User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
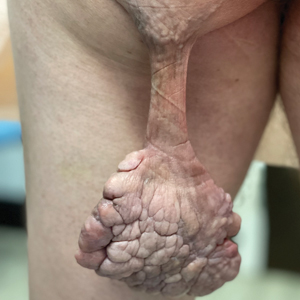
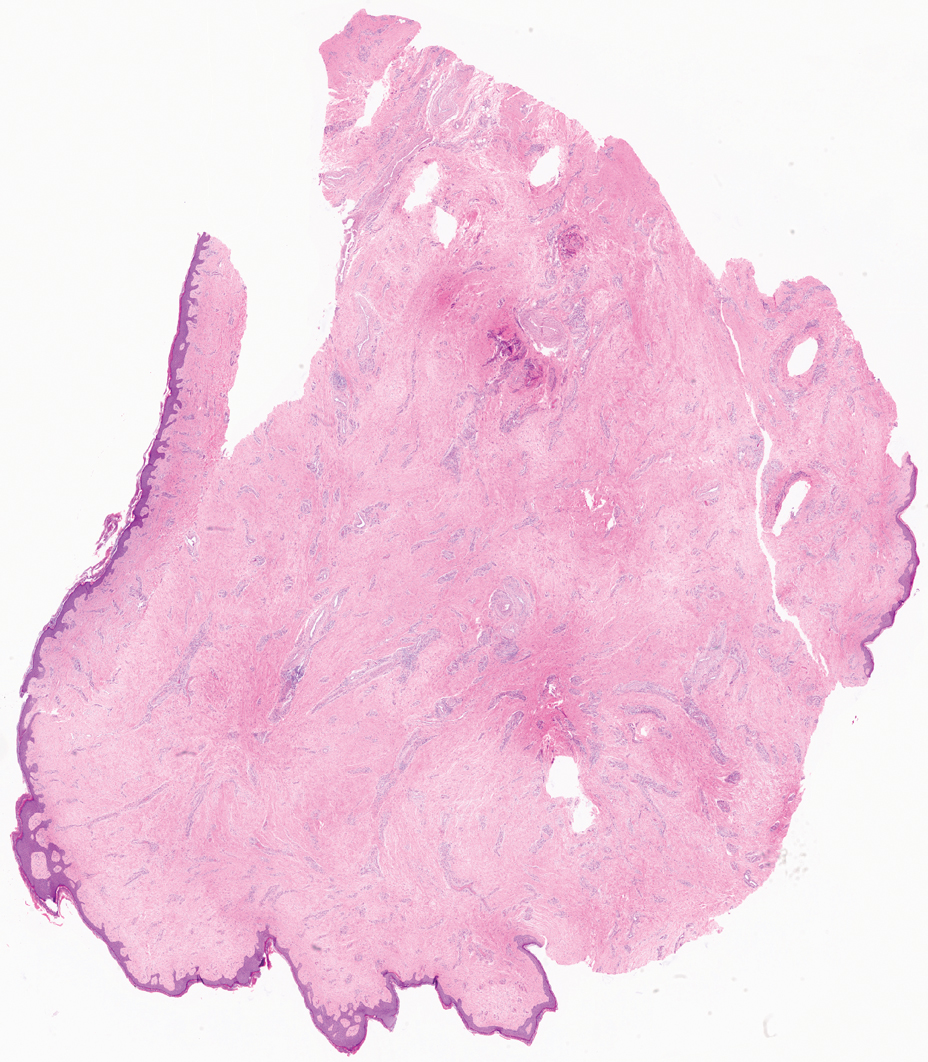
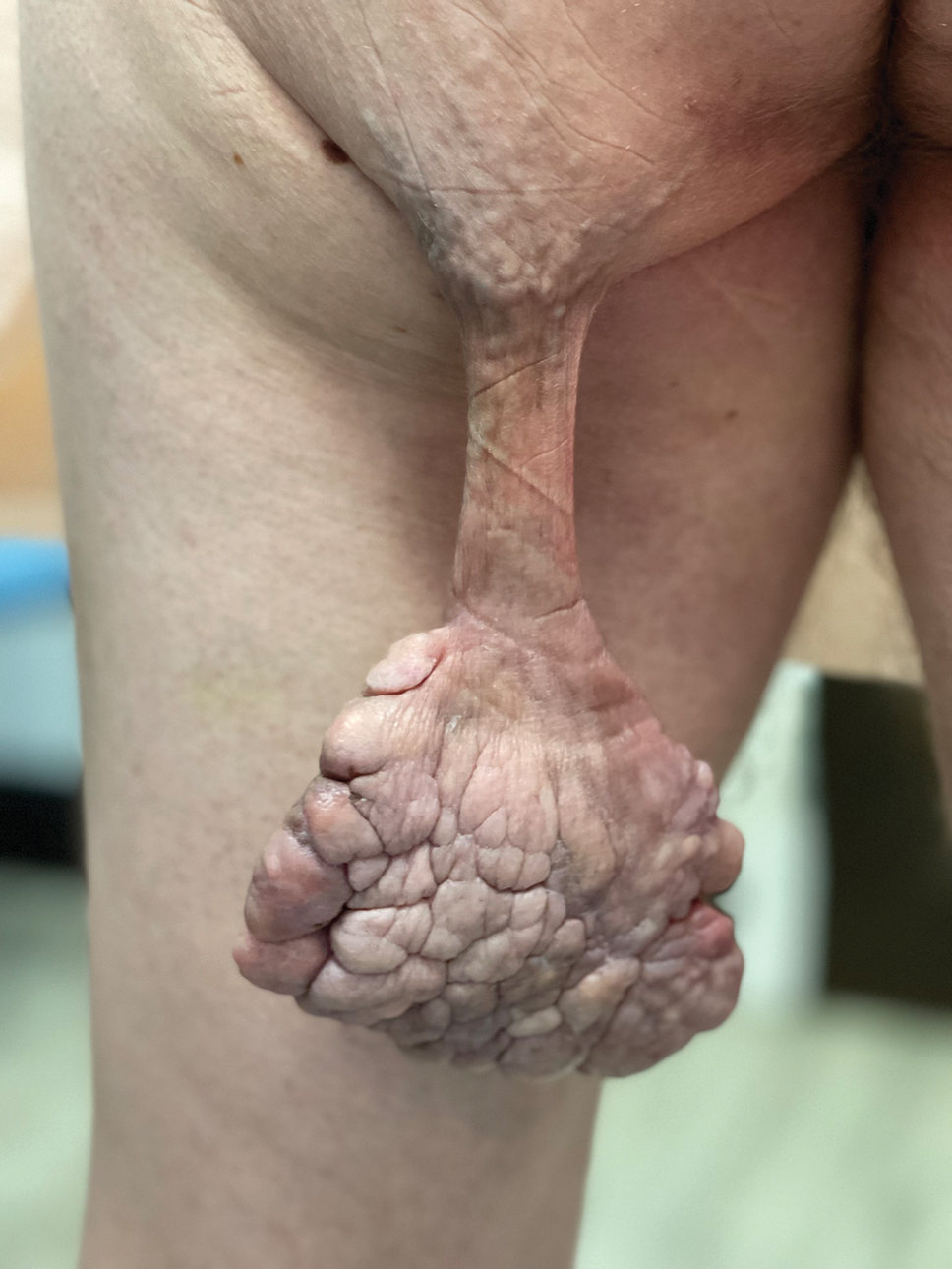
Pedunculated Verrucous Tumor on the Buttock
The Diagnosis: Giant Acrochordon
Based on the clinical and histologic findings, our patient was diagnosed with a giant acrochordon. Acrochordons (also known as fibroepithelial polyps or skin tags) are among the most commonly identified skin lesions and are believed to affect up to 46% of the general population.1,2 These benign growths typically appear after middle age in men and women alike and are believed to be of ectodermal and mesenchymal origin.3 The most common locations include the axillae, neck, and inguinal folds. They generally are small, measuring only a few millimeters, and frequently present as multiple lesions that are called giant acrochordons when their size exceeds 5 cm in length.2 Acrochordons are benign lesions with only rare reports of the presence of basal or squamous cell carcinoma within the lesion on pathology.4 In addition to being cosmetically unsightly, patients with acrochordons often report pruritus. These lesions are easily removed in an outpatient setting via snip excision, cryosurgery, or electrodesiccation. Once removed, recurrence is unlikely. Despite the prevalence of fibroepithelial polyps worldwide, reports of giant acrochordons are limited. The histopathology of giant acrochordons is similar to smaller acrochordons, with features including epidermal acanthosis and a central core of fibrovascular tissue without adnexal structures (Figure).4
The differential diagnosis of giant acrochordon includes neurofibroma, nodular melanoma, squamous cell carcinoma, and giant condylomata acuminata (Buschke-Löwenstein tumor).1 It is important to consider the clinical presentation and histopathologic findings to differentiate giant acrochordons from these other entities.
Neurofibromas typically present as multiple flesh-colored to brown nodules that invaginate into the skin when minimal external pressure is applied.5 Histopathology demonstrates a discrete, nonencapsulated, dermal collection of small nerve fibers and loosely arranged spindle cells. In contrast, giant acrochordons typically present as large, fleshcolored, pedunculated, verrucous tumors with a central stalk. Histopathology reveals epidermal acanthosis and a central core of fibrovascular tissue without adnexal structures.
Nodular melanomas usually are blue to black and grow rapidly over the course of several months.6 They have signs of hemorrhagic crust, and histopathology reveals atypical melanocytes, frequent mitoses, pleomorphic tumor cells, and irregular clumping of chromatin within the nuclei. Giant acrochordons are flesh colored, benign, and do not have these malignant features.
Squamous cell carcinoma often presents as an erythematous scaly patch or red plaque on sun-exposed areas of the skin.1 Histopathology of squamous cell carcinoma shows atypical keratinocytes with an invasive growth pattern; giant acrochordon does not show keratinocytic atypia or invasive epidermal growth.
Giant condylomata acuminata (Buschke-Löwenstein tumor) is a locally destructive verrucous plaque that typically appears on the penis but can occur elsewhere in the anogenital region.7 Histopathologic features include epidermal hyperplasia, papillomatosis, and koilocytes. In contrast, giant acrochordons typically are located on the buttocks and do not present with these epidermal changes.
Based on the clinical and histologic findings, our patient was diagnosed with a giant acrochordon, a rare variant of the common skin lesion. Excisional removal was critical for both diagnostic and treatment purposes. By considering the clinical presentation and histopathologic features of other conditions in the differential, giant acrochordons can be distinguished from other similar entities. Diagnosis and prompt surgical removal are important for management of these neoplasms and prevention of misdiagnosis.
- Alkhalili E, Prapasiri S, Russell J. Giant acrochordon of the axilla. BMJ Case Rep. 2015:bcr2015210623. doi:10.1136/bcr-2015-210623
- Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi:10.1159/000249169
- Can B, Yildrim Ozluk A. Giant fibroepithelial polyps: why do they grow excessively? Med Bull Sisli Etfal Hastan Tip Bul. 2020;54:257-260. doi:10.14744/SEMB.2018.33603
- Ghosh SK, Bandyopadhyay D, Chatterjee G, et al. Giant skin tags on unusual locations. J Eur Acad Dermatol Venereol. 2009;23:233. doi:10.1111/j.1468-3083.2008.02816.x
- Messersmith L, Krauland K. Neurofibroma. StatPearls [Internet]. StatPearls Publishing; 2023.
- Saaiq M, Ashraf B, Siddiqui S. Nodular melanoma. Iran J Med Sci. 2016;41:164-165.
- Spinu D, Ra˘dulescu A, Bratu O, et al. Giant condyloma acuminatum. Buschke-Lowenstein disease: a literature review. Chirurgia (Bucur). 2014;109:445-450.
The Diagnosis: Giant Acrochordon
Based on the clinical and histologic findings, our patient was diagnosed with a giant acrochordon. Acrochordons (also known as fibroepithelial polyps or skin tags) are among the most commonly identified skin lesions and are believed to affect up to 46% of the general population.1,2 These benign growths typically appear after middle age in men and women alike and are believed to be of ectodermal and mesenchymal origin.3 The most common locations include the axillae, neck, and inguinal folds. They generally are small, measuring only a few millimeters, and frequently present as multiple lesions that are called giant acrochordons when their size exceeds 5 cm in length.2 Acrochordons are benign lesions with only rare reports of the presence of basal or squamous cell carcinoma within the lesion on pathology.4 In addition to being cosmetically unsightly, patients with acrochordons often report pruritus. These lesions are easily removed in an outpatient setting via snip excision, cryosurgery, or electrodesiccation. Once removed, recurrence is unlikely. Despite the prevalence of fibroepithelial polyps worldwide, reports of giant acrochordons are limited. The histopathology of giant acrochordons is similar to smaller acrochordons, with features including epidermal acanthosis and a central core of fibrovascular tissue without adnexal structures (Figure).4

The differential diagnosis of giant acrochordon includes neurofibroma, nodular melanoma, squamous cell carcinoma, and giant condylomata acuminata (Buschke-Löwenstein tumor).1 It is important to consider the clinical presentation and histopathologic findings to differentiate giant acrochordons from these other entities.
Neurofibromas typically present as multiple flesh-colored to brown nodules that invaginate into the skin when minimal external pressure is applied.5 Histopathology demonstrates a discrete, nonencapsulated, dermal collection of small nerve fibers and loosely arranged spindle cells. In contrast, giant acrochordons typically present as large, fleshcolored, pedunculated, verrucous tumors with a central stalk. Histopathology reveals epidermal acanthosis and a central core of fibrovascular tissue without adnexal structures.
Nodular melanomas usually are blue to black and grow rapidly over the course of several months.6 They have signs of hemorrhagic crust, and histopathology reveals atypical melanocytes, frequent mitoses, pleomorphic tumor cells, and irregular clumping of chromatin within the nuclei. Giant acrochordons are flesh colored, benign, and do not have these malignant features.
Squamous cell carcinoma often presents as an erythematous scaly patch or red plaque on sun-exposed areas of the skin.1 Histopathology of squamous cell carcinoma shows atypical keratinocytes with an invasive growth pattern; giant acrochordon does not show keratinocytic atypia or invasive epidermal growth.
Giant condylomata acuminata (Buschke-Löwenstein tumor) is a locally destructive verrucous plaque that typically appears on the penis but can occur elsewhere in the anogenital region.7 Histopathologic features include epidermal hyperplasia, papillomatosis, and koilocytes. In contrast, giant acrochordons typically are located on the buttocks and do not present with these epidermal changes.
Based on the clinical and histologic findings, our patient was diagnosed with a giant acrochordon, a rare variant of the common skin lesion. Excisional removal was critical for both diagnostic and treatment purposes. By considering the clinical presentation and histopathologic features of other conditions in the differential, giant acrochordons can be distinguished from other similar entities. Diagnosis and prompt surgical removal are important for management of these neoplasms and prevention of misdiagnosis.
The Diagnosis: Giant Acrochordon
Based on the clinical and histologic findings, our patient was diagnosed with a giant acrochordon. Acrochordons (also known as fibroepithelial polyps or skin tags) are among the most commonly identified skin lesions and are believed to affect up to 46% of the general population.1,2 These benign growths typically appear after middle age in men and women alike and are believed to be of ectodermal and mesenchymal origin.3 The most common locations include the axillae, neck, and inguinal folds. They generally are small, measuring only a few millimeters, and frequently present as multiple lesions that are called giant acrochordons when their size exceeds 5 cm in length.2 Acrochordons are benign lesions with only rare reports of the presence of basal or squamous cell carcinoma within the lesion on pathology.4 In addition to being cosmetically unsightly, patients with acrochordons often report pruritus. These lesions are easily removed in an outpatient setting via snip excision, cryosurgery, or electrodesiccation. Once removed, recurrence is unlikely. Despite the prevalence of fibroepithelial polyps worldwide, reports of giant acrochordons are limited. The histopathology of giant acrochordons is similar to smaller acrochordons, with features including epidermal acanthosis and a central core of fibrovascular tissue without adnexal structures (Figure).4

The differential diagnosis of giant acrochordon includes neurofibroma, nodular melanoma, squamous cell carcinoma, and giant condylomata acuminata (Buschke-Löwenstein tumor).1 It is important to consider the clinical presentation and histopathologic findings to differentiate giant acrochordons from these other entities.
Neurofibromas typically present as multiple flesh-colored to brown nodules that invaginate into the skin when minimal external pressure is applied.5 Histopathology demonstrates a discrete, nonencapsulated, dermal collection of small nerve fibers and loosely arranged spindle cells. In contrast, giant acrochordons typically present as large, fleshcolored, pedunculated, verrucous tumors with a central stalk. Histopathology reveals epidermal acanthosis and a central core of fibrovascular tissue without adnexal structures.
Nodular melanomas usually are blue to black and grow rapidly over the course of several months.6 They have signs of hemorrhagic crust, and histopathology reveals atypical melanocytes, frequent mitoses, pleomorphic tumor cells, and irregular clumping of chromatin within the nuclei. Giant acrochordons are flesh colored, benign, and do not have these malignant features.
Squamous cell carcinoma often presents as an erythematous scaly patch or red plaque on sun-exposed areas of the skin.1 Histopathology of squamous cell carcinoma shows atypical keratinocytes with an invasive growth pattern; giant acrochordon does not show keratinocytic atypia or invasive epidermal growth.
Giant condylomata acuminata (Buschke-Löwenstein tumor) is a locally destructive verrucous plaque that typically appears on the penis but can occur elsewhere in the anogenital region.7 Histopathologic features include epidermal hyperplasia, papillomatosis, and koilocytes. In contrast, giant acrochordons typically are located on the buttocks and do not present with these epidermal changes.
Based on the clinical and histologic findings, our patient was diagnosed with a giant acrochordon, a rare variant of the common skin lesion. Excisional removal was critical for both diagnostic and treatment purposes. By considering the clinical presentation and histopathologic features of other conditions in the differential, giant acrochordons can be distinguished from other similar entities. Diagnosis and prompt surgical removal are important for management of these neoplasms and prevention of misdiagnosis.
- Alkhalili E, Prapasiri S, Russell J. Giant acrochordon of the axilla. BMJ Case Rep. 2015:bcr2015210623. doi:10.1136/bcr-2015-210623
- Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi:10.1159/000249169
- Can B, Yildrim Ozluk A. Giant fibroepithelial polyps: why do they grow excessively? Med Bull Sisli Etfal Hastan Tip Bul. 2020;54:257-260. doi:10.14744/SEMB.2018.33603
- Ghosh SK, Bandyopadhyay D, Chatterjee G, et al. Giant skin tags on unusual locations. J Eur Acad Dermatol Venereol. 2009;23:233. doi:10.1111/j.1468-3083.2008.02816.x
- Messersmith L, Krauland K. Neurofibroma. StatPearls [Internet]. StatPearls Publishing; 2023.
- Saaiq M, Ashraf B, Siddiqui S. Nodular melanoma. Iran J Med Sci. 2016;41:164-165.
- Spinu D, Ra˘dulescu A, Bratu O, et al. Giant condyloma acuminatum. Buschke-Lowenstein disease: a literature review. Chirurgia (Bucur). 2014;109:445-450.
- Alkhalili E, Prapasiri S, Russell J. Giant acrochordon of the axilla. BMJ Case Rep. 2015:bcr2015210623. doi:10.1136/bcr-2015-210623
- Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi:10.1159/000249169
- Can B, Yildrim Ozluk A. Giant fibroepithelial polyps: why do they grow excessively? Med Bull Sisli Etfal Hastan Tip Bul. 2020;54:257-260. doi:10.14744/SEMB.2018.33603
- Ghosh SK, Bandyopadhyay D, Chatterjee G, et al. Giant skin tags on unusual locations. J Eur Acad Dermatol Venereol. 2009;23:233. doi:10.1111/j.1468-3083.2008.02816.x
- Messersmith L, Krauland K. Neurofibroma. StatPearls [Internet]. StatPearls Publishing; 2023.
- Saaiq M, Ashraf B, Siddiqui S. Nodular melanoma. Iran J Med Sci. 2016;41:164-165.
- Spinu D, Ra˘dulescu A, Bratu O, et al. Giant condyloma acuminatum. Buschke-Lowenstein disease: a literature review. Chirurgia (Bucur). 2014;109:445-450.
A 40-year-old man presented to our dermatology clinic with a growth on the left buttock of more than 22 years’ duration that progressively increased in size. He was otherwise in good health and reported no ongoing medical problems. Physical examination revealed a 19×12-cm, flesh-colored, pedunculated, verrucous tumor with a central stalk. The patient underwent an excisional removal, and the specimen was sent for histopathologic evaluation.
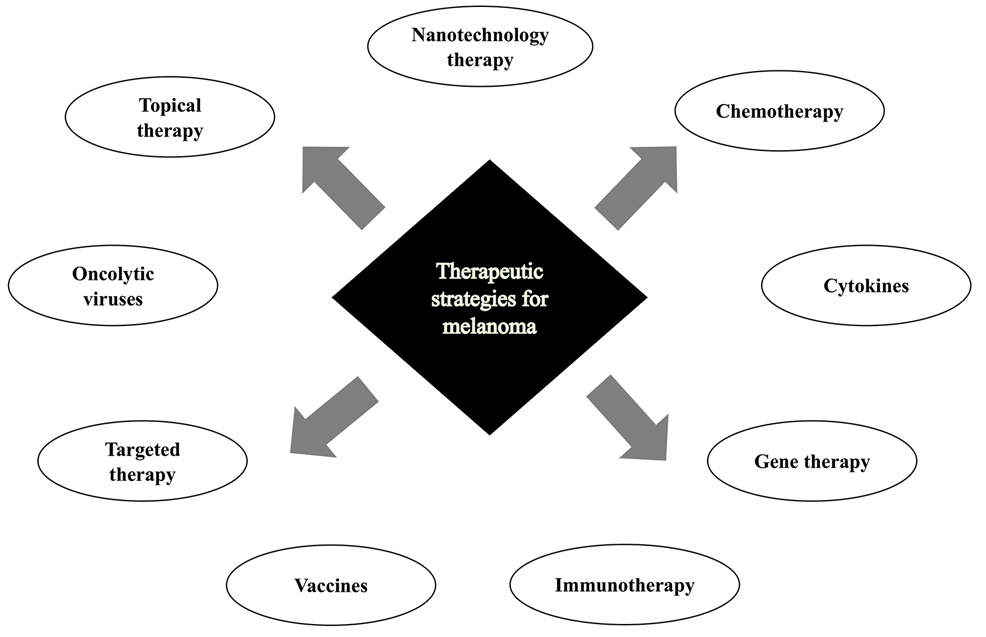
New Therapies in Melanoma: Current Trends, Evolving Paradigms, and Future Perspectives
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2
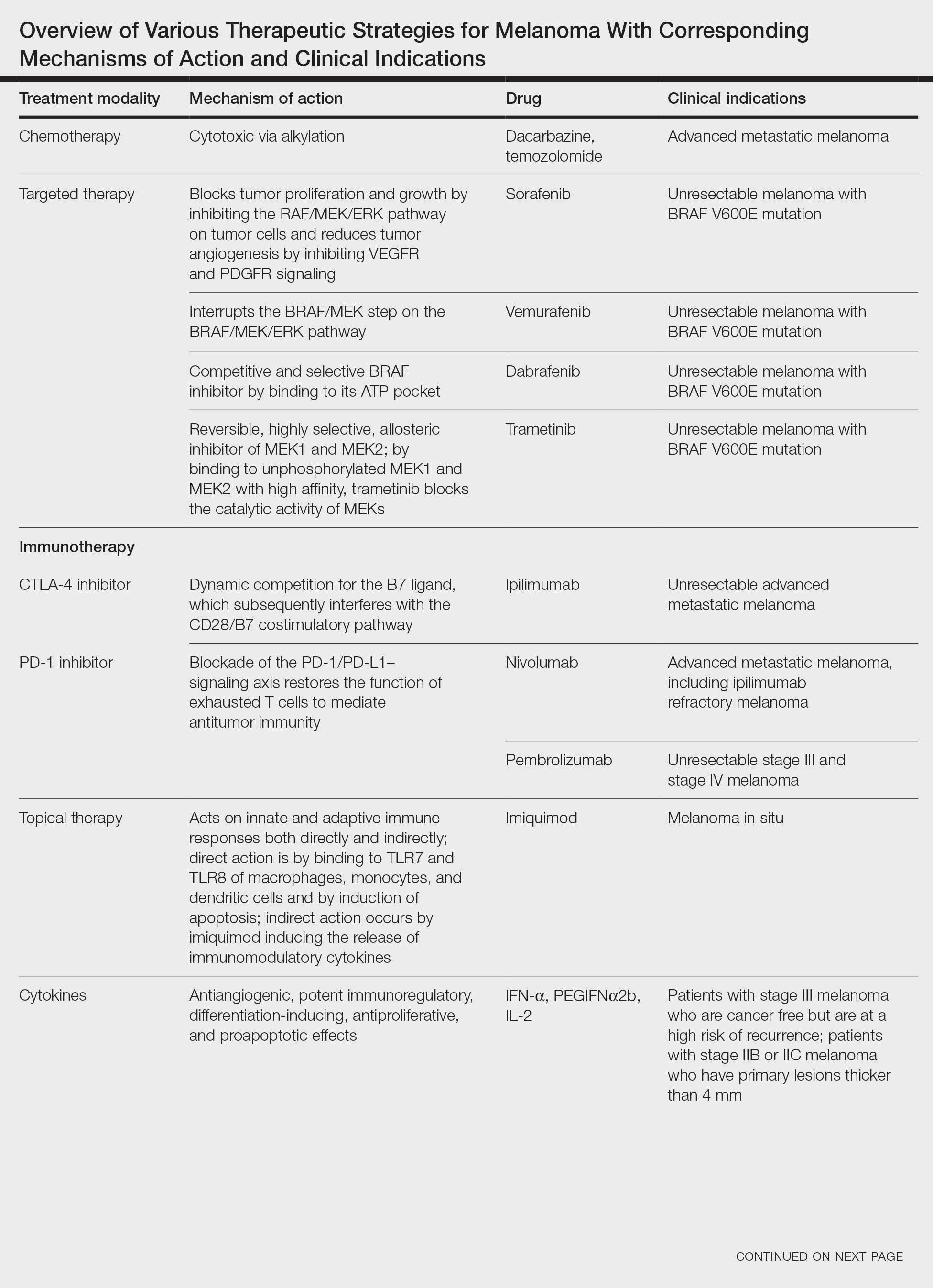
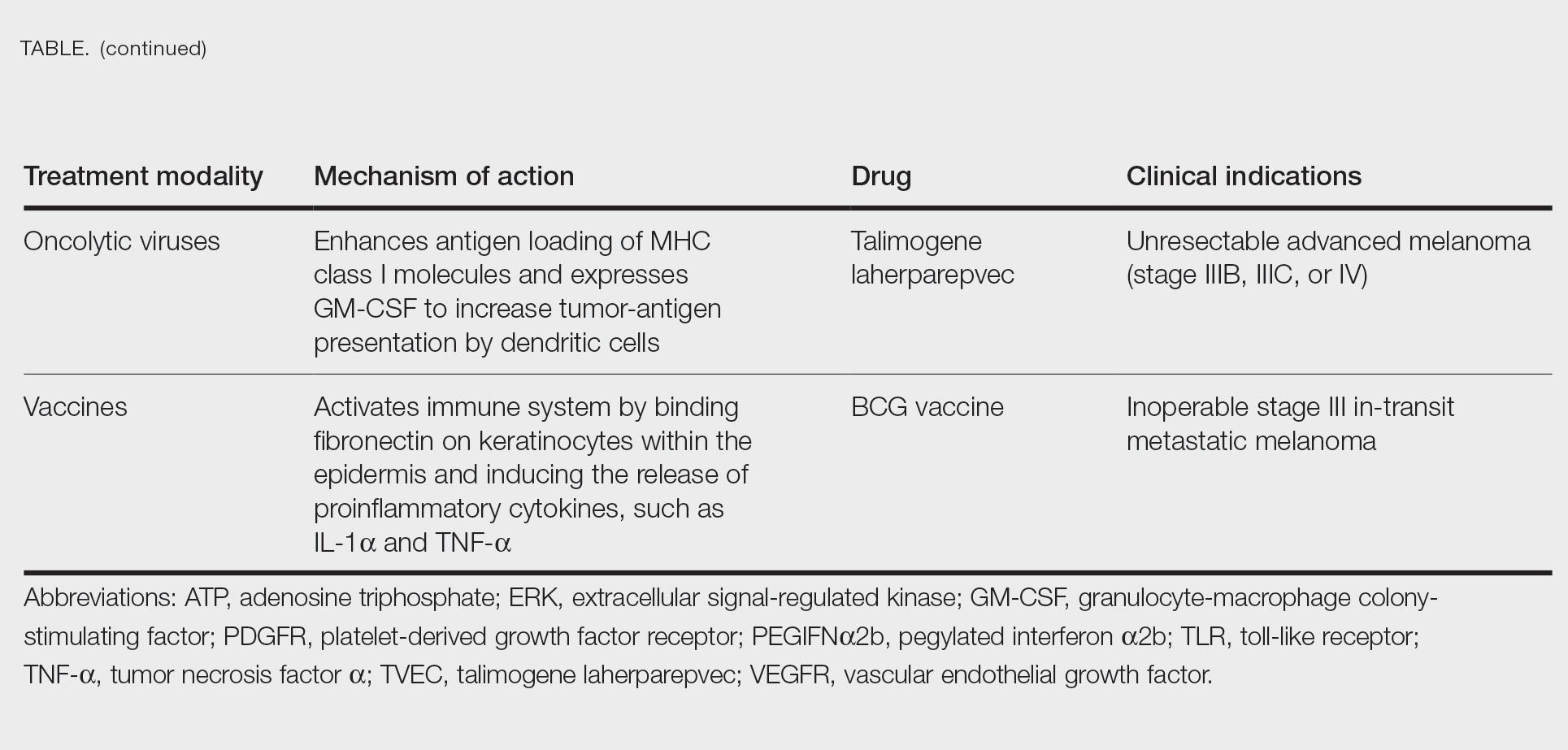
In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.
Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
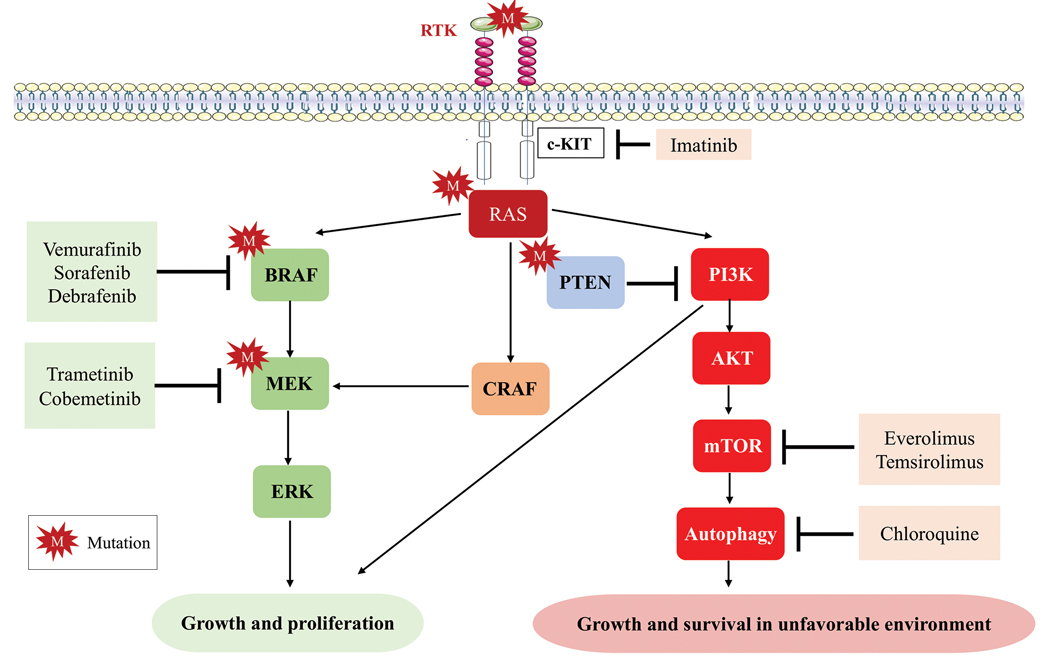
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8
Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2

In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.


Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8

Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2

In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.


Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8

Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
Practice Points
- Immune checkpoint inhibition has resulted in a paradigm shift for the treatment of metastatic melanoma.
- Alternative therapies with novel targets such as lymphocyte-activated gene 3 aim to overcome resistance to the usual immune targets such asPD-1/PD-L1 and CTLA-4.
- Newer promising tools such as nanotechnology are being added to the growing armamentarium of melanoma treatment strategies.
Neutrophilic Dermatosis of the Dorsal Hand: A Distinctive Variant of Sweet Syndrome
To the Editor:
Neutrophilic dermatosis of the dorsal hand (NDDH) is an uncommon reactive neutrophilic dermatosis that presents as a painful, enlarging, ulcerative nodule. It often is misdiagnosed and initially treated as an infection. Similar to other neutrophilic dermatoses, it is associated with underlying infections, inflammatory conditions, and malignancies. Neutrophilic dermatosis of the dorsal hand is considered a subset of Sweet syndrome (SS); we highlight similarities and differences between NDDH and SS, reporting the case of a 66-year-old man without systemic symptoms who developed NDDH on the right hand.
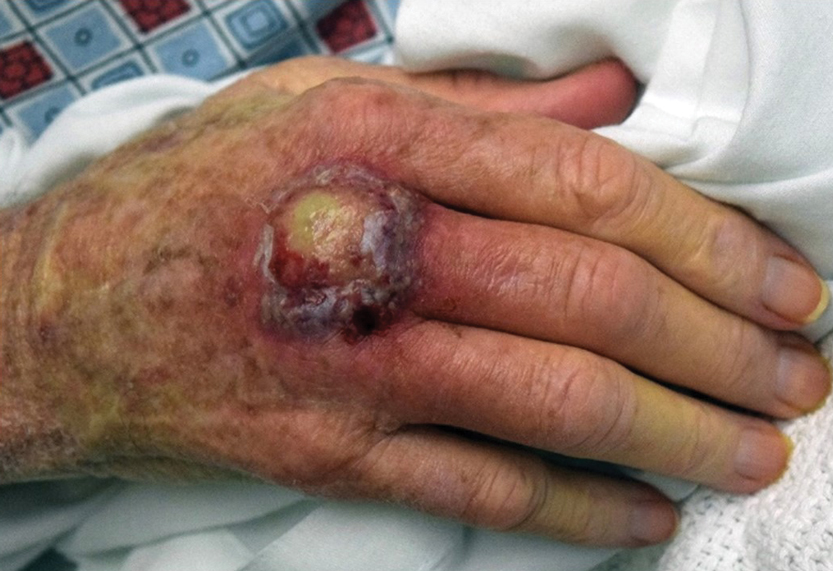
A 66-year-old man presented with a progressively enlarging, painful, ulcerative, 2-cm nodule on the right hand following mechanical trauma 2 weeks prior (Figure 1). He was afebrile with no remarkable medical history. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 20 mm/h (reference range, 0-10 mm/h) and C-reactive protein (CRP) level of 3.52 mg/dL (reference range, 0-0.5 mg/dL) without leukocytosis; both were not remarkably elevated when adjusted for age.1,2 The clinical differential diagnosis was broad and included pyoderma with evolving cellulitis, neutrophilic dermatosis, atypical mycobacterial infection, subcutaneous or deep fungal infection, squamous cell carcinoma, cutaneous lymphoma, and metastasis. Due to the rapid development of the lesion, initial treatment focused on a bacterial infection, but there was no improvement on antibiotics and wound cultures were negative. The ulcerative nodule was biopsied, and histopathology demonstrated abundant neutrophilic inflammation, endothelial swelling, and leukocytoclasis without microorganisms (Figure 2). Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. A diagnosis of NDDH was made based on clinical and histologic findings. The wound improved with a 3-week course of oral prednisone.
Neutrophilic dermatosis of the dorsal hand is a subset of reactive neutrophilic dermatoses, which includes SS (acute febrile neutrophilic dermatosis) and pyoderma gangrenosum. It is described as a localized variant of SS, with similar associated underlying inflammatory, neoplastic conditions and laboratory findings.3 However, NDDH has characteristic features that differ from classic SS. Neutrophilic dermatosis of the dorsal hand typically presents as painful papules, pustules, or ulcers that progress to become larger ulcers, plaques, and nodules. The clinical appearance may more closely resemble pyoderma gangrenosum or atypical SS, with ulceration frequently present. Pathergy also may be demonstrated in NDDH, similar to our patient. The average age of presentation for NDDH is 60 years, which is older than the average age for SS or pyoderma gangrenosum.3 Similar to other neutrophilic dermatoses, NDDH responds well to oral steroids or steroid-sparing immunosuppressants such as dapsone, colchicine, azathioprine, or tetracycline antibiotics.4
The criteria for SS are well established5,6 and may be used for the diagnosis of NDDH, taking into account the localization of lesions to the dorsal aspect of the hands. The diagnostic criteria for SS include fulfillment of both major and at least 2 of 4 minor criteria. The 2 major criteria include rapid presentation of skin lesions and neutrophilic dermal infiltrate on biopsy. Minor criteria are defined as the following: (1) preceding nonspecific respiratory or gastrointestinal tract infection, inflammatory conditions, underlying malignancy, or pregnancy; (2) fever; (3) excellent response to steroids; and (4) 3 of the 4 of the following laboratory abnormalities: elevated CRP, ESR, leukocytosis, or left shift in complete blood cell count. Our patient met both major criteria and only 1 minor criterion—excellent response to systemic corticosteroids. Nofal et al7 advocated for revised diagnostic criteria for SS, with one suggestion utilizing only the 2 major criteria being necessary for diagnosis. Given that serum inflammatory markers may not be as elevated in NDDH compared to SS,3,7,8 meeting the major criteria alone may be a better way to diagnose NDDH, as in our patient.
Our patient presented with an expanding ulcerating nodule on the hand that elicited a wide list of differential diagnoses to include infections and neoplasms. Rapid development, localization to the dorsal aspect of the hand, and treatment resistance to antibiotics may help the clinician consider a diagnosis of NDDH, which should be confirmed by a biopsy. Similar to other neutrophilic dermatoses, an underlying malignancy or inflammatory condition should be sought out. Neutrophilic dermatosis of the dorsal hand responds well to systemic steroids, though recurrences may occur.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med (Clinical Res Ed). 1983;286:226.
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, et al. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249-254.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Gaulding J, Kohen LL. Neutrophilic dermatosis of the dorsal hands. J Am Acad Dermatol. 2017; 76(6 suppl 1):AB178.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Nofal A, Abdelmaksoud A, Amer H, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15:1081-1088.
- Wolf R, Tüzün Y. Acral manifestations of Sweet syndrome (neutrophilic dermatosis of the hands). Clin Dermatol. 2017;35:81-84.
To the Editor:
Neutrophilic dermatosis of the dorsal hand (NDDH) is an uncommon reactive neutrophilic dermatosis that presents as a painful, enlarging, ulcerative nodule. It often is misdiagnosed and initially treated as an infection. Similar to other neutrophilic dermatoses, it is associated with underlying infections, inflammatory conditions, and malignancies. Neutrophilic dermatosis of the dorsal hand is considered a subset of Sweet syndrome (SS); we highlight similarities and differences between NDDH and SS, reporting the case of a 66-year-old man without systemic symptoms who developed NDDH on the right hand.

A 66-year-old man presented with a progressively enlarging, painful, ulcerative, 2-cm nodule on the right hand following mechanical trauma 2 weeks prior (Figure 1). He was afebrile with no remarkable medical history. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 20 mm/h (reference range, 0-10 mm/h) and C-reactive protein (CRP) level of 3.52 mg/dL (reference range, 0-0.5 mg/dL) without leukocytosis; both were not remarkably elevated when adjusted for age.1,2 The clinical differential diagnosis was broad and included pyoderma with evolving cellulitis, neutrophilic dermatosis, atypical mycobacterial infection, subcutaneous or deep fungal infection, squamous cell carcinoma, cutaneous lymphoma, and metastasis. Due to the rapid development of the lesion, initial treatment focused on a bacterial infection, but there was no improvement on antibiotics and wound cultures were negative. The ulcerative nodule was biopsied, and histopathology demonstrated abundant neutrophilic inflammation, endothelial swelling, and leukocytoclasis without microorganisms (Figure 2). Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. A diagnosis of NDDH was made based on clinical and histologic findings. The wound improved with a 3-week course of oral prednisone.

Neutrophilic dermatosis of the dorsal hand is a subset of reactive neutrophilic dermatoses, which includes SS (acute febrile neutrophilic dermatosis) and pyoderma gangrenosum. It is described as a localized variant of SS, with similar associated underlying inflammatory, neoplastic conditions and laboratory findings.3 However, NDDH has characteristic features that differ from classic SS. Neutrophilic dermatosis of the dorsal hand typically presents as painful papules, pustules, or ulcers that progress to become larger ulcers, plaques, and nodules. The clinical appearance may more closely resemble pyoderma gangrenosum or atypical SS, with ulceration frequently present. Pathergy also may be demonstrated in NDDH, similar to our patient. The average age of presentation for NDDH is 60 years, which is older than the average age for SS or pyoderma gangrenosum.3 Similar to other neutrophilic dermatoses, NDDH responds well to oral steroids or steroid-sparing immunosuppressants such as dapsone, colchicine, azathioprine, or tetracycline antibiotics.4
The criteria for SS are well established5,6 and may be used for the diagnosis of NDDH, taking into account the localization of lesions to the dorsal aspect of the hands. The diagnostic criteria for SS include fulfillment of both major and at least 2 of 4 minor criteria. The 2 major criteria include rapid presentation of skin lesions and neutrophilic dermal infiltrate on biopsy. Minor criteria are defined as the following: (1) preceding nonspecific respiratory or gastrointestinal tract infection, inflammatory conditions, underlying malignancy, or pregnancy; (2) fever; (3) excellent response to steroids; and (4) 3 of the 4 of the following laboratory abnormalities: elevated CRP, ESR, leukocytosis, or left shift in complete blood cell count. Our patient met both major criteria and only 1 minor criterion—excellent response to systemic corticosteroids. Nofal et al7 advocated for revised diagnostic criteria for SS, with one suggestion utilizing only the 2 major criteria being necessary for diagnosis. Given that serum inflammatory markers may not be as elevated in NDDH compared to SS,3,7,8 meeting the major criteria alone may be a better way to diagnose NDDH, as in our patient.
Our patient presented with an expanding ulcerating nodule on the hand that elicited a wide list of differential diagnoses to include infections and neoplasms. Rapid development, localization to the dorsal aspect of the hand, and treatment resistance to antibiotics may help the clinician consider a diagnosis of NDDH, which should be confirmed by a biopsy. Similar to other neutrophilic dermatoses, an underlying malignancy or inflammatory condition should be sought out. Neutrophilic dermatosis of the dorsal hand responds well to systemic steroids, though recurrences may occur.
To the Editor:
Neutrophilic dermatosis of the dorsal hand (NDDH) is an uncommon reactive neutrophilic dermatosis that presents as a painful, enlarging, ulcerative nodule. It often is misdiagnosed and initially treated as an infection. Similar to other neutrophilic dermatoses, it is associated with underlying infections, inflammatory conditions, and malignancies. Neutrophilic dermatosis of the dorsal hand is considered a subset of Sweet syndrome (SS); we highlight similarities and differences between NDDH and SS, reporting the case of a 66-year-old man without systemic symptoms who developed NDDH on the right hand.

A 66-year-old man presented with a progressively enlarging, painful, ulcerative, 2-cm nodule on the right hand following mechanical trauma 2 weeks prior (Figure 1). He was afebrile with no remarkable medical history. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 20 mm/h (reference range, 0-10 mm/h) and C-reactive protein (CRP) level of 3.52 mg/dL (reference range, 0-0.5 mg/dL) without leukocytosis; both were not remarkably elevated when adjusted for age.1,2 The clinical differential diagnosis was broad and included pyoderma with evolving cellulitis, neutrophilic dermatosis, atypical mycobacterial infection, subcutaneous or deep fungal infection, squamous cell carcinoma, cutaneous lymphoma, and metastasis. Due to the rapid development of the lesion, initial treatment focused on a bacterial infection, but there was no improvement on antibiotics and wound cultures were negative. The ulcerative nodule was biopsied, and histopathology demonstrated abundant neutrophilic inflammation, endothelial swelling, and leukocytoclasis without microorganisms (Figure 2). Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. A diagnosis of NDDH was made based on clinical and histologic findings. The wound improved with a 3-week course of oral prednisone.

Neutrophilic dermatosis of the dorsal hand is a subset of reactive neutrophilic dermatoses, which includes SS (acute febrile neutrophilic dermatosis) and pyoderma gangrenosum. It is described as a localized variant of SS, with similar associated underlying inflammatory, neoplastic conditions and laboratory findings.3 However, NDDH has characteristic features that differ from classic SS. Neutrophilic dermatosis of the dorsal hand typically presents as painful papules, pustules, or ulcers that progress to become larger ulcers, plaques, and nodules. The clinical appearance may more closely resemble pyoderma gangrenosum or atypical SS, with ulceration frequently present. Pathergy also may be demonstrated in NDDH, similar to our patient. The average age of presentation for NDDH is 60 years, which is older than the average age for SS or pyoderma gangrenosum.3 Similar to other neutrophilic dermatoses, NDDH responds well to oral steroids or steroid-sparing immunosuppressants such as dapsone, colchicine, azathioprine, or tetracycline antibiotics.4
The criteria for SS are well established5,6 and may be used for the diagnosis of NDDH, taking into account the localization of lesions to the dorsal aspect of the hands. The diagnostic criteria for SS include fulfillment of both major and at least 2 of 4 minor criteria. The 2 major criteria include rapid presentation of skin lesions and neutrophilic dermal infiltrate on biopsy. Minor criteria are defined as the following: (1) preceding nonspecific respiratory or gastrointestinal tract infection, inflammatory conditions, underlying malignancy, or pregnancy; (2) fever; (3) excellent response to steroids; and (4) 3 of the 4 of the following laboratory abnormalities: elevated CRP, ESR, leukocytosis, or left shift in complete blood cell count. Our patient met both major criteria and only 1 minor criterion—excellent response to systemic corticosteroids. Nofal et al7 advocated for revised diagnostic criteria for SS, with one suggestion utilizing only the 2 major criteria being necessary for diagnosis. Given that serum inflammatory markers may not be as elevated in NDDH compared to SS,3,7,8 meeting the major criteria alone may be a better way to diagnose NDDH, as in our patient.
Our patient presented with an expanding ulcerating nodule on the hand that elicited a wide list of differential diagnoses to include infections and neoplasms. Rapid development, localization to the dorsal aspect of the hand, and treatment resistance to antibiotics may help the clinician consider a diagnosis of NDDH, which should be confirmed by a biopsy. Similar to other neutrophilic dermatoses, an underlying malignancy or inflammatory condition should be sought out. Neutrophilic dermatosis of the dorsal hand responds well to systemic steroids, though recurrences may occur.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med (Clinical Res Ed). 1983;286:226.
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, et al. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249-254.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Gaulding J, Kohen LL. Neutrophilic dermatosis of the dorsal hands. J Am Acad Dermatol. 2017; 76(6 suppl 1):AB178.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Nofal A, Abdelmaksoud A, Amer H, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15:1081-1088.
- Wolf R, Tüzün Y. Acral manifestations of Sweet syndrome (neutrophilic dermatosis of the hands). Clin Dermatol. 2017;35:81-84.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med (Clinical Res Ed). 1983;286:226.
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, et al. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249-254.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Gaulding J, Kohen LL. Neutrophilic dermatosis of the dorsal hands. J Am Acad Dermatol. 2017; 76(6 suppl 1):AB178.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Nofal A, Abdelmaksoud A, Amer H, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15:1081-1088.
- Wolf R, Tüzün Y. Acral manifestations of Sweet syndrome (neutrophilic dermatosis of the hands). Clin Dermatol. 2017;35:81-84.
Practice Points
- Neutrophilic dermatosis of the dorsal hand (NDDH) is a reactive neutrophilic dermatosis that includes Sweet syndrome (SS) and pyoderma gangrenosum.
- Localization to the dorsal aspect of the hand, presence of ulcerative nodules, and older age at onset are characteristic features of NDDH.
- Meeting the major criteria alone for SS may be a more sensitive way to diagnose NDDH, as serum inflammatory markers may not be remarkably elevated in this condition.
Hyperpigmented Flexural Plaques, Hypohidrosis, and Hypotrichosis
The Diagnosis: Lelis Syndrome
Histopathology revealed spongiotic dermatitis with marked acanthosis and hyperkeratosis (Figure, A) with fungal colonization of the stratum corneum (Figure, B). Our patient was diagnosed with Lelis syndrome (also referred to as ectodermal dysplasia with acanthosis nigricans syndrome), a rare condition with hypotrichosis and hypohidrosis resulting from ectodermal dysplasia.1,2 The pruritic rash was diagnosed as chronic dermatitis due to fungal colonization in the setting of acanthosis nigricans. The fungal infection was treated with a 4-week course of oral fluconazole 200 mg/wk, ketoconazole cream 2% twice daily, and discontinuation of topical steroids, resulting in the thinning of the plaques on the neck and antecubital fossae as well as resolution of the pruritus. Following antifungal treatment, our patient was started on tazarotene cream 0.1% for acanthosis nigricans.
Ectodermal dysplasias are inherited disorders with abnormalities of the skin, hair, sweat glands, nails, teeth, and sometimes internal organs.3 Patients with Lelis syndrome may have other manifestations of ectodermal dysplasia in addition to hypohidrosis and hypotrichosis, including deafness and abnormal dentition,1,3 as seen in our patient. Intellectual disability has been described in many types of ectodermal dysplasia, including Lelis syndrome, but the association may be obscured by neurologic damage after repeat episodes of hyperthermia in infancy due to anhidrosis or hypohidrosis.4
When evaluating the differential diagnoses, the presence of hypotrichosis and hypohidrosis indicating ectodermal dysplasia is key. Confluent and reticulated papillomatosis presents with hyperkeratosis, papillomatosis, and focal acanthosis on histopathology. It can present on the neck and antecubital fossae; however, it is not associated with hypohidrosis and hypotrichosis.5 Although activating fibroblast growth factor receptor, FGFR, mutations have been implicated in the development of acanthosis nigricans in a variety of syndromes, these diagnoses are associated with abnormalities in skeletal development such as craniosynostosis and short stature; hypotrichosis and hypohidrosis are not seen.6,7 HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome typically presents in the prepubertal period with obesity and insulin resistance; acanthosis nigricans and alopecia can occur due to insulin resistance and hyperandrogenism, but concurrent clitoromegaly and hirsutism are common.6 Sudden onset of extensive acanthosis nigricans also is among the paraneoplastic dermatoses; it has been associated with multiple malignancies, but in these cases, hypotrichosis and hypohidrosis are not observed. Adenocarcinomas are the most common neoplasms associated with paraneoplastic acanthosis nigricans, which occurs through growth factor secretion by tumor cells stimulating hyperkeratosis and papillomatosis.6
Lelis syndrome is rare, and our case is unique because the patient had severe manifestations of acanthosis nigricans and hypotrichosis. Because the inheritance pattern and specific genetics of the condition have not been fully elucidated, the diagnosis primarily is clinical.1,8 Diagnosis may be complicated by the variety of other signs that can accompany acanthosis nigricans, hypohidrosis, and hypotrichosis.1,2 The condition also may alter or obscure presentation of other dermatologic conditions, as in our case.
Although there is no cure for Lelis syndrome, one case report described treatment with acitretin that resulted in marked improvement of the patient’s hyperkeratosis and acanthosis nigricans.9 Due to lack of health insurance coverage of acitretin, our patient was started on tazarotene cream 0.1% for acanthosis nigricans. General treatment of ectodermal dysplasia primarily consists of multidisciplinary symptom management, including careful monitoring of temperature and heat intolerance as well as provision of dental prosthetics.4,10 For ectodermal dysplasias caused by identified genetic mutations, prenatal interventions targeting gene pathways offer potentially curative treatment.10 However, for Lelis syndrome, along with many other disorders of ectodermal dysplasia, mitigation of signs and symptoms remains the primary treatment objective. Despite its rarity, increased awareness of Lelis syndrome is important to increase knowledge of ectodermal dysplasia syndromes and allow for the investigation of potential treatment options.
- Steiner CE, Cintra ML, Marques-de-Faria AP. Ectodermal dysplasia with acanthosis nigricans (Lelis syndrome). Am J Med Genet. 2002;113:381-384. doi:10.1002/ajmg.b.10787
- Lelis J. Autosomal recessive ectodermal dysplasia. Cutis. 1992; 49:435-437.
- Itin PH, Fistarol SK. Ectodermal dysplasias. Am J Med Genet C Semin Med Genet. 2004;131C:45-51. doi:10.1002/ajmg.c.30033
- Blüschke G, Nüsken KD, Schneider H. Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev. 2010;86:397-399. doi:10.1016/j .earlhumdev.2010.04.008
- Le C, Bedocs PM. Confluent and reticulated papillomatosis. StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459130/
- Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19:1857-1865. doi:10.1111/jocd.13544
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101. doi:10 .1046/j.1365-2133.2002.05150.x
- van Steensel MAM, van der Hout AH. Lelis syndrome may be a manifestation of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A:1612-1613. doi:10.1002/ajmg.a.32945
- Yoshimura AM, Neves Ferreira Velho PE, Ferreira Magalhães R, et al. Lelis’ syndrome: treatment with acitretin. Int J Dermatol. 2008;47: 1330-1331. doi:10.1111/j.1365-4632.2008.03874.x
- Schneider H. Ectodermal dysplasias: new perspectives on the treatment of so far immedicable genetic disorders. Front Genet. 2022;13:1000744. doi:10.3389/fgene.2022.1000744
The Diagnosis: Lelis Syndrome
Histopathology revealed spongiotic dermatitis with marked acanthosis and hyperkeratosis (Figure, A) with fungal colonization of the stratum corneum (Figure, B). Our patient was diagnosed with Lelis syndrome (also referred to as ectodermal dysplasia with acanthosis nigricans syndrome), a rare condition with hypotrichosis and hypohidrosis resulting from ectodermal dysplasia.1,2 The pruritic rash was diagnosed as chronic dermatitis due to fungal colonization in the setting of acanthosis nigricans. The fungal infection was treated with a 4-week course of oral fluconazole 200 mg/wk, ketoconazole cream 2% twice daily, and discontinuation of topical steroids, resulting in the thinning of the plaques on the neck and antecubital fossae as well as resolution of the pruritus. Following antifungal treatment, our patient was started on tazarotene cream 0.1% for acanthosis nigricans.

Ectodermal dysplasias are inherited disorders with abnormalities of the skin, hair, sweat glands, nails, teeth, and sometimes internal organs.3 Patients with Lelis syndrome may have other manifestations of ectodermal dysplasia in addition to hypohidrosis and hypotrichosis, including deafness and abnormal dentition,1,3 as seen in our patient. Intellectual disability has been described in many types of ectodermal dysplasia, including Lelis syndrome, but the association may be obscured by neurologic damage after repeat episodes of hyperthermia in infancy due to anhidrosis or hypohidrosis.4
When evaluating the differential diagnoses, the presence of hypotrichosis and hypohidrosis indicating ectodermal dysplasia is key. Confluent and reticulated papillomatosis presents with hyperkeratosis, papillomatosis, and focal acanthosis on histopathology. It can present on the neck and antecubital fossae; however, it is not associated with hypohidrosis and hypotrichosis.5 Although activating fibroblast growth factor receptor, FGFR, mutations have been implicated in the development of acanthosis nigricans in a variety of syndromes, these diagnoses are associated with abnormalities in skeletal development such as craniosynostosis and short stature; hypotrichosis and hypohidrosis are not seen.6,7 HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome typically presents in the prepubertal period with obesity and insulin resistance; acanthosis nigricans and alopecia can occur due to insulin resistance and hyperandrogenism, but concurrent clitoromegaly and hirsutism are common.6 Sudden onset of extensive acanthosis nigricans also is among the paraneoplastic dermatoses; it has been associated with multiple malignancies, but in these cases, hypotrichosis and hypohidrosis are not observed. Adenocarcinomas are the most common neoplasms associated with paraneoplastic acanthosis nigricans, which occurs through growth factor secretion by tumor cells stimulating hyperkeratosis and papillomatosis.6
Lelis syndrome is rare, and our case is unique because the patient had severe manifestations of acanthosis nigricans and hypotrichosis. Because the inheritance pattern and specific genetics of the condition have not been fully elucidated, the diagnosis primarily is clinical.1,8 Diagnosis may be complicated by the variety of other signs that can accompany acanthosis nigricans, hypohidrosis, and hypotrichosis.1,2 The condition also may alter or obscure presentation of other dermatologic conditions, as in our case.
Although there is no cure for Lelis syndrome, one case report described treatment with acitretin that resulted in marked improvement of the patient’s hyperkeratosis and acanthosis nigricans.9 Due to lack of health insurance coverage of acitretin, our patient was started on tazarotene cream 0.1% for acanthosis nigricans. General treatment of ectodermal dysplasia primarily consists of multidisciplinary symptom management, including careful monitoring of temperature and heat intolerance as well as provision of dental prosthetics.4,10 For ectodermal dysplasias caused by identified genetic mutations, prenatal interventions targeting gene pathways offer potentially curative treatment.10 However, for Lelis syndrome, along with many other disorders of ectodermal dysplasia, mitigation of signs and symptoms remains the primary treatment objective. Despite its rarity, increased awareness of Lelis syndrome is important to increase knowledge of ectodermal dysplasia syndromes and allow for the investigation of potential treatment options.
The Diagnosis: Lelis Syndrome
Histopathology revealed spongiotic dermatitis with marked acanthosis and hyperkeratosis (Figure, A) with fungal colonization of the stratum corneum (Figure, B). Our patient was diagnosed with Lelis syndrome (also referred to as ectodermal dysplasia with acanthosis nigricans syndrome), a rare condition with hypotrichosis and hypohidrosis resulting from ectodermal dysplasia.1,2 The pruritic rash was diagnosed as chronic dermatitis due to fungal colonization in the setting of acanthosis nigricans. The fungal infection was treated with a 4-week course of oral fluconazole 200 mg/wk, ketoconazole cream 2% twice daily, and discontinuation of topical steroids, resulting in the thinning of the plaques on the neck and antecubital fossae as well as resolution of the pruritus. Following antifungal treatment, our patient was started on tazarotene cream 0.1% for acanthosis nigricans.

Ectodermal dysplasias are inherited disorders with abnormalities of the skin, hair, sweat glands, nails, teeth, and sometimes internal organs.3 Patients with Lelis syndrome may have other manifestations of ectodermal dysplasia in addition to hypohidrosis and hypotrichosis, including deafness and abnormal dentition,1,3 as seen in our patient. Intellectual disability has been described in many types of ectodermal dysplasia, including Lelis syndrome, but the association may be obscured by neurologic damage after repeat episodes of hyperthermia in infancy due to anhidrosis or hypohidrosis.4
When evaluating the differential diagnoses, the presence of hypotrichosis and hypohidrosis indicating ectodermal dysplasia is key. Confluent and reticulated papillomatosis presents with hyperkeratosis, papillomatosis, and focal acanthosis on histopathology. It can present on the neck and antecubital fossae; however, it is not associated with hypohidrosis and hypotrichosis.5 Although activating fibroblast growth factor receptor, FGFR, mutations have been implicated in the development of acanthosis nigricans in a variety of syndromes, these diagnoses are associated with abnormalities in skeletal development such as craniosynostosis and short stature; hypotrichosis and hypohidrosis are not seen.6,7 HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome typically presents in the prepubertal period with obesity and insulin resistance; acanthosis nigricans and alopecia can occur due to insulin resistance and hyperandrogenism, but concurrent clitoromegaly and hirsutism are common.6 Sudden onset of extensive acanthosis nigricans also is among the paraneoplastic dermatoses; it has been associated with multiple malignancies, but in these cases, hypotrichosis and hypohidrosis are not observed. Adenocarcinomas are the most common neoplasms associated with paraneoplastic acanthosis nigricans, which occurs through growth factor secretion by tumor cells stimulating hyperkeratosis and papillomatosis.6
Lelis syndrome is rare, and our case is unique because the patient had severe manifestations of acanthosis nigricans and hypotrichosis. Because the inheritance pattern and specific genetics of the condition have not been fully elucidated, the diagnosis primarily is clinical.1,8 Diagnosis may be complicated by the variety of other signs that can accompany acanthosis nigricans, hypohidrosis, and hypotrichosis.1,2 The condition also may alter or obscure presentation of other dermatologic conditions, as in our case.
Although there is no cure for Lelis syndrome, one case report described treatment with acitretin that resulted in marked improvement of the patient’s hyperkeratosis and acanthosis nigricans.9 Due to lack of health insurance coverage of acitretin, our patient was started on tazarotene cream 0.1% for acanthosis nigricans. General treatment of ectodermal dysplasia primarily consists of multidisciplinary symptom management, including careful monitoring of temperature and heat intolerance as well as provision of dental prosthetics.4,10 For ectodermal dysplasias caused by identified genetic mutations, prenatal interventions targeting gene pathways offer potentially curative treatment.10 However, for Lelis syndrome, along with many other disorders of ectodermal dysplasia, mitigation of signs and symptoms remains the primary treatment objective. Despite its rarity, increased awareness of Lelis syndrome is important to increase knowledge of ectodermal dysplasia syndromes and allow for the investigation of potential treatment options.
- Steiner CE, Cintra ML, Marques-de-Faria AP. Ectodermal dysplasia with acanthosis nigricans (Lelis syndrome). Am J Med Genet. 2002;113:381-384. doi:10.1002/ajmg.b.10787
- Lelis J. Autosomal recessive ectodermal dysplasia. Cutis. 1992; 49:435-437.
- Itin PH, Fistarol SK. Ectodermal dysplasias. Am J Med Genet C Semin Med Genet. 2004;131C:45-51. doi:10.1002/ajmg.c.30033
- Blüschke G, Nüsken KD, Schneider H. Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev. 2010;86:397-399. doi:10.1016/j .earlhumdev.2010.04.008
- Le C, Bedocs PM. Confluent and reticulated papillomatosis. StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459130/
- Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19:1857-1865. doi:10.1111/jocd.13544
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101. doi:10 .1046/j.1365-2133.2002.05150.x
- van Steensel MAM, van der Hout AH. Lelis syndrome may be a manifestation of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A:1612-1613. doi:10.1002/ajmg.a.32945
- Yoshimura AM, Neves Ferreira Velho PE, Ferreira Magalhães R, et al. Lelis’ syndrome: treatment with acitretin. Int J Dermatol. 2008;47: 1330-1331. doi:10.1111/j.1365-4632.2008.03874.x
- Schneider H. Ectodermal dysplasias: new perspectives on the treatment of so far immedicable genetic disorders. Front Genet. 2022;13:1000744. doi:10.3389/fgene.2022.1000744
- Steiner CE, Cintra ML, Marques-de-Faria AP. Ectodermal dysplasia with acanthosis nigricans (Lelis syndrome). Am J Med Genet. 2002;113:381-384. doi:10.1002/ajmg.b.10787
- Lelis J. Autosomal recessive ectodermal dysplasia. Cutis. 1992; 49:435-437.
- Itin PH, Fistarol SK. Ectodermal dysplasias. Am J Med Genet C Semin Med Genet. 2004;131C:45-51. doi:10.1002/ajmg.c.30033
- Blüschke G, Nüsken KD, Schneider H. Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev. 2010;86:397-399. doi:10.1016/j .earlhumdev.2010.04.008
- Le C, Bedocs PM. Confluent and reticulated papillomatosis. StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459130/
- Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19:1857-1865. doi:10.1111/jocd.13544
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101. doi:10 .1046/j.1365-2133.2002.05150.x
- van Steensel MAM, van der Hout AH. Lelis syndrome may be a manifestation of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A:1612-1613. doi:10.1002/ajmg.a.32945
- Yoshimura AM, Neves Ferreira Velho PE, Ferreira Magalhães R, et al. Lelis’ syndrome: treatment with acitretin. Int J Dermatol. 2008;47: 1330-1331. doi:10.1111/j.1365-4632.2008.03874.x
- Schneider H. Ectodermal dysplasias: new perspectives on the treatment of so far immedicable genetic disorders. Front Genet. 2022;13:1000744. doi:10.3389/fgene.2022.1000744
A 61-year-old woman with a history of hypohidrosis and deafness presented with a pruritic rash on the neck and antecubital fossae of several years’ duration. Prior treatment with topical corticosteroids failed to resolve the rash. Physical examination revealed thick, velvety, hyperpigmented plaques on the inframammary folds, axillae, groin, posterior neck, and antecubital fossae with lichenification of the latter 2 areas. Many pedunculated papules were seen on the face, chest, shoulders, and trunk, as well as diffuse hair thinning, particularly of the frontal and vertex scalp. Eyebrows, eyelashes, and axillary hair were absent. Two 5-mm punch biopsies of the antecubital fossa and inframammary fold were obtained for histopathologic analysis.
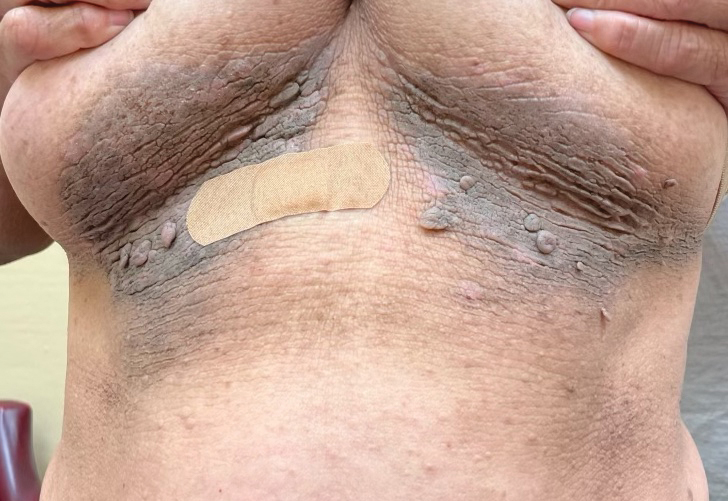
Dupilumab for Dyshidrotic Eczema With Secondary Improvement in Eosinophilic Interstitial Lung Disease
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).
Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
To the Editor:
Biologic medications are increasingly utilized in adults with moderate to severe atopic dermatitis (AD) that is inadequately controlled with topical medication. By targeting the IL-4 receptor alpha subunit, dupilumab inhibits the biologic effects of IL-4 and IL-13, resulting in remarkable improvement in disease and quality of life for many patients with refractory AD.1
In 2017, the US Food and Drug Administration approved dupilumab for use in AD, asthma, and chronic rhinosinusitis. However, there is evidence of the drug’s off-label efficacy in conditions such as eosinophilic annular erythema.2 We present a patient with dyshidrotic eczema treated with dupilumab who experienced contemporaneous secondary improvement in chronic eosinophilic pneumonia (CEP) and interstitial lung disease (ILD).
A 45-year-old man was referred to our dermatology clinic for chronic hand dermatitis refractory to increasing strengths of topical corticosteroids. He had a history of progressive shortness of breath of unknown cause, which began 2 years prior, and he was being followed at our institution’s ILD clinic. Earlier pulmonary function testing revealed a restrictive pattern with interstitial infiltrates seen on chest computed tomography. A lung biopsy demonstrated features of fibrotic nonspecific interstitial pneumonitis with superimposed eosinophilic pneumonia. His pulmonary symptoms had progressively worsened; over a period of several months, the supplemental oxygen requirement had increased to 6 L at rest and 12 L upon exertion. Prednisone therapy was initiated, which alleviated respiratory symptoms; however, the patient was unable to tolerate a gradual wean of the medication, which rendered him steroid dependent at 30 mg/d.
Along with respiratory symptoms, the patient reported symptoms consistent with an autoimmune process, including dry eyes. Muscle weakness and tenderness also were noted. Ultimately, a diagnosis of anti–PL-7 (anti-threonyl-transfer RNA synthetase) antisynthetase syndrome was rendered by identification of anti–PL-7 antibodies and an elevated level of creatinine kinase.
Physical examination at our clinic revealed subtle palmar scaling on the hands and multiple small clear vesicles on the lateral aspects of the digits (Figure, A), consistent with dyshidrotic eczema. He initially was treated with clobetasol propionate ointment 0.05%. Despite adherence to this high-potency topical corticosteroid, he experienced only minimal improvement over a period of 3 months. Dupilumab was started at standard dosing—600 mg at initiation, followed by 300 mg every 2 weeks. The patient reported rapid improvement in dyshidrotic eczema over several months with near-complete resolution (Figure, B).

Concurrent with initiation and continued use of dupilumab, without other changes in his medication regimen, the patient noted gradual improvement in respiratory symptoms. At 6-month follow-up he reported notable improvement in respiratory function and quality of life. He then tolerated a gradual wean of prednisone to 10 mg/d, with a similar reduction in supplemental oxygen.
Off-label use of dupilumab for various eosinophilic conditions has shown promising efficacy. Our patient experienced improvement in CEP shortly after initiation of dupilumab, enabling weaning of prednisone, which has a well established adverse effect profile associated with long term use.3,4 In comparison, dupilumab generally is well tolerated, with rare ophthalmologic complications and injection-site reactions.5
One case report suggested that CEP may represent a potential rare adverse effect of dupilumab initiation.6 However, prior to initiation of dupilumab, that patient had poorly controlled asthma requiring frequent oral corticosteroid therapy. It is possible that CEP was subclinical prior to initiation of dupilumab and became more noticeable once the patient was weaned from corticosteroids, which had served as an indirect treatment.6 Nonetheless, more research is needed to definitively establish the efficacy of dupilumab in CEP prior to more widespread use.
Irrespective of the potential efficacy of dupilumab for the treatment of CEP, our case highlights the growing body of evidence that dupilumab should be considered in the treatment of dyshidrotic eczema, particularly in cases refractory to topical treatment.7 When a systemic medication is preferred, dupilumab likely represents an option with a relatively well-tolerated adverse effect profile compared to traditional systemic treatments for dyshidrotic eczema.
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
1. Barbarot S, Wollenberg A, Silverberg JI, et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: combined results ofour randomized phase 3 trials. J Dermatolog Treat. 2022;33:266-277. doi:10.1080/09546634.2020.1750550
2. Gordon SC, Robinson SN, Abudu M, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35:E255-E256. doi:10.1111/pde.13533
3. Callaghan DJ 3rd. Use of Google Trends to examine interest in Mohs micrographic surgery: 2004 to 2016. Dermatol Surg. 2018;44:186-192. doi:10.1097/DSS.0000000000001270
4. Fowler C, Hoover W. Dupilumab for chronic eosinophilic pneumonia. Pediatr Pulmonol. 2020;55:3229-3230. doi:10.1002/ppul.25096
5. Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017;376:1090-1091. doi:10.1056/NEJMc1700366
6. Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869-875. doi:10.2147/TCRM.S207402
7. Waldman RA, DeWane ME, Sloan B, et al. Dupilumab for the treatment of dyshidrotic eczema in 15 consecutive patients. J Am Acad Dermatol. 2020;82:1251-1252. doi:10.1016/j.jaad.2019.12.053
Practice Points
- Dupilumab can be considered for treatment of refractory dyshidrotic eczema.
- Dupilumab may provide secondary efficacy in patients with dyshidrotic eczema who also have an eosinophilic condition such as eosinophilic pneumonia.
Paradoxical Reaction to TNF-α Inhibitor Therapy in a Patient With Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.
Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.
Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
Practice Points
- Clinicians need to be aware of the potential risk for a paradoxical reaction in patients receiving a tumor necrosis factor α (TNF-α) inhibitor for hidradenitis suppurativa.
- Although uncommon, developing more than 1 type of paradoxical skin reaction is possible with a TNF-α inhibitor.
- Early recognition and appropriate management of these paradoxical reactions are critical.
Impact of the COVID-19 Pandemic on Care for Patients With Atopic Dermatitis
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
Practice Points
- The landscape of dermatology has seen major changes due to the COVID-19 pandemic, as many patients now utilize telemedicine to receive care.
- Understanding accessibility to in-person care for patients with atopic dermatitis during the COVID-19 pandemic can assist with the development of methods to enhance management.
Aberrant Expression of CD56 in Metastatic Malignant Melanoma
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.
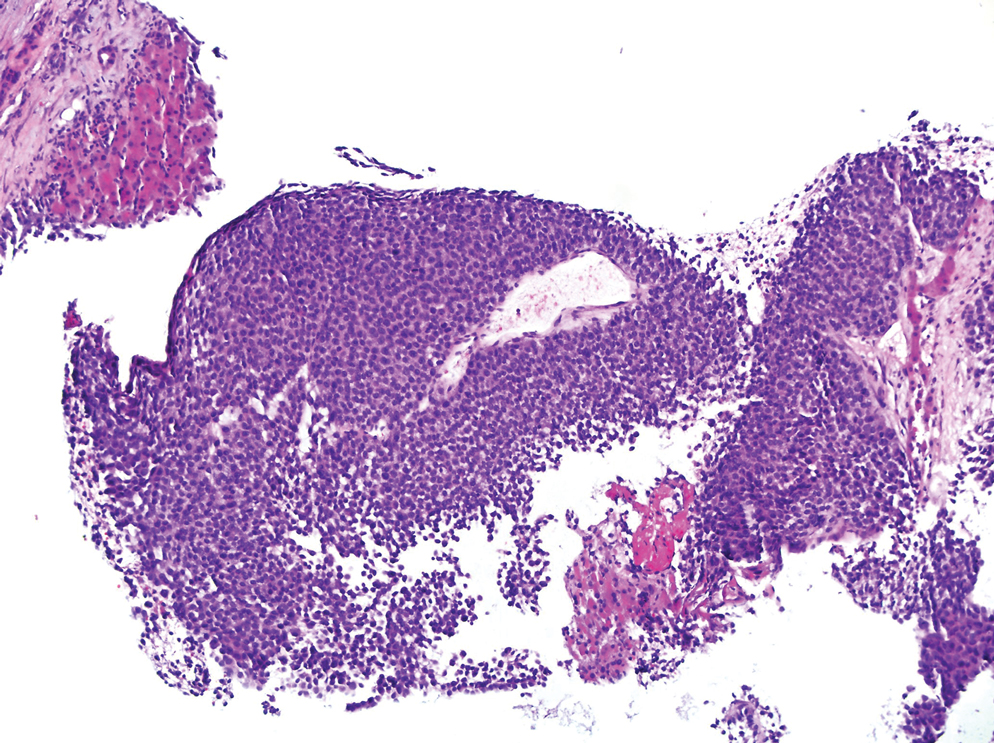
BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.
More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5
Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
Practice Points
- The diagnosis of melanoma often is challenging as tumors can show notable histologic diversity and have the potential to express aberrant immunophenotypes including CD56 expression.
- Because expression of CD56 in melanoma is rare, it is important to be aware of this potential aberrant staining pattern.
- Recognizing this heterogeneity and divergent differentiation as well as knowing potential aberrant immunohistochemical staining patterns are imperative for accurate and timely diagnosis.
Asymptomatic Hair Loss in a Patient With Systemic Lupus Erythematosus
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4
Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4

Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4

Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
A 51-year-old woman residing in the Hainan Province, China, was referred to our hospital for treatment of recurrent joint pain that could not be controlled at the local hospital. She had a history of systemic lupus erythematosus with a Systemic Lupus Erythematosus Disease Activity Index score of 8 (mild activity). Physical examination revealed irregular patches of hair loss on the head. There also were remnants of hair in some areas with black dots at the follicular opening and perifollicular keratotic papules interspersed as well as a few pale erythematous spots and white adherent scales.
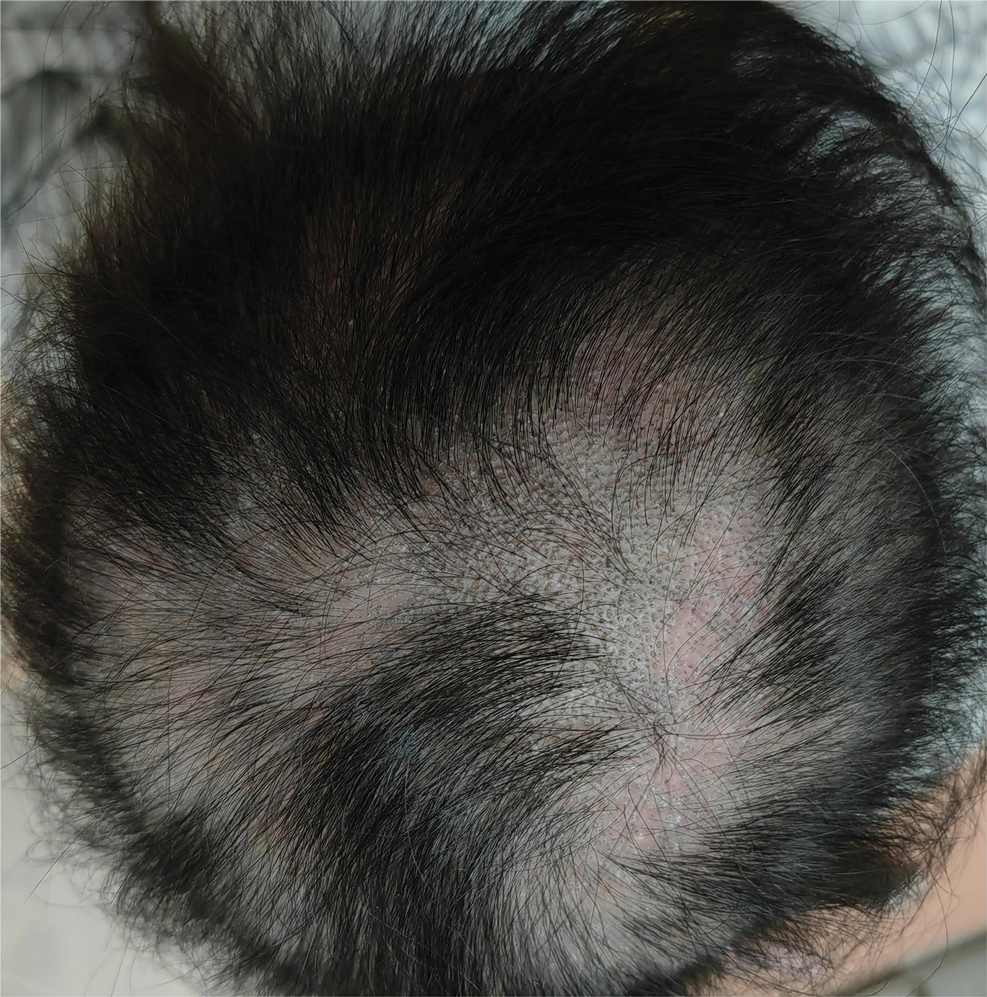
Alopecia Universalis Treated With Tofacitinib: The Role of JAK/STAT Inhibitors in Hair Regrowth
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).
The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.
Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.
Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4
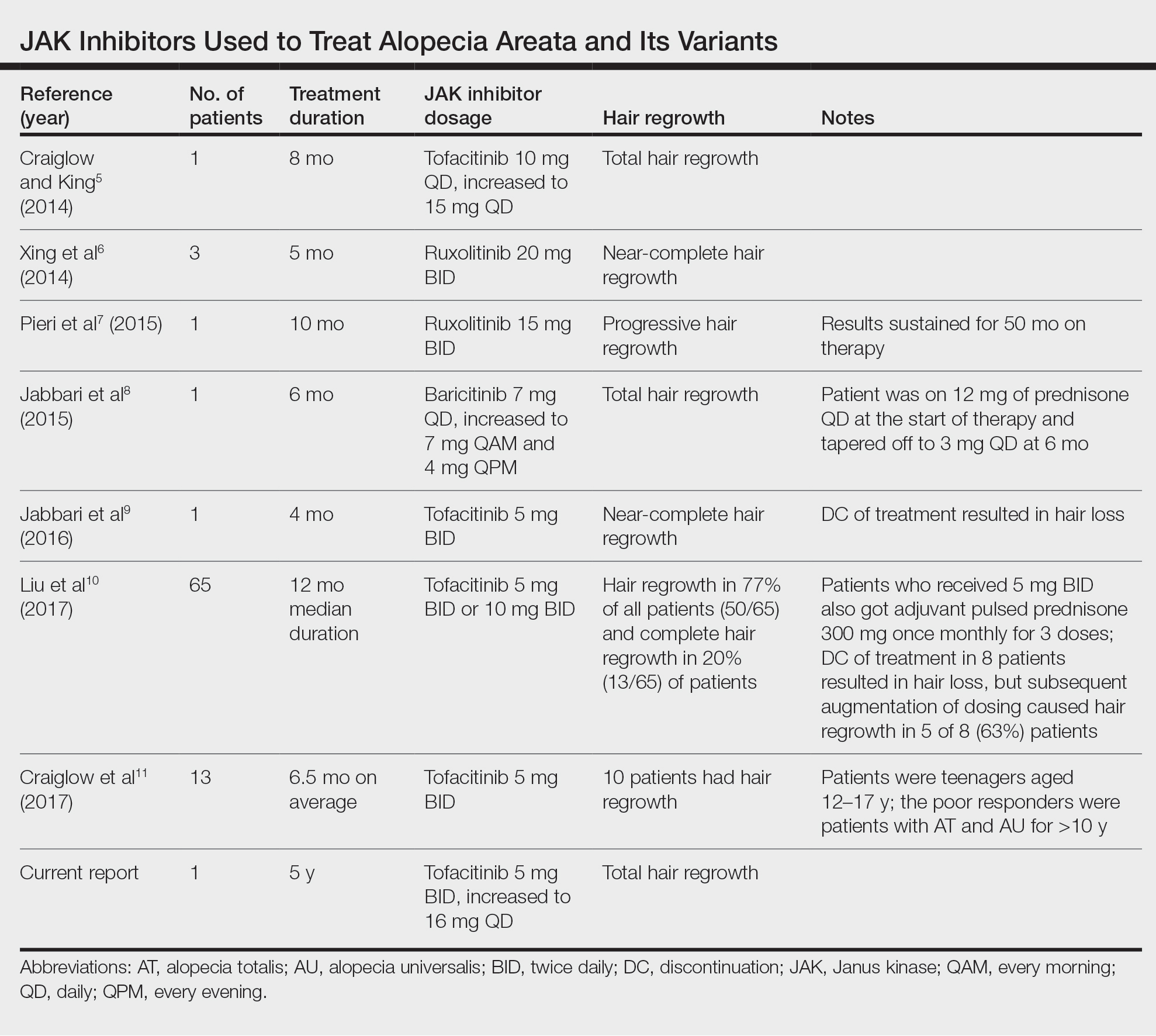
In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).

The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.

Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.

Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4

In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).

The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.

Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.

Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4

In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
Practice Points
- Janus kinase inhibitors target one of the cellular pathogeneses of alopecia areata.
- Janus kinase inhibitors may be an option for patients who have exhausted other treatment modalities for alopecia.