User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Virtual Dermatology: A COVID-19 Update
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
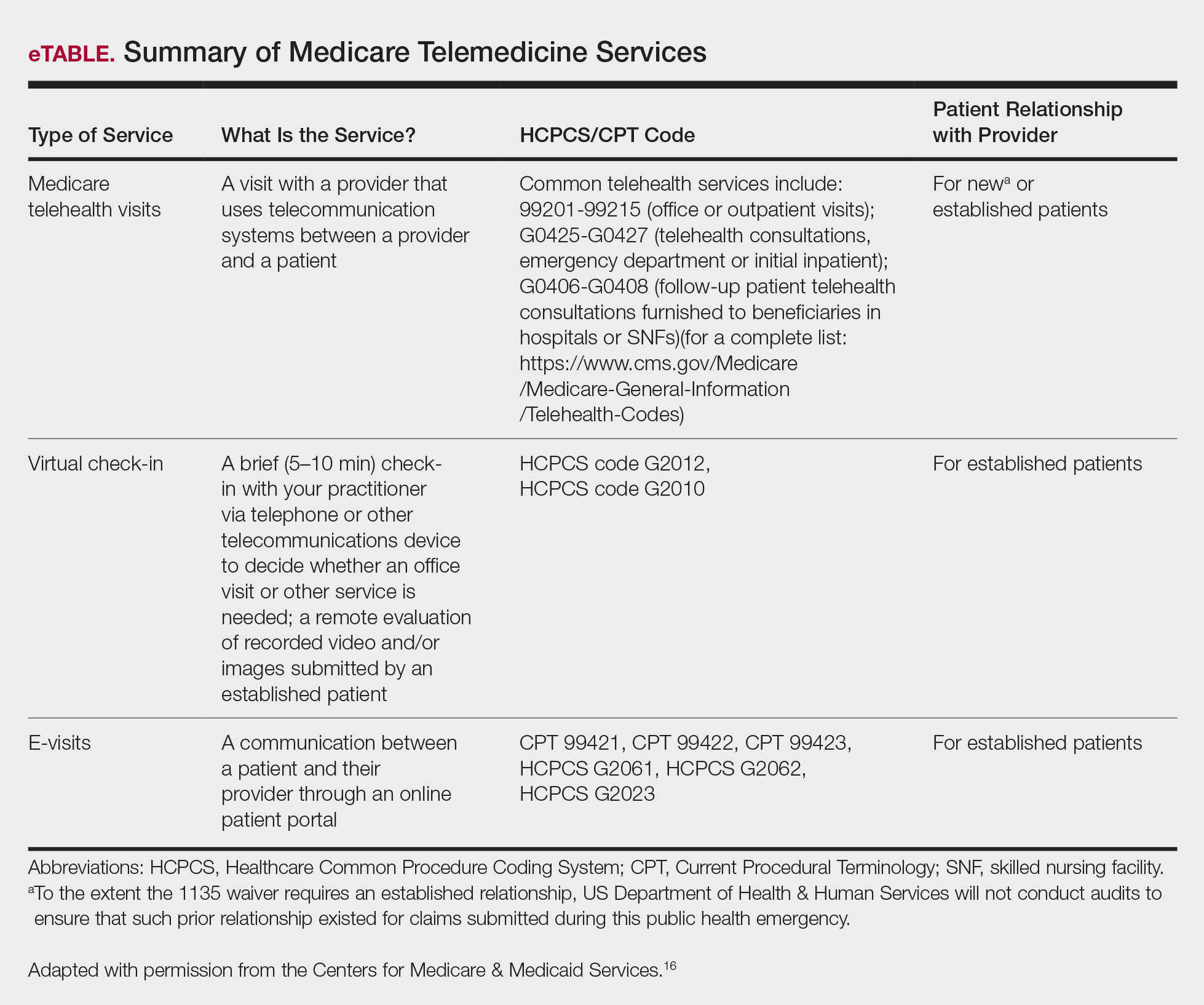
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.
Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.

Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.

Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
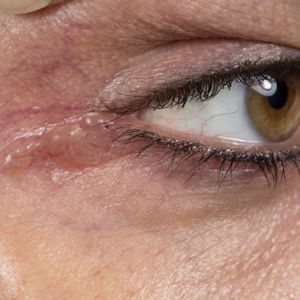
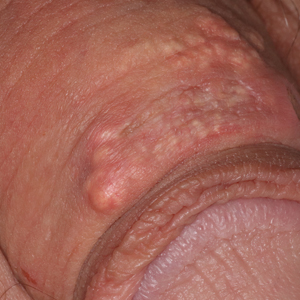
Translucent Periorbital Papules
The Diagnosis: Apocrine Hidrocystoma
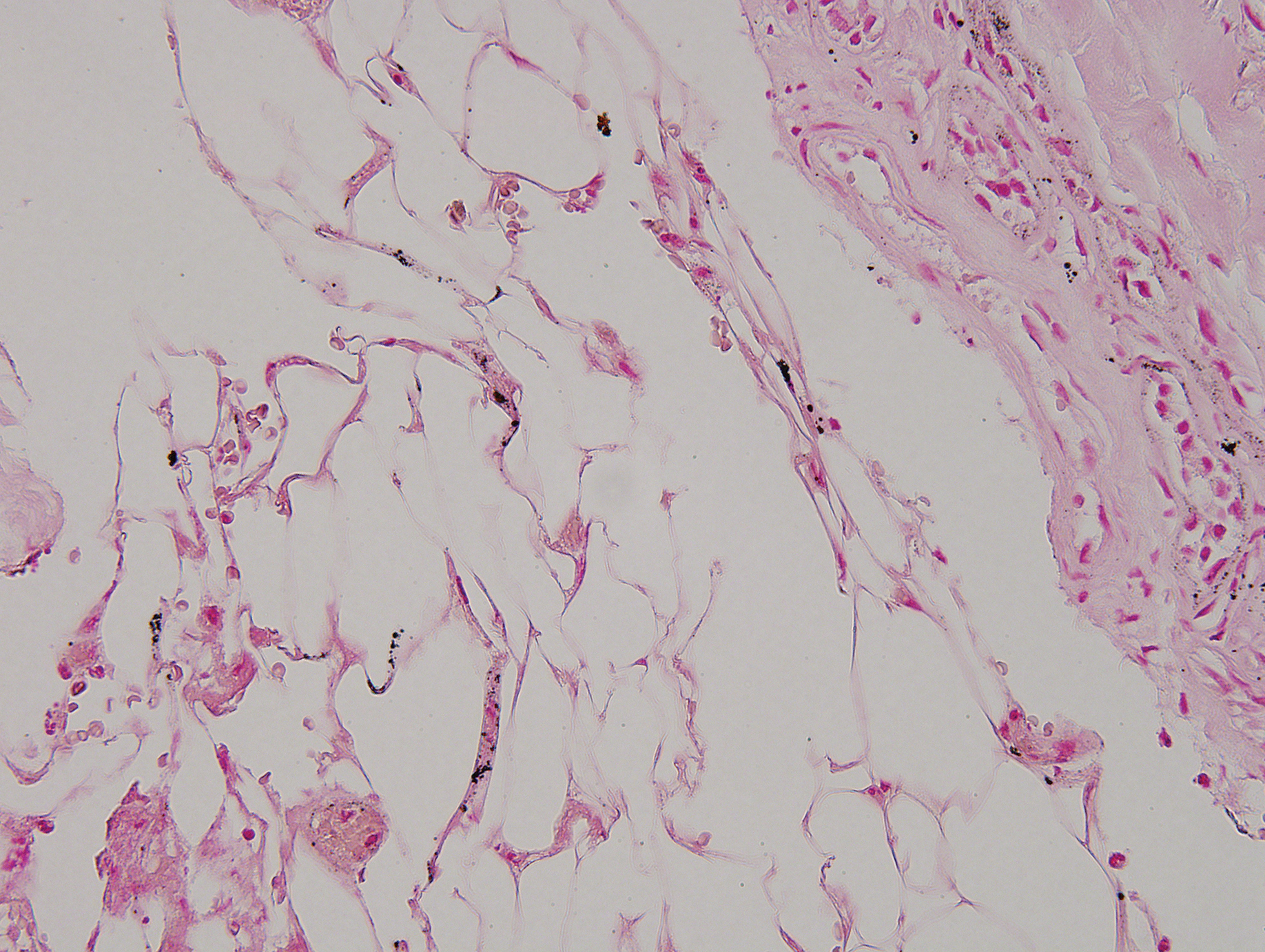
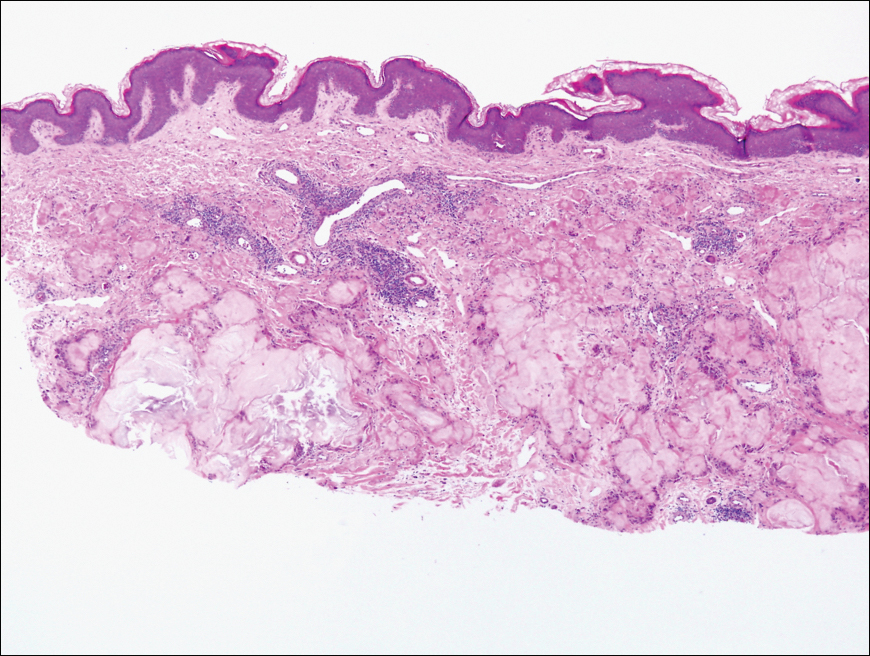
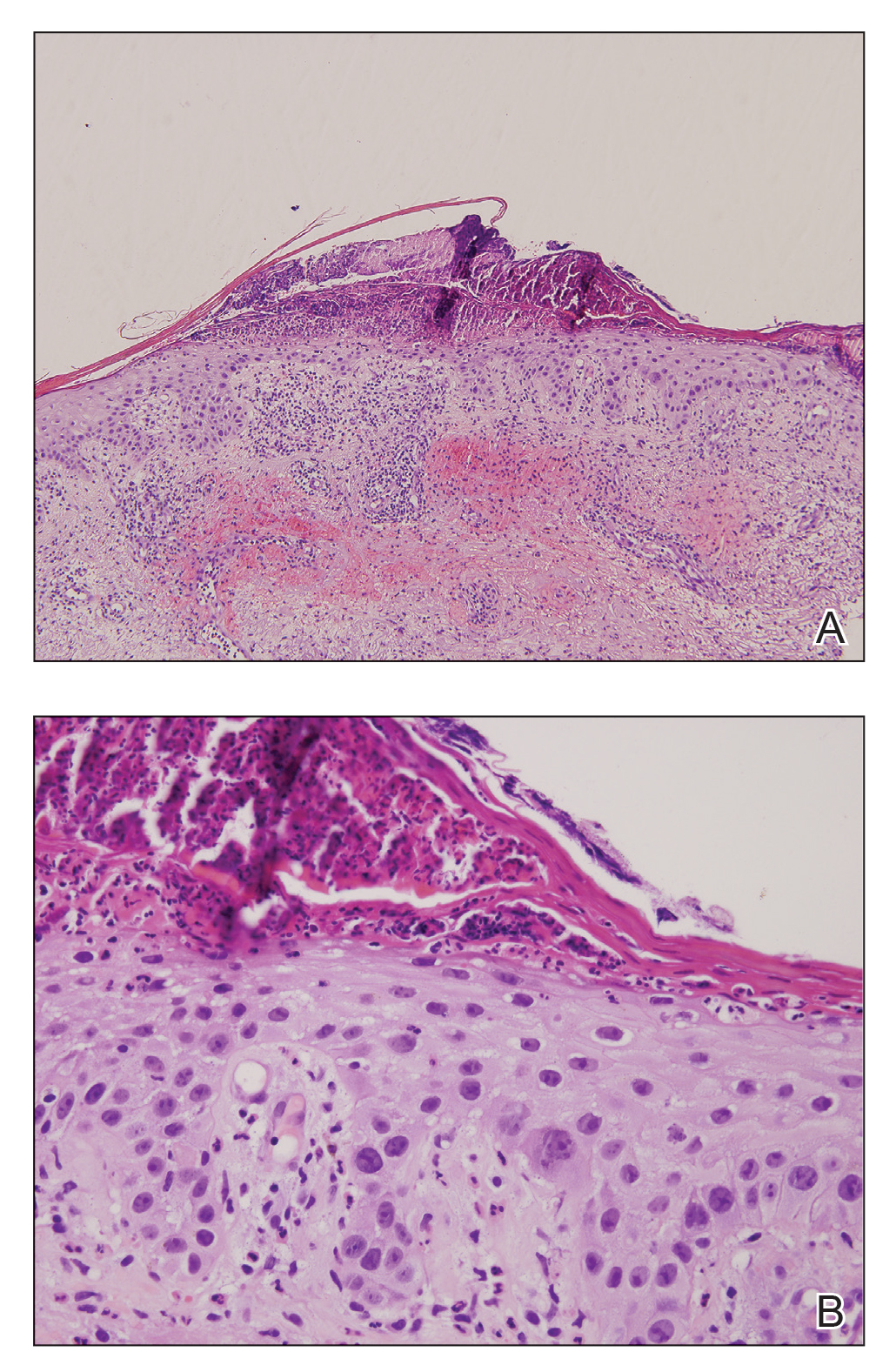
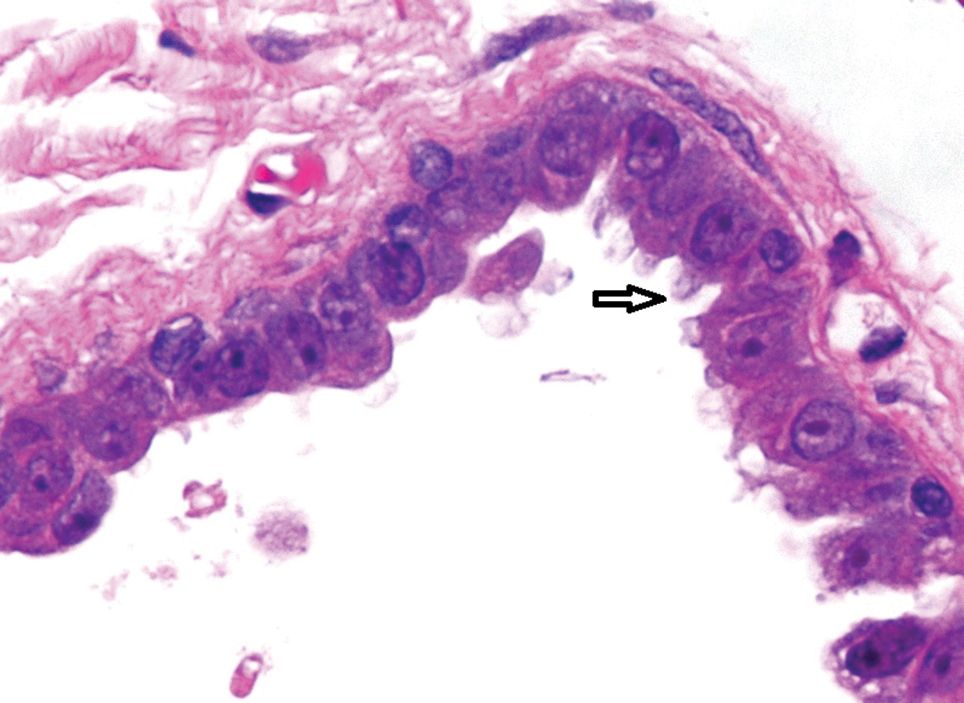
Histopathologic examination of one of the papules revealed cystic cavities located within the dermis (Figure 1) lined by a cuboidal epithelium demonstrating decapitation secretion (Figure 2), confirming the diagnosis of apocrine hidrocystomas. The presence of multiple lesions prompted further examination for an underlying genetic disorder; however, the patient's hair, nails, and teeth were normal. There also was no evidence of palmoplantar keratoderma or blaschkoid dermatosis.
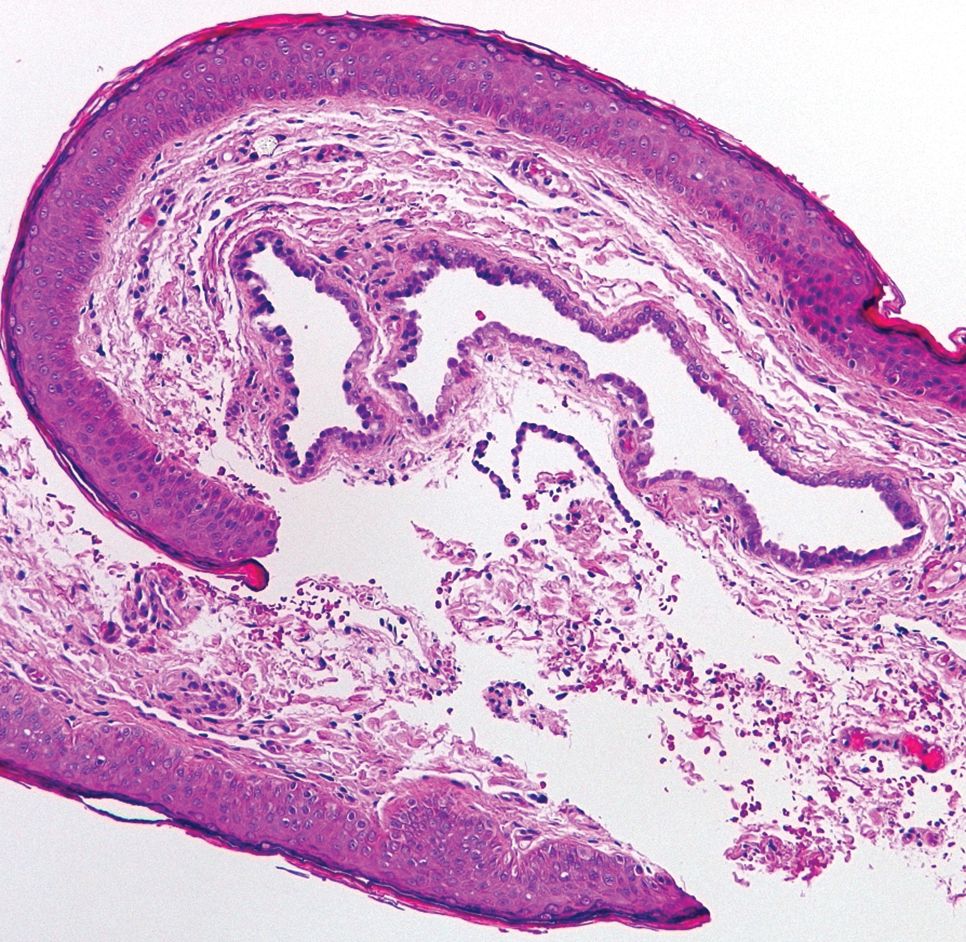
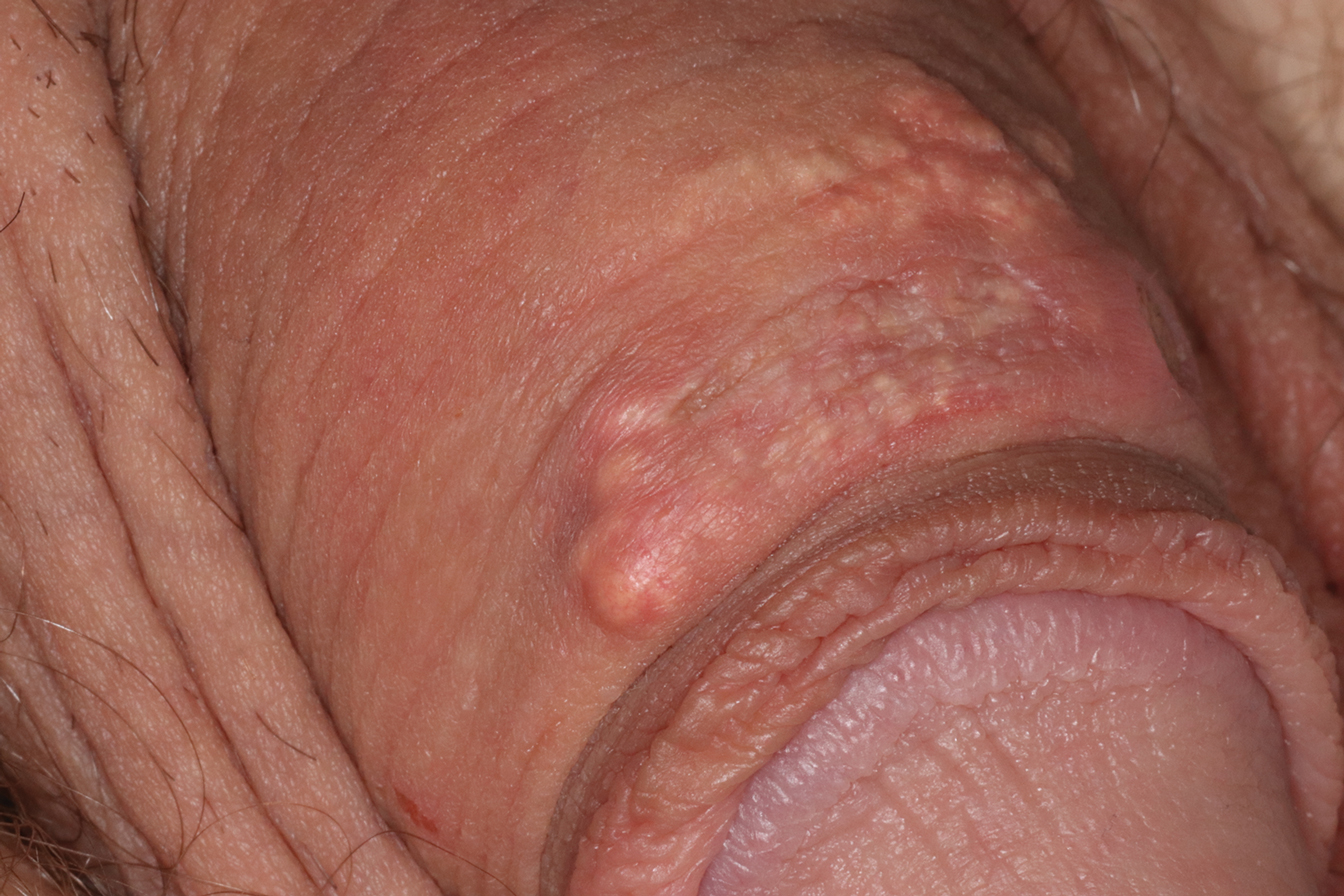
Hidrocystomas are benign cysts of the sudoriferous apparatus that can be subdivided based on histogenesis (apocrine vs eccrine) or lesion count (single vs multiple).1 Multiple lesions may be associated with disorders of ectodermal dysplasia, including Goltz syndrome and Schopf-Schulz-Passarge syndrome. Apocrine hidrocystomas tend to present as solitary, translucent, flesh-colored to bluish facial papules, and the occurrence of multiple lesions is rare in contrast to its eccrine counterpart.2 Various extrafacial sites have been described including the trunk, axillae, umbilicus, genitalia, and digits.3 Apocrine hidrocystomas do not demonstrate aggravation with exposure to heat, unlike their eccrine counterparts.2
A review of 107 patients with 215 histologically proven hidrocystomas demonstrated a preponderance for women in their mid 50s; 74.8% of patients had unilateral disease, and 69.8% of all lesions affected either the lower eyelid or lateral canthus. Recurrence following conventional surgical excision was observed in 2.3% of lesions.1
A review from Japan recounted an incidence of 5 cases per year from 1999 to 2003.4 Patients ranged in age from 30 to 70 years, but there was no gender predilection. Individual apocrine hidrocystomas were mostly less than 2 cm and varied from flesh colored to light red, brown, blue, or purple; 61% of lesions arose periorbitally. Within their cohort, patients with multiple lesions were uncommon, with only 2 cases presenting with 2 lesions simultaneously.4
Apocrine hidrocystomas are thought to result from a cystic proliferation of the secretory component of apocrine sweat glands, though the exact pathogenesis still is unclear.3 Histologic features include a unilocular or multilocular cystic cavity within the dermis lined by columnar cells demonstrating decapitation secretion, followed by a peripheral rim of flattened myoepithelial cells.
Treatment of apocrine hidrocystomas includes topical anticholinergics, surgical excision, electrodesiccation, 1450-nm diode or CO2 lasers, and trichloroacetic acid.2 The novel use of cryotherapy,5 botulinum toxin,2 and intralesional injections of 50% glucose (as a sclerosant)6 also have been reported. Caution should be exercised when managing digital lesions, as digital papillary carcinoma has been described as a clinical and histopathologic mimicker.7
Lipoid proteinosis is a rare autosomal-recessive disorder. Cutaneous lesions manifest in 2 overlapping stages, typically within the first 2 years of life. The first stage consists of vesicles and hemorrhagic crusts on the face and extremities and intraorally, which may heal with scarring. In the second stage, the skin becomes diffusely thickened and waxy, with the appearance of papules, nodules, or plaques along the eyelid margins (moniliform blepharosis), face, axillae, or scrotum. Verrucous lesions also may develop on the knee or elbow extensors.8
Lymphangioma circumscriptum represents microcystic lymphatic malformations that can arise anywhere on the skin or oral mucosa. They present as clusters of clear or hemorrhagic vesicles of variable size and number favoring the proximal extremities and chest. Histologically, dilated lymphatic channels are seen in the upper dermis.8
Syringomas are common benign tumors of the sweat ducts characterized histologically by superficial dermal proliferations of small comma-shaped ducts set in a fibrotic stroma. Clinically, syringomas appear as small, firm, flesh-colored papules with a predilection for the periorbital area. An eruptive onset may be observed, most commonly affecting the trunk. Syringomas may be associated with Down syndrome, while the clear cell variant may be associated with diabetes mellitus.8
Primary systemic amyloidosis may present with a variety of systemic manifestations. Skin involvement can present as waxy, translucent, or purpuric papulonodules or plaques characteristically affecting the periorbital region. Other mucocutaneous signs include macroglossia with or without translucent to hemorrhagic papulovesicles; bruising, especially on the eyelids, neck, axillae, or anogenital area; vesiculobullous skin lesions; or diffuse cutaneous infiltration imparting a sclerodermoid appearance.8
- Maeng M, Petrakos P, Zhou M, et al. Bi-institutional retrospective study on the demographics and basic clinical presentation of hidrocystomas. Orbit. 2017;36:433-435.
- Bordelon JR, Tang N, Elston D, et al. Multiple apocrine hidrocystomas successfully treated with botulinum toxin A. Br J Dermatol. 2017;176:488-490.
- Hafsi W, Badri T. Apocrine hidrocystoma. StatPearls. Treasure Island, FL: StatPearls Publishing; 2017.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Panagiotopoulos A, Vasalou V, Sgontzou T, et al. Multiple apocrine hidrocystomas successfully treated with cryotherapy. Dermatol Surg. 2017;43:993-995.
- Osaki TH, Osaki MH, Osaki T, et al. A minimally invasive approach for apocrine hidrocystomas of the eyelid. Dermatol Surg. 2016;42:134-136.
- Molina-Ruiz AM, Llamas-Velasco M, Rütten A, et al. 'Apocrine hidrocystoma and cystadenoma'-like tumor of the digits or toes: a potential diagnostic pitfall of digital papillary adenocarcinoma. Am J Surg Pathol. 2016;40:410-418.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
The Diagnosis: Apocrine Hidrocystoma
Histopathologic examination of one of the papules revealed cystic cavities located within the dermis (Figure 1) lined by a cuboidal epithelium demonstrating decapitation secretion (Figure 2), confirming the diagnosis of apocrine hidrocystomas. The presence of multiple lesions prompted further examination for an underlying genetic disorder; however, the patient's hair, nails, and teeth were normal. There also was no evidence of palmoplantar keratoderma or blaschkoid dermatosis.


Hidrocystomas are benign cysts of the sudoriferous apparatus that can be subdivided based on histogenesis (apocrine vs eccrine) or lesion count (single vs multiple).1 Multiple lesions may be associated with disorders of ectodermal dysplasia, including Goltz syndrome and Schopf-Schulz-Passarge syndrome. Apocrine hidrocystomas tend to present as solitary, translucent, flesh-colored to bluish facial papules, and the occurrence of multiple lesions is rare in contrast to its eccrine counterpart.2 Various extrafacial sites have been described including the trunk, axillae, umbilicus, genitalia, and digits.3 Apocrine hidrocystomas do not demonstrate aggravation with exposure to heat, unlike their eccrine counterparts.2
A review of 107 patients with 215 histologically proven hidrocystomas demonstrated a preponderance for women in their mid 50s; 74.8% of patients had unilateral disease, and 69.8% of all lesions affected either the lower eyelid or lateral canthus. Recurrence following conventional surgical excision was observed in 2.3% of lesions.1
A review from Japan recounted an incidence of 5 cases per year from 1999 to 2003.4 Patients ranged in age from 30 to 70 years, but there was no gender predilection. Individual apocrine hidrocystomas were mostly less than 2 cm and varied from flesh colored to light red, brown, blue, or purple; 61% of lesions arose periorbitally. Within their cohort, patients with multiple lesions were uncommon, with only 2 cases presenting with 2 lesions simultaneously.4
Apocrine hidrocystomas are thought to result from a cystic proliferation of the secretory component of apocrine sweat glands, though the exact pathogenesis still is unclear.3 Histologic features include a unilocular or multilocular cystic cavity within the dermis lined by columnar cells demonstrating decapitation secretion, followed by a peripheral rim of flattened myoepithelial cells.
Treatment of apocrine hidrocystomas includes topical anticholinergics, surgical excision, electrodesiccation, 1450-nm diode or CO2 lasers, and trichloroacetic acid.2 The novel use of cryotherapy,5 botulinum toxin,2 and intralesional injections of 50% glucose (as a sclerosant)6 also have been reported. Caution should be exercised when managing digital lesions, as digital papillary carcinoma has been described as a clinical and histopathologic mimicker.7
Lipoid proteinosis is a rare autosomal-recessive disorder. Cutaneous lesions manifest in 2 overlapping stages, typically within the first 2 years of life. The first stage consists of vesicles and hemorrhagic crusts on the face and extremities and intraorally, which may heal with scarring. In the second stage, the skin becomes diffusely thickened and waxy, with the appearance of papules, nodules, or plaques along the eyelid margins (moniliform blepharosis), face, axillae, or scrotum. Verrucous lesions also may develop on the knee or elbow extensors.8
Lymphangioma circumscriptum represents microcystic lymphatic malformations that can arise anywhere on the skin or oral mucosa. They present as clusters of clear or hemorrhagic vesicles of variable size and number favoring the proximal extremities and chest. Histologically, dilated lymphatic channels are seen in the upper dermis.8
Syringomas are common benign tumors of the sweat ducts characterized histologically by superficial dermal proliferations of small comma-shaped ducts set in a fibrotic stroma. Clinically, syringomas appear as small, firm, flesh-colored papules with a predilection for the periorbital area. An eruptive onset may be observed, most commonly affecting the trunk. Syringomas may be associated with Down syndrome, while the clear cell variant may be associated with diabetes mellitus.8
Primary systemic amyloidosis may present with a variety of systemic manifestations. Skin involvement can present as waxy, translucent, or purpuric papulonodules or plaques characteristically affecting the periorbital region. Other mucocutaneous signs include macroglossia with or without translucent to hemorrhagic papulovesicles; bruising, especially on the eyelids, neck, axillae, or anogenital area; vesiculobullous skin lesions; or diffuse cutaneous infiltration imparting a sclerodermoid appearance.8
The Diagnosis: Apocrine Hidrocystoma
Histopathologic examination of one of the papules revealed cystic cavities located within the dermis (Figure 1) lined by a cuboidal epithelium demonstrating decapitation secretion (Figure 2), confirming the diagnosis of apocrine hidrocystomas. The presence of multiple lesions prompted further examination for an underlying genetic disorder; however, the patient's hair, nails, and teeth were normal. There also was no evidence of palmoplantar keratoderma or blaschkoid dermatosis.


Hidrocystomas are benign cysts of the sudoriferous apparatus that can be subdivided based on histogenesis (apocrine vs eccrine) or lesion count (single vs multiple).1 Multiple lesions may be associated with disorders of ectodermal dysplasia, including Goltz syndrome and Schopf-Schulz-Passarge syndrome. Apocrine hidrocystomas tend to present as solitary, translucent, flesh-colored to bluish facial papules, and the occurrence of multiple lesions is rare in contrast to its eccrine counterpart.2 Various extrafacial sites have been described including the trunk, axillae, umbilicus, genitalia, and digits.3 Apocrine hidrocystomas do not demonstrate aggravation with exposure to heat, unlike their eccrine counterparts.2
A review of 107 patients with 215 histologically proven hidrocystomas demonstrated a preponderance for women in their mid 50s; 74.8% of patients had unilateral disease, and 69.8% of all lesions affected either the lower eyelid or lateral canthus. Recurrence following conventional surgical excision was observed in 2.3% of lesions.1
A review from Japan recounted an incidence of 5 cases per year from 1999 to 2003.4 Patients ranged in age from 30 to 70 years, but there was no gender predilection. Individual apocrine hidrocystomas were mostly less than 2 cm and varied from flesh colored to light red, brown, blue, or purple; 61% of lesions arose periorbitally. Within their cohort, patients with multiple lesions were uncommon, with only 2 cases presenting with 2 lesions simultaneously.4
Apocrine hidrocystomas are thought to result from a cystic proliferation of the secretory component of apocrine sweat glands, though the exact pathogenesis still is unclear.3 Histologic features include a unilocular or multilocular cystic cavity within the dermis lined by columnar cells demonstrating decapitation secretion, followed by a peripheral rim of flattened myoepithelial cells.
Treatment of apocrine hidrocystomas includes topical anticholinergics, surgical excision, electrodesiccation, 1450-nm diode or CO2 lasers, and trichloroacetic acid.2 The novel use of cryotherapy,5 botulinum toxin,2 and intralesional injections of 50% glucose (as a sclerosant)6 also have been reported. Caution should be exercised when managing digital lesions, as digital papillary carcinoma has been described as a clinical and histopathologic mimicker.7
Lipoid proteinosis is a rare autosomal-recessive disorder. Cutaneous lesions manifest in 2 overlapping stages, typically within the first 2 years of life. The first stage consists of vesicles and hemorrhagic crusts on the face and extremities and intraorally, which may heal with scarring. In the second stage, the skin becomes diffusely thickened and waxy, with the appearance of papules, nodules, or plaques along the eyelid margins (moniliform blepharosis), face, axillae, or scrotum. Verrucous lesions also may develop on the knee or elbow extensors.8
Lymphangioma circumscriptum represents microcystic lymphatic malformations that can arise anywhere on the skin or oral mucosa. They present as clusters of clear or hemorrhagic vesicles of variable size and number favoring the proximal extremities and chest. Histologically, dilated lymphatic channels are seen in the upper dermis.8
Syringomas are common benign tumors of the sweat ducts characterized histologically by superficial dermal proliferations of small comma-shaped ducts set in a fibrotic stroma. Clinically, syringomas appear as small, firm, flesh-colored papules with a predilection for the periorbital area. An eruptive onset may be observed, most commonly affecting the trunk. Syringomas may be associated with Down syndrome, while the clear cell variant may be associated with diabetes mellitus.8
Primary systemic amyloidosis may present with a variety of systemic manifestations. Skin involvement can present as waxy, translucent, or purpuric papulonodules or plaques characteristically affecting the periorbital region. Other mucocutaneous signs include macroglossia with or without translucent to hemorrhagic papulovesicles; bruising, especially on the eyelids, neck, axillae, or anogenital area; vesiculobullous skin lesions; or diffuse cutaneous infiltration imparting a sclerodermoid appearance.8
- Maeng M, Petrakos P, Zhou M, et al. Bi-institutional retrospective study on the demographics and basic clinical presentation of hidrocystomas. Orbit. 2017;36:433-435.
- Bordelon JR, Tang N, Elston D, et al. Multiple apocrine hidrocystomas successfully treated with botulinum toxin A. Br J Dermatol. 2017;176:488-490.
- Hafsi W, Badri T. Apocrine hidrocystoma. StatPearls. Treasure Island, FL: StatPearls Publishing; 2017.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Panagiotopoulos A, Vasalou V, Sgontzou T, et al. Multiple apocrine hidrocystomas successfully treated with cryotherapy. Dermatol Surg. 2017;43:993-995.
- Osaki TH, Osaki MH, Osaki T, et al. A minimally invasive approach for apocrine hidrocystomas of the eyelid. Dermatol Surg. 2016;42:134-136.
- Molina-Ruiz AM, Llamas-Velasco M, Rütten A, et al. 'Apocrine hidrocystoma and cystadenoma'-like tumor of the digits or toes: a potential diagnostic pitfall of digital papillary adenocarcinoma. Am J Surg Pathol. 2016;40:410-418.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
- Maeng M, Petrakos P, Zhou M, et al. Bi-institutional retrospective study on the demographics and basic clinical presentation of hidrocystomas. Orbit. 2017;36:433-435.
- Bordelon JR, Tang N, Elston D, et al. Multiple apocrine hidrocystomas successfully treated with botulinum toxin A. Br J Dermatol. 2017;176:488-490.
- Hafsi W, Badri T. Apocrine hidrocystoma. StatPearls. Treasure Island, FL: StatPearls Publishing; 2017.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Panagiotopoulos A, Vasalou V, Sgontzou T, et al. Multiple apocrine hidrocystomas successfully treated with cryotherapy. Dermatol Surg. 2017;43:993-995.
- Osaki TH, Osaki MH, Osaki T, et al. A minimally invasive approach for apocrine hidrocystomas of the eyelid. Dermatol Surg. 2016;42:134-136.
- Molina-Ruiz AM, Llamas-Velasco M, Rütten A, et al. 'Apocrine hidrocystoma and cystadenoma'-like tumor of the digits or toes: a potential diagnostic pitfall of digital papillary adenocarcinoma. Am J Surg Pathol. 2016;40:410-418.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
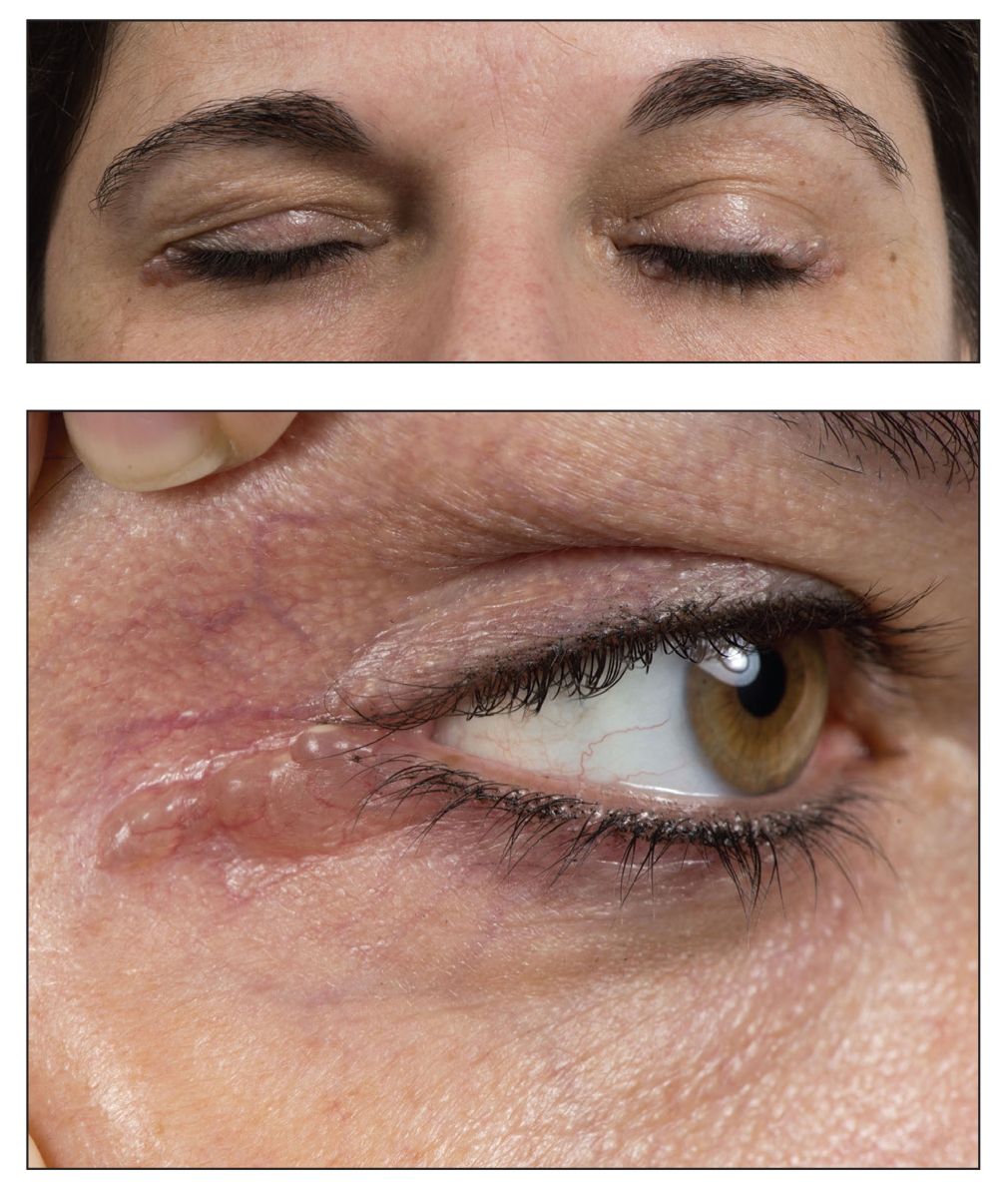
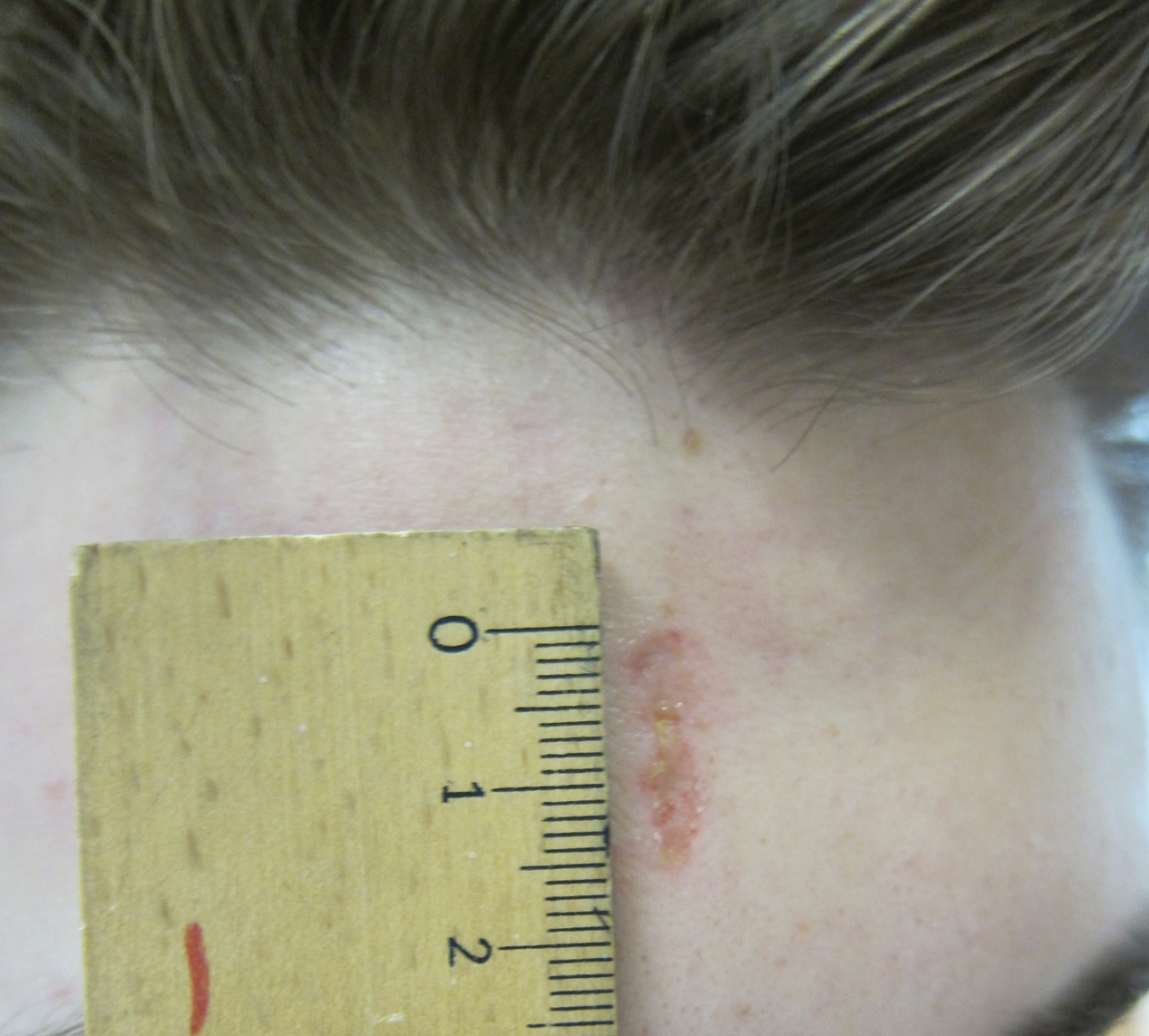
A 40-year-old woman was referred to dermatology for evaluation of occasionally pruritic periorbital papules that had gradually increased in size and number over the last 7 to 8 months (top). She had a similar solitary lesion on the left lower eyelid that was removed twice: 10 years and 10 months prior. She was taking an oral contraceptive (desogestrel) but otherwise had no notable medical history or drug allergies. Physical examination revealed individual and clustered translucent papules along the eyelid margins, left medial canthus, and both lateral canthi (bottom).
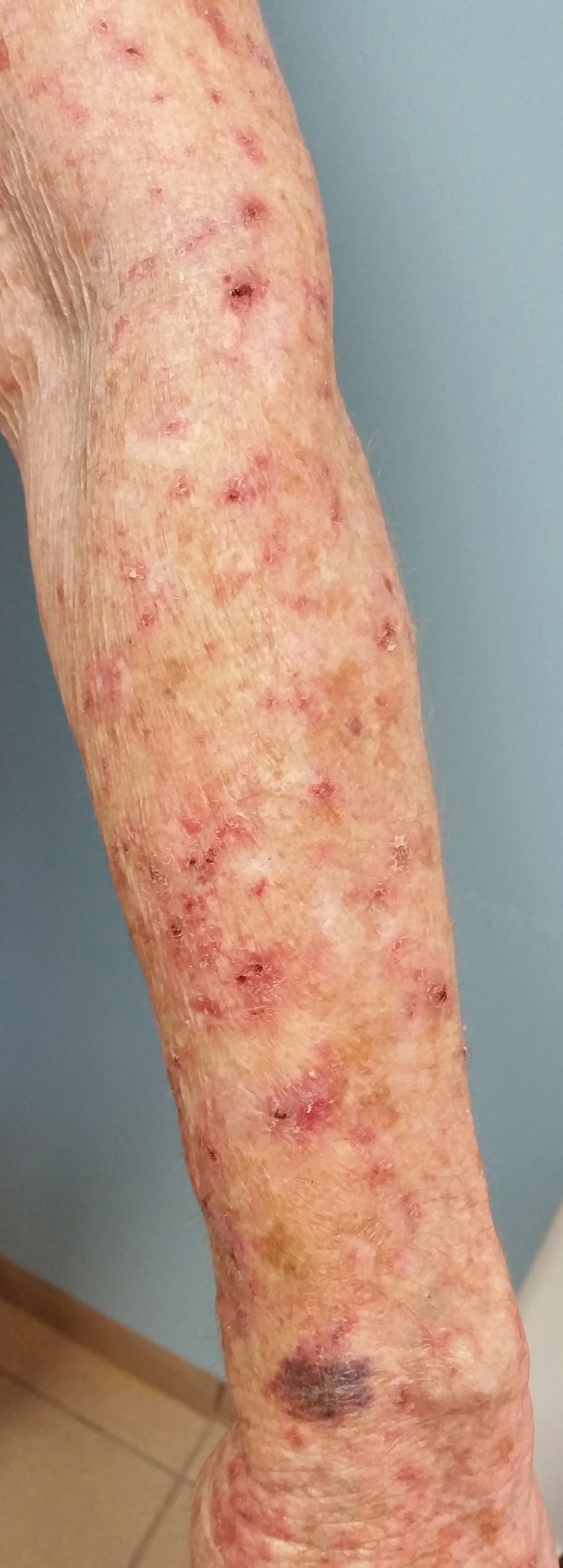
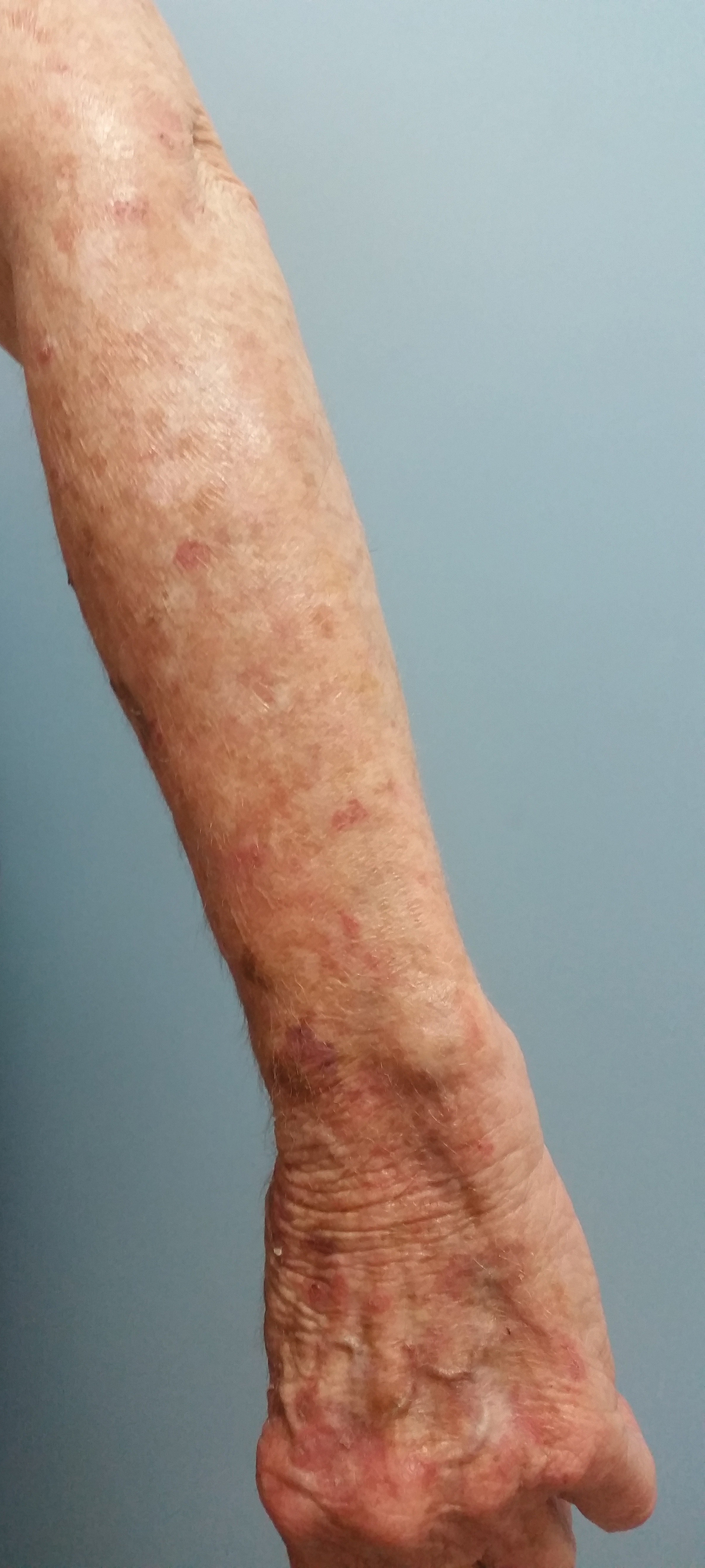
An Unusual Presentation of Calciphylaxis
To the Editor:
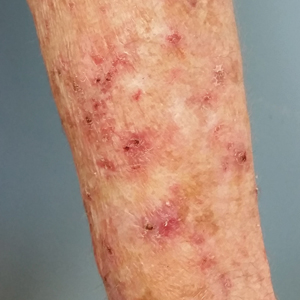
Calciphylaxis (also known as calcific uremic arteriolopathy and calcifying panniculitis) is a rare vasculopathy affecting the small vessels.1 It is characterized by cutaneous ischemia and necrosis secondary to calcification. It is most commonly seen in patients with end-stage renal disease (ESRD) and hyperparathyroidism.1-3 Histopathologic features that are consistent with the diagnosis of calciphylaxis include calcification of medium-sized vessels in the deep dermis or subcutaneous fat as well as smaller distal vessels that supply the papillary dermis and epidermis.4,5 Although it commonly presents as well-demarcated, painful, purplish lesions that evolve into necrotic eschars, calciphylaxis rarely can present with hemorrhagic or serous bullous lesions followed by ulceration, as was seen in our patient.1,5,6 We report this uncommon presentation to highlight the variety in clinical appearance of calciphylaxis and the importance of early diagnosis.
A 43-year-old woman presented to the emergency department for evaluation of chest and abdominal pain that began 1 day prior to presentation. She had a history of systemic lupus erythematosus and ESRD secondary to poststreptococcal glomerulonephritis and was currently on peritoneal dialysis. The patient was admitted for peritonitis and treated with broad-spectrum antibiotics. At the time of admission, the patient also was noted to have several painful bullae on the legs. Her medical history also was remarkable for cerebral infarction, fibromyalgia, cerebral artery occlusion with cerebral infarction, sciatica, hyperlipidemia, deep vein thrombosis, and seizures. She had no history of herpes simplex virus. Surgical history was remarkable for tubal ligation, nephrectomy and kidney transplant, parathyroidectomy, and cholecystectomy. The patient’s medications included sevelamer carbonate, prednisone, epogen, calcium carbonate, esomeprazole, ondansetron, topical gentamicin, and atorvastatin.
Skin examination was performed by the inpatient dermatology service and revealed several tense, 1- to 5-cm, nonhemorrhagic bullae on the thighs and lower legs, some that had ruptured. The lesions were notably tender to palpation. No surrounding erythema, ecchymosis, or warmth was appreciated. The Nikolsky sign was negative. The patient also was noted to have at least grade 2 to 3+ pitting edema of the bilateral legs. The oral and conjunctival mucosae were unremarkable.
Antinuclear antibody, double-stranded DNA, and anti-Smith antibody levels were negative. A punch biopsy of the left lateral thigh revealed intraepidermal vesicular dermatitis with dermal edema suggestive of edema bullae and direct immunofluorescence was negative for immune complex and complement deposition.
Conservative therapy with wound care was recommended. The patient continued to report persistent severe skin pain and developed a subcutaneous nodule on the right inner thigh 1 week later, prompting a second biopsy. Results of the excisional biopsy were nondiagnostic but were suggestive of calciphylaxis, revealing subepidermal bullae with epidermal necrosis, a scant perivascular lymphocytic infiltrate, and extravasated erythrocytes. No evidence of calcification was seen within the vessels. The patient was then started on sodium thiosulfate with hemodialysis for treatment of presumed calciphylaxis.
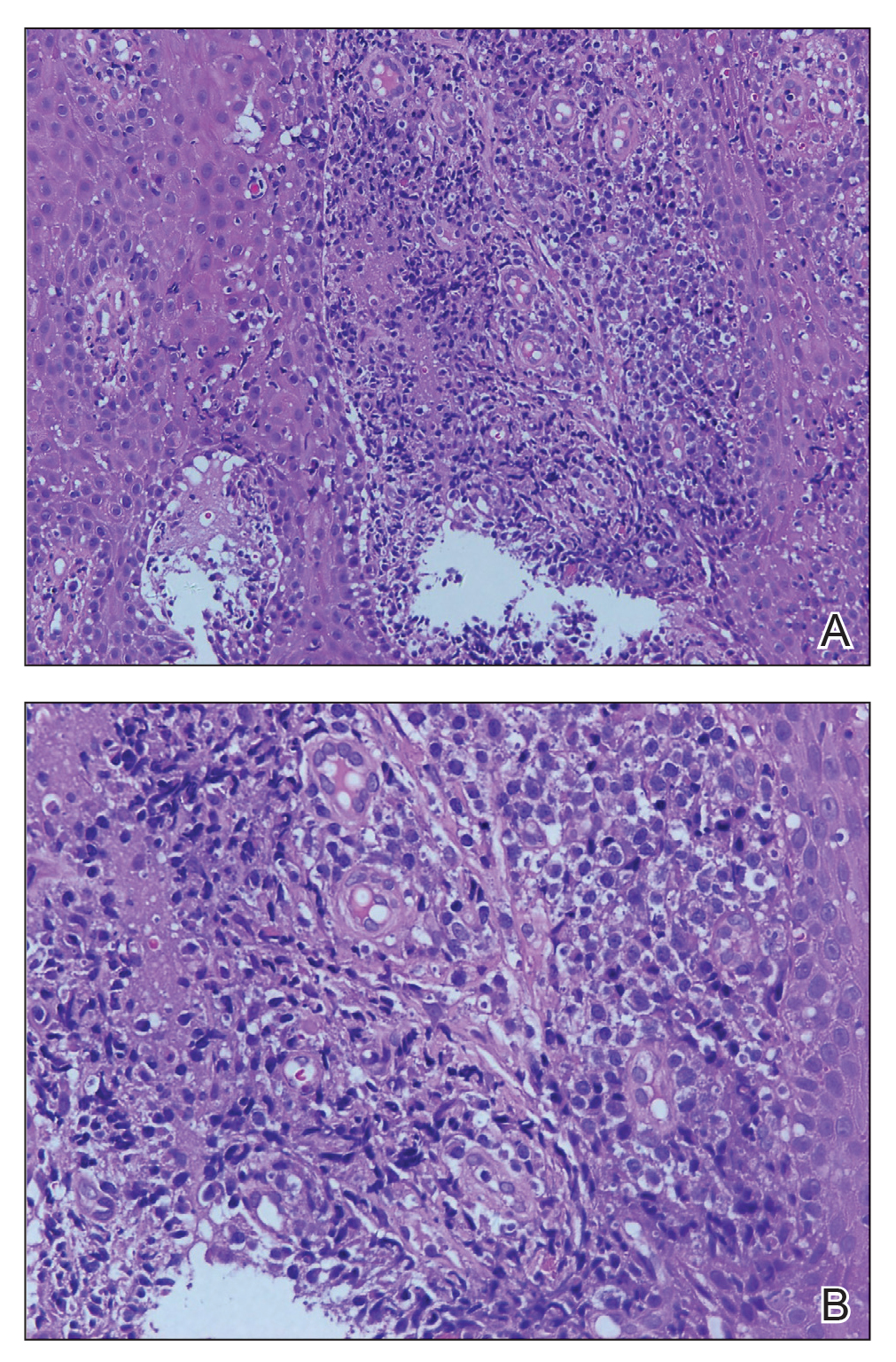
Despite meticulous wound care and treatment with sodium thiosulfate, the patient developed ulcerations with necrotic eschars on the bilateral buttocks, hips, and thighs 1 month later (Figure 1). She subsequently worsened over the next few weeks. She developed sepsis and was transferred to the intensive care unit. A third biopsy was performed, finally confirming the diagnosis of calciphylaxis. Histopathology revealed small blood vessels with basophilic granular deposits in the walls consistent with calcium in the subcutaneous tissue (highlighted with the von Kossa stain), as well as thrombi in the lumens of some vessels; early fat necrosis; focal epidermal necrosis with underlying congested blood vessels with deposits in their walls; a perivascular infiltrate predominately of lymphocytes and neutrophils with scattered nuclear dust; and thick, hyalinized, closely crowded collagen bundles in the reticular dermis and in a widened subcutaneous septum (Figures 2 and 3).
Supportive care and pain control were continued, but the overall prognosis was determined to be very poor, and the patient eventually was discharged to hospice and died.
Although calciphylaxis is commonly seen in patients with ESRD and hyperparathyroidism, patients without renal disease also may develop the condition.2,3 Prior epidemiologic studies have shown a prevalence of 1% in patients with chronic kidney disease and up to 4% in those receiving dialysis.2-5 The average age at presentation is 48 years.6,7 Although calciphylaxis has been noted to affect males and females equally, some studies have suggested a female predominance.5-8
The etiology of calciphylaxis is unknown, but ESRD requiring dialysis, primary or secondary hyperparathyroidism, obesity, diabetes mellitus, skin trauma, and/or a hypercoagulable state may put patients at increased risk for developing this disease.2,3 Other risk factors include systemic corticosteroids, liver disease, increased serum aluminum, and increased erythrocyte sedimentation rate. Although high calcium-phosphate product has been noted as a risk factor in prior studies, one retrospective study found that it does not reliably confirm or exclude a diagnosis of calciphylaxis.8
The pathogenesis of calciphylaxis is not well understood; however, some researchers suggest that an imbalance in calcium-phosphate homeostasis may lead to calciphylaxis; that is, elevated calcium and phosphate levels exceed their solubility and deposit in the walls of small- and medium-sized arteries, which consequently leads to ischemic necrosis and gangrene of the surrounding tissue.9
Clinically, calciphylaxis has an indolent onset and usually presents as well-demarcated, painful, purplish, mottled lesions that evolve into necrotic gray-black eschars and gangrene in adjacent tissues.1,5,6 The ischemic process may even extend to the muscle layer.5 Other common presentations include mild erythematous patches; livedo reticularis; painful nodules; necrotic ulcerating lesions; and more rarely flaccid, hemorrhagic, or serous bullous lesions followed by ulceration, as was seen in our patient.6,9,10 Lesions usually begin at sites of trauma and seem to be distributed symmetrically.5,6 The most commonly affected locations are the legs, specifically the medial thighs, as well as the abdomen and buttocks, but lesions also can be found at more distal sites such as the breasts, tongue, vulva, penis, fingers, and toes.5,6,10 The head and neck region rarely is affected. Although uncommon, calciphylaxis may affect other organs, including the lungs, stomach, kidneys, and adrenal glands.5 The accompanying systemic symptoms and findings may include muscle weakness, tenderness, or myositis with rhabdomyolysis; calcific cerebral embolism; dementia and infarction of the central nervous system; acute respiratory failure; heart disease; atrioventricular block; and calcification of the cardiac conduction system.6 Unlike other forms of peripheral vascular disease, distal pulses are present in calciphylaxis, as blood flow usually is preserved distal and deep to the areas of necrosis.5,6
A careful history and thorough physical examination are important first steps in the diagnosis of this condition.2,10 Although there are no definitive laboratory tests, elevated serum calcium, phosphorous, and calcium-phosphate product levels, as well as parathyroid hormone level, may be suggestive of calciphylaxis.2,5 Leukocytosis may occur if an infection is present.5
The most accurate method to confirm the diagnosis is a deep incisional biopsy from an erythematous, slightly purpuric area adjacent to the necrotic lesion.2,10,11 The histopathologic features used to make the diagnosis include calcification of medium-sized vessels, particularly the intimal or medial layers, in the deep dermis and subcutaneous fat in addition to lobular capillaries of the subcutaneous fat.5,10 These vessels, including the smaller distal vessels that supply the papillary dermis and epidermis, also may be thrombosed due to calcification, leading to vascular occlusion and subsequently ischemic necrosis of the overlying epidermis.10 Other findings may include pseudoxanthoma elasticum changes, panniculitis, and subcutaneous fat necrosis.4,10
The differential diagnosis for calciphylaxis includes peripheral vascular disease, vasculitis, juvenile dermatomyositis, proteins C and S deficiencies, cryofibrinogenemia, calcinosis cutis, and tumoral calcinosis.2 Polyarteritis nodosa, Sjögren syndrome, atherosclerotic peripheral vascular disease, pyoderma gangrenosum, systemic lupus erythematosus, necrotizing fasciitis, septic embolism, and necrosis secondary to warfarin and heparin may mimic calciphylaxis.5
Treatment of calciphylaxis is multidimensional but primarily is supportive.6,11 Controlling calcium and phosphate levels and secondary hyperparathyroidism through diet and phosphate binders (eg, sevelamer hydrochloride) has been shown to be effective.6 Pamidronate, a bisphosphonate, inhibits arterial calcification in animal models and has been reported to treat calciphylaxis, resulting in marked pain reduction and ulcer healing.4,6 Cinacalcet, which functions as a calcimimetic, has been implicated in the treatment of calciphylaxis. It has been used to treat primary and secondary hyperparathyroidism and to normalize serum calcium levels; it also may be used as an alternative to parathyroidectomy.4,6 Intravenous administration of sodium thiosulfate, a potent antioxidant and chelator of calcium, has been helpful in reversing signs and symptoms of calciphylaxis.6,12 It also has been shown to effectively remove extra calcium during peritoneal dialysis.6 Parathyroidectomy has been useful in patients with markedly elevated parathyroid hormone levels, as it suppresses or eliminates the sensitizing agent causing hypercalcemia, elevated calcium-phosphate product, and hyperparathyroidism.1,2,6,13
Wound care and prevention of sepsis are essential in the treatment of calciphylaxis. Management options include surgical debridement, hydrocolloid and biologic dressings, skin grafts, systemic antibiotics, oral pentoxifylline combined with maggot therapy, nutritional support, hyperbaric oxygen therapy, and revascularization and amputation when other interventions have failed. Pain control with analgesics and correction of thrombosis in the skin and blood vessels via anticoagulation therapy also are important complementary treatments.6
The clinical outcome of calciphylaxis is dependent on early diagnosis, antimicrobial therapy, and wound management,9 but overall, the prognosis usually is poor and has a high mortality rate. The most common causes of death are infection and sepsis.1,9 A study of 7 cases reported 100% mortality,14 but other studies have suggested a mortality rate of 60% to 80%.4,10 Female sex and obesity are poor prognostic indicators.2 A better prognosis has been appreciated in cases in which lesions occur at distal sites (eg, lower legs, hands) compared to more proximal sites (eg, abdomen), where 25% and 75% mortalities have been noted, respectively.10,14,15 In one study, the overall mortality rate was 45% in patients with calciphylaxis at 1 year.6 The rate was 41% in patients with plaques only and 67% in those who presented with ulceration. Patients who survive often experience a high degree of morbidity and prolonged hospitalization; these patients often are severely debilitated, especially in the case of limb amputation.6
Our report of calciphylaxis demonstrates the diversity in clinical presentation and emphasizes the importance of early and accurate diagnosis in reducing morbidity and mortality. In our case, the patient presented with skin pain and tense nonhemorrhagic bullae without underlying ecchymotic or erythematous lesions as the earliest sign of calciphylaxis. Physicians should have a high degree of suspicion in the setting of dialysis-dependent ESRD patients with bullae, extreme pain, and continuous decline. We hope that this case will help increase awareness of the varying presentations of this condition.
- Hanafusa T, Yamaguchi Y, Tani M, et al. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol. 2001;57:1021-1025.
- Somorin AO, Harbi AA, Subaity Y, et al. Calciphylaxis: case report and literature review. Afr J Med Sci. 2002;31:175-178.
- Barreiros HM, Goulão J, Cunha H, et al. Calciphylaxis: a diagnostic and therapeutic challenge. J Dermatol Case Rep. 2013;2:69-70.
- Vedvyas C, Winterfield LS, Vleugels RA. Calciphylaxis: a systematic review of existing and emerging therapies. J Am Acad Dermatol. 2012;67:E253-E260.
- Beitz JM. Calciphylaxis: a case study with differential diagnosis. Ostomy Wound Manag. 2003;49:28-38.
- Daudén E, Oñate M. Calciphylaxis. Dermatol Clin. 2008;26:557-568.
- Oh DH, Eulau D, Tokugawa DA, et al. Five cases of calciphylaxis and a review of the literature. J Am Acad Dermatol. 1999;40:979-987.
- Weenig RH, Sewell LD, Davis MDP, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-578.
- Hanvesakul R, Silva MA, Hejmadi R, et al. Calciphylaxis following kidney transplantation: a case report. J Med Cases. 2009;3:9297.
- Kouba DJ, Owens NM, Barrett TL, et al. An unusual case of calciphylaxis. J Cutan Med Surg. 2004;8:19-22.
- Arch-Ferrer JE, Beenken SW, Rue LW, et al. Therapy for calciphylaxis: an outcome analysis. Surgery. 2003;134:941-945.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Mirza I, Chaubay D, Gunderia H, et al. An unusual presentation of calciphylaxis due to primary hyperparathyroidism. Arch Pathol Lab Med. 2001;125:1351-1353.
- Alain J, Poulin YP, Cloutier RA, et al. Calciphylaxis: seven new cases. J Cutan Med Surg. 2000;4:213-218.
- Hafner J, Keusch G, Wahl C, et al. Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa. 1998;27:137-143.
To the Editor:
Calciphylaxis (also known as calcific uremic arteriolopathy and calcifying panniculitis) is a rare vasculopathy affecting the small vessels.1 It is characterized by cutaneous ischemia and necrosis secondary to calcification. It is most commonly seen in patients with end-stage renal disease (ESRD) and hyperparathyroidism.1-3 Histopathologic features that are consistent with the diagnosis of calciphylaxis include calcification of medium-sized vessels in the deep dermis or subcutaneous fat as well as smaller distal vessels that supply the papillary dermis and epidermis.4,5 Although it commonly presents as well-demarcated, painful, purplish lesions that evolve into necrotic eschars, calciphylaxis rarely can present with hemorrhagic or serous bullous lesions followed by ulceration, as was seen in our patient.1,5,6 We report this uncommon presentation to highlight the variety in clinical appearance of calciphylaxis and the importance of early diagnosis.
A 43-year-old woman presented to the emergency department for evaluation of chest and abdominal pain that began 1 day prior to presentation. She had a history of systemic lupus erythematosus and ESRD secondary to poststreptococcal glomerulonephritis and was currently on peritoneal dialysis. The patient was admitted for peritonitis and treated with broad-spectrum antibiotics. At the time of admission, the patient also was noted to have several painful bullae on the legs. Her medical history also was remarkable for cerebral infarction, fibromyalgia, cerebral artery occlusion with cerebral infarction, sciatica, hyperlipidemia, deep vein thrombosis, and seizures. She had no history of herpes simplex virus. Surgical history was remarkable for tubal ligation, nephrectomy and kidney transplant, parathyroidectomy, and cholecystectomy. The patient’s medications included sevelamer carbonate, prednisone, epogen, calcium carbonate, esomeprazole, ondansetron, topical gentamicin, and atorvastatin.
Skin examination was performed by the inpatient dermatology service and revealed several tense, 1- to 5-cm, nonhemorrhagic bullae on the thighs and lower legs, some that had ruptured. The lesions were notably tender to palpation. No surrounding erythema, ecchymosis, or warmth was appreciated. The Nikolsky sign was negative. The patient also was noted to have at least grade 2 to 3+ pitting edema of the bilateral legs. The oral and conjunctival mucosae were unremarkable.
Antinuclear antibody, double-stranded DNA, and anti-Smith antibody levels were negative. A punch biopsy of the left lateral thigh revealed intraepidermal vesicular dermatitis with dermal edema suggestive of edema bullae and direct immunofluorescence was negative for immune complex and complement deposition.
Conservative therapy with wound care was recommended. The patient continued to report persistent severe skin pain and developed a subcutaneous nodule on the right inner thigh 1 week later, prompting a second biopsy. Results of the excisional biopsy were nondiagnostic but were suggestive of calciphylaxis, revealing subepidermal bullae with epidermal necrosis, a scant perivascular lymphocytic infiltrate, and extravasated erythrocytes. No evidence of calcification was seen within the vessels. The patient was then started on sodium thiosulfate with hemodialysis for treatment of presumed calciphylaxis.
Despite meticulous wound care and treatment with sodium thiosulfate, the patient developed ulcerations with necrotic eschars on the bilateral buttocks, hips, and thighs 1 month later (Figure 1). She subsequently worsened over the next few weeks. She developed sepsis and was transferred to the intensive care unit. A third biopsy was performed, finally confirming the diagnosis of calciphylaxis. Histopathology revealed small blood vessels with basophilic granular deposits in the walls consistent with calcium in the subcutaneous tissue (highlighted with the von Kossa stain), as well as thrombi in the lumens of some vessels; early fat necrosis; focal epidermal necrosis with underlying congested blood vessels with deposits in their walls; a perivascular infiltrate predominately of lymphocytes and neutrophils with scattered nuclear dust; and thick, hyalinized, closely crowded collagen bundles in the reticular dermis and in a widened subcutaneous septum (Figures 2 and 3).
Supportive care and pain control were continued, but the overall prognosis was determined to be very poor, and the patient eventually was discharged to hospice and died.
Although calciphylaxis is commonly seen in patients with ESRD and hyperparathyroidism, patients without renal disease also may develop the condition.2,3 Prior epidemiologic studies have shown a prevalence of 1% in patients with chronic kidney disease and up to 4% in those receiving dialysis.2-5 The average age at presentation is 48 years.6,7 Although calciphylaxis has been noted to affect males and females equally, some studies have suggested a female predominance.5-8
The etiology of calciphylaxis is unknown, but ESRD requiring dialysis, primary or secondary hyperparathyroidism, obesity, diabetes mellitus, skin trauma, and/or a hypercoagulable state may put patients at increased risk for developing this disease.2,3 Other risk factors include systemic corticosteroids, liver disease, increased serum aluminum, and increased erythrocyte sedimentation rate. Although high calcium-phosphate product has been noted as a risk factor in prior studies, one retrospective study found that it does not reliably confirm or exclude a diagnosis of calciphylaxis.8
The pathogenesis of calciphylaxis is not well understood; however, some researchers suggest that an imbalance in calcium-phosphate homeostasis may lead to calciphylaxis; that is, elevated calcium and phosphate levels exceed their solubility and deposit in the walls of small- and medium-sized arteries, which consequently leads to ischemic necrosis and gangrene of the surrounding tissue.9
Clinically, calciphylaxis has an indolent onset and usually presents as well-demarcated, painful, purplish, mottled lesions that evolve into necrotic gray-black eschars and gangrene in adjacent tissues.1,5,6 The ischemic process may even extend to the muscle layer.5 Other common presentations include mild erythematous patches; livedo reticularis; painful nodules; necrotic ulcerating lesions; and more rarely flaccid, hemorrhagic, or serous bullous lesions followed by ulceration, as was seen in our patient.6,9,10 Lesions usually begin at sites of trauma and seem to be distributed symmetrically.5,6 The most commonly affected locations are the legs, specifically the medial thighs, as well as the abdomen and buttocks, but lesions also can be found at more distal sites such as the breasts, tongue, vulva, penis, fingers, and toes.5,6,10 The head and neck region rarely is affected. Although uncommon, calciphylaxis may affect other organs, including the lungs, stomach, kidneys, and adrenal glands.5 The accompanying systemic symptoms and findings may include muscle weakness, tenderness, or myositis with rhabdomyolysis; calcific cerebral embolism; dementia and infarction of the central nervous system; acute respiratory failure; heart disease; atrioventricular block; and calcification of the cardiac conduction system.6 Unlike other forms of peripheral vascular disease, distal pulses are present in calciphylaxis, as blood flow usually is preserved distal and deep to the areas of necrosis.5,6
A careful history and thorough physical examination are important first steps in the diagnosis of this condition.2,10 Although there are no definitive laboratory tests, elevated serum calcium, phosphorous, and calcium-phosphate product levels, as well as parathyroid hormone level, may be suggestive of calciphylaxis.2,5 Leukocytosis may occur if an infection is present.5
The most accurate method to confirm the diagnosis is a deep incisional biopsy from an erythematous, slightly purpuric area adjacent to the necrotic lesion.2,10,11 The histopathologic features used to make the diagnosis include calcification of medium-sized vessels, particularly the intimal or medial layers, in the deep dermis and subcutaneous fat in addition to lobular capillaries of the subcutaneous fat.5,10 These vessels, including the smaller distal vessels that supply the papillary dermis and epidermis, also may be thrombosed due to calcification, leading to vascular occlusion and subsequently ischemic necrosis of the overlying epidermis.10 Other findings may include pseudoxanthoma elasticum changes, panniculitis, and subcutaneous fat necrosis.4,10
The differential diagnosis for calciphylaxis includes peripheral vascular disease, vasculitis, juvenile dermatomyositis, proteins C and S deficiencies, cryofibrinogenemia, calcinosis cutis, and tumoral calcinosis.2 Polyarteritis nodosa, Sjögren syndrome, atherosclerotic peripheral vascular disease, pyoderma gangrenosum, systemic lupus erythematosus, necrotizing fasciitis, septic embolism, and necrosis secondary to warfarin and heparin may mimic calciphylaxis.5
Treatment of calciphylaxis is multidimensional but primarily is supportive.6,11 Controlling calcium and phosphate levels and secondary hyperparathyroidism through diet and phosphate binders (eg, sevelamer hydrochloride) has been shown to be effective.6 Pamidronate, a bisphosphonate, inhibits arterial calcification in animal models and has been reported to treat calciphylaxis, resulting in marked pain reduction and ulcer healing.4,6 Cinacalcet, which functions as a calcimimetic, has been implicated in the treatment of calciphylaxis. It has been used to treat primary and secondary hyperparathyroidism and to normalize serum calcium levels; it also may be used as an alternative to parathyroidectomy.4,6 Intravenous administration of sodium thiosulfate, a potent antioxidant and chelator of calcium, has been helpful in reversing signs and symptoms of calciphylaxis.6,12 It also has been shown to effectively remove extra calcium during peritoneal dialysis.6 Parathyroidectomy has been useful in patients with markedly elevated parathyroid hormone levels, as it suppresses or eliminates the sensitizing agent causing hypercalcemia, elevated calcium-phosphate product, and hyperparathyroidism.1,2,6,13
Wound care and prevention of sepsis are essential in the treatment of calciphylaxis. Management options include surgical debridement, hydrocolloid and biologic dressings, skin grafts, systemic antibiotics, oral pentoxifylline combined with maggot therapy, nutritional support, hyperbaric oxygen therapy, and revascularization and amputation when other interventions have failed. Pain control with analgesics and correction of thrombosis in the skin and blood vessels via anticoagulation therapy also are important complementary treatments.6
The clinical outcome of calciphylaxis is dependent on early diagnosis, antimicrobial therapy, and wound management,9 but overall, the prognosis usually is poor and has a high mortality rate. The most common causes of death are infection and sepsis.1,9 A study of 7 cases reported 100% mortality,14 but other studies have suggested a mortality rate of 60% to 80%.4,10 Female sex and obesity are poor prognostic indicators.2 A better prognosis has been appreciated in cases in which lesions occur at distal sites (eg, lower legs, hands) compared to more proximal sites (eg, abdomen), where 25% and 75% mortalities have been noted, respectively.10,14,15 In one study, the overall mortality rate was 45% in patients with calciphylaxis at 1 year.6 The rate was 41% in patients with plaques only and 67% in those who presented with ulceration. Patients who survive often experience a high degree of morbidity and prolonged hospitalization; these patients often are severely debilitated, especially in the case of limb amputation.6
Our report of calciphylaxis demonstrates the diversity in clinical presentation and emphasizes the importance of early and accurate diagnosis in reducing morbidity and mortality. In our case, the patient presented with skin pain and tense nonhemorrhagic bullae without underlying ecchymotic or erythematous lesions as the earliest sign of calciphylaxis. Physicians should have a high degree of suspicion in the setting of dialysis-dependent ESRD patients with bullae, extreme pain, and continuous decline. We hope that this case will help increase awareness of the varying presentations of this condition.
To the Editor:
Calciphylaxis (also known as calcific uremic arteriolopathy and calcifying panniculitis) is a rare vasculopathy affecting the small vessels.1 It is characterized by cutaneous ischemia and necrosis secondary to calcification. It is most commonly seen in patients with end-stage renal disease (ESRD) and hyperparathyroidism.1-3 Histopathologic features that are consistent with the diagnosis of calciphylaxis include calcification of medium-sized vessels in the deep dermis or subcutaneous fat as well as smaller distal vessels that supply the papillary dermis and epidermis.4,5 Although it commonly presents as well-demarcated, painful, purplish lesions that evolve into necrotic eschars, calciphylaxis rarely can present with hemorrhagic or serous bullous lesions followed by ulceration, as was seen in our patient.1,5,6 We report this uncommon presentation to highlight the variety in clinical appearance of calciphylaxis and the importance of early diagnosis.
A 43-year-old woman presented to the emergency department for evaluation of chest and abdominal pain that began 1 day prior to presentation. She had a history of systemic lupus erythematosus and ESRD secondary to poststreptococcal glomerulonephritis and was currently on peritoneal dialysis. The patient was admitted for peritonitis and treated with broad-spectrum antibiotics. At the time of admission, the patient also was noted to have several painful bullae on the legs. Her medical history also was remarkable for cerebral infarction, fibromyalgia, cerebral artery occlusion with cerebral infarction, sciatica, hyperlipidemia, deep vein thrombosis, and seizures. She had no history of herpes simplex virus. Surgical history was remarkable for tubal ligation, nephrectomy and kidney transplant, parathyroidectomy, and cholecystectomy. The patient’s medications included sevelamer carbonate, prednisone, epogen, calcium carbonate, esomeprazole, ondansetron, topical gentamicin, and atorvastatin.
Skin examination was performed by the inpatient dermatology service and revealed several tense, 1- to 5-cm, nonhemorrhagic bullae on the thighs and lower legs, some that had ruptured. The lesions were notably tender to palpation. No surrounding erythema, ecchymosis, or warmth was appreciated. The Nikolsky sign was negative. The patient also was noted to have at least grade 2 to 3+ pitting edema of the bilateral legs. The oral and conjunctival mucosae were unremarkable.
Antinuclear antibody, double-stranded DNA, and anti-Smith antibody levels were negative. A punch biopsy of the left lateral thigh revealed intraepidermal vesicular dermatitis with dermal edema suggestive of edema bullae and direct immunofluorescence was negative for immune complex and complement deposition.
Conservative therapy with wound care was recommended. The patient continued to report persistent severe skin pain and developed a subcutaneous nodule on the right inner thigh 1 week later, prompting a second biopsy. Results of the excisional biopsy were nondiagnostic but were suggestive of calciphylaxis, revealing subepidermal bullae with epidermal necrosis, a scant perivascular lymphocytic infiltrate, and extravasated erythrocytes. No evidence of calcification was seen within the vessels. The patient was then started on sodium thiosulfate with hemodialysis for treatment of presumed calciphylaxis.
Despite meticulous wound care and treatment with sodium thiosulfate, the patient developed ulcerations with necrotic eschars on the bilateral buttocks, hips, and thighs 1 month later (Figure 1). She subsequently worsened over the next few weeks. She developed sepsis and was transferred to the intensive care unit. A third biopsy was performed, finally confirming the diagnosis of calciphylaxis. Histopathology revealed small blood vessels with basophilic granular deposits in the walls consistent with calcium in the subcutaneous tissue (highlighted with the von Kossa stain), as well as thrombi in the lumens of some vessels; early fat necrosis; focal epidermal necrosis with underlying congested blood vessels with deposits in their walls; a perivascular infiltrate predominately of lymphocytes and neutrophils with scattered nuclear dust; and thick, hyalinized, closely crowded collagen bundles in the reticular dermis and in a widened subcutaneous septum (Figures 2 and 3).
Supportive care and pain control were continued, but the overall prognosis was determined to be very poor, and the patient eventually was discharged to hospice and died.
Although calciphylaxis is commonly seen in patients with ESRD and hyperparathyroidism, patients without renal disease also may develop the condition.2,3 Prior epidemiologic studies have shown a prevalence of 1% in patients with chronic kidney disease and up to 4% in those receiving dialysis.2-5 The average age at presentation is 48 years.6,7 Although calciphylaxis has been noted to affect males and females equally, some studies have suggested a female predominance.5-8
The etiology of calciphylaxis is unknown, but ESRD requiring dialysis, primary or secondary hyperparathyroidism, obesity, diabetes mellitus, skin trauma, and/or a hypercoagulable state may put patients at increased risk for developing this disease.2,3 Other risk factors include systemic corticosteroids, liver disease, increased serum aluminum, and increased erythrocyte sedimentation rate. Although high calcium-phosphate product has been noted as a risk factor in prior studies, one retrospective study found that it does not reliably confirm or exclude a diagnosis of calciphylaxis.8
The pathogenesis of calciphylaxis is not well understood; however, some researchers suggest that an imbalance in calcium-phosphate homeostasis may lead to calciphylaxis; that is, elevated calcium and phosphate levels exceed their solubility and deposit in the walls of small- and medium-sized arteries, which consequently leads to ischemic necrosis and gangrene of the surrounding tissue.9
Clinically, calciphylaxis has an indolent onset and usually presents as well-demarcated, painful, purplish, mottled lesions that evolve into necrotic gray-black eschars and gangrene in adjacent tissues.1,5,6 The ischemic process may even extend to the muscle layer.5 Other common presentations include mild erythematous patches; livedo reticularis; painful nodules; necrotic ulcerating lesions; and more rarely flaccid, hemorrhagic, or serous bullous lesions followed by ulceration, as was seen in our patient.6,9,10 Lesions usually begin at sites of trauma and seem to be distributed symmetrically.5,6 The most commonly affected locations are the legs, specifically the medial thighs, as well as the abdomen and buttocks, but lesions also can be found at more distal sites such as the breasts, tongue, vulva, penis, fingers, and toes.5,6,10 The head and neck region rarely is affected. Although uncommon, calciphylaxis may affect other organs, including the lungs, stomach, kidneys, and adrenal glands.5 The accompanying systemic symptoms and findings may include muscle weakness, tenderness, or myositis with rhabdomyolysis; calcific cerebral embolism; dementia and infarction of the central nervous system; acute respiratory failure; heart disease; atrioventricular block; and calcification of the cardiac conduction system.6 Unlike other forms of peripheral vascular disease, distal pulses are present in calciphylaxis, as blood flow usually is preserved distal and deep to the areas of necrosis.5,6
A careful history and thorough physical examination are important first steps in the diagnosis of this condition.2,10 Although there are no definitive laboratory tests, elevated serum calcium, phosphorous, and calcium-phosphate product levels, as well as parathyroid hormone level, may be suggestive of calciphylaxis.2,5 Leukocytosis may occur if an infection is present.5
The most accurate method to confirm the diagnosis is a deep incisional biopsy from an erythematous, slightly purpuric area adjacent to the necrotic lesion.2,10,11 The histopathologic features used to make the diagnosis include calcification of medium-sized vessels, particularly the intimal or medial layers, in the deep dermis and subcutaneous fat in addition to lobular capillaries of the subcutaneous fat.5,10 These vessels, including the smaller distal vessels that supply the papillary dermis and epidermis, also may be thrombosed due to calcification, leading to vascular occlusion and subsequently ischemic necrosis of the overlying epidermis.10 Other findings may include pseudoxanthoma elasticum changes, panniculitis, and subcutaneous fat necrosis.4,10
The differential diagnosis for calciphylaxis includes peripheral vascular disease, vasculitis, juvenile dermatomyositis, proteins C and S deficiencies, cryofibrinogenemia, calcinosis cutis, and tumoral calcinosis.2 Polyarteritis nodosa, Sjögren syndrome, atherosclerotic peripheral vascular disease, pyoderma gangrenosum, systemic lupus erythematosus, necrotizing fasciitis, septic embolism, and necrosis secondary to warfarin and heparin may mimic calciphylaxis.5
Treatment of calciphylaxis is multidimensional but primarily is supportive.6,11 Controlling calcium and phosphate levels and secondary hyperparathyroidism through diet and phosphate binders (eg, sevelamer hydrochloride) has been shown to be effective.6 Pamidronate, a bisphosphonate, inhibits arterial calcification in animal models and has been reported to treat calciphylaxis, resulting in marked pain reduction and ulcer healing.4,6 Cinacalcet, which functions as a calcimimetic, has been implicated in the treatment of calciphylaxis. It has been used to treat primary and secondary hyperparathyroidism and to normalize serum calcium levels; it also may be used as an alternative to parathyroidectomy.4,6 Intravenous administration of sodium thiosulfate, a potent antioxidant and chelator of calcium, has been helpful in reversing signs and symptoms of calciphylaxis.6,12 It also has been shown to effectively remove extra calcium during peritoneal dialysis.6 Parathyroidectomy has been useful in patients with markedly elevated parathyroid hormone levels, as it suppresses or eliminates the sensitizing agent causing hypercalcemia, elevated calcium-phosphate product, and hyperparathyroidism.1,2,6,13
Wound care and prevention of sepsis are essential in the treatment of calciphylaxis. Management options include surgical debridement, hydrocolloid and biologic dressings, skin grafts, systemic antibiotics, oral pentoxifylline combined with maggot therapy, nutritional support, hyperbaric oxygen therapy, and revascularization and amputation when other interventions have failed. Pain control with analgesics and correction of thrombosis in the skin and blood vessels via anticoagulation therapy also are important complementary treatments.6
The clinical outcome of calciphylaxis is dependent on early diagnosis, antimicrobial therapy, and wound management,9 but overall, the prognosis usually is poor and has a high mortality rate. The most common causes of death are infection and sepsis.1,9 A study of 7 cases reported 100% mortality,14 but other studies have suggested a mortality rate of 60% to 80%.4,10 Female sex and obesity are poor prognostic indicators.2 A better prognosis has been appreciated in cases in which lesions occur at distal sites (eg, lower legs, hands) compared to more proximal sites (eg, abdomen), where 25% and 75% mortalities have been noted, respectively.10,14,15 In one study, the overall mortality rate was 45% in patients with calciphylaxis at 1 year.6 The rate was 41% in patients with plaques only and 67% in those who presented with ulceration. Patients who survive often experience a high degree of morbidity and prolonged hospitalization; these patients often are severely debilitated, especially in the case of limb amputation.6
Our report of calciphylaxis demonstrates the diversity in clinical presentation and emphasizes the importance of early and accurate diagnosis in reducing morbidity and mortality. In our case, the patient presented with skin pain and tense nonhemorrhagic bullae without underlying ecchymotic or erythematous lesions as the earliest sign of calciphylaxis. Physicians should have a high degree of suspicion in the setting of dialysis-dependent ESRD patients with bullae, extreme pain, and continuous decline. We hope that this case will help increase awareness of the varying presentations of this condition.
- Hanafusa T, Yamaguchi Y, Tani M, et al. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol. 2001;57:1021-1025.
- Somorin AO, Harbi AA, Subaity Y, et al. Calciphylaxis: case report and literature review. Afr J Med Sci. 2002;31:175-178.
- Barreiros HM, Goulão J, Cunha H, et al. Calciphylaxis: a diagnostic and therapeutic challenge. J Dermatol Case Rep. 2013;2:69-70.
- Vedvyas C, Winterfield LS, Vleugels RA. Calciphylaxis: a systematic review of existing and emerging therapies. J Am Acad Dermatol. 2012;67:E253-E260.
- Beitz JM. Calciphylaxis: a case study with differential diagnosis. Ostomy Wound Manag. 2003;49:28-38.
- Daudén E, Oñate M. Calciphylaxis. Dermatol Clin. 2008;26:557-568.
- Oh DH, Eulau D, Tokugawa DA, et al. Five cases of calciphylaxis and a review of the literature. J Am Acad Dermatol. 1999;40:979-987.
- Weenig RH, Sewell LD, Davis MDP, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-578.
- Hanvesakul R, Silva MA, Hejmadi R, et al. Calciphylaxis following kidney transplantation: a case report. J Med Cases. 2009;3:9297.
- Kouba DJ, Owens NM, Barrett TL, et al. An unusual case of calciphylaxis. J Cutan Med Surg. 2004;8:19-22.
- Arch-Ferrer JE, Beenken SW, Rue LW, et al. Therapy for calciphylaxis: an outcome analysis. Surgery. 2003;134:941-945.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Mirza I, Chaubay D, Gunderia H, et al. An unusual presentation of calciphylaxis due to primary hyperparathyroidism. Arch Pathol Lab Med. 2001;125:1351-1353.
- Alain J, Poulin YP, Cloutier RA, et al. Calciphylaxis: seven new cases. J Cutan Med Surg. 2000;4:213-218.
- Hafner J, Keusch G, Wahl C, et al. Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa. 1998;27:137-143.
- Hanafusa T, Yamaguchi Y, Tani M, et al. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol. 2001;57:1021-1025.
- Somorin AO, Harbi AA, Subaity Y, et al. Calciphylaxis: case report and literature review. Afr J Med Sci. 2002;31:175-178.
- Barreiros HM, Goulão J, Cunha H, et al. Calciphylaxis: a diagnostic and therapeutic challenge. J Dermatol Case Rep. 2013;2:69-70.
- Vedvyas C, Winterfield LS, Vleugels RA. Calciphylaxis: a systematic review of existing and emerging therapies. J Am Acad Dermatol. 2012;67:E253-E260.
- Beitz JM. Calciphylaxis: a case study with differential diagnosis. Ostomy Wound Manag. 2003;49:28-38.
- Daudén E, Oñate M. Calciphylaxis. Dermatol Clin. 2008;26:557-568.
- Oh DH, Eulau D, Tokugawa DA, et al. Five cases of calciphylaxis and a review of the literature. J Am Acad Dermatol. 1999;40:979-987.
- Weenig RH, Sewell LD, Davis MDP, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-578.
- Hanvesakul R, Silva MA, Hejmadi R, et al. Calciphylaxis following kidney transplantation: a case report. J Med Cases. 2009;3:9297.
- Kouba DJ, Owens NM, Barrett TL, et al. An unusual case of calciphylaxis. J Cutan Med Surg. 2004;8:19-22.
- Arch-Ferrer JE, Beenken SW, Rue LW, et al. Therapy for calciphylaxis: an outcome analysis. Surgery. 2003;134:941-945.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Mirza I, Chaubay D, Gunderia H, et al. An unusual presentation of calciphylaxis due to primary hyperparathyroidism. Arch Pathol Lab Med. 2001;125:1351-1353.
- Alain J, Poulin YP, Cloutier RA, et al. Calciphylaxis: seven new cases. J Cutan Med Surg. 2000;4:213-218.
- Hafner J, Keusch G, Wahl C, et al. Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa. 1998;27:137-143.
Practice Points
- Calciphylaxis is a rare microvascular occlusion syndrome characterized by cutaneous ischemia and necrosis secondary to calcification.
- Clinically, lesions present with severely painful, violaceous, retiform patches and plaques, and less commonly bullae that progress to necrotic ulcers on the buttocks, legs, or abdomen, which is most often associated with end-stage renal disease and hyperparathyroidism.
- The diagnosis is made through deep wedge or excisional biopsy and shows calcification of medium-sized vessels in the deep dermis and subcutaneous fat. Treatment requires a multidisciplinary approach, but morbidity and mortality remain high.
Solitary Warty Mucosal Lesion on the Hard Palate
The Diagnosis: Solitary Oral Condyloma Lata of Secondary Syphilis
A punch biopsy of the lesion revealed acanthosis with elongation of rete ridges; interface dermatitis; and a moderately dense, predominantly lymphoid dermal infiltration (Figure). Based on a serologic toluidine red unheated serum test (TRUST) titer of 1:64 and positive Treponema antibodies, a diagnosis of secondary syphilitic infection was made. A test for human immunodeficiency virus infection was negative, and the patient was not immunocompromised. Due to allergy to benzathine penicillin G, she was prescribed oral minocycline 100 mg twice daily for 15 days. (See the Table for current recommended regimens from the Centers for Disease Control and Prevention for the treatment of syphilis.1) The hard palate plaque began to fade after 2 days of treatment and completely regressed 2 weeks later. The TRUST titer decreased to 1:4 after 6 months.
The patient's husband was examined following confirmation of his wife's infection; his TRUST titer was 1:64 and Treponema antibodies were positive. No skin lesions were detected. A test for human immunodeficiency virus infection also was negative. Further inquiry revealed that he had had sexual intercourse with a prostitute about 3 months prior. He was diagnosed with latent syphilis and prescribed the same medication regimen as his wife. However, after 6 months, his TRUST titer was still 1:64, possibly due to irregular medication use.
Secondary syphilis often is preceded by flulike symptoms of fever, sore throat, headache, malaise, generalized painless lymphadenopathy, and myalgia 4 to 10 weeks after onset of infection.2-5 Condyloma lata can be one of the characteristic mucosal signs of secondary syphilis; however, it is typically located in the anogenital area or less commonly in atypical areas such as the umbilicus, axillae, inframammary folds, and toe web spaces.6 Condyloma lata in the oral cavity is rare. In fact, this unusual manifestation prompted the patient to suspect cancer and she initially presented to a local tumor hospital. However, oral computed tomography did not detect any tumor cells, and subsequent testing yielded the diagnosis of secondary syphilis.
The differential diagnosis for a warty oral mass includes squamous cell carcinoma, condyloma acuminatum, oral submucous fibrosis, and Wegener granulomatosis.
Similar to other nontreponemal tests, TRUST is a flocculation-based quantitative test that can be used to follow treatment response, as its antibody titers may correlate with disease activity.7 Clinically, a 4-fold change in titer (equivalent to a change of 2 dilutions) is considered necessary to demonstrate a notable difference between 2 nontreponemal test results obtained using the same serologic test. The TRUST titers for the case patient decreased from 1:64 to 1:4, indicating a good response to minocycline. In contrast, the TRUST of her husband remained as high at 6-month follow-up as it had been at initial examination. This serofast state was most likely related to his irregular medication use; however, other possibilities should be considered, including confounding nontreponemal inflammatory conditions in the host, the variability of host response to infection, or even persistent low-level infection with Treponema pallidum.8 Because treponemal antibodies typically remain positive for life and most patients who have a reactive treponemal test will have a reactive report for the remainder of their lives, regardless of treatment or disease activity, treponemal antibody titers should not be used to monitor treatment response.9
China has experienced a resurgence in the incidence and prevalence of syphilis in recent decades. According to the national reporting database, the annual rate of syphilis in China has increased 14.3% since 2009 (6.5 cases per 100,000 population in 1999 vs 24.66 cases per 100,000 population in 2009).10 This re-emergence is truly remarkable, given this infection was virtually eradicated in the country 60 years ago. Recognizing this syphilis epidemic as a public health threat, the Ministry of Health of the People's Republic of China in 2010 announced a 10-year plan for syphilis control and prevention to curb the spread of syphilis and other sexually transmitted diseases. Currently, the syphilis burden is still great, with 25.54 cases per 100,000 population in 2016,11 but the situation has been stabilized and the annual increase is less than 1% since the plan's introduction.
Globally, there has been a marked resurgence of syphilis in the last decade, largely attributed to changing social and behavioral factors, especially among the population of men who have sex with men. Despite the availability of effective treatments and previously reliable prevention strategies, there are an estimated 6 million new cases of syphilis in those aged 15 to 49 years, and congenital syphilis causes more than 300,000 fetal and neonatal deaths each year.12 Continued vigilance and investment is needed to combat syphilis worldwide, and recognition of syphilis, with its versatile presentations, is of vital importance today.13
The presentation of secondary syphilis can be highly variable and requires a high level of awareness.4-6 Solitary oral involvement in secondary syphilis is rare and can lead to misdiagnosis; therefore, a high level of suspicion for syphilis should be maintained when evaluating oral lesions.
- Centers for Disease Control and Prevention. 2015 SexuallyTransmitted Diseases Treatment Guidelines: Syphilis. https://www.cdc.gov/std/tg2015/syphilis.htm. Accessed March 25, 2020.
- Lombardo J, Alhashim M. Secondary syphilis: an atypical presentation complicated by a false negative rapid plasma reagin test. Cutis. 2018;101:E11-E13.
- Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283-290.
- Dourmishev LA, Assen L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Martin DH, Mroczkowski TF. Dermatological manifestations of sexually transmitted diseases other than HIV. Infect Dis Clin North Am. 1994;8:533-583.
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277.
- Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015;22:137-147.
- Seña AC, Wolff M, Behets F, et al. Response to therapy following retreatment of serofast early syphilis patients with benzathine penicillin. Clin Infect Dis. 2013;56:420-422.
- Rhoads DD, Genzen JR, Bashleben CP, et al. Prevalence of traditional and reverse-algorithm syphilis screening in laboratory practice: a survey of participants in the College of American Pathologists syphilis serology proficiency testing program. Arch Pathol Lab Med. 2017;141:93-97.
- Tucker JD, Cohen MS. China's syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011;24:50-55.
- Yang S, Wu J, Ding C, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. 2016;17:716-725.
- Noah K, Jeffrey DK. An update on the global epidemiology of syphilis. Curr Epidemiol Rep. 2018;5:24-38.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
The Diagnosis: Solitary Oral Condyloma Lata of Secondary Syphilis
A punch biopsy of the lesion revealed acanthosis with elongation of rete ridges; interface dermatitis; and a moderately dense, predominantly lymphoid dermal infiltration (Figure). Based on a serologic toluidine red unheated serum test (TRUST) titer of 1:64 and positive Treponema antibodies, a diagnosis of secondary syphilitic infection was made. A test for human immunodeficiency virus infection was negative, and the patient was not immunocompromised. Due to allergy to benzathine penicillin G, she was prescribed oral minocycline 100 mg twice daily for 15 days. (See the Table for current recommended regimens from the Centers for Disease Control and Prevention for the treatment of syphilis.1) The hard palate plaque began to fade after 2 days of treatment and completely regressed 2 weeks later. The TRUST titer decreased to 1:4 after 6 months.
The patient's husband was examined following confirmation of his wife's infection; his TRUST titer was 1:64 and Treponema antibodies were positive. No skin lesions were detected. A test for human immunodeficiency virus infection also was negative. Further inquiry revealed that he had had sexual intercourse with a prostitute about 3 months prior. He was diagnosed with latent syphilis and prescribed the same medication regimen as his wife. However, after 6 months, his TRUST titer was still 1:64, possibly due to irregular medication use.
Secondary syphilis often is preceded by flulike symptoms of fever, sore throat, headache, malaise, generalized painless lymphadenopathy, and myalgia 4 to 10 weeks after onset of infection.2-5 Condyloma lata can be one of the characteristic mucosal signs of secondary syphilis; however, it is typically located in the anogenital area or less commonly in atypical areas such as the umbilicus, axillae, inframammary folds, and toe web spaces.6 Condyloma lata in the oral cavity is rare. In fact, this unusual manifestation prompted the patient to suspect cancer and she initially presented to a local tumor hospital. However, oral computed tomography did not detect any tumor cells, and subsequent testing yielded the diagnosis of secondary syphilis.
The differential diagnosis for a warty oral mass includes squamous cell carcinoma, condyloma acuminatum, oral submucous fibrosis, and Wegener granulomatosis.
Similar to other nontreponemal tests, TRUST is a flocculation-based quantitative test that can be used to follow treatment response, as its antibody titers may correlate with disease activity.7 Clinically, a 4-fold change in titer (equivalent to a change of 2 dilutions) is considered necessary to demonstrate a notable difference between 2 nontreponemal test results obtained using the same serologic test. The TRUST titers for the case patient decreased from 1:64 to 1:4, indicating a good response to minocycline. In contrast, the TRUST of her husband remained as high at 6-month follow-up as it had been at initial examination. This serofast state was most likely related to his irregular medication use; however, other possibilities should be considered, including confounding nontreponemal inflammatory conditions in the host, the variability of host response to infection, or even persistent low-level infection with Treponema pallidum.8 Because treponemal antibodies typically remain positive for life and most patients who have a reactive treponemal test will have a reactive report for the remainder of their lives, regardless of treatment or disease activity, treponemal antibody titers should not be used to monitor treatment response.9
China has experienced a resurgence in the incidence and prevalence of syphilis in recent decades. According to the national reporting database, the annual rate of syphilis in China has increased 14.3% since 2009 (6.5 cases per 100,000 population in 1999 vs 24.66 cases per 100,000 population in 2009).10 This re-emergence is truly remarkable, given this infection was virtually eradicated in the country 60 years ago. Recognizing this syphilis epidemic as a public health threat, the Ministry of Health of the People's Republic of China in 2010 announced a 10-year plan for syphilis control and prevention to curb the spread of syphilis and other sexually transmitted diseases. Currently, the syphilis burden is still great, with 25.54 cases per 100,000 population in 2016,11 but the situation has been stabilized and the annual increase is less than 1% since the plan's introduction.
Globally, there has been a marked resurgence of syphilis in the last decade, largely attributed to changing social and behavioral factors, especially among the population of men who have sex with men. Despite the availability of effective treatments and previously reliable prevention strategies, there are an estimated 6 million new cases of syphilis in those aged 15 to 49 years, and congenital syphilis causes more than 300,000 fetal and neonatal deaths each year.12 Continued vigilance and investment is needed to combat syphilis worldwide, and recognition of syphilis, with its versatile presentations, is of vital importance today.13
The presentation of secondary syphilis can be highly variable and requires a high level of awareness.4-6 Solitary oral involvement in secondary syphilis is rare and can lead to misdiagnosis; therefore, a high level of suspicion for syphilis should be maintained when evaluating oral lesions.
The Diagnosis: Solitary Oral Condyloma Lata of Secondary Syphilis
A punch biopsy of the lesion revealed acanthosis with elongation of rete ridges; interface dermatitis; and a moderately dense, predominantly lymphoid dermal infiltration (Figure). Based on a serologic toluidine red unheated serum test (TRUST) titer of 1:64 and positive Treponema antibodies, a diagnosis of secondary syphilitic infection was made. A test for human immunodeficiency virus infection was negative, and the patient was not immunocompromised. Due to allergy to benzathine penicillin G, she was prescribed oral minocycline 100 mg twice daily for 15 days. (See the Table for current recommended regimens from the Centers for Disease Control and Prevention for the treatment of syphilis.1) The hard palate plaque began to fade after 2 days of treatment and completely regressed 2 weeks later. The TRUST titer decreased to 1:4 after 6 months.
The patient's husband was examined following confirmation of his wife's infection; his TRUST titer was 1:64 and Treponema antibodies were positive. No skin lesions were detected. A test for human immunodeficiency virus infection also was negative. Further inquiry revealed that he had had sexual intercourse with a prostitute about 3 months prior. He was diagnosed with latent syphilis and prescribed the same medication regimen as his wife. However, after 6 months, his TRUST titer was still 1:64, possibly due to irregular medication use.
Secondary syphilis often is preceded by flulike symptoms of fever, sore throat, headache, malaise, generalized painless lymphadenopathy, and myalgia 4 to 10 weeks after onset of infection.2-5 Condyloma lata can be one of the characteristic mucosal signs of secondary syphilis; however, it is typically located in the anogenital area or less commonly in atypical areas such as the umbilicus, axillae, inframammary folds, and toe web spaces.6 Condyloma lata in the oral cavity is rare. In fact, this unusual manifestation prompted the patient to suspect cancer and she initially presented to a local tumor hospital. However, oral computed tomography did not detect any tumor cells, and subsequent testing yielded the diagnosis of secondary syphilis.
The differential diagnosis for a warty oral mass includes squamous cell carcinoma, condyloma acuminatum, oral submucous fibrosis, and Wegener granulomatosis.
Similar to other nontreponemal tests, TRUST is a flocculation-based quantitative test that can be used to follow treatment response, as its antibody titers may correlate with disease activity.7 Clinically, a 4-fold change in titer (equivalent to a change of 2 dilutions) is considered necessary to demonstrate a notable difference between 2 nontreponemal test results obtained using the same serologic test. The TRUST titers for the case patient decreased from 1:64 to 1:4, indicating a good response to minocycline. In contrast, the TRUST of her husband remained as high at 6-month follow-up as it had been at initial examination. This serofast state was most likely related to his irregular medication use; however, other possibilities should be considered, including confounding nontreponemal inflammatory conditions in the host, the variability of host response to infection, or even persistent low-level infection with Treponema pallidum.8 Because treponemal antibodies typically remain positive for life and most patients who have a reactive treponemal test will have a reactive report for the remainder of their lives, regardless of treatment or disease activity, treponemal antibody titers should not be used to monitor treatment response.9
China has experienced a resurgence in the incidence and prevalence of syphilis in recent decades. According to the national reporting database, the annual rate of syphilis in China has increased 14.3% since 2009 (6.5 cases per 100,000 population in 1999 vs 24.66 cases per 100,000 population in 2009).10 This re-emergence is truly remarkable, given this infection was virtually eradicated in the country 60 years ago. Recognizing this syphilis epidemic as a public health threat, the Ministry of Health of the People's Republic of China in 2010 announced a 10-year plan for syphilis control and prevention to curb the spread of syphilis and other sexually transmitted diseases. Currently, the syphilis burden is still great, with 25.54 cases per 100,000 population in 2016,11 but the situation has been stabilized and the annual increase is less than 1% since the plan's introduction.
Globally, there has been a marked resurgence of syphilis in the last decade, largely attributed to changing social and behavioral factors, especially among the population of men who have sex with men. Despite the availability of effective treatments and previously reliable prevention strategies, there are an estimated 6 million new cases of syphilis in those aged 15 to 49 years, and congenital syphilis causes more than 300,000 fetal and neonatal deaths each year.12 Continued vigilance and investment is needed to combat syphilis worldwide, and recognition of syphilis, with its versatile presentations, is of vital importance today.13
The presentation of secondary syphilis can be highly variable and requires a high level of awareness.4-6 Solitary oral involvement in secondary syphilis is rare and can lead to misdiagnosis; therefore, a high level of suspicion for syphilis should be maintained when evaluating oral lesions.
- Centers for Disease Control and Prevention. 2015 SexuallyTransmitted Diseases Treatment Guidelines: Syphilis. https://www.cdc.gov/std/tg2015/syphilis.htm. Accessed March 25, 2020.
- Lombardo J, Alhashim M. Secondary syphilis: an atypical presentation complicated by a false negative rapid plasma reagin test. Cutis. 2018;101:E11-E13.
- Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283-290.
- Dourmishev LA, Assen L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Martin DH, Mroczkowski TF. Dermatological manifestations of sexually transmitted diseases other than HIV. Infect Dis Clin North Am. 1994;8:533-583.
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277.
- Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015;22:137-147.
- Seña AC, Wolff M, Behets F, et al. Response to therapy following retreatment of serofast early syphilis patients with benzathine penicillin. Clin Infect Dis. 2013;56:420-422.
- Rhoads DD, Genzen JR, Bashleben CP, et al. Prevalence of traditional and reverse-algorithm syphilis screening in laboratory practice: a survey of participants in the College of American Pathologists syphilis serology proficiency testing program. Arch Pathol Lab Med. 2017;141:93-97.
- Tucker JD, Cohen MS. China's syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011;24:50-55.
- Yang S, Wu J, Ding C, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. 2016;17:716-725.
- Noah K, Jeffrey DK. An update on the global epidemiology of syphilis. Curr Epidemiol Rep. 2018;5:24-38.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Centers for Disease Control and Prevention. 2015 SexuallyTransmitted Diseases Treatment Guidelines: Syphilis. https://www.cdc.gov/std/tg2015/syphilis.htm. Accessed March 25, 2020.
- Lombardo J, Alhashim M. Secondary syphilis: an atypical presentation complicated by a false negative rapid plasma reagin test. Cutis. 2018;101:E11-E13.
- Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283-290.
- Dourmishev LA, Assen L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Martin DH, Mroczkowski TF. Dermatological manifestations of sexually transmitted diseases other than HIV. Infect Dis Clin North Am. 1994;8:533-583.
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277.
- Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015;22:137-147.
- Seña AC, Wolff M, Behets F, et al. Response to therapy following retreatment of serofast early syphilis patients with benzathine penicillin. Clin Infect Dis. 2013;56:420-422.
- Rhoads DD, Genzen JR, Bashleben CP, et al. Prevalence of traditional and reverse-algorithm syphilis screening in laboratory practice: a survey of participants in the College of American Pathologists syphilis serology proficiency testing program. Arch Pathol Lab Med. 2017;141:93-97.
- Tucker JD, Cohen MS. China's syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011;24:50-55.
- Yang S, Wu J, Ding C, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. 2016;17:716-725.
- Noah K, Jeffrey DK. An update on the global epidemiology of syphilis. Curr Epidemiol Rep. 2018;5:24-38.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
A 50-year-old Chinese woman presented with a painless, well-demarcated, nontender, elevated, flat-topped verrucous plaque on the hard palate of 1 month's duration. The lesion measured 2 cm in diameter. The patient reported no other dermatologic or systemic concerns, and no other skin or genital lesions were observed.
Coronavirus and Dermatology: A Resident’s Perspective
On January 30, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a public health emergency of international concern.1 Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is an enveloped, single-stranded RNA virus. It is the seventh known coronavirus to infect humans and third zoonotic Coronaviridae to cause fatal respiratory illness, along with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).2 There has been a rapid shift in the geographic center of the outbreak as well as the numbers of confirmed cases and deaths. Although the first cases in late 2019 and early 2020 were in China, by mid-March Italy became the center of the pandemic, with a steep increase in the number of cases in other European countries and the United States.3 Although COVID-19 does not have known dermatologic manifestations, it has the potential for wide-reaching impact on our field.
Strained Resources
In the United States, COVID-19 initially was associated with international travel but is now rapidly spreading throughout the community. I am currently a dermatology resident at New York-Presbyterian, Columbia campus, in New York, New York, a city that unfortunately finds itself underprepared to handle this unprecedented crisis. As of Monday, March 16, 2020, New York-Presbyterian made the decision to postpone all elective procedures, including Mohs micrographic surgery, to preserve hospital resources, including trained personnel, personal protective equipment, ventilators, and hospital beds. There have not been clear-cut guidelines regarding how to approach other dermatologic care for our patients, including routine clinic visits and inpatient dermatology consultations, leaving decisions up to individual departments and providers.
It would be prudent to learn from our colleagues in China who report steps that have been successful in preventing nosocomial spread of COVID-19 in the dermatologic setting. Tao et al4 described their protocols in both the outpatient and inpatient dermatologic setting, beginning with strict triage before patients can even enter a clinic building for their outpatient appointment. Those who screen positive are sent to a fever clinic for further evaluation, which may include a rapid computed tomography scan (using a machine that may perform 200 chest computed tomography scans per day) and SARS-CoV-2 polymerase chain reaction.5 For inpatient dermatologic consultation of patients with known COVID-19, telehealth and multidisciplinary meetings are first and second line, respectively, with bedside dermatologic consultation as a last resort.4 Chen et al6 described similarly strict triage protocols as well as physician use of full-body personal protective equipment during all patient encounters. These measures are taken in light of the well-documented phenomenon of asymptomatic carriage and transmission, as all patients entering their dermatologic clinics have screened negative for symptomatic SARS-CoV-2 infection.6
Conferences and Education
Coronavirus is impacting the education of millions of individuals worldwide, including that of dermatology residents. The Annual Meeting of the American Academy of Dermatology, which was scheduled to take place in March 2020, was canceled due to COVID-19.7 The American Board of Dermatology has released a statement indicating that for all dermatology residents, time spent in COVID-19–mandated quarantine will count as clinical education if residents are able to work with their program to complete independent structured academic activity during that time.8 We also must consider the possibility that dermatology residents are reassigned to work outside of our specialty, resulting in less time and experience caring for patients with dermatologic conditions. Dermatologists in other countries have been called upon to care for COVID-19 patients, even reported to be working in intensive care units in Italy.9 Virtual technologies may be used in novel ways to support dermatology resident education throughout this process.
Final Thoughts
As physicians, dermatologists are in the position to educate their patients regarding prevention strategies, especially given that the lay press disseminates confusing and inaccurate information. The World Health Organization provides specific guidance, focusing on handwashing, respiratory hygiene, social distancing, and encouraging symptomatic patients to seek remote care whenever possible.10 Many of our patients are at high risk of complications due to COVID-19, whether due to their age or because they are immunocompromised. As the situation unfolds, the impact on and role of dermatology in this crisis will continue to evolve.
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[news release]. Geneva, Switzerland: World Health Organization; January 30, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29. Accessed March 16, 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- World Health Organization. Coronavirus disease 2019: Situation Report—58. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 19, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- McNeil DG. Jr. Inside China’s all out war on the coronavirus. The New York Times. March 4, 2020. https://www.nytimes.com/2020/03/04/health/coronavirus-china-aylward.html?smid=tw-nytimes&smtyp=cur. Accessed March 19, 2020.
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol. 2020;82:1034.
- Hruza GJ. 2020 annual AAD meeting is canceled due to COVID-19 outbreak. American Academy of Dermatology website. https://www.aad.org/member/meetings/am2020/faqs/coronavirus. Accessed March 16, 2020.
- American Board of Dermatology. Impact of COVID-19 on dermatology resident education. March 6, 2020. https://www.abderm.org/2978.aspx. Accessed March 16, 2020.
- “It’s Like a War” [podcast]. The Daily. March 17, 2020. https://www.nytimes.com/2020/03/17/podcasts/the-daily/italy-coronavirus.html?action=click&module=audio-series-bar®ion=header&pgtype=Article. Accessed March 20, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for public. March 18, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 19, 2020.
On January 30, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a public health emergency of international concern.1 Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is an enveloped, single-stranded RNA virus. It is the seventh known coronavirus to infect humans and third zoonotic Coronaviridae to cause fatal respiratory illness, along with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).2 There has been a rapid shift in the geographic center of the outbreak as well as the numbers of confirmed cases and deaths. Although the first cases in late 2019 and early 2020 were in China, by mid-March Italy became the center of the pandemic, with a steep increase in the number of cases in other European countries and the United States.3 Although COVID-19 does not have known dermatologic manifestations, it has the potential for wide-reaching impact on our field.
Strained Resources
In the United States, COVID-19 initially was associated with international travel but is now rapidly spreading throughout the community. I am currently a dermatology resident at New York-Presbyterian, Columbia campus, in New York, New York, a city that unfortunately finds itself underprepared to handle this unprecedented crisis. As of Monday, March 16, 2020, New York-Presbyterian made the decision to postpone all elective procedures, including Mohs micrographic surgery, to preserve hospital resources, including trained personnel, personal protective equipment, ventilators, and hospital beds. There have not been clear-cut guidelines regarding how to approach other dermatologic care for our patients, including routine clinic visits and inpatient dermatology consultations, leaving decisions up to individual departments and providers.
It would be prudent to learn from our colleagues in China who report steps that have been successful in preventing nosocomial spread of COVID-19 in the dermatologic setting. Tao et al4 described their protocols in both the outpatient and inpatient dermatologic setting, beginning with strict triage before patients can even enter a clinic building for their outpatient appointment. Those who screen positive are sent to a fever clinic for further evaluation, which may include a rapid computed tomography scan (using a machine that may perform 200 chest computed tomography scans per day) and SARS-CoV-2 polymerase chain reaction.5 For inpatient dermatologic consultation of patients with known COVID-19, telehealth and multidisciplinary meetings are first and second line, respectively, with bedside dermatologic consultation as a last resort.4 Chen et al6 described similarly strict triage protocols as well as physician use of full-body personal protective equipment during all patient encounters. These measures are taken in light of the well-documented phenomenon of asymptomatic carriage and transmission, as all patients entering their dermatologic clinics have screened negative for symptomatic SARS-CoV-2 infection.6
Conferences and Education
Coronavirus is impacting the education of millions of individuals worldwide, including that of dermatology residents. The Annual Meeting of the American Academy of Dermatology, which was scheduled to take place in March 2020, was canceled due to COVID-19.7 The American Board of Dermatology has released a statement indicating that for all dermatology residents, time spent in COVID-19–mandated quarantine will count as clinical education if residents are able to work with their program to complete independent structured academic activity during that time.8 We also must consider the possibility that dermatology residents are reassigned to work outside of our specialty, resulting in less time and experience caring for patients with dermatologic conditions. Dermatologists in other countries have been called upon to care for COVID-19 patients, even reported to be working in intensive care units in Italy.9 Virtual technologies may be used in novel ways to support dermatology resident education throughout this process.
Final Thoughts
As physicians, dermatologists are in the position to educate their patients regarding prevention strategies, especially given that the lay press disseminates confusing and inaccurate information. The World Health Organization provides specific guidance, focusing on handwashing, respiratory hygiene, social distancing, and encouraging symptomatic patients to seek remote care whenever possible.10 Many of our patients are at high risk of complications due to COVID-19, whether due to their age or because they are immunocompromised. As the situation unfolds, the impact on and role of dermatology in this crisis will continue to evolve.
On January 30, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a public health emergency of international concern.1 Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is an enveloped, single-stranded RNA virus. It is the seventh known coronavirus to infect humans and third zoonotic Coronaviridae to cause fatal respiratory illness, along with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).2 There has been a rapid shift in the geographic center of the outbreak as well as the numbers of confirmed cases and deaths. Although the first cases in late 2019 and early 2020 were in China, by mid-March Italy became the center of the pandemic, with a steep increase in the number of cases in other European countries and the United States.3 Although COVID-19 does not have known dermatologic manifestations, it has the potential for wide-reaching impact on our field.
Strained Resources
In the United States, COVID-19 initially was associated with international travel but is now rapidly spreading throughout the community. I am currently a dermatology resident at New York-Presbyterian, Columbia campus, in New York, New York, a city that unfortunately finds itself underprepared to handle this unprecedented crisis. As of Monday, March 16, 2020, New York-Presbyterian made the decision to postpone all elective procedures, including Mohs micrographic surgery, to preserve hospital resources, including trained personnel, personal protective equipment, ventilators, and hospital beds. There have not been clear-cut guidelines regarding how to approach other dermatologic care for our patients, including routine clinic visits and inpatient dermatology consultations, leaving decisions up to individual departments and providers.
It would be prudent to learn from our colleagues in China who report steps that have been successful in preventing nosocomial spread of COVID-19 in the dermatologic setting. Tao et al4 described their protocols in both the outpatient and inpatient dermatologic setting, beginning with strict triage before patients can even enter a clinic building for their outpatient appointment. Those who screen positive are sent to a fever clinic for further evaluation, which may include a rapid computed tomography scan (using a machine that may perform 200 chest computed tomography scans per day) and SARS-CoV-2 polymerase chain reaction.5 For inpatient dermatologic consultation of patients with known COVID-19, telehealth and multidisciplinary meetings are first and second line, respectively, with bedside dermatologic consultation as a last resort.4 Chen et al6 described similarly strict triage protocols as well as physician use of full-body personal protective equipment during all patient encounters. These measures are taken in light of the well-documented phenomenon of asymptomatic carriage and transmission, as all patients entering their dermatologic clinics have screened negative for symptomatic SARS-CoV-2 infection.6
Conferences and Education
Coronavirus is impacting the education of millions of individuals worldwide, including that of dermatology residents. The Annual Meeting of the American Academy of Dermatology, which was scheduled to take place in March 2020, was canceled due to COVID-19.7 The American Board of Dermatology has released a statement indicating that for all dermatology residents, time spent in COVID-19–mandated quarantine will count as clinical education if residents are able to work with their program to complete independent structured academic activity during that time.8 We also must consider the possibility that dermatology residents are reassigned to work outside of our specialty, resulting in less time and experience caring for patients with dermatologic conditions. Dermatologists in other countries have been called upon to care for COVID-19 patients, even reported to be working in intensive care units in Italy.9 Virtual technologies may be used in novel ways to support dermatology resident education throughout this process.
Final Thoughts
As physicians, dermatologists are in the position to educate their patients regarding prevention strategies, especially given that the lay press disseminates confusing and inaccurate information. The World Health Organization provides specific guidance, focusing on handwashing, respiratory hygiene, social distancing, and encouraging symptomatic patients to seek remote care whenever possible.10 Many of our patients are at high risk of complications due to COVID-19, whether due to their age or because they are immunocompromised. As the situation unfolds, the impact on and role of dermatology in this crisis will continue to evolve.
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[news release]. Geneva, Switzerland: World Health Organization; January 30, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29. Accessed March 16, 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- World Health Organization. Coronavirus disease 2019: Situation Report—58. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 19, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- McNeil DG. Jr. Inside China’s all out war on the coronavirus. The New York Times. March 4, 2020. https://www.nytimes.com/2020/03/04/health/coronavirus-china-aylward.html?smid=tw-nytimes&smtyp=cur. Accessed March 19, 2020.
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol. 2020;82:1034.
- Hruza GJ. 2020 annual AAD meeting is canceled due to COVID-19 outbreak. American Academy of Dermatology website. https://www.aad.org/member/meetings/am2020/faqs/coronavirus. Accessed March 16, 2020.
- American Board of Dermatology. Impact of COVID-19 on dermatology resident education. March 6, 2020. https://www.abderm.org/2978.aspx. Accessed March 16, 2020.
- “It’s Like a War” [podcast]. The Daily. March 17, 2020. https://www.nytimes.com/2020/03/17/podcasts/the-daily/italy-coronavirus.html?action=click&module=audio-series-bar®ion=header&pgtype=Article. Accessed March 20, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for public. March 18, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 19, 2020.
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[news release]. Geneva, Switzerland: World Health Organization; January 30, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29. Accessed March 16, 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- World Health Organization. Coronavirus disease 2019: Situation Report—58. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 19, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- McNeil DG. Jr. Inside China’s all out war on the coronavirus. The New York Times. March 4, 2020. https://www.nytimes.com/2020/03/04/health/coronavirus-china-aylward.html?smid=tw-nytimes&smtyp=cur. Accessed March 19, 2020.
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol. 2020;82:1034.
- Hruza GJ. 2020 annual AAD meeting is canceled due to COVID-19 outbreak. American Academy of Dermatology website. https://www.aad.org/member/meetings/am2020/faqs/coronavirus. Accessed March 16, 2020.
- American Board of Dermatology. Impact of COVID-19 on dermatology resident education. March 6, 2020. https://www.abderm.org/2978.aspx. Accessed March 16, 2020.
- “It’s Like a War” [podcast]. The Daily. March 17, 2020. https://www.nytimes.com/2020/03/17/podcasts/the-daily/italy-coronavirus.html?action=click&module=audio-series-bar®ion=header&pgtype=Article. Accessed March 20, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for public. March 18, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 19, 2020.
Skin Burns From Transcranial Electrical Stimulation
To the Editor:
In recent years, noninvasive brain stimulation techniques have gained growing importance in the treatment of psychiatric1 and neurologic disorders as well as in neurologic rehabilitation (eg, after a stroke).2 One of these techniques is transcranial electrical stimulation (tES), which includes transcranial direct current stimulation (tDCS), transcranial random noise stimulation, and transcranial alternating current stimulation. The current is administered through rubber electrodes covered by saline-soaked sponges that are attached to the skull over the dysfunctional brain areas using broad rubber bands.
Transcranial direct current stimulation ameliorates brain function by anodal stimulation after a series of 5 to 10 stimulations.1 Recently, commercially available brain stimulation devices have been developed to improve working memory for online/video gaming3; however, application of a direct current (eg, 1–2 mA) over longer periods of time (15–20 minutes) can cause skin burns due to drying out of the electrode.4 Inhomogeneities in skin-electrode contact can lead to current bridges, resulting in quick evaporation of the contact medium (sodium chloride solution) and subsequent thermic damage of the skin. Another possible cause of skin lesions associated with tES is skin contact with the rubber electrode due to incorrect positioning of the electrode in the sponge covering. We report 2 cases of burns caused by tDCS.
A 55-year-old woman who was treated with tDCS (2 mA; 20 minutes) for recurrent depressive disorder developed a 0.5-cm, round, erosive, second-degree burn with hemorrhagic crust on the skin in the middle of the area where a 5×7-cm electrode (cathode) was positioned over the right orbit (Figure 1). It was the fifth stimulation with tDCS. The anode was positioned over the left dorsolateral prefrontal cortex. It was determined that the saline-soaked sponge covering the rubber electrode dried out during treatment and caused the burn. Transcranial direct current stimulation subsequently was stopped, and the lesion healed without intervention within 4 to 5 weeks, resulting in a small scar.
A 20-year-old man who was treated with tDCS (2 mA; 20 minutes) for schizophrenia developed a superficial stripe-shaped burn on the skin over the right orbit after the eighth stimulation because the 5×7-cm rubber electrode (cathode) was not fully covered by the saline-soaked sponge and the skin came into direct contact with the short side of the electrode (Figure 2). Transcranial direct current stimulation was stopped, and the skin lesion healed without intervention within 4 to 5 weeks with no scar.
The main factor associated with thermic skin damage in tES is incorrect application of the electrodes; therefore, a high standard should be applied when soaking sponges and placing and fixing the electrodes but likely is only guaranteed in specialized services and not when utilizing tES at home. Dermatologists may be confronted with an increasing number of burns due to the widespread use of tES for modulating neuropsychiatric disorders but also due to the use of tES as a lifestyle brain tuning instrument.3
- Mondino M, Bennabi D, Poulet E, et al. Can transcranial direct current stimulation (tDCS) alleviate symptoms and improve cognition in psychiatric disorders? World J Biol Psychiatry. 2014;15:261-275.
- Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev. 2013;11:CD009645.
- Steenbergen L, Sellaro R, Hommel B, et al. “Unfocus” on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res. 2016;234:637-643.
- Palm U, Keeser D, Schiller C, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS). Brain Stimul. 2008;1:386-387.
To the Editor:
In recent years, noninvasive brain stimulation techniques have gained growing importance in the treatment of psychiatric1 and neurologic disorders as well as in neurologic rehabilitation (eg, after a stroke).2 One of these techniques is transcranial electrical stimulation (tES), which includes transcranial direct current stimulation (tDCS), transcranial random noise stimulation, and transcranial alternating current stimulation. The current is administered through rubber electrodes covered by saline-soaked sponges that are attached to the skull over the dysfunctional brain areas using broad rubber bands.
Transcranial direct current stimulation ameliorates brain function by anodal stimulation after a series of 5 to 10 stimulations.1 Recently, commercially available brain stimulation devices have been developed to improve working memory for online/video gaming3; however, application of a direct current (eg, 1–2 mA) over longer periods of time (15–20 minutes) can cause skin burns due to drying out of the electrode.4 Inhomogeneities in skin-electrode contact can lead to current bridges, resulting in quick evaporation of the contact medium (sodium chloride solution) and subsequent thermic damage of the skin. Another possible cause of skin lesions associated with tES is skin contact with the rubber electrode due to incorrect positioning of the electrode in the sponge covering. We report 2 cases of burns caused by tDCS.
A 55-year-old woman who was treated with tDCS (2 mA; 20 minutes) for recurrent depressive disorder developed a 0.5-cm, round, erosive, second-degree burn with hemorrhagic crust on the skin in the middle of the area where a 5×7-cm electrode (cathode) was positioned over the right orbit (Figure 1). It was the fifth stimulation with tDCS. The anode was positioned over the left dorsolateral prefrontal cortex. It was determined that the saline-soaked sponge covering the rubber electrode dried out during treatment and caused the burn. Transcranial direct current stimulation subsequently was stopped, and the lesion healed without intervention within 4 to 5 weeks, resulting in a small scar.
A 20-year-old man who was treated with tDCS (2 mA; 20 minutes) for schizophrenia developed a superficial stripe-shaped burn on the skin over the right orbit after the eighth stimulation because the 5×7-cm rubber electrode (cathode) was not fully covered by the saline-soaked sponge and the skin came into direct contact with the short side of the electrode (Figure 2). Transcranial direct current stimulation was stopped, and the skin lesion healed without intervention within 4 to 5 weeks with no scar.
The main factor associated with thermic skin damage in tES is incorrect application of the electrodes; therefore, a high standard should be applied when soaking sponges and placing and fixing the electrodes but likely is only guaranteed in specialized services and not when utilizing tES at home. Dermatologists may be confronted with an increasing number of burns due to the widespread use of tES for modulating neuropsychiatric disorders but also due to the use of tES as a lifestyle brain tuning instrument.3
To the Editor:
In recent years, noninvasive brain stimulation techniques have gained growing importance in the treatment of psychiatric1 and neurologic disorders as well as in neurologic rehabilitation (eg, after a stroke).2 One of these techniques is transcranial electrical stimulation (tES), which includes transcranial direct current stimulation (tDCS), transcranial random noise stimulation, and transcranial alternating current stimulation. The current is administered through rubber electrodes covered by saline-soaked sponges that are attached to the skull over the dysfunctional brain areas using broad rubber bands.
Transcranial direct current stimulation ameliorates brain function by anodal stimulation after a series of 5 to 10 stimulations.1 Recently, commercially available brain stimulation devices have been developed to improve working memory for online/video gaming3; however, application of a direct current (eg, 1–2 mA) over longer periods of time (15–20 minutes) can cause skin burns due to drying out of the electrode.4 Inhomogeneities in skin-electrode contact can lead to current bridges, resulting in quick evaporation of the contact medium (sodium chloride solution) and subsequent thermic damage of the skin. Another possible cause of skin lesions associated with tES is skin contact with the rubber electrode due to incorrect positioning of the electrode in the sponge covering. We report 2 cases of burns caused by tDCS.
A 55-year-old woman who was treated with tDCS (2 mA; 20 minutes) for recurrent depressive disorder developed a 0.5-cm, round, erosive, second-degree burn with hemorrhagic crust on the skin in the middle of the area where a 5×7-cm electrode (cathode) was positioned over the right orbit (Figure 1). It was the fifth stimulation with tDCS. The anode was positioned over the left dorsolateral prefrontal cortex. It was determined that the saline-soaked sponge covering the rubber electrode dried out during treatment and caused the burn. Transcranial direct current stimulation subsequently was stopped, and the lesion healed without intervention within 4 to 5 weeks, resulting in a small scar.
A 20-year-old man who was treated with tDCS (2 mA; 20 minutes) for schizophrenia developed a superficial stripe-shaped burn on the skin over the right orbit after the eighth stimulation because the 5×7-cm rubber electrode (cathode) was not fully covered by the saline-soaked sponge and the skin came into direct contact with the short side of the electrode (Figure 2). Transcranial direct current stimulation was stopped, and the skin lesion healed without intervention within 4 to 5 weeks with no scar.
The main factor associated with thermic skin damage in tES is incorrect application of the electrodes; therefore, a high standard should be applied when soaking sponges and placing and fixing the electrodes but likely is only guaranteed in specialized services and not when utilizing tES at home. Dermatologists may be confronted with an increasing number of burns due to the widespread use of tES for modulating neuropsychiatric disorders but also due to the use of tES as a lifestyle brain tuning instrument.3
- Mondino M, Bennabi D, Poulet E, et al. Can transcranial direct current stimulation (tDCS) alleviate symptoms and improve cognition in psychiatric disorders? World J Biol Psychiatry. 2014;15:261-275.
- Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev. 2013;11:CD009645.
- Steenbergen L, Sellaro R, Hommel B, et al. “Unfocus” on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res. 2016;234:637-643.
- Palm U, Keeser D, Schiller C, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS). Brain Stimul. 2008;1:386-387.
- Mondino M, Bennabi D, Poulet E, et al. Can transcranial direct current stimulation (tDCS) alleviate symptoms and improve cognition in psychiatric disorders? World J Biol Psychiatry. 2014;15:261-275.
- Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev. 2013;11:CD009645.
- Steenbergen L, Sellaro R, Hommel B, et al. “Unfocus” on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res. 2016;234:637-643.
- Palm U, Keeser D, Schiller C, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS). Brain Stimul. 2008;1:386-387.
Practice Point
- Cranial skin burns can point to misuse of electrical stimulation.
Multinodular Plaque on the Penis
The Diagnosis: Tophaceous Gout
Biopsy revealed amorphous pink material within the center of palisading granulomas lined by histiocytes and giant cells. Scattered crystal remnants also were identified within the center of the granulomas; however, the majority of the crystals were dissolved during the formalin processing of the tissue to become the amorphous material. A perivascular mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and plasma cells surrounded the tophi nodules. A biopsy confirmed the diagnosis of tophaceous gout (Figure).
Gout is a systemic metabolic disease characterized by the supersaturation of monosodium urate (MSU) crystals in joints and bursae. Peripheral joints most commonly are affected due to the poor solubility of MSU crystals at low temperatures.1 It is one of the most common forms of inflammatory arthritis, with an estimated prevalence of 4% of adults in the United States.2 An estimated $1 billion is spent each year on ambulatory care for gout.3 Gout occurs most commonly in men and usually manifests in the fifth or sixth decades of life.4 Risk factors for the development of gout include obesity, hypertension, poor dietary habits and kidney function, excessive alcohol intake, and diuretic use.3
Disease manifestations range from asymptomatic hyperuricemia to acute gouty arthritis and chronic tophaceous gout. Patients may present with chronic tophaceous gout without a prior clinically apparent acute gout episode.5,6 Uncontrolled gout may result in large accumulations of MSU crystals, leading to well-circumscribed masses (known as tophi), as demonstrated in our patient.1 Tophi are pathognomonic features of gout and are the sine qua non of advanced gout (also known as chronic tophaceous gout).2 Clinically, these tophi appear as subcutaneous, yellowish white, firm and smooth nodules that are highlighted on the skin.4 Tophi most commonly are found on the helix, articular and periarticular tissue, and the tissue of the hands and feet. They usually are visible on physical examination but also may be detected on imaging studies.2,4
Gouty tophi have been reported in extraordinary locations, such as in sclerae; vocal cords; heart valves; abdominal striae; nerves; axial skeleton4,7; and the penis, as in our patient and one other case.2 These gouty deposits can appear similarly to lipomas, rheumatoid and osteoarthritic nodules, and infectious and malignant processes.1,5 When tophi present in unusual locations, tissue biopsy often is necessary to confirm the diagnosis. Tissue preservation in alcohol is required to preserve the urate crystals. Microscopically, urate crystals appear as tightly packed, brown, needle-shaped crystals surrounded by granulomatous inflammation with foreign body giant cells, macrophages, and possibly some fibrosis. When examined under polarized light, the MSU crystals are negatively birefringent. However, when clinical suspicion for gout is low and the tissue is instead formalin fixed, as was performed in our case, the crystals dissolve into fibrillary amorphous deposits within the center of the granulomatous inflammation, which is another characteristic histologic finding in tophaceous gout.8
Management of gout focuses on urate-lowering therapy including lifestyle changes. Lower serum urate levels are associated with a decreased incidence of acute gout attacks and chronic tophaceous gout.2 Urate-lowering drugs often are combined with anti-inflammatory drugs during acute attacks. Lifestyle changes, such as weight loss, exercise, reduced alcohol consumption, high fluid intake, and a low-purine diet also are beneficial.3,4 Although gout cannot be cured, it can be effectively managed, and appropriate treatment can improve quality of life and reduce the risk for permanent joint damage and structural deformities. If medical treatment and lifestyle changes fail to adequately control tophaceous gout or if tophi become symptomatic, surgical removal of tophi is appropriate.4
At follow-up, our patient opted for surgical removal of the penile tophi. Using local anesthesia, surgical debulking via curettage was performed. Open defects were closed with fine absorbable sutures, and prophylactic antibiotics were given. Allopurinol also was started. Six weeks following extraction, the patient reported no complications and the area was continuing to heal.
Tophaceous gout would be distinguished from conditions in the differential diagnosis based on histologic findings from hematoxylin and eosin (H&E)-stained sections. Actinomycotic mycetoma is rare in the United States and is characterized by a seropurulent or stringy exudate with grains, ulcerations, melicerous scabs, and retractable scarring.9 On H&E-stained sections, actinomyces appear filamentous with deeply basophilic staining and radially oriented acidophilic projections.10 Calcinosis cutis of the penis has been reported to appear as asymptomatic papules; however, microscopic sections reveal deeply basophilic calcium deposits within the tissue.11 Multinodular syphilis shows characteristic histology with lichenoid or vacuolar interface dermatitis, slender acanthosis, plasma cells, and endothelial swelling of the small vessels. A Treponema pallidum immunoperoxidase stain shows numerous organisms. Planar xanthoma shows xanthomatous or foamy histiocytes throughout the dermis on H&E-stained sections.12
- Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective--a review. J Adv Res. 2007;8:495-511.
- Flores Martín JF, Vázquez Alonso F, Puche Sanz I, et al. Gouty tophi in the penis: a case report and review of the literature. Case Rep Urol. 2012;2012:594905.
- Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68.
- Forbess LJ, Fields TR. The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42:146-154.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Gaviria JL, Ortega VG, Gaona J. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Patterson JW, Hosler GA, Weedon D. Weedon's Skin Pathology. Edinburgh, Scotland: Churchill Livingstone/Elsevier; 2016.
- Guerra-Leal JD, Medrano-Danés LA, Montemayor-Martinez A, et al. The importance of diagnostic imaging of mycetoma in the foot [published online December 18, 2018]. Int J Dermatol. 2019;58:600-604.
- Fazeli MS, Bateni H. Actinomycosis: a rare soft tissue infection. Dermatol Online J. 2005;11:18.
- Cohen PR, Tschen JA. Idiopathic calcinosis cutis of the penis. J Clin Aesthet Dermatol. 2012;5:23-30.
- Ko C, Elston DM, Ferringer T. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019.
The Diagnosis: Tophaceous Gout
Biopsy revealed amorphous pink material within the center of palisading granulomas lined by histiocytes and giant cells. Scattered crystal remnants also were identified within the center of the granulomas; however, the majority of the crystals were dissolved during the formalin processing of the tissue to become the amorphous material. A perivascular mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and plasma cells surrounded the tophi nodules. A biopsy confirmed the diagnosis of tophaceous gout (Figure).
Gout is a systemic metabolic disease characterized by the supersaturation of monosodium urate (MSU) crystals in joints and bursae. Peripheral joints most commonly are affected due to the poor solubility of MSU crystals at low temperatures.1 It is one of the most common forms of inflammatory arthritis, with an estimated prevalence of 4% of adults in the United States.2 An estimated $1 billion is spent each year on ambulatory care for gout.3 Gout occurs most commonly in men and usually manifests in the fifth or sixth decades of life.4 Risk factors for the development of gout include obesity, hypertension, poor dietary habits and kidney function, excessive alcohol intake, and diuretic use.3
Disease manifestations range from asymptomatic hyperuricemia to acute gouty arthritis and chronic tophaceous gout. Patients may present with chronic tophaceous gout without a prior clinically apparent acute gout episode.5,6 Uncontrolled gout may result in large accumulations of MSU crystals, leading to well-circumscribed masses (known as tophi), as demonstrated in our patient.1 Tophi are pathognomonic features of gout and are the sine qua non of advanced gout (also known as chronic tophaceous gout).2 Clinically, these tophi appear as subcutaneous, yellowish white, firm and smooth nodules that are highlighted on the skin.4 Tophi most commonly are found on the helix, articular and periarticular tissue, and the tissue of the hands and feet. They usually are visible on physical examination but also may be detected on imaging studies.2,4
Gouty tophi have been reported in extraordinary locations, such as in sclerae; vocal cords; heart valves; abdominal striae; nerves; axial skeleton4,7; and the penis, as in our patient and one other case.2 These gouty deposits can appear similarly to lipomas, rheumatoid and osteoarthritic nodules, and infectious and malignant processes.1,5 When tophi present in unusual locations, tissue biopsy often is necessary to confirm the diagnosis. Tissue preservation in alcohol is required to preserve the urate crystals. Microscopically, urate crystals appear as tightly packed, brown, needle-shaped crystals surrounded by granulomatous inflammation with foreign body giant cells, macrophages, and possibly some fibrosis. When examined under polarized light, the MSU crystals are negatively birefringent. However, when clinical suspicion for gout is low and the tissue is instead formalin fixed, as was performed in our case, the crystals dissolve into fibrillary amorphous deposits within the center of the granulomatous inflammation, which is another characteristic histologic finding in tophaceous gout.8
Management of gout focuses on urate-lowering therapy including lifestyle changes. Lower serum urate levels are associated with a decreased incidence of acute gout attacks and chronic tophaceous gout.2 Urate-lowering drugs often are combined with anti-inflammatory drugs during acute attacks. Lifestyle changes, such as weight loss, exercise, reduced alcohol consumption, high fluid intake, and a low-purine diet also are beneficial.3,4 Although gout cannot be cured, it can be effectively managed, and appropriate treatment can improve quality of life and reduce the risk for permanent joint damage and structural deformities. If medical treatment and lifestyle changes fail to adequately control tophaceous gout or if tophi become symptomatic, surgical removal of tophi is appropriate.4
At follow-up, our patient opted for surgical removal of the penile tophi. Using local anesthesia, surgical debulking via curettage was performed. Open defects were closed with fine absorbable sutures, and prophylactic antibiotics were given. Allopurinol also was started. Six weeks following extraction, the patient reported no complications and the area was continuing to heal.
Tophaceous gout would be distinguished from conditions in the differential diagnosis based on histologic findings from hematoxylin and eosin (H&E)-stained sections. Actinomycotic mycetoma is rare in the United States and is characterized by a seropurulent or stringy exudate with grains, ulcerations, melicerous scabs, and retractable scarring.9 On H&E-stained sections, actinomyces appear filamentous with deeply basophilic staining and radially oriented acidophilic projections.10 Calcinosis cutis of the penis has been reported to appear as asymptomatic papules; however, microscopic sections reveal deeply basophilic calcium deposits within the tissue.11 Multinodular syphilis shows characteristic histology with lichenoid or vacuolar interface dermatitis, slender acanthosis, plasma cells, and endothelial swelling of the small vessels. A Treponema pallidum immunoperoxidase stain shows numerous organisms. Planar xanthoma shows xanthomatous or foamy histiocytes throughout the dermis on H&E-stained sections.12
The Diagnosis: Tophaceous Gout
Biopsy revealed amorphous pink material within the center of palisading granulomas lined by histiocytes and giant cells. Scattered crystal remnants also were identified within the center of the granulomas; however, the majority of the crystals were dissolved during the formalin processing of the tissue to become the amorphous material. A perivascular mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and plasma cells surrounded the tophi nodules. A biopsy confirmed the diagnosis of tophaceous gout (Figure).
Gout is a systemic metabolic disease characterized by the supersaturation of monosodium urate (MSU) crystals in joints and bursae. Peripheral joints most commonly are affected due to the poor solubility of MSU crystals at low temperatures.1 It is one of the most common forms of inflammatory arthritis, with an estimated prevalence of 4% of adults in the United States.2 An estimated $1 billion is spent each year on ambulatory care for gout.3 Gout occurs most commonly in men and usually manifests in the fifth or sixth decades of life.4 Risk factors for the development of gout include obesity, hypertension, poor dietary habits and kidney function, excessive alcohol intake, and diuretic use.3
Disease manifestations range from asymptomatic hyperuricemia to acute gouty arthritis and chronic tophaceous gout. Patients may present with chronic tophaceous gout without a prior clinically apparent acute gout episode.5,6 Uncontrolled gout may result in large accumulations of MSU crystals, leading to well-circumscribed masses (known as tophi), as demonstrated in our patient.1 Tophi are pathognomonic features of gout and are the sine qua non of advanced gout (also known as chronic tophaceous gout).2 Clinically, these tophi appear as subcutaneous, yellowish white, firm and smooth nodules that are highlighted on the skin.4 Tophi most commonly are found on the helix, articular and periarticular tissue, and the tissue of the hands and feet. They usually are visible on physical examination but also may be detected on imaging studies.2,4
Gouty tophi have been reported in extraordinary locations, such as in sclerae; vocal cords; heart valves; abdominal striae; nerves; axial skeleton4,7; and the penis, as in our patient and one other case.2 These gouty deposits can appear similarly to lipomas, rheumatoid and osteoarthritic nodules, and infectious and malignant processes.1,5 When tophi present in unusual locations, tissue biopsy often is necessary to confirm the diagnosis. Tissue preservation in alcohol is required to preserve the urate crystals. Microscopically, urate crystals appear as tightly packed, brown, needle-shaped crystals surrounded by granulomatous inflammation with foreign body giant cells, macrophages, and possibly some fibrosis. When examined under polarized light, the MSU crystals are negatively birefringent. However, when clinical suspicion for gout is low and the tissue is instead formalin fixed, as was performed in our case, the crystals dissolve into fibrillary amorphous deposits within the center of the granulomatous inflammation, which is another characteristic histologic finding in tophaceous gout.8
Management of gout focuses on urate-lowering therapy including lifestyle changes. Lower serum urate levels are associated with a decreased incidence of acute gout attacks and chronic tophaceous gout.2 Urate-lowering drugs often are combined with anti-inflammatory drugs during acute attacks. Lifestyle changes, such as weight loss, exercise, reduced alcohol consumption, high fluid intake, and a low-purine diet also are beneficial.3,4 Although gout cannot be cured, it can be effectively managed, and appropriate treatment can improve quality of life and reduce the risk for permanent joint damage and structural deformities. If medical treatment and lifestyle changes fail to adequately control tophaceous gout or if tophi become symptomatic, surgical removal of tophi is appropriate.4
At follow-up, our patient opted for surgical removal of the penile tophi. Using local anesthesia, surgical debulking via curettage was performed. Open defects were closed with fine absorbable sutures, and prophylactic antibiotics were given. Allopurinol also was started. Six weeks following extraction, the patient reported no complications and the area was continuing to heal.
Tophaceous gout would be distinguished from conditions in the differential diagnosis based on histologic findings from hematoxylin and eosin (H&E)-stained sections. Actinomycotic mycetoma is rare in the United States and is characterized by a seropurulent or stringy exudate with grains, ulcerations, melicerous scabs, and retractable scarring.9 On H&E-stained sections, actinomyces appear filamentous with deeply basophilic staining and radially oriented acidophilic projections.10 Calcinosis cutis of the penis has been reported to appear as asymptomatic papules; however, microscopic sections reveal deeply basophilic calcium deposits within the tissue.11 Multinodular syphilis shows characteristic histology with lichenoid or vacuolar interface dermatitis, slender acanthosis, plasma cells, and endothelial swelling of the small vessels. A Treponema pallidum immunoperoxidase stain shows numerous organisms. Planar xanthoma shows xanthomatous or foamy histiocytes throughout the dermis on H&E-stained sections.12
- Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective--a review. J Adv Res. 2007;8:495-511.
- Flores Martín JF, Vázquez Alonso F, Puche Sanz I, et al. Gouty tophi in the penis: a case report and review of the literature. Case Rep Urol. 2012;2012:594905.
- Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68.
- Forbess LJ, Fields TR. The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42:146-154.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Gaviria JL, Ortega VG, Gaona J. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Patterson JW, Hosler GA, Weedon D. Weedon's Skin Pathology. Edinburgh, Scotland: Churchill Livingstone/Elsevier; 2016.
- Guerra-Leal JD, Medrano-Danés LA, Montemayor-Martinez A, et al. The importance of diagnostic imaging of mycetoma in the foot [published online December 18, 2018]. Int J Dermatol. 2019;58:600-604.
- Fazeli MS, Bateni H. Actinomycosis: a rare soft tissue infection. Dermatol Online J. 2005;11:18.
- Cohen PR, Tschen JA. Idiopathic calcinosis cutis of the penis. J Clin Aesthet Dermatol. 2012;5:23-30.
- Ko C, Elston DM, Ferringer T. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019.
- Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective--a review. J Adv Res. 2007;8:495-511.
- Flores Martín JF, Vázquez Alonso F, Puche Sanz I, et al. Gouty tophi in the penis: a case report and review of the literature. Case Rep Urol. 2012;2012:594905.
- Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68.
- Forbess LJ, Fields TR. The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42:146-154.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Gaviria JL, Ortega VG, Gaona J. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Patterson JW, Hosler GA, Weedon D. Weedon's Skin Pathology. Edinburgh, Scotland: Churchill Livingstone/Elsevier; 2016.
- Guerra-Leal JD, Medrano-Danés LA, Montemayor-Martinez A, et al. The importance of diagnostic imaging of mycetoma in the foot [published online December 18, 2018]. Int J Dermatol. 2019;58:600-604.
- Fazeli MS, Bateni H. Actinomycosis: a rare soft tissue infection. Dermatol Online J. 2005;11:18.
- Cohen PR, Tschen JA. Idiopathic calcinosis cutis of the penis. J Clin Aesthet Dermatol. 2012;5:23-30.
- Ko C, Elston DM, Ferringer T. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019.
A 34-year-old man presented for evaluation of a slowly growing group of firm white bumps on the penis. The lesions were nontender and asymptomatic. Medical and family history was notable for gout, though he was not being treated. Physical examination revealed a 3-cm, firm, multinodular, chalky white plaque on the dorsal aspect of the penile shaft. A tangential biopsy was performed and sent for hematoxylin and eosin staining.
Psoriasis Therapy During the COVID-19 Pandemic: Should Patients Continue Biologics?



Inflammatory Changes in Actinic Keratoses Associated With Afatinib Therapy
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).
In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).
Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).
In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).
Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
To the Editor:
Afatinib is a small molecule covalently binding and inhibiting the epidermal growth factor receptor (EGFR) as well as HER2 and HER4 receptor tyrosine kinases.1 The EGFR family is part of a complex signal transduction network that is central to several critical cellular processes.2 The human EGFR family is dysregulated in many solid tumors, making it an attractive target for anticancer therapy.2 In 2013, the US Food and Drug Administration approved afatinib as a first-line treatment of patients with metastatic non–small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.3
Treatment with afatinib and other EGFR inhibitors is frequently associated with cutaneous adverse effects that occur in up to 90% of patients. These cutaneous reactions are typical for this drug family and distinct from the skin adverse effects related to other types of anticancer chemotherapy.4 The most frequent skin manifestations following afatinib treatment consist of an acneform pustular eruption in up to 90% of patients.5,6 Other dermatologic reactions include nonspecific maculopapular rashes (90%), stomatitis (71%), paronychia with some nail changes (58%), xerosis (31%), pruritus (21%), and hand-foot syndrome (7%)5,6; however, grade 3 dermatologic reactions occurred in only 0.15% of patients.
Inflammatory changes in both preexisting and undetected actinic keratoses (AKs) and even progression to squamous cell carcinoma (SCC) have been previously described as uncommon dermatologic adverse effects of 2 EGFR inhibitors, sorafenib and erlotinib.7-9 Seven of 131 patients with metastatic renal cell carcinoma treated with single-agent sorafenib developed cutaneous SCC and 3 more had AKs.9 One patient demonstrated self-limited inflammatory flare-up of AKs during erlotinib treatment.8 We report acute inflammation of AKs from afatinib treatment.
A 78-year-old woman with fair skin who was previously treated for several AKs in sun-exposed areas presented with inflammatory changes that appeared at the site of AKs on photoexposed areas 110 days after initiating afatinib therapy (40 mg/d). Physical examination revealed multiple erythematous scaly plaques on the face, neck, chest, and forearms (Figure 1).
In the previous 2 decades, lesions that were surgically removed and histopathologically examined included Bowen disease (2 lesions), 2 basal cell carcinomas, 2 blue nevi, and a seborrheic keratosis. Several AKs also were surgically removed and confirmed histopathologically.
Eighteen months prior to the current presentation, the patient was diagnosed with locally advanced, inoperable, stage IIIA adenocarcinoma of the lung with deletion in exon 19 of the EGFR gene. She received definitive concomitant chemoradiation with the carboplatin-vinorelbine regimen and 60-Gy radiation. Four months later, a positron emission tomography (PET)–fludeoxyglucose scan revealed a single bone lesion in the L5 vertebra leading to irradiation to the lumbar spine. Subsequently, new metastases to the neck, right lung, T5 vertebra, and left acetabulum were detected by PET–computed tomography. One year later, afatinib 40 mg/d was initiated. A PET scan after 2 months of treatment showed excellent response.
At the current presentation, a punch biopsy obtained from an inflammatory lesion on the left dorsal forearm revealed findings consistent with an eroded and inflamed AK; the biopsy showed marked dysplasia of the keratinocytes that was predominately located in the basal layer of the epidermis. The lesion was accompanied by a dense mixed inflammatory cell infiltrate that was centered in the papillary dermis and extended to the epidermis (Figure 2). Because of this grade 3 skin toxicity, the afatinib dosage was reduced to 20 mg/d, and betamethasone cream 0.1% and emollients were applied locally for 2 weeks. A reduction in the number of AKs and clinical regression of the inflammatory changes was observed 2 weeks later (Figure 3).
Chronically sun-exposed skin is prone to develop AKs that are at risk to progress to SCC.10-12 These lesions are increasingly diagnosed in older patients when internal cancers also are prevalent.13 Inflammatory flare-up of AKs is typically present during the regression phase14,15 but also during progression to SCC.16
There are many strategies for treating AKs. Physical procedures for destroying the lesions are commonly used. Some topical drugs, including imiquimod, 5-fluorouracil, and diclofenac sodium, also have proven efficacy.17
Conventional chemotherapeutic agents that have been described to be associated with the inflammation of AKs include docetaxel; doxorubicin; capecitabine; pentostatin; and the combination of dactinomycin, vincristine, dacarbazine and doxorubicin, cytarabine, and 6-thioguanine.7,18 The mechanism leading to this effect is unknown, though abnormal DNA synthesis and a type of radiation recall phenomenon have been postulated.7
We described inflammatory changes in AKs associated with afatinib treatment. The precise mechanism by which afatinib induces inflammation in AK has not been elucidated; however, it is known that EGFR normally downregulates chemokine expression in keratinocytes. Conversely, EGFR signaling blockade produces opposite effects, with increased CCL2, CCL5, and CXCL10, as well as reduced CXCL8 expression, leading to enhanced skin inflammation.19 Afatinib is a targeted agent that modulates the Ras/Raf/MEK/ERK signaling circuit, which is a key intracellular signal transduction pathway.20 This pathway and its downstream effectors have been implicated in cutaneous squamous cell carcinogenesis that might be accompanied by inflammatory changes.21,22 The remarkable clinical improvement of the AKs in our patient following the inflammatory flare-up supports the notion that the anticancer effect on intraepidermal neoplasms might be mediated by inflammation.23
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
- Katakami N, Atagi S, Goto K, et al. LUX-lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3342.
- Liao BC, Lin CC, Yang JCH. First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. Drugs. 2013;73:357-369.
- Jain P, Khanal R, Sharma A, et al. Afatinib and lung cancer. Expert Rev Anticancer Ther. 2014;14:1391-1406.
- Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45-63.
- Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-1433.
- Agero ALC, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670.
- Lacouture ME, Desai A, Soltani K, et al. Inflammation of actinic keratoses subsequent to therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. Clin Exp Dermatol. 2006;31:783-785.
- Hermanns JF, Piérard GE, Quatresooz P. Erlotinib-responsive actinic keratoses. Oncol Rep. 2007;18:581-584.
- Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23.
- Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6:207-209.
- Ehrig T, Cockerell C, Piacquadio D, et al. Actinic keratoses and the incidence of occult squamous cell carcinoma: a clinical-histopathologic correlation. Dermatolog Surg. 2006;32:1261-1265.
- Quaedvlieg PJF, Tirsi E, Thissen MRTM, et al. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16:335-339.
- Atkins D, Bang RH, Sternberg MR, et al. Reliable methods to evaluate the burden of actinic keratoses. J Invest Dermatol. 2006;126:591-594.
- Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72-78.
- Quatresooz P, Piérard GE. Imiquimod-responsive basal cell carcinomas and factor XIIIa enriched dendrocytes. Clin Exp Dermatol. 2003;28(suppl 1):27-29.
- Berhane T, Halliday GM, Cooke B, et al. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810-815.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 suppl 1):S28-S38.
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367-398.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- Zebisch A, Czernilofsky AP, Keri G, et al. Signaling through RAS-RAF-MEK ERK: from basics to bedside. Curr Med Chem. 2007;14:601-623.
- Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657-1667.
- Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583-589.
- Kumar S, Kumar R, Medhi B, et al. Novel strategies for effective actinic keratosis treatment: a review. Curr Cancer Ther Rev. 2015;11:119-1132.
Practice Points
- One of the underreported adverse events of afatinibis is the induction of inflammatory changes in actinic keratoses (AKs).
- Our cases showed that inflammatory changes eventually led to shrinkage and resolution of the underlying AK.
Cutaneous Id Reaction After Using Cyanoacrylate for Wound Closure
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.
Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.
Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.
Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.
Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
To the Editor:
In 1998, 2-octyl-cyanoacrylate (2-CA) tissue adhesive gained US Food and Drug Administration approval for topical application to easily hold closed approximated skin edges from surgical excisions and simple trauma-induced lacerations.1 It has since been employed for a number of off-label indications, including sutureless circumcision,2 skin graft fixation,3 pericatheter leakage,4 and intracorporeal use to control air leaks during lung resection.5 Animal investigations additionally have attempted to elucidate potential future uses of 2-CA for procedures such as inguinal hernia repair,6 bowel anastomosis,7 incisional hernia repair with mesh,8 and microvascular anastomosis.9 Compared to sutures, 2-CA offers ease and rapidity of application, a water-resistant barrier, and equivalent cosmetic results, as well as eliminates the need for suture removal.10 As 2-CA is used with increasing frequency across a variety of settings, there arises a greater need to be mindful of the potential complications of its use, such as irritant contact dermatitis (ICD), allergic contact dermatitis (ACD), and cutaneous id reaction.
A 14-year-old adolescent boy with no notable medical history and no known allergies underwent a minimally invasive Nuss procedure11 (performed by P.L.G.) for the repair of severe pectus excavatum. Two 4-cm incisions were made—one in each lateral chest wall at the approximately eighth intercostal space—to facilitate the introduction of the Nuss bar. The surgical wounds were closed with 2 layers of running polyglactin 910 suture before 2-CA was applied topically to the incision sites. The surgery was well tolerated, and the patient’s wounds healed without incident. When the patient was evaluated for Nuss bar removal 3 years later, incision sites were noted to be well healed, and he exhibited no other skin lesions. The original incision sites (bilateral chest walls) were utilized to facilitate surgical Nuss bar removal. The wounds were closed in 4 layers and 2-CA was again applied topically to the incision sites. There were no intraoperative complications; no devices, drains, or tissue implants were left in the patient at the conclusion of the procedure.
One week later, via text message and digital photographs, the patient reported intense pruritus at the bilateral chest wall incision sites, which were now surrounded by symmetric 1-cm erythematous plaques and associated sparse erythematous satellite papules (Figure 1). The patient denied any fevers, pain, swelling, or purulent discharge from the wounds. He was started on hydrocortisone cream 1% twice daily as well as oral diphenhydramine 25 mg at bedtime with initial good effect.
Three days later, the patient sent digital photographs of a morphologically similar–appearing rash that had progressed beyond the lateral chest walls to include the central chest and bilateral upper and lower extremities (Figure 2). He continued to deny any local or systemic signs of infection. Dermatology was consulted, and a diagnosis of ACD with cutaneous id reaction was made. The patient’s medication regimen was modified to include triamcinolone acetonide cream 0.1% applied twice daily to the rash away from the wounds, clobetasol propionate ointment 0.05% applied twice daily to the rash at the wound sites, oral levocetirizine 5 mg once daily, and oral hydroxyzine 25 to 50 mg every 6 hours as needed for pruritus. Additional recommendations included the use of a fragrance-free soap and application of an over-the-counter anti-itch lotion containing menthol and camphor applied as needed. Within 24 hours of starting this modified treatment regimen, the patient began to notice an improvement in symptoms, with full resolution over the course of the ensuing 2 weeks. The patient was counseled to inform his physicians—present and future—of his allergy to 2-CA.
Contact dermatitis associated with the use of 2-CA has been described in the literature.12-15 We report progression to an id reaction, which is characterized by the diffuse symmetric spread of a cutaneous eruption at a site distant from the primary localized dermatitis that develops within a few days of the primary lesion and exhibits the same morphologic and histopathologic findings.16,17 In our patient, pruritic erythematous papules and plaques symmetrically distributed on the arms, legs, and chest appeared 3 days after he first reported a similar eruption at the 2-CA application sites. It is theorized that id reactions develop when the sensitization phase of a type IV hypersensitivity reaction generates a population of T cells that not only recognizes a hapten but also recognizes keratinocyte-derived epitopes.16 A hapten is a small molecule (<500 Da) that is capable of penetrating the stratum corneum and binding skin components. A contact allergen is a hapten that has bound epidermal proteins to create a new antigenic determinant.18 The secondary dermatitis that characterizes id reactions results from an abnormal autoimmune response. Id reactions associated with exposure to adhesive material are rare.19
Allergic contact dermatitis is a type IV hypersensitivity reaction that appears after initial sensitization to an allergen followed by re-exposure. Our patient presented with symmetric erythematous plaques at the surgical incision sites 1 week after 2-CA had been applied. During this interval, sensitization to the inciting allergen occurred. The allergen is taken up by antigen-presenting cells, which then migrate to lymph nodes where they encounter naïve T lymphocytes that subsequently undergo clonal expansion to produce a cohort of T cells that are capable of recognizing the allergen. If subsequent exposure to the specific allergen takes place, an elicitation phase occurs in which primed T cells are incited to release mediators of inflammation that engender the manifestations of ACD within 24 to 72 hours.18,20 Sensitization may be promoted by skin barrier impairments such as dermatitis or a frank wound.12,20 In most cases, the patient is unaware that sensitization has occurred, though a primary ACD within 5 to 15 days after initial exposure to the inciting allergen rarely may be observed.18 Although our patient had 2-CA applied to his surgical wounds at 14 years of age, it was unlikely that sensitization took place at that time, as it was 1 week rather than 1 to 3 days before he experienced the cutaneous eruption associated with his second 2-CA exposure at 17 years of age.
Cyanoacrylate tissue adhesive also may cause ICD resulting from histotoxic degradation products such as formaldehyde and cyanoacetate that are capable of compromising cutaneous barrier function. Keratinocytes that have had their membranes disturbed release proinflammatory cytokines, which recruit cells of the innate immune system as well as T lymphocytes to the site of insult to facilitate the inflammatory response. The manifestations of ICD include erythema, edema, and local necrosis that can compromise wound healing.20 The speed at which a given cyanoacrylate adhesive degrades is proportional to the length of its carbon side chain. Those with shorter side chains—ethyl and methyl cyanoacrylate—degrade more rapidly into formaldehyde and cyanoacetate; 2-CA possesses a longer side chain and therefore degrades more slowly, which should, in theory, lessen its potential to cause ICD.20 Because it may take 7 to 14 days before 2-CA will spontaneously peel from the application site, however, its potential to evoke ICD nevertheless exists.
Treatment of ICD entails removing the irritant while concurrently working to restore the skin’s barrier with emollients. Although topical corticosteroids often are reflexively prescribed to treat rashes, some believe that their use should be avoided in cases of ICD, as their inhibitory effects on epidermal lipid synthesis may further impair the skin’s barrier.21 For cases of ACD, with or without an accompanying id reaction, topical corticosteroids are the mainstay of therapy. It is customary to start with a higher-potency topical steroid such as clobetasol and taper to lower-potency steroids as the patient’s condition improves. Steroid ointments are petroleum based and are capable of causing 2-CA to separate from the skin.10 As a result, they should be used with care when being applied to an area where 2-CA is maintaining dermal closure. Systemic corticosteroids may be warranted in cases with involvement of more than 20% of the body surface area and should start to provide relief within 12 to 24 hours.22 Oral antihistamines and cold water compresses can be added to help address pruritus and discomfort in both ACD and ICD.
Instances of contact dermatitis caused by 2-CA are rare, and progression to an id reaction is rarer still. Physicians should be aware of the possibility of encountering a patient that manifests one or both of these complications whenever 2-CA is employed for skin closure. Physicians who employ 2-CA for skin closure should first ask patients about prior cutaneous reactions to cyanoacrylates including 2-CA and other commonly encountered acrylate-containing products including adhesive wound dressings, dental cements and prostheses, superglue, artificial nails, and adhesives for wigs and false eyelashes. Still, many patients who exhibit acrylate-induced contact dermatitis, with or without an associated id reaction, will not attest to a history of adverse reactions; they simply may not recognize acrylate as the inciting agent. Practitioners across a range of specialties outside of dermatology—surgeons, emergency physicians, and primary care providers—should be prepared to both recognize contact dermatitis and id reaction arising from the use of 2-CA and implement a basic treatment plan that will bring the patient relief without compromising wound closure.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
- US Food and Drug Administration. Premarket approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p960052. Accessed March 4, 2020.
- Elmore JM, Smith EA, Kirsch AJ. Sutureless circumcision using 2-octyl cyanoacrylate (Dermabond): appraisal after 18-month experience. Urology. 2007;70:803-806.
- Kilic A, Ozdengil E. Skin graft fixation by applying cyanoacrylate without any complication. Plast Reconstr Surg. 2002;110:370-371.
- Gurnaney H, Kraemer FW, Ganesh A. Dermabond decreases pericatheter local anesthetic leakage after continuous perineural infusions. Anesth Analg. 2011;113:206.
- Carr JA. The intracorporeal use of 2-octyl cyanoacrylate resin to control air leaks after lung resection. Eur J Cardiothorac Surg. 2011;39:579-583.
- Miyano G, Yamataka A, Kato Y, et al. Laparoscopic injection of Dermabond tissue adhesive for the repair of inguinal hernia: short- and long-term follow-up. J Pediatr Surg. 2004;39:1867-1870.
- Paral J, Subrt Z, Lochman P, et al. Suture-free anastomosis of the colon. experimental comparison of two cyanoacrylate adhesives. J Gastrointest Surg. 2011;15:451-459.
- Birch DW, Park A. Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg. 2001;67:974-978.
- Ang ES, Tan KC, Tan LH, et al. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193-201.
- Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Physician. 2000;61:1383-1388.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545-552.
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol. 2008;144:814-815.
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
- Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
- Sachse MM, Junghans T, Rose C, et al. Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis. 2013;68:317-319.
- Fehr BS, Takashima A, Bergstresser PR, et al. T cells reactive to keratinocyte antigens are generated during induction of contact hypersensitivity in mice. a model for autoeczematization in humans? Am J Contact Dermat. 2000;11:145-154.
- Gonzalez-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Vocanson M, Hennino A, Rozières A, et al. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699-1714.
- Sommer LL, Hejazi EZ, Heymann WR. An acute linear pruritic eruption following allergic contact dermatitis. J Clin Aesthet Dermatol. 2014;7:42-44.
- Rietschel RL, Fowler JF. Plastics, adhesives, and synthetic resins. In: Rietschek RL, Fowler JF, eds. Fisher’s Contact Dermatitis. Hamilton, BC: Decker Inc; 2008:542-560.
- Kao JS, Fluhr JW, Man M, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456-464.
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: a practice parameter. Ann Allergy Asthma Immunol. 2006;97(3 suppl 2):S1-S38.
Practice Points
- 2-Octyl-cyanoacrylate (2-CA) tissue adhesive has been reported to cause contact dermatitis when applied topically for surgical site closure.
- Id reactions resulting from the use of 2-CA tissue adhesive are possible, though less commonly observed.
- Id reactions caused by 2-CA tissue adhesive respond well to treatment with a combination of topical steroids and oral antihistamines. Systemic corticosteroids may be warranted in cases involving greater than 20% body surface area.