User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
‘Category 5’ COVID hurricane approaches, expert says
The United States is facing a “Category 5” storm as coronavirus variants begin to spread across the country, one of the nation’s top infectious disease experts said Sunday.
“We are going to see something like we have not seen yet in this country,” Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said on NBC’s Meet the Press.
The United States has reported 467 cases of the coronavirus variant first identified in the United Kingdom, across 32 states, according to the CDC variant tracker. The United States has also reported three cases of the variant first identified in South Africa in South Carolina and Maryland. One case of the variant first identified in Brazil has been found in Minnesota.
Although overall COVID-19 cases and hospitalizations have declined during the past few weeks, another storm is brewing on the horizon with the variants, Dr. Osterholm told host Chuck Todd. The U.K. variant will likely cause a surge in COVID-19 cases during the next 6-14 weeks, he said. “You and I are sitting on this beach where it’s 70 degrees, perfectly blue skies, gentle breeze. But I see that hurricane 5, Category 5 or higher, 450 miles offshore. And telling people to evacuate on that nice blue sky day is going to be hard. But I can also tell you that hurricane is coming.”
Dr. Osterholm urged federal and state officials to vaccinate as many people as possible to reduce the oncoming storm. The United States has distributed 49.9 million doses and administered 31.1 million doses, according to the latest CDC data updated Sunday, including 25.2 million first doses and 5.6 million second doses.
Doling out more doses to older Americans, rather than holding onto the second dose of the two-shot regimen, is an urgent decision, Dr. Osterholm said.
“I think right now, in advance of this surge, we need to get as many one doses in as many people over 65 as we possibly can to reduce serious illnesses and deaths that are going to occur over the weeks ahead,” he said.
The U.K. variant will likely become the dominant coronavirus strain in the United States in coming weeks, Dr. Osterholm said, adding that COVID-19 vaccines should be able to protect against it. In the meantime, however, he’s worried that the variant will cause more infections and deaths until more people get vaccinated.
“What we have to do now is also anticipate this and understand that we’re going to have change quickly,” he said. “As fast as we’re opening restaurants, we’re likely going to be closing them in the near term.”
A version of this article first appeared on WebMD.com.
The United States is facing a “Category 5” storm as coronavirus variants begin to spread across the country, one of the nation’s top infectious disease experts said Sunday.
“We are going to see something like we have not seen yet in this country,” Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said on NBC’s Meet the Press.
The United States has reported 467 cases of the coronavirus variant first identified in the United Kingdom, across 32 states, according to the CDC variant tracker. The United States has also reported three cases of the variant first identified in South Africa in South Carolina and Maryland. One case of the variant first identified in Brazil has been found in Minnesota.
Although overall COVID-19 cases and hospitalizations have declined during the past few weeks, another storm is brewing on the horizon with the variants, Dr. Osterholm told host Chuck Todd. The U.K. variant will likely cause a surge in COVID-19 cases during the next 6-14 weeks, he said. “You and I are sitting on this beach where it’s 70 degrees, perfectly blue skies, gentle breeze. But I see that hurricane 5, Category 5 or higher, 450 miles offshore. And telling people to evacuate on that nice blue sky day is going to be hard. But I can also tell you that hurricane is coming.”
Dr. Osterholm urged federal and state officials to vaccinate as many people as possible to reduce the oncoming storm. The United States has distributed 49.9 million doses and administered 31.1 million doses, according to the latest CDC data updated Sunday, including 25.2 million first doses and 5.6 million second doses.
Doling out more doses to older Americans, rather than holding onto the second dose of the two-shot regimen, is an urgent decision, Dr. Osterholm said.
“I think right now, in advance of this surge, we need to get as many one doses in as many people over 65 as we possibly can to reduce serious illnesses and deaths that are going to occur over the weeks ahead,” he said.
The U.K. variant will likely become the dominant coronavirus strain in the United States in coming weeks, Dr. Osterholm said, adding that COVID-19 vaccines should be able to protect against it. In the meantime, however, he’s worried that the variant will cause more infections and deaths until more people get vaccinated.
“What we have to do now is also anticipate this and understand that we’re going to have change quickly,” he said. “As fast as we’re opening restaurants, we’re likely going to be closing them in the near term.”
A version of this article first appeared on WebMD.com.
The United States is facing a “Category 5” storm as coronavirus variants begin to spread across the country, one of the nation’s top infectious disease experts said Sunday.
“We are going to see something like we have not seen yet in this country,” Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said on NBC’s Meet the Press.
The United States has reported 467 cases of the coronavirus variant first identified in the United Kingdom, across 32 states, according to the CDC variant tracker. The United States has also reported three cases of the variant first identified in South Africa in South Carolina and Maryland. One case of the variant first identified in Brazil has been found in Minnesota.
Although overall COVID-19 cases and hospitalizations have declined during the past few weeks, another storm is brewing on the horizon with the variants, Dr. Osterholm told host Chuck Todd. The U.K. variant will likely cause a surge in COVID-19 cases during the next 6-14 weeks, he said. “You and I are sitting on this beach where it’s 70 degrees, perfectly blue skies, gentle breeze. But I see that hurricane 5, Category 5 or higher, 450 miles offshore. And telling people to evacuate on that nice blue sky day is going to be hard. But I can also tell you that hurricane is coming.”
Dr. Osterholm urged federal and state officials to vaccinate as many people as possible to reduce the oncoming storm. The United States has distributed 49.9 million doses and administered 31.1 million doses, according to the latest CDC data updated Sunday, including 25.2 million first doses and 5.6 million second doses.
Doling out more doses to older Americans, rather than holding onto the second dose of the two-shot regimen, is an urgent decision, Dr. Osterholm said.
“I think right now, in advance of this surge, we need to get as many one doses in as many people over 65 as we possibly can to reduce serious illnesses and deaths that are going to occur over the weeks ahead,” he said.
The U.K. variant will likely become the dominant coronavirus strain in the United States in coming weeks, Dr. Osterholm said, adding that COVID-19 vaccines should be able to protect against it. In the meantime, however, he’s worried that the variant will cause more infections and deaths until more people get vaccinated.
“What we have to do now is also anticipate this and understand that we’re going to have change quickly,” he said. “As fast as we’re opening restaurants, we’re likely going to be closing them in the near term.”
A version of this article first appeared on WebMD.com.
Tough pain relief choices in the COVID-19 pandemic
More people with fever and body aches are turning to NSAIDs to ease symptoms, but the drugs have come under new scrutiny as investigators work to determine whether they are a safe way to relieve the pain of COVID-19 vaccination or symptoms of the disease.
Early on in the pandemic, French health officials warned that NSAIDs, such as ibuprofen, could worsen coronavirus disease, and they recommended switching to acetaminophen instead.
The National Health Service in the United Kingdom followed with a similar recommendation for acetaminophen.
But the European Medicines Agency took a different approach, reporting “no scientific evidence” that NSAIDs could worsen COVID-19. The U.S. Food and Drug Administration also opted not to take a stance.
The debate prompted discussion on social media, with various reactions from around the world. It also inspired Craig Wilen, MD, PhD, from Yale University, New Haven, Conn., and associates to examine the effect of NSAIDs on COVID-19 infection and immune response. Their findings were published online Jan.20 in the Journal of Virology.
“It really bothered me that non–evidence-based decisions were driving the conversation,” Dr. Wilen said. “Millions of people are taking NSAIDs every day and clinical decisions about their care shouldn’t be made on a hypothesis.”
One theory is that NSAIDs alter susceptibility to infection by modifying ACE2. The drugs might also change the cell entry receptor for SARS-CoV-2, alter virus replication, or even modify the immune response.
British researchers, also questioning the safety of NSAIDs in patients with COVID-19, delved into National Health Service records to study two large groups of patients, some of whom were taking the pain relievers.
“We were watching the controversy and the lack of evidence and wanted to contribute,” lead investigator Angel Wong, PhD, from the London School of Hygiene and Tropical Medicine, said in an interview.
And with nearly 11 million NSAID prescriptions dispensed in primary care in England alone in the past 12 months, the inconsistency was concerning.
The team compared COVID-19–related deaths in two groups: one group of more than 700,000 people taking NSAIDs, including patients with rheumatoid arthritis and osteoarthritis; and another of almost 3.5 million people not on the medication.
NSAIDs work by inhibiting cyclooxygenase-1 and COX-2 enzymes in the body, which are crucial for the generation of prostaglandins. These lipid molecules play a role in inflammation and are blocked by NSAIDs.
The investigators found no evidence of a harmful effect of NSAIDs on COVID-19-related deaths; their results were published online Jan. 21 in the Annals of the Rheumatic Diseases.
The results, they pointed out, are in line with a Danish study that also showed no evidence of a higher risk for severe COVID-19 outcomes with NSAID use.
“It’s reassuring,” Dr. Wong said, “that patients can safely continue treatment.”
More new evidence
Dr. Wilen’s team found that SARS-CoV-2 infection stimulated COX-2 expression in human and mice cells. However, suppression of COX-2 by two commonly used NSAIDs, ibuprofen and meloxicam, had no effect on ACE2 expression, viral entry, or viral replication.
In their mouse model of SARS-CoV-2 infection, the investigators saw that NSAIDs impaired the production of proinflammatory cytokines and neutralizing antibodies. The findings suggest that NSAIDs influence COVID-19 outcomes by dampening the inflammatory response and production of protective antibodies, rather than modifying susceptibility to infection or viral replication.
Understanding the effect of NSAIDs on cytokine production is critical, Dr. Wilen pointed out, because they might be protective early in COVID-19 but pathologic at later stages.
Timing is crucial in the case of other immunomodulatory drugs. For example, dexamethasone lowers mortality in COVID-19 patients on respiratory support but is potentially harmful for those with milder disease.
There still is a lot to learn, Dr. Wilen acknowledged. “We may be seeing something similar going on with NSAIDs, where the timing of treatment is important.”
A version of this article first appeared on Medscape.com.
More people with fever and body aches are turning to NSAIDs to ease symptoms, but the drugs have come under new scrutiny as investigators work to determine whether they are a safe way to relieve the pain of COVID-19 vaccination or symptoms of the disease.
Early on in the pandemic, French health officials warned that NSAIDs, such as ibuprofen, could worsen coronavirus disease, and they recommended switching to acetaminophen instead.
The National Health Service in the United Kingdom followed with a similar recommendation for acetaminophen.
But the European Medicines Agency took a different approach, reporting “no scientific evidence” that NSAIDs could worsen COVID-19. The U.S. Food and Drug Administration also opted not to take a stance.
The debate prompted discussion on social media, with various reactions from around the world. It also inspired Craig Wilen, MD, PhD, from Yale University, New Haven, Conn., and associates to examine the effect of NSAIDs on COVID-19 infection and immune response. Their findings were published online Jan.20 in the Journal of Virology.
“It really bothered me that non–evidence-based decisions were driving the conversation,” Dr. Wilen said. “Millions of people are taking NSAIDs every day and clinical decisions about their care shouldn’t be made on a hypothesis.”
One theory is that NSAIDs alter susceptibility to infection by modifying ACE2. The drugs might also change the cell entry receptor for SARS-CoV-2, alter virus replication, or even modify the immune response.
British researchers, also questioning the safety of NSAIDs in patients with COVID-19, delved into National Health Service records to study two large groups of patients, some of whom were taking the pain relievers.
“We were watching the controversy and the lack of evidence and wanted to contribute,” lead investigator Angel Wong, PhD, from the London School of Hygiene and Tropical Medicine, said in an interview.
And with nearly 11 million NSAID prescriptions dispensed in primary care in England alone in the past 12 months, the inconsistency was concerning.
The team compared COVID-19–related deaths in two groups: one group of more than 700,000 people taking NSAIDs, including patients with rheumatoid arthritis and osteoarthritis; and another of almost 3.5 million people not on the medication.
NSAIDs work by inhibiting cyclooxygenase-1 and COX-2 enzymes in the body, which are crucial for the generation of prostaglandins. These lipid molecules play a role in inflammation and are blocked by NSAIDs.
The investigators found no evidence of a harmful effect of NSAIDs on COVID-19-related deaths; their results were published online Jan. 21 in the Annals of the Rheumatic Diseases.
The results, they pointed out, are in line with a Danish study that also showed no evidence of a higher risk for severe COVID-19 outcomes with NSAID use.
“It’s reassuring,” Dr. Wong said, “that patients can safely continue treatment.”
More new evidence
Dr. Wilen’s team found that SARS-CoV-2 infection stimulated COX-2 expression in human and mice cells. However, suppression of COX-2 by two commonly used NSAIDs, ibuprofen and meloxicam, had no effect on ACE2 expression, viral entry, or viral replication.
In their mouse model of SARS-CoV-2 infection, the investigators saw that NSAIDs impaired the production of proinflammatory cytokines and neutralizing antibodies. The findings suggest that NSAIDs influence COVID-19 outcomes by dampening the inflammatory response and production of protective antibodies, rather than modifying susceptibility to infection or viral replication.
Understanding the effect of NSAIDs on cytokine production is critical, Dr. Wilen pointed out, because they might be protective early in COVID-19 but pathologic at later stages.
Timing is crucial in the case of other immunomodulatory drugs. For example, dexamethasone lowers mortality in COVID-19 patients on respiratory support but is potentially harmful for those with milder disease.
There still is a lot to learn, Dr. Wilen acknowledged. “We may be seeing something similar going on with NSAIDs, where the timing of treatment is important.”
A version of this article first appeared on Medscape.com.
More people with fever and body aches are turning to NSAIDs to ease symptoms, but the drugs have come under new scrutiny as investigators work to determine whether they are a safe way to relieve the pain of COVID-19 vaccination or symptoms of the disease.
Early on in the pandemic, French health officials warned that NSAIDs, such as ibuprofen, could worsen coronavirus disease, and they recommended switching to acetaminophen instead.
The National Health Service in the United Kingdom followed with a similar recommendation for acetaminophen.
But the European Medicines Agency took a different approach, reporting “no scientific evidence” that NSAIDs could worsen COVID-19. The U.S. Food and Drug Administration also opted not to take a stance.
The debate prompted discussion on social media, with various reactions from around the world. It also inspired Craig Wilen, MD, PhD, from Yale University, New Haven, Conn., and associates to examine the effect of NSAIDs on COVID-19 infection and immune response. Their findings were published online Jan.20 in the Journal of Virology.
“It really bothered me that non–evidence-based decisions were driving the conversation,” Dr. Wilen said. “Millions of people are taking NSAIDs every day and clinical decisions about their care shouldn’t be made on a hypothesis.”
One theory is that NSAIDs alter susceptibility to infection by modifying ACE2. The drugs might also change the cell entry receptor for SARS-CoV-2, alter virus replication, or even modify the immune response.
British researchers, also questioning the safety of NSAIDs in patients with COVID-19, delved into National Health Service records to study two large groups of patients, some of whom were taking the pain relievers.
“We were watching the controversy and the lack of evidence and wanted to contribute,” lead investigator Angel Wong, PhD, from the London School of Hygiene and Tropical Medicine, said in an interview.
And with nearly 11 million NSAID prescriptions dispensed in primary care in England alone in the past 12 months, the inconsistency was concerning.
The team compared COVID-19–related deaths in two groups: one group of more than 700,000 people taking NSAIDs, including patients with rheumatoid arthritis and osteoarthritis; and another of almost 3.5 million people not on the medication.
NSAIDs work by inhibiting cyclooxygenase-1 and COX-2 enzymes in the body, which are crucial for the generation of prostaglandins. These lipid molecules play a role in inflammation and are blocked by NSAIDs.
The investigators found no evidence of a harmful effect of NSAIDs on COVID-19-related deaths; their results were published online Jan. 21 in the Annals of the Rheumatic Diseases.
The results, they pointed out, are in line with a Danish study that also showed no evidence of a higher risk for severe COVID-19 outcomes with NSAID use.
“It’s reassuring,” Dr. Wong said, “that patients can safely continue treatment.”
More new evidence
Dr. Wilen’s team found that SARS-CoV-2 infection stimulated COX-2 expression in human and mice cells. However, suppression of COX-2 by two commonly used NSAIDs, ibuprofen and meloxicam, had no effect on ACE2 expression, viral entry, or viral replication.
In their mouse model of SARS-CoV-2 infection, the investigators saw that NSAIDs impaired the production of proinflammatory cytokines and neutralizing antibodies. The findings suggest that NSAIDs influence COVID-19 outcomes by dampening the inflammatory response and production of protective antibodies, rather than modifying susceptibility to infection or viral replication.
Understanding the effect of NSAIDs on cytokine production is critical, Dr. Wilen pointed out, because they might be protective early in COVID-19 but pathologic at later stages.
Timing is crucial in the case of other immunomodulatory drugs. For example, dexamethasone lowers mortality in COVID-19 patients on respiratory support but is potentially harmful for those with milder disease.
There still is a lot to learn, Dr. Wilen acknowledged. “We may be seeing something similar going on with NSAIDs, where the timing of treatment is important.”
A version of this article first appeared on Medscape.com.
Dr. Fauci sees ‘wake-up call’ in emergence of new virus variants
New data on COVID-19 vaccines should serve as a “wake-up call” about the need to stop the spread of the SARS-CoV-2 virus among people and thus deprive it of opportunities to evolve its defenses, the top federal expert on infectious diseases said.
“The virus will continue to mutate and will mutate for its own selective advantage,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, at a Friday news conference organized by the White House.
The continued transmission of SARS-CoV-2 “gives the virus the chance to adapt to the forces, in this case the immune response, that’s trying to get rid of it,” Dr. Fauci said. “That’s where you get mutations.”
Federal health officials are working to boost the U.S. supply of COVID-19 vaccines, even as signals emerge about the extent that the virus is already evolving.
Data released this week about the Janssen/Johnson & Johnson (J&J) and Novavax COVID-19 vaccines in late-stage development provides further evidence that they may not protect as well against emerging variants, Dr. Fauci said.
“Mutations that lead to different lineage do have clinical consequences,” he said, while also emphasizing that the emerging vaccines appear to confer broad protection. Dr. Fauci earlier in the day addressed the “messaging challenge” for clinicians and researchers in discussing the results of the J&J vaccine trial, which appear to fall short of those reported for the two vaccines already approved and in use in the United States. He noted the benefits of possibly soon having more authorized vaccines to combat COVID-19. But continued community spread of the infection will foster conditions that can undermine the vaccines’ effectiveness.
“Even though the long-range effect in the sense of severe disease is still handled reasonably well by the vaccines, this is a wake-up call to all of us,” Dr. Fauci said.
Pharmaceutical scientists and executives and government health officials will need to work together to continue to develop vaccines that can outwit the emerging variants, he said.
On Jan. 29, J&J reported that its highly anticipated single-dose vaccine had shown its worst results in South Africa where many cases of COVID-19 were caused by infection with a SARS-CoV-2 variant from the B.1.351 lineage. The overall efficacy was 66% globally, 72% in the United States, and 57% in South Africa against moderate to severe SARS-CoV-2, J&J said.
Novavax on Jan. 28 reported an efficacy rate for its COVID-19 vaccine of 49.4% from a clinical trial conducted in South Africa, compared with an 89.3% rate from a U.K. study. There already have been attempts to estimate how well the Pfizer/BioNTech and Moderna vaccines can handle new variants of the virus. They both have been granted emergency-use authorization by the U.S. Food and Drug Administration.
‘Genomic surveillance’
The Centers for Disease Control and Prevention on Thursday reported the first U.S.-documented cases of the B.1.351 variant of SARS-CoV-2 in South Carolina. On Jan. 26, the first confirmed U.S. case of a highly transmissible Brazilian coronavirus variant was detected in Minnesota, state health officials said.
The CDC’s stepped-up “genomic surveillance” will help keep clinicians and researchers aware of how SARS-CoV-2 is changing, Dr. Fauci said.
Speaking at the same White House news conference, CDC director Rochelle Walensky, MD, MPH, said the two South Carolina cases of the B.1.351 variant were reported in different parts of the state and not believed to be epidemiologically linked. The people involved “did not have any travel history,” she added.
The SARS-CoV-2 mutations were expected to emerge at some point, as with any virus, but their appearance underscores the need for people to remain vigilant about precautions that can stop its spread, Dr. Walensky said.
She and Dr. Fauci both stressed the need for continued use of masks and social distancing and urged people to get COVID-19 vaccines as they become available. Continued community spread of the virus allows this global health threat to keep replicating, and thus increases its chances to thwart medical interventions, Dr. Fauci said.
“The virus has a playing field, as it were, to mutate,” Dr. Fauci said. “If you stop that and stop the replication, the viruses cannot mutate if they don’t replicate.”
A version of this article first appeared on Medscape.com.
New data on COVID-19 vaccines should serve as a “wake-up call” about the need to stop the spread of the SARS-CoV-2 virus among people and thus deprive it of opportunities to evolve its defenses, the top federal expert on infectious diseases said.
“The virus will continue to mutate and will mutate for its own selective advantage,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, at a Friday news conference organized by the White House.
The continued transmission of SARS-CoV-2 “gives the virus the chance to adapt to the forces, in this case the immune response, that’s trying to get rid of it,” Dr. Fauci said. “That’s where you get mutations.”
Federal health officials are working to boost the U.S. supply of COVID-19 vaccines, even as signals emerge about the extent that the virus is already evolving.
Data released this week about the Janssen/Johnson & Johnson (J&J) and Novavax COVID-19 vaccines in late-stage development provides further evidence that they may not protect as well against emerging variants, Dr. Fauci said.
“Mutations that lead to different lineage do have clinical consequences,” he said, while also emphasizing that the emerging vaccines appear to confer broad protection. Dr. Fauci earlier in the day addressed the “messaging challenge” for clinicians and researchers in discussing the results of the J&J vaccine trial, which appear to fall short of those reported for the two vaccines already approved and in use in the United States. He noted the benefits of possibly soon having more authorized vaccines to combat COVID-19. But continued community spread of the infection will foster conditions that can undermine the vaccines’ effectiveness.
“Even though the long-range effect in the sense of severe disease is still handled reasonably well by the vaccines, this is a wake-up call to all of us,” Dr. Fauci said.
Pharmaceutical scientists and executives and government health officials will need to work together to continue to develop vaccines that can outwit the emerging variants, he said.
On Jan. 29, J&J reported that its highly anticipated single-dose vaccine had shown its worst results in South Africa where many cases of COVID-19 were caused by infection with a SARS-CoV-2 variant from the B.1.351 lineage. The overall efficacy was 66% globally, 72% in the United States, and 57% in South Africa against moderate to severe SARS-CoV-2, J&J said.
Novavax on Jan. 28 reported an efficacy rate for its COVID-19 vaccine of 49.4% from a clinical trial conducted in South Africa, compared with an 89.3% rate from a U.K. study. There already have been attempts to estimate how well the Pfizer/BioNTech and Moderna vaccines can handle new variants of the virus. They both have been granted emergency-use authorization by the U.S. Food and Drug Administration.
‘Genomic surveillance’
The Centers for Disease Control and Prevention on Thursday reported the first U.S.-documented cases of the B.1.351 variant of SARS-CoV-2 in South Carolina. On Jan. 26, the first confirmed U.S. case of a highly transmissible Brazilian coronavirus variant was detected in Minnesota, state health officials said.
The CDC’s stepped-up “genomic surveillance” will help keep clinicians and researchers aware of how SARS-CoV-2 is changing, Dr. Fauci said.
Speaking at the same White House news conference, CDC director Rochelle Walensky, MD, MPH, said the two South Carolina cases of the B.1.351 variant were reported in different parts of the state and not believed to be epidemiologically linked. The people involved “did not have any travel history,” she added.
The SARS-CoV-2 mutations were expected to emerge at some point, as with any virus, but their appearance underscores the need for people to remain vigilant about precautions that can stop its spread, Dr. Walensky said.
She and Dr. Fauci both stressed the need for continued use of masks and social distancing and urged people to get COVID-19 vaccines as they become available. Continued community spread of the virus allows this global health threat to keep replicating, and thus increases its chances to thwart medical interventions, Dr. Fauci said.
“The virus has a playing field, as it were, to mutate,” Dr. Fauci said. “If you stop that and stop the replication, the viruses cannot mutate if they don’t replicate.”
A version of this article first appeared on Medscape.com.
New data on COVID-19 vaccines should serve as a “wake-up call” about the need to stop the spread of the SARS-CoV-2 virus among people and thus deprive it of opportunities to evolve its defenses, the top federal expert on infectious diseases said.
“The virus will continue to mutate and will mutate for its own selective advantage,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, at a Friday news conference organized by the White House.
The continued transmission of SARS-CoV-2 “gives the virus the chance to adapt to the forces, in this case the immune response, that’s trying to get rid of it,” Dr. Fauci said. “That’s where you get mutations.”
Federal health officials are working to boost the U.S. supply of COVID-19 vaccines, even as signals emerge about the extent that the virus is already evolving.
Data released this week about the Janssen/Johnson & Johnson (J&J) and Novavax COVID-19 vaccines in late-stage development provides further evidence that they may not protect as well against emerging variants, Dr. Fauci said.
“Mutations that lead to different lineage do have clinical consequences,” he said, while also emphasizing that the emerging vaccines appear to confer broad protection. Dr. Fauci earlier in the day addressed the “messaging challenge” for clinicians and researchers in discussing the results of the J&J vaccine trial, which appear to fall short of those reported for the two vaccines already approved and in use in the United States. He noted the benefits of possibly soon having more authorized vaccines to combat COVID-19. But continued community spread of the infection will foster conditions that can undermine the vaccines’ effectiveness.
“Even though the long-range effect in the sense of severe disease is still handled reasonably well by the vaccines, this is a wake-up call to all of us,” Dr. Fauci said.
Pharmaceutical scientists and executives and government health officials will need to work together to continue to develop vaccines that can outwit the emerging variants, he said.
On Jan. 29, J&J reported that its highly anticipated single-dose vaccine had shown its worst results in South Africa where many cases of COVID-19 were caused by infection with a SARS-CoV-2 variant from the B.1.351 lineage. The overall efficacy was 66% globally, 72% in the United States, and 57% in South Africa against moderate to severe SARS-CoV-2, J&J said.
Novavax on Jan. 28 reported an efficacy rate for its COVID-19 vaccine of 49.4% from a clinical trial conducted in South Africa, compared with an 89.3% rate from a U.K. study. There already have been attempts to estimate how well the Pfizer/BioNTech and Moderna vaccines can handle new variants of the virus. They both have been granted emergency-use authorization by the U.S. Food and Drug Administration.
‘Genomic surveillance’
The Centers for Disease Control and Prevention on Thursday reported the first U.S.-documented cases of the B.1.351 variant of SARS-CoV-2 in South Carolina. On Jan. 26, the first confirmed U.S. case of a highly transmissible Brazilian coronavirus variant was detected in Minnesota, state health officials said.
The CDC’s stepped-up “genomic surveillance” will help keep clinicians and researchers aware of how SARS-CoV-2 is changing, Dr. Fauci said.
Speaking at the same White House news conference, CDC director Rochelle Walensky, MD, MPH, said the two South Carolina cases of the B.1.351 variant were reported in different parts of the state and not believed to be epidemiologically linked. The people involved “did not have any travel history,” she added.
The SARS-CoV-2 mutations were expected to emerge at some point, as with any virus, but their appearance underscores the need for people to remain vigilant about precautions that can stop its spread, Dr. Walensky said.
She and Dr. Fauci both stressed the need for continued use of masks and social distancing and urged people to get COVID-19 vaccines as they become available. Continued community spread of the virus allows this global health threat to keep replicating, and thus increases its chances to thwart medical interventions, Dr. Fauci said.
“The virus has a playing field, as it were, to mutate,” Dr. Fauci said. “If you stop that and stop the replication, the viruses cannot mutate if they don’t replicate.”
A version of this article first appeared on Medscape.com.
Gene expression profile test helps inform management of high-risk SCC patients
, according to Anna A. Bar, MD.
“The incidence of SCC has been growing rapidly, and the disease-related mortality is actually more than that of melanoma,” Dr. Bar, associate professor of dermatology at Oregon Health & Science University, Portland, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
“Like many cancers, SCC management plans are guided by the risk of metastasis. The current staging systems, like NCCN, AJCC, or Brigham and Women’s systems, struggle to provide accurate data of the metastatic potential of an individual’s SCC,” she said. “Furthermore, the predictive accuracy of these systems in SCC is variable, and many patients who have high risk factors do not experience poor outcomes, while others initially classified as having less concerning tumors will go on to have metastatic disease. That is where new gene expression tests come into play.”
Developed by and commercially available from Castle Biosciences, DecisionDx-SCC classifies an individual SCC patient’s tumor into one of the categories: low (class 1), moderate (class 2A), or high (class 2B) biologic risk of metastasis. “We’re hoping that DecisionDx results can help make management decisions within established guidelines,” Dr. Bar said. The test is indicated for patients with high-risk features including tumor size greater than 2 cm; tumor location on the head, neck, hands, genitals, feet, or pretibial surface; immunosuppression; a rapidly growing tumor; a tumor with poorly defined borders; a tumor at the site of prior radiation or chronic inflammation; perineural invasion; poorly defined tumor grade, and a deep tumor beyond the subcutaneous fat.
One validity study and three clinical utility studies of DecisionDx-SCC have been published that include data from more than 1,100 patients (see Curr Med Res Opin. 2020 Aug;36[8]:1301-7; Curr Med Res Opin. 2020 Aug;36[8]:1295-1300, and J Drugs Dermatol. 2019 Oct 1;18[10]:980-4). “This is a work in progress,” said Dr. Bar, director of the university’s Mohs micrographic surgery and cutaneous oncology fellowship.
The test was validated in an another study, which was prospectively designed and used archival tissue from 33 independent academic and community centers, including Oregon Health & Science University. All 420 patients in the clinical validation study had one or more high-risk factors, meeting the definition of high risk by NCCN or Mohs Appropriate Use Criteria (AUC). Their mean age was 71 years, 73% were male, 99% were White, and 25% were immune deficient.
Of the 420 patients, 63 had metastasis, and 86% of metastases were located on the head and neck. About 30% of metastasized lesions had perineural involvement, 27% had invasion beyond subcutaneous fat, and metastasized lesions were about 1 cm wider compared with lesions that were not. The overall metastasis rate at 3 years was 15%, “which is similar to that seen in the medical literature for high-risk populations,” Dr. Bar said.
The median time to metastasis was 0.9 years and the 95th percentile was 2.7 years. “This means that the 3-year horizon for identifying events in this study enabled identification of most patients who eventually experienced metastatic events,” she said. In this cohort, approximately half of the metastatic events occurred around 11 months post diagnosis, which “may provide guidance about the timeline and duration of high-intensity follow-up with frequency of clinical visits and imaging for patients at highest risk within the first year.”
The positive predictive value of the DecisionDx-SCC is 52%, meaning that half of class 2B lesions will metastasize. “This compares favorably when you look at the lower positive predictive value of the other staging systems,” Dr. Bar said. “The negative predictive value is 93%, meaning there are not a lot of false negatives. This also compares favorably to the other staging systems.”
Kaplan-Meier analysis of metastasis-free survival showed strong separation between patients with class 1, class 2A, and class 2B results, Dr. Bar said. While the overall risk of metastasis in this patient cohort was 15%, the risk among those with a class 1 result was less than half of that. “Patients with a class 2A result behave similarly to those with traditional risk factors such as deep invasion and poor differentiation, having about a 20% risk of metastasis,” she said. “The class 2B result identifies the most worrisome SCCs, with a greater than 50% risk of metastasis. While the results distribution from routine clinical testing is not yet known, this large validation study of high-risk SCC revealed that approximately half of the patients were class 1, less than half were class 2A, and about 1 in 18 had a class 2B result.”
On univariate analyses with traditional risk factors and use of the Brigham and Women’s staging system, the hazard ratio (HR) for class 2A lesions was 3.2, “which is similar to deep invasion, poor differentiation, or perineural involvement,” Dr. Bar said. At the same time, the HR for class 2B lesions was 11.6, “so class 2B is the strongest predictor of metastasis. The class 2B HR remained statistically significant in the multivariate analysis and is three times higher than that of the next highest HR in this cohort. For example, a high-risk SCC with deep invasion is already two times more likely to metastasize. Adding a class 2B score would be over 14 times more likely to metastasize than a tumor with a class 1 result.”
DecisionDx-SCC test results can inform management decisions within established guidelines. For example, for a high-risk SCC patient who has a class 1 result, or low risk of metastasis, “you may proceed with surgery and clinical nodal exam, and then follow up a couple of times a year,” Dr. Bar said. “For a high-risk patient with a 2A or moderate risk result, you might proceed with surgical treatment plus consider imaging studies such as ultrasound, CT, PET CT, and consider referral to other specialties.”
For a high-risk patient with a 2B or high risk result, she continued, “you may want to proceed with imaging studies right away in addition to surgery and consider consultation with radiation oncology or medical oncology, as well as more frequent follow-up with nodal exams, because the class 2B patients have been shown to have a greater than 50% risk of metastasis.”
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Bar disclosed that Oregon Health & Science University has received research funding from Castle Biosciences.
, according to Anna A. Bar, MD.
“The incidence of SCC has been growing rapidly, and the disease-related mortality is actually more than that of melanoma,” Dr. Bar, associate professor of dermatology at Oregon Health & Science University, Portland, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
“Like many cancers, SCC management plans are guided by the risk of metastasis. The current staging systems, like NCCN, AJCC, or Brigham and Women’s systems, struggle to provide accurate data of the metastatic potential of an individual’s SCC,” she said. “Furthermore, the predictive accuracy of these systems in SCC is variable, and many patients who have high risk factors do not experience poor outcomes, while others initially classified as having less concerning tumors will go on to have metastatic disease. That is where new gene expression tests come into play.”
Developed by and commercially available from Castle Biosciences, DecisionDx-SCC classifies an individual SCC patient’s tumor into one of the categories: low (class 1), moderate (class 2A), or high (class 2B) biologic risk of metastasis. “We’re hoping that DecisionDx results can help make management decisions within established guidelines,” Dr. Bar said. The test is indicated for patients with high-risk features including tumor size greater than 2 cm; tumor location on the head, neck, hands, genitals, feet, or pretibial surface; immunosuppression; a rapidly growing tumor; a tumor with poorly defined borders; a tumor at the site of prior radiation or chronic inflammation; perineural invasion; poorly defined tumor grade, and a deep tumor beyond the subcutaneous fat.
One validity study and three clinical utility studies of DecisionDx-SCC have been published that include data from more than 1,100 patients (see Curr Med Res Opin. 2020 Aug;36[8]:1301-7; Curr Med Res Opin. 2020 Aug;36[8]:1295-1300, and J Drugs Dermatol. 2019 Oct 1;18[10]:980-4). “This is a work in progress,” said Dr. Bar, director of the university’s Mohs micrographic surgery and cutaneous oncology fellowship.
The test was validated in an another study, which was prospectively designed and used archival tissue from 33 independent academic and community centers, including Oregon Health & Science University. All 420 patients in the clinical validation study had one or more high-risk factors, meeting the definition of high risk by NCCN or Mohs Appropriate Use Criteria (AUC). Their mean age was 71 years, 73% were male, 99% were White, and 25% were immune deficient.
Of the 420 patients, 63 had metastasis, and 86% of metastases were located on the head and neck. About 30% of metastasized lesions had perineural involvement, 27% had invasion beyond subcutaneous fat, and metastasized lesions were about 1 cm wider compared with lesions that were not. The overall metastasis rate at 3 years was 15%, “which is similar to that seen in the medical literature for high-risk populations,” Dr. Bar said.
The median time to metastasis was 0.9 years and the 95th percentile was 2.7 years. “This means that the 3-year horizon for identifying events in this study enabled identification of most patients who eventually experienced metastatic events,” she said. In this cohort, approximately half of the metastatic events occurred around 11 months post diagnosis, which “may provide guidance about the timeline and duration of high-intensity follow-up with frequency of clinical visits and imaging for patients at highest risk within the first year.”
The positive predictive value of the DecisionDx-SCC is 52%, meaning that half of class 2B lesions will metastasize. “This compares favorably when you look at the lower positive predictive value of the other staging systems,” Dr. Bar said. “The negative predictive value is 93%, meaning there are not a lot of false negatives. This also compares favorably to the other staging systems.”
Kaplan-Meier analysis of metastasis-free survival showed strong separation between patients with class 1, class 2A, and class 2B results, Dr. Bar said. While the overall risk of metastasis in this patient cohort was 15%, the risk among those with a class 1 result was less than half of that. “Patients with a class 2A result behave similarly to those with traditional risk factors such as deep invasion and poor differentiation, having about a 20% risk of metastasis,” she said. “The class 2B result identifies the most worrisome SCCs, with a greater than 50% risk of metastasis. While the results distribution from routine clinical testing is not yet known, this large validation study of high-risk SCC revealed that approximately half of the patients were class 1, less than half were class 2A, and about 1 in 18 had a class 2B result.”
On univariate analyses with traditional risk factors and use of the Brigham and Women’s staging system, the hazard ratio (HR) for class 2A lesions was 3.2, “which is similar to deep invasion, poor differentiation, or perineural involvement,” Dr. Bar said. At the same time, the HR for class 2B lesions was 11.6, “so class 2B is the strongest predictor of metastasis. The class 2B HR remained statistically significant in the multivariate analysis and is three times higher than that of the next highest HR in this cohort. For example, a high-risk SCC with deep invasion is already two times more likely to metastasize. Adding a class 2B score would be over 14 times more likely to metastasize than a tumor with a class 1 result.”
DecisionDx-SCC test results can inform management decisions within established guidelines. For example, for a high-risk SCC patient who has a class 1 result, or low risk of metastasis, “you may proceed with surgery and clinical nodal exam, and then follow up a couple of times a year,” Dr. Bar said. “For a high-risk patient with a 2A or moderate risk result, you might proceed with surgical treatment plus consider imaging studies such as ultrasound, CT, PET CT, and consider referral to other specialties.”
For a high-risk patient with a 2B or high risk result, she continued, “you may want to proceed with imaging studies right away in addition to surgery and consider consultation with radiation oncology or medical oncology, as well as more frequent follow-up with nodal exams, because the class 2B patients have been shown to have a greater than 50% risk of metastasis.”
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Bar disclosed that Oregon Health & Science University has received research funding from Castle Biosciences.
, according to Anna A. Bar, MD.
“The incidence of SCC has been growing rapidly, and the disease-related mortality is actually more than that of melanoma,” Dr. Bar, associate professor of dermatology at Oregon Health & Science University, Portland, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
“Like many cancers, SCC management plans are guided by the risk of metastasis. The current staging systems, like NCCN, AJCC, or Brigham and Women’s systems, struggle to provide accurate data of the metastatic potential of an individual’s SCC,” she said. “Furthermore, the predictive accuracy of these systems in SCC is variable, and many patients who have high risk factors do not experience poor outcomes, while others initially classified as having less concerning tumors will go on to have metastatic disease. That is where new gene expression tests come into play.”
Developed by and commercially available from Castle Biosciences, DecisionDx-SCC classifies an individual SCC patient’s tumor into one of the categories: low (class 1), moderate (class 2A), or high (class 2B) biologic risk of metastasis. “We’re hoping that DecisionDx results can help make management decisions within established guidelines,” Dr. Bar said. The test is indicated for patients with high-risk features including tumor size greater than 2 cm; tumor location on the head, neck, hands, genitals, feet, or pretibial surface; immunosuppression; a rapidly growing tumor; a tumor with poorly defined borders; a tumor at the site of prior radiation or chronic inflammation; perineural invasion; poorly defined tumor grade, and a deep tumor beyond the subcutaneous fat.
One validity study and three clinical utility studies of DecisionDx-SCC have been published that include data from more than 1,100 patients (see Curr Med Res Opin. 2020 Aug;36[8]:1301-7; Curr Med Res Opin. 2020 Aug;36[8]:1295-1300, and J Drugs Dermatol. 2019 Oct 1;18[10]:980-4). “This is a work in progress,” said Dr. Bar, director of the university’s Mohs micrographic surgery and cutaneous oncology fellowship.
The test was validated in an another study, which was prospectively designed and used archival tissue from 33 independent academic and community centers, including Oregon Health & Science University. All 420 patients in the clinical validation study had one or more high-risk factors, meeting the definition of high risk by NCCN or Mohs Appropriate Use Criteria (AUC). Their mean age was 71 years, 73% were male, 99% were White, and 25% were immune deficient.
Of the 420 patients, 63 had metastasis, and 86% of metastases were located on the head and neck. About 30% of metastasized lesions had perineural involvement, 27% had invasion beyond subcutaneous fat, and metastasized lesions were about 1 cm wider compared with lesions that were not. The overall metastasis rate at 3 years was 15%, “which is similar to that seen in the medical literature for high-risk populations,” Dr. Bar said.
The median time to metastasis was 0.9 years and the 95th percentile was 2.7 years. “This means that the 3-year horizon for identifying events in this study enabled identification of most patients who eventually experienced metastatic events,” she said. In this cohort, approximately half of the metastatic events occurred around 11 months post diagnosis, which “may provide guidance about the timeline and duration of high-intensity follow-up with frequency of clinical visits and imaging for patients at highest risk within the first year.”
The positive predictive value of the DecisionDx-SCC is 52%, meaning that half of class 2B lesions will metastasize. “This compares favorably when you look at the lower positive predictive value of the other staging systems,” Dr. Bar said. “The negative predictive value is 93%, meaning there are not a lot of false negatives. This also compares favorably to the other staging systems.”
Kaplan-Meier analysis of metastasis-free survival showed strong separation between patients with class 1, class 2A, and class 2B results, Dr. Bar said. While the overall risk of metastasis in this patient cohort was 15%, the risk among those with a class 1 result was less than half of that. “Patients with a class 2A result behave similarly to those with traditional risk factors such as deep invasion and poor differentiation, having about a 20% risk of metastasis,” she said. “The class 2B result identifies the most worrisome SCCs, with a greater than 50% risk of metastasis. While the results distribution from routine clinical testing is not yet known, this large validation study of high-risk SCC revealed that approximately half of the patients were class 1, less than half were class 2A, and about 1 in 18 had a class 2B result.”
On univariate analyses with traditional risk factors and use of the Brigham and Women’s staging system, the hazard ratio (HR) for class 2A lesions was 3.2, “which is similar to deep invasion, poor differentiation, or perineural involvement,” Dr. Bar said. At the same time, the HR for class 2B lesions was 11.6, “so class 2B is the strongest predictor of metastasis. The class 2B HR remained statistically significant in the multivariate analysis and is three times higher than that of the next highest HR in this cohort. For example, a high-risk SCC with deep invasion is already two times more likely to metastasize. Adding a class 2B score would be over 14 times more likely to metastasize than a tumor with a class 1 result.”
DecisionDx-SCC test results can inform management decisions within established guidelines. For example, for a high-risk SCC patient who has a class 1 result, or low risk of metastasis, “you may proceed with surgery and clinical nodal exam, and then follow up a couple of times a year,” Dr. Bar said. “For a high-risk patient with a 2A or moderate risk result, you might proceed with surgical treatment plus consider imaging studies such as ultrasound, CT, PET CT, and consider referral to other specialties.”
For a high-risk patient with a 2B or high risk result, she continued, “you may want to proceed with imaging studies right away in addition to surgery and consider consultation with radiation oncology or medical oncology, as well as more frequent follow-up with nodal exams, because the class 2B patients have been shown to have a greater than 50% risk of metastasis.”
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Bar disclosed that Oregon Health & Science University has received research funding from Castle Biosciences.
FROM THE CUTANEOUS MALIGNANCIES FORUM
The COVID-19 virus may prompt the body to attack itself
An international team of researchers studying COVID-19 has made a startling and pivotal discovery: The virus appears to cause the body to make weapons to attack its own tissues.
The finding could unlock a number of COVID-19’s clinical mysteries. They include the puzzling collection of symptoms that can come with the infection; the persistence of symptoms in some people for months after they clear the virus, a phenomenon dubbed long COVID-19; and why some children and adults have a serious inflammatory syndrome, called multisystem inflammatory syndrome in children (MIS-C) or MIS in adults (MIS-A), after their infections.
“It suggests that the virus might be directly causing autoimmunity, which would be fascinating,” says lead study author Paul Utz, MD, who studies immunology and autoimmunity at Stanford (Calif.) University.
The study also deepens the question of whether other respiratory viruses might also break the body’s tolerance to itself, setting people up for autoimmune diseases like multiple sclerosis, rheumatoid arthritis, and lupus later in life.
Dr. Utz said he and his team are next going to study flu patients to see if that virus might also cause this phenomenon.
“My prediction is that it isn’t going to be specific just to SARS-CoV-2. I’m willing to bet that we will find this with other respiratory viruses,” he said.
The study comes on the heels of a handful of smaller, detailed investigations that have come to similar conclusions.
The study included data from more than 300 patients from four hospitals: two in California, one in Pennsylvania, and another in Germany.
Researchers used blood tests to study their immune responses as their infections progressed. Researchers looked for autoantibodies – weapons of the immune system that go rogue and launch an attack against the body’s own tissues. They compared these autoantibodies with those found in people who were not infected with the virus that causes COVID.
As previous studies have found, autoantibodies were more common after COVID – 50% of people hospitalized for their infections had autoantibodies, compared with less than 15% of those who were healthy and uninfected.
Some people with autoantibodies had little change in them as their infections progressed. That suggests the autoantibodies were there to begin with, possibly allowing the infection to burn out of control in the body.
“Their body is set up to get bad COVID, and it’s probably caused by the autoantibodies,” Dr. Utz said.
But in others, about 20% of people who had them, the autoantibodies became more common as the infection progressed, suggesting they were directly related to the viral infection, instead of being a preexisting condition.
Some of these were antibodies that attack key components of the immune system’s weapons against the virus, like interferon. Interferons are proteins that help infected cells call for reinforcements and can also interfere with a virus’s ability to copy itself. Taking them out is a powerful evasive tactic, and previous studies have shown that people who are born with genes that cause them to have lower interferon function, or who make autoantibodies against these proteins, appear to be at higher risk for life-threatening COVID infections.
“It seems to give the virus a powerful advantage,” said study author, John Wherry, PhD, who directs the Institute for Immunology at the University of Pennsylvania, Philadelphia. “Now your immune system, instead of having a tiny little hill to climb, is staring at Mount Everest. That really is devious.”
In addition to those that sabotage the immune system, some people in the study had autoantibodies against muscles and connective tissues that are seen in some rare disorders.
Dr. Utz said they started the study after seeing COVID patients with strange collections of symptoms that looked more like autoimmune diseases than viral infections – skin rashes, joint pain, fatigue, aching muscles, brain swelling, dry eyes, blood that clots easily, and inflamed blood vessels.
“One thing that’s very important to note is that we don’t know if these patients are going to go on to develop autoimmune disease,” Dr. Utz said. “I think we’ll be able to answer that question in the next 6-12 months as we follow the long haulers and study their samples.”
Dr. Utz said it will be important to study autoantibodies in long haulers to see if they can identify exactly which ones seem to be at work in the condition. If you can catch them early, it might be possible to treat those at risk for enduring symptoms with drugs that suppress the immune system.
What this means, he said, is that COVID will be with us for a long, long time.
“We have to realize that there’s going to be long-term damage from this virus for the survivors. Not just the long haulers, but all the people who have lung damage and heart damage and everything else. We’re going to be studying this virus and it’s badness for decades,” Dr. Utz said.
A version of this article first appeared on WebMD.com.
An international team of researchers studying COVID-19 has made a startling and pivotal discovery: The virus appears to cause the body to make weapons to attack its own tissues.
The finding could unlock a number of COVID-19’s clinical mysteries. They include the puzzling collection of symptoms that can come with the infection; the persistence of symptoms in some people for months after they clear the virus, a phenomenon dubbed long COVID-19; and why some children and adults have a serious inflammatory syndrome, called multisystem inflammatory syndrome in children (MIS-C) or MIS in adults (MIS-A), after their infections.
“It suggests that the virus might be directly causing autoimmunity, which would be fascinating,” says lead study author Paul Utz, MD, who studies immunology and autoimmunity at Stanford (Calif.) University.
The study also deepens the question of whether other respiratory viruses might also break the body’s tolerance to itself, setting people up for autoimmune diseases like multiple sclerosis, rheumatoid arthritis, and lupus later in life.
Dr. Utz said he and his team are next going to study flu patients to see if that virus might also cause this phenomenon.
“My prediction is that it isn’t going to be specific just to SARS-CoV-2. I’m willing to bet that we will find this with other respiratory viruses,” he said.
The study comes on the heels of a handful of smaller, detailed investigations that have come to similar conclusions.
The study included data from more than 300 patients from four hospitals: two in California, one in Pennsylvania, and another in Germany.
Researchers used blood tests to study their immune responses as their infections progressed. Researchers looked for autoantibodies – weapons of the immune system that go rogue and launch an attack against the body’s own tissues. They compared these autoantibodies with those found in people who were not infected with the virus that causes COVID.
As previous studies have found, autoantibodies were more common after COVID – 50% of people hospitalized for their infections had autoantibodies, compared with less than 15% of those who were healthy and uninfected.
Some people with autoantibodies had little change in them as their infections progressed. That suggests the autoantibodies were there to begin with, possibly allowing the infection to burn out of control in the body.
“Their body is set up to get bad COVID, and it’s probably caused by the autoantibodies,” Dr. Utz said.
But in others, about 20% of people who had them, the autoantibodies became more common as the infection progressed, suggesting they were directly related to the viral infection, instead of being a preexisting condition.
Some of these were antibodies that attack key components of the immune system’s weapons against the virus, like interferon. Interferons are proteins that help infected cells call for reinforcements and can also interfere with a virus’s ability to copy itself. Taking them out is a powerful evasive tactic, and previous studies have shown that people who are born with genes that cause them to have lower interferon function, or who make autoantibodies against these proteins, appear to be at higher risk for life-threatening COVID infections.
“It seems to give the virus a powerful advantage,” said study author, John Wherry, PhD, who directs the Institute for Immunology at the University of Pennsylvania, Philadelphia. “Now your immune system, instead of having a tiny little hill to climb, is staring at Mount Everest. That really is devious.”
In addition to those that sabotage the immune system, some people in the study had autoantibodies against muscles and connective tissues that are seen in some rare disorders.
Dr. Utz said they started the study after seeing COVID patients with strange collections of symptoms that looked more like autoimmune diseases than viral infections – skin rashes, joint pain, fatigue, aching muscles, brain swelling, dry eyes, blood that clots easily, and inflamed blood vessels.
“One thing that’s very important to note is that we don’t know if these patients are going to go on to develop autoimmune disease,” Dr. Utz said. “I think we’ll be able to answer that question in the next 6-12 months as we follow the long haulers and study their samples.”
Dr. Utz said it will be important to study autoantibodies in long haulers to see if they can identify exactly which ones seem to be at work in the condition. If you can catch them early, it might be possible to treat those at risk for enduring symptoms with drugs that suppress the immune system.
What this means, he said, is that COVID will be with us for a long, long time.
“We have to realize that there’s going to be long-term damage from this virus for the survivors. Not just the long haulers, but all the people who have lung damage and heart damage and everything else. We’re going to be studying this virus and it’s badness for decades,” Dr. Utz said.
A version of this article first appeared on WebMD.com.
An international team of researchers studying COVID-19 has made a startling and pivotal discovery: The virus appears to cause the body to make weapons to attack its own tissues.
The finding could unlock a number of COVID-19’s clinical mysteries. They include the puzzling collection of symptoms that can come with the infection; the persistence of symptoms in some people for months after they clear the virus, a phenomenon dubbed long COVID-19; and why some children and adults have a serious inflammatory syndrome, called multisystem inflammatory syndrome in children (MIS-C) or MIS in adults (MIS-A), after their infections.
“It suggests that the virus might be directly causing autoimmunity, which would be fascinating,” says lead study author Paul Utz, MD, who studies immunology and autoimmunity at Stanford (Calif.) University.
The study also deepens the question of whether other respiratory viruses might also break the body’s tolerance to itself, setting people up for autoimmune diseases like multiple sclerosis, rheumatoid arthritis, and lupus later in life.
Dr. Utz said he and his team are next going to study flu patients to see if that virus might also cause this phenomenon.
“My prediction is that it isn’t going to be specific just to SARS-CoV-2. I’m willing to bet that we will find this with other respiratory viruses,” he said.
The study comes on the heels of a handful of smaller, detailed investigations that have come to similar conclusions.
The study included data from more than 300 patients from four hospitals: two in California, one in Pennsylvania, and another in Germany.
Researchers used blood tests to study their immune responses as their infections progressed. Researchers looked for autoantibodies – weapons of the immune system that go rogue and launch an attack against the body’s own tissues. They compared these autoantibodies with those found in people who were not infected with the virus that causes COVID.
As previous studies have found, autoantibodies were more common after COVID – 50% of people hospitalized for their infections had autoantibodies, compared with less than 15% of those who were healthy and uninfected.
Some people with autoantibodies had little change in them as their infections progressed. That suggests the autoantibodies were there to begin with, possibly allowing the infection to burn out of control in the body.
“Their body is set up to get bad COVID, and it’s probably caused by the autoantibodies,” Dr. Utz said.
But in others, about 20% of people who had them, the autoantibodies became more common as the infection progressed, suggesting they were directly related to the viral infection, instead of being a preexisting condition.
Some of these were antibodies that attack key components of the immune system’s weapons against the virus, like interferon. Interferons are proteins that help infected cells call for reinforcements and can also interfere with a virus’s ability to copy itself. Taking them out is a powerful evasive tactic, and previous studies have shown that people who are born with genes that cause them to have lower interferon function, or who make autoantibodies against these proteins, appear to be at higher risk for life-threatening COVID infections.
“It seems to give the virus a powerful advantage,” said study author, John Wherry, PhD, who directs the Institute for Immunology at the University of Pennsylvania, Philadelphia. “Now your immune system, instead of having a tiny little hill to climb, is staring at Mount Everest. That really is devious.”
In addition to those that sabotage the immune system, some people in the study had autoantibodies against muscles and connective tissues that are seen in some rare disorders.
Dr. Utz said they started the study after seeing COVID patients with strange collections of symptoms that looked more like autoimmune diseases than viral infections – skin rashes, joint pain, fatigue, aching muscles, brain swelling, dry eyes, blood that clots easily, and inflamed blood vessels.
“One thing that’s very important to note is that we don’t know if these patients are going to go on to develop autoimmune disease,” Dr. Utz said. “I think we’ll be able to answer that question in the next 6-12 months as we follow the long haulers and study their samples.”
Dr. Utz said it will be important to study autoantibodies in long haulers to see if they can identify exactly which ones seem to be at work in the condition. If you can catch them early, it might be possible to treat those at risk for enduring symptoms with drugs that suppress the immune system.
What this means, he said, is that COVID will be with us for a long, long time.
“We have to realize that there’s going to be long-term damage from this virus for the survivors. Not just the long haulers, but all the people who have lung damage and heart damage and everything else. We’re going to be studying this virus and it’s badness for decades,” Dr. Utz said.
A version of this article first appeared on WebMD.com.
Expert offers tips for sorting out pink lesions on dermoscopy
Even in the most experienced hands, .
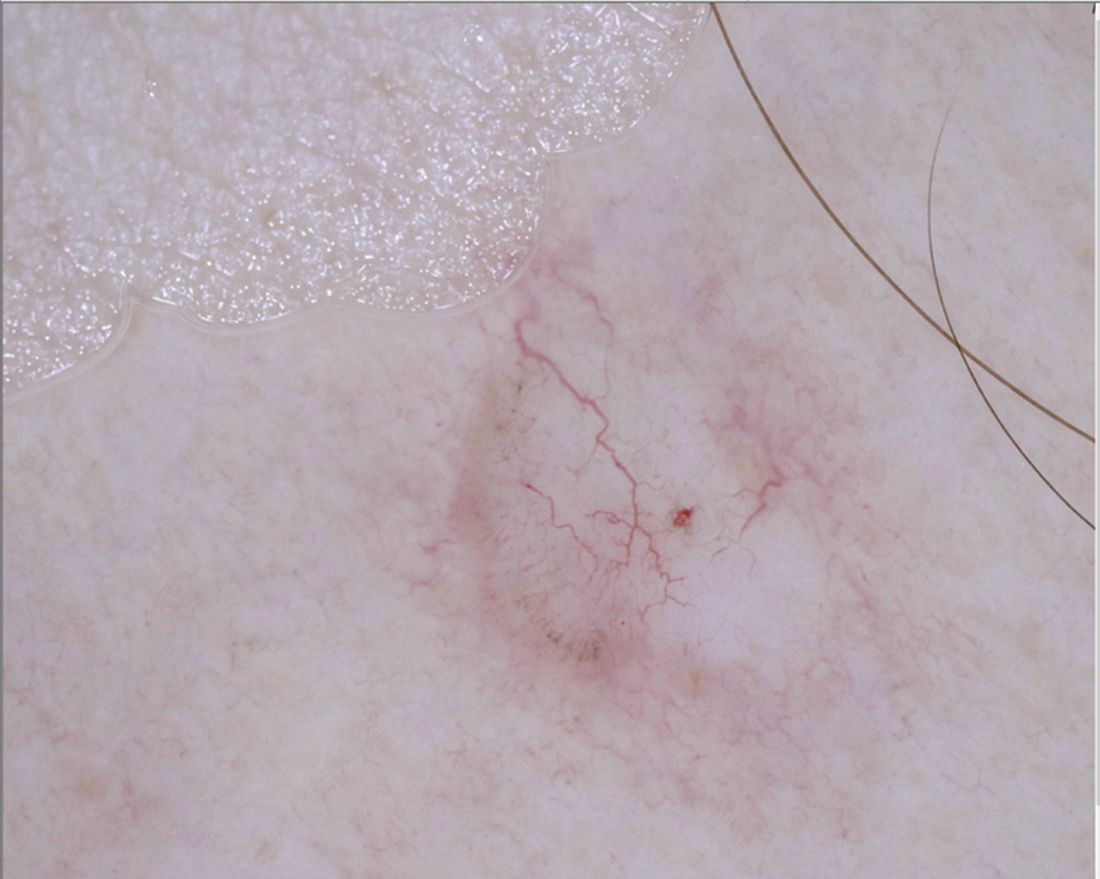
“For me, pink lesions are challenging,” Jennifer A. Stein, MD, PhD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference. “How can dermoscopy help us distinguish between Spitz nevus, melanoma, clear cell acanthoma, psoriasis, basal cell carcinoma, and squamous cell carcinoma?”
Dr. Stein, professor of dermatology at New York University, offered four tips. First, look for the shiny white perpendicular lines, otherwise known as the chrysalis or crystalline pattern. “You can only see this feature when you’re looking with polarized light,” she said. “This is why you want a dermatoscope that has polarized light, and better yet, one that you’re able to turn on and off, the hybrid kind, because then you can convince yourself that you’re looking at this feature, because it blinks on and off.”
The differential diagnosis for white shiny perpendicular lines includes dermatofibroma/scars (which is most common), Spitz and atypical genital nevi, BCC, and melanoma. “Dermatofibromas sometimes have white circles or rings in the center,” Dr. Stein said. “In BCC, the lines aren’t always perpendicular. Sometimes it’s more of a blotch or strands.”
A second tip for managing a pink lesion on dermoscopy is to look for any brown color. “When you see that combo together you have to worry,” she said. “When you see pigment network on dermoscopy, you have to put melanoma in your differential. If you see shiny white lines in something that is melanocytic, there’s a 98% specificity for melanoma.”
A third tip she offered for managing pink lesions is to check the blood vessels for clues. “For years, I was just naming the vessels based on making the diagnosis and then deciding, ‘that’s a basal cell carcinoma; those must be branching vessels,’ ” said Dr. Stein, who manages NYU’s medical dermatology faculty group practice.
However, blood vessel patterns differ. For example, branching or arborizing vessels are suggestive of BCC. “These vessels are very crisp-looking on dermoscopy,” she said. “They’re all in the same plane of focus and they look like they were drawn in with a fine point marker. That’s different from other blood vessel patterns.” She also pointed out that superficial basal cells have short, fine telangiectasias. “When you put on the polarized light, the clue is the white, shiny structures,” she said.
Dotted vessels, meanwhile, appear on dermoscopy as small red dots aligned perpendicular to the skin surface. The differential includes inflammatory lesions like psoriasis, stasis, and trauma; clear cell acanthoma (characterized by a “string of pearls” arrangement), nevi, and melanoma. “I find dermoscopy most useful in diagnosing SCC – especially squamous cell in situ,” she said. “Important clinical clues suggestive of SCC or melanoma include a solitary lesion, it’s new, it’s growing, and it’s not going away with a topical steroid.”
An additional pattern to be aware of are hairpin vessels, which are looped and feature a sharp bend at one end. These are often seen in seborrheic keratoses. “You can’t count on the hairpin vessels alone, because you can see this in anything keratotic, such as in keratoacanthoma (at the periphery with a yellow keratotic center), warts, SCC, BCC, as well as in dermal nevi and Spitz nevi,” said Dr. Stein, who recommended dermoscopedia.org as resource.
Comma vessels, meanwhile, appear in dermal or compound nevi. She described these as “slightly curved vessels that are much less in focus than branched vessels, because they come in and out of the plane of focus,” she said. “If you put your dermatoscope on top of the nevus and wobble it around you can appreciate the curve. If you look at it from the side, it looks like a curve. If you look at it straight on it will look more like a line. If you look at from the end it will look like a dot.”
Another vessel type she discussed are linear irregular and polymorphous vessels, which she described as “any combination of different types of vessels. We get most worried when we see dotted and linear irregular vessels together. In that case, you worry about melanoma. These can also be seen in nevi and other tumors, such as BCC.”
Dr. Stein’s fourth tip of the presentation was a reminder to consider dermoscopy as one piece of the clinical exam. “Always think about the lesion in context of the rest of the clinical picture and history,” she said. “Don’t get discouraged if it’s hard; just keep practicing. Look for any brown and use your clinical clues to put together to make the right decision.”
She disclosed that NYU receives compensation from MoleSafe for her telemedicine dermoscopic diagnoses.
Even in the most experienced hands, .

“For me, pink lesions are challenging,” Jennifer A. Stein, MD, PhD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference. “How can dermoscopy help us distinguish between Spitz nevus, melanoma, clear cell acanthoma, psoriasis, basal cell carcinoma, and squamous cell carcinoma?”
Dr. Stein, professor of dermatology at New York University, offered four tips. First, look for the shiny white perpendicular lines, otherwise known as the chrysalis or crystalline pattern. “You can only see this feature when you’re looking with polarized light,” she said. “This is why you want a dermatoscope that has polarized light, and better yet, one that you’re able to turn on and off, the hybrid kind, because then you can convince yourself that you’re looking at this feature, because it blinks on and off.”
The differential diagnosis for white shiny perpendicular lines includes dermatofibroma/scars (which is most common), Spitz and atypical genital nevi, BCC, and melanoma. “Dermatofibromas sometimes have white circles or rings in the center,” Dr. Stein said. “In BCC, the lines aren’t always perpendicular. Sometimes it’s more of a blotch or strands.”
A second tip for managing a pink lesion on dermoscopy is to look for any brown color. “When you see that combo together you have to worry,” she said. “When you see pigment network on dermoscopy, you have to put melanoma in your differential. If you see shiny white lines in something that is melanocytic, there’s a 98% specificity for melanoma.”
A third tip she offered for managing pink lesions is to check the blood vessels for clues. “For years, I was just naming the vessels based on making the diagnosis and then deciding, ‘that’s a basal cell carcinoma; those must be branching vessels,’ ” said Dr. Stein, who manages NYU’s medical dermatology faculty group practice.
However, blood vessel patterns differ. For example, branching or arborizing vessels are suggestive of BCC. “These vessels are very crisp-looking on dermoscopy,” she said. “They’re all in the same plane of focus and they look like they were drawn in with a fine point marker. That’s different from other blood vessel patterns.” She also pointed out that superficial basal cells have short, fine telangiectasias. “When you put on the polarized light, the clue is the white, shiny structures,” she said.
Dotted vessels, meanwhile, appear on dermoscopy as small red dots aligned perpendicular to the skin surface. The differential includes inflammatory lesions like psoriasis, stasis, and trauma; clear cell acanthoma (characterized by a “string of pearls” arrangement), nevi, and melanoma. “I find dermoscopy most useful in diagnosing SCC – especially squamous cell in situ,” she said. “Important clinical clues suggestive of SCC or melanoma include a solitary lesion, it’s new, it’s growing, and it’s not going away with a topical steroid.”
An additional pattern to be aware of are hairpin vessels, which are looped and feature a sharp bend at one end. These are often seen in seborrheic keratoses. “You can’t count on the hairpin vessels alone, because you can see this in anything keratotic, such as in keratoacanthoma (at the periphery with a yellow keratotic center), warts, SCC, BCC, as well as in dermal nevi and Spitz nevi,” said Dr. Stein, who recommended dermoscopedia.org as resource.
Comma vessels, meanwhile, appear in dermal or compound nevi. She described these as “slightly curved vessels that are much less in focus than branched vessels, because they come in and out of the plane of focus,” she said. “If you put your dermatoscope on top of the nevus and wobble it around you can appreciate the curve. If you look at it from the side, it looks like a curve. If you look at it straight on it will look more like a line. If you look at from the end it will look like a dot.”
Another vessel type she discussed are linear irregular and polymorphous vessels, which she described as “any combination of different types of vessels. We get most worried when we see dotted and linear irregular vessels together. In that case, you worry about melanoma. These can also be seen in nevi and other tumors, such as BCC.”
Dr. Stein’s fourth tip of the presentation was a reminder to consider dermoscopy as one piece of the clinical exam. “Always think about the lesion in context of the rest of the clinical picture and history,” she said. “Don’t get discouraged if it’s hard; just keep practicing. Look for any brown and use your clinical clues to put together to make the right decision.”
She disclosed that NYU receives compensation from MoleSafe for her telemedicine dermoscopic diagnoses.
Even in the most experienced hands, .

“For me, pink lesions are challenging,” Jennifer A. Stein, MD, PhD, said during the virtual Orlando Dermatology Aesthetic and Clinical Conference. “How can dermoscopy help us distinguish between Spitz nevus, melanoma, clear cell acanthoma, psoriasis, basal cell carcinoma, and squamous cell carcinoma?”
Dr. Stein, professor of dermatology at New York University, offered four tips. First, look for the shiny white perpendicular lines, otherwise known as the chrysalis or crystalline pattern. “You can only see this feature when you’re looking with polarized light,” she said. “This is why you want a dermatoscope that has polarized light, and better yet, one that you’re able to turn on and off, the hybrid kind, because then you can convince yourself that you’re looking at this feature, because it blinks on and off.”
The differential diagnosis for white shiny perpendicular lines includes dermatofibroma/scars (which is most common), Spitz and atypical genital nevi, BCC, and melanoma. “Dermatofibromas sometimes have white circles or rings in the center,” Dr. Stein said. “In BCC, the lines aren’t always perpendicular. Sometimes it’s more of a blotch or strands.”
A second tip for managing a pink lesion on dermoscopy is to look for any brown color. “When you see that combo together you have to worry,” she said. “When you see pigment network on dermoscopy, you have to put melanoma in your differential. If you see shiny white lines in something that is melanocytic, there’s a 98% specificity for melanoma.”
A third tip she offered for managing pink lesions is to check the blood vessels for clues. “For years, I was just naming the vessels based on making the diagnosis and then deciding, ‘that’s a basal cell carcinoma; those must be branching vessels,’ ” said Dr. Stein, who manages NYU’s medical dermatology faculty group practice.
However, blood vessel patterns differ. For example, branching or arborizing vessels are suggestive of BCC. “These vessels are very crisp-looking on dermoscopy,” she said. “They’re all in the same plane of focus and they look like they were drawn in with a fine point marker. That’s different from other blood vessel patterns.” She also pointed out that superficial basal cells have short, fine telangiectasias. “When you put on the polarized light, the clue is the white, shiny structures,” she said.
Dotted vessels, meanwhile, appear on dermoscopy as small red dots aligned perpendicular to the skin surface. The differential includes inflammatory lesions like psoriasis, stasis, and trauma; clear cell acanthoma (characterized by a “string of pearls” arrangement), nevi, and melanoma. “I find dermoscopy most useful in diagnosing SCC – especially squamous cell in situ,” she said. “Important clinical clues suggestive of SCC or melanoma include a solitary lesion, it’s new, it’s growing, and it’s not going away with a topical steroid.”
An additional pattern to be aware of are hairpin vessels, which are looped and feature a sharp bend at one end. These are often seen in seborrheic keratoses. “You can’t count on the hairpin vessels alone, because you can see this in anything keratotic, such as in keratoacanthoma (at the periphery with a yellow keratotic center), warts, SCC, BCC, as well as in dermal nevi and Spitz nevi,” said Dr. Stein, who recommended dermoscopedia.org as resource.
Comma vessels, meanwhile, appear in dermal or compound nevi. She described these as “slightly curved vessels that are much less in focus than branched vessels, because they come in and out of the plane of focus,” she said. “If you put your dermatoscope on top of the nevus and wobble it around you can appreciate the curve. If you look at it from the side, it looks like a curve. If you look at it straight on it will look more like a line. If you look at from the end it will look like a dot.”
Another vessel type she discussed are linear irregular and polymorphous vessels, which she described as “any combination of different types of vessels. We get most worried when we see dotted and linear irregular vessels together. In that case, you worry about melanoma. These can also be seen in nevi and other tumors, such as BCC.”
Dr. Stein’s fourth tip of the presentation was a reminder to consider dermoscopy as one piece of the clinical exam. “Always think about the lesion in context of the rest of the clinical picture and history,” she said. “Don’t get discouraged if it’s hard; just keep practicing. Look for any brown and use your clinical clues to put together to make the right decision.”
She disclosed that NYU receives compensation from MoleSafe for her telemedicine dermoscopic diagnoses.
FROM ODAC 2021
Dermatology history: University Hospital ‘Saint Louis,’ Paris
The Hospital “Saint Louis” was founded in 1607 by King Henry IV of France to relieve overcrowding of Parisian hospitals during the plague epidemic of 1605-1606. He named it Saint-Louis in memory of his grandfather, King Louis IX.
Today, the Hospital Saint-Louis, a registered historic monument, is used for administrative activities.

Since 1980, a modern building has hosted all the activities of the University Hospital Center, which belongs to the University of Paris 7.

In addition to dermatology, the main departments include hematology and bone marrow transplantation, hemato-oncology, general surgery, endocrinology, gastroenterology, clinical immunology, internal medicine, and nephrology. Saint-Louis Hospital employs 2,500 people, including a medical staff of 1,000. It houses the Institute Inserm U976 – a public research unit that is part of the National Health and Medical Research Institute, which focuses on human immunology, physiopathology and immunotherapy – as well as the René-Touraine Foundation, a private non-profit organization that brings together dermatologists, scientists, pharmaceutical companies, and health authorities to support therapeutic progress in dermatology.
Saint-Louis Hospital is known for its long tradition in hematology; it is the site of the first successful allogeneic bone marrow transplant in 1958, performed by Georges Mathé, MD, Professor Jean Bernard, and one of the recipients of the 1980 Nobel Prize in Medicine, Professor Jean Dausset. The hospital is known for not only its activity in dermatology care and research (such as oncodermatology and inflammatory diseases) but also its long tradition of teaching in dermatology and venereology.
Over the last four centuries, great physicians have practiced their art here and many professors, and clinicians at Saint-Louis Hospital have authored publications and developed manuals of dermatology that have been translated across five continents. Many diseases and semiology signs in dermatology were first described by physicians from this hospital, their names familiar to dermatologists worldwide: Jean-Louis-Marc Alibert, MD; Jean Guillaume Auguste Lugol, MD; Laurent-Théodore Biett, MD; Pierre-Antoine-Ernest Bazin, MD; Pierre Louis Alphée Cazenave, MD; François Henri Hallopeau, MD; Léon Lortat-Jacob, MD; Henri-Alexandre Danlos, MD; Ernest Besnier, MD; Jean Baptiste Emile Vidal, MD; Ferdinand-Jean Darier, MD; Louis Brocq, MD; Bernard Felix Duperrat, MD; Gaston Auguste Milian, MD; Albert Sézary, MD; Achille Civatte, MD; Raymond Sabouraud, MD; Henri Gougerot, MD; Albert Touraine, MD; Arnault Tzanck, MD; and Robert Degos, MD, among others.
The Henri-Feulard library – known as the “Dermatology Wax Museum” – is a fascinating place that houses the world’s largest collection of 4,807 wax casts dedicated to teaching skin diseases and venereal diseases.

The library next to the museum contains numerous outstanding ancient works on dermatology and sexually transmitted diseases, including first issues of dermatology journals from the 19th century and rare dermatology textbooks published in the last 2 centuries.
The recently renovated museum hosts national and international dermatological meetings and is also where the hospital’s dermatology staff meets weekly.
In the dermatology department at Saint-Louis Hospital, patient care is provided in two hospital areas with 18 beds each and a day hospital with 8 beds for patients with inflammatory and dysimmune dermatoses, including a special room with a bathtub for the management of patients with severe genodermatoses. The department is a referral center for genodermatoses and a dedicated center for autoimmune bullous diseases.
Patients with all types of skin tumors, particularly melanomas, carcinomas, sarcomas, and cutaneous lymphomas, are treated at the oncodermatology center, which has a 10-bed day hospital and a very active consultation service. The Saint-Louis Hospital dermatology department is also a National Reference Center for cutaneous lymphomas, providing four Multidisciplinary Consultation Meetings, a national MCM for cutaneous lymphomas, and a multidisciplinary MCM for the diagnosis and treatment of side effects of new targeted therapies and immunotherapies for cancers.
The dermatology polyclinic, an outpatient clinic, provides 54,000 consultations per year. It includes a very active general consultation service, including a wide variety of specialized consultations for atopic dermatitis, psoriasis, hand dermatitis, hidradenitis suppurativa, internal medicine/dermatology, bullous diseases, keloids, angiomas, leprosy, genodermatoses, and medical mycology.
Anonymous, free screening services are available at the Sexually Transmitted Diseases Center through “CeGIDD,” a free center for HIV/AIDS screening and specialized consultations in venereology and mucosal pathologies.
The surgical activity of the department is provided in the Center of Dermo-Surgery. Dedicated medical and paramedical consultations ensure the management of ulcers and therapeutic baths for patients and families with refractory scabies.
The technical platform includes an allergology consultation, a phototherapy center, a Fotofinder diagnosis, a photodynamic therapy unit, and a confocal microscopy unit. The department, completed in September 2019 with a laser center with four devices, also works in close collaboration with the Sabouraud Center, created by Dr. Sabouraud and dedicated to the investigation and treatment of scalp diseases.
We are absolutely aware that working in a hospital so rich in past personalities and discoveries and part of the history of dermatology is not only a huge honor requiring a special commitment to continue the tradition of research and excellence in dermatology initiated hundreds of years ago, but also an important responsibility to focus all our efforts on teaching dermatology to next generations in France and around the world. It is also our responsibility to pursue this historic tradition of excellence by developing dynamic translational research activities that lead to innovations in the field of dermatology.
Professor Bagot is head of the dermatology department of University Hospital Saint-Louis, Paris. Dr. Ionescu is a specialist in dermatology and venereology in the department of dermatology at University Hospital Saint-Louis in Paris and is a member of the Dermatology News editorial board. Write to them at dermnews@mdedge.com.
The Hospital “Saint Louis” was founded in 1607 by King Henry IV of France to relieve overcrowding of Parisian hospitals during the plague epidemic of 1605-1606. He named it Saint-Louis in memory of his grandfather, King Louis IX.
Today, the Hospital Saint-Louis, a registered historic monument, is used for administrative activities.

Since 1980, a modern building has hosted all the activities of the University Hospital Center, which belongs to the University of Paris 7.

In addition to dermatology, the main departments include hematology and bone marrow transplantation, hemato-oncology, general surgery, endocrinology, gastroenterology, clinical immunology, internal medicine, and nephrology. Saint-Louis Hospital employs 2,500 people, including a medical staff of 1,000. It houses the Institute Inserm U976 – a public research unit that is part of the National Health and Medical Research Institute, which focuses on human immunology, physiopathology and immunotherapy – as well as the René-Touraine Foundation, a private non-profit organization that brings together dermatologists, scientists, pharmaceutical companies, and health authorities to support therapeutic progress in dermatology.
Saint-Louis Hospital is known for its long tradition in hematology; it is the site of the first successful allogeneic bone marrow transplant in 1958, performed by Georges Mathé, MD, Professor Jean Bernard, and one of the recipients of the 1980 Nobel Prize in Medicine, Professor Jean Dausset. The hospital is known for not only its activity in dermatology care and research (such as oncodermatology and inflammatory diseases) but also its long tradition of teaching in dermatology and venereology.
Over the last four centuries, great physicians have practiced their art here and many professors, and clinicians at Saint-Louis Hospital have authored publications and developed manuals of dermatology that have been translated across five continents. Many diseases and semiology signs in dermatology were first described by physicians from this hospital, their names familiar to dermatologists worldwide: Jean-Louis-Marc Alibert, MD; Jean Guillaume Auguste Lugol, MD; Laurent-Théodore Biett, MD; Pierre-Antoine-Ernest Bazin, MD; Pierre Louis Alphée Cazenave, MD; François Henri Hallopeau, MD; Léon Lortat-Jacob, MD; Henri-Alexandre Danlos, MD; Ernest Besnier, MD; Jean Baptiste Emile Vidal, MD; Ferdinand-Jean Darier, MD; Louis Brocq, MD; Bernard Felix Duperrat, MD; Gaston Auguste Milian, MD; Albert Sézary, MD; Achille Civatte, MD; Raymond Sabouraud, MD; Henri Gougerot, MD; Albert Touraine, MD; Arnault Tzanck, MD; and Robert Degos, MD, among others.
The Henri-Feulard library – known as the “Dermatology Wax Museum” – is a fascinating place that houses the world’s largest collection of 4,807 wax casts dedicated to teaching skin diseases and venereal diseases.

The library next to the museum contains numerous outstanding ancient works on dermatology and sexually transmitted diseases, including first issues of dermatology journals from the 19th century and rare dermatology textbooks published in the last 2 centuries.
The recently renovated museum hosts national and international dermatological meetings and is also where the hospital’s dermatology staff meets weekly.
In the dermatology department at Saint-Louis Hospital, patient care is provided in two hospital areas with 18 beds each and a day hospital with 8 beds for patients with inflammatory and dysimmune dermatoses, including a special room with a bathtub for the management of patients with severe genodermatoses. The department is a referral center for genodermatoses and a dedicated center for autoimmune bullous diseases.
Patients with all types of skin tumors, particularly melanomas, carcinomas, sarcomas, and cutaneous lymphomas, are treated at the oncodermatology center, which has a 10-bed day hospital and a very active consultation service. The Saint-Louis Hospital dermatology department is also a National Reference Center for cutaneous lymphomas, providing four Multidisciplinary Consultation Meetings, a national MCM for cutaneous lymphomas, and a multidisciplinary MCM for the diagnosis and treatment of side effects of new targeted therapies and immunotherapies for cancers.
The dermatology polyclinic, an outpatient clinic, provides 54,000 consultations per year. It includes a very active general consultation service, including a wide variety of specialized consultations for atopic dermatitis, psoriasis, hand dermatitis, hidradenitis suppurativa, internal medicine/dermatology, bullous diseases, keloids, angiomas, leprosy, genodermatoses, and medical mycology.
Anonymous, free screening services are available at the Sexually Transmitted Diseases Center through “CeGIDD,” a free center for HIV/AIDS screening and specialized consultations in venereology and mucosal pathologies.
The surgical activity of the department is provided in the Center of Dermo-Surgery. Dedicated medical and paramedical consultations ensure the management of ulcers and therapeutic baths for patients and families with refractory scabies.
The technical platform includes an allergology consultation, a phototherapy center, a Fotofinder diagnosis, a photodynamic therapy unit, and a confocal microscopy unit. The department, completed in September 2019 with a laser center with four devices, also works in close collaboration with the Sabouraud Center, created by Dr. Sabouraud and dedicated to the investigation and treatment of scalp diseases.
We are absolutely aware that working in a hospital so rich in past personalities and discoveries and part of the history of dermatology is not only a huge honor requiring a special commitment to continue the tradition of research and excellence in dermatology initiated hundreds of years ago, but also an important responsibility to focus all our efforts on teaching dermatology to next generations in France and around the world. It is also our responsibility to pursue this historic tradition of excellence by developing dynamic translational research activities that lead to innovations in the field of dermatology.
Professor Bagot is head of the dermatology department of University Hospital Saint-Louis, Paris. Dr. Ionescu is a specialist in dermatology and venereology in the department of dermatology at University Hospital Saint-Louis in Paris and is a member of the Dermatology News editorial board. Write to them at dermnews@mdedge.com.
The Hospital “Saint Louis” was founded in 1607 by King Henry IV of France to relieve overcrowding of Parisian hospitals during the plague epidemic of 1605-1606. He named it Saint-Louis in memory of his grandfather, King Louis IX.
Today, the Hospital Saint-Louis, a registered historic monument, is used for administrative activities.

Since 1980, a modern building has hosted all the activities of the University Hospital Center, which belongs to the University of Paris 7.

In addition to dermatology, the main departments include hematology and bone marrow transplantation, hemato-oncology, general surgery, endocrinology, gastroenterology, clinical immunology, internal medicine, and nephrology. Saint-Louis Hospital employs 2,500 people, including a medical staff of 1,000. It houses the Institute Inserm U976 – a public research unit that is part of the National Health and Medical Research Institute, which focuses on human immunology, physiopathology and immunotherapy – as well as the René-Touraine Foundation, a private non-profit organization that brings together dermatologists, scientists, pharmaceutical companies, and health authorities to support therapeutic progress in dermatology.
Saint-Louis Hospital is known for its long tradition in hematology; it is the site of the first successful allogeneic bone marrow transplant in 1958, performed by Georges Mathé, MD, Professor Jean Bernard, and one of the recipients of the 1980 Nobel Prize in Medicine, Professor Jean Dausset. The hospital is known for not only its activity in dermatology care and research (such as oncodermatology and inflammatory diseases) but also its long tradition of teaching in dermatology and venereology.
Over the last four centuries, great physicians have practiced their art here and many professors, and clinicians at Saint-Louis Hospital have authored publications and developed manuals of dermatology that have been translated across five continents. Many diseases and semiology signs in dermatology were first described by physicians from this hospital, their names familiar to dermatologists worldwide: Jean-Louis-Marc Alibert, MD; Jean Guillaume Auguste Lugol, MD; Laurent-Théodore Biett, MD; Pierre-Antoine-Ernest Bazin, MD; Pierre Louis Alphée Cazenave, MD; François Henri Hallopeau, MD; Léon Lortat-Jacob, MD; Henri-Alexandre Danlos, MD; Ernest Besnier, MD; Jean Baptiste Emile Vidal, MD; Ferdinand-Jean Darier, MD; Louis Brocq, MD; Bernard Felix Duperrat, MD; Gaston Auguste Milian, MD; Albert Sézary, MD; Achille Civatte, MD; Raymond Sabouraud, MD; Henri Gougerot, MD; Albert Touraine, MD; Arnault Tzanck, MD; and Robert Degos, MD, among others.
The Henri-Feulard library – known as the “Dermatology Wax Museum” – is a fascinating place that houses the world’s largest collection of 4,807 wax casts dedicated to teaching skin diseases and venereal diseases.

The library next to the museum contains numerous outstanding ancient works on dermatology and sexually transmitted diseases, including first issues of dermatology journals from the 19th century and rare dermatology textbooks published in the last 2 centuries.
The recently renovated museum hosts national and international dermatological meetings and is also where the hospital’s dermatology staff meets weekly.
In the dermatology department at Saint-Louis Hospital, patient care is provided in two hospital areas with 18 beds each and a day hospital with 8 beds for patients with inflammatory and dysimmune dermatoses, including a special room with a bathtub for the management of patients with severe genodermatoses. The department is a referral center for genodermatoses and a dedicated center for autoimmune bullous diseases.
Patients with all types of skin tumors, particularly melanomas, carcinomas, sarcomas, and cutaneous lymphomas, are treated at the oncodermatology center, which has a 10-bed day hospital and a very active consultation service. The Saint-Louis Hospital dermatology department is also a National Reference Center for cutaneous lymphomas, providing four Multidisciplinary Consultation Meetings, a national MCM for cutaneous lymphomas, and a multidisciplinary MCM for the diagnosis and treatment of side effects of new targeted therapies and immunotherapies for cancers.
The dermatology polyclinic, an outpatient clinic, provides 54,000 consultations per year. It includes a very active general consultation service, including a wide variety of specialized consultations for atopic dermatitis, psoriasis, hand dermatitis, hidradenitis suppurativa, internal medicine/dermatology, bullous diseases, keloids, angiomas, leprosy, genodermatoses, and medical mycology.
Anonymous, free screening services are available at the Sexually Transmitted Diseases Center through “CeGIDD,” a free center for HIV/AIDS screening and specialized consultations in venereology and mucosal pathologies.
The surgical activity of the department is provided in the Center of Dermo-Surgery. Dedicated medical and paramedical consultations ensure the management of ulcers and therapeutic baths for patients and families with refractory scabies.
The technical platform includes an allergology consultation, a phototherapy center, a Fotofinder diagnosis, a photodynamic therapy unit, and a confocal microscopy unit. The department, completed in September 2019 with a laser center with four devices, also works in close collaboration with the Sabouraud Center, created by Dr. Sabouraud and dedicated to the investigation and treatment of scalp diseases.
We are absolutely aware that working in a hospital so rich in past personalities and discoveries and part of the history of dermatology is not only a huge honor requiring a special commitment to continue the tradition of research and excellence in dermatology initiated hundreds of years ago, but also an important responsibility to focus all our efforts on teaching dermatology to next generations in France and around the world. It is also our responsibility to pursue this historic tradition of excellence by developing dynamic translational research activities that lead to innovations in the field of dermatology.
Professor Bagot is head of the dermatology department of University Hospital Saint-Louis, Paris. Dr. Ionescu is a specialist in dermatology and venereology in the department of dermatology at University Hospital Saint-Louis in Paris and is a member of the Dermatology News editorial board. Write to them at dermnews@mdedge.com.
Meta-analysis finds much less lupus than expected
The prevalence of systemic lupus erythematosus (SLE) appears to be much lower than previously believed and may pose “a potential risk to research funding for the disease,” according to results of a meta-analysis involving a network of population-based registries.
“When we started this study, a widely cited lupus statistic was that approximately 1.5 million Americans were affected. Our meta-analysis found the actual prevalence to be slightly more than 200,000: a number that approaches the [Food and Drug Administration’s] definition of a rare disease,” Emily Somers, PhD, ScM, senior author and associate professor of rheumatology and environmental health sciences at the University of Michigan, Ann Arbor, said in a written statement.
Their estimates, published online in Arthritis & Rheumatology, put the overall SLE prevalence in the United States at 72.8 per 100,000 person-years in 2018, with nearly nine times more females affected (128.7 cases per 100,000) than males (14.6 per 100,000). Race and ethnicity also play a role, as prevalence was highest among American Indian/Alaska Native and Black females, with Hispanic females lower but still higher than White and Asian/Pacific Islander females, Peter M. Izmirly, MD, MSc, of New York University, the lead author, and associates said.
SLE prevalence was distributed similarly in men, although there was a greater relative margin between American Indians/Alaska Natives (53.8 cases per 100,000 person-years) and Blacks (26.7 per 100,000), and Asians/Pacific Islanders were higher than Whites (11.2 vs. 8.9), the investigators reported.
The meta-analysis leveraged data from the Centers for Disease Control and Prevention’s national lupus registries, which include four state-specific SLE registries and a fifth in the Indian Health Service. All cases of SLE occurred in 2002-2009, and the data were age adjusted to the 2000 U.S. population and separately extrapolated to the 2018 U.S. Census population, they explained.
The analysis was funded by cooperative agreements between the New York City Department of Health and Mental Hygiene and New York University, and the CDC and National Institute of Health.
The prevalence of systemic lupus erythematosus (SLE) appears to be much lower than previously believed and may pose “a potential risk to research funding for the disease,” according to results of a meta-analysis involving a network of population-based registries.

“When we started this study, a widely cited lupus statistic was that approximately 1.5 million Americans were affected. Our meta-analysis found the actual prevalence to be slightly more than 200,000: a number that approaches the [Food and Drug Administration’s] definition of a rare disease,” Emily Somers, PhD, ScM, senior author and associate professor of rheumatology and environmental health sciences at the University of Michigan, Ann Arbor, said in a written statement.
Their estimates, published online in Arthritis & Rheumatology, put the overall SLE prevalence in the United States at 72.8 per 100,000 person-years in 2018, with nearly nine times more females affected (128.7 cases per 100,000) than males (14.6 per 100,000). Race and ethnicity also play a role, as prevalence was highest among American Indian/Alaska Native and Black females, with Hispanic females lower but still higher than White and Asian/Pacific Islander females, Peter M. Izmirly, MD, MSc, of New York University, the lead author, and associates said.
SLE prevalence was distributed similarly in men, although there was a greater relative margin between American Indians/Alaska Natives (53.8 cases per 100,000 person-years) and Blacks (26.7 per 100,000), and Asians/Pacific Islanders were higher than Whites (11.2 vs. 8.9), the investigators reported.
The meta-analysis leveraged data from the Centers for Disease Control and Prevention’s national lupus registries, which include four state-specific SLE registries and a fifth in the Indian Health Service. All cases of SLE occurred in 2002-2009, and the data were age adjusted to the 2000 U.S. population and separately extrapolated to the 2018 U.S. Census population, they explained.
The analysis was funded by cooperative agreements between the New York City Department of Health and Mental Hygiene and New York University, and the CDC and National Institute of Health.
The prevalence of systemic lupus erythematosus (SLE) appears to be much lower than previously believed and may pose “a potential risk to research funding for the disease,” according to results of a meta-analysis involving a network of population-based registries.

“When we started this study, a widely cited lupus statistic was that approximately 1.5 million Americans were affected. Our meta-analysis found the actual prevalence to be slightly more than 200,000: a number that approaches the [Food and Drug Administration’s] definition of a rare disease,” Emily Somers, PhD, ScM, senior author and associate professor of rheumatology and environmental health sciences at the University of Michigan, Ann Arbor, said in a written statement.
Their estimates, published online in Arthritis & Rheumatology, put the overall SLE prevalence in the United States at 72.8 per 100,000 person-years in 2018, with nearly nine times more females affected (128.7 cases per 100,000) than males (14.6 per 100,000). Race and ethnicity also play a role, as prevalence was highest among American Indian/Alaska Native and Black females, with Hispanic females lower but still higher than White and Asian/Pacific Islander females, Peter M. Izmirly, MD, MSc, of New York University, the lead author, and associates said.
SLE prevalence was distributed similarly in men, although there was a greater relative margin between American Indians/Alaska Natives (53.8 cases per 100,000 person-years) and Blacks (26.7 per 100,000), and Asians/Pacific Islanders were higher than Whites (11.2 vs. 8.9), the investigators reported.
The meta-analysis leveraged data from the Centers for Disease Control and Prevention’s national lupus registries, which include four state-specific SLE registries and a fifth in the Indian Health Service. All cases of SLE occurred in 2002-2009, and the data were age adjusted to the 2000 U.S. population and separately extrapolated to the 2018 U.S. Census population, they explained.
The analysis was funded by cooperative agreements between the New York City Department of Health and Mental Hygiene and New York University, and the CDC and National Institute of Health.
FROM ARTHRITIS & RHEUMATOLOGY
Topical brepocitinib for atopic dermatitis meets endpoints in phase 2b study
, and with a safety profile essentially indistinguishable from vehicle cream in a phase 2b randomized trial, Megan N. Landis, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The study included 240 adolescents and adults with mild to moderate AD at 70 sites in the United States and nine other countries. Patients’ mean baseline Eczema Area and Severity Index (EASI) score was 7.3, with 9.2% of their body surface area being involved. Participants were equally split between mild and moderate disease. They were randomized to 6 weeks of double-blind treatment in one of eight study arms: once-daily topical brepocitinib at a concentration of 0.1%, 0.3%, 1%, or 3%; twice-daily brepocitinib at 1% or 3%; or once- or twice-daily vehicle cream.
The primary endpoint was change in EASI score from baseline to week 6. Brepocitinib 1% and 3% once daily and 1% twice daily outperformed vehicle, with EASI score reductions of 70.1%, 67.9%, and 75%, respectively, compared with a 44.4% decrease among those in the once-daily vehicle control group and a 47.6% reduction among those in the twice-daily vehicle control group, according to Dr. Landis, a dermatologist at the University of Louisville (Ky).
The key secondary efficacy endpoint was the proportion of patients achieving an Investigator’s Global Assessment (IGA) score of 0 or 1 – clear or almost clear skin – plus at least a 2-point reduction at week 6. This occurred in a dose-dependent fashion in 27.8%-44.4% of patients on once-daily brepocitinib, all significantly better results than the 10.8% rate in once-daily controls. Patients on the TYK2/JAK1 inhibitor at 0.3% twice daily had a 33.3% IGA response rate, versus 13.9% with twice-daily vehicle, also a significant difference.
A 90% reduction in EASI score at week 6, or EASI 90 response, occurred in a dose-dependent fashion in 27.8%-41.7% of patients on 0.3%, 1%, and 3% of patients on once-daily brepocitinib, all significantly better than the 10.8% rate with once-daily vehicle, and in 27% of patients on brepocitinib 1% twice daily, versus 8.3% with twice-daily vehicle.
Improvement in itch was another secondary endpoint. A clinically meaningful week-6 improvement of at least 4 points on the Peak Pruritus Numerical Rating Scale was documented in 45.2% of patients on 1% brepocitinib once daily, 50% on 3% once daily, and 40.7% on 1% brepocitinib twice daily, all significantly better than the roughly 17% itch response rate in controls.
Treatment-emergent adverse events were about one-third more frequent in controls than in brepocitinib-treated patients. These events were overwhelmingly mild and were similar in nature in the two groups. There was no dose-dependent increase in treatment-emergent adverse events in the brepocitinib patients. Moreover, no serious treatment-emergent adverse events occurred during the study, nor were there any cases of herpes zoster or malignancies, and no changes in laboratory parameters or ECG findings.
Pfizer sponsored the phase 2b AD trial of the topical TYK2/JAK1 inhibitor, which is also in phase 2 studies for psoriatic arthritis, psoriasis, lupus, and alopecia areata.
Dr. Landis reported serving as a paid investigator for Pfizer and numerous other pharmaceutical companies.
, and with a safety profile essentially indistinguishable from vehicle cream in a phase 2b randomized trial, Megan N. Landis, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The study included 240 adolescents and adults with mild to moderate AD at 70 sites in the United States and nine other countries. Patients’ mean baseline Eczema Area and Severity Index (EASI) score was 7.3, with 9.2% of their body surface area being involved. Participants were equally split between mild and moderate disease. They were randomized to 6 weeks of double-blind treatment in one of eight study arms: once-daily topical brepocitinib at a concentration of 0.1%, 0.3%, 1%, or 3%; twice-daily brepocitinib at 1% or 3%; or once- or twice-daily vehicle cream.
The primary endpoint was change in EASI score from baseline to week 6. Brepocitinib 1% and 3% once daily and 1% twice daily outperformed vehicle, with EASI score reductions of 70.1%, 67.9%, and 75%, respectively, compared with a 44.4% decrease among those in the once-daily vehicle control group and a 47.6% reduction among those in the twice-daily vehicle control group, according to Dr. Landis, a dermatologist at the University of Louisville (Ky).
The key secondary efficacy endpoint was the proportion of patients achieving an Investigator’s Global Assessment (IGA) score of 0 or 1 – clear or almost clear skin – plus at least a 2-point reduction at week 6. This occurred in a dose-dependent fashion in 27.8%-44.4% of patients on once-daily brepocitinib, all significantly better results than the 10.8% rate in once-daily controls. Patients on the TYK2/JAK1 inhibitor at 0.3% twice daily had a 33.3% IGA response rate, versus 13.9% with twice-daily vehicle, also a significant difference.
A 90% reduction in EASI score at week 6, or EASI 90 response, occurred in a dose-dependent fashion in 27.8%-41.7% of patients on 0.3%, 1%, and 3% of patients on once-daily brepocitinib, all significantly better than the 10.8% rate with once-daily vehicle, and in 27% of patients on brepocitinib 1% twice daily, versus 8.3% with twice-daily vehicle.
Improvement in itch was another secondary endpoint. A clinically meaningful week-6 improvement of at least 4 points on the Peak Pruritus Numerical Rating Scale was documented in 45.2% of patients on 1% brepocitinib once daily, 50% on 3% once daily, and 40.7% on 1% brepocitinib twice daily, all significantly better than the roughly 17% itch response rate in controls.
Treatment-emergent adverse events were about one-third more frequent in controls than in brepocitinib-treated patients. These events were overwhelmingly mild and were similar in nature in the two groups. There was no dose-dependent increase in treatment-emergent adverse events in the brepocitinib patients. Moreover, no serious treatment-emergent adverse events occurred during the study, nor were there any cases of herpes zoster or malignancies, and no changes in laboratory parameters or ECG findings.
Pfizer sponsored the phase 2b AD trial of the topical TYK2/JAK1 inhibitor, which is also in phase 2 studies for psoriatic arthritis, psoriasis, lupus, and alopecia areata.
Dr. Landis reported serving as a paid investigator for Pfizer and numerous other pharmaceutical companies.
, and with a safety profile essentially indistinguishable from vehicle cream in a phase 2b randomized trial, Megan N. Landis, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The study included 240 adolescents and adults with mild to moderate AD at 70 sites in the United States and nine other countries. Patients’ mean baseline Eczema Area and Severity Index (EASI) score was 7.3, with 9.2% of their body surface area being involved. Participants were equally split between mild and moderate disease. They were randomized to 6 weeks of double-blind treatment in one of eight study arms: once-daily topical brepocitinib at a concentration of 0.1%, 0.3%, 1%, or 3%; twice-daily brepocitinib at 1% or 3%; or once- or twice-daily vehicle cream.
The primary endpoint was change in EASI score from baseline to week 6. Brepocitinib 1% and 3% once daily and 1% twice daily outperformed vehicle, with EASI score reductions of 70.1%, 67.9%, and 75%, respectively, compared with a 44.4% decrease among those in the once-daily vehicle control group and a 47.6% reduction among those in the twice-daily vehicle control group, according to Dr. Landis, a dermatologist at the University of Louisville (Ky).
The key secondary efficacy endpoint was the proportion of patients achieving an Investigator’s Global Assessment (IGA) score of 0 or 1 – clear or almost clear skin – plus at least a 2-point reduction at week 6. This occurred in a dose-dependent fashion in 27.8%-44.4% of patients on once-daily brepocitinib, all significantly better results than the 10.8% rate in once-daily controls. Patients on the TYK2/JAK1 inhibitor at 0.3% twice daily had a 33.3% IGA response rate, versus 13.9% with twice-daily vehicle, also a significant difference.
A 90% reduction in EASI score at week 6, or EASI 90 response, occurred in a dose-dependent fashion in 27.8%-41.7% of patients on 0.3%, 1%, and 3% of patients on once-daily brepocitinib, all significantly better than the 10.8% rate with once-daily vehicle, and in 27% of patients on brepocitinib 1% twice daily, versus 8.3% with twice-daily vehicle.
Improvement in itch was another secondary endpoint. A clinically meaningful week-6 improvement of at least 4 points on the Peak Pruritus Numerical Rating Scale was documented in 45.2% of patients on 1% brepocitinib once daily, 50% on 3% once daily, and 40.7% on 1% brepocitinib twice daily, all significantly better than the roughly 17% itch response rate in controls.
Treatment-emergent adverse events were about one-third more frequent in controls than in brepocitinib-treated patients. These events were overwhelmingly mild and were similar in nature in the two groups. There was no dose-dependent increase in treatment-emergent adverse events in the brepocitinib patients. Moreover, no serious treatment-emergent adverse events occurred during the study, nor were there any cases of herpes zoster or malignancies, and no changes in laboratory parameters or ECG findings.
Pfizer sponsored the phase 2b AD trial of the topical TYK2/JAK1 inhibitor, which is also in phase 2 studies for psoriatic arthritis, psoriasis, lupus, and alopecia areata.
Dr. Landis reported serving as a paid investigator for Pfizer and numerous other pharmaceutical companies.
FROM THE EADV CONGRESS
Are pediatric and adult dermatitis the same disease?
“Maybe not,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis symposium.
Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington, based his comments largely on a review that he and his colleagues carried out to understand how features of atopic dermatitis (AD) vary by region globally as well as by age. They identified 101 studies with sufficient data for meta-analysis and stratified the results by pediatric and adult age groups.
Several signs and symptoms occurred with similar frequency among pediatric and adult patients, including pruritus, xerosis, flexural involvement, extensor involvement, early onset of disease, comorbid atopy, head and neck involvement, and ophthalmic comorbidities. However, adults were found to have more signs of chronic disease, more hand eczema, different patterns of hand eczema, and a stronger relationship of disease activity with emotional factors. Meanwhile, children were found to have more exudative or weeping lesions, more perifollicular eczema, and more pityriasis alba.
Dr. Silverberg showed photos of three adults with varied presentations of extensor involvement, including one “who had a lot of lichenification and thickening of the skin, but over knees where you might think about psoriasis,” he said. “All three of these patients were of Southeast Asian descent. That happens to be a region where this feature was reported much more commonly. It may even tie to some underlying immunopathophysiologic differences of the disease across different patient populations.”
AD signs that occur more commonly in adults than children include lichenification (100% vs. 48%), urticaria (32% vs. 20%), popular lichenoid lesions (46% vs. 8%), Hertoghe’s sign (25% vs. 2%), erythroderma (29% vs. 1%), and nodular prurigo (18% vs. 4%).
Hand eczema features also differ between adults and children, including hand or foot dermatitis (44% vs. 25%), dyshidrosis/pompholyx (21% vs. 3%), knuckle dermatitis (25% vs. 8%), nail involvement (15% vs. 8%), and fissured heels. However, ventral wrist dermatitis was found to be more than twice as common in children, compared with adults (34% vs. 15%).
Other signs of AD were more common in children, compared with adults, including exudative eczema (61% vs. 42%), pityriasis alba (28% vs. 18%), Dennie-Morgan infraorbital folds (47% vs. 36%), seborrheic dermatitis–like lesions (40% vs. 18%), and perifollicular accentuation (37% vs. 21%). “This is such an important sign to wrap your head around and get comfortable assessing,” he said. “I have seen patients who are erythrodermic with follicular eczema who were told that they were crazy and had psychogenic itch, and they should go to a shrink.”
AD triggers can differ between adults and children as well, including course influenced by emotions/environmental factors (72% vs. 32%), worsening itch worse (65% vs. 49%), course influenced by environment (62% vs. 37%), and course influenced by emotions (70% vs. 15%).
According to Dr. Silverberg, emerging research suggests that there may be differences in the immune pathways activated in pediatric versus adult AD. Specifically, more Th17 and interferon-gamma in AD lesions have been observed in children, compared with adults, and more Th22 and Th17 in nonlesional AD have been seen in children, compared with adults. “This leads to a question: Will children respond differently than adults to treatment?” Dr. Silverberg said. “We see that omalizumab doesn’t seem to help much in adults, yet a recent study suggested that it might work reasonably well for children. Dupilumab has different dosing requirements and potentially different responses between the pediatric and adult populations.”
Age differences in AD may also be related to differences in the skin microbiome. In 2016, researchers led by Richard L. Gallo, MD, PhD, professor of dermatology, University of California, San Diego, compared the skin microbiome between adults and children with AD by swabbing the volar forearm and performing 16S rRNA gene sequencing. The study included 59 young children, 13 teenagers, and 56 adults with AD as well as 68 age-matched non-atopic healthy controls. The researchers found a greater abundance of Streptococcus, Granulicatella, Gemella, Rothia, and Haemophilus in young children, compared with adults, while Propionibacterium, Corynebacterium, Staphylococcus, Lactobacillus, Finegoldia, and Anaerococcus were more abundant in adults, compared with children.
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Maybe not,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis symposium.
Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington, based his comments largely on a review that he and his colleagues carried out to understand how features of atopic dermatitis (AD) vary by region globally as well as by age. They identified 101 studies with sufficient data for meta-analysis and stratified the results by pediatric and adult age groups.
Several signs and symptoms occurred with similar frequency among pediatric and adult patients, including pruritus, xerosis, flexural involvement, extensor involvement, early onset of disease, comorbid atopy, head and neck involvement, and ophthalmic comorbidities. However, adults were found to have more signs of chronic disease, more hand eczema, different patterns of hand eczema, and a stronger relationship of disease activity with emotional factors. Meanwhile, children were found to have more exudative or weeping lesions, more perifollicular eczema, and more pityriasis alba.
Dr. Silverberg showed photos of three adults with varied presentations of extensor involvement, including one “who had a lot of lichenification and thickening of the skin, but over knees where you might think about psoriasis,” he said. “All three of these patients were of Southeast Asian descent. That happens to be a region where this feature was reported much more commonly. It may even tie to some underlying immunopathophysiologic differences of the disease across different patient populations.”
AD signs that occur more commonly in adults than children include lichenification (100% vs. 48%), urticaria (32% vs. 20%), popular lichenoid lesions (46% vs. 8%), Hertoghe’s sign (25% vs. 2%), erythroderma (29% vs. 1%), and nodular prurigo (18% vs. 4%).
Hand eczema features also differ between adults and children, including hand or foot dermatitis (44% vs. 25%), dyshidrosis/pompholyx (21% vs. 3%), knuckle dermatitis (25% vs. 8%), nail involvement (15% vs. 8%), and fissured heels. However, ventral wrist dermatitis was found to be more than twice as common in children, compared with adults (34% vs. 15%).
Other signs of AD were more common in children, compared with adults, including exudative eczema (61% vs. 42%), pityriasis alba (28% vs. 18%), Dennie-Morgan infraorbital folds (47% vs. 36%), seborrheic dermatitis–like lesions (40% vs. 18%), and perifollicular accentuation (37% vs. 21%). “This is such an important sign to wrap your head around and get comfortable assessing,” he said. “I have seen patients who are erythrodermic with follicular eczema who were told that they were crazy and had psychogenic itch, and they should go to a shrink.”
AD triggers can differ between adults and children as well, including course influenced by emotions/environmental factors (72% vs. 32%), worsening itch worse (65% vs. 49%), course influenced by environment (62% vs. 37%), and course influenced by emotions (70% vs. 15%).
According to Dr. Silverberg, emerging research suggests that there may be differences in the immune pathways activated in pediatric versus adult AD. Specifically, more Th17 and interferon-gamma in AD lesions have been observed in children, compared with adults, and more Th22 and Th17 in nonlesional AD have been seen in children, compared with adults. “This leads to a question: Will children respond differently than adults to treatment?” Dr. Silverberg said. “We see that omalizumab doesn’t seem to help much in adults, yet a recent study suggested that it might work reasonably well for children. Dupilumab has different dosing requirements and potentially different responses between the pediatric and adult populations.”
Age differences in AD may also be related to differences in the skin microbiome. In 2016, researchers led by Richard L. Gallo, MD, PhD, professor of dermatology, University of California, San Diego, compared the skin microbiome between adults and children with AD by swabbing the volar forearm and performing 16S rRNA gene sequencing. The study included 59 young children, 13 teenagers, and 56 adults with AD as well as 68 age-matched non-atopic healthy controls. The researchers found a greater abundance of Streptococcus, Granulicatella, Gemella, Rothia, and Haemophilus in young children, compared with adults, while Propionibacterium, Corynebacterium, Staphylococcus, Lactobacillus, Finegoldia, and Anaerococcus were more abundant in adults, compared with children.
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Maybe not,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis symposium.
Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington, based his comments largely on a review that he and his colleagues carried out to understand how features of atopic dermatitis (AD) vary by region globally as well as by age. They identified 101 studies with sufficient data for meta-analysis and stratified the results by pediatric and adult age groups.
Several signs and symptoms occurred with similar frequency among pediatric and adult patients, including pruritus, xerosis, flexural involvement, extensor involvement, early onset of disease, comorbid atopy, head and neck involvement, and ophthalmic comorbidities. However, adults were found to have more signs of chronic disease, more hand eczema, different patterns of hand eczema, and a stronger relationship of disease activity with emotional factors. Meanwhile, children were found to have more exudative or weeping lesions, more perifollicular eczema, and more pityriasis alba.
Dr. Silverberg showed photos of three adults with varied presentations of extensor involvement, including one “who had a lot of lichenification and thickening of the skin, but over knees where you might think about psoriasis,” he said. “All three of these patients were of Southeast Asian descent. That happens to be a region where this feature was reported much more commonly. It may even tie to some underlying immunopathophysiologic differences of the disease across different patient populations.”
AD signs that occur more commonly in adults than children include lichenification (100% vs. 48%), urticaria (32% vs. 20%), popular lichenoid lesions (46% vs. 8%), Hertoghe’s sign (25% vs. 2%), erythroderma (29% vs. 1%), and nodular prurigo (18% vs. 4%).
Hand eczema features also differ between adults and children, including hand or foot dermatitis (44% vs. 25%), dyshidrosis/pompholyx (21% vs. 3%), knuckle dermatitis (25% vs. 8%), nail involvement (15% vs. 8%), and fissured heels. However, ventral wrist dermatitis was found to be more than twice as common in children, compared with adults (34% vs. 15%).
Other signs of AD were more common in children, compared with adults, including exudative eczema (61% vs. 42%), pityriasis alba (28% vs. 18%), Dennie-Morgan infraorbital folds (47% vs. 36%), seborrheic dermatitis–like lesions (40% vs. 18%), and perifollicular accentuation (37% vs. 21%). “This is such an important sign to wrap your head around and get comfortable assessing,” he said. “I have seen patients who are erythrodermic with follicular eczema who were told that they were crazy and had psychogenic itch, and they should go to a shrink.”
AD triggers can differ between adults and children as well, including course influenced by emotions/environmental factors (72% vs. 32%), worsening itch worse (65% vs. 49%), course influenced by environment (62% vs. 37%), and course influenced by emotions (70% vs. 15%).
According to Dr. Silverberg, emerging research suggests that there may be differences in the immune pathways activated in pediatric versus adult AD. Specifically, more Th17 and interferon-gamma in AD lesions have been observed in children, compared with adults, and more Th22 and Th17 in nonlesional AD have been seen in children, compared with adults. “This leads to a question: Will children respond differently than adults to treatment?” Dr. Silverberg said. “We see that omalizumab doesn’t seem to help much in adults, yet a recent study suggested that it might work reasonably well for children. Dupilumab has different dosing requirements and potentially different responses between the pediatric and adult populations.”
Age differences in AD may also be related to differences in the skin microbiome. In 2016, researchers led by Richard L. Gallo, MD, PhD, professor of dermatology, University of California, San Diego, compared the skin microbiome between adults and children with AD by swabbing the volar forearm and performing 16S rRNA gene sequencing. The study included 59 young children, 13 teenagers, and 56 adults with AD as well as 68 age-matched non-atopic healthy controls. The researchers found a greater abundance of Streptococcus, Granulicatella, Gemella, Rothia, and Haemophilus in young children, compared with adults, while Propionibacterium, Corynebacterium, Staphylococcus, Lactobacillus, Finegoldia, and Anaerococcus were more abundant in adults, compared with children.
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2020


