User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Skin Cancer in the US Military
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
Practice Points
- An increased risk for melanoma and keratinocyte carcinomas has been identified in those who have served in the US Military.
- UV radiation exposure, low utilization of sun-protective strategies, and low overall education regarding the risks of UV exposure appear to be the primary contributors to increased risks of skin cancer in this population.
- Improving education for military servicemembers on the risks of UV exposure, increasing utilization of sun-protective clothing, and improving access and utilization of sunscreen are viable options to decrease the risk for cutaneous malignancies in US Military servicemembers.
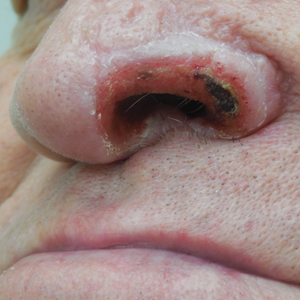
Unilateral Alar Ulceration
The Diagnosis: Trigeminal Trophic Syndrome (Self-induced Trauma)
The patient admitted to manipulation of the ala in response to persistent pain despite resolution of the herpes zoster, for which he recently had completed a course of oral acyclovir. A preliminary diagnosis of trigeminal trophic syndrome (TTS) was made, and a subsequent punch biopsy revealed no evidence of malignancy. Topical antibiotic prophylaxis was prescribed, and he was instructed to avoid manipulation of the affected area. Treatment was initiated in consultation with pain specialists, and over the following 3 years our patient experienced a waxing and waning course of persistent pain complicated by new scalp and oral ulcers as well as alar impetigo. His condition eventually stabilized with tolerable pain on oral gabapentin and doxepin cream 5% applied up to 4 times daily. The alar lesion healed following sufficient abstinence from manipulation, leaving a crescent-shaped rim defect.
Trigeminal trophic syndrome classically is characterized by a triad of cutaneous anesthesia, paresthesia and/or pain, and ulceration secondary to pathology of trigeminal nerve sensory branches. Ulceration arises primarily through excoriation in response to paresthetic pruritus or pain. The differential diagnosis for TTS includes ulcerating cutaneous neoplasms (eg, basal cell carcinoma); mycobacterial, fungal, and viral infections (especially herpetic lesions); and cutaneous involvement of systemic vasculitides (eg, granulomatosis with polyangiitis).1 Biopsy is necessary to exclude malignancy, and ulcers may be scraped for viral diagnosis. Complete blood cell count and serologic testing also may help to exclude immunodeficiencies or disorders. Apart from viral neuropathy, common etiologies of TTS include iatrogenic trigeminal injury (eg, in ablation treatment for trigeminal neuralgia) and stroke (eg, lateral medullary syndrome).
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019;58:530-537.
The Diagnosis: Trigeminal Trophic Syndrome (Self-induced Trauma)
The patient admitted to manipulation of the ala in response to persistent pain despite resolution of the herpes zoster, for which he recently had completed a course of oral acyclovir. A preliminary diagnosis of trigeminal trophic syndrome (TTS) was made, and a subsequent punch biopsy revealed no evidence of malignancy. Topical antibiotic prophylaxis was prescribed, and he was instructed to avoid manipulation of the affected area. Treatment was initiated in consultation with pain specialists, and over the following 3 years our patient experienced a waxing and waning course of persistent pain complicated by new scalp and oral ulcers as well as alar impetigo. His condition eventually stabilized with tolerable pain on oral gabapentin and doxepin cream 5% applied up to 4 times daily. The alar lesion healed following sufficient abstinence from manipulation, leaving a crescent-shaped rim defect.
Trigeminal trophic syndrome classically is characterized by a triad of cutaneous anesthesia, paresthesia and/or pain, and ulceration secondary to pathology of trigeminal nerve sensory branches. Ulceration arises primarily through excoriation in response to paresthetic pruritus or pain. The differential diagnosis for TTS includes ulcerating cutaneous neoplasms (eg, basal cell carcinoma); mycobacterial, fungal, and viral infections (especially herpetic lesions); and cutaneous involvement of systemic vasculitides (eg, granulomatosis with polyangiitis).1 Biopsy is necessary to exclude malignancy, and ulcers may be scraped for viral diagnosis. Complete blood cell count and serologic testing also may help to exclude immunodeficiencies or disorders. Apart from viral neuropathy, common etiologies of TTS include iatrogenic trigeminal injury (eg, in ablation treatment for trigeminal neuralgia) and stroke (eg, lateral medullary syndrome).
The Diagnosis: Trigeminal Trophic Syndrome (Self-induced Trauma)
The patient admitted to manipulation of the ala in response to persistent pain despite resolution of the herpes zoster, for which he recently had completed a course of oral acyclovir. A preliminary diagnosis of trigeminal trophic syndrome (TTS) was made, and a subsequent punch biopsy revealed no evidence of malignancy. Topical antibiotic prophylaxis was prescribed, and he was instructed to avoid manipulation of the affected area. Treatment was initiated in consultation with pain specialists, and over the following 3 years our patient experienced a waxing and waning course of persistent pain complicated by new scalp and oral ulcers as well as alar impetigo. His condition eventually stabilized with tolerable pain on oral gabapentin and doxepin cream 5% applied up to 4 times daily. The alar lesion healed following sufficient abstinence from manipulation, leaving a crescent-shaped rim defect.
Trigeminal trophic syndrome classically is characterized by a triad of cutaneous anesthesia, paresthesia and/or pain, and ulceration secondary to pathology of trigeminal nerve sensory branches. Ulceration arises primarily through excoriation in response to paresthetic pruritus or pain. The differential diagnosis for TTS includes ulcerating cutaneous neoplasms (eg, basal cell carcinoma); mycobacterial, fungal, and viral infections (especially herpetic lesions); and cutaneous involvement of systemic vasculitides (eg, granulomatosis with polyangiitis).1 Biopsy is necessary to exclude malignancy, and ulcers may be scraped for viral diagnosis. Complete blood cell count and serologic testing also may help to exclude immunodeficiencies or disorders. Apart from viral neuropathy, common etiologies of TTS include iatrogenic trigeminal injury (eg, in ablation treatment for trigeminal neuralgia) and stroke (eg, lateral medullary syndrome).
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019;58:530-537.
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019;58:530-537.
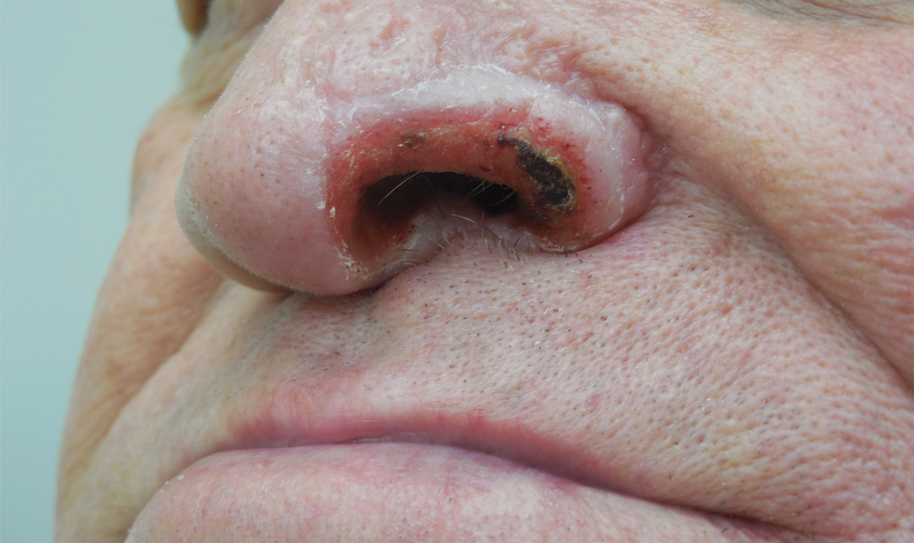
A 68-year-old man presented with a new left nasal alar ulcer following a recent episode of primary herpes zoster. Physical examination revealed erythema, erosion, and necrosis of the left naris with partial loss of the alar rim. Additional erythema was present without vesicles around the left eye and on the forehead.
Telangiectatic Patch on the Forehead
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3
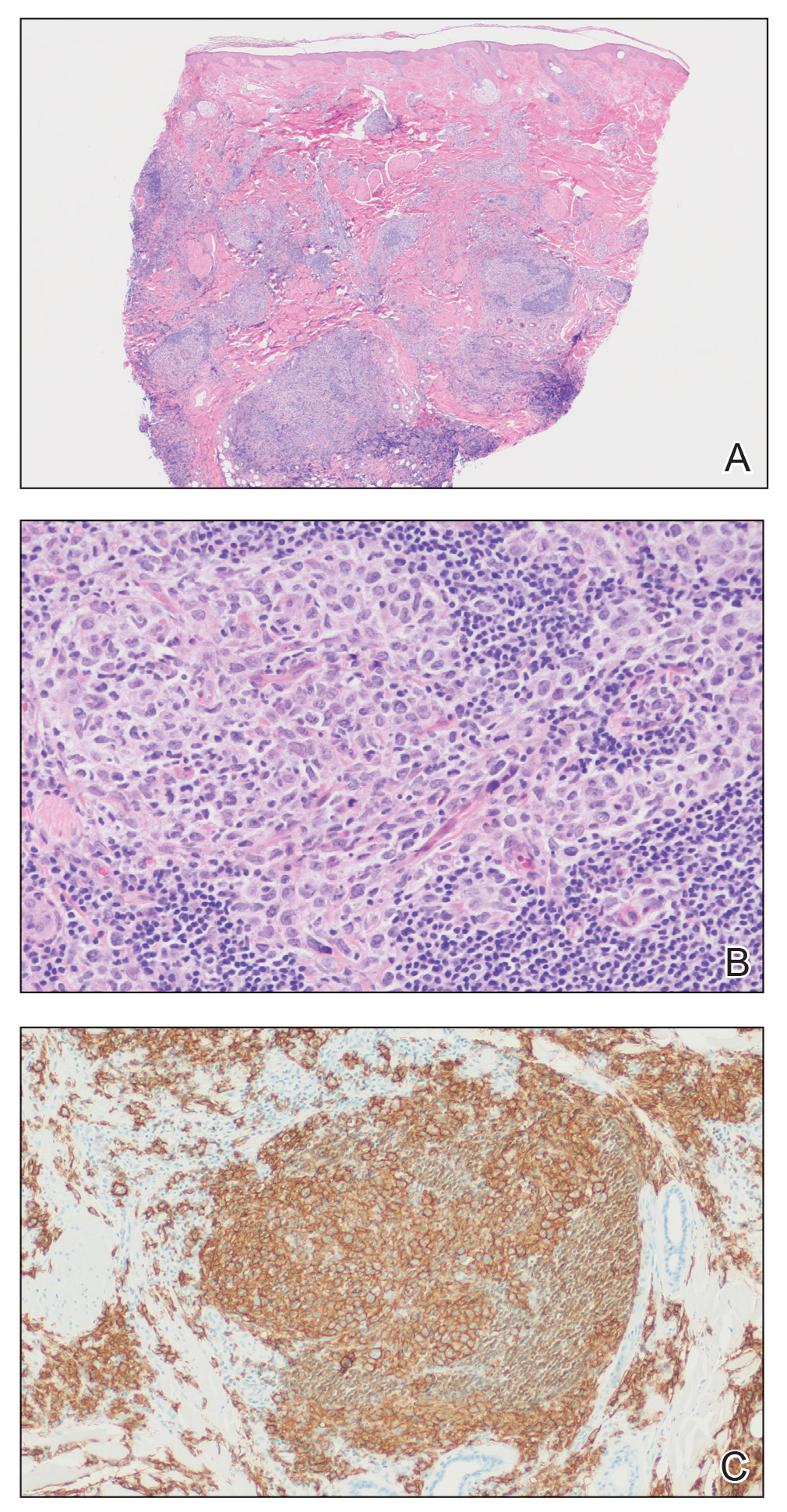
Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3

Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3

Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
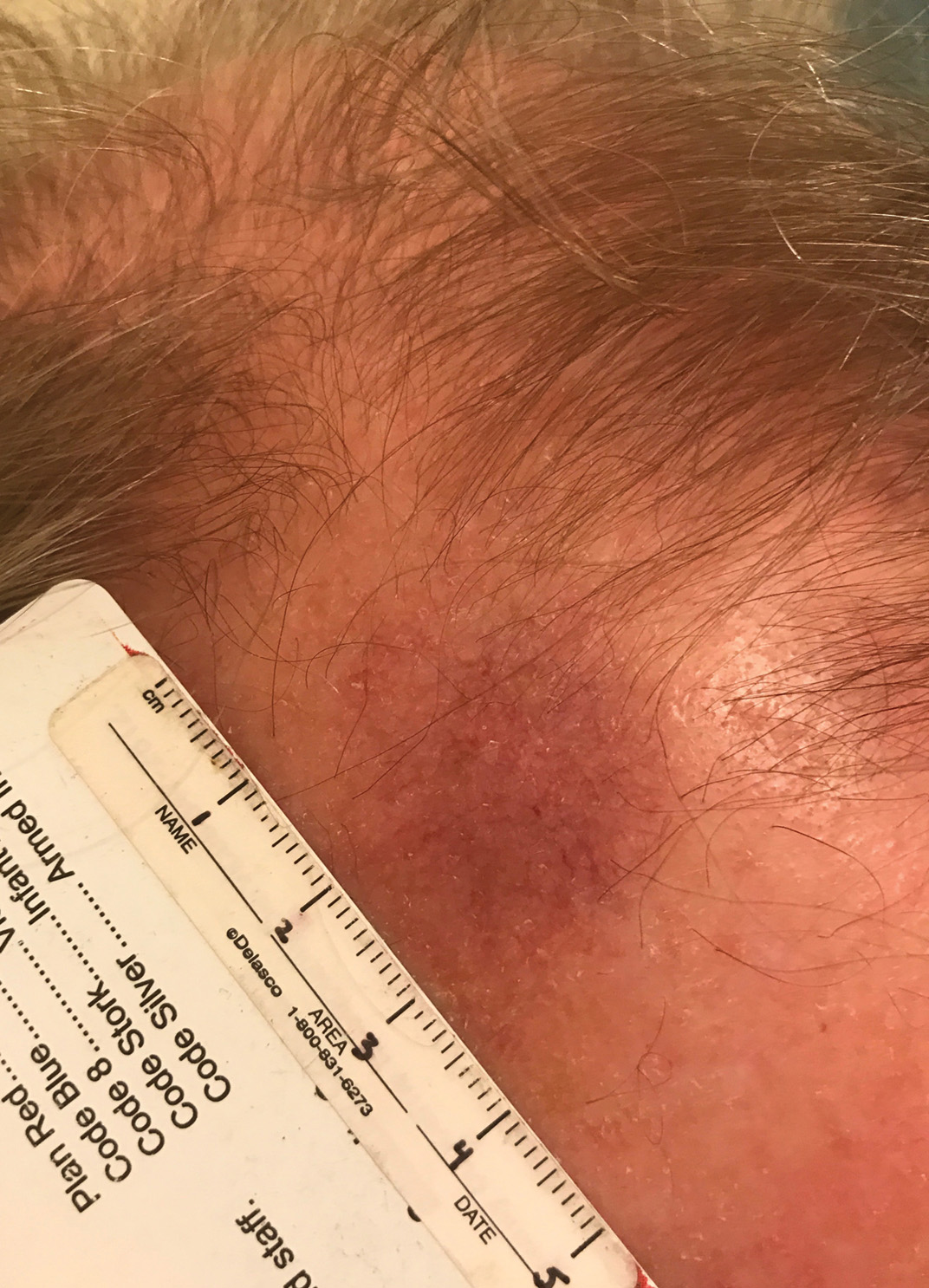
Diet and Skin: A Primer
Dermatologists frequently learn about skin conditions that are directly linked to diet. For example, we know that nutritional deficiencies can impact the hair, skin, and nails, and that celiac disease manifests with dermatitis herpetiformis of the skin. Patients commonly ask their dermatologists about the impact of diet on their skin. There are many outdated myths, but research on the subject is increasingly demonstrating important associations. Dermatologists must become familiar with the data on this topic so that we can provide informed counseling for our patients. This article reviews the current literature on associations between diet and 3 common cutaneous conditions—acne, psoriasis, and atopic dermatitis [AD]—and provides tips on how to best address our patients’ questions on this topic.
Acne
Studies increasingly support an association between a high glycemic diet (foods that lead to a spike in serum glucose) and acne; Bowe et al1 provided an excellent summary of the topic in 2010. This year, a large prospective cohort study of more than 24,000 participants demonstrated an association between adult acne and a diet high in milk, sugary beverages and foods, and fatty foods.2 In prospective cohort studies of more than 6000 adolescent girls and 4000 adolescent boys, Adebamowo et al3,4 demonstrated a correlation between skim milk consumption and acne. Whey protein supplementation also has been implicated in acne flares.5,6 The biological mechanism of the impact of high glycemic index foods and acne is believed to be mainly via activation of the insulinlike growth factor 1 (IGF-1) pathway, which promotes androgen synthesis and increases androgen bioavailability via decreased synthesis of sex hormone binding globulin.1,2 Insulinlike growth factor 1 also stimulates its downstream target, mammalian target of rapamycin (mTOR), leading to activation of antiapoptotic and proliferation signaling, ultimately resulting in oxidative stress and inflammation causing acne.2 Penso et al2 noted that patients with IGF-1 deficiency (Laron syndrome) never develop acne unless treated with exogenous IGF-1, further supporting its role in acne formation.7 There currently is a paucity of randomized controlled trials assessing the impact of diet on acne.
Psoriasis
The literature consistently shows that obesity is a predisposing factor for psoriasis. Additionally, weight gain may cause flares of existing psoriasis.8 Promotion of a healthy diet is an important factor in the management of obesity, alongside physical activity and, in some cases, medication and bariatric surgery.9 Patients with psoriasis who are overweight have been shown to experience improvement in their psoriasis after weight loss secondary to diet and exercise.8,10 The joint American Academy of Dermatology and National Psoriasis Foundation guidelines recommend that dermatologists advise patients to practice a healthy lifestyle including a healthy diet and communicate with a patient’s primary care provider so they can be appropriately evaluated and treated for comorbidities including metabolic syndrome, diabetes, and hyperlipidemia.11 In the NutriNet-Santé cohort study, investigators found an inverse correlation between psoriasis severity and adherence to a Mediterranean diet, which the authors conclude supports the hypothesis that this may slow the progression of psoriasis.12 In a single meta-analysis, it was reported that patients with psoriasis have a 3-fold increased risk for celiac disease compared to the general population.13 It remains unknown if these data are generalizable to the US population. Dermatologists should consider screening patients with psoriasis for celiac disease based on reported symptoms. When suspected, it is necessary to order appropriate serologies and consider referral to gastroenterology prior to recommending a gluten-free diet, as elimination of gluten prior to testing may lead to false-negative results.
Atopic Dermatitis
Patients and parents/guardians of children with AD often ask about the impact of diet on the condition. A small minority of patients may experience flares of AD due to ongoing, non–IgE-mediated allergen exposure.14 Diet as a trigger for flares should be suspected in children with persistent, moderate to severe AD. In these patients, allergen avoidance may lead to improvement but not resolution of AD. Allergens ordered from most common to least common are the following: eggs, milk, peanuts/tree nuts, shellfish, soy, and wheat.15 Additionally, it is important to note that children with AD are at higher risk for developing life-threatening, IgE-mediated food allergies compared to the general population (37% vs 6.8%).16,17 The LEAP (Learning Early about Peanut Allergy) study led to a paradigm shift in prevention of peanut allergies in high-risk children (ie, those with severe AD and/or egg allergy), providing data to support the idea that early introduction of allergenic foods such as peanuts may prevent severe allergies.18 Further studies are necessary to clarify the population in which allergen testing and recommendations on food avoidance are warranted vs early introduction.19
Conclusion
Early data support the relationship between diet and many common dermatologic conditions, including acne, psoriasis, and AD. Dermatologists should be familiar with the evidence supporting the relationship between diet and various skin conditions to best answer patients’ questions and counsel as appropriate. It is important for dermatologists to continue to stay up-to-date on the literature on this subject as new data emerge. Knowledge about the relationship between diet and skin allows dermatologists to not only support our patients’ skin health but their overall health as well.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
- Cengiz FP, Cemil BC, Emiroglu N, et al. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect. 2017;7:106-108.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Jensen P, Skov L. Psoriasis and obesity [published online February 23, 2017]. Dermatology. 2016;232:633-639.
- Extreme obesity, and what you can do. American Heart Association website. https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/extreme-obesity-and-what-you-can-do. Updated April 18, 2014. Accessed November 30, 2020.
- Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634-642.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:203-218.
- Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8.
- Age-adjusted percentages (with standard errors) of hay fever, respiratory allergies, food allergies, and skin allergies in the past 12 months for children under age 18 years, by selected characteristics: United States, 2016. CDC website. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf. Accessed December 8, 2020.
- Du Toit G, Roberts G, Sayre PH, et al; LEAP study team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy [published online October 22, 2019]. Allergol Int. 2020;69:204-214.
Dermatologists frequently learn about skin conditions that are directly linked to diet. For example, we know that nutritional deficiencies can impact the hair, skin, and nails, and that celiac disease manifests with dermatitis herpetiformis of the skin. Patients commonly ask their dermatologists about the impact of diet on their skin. There are many outdated myths, but research on the subject is increasingly demonstrating important associations. Dermatologists must become familiar with the data on this topic so that we can provide informed counseling for our patients. This article reviews the current literature on associations between diet and 3 common cutaneous conditions—acne, psoriasis, and atopic dermatitis [AD]—and provides tips on how to best address our patients’ questions on this topic.
Acne
Studies increasingly support an association between a high glycemic diet (foods that lead to a spike in serum glucose) and acne; Bowe et al1 provided an excellent summary of the topic in 2010. This year, a large prospective cohort study of more than 24,000 participants demonstrated an association between adult acne and a diet high in milk, sugary beverages and foods, and fatty foods.2 In prospective cohort studies of more than 6000 adolescent girls and 4000 adolescent boys, Adebamowo et al3,4 demonstrated a correlation between skim milk consumption and acne. Whey protein supplementation also has been implicated in acne flares.5,6 The biological mechanism of the impact of high glycemic index foods and acne is believed to be mainly via activation of the insulinlike growth factor 1 (IGF-1) pathway, which promotes androgen synthesis and increases androgen bioavailability via decreased synthesis of sex hormone binding globulin.1,2 Insulinlike growth factor 1 also stimulates its downstream target, mammalian target of rapamycin (mTOR), leading to activation of antiapoptotic and proliferation signaling, ultimately resulting in oxidative stress and inflammation causing acne.2 Penso et al2 noted that patients with IGF-1 deficiency (Laron syndrome) never develop acne unless treated with exogenous IGF-1, further supporting its role in acne formation.7 There currently is a paucity of randomized controlled trials assessing the impact of diet on acne.
Psoriasis
The literature consistently shows that obesity is a predisposing factor for psoriasis. Additionally, weight gain may cause flares of existing psoriasis.8 Promotion of a healthy diet is an important factor in the management of obesity, alongside physical activity and, in some cases, medication and bariatric surgery.9 Patients with psoriasis who are overweight have been shown to experience improvement in their psoriasis after weight loss secondary to diet and exercise.8,10 The joint American Academy of Dermatology and National Psoriasis Foundation guidelines recommend that dermatologists advise patients to practice a healthy lifestyle including a healthy diet and communicate with a patient’s primary care provider so they can be appropriately evaluated and treated for comorbidities including metabolic syndrome, diabetes, and hyperlipidemia.11 In the NutriNet-Santé cohort study, investigators found an inverse correlation between psoriasis severity and adherence to a Mediterranean diet, which the authors conclude supports the hypothesis that this may slow the progression of psoriasis.12 In a single meta-analysis, it was reported that patients with psoriasis have a 3-fold increased risk for celiac disease compared to the general population.13 It remains unknown if these data are generalizable to the US population. Dermatologists should consider screening patients with psoriasis for celiac disease based on reported symptoms. When suspected, it is necessary to order appropriate serologies and consider referral to gastroenterology prior to recommending a gluten-free diet, as elimination of gluten prior to testing may lead to false-negative results.
Atopic Dermatitis
Patients and parents/guardians of children with AD often ask about the impact of diet on the condition. A small minority of patients may experience flares of AD due to ongoing, non–IgE-mediated allergen exposure.14 Diet as a trigger for flares should be suspected in children with persistent, moderate to severe AD. In these patients, allergen avoidance may lead to improvement but not resolution of AD. Allergens ordered from most common to least common are the following: eggs, milk, peanuts/tree nuts, shellfish, soy, and wheat.15 Additionally, it is important to note that children with AD are at higher risk for developing life-threatening, IgE-mediated food allergies compared to the general population (37% vs 6.8%).16,17 The LEAP (Learning Early about Peanut Allergy) study led to a paradigm shift in prevention of peanut allergies in high-risk children (ie, those with severe AD and/or egg allergy), providing data to support the idea that early introduction of allergenic foods such as peanuts may prevent severe allergies.18 Further studies are necessary to clarify the population in which allergen testing and recommendations on food avoidance are warranted vs early introduction.19
Conclusion
Early data support the relationship between diet and many common dermatologic conditions, including acne, psoriasis, and AD. Dermatologists should be familiar with the evidence supporting the relationship between diet and various skin conditions to best answer patients’ questions and counsel as appropriate. It is important for dermatologists to continue to stay up-to-date on the literature on this subject as new data emerge. Knowledge about the relationship between diet and skin allows dermatologists to not only support our patients’ skin health but their overall health as well.
Dermatologists frequently learn about skin conditions that are directly linked to diet. For example, we know that nutritional deficiencies can impact the hair, skin, and nails, and that celiac disease manifests with dermatitis herpetiformis of the skin. Patients commonly ask their dermatologists about the impact of diet on their skin. There are many outdated myths, but research on the subject is increasingly demonstrating important associations. Dermatologists must become familiar with the data on this topic so that we can provide informed counseling for our patients. This article reviews the current literature on associations between diet and 3 common cutaneous conditions—acne, psoriasis, and atopic dermatitis [AD]—and provides tips on how to best address our patients’ questions on this topic.
Acne
Studies increasingly support an association between a high glycemic diet (foods that lead to a spike in serum glucose) and acne; Bowe et al1 provided an excellent summary of the topic in 2010. This year, a large prospective cohort study of more than 24,000 participants demonstrated an association between adult acne and a diet high in milk, sugary beverages and foods, and fatty foods.2 In prospective cohort studies of more than 6000 adolescent girls and 4000 adolescent boys, Adebamowo et al3,4 demonstrated a correlation between skim milk consumption and acne. Whey protein supplementation also has been implicated in acne flares.5,6 The biological mechanism of the impact of high glycemic index foods and acne is believed to be mainly via activation of the insulinlike growth factor 1 (IGF-1) pathway, which promotes androgen synthesis and increases androgen bioavailability via decreased synthesis of sex hormone binding globulin.1,2 Insulinlike growth factor 1 also stimulates its downstream target, mammalian target of rapamycin (mTOR), leading to activation of antiapoptotic and proliferation signaling, ultimately resulting in oxidative stress and inflammation causing acne.2 Penso et al2 noted that patients with IGF-1 deficiency (Laron syndrome) never develop acne unless treated with exogenous IGF-1, further supporting its role in acne formation.7 There currently is a paucity of randomized controlled trials assessing the impact of diet on acne.
Psoriasis
The literature consistently shows that obesity is a predisposing factor for psoriasis. Additionally, weight gain may cause flares of existing psoriasis.8 Promotion of a healthy diet is an important factor in the management of obesity, alongside physical activity and, in some cases, medication and bariatric surgery.9 Patients with psoriasis who are overweight have been shown to experience improvement in their psoriasis after weight loss secondary to diet and exercise.8,10 The joint American Academy of Dermatology and National Psoriasis Foundation guidelines recommend that dermatologists advise patients to practice a healthy lifestyle including a healthy diet and communicate with a patient’s primary care provider so they can be appropriately evaluated and treated for comorbidities including metabolic syndrome, diabetes, and hyperlipidemia.11 In the NutriNet-Santé cohort study, investigators found an inverse correlation between psoriasis severity and adherence to a Mediterranean diet, which the authors conclude supports the hypothesis that this may slow the progression of psoriasis.12 In a single meta-analysis, it was reported that patients with psoriasis have a 3-fold increased risk for celiac disease compared to the general population.13 It remains unknown if these data are generalizable to the US population. Dermatologists should consider screening patients with psoriasis for celiac disease based on reported symptoms. When suspected, it is necessary to order appropriate serologies and consider referral to gastroenterology prior to recommending a gluten-free diet, as elimination of gluten prior to testing may lead to false-negative results.
Atopic Dermatitis
Patients and parents/guardians of children with AD often ask about the impact of diet on the condition. A small minority of patients may experience flares of AD due to ongoing, non–IgE-mediated allergen exposure.14 Diet as a trigger for flares should be suspected in children with persistent, moderate to severe AD. In these patients, allergen avoidance may lead to improvement but not resolution of AD. Allergens ordered from most common to least common are the following: eggs, milk, peanuts/tree nuts, shellfish, soy, and wheat.15 Additionally, it is important to note that children with AD are at higher risk for developing life-threatening, IgE-mediated food allergies compared to the general population (37% vs 6.8%).16,17 The LEAP (Learning Early about Peanut Allergy) study led to a paradigm shift in prevention of peanut allergies in high-risk children (ie, those with severe AD and/or egg allergy), providing data to support the idea that early introduction of allergenic foods such as peanuts may prevent severe allergies.18 Further studies are necessary to clarify the population in which allergen testing and recommendations on food avoidance are warranted vs early introduction.19
Conclusion
Early data support the relationship between diet and many common dermatologic conditions, including acne, psoriasis, and AD. Dermatologists should be familiar with the evidence supporting the relationship between diet and various skin conditions to best answer patients’ questions and counsel as appropriate. It is important for dermatologists to continue to stay up-to-date on the literature on this subject as new data emerge. Knowledge about the relationship between diet and skin allows dermatologists to not only support our patients’ skin health but their overall health as well.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
- Cengiz FP, Cemil BC, Emiroglu N, et al. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect. 2017;7:106-108.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Jensen P, Skov L. Psoriasis and obesity [published online February 23, 2017]. Dermatology. 2016;232:633-639.
- Extreme obesity, and what you can do. American Heart Association website. https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/extreme-obesity-and-what-you-can-do. Updated April 18, 2014. Accessed November 30, 2020.
- Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634-642.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:203-218.
- Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8.
- Age-adjusted percentages (with standard errors) of hay fever, respiratory allergies, food allergies, and skin allergies in the past 12 months for children under age 18 years, by selected characteristics: United States, 2016. CDC website. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf. Accessed December 8, 2020.
- Du Toit G, Roberts G, Sayre PH, et al; LEAP study team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy [published online October 22, 2019]. Allergol Int. 2020;69:204-214.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
- Cengiz FP, Cemil BC, Emiroglu N, et al. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect. 2017;7:106-108.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Jensen P, Skov L. Psoriasis and obesity [published online February 23, 2017]. Dermatology. 2016;232:633-639.
- Extreme obesity, and what you can do. American Heart Association website. https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/extreme-obesity-and-what-you-can-do. Updated April 18, 2014. Accessed November 30, 2020.
- Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634-642.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:203-218.
- Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8.
- Age-adjusted percentages (with standard errors) of hay fever, respiratory allergies, food allergies, and skin allergies in the past 12 months for children under age 18 years, by selected characteristics: United States, 2016. CDC website. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf. Accessed December 8, 2020.
- Du Toit G, Roberts G, Sayre PH, et al; LEAP study team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy [published online October 22, 2019]. Allergol Int. 2020;69:204-214.
Resident Pearls
- There are strong data on the relationship between dietary patterns and skin conditions.
- High glycemic index foods (eg, skim milk, whey protein, sugary beverages, fatty foods) are associated with acne vulgaris.
- Obesity is a risk factor for psoriasis; weight loss interventions such as improved dietary patterns can improve psoriasis.
- Children with atopic dermatitis (AD) are at higher risk for food allergies (both IgE and non–IgE-mediated allergies). A small subset may experience flares in their AD in relation to non–IgE-mediated food allergies.
Multiple Nontender Subcutaneous Nodules on the Finger
The Diagnosis: Subcutaneous Granuloma Annulare
Subcutaneous granuloma annulare (SGA), also known as deep GA, is a rare variant of GA that usually occurs in children and young adults. It presents as single or multiple, nontender, deep dermal and/or subcutaneous nodules with normal-appearing skin usually on the anterior lower legs, dorsal aspects of the hands and fingers, scalp, or buttocks.1-3 The pathogenesis of SGA as well as GA is not fully understood, and proposed inciting factors include trauma, insect bite reactions, tuberculin skin testing, vaccines, UV exposure, medications, and viral infections.3-6 A cell-mediated, delayed-type hypersensitivity reaction to an unknown antigen also has been postulated as a possible mechanism.7 Treatment usually is not necessary, as the nature of the condition is benign and the course often is self-limited. Spontaneous resolution occurs within 2 years in 50% of patients with localized GA.4,8 Surgery usually is not recommended due to the high recurrence rate (40%-75%).4,9
Absence of epidermal change in this entity obfuscates clinical recognition, and accurate diagnosis often depends on punch or excisional biopsies revealing characteristic histopathology. The histology of SGA consists of palisaded granulomas with central areas of necrobiosis composed of degenerated collagen, mucin deposition, and nuclear dust from neutrophils that extend into the deep dermis and subcutis.2 The periphery of the granulomas is lined by palisading epithelioid histiocytes with occasional multinucleated giant cells.10,11 Eosinophils often are present.12 Colloidal iron and Alcian blue stains can be used to highlight the abundant connective tissue mucin of the granulomas.4
The histologic differential diagnosis of SGA includes rheumatoid nodule, necrobiosis lipoidica, epithelioid sarcoma, and tophaceous gout.2 Rheumatoid nodules are the most common dermatologic presentation of rheumatoid arthritis and are found in up to 30% to 40% of patients with the disease.13-15 They present as firm, painless, subcutaneous papulonodules on the extensor surfaces and at sites of trauma or pressure. Histologically, rheumatoid nodules exhibit a homogenous and eosinophilic central area of necrobiosis with fibrin deposition and absent mucin deep within the dermis and subcutaneous tissue (Figure 1). In contrast, granulomas in SGA usually are pale and basophilic with abundant mucin.2
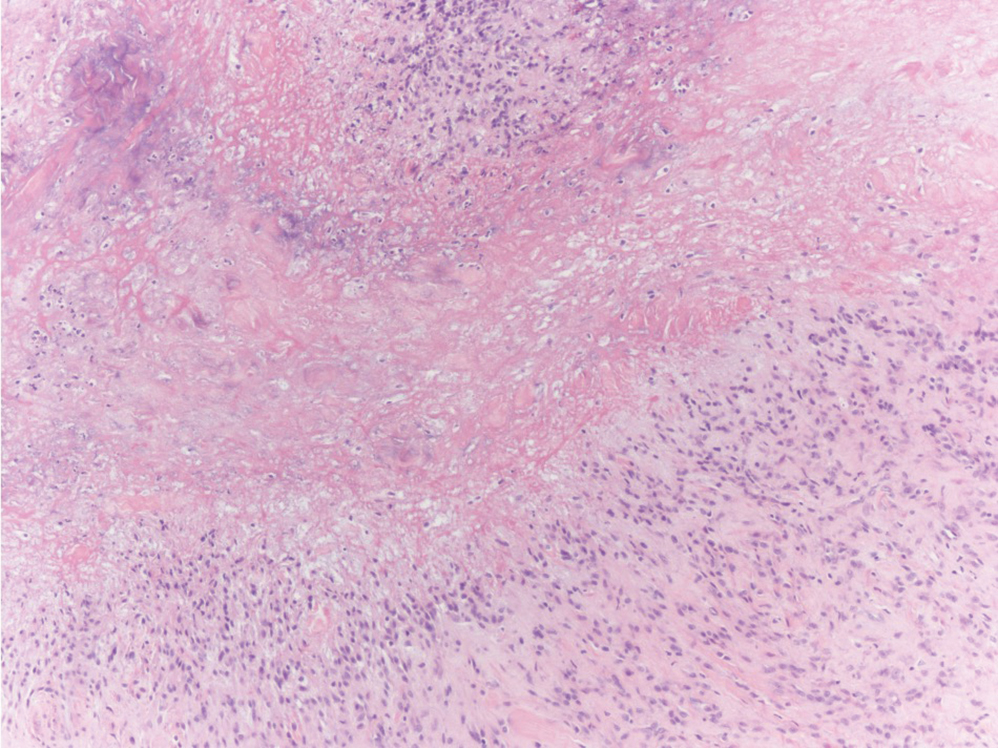
Necrobiosis lipoidica is a rare chronic granulomatous disease of the skin that most commonly occurs in young to middle-aged adults and is strongly associated with diabetes mellitus.16 It clinically presents as yellow to red-brown papules and plaques with a peripheral erythematous to violaceous rim usually on the pretibial area. Over time, lesions become yellowish atrophic patches and plaques that sometimes can ulcerate. Histopathology reveals areas of horizontally arranged, palisaded, and interstitial granulomatous dermatitis intermixed with areas of degenerated collagen and widespread fibrosis extending from the superficial dermis into the subcutis (Figure 2).2 These areas lack mucin and have an increased number of plasma cells. Eosinophils and/or lymphoid nodules occasionally can be seen.17,18
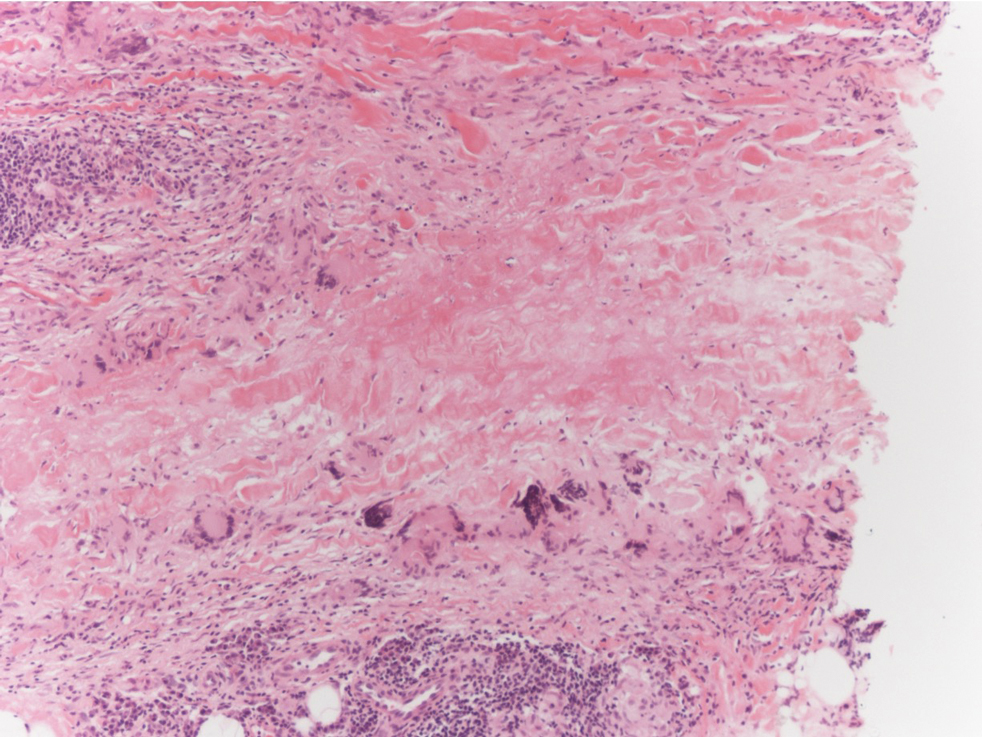
Epithelioid sarcoma is a rare malignant soft tissue sarcoma that tends to occur on the distal extremities in younger patients, typically aged 20 to 40 years, often with preceding trauma to the area. It usually presents as a solitary, poorly defined, hard, subcutaneous nodule. Histologic analysis shows central areas of necrosis and degenerated collagen surrounded by epithelioid and spindle cells with hyperchromatic and pleomorphic nuclei and mitoses (Figure 3).2 These tumor cells express positivity for keratins, vimentin, epithelial membrane antigen, and CD34, while they usually are negative for desmin, S-100, and FLI-1 nuclear transcription factor.2,4,19
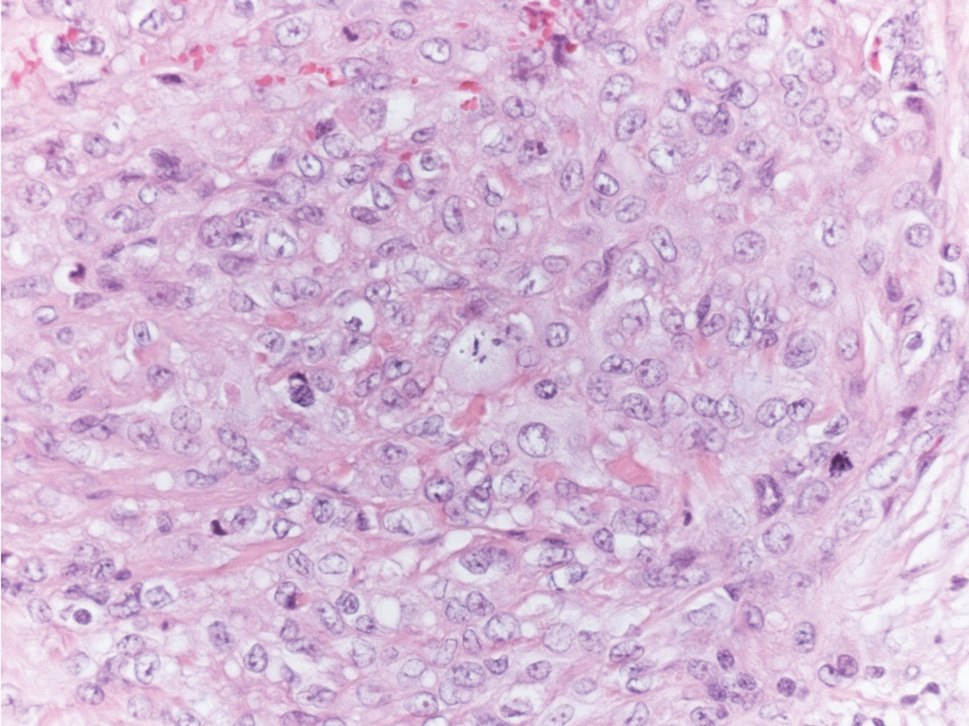
Tophaceous gout results from the accumulation of monosodium urate crystals in the skin. It clinically presents as firm, white-yellow, dermal and subcutaneous papulonodules on the helix of the ear and the skin overlying joints. Histopathology reveals palisaded granulomas surrounding an amorphous feathery material that corresponds to the urate crystals that were destroyed with formalin fixation (Figure 4). When the tissue is fixed with ethanol or is incompletely fixed in formalin, birefringent urate crystals are evident with polarization.20
- Felner EI, Steinberg JB, Weinberg AG. Subcutaneous granuloma annulare: a review of 47 cases. Pediatrics. 1997;100:965-967.
- Requena L, Fernández-Figueras MT. Subcutaneous granuloma annulare. Semin Cutan Med Surg. 2007;26:96-99.
- Taranu T, Grigorovici M, Constantin M, et al. Subcutaneous granuloma annulare. Acta Dermatovenerol Croat. 2017;25:292-294.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1644-1663.
- Mills A, Chetty R. Auricular granuloma annulare: a consequence of trauma? Am J Dermatopathol. 1992;14:431-433.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Buechner SA, Winkelmann RK, Banks PM. Identification of T-cell subpopulations in granuloma annulare. Arch Dermatol. 1983;119:125-128.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199.
- Davids JR, Kolman BH, Billman GF, et al. Subcutaneous granuloma annulare: recognition and treatment. J Pediatr Orthop. 1993;13:582-586.
- Evans MJ, Blessing K, Gray ES. Pseudorheumatoid nodule (deep granuloma annulare) of childhood: clinicopathologic features of twenty patients. Pediatr Dermatol. 1994;11:6-9.
- Patterson JW. Rheumatoid nodule and subcutaneous granuloma annulare: a comparative histologic study. Am J Dermatopathol. 1988;10:1-8.
- Weedon D. Granuloma annulare. Skin Pathology. Edinburgh, Scotland: Churchill-Livingstone; 1997:167-170.
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209.
- Highton J, Hessian PA, Stamp L. The rheumatoid nodule: peripheral or central to rheumatoid arthritis? Rheumatology (Oxford). 2007;46:1385-1387.
- Turesson C, Jacobsson LT. Epidemiology of extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol. 2004;33:65-72.
- Erfurt-Berge C, Dissemond J, Schwede K, et al. Updated results of 100 patients on clinical features and therapeutic options in necrobiosis lipoidica in a retrospective multicenter study. Eur J Dermatol. 2015;25:595-601.
- Kota SK, Jammula S, Kota SK, et al. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocrinol Metab. 2012;16:614-620.
- Alegre VA, Winkelmann RK. A new histopathologic feature of necrobiosis lipoidica diabeticorum: lymphoid nodules. J Cutan Pathol. 1988;15:75-77.
- Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med. 2009;133:814-819.
- Shidham V, Chivukula M, Basir Z, et al. Evaluation of crystals in formalin-fixed, paraffin-embedded tissue sections for the differential diagnosis pseudogout, gout, and tumoral calcinosis. Mod Pathol. 2001;14:806-810.
The Diagnosis: Subcutaneous Granuloma Annulare
Subcutaneous granuloma annulare (SGA), also known as deep GA, is a rare variant of GA that usually occurs in children and young adults. It presents as single or multiple, nontender, deep dermal and/or subcutaneous nodules with normal-appearing skin usually on the anterior lower legs, dorsal aspects of the hands and fingers, scalp, or buttocks.1-3 The pathogenesis of SGA as well as GA is not fully understood, and proposed inciting factors include trauma, insect bite reactions, tuberculin skin testing, vaccines, UV exposure, medications, and viral infections.3-6 A cell-mediated, delayed-type hypersensitivity reaction to an unknown antigen also has been postulated as a possible mechanism.7 Treatment usually is not necessary, as the nature of the condition is benign and the course often is self-limited. Spontaneous resolution occurs within 2 years in 50% of patients with localized GA.4,8 Surgery usually is not recommended due to the high recurrence rate (40%-75%).4,9
Absence of epidermal change in this entity obfuscates clinical recognition, and accurate diagnosis often depends on punch or excisional biopsies revealing characteristic histopathology. The histology of SGA consists of palisaded granulomas with central areas of necrobiosis composed of degenerated collagen, mucin deposition, and nuclear dust from neutrophils that extend into the deep dermis and subcutis.2 The periphery of the granulomas is lined by palisading epithelioid histiocytes with occasional multinucleated giant cells.10,11 Eosinophils often are present.12 Colloidal iron and Alcian blue stains can be used to highlight the abundant connective tissue mucin of the granulomas.4
The histologic differential diagnosis of SGA includes rheumatoid nodule, necrobiosis lipoidica, epithelioid sarcoma, and tophaceous gout.2 Rheumatoid nodules are the most common dermatologic presentation of rheumatoid arthritis and are found in up to 30% to 40% of patients with the disease.13-15 They present as firm, painless, subcutaneous papulonodules on the extensor surfaces and at sites of trauma or pressure. Histologically, rheumatoid nodules exhibit a homogenous and eosinophilic central area of necrobiosis with fibrin deposition and absent mucin deep within the dermis and subcutaneous tissue (Figure 1). In contrast, granulomas in SGA usually are pale and basophilic with abundant mucin.2

Necrobiosis lipoidica is a rare chronic granulomatous disease of the skin that most commonly occurs in young to middle-aged adults and is strongly associated with diabetes mellitus.16 It clinically presents as yellow to red-brown papules and plaques with a peripheral erythematous to violaceous rim usually on the pretibial area. Over time, lesions become yellowish atrophic patches and plaques that sometimes can ulcerate. Histopathology reveals areas of horizontally arranged, palisaded, and interstitial granulomatous dermatitis intermixed with areas of degenerated collagen and widespread fibrosis extending from the superficial dermis into the subcutis (Figure 2).2 These areas lack mucin and have an increased number of plasma cells. Eosinophils and/or lymphoid nodules occasionally can be seen.17,18

Epithelioid sarcoma is a rare malignant soft tissue sarcoma that tends to occur on the distal extremities in younger patients, typically aged 20 to 40 years, often with preceding trauma to the area. It usually presents as a solitary, poorly defined, hard, subcutaneous nodule. Histologic analysis shows central areas of necrosis and degenerated collagen surrounded by epithelioid and spindle cells with hyperchromatic and pleomorphic nuclei and mitoses (Figure 3).2 These tumor cells express positivity for keratins, vimentin, epithelial membrane antigen, and CD34, while they usually are negative for desmin, S-100, and FLI-1 nuclear transcription factor.2,4,19

Tophaceous gout results from the accumulation of monosodium urate crystals in the skin. It clinically presents as firm, white-yellow, dermal and subcutaneous papulonodules on the helix of the ear and the skin overlying joints. Histopathology reveals palisaded granulomas surrounding an amorphous feathery material that corresponds to the urate crystals that were destroyed with formalin fixation (Figure 4). When the tissue is fixed with ethanol or is incompletely fixed in formalin, birefringent urate crystals are evident with polarization.20

The Diagnosis: Subcutaneous Granuloma Annulare
Subcutaneous granuloma annulare (SGA), also known as deep GA, is a rare variant of GA that usually occurs in children and young adults. It presents as single or multiple, nontender, deep dermal and/or subcutaneous nodules with normal-appearing skin usually on the anterior lower legs, dorsal aspects of the hands and fingers, scalp, or buttocks.1-3 The pathogenesis of SGA as well as GA is not fully understood, and proposed inciting factors include trauma, insect bite reactions, tuberculin skin testing, vaccines, UV exposure, medications, and viral infections.3-6 A cell-mediated, delayed-type hypersensitivity reaction to an unknown antigen also has been postulated as a possible mechanism.7 Treatment usually is not necessary, as the nature of the condition is benign and the course often is self-limited. Spontaneous resolution occurs within 2 years in 50% of patients with localized GA.4,8 Surgery usually is not recommended due to the high recurrence rate (40%-75%).4,9
Absence of epidermal change in this entity obfuscates clinical recognition, and accurate diagnosis often depends on punch or excisional biopsies revealing characteristic histopathology. The histology of SGA consists of palisaded granulomas with central areas of necrobiosis composed of degenerated collagen, mucin deposition, and nuclear dust from neutrophils that extend into the deep dermis and subcutis.2 The periphery of the granulomas is lined by palisading epithelioid histiocytes with occasional multinucleated giant cells.10,11 Eosinophils often are present.12 Colloidal iron and Alcian blue stains can be used to highlight the abundant connective tissue mucin of the granulomas.4
The histologic differential diagnosis of SGA includes rheumatoid nodule, necrobiosis lipoidica, epithelioid sarcoma, and tophaceous gout.2 Rheumatoid nodules are the most common dermatologic presentation of rheumatoid arthritis and are found in up to 30% to 40% of patients with the disease.13-15 They present as firm, painless, subcutaneous papulonodules on the extensor surfaces and at sites of trauma or pressure. Histologically, rheumatoid nodules exhibit a homogenous and eosinophilic central area of necrobiosis with fibrin deposition and absent mucin deep within the dermis and subcutaneous tissue (Figure 1). In contrast, granulomas in SGA usually are pale and basophilic with abundant mucin.2

Necrobiosis lipoidica is a rare chronic granulomatous disease of the skin that most commonly occurs in young to middle-aged adults and is strongly associated with diabetes mellitus.16 It clinically presents as yellow to red-brown papules and plaques with a peripheral erythematous to violaceous rim usually on the pretibial area. Over time, lesions become yellowish atrophic patches and plaques that sometimes can ulcerate. Histopathology reveals areas of horizontally arranged, palisaded, and interstitial granulomatous dermatitis intermixed with areas of degenerated collagen and widespread fibrosis extending from the superficial dermis into the subcutis (Figure 2).2 These areas lack mucin and have an increased number of plasma cells. Eosinophils and/or lymphoid nodules occasionally can be seen.17,18

Epithelioid sarcoma is a rare malignant soft tissue sarcoma that tends to occur on the distal extremities in younger patients, typically aged 20 to 40 years, often with preceding trauma to the area. It usually presents as a solitary, poorly defined, hard, subcutaneous nodule. Histologic analysis shows central areas of necrosis and degenerated collagen surrounded by epithelioid and spindle cells with hyperchromatic and pleomorphic nuclei and mitoses (Figure 3).2 These tumor cells express positivity for keratins, vimentin, epithelial membrane antigen, and CD34, while they usually are negative for desmin, S-100, and FLI-1 nuclear transcription factor.2,4,19

Tophaceous gout results from the accumulation of monosodium urate crystals in the skin. It clinically presents as firm, white-yellow, dermal and subcutaneous papulonodules on the helix of the ear and the skin overlying joints. Histopathology reveals palisaded granulomas surrounding an amorphous feathery material that corresponds to the urate crystals that were destroyed with formalin fixation (Figure 4). When the tissue is fixed with ethanol or is incompletely fixed in formalin, birefringent urate crystals are evident with polarization.20

- Felner EI, Steinberg JB, Weinberg AG. Subcutaneous granuloma annulare: a review of 47 cases. Pediatrics. 1997;100:965-967.
- Requena L, Fernández-Figueras MT. Subcutaneous granuloma annulare. Semin Cutan Med Surg. 2007;26:96-99.
- Taranu T, Grigorovici M, Constantin M, et al. Subcutaneous granuloma annulare. Acta Dermatovenerol Croat. 2017;25:292-294.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1644-1663.
- Mills A, Chetty R. Auricular granuloma annulare: a consequence of trauma? Am J Dermatopathol. 1992;14:431-433.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Buechner SA, Winkelmann RK, Banks PM. Identification of T-cell subpopulations in granuloma annulare. Arch Dermatol. 1983;119:125-128.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199.
- Davids JR, Kolman BH, Billman GF, et al. Subcutaneous granuloma annulare: recognition and treatment. J Pediatr Orthop. 1993;13:582-586.
- Evans MJ, Blessing K, Gray ES. Pseudorheumatoid nodule (deep granuloma annulare) of childhood: clinicopathologic features of twenty patients. Pediatr Dermatol. 1994;11:6-9.
- Patterson JW. Rheumatoid nodule and subcutaneous granuloma annulare: a comparative histologic study. Am J Dermatopathol. 1988;10:1-8.
- Weedon D. Granuloma annulare. Skin Pathology. Edinburgh, Scotland: Churchill-Livingstone; 1997:167-170.
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209.
- Highton J, Hessian PA, Stamp L. The rheumatoid nodule: peripheral or central to rheumatoid arthritis? Rheumatology (Oxford). 2007;46:1385-1387.
- Turesson C, Jacobsson LT. Epidemiology of extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol. 2004;33:65-72.
- Erfurt-Berge C, Dissemond J, Schwede K, et al. Updated results of 100 patients on clinical features and therapeutic options in necrobiosis lipoidica in a retrospective multicenter study. Eur J Dermatol. 2015;25:595-601.
- Kota SK, Jammula S, Kota SK, et al. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocrinol Metab. 2012;16:614-620.
- Alegre VA, Winkelmann RK. A new histopathologic feature of necrobiosis lipoidica diabeticorum: lymphoid nodules. J Cutan Pathol. 1988;15:75-77.
- Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med. 2009;133:814-819.
- Shidham V, Chivukula M, Basir Z, et al. Evaluation of crystals in formalin-fixed, paraffin-embedded tissue sections for the differential diagnosis pseudogout, gout, and tumoral calcinosis. Mod Pathol. 2001;14:806-810.
- Felner EI, Steinberg JB, Weinberg AG. Subcutaneous granuloma annulare: a review of 47 cases. Pediatrics. 1997;100:965-967.
- Requena L, Fernández-Figueras MT. Subcutaneous granuloma annulare. Semin Cutan Med Surg. 2007;26:96-99.
- Taranu T, Grigorovici M, Constantin M, et al. Subcutaneous granuloma annulare. Acta Dermatovenerol Croat. 2017;25:292-294.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1644-1663.
- Mills A, Chetty R. Auricular granuloma annulare: a consequence of trauma? Am J Dermatopathol. 1992;14:431-433.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Buechner SA, Winkelmann RK, Banks PM. Identification of T-cell subpopulations in granuloma annulare. Arch Dermatol. 1983;119:125-128.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199.
- Davids JR, Kolman BH, Billman GF, et al. Subcutaneous granuloma annulare: recognition and treatment. J Pediatr Orthop. 1993;13:582-586.
- Evans MJ, Blessing K, Gray ES. Pseudorheumatoid nodule (deep granuloma annulare) of childhood: clinicopathologic features of twenty patients. Pediatr Dermatol. 1994;11:6-9.
- Patterson JW. Rheumatoid nodule and subcutaneous granuloma annulare: a comparative histologic study. Am J Dermatopathol. 1988;10:1-8.
- Weedon D. Granuloma annulare. Skin Pathology. Edinburgh, Scotland: Churchill-Livingstone; 1997:167-170.
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209.
- Highton J, Hessian PA, Stamp L. The rheumatoid nodule: peripheral or central to rheumatoid arthritis? Rheumatology (Oxford). 2007;46:1385-1387.
- Turesson C, Jacobsson LT. Epidemiology of extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol. 2004;33:65-72.
- Erfurt-Berge C, Dissemond J, Schwede K, et al. Updated results of 100 patients on clinical features and therapeutic options in necrobiosis lipoidica in a retrospective multicenter study. Eur J Dermatol. 2015;25:595-601.
- Kota SK, Jammula S, Kota SK, et al. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocrinol Metab. 2012;16:614-620.
- Alegre VA, Winkelmann RK. A new histopathologic feature of necrobiosis lipoidica diabeticorum: lymphoid nodules. J Cutan Pathol. 1988;15:75-77.
- Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med. 2009;133:814-819.
- Shidham V, Chivukula M, Basir Z, et al. Evaluation of crystals in formalin-fixed, paraffin-embedded tissue sections for the differential diagnosis pseudogout, gout, and tumoral calcinosis. Mod Pathol. 2001;14:806-810.
Widespread Purple Plaques
The Diagnosis: Kaposi Sarcoma
On initial presentation, the differential diagnosis included secondary syphilis, Kaposi sarcoma (KS), lichen planus pigmentosus, sarcoidosis, and psoriasis. A laboratory workup was ordered, which included complete blood cell count, comprehensive metabolic panel, antinuclear antibodies, anti-Ro/Sjögren syndrome antigen A and anti-La/Sjögren syndrome antigen B autoantibodies, angiotensin-converting enzyme, rapid plasma reagin, and human immunodeficiency virus (HIV) antibodies. A 4-mm punch biopsy of the rash also was performed from the right upper back. Histology revealed a vascular proliferation that was diffusely positive for human herpesvirus 8 (HHV-8)(Figure 1). The patient was informed of the diagnosis, at which time he revealed he had a history of homosexual relationships, with his last sexual contact being more than 1 year prior to presentation. The laboratory workup confirmed a diagnosis of HIV, and the remainder of the tests were unremarkable.
He was referred to our university's HIV clinic where he was started on highly active antiretroviral therapy (HAART). His facial swelling worsened, leading to hospital admission. Computed tomography (CT) of the chest, abdomen, and pelvis showed diffuse lymphadenopathy and lung nodules concerning for visceral involvement of KS. Hematology and oncology was consulted for further evaluation, and he was treated with 6 cycles of doxorubicin 20 mg/m2, which led to resolution of the lung nodules on CT and improvement of the rash burden. He was then started on alitretinoin gel 0.1% twice daily, which led to continued slow improvement (Figure 2).

Kaposi sarcoma is a vascular neoplasm that occurs from infection with HHV-8. It typically presents as painless, reddish to violaceous macules or patches involving the skin and mucosa that often progress to plaques or nodules with possible visceral involvement. Kaposi sarcoma is classified into 4 subtypes based on epidemiology and clinical presentation: classic, endemic, iatrogenic, and AIDS associated.1,2
Classic KS primarily affects elderly males of Mediterranean or Eastern European descent, with a mean age of 64.1 years and a male to female ratio of 3 to 1. It has an indolent course and a strong predilection for the skin of the lower extremities. The endemic form occurs mainly in Africa and has a more aggressive course, especially the lymphadenopathic type that affects children younger than 10 years.3 Iatrogenic KS develops in immunosuppressed patients, such as transplant recipients, and may regress if the immunosuppressive agent is stopped.1 Kaposi sarcoma is an AIDS-defining illness and is the most common malignancy in AIDS patients. It is strongly associated with a low CD4 count, which accounts for the notable decline in its incidence after the widespread introduction of HAART.1 Among HIV patients, KS has the highest incidence in men who have sex with men. This population has a higher seroprevalence of HHV-8, which suggests possible sexual transmission of HHV-8. AIDS-associated KS most commonly involves the lower extremities, face, and oral mucosa. It may have visceral involvement, particularly of the gastrointestinal and respiratory systems, which carries a poor prognosis.4,5
Approximately 40% of patients presenting with KS have gastrointestinal tract involvement.6 Of these patients, up to 80% are asymptomatic, with diagnosis usually being made on endoscopy.7 In contrast, pulmonary KS is less common and typically is symptomatic. It can involve the lung parenchyma, airways, or pleura and is diagnosed by chest radiography or CT scans. Glucocorticoid therapy is a known trigger for pulmonary KS exacerbation.8
All 4 subtypes share the same histopathologic findings consisting of spindled endothelial cell proliferation, inflammation, and angiogenesis. Immunohistochemistry reveals tumor cells that are CD34 and CD31 positive but are factor VIII negative. Staining for HHV-8 antigen is used to confirm the diagnosis. The inflammatory infiltrate predominantly is lymphocytic with scattered plasma cells.9
The laboratory results and histopathologic findings clearly indicated a diagnosis of KS in our patient. Other entities in the clinical differential would have shown notably different histopathologic findings and laboratory results. Lichen planus pigmentosus displays a lichenoid infiltrate and pigment dropout on histology. Histologic findings of psoriasis include psoriasiform acanthosis, dilated vessels in the dermal papillae, thinning of suprapapillary plates, and neutrophilic microabscesses. Sarcoidosis would demonstrate naked granulomas on histopathology. Syphilis displays variable but often psoriasiform or lichenoid findings on histology, and a positive rapid plasma reagin also would be noted.
First-line treatment of AIDS-related KS is HAART. For patients with severe and rapidly progressive KS or with visceral involvement, cytotoxic chemotherapy with doxorubicin or taxanes often is required. Additional therapies include radiotherapy, topical alitretinoin, and cryotherapy.1,10
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206; quiz 207-208.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:239-250.
- Beral V, Peterman TA, Berkelman RL, et al. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
- Smith NA, Sabin CA, Gopal R, et al. Serologic evidence of human herpesvirus 8 transmission by homosexual but not heterosexual sex. J Infect Dis. 1999;180:600-606.
- Arora M, Goldberg EM. Kaposi sarcoma involving the gastrointestinal tract. Gastroenterol Hepatol (N Y). 2010;6:459-462.
- Parente F, Cernuschi M, Orlando G, et al. Kaposi’s sarcoma and AIDS: frequency of gastrointestinal involvement and its effect on survival. a prospective study in a heterogeneous population. Scand J Gastroenterol. 1991;26:1007-1012.
- Gasparetto TD, Marchiori E, Lourenco S, et al. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis. 2009;4:18.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Regnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
The Diagnosis: Kaposi Sarcoma
On initial presentation, the differential diagnosis included secondary syphilis, Kaposi sarcoma (KS), lichen planus pigmentosus, sarcoidosis, and psoriasis. A laboratory workup was ordered, which included complete blood cell count, comprehensive metabolic panel, antinuclear antibodies, anti-Ro/Sjögren syndrome antigen A and anti-La/Sjögren syndrome antigen B autoantibodies, angiotensin-converting enzyme, rapid plasma reagin, and human immunodeficiency virus (HIV) antibodies. A 4-mm punch biopsy of the rash also was performed from the right upper back. Histology revealed a vascular proliferation that was diffusely positive for human herpesvirus 8 (HHV-8)(Figure 1). The patient was informed of the diagnosis, at which time he revealed he had a history of homosexual relationships, with his last sexual contact being more than 1 year prior to presentation. The laboratory workup confirmed a diagnosis of HIV, and the remainder of the tests were unremarkable.

He was referred to our university's HIV clinic where he was started on highly active antiretroviral therapy (HAART). His facial swelling worsened, leading to hospital admission. Computed tomography (CT) of the chest, abdomen, and pelvis showed diffuse lymphadenopathy and lung nodules concerning for visceral involvement of KS. Hematology and oncology was consulted for further evaluation, and he was treated with 6 cycles of doxorubicin 20 mg/m2, which led to resolution of the lung nodules on CT and improvement of the rash burden. He was then started on alitretinoin gel 0.1% twice daily, which led to continued slow improvement (Figure 2).

Kaposi sarcoma is a vascular neoplasm that occurs from infection with HHV-8. It typically presents as painless, reddish to violaceous macules or patches involving the skin and mucosa that often progress to plaques or nodules with possible visceral involvement. Kaposi sarcoma is classified into 4 subtypes based on epidemiology and clinical presentation: classic, endemic, iatrogenic, and AIDS associated.1,2
Classic KS primarily affects elderly males of Mediterranean or Eastern European descent, with a mean age of 64.1 years and a male to female ratio of 3 to 1. It has an indolent course and a strong predilection for the skin of the lower extremities. The endemic form occurs mainly in Africa and has a more aggressive course, especially the lymphadenopathic type that affects children younger than 10 years.3 Iatrogenic KS develops in immunosuppressed patients, such as transplant recipients, and may regress if the immunosuppressive agent is stopped.1 Kaposi sarcoma is an AIDS-defining illness and is the most common malignancy in AIDS patients. It is strongly associated with a low CD4 count, which accounts for the notable decline in its incidence after the widespread introduction of HAART.1 Among HIV patients, KS has the highest incidence in men who have sex with men. This population has a higher seroprevalence of HHV-8, which suggests possible sexual transmission of HHV-8. AIDS-associated KS most commonly involves the lower extremities, face, and oral mucosa. It may have visceral involvement, particularly of the gastrointestinal and respiratory systems, which carries a poor prognosis.4,5
Approximately 40% of patients presenting with KS have gastrointestinal tract involvement.6 Of these patients, up to 80% are asymptomatic, with diagnosis usually being made on endoscopy.7 In contrast, pulmonary KS is less common and typically is symptomatic. It can involve the lung parenchyma, airways, or pleura and is diagnosed by chest radiography or CT scans. Glucocorticoid therapy is a known trigger for pulmonary KS exacerbation.8
All 4 subtypes share the same histopathologic findings consisting of spindled endothelial cell proliferation, inflammation, and angiogenesis. Immunohistochemistry reveals tumor cells that are CD34 and CD31 positive but are factor VIII negative. Staining for HHV-8 antigen is used to confirm the diagnosis. The inflammatory infiltrate predominantly is lymphocytic with scattered plasma cells.9
The laboratory results and histopathologic findings clearly indicated a diagnosis of KS in our patient. Other entities in the clinical differential would have shown notably different histopathologic findings and laboratory results. Lichen planus pigmentosus displays a lichenoid infiltrate and pigment dropout on histology. Histologic findings of psoriasis include psoriasiform acanthosis, dilated vessels in the dermal papillae, thinning of suprapapillary plates, and neutrophilic microabscesses. Sarcoidosis would demonstrate naked granulomas on histopathology. Syphilis displays variable but often psoriasiform or lichenoid findings on histology, and a positive rapid plasma reagin also would be noted.
First-line treatment of AIDS-related KS is HAART. For patients with severe and rapidly progressive KS or with visceral involvement, cytotoxic chemotherapy with doxorubicin or taxanes often is required. Additional therapies include radiotherapy, topical alitretinoin, and cryotherapy.1,10
The Diagnosis: Kaposi Sarcoma
On initial presentation, the differential diagnosis included secondary syphilis, Kaposi sarcoma (KS), lichen planus pigmentosus, sarcoidosis, and psoriasis. A laboratory workup was ordered, which included complete blood cell count, comprehensive metabolic panel, antinuclear antibodies, anti-Ro/Sjögren syndrome antigen A and anti-La/Sjögren syndrome antigen B autoantibodies, angiotensin-converting enzyme, rapid plasma reagin, and human immunodeficiency virus (HIV) antibodies. A 4-mm punch biopsy of the rash also was performed from the right upper back. Histology revealed a vascular proliferation that was diffusely positive for human herpesvirus 8 (HHV-8)(Figure 1). The patient was informed of the diagnosis, at which time he revealed he had a history of homosexual relationships, with his last sexual contact being more than 1 year prior to presentation. The laboratory workup confirmed a diagnosis of HIV, and the remainder of the tests were unremarkable.

He was referred to our university's HIV clinic where he was started on highly active antiretroviral therapy (HAART). His facial swelling worsened, leading to hospital admission. Computed tomography (CT) of the chest, abdomen, and pelvis showed diffuse lymphadenopathy and lung nodules concerning for visceral involvement of KS. Hematology and oncology was consulted for further evaluation, and he was treated with 6 cycles of doxorubicin 20 mg/m2, which led to resolution of the lung nodules on CT and improvement of the rash burden. He was then started on alitretinoin gel 0.1% twice daily, which led to continued slow improvement (Figure 2).

Kaposi sarcoma is a vascular neoplasm that occurs from infection with HHV-8. It typically presents as painless, reddish to violaceous macules or patches involving the skin and mucosa that often progress to plaques or nodules with possible visceral involvement. Kaposi sarcoma is classified into 4 subtypes based on epidemiology and clinical presentation: classic, endemic, iatrogenic, and AIDS associated.1,2
Classic KS primarily affects elderly males of Mediterranean or Eastern European descent, with a mean age of 64.1 years and a male to female ratio of 3 to 1. It has an indolent course and a strong predilection for the skin of the lower extremities. The endemic form occurs mainly in Africa and has a more aggressive course, especially the lymphadenopathic type that affects children younger than 10 years.3 Iatrogenic KS develops in immunosuppressed patients, such as transplant recipients, and may regress if the immunosuppressive agent is stopped.1 Kaposi sarcoma is an AIDS-defining illness and is the most common malignancy in AIDS patients. It is strongly associated with a low CD4 count, which accounts for the notable decline in its incidence after the widespread introduction of HAART.1 Among HIV patients, KS has the highest incidence in men who have sex with men. This population has a higher seroprevalence of HHV-8, which suggests possible sexual transmission of HHV-8. AIDS-associated KS most commonly involves the lower extremities, face, and oral mucosa. It may have visceral involvement, particularly of the gastrointestinal and respiratory systems, which carries a poor prognosis.4,5
Approximately 40% of patients presenting with KS have gastrointestinal tract involvement.6 Of these patients, up to 80% are asymptomatic, with diagnosis usually being made on endoscopy.7 In contrast, pulmonary KS is less common and typically is symptomatic. It can involve the lung parenchyma, airways, or pleura and is diagnosed by chest radiography or CT scans. Glucocorticoid therapy is a known trigger for pulmonary KS exacerbation.8
All 4 subtypes share the same histopathologic findings consisting of spindled endothelial cell proliferation, inflammation, and angiogenesis. Immunohistochemistry reveals tumor cells that are CD34 and CD31 positive but are factor VIII negative. Staining for HHV-8 antigen is used to confirm the diagnosis. The inflammatory infiltrate predominantly is lymphocytic with scattered plasma cells.9
The laboratory results and histopathologic findings clearly indicated a diagnosis of KS in our patient. Other entities in the clinical differential would have shown notably different histopathologic findings and laboratory results. Lichen planus pigmentosus displays a lichenoid infiltrate and pigment dropout on histology. Histologic findings of psoriasis include psoriasiform acanthosis, dilated vessels in the dermal papillae, thinning of suprapapillary plates, and neutrophilic microabscesses. Sarcoidosis would demonstrate naked granulomas on histopathology. Syphilis displays variable but often psoriasiform or lichenoid findings on histology, and a positive rapid plasma reagin also would be noted.
First-line treatment of AIDS-related KS is HAART. For patients with severe and rapidly progressive KS or with visceral involvement, cytotoxic chemotherapy with doxorubicin or taxanes often is required. Additional therapies include radiotherapy, topical alitretinoin, and cryotherapy.1,10
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206; quiz 207-208.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:239-250.
- Beral V, Peterman TA, Berkelman RL, et al. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
- Smith NA, Sabin CA, Gopal R, et al. Serologic evidence of human herpesvirus 8 transmission by homosexual but not heterosexual sex. J Infect Dis. 1999;180:600-606.
- Arora M, Goldberg EM. Kaposi sarcoma involving the gastrointestinal tract. Gastroenterol Hepatol (N Y). 2010;6:459-462.
- Parente F, Cernuschi M, Orlando G, et al. Kaposi’s sarcoma and AIDS: frequency of gastrointestinal involvement and its effect on survival. a prospective study in a heterogeneous population. Scand J Gastroenterol. 1991;26:1007-1012.
- Gasparetto TD, Marchiori E, Lourenco S, et al. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis. 2009;4:18.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Regnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206; quiz 207-208.
- Mohanna S, Maco V, Bravo F, et al. Epidemiology and clinical characteristics of classic Kaposi’s sarcoma, seroprevalence, and variants of human herpesvirus 8 in South America: a critical review of an old disease. Int J Infect Dis. 2005;9:239-250.
- Beral V, Peterman TA, Berkelman RL, et al. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
- Smith NA, Sabin CA, Gopal R, et al. Serologic evidence of human herpesvirus 8 transmission by homosexual but not heterosexual sex. J Infect Dis. 1999;180:600-606.
- Arora M, Goldberg EM. Kaposi sarcoma involving the gastrointestinal tract. Gastroenterol Hepatol (N Y). 2010;6:459-462.
- Parente F, Cernuschi M, Orlando G, et al. Kaposi’s sarcoma and AIDS: frequency of gastrointestinal involvement and its effect on survival. a prospective study in a heterogeneous population. Scand J Gastroenterol. 1991;26:1007-1012.
- Gasparetto TD, Marchiori E, Lourenco S, et al. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis. 2009;4:18.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Regnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
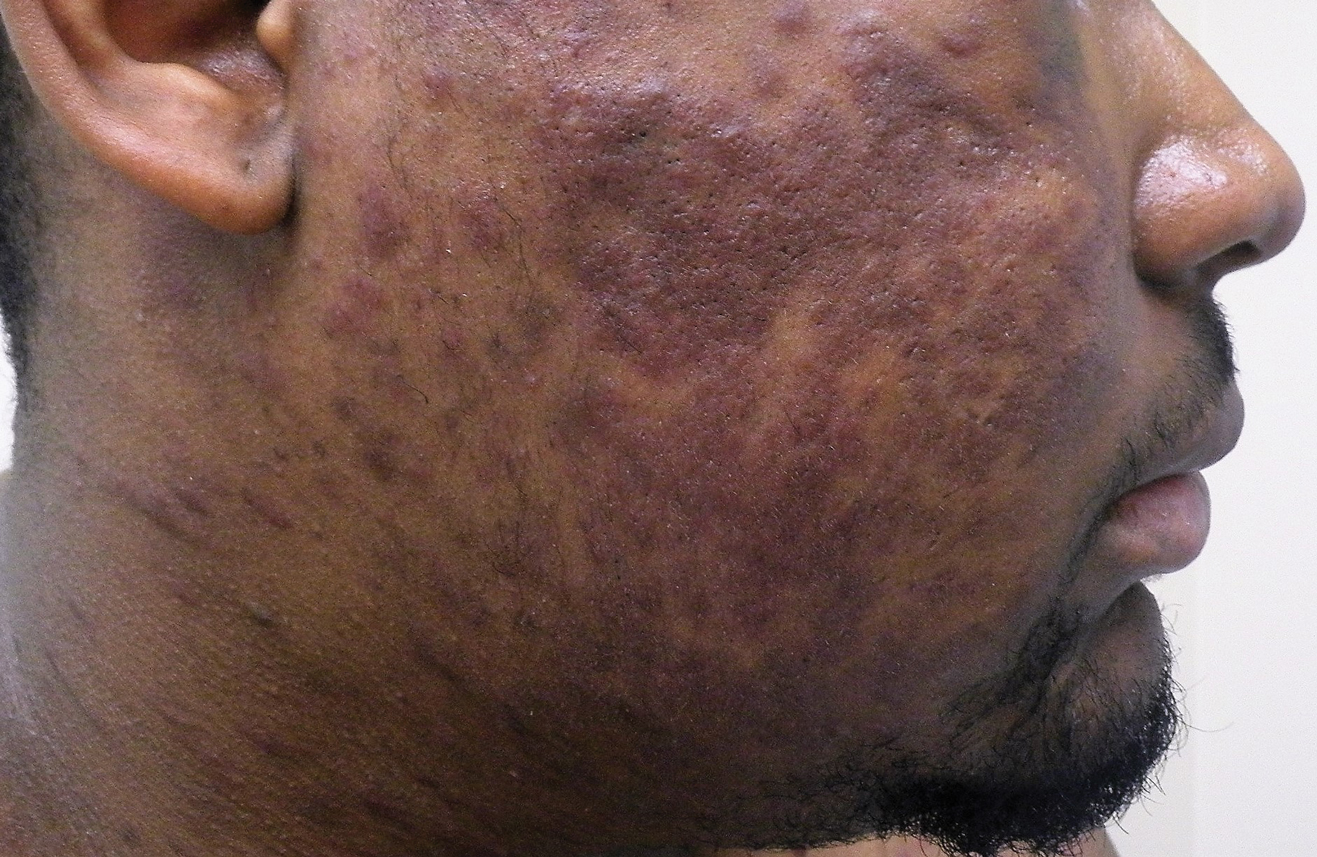
A 24-year-old Black man presented for evaluation of an asymptomatic rash on the face, chest, back, and arms that had been progressively spreading over the course of 3 months. He had some swelling of the lips prior to the onset of the rash and was prescribed prednisone 10 mg daily by an outside physician. He had no known medical problems and was taking no medications. Physical examination revealed numerous violaceous plaques scattered symmetrically on the trunk, arms, legs, and face. His family history was negative for autoimmune disease, and a review of systems was unremarkable. He denied any recent sexual contacts.
Umbilicated Keratotic Papule on the Scalp
The Diagnosis: Warty Dyskeratoma
Warty dyskeratoma (WD) is a benign cutaneous tumor that was first described in 1954 as isolated Darier disease (DD). In 1957, Szymanski1 renamed it warty dyskeratoma as a distinct condition from DD. Warty dyskeratoma typically presents as a flesh-colored to brownish, round, well-demarcated, and slightly elevated papule or nodule accompanied by an umbilical invagination at the center. It most commonly arises on the scalp, face, or neck.2 In contrast to DD, familial occurrence is uncommon. It usually is difficult to distinguish WD from other conditions such as seborrheic keratosis, verruca vulgaris, or keratoacanthoma due to its macroscopic features. Therefore, histopathologic investigation is necessary for a precise diagnosis.
In our case, histologic investigation revealed a symmetric cup-shaped invagination filled with acantholytic and dyskeratotic keratinocytes with no atypia or mitotic figures (Figure, A). The bottom of the invagination was occupied with numerous villi covered by a single layer of basal cells (Figure, B). At the edge of the invagination, corps ronds and grains were observed in the granular and cornified layers, respectively (Figure, C).
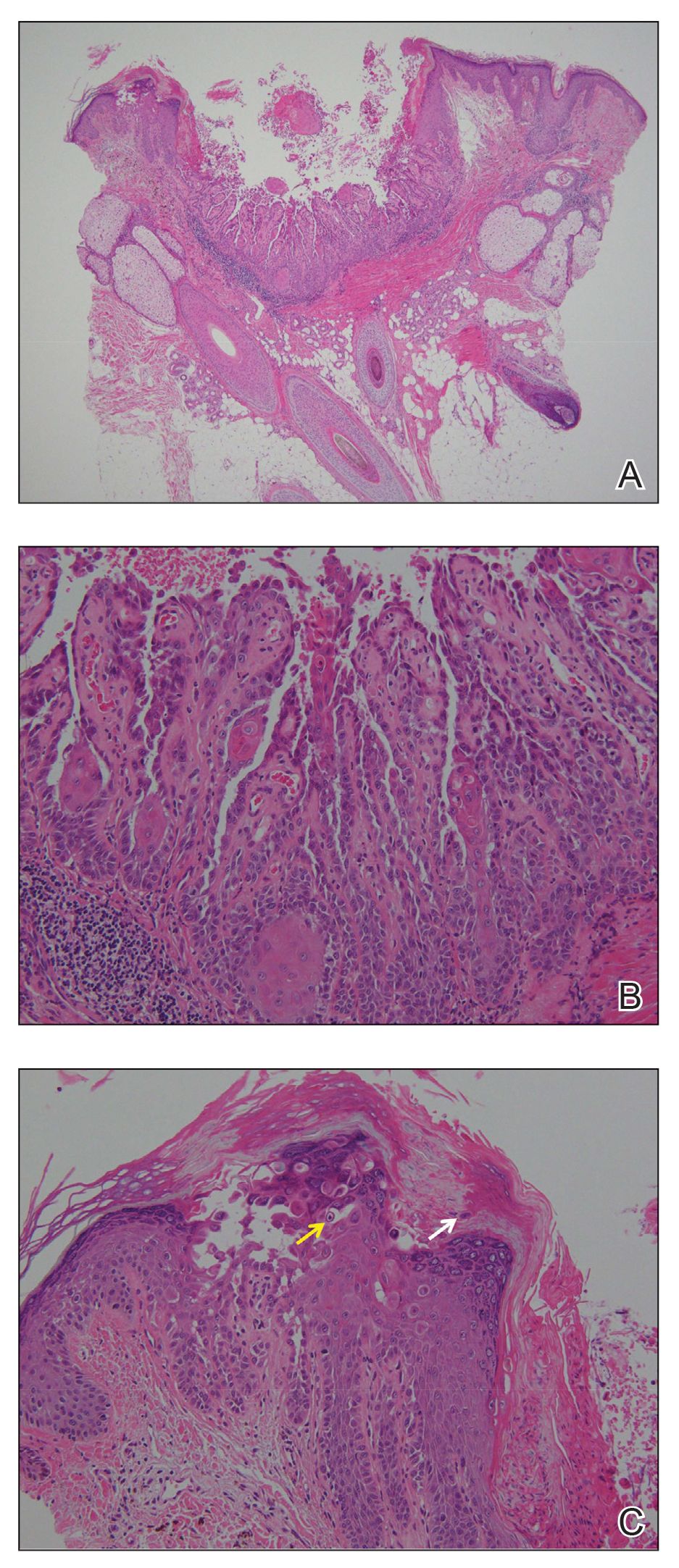
The hallmark histopathologic findings are acantholysis and dyskeratosis just above the basal cell layer, called focal acantholytic dyskeratosis. The differential diagnosis includes other disorders associated with focal acantholytic dyskeratosis, such as DD and acantholytic squamous cell carcinoma.3 Distinguishing WD from DD may be difficult in rare cases with multiple lesions.4 In such cases, an autosomal-dominant inheritance pattern and younger age of onset should prompt clinicians to seek for mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene, ATP2A2, for the diagnosis of DD.5 Additionally, the presence of atypia or mitotic figures will rule out malignant disorders such as squamous cell carcinoma.
Although the pathogenesis of WD is not fully understood, most clinicians consider it a follicular adnexal neoplasm because the lesions often are connected to the pilosebaceous unit on microscopic observation.6 Although WD-like lesions arising from the oral mucosa have been reported,7 their etiology may be different from WD because the oral mucosa lacks hair follicles.8 The term warty leads to speculation of the contribution of human papillomavirus to the pathogenesis of WD, but this has been questioned due to the negative result of viral DNA detection from WD lesions by polymerase chain reaction analysis.2 Therefore, the term follicular dyskeratoma has been suggested as a novel denomination that reflects its etiology more precisely.2
The efficacy of topical treatment has not yet been established. Cryosurgery is another therapeutic option, but it sometimes fails.9 As performed in our patient, excisional biopsy is the most reasonable treatment option to obtain both complete removal and precise diagnosis.
The Diagnosis: Warty Dyskeratoma
Warty dyskeratoma (WD) is a benign cutaneous tumor that was first described in 1954 as isolated Darier disease (DD). In 1957, Szymanski1 renamed it warty dyskeratoma as a distinct condition from DD. Warty dyskeratoma typically presents as a flesh-colored to brownish, round, well-demarcated, and slightly elevated papule or nodule accompanied by an umbilical invagination at the center. It most commonly arises on the scalp, face, or neck.2 In contrast to DD, familial occurrence is uncommon. It usually is difficult to distinguish WD from other conditions such as seborrheic keratosis, verruca vulgaris, or keratoacanthoma due to its macroscopic features. Therefore, histopathologic investigation is necessary for a precise diagnosis.
In our case, histologic investigation revealed a symmetric cup-shaped invagination filled with acantholytic and dyskeratotic keratinocytes with no atypia or mitotic figures (Figure, A). The bottom of the invagination was occupied with numerous villi covered by a single layer of basal cells (Figure, B). At the edge of the invagination, corps ronds and grains were observed in the granular and cornified layers, respectively (Figure, C).

The hallmark histopathologic findings are acantholysis and dyskeratosis just above the basal cell layer, called focal acantholytic dyskeratosis. The differential diagnosis includes other disorders associated with focal acantholytic dyskeratosis, such as DD and acantholytic squamous cell carcinoma.3 Distinguishing WD from DD may be difficult in rare cases with multiple lesions.4 In such cases, an autosomal-dominant inheritance pattern and younger age of onset should prompt clinicians to seek for mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene, ATP2A2, for the diagnosis of DD.5 Additionally, the presence of atypia or mitotic figures will rule out malignant disorders such as squamous cell carcinoma.
Although the pathogenesis of WD is not fully understood, most clinicians consider it a follicular adnexal neoplasm because the lesions often are connected to the pilosebaceous unit on microscopic observation.6 Although WD-like lesions arising from the oral mucosa have been reported,7 their etiology may be different from WD because the oral mucosa lacks hair follicles.8 The term warty leads to speculation of the contribution of human papillomavirus to the pathogenesis of WD, but this has been questioned due to the negative result of viral DNA detection from WD lesions by polymerase chain reaction analysis.2 Therefore, the term follicular dyskeratoma has been suggested as a novel denomination that reflects its etiology more precisely.2
The efficacy of topical treatment has not yet been established. Cryosurgery is another therapeutic option, but it sometimes fails.9 As performed in our patient, excisional biopsy is the most reasonable treatment option to obtain both complete removal and precise diagnosis.
The Diagnosis: Warty Dyskeratoma
Warty dyskeratoma (WD) is a benign cutaneous tumor that was first described in 1954 as isolated Darier disease (DD). In 1957, Szymanski1 renamed it warty dyskeratoma as a distinct condition from DD. Warty dyskeratoma typically presents as a flesh-colored to brownish, round, well-demarcated, and slightly elevated papule or nodule accompanied by an umbilical invagination at the center. It most commonly arises on the scalp, face, or neck.2 In contrast to DD, familial occurrence is uncommon. It usually is difficult to distinguish WD from other conditions such as seborrheic keratosis, verruca vulgaris, or keratoacanthoma due to its macroscopic features. Therefore, histopathologic investigation is necessary for a precise diagnosis.
In our case, histologic investigation revealed a symmetric cup-shaped invagination filled with acantholytic and dyskeratotic keratinocytes with no atypia or mitotic figures (Figure, A). The bottom of the invagination was occupied with numerous villi covered by a single layer of basal cells (Figure, B). At the edge of the invagination, corps ronds and grains were observed in the granular and cornified layers, respectively (Figure, C).

The hallmark histopathologic findings are acantholysis and dyskeratosis just above the basal cell layer, called focal acantholytic dyskeratosis. The differential diagnosis includes other disorders associated with focal acantholytic dyskeratosis, such as DD and acantholytic squamous cell carcinoma.3 Distinguishing WD from DD may be difficult in rare cases with multiple lesions.4 In such cases, an autosomal-dominant inheritance pattern and younger age of onset should prompt clinicians to seek for mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene, ATP2A2, for the diagnosis of DD.5 Additionally, the presence of atypia or mitotic figures will rule out malignant disorders such as squamous cell carcinoma.
Although the pathogenesis of WD is not fully understood, most clinicians consider it a follicular adnexal neoplasm because the lesions often are connected to the pilosebaceous unit on microscopic observation.6 Although WD-like lesions arising from the oral mucosa have been reported,7 their etiology may be different from WD because the oral mucosa lacks hair follicles.8 The term warty leads to speculation of the contribution of human papillomavirus to the pathogenesis of WD, but this has been questioned due to the negative result of viral DNA detection from WD lesions by polymerase chain reaction analysis.2 Therefore, the term follicular dyskeratoma has been suggested as a novel denomination that reflects its etiology more precisely.2
The efficacy of topical treatment has not yet been established. Cryosurgery is another therapeutic option, but it sometimes fails.9 As performed in our patient, excisional biopsy is the most reasonable treatment option to obtain both complete removal and precise diagnosis.
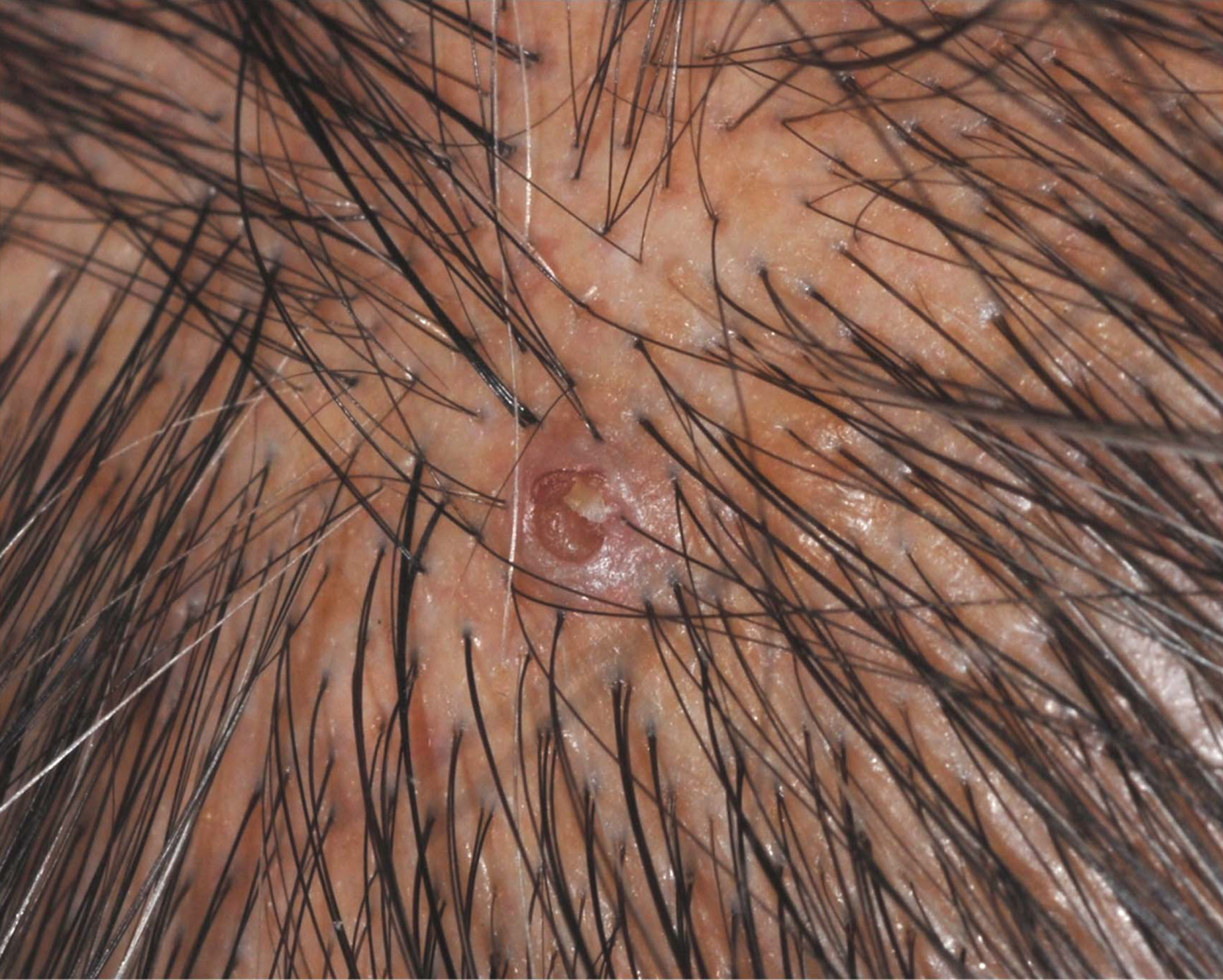
A 72-year-old man was referred to our dermatology clinic for evaluation of a solitary papule on the scalp measuring 3.2 mm in diameter with a keratotic umbilicated center of 1 year’s duration. His medical history included acute appendicitis. Treatment with fusidic acid ointment 2% was unsuccessful. The papule was hard without tenderness on palpation. An excisional biopsy was performed under local anesthesia.
Herpes Zoster May Be a Marker for COVID-19 Infection During Pregnancy
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.
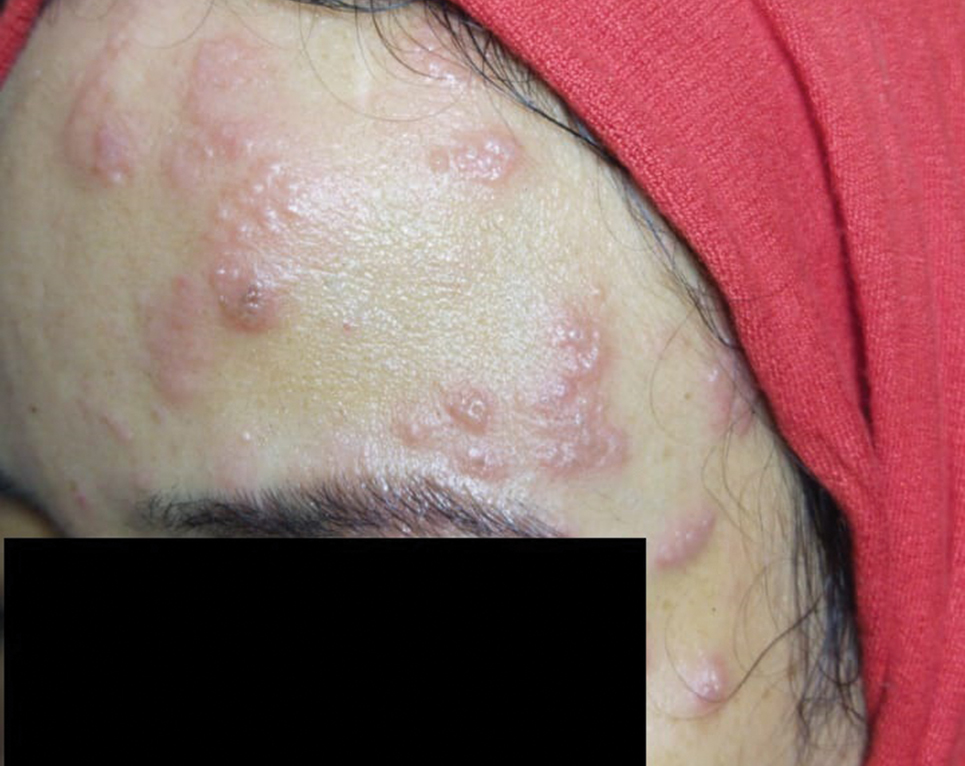
Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.

Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.

Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
Practice Points
- The vesicular rash of coronavirus disease 2019 (COVID-19) has been reported to have different forms of presentation.
- Pregnant women appear to be at increased risk for complications from COVID-19 infection.
- The clinical presentation of herpes zoster should be carefully monitored and reported for further assessment, especially if associated with other signs of COVID-19 infection.
Racial Disparities in Dermatology Training: The Impact on Black Patients
Although physicians commit themselves to providing equitable treatment to all patients, significant disparities remain in the dermatologic care of Black patients, who constitute 13% of the US population, which continues to grow increasingly diverse.1 Despite these changes in the population, the literature demonstrates that dermatologic training does not adequately focus on unique presentations of cutaneous pathology in the Black population.2,3 Accordingly, medical students lack proper training in how skin disorders manifest in people of color. Compounding the problem, only 3% of dermatologists are Black, creating a cultural barrier that can compromise care for Black patients.2,4 Racial disparities in dermatology training can compromise treatment, patient satisfaction, and outcomes.3
Issues in Medical Education Training and Resources
Lack of diversity in the resources used for dermatology training in medical schools affects diagnosis and treatment, as skin manifestations such as hypersensitivity reactions, rashes, and cancer can appear differently on different skin tones.5 A study of medical students’ ability to diagnose common dermatologic pathologies found that when trainees were presented with photographs of dark skin, their accuracy in identifying urticaria, squamous cell carcinoma, and even atopic dermatitis was reduced, despite these diseases being more prevalent in children of African American ancestry.4,6
Dermatologic diseases also can have different distributions in different races; for example, on non–sun-exposed sites, squamous cell carcinoma in Black patients occurs at 8.5 times the frequency of White patients.7 Failure to identify diseases accurately due to insufficient training can have grave consequences for patients. Although skin cancer is less common in individuals with skin of color, it is associated with greater morbidity and mortality, in part due to delayed diagnosis.7
Inadequate research, reporting, and instruction on dermatologic findings in patients with darker complexions further compound racial disparities in dermatology. A 2006 study of the representation of darker skin in major dermatology educational resources found that only 2% of teaching events at American Academy of Dermatology annual meetings focused on skin of color. Furthermore, the study determined that many common diseases in patients with dark skin, such as acne vulgaris and pityriasis rosea, were completely absent or limited in dermatology textbooks.8
Impact on the Black Patient Experience
Patients’ therapeutic relationship with their physician also is damaged by limitations in training in diverse skin color. A study that assessed Black patients seen in a skin of color clinic (SOCC) compared to Black patients seen in a non-SOCC found that non-SOCC patients reported a lower degree of respect, dignity, understanding, and trust compared to the patients seen in a SOCC. Black patients expressed specific concerns about non-SOCC dermatologists’ knowledge of abnormalities that present in darker skin and Black hair.3 These findings are compounded by reports suggesting that, independent of care, structural racism contributes to dermatologic disease severity by influencing patient education level, household income, and degree of exposure to harmful environmental irritants.6
Racial disparities continue to be seen in the makeup of the universe of dermatologists and skin researchers. As of 2016, only 3% of dermatologists were Black, making dermatology one of the least diverse medical specialties.2 Increasing the diversity of the dermatology workforce is important to improve patient satisfaction and treatment, both for minority and nonminority patients. Compared to race-discordant medical visits, race-concordant visits were shown to have a higher rate of satisfaction and better shared decision-making.9 Also, minority physicians are more likely to practice health care in areas that are traditionally underserved and to care for patients who do not have health insurance, making their participation essential in addressing some of the baseline disparities Black patients face in securing quality dermatologic care.1
Structural Racism in Medicine
Changing dermatology training to ensure improved treatment of Black patients requires not only increased attention to differences in disease presentation but also heightened awareness of underlying genetic, environmental, and structural factors that contribute to the disease course.6 For example, there is evidence suggesting that structural racism in the form of residential segregation, lower socioeconomic status, and lower educational attainment contribute to disease severity in conditions such as atopic dermatitis. There is additional evidence suggesting that White patients are more readily offered therapeutic options than Black patients. A study of racial disparities in psoriasis treatment found that Black patients with moderate to severe psoriasis were 70% less likely to receive treatment with a biologic than White patients, independent of socioeconomic factors, comorbidities, and insurance plans.10
Moving Forward
Although research continues to underscore racial disparities in dermatology, some leaders in the field are actively combating these problems. A recent study that looked at representations of dark skin images in medical educational resources found far greater representation of dark pigmented skin in web-based resources than in traditional printed texts. Specifically, the online resource VisualDx (https://www.visualdx.com/) features 28.5% dark skin images compared to 10.3% (on average) in printed dermatology books.11 There also is increasing public awareness of these issues, with organizations such as the Skin of Color Society (http://skinofcolorsociety.org/) helping to promote interest in racial disparities in dermatology. Physicians also have created textbooks and social media accounts focused on dermatologic manifestations in skin of color.12 The Instagram account Brown Skin Matters (@brownskinmatters) has created a publicly accessible online resource where physicians and patients can see and post dermatologic diseases in skin of color.5
Final Thoughts
It is critical that physicians be trained to identify skin and hair manifestations of disease and disorders in Black patients. Training can be improved by including more images of skin manifestations in dark skin, both in medical school curricula and in new editions of dermatology textbooks. Training also must teach students about hair in Black individuals and how to properly treat it as well as related conditions of the hair and scalp.13 More research also is needed to better understand how dermatologists can improve the patient experience for Black patients. Residency programs must work to increase diversity among dermatology trainees.
Lastly, dermatology education should increasingly be supplemented with newer, web-based resources that show dermatologic manifestations across the spectrum of skin tones. Dermatology training must be adapted to better account for diverse patient populations and increase its focus on the systems that produce baseline disparities in disease morbidity and mortality.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Gallegos A. Dermatology lacks diversity. Dermatology News. June 1, 2016. Accessed November 18, 2020. https://www.mdedge.com/dermatology/article/108920/practice-management/dermatology-lacks-diversity.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958.
- Prichep D. Diagnostic gaps: skin comes in many shades and so do rashes. NPR website. November 14, 2019. Accessed November 19, 2020. https://www.npr.org/sections/health-shots/2019/11/04/774910915/diagnostic-gaps-skin-comes-in-many-shades-and-so-do-rashes.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Rabin RC. Dermatology has a problem with skin color. The New York Times. August 30, 2020. http://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed November 19, 2020.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80.
Although physicians commit themselves to providing equitable treatment to all patients, significant disparities remain in the dermatologic care of Black patients, who constitute 13% of the US population, which continues to grow increasingly diverse.1 Despite these changes in the population, the literature demonstrates that dermatologic training does not adequately focus on unique presentations of cutaneous pathology in the Black population.2,3 Accordingly, medical students lack proper training in how skin disorders manifest in people of color. Compounding the problem, only 3% of dermatologists are Black, creating a cultural barrier that can compromise care for Black patients.2,4 Racial disparities in dermatology training can compromise treatment, patient satisfaction, and outcomes.3
Issues in Medical Education Training and Resources
Lack of diversity in the resources used for dermatology training in medical schools affects diagnosis and treatment, as skin manifestations such as hypersensitivity reactions, rashes, and cancer can appear differently on different skin tones.5 A study of medical students’ ability to diagnose common dermatologic pathologies found that when trainees were presented with photographs of dark skin, their accuracy in identifying urticaria, squamous cell carcinoma, and even atopic dermatitis was reduced, despite these diseases being more prevalent in children of African American ancestry.4,6
Dermatologic diseases also can have different distributions in different races; for example, on non–sun-exposed sites, squamous cell carcinoma in Black patients occurs at 8.5 times the frequency of White patients.7 Failure to identify diseases accurately due to insufficient training can have grave consequences for patients. Although skin cancer is less common in individuals with skin of color, it is associated with greater morbidity and mortality, in part due to delayed diagnosis.7
Inadequate research, reporting, and instruction on dermatologic findings in patients with darker complexions further compound racial disparities in dermatology. A 2006 study of the representation of darker skin in major dermatology educational resources found that only 2% of teaching events at American Academy of Dermatology annual meetings focused on skin of color. Furthermore, the study determined that many common diseases in patients with dark skin, such as acne vulgaris and pityriasis rosea, were completely absent or limited in dermatology textbooks.8
Impact on the Black Patient Experience
Patients’ therapeutic relationship with their physician also is damaged by limitations in training in diverse skin color. A study that assessed Black patients seen in a skin of color clinic (SOCC) compared to Black patients seen in a non-SOCC found that non-SOCC patients reported a lower degree of respect, dignity, understanding, and trust compared to the patients seen in a SOCC. Black patients expressed specific concerns about non-SOCC dermatologists’ knowledge of abnormalities that present in darker skin and Black hair.3 These findings are compounded by reports suggesting that, independent of care, structural racism contributes to dermatologic disease severity by influencing patient education level, household income, and degree of exposure to harmful environmental irritants.6
Racial disparities continue to be seen in the makeup of the universe of dermatologists and skin researchers. As of 2016, only 3% of dermatologists were Black, making dermatology one of the least diverse medical specialties.2 Increasing the diversity of the dermatology workforce is important to improve patient satisfaction and treatment, both for minority and nonminority patients. Compared to race-discordant medical visits, race-concordant visits were shown to have a higher rate of satisfaction and better shared decision-making.9 Also, minority physicians are more likely to practice health care in areas that are traditionally underserved and to care for patients who do not have health insurance, making their participation essential in addressing some of the baseline disparities Black patients face in securing quality dermatologic care.1
Structural Racism in Medicine
Changing dermatology training to ensure improved treatment of Black patients requires not only increased attention to differences in disease presentation but also heightened awareness of underlying genetic, environmental, and structural factors that contribute to the disease course.6 For example, there is evidence suggesting that structural racism in the form of residential segregation, lower socioeconomic status, and lower educational attainment contribute to disease severity in conditions such as atopic dermatitis. There is additional evidence suggesting that White patients are more readily offered therapeutic options than Black patients. A study of racial disparities in psoriasis treatment found that Black patients with moderate to severe psoriasis were 70% less likely to receive treatment with a biologic than White patients, independent of socioeconomic factors, comorbidities, and insurance plans.10
Moving Forward
Although research continues to underscore racial disparities in dermatology, some leaders in the field are actively combating these problems. A recent study that looked at representations of dark skin images in medical educational resources found far greater representation of dark pigmented skin in web-based resources than in traditional printed texts. Specifically, the online resource VisualDx (https://www.visualdx.com/) features 28.5% dark skin images compared to 10.3% (on average) in printed dermatology books.11 There also is increasing public awareness of these issues, with organizations such as the Skin of Color Society (http://skinofcolorsociety.org/) helping to promote interest in racial disparities in dermatology. Physicians also have created textbooks and social media accounts focused on dermatologic manifestations in skin of color.12 The Instagram account Brown Skin Matters (@brownskinmatters) has created a publicly accessible online resource where physicians and patients can see and post dermatologic diseases in skin of color.5
Final Thoughts
It is critical that physicians be trained to identify skin and hair manifestations of disease and disorders in Black patients. Training can be improved by including more images of skin manifestations in dark skin, both in medical school curricula and in new editions of dermatology textbooks. Training also must teach students about hair in Black individuals and how to properly treat it as well as related conditions of the hair and scalp.13 More research also is needed to better understand how dermatologists can improve the patient experience for Black patients. Residency programs must work to increase diversity among dermatology trainees.
Lastly, dermatology education should increasingly be supplemented with newer, web-based resources that show dermatologic manifestations across the spectrum of skin tones. Dermatology training must be adapted to better account for diverse patient populations and increase its focus on the systems that produce baseline disparities in disease morbidity and mortality.
Although physicians commit themselves to providing equitable treatment to all patients, significant disparities remain in the dermatologic care of Black patients, who constitute 13% of the US population, which continues to grow increasingly diverse.1 Despite these changes in the population, the literature demonstrates that dermatologic training does not adequately focus on unique presentations of cutaneous pathology in the Black population.2,3 Accordingly, medical students lack proper training in how skin disorders manifest in people of color. Compounding the problem, only 3% of dermatologists are Black, creating a cultural barrier that can compromise care for Black patients.2,4 Racial disparities in dermatology training can compromise treatment, patient satisfaction, and outcomes.3
Issues in Medical Education Training and Resources
Lack of diversity in the resources used for dermatology training in medical schools affects diagnosis and treatment, as skin manifestations such as hypersensitivity reactions, rashes, and cancer can appear differently on different skin tones.5 A study of medical students’ ability to diagnose common dermatologic pathologies found that when trainees were presented with photographs of dark skin, their accuracy in identifying urticaria, squamous cell carcinoma, and even atopic dermatitis was reduced, despite these diseases being more prevalent in children of African American ancestry.4,6
Dermatologic diseases also can have different distributions in different races; for example, on non–sun-exposed sites, squamous cell carcinoma in Black patients occurs at 8.5 times the frequency of White patients.7 Failure to identify diseases accurately due to insufficient training can have grave consequences for patients. Although skin cancer is less common in individuals with skin of color, it is associated with greater morbidity and mortality, in part due to delayed diagnosis.7
Inadequate research, reporting, and instruction on dermatologic findings in patients with darker complexions further compound racial disparities in dermatology. A 2006 study of the representation of darker skin in major dermatology educational resources found that only 2% of teaching events at American Academy of Dermatology annual meetings focused on skin of color. Furthermore, the study determined that many common diseases in patients with dark skin, such as acne vulgaris and pityriasis rosea, were completely absent or limited in dermatology textbooks.8
Impact on the Black Patient Experience
Patients’ therapeutic relationship with their physician also is damaged by limitations in training in diverse skin color. A study that assessed Black patients seen in a skin of color clinic (SOCC) compared to Black patients seen in a non-SOCC found that non-SOCC patients reported a lower degree of respect, dignity, understanding, and trust compared to the patients seen in a SOCC. Black patients expressed specific concerns about non-SOCC dermatologists’ knowledge of abnormalities that present in darker skin and Black hair.3 These findings are compounded by reports suggesting that, independent of care, structural racism contributes to dermatologic disease severity by influencing patient education level, household income, and degree of exposure to harmful environmental irritants.6
Racial disparities continue to be seen in the makeup of the universe of dermatologists and skin researchers. As of 2016, only 3% of dermatologists were Black, making dermatology one of the least diverse medical specialties.2 Increasing the diversity of the dermatology workforce is important to improve patient satisfaction and treatment, both for minority and nonminority patients. Compared to race-discordant medical visits, race-concordant visits were shown to have a higher rate of satisfaction and better shared decision-making.9 Also, minority physicians are more likely to practice health care in areas that are traditionally underserved and to care for patients who do not have health insurance, making their participation essential in addressing some of the baseline disparities Black patients face in securing quality dermatologic care.1
Structural Racism in Medicine
Changing dermatology training to ensure improved treatment of Black patients requires not only increased attention to differences in disease presentation but also heightened awareness of underlying genetic, environmental, and structural factors that contribute to the disease course.6 For example, there is evidence suggesting that structural racism in the form of residential segregation, lower socioeconomic status, and lower educational attainment contribute to disease severity in conditions such as atopic dermatitis. There is additional evidence suggesting that White patients are more readily offered therapeutic options than Black patients. A study of racial disparities in psoriasis treatment found that Black patients with moderate to severe psoriasis were 70% less likely to receive treatment with a biologic than White patients, independent of socioeconomic factors, comorbidities, and insurance plans.10
Moving Forward
Although research continues to underscore racial disparities in dermatology, some leaders in the field are actively combating these problems. A recent study that looked at representations of dark skin images in medical educational resources found far greater representation of dark pigmented skin in web-based resources than in traditional printed texts. Specifically, the online resource VisualDx (https://www.visualdx.com/) features 28.5% dark skin images compared to 10.3% (on average) in printed dermatology books.11 There also is increasing public awareness of these issues, with organizations such as the Skin of Color Society (http://skinofcolorsociety.org/) helping to promote interest in racial disparities in dermatology. Physicians also have created textbooks and social media accounts focused on dermatologic manifestations in skin of color.12 The Instagram account Brown Skin Matters (@brownskinmatters) has created a publicly accessible online resource where physicians and patients can see and post dermatologic diseases in skin of color.5
Final Thoughts
It is critical that physicians be trained to identify skin and hair manifestations of disease and disorders in Black patients. Training can be improved by including more images of skin manifestations in dark skin, both in medical school curricula and in new editions of dermatology textbooks. Training also must teach students about hair in Black individuals and how to properly treat it as well as related conditions of the hair and scalp.13 More research also is needed to better understand how dermatologists can improve the patient experience for Black patients. Residency programs must work to increase diversity among dermatology trainees.
Lastly, dermatology education should increasingly be supplemented with newer, web-based resources that show dermatologic manifestations across the spectrum of skin tones. Dermatology training must be adapted to better account for diverse patient populations and increase its focus on the systems that produce baseline disparities in disease morbidity and mortality.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Gallegos A. Dermatology lacks diversity. Dermatology News. June 1, 2016. Accessed November 18, 2020. https://www.mdedge.com/dermatology/article/108920/practice-management/dermatology-lacks-diversity.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958.
- Prichep D. Diagnostic gaps: skin comes in many shades and so do rashes. NPR website. November 14, 2019. Accessed November 19, 2020. https://www.npr.org/sections/health-shots/2019/11/04/774910915/diagnostic-gaps-skin-comes-in-many-shades-and-so-do-rashes.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Rabin RC. Dermatology has a problem with skin color. The New York Times. August 30, 2020. http://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed November 19, 2020.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Gallegos A. Dermatology lacks diversity. Dermatology News. June 1, 2016. Accessed November 18, 2020. https://www.mdedge.com/dermatology/article/108920/practice-management/dermatology-lacks-diversity.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134.
- Fenton A, Elliott E, Shahbandi A, et al. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J Am Acad Dermatol. 2020;83:957-958.
- Prichep D. Diagnostic gaps: skin comes in many shades and so do rashes. NPR website. November 14, 2019. Accessed November 19, 2020. https://www.npr.org/sections/health-shots/2019/11/04/774910915/diagnostic-gaps-skin-comes-in-many-shades-and-so-do-rashes.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatr Dermatol. 2020;37:142-146.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis [published online June 18, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.041.
- Rabin RC. Dermatology has a problem with skin color. The New York Times. August 30, 2020. http://www.nytimes.com/2020/08/30/health/skin-diseases-black-hispanic.html. Accessed November 19, 2020.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80.
Practice Points
- Dermatologists should be aware of the existing health disparities in dermatology training, including lack of representation among dermatologists, treatment, patient satisfaction, and outcomes.
- Dermatologic diseases can present differently in different skin tones, and current dermatology training does not reflect these differences.
- We must continue to work toward increasing diversity of the dermatology workforce, including a diverse range of skin tones in images used in dermatology training, and teaching trainees how diseases present differently in different skin tones.