User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
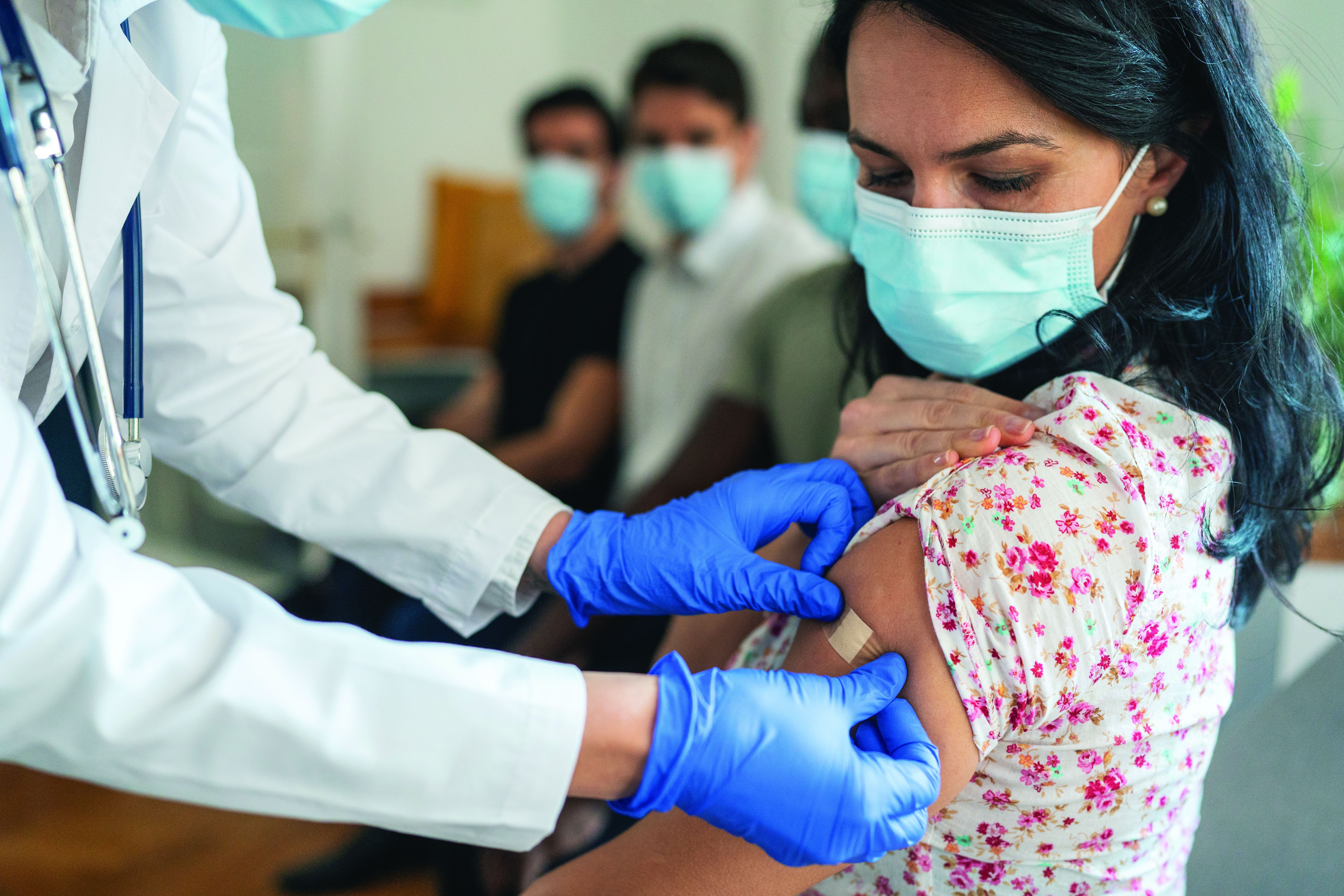
Breakthrough COVID studies lend support to use of new boosters in immunosuppressed patients
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
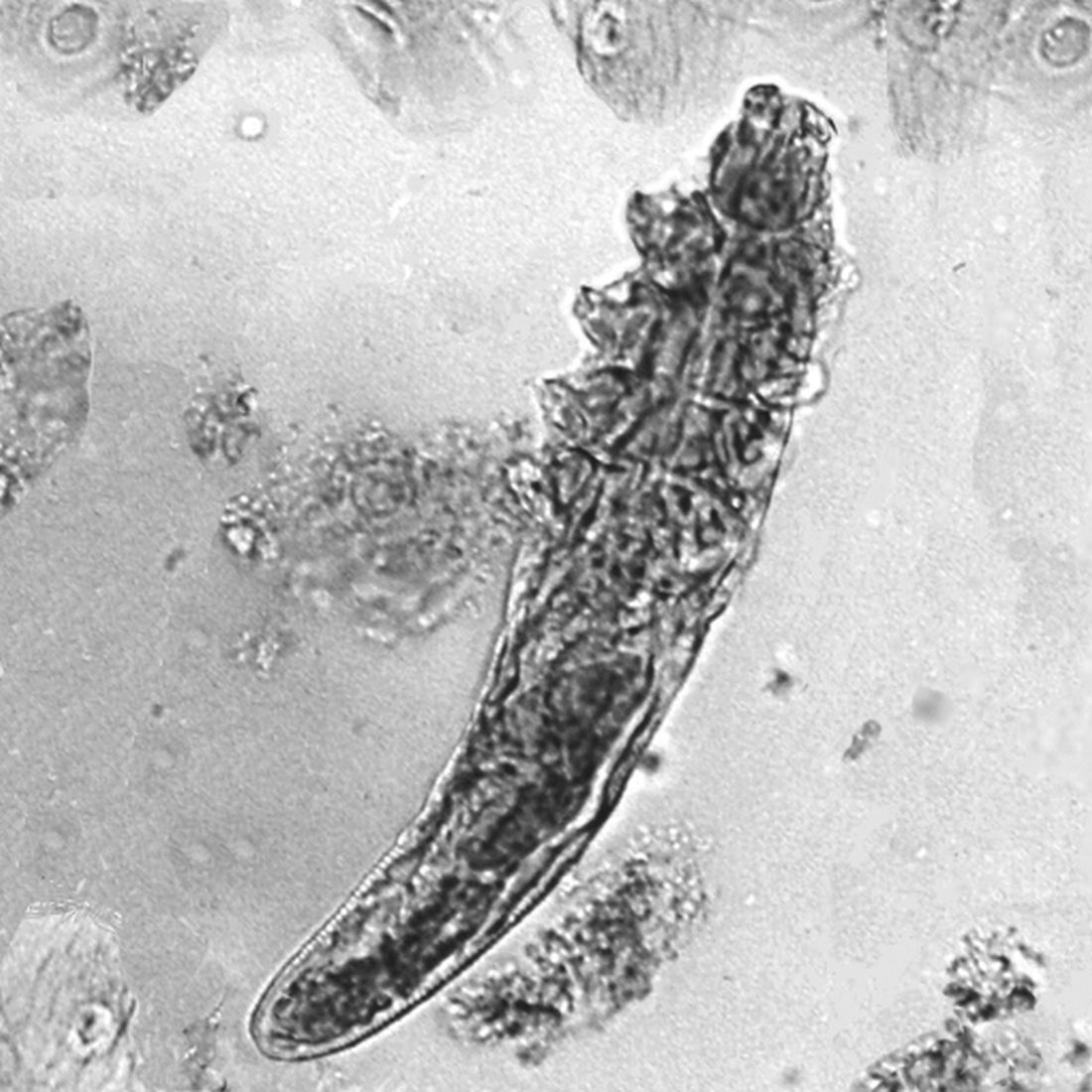
Don’t make children with head lice leave school, report says
The American Academy of Pediatrics says children with head lice don’t need to be sent home from school.
Head lice infestations aren’t really a health hazard because of low transmission rates, a new report from the academy says, and sending students home “may stigmatize children suspected of having head lice.” The group says schools should instead offer education programs to help families understand how to manage head lice.
“Head lice are an unpleasant part of the human experience, but they can be successfully managed and are no reason for a child to miss school,” Dawn Nolt, MD, lead author of the report on head lice, said in a news release.
The report advises schools to abandon “no-nit” policies, which call for a student to be lice-free before being allowed back in class.
“A child or adolescent should not be restricted from school attendance because of head lice, given the low contagion within classrooms. ‘No-nit’ policies that exclude children or adolescents until all nits are removed may violate a child’s or adolescent’s civil liberties and are best addressed with legal counsel for schools,” the report says.
The report notes that lice almost always spread through head-to-head contact, not by “jumping” from one person to another. It’s possible for lice to spread by touching the belongings of a person with lice, such as combs or sports helmets, but the chances of that happening are very low, the academy said.
“Lice found on combs are likely to be injured or dead, and a louse is not likely to leave a healthy head unless there is a heavy infestation,” the report says.
The report lists new medications for treatment and gives an algorithm for managing head lice cases.
“The ideal treatment of head lice should be safe, free of toxic chemicals, readily available, simple to apply, effective, and inexpensive,” the report says.
This is the first updated guidance on head lice from the American Academy of Pediatrics since 2015. The CDC also says students with head lice don’t need to be sent home.
“Students diagnosed with live head lice do not need to be sent home early from school; they can go home at the end of the day, be treated, and return to class after appropriate treatment has begun. Nits may persist after treatment, but successful treatment should kill crawling lice,” the CDC says.
A version of this article first appeared on WebMD.com.
The American Academy of Pediatrics says children with head lice don’t need to be sent home from school.
Head lice infestations aren’t really a health hazard because of low transmission rates, a new report from the academy says, and sending students home “may stigmatize children suspected of having head lice.” The group says schools should instead offer education programs to help families understand how to manage head lice.
“Head lice are an unpleasant part of the human experience, but they can be successfully managed and are no reason for a child to miss school,” Dawn Nolt, MD, lead author of the report on head lice, said in a news release.
The report advises schools to abandon “no-nit” policies, which call for a student to be lice-free before being allowed back in class.
“A child or adolescent should not be restricted from school attendance because of head lice, given the low contagion within classrooms. ‘No-nit’ policies that exclude children or adolescents until all nits are removed may violate a child’s or adolescent’s civil liberties and are best addressed with legal counsel for schools,” the report says.
The report notes that lice almost always spread through head-to-head contact, not by “jumping” from one person to another. It’s possible for lice to spread by touching the belongings of a person with lice, such as combs or sports helmets, but the chances of that happening are very low, the academy said.
“Lice found on combs are likely to be injured or dead, and a louse is not likely to leave a healthy head unless there is a heavy infestation,” the report says.
The report lists new medications for treatment and gives an algorithm for managing head lice cases.
“The ideal treatment of head lice should be safe, free of toxic chemicals, readily available, simple to apply, effective, and inexpensive,” the report says.
This is the first updated guidance on head lice from the American Academy of Pediatrics since 2015. The CDC also says students with head lice don’t need to be sent home.
“Students diagnosed with live head lice do not need to be sent home early from school; they can go home at the end of the day, be treated, and return to class after appropriate treatment has begun. Nits may persist after treatment, but successful treatment should kill crawling lice,” the CDC says.
A version of this article first appeared on WebMD.com.
The American Academy of Pediatrics says children with head lice don’t need to be sent home from school.
Head lice infestations aren’t really a health hazard because of low transmission rates, a new report from the academy says, and sending students home “may stigmatize children suspected of having head lice.” The group says schools should instead offer education programs to help families understand how to manage head lice.
“Head lice are an unpleasant part of the human experience, but they can be successfully managed and are no reason for a child to miss school,” Dawn Nolt, MD, lead author of the report on head lice, said in a news release.
The report advises schools to abandon “no-nit” policies, which call for a student to be lice-free before being allowed back in class.
“A child or adolescent should not be restricted from school attendance because of head lice, given the low contagion within classrooms. ‘No-nit’ policies that exclude children or adolescents until all nits are removed may violate a child’s or adolescent’s civil liberties and are best addressed with legal counsel for schools,” the report says.
The report notes that lice almost always spread through head-to-head contact, not by “jumping” from one person to another. It’s possible for lice to spread by touching the belongings of a person with lice, such as combs or sports helmets, but the chances of that happening are very low, the academy said.
“Lice found on combs are likely to be injured or dead, and a louse is not likely to leave a healthy head unless there is a heavy infestation,” the report says.
The report lists new medications for treatment and gives an algorithm for managing head lice cases.
“The ideal treatment of head lice should be safe, free of toxic chemicals, readily available, simple to apply, effective, and inexpensive,” the report says.
This is the first updated guidance on head lice from the American Academy of Pediatrics since 2015. The CDC also says students with head lice don’t need to be sent home.
“Students diagnosed with live head lice do not need to be sent home early from school; they can go home at the end of the day, be treated, and return to class after appropriate treatment has begun. Nits may persist after treatment, but successful treatment should kill crawling lice,” the CDC says.
A version of this article first appeared on WebMD.com.
Physician bias may prevent quality care for patients with disabilities
For Tara Lagu, MD, the realization that the health care system was broken for patients with disabilities came when a woman she had been treating seemed to keep ignoring Dr. Lagu’s request to see a urologist.
When Dr. Lagu asked the patient’s two attentive daughters about the delay, their response surprised her. The women said they couldn’t find a urologist who was willing to see a patient in a wheelchair.
Surprised and a bit doubtful, Dr. Lagu checked around. She found that, indeed, the only way to get her patient in to see the type of physician required was to send her by ambulance.
“It opened my eyes to how hard it is for patients with disabilities to navigate the health care system,” Dr. Lagu said.
Dr. Lagu, director of the Center for Health Services and Outcomes Research at Northwestern University in Chicago, decided to take a closer look at how her colleagues in medicine care for – or not, as the case proved – the roughly one in four American adults, and millions of children, with disabilities.
In a series of three focus groups, Dr. Lagu and colleagues identified a range of obstacles – including some physician attitudes – that prevent people with disabilities from getting adequate care.
For the study, published in Health Affairs, the researchers interviewed 22 physicians in three groups: Nonrural primary care physicians, rural primary care physicians, and specialists in rheumatology, neurology, obstetrics/gynecology, orthopedics, and ophthalmology.
During the interviews, conducted in the fall of 2018, participants were asked about providing care for five specific types of disabilities: mobility, hearing, vision, mental health, and intellectual limitations.
Lack of experience, logistics often cited
Some physicians admitted that limited resources and training left them without the space and necessary knowledge to properly care for patients with disabilities. They felt they lacked the expertise or exposure to care for individuals with disabilities, nor did they have enough time and space to properly accommodate these patients, according to the researchers. Some said they struggled to coordinate care for individuals with disabilities and did not know which types of accessible equipment, such as adjustable tables and chair scales, were needed or how to use them.
Several physicians also noted that they are inadequately reimbursed for the special accommodations – including additional staff, equipment, and time – required to care for these patients. One primary care physician said he hired a sign-language interpreter for a patient but the bill for the services exceeded the amount insurance reimbursed. As a result, he said, he spent $30 of his own money per visit to see the patient.
Because of these limitations, some physicians in the focus groups said they try to turn away patients with disabilities. Both specialists and general practitioners said they had told patients with disabilities that they didn’t feel they could provide the care needed, and suggested they look elsewhere. A few were surprisingly – even upsettingly – honest, Dr. Lagu said, making statements such as: “I am not the doctor for you.”
‘We really need a rewrite’
Previous work has shown that people with disabilities have worse health outcomes, such as undetected cancer, obesity, and cardiovascular disease.
But “the disability itself isn’t what leads to worse outcomes,” said Allison Kessler, MD, section chief of the Renée Crown Center for Spinal Cord Innovation and associate director of the Shirley Ryan AbilityLab in Chicago*. This study does a good job at highlighting “the need for change on multiple levels,” said Dr. Kessler, who was not a member of the study team.
“People with disabilities have all these disparities in access and outcomes. We’ve never understood why. I think the why is complicated,” Dr. Lagu added. “I think this study suggests some of the negative outcomes are due to explicit bias.”
“It’s also clear that the current framework of health care in the United States does not lend to allowing physicians and medical providers the time needed to adequately address patient issues – those with disabilities or just multiple complex problems,” Colin O’Reilly, DO, vice president and chief medical officer at Children’s Specialized Hospital, an acute rehabilitation facility affiliated with RWJBarnabas Health, in New Brunswick, N.J. “We really need a rewrite.”
However, Dr. O’Reilly said, such a small study population with no control group and no mention of physician resources makes it difficult to come to a strong conclusion about physician bias and discriminatory attitudes against individuals with disabilities.
Dr. Lagu agreed, saying this research “is not conclusive in any way.” The excuses doctors use to discharge patients with disabilities, such as “we don’t accept your insurance,” “we aren’t taking new patients,” and “we can’t provide you with the appropriate care,” could be legitimate, the study authors wrote. But the “disparities in care for people with disabilities suggest that there is a pattern of more frequently denying care to them than people without a disability,” they added.
Dr. Kessler said many of her patients have told her they experience barriers to care. Some say finding an office with the necessary equipment is a challenge or that they often don’t feel welcome.
The Americans With Disabilities Act (ADA) is a federal civil rights law that prohibits discrimination against individuals with disabilities in all public and private places that are open to the general public, including medical offices.
“It is difficult to enforce the ADA in medical settings,” the researchers noted. “Explanations physicians gave in this study could, for any single case of denying care, be legitimate.” Knowing whether a particular instance of denial of care represents discrimination related to disability is “nearly impossible,” they wrote.
All the experts agreed that the study adds valuable insight into an ongoing health disparity. And while system and policy changes are required, Dr. Kessler said, individual physicians can take steps to improve the situation.
A physician in an academic setting can look at the curriculum and the medical school and see about increasing exposure to patients with disabilities earlier in training. In a practice, physicians can retrain staff to ask every patient if an accommodation is needed. “Each one of those changes can only help us move our system in the right direction,” Dr. Kessler said.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
*Correction, 10/5/22: This article includes a corrected title for Dr. Allison Kessler.
A version of this article first appeared on Medscape.com.
For Tara Lagu, MD, the realization that the health care system was broken for patients with disabilities came when a woman she had been treating seemed to keep ignoring Dr. Lagu’s request to see a urologist.
When Dr. Lagu asked the patient’s two attentive daughters about the delay, their response surprised her. The women said they couldn’t find a urologist who was willing to see a patient in a wheelchair.
Surprised and a bit doubtful, Dr. Lagu checked around. She found that, indeed, the only way to get her patient in to see the type of physician required was to send her by ambulance.
“It opened my eyes to how hard it is for patients with disabilities to navigate the health care system,” Dr. Lagu said.
Dr. Lagu, director of the Center for Health Services and Outcomes Research at Northwestern University in Chicago, decided to take a closer look at how her colleagues in medicine care for – or not, as the case proved – the roughly one in four American adults, and millions of children, with disabilities.
In a series of three focus groups, Dr. Lagu and colleagues identified a range of obstacles – including some physician attitudes – that prevent people with disabilities from getting adequate care.
For the study, published in Health Affairs, the researchers interviewed 22 physicians in three groups: Nonrural primary care physicians, rural primary care physicians, and specialists in rheumatology, neurology, obstetrics/gynecology, orthopedics, and ophthalmology.
During the interviews, conducted in the fall of 2018, participants were asked about providing care for five specific types of disabilities: mobility, hearing, vision, mental health, and intellectual limitations.
Lack of experience, logistics often cited
Some physicians admitted that limited resources and training left them without the space and necessary knowledge to properly care for patients with disabilities. They felt they lacked the expertise or exposure to care for individuals with disabilities, nor did they have enough time and space to properly accommodate these patients, according to the researchers. Some said they struggled to coordinate care for individuals with disabilities and did not know which types of accessible equipment, such as adjustable tables and chair scales, were needed or how to use them.
Several physicians also noted that they are inadequately reimbursed for the special accommodations – including additional staff, equipment, and time – required to care for these patients. One primary care physician said he hired a sign-language interpreter for a patient but the bill for the services exceeded the amount insurance reimbursed. As a result, he said, he spent $30 of his own money per visit to see the patient.
Because of these limitations, some physicians in the focus groups said they try to turn away patients with disabilities. Both specialists and general practitioners said they had told patients with disabilities that they didn’t feel they could provide the care needed, and suggested they look elsewhere. A few were surprisingly – even upsettingly – honest, Dr. Lagu said, making statements such as: “I am not the doctor for you.”
‘We really need a rewrite’
Previous work has shown that people with disabilities have worse health outcomes, such as undetected cancer, obesity, and cardiovascular disease.
But “the disability itself isn’t what leads to worse outcomes,” said Allison Kessler, MD, section chief of the Renée Crown Center for Spinal Cord Innovation and associate director of the Shirley Ryan AbilityLab in Chicago*. This study does a good job at highlighting “the need for change on multiple levels,” said Dr. Kessler, who was not a member of the study team.
“People with disabilities have all these disparities in access and outcomes. We’ve never understood why. I think the why is complicated,” Dr. Lagu added. “I think this study suggests some of the negative outcomes are due to explicit bias.”
“It’s also clear that the current framework of health care in the United States does not lend to allowing physicians and medical providers the time needed to adequately address patient issues – those with disabilities or just multiple complex problems,” Colin O’Reilly, DO, vice president and chief medical officer at Children’s Specialized Hospital, an acute rehabilitation facility affiliated with RWJBarnabas Health, in New Brunswick, N.J. “We really need a rewrite.”
However, Dr. O’Reilly said, such a small study population with no control group and no mention of physician resources makes it difficult to come to a strong conclusion about physician bias and discriminatory attitudes against individuals with disabilities.
Dr. Lagu agreed, saying this research “is not conclusive in any way.” The excuses doctors use to discharge patients with disabilities, such as “we don’t accept your insurance,” “we aren’t taking new patients,” and “we can’t provide you with the appropriate care,” could be legitimate, the study authors wrote. But the “disparities in care for people with disabilities suggest that there is a pattern of more frequently denying care to them than people without a disability,” they added.
Dr. Kessler said many of her patients have told her they experience barriers to care. Some say finding an office with the necessary equipment is a challenge or that they often don’t feel welcome.
The Americans With Disabilities Act (ADA) is a federal civil rights law that prohibits discrimination against individuals with disabilities in all public and private places that are open to the general public, including medical offices.
“It is difficult to enforce the ADA in medical settings,” the researchers noted. “Explanations physicians gave in this study could, for any single case of denying care, be legitimate.” Knowing whether a particular instance of denial of care represents discrimination related to disability is “nearly impossible,” they wrote.
All the experts agreed that the study adds valuable insight into an ongoing health disparity. And while system and policy changes are required, Dr. Kessler said, individual physicians can take steps to improve the situation.
A physician in an academic setting can look at the curriculum and the medical school and see about increasing exposure to patients with disabilities earlier in training. In a practice, physicians can retrain staff to ask every patient if an accommodation is needed. “Each one of those changes can only help us move our system in the right direction,” Dr. Kessler said.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
*Correction, 10/5/22: This article includes a corrected title for Dr. Allison Kessler.
A version of this article first appeared on Medscape.com.
For Tara Lagu, MD, the realization that the health care system was broken for patients with disabilities came when a woman she had been treating seemed to keep ignoring Dr. Lagu’s request to see a urologist.
When Dr. Lagu asked the patient’s two attentive daughters about the delay, their response surprised her. The women said they couldn’t find a urologist who was willing to see a patient in a wheelchair.
Surprised and a bit doubtful, Dr. Lagu checked around. She found that, indeed, the only way to get her patient in to see the type of physician required was to send her by ambulance.
“It opened my eyes to how hard it is for patients with disabilities to navigate the health care system,” Dr. Lagu said.
Dr. Lagu, director of the Center for Health Services and Outcomes Research at Northwestern University in Chicago, decided to take a closer look at how her colleagues in medicine care for – or not, as the case proved – the roughly one in four American adults, and millions of children, with disabilities.
In a series of three focus groups, Dr. Lagu and colleagues identified a range of obstacles – including some physician attitudes – that prevent people with disabilities from getting adequate care.
For the study, published in Health Affairs, the researchers interviewed 22 physicians in three groups: Nonrural primary care physicians, rural primary care physicians, and specialists in rheumatology, neurology, obstetrics/gynecology, orthopedics, and ophthalmology.
During the interviews, conducted in the fall of 2018, participants were asked about providing care for five specific types of disabilities: mobility, hearing, vision, mental health, and intellectual limitations.
Lack of experience, logistics often cited
Some physicians admitted that limited resources and training left them without the space and necessary knowledge to properly care for patients with disabilities. They felt they lacked the expertise or exposure to care for individuals with disabilities, nor did they have enough time and space to properly accommodate these patients, according to the researchers. Some said they struggled to coordinate care for individuals with disabilities and did not know which types of accessible equipment, such as adjustable tables and chair scales, were needed or how to use them.
Several physicians also noted that they are inadequately reimbursed for the special accommodations – including additional staff, equipment, and time – required to care for these patients. One primary care physician said he hired a sign-language interpreter for a patient but the bill for the services exceeded the amount insurance reimbursed. As a result, he said, he spent $30 of his own money per visit to see the patient.
Because of these limitations, some physicians in the focus groups said they try to turn away patients with disabilities. Both specialists and general practitioners said they had told patients with disabilities that they didn’t feel they could provide the care needed, and suggested they look elsewhere. A few were surprisingly – even upsettingly – honest, Dr. Lagu said, making statements such as: “I am not the doctor for you.”
‘We really need a rewrite’
Previous work has shown that people with disabilities have worse health outcomes, such as undetected cancer, obesity, and cardiovascular disease.
But “the disability itself isn’t what leads to worse outcomes,” said Allison Kessler, MD, section chief of the Renée Crown Center for Spinal Cord Innovation and associate director of the Shirley Ryan AbilityLab in Chicago*. This study does a good job at highlighting “the need for change on multiple levels,” said Dr. Kessler, who was not a member of the study team.
“People with disabilities have all these disparities in access and outcomes. We’ve never understood why. I think the why is complicated,” Dr. Lagu added. “I think this study suggests some of the negative outcomes are due to explicit bias.”
“It’s also clear that the current framework of health care in the United States does not lend to allowing physicians and medical providers the time needed to adequately address patient issues – those with disabilities or just multiple complex problems,” Colin O’Reilly, DO, vice president and chief medical officer at Children’s Specialized Hospital, an acute rehabilitation facility affiliated with RWJBarnabas Health, in New Brunswick, N.J. “We really need a rewrite.”
However, Dr. O’Reilly said, such a small study population with no control group and no mention of physician resources makes it difficult to come to a strong conclusion about physician bias and discriminatory attitudes against individuals with disabilities.
Dr. Lagu agreed, saying this research “is not conclusive in any way.” The excuses doctors use to discharge patients with disabilities, such as “we don’t accept your insurance,” “we aren’t taking new patients,” and “we can’t provide you with the appropriate care,” could be legitimate, the study authors wrote. But the “disparities in care for people with disabilities suggest that there is a pattern of more frequently denying care to them than people without a disability,” they added.
Dr. Kessler said many of her patients have told her they experience barriers to care. Some say finding an office with the necessary equipment is a challenge or that they often don’t feel welcome.
The Americans With Disabilities Act (ADA) is a federal civil rights law that prohibits discrimination against individuals with disabilities in all public and private places that are open to the general public, including medical offices.
“It is difficult to enforce the ADA in medical settings,” the researchers noted. “Explanations physicians gave in this study could, for any single case of denying care, be legitimate.” Knowing whether a particular instance of denial of care represents discrimination related to disability is “nearly impossible,” they wrote.
All the experts agreed that the study adds valuable insight into an ongoing health disparity. And while system and policy changes are required, Dr. Kessler said, individual physicians can take steps to improve the situation.
A physician in an academic setting can look at the curriculum and the medical school and see about increasing exposure to patients with disabilities earlier in training. In a practice, physicians can retrain staff to ask every patient if an accommodation is needed. “Each one of those changes can only help us move our system in the right direction,” Dr. Kessler said.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
*Correction, 10/5/22: This article includes a corrected title for Dr. Allison Kessler.
A version of this article first appeared on Medscape.com.
BREEZE-AD-PEDS: First data for baricitinib in childhood eczema reported
The oral Janus kinase
After 16 weeks of treatment, the primary endpoint – an Investigators Global Assessment (IGA) score of 0 or 1 with at least a 2-point improvement from baseline – was met by 41.7% of patients given 2 mg (those younger than age 10) or 4 mg of baricitinib (those aged 10-17 years), the highest dose studied in each of those two age groups.
By comparison, the primary endpoint was met in 16.4% of children in the placebo group (P < .001).
Baricitinib is approved for the treatment of AD in adults in many countries, Antonio Torrelo, MD, of the Hospital Infantil Niño Jesús, Madrid, said at the annual congress of the European Academy of Dermatology and Venereology. It was approved by the U.S. Food and Drug Administration for treating adults with severe alopecia areata in June and is under FDA review for the treatment of AD.
The phase 3 BREEZE-AD-PEDS trial
BREEZE-AD-PEDS was a randomized, double-blind trial that evaluated the safety and efficacy of baricitinib in 483 children and adolescents with moderate to severe AD. Participants were aged 2-17 years. Those aged 2-5 years had been diagnosed with AD for at least 6 months; if they were older, they had been diagnosed for at least 12 months.
Three dosing levels of baricitinib were tested: 121 patients were given a low dose, which was 0.5 mg/day in children aged 2 to less than 10 years and 1 mg/day in those aged 10 to less than 18 years. A medium dose – 1 mg/day in the younger children and 2 mg/day in the older children – was given to 120 children, while a high dose – 2 mg/day and 4 mg/day, respectively – was given to another 120 children.
Topical treatments were permitted, although for entry into the trial, participants had to have had an inadequate response to steroids and an inadequate or no response to topical calcineurin inhibitors. In all groups, age, gender, race, geographic region, age at diagnosis of AD, and duration of AD “were more or less similar,” Dr. Torello said.
Good results, but only with highest dose
The primary IGA endpoint was reached by 25.8% of children in the medium-dose group and by 18.2% in the low-dose group. Neither result was statistically significant in comparison with placebo (16.4%).
When breaking down the results between different ages, “the results in the IGA scores are consistent in both age subgroups – below 10 years and over 10 years,” Dr. Torello noted. The results are also consistent across body weights (< 20 kg, 20-60 kg, and > 60 kg), he added.
Among those treated with the high dose of baricitinib, Eczema Area and Severity Index (EASI) 75% and 95% improvement scores were reached in 52.5% and 30% of patients, respectively. Corresponding figures for the medium dose were 40% and 21.7%; for the low baricitinib dose, 32.2% and 11.6%; and for placebo, 32% and 12.3%. Again, only the results for the highest baricitinib dose were significant in comparison with placebo.
A similar pattern was seen for improvement in itch, and there was a 75% improvement in Scoring Atopic Dermatitis (SCORAD75) results.
Safety of baricitinib in children
The labeling for JAK inhibitors that have been approved to date, including baricitinib, include a boxed warning regarding risks for thrombosis, major adverse cardiovascular events, and all-cause mortality. The warning is based on use by patients with rheumatoid arthritis.
Dr. Torello summarized baricitinib’s safety profile in the trial as being “consistent with the well-known safety profile for baricitinib in adults with moderate to severe atopic dermatitis.”
In the study, no severe adverse effects were noted, and no new safety signals were observed, he said. The rate of any treatment-emergent effect among patients was around 50% and was similar across all baricitinib and placebo groups. Study discontinuations because of a side effect were more frequent in the placebo arm (1.6% of patients) than in the baricitinib low-, medium-, and high-dose arms (0.8%, 0%, and 0.8%, respectively).
There were no cases of deep-vein thrombosis, pulmonary embolism, or other adverse effects of special interest, including major adverse cardiovascular events, gastrointestinal perforations, and opportunistic infections, Dr. Torrelo said.
No patient experienced elevations in liver enzyme levels, although there were some cases of elevated creatinine phosphokinase levels (16% in the placebo group and 19% in the baricitinib arms altogether) that were not from muscle injury. There was a possible increase in low-density cholesterol level (3.3% of those taking placebo vs. 10.1% of baricitinib-treated patients).
Is there a role for baricitinib?
“Baricitinib is a potential therapeutic option with a favorable benefit-to-risk profile for children between 2 and 18 years who have moderate to severe atopic dermatitis, and candidates for systemic therapy,” Dr. Torrelo said. “No single drug is capable to treat every patient with atopic dermatitis,” he added in discussing the possible place of baricitinib in pediatric practice.
“There are patients who do not respond to dupilumab, who apparently respond later to JAK inhibitors,” he noted.
“We are trying to work phenotypically, trying to learn what kind of patients – especially children who have a more heterogeneous disease than adults – can be better treated with JAK inhibitors or dupilumab.” There may be other important considerations in choosing a treatment in children, Dr. Torrelo said, including that JAK inhibitors can be given orally, while dupilumab is administered by injection.
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation in Irvine, Calif., pointed out that “only the higher dose is significantly more effective than placebo.”
In his view, “the potentially severe adverse events are not worth the risk compared to more effective agents, such as dupilumab, in this pediatric population,” added Dr. Wu, who recently authored a review of the role of JAK inhibitors in skin disease. He was not involved with the baricitinib study.
The study was funded by Eli Lilly in collaboration with Incyte. Dr. Torello has participated in advisory boards and/or has served as a principal investigator in clinical trials for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Pierre Fabre, and Sanofi. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The oral Janus kinase
After 16 weeks of treatment, the primary endpoint – an Investigators Global Assessment (IGA) score of 0 or 1 with at least a 2-point improvement from baseline – was met by 41.7% of patients given 2 mg (those younger than age 10) or 4 mg of baricitinib (those aged 10-17 years), the highest dose studied in each of those two age groups.
By comparison, the primary endpoint was met in 16.4% of children in the placebo group (P < .001).
Baricitinib is approved for the treatment of AD in adults in many countries, Antonio Torrelo, MD, of the Hospital Infantil Niño Jesús, Madrid, said at the annual congress of the European Academy of Dermatology and Venereology. It was approved by the U.S. Food and Drug Administration for treating adults with severe alopecia areata in June and is under FDA review for the treatment of AD.
The phase 3 BREEZE-AD-PEDS trial
BREEZE-AD-PEDS was a randomized, double-blind trial that evaluated the safety and efficacy of baricitinib in 483 children and adolescents with moderate to severe AD. Participants were aged 2-17 years. Those aged 2-5 years had been diagnosed with AD for at least 6 months; if they were older, they had been diagnosed for at least 12 months.
Three dosing levels of baricitinib were tested: 121 patients were given a low dose, which was 0.5 mg/day in children aged 2 to less than 10 years and 1 mg/day in those aged 10 to less than 18 years. A medium dose – 1 mg/day in the younger children and 2 mg/day in the older children – was given to 120 children, while a high dose – 2 mg/day and 4 mg/day, respectively – was given to another 120 children.
Topical treatments were permitted, although for entry into the trial, participants had to have had an inadequate response to steroids and an inadequate or no response to topical calcineurin inhibitors. In all groups, age, gender, race, geographic region, age at diagnosis of AD, and duration of AD “were more or less similar,” Dr. Torello said.
Good results, but only with highest dose
The primary IGA endpoint was reached by 25.8% of children in the medium-dose group and by 18.2% in the low-dose group. Neither result was statistically significant in comparison with placebo (16.4%).
When breaking down the results between different ages, “the results in the IGA scores are consistent in both age subgroups – below 10 years and over 10 years,” Dr. Torello noted. The results are also consistent across body weights (< 20 kg, 20-60 kg, and > 60 kg), he added.
Among those treated with the high dose of baricitinib, Eczema Area and Severity Index (EASI) 75% and 95% improvement scores were reached in 52.5% and 30% of patients, respectively. Corresponding figures for the medium dose were 40% and 21.7%; for the low baricitinib dose, 32.2% and 11.6%; and for placebo, 32% and 12.3%. Again, only the results for the highest baricitinib dose were significant in comparison with placebo.
A similar pattern was seen for improvement in itch, and there was a 75% improvement in Scoring Atopic Dermatitis (SCORAD75) results.
Safety of baricitinib in children
The labeling for JAK inhibitors that have been approved to date, including baricitinib, include a boxed warning regarding risks for thrombosis, major adverse cardiovascular events, and all-cause mortality. The warning is based on use by patients with rheumatoid arthritis.
Dr. Torello summarized baricitinib’s safety profile in the trial as being “consistent with the well-known safety profile for baricitinib in adults with moderate to severe atopic dermatitis.”
In the study, no severe adverse effects were noted, and no new safety signals were observed, he said. The rate of any treatment-emergent effect among patients was around 50% and was similar across all baricitinib and placebo groups. Study discontinuations because of a side effect were more frequent in the placebo arm (1.6% of patients) than in the baricitinib low-, medium-, and high-dose arms (0.8%, 0%, and 0.8%, respectively).
There were no cases of deep-vein thrombosis, pulmonary embolism, or other adverse effects of special interest, including major adverse cardiovascular events, gastrointestinal perforations, and opportunistic infections, Dr. Torrelo said.
No patient experienced elevations in liver enzyme levels, although there were some cases of elevated creatinine phosphokinase levels (16% in the placebo group and 19% in the baricitinib arms altogether) that were not from muscle injury. There was a possible increase in low-density cholesterol level (3.3% of those taking placebo vs. 10.1% of baricitinib-treated patients).
Is there a role for baricitinib?
“Baricitinib is a potential therapeutic option with a favorable benefit-to-risk profile for children between 2 and 18 years who have moderate to severe atopic dermatitis, and candidates for systemic therapy,” Dr. Torrelo said. “No single drug is capable to treat every patient with atopic dermatitis,” he added in discussing the possible place of baricitinib in pediatric practice.
“There are patients who do not respond to dupilumab, who apparently respond later to JAK inhibitors,” he noted.
“We are trying to work phenotypically, trying to learn what kind of patients – especially children who have a more heterogeneous disease than adults – can be better treated with JAK inhibitors or dupilumab.” There may be other important considerations in choosing a treatment in children, Dr. Torrelo said, including that JAK inhibitors can be given orally, while dupilumab is administered by injection.
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation in Irvine, Calif., pointed out that “only the higher dose is significantly more effective than placebo.”
In his view, “the potentially severe adverse events are not worth the risk compared to more effective agents, such as dupilumab, in this pediatric population,” added Dr. Wu, who recently authored a review of the role of JAK inhibitors in skin disease. He was not involved with the baricitinib study.
The study was funded by Eli Lilly in collaboration with Incyte. Dr. Torello has participated in advisory boards and/or has served as a principal investigator in clinical trials for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Pierre Fabre, and Sanofi. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The oral Janus kinase
After 16 weeks of treatment, the primary endpoint – an Investigators Global Assessment (IGA) score of 0 or 1 with at least a 2-point improvement from baseline – was met by 41.7% of patients given 2 mg (those younger than age 10) or 4 mg of baricitinib (those aged 10-17 years), the highest dose studied in each of those two age groups.
By comparison, the primary endpoint was met in 16.4% of children in the placebo group (P < .001).
Baricitinib is approved for the treatment of AD in adults in many countries, Antonio Torrelo, MD, of the Hospital Infantil Niño Jesús, Madrid, said at the annual congress of the European Academy of Dermatology and Venereology. It was approved by the U.S. Food and Drug Administration for treating adults with severe alopecia areata in June and is under FDA review for the treatment of AD.
The phase 3 BREEZE-AD-PEDS trial
BREEZE-AD-PEDS was a randomized, double-blind trial that evaluated the safety and efficacy of baricitinib in 483 children and adolescents with moderate to severe AD. Participants were aged 2-17 years. Those aged 2-5 years had been diagnosed with AD for at least 6 months; if they were older, they had been diagnosed for at least 12 months.
Three dosing levels of baricitinib were tested: 121 patients were given a low dose, which was 0.5 mg/day in children aged 2 to less than 10 years and 1 mg/day in those aged 10 to less than 18 years. A medium dose – 1 mg/day in the younger children and 2 mg/day in the older children – was given to 120 children, while a high dose – 2 mg/day and 4 mg/day, respectively – was given to another 120 children.
Topical treatments were permitted, although for entry into the trial, participants had to have had an inadequate response to steroids and an inadequate or no response to topical calcineurin inhibitors. In all groups, age, gender, race, geographic region, age at diagnosis of AD, and duration of AD “were more or less similar,” Dr. Torello said.
Good results, but only with highest dose
The primary IGA endpoint was reached by 25.8% of children in the medium-dose group and by 18.2% in the low-dose group. Neither result was statistically significant in comparison with placebo (16.4%).
When breaking down the results between different ages, “the results in the IGA scores are consistent in both age subgroups – below 10 years and over 10 years,” Dr. Torello noted. The results are also consistent across body weights (< 20 kg, 20-60 kg, and > 60 kg), he added.
Among those treated with the high dose of baricitinib, Eczema Area and Severity Index (EASI) 75% and 95% improvement scores were reached in 52.5% and 30% of patients, respectively. Corresponding figures for the medium dose were 40% and 21.7%; for the low baricitinib dose, 32.2% and 11.6%; and for placebo, 32% and 12.3%. Again, only the results for the highest baricitinib dose were significant in comparison with placebo.
A similar pattern was seen for improvement in itch, and there was a 75% improvement in Scoring Atopic Dermatitis (SCORAD75) results.
Safety of baricitinib in children
The labeling for JAK inhibitors that have been approved to date, including baricitinib, include a boxed warning regarding risks for thrombosis, major adverse cardiovascular events, and all-cause mortality. The warning is based on use by patients with rheumatoid arthritis.
Dr. Torello summarized baricitinib’s safety profile in the trial as being “consistent with the well-known safety profile for baricitinib in adults with moderate to severe atopic dermatitis.”
In the study, no severe adverse effects were noted, and no new safety signals were observed, he said. The rate of any treatment-emergent effect among patients was around 50% and was similar across all baricitinib and placebo groups. Study discontinuations because of a side effect were more frequent in the placebo arm (1.6% of patients) than in the baricitinib low-, medium-, and high-dose arms (0.8%, 0%, and 0.8%, respectively).
There were no cases of deep-vein thrombosis, pulmonary embolism, or other adverse effects of special interest, including major adverse cardiovascular events, gastrointestinal perforations, and opportunistic infections, Dr. Torrelo said.
No patient experienced elevations in liver enzyme levels, although there were some cases of elevated creatinine phosphokinase levels (16% in the placebo group and 19% in the baricitinib arms altogether) that were not from muscle injury. There was a possible increase in low-density cholesterol level (3.3% of those taking placebo vs. 10.1% of baricitinib-treated patients).
Is there a role for baricitinib?
“Baricitinib is a potential therapeutic option with a favorable benefit-to-risk profile for children between 2 and 18 years who have moderate to severe atopic dermatitis, and candidates for systemic therapy,” Dr. Torrelo said. “No single drug is capable to treat every patient with atopic dermatitis,” he added in discussing the possible place of baricitinib in pediatric practice.
“There are patients who do not respond to dupilumab, who apparently respond later to JAK inhibitors,” he noted.
“We are trying to work phenotypically, trying to learn what kind of patients – especially children who have a more heterogeneous disease than adults – can be better treated with JAK inhibitors or dupilumab.” There may be other important considerations in choosing a treatment in children, Dr. Torrelo said, including that JAK inhibitors can be given orally, while dupilumab is administered by injection.
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation in Irvine, Calif., pointed out that “only the higher dose is significantly more effective than placebo.”
In his view, “the potentially severe adverse events are not worth the risk compared to more effective agents, such as dupilumab, in this pediatric population,” added Dr. Wu, who recently authored a review of the role of JAK inhibitors in skin disease. He was not involved with the baricitinib study.
The study was funded by Eli Lilly in collaboration with Incyte. Dr. Torello has participated in advisory boards and/or has served as a principal investigator in clinical trials for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Pierre Fabre, and Sanofi. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Instagram: Cosmetic procedures discussed without cosmetic experts
according to an analysis of related posts on the photo-sharing service.
“Given that there is little to no oversight on social networking sites, unqualified sources can widely disseminate misinformation resulting in misguided management or unnecessary procedures,” Taryn N. Murray, MD, and associates said in Lasers in Surgery and Medicine.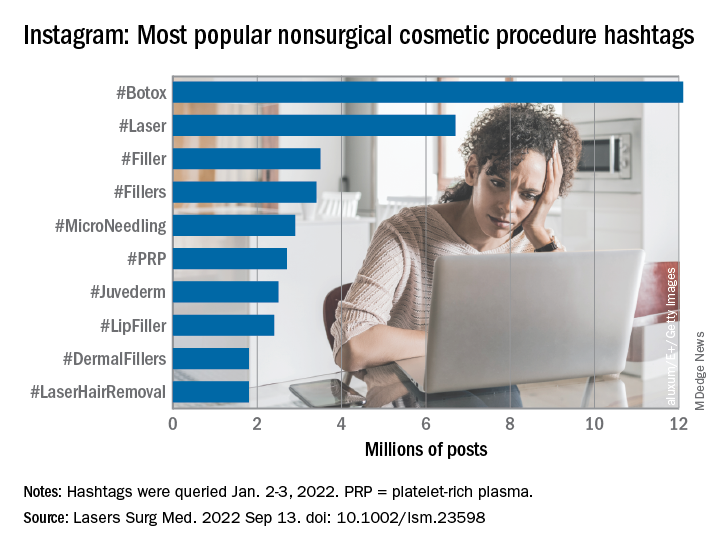
They generated a list of 25 hashtags related to nonsurgical cosmetic procedures, which were queried on Instagram on Jan. 2-3, 2022. The most popular was #Botox, with 12.1 million posts, followed by #Laser with 6.7 million, and #Filler with 3.5 million. Each of the 25 hashtags had at least 250,000 posts, they reported.
“Studies have shown that cosmetic patients and younger patients value social media when selecting a provider and patients often make treatment decisions based on social media,” they noted.
The bulk of the study involved “the first 10 posts displayed under the ‘Top’ section for each hashtag, as sorted by Instagram’s proprietary algorithm,” explained Dr. Murray and associates at the Dermatology and Laser Surgery Center in Houston. The 250 posts eventually selected for inclusion each received scrutiny in terms of content type and creator credentials.
Physicians in core cosmetic specialties – dermatology, plastic surgery, facial plastic surgery, and oculoplastic surgery – created just 12% of those 250 posts, compared with 68% for nonphysician providers, 13% for consumers/others, and 8% for other physicians, they said.
Educational content made up the largest share (38%) of posts by core cosmetic physicians, with before-and-after next at 31%, self-promotional at 21%, and personal at 10%. Nonphysician providers were the most likely to create before-and-after (49% of their total) and self-promotional (28%) content, while consumers had the largest share of promotional posts (31%) and other physicians posted the most entertainment content (25%), the researchers said.
An overall look at the content shows that the largest proportion of all 250 posts included in the analysis involved before-and-after photos (45%), with self-promotion next at 23%. Education represented just 17% of the posting total for nonsurgical cosmetic procedures, with personal, entertainment, and promotional each at 5%, Dr. Murray and associates reported.
By recognizing “the role social media plays in patients’ understanding of and desire to undergo nonsurgical cosmetic procedures” and “increasing their presence on Instagram, core cosmetic physicians can provide patient education, counteract misinformation, and raise awareness on training and qualifications,” they wrote.
The study authors did not provide any disclosures regarding funding or financial conflicts.
according to an analysis of related posts on the photo-sharing service.
“Given that there is little to no oversight on social networking sites, unqualified sources can widely disseminate misinformation resulting in misguided management or unnecessary procedures,” Taryn N. Murray, MD, and associates said in Lasers in Surgery and Medicine.
They generated a list of 25 hashtags related to nonsurgical cosmetic procedures, which were queried on Instagram on Jan. 2-3, 2022. The most popular was #Botox, with 12.1 million posts, followed by #Laser with 6.7 million, and #Filler with 3.5 million. Each of the 25 hashtags had at least 250,000 posts, they reported.
“Studies have shown that cosmetic patients and younger patients value social media when selecting a provider and patients often make treatment decisions based on social media,” they noted.
The bulk of the study involved “the first 10 posts displayed under the ‘Top’ section for each hashtag, as sorted by Instagram’s proprietary algorithm,” explained Dr. Murray and associates at the Dermatology and Laser Surgery Center in Houston. The 250 posts eventually selected for inclusion each received scrutiny in terms of content type and creator credentials.
Physicians in core cosmetic specialties – dermatology, plastic surgery, facial plastic surgery, and oculoplastic surgery – created just 12% of those 250 posts, compared with 68% for nonphysician providers, 13% for consumers/others, and 8% for other physicians, they said.
Educational content made up the largest share (38%) of posts by core cosmetic physicians, with before-and-after next at 31%, self-promotional at 21%, and personal at 10%. Nonphysician providers were the most likely to create before-and-after (49% of their total) and self-promotional (28%) content, while consumers had the largest share of promotional posts (31%) and other physicians posted the most entertainment content (25%), the researchers said.
An overall look at the content shows that the largest proportion of all 250 posts included in the analysis involved before-and-after photos (45%), with self-promotion next at 23%. Education represented just 17% of the posting total for nonsurgical cosmetic procedures, with personal, entertainment, and promotional each at 5%, Dr. Murray and associates reported.
By recognizing “the role social media plays in patients’ understanding of and desire to undergo nonsurgical cosmetic procedures” and “increasing their presence on Instagram, core cosmetic physicians can provide patient education, counteract misinformation, and raise awareness on training and qualifications,” they wrote.
The study authors did not provide any disclosures regarding funding or financial conflicts.
according to an analysis of related posts on the photo-sharing service.
“Given that there is little to no oversight on social networking sites, unqualified sources can widely disseminate misinformation resulting in misguided management or unnecessary procedures,” Taryn N. Murray, MD, and associates said in Lasers in Surgery and Medicine.
They generated a list of 25 hashtags related to nonsurgical cosmetic procedures, which were queried on Instagram on Jan. 2-3, 2022. The most popular was #Botox, with 12.1 million posts, followed by #Laser with 6.7 million, and #Filler with 3.5 million. Each of the 25 hashtags had at least 250,000 posts, they reported.
“Studies have shown that cosmetic patients and younger patients value social media when selecting a provider and patients often make treatment decisions based on social media,” they noted.
The bulk of the study involved “the first 10 posts displayed under the ‘Top’ section for each hashtag, as sorted by Instagram’s proprietary algorithm,” explained Dr. Murray and associates at the Dermatology and Laser Surgery Center in Houston. The 250 posts eventually selected for inclusion each received scrutiny in terms of content type and creator credentials.
Physicians in core cosmetic specialties – dermatology, plastic surgery, facial plastic surgery, and oculoplastic surgery – created just 12% of those 250 posts, compared with 68% for nonphysician providers, 13% for consumers/others, and 8% for other physicians, they said.
Educational content made up the largest share (38%) of posts by core cosmetic physicians, with before-and-after next at 31%, self-promotional at 21%, and personal at 10%. Nonphysician providers were the most likely to create before-and-after (49% of their total) and self-promotional (28%) content, while consumers had the largest share of promotional posts (31%) and other physicians posted the most entertainment content (25%), the researchers said.
An overall look at the content shows that the largest proportion of all 250 posts included in the analysis involved before-and-after photos (45%), with self-promotion next at 23%. Education represented just 17% of the posting total for nonsurgical cosmetic procedures, with personal, entertainment, and promotional each at 5%, Dr. Murray and associates reported.
By recognizing “the role social media plays in patients’ understanding of and desire to undergo nonsurgical cosmetic procedures” and “increasing their presence on Instagram, core cosmetic physicians can provide patient education, counteract misinformation, and raise awareness on training and qualifications,” they wrote.
The study authors did not provide any disclosures regarding funding or financial conflicts.
FROM LASERS IN SURGERY AND MEDICINE
What’s the true role of Demodex mites in the development of papulopustular rosacea?
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
FROM JEADV
Image-guided superficial radiation as first-line in skin cancer?
The study covered in this summary was published on medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaway
- Absolute lesion control rate with image-guided superficial radiation therapy (IGSRT) for early-stage nonmelanoma skin cancer was achieved in nearly all patients.
Why this matters
- IGSRT is a newer radiation technique for skin cancer, an alternative to Mohs micrographic surgery and other surgical options.
- The ultrasound imaging used during IGSRT allows for precise targeting of cancer cells while sparing surrounding tissue.
- IGSRT is currently recommended for early-stage nonmelanoma skin cancer among patients who refuse or cannot tolerate surgery.
- Given the safety, lack of surgical disfigurement, cost-effectiveness, and high cure rate, IGSRT should be considered more broadly as a first-line option for early-stage nonmelanoma skin cancer, the researchers concluded.
Study design
- The investigators reviewed 1,899 early-stage nonmelanoma skin cancer lesions in 1,243 patients treated with IGSRT at an outpatient dermatology clinic in Dallas.
- Energies ranged from 50 to 100 kV, with a mean treatment dose of 5,364.4 cGy over an average of 20.2 fractions.
- Treatment duration was a mean of 7.5 weeks and followed for a mean of 65.5 weeks.
Key results
- Absolute lesion control was achieved in 99.7% of patients, with a stable control rate of 99.6% past 12 months.
- At a 5-year follow-up, local control was 99.4%.
- Local control for both basal and squamous cell carcinoma at 5 years was 99%; local control for squamous cell carcinoma in situ was 100% at 5 years.
- The most common side effects were erythema, dryness, and dry desquamation. Some patients had ulceration and moist desquamation, but it did not affect lesion control.
- The procedure was well tolerated, with a grade 1 Radiation Treatment Oncology Group toxicity score in 72% of lesions.
- The results compare favorably with Mohs surgery.
Limitations
- No study limitations were noted.
Disclosures
- No funding source was reported.
- Senior investigator Lio Yu, MD, reported research, speaking and/or consulting for SkinCure Oncology, a developer of IGSRT technology.
This is a summary of a preprint research study, “Analysis of Image-Guided Superficial Radiation Therapy (IGSRT) on the Treatment of Early Stage Non-Melanoma Skin Cancer (NMSC) in the Outpatient Dermatology Setting,” led by Alison Tran, MD, of Baylor University Medical Center, Dallas. The study has not been peer reviewed. The full text can be found at medRxiv.org.
A version of this article first appeared on Medscape.com.
The study covered in this summary was published on medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaway
- Absolute lesion control rate with image-guided superficial radiation therapy (IGSRT) for early-stage nonmelanoma skin cancer was achieved in nearly all patients.
Why this matters
- IGSRT is a newer radiation technique for skin cancer, an alternative to Mohs micrographic surgery and other surgical options.
- The ultrasound imaging used during IGSRT allows for precise targeting of cancer cells while sparing surrounding tissue.
- IGSRT is currently recommended for early-stage nonmelanoma skin cancer among patients who refuse or cannot tolerate surgery.
- Given the safety, lack of surgical disfigurement, cost-effectiveness, and high cure rate, IGSRT should be considered more broadly as a first-line option for early-stage nonmelanoma skin cancer, the researchers concluded.
Study design
- The investigators reviewed 1,899 early-stage nonmelanoma skin cancer lesions in 1,243 patients treated with IGSRT at an outpatient dermatology clinic in Dallas.
- Energies ranged from 50 to 100 kV, with a mean treatment dose of 5,364.4 cGy over an average of 20.2 fractions.
- Treatment duration was a mean of 7.5 weeks and followed for a mean of 65.5 weeks.
Key results
- Absolute lesion control was achieved in 99.7% of patients, with a stable control rate of 99.6% past 12 months.
- At a 5-year follow-up, local control was 99.4%.
- Local control for both basal and squamous cell carcinoma at 5 years was 99%; local control for squamous cell carcinoma in situ was 100% at 5 years.
- The most common side effects were erythema, dryness, and dry desquamation. Some patients had ulceration and moist desquamation, but it did not affect lesion control.
- The procedure was well tolerated, with a grade 1 Radiation Treatment Oncology Group toxicity score in 72% of lesions.
- The results compare favorably with Mohs surgery.
Limitations
- No study limitations were noted.
Disclosures
- No funding source was reported.
- Senior investigator Lio Yu, MD, reported research, speaking and/or consulting for SkinCure Oncology, a developer of IGSRT technology.
This is a summary of a preprint research study, “Analysis of Image-Guided Superficial Radiation Therapy (IGSRT) on the Treatment of Early Stage Non-Melanoma Skin Cancer (NMSC) in the Outpatient Dermatology Setting,” led by Alison Tran, MD, of Baylor University Medical Center, Dallas. The study has not been peer reviewed. The full text can be found at medRxiv.org.
A version of this article first appeared on Medscape.com.
The study covered in this summary was published on medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaway
- Absolute lesion control rate with image-guided superficial radiation therapy (IGSRT) for early-stage nonmelanoma skin cancer was achieved in nearly all patients.
Why this matters
- IGSRT is a newer radiation technique for skin cancer, an alternative to Mohs micrographic surgery and other surgical options.
- The ultrasound imaging used during IGSRT allows for precise targeting of cancer cells while sparing surrounding tissue.
- IGSRT is currently recommended for early-stage nonmelanoma skin cancer among patients who refuse or cannot tolerate surgery.
- Given the safety, lack of surgical disfigurement, cost-effectiveness, and high cure rate, IGSRT should be considered more broadly as a first-line option for early-stage nonmelanoma skin cancer, the researchers concluded.
Study design
- The investigators reviewed 1,899 early-stage nonmelanoma skin cancer lesions in 1,243 patients treated with IGSRT at an outpatient dermatology clinic in Dallas.
- Energies ranged from 50 to 100 kV, with a mean treatment dose of 5,364.4 cGy over an average of 20.2 fractions.
- Treatment duration was a mean of 7.5 weeks and followed for a mean of 65.5 weeks.
Key results
- Absolute lesion control was achieved in 99.7% of patients, with a stable control rate of 99.6% past 12 months.
- At a 5-year follow-up, local control was 99.4%.
- Local control for both basal and squamous cell carcinoma at 5 years was 99%; local control for squamous cell carcinoma in situ was 100% at 5 years.
- The most common side effects were erythema, dryness, and dry desquamation. Some patients had ulceration and moist desquamation, but it did not affect lesion control.
- The procedure was well tolerated, with a grade 1 Radiation Treatment Oncology Group toxicity score in 72% of lesions.
- The results compare favorably with Mohs surgery.
Limitations
- No study limitations were noted.
Disclosures
- No funding source was reported.
- Senior investigator Lio Yu, MD, reported research, speaking and/or consulting for SkinCure Oncology, a developer of IGSRT technology.
This is a summary of a preprint research study, “Analysis of Image-Guided Superficial Radiation Therapy (IGSRT) on the Treatment of Early Stage Non-Melanoma Skin Cancer (NMSC) in the Outpatient Dermatology Setting,” led by Alison Tran, MD, of Baylor University Medical Center, Dallas. The study has not been peer reviewed. The full text can be found at medRxiv.org.
A version of this article first appeared on Medscape.com.
Monkeypox features include mucocutaneous involvement in almost all cases
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
AT THE EADV CONGRESS
Alopecia areata: Positive results reported for two investigational JAK inhibitors
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Training program linked to less hand eczema for hairdressers
The study was conducted in Denmark, where about 40% of hairdressers develop occupational hand eczema (OHE), according to researchers. Hairdressers globally are exposed to wet work and myriad skin irritants and allergens, including dyes, permanent-wave solutions, persulfates, preservatives, and fragrances. The study, which was funded by the Danish hairdressers and beauticians union, was published in Contact Dermatitis.
Lead author Martin Havmose, BSc, of the National Allergy Research Center, department of dermatology and allergy, University of Copenhagen, Hellerup, Denmark, and colleagues wrote that prevention is critical, inasmuch as eczema can cut careers short and have lasting health effects.
Up to 70% of hairdressers experience some sort of work-related skin damage in their careers, as reported by this news organization.
Hand eczema also is common among hairdressers in the United States, Mark Denis Davis, MD, chair of dermatology at the Mayo Clinic in Rochester, Minn., told this news organization. It can be quite debilitating, itchy, and painful, he said.
“Often it is associated with painful fissuring, cracks in the skin, particularly involving the fingers. It may also be unsightly,” he said.
Dr. Davis said he hears anecdotally in his practice that many hairdressers are reluctant to wear gloves because of the touch and dexterity needed in their work.
The researchers evaluated the risk of OHE and compliance with skin protection measures among hairdressers who were trained before Denmark rolled out a nationwide skin protection program in hairdressing vocational schools in 2011.
Questionnaires were sent in May 2009 to all hairdressers (96.4% women; average age, 26) who had graduated from 1985 to 2007; in May 2020, questionnaires were sent to all hairdressers who had graduated from 2008 to 2018.
The average time worked in the trade was 8 years, and 28.8% no longer worked as hairdressers, data show.
The response rate was 66.6% (305/460) for the 2009 survey and 29.9% (363/1215) for the 2020 survey.
Prevalence of OHE dropped after program
The prevalence of OHE during career time dropped from 42.8% to 29% (adjusted odds ratio, 0.55; 95% confidence interval [CI], 0.40-0.77) between the two surveys.
In addition, the incidence rate of OHE decreased from 57.5 (95% CI, 48.4-68.4) to 42.0 (95% CI, 34.6-50.9) per 1,000 person-years (incidence rate ratio, 0.73; 95% CI, 0.560.95) in that period.
There was an increase in the use of gloves between the two surveys. There was more glove use when the hairdressers engaged in wet work and handled dyes, products with bleach, and permanent-wave solutions (P < .05).
The nationwide program educates hairdressing apprentices on contact allergy/urticaria, how to prevent occupational skin disease, and skin biology. Teaching materials focus on 11 recommendations, 7 of which are related to glove use.
“The lack of primary prevention of OHE in hairdressing vocational schools may be a missed opportunity in the prevention of the disease,” the authors concluded.
Dr. Davis said hairdressers with hand eczema should know that in the short term, topical corticosteroids can be used to decrease the inflammation of the skin.
He highlighted the following advice from the authors:
- Gloves should be used when washing, dyeing, bleaching, and creating perms.
- Disposable gloves should never be reused.
- Gloves should be used only as long as necessary.
- Rings should not be worn at work.
- Cotton gloves should be worn underneath protective gloves.
- For clients who are having their hair both cut and dyed, the hair should be cut before it is dyed.
- Nitrile gloves should be used without rubber accelerators.
“In the longer term,” said Dr. Davis, “the most important thing is to avoid exposure to the precipitating factors, such as wet work – working with water, which irritates the skin – and avoiding any allergens that are contributing to the eczema.”
The study was funded by an unrestricted grant from the Danish hairdressers and beauticians union. Two coauthors have links to industry, as listed in the original article. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study was conducted in Denmark, where about 40% of hairdressers develop occupational hand eczema (OHE), according to researchers. Hairdressers globally are exposed to wet work and myriad skin irritants and allergens, including dyes, permanent-wave solutions, persulfates, preservatives, and fragrances. The study, which was funded by the Danish hairdressers and beauticians union, was published in Contact Dermatitis.
Lead author Martin Havmose, BSc, of the National Allergy Research Center, department of dermatology and allergy, University of Copenhagen, Hellerup, Denmark, and colleagues wrote that prevention is critical, inasmuch as eczema can cut careers short and have lasting health effects.
Up to 70% of hairdressers experience some sort of work-related skin damage in their careers, as reported by this news organization.
Hand eczema also is common among hairdressers in the United States, Mark Denis Davis, MD, chair of dermatology at the Mayo Clinic in Rochester, Minn., told this news organization. It can be quite debilitating, itchy, and painful, he said.
“Often it is associated with painful fissuring, cracks in the skin, particularly involving the fingers. It may also be unsightly,” he said.
Dr. Davis said he hears anecdotally in his practice that many hairdressers are reluctant to wear gloves because of the touch and dexterity needed in their work.
The researchers evaluated the risk of OHE and compliance with skin protection measures among hairdressers who were trained before Denmark rolled out a nationwide skin protection program in hairdressing vocational schools in 2011.
Questionnaires were sent in May 2009 to all hairdressers (96.4% women; average age, 26) who had graduated from 1985 to 2007; in May 2020, questionnaires were sent to all hairdressers who had graduated from 2008 to 2018.
The average time worked in the trade was 8 years, and 28.8% no longer worked as hairdressers, data show.
The response rate was 66.6% (305/460) for the 2009 survey and 29.9% (363/1215) for the 2020 survey.
Prevalence of OHE dropped after program
The prevalence of OHE during career time dropped from 42.8% to 29% (adjusted odds ratio, 0.55; 95% confidence interval [CI], 0.40-0.77) between the two surveys.
In addition, the incidence rate of OHE decreased from 57.5 (95% CI, 48.4-68.4) to 42.0 (95% CI, 34.6-50.9) per 1,000 person-years (incidence rate ratio, 0.73; 95% CI, 0.560.95) in that period.
There was an increase in the use of gloves between the two surveys. There was more glove use when the hairdressers engaged in wet work and handled dyes, products with bleach, and permanent-wave solutions (P < .05).
The nationwide program educates hairdressing apprentices on contact allergy/urticaria, how to prevent occupational skin disease, and skin biology. Teaching materials focus on 11 recommendations, 7 of which are related to glove use.
“The lack of primary prevention of OHE in hairdressing vocational schools may be a missed opportunity in the prevention of the disease,” the authors concluded.
Dr. Davis said hairdressers with hand eczema should know that in the short term, topical corticosteroids can be used to decrease the inflammation of the skin.
He highlighted the following advice from the authors:
- Gloves should be used when washing, dyeing, bleaching, and creating perms.
- Disposable gloves should never be reused.
- Gloves should be used only as long as necessary.
- Rings should not be worn at work.
- Cotton gloves should be worn underneath protective gloves.
- For clients who are having their hair both cut and dyed, the hair should be cut before it is dyed.
- Nitrile gloves should be used without rubber accelerators.
“In the longer term,” said Dr. Davis, “the most important thing is to avoid exposure to the precipitating factors, such as wet work – working with water, which irritates the skin – and avoiding any allergens that are contributing to the eczema.”
The study was funded by an unrestricted grant from the Danish hairdressers and beauticians union. Two coauthors have links to industry, as listed in the original article. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study was conducted in Denmark, where about 40% of hairdressers develop occupational hand eczema (OHE), according to researchers. Hairdressers globally are exposed to wet work and myriad skin irritants and allergens, including dyes, permanent-wave solutions, persulfates, preservatives, and fragrances. The study, which was funded by the Danish hairdressers and beauticians union, was published in Contact Dermatitis.
Lead author Martin Havmose, BSc, of the National Allergy Research Center, department of dermatology and allergy, University of Copenhagen, Hellerup, Denmark, and colleagues wrote that prevention is critical, inasmuch as eczema can cut careers short and have lasting health effects.
Up to 70% of hairdressers experience some sort of work-related skin damage in their careers, as reported by this news organization.
Hand eczema also is common among hairdressers in the United States, Mark Denis Davis, MD, chair of dermatology at the Mayo Clinic in Rochester, Minn., told this news organization. It can be quite debilitating, itchy, and painful, he said.
“Often it is associated with painful fissuring, cracks in the skin, particularly involving the fingers. It may also be unsightly,” he said.
Dr. Davis said he hears anecdotally in his practice that many hairdressers are reluctant to wear gloves because of the touch and dexterity needed in their work.
The researchers evaluated the risk of OHE and compliance with skin protection measures among hairdressers who were trained before Denmark rolled out a nationwide skin protection program in hairdressing vocational schools in 2011.
Questionnaires were sent in May 2009 to all hairdressers (96.4% women; average age, 26) who had graduated from 1985 to 2007; in May 2020, questionnaires were sent to all hairdressers who had graduated from 2008 to 2018.
The average time worked in the trade was 8 years, and 28.8% no longer worked as hairdressers, data show.
The response rate was 66.6% (305/460) for the 2009 survey and 29.9% (363/1215) for the 2020 survey.
Prevalence of OHE dropped after program
The prevalence of OHE during career time dropped from 42.8% to 29% (adjusted odds ratio, 0.55; 95% confidence interval [CI], 0.40-0.77) between the two surveys.
In addition, the incidence rate of OHE decreased from 57.5 (95% CI, 48.4-68.4) to 42.0 (95% CI, 34.6-50.9) per 1,000 person-years (incidence rate ratio, 0.73; 95% CI, 0.560.95) in that period.
There was an increase in the use of gloves between the two surveys. There was more glove use when the hairdressers engaged in wet work and handled dyes, products with bleach, and permanent-wave solutions (P < .05).
The nationwide program educates hairdressing apprentices on contact allergy/urticaria, how to prevent occupational skin disease, and skin biology. Teaching materials focus on 11 recommendations, 7 of which are related to glove use.
“The lack of primary prevention of OHE in hairdressing vocational schools may be a missed opportunity in the prevention of the disease,” the authors concluded.
Dr. Davis said hairdressers with hand eczema should know that in the short term, topical corticosteroids can be used to decrease the inflammation of the skin.
He highlighted the following advice from the authors:
- Gloves should be used when washing, dyeing, bleaching, and creating perms.
- Disposable gloves should never be reused.
- Gloves should be used only as long as necessary.
- Rings should not be worn at work.
- Cotton gloves should be worn underneath protective gloves.
- For clients who are having their hair both cut and dyed, the hair should be cut before it is dyed.
- Nitrile gloves should be used without rubber accelerators.
“In the longer term,” said Dr. Davis, “the most important thing is to avoid exposure to the precipitating factors, such as wet work – working with water, which irritates the skin – and avoiding any allergens that are contributing to the eczema.”
The study was funded by an unrestricted grant from the Danish hairdressers and beauticians union. Two coauthors have links to industry, as listed in the original article. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS