User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Cutaneous Id Reaction After Using Cyanoacrylate for Wound Closure
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
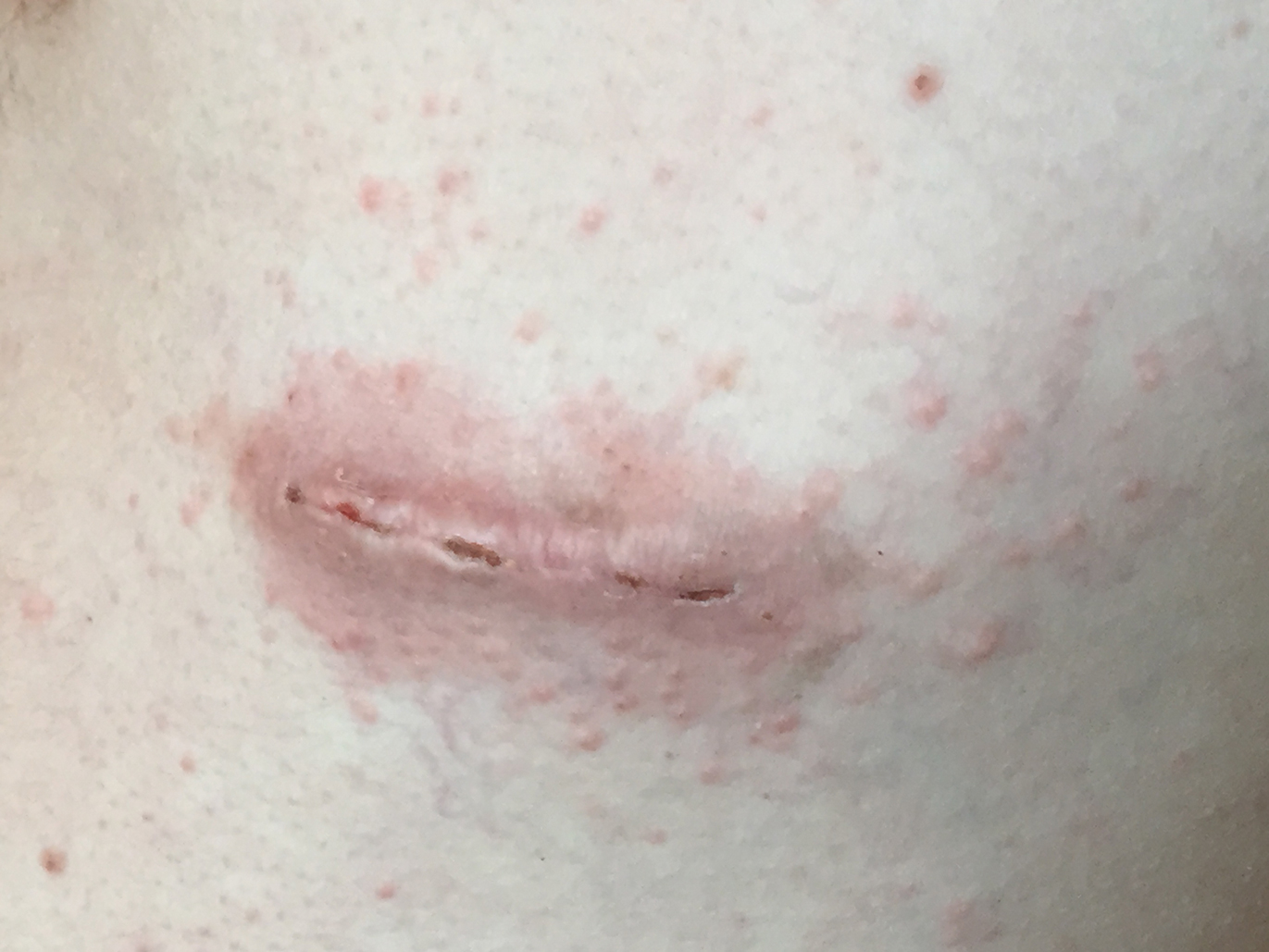
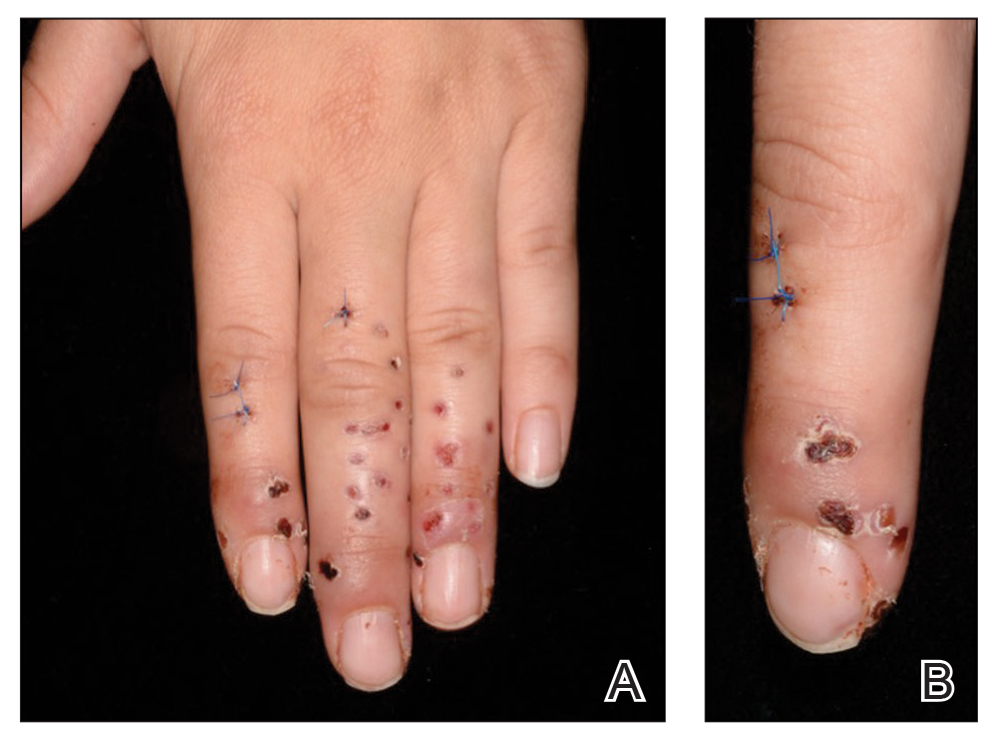
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.
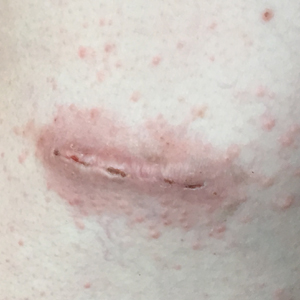
Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.
Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.
Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.
Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.
Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.
Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
Practice Points
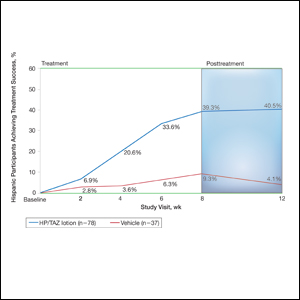
- 2-Octyl-cyanoacrylate (2-CA) tissue adhesive has been reported to cause contact dermatitis when applied topically for surgical site closure.
- Id reactions resulting from the use of 2-CA tissue adhesive are possible, though less commonly observed.
- Id reactions caused by 2-CA tissue adhesive respond well to treatment with a combination of topical steroids and oral antihistamines. Systemic corticosteroids may be warranted in cases involving greater than 20% body surface area.
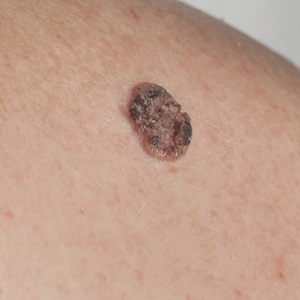
Tense Bullae on the Hands
The Diagnosis: Epidermolysis Bullosa Acquisita
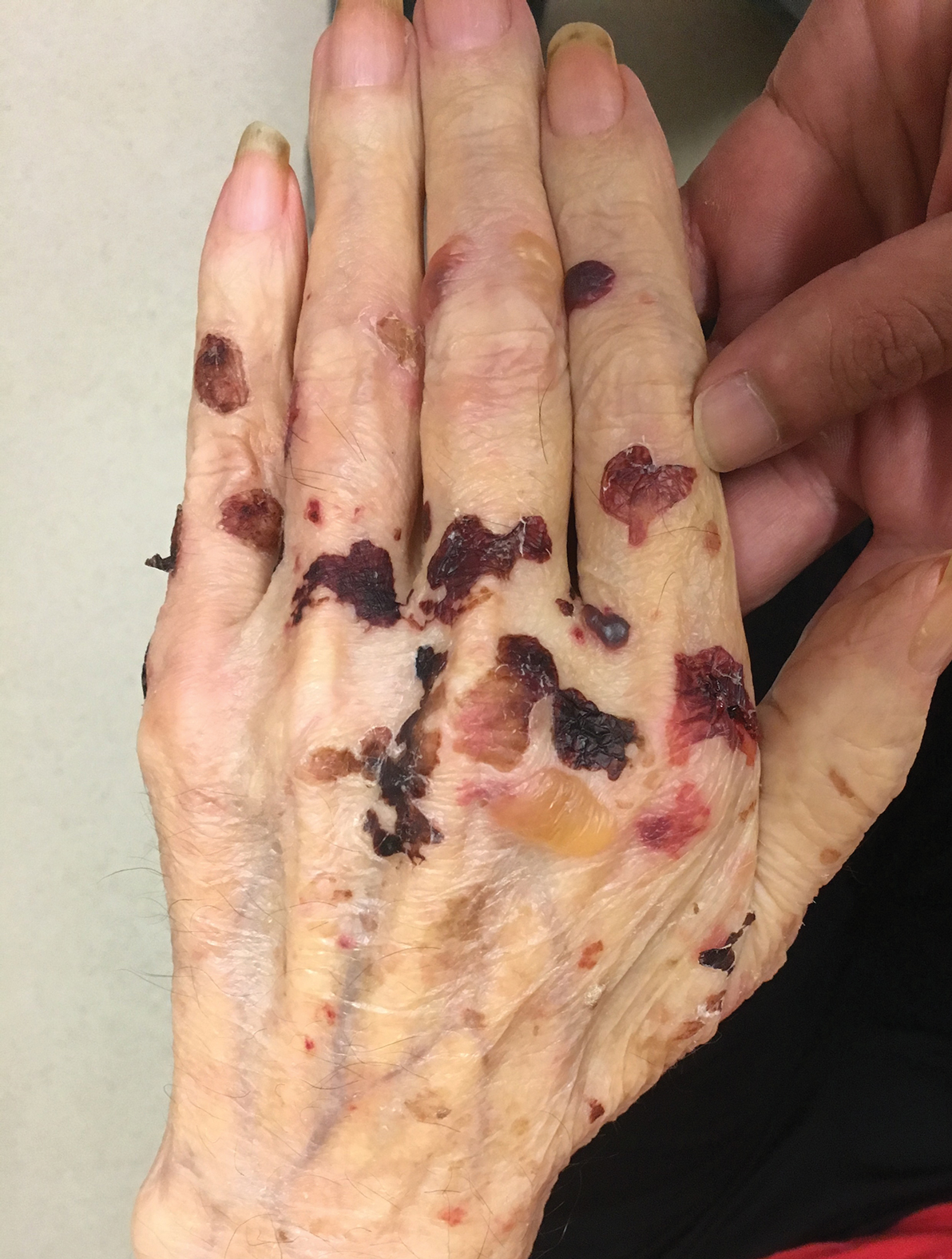
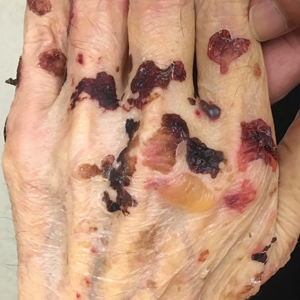
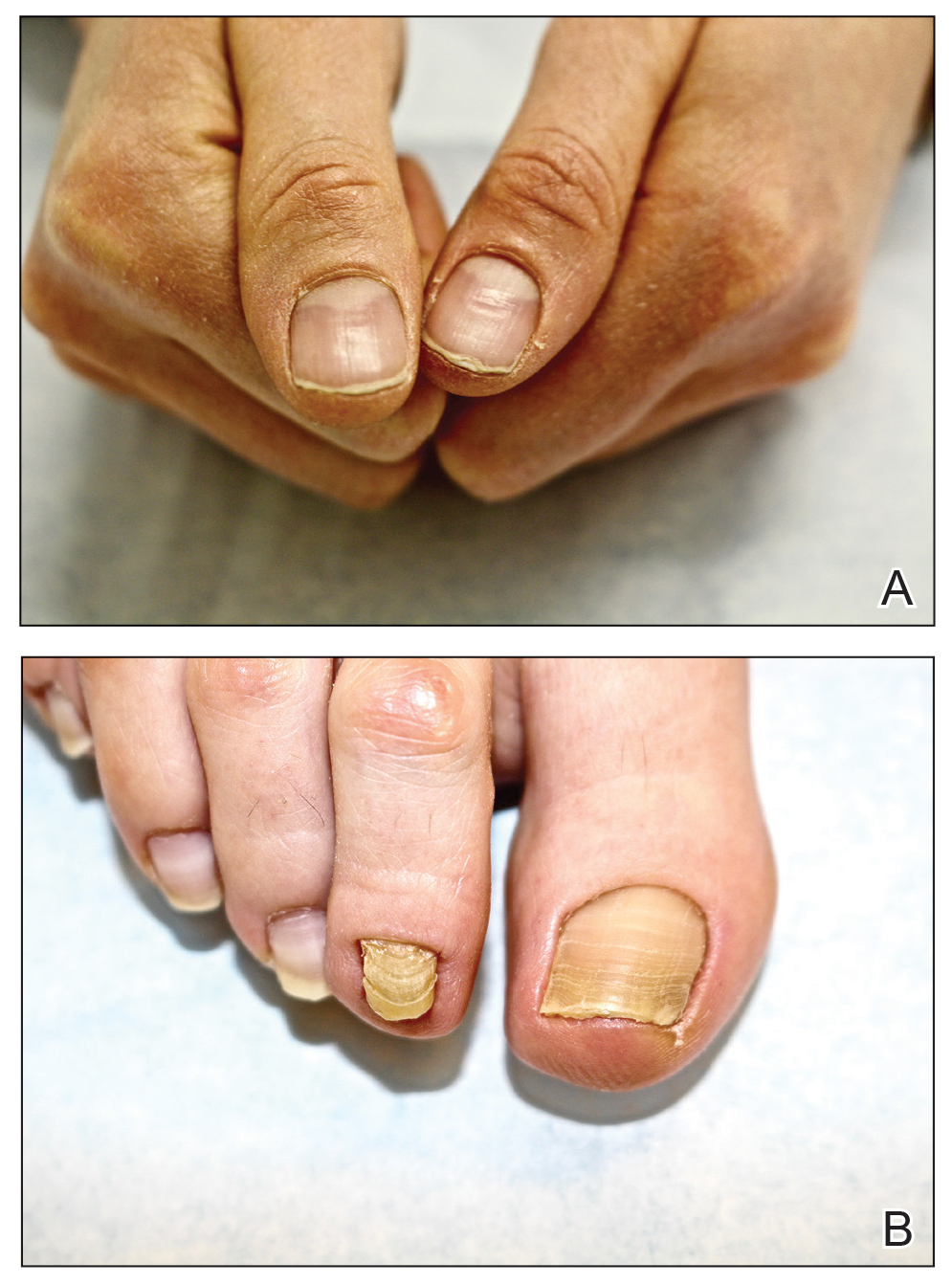
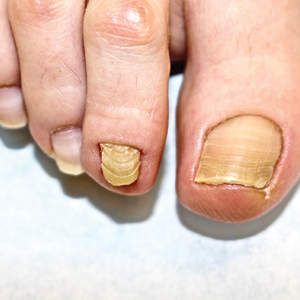
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune blistering disorder characterized by tense bullae, skin fragility, atrophic scarring, and milia formation.1 Blisters occur on a noninflammatory base in the classic variant and are trauma induced, hence the predilection for the extensor surfaces.2 Mucosal involvement also has been described.1 The characteristic findings in EBA are IgG autoantibodies directed at the N-terminal collagenous domain of type VII collagen, which composes the anchoring fibrils in the basement membrane zone.1 Differentiating EBA from other subepidermal bullous diseases, especially bullous pemphigoid (BP), can be difficult, necessitating specialized tests.
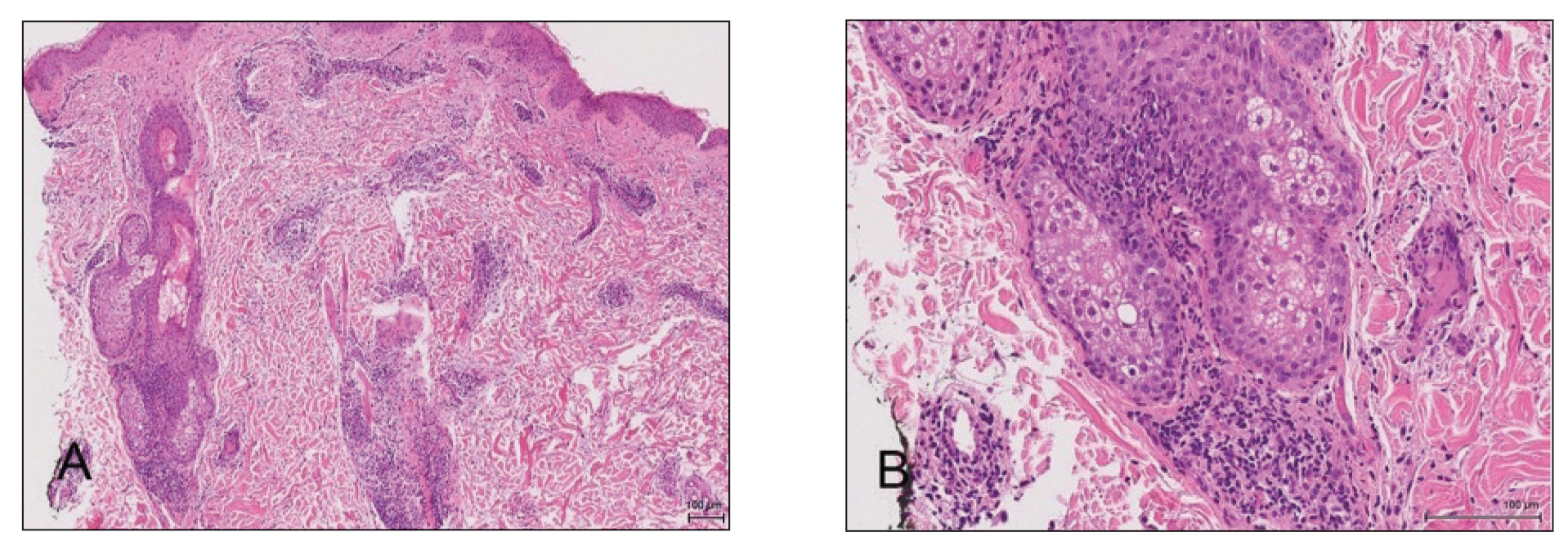
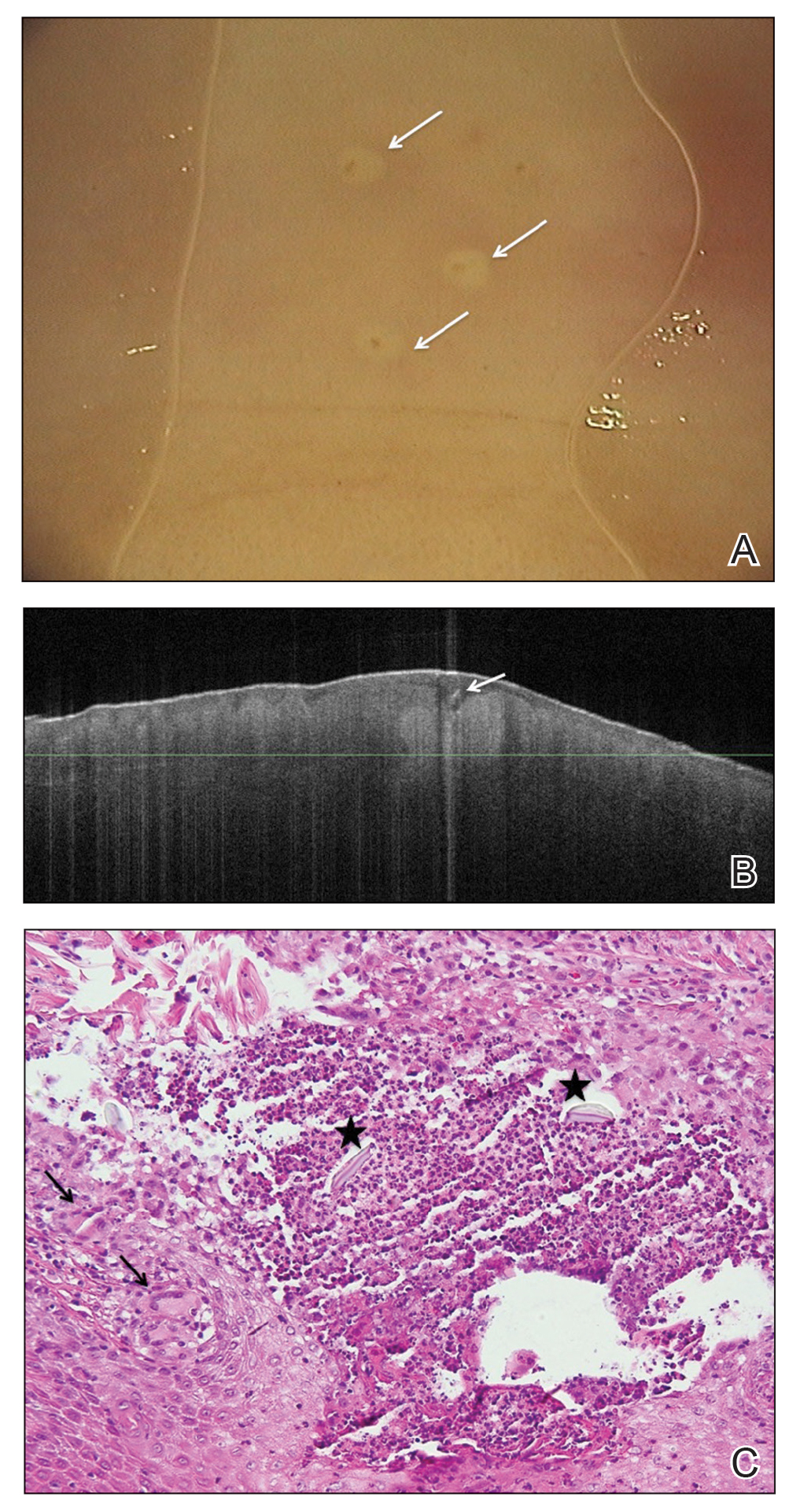
Biopsy of the perilesional skin can help identify the location of the blister formation. Our patient's biopsy showed a subepidermal blister with granulocytes. The differential diagnosis of a subepidermal blister includes BP, herpes gestationis, cicatricial pemphigoid, EBA, bullous systemic lupus erythematosus, dermatitis herpetiformis, linear IgA disease, and porphyria cutanea tarda.
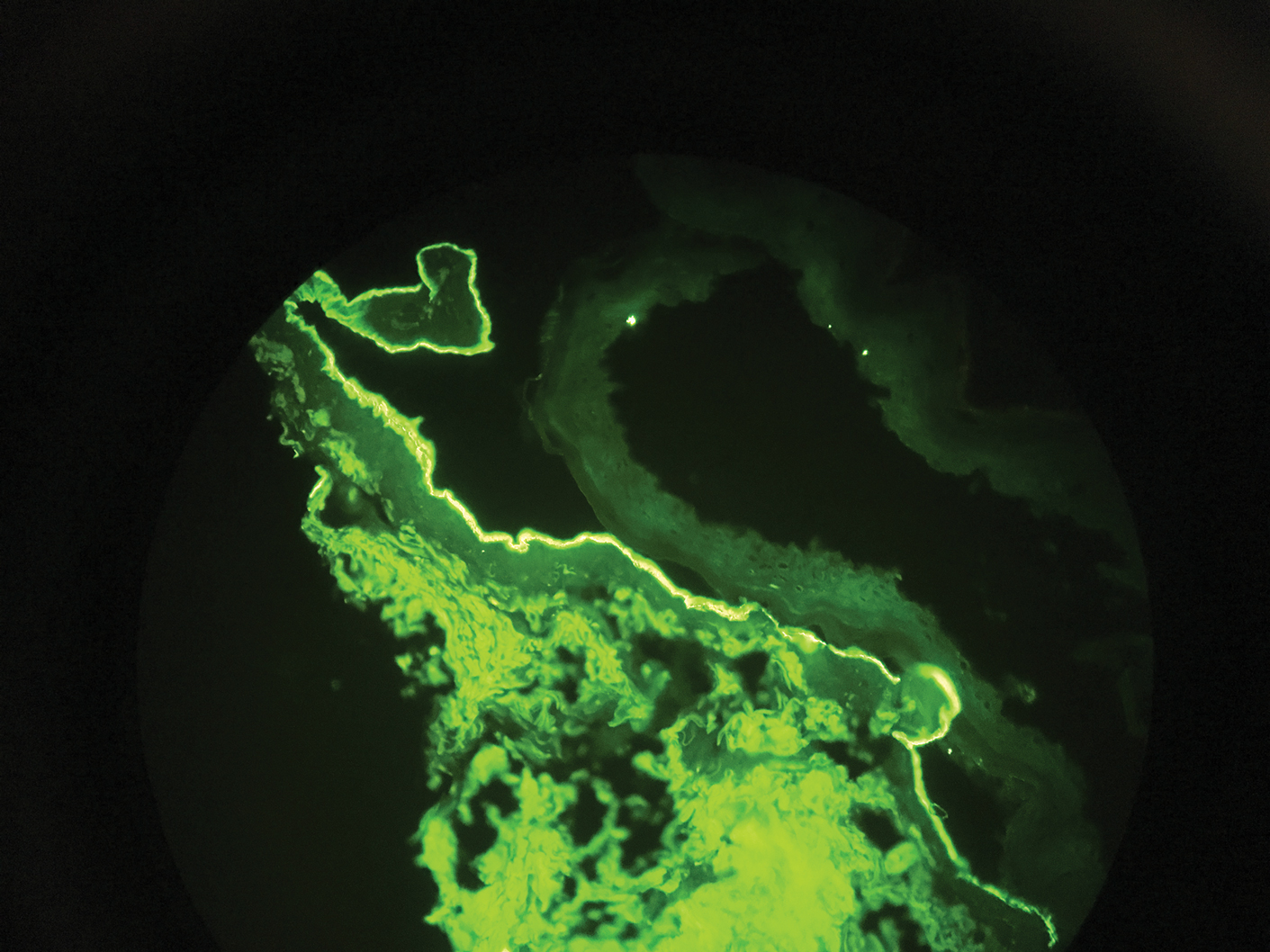
Direct immunofluorescence (DIF) was performed on the biopsy from our patient, which showed linear/particulate IgG, C3, and IgA deposits in the basement membrane zone, narrowing the differential diagnosis to BP or EBA. To differentiate EBA from BP, DIF of perilesional skin using a salt-split preparation was performed. This test distinguishes the location of the immunoreactants at the basement membrane zone. The antibody complexes in BP are found on the epidermal side of the split, while the antibody complexes in EBA are found on the dermal side of the split. Indirect immunofluorescence on salt-split skin also has been used to distinguish EBA from BP but is only conclusive if there are circulating autoantibodies to the basement membrane zone in the serum, which occurs in approximately 50% of patients with EBA and 15% of patients with BP.3 The immune complexes in our patient were found to be on the dermal side of the split after DIF on salt-split skin, confirming the diagnosis of EBA (Figure).
Differentiating EBA from BP has great value, as the diagnosis affects treatment options. Bullous pemphigoid is fairly easy to treat, with most patients responding to prednisone.3 Epidermolysis bullosa acquisita usually is resistant to therapy. The disease course is chronic with exacerbations and remissions. Dapsone often is used to control the disease, though this therapy for EBA is not currently approved by the US Food and Drug Administration. The recommended initial dose of dapsone is 50 mg daily and should be increased by 50 mg each week until remission, usually 100 to 250 mg.4 We prescribed dapsone for our patient upon clinical suspicion of EBA before the DIF on salt-split skin was completed. A trial of prednisone may be warranted for EBA if there is no response to dapsone or colchicine, but the response is unpredictable. Cyclosporine usually results in a quick response and may be considered if there is clinically severe disease and other treatment alternatives have failed.4
- Ishii N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what's new. J Dermatol. 2010;37:220-230.
- Lehman JS, Camilleri MJ, Gibsom LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227-236.
- Woodley D. Immunofluorescence on the salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229-231.
- Mutasim DF. Bullous diseases. In: Kellerman RD, Rakel DP, eds. Conn's Current Therapy. Philadelphia, PA: Elsevier; 2020:978-982.
The Diagnosis: Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune blistering disorder characterized by tense bullae, skin fragility, atrophic scarring, and milia formation.1 Blisters occur on a noninflammatory base in the classic variant and are trauma induced, hence the predilection for the extensor surfaces.2 Mucosal involvement also has been described.1 The characteristic findings in EBA are IgG autoantibodies directed at the N-terminal collagenous domain of type VII collagen, which composes the anchoring fibrils in the basement membrane zone.1 Differentiating EBA from other subepidermal bullous diseases, especially bullous pemphigoid (BP), can be difficult, necessitating specialized tests.
Biopsy of the perilesional skin can help identify the location of the blister formation. Our patient's biopsy showed a subepidermal blister with granulocytes. The differential diagnosis of a subepidermal blister includes BP, herpes gestationis, cicatricial pemphigoid, EBA, bullous systemic lupus erythematosus, dermatitis herpetiformis, linear IgA disease, and porphyria cutanea tarda.
Direct immunofluorescence (DIF) was performed on the biopsy from our patient, which showed linear/particulate IgG, C3, and IgA deposits in the basement membrane zone, narrowing the differential diagnosis to BP or EBA. To differentiate EBA from BP, DIF of perilesional skin using a salt-split preparation was performed. This test distinguishes the location of the immunoreactants at the basement membrane zone. The antibody complexes in BP are found on the epidermal side of the split, while the antibody complexes in EBA are found on the dermal side of the split. Indirect immunofluorescence on salt-split skin also has been used to distinguish EBA from BP but is only conclusive if there are circulating autoantibodies to the basement membrane zone in the serum, which occurs in approximately 50% of patients with EBA and 15% of patients with BP.3 The immune complexes in our patient were found to be on the dermal side of the split after DIF on salt-split skin, confirming the diagnosis of EBA (Figure).
Differentiating EBA from BP has great value, as the diagnosis affects treatment options. Bullous pemphigoid is fairly easy to treat, with most patients responding to prednisone.3 Epidermolysis bullosa acquisita usually is resistant to therapy. The disease course is chronic with exacerbations and remissions. Dapsone often is used to control the disease, though this therapy for EBA is not currently approved by the US Food and Drug Administration. The recommended initial dose of dapsone is 50 mg daily and should be increased by 50 mg each week until remission, usually 100 to 250 mg.4 We prescribed dapsone for our patient upon clinical suspicion of EBA before the DIF on salt-split skin was completed. A trial of prednisone may be warranted for EBA if there is no response to dapsone or colchicine, but the response is unpredictable. Cyclosporine usually results in a quick response and may be considered if there is clinically severe disease and other treatment alternatives have failed.4
The Diagnosis: Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune blistering disorder characterized by tense bullae, skin fragility, atrophic scarring, and milia formation.1 Blisters occur on a noninflammatory base in the classic variant and are trauma induced, hence the predilection for the extensor surfaces.2 Mucosal involvement also has been described.1 The characteristic findings in EBA are IgG autoantibodies directed at the N-terminal collagenous domain of type VII collagen, which composes the anchoring fibrils in the basement membrane zone.1 Differentiating EBA from other subepidermal bullous diseases, especially bullous pemphigoid (BP), can be difficult, necessitating specialized tests.
Biopsy of the perilesional skin can help identify the location of the blister formation. Our patient's biopsy showed a subepidermal blister with granulocytes. The differential diagnosis of a subepidermal blister includes BP, herpes gestationis, cicatricial pemphigoid, EBA, bullous systemic lupus erythematosus, dermatitis herpetiformis, linear IgA disease, and porphyria cutanea tarda.
Direct immunofluorescence (DIF) was performed on the biopsy from our patient, which showed linear/particulate IgG, C3, and IgA deposits in the basement membrane zone, narrowing the differential diagnosis to BP or EBA. To differentiate EBA from BP, DIF of perilesional skin using a salt-split preparation was performed. This test distinguishes the location of the immunoreactants at the basement membrane zone. The antibody complexes in BP are found on the epidermal side of the split, while the antibody complexes in EBA are found on the dermal side of the split. Indirect immunofluorescence on salt-split skin also has been used to distinguish EBA from BP but is only conclusive if there are circulating autoantibodies to the basement membrane zone in the serum, which occurs in approximately 50% of patients with EBA and 15% of patients with BP.3 The immune complexes in our patient were found to be on the dermal side of the split after DIF on salt-split skin, confirming the diagnosis of EBA (Figure).
Differentiating EBA from BP has great value, as the diagnosis affects treatment options. Bullous pemphigoid is fairly easy to treat, with most patients responding to prednisone.3 Epidermolysis bullosa acquisita usually is resistant to therapy. The disease course is chronic with exacerbations and remissions. Dapsone often is used to control the disease, though this therapy for EBA is not currently approved by the US Food and Drug Administration. The recommended initial dose of dapsone is 50 mg daily and should be increased by 50 mg each week until remission, usually 100 to 250 mg.4 We prescribed dapsone for our patient upon clinical suspicion of EBA before the DIF on salt-split skin was completed. A trial of prednisone may be warranted for EBA if there is no response to dapsone or colchicine, but the response is unpredictable. Cyclosporine usually results in a quick response and may be considered if there is clinically severe disease and other treatment alternatives have failed.4
- Ishii N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what's new. J Dermatol. 2010;37:220-230.
- Lehman JS, Camilleri MJ, Gibsom LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227-236.
- Woodley D. Immunofluorescence on the salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229-231.
- Mutasim DF. Bullous diseases. In: Kellerman RD, Rakel DP, eds. Conn's Current Therapy. Philadelphia, PA: Elsevier; 2020:978-982.
- Ishii N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what's new. J Dermatol. 2010;37:220-230.
- Lehman JS, Camilleri MJ, Gibsom LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227-236.
- Woodley D. Immunofluorescence on the salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229-231.
- Mutasim DF. Bullous diseases. In: Kellerman RD, Rakel DP, eds. Conn's Current Therapy. Philadelphia, PA: Elsevier; 2020:978-982.
A 75-year-old man presented to our clinic with nonpainful, nonpruritic, tense bullae and erosions on the dorsal aspects of the hands and extensor surfaces of the elbows of 1 month's duration. The patient also had erythematous erosions and crusted papules on the left cheek and surrounding the left eye. He denied any new medications, history of liver or kidney disease, or history of hepatitis or human immunodeficiency virus. There were no obvious exacerbating factors, including exposure to sunlight. Direct immunofluorescence using a salt-split preparation was performed on a biopsy of the perilesional skin.
Rapid Development of Perifolliculitis Following Mesotherapy
To the Editor:
Mesotherapy, also known as intradermotherapy, is a cosmetic procedure in which multiple intradermal or subcutaneous injections of homeopathic substances, vitamins, chemicals, and plant extracts are administered.1 First conceived in Europe, mesotherapy is not approved by the US Food and Drug Administration but is gaining popularity in the United States as an alternative cosmetic procedure for various purposes, including lipolysis, body contouring, stretch marks, acne scars, actinic damage, and skin rejuvenation.1,2 We report a case of a healthy woman who developed perifolliculitis, transaminitis, and neutropenia 2 weeks after mesotherapy administration to the face, neck, and chest. We also review other potential side effects of this procedure.
A 36-year-old woman with no notable medical history presented to the emergency department with a worsening pruritic and painful rash on the face, chest, and neck of 2 weeks’ duration. The rash had developed 3 days after the patient received mesotherapy with an unknown substance for cosmetic rejuvenation; the rash was localized only to the injection sites. She did not note any fever, chills, nausea, vomiting, diarrhea, headache, arthralgia, or upper respiratory tract symptoms. She further denied starting any new medications, herbal products, or topical therapies apart from the procedure she had received 2 weeks prior.
The patient was found to be in no acute distress and vital signs were stable. Laboratory testing was remarkable for elevations in alanine aminotransferase (62 U/L [reference range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had an absolute neutrophil count of 0.5×103 cells/µL (reference range 1.8–8.0×103 cells/µL). An electrolyte panel, creatinine level, and urinalysis were normal. Physical examination revealed numerous 4- to 5-mm erythematous papules in a gridlike distribution across the face, neck, and chest (Figure 1). No pustules or nodules were present. There was no discharge, crust, excoriations, or secondary lesions. Additionally, there was no lymphadenopathy and no mucous membrane or ocular involvement.
A 4-mm punch biopsy from a representative papule on the right lateral aspect of the neck demonstrated a perifollicular and perivascular lymphohistiocytic infiltrate with some focal granulomatous changes. No polarizable foreign body material was found (Figure 2). Bacterial, fungal, mycobacterial, and skin cultures were obtained, and results were all negative after several weeks.
A diagnosis of perifolliculitis from the mesotherapy procedure was on the top of the differential vs a fast-growing mycobacterial or granulomatous reaction. The patient was started on a prednisone taper at 40 mg once daily tapered down completely over 3 weeks in addition to triamcinolone cream 0.1% applied 2 to 4 times daily as needed. Although she did not return to our outpatient clinic for follow-up, she informed us that her rash had improved 1 month after starting the prednisone taper. She was later lost to follow-up. It is unclear if the transaminitis and neutropenia were related to the materials injected during the mesotherapy procedure or from long-standing health issues.
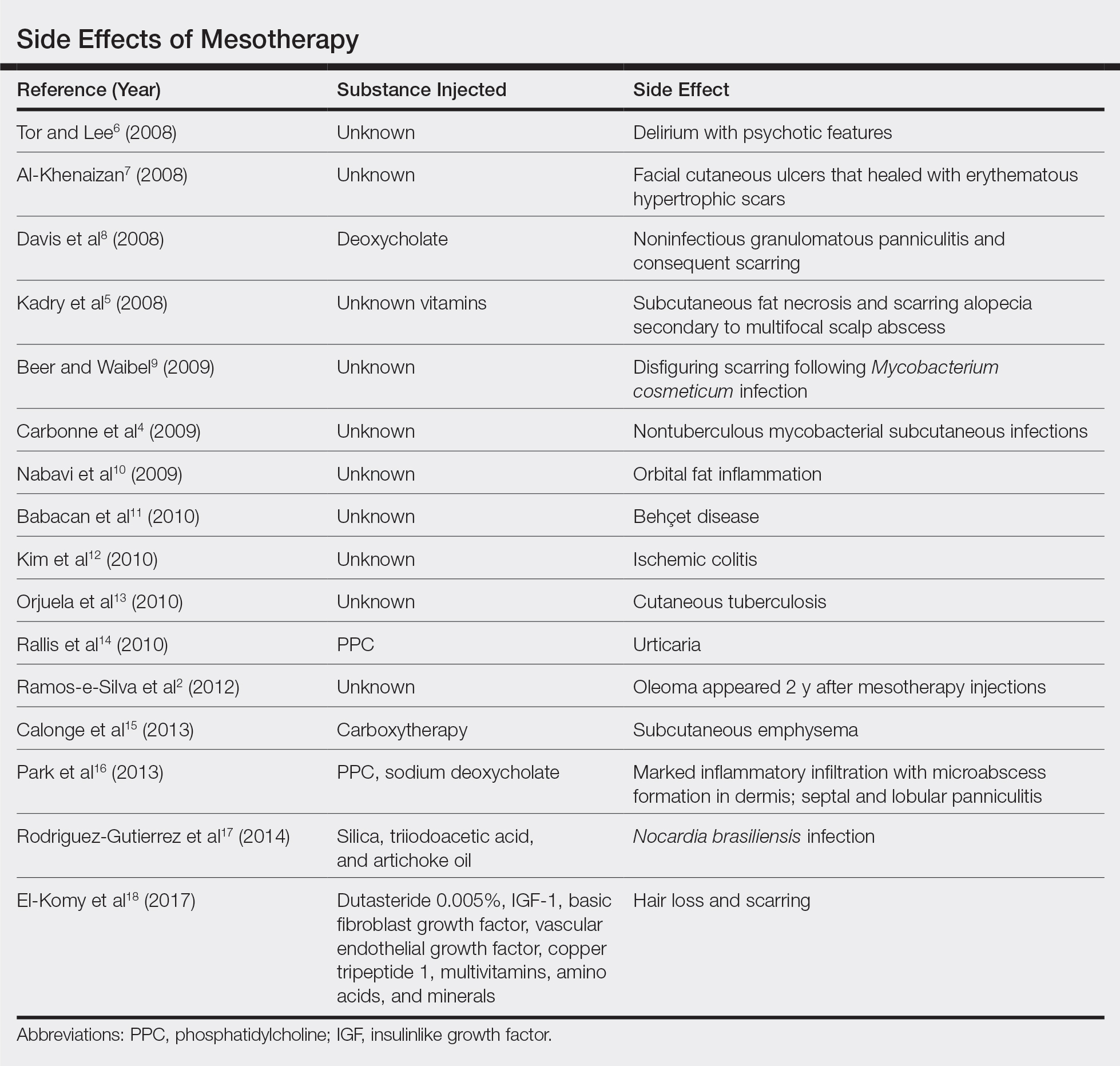
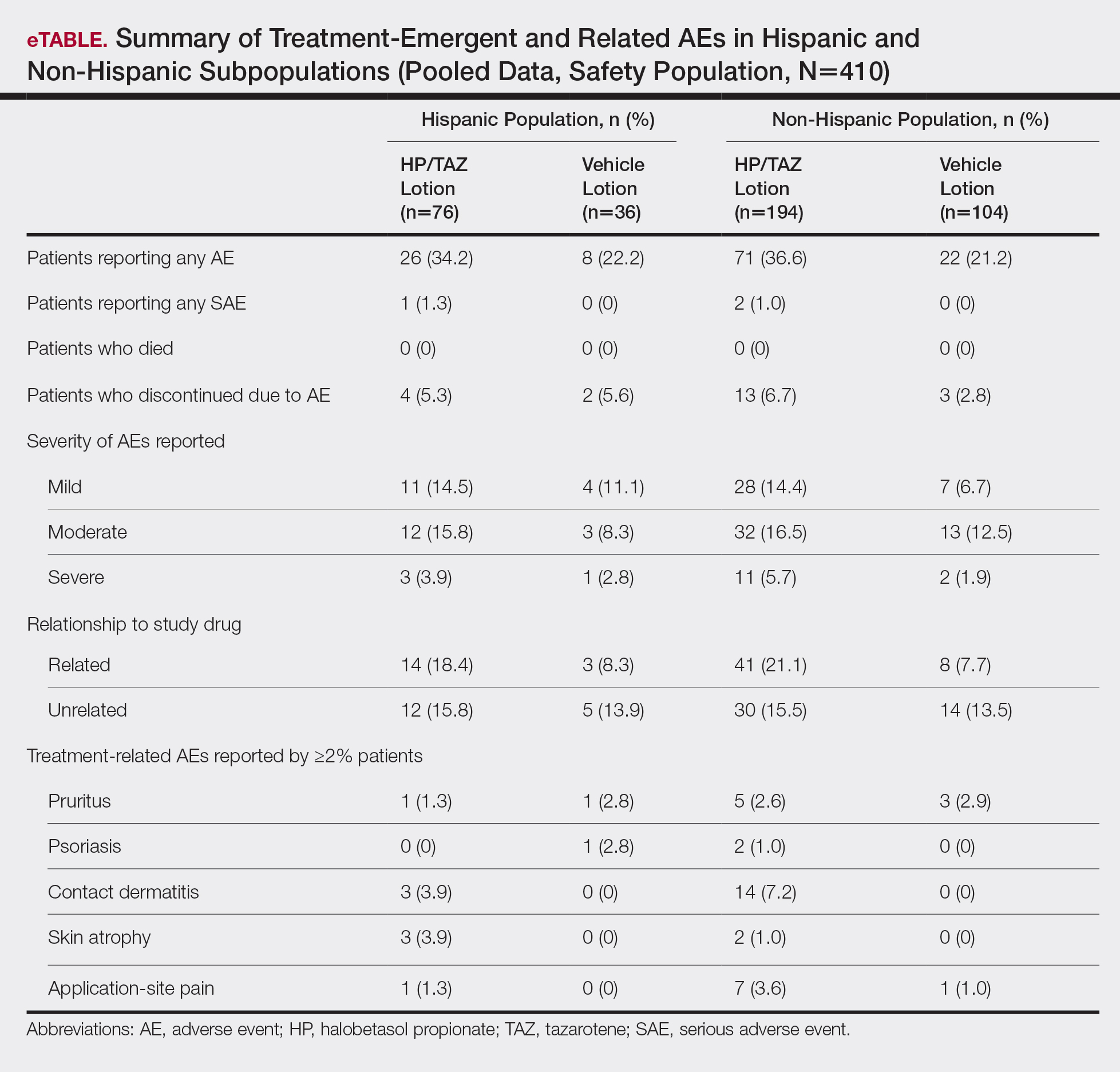
Mesotherapy promises aesthetic benefits through a minimally invasive procedure and therefore is rapidly gaining popularity in aesthetic spas and treatment centers. Due to the lack of regulation in treatment protocols and substances used, there have been numerous reported cases of adverse side effects following mesotherapy, such as pain, allergic reactions, urticaria, panniculitis, ulceration, hair loss, necrosis, paraffinoma, cutaneous tuberculosis, and rapidly growing nontuberculous mycobacterial infections.1-5 More serious side effects also have been reported, such as permanent scarring, deformities, delirium, and massive subcutaneous emphysema (Table).2,4-18
Given the potential complications of mesotherapy documented in the literature, we believe clinical investigations and trials must be performed to appropriately assess the safety and efficacy of this potentially hazardous procedure. Because there currently is insufficient research showing why certain patients are developing these adverse side effects, aesthetic spas and treatment centers should inform patients of all potential side effects associated with mesotherapy for the patient to make an informed decision about the procedure. Mesotherapy should be a point of focus for both the US Food and Drug Administration and researchers to determine its efficacy, safety, and standardization of the procedure.
- Bishara AS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842-849.
- Ramos-e-Silva M, Pereira AL, Ramos-e-Silva S, et al. Oleoma: a rare complication of mesotherapy for cellulite. Int J Dermatol. 2012;51:162-167.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-480.
- Carbonne A, Brossier F, Arnaud I, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. 2009;47:1961-1964.
- Kadry R, Hamadah I, Al-Issa A, et al. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol. 2008;7:72-73.
- Tor PC, Lee TS. Delirium with psychotic features possibly associated with mesotherapy. Psychosomatics. 2008;49:273-274.
- Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg. 2008;34:832-834.
- Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-809.
- Beer K, Waibel J. Disfiguring scarring following mesotherapy-associated Mycobacterium cosmeticum infection. J Drugs Dermatol. 2009;8:391-393.
- Nabavi CB, Minckler DS, Tao JP. Histologic features of mesotherapy-induced orbital fat inflammation. Opthalmic Plast Reconstr Surg. 2009;25:69-70.
- Babacan T, Onat AM, Pehlivan Y, et al. A case of Behçet’s disease diagnosed by the panniculitis after mesotherapy. Rheumatol Int. 2010;30:1657-1659.
- Kim JB, Moon W, Park SJ, et al. Ischemic colitis after mesotherapy combined with anti-obesity medications. World J Gastroenterol. 2010;16:1537-1540.
- Orjuela D, Puerto G, Mejia G, et al. Cutaneous tuberculosis after mesotherapy: report of six cases. Biomedica. 2010;30:321-326.
- Rallis E, Kintzoglou S, Moussatou V, et al. Mesotherapy-induced urticaria. Dermatol Surg. 2010;36:1355-1356.
- Calonge WM, Lesbros-Pantoflickova D, Hodina M, et al. Massive subcutaneous emphysema after carbon dioxide mesotherapy. Aesthetic Plast Surg. 2013;37:194-197.
- Park EJ, Kim HS, Kim M, et al. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J Cosmet Dermatol. 2013;3:240-243.
- Rodriguez-Gutierrez G, Toussaint S, Hernandez-Castro R, et al. Norcardia brasiliensis infection: an emergent suppurative granuloma after mesotherapy. Int J Dermatol. 2014;53:888-890.
- El-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia [published online February 3, 2017]. J Cosmet Dermatol. 2017;16:E28-E30.
To the Editor:
Mesotherapy, also known as intradermotherapy, is a cosmetic procedure in which multiple intradermal or subcutaneous injections of homeopathic substances, vitamins, chemicals, and plant extracts are administered.1 First conceived in Europe, mesotherapy is not approved by the US Food and Drug Administration but is gaining popularity in the United States as an alternative cosmetic procedure for various purposes, including lipolysis, body contouring, stretch marks, acne scars, actinic damage, and skin rejuvenation.1,2 We report a case of a healthy woman who developed perifolliculitis, transaminitis, and neutropenia 2 weeks after mesotherapy administration to the face, neck, and chest. We also review other potential side effects of this procedure.
A 36-year-old woman with no notable medical history presented to the emergency department with a worsening pruritic and painful rash on the face, chest, and neck of 2 weeks’ duration. The rash had developed 3 days after the patient received mesotherapy with an unknown substance for cosmetic rejuvenation; the rash was localized only to the injection sites. She did not note any fever, chills, nausea, vomiting, diarrhea, headache, arthralgia, or upper respiratory tract symptoms. She further denied starting any new medications, herbal products, or topical therapies apart from the procedure she had received 2 weeks prior.
The patient was found to be in no acute distress and vital signs were stable. Laboratory testing was remarkable for elevations in alanine aminotransferase (62 U/L [reference range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had an absolute neutrophil count of 0.5×103 cells/µL (reference range 1.8–8.0×103 cells/µL). An electrolyte panel, creatinine level, and urinalysis were normal. Physical examination revealed numerous 4- to 5-mm erythematous papules in a gridlike distribution across the face, neck, and chest (Figure 1). No pustules or nodules were present. There was no discharge, crust, excoriations, or secondary lesions. Additionally, there was no lymphadenopathy and no mucous membrane or ocular involvement.
A 4-mm punch biopsy from a representative papule on the right lateral aspect of the neck demonstrated a perifollicular and perivascular lymphohistiocytic infiltrate with some focal granulomatous changes. No polarizable foreign body material was found (Figure 2). Bacterial, fungal, mycobacterial, and skin cultures were obtained, and results were all negative after several weeks.
A diagnosis of perifolliculitis from the mesotherapy procedure was on the top of the differential vs a fast-growing mycobacterial or granulomatous reaction. The patient was started on a prednisone taper at 40 mg once daily tapered down completely over 3 weeks in addition to triamcinolone cream 0.1% applied 2 to 4 times daily as needed. Although she did not return to our outpatient clinic for follow-up, she informed us that her rash had improved 1 month after starting the prednisone taper. She was later lost to follow-up. It is unclear if the transaminitis and neutropenia were related to the materials injected during the mesotherapy procedure or from long-standing health issues.
Mesotherapy promises aesthetic benefits through a minimally invasive procedure and therefore is rapidly gaining popularity in aesthetic spas and treatment centers. Due to the lack of regulation in treatment protocols and substances used, there have been numerous reported cases of adverse side effects following mesotherapy, such as pain, allergic reactions, urticaria, panniculitis, ulceration, hair loss, necrosis, paraffinoma, cutaneous tuberculosis, and rapidly growing nontuberculous mycobacterial infections.1-5 More serious side effects also have been reported, such as permanent scarring, deformities, delirium, and massive subcutaneous emphysema (Table).2,4-18
Given the potential complications of mesotherapy documented in the literature, we believe clinical investigations and trials must be performed to appropriately assess the safety and efficacy of this potentially hazardous procedure. Because there currently is insufficient research showing why certain patients are developing these adverse side effects, aesthetic spas and treatment centers should inform patients of all potential side effects associated with mesotherapy for the patient to make an informed decision about the procedure. Mesotherapy should be a point of focus for both the US Food and Drug Administration and researchers to determine its efficacy, safety, and standardization of the procedure.
To the Editor:
Mesotherapy, also known as intradermotherapy, is a cosmetic procedure in which multiple intradermal or subcutaneous injections of homeopathic substances, vitamins, chemicals, and plant extracts are administered.1 First conceived in Europe, mesotherapy is not approved by the US Food and Drug Administration but is gaining popularity in the United States as an alternative cosmetic procedure for various purposes, including lipolysis, body contouring, stretch marks, acne scars, actinic damage, and skin rejuvenation.1,2 We report a case of a healthy woman who developed perifolliculitis, transaminitis, and neutropenia 2 weeks after mesotherapy administration to the face, neck, and chest. We also review other potential side effects of this procedure.
A 36-year-old woman with no notable medical history presented to the emergency department with a worsening pruritic and painful rash on the face, chest, and neck of 2 weeks’ duration. The rash had developed 3 days after the patient received mesotherapy with an unknown substance for cosmetic rejuvenation; the rash was localized only to the injection sites. She did not note any fever, chills, nausea, vomiting, diarrhea, headache, arthralgia, or upper respiratory tract symptoms. She further denied starting any new medications, herbal products, or topical therapies apart from the procedure she had received 2 weeks prior.
The patient was found to be in no acute distress and vital signs were stable. Laboratory testing was remarkable for elevations in alanine aminotransferase (62 U/L [reference range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had an absolute neutrophil count of 0.5×103 cells/µL (reference range 1.8–8.0×103 cells/µL). An electrolyte panel, creatinine level, and urinalysis were normal. Physical examination revealed numerous 4- to 5-mm erythematous papules in a gridlike distribution across the face, neck, and chest (Figure 1). No pustules or nodules were present. There was no discharge, crust, excoriations, or secondary lesions. Additionally, there was no lymphadenopathy and no mucous membrane or ocular involvement.
A 4-mm punch biopsy from a representative papule on the right lateral aspect of the neck demonstrated a perifollicular and perivascular lymphohistiocytic infiltrate with some focal granulomatous changes. No polarizable foreign body material was found (Figure 2). Bacterial, fungal, mycobacterial, and skin cultures were obtained, and results were all negative after several weeks.
A diagnosis of perifolliculitis from the mesotherapy procedure was on the top of the differential vs a fast-growing mycobacterial or granulomatous reaction. The patient was started on a prednisone taper at 40 mg once daily tapered down completely over 3 weeks in addition to triamcinolone cream 0.1% applied 2 to 4 times daily as needed. Although she did not return to our outpatient clinic for follow-up, she informed us that her rash had improved 1 month after starting the prednisone taper. She was later lost to follow-up. It is unclear if the transaminitis and neutropenia were related to the materials injected during the mesotherapy procedure or from long-standing health issues.
Mesotherapy promises aesthetic benefits through a minimally invasive procedure and therefore is rapidly gaining popularity in aesthetic spas and treatment centers. Due to the lack of regulation in treatment protocols and substances used, there have been numerous reported cases of adverse side effects following mesotherapy, such as pain, allergic reactions, urticaria, panniculitis, ulceration, hair loss, necrosis, paraffinoma, cutaneous tuberculosis, and rapidly growing nontuberculous mycobacterial infections.1-5 More serious side effects also have been reported, such as permanent scarring, deformities, delirium, and massive subcutaneous emphysema (Table).2,4-18
Given the potential complications of mesotherapy documented in the literature, we believe clinical investigations and trials must be performed to appropriately assess the safety and efficacy of this potentially hazardous procedure. Because there currently is insufficient research showing why certain patients are developing these adverse side effects, aesthetic spas and treatment centers should inform patients of all potential side effects associated with mesotherapy for the patient to make an informed decision about the procedure. Mesotherapy should be a point of focus for both the US Food and Drug Administration and researchers to determine its efficacy, safety, and standardization of the procedure.
- Bishara AS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842-849.
- Ramos-e-Silva M, Pereira AL, Ramos-e-Silva S, et al. Oleoma: a rare complication of mesotherapy for cellulite. Int J Dermatol. 2012;51:162-167.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-480.
- Carbonne A, Brossier F, Arnaud I, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. 2009;47:1961-1964.
- Kadry R, Hamadah I, Al-Issa A, et al. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol. 2008;7:72-73.
- Tor PC, Lee TS. Delirium with psychotic features possibly associated with mesotherapy. Psychosomatics. 2008;49:273-274.
- Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg. 2008;34:832-834.
- Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-809.
- Beer K, Waibel J. Disfiguring scarring following mesotherapy-associated Mycobacterium cosmeticum infection. J Drugs Dermatol. 2009;8:391-393.
- Nabavi CB, Minckler DS, Tao JP. Histologic features of mesotherapy-induced orbital fat inflammation. Opthalmic Plast Reconstr Surg. 2009;25:69-70.
- Babacan T, Onat AM, Pehlivan Y, et al. A case of Behçet’s disease diagnosed by the panniculitis after mesotherapy. Rheumatol Int. 2010;30:1657-1659.
- Kim JB, Moon W, Park SJ, et al. Ischemic colitis after mesotherapy combined with anti-obesity medications. World J Gastroenterol. 2010;16:1537-1540.
- Orjuela D, Puerto G, Mejia G, et al. Cutaneous tuberculosis after mesotherapy: report of six cases. Biomedica. 2010;30:321-326.
- Rallis E, Kintzoglou S, Moussatou V, et al. Mesotherapy-induced urticaria. Dermatol Surg. 2010;36:1355-1356.
- Calonge WM, Lesbros-Pantoflickova D, Hodina M, et al. Massive subcutaneous emphysema after carbon dioxide mesotherapy. Aesthetic Plast Surg. 2013;37:194-197.
- Park EJ, Kim HS, Kim M, et al. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J Cosmet Dermatol. 2013;3:240-243.
- Rodriguez-Gutierrez G, Toussaint S, Hernandez-Castro R, et al. Norcardia brasiliensis infection: an emergent suppurative granuloma after mesotherapy. Int J Dermatol. 2014;53:888-890.
- El-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia [published online February 3, 2017]. J Cosmet Dermatol. 2017;16:E28-E30.
- Bishara AS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842-849.
- Ramos-e-Silva M, Pereira AL, Ramos-e-Silva S, et al. Oleoma: a rare complication of mesotherapy for cellulite. Int J Dermatol. 2012;51:162-167.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-480.
- Carbonne A, Brossier F, Arnaud I, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. 2009;47:1961-1964.
- Kadry R, Hamadah I, Al-Issa A, et al. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol. 2008;7:72-73.
- Tor PC, Lee TS. Delirium with psychotic features possibly associated with mesotherapy. Psychosomatics. 2008;49:273-274.
- Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg. 2008;34:832-834.
- Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-809.
- Beer K, Waibel J. Disfiguring scarring following mesotherapy-associated Mycobacterium cosmeticum infection. J Drugs Dermatol. 2009;8:391-393.
- Nabavi CB, Minckler DS, Tao JP. Histologic features of mesotherapy-induced orbital fat inflammation. Opthalmic Plast Reconstr Surg. 2009;25:69-70.
- Babacan T, Onat AM, Pehlivan Y, et al. A case of Behçet’s disease diagnosed by the panniculitis after mesotherapy. Rheumatol Int. 2010;30:1657-1659.
- Kim JB, Moon W, Park SJ, et al. Ischemic colitis after mesotherapy combined with anti-obesity medications. World J Gastroenterol. 2010;16:1537-1540.
- Orjuela D, Puerto G, Mejia G, et al. Cutaneous tuberculosis after mesotherapy: report of six cases. Biomedica. 2010;30:321-326.
- Rallis E, Kintzoglou S, Moussatou V, et al. Mesotherapy-induced urticaria. Dermatol Surg. 2010;36:1355-1356.
- Calonge WM, Lesbros-Pantoflickova D, Hodina M, et al. Massive subcutaneous emphysema after carbon dioxide mesotherapy. Aesthetic Plast Surg. 2013;37:194-197.
- Park EJ, Kim HS, Kim M, et al. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J Cosmet Dermatol. 2013;3:240-243.
- Rodriguez-Gutierrez G, Toussaint S, Hernandez-Castro R, et al. Norcardia brasiliensis infection: an emergent suppurative granuloma after mesotherapy. Int J Dermatol. 2014;53:888-890.
- El-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia [published online February 3, 2017]. J Cosmet Dermatol. 2017;16:E28-E30.
Practice Points
- Mesotherapy—the delivery of vitamins, chemicals, and plant extracts directly into the dermis via injections—is a common procedure performed in both medical and nonmedical settings for cosmetic rejuvenation.
- Complications can occur from mesotherapy treatment.
- Patients should be advised to seek medical care with US Food and Drug Administration–approved cosmetic techniques and substances only
Human Immunodeficiency Virus Infection in a Hepatitis B Virus–Positive Psoriasis Patient Treated With Ustekinumab
To the Editor:
The incidence of psoriasis in human immunodeficiency virus (HIV)–infected patients is similar to the general population, but it usually becomes more severe as immunosuppression increases. Additionally, it tends to be more resistant to conventional therapies, and the incidence and severity of psoriatic arthropathy is increased. Psoriasis often worsens at the time of HIV primary infection.1 We describe a case of a patient with hepatitis B virus (HBV) whose severe plaque psoriasis was controlled on ustekinumab; he was later diagnosed with HIV infection.
A 42-year-old man with HBV treated with entecavir (HBV DNA viral load, <20 copies/mL [inactive carrier, <2000 copies/mL]) presented to our dermatology unit with severe plaque psoriasis (psoriasis area and severity index 23) that caused notable psychologic difficulties such as anxiety and depression. Treatment was attempted with cyclosporine; acitretin; psoralen plus UVA; infliximab; adalimumab; and eventually ustekinumab (45 mg every 3 months), which controlled the condition well (psoriasis area and severity index 0) in an almost completely sustained manner.
Serologic tests requested at one of his analytical control appointments 2 years after initiating treatment with ustekinumab revealed he was HIV positive. The patient reported unprotected sexual intercourse 4 months prior. He was referred to the infectious disease unit and was classified in subtype A1 of HIV infection (CD4 count, 583 cells/µL [reference range, 500-1200 cells/µL]; viral load, 159,268 copies/mL [rapid progression to AIDS, >100,000 copies/mL]). Treatment was initiated with raltegravir, ritonavir, darunavir, and abacavir; tolerance was suitable. Because of the patient’s history of severe psoriasis, treatment with ustekinumab was maintained. Normally, treatment with this drug would be contraindicated in patients with HIV, as it can lead to viral reactivation. Four years after his HIV diagnosis, neither the patient’s cutaneous nor HIV-associated condition had worsened.
For patients with HIV and mild or moderate psoriasis, topical therapies (eg, corticosteroids, vitamin D analogues, tazarotene) are recommended, similar to patients who are HIV negative. Human immunodeficiency virus–positive patients with severe psoriasis who do not respond to topical treatment should receive phototherapy (UVB or psoralen plus UVA) or acitretin along with their antiretroviral drugs.2 In refractory cases, immunosuppressants, including cyclosporine, methotrexate, or tumor necrosis factor α inhibitors, might be used, though experience with them is limited.3,4 Maintaining antiretroviral therapy and prophylaxis against opportunist disease is important in patients who receive such immunosuppressants, as is close monitoring of the viral load.
Ustekinumab is an IL-12/IL-23 monoclonal antibody indicated for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, and inflammatory bowel disease. It is contraindicated in patients with clinically important active infections, such as HBV and hepatitis C virus infections.5 However, it was shown to be safe in a group of 18 patients with HBV who had received antiviral prophylaxis6; a degree of reactivation was observed in similar patients who received no such prophylaxis and in others with hepatitis C virus infection.7 The simultaneous use of methotrexate with ustekinumab in the treatment of psoriatic arthritis does not appear to affect the safety of the latter drug.8 Paparizos et al9 described a patient with HIV controlled with antiretroviral drugs who was treated with ustekinumab for psoriasis; no adverse effects were observed.
We report the case of a patient with HBV and psoriasis who was treated with ustekinumab and later became infected with HIV. His ustekinumab treatment was maintained without subsequent cutaneous or systemic complications.
- Menon K, Van Voorhees V, Bebo B, et al. Psoriasis in patients with HIV infection: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Chiricozzi A, Saraceno R, Cannizzaro MV. Complete resolution of erythrodermic psoriasis in an HIV and HCV patient unresponsive to antipsoriatic treatments after highly active antiretroviral therapy. Dermatology. 2012;225:333-337.
- Barco D, Puig L, Alomar A. Treatment of moderate-severe psoriasis with etanercept in patients with chronic human immunodeficiency virus infection. Actas Dermosifiliogr. 2010;101(suppl 1):77-81.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV positive patient. J Drugs Dermatol. 2014;13:869-871.
- Rustin MH. Long-term safety of biologics in the treatment of moderate to severe plaque psoriasis: review of the current data. Br J Dermatol. 2012;167(suppl 3):3-11.
- Navarro R, Vilarrasa E, Herranz P, et al. Safety and effectiveness of ustekinumab and antitumour necrosis factor therapy in patients with psoriasis and chronic viral hepatitis B or C: a retrospective, multicentre study in a clinical setting. Br J Dermatol. 2013;168:609-616.
- Chiu HY, Chen CH, Wu MS, et al. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013;169:1295-1303.
- Weitz JE, Ritchlin CT. Ustekinumab: targeting the IL-17 pathway to improve outcomes in psoriatic arthritis. Expert Opin Biol Ther. 2014;14:515-526.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399.
To the Editor:
The incidence of psoriasis in human immunodeficiency virus (HIV)–infected patients is similar to the general population, but it usually becomes more severe as immunosuppression increases. Additionally, it tends to be more resistant to conventional therapies, and the incidence and severity of psoriatic arthropathy is increased. Psoriasis often worsens at the time of HIV primary infection.1 We describe a case of a patient with hepatitis B virus (HBV) whose severe plaque psoriasis was controlled on ustekinumab; he was later diagnosed with HIV infection.
A 42-year-old man with HBV treated with entecavir (HBV DNA viral load, <20 copies/mL [inactive carrier, <2000 copies/mL]) presented to our dermatology unit with severe plaque psoriasis (psoriasis area and severity index 23) that caused notable psychologic difficulties such as anxiety and depression. Treatment was attempted with cyclosporine; acitretin; psoralen plus UVA; infliximab; adalimumab; and eventually ustekinumab (45 mg every 3 months), which controlled the condition well (psoriasis area and severity index 0) in an almost completely sustained manner.
Serologic tests requested at one of his analytical control appointments 2 years after initiating treatment with ustekinumab revealed he was HIV positive. The patient reported unprotected sexual intercourse 4 months prior. He was referred to the infectious disease unit and was classified in subtype A1 of HIV infection (CD4 count, 583 cells/µL [reference range, 500-1200 cells/µL]; viral load, 159,268 copies/mL [rapid progression to AIDS, >100,000 copies/mL]). Treatment was initiated with raltegravir, ritonavir, darunavir, and abacavir; tolerance was suitable. Because of the patient’s history of severe psoriasis, treatment with ustekinumab was maintained. Normally, treatment with this drug would be contraindicated in patients with HIV, as it can lead to viral reactivation. Four years after his HIV diagnosis, neither the patient’s cutaneous nor HIV-associated condition had worsened.
For patients with HIV and mild or moderate psoriasis, topical therapies (eg, corticosteroids, vitamin D analogues, tazarotene) are recommended, similar to patients who are HIV negative. Human immunodeficiency virus–positive patients with severe psoriasis who do not respond to topical treatment should receive phototherapy (UVB or psoralen plus UVA) or acitretin along with their antiretroviral drugs.2 In refractory cases, immunosuppressants, including cyclosporine, methotrexate, or tumor necrosis factor α inhibitors, might be used, though experience with them is limited.3,4 Maintaining antiretroviral therapy and prophylaxis against opportunist disease is important in patients who receive such immunosuppressants, as is close monitoring of the viral load.
Ustekinumab is an IL-12/IL-23 monoclonal antibody indicated for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, and inflammatory bowel disease. It is contraindicated in patients with clinically important active infections, such as HBV and hepatitis C virus infections.5 However, it was shown to be safe in a group of 18 patients with HBV who had received antiviral prophylaxis6; a degree of reactivation was observed in similar patients who received no such prophylaxis and in others with hepatitis C virus infection.7 The simultaneous use of methotrexate with ustekinumab in the treatment of psoriatic arthritis does not appear to affect the safety of the latter drug.8 Paparizos et al9 described a patient with HIV controlled with antiretroviral drugs who was treated with ustekinumab for psoriasis; no adverse effects were observed.
We report the case of a patient with HBV and psoriasis who was treated with ustekinumab and later became infected with HIV. His ustekinumab treatment was maintained without subsequent cutaneous or systemic complications.
To the Editor:
The incidence of psoriasis in human immunodeficiency virus (HIV)–infected patients is similar to the general population, but it usually becomes more severe as immunosuppression increases. Additionally, it tends to be more resistant to conventional therapies, and the incidence and severity of psoriatic arthropathy is increased. Psoriasis often worsens at the time of HIV primary infection.1 We describe a case of a patient with hepatitis B virus (HBV) whose severe plaque psoriasis was controlled on ustekinumab; he was later diagnosed with HIV infection.
A 42-year-old man with HBV treated with entecavir (HBV DNA viral load, <20 copies/mL [inactive carrier, <2000 copies/mL]) presented to our dermatology unit with severe plaque psoriasis (psoriasis area and severity index 23) that caused notable psychologic difficulties such as anxiety and depression. Treatment was attempted with cyclosporine; acitretin; psoralen plus UVA; infliximab; adalimumab; and eventually ustekinumab (45 mg every 3 months), which controlled the condition well (psoriasis area and severity index 0) in an almost completely sustained manner.
Serologic tests requested at one of his analytical control appointments 2 years after initiating treatment with ustekinumab revealed he was HIV positive. The patient reported unprotected sexual intercourse 4 months prior. He was referred to the infectious disease unit and was classified in subtype A1 of HIV infection (CD4 count, 583 cells/µL [reference range, 500-1200 cells/µL]; viral load, 159,268 copies/mL [rapid progression to AIDS, >100,000 copies/mL]). Treatment was initiated with raltegravir, ritonavir, darunavir, and abacavir; tolerance was suitable. Because of the patient’s history of severe psoriasis, treatment with ustekinumab was maintained. Normally, treatment with this drug would be contraindicated in patients with HIV, as it can lead to viral reactivation. Four years after his HIV diagnosis, neither the patient’s cutaneous nor HIV-associated condition had worsened.
For patients with HIV and mild or moderate psoriasis, topical therapies (eg, corticosteroids, vitamin D analogues, tazarotene) are recommended, similar to patients who are HIV negative. Human immunodeficiency virus–positive patients with severe psoriasis who do not respond to topical treatment should receive phototherapy (UVB or psoralen plus UVA) or acitretin along with their antiretroviral drugs.2 In refractory cases, immunosuppressants, including cyclosporine, methotrexate, or tumor necrosis factor α inhibitors, might be used, though experience with them is limited.3,4 Maintaining antiretroviral therapy and prophylaxis against opportunist disease is important in patients who receive such immunosuppressants, as is close monitoring of the viral load.
Ustekinumab is an IL-12/IL-23 monoclonal antibody indicated for the treatment of moderate to severe plaque psoriasis, active psoriatic arthritis, and inflammatory bowel disease. It is contraindicated in patients with clinically important active infections, such as HBV and hepatitis C virus infections.5 However, it was shown to be safe in a group of 18 patients with HBV who had received antiviral prophylaxis6; a degree of reactivation was observed in similar patients who received no such prophylaxis and in others with hepatitis C virus infection.7 The simultaneous use of methotrexate with ustekinumab in the treatment of psoriatic arthritis does not appear to affect the safety of the latter drug.8 Paparizos et al9 described a patient with HIV controlled with antiretroviral drugs who was treated with ustekinumab for psoriasis; no adverse effects were observed.
We report the case of a patient with HBV and psoriasis who was treated with ustekinumab and later became infected with HIV. His ustekinumab treatment was maintained without subsequent cutaneous or systemic complications.
- Menon K, Van Voorhees V, Bebo B, et al. Psoriasis in patients with HIV infection: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Chiricozzi A, Saraceno R, Cannizzaro MV. Complete resolution of erythrodermic psoriasis in an HIV and HCV patient unresponsive to antipsoriatic treatments after highly active antiretroviral therapy. Dermatology. 2012;225:333-337.
- Barco D, Puig L, Alomar A. Treatment of moderate-severe psoriasis with etanercept in patients with chronic human immunodeficiency virus infection. Actas Dermosifiliogr. 2010;101(suppl 1):77-81.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV positive patient. J Drugs Dermatol. 2014;13:869-871.
- Rustin MH. Long-term safety of biologics in the treatment of moderate to severe plaque psoriasis: review of the current data. Br J Dermatol. 2012;167(suppl 3):3-11.
- Navarro R, Vilarrasa E, Herranz P, et al. Safety and effectiveness of ustekinumab and antitumour necrosis factor therapy in patients with psoriasis and chronic viral hepatitis B or C: a retrospective, multicentre study in a clinical setting. Br J Dermatol. 2013;168:609-616.
- Chiu HY, Chen CH, Wu MS, et al. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013;169:1295-1303.
- Weitz JE, Ritchlin CT. Ustekinumab: targeting the IL-17 pathway to improve outcomes in psoriatic arthritis. Expert Opin Biol Ther. 2014;14:515-526.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399.
- Menon K, Van Voorhees V, Bebo B, et al. Psoriasis in patients with HIV infection: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Chiricozzi A, Saraceno R, Cannizzaro MV. Complete resolution of erythrodermic psoriasis in an HIV and HCV patient unresponsive to antipsoriatic treatments after highly active antiretroviral therapy. Dermatology. 2012;225:333-337.
- Barco D, Puig L, Alomar A. Treatment of moderate-severe psoriasis with etanercept in patients with chronic human immunodeficiency virus infection. Actas Dermosifiliogr. 2010;101(suppl 1):77-81.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV positive patient. J Drugs Dermatol. 2014;13:869-871.
- Rustin MH. Long-term safety of biologics in the treatment of moderate to severe plaque psoriasis: review of the current data. Br J Dermatol. 2012;167(suppl 3):3-11.
- Navarro R, Vilarrasa E, Herranz P, et al. Safety and effectiveness of ustekinumab and antitumour necrosis factor therapy in patients with psoriasis and chronic viral hepatitis B or C: a retrospective, multicentre study in a clinical setting. Br J Dermatol. 2013;168:609-616.
- Chiu HY, Chen CH, Wu MS, et al. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013;169:1295-1303.
- Weitz JE, Ritchlin CT. Ustekinumab: targeting the IL-17 pathway to improve outcomes in psoriatic arthritis. Expert Opin Biol Ther. 2014;14:515-526.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399.
Practice Points
- Psoriasis in patients with human immunodeficiency virus (HIV) tends to be more resistant to conventional therapies.
- Experience is limited in the use of immunosuppressants and biologics to treat psoriasis in HIV patients.
- Maintaining antiretroviral therapy and prophylaxis against opportunist disease is important in HIV patients who receive biologics, as is close monitoring of the viral load.
Residency Training During the #MeToo Movement
The #MeToo movement that took hold in the wake of the Harvey Weinstein allegations in 2017 likely will be considered one of the major cultural touchpoints of the 2010s. Although activism within the entertainment industry initially drew attention to this movement, it is understood that virtually no workplace is immune to sexual misconduct. Many medical professionals acknowledge #MeToo as a catchy hashtag summarizing a problem that has long been recognized in the field of medicine but often has been inadequately addressed.1 As dermatology residency program directors (PDs) at the University of Southern California (USC) Keck School of Medicine (Los Angeles, California), we have seen the considerable impact that recent high-profile allegations of sexual assault have had at our institution, leading us to take part in institutional and departmental initiatives and reflections that we believe have strengthened the culture within our residency program and positioned us to be proactive in addressing this critical issue.
Before we discuss the efforts to combat sexual misconduct and gender inequality at USC and within our dermatology department, it is worth reflecting on where we stand as a specialty with regard to gender representation. A recent JAMA Dermatology article reported that in 1970 only 10.8% of dermatology academic faculty were women but by 2018 that number had skyrocketed to 51.2%; however, in contrast to this overall increase, only 19.4% of dermatology department chairs in 2018 were women.2 Although we have made large strides as a field, this discrepancy indicates that we still have a long way to go to achieve gender equality.
Although dermatology as a specialty is working toward gender equality, we believe it is crucial to consider this issue in the context of the entire field of medicine, particularly because academic physicians and trainees often interface with a myriad of specialties. It is well known that women in medicine are more likely to be victims of sexual harassment or assault in the workplace and that subsequent issues with imposter syndrome and/or depression are more prevalent in female physicians.3,4 Gender inequality and sexism, among other factors, can make it difficult for women to obtain and maintain leadership positions and can negatively impact the culture of an academic institution in numerous downstream ways.
We also know that academic environments in medicine have a higher prevalence of gender equality issues than in private practice or in settings where medicine is practiced without trainees due to the hierarchical nature of training and the necessary differences in experience between trainees and faculty.3 Furthermore, because trainees form and solidify their professional identities during graduate medical education (GME) training, it is a prime time to emphasize the importance of gender equality and establish zero tolerance policies for workplace abuse and transgressions.5
The data and our personal experiences delineate a clear need for continued vigilance regarding gender equality issues both in dermatology as a specialty and in medicine in general. As PDs, we feel fortunate to have worked in conjunction with our GME committee and our dermatology department to solidify and create policies that work to promote a culture of gender equality. Herein, we will outline some of these efforts with the hope that other academic institutions may consider implementing these programs to protect members of their community from harassment, sexual violence, and gender discrimination.
Create a SAFE Committee
At the institutional level, our GME committee has created the SAFE (Safety, Fairness & Equity) committee under the leadership of Lawrence Opas, MD. The SAFE committee is headed by a female faculty physician and includes members of the medical community who have the influence to affect change and a commitment to protect vulnerable populations. Members include the Chief Medical Officer, the Designated Institutional Officer, the Director of Resident Wellness, and the Dean of the Keck School of Medicine at USC. The SAFE committee serves as a 24/7 reporting resource whereby trainees can report any issues relating to harassment in the workplace via a telephone hotline or online platform. Issues brought to this committee are immediately dealt with and reviewed at monthly GME meetings to keep institutional PDs up-to-date on issues pertaining to sexual harassment and assault within our workplace. The SAFE committee also has departmental resident liaisons who bring information to residents and help guide them to appropriate resources.
Emphasize Resident Wellness
Along with the development of robust reporting resources, our institution has continued to build upon a culture that places a strong emphasis on resident wellness. One of the most meaningful efforts over the last 5 years has included recruitment of a clinical psychologist, Tobi Fishel, PhD, to serve as our institution’s Director of Wellness. She is available to meet confidentially with our residents and helps to serve as a link between trainees and the GME committee.
Our dermatology department takes a tremendous amount of pride in its culture. We are fortunate to have David Peng, MD, MPH, Chair, and Stefani Takahashi, MD, Vice Chair of Education, working daily to create an environment that values teamwork, selflessness, and wellness. We have been continuously grateful for their leadership and guidance in addressing the allegations of sexual assault and harassment that arose at USC over the past several years. Our department has a zero tolerance policy for sexual harassment or harassment of any kind, and we have taken important steps to ensure and promote a safe environment for our trainees, many of which are focused on communication. We try to avoid assumptions and encourage both residents and faculty to explicitly state their experiences and opinions in general but also in relation to instances of potential misconduct.
Encourage Communication
When allegations of sexual misconduct in the workplace were made at our institution, we prioritized immediate in-person communication with our residents to reinforce our zero tolerance policy and to remind them that we are available should any similar issues arise in our department. It was of equal value to remind our trainees of potential resources, such as the SAFE committee, to whom they could bring their concerns if they were not comfortable communicating directly with us. Although we hoped that our trainees understood that we would not be tolerant of any form of harassment based on our past actions and communications, we felt that it was helpful to explicitly delineate this by laying out other avenues of support on a regular basis with them. By ensuring there is a space for a dialogue with others, if needed, our institution and department have provided an extra layer of security for our trainees. Multiple channels of support are crucial to ensure trainee safety.
Dr. Peng also created a workplace safety committee that includes several female faculty members. The committee regularly shares and highlights institutional and departmental resources as they pertain to gender equality and safety within the workplace and also has considerable faculty overlap with our departmental diversity committee. Together, these committees work toward the common goal of fostering an environment in which all members of our department feel comfortable voicing concerns, and we are best able to recruit and retain a diverse faculty.
As PDs, we work to reinforce departmental and institutional messages in our daily communication with residents. We have found that ensuring frequent and varied interactions—quarterly meetings, biannual evaluations, faculty-led didactics 2 half-days per week, and weekly clinical interactions—with our trainees can help to create a culture where they feel comfortable bringing up issues, be they routine clinical operations questions or issues relating to their professional identity. We hope it also has created the space for them to approach us with any issues pertaining to harassment should they ever arise, and we are grateful to know that even if this comfort does not exist, our institution and department have other resources for them.
Final Thoughts
Although some of the measures discussed here were reactionary, many predated the recent institutional concerns and allegations at USC. We hope and believe that the culture we foster within our department has helped our trainees feel safe and cared for during a time of institutional turbulence. We also believe that taking similar proactive measures may benefit the overall culture and foster the development of diverse physicians and leadership at other institutions. In conjunction with reworking legislation and implementing institutional safeguards, the long-term goals of taking these proactive measures are to promote gender equality and workplace safety and to cultivate and retain effective female leadership in medical institutions and training programs.
We feel incredibly fortunate to be part of a specialty in which gender equality has long been considered and sought after. We also are proud to be members of the Association of Professors of Dermatology, which has addressed issues such as diversity and gender equality in a transparent and head-on manner and continues to do so. As a specialty, we hope we can support our trainees in their professional growth and help to cultivate sensitive physicians who will care for an increasingly diverse population and better support each other in their own career development.
- Ladika S. Sexual harassment: health care, it is #youtoo. Manag Care. 2018;27:14-17.
- Xierali IM, Nivet MA, Pandya AG. US dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970 to 2018 [published online January 8, 2020]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.4297.
- Minkina N. Can #MeToo abolish sexual harassment and discrimination in medicine? Lancet. 2019;394:383-384.
- Dzau VJ, Johnson PA. Ending sexual harassment in academic medicine. N Engl J Med. 2018;379:1589-1591.
- Nothnagle M, Reis S, Goldman RE, et al. Fostering professional formation in residency: development and evaluation of the “forum” seminar series. Teach Learn Med. 2014;26:230-238.
The #MeToo movement that took hold in the wake of the Harvey Weinstein allegations in 2017 likely will be considered one of the major cultural touchpoints of the 2010s. Although activism within the entertainment industry initially drew attention to this movement, it is understood that virtually no workplace is immune to sexual misconduct. Many medical professionals acknowledge #MeToo as a catchy hashtag summarizing a problem that has long been recognized in the field of medicine but often has been inadequately addressed.1 As dermatology residency program directors (PDs) at the University of Southern California (USC) Keck School of Medicine (Los Angeles, California), we have seen the considerable impact that recent high-profile allegations of sexual assault have had at our institution, leading us to take part in institutional and departmental initiatives and reflections that we believe have strengthened the culture within our residency program and positioned us to be proactive in addressing this critical issue.
Before we discuss the efforts to combat sexual misconduct and gender inequality at USC and within our dermatology department, it is worth reflecting on where we stand as a specialty with regard to gender representation. A recent JAMA Dermatology article reported that in 1970 only 10.8% of dermatology academic faculty were women but by 2018 that number had skyrocketed to 51.2%; however, in contrast to this overall increase, only 19.4% of dermatology department chairs in 2018 were women.2 Although we have made large strides as a field, this discrepancy indicates that we still have a long way to go to achieve gender equality.
Although dermatology as a specialty is working toward gender equality, we believe it is crucial to consider this issue in the context of the entire field of medicine, particularly because academic physicians and trainees often interface with a myriad of specialties. It is well known that women in medicine are more likely to be victims of sexual harassment or assault in the workplace and that subsequent issues with imposter syndrome and/or depression are more prevalent in female physicians.3,4 Gender inequality and sexism, among other factors, can make it difficult for women to obtain and maintain leadership positions and can negatively impact the culture of an academic institution in numerous downstream ways.
We also know that academic environments in medicine have a higher prevalence of gender equality issues than in private practice or in settings where medicine is practiced without trainees due to the hierarchical nature of training and the necessary differences in experience between trainees and faculty.3 Furthermore, because trainees form and solidify their professional identities during graduate medical education (GME) training, it is a prime time to emphasize the importance of gender equality and establish zero tolerance policies for workplace abuse and transgressions.5
The data and our personal experiences delineate a clear need for continued vigilance regarding gender equality issues both in dermatology as a specialty and in medicine in general. As PDs, we feel fortunate to have worked in conjunction with our GME committee and our dermatology department to solidify and create policies that work to promote a culture of gender equality. Herein, we will outline some of these efforts with the hope that other academic institutions may consider implementing these programs to protect members of their community from harassment, sexual violence, and gender discrimination.
Create a SAFE Committee
At the institutional level, our GME committee has created the SAFE (Safety, Fairness & Equity) committee under the leadership of Lawrence Opas, MD. The SAFE committee is headed by a female faculty physician and includes members of the medical community who have the influence to affect change and a commitment to protect vulnerable populations. Members include the Chief Medical Officer, the Designated Institutional Officer, the Director of Resident Wellness, and the Dean of the Keck School of Medicine at USC. The SAFE committee serves as a 24/7 reporting resource whereby trainees can report any issues relating to harassment in the workplace via a telephone hotline or online platform. Issues brought to this committee are immediately dealt with and reviewed at monthly GME meetings to keep institutional PDs up-to-date on issues pertaining to sexual harassment and assault within our workplace. The SAFE committee also has departmental resident liaisons who bring information to residents and help guide them to appropriate resources.
Emphasize Resident Wellness
Along with the development of robust reporting resources, our institution has continued to build upon a culture that places a strong emphasis on resident wellness. One of the most meaningful efforts over the last 5 years has included recruitment of a clinical psychologist, Tobi Fishel, PhD, to serve as our institution’s Director of Wellness. She is available to meet confidentially with our residents and helps to serve as a link between trainees and the GME committee.
Our dermatology department takes a tremendous amount of pride in its culture. We are fortunate to have David Peng, MD, MPH, Chair, and Stefani Takahashi, MD, Vice Chair of Education, working daily to create an environment that values teamwork, selflessness, and wellness. We have been continuously grateful for their leadership and guidance in addressing the allegations of sexual assault and harassment that arose at USC over the past several years. Our department has a zero tolerance policy for sexual harassment or harassment of any kind, and we have taken important steps to ensure and promote a safe environment for our trainees, many of which are focused on communication. We try to avoid assumptions and encourage both residents and faculty to explicitly state their experiences and opinions in general but also in relation to instances of potential misconduct.
Encourage Communication
When allegations of sexual misconduct in the workplace were made at our institution, we prioritized immediate in-person communication with our residents to reinforce our zero tolerance policy and to remind them that we are available should any similar issues arise in our department. It was of equal value to remind our trainees of potential resources, such as the SAFE committee, to whom they could bring their concerns if they were not comfortable communicating directly with us. Although we hoped that our trainees understood that we would not be tolerant of any form of harassment based on our past actions and communications, we felt that it was helpful to explicitly delineate this by laying out other avenues of support on a regular basis with them. By ensuring there is a space for a dialogue with others, if needed, our institution and department have provided an extra layer of security for our trainees. Multiple channels of support are crucial to ensure trainee safety.
Dr. Peng also created a workplace safety committee that includes several female faculty members. The committee regularly shares and highlights institutional and departmental resources as they pertain to gender equality and safety within the workplace and also has considerable faculty overlap with our departmental diversity committee. Together, these committees work toward the common goal of fostering an environment in which all members of our department feel comfortable voicing concerns, and we are best able to recruit and retain a diverse faculty.
As PDs, we work to reinforce departmental and institutional messages in our daily communication with residents. We have found that ensuring frequent and varied interactions—quarterly meetings, biannual evaluations, faculty-led didactics 2 half-days per week, and weekly clinical interactions—with our trainees can help to create a culture where they feel comfortable bringing up issues, be they routine clinical operations questions or issues relating to their professional identity. We hope it also has created the space for them to approach us with any issues pertaining to harassment should they ever arise, and we are grateful to know that even if this comfort does not exist, our institution and department have other resources for them.
Final Thoughts
Although some of the measures discussed here were reactionary, many predated the recent institutional concerns and allegations at USC. We hope and believe that the culture we foster within our department has helped our trainees feel safe and cared for during a time of institutional turbulence. We also believe that taking similar proactive measures may benefit the overall culture and foster the development of diverse physicians and leadership at other institutions. In conjunction with reworking legislation and implementing institutional safeguards, the long-term goals of taking these proactive measures are to promote gender equality and workplace safety and to cultivate and retain effective female leadership in medical institutions and training programs.
We feel incredibly fortunate to be part of a specialty in which gender equality has long been considered and sought after. We also are proud to be members of the Association of Professors of Dermatology, which has addressed issues such as diversity and gender equality in a transparent and head-on manner and continues to do so. As a specialty, we hope we can support our trainees in their professional growth and help to cultivate sensitive physicians who will care for an increasingly diverse population and better support each other in their own career development.
The #MeToo movement that took hold in the wake of the Harvey Weinstein allegations in 2017 likely will be considered one of the major cultural touchpoints of the 2010s. Although activism within the entertainment industry initially drew attention to this movement, it is understood that virtually no workplace is immune to sexual misconduct. Many medical professionals acknowledge #MeToo as a catchy hashtag summarizing a problem that has long been recognized in the field of medicine but often has been inadequately addressed.1 As dermatology residency program directors (PDs) at the University of Southern California (USC) Keck School of Medicine (Los Angeles, California), we have seen the considerable impact that recent high-profile allegations of sexual assault have had at our institution, leading us to take part in institutional and departmental initiatives and reflections that we believe have strengthened the culture within our residency program and positioned us to be proactive in addressing this critical issue.
Before we discuss the efforts to combat sexual misconduct and gender inequality at USC and within our dermatology department, it is worth reflecting on where we stand as a specialty with regard to gender representation. A recent JAMA Dermatology article reported that in 1970 only 10.8% of dermatology academic faculty were women but by 2018 that number had skyrocketed to 51.2%; however, in contrast to this overall increase, only 19.4% of dermatology department chairs in 2018 were women.2 Although we have made large strides as a field, this discrepancy indicates that we still have a long way to go to achieve gender equality.
Although dermatology as a specialty is working toward gender equality, we believe it is crucial to consider this issue in the context of the entire field of medicine, particularly because academic physicians and trainees often interface with a myriad of specialties. It is well known that women in medicine are more likely to be victims of sexual harassment or assault in the workplace and that subsequent issues with imposter syndrome and/or depression are more prevalent in female physicians.3,4 Gender inequality and sexism, among other factors, can make it difficult for women to obtain and maintain leadership positions and can negatively impact the culture of an academic institution in numerous downstream ways.
We also know that academic environments in medicine have a higher prevalence of gender equality issues than in private practice or in settings where medicine is practiced without trainees due to the hierarchical nature of training and the necessary differences in experience between trainees and faculty.3 Furthermore, because trainees form and solidify their professional identities during graduate medical education (GME) training, it is a prime time to emphasize the importance of gender equality and establish zero tolerance policies for workplace abuse and transgressions.5
The data and our personal experiences delineate a clear need for continued vigilance regarding gender equality issues both in dermatology as a specialty and in medicine in general. As PDs, we feel fortunate to have worked in conjunction with our GME committee and our dermatology department to solidify and create policies that work to promote a culture of gender equality. Herein, we will outline some of these efforts with the hope that other academic institutions may consider implementing these programs to protect members of their community from harassment, sexual violence, and gender discrimination.
Create a SAFE Committee
At the institutional level, our GME committee has created the SAFE (Safety, Fairness & Equity) committee under the leadership of Lawrence Opas, MD. The SAFE committee is headed by a female faculty physician and includes members of the medical community who have the influence to affect change and a commitment to protect vulnerable populations. Members include the Chief Medical Officer, the Designated Institutional Officer, the Director of Resident Wellness, and the Dean of the Keck School of Medicine at USC. The SAFE committee serves as a 24/7 reporting resource whereby trainees can report any issues relating to harassment in the workplace via a telephone hotline or online platform. Issues brought to this committee are immediately dealt with and reviewed at monthly GME meetings to keep institutional PDs up-to-date on issues pertaining to sexual harassment and assault within our workplace. The SAFE committee also has departmental resident liaisons who bring information to residents and help guide them to appropriate resources.
Emphasize Resident Wellness
Along with the development of robust reporting resources, our institution has continued to build upon a culture that places a strong emphasis on resident wellness. One of the most meaningful efforts over the last 5 years has included recruitment of a clinical psychologist, Tobi Fishel, PhD, to serve as our institution’s Director of Wellness. She is available to meet confidentially with our residents and helps to serve as a link between trainees and the GME committee.
Our dermatology department takes a tremendous amount of pride in its culture. We are fortunate to have David Peng, MD, MPH, Chair, and Stefani Takahashi, MD, Vice Chair of Education, working daily to create an environment that values teamwork, selflessness, and wellness. We have been continuously grateful for their leadership and guidance in addressing the allegations of sexual assault and harassment that arose at USC over the past several years. Our department has a zero tolerance policy for sexual harassment or harassment of any kind, and we have taken important steps to ensure and promote a safe environment for our trainees, many of which are focused on communication. We try to avoid assumptions and encourage both residents and faculty to explicitly state their experiences and opinions in general but also in relation to instances of potential misconduct.
Encourage Communication
When allegations of sexual misconduct in the workplace were made at our institution, we prioritized immediate in-person communication with our residents to reinforce our zero tolerance policy and to remind them that we are available should any similar issues arise in our department. It was of equal value to remind our trainees of potential resources, such as the SAFE committee, to whom they could bring their concerns if they were not comfortable communicating directly with us. Although we hoped that our trainees understood that we would not be tolerant of any form of harassment based on our past actions and communications, we felt that it was helpful to explicitly delineate this by laying out other avenues of support on a regular basis with them. By ensuring there is a space for a dialogue with others, if needed, our institution and department have provided an extra layer of security for our trainees. Multiple channels of support are crucial to ensure trainee safety.
Dr. Peng also created a workplace safety committee that includes several female faculty members. The committee regularly shares and highlights institutional and departmental resources as they pertain to gender equality and safety within the workplace and also has considerable faculty overlap with our departmental diversity committee. Together, these committees work toward the common goal of fostering an environment in which all members of our department feel comfortable voicing concerns, and we are best able to recruit and retain a diverse faculty.
As PDs, we work to reinforce departmental and institutional messages in our daily communication with residents. We have found that ensuring frequent and varied interactions—quarterly meetings, biannual evaluations, faculty-led didactics 2 half-days per week, and weekly clinical interactions—with our trainees can help to create a culture where they feel comfortable bringing up issues, be they routine clinical operations questions or issues relating to their professional identity. We hope it also has created the space for them to approach us with any issues pertaining to harassment should they ever arise, and we are grateful to know that even if this comfort does not exist, our institution and department have other resources for them.
Final Thoughts
Although some of the measures discussed here were reactionary, many predated the recent institutional concerns and allegations at USC. We hope and believe that the culture we foster within our department has helped our trainees feel safe and cared for during a time of institutional turbulence. We also believe that taking similar proactive measures may benefit the overall culture and foster the development of diverse physicians and leadership at other institutions. In conjunction with reworking legislation and implementing institutional safeguards, the long-term goals of taking these proactive measures are to promote gender equality and workplace safety and to cultivate and retain effective female leadership in medical institutions and training programs.
We feel incredibly fortunate to be part of a specialty in which gender equality has long been considered and sought after. We also are proud to be members of the Association of Professors of Dermatology, which has addressed issues such as diversity and gender equality in a transparent and head-on manner and continues to do so. As a specialty, we hope we can support our trainees in their professional growth and help to cultivate sensitive physicians who will care for an increasingly diverse population and better support each other in their own career development.
- Ladika S. Sexual harassment: health care, it is #youtoo. Manag Care. 2018;27:14-17.
- Xierali IM, Nivet MA, Pandya AG. US dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970 to 2018 [published online January 8, 2020]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.4297.
- Minkina N. Can #MeToo abolish sexual harassment and discrimination in medicine? Lancet. 2019;394:383-384.
- Dzau VJ, Johnson PA. Ending sexual harassment in academic medicine. N Engl J Med. 2018;379:1589-1591.
- Nothnagle M, Reis S, Goldman RE, et al. Fostering professional formation in residency: development and evaluation of the “forum” seminar series. Teach Learn Med. 2014;26:230-238.
- Ladika S. Sexual harassment: health care, it is #youtoo. Manag Care. 2018;27:14-17.
- Xierali IM, Nivet MA, Pandya AG. US dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970 to 2018 [published online January 8, 2020]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.4297.
- Minkina N. Can #MeToo abolish sexual harassment and discrimination in medicine? Lancet. 2019;394:383-384.
- Dzau VJ, Johnson PA. Ending sexual harassment in academic medicine. N Engl J Med. 2018;379:1589-1591.
- Nothnagle M, Reis S, Goldman RE, et al. Fostering professional formation in residency: development and evaluation of the “forum” seminar series. Teach Learn Med. 2014;26:230-238.
Evaluating the Impact and Educational Value of YouTube Videos on Nail Biopsy Procedures
To the Editor:
Nail biopsy is an important surgical procedure for diagnosis of nail pathology. YouTube has become a potential instrument for physicians and patients to learn about medical procedures.1,2 However, the sources, content, and quality of the information available have not been fully studied or characterized. Our objective was to analyze the efficiency of information and quality of YouTube videos on nail biopsies. We hypothesized that the quality of patient education and physician training videos would be unrepresentative on YouTube.
The term nail biopsy was searched on January 29, 2019, and filtered by relevance and rating using the default YouTube algorithm. Data were collected from the top 40 hits for the search term and filter coupling. All videos were viewed and sorted for nail biopsy procedures, and then those videos were categorized as being produced by a physician or other health care provider. The US medical board status of each physician videographer was determined using the American Board of Medical Specialties database.3 DISCERN criteria for assessing consumer health care information4 were used to interpret the videos by researchers (S.I. and S.R.L.) in this study.
From the queried search term collection, only 10 videos (1,023,423 combined views) were analyzed in this study (eTable). Although the other resulting videos were tagged as nail biopsy, they were excluded due to irrelevant content (eg, patient blogs, PowerPoints, nail avulsions). The mean age of the videos was 4 years (range, 4 days to 11 years), with a mean video length of 3.30 minutes (range, 49 seconds to 9.03 minutes). Four of 10 videos were performed for longitudinal melanonychia, and 5 of 10 videos were performed for melanonychia, clinically consistent with subungual hematoma. Dermatologists, plastic surgeons, and podiatrists produced the majority of the nail biopsy videos. The overall mean DISCERN rating was 1.60/5.00 (range, 1–3), meaning that the quality of content on nail biopsies was poor. This low DISCERN score signifies poor consumer health information. Video 2 (published in 2015) received a DISCERN score of 2 and received almost 1 million views, which is likely because the specific channel has a well-established subscriber pool (4.9 million subscribers). The highest DISCERN score of 3, demonstrating a tangential shave biopsy, was given to video 4 (published in 2010) and only received about 17,400 views (56 subscribers). Additionally, many videos lacked important information about the procedure. For instance, only 3 of 10 videos demonstrated the anesthetic technique and only 5 videos showed repair methods.
YouTube is an electronic learning source for general information; however, the content and quality of information on nail biopsy is not updated, reliable, or abundant. Patients undergoing nail biopsies are unlikely to find reliable and comprehensible information on YouTube; thus, there is a strong need for patient education in this area. In addition, physicians who did not learn to perform a nail biopsy during training are unlikely to learn this procedure from YouTube. Therefore, there is an unmet need for an outlet that will provide updated reliable content on nail biopsies geared toward both patients and physicians.
- Kwok TM, Singla AA, Phang K, et al. YouTube as a source of patient information for varicose vein treatment options. J Vasc Surg Venous Lymphat Disord. 2017;5:238-243.
- Ward B, Ward M, Nicheporuck A, et al. Assessment of YouTube as an informative resource on facial plastic surgery procedures. JAMA Facial Plastic Surgery. 2019;21:75-76.
- American Board of Medical Specialties. Certification Matters. https://www.certificationmatters.org. Accessed February 7, 2020.
- The DISCERN Instrument. DISCERN Online. http://www.discern.org.uk/discern_instrument.php. Published October 1999. Accessed February 7, 2020.
To the Editor:
Nail biopsy is an important surgical procedure for diagnosis of nail pathology. YouTube has become a potential instrument for physicians and patients to learn about medical procedures.1,2 However, the sources, content, and quality of the information available have not been fully studied or characterized. Our objective was to analyze the efficiency of information and quality of YouTube videos on nail biopsies. We hypothesized that the quality of patient education and physician training videos would be unrepresentative on YouTube.
The term nail biopsy was searched on January 29, 2019, and filtered by relevance and rating using the default YouTube algorithm. Data were collected from the top 40 hits for the search term and filter coupling. All videos were viewed and sorted for nail biopsy procedures, and then those videos were categorized as being produced by a physician or other health care provider. The US medical board status of each physician videographer was determined using the American Board of Medical Specialties database.3 DISCERN criteria for assessing consumer health care information4 were used to interpret the videos by researchers (S.I. and S.R.L.) in this study.
From the queried search term collection, only 10 videos (1,023,423 combined views) were analyzed in this study (eTable). Although the other resulting videos were tagged as nail biopsy, they were excluded due to irrelevant content (eg, patient blogs, PowerPoints, nail avulsions). The mean age of the videos was 4 years (range, 4 days to 11 years), with a mean video length of 3.30 minutes (range, 49 seconds to 9.03 minutes). Four of 10 videos were performed for longitudinal melanonychia, and 5 of 10 videos were performed for melanonychia, clinically consistent with subungual hematoma. Dermatologists, plastic surgeons, and podiatrists produced the majority of the nail biopsy videos. The overall mean DISCERN rating was 1.60/5.00 (range, 1–3), meaning that the quality of content on nail biopsies was poor. This low DISCERN score signifies poor consumer health information. Video 2 (published in 2015) received a DISCERN score of 2 and received almost 1 million views, which is likely because the specific channel has a well-established subscriber pool (4.9 million subscribers). The highest DISCERN score of 3, demonstrating a tangential shave biopsy, was given to video 4 (published in 2010) and only received about 17,400 views (56 subscribers). Additionally, many videos lacked important information about the procedure. For instance, only 3 of 10 videos demonstrated the anesthetic technique and only 5 videos showed repair methods.
YouTube is an electronic learning source for general information; however, the content and quality of information on nail biopsy is not updated, reliable, or abundant. Patients undergoing nail biopsies are unlikely to find reliable and comprehensible information on YouTube; thus, there is a strong need for patient education in this area. In addition, physicians who did not learn to perform a nail biopsy during training are unlikely to learn this procedure from YouTube. Therefore, there is an unmet need for an outlet that will provide updated reliable content on nail biopsies geared toward both patients and physicians.
To the Editor:
Nail biopsy is an important surgical procedure for diagnosis of nail pathology. YouTube has become a potential instrument for physicians and patients to learn about medical procedures.1,2 However, the sources, content, and quality of the information available have not been fully studied or characterized. Our objective was to analyze the efficiency of information and quality of YouTube videos on nail biopsies. We hypothesized that the quality of patient education and physician training videos would be unrepresentative on YouTube.
The term nail biopsy was searched on January 29, 2019, and filtered by relevance and rating using the default YouTube algorithm. Data were collected from the top 40 hits for the search term and filter coupling. All videos were viewed and sorted for nail biopsy procedures, and then those videos were categorized as being produced by a physician or other health care provider. The US medical board status of each physician videographer was determined using the American Board of Medical Specialties database.3 DISCERN criteria for assessing consumer health care information4 were used to interpret the videos by researchers (S.I. and S.R.L.) in this study.
From the queried search term collection, only 10 videos (1,023,423 combined views) were analyzed in this study (eTable). Although the other resulting videos were tagged as nail biopsy, they were excluded due to irrelevant content (eg, patient blogs, PowerPoints, nail avulsions). The mean age of the videos was 4 years (range, 4 days to 11 years), with a mean video length of 3.30 minutes (range, 49 seconds to 9.03 minutes). Four of 10 videos were performed for longitudinal melanonychia, and 5 of 10 videos were performed for melanonychia, clinically consistent with subungual hematoma. Dermatologists, plastic surgeons, and podiatrists produced the majority of the nail biopsy videos. The overall mean DISCERN rating was 1.60/5.00 (range, 1–3), meaning that the quality of content on nail biopsies was poor. This low DISCERN score signifies poor consumer health information. Video 2 (published in 2015) received a DISCERN score of 2 and received almost 1 million views, which is likely because the specific channel has a well-established subscriber pool (4.9 million subscribers). The highest DISCERN score of 3, demonstrating a tangential shave biopsy, was given to video 4 (published in 2010) and only received about 17,400 views (56 subscribers). Additionally, many videos lacked important information about the procedure. For instance, only 3 of 10 videos demonstrated the anesthetic technique and only 5 videos showed repair methods.
YouTube is an electronic learning source for general information; however, the content and quality of information on nail biopsy is not updated, reliable, or abundant. Patients undergoing nail biopsies are unlikely to find reliable and comprehensible information on YouTube; thus, there is a strong need for patient education in this area. In addition, physicians who did not learn to perform a nail biopsy during training are unlikely to learn this procedure from YouTube. Therefore, there is an unmet need for an outlet that will provide updated reliable content on nail biopsies geared toward both patients and physicians.
- Kwok TM, Singla AA, Phang K, et al. YouTube as a source of patient information for varicose vein treatment options. J Vasc Surg Venous Lymphat Disord. 2017;5:238-243.
- Ward B, Ward M, Nicheporuck A, et al. Assessment of YouTube as an informative resource on facial plastic surgery procedures. JAMA Facial Plastic Surgery. 2019;21:75-76.
- American Board of Medical Specialties. Certification Matters. https://www.certificationmatters.org. Accessed February 7, 2020.
- The DISCERN Instrument. DISCERN Online. http://www.discern.org.uk/discern_instrument.php. Published October 1999. Accessed February 7, 2020.
- Kwok TM, Singla AA, Phang K, et al. YouTube as a source of patient information for varicose vein treatment options. J Vasc Surg Venous Lymphat Disord. 2017;5:238-243.
- Ward B, Ward M, Nicheporuck A, et al. Assessment of YouTube as an informative resource on facial plastic surgery procedures. JAMA Facial Plastic Surgery. 2019;21:75-76.
- American Board of Medical Specialties. Certification Matters. https://www.certificationmatters.org. Accessed February 7, 2020.
- The DISCERN Instrument. DISCERN Online. http://www.discern.org.uk/discern_instrument.php. Published October 1999. Accessed February 7, 2020.
Practice Points
- A nail biopsy is sometimes necessary for histopathologic confirmation of a clinical diagnosis.
- YouTube has become a potential educational platform for physicians and patients to learn about nail biopsy procedures.
- Physicians and patients interested in learning more about nail biopsies are unlikely to find reliable and comprehensible information on YouTube; therefore, there is a need for updated reliable video content on nail biopsies geared toward both physicians and patients.
Efficacy, Safety, and Tolerability of Halobetasol Propionate 0.01%–Tazarotene 0.045% Lotion for Moderate to Severe Plaque Psoriasis in the Hispanic Population: Post Hoc Analysis
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).
Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).
Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).
Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.
Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).
Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10
A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).
For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.
Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).
Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).
Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).
Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.
Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).
Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10
A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).
For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.
Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).
Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).
Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).
Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.
Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).
Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10
A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).
For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.
Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
Practice Points
- Although psoriasis is a common inflammatory disease, data in the Hispanic population are sparse and disease may be more severe.
- A recent clinical investigation with halobetasol propionate 0.01%–tazarotene 0.045% lotion included a number of Hispanic patients, affording an ideal opportunity to provide important data on this population.
- This fixed-combination therapy was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Concurrent Beau Lines, Onychomadesis, and Retronychia Following Scurvy
Beau lines are palpable transverse depressions on the dorsal aspect of the nail plate that result from a temporary slowing of nail plate production by the proximal nail matrix. Onychomadesis is a separation of the proximal nail plate from the distal nail plate leading to shedding of the nail. It occurs due to a complete growth arrest in the nail matrix and is thought to be on a continuum with Beau lines. The etiologies of these 2 conditions overlap and include trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.1-5 In almost all cases of both conditions, normal nail plate production ensues upon identification and removal of the inciting agent or recuperation from the causal illness.3,4,6 Beau lines will move distally as the nail grows out and can be clipped. In onychomadesis, the affected nails will be shed with time. Resolution of these nail defects can be estimated from average nail growth rates (1 mm/mo for fingernails and 2–3 mm/mo for toenails).7
Retronychia is defined as a proximal ingrowing of the nail plate into the ventral surface of the proximal nail fold.4,6 It is thought to occur via vertical progression of the nail plate into the proximal nail fold, repetitive nail matrix trauma, or shearing forces, resulting in inflammation that leads to nail plate stacking.8,9 Although conservative treatment using topical corticosteroids may be attempted, proximal nail plate avulsion typically is required for treatment.10
Braswell et al1 suggested a unifying hypothesis for a common pathophysiologic basis for these 3 conditions; that is, nail matrix injury results in slowing and/or cessation of nail plate production, followed by recommencement of nail plate production by the nail matrix after removal of the insult. We report a case of a patient presenting with concurrent Beau lines, onychomadesis, and retronychia following scurvy, thus supporting the hypothesis that these 3 nail conditions lie on a continuum.
Case Report
A 41-year-old woman with a history of thyroiditis, gastroesophageal reflux disease, endometriosis, osteoarthritis, gastric ulcer, pancreatitis, fatty liver, and polycystic ovarian syndrome presented with lines on the toenails and no growth of the right second toenail of several months’ duration. She denied any pain or prior trauma to the nails, participation in sports activities, or wearing tight or high-heeled shoes. She had presented 6 months prior for evaluation of perifollicular erythema on the posterior thighs, legs, and abdomen, as well as gingival bleeding.11 At that time, one of the authors (S.R.L.) found that she was vitamin C deficient, and a diagnosis of scurvy was made. The rash and gingival bleeding resolved with vitamin C supplementation.11
At the current presentation, physical examination revealed transverse grooves involving several fingernails but most evident on the left thumbnail (Figure, A). The grooves did not span the entire breadth of the nail, which was consistent with Beau lines. Several toenails had parallel transverse grooves spanning the entire width of the nail plate such that the proximal nail plate was discontinuous with the distal nail plate, which was consistent with onychomadesis (Figure, B). The right second toenail was yellow and thickened with layered nail plates, indicative of retronychia (Figure, B). Histopathology of a nail plate clipping from the right second toenail was negative for fungal hyphae, and a radiograph was negative for bony changes or exostosis.
Comment
The nail matrix is responsible for nail plate production, and the newly formed nail plate then moves outward over the nail bed. It is hypothesized that the pathophysiologic basis for Beau lines, onychomadesis, and retronychia lies on a continuum such that all 3 conditions are caused by an insult to the nail matrix that results in slowing and/or halting of nail plate growth. Beau lines result from slowing or disruption in cell growth from the nail matrix, whereas onychomadesis is associated with a complete halt in nail plate production.1,3 In retronychia, the new nail growing from the matrix pushes the old one upward, interrupting the longitudinal growth of the nail and leading to nail plate stacking.10
Our patient presented with concurrent Beau lines, onychomadesis, and retronychia. Although Beau lines and onychomadesis have been reported together in some instances,12-14 retronychia is not commonly reported with either of these conditions. The exact incidence of each condition has not been studied, but Beau lines are relatively common, onychomadesis is less common, and retronychia is seen infrequently; therefore, the concurrent presentation of these 3 conditions in the same patient is exceedingly rare. Thus, it was most likely that one etiology accounted for all 3 nail findings.
Because the patient had been diagnosed with scurvy 6 months prior to presentation, we hypothesized that the associated vitamin C deficiency caused a systemic insult to the nail matrix, which resulted in cessation of nail growth. The mechanism of nail matrix arrest in the setting of systemic disease is thought to be due to inhibition of cellular proliferation or a change in the quality of the newly manufactured nail plate, which becomes thinner and more dystrophic.15 Vitamin C (ascorbic acid) deficiency causes scurvy, which is characterized by cutaneous signs such as perifollicular hemorrhage and purpura, corkscrew hairs, bruising, gingivitis, arthralgia, and impaired wound healing.16 These clinical manifestations are due to impaired collagen synthesis and disordered connective tissue. Ascorbic acid also is involved in fatty acid transport, neurotransmitter synthesis, prostaglandin metabolism, and nitric oxide synthesis.17 Ascorbic acid has not been studied for its role in nail plate synthesis18; however, given the role that ascorbic acid plays in a myriad of biologic processes, the deficiency associated with scurvy likely had a considerable systemic effect in our patient that halted nail plate synthesis and resulted in the concurrent presentation of Beau lines, onychomadesis, and retronychia.
- Braswell MA, Daniel CR III, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Lipner SR. Onychomadesis following a fish pedicure. JAMA Dermatol. 2018;154:1091-1092.
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Lawry M, Daniel CR III. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:147-176.
- Lipner SR, Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385.
- Rich P. Nail signs and symptoms. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:1-6.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach H, et al, eds. Agache’s Measuring the Skin. Cham, Switzerland: Springer; 2017:1-15.
- de Berker DA, Richert B, Duhard E, et al. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol. 2008;58:978-983.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Piraccini BM, Richert B, de Berker DA, et al. Retronychia in children, adolescents, and young adults: a case series. J Am Acad Dermatol. 2014;70:388-390.
- Lipner S. A classic case of scurvy. Lancet. 2018;392:431.
- Jacobsen L, Zimmerman S, Lohr J. Nail findings in hand-foot-and-mouth disease. Pediatr Infect Dis J. 2015;34:449-450.
- Damevska K, Gocev G, Pollozhani N, et al. Onychomadesis following cutaneous vasculitis. Acta Dermatovenerol Croat. 2017;25:77-79.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand‐foot‐mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Weismann K. J.H.S Beau and his descriptions of transverse depressions on nails. Br J Dermatol. 1977;97:571-572.
- Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid). Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499877/. Updated October 21, 2019. Accessed February 24, 2020.
- Pazirandeh S, Burns DL. Overview of water-soluble vitamins. UpToDate. https://www.uptodate.com/contents/overview-of-water-soluble-vitamins. Updated January 29, 2020. Accessed February 24, 2020.
- Scheinfeld N, Dahdah MJ, Scher RK. Vitamins and minerals: their role in nail health and disease. J Drugs Dermatol. 2007;6:782-787.
Beau lines are palpable transverse depressions on the dorsal aspect of the nail plate that result from a temporary slowing of nail plate production by the proximal nail matrix. Onychomadesis is a separation of the proximal nail plate from the distal nail plate leading to shedding of the nail. It occurs due to a complete growth arrest in the nail matrix and is thought to be on a continuum with Beau lines. The etiologies of these 2 conditions overlap and include trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.1-5 In almost all cases of both conditions, normal nail plate production ensues upon identification and removal of the inciting agent or recuperation from the causal illness.3,4,6 Beau lines will move distally as the nail grows out and can be clipped. In onychomadesis, the affected nails will be shed with time. Resolution of these nail defects can be estimated from average nail growth rates (1 mm/mo for fingernails and 2–3 mm/mo for toenails).7
Retronychia is defined as a proximal ingrowing of the nail plate into the ventral surface of the proximal nail fold.4,6 It is thought to occur via vertical progression of the nail plate into the proximal nail fold, repetitive nail matrix trauma, or shearing forces, resulting in inflammation that leads to nail plate stacking.8,9 Although conservative treatment using topical corticosteroids may be attempted, proximal nail plate avulsion typically is required for treatment.10
Braswell et al1 suggested a unifying hypothesis for a common pathophysiologic basis for these 3 conditions; that is, nail matrix injury results in slowing and/or cessation of nail plate production, followed by recommencement of nail plate production by the nail matrix after removal of the insult. We report a case of a patient presenting with concurrent Beau lines, onychomadesis, and retronychia following scurvy, thus supporting the hypothesis that these 3 nail conditions lie on a continuum.
Case Report
A 41-year-old woman with a history of thyroiditis, gastroesophageal reflux disease, endometriosis, osteoarthritis, gastric ulcer, pancreatitis, fatty liver, and polycystic ovarian syndrome presented with lines on the toenails and no growth of the right second toenail of several months’ duration. She denied any pain or prior trauma to the nails, participation in sports activities, or wearing tight or high-heeled shoes. She had presented 6 months prior for evaluation of perifollicular erythema on the posterior thighs, legs, and abdomen, as well as gingival bleeding.11 At that time, one of the authors (S.R.L.) found that she was vitamin C deficient, and a diagnosis of scurvy was made. The rash and gingival bleeding resolved with vitamin C supplementation.11
At the current presentation, physical examination revealed transverse grooves involving several fingernails but most evident on the left thumbnail (Figure, A). The grooves did not span the entire breadth of the nail, which was consistent with Beau lines. Several toenails had parallel transverse grooves spanning the entire width of the nail plate such that the proximal nail plate was discontinuous with the distal nail plate, which was consistent with onychomadesis (Figure, B). The right second toenail was yellow and thickened with layered nail plates, indicative of retronychia (Figure, B). Histopathology of a nail plate clipping from the right second toenail was negative for fungal hyphae, and a radiograph was negative for bony changes or exostosis.
Comment
The nail matrix is responsible for nail plate production, and the newly formed nail plate then moves outward over the nail bed. It is hypothesized that the pathophysiologic basis for Beau lines, onychomadesis, and retronychia lies on a continuum such that all 3 conditions are caused by an insult to the nail matrix that results in slowing and/or halting of nail plate growth. Beau lines result from slowing or disruption in cell growth from the nail matrix, whereas onychomadesis is associated with a complete halt in nail plate production.1,3 In retronychia, the new nail growing from the matrix pushes the old one upward, interrupting the longitudinal growth of the nail and leading to nail plate stacking.10
Our patient presented with concurrent Beau lines, onychomadesis, and retronychia. Although Beau lines and onychomadesis have been reported together in some instances,12-14 retronychia is not commonly reported with either of these conditions. The exact incidence of each condition has not been studied, but Beau lines are relatively common, onychomadesis is less common, and retronychia is seen infrequently; therefore, the concurrent presentation of these 3 conditions in the same patient is exceedingly rare. Thus, it was most likely that one etiology accounted for all 3 nail findings.
Because the patient had been diagnosed with scurvy 6 months prior to presentation, we hypothesized that the associated vitamin C deficiency caused a systemic insult to the nail matrix, which resulted in cessation of nail growth. The mechanism of nail matrix arrest in the setting of systemic disease is thought to be due to inhibition of cellular proliferation or a change in the quality of the newly manufactured nail plate, which becomes thinner and more dystrophic.15 Vitamin C (ascorbic acid) deficiency causes scurvy, which is characterized by cutaneous signs such as perifollicular hemorrhage and purpura, corkscrew hairs, bruising, gingivitis, arthralgia, and impaired wound healing.16 These clinical manifestations are due to impaired collagen synthesis and disordered connective tissue. Ascorbic acid also is involved in fatty acid transport, neurotransmitter synthesis, prostaglandin metabolism, and nitric oxide synthesis.17 Ascorbic acid has not been studied for its role in nail plate synthesis18; however, given the role that ascorbic acid plays in a myriad of biologic processes, the deficiency associated with scurvy likely had a considerable systemic effect in our patient that halted nail plate synthesis and resulted in the concurrent presentation of Beau lines, onychomadesis, and retronychia.
Beau lines are palpable transverse depressions on the dorsal aspect of the nail plate that result from a temporary slowing of nail plate production by the proximal nail matrix. Onychomadesis is a separation of the proximal nail plate from the distal nail plate leading to shedding of the nail. It occurs due to a complete growth arrest in the nail matrix and is thought to be on a continuum with Beau lines. The etiologies of these 2 conditions overlap and include trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.1-5 In almost all cases of both conditions, normal nail plate production ensues upon identification and removal of the inciting agent or recuperation from the causal illness.3,4,6 Beau lines will move distally as the nail grows out and can be clipped. In onychomadesis, the affected nails will be shed with time. Resolution of these nail defects can be estimated from average nail growth rates (1 mm/mo for fingernails and 2–3 mm/mo for toenails).7
Retronychia is defined as a proximal ingrowing of the nail plate into the ventral surface of the proximal nail fold.4,6 It is thought to occur via vertical progression of the nail plate into the proximal nail fold, repetitive nail matrix trauma, or shearing forces, resulting in inflammation that leads to nail plate stacking.8,9 Although conservative treatment using topical corticosteroids may be attempted, proximal nail plate avulsion typically is required for treatment.10
Braswell et al1 suggested a unifying hypothesis for a common pathophysiologic basis for these 3 conditions; that is, nail matrix injury results in slowing and/or cessation of nail plate production, followed by recommencement of nail plate production by the nail matrix after removal of the insult. We report a case of a patient presenting with concurrent Beau lines, onychomadesis, and retronychia following scurvy, thus supporting the hypothesis that these 3 nail conditions lie on a continuum.
Case Report
A 41-year-old woman with a history of thyroiditis, gastroesophageal reflux disease, endometriosis, osteoarthritis, gastric ulcer, pancreatitis, fatty liver, and polycystic ovarian syndrome presented with lines on the toenails and no growth of the right second toenail of several months’ duration. She denied any pain or prior trauma to the nails, participation in sports activities, or wearing tight or high-heeled shoes. She had presented 6 months prior for evaluation of perifollicular erythema on the posterior thighs, legs, and abdomen, as well as gingival bleeding.11 At that time, one of the authors (S.R.L.) found that she was vitamin C deficient, and a diagnosis of scurvy was made. The rash and gingival bleeding resolved with vitamin C supplementation.11
At the current presentation, physical examination revealed transverse grooves involving several fingernails but most evident on the left thumbnail (Figure, A). The grooves did not span the entire breadth of the nail, which was consistent with Beau lines. Several toenails had parallel transverse grooves spanning the entire width of the nail plate such that the proximal nail plate was discontinuous with the distal nail plate, which was consistent with onychomadesis (Figure, B). The right second toenail was yellow and thickened with layered nail plates, indicative of retronychia (Figure, B). Histopathology of a nail plate clipping from the right second toenail was negative for fungal hyphae, and a radiograph was negative for bony changes or exostosis.
Comment
The nail matrix is responsible for nail plate production, and the newly formed nail plate then moves outward over the nail bed. It is hypothesized that the pathophysiologic basis for Beau lines, onychomadesis, and retronychia lies on a continuum such that all 3 conditions are caused by an insult to the nail matrix that results in slowing and/or halting of nail plate growth. Beau lines result from slowing or disruption in cell growth from the nail matrix, whereas onychomadesis is associated with a complete halt in nail plate production.1,3 In retronychia, the new nail growing from the matrix pushes the old one upward, interrupting the longitudinal growth of the nail and leading to nail plate stacking.10
Our patient presented with concurrent Beau lines, onychomadesis, and retronychia. Although Beau lines and onychomadesis have been reported together in some instances,12-14 retronychia is not commonly reported with either of these conditions. The exact incidence of each condition has not been studied, but Beau lines are relatively common, onychomadesis is less common, and retronychia is seen infrequently; therefore, the concurrent presentation of these 3 conditions in the same patient is exceedingly rare. Thus, it was most likely that one etiology accounted for all 3 nail findings.
Because the patient had been diagnosed with scurvy 6 months prior to presentation, we hypothesized that the associated vitamin C deficiency caused a systemic insult to the nail matrix, which resulted in cessation of nail growth. The mechanism of nail matrix arrest in the setting of systemic disease is thought to be due to inhibition of cellular proliferation or a change in the quality of the newly manufactured nail plate, which becomes thinner and more dystrophic.15 Vitamin C (ascorbic acid) deficiency causes scurvy, which is characterized by cutaneous signs such as perifollicular hemorrhage and purpura, corkscrew hairs, bruising, gingivitis, arthralgia, and impaired wound healing.16 These clinical manifestations are due to impaired collagen synthesis and disordered connective tissue. Ascorbic acid also is involved in fatty acid transport, neurotransmitter synthesis, prostaglandin metabolism, and nitric oxide synthesis.17 Ascorbic acid has not been studied for its role in nail plate synthesis18; however, given the role that ascorbic acid plays in a myriad of biologic processes, the deficiency associated with scurvy likely had a considerable systemic effect in our patient that halted nail plate synthesis and resulted in the concurrent presentation of Beau lines, onychomadesis, and retronychia.
- Braswell MA, Daniel CR III, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Lipner SR. Onychomadesis following a fish pedicure. JAMA Dermatol. 2018;154:1091-1092.
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Lawry M, Daniel CR III. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:147-176.
- Lipner SR, Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385.
- Rich P. Nail signs and symptoms. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:1-6.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach H, et al, eds. Agache’s Measuring the Skin. Cham, Switzerland: Springer; 2017:1-15.
- de Berker DA, Richert B, Duhard E, et al. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol. 2008;58:978-983.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Piraccini BM, Richert B, de Berker DA, et al. Retronychia in children, adolescents, and young adults: a case series. J Am Acad Dermatol. 2014;70:388-390.
- Lipner S. A classic case of scurvy. Lancet. 2018;392:431.
- Jacobsen L, Zimmerman S, Lohr J. Nail findings in hand-foot-and-mouth disease. Pediatr Infect Dis J. 2015;34:449-450.
- Damevska K, Gocev G, Pollozhani N, et al. Onychomadesis following cutaneous vasculitis. Acta Dermatovenerol Croat. 2017;25:77-79.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand‐foot‐mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Weismann K. J.H.S Beau and his descriptions of transverse depressions on nails. Br J Dermatol. 1977;97:571-572.
- Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid). Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499877/. Updated October 21, 2019. Accessed February 24, 2020.
- Pazirandeh S, Burns DL. Overview of water-soluble vitamins. UpToDate. https://www.uptodate.com/contents/overview-of-water-soluble-vitamins. Updated January 29, 2020. Accessed February 24, 2020.
- Scheinfeld N, Dahdah MJ, Scher RK. Vitamins and minerals: their role in nail health and disease. J Drugs Dermatol. 2007;6:782-787.
- Braswell MA, Daniel CR III, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Lipner SR. Onychomadesis following a fish pedicure. JAMA Dermatol. 2018;154:1091-1092.
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Lawry M, Daniel CR III. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:147-176.
- Lipner SR, Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385.
- Rich P. Nail signs and symptoms. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:1-6.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach H, et al, eds. Agache’s Measuring the Skin. Cham, Switzerland: Springer; 2017:1-15.
- de Berker DA, Richert B, Duhard E, et al. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol. 2008;58:978-983.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Piraccini BM, Richert B, de Berker DA, et al. Retronychia in children, adolescents, and young adults: a case series. J Am Acad Dermatol. 2014;70:388-390.
- Lipner S. A classic case of scurvy. Lancet. 2018;392:431.
- Jacobsen L, Zimmerman S, Lohr J. Nail findings in hand-foot-and-mouth disease. Pediatr Infect Dis J. 2015;34:449-450.
- Damevska K, Gocev G, Pollozhani N, et al. Onychomadesis following cutaneous vasculitis. Acta Dermatovenerol Croat. 2017;25:77-79.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand‐foot‐mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Weismann K. J.H.S Beau and his descriptions of transverse depressions on nails. Br J Dermatol. 1977;97:571-572.
- Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid). Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499877/. Updated October 21, 2019. Accessed February 24, 2020.
- Pazirandeh S, Burns DL. Overview of water-soluble vitamins. UpToDate. https://www.uptodate.com/contents/overview-of-water-soluble-vitamins. Updated January 29, 2020. Accessed February 24, 2020.
- Scheinfeld N, Dahdah MJ, Scher RK. Vitamins and minerals: their role in nail health and disease. J Drugs Dermatol. 2007;6:782-787.
Practice Points
- Beau lines, onychomadesis, and retronychia are nail conditions with distinct clinical findings.
- Beau lines and onychomadesis may be seen concurrently following trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.
- Retronychia shares a common pathophysiology with Beau lines and onychomadesis, and all reflect slowing or cessation of nail plate production.
Granulomatous Reaction After Cholla Cactus Spine Injury
Skin injuries caused by spines of various species of cactus are common in the southwestern United States and Mexico and have been described worldwide.1 Effects of injury vary depending on localization, surface extension, and skin conditions (eg, preexisting erosions, ulcerations, sunburns).
Case Report
A 22-year-old woman presented to the outpatient department with extremely painful, erythematous papules on the second, third, and fourth fingers of the left hand, as well as diffuse swelling of the entire metacarpophalangeal and interphalangeal joints (Figure 1). She reported accidentally falling on a cholla cactus (genus Cylindropuntia) 2 weeks earlier while walking on a cholla cactus trail during a vacation in California. She reported that the symptoms had worsened over the last week. Class 3 corticosteroid ointments did not provide benefit. The patient had no comorbidities and was allergic to penicillin.
Radiographs of the left hand excluded concomitant fracture. Digital dermoscopy showed multiple white homogeneous areas with a central pustule (Figure 2A). Frequency-domain optical coherence tomography (OCT) displayed round hyperrefractive structures in the dermis suggestive of granulomas, as well as a small needlelike hyperrefractive structure, a foreign body (Figure 2B).
The few visible spines were immediately removed with tweezers; the patient remained symptom free for approximately 2 weeks. Subsequently, extreme pain developed in the left hand; the clinical presentation and pain did not respond to empiric intravenous antibiotic therapy with weight-calculated clarithromycin (500 mg twice daily), systemic analgesia with nonsteroidal anti-inflammatory drugs, and local therapy with antiseptics and class 3 corticosteroid ointment. Four days later, all 27 papules were excised with 3- and 4-mm punch biopsies using digital nerve blocks. Histology showed classic foreign body granulomas with hematoxylin and eosin stain (Figure 2C).
One week later, pain, erythema, and swelling had disappeared; no additional lesions had developed (Figure 3). Follow-up OCT showed no foreign bodies. At 4-week follow-up, the inflammatory component had disappeared, and no granulomas were evident. Six months later, the lesions healed with minimal scarring that could later be treated with fractional laser therapy (Figure 4).
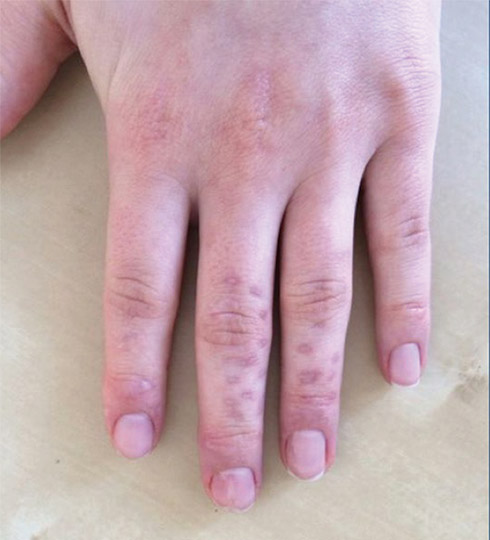
Comment
Pathogenesis and Presentation
Cactus spines are included in the possible causes of foreign body granulomas of the skin (eTable).2,3 However, granulomatous inflammation after cactus spine injury rarely has been described in the medical literature. In the first known case report in 1955, Winer and Zeilenga4 described a woman who developed multiple hand granulomas that were partially removed by curettage, while the spines underwent slow spontaneous expulsion.
In 1971, Schreiber et al5 hypothesized a type 2 allergic response to cactus spines based on the variability of reactions in different cases. Doctoroff et al6 proposed an unroofing technique based on the removal of spines under microscopy, which brought faster (2–4 months) healing. Madkan et al7 reported that complete response is possible only with punch excision of the largest lesions.
The cholla (Cylindropuntia) cactus has been described as the species most commonly implicated in granulomatous reactions to cactus spines.8,9 Two principal pathogenic mechanisms have been described—foreign body granuloma and allergic reaction to cactus antigens—because not every patient develops granulomatous lesions.
Sequelae
Complications of injury from cactus spines are common, especially when spines are not completely removed, including local inflammation, superinfection, necrosis, allergic reactions, granulomas, scarring, and chronic pain. Rare consequences of cactus injury include bacterial infection with Staphylococcus aureus; Enterobacter species; atypical mycobacteria, including Mycobacterium marinum; Nocardia species; and Clostridium tetani, as well as deep fungal infection, especially in immunocompromised patients.10 In our case, bacterial culture and polymerase chain reaction testing for mycobacteria were negative.
Diagnosis
Cactus spine injuries usually are easy to diagnose based on the clear-cut anamnesis and clinical picture; however, it might be interesting to assess the presence of foreign body granulomas without biopsy. Optical coherence tomography is a noninvasive optical imaging technique based on low-coherence interferometry that uses a low-intensity, 1310-nm infrared laser. Widespread in ophthalmology, OCT has gained importance in dermatologic diagnostics, especially for nonmelanoma skin cancer.11 Moreover, it has demonstrated its usefulness in various dermatologic fields, including granulomatous lesions.12 Further methods include reflectance confocal microscopy, based on a near-infrared laser, and 7.5-MHz ultrasonography. In our experience, however, 7.5-MHz ultrasonography has been ineffective in detecting cactus spines in the current patient as well as others. Preoperative and postoperative monitoring with dermoscopy and OCT helped us evaluate the nature, size, and location of spines and lesions and effective healing.
Treatment
Management strategies are still debated and include watchful waiting, corticosteroid ointment, partial removal of spines, and unroofing.1,2,4-10,13-18 We treated our patient with an innovative radical surgical approach using punch excision for granulomas that developed after cholla cactus spine injury. Our approach resulted in rapid relief of pain and reduced complications, a good aesthetic result, and no recurrence.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523. - Patterson JW. Weedon’s Skin Pathology. 4th ed. New York, NY: Elsevier; 2016.
- Winer LH, Zeilenga RH. Cactus granulomas of the skin; report of a case. AMA Arch Derm. 1955;72:566-569.
- Schreiber MM, Shapiro SI, Berry CZ. Cactus granulomas of the skin. an allergic phenomenon. Arch Dermatol. 1971;104:374-379.
- Doctoroff A, Vidimos AT, Taylor JS. Cactus skin injuries. Cutis. 2000;65:290-292.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Burrell SR, Ostlie DJ, Saubolle M, et al. Apophysomyces elegans infection associated with cactus spine injury in an immunocompetent pediatric patient. Pediatr Infect Dis J. 1998;17:663-664.
- von BraunmT. Optical coherence tomography. Hautarzt. 2015;66:499-503.
- Banzhaf C, Jemec GB. Imaging granulomatous lesions with optical coherence tomography. Case Rep Dermatol. 2012;4:14-18.
- Putnam MH. Simple cactus spine removal. J Pediatr. 1981;98:333.
- Snyder RA, Schwartz RA. Cactus bristle implantation. Report of an unusual case initially seen with rows of yellow hairs. Arch Dermatol. 1983;119:152-154.
- Schunk JE, Corneli HM. Cactus spine removal. J Pediatr. 1987;110:667.
- Gutierrez Ortega MC, Martin Moreno L, Arias Palomo D, et al. Facial granuloma caused by cactus bristles. Med Cutan Ibero Lat Am. 1990;18:197-200.
- Dieter RA Jr, Whitehouse LR, Gulliver R. Cactus spine wounds: a case report and short review of the literature. Wounds. 2017;29:E18-E21.
- O’Neill PJ, Sinha M, McArthur RA, et al. Penetrating cactus spine injury to the mediastinum of a child. J Pediatr Surg. 2008;43:E33-E35.
Skin injuries caused by spines of various species of cactus are common in the southwestern United States and Mexico and have been described worldwide.1 Effects of injury vary depending on localization, surface extension, and skin conditions (eg, preexisting erosions, ulcerations, sunburns).
Case Report
A 22-year-old woman presented to the outpatient department with extremely painful, erythematous papules on the second, third, and fourth fingers of the left hand, as well as diffuse swelling of the entire metacarpophalangeal and interphalangeal joints (Figure 1). She reported accidentally falling on a cholla cactus (genus Cylindropuntia) 2 weeks earlier while walking on a cholla cactus trail during a vacation in California. She reported that the symptoms had worsened over the last week. Class 3 corticosteroid ointments did not provide benefit. The patient had no comorbidities and was allergic to penicillin.
Radiographs of the left hand excluded concomitant fracture. Digital dermoscopy showed multiple white homogeneous areas with a central pustule (Figure 2A). Frequency-domain optical coherence tomography (OCT) displayed round hyperrefractive structures in the dermis suggestive of granulomas, as well as a small needlelike hyperrefractive structure, a foreign body (Figure 2B).
The few visible spines were immediately removed with tweezers; the patient remained symptom free for approximately 2 weeks. Subsequently, extreme pain developed in the left hand; the clinical presentation and pain did not respond to empiric intravenous antibiotic therapy with weight-calculated clarithromycin (500 mg twice daily), systemic analgesia with nonsteroidal anti-inflammatory drugs, and local therapy with antiseptics and class 3 corticosteroid ointment. Four days later, all 27 papules were excised with 3- and 4-mm punch biopsies using digital nerve blocks. Histology showed classic foreign body granulomas with hematoxylin and eosin stain (Figure 2C).
One week later, pain, erythema, and swelling had disappeared; no additional lesions had developed (Figure 3). Follow-up OCT showed no foreign bodies. At 4-week follow-up, the inflammatory component had disappeared, and no granulomas were evident. Six months later, the lesions healed with minimal scarring that could later be treated with fractional laser therapy (Figure 4).

Comment
Pathogenesis and Presentation
Cactus spines are included in the possible causes of foreign body granulomas of the skin (eTable).2,3 However, granulomatous inflammation after cactus spine injury rarely has been described in the medical literature. In the first known case report in 1955, Winer and Zeilenga4 described a woman who developed multiple hand granulomas that were partially removed by curettage, while the spines underwent slow spontaneous expulsion.
In 1971, Schreiber et al5 hypothesized a type 2 allergic response to cactus spines based on the variability of reactions in different cases. Doctoroff et al6 proposed an unroofing technique based on the removal of spines under microscopy, which brought faster (2–4 months) healing. Madkan et al7 reported that complete response is possible only with punch excision of the largest lesions.
The cholla (Cylindropuntia) cactus has been described as the species most commonly implicated in granulomatous reactions to cactus spines.8,9 Two principal pathogenic mechanisms have been described—foreign body granuloma and allergic reaction to cactus antigens—because not every patient develops granulomatous lesions.
Sequelae
Complications of injury from cactus spines are common, especially when spines are not completely removed, including local inflammation, superinfection, necrosis, allergic reactions, granulomas, scarring, and chronic pain. Rare consequences of cactus injury include bacterial infection with Staphylococcus aureus; Enterobacter species; atypical mycobacteria, including Mycobacterium marinum; Nocardia species; and Clostridium tetani, as well as deep fungal infection, especially in immunocompromised patients.10 In our case, bacterial culture and polymerase chain reaction testing for mycobacteria were negative.
Diagnosis
Cactus spine injuries usually are easy to diagnose based on the clear-cut anamnesis and clinical picture; however, it might be interesting to assess the presence of foreign body granulomas without biopsy. Optical coherence tomography is a noninvasive optical imaging technique based on low-coherence interferometry that uses a low-intensity, 1310-nm infrared laser. Widespread in ophthalmology, OCT has gained importance in dermatologic diagnostics, especially for nonmelanoma skin cancer.11 Moreover, it has demonstrated its usefulness in various dermatologic fields, including granulomatous lesions.12 Further methods include reflectance confocal microscopy, based on a near-infrared laser, and 7.5-MHz ultrasonography. In our experience, however, 7.5-MHz ultrasonography has been ineffective in detecting cactus spines in the current patient as well as others. Preoperative and postoperative monitoring with dermoscopy and OCT helped us evaluate the nature, size, and location of spines and lesions and effective healing.
Treatment
Management strategies are still debated and include watchful waiting, corticosteroid ointment, partial removal of spines, and unroofing.1,2,4-10,13-18 We treated our patient with an innovative radical surgical approach using punch excision for granulomas that developed after cholla cactus spine injury. Our approach resulted in rapid relief of pain and reduced complications, a good aesthetic result, and no recurrence.
Skin injuries caused by spines of various species of cactus are common in the southwestern United States and Mexico and have been described worldwide.1 Effects of injury vary depending on localization, surface extension, and skin conditions (eg, preexisting erosions, ulcerations, sunburns).
Case Report
A 22-year-old woman presented to the outpatient department with extremely painful, erythematous papules on the second, third, and fourth fingers of the left hand, as well as diffuse swelling of the entire metacarpophalangeal and interphalangeal joints (Figure 1). She reported accidentally falling on a cholla cactus (genus Cylindropuntia) 2 weeks earlier while walking on a cholla cactus trail during a vacation in California. She reported that the symptoms had worsened over the last week. Class 3 corticosteroid ointments did not provide benefit. The patient had no comorbidities and was allergic to penicillin.
Radiographs of the left hand excluded concomitant fracture. Digital dermoscopy showed multiple white homogeneous areas with a central pustule (Figure 2A). Frequency-domain optical coherence tomography (OCT) displayed round hyperrefractive structures in the dermis suggestive of granulomas, as well as a small needlelike hyperrefractive structure, a foreign body (Figure 2B).
The few visible spines were immediately removed with tweezers; the patient remained symptom free for approximately 2 weeks. Subsequently, extreme pain developed in the left hand; the clinical presentation and pain did not respond to empiric intravenous antibiotic therapy with weight-calculated clarithromycin (500 mg twice daily), systemic analgesia with nonsteroidal anti-inflammatory drugs, and local therapy with antiseptics and class 3 corticosteroid ointment. Four days later, all 27 papules were excised with 3- and 4-mm punch biopsies using digital nerve blocks. Histology showed classic foreign body granulomas with hematoxylin and eosin stain (Figure 2C).
One week later, pain, erythema, and swelling had disappeared; no additional lesions had developed (Figure 3). Follow-up OCT showed no foreign bodies. At 4-week follow-up, the inflammatory component had disappeared, and no granulomas were evident. Six months later, the lesions healed with minimal scarring that could later be treated with fractional laser therapy (Figure 4).

Comment
Pathogenesis and Presentation
Cactus spines are included in the possible causes of foreign body granulomas of the skin (eTable).2,3 However, granulomatous inflammation after cactus spine injury rarely has been described in the medical literature. In the first known case report in 1955, Winer and Zeilenga4 described a woman who developed multiple hand granulomas that were partially removed by curettage, while the spines underwent slow spontaneous expulsion.
In 1971, Schreiber et al5 hypothesized a type 2 allergic response to cactus spines based on the variability of reactions in different cases. Doctoroff et al6 proposed an unroofing technique based on the removal of spines under microscopy, which brought faster (2–4 months) healing. Madkan et al7 reported that complete response is possible only with punch excision of the largest lesions.
The cholla (Cylindropuntia) cactus has been described as the species most commonly implicated in granulomatous reactions to cactus spines.8,9 Two principal pathogenic mechanisms have been described—foreign body granuloma and allergic reaction to cactus antigens—because not every patient develops granulomatous lesions.
Sequelae
Complications of injury from cactus spines are common, especially when spines are not completely removed, including local inflammation, superinfection, necrosis, allergic reactions, granulomas, scarring, and chronic pain. Rare consequences of cactus injury include bacterial infection with Staphylococcus aureus; Enterobacter species; atypical mycobacteria, including Mycobacterium marinum; Nocardia species; and Clostridium tetani, as well as deep fungal infection, especially in immunocompromised patients.10 In our case, bacterial culture and polymerase chain reaction testing for mycobacteria were negative.
Diagnosis
Cactus spine injuries usually are easy to diagnose based on the clear-cut anamnesis and clinical picture; however, it might be interesting to assess the presence of foreign body granulomas without biopsy. Optical coherence tomography is a noninvasive optical imaging technique based on low-coherence interferometry that uses a low-intensity, 1310-nm infrared laser. Widespread in ophthalmology, OCT has gained importance in dermatologic diagnostics, especially for nonmelanoma skin cancer.11 Moreover, it has demonstrated its usefulness in various dermatologic fields, including granulomatous lesions.12 Further methods include reflectance confocal microscopy, based on a near-infrared laser, and 7.5-MHz ultrasonography. In our experience, however, 7.5-MHz ultrasonography has been ineffective in detecting cactus spines in the current patient as well as others. Preoperative and postoperative monitoring with dermoscopy and OCT helped us evaluate the nature, size, and location of spines and lesions and effective healing.
Treatment
Management strategies are still debated and include watchful waiting, corticosteroid ointment, partial removal of spines, and unroofing.1,2,4-10,13-18 We treated our patient with an innovative radical surgical approach using punch excision for granulomas that developed after cholla cactus spine injury. Our approach resulted in rapid relief of pain and reduced complications, a good aesthetic result, and no recurrence.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523. - Patterson JW. Weedon’s Skin Pathology. 4th ed. New York, NY: Elsevier; 2016.
- Winer LH, Zeilenga RH. Cactus granulomas of the skin; report of a case. AMA Arch Derm. 1955;72:566-569.
- Schreiber MM, Shapiro SI, Berry CZ. Cactus granulomas of the skin. an allergic phenomenon. Arch Dermatol. 1971;104:374-379.
- Doctoroff A, Vidimos AT, Taylor JS. Cactus skin injuries. Cutis. 2000;65:290-292.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Burrell SR, Ostlie DJ, Saubolle M, et al. Apophysomyces elegans infection associated with cactus spine injury in an immunocompetent pediatric patient. Pediatr Infect Dis J. 1998;17:663-664.
- von BraunmT. Optical coherence tomography. Hautarzt. 2015;66:499-503.
- Banzhaf C, Jemec GB. Imaging granulomatous lesions with optical coherence tomography. Case Rep Dermatol. 2012;4:14-18.
- Putnam MH. Simple cactus spine removal. J Pediatr. 1981;98:333.
- Snyder RA, Schwartz RA. Cactus bristle implantation. Report of an unusual case initially seen with rows of yellow hairs. Arch Dermatol. 1983;119:152-154.
- Schunk JE, Corneli HM. Cactus spine removal. J Pediatr. 1987;110:667.
- Gutierrez Ortega MC, Martin Moreno L, Arias Palomo D, et al. Facial granuloma caused by cactus bristles. Med Cutan Ibero Lat Am. 1990;18:197-200.
- Dieter RA Jr, Whitehouse LR, Gulliver R. Cactus spine wounds: a case report and short review of the literature. Wounds. 2017;29:E18-E21.
- O’Neill PJ, Sinha M, McArthur RA, et al. Penetrating cactus spine injury to the mediastinum of a child. J Pediatr Surg. 2008;43:E33-E35.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523. - Patterson JW. Weedon’s Skin Pathology. 4th ed. New York, NY: Elsevier; 2016.
- Winer LH, Zeilenga RH. Cactus granulomas of the skin; report of a case. AMA Arch Derm. 1955;72:566-569.
- Schreiber MM, Shapiro SI, Berry CZ. Cactus granulomas of the skin. an allergic phenomenon. Arch Dermatol. 1971;104:374-379.
- Doctoroff A, Vidimos AT, Taylor JS. Cactus skin injuries. Cutis. 2000;65:290-292.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Burrell SR, Ostlie DJ, Saubolle M, et al. Apophysomyces elegans infection associated with cactus spine injury in an immunocompetent pediatric patient. Pediatr Infect Dis J. 1998;17:663-664.
- von BraunmT. Optical coherence tomography. Hautarzt. 2015;66:499-503.
- Banzhaf C, Jemec GB. Imaging granulomatous lesions with optical coherence tomography. Case Rep Dermatol. 2012;4:14-18.
- Putnam MH. Simple cactus spine removal. J Pediatr. 1981;98:333.
- Snyder RA, Schwartz RA. Cactus bristle implantation. Report of an unusual case initially seen with rows of yellow hairs. Arch Dermatol. 1983;119:152-154.
- Schunk JE, Corneli HM. Cactus spine removal. J Pediatr. 1987;110:667.
- Gutierrez Ortega MC, Martin Moreno L, Arias Palomo D, et al. Facial granuloma caused by cactus bristles. Med Cutan Ibero Lat Am. 1990;18:197-200.
- Dieter RA Jr, Whitehouse LR, Gulliver R. Cactus spine wounds: a case report and short review of the literature. Wounds. 2017;29:E18-E21.
- O’Neill PJ, Sinha M, McArthur RA, et al. Penetrating cactus spine injury to the mediastinum of a child. J Pediatr Surg. 2008;43:E33-E35.
Practice Points
- Cactus spine injuries are an important source of morbidity in sports and leisure.
- Even after removal of cactus spines, painful granulomas can develop and persist for a long period of time. Patient education on early treatment can prevent further complications.
- Immediate and complete removal of spines as well as avoidance of bacterial superinfections should be given priority in cactus spine injuries. In case of granulomas, a surgical approach can result in rapid relief of symptoms.
Emerging Noninvasive Treatments of Nonmelanoma Skin Cancers
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Practice Points
- As of 2018, there has been an increase in options for the noninvasive management of nonmelanoma skin cancers that should be considered.
- Recently, approved advances in treatment options have included not only advanced basal cell carcinoma but also advanced squamous cell carcinoma such as cemiplimab.