User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Palmoplantar Eruption in a Patient With Mercury Poisoning
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
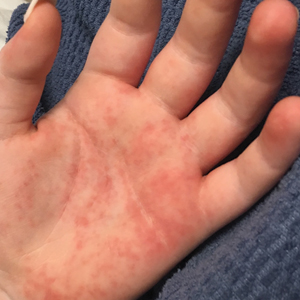
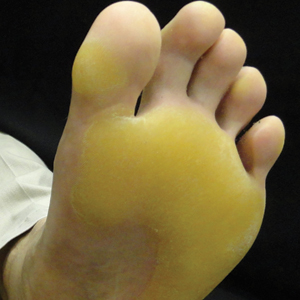
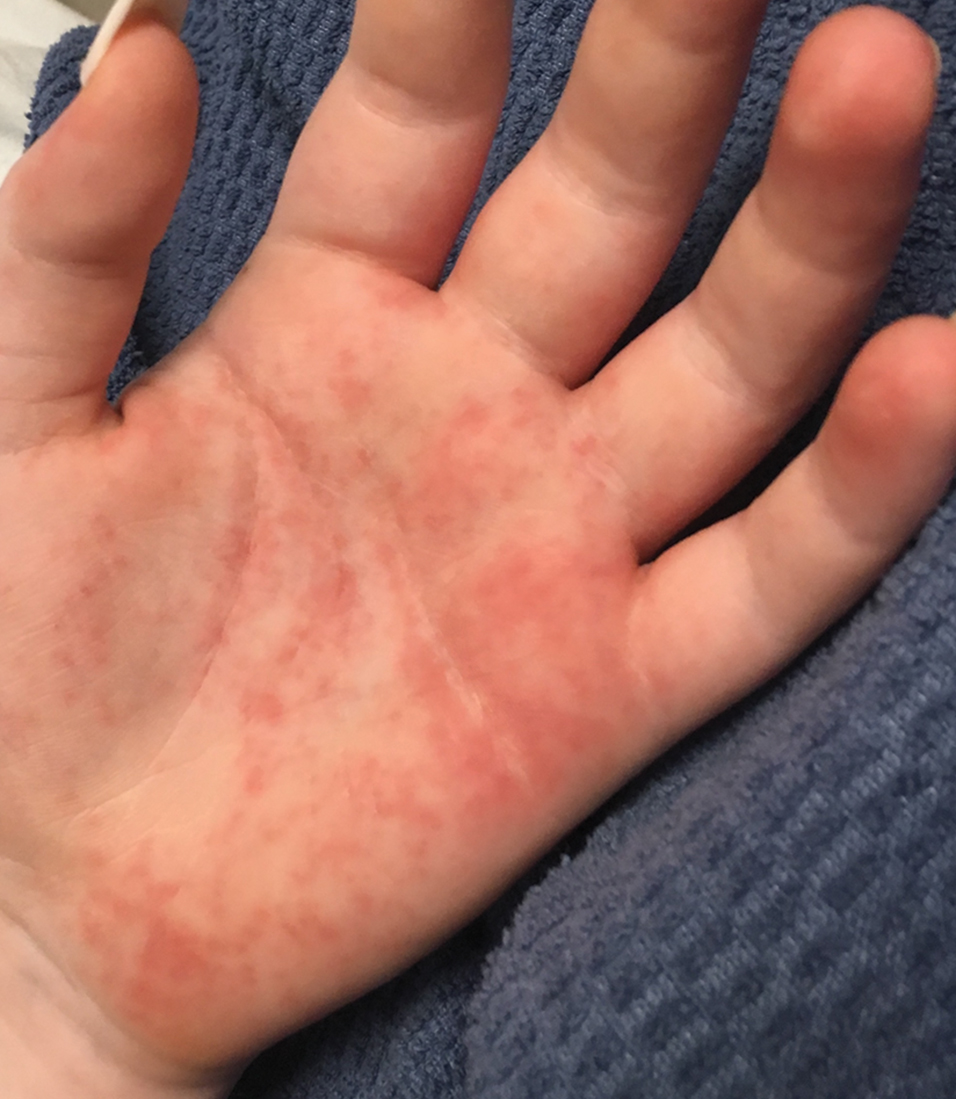
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.
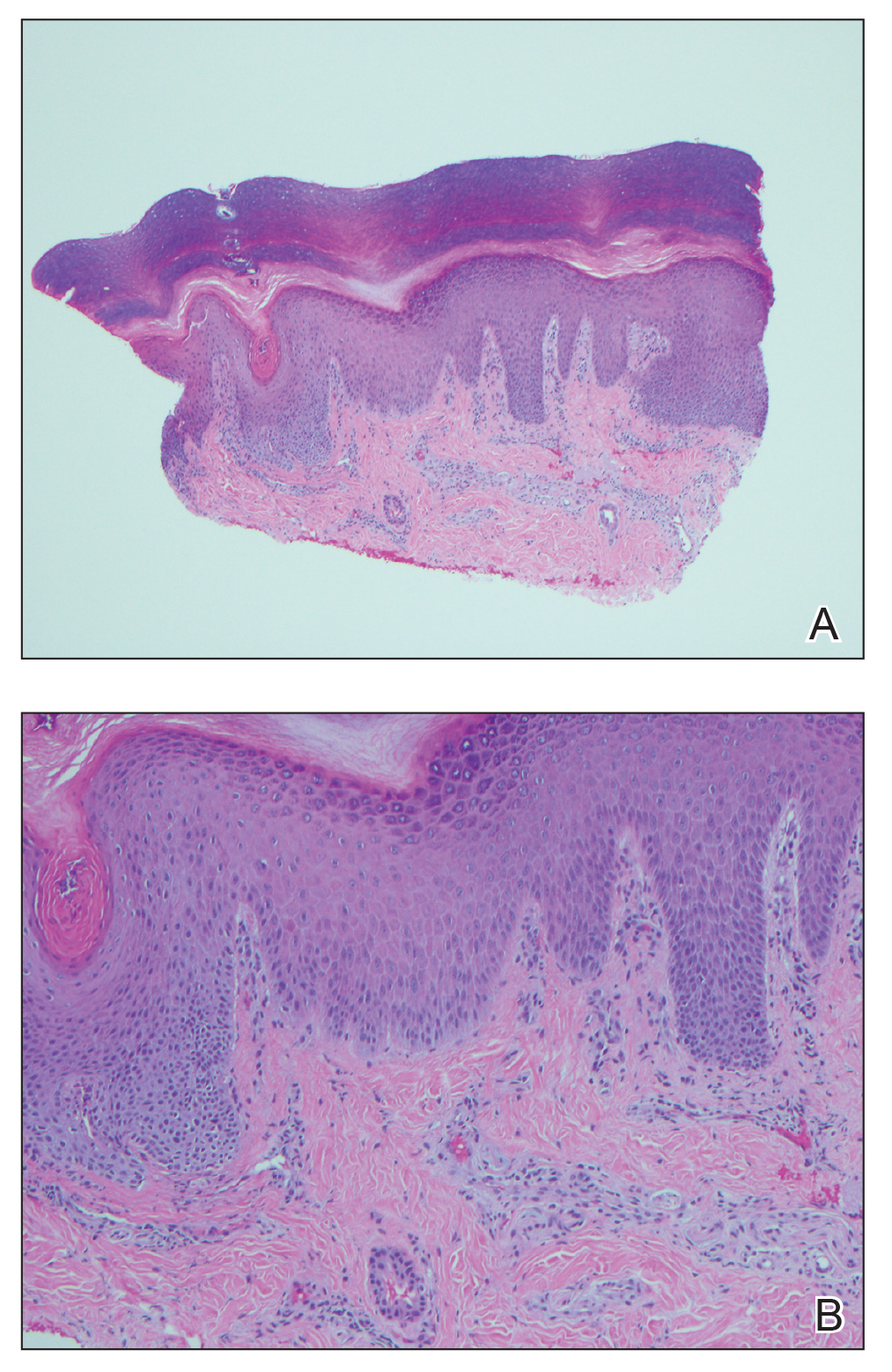
An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.
Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.

An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.

Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.

An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.

Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
Practice Points
- The dermatologic and histologic presentation of mercury exposure may be nonspecific, requiring a high degree of clinical suspicion to make a diagnosis.
- Mercury exposure should be included in the differential diagnosis in patients presenting with a rash of the palms and soles, especially in young patients with systemic symptoms.
What’s Eating You? Human Flea (Pulex irritans)
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.

Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
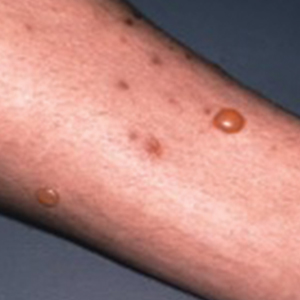
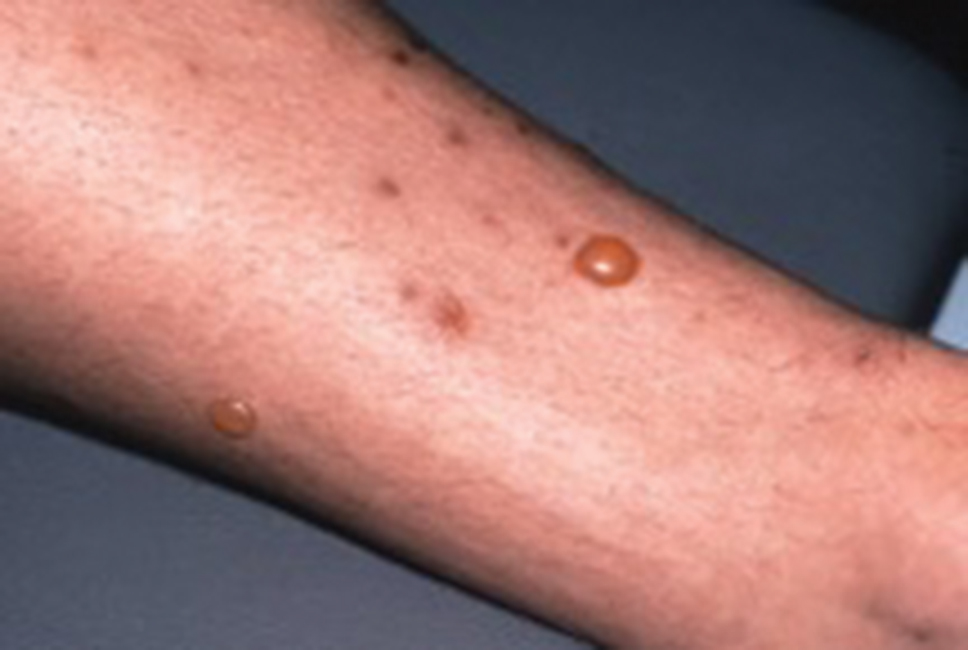
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.

Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.

Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.

Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.

Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Practice Points
- The human flea, Pulex irritans, is a vector for various human diseases including the bubonic plague, bartonellosis, and rickettsioses.
- Presenting symptoms of flea bites include intensely pruritic, urticarial to vesicular papules on exposed areas of skin.
- The primary method of flea control includes a combination of insecticidal products and insect growth regulators.
Intraoperative Tissue Expansion to Allow Primary Linear Closure of 2 Large Adjacent Surgical Defects
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3
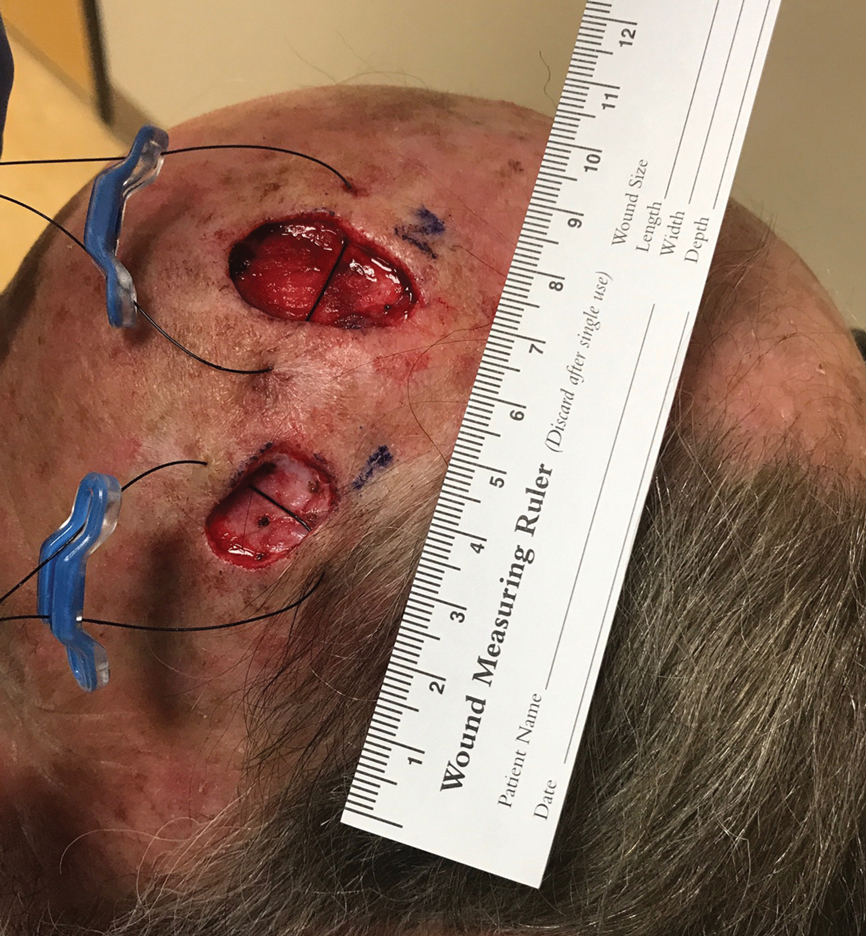
After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.

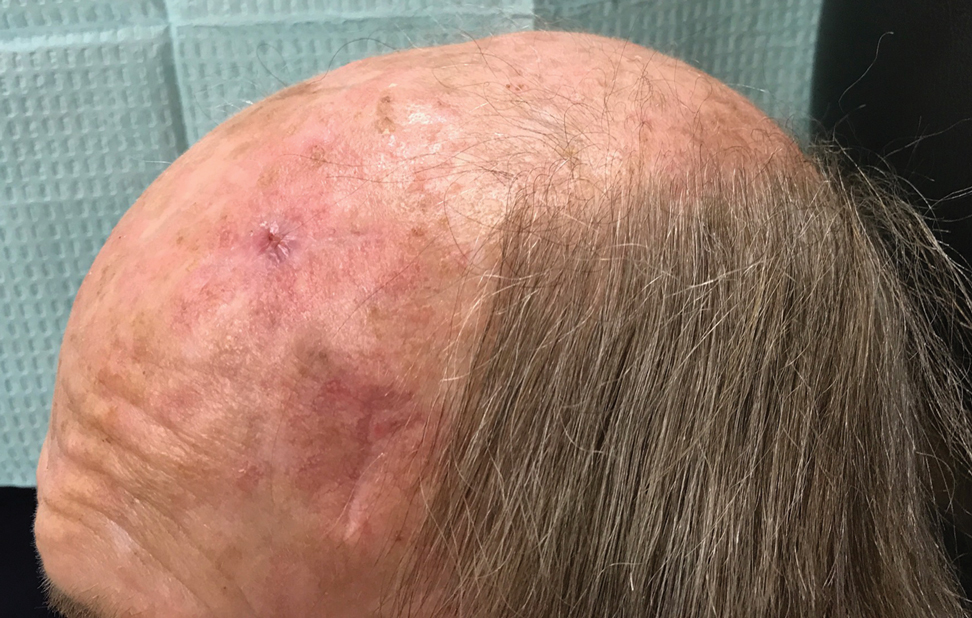
The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).
Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3

After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.

The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).

Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3

After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.

The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).

Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
The Gips Procedure for Pilonidal Disease: A Retrospective Review of Adolescent Patients
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.
Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.

Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.

Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
Practice Points
- The Gips procedure is an easy-to-use outpatient procedure for adolescents with pilonidal disease.
- This procedure has a high success rate and does not restrict school or social activities.
Biologics in Pediatric Psoriasis and Atopic Dermatitis: Revolutionizing the Treatment Landscape
Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.
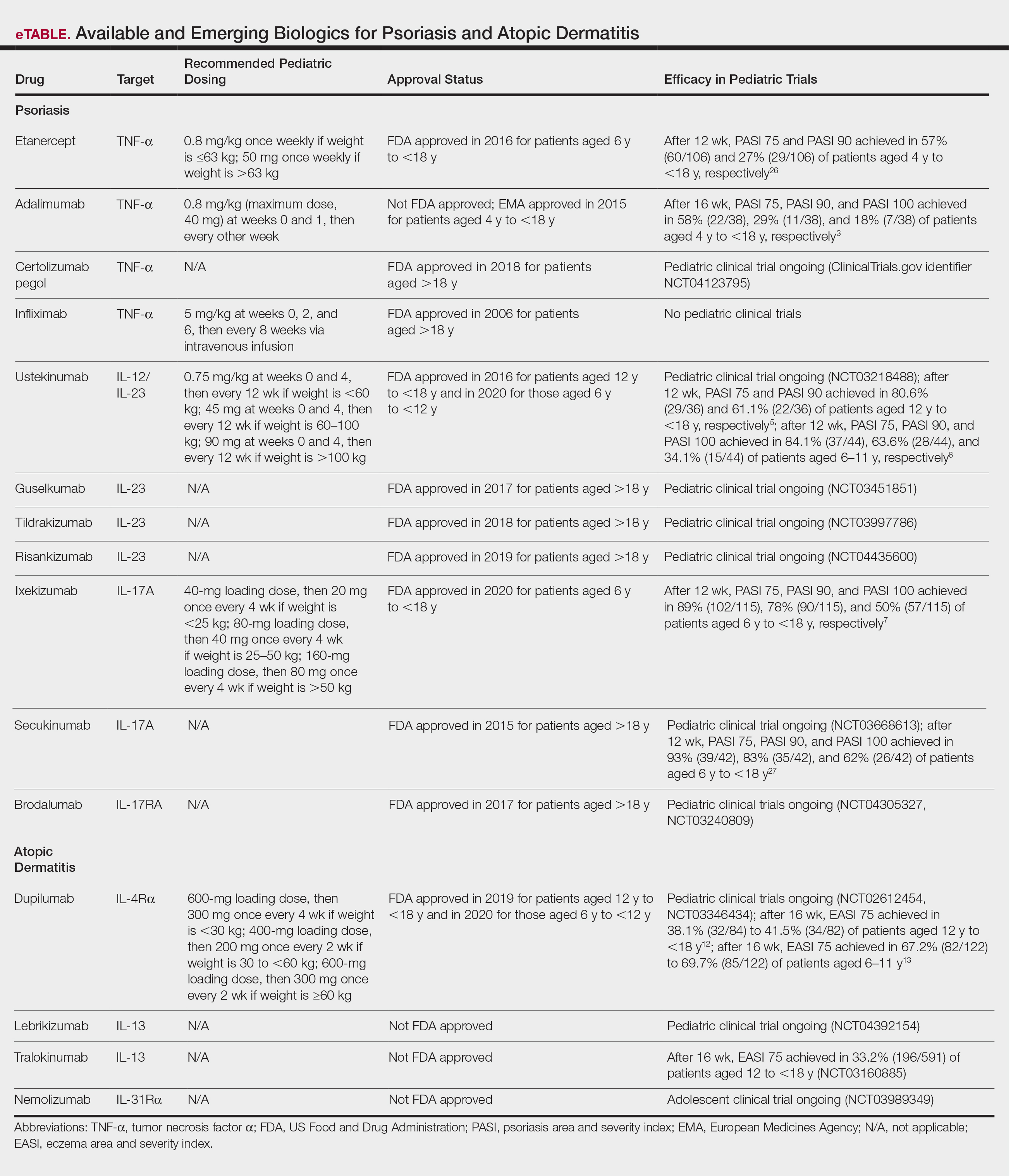
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.

Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.

- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
Dermatologists as Social Media Contributors During the COVID-19 Pandemic
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
Practice Points
- With the coronavirus disease 2019 (COVID-19) pandemic, strict physical distancing measures have made patients and providers alike reliant on global digital social networks such as Instagram, Twitter, and Facebook to facilitate information sharing about COVID-19.
- Dermatologists should utilize social media as a platform to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during the global pandemic.
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Overlap Syndrome in a Patient With Relapsing Polychondritis
To the Editor:
Relapsing polychondritis (RP) is a chronic, progressive, and episodic systemic inflammatory disease that primarily affects the cartilaginous structures of the ears and nose. Involvement of other proteoglycan-rich structures such as the joints, eyes, inner ears, blood vessels, heart, and kidneys also may be seen. Dermatologic manifestations occur in 35% to 50% of patients and may be the presenting sign in up to 12% of cases.1 The most commonly reported dermatologic findings include oral aphthosis, erythema nodosum, and purpura with vasculitic changes. Less commonly reported associations include Sweet syndrome, pyoderma gangrenosum, panniculitis, erythema elevatum diutinum, erythema annulare centrifugum, and erythema multiforme.1
A 43-year-old woman who was otherwise healthy developed new-onset tenderness and swelling of the left pinna while on vacation. She was treated with trimethoprim-sulfamethoxazole, clindamycin, and levofloxacin for presumed auricular cellulitis. The patient developed a fever; sore throat; and a progressive, pruritic, blistering rash on the face, torso, bilateral extremities, palms, and soles 1 day after completing the antibiotic course. After 5 days of unremitting symptoms despite oral, intramuscular, and topical steroids, the patient presented to the emergency department. Physical examination revealed diffuse, tender, erythematous to violaceous macules with varying degrees of coalescence on the chest, back, and extremities. Scattered flaccid bullae and erosions of the oral and genital mucosa also were seen. Laboratory analysis was notable only for a urinary tract infection with Klebsiella pneumoniae. A punch biopsy demonstrated full-thickness necrosis of the epidermis with subepidermal bullae and a mild to moderate lymphocytic infiltrate with rare eosinophils, consistent with a diagnosis of Stevens-Johnson syndrome (SJS). Because of the body surface area involved (20%) and the recent history of trimethoprim-sulfamethoxazole use, a diagnosis of SJS/toxic epidermal necrolysis (TEN) overlap syndrome was made. The patient was successfully treated with subcutaneous etanercept (50 mg), supportive care, and cephalexin for the urinary tract infection.
Approximately 5 weeks after discharge from the hospital, the patient was evaluated for new-onset pain and swelling of the right ear (Figure) in conjunction with recent tenderness and depression of the superior septal structure of the nose. A punch biopsy of the ear revealed mild perichondral inflammation without vasculitic changes and a superficial, deep perivascular, and periadnexal lymphoplasmacytic inflammatory infiltrate with scattered eosinophils. A diagnosis of RP was made, as the patient met Damiani and Levine’s2 criteria with bilateral auricular inflammation, ocular inflammation, and nasal chondritis.

Although the exact pathogenesis of RP remains unclear, there is strong evidence to suggest an underlying autoimmune etiology.3 Autoantibodies against type II collagen, in addition to other minor collagen and cartilage proteins, such as cartilage oligomeric matrix proteins and matrilin-1, are seen in a subset of patients. Titers have been reported to correlate with disease activity.3,4 Direct immunofluorescence also has demonstrated plentiful CD4+ T cells, as well as IgM, IgA, IgG, and C3 deposits in the inflamed cartilage of patients with RP.3 Additionally, approximately 30% of patients with RP will have another autoimmune disease, and more than 50% of patients with RP carry the HLA-DR4 antigen.3 Alternatively, SJS and TEN are not reported in association with autoimmune diseases and are believed to be CD8+ T-cell driven. Some HLA-B subtypes have been found in strong association with SJS and TEN, suggesting the role of a potential genetic susceptibility.5
We report a unique case of SJS/TEN overlap syndrome occurring in a patient with RP.1 Although the association may be coincidental, it is well known that patients with lupus erythematosus are predisposed to the development of SJS and TEN. Therefore, a shared underlying genetic predisposition or immune system hyperactivity secondary to active RP is a possible explanation for our patient’s unique presentation.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Damiani JM, Levine HL. Relapsing polychondritis—report of ten cases. Laryngoscope. 1979;89:929-46.
- Puéchal X, Terrier B, Mouthon L, et al. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-24.
- Arnaud L, Mathian A, Haroche J, et al. Pathogenesis of relapsing polychondritis. Autoimmun Rev. 2014;13:90-95.
- Harr T, French L. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16;5:39.
To the Editor:
Relapsing polychondritis (RP) is a chronic, progressive, and episodic systemic inflammatory disease that primarily affects the cartilaginous structures of the ears and nose. Involvement of other proteoglycan-rich structures such as the joints, eyes, inner ears, blood vessels, heart, and kidneys also may be seen. Dermatologic manifestations occur in 35% to 50% of patients and may be the presenting sign in up to 12% of cases.1 The most commonly reported dermatologic findings include oral aphthosis, erythema nodosum, and purpura with vasculitic changes. Less commonly reported associations include Sweet syndrome, pyoderma gangrenosum, panniculitis, erythema elevatum diutinum, erythema annulare centrifugum, and erythema multiforme.1
A 43-year-old woman who was otherwise healthy developed new-onset tenderness and swelling of the left pinna while on vacation. She was treated with trimethoprim-sulfamethoxazole, clindamycin, and levofloxacin for presumed auricular cellulitis. The patient developed a fever; sore throat; and a progressive, pruritic, blistering rash on the face, torso, bilateral extremities, palms, and soles 1 day after completing the antibiotic course. After 5 days of unremitting symptoms despite oral, intramuscular, and topical steroids, the patient presented to the emergency department. Physical examination revealed diffuse, tender, erythematous to violaceous macules with varying degrees of coalescence on the chest, back, and extremities. Scattered flaccid bullae and erosions of the oral and genital mucosa also were seen. Laboratory analysis was notable only for a urinary tract infection with Klebsiella pneumoniae. A punch biopsy demonstrated full-thickness necrosis of the epidermis with subepidermal bullae and a mild to moderate lymphocytic infiltrate with rare eosinophils, consistent with a diagnosis of Stevens-Johnson syndrome (SJS). Because of the body surface area involved (20%) and the recent history of trimethoprim-sulfamethoxazole use, a diagnosis of SJS/toxic epidermal necrolysis (TEN) overlap syndrome was made. The patient was successfully treated with subcutaneous etanercept (50 mg), supportive care, and cephalexin for the urinary tract infection.
Approximately 5 weeks after discharge from the hospital, the patient was evaluated for new-onset pain and swelling of the right ear (Figure) in conjunction with recent tenderness and depression of the superior septal structure of the nose. A punch biopsy of the ear revealed mild perichondral inflammation without vasculitic changes and a superficial, deep perivascular, and periadnexal lymphoplasmacytic inflammatory infiltrate with scattered eosinophils. A diagnosis of RP was made, as the patient met Damiani and Levine’s2 criteria with bilateral auricular inflammation, ocular inflammation, and nasal chondritis.

Although the exact pathogenesis of RP remains unclear, there is strong evidence to suggest an underlying autoimmune etiology.3 Autoantibodies against type II collagen, in addition to other minor collagen and cartilage proteins, such as cartilage oligomeric matrix proteins and matrilin-1, are seen in a subset of patients. Titers have been reported to correlate with disease activity.3,4 Direct immunofluorescence also has demonstrated plentiful CD4+ T cells, as well as IgM, IgA, IgG, and C3 deposits in the inflamed cartilage of patients with RP.3 Additionally, approximately 30% of patients with RP will have another autoimmune disease, and more than 50% of patients with RP carry the HLA-DR4 antigen.3 Alternatively, SJS and TEN are not reported in association with autoimmune diseases and are believed to be CD8+ T-cell driven. Some HLA-B subtypes have been found in strong association with SJS and TEN, suggesting the role of a potential genetic susceptibility.5
We report a unique case of SJS/TEN overlap syndrome occurring in a patient with RP.1 Although the association may be coincidental, it is well known that patients with lupus erythematosus are predisposed to the development of SJS and TEN. Therefore, a shared underlying genetic predisposition or immune system hyperactivity secondary to active RP is a possible explanation for our patient’s unique presentation.
To the Editor:
Relapsing polychondritis (RP) is a chronic, progressive, and episodic systemic inflammatory disease that primarily affects the cartilaginous structures of the ears and nose. Involvement of other proteoglycan-rich structures such as the joints, eyes, inner ears, blood vessels, heart, and kidneys also may be seen. Dermatologic manifestations occur in 35% to 50% of patients and may be the presenting sign in up to 12% of cases.1 The most commonly reported dermatologic findings include oral aphthosis, erythema nodosum, and purpura with vasculitic changes. Less commonly reported associations include Sweet syndrome, pyoderma gangrenosum, panniculitis, erythema elevatum diutinum, erythema annulare centrifugum, and erythema multiforme.1
A 43-year-old woman who was otherwise healthy developed new-onset tenderness and swelling of the left pinna while on vacation. She was treated with trimethoprim-sulfamethoxazole, clindamycin, and levofloxacin for presumed auricular cellulitis. The patient developed a fever; sore throat; and a progressive, pruritic, blistering rash on the face, torso, bilateral extremities, palms, and soles 1 day after completing the antibiotic course. After 5 days of unremitting symptoms despite oral, intramuscular, and topical steroids, the patient presented to the emergency department. Physical examination revealed diffuse, tender, erythematous to violaceous macules with varying degrees of coalescence on the chest, back, and extremities. Scattered flaccid bullae and erosions of the oral and genital mucosa also were seen. Laboratory analysis was notable only for a urinary tract infection with Klebsiella pneumoniae. A punch biopsy demonstrated full-thickness necrosis of the epidermis with subepidermal bullae and a mild to moderate lymphocytic infiltrate with rare eosinophils, consistent with a diagnosis of Stevens-Johnson syndrome (SJS). Because of the body surface area involved (20%) and the recent history of trimethoprim-sulfamethoxazole use, a diagnosis of SJS/toxic epidermal necrolysis (TEN) overlap syndrome was made. The patient was successfully treated with subcutaneous etanercept (50 mg), supportive care, and cephalexin for the urinary tract infection.
Approximately 5 weeks after discharge from the hospital, the patient was evaluated for new-onset pain and swelling of the right ear (Figure) in conjunction with recent tenderness and depression of the superior septal structure of the nose. A punch biopsy of the ear revealed mild perichondral inflammation without vasculitic changes and a superficial, deep perivascular, and periadnexal lymphoplasmacytic inflammatory infiltrate with scattered eosinophils. A diagnosis of RP was made, as the patient met Damiani and Levine’s2 criteria with bilateral auricular inflammation, ocular inflammation, and nasal chondritis.

Although the exact pathogenesis of RP remains unclear, there is strong evidence to suggest an underlying autoimmune etiology.3 Autoantibodies against type II collagen, in addition to other minor collagen and cartilage proteins, such as cartilage oligomeric matrix proteins and matrilin-1, are seen in a subset of patients. Titers have been reported to correlate with disease activity.3,4 Direct immunofluorescence also has demonstrated plentiful CD4+ T cells, as well as IgM, IgA, IgG, and C3 deposits in the inflamed cartilage of patients with RP.3 Additionally, approximately 30% of patients with RP will have another autoimmune disease, and more than 50% of patients with RP carry the HLA-DR4 antigen.3 Alternatively, SJS and TEN are not reported in association with autoimmune diseases and are believed to be CD8+ T-cell driven. Some HLA-B subtypes have been found in strong association with SJS and TEN, suggesting the role of a potential genetic susceptibility.5
We report a unique case of SJS/TEN overlap syndrome occurring in a patient with RP.1 Although the association may be coincidental, it is well known that patients with lupus erythematosus are predisposed to the development of SJS and TEN. Therefore, a shared underlying genetic predisposition or immune system hyperactivity secondary to active RP is a possible explanation for our patient’s unique presentation.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Damiani JM, Levine HL. Relapsing polychondritis—report of ten cases. Laryngoscope. 1979;89:929-46.
- Puéchal X, Terrier B, Mouthon L, et al. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-24.
- Arnaud L, Mathian A, Haroche J, et al. Pathogenesis of relapsing polychondritis. Autoimmun Rev. 2014;13:90-95.
- Harr T, French L. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16;5:39.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Damiani JM, Levine HL. Relapsing polychondritis—report of ten cases. Laryngoscope. 1979;89:929-46.
- Puéchal X, Terrier B, Mouthon L, et al. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-24.
- Arnaud L, Mathian A, Haroche J, et al. Pathogenesis of relapsing polychondritis. Autoimmun Rev. 2014;13:90-95.
- Harr T, French L. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16;5:39.
Practice Points
- The clinical presentation of relapsing polychondritis (RP) may demonstrate cutaneous manifestations other than the typical inflammation of cartilage-rich structures.
- Approximately 30% of patients with RP will have another autoimmune disease.
Orbital Granuloma Formation Following Autoinjection of Paraffin Oil: Management Considerations
To the Editor:
Injectable fillers are an increasingly common means of achieving minimally invasive facial rejuvenation. In the hands of well-trained practitioners, these compounds typically are well tolerated, effective, and have a strong safety profile1; however, there have been reports of complications, including vision loss,2 orbital infarction,3 persistent inflammatory nodules,4 and infection.4,5 Paraffin, a derivative of mineral oil, currently is used in cosmetic products and medical ointments.6 In the early 1900s, it often was injected into the body for various medical procedures, such as to create prosthetic testicles, to treat bladder incontinence, and eventually to correct facial contour defects.7,8 Due to adverse effects, injection of paraffin oil was discontinued in the Western medical community around the time of World War I.7 Unfortunately, some patients continue to self-inject paraffin oil for cosmetic purposes today. We present a case of foreign-body granuloma formation mimicking periorbital cellulitis following self-injection of paraffin oil. Our patient developed serious periorbital sequelae that required surgical intervention to restore normal anatomic function.
A 60-year-old woman who was otherwise healthy presented to the emergency department with facial swelling and a rash of 2 weeks’ duration. She reported that she had purchased what she believed was a cosmetic product at a local flea market 2 weeks prior to presentation. Her purchase included needles and a syringe with verbal instructions for injection into the face. She was told the product was used to treat wrinkles and referred to the injectable material as “oil” when providing her history. She reported that she had injected the material into the bilateral lower eyelids, left lateral lip, and left lateral chin. Three days later, she developed tingling and itching with swelling and redness at the injection sites. The patient was evaluated by the emergency department team and was prescribed a 10-day course of clindamycin empirically for suspected facial cellulitis.
The patient returned to the emergency department 12 days later upon completion of the antibiotic course with worsening edema and erythema. Examination revealed indurated, erythematous, and edematous warm plaques on the face that were concentrated around the prior injection sites with substantial periorbital erythema and edema (Figure 1). A consultation with oculoplastic surgery was obtained. Mechanical ptosis of the right eyelid was noted. Visual acuity was 20/30 in both eyes with habitual correction. Intraocular pressure was soft to palpation, and the pupils were round and reactive with no evidence of a relative afferent pupillary defect. Extraocular motility was intact bilaterally. Examination of the conjunctiva and sclera revealed bilateral conjunctival injection with chemosis of the right eye. The remainder of the anterior and posterior segment examination was within normal limits bilaterally.
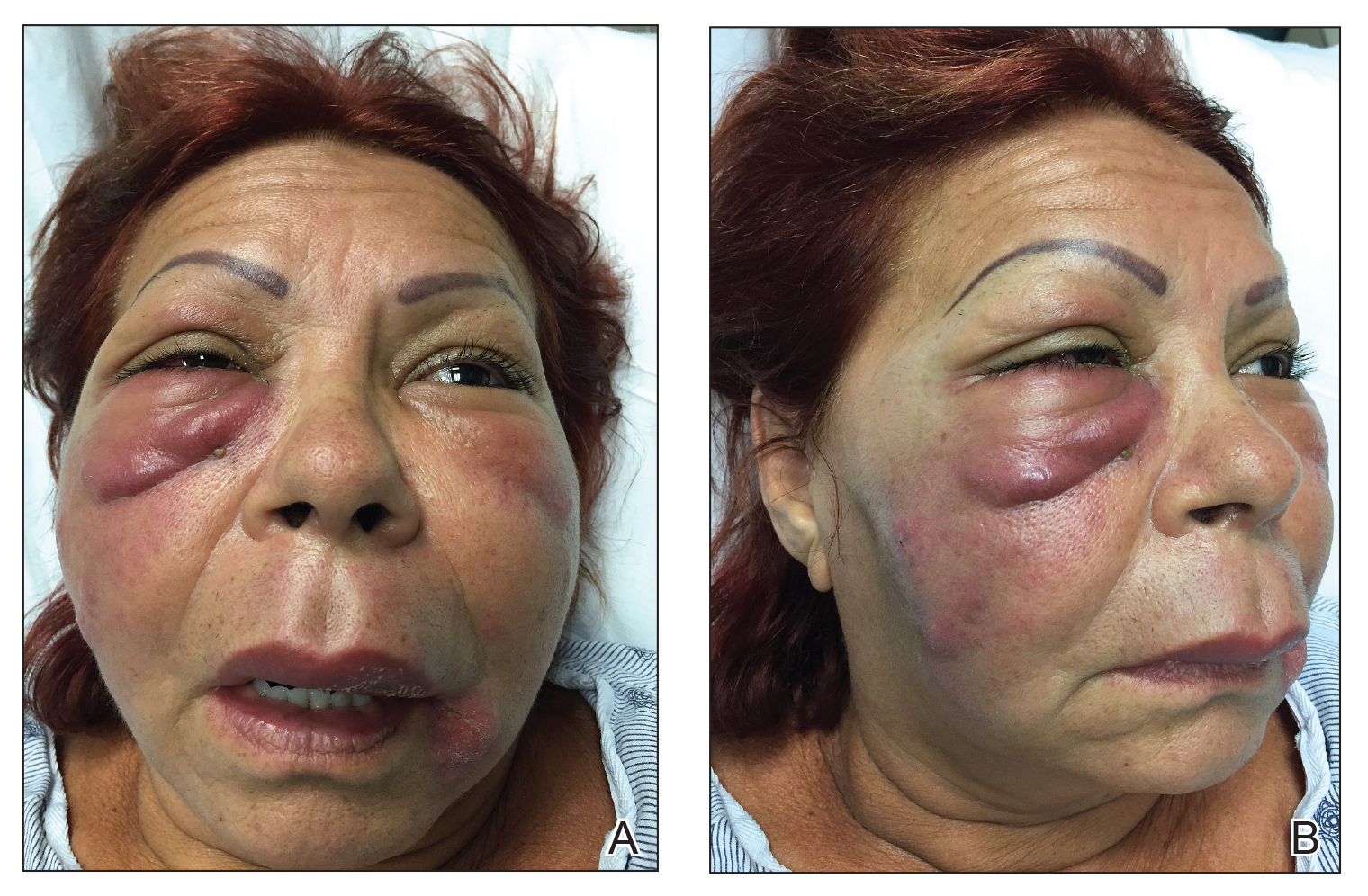
Computed tomography of the face showed extensive facial and periorbital swelling without abscess. A dermatology consultation was obtained. Two 4-mm punch biopsies were obtained from the left lower face and were sent for hematoxylin and eosin stain and tissue culture (bacterial, fungal, and acid-fast bacillus). Given the possibility of facial and periorbital cellulitis, empiric intravenous antibiotic therapy was initiated.
The tissue culture revealed normal skin flora. The biopsy results indicated a foreign-body reaction consistent with paraffin granuloma (Figures 2 and 3). Fite-Faraco, Grocott-Gomori methenamine-silver, and periodic acid–Schiff stains were all negative for infection. A diagnosis of foreign-body granuloma was established. Oral minocycline at a dosage of 100 mg twice daily was started, and the patient was discharged.


After 4 weeks of minocycline therapy, the patient showed no improvement and returned to the emergency department with worsening symptoms. She was readmitted and started on intravenous prednisone (1.5 mg/kg/d). Over the ensuing 5 days, the edema, erythema, conjunctival injection, and chemosis demonstrated notable improvement. She was subsequently discharged on an oral prednisone taper. Unfortunately, she did not respond to a trial of intralesional steroid injections to an area of granuloma formation on the left chin performed in the hospital before she was discharged.
In the ensuing months, she began to develop cicatricial ectropion of the right lower eyelid and mechanical ptosis of the right upper eyelid. Ten months after initial self-injection, staged surgical excision was initiated by an oculoplastic surgeon (I.V.) with the goal of debulking the periorbital region to correct the ectropion and mechanical ptosis. A transconjunctival approach was used to carefully excise the material while still maintaining the architecture of the lower eyelid. The ectropion was surgically corrected concurrently.
One month after excision, serial injections of 5-fluorouracil (5-FU) and triamcinolone acetonide 40 mg/mL were administered to the right lower eyelid and anterior orbit for 3 months. Fifteen weeks after the first surgery, a second surgery was performed to address residual medial right lower eyelid induration, right upper eyelid mechanical ptosis, and left orbital inflammation. During the postoperative period, serial monthly injections of 5-FU and triamcinolone acetonide were again performed beginning at the first postoperative month.
The surgical excisions resulted in notable improvement 3 months following excision (Figure 4). The patient noted improved ocular surface comfort with decreased foreign-body sensation and tearing. She also was pleased with the improved cosmetic outcome.
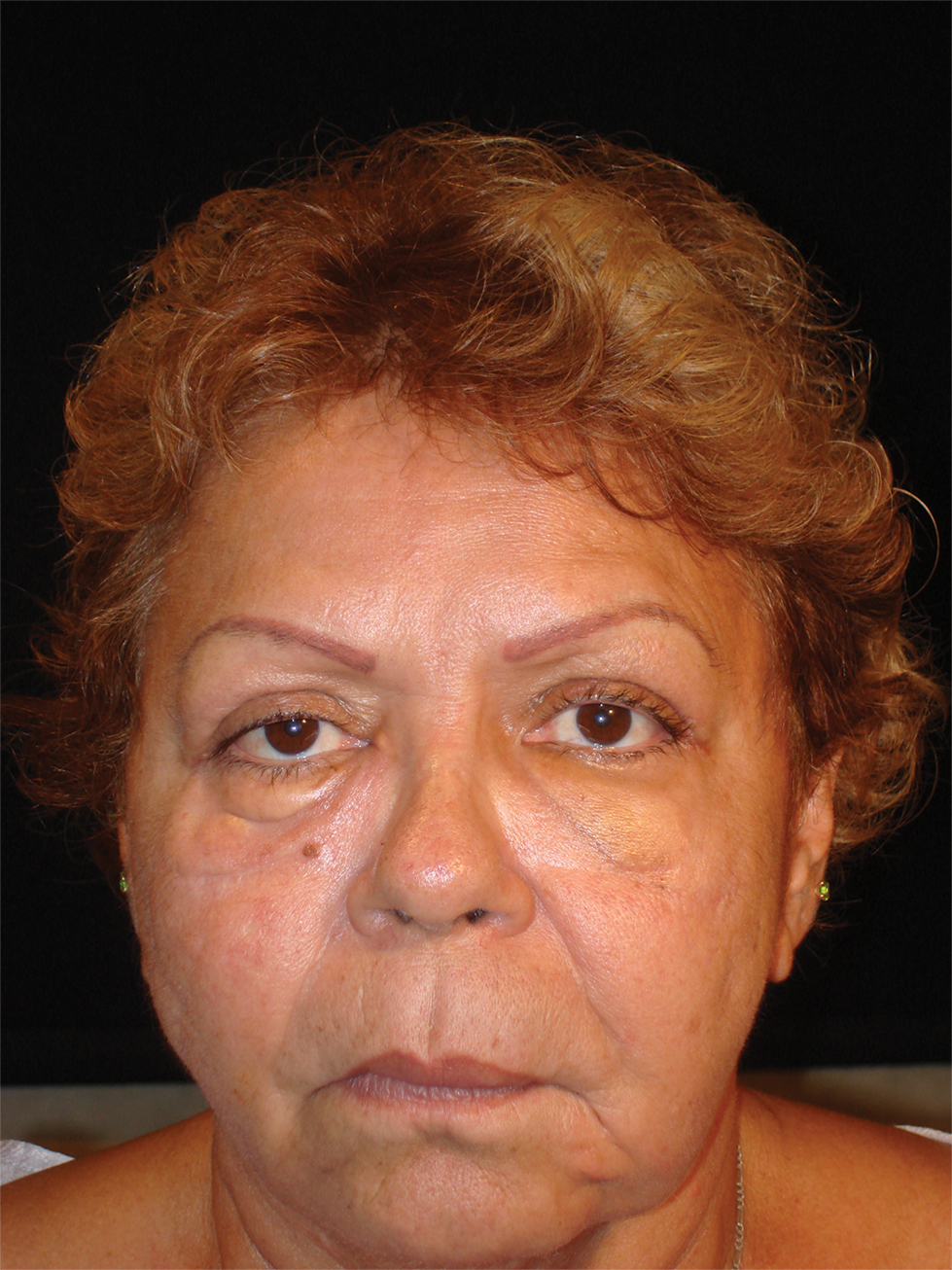
Crude substances such as paraffin, petroleum jelly, and lanolin were used for aesthetic purposes in the late 19th and early 20th centuries, initially with satisfying results; however, long-term adverse effects such as hardening of the skin, swelling, granuloma formation, ulceration, infections, and abscesses have discouraged its use by medical professionals today.5 Since paraffin is resistant to degradation and absorption, foreign-body reactions may occur upon injection. These reactions are characterized by replacement of normal subcutaneous tissue by cystic spaces of paraffin oil and/or calcification, similar to the appearance of Swiss cheese on histology and surrounded by various inflammatory cells and fibrous tissue.9,10
Clinically, there is an acute inflammatory phase followed by a latent phase of chronic granulomatous inflammation that can last for years.10 Our patient presented during the acute phase, with erythematous and edematous warm plaques around the eye mimicking an orbital infection.
The treatment of choice for paraffin granuloma is complete surgical excision to prevent recurrence.6,9 However, intralesional corticosteroids are preferred in the facial area, especially if complete removal is not possible.10 Intralesional corticosteroid injections inhibit fibroblast and macrophage activity as well as the deposition of collagen, leading to reduced pain and swelling in most cases.11 Additionally, combining antimitotic agents such as 5-FU with a corticosteroid might reduce the risk for cortisone skin atrophy.12 In our case, the patient did not respond to combined 5-FU with intralesional steroids and required oral corticosteroids while awaiting serial excisions.
Our case highlights several important points in the management of paraffin granuloma. First, the clinician must perform a thorough patient history, as surreptitious use of non–medical-grade fillers is more common than one might think.13 Second, the initial presentation of these patients can mimic an infectious process. Careful history, testing, and observation can aid in making the appropriate diagnosis. Finally, treatment of these patients is complex. The mainstays of therapy are systemic anti-inflammatory medications, time, and supportive care. In some cases, surgery may be required. When processes such as paraffin granulomas involve the periorbital region, particular care is required to avoid cicatricial lagophthalmos, ectropion, or retraction. Thoughtful surgical manipulation is required to avoid these complications, which indeed may occur even with the most appropriate interventions.
- Duker D, Erdmann R, Hartmann V, et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS)—study. J Eur Acad Dermatol Venereol. 2016;30:1013-1020.
- Prado G, Rodriguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? [published online December 28, 2016]. Aesthetic Plast Surg. 2017;41:199-203.
- Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28:E68-E70.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215E-227E.
- Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30:599-614.
- Friedrich RE, Zustin J. Paraffinoma of lips and oral mucosa: case report and brief review of literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc05.
- Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133-140.
- Glicenstein J. Les premiers fillers, Vaseline et paraffine. du miracle a la catastrope. Ann Chir Plast Esthet. 2007;52:157-161.
- Cohen JL, Keoleian CM, Krull EA. Penile paraffinoma: self-injection with mineral oil. J Am Acad Dermatol 2002;47:S251-S253.
- Legaspi-Vicerra ME, Field LM. Paraffin granulomata, “witch’s chin,” and nasal deformities excision and reconstruction with reduction chinplasty and open rhinotomy resection. J Clin Aesthet Dermatol 2010;3:54-58.
- Carlos-Fabuel L, Marzal-Gamarra C, Marti-Alamo S, et al. Foreign body granulomatous reactions to cosmetic fillers. J Clin Exp Dent. 2012;4:E244-E247.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. treatment options. Plast Reconstr Surg. 2009;123:1864-1873.
- Seok J, Hong JY, Park KY, et al. Delayed immunologic complications due to injectable fillers by unlicensed practitioners: our experiences and a review of the literature. Dermatol Ther. 2016;29:41-44.
To the Editor:
Injectable fillers are an increasingly common means of achieving minimally invasive facial rejuvenation. In the hands of well-trained practitioners, these compounds typically are well tolerated, effective, and have a strong safety profile1; however, there have been reports of complications, including vision loss,2 orbital infarction,3 persistent inflammatory nodules,4 and infection.4,5 Paraffin, a derivative of mineral oil, currently is used in cosmetic products and medical ointments.6 In the early 1900s, it often was injected into the body for various medical procedures, such as to create prosthetic testicles, to treat bladder incontinence, and eventually to correct facial contour defects.7,8 Due to adverse effects, injection of paraffin oil was discontinued in the Western medical community around the time of World War I.7 Unfortunately, some patients continue to self-inject paraffin oil for cosmetic purposes today. We present a case of foreign-body granuloma formation mimicking periorbital cellulitis following self-injection of paraffin oil. Our patient developed serious periorbital sequelae that required surgical intervention to restore normal anatomic function.
A 60-year-old woman who was otherwise healthy presented to the emergency department with facial swelling and a rash of 2 weeks’ duration. She reported that she had purchased what she believed was a cosmetic product at a local flea market 2 weeks prior to presentation. Her purchase included needles and a syringe with verbal instructions for injection into the face. She was told the product was used to treat wrinkles and referred to the injectable material as “oil” when providing her history. She reported that she had injected the material into the bilateral lower eyelids, left lateral lip, and left lateral chin. Three days later, she developed tingling and itching with swelling and redness at the injection sites. The patient was evaluated by the emergency department team and was prescribed a 10-day course of clindamycin empirically for suspected facial cellulitis.
The patient returned to the emergency department 12 days later upon completion of the antibiotic course with worsening edema and erythema. Examination revealed indurated, erythematous, and edematous warm plaques on the face that were concentrated around the prior injection sites with substantial periorbital erythema and edema (Figure 1). A consultation with oculoplastic surgery was obtained. Mechanical ptosis of the right eyelid was noted. Visual acuity was 20/30 in both eyes with habitual correction. Intraocular pressure was soft to palpation, and the pupils were round and reactive with no evidence of a relative afferent pupillary defect. Extraocular motility was intact bilaterally. Examination of the conjunctiva and sclera revealed bilateral conjunctival injection with chemosis of the right eye. The remainder of the anterior and posterior segment examination was within normal limits bilaterally.

Computed tomography of the face showed extensive facial and periorbital swelling without abscess. A dermatology consultation was obtained. Two 4-mm punch biopsies were obtained from the left lower face and were sent for hematoxylin and eosin stain and tissue culture (bacterial, fungal, and acid-fast bacillus). Given the possibility of facial and periorbital cellulitis, empiric intravenous antibiotic therapy was initiated.
The tissue culture revealed normal skin flora. The biopsy results indicated a foreign-body reaction consistent with paraffin granuloma (Figures 2 and 3). Fite-Faraco, Grocott-Gomori methenamine-silver, and periodic acid–Schiff stains were all negative for infection. A diagnosis of foreign-body granuloma was established. Oral minocycline at a dosage of 100 mg twice daily was started, and the patient was discharged.


After 4 weeks of minocycline therapy, the patient showed no improvement and returned to the emergency department with worsening symptoms. She was readmitted and started on intravenous prednisone (1.5 mg/kg/d). Over the ensuing 5 days, the edema, erythema, conjunctival injection, and chemosis demonstrated notable improvement. She was subsequently discharged on an oral prednisone taper. Unfortunately, she did not respond to a trial of intralesional steroid injections to an area of granuloma formation on the left chin performed in the hospital before she was discharged.
In the ensuing months, she began to develop cicatricial ectropion of the right lower eyelid and mechanical ptosis of the right upper eyelid. Ten months after initial self-injection, staged surgical excision was initiated by an oculoplastic surgeon (I.V.) with the goal of debulking the periorbital region to correct the ectropion and mechanical ptosis. A transconjunctival approach was used to carefully excise the material while still maintaining the architecture of the lower eyelid. The ectropion was surgically corrected concurrently.
One month after excision, serial injections of 5-fluorouracil (5-FU) and triamcinolone acetonide 40 mg/mL were administered to the right lower eyelid and anterior orbit for 3 months. Fifteen weeks after the first surgery, a second surgery was performed to address residual medial right lower eyelid induration, right upper eyelid mechanical ptosis, and left orbital inflammation. During the postoperative period, serial monthly injections of 5-FU and triamcinolone acetonide were again performed beginning at the first postoperative month.
The surgical excisions resulted in notable improvement 3 months following excision (Figure 4). The patient noted improved ocular surface comfort with decreased foreign-body sensation and tearing. She also was pleased with the improved cosmetic outcome.

Crude substances such as paraffin, petroleum jelly, and lanolin were used for aesthetic purposes in the late 19th and early 20th centuries, initially with satisfying results; however, long-term adverse effects such as hardening of the skin, swelling, granuloma formation, ulceration, infections, and abscesses have discouraged its use by medical professionals today.5 Since paraffin is resistant to degradation and absorption, foreign-body reactions may occur upon injection. These reactions are characterized by replacement of normal subcutaneous tissue by cystic spaces of paraffin oil and/or calcification, similar to the appearance of Swiss cheese on histology and surrounded by various inflammatory cells and fibrous tissue.9,10
Clinically, there is an acute inflammatory phase followed by a latent phase of chronic granulomatous inflammation that can last for years.10 Our patient presented during the acute phase, with erythematous and edematous warm plaques around the eye mimicking an orbital infection.
The treatment of choice for paraffin granuloma is complete surgical excision to prevent recurrence.6,9 However, intralesional corticosteroids are preferred in the facial area, especially if complete removal is not possible.10 Intralesional corticosteroid injections inhibit fibroblast and macrophage activity as well as the deposition of collagen, leading to reduced pain and swelling in most cases.11 Additionally, combining antimitotic agents such as 5-FU with a corticosteroid might reduce the risk for cortisone skin atrophy.12 In our case, the patient did not respond to combined 5-FU with intralesional steroids and required oral corticosteroids while awaiting serial excisions.
Our case highlights several important points in the management of paraffin granuloma. First, the clinician must perform a thorough patient history, as surreptitious use of non–medical-grade fillers is more common than one might think.13 Second, the initial presentation of these patients can mimic an infectious process. Careful history, testing, and observation can aid in making the appropriate diagnosis. Finally, treatment of these patients is complex. The mainstays of therapy are systemic anti-inflammatory medications, time, and supportive care. In some cases, surgery may be required. When processes such as paraffin granulomas involve the periorbital region, particular care is required to avoid cicatricial lagophthalmos, ectropion, or retraction. Thoughtful surgical manipulation is required to avoid these complications, which indeed may occur even with the most appropriate interventions.
To the Editor:
Injectable fillers are an increasingly common means of achieving minimally invasive facial rejuvenation. In the hands of well-trained practitioners, these compounds typically are well tolerated, effective, and have a strong safety profile1; however, there have been reports of complications, including vision loss,2 orbital infarction,3 persistent inflammatory nodules,4 and infection.4,5 Paraffin, a derivative of mineral oil, currently is used in cosmetic products and medical ointments.6 In the early 1900s, it often was injected into the body for various medical procedures, such as to create prosthetic testicles, to treat bladder incontinence, and eventually to correct facial contour defects.7,8 Due to adverse effects, injection of paraffin oil was discontinued in the Western medical community around the time of World War I.7 Unfortunately, some patients continue to self-inject paraffin oil for cosmetic purposes today. We present a case of foreign-body granuloma formation mimicking periorbital cellulitis following self-injection of paraffin oil. Our patient developed serious periorbital sequelae that required surgical intervention to restore normal anatomic function.
A 60-year-old woman who was otherwise healthy presented to the emergency department with facial swelling and a rash of 2 weeks’ duration. She reported that she had purchased what she believed was a cosmetic product at a local flea market 2 weeks prior to presentation. Her purchase included needles and a syringe with verbal instructions for injection into the face. She was told the product was used to treat wrinkles and referred to the injectable material as “oil” when providing her history. She reported that she had injected the material into the bilateral lower eyelids, left lateral lip, and left lateral chin. Three days later, she developed tingling and itching with swelling and redness at the injection sites. The patient was evaluated by the emergency department team and was prescribed a 10-day course of clindamycin empirically for suspected facial cellulitis.
The patient returned to the emergency department 12 days later upon completion of the antibiotic course with worsening edema and erythema. Examination revealed indurated, erythematous, and edematous warm plaques on the face that were concentrated around the prior injection sites with substantial periorbital erythema and edema (Figure 1). A consultation with oculoplastic surgery was obtained. Mechanical ptosis of the right eyelid was noted. Visual acuity was 20/30 in both eyes with habitual correction. Intraocular pressure was soft to palpation, and the pupils were round and reactive with no evidence of a relative afferent pupillary defect. Extraocular motility was intact bilaterally. Examination of the conjunctiva and sclera revealed bilateral conjunctival injection with chemosis of the right eye. The remainder of the anterior and posterior segment examination was within normal limits bilaterally.

Computed tomography of the face showed extensive facial and periorbital swelling without abscess. A dermatology consultation was obtained. Two 4-mm punch biopsies were obtained from the left lower face and were sent for hematoxylin and eosin stain and tissue culture (bacterial, fungal, and acid-fast bacillus). Given the possibility of facial and periorbital cellulitis, empiric intravenous antibiotic therapy was initiated.
The tissue culture revealed normal skin flora. The biopsy results indicated a foreign-body reaction consistent with paraffin granuloma (Figures 2 and 3). Fite-Faraco, Grocott-Gomori methenamine-silver, and periodic acid–Schiff stains were all negative for infection. A diagnosis of foreign-body granuloma was established. Oral minocycline at a dosage of 100 mg twice daily was started, and the patient was discharged.


After 4 weeks of minocycline therapy, the patient showed no improvement and returned to the emergency department with worsening symptoms. She was readmitted and started on intravenous prednisone (1.5 mg/kg/d). Over the ensuing 5 days, the edema, erythema, conjunctival injection, and chemosis demonstrated notable improvement. She was subsequently discharged on an oral prednisone taper. Unfortunately, she did not respond to a trial of intralesional steroid injections to an area of granuloma formation on the left chin performed in the hospital before she was discharged.
In the ensuing months, she began to develop cicatricial ectropion of the right lower eyelid and mechanical ptosis of the right upper eyelid. Ten months after initial self-injection, staged surgical excision was initiated by an oculoplastic surgeon (I.V.) with the goal of debulking the periorbital region to correct the ectropion and mechanical ptosis. A transconjunctival approach was used to carefully excise the material while still maintaining the architecture of the lower eyelid. The ectropion was surgically corrected concurrently.
One month after excision, serial injections of 5-fluorouracil (5-FU) and triamcinolone acetonide 40 mg/mL were administered to the right lower eyelid and anterior orbit for 3 months. Fifteen weeks after the first surgery, a second surgery was performed to address residual medial right lower eyelid induration, right upper eyelid mechanical ptosis, and left orbital inflammation. During the postoperative period, serial monthly injections of 5-FU and triamcinolone acetonide were again performed beginning at the first postoperative month.
The surgical excisions resulted in notable improvement 3 months following excision (Figure 4). The patient noted improved ocular surface comfort with decreased foreign-body sensation and tearing. She also was pleased with the improved cosmetic outcome.

Crude substances such as paraffin, petroleum jelly, and lanolin were used for aesthetic purposes in the late 19th and early 20th centuries, initially with satisfying results; however, long-term adverse effects such as hardening of the skin, swelling, granuloma formation, ulceration, infections, and abscesses have discouraged its use by medical professionals today.5 Since paraffin is resistant to degradation and absorption, foreign-body reactions may occur upon injection. These reactions are characterized by replacement of normal subcutaneous tissue by cystic spaces of paraffin oil and/or calcification, similar to the appearance of Swiss cheese on histology and surrounded by various inflammatory cells and fibrous tissue.9,10
Clinically, there is an acute inflammatory phase followed by a latent phase of chronic granulomatous inflammation that can last for years.10 Our patient presented during the acute phase, with erythematous and edematous warm plaques around the eye mimicking an orbital infection.
The treatment of choice for paraffin granuloma is complete surgical excision to prevent recurrence.6,9 However, intralesional corticosteroids are preferred in the facial area, especially if complete removal is not possible.10 Intralesional corticosteroid injections inhibit fibroblast and macrophage activity as well as the deposition of collagen, leading to reduced pain and swelling in most cases.11 Additionally, combining antimitotic agents such as 5-FU with a corticosteroid might reduce the risk for cortisone skin atrophy.12 In our case, the patient did not respond to combined 5-FU with intralesional steroids and required oral corticosteroids while awaiting serial excisions.
Our case highlights several important points in the management of paraffin granuloma. First, the clinician must perform a thorough patient history, as surreptitious use of non–medical-grade fillers is more common than one might think.13 Second, the initial presentation of these patients can mimic an infectious process. Careful history, testing, and observation can aid in making the appropriate diagnosis. Finally, treatment of these patients is complex. The mainstays of therapy are systemic anti-inflammatory medications, time, and supportive care. In some cases, surgery may be required. When processes such as paraffin granulomas involve the periorbital region, particular care is required to avoid cicatricial lagophthalmos, ectropion, or retraction. Thoughtful surgical manipulation is required to avoid these complications, which indeed may occur even with the most appropriate interventions.
- Duker D, Erdmann R, Hartmann V, et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS)—study. J Eur Acad Dermatol Venereol. 2016;30:1013-1020.
- Prado G, Rodriguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? [published online December 28, 2016]. Aesthetic Plast Surg. 2017;41:199-203.
- Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28:E68-E70.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215E-227E.
- Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30:599-614.
- Friedrich RE, Zustin J. Paraffinoma of lips and oral mucosa: case report and brief review of literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc05.
- Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133-140.
- Glicenstein J. Les premiers fillers, Vaseline et paraffine. du miracle a la catastrope. Ann Chir Plast Esthet. 2007;52:157-161.
- Cohen JL, Keoleian CM, Krull EA. Penile paraffinoma: self-injection with mineral oil. J Am Acad Dermatol 2002;47:S251-S253.
- Legaspi-Vicerra ME, Field LM. Paraffin granulomata, “witch’s chin,” and nasal deformities excision and reconstruction with reduction chinplasty and open rhinotomy resection. J Clin Aesthet Dermatol 2010;3:54-58.
- Carlos-Fabuel L, Marzal-Gamarra C, Marti-Alamo S, et al. Foreign body granulomatous reactions to cosmetic fillers. J Clin Exp Dent. 2012;4:E244-E247.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. treatment options. Plast Reconstr Surg. 2009;123:1864-1873.
- Seok J, Hong JY, Park KY, et al. Delayed immunologic complications due to injectable fillers by unlicensed practitioners: our experiences and a review of the literature. Dermatol Ther. 2016;29:41-44.
- Duker D, Erdmann R, Hartmann V, et al. The impact of adverse reactions to injectable filler substances on quality of life: results from the Berlin Injectable Filler Safety (IFS)—study. J Eur Acad Dermatol Venereol. 2016;30:1013-1020.
- Prado G, Rodriguez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? [published online December 28, 2016]. Aesthetic Plast Surg. 2017;41:199-203.
- Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lactic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28:E68-E70.
- Cassuto D, Pignatti M, Pacchioni L, et al. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138:215E-227E.
- Haneke E. Adverse effects of fillers and their histopathology. Facial Plast Surg. 2014;30:599-614.
- Friedrich RE, Zustin J. Paraffinoma of lips and oral mucosa: case report and brief review of literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc05.
- Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133-140.
- Glicenstein J. Les premiers fillers, Vaseline et paraffine. du miracle a la catastrope. Ann Chir Plast Esthet. 2007;52:157-161.
- Cohen JL, Keoleian CM, Krull EA. Penile paraffinoma: self-injection with mineral oil. J Am Acad Dermatol 2002;47:S251-S253.
- Legaspi-Vicerra ME, Field LM. Paraffin granulomata, “witch’s chin,” and nasal deformities excision and reconstruction with reduction chinplasty and open rhinotomy resection. J Clin Aesthet Dermatol 2010;3:54-58.
- Carlos-Fabuel L, Marzal-Gamarra C, Marti-Alamo S, et al. Foreign body granulomatous reactions to cosmetic fillers. J Clin Exp Dent. 2012;4:E244-E247.
- Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. treatment options. Plast Reconstr Surg. 2009;123:1864-1873.
- Seok J, Hong JY, Park KY, et al. Delayed immunologic complications due to injectable fillers by unlicensed practitioners: our experiences and a review of the literature. Dermatol Ther. 2016;29:41-44.
Practice Points
- The initial presentation of a foreign-body granulomatous process in a patient with surreptitious use of nonmedical filler can mimic infection; thus, careful history and diagnostic measures are paramount.
- Treatment of paraffin oil granuloma can be multifactorial and involves supportive care, systemic anti-inflammatory medications, time, and surgery.
- When a paraffin granuloma involves the orbital region, particular care is required to avoid long-term complications including cicatricial lagophthalmos, ectropion, or retractions, which can be mitigated with the help of oculoplastic surgery.
Tylosis in a Patient With Howel-Evans Syndrome: Management With Acitretin
To the Editor:
Tylosis with esophageal cancer was first described by Howel-Evans et al1 in 1958 in a family from Liverpool, England. The disease is inherited in an autosomal-dominant fashion with a mutation in the tylosis with esophageal cancer gene, TOC.2 The keratoderma associated with this syndrome has been reported to be focal in nature, painful, and primarily involving the plantar surfaces.3 Palmar involvement has been reported to manifest as calluses in patients who use their hands for manual labor.4 Oral leukoplakia also has been described in this syndrome5; however, long-term follow-up in one family demonstrated a benign course.6 Herein, we describe a case of painful tylosis in a patient with Howel-Evans syndrome who was successfully treated with acitretin.
A 50-year-old man presented to clinic for evaluation of hyperkeratosis of the palms and soles that began when he was a teenager. He reported the soles of the feet often were painful, especially without shoes (Figure, A). He used many over-the-counter emollients and tried both prescription and nonprescription keratolytics. At presentation, he was mechanically paring down some of the thickness of the calluses to decrease the pain.
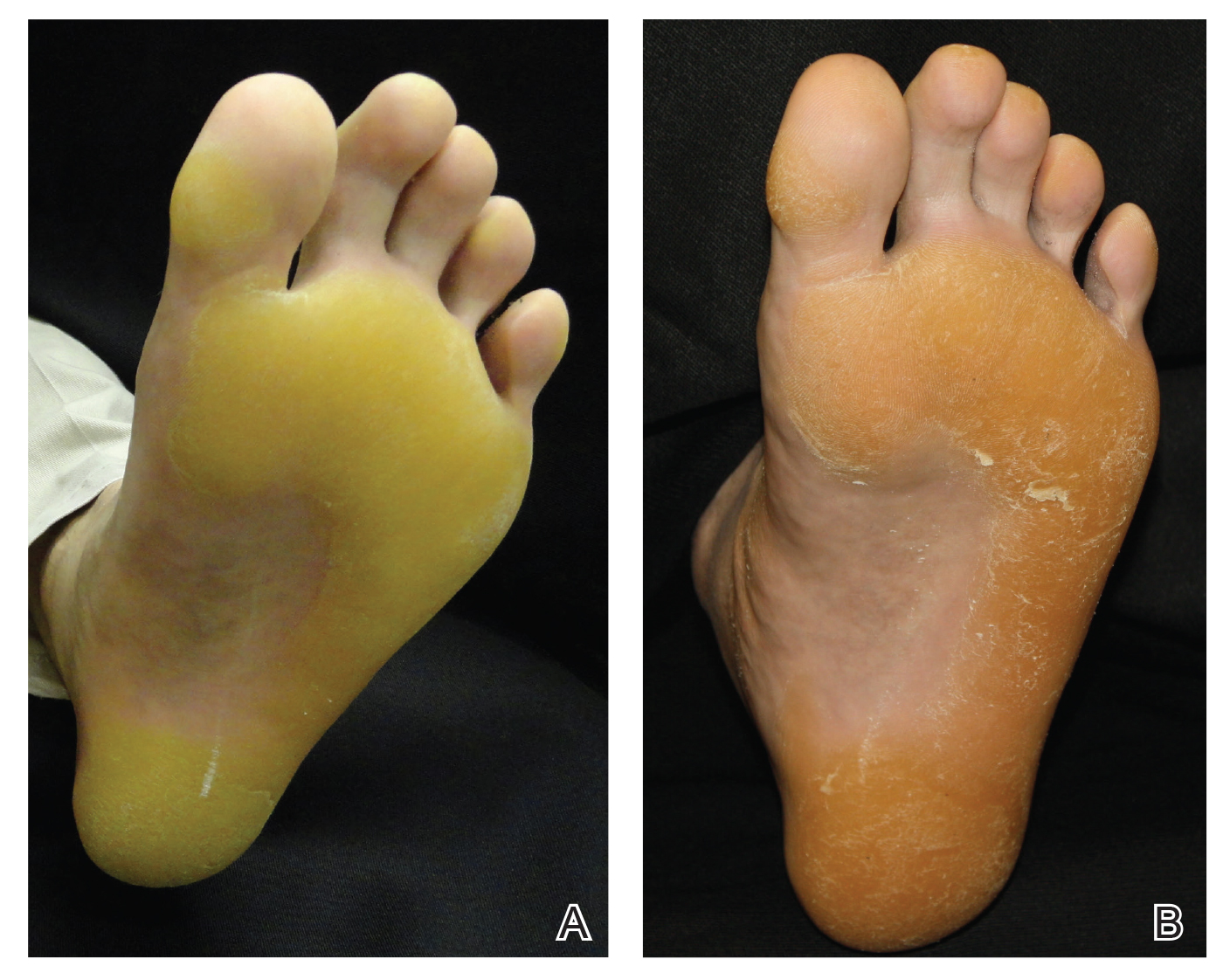
There was no relevant medical history, he had no history of smoking, he consumed more than 1 alcoholic drink per day, and he denied illicit drug use. The patient was not on any other medications. His family history revealed that his father also had the same hyperkeratosis of the palms and soles and died from esophageal carcinoma at an early age. It was determined that his father had tylosis with esophageal carcinoma (Howel-Evans syndrome). (The patient’s pedigree previously was published.3,4) Physical examination at presentation revealed plantar hyperkeratosis limited mainly to areas of pressure. His hands had mild hyperkeratosis on the distal fingers. No mucosa leukoplakia was identified.
Treatment options were discussed, and because the pain associated with the plantar keratoderma was interfering with his quality of life (QOL), acitretin was started. The initial dosage was 10 mg daily for 2 weeks and subsequently was increased to 25 mg daily. He has been maintained on this dosage for more than a year. An attempt was made to increase acitretin to 50 mg daily; however, he could not tolerate the dryness and peeling of the hands caused by the higher dosage. A fasting lipid panel and hepatic function panel performed every 3 months was within reference range. He had a remarkable decrease in the hyperkeratosis 2 months after starting therapy (Figure, B) and most importantly a decrease in pain associated with it. His QOL notably improved, enabling him to participate in sporting events with his children without severe pain. This patient was referred to gastroenterology where an esophagogastroduodenoscopy was performed and no concerning lesions were found. He was continued on this dose for 2 years. He moved to a new town, and our most recent update from him was that he was taking acitretin intermittently before big sporting events with his children.
The use of systemic retinoids has long been known to be effective in the treatment of disorders of keratinization. Recommended monitoring guidelines include a baseline complete blood cell count, renal function, hepatic function, and fasting lipid panel, which should be repeated every 3 months focusing on the hepatic function and lipid panel, as retinoids rarely cause hematologic or renal abnormalities.7 Our patient’s baseline laboratory test results were within reference range, and we repeated a fasting lipid and hepatic function panel every 3 months without any abnormalities.
Diffuse idiopathic skeletal hyperostosis (DISH), the ossification of ligaments and entheses often of the spine, is a potential complication of long-term use of oral retinoids. There are no consensus guidelines on screening for this complication, but baseline and annual radiographs seem reasonable. A 1996 study concluded that if DISH occurs, it is likely to be sporadic in a predisposed patient, as their data did not find any statistically significant relationship between the treatment or the cumulative dose and the prevalence and severity of DISH, degenerative changes, and osteoporosis.8 When annual screening is declined, imaging could be performed if a new skeletal concern were to arise in patients on long-term therapy.7 We discussed the skeletal concerns with our patient and he declined baseline or annual radiographs, but we will follow him with a rheumatologic review of systems. We feel this approach is reasonable, as our patient is a healthy adult in his 50s with no prior retinoid exposure and is on a low to moderate dose.
We report a case of Howel-Evans keratoderma successfully managed with acitretin. In patients with painful keratoderma that is interfering with QOL, low-dose acitretin can be used to diminish these symptoms.
- Howel-Evans W, McConnell RB, Clarke CA, et al. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958;27:413-429.
- Rogaev EI, Rogaeva EA, Ginter EK, et al. Identification of the genetic locus for keratosis palmaris et plantaris on chromosome 17 near the RARA and keratin type I genes. Nat Genet. 1993;5:158-162.
- Stevens HP, Kelsell DP, Bryant SP, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996;132:640-651.
- Marger RS, Marger D. Carcinoma of the esophagus and tylosis. a lethal genetic combination. Cancer. 1993;72:17-19.
- Tyldesley WR. Oral leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1974;3:62-70.
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B:102-112.
- Wu J, Wolverton S. Systemic retinoids. In: Wolverton S, ed. Comprehensive Dermatologic Drug Therapy. 4th ed. Edinburgh, Scotland: Elsevier; 2020:245-262.
- Van Dooren-Greebe RJ, Lemmens JA, De Boo T, et al. Prolonged treatment with oral retinoids in adults: no influence on the frequency and severity of spinal abnormalities. Br J Dermatol. 1996;134:71-76.
To the Editor:
Tylosis with esophageal cancer was first described by Howel-Evans et al1 in 1958 in a family from Liverpool, England. The disease is inherited in an autosomal-dominant fashion with a mutation in the tylosis with esophageal cancer gene, TOC.2 The keratoderma associated with this syndrome has been reported to be focal in nature, painful, and primarily involving the plantar surfaces.3 Palmar involvement has been reported to manifest as calluses in patients who use their hands for manual labor.4 Oral leukoplakia also has been described in this syndrome5; however, long-term follow-up in one family demonstrated a benign course.6 Herein, we describe a case of painful tylosis in a patient with Howel-Evans syndrome who was successfully treated with acitretin.
A 50-year-old man presented to clinic for evaluation of hyperkeratosis of the palms and soles that began when he was a teenager. He reported the soles of the feet often were painful, especially without shoes (Figure, A). He used many over-the-counter emollients and tried both prescription and nonprescription keratolytics. At presentation, he was mechanically paring down some of the thickness of the calluses to decrease the pain.

There was no relevant medical history, he had no history of smoking, he consumed more than 1 alcoholic drink per day, and he denied illicit drug use. The patient was not on any other medications. His family history revealed that his father also had the same hyperkeratosis of the palms and soles and died from esophageal carcinoma at an early age. It was determined that his father had tylosis with esophageal carcinoma (Howel-Evans syndrome). (The patient’s pedigree previously was published.3,4) Physical examination at presentation revealed plantar hyperkeratosis limited mainly to areas of pressure. His hands had mild hyperkeratosis on the distal fingers. No mucosa leukoplakia was identified.
Treatment options were discussed, and because the pain associated with the plantar keratoderma was interfering with his quality of life (QOL), acitretin was started. The initial dosage was 10 mg daily for 2 weeks and subsequently was increased to 25 mg daily. He has been maintained on this dosage for more than a year. An attempt was made to increase acitretin to 50 mg daily; however, he could not tolerate the dryness and peeling of the hands caused by the higher dosage. A fasting lipid panel and hepatic function panel performed every 3 months was within reference range. He had a remarkable decrease in the hyperkeratosis 2 months after starting therapy (Figure, B) and most importantly a decrease in pain associated with it. His QOL notably improved, enabling him to participate in sporting events with his children without severe pain. This patient was referred to gastroenterology where an esophagogastroduodenoscopy was performed and no concerning lesions were found. He was continued on this dose for 2 years. He moved to a new town, and our most recent update from him was that he was taking acitretin intermittently before big sporting events with his children.
The use of systemic retinoids has long been known to be effective in the treatment of disorders of keratinization. Recommended monitoring guidelines include a baseline complete blood cell count, renal function, hepatic function, and fasting lipid panel, which should be repeated every 3 months focusing on the hepatic function and lipid panel, as retinoids rarely cause hematologic or renal abnormalities.7 Our patient’s baseline laboratory test results were within reference range, and we repeated a fasting lipid and hepatic function panel every 3 months without any abnormalities.
Diffuse idiopathic skeletal hyperostosis (DISH), the ossification of ligaments and entheses often of the spine, is a potential complication of long-term use of oral retinoids. There are no consensus guidelines on screening for this complication, but baseline and annual radiographs seem reasonable. A 1996 study concluded that if DISH occurs, it is likely to be sporadic in a predisposed patient, as their data did not find any statistically significant relationship between the treatment or the cumulative dose and the prevalence and severity of DISH, degenerative changes, and osteoporosis.8 When annual screening is declined, imaging could be performed if a new skeletal concern were to arise in patients on long-term therapy.7 We discussed the skeletal concerns with our patient and he declined baseline or annual radiographs, but we will follow him with a rheumatologic review of systems. We feel this approach is reasonable, as our patient is a healthy adult in his 50s with no prior retinoid exposure and is on a low to moderate dose.
We report a case of Howel-Evans keratoderma successfully managed with acitretin. In patients with painful keratoderma that is interfering with QOL, low-dose acitretin can be used to diminish these symptoms.
To the Editor:
Tylosis with esophageal cancer was first described by Howel-Evans et al1 in 1958 in a family from Liverpool, England. The disease is inherited in an autosomal-dominant fashion with a mutation in the tylosis with esophageal cancer gene, TOC.2 The keratoderma associated with this syndrome has been reported to be focal in nature, painful, and primarily involving the plantar surfaces.3 Palmar involvement has been reported to manifest as calluses in patients who use their hands for manual labor.4 Oral leukoplakia also has been described in this syndrome5; however, long-term follow-up in one family demonstrated a benign course.6 Herein, we describe a case of painful tylosis in a patient with Howel-Evans syndrome who was successfully treated with acitretin.
A 50-year-old man presented to clinic for evaluation of hyperkeratosis of the palms and soles that began when he was a teenager. He reported the soles of the feet often were painful, especially without shoes (Figure, A). He used many over-the-counter emollients and tried both prescription and nonprescription keratolytics. At presentation, he was mechanically paring down some of the thickness of the calluses to decrease the pain.

There was no relevant medical history, he had no history of smoking, he consumed more than 1 alcoholic drink per day, and he denied illicit drug use. The patient was not on any other medications. His family history revealed that his father also had the same hyperkeratosis of the palms and soles and died from esophageal carcinoma at an early age. It was determined that his father had tylosis with esophageal carcinoma (Howel-Evans syndrome). (The patient’s pedigree previously was published.3,4) Physical examination at presentation revealed plantar hyperkeratosis limited mainly to areas of pressure. His hands had mild hyperkeratosis on the distal fingers. No mucosa leukoplakia was identified.
Treatment options were discussed, and because the pain associated with the plantar keratoderma was interfering with his quality of life (QOL), acitretin was started. The initial dosage was 10 mg daily for 2 weeks and subsequently was increased to 25 mg daily. He has been maintained on this dosage for more than a year. An attempt was made to increase acitretin to 50 mg daily; however, he could not tolerate the dryness and peeling of the hands caused by the higher dosage. A fasting lipid panel and hepatic function panel performed every 3 months was within reference range. He had a remarkable decrease in the hyperkeratosis 2 months after starting therapy (Figure, B) and most importantly a decrease in pain associated with it. His QOL notably improved, enabling him to participate in sporting events with his children without severe pain. This patient was referred to gastroenterology where an esophagogastroduodenoscopy was performed and no concerning lesions were found. He was continued on this dose for 2 years. He moved to a new town, and our most recent update from him was that he was taking acitretin intermittently before big sporting events with his children.
The use of systemic retinoids has long been known to be effective in the treatment of disorders of keratinization. Recommended monitoring guidelines include a baseline complete blood cell count, renal function, hepatic function, and fasting lipid panel, which should be repeated every 3 months focusing on the hepatic function and lipid panel, as retinoids rarely cause hematologic or renal abnormalities.7 Our patient’s baseline laboratory test results were within reference range, and we repeated a fasting lipid and hepatic function panel every 3 months without any abnormalities.
Diffuse idiopathic skeletal hyperostosis (DISH), the ossification of ligaments and entheses often of the spine, is a potential complication of long-term use of oral retinoids. There are no consensus guidelines on screening for this complication, but baseline and annual radiographs seem reasonable. A 1996 study concluded that if DISH occurs, it is likely to be sporadic in a predisposed patient, as their data did not find any statistically significant relationship between the treatment or the cumulative dose and the prevalence and severity of DISH, degenerative changes, and osteoporosis.8 When annual screening is declined, imaging could be performed if a new skeletal concern were to arise in patients on long-term therapy.7 We discussed the skeletal concerns with our patient and he declined baseline or annual radiographs, but we will follow him with a rheumatologic review of systems. We feel this approach is reasonable, as our patient is a healthy adult in his 50s with no prior retinoid exposure and is on a low to moderate dose.
We report a case of Howel-Evans keratoderma successfully managed with acitretin. In patients with painful keratoderma that is interfering with QOL, low-dose acitretin can be used to diminish these symptoms.
- Howel-Evans W, McConnell RB, Clarke CA, et al. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958;27:413-429.
- Rogaev EI, Rogaeva EA, Ginter EK, et al. Identification of the genetic locus for keratosis palmaris et plantaris on chromosome 17 near the RARA and keratin type I genes. Nat Genet. 1993;5:158-162.
- Stevens HP, Kelsell DP, Bryant SP, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996;132:640-651.
- Marger RS, Marger D. Carcinoma of the esophagus and tylosis. a lethal genetic combination. Cancer. 1993;72:17-19.
- Tyldesley WR. Oral leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1974;3:62-70.
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B:102-112.
- Wu J, Wolverton S. Systemic retinoids. In: Wolverton S, ed. Comprehensive Dermatologic Drug Therapy. 4th ed. Edinburgh, Scotland: Elsevier; 2020:245-262.
- Van Dooren-Greebe RJ, Lemmens JA, De Boo T, et al. Prolonged treatment with oral retinoids in adults: no influence on the frequency and severity of spinal abnormalities. Br J Dermatol. 1996;134:71-76.
- Howel-Evans W, McConnell RB, Clarke CA, et al. Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med. 1958;27:413-429.
- Rogaev EI, Rogaeva EA, Ginter EK, et al. Identification of the genetic locus for keratosis palmaris et plantaris on chromosome 17 near the RARA and keratin type I genes. Nat Genet. 1993;5:158-162.
- Stevens HP, Kelsell DP, Bryant SP, et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. literature survey and proposed updated classification of the keratodermas. Arch Dermatol. 1996;132:640-651.
- Marger RS, Marger D. Carcinoma of the esophagus and tylosis. a lethal genetic combination. Cancer. 1993;72:17-19.
- Tyldesley WR. Oral leukoplakia associated with tylosis and esophageal carcinoma. J Oral Pathol. 1974;3:62-70.
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family—a review of six generations. Eur J Cancer B Oral Oncol. 1994;30B:102-112.
- Wu J, Wolverton S. Systemic retinoids. In: Wolverton S, ed. Comprehensive Dermatologic Drug Therapy. 4th ed. Edinburgh, Scotland: Elsevier; 2020:245-262.
- Van Dooren-Greebe RJ, Lemmens JA, De Boo T, et al. Prolonged treatment with oral retinoids in adults: no influence on the frequency and severity of spinal abnormalities. Br J Dermatol. 1996;134:71-76.
Practice Points
- Keratoderma can be especially painful for patients and can have a great impact on their quality of life. For these patients, acitretin should be considered when topical therapies have failed.
- Howel-Evans syndrome is an autosomal-dominant condition that predominantly presents with plantar keratoderma and has a high risk for esophageal cancer.