User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Semaglutide win in HFpEF with obesity regardless of ejection fraction: STEP-HFpEF
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.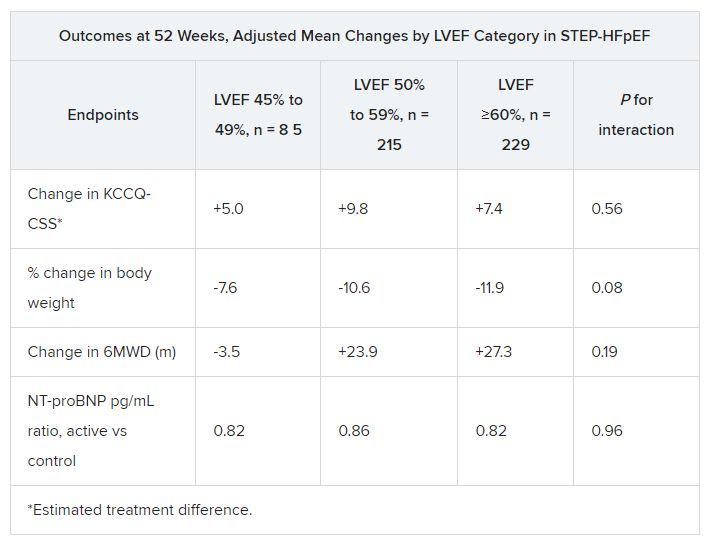
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
AT HFSA 2023
Burnout in medical profession higher among women, younger clinicians
The poster child for a burned-out physician is a young woman practicing in primary care, according to a new study of more than 1,300 clinicians.
The study, published in JAMA Network Open. investigated patterns in physician burnout among 1,373 physicians at Massachusetts General Physicians Organization, a hospital-owned group practice. It assessed burnout in 3 years: 2017, 2019, and 2021.
Respondents were queried about their satisfaction with their career and compensation, as well as their well-being, administrative workload, and leadership and diversity.
Female physicians exhibited a higher burnout rate than male physicians (odds ratio, 1.47; 95% confidence interval, 1.02-2.12), while among primary care physicians (PCPs), the burnout rate was almost three times higher than among those in internal medicine (OR, 2.82; 95% CI, 1.76-4.50). Among physicians with 30 or more years of experience, the burnout rate was lower than among those with 10 years of experience or less (OR, 0.21; 95% CI, 0.13-0.35).
The fact that burnout disproportionately affects female physicians could reflect the additional household and family obligations women are often expected to handle, as well as their desire to form relationships with their patients, according to Timothy Hoff, PhD, a professor of management, healthcare systems, and health policy at Northeastern University, Boston.
“Female physicians tend to practice differently than their male counterparts,” said Dr. Hoff, who studies primary care. “They may focus more on the relational aspects of care, and that could lead to a higher rate of burnout.”
The study used the Maslach Burnout Inventory and three burnout subscales: exhaustion, cynicism, and reduced personal efficacy. The cohort was composed of 50% men, 67% White respondents, and 87% non-Hispanic respondents. A little over two-thirds of physicians had from 11 to 20 years of experience.
About 93% of those surveyed responded; by comparison, response rates were between 27% and 32% in previous analyses of physician burnout, the study authors say. They attribute this high participation rate to the fact that they compensated each participant with $850, more than is usually offered.
Hilton Gomes, MD, a partner at a concierge primary care practice in Miami – who has been practicing medicine for more than 15 years – said the increased rates of burnout among his younger colleagues are partly the result of a recent shift in what is considered the ideal work-life balance.
“Younger generations of doctors enter the profession with a strong desire for a better work-life balance. Unfortunately, medicine does not typically lend itself to achieving this balance,” he said.
Dr. Gomes recalled a time in medical school when he tried to visit his former pediatrician, who couldn’t be found at home.
“His wife informed me that he was tending to an urgent sick visit at the hospital, while his wife had to deal with their own grandson’s fracture being treated at urgent care,” Dr. Gomes said. “This illustrates, in my experience, how older generations of physicians accepted the demands of the profession as part of their commitment, and this often involved putting our own families second.”
Dr. Gomes, like many other PCPs who have converted to concierge medicine, previously worked at a practice where he saw nearly two dozen patients a day for a maximum of 15 minutes each.
“The structure of managed care often results in primary care physicians spending less time with patients and more time on paperwork, which is not the reason why physicians enter the field of medicine,” Dr. Gomes said.
Physicians are not alone in their feelings of physical and mental exhaustion. In the Medscape Physician Assistant Burnout Report 2023, 16% of respondents said the burnout they experienced was so severe that they were thinking of leaving medicine.
In 2022, PCP burnout cost the United States $260 million in excess health care expenditures. Burnout has also increased rates of physician suicide over the past 50 years and has led to a rise in medical errors.
Physicians say that programs that teach them to perform yoga and take deep breaths – which are offered by their employers – are not the solution.
“We sort of know what the realities of physician burnout are now; the imperative is to address it,” Dr. Hoff said. “We need studies that focus on the concepts of sustainability.”
The study was funded by the Massachusetts General Physicians Organization. A coauthor reports receiving a grant from the American Heart Association. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
The poster child for a burned-out physician is a young woman practicing in primary care, according to a new study of more than 1,300 clinicians.
The study, published in JAMA Network Open. investigated patterns in physician burnout among 1,373 physicians at Massachusetts General Physicians Organization, a hospital-owned group practice. It assessed burnout in 3 years: 2017, 2019, and 2021.
Respondents were queried about their satisfaction with their career and compensation, as well as their well-being, administrative workload, and leadership and diversity.
Female physicians exhibited a higher burnout rate than male physicians (odds ratio, 1.47; 95% confidence interval, 1.02-2.12), while among primary care physicians (PCPs), the burnout rate was almost three times higher than among those in internal medicine (OR, 2.82; 95% CI, 1.76-4.50). Among physicians with 30 or more years of experience, the burnout rate was lower than among those with 10 years of experience or less (OR, 0.21; 95% CI, 0.13-0.35).
The fact that burnout disproportionately affects female physicians could reflect the additional household and family obligations women are often expected to handle, as well as their desire to form relationships with their patients, according to Timothy Hoff, PhD, a professor of management, healthcare systems, and health policy at Northeastern University, Boston.
“Female physicians tend to practice differently than their male counterparts,” said Dr. Hoff, who studies primary care. “They may focus more on the relational aspects of care, and that could lead to a higher rate of burnout.”
The study used the Maslach Burnout Inventory and three burnout subscales: exhaustion, cynicism, and reduced personal efficacy. The cohort was composed of 50% men, 67% White respondents, and 87% non-Hispanic respondents. A little over two-thirds of physicians had from 11 to 20 years of experience.
About 93% of those surveyed responded; by comparison, response rates were between 27% and 32% in previous analyses of physician burnout, the study authors say. They attribute this high participation rate to the fact that they compensated each participant with $850, more than is usually offered.
Hilton Gomes, MD, a partner at a concierge primary care practice in Miami – who has been practicing medicine for more than 15 years – said the increased rates of burnout among his younger colleagues are partly the result of a recent shift in what is considered the ideal work-life balance.
“Younger generations of doctors enter the profession with a strong desire for a better work-life balance. Unfortunately, medicine does not typically lend itself to achieving this balance,” he said.
Dr. Gomes recalled a time in medical school when he tried to visit his former pediatrician, who couldn’t be found at home.
“His wife informed me that he was tending to an urgent sick visit at the hospital, while his wife had to deal with their own grandson’s fracture being treated at urgent care,” Dr. Gomes said. “This illustrates, in my experience, how older generations of physicians accepted the demands of the profession as part of their commitment, and this often involved putting our own families second.”
Dr. Gomes, like many other PCPs who have converted to concierge medicine, previously worked at a practice where he saw nearly two dozen patients a day for a maximum of 15 minutes each.
“The structure of managed care often results in primary care physicians spending less time with patients and more time on paperwork, which is not the reason why physicians enter the field of medicine,” Dr. Gomes said.
Physicians are not alone in their feelings of physical and mental exhaustion. In the Medscape Physician Assistant Burnout Report 2023, 16% of respondents said the burnout they experienced was so severe that they were thinking of leaving medicine.
In 2022, PCP burnout cost the United States $260 million in excess health care expenditures. Burnout has also increased rates of physician suicide over the past 50 years and has led to a rise in medical errors.
Physicians say that programs that teach them to perform yoga and take deep breaths – which are offered by their employers – are not the solution.
“We sort of know what the realities of physician burnout are now; the imperative is to address it,” Dr. Hoff said. “We need studies that focus on the concepts of sustainability.”
The study was funded by the Massachusetts General Physicians Organization. A coauthor reports receiving a grant from the American Heart Association. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
The poster child for a burned-out physician is a young woman practicing in primary care, according to a new study of more than 1,300 clinicians.
The study, published in JAMA Network Open. investigated patterns in physician burnout among 1,373 physicians at Massachusetts General Physicians Organization, a hospital-owned group practice. It assessed burnout in 3 years: 2017, 2019, and 2021.
Respondents were queried about their satisfaction with their career and compensation, as well as their well-being, administrative workload, and leadership and diversity.
Female physicians exhibited a higher burnout rate than male physicians (odds ratio, 1.47; 95% confidence interval, 1.02-2.12), while among primary care physicians (PCPs), the burnout rate was almost three times higher than among those in internal medicine (OR, 2.82; 95% CI, 1.76-4.50). Among physicians with 30 or more years of experience, the burnout rate was lower than among those with 10 years of experience or less (OR, 0.21; 95% CI, 0.13-0.35).
The fact that burnout disproportionately affects female physicians could reflect the additional household and family obligations women are often expected to handle, as well as their desire to form relationships with their patients, according to Timothy Hoff, PhD, a professor of management, healthcare systems, and health policy at Northeastern University, Boston.
“Female physicians tend to practice differently than their male counterparts,” said Dr. Hoff, who studies primary care. “They may focus more on the relational aspects of care, and that could lead to a higher rate of burnout.”
The study used the Maslach Burnout Inventory and three burnout subscales: exhaustion, cynicism, and reduced personal efficacy. The cohort was composed of 50% men, 67% White respondents, and 87% non-Hispanic respondents. A little over two-thirds of physicians had from 11 to 20 years of experience.
About 93% of those surveyed responded; by comparison, response rates were between 27% and 32% in previous analyses of physician burnout, the study authors say. They attribute this high participation rate to the fact that they compensated each participant with $850, more than is usually offered.
Hilton Gomes, MD, a partner at a concierge primary care practice in Miami – who has been practicing medicine for more than 15 years – said the increased rates of burnout among his younger colleagues are partly the result of a recent shift in what is considered the ideal work-life balance.
“Younger generations of doctors enter the profession with a strong desire for a better work-life balance. Unfortunately, medicine does not typically lend itself to achieving this balance,” he said.
Dr. Gomes recalled a time in medical school when he tried to visit his former pediatrician, who couldn’t be found at home.
“His wife informed me that he was tending to an urgent sick visit at the hospital, while his wife had to deal with their own grandson’s fracture being treated at urgent care,” Dr. Gomes said. “This illustrates, in my experience, how older generations of physicians accepted the demands of the profession as part of their commitment, and this often involved putting our own families second.”
Dr. Gomes, like many other PCPs who have converted to concierge medicine, previously worked at a practice where he saw nearly two dozen patients a day for a maximum of 15 minutes each.
“The structure of managed care often results in primary care physicians spending less time with patients and more time on paperwork, which is not the reason why physicians enter the field of medicine,” Dr. Gomes said.
Physicians are not alone in their feelings of physical and mental exhaustion. In the Medscape Physician Assistant Burnout Report 2023, 16% of respondents said the burnout they experienced was so severe that they were thinking of leaving medicine.
In 2022, PCP burnout cost the United States $260 million in excess health care expenditures. Burnout has also increased rates of physician suicide over the past 50 years and has led to a rise in medical errors.
Physicians say that programs that teach them to perform yoga and take deep breaths – which are offered by their employers – are not the solution.
“We sort of know what the realities of physician burnout are now; the imperative is to address it,” Dr. Hoff said. “We need studies that focus on the concepts of sustainability.”
The study was funded by the Massachusetts General Physicians Organization. A coauthor reports receiving a grant from the American Heart Association. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Home-based exercise benefits patients with PAD
TOPLINE:
Compared with supervised treadmill workouts at a gym, which is considered first-line therapy for walking impairment in lower extremity peripheral artery disease (PAD), exercising at home significantly improves 6-minute walking (6MW) distance, but not maximal treadmill walking distance, results of a new meta-analysis show.
METHODOLOGY:
- The analysis included five randomized clinical trials with a total of 719 participants, mean age 68.6 years, all led by researchers at Northwestern University, Chicago, that compared either supervised treadmill or home-based walking exercise with a nonexercise control group in people with PAD (defined as Ankle Brachial Index ≤ 0.90).
- All trials measured 6-minute walk (6MW) distance (walking as far as possible in 6 minutes), treadmill walking performance, and outcomes from the Walking Impairment Questionnaire (WIQ), which includes distance, walking speed, and stair-climbing domains, at baseline and at 6 months.
- Supervised treadmill exercise interventions included three individualized exercise sessions per week with an exercise physiologist at an exercise center, and home-based exercises involved walking near home 5 days per week, both for up to 50 minutes per session.
TAKEAWAY:
- After adjusting for study, age, sex, race, smoking, history of myocardial infarction, heart failure, and baseline 6MW distance, the study found both exercise interventions were better than nonexercise controls for 6MW distance.
- Compared with supervised treadmill exercise, home-based walking was associated with significantly improved mean 6MW distance (31.8 m vs. 55.6 m; adjusted between-group difference: −23.8 m; 95% confidence interval, −44.0 to −3.6; P = .021), and significantly improved WIQ walking speed score.
- However, home-based walking was associated with significantly less improvement in maximal treadmill walking distance, compared with supervised treadmill exercise (adjusted between-group difference: 132.5 m; 95% CI, 72.1-192.9; P < .001).
IN PRACTICE:
Home-based walking exercise “circumvents” barriers to accessing supervised exercise such as having to travel to a facility, said the authors, who noted the new data “demonstrated a large and consistent effect of home-based walking exercise on improved 6MW distance and also significantly improved the WIQ walking speed score, compared with supervised treadmill exercise.”
SOURCE:
The study was conducted by Neela D. Thangada, MD, Northwestern University, Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
Data were combined from different randomized clinical trials that were led by one investigative team, and reported comparisons were not prespecified. Comparisons between supervised and home-based exercise lacked statistical power for the WIQ distance and stair-climbing measures.
DISCLOSURES:
The study was sponsored by the National Center for Research Resources and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Thangada reports no relevant financial relationships. Disclosures for study coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with supervised treadmill workouts at a gym, which is considered first-line therapy for walking impairment in lower extremity peripheral artery disease (PAD), exercising at home significantly improves 6-minute walking (6MW) distance, but not maximal treadmill walking distance, results of a new meta-analysis show.
METHODOLOGY:
- The analysis included five randomized clinical trials with a total of 719 participants, mean age 68.6 years, all led by researchers at Northwestern University, Chicago, that compared either supervised treadmill or home-based walking exercise with a nonexercise control group in people with PAD (defined as Ankle Brachial Index ≤ 0.90).
- All trials measured 6-minute walk (6MW) distance (walking as far as possible in 6 minutes), treadmill walking performance, and outcomes from the Walking Impairment Questionnaire (WIQ), which includes distance, walking speed, and stair-climbing domains, at baseline and at 6 months.
- Supervised treadmill exercise interventions included three individualized exercise sessions per week with an exercise physiologist at an exercise center, and home-based exercises involved walking near home 5 days per week, both for up to 50 minutes per session.
TAKEAWAY:
- After adjusting for study, age, sex, race, smoking, history of myocardial infarction, heart failure, and baseline 6MW distance, the study found both exercise interventions were better than nonexercise controls for 6MW distance.
- Compared with supervised treadmill exercise, home-based walking was associated with significantly improved mean 6MW distance (31.8 m vs. 55.6 m; adjusted between-group difference: −23.8 m; 95% confidence interval, −44.0 to −3.6; P = .021), and significantly improved WIQ walking speed score.
- However, home-based walking was associated with significantly less improvement in maximal treadmill walking distance, compared with supervised treadmill exercise (adjusted between-group difference: 132.5 m; 95% CI, 72.1-192.9; P < .001).
IN PRACTICE:
Home-based walking exercise “circumvents” barriers to accessing supervised exercise such as having to travel to a facility, said the authors, who noted the new data “demonstrated a large and consistent effect of home-based walking exercise on improved 6MW distance and also significantly improved the WIQ walking speed score, compared with supervised treadmill exercise.”
SOURCE:
The study was conducted by Neela D. Thangada, MD, Northwestern University, Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
Data were combined from different randomized clinical trials that were led by one investigative team, and reported comparisons were not prespecified. Comparisons between supervised and home-based exercise lacked statistical power for the WIQ distance and stair-climbing measures.
DISCLOSURES:
The study was sponsored by the National Center for Research Resources and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Thangada reports no relevant financial relationships. Disclosures for study coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with supervised treadmill workouts at a gym, which is considered first-line therapy for walking impairment in lower extremity peripheral artery disease (PAD), exercising at home significantly improves 6-minute walking (6MW) distance, but not maximal treadmill walking distance, results of a new meta-analysis show.
METHODOLOGY:
- The analysis included five randomized clinical trials with a total of 719 participants, mean age 68.6 years, all led by researchers at Northwestern University, Chicago, that compared either supervised treadmill or home-based walking exercise with a nonexercise control group in people with PAD (defined as Ankle Brachial Index ≤ 0.90).
- All trials measured 6-minute walk (6MW) distance (walking as far as possible in 6 minutes), treadmill walking performance, and outcomes from the Walking Impairment Questionnaire (WIQ), which includes distance, walking speed, and stair-climbing domains, at baseline and at 6 months.
- Supervised treadmill exercise interventions included three individualized exercise sessions per week with an exercise physiologist at an exercise center, and home-based exercises involved walking near home 5 days per week, both for up to 50 minutes per session.
TAKEAWAY:
- After adjusting for study, age, sex, race, smoking, history of myocardial infarction, heart failure, and baseline 6MW distance, the study found both exercise interventions were better than nonexercise controls for 6MW distance.
- Compared with supervised treadmill exercise, home-based walking was associated with significantly improved mean 6MW distance (31.8 m vs. 55.6 m; adjusted between-group difference: −23.8 m; 95% confidence interval, −44.0 to −3.6; P = .021), and significantly improved WIQ walking speed score.
- However, home-based walking was associated with significantly less improvement in maximal treadmill walking distance, compared with supervised treadmill exercise (adjusted between-group difference: 132.5 m; 95% CI, 72.1-192.9; P < .001).
IN PRACTICE:
Home-based walking exercise “circumvents” barriers to accessing supervised exercise such as having to travel to a facility, said the authors, who noted the new data “demonstrated a large and consistent effect of home-based walking exercise on improved 6MW distance and also significantly improved the WIQ walking speed score, compared with supervised treadmill exercise.”
SOURCE:
The study was conducted by Neela D. Thangada, MD, Northwestern University, Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
Data were combined from different randomized clinical trials that were led by one investigative team, and reported comparisons were not prespecified. Comparisons between supervised and home-based exercise lacked statistical power for the WIQ distance and stair-climbing measures.
DISCLOSURES:
The study was sponsored by the National Center for Research Resources and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Thangada reports no relevant financial relationships. Disclosures for study coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
Lead pollutants as harmful to health as particulate matter
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
in a presentation to the World Bank. Their work was published in The Lancet Planetary Health.
As Mr. Larsen and Mr. Sánchez-Triana report, the economic consequences of increased exposure to lead are already immense, especially in low- and middle-income countries (LMICs). The study was financed by the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program.
Intellectual, cardiovascular effects
“It is a very important publication that affects all of us,” pediatrician Stephan Böse-O’Reilly, MD, of the Institute and Polyclinic for Occupational, Social, and Environmental Health at Ludwig Maximilian University Hospital in Munich, Germany, said in an interview. “The study, the results of which I think are very reliable, shows that elevated levels of lead in the blood have a much more drastic effect on children’s intelligence than we previously thought.”
It is well known that lead affects the antenatal and postnatal cognitive development of children, Dr. Böse-O’Reilly explained. But the extent of this effect has quite clearly been underestimated before now.
On the other hand, Mr. Larsen and Mr. Sánchez-Triana’s work could prove that lead may lead to more cardiovascular diseases in adulthood. “We already knew that increased exposure to lead increased the risk of high blood pressure and, as a result, mortality,” said Dr. Böse-O’Reilly. “This study now very clearly shows that the risk of arteriosclerosis, for example, also increases through lead exposure.”
Figures from 2019
“For the first time, to our knowledge, we aimed to estimate the global burden and cost of IQ loss and cardiovascular disease mortality from lead exposure,” wrote Mr. Larsen and Mr. Sánchez-Triana. For their calculations, the scientists used blood lead level estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.
They estimated IQ loss in children younger than 5 years using the internationally recognized blood lead level–IQ loss function. The researchers subsequently estimated the cost of this IQ loss based on the loss in lifetime income, presented as cost in U.S. dollars and percentage of gross domestic product (GDP).
Mr. Larsen and Mr. Sánchez-Triana estimated cardiovascular deaths caused by lead exposure in adults aged 25 years or older using a model that captures the effects of lead exposure on cardiovascular disease mortality that is mediated through mechanisms other than hypertension.
Finally, they used the statistical life expectancy to estimate the welfare cost of premature mortality, also presented as cost in U.S. dollars and percentage of GDP. All estimates were calculated according to the World Bank income classification for 2019.
Millions of deaths
As reported by Mr. Larsen and Mr. Sánchez-Triana, children younger than 5 years lost an estimated 765 million IQ points worldwide because of lead exposure in this period. In 2019, 5,545,000 adults died from cardiovascular diseases caused by lead exposure. The scientists recorded 729 million of the IQ points lost (95.3%) and 5,004,000 (90.2%) of the deaths as occurring in LMICs.
The IQ loss here was nearly 80% higher than a previous estimate, wrote Mr. Larsen and Mr. Sánchez-Triana. The number of cardiovascular disease deaths they determined was six times higher than the GBD 2019 estimate.
“These are results with which the expert societies, especially the German Society of Pediatrics and Adolescent Medicine and the German Cardiac Society, and the corresponding professional associations need to concern themselves,” said Dr. Böse-O’Reilly.
Although blood lead concentrations have declined substantially since the phase-out of leaded gasoline, especially in Western countries, lead still represents a major health issue because it stays in the bones for decades.
European situation moderate
“We need a broad discussion on questions such as whether lead levels should be included in prophylactic assessments in certain age groups, what blood level is even tolerable, and in what situation medicinal therapy with chelating agents would possibly be appropriate,” said Dr. Böse-O’Reilly.
“Of course, we cannot answer these questions on the basis of one individual study,” he added. “However, the work in question definitely illustrates how dangerous lead can be and that we need further research into the actual burden and the best preventive measures.”
In this respect, the situation in Europe is still comparatively moderate. “Globally, lead exposure has risen in recent years,” said Dr. Böse-O’Reilly. According to an investigation by the Planet Earth Foundation, outside of the European Union, lead can increasingly be found in toys, spices, and cooking utensils, for example.
“Especially in lower-income countries, there is a lack of consumer protection or a good monitoring program like we have here in the EU,” said Dr. Böse-O’Reilly. In these countries, lead is sometimes added to spices by unscrupulous retailers to make the color more intense or to simply add to its weight to gain more profit.
Recycling lead-acid batteries or other electrical waste, often transferred to poorer countries, constitutes a large problem. “In general, children in Germany have a blood lead level of less than 1 mcg/dL,” explained Dr. Böse-O’Reilly. “In some regions of Indonesia, where these recycling factories are located, more than 50% of children have levels of more than 20 mcg/dL.”
Particulate matter
According to Mr. Larsen and Mr. Sánchez-Triana, the global cost of increased lead exposure was around $6 trillion USD in 2019, which was equivalent to 6.9% of global GDP. About 77% of the cost ($4.62 trillion USD) comprised the welfare costs of cardiovascular disease mortality, and 23% ($1.38 trillion USD) comprised the present value of future income losses because of IQ loss in children.
“Our findings suggest that global lead exposure has health and economic costs on par with PM2.5 air pollution,” wrote the authors. This places lead as an environmental risk factor on par with particulate matter and above that of air pollution from solid fuels, ahead of unsafe drinking water, unhygienic sanitation, or insufficient handwashing.
“This finding is in contrast to that of GBD 2019, which ranked lead exposure as a distant fourth environmental risk factor, due to not accounting for IQ loss in children – other than idiopathic developmental intellectual disability in a small subset of children – and reporting a substantially lower estimate of adult cardiovascular disease mortality,” wrote Mr. Larsen and Mr. Sánchez-Triana.
“A central implication for future research and policy is that LMICs bear an extraordinarily large share of the health and cost burden of lead exposure,” wrote the authors. Consequently, improved quality of blood lead level measurements and identification of sources containing lead are urgently needed there.
Improved recycling methods
Dr. Böse-O’Reilly would like an increased focus on children. “If children’s cognitive skills are lost, this of course has a long-term effect on a country’s economic position,” he said. “Precisely that which LMICs actually need for their development is being stripped from them.
“We should think long and hard about whether we really need to send so much of our electrical waste and so many old cars to poorer countries, where they are incorrectly recycled,” he warned. “We should at least give the LMICs the support necessary for them to be able to process lead-containing products in the future so that less lead makes it into the environment.
“Through these global cycles, we all contribute a lot toward the worldwide lead burden,” Dr. Böse-O’Reilly said. “In my opinion, the German Supply Chain Act is therefore definitely sensible. Not only does it protect our own economy, but it also protects the health of people in other countries.”
This article was translated from Medscape’s German Edition. A version of this article appeared on Medscape.com.
FROM THE LANCET PLANETARY HEALTH
Depression tied to higher all-cause and cardiovascular mortality
In a large prospective study, a graded higher risk of all-cause mortality and mortality from cardiovascular disease (CVD) and ischemic heart disease (IHD) emerged in adults with moderate to severe depressive symptoms, compared with those with no such symptoms.
Participants with mild depressive symptoms had a 35%-49% higher risk of all-cause and CVD mortality, respectively, while for those with moderate to severe depressive symptoms, the risk of all-cause, CVD, and IHD mortality was 62%, 79%, and 121% higher, respectively.
“This information highlights the importance for clinicians to identify patients with depressive symptoms and help them engage in treatment,” lead author Zefeng Zhang, MD, PhD, of the division for heart disease and stroke prevention at the U.S. Centers for Disease Control and Prevention, Atlanta, said in an interview.
The study appears in JAMA Network Open.
A nonclassic risk factor for CVD death
This graded positive association between depressive symptoms and CVD death was observed in data from the National Health and Nutrition Examination Survey 2005-2018, which were linked with the National Death Index through 2019 for adults aged 20 and older. Data analysis occurred from March 1 to May 26, 2023. According to the authors, their analyses extend findings from previous research by assessing these associations in a large, diverse, and nationally representative sample. Using more nuanced CVD-related causes of death, depressive symptoms emerged as a nontraditional risk factor for CVD mortality.
The study
In a total cohort of 23,694, about half male, mean overall age 44.7 years, prevalences of mild and moderate to severe depression were 14.9% and 7.2%, respectively, with depressive symptoms assessed by the nine-item Patient Health Questionnaire asking about symptoms over the past 2 weeks.
Adults with depression had significantly lower CV health scores in six of the American Heart Association Life’s Essential 8 metrics for heart health. For all-cause mortality, hazard ratios were 1.35 (95% confidence interval, 1.07-1.72) for mild depressive symptoms vs. none and 1.62 (95% CI, 1.24-2.12) for moderate to severe depressive symptoms vs. none.
The corresponding hazard ratios were 1.49 (95% CI, 1.11-2.0) and 1.79 (95% CI,1.22-2.62) for CVD mortality and 0.96 (95% CI, 0.58-1.60) and 2.21 (95% CI, 1.24-3.91) for IHD death, with associations largely consistent across subgroups.
At the highest severity of depressive symptoms (almost daily for past 2 weeks), feeling tired or having little energy, poor appetite or overeating, and having little interest in doing things were significantly associated with all-cause and CVD mortality after adjusting for potential confounders.
Approximately 11%-16% of the positive associations could be explained by lifestyle factors such as excess alcohol consumption, overeating, and inactivity as per the AHA’s Life’s Essential 8 metrics.
“Taken together with the body of literature on associations between depression and CVD mortality, these findings can support public health efforts to develop a comprehensive, nationwide strategy to improve well-being, including both mental and cardiovascular health,” Dr. Zhang and associates wrote.
This research was funded by the U.S. Centers for Disease Control and Prevention. The authors had no conflicts of interest to disclose.
In a large prospective study, a graded higher risk of all-cause mortality and mortality from cardiovascular disease (CVD) and ischemic heart disease (IHD) emerged in adults with moderate to severe depressive symptoms, compared with those with no such symptoms.
Participants with mild depressive symptoms had a 35%-49% higher risk of all-cause and CVD mortality, respectively, while for those with moderate to severe depressive symptoms, the risk of all-cause, CVD, and IHD mortality was 62%, 79%, and 121% higher, respectively.
“This information highlights the importance for clinicians to identify patients with depressive symptoms and help them engage in treatment,” lead author Zefeng Zhang, MD, PhD, of the division for heart disease and stroke prevention at the U.S. Centers for Disease Control and Prevention, Atlanta, said in an interview.
The study appears in JAMA Network Open.
A nonclassic risk factor for CVD death
This graded positive association between depressive symptoms and CVD death was observed in data from the National Health and Nutrition Examination Survey 2005-2018, which were linked with the National Death Index through 2019 for adults aged 20 and older. Data analysis occurred from March 1 to May 26, 2023. According to the authors, their analyses extend findings from previous research by assessing these associations in a large, diverse, and nationally representative sample. Using more nuanced CVD-related causes of death, depressive symptoms emerged as a nontraditional risk factor for CVD mortality.
The study
In a total cohort of 23,694, about half male, mean overall age 44.7 years, prevalences of mild and moderate to severe depression were 14.9% and 7.2%, respectively, with depressive symptoms assessed by the nine-item Patient Health Questionnaire asking about symptoms over the past 2 weeks.
Adults with depression had significantly lower CV health scores in six of the American Heart Association Life’s Essential 8 metrics for heart health. For all-cause mortality, hazard ratios were 1.35 (95% confidence interval, 1.07-1.72) for mild depressive symptoms vs. none and 1.62 (95% CI, 1.24-2.12) for moderate to severe depressive symptoms vs. none.
The corresponding hazard ratios were 1.49 (95% CI, 1.11-2.0) and 1.79 (95% CI,1.22-2.62) for CVD mortality and 0.96 (95% CI, 0.58-1.60) and 2.21 (95% CI, 1.24-3.91) for IHD death, with associations largely consistent across subgroups.
At the highest severity of depressive symptoms (almost daily for past 2 weeks), feeling tired or having little energy, poor appetite or overeating, and having little interest in doing things were significantly associated with all-cause and CVD mortality after adjusting for potential confounders.
Approximately 11%-16% of the positive associations could be explained by lifestyle factors such as excess alcohol consumption, overeating, and inactivity as per the AHA’s Life’s Essential 8 metrics.
“Taken together with the body of literature on associations between depression and CVD mortality, these findings can support public health efforts to develop a comprehensive, nationwide strategy to improve well-being, including both mental and cardiovascular health,” Dr. Zhang and associates wrote.
This research was funded by the U.S. Centers for Disease Control and Prevention. The authors had no conflicts of interest to disclose.
In a large prospective study, a graded higher risk of all-cause mortality and mortality from cardiovascular disease (CVD) and ischemic heart disease (IHD) emerged in adults with moderate to severe depressive symptoms, compared with those with no such symptoms.
Participants with mild depressive symptoms had a 35%-49% higher risk of all-cause and CVD mortality, respectively, while for those with moderate to severe depressive symptoms, the risk of all-cause, CVD, and IHD mortality was 62%, 79%, and 121% higher, respectively.
“This information highlights the importance for clinicians to identify patients with depressive symptoms and help them engage in treatment,” lead author Zefeng Zhang, MD, PhD, of the division for heart disease and stroke prevention at the U.S. Centers for Disease Control and Prevention, Atlanta, said in an interview.
The study appears in JAMA Network Open.
A nonclassic risk factor for CVD death
This graded positive association between depressive symptoms and CVD death was observed in data from the National Health and Nutrition Examination Survey 2005-2018, which were linked with the National Death Index through 2019 for adults aged 20 and older. Data analysis occurred from March 1 to May 26, 2023. According to the authors, their analyses extend findings from previous research by assessing these associations in a large, diverse, and nationally representative sample. Using more nuanced CVD-related causes of death, depressive symptoms emerged as a nontraditional risk factor for CVD mortality.
The study
In a total cohort of 23,694, about half male, mean overall age 44.7 years, prevalences of mild and moderate to severe depression were 14.9% and 7.2%, respectively, with depressive symptoms assessed by the nine-item Patient Health Questionnaire asking about symptoms over the past 2 weeks.
Adults with depression had significantly lower CV health scores in six of the American Heart Association Life’s Essential 8 metrics for heart health. For all-cause mortality, hazard ratios were 1.35 (95% confidence interval, 1.07-1.72) for mild depressive symptoms vs. none and 1.62 (95% CI, 1.24-2.12) for moderate to severe depressive symptoms vs. none.
The corresponding hazard ratios were 1.49 (95% CI, 1.11-2.0) and 1.79 (95% CI,1.22-2.62) for CVD mortality and 0.96 (95% CI, 0.58-1.60) and 2.21 (95% CI, 1.24-3.91) for IHD death, with associations largely consistent across subgroups.
At the highest severity of depressive symptoms (almost daily for past 2 weeks), feeling tired or having little energy, poor appetite or overeating, and having little interest in doing things were significantly associated with all-cause and CVD mortality after adjusting for potential confounders.
Approximately 11%-16% of the positive associations could be explained by lifestyle factors such as excess alcohol consumption, overeating, and inactivity as per the AHA’s Life’s Essential 8 metrics.
“Taken together with the body of literature on associations between depression and CVD mortality, these findings can support public health efforts to develop a comprehensive, nationwide strategy to improve well-being, including both mental and cardiovascular health,” Dr. Zhang and associates wrote.
This research was funded by the U.S. Centers for Disease Control and Prevention. The authors had no conflicts of interest to disclose.
FROM JAMA NETWORK OPEN
Optimal antiplatelet regimen in ‘bi-risk’ ACS?
Among “bi-risk” patients with acute coronary syndrome (ACS) who received a stent and completed 9-12 months of dual-antiplatelet therapy (DAPT), those who de-escalated therapy to clopidogrel alone as opposed to continuing on clopidogrel and aspirin for 9 months had 25% less bleeding without increased ischemic risk.
The findings are from the OPT-BIRISK trial in more than 7,700 patients in China deemed “bi-risk” because they had both a high risk of clinically relevant bleeding and a high risk of major adverse cardiac and cerebral events (MACCE).
Yaling Han, MD, from General Hospital of Northern Theater Command in Shenyang, China, presented the trial in a hotline session at the annual congress of the European Society of Cardiology.
Dr. Han said in an interview.
She acknowledged that the findings may not be generalizable to non-Asian cohorts. Also, these patients were event-free after 9 months on DAPT, so they were relatively stable. Moreover, the finding that clopidogrel monotherapy was superior to DAPT for MACCE is only hypothesis-generating.
Renato D. Lopes, MD, PhD, Duke University, Durham, N.C., the assigned discussant at the session, congratulated the authors “for an important trial in the understudied East Asian population. The OPT-BIRISK trial adds information to the complex puzzle of antithrombotic therapy after ACS,” he said.
However, he brought up a few points that should be taken into consideration when interpreting this trial, including the ones noted by Dr. Han.
In an interview, Dr. Lopes cautioned that OPT-BIRISK tested an antiplatelet strategy “in challenging patients at increased risk for bleeding and ischemic events, but I don’t think we can say this is truly a high-risk population.” Invited to reply, Dr. Han conceded that these patients constituted a relatively low-risk subset of bi-risk patients.
Double-edged sword
“Antiplatelet therapy is a double-edged sword: it reduces ischemic risk but increases bleeding risk. Optimal antiplatelet therapy for bi-risk ACS patients remains a clinical challenge, and unsolved problem for the cardiovascular physician,” Dr. Han said in a press briefing.
The rationale and design of OPT-BIRISK were published in the American Heart Journal in 2020.
Between February 2018 and December 2020, the researchers enrolled and randomly assigned 7,758 bi-risk patients in 101 centers in China who had completed 9-12 months of DAPT (aspirin plus either clopidogrel or ticagrelor) after drug-eluting stent implantation for ACS.
The patients were randomly assigned to receive either clopidogrel plus aspirin or clopidogrel plus placebo for 9 months, followed by 3 months of aspirin.
The primary endpoint was clinically relevant Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding, at 9 months after randomization.
Key secondary endpoints were MACCE (all-cause mortality, MI, stroke, or clinically driven revascularization), individual components of MACCE, any bleeding, and stent thrombosis at 9 months after randomization.
The patient criteria for having bi-risk ACS were:
- < 65 years old with at least one high-bleeding risk criterion and at least one high-ischemia risk criterion.
- 65-78 years old with at least one high-bleeding risk criterion or at least one high-ischemia risk criterion.
- > 75 years old.
The high bleeding risk criteria were female gender, iron deficiency anemia, stroke, taking a type 2 diabetes medication, and chronic kidney disease.
The high ischemic risk criteria included troponin-positive ACS, previous stent thrombosis, previous CV events (MI, stroke, peripheral artery disease [PAD], percutaneous coronary intervention [PCI]), on a type 2 diabetes medication, chronic kidney disease, and certain lesion characteristics.
The patients had a mean age of about 65 years and 41% were female.
About half (52%) had type 2 diabetes, 18% had previous MI, and 15% had previous ischemic stroke. The ACS was mainly unstable angina (62%), followed by NSTEMI (17%) or STEMI (21%).
The patients had a mean high ischemic risk criteria of 3.2 and a mean high bleeding risk criteria of 1.4.
The initial DAPT treatment was aspirin and clopidogrel in three quarters of the patients and aspirin and ticagrelor in the remaining patients.
At 9 months, the primary endpoint of BARC type 2-5 bleeding occurred in 2.5% of patients in the clopidogrel plus placebo group and in 3.3% of patients in the clopidogrel plus aspirin group (hazard ratio, 0.75; 95% confidence interval, 0.57-0.97, P = .03).
“The bleeding results are not surprising,” Dr. Lopes said. Monotherapy vs. DAPT will cause less bleeding, Dr. Han agreed.
At 9 months, MACCE occurred in 2.6% of patients in the clopidogrel plus placebo group and in 3.5% of patients in the clopidogrel plus aspirin group (HR, 0.74; 95% CI, 0.57-0.96, P = .02).
Interpreting this latter finding as “reduced risk” of MACCE “is a stretch,” Dr. Lopes cautioned.
A potential explanation for this finding in the trial is that in the comparison group (aspirin plus clopidogrel), when patients had bleeding, they might have stopped all antiplatelet therapy, and this may have led to more ischemic events, he speculated.
“The observed reduction in MACCE is plausible,” Dr. Han said. “However, according to study protocol, we assumed that clopidogrel monotherapy would be noninferior to DAPT on the risk of MACCE. The superiority of clopidogrel alone vs. DAPT on MACCE should therefore be hypothesis-generating.”
“The increased rate of MACCE in the clopidogrel plus aspirin group was surprising,” she said in a press release from the ESC, “and may be because hemorrhagic events, which are more common with ongoing DAPT, could be associated with an adrenergic state with increased platelet aggregation due to hypotension, remedial procedures to treat bleeding, and the cessation of anti-ischemic medications.”
A low-risk subset of bi-risk patients, commonly seen in clinical practice
At the time of the index ACS, more than 60% of the patients had unstable angina, Dr. Lopes observed, “and we know these patients are lower risk.” Also, more than 1,000 of the patients did not have at least one high-risk factor for bleeding or ischemia. Moreover, these patients had not had any clinical events in the past 9-12 months on DAPT, “so they were not truly high risk when they were randomized.
“Patients aged 75 years and above are definitely bi-risk (even without any bleeding/ischemic criteria), especially post ACS, according to much literature,” Dr. Han said.
“Although patients met the bi-risk criteria for increased ischemia and bleeding at the time of index ACS and PCI, they were free from major events for at least 6 months on DAPT, thus constituting a relatively low-risk subset of bi-risk patients,” she conceded.
“Nonetheless, these patients (mean age nearly 65 years, 41% female, 52% diabetes, 18% MI history and 15% ischemic stroke history in bi-risk study) represent a large cohort seen in clinical practice,” she said. And “according to a real-world, nationwide registry from China (the OPT-CAD study), unstable angina accounted for about 50% of all ACS patients.”
There have been more data with shorter times for stopping aspirin, so it’s difficult to reconcile those studies with data from OPT-BIRISK, according to Dr. Lopes.
For example, the 2019 TWILIGHT study in patients undergoing PCI at high risk for bleeding showed that it seems to be safe to stop aspirin after 3 months and continue ticagrelor, without an increase in ischemic events.
“The question is almost in the wrong time,” he said, noting that the field is moving in the direction of stopping aspirin earlier, according to five or six recently published trials.
It is hard to generalize from an Asian population, he agreed. “In the U.S., we have other data that suggests that for high-risk patients, you can stop aspirin earlier than 9 months. That’s what most practices are doing.”
“When you look at different drugs, different doses, different duration,” Dr. Lopes summarized, “you have thousands of different permutations,” for antiplatelet therapy strategies. “Every time we have some data in large studies it adds a piece to the puzzle.”
The study was funded by the National Key Research and Development Project in China, and by a grant from Sanofi-Aventis. Dr. Han reports no relevant financial relationships. Disclosures for the other coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
Among “bi-risk” patients with acute coronary syndrome (ACS) who received a stent and completed 9-12 months of dual-antiplatelet therapy (DAPT), those who de-escalated therapy to clopidogrel alone as opposed to continuing on clopidogrel and aspirin for 9 months had 25% less bleeding without increased ischemic risk.
The findings are from the OPT-BIRISK trial in more than 7,700 patients in China deemed “bi-risk” because they had both a high risk of clinically relevant bleeding and a high risk of major adverse cardiac and cerebral events (MACCE).
Yaling Han, MD, from General Hospital of Northern Theater Command in Shenyang, China, presented the trial in a hotline session at the annual congress of the European Society of Cardiology.
Dr. Han said in an interview.
She acknowledged that the findings may not be generalizable to non-Asian cohorts. Also, these patients were event-free after 9 months on DAPT, so they were relatively stable. Moreover, the finding that clopidogrel monotherapy was superior to DAPT for MACCE is only hypothesis-generating.
Renato D. Lopes, MD, PhD, Duke University, Durham, N.C., the assigned discussant at the session, congratulated the authors “for an important trial in the understudied East Asian population. The OPT-BIRISK trial adds information to the complex puzzle of antithrombotic therapy after ACS,” he said.
However, he brought up a few points that should be taken into consideration when interpreting this trial, including the ones noted by Dr. Han.
In an interview, Dr. Lopes cautioned that OPT-BIRISK tested an antiplatelet strategy “in challenging patients at increased risk for bleeding and ischemic events, but I don’t think we can say this is truly a high-risk population.” Invited to reply, Dr. Han conceded that these patients constituted a relatively low-risk subset of bi-risk patients.
Double-edged sword
“Antiplatelet therapy is a double-edged sword: it reduces ischemic risk but increases bleeding risk. Optimal antiplatelet therapy for bi-risk ACS patients remains a clinical challenge, and unsolved problem for the cardiovascular physician,” Dr. Han said in a press briefing.
The rationale and design of OPT-BIRISK were published in the American Heart Journal in 2020.
Between February 2018 and December 2020, the researchers enrolled and randomly assigned 7,758 bi-risk patients in 101 centers in China who had completed 9-12 months of DAPT (aspirin plus either clopidogrel or ticagrelor) after drug-eluting stent implantation for ACS.
The patients were randomly assigned to receive either clopidogrel plus aspirin or clopidogrel plus placebo for 9 months, followed by 3 months of aspirin.
The primary endpoint was clinically relevant Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding, at 9 months after randomization.
Key secondary endpoints were MACCE (all-cause mortality, MI, stroke, or clinically driven revascularization), individual components of MACCE, any bleeding, and stent thrombosis at 9 months after randomization.
The patient criteria for having bi-risk ACS were:
- < 65 years old with at least one high-bleeding risk criterion and at least one high-ischemia risk criterion.
- 65-78 years old with at least one high-bleeding risk criterion or at least one high-ischemia risk criterion.
- > 75 years old.
The high bleeding risk criteria were female gender, iron deficiency anemia, stroke, taking a type 2 diabetes medication, and chronic kidney disease.
The high ischemic risk criteria included troponin-positive ACS, previous stent thrombosis, previous CV events (MI, stroke, peripheral artery disease [PAD], percutaneous coronary intervention [PCI]), on a type 2 diabetes medication, chronic kidney disease, and certain lesion characteristics.
The patients had a mean age of about 65 years and 41% were female.
About half (52%) had type 2 diabetes, 18% had previous MI, and 15% had previous ischemic stroke. The ACS was mainly unstable angina (62%), followed by NSTEMI (17%) or STEMI (21%).
The patients had a mean high ischemic risk criteria of 3.2 and a mean high bleeding risk criteria of 1.4.
The initial DAPT treatment was aspirin and clopidogrel in three quarters of the patients and aspirin and ticagrelor in the remaining patients.
At 9 months, the primary endpoint of BARC type 2-5 bleeding occurred in 2.5% of patients in the clopidogrel plus placebo group and in 3.3% of patients in the clopidogrel plus aspirin group (hazard ratio, 0.75; 95% confidence interval, 0.57-0.97, P = .03).
“The bleeding results are not surprising,” Dr. Lopes said. Monotherapy vs. DAPT will cause less bleeding, Dr. Han agreed.
At 9 months, MACCE occurred in 2.6% of patients in the clopidogrel plus placebo group and in 3.5% of patients in the clopidogrel plus aspirin group (HR, 0.74; 95% CI, 0.57-0.96, P = .02).
Interpreting this latter finding as “reduced risk” of MACCE “is a stretch,” Dr. Lopes cautioned.
A potential explanation for this finding in the trial is that in the comparison group (aspirin plus clopidogrel), when patients had bleeding, they might have stopped all antiplatelet therapy, and this may have led to more ischemic events, he speculated.
“The observed reduction in MACCE is plausible,” Dr. Han said. “However, according to study protocol, we assumed that clopidogrel monotherapy would be noninferior to DAPT on the risk of MACCE. The superiority of clopidogrel alone vs. DAPT on MACCE should therefore be hypothesis-generating.”
“The increased rate of MACCE in the clopidogrel plus aspirin group was surprising,” she said in a press release from the ESC, “and may be because hemorrhagic events, which are more common with ongoing DAPT, could be associated with an adrenergic state with increased platelet aggregation due to hypotension, remedial procedures to treat bleeding, and the cessation of anti-ischemic medications.”
A low-risk subset of bi-risk patients, commonly seen in clinical practice
At the time of the index ACS, more than 60% of the patients had unstable angina, Dr. Lopes observed, “and we know these patients are lower risk.” Also, more than 1,000 of the patients did not have at least one high-risk factor for bleeding or ischemia. Moreover, these patients had not had any clinical events in the past 9-12 months on DAPT, “so they were not truly high risk when they were randomized.
“Patients aged 75 years and above are definitely bi-risk (even without any bleeding/ischemic criteria), especially post ACS, according to much literature,” Dr. Han said.
“Although patients met the bi-risk criteria for increased ischemia and bleeding at the time of index ACS and PCI, they were free from major events for at least 6 months on DAPT, thus constituting a relatively low-risk subset of bi-risk patients,” she conceded.
“Nonetheless, these patients (mean age nearly 65 years, 41% female, 52% diabetes, 18% MI history and 15% ischemic stroke history in bi-risk study) represent a large cohort seen in clinical practice,” she said. And “according to a real-world, nationwide registry from China (the OPT-CAD study), unstable angina accounted for about 50% of all ACS patients.”
There have been more data with shorter times for stopping aspirin, so it’s difficult to reconcile those studies with data from OPT-BIRISK, according to Dr. Lopes.
For example, the 2019 TWILIGHT study in patients undergoing PCI at high risk for bleeding showed that it seems to be safe to stop aspirin after 3 months and continue ticagrelor, without an increase in ischemic events.
“The question is almost in the wrong time,” he said, noting that the field is moving in the direction of stopping aspirin earlier, according to five or six recently published trials.
It is hard to generalize from an Asian population, he agreed. “In the U.S., we have other data that suggests that for high-risk patients, you can stop aspirin earlier than 9 months. That’s what most practices are doing.”
“When you look at different drugs, different doses, different duration,” Dr. Lopes summarized, “you have thousands of different permutations,” for antiplatelet therapy strategies. “Every time we have some data in large studies it adds a piece to the puzzle.”
The study was funded by the National Key Research and Development Project in China, and by a grant from Sanofi-Aventis. Dr. Han reports no relevant financial relationships. Disclosures for the other coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
Among “bi-risk” patients with acute coronary syndrome (ACS) who received a stent and completed 9-12 months of dual-antiplatelet therapy (DAPT), those who de-escalated therapy to clopidogrel alone as opposed to continuing on clopidogrel and aspirin for 9 months had 25% less bleeding without increased ischemic risk.
The findings are from the OPT-BIRISK trial in more than 7,700 patients in China deemed “bi-risk” because they had both a high risk of clinically relevant bleeding and a high risk of major adverse cardiac and cerebral events (MACCE).
Yaling Han, MD, from General Hospital of Northern Theater Command in Shenyang, China, presented the trial in a hotline session at the annual congress of the European Society of Cardiology.
Dr. Han said in an interview.
She acknowledged that the findings may not be generalizable to non-Asian cohorts. Also, these patients were event-free after 9 months on DAPT, so they were relatively stable. Moreover, the finding that clopidogrel monotherapy was superior to DAPT for MACCE is only hypothesis-generating.
Renato D. Lopes, MD, PhD, Duke University, Durham, N.C., the assigned discussant at the session, congratulated the authors “for an important trial in the understudied East Asian population. The OPT-BIRISK trial adds information to the complex puzzle of antithrombotic therapy after ACS,” he said.
However, he brought up a few points that should be taken into consideration when interpreting this trial, including the ones noted by Dr. Han.
In an interview, Dr. Lopes cautioned that OPT-BIRISK tested an antiplatelet strategy “in challenging patients at increased risk for bleeding and ischemic events, but I don’t think we can say this is truly a high-risk population.” Invited to reply, Dr. Han conceded that these patients constituted a relatively low-risk subset of bi-risk patients.
Double-edged sword
“Antiplatelet therapy is a double-edged sword: it reduces ischemic risk but increases bleeding risk. Optimal antiplatelet therapy for bi-risk ACS patients remains a clinical challenge, and unsolved problem for the cardiovascular physician,” Dr. Han said in a press briefing.
The rationale and design of OPT-BIRISK were published in the American Heart Journal in 2020.
Between February 2018 and December 2020, the researchers enrolled and randomly assigned 7,758 bi-risk patients in 101 centers in China who had completed 9-12 months of DAPT (aspirin plus either clopidogrel or ticagrelor) after drug-eluting stent implantation for ACS.
The patients were randomly assigned to receive either clopidogrel plus aspirin or clopidogrel plus placebo for 9 months, followed by 3 months of aspirin.
The primary endpoint was clinically relevant Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding, at 9 months after randomization.
Key secondary endpoints were MACCE (all-cause mortality, MI, stroke, or clinically driven revascularization), individual components of MACCE, any bleeding, and stent thrombosis at 9 months after randomization.
The patient criteria for having bi-risk ACS were:
- < 65 years old with at least one high-bleeding risk criterion and at least one high-ischemia risk criterion.
- 65-78 years old with at least one high-bleeding risk criterion or at least one high-ischemia risk criterion.
- > 75 years old.
The high bleeding risk criteria were female gender, iron deficiency anemia, stroke, taking a type 2 diabetes medication, and chronic kidney disease.
The high ischemic risk criteria included troponin-positive ACS, previous stent thrombosis, previous CV events (MI, stroke, peripheral artery disease [PAD], percutaneous coronary intervention [PCI]), on a type 2 diabetes medication, chronic kidney disease, and certain lesion characteristics.
The patients had a mean age of about 65 years and 41% were female.
About half (52%) had type 2 diabetes, 18% had previous MI, and 15% had previous ischemic stroke. The ACS was mainly unstable angina (62%), followed by NSTEMI (17%) or STEMI (21%).
The patients had a mean high ischemic risk criteria of 3.2 and a mean high bleeding risk criteria of 1.4.
The initial DAPT treatment was aspirin and clopidogrel in three quarters of the patients and aspirin and ticagrelor in the remaining patients.
At 9 months, the primary endpoint of BARC type 2-5 bleeding occurred in 2.5% of patients in the clopidogrel plus placebo group and in 3.3% of patients in the clopidogrel plus aspirin group (hazard ratio, 0.75; 95% confidence interval, 0.57-0.97, P = .03).
“The bleeding results are not surprising,” Dr. Lopes said. Monotherapy vs. DAPT will cause less bleeding, Dr. Han agreed.
At 9 months, MACCE occurred in 2.6% of patients in the clopidogrel plus placebo group and in 3.5% of patients in the clopidogrel plus aspirin group (HR, 0.74; 95% CI, 0.57-0.96, P = .02).
Interpreting this latter finding as “reduced risk” of MACCE “is a stretch,” Dr. Lopes cautioned.
A potential explanation for this finding in the trial is that in the comparison group (aspirin plus clopidogrel), when patients had bleeding, they might have stopped all antiplatelet therapy, and this may have led to more ischemic events, he speculated.
“The observed reduction in MACCE is plausible,” Dr. Han said. “However, according to study protocol, we assumed that clopidogrel monotherapy would be noninferior to DAPT on the risk of MACCE. The superiority of clopidogrel alone vs. DAPT on MACCE should therefore be hypothesis-generating.”
“The increased rate of MACCE in the clopidogrel plus aspirin group was surprising,” she said in a press release from the ESC, “and may be because hemorrhagic events, which are more common with ongoing DAPT, could be associated with an adrenergic state with increased platelet aggregation due to hypotension, remedial procedures to treat bleeding, and the cessation of anti-ischemic medications.”
A low-risk subset of bi-risk patients, commonly seen in clinical practice
At the time of the index ACS, more than 60% of the patients had unstable angina, Dr. Lopes observed, “and we know these patients are lower risk.” Also, more than 1,000 of the patients did not have at least one high-risk factor for bleeding or ischemia. Moreover, these patients had not had any clinical events in the past 9-12 months on DAPT, “so they were not truly high risk when they were randomized.
“Patients aged 75 years and above are definitely bi-risk (even without any bleeding/ischemic criteria), especially post ACS, according to much literature,” Dr. Han said.
“Although patients met the bi-risk criteria for increased ischemia and bleeding at the time of index ACS and PCI, they were free from major events for at least 6 months on DAPT, thus constituting a relatively low-risk subset of bi-risk patients,” she conceded.
“Nonetheless, these patients (mean age nearly 65 years, 41% female, 52% diabetes, 18% MI history and 15% ischemic stroke history in bi-risk study) represent a large cohort seen in clinical practice,” she said. And “according to a real-world, nationwide registry from China (the OPT-CAD study), unstable angina accounted for about 50% of all ACS patients.”
There have been more data with shorter times for stopping aspirin, so it’s difficult to reconcile those studies with data from OPT-BIRISK, according to Dr. Lopes.
For example, the 2019 TWILIGHT study in patients undergoing PCI at high risk for bleeding showed that it seems to be safe to stop aspirin after 3 months and continue ticagrelor, without an increase in ischemic events.
“The question is almost in the wrong time,” he said, noting that the field is moving in the direction of stopping aspirin earlier, according to five or six recently published trials.
It is hard to generalize from an Asian population, he agreed. “In the U.S., we have other data that suggests that for high-risk patients, you can stop aspirin earlier than 9 months. That’s what most practices are doing.”
“When you look at different drugs, different doses, different duration,” Dr. Lopes summarized, “you have thousands of different permutations,” for antiplatelet therapy strategies. “Every time we have some data in large studies it adds a piece to the puzzle.”
The study was funded by the National Key Research and Development Project in China, and by a grant from Sanofi-Aventis. Dr. Han reports no relevant financial relationships. Disclosures for the other coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
The how and why of quad therapy in reduced-EF heart failure
It’s as if hospitals, clinicians, and the health care system itself were unprepared for such success as a powerful multiple-drug regimen emerged for hospitalized patients with heart failure with reduced ejection fraction (HFrEF).
Uptake in practice has been sluggish for the management strategy driven by a quartet of medications, each with its own mechanisms of action, started in the hospital simultaneously or in rapid succession over a few days. Key to the regimen, dosages are at least partly uptitrated in the hospital then optimized during close postdischarge follow-up.
The so-called four pillars of medical therapy for HFrEF, defined by a left ventricular ejection fraction (LVEF) of 40% or lower, include an SGLT2 inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin-system (RAS) inhibitor – preferably sacubitril-valsartan (Entresto) or, as a backup, an ACE inhibitor or angiotensin receptor blocker (ARB).
Academic consensus on the strategy is strong. The approach is consistent with heart failure (HF) guidelines on both sides of the Atlantic and is backed by solid trial evidence suggesting striking improvements in survival, readmission risk, and quality of life.
Gregg C. Fonarow, MD, University of California, Los Angeles, said in an interview.
“Yet, when we look at their actual implementation in clinical practice, we’ve seen this slow and variable uptake.”
So, why is that?
The STRONG-HF trial tested a version of the multiple-drug strategy and demonstrated what it could achieve even without a contribution from SGLT2 inhibitors, which weren’t yet indicated for HF. Eligibility for the trial, with more than 1,000 patients, wasn’t dependent on their LVEF.
Patients assigned to early and rapidly sequential initiation of a beta-blocker, an MRA, and a RAS inhibitor, compared with a standard-care control group, benefited with a 34% drop (P = .002) in risk for death or HF readmission over the next 6 months.
Few doubt – and the bulk of evidence suggests – that adding an SGLT2 inhibitor to round out the four-pillar strategy would safely boost its clinical potential in HFrEF.
The strategy’s smooth adoption in practice likely has multiple confounders that include clinical inertia, perceptions of HF medical management as a long-term outpatient process, and the onerous and Kafkaesque systems of care and reimbursement in the United States.
For example, the drug initiation and uptitration process may seem too complex for integration into slow-to-change hospital practices. And there could be a misguided sense that the regimen and follow-up must abide by the same exacting detail and standards set forth in, for example, the STRONG-HF protocol.
But starting hospitalized patients with HFrEF on the quartet of drugs and optimizing their dosages in hospital and after discharge can be simpler and more straightforward than that, Dr. Fonarow and other experts explain.
The academic community’s buy-in is a first step, but broader acceptance is frustrated by an “overwhelming culture of clinical care for heart failure” that encourages a more drawn-out process for adding medications, said Stephen J. Greene, MD, Duke Clinical Research Institute, Durham, N.C. “We need to turn our thinking on its head about heart failure in clinical practice.”
The “dramatic” underuse of the four pillars in the hospital stems in part from “outmoded” treatment algorithms that clinicians are following, Dr. Fonarow said. And they have “no sense of urgency,” sometimes wrongly believing “that it takes months for these medications to ultimately kick in.”
For hospitalized patients with HFrEF, “there is an imperative to overcome these timid algorithms and timid thinking,” he said. They should be on “full quadruple therapy” before discharge.
“And for newly diagnosed outpatients, you should essentially give yourself 7 days to get these drugs on board,” he added, either simultaneously or in “very rapid sequence.”
What’s needed is a “cultural shift” in medicine that “elevates heart failure to the same level of urgency that we have in the care of some other disease states,” agreed Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital as opportunity
The patient’s 4-7 days in the hospital typically represent a “wonderful opportunity” to initiate all four drug classes in rapid succession and start uptitrations. But most hospitals and other health care settings, Dr. Vaduganathan observed, lack the structure and systems to support the process. Broad application will require “buy-in from multiple parties – from the clinician, from the patient, their caregivers, and their partners as well as the health system.”
Physician awareness and support for the strategy, suggests at least one of these experts, is probably much less of a challenge to its broad adoption than the bewildering mechanics of health care delivery and reimbursement.
“The problem is not education. The problem is the way that our health care system is structured,” said Milton Packer, MD, Baylor Heart and Vascular Institute, Dallas.
For example, sacubitril-valsartan and the SGLT2 inhibitors are still under patent and are far more expensive than longtime generic beta-blockers and MRAs. That means physicians typically spend valuable time pursuing prior authorizations for the brand-name drugs under pressure to eventually discharge the patient because of limits on hospital reimbursement.
Clinicians in the hospital are “almost disincentivized by the system” to implement management plans that call for early and rapid initiation of multiple drugs, Dr. Vaduganathan pointed out.
One change per day
There’s no one formula for carrying out the quadruple drug strategy, Dr. Vaduganathan noted. “I make only a single change per day” to the regimen, such as uptitration or addition of a single agent. That way, tolerability can be evaluated one drug at a time, “and then the following day, I can make the next therapeutic change.”
The order in which the drugs are started mostly does not matter, in contrast to a traditional approach that might have added new drugs in the sequence of their approval for HFrEF or adoption in guidelines. Under that scenario, each successive agent might be fully uptitrated before the next could be brought on board.
Historically, Dr. Packer observed, “you would start with an ACE inhibitor, add a beta-blocker, add an MRA, switch to sacubitril-valsartan, add an SGLT2 inhibitor – and it would take 8 months.” Any prescribed sequence is pointless given the short time frame that is ideal for initiating all the drugs, he said.
Hypothetically, however, there is some rationale for starting them in an order that leverages their unique actions and side effects. For example, Dr. Vaduganathan and others observed, it may be helpful to start an SGLT2 inhibitor and sacubitril-valsartan early in the process, because they can mitigate any hyperkalemia from the subsequent addition of an MRA.
That being said, “I don’t think we have firm evidence that any particular order is more efficacious than another,” Dr. Vaduganathan said. “It’s really about getting patients on all four drugs as quickly as possible, regardless of the sequence.”
Discussions about sequencing the drugs are “a distraction for our field,” Dr. Greene said. In trials, clinical benefit from the multiple-drug regimen has emerged almost right away once the drugs were on board. “The data clearly show that initiating all four, at least at low doses, gives the best bang for your buck and would be a high-yield strategy.”
Best evidence suggests that once all four agents have been started, attention can turn to uptitration, “with the beta-blocker as the higher priority,” Dr. Greene said. “The bottom line is to keep it simple: four drugs, simultaneously or within 1 week, and prioritize initiation at low doses to maximize tolerability.”
The four-drug approach yields survival and rehospitalization benefits even when uptitrations don’t reach prespecified goals, Dr. Fonarow observed. The SGLT2 inhibitors are started and maintained at the same dosage. But for the other three agents, uptitration should aim for the highest well-tolerated level, up to the target, even if the highest tolerated is the initial dosage.
‘Challenging to generalize’
The goal in STRONG-HF was to start and at least partly uptitrate a beta-blocker, an MRA, and sacubitril-valsartan in the hospital and fully optimize their dosages within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were closely monitored at four in-person evaluations during the first 6 outpatient weeks.
But few believe the trial’s intensive drug regimen and postdischarge follow-up, as stipulated in the protocol, would be tolerated by current systems of care and reimbursement.
STRONG-HF “affirms the strategy in a rigorous, well conducted way,” Dr. Vaduganathan said, but would be “challenging to generalize to all health care systems.”
As a result, some in the field are “quick to almost disregard STRONG-HF in its entirety” and consider it “wishful thinking,” Dr. Greene said. Better that providers not become distracted by the precise details of its protocol.
At Duke, he said, “we see all our patients within 1 week of discharge to ensure they’re doing okay in terms of volume status and look for opportunities to escalate their guideline-directed medical therapy.”
But that can be done without in-person visits. A lot of the follow-up and uptitrations, Dr. Greene said, can be achieved by telephone or at virtual appointments in conjunction with regular laboratory testing. “That, I think, really is the path for the future, in this age when clinics are overwhelmed by in-person visits.”
Mildly reduced and preserved EF
STRONG-HF, in which patients were enrolled without regard to ejection fraction, suggests that its rapidly sequential drug regimen and intensive management protocol improves outcomes for patients with HF at any level of LVEF.
Those findings and others, along with DELIVER, EMPEROR-Preserved and other studies, make a tantalizing case for the quadruple drug approach in patients with HF and LVEF >40% – that is, those with mildly reduced (LVEF > 40% to < 50%, HFmrEF) or preserved LVEF > 50%, HFpEF) ejection fraction.
But the case isn’t solid enough to declare the four agents as core therapy for HF and LVEF > 40%, observed Dr. Vaduganathan. Currently, SGLT2 inhibitors “are the only drug class that we are routinely implementing” in HFmrEF and HFpEF.
There have been suggestions of clinical benefit for such patients with sacubitril-valsartan and MRAs, especially in PARAGON-HF and TOPCAT, respectively. The evidence is stronger in HFmrEF than in HFpEF, but in either case it’s weaker than the clear-cut trial support for SGLT2 inhibitors in those HF categories.
Trials also suggest that in HF with LVEF > 40%, clinical benefits from RAS inhibitors and MRAs taper off with increasing ejection fraction, especially into the > 60% range.
In both HFmrEF and HFpEF, “I routinely try to get the patient on an SGLT2 inhibitor rapidly and then treat with some of the other agents on a more individual basis,” Dr. Vaduganathan said. An LVEF in the HFmrEF range, for example, would likely call for the addition of an MRA and sacubitril-valsartan.
Dr. Packer said he would likely recommend all four agents for patients with HF and LVEF up to 60%, which he considers a more appropriate definition of HFrEF. Their clinical benefits appear consistent across that LVEF range, he said, although they thin out somewhat at the higher end.
Evidence supporting the four pillars in HF with LV > 40% and < 60% is weakest for beta-blockers, Dr. Packer noted, so arguably those drugs could be left out of the mix for patients with ejection fractions in that range.
Dr. Fonarow reported ties with Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene disclosed ties with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb, Corteria, CSL Vifor, Cytokinetics, Lexicon Merck, Novartis, Pfizer, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, and Urovant Pharmaceuticals. Dr. Vaduganathan disclosed ties with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Galmed, Impulse Dynamics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Occlutech, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health. Dr. Packer disclosed relationships with 89bio, AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Attralus, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Pharmacosmos, Reata, Regeneron, Relypsa, and Salamandra.
A version of this article first appeared on Medscape.com.
It’s as if hospitals, clinicians, and the health care system itself were unprepared for such success as a powerful multiple-drug regimen emerged for hospitalized patients with heart failure with reduced ejection fraction (HFrEF).
Uptake in practice has been sluggish for the management strategy driven by a quartet of medications, each with its own mechanisms of action, started in the hospital simultaneously or in rapid succession over a few days. Key to the regimen, dosages are at least partly uptitrated in the hospital then optimized during close postdischarge follow-up.
The so-called four pillars of medical therapy for HFrEF, defined by a left ventricular ejection fraction (LVEF) of 40% or lower, include an SGLT2 inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin-system (RAS) inhibitor – preferably sacubitril-valsartan (Entresto) or, as a backup, an ACE inhibitor or angiotensin receptor blocker (ARB).
Academic consensus on the strategy is strong. The approach is consistent with heart failure (HF) guidelines on both sides of the Atlantic and is backed by solid trial evidence suggesting striking improvements in survival, readmission risk, and quality of life.
Gregg C. Fonarow, MD, University of California, Los Angeles, said in an interview.
“Yet, when we look at their actual implementation in clinical practice, we’ve seen this slow and variable uptake.”
So, why is that?
The STRONG-HF trial tested a version of the multiple-drug strategy and demonstrated what it could achieve even without a contribution from SGLT2 inhibitors, which weren’t yet indicated for HF. Eligibility for the trial, with more than 1,000 patients, wasn’t dependent on their LVEF.
Patients assigned to early and rapidly sequential initiation of a beta-blocker, an MRA, and a RAS inhibitor, compared with a standard-care control group, benefited with a 34% drop (P = .002) in risk for death or HF readmission over the next 6 months.
Few doubt – and the bulk of evidence suggests – that adding an SGLT2 inhibitor to round out the four-pillar strategy would safely boost its clinical potential in HFrEF.
The strategy’s smooth adoption in practice likely has multiple confounders that include clinical inertia, perceptions of HF medical management as a long-term outpatient process, and the onerous and Kafkaesque systems of care and reimbursement in the United States.
For example, the drug initiation and uptitration process may seem too complex for integration into slow-to-change hospital practices. And there could be a misguided sense that the regimen and follow-up must abide by the same exacting detail and standards set forth in, for example, the STRONG-HF protocol.
But starting hospitalized patients with HFrEF on the quartet of drugs and optimizing their dosages in hospital and after discharge can be simpler and more straightforward than that, Dr. Fonarow and other experts explain.
The academic community’s buy-in is a first step, but broader acceptance is frustrated by an “overwhelming culture of clinical care for heart failure” that encourages a more drawn-out process for adding medications, said Stephen J. Greene, MD, Duke Clinical Research Institute, Durham, N.C. “We need to turn our thinking on its head about heart failure in clinical practice.”
The “dramatic” underuse of the four pillars in the hospital stems in part from “outmoded” treatment algorithms that clinicians are following, Dr. Fonarow said. And they have “no sense of urgency,” sometimes wrongly believing “that it takes months for these medications to ultimately kick in.”
For hospitalized patients with HFrEF, “there is an imperative to overcome these timid algorithms and timid thinking,” he said. They should be on “full quadruple therapy” before discharge.
“And for newly diagnosed outpatients, you should essentially give yourself 7 days to get these drugs on board,” he added, either simultaneously or in “very rapid sequence.”
What’s needed is a “cultural shift” in medicine that “elevates heart failure to the same level of urgency that we have in the care of some other disease states,” agreed Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital as opportunity
The patient’s 4-7 days in the hospital typically represent a “wonderful opportunity” to initiate all four drug classes in rapid succession and start uptitrations. But most hospitals and other health care settings, Dr. Vaduganathan observed, lack the structure and systems to support the process. Broad application will require “buy-in from multiple parties – from the clinician, from the patient, their caregivers, and their partners as well as the health system.”
Physician awareness and support for the strategy, suggests at least one of these experts, is probably much less of a challenge to its broad adoption than the bewildering mechanics of health care delivery and reimbursement.
“The problem is not education. The problem is the way that our health care system is structured,” said Milton Packer, MD, Baylor Heart and Vascular Institute, Dallas.
For example, sacubitril-valsartan and the SGLT2 inhibitors are still under patent and are far more expensive than longtime generic beta-blockers and MRAs. That means physicians typically spend valuable time pursuing prior authorizations for the brand-name drugs under pressure to eventually discharge the patient because of limits on hospital reimbursement.
Clinicians in the hospital are “almost disincentivized by the system” to implement management plans that call for early and rapid initiation of multiple drugs, Dr. Vaduganathan pointed out.
One change per day
There’s no one formula for carrying out the quadruple drug strategy, Dr. Vaduganathan noted. “I make only a single change per day” to the regimen, such as uptitration or addition of a single agent. That way, tolerability can be evaluated one drug at a time, “and then the following day, I can make the next therapeutic change.”
The order in which the drugs are started mostly does not matter, in contrast to a traditional approach that might have added new drugs in the sequence of their approval for HFrEF or adoption in guidelines. Under that scenario, each successive agent might be fully uptitrated before the next could be brought on board.
Historically, Dr. Packer observed, “you would start with an ACE inhibitor, add a beta-blocker, add an MRA, switch to sacubitril-valsartan, add an SGLT2 inhibitor – and it would take 8 months.” Any prescribed sequence is pointless given the short time frame that is ideal for initiating all the drugs, he said.
Hypothetically, however, there is some rationale for starting them in an order that leverages their unique actions and side effects. For example, Dr. Vaduganathan and others observed, it may be helpful to start an SGLT2 inhibitor and sacubitril-valsartan early in the process, because they can mitigate any hyperkalemia from the subsequent addition of an MRA.
That being said, “I don’t think we have firm evidence that any particular order is more efficacious than another,” Dr. Vaduganathan said. “It’s really about getting patients on all four drugs as quickly as possible, regardless of the sequence.”
Discussions about sequencing the drugs are “a distraction for our field,” Dr. Greene said. In trials, clinical benefit from the multiple-drug regimen has emerged almost right away once the drugs were on board. “The data clearly show that initiating all four, at least at low doses, gives the best bang for your buck and would be a high-yield strategy.”
Best evidence suggests that once all four agents have been started, attention can turn to uptitration, “with the beta-blocker as the higher priority,” Dr. Greene said. “The bottom line is to keep it simple: four drugs, simultaneously or within 1 week, and prioritize initiation at low doses to maximize tolerability.”
The four-drug approach yields survival and rehospitalization benefits even when uptitrations don’t reach prespecified goals, Dr. Fonarow observed. The SGLT2 inhibitors are started and maintained at the same dosage. But for the other three agents, uptitration should aim for the highest well-tolerated level, up to the target, even if the highest tolerated is the initial dosage.
‘Challenging to generalize’
The goal in STRONG-HF was to start and at least partly uptitrate a beta-blocker, an MRA, and sacubitril-valsartan in the hospital and fully optimize their dosages within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were closely monitored at four in-person evaluations during the first 6 outpatient weeks.
But few believe the trial’s intensive drug regimen and postdischarge follow-up, as stipulated in the protocol, would be tolerated by current systems of care and reimbursement.
STRONG-HF “affirms the strategy in a rigorous, well conducted way,” Dr. Vaduganathan said, but would be “challenging to generalize to all health care systems.”
As a result, some in the field are “quick to almost disregard STRONG-HF in its entirety” and consider it “wishful thinking,” Dr. Greene said. Better that providers not become distracted by the precise details of its protocol.
At Duke, he said, “we see all our patients within 1 week of discharge to ensure they’re doing okay in terms of volume status and look for opportunities to escalate their guideline-directed medical therapy.”
But that can be done without in-person visits. A lot of the follow-up and uptitrations, Dr. Greene said, can be achieved by telephone or at virtual appointments in conjunction with regular laboratory testing. “That, I think, really is the path for the future, in this age when clinics are overwhelmed by in-person visits.”
Mildly reduced and preserved EF
STRONG-HF, in which patients were enrolled without regard to ejection fraction, suggests that its rapidly sequential drug regimen and intensive management protocol improves outcomes for patients with HF at any level of LVEF.
Those findings and others, along with DELIVER, EMPEROR-Preserved and other studies, make a tantalizing case for the quadruple drug approach in patients with HF and LVEF >40% – that is, those with mildly reduced (LVEF > 40% to < 50%, HFmrEF) or preserved LVEF > 50%, HFpEF) ejection fraction.
But the case isn’t solid enough to declare the four agents as core therapy for HF and LVEF > 40%, observed Dr. Vaduganathan. Currently, SGLT2 inhibitors “are the only drug class that we are routinely implementing” in HFmrEF and HFpEF.
There have been suggestions of clinical benefit for such patients with sacubitril-valsartan and MRAs, especially in PARAGON-HF and TOPCAT, respectively. The evidence is stronger in HFmrEF than in HFpEF, but in either case it’s weaker than the clear-cut trial support for SGLT2 inhibitors in those HF categories.
Trials also suggest that in HF with LVEF > 40%, clinical benefits from RAS inhibitors and MRAs taper off with increasing ejection fraction, especially into the > 60% range.
In both HFmrEF and HFpEF, “I routinely try to get the patient on an SGLT2 inhibitor rapidly and then treat with some of the other agents on a more individual basis,” Dr. Vaduganathan said. An LVEF in the HFmrEF range, for example, would likely call for the addition of an MRA and sacubitril-valsartan.
Dr. Packer said he would likely recommend all four agents for patients with HF and LVEF up to 60%, which he considers a more appropriate definition of HFrEF. Their clinical benefits appear consistent across that LVEF range, he said, although they thin out somewhat at the higher end.
Evidence supporting the four pillars in HF with LV > 40% and < 60% is weakest for beta-blockers, Dr. Packer noted, so arguably those drugs could be left out of the mix for patients with ejection fractions in that range.
Dr. Fonarow reported ties with Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene disclosed ties with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb, Corteria, CSL Vifor, Cytokinetics, Lexicon Merck, Novartis, Pfizer, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, and Urovant Pharmaceuticals. Dr. Vaduganathan disclosed ties with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Galmed, Impulse Dynamics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Occlutech, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health. Dr. Packer disclosed relationships with 89bio, AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Attralus, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Pharmacosmos, Reata, Regeneron, Relypsa, and Salamandra.
A version of this article first appeared on Medscape.com.
It’s as if hospitals, clinicians, and the health care system itself were unprepared for such success as a powerful multiple-drug regimen emerged for hospitalized patients with heart failure with reduced ejection fraction (HFrEF).
Uptake in practice has been sluggish for the management strategy driven by a quartet of medications, each with its own mechanisms of action, started in the hospital simultaneously or in rapid succession over a few days. Key to the regimen, dosages are at least partly uptitrated in the hospital then optimized during close postdischarge follow-up.
The so-called four pillars of medical therapy for HFrEF, defined by a left ventricular ejection fraction (LVEF) of 40% or lower, include an SGLT2 inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin-system (RAS) inhibitor – preferably sacubitril-valsartan (Entresto) or, as a backup, an ACE inhibitor or angiotensin receptor blocker (ARB).
Academic consensus on the strategy is strong. The approach is consistent with heart failure (HF) guidelines on both sides of the Atlantic and is backed by solid trial evidence suggesting striking improvements in survival, readmission risk, and quality of life.
Gregg C. Fonarow, MD, University of California, Los Angeles, said in an interview.
“Yet, when we look at their actual implementation in clinical practice, we’ve seen this slow and variable uptake.”
So, why is that?
The STRONG-HF trial tested a version of the multiple-drug strategy and demonstrated what it could achieve even without a contribution from SGLT2 inhibitors, which weren’t yet indicated for HF. Eligibility for the trial, with more than 1,000 patients, wasn’t dependent on their LVEF.
Patients assigned to early and rapidly sequential initiation of a beta-blocker, an MRA, and a RAS inhibitor, compared with a standard-care control group, benefited with a 34% drop (P = .002) in risk for death or HF readmission over the next 6 months.
Few doubt – and the bulk of evidence suggests – that adding an SGLT2 inhibitor to round out the four-pillar strategy would safely boost its clinical potential in HFrEF.
The strategy’s smooth adoption in practice likely has multiple confounders that include clinical inertia, perceptions of HF medical management as a long-term outpatient process, and the onerous and Kafkaesque systems of care and reimbursement in the United States.
For example, the drug initiation and uptitration process may seem too complex for integration into slow-to-change hospital practices. And there could be a misguided sense that the regimen and follow-up must abide by the same exacting detail and standards set forth in, for example, the STRONG-HF protocol.
But starting hospitalized patients with HFrEF on the quartet of drugs and optimizing their dosages in hospital and after discharge can be simpler and more straightforward than that, Dr. Fonarow and other experts explain.
The academic community’s buy-in is a first step, but broader acceptance is frustrated by an “overwhelming culture of clinical care for heart failure” that encourages a more drawn-out process for adding medications, said Stephen J. Greene, MD, Duke Clinical Research Institute, Durham, N.C. “We need to turn our thinking on its head about heart failure in clinical practice.”
The “dramatic” underuse of the four pillars in the hospital stems in part from “outmoded” treatment algorithms that clinicians are following, Dr. Fonarow said. And they have “no sense of urgency,” sometimes wrongly believing “that it takes months for these medications to ultimately kick in.”
For hospitalized patients with HFrEF, “there is an imperative to overcome these timid algorithms and timid thinking,” he said. They should be on “full quadruple therapy” before discharge.
“And for newly diagnosed outpatients, you should essentially give yourself 7 days to get these drugs on board,” he added, either simultaneously or in “very rapid sequence.”
What’s needed is a “cultural shift” in medicine that “elevates heart failure to the same level of urgency that we have in the care of some other disease states,” agreed Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital as opportunity
The patient’s 4-7 days in the hospital typically represent a “wonderful opportunity” to initiate all four drug classes in rapid succession and start uptitrations. But most hospitals and other health care settings, Dr. Vaduganathan observed, lack the structure and systems to support the process. Broad application will require “buy-in from multiple parties – from the clinician, from the patient, their caregivers, and their partners as well as the health system.”
Physician awareness and support for the strategy, suggests at least one of these experts, is probably much less of a challenge to its broad adoption than the bewildering mechanics of health care delivery and reimbursement.
“The problem is not education. The problem is the way that our health care system is structured,” said Milton Packer, MD, Baylor Heart and Vascular Institute, Dallas.
For example, sacubitril-valsartan and the SGLT2 inhibitors are still under patent and are far more expensive than longtime generic beta-blockers and MRAs. That means physicians typically spend valuable time pursuing prior authorizations for the brand-name drugs under pressure to eventually discharge the patient because of limits on hospital reimbursement.
Clinicians in the hospital are “almost disincentivized by the system” to implement management plans that call for early and rapid initiation of multiple drugs, Dr. Vaduganathan pointed out.
One change per day
There’s no one formula for carrying out the quadruple drug strategy, Dr. Vaduganathan noted. “I make only a single change per day” to the regimen, such as uptitration or addition of a single agent. That way, tolerability can be evaluated one drug at a time, “and then the following day, I can make the next therapeutic change.”
The order in which the drugs are started mostly does not matter, in contrast to a traditional approach that might have added new drugs in the sequence of their approval for HFrEF or adoption in guidelines. Under that scenario, each successive agent might be fully uptitrated before the next could be brought on board.
Historically, Dr. Packer observed, “you would start with an ACE inhibitor, add a beta-blocker, add an MRA, switch to sacubitril-valsartan, add an SGLT2 inhibitor – and it would take 8 months.” Any prescribed sequence is pointless given the short time frame that is ideal for initiating all the drugs, he said.
Hypothetically, however, there is some rationale for starting them in an order that leverages their unique actions and side effects. For example, Dr. Vaduganathan and others observed, it may be helpful to start an SGLT2 inhibitor and sacubitril-valsartan early in the process, because they can mitigate any hyperkalemia from the subsequent addition of an MRA.
That being said, “I don’t think we have firm evidence that any particular order is more efficacious than another,” Dr. Vaduganathan said. “It’s really about getting patients on all four drugs as quickly as possible, regardless of the sequence.”
Discussions about sequencing the drugs are “a distraction for our field,” Dr. Greene said. In trials, clinical benefit from the multiple-drug regimen has emerged almost right away once the drugs were on board. “The data clearly show that initiating all four, at least at low doses, gives the best bang for your buck and would be a high-yield strategy.”
Best evidence suggests that once all four agents have been started, attention can turn to uptitration, “with the beta-blocker as the higher priority,” Dr. Greene said. “The bottom line is to keep it simple: four drugs, simultaneously or within 1 week, and prioritize initiation at low doses to maximize tolerability.”
The four-drug approach yields survival and rehospitalization benefits even when uptitrations don’t reach prespecified goals, Dr. Fonarow observed. The SGLT2 inhibitors are started and maintained at the same dosage. But for the other three agents, uptitration should aim for the highest well-tolerated level, up to the target, even if the highest tolerated is the initial dosage.
‘Challenging to generalize’
The goal in STRONG-HF was to start and at least partly uptitrate a beta-blocker, an MRA, and sacubitril-valsartan in the hospital and fully optimize their dosages within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were closely monitored at four in-person evaluations during the first 6 outpatient weeks.
But few believe the trial’s intensive drug regimen and postdischarge follow-up, as stipulated in the protocol, would be tolerated by current systems of care and reimbursement.
STRONG-HF “affirms the strategy in a rigorous, well conducted way,” Dr. Vaduganathan said, but would be “challenging to generalize to all health care systems.”
As a result, some in the field are “quick to almost disregard STRONG-HF in its entirety” and consider it “wishful thinking,” Dr. Greene said. Better that providers not become distracted by the precise details of its protocol.
At Duke, he said, “we see all our patients within 1 week of discharge to ensure they’re doing okay in terms of volume status and look for opportunities to escalate their guideline-directed medical therapy.”
But that can be done without in-person visits. A lot of the follow-up and uptitrations, Dr. Greene said, can be achieved by telephone or at virtual appointments in conjunction with regular laboratory testing. “That, I think, really is the path for the future, in this age when clinics are overwhelmed by in-person visits.”
Mildly reduced and preserved EF
STRONG-HF, in which patients were enrolled without regard to ejection fraction, suggests that its rapidly sequential drug regimen and intensive management protocol improves outcomes for patients with HF at any level of LVEF.
Those findings and others, along with DELIVER, EMPEROR-Preserved and other studies, make a tantalizing case for the quadruple drug approach in patients with HF and LVEF >40% – that is, those with mildly reduced (LVEF > 40% to < 50%, HFmrEF) or preserved LVEF > 50%, HFpEF) ejection fraction.
But the case isn’t solid enough to declare the four agents as core therapy for HF and LVEF > 40%, observed Dr. Vaduganathan. Currently, SGLT2 inhibitors “are the only drug class that we are routinely implementing” in HFmrEF and HFpEF.
There have been suggestions of clinical benefit for such patients with sacubitril-valsartan and MRAs, especially in PARAGON-HF and TOPCAT, respectively. The evidence is stronger in HFmrEF than in HFpEF, but in either case it’s weaker than the clear-cut trial support for SGLT2 inhibitors in those HF categories.
Trials also suggest that in HF with LVEF > 40%, clinical benefits from RAS inhibitors and MRAs taper off with increasing ejection fraction, especially into the > 60% range.
In both HFmrEF and HFpEF, “I routinely try to get the patient on an SGLT2 inhibitor rapidly and then treat with some of the other agents on a more individual basis,” Dr. Vaduganathan said. An LVEF in the HFmrEF range, for example, would likely call for the addition of an MRA and sacubitril-valsartan.
Dr. Packer said he would likely recommend all four agents for patients with HF and LVEF up to 60%, which he considers a more appropriate definition of HFrEF. Their clinical benefits appear consistent across that LVEF range, he said, although they thin out somewhat at the higher end.
Evidence supporting the four pillars in HF with LV > 40% and < 60% is weakest for beta-blockers, Dr. Packer noted, so arguably those drugs could be left out of the mix for patients with ejection fractions in that range.
Dr. Fonarow reported ties with Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene disclosed ties with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb, Corteria, CSL Vifor, Cytokinetics, Lexicon Merck, Novartis, Pfizer, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, and Urovant Pharmaceuticals. Dr. Vaduganathan disclosed ties with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Galmed, Impulse Dynamics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Occlutech, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health. Dr. Packer disclosed relationships with 89bio, AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Attralus, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Pharmacosmos, Reata, Regeneron, Relypsa, and Salamandra.
A version of this article first appeared on Medscape.com.
History of heart transplant tied to worse pregnancy outcome
TOPLINE:
than do other pregnant women, results of a large study with a nationwide sample suggest.
METHODOLOGY:
- The retrospective cohort study included 2010-2020 information from the Nationwide Readmissions Database (NRD), a large, all-payer administrative dataset that allows for tracking of patient hospital readmissions in the same U.S. state within the same calendar year and includes patient demographics, hospital characteristics, diagnosis and procedure codes (including for cardiac transplants), length of stay, and discharge disposition.
- The primary outcome was nontransfusion SMM which, among other conditions, included acute myocardial infarction, aortic aneurysm, acute renal failure, adult respiratory distress syndrome, amniotic fluid embolism, cardiac arrest/ventricular fibrillation, and heart failure/arrest, during the delivery hospitalization.
- Additional outcomes included rates of all SMMs (including transfusion), a composite cardiovascular SMM (cSMM) outcome that included acute myocardial infarction, aortic aneurysm, cardiac arrest/ventricular fibrillation, cardioversion, and acute heart failure, preterm birth, and readmission rates.
TAKEAWAY:
- From 2010 to 2020, there were 19,399,521 hospital deliveries, of which, 105 were in HT recipients.
- In unadjusted comparisons, rates of all outcomes were higher in HT, compared with non-HT delivery hospitalizations, and after adjusting for age, demographic and facility characteristics, comorbid conditions, and calendar year, HT recipients continued to have higher odds of adverse maternal outcomes. For example, HT recipients had higher rates of nontransfusion SMM (adjusted odds ratio, 28.12; 95% confidence interval, 15.65-50.53), all SMM (aOR, 15.73; 95% CI, 9.17-27.00), cSMM (aOR, 37.7; 95% CI, 17.39-82.01), and preterm birth (aOR, 7.15; 95%, CI 4.75-10.77).
- HT recipients also had longer hospital stays and higher rates of cesarean delivery, although the authors noted that it’s unclear whether this increase was caused by the HT or complications of pregnancy because data were unavailable regarding indication for cesareans.
- Patients with HT were also at increased risk for hospital readmission within the first year after delivery, particularly within the first 6 months, including for HT-related complications, a finding that supports guidelines recommending an initial postpartum visit within 7-14 days of discharge for patients with cardiac conditions, write the authors.
IN PRACTICE:
The findings demonstrate the importance of counseling HT patients at early gestational ages “to provide information about anticipated risks in pregnancy and the postpartum period to allow patients the opportunity to make informed choices regarding their reproductive options,” the authors conclude.
SOURCE:
The study was conducted by Amanda M. Craig, MD, division of maternal fetal medicine, department of obstetrics and gynecology, Duke University Medical Center, Durham, N.C., and colleagues. It was published online in JACC Heart Failure.
LIMITATIONS:
Relying on diagnosis and procedure codes in administrative datasets like NRD may result in underestimation of outcomes. In this study, outcomes were limited to delivery hospitalizations, which may underestimate the true incidence of complications or fail to include pregnancies that didn’t end in a delivery, including pregnancy terminations or spontaneous abortions. Information related to race, ethnicity, hospital regions, and cause of death are not captured in the NRD dataset.
DISCLOSURES:
The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
than do other pregnant women, results of a large study with a nationwide sample suggest.
METHODOLOGY:
- The retrospective cohort study included 2010-2020 information from the Nationwide Readmissions Database (NRD), a large, all-payer administrative dataset that allows for tracking of patient hospital readmissions in the same U.S. state within the same calendar year and includes patient demographics, hospital characteristics, diagnosis and procedure codes (including for cardiac transplants), length of stay, and discharge disposition.
- The primary outcome was nontransfusion SMM which, among other conditions, included acute myocardial infarction, aortic aneurysm, acute renal failure, adult respiratory distress syndrome, amniotic fluid embolism, cardiac arrest/ventricular fibrillation, and heart failure/arrest, during the delivery hospitalization.
- Additional outcomes included rates of all SMMs (including transfusion), a composite cardiovascular SMM (cSMM) outcome that included acute myocardial infarction, aortic aneurysm, cardiac arrest/ventricular fibrillation, cardioversion, and acute heart failure, preterm birth, and readmission rates.
TAKEAWAY:
- From 2010 to 2020, there were 19,399,521 hospital deliveries, of which, 105 were in HT recipients.
- In unadjusted comparisons, rates of all outcomes were higher in HT, compared with non-HT delivery hospitalizations, and after adjusting for age, demographic and facility characteristics, comorbid conditions, and calendar year, HT recipients continued to have higher odds of adverse maternal outcomes. For example, HT recipients had higher rates of nontransfusion SMM (adjusted odds ratio, 28.12; 95% confidence interval, 15.65-50.53), all SMM (aOR, 15.73; 95% CI, 9.17-27.00), cSMM (aOR, 37.7; 95% CI, 17.39-82.01), and preterm birth (aOR, 7.15; 95%, CI 4.75-10.77).
- HT recipients also had longer hospital stays and higher rates of cesarean delivery, although the authors noted that it’s unclear whether this increase was caused by the HT or complications of pregnancy because data were unavailable regarding indication for cesareans.
- Patients with HT were also at increased risk for hospital readmission within the first year after delivery, particularly within the first 6 months, including for HT-related complications, a finding that supports guidelines recommending an initial postpartum visit within 7-14 days of discharge for patients with cardiac conditions, write the authors.
IN PRACTICE:
The findings demonstrate the importance of counseling HT patients at early gestational ages “to provide information about anticipated risks in pregnancy and the postpartum period to allow patients the opportunity to make informed choices regarding their reproductive options,” the authors conclude.
SOURCE:
The study was conducted by Amanda M. Craig, MD, division of maternal fetal medicine, department of obstetrics and gynecology, Duke University Medical Center, Durham, N.C., and colleagues. It was published online in JACC Heart Failure.
LIMITATIONS:
Relying on diagnosis and procedure codes in administrative datasets like NRD may result in underestimation of outcomes. In this study, outcomes were limited to delivery hospitalizations, which may underestimate the true incidence of complications or fail to include pregnancies that didn’t end in a delivery, including pregnancy terminations or spontaneous abortions. Information related to race, ethnicity, hospital regions, and cause of death are not captured in the NRD dataset.
DISCLOSURES:
The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
than do other pregnant women, results of a large study with a nationwide sample suggest.
METHODOLOGY:
- The retrospective cohort study included 2010-2020 information from the Nationwide Readmissions Database (NRD), a large, all-payer administrative dataset that allows for tracking of patient hospital readmissions in the same U.S. state within the same calendar year and includes patient demographics, hospital characteristics, diagnosis and procedure codes (including for cardiac transplants), length of stay, and discharge disposition.
- The primary outcome was nontransfusion SMM which, among other conditions, included acute myocardial infarction, aortic aneurysm, acute renal failure, adult respiratory distress syndrome, amniotic fluid embolism, cardiac arrest/ventricular fibrillation, and heart failure/arrest, during the delivery hospitalization.
- Additional outcomes included rates of all SMMs (including transfusion), a composite cardiovascular SMM (cSMM) outcome that included acute myocardial infarction, aortic aneurysm, cardiac arrest/ventricular fibrillation, cardioversion, and acute heart failure, preterm birth, and readmission rates.
TAKEAWAY:
- From 2010 to 2020, there were 19,399,521 hospital deliveries, of which, 105 were in HT recipients.
- In unadjusted comparisons, rates of all outcomes were higher in HT, compared with non-HT delivery hospitalizations, and after adjusting for age, demographic and facility characteristics, comorbid conditions, and calendar year, HT recipients continued to have higher odds of adverse maternal outcomes. For example, HT recipients had higher rates of nontransfusion SMM (adjusted odds ratio, 28.12; 95% confidence interval, 15.65-50.53), all SMM (aOR, 15.73; 95% CI, 9.17-27.00), cSMM (aOR, 37.7; 95% CI, 17.39-82.01), and preterm birth (aOR, 7.15; 95%, CI 4.75-10.77).
- HT recipients also had longer hospital stays and higher rates of cesarean delivery, although the authors noted that it’s unclear whether this increase was caused by the HT or complications of pregnancy because data were unavailable regarding indication for cesareans.
- Patients with HT were also at increased risk for hospital readmission within the first year after delivery, particularly within the first 6 months, including for HT-related complications, a finding that supports guidelines recommending an initial postpartum visit within 7-14 days of discharge for patients with cardiac conditions, write the authors.
IN PRACTICE:
The findings demonstrate the importance of counseling HT patients at early gestational ages “to provide information about anticipated risks in pregnancy and the postpartum period to allow patients the opportunity to make informed choices regarding their reproductive options,” the authors conclude.
SOURCE:
The study was conducted by Amanda M. Craig, MD, division of maternal fetal medicine, department of obstetrics and gynecology, Duke University Medical Center, Durham, N.C., and colleagues. It was published online in JACC Heart Failure.
LIMITATIONS:
Relying on diagnosis and procedure codes in administrative datasets like NRD may result in underestimation of outcomes. In this study, outcomes were limited to delivery hospitalizations, which may underestimate the true incidence of complications or fail to include pregnancies that didn’t end in a delivery, including pregnancy terminations or spontaneous abortions. Information related to race, ethnicity, hospital regions, and cause of death are not captured in the NRD dataset.
DISCLOSURES:
The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Training more doctors should be our first priority, says ethicist
This transcript has been edited for clarity.
Recently, the Supreme Court of the United States struck down the use of affirmative action in admissions to colleges, universities, medical schools, and nursing schools. This has led to an enormous amount of worry and concern, particularly in medical school admissions in the world I’m in, where people start to say that diversity matters. Diversity is important.
I know many deans of medical schools immediately sent out messages of reassurance to their students, saying New York University or Stanford or Harvard or Minnesota or Case Western is still deeply concerned about diversity, and we’re going to do what we can to preserve attention to diversity.
I’ve served on admissions at a number of schools over the years for med school. I understand – and have been told – that diversity is important, and according to the Supreme Court, not explicitly by race. There are obviously many variables to take into account when trying to keep diversity at the forefront of admissions.
At the schools I’ve been at, including Columbia, NYU, University of Pittsburgh, University of Minnesota, and University of Pennsylvania, there are plenty of qualified students. Happily, we’ve always been engaged in some effort to try and whittle down the class to the size that we can manage and accept, and many qualified students don’t get admitted.
The first order of business for me is not to worry about how to maintain diversity. It’s to recognize that we need more doctors, nurses, and mental health care providers. I will, in a second, say a few words about diversity and where it fits into admissions, but I want to make the point clearly that what we should be doing is trying to expand the pool of students who are going to become doctors, nurses, mental health care providers, and social workers.
There are too many early retirements. We don’t have the person power we need to manage the health care challenges of an aging population. Let’s not get lost in arguing about what characteristics ought to get you into the finest medical schools. Let’s realize that we have to expand the number of schools we have.
We better be working pretty hard to expand our physician assistant programs, to make sure that we give full authority to qualified dentists and nurses who can help deliver some clinical care. We need more folks. That’s really where the battle ought to be: How do we get that done and how do we get it done quickly, not arguing about who’s in, who’s out, and why.
That said, diversity to me has never meant just race. I’m always interested in gender orientation, disability, and geographic input. Sometimes in decisions that you’re looking at, when I have students in front of me, they tell me they play a musical instrument or about the obstacles they had to overcome to get to medical school. Some of them will say they were involved in 4-H and did rodeo in high school or junior high school, which makes them a diverse potential student with characteristics that maybe some others don’t bring.
I’m not against diversity. I think having a rich set of experiences in any class – medicine, nursing, whatever it’s going to be – is beneficial to the students. They learn from each other. It is sometimes said that it’s also good for patients. I’m a little less excited about that, because I think our training goal should be to make every medical student and nursing student qualified to treat anybody.
I don’t think that, just because you’re Latinx or gay, that’s going to make a gay patient feel better. I think we should teach our students how to give care to everybody that they encounter. They shouldn’t have to match up characteristics to feel like they’re going to get quality care. That isn’t the right reason.
When you have a diverse set of providers, they can call that out and be on the alert for it, and that’s very important.
I also believe that we should think widely and broadly about diversity. Maybe race is out, but certainly other experiences related to income, background, struggle that got you to the point where you’re applying to medical school, motivation, the kinds of experiences you might have had caring for an elderly person, dealing with a disability or learning disability, and trying to overcome, let’s say, going to school in a poor area with not such a wonderful school, really help in terms of forming professionalism, empathy, and a caring point of view.
To me, the main goal is to expand our workforce. The secondary goal is to stay diverse, because we get better providers when we do so.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, the Supreme Court of the United States struck down the use of affirmative action in admissions to colleges, universities, medical schools, and nursing schools. This has led to an enormous amount of worry and concern, particularly in medical school admissions in the world I’m in, where people start to say that diversity matters. Diversity is important.
I know many deans of medical schools immediately sent out messages of reassurance to their students, saying New York University or Stanford or Harvard or Minnesota or Case Western is still deeply concerned about diversity, and we’re going to do what we can to preserve attention to diversity.
I’ve served on admissions at a number of schools over the years for med school. I understand – and have been told – that diversity is important, and according to the Supreme Court, not explicitly by race. There are obviously many variables to take into account when trying to keep diversity at the forefront of admissions.
At the schools I’ve been at, including Columbia, NYU, University of Pittsburgh, University of Minnesota, and University of Pennsylvania, there are plenty of qualified students. Happily, we’ve always been engaged in some effort to try and whittle down the class to the size that we can manage and accept, and many qualified students don’t get admitted.
The first order of business for me is not to worry about how to maintain diversity. It’s to recognize that we need more doctors, nurses, and mental health care providers. I will, in a second, say a few words about diversity and where it fits into admissions, but I want to make the point clearly that what we should be doing is trying to expand the pool of students who are going to become doctors, nurses, mental health care providers, and social workers.
There are too many early retirements. We don’t have the person power we need to manage the health care challenges of an aging population. Let’s not get lost in arguing about what characteristics ought to get you into the finest medical schools. Let’s realize that we have to expand the number of schools we have.
We better be working pretty hard to expand our physician assistant programs, to make sure that we give full authority to qualified dentists and nurses who can help deliver some clinical care. We need more folks. That’s really where the battle ought to be: How do we get that done and how do we get it done quickly, not arguing about who’s in, who’s out, and why.
That said, diversity to me has never meant just race. I’m always interested in gender orientation, disability, and geographic input. Sometimes in decisions that you’re looking at, when I have students in front of me, they tell me they play a musical instrument or about the obstacles they had to overcome to get to medical school. Some of them will say they were involved in 4-H and did rodeo in high school or junior high school, which makes them a diverse potential student with characteristics that maybe some others don’t bring.
I’m not against diversity. I think having a rich set of experiences in any class – medicine, nursing, whatever it’s going to be – is beneficial to the students. They learn from each other. It is sometimes said that it’s also good for patients. I’m a little less excited about that, because I think our training goal should be to make every medical student and nursing student qualified to treat anybody.
I don’t think that, just because you’re Latinx or gay, that’s going to make a gay patient feel better. I think we should teach our students how to give care to everybody that they encounter. They shouldn’t have to match up characteristics to feel like they’re going to get quality care. That isn’t the right reason.
When you have a diverse set of providers, they can call that out and be on the alert for it, and that’s very important.
I also believe that we should think widely and broadly about diversity. Maybe race is out, but certainly other experiences related to income, background, struggle that got you to the point where you’re applying to medical school, motivation, the kinds of experiences you might have had caring for an elderly person, dealing with a disability or learning disability, and trying to overcome, let’s say, going to school in a poor area with not such a wonderful school, really help in terms of forming professionalism, empathy, and a caring point of view.
To me, the main goal is to expand our workforce. The secondary goal is to stay diverse, because we get better providers when we do so.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, the Supreme Court of the United States struck down the use of affirmative action in admissions to colleges, universities, medical schools, and nursing schools. This has led to an enormous amount of worry and concern, particularly in medical school admissions in the world I’m in, where people start to say that diversity matters. Diversity is important.
I know many deans of medical schools immediately sent out messages of reassurance to their students, saying New York University or Stanford or Harvard or Minnesota or Case Western is still deeply concerned about diversity, and we’re going to do what we can to preserve attention to diversity.
I’ve served on admissions at a number of schools over the years for med school. I understand – and have been told – that diversity is important, and according to the Supreme Court, not explicitly by race. There are obviously many variables to take into account when trying to keep diversity at the forefront of admissions.
At the schools I’ve been at, including Columbia, NYU, University of Pittsburgh, University of Minnesota, and University of Pennsylvania, there are plenty of qualified students. Happily, we’ve always been engaged in some effort to try and whittle down the class to the size that we can manage and accept, and many qualified students don’t get admitted.
The first order of business for me is not to worry about how to maintain diversity. It’s to recognize that we need more doctors, nurses, and mental health care providers. I will, in a second, say a few words about diversity and where it fits into admissions, but I want to make the point clearly that what we should be doing is trying to expand the pool of students who are going to become doctors, nurses, mental health care providers, and social workers.
There are too many early retirements. We don’t have the person power we need to manage the health care challenges of an aging population. Let’s not get lost in arguing about what characteristics ought to get you into the finest medical schools. Let’s realize that we have to expand the number of schools we have.
We better be working pretty hard to expand our physician assistant programs, to make sure that we give full authority to qualified dentists and nurses who can help deliver some clinical care. We need more folks. That’s really where the battle ought to be: How do we get that done and how do we get it done quickly, not arguing about who’s in, who’s out, and why.
That said, diversity to me has never meant just race. I’m always interested in gender orientation, disability, and geographic input. Sometimes in decisions that you’re looking at, when I have students in front of me, they tell me they play a musical instrument or about the obstacles they had to overcome to get to medical school. Some of them will say they were involved in 4-H and did rodeo in high school or junior high school, which makes them a diverse potential student with characteristics that maybe some others don’t bring.
I’m not against diversity. I think having a rich set of experiences in any class – medicine, nursing, whatever it’s going to be – is beneficial to the students. They learn from each other. It is sometimes said that it’s also good for patients. I’m a little less excited about that, because I think our training goal should be to make every medical student and nursing student qualified to treat anybody.
I don’t think that, just because you’re Latinx or gay, that’s going to make a gay patient feel better. I think we should teach our students how to give care to everybody that they encounter. They shouldn’t have to match up characteristics to feel like they’re going to get quality care. That isn’t the right reason.
When you have a diverse set of providers, they can call that out and be on the alert for it, and that’s very important.
I also believe that we should think widely and broadly about diversity. Maybe race is out, but certainly other experiences related to income, background, struggle that got you to the point where you’re applying to medical school, motivation, the kinds of experiences you might have had caring for an elderly person, dealing with a disability or learning disability, and trying to overcome, let’s say, going to school in a poor area with not such a wonderful school, really help in terms of forming professionalism, empathy, and a caring point of view.
To me, the main goal is to expand our workforce. The secondary goal is to stay diverse, because we get better providers when we do so.
A version of this article first appeared on Medscape.com.
CPAP adherence curbs severe cardiovascular disease outcomes
, based on data from more than 4,000 individuals.
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular diseases, but the association between management of OSA with a continuous positive-airway pressure device (CPAP) and major adverse cardiac or cerebrovascular events (MACCEs) remains unclear, wrote Manuel Sánchez-de-la-Torre, PhD, of the University of Lleida, Spain, and colleagues.
In a meta-analysis published in JAMA, the researchers reviewed data from 4,186 individuals with a mean age of 61.2 years; 82.1% were men. The study population included 2,097 patients who used CPAP and 2,089 who did not. The mean apnea-hypopnea index (AHI) was 31.2 events per hour, and OSA was defined as an oxygen desaturation index of 12 events or more per hour or an AHI of 15 events or more per hour. The composite primary outcome included the first MACCE, or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Each of these components was a secondary endpoint.
Overall, the primary outcome of MACCE was similar for CPAP and non-CPAP using patients (hazard ratio, 1.01) with a total of 349 MACCE events in the CPAP group and 342 in the non-CPAP group. The mean adherence to CPAP was 3.1 hours per day. A total of 38.5% of patients in the CPAP group met the criteria for good adherence, defined as a mean of 4 or more hours per day.
However, as defined, good adherence to CPAP significantly reduced the risk of MACCE, compared with no CPAP use (HR, 0.69), and a sensitivity analysis showed a significant risk reduction, compared with patients who did not meet the criteria for good adherence (HR, 0.55; P = .005).
“Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features,” the researchers wrote in their discussion.
The findings were limited by several factors including the evaluation only of CPAP as a treatment for OSA, and the inability to assess separate components of the composite endpoint, the researchers noted. Other limitations included the relatively small number of female patients, reliance mainly on at-home sleep apnea tests, and the potential for selection bias, they said.
However, the results suggest that CPAP adherence is important to prevention of secondary cardiovascular outcomes in OSA patients, and that implementation of specific and personalized strategies to improve adherence to treatment should be a clinical priority, they concluded.
The study was funded by the Instituto de Salud Carlos III, the European Union and FEDER, IRBLleida–Fundació Dr Pifarré, SEPAR, ResMed Ltd. (Australia), Associació Lleidatana de Respiratori, and CIBERES. Dr Sánchez-de-la-Torre also disclosed financial support from a Ramón y Cajal grant.
, based on data from more than 4,000 individuals.
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular diseases, but the association between management of OSA with a continuous positive-airway pressure device (CPAP) and major adverse cardiac or cerebrovascular events (MACCEs) remains unclear, wrote Manuel Sánchez-de-la-Torre, PhD, of the University of Lleida, Spain, and colleagues.
In a meta-analysis published in JAMA, the researchers reviewed data from 4,186 individuals with a mean age of 61.2 years; 82.1% were men. The study population included 2,097 patients who used CPAP and 2,089 who did not. The mean apnea-hypopnea index (AHI) was 31.2 events per hour, and OSA was defined as an oxygen desaturation index of 12 events or more per hour or an AHI of 15 events or more per hour. The composite primary outcome included the first MACCE, or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Each of these components was a secondary endpoint.
Overall, the primary outcome of MACCE was similar for CPAP and non-CPAP using patients (hazard ratio, 1.01) with a total of 349 MACCE events in the CPAP group and 342 in the non-CPAP group. The mean adherence to CPAP was 3.1 hours per day. A total of 38.5% of patients in the CPAP group met the criteria for good adherence, defined as a mean of 4 or more hours per day.
However, as defined, good adherence to CPAP significantly reduced the risk of MACCE, compared with no CPAP use (HR, 0.69), and a sensitivity analysis showed a significant risk reduction, compared with patients who did not meet the criteria for good adherence (HR, 0.55; P = .005).
“Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features,” the researchers wrote in their discussion.
The findings were limited by several factors including the evaluation only of CPAP as a treatment for OSA, and the inability to assess separate components of the composite endpoint, the researchers noted. Other limitations included the relatively small number of female patients, reliance mainly on at-home sleep apnea tests, and the potential for selection bias, they said.
However, the results suggest that CPAP adherence is important to prevention of secondary cardiovascular outcomes in OSA patients, and that implementation of specific and personalized strategies to improve adherence to treatment should be a clinical priority, they concluded.
The study was funded by the Instituto de Salud Carlos III, the European Union and FEDER, IRBLleida–Fundació Dr Pifarré, SEPAR, ResMed Ltd. (Australia), Associació Lleidatana de Respiratori, and CIBERES. Dr Sánchez-de-la-Torre also disclosed financial support from a Ramón y Cajal grant.
, based on data from more than 4,000 individuals.
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular diseases, but the association between management of OSA with a continuous positive-airway pressure device (CPAP) and major adverse cardiac or cerebrovascular events (MACCEs) remains unclear, wrote Manuel Sánchez-de-la-Torre, PhD, of the University of Lleida, Spain, and colleagues.
In a meta-analysis published in JAMA, the researchers reviewed data from 4,186 individuals with a mean age of 61.2 years; 82.1% were men. The study population included 2,097 patients who used CPAP and 2,089 who did not. The mean apnea-hypopnea index (AHI) was 31.2 events per hour, and OSA was defined as an oxygen desaturation index of 12 events or more per hour or an AHI of 15 events or more per hour. The composite primary outcome included the first MACCE, or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Each of these components was a secondary endpoint.
Overall, the primary outcome of MACCE was similar for CPAP and non-CPAP using patients (hazard ratio, 1.01) with a total of 349 MACCE events in the CPAP group and 342 in the non-CPAP group. The mean adherence to CPAP was 3.1 hours per day. A total of 38.5% of patients in the CPAP group met the criteria for good adherence, defined as a mean of 4 or more hours per day.
However, as defined, good adherence to CPAP significantly reduced the risk of MACCE, compared with no CPAP use (HR, 0.69), and a sensitivity analysis showed a significant risk reduction, compared with patients who did not meet the criteria for good adherence (HR, 0.55; P = .005).
“Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features,” the researchers wrote in their discussion.
The findings were limited by several factors including the evaluation only of CPAP as a treatment for OSA, and the inability to assess separate components of the composite endpoint, the researchers noted. Other limitations included the relatively small number of female patients, reliance mainly on at-home sleep apnea tests, and the potential for selection bias, they said.
However, the results suggest that CPAP adherence is important to prevention of secondary cardiovascular outcomes in OSA patients, and that implementation of specific and personalized strategies to improve adherence to treatment should be a clinical priority, they concluded.
The study was funded by the Instituto de Salud Carlos III, the European Union and FEDER, IRBLleida–Fundació Dr Pifarré, SEPAR, ResMed Ltd. (Australia), Associació Lleidatana de Respiratori, and CIBERES. Dr Sánchez-de-la-Torre also disclosed financial support from a Ramón y Cajal grant.
FROM JAMA

