User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
To the Editor:
Scabies infestation, which has been recognized as a neglected tropical disease by the World Health Organization since 2017, is caused by the human itch mite (Sarcoptes scabiei var hominis).1 Infected individuals experience a pruritic papular rash when the mite burrows into the epidermis, where it lives and lays eggs.2,3 Infected individuals also may develop bacterial superinfections if the skin barrier becomes compromised, leading to systemic complications and considerable morbidity.3
In countries with high human development indices, scabies outbreaks are linked to densely populated living conditions, such as those found in nursing homes or prisons.3,4 Scabies also is transmitted via sexual contact in adults. Beyond immunosuppression, little is known about other comorbid conditions or lifestyle risk factors associated with scabies infestation.2 Because scabies can mimic a range of other dermatologic conditions such as folliculitis, atopic dermatitis, and arthropod bites, misdiagnosis is common and can lead to delayed treatment and increased transmission risk.4 In this study, we sought to examine comorbid conditions and/or lifestyle risk factors associated with scabies infestation.
A matched case-control study was performed using the Registered Tier dataset of the National Institutes of Health All of Us Research Program Curated Data Repository version 7, which includes more than 400,000 unique participants aged 18 years or older from across the United States. The All of Us Research Program excludes adults who are unable to consent independently as well as incarcerated populations and children younger than 18 years. Participants diagnosed with scabies were identified using SNOMED code 62752005 and compared to a control group matched 1:4 based on age, sex, and selfidentified race. SNOMED codes also were used to identify various comorbidities and lifestyle risk factors, including depression, bipolar disorder, anxiety, schizophrenia, peripheral vascular disease (PVD), HIV, type 2 diabetes mellitus (T2DM), unsheltered status, tobacco use, difficulty with activities of daily living, insurance status, and any recent travel history. Logistic regression models were used to calculate odds ratios (ORs) and estimate effect sizes, with statistical significance set at P<.05.
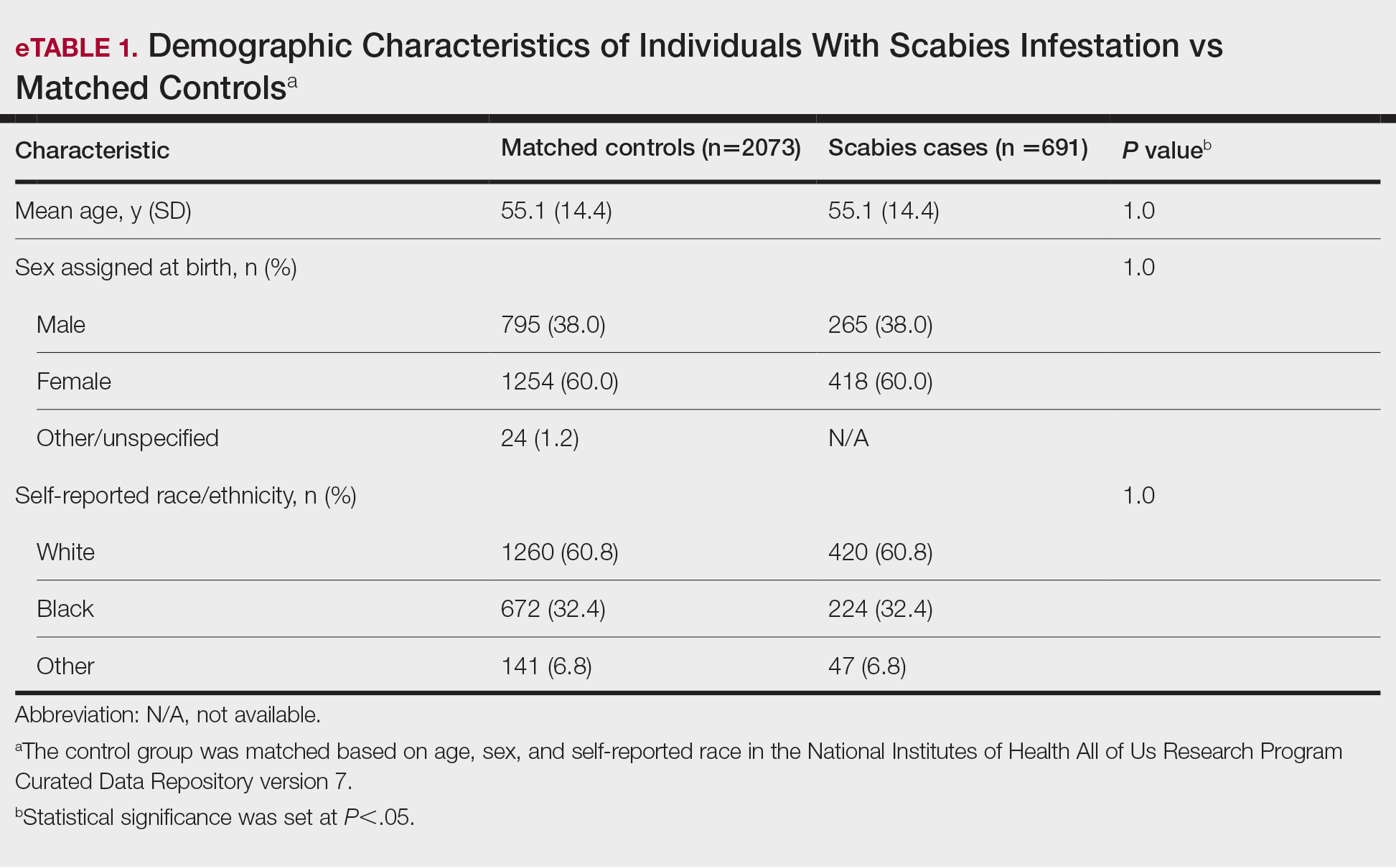
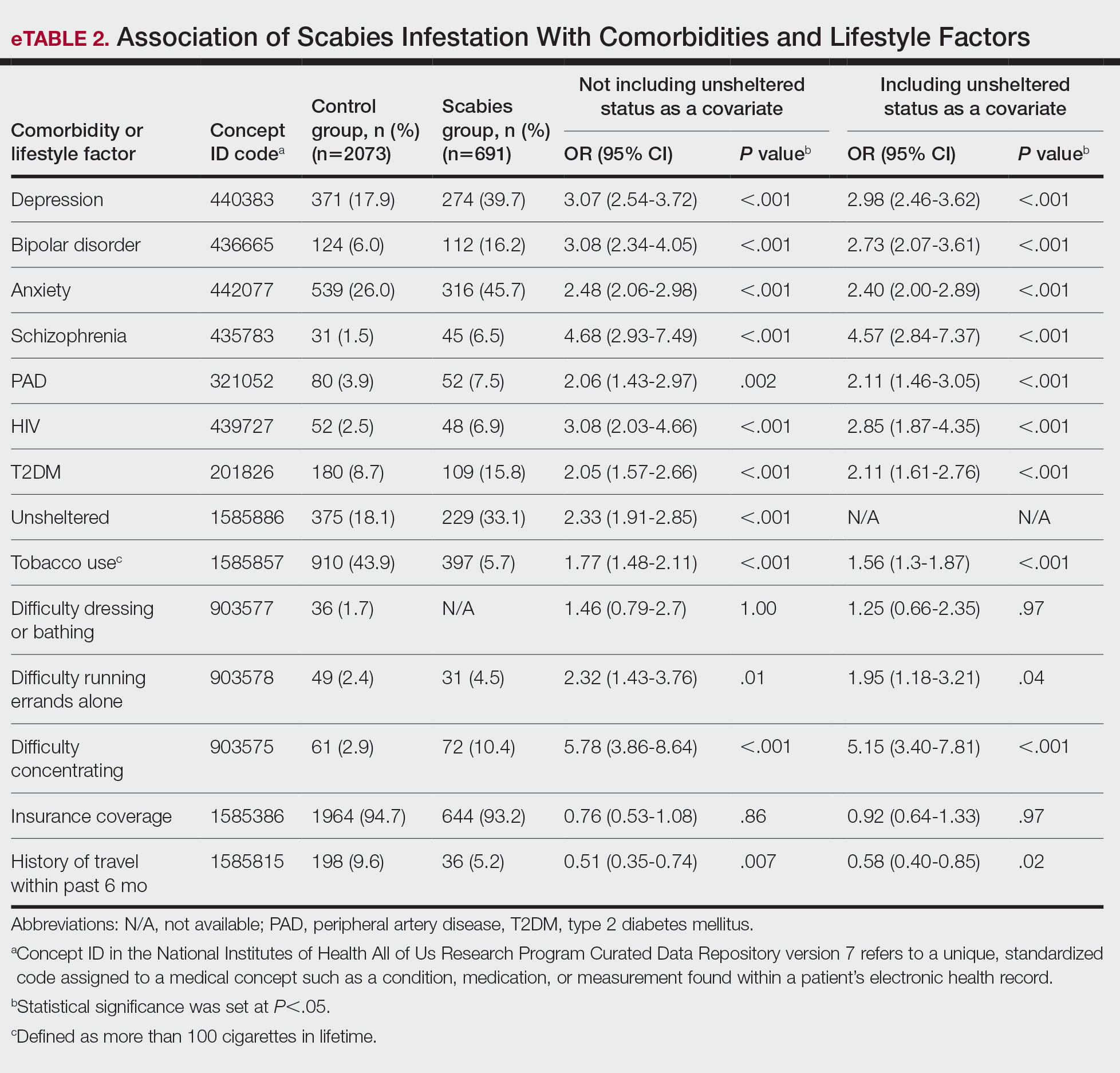
We identified 691 cases of scabies infestation and 2073 controls. The average age of the patients diagnosed with scabies was 55.1 years. Seventy percent (481/691) identified as female and 32.4% (224/491) identified as Black or African American. Matched controls were similar for all analyzed demographic characteristics (P=1.0)(eTable 1). Patients diagnosed with scabies were more likely to be unsheltered (OR, 2.33 [95% CI, 1.91-2.85]), use tobacco (OR 1.77 [95% CI, 1.48-2.11]) and have a comorbid diagnosis of HIV (OR, 3.08 [95% CI, 2.03-4.66]), T2DM (OR, 2.05 [95% CI, 1.57- 2.66]) or PVD (OR, 2.06 [95% CI, 1.43-2.97]) compared with controls (P<.001). Psychiatric comorbidities were more common in the patients diagnosed with scabies, including depression (OR, 3.07 [95% CI, 2.54-3.72]), anxiety (OR, 2.48 [95% CI, 2.06-2.98]), bipolar disorder (OR, 3.08 [95% CI, 2.34-4.05]), and schizophrenia (OR, 4.68 [95% CI, 2.93-7.49])(P<.001). Difficulties with activities of daily living, including running errands alone (OR, 2.32 [95% CI, 1.43-3.76]) and concentrating (OR, 5.78; 95% CI, 3.86-8.64), were more prevalent in the scabies group compared to controls (both P<.05). In a multivariate logistic regression model including unsheltered status as a covariate, all associations remained statistically significant (P<.05)(eTable 2).
This large diverse study demonstrated an association between scabies infestation and unsheltered status. Previous studies have shown that unsheltered populations are at increased risk for many dermatologic conditions, perhaps due to decreased access to health care and social support, lack of access to hygiene facilities (eg, public showers), and increased prevalence of substance use and psychiatric disorders among this population.5 In a cross-sectional analysis of hospitalized patients, 8.6% of unsheltered patients (n=197) had an ectoparasitic disease (including scabies) compared with 1.0% of patients with stable housing (n=1018), with a 9.43-fold increased risk for ectoparasitic infestation among unsheltered patients (95% CI, 3.79-23.47; P<.001).6 Increased attention to public health initiatives among unsheltered populations— including access to hygiene facilities and increased dermatologic services—are needed, as ectoparasitic infections are both preventable and treatable, and these initiatives could reduce morbidity associated with superimposed bacterial infections for which unsheltered patients are at increased risk.6
Our results also showed that individuals diagnosed with scabies were more likely than the controls to have been diagnosed with HIV, T2DM, and PVD. Our findings are similar to those of a systematic review of immunosuppressive factors associated with crusted scabies (a severe form of scabies infestation) in which 10.2% and 15.7% of patients (n=683) had comorbid HIV and T2DM, respectively.7 A functioning cell-mediated response to scabies mite antigens limits proliferation of the human itch mite; thus, infection with HIV/AIDS, which induces the destruction of CD4+ T cells, limits the immune system’s ability to mount an effective response against these antigens. The association of scabies with T2DM likely is multifactorial; for example, chronic hyperglycemia may lead to immune system impairment, and peripheral neuropathy may reduce the itch sensation, allowing scabies mites to proliferate without removal by scratching.7 In a descriptive epidemiologic study in Japan, 11.7% of patients with scabies (N=857) had comorbid PVD.8 Peripheral vascular disease can lead to the development of ulcers, gangrene, and stasis dermatitis, all of which compromise the skin barrier and increase susceptibility to infection.9 Notably, these associations remained even when unsheltered status was considered as a confounding variable. Because individuals with HIV, T2DM, and PVD may be at higher risk for serious complications of scabies infestation (eg, secondary bacterial infections, invasive group A streptococcal infections), prompt detection and treatment of scabies are crucial in curbing morbidity in these at-risk populations.
Our study also demonstrated that psychiatric comorbidities including depression, anxiety, bipolar disorder, and schizophrenia were associated with scabies infestation, even when controlling for unsheltered status, which may have a bidirectional relationship with mental health disorders.10 In a cross-sectional study of 83 adult patients diagnosed with scabies, 72.2% (60/83) reported moderate to extremely large effect of scabies infestation on quality of life using the Dermatology Life Quality Index, and these scores positively correlated with increased Beck Depression Scale and Beck Anxiety Scale scores (rs=0.448 and rs=0.456 0.456, respectively; both P=.000). The results of this study suggest that scabies negatively impacts quality of life, which might increase symptoms of depression and anxiety.11
Studies are needed to assess whether patients with pre-existing depression and anxiety face increased risk for scabies infestation. In a retrospective case-control study using data from the National Health Insurance Research Database of Taiwan, 0.8% (58/7096) of patients with scabies (n=7096) and 0.4% of controls (n=28,375) were newly diagnosed with bipolar disorder over a 7-year period, indicating a 1.55-fold increased risk for bipolar disorder in patients with scabies compared to those without (95% CI, 1.12-2.09; P<.05).12 Future studies are needed to determine whether the relationship between bipolar disorder and scabies is bidirectional, with pre-existing bipolar disorder evaluated as a risk factor for subsequent scabies infestation. Increased difficulties with activities of daily living, including running errands independently and concentrating, were associated with scabies. These difficulties may reflect sequelae of psychiatric illness or pruritus associated with scabies affecting daily living.
Physician awareness of comorbidities and lifestyle risk factors associated with scabies infestation may improve diagnosis and prevent treatment delays. In a retrospective study at a single dermatology outpatient clinic, 45.3% of patients with scabies (n=428) had previously been misdiagnosed with another dermatologic condition, and the most common erroneous diagnosis was atopic dermatitis.13 Our study provides a framework of comorbidities and lifestyle risk factors associated with scabies infestation that dermatologists can use to stratify patients who may be at greater risk for this condition, allowing dermatologists to select appropriate treatment when clinical signs are ambiguous.
Limitations of our study included the potential for miscoding in the database, lack of information about treatment regimens employed (if any), and lack of information about the temporal relationship between associations.
In summary, it is recommended that patients with pruritus and other characteristic clinical findings of scabies receive appropriate workup for scabies regardless of risk factors; however, the medical and psychiatric comorbidities and lifestyle risk factors identified in this study may help to identify at-risk patients. Our study showed that unsheltered patients are at increased risk for scabies, potentially due to unique dermatologic challenges and lack of access to health care and hygiene facilities. Positive correlations between scabies and HIV, T2DM, and PVD suggest that patients with chronic immunocompromising illnesses who live in group homes or other crowded quarters and present with symptoms could be evaluated for scabies infestation to prevent widespread and difficult- to-control outbreaks in these communities. Based on our findings, scabies also should be included in the differential diagnosis for patients with psychiatric illness and suggestive symptoms. Early identification and treatment of scabies infestation could prevent misdiagnosis and treatment delays.
- World Health Organization. Scabies fact sheet. May 31, 2023. Accessed February 13, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Schneider S, Wu J, Tizek L, et al. Prevalence of scabies worldwidean updated systematic literature review in 2022. J Eur Acad Dermatol Venereol. 2023;37:1749-1757. doi:10.1111/jdv.19167
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Henry T, Khachemoune A. Dermatologic conditions and risk factors in people experiencing homelessness (PEH): systematic review. Arch Dermatol Res. 2023;315:2795-2803. doi:10.1007/s00403-023-02722-2
- Zakaria A, Amerson EH, Kim-Lim P, et al. Characterization of dermatological diagnoses among hospitalized patients experiencing homelessness. Clin Exp Dermatol. 2022;47:117-120. doi:10.1111/ced.14828
- Bergamin G, Hudson J, Currie BJ, et al. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int J Infect Dis. 2024;143:107036. doi:10.1016/j.ijid.2024.107036
- Yamaguchi Y, Murata F, Maeda M, et al. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. 2024;11:100353. doi:10.1016 /j.ijregi.2024.03.008
- Raja A, Karch J, Shih AF, et al. Part II: Cutaneous manifestations of peripheral vascular disease. J Am Acad Dermatol. 2023;89:211-226. doi:10.1016/j.jaad.2021.05.077
- Barry R, Anderson J, Tran L, et al. Prevalence of mental health disorders among individuals experiencing homelessness: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:691-699. doi:10.1001 /jamapsychiatry.2024.0426
- Koc Y.ld.r.m S, Demirel Og. ut N, Erbag. c. E, et al. Scabies affects quality of life in correlation with depression and anxiety. Dermatol Pract Concept. 2023;13:E2023144. doi:10.5826/dpc.1302a144
- Lin CY, Chang FW, Yang JJ, et al. Increased risk of bipolar disorder in patients with scabies: a nationwide population-based matched-cohort study. Psychiatry Res. 2017;257:14-20. doi:10.1016 /j.psychres.2017.07.013
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017;30:78-84. doi:10.3122/jabfm.2017.01.160190
To the Editor:
Scabies infestation, which has been recognized as a neglected tropical disease by the World Health Organization since 2017, is caused by the human itch mite (Sarcoptes scabiei var hominis).1 Infected individuals experience a pruritic papular rash when the mite burrows into the epidermis, where it lives and lays eggs.2,3 Infected individuals also may develop bacterial superinfections if the skin barrier becomes compromised, leading to systemic complications and considerable morbidity.3
In countries with high human development indices, scabies outbreaks are linked to densely populated living conditions, such as those found in nursing homes or prisons.3,4 Scabies also is transmitted via sexual contact in adults. Beyond immunosuppression, little is known about other comorbid conditions or lifestyle risk factors associated with scabies infestation.2 Because scabies can mimic a range of other dermatologic conditions such as folliculitis, atopic dermatitis, and arthropod bites, misdiagnosis is common and can lead to delayed treatment and increased transmission risk.4 In this study, we sought to examine comorbid conditions and/or lifestyle risk factors associated with scabies infestation.
A matched case-control study was performed using the Registered Tier dataset of the National Institutes of Health All of Us Research Program Curated Data Repository version 7, which includes more than 400,000 unique participants aged 18 years or older from across the United States. The All of Us Research Program excludes adults who are unable to consent independently as well as incarcerated populations and children younger than 18 years. Participants diagnosed with scabies were identified using SNOMED code 62752005 and compared to a control group matched 1:4 based on age, sex, and selfidentified race. SNOMED codes also were used to identify various comorbidities and lifestyle risk factors, including depression, bipolar disorder, anxiety, schizophrenia, peripheral vascular disease (PVD), HIV, type 2 diabetes mellitus (T2DM), unsheltered status, tobacco use, difficulty with activities of daily living, insurance status, and any recent travel history. Logistic regression models were used to calculate odds ratios (ORs) and estimate effect sizes, with statistical significance set at P<.05.
We identified 691 cases of scabies infestation and 2073 controls. The average age of the patients diagnosed with scabies was 55.1 years. Seventy percent (481/691) identified as female and 32.4% (224/491) identified as Black or African American. Matched controls were similar for all analyzed demographic characteristics (P=1.0)(eTable 1). Patients diagnosed with scabies were more likely to be unsheltered (OR, 2.33 [95% CI, 1.91-2.85]), use tobacco (OR 1.77 [95% CI, 1.48-2.11]) and have a comorbid diagnosis of HIV (OR, 3.08 [95% CI, 2.03-4.66]), T2DM (OR, 2.05 [95% CI, 1.57- 2.66]) or PVD (OR, 2.06 [95% CI, 1.43-2.97]) compared with controls (P<.001). Psychiatric comorbidities were more common in the patients diagnosed with scabies, including depression (OR, 3.07 [95% CI, 2.54-3.72]), anxiety (OR, 2.48 [95% CI, 2.06-2.98]), bipolar disorder (OR, 3.08 [95% CI, 2.34-4.05]), and schizophrenia (OR, 4.68 [95% CI, 2.93-7.49])(P<.001). Difficulties with activities of daily living, including running errands alone (OR, 2.32 [95% CI, 1.43-3.76]) and concentrating (OR, 5.78; 95% CI, 3.86-8.64), were more prevalent in the scabies group compared to controls (both P<.05). In a multivariate logistic regression model including unsheltered status as a covariate, all associations remained statistically significant (P<.05)(eTable 2).


This large diverse study demonstrated an association between scabies infestation and unsheltered status. Previous studies have shown that unsheltered populations are at increased risk for many dermatologic conditions, perhaps due to decreased access to health care and social support, lack of access to hygiene facilities (eg, public showers), and increased prevalence of substance use and psychiatric disorders among this population.5 In a cross-sectional analysis of hospitalized patients, 8.6% of unsheltered patients (n=197) had an ectoparasitic disease (including scabies) compared with 1.0% of patients with stable housing (n=1018), with a 9.43-fold increased risk for ectoparasitic infestation among unsheltered patients (95% CI, 3.79-23.47; P<.001).6 Increased attention to public health initiatives among unsheltered populations— including access to hygiene facilities and increased dermatologic services—are needed, as ectoparasitic infections are both preventable and treatable, and these initiatives could reduce morbidity associated with superimposed bacterial infections for which unsheltered patients are at increased risk.6
Our results also showed that individuals diagnosed with scabies were more likely than the controls to have been diagnosed with HIV, T2DM, and PVD. Our findings are similar to those of a systematic review of immunosuppressive factors associated with crusted scabies (a severe form of scabies infestation) in which 10.2% and 15.7% of patients (n=683) had comorbid HIV and T2DM, respectively.7 A functioning cell-mediated response to scabies mite antigens limits proliferation of the human itch mite; thus, infection with HIV/AIDS, which induces the destruction of CD4+ T cells, limits the immune system’s ability to mount an effective response against these antigens. The association of scabies with T2DM likely is multifactorial; for example, chronic hyperglycemia may lead to immune system impairment, and peripheral neuropathy may reduce the itch sensation, allowing scabies mites to proliferate without removal by scratching.7 In a descriptive epidemiologic study in Japan, 11.7% of patients with scabies (N=857) had comorbid PVD.8 Peripheral vascular disease can lead to the development of ulcers, gangrene, and stasis dermatitis, all of which compromise the skin barrier and increase susceptibility to infection.9 Notably, these associations remained even when unsheltered status was considered as a confounding variable. Because individuals with HIV, T2DM, and PVD may be at higher risk for serious complications of scabies infestation (eg, secondary bacterial infections, invasive group A streptococcal infections), prompt detection and treatment of scabies are crucial in curbing morbidity in these at-risk populations.
Our study also demonstrated that psychiatric comorbidities including depression, anxiety, bipolar disorder, and schizophrenia were associated with scabies infestation, even when controlling for unsheltered status, which may have a bidirectional relationship with mental health disorders.10 In a cross-sectional study of 83 adult patients diagnosed with scabies, 72.2% (60/83) reported moderate to extremely large effect of scabies infestation on quality of life using the Dermatology Life Quality Index, and these scores positively correlated with increased Beck Depression Scale and Beck Anxiety Scale scores (rs=0.448 and rs=0.456 0.456, respectively; both P=.000). The results of this study suggest that scabies negatively impacts quality of life, which might increase symptoms of depression and anxiety.11
Studies are needed to assess whether patients with pre-existing depression and anxiety face increased risk for scabies infestation. In a retrospective case-control study using data from the National Health Insurance Research Database of Taiwan, 0.8% (58/7096) of patients with scabies (n=7096) and 0.4% of controls (n=28,375) were newly diagnosed with bipolar disorder over a 7-year period, indicating a 1.55-fold increased risk for bipolar disorder in patients with scabies compared to those without (95% CI, 1.12-2.09; P<.05).12 Future studies are needed to determine whether the relationship between bipolar disorder and scabies is bidirectional, with pre-existing bipolar disorder evaluated as a risk factor for subsequent scabies infestation. Increased difficulties with activities of daily living, including running errands independently and concentrating, were associated with scabies. These difficulties may reflect sequelae of psychiatric illness or pruritus associated with scabies affecting daily living.
Physician awareness of comorbidities and lifestyle risk factors associated with scabies infestation may improve diagnosis and prevent treatment delays. In a retrospective study at a single dermatology outpatient clinic, 45.3% of patients with scabies (n=428) had previously been misdiagnosed with another dermatologic condition, and the most common erroneous diagnosis was atopic dermatitis.13 Our study provides a framework of comorbidities and lifestyle risk factors associated with scabies infestation that dermatologists can use to stratify patients who may be at greater risk for this condition, allowing dermatologists to select appropriate treatment when clinical signs are ambiguous.
Limitations of our study included the potential for miscoding in the database, lack of information about treatment regimens employed (if any), and lack of information about the temporal relationship between associations.
In summary, it is recommended that patients with pruritus and other characteristic clinical findings of scabies receive appropriate workup for scabies regardless of risk factors; however, the medical and psychiatric comorbidities and lifestyle risk factors identified in this study may help to identify at-risk patients. Our study showed that unsheltered patients are at increased risk for scabies, potentially due to unique dermatologic challenges and lack of access to health care and hygiene facilities. Positive correlations between scabies and HIV, T2DM, and PVD suggest that patients with chronic immunocompromising illnesses who live in group homes or other crowded quarters and present with symptoms could be evaluated for scabies infestation to prevent widespread and difficult- to-control outbreaks in these communities. Based on our findings, scabies also should be included in the differential diagnosis for patients with psychiatric illness and suggestive symptoms. Early identification and treatment of scabies infestation could prevent misdiagnosis and treatment delays.
To the Editor:
Scabies infestation, which has been recognized as a neglected tropical disease by the World Health Organization since 2017, is caused by the human itch mite (Sarcoptes scabiei var hominis).1 Infected individuals experience a pruritic papular rash when the mite burrows into the epidermis, where it lives and lays eggs.2,3 Infected individuals also may develop bacterial superinfections if the skin barrier becomes compromised, leading to systemic complications and considerable morbidity.3
In countries with high human development indices, scabies outbreaks are linked to densely populated living conditions, such as those found in nursing homes or prisons.3,4 Scabies also is transmitted via sexual contact in adults. Beyond immunosuppression, little is known about other comorbid conditions or lifestyle risk factors associated with scabies infestation.2 Because scabies can mimic a range of other dermatologic conditions such as folliculitis, atopic dermatitis, and arthropod bites, misdiagnosis is common and can lead to delayed treatment and increased transmission risk.4 In this study, we sought to examine comorbid conditions and/or lifestyle risk factors associated with scabies infestation.
A matched case-control study was performed using the Registered Tier dataset of the National Institutes of Health All of Us Research Program Curated Data Repository version 7, which includes more than 400,000 unique participants aged 18 years or older from across the United States. The All of Us Research Program excludes adults who are unable to consent independently as well as incarcerated populations and children younger than 18 years. Participants diagnosed with scabies were identified using SNOMED code 62752005 and compared to a control group matched 1:4 based on age, sex, and selfidentified race. SNOMED codes also were used to identify various comorbidities and lifestyle risk factors, including depression, bipolar disorder, anxiety, schizophrenia, peripheral vascular disease (PVD), HIV, type 2 diabetes mellitus (T2DM), unsheltered status, tobacco use, difficulty with activities of daily living, insurance status, and any recent travel history. Logistic regression models were used to calculate odds ratios (ORs) and estimate effect sizes, with statistical significance set at P<.05.
We identified 691 cases of scabies infestation and 2073 controls. The average age of the patients diagnosed with scabies was 55.1 years. Seventy percent (481/691) identified as female and 32.4% (224/491) identified as Black or African American. Matched controls were similar for all analyzed demographic characteristics (P=1.0)(eTable 1). Patients diagnosed with scabies were more likely to be unsheltered (OR, 2.33 [95% CI, 1.91-2.85]), use tobacco (OR 1.77 [95% CI, 1.48-2.11]) and have a comorbid diagnosis of HIV (OR, 3.08 [95% CI, 2.03-4.66]), T2DM (OR, 2.05 [95% CI, 1.57- 2.66]) or PVD (OR, 2.06 [95% CI, 1.43-2.97]) compared with controls (P<.001). Psychiatric comorbidities were more common in the patients diagnosed with scabies, including depression (OR, 3.07 [95% CI, 2.54-3.72]), anxiety (OR, 2.48 [95% CI, 2.06-2.98]), bipolar disorder (OR, 3.08 [95% CI, 2.34-4.05]), and schizophrenia (OR, 4.68 [95% CI, 2.93-7.49])(P<.001). Difficulties with activities of daily living, including running errands alone (OR, 2.32 [95% CI, 1.43-3.76]) and concentrating (OR, 5.78; 95% CI, 3.86-8.64), were more prevalent in the scabies group compared to controls (both P<.05). In a multivariate logistic regression model including unsheltered status as a covariate, all associations remained statistically significant (P<.05)(eTable 2).


This large diverse study demonstrated an association between scabies infestation and unsheltered status. Previous studies have shown that unsheltered populations are at increased risk for many dermatologic conditions, perhaps due to decreased access to health care and social support, lack of access to hygiene facilities (eg, public showers), and increased prevalence of substance use and psychiatric disorders among this population.5 In a cross-sectional analysis of hospitalized patients, 8.6% of unsheltered patients (n=197) had an ectoparasitic disease (including scabies) compared with 1.0% of patients with stable housing (n=1018), with a 9.43-fold increased risk for ectoparasitic infestation among unsheltered patients (95% CI, 3.79-23.47; P<.001).6 Increased attention to public health initiatives among unsheltered populations— including access to hygiene facilities and increased dermatologic services—are needed, as ectoparasitic infections are both preventable and treatable, and these initiatives could reduce morbidity associated with superimposed bacterial infections for which unsheltered patients are at increased risk.6
Our results also showed that individuals diagnosed with scabies were more likely than the controls to have been diagnosed with HIV, T2DM, and PVD. Our findings are similar to those of a systematic review of immunosuppressive factors associated with crusted scabies (a severe form of scabies infestation) in which 10.2% and 15.7% of patients (n=683) had comorbid HIV and T2DM, respectively.7 A functioning cell-mediated response to scabies mite antigens limits proliferation of the human itch mite; thus, infection with HIV/AIDS, which induces the destruction of CD4+ T cells, limits the immune system’s ability to mount an effective response against these antigens. The association of scabies with T2DM likely is multifactorial; for example, chronic hyperglycemia may lead to immune system impairment, and peripheral neuropathy may reduce the itch sensation, allowing scabies mites to proliferate without removal by scratching.7 In a descriptive epidemiologic study in Japan, 11.7% of patients with scabies (N=857) had comorbid PVD.8 Peripheral vascular disease can lead to the development of ulcers, gangrene, and stasis dermatitis, all of which compromise the skin barrier and increase susceptibility to infection.9 Notably, these associations remained even when unsheltered status was considered as a confounding variable. Because individuals with HIV, T2DM, and PVD may be at higher risk for serious complications of scabies infestation (eg, secondary bacterial infections, invasive group A streptococcal infections), prompt detection and treatment of scabies are crucial in curbing morbidity in these at-risk populations.
Our study also demonstrated that psychiatric comorbidities including depression, anxiety, bipolar disorder, and schizophrenia were associated with scabies infestation, even when controlling for unsheltered status, which may have a bidirectional relationship with mental health disorders.10 In a cross-sectional study of 83 adult patients diagnosed with scabies, 72.2% (60/83) reported moderate to extremely large effect of scabies infestation on quality of life using the Dermatology Life Quality Index, and these scores positively correlated with increased Beck Depression Scale and Beck Anxiety Scale scores (rs=0.448 and rs=0.456 0.456, respectively; both P=.000). The results of this study suggest that scabies negatively impacts quality of life, which might increase symptoms of depression and anxiety.11
Studies are needed to assess whether patients with pre-existing depression and anxiety face increased risk for scabies infestation. In a retrospective case-control study using data from the National Health Insurance Research Database of Taiwan, 0.8% (58/7096) of patients with scabies (n=7096) and 0.4% of controls (n=28,375) were newly diagnosed with bipolar disorder over a 7-year period, indicating a 1.55-fold increased risk for bipolar disorder in patients with scabies compared to those without (95% CI, 1.12-2.09; P<.05).12 Future studies are needed to determine whether the relationship between bipolar disorder and scabies is bidirectional, with pre-existing bipolar disorder evaluated as a risk factor for subsequent scabies infestation. Increased difficulties with activities of daily living, including running errands independently and concentrating, were associated with scabies. These difficulties may reflect sequelae of psychiatric illness or pruritus associated with scabies affecting daily living.
Physician awareness of comorbidities and lifestyle risk factors associated with scabies infestation may improve diagnosis and prevent treatment delays. In a retrospective study at a single dermatology outpatient clinic, 45.3% of patients with scabies (n=428) had previously been misdiagnosed with another dermatologic condition, and the most common erroneous diagnosis was atopic dermatitis.13 Our study provides a framework of comorbidities and lifestyle risk factors associated with scabies infestation that dermatologists can use to stratify patients who may be at greater risk for this condition, allowing dermatologists to select appropriate treatment when clinical signs are ambiguous.
Limitations of our study included the potential for miscoding in the database, lack of information about treatment regimens employed (if any), and lack of information about the temporal relationship between associations.
In summary, it is recommended that patients with pruritus and other characteristic clinical findings of scabies receive appropriate workup for scabies regardless of risk factors; however, the medical and psychiatric comorbidities and lifestyle risk factors identified in this study may help to identify at-risk patients. Our study showed that unsheltered patients are at increased risk for scabies, potentially due to unique dermatologic challenges and lack of access to health care and hygiene facilities. Positive correlations between scabies and HIV, T2DM, and PVD suggest that patients with chronic immunocompromising illnesses who live in group homes or other crowded quarters and present with symptoms could be evaluated for scabies infestation to prevent widespread and difficult- to-control outbreaks in these communities. Based on our findings, scabies also should be included in the differential diagnosis for patients with psychiatric illness and suggestive symptoms. Early identification and treatment of scabies infestation could prevent misdiagnosis and treatment delays.
- World Health Organization. Scabies fact sheet. May 31, 2023. Accessed February 13, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Schneider S, Wu J, Tizek L, et al. Prevalence of scabies worldwidean updated systematic literature review in 2022. J Eur Acad Dermatol Venereol. 2023;37:1749-1757. doi:10.1111/jdv.19167
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Henry T, Khachemoune A. Dermatologic conditions and risk factors in people experiencing homelessness (PEH): systematic review. Arch Dermatol Res. 2023;315:2795-2803. doi:10.1007/s00403-023-02722-2
- Zakaria A, Amerson EH, Kim-Lim P, et al. Characterization of dermatological diagnoses among hospitalized patients experiencing homelessness. Clin Exp Dermatol. 2022;47:117-120. doi:10.1111/ced.14828
- Bergamin G, Hudson J, Currie BJ, et al. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int J Infect Dis. 2024;143:107036. doi:10.1016/j.ijid.2024.107036
- Yamaguchi Y, Murata F, Maeda M, et al. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. 2024;11:100353. doi:10.1016 /j.ijregi.2024.03.008
- Raja A, Karch J, Shih AF, et al. Part II: Cutaneous manifestations of peripheral vascular disease. J Am Acad Dermatol. 2023;89:211-226. doi:10.1016/j.jaad.2021.05.077
- Barry R, Anderson J, Tran L, et al. Prevalence of mental health disorders among individuals experiencing homelessness: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:691-699. doi:10.1001 /jamapsychiatry.2024.0426
- Koc Y.ld.r.m S, Demirel Og. ut N, Erbag. c. E, et al. Scabies affects quality of life in correlation with depression and anxiety. Dermatol Pract Concept. 2023;13:E2023144. doi:10.5826/dpc.1302a144
- Lin CY, Chang FW, Yang JJ, et al. Increased risk of bipolar disorder in patients with scabies: a nationwide population-based matched-cohort study. Psychiatry Res. 2017;257:14-20. doi:10.1016 /j.psychres.2017.07.013
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017;30:78-84. doi:10.3122/jabfm.2017.01.160190
- World Health Organization. Scabies fact sheet. May 31, 2023. Accessed February 13, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Schneider S, Wu J, Tizek L, et al. Prevalence of scabies worldwidean updated systematic literature review in 2022. J Eur Acad Dermatol Venereol. 2023;37:1749-1757. doi:10.1111/jdv.19167
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Henry T, Khachemoune A. Dermatologic conditions and risk factors in people experiencing homelessness (PEH): systematic review. Arch Dermatol Res. 2023;315:2795-2803. doi:10.1007/s00403-023-02722-2
- Zakaria A, Amerson EH, Kim-Lim P, et al. Characterization of dermatological diagnoses among hospitalized patients experiencing homelessness. Clin Exp Dermatol. 2022;47:117-120. doi:10.1111/ced.14828
- Bergamin G, Hudson J, Currie BJ, et al. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int J Infect Dis. 2024;143:107036. doi:10.1016/j.ijid.2024.107036
- Yamaguchi Y, Murata F, Maeda M, et al. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. 2024;11:100353. doi:10.1016 /j.ijregi.2024.03.008
- Raja A, Karch J, Shih AF, et al. Part II: Cutaneous manifestations of peripheral vascular disease. J Am Acad Dermatol. 2023;89:211-226. doi:10.1016/j.jaad.2021.05.077
- Barry R, Anderson J, Tran L, et al. Prevalence of mental health disorders among individuals experiencing homelessness: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:691-699. doi:10.1001 /jamapsychiatry.2024.0426
- Koc Y.ld.r.m S, Demirel Og. ut N, Erbag. c. E, et al. Scabies affects quality of life in correlation with depression and anxiety. Dermatol Pract Concept. 2023;13:E2023144. doi:10.5826/dpc.1302a144
- Lin CY, Chang FW, Yang JJ, et al. Increased risk of bipolar disorder in patients with scabies: a nationwide population-based matched-cohort study. Psychiatry Res. 2017;257:14-20. doi:10.1016 /j.psychres.2017.07.013
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017;30:78-84. doi:10.3122/jabfm.2017.01.160190
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
PRACTICE POINTS
- Scabies infestation is caused by the human itch mite (Sarcoptes scabiei var hominis) and can be spread via sexual contact in adults.
- Crowded living conditions are associated with scabies infestation in countries with high human development indices, such as the United States.
- Patients with certain comorbid conditions or lifestyle risk factors should be screened for scabies infestation when presenting with pruritus and other characteristic clinical findings.
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11
Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5
Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13
Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4
Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
A 57-year-old man with no notable medical history presented to the dermatology clinic for evaluation of an asymptomatic papule on the left lower eyelid. The patient reported that the lesion seemed to wax and wane in size over time. Physical examination revealed a small, pink, verrucous papule on the left lower eyelid. A shave biopsy of the lesion revealed a well-circumscribed collection of small, monomorphic, cuboidal cells with basophilic round nuclei, inconspicuous nucleoli, and compact eosinophilic cytoplasm (top) with focal areas of duct formation (bottom) that was sharply demarcated from normal keratinocytes.
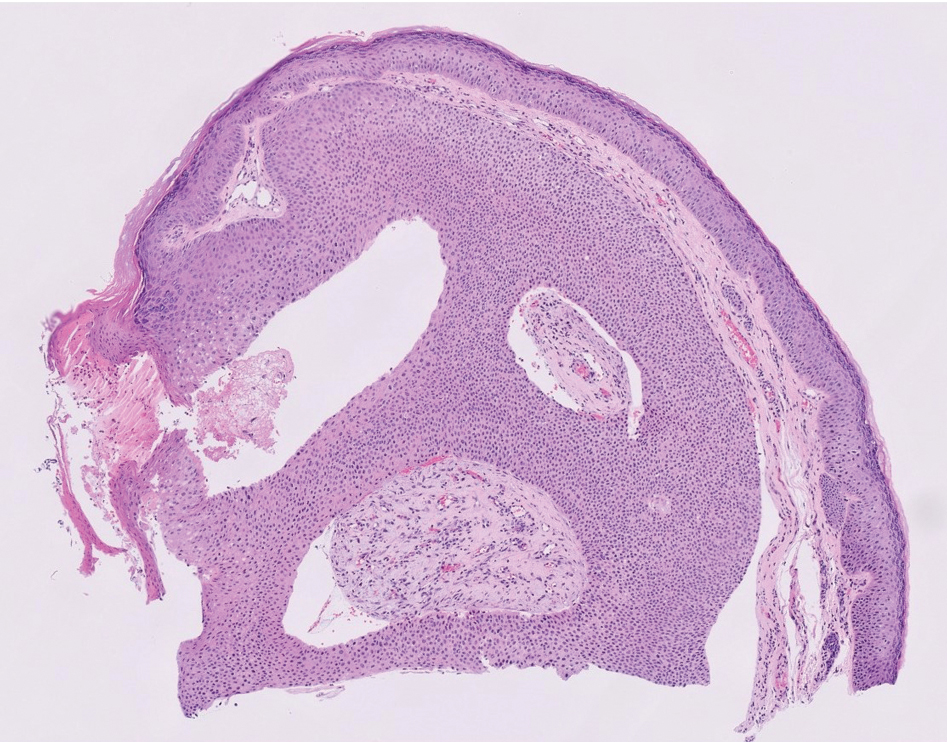
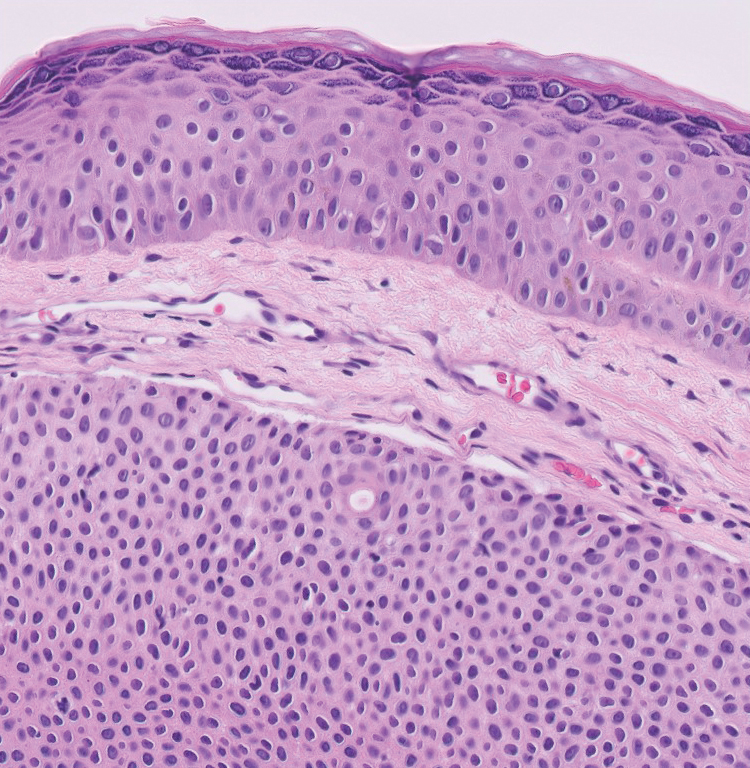
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
THE DIAGNOSIS: Granuloma Faciale
Histology revealed a dense mixed inflammatory cell infiltrate with conspicuous neutrophils and eosinophils in the upper to mid dermis with a narrow uninvolved grenz zone beneath the epidermis (Figures 1 and 2). These findings along with the clinical presentation (Figure 3) were consistent with a diagnosis of granuloma faciale (GF). Most often seen in middle-aged White men, GF is an uncommon localized inflammatory skin condition that often manifests as a single, well-defined, red-to-brown papule, nodule, or plaque on the face or other sun-exposed areas of the skin. Since numerous other skin diseases manifest similarly to GF, biopsy is necessary for definitive diagnosis.1 Histopathology of GF classically shows a mixed inflammatory infiltrate with a narrow band of uninvolved dermis separating it from the epidermis (grenz zone). Dilated follicular plugs and vascular changes frequently are appreciated. Despite its name, GF does not include granulomas and is thought to be similar to leukocytoclastic vasculitis.1 Reports of GF in the literature have shown immunohistochemical staining with the presence of CD4+ lymphocytes that secrete IL-5, a chemotactic agent responsible for attracting eosinophils that contributes to the eosinophilic infiltrate on histology.2
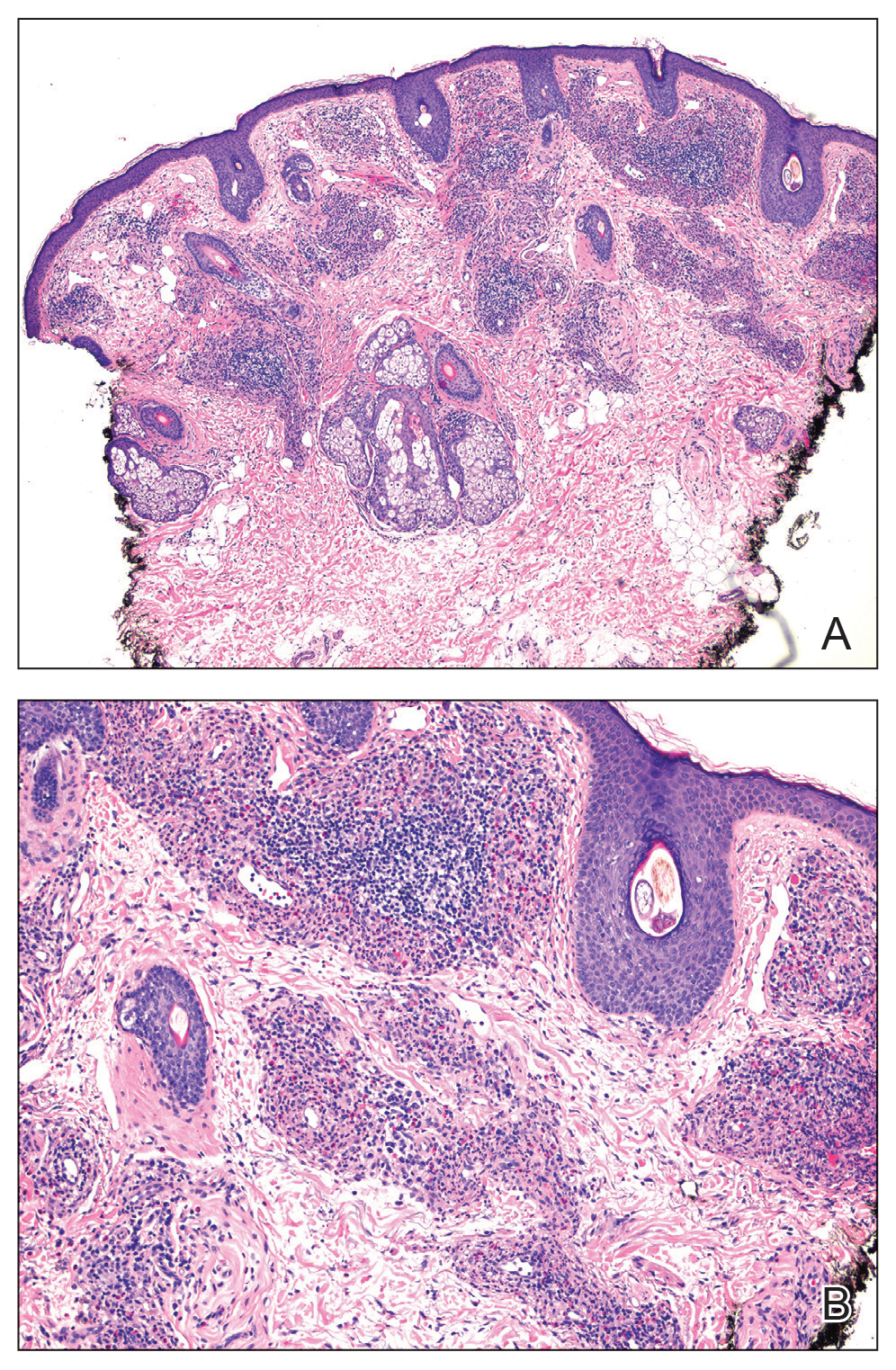
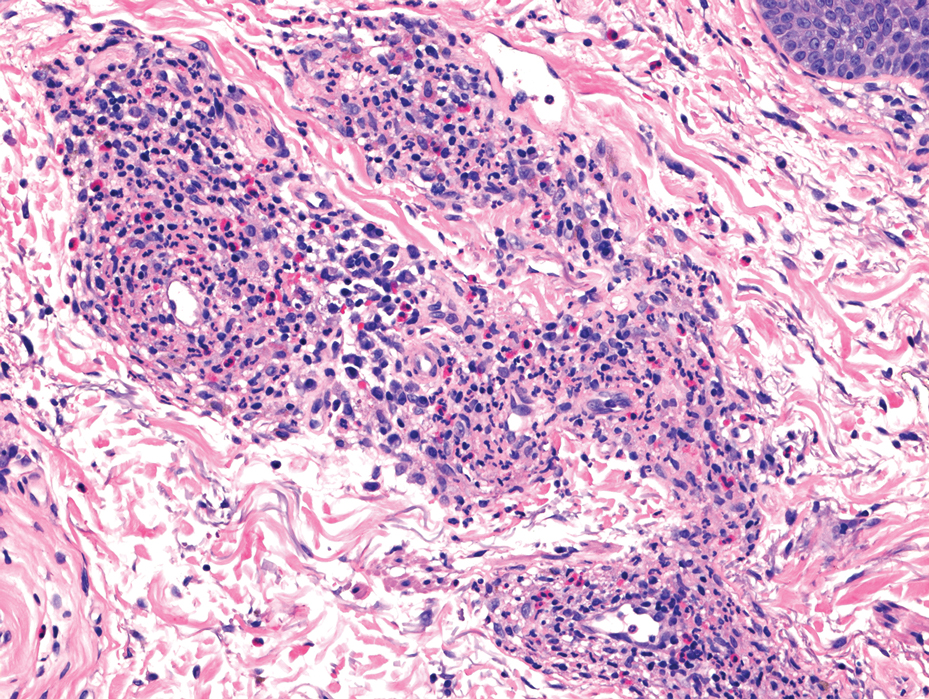
Topical corticosteroids and topical tacrolimus are the first-line treatments for GF. Intralesional corticosteroids also are a treatment option and can be used in combination with cryotherapy.1,3 Additionally, both topical and oral dapsone have been shown to be effective for GF.1 Oral dapsone is given at a dose of 50 mg to 150 mg once daily.1 Clofazimine, typically used as an antileprosy treatment, also has been efficacious in treating GF. Clofazimine has anti-inflammatory and antiproliferative effects on lymphocytes that may attenuate the inflammation underlying GF. It is prescribed at a dose of 300 mg once daily for 3 to 5 months.1
The differential diagnosis for GF is broad and includes tumid lupus erythematosus, Jessner lymphocytic infiltrate (JLI), cutaneous sarcoidosis, and mycosis fungoides. Tumid lupus erythematosus is a subtype of cutaneous lupus erythematosus that rarely is associated with systemic lupus manifestations. Tumid lupus erythematosus manifests as annular, indurated, erythematous plaques, whereas JLI manifests with erythematous papular to nodular lesions without scale on the upper back or face.4 Jessner lymphocytic infiltrate and tumid lupus erythematosus are histopathologically identical, with abundant dermal mucin deposition and a superficial and deep perivascular and periadnexal lymphocytic infiltrate. It is debatable whether JLI is a separate entity or a variant of tumid lupus erythematosus. Sarcoidosis is a granulomatous disease that manifests with a myriad of clinical features. The skin is the second most commonly involved organ.5 The most common morphology is numerous small, firm, nonscaly papules, typically on the face. Histology in cutaneous sarcoidosis will show lymphocyte-poor, noncaseating epithelioid cell granulomas with positive reticulin staining, which were not seen in our patient.6 Lastly, mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It can manifest as patches, plaques, or tumors. The plaque stage may mimic GF as lesions are infiltrative, annular, and raised, with well-defined margins. Histopathology will show intraepidermal lymphocytes out of proportion with spongiosis.7
- Al Dhafiri M, Kaliyadan F. Granuloma faciale. StatPearls Publishing. Updated July 4, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539832/
- Chen A, Harview CL, Rand SE, et al. Refractory granuloma faciale successfully treated with adjunct topical JAK inhibitor. JAAD Case Rep. 2023;33:91-94. doi:10.1016/j.jdcr.2023.01.016
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551. doi:10.1046 /j.1365-4362.1997.00161.x
- Koritala T, Grubbs H, Crane J. Tumid lupus erythematosus. StatPearls Publishing. Updated June 28, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482515/
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689-699. doi:10.1055/s-0040-1713130
- Singh P, Jain E, Dhingra H, et al. Clinico-pathological spectrum of cutaneous sarcoidosis: an experience from a government institute in North India. Med Pharm Rep. 2020;93:241-245. doi:10.15386 /mpr-1384
- Vaidya T, Badri T. Mycosis fungoides. StatPearls Publishing. Updated July 31, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519572/
THE DIAGNOSIS: Granuloma Faciale
Histology revealed a dense mixed inflammatory cell infiltrate with conspicuous neutrophils and eosinophils in the upper to mid dermis with a narrow uninvolved grenz zone beneath the epidermis (Figures 1 and 2). These findings along with the clinical presentation (Figure 3) were consistent with a diagnosis of granuloma faciale (GF). Most often seen in middle-aged White men, GF is an uncommon localized inflammatory skin condition that often manifests as a single, well-defined, red-to-brown papule, nodule, or plaque on the face or other sun-exposed areas of the skin. Since numerous other skin diseases manifest similarly to GF, biopsy is necessary for definitive diagnosis.1 Histopathology of GF classically shows a mixed inflammatory infiltrate with a narrow band of uninvolved dermis separating it from the epidermis (grenz zone). Dilated follicular plugs and vascular changes frequently are appreciated. Despite its name, GF does not include granulomas and is thought to be similar to leukocytoclastic vasculitis.1 Reports of GF in the literature have shown immunohistochemical staining with the presence of CD4+ lymphocytes that secrete IL-5, a chemotactic agent responsible for attracting eosinophils that contributes to the eosinophilic infiltrate on histology.2



Topical corticosteroids and topical tacrolimus are the first-line treatments for GF. Intralesional corticosteroids also are a treatment option and can be used in combination with cryotherapy.1,3 Additionally, both topical and oral dapsone have been shown to be effective for GF.1 Oral dapsone is given at a dose of 50 mg to 150 mg once daily.1 Clofazimine, typically used as an antileprosy treatment, also has been efficacious in treating GF. Clofazimine has anti-inflammatory and antiproliferative effects on lymphocytes that may attenuate the inflammation underlying GF. It is prescribed at a dose of 300 mg once daily for 3 to 5 months.1
The differential diagnosis for GF is broad and includes tumid lupus erythematosus, Jessner lymphocytic infiltrate (JLI), cutaneous sarcoidosis, and mycosis fungoides. Tumid lupus erythematosus is a subtype of cutaneous lupus erythematosus that rarely is associated with systemic lupus manifestations. Tumid lupus erythematosus manifests as annular, indurated, erythematous plaques, whereas JLI manifests with erythematous papular to nodular lesions without scale on the upper back or face.4 Jessner lymphocytic infiltrate and tumid lupus erythematosus are histopathologically identical, with abundant dermal mucin deposition and a superficial and deep perivascular and periadnexal lymphocytic infiltrate. It is debatable whether JLI is a separate entity or a variant of tumid lupus erythematosus. Sarcoidosis is a granulomatous disease that manifests with a myriad of clinical features. The skin is the second most commonly involved organ.5 The most common morphology is numerous small, firm, nonscaly papules, typically on the face. Histology in cutaneous sarcoidosis will show lymphocyte-poor, noncaseating epithelioid cell granulomas with positive reticulin staining, which were not seen in our patient.6 Lastly, mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It can manifest as patches, plaques, or tumors. The plaque stage may mimic GF as lesions are infiltrative, annular, and raised, with well-defined margins. Histopathology will show intraepidermal lymphocytes out of proportion with spongiosis.7
THE DIAGNOSIS: Granuloma Faciale
Histology revealed a dense mixed inflammatory cell infiltrate with conspicuous neutrophils and eosinophils in the upper to mid dermis with a narrow uninvolved grenz zone beneath the epidermis (Figures 1 and 2). These findings along with the clinical presentation (Figure 3) were consistent with a diagnosis of granuloma faciale (GF). Most often seen in middle-aged White men, GF is an uncommon localized inflammatory skin condition that often manifests as a single, well-defined, red-to-brown papule, nodule, or plaque on the face or other sun-exposed areas of the skin. Since numerous other skin diseases manifest similarly to GF, biopsy is necessary for definitive diagnosis.1 Histopathology of GF classically shows a mixed inflammatory infiltrate with a narrow band of uninvolved dermis separating it from the epidermis (grenz zone). Dilated follicular plugs and vascular changes frequently are appreciated. Despite its name, GF does not include granulomas and is thought to be similar to leukocytoclastic vasculitis.1 Reports of GF in the literature have shown immunohistochemical staining with the presence of CD4+ lymphocytes that secrete IL-5, a chemotactic agent responsible for attracting eosinophils that contributes to the eosinophilic infiltrate on histology.2



Topical corticosteroids and topical tacrolimus are the first-line treatments for GF. Intralesional corticosteroids also are a treatment option and can be used in combination with cryotherapy.1,3 Additionally, both topical and oral dapsone have been shown to be effective for GF.1 Oral dapsone is given at a dose of 50 mg to 150 mg once daily.1 Clofazimine, typically used as an antileprosy treatment, also has been efficacious in treating GF. Clofazimine has anti-inflammatory and antiproliferative effects on lymphocytes that may attenuate the inflammation underlying GF. It is prescribed at a dose of 300 mg once daily for 3 to 5 months.1
The differential diagnosis for GF is broad and includes tumid lupus erythematosus, Jessner lymphocytic infiltrate (JLI), cutaneous sarcoidosis, and mycosis fungoides. Tumid lupus erythematosus is a subtype of cutaneous lupus erythematosus that rarely is associated with systemic lupus manifestations. Tumid lupus erythematosus manifests as annular, indurated, erythematous plaques, whereas JLI manifests with erythematous papular to nodular lesions without scale on the upper back or face.4 Jessner lymphocytic infiltrate and tumid lupus erythematosus are histopathologically identical, with abundant dermal mucin deposition and a superficial and deep perivascular and periadnexal lymphocytic infiltrate. It is debatable whether JLI is a separate entity or a variant of tumid lupus erythematosus. Sarcoidosis is a granulomatous disease that manifests with a myriad of clinical features. The skin is the second most commonly involved organ.5 The most common morphology is numerous small, firm, nonscaly papules, typically on the face. Histology in cutaneous sarcoidosis will show lymphocyte-poor, noncaseating epithelioid cell granulomas with positive reticulin staining, which were not seen in our patient.6 Lastly, mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It can manifest as patches, plaques, or tumors. The plaque stage may mimic GF as lesions are infiltrative, annular, and raised, with well-defined margins. Histopathology will show intraepidermal lymphocytes out of proportion with spongiosis.7
- Al Dhafiri M, Kaliyadan F. Granuloma faciale. StatPearls Publishing. Updated July 4, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539832/
- Chen A, Harview CL, Rand SE, et al. Refractory granuloma faciale successfully treated with adjunct topical JAK inhibitor. JAAD Case Rep. 2023;33:91-94. doi:10.1016/j.jdcr.2023.01.016
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551. doi:10.1046 /j.1365-4362.1997.00161.x
- Koritala T, Grubbs H, Crane J. Tumid lupus erythematosus. StatPearls Publishing. Updated June 28, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482515/
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689-699. doi:10.1055/s-0040-1713130
- Singh P, Jain E, Dhingra H, et al. Clinico-pathological spectrum of cutaneous sarcoidosis: an experience from a government institute in North India. Med Pharm Rep. 2020;93:241-245. doi:10.15386 /mpr-1384
- Vaidya T, Badri T. Mycosis fungoides. StatPearls Publishing. Updated July 31, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519572/
- Al Dhafiri M, Kaliyadan F. Granuloma faciale. StatPearls Publishing. Updated July 4, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539832/
- Chen A, Harview CL, Rand SE, et al. Refractory granuloma faciale successfully treated with adjunct topical JAK inhibitor. JAAD Case Rep. 2023;33:91-94. doi:10.1016/j.jdcr.2023.01.016
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551. doi:10.1046 /j.1365-4362.1997.00161.x
- Koritala T, Grubbs H, Crane J. Tumid lupus erythematosus. StatPearls Publishing. Updated June 28, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482515/
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689-699. doi:10.1055/s-0040-1713130
- Singh P, Jain E, Dhingra H, et al. Clinico-pathological spectrum of cutaneous sarcoidosis: an experience from a government institute in North India. Med Pharm Rep. 2020;93:241-245. doi:10.15386 /mpr-1384
- Vaidya T, Badri T. Mycosis fungoides. StatPearls Publishing. Updated July 31, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519572/
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
A 44-year-old man presented to the dermatology clinic with a facial rash of 2 years’ duration. The patient reported associated pruritus but no systemic symptoms. His medical history was relevant for childhood eczema. He had tried various over-the-counter treatments for the facial rash, including topical hydrocortisone, neomycin/bacitracin/polymyxin antibiotic ointment, moisturizers, and antihistamines, with no success. Physical examination demonstrated symmetric, well-circumscribed, circinate, brownish-red, indurated plaques without scaling on the cheeks. A 4-mm punch biopsy was obtained from a plaque on the left cheek.
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8
Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8

Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8

Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
A 14-year-old boy with a history of toe walking, attention-deficit/hyperactivity disorder, and mixed receptive expressive language disorder presented to our pediatric dermatology clinic with fingernail abnormalities that had been present since birth. Physical examination revealed narrowing and longitudinal splitting of the nail plates with triangular lacunae and progressive improvement appreciated toward the fifth digits. The nail changes were most prominent on the first digits. A review of the patient’s medical record revealed incidental bilateral iliac horns of the pelvis on radiographs taken at age 18 months. The patient reported waxing and waning knee pain that worsened with prolonged activity and when climbing stairs. Urinalysis demonstrated mild hematuria without proteinuria. The patient was normotensive. There was no evidence of glaucoma, cataracts, or hyperpigmentation of the pupillary margin (Lester iris) on ophthalmologic examination. Genetic testing was performed.
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.
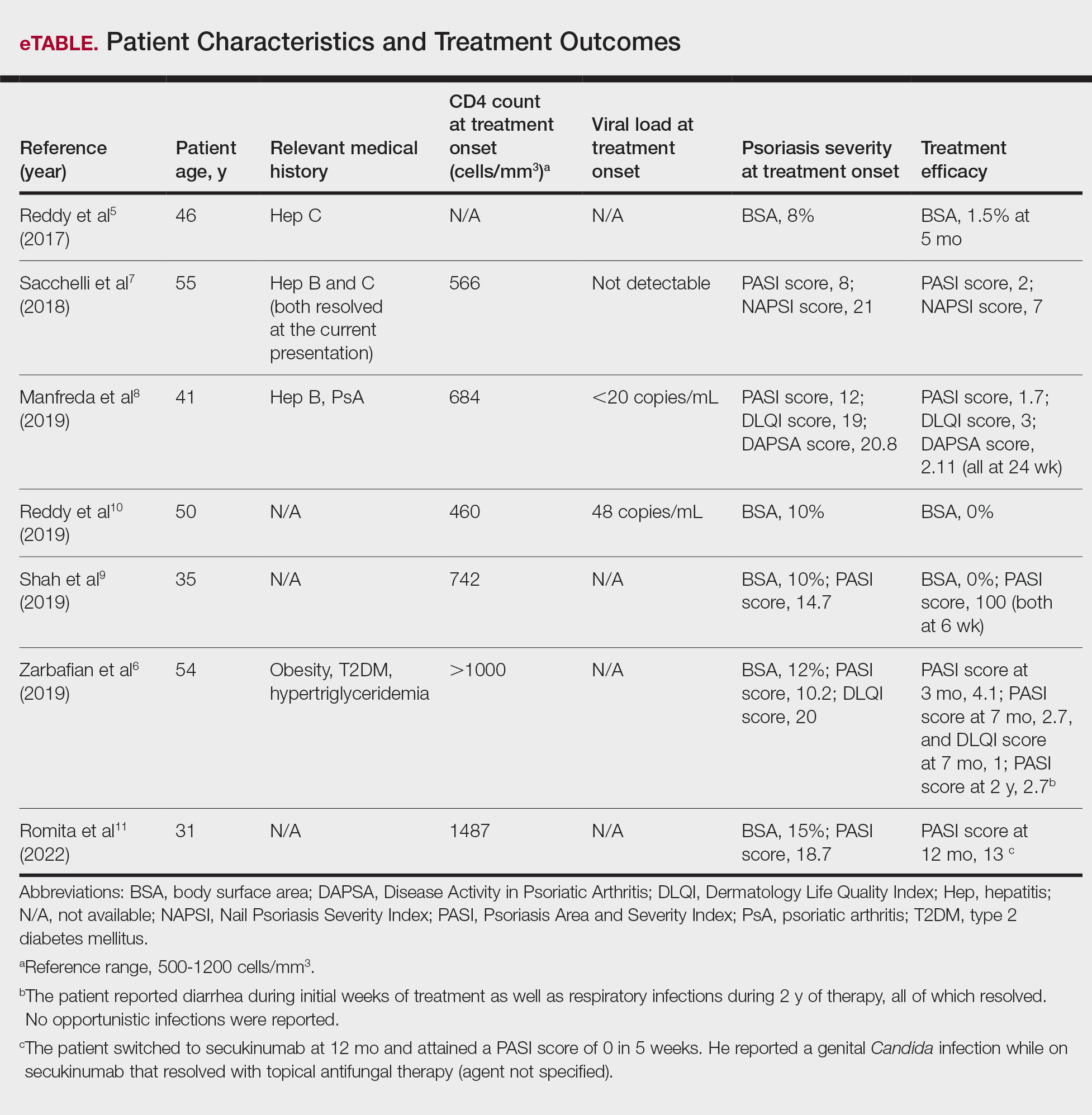
The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.

The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.

The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
PRACTICE POINT
- For patients with HIV who require systemic therapy for psoriasis, apremilast may provide an effective and safe therapeutic option, with minimal immunosuppressive adverse effects.
Oral Biologics: The New Wave for Treating Psoriasis
Oral Biologics: The New Wave for Treating Psoriasis
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.
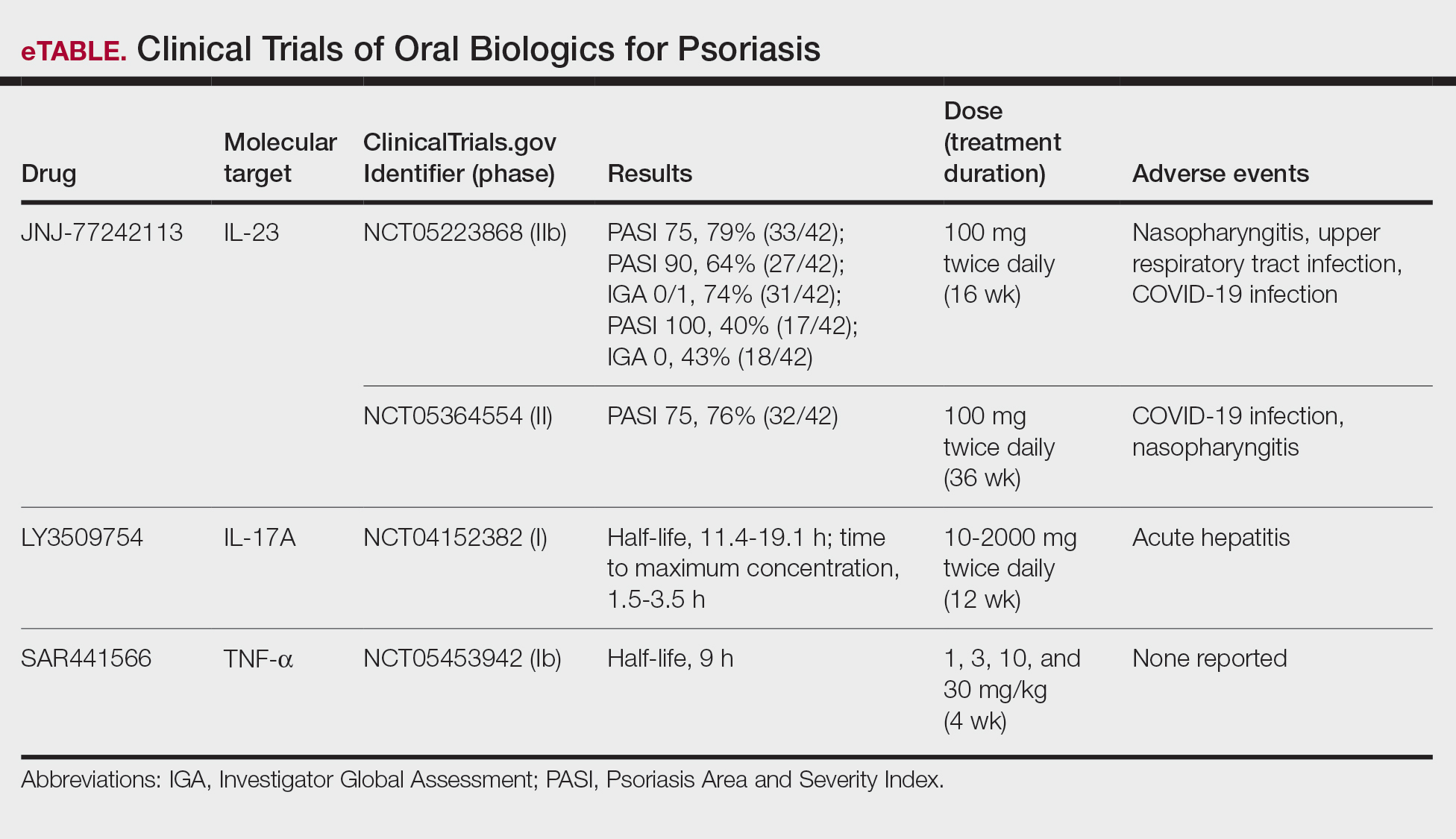
A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Oral Biologics: The New Wave for Treating Psoriasis
Oral Biologics: The New Wave for Treating Psoriasis
PRACTICE POINTS
- The biologics that currently are approved for psoriasis are expensive and must be administered via injection due to their large molecule size.
- Emerging small-molecule oral therapies for psoriasis are effective and affordable and may represent the future for psoriasis patients.
Legislative, Practice Management, and Coding Updates for 2025
Legislative, Practice Management, and Coding Updates for 2025
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11
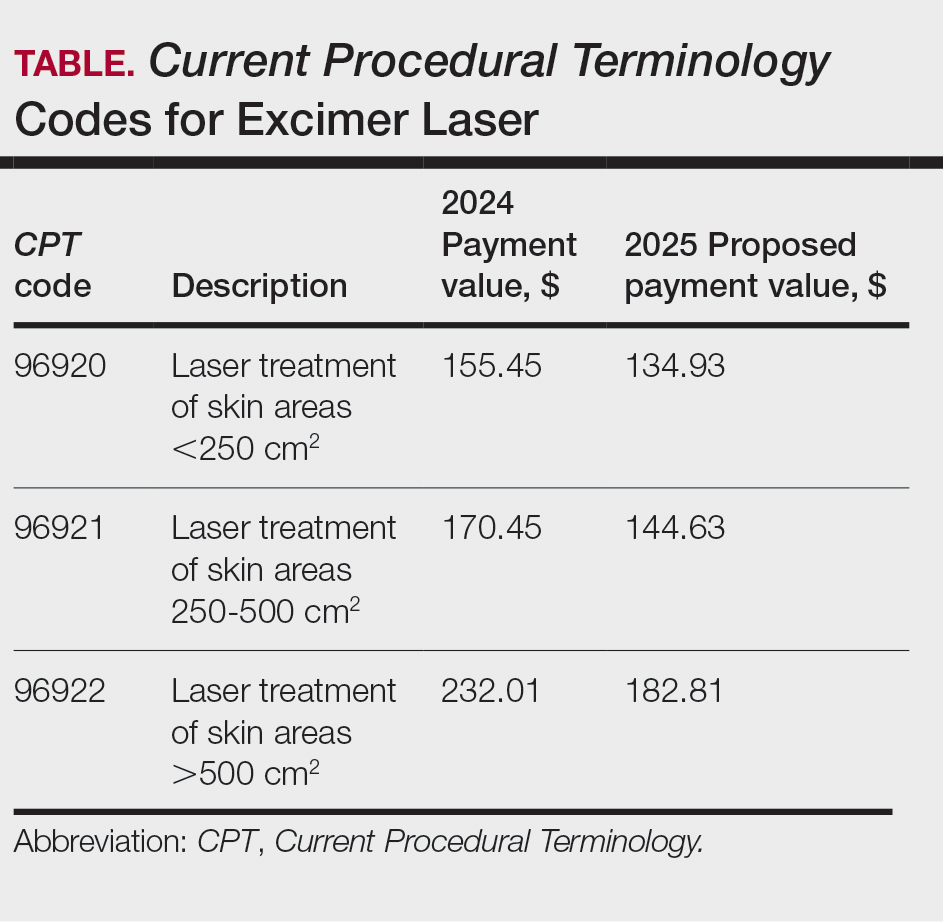
Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13
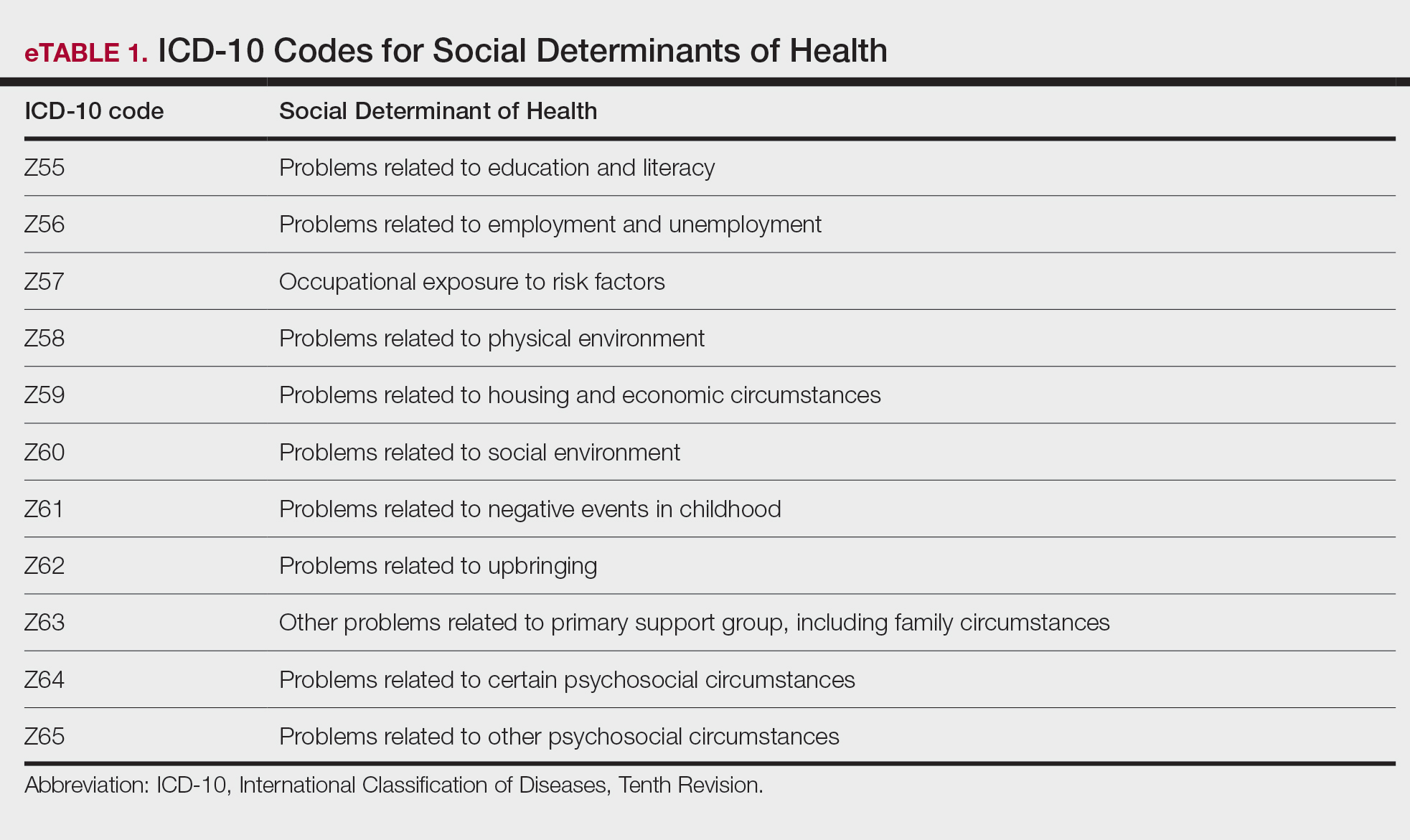
New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14
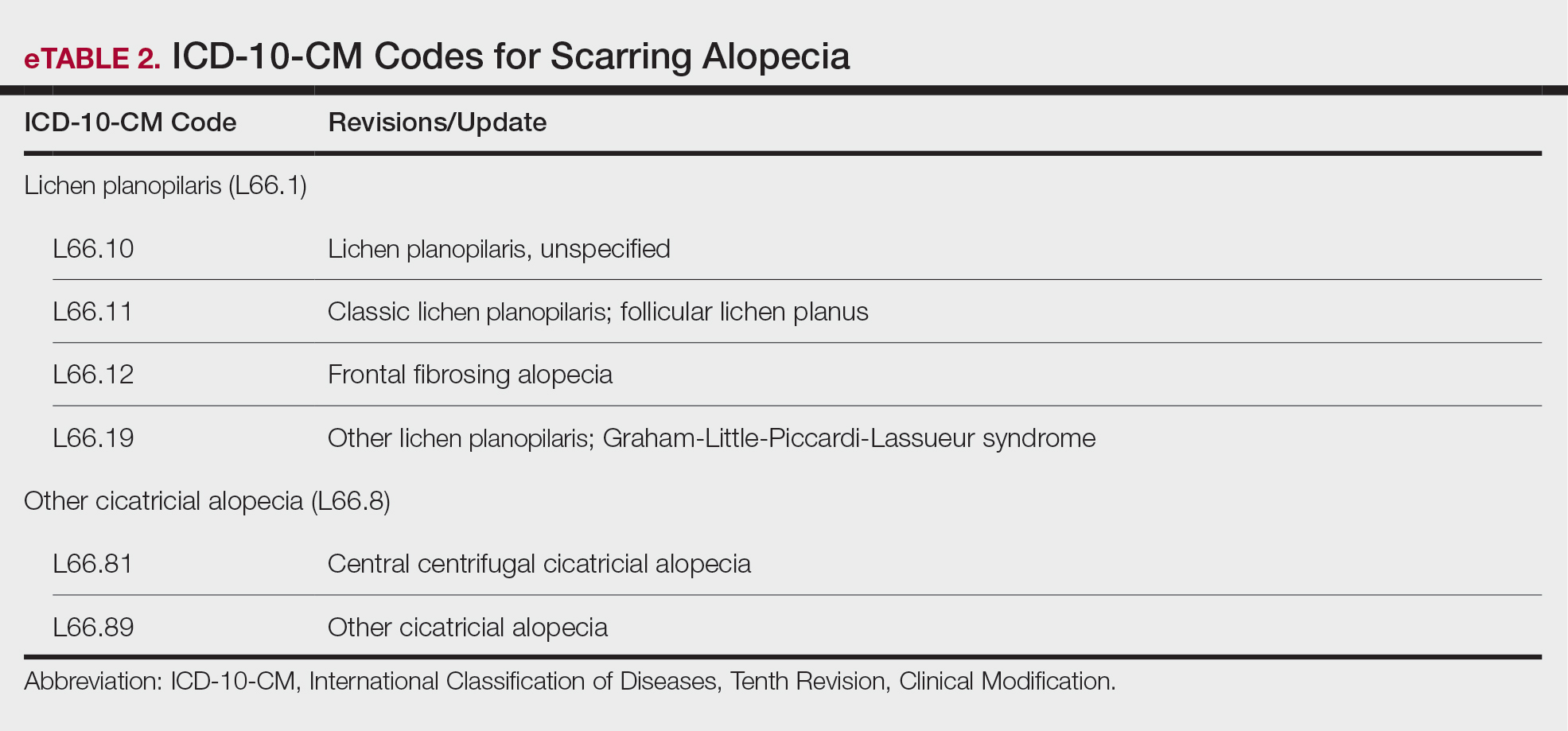
Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11

Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13

New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14

Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11

Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13

New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14

Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
Legislative, Practice Management, and Coding Updates for 2025
Legislative, Practice Management, and Coding Updates for 2025
PRACTICE POINTS
- The Centers for Medicare & Medicaid Services released the 2025 Medicare Physician Fee Schedule final rule on November 1, 2024, setting the 2025 conversion factor at $32.35—a 2.83% reduction from 2024.
- With this change, dermatology practices may see an overall 2.83% reduction in payments in 2025 compared to 2024, although individual outcomes will vary based on practice mix.
- The American Academy of Dermatology Association continues to advocate for change, and members need to urge their federal legislators to support critical bills aimed at reforming Medicare physician payment.
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.
The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6
Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9
Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
A 46-year-old woman with a history of systemic lupus erythematosus and end-stage renal disease presented to the dermatology department with painful ulcers on the extensor surfaces of the elbows, knees, and ankles of 2 months’ duration. Physical examination revealed angulated ulcers with surrounding pink erythema. A 4-mm punch biopsy and CD31 immunostaining of the left knee revealed dystrophic elastic fibers and purplish calciumlike depositions on connective tissue fibers in the mid to deep dermis.