User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Purpuric Bullae on the Lower Extremities
The Diagnosis: Bullous Leukocytoclastic Vasculitis
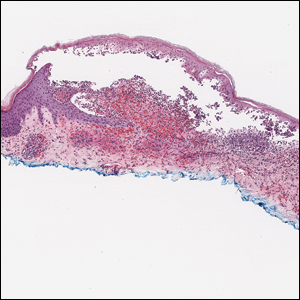
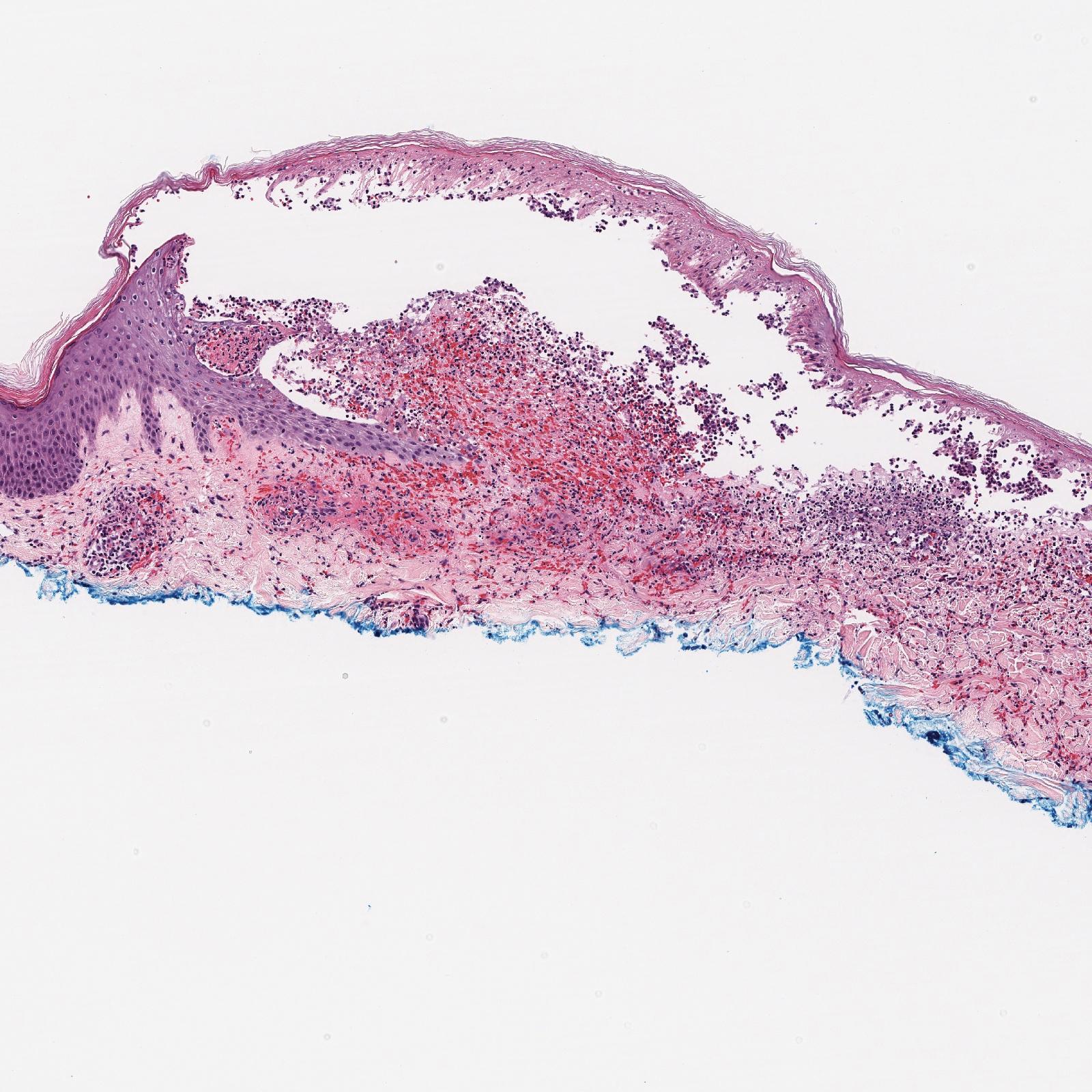
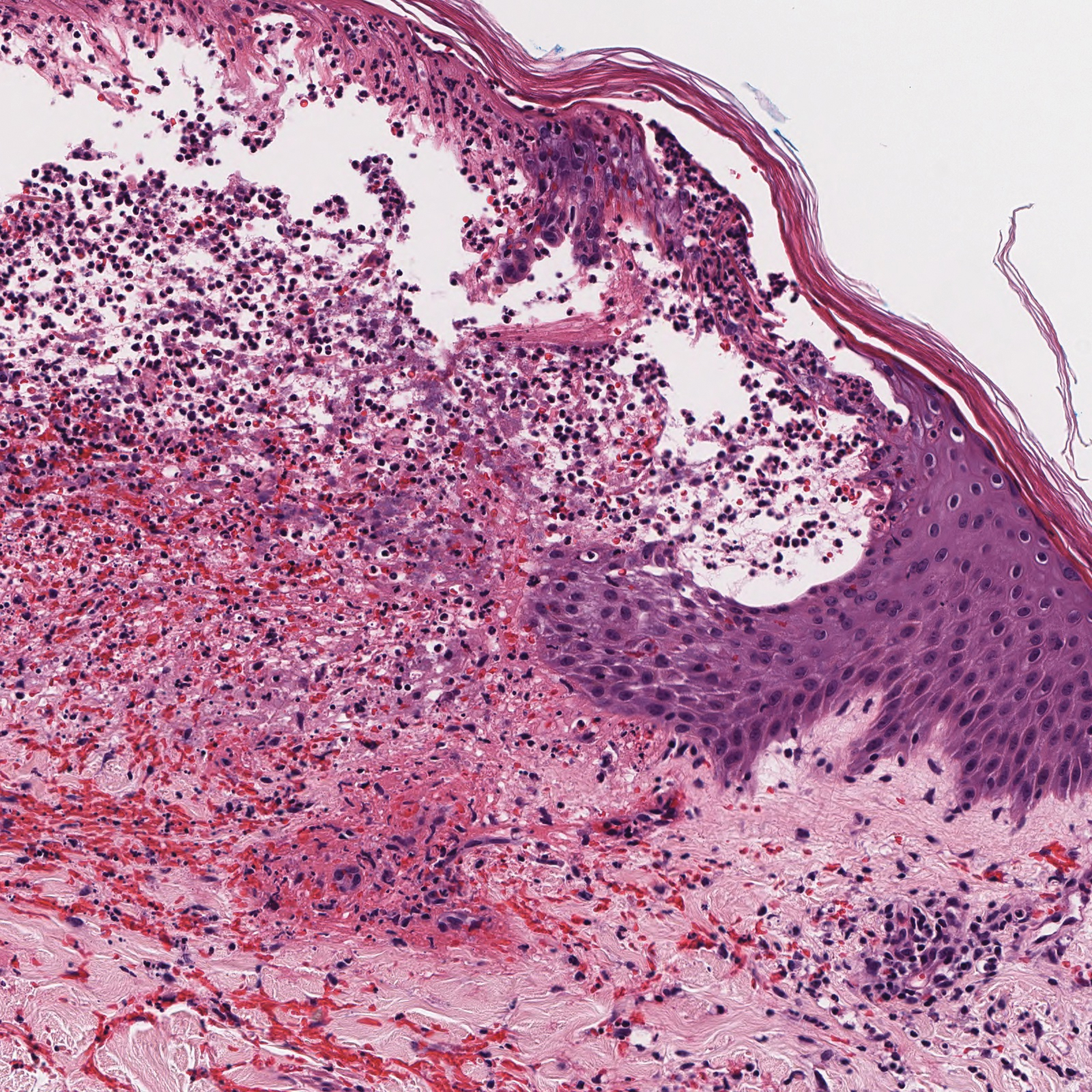
Histopathology with hematoxylin and eosin (H&E) stain showed a perivascular neutrophilic infiltrate, karyorrhexis, red blood cell extravasation, and fibrin deposition in the vessel wall (quiz images). Direct immunofluorescence (DIF) showed fibrin surrounding the vasculature, consistent with vasculitis. The clinical and histopathological evaluation supported the diagnosis of bullous leukocytoclastic vasculitis (LCV). The patient had a full LCV workup including antinuclear antibody, rheumatoid factor, hepatitis B and hepatitis C screening, erythrocyte sedimentation rate, C-reactive protein, and C3/C4/total complement level, which were all within reference range. The patient denied that she had taken any medications prior to the onset of the rash. She was started on a 12-day prednisone taper starting at 60 mg, and the rash resolved in 1 week.
Although the incidence of LCV is estimated to be 30 cases per million individuals per year,1 bullous LCV is a rarer entity with only a few cases reported in the literature.2,3 As in our patient's case, up to 50% of LCV cases are idiopathic or the etiology cannot be determined despite laboratory workup and medication review. Other cases can be secondary to medication, infection, collagen vascular disease, or malignancy.3 Despite the exact pathogenesis of bullous LCV being unknown,4 it likely is related to a type III hypersensitivity reaction with immune complex deposition in postcapillary venules leading to endothelial injury, activation of the complement cascade, and development of intraepidermal or subepidermal blister formation depending on location of inflammation and edema.2 Clinically, an intraepidermal split would be more flaccid, similar to pemphigus vulgaris, while a subepidermal split, as in our patient, would be taut bullae. The subepidermal split more commonly is seen in bullous LCV.2
Leukocytoclastic vasculitis on H&E staining characteristically has a perivascular inflammatory infiltrate, neutrophilic fragments called leukocytoclasis, and blood extravasation.3 Extravasated blood presents clinically as petechiae. In this case, the petechiae helped distinguish this entity from the differential diagnosis. Furthermore, DIF would be helpful in distinguishing bullous diseases such as bullous pemphigoid (BP) and pemphigus vulgaris from LCV.2 Direct immunofluorescence in bullous LCV would have fibrinogen surrounding the vasculature without C3 and IgG deposition (intraepidermal or subepidermal).
Mild cases of LCV often resolve with supportive measures including elevation of the legs, ice packs applied to the affected area, and removal of the inciting drug or event.4 In the few cases reported in the literature, bullous LCV presented more diffusely than classic LCV with bullous lesions on the forearms and the lower extremities. Oral steroids are efficacious for extensive bullous LCV.4
The differential diagnosis of bullous LCV includes bullous diseases with subepidermal split including BP and linear IgA bullous dermatosis (LABD). Bullous pemphigoid is an autoimmune subepidermal blistering disease typically affecting patients older than 60 years.5 The pathogenesis of BP is related to development of autoantibodies directed against hemidesmosome components, bullous pemphigoid antigen (BPAG) 1 or BPAG2.5 Bullous pemphigoid presents clinically as widespread, generally pruritic, erythematous, urticarial plaques with bullae. Histologically, BP characteristically has a subepidermal split with superficial dermal edema and eosinophils at the dermoepidermal junction (Figure 1). Direct immunofluorescence confirms the diagnosis with IgG and C3 deposition in an n-serrated pattern at the dermoepidermal junction.6 Bullous pemphigoid can be distinguished from bullous LCV by the older age of presentation, DIF findings, and the absence of purpura.

Linear IgA bullous dermatosis represents a rare subepidermal vesiculobullous disease occurring in patients in their 60s.7 Clinically, this entity presents as tense bullae often located on the periphery of an urticarial plaque, classically called the "string of pearls sign." Histologically, LABD also presents with subepidermal split; however, neutrophils are the predominant cell type vs eosinophils in BP (Figure 2).7 Direct immunofluorescence is specific with a linear deposition of IgA at the dermoepidermal junction. Linear IgA bullous dermatosis most commonly is induced by vancomycin. Unlike bullous LCV, the bullae of LABD have an annular peripheral pattern on an erythematous base and lack purpura.

Stasis dermatitis is inflammation of the dermis due to venous insufficiency that often is present in the bilateral lower extremities. The disorder affects approximately 7% of adults older than 50 years, but it also can occur in younger patients.8 The pathophysiology of stasis dermatitis is caused by edema, which leads to extracellular fluid, plasma proteins, macrophages, and erythrocytes passing into the interstitial space. Patients with stasis dermatitis present with scaly erythematous papules and plaques or edematous blisters on the lower extremities. Diagnosis usually can be made clinically; however, a skin biopsy also can be helpful. Hematoxylin and eosin shows a pauci-inflammatory subepidermal bulla with fibrin (Figure 3).8 The overlying epidermis is intact. The dermis has cannon ball angiomatosis, red blood cell extravasation, and fibrosis typical of stasis dermatitis. Stasis dermatitis with bullae is cell poor and lacks the perivascular inflammatory infiltrate and neutrophilic fragments that often are present in LCV, making the 2 entities distinguishable.

Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) lies on a spectrum of severe cutaneous drug reactions involving the skin and mucous membranes. Cutaneous involvement typically begins on the trunk and face and later can involve the palms and soles.9 Similar drugs have been implicated in bullous LCV and SJS/TEN, including nonsteroidal anti-inflammatory drugs and antibiotics. Histologically, SJS/TEN has full-thickness epidermal necrolysis, vacuolar interface, and keratinocyte apoptosis (Figure 4).9 The clinical presentation of sloughing of skin with positive Nikolsky sign, oral involvement, and H&E and DIF findings can help differentiate this entity from bullous LCV.

- Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77-78.
- Davidson KA, Ringpfeil F, Lee JB. Ibuprofen-induced bullous leukocytoclastic vasculitis. Cutis. 2001;67:303-307.
- Lazic T, Fonder M, Robinson-Bostom L, et al. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. 2013;91:148-149.
- Mericliler M, Shnawa A, Al-Qaysi D, et al. Oxacillin-induced leukocytoclastic vasculitis. IDCases. 2019;17:E00539.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513-528.
- High WA. Blistering disorders. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019:161-171.
- Visentainer L, Massuda JY, Cintra ML, et al. Vancomycin-induced linear IgA bullous dermatosis (LABD)--an atypical presentation. Clin Case Rep. 2019;7:1091-1093.
- Hyman DA, Cohen PR. Stasis dermatitis as a complication of recurrent levofloxacin-associated bilateral leg edema. Dermatol Online J. 2013;19:20399.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
The Diagnosis: Bullous Leukocytoclastic Vasculitis
Histopathology with hematoxylin and eosin (H&E) stain showed a perivascular neutrophilic infiltrate, karyorrhexis, red blood cell extravasation, and fibrin deposition in the vessel wall (quiz images). Direct immunofluorescence (DIF) showed fibrin surrounding the vasculature, consistent with vasculitis. The clinical and histopathological evaluation supported the diagnosis of bullous leukocytoclastic vasculitis (LCV). The patient had a full LCV workup including antinuclear antibody, rheumatoid factor, hepatitis B and hepatitis C screening, erythrocyte sedimentation rate, C-reactive protein, and C3/C4/total complement level, which were all within reference range. The patient denied that she had taken any medications prior to the onset of the rash. She was started on a 12-day prednisone taper starting at 60 mg, and the rash resolved in 1 week.
Although the incidence of LCV is estimated to be 30 cases per million individuals per year,1 bullous LCV is a rarer entity with only a few cases reported in the literature.2,3 As in our patient's case, up to 50% of LCV cases are idiopathic or the etiology cannot be determined despite laboratory workup and medication review. Other cases can be secondary to medication, infection, collagen vascular disease, or malignancy.3 Despite the exact pathogenesis of bullous LCV being unknown,4 it likely is related to a type III hypersensitivity reaction with immune complex deposition in postcapillary venules leading to endothelial injury, activation of the complement cascade, and development of intraepidermal or subepidermal blister formation depending on location of inflammation and edema.2 Clinically, an intraepidermal split would be more flaccid, similar to pemphigus vulgaris, while a subepidermal split, as in our patient, would be taut bullae. The subepidermal split more commonly is seen in bullous LCV.2
Leukocytoclastic vasculitis on H&E staining characteristically has a perivascular inflammatory infiltrate, neutrophilic fragments called leukocytoclasis, and blood extravasation.3 Extravasated blood presents clinically as petechiae. In this case, the petechiae helped distinguish this entity from the differential diagnosis. Furthermore, DIF would be helpful in distinguishing bullous diseases such as bullous pemphigoid (BP) and pemphigus vulgaris from LCV.2 Direct immunofluorescence in bullous LCV would have fibrinogen surrounding the vasculature without C3 and IgG deposition (intraepidermal or subepidermal).
Mild cases of LCV often resolve with supportive measures including elevation of the legs, ice packs applied to the affected area, and removal of the inciting drug or event.4 In the few cases reported in the literature, bullous LCV presented more diffusely than classic LCV with bullous lesions on the forearms and the lower extremities. Oral steroids are efficacious for extensive bullous LCV.4
The differential diagnosis of bullous LCV includes bullous diseases with subepidermal split including BP and linear IgA bullous dermatosis (LABD). Bullous pemphigoid is an autoimmune subepidermal blistering disease typically affecting patients older than 60 years.5 The pathogenesis of BP is related to development of autoantibodies directed against hemidesmosome components, bullous pemphigoid antigen (BPAG) 1 or BPAG2.5 Bullous pemphigoid presents clinically as widespread, generally pruritic, erythematous, urticarial plaques with bullae. Histologically, BP characteristically has a subepidermal split with superficial dermal edema and eosinophils at the dermoepidermal junction (Figure 1). Direct immunofluorescence confirms the diagnosis with IgG and C3 deposition in an n-serrated pattern at the dermoepidermal junction.6 Bullous pemphigoid can be distinguished from bullous LCV by the older age of presentation, DIF findings, and the absence of purpura.

Linear IgA bullous dermatosis represents a rare subepidermal vesiculobullous disease occurring in patients in their 60s.7 Clinically, this entity presents as tense bullae often located on the periphery of an urticarial plaque, classically called the "string of pearls sign." Histologically, LABD also presents with subepidermal split; however, neutrophils are the predominant cell type vs eosinophils in BP (Figure 2).7 Direct immunofluorescence is specific with a linear deposition of IgA at the dermoepidermal junction. Linear IgA bullous dermatosis most commonly is induced by vancomycin. Unlike bullous LCV, the bullae of LABD have an annular peripheral pattern on an erythematous base and lack purpura.

Stasis dermatitis is inflammation of the dermis due to venous insufficiency that often is present in the bilateral lower extremities. The disorder affects approximately 7% of adults older than 50 years, but it also can occur in younger patients.8 The pathophysiology of stasis dermatitis is caused by edema, which leads to extracellular fluid, plasma proteins, macrophages, and erythrocytes passing into the interstitial space. Patients with stasis dermatitis present with scaly erythematous papules and plaques or edematous blisters on the lower extremities. Diagnosis usually can be made clinically; however, a skin biopsy also can be helpful. Hematoxylin and eosin shows a pauci-inflammatory subepidermal bulla with fibrin (Figure 3).8 The overlying epidermis is intact. The dermis has cannon ball angiomatosis, red blood cell extravasation, and fibrosis typical of stasis dermatitis. Stasis dermatitis with bullae is cell poor and lacks the perivascular inflammatory infiltrate and neutrophilic fragments that often are present in LCV, making the 2 entities distinguishable.

Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) lies on a spectrum of severe cutaneous drug reactions involving the skin and mucous membranes. Cutaneous involvement typically begins on the trunk and face and later can involve the palms and soles.9 Similar drugs have been implicated in bullous LCV and SJS/TEN, including nonsteroidal anti-inflammatory drugs and antibiotics. Histologically, SJS/TEN has full-thickness epidermal necrolysis, vacuolar interface, and keratinocyte apoptosis (Figure 4).9 The clinical presentation of sloughing of skin with positive Nikolsky sign, oral involvement, and H&E and DIF findings can help differentiate this entity from bullous LCV.

The Diagnosis: Bullous Leukocytoclastic Vasculitis
Histopathology with hematoxylin and eosin (H&E) stain showed a perivascular neutrophilic infiltrate, karyorrhexis, red blood cell extravasation, and fibrin deposition in the vessel wall (quiz images). Direct immunofluorescence (DIF) showed fibrin surrounding the vasculature, consistent with vasculitis. The clinical and histopathological evaluation supported the diagnosis of bullous leukocytoclastic vasculitis (LCV). The patient had a full LCV workup including antinuclear antibody, rheumatoid factor, hepatitis B and hepatitis C screening, erythrocyte sedimentation rate, C-reactive protein, and C3/C4/total complement level, which were all within reference range. The patient denied that she had taken any medications prior to the onset of the rash. She was started on a 12-day prednisone taper starting at 60 mg, and the rash resolved in 1 week.
Although the incidence of LCV is estimated to be 30 cases per million individuals per year,1 bullous LCV is a rarer entity with only a few cases reported in the literature.2,3 As in our patient's case, up to 50% of LCV cases are idiopathic or the etiology cannot be determined despite laboratory workup and medication review. Other cases can be secondary to medication, infection, collagen vascular disease, or malignancy.3 Despite the exact pathogenesis of bullous LCV being unknown,4 it likely is related to a type III hypersensitivity reaction with immune complex deposition in postcapillary venules leading to endothelial injury, activation of the complement cascade, and development of intraepidermal or subepidermal blister formation depending on location of inflammation and edema.2 Clinically, an intraepidermal split would be more flaccid, similar to pemphigus vulgaris, while a subepidermal split, as in our patient, would be taut bullae. The subepidermal split more commonly is seen in bullous LCV.2
Leukocytoclastic vasculitis on H&E staining characteristically has a perivascular inflammatory infiltrate, neutrophilic fragments called leukocytoclasis, and blood extravasation.3 Extravasated blood presents clinically as petechiae. In this case, the petechiae helped distinguish this entity from the differential diagnosis. Furthermore, DIF would be helpful in distinguishing bullous diseases such as bullous pemphigoid (BP) and pemphigus vulgaris from LCV.2 Direct immunofluorescence in bullous LCV would have fibrinogen surrounding the vasculature without C3 and IgG deposition (intraepidermal or subepidermal).
Mild cases of LCV often resolve with supportive measures including elevation of the legs, ice packs applied to the affected area, and removal of the inciting drug or event.4 In the few cases reported in the literature, bullous LCV presented more diffusely than classic LCV with bullous lesions on the forearms and the lower extremities. Oral steroids are efficacious for extensive bullous LCV.4
The differential diagnosis of bullous LCV includes bullous diseases with subepidermal split including BP and linear IgA bullous dermatosis (LABD). Bullous pemphigoid is an autoimmune subepidermal blistering disease typically affecting patients older than 60 years.5 The pathogenesis of BP is related to development of autoantibodies directed against hemidesmosome components, bullous pemphigoid antigen (BPAG) 1 or BPAG2.5 Bullous pemphigoid presents clinically as widespread, generally pruritic, erythematous, urticarial plaques with bullae. Histologically, BP characteristically has a subepidermal split with superficial dermal edema and eosinophils at the dermoepidermal junction (Figure 1). Direct immunofluorescence confirms the diagnosis with IgG and C3 deposition in an n-serrated pattern at the dermoepidermal junction.6 Bullous pemphigoid can be distinguished from bullous LCV by the older age of presentation, DIF findings, and the absence of purpura.

Linear IgA bullous dermatosis represents a rare subepidermal vesiculobullous disease occurring in patients in their 60s.7 Clinically, this entity presents as tense bullae often located on the periphery of an urticarial plaque, classically called the "string of pearls sign." Histologically, LABD also presents with subepidermal split; however, neutrophils are the predominant cell type vs eosinophils in BP (Figure 2).7 Direct immunofluorescence is specific with a linear deposition of IgA at the dermoepidermal junction. Linear IgA bullous dermatosis most commonly is induced by vancomycin. Unlike bullous LCV, the bullae of LABD have an annular peripheral pattern on an erythematous base and lack purpura.

Stasis dermatitis is inflammation of the dermis due to venous insufficiency that often is present in the bilateral lower extremities. The disorder affects approximately 7% of adults older than 50 years, but it also can occur in younger patients.8 The pathophysiology of stasis dermatitis is caused by edema, which leads to extracellular fluid, plasma proteins, macrophages, and erythrocytes passing into the interstitial space. Patients with stasis dermatitis present with scaly erythematous papules and plaques or edematous blisters on the lower extremities. Diagnosis usually can be made clinically; however, a skin biopsy also can be helpful. Hematoxylin and eosin shows a pauci-inflammatory subepidermal bulla with fibrin (Figure 3).8 The overlying epidermis is intact. The dermis has cannon ball angiomatosis, red blood cell extravasation, and fibrosis typical of stasis dermatitis. Stasis dermatitis with bullae is cell poor and lacks the perivascular inflammatory infiltrate and neutrophilic fragments that often are present in LCV, making the 2 entities distinguishable.

Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) lies on a spectrum of severe cutaneous drug reactions involving the skin and mucous membranes. Cutaneous involvement typically begins on the trunk and face and later can involve the palms and soles.9 Similar drugs have been implicated in bullous LCV and SJS/TEN, including nonsteroidal anti-inflammatory drugs and antibiotics. Histologically, SJS/TEN has full-thickness epidermal necrolysis, vacuolar interface, and keratinocyte apoptosis (Figure 4).9 The clinical presentation of sloughing of skin with positive Nikolsky sign, oral involvement, and H&E and DIF findings can help differentiate this entity from bullous LCV.

- Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77-78.
- Davidson KA, Ringpfeil F, Lee JB. Ibuprofen-induced bullous leukocytoclastic vasculitis. Cutis. 2001;67:303-307.
- Lazic T, Fonder M, Robinson-Bostom L, et al. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. 2013;91:148-149.
- Mericliler M, Shnawa A, Al-Qaysi D, et al. Oxacillin-induced leukocytoclastic vasculitis. IDCases. 2019;17:E00539.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513-528.
- High WA. Blistering disorders. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019:161-171.
- Visentainer L, Massuda JY, Cintra ML, et al. Vancomycin-induced linear IgA bullous dermatosis (LABD)--an atypical presentation. Clin Case Rep. 2019;7:1091-1093.
- Hyman DA, Cohen PR. Stasis dermatitis as a complication of recurrent levofloxacin-associated bilateral leg edema. Dermatol Online J. 2013;19:20399.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
- Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77-78.
- Davidson KA, Ringpfeil F, Lee JB. Ibuprofen-induced bullous leukocytoclastic vasculitis. Cutis. 2001;67:303-307.
- Lazic T, Fonder M, Robinson-Bostom L, et al. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. 2013;91:148-149.
- Mericliler M, Shnawa A, Al-Qaysi D, et al. Oxacillin-induced leukocytoclastic vasculitis. IDCases. 2019;17:E00539.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513-528.
- High WA. Blistering disorders. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019:161-171.
- Visentainer L, Massuda JY, Cintra ML, et al. Vancomycin-induced linear IgA bullous dermatosis (LABD)--an atypical presentation. Clin Case Rep. 2019;7:1091-1093.
- Hyman DA, Cohen PR. Stasis dermatitis as a complication of recurrent levofloxacin-associated bilateral leg edema. Dermatol Online J. 2013;19:20399.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
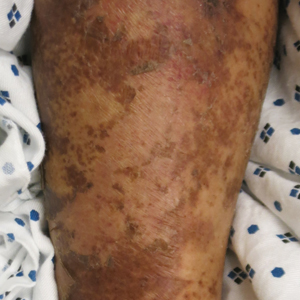
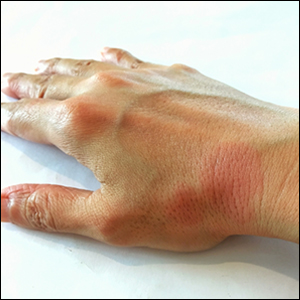
A 30-year-old woman with a medical history of uncontrolled type 2 diabetes mellitus and morbid obesity presented to the dermatology clinic with a painful blistering rash on the lower extremities with scattered red-purple papules of 1 week's duration. The rash began on the left dorsal foot. Physical examination showed nonblanching, 2- to 4-mm, violaceous papules with numerous vesiculopustular bullae on the lower extremities from the dorsal feet to the proximal knee. A shave biopsy with hematoxylin and eosin stain and a punch biopsy for direct immunofluorescence were performed.
Nutritional Dermatoses in the Hospitalized Patient
The World Health Organization defines malnutrition as deficiencies, excesses, or imbalances in an individual’s intake of energy and/or nutrients.1 This review will focus on undernutrition, which may result from macronutrient or micronutrient deficiencies. Undernutrition in the hospitalized patient is a common yet underrecognized phenomenon, with an estimated prevalence of 20% to 50% worldwide.2 Malnutrition is an independent risk factor for patient morbidity and mortality and has been associated with increased health care costs.3 Nutritional deficiencies may arise from inadequate nutrient intake, abnormal nutrient absorption, or improper nutrient utilization.4 Unfortunately, no standardized algorithm for screening and diagnosing patients with malnutrition exists, making early physical examination findings of utmost importance. Herein, we present a review of acquired nutritional deficiency dermatoses in the inpatient setting.
Protein-Energy Malnutrition
Protein-energy malnutrition (PEM) refers to a set of related disorders that include marasmus, kwashiorkor (KW), and marasmic KW. These conditions frequently are seen in developing countries but also have been reported in developed nations.5 Marasmus occurs from a chronic deficiency of protein and calories. Decreased insulin production and unopposed catabolism result in sarcopenia and loss of bone and subcutaneous fat.6 Affected patients include children who are less than 60% ideal body weight (IBW) without edema or hypoproteinemia.7 Kwashiorkor is the edematous form of PEM that develops from isolated protein deficiency, resulting in edema, diarrhea, and immunosuppression.6 Micronutrient deficiencies, oxidative stress, slow protein catabolism, and excess antidiuretic hormone have been proposed as potential drivers of KW.8 Kwashiorkor affects children between 60% and 80% IBW. Marasmic KW has features of both diseases, including children who are less than 60% IBW but with associated edema and/or hypoproteinemia.9
Although PEM is uncommon in adults, hospitalized patients carry many predisposing risk factors, including infections, malabsorptive conditions, psychiatric disease, and chronic illness (eTable). Patients with chronic infections present with findings consistent with marasmic KW due to lean body mass loss.
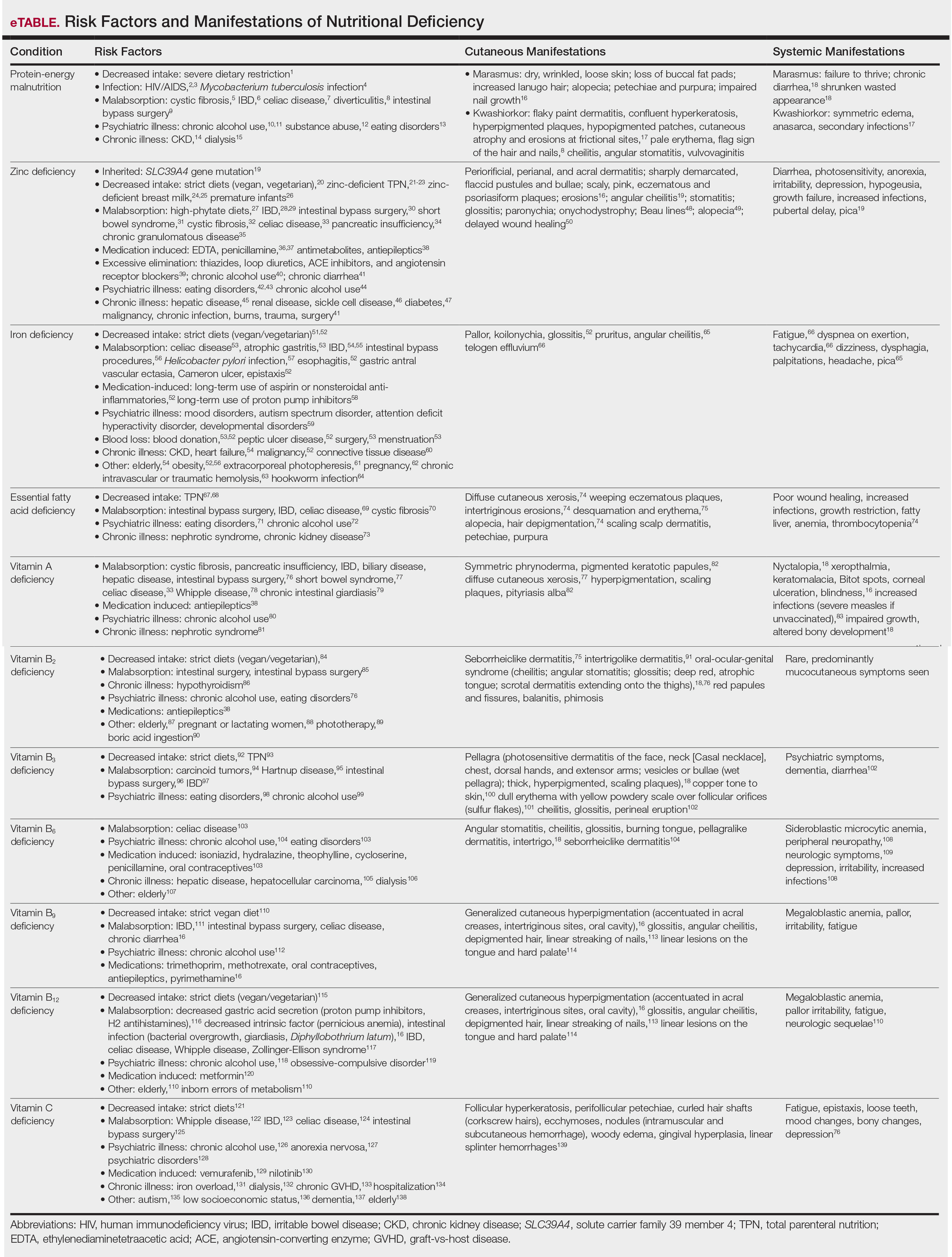
The cutaneous findings in PEM are related to dysmaturation of epidermal keratinocytes and resultant epidermal atrophy.10 Patients with marasmus exhibit dry, wrinkled, loose skin due to subcutaneous fat loss. Emaciated children often lose their buccal fat pads, and reduced perianal adipose may lead to rectal prolapse. Increased lanugo hair may be present on the face, and alopecia of the scalp may occur.6 In KW, cutaneous disease progresses from confluent hyperkeratosis to a dry atrophic epidermis that erodes easily, leaving underlying pale erythema. The resultant pattern is one of hyperpigmented plaques with slightly raised borders, and hypopigmented patches and erosions described as flaky paint dermatitis (Figure 1).5 Lesions appear first in areas of friction. The hair often is dry and brittle; curly hair may straighten and scale.11 Red-yellow to gray-white hypopigmentation may develop, denoting periods of inadequate nutrition. The flag sign describes alternating horizontal bands of hypopigmentation interspersed with bands of pigmented hair. The nails usually are thin and soft and may exhibit the nail flag sign, characterized by horizontal bands of white and red.12 Cheilitis, angular stomatitis, and vulvovaginitis may be present.6
In adults, weight loss and body mass index can be used to assess nutritional status, along with a focused history and physical examination. Complete blood cell count, electrolyte levels, and blood urea nitrogen should be assessed, as hypoglycemia and anemia often accompany PEM.13 In KW, hypoalbuminemia and hypoproteinemia are invariably present. Although prealbumin may be a valid prognostic indicator of disease outcomes and mortality in patients at risk for malnutrition, checking other serum biomarkers remains controversial.14 Focused testing may be warranted in patients with risk factors for chronic infectious processes, such as human immunodeficiency virus or tuberculosis.6 Skin biopsy may solidify the diagnosis of PEM. Hypertrophy of the stratum corneum, atrophy of the stratum spinosum and stratum granulosum, and increased basal layer melanin have been reported.15
Treatment involves initial fluid resuscitation and correction of electrolyte imbalances, followed by nutritional replacement.13 Oral or enteral tube feedings are preferred over total parenteral nutrition (TPN), as they enhance recovery of the gastrointestinal tract.16 Refeeding should occur in small amounts and frequent intervals.5 Skin-directed therapy is aimed at restoring epidermal function and hydration, with regular moisturization and application of barrier creams, such as zinc oxide ointment or petrolatum.10
Zinc Deficiency
Zinc is an essential trace element that provides regulatory, structural, and catalytic functions across multiple biochemical pathways6 and serves as an enzymatic cofactor and key component for numerous transcription factors.17 Zinc is derived from food sources, and its concentration correlates with protein content.18 Zinc is found in both animal and plant-based proteins, albeit with a lower oral bioavailability in the latter. Zinc deficiency may be inherited or acquired. Primary acrodermatitis enteropathica is an autosomal-recessive disorder of the solute carrier family 39 member 4 gene, SLC39A4 (encodes zinc transporter ZIP4 on enterocytes); the result is abnormal zinc absorption from the small intestine.18
Acquired zinc deficiency occurs from decreased dietary zinc intake, impaired intestinal zinc absorption, excessive zinc elimination, or systemic states of high catabolism or low albumin (eTable). Total parenteral nutrition–associated deficiency has arisen when nutritional formulations did not contain trace elements during national shortages or when prolonged TPN was not anticipated and trace elements were removed.19 Zinc levels may already be low in patients with chronic illness or inflammation, so even a short period on TPN can precipitate deficiency.18,19 Diets high in phytate may result in zinc deficiency, as phytate impairs intestinal zinc absorption.20 Approximately 15% of patients with inflammatory bowel disease experienced zinc deficiency worldwide.21 In Crohn disease, zinc deficiency has been associated with active intestinal inflammation, increased risk for hospitalization, surgeries, and disease-related complications.22,23
Medications such as antiepileptics, antimetabolites, or penicillamine may induce zinc deficiency, highlighting the importance of medication review for hospitalized patients (eTable). Catabolic states, frequently encountered in hospitalized patients, increase the risk for zinc deficiency.24 Patients with necrolytic migratory erythema (associated with pancreatic glucagonomas) often experience low serum zinc levels.25
The skin is the third most zinc-abundant tissue in the human body. Within keratinocytes, zinc is critical to normal proliferation and suppression of inflammation.17 Zinc also plays an important role in cutaneous immune function.26 Zinc deficiency presents with sharply demarcated, flaccid pustules and bullae that erode into scaly, pink, eczematous or psoriasiform plaques. Lesions are found preferentially in acral and periorificial sites, often with crusting and exudate. The groin and flexural surfaces may be affected. Erosions often become secondarily impetiginized. Other cutaneous findings include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.26 Histopathology of skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.27 Acquired bullous acrodermatitis enteropathica has been reported as a histologic mimicker of pemphigus foliaceous in patients on TPN.28
Diagnosis of zinc deficiency is made by measuring plasma zinc levels. Fasting levels should be drawn in the morning, as they can fluctuate based on the time of day, stress levels, or inflammation.6 Sample hemolysis and anticoagulants high in zinc may falsely elevate plasma zinc. A normal zinc level is greater than 70 µg/dL; however, normal levels do not rule out deficiency.18 Measurement of zinc-dependent enzymes, such as alkaline phosphatase, can be a quick way to assess zinc status. Serum albumin also should be measured; because zinc is carried by albumin in the blood, hypoalbuminemia may result in secondary zinc deficiency.18
Zinc replacement therapy is largely through oral supplementation and should start at 0.5 to 2.0 mg/kg/d in adults with acquired disease.29,30 Zinc sulfate is the most affordable and is the supplement of choice, with 50 mg of elemental zinc per 220 mg of zinc sulfate (~23% elemental zinc).31 Alternative zinc salts, such as zinc gluconate (13% elemental zinc), may be used. Patients with malabsorptive disorders often require parenteral supplementation.32 Clinical symptoms often will resolve within 1 to 2 weeks of supplementation.29 In patients with primary acrodermatitis enteropathica, lifelong supplementation with 3 mg/kg/d elemental zinc should occur.6 Calcium and folate may reduce zinc absorption, while zinc supplementation can interfere with copper and iron absorption.33
Iron Deficiency
Iron is an essential component of the hemoglobin molecule. Iron homeostasis and metabolism are tightly regulated processes that drive erythropoiesis. Only 5% to 10% of dietary iron is absorbed through nutrition, while the remainder is recycled from red cell breakdown. Both normal iron levels and iron deficiency (ID) are defined by age and gender.34 Iron-deficiency anemia (IDA) is one of the most common cause-specific anemias worldwide.35
Fatigue is the most common and earliest symptom of ID. In a single study, pallor was predictive of anemia in hospitalized patients; however, absence of pallor did not rule out anemia.34 Dyspnea on exertion, tachycardia, dysphagia, and pica also may be reported. Cutaneous manifestations include koilonychia (Figure 2), glossitis, pruritus, angular cheilitis, and telogen effluvium. Plummer-Vinson syndrome is characterized by microcytic anemia, glossitis, and dysphagia.
Risk factors for ID include insufficient dietary consumption,36 blood loss, malabsorptive states,37,38 and increased iron requirements (eTable). Patient fragility (eg, elderly, chronic disease) is a newly described risk factor where correction of ID may impact morbidity, mortality, and quality of life.35
Iron deficiency can be present despite a normal hemoglobin level. Serum ferritin and percentage transferrin saturation are key to early identification of IDA.35 Ferritin levels lower than 30 µg/L confirm the diagnosis. Decreased transferrin saturation and increased total iron binding capacity aid in the diagnosis of IDA. Serum ferritin is an acute-phase reactant, and levels may be falsely elevated in the setting of inflammation or infection.
Treatment includes reversing the cause of deficiency and supplementing iron. Calculation of the total iron deficit can help inform iron supplementation. First-line therapy for IDA is oral ferrous sulfate 325 mg (65 mg elemental iron) 3 times daily. Newer studies suggest 40 to 80 mg oral iron should be taken every other day to increase absorption.39 Other iron salts, such as ferrous gluconate (325 mg is equivalent to 38 mg elemental iron), have been used. Iron absorption is enhanced by an acidic environment. Parenteral iron is utilized in patients with uncorrectable blood loss, malabsorption, renal failure, intolerance to oral iron, and nonadherence in those who are unable to receive transfusions. Iron infusions are favored in frail patients, such as the elderly and those with chronic kidney disease or heart failure.35 Multiple parenteral iron formulations exist, and their use should be driven by underlying patient comorbidities and potential risks. Packed red blood cell transfusions should be considered in acute blood loss, hypoxia, or cardiac insufficiency.
Essential Fatty Acid Deficiency
Essential fatty acids (EFAs) including linoleic and α-linolenic acid cannot be synthesized by the human body and must be obtained through diet (mostly plant oils). Essential fatty acids have various functions, including maintaining phospholipid membrane integrity, forming prostaglandins and leukotrienes, and storing energy.40 Essential fatty acids are important in the structure and function of the stratum corneum and are crucial in maintaining epidermal barrier function.41 Increased epidermal permeability and transepidermal water loss may be the first signs of EFA deficiency (EFAD).42
The cutaneous manifestations of EFAD include xerosis, weeping eczematous plaques, and erosions in intertriginous sites. The lesions may progress to widespread desquamation and erythema. With time, the skin can become thick and leathery. Alopecia may occur, and hair may depigment.7 Additional findings include poor wound healing and increased susceptibility to infections.43,44
Essential fatty acid deficiency may occur when dietary fat intake is severely restricted or in malabsorptive states.45,46 It develops in patients on prolonged TPN, typically when receiving fat-restricted nutrition,47,48 as occurs in hypertriglyceridemia.47 Essential fatty acid deficiency has developed in patients on TPN containing EFAs,47 as the introduction of novel intravenous lipid emulsions has resulted in varying proportions of EFA.40 Premature neonates are particularly at risk for EFAD.49
The diagnosis of EFAD involves the measurement of the triene to tetraene ratio. A ratio of more than 0.2 suggests EFAD, but the clinical signs are not seen until the ratio is over 0.4.40 Low plasma levels of linoleic, linolenic, and arachidonic acids also are seen. Elevated liver function tests are supportive of the diagnosis. Biochemical findings typically are seen before cutaneous manifestations.40
Treatment of EFAD includes topical, oral, or intravenous replacement of EFAs. Improvement of EFAD with the application of topical linoleic acid to the skin has been reported.50 Patients receiving TPN should undergo assessment of parenteral lipid emulsion to ensure adequate fatty acid composition.
Vitamin A Deficiency
Vitamin A (retinol) is a fat-soluble vitamin that plays a critical role in keratinization, epithelial proliferation, and cellular differentiation.6 Vitamin A is found in animal products as retinyl esters and in plants as beta-carotene. Vitamin A has 2 clinically important forms: all-trans retinoic acid and 11-cis-retinal. All-trans retinoic acid is involved in cellular differentiation and regulating gene transcription, while 11-cis-retinal is key to rhodopsin generation required for vision. Vitamin A deficiency presents with early ophthalmologic findings, specifically nyctalopia, or delayed adaptation to the dark.51 Xerophthalmia, abnormal conjunctival keratinization, and Bitot spots subsequently develop and may progress to corneal ulceration and blindness.6
Vitamin A deficiency manifests in the skin as follicular hyperkeratosis, or phrynoderma. Notably, numerous other micronutrient deficiencies may result in phrynoderma. Clinically, multiple pigmented keratotic papules of various sizes, many with a central keratinous plug, are distributed symmetrically on the extensor elbows, knees, shoulders, buttocks, and extremities. The skin surrounding these lesions may be scaly and hyperpigmented.52 Generalized xerosis without preceding nyctalopia has been reported.53 Accompanying pityriasis alba may develop.52 Lesions on the face may mimic acne, while lesions on the extremities may simulate a perforating disorder. Histopathology of phrynoderma reveals epidermal hyperkeratosis, follicular hyperkeratosis, and follicular plugging.52
Patients at risk for vitamin A deficiency include those with conditions that affect intestinal fat absorption, underlying psychiatric illness, or chronic disease (eTable). Chronic alcohol use predisposes patients to a multitude of micronutrient deficiencies, including vitamin A deficiency.54 In chronic alcohol use, even mild cutaneous changes may be the first clue to low serum retinol.55
Vitamin A deficiency can be diagnosed by measuring serum retinol levels, with levels lower than 20 µg/dL being diagnostic of deficiency.56 Decreased serum retinol in patients hospitalized with flaring irritable bowel disorder has been repeatedly reported.57-59 Notably, serum retinol concentration does not decline until liver reserves of vitamin A are nearing exhaustion.33
The US Food and Drug Administration requires manufacturers to list retinol activity equivalents on labels. One international unit of retinol is equivalent to 0.3 µg of retinol activity equivalents.60 The treatment of vitamin A deficiency involves high-dose oral supplementation when possible.61 Although dependent on age, the treatment dose for most adults with vitamin A deficiency is 3000 µg (10,000 IU) once daily.
Phrynoderma has been specifically treated with salicylic acid ointment 3% and intramuscular vitamin A.62 Topical urea cream also may treat phrynoderma.63
Vitamin B2
Vitamin B2 (riboflavin) is absorbed in the small intestine and converted into 2 biologically active forms—flavin adenine dinucleotide and flavin mononucleotide—which serve as cofactors in metabolic and oxidation-reduction reactions. Malabsorptive disorders and bowel resection can lead to riboflavin deficiency.64 Other at-risk populations include those with restrictive diets,65 psychiatric illness, or systemic illness (eTable). Riboflavin can be degraded by light (deficiency has been reported after phototherapy for neonatal jaundice66) and following boric acid ingestion.67 Medications, including long-term treatment with antiepileptics, may lead to riboflavin deficiency.68
Riboflavin is critical to maintaining collagen production. Riboflavin deficiency may manifest clinically with extensive seborrheiclike dermatitis,44 intertrigolike dermatitis,69 or oral-ocular-genital syndrome.70 Angular cheilitis may accompany an atrophic tongue that is deep red in color. The scrotum is characteristically involved in men, with confluent dermatitis extending onto the thighs and sparing the midline. Red papules and painful fissures may develop. Balanitis and phimosis have been reported. Testing for riboflavin deficiency should be considered in patients with refractory seborrheic dermatitis.
Riboflavin stores are assessed by the erythrocyte glutathione reductase activity coefficient.44 A level of 1.4 or higher is consistent with deficiency. Serum riboflavin levels, performed after a 12-hour fast, may support the diagnosis but are less sensitive. Patients with glucose-6-phosphate deficiency cannot be assessed via the erythrocyte glutathione reductase activity coefficient and may instead require evaluation of 24-hour urine riboflavin level.44
Vitamin B3
Vitamin B3 (niacin, nicotinamide, nicotinic acid) is found in plant and animal products or can be derived from its amino acid precursor tryptophan. Niacin deficiency results in pellagra, characterized by dermatitis, dementia, and diarrhea.71 The most prominent feature is a symmetrically distributed photosensitive dermatitis of the face, neck (called Casal necklace)(Figure 3), chest, dorsal hands, and extensor arms. The eruption may begin with erythema, vesicles, or bullae (wet pellagra) and evolve into thick, hyperpigmented, scaling plaques.71 The skin may take on a copper tone and become atrophic.72 Dull erythema with overlying yellow powdery scale (called sulfur flakes) at follicular orifices has been described on the nasal bridge.73
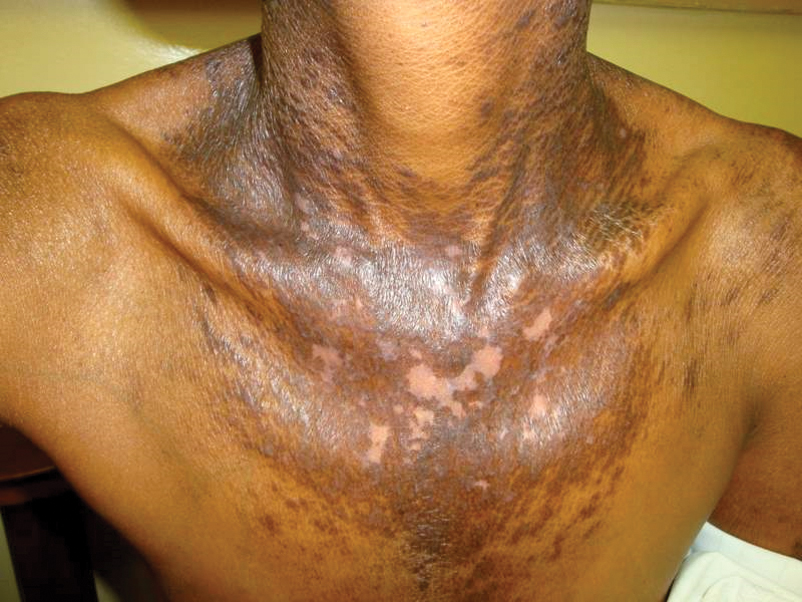
Causes of niacin deficiency include malabsorptive conditions, malignancy (including carcinoid tumors), parenteral nutrition, psychiatric disease,74,75 and restrictive diets (eTable).76 Carcinoid tumors divert tryptophan to serotonin resulting in niacin deficiency.77
The diagnosis of niacin deficiency is based on clinical findings and response to supplementation.75 Low niacin urinary metabolites (N-methylnicotinamide and 2-pyridone) may aid in diagnosis.6 Treatment generally includes oral nicotinamide 100 mg every 6 hours; the dose can then be tapered to 50 mg every 8 to 12 hours until symptoms resolve. Severe deficiency may require parenteral nicotinamide 1 g 3 to 4 times daily.75
Vitamin B6
Vitamin B6 (pyridoxine, pyridoxamine, pyridoxal) is found in whole grains and plant and animal products. Vitamin B6 functions as a coenzyme in many metabolic pathways and is involved in the conversion of tryptophan to niacin.44 Absorption requires hydrolysis by intestinal phosphates and transport to the liver for rephosphorylation prior to release in active form.6
Cutaneous findings associated with vitamin B6 deficiency include periorificial and perineal seborrheic dermatitis,78 angular stomatitis, and cheilitis, with associated burning, redness, and tongue edema.6 Vitamin B6 deficiency is a rarely reported cause of burning mouth syndrome.79 Because vitamin B6 is involved in the conversion of tryptophan to niacin, deficiency also may present with pellagralike findings.70 Other clinical symptoms are outlined in the eTable.80,81
Conditions that increase risk for vitamin B6 deficiency are highlighted in the eTable and include malabsorptive disorders; psychiatric illness82; and chronic disease, especially end-stage renal disease.83 Vitamin B6 deficiency associated with chronic alcohol use is due to both inadequate vitamin B6 intake as well as reduced hepatic storage.78 Medications such as isoniazid, hydralazine, and oral contraceptives may decrease vitamin B6 levels (eTable).82
Vitamin B6 can be measured in the plasma as pyridoxal 5′-phosphate. Plasma concentrations of less than 20 nmol/L are suggestive of deficiency.82 Indirect tests include tryptophan and methionine loading.6 The treatment of vitamin B6 deficiency is determined by symptom severity. Recommendations for oral supplementation range from 25 to 600 mg daily.82 Symptoms typically improve on 100 mg daily.6
Vitamins B9 and B12
Deficiencies of vitamins B9 (folic acid, folate) and B12 (cobalamin) have similar clinical presentations. Folate is essential in the metabolism of amino acids, purines, and pyrimidines.6 Cobalamin, found in animal products, is a cofactor for methionine synthase and methylmalonyl-CoA mutase.84 Megaloblastic anemia is the main finding in folate or cobalamin deficiency. Neurologic findings only accompany cobalamin deficiency. Risk factors for folate deficiency include malabsorptive conditions,6 chronic alcohol use,85 and antifolate medication use (eTable).6
Cobalamin absorption requires gastric acid and intrinsic factor binding in the duodenum. Deficiency may occur from strict diets, psychiatric illness, old age,86 decreased gastric acid secretion,87 abnormal intrinsic factor function, or intestinal infections.6
Generalized cutaneous hyperpigmentation may be the first manifestation of vitamins B9 and B12 deficiency.88 Typically accentuated in acral creases and the oral cavity, pigmentation may mimic Addison disease. Hair depigmentation and linear streaking of the nails are reported.84 The tongue becomes painful and red with atrophy of the filiform papillae (Hunter glossitis).78 Linear lesions on the tongue and hard palate may serve as an early sign of cobalamin deficiency.89
Folate deficiency is diagnosed by measuring the plasma folate level; coincidental cobalamin deficiency should be excluded. Deficiency is managed with oral supplementation (when possible) with 1 to 5 mg of folate daily.6 Cobalamin deficiency is based on low serum levels (<150 pg/mL is diagnostic).86 Cobalamin deficiency may take years to develop, as vitamin B12 exists in large body stores.6 Serum methylmalonic acid may be elevated in patients with clinical features but normal-low serum vitamin B12 level.86 Treatment of vitamin B12 deficiency is with oral (2 mg once daily) or parenteral (1 mg every 4 weeks then maintained at once monthly) cyanocobalamin. For patients with neurologic symptoms, intramuscular injection should be given.86 The underlying cause of deficiency must be elucidated and treated.
Vitamin C Deficiency
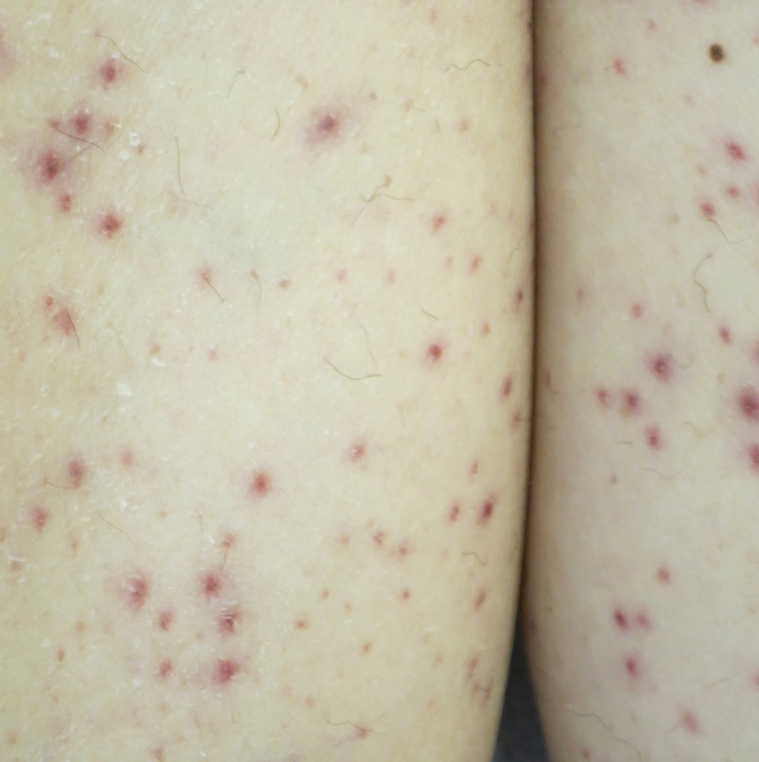
Vitamin C (ascorbic acid) is an essential cofactor for the hydroxylation of proline and lysine residues in collagen synthesis. Plant-based foods are the main dietary source of vitamin C, and deficiency presents clinically as scurvy. Cutaneous findings include follicular hyperkeratosis, perifollicular petechiae, and curled hair shafts (corkscrew hairs)(Figure 4). Ecchymoses of the lower extremities, forearms, and abdomen may be seen. Nodules representing intramuscular and subcutaneous hemorrhage can be present.90 Woody edema may mimic cellulitis, while lower extremity hemorrhage may mimic vasculitis. Gingival hyperplasia, hemorrhage, and edema may occur,90 along with linear splinter hemorrhages.91
Hypovitaminosis C has been routinely demonstrated in hospitalized patients.92 Scurvy may occur in patients on strict diets,93 chronic alcohol use,94 psychiatric illness,95 or gastrointestinal tract disease (eTable).96-99 Those with low socioeconomic status70 or dementia100 as well as the elderly also are at risk.101 Scurvy has developed in patients with iron overload and those who are on hemodialysis44 as well as in association with nilotinib use.102 Patients with chronic mucous membrane graft-vs-host disease may exhibit vitamin C deficiency.103
Scurvy is a clinical diagnosis. Vitamin C levels normalize quickly with supplementation. Cutaneous biopsy will exhibit follicular hyperkeratosis, perifollicular hemorrhage, and fibrosis.91
Oral ascorbic acid supplementation should be initiated at 500 to 1000 mg daily in adults.104 The cause of deficiency should be identified, and further supplementation should be decided based on patient risk factors. Lifestyle modifications, such as cessation of smoking and chronic alcohol use, is recommended. The diagnosis of scurvy should prompt workup for additional nutrient deficiencies.
Final Thoughts
Dermatologists play an important role in the early recognition of nutritional deficiencies, as cutaneous manifestations often are the first clue to diagnosis. Nutritional deficiencies are common yet underrecognized in the hospitalized patient and serve as an independent risk factor for patient morbidity and mortality.3 Awareness of the cutaneous manifestations of undernutrition as well as the risk factors for nutritional deficiency may expedite diagnosis and supplementation, thereby improving outcomes for hospitalized patients.
- Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37:460-481.
- Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8:514-527.
- Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf). 2016;4:272-280.
- Basavaraj KH, Seemanthini C, Rashmi R. Diet in dermatology: present perspectives. Indian J Dermatol. 2010;55:205-210.
- Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56:1055-1068.
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685.
- Lekwuttikarn R, Teng JMC. Cutaneous manifestations of nutritional deficiency. Curr Opin Pediatr. 2018;30:505-513.
- Jaffe AT, Heymann WR. Kwashiorkor/zinc deficiency overlap following partial gastrectomy. Int J Dermatol. 1998;37:134-137.
- Listernick R, Christoffel K, Pace J, et al. Severe primary malnutrition in US children. Am J Dis Child. 1985;139:1157-1160.
- Heilskov S, Rytter MJ, Vestergaard C, et al. Dermatosis in children with oedematous malnutrition (Kwashiorkor): a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:995-1001.
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Cohen PR. The nail flag sign: case report in a man with diverticulitis and review of dermatology flag sign of the hair, skin, and nails. Cureus. 2018;10:e2929.
- Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. Geneva, Switzerland: World Health Organization; 1999. https://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf. Accessed May 19, 2020.
- Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8:775.
- Thavaraj V, Sesikeran B. Histopathological changes in skin of children with clinical protein energy malnutrition before and after recovery. J Trop Pediatr. 1989;35:105-108.
- McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305-315.
- Ogawa Y, Kinoshita M, Shimada S, et al. Zinc and skin disorders. Nutrients. 2018;10:199.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Wiznia LE, Bhansali S, Brinster N, et al. Acquired acrodermatitis enteropathica due to zinc-depleted parenteral nutrition. Pediatr Dermatol. 2019;36:520-523.
- Sandstead HH, Freeland-Graves JH. Dietary phytate, zinc and hidden zinc deficiency. J Trace Elem Med Biol. 2014;28:414-417.
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319.
- Schoelmerich J, Becher MS, Hoppe-Seyler P, et al. Zinc and vitamin A deficiency in patients with Crohn’s disease is correlated with activity but not with localization or extent of the disease. Hepatogastroenterology. 1985;32:34-38.
- Siva S, Rubin DT, Gulotta G, et al. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:152-157.
- Semrad CE. Zinc and intestinal function. Curr Gastroenterol Rep. 1999;1:398-403.
- Sinclair SA, Reynolds NJ. Necrolytic migratory erythema and zinc deficiency. Br J Dermatol. 1997;136:783-785.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Wu D, Fung MA, Kiuru M, et al. Acquired bullous acrodermatitis enteropathica as a histologic mimic of pemphigus foliaceus in a patient on parenteral nutrition. Dermatol Online J. 2018;24:20.
- Maxfield L, Crane J. Zinc Deficiency. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493231/Updated November 14, 2019. Accessed May 19, 2020.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Wegmüller R, Tay F, Zeder C, et al. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J Nutr. 2014;144:132-136.
- Vick G, Mahmoudizad R, Fiala K. Intravenous zinc therapy for acquired zinc deficiency secondary to gastric bypass surgery: a case report. Dermatol Ther. 2015;28:222-225.
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808.
- Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75:671-678.
- De Franceschi L, Iolascon A, Taher A, et al. Clinical management of iron deficiency anemia in adults: systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017;42:16-23.
- Haider LM, Schwingshackl L, Hoffmann G, et al. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;58:1359-1374.
- Enani G, Bilgic E, Lebedeva E, et al. The incidence of iron deficiency anemia post-Roux-en-Y gastric bypass and sleeve gastrectomy: a systematic review [published online September 4, 2019]. Surg Endosc. doi:10.1007/s00464-019-07092-3.
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981-1989.
- Gramlich L, Meddings L, Alberda C, et al. Essential fatty acid deficiency in 2015: the impact of novel intravenous lipid emulsions. JPEN J Parenter Enteral Nutr. 2015;39(1 suppl):61S-66S.
- Khnykin D, Miner JH, Jahnsen F. Role of fatty acid transporters in epidermis: implications for health and disease. Dermatoendocrinol. 2011;3:53-61.
- Wright S. Essential fatty acids and the skin. Br J Dermatol. 1991;125:503-515.
- Lakdawala N, Grant-Kels JM. Acrodermatitis caused by nutritional deficiency and metabolic disorders. Clin Dermatol. 2017;35:64-67.
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503.
- Aldámiz-Echevarría L, Bilbao A, Andrade F, et al. Fatty acid deficiency profile in children with food allergy managed with elimination diets. Acta Paediatr. 2008;97:1572-1576.
- Jeppesen PB, Christensen MS, Høy CE, et al. Essential fatty acid deficiency in patients with severe fat malabsorption. Am J Clin Nutr. 1997;65:837-843.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published online June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
- Fleming CR, Smith LM, Hodges RE. Essential fatty acid deficiency in adults receiving total parenteral nutrition. Am J Clin Nutr. 1976;29:976-983.
- Cooke RJ, Zee P, Yeh YY. Essential fatty acid status of the premature infant during short-term fat-free parenteral nutrition. J Pediatr Gastroenterol Nutr. 1984;3:446-449.
- Skolnik P, Eaglstein WH, Ziboh VA. Human essential fatty acid deficiency: treatment by topical application of linoleic acid. Arch Dermatol. 1977;113:939-941.
- Vahlquist A. Clinical use of vitamin A and its derivatives—physiological and pharmacological aspects. Clin Exp Dermatol. 1985;10:133-143.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56:389-392.
- Phanachet P, Shantavasinkul PC, Chantrathammachart P, et al. Unusual manifestation of vitamin A deficiency presenting with generalized xerosis without night blindness. Clin Case Rep. 2018;6:878-882.
- Fuchs J. Alcoholism, malnutrition, vitamin deficiencies, and the skin. Clin Dermatol. 1999;17:457-461.
- Uhoda E, Petit L, Piérard-Franchimont C, et al. Ultraviolet light-enhanced visualization of cutaneous signs of carotene and vitamin A dietary deficiency. Acta Clin Belg. 2004;59:97-101.
- de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132(9 suppl):2895S-2901S.
- Fernandez-Banares F, Abad-Lacruz A, Xiol X, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744-748.
- Main AN, Mills PR, Russell RI, et al. Vitamin A deficiency in Crohn’s disease. Gut. 1983;24:1169-1175.
- Cobos G, Cornejo C, McMahon P. A case of phrynoderma in a patient with Crohn’s disease. Pediatr Dermatol. 2015;32:234-236.
- Trumbo P, Yates AA, Schlicker S, et al. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294-301.
- Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;132(9 suppl):2902S-2906S.
- Ragunatha S, Jagannath Kumar V, Murugesh SB, et al. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res. 2014;8:116-118.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol. 1988;15:531-534.
- Pinto JT, Zempleni J. Riboflavin. Adv Nutr. 2016;7:973-975.
- Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr. 2002;76:100-106.
- Gromisch DS, Lopez R, Cole HS, et al. Light (phototherapy)—induced riboflavin deficiency in the neonate. J Pediatr. 1977;90:118-122.
- Pinto J, Huang YP, McConnell RJ, et al. Increased urinary riboflavin excretion resulting from boric acid ingestion. J Lab Clin Med. 1978;92:126-134.
- Soltani D, Ghaffar Pour M, et al. Nutritional aspects of treatment in epileptic patients. Iran J Child Neurol. 2016;10:1-12.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol. 1991;10:293-295.
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481.
- Nogueira A, Duarte AF, Magina S, et al. Pellagra associated with esophageal carcinoma and alcoholism. Dermatol Online J. 2009;15:8.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol. 2011;164:1188-1200.
- Jagielska G, Tomaszewicz-Libudzic EC, Brzozowska A. Pellagra: a rare complication of anorexia nervosa. Eur Child Adolesc Psychiatry. 2007;16:417-420.
- Li R, Yu K, Wang Q, et al. Pellagra secondary to medication and alcoholism: a case report and review of the literature. Nutr Clin Pract. 2016;31:785-789.
- Ladoyanni E, Cheung ST, North J, et al. Pellagra occurring in a patient with atopic dermatitis and food allergy. J Eur Acad Dermatol Venereol. 2007;21:394-396.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274.
- Lamey PJ, Hammond A, Allam BF, et al. Vitamin status of patients with burning mouth syndrome and the response to replacement therapy. Br Dent J. 1986;160:81-84.
- Stover PJ, Field MS. Vitamin B-6. Adv Nutr. 2015;6:132-133.
- Gerlach AT, Thomas S, Stawicki SP, et al. Vitamin B6 deficiency: a potential cause of refractory seizures in adults. JPEN J Parenter Enteral Nutr. 2011;35:272-275.
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Ross EA, Shah GM, Reynolds RD, et al. Vitamin B6 requirements of patients on chronic peritoneal dialysis. Kidney Int. 1989;36:702-706.
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33.
- Sanvisens A, Zuluaga P, Pineda M, et al. Folate deficiency in patients seeking treatment of alcohol use disorder. Drug Alcohol Depend. 2017;180:417-422.
- Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. 2017;96:384-389.
- Bradford GS, Taylor CT. Omeprazole and vitamin B12 deficiency. Ann Pharmacother. 1999;33:641-643.
- Srivastava N, Chand S, Bansal M, et al. Reversible hyperpigmentation as the first manifestation of dietary vitamin B12 deficiency. Indian J Dermatol Venereol Leprol. 2006;72:389-390.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498-500.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906; quiz 907-810.
- Shaath T, Fischer R, Goeser M, et al. Scurvy in the present times: vitamin C allergy leading to strict fast food diet. Dermatol Online J. 2016;22:13030/qt50b8w28b.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Ahmad SA, Al Thobiti TA, El Toum M, et al. Florid scurvy in an autistic child on a ketogenic diet [published online November 19, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001695.
- Lux-Battistelli C, Battistelli D. Latent scurvy with tiredness and leg pain in alcoholics: an underestimated disease three case reports. Medicine (Baltimore). 2017;96:e8861.
- Christopher K, Tammaro D, Wing EJ. Early scurvy complicating anorexia nervosa. South Med J. 2002;95:1065-1066.
- Berger ML, Siegel DM, Lee EL. Scurvy as an initial manifestation of Whipple’s disease. Ann Intern Med. 1984;101:58-59.
- Imes S, Dinwoodie A, Walker K, et al. Vitamin C status in 137 outpatients with Crohn’s disease. effect of diet counseling. J Clin Gastroenterol. 1986;8:443-446.
- Echeverría Zudaire L, García Cuartero B, Campelo Moreno O, et al. Scurvy associated with celiac disease [in Spanish]. An Esp Pediatr. 2002;57:587.
- Hansen EP, Metzsche C, Henningsen E, et al. Severe scurvy after gastric bypass surgery and a poor postoperative diet. J Clin Med Res. 2012;4:135-137.
- Rivière S, Birlouez-Aragon I, Nourhashémi F, et al. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int J Geriatr Psychiatry. 1998;13:749-754.
- Bhattacharyya P, Giannoutsos J, Eslick GD, et al. Scurvy: an unrecognized and emerging public health issue in developed economies. Mayo Clin Proc. 2019;94:2594-2597.
- Oak AS, Jaleel T, Fening K, et al. A case of scurvy associated with nilotinib. J Cutan Pathol. 2016;43:725-726.
- Kletzel M, Powers K, Hayes M. Scurvy: a new problem for patients with chronic GVHD involving mucous membranes; an easy problem to resolve. Pediatr Transplant. 2014;18:524-526.
- Maxfield L, Crane JS. Vitamin C Deficiency (Scurvy). Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493187/. Updated November 19, 2019. Accessed May 19, 2020.
The World Health Organization defines malnutrition as deficiencies, excesses, or imbalances in an individual’s intake of energy and/or nutrients.1 This review will focus on undernutrition, which may result from macronutrient or micronutrient deficiencies. Undernutrition in the hospitalized patient is a common yet underrecognized phenomenon, with an estimated prevalence of 20% to 50% worldwide.2 Malnutrition is an independent risk factor for patient morbidity and mortality and has been associated with increased health care costs.3 Nutritional deficiencies may arise from inadequate nutrient intake, abnormal nutrient absorption, or improper nutrient utilization.4 Unfortunately, no standardized algorithm for screening and diagnosing patients with malnutrition exists, making early physical examination findings of utmost importance. Herein, we present a review of acquired nutritional deficiency dermatoses in the inpatient setting.
Protein-Energy Malnutrition
Protein-energy malnutrition (PEM) refers to a set of related disorders that include marasmus, kwashiorkor (KW), and marasmic KW. These conditions frequently are seen in developing countries but also have been reported in developed nations.5 Marasmus occurs from a chronic deficiency of protein and calories. Decreased insulin production and unopposed catabolism result in sarcopenia and loss of bone and subcutaneous fat.6 Affected patients include children who are less than 60% ideal body weight (IBW) without edema or hypoproteinemia.7 Kwashiorkor is the edematous form of PEM that develops from isolated protein deficiency, resulting in edema, diarrhea, and immunosuppression.6 Micronutrient deficiencies, oxidative stress, slow protein catabolism, and excess antidiuretic hormone have been proposed as potential drivers of KW.8 Kwashiorkor affects children between 60% and 80% IBW. Marasmic KW has features of both diseases, including children who are less than 60% IBW but with associated edema and/or hypoproteinemia.9
Although PEM is uncommon in adults, hospitalized patients carry many predisposing risk factors, including infections, malabsorptive conditions, psychiatric disease, and chronic illness (eTable). Patients with chronic infections present with findings consistent with marasmic KW due to lean body mass loss.

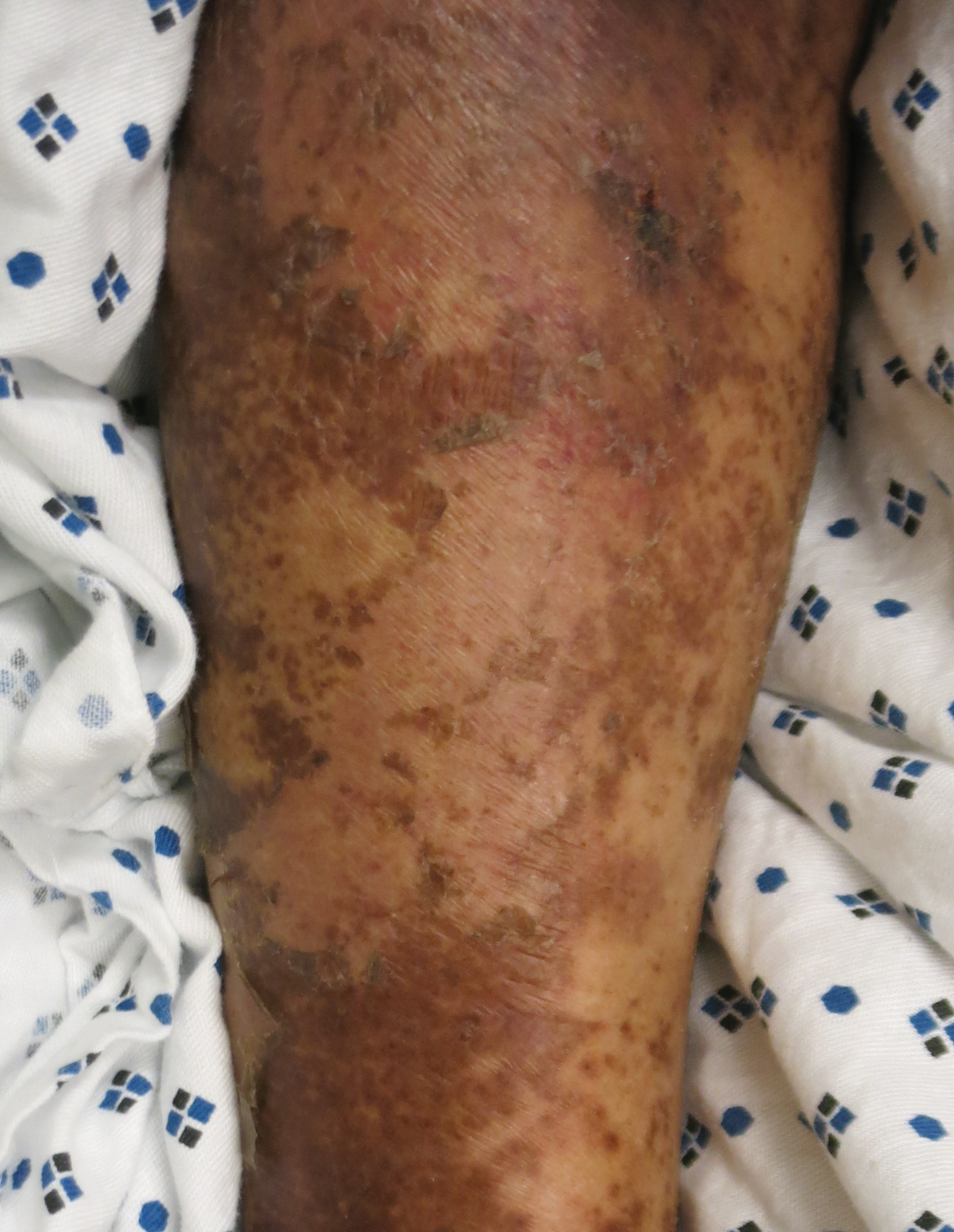
The cutaneous findings in PEM are related to dysmaturation of epidermal keratinocytes and resultant epidermal atrophy.10 Patients with marasmus exhibit dry, wrinkled, loose skin due to subcutaneous fat loss. Emaciated children often lose their buccal fat pads, and reduced perianal adipose may lead to rectal prolapse. Increased lanugo hair may be present on the face, and alopecia of the scalp may occur.6 In KW, cutaneous disease progresses from confluent hyperkeratosis to a dry atrophic epidermis that erodes easily, leaving underlying pale erythema. The resultant pattern is one of hyperpigmented plaques with slightly raised borders, and hypopigmented patches and erosions described as flaky paint dermatitis (Figure 1).5 Lesions appear first in areas of friction. The hair often is dry and brittle; curly hair may straighten and scale.11 Red-yellow to gray-white hypopigmentation may develop, denoting periods of inadequate nutrition. The flag sign describes alternating horizontal bands of hypopigmentation interspersed with bands of pigmented hair. The nails usually are thin and soft and may exhibit the nail flag sign, characterized by horizontal bands of white and red.12 Cheilitis, angular stomatitis, and vulvovaginitis may be present.6

In adults, weight loss and body mass index can be used to assess nutritional status, along with a focused history and physical examination. Complete blood cell count, electrolyte levels, and blood urea nitrogen should be assessed, as hypoglycemia and anemia often accompany PEM.13 In KW, hypoalbuminemia and hypoproteinemia are invariably present. Although prealbumin may be a valid prognostic indicator of disease outcomes and mortality in patients at risk for malnutrition, checking other serum biomarkers remains controversial.14 Focused testing may be warranted in patients with risk factors for chronic infectious processes, such as human immunodeficiency virus or tuberculosis.6 Skin biopsy may solidify the diagnosis of PEM. Hypertrophy of the stratum corneum, atrophy of the stratum spinosum and stratum granulosum, and increased basal layer melanin have been reported.15
Treatment involves initial fluid resuscitation and correction of electrolyte imbalances, followed by nutritional replacement.13 Oral or enteral tube feedings are preferred over total parenteral nutrition (TPN), as they enhance recovery of the gastrointestinal tract.16 Refeeding should occur in small amounts and frequent intervals.5 Skin-directed therapy is aimed at restoring epidermal function and hydration, with regular moisturization and application of barrier creams, such as zinc oxide ointment or petrolatum.10
Zinc Deficiency
Zinc is an essential trace element that provides regulatory, structural, and catalytic functions across multiple biochemical pathways6 and serves as an enzymatic cofactor and key component for numerous transcription factors.17 Zinc is derived from food sources, and its concentration correlates with protein content.18 Zinc is found in both animal and plant-based proteins, albeit with a lower oral bioavailability in the latter. Zinc deficiency may be inherited or acquired. Primary acrodermatitis enteropathica is an autosomal-recessive disorder of the solute carrier family 39 member 4 gene, SLC39A4 (encodes zinc transporter ZIP4 on enterocytes); the result is abnormal zinc absorption from the small intestine.18
Acquired zinc deficiency occurs from decreased dietary zinc intake, impaired intestinal zinc absorption, excessive zinc elimination, or systemic states of high catabolism or low albumin (eTable). Total parenteral nutrition–associated deficiency has arisen when nutritional formulations did not contain trace elements during national shortages or when prolonged TPN was not anticipated and trace elements were removed.19 Zinc levels may already be low in patients with chronic illness or inflammation, so even a short period on TPN can precipitate deficiency.18,19 Diets high in phytate may result in zinc deficiency, as phytate impairs intestinal zinc absorption.20 Approximately 15% of patients with inflammatory bowel disease experienced zinc deficiency worldwide.21 In Crohn disease, zinc deficiency has been associated with active intestinal inflammation, increased risk for hospitalization, surgeries, and disease-related complications.22,23
Medications such as antiepileptics, antimetabolites, or penicillamine may induce zinc deficiency, highlighting the importance of medication review for hospitalized patients (eTable). Catabolic states, frequently encountered in hospitalized patients, increase the risk for zinc deficiency.24 Patients with necrolytic migratory erythema (associated with pancreatic glucagonomas) often experience low serum zinc levels.25
The skin is the third most zinc-abundant tissue in the human body. Within keratinocytes, zinc is critical to normal proliferation and suppression of inflammation.17 Zinc also plays an important role in cutaneous immune function.26 Zinc deficiency presents with sharply demarcated, flaccid pustules and bullae that erode into scaly, pink, eczematous or psoriasiform plaques. Lesions are found preferentially in acral and periorificial sites, often with crusting and exudate. The groin and flexural surfaces may be affected. Erosions often become secondarily impetiginized. Other cutaneous findings include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.26 Histopathology of skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.27 Acquired bullous acrodermatitis enteropathica has been reported as a histologic mimicker of pemphigus foliaceous in patients on TPN.28
Diagnosis of zinc deficiency is made by measuring plasma zinc levels. Fasting levels should be drawn in the morning, as they can fluctuate based on the time of day, stress levels, or inflammation.6 Sample hemolysis and anticoagulants high in zinc may falsely elevate plasma zinc. A normal zinc level is greater than 70 µg/dL; however, normal levels do not rule out deficiency.18 Measurement of zinc-dependent enzymes, such as alkaline phosphatase, can be a quick way to assess zinc status. Serum albumin also should be measured; because zinc is carried by albumin in the blood, hypoalbuminemia may result in secondary zinc deficiency.18
Zinc replacement therapy is largely through oral supplementation and should start at 0.5 to 2.0 mg/kg/d in adults with acquired disease.29,30 Zinc sulfate is the most affordable and is the supplement of choice, with 50 mg of elemental zinc per 220 mg of zinc sulfate (~23% elemental zinc).31 Alternative zinc salts, such as zinc gluconate (13% elemental zinc), may be used. Patients with malabsorptive disorders often require parenteral supplementation.32 Clinical symptoms often will resolve within 1 to 2 weeks of supplementation.29 In patients with primary acrodermatitis enteropathica, lifelong supplementation with 3 mg/kg/d elemental zinc should occur.6 Calcium and folate may reduce zinc absorption, while zinc supplementation can interfere with copper and iron absorption.33
Iron Deficiency
Iron is an essential component of the hemoglobin molecule. Iron homeostasis and metabolism are tightly regulated processes that drive erythropoiesis. Only 5% to 10% of dietary iron is absorbed through nutrition, while the remainder is recycled from red cell breakdown. Both normal iron levels and iron deficiency (ID) are defined by age and gender.34 Iron-deficiency anemia (IDA) is one of the most common cause-specific anemias worldwide.35
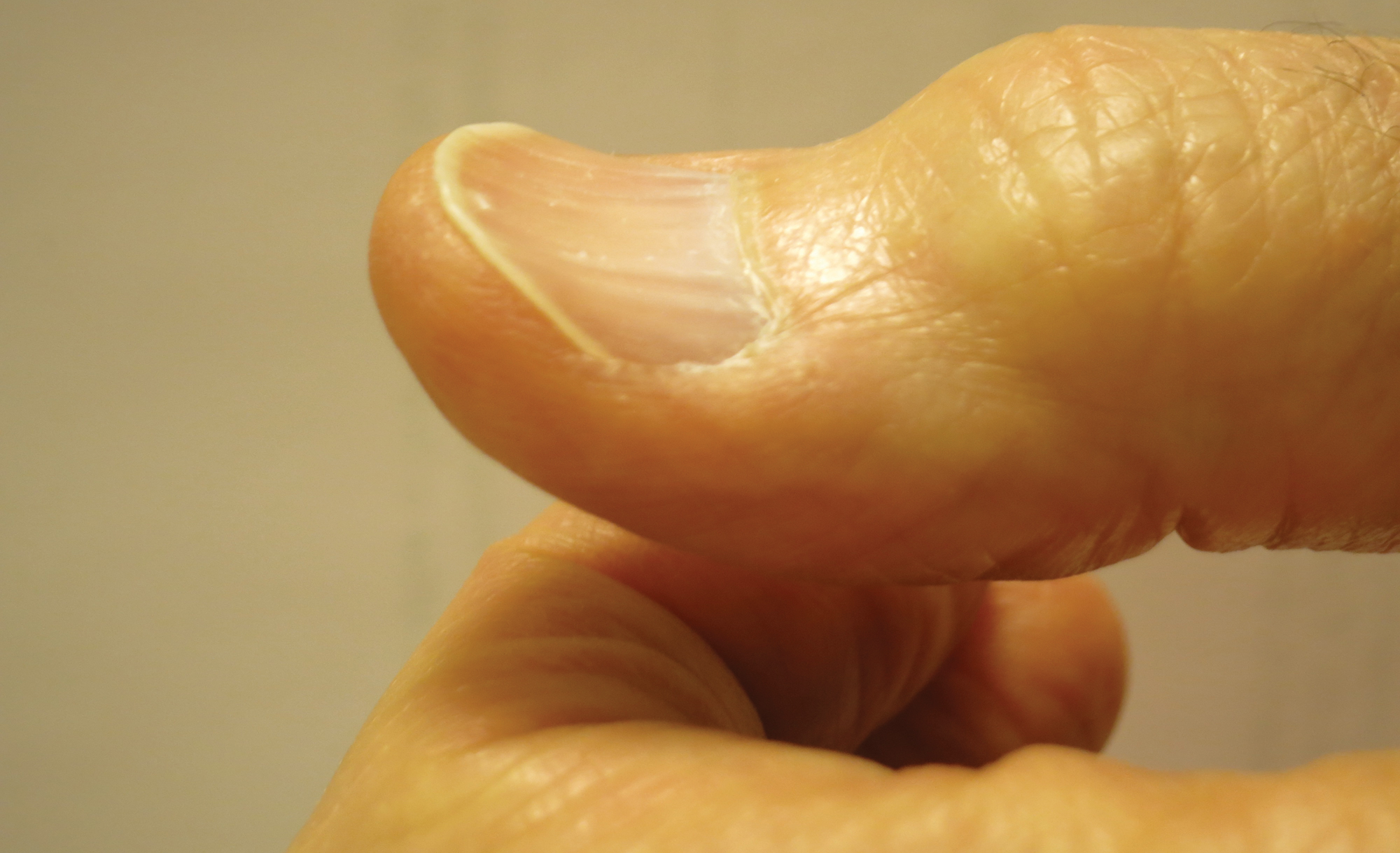
Fatigue is the most common and earliest symptom of ID. In a single study, pallor was predictive of anemia in hospitalized patients; however, absence of pallor did not rule out anemia.34 Dyspnea on exertion, tachycardia, dysphagia, and pica also may be reported. Cutaneous manifestations include koilonychia (Figure 2), glossitis, pruritus, angular cheilitis, and telogen effluvium. Plummer-Vinson syndrome is characterized by microcytic anemia, glossitis, and dysphagia.

Risk factors for ID include insufficient dietary consumption,36 blood loss, malabsorptive states,37,38 and increased iron requirements (eTable). Patient fragility (eg, elderly, chronic disease) is a newly described risk factor where correction of ID may impact morbidity, mortality, and quality of life.35
Iron deficiency can be present despite a normal hemoglobin level. Serum ferritin and percentage transferrin saturation are key to early identification of IDA.35 Ferritin levels lower than 30 µg/L confirm the diagnosis. Decreased transferrin saturation and increased total iron binding capacity aid in the diagnosis of IDA. Serum ferritin is an acute-phase reactant, and levels may be falsely elevated in the setting of inflammation or infection.
Treatment includes reversing the cause of deficiency and supplementing iron. Calculation of the total iron deficit can help inform iron supplementation. First-line therapy for IDA is oral ferrous sulfate 325 mg (65 mg elemental iron) 3 times daily. Newer studies suggest 40 to 80 mg oral iron should be taken every other day to increase absorption.39 Other iron salts, such as ferrous gluconate (325 mg is equivalent to 38 mg elemental iron), have been used. Iron absorption is enhanced by an acidic environment. Parenteral iron is utilized in patients with uncorrectable blood loss, malabsorption, renal failure, intolerance to oral iron, and nonadherence in those who are unable to receive transfusions. Iron infusions are favored in frail patients, such as the elderly and those with chronic kidney disease or heart failure.35 Multiple parenteral iron formulations exist, and their use should be driven by underlying patient comorbidities and potential risks. Packed red blood cell transfusions should be considered in acute blood loss, hypoxia, or cardiac insufficiency.
Essential Fatty Acid Deficiency
Essential fatty acids (EFAs) including linoleic and α-linolenic acid cannot be synthesized by the human body and must be obtained through diet (mostly plant oils). Essential fatty acids have various functions, including maintaining phospholipid membrane integrity, forming prostaglandins and leukotrienes, and storing energy.40 Essential fatty acids are important in the structure and function of the stratum corneum and are crucial in maintaining epidermal barrier function.41 Increased epidermal permeability and transepidermal water loss may be the first signs of EFA deficiency (EFAD).42
The cutaneous manifestations of EFAD include xerosis, weeping eczematous plaques, and erosions in intertriginous sites. The lesions may progress to widespread desquamation and erythema. With time, the skin can become thick and leathery. Alopecia may occur, and hair may depigment.7 Additional findings include poor wound healing and increased susceptibility to infections.43,44
Essential fatty acid deficiency may occur when dietary fat intake is severely restricted or in malabsorptive states.45,46 It develops in patients on prolonged TPN, typically when receiving fat-restricted nutrition,47,48 as occurs in hypertriglyceridemia.47 Essential fatty acid deficiency has developed in patients on TPN containing EFAs,47 as the introduction of novel intravenous lipid emulsions has resulted in varying proportions of EFA.40 Premature neonates are particularly at risk for EFAD.49
The diagnosis of EFAD involves the measurement of the triene to tetraene ratio. A ratio of more than 0.2 suggests EFAD, but the clinical signs are not seen until the ratio is over 0.4.40 Low plasma levels of linoleic, linolenic, and arachidonic acids also are seen. Elevated liver function tests are supportive of the diagnosis. Biochemical findings typically are seen before cutaneous manifestations.40
Treatment of EFAD includes topical, oral, or intravenous replacement of EFAs. Improvement of EFAD with the application of topical linoleic acid to the skin has been reported.50 Patients receiving TPN should undergo assessment of parenteral lipid emulsion to ensure adequate fatty acid composition.
Vitamin A Deficiency
Vitamin A (retinol) is a fat-soluble vitamin that plays a critical role in keratinization, epithelial proliferation, and cellular differentiation.6 Vitamin A is found in animal products as retinyl esters and in plants as beta-carotene. Vitamin A has 2 clinically important forms: all-trans retinoic acid and 11-cis-retinal. All-trans retinoic acid is involved in cellular differentiation and regulating gene transcription, while 11-cis-retinal is key to rhodopsin generation required for vision. Vitamin A deficiency presents with early ophthalmologic findings, specifically nyctalopia, or delayed adaptation to the dark.51 Xerophthalmia, abnormal conjunctival keratinization, and Bitot spots subsequently develop and may progress to corneal ulceration and blindness.6
Vitamin A deficiency manifests in the skin as follicular hyperkeratosis, or phrynoderma. Notably, numerous other micronutrient deficiencies may result in phrynoderma. Clinically, multiple pigmented keratotic papules of various sizes, many with a central keratinous plug, are distributed symmetrically on the extensor elbows, knees, shoulders, buttocks, and extremities. The skin surrounding these lesions may be scaly and hyperpigmented.52 Generalized xerosis without preceding nyctalopia has been reported.53 Accompanying pityriasis alba may develop.52 Lesions on the face may mimic acne, while lesions on the extremities may simulate a perforating disorder. Histopathology of phrynoderma reveals epidermal hyperkeratosis, follicular hyperkeratosis, and follicular plugging.52
Patients at risk for vitamin A deficiency include those with conditions that affect intestinal fat absorption, underlying psychiatric illness, or chronic disease (eTable). Chronic alcohol use predisposes patients to a multitude of micronutrient deficiencies, including vitamin A deficiency.54 In chronic alcohol use, even mild cutaneous changes may be the first clue to low serum retinol.55
Vitamin A deficiency can be diagnosed by measuring serum retinol levels, with levels lower than 20 µg/dL being diagnostic of deficiency.56 Decreased serum retinol in patients hospitalized with flaring irritable bowel disorder has been repeatedly reported.57-59 Notably, serum retinol concentration does not decline until liver reserves of vitamin A are nearing exhaustion.33
The US Food and Drug Administration requires manufacturers to list retinol activity equivalents on labels. One international unit of retinol is equivalent to 0.3 µg of retinol activity equivalents.60 The treatment of vitamin A deficiency involves high-dose oral supplementation when possible.61 Although dependent on age, the treatment dose for most adults with vitamin A deficiency is 3000 µg (10,000 IU) once daily.
Phrynoderma has been specifically treated with salicylic acid ointment 3% and intramuscular vitamin A.62 Topical urea cream also may treat phrynoderma.63
Vitamin B2
Vitamin B2 (riboflavin) is absorbed in the small intestine and converted into 2 biologically active forms—flavin adenine dinucleotide and flavin mononucleotide—which serve as cofactors in metabolic and oxidation-reduction reactions. Malabsorptive disorders and bowel resection can lead to riboflavin deficiency.64 Other at-risk populations include those with restrictive diets,65 psychiatric illness, or systemic illness (eTable). Riboflavin can be degraded by light (deficiency has been reported after phototherapy for neonatal jaundice66) and following boric acid ingestion.67 Medications, including long-term treatment with antiepileptics, may lead to riboflavin deficiency.68
Riboflavin is critical to maintaining collagen production. Riboflavin deficiency may manifest clinically with extensive seborrheiclike dermatitis,44 intertrigolike dermatitis,69 or oral-ocular-genital syndrome.70 Angular cheilitis may accompany an atrophic tongue that is deep red in color. The scrotum is characteristically involved in men, with confluent dermatitis extending onto the thighs and sparing the midline. Red papules and painful fissures may develop. Balanitis and phimosis have been reported. Testing for riboflavin deficiency should be considered in patients with refractory seborrheic dermatitis.
Riboflavin stores are assessed by the erythrocyte glutathione reductase activity coefficient.44 A level of 1.4 or higher is consistent with deficiency. Serum riboflavin levels, performed after a 12-hour fast, may support the diagnosis but are less sensitive. Patients with glucose-6-phosphate deficiency cannot be assessed via the erythrocyte glutathione reductase activity coefficient and may instead require evaluation of 24-hour urine riboflavin level.44
Vitamin B3
Vitamin B3 (niacin, nicotinamide, nicotinic acid) is found in plant and animal products or can be derived from its amino acid precursor tryptophan. Niacin deficiency results in pellagra, characterized by dermatitis, dementia, and diarrhea.71 The most prominent feature is a symmetrically distributed photosensitive dermatitis of the face, neck (called Casal necklace)(Figure 3), chest, dorsal hands, and extensor arms. The eruption may begin with erythema, vesicles, or bullae (wet pellagra) and evolve into thick, hyperpigmented, scaling plaques.71 The skin may take on a copper tone and become atrophic.72 Dull erythema with overlying yellow powdery scale (called sulfur flakes) at follicular orifices has been described on the nasal bridge.73

Causes of niacin deficiency include malabsorptive conditions, malignancy (including carcinoid tumors), parenteral nutrition, psychiatric disease,74,75 and restrictive diets (eTable).76 Carcinoid tumors divert tryptophan to serotonin resulting in niacin deficiency.77
The diagnosis of niacin deficiency is based on clinical findings and response to supplementation.75 Low niacin urinary metabolites (N-methylnicotinamide and 2-pyridone) may aid in diagnosis.6 Treatment generally includes oral nicotinamide 100 mg every 6 hours; the dose can then be tapered to 50 mg every 8 to 12 hours until symptoms resolve. Severe deficiency may require parenteral nicotinamide 1 g 3 to 4 times daily.75
Vitamin B6
Vitamin B6 (pyridoxine, pyridoxamine, pyridoxal) is found in whole grains and plant and animal products. Vitamin B6 functions as a coenzyme in many metabolic pathways and is involved in the conversion of tryptophan to niacin.44 Absorption requires hydrolysis by intestinal phosphates and transport to the liver for rephosphorylation prior to release in active form.6
Cutaneous findings associated with vitamin B6 deficiency include periorificial and perineal seborrheic dermatitis,78 angular stomatitis, and cheilitis, with associated burning, redness, and tongue edema.6 Vitamin B6 deficiency is a rarely reported cause of burning mouth syndrome.79 Because vitamin B6 is involved in the conversion of tryptophan to niacin, deficiency also may present with pellagralike findings.70 Other clinical symptoms are outlined in the eTable.80,81
Conditions that increase risk for vitamin B6 deficiency are highlighted in the eTable and include malabsorptive disorders; psychiatric illness82; and chronic disease, especially end-stage renal disease.83 Vitamin B6 deficiency associated with chronic alcohol use is due to both inadequate vitamin B6 intake as well as reduced hepatic storage.78 Medications such as isoniazid, hydralazine, and oral contraceptives may decrease vitamin B6 levels (eTable).82
Vitamin B6 can be measured in the plasma as pyridoxal 5′-phosphate. Plasma concentrations of less than 20 nmol/L are suggestive of deficiency.82 Indirect tests include tryptophan and methionine loading.6 The treatment of vitamin B6 deficiency is determined by symptom severity. Recommendations for oral supplementation range from 25 to 600 mg daily.82 Symptoms typically improve on 100 mg daily.6
Vitamins B9 and B12
Deficiencies of vitamins B9 (folic acid, folate) and B12 (cobalamin) have similar clinical presentations. Folate is essential in the metabolism of amino acids, purines, and pyrimidines.6 Cobalamin, found in animal products, is a cofactor for methionine synthase and methylmalonyl-CoA mutase.84 Megaloblastic anemia is the main finding in folate or cobalamin deficiency. Neurologic findings only accompany cobalamin deficiency. Risk factors for folate deficiency include malabsorptive conditions,6 chronic alcohol use,85 and antifolate medication use (eTable).6
Cobalamin absorption requires gastric acid and intrinsic factor binding in the duodenum. Deficiency may occur from strict diets, psychiatric illness, old age,86 decreased gastric acid secretion,87 abnormal intrinsic factor function, or intestinal infections.6
Generalized cutaneous hyperpigmentation may be the first manifestation of vitamins B9 and B12 deficiency.88 Typically accentuated in acral creases and the oral cavity, pigmentation may mimic Addison disease. Hair depigmentation and linear streaking of the nails are reported.84 The tongue becomes painful and red with atrophy of the filiform papillae (Hunter glossitis).78 Linear lesions on the tongue and hard palate may serve as an early sign of cobalamin deficiency.89
Folate deficiency is diagnosed by measuring the plasma folate level; coincidental cobalamin deficiency should be excluded. Deficiency is managed with oral supplementation (when possible) with 1 to 5 mg of folate daily.6 Cobalamin deficiency is based on low serum levels (<150 pg/mL is diagnostic).86 Cobalamin deficiency may take years to develop, as vitamin B12 exists in large body stores.6 Serum methylmalonic acid may be elevated in patients with clinical features but normal-low serum vitamin B12 level.86 Treatment of vitamin B12 deficiency is with oral (2 mg once daily) or parenteral (1 mg every 4 weeks then maintained at once monthly) cyanocobalamin. For patients with neurologic symptoms, intramuscular injection should be given.86 The underlying cause of deficiency must be elucidated and treated.
Vitamin C Deficiency
Vitamin C (ascorbic acid) is an essential cofactor for the hydroxylation of proline and lysine residues in collagen synthesis. Plant-based foods are the main dietary source of vitamin C, and deficiency presents clinically as scurvy. Cutaneous findings include follicular hyperkeratosis, perifollicular petechiae, and curled hair shafts (corkscrew hairs)(Figure 4). Ecchymoses of the lower extremities, forearms, and abdomen may be seen. Nodules representing intramuscular and subcutaneous hemorrhage can be present.90 Woody edema may mimic cellulitis, while lower extremity hemorrhage may mimic vasculitis. Gingival hyperplasia, hemorrhage, and edema may occur,90 along with linear splinter hemorrhages.91

Hypovitaminosis C has been routinely demonstrated in hospitalized patients.92 Scurvy may occur in patients on strict diets,93 chronic alcohol use,94 psychiatric illness,95 or gastrointestinal tract disease (eTable).96-99 Those with low socioeconomic status70 or dementia100 as well as the elderly also are at risk.101 Scurvy has developed in patients with iron overload and those who are on hemodialysis44 as well as in association with nilotinib use.102 Patients with chronic mucous membrane graft-vs-host disease may exhibit vitamin C deficiency.103
Scurvy is a clinical diagnosis. Vitamin C levels normalize quickly with supplementation. Cutaneous biopsy will exhibit follicular hyperkeratosis, perifollicular hemorrhage, and fibrosis.91
Oral ascorbic acid supplementation should be initiated at 500 to 1000 mg daily in adults.104 The cause of deficiency should be identified, and further supplementation should be decided based on patient risk factors. Lifestyle modifications, such as cessation of smoking and chronic alcohol use, is recommended. The diagnosis of scurvy should prompt workup for additional nutrient deficiencies.
Final Thoughts
Dermatologists play an important role in the early recognition of nutritional deficiencies, as cutaneous manifestations often are the first clue to diagnosis. Nutritional deficiencies are common yet underrecognized in the hospitalized patient and serve as an independent risk factor for patient morbidity and mortality.3 Awareness of the cutaneous manifestations of undernutrition as well as the risk factors for nutritional deficiency may expedite diagnosis and supplementation, thereby improving outcomes for hospitalized patients.
The World Health Organization defines malnutrition as deficiencies, excesses, or imbalances in an individual’s intake of energy and/or nutrients.1 This review will focus on undernutrition, which may result from macronutrient or micronutrient deficiencies. Undernutrition in the hospitalized patient is a common yet underrecognized phenomenon, with an estimated prevalence of 20% to 50% worldwide.2 Malnutrition is an independent risk factor for patient morbidity and mortality and has been associated with increased health care costs.3 Nutritional deficiencies may arise from inadequate nutrient intake, abnormal nutrient absorption, or improper nutrient utilization.4 Unfortunately, no standardized algorithm for screening and diagnosing patients with malnutrition exists, making early physical examination findings of utmost importance. Herein, we present a review of acquired nutritional deficiency dermatoses in the inpatient setting.
Protein-Energy Malnutrition
Protein-energy malnutrition (PEM) refers to a set of related disorders that include marasmus, kwashiorkor (KW), and marasmic KW. These conditions frequently are seen in developing countries but also have been reported in developed nations.5 Marasmus occurs from a chronic deficiency of protein and calories. Decreased insulin production and unopposed catabolism result in sarcopenia and loss of bone and subcutaneous fat.6 Affected patients include children who are less than 60% ideal body weight (IBW) without edema or hypoproteinemia.7 Kwashiorkor is the edematous form of PEM that develops from isolated protein deficiency, resulting in edema, diarrhea, and immunosuppression.6 Micronutrient deficiencies, oxidative stress, slow protein catabolism, and excess antidiuretic hormone have been proposed as potential drivers of KW.8 Kwashiorkor affects children between 60% and 80% IBW. Marasmic KW has features of both diseases, including children who are less than 60% IBW but with associated edema and/or hypoproteinemia.9
Although PEM is uncommon in adults, hospitalized patients carry many predisposing risk factors, including infections, malabsorptive conditions, psychiatric disease, and chronic illness (eTable). Patients with chronic infections present with findings consistent with marasmic KW due to lean body mass loss.

The cutaneous findings in PEM are related to dysmaturation of epidermal keratinocytes and resultant epidermal atrophy.10 Patients with marasmus exhibit dry, wrinkled, loose skin due to subcutaneous fat loss. Emaciated children often lose their buccal fat pads, and reduced perianal adipose may lead to rectal prolapse. Increased lanugo hair may be present on the face, and alopecia of the scalp may occur.6 In KW, cutaneous disease progresses from confluent hyperkeratosis to a dry atrophic epidermis that erodes easily, leaving underlying pale erythema. The resultant pattern is one of hyperpigmented plaques with slightly raised borders, and hypopigmented patches and erosions described as flaky paint dermatitis (Figure 1).5 Lesions appear first in areas of friction. The hair often is dry and brittle; curly hair may straighten and scale.11 Red-yellow to gray-white hypopigmentation may develop, denoting periods of inadequate nutrition. The flag sign describes alternating horizontal bands of hypopigmentation interspersed with bands of pigmented hair. The nails usually are thin and soft and may exhibit the nail flag sign, characterized by horizontal bands of white and red.12 Cheilitis, angular stomatitis, and vulvovaginitis may be present.6

In adults, weight loss and body mass index can be used to assess nutritional status, along with a focused history and physical examination. Complete blood cell count, electrolyte levels, and blood urea nitrogen should be assessed, as hypoglycemia and anemia often accompany PEM.13 In KW, hypoalbuminemia and hypoproteinemia are invariably present. Although prealbumin may be a valid prognostic indicator of disease outcomes and mortality in patients at risk for malnutrition, checking other serum biomarkers remains controversial.14 Focused testing may be warranted in patients with risk factors for chronic infectious processes, such as human immunodeficiency virus or tuberculosis.6 Skin biopsy may solidify the diagnosis of PEM. Hypertrophy of the stratum corneum, atrophy of the stratum spinosum and stratum granulosum, and increased basal layer melanin have been reported.15
Treatment involves initial fluid resuscitation and correction of electrolyte imbalances, followed by nutritional replacement.13 Oral or enteral tube feedings are preferred over total parenteral nutrition (TPN), as they enhance recovery of the gastrointestinal tract.16 Refeeding should occur in small amounts and frequent intervals.5 Skin-directed therapy is aimed at restoring epidermal function and hydration, with regular moisturization and application of barrier creams, such as zinc oxide ointment or petrolatum.10
Zinc Deficiency
Zinc is an essential trace element that provides regulatory, structural, and catalytic functions across multiple biochemical pathways6 and serves as an enzymatic cofactor and key component for numerous transcription factors.17 Zinc is derived from food sources, and its concentration correlates with protein content.18 Zinc is found in both animal and plant-based proteins, albeit with a lower oral bioavailability in the latter. Zinc deficiency may be inherited or acquired. Primary acrodermatitis enteropathica is an autosomal-recessive disorder of the solute carrier family 39 member 4 gene, SLC39A4 (encodes zinc transporter ZIP4 on enterocytes); the result is abnormal zinc absorption from the small intestine.18
Acquired zinc deficiency occurs from decreased dietary zinc intake, impaired intestinal zinc absorption, excessive zinc elimination, or systemic states of high catabolism or low albumin (eTable). Total parenteral nutrition–associated deficiency has arisen when nutritional formulations did not contain trace elements during national shortages or when prolonged TPN was not anticipated and trace elements were removed.19 Zinc levels may already be low in patients with chronic illness or inflammation, so even a short period on TPN can precipitate deficiency.18,19 Diets high in phytate may result in zinc deficiency, as phytate impairs intestinal zinc absorption.20 Approximately 15% of patients with inflammatory bowel disease experienced zinc deficiency worldwide.21 In Crohn disease, zinc deficiency has been associated with active intestinal inflammation, increased risk for hospitalization, surgeries, and disease-related complications.22,23
Medications such as antiepileptics, antimetabolites, or penicillamine may induce zinc deficiency, highlighting the importance of medication review for hospitalized patients (eTable). Catabolic states, frequently encountered in hospitalized patients, increase the risk for zinc deficiency.24 Patients with necrolytic migratory erythema (associated with pancreatic glucagonomas) often experience low serum zinc levels.25
The skin is the third most zinc-abundant tissue in the human body. Within keratinocytes, zinc is critical to normal proliferation and suppression of inflammation.17 Zinc also plays an important role in cutaneous immune function.26 Zinc deficiency presents with sharply demarcated, flaccid pustules and bullae that erode into scaly, pink, eczematous or psoriasiform plaques. Lesions are found preferentially in acral and periorificial sites, often with crusting and exudate. The groin and flexural surfaces may be affected. Erosions often become secondarily impetiginized. Other cutaneous findings include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.26 Histopathology of skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.27 Acquired bullous acrodermatitis enteropathica has been reported as a histologic mimicker of pemphigus foliaceous in patients on TPN.28
Diagnosis of zinc deficiency is made by measuring plasma zinc levels. Fasting levels should be drawn in the morning, as they can fluctuate based on the time of day, stress levels, or inflammation.6 Sample hemolysis and anticoagulants high in zinc may falsely elevate plasma zinc. A normal zinc level is greater than 70 µg/dL; however, normal levels do not rule out deficiency.18 Measurement of zinc-dependent enzymes, such as alkaline phosphatase, can be a quick way to assess zinc status. Serum albumin also should be measured; because zinc is carried by albumin in the blood, hypoalbuminemia may result in secondary zinc deficiency.18
Zinc replacement therapy is largely through oral supplementation and should start at 0.5 to 2.0 mg/kg/d in adults with acquired disease.29,30 Zinc sulfate is the most affordable and is the supplement of choice, with 50 mg of elemental zinc per 220 mg of zinc sulfate (~23% elemental zinc).31 Alternative zinc salts, such as zinc gluconate (13% elemental zinc), may be used. Patients with malabsorptive disorders often require parenteral supplementation.32 Clinical symptoms often will resolve within 1 to 2 weeks of supplementation.29 In patients with primary acrodermatitis enteropathica, lifelong supplementation with 3 mg/kg/d elemental zinc should occur.6 Calcium and folate may reduce zinc absorption, while zinc supplementation can interfere with copper and iron absorption.33
Iron Deficiency
Iron is an essential component of the hemoglobin molecule. Iron homeostasis and metabolism are tightly regulated processes that drive erythropoiesis. Only 5% to 10% of dietary iron is absorbed through nutrition, while the remainder is recycled from red cell breakdown. Both normal iron levels and iron deficiency (ID) are defined by age and gender.34 Iron-deficiency anemia (IDA) is one of the most common cause-specific anemias worldwide.35
Fatigue is the most common and earliest symptom of ID. In a single study, pallor was predictive of anemia in hospitalized patients; however, absence of pallor did not rule out anemia.34 Dyspnea on exertion, tachycardia, dysphagia, and pica also may be reported. Cutaneous manifestations include koilonychia (Figure 2), glossitis, pruritus, angular cheilitis, and telogen effluvium. Plummer-Vinson syndrome is characterized by microcytic anemia, glossitis, and dysphagia.

Risk factors for ID include insufficient dietary consumption,36 blood loss, malabsorptive states,37,38 and increased iron requirements (eTable). Patient fragility (eg, elderly, chronic disease) is a newly described risk factor where correction of ID may impact morbidity, mortality, and quality of life.35
Iron deficiency can be present despite a normal hemoglobin level. Serum ferritin and percentage transferrin saturation are key to early identification of IDA.35 Ferritin levels lower than 30 µg/L confirm the diagnosis. Decreased transferrin saturation and increased total iron binding capacity aid in the diagnosis of IDA. Serum ferritin is an acute-phase reactant, and levels may be falsely elevated in the setting of inflammation or infection.
Treatment includes reversing the cause of deficiency and supplementing iron. Calculation of the total iron deficit can help inform iron supplementation. First-line therapy for IDA is oral ferrous sulfate 325 mg (65 mg elemental iron) 3 times daily. Newer studies suggest 40 to 80 mg oral iron should be taken every other day to increase absorption.39 Other iron salts, such as ferrous gluconate (325 mg is equivalent to 38 mg elemental iron), have been used. Iron absorption is enhanced by an acidic environment. Parenteral iron is utilized in patients with uncorrectable blood loss, malabsorption, renal failure, intolerance to oral iron, and nonadherence in those who are unable to receive transfusions. Iron infusions are favored in frail patients, such as the elderly and those with chronic kidney disease or heart failure.35 Multiple parenteral iron formulations exist, and their use should be driven by underlying patient comorbidities and potential risks. Packed red blood cell transfusions should be considered in acute blood loss, hypoxia, or cardiac insufficiency.
Essential Fatty Acid Deficiency
Essential fatty acids (EFAs) including linoleic and α-linolenic acid cannot be synthesized by the human body and must be obtained through diet (mostly plant oils). Essential fatty acids have various functions, including maintaining phospholipid membrane integrity, forming prostaglandins and leukotrienes, and storing energy.40 Essential fatty acids are important in the structure and function of the stratum corneum and are crucial in maintaining epidermal barrier function.41 Increased epidermal permeability and transepidermal water loss may be the first signs of EFA deficiency (EFAD).42
The cutaneous manifestations of EFAD include xerosis, weeping eczematous plaques, and erosions in intertriginous sites. The lesions may progress to widespread desquamation and erythema. With time, the skin can become thick and leathery. Alopecia may occur, and hair may depigment.7 Additional findings include poor wound healing and increased susceptibility to infections.43,44
Essential fatty acid deficiency may occur when dietary fat intake is severely restricted or in malabsorptive states.45,46 It develops in patients on prolonged TPN, typically when receiving fat-restricted nutrition,47,48 as occurs in hypertriglyceridemia.47 Essential fatty acid deficiency has developed in patients on TPN containing EFAs,47 as the introduction of novel intravenous lipid emulsions has resulted in varying proportions of EFA.40 Premature neonates are particularly at risk for EFAD.49
The diagnosis of EFAD involves the measurement of the triene to tetraene ratio. A ratio of more than 0.2 suggests EFAD, but the clinical signs are not seen until the ratio is over 0.4.40 Low plasma levels of linoleic, linolenic, and arachidonic acids also are seen. Elevated liver function tests are supportive of the diagnosis. Biochemical findings typically are seen before cutaneous manifestations.40
Treatment of EFAD includes topical, oral, or intravenous replacement of EFAs. Improvement of EFAD with the application of topical linoleic acid to the skin has been reported.50 Patients receiving TPN should undergo assessment of parenteral lipid emulsion to ensure adequate fatty acid composition.
Vitamin A Deficiency
Vitamin A (retinol) is a fat-soluble vitamin that plays a critical role in keratinization, epithelial proliferation, and cellular differentiation.6 Vitamin A is found in animal products as retinyl esters and in plants as beta-carotene. Vitamin A has 2 clinically important forms: all-trans retinoic acid and 11-cis-retinal. All-trans retinoic acid is involved in cellular differentiation and regulating gene transcription, while 11-cis-retinal is key to rhodopsin generation required for vision. Vitamin A deficiency presents with early ophthalmologic findings, specifically nyctalopia, or delayed adaptation to the dark.51 Xerophthalmia, abnormal conjunctival keratinization, and Bitot spots subsequently develop and may progress to corneal ulceration and blindness.6
Vitamin A deficiency manifests in the skin as follicular hyperkeratosis, or phrynoderma. Notably, numerous other micronutrient deficiencies may result in phrynoderma. Clinically, multiple pigmented keratotic papules of various sizes, many with a central keratinous plug, are distributed symmetrically on the extensor elbows, knees, shoulders, buttocks, and extremities. The skin surrounding these lesions may be scaly and hyperpigmented.52 Generalized xerosis without preceding nyctalopia has been reported.53 Accompanying pityriasis alba may develop.52 Lesions on the face may mimic acne, while lesions on the extremities may simulate a perforating disorder. Histopathology of phrynoderma reveals epidermal hyperkeratosis, follicular hyperkeratosis, and follicular plugging.52
Patients at risk for vitamin A deficiency include those with conditions that affect intestinal fat absorption, underlying psychiatric illness, or chronic disease (eTable). Chronic alcohol use predisposes patients to a multitude of micronutrient deficiencies, including vitamin A deficiency.54 In chronic alcohol use, even mild cutaneous changes may be the first clue to low serum retinol.55
Vitamin A deficiency can be diagnosed by measuring serum retinol levels, with levels lower than 20 µg/dL being diagnostic of deficiency.56 Decreased serum retinol in patients hospitalized with flaring irritable bowel disorder has been repeatedly reported.57-59 Notably, serum retinol concentration does not decline until liver reserves of vitamin A are nearing exhaustion.33
The US Food and Drug Administration requires manufacturers to list retinol activity equivalents on labels. One international unit of retinol is equivalent to 0.3 µg of retinol activity equivalents.60 The treatment of vitamin A deficiency involves high-dose oral supplementation when possible.61 Although dependent on age, the treatment dose for most adults with vitamin A deficiency is 3000 µg (10,000 IU) once daily.
Phrynoderma has been specifically treated with salicylic acid ointment 3% and intramuscular vitamin A.62 Topical urea cream also may treat phrynoderma.63
Vitamin B2
Vitamin B2 (riboflavin) is absorbed in the small intestine and converted into 2 biologically active forms—flavin adenine dinucleotide and flavin mononucleotide—which serve as cofactors in metabolic and oxidation-reduction reactions. Malabsorptive disorders and bowel resection can lead to riboflavin deficiency.64 Other at-risk populations include those with restrictive diets,65 psychiatric illness, or systemic illness (eTable). Riboflavin can be degraded by light (deficiency has been reported after phototherapy for neonatal jaundice66) and following boric acid ingestion.67 Medications, including long-term treatment with antiepileptics, may lead to riboflavin deficiency.68
Riboflavin is critical to maintaining collagen production. Riboflavin deficiency may manifest clinically with extensive seborrheiclike dermatitis,44 intertrigolike dermatitis,69 or oral-ocular-genital syndrome.70 Angular cheilitis may accompany an atrophic tongue that is deep red in color. The scrotum is characteristically involved in men, with confluent dermatitis extending onto the thighs and sparing the midline. Red papules and painful fissures may develop. Balanitis and phimosis have been reported. Testing for riboflavin deficiency should be considered in patients with refractory seborrheic dermatitis.
Riboflavin stores are assessed by the erythrocyte glutathione reductase activity coefficient.44 A level of 1.4 or higher is consistent with deficiency. Serum riboflavin levels, performed after a 12-hour fast, may support the diagnosis but are less sensitive. Patients with glucose-6-phosphate deficiency cannot be assessed via the erythrocyte glutathione reductase activity coefficient and may instead require evaluation of 24-hour urine riboflavin level.44
Vitamin B3
Vitamin B3 (niacin, nicotinamide, nicotinic acid) is found in plant and animal products or can be derived from its amino acid precursor tryptophan. Niacin deficiency results in pellagra, characterized by dermatitis, dementia, and diarrhea.71 The most prominent feature is a symmetrically distributed photosensitive dermatitis of the face, neck (called Casal necklace)(Figure 3), chest, dorsal hands, and extensor arms. The eruption may begin with erythema, vesicles, or bullae (wet pellagra) and evolve into thick, hyperpigmented, scaling plaques.71 The skin may take on a copper tone and become atrophic.72 Dull erythema with overlying yellow powdery scale (called sulfur flakes) at follicular orifices has been described on the nasal bridge.73

Causes of niacin deficiency include malabsorptive conditions, malignancy (including carcinoid tumors), parenteral nutrition, psychiatric disease,74,75 and restrictive diets (eTable).76 Carcinoid tumors divert tryptophan to serotonin resulting in niacin deficiency.77
The diagnosis of niacin deficiency is based on clinical findings and response to supplementation.75 Low niacin urinary metabolites (N-methylnicotinamide and 2-pyridone) may aid in diagnosis.6 Treatment generally includes oral nicotinamide 100 mg every 6 hours; the dose can then be tapered to 50 mg every 8 to 12 hours until symptoms resolve. Severe deficiency may require parenteral nicotinamide 1 g 3 to 4 times daily.75
Vitamin B6
Vitamin B6 (pyridoxine, pyridoxamine, pyridoxal) is found in whole grains and plant and animal products. Vitamin B6 functions as a coenzyme in many metabolic pathways and is involved in the conversion of tryptophan to niacin.44 Absorption requires hydrolysis by intestinal phosphates and transport to the liver for rephosphorylation prior to release in active form.6
Cutaneous findings associated with vitamin B6 deficiency include periorificial and perineal seborrheic dermatitis,78 angular stomatitis, and cheilitis, with associated burning, redness, and tongue edema.6 Vitamin B6 deficiency is a rarely reported cause of burning mouth syndrome.79 Because vitamin B6 is involved in the conversion of tryptophan to niacin, deficiency also may present with pellagralike findings.70 Other clinical symptoms are outlined in the eTable.80,81
Conditions that increase risk for vitamin B6 deficiency are highlighted in the eTable and include malabsorptive disorders; psychiatric illness82; and chronic disease, especially end-stage renal disease.83 Vitamin B6 deficiency associated with chronic alcohol use is due to both inadequate vitamin B6 intake as well as reduced hepatic storage.78 Medications such as isoniazid, hydralazine, and oral contraceptives may decrease vitamin B6 levels (eTable).82
Vitamin B6 can be measured in the plasma as pyridoxal 5′-phosphate. Plasma concentrations of less than 20 nmol/L are suggestive of deficiency.82 Indirect tests include tryptophan and methionine loading.6 The treatment of vitamin B6 deficiency is determined by symptom severity. Recommendations for oral supplementation range from 25 to 600 mg daily.82 Symptoms typically improve on 100 mg daily.6
Vitamins B9 and B12
Deficiencies of vitamins B9 (folic acid, folate) and B12 (cobalamin) have similar clinical presentations. Folate is essential in the metabolism of amino acids, purines, and pyrimidines.6 Cobalamin, found in animal products, is a cofactor for methionine synthase and methylmalonyl-CoA mutase.84 Megaloblastic anemia is the main finding in folate or cobalamin deficiency. Neurologic findings only accompany cobalamin deficiency. Risk factors for folate deficiency include malabsorptive conditions,6 chronic alcohol use,85 and antifolate medication use (eTable).6
Cobalamin absorption requires gastric acid and intrinsic factor binding in the duodenum. Deficiency may occur from strict diets, psychiatric illness, old age,86 decreased gastric acid secretion,87 abnormal intrinsic factor function, or intestinal infections.6
Generalized cutaneous hyperpigmentation may be the first manifestation of vitamins B9 and B12 deficiency.88 Typically accentuated in acral creases and the oral cavity, pigmentation may mimic Addison disease. Hair depigmentation and linear streaking of the nails are reported.84 The tongue becomes painful and red with atrophy of the filiform papillae (Hunter glossitis).78 Linear lesions on the tongue and hard palate may serve as an early sign of cobalamin deficiency.89
Folate deficiency is diagnosed by measuring the plasma folate level; coincidental cobalamin deficiency should be excluded. Deficiency is managed with oral supplementation (when possible) with 1 to 5 mg of folate daily.6 Cobalamin deficiency is based on low serum levels (<150 pg/mL is diagnostic).86 Cobalamin deficiency may take years to develop, as vitamin B12 exists in large body stores.6 Serum methylmalonic acid may be elevated in patients with clinical features but normal-low serum vitamin B12 level.86 Treatment of vitamin B12 deficiency is with oral (2 mg once daily) or parenteral (1 mg every 4 weeks then maintained at once monthly) cyanocobalamin. For patients with neurologic symptoms, intramuscular injection should be given.86 The underlying cause of deficiency must be elucidated and treated.
Vitamin C Deficiency
Vitamin C (ascorbic acid) is an essential cofactor for the hydroxylation of proline and lysine residues in collagen synthesis. Plant-based foods are the main dietary source of vitamin C, and deficiency presents clinically as scurvy. Cutaneous findings include follicular hyperkeratosis, perifollicular petechiae, and curled hair shafts (corkscrew hairs)(Figure 4). Ecchymoses of the lower extremities, forearms, and abdomen may be seen. Nodules representing intramuscular and subcutaneous hemorrhage can be present.90 Woody edema may mimic cellulitis, while lower extremity hemorrhage may mimic vasculitis. Gingival hyperplasia, hemorrhage, and edema may occur,90 along with linear splinter hemorrhages.91

Hypovitaminosis C has been routinely demonstrated in hospitalized patients.92 Scurvy may occur in patients on strict diets,93 chronic alcohol use,94 psychiatric illness,95 or gastrointestinal tract disease (eTable).96-99 Those with low socioeconomic status70 or dementia100 as well as the elderly also are at risk.101 Scurvy has developed in patients with iron overload and those who are on hemodialysis44 as well as in association with nilotinib use.102 Patients with chronic mucous membrane graft-vs-host disease may exhibit vitamin C deficiency.103
Scurvy is a clinical diagnosis. Vitamin C levels normalize quickly with supplementation. Cutaneous biopsy will exhibit follicular hyperkeratosis, perifollicular hemorrhage, and fibrosis.91
Oral ascorbic acid supplementation should be initiated at 500 to 1000 mg daily in adults.104 The cause of deficiency should be identified, and further supplementation should be decided based on patient risk factors. Lifestyle modifications, such as cessation of smoking and chronic alcohol use, is recommended. The diagnosis of scurvy should prompt workup for additional nutrient deficiencies.
Final Thoughts
Dermatologists play an important role in the early recognition of nutritional deficiencies, as cutaneous manifestations often are the first clue to diagnosis. Nutritional deficiencies are common yet underrecognized in the hospitalized patient and serve as an independent risk factor for patient morbidity and mortality.3 Awareness of the cutaneous manifestations of undernutrition as well as the risk factors for nutritional deficiency may expedite diagnosis and supplementation, thereby improving outcomes for hospitalized patients.
- Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37:460-481.
- Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8:514-527.
- Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf). 2016;4:272-280.
- Basavaraj KH, Seemanthini C, Rashmi R. Diet in dermatology: present perspectives. Indian J Dermatol. 2010;55:205-210.
- Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56:1055-1068.
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685.
- Lekwuttikarn R, Teng JMC. Cutaneous manifestations of nutritional deficiency. Curr Opin Pediatr. 2018;30:505-513.
- Jaffe AT, Heymann WR. Kwashiorkor/zinc deficiency overlap following partial gastrectomy. Int J Dermatol. 1998;37:134-137.
- Listernick R, Christoffel K, Pace J, et al. Severe primary malnutrition in US children. Am J Dis Child. 1985;139:1157-1160.
- Heilskov S, Rytter MJ, Vestergaard C, et al. Dermatosis in children with oedematous malnutrition (Kwashiorkor): a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:995-1001.
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Cohen PR. The nail flag sign: case report in a man with diverticulitis and review of dermatology flag sign of the hair, skin, and nails. Cureus. 2018;10:e2929.
- Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. Geneva, Switzerland: World Health Organization; 1999. https://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf. Accessed May 19, 2020.
- Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8:775.
- Thavaraj V, Sesikeran B. Histopathological changes in skin of children with clinical protein energy malnutrition before and after recovery. J Trop Pediatr. 1989;35:105-108.
- McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305-315.
- Ogawa Y, Kinoshita M, Shimada S, et al. Zinc and skin disorders. Nutrients. 2018;10:199.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Wiznia LE, Bhansali S, Brinster N, et al. Acquired acrodermatitis enteropathica due to zinc-depleted parenteral nutrition. Pediatr Dermatol. 2019;36:520-523.
- Sandstead HH, Freeland-Graves JH. Dietary phytate, zinc and hidden zinc deficiency. J Trace Elem Med Biol. 2014;28:414-417.
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319.
- Schoelmerich J, Becher MS, Hoppe-Seyler P, et al. Zinc and vitamin A deficiency in patients with Crohn’s disease is correlated with activity but not with localization or extent of the disease. Hepatogastroenterology. 1985;32:34-38.
- Siva S, Rubin DT, Gulotta G, et al. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:152-157.
- Semrad CE. Zinc and intestinal function. Curr Gastroenterol Rep. 1999;1:398-403.
- Sinclair SA, Reynolds NJ. Necrolytic migratory erythema and zinc deficiency. Br J Dermatol. 1997;136:783-785.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Wu D, Fung MA, Kiuru M, et al. Acquired bullous acrodermatitis enteropathica as a histologic mimic of pemphigus foliaceus in a patient on parenteral nutrition. Dermatol Online J. 2018;24:20.
- Maxfield L, Crane J. Zinc Deficiency. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493231/Updated November 14, 2019. Accessed May 19, 2020.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Wegmüller R, Tay F, Zeder C, et al. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J Nutr. 2014;144:132-136.
- Vick G, Mahmoudizad R, Fiala K. Intravenous zinc therapy for acquired zinc deficiency secondary to gastric bypass surgery: a case report. Dermatol Ther. 2015;28:222-225.
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808.
- Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75:671-678.
- De Franceschi L, Iolascon A, Taher A, et al. Clinical management of iron deficiency anemia in adults: systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017;42:16-23.
- Haider LM, Schwingshackl L, Hoffmann G, et al. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;58:1359-1374.
- Enani G, Bilgic E, Lebedeva E, et al. The incidence of iron deficiency anemia post-Roux-en-Y gastric bypass and sleeve gastrectomy: a systematic review [published online September 4, 2019]. Surg Endosc. doi:10.1007/s00464-019-07092-3.
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981-1989.
- Gramlich L, Meddings L, Alberda C, et al. Essential fatty acid deficiency in 2015: the impact of novel intravenous lipid emulsions. JPEN J Parenter Enteral Nutr. 2015;39(1 suppl):61S-66S.
- Khnykin D, Miner JH, Jahnsen F. Role of fatty acid transporters in epidermis: implications for health and disease. Dermatoendocrinol. 2011;3:53-61.
- Wright S. Essential fatty acids and the skin. Br J Dermatol. 1991;125:503-515.
- Lakdawala N, Grant-Kels JM. Acrodermatitis caused by nutritional deficiency and metabolic disorders. Clin Dermatol. 2017;35:64-67.
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503.
- Aldámiz-Echevarría L, Bilbao A, Andrade F, et al. Fatty acid deficiency profile in children with food allergy managed with elimination diets. Acta Paediatr. 2008;97:1572-1576.
- Jeppesen PB, Christensen MS, Høy CE, et al. Essential fatty acid deficiency in patients with severe fat malabsorption. Am J Clin Nutr. 1997;65:837-843.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published online June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
- Fleming CR, Smith LM, Hodges RE. Essential fatty acid deficiency in adults receiving total parenteral nutrition. Am J Clin Nutr. 1976;29:976-983.
- Cooke RJ, Zee P, Yeh YY. Essential fatty acid status of the premature infant during short-term fat-free parenteral nutrition. J Pediatr Gastroenterol Nutr. 1984;3:446-449.
- Skolnik P, Eaglstein WH, Ziboh VA. Human essential fatty acid deficiency: treatment by topical application of linoleic acid. Arch Dermatol. 1977;113:939-941.
- Vahlquist A. Clinical use of vitamin A and its derivatives—physiological and pharmacological aspects. Clin Exp Dermatol. 1985;10:133-143.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56:389-392.
- Phanachet P, Shantavasinkul PC, Chantrathammachart P, et al. Unusual manifestation of vitamin A deficiency presenting with generalized xerosis without night blindness. Clin Case Rep. 2018;6:878-882.
- Fuchs J. Alcoholism, malnutrition, vitamin deficiencies, and the skin. Clin Dermatol. 1999;17:457-461.
- Uhoda E, Petit L, Piérard-Franchimont C, et al. Ultraviolet light-enhanced visualization of cutaneous signs of carotene and vitamin A dietary deficiency. Acta Clin Belg. 2004;59:97-101.
- de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132(9 suppl):2895S-2901S.
- Fernandez-Banares F, Abad-Lacruz A, Xiol X, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744-748.
- Main AN, Mills PR, Russell RI, et al. Vitamin A deficiency in Crohn’s disease. Gut. 1983;24:1169-1175.
- Cobos G, Cornejo C, McMahon P. A case of phrynoderma in a patient with Crohn’s disease. Pediatr Dermatol. 2015;32:234-236.
- Trumbo P, Yates AA, Schlicker S, et al. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294-301.
- Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;132(9 suppl):2902S-2906S.
- Ragunatha S, Jagannath Kumar V, Murugesh SB, et al. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res. 2014;8:116-118.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol. 1988;15:531-534.
- Pinto JT, Zempleni J. Riboflavin. Adv Nutr. 2016;7:973-975.
- Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr. 2002;76:100-106.
- Gromisch DS, Lopez R, Cole HS, et al. Light (phototherapy)—induced riboflavin deficiency in the neonate. J Pediatr. 1977;90:118-122.
- Pinto J, Huang YP, McConnell RJ, et al. Increased urinary riboflavin excretion resulting from boric acid ingestion. J Lab Clin Med. 1978;92:126-134.
- Soltani D, Ghaffar Pour M, et al. Nutritional aspects of treatment in epileptic patients. Iran J Child Neurol. 2016;10:1-12.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol. 1991;10:293-295.
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481.
- Nogueira A, Duarte AF, Magina S, et al. Pellagra associated with esophageal carcinoma and alcoholism. Dermatol Online J. 2009;15:8.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol. 2011;164:1188-1200.
- Jagielska G, Tomaszewicz-Libudzic EC, Brzozowska A. Pellagra: a rare complication of anorexia nervosa. Eur Child Adolesc Psychiatry. 2007;16:417-420.
- Li R, Yu K, Wang Q, et al. Pellagra secondary to medication and alcoholism: a case report and review of the literature. Nutr Clin Pract. 2016;31:785-789.
- Ladoyanni E, Cheung ST, North J, et al. Pellagra occurring in a patient with atopic dermatitis and food allergy. J Eur Acad Dermatol Venereol. 2007;21:394-396.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274.
- Lamey PJ, Hammond A, Allam BF, et al. Vitamin status of patients with burning mouth syndrome and the response to replacement therapy. Br Dent J. 1986;160:81-84.
- Stover PJ, Field MS. Vitamin B-6. Adv Nutr. 2015;6:132-133.
- Gerlach AT, Thomas S, Stawicki SP, et al. Vitamin B6 deficiency: a potential cause of refractory seizures in adults. JPEN J Parenter Enteral Nutr. 2011;35:272-275.
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Ross EA, Shah GM, Reynolds RD, et al. Vitamin B6 requirements of patients on chronic peritoneal dialysis. Kidney Int. 1989;36:702-706.
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33.
- Sanvisens A, Zuluaga P, Pineda M, et al. Folate deficiency in patients seeking treatment of alcohol use disorder. Drug Alcohol Depend. 2017;180:417-422.
- Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. 2017;96:384-389.
- Bradford GS, Taylor CT. Omeprazole and vitamin B12 deficiency. Ann Pharmacother. 1999;33:641-643.
- Srivastava N, Chand S, Bansal M, et al. Reversible hyperpigmentation as the first manifestation of dietary vitamin B12 deficiency. Indian J Dermatol Venereol Leprol. 2006;72:389-390.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498-500.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906; quiz 907-810.
- Shaath T, Fischer R, Goeser M, et al. Scurvy in the present times: vitamin C allergy leading to strict fast food diet. Dermatol Online J. 2016;22:13030/qt50b8w28b.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Ahmad SA, Al Thobiti TA, El Toum M, et al. Florid scurvy in an autistic child on a ketogenic diet [published online November 19, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001695.
- Lux-Battistelli C, Battistelli D. Latent scurvy with tiredness and leg pain in alcoholics: an underestimated disease three case reports. Medicine (Baltimore). 2017;96:e8861.
- Christopher K, Tammaro D, Wing EJ. Early scurvy complicating anorexia nervosa. South Med J. 2002;95:1065-1066.
- Berger ML, Siegel DM, Lee EL. Scurvy as an initial manifestation of Whipple’s disease. Ann Intern Med. 1984;101:58-59.
- Imes S, Dinwoodie A, Walker K, et al. Vitamin C status in 137 outpatients with Crohn’s disease. effect of diet counseling. J Clin Gastroenterol. 1986;8:443-446.
- Echeverría Zudaire L, García Cuartero B, Campelo Moreno O, et al. Scurvy associated with celiac disease [in Spanish]. An Esp Pediatr. 2002;57:587.
- Hansen EP, Metzsche C, Henningsen E, et al. Severe scurvy after gastric bypass surgery and a poor postoperative diet. J Clin Med Res. 2012;4:135-137.
- Rivière S, Birlouez-Aragon I, Nourhashémi F, et al. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int J Geriatr Psychiatry. 1998;13:749-754.
- Bhattacharyya P, Giannoutsos J, Eslick GD, et al. Scurvy: an unrecognized and emerging public health issue in developed economies. Mayo Clin Proc. 2019;94:2594-2597.
- Oak AS, Jaleel T, Fening K, et al. A case of scurvy associated with nilotinib. J Cutan Pathol. 2016;43:725-726.
- Kletzel M, Powers K, Hayes M. Scurvy: a new problem for patients with chronic GVHD involving mucous membranes; an easy problem to resolve. Pediatr Transplant. 2014;18:524-526.
- Maxfield L, Crane JS. Vitamin C Deficiency (Scurvy). Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493187/. Updated November 19, 2019. Accessed May 19, 2020.
- Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37:460-481.
- Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8:514-527.
- Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf). 2016;4:272-280.
- Basavaraj KH, Seemanthini C, Rashmi R. Diet in dermatology: present perspectives. Indian J Dermatol. 2010;55:205-210.
- Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56:1055-1068.
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685.
- Lekwuttikarn R, Teng JMC. Cutaneous manifestations of nutritional deficiency. Curr Opin Pediatr. 2018;30:505-513.
- Jaffe AT, Heymann WR. Kwashiorkor/zinc deficiency overlap following partial gastrectomy. Int J Dermatol. 1998;37:134-137.
- Listernick R, Christoffel K, Pace J, et al. Severe primary malnutrition in US children. Am J Dis Child. 1985;139:1157-1160.
- Heilskov S, Rytter MJ, Vestergaard C, et al. Dermatosis in children with oedematous malnutrition (Kwashiorkor): a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:995-1001.
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Cohen PR. The nail flag sign: case report in a man with diverticulitis and review of dermatology flag sign of the hair, skin, and nails. Cureus. 2018;10:e2929.
- Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. Geneva, Switzerland: World Health Organization; 1999. https://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf. Accessed May 19, 2020.
- Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8:775.
- Thavaraj V, Sesikeran B. Histopathological changes in skin of children with clinical protein energy malnutrition before and after recovery. J Trop Pediatr. 1989;35:105-108.
- McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305-315.
- Ogawa Y, Kinoshita M, Shimada S, et al. Zinc and skin disorders. Nutrients. 2018;10:199.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Wiznia LE, Bhansali S, Brinster N, et al. Acquired acrodermatitis enteropathica due to zinc-depleted parenteral nutrition. Pediatr Dermatol. 2019;36:520-523.
- Sandstead HH, Freeland-Graves JH. Dietary phytate, zinc and hidden zinc deficiency. J Trace Elem Med Biol. 2014;28:414-417.
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319.
- Schoelmerich J, Becher MS, Hoppe-Seyler P, et al. Zinc and vitamin A deficiency in patients with Crohn’s disease is correlated with activity but not with localization or extent of the disease. Hepatogastroenterology. 1985;32:34-38.
- Siva S, Rubin DT, Gulotta G, et al. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:152-157.
- Semrad CE. Zinc and intestinal function. Curr Gastroenterol Rep. 1999;1:398-403.
- Sinclair SA, Reynolds NJ. Necrolytic migratory erythema and zinc deficiency. Br J Dermatol. 1997;136:783-785.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Wu D, Fung MA, Kiuru M, et al. Acquired bullous acrodermatitis enteropathica as a histologic mimic of pemphigus foliaceus in a patient on parenteral nutrition. Dermatol Online J. 2018;24:20.
- Maxfield L, Crane J. Zinc Deficiency. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493231/Updated November 14, 2019. Accessed May 19, 2020.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Wegmüller R, Tay F, Zeder C, et al. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J Nutr. 2014;144:132-136.
- Vick G, Mahmoudizad R, Fiala K. Intravenous zinc therapy for acquired zinc deficiency secondary to gastric bypass surgery: a case report. Dermatol Ther. 2015;28:222-225.
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808.
- Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75:671-678.
- De Franceschi L, Iolascon A, Taher A, et al. Clinical management of iron deficiency anemia in adults: systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017;42:16-23.
- Haider LM, Schwingshackl L, Hoffmann G, et al. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;58:1359-1374.
- Enani G, Bilgic E, Lebedeva E, et al. The incidence of iron deficiency anemia post-Roux-en-Y gastric bypass and sleeve gastrectomy: a systematic review [published online September 4, 2019]. Surg Endosc. doi:10.1007/s00464-019-07092-3.
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981-1989.
- Gramlich L, Meddings L, Alberda C, et al. Essential fatty acid deficiency in 2015: the impact of novel intravenous lipid emulsions. JPEN J Parenter Enteral Nutr. 2015;39(1 suppl):61S-66S.
- Khnykin D, Miner JH, Jahnsen F. Role of fatty acid transporters in epidermis: implications for health and disease. Dermatoendocrinol. 2011;3:53-61.
- Wright S. Essential fatty acids and the skin. Br J Dermatol. 1991;125:503-515.
- Lakdawala N, Grant-Kels JM. Acrodermatitis caused by nutritional deficiency and metabolic disorders. Clin Dermatol. 2017;35:64-67.
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503.
- Aldámiz-Echevarría L, Bilbao A, Andrade F, et al. Fatty acid deficiency profile in children with food allergy managed with elimination diets. Acta Paediatr. 2008;97:1572-1576.
- Jeppesen PB, Christensen MS, Høy CE, et al. Essential fatty acid deficiency in patients with severe fat malabsorption. Am J Clin Nutr. 1997;65:837-843.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published online June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
- Fleming CR, Smith LM, Hodges RE. Essential fatty acid deficiency in adults receiving total parenteral nutrition. Am J Clin Nutr. 1976;29:976-983.
- Cooke RJ, Zee P, Yeh YY. Essential fatty acid status of the premature infant during short-term fat-free parenteral nutrition. J Pediatr Gastroenterol Nutr. 1984;3:446-449.
- Skolnik P, Eaglstein WH, Ziboh VA. Human essential fatty acid deficiency: treatment by topical application of linoleic acid. Arch Dermatol. 1977;113:939-941.
- Vahlquist A. Clinical use of vitamin A and its derivatives—physiological and pharmacological aspects. Clin Exp Dermatol. 1985;10:133-143.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56:389-392.
- Phanachet P, Shantavasinkul PC, Chantrathammachart P, et al. Unusual manifestation of vitamin A deficiency presenting with generalized xerosis without night blindness. Clin Case Rep. 2018;6:878-882.
- Fuchs J. Alcoholism, malnutrition, vitamin deficiencies, and the skin. Clin Dermatol. 1999;17:457-461.
- Uhoda E, Petit L, Piérard-Franchimont C, et al. Ultraviolet light-enhanced visualization of cutaneous signs of carotene and vitamin A dietary deficiency. Acta Clin Belg. 2004;59:97-101.
- de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132(9 suppl):2895S-2901S.
- Fernandez-Banares F, Abad-Lacruz A, Xiol X, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744-748.
- Main AN, Mills PR, Russell RI, et al. Vitamin A deficiency in Crohn’s disease. Gut. 1983;24:1169-1175.
- Cobos G, Cornejo C, McMahon P. A case of phrynoderma in a patient with Crohn’s disease. Pediatr Dermatol. 2015;32:234-236.
- Trumbo P, Yates AA, Schlicker S, et al. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294-301.
- Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;132(9 suppl):2902S-2906S.
- Ragunatha S, Jagannath Kumar V, Murugesh SB, et al. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res. 2014;8:116-118.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol. 1988;15:531-534.
- Pinto JT, Zempleni J. Riboflavin. Adv Nutr. 2016;7:973-975.
- Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr. 2002;76:100-106.
- Gromisch DS, Lopez R, Cole HS, et al. Light (phototherapy)—induced riboflavin deficiency in the neonate. J Pediatr. 1977;90:118-122.
- Pinto J, Huang YP, McConnell RJ, et al. Increased urinary riboflavin excretion resulting from boric acid ingestion. J Lab Clin Med. 1978;92:126-134.
- Soltani D, Ghaffar Pour M, et al. Nutritional aspects of treatment in epileptic patients. Iran J Child Neurol. 2016;10:1-12.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol. 1991;10:293-295.
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481.
- Nogueira A, Duarte AF, Magina S, et al. Pellagra associated with esophageal carcinoma and alcoholism. Dermatol Online J. 2009;15:8.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol. 2011;164:1188-1200.
- Jagielska G, Tomaszewicz-Libudzic EC, Brzozowska A. Pellagra: a rare complication of anorexia nervosa. Eur Child Adolesc Psychiatry. 2007;16:417-420.
- Li R, Yu K, Wang Q, et al. Pellagra secondary to medication and alcoholism: a case report and review of the literature. Nutr Clin Pract. 2016;31:785-789.
- Ladoyanni E, Cheung ST, North J, et al. Pellagra occurring in a patient with atopic dermatitis and food allergy. J Eur Acad Dermatol Venereol. 2007;21:394-396.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274.
- Lamey PJ, Hammond A, Allam BF, et al. Vitamin status of patients with burning mouth syndrome and the response to replacement therapy. Br Dent J. 1986;160:81-84.
- Stover PJ, Field MS. Vitamin B-6. Adv Nutr. 2015;6:132-133.
- Gerlach AT, Thomas S, Stawicki SP, et al. Vitamin B6 deficiency: a potential cause of refractory seizures in adults. JPEN J Parenter Enteral Nutr. 2011;35:272-275.
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Ross EA, Shah GM, Reynolds RD, et al. Vitamin B6 requirements of patients on chronic peritoneal dialysis. Kidney Int. 1989;36:702-706.
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33.
- Sanvisens A, Zuluaga P, Pineda M, et al. Folate deficiency in patients seeking treatment of alcohol use disorder. Drug Alcohol Depend. 2017;180:417-422.
- Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. 2017;96:384-389.
- Bradford GS, Taylor CT. Omeprazole and vitamin B12 deficiency. Ann Pharmacother. 1999;33:641-643.
- Srivastava N, Chand S, Bansal M, et al. Reversible hyperpigmentation as the first manifestation of dietary vitamin B12 deficiency. Indian J Dermatol Venereol Leprol. 2006;72:389-390.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498-500.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906; quiz 907-810.
- Shaath T, Fischer R, Goeser M, et al. Scurvy in the present times: vitamin C allergy leading to strict fast food diet. Dermatol Online J. 2016;22:13030/qt50b8w28b.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Ahmad SA, Al Thobiti TA, El Toum M, et al. Florid scurvy in an autistic child on a ketogenic diet [published online November 19, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001695.
- Lux-Battistelli C, Battistelli D. Latent scurvy with tiredness and leg pain in alcoholics: an underestimated disease three case reports. Medicine (Baltimore). 2017;96:e8861.
- Christopher K, Tammaro D, Wing EJ. Early scurvy complicating anorexia nervosa. South Med J. 2002;95:1065-1066.
- Berger ML, Siegel DM, Lee EL. Scurvy as an initial manifestation of Whipple’s disease. Ann Intern Med. 1984;101:58-59.
- Imes S, Dinwoodie A, Walker K, et al. Vitamin C status in 137 outpatients with Crohn’s disease. effect of diet counseling. J Clin Gastroenterol. 1986;8:443-446.
- Echeverría Zudaire L, García Cuartero B, Campelo Moreno O, et al. Scurvy associated with celiac disease [in Spanish]. An Esp Pediatr. 2002;57:587.
- Hansen EP, Metzsche C, Henningsen E, et al. Severe scurvy after gastric bypass surgery and a poor postoperative diet. J Clin Med Res. 2012;4:135-137.
- Rivière S, Birlouez-Aragon I, Nourhashémi F, et al. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int J Geriatr Psychiatry. 1998;13:749-754.
- Bhattacharyya P, Giannoutsos J, Eslick GD, et al. Scurvy: an unrecognized and emerging public health issue in developed economies. Mayo Clin Proc. 2019;94:2594-2597.
- Oak AS, Jaleel T, Fening K, et al. A case of scurvy associated with nilotinib. J Cutan Pathol. 2016;43:725-726.
- Kletzel M, Powers K, Hayes M. Scurvy: a new problem for patients with chronic GVHD involving mucous membranes; an easy problem to resolve. Pediatr Transplant. 2014;18:524-526.
- Maxfield L, Crane JS. Vitamin C Deficiency (Scurvy). Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493187/. Updated November 19, 2019. Accessed May 19, 2020.
Practice Points
- Nutritional deficiencies are common in hospitalized patients and often go unrecognized.
- Awareness of the risk factors predisposing patients to nutritional deficiencies and the cutaneous manifestations associated with undernutrition can promote early diagnosis.
- When investigating cutaneous findings, undernutrition should be considered in patients with chronic infections, malabsorptive states, psychiatric illness, and strict dietary practices, as well as in those using certain medications.
- Prompt nutritional supplementation can prevent patient morbidity and mortality and reverse skin disease.
Brilliant Green Staining of the Fingernails
Case Report
A 92-year-old Eastern European woman presented to our nail clinic with a history of onychodystrophy and arthralgia of the digits of several months’ duration. Her dermatologic history was notable for irritant hand dermatitis. A prior nail plate clipping with histopathologic examination was negative for fungal elements. Physical examination revealed onychorrhexis of all fingernails as well as onycholysis and subungual hyperkeratosis of the right fourth fingernail. Blue-green staining was incidentally noted on the right second and third fingernails and nail folds (Figure 1). Contact dermoscopy using ultrasound gel revealed translucent areas with sparse pigment, though denser areas had a fine branching pattern (Figure 2). When questioned, the patient reported use of “zelyonka,” a brilliant green solution, to self-treat the nails. Histopathology on repeat nail clippings showed parakeratosis and serum, which was most consistent with her known history of irritant hand dermatitis. Radiographs of the hands revealed osteoarthritis that was most prominent at the distal interphalangeal joints.
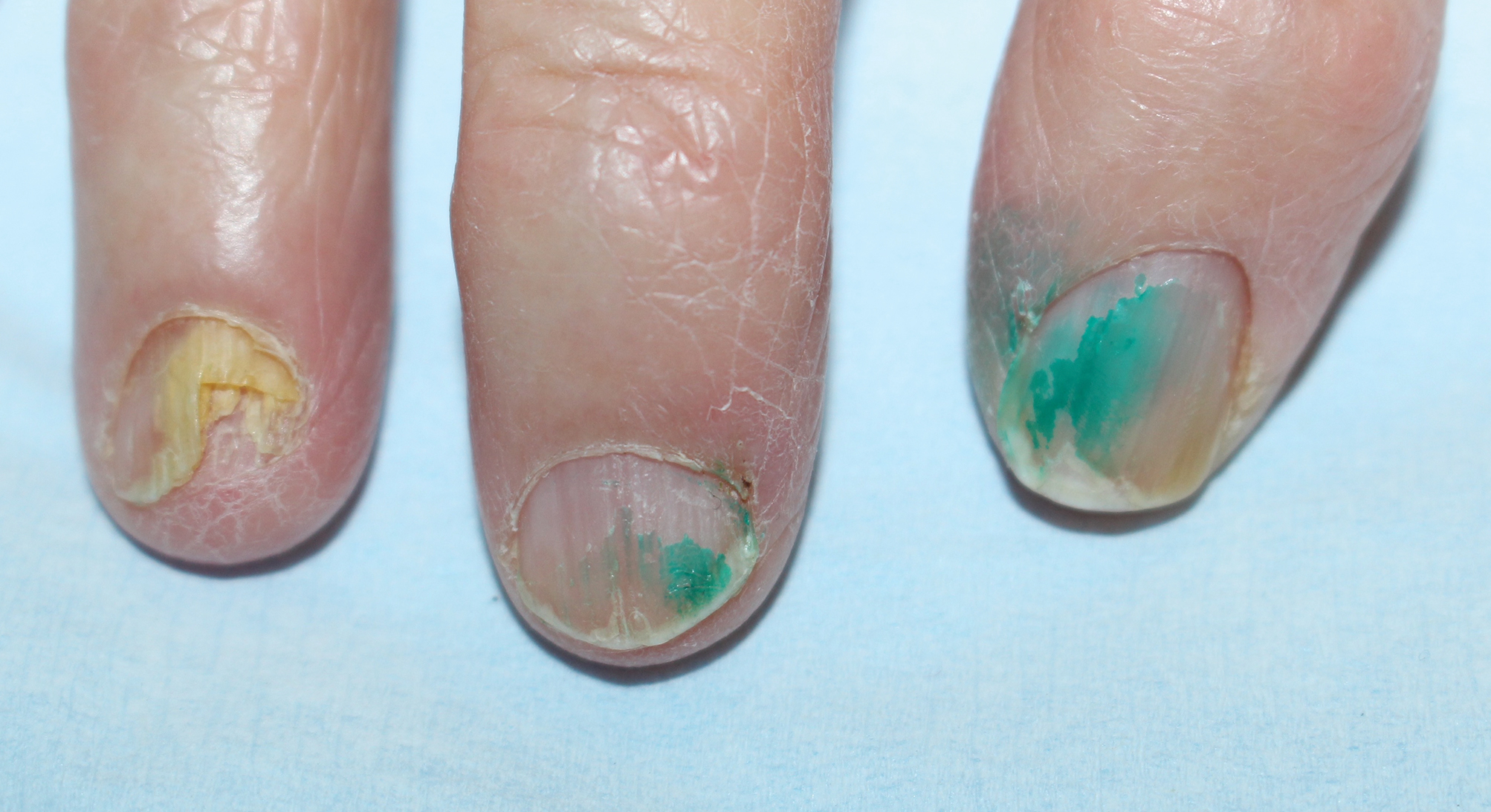
Comment
Brilliant green is a triphenylmethane dye commonly used in Eastern Europe and other regions for the treatment of superficial skin infections and onychomycosis.1 Its use as an antiseptic and wound healing agent has been investigated in the scientific literature since at least the early 20th century.2 Brilliant green typically is applied in a 0.1% to 2% ethanol solution.1 The dye has bactericidal activity against gram-positive organisms, particularly staphylococci and streptococci.2,3 It has been used for the treatment of fungal skin and nail infections since at least the early 20th century, with anecdotal success.4 Although there have been no studies investigating use of brilliant green alone for the treatment of onychomycosis, it is sometimes used in combination with conventional oral agents for this purpose.5 Because of its availability, safety, ease of use, and low cost, brilliant green has been promoted as an antiseptic in resource-poor settings.3 The revival of brilliant green and other antiseptic dyes in these settings has been suggested as an alternative to oral antibiotic agents, to which resistance is rising, and as a potential cancer therapy.6,7 Although brilliant green’s mechanism of action in treating skin infections is unclear, it has been shown to form covalent adducts with thioredoxin reductase 2, a protein conserved from bacteria to humans with an essential function for cellular activity.7
Early case studies suggested that brilliant green was beneficial in treating wounds2; however, this indication is controversial. In a guinea pig study, brilliant green was shown to inhibit wound healing and the formation of granulation tissue.8 It also should be noted that when used topically, brilliant green may cause skin sensitization, necrotic skin reactions, and permanent staining of clothing. It has no known anti-inflammatory properties and also may cause skin irritation.8 Brilliant green may cause blindness if it comes in contact with the eyes.1
Brilliant green has other potential dermatologic indications. For example, a combination of brilliant green and gentian violet, a related dye, has demonstrated efficacy in the treatment of cutaneous hemangiomas in mouse models by blocking expression of angiopoietin-2.7
Dermatologists should be familiar with brilliant green and its common uses as well as adverse effects. Brilliant green is commercially available for a low cost ($5 to $20) in specialty pharmacies or online (eg, Amazon). It is sold alone or in combination with gentian violet and proflavine hemisulfate, and a prescription is not required. Due to its low cost and accessibility, patients may use brilliant green to self-treat dermatologic conditions. Green nails due to staining with brilliant green dye must be distinguished from other etiologies causing green nail discoloration, such as infection with Pseudomonas aeruginosa or Aspergillus, bullous disorders, jaundice, “old” hematomas, nail polish, and other exogenous pigments.
- Balabanova M, Popova L, Tchipeva R. Dyes in dermatology. Clin Dermatol. 2003;21:2-6.
- Browning CH, Gulbransen R, Kennaway EL, et al. Flavine and brilliant green, powerful antiseptics with low toxicity to the tissues: their use in the treatment of infected wounds. Br Med J. 1917;1:73-78.
- Bakker P, Doorne H, Gooskens V, et al. Activity of gentian violet and brilliant green against some microorganisms associated with skin infections. Int J Dermatol. 1992;31:210-213.
- Montgomery RM, Casper EA. Cutaneous manifestations of the fungi causing dermatophytosis and onychomycosis and their treatment. J Am Med Assoc. 1945;128:77-83.
- Tchernev G, Cardoso JC, Ali MM, et al. Primary onychomycosis with granulomatous Tinea faciei. Braz J Infect Dis. 2010;14:546-547.
- Berrios RL, Arbiser JL. Effectiveness of gentian violet and similar products commonly used to treat pyodermas. Dermatol Clin. 2011;29:69-73.
- Maley AM, Arbiser JL. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol. 2013;22:775-80.
- Niedner R, Schöpf E. Inhibition of wound healing by antiseptics. Br J Dermatol. 1986;115:41-44
Case Report
A 92-year-old Eastern European woman presented to our nail clinic with a history of onychodystrophy and arthralgia of the digits of several months’ duration. Her dermatologic history was notable for irritant hand dermatitis. A prior nail plate clipping with histopathologic examination was negative for fungal elements. Physical examination revealed onychorrhexis of all fingernails as well as onycholysis and subungual hyperkeratosis of the right fourth fingernail. Blue-green staining was incidentally noted on the right second and third fingernails and nail folds (Figure 1). Contact dermoscopy using ultrasound gel revealed translucent areas with sparse pigment, though denser areas had a fine branching pattern (Figure 2). When questioned, the patient reported use of “zelyonka,” a brilliant green solution, to self-treat the nails. Histopathology on repeat nail clippings showed parakeratosis and serum, which was most consistent with her known history of irritant hand dermatitis. Radiographs of the hands revealed osteoarthritis that was most prominent at the distal interphalangeal joints.


Comment
Brilliant green is a triphenylmethane dye commonly used in Eastern Europe and other regions for the treatment of superficial skin infections and onychomycosis.1 Its use as an antiseptic and wound healing agent has been investigated in the scientific literature since at least the early 20th century.2 Brilliant green typically is applied in a 0.1% to 2% ethanol solution.1 The dye has bactericidal activity against gram-positive organisms, particularly staphylococci and streptococci.2,3 It has been used for the treatment of fungal skin and nail infections since at least the early 20th century, with anecdotal success.4 Although there have been no studies investigating use of brilliant green alone for the treatment of onychomycosis, it is sometimes used in combination with conventional oral agents for this purpose.5 Because of its availability, safety, ease of use, and low cost, brilliant green has been promoted as an antiseptic in resource-poor settings.3 The revival of brilliant green and other antiseptic dyes in these settings has been suggested as an alternative to oral antibiotic agents, to which resistance is rising, and as a potential cancer therapy.6,7 Although brilliant green’s mechanism of action in treating skin infections is unclear, it has been shown to form covalent adducts with thioredoxin reductase 2, a protein conserved from bacteria to humans with an essential function for cellular activity.7
Early case studies suggested that brilliant green was beneficial in treating wounds2; however, this indication is controversial. In a guinea pig study, brilliant green was shown to inhibit wound healing and the formation of granulation tissue.8 It also should be noted that when used topically, brilliant green may cause skin sensitization, necrotic skin reactions, and permanent staining of clothing. It has no known anti-inflammatory properties and also may cause skin irritation.8 Brilliant green may cause blindness if it comes in contact with the eyes.1
Brilliant green has other potential dermatologic indications. For example, a combination of brilliant green and gentian violet, a related dye, has demonstrated efficacy in the treatment of cutaneous hemangiomas in mouse models by blocking expression of angiopoietin-2.7
Dermatologists should be familiar with brilliant green and its common uses as well as adverse effects. Brilliant green is commercially available for a low cost ($5 to $20) in specialty pharmacies or online (eg, Amazon). It is sold alone or in combination with gentian violet and proflavine hemisulfate, and a prescription is not required. Due to its low cost and accessibility, patients may use brilliant green to self-treat dermatologic conditions. Green nails due to staining with brilliant green dye must be distinguished from other etiologies causing green nail discoloration, such as infection with Pseudomonas aeruginosa or Aspergillus, bullous disorders, jaundice, “old” hematomas, nail polish, and other exogenous pigments.
Case Report
A 92-year-old Eastern European woman presented to our nail clinic with a history of onychodystrophy and arthralgia of the digits of several months’ duration. Her dermatologic history was notable for irritant hand dermatitis. A prior nail plate clipping with histopathologic examination was negative for fungal elements. Physical examination revealed onychorrhexis of all fingernails as well as onycholysis and subungual hyperkeratosis of the right fourth fingernail. Blue-green staining was incidentally noted on the right second and third fingernails and nail folds (Figure 1). Contact dermoscopy using ultrasound gel revealed translucent areas with sparse pigment, though denser areas had a fine branching pattern (Figure 2). When questioned, the patient reported use of “zelyonka,” a brilliant green solution, to self-treat the nails. Histopathology on repeat nail clippings showed parakeratosis and serum, which was most consistent with her known history of irritant hand dermatitis. Radiographs of the hands revealed osteoarthritis that was most prominent at the distal interphalangeal joints.


Comment
Brilliant green is a triphenylmethane dye commonly used in Eastern Europe and other regions for the treatment of superficial skin infections and onychomycosis.1 Its use as an antiseptic and wound healing agent has been investigated in the scientific literature since at least the early 20th century.2 Brilliant green typically is applied in a 0.1% to 2% ethanol solution.1 The dye has bactericidal activity against gram-positive organisms, particularly staphylococci and streptococci.2,3 It has been used for the treatment of fungal skin and nail infections since at least the early 20th century, with anecdotal success.4 Although there have been no studies investigating use of brilliant green alone for the treatment of onychomycosis, it is sometimes used in combination with conventional oral agents for this purpose.5 Because of its availability, safety, ease of use, and low cost, brilliant green has been promoted as an antiseptic in resource-poor settings.3 The revival of brilliant green and other antiseptic dyes in these settings has been suggested as an alternative to oral antibiotic agents, to which resistance is rising, and as a potential cancer therapy.6,7 Although brilliant green’s mechanism of action in treating skin infections is unclear, it has been shown to form covalent adducts with thioredoxin reductase 2, a protein conserved from bacteria to humans with an essential function for cellular activity.7
Early case studies suggested that brilliant green was beneficial in treating wounds2; however, this indication is controversial. In a guinea pig study, brilliant green was shown to inhibit wound healing and the formation of granulation tissue.8 It also should be noted that when used topically, brilliant green may cause skin sensitization, necrotic skin reactions, and permanent staining of clothing. It has no known anti-inflammatory properties and also may cause skin irritation.8 Brilliant green may cause blindness if it comes in contact with the eyes.1
Brilliant green has other potential dermatologic indications. For example, a combination of brilliant green and gentian violet, a related dye, has demonstrated efficacy in the treatment of cutaneous hemangiomas in mouse models by blocking expression of angiopoietin-2.7
Dermatologists should be familiar with brilliant green and its common uses as well as adverse effects. Brilliant green is commercially available for a low cost ($5 to $20) in specialty pharmacies or online (eg, Amazon). It is sold alone or in combination with gentian violet and proflavine hemisulfate, and a prescription is not required. Due to its low cost and accessibility, patients may use brilliant green to self-treat dermatologic conditions. Green nails due to staining with brilliant green dye must be distinguished from other etiologies causing green nail discoloration, such as infection with Pseudomonas aeruginosa or Aspergillus, bullous disorders, jaundice, “old” hematomas, nail polish, and other exogenous pigments.
- Balabanova M, Popova L, Tchipeva R. Dyes in dermatology. Clin Dermatol. 2003;21:2-6.
- Browning CH, Gulbransen R, Kennaway EL, et al. Flavine and brilliant green, powerful antiseptics with low toxicity to the tissues: their use in the treatment of infected wounds. Br Med J. 1917;1:73-78.
- Bakker P, Doorne H, Gooskens V, et al. Activity of gentian violet and brilliant green against some microorganisms associated with skin infections. Int J Dermatol. 1992;31:210-213.
- Montgomery RM, Casper EA. Cutaneous manifestations of the fungi causing dermatophytosis and onychomycosis and their treatment. J Am Med Assoc. 1945;128:77-83.
- Tchernev G, Cardoso JC, Ali MM, et al. Primary onychomycosis with granulomatous Tinea faciei. Braz J Infect Dis. 2010;14:546-547.
- Berrios RL, Arbiser JL. Effectiveness of gentian violet and similar products commonly used to treat pyodermas. Dermatol Clin. 2011;29:69-73.
- Maley AM, Arbiser JL. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol. 2013;22:775-80.
- Niedner R, Schöpf E. Inhibition of wound healing by antiseptics. Br J Dermatol. 1986;115:41-44
- Balabanova M, Popova L, Tchipeva R. Dyes in dermatology. Clin Dermatol. 2003;21:2-6.
- Browning CH, Gulbransen R, Kennaway EL, et al. Flavine and brilliant green, powerful antiseptics with low toxicity to the tissues: their use in the treatment of infected wounds. Br Med J. 1917;1:73-78.
- Bakker P, Doorne H, Gooskens V, et al. Activity of gentian violet and brilliant green against some microorganisms associated with skin infections. Int J Dermatol. 1992;31:210-213.
- Montgomery RM, Casper EA. Cutaneous manifestations of the fungi causing dermatophytosis and onychomycosis and their treatment. J Am Med Assoc. 1945;128:77-83.
- Tchernev G, Cardoso JC, Ali MM, et al. Primary onychomycosis with granulomatous Tinea faciei. Braz J Infect Dis. 2010;14:546-547.
- Berrios RL, Arbiser JL. Effectiveness of gentian violet and similar products commonly used to treat pyodermas. Dermatol Clin. 2011;29:69-73.
- Maley AM, Arbiser JL. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol. 2013;22:775-80.
- Niedner R, Schöpf E. Inhibition of wound healing by antiseptics. Br J Dermatol. 1986;115:41-44
Practice Points
- Chloronychia, or green nail syndrome, is due to Pseudomonas aeruginosaPalatino LT Std infection and is a common etiology of green nail discoloration. Green nail discoloration also may be secondary to use of the antiseptic dye brilliant green.
- Brilliant green is bactericidal but has no known antifungal or anti-inflammatory activity; it should be considered in the differential diagnosis of green nail discoloration and also may cause blindness with eye contact.
Isobornyl Acrylate and Diabetic Devices Steal the Show for the 2020 American Contact Dermatitis Society Allergen of the Year
Each year, the American Contact Dermatitis Society names an Allergen of the Year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the Allergen of the Year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD).In 2020, the American Contact Dermatitis Society chose isobornyl acrylate as the Allergen of the Year.1 Not only has isobornyl acrylate been implicated in an epidemic of contact allergy to diabetic devices, but it also illustrates the challenges of investigating contact allergy to medical devices in general.
What Is Isobornyl Acrylate?
Isobornyl acrylate, also known as the isobornyl ester of acrylic acid, is a chemical used in glues, adhesives, coatings, sealants, inks, and paints. Similar to other acrylates, such as those involved in gel nail treatments, it is photopolymerizable; that is, when exposed to UV light, it can transform from a liquid monomer into a hard polymer, contributing to its utility as an adhesive. Prior to its recent implication in diabetic device contact allergy, isobornyl acrylate was not thought to be a common skin sensitizer. In a 2013 Dutch study of patients with acrylate allergy, only 1 of 14 patients with a contact allergy to other acrylates had a positive patch test reaction to isobornyl acrylate, which led the authors to conclude that adding it to their acrylate patch test series was not indicated.2
Isobornyl Acrylate in Diabetic Devices
Devices such as glucose monitoring systems and insulin pumps are used by millions of patients with diabetes worldwide. Not only are continuous glucose monitoring devices more convenient than self-monitoring of blood glucose, but they also are associated with a reduction in hemoglobin A1c levels and lower risk for hypoglycemia.3 However, these devices have been increasingly recognized as a source of irritant contact dermatitis and ACD.
Early cases of contact allergy to isobornyl acrylate in diabetic devices were reported in 1995 when 2 Belgian patients using insulin pumps developed ACD.4 The patients had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum and other allergens including acrylates. In addition, patch testing with plastic scrapings from their insulin pumps also was positive, and it was determined that the glue affixing the needle to the plastic had diffused into the plastic. The patients were switched to insulin pumps produced by heat staking instead of glue, and their symptoms resolved. In retrospect, this case series may seem prescient, as it was written 2 decades before isobornyl acrylate became recognized as a widespread cause of ACD in users of diabetic devices. Admittedly, other acrylate components of the glue also were positive on patch testing in these patients, so it was not until much later that the focus turned more exclusively to isobornyl acrylate.4
Similar to the insulin pumps in the 1995 Belgian series, diffusion of glue to other parts of modern glucose sensors also appears to cause isobornyl acrylate contact allergy. This theory was supported by a 2017 study from Belgian and Swedish investigators in which gas chromatography–mass spectrometry was used to identify concentrations of isobornyl acrylate in various components of a popular continuous glucose monitoring sensor.5 The concentration of isobornyl acrylate was approximately 100-fold higher at the site where the top and bottom plastic components of the sensor were joined as compared to the adhesive patch in contact with the patient’s skin. Therefore, the adhesive patch itself was not the source of the isobornyl acrylate exposure; rather, the isobornyl acrylate diffused into the adhesive patch from the glue used to join the components of the sensor together.5 One ramification is that patients with diabetic device contact allergy can have a false-negative patch test result if the adhesive patch is tested by itself, whereas they may react to patch testing with the whole sensor or an acetonic extract thereof.
Frequency of Sensitization to Isobornyl Acrylate
It is difficult to estimate the frequency of sensitization to isobornyl acrylate among users of diabetic devices, in part because those with mild allergy may not seek medical treatment. Nevertheless, there are studies that demonstrate a high prevalence of sensitization among users with suspected allergy. In a 2019 Finnish study of 6567 patients using an isobornyl acrylate–containing glucose sensor, 63 were patch tested for suspected ACD.6 Of these 63 patients, 51 (81%) had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum. These findings were consistent with the original 2017 study from Belgium and Sweden, in which 10 of 11 (91%) patients who used an isobornyl acrylate–containing glucose sensor and had suspected contact allergy had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum compared to no positive reactions in the 14 control patients.5 Given that there are more than 1.5 million users of this isobornyl acrylate–containing glucose sensor across 46 countries,7 it requires no stretch of the imagination to understand why investigators refer to isobornyl acrylate allergy as an epidemic, even if only a small percentage of users are sensitized to the device.
The Journey to Discover Isobornyl Acrylate as a Culprit Allergen
Similar to the discoveries of radiography and penicillin, the discovery of isobornyl acrylate as a culprit allergen in a modern glucose sensor was purely accidental. In 2016, a 9-year-old boy with diabetes presented to a Belgian dermatology department with ACD to a glucose sensor.1 A patch test nurse serendipitously applied isobornyl acrylate—0.01%, 0.05%, and 0.1% in petrolatum—which was not intended to be applied as part of the typical acrylate series. The only positive patch test reactions in this patient were to isobornyl acrylate at all 3 concentrations. This lucky error inspired isobornyl acrylate to be tested at multiple other dermatology departments in Europe in patients with ACD to their glucose sensors, leading to its discovery as a culprit allergen.1
One challenge facing investigators was obtaining information and materials from the diabetic device industry. Medical device manufacturers are not required to disclose chemicals present in a device on its label.8 Therefore, for patients or investigators to determine whether a potential allergen is present in a given device, they must request that information from the manufacturer, which can be a time-consuming and frustrating effort. Luckily, investigators collaborated with one another, and Belgian investigators suggested that Swedish investigators performing chemical analyses on a glucose monitoring device should focus on isobornyl acrylate, which enabled its detection in an extract from the device.5
Testing for Isobornyl Acrylate Allergy in Your Clinic
Patients with suspected ACD to a diabetic device—insulin pump or glucose sensor—should be patch tested with isobornyl acrylate, in addition to other previously reported allergens. The vehicle typically is petrolatum, and the commonly tested concentration is 0.1%. Testing with lower concentrations such as 0.01% can result in false-negative reactions,9 and testing at higher concentrations such as 0.3% can result in irritant skin reactions.2 Isobornyl acrylate 0.1% in petrolatum currently is available from one commercial allergen supplier (Chemotechnique Diagnostics). A positive patch test reaction to isobornyl acrylate 0.1% in petrolatum is shown in the Figure.
Management of Diabetic Device ACD
For patients with diabetic device ACD, there are several strategies that can reduce direct contact between the device and the patient’s skin. Methods that have been tried with varying success to allow patients to continue using their glucose sensors include barrier sprays (eg, Cavilon [3M], Silesse Skin Barrier [ConvaTec]); barrier pads (eg, Compeed [HRA Pharma], Surround skin protectors [Eakin], DuoDERM dressings [ConvaTec], Tegaderm dressings [3M]); and topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 inhibitors. Nevertheless, a 2019 Finnish study showed that only 14 of 63 (22%) patients with ACD to their isobornyl acrylate–containing glucose sensor were able to continue using the device, with all 14 requiring use of a barrier agent. Despite using the barrier agent, 13 (93%) of these patients had residual dermatitis.6 There also is concern that use of barrier methods might hamper the proper functioning of glucose sensors and related devices.
Patients with known isobornyl acrylate contact allergy also may switch to a different diabetic device. A 2019 German study showed that in 5 patients with isobornyl acrylate ACD, none had reactions to the one particular system that has been shown by gas chromatography–mass spectrometry to not contain isobornyl acrylate.10 However, as a word of caution, the same device also has been associated with ACD11,12 but has been resolved by using heat staking during the production process.13 As manufacturers update device components, identification of other isobornyl acrylate–free devices may require a degree of trial and error, as neither isobornyl acrylate nor any other potential allergen is listed on device labels.
Final Interpretation
Isobornyl acrylate is not a common sensitizer in general patch test populations but is a recently identified major culprit in ACD to diabetic devices. Patch testing with isobornyl acrylate 0.1% in petrolatum is not necessary in standard screening panels but should be considered in patients with suspected ACD to glucose sensors or insulin pumps. If a patient with ACD wants to continue to experience the convenience provided by a diabetic device, options include using topical steroids or barrier agents and/or changing the brand of the diabetic device, though none of these methods are foolproof. Hopefully, the identification of isobornyl acrylate as a culprit allergen will help to improve the lives of patients who use diabetic devices worldwide.
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis. 2013;69:86-92.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Busschots AM, Meuleman V, Poesen N, et al. Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis. 1995;33:205-206.
- Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis. 2017;77:367-373.
- Hyry HSI, Liippo JP, Virtanen HM. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis. 2019;81:161-166.
- Abbott’s Revolutionary FreeStyle® Libre system now reimbursed in the two largest provinces in Canada [press release]. Abbott Park, IL: Abbott; September 13, 2019. https://abbott.mediaroom.com/2019-09-13-Abbotts-Revolutionary-FreeStyle-R-Libre-System-Now-Reimbursed-in-the-Two-Largest-Provinces-in-Canada. Accessed May 14, 2020.
- Herman A, Goossens A. The need to disclose the composition of medical devices at the European level. Contact Dermatitis. 2019;81:159-160.
- Raison-Peyron N, Mowitz M, Bonardel N, et al. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis. 2018;79:76-80.
- Oppel E, Kamann S, Reichl FX, et al. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81:32-36.
- Peeters C, Herman A, Goossens A, et al. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426-429.
- Aschenbeck KA, Hylwa SA. A diabetic’s allergy: ethyl cyanoacrylate in glucose sensor adhesive. Dermatitis. 2017;28:289-291.
- Gisin V, Chan A, Welsh B. Manufacturing process changes and reduced skin irritations of an adhesive patch used for continuous glucose monitoring devices. J Diabetes Sci Technol. 2018;12:725-726.
Each year, the American Contact Dermatitis Society names an Allergen of the Year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the Allergen of the Year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD).In 2020, the American Contact Dermatitis Society chose isobornyl acrylate as the Allergen of the Year.1 Not only has isobornyl acrylate been implicated in an epidemic of contact allergy to diabetic devices, but it also illustrates the challenges of investigating contact allergy to medical devices in general.
What Is Isobornyl Acrylate?
Isobornyl acrylate, also known as the isobornyl ester of acrylic acid, is a chemical used in glues, adhesives, coatings, sealants, inks, and paints. Similar to other acrylates, such as those involved in gel nail treatments, it is photopolymerizable; that is, when exposed to UV light, it can transform from a liquid monomer into a hard polymer, contributing to its utility as an adhesive. Prior to its recent implication in diabetic device contact allergy, isobornyl acrylate was not thought to be a common skin sensitizer. In a 2013 Dutch study of patients with acrylate allergy, only 1 of 14 patients with a contact allergy to other acrylates had a positive patch test reaction to isobornyl acrylate, which led the authors to conclude that adding it to their acrylate patch test series was not indicated.2
Isobornyl Acrylate in Diabetic Devices
Devices such as glucose monitoring systems and insulin pumps are used by millions of patients with diabetes worldwide. Not only are continuous glucose monitoring devices more convenient than self-monitoring of blood glucose, but they also are associated with a reduction in hemoglobin A1c levels and lower risk for hypoglycemia.3 However, these devices have been increasingly recognized as a source of irritant contact dermatitis and ACD.
Early cases of contact allergy to isobornyl acrylate in diabetic devices were reported in 1995 when 2 Belgian patients using insulin pumps developed ACD.4 The patients had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum and other allergens including acrylates. In addition, patch testing with plastic scrapings from their insulin pumps also was positive, and it was determined that the glue affixing the needle to the plastic had diffused into the plastic. The patients were switched to insulin pumps produced by heat staking instead of glue, and their symptoms resolved. In retrospect, this case series may seem prescient, as it was written 2 decades before isobornyl acrylate became recognized as a widespread cause of ACD in users of diabetic devices. Admittedly, other acrylate components of the glue also were positive on patch testing in these patients, so it was not until much later that the focus turned more exclusively to isobornyl acrylate.4
Similar to the insulin pumps in the 1995 Belgian series, diffusion of glue to other parts of modern glucose sensors also appears to cause isobornyl acrylate contact allergy. This theory was supported by a 2017 study from Belgian and Swedish investigators in which gas chromatography–mass spectrometry was used to identify concentrations of isobornyl acrylate in various components of a popular continuous glucose monitoring sensor.5 The concentration of isobornyl acrylate was approximately 100-fold higher at the site where the top and bottom plastic components of the sensor were joined as compared to the adhesive patch in contact with the patient’s skin. Therefore, the adhesive patch itself was not the source of the isobornyl acrylate exposure; rather, the isobornyl acrylate diffused into the adhesive patch from the glue used to join the components of the sensor together.5 One ramification is that patients with diabetic device contact allergy can have a false-negative patch test result if the adhesive patch is tested by itself, whereas they may react to patch testing with the whole sensor or an acetonic extract thereof.
Frequency of Sensitization to Isobornyl Acrylate
It is difficult to estimate the frequency of sensitization to isobornyl acrylate among users of diabetic devices, in part because those with mild allergy may not seek medical treatment. Nevertheless, there are studies that demonstrate a high prevalence of sensitization among users with suspected allergy. In a 2019 Finnish study of 6567 patients using an isobornyl acrylate–containing glucose sensor, 63 were patch tested for suspected ACD.6 Of these 63 patients, 51 (81%) had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum. These findings were consistent with the original 2017 study from Belgium and Sweden, in which 10 of 11 (91%) patients who used an isobornyl acrylate–containing glucose sensor and had suspected contact allergy had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum compared to no positive reactions in the 14 control patients.5 Given that there are more than 1.5 million users of this isobornyl acrylate–containing glucose sensor across 46 countries,7 it requires no stretch of the imagination to understand why investigators refer to isobornyl acrylate allergy as an epidemic, even if only a small percentage of users are sensitized to the device.
The Journey to Discover Isobornyl Acrylate as a Culprit Allergen
Similar to the discoveries of radiography and penicillin, the discovery of isobornyl acrylate as a culprit allergen in a modern glucose sensor was purely accidental. In 2016, a 9-year-old boy with diabetes presented to a Belgian dermatology department with ACD to a glucose sensor.1 A patch test nurse serendipitously applied isobornyl acrylate—0.01%, 0.05%, and 0.1% in petrolatum—which was not intended to be applied as part of the typical acrylate series. The only positive patch test reactions in this patient were to isobornyl acrylate at all 3 concentrations. This lucky error inspired isobornyl acrylate to be tested at multiple other dermatology departments in Europe in patients with ACD to their glucose sensors, leading to its discovery as a culprit allergen.1
One challenge facing investigators was obtaining information and materials from the diabetic device industry. Medical device manufacturers are not required to disclose chemicals present in a device on its label.8 Therefore, for patients or investigators to determine whether a potential allergen is present in a given device, they must request that information from the manufacturer, which can be a time-consuming and frustrating effort. Luckily, investigators collaborated with one another, and Belgian investigators suggested that Swedish investigators performing chemical analyses on a glucose monitoring device should focus on isobornyl acrylate, which enabled its detection in an extract from the device.5
Testing for Isobornyl Acrylate Allergy in Your Clinic
Patients with suspected ACD to a diabetic device—insulin pump or glucose sensor—should be patch tested with isobornyl acrylate, in addition to other previously reported allergens. The vehicle typically is petrolatum, and the commonly tested concentration is 0.1%. Testing with lower concentrations such as 0.01% can result in false-negative reactions,9 and testing at higher concentrations such as 0.3% can result in irritant skin reactions.2 Isobornyl acrylate 0.1% in petrolatum currently is available from one commercial allergen supplier (Chemotechnique Diagnostics). A positive patch test reaction to isobornyl acrylate 0.1% in petrolatum is shown in the Figure.

Management of Diabetic Device ACD
For patients with diabetic device ACD, there are several strategies that can reduce direct contact between the device and the patient’s skin. Methods that have been tried with varying success to allow patients to continue using their glucose sensors include barrier sprays (eg, Cavilon [3M], Silesse Skin Barrier [ConvaTec]); barrier pads (eg, Compeed [HRA Pharma], Surround skin protectors [Eakin], DuoDERM dressings [ConvaTec], Tegaderm dressings [3M]); and topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 inhibitors. Nevertheless, a 2019 Finnish study showed that only 14 of 63 (22%) patients with ACD to their isobornyl acrylate–containing glucose sensor were able to continue using the device, with all 14 requiring use of a barrier agent. Despite using the barrier agent, 13 (93%) of these patients had residual dermatitis.6 There also is concern that use of barrier methods might hamper the proper functioning of glucose sensors and related devices.
Patients with known isobornyl acrylate contact allergy also may switch to a different diabetic device. A 2019 German study showed that in 5 patients with isobornyl acrylate ACD, none had reactions to the one particular system that has been shown by gas chromatography–mass spectrometry to not contain isobornyl acrylate.10 However, as a word of caution, the same device also has been associated with ACD11,12 but has been resolved by using heat staking during the production process.13 As manufacturers update device components, identification of other isobornyl acrylate–free devices may require a degree of trial and error, as neither isobornyl acrylate nor any other potential allergen is listed on device labels.
Final Interpretation
Isobornyl acrylate is not a common sensitizer in general patch test populations but is a recently identified major culprit in ACD to diabetic devices. Patch testing with isobornyl acrylate 0.1% in petrolatum is not necessary in standard screening panels but should be considered in patients with suspected ACD to glucose sensors or insulin pumps. If a patient with ACD wants to continue to experience the convenience provided by a diabetic device, options include using topical steroids or barrier agents and/or changing the brand of the diabetic device, though none of these methods are foolproof. Hopefully, the identification of isobornyl acrylate as a culprit allergen will help to improve the lives of patients who use diabetic devices worldwide.
Each year, the American Contact Dermatitis Society names an Allergen of the Year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the Allergen of the Year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD).In 2020, the American Contact Dermatitis Society chose isobornyl acrylate as the Allergen of the Year.1 Not only has isobornyl acrylate been implicated in an epidemic of contact allergy to diabetic devices, but it also illustrates the challenges of investigating contact allergy to medical devices in general.
What Is Isobornyl Acrylate?
Isobornyl acrylate, also known as the isobornyl ester of acrylic acid, is a chemical used in glues, adhesives, coatings, sealants, inks, and paints. Similar to other acrylates, such as those involved in gel nail treatments, it is photopolymerizable; that is, when exposed to UV light, it can transform from a liquid monomer into a hard polymer, contributing to its utility as an adhesive. Prior to its recent implication in diabetic device contact allergy, isobornyl acrylate was not thought to be a common skin sensitizer. In a 2013 Dutch study of patients with acrylate allergy, only 1 of 14 patients with a contact allergy to other acrylates had a positive patch test reaction to isobornyl acrylate, which led the authors to conclude that adding it to their acrylate patch test series was not indicated.2
Isobornyl Acrylate in Diabetic Devices
Devices such as glucose monitoring systems and insulin pumps are used by millions of patients with diabetes worldwide. Not only are continuous glucose monitoring devices more convenient than self-monitoring of blood glucose, but they also are associated with a reduction in hemoglobin A1c levels and lower risk for hypoglycemia.3 However, these devices have been increasingly recognized as a source of irritant contact dermatitis and ACD.
Early cases of contact allergy to isobornyl acrylate in diabetic devices were reported in 1995 when 2 Belgian patients using insulin pumps developed ACD.4 The patients had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum and other allergens including acrylates. In addition, patch testing with plastic scrapings from their insulin pumps also was positive, and it was determined that the glue affixing the needle to the plastic had diffused into the plastic. The patients were switched to insulin pumps produced by heat staking instead of glue, and their symptoms resolved. In retrospect, this case series may seem prescient, as it was written 2 decades before isobornyl acrylate became recognized as a widespread cause of ACD in users of diabetic devices. Admittedly, other acrylate components of the glue also were positive on patch testing in these patients, so it was not until much later that the focus turned more exclusively to isobornyl acrylate.4
Similar to the insulin pumps in the 1995 Belgian series, diffusion of glue to other parts of modern glucose sensors also appears to cause isobornyl acrylate contact allergy. This theory was supported by a 2017 study from Belgian and Swedish investigators in which gas chromatography–mass spectrometry was used to identify concentrations of isobornyl acrylate in various components of a popular continuous glucose monitoring sensor.5 The concentration of isobornyl acrylate was approximately 100-fold higher at the site where the top and bottom plastic components of the sensor were joined as compared to the adhesive patch in contact with the patient’s skin. Therefore, the adhesive patch itself was not the source of the isobornyl acrylate exposure; rather, the isobornyl acrylate diffused into the adhesive patch from the glue used to join the components of the sensor together.5 One ramification is that patients with diabetic device contact allergy can have a false-negative patch test result if the adhesive patch is tested by itself, whereas they may react to patch testing with the whole sensor or an acetonic extract thereof.
Frequency of Sensitization to Isobornyl Acrylate
It is difficult to estimate the frequency of sensitization to isobornyl acrylate among users of diabetic devices, in part because those with mild allergy may not seek medical treatment. Nevertheless, there are studies that demonstrate a high prevalence of sensitization among users with suspected allergy. In a 2019 Finnish study of 6567 patients using an isobornyl acrylate–containing glucose sensor, 63 were patch tested for suspected ACD.6 Of these 63 patients, 51 (81%) had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum. These findings were consistent with the original 2017 study from Belgium and Sweden, in which 10 of 11 (91%) patients who used an isobornyl acrylate–containing glucose sensor and had suspected contact allergy had positive patch test reactions to isobornyl acrylate 0.1% in petrolatum compared to no positive reactions in the 14 control patients.5 Given that there are more than 1.5 million users of this isobornyl acrylate–containing glucose sensor across 46 countries,7 it requires no stretch of the imagination to understand why investigators refer to isobornyl acrylate allergy as an epidemic, even if only a small percentage of users are sensitized to the device.
The Journey to Discover Isobornyl Acrylate as a Culprit Allergen
Similar to the discoveries of radiography and penicillin, the discovery of isobornyl acrylate as a culprit allergen in a modern glucose sensor was purely accidental. In 2016, a 9-year-old boy with diabetes presented to a Belgian dermatology department with ACD to a glucose sensor.1 A patch test nurse serendipitously applied isobornyl acrylate—0.01%, 0.05%, and 0.1% in petrolatum—which was not intended to be applied as part of the typical acrylate series. The only positive patch test reactions in this patient were to isobornyl acrylate at all 3 concentrations. This lucky error inspired isobornyl acrylate to be tested at multiple other dermatology departments in Europe in patients with ACD to their glucose sensors, leading to its discovery as a culprit allergen.1
One challenge facing investigators was obtaining information and materials from the diabetic device industry. Medical device manufacturers are not required to disclose chemicals present in a device on its label.8 Therefore, for patients or investigators to determine whether a potential allergen is present in a given device, they must request that information from the manufacturer, which can be a time-consuming and frustrating effort. Luckily, investigators collaborated with one another, and Belgian investigators suggested that Swedish investigators performing chemical analyses on a glucose monitoring device should focus on isobornyl acrylate, which enabled its detection in an extract from the device.5
Testing for Isobornyl Acrylate Allergy in Your Clinic
Patients with suspected ACD to a diabetic device—insulin pump or glucose sensor—should be patch tested with isobornyl acrylate, in addition to other previously reported allergens. The vehicle typically is petrolatum, and the commonly tested concentration is 0.1%. Testing with lower concentrations such as 0.01% can result in false-negative reactions,9 and testing at higher concentrations such as 0.3% can result in irritant skin reactions.2 Isobornyl acrylate 0.1% in petrolatum currently is available from one commercial allergen supplier (Chemotechnique Diagnostics). A positive patch test reaction to isobornyl acrylate 0.1% in petrolatum is shown in the Figure.

Management of Diabetic Device ACD
For patients with diabetic device ACD, there are several strategies that can reduce direct contact between the device and the patient’s skin. Methods that have been tried with varying success to allow patients to continue using their glucose sensors include barrier sprays (eg, Cavilon [3M], Silesse Skin Barrier [ConvaTec]); barrier pads (eg, Compeed [HRA Pharma], Surround skin protectors [Eakin], DuoDERM dressings [ConvaTec], Tegaderm dressings [3M]); and topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 inhibitors. Nevertheless, a 2019 Finnish study showed that only 14 of 63 (22%) patients with ACD to their isobornyl acrylate–containing glucose sensor were able to continue using the device, with all 14 requiring use of a barrier agent. Despite using the barrier agent, 13 (93%) of these patients had residual dermatitis.6 There also is concern that use of barrier methods might hamper the proper functioning of glucose sensors and related devices.
Patients with known isobornyl acrylate contact allergy also may switch to a different diabetic device. A 2019 German study showed that in 5 patients with isobornyl acrylate ACD, none had reactions to the one particular system that has been shown by gas chromatography–mass spectrometry to not contain isobornyl acrylate.10 However, as a word of caution, the same device also has been associated with ACD11,12 but has been resolved by using heat staking during the production process.13 As manufacturers update device components, identification of other isobornyl acrylate–free devices may require a degree of trial and error, as neither isobornyl acrylate nor any other potential allergen is listed on device labels.
Final Interpretation
Isobornyl acrylate is not a common sensitizer in general patch test populations but is a recently identified major culprit in ACD to diabetic devices. Patch testing with isobornyl acrylate 0.1% in petrolatum is not necessary in standard screening panels but should be considered in patients with suspected ACD to glucose sensors or insulin pumps. If a patient with ACD wants to continue to experience the convenience provided by a diabetic device, options include using topical steroids or barrier agents and/or changing the brand of the diabetic device, though none of these methods are foolproof. Hopefully, the identification of isobornyl acrylate as a culprit allergen will help to improve the lives of patients who use diabetic devices worldwide.
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis. 2013;69:86-92.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Busschots AM, Meuleman V, Poesen N, et al. Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis. 1995;33:205-206.
- Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis. 2017;77:367-373.
- Hyry HSI, Liippo JP, Virtanen HM. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis. 2019;81:161-166.
- Abbott’s Revolutionary FreeStyle® Libre system now reimbursed in the two largest provinces in Canada [press release]. Abbott Park, IL: Abbott; September 13, 2019. https://abbott.mediaroom.com/2019-09-13-Abbotts-Revolutionary-FreeStyle-R-Libre-System-Now-Reimbursed-in-the-Two-Largest-Provinces-in-Canada. Accessed May 14, 2020.
- Herman A, Goossens A. The need to disclose the composition of medical devices at the European level. Contact Dermatitis. 2019;81:159-160.
- Raison-Peyron N, Mowitz M, Bonardel N, et al. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis. 2018;79:76-80.
- Oppel E, Kamann S, Reichl FX, et al. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81:32-36.
- Peeters C, Herman A, Goossens A, et al. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426-429.
- Aschenbeck KA, Hylwa SA. A diabetic’s allergy: ethyl cyanoacrylate in glucose sensor adhesive. Dermatitis. 2017;28:289-291.
- Gisin V, Chan A, Welsh B. Manufacturing process changes and reduced skin irritations of an adhesive patch used for continuous glucose monitoring devices. J Diabetes Sci Technol. 2018;12:725-726.
- Aerts O, Herman A, Mowitz M, et al. Isobornyl acrylate. Dermatitis. 2020;31:4-12.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis. 2013;69:86-92.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Busschots AM, Meuleman V, Poesen N, et al. Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis. 1995;33:205-206.
- Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis. 2017;77:367-373.
- Hyry HSI, Liippo JP, Virtanen HM. Allergic contact dermatitis caused by glucose sensors in type 1 diabetes patients. Contact Dermatitis. 2019;81:161-166.
- Abbott’s Revolutionary FreeStyle® Libre system now reimbursed in the two largest provinces in Canada [press release]. Abbott Park, IL: Abbott; September 13, 2019. https://abbott.mediaroom.com/2019-09-13-Abbotts-Revolutionary-FreeStyle-R-Libre-System-Now-Reimbursed-in-the-Two-Largest-Provinces-in-Canada. Accessed May 14, 2020.
- Herman A, Goossens A. The need to disclose the composition of medical devices at the European level. Contact Dermatitis. 2019;81:159-160.
- Raison-Peyron N, Mowitz M, Bonardel N, et al. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis. 2018;79:76-80.
- Oppel E, Kamann S, Reichl FX, et al. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81:32-36.
- Peeters C, Herman A, Goossens A, et al. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426-429.
- Aschenbeck KA, Hylwa SA. A diabetic’s allergy: ethyl cyanoacrylate in glucose sensor adhesive. Dermatitis. 2017;28:289-291.
- Gisin V, Chan A, Welsh B. Manufacturing process changes and reduced skin irritations of an adhesive patch used for continuous glucose monitoring devices. J Diabetes Sci Technol. 2018;12:725-726.
Practice Points
- In patients with suspected allergic contact dermatitis (ACD) to a diabetic device, patch testing with isobornyl acrylate 0.1% in petrolatum should be considered.
- If patients with ACD to their diabetic device want to continue using the device, options include utilizing topical steroids or barrier agents and/or changing the brand of the diabetic device, though these steps may not be effective for every patient.
Utilization of a Stress Ball to Diminish Anxiety During Nail Surgery
Practice Gap
Anxiety is common in patients undergoing surgery with general anesthesia and may be exacerbated in patients undergoing dermatologic surgery with local anesthesia. Apprehension might be worse for nail surgery patients because the nail unit is highly innervated and vascular. Many patients fear the anesthetic injections, and there often is pain postoperatively. Perioperative anxiety correlates with increased postoperative pain,1 analgesic use,2 and delayed recovery.3 Several alternatives have been proposed to decrease perioperative anxiety, including nonpharmacologic interventions such as using educational videos, personalized music, hand holding, art activities, and virtual reality, as well as pharmacologic interventions such as benzodiazepines. However, these techniques have not been well studied for nail surgery.
The Technique
Patients generally are anxious about nail surgery secondary to the pain associated with the local anesthetic infiltration; hence, it is crucial to decrease anxiety during this initial step. In our practice, we provide patients with a palm-sized stress ball made of closed-cell polyurethane foam rubber before surgery. Patients are then instructed to hold the stress ball with the free hand and squeeze it whenever they feel anxious or when they feel any discomfort related to the procedure (Figure). A variety of balls can be bought for less than $1 each, thus making it a cost-effective option.
Practice Implications
Holding a stress ball has been found to reduce both pain and anxiety in patients undergoing conscious surgery.4 Furthermore, squeezing a stress ball perioperatively may increase feelings of empowerment, given that patients have direct control over the object, which in turn may have a positive effect on anxiety and patient satisfaction without interfering with the surgical procedure.5 Holding a stress ball is a safe, widely accessible, and inexpensive technique that may aid in decreasing patients’ anxiety related to nail surgery. Nonetheless, controlled clinical trials assessing the efficacy of this method in reducing anxiety related to nail surgery are needed to determine its benefit compared to other methods.
- Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521-530.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16:600-610.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:e20306.
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455.
- Foy CR, Timmins F. Improving communication in day surgery settings. Nurs Stand. 2004;19:37-42.
Practice Gap
Anxiety is common in patients undergoing surgery with general anesthesia and may be exacerbated in patients undergoing dermatologic surgery with local anesthesia. Apprehension might be worse for nail surgery patients because the nail unit is highly innervated and vascular. Many patients fear the anesthetic injections, and there often is pain postoperatively. Perioperative anxiety correlates with increased postoperative pain,1 analgesic use,2 and delayed recovery.3 Several alternatives have been proposed to decrease perioperative anxiety, including nonpharmacologic interventions such as using educational videos, personalized music, hand holding, art activities, and virtual reality, as well as pharmacologic interventions such as benzodiazepines. However, these techniques have not been well studied for nail surgery.
The Technique
Patients generally are anxious about nail surgery secondary to the pain associated with the local anesthetic infiltration; hence, it is crucial to decrease anxiety during this initial step. In our practice, we provide patients with a palm-sized stress ball made of closed-cell polyurethane foam rubber before surgery. Patients are then instructed to hold the stress ball with the free hand and squeeze it whenever they feel anxious or when they feel any discomfort related to the procedure (Figure). A variety of balls can be bought for less than $1 each, thus making it a cost-effective option.

Practice Implications
Holding a stress ball has been found to reduce both pain and anxiety in patients undergoing conscious surgery.4 Furthermore, squeezing a stress ball perioperatively may increase feelings of empowerment, given that patients have direct control over the object, which in turn may have a positive effect on anxiety and patient satisfaction without interfering with the surgical procedure.5 Holding a stress ball is a safe, widely accessible, and inexpensive technique that may aid in decreasing patients’ anxiety related to nail surgery. Nonetheless, controlled clinical trials assessing the efficacy of this method in reducing anxiety related to nail surgery are needed to determine its benefit compared to other methods.
Practice Gap
Anxiety is common in patients undergoing surgery with general anesthesia and may be exacerbated in patients undergoing dermatologic surgery with local anesthesia. Apprehension might be worse for nail surgery patients because the nail unit is highly innervated and vascular. Many patients fear the anesthetic injections, and there often is pain postoperatively. Perioperative anxiety correlates with increased postoperative pain,1 analgesic use,2 and delayed recovery.3 Several alternatives have been proposed to decrease perioperative anxiety, including nonpharmacologic interventions such as using educational videos, personalized music, hand holding, art activities, and virtual reality, as well as pharmacologic interventions such as benzodiazepines. However, these techniques have not been well studied for nail surgery.
The Technique
Patients generally are anxious about nail surgery secondary to the pain associated with the local anesthetic infiltration; hence, it is crucial to decrease anxiety during this initial step. In our practice, we provide patients with a palm-sized stress ball made of closed-cell polyurethane foam rubber before surgery. Patients are then instructed to hold the stress ball with the free hand and squeeze it whenever they feel anxious or when they feel any discomfort related to the procedure (Figure). A variety of balls can be bought for less than $1 each, thus making it a cost-effective option.

Practice Implications
Holding a stress ball has been found to reduce both pain and anxiety in patients undergoing conscious surgery.4 Furthermore, squeezing a stress ball perioperatively may increase feelings of empowerment, given that patients have direct control over the object, which in turn may have a positive effect on anxiety and patient satisfaction without interfering with the surgical procedure.5 Holding a stress ball is a safe, widely accessible, and inexpensive technique that may aid in decreasing patients’ anxiety related to nail surgery. Nonetheless, controlled clinical trials assessing the efficacy of this method in reducing anxiety related to nail surgery are needed to determine its benefit compared to other methods.
- Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521-530.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16:600-610.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:e20306.
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455.
- Foy CR, Timmins F. Improving communication in day surgery settings. Nurs Stand. 2004;19:37-42.
- Carr EC, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521-530.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16:600-610.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:e20306.
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455.
- Foy CR, Timmins F. Improving communication in day surgery settings. Nurs Stand. 2004;19:37-42.
Multiethnic Training in Residency: A Survey of Dermatology Residents
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Practice Points
- To treat the ever-changing demographics of patients in the United States, dermatologists must receive adequate exposure and education regarding dermatologic conditions in patients from various ethnic backgrounds.
- Dermatology residents from less diverse regions are more likely to agree that dedicated clinics and rotations are important to gain competence compared to those from more diverse regions.
- In areas with less diversity, dedicated multiethnic skin clinics and faculty may be more important for assuring an adequate residency experience.
Bullous Eruption Caused by an Exotic Hedgehog Purchased as a Household Pet
Case Report
A 37-year-old woman presented to the dermatology clinic with an itchy rash involving the right hand. The rash had been present for 10 days but had become increasingly pruritic and vesicular over the last 5 days. She denied new exposures or other household members with similar symptoms. The patient reported that she had purchased a 4-toed, white-bellied African pygmy hedgehog (Atelerix albiventris) approximately 4 months prior. Upon questioning, she stated that she handled the hedgehog a couple of times a week and always washed her hands with soap and water immediately after. The patient’s medical and personal history were otherwise unremarkable.
Review of systems, including fevers, chills, and night sweats, was negative. Clinical examination revealed erythema with overlying vesicles and pustules on the right radial palm, radial dorsal hand, and interdigital web space of the first and second digit (Figure 1). The eruption was actively discharging serous exudate. No other lesions were present.
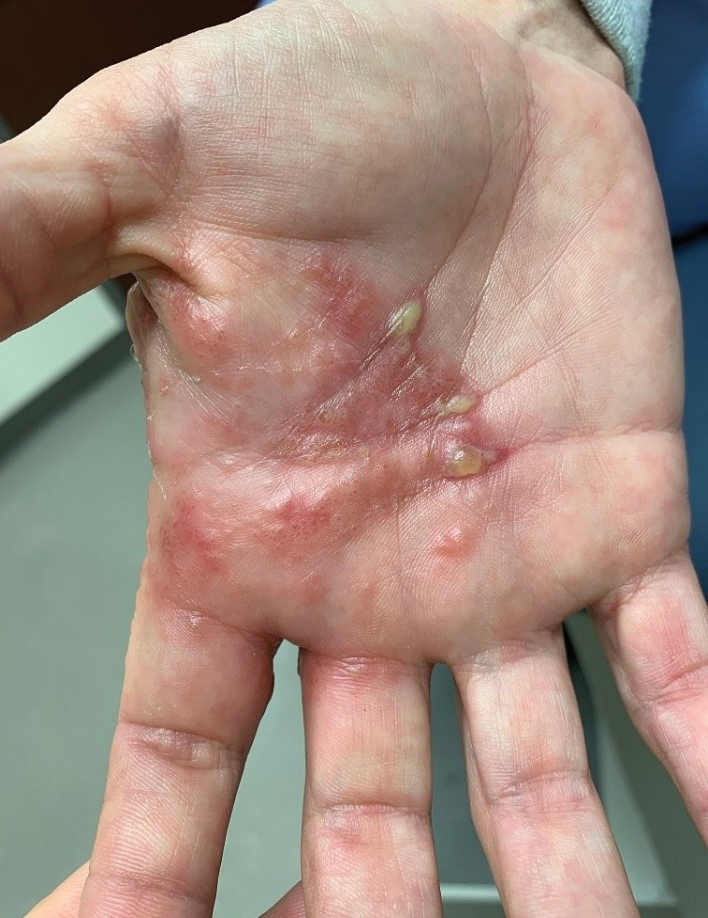
Unspecified acute contact dermatitis was the preliminary diagnosis based on clinical presentation and history. Other entities considered before making the diagnosis included psoriasis, eczema, and an infectious cause. Specimens were taken for bacterial and fungal cultures as well as a specimen for herpes simplex virus by polymerase chain reaction. Due to the intense pruritus and vesicular nature of the rash, the patient was treatedwith a 2-week, 60-40-20 prednisone taper and clobetasol propionate ointment 0.05% twice daily.
At 1-week follow-up, the eruption had improved, but the patient was still experiencing mild pruritus. Physical examination of the affected areas showed erythematous, violaceous, annular patches with slight scale at the periphery; all bullous lesions had resolved (Figure 2). Bacterial culture and herpes simplex virus by polymerase chain reaction were negative.
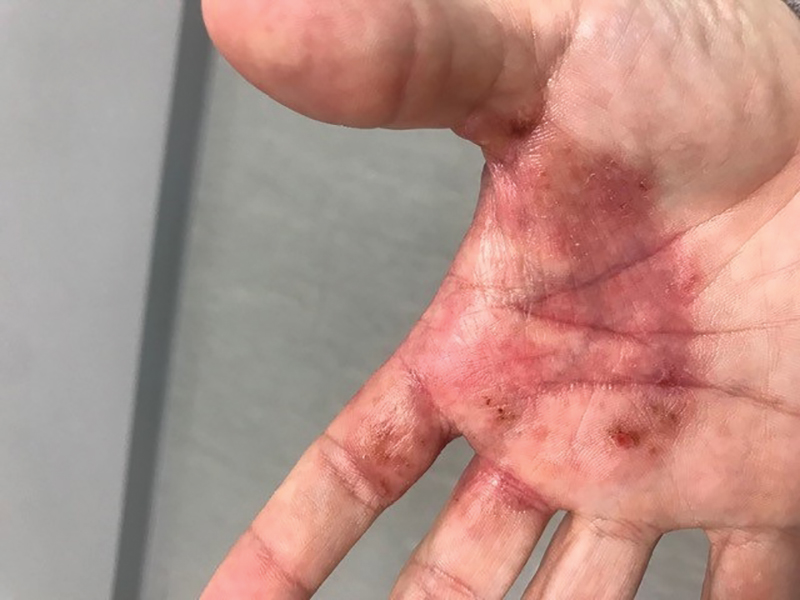
Two weeks after initial consultation, the fungal culture returned positive and showed growth of Trichophyton mentagrophytes. The patient was contacted and returned for re-evaluation. Physical examination showed decreased erythema and no bullous lesions; however, there was increased fine scale throughout the affected area on the right palm and first and second interdigital spaces (Figure 3). She reported mild pruritus. A confirmatory potassium hydroxide (KOH) preparation was positive for fungal hyphae. The patient was subsequently diagnosed with bullous tinea secondary to domestic hedgehog exposure that was now presenting as tinea manuum incognita. After 2 weeks of appropriate systemic and topical antifungal therapy, the patient’s skin eruption markedly improved (Figure 4).
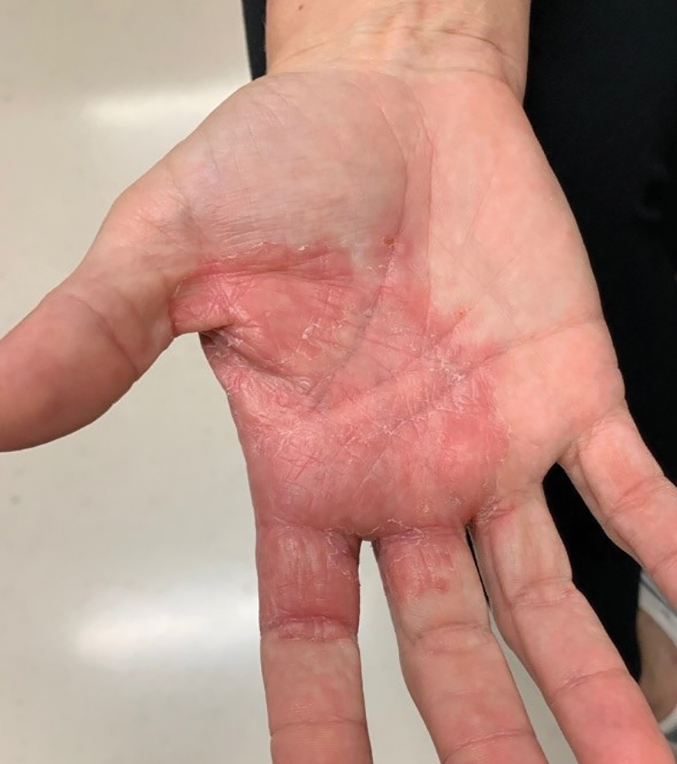
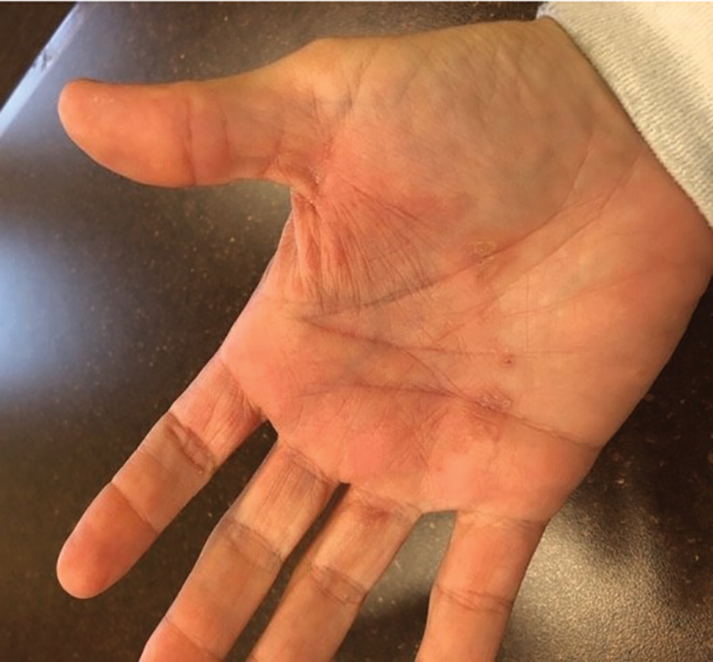
Comment
Tinea manuum is a dermatophytic epidermal infection of the hand. The most common causative organisms are Trichophyton rubrum, T mentagrophytes, and Epidermophyton floccosum. Infection can be acquired from contact with an infected person or animal, fomites, soil, or autoinoculation. Tinea manuum often is associated with tinea pedis. The hand that is used to excoriate the pruritic feet becomes infected, resulting in the classic two feet–one hand syndrome, which this patient did not have.1
Dermatophytes colonize keratin-containing tissues—skin, hair, and nails—utilizing the keratin for nutrients, and they do not invade living tissue in immunocompetent hosts. Dermatophytes cause clinical disease from an allergic host response to fungal antigens or their metabolic products.1 Tinea incognito results from the use of corticosteroids to treat a cutaneous fungal infection. The immunomodulatory effects of corticosteroids alter the appearance of the lesion. Hallmark signs and symptoms of a tinea infection, including scale, prominent border, erythema, and pruritus, can be reduced with corticosteroid use, giving the false impression that the lesion is resolving.2,3
The diagnosis of tinea manuum can be made clinically and often is supported with the findings of a KOH preparation. Scraping from an active scaling border generally provides the best results for obtaining fungal elements. For vesiculobullous lesions, the roof of a vesicle can provide an adequate specimen. Fungal culture and specific dermatophyte testing mediums can be used as confirmatory tests or allow for speciation, which help establish the diagnosis.1
Trichophyton mentagrophytes is a species complex—a group of closely related organisms that share morphologic appearance to the point that boundaries between them often are unclear. It can be identified by gross and microscopic morphology; however, variants of T mentagrophytes (eg, Trichophyton interdigitale, Trichophyton erinacei) require a confirmatory test or molecular analysis to be correctly identified.4-6 The laboratory used at our facility does not routinely attempt to identify the variant due to of lack of clinical significance.7,8
Anthropophilic fungi such as T rubrum, E floccosum, and T interdigitale generally do not cause a robust immunologic reaction. Infection usually is chronic in nature, though cases of pustular and vesicular tinea have been described.9,10Trichophyton erinacei and T mentagrophytes are zoophilic dermatophytes that cause an acute host response and are more likely to present with vesiculobullous lesions. Trichophyton erinacei is the most common fungal pathogen associated with A albiventris and has been isolated from its epidermal mites and quills,11,12 which likely facilitates interspecies transmission and compromises the cutaneous barrier of human hosts when the hedgehog is handled.
Atelerix albiventris is the most common domesticated hedgehog in the United States. These mild-mannered, nocturnal insectivores are unique, low-maintenance pets that have recently gained popularity. They are notable for their propensity to curl into a ball when frightened (Figure 5). The spines are not barbed and do not detach, as those of a porcupine do, but are still capable of piercing the skin. Atelerix albiventris is known to cause zoonotic dermatosis in humans and should be handled with gloves.13 Performing a KOH preparation early in the diagnostic workup can help initiate antifungal therapy, as results of fungal culture can take several weeks.

Conclusion
This case illustrates the importance of close follow-up of skin lesions that only partially respond to initial treatment and maintaining a high index of suspicion as exotic pets become popular.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1329-1363.
- Habif T. Superficial fungal infections. In: Habif T. Clinical Dermatology. 6th ed. Philadelphia, PA: Elsevier; 2016:487-533.
- Lange M, Jasiel‐Walikowska E, Nowicki R, et al. Tinea incognito due to Trichophyton mentagrophytes. Mycoses. 2010;53:455-457.
- Pchelin IM, Azarov DV, Churina MA, et al. Species boundaries in the Trichophyton mentagrophytes/T. interdigitale species complex. Med Mycol. 2019;57:781-789.
- Makimura K, Mochizuki T, Hasegawa A, et al. Phylogenetic classification of Trichophyton mentagrophytes complex strains based on DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Clin Microbiol. 1998;36:2629-2633.
- de Hoog GS, Dukik K, Monod M, et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5-31.
- Rudramurthy SM, Shankarnarayan SA, Dogra S, et al. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:e02522-17.
- Singh A, Masih A, Khurana A, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61:477-484.
- Kawakami Y, Oyama N, Sakai E, et al. Childhood tinea incognito caused by Trichophyton mentagrophytes var. interdigitale mimicking pustular psoriasis. Pediatr Dermatol. 2011;28:738-739.
- Neri I, Piraccini BM, Guareschi E, et al. Bullous tinea pedis in two children. Mycoses. 2004;47:475-478.
- Abarca ML, Castellá G, Martorell J, et al. Trichophyton erinacei in pet hedgehogs in Spain: occurrence and revision of its taxonomic status. Med Mycol. 2016;55:164-172.
- Morris P, English MP. Transmission and course of Trichophyton erinacei infections in British hedgehogs. Sabouraudia. 1973;11:42-47.
- Riley PY, Chomel BB. Hedgehog zoonoses. Emerg Infect Dis. 2005;11:1-5.
Case Report
A 37-year-old woman presented to the dermatology clinic with an itchy rash involving the right hand. The rash had been present for 10 days but had become increasingly pruritic and vesicular over the last 5 days. She denied new exposures or other household members with similar symptoms. The patient reported that she had purchased a 4-toed, white-bellied African pygmy hedgehog (Atelerix albiventris) approximately 4 months prior. Upon questioning, she stated that she handled the hedgehog a couple of times a week and always washed her hands with soap and water immediately after. The patient’s medical and personal history were otherwise unremarkable.
Review of systems, including fevers, chills, and night sweats, was negative. Clinical examination revealed erythema with overlying vesicles and pustules on the right radial palm, radial dorsal hand, and interdigital web space of the first and second digit (Figure 1). The eruption was actively discharging serous exudate. No other lesions were present.

Unspecified acute contact dermatitis was the preliminary diagnosis based on clinical presentation and history. Other entities considered before making the diagnosis included psoriasis, eczema, and an infectious cause. Specimens were taken for bacterial and fungal cultures as well as a specimen for herpes simplex virus by polymerase chain reaction. Due to the intense pruritus and vesicular nature of the rash, the patient was treatedwith a 2-week, 60-40-20 prednisone taper and clobetasol propionate ointment 0.05% twice daily.
At 1-week follow-up, the eruption had improved, but the patient was still experiencing mild pruritus. Physical examination of the affected areas showed erythematous, violaceous, annular patches with slight scale at the periphery; all bullous lesions had resolved (Figure 2). Bacterial culture and herpes simplex virus by polymerase chain reaction were negative.

Two weeks after initial consultation, the fungal culture returned positive and showed growth of Trichophyton mentagrophytes. The patient was contacted and returned for re-evaluation. Physical examination showed decreased erythema and no bullous lesions; however, there was increased fine scale throughout the affected area on the right palm and first and second interdigital spaces (Figure 3). She reported mild pruritus. A confirmatory potassium hydroxide (KOH) preparation was positive for fungal hyphae. The patient was subsequently diagnosed with bullous tinea secondary to domestic hedgehog exposure that was now presenting as tinea manuum incognita. After 2 weeks of appropriate systemic and topical antifungal therapy, the patient’s skin eruption markedly improved (Figure 4).


Comment
Tinea manuum is a dermatophytic epidermal infection of the hand. The most common causative organisms are Trichophyton rubrum, T mentagrophytes, and Epidermophyton floccosum. Infection can be acquired from contact with an infected person or animal, fomites, soil, or autoinoculation. Tinea manuum often is associated with tinea pedis. The hand that is used to excoriate the pruritic feet becomes infected, resulting in the classic two feet–one hand syndrome, which this patient did not have.1
Dermatophytes colonize keratin-containing tissues—skin, hair, and nails—utilizing the keratin for nutrients, and they do not invade living tissue in immunocompetent hosts. Dermatophytes cause clinical disease from an allergic host response to fungal antigens or their metabolic products.1 Tinea incognito results from the use of corticosteroids to treat a cutaneous fungal infection. The immunomodulatory effects of corticosteroids alter the appearance of the lesion. Hallmark signs and symptoms of a tinea infection, including scale, prominent border, erythema, and pruritus, can be reduced with corticosteroid use, giving the false impression that the lesion is resolving.2,3
The diagnosis of tinea manuum can be made clinically and often is supported with the findings of a KOH preparation. Scraping from an active scaling border generally provides the best results for obtaining fungal elements. For vesiculobullous lesions, the roof of a vesicle can provide an adequate specimen. Fungal culture and specific dermatophyte testing mediums can be used as confirmatory tests or allow for speciation, which help establish the diagnosis.1
Trichophyton mentagrophytes is a species complex—a group of closely related organisms that share morphologic appearance to the point that boundaries between them often are unclear. It can be identified by gross and microscopic morphology; however, variants of T mentagrophytes (eg, Trichophyton interdigitale, Trichophyton erinacei) require a confirmatory test or molecular analysis to be correctly identified.4-6 The laboratory used at our facility does not routinely attempt to identify the variant due to of lack of clinical significance.7,8
Anthropophilic fungi such as T rubrum, E floccosum, and T interdigitale generally do not cause a robust immunologic reaction. Infection usually is chronic in nature, though cases of pustular and vesicular tinea have been described.9,10Trichophyton erinacei and T mentagrophytes are zoophilic dermatophytes that cause an acute host response and are more likely to present with vesiculobullous lesions. Trichophyton erinacei is the most common fungal pathogen associated with A albiventris and has been isolated from its epidermal mites and quills,11,12 which likely facilitates interspecies transmission and compromises the cutaneous barrier of human hosts when the hedgehog is handled.
Atelerix albiventris is the most common domesticated hedgehog in the United States. These mild-mannered, nocturnal insectivores are unique, low-maintenance pets that have recently gained popularity. They are notable for their propensity to curl into a ball when frightened (Figure 5). The spines are not barbed and do not detach, as those of a porcupine do, but are still capable of piercing the skin. Atelerix albiventris is known to cause zoonotic dermatosis in humans and should be handled with gloves.13 Performing a KOH preparation early in the diagnostic workup can help initiate antifungal therapy, as results of fungal culture can take several weeks.

Conclusion
This case illustrates the importance of close follow-up of skin lesions that only partially respond to initial treatment and maintaining a high index of suspicion as exotic pets become popular.
Case Report
A 37-year-old woman presented to the dermatology clinic with an itchy rash involving the right hand. The rash had been present for 10 days but had become increasingly pruritic and vesicular over the last 5 days. She denied new exposures or other household members with similar symptoms. The patient reported that she had purchased a 4-toed, white-bellied African pygmy hedgehog (Atelerix albiventris) approximately 4 months prior. Upon questioning, she stated that she handled the hedgehog a couple of times a week and always washed her hands with soap and water immediately after. The patient’s medical and personal history were otherwise unremarkable.
Review of systems, including fevers, chills, and night sweats, was negative. Clinical examination revealed erythema with overlying vesicles and pustules on the right radial palm, radial dorsal hand, and interdigital web space of the first and second digit (Figure 1). The eruption was actively discharging serous exudate. No other lesions were present.

Unspecified acute contact dermatitis was the preliminary diagnosis based on clinical presentation and history. Other entities considered before making the diagnosis included psoriasis, eczema, and an infectious cause. Specimens were taken for bacterial and fungal cultures as well as a specimen for herpes simplex virus by polymerase chain reaction. Due to the intense pruritus and vesicular nature of the rash, the patient was treatedwith a 2-week, 60-40-20 prednisone taper and clobetasol propionate ointment 0.05% twice daily.
At 1-week follow-up, the eruption had improved, but the patient was still experiencing mild pruritus. Physical examination of the affected areas showed erythematous, violaceous, annular patches with slight scale at the periphery; all bullous lesions had resolved (Figure 2). Bacterial culture and herpes simplex virus by polymerase chain reaction were negative.

Two weeks after initial consultation, the fungal culture returned positive and showed growth of Trichophyton mentagrophytes. The patient was contacted and returned for re-evaluation. Physical examination showed decreased erythema and no bullous lesions; however, there was increased fine scale throughout the affected area on the right palm and first and second interdigital spaces (Figure 3). She reported mild pruritus. A confirmatory potassium hydroxide (KOH) preparation was positive for fungal hyphae. The patient was subsequently diagnosed with bullous tinea secondary to domestic hedgehog exposure that was now presenting as tinea manuum incognita. After 2 weeks of appropriate systemic and topical antifungal therapy, the patient’s skin eruption markedly improved (Figure 4).


Comment
Tinea manuum is a dermatophytic epidermal infection of the hand. The most common causative organisms are Trichophyton rubrum, T mentagrophytes, and Epidermophyton floccosum. Infection can be acquired from contact with an infected person or animal, fomites, soil, or autoinoculation. Tinea manuum often is associated with tinea pedis. The hand that is used to excoriate the pruritic feet becomes infected, resulting in the classic two feet–one hand syndrome, which this patient did not have.1
Dermatophytes colonize keratin-containing tissues—skin, hair, and nails—utilizing the keratin for nutrients, and they do not invade living tissue in immunocompetent hosts. Dermatophytes cause clinical disease from an allergic host response to fungal antigens or their metabolic products.1 Tinea incognito results from the use of corticosteroids to treat a cutaneous fungal infection. The immunomodulatory effects of corticosteroids alter the appearance of the lesion. Hallmark signs and symptoms of a tinea infection, including scale, prominent border, erythema, and pruritus, can be reduced with corticosteroid use, giving the false impression that the lesion is resolving.2,3
The diagnosis of tinea manuum can be made clinically and often is supported with the findings of a KOH preparation. Scraping from an active scaling border generally provides the best results for obtaining fungal elements. For vesiculobullous lesions, the roof of a vesicle can provide an adequate specimen. Fungal culture and specific dermatophyte testing mediums can be used as confirmatory tests or allow for speciation, which help establish the diagnosis.1
Trichophyton mentagrophytes is a species complex—a group of closely related organisms that share morphologic appearance to the point that boundaries between them often are unclear. It can be identified by gross and microscopic morphology; however, variants of T mentagrophytes (eg, Trichophyton interdigitale, Trichophyton erinacei) require a confirmatory test or molecular analysis to be correctly identified.4-6 The laboratory used at our facility does not routinely attempt to identify the variant due to of lack of clinical significance.7,8
Anthropophilic fungi such as T rubrum, E floccosum, and T interdigitale generally do not cause a robust immunologic reaction. Infection usually is chronic in nature, though cases of pustular and vesicular tinea have been described.9,10Trichophyton erinacei and T mentagrophytes are zoophilic dermatophytes that cause an acute host response and are more likely to present with vesiculobullous lesions. Trichophyton erinacei is the most common fungal pathogen associated with A albiventris and has been isolated from its epidermal mites and quills,11,12 which likely facilitates interspecies transmission and compromises the cutaneous barrier of human hosts when the hedgehog is handled.
Atelerix albiventris is the most common domesticated hedgehog in the United States. These mild-mannered, nocturnal insectivores are unique, low-maintenance pets that have recently gained popularity. They are notable for their propensity to curl into a ball when frightened (Figure 5). The spines are not barbed and do not detach, as those of a porcupine do, but are still capable of piercing the skin. Atelerix albiventris is known to cause zoonotic dermatosis in humans and should be handled with gloves.13 Performing a KOH preparation early in the diagnostic workup can help initiate antifungal therapy, as results of fungal culture can take several weeks.

Conclusion
This case illustrates the importance of close follow-up of skin lesions that only partially respond to initial treatment and maintaining a high index of suspicion as exotic pets become popular.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1329-1363.
- Habif T. Superficial fungal infections. In: Habif T. Clinical Dermatology. 6th ed. Philadelphia, PA: Elsevier; 2016:487-533.
- Lange M, Jasiel‐Walikowska E, Nowicki R, et al. Tinea incognito due to Trichophyton mentagrophytes. Mycoses. 2010;53:455-457.
- Pchelin IM, Azarov DV, Churina MA, et al. Species boundaries in the Trichophyton mentagrophytes/T. interdigitale species complex. Med Mycol. 2019;57:781-789.
- Makimura K, Mochizuki T, Hasegawa A, et al. Phylogenetic classification of Trichophyton mentagrophytes complex strains based on DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Clin Microbiol. 1998;36:2629-2633.
- de Hoog GS, Dukik K, Monod M, et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5-31.
- Rudramurthy SM, Shankarnarayan SA, Dogra S, et al. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:e02522-17.
- Singh A, Masih A, Khurana A, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61:477-484.
- Kawakami Y, Oyama N, Sakai E, et al. Childhood tinea incognito caused by Trichophyton mentagrophytes var. interdigitale mimicking pustular psoriasis. Pediatr Dermatol. 2011;28:738-739.
- Neri I, Piraccini BM, Guareschi E, et al. Bullous tinea pedis in two children. Mycoses. 2004;47:475-478.
- Abarca ML, Castellá G, Martorell J, et al. Trichophyton erinacei in pet hedgehogs in Spain: occurrence and revision of its taxonomic status. Med Mycol. 2016;55:164-172.
- Morris P, English MP. Transmission and course of Trichophyton erinacei infections in British hedgehogs. Sabouraudia. 1973;11:42-47.
- Riley PY, Chomel BB. Hedgehog zoonoses. Emerg Infect Dis. 2005;11:1-5.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1329-1363.
- Habif T. Superficial fungal infections. In: Habif T. Clinical Dermatology. 6th ed. Philadelphia, PA: Elsevier; 2016:487-533.
- Lange M, Jasiel‐Walikowska E, Nowicki R, et al. Tinea incognito due to Trichophyton mentagrophytes. Mycoses. 2010;53:455-457.
- Pchelin IM, Azarov DV, Churina MA, et al. Species boundaries in the Trichophyton mentagrophytes/T. interdigitale species complex. Med Mycol. 2019;57:781-789.
- Makimura K, Mochizuki T, Hasegawa A, et al. Phylogenetic classification of Trichophyton mentagrophytes complex strains based on DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Clin Microbiol. 1998;36:2629-2633.
- de Hoog GS, Dukik K, Monod M, et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5-31.
- Rudramurthy SM, Shankarnarayan SA, Dogra S, et al. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:e02522-17.
- Singh A, Masih A, Khurana A, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61:477-484.
- Kawakami Y, Oyama N, Sakai E, et al. Childhood tinea incognito caused by Trichophyton mentagrophytes var. interdigitale mimicking pustular psoriasis. Pediatr Dermatol. 2011;28:738-739.
- Neri I, Piraccini BM, Guareschi E, et al. Bullous tinea pedis in two children. Mycoses. 2004;47:475-478.
- Abarca ML, Castellá G, Martorell J, et al. Trichophyton erinacei in pet hedgehogs in Spain: occurrence and revision of its taxonomic status. Med Mycol. 2016;55:164-172.
- Morris P, English MP. Transmission and course of Trichophyton erinacei infections in British hedgehogs. Sabouraudia. 1973;11:42-47.
- Riley PY, Chomel BB. Hedgehog zoonoses. Emerg Infect Dis. 2005;11:1-5.
Practice Points
- Bullous tinea may present with little or no scale, which can lead to confusion with acute contact dermatitis.
- The recent popularity of exotic pets may increase the incidence of fungal zoonotic dermatitis.
- Prompt recognition of tinea incognito is essential when treating lesions with corticosteroids.
- Skin lesions not responding appropriately to therapy warrant reassessment and further evaluation.
Comment on “Racial Limitations of Fitzpatrick Skin Type”
To the Editor:
It is with great interest that I read the article by Ware et al,1 “Racial Limitations of Fitzpatrick Skin Type.” Within my own department, the issue of the appropriateness of using Fitzpatrick skin type (FST) as a surrogate to describe skin color has been raised with mixed responses.
As in many dermatology residency programs across the country, first-year dermatology residents are asked to describe the morphology of a lesion/eruption seen on a patient during Grand Rounds. Preceding the morphologic description, many providers describe the appearance of the patient including their skin color, as constitutive skin color can impact understanding of the morphologic descriptions, favor different diagnoses based on disease epidemiology, and guide subsequent treatment recommendations.2,3 During one of my first Grand Rounds as an early dermatology resident, a patient was described as a “well-appearing brown boy,” which led to a lively discussion regarding the terms that should be used to describe skin color, with some in the audience preferring FST, others including myself preferring degree of pigmentation (eg, light, moderate, dark), and lastly others preferring an inferred ethnicity based on the patient’s appearance. One audience member commented, “I am brown, therefore I think it is fine to say ‘brown boy,’” which adds to findings from Ware et al1 that there may be differences in what providers prefer to utilize to describe a patient’s skin color based on their own constitutive skin color.
I inquired with 2 other first-year dermatology residents with skin of color at other programs. When asked what terminology they use to describe a patient for Grand Rounds or in clinic, one resident replied, “It’s stylistic but if it’s your one liner [for assessment and plan] use their ethnicity [whereas] if it’s [for] a physical exam use their Fitzpatrick skin type.” The other resident replied, “I use Fitzpatrick skin type even though it’s technically subjective and therefore not appropriate for use within objective data, such as the physical exam, however it’s a language that most colleagues understand as a substitute for skin color.” I also raised the same question to an attending dermatologist at a primarily skin-of-color community hospital. She replied, “I think when unsure about ethnicity, Fitzpatrick type is an appropriate way to describe someone. It’s not really correct to say [a patient’s ethnicity] when you don’t know for sure.”
Unfortunately, as Ware and colleagues1 indicated, there is no consensus by which to objectively classify nonwhite skin color. Within the dermatology literature, it has been proposed that race should not be used to express skin color, and this article proposes that FST is an inappropriate surrogate for race/ethnicity.4 Although I agree that appropriate use of FST should be emphasized in training, is there a vocabulary that Ware et al1 recommend we use instead? Does the Skin of Color Society have suggestions on preferred language among its members? Finally, what efforts are being made to develop “culturally appropriate and clinically relevant methods for describing skin of color,” as the authors stated, within our own Skin of Color Society, or to whom does this responsibility ultimately fall?
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
3. Kelly AP, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
4. Bigby M, Thaler D. Describing patients’ “race” in clinical presentations should be abandoned. J Am Acad Dermatol. 2006;54:1074-1076.
Author’s Response
My colleagues and I thank Dr. Pimentel for his insights regarding the article, “Racial Limitations of Fitzpatrick Skin Type.”1 The conundrum on how to appropriately categorize skin color for descriptive and epidemiologic purposes continues to remain unsolved today. However, attempts have been made in the past. For example, in September 2006, Dr. Susan C. Taylor (Philadelphia, Pennsylvania), formed and chaired a workshop session titled “A New Classification System for All Skin Types.” Dermatology leaders with skin of color expertise were invited from around the world for a weekend in New York, New York, to brainstorm a new skin color classification system. This endeavor did not produce any successful alternatives, but it has remained a pertinent topic of discussion in academic dermatology, including the Skin of Color Society, since then.
When unsure about ethnicity, my colleagues and I continue to advocate that the Fitzpatrick scale is not an appropriate substitute to describe skin color. This usage of Fitzpatrick skin type (FST) perpetuates the idea that the Fitzpatrick scale is a suitable proxy to describe ethnicity or race, which it is not. It is important to remember that race is a social classification construct, not a biological one.2 The topic of race in contemporary culture undoubtedly invokes strong emotional connotations. The language around race is constantly evolving. I would argue that fear and discomfort of using incorrect racial language promotes the inappropriate use of FST, as the FST may be perceived as a more scientific and pseudoapplicable form of classification. To gain knowledge about a patient’s ethnicity/race to assess epidemiologic ethnic trends, we recommend asking the patient in an intake form or during consultation to self-identify his/her ethnicity or race,3 which takes the guesswork out for providers. However, caution must be exercised to avoid using race and ethnicity to later describe skin color.
Until a more culturally and medically relevant method of skin color classification is created, my colleagues and I recommend using basic color adjectives such as brown, black, pink, tan, or white supplemented with light, medium, or dark predescriptors. For example, “A 35-year-old self-identified African American woman with a dark brown skin hue presents with a 2-week flare of itchy, dark purple plaques with white scale on the scalp and extensor surfaces of the knees and elbows.” These basic descriptions for constitutive skin color conjure ample visual information for the listener/reader to understand morphologic descriptions, presentation of erythema, changes in pigmentation, and more. For a more specific skin color classification, we recommend developing a user-friendly Pantone-like color system to classify constitutive skin color.4
Jessica E. Dawson, MD
From the University of Washington School of Medicine, Seattle.
The author reports no conflict of interest.
Correspondence: Jessica E. Dawson, MD, University of Washington School of Medicine, 1959 NE Pacific St, Seattle, WA 98195 (jessdawsonmed@gmail.com).
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Ifekwunigwe JO, Wagner JK, Yu JH, et al. A qualitative analysis of how anthropologists interpret the race construct. Am Anthropol. 2017;119:422-434.
3. Hasnain-Wynia R, Baker DW. Obtaining data on patient race, ethnicity, and primary language in health care organizations: current challenges and proposed solutions. Health Serv Res. 2006;41:1501-1518.
4. What is the Pantone color system? Pantone website. https://www.pantone.com/color-systems/pantone-color-systems-explained. Accesed May 13, 2020.
To the Editor:
It is with great interest that I read the article by Ware et al,1 “Racial Limitations of Fitzpatrick Skin Type.” Within my own department, the issue of the appropriateness of using Fitzpatrick skin type (FST) as a surrogate to describe skin color has been raised with mixed responses.
As in many dermatology residency programs across the country, first-year dermatology residents are asked to describe the morphology of a lesion/eruption seen on a patient during Grand Rounds. Preceding the morphologic description, many providers describe the appearance of the patient including their skin color, as constitutive skin color can impact understanding of the morphologic descriptions, favor different diagnoses based on disease epidemiology, and guide subsequent treatment recommendations.2,3 During one of my first Grand Rounds as an early dermatology resident, a patient was described as a “well-appearing brown boy,” which led to a lively discussion regarding the terms that should be used to describe skin color, with some in the audience preferring FST, others including myself preferring degree of pigmentation (eg, light, moderate, dark), and lastly others preferring an inferred ethnicity based on the patient’s appearance. One audience member commented, “I am brown, therefore I think it is fine to say ‘brown boy,’” which adds to findings from Ware et al1 that there may be differences in what providers prefer to utilize to describe a patient’s skin color based on their own constitutive skin color.
I inquired with 2 other first-year dermatology residents with skin of color at other programs. When asked what terminology they use to describe a patient for Grand Rounds or in clinic, one resident replied, “It’s stylistic but if it’s your one liner [for assessment and plan] use their ethnicity [whereas] if it’s [for] a physical exam use their Fitzpatrick skin type.” The other resident replied, “I use Fitzpatrick skin type even though it’s technically subjective and therefore not appropriate for use within objective data, such as the physical exam, however it’s a language that most colleagues understand as a substitute for skin color.” I also raised the same question to an attending dermatologist at a primarily skin-of-color community hospital. She replied, “I think when unsure about ethnicity, Fitzpatrick type is an appropriate way to describe someone. It’s not really correct to say [a patient’s ethnicity] when you don’t know for sure.”
Unfortunately, as Ware and colleagues1 indicated, there is no consensus by which to objectively classify nonwhite skin color. Within the dermatology literature, it has been proposed that race should not be used to express skin color, and this article proposes that FST is an inappropriate surrogate for race/ethnicity.4 Although I agree that appropriate use of FST should be emphasized in training, is there a vocabulary that Ware et al1 recommend we use instead? Does the Skin of Color Society have suggestions on preferred language among its members? Finally, what efforts are being made to develop “culturally appropriate and clinically relevant methods for describing skin of color,” as the authors stated, within our own Skin of Color Society, or to whom does this responsibility ultimately fall?
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
3. Kelly AP, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
4. Bigby M, Thaler D. Describing patients’ “race” in clinical presentations should be abandoned. J Am Acad Dermatol. 2006;54:1074-1076.
Author’s Response
My colleagues and I thank Dr. Pimentel for his insights regarding the article, “Racial Limitations of Fitzpatrick Skin Type.”1 The conundrum on how to appropriately categorize skin color for descriptive and epidemiologic purposes continues to remain unsolved today. However, attempts have been made in the past. For example, in September 2006, Dr. Susan C. Taylor (Philadelphia, Pennsylvania), formed and chaired a workshop session titled “A New Classification System for All Skin Types.” Dermatology leaders with skin of color expertise were invited from around the world for a weekend in New York, New York, to brainstorm a new skin color classification system. This endeavor did not produce any successful alternatives, but it has remained a pertinent topic of discussion in academic dermatology, including the Skin of Color Society, since then.
When unsure about ethnicity, my colleagues and I continue to advocate that the Fitzpatrick scale is not an appropriate substitute to describe skin color. This usage of Fitzpatrick skin type (FST) perpetuates the idea that the Fitzpatrick scale is a suitable proxy to describe ethnicity or race, which it is not. It is important to remember that race is a social classification construct, not a biological one.2 The topic of race in contemporary culture undoubtedly invokes strong emotional connotations. The language around race is constantly evolving. I would argue that fear and discomfort of using incorrect racial language promotes the inappropriate use of FST, as the FST may be perceived as a more scientific and pseudoapplicable form of classification. To gain knowledge about a patient’s ethnicity/race to assess epidemiologic ethnic trends, we recommend asking the patient in an intake form or during consultation to self-identify his/her ethnicity or race,3 which takes the guesswork out for providers. However, caution must be exercised to avoid using race and ethnicity to later describe skin color.
Until a more culturally and medically relevant method of skin color classification is created, my colleagues and I recommend using basic color adjectives such as brown, black, pink, tan, or white supplemented with light, medium, or dark predescriptors. For example, “A 35-year-old self-identified African American woman with a dark brown skin hue presents with a 2-week flare of itchy, dark purple plaques with white scale on the scalp and extensor surfaces of the knees and elbows.” These basic descriptions for constitutive skin color conjure ample visual information for the listener/reader to understand morphologic descriptions, presentation of erythema, changes in pigmentation, and more. For a more specific skin color classification, we recommend developing a user-friendly Pantone-like color system to classify constitutive skin color.4
Jessica E. Dawson, MD
From the University of Washington School of Medicine, Seattle.
The author reports no conflict of interest.
Correspondence: Jessica E. Dawson, MD, University of Washington School of Medicine, 1959 NE Pacific St, Seattle, WA 98195 (jessdawsonmed@gmail.com).
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Ifekwunigwe JO, Wagner JK, Yu JH, et al. A qualitative analysis of how anthropologists interpret the race construct. Am Anthropol. 2017;119:422-434.
3. Hasnain-Wynia R, Baker DW. Obtaining data on patient race, ethnicity, and primary language in health care organizations: current challenges and proposed solutions. Health Serv Res. 2006;41:1501-1518.
4. What is the Pantone color system? Pantone website. https://www.pantone.com/color-systems/pantone-color-systems-explained. Accesed May 13, 2020.
To the Editor:
It is with great interest that I read the article by Ware et al,1 “Racial Limitations of Fitzpatrick Skin Type.” Within my own department, the issue of the appropriateness of using Fitzpatrick skin type (FST) as a surrogate to describe skin color has been raised with mixed responses.
As in many dermatology residency programs across the country, first-year dermatology residents are asked to describe the morphology of a lesion/eruption seen on a patient during Grand Rounds. Preceding the morphologic description, many providers describe the appearance of the patient including their skin color, as constitutive skin color can impact understanding of the morphologic descriptions, favor different diagnoses based on disease epidemiology, and guide subsequent treatment recommendations.2,3 During one of my first Grand Rounds as an early dermatology resident, a patient was described as a “well-appearing brown boy,” which led to a lively discussion regarding the terms that should be used to describe skin color, with some in the audience preferring FST, others including myself preferring degree of pigmentation (eg, light, moderate, dark), and lastly others preferring an inferred ethnicity based on the patient’s appearance. One audience member commented, “I am brown, therefore I think it is fine to say ‘brown boy,’” which adds to findings from Ware et al1 that there may be differences in what providers prefer to utilize to describe a patient’s skin color based on their own constitutive skin color.
I inquired with 2 other first-year dermatology residents with skin of color at other programs. When asked what terminology they use to describe a patient for Grand Rounds or in clinic, one resident replied, “It’s stylistic but if it’s your one liner [for assessment and plan] use their ethnicity [whereas] if it’s [for] a physical exam use their Fitzpatrick skin type.” The other resident replied, “I use Fitzpatrick skin type even though it’s technically subjective and therefore not appropriate for use within objective data, such as the physical exam, however it’s a language that most colleagues understand as a substitute for skin color.” I also raised the same question to an attending dermatologist at a primarily skin-of-color community hospital. She replied, “I think when unsure about ethnicity, Fitzpatrick type is an appropriate way to describe someone. It’s not really correct to say [a patient’s ethnicity] when you don’t know for sure.”
Unfortunately, as Ware and colleagues1 indicated, there is no consensus by which to objectively classify nonwhite skin color. Within the dermatology literature, it has been proposed that race should not be used to express skin color, and this article proposes that FST is an inappropriate surrogate for race/ethnicity.4 Although I agree that appropriate use of FST should be emphasized in training, is there a vocabulary that Ware et al1 recommend we use instead? Does the Skin of Color Society have suggestions on preferred language among its members? Finally, what efforts are being made to develop “culturally appropriate and clinically relevant methods for describing skin of color,” as the authors stated, within our own Skin of Color Society, or to whom does this responsibility ultimately fall?
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
3. Kelly AP, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
4. Bigby M, Thaler D. Describing patients’ “race” in clinical presentations should be abandoned. J Am Acad Dermatol. 2006;54:1074-1076.
Author’s Response
My colleagues and I thank Dr. Pimentel for his insights regarding the article, “Racial Limitations of Fitzpatrick Skin Type.”1 The conundrum on how to appropriately categorize skin color for descriptive and epidemiologic purposes continues to remain unsolved today. However, attempts have been made in the past. For example, in September 2006, Dr. Susan C. Taylor (Philadelphia, Pennsylvania), formed and chaired a workshop session titled “A New Classification System for All Skin Types.” Dermatology leaders with skin of color expertise were invited from around the world for a weekend in New York, New York, to brainstorm a new skin color classification system. This endeavor did not produce any successful alternatives, but it has remained a pertinent topic of discussion in academic dermatology, including the Skin of Color Society, since then.
When unsure about ethnicity, my colleagues and I continue to advocate that the Fitzpatrick scale is not an appropriate substitute to describe skin color. This usage of Fitzpatrick skin type (FST) perpetuates the idea that the Fitzpatrick scale is a suitable proxy to describe ethnicity or race, which it is not. It is important to remember that race is a social classification construct, not a biological one.2 The topic of race in contemporary culture undoubtedly invokes strong emotional connotations. The language around race is constantly evolving. I would argue that fear and discomfort of using incorrect racial language promotes the inappropriate use of FST, as the FST may be perceived as a more scientific and pseudoapplicable form of classification. To gain knowledge about a patient’s ethnicity/race to assess epidemiologic ethnic trends, we recommend asking the patient in an intake form or during consultation to self-identify his/her ethnicity or race,3 which takes the guesswork out for providers. However, caution must be exercised to avoid using race and ethnicity to later describe skin color.
Until a more culturally and medically relevant method of skin color classification is created, my colleagues and I recommend using basic color adjectives such as brown, black, pink, tan, or white supplemented with light, medium, or dark predescriptors. For example, “A 35-year-old self-identified African American woman with a dark brown skin hue presents with a 2-week flare of itchy, dark purple plaques with white scale on the scalp and extensor surfaces of the knees and elbows.” These basic descriptions for constitutive skin color conjure ample visual information for the listener/reader to understand morphologic descriptions, presentation of erythema, changes in pigmentation, and more. For a more specific skin color classification, we recommend developing a user-friendly Pantone-like color system to classify constitutive skin color.4
Jessica E. Dawson, MD
From the University of Washington School of Medicine, Seattle.
The author reports no conflict of interest.
Correspondence: Jessica E. Dawson, MD, University of Washington School of Medicine, 1959 NE Pacific St, Seattle, WA 98195 (jessdawsonmed@gmail.com).
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Ifekwunigwe JO, Wagner JK, Yu JH, et al. A qualitative analysis of how anthropologists interpret the race construct. Am Anthropol. 2017;119:422-434.
3. Hasnain-Wynia R, Baker DW. Obtaining data on patient race, ethnicity, and primary language in health care organizations: current challenges and proposed solutions. Health Serv Res. 2006;41:1501-1518.
4. What is the Pantone color system? Pantone website. https://www.pantone.com/color-systems/pantone-color-systems-explained. Accesed May 13, 2020.
Emerging Therapies for Cutaneous Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease that can have devastating effects on many organs. Despite the considerable morbidity and mortality associated with SLE, treatment options have been largely unchanged since the 1950s.1 It was not until the last decade that a new biologic medication was approved, and several other promising treatments currently are being evaluated in clinical trials. Dermatologists are most likely to encounter cutaneous lupus erythematosus (CLE) with or without SLE, which can present with a variety of skin manifestations. Cutaneous lupus erythematosus can have devastating effects on quality of life and can be a visible sign of the internal activity and damage of SLE.2,3 Although many trials have been completed evaluating SLE treatments, few medications have been evaluated specifically for CLE despite the availability of validated measures of CLE skin activity.4 There is a recent shortage of antimalarial medications, the current first-line therapy for CLE, due to both an import alert in the United States on quinacrine placed in 2019 as well as the use of hydroxychloroquine and chloroquine in treating coronavirus disease 2019.5,6 Due to this shortage, the need for new and effective treatments is more critical than ever, as alternatives to first-line therapy frequently require immunosuppression. We review recent drug approvals for SLE and their efficacy in CLE. We also provide an update on new agents currently being studied to treat this disease.
Belimumab
Belimumab is a B-lymphocyte stimulator–specific inhibitor that was first approved for treatment of SLE in 2011. It was the first monoclonal antibody approved to treat SLE.7 B-lymphocyte stimulator plays a critical role in B-cell survival; thus, its inhibition increases apoptosis of autoreactive B cells involved in the pathogenesis of SLE. More recently, belimumab was approved for pediatric SLE in April 2019 based on the PLUTO study, a phase 2 randomized, double-blind study of 93 patients.8 Although patients with cutaneous manifestations of lupus were included in trials for belimumab, they lacked CLE-specific outcome measurements to truly evaluate the efficacy in treating skin disease.9 This medication currently is not approved by the US Food and Drug Administration (FDA) for CLE; however, it is used off label in some cases for recalcitrant disease.10
Baricitinib
Baricitinib is a selective and reversible inhibitor of JAK1 and JAK2 that was granted fast-track status by the FDA in December 2018. In a phase 2 trial, baricitinib was superior to placebo plus standard of care, primarily for arthritis and lupus nephritis.11 Although improvement of cutaneous disease was measured as an end point, it did not show significant improvement in disease. The presence of skin disease was high, but the activity of disease was low, which can make it difficult to show meaningful improvement, as there is not much room for patients to objectively improve.12 Showing meaningful improvement in skin disease often is difficult in phase 2 trials, especially when the trial design is focused on SLE rather than CLE activity. Further studies of baricitinib that include more severe patients with CLE disease are needed to truly understand its effects on the skin.
Lenalidomide
There have been several CLE studies in the last several years surrounding lenalidomide, an analog of thalidomide.13-15 This molecule has a number of immunomodulatory effects including antiangiogenic effects, increased natural killer cell–dependent cytotoxicity, and cytokine and interleukin inhibition. Lenalidomide is of particular interest in treating CLE, as it was shown to be more potent than thalidomide at low doses and with a better side-effect profile. Multiple small, open-label trials have shown lenalidomide to be both safe and efficacious in the treatment of CLE.13,14 In addition, iberdomide, a derivative of lenalidomide, recently completed a phase 2 dose-escalation study showing improvement in both SLE and CLE end points.16 A phase 2b proof-of-concept study currently is underway (ClinicalTrials.gov Identifier NCT03161483).
Monoclonal Antibodies
Many developing therapies target specific components of the type I interferon pathway, which is a primary driver of CLE lesions. Innate immune system pathways involving type I interferon were shown to be active in the pathogenesis of CLE, and levels of interferon correlate with skin disease activity.17 One molecule in development that targets this pathway is BIIB059, a humanized IgG1 monoclonal antibody that binds to blood dendritic cell antigen 2. This cell surface protein is uniquely expressed on plasmacytoid dendritic cells, which are the main source of type I interferon overproduction in SLE. The binding of this antibody to the blood dendritic cell antigen 2 receptor both blocks type I interferon production and decreases the overall number of active plasmacytoid dendritic cells present.18 In the completed phase 1b study, a response in cutaneous disease was shown through a reduction in the CLE disease area and severity index score following single-dose administration.19 More recently, a phase 2 study met primary end points in both SLE and CLE compared to placebo.20
Anifrolumab is a human IgG1k monoclonal antibody that binds to type I interferon receptor, blocking all type I interferon signaling. Following a successful phase 2 trial, it failed to meet its primary end point in its first phase 3 trial.21 Several secondary end points suggested a clinical benefit. A second phase 3 trial of 362 patients randomized to treatment with anifrolumab or placebo over 48 weeks showed anifrolumab to be superior to placebo for multiple end points, including the overall disease primary end point as well as a notable reduction in skin activity.22
Final Thoughts
Outside of the approval of belimumab, there have been no new FDA-approved treatments for SLE since the approval of antimalarial agents nearly 50 years ago. For CLE specifically, there is an even greater scarcity of evidence-based treatments. Recently studied medications, such as belimumab and lenalidomide, are available off label for CLE patients when other options have failed. Recent studies have evaluated the efficacy of these agents in the treatment of CLE using the CLE disease area and severity index.10,13,14 Enrollment in CLE trials is difficult due to the rarity of the disease, and careful attention must be paid to evaluating skin end points. As experts in CLE and the nuances of these assessments, it is critical that dermatologists be involved in clinical trials. Future SLE trials must consider CLE as an important end point for CLE patients to get access to much-needed novel therapies.
- Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550-2557.
- Vasquez R, Wang D, Tran QP, et al. A multicentre, cross-sectional study on quality of life in patients with cutaneous lupus erythematosus. Br J Dermatol. 2013;168:145-153.
- Klein R, Moghadam-Kia S, Taylor L, et al. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;64:849-858.
- Klein R, Moghadam-Kia S, LoMonico J, et al. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol. 2011;147:203-208.
- Jakhar D, Kaur I. Potential of chloroquine and hydroxychloroquine to treat COVID-19 causes fears of shortages among people with systemic lupus erythematosus. Nat Med. 2020;26:632.
- American College of Rheumatology. Quinacrine shortage & what the ACR is doing about it. https://www.the-rheumatologist.org/article/quinacrine-shortage-what-the-acr-is-doing-about-it/. Published February 8, 2019. Accessed May 15, 2020.
- Dubey AK, Handu SS, Dubey S, et al. Belimumab: first targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. 2011;2:317-319.
- Brunner H, Abud-Mendoza C, Viola D, et al. Efficacy and safety of intravenous belimumab in children with systemic lupus erythematosus [abstract]. Arthritis Rheumatol. 2018;70(suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-intravenous-belimumab-in-children-with-systemic-lupus-erythematosus/. Accessed May 7, 2020.
- Hui-Yuen JS, Reddy A, Taylor J, et al. Safety and efficacy of belimumab to treat systemic lupus erythematosus in academic clinical practices. J Rheumatol. 2015;42:2288-2295.
- Vashisht P, Borghoff K, O’Dell JR, et al. Belimumab for the treatment of recalcitrant cutaneous lupus. Lupus. 2017;26:857-864.
- Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392:222-231.
- Werth VP, Merrill JT. A double-blind, randomized, placebo-controlled, phase II trial of baricitinib for systemic lupus erythematosus: how to optimize lupus trials to examine effects on cutaneous lupus erythematosus. Br J Dermatol. 2019;180:964-965.
- Cortés-Hernández J, Ávila G, Vilardell-Tarrés M, et al. Efficacy and safety of lenalidomide for refractory cutaneous lupus erythematosus. Arthritis Res Ther. 2012;14:R265.
- Okon L, Rosenbach M, Krathen M, et al. Lenalidomide in treatment-refractory cutaneous lupus erythematosus: efficacy and safety in a 52-week trial. J Am Acad Dermatol. 2014;70:583-584.
- Fennira F, Chasset F, Soubrier M, et al. Lenalidomide for refractory chronic and subacute cutaneous lupus erythematosus: 16 patients. J Am Acad Dermatol. 2016;74:1248-1251.
- Furie R, Werth V, Gaudy A, et al. A randomized, placebo-controlled, double-blind, ascending-dose, safety, and pharmacokinetics study of CC-220 in subjects with systemic LUPUS erythematosus [abstract]. Arthritis Rheumatol. 2017;69(suppl 10). https://acrabstracts.org/abstract/a-randomized-placebo-controlled-double-blind-ascending-dose-safety-and-pharmacokinetics-study-of-cc-220-in-subjects-with-systemic-lupus-erythematosus/. Accessed May 7, 2020.
- Braunstein I, Klein R, Okawa J, et al. The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br J Dermatol. 2012;166:971-975.
- Kim JM, Park SH, Kim HY, et al. A plasmacytoid dendritic cells-type I interferon axis is critically implicated in the pathogenesis of systemic lupus erythematosus. Int J Mol Sci. 2015;16:14158-14170.
- Furie R, Werth VP, Merola JF, et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest. 2019;129:1359-1371.
- Werth V, Musselli C, Furie R, et al. BIIB059, a humanized monoclonal antibody targeting BDCA2 on plasmacytoid dendritic cells (pDC), shows dose-related efficacy in the phase 2 LILAC study in patients (pts) with active cutaneous lupus erythematosus (CLE). Ann Rheum Dis. In press.
- Furie R, Morand EF, Bruce I, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019;1:E208-E219.
- Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382:211-221.
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease that can have devastating effects on many organs. Despite the considerable morbidity and mortality associated with SLE, treatment options have been largely unchanged since the 1950s.1 It was not until the last decade that a new biologic medication was approved, and several other promising treatments currently are being evaluated in clinical trials. Dermatologists are most likely to encounter cutaneous lupus erythematosus (CLE) with or without SLE, which can present with a variety of skin manifestations. Cutaneous lupus erythematosus can have devastating effects on quality of life and can be a visible sign of the internal activity and damage of SLE.2,3 Although many trials have been completed evaluating SLE treatments, few medications have been evaluated specifically for CLE despite the availability of validated measures of CLE skin activity.4 There is a recent shortage of antimalarial medications, the current first-line therapy for CLE, due to both an import alert in the United States on quinacrine placed in 2019 as well as the use of hydroxychloroquine and chloroquine in treating coronavirus disease 2019.5,6 Due to this shortage, the need for new and effective treatments is more critical than ever, as alternatives to first-line therapy frequently require immunosuppression. We review recent drug approvals for SLE and their efficacy in CLE. We also provide an update on new agents currently being studied to treat this disease.
Belimumab
Belimumab is a B-lymphocyte stimulator–specific inhibitor that was first approved for treatment of SLE in 2011. It was the first monoclonal antibody approved to treat SLE.7 B-lymphocyte stimulator plays a critical role in B-cell survival; thus, its inhibition increases apoptosis of autoreactive B cells involved in the pathogenesis of SLE. More recently, belimumab was approved for pediatric SLE in April 2019 based on the PLUTO study, a phase 2 randomized, double-blind study of 93 patients.8 Although patients with cutaneous manifestations of lupus were included in trials for belimumab, they lacked CLE-specific outcome measurements to truly evaluate the efficacy in treating skin disease.9 This medication currently is not approved by the US Food and Drug Administration (FDA) for CLE; however, it is used off label in some cases for recalcitrant disease.10
Baricitinib
Baricitinib is a selective and reversible inhibitor of JAK1 and JAK2 that was granted fast-track status by the FDA in December 2018. In a phase 2 trial, baricitinib was superior to placebo plus standard of care, primarily for arthritis and lupus nephritis.11 Although improvement of cutaneous disease was measured as an end point, it did not show significant improvement in disease. The presence of skin disease was high, but the activity of disease was low, which can make it difficult to show meaningful improvement, as there is not much room for patients to objectively improve.12 Showing meaningful improvement in skin disease often is difficult in phase 2 trials, especially when the trial design is focused on SLE rather than CLE activity. Further studies of baricitinib that include more severe patients with CLE disease are needed to truly understand its effects on the skin.
Lenalidomide
There have been several CLE studies in the last several years surrounding lenalidomide, an analog of thalidomide.13-15 This molecule has a number of immunomodulatory effects including antiangiogenic effects, increased natural killer cell–dependent cytotoxicity, and cytokine and interleukin inhibition. Lenalidomide is of particular interest in treating CLE, as it was shown to be more potent than thalidomide at low doses and with a better side-effect profile. Multiple small, open-label trials have shown lenalidomide to be both safe and efficacious in the treatment of CLE.13,14 In addition, iberdomide, a derivative of lenalidomide, recently completed a phase 2 dose-escalation study showing improvement in both SLE and CLE end points.16 A phase 2b proof-of-concept study currently is underway (ClinicalTrials.gov Identifier NCT03161483).
Monoclonal Antibodies
Many developing therapies target specific components of the type I interferon pathway, which is a primary driver of CLE lesions. Innate immune system pathways involving type I interferon were shown to be active in the pathogenesis of CLE, and levels of interferon correlate with skin disease activity.17 One molecule in development that targets this pathway is BIIB059, a humanized IgG1 monoclonal antibody that binds to blood dendritic cell antigen 2. This cell surface protein is uniquely expressed on plasmacytoid dendritic cells, which are the main source of type I interferon overproduction in SLE. The binding of this antibody to the blood dendritic cell antigen 2 receptor both blocks type I interferon production and decreases the overall number of active plasmacytoid dendritic cells present.18 In the completed phase 1b study, a response in cutaneous disease was shown through a reduction in the CLE disease area and severity index score following single-dose administration.19 More recently, a phase 2 study met primary end points in both SLE and CLE compared to placebo.20
Anifrolumab is a human IgG1k monoclonal antibody that binds to type I interferon receptor, blocking all type I interferon signaling. Following a successful phase 2 trial, it failed to meet its primary end point in its first phase 3 trial.21 Several secondary end points suggested a clinical benefit. A second phase 3 trial of 362 patients randomized to treatment with anifrolumab or placebo over 48 weeks showed anifrolumab to be superior to placebo for multiple end points, including the overall disease primary end point as well as a notable reduction in skin activity.22
Final Thoughts
Outside of the approval of belimumab, there have been no new FDA-approved treatments for SLE since the approval of antimalarial agents nearly 50 years ago. For CLE specifically, there is an even greater scarcity of evidence-based treatments. Recently studied medications, such as belimumab and lenalidomide, are available off label for CLE patients when other options have failed. Recent studies have evaluated the efficacy of these agents in the treatment of CLE using the CLE disease area and severity index.10,13,14 Enrollment in CLE trials is difficult due to the rarity of the disease, and careful attention must be paid to evaluating skin end points. As experts in CLE and the nuances of these assessments, it is critical that dermatologists be involved in clinical trials. Future SLE trials must consider CLE as an important end point for CLE patients to get access to much-needed novel therapies.
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease that can have devastating effects on many organs. Despite the considerable morbidity and mortality associated with SLE, treatment options have been largely unchanged since the 1950s.1 It was not until the last decade that a new biologic medication was approved, and several other promising treatments currently are being evaluated in clinical trials. Dermatologists are most likely to encounter cutaneous lupus erythematosus (CLE) with or without SLE, which can present with a variety of skin manifestations. Cutaneous lupus erythematosus can have devastating effects on quality of life and can be a visible sign of the internal activity and damage of SLE.2,3 Although many trials have been completed evaluating SLE treatments, few medications have been evaluated specifically for CLE despite the availability of validated measures of CLE skin activity.4 There is a recent shortage of antimalarial medications, the current first-line therapy for CLE, due to both an import alert in the United States on quinacrine placed in 2019 as well as the use of hydroxychloroquine and chloroquine in treating coronavirus disease 2019.5,6 Due to this shortage, the need for new and effective treatments is more critical than ever, as alternatives to first-line therapy frequently require immunosuppression. We review recent drug approvals for SLE and their efficacy in CLE. We also provide an update on new agents currently being studied to treat this disease.
Belimumab
Belimumab is a B-lymphocyte stimulator–specific inhibitor that was first approved for treatment of SLE in 2011. It was the first monoclonal antibody approved to treat SLE.7 B-lymphocyte stimulator plays a critical role in B-cell survival; thus, its inhibition increases apoptosis of autoreactive B cells involved in the pathogenesis of SLE. More recently, belimumab was approved for pediatric SLE in April 2019 based on the PLUTO study, a phase 2 randomized, double-blind study of 93 patients.8 Although patients with cutaneous manifestations of lupus were included in trials for belimumab, they lacked CLE-specific outcome measurements to truly evaluate the efficacy in treating skin disease.9 This medication currently is not approved by the US Food and Drug Administration (FDA) for CLE; however, it is used off label in some cases for recalcitrant disease.10
Baricitinib
Baricitinib is a selective and reversible inhibitor of JAK1 and JAK2 that was granted fast-track status by the FDA in December 2018. In a phase 2 trial, baricitinib was superior to placebo plus standard of care, primarily for arthritis and lupus nephritis.11 Although improvement of cutaneous disease was measured as an end point, it did not show significant improvement in disease. The presence of skin disease was high, but the activity of disease was low, which can make it difficult to show meaningful improvement, as there is not much room for patients to objectively improve.12 Showing meaningful improvement in skin disease often is difficult in phase 2 trials, especially when the trial design is focused on SLE rather than CLE activity. Further studies of baricitinib that include more severe patients with CLE disease are needed to truly understand its effects on the skin.
Lenalidomide
There have been several CLE studies in the last several years surrounding lenalidomide, an analog of thalidomide.13-15 This molecule has a number of immunomodulatory effects including antiangiogenic effects, increased natural killer cell–dependent cytotoxicity, and cytokine and interleukin inhibition. Lenalidomide is of particular interest in treating CLE, as it was shown to be more potent than thalidomide at low doses and with a better side-effect profile. Multiple small, open-label trials have shown lenalidomide to be both safe and efficacious in the treatment of CLE.13,14 In addition, iberdomide, a derivative of lenalidomide, recently completed a phase 2 dose-escalation study showing improvement in both SLE and CLE end points.16 A phase 2b proof-of-concept study currently is underway (ClinicalTrials.gov Identifier NCT03161483).
Monoclonal Antibodies
Many developing therapies target specific components of the type I interferon pathway, which is a primary driver of CLE lesions. Innate immune system pathways involving type I interferon were shown to be active in the pathogenesis of CLE, and levels of interferon correlate with skin disease activity.17 One molecule in development that targets this pathway is BIIB059, a humanized IgG1 monoclonal antibody that binds to blood dendritic cell antigen 2. This cell surface protein is uniquely expressed on plasmacytoid dendritic cells, which are the main source of type I interferon overproduction in SLE. The binding of this antibody to the blood dendritic cell antigen 2 receptor both blocks type I interferon production and decreases the overall number of active plasmacytoid dendritic cells present.18 In the completed phase 1b study, a response in cutaneous disease was shown through a reduction in the CLE disease area and severity index score following single-dose administration.19 More recently, a phase 2 study met primary end points in both SLE and CLE compared to placebo.20
Anifrolumab is a human IgG1k monoclonal antibody that binds to type I interferon receptor, blocking all type I interferon signaling. Following a successful phase 2 trial, it failed to meet its primary end point in its first phase 3 trial.21 Several secondary end points suggested a clinical benefit. A second phase 3 trial of 362 patients randomized to treatment with anifrolumab or placebo over 48 weeks showed anifrolumab to be superior to placebo for multiple end points, including the overall disease primary end point as well as a notable reduction in skin activity.22
Final Thoughts
Outside of the approval of belimumab, there have been no new FDA-approved treatments for SLE since the approval of antimalarial agents nearly 50 years ago. For CLE specifically, there is an even greater scarcity of evidence-based treatments. Recently studied medications, such as belimumab and lenalidomide, are available off label for CLE patients when other options have failed. Recent studies have evaluated the efficacy of these agents in the treatment of CLE using the CLE disease area and severity index.10,13,14 Enrollment in CLE trials is difficult due to the rarity of the disease, and careful attention must be paid to evaluating skin end points. As experts in CLE and the nuances of these assessments, it is critical that dermatologists be involved in clinical trials. Future SLE trials must consider CLE as an important end point for CLE patients to get access to much-needed novel therapies.
- Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550-2557.
- Vasquez R, Wang D, Tran QP, et al. A multicentre, cross-sectional study on quality of life in patients with cutaneous lupus erythematosus. Br J Dermatol. 2013;168:145-153.
- Klein R, Moghadam-Kia S, Taylor L, et al. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;64:849-858.
- Klein R, Moghadam-Kia S, LoMonico J, et al. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol. 2011;147:203-208.
- Jakhar D, Kaur I. Potential of chloroquine and hydroxychloroquine to treat COVID-19 causes fears of shortages among people with systemic lupus erythematosus. Nat Med. 2020;26:632.
- American College of Rheumatology. Quinacrine shortage & what the ACR is doing about it. https://www.the-rheumatologist.org/article/quinacrine-shortage-what-the-acr-is-doing-about-it/. Published February 8, 2019. Accessed May 15, 2020.
- Dubey AK, Handu SS, Dubey S, et al. Belimumab: first targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. 2011;2:317-319.
- Brunner H, Abud-Mendoza C, Viola D, et al. Efficacy and safety of intravenous belimumab in children with systemic lupus erythematosus [abstract]. Arthritis Rheumatol. 2018;70(suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-intravenous-belimumab-in-children-with-systemic-lupus-erythematosus/. Accessed May 7, 2020.
- Hui-Yuen JS, Reddy A, Taylor J, et al. Safety and efficacy of belimumab to treat systemic lupus erythematosus in academic clinical practices. J Rheumatol. 2015;42:2288-2295.
- Vashisht P, Borghoff K, O’Dell JR, et al. Belimumab for the treatment of recalcitrant cutaneous lupus. Lupus. 2017;26:857-864.
- Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392:222-231.
- Werth VP, Merrill JT. A double-blind, randomized, placebo-controlled, phase II trial of baricitinib for systemic lupus erythematosus: how to optimize lupus trials to examine effects on cutaneous lupus erythematosus. Br J Dermatol. 2019;180:964-965.
- Cortés-Hernández J, Ávila G, Vilardell-Tarrés M, et al. Efficacy and safety of lenalidomide for refractory cutaneous lupus erythematosus. Arthritis Res Ther. 2012;14:R265.
- Okon L, Rosenbach M, Krathen M, et al. Lenalidomide in treatment-refractory cutaneous lupus erythematosus: efficacy and safety in a 52-week trial. J Am Acad Dermatol. 2014;70:583-584.
- Fennira F, Chasset F, Soubrier M, et al. Lenalidomide for refractory chronic and subacute cutaneous lupus erythematosus: 16 patients. J Am Acad Dermatol. 2016;74:1248-1251.
- Furie R, Werth V, Gaudy A, et al. A randomized, placebo-controlled, double-blind, ascending-dose, safety, and pharmacokinetics study of CC-220 in subjects with systemic LUPUS erythematosus [abstract]. Arthritis Rheumatol. 2017;69(suppl 10). https://acrabstracts.org/abstract/a-randomized-placebo-controlled-double-blind-ascending-dose-safety-and-pharmacokinetics-study-of-cc-220-in-subjects-with-systemic-lupus-erythematosus/. Accessed May 7, 2020.
- Braunstein I, Klein R, Okawa J, et al. The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br J Dermatol. 2012;166:971-975.
- Kim JM, Park SH, Kim HY, et al. A plasmacytoid dendritic cells-type I interferon axis is critically implicated in the pathogenesis of systemic lupus erythematosus. Int J Mol Sci. 2015;16:14158-14170.
- Furie R, Werth VP, Merola JF, et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest. 2019;129:1359-1371.
- Werth V, Musselli C, Furie R, et al. BIIB059, a humanized monoclonal antibody targeting BDCA2 on plasmacytoid dendritic cells (pDC), shows dose-related efficacy in the phase 2 LILAC study in patients (pts) with active cutaneous lupus erythematosus (CLE). Ann Rheum Dis. In press.
- Furie R, Morand EF, Bruce I, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019;1:E208-E219.
- Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382:211-221.
- Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550-2557.
- Vasquez R, Wang D, Tran QP, et al. A multicentre, cross-sectional study on quality of life in patients with cutaneous lupus erythematosus. Br J Dermatol. 2013;168:145-153.
- Klein R, Moghadam-Kia S, Taylor L, et al. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;64:849-858.
- Klein R, Moghadam-Kia S, LoMonico J, et al. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol. 2011;147:203-208.
- Jakhar D, Kaur I. Potential of chloroquine and hydroxychloroquine to treat COVID-19 causes fears of shortages among people with systemic lupus erythematosus. Nat Med. 2020;26:632.
- American College of Rheumatology. Quinacrine shortage & what the ACR is doing about it. https://www.the-rheumatologist.org/article/quinacrine-shortage-what-the-acr-is-doing-about-it/. Published February 8, 2019. Accessed May 15, 2020.
- Dubey AK, Handu SS, Dubey S, et al. Belimumab: first targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. 2011;2:317-319.
- Brunner H, Abud-Mendoza C, Viola D, et al. Efficacy and safety of intravenous belimumab in children with systemic lupus erythematosus [abstract]. Arthritis Rheumatol. 2018;70(suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-intravenous-belimumab-in-children-with-systemic-lupus-erythematosus/. Accessed May 7, 2020.
- Hui-Yuen JS, Reddy A, Taylor J, et al. Safety and efficacy of belimumab to treat systemic lupus erythematosus in academic clinical practices. J Rheumatol. 2015;42:2288-2295.
- Vashisht P, Borghoff K, O’Dell JR, et al. Belimumab for the treatment of recalcitrant cutaneous lupus. Lupus. 2017;26:857-864.
- Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392:222-231.
- Werth VP, Merrill JT. A double-blind, randomized, placebo-controlled, phase II trial of baricitinib for systemic lupus erythematosus: how to optimize lupus trials to examine effects on cutaneous lupus erythematosus. Br J Dermatol. 2019;180:964-965.
- Cortés-Hernández J, Ávila G, Vilardell-Tarrés M, et al. Efficacy and safety of lenalidomide for refractory cutaneous lupus erythematosus. Arthritis Res Ther. 2012;14:R265.
- Okon L, Rosenbach M, Krathen M, et al. Lenalidomide in treatment-refractory cutaneous lupus erythematosus: efficacy and safety in a 52-week trial. J Am Acad Dermatol. 2014;70:583-584.
- Fennira F, Chasset F, Soubrier M, et al. Lenalidomide for refractory chronic and subacute cutaneous lupus erythematosus: 16 patients. J Am Acad Dermatol. 2016;74:1248-1251.
- Furie R, Werth V, Gaudy A, et al. A randomized, placebo-controlled, double-blind, ascending-dose, safety, and pharmacokinetics study of CC-220 in subjects with systemic LUPUS erythematosus [abstract]. Arthritis Rheumatol. 2017;69(suppl 10). https://acrabstracts.org/abstract/a-randomized-placebo-controlled-double-blind-ascending-dose-safety-and-pharmacokinetics-study-of-cc-220-in-subjects-with-systemic-lupus-erythematosus/. Accessed May 7, 2020.
- Braunstein I, Klein R, Okawa J, et al. The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br J Dermatol. 2012;166:971-975.
- Kim JM, Park SH, Kim HY, et al. A plasmacytoid dendritic cells-type I interferon axis is critically implicated in the pathogenesis of systemic lupus erythematosus. Int J Mol Sci. 2015;16:14158-14170.
- Furie R, Werth VP, Merola JF, et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest. 2019;129:1359-1371.
- Werth V, Musselli C, Furie R, et al. BIIB059, a humanized monoclonal antibody targeting BDCA2 on plasmacytoid dendritic cells (pDC), shows dose-related efficacy in the phase 2 LILAC study in patients (pts) with active cutaneous lupus erythematosus (CLE). Ann Rheum Dis. In press.
- Furie R, Morand EF, Bruce I, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019;1:E208-E219.
- Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382:211-221.
Compounding Topicals in Dermatology
Compounding is a way of mixing or combining medications in formulations that are not widely available. Because dermatology is a field that includes a variety of topical treatments, compounding topicals is a way to create unique and customized treatment options for patients.
Advantages
Custom compounding topical medications has many benefits in comparison to traditional topical formulations. Compounding is a way of personalizing prescriptions to best suit the individual needs of each patient. Multiple ingredients with different mechanisms of action can be combined in a single medication for patients to use, which ultimately can simplify their treatment regimen.1 For rare conditions with uncommon treatments, compounding pharmacies can provide medications that are not widely available in retail pharmacies. Compounding topical medications also can be an efficient way of prescribing medications without dealing with the uncertainty of prior authorizations or how much the co-pay will be.
Disadvantages
One of the major disadvantages of compounding topical medications is the lack of safety data. Although most active drugs have been tested independently, there is little data on the safety of compounding 2 or more active drugs. Furthermore, the vehicle used may change the permeability of the topical formulation, and systemic absorption may be possible. Two deaths were reported with the application of compounded topical lidocaine and tetracaine gel due to systemic absorption. In these cases, the gel was used before laser hair removal, and it was applied under occlusion to greater than 50% of the body surface area, leading to fatal systemic absorption.1,2
One of the hypothetical benefits of compounding topicals is being able to avoid side effects of systemic medications. However, depending on the skin intactness and the strength of the medication used, systemic adverse effects have been reported.1 In a case series of 2 patients detailing the use of amitriptyline cream 5% and 10% for neuropathic pain, the patient using 10% cream experienced systemic effects of drowsiness and discontinued treatment.3
Another major disadvantage of compounding topicals is a lack of published data about the efficacy, especially given the unique nature of what is being compounded. When combining multiple medications, there are little to no published data about the efficacy of these formulations and how they compare to monotherapy. Although there may be data about the efficacy of an oral agent, it does not translate to the topical form being safe and efficacious. Much of the published data of topical formulations is limited to case reports and case series.
Finally, many compounded medications are not covered by insurance, and the out-of-pocket cost may be prohibitive for some patients. Compounding pharmacies typically will give patients a price estimate before the prescription is filled. When compounding topicals for patient use, it is important to counsel patients about the following:the unknown safety profile; lack of data regarding efficacy; and cost, as the medication likely will not be covered by insurance.
Pharmaceutical Regulations
After a contaminated product at a compounding pharmacy in New England led to an outbreak of fungal meningitis, there has been increased regulation by the US Food and Drug Administration.4 To meet safety regulations, compounding pharmacies must adhere to the standards set by the US Pharmacopeia. The US Food and Drug Administration says that physicians are not to prescribe compounded medications that are “unapproved, adulterated, or misbranded drugs,” which has been interpreted to mean that compounded medications should not mimic a branded medication but should instead be a unique formulation or strength.4,5 Thus, while compounding topicals may provide an alternative when a specific medication is not covered by insurance, it cannot be the same as a branded medication.
Pharmaceutical Options
Most major cities have custom compounding pharmacies or apothecaries. One of the benefits of using a local compounding pharmacy is that you typically can speak directly with the pharmacist about your patient’s diagnosis and his/her specific needs. The pharmacist can guide you through which formulations to compound, which strength to choose, and the best vehicle to use as a base. This expertise is invaluable in the compounding process. There also are online compounding pharmacies available.
Options for Bases
Dermatologists can request for their medications to be compounded in traditional over-the-counter emollients or petrolatum-based products, which work by passively diffusing through the stratum corneum into the superficial epidermis to treat skin conditions.1 For a topical drug to be absorbed effectively through the skin and into the general circulation, the vehicle needs to have affinity for both lipid and aqueous environments. Lipophilic drugs will absorb better through the stratum corneum, while hydrophilic drugs will absorb better through the aqueous layer of the epidermis. For a topical formulation to be both hydrophobic and hydrophilic, components such as viscosity enhancers and permeation enhancers can be added.1 Many compounding pharmacies also have proprietary bases that can be used.
Final Thoughts
Compounding topical medications in dermatology provides dermatologists with the ability to provide unique formulations to best suit their patients’ individual needs. However, dermatologists must keep in mind the limitations of compounding topicals, including a lack of data on efficacy and safety.
- Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271.
- Ukens C. Coed death tied to compounded drug. Drug Topics. March 7, 2005. https://www.drugtopics.com/community-pharmacy/coed-death-tied-compounded-drug. Accessed May 31, 2020.
- Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: 2 cases and literature review. Pain Pract. 2012;12:148-153.
- Campbell EH, Elston DM, Straughan CL, et al. Regulations, liability, safety, and economics related to compounding [published online December 9, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.11.061.
- US Food and Drug Administration. Administrative Destruction of Certain Drugs Refused Admission to the United States; Final Rule: Docket No. FDA-2014-N-0504. https://www.fda.gov/media/93525/download. Accessed May 31, 2020.
Compounding is a way of mixing or combining medications in formulations that are not widely available. Because dermatology is a field that includes a variety of topical treatments, compounding topicals is a way to create unique and customized treatment options for patients.
Advantages
Custom compounding topical medications has many benefits in comparison to traditional topical formulations. Compounding is a way of personalizing prescriptions to best suit the individual needs of each patient. Multiple ingredients with different mechanisms of action can be combined in a single medication for patients to use, which ultimately can simplify their treatment regimen.1 For rare conditions with uncommon treatments, compounding pharmacies can provide medications that are not widely available in retail pharmacies. Compounding topical medications also can be an efficient way of prescribing medications without dealing with the uncertainty of prior authorizations or how much the co-pay will be.
Disadvantages
One of the major disadvantages of compounding topical medications is the lack of safety data. Although most active drugs have been tested independently, there is little data on the safety of compounding 2 or more active drugs. Furthermore, the vehicle used may change the permeability of the topical formulation, and systemic absorption may be possible. Two deaths were reported with the application of compounded topical lidocaine and tetracaine gel due to systemic absorption. In these cases, the gel was used before laser hair removal, and it was applied under occlusion to greater than 50% of the body surface area, leading to fatal systemic absorption.1,2
One of the hypothetical benefits of compounding topicals is being able to avoid side effects of systemic medications. However, depending on the skin intactness and the strength of the medication used, systemic adverse effects have been reported.1 In a case series of 2 patients detailing the use of amitriptyline cream 5% and 10% for neuropathic pain, the patient using 10% cream experienced systemic effects of drowsiness and discontinued treatment.3
Another major disadvantage of compounding topicals is a lack of published data about the efficacy, especially given the unique nature of what is being compounded. When combining multiple medications, there are little to no published data about the efficacy of these formulations and how they compare to monotherapy. Although there may be data about the efficacy of an oral agent, it does not translate to the topical form being safe and efficacious. Much of the published data of topical formulations is limited to case reports and case series.
Finally, many compounded medications are not covered by insurance, and the out-of-pocket cost may be prohibitive for some patients. Compounding pharmacies typically will give patients a price estimate before the prescription is filled. When compounding topicals for patient use, it is important to counsel patients about the following:the unknown safety profile; lack of data regarding efficacy; and cost, as the medication likely will not be covered by insurance.
Pharmaceutical Regulations
After a contaminated product at a compounding pharmacy in New England led to an outbreak of fungal meningitis, there has been increased regulation by the US Food and Drug Administration.4 To meet safety regulations, compounding pharmacies must adhere to the standards set by the US Pharmacopeia. The US Food and Drug Administration says that physicians are not to prescribe compounded medications that are “unapproved, adulterated, or misbranded drugs,” which has been interpreted to mean that compounded medications should not mimic a branded medication but should instead be a unique formulation or strength.4,5 Thus, while compounding topicals may provide an alternative when a specific medication is not covered by insurance, it cannot be the same as a branded medication.
Pharmaceutical Options
Most major cities have custom compounding pharmacies or apothecaries. One of the benefits of using a local compounding pharmacy is that you typically can speak directly with the pharmacist about your patient’s diagnosis and his/her specific needs. The pharmacist can guide you through which formulations to compound, which strength to choose, and the best vehicle to use as a base. This expertise is invaluable in the compounding process. There also are online compounding pharmacies available.
Options for Bases
Dermatologists can request for their medications to be compounded in traditional over-the-counter emollients or petrolatum-based products, which work by passively diffusing through the stratum corneum into the superficial epidermis to treat skin conditions.1 For a topical drug to be absorbed effectively through the skin and into the general circulation, the vehicle needs to have affinity for both lipid and aqueous environments. Lipophilic drugs will absorb better through the stratum corneum, while hydrophilic drugs will absorb better through the aqueous layer of the epidermis. For a topical formulation to be both hydrophobic and hydrophilic, components such as viscosity enhancers and permeation enhancers can be added.1 Many compounding pharmacies also have proprietary bases that can be used.
Final Thoughts
Compounding topical medications in dermatology provides dermatologists with the ability to provide unique formulations to best suit their patients’ individual needs. However, dermatologists must keep in mind the limitations of compounding topicals, including a lack of data on efficacy and safety.
Compounding is a way of mixing or combining medications in formulations that are not widely available. Because dermatology is a field that includes a variety of topical treatments, compounding topicals is a way to create unique and customized treatment options for patients.
Advantages
Custom compounding topical medications has many benefits in comparison to traditional topical formulations. Compounding is a way of personalizing prescriptions to best suit the individual needs of each patient. Multiple ingredients with different mechanisms of action can be combined in a single medication for patients to use, which ultimately can simplify their treatment regimen.1 For rare conditions with uncommon treatments, compounding pharmacies can provide medications that are not widely available in retail pharmacies. Compounding topical medications also can be an efficient way of prescribing medications without dealing with the uncertainty of prior authorizations or how much the co-pay will be.
Disadvantages
One of the major disadvantages of compounding topical medications is the lack of safety data. Although most active drugs have been tested independently, there is little data on the safety of compounding 2 or more active drugs. Furthermore, the vehicle used may change the permeability of the topical formulation, and systemic absorption may be possible. Two deaths were reported with the application of compounded topical lidocaine and tetracaine gel due to systemic absorption. In these cases, the gel was used before laser hair removal, and it was applied under occlusion to greater than 50% of the body surface area, leading to fatal systemic absorption.1,2
One of the hypothetical benefits of compounding topicals is being able to avoid side effects of systemic medications. However, depending on the skin intactness and the strength of the medication used, systemic adverse effects have been reported.1 In a case series of 2 patients detailing the use of amitriptyline cream 5% and 10% for neuropathic pain, the patient using 10% cream experienced systemic effects of drowsiness and discontinued treatment.3
Another major disadvantage of compounding topicals is a lack of published data about the efficacy, especially given the unique nature of what is being compounded. When combining multiple medications, there are little to no published data about the efficacy of these formulations and how they compare to monotherapy. Although there may be data about the efficacy of an oral agent, it does not translate to the topical form being safe and efficacious. Much of the published data of topical formulations is limited to case reports and case series.
Finally, many compounded medications are not covered by insurance, and the out-of-pocket cost may be prohibitive for some patients. Compounding pharmacies typically will give patients a price estimate before the prescription is filled. When compounding topicals for patient use, it is important to counsel patients about the following:the unknown safety profile; lack of data regarding efficacy; and cost, as the medication likely will not be covered by insurance.
Pharmaceutical Regulations
After a contaminated product at a compounding pharmacy in New England led to an outbreak of fungal meningitis, there has been increased regulation by the US Food and Drug Administration.4 To meet safety regulations, compounding pharmacies must adhere to the standards set by the US Pharmacopeia. The US Food and Drug Administration says that physicians are not to prescribe compounded medications that are “unapproved, adulterated, or misbranded drugs,” which has been interpreted to mean that compounded medications should not mimic a branded medication but should instead be a unique formulation or strength.4,5 Thus, while compounding topicals may provide an alternative when a specific medication is not covered by insurance, it cannot be the same as a branded medication.
Pharmaceutical Options
Most major cities have custom compounding pharmacies or apothecaries. One of the benefits of using a local compounding pharmacy is that you typically can speak directly with the pharmacist about your patient’s diagnosis and his/her specific needs. The pharmacist can guide you through which formulations to compound, which strength to choose, and the best vehicle to use as a base. This expertise is invaluable in the compounding process. There also are online compounding pharmacies available.
Options for Bases
Dermatologists can request for their medications to be compounded in traditional over-the-counter emollients or petrolatum-based products, which work by passively diffusing through the stratum corneum into the superficial epidermis to treat skin conditions.1 For a topical drug to be absorbed effectively through the skin and into the general circulation, the vehicle needs to have affinity for both lipid and aqueous environments. Lipophilic drugs will absorb better through the stratum corneum, while hydrophilic drugs will absorb better through the aqueous layer of the epidermis. For a topical formulation to be both hydrophobic and hydrophilic, components such as viscosity enhancers and permeation enhancers can be added.1 Many compounding pharmacies also have proprietary bases that can be used.
Final Thoughts
Compounding topical medications in dermatology provides dermatologists with the ability to provide unique formulations to best suit their patients’ individual needs. However, dermatologists must keep in mind the limitations of compounding topicals, including a lack of data on efficacy and safety.
- Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271.
- Ukens C. Coed death tied to compounded drug. Drug Topics. March 7, 2005. https://www.drugtopics.com/community-pharmacy/coed-death-tied-compounded-drug. Accessed May 31, 2020.
- Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: 2 cases and literature review. Pain Pract. 2012;12:148-153.
- Campbell EH, Elston DM, Straughan CL, et al. Regulations, liability, safety, and economics related to compounding [published online December 9, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.11.061.
- US Food and Drug Administration. Administrative Destruction of Certain Drugs Refused Admission to the United States; Final Rule: Docket No. FDA-2014-N-0504. https://www.fda.gov/media/93525/download. Accessed May 31, 2020.
- Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271.
- Ukens C. Coed death tied to compounded drug. Drug Topics. March 7, 2005. https://www.drugtopics.com/community-pharmacy/coed-death-tied-compounded-drug. Accessed May 31, 2020.
- Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: 2 cases and literature review. Pain Pract. 2012;12:148-153.
- Campbell EH, Elston DM, Straughan CL, et al. Regulations, liability, safety, and economics related to compounding [published online December 9, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.11.061.
- US Food and Drug Administration. Administrative Destruction of Certain Drugs Refused Admission to the United States; Final Rule: Docket No. FDA-2014-N-0504. https://www.fda.gov/media/93525/download. Accessed May 31, 2020.
Resident Pearls
- Compounding topical medications provides dermatologists with the ability to create custom formulations that cater to the individual needs of each patient.
- Dermatologists must keep in mind that data are limited regarding both safety and efficacy of compounded medications.