User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Pediatric Dermatology Emergencies
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
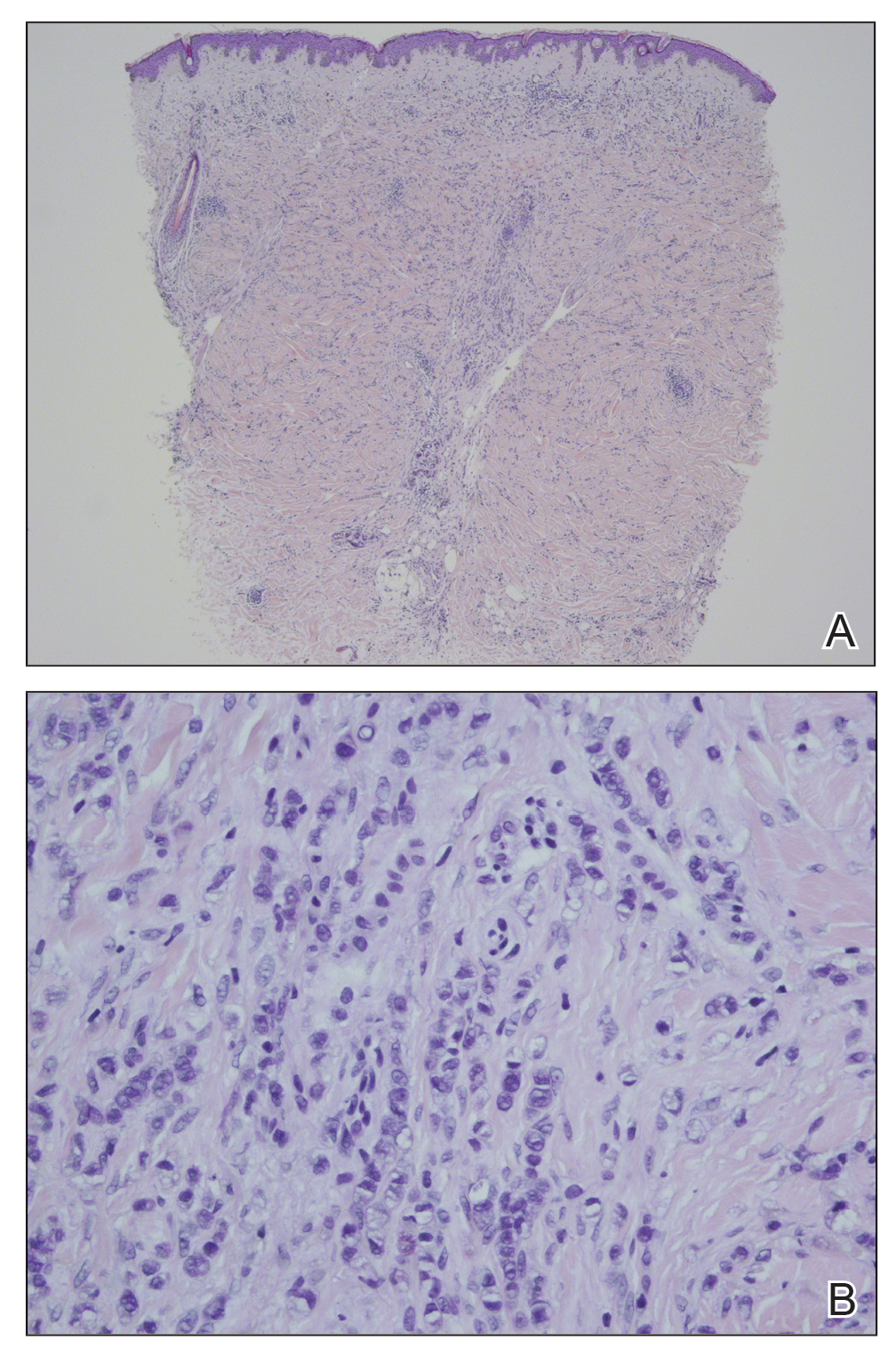
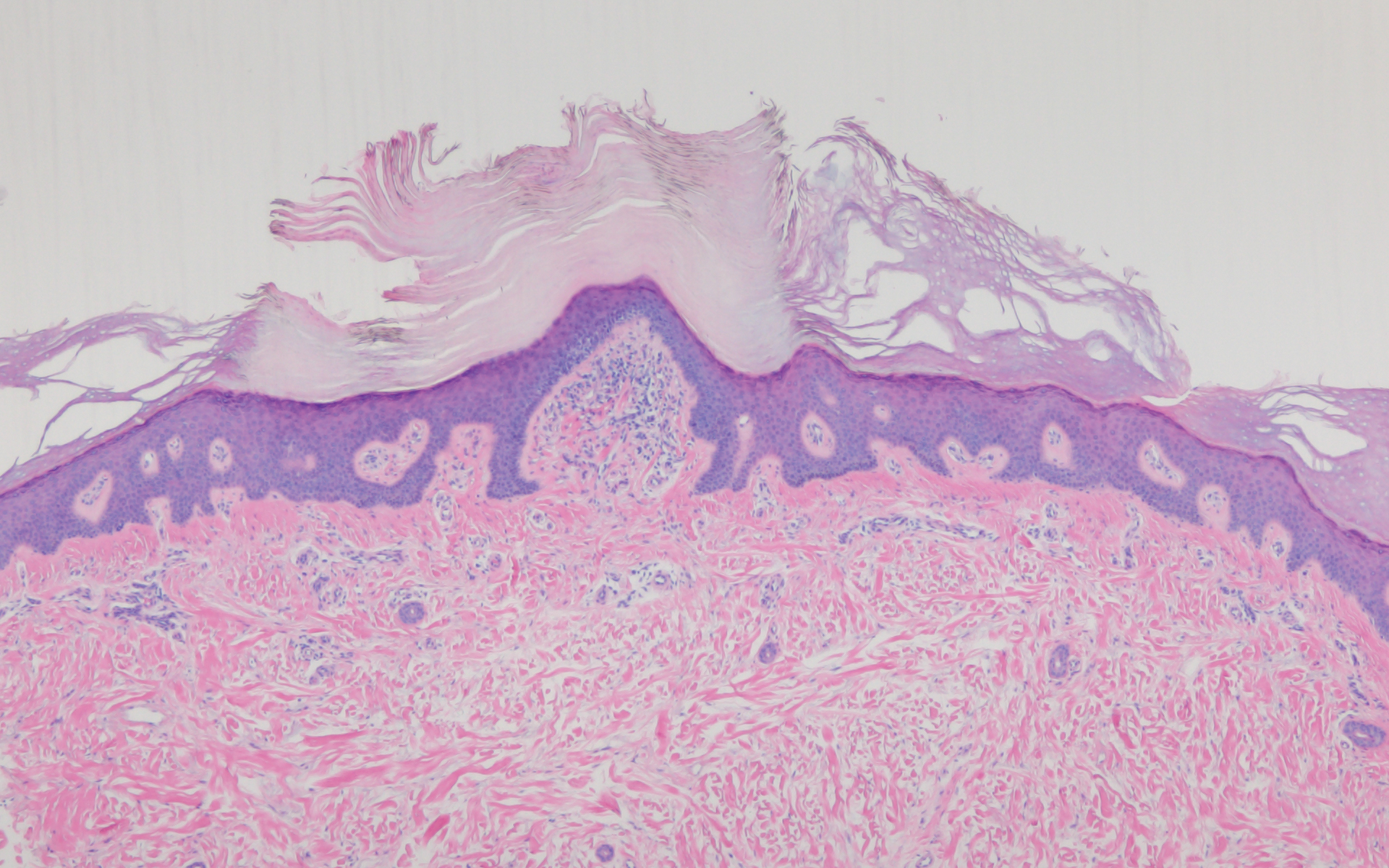
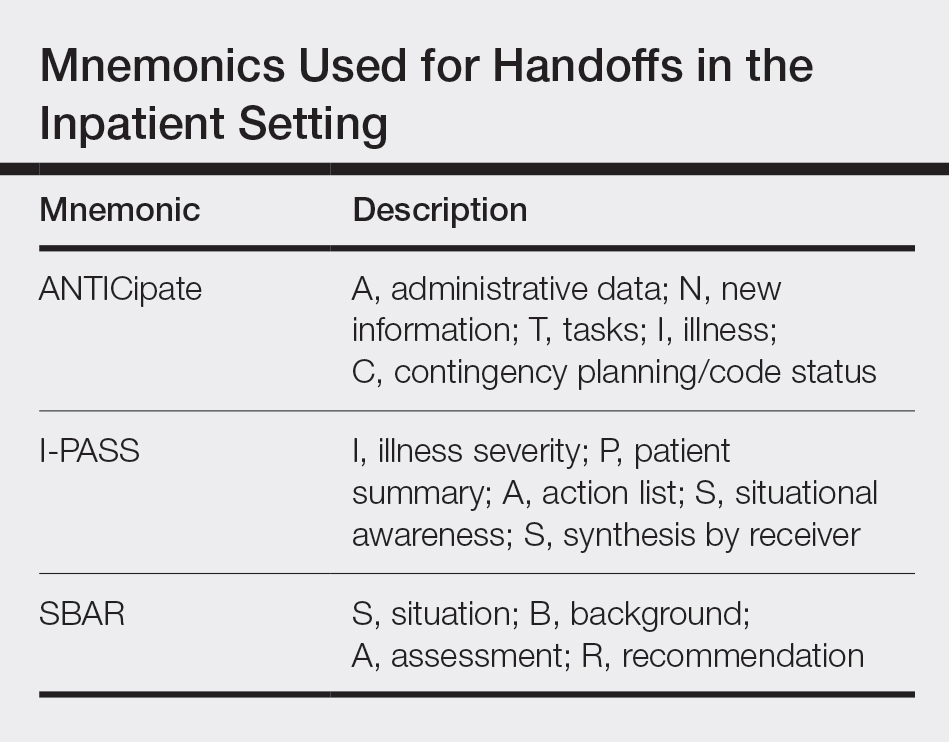
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
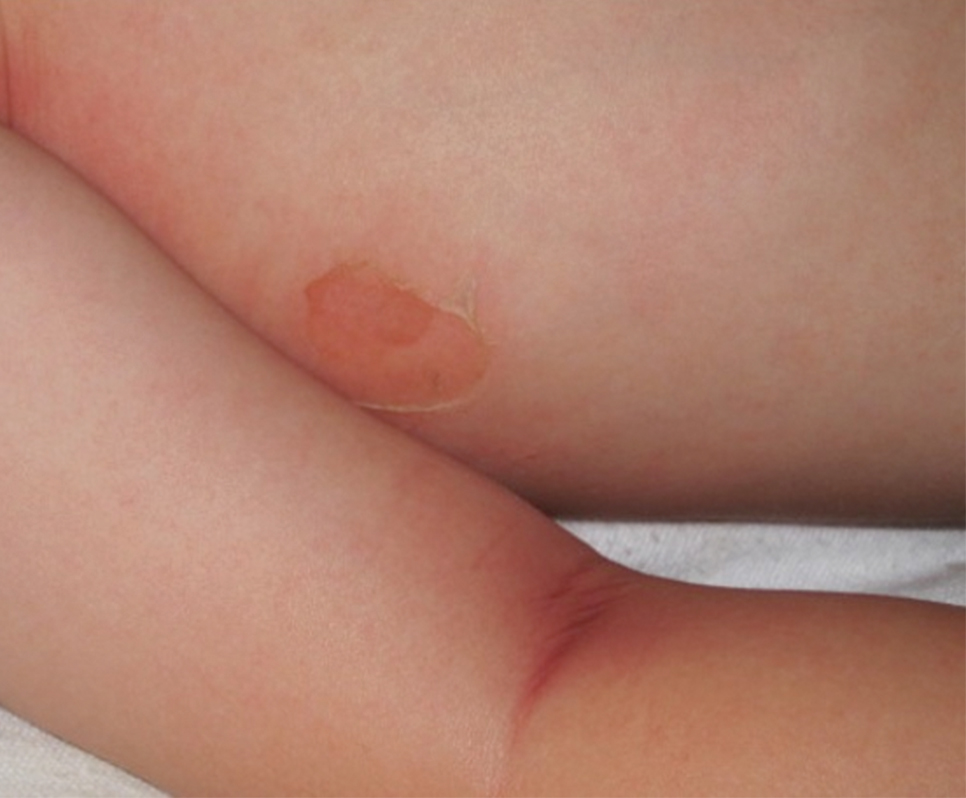
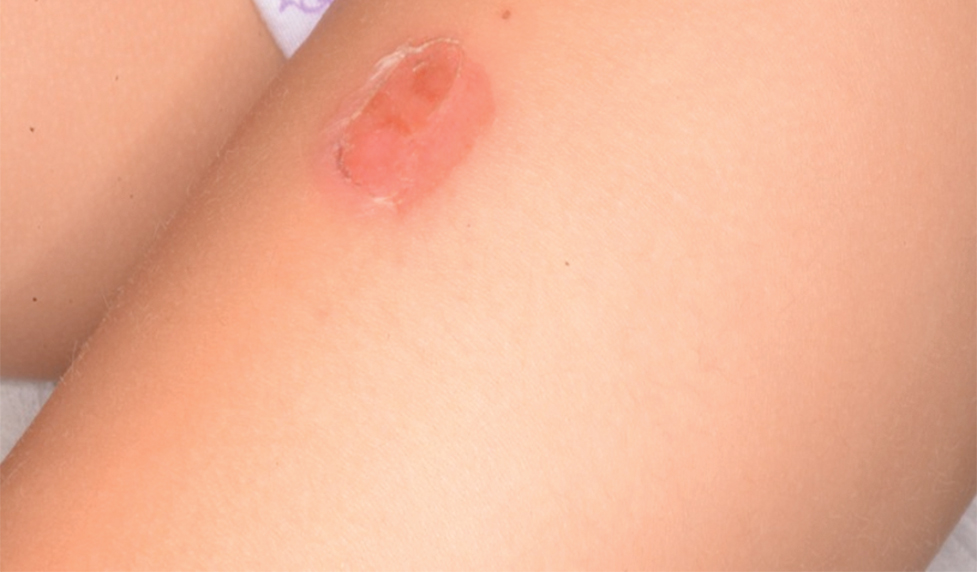
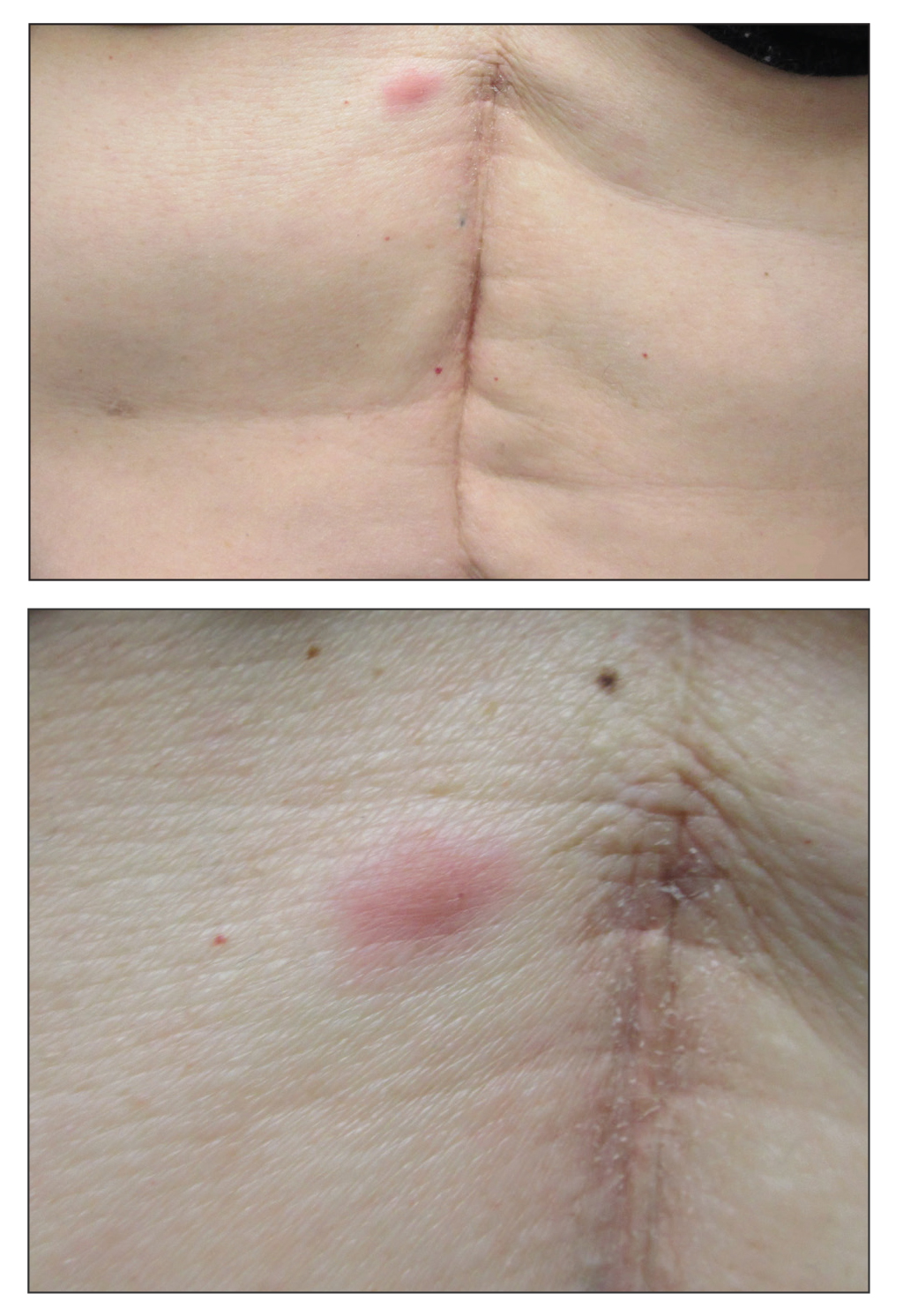
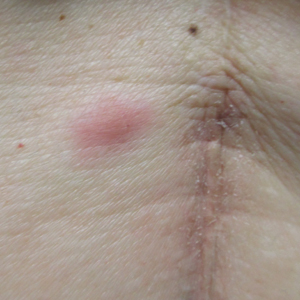
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
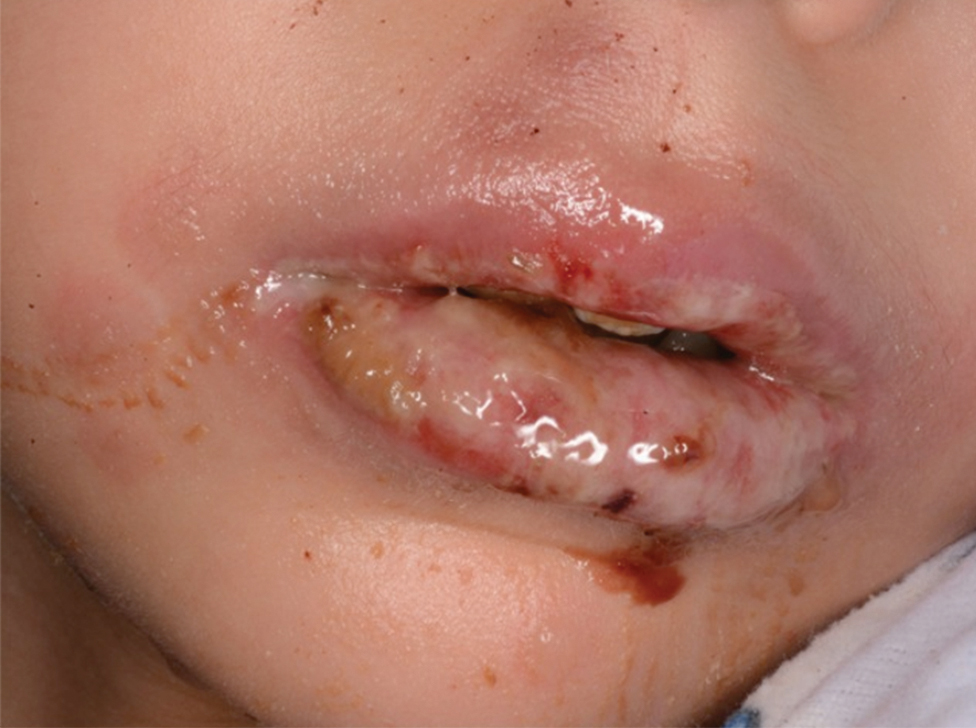
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
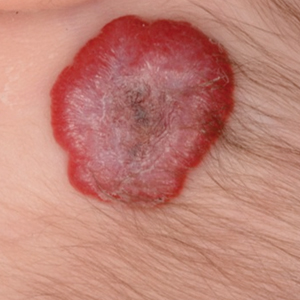
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24
The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
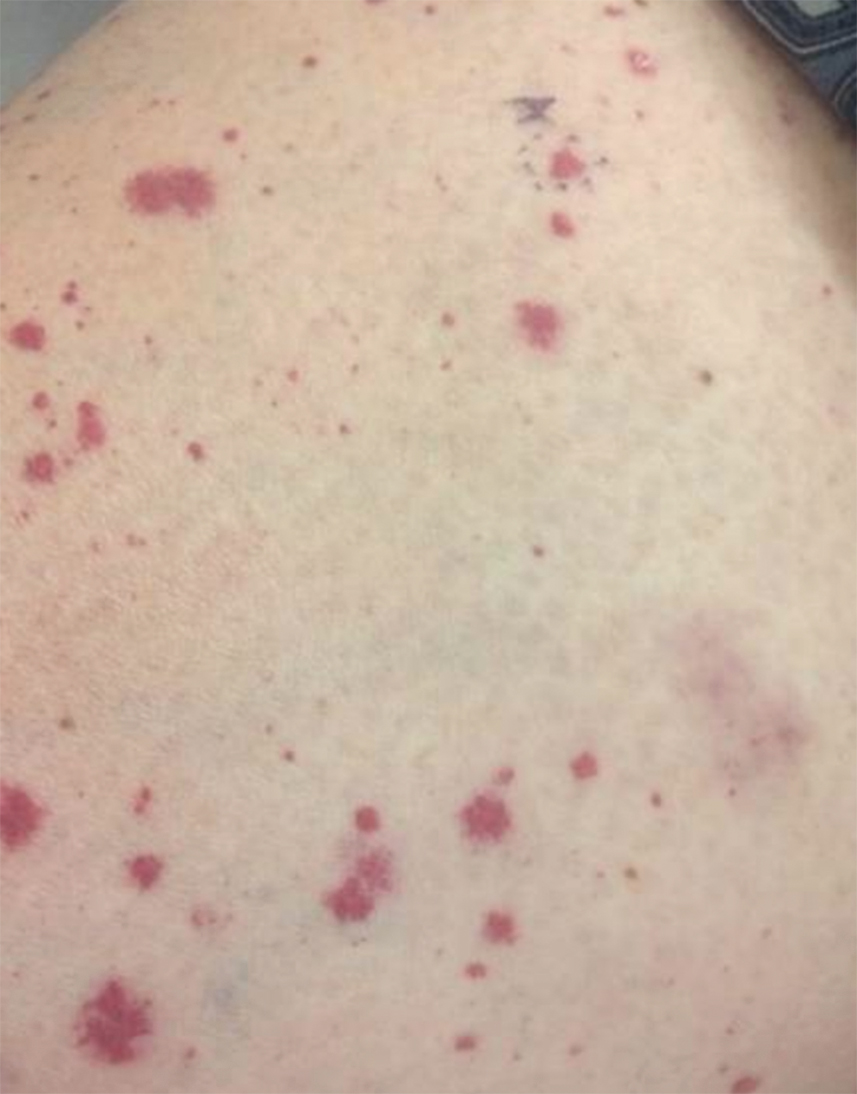
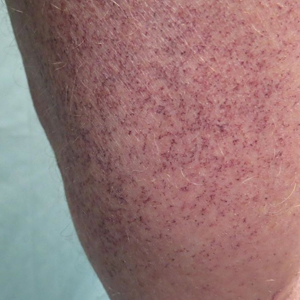
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
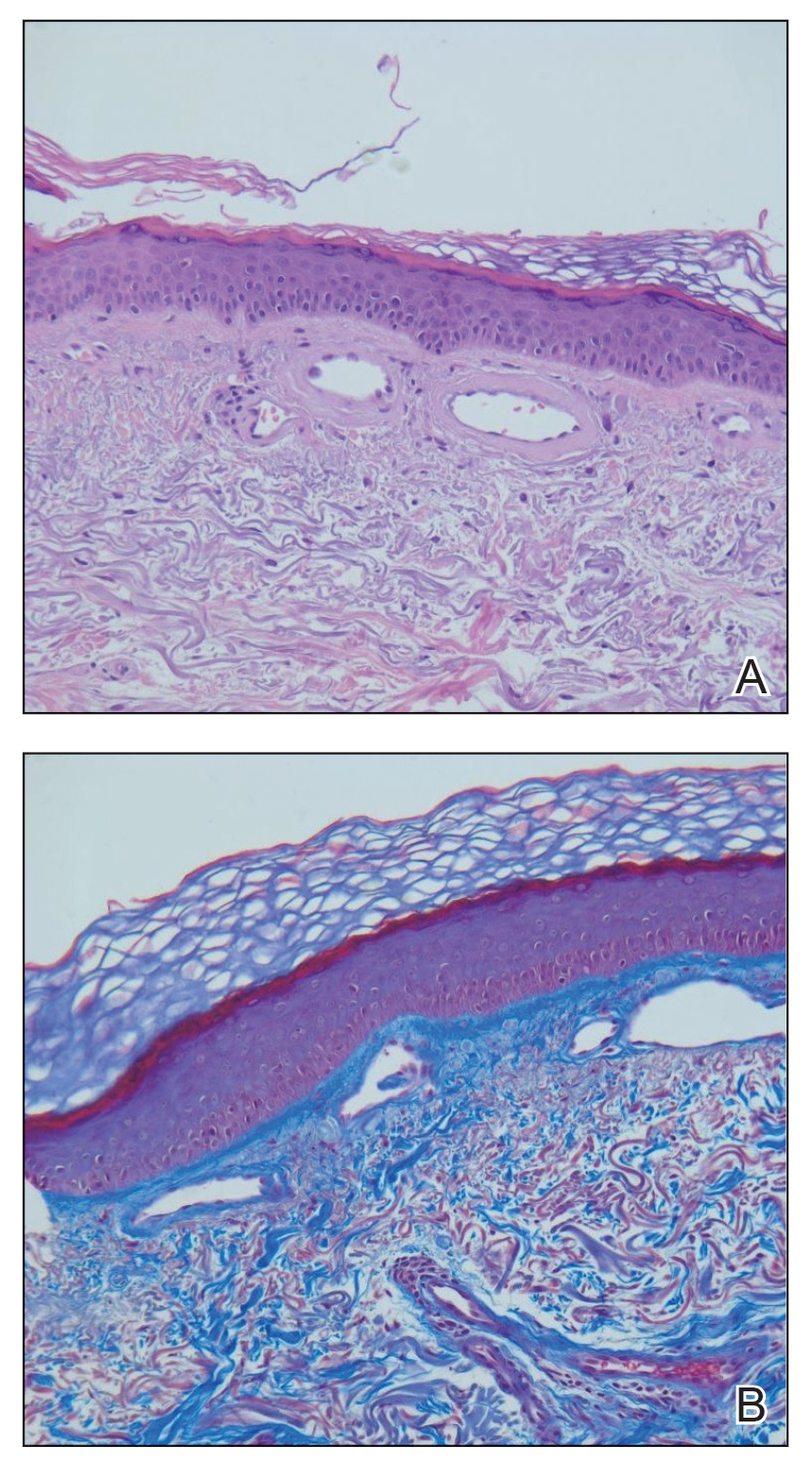
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24
The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24
The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
Practice Points
- Staphylococcal scalded skin syndrome, impetigo, eczema herpeticum, Langerhans cell histiocytosis, infantile hemangiomas, and IgA vasculitis all present potential emergencies in pediatric patients in dermatologic settings.
- Early and accurate identification and management of these entities is critical to avoid short-term and long-term negative sequalae.
What’s Eating You? Human Body Lice (Pediculus humanus corporis)
Epidemiology and Transmission
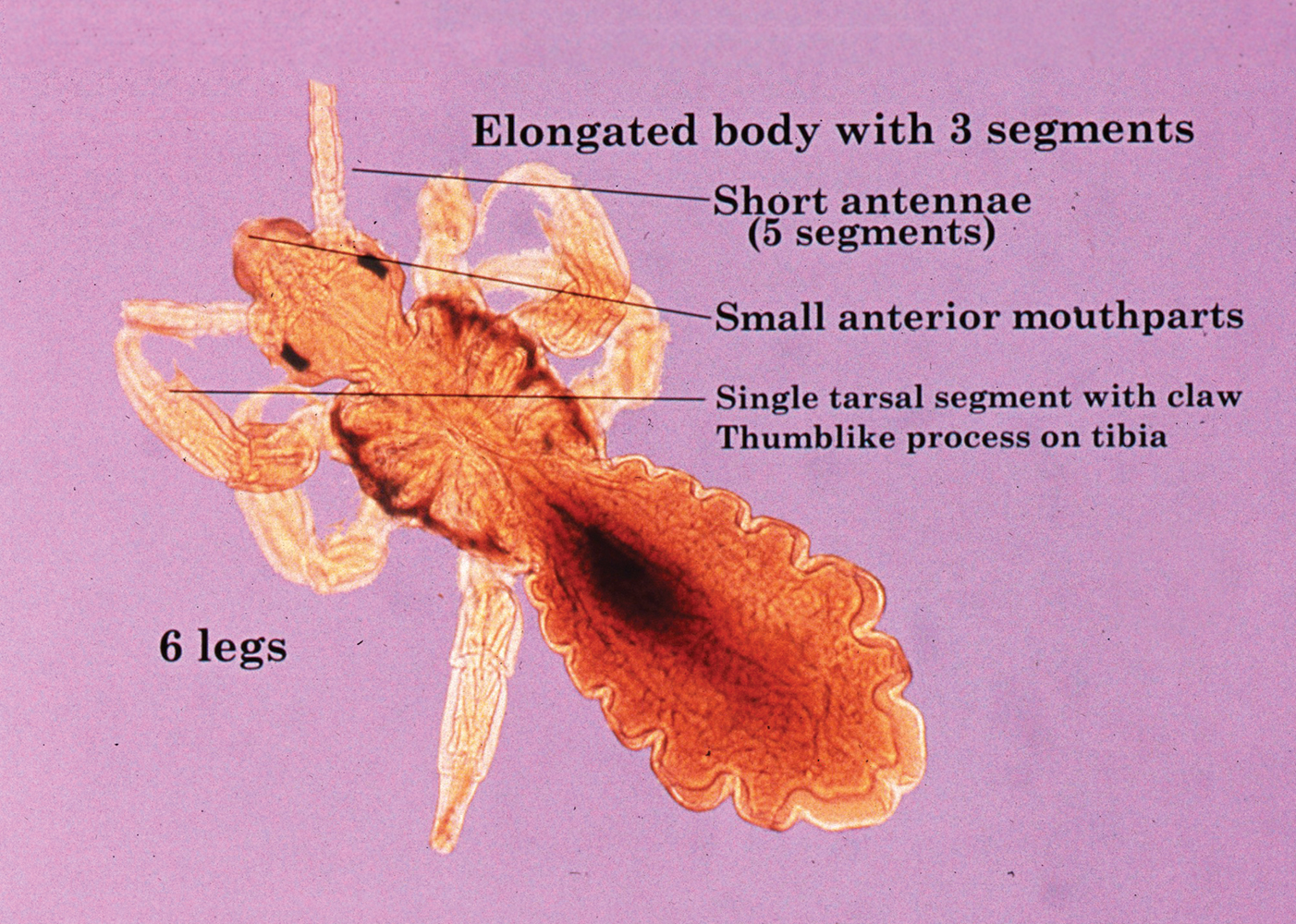
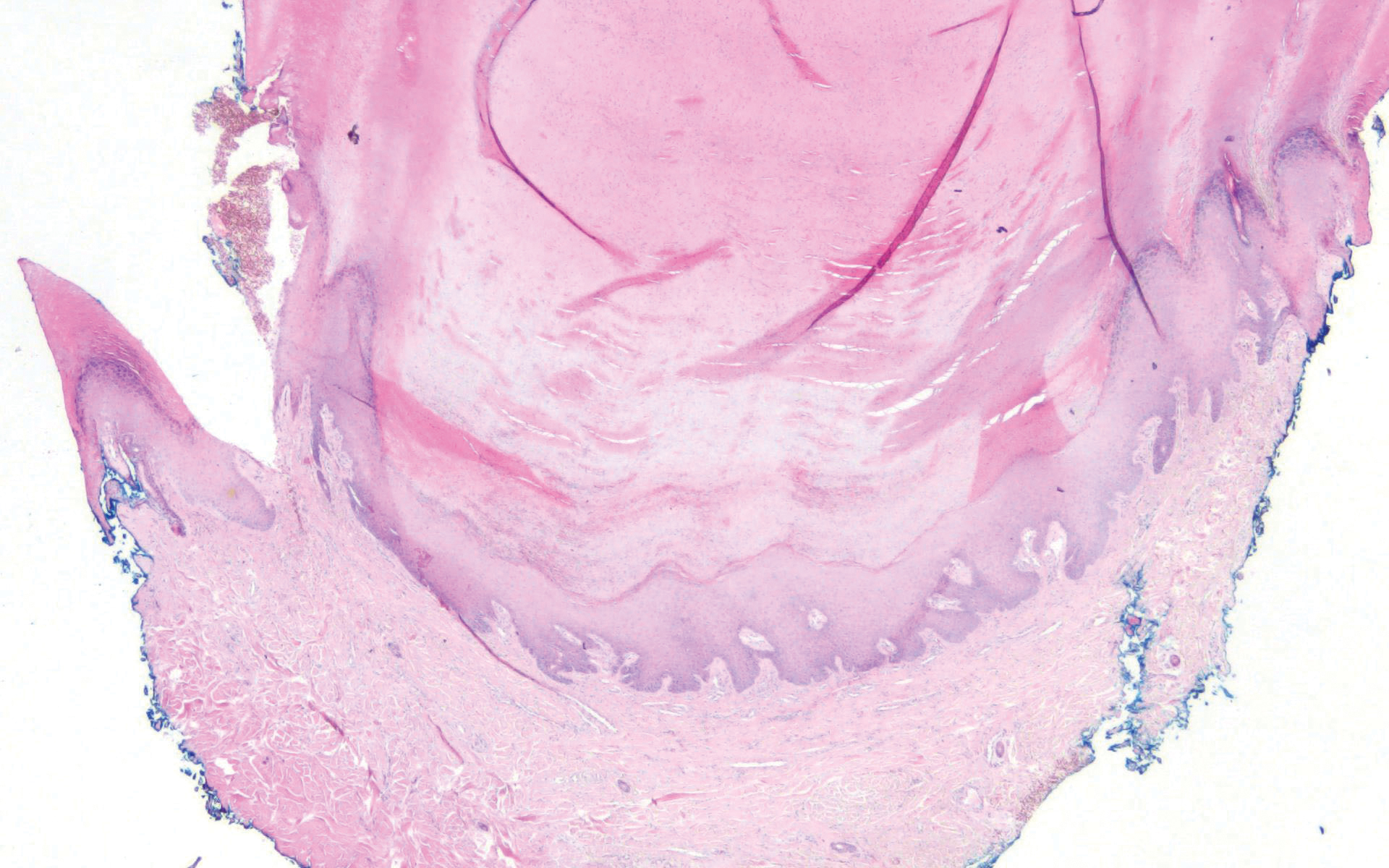
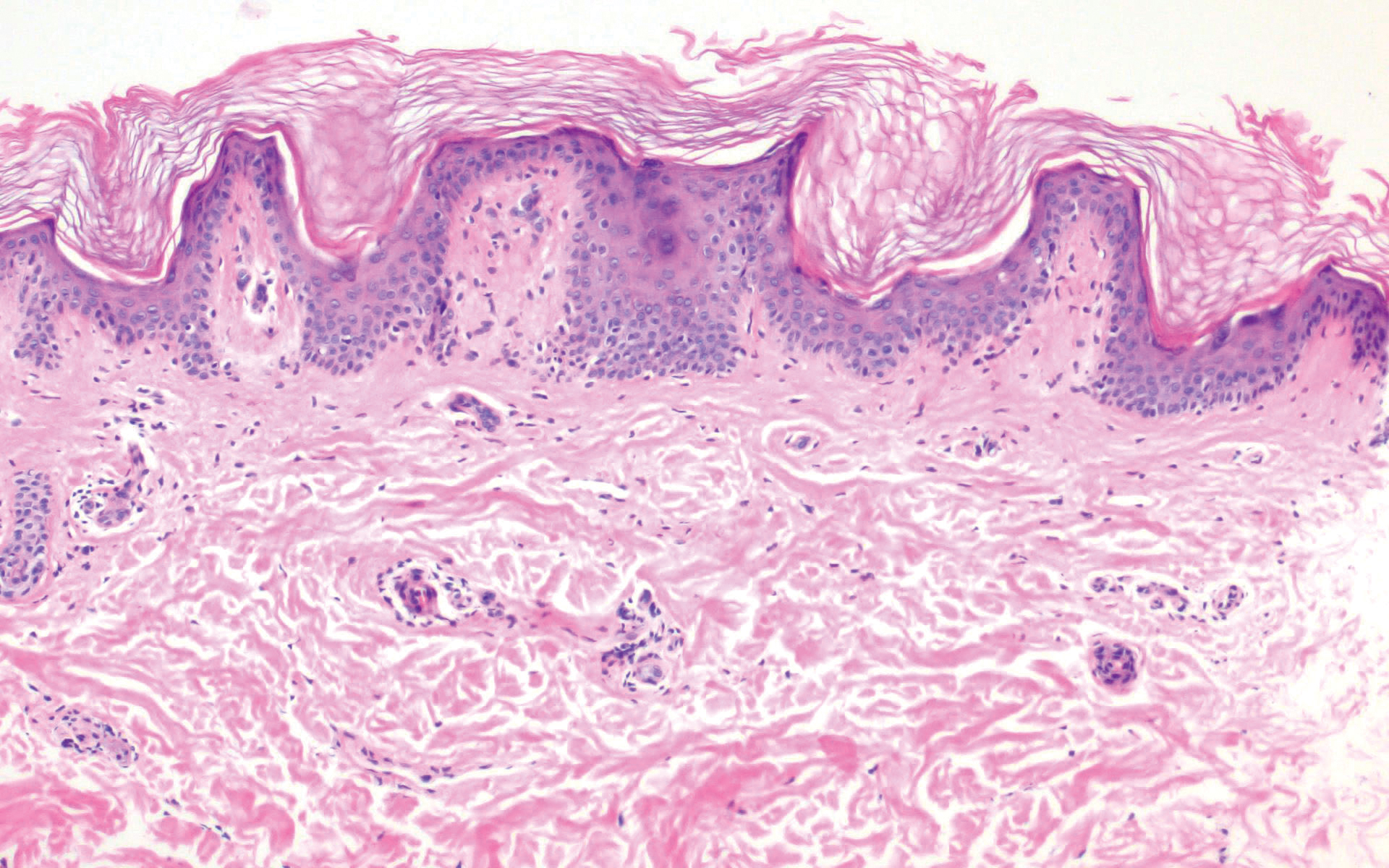
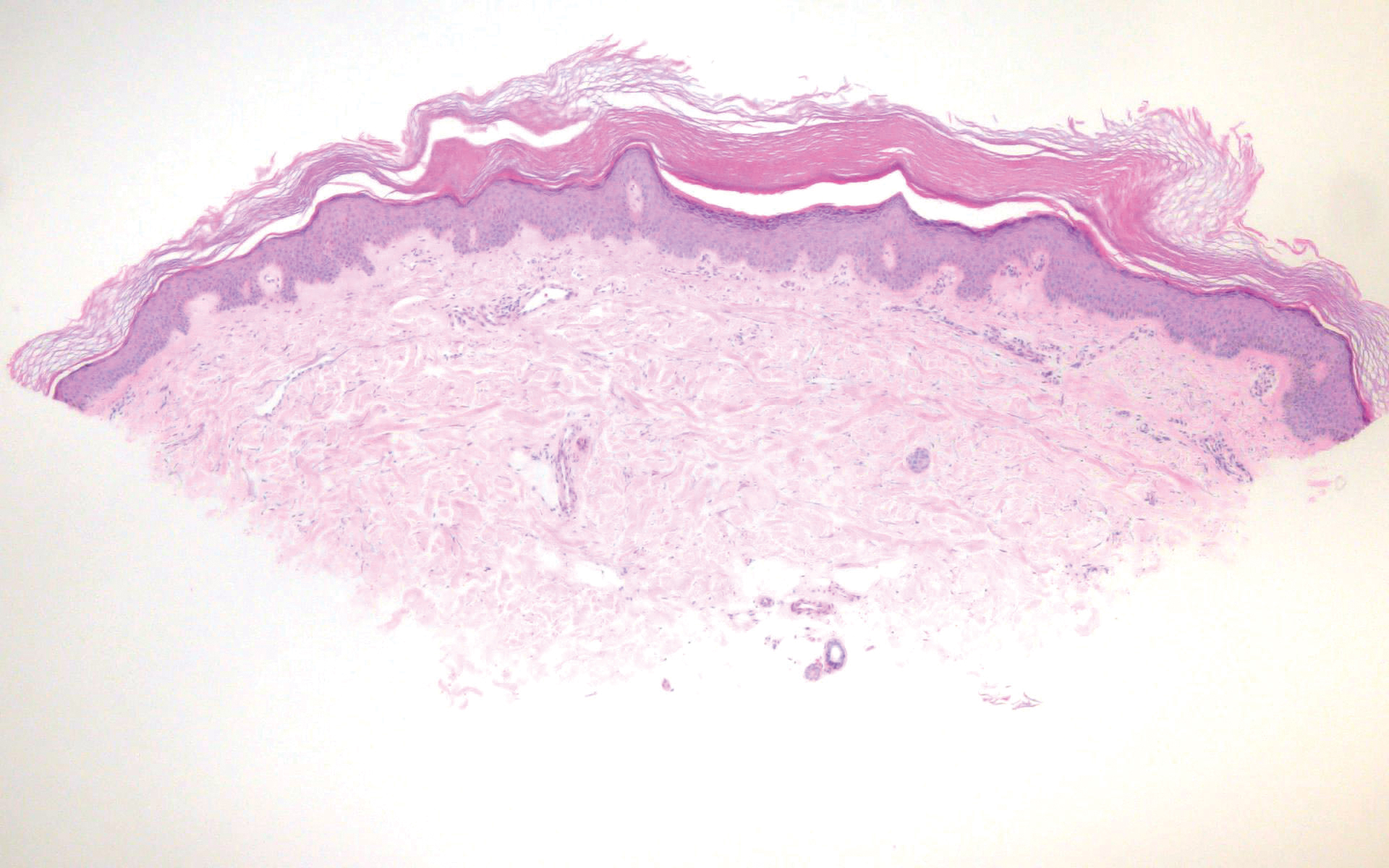
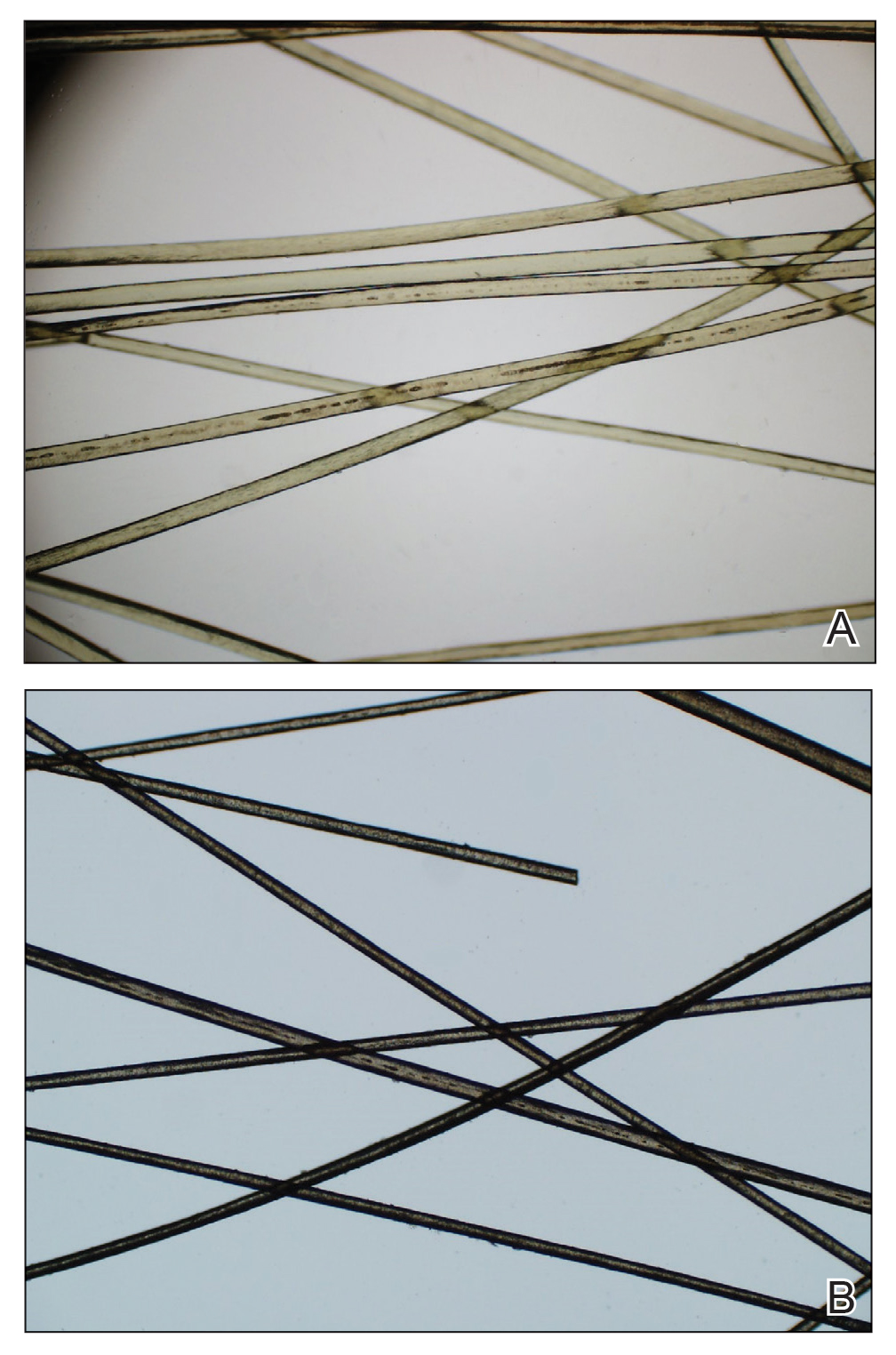
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3
Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
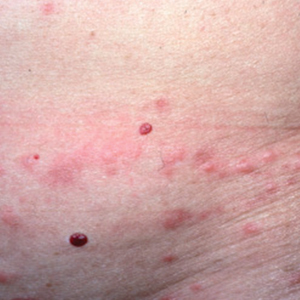
Clinical Manifestations
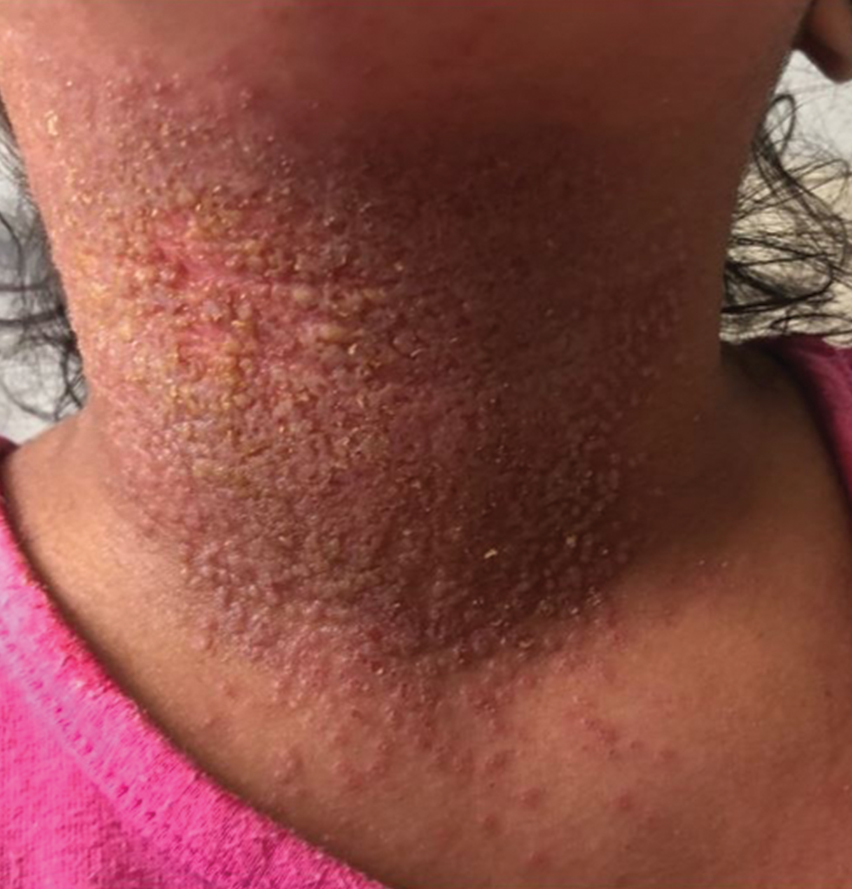
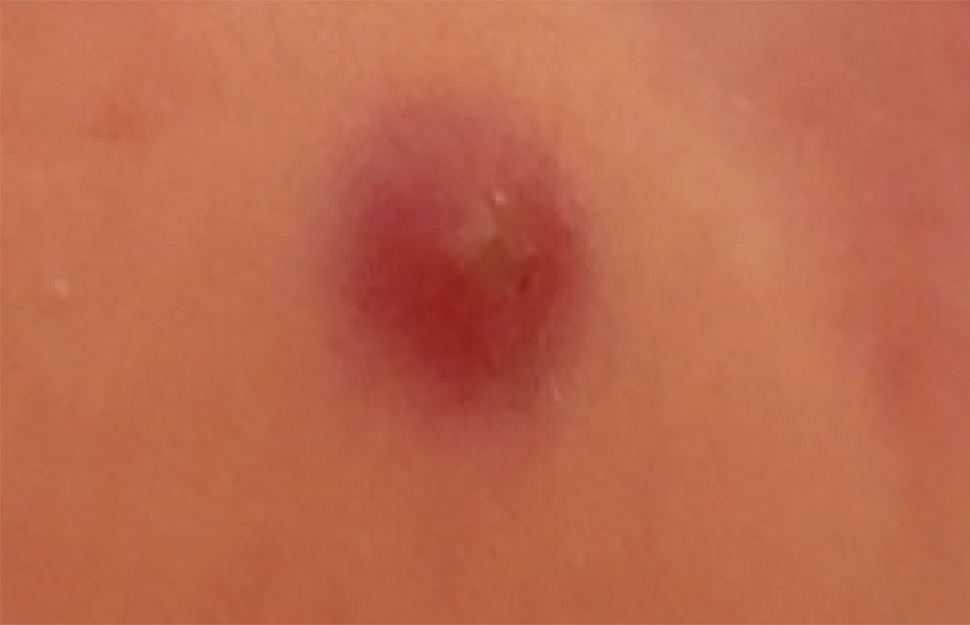
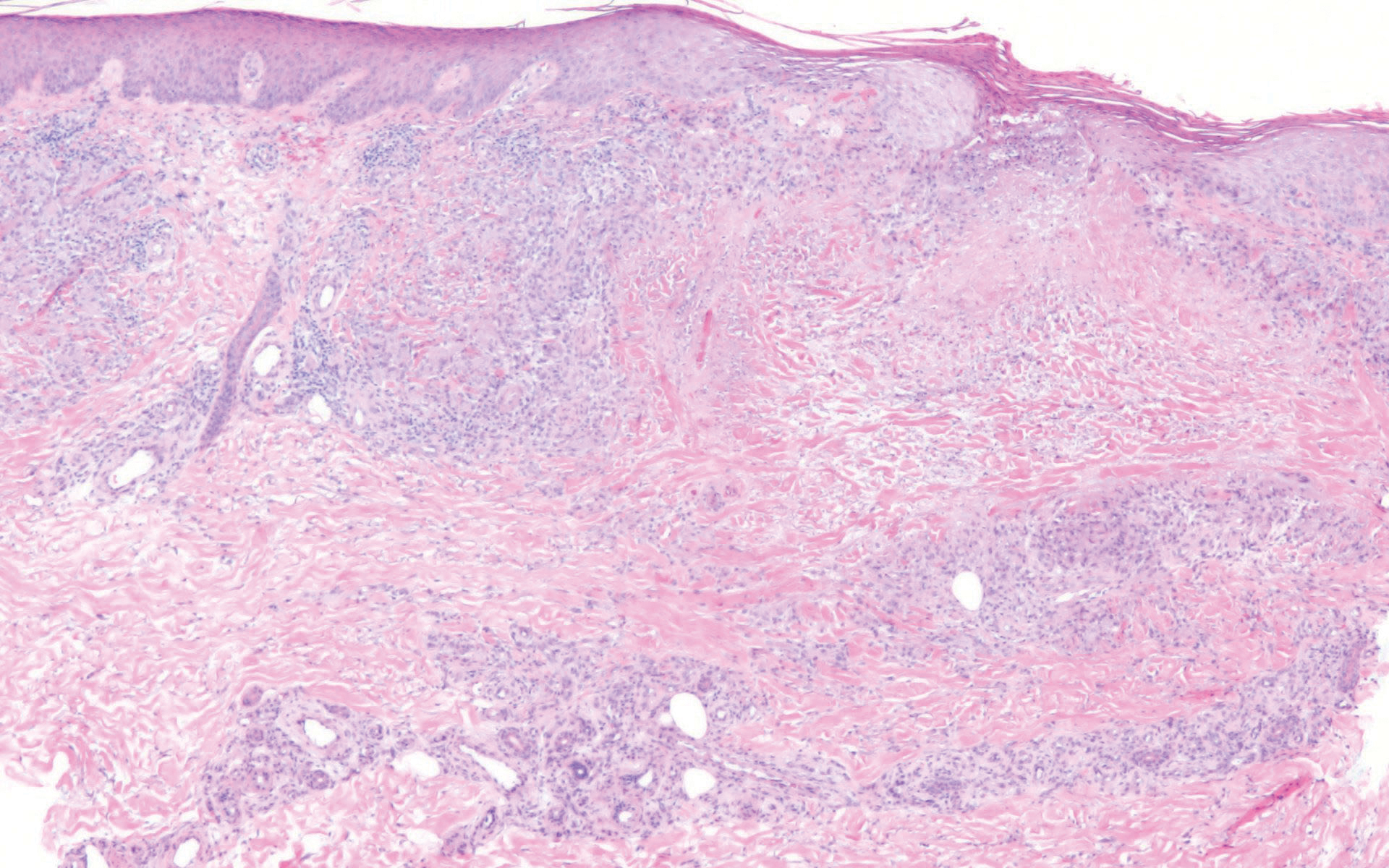
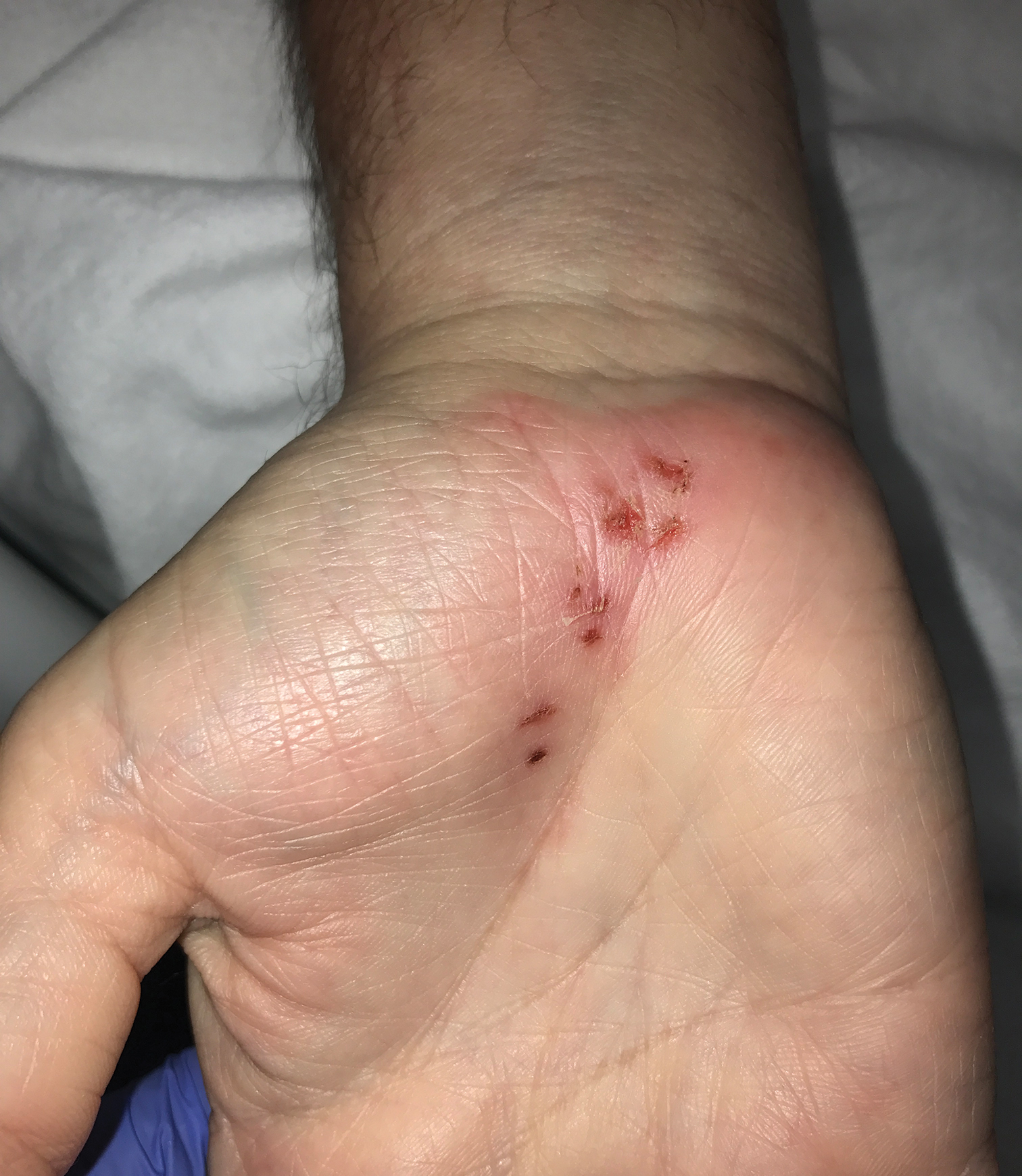
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15
Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3
Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15
Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
Epidemiology and Transmission
Pediculus humanus corporis, commonly known as the human body louse, is one in a family of 3 ectoparasites of the same suborder that also encompasses pubic lice (Phthirus pubis) and head lice (Pediculus humanus capitis). Adults are approximately 2 mm in size, with the same life cycle as head lice (Figure 1). They require blood meals roughly 5 times per day and cannot survive longer than 2 days without feeding.1 Although similar in structure to head lice, body lice differ behaviorally in that they do not reside on their human host’s body; instead, they infest the host’s clothing, localizing to seams (Figure 2), and migrate to the host for blood meals. In fact, based on this behavior, genetic analysis of early human body lice has been used to postulate when clothing was first used by humans as well as to determine early human migration patterns.2,3
Although clinicians in developed countries may be less familiar with body lice compared to their counterparts, body lice nevertheless remain a global health concern in impoverished, densely populated areas, as well as in homeless populations due to poor hygiene. Transmission frequently occurs via physical contact with an affected individual and his/her personal items (eg, linens) via fomites.4,5 Body louse infestation is more prevalent in homeless individuals who sleep outside vs in shelters; a history of pubic lice and lack of regular bathing have been reported as additional risk factors.6 Outbreaks have been noted in the wake of natural disasters, in the setting of political upheavals, and in refugee camps, as well as in individuals seeking political asylum.7 Unlike head and pubic lice, body lice can serve as vectors for infectious diseases including Rickettsia prowazekii (epidemic typhus), Borrelia recurrentis (louse-borne relapsing fever), Bartonella quintana (trench fever), and Yersinia pestis (plague).5,8,9 Several Acinetobacter species were isolated from nearly one-third of collected body louse specimens in a French study.10 Additionally, serology for B quintana was found to be positive in up to 30% of cases in one United States urban homeless population.4
Clinical Manifestations
Patients often present with generalized pruritus, usually considerably more severe than with P humanus capitis, with lesions concentrated on the trunk.11 In addition to often impetiginized, self-inflicted excoriations, feeding sites may present as erythematous macules (Figure 3), papules, or papular urticaria with a central hemorrhagic punctum. Extensive infestation also can manifest as the colloquial vagabond disease, characterized by postinflammatory hyperpigmentation and thickening of the involved skin. Remarkably, patients also may present with considerable iron-deficiency anemia secondary to high parasite load and large volume blood feeding. Multiple case reports have demonstrated associated morbidity.12-14 The differential diagnosis for pediculosis may include scabies, lichen simplex chronicus, and eczematous dermatitis, though the clinician should prudently consider whether both scabies and pediculosis may be present, as coexistence is possible.4,15
Diagnosis
Diagnosis can be reached by visualizing adult lice, nymphs, or viable nits on the body or more commonly within inner clothing seams; nits also fluoresce under Wood light.15 Although dermoscopy has proven useful for increased sensitivity and differentiation between viable and hatched nits, the insects also can be viewed with the unaided eye.16
Treatment: New Concerns and Strategies
The mainstay of treatment for body lice has long consisted of thorough washing and drying of all clothing and linens in a hot dryer. Treatment can be augmented with the addition of pharmacotherapy, plus antibiotics as warranted for louse-borne disease. Pharmacologic intervention often is used in cases of mass infestation and is similar to head lice.
Options for head lice include topical permethrin, malathion, lindane, spinosad, benzyl alcohol, and ivermectin. Pyrethroids, derived from the chrysanthemum, generally are considered safe for human use with a side-effect profile limited to irritation and allergy17; however, neurotoxicity and leukemia are clinical concerns, with an association more recently shown between large-volume use of pyrethroids and acute lymphoblastic leukemia.18,19 Use of lindane is not recommended due to a greater potential for central nervous system neurotoxicity, manifested by seizures, with repeated large surface application. Malathion is problematic due to the risk for mucosal irritation, flammability of some formulations, and theoretical organophosphate poisoning, as its mechanism of action involves inhibition of acetylcholinesterase.15 However, in the context of head lice treatment, a randomized controlled trial reported no incidence of acetylcholinesterase inhibition.20 Spinosad, manufactured from the soil bacterium Saccharopolyspora spinosa, functions similarly by interfering with the nicotinic acetylcholine receptor and also carries a risk for skin irritation.21 Among all the treatment options, we prefer benzyl alcohol, particularly in the context of resistance, as it is effective via a physical mechanism of action and lacks notable neurotoxic effects to the host. Use of benzyl alcohol is approved for patients as young as 6 months; it functions by asphyxiating the lice via paralysis of the respiratory spiracle with occlusion by inert ingredients. Itching, episodic numbness, and scalp or mucosal irritation are possible complications of treatment.22
Treatment resistance of body lice has increased in recent years, warranting exploration of additional management strategies. Moreover, developing resistance to lindane and malathion has been reported.23 Resistance to pyrethroids has been attributed to mutations in a voltage-gated sodium channel, one of which was universally present in the sampling of a single population.24 A randomized controlled trial showed that off-label oral ivermectin 400 μg/kg was superior to malathion lotion 0.5% in difficult-to-treat cases of head lice25; utility of oral ivermectin also has been reported in body lice.26 In vitro studies also have shown promise for pursuing synergistic treatment of body lice with both ivermectin and antibiotics.27
A novel primary prophylaxis approach for at-risk homeless individuals recently utilized permethrin-impregnated underwear. Although the intervention provided short-term infestation improvement, longer-term use did not show improvement from placebo and also increased prevalence of permethrin-resistant haplotypes.2
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
- Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571.
- Kittler R, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origin of clothing. Curr Biol. 2003;13:1414-1417.
- Drali R, Mumcuoglu KY, Yesilyurt G, et al. Studies of ancient lice reveal unsuspected past migrations of vectors. Am J Trop Med Hyg. 2015;93:623-625.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Feldmeier H, Heukelbach J. Epidermal parasitic skin diseases: a neglected category of poverty-associated plagues. Bull World Health Organ. 2009;87:152-159.
- Arnaud A, Chosidow O, Detrez MA, et al. Prevalence of scabies and Pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112.
- Hytonen J, Khawaja T, Gronroos JO, et al. Louse-borne relapsing fever in Finland in two asylum seekers from Somalia. APMIS. 2017;125:59-62.
- Nordmann T, Feldt T, Bosselmann M, et al. Outbreak of louse-borne relapsing fever among urban dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am J Trop Med Hyg. 2018;98:1599-1602.
- Louni M, Mana N, Bitam I, et al. Body lice of homeless people reveal the presence of several emerging bacterial pathogens in northern Algeria. PLoS Negl Trop Dis. 2018;12:E0006397.
- Candy K, Amanzougaghene N, Izri A, et al. Molecular survey of head and body lice, Pediculus humanus, in France. Vector Borne Zoonotic Dis. 2018;18:243-251.
Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier Limited; 2018. - Nara A, Nagai H, Yamaguchi R, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130:765-769.
- Althomali SA, Alzubaidi LM, Alkhaldi DM. Severe iron deficiency anaemia associated with heavy lice infestation in a young woman [published online November 5, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-212207.
- Hau V, Muhi-Iddin N. A ghost covered in lice: a case of severe blood loss with long-standing heavy pediculosis capitis infestation [published online December 19, 2014]. BMJ Case Rep. doi:10.1136/bcr-2014-206623.
- Diaz JH. Lice (Pediculosis). In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. New York, NY: Elsevier; 2020:3482-3486.
- Martins LG, Bernardes Filho F, Quaresma MV, et al. Dermoscopy applied to pediculosis corporis diagnosis. An Bras Dermatol. 2014;89:513-514.
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113:123-136.
- Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46:13480-13487.
- Meinking TL, Vicaria M, Eyerdam DH, et al. A randomized, investigator-blinded, time-ranging study of the comparative efficacy of 0.5% malathion gel versus Ovide Lotion (0.5% malathion) or Nix Crème Rinse (1% permethrin) used as labeled, for the treatment of head lice. Pediatr Dermatol. 2007;24:405-411.
- McCormack PL. Spinosad: in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis). Pediatr Dermatol. 2010;27:19-24.
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965-974
- Drali R, Benkouiten S, Badiaga S, et al. Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol. 2012;50:2229-2233.
- Chosidow O, Giraudeau B, Cottrell J, et al. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med. 2010;362:896-905.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
- Sangaré AK, Doumbo OK, Raoult D. Management and treatment of human lice [published online July 27, 2016]. Biomed Res Int. doi:10.1155/2016/8962685.
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279.
Practice Points
- Body lice reside in clothing, particularly folds and seams, and migrate to the host for blood meals. To evaluate for infestation, the clinician should not only look at the skin but also closely examine the patient’s clothing. Clothes also are a target for treatment via washing in hot water.
- Due to observed and theoretical adverse effects of other chemical treatments, benzyl alcohol is the authors’ choice for treatment of head lice.
- Oral ivermectin is a promising future treatment for body lice.
Firm Abdominal Papule
The Diagnosis: Cutaneous Metastatic Gastric Carcinoma
Cutaneous metastasis of primary gastric carcinoma is a rare occurrence, with the more common metastatic sites being the lymph nodes, liver, and peritoneal cavity. The incidence of visceral neoplasm metastasis to the skin ranges from 0.7% to 9% and is less than 1% for upper digestive tract carcinomas.1 Cutaneous metastases make up 2% of all tumors of the skin and commonly are located near the site of the primary tumor.2 The most common cutaneous metastasis sites for gastric carcinoma include the neck, chest, and head.3 One of the more typical sites of cutaneous metastasis from gastric cancer is the umbilicus (ie, Sister Mary Joseph nodule). Cutaneous metastases from gastric carcinoma commonly present as asymptomatic hyperpigmented nodules.1,3
In our patient, histopathologic sections showed diffuse infiltration of the dermis by atypical polygonal/round cells arranged in cords and small aggregates. Some of the neoplastic cells had signet ring morphology (Figure). Tumor cells demonstrated positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen; they were negative for S-100, MART-1 (melanoma-associated antigen recognized by T cells 1), leukocyte common antigen, gross cystic disease fluid protein 15, estrogen and progesterone receptor, and HER2/neu (human epidermal growth factor receptor 2).
Our patient's presentation was rare in that she developed an asymptomatic erythematous papule on the skin of the abdomen. However, her history of stage IIIB gastric adenocarcinoma in conjunction with the clinical picture and microscopic findings were most consistent with metastatic carcinoma of gastrointestinal origin. The histologic hallmarks of cutaneous metastatic gastric carcinoma include aggregates of neoplastic cells arranged in cords, sometimes forming glands, embedded in a fibrous stroma. Tumor cells may demonstrate signet ring morphology. These unique histologic findings, as well as positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen, rule out other potential diagnoses for an asymptomatic solitary papule.
Dermatofibrosarcoma protuberans presents as an asymptomatic, slow-growing, indurated papule or plaque that develops into a red or brownish nodule. Histologically, dermatofibrosarcoma protuberans is characterized by spindled cells, few mitotic figures, infiltration of the subcutaneous tissue in a honeycomblike pattern, and obliteration of the adnexal structures.4
Cutaneous B-cell lymphoma (CBCL) can present as single or multiple red papules or nodules located on the trunk, face, or extremities. Histologically, CBCL would show a nodular or diffuse infiltrate throughout the dermis, frequently with accentuation in the deep reticular dermis, sparing of the epidermis, and the presence of a grenz zone. The infiltrate in CBCL consists of CD20+, CD19+, and CD79a+ B cells. Identification of a monoclonal B-cell population either by immunohistochemistry or polymerase chain reaction would further support a diagnosis of CBCL.4 These specific histologic findings and the immunohistochemical staining pattern helped rule out CBCL as the diagnosis in our patient.
Amelanotic melanomas present as flesh-colored to light pink papules, making them especially challenging to diagnose clinically. Asymmetrical, poorly circumscribed nests of atypical melanocytes as well as single melanocytes within the epidermis and dermis are seen histologically; mitotic figures are common. Immunohistochemical staining for melanoma includes S-100, human melanoma black 45, MART-1/Melan-A, tyrosinase, and microphthalmia-associated transcription factor 1.4
Neurothekeomas can present as asymptomatic, solitary, flesh-colored papules located on the head, neck, and upper trunk. Histologically, neurothekeomas have a distinct appearance consisting of a well-defined mass composed of variable-sized lobules of spindled and epithelioid cells dispersed in a myxoid stroma within the reticular dermis.4 These specific histologic findings helped rule out neurothekeoma in our patient.
Following the diagnosis of cutaneous metastatic gastric carcinoma in our patient, positron emission tomography and computed tomography of the chest, abdomen, and pelvis were unremarkable for distant disease. Subsequently, the patient underwent surgical excision of the papule with clear margins, followed by a short course of radiation therapy. She currently is under close monitoring but remains in remission with no new cutaneous manifestations of the gastric carcinoma.
- Erdemir A, Atilganoglu U, Onsun N, et al. Cutaneous metastases from gastric adenocarcinoma. Indian J Dermatol. 2011;56:236-237.
- Junqueira AL, Corbett AM, Oliveira Filho Jd, et al. Cutaneous metastasis from gastrointestinal adenocarcinoma of unknown primary origin. An Bras Dermatol. 2015;90:564-566.
- Cesaretti M, Malerba M, Basso V, et al. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
The Diagnosis: Cutaneous Metastatic Gastric Carcinoma
Cutaneous metastasis of primary gastric carcinoma is a rare occurrence, with the more common metastatic sites being the lymph nodes, liver, and peritoneal cavity. The incidence of visceral neoplasm metastasis to the skin ranges from 0.7% to 9% and is less than 1% for upper digestive tract carcinomas.1 Cutaneous metastases make up 2% of all tumors of the skin and commonly are located near the site of the primary tumor.2 The most common cutaneous metastasis sites for gastric carcinoma include the neck, chest, and head.3 One of the more typical sites of cutaneous metastasis from gastric cancer is the umbilicus (ie, Sister Mary Joseph nodule). Cutaneous metastases from gastric carcinoma commonly present as asymptomatic hyperpigmented nodules.1,3
In our patient, histopathologic sections showed diffuse infiltration of the dermis by atypical polygonal/round cells arranged in cords and small aggregates. Some of the neoplastic cells had signet ring morphology (Figure). Tumor cells demonstrated positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen; they were negative for S-100, MART-1 (melanoma-associated antigen recognized by T cells 1), leukocyte common antigen, gross cystic disease fluid protein 15, estrogen and progesterone receptor, and HER2/neu (human epidermal growth factor receptor 2).
Our patient's presentation was rare in that she developed an asymptomatic erythematous papule on the skin of the abdomen. However, her history of stage IIIB gastric adenocarcinoma in conjunction with the clinical picture and microscopic findings were most consistent with metastatic carcinoma of gastrointestinal origin. The histologic hallmarks of cutaneous metastatic gastric carcinoma include aggregates of neoplastic cells arranged in cords, sometimes forming glands, embedded in a fibrous stroma. Tumor cells may demonstrate signet ring morphology. These unique histologic findings, as well as positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen, rule out other potential diagnoses for an asymptomatic solitary papule.
Dermatofibrosarcoma protuberans presents as an asymptomatic, slow-growing, indurated papule or plaque that develops into a red or brownish nodule. Histologically, dermatofibrosarcoma protuberans is characterized by spindled cells, few mitotic figures, infiltration of the subcutaneous tissue in a honeycomblike pattern, and obliteration of the adnexal structures.4
Cutaneous B-cell lymphoma (CBCL) can present as single or multiple red papules or nodules located on the trunk, face, or extremities. Histologically, CBCL would show a nodular or diffuse infiltrate throughout the dermis, frequently with accentuation in the deep reticular dermis, sparing of the epidermis, and the presence of a grenz zone. The infiltrate in CBCL consists of CD20+, CD19+, and CD79a+ B cells. Identification of a monoclonal B-cell population either by immunohistochemistry or polymerase chain reaction would further support a diagnosis of CBCL.4 These specific histologic findings and the immunohistochemical staining pattern helped rule out CBCL as the diagnosis in our patient.
Amelanotic melanomas present as flesh-colored to light pink papules, making them especially challenging to diagnose clinically. Asymmetrical, poorly circumscribed nests of atypical melanocytes as well as single melanocytes within the epidermis and dermis are seen histologically; mitotic figures are common. Immunohistochemical staining for melanoma includes S-100, human melanoma black 45, MART-1/Melan-A, tyrosinase, and microphthalmia-associated transcription factor 1.4
Neurothekeomas can present as asymptomatic, solitary, flesh-colored papules located on the head, neck, and upper trunk. Histologically, neurothekeomas have a distinct appearance consisting of a well-defined mass composed of variable-sized lobules of spindled and epithelioid cells dispersed in a myxoid stroma within the reticular dermis.4 These specific histologic findings helped rule out neurothekeoma in our patient.
Following the diagnosis of cutaneous metastatic gastric carcinoma in our patient, positron emission tomography and computed tomography of the chest, abdomen, and pelvis were unremarkable for distant disease. Subsequently, the patient underwent surgical excision of the papule with clear margins, followed by a short course of radiation therapy. She currently is under close monitoring but remains in remission with no new cutaneous manifestations of the gastric carcinoma.
The Diagnosis: Cutaneous Metastatic Gastric Carcinoma
Cutaneous metastasis of primary gastric carcinoma is a rare occurrence, with the more common metastatic sites being the lymph nodes, liver, and peritoneal cavity. The incidence of visceral neoplasm metastasis to the skin ranges from 0.7% to 9% and is less than 1% for upper digestive tract carcinomas.1 Cutaneous metastases make up 2% of all tumors of the skin and commonly are located near the site of the primary tumor.2 The most common cutaneous metastasis sites for gastric carcinoma include the neck, chest, and head.3 One of the more typical sites of cutaneous metastasis from gastric cancer is the umbilicus (ie, Sister Mary Joseph nodule). Cutaneous metastases from gastric carcinoma commonly present as asymptomatic hyperpigmented nodules.1,3
In our patient, histopathologic sections showed diffuse infiltration of the dermis by atypical polygonal/round cells arranged in cords and small aggregates. Some of the neoplastic cells had signet ring morphology (Figure). Tumor cells demonstrated positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen; they were negative for S-100, MART-1 (melanoma-associated antigen recognized by T cells 1), leukocyte common antigen, gross cystic disease fluid protein 15, estrogen and progesterone receptor, and HER2/neu (human epidermal growth factor receptor 2).
Our patient's presentation was rare in that she developed an asymptomatic erythematous papule on the skin of the abdomen. However, her history of stage IIIB gastric adenocarcinoma in conjunction with the clinical picture and microscopic findings were most consistent with metastatic carcinoma of gastrointestinal origin. The histologic hallmarks of cutaneous metastatic gastric carcinoma include aggregates of neoplastic cells arranged in cords, sometimes forming glands, embedded in a fibrous stroma. Tumor cells may demonstrate signet ring morphology. These unique histologic findings, as well as positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen, rule out other potential diagnoses for an asymptomatic solitary papule.
Dermatofibrosarcoma protuberans presents as an asymptomatic, slow-growing, indurated papule or plaque that develops into a red or brownish nodule. Histologically, dermatofibrosarcoma protuberans is characterized by spindled cells, few mitotic figures, infiltration of the subcutaneous tissue in a honeycomblike pattern, and obliteration of the adnexal structures.4
Cutaneous B-cell lymphoma (CBCL) can present as single or multiple red papules or nodules located on the trunk, face, or extremities. Histologically, CBCL would show a nodular or diffuse infiltrate throughout the dermis, frequently with accentuation in the deep reticular dermis, sparing of the epidermis, and the presence of a grenz zone. The infiltrate in CBCL consists of CD20+, CD19+, and CD79a+ B cells. Identification of a monoclonal B-cell population either by immunohistochemistry or polymerase chain reaction would further support a diagnosis of CBCL.4 These specific histologic findings and the immunohistochemical staining pattern helped rule out CBCL as the diagnosis in our patient.
Amelanotic melanomas present as flesh-colored to light pink papules, making them especially challenging to diagnose clinically. Asymmetrical, poorly circumscribed nests of atypical melanocytes as well as single melanocytes within the epidermis and dermis are seen histologically; mitotic figures are common. Immunohistochemical staining for melanoma includes S-100, human melanoma black 45, MART-1/Melan-A, tyrosinase, and microphthalmia-associated transcription factor 1.4
Neurothekeomas can present as asymptomatic, solitary, flesh-colored papules located on the head, neck, and upper trunk. Histologically, neurothekeomas have a distinct appearance consisting of a well-defined mass composed of variable-sized lobules of spindled and epithelioid cells dispersed in a myxoid stroma within the reticular dermis.4 These specific histologic findings helped rule out neurothekeoma in our patient.
Following the diagnosis of cutaneous metastatic gastric carcinoma in our patient, positron emission tomography and computed tomography of the chest, abdomen, and pelvis were unremarkable for distant disease. Subsequently, the patient underwent surgical excision of the papule with clear margins, followed by a short course of radiation therapy. She currently is under close monitoring but remains in remission with no new cutaneous manifestations of the gastric carcinoma.
- Erdemir A, Atilganoglu U, Onsun N, et al. Cutaneous metastases from gastric adenocarcinoma. Indian J Dermatol. 2011;56:236-237.
- Junqueira AL, Corbett AM, Oliveira Filho Jd, et al. Cutaneous metastasis from gastrointestinal adenocarcinoma of unknown primary origin. An Bras Dermatol. 2015;90:564-566.
- Cesaretti M, Malerba M, Basso V, et al. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
- Erdemir A, Atilganoglu U, Onsun N, et al. Cutaneous metastases from gastric adenocarcinoma. Indian J Dermatol. 2011;56:236-237.
- Junqueira AL, Corbett AM, Oliveira Filho Jd, et al. Cutaneous metastasis from gastrointestinal adenocarcinoma of unknown primary origin. An Bras Dermatol. 2015;90:564-566.
- Cesaretti M, Malerba M, Basso V, et al. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
A 53-year-old woman with a history of melanoma on the right thigh, stage II Hodgkin lymphoma, and stage IIIB gastric adenocarcinoma treated with a distal gastrectomy presented with an asymptomatic but persistent skin lesion on the abdomen of 2 months' duration. The lesion arose spontaneously 6 months prior and had increased in size during that time. Physical examination revealed a 6-mm, solitary, firm, erythematous papule on the skin of the right upper quadrant of the abdomen. The patient was otherwise healthy, and a review of systems did not reveal any abnormalities. A punch biopsy was submitted for histopathologic review.
Spiky Papules on the Dorsal Feet
The Diagnosis: Hyperkeratosis Lenticularis Perstans (Flegel Disease)
Hyperkeratosis lenticularis perstans, also known as Flegel disease, is a rare dermatosis first described by Flegel1 in 1958. This benign disorder is characterized by multiple asymptomatic 1- to 5-mm keratotic papules in a symmetric distribution favoring the dorsal aspects of the feet and distal extremities in adults. An autosomal-dominant inheritance pattern has been postulated, though many cases sporadically occur.2 The characteristic spiky papules typically appear during mid to late adulthood and tend to persist. Treatment options are lacking, with reports of partial or no response to topical calcipotriol, topical 5-fluorouracil, cryotherapy, and topical and oral retinoids.3,4
The histopathology of hyperkeratosis lenticularis perstans is distinct, showing a central discrete area of orthohyperkeratosis with patchy parakeratosis flanked by a normal stratum corneum. The underlying epidermis typically shows effacement of the rete ridge pattern with subtle basal zone vacuolization and rare necrotic keratinocytes with an underlying lichenoid infiltrate within the papillary dermis comprised of lymphomononuclear cells.
In contrast, punctate porokeratosis clinically tends to involve the palms and soles, though the arms and legs also may be involved. This entity tends to occur during adolescence. A raised hyperkeratotic papule clinically is present. Histopathologically, the epidermis has a cup-shaped depression filled with hyperkeratosis and a column of parakeratosis (coronoid lamellae)(Figure 1).
Acrokeratosis verruciformis of Hopf clinically appears on the dorsal aspects of the hands and feet as small warty papules in association with Darier disease. It typically presents during early childhood. Histopathology shows tiered hyperkeratosis, papillomatosis, and acanthosis (Figure 2).
Perforating granuloma annulare presents on the dorsal aspects of the hands and fingers as scaly papules with either central umbilication or keratotic plugs. Histopathology shows transepidermal elimination of degenerated collagen (Figure 3).
Stucco keratoses present on the dorsal aspects of the feet and ankles but are waxy smooth papules as opposed to hyperkeratotic spiky papules. Histologically, they are characterized by retention hyperkeratosis with lack of parakeratosis and regular acanthosis with a "string sign" indicating that the lesion extends to a uniform depth. (Figure 4).
- Flegel H. Hyperkeratosis lenticularis perstans. Hautzarzt. 1958;9:363-364.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel's disease). Am J Dermatopathol. 2006;28:122-126.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)--lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.
The Diagnosis: Hyperkeratosis Lenticularis Perstans (Flegel Disease)
Hyperkeratosis lenticularis perstans, also known as Flegel disease, is a rare dermatosis first described by Flegel1 in 1958. This benign disorder is characterized by multiple asymptomatic 1- to 5-mm keratotic papules in a symmetric distribution favoring the dorsal aspects of the feet and distal extremities in adults. An autosomal-dominant inheritance pattern has been postulated, though many cases sporadically occur.2 The characteristic spiky papules typically appear during mid to late adulthood and tend to persist. Treatment options are lacking, with reports of partial or no response to topical calcipotriol, topical 5-fluorouracil, cryotherapy, and topical and oral retinoids.3,4
The histopathology of hyperkeratosis lenticularis perstans is distinct, showing a central discrete area of orthohyperkeratosis with patchy parakeratosis flanked by a normal stratum corneum. The underlying epidermis typically shows effacement of the rete ridge pattern with subtle basal zone vacuolization and rare necrotic keratinocytes with an underlying lichenoid infiltrate within the papillary dermis comprised of lymphomononuclear cells.
In contrast, punctate porokeratosis clinically tends to involve the palms and soles, though the arms and legs also may be involved. This entity tends to occur during adolescence. A raised hyperkeratotic papule clinically is present. Histopathologically, the epidermis has a cup-shaped depression filled with hyperkeratosis and a column of parakeratosis (coronoid lamellae)(Figure 1).
Acrokeratosis verruciformis of Hopf clinically appears on the dorsal aspects of the hands and feet as small warty papules in association with Darier disease. It typically presents during early childhood. Histopathology shows tiered hyperkeratosis, papillomatosis, and acanthosis (Figure 2).
Perforating granuloma annulare presents on the dorsal aspects of the hands and fingers as scaly papules with either central umbilication or keratotic plugs. Histopathology shows transepidermal elimination of degenerated collagen (Figure 3).
Stucco keratoses present on the dorsal aspects of the feet and ankles but are waxy smooth papules as opposed to hyperkeratotic spiky papules. Histologically, they are characterized by retention hyperkeratosis with lack of parakeratosis and regular acanthosis with a "string sign" indicating that the lesion extends to a uniform depth. (Figure 4).
The Diagnosis: Hyperkeratosis Lenticularis Perstans (Flegel Disease)
Hyperkeratosis lenticularis perstans, also known as Flegel disease, is a rare dermatosis first described by Flegel1 in 1958. This benign disorder is characterized by multiple asymptomatic 1- to 5-mm keratotic papules in a symmetric distribution favoring the dorsal aspects of the feet and distal extremities in adults. An autosomal-dominant inheritance pattern has been postulated, though many cases sporadically occur.2 The characteristic spiky papules typically appear during mid to late adulthood and tend to persist. Treatment options are lacking, with reports of partial or no response to topical calcipotriol, topical 5-fluorouracil, cryotherapy, and topical and oral retinoids.3,4
The histopathology of hyperkeratosis lenticularis perstans is distinct, showing a central discrete area of orthohyperkeratosis with patchy parakeratosis flanked by a normal stratum corneum. The underlying epidermis typically shows effacement of the rete ridge pattern with subtle basal zone vacuolization and rare necrotic keratinocytes with an underlying lichenoid infiltrate within the papillary dermis comprised of lymphomononuclear cells.
In contrast, punctate porokeratosis clinically tends to involve the palms and soles, though the arms and legs also may be involved. This entity tends to occur during adolescence. A raised hyperkeratotic papule clinically is present. Histopathologically, the epidermis has a cup-shaped depression filled with hyperkeratosis and a column of parakeratosis (coronoid lamellae)(Figure 1).
Acrokeratosis verruciformis of Hopf clinically appears on the dorsal aspects of the hands and feet as small warty papules in association with Darier disease. It typically presents during early childhood. Histopathology shows tiered hyperkeratosis, papillomatosis, and acanthosis (Figure 2).
Perforating granuloma annulare presents on the dorsal aspects of the hands and fingers as scaly papules with either central umbilication or keratotic plugs. Histopathology shows transepidermal elimination of degenerated collagen (Figure 3).
Stucco keratoses present on the dorsal aspects of the feet and ankles but are waxy smooth papules as opposed to hyperkeratotic spiky papules. Histologically, they are characterized by retention hyperkeratosis with lack of parakeratosis and regular acanthosis with a "string sign" indicating that the lesion extends to a uniform depth. (Figure 4).
- Flegel H. Hyperkeratosis lenticularis perstans. Hautzarzt. 1958;9:363-364.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel's disease). Am J Dermatopathol. 2006;28:122-126.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)--lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.
- Flegel H. Hyperkeratosis lenticularis perstans. Hautzarzt. 1958;9:363-364.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel's disease). Am J Dermatopathol. 2006;28:122-126.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)--lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.
A 54-year-old man who was otherwise healthy presented with asymptomatic, discrete, rough, red-brown, hyperkeratotic papules on the dorsal aspects of the feet of several years' duration. The lesions spared the soles of the feet and hands. A diagnosis of eczema previously was made by his general practitioner, and he was using moisturizer. No prescription treatments were pursued, and no other rashes or lesions were noted on physical examination. A punch biopsy of a spiky papule was performed.
Recurrent Vesicles on the Palm
The Diagnosis: Herpes Simplex Virus Dermatitis
A swab of the lesions yielded negative varicella-zoster virus and herpes simplex virus (HSV) cultures, but polymerase chain reaction (PCR) was positive for HSV DNA. The patient was started on acyclovir, which resulted in resolution of the lesions.
Recurrent HSV dermatitis most frequently is encountered in the orolabial or genital regions. After primary infection, HSV is retrogradely taken up into the dorsal root ganglion and may be reactivated in the same dermatome upon stress induction, forming clustered vesicles that rupture to form painful erosions.1 Our patient's history of numerous recurrent episodes in the same area of the palm in the distribution of the median nerve suggests viral latency in the C5 through T1 dorsal root ganglia with reactivation rather than autoinoculation or external infection from another source. The incidence of HSV involving the hand has been estimated at 2.4 cases per 100,000 individuals per year, with finger, thumb, or palm/wrist involvement accounting for 67%, 22%, and 11% of cases, respectively.2 Of the palmar cases that have been reported, most have a positive history for genital or orolabial HSV infection.2-5
In cases of suspected HSV dermatitis with atypical presentations, diagnostic studies are of importance. Although viral culture is the diagnostic gold standard in active lesions, it has lower sensitivity in improperly handled specimens; cases of recurrent disease; and specimens from dried, crusted, or aged lesions,1 which helps to explain the negative culture result in our patient. Viral culture has been largely replaced in clinical practice by nucleic acid amplification tests using PCR, which is fast and type specific.6,7 The sensitivity of PCR approaches 100% when vesicles or wet ulcers are sampled, and PCR has better yields from dry ulcers or crusts compared to viral culture.6 However, because viral shedding is intermittent, a negative PCR result does not rule out HSV infection.8 Additional bedside diagnostic techniques include Tzanck smear, a rapid and inexpensive test in which lesions are scraped and stained with Giemsa, Wright, or Papanicolaou stains. Under light microscopy, multinucleated giant cells are seen in 60% to 75% of cases.9 This method, however, cannot distinguish HSV from varicella-zoster virus and must be followed by direct fluorescent antibody testing or immunohistochemistry for viral typing.1,9 Serologic testing also may be useful in patients who have a suspicious history for HSV infection but do not have lesions on physical examination to diagnose clinically or sample for PCR. Enzyme-linked immunosorbent assay testing can detect IgG starting 3 weeks after infection, and newer type-specific assays can distinguish between HSV types 1 and 2.6 In low-incidence populations, false positives from enzyme-linked immunosorbent assay can be seen and should be confirmed by western blot.6,7
Preferred treatment of HSV includes antiviral medications such as acyclovir, valacyclovir, and famciclovir. Regimens vary based on the site of infection, primary or recurrent nature of the infection, immune status of the patient, and whether or not viral suppression is desired to prevent recurrent outbreaks.7,10
Tinea manuum also may present with unilateral vesicles and erosions involving the palms11; however, it was less likely than HSV dermatitis in this patient presenting with a history of numerous recurrent episodes and without scaling on physical examination. Dyshidrotic eczema, contact dermatitis, and scabies are more characteristically pruritic rather than painful. Additionally, dyshidrotic eczema and scabies would be more likely to have symmetric involvement of the arms. Although vesicles are seen in both dyshidrotic eczema and HSV dermatitis, the vesicles of dyshidrotic eczema usually are noninflammatory compared to the painful vesicles on an erythematous base classically seen in HSV dermatitis.
Acknowledgment
The authors thank Elizabeth Ergen, MD (Knoxville, Tennessee), for her assistance with this case.
- Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57:737-763; quiz 764-766.
- Gill MJ, Arlette J, Buchan K. Herpes simplex virus infection of the hand. a profile of 79 cases. Am J Med. 1988;84:89-93.
- Widenfalk B, Wallin J. Recurrent herpes simplex virus infections in the adult hand. Scand J Plast Reconstr Surg Hand Surg. 1988;22:177-180.
- Gill MJ, Arlette J, Buchan KA. Herpes simplex virus infection of the hand. J Am Acad Dermatol. 1990;22:111-116.
- Osio A, Fremont G, Petit A, et al. An unusual bipolar primary herpes simplex virus 1 infection. J Clin Virol. 2008;43:230-232.
- Gnann JW Jr, Whitley RJ. Clinical practice. genital herpes. N Engl J Med. 2016;375:666-674.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
- Downing C, Mendoza N, Sra K, et al. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1400-1424.
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines for the Treatment of Genital Herpes Simplex Virus. Geneva, Switzerland: World Health Organization; 2016.
- Veraldi S, Schianchi R, Benzecry V, et al. Tinea manuum: a report of 18 cases observed in the metropolitan area of Milan and review of the literature. Mycoses. 2019;62:604-608.
The Diagnosis: Herpes Simplex Virus Dermatitis
A swab of the lesions yielded negative varicella-zoster virus and herpes simplex virus (HSV) cultures, but polymerase chain reaction (PCR) was positive for HSV DNA. The patient was started on acyclovir, which resulted in resolution of the lesions.
Recurrent HSV dermatitis most frequently is encountered in the orolabial or genital regions. After primary infection, HSV is retrogradely taken up into the dorsal root ganglion and may be reactivated in the same dermatome upon stress induction, forming clustered vesicles that rupture to form painful erosions.1 Our patient's history of numerous recurrent episodes in the same area of the palm in the distribution of the median nerve suggests viral latency in the C5 through T1 dorsal root ganglia with reactivation rather than autoinoculation or external infection from another source. The incidence of HSV involving the hand has been estimated at 2.4 cases per 100,000 individuals per year, with finger, thumb, or palm/wrist involvement accounting for 67%, 22%, and 11% of cases, respectively.2 Of the palmar cases that have been reported, most have a positive history for genital or orolabial HSV infection.2-5
In cases of suspected HSV dermatitis with atypical presentations, diagnostic studies are of importance. Although viral culture is the diagnostic gold standard in active lesions, it has lower sensitivity in improperly handled specimens; cases of recurrent disease; and specimens from dried, crusted, or aged lesions,1 which helps to explain the negative culture result in our patient. Viral culture has been largely replaced in clinical practice by nucleic acid amplification tests using PCR, which is fast and type specific.6,7 The sensitivity of PCR approaches 100% when vesicles or wet ulcers are sampled, and PCR has better yields from dry ulcers or crusts compared to viral culture.6 However, because viral shedding is intermittent, a negative PCR result does not rule out HSV infection.8 Additional bedside diagnostic techniques include Tzanck smear, a rapid and inexpensive test in which lesions are scraped and stained with Giemsa, Wright, or Papanicolaou stains. Under light microscopy, multinucleated giant cells are seen in 60% to 75% of cases.9 This method, however, cannot distinguish HSV from varicella-zoster virus and must be followed by direct fluorescent antibody testing or immunohistochemistry for viral typing.1,9 Serologic testing also may be useful in patients who have a suspicious history for HSV infection but do not have lesions on physical examination to diagnose clinically or sample for PCR. Enzyme-linked immunosorbent assay testing can detect IgG starting 3 weeks after infection, and newer type-specific assays can distinguish between HSV types 1 and 2.6 In low-incidence populations, false positives from enzyme-linked immunosorbent assay can be seen and should be confirmed by western blot.6,7
Preferred treatment of HSV includes antiviral medications such as acyclovir, valacyclovir, and famciclovir. Regimens vary based on the site of infection, primary or recurrent nature of the infection, immune status of the patient, and whether or not viral suppression is desired to prevent recurrent outbreaks.7,10
Tinea manuum also may present with unilateral vesicles and erosions involving the palms11; however, it was less likely than HSV dermatitis in this patient presenting with a history of numerous recurrent episodes and without scaling on physical examination. Dyshidrotic eczema, contact dermatitis, and scabies are more characteristically pruritic rather than painful. Additionally, dyshidrotic eczema and scabies would be more likely to have symmetric involvement of the arms. Although vesicles are seen in both dyshidrotic eczema and HSV dermatitis, the vesicles of dyshidrotic eczema usually are noninflammatory compared to the painful vesicles on an erythematous base classically seen in HSV dermatitis.
Acknowledgment
The authors thank Elizabeth Ergen, MD (Knoxville, Tennessee), for her assistance with this case.
The Diagnosis: Herpes Simplex Virus Dermatitis
A swab of the lesions yielded negative varicella-zoster virus and herpes simplex virus (HSV) cultures, but polymerase chain reaction (PCR) was positive for HSV DNA. The patient was started on acyclovir, which resulted in resolution of the lesions.
Recurrent HSV dermatitis most frequently is encountered in the orolabial or genital regions. After primary infection, HSV is retrogradely taken up into the dorsal root ganglion and may be reactivated in the same dermatome upon stress induction, forming clustered vesicles that rupture to form painful erosions.1 Our patient's history of numerous recurrent episodes in the same area of the palm in the distribution of the median nerve suggests viral latency in the C5 through T1 dorsal root ganglia with reactivation rather than autoinoculation or external infection from another source. The incidence of HSV involving the hand has been estimated at 2.4 cases per 100,000 individuals per year, with finger, thumb, or palm/wrist involvement accounting for 67%, 22%, and 11% of cases, respectively.2 Of the palmar cases that have been reported, most have a positive history for genital or orolabial HSV infection.2-5
In cases of suspected HSV dermatitis with atypical presentations, diagnostic studies are of importance. Although viral culture is the diagnostic gold standard in active lesions, it has lower sensitivity in improperly handled specimens; cases of recurrent disease; and specimens from dried, crusted, or aged lesions,1 which helps to explain the negative culture result in our patient. Viral culture has been largely replaced in clinical practice by nucleic acid amplification tests using PCR, which is fast and type specific.6,7 The sensitivity of PCR approaches 100% when vesicles or wet ulcers are sampled, and PCR has better yields from dry ulcers or crusts compared to viral culture.6 However, because viral shedding is intermittent, a negative PCR result does not rule out HSV infection.8 Additional bedside diagnostic techniques include Tzanck smear, a rapid and inexpensive test in which lesions are scraped and stained with Giemsa, Wright, or Papanicolaou stains. Under light microscopy, multinucleated giant cells are seen in 60% to 75% of cases.9 This method, however, cannot distinguish HSV from varicella-zoster virus and must be followed by direct fluorescent antibody testing or immunohistochemistry for viral typing.1,9 Serologic testing also may be useful in patients who have a suspicious history for HSV infection but do not have lesions on physical examination to diagnose clinically or sample for PCR. Enzyme-linked immunosorbent assay testing can detect IgG starting 3 weeks after infection, and newer type-specific assays can distinguish between HSV types 1 and 2.6 In low-incidence populations, false positives from enzyme-linked immunosorbent assay can be seen and should be confirmed by western blot.6,7
Preferred treatment of HSV includes antiviral medications such as acyclovir, valacyclovir, and famciclovir. Regimens vary based on the site of infection, primary or recurrent nature of the infection, immune status of the patient, and whether or not viral suppression is desired to prevent recurrent outbreaks.7,10
Tinea manuum also may present with unilateral vesicles and erosions involving the palms11; however, it was less likely than HSV dermatitis in this patient presenting with a history of numerous recurrent episodes and without scaling on physical examination. Dyshidrotic eczema, contact dermatitis, and scabies are more characteristically pruritic rather than painful. Additionally, dyshidrotic eczema and scabies would be more likely to have symmetric involvement of the arms. Although vesicles are seen in both dyshidrotic eczema and HSV dermatitis, the vesicles of dyshidrotic eczema usually are noninflammatory compared to the painful vesicles on an erythematous base classically seen in HSV dermatitis.
Acknowledgment
The authors thank Elizabeth Ergen, MD (Knoxville, Tennessee), for her assistance with this case.
- Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57:737-763; quiz 764-766.
- Gill MJ, Arlette J, Buchan K. Herpes simplex virus infection of the hand. a profile of 79 cases. Am J Med. 1988;84:89-93.
- Widenfalk B, Wallin J. Recurrent herpes simplex virus infections in the adult hand. Scand J Plast Reconstr Surg Hand Surg. 1988;22:177-180.
- Gill MJ, Arlette J, Buchan KA. Herpes simplex virus infection of the hand. J Am Acad Dermatol. 1990;22:111-116.
- Osio A, Fremont G, Petit A, et al. An unusual bipolar primary herpes simplex virus 1 infection. J Clin Virol. 2008;43:230-232.
- Gnann JW Jr, Whitley RJ. Clinical practice. genital herpes. N Engl J Med. 2016;375:666-674.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
- Downing C, Mendoza N, Sra K, et al. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1400-1424.
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines for the Treatment of Genital Herpes Simplex Virus. Geneva, Switzerland: World Health Organization; 2016.
- Veraldi S, Schianchi R, Benzecry V, et al. Tinea manuum: a report of 18 cases observed in the metropolitan area of Milan and review of the literature. Mycoses. 2019;62:604-608.
- Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57:737-763; quiz 764-766.
- Gill MJ, Arlette J, Buchan K. Herpes simplex virus infection of the hand. a profile of 79 cases. Am J Med. 1988;84:89-93.
- Widenfalk B, Wallin J. Recurrent herpes simplex virus infections in the adult hand. Scand J Plast Reconstr Surg Hand Surg. 1988;22:177-180.
- Gill MJ, Arlette J, Buchan KA. Herpes simplex virus infection of the hand. J Am Acad Dermatol. 1990;22:111-116.
- Osio A, Fremont G, Petit A, et al. An unusual bipolar primary herpes simplex virus 1 infection. J Clin Virol. 2008;43:230-232.
- Gnann JW Jr, Whitley RJ. Clinical practice. genital herpes. N Engl J Med. 2016;375:666-674.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
- Downing C, Mendoza N, Sra K, et al. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1400-1424.
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines for the Treatment of Genital Herpes Simplex Virus. Geneva, Switzerland: World Health Organization; 2016.
- Veraldi S, Schianchi R, Benzecry V, et al. Tinea manuum: a report of 18 cases observed in the metropolitan area of Milan and review of the literature. Mycoses. 2019;62:604-608.
A 54-year-old man presented to the emergency department with painful lesions at the base of the right palm that had progressed to include areas of erythema and warmth migrating proximally along the right forearm and distal right arm. He stated that similar lesions had occurred episodically in the same location approximately 100 times over the last 20 years. Each time, the lesions began as painful vesicles that he subsequently popped with a sewing needle. He denied any history of orolabial or genital herpes simplex virus infection. Physical examination revealed erythematous scattered papules with dry hemorrhagic crust over the base of the right palm with expressible serous fluid upon forceful pressure. Swelling, erythema, and warmth of the distal right forearm also were observed.
Angiosarcoma Imitating a Morpheaform Basal Cell Carcinoma
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
Practice Points
- Angiosarcoma is an aggressive vascular tumor with a poor prognosis.
- Angiosarcomas can arise in the setting of chronic lymphedema or prior radiation therapy or can arise spontaneously.
- Classically, angiosarcoma presents as a violaceous patch or plaque but occasionally can exhibit atypical clinical features. Angiosarcomas should be considered on the differential for any changing plaque on the head or neck.
Handoffs in Dermatology Residency
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
Resident Pearl
- For dermatology residents, ensuring organized handoff and follow-up practices is essential. Residency provides an opportunity to become familiar with different practices to take forward in one’s career.
Cutaneous Collagenous Vasculopathy
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is a rare idiopathic microangiopathy characterized by diffuse blanchable telangiectases that usually develop in late adulthood. It appears morphologically identical to generalized essential telangiectasia (GET), but skin biopsy characteristically shows dilated superficial blood vessels in the papillary dermis that are surrounded by a thickened layer of type IV collagen.1 We report a case of CCV occurring in an elderly white man.
A 72-year-old man presented with an asymptomatic rash on the arms, legs, and abdomen of 3 years’ duration. His medical history was remarkable for hypothyroidism, hypertension, reflex sympathetic dystrophy syndrome, coronary artery disease, and nonmelanoma skin cancer. He denied any changes in medications or illnesses prior to onset of the rash. Physical examination revealed diffuse, erythematous, blanchable telangiectases on the arms, legs, and trunk (Figure 1). No petechiae, atrophy, or epidermal changes were appreciated. Darier sign was negative.
Hematoxylin and eosin–stained sections of skin from the abdomen showed an unremarkable epidermis overlying a superficial dermis with dilated blood vessels with thickened walls that contained eosinophilic amorphous hyaline material (Figure 2A). This material stained positive with Masson trichrome (Figure 2B), a finding that was consistent with increased collagen fiber deposition within the vessel walls. Phosphotungstic acid–hematoxylin and Congo red stains were negative. No histologic features of a vaso-occlusive disorder or vasculitis were identified. These histologic findings were consistent with the rare diagnosis of CCV.
Cutaneous collagenous vasculopathy is a rare idiopathic microangiopathy that was first reported by Salama and Rosenthal1 in 2000. They reported the case of a 54-year-old man with spreading, asymptomatic, generalized cutaneous telangiectases of 5 years’ duration. Similar to our patient, skin biopsy showed dilated superficial dermal vasculature with deposition of eosinophilic hyaline material, which stained positive with periodic acid–Schiff with diastase and exhibited immunoreactivity to type IV collagen.1
A PubMed search of articles indexed for MEDLINE using the search term cutaneous collagenous vasculopathy yielded 19 additional patients with biopsy-proven CCV.2-6 The condition has shown no gender prevalence but generally is seen in middle-aged or elderly white individuals, with the exception of a white pediatric patient.4 Cutaneous collagenous vasculopathy usually presents as telangiectases on the legs that progress to involve the trunk and arms while sparing the head and neck, nail beds, and mucous membranes.5 However, it also has been described as first presenting on the bilateral breasts2 as well as a nonprogressive localization on the thigh.6
Skin biopsy is essential to differentiate CCV from GET, which appears morphologically identical. Cutaneous collagenous vasculopathy may be underreported as a result of clinician choice not to biopsy due to a presumptive diagnosis of GET.3 Successful treatment with a pulsed dye laser has been reported,7 though the extent of disease may make complete destruction of the lesions difficult to accomplish. Although it is theorized that CCV may be a marker for underlying systemic disease or even a genetic defect causing abnormal collagen deposition, its cause has yet to be ascertained.5 Previously reported patients have had a variety of comorbidities, including several cases of type 2 diabetes mellitus.6 Another patient was reported to have recently started treatment with an angiotensin receptor blocker prior to onset of CCV.5
Our case contributes to the small series of reported patients with this rare diagnosis and further suggests that it may be underreported at this time. Similar to previously reported cases, our patient was an elderly white individual. Although our patient had long-standing iatrogenic hypothyroidism, no recent medication changes or underlying comorbidities could be tied to the development of CCV. Further studies are needed to determine if this disease process is associated with any underlying systemic illnesses, medications, or family history.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Borroni RG, Derlino F, Agozzino M, et al. Hypothermic cutaneous collagenous vasculopathy with centrifugal spreading [published online March 31, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1444-1446.
- Moulonguet I, Hershkovitch D, Fraitag S. Widespread cutaneous telangiectasias: challenge. Am J Dermatopathol. 2013;35:661-662, 688-669.
- Lloyd BM, Pruden SJ 2nd, Lind AC, et al. Cutaneous collagenous vasculopathy: report of the first pediatric case. Pediatr Dermatol. 2011;28:598-599.
- Kanitakis J, Faisant M, Wagschal D, et al. Cutaneous collagenous vasculopathy: ultrastructural and immunohistochemical study of a new case. Am J Clin Dermatol. 2010;11:63-66.
- Davis TL, Mandal RV, Bevona C, et al. Collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2008;35:967-970.
- Echeverría B, Sanmartín O, Botella-Estrada R, et al. Cutaneous collagenous vasculopathy successfully treated with pulsed dye laser. Int J Dermatol. 2012;51:1359-1362.
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is a rare idiopathic microangiopathy characterized by diffuse blanchable telangiectases that usually develop in late adulthood. It appears morphologically identical to generalized essential telangiectasia (GET), but skin biopsy characteristically shows dilated superficial blood vessels in the papillary dermis that are surrounded by a thickened layer of type IV collagen.1 We report a case of CCV occurring in an elderly white man.
A 72-year-old man presented with an asymptomatic rash on the arms, legs, and abdomen of 3 years’ duration. His medical history was remarkable for hypothyroidism, hypertension, reflex sympathetic dystrophy syndrome, coronary artery disease, and nonmelanoma skin cancer. He denied any changes in medications or illnesses prior to onset of the rash. Physical examination revealed diffuse, erythematous, blanchable telangiectases on the arms, legs, and trunk (Figure 1). No petechiae, atrophy, or epidermal changes were appreciated. Darier sign was negative.
Hematoxylin and eosin–stained sections of skin from the abdomen showed an unremarkable epidermis overlying a superficial dermis with dilated blood vessels with thickened walls that contained eosinophilic amorphous hyaline material (Figure 2A). This material stained positive with Masson trichrome (Figure 2B), a finding that was consistent with increased collagen fiber deposition within the vessel walls. Phosphotungstic acid–hematoxylin and Congo red stains were negative. No histologic features of a vaso-occlusive disorder or vasculitis were identified. These histologic findings were consistent with the rare diagnosis of CCV.
Cutaneous collagenous vasculopathy is a rare idiopathic microangiopathy that was first reported by Salama and Rosenthal1 in 2000. They reported the case of a 54-year-old man with spreading, asymptomatic, generalized cutaneous telangiectases of 5 years’ duration. Similar to our patient, skin biopsy showed dilated superficial dermal vasculature with deposition of eosinophilic hyaline material, which stained positive with periodic acid–Schiff with diastase and exhibited immunoreactivity to type IV collagen.1
A PubMed search of articles indexed for MEDLINE using the search term cutaneous collagenous vasculopathy yielded 19 additional patients with biopsy-proven CCV.2-6 The condition has shown no gender prevalence but generally is seen in middle-aged or elderly white individuals, with the exception of a white pediatric patient.4 Cutaneous collagenous vasculopathy usually presents as telangiectases on the legs that progress to involve the trunk and arms while sparing the head and neck, nail beds, and mucous membranes.5 However, it also has been described as first presenting on the bilateral breasts2 as well as a nonprogressive localization on the thigh.6
Skin biopsy is essential to differentiate CCV from GET, which appears morphologically identical. Cutaneous collagenous vasculopathy may be underreported as a result of clinician choice not to biopsy due to a presumptive diagnosis of GET.3 Successful treatment with a pulsed dye laser has been reported,7 though the extent of disease may make complete destruction of the lesions difficult to accomplish. Although it is theorized that CCV may be a marker for underlying systemic disease or even a genetic defect causing abnormal collagen deposition, its cause has yet to be ascertained.5 Previously reported patients have had a variety of comorbidities, including several cases of type 2 diabetes mellitus.6 Another patient was reported to have recently started treatment with an angiotensin receptor blocker prior to onset of CCV.5
Our case contributes to the small series of reported patients with this rare diagnosis and further suggests that it may be underreported at this time. Similar to previously reported cases, our patient was an elderly white individual. Although our patient had long-standing iatrogenic hypothyroidism, no recent medication changes or underlying comorbidities could be tied to the development of CCV. Further studies are needed to determine if this disease process is associated with any underlying systemic illnesses, medications, or family history.
To the Editor:
Cutaneous collagenous vasculopathy (CCV) is a rare idiopathic microangiopathy characterized by diffuse blanchable telangiectases that usually develop in late adulthood. It appears morphologically identical to generalized essential telangiectasia (GET), but skin biopsy characteristically shows dilated superficial blood vessels in the papillary dermis that are surrounded by a thickened layer of type IV collagen.1 We report a case of CCV occurring in an elderly white man.
A 72-year-old man presented with an asymptomatic rash on the arms, legs, and abdomen of 3 years’ duration. His medical history was remarkable for hypothyroidism, hypertension, reflex sympathetic dystrophy syndrome, coronary artery disease, and nonmelanoma skin cancer. He denied any changes in medications or illnesses prior to onset of the rash. Physical examination revealed diffuse, erythematous, blanchable telangiectases on the arms, legs, and trunk (Figure 1). No petechiae, atrophy, or epidermal changes were appreciated. Darier sign was negative.
Hematoxylin and eosin–stained sections of skin from the abdomen showed an unremarkable epidermis overlying a superficial dermis with dilated blood vessels with thickened walls that contained eosinophilic amorphous hyaline material (Figure 2A). This material stained positive with Masson trichrome (Figure 2B), a finding that was consistent with increased collagen fiber deposition within the vessel walls. Phosphotungstic acid–hematoxylin and Congo red stains were negative. No histologic features of a vaso-occlusive disorder or vasculitis were identified. These histologic findings were consistent with the rare diagnosis of CCV.
Cutaneous collagenous vasculopathy is a rare idiopathic microangiopathy that was first reported by Salama and Rosenthal1 in 2000. They reported the case of a 54-year-old man with spreading, asymptomatic, generalized cutaneous telangiectases of 5 years’ duration. Similar to our patient, skin biopsy showed dilated superficial dermal vasculature with deposition of eosinophilic hyaline material, which stained positive with periodic acid–Schiff with diastase and exhibited immunoreactivity to type IV collagen.1
A PubMed search of articles indexed for MEDLINE using the search term cutaneous collagenous vasculopathy yielded 19 additional patients with biopsy-proven CCV.2-6 The condition has shown no gender prevalence but generally is seen in middle-aged or elderly white individuals, with the exception of a white pediatric patient.4 Cutaneous collagenous vasculopathy usually presents as telangiectases on the legs that progress to involve the trunk and arms while sparing the head and neck, nail beds, and mucous membranes.5 However, it also has been described as first presenting on the bilateral breasts2 as well as a nonprogressive localization on the thigh.6
Skin biopsy is essential to differentiate CCV from GET, which appears morphologically identical. Cutaneous collagenous vasculopathy may be underreported as a result of clinician choice not to biopsy due to a presumptive diagnosis of GET.3 Successful treatment with a pulsed dye laser has been reported,7 though the extent of disease may make complete destruction of the lesions difficult to accomplish. Although it is theorized that CCV may be a marker for underlying systemic disease or even a genetic defect causing abnormal collagen deposition, its cause has yet to be ascertained.5 Previously reported patients have had a variety of comorbidities, including several cases of type 2 diabetes mellitus.6 Another patient was reported to have recently started treatment with an angiotensin receptor blocker prior to onset of CCV.5
Our case contributes to the small series of reported patients with this rare diagnosis and further suggests that it may be underreported at this time. Similar to previously reported cases, our patient was an elderly white individual. Although our patient had long-standing iatrogenic hypothyroidism, no recent medication changes or underlying comorbidities could be tied to the development of CCV. Further studies are needed to determine if this disease process is associated with any underlying systemic illnesses, medications, or family history.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Borroni RG, Derlino F, Agozzino M, et al. Hypothermic cutaneous collagenous vasculopathy with centrifugal spreading [published online March 31, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1444-1446.
- Moulonguet I, Hershkovitch D, Fraitag S. Widespread cutaneous telangiectasias: challenge. Am J Dermatopathol. 2013;35:661-662, 688-669.
- Lloyd BM, Pruden SJ 2nd, Lind AC, et al. Cutaneous collagenous vasculopathy: report of the first pediatric case. Pediatr Dermatol. 2011;28:598-599.
- Kanitakis J, Faisant M, Wagschal D, et al. Cutaneous collagenous vasculopathy: ultrastructural and immunohistochemical study of a new case. Am J Clin Dermatol. 2010;11:63-66.
- Davis TL, Mandal RV, Bevona C, et al. Collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2008;35:967-970.
- Echeverría B, Sanmartín O, Botella-Estrada R, et al. Cutaneous collagenous vasculopathy successfully treated with pulsed dye laser. Int J Dermatol. 2012;51:1359-1362.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Borroni RG, Derlino F, Agozzino M, et al. Hypothermic cutaneous collagenous vasculopathy with centrifugal spreading [published online March 31, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1444-1446.
- Moulonguet I, Hershkovitch D, Fraitag S. Widespread cutaneous telangiectasias: challenge. Am J Dermatopathol. 2013;35:661-662, 688-669.
- Lloyd BM, Pruden SJ 2nd, Lind AC, et al. Cutaneous collagenous vasculopathy: report of the first pediatric case. Pediatr Dermatol. 2011;28:598-599.
- Kanitakis J, Faisant M, Wagschal D, et al. Cutaneous collagenous vasculopathy: ultrastructural and immunohistochemical study of a new case. Am J Clin Dermatol. 2010;11:63-66.
- Davis TL, Mandal RV, Bevona C, et al. Collagenous vasculopathy: a report of three cases. J Cutan Pathol. 2008;35:967-970.
- Echeverría B, Sanmartín O, Botella-Estrada R, et al. Cutaneous collagenous vasculopathy successfully treated with pulsed dye laser. Int J Dermatol. 2012;51:1359-1362.
Practice Points
- Cutaneous collagenous vasculopathy (CCV) should be in the differential diagnosis of widespread telangiectases.
- Biopsy is needed to differentiate between CCV and generalized essential telangiectasia because of their similar clinical features.
- There may be underlying comorbidities associated with CCV, but the exact cause of the condition has yet to be found.
Persistent Chlorotrichosis With Chronic Sun Exposure
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
To the Editor:
Chlorotrichosis, or green hair discoloration, is a dermatologic condition secondary to copper deposition on the hair. It most often is seen among swimmers who have prolonged exposure to chlorinated pools. The classic patient has predisposing chemical, heat, or mechanical damage to the hair shaft and usually lighter-colored hair.1-3 We present a case of chlorotrichosis in a young brunette patient who did not have predisposing factors except for chronic sun exposure.
A 13-year-old healthy adolescent girl with brown hair presented with persistent green hair for 2 years (Figure 1A). She had first noted hair discoloration after swimming in a neighbor’s chlorinated outdoor pool during summertime but experienced year-round persistence even without swimming. She denied any history of typical risk factors for hair damage, including exposure to hair dye or bleach, styling products, heat, or mechanical damage from excessive brushing. Her sister had blonde hair with a history of similar activities and exposures, and although she did style her hair with heat, she did not develop hair discoloration. The patient lived in a newer home, and prior tap water testing did not show elevated levels of copper. She admitted to strictly wearing her hair down at all times, including during strenuous activity and swimming. Excessive teasing at school prompted her mother to seek advice from hair salons. Bleaching test strips of hair reportedly caused paradoxical intensification of green, and the patient declined recommendations for red hair dye. The patient also tried Internet-based suggestions such as topically applying crushed aspirin, lemon juice, tea tree oil, and clarifying shampoos, which all failed to result in notable improvement.
Physical examination revealed a sun-exposed distribution of ashy green hair that was worse at the distal hair ends and completely spared the roots. Trichoscopy of discolored hair (Figure 2A) revealed diffuse cuticle thinning, whereas unaffected hair appeared normal (Figure 2B). Because the patient reported slight improvement with tea tree oil, treatment was initiated with twice-weekly hot vegetable oil treatments applied for 20 minutes, which ultimately proved unsuccessful. Penicillamine shampoo (250-mg capsule of penicillamine into 5-mL purified water and 5-mL pH-balanced clear shampoo) was then recommended. At 3-month follow-up, the patient exhibited notable improvement of the hair discoloration, with only mild persistence at the distal ends of sun-damaged hair, visible only under fluorescent lighting (Figure 1B). Our recommendations thereafter were focused on prevention (Table).
The source of exogenous copper in chlorotrichosis commonly is tap water flowing through copper pipes or swimming pools rich in chlorine and copper-containing algaecides.2,4,8 The acidity of tap water is thought to cause the release of copper from the pipes.2,5 Such acidity could result from the effects of acid rain on water reservoirs or from water additives such as fluoride2 or those used in decalcification systems.5 Additionally, the attachment of electrical grounds to copper piping can cause copper to solubilize through an electric current, increasing water levels of copper.3 Although low pH facilitates copper solubility, high pH within the hair facilitates copper precipitation, which is quickly followed by adhesion to anionic molecules within hair shafts. Therefore, it is postulated that chlorotrichosis may persist in insufficiently rinsed hair with residual alkaline shampoo.6
Beyond pH flux in the induction of chlorotrichosis, other environmental agents have been suspected to play a role. A case report of green hair in a black patient following use of selenium sulfide 2.5% shampoo identified hair damage from tinea capitis infection as predisposing to chlorotrichosis.9 Other reports have cited tar shampoo and industrial exposure to cobalt, nickel, brass, mercury, or chromium as causative factors.2,3,6,7 Interestingly, green hair discoloration also has been observed in the metabolic disorder phenylketonuria.1
Few individuals exposed to elevated levels of copper will develop chlorotrichosis, which emphasizes the critical role of predisposing hair damage in its pathogenesis. With violation of the hair cuticle, chlorine can crystallize and copper can adhere to the hair shaft.10 Bleaching and waving of the hair also appear to alter the composition of keratin by increasing the number of cysteic acid and similar anionic sulfonate groups, which can bind copper.8
Although not harmful, chlorotrichosis may be aesthetically undesirable and lead to considerable social ostracism. Without intrinsic hair defects or obvious differences in predisposing factors, the question was raised as to why our patient, as a brunette, experienced dramatic hair discoloration while her blonde sister was entirely unaffected. We postulated that our patient’s persistent green hair may have been due to her unique predisposition to extensive sun-induced and mechanical hair damage because of her unwavering tendency to wear her hair down at all times. A variety of treatments of variable reported efficacy have been proposed (Table); fortunately, if treatments fail, the discoloration resolves with hair growth.
This case is unique in that it presented in a brunette patient with seemingly minimal hair damage with an unaffected blonde-haired sibling and with persistence over years. Furthermore, it lends credence to the use of penicillamine shampoo in treating chlorotrichosis, even in particularly difficult cases in which other treatments have failed.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
- Holmes LB, Goldsmith LA. The man with green hair [letter]. N Engl J Med. 1974;291:1037.
Lampe RM, Henderson AL, Hansen GH. Green hair. JAMA. 1977;237:2092. - Nordlund JJ, Hartley C, Fister J. On the cause of green hair. Arch Dermatol. 1977;113:1700.
- Goldschmidt H. Green hair. Arch Dermatol. 1979;115:1288.
- Hinz T, Klingmuller K, Bieber T, et al. The mystery of green hair. Eur J Dermatol. 2009;19:409-410.
- Mascaro JM Jr, Ferrando J, Fontarnau R, et al. Green hair. Cutis. 1995;56:37-40.
- Bhat GR, Lukenbach ER, Kennedy RR, et al. The green hair problem: a preliminary investigation. J Soc Cosmet Chem. 1979;30:1-8.
- Blanc D, Zultak M, Rochefort A, et al. Green hair: clinical, chemical and epidemiologic study. apropos of a case. Ann Dermatol Venereol. 1988;115:807-812.
- Fitzgerald EA, Purcell SM, Goldman HM. Green hair discoloration due to selenium sulfide. Int J Dermatol. 1997;36:238-239.
- Fair NB, Gupta BS. The chlorine-hair interaction. II. effect of chlorination at varied pH levels on hair properties. J Soc Cosmet Chem. 1987;38:371-384.
Practice Points
- Chlorotrichosis is the deposition of copper onto hair, which causes a green discoloration and most commonly occurs in blonde patients with excessive exposure to chlorinated water.
- Hair cuticle damage from hair care practices, such as use of heat or chemicals, can predispose patients to the development of chlorotrichosis.
- Although a number of treatments have been proposed, the use of penicillamine shampoo seems to be particularly effective and works via chelation of the adherent copper molecules.