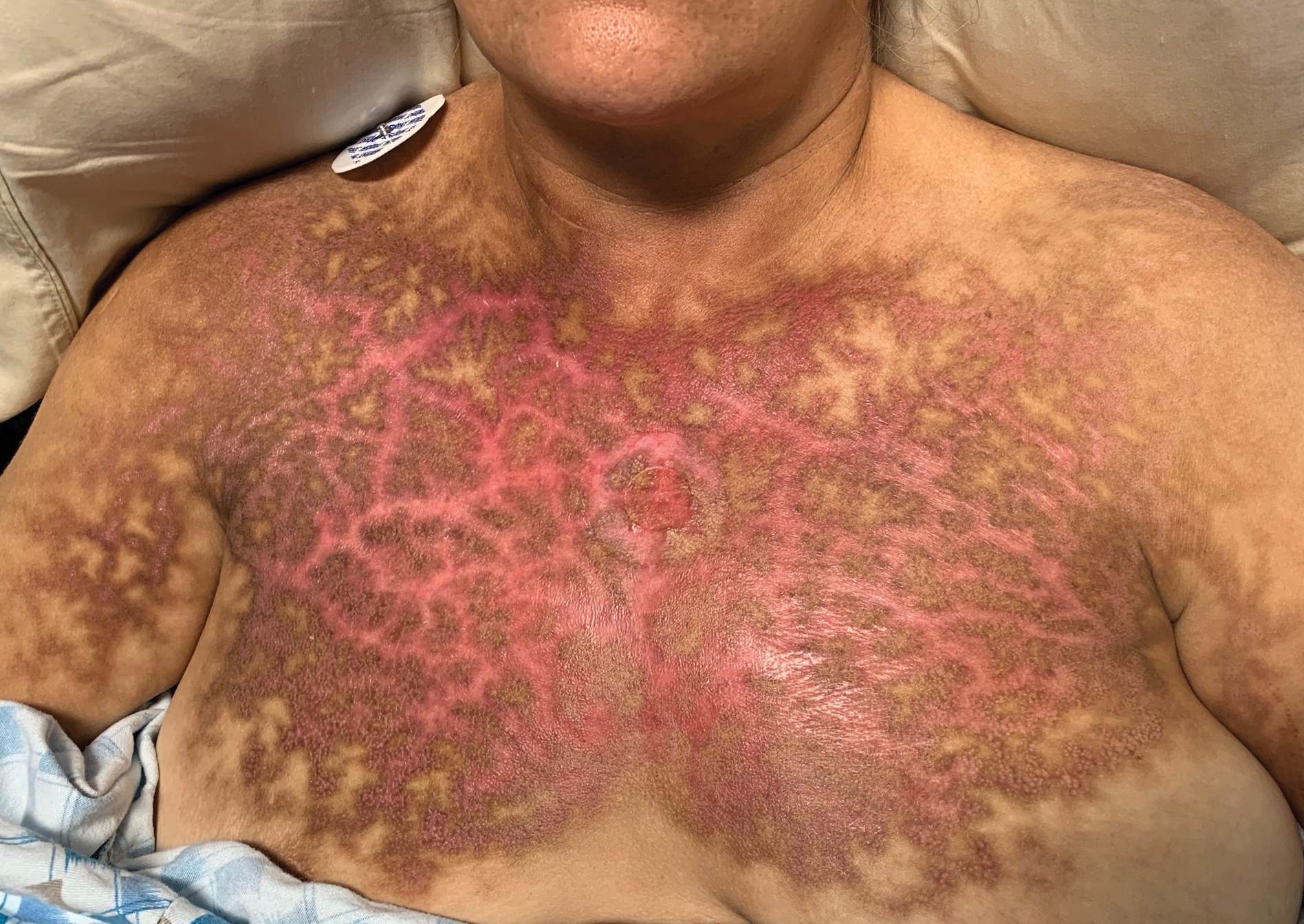
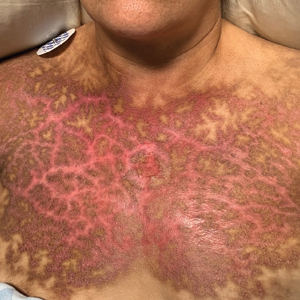
A 36-year-old man presented to an emergency department in the southwestern United States with a cough, fatigue, and worsening back pain associated with night sweats of 1 month’s duration. He experienced a 9.07-kg weight loss, as well as development of a rough, nontender, nonpruritic rash along the left upper chest over the prior month. The patient was born in West Africa and reported that he had moved to the southwestern United States from the eastern United States approximately 6 years prior to presentation. Physical examination on admission revealed a 5×3-cm, purple-brown, verrucous plaque with a central pink cobblestone appearance and ulceration. Chest radiography was notable for perihilar adenopathy with no focal infiltrates or cavitary lesions. Computed tomography and magnetic resonance imaging of the chest were notable for miliary nodules throughout the lungs; extensive lytic spine lesions of cervical, thoracic, and lumbar vertebral bodies and left twelfth rib; and a left paraspinal thoracic epidural soft tissue phlegmon. Initial laboratory investigations revealed peripheral eosinophilia without absolute leukocytosis and a microcytic anemia.
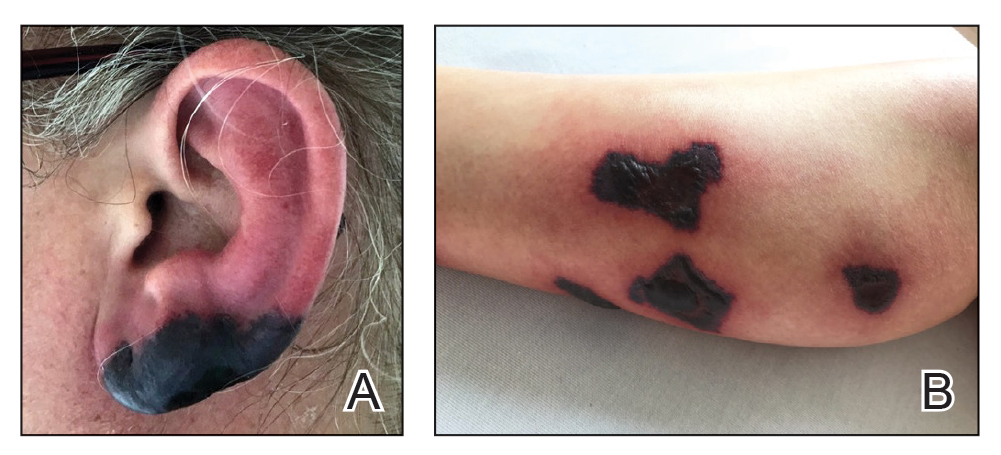
Bullous Retiform Purpura on the Ears and Legs
The Diagnosis: Levamisole-Induced Vasculopathy
Biopsy of one of the bullous retiform purpura on the leg (Figure 1) revealed a combined leukocytoclastic vasculitis and thrombotic vasculopathy (quiz images). Periodic acid-Schiff and Gram stains, with adequate controls, were negative for pathogenic fungal and bacterial organisms. Although this reaction pattern has an extensive differential, in this clinical setting with associated cocaine-positive urine toxicologic analysis, perinuclear antineutrophil cytoplasmic antibodies (p-ANCA), and leukopenia, the histopathologic findings were consistent with levamisole-induced vasculopathy (LIV).1,2 Although not specific, leukocytoclastic vasculitis and thrombotic vasculopathy have been reported as the classic histopathologic findings of LIV. In addition, interstitial and perivascular neovascularization have been reported as a potential histopathologic finding associated with this entity but was not seen in our case.3
Levamisole is an anthelminthic agent used to adulterate cocaine, a practice first noted in 2003 with increasing incidence.1 Both levamisole and cocaine stimulate the sympathetic nervous system by increasing dopamine in the euphoric areas of the brain.1,3 By combining the 2 substances, preparation costs are reduced and stimulant effects are enhanced. It is estimated that 69% to 80% of cocaine in the United States is contaminated with levamisole.2,4,5 The constellation of findings seen in patients abusing levamisole-contaminated cocaine include agranulocytosis; p-ANCA; and a tender, vasculitic, retiform purpura presentation. The most common sites for the purpura include the cheeks and ears. The purpura can progress to bullous lesions, as seen in our patient, followed by necrosis.4,6 Recurrent use of levamisole-contaminated cocaine is associated with recurrent agranulocytosis and classic skin findings, which is suggestive of a causal relationship.6
Serologic testing for levamisole exposure presents a challenge. The half-life of levamisole is relatively short (estimated at 5.6 hours) and is found in urine samples approximately 3% of the time.1,3,6 The volatile diagnostic characteristics of levamisole make concrete laboratory confirmation difficult. Although a skin biopsy can be helpful to rule out other causes of vasculitislike presentations, it is not specific for LIV. Therefore, clinical suspicion for LIV should remain high in patients who present with the cutaneous findings described as well as agranulocytosis, positive p-ANCA, and a history of cocaine use with a skin biopsy showing leukocytoclastic vasculitis and thrombotic vasculopathy.
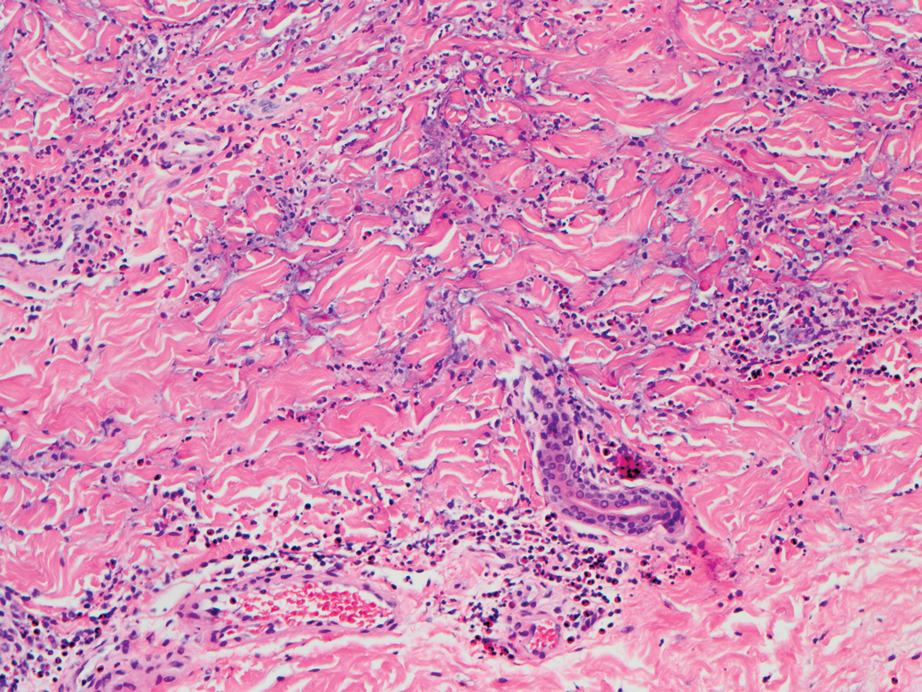
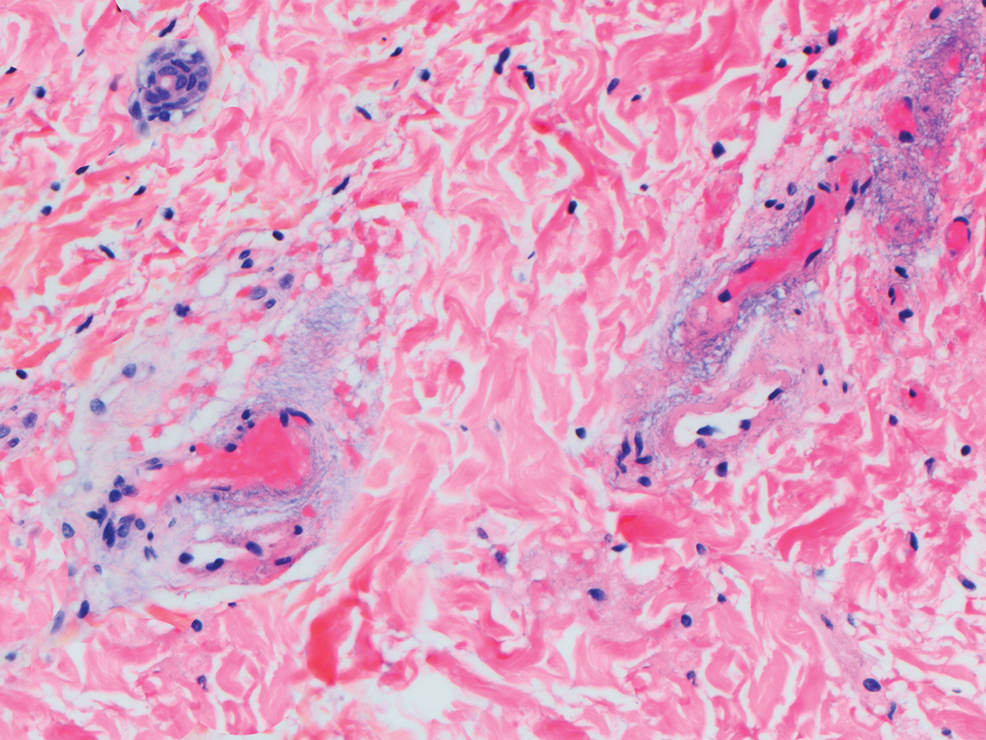
The differential diagnosis for LIV with retiform bullous lesions includes several other vasculitides and vesiculobullous diseases. Eosinophilic granulomatosis with polyangiitis (EGPA) is a multisystem vasculitis that is characterized by eosinophilia, asthma, and rhinosinusitis. Eosinophilic granulomatosis with polyangiitis primarily affects small and medium arteries in the skin and respiratory tract and occurs in 3 stages: prodromal, eosinophilic, and vasculitic. These stages are characterized by mild asthma or rhinitis, eosinophilia with multiorgan infiltration, and vasculitis with extravascular granulomatosis, respectively. Diagnosis often is clinical based on these findings and laboratory evaluation. Eosinophilic granulomatosis with polyangiitis presents with positive p-ANCA in 40% to 60% of patients.7 The vasculitis stage of EGPA presents with cutaneous findings in 60% of cases, including palpable purpura, infiltrated papules and plaques, urticaria, necrotizing lesions, and rarely vesicles and bullae.8 Classic histopathologic features include leukocytoclastic or eosinophilic vasculitis, an eosinophilic infiltrate, granuloma formation, and eosinophilic granule deposition onto collagen fibrils (otherwise known as flame figures)(Figure 2). Biopsy of these lesions with the aforementioned findings, in constellation with the described systemic signs and symptoms, can aid in diagnosis of EGPA.
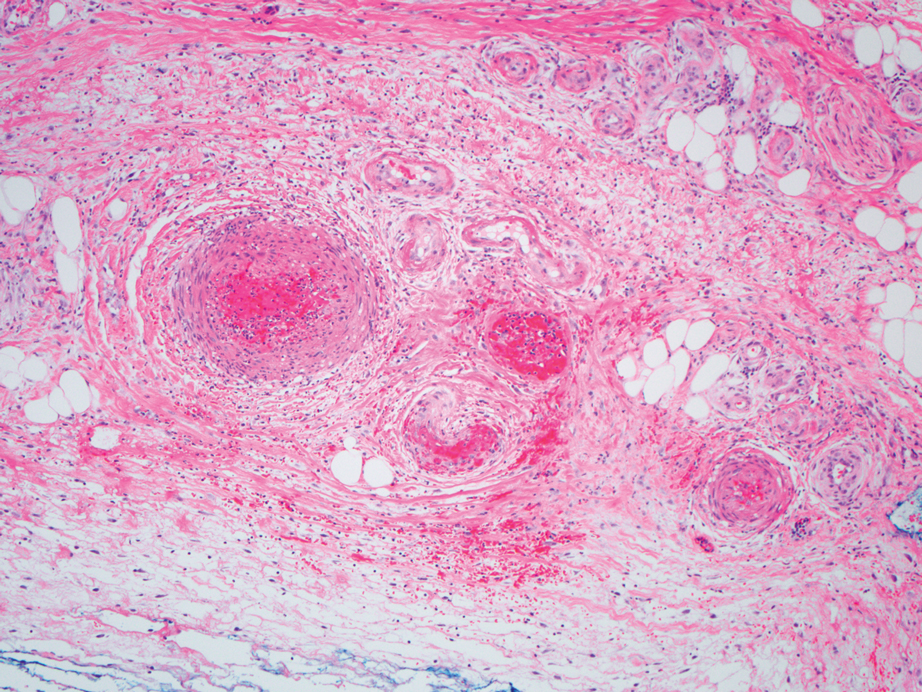
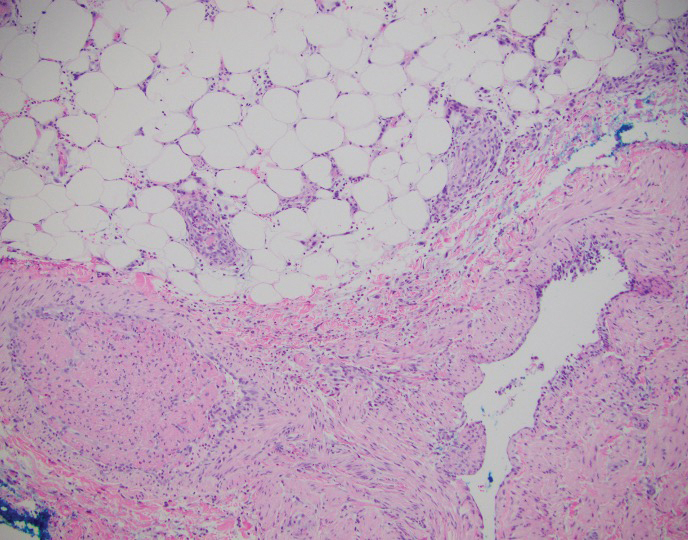
Polyarteritis nodosa (PAN) is a vasculitis that can be either multisystem or limited to one organ. Classic PAN affects the small- to medium-sized vessels. When there is multisystem involvement, it most often affects the skin, gastrointestinal tract, and kidneys. It presents with subcutaneous or dermal nodules, necrotic lesions, livedo reticularis, hypertension, abdominal pain, and an acute abdomen.9 When PAN is in its limited form, it most commonly occurs in the skin. The cutaneous manifestations of skin-limited PAN are identical to classic PAN, most commonly occurring on the legs and arms and less often on the trunk, head, and neck.10 To aid in diagnosis, biopsies of cutaneous lesions are beneficial. Dermatopathologic examination of PAN reveals fibrinoid necrosis of small and medium vessels with a perivascular mononuclear inflammatory infiltrate (Figure 3). Cutaneous PAN rarely progresses to multisystem classic PAN and carries a more favorable prognosis.
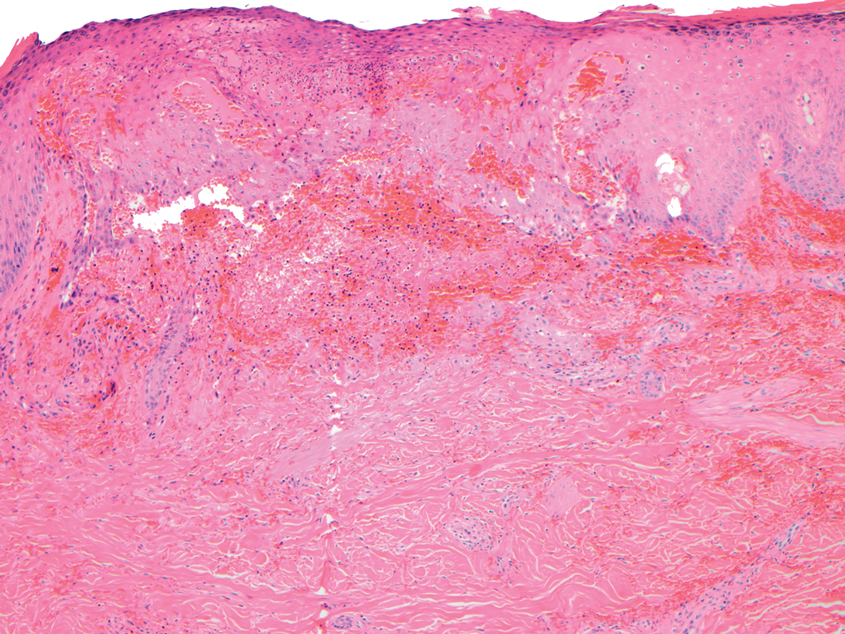
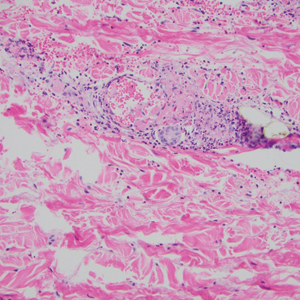
Microvascular occlusion syndromes can result in clinical presentations that resemble LIV. Idiopathic thrombocytopenic purpura is a hematologic autoimmune condition resulting in destruction of platelets and subsequent thrombocytopenia. Idiopathic thrombocytopenic purpura can be either primary or secondary to infections, drugs, malignancy, or other autoimmune conditions. Clinically, it presents as mucosal or cutaneous bleeding, epistaxis, hematochezia, or hematuria and can result in substantial hemorrhage. On the skin, it can appear as petechiae and ecchymoses in dependent areas and rarely hemorrhagic bullae of the skin and mucous membranes in cases of severe thrombocytopenia.11,12 Biopsies of these lesions will show notable extravasation of red blood cells with incipient hemorrhagic bullae formation (Figure 4). Recognition of hemorrhagic bullae as a presentation of idiopathic thrombocytopenic purpura is critical to identifying severe underlying disease.
Beyond other vasculitides and microvascular occlusion syndromes, vessel-invasive microorganisms can result in similar histopathologic and clinical presentations to LIV. Ecthyma gangrenosum (EG) is a septic vasculitis, often caused by Pseudomonas aeruginosa, usually affecting immunocompromised patients. Ecthyma gangrenosum presents with vesiculobullous lesions with erythematous violaceous borders that develop into hemorrhagic bullae with necrotic centers.13 Biopsy of EG will show vascular occlusion and basophilic granular material within or around vessels, suggestive of bacterial sepsis (Figure 5). The detection of an infectious agent on histopathology allows one to easily distinguish between EG and LIV.
- Bajaj S, Hibler B, Rossi A. Painful violaceous purpura on a 44-year-old woman. Am J Med. 2016;129:E5-E7.
- Munoz-Vahos CH, Herrera-Uribe S, Arbelaez-Cortes A, et al. Clinical profile of levamisole-adulterated cocaine-induced vasculitis/vasculopathy. J Clin Rheumatol. 2019;25:E16-E26.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Gillis JA, Green P, Williams J. Levamisole-induced vasculopathy: staging and management. J Plast Reconstr Aesthet Surg. 2014;67:E29-E31.
- Farhat EK, Muirhead TT, Chafins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;146:1320-1321.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia-a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2010;65:722-725.
- Negbenebor NA, Khalifian S, Foreman RK, et al. A 92-year-old male with eosinophilic asthma presenting with recurrent palpable purpuric plaques. Dermatopathology (Basel). 2018;5:44-48.
- Sherman S, Gal N, Didkovsky E, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) relapsing as bullous eruption. Acta Derm Venereol. 2017;97:406-407.
- Braungart S, Campbell A, Besarovic S. Atypical Henoch-Schonlein purpura? consider polyarteritis nodosa! BMJ Case Rep. 2014. doi:10.1136/bcr-2013-201764
- Alquorain NAA, Aljabr ASH, Alghamdi NJ. Cutaneous polyarteritis nodosa treated with pentoxifylline and clobetasol propionate: a case report. Saudi J Med Sci. 2018;6:104-107.
- Helms AE, Schaffer RI. Idiopathic thrombocytopenic purpura with black oral mucosal lesions. Cutis. 2007;79:456-458.
- Lountzis N, Maroon M, Tyler W. Mucocutaneous hemorrhagic bullae in idiopathic thrombocytopenic purpura. J Am Acad Dermatol. 2009;60:AB124.
- Llamas-Velasco M, Alegeria V, Santos-Briz A, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
The Diagnosis: Levamisole-Induced Vasculopathy
Biopsy of one of the bullous retiform purpura on the leg (Figure 1) revealed a combined leukocytoclastic vasculitis and thrombotic vasculopathy (quiz images). Periodic acid-Schiff and Gram stains, with adequate controls, were negative for pathogenic fungal and bacterial organisms. Although this reaction pattern has an extensive differential, in this clinical setting with associated cocaine-positive urine toxicologic analysis, perinuclear antineutrophil cytoplasmic antibodies (p-ANCA), and leukopenia, the histopathologic findings were consistent with levamisole-induced vasculopathy (LIV).1,2 Although not specific, leukocytoclastic vasculitis and thrombotic vasculopathy have been reported as the classic histopathologic findings of LIV. In addition, interstitial and perivascular neovascularization have been reported as a potential histopathologic finding associated with this entity but was not seen in our case.3
Levamisole is an anthelminthic agent used to adulterate cocaine, a practice first noted in 2003 with increasing incidence.1 Both levamisole and cocaine stimulate the sympathetic nervous system by increasing dopamine in the euphoric areas of the brain.1,3 By combining the 2 substances, preparation costs are reduced and stimulant effects are enhanced. It is estimated that 69% to 80% of cocaine in the United States is contaminated with levamisole.2,4,5 The constellation of findings seen in patients abusing levamisole-contaminated cocaine include agranulocytosis; p-ANCA; and a tender, vasculitic, retiform purpura presentation. The most common sites for the purpura include the cheeks and ears. The purpura can progress to bullous lesions, as seen in our patient, followed by necrosis.4,6 Recurrent use of levamisole-contaminated cocaine is associated with recurrent agranulocytosis and classic skin findings, which is suggestive of a causal relationship.6
Serologic testing for levamisole exposure presents a challenge. The half-life of levamisole is relatively short (estimated at 5.6 hours) and is found in urine samples approximately 3% of the time.1,3,6 The volatile diagnostic characteristics of levamisole make concrete laboratory confirmation difficult. Although a skin biopsy can be helpful to rule out other causes of vasculitislike presentations, it is not specific for LIV. Therefore, clinical suspicion for LIV should remain high in patients who present with the cutaneous findings described as well as agranulocytosis, positive p-ANCA, and a history of cocaine use with a skin biopsy showing leukocytoclastic vasculitis and thrombotic vasculopathy.
The differential diagnosis for LIV with retiform bullous lesions includes several other vasculitides and vesiculobullous diseases. Eosinophilic granulomatosis with polyangiitis (EGPA) is a multisystem vasculitis that is characterized by eosinophilia, asthma, and rhinosinusitis. Eosinophilic granulomatosis with polyangiitis primarily affects small and medium arteries in the skin and respiratory tract and occurs in 3 stages: prodromal, eosinophilic, and vasculitic. These stages are characterized by mild asthma or rhinitis, eosinophilia with multiorgan infiltration, and vasculitis with extravascular granulomatosis, respectively. Diagnosis often is clinical based on these findings and laboratory evaluation. Eosinophilic granulomatosis with polyangiitis presents with positive p-ANCA in 40% to 60% of patients.7 The vasculitis stage of EGPA presents with cutaneous findings in 60% of cases, including palpable purpura, infiltrated papules and plaques, urticaria, necrotizing lesions, and rarely vesicles and bullae.8 Classic histopathologic features include leukocytoclastic or eosinophilic vasculitis, an eosinophilic infiltrate, granuloma formation, and eosinophilic granule deposition onto collagen fibrils (otherwise known as flame figures)(Figure 2). Biopsy of these lesions with the aforementioned findings, in constellation with the described systemic signs and symptoms, can aid in diagnosis of EGPA.
Polyarteritis nodosa (PAN) is a vasculitis that can be either multisystem or limited to one organ. Classic PAN affects the small- to medium-sized vessels. When there is multisystem involvement, it most often affects the skin, gastrointestinal tract, and kidneys. It presents with subcutaneous or dermal nodules, necrotic lesions, livedo reticularis, hypertension, abdominal pain, and an acute abdomen.9 When PAN is in its limited form, it most commonly occurs in the skin. The cutaneous manifestations of skin-limited PAN are identical to classic PAN, most commonly occurring on the legs and arms and less often on the trunk, head, and neck.10 To aid in diagnosis, biopsies of cutaneous lesions are beneficial. Dermatopathologic examination of PAN reveals fibrinoid necrosis of small and medium vessels with a perivascular mononuclear inflammatory infiltrate (Figure 3). Cutaneous PAN rarely progresses to multisystem classic PAN and carries a more favorable prognosis.
Microvascular occlusion syndromes can result in clinical presentations that resemble LIV. Idiopathic thrombocytopenic purpura is a hematologic autoimmune condition resulting in destruction of platelets and subsequent thrombocytopenia. Idiopathic thrombocytopenic purpura can be either primary or secondary to infections, drugs, malignancy, or other autoimmune conditions. Clinically, it presents as mucosal or cutaneous bleeding, epistaxis, hematochezia, or hematuria and can result in substantial hemorrhage. On the skin, it can appear as petechiae and ecchymoses in dependent areas and rarely hemorrhagic bullae of the skin and mucous membranes in cases of severe thrombocytopenia.11,12 Biopsies of these lesions will show notable extravasation of red blood cells with incipient hemorrhagic bullae formation (Figure 4). Recognition of hemorrhagic bullae as a presentation of idiopathic thrombocytopenic purpura is critical to identifying severe underlying disease.
Beyond other vasculitides and microvascular occlusion syndromes, vessel-invasive microorganisms can result in similar histopathologic and clinical presentations to LIV. Ecthyma gangrenosum (EG) is a septic vasculitis, often caused by Pseudomonas aeruginosa, usually affecting immunocompromised patients. Ecthyma gangrenosum presents with vesiculobullous lesions with erythematous violaceous borders that develop into hemorrhagic bullae with necrotic centers.13 Biopsy of EG will show vascular occlusion and basophilic granular material within or around vessels, suggestive of bacterial sepsis (Figure 5). The detection of an infectious agent on histopathology allows one to easily distinguish between EG and LIV.
The Diagnosis: Levamisole-Induced Vasculopathy
Biopsy of one of the bullous retiform purpura on the leg (Figure 1) revealed a combined leukocytoclastic vasculitis and thrombotic vasculopathy (quiz images). Periodic acid-Schiff and Gram stains, with adequate controls, were negative for pathogenic fungal and bacterial organisms. Although this reaction pattern has an extensive differential, in this clinical setting with associated cocaine-positive urine toxicologic analysis, perinuclear antineutrophil cytoplasmic antibodies (p-ANCA), and leukopenia, the histopathologic findings were consistent with levamisole-induced vasculopathy (LIV).1,2 Although not specific, leukocytoclastic vasculitis and thrombotic vasculopathy have been reported as the classic histopathologic findings of LIV. In addition, interstitial and perivascular neovascularization have been reported as a potential histopathologic finding associated with this entity but was not seen in our case.3
Levamisole is an anthelminthic agent used to adulterate cocaine, a practice first noted in 2003 with increasing incidence.1 Both levamisole and cocaine stimulate the sympathetic nervous system by increasing dopamine in the euphoric areas of the brain.1,3 By combining the 2 substances, preparation costs are reduced and stimulant effects are enhanced. It is estimated that 69% to 80% of cocaine in the United States is contaminated with levamisole.2,4,5 The constellation of findings seen in patients abusing levamisole-contaminated cocaine include agranulocytosis; p-ANCA; and a tender, vasculitic, retiform purpura presentation. The most common sites for the purpura include the cheeks and ears. The purpura can progress to bullous lesions, as seen in our patient, followed by necrosis.4,6 Recurrent use of levamisole-contaminated cocaine is associated with recurrent agranulocytosis and classic skin findings, which is suggestive of a causal relationship.6
Serologic testing for levamisole exposure presents a challenge. The half-life of levamisole is relatively short (estimated at 5.6 hours) and is found in urine samples approximately 3% of the time.1,3,6 The volatile diagnostic characteristics of levamisole make concrete laboratory confirmation difficult. Although a skin biopsy can be helpful to rule out other causes of vasculitislike presentations, it is not specific for LIV. Therefore, clinical suspicion for LIV should remain high in patients who present with the cutaneous findings described as well as agranulocytosis, positive p-ANCA, and a history of cocaine use with a skin biopsy showing leukocytoclastic vasculitis and thrombotic vasculopathy.
The differential diagnosis for LIV with retiform bullous lesions includes several other vasculitides and vesiculobullous diseases. Eosinophilic granulomatosis with polyangiitis (EGPA) is a multisystem vasculitis that is characterized by eosinophilia, asthma, and rhinosinusitis. Eosinophilic granulomatosis with polyangiitis primarily affects small and medium arteries in the skin and respiratory tract and occurs in 3 stages: prodromal, eosinophilic, and vasculitic. These stages are characterized by mild asthma or rhinitis, eosinophilia with multiorgan infiltration, and vasculitis with extravascular granulomatosis, respectively. Diagnosis often is clinical based on these findings and laboratory evaluation. Eosinophilic granulomatosis with polyangiitis presents with positive p-ANCA in 40% to 60% of patients.7 The vasculitis stage of EGPA presents with cutaneous findings in 60% of cases, including palpable purpura, infiltrated papules and plaques, urticaria, necrotizing lesions, and rarely vesicles and bullae.8 Classic histopathologic features include leukocytoclastic or eosinophilic vasculitis, an eosinophilic infiltrate, granuloma formation, and eosinophilic granule deposition onto collagen fibrils (otherwise known as flame figures)(Figure 2). Biopsy of these lesions with the aforementioned findings, in constellation with the described systemic signs and symptoms, can aid in diagnosis of EGPA.
Polyarteritis nodosa (PAN) is a vasculitis that can be either multisystem or limited to one organ. Classic PAN affects the small- to medium-sized vessels. When there is multisystem involvement, it most often affects the skin, gastrointestinal tract, and kidneys. It presents with subcutaneous or dermal nodules, necrotic lesions, livedo reticularis, hypertension, abdominal pain, and an acute abdomen.9 When PAN is in its limited form, it most commonly occurs in the skin. The cutaneous manifestations of skin-limited PAN are identical to classic PAN, most commonly occurring on the legs and arms and less often on the trunk, head, and neck.10 To aid in diagnosis, biopsies of cutaneous lesions are beneficial. Dermatopathologic examination of PAN reveals fibrinoid necrosis of small and medium vessels with a perivascular mononuclear inflammatory infiltrate (Figure 3). Cutaneous PAN rarely progresses to multisystem classic PAN and carries a more favorable prognosis.
Microvascular occlusion syndromes can result in clinical presentations that resemble LIV. Idiopathic thrombocytopenic purpura is a hematologic autoimmune condition resulting in destruction of platelets and subsequent thrombocytopenia. Idiopathic thrombocytopenic purpura can be either primary or secondary to infections, drugs, malignancy, or other autoimmune conditions. Clinically, it presents as mucosal or cutaneous bleeding, epistaxis, hematochezia, or hematuria and can result in substantial hemorrhage. On the skin, it can appear as petechiae and ecchymoses in dependent areas and rarely hemorrhagic bullae of the skin and mucous membranes in cases of severe thrombocytopenia.11,12 Biopsies of these lesions will show notable extravasation of red blood cells with incipient hemorrhagic bullae formation (Figure 4). Recognition of hemorrhagic bullae as a presentation of idiopathic thrombocytopenic purpura is critical to identifying severe underlying disease.
Beyond other vasculitides and microvascular occlusion syndromes, vessel-invasive microorganisms can result in similar histopathologic and clinical presentations to LIV. Ecthyma gangrenosum (EG) is a septic vasculitis, often caused by Pseudomonas aeruginosa, usually affecting immunocompromised patients. Ecthyma gangrenosum presents with vesiculobullous lesions with erythematous violaceous borders that develop into hemorrhagic bullae with necrotic centers.13 Biopsy of EG will show vascular occlusion and basophilic granular material within or around vessels, suggestive of bacterial sepsis (Figure 5). The detection of an infectious agent on histopathology allows one to easily distinguish between EG and LIV.
- Bajaj S, Hibler B, Rossi A. Painful violaceous purpura on a 44-year-old woman. Am J Med. 2016;129:E5-E7.
- Munoz-Vahos CH, Herrera-Uribe S, Arbelaez-Cortes A, et al. Clinical profile of levamisole-adulterated cocaine-induced vasculitis/vasculopathy. J Clin Rheumatol. 2019;25:E16-E26.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Gillis JA, Green P, Williams J. Levamisole-induced vasculopathy: staging and management. J Plast Reconstr Aesthet Surg. 2014;67:E29-E31.
- Farhat EK, Muirhead TT, Chafins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;146:1320-1321.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia-a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2010;65:722-725.
- Negbenebor NA, Khalifian S, Foreman RK, et al. A 92-year-old male with eosinophilic asthma presenting with recurrent palpable purpuric plaques. Dermatopathology (Basel). 2018;5:44-48.
- Sherman S, Gal N, Didkovsky E, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) relapsing as bullous eruption. Acta Derm Venereol. 2017;97:406-407.
- Braungart S, Campbell A, Besarovic S. Atypical Henoch-Schonlein purpura? consider polyarteritis nodosa! BMJ Case Rep. 2014. doi:10.1136/bcr-2013-201764
- Alquorain NAA, Aljabr ASH, Alghamdi NJ. Cutaneous polyarteritis nodosa treated with pentoxifylline and clobetasol propionate: a case report. Saudi J Med Sci. 2018;6:104-107.
- Helms AE, Schaffer RI. Idiopathic thrombocytopenic purpura with black oral mucosal lesions. Cutis. 2007;79:456-458.
- Lountzis N, Maroon M, Tyler W. Mucocutaneous hemorrhagic bullae in idiopathic thrombocytopenic purpura. J Am Acad Dermatol. 2009;60:AB124.
- Llamas-Velasco M, Alegeria V, Santos-Briz A, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Bajaj S, Hibler B, Rossi A. Painful violaceous purpura on a 44-year-old woman. Am J Med. 2016;129:E5-E7.
- Munoz-Vahos CH, Herrera-Uribe S, Arbelaez-Cortes A, et al. Clinical profile of levamisole-adulterated cocaine-induced vasculitis/vasculopathy. J Clin Rheumatol. 2019;25:E16-E26.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Gillis JA, Green P, Williams J. Levamisole-induced vasculopathy: staging and management. J Plast Reconstr Aesthet Surg. 2014;67:E29-E31.
- Farhat EK, Muirhead TT, Chafins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;146:1320-1321.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia-a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2010;65:722-725.
- Negbenebor NA, Khalifian S, Foreman RK, et al. A 92-year-old male with eosinophilic asthma presenting with recurrent palpable purpuric plaques. Dermatopathology (Basel). 2018;5:44-48.
- Sherman S, Gal N, Didkovsky E, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) relapsing as bullous eruption. Acta Derm Venereol. 2017;97:406-407.
- Braungart S, Campbell A, Besarovic S. Atypical Henoch-Schonlein purpura? consider polyarteritis nodosa! BMJ Case Rep. 2014. doi:10.1136/bcr-2013-201764
- Alquorain NAA, Aljabr ASH, Alghamdi NJ. Cutaneous polyarteritis nodosa treated with pentoxifylline and clobetasol propionate: a case report. Saudi J Med Sci. 2018;6:104-107.
- Helms AE, Schaffer RI. Idiopathic thrombocytopenic purpura with black oral mucosal lesions. Cutis. 2007;79:456-458.
- Lountzis N, Maroon M, Tyler W. Mucocutaneous hemorrhagic bullae in idiopathic thrombocytopenic purpura. J Am Acad Dermatol. 2009;60:AB124.
- Llamas-Velasco M, Alegeria V, Santos-Briz A, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
A 40-year-old woman presented with a progressive painful rash on the ears and legs of 2 weeks’ duration. She described the rash as initially red and nonpainful; it started on the right leg and progressed to the left leg, eventually involving the earlobes 4 days prior to presentation. Physical examination revealed edematous purpura of the earlobes and bullous retiform purpura on the lower extremities. Laboratory studies revealed leukopenia (3.6×103 /cm2 [reference range, 4.0–10.5×103 /cm2 ]) and elevated antineutrophil cytoplasmic antibodies (1:320 titer [reference range, <1:40]) in a perinuclear pattern (perinuclear antineutrophil cytoplasmic antibodies). Urine toxicology screening was positive for cocaine and opiates. A punch biopsy of a bullous retiform purpura on the right thigh was obtained for standard hematoxylin and eosin staining.
Exploring the Utility of Artificial Intelligence During COVID-19 in Dermatology Practice
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
Practice Points
- Dermatologists should amass pictures of dermatologic conditions in skin of color to contribute to growing awareness and knowledge of presentation of disease in this population.
- Dermatologists should use artificial intelligence as a tool for delivering more efficient and beneficial patient care.
Mobile App Usage Among Dermatology Residents in America
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Practice Points
- Virtual resources, including mobile apps, have become critical tools for learning and patient care during dermatology resident training for reasons that should be elucidated.
- Dermatology residents of different years and sexes utilize mobile apps in different amounts and for different purposes.
Aquatic Antagonists: Sea Cucumbers (Holothuroidea)
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Practice Points
- Sea cucumbers produce a toxin known as holothurin, which is contained in specialized structures called Cuvierian tubules and secreted onto the outer surface of the body wall. Some species also eject portions of their toxic inner organs through the anus as a defensive mechanism.
- In humans, the holothurin toxins cause an acute irritant dermatitis upon contact with the skin and a painful chemical conjunctivitis upon contact with the eyes.
- In addition to their own toxin, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through their integument, causing additional effects on human skin.
- The dermatologic effects of sea cucumbers can be prevented with the use of gloves and swim masks or goggles.
The Top 100 Most-Cited Articles on Nail Psoriasis: A Bibliometric Analysis
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
Reticular Rash on the Chest
The Diagnosis: Erythema Ab Igne
Based on the clinical findings and history, a diagnosis of erythema ab igne (EAI), a skin reaction to chronic infrared radiation exposure, was made. The name of this condition translates from Latin as “redness from fire”; other names include toasted skin syndrome and fire stains. The most common presentation is reticulated hyperpigmentation, erythema, and cutaneous atrophy, as well as possible crusting, scaling, or telangiectasia. The rash also typically presents in areas of heat exposure—from heated blankets, heating pads, or the use of infrared heaters or lamps.1,2 The patient usually will have pain and pruritus over the affected areas. The diagnosis of EAI largely is clinical and based on the patient’s history of exposure; it rarely requires biopsy and histologic analysis. However, some of the common histopathologic findings include hyperkeratosis, a hyperpigmented basal layer, hemosiderin deposits, prominent melanophages, basal cell degeneration, course collagen, and elastosis.2,3 These changes are common with UV radiation exposure and thermal damage. The primary treatment in all cases is to remove or reduce the source of infrared radiation. However, EAI has been reported to be successfully treated with removal of the insult as well as topical agents such as imiquimod and 5-fluorouracil.4 Possible complications include increased risk for malignancies such as squamous cell carcinoma in the affected area.1
The possible differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectatica congenita. All of these conditions are related to dysfunction of the cutaneous vasculature that creates a reticular, mottled, reddish purple rash. When the livedo is reversible and idiopathic, it is referred to as livedo reticularis, but when it is generalized and permanent it is referred to as livedo racemosa. Livedo racemosa can be caused by a variety of conditions, including systemic lupus erythematosus and antiphospholipid syndrome.1 Physiologic livedo reticularis that is more transient and can be reversed by warming is referred to as cutis marmorata. Finally, cutis marmorata telangiectatica congenita primarily is found in neonates, and although persistent, it usually improves with age. Erythema ab igne also is a type of livedo with a known heat exposure and localized distribution.
Our patient was educated on the etiology of the rash, specifically related to heating pad usage for multiple years, and the risk for cutaneous malignancy after longstanding EAI. It was recommended that she discontinue use of a heating pad on the affected areas to allow them to properly heal. If she found that heating pad usage was necessary, she was advised to limit use to 5 to 10 minutes with 2 to 3 hours in between applications. In addition, she was advised to apply petroleum jelly daily for assistance with wound healing as well as anti-itch sensitive lotion twice daily on the arms and back to alleviate some of the tingling pain. We explained that areas of hyperpigmentation may improve with time; however, areas of erythema/ atrophy may be long-lasting.
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635.
- Dellavalle RP, Gillum P. Erythema ab igne following heating/cooling blanket use in the intensive care unit. Cutis. 2000;66:136-138.
- Finlayson GR, Sams WM Jr, Smith JG Jr. Erythema ab igne: a histopathological study. J Invest Dermatol. 1966;46:104-108.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
The Diagnosis: Erythema Ab Igne
Based on the clinical findings and history, a diagnosis of erythema ab igne (EAI), a skin reaction to chronic infrared radiation exposure, was made. The name of this condition translates from Latin as “redness from fire”; other names include toasted skin syndrome and fire stains. The most common presentation is reticulated hyperpigmentation, erythema, and cutaneous atrophy, as well as possible crusting, scaling, or telangiectasia. The rash also typically presents in areas of heat exposure—from heated blankets, heating pads, or the use of infrared heaters or lamps.1,2 The patient usually will have pain and pruritus over the affected areas. The diagnosis of EAI largely is clinical and based on the patient’s history of exposure; it rarely requires biopsy and histologic analysis. However, some of the common histopathologic findings include hyperkeratosis, a hyperpigmented basal layer, hemosiderin deposits, prominent melanophages, basal cell degeneration, course collagen, and elastosis.2,3 These changes are common with UV radiation exposure and thermal damage. The primary treatment in all cases is to remove or reduce the source of infrared radiation. However, EAI has been reported to be successfully treated with removal of the insult as well as topical agents such as imiquimod and 5-fluorouracil.4 Possible complications include increased risk for malignancies such as squamous cell carcinoma in the affected area.1
The possible differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectatica congenita. All of these conditions are related to dysfunction of the cutaneous vasculature that creates a reticular, mottled, reddish purple rash. When the livedo is reversible and idiopathic, it is referred to as livedo reticularis, but when it is generalized and permanent it is referred to as livedo racemosa. Livedo racemosa can be caused by a variety of conditions, including systemic lupus erythematosus and antiphospholipid syndrome.1 Physiologic livedo reticularis that is more transient and can be reversed by warming is referred to as cutis marmorata. Finally, cutis marmorata telangiectatica congenita primarily is found in neonates, and although persistent, it usually improves with age. Erythema ab igne also is a type of livedo with a known heat exposure and localized distribution.
Our patient was educated on the etiology of the rash, specifically related to heating pad usage for multiple years, and the risk for cutaneous malignancy after longstanding EAI. It was recommended that she discontinue use of a heating pad on the affected areas to allow them to properly heal. If she found that heating pad usage was necessary, she was advised to limit use to 5 to 10 minutes with 2 to 3 hours in between applications. In addition, she was advised to apply petroleum jelly daily for assistance with wound healing as well as anti-itch sensitive lotion twice daily on the arms and back to alleviate some of the tingling pain. We explained that areas of hyperpigmentation may improve with time; however, areas of erythema/ atrophy may be long-lasting.
The Diagnosis: Erythema Ab Igne
Based on the clinical findings and history, a diagnosis of erythema ab igne (EAI), a skin reaction to chronic infrared radiation exposure, was made. The name of this condition translates from Latin as “redness from fire”; other names include toasted skin syndrome and fire stains. The most common presentation is reticulated hyperpigmentation, erythema, and cutaneous atrophy, as well as possible crusting, scaling, or telangiectasia. The rash also typically presents in areas of heat exposure—from heated blankets, heating pads, or the use of infrared heaters or lamps.1,2 The patient usually will have pain and pruritus over the affected areas. The diagnosis of EAI largely is clinical and based on the patient’s history of exposure; it rarely requires biopsy and histologic analysis. However, some of the common histopathologic findings include hyperkeratosis, a hyperpigmented basal layer, hemosiderin deposits, prominent melanophages, basal cell degeneration, course collagen, and elastosis.2,3 These changes are common with UV radiation exposure and thermal damage. The primary treatment in all cases is to remove or reduce the source of infrared radiation. However, EAI has been reported to be successfully treated with removal of the insult as well as topical agents such as imiquimod and 5-fluorouracil.4 Possible complications include increased risk for malignancies such as squamous cell carcinoma in the affected area.1
The possible differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectatica congenita. All of these conditions are related to dysfunction of the cutaneous vasculature that creates a reticular, mottled, reddish purple rash. When the livedo is reversible and idiopathic, it is referred to as livedo reticularis, but when it is generalized and permanent it is referred to as livedo racemosa. Livedo racemosa can be caused by a variety of conditions, including systemic lupus erythematosus and antiphospholipid syndrome.1 Physiologic livedo reticularis that is more transient and can be reversed by warming is referred to as cutis marmorata. Finally, cutis marmorata telangiectatica congenita primarily is found in neonates, and although persistent, it usually improves with age. Erythema ab igne also is a type of livedo with a known heat exposure and localized distribution.
Our patient was educated on the etiology of the rash, specifically related to heating pad usage for multiple years, and the risk for cutaneous malignancy after longstanding EAI. It was recommended that she discontinue use of a heating pad on the affected areas to allow them to properly heal. If she found that heating pad usage was necessary, she was advised to limit use to 5 to 10 minutes with 2 to 3 hours in between applications. In addition, she was advised to apply petroleum jelly daily for assistance with wound healing as well as anti-itch sensitive lotion twice daily on the arms and back to alleviate some of the tingling pain. We explained that areas of hyperpigmentation may improve with time; however, areas of erythema/ atrophy may be long-lasting.
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635.
- Dellavalle RP, Gillum P. Erythema ab igne following heating/cooling blanket use in the intensive care unit. Cutis. 2000;66:136-138.
- Finlayson GR, Sams WM Jr, Smith JG Jr. Erythema ab igne: a histopathological study. J Invest Dermatol. 1966;46:104-108.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635.
- Dellavalle RP, Gillum P. Erythema ab igne following heating/cooling blanket use in the intensive care unit. Cutis. 2000;66:136-138.
- Finlayson GR, Sams WM Jr, Smith JG Jr. Erythema ab igne: a histopathological study. J Invest Dermatol. 1966;46:104-108.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
A 53-year-old woman with a history of diabetes mellitus, hypertension, chronic complex regional pain syndrome type 1, and chronic prescription opiate use presented to the hospital with a pruritic rash on the chest of 15 years’ duration that started a few weeks after a left shoulder repair. The patient was using fentanyl patches and acetaminophen with oxycodone as well as a heating pad for 20 to 22 hours per day for many years to help with her chronic pain. She also described similar lesions on the abdomen and back when she used the heating pad on those areas for weeks at a time. Vital signs were within normal limits. Physical examination revealed a lacy, reticular, eroded, well-demarcated rash on the chest along with areas of cracking. Laboratory evaluation did not reveal any abnormalities.