User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children and COVID: Weekly cases top 95,000, admissions continue to rise
New pediatric COVID-19 cases increased for the third straight week as a substantial number of children under age 5 years started to receive their second doses of the vaccine.
Despite the 3-week trend, however, there are some positive signs. The new-case count for the latest reporting week (July 22-28) was over 95,000, but the 3.9% increase over the previous week’s 92,000 cases is much smaller than that week’s (July 15-21) corresponding jump of almost 22% over the July 8-14 total (75,000), according to the American Academy of Pediatrics and the Children’s Hospital Association.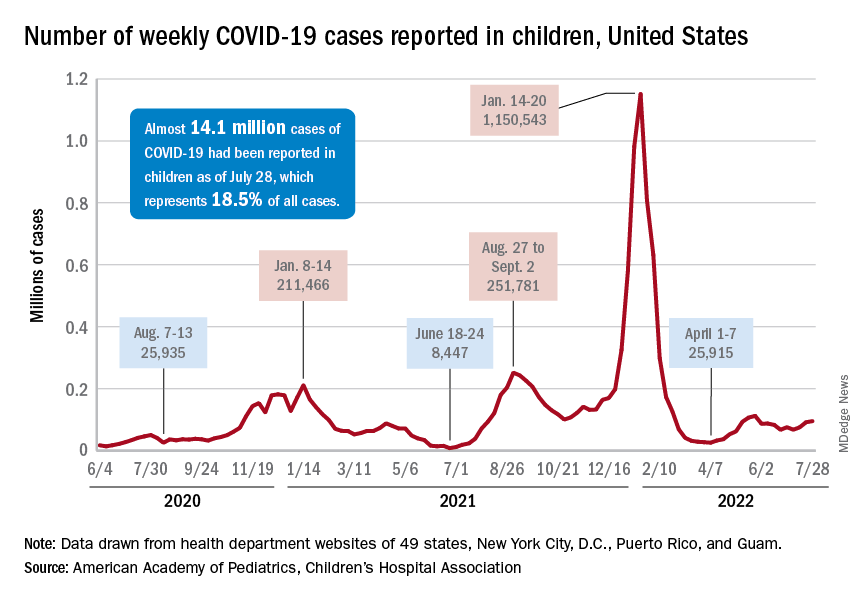
On the not-so-positive side is the trend in admissions among children aged 0-17 years, which continue to climb steadily and have nearly equaled the highest rate seen during the Delta surge in 2021. The rate on July 29 was 0.46 admissions per 100,000 population, and the highest rate over the course of the Delta surge was 0.47 per 100,000, but the all-time high from the Omicron surge – 1.25 per 100,000 in mid-January – is still a long way off, based on data from the Centers for Disease Control and Prevention.
A similar situation is occurring with emergency department visits, but there is differentiation by age group. Among those aged 0-11 years, visits with diagnosed COVID made up 6.5% of all their ED visits on July 25, which was well above the high (4.0%) during the Delta surge, the CDC said.
That is not the case, however, for the older children, for whom rates are rising more slowly. Those aged 12-15 have reached 3.4% so far this summer, as have the 16- to 17-years-olds, versus Delta highs last year of around 7%, the CDC said on its COVID Data Tracker. As with admissions, though, current rates are well below the all-time Omicron high points, the CDC data show.
Joining the ranks of the fully vaccinated
Over the last 2 weeks, the first children to receive the COVID vaccine after its approval for those under age 5 years have been coming back for their second doses. Almost 50,000, about 0.3% of all those in that age group, had done so by July 27. Just over 662,000, about 3.4% of the total under-5 population, have received at least one dose, the CDC said.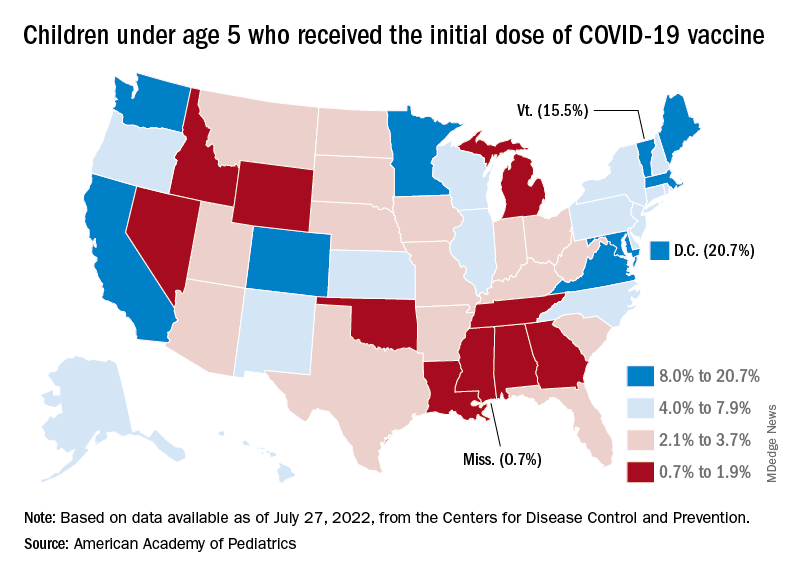
Meanwhile, analysis of “data from the first several weeks following availability of the vaccine in this age group indicate high variability across states,” the AAP said in its weekly vaccination report. In the District of Columbia, 20.7% of all children under age 5 have received an initial dose as of July 27, as have 15.5% of those in Vermont and 12.5% in Massachusetts. No other state was above 10%, but Mississippi, at 0.7%, was the only one below 1%.
The older children, obviously, have a head start, so their numbers are much higher. At the state level, Vermont has the highest initial dose rate, 69%, for those aged 5-11 years, while Alabama, Mississippi, and Wyoming, at 17%, are looking up at everyone else in the country. Among children aged 12-17 years, D.C. is the highest with 100% vaccination – Massachusetts and Rhode Island are at 98% – and Wyoming is the lowest with 40%, the AAP said.
New pediatric COVID-19 cases increased for the third straight week as a substantial number of children under age 5 years started to receive their second doses of the vaccine.
Despite the 3-week trend, however, there are some positive signs. The new-case count for the latest reporting week (July 22-28) was over 95,000, but the 3.9% increase over the previous week’s 92,000 cases is much smaller than that week’s (July 15-21) corresponding jump of almost 22% over the July 8-14 total (75,000), according to the American Academy of Pediatrics and the Children’s Hospital Association.
On the not-so-positive side is the trend in admissions among children aged 0-17 years, which continue to climb steadily and have nearly equaled the highest rate seen during the Delta surge in 2021. The rate on July 29 was 0.46 admissions per 100,000 population, and the highest rate over the course of the Delta surge was 0.47 per 100,000, but the all-time high from the Omicron surge – 1.25 per 100,000 in mid-January – is still a long way off, based on data from the Centers for Disease Control and Prevention.
A similar situation is occurring with emergency department visits, but there is differentiation by age group. Among those aged 0-11 years, visits with diagnosed COVID made up 6.5% of all their ED visits on July 25, which was well above the high (4.0%) during the Delta surge, the CDC said.
That is not the case, however, for the older children, for whom rates are rising more slowly. Those aged 12-15 have reached 3.4% so far this summer, as have the 16- to 17-years-olds, versus Delta highs last year of around 7%, the CDC said on its COVID Data Tracker. As with admissions, though, current rates are well below the all-time Omicron high points, the CDC data show.
Joining the ranks of the fully vaccinated
Over the last 2 weeks, the first children to receive the COVID vaccine after its approval for those under age 5 years have been coming back for their second doses. Almost 50,000, about 0.3% of all those in that age group, had done so by July 27. Just over 662,000, about 3.4% of the total under-5 population, have received at least one dose, the CDC said.
Meanwhile, analysis of “data from the first several weeks following availability of the vaccine in this age group indicate high variability across states,” the AAP said in its weekly vaccination report. In the District of Columbia, 20.7% of all children under age 5 have received an initial dose as of July 27, as have 15.5% of those in Vermont and 12.5% in Massachusetts. No other state was above 10%, but Mississippi, at 0.7%, was the only one below 1%.
The older children, obviously, have a head start, so their numbers are much higher. At the state level, Vermont has the highest initial dose rate, 69%, for those aged 5-11 years, while Alabama, Mississippi, and Wyoming, at 17%, are looking up at everyone else in the country. Among children aged 12-17 years, D.C. is the highest with 100% vaccination – Massachusetts and Rhode Island are at 98% – and Wyoming is the lowest with 40%, the AAP said.
New pediatric COVID-19 cases increased for the third straight week as a substantial number of children under age 5 years started to receive their second doses of the vaccine.
Despite the 3-week trend, however, there are some positive signs. The new-case count for the latest reporting week (July 22-28) was over 95,000, but the 3.9% increase over the previous week’s 92,000 cases is much smaller than that week’s (July 15-21) corresponding jump of almost 22% over the July 8-14 total (75,000), according to the American Academy of Pediatrics and the Children’s Hospital Association.
On the not-so-positive side is the trend in admissions among children aged 0-17 years, which continue to climb steadily and have nearly equaled the highest rate seen during the Delta surge in 2021. The rate on July 29 was 0.46 admissions per 100,000 population, and the highest rate over the course of the Delta surge was 0.47 per 100,000, but the all-time high from the Omicron surge – 1.25 per 100,000 in mid-January – is still a long way off, based on data from the Centers for Disease Control and Prevention.
A similar situation is occurring with emergency department visits, but there is differentiation by age group. Among those aged 0-11 years, visits with diagnosed COVID made up 6.5% of all their ED visits on July 25, which was well above the high (4.0%) during the Delta surge, the CDC said.
That is not the case, however, for the older children, for whom rates are rising more slowly. Those aged 12-15 have reached 3.4% so far this summer, as have the 16- to 17-years-olds, versus Delta highs last year of around 7%, the CDC said on its COVID Data Tracker. As with admissions, though, current rates are well below the all-time Omicron high points, the CDC data show.
Joining the ranks of the fully vaccinated
Over the last 2 weeks, the first children to receive the COVID vaccine after its approval for those under age 5 years have been coming back for their second doses. Almost 50,000, about 0.3% of all those in that age group, had done so by July 27. Just over 662,000, about 3.4% of the total under-5 population, have received at least one dose, the CDC said.
Meanwhile, analysis of “data from the first several weeks following availability of the vaccine in this age group indicate high variability across states,” the AAP said in its weekly vaccination report. In the District of Columbia, 20.7% of all children under age 5 have received an initial dose as of July 27, as have 15.5% of those in Vermont and 12.5% in Massachusetts. No other state was above 10%, but Mississippi, at 0.7%, was the only one below 1%.
The older children, obviously, have a head start, so their numbers are much higher. At the state level, Vermont has the highest initial dose rate, 69%, for those aged 5-11 years, while Alabama, Mississippi, and Wyoming, at 17%, are looking up at everyone else in the country. Among children aged 12-17 years, D.C. is the highest with 100% vaccination – Massachusetts and Rhode Island are at 98% – and Wyoming is the lowest with 40%, the AAP said.
Low calcium, potassium key risk factors for kidney stones
as well as their symptomatic recurrence, a population-based study of dietary factors shows.
“Our research is of particular importance as recommendations for preventing symptomatic recurrence of kidney stones has largely been based on dietary factors associated with the incidence rather than the recurrence of stone formation,” Api Chewcharat, MD, Mayo Clinic, Rochester, Minn., said in a video discussing the study.
“We recommend a daily intake of calcium of approximately 1,200 mg and a diet that is high in potassium, especially high in fruits and vegetables, in order to prevent both incident and recurrent symptomatic kidney stone formation,” he stressed.
The study was published online in Mayo Clinic Proceedings.
Lower dietary calcium, potassium, and fluid associated with increased incidence
Some 411 patients with incident symptomatic kidney stone formation were recruited. Diets were compared between them and 384 controls. Patients were seen at the Mayo Clinic in either Minnesota or Florida between Jan. 1, 2009, and Aug. 31, 2018. “Dietary factors were based on a Viocare food frequency questionnaire administered during a baseline in-person study visit,” Dr. Chewcharat and colleagues observed.
During a median follow-up of 4.1 years, 73 patients experienced a symptomatic recurrence. In a fully adjusted analysis, a dietary calcium intake less than 1,200 mg/d was associated with incident stone formation. Similarly, among participants with a fluid intake less than 3,400 mL/d – about nine 12-oz glasses of fluid – was also associated with incident stone formation, as was a lower intake of dietary potassium, caffeine, and phytate. Phytate is an antioxidant found in whole grains, nuts, and other foods that can increase calcium absorption and urinary calcium excretion.
After excluding patients who were taking either a thiazide diuretic or a calcium supplement, lower dietary calcium and potassium, fluid, and phytate intake remained significantly associated with incident stone formation.
However, only lower dietary calcium intake was associated with a higher risk for symptomatic recurrence, although a lower dietary potassium intake was also associated with a higher risk for symptomatic recurrence in an analysis that adjusted for body mass index, fluid, and energy intake.
As the authors suggested, patients may be less keen to adjust their diet to prevent the development of incident kidney stones. On the other hand, they may be much more willing to adjust their diet to prevent their symptomatic recurrence. The Department of Agriculture currently recommends that individuals get approximately 1,200 mg/d of dietary calcium which, given the study results, appears to be justified for the prevention of symptomatic stone recurrence.
A higher-calcium diet is associated with a higher urinary pH, and citrate confers an alkali load which helps protect against the formation of calcium oxalate stones. Foods that are high in potassium also contain more fluid, citrate, and phytate, which, again, have been reported to be protective against kidney stones. “Changing your diet to prevent kidney stones can be very difficult,” Andrew Rule, MD, a nephrologist at the Mayo Clinic said in a statement.
“Thus, knowing the dietary factors that are most important for preventing kidney stone recurrence can help patients and providers know what to prioritize,” he added.
The authors have no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
as well as their symptomatic recurrence, a population-based study of dietary factors shows.
“Our research is of particular importance as recommendations for preventing symptomatic recurrence of kidney stones has largely been based on dietary factors associated with the incidence rather than the recurrence of stone formation,” Api Chewcharat, MD, Mayo Clinic, Rochester, Minn., said in a video discussing the study.
“We recommend a daily intake of calcium of approximately 1,200 mg and a diet that is high in potassium, especially high in fruits and vegetables, in order to prevent both incident and recurrent symptomatic kidney stone formation,” he stressed.
The study was published online in Mayo Clinic Proceedings.
Lower dietary calcium, potassium, and fluid associated with increased incidence
Some 411 patients with incident symptomatic kidney stone formation were recruited. Diets were compared between them and 384 controls. Patients were seen at the Mayo Clinic in either Minnesota or Florida between Jan. 1, 2009, and Aug. 31, 2018. “Dietary factors were based on a Viocare food frequency questionnaire administered during a baseline in-person study visit,” Dr. Chewcharat and colleagues observed.
During a median follow-up of 4.1 years, 73 patients experienced a symptomatic recurrence. In a fully adjusted analysis, a dietary calcium intake less than 1,200 mg/d was associated with incident stone formation. Similarly, among participants with a fluid intake less than 3,400 mL/d – about nine 12-oz glasses of fluid – was also associated with incident stone formation, as was a lower intake of dietary potassium, caffeine, and phytate. Phytate is an antioxidant found in whole grains, nuts, and other foods that can increase calcium absorption and urinary calcium excretion.
After excluding patients who were taking either a thiazide diuretic or a calcium supplement, lower dietary calcium and potassium, fluid, and phytate intake remained significantly associated with incident stone formation.
However, only lower dietary calcium intake was associated with a higher risk for symptomatic recurrence, although a lower dietary potassium intake was also associated with a higher risk for symptomatic recurrence in an analysis that adjusted for body mass index, fluid, and energy intake.
As the authors suggested, patients may be less keen to adjust their diet to prevent the development of incident kidney stones. On the other hand, they may be much more willing to adjust their diet to prevent their symptomatic recurrence. The Department of Agriculture currently recommends that individuals get approximately 1,200 mg/d of dietary calcium which, given the study results, appears to be justified for the prevention of symptomatic stone recurrence.
A higher-calcium diet is associated with a higher urinary pH, and citrate confers an alkali load which helps protect against the formation of calcium oxalate stones. Foods that are high in potassium also contain more fluid, citrate, and phytate, which, again, have been reported to be protective against kidney stones. “Changing your diet to prevent kidney stones can be very difficult,” Andrew Rule, MD, a nephrologist at the Mayo Clinic said in a statement.
“Thus, knowing the dietary factors that are most important for preventing kidney stone recurrence can help patients and providers know what to prioritize,” he added.
The authors have no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
as well as their symptomatic recurrence, a population-based study of dietary factors shows.
“Our research is of particular importance as recommendations for preventing symptomatic recurrence of kidney stones has largely been based on dietary factors associated with the incidence rather than the recurrence of stone formation,” Api Chewcharat, MD, Mayo Clinic, Rochester, Minn., said in a video discussing the study.
“We recommend a daily intake of calcium of approximately 1,200 mg and a diet that is high in potassium, especially high in fruits and vegetables, in order to prevent both incident and recurrent symptomatic kidney stone formation,” he stressed.
The study was published online in Mayo Clinic Proceedings.
Lower dietary calcium, potassium, and fluid associated with increased incidence
Some 411 patients with incident symptomatic kidney stone formation were recruited. Diets were compared between them and 384 controls. Patients were seen at the Mayo Clinic in either Minnesota or Florida between Jan. 1, 2009, and Aug. 31, 2018. “Dietary factors were based on a Viocare food frequency questionnaire administered during a baseline in-person study visit,” Dr. Chewcharat and colleagues observed.
During a median follow-up of 4.1 years, 73 patients experienced a symptomatic recurrence. In a fully adjusted analysis, a dietary calcium intake less than 1,200 mg/d was associated with incident stone formation. Similarly, among participants with a fluid intake less than 3,400 mL/d – about nine 12-oz glasses of fluid – was also associated with incident stone formation, as was a lower intake of dietary potassium, caffeine, and phytate. Phytate is an antioxidant found in whole grains, nuts, and other foods that can increase calcium absorption and urinary calcium excretion.
After excluding patients who were taking either a thiazide diuretic or a calcium supplement, lower dietary calcium and potassium, fluid, and phytate intake remained significantly associated with incident stone formation.
However, only lower dietary calcium intake was associated with a higher risk for symptomatic recurrence, although a lower dietary potassium intake was also associated with a higher risk for symptomatic recurrence in an analysis that adjusted for body mass index, fluid, and energy intake.
As the authors suggested, patients may be less keen to adjust their diet to prevent the development of incident kidney stones. On the other hand, they may be much more willing to adjust their diet to prevent their symptomatic recurrence. The Department of Agriculture currently recommends that individuals get approximately 1,200 mg/d of dietary calcium which, given the study results, appears to be justified for the prevention of symptomatic stone recurrence.
A higher-calcium diet is associated with a higher urinary pH, and citrate confers an alkali load which helps protect against the formation of calcium oxalate stones. Foods that are high in potassium also contain more fluid, citrate, and phytate, which, again, have been reported to be protective against kidney stones. “Changing your diet to prevent kidney stones can be very difficult,” Andrew Rule, MD, a nephrologist at the Mayo Clinic said in a statement.
“Thus, knowing the dietary factors that are most important for preventing kidney stone recurrence can help patients and providers know what to prioritize,” he added.
The authors have no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
FROM MAYO CLINIC PRECEEDINGS
COVID skin manifestations vary by type of variant, U.K. study finds
during the Omicron and Delta waves.
Among the key findings, the study shows that skin involvement during the Omicron wave was less frequent than during the Delta wave (11.4% vs. 17.6%), skin symptoms generally resolved more quickly, and that the risk for skin symptoms was similar whether patients had or had not been vaccinated, according to a team led by Alessia Visconti, PhD, a research fellow in the department of twin research and genetic epidemiology, King’s College, London.
These data are consistent with the experience of those dermatologists who have been following this area closely, according to Esther Freeman, MD, PhD, associate professor of dermatology at Harvard Medical School and director of MGH Global Health Dermatology at Massachusetts General Hospital, both in Boston.
“Anecdotally, we thought we were seeing fewer skin symptoms with Omicron versus Delta and the ancestral strains, and now this study shows it is true,” said Dr. Freeman, who is also principal investigator of the American Academy of Dermatology’s International Dermatology COVID-19 Registry.
The data also confirm that the skin is less likely to be involved than in past waves of COVID-19 infections.
“Up to this point, it was hard to know if we were seeing fewer referrals for COVID-related skin rashes or if clinicians had just become more comfortable with these rashes and were not referring them as often,” added Dr. Freeman, who was among the study coauthors.
Data captured from 348,691 patients
The data from the study was generated by 348,691 users in the United Kingdom of the ZOE COVID study app, a smartphone-based tool introduced relatively early in the pandemic. It asked users to provide demographic data, information on COVID-19 symptoms, including those involving the skin, and treatments. Of 33 COVID-related symptoms included in the app, five related to the skin (acral rash, burning rash, erythematopapular rash, urticarial rash, and unusual hair loss).
While the focus of this study was to compare skin manifestations during the Omicron wave with the Delta wave of COVID-19, the investigators also had data on the experience in 2020 with wild-type COVID-19 that preceded both variants. Overall, this showed a stepwise decline in skin symptoms overall, as well in as skin symptoms that occurred in the absence of systemic symptoms.
“The shift in the skin manifestations makes sense when you think about the change that is also being seen in the systemic symptoms,” said Dr. Freeman, referring to lower rates of cough and loss of smell but higher rates of sore throat and fatigue. “Omicron is achieving immune escape, which is why there is a shift in involved tissues,” she said in an interview.
Previous data collected during the wild-type COVID-19 stage of the pandemic by the same group of investigators showed that 17% of patients reported skin rash as the first symptom of COVID-19 infection, and 21% reported skin rash as the only clinical sign of infection.
In the Delta and Omicron waves, skin rash was an isolated initial symptom in only 0.8% and 0.5% of patients, respectively. (The authors noted that, in the United Kingdom, the first documented samples of the Delta variant were detected in October 2020, and the first documented samples of the Omicron variant were detected in November 2021.)
During the early stages of wild-type COVID, an acral rash was characteristic, occurring in 3.1% of patients, according to the U.K. data. In the Delta wave, acral rashes, at an incidence of 1.1% remained positively correlated with a diagnosis of COVID-19 infection. In the Omicron wave, acral rashes were observed in only 0.7% of patients and were no longer statistically correlated with a positive COVID diagnosis.
Characteristic cutaneous symptoms are evolving
Early in the course of the COVID-19 epidemic, more than 30 types of rashes were observed in patients with COVID-19 infection. Cutaneous symptoms continue to be diverse, but some, such as acral rash, are being seen less frequently. For example, the odds ratio of a positive COVID-19 diagnosis among those with an erythematopapular rash fell from 1.76 to 1.08 between the Delta and Omicron waves.
While specific cutaneous symptoms are less predictive of a diagnosis of COVID-19, clinicians should not discount cutaneous symptoms as a sign of disease, according to Veronique Bataille, MD, PhD, a consultant dermatologist at King’s College.
“You need to keep an open mind” regarding cutaneous signs and a diagnosis of COVID-19, Dr. Bataille, one of the coauthors of the U.K. report, said in an interview. In general, she considers a low threshold of suspicion appropriate. “If the patient has no past history of skin disease and no other triggers for a rash, then, in a high prevalence area, COVID must be suspected.”
In most cases, the rash resolves on its own, but Dr. Bataille emphasized the need for individualized care. Even as the risk of life-threatening COVID-19 infections appears to be diminishing with current variants, cutaneous manifestations can be severe.
“There are cases of long COVID affecting the skin, such as urticaria or a lichenoid erythematopapular rash, both of which can be very pruritic and difficult to control,” she said.
Dr. Freeman echoed the importance of an individualized approach. She agreed that most cutaneous symptoms are self-limited, but there are exceptions and treatments vary for the different types of skin involvement. “I think another point to consider when examining skin lesions is monkey pox. The fact that these are overlapping outbreaks should not be ignored. You need to be alert for both.”
Dr. Visconti, Dr. Freeman, and Dr. Bataille reported no potential conflicts of interest.
during the Omicron and Delta waves.
Among the key findings, the study shows that skin involvement during the Omicron wave was less frequent than during the Delta wave (11.4% vs. 17.6%), skin symptoms generally resolved more quickly, and that the risk for skin symptoms was similar whether patients had or had not been vaccinated, according to a team led by Alessia Visconti, PhD, a research fellow in the department of twin research and genetic epidemiology, King’s College, London.
These data are consistent with the experience of those dermatologists who have been following this area closely, according to Esther Freeman, MD, PhD, associate professor of dermatology at Harvard Medical School and director of MGH Global Health Dermatology at Massachusetts General Hospital, both in Boston.
“Anecdotally, we thought we were seeing fewer skin symptoms with Omicron versus Delta and the ancestral strains, and now this study shows it is true,” said Dr. Freeman, who is also principal investigator of the American Academy of Dermatology’s International Dermatology COVID-19 Registry.
The data also confirm that the skin is less likely to be involved than in past waves of COVID-19 infections.
“Up to this point, it was hard to know if we were seeing fewer referrals for COVID-related skin rashes or if clinicians had just become more comfortable with these rashes and were not referring them as often,” added Dr. Freeman, who was among the study coauthors.
Data captured from 348,691 patients
The data from the study was generated by 348,691 users in the United Kingdom of the ZOE COVID study app, a smartphone-based tool introduced relatively early in the pandemic. It asked users to provide demographic data, information on COVID-19 symptoms, including those involving the skin, and treatments. Of 33 COVID-related symptoms included in the app, five related to the skin (acral rash, burning rash, erythematopapular rash, urticarial rash, and unusual hair loss).
While the focus of this study was to compare skin manifestations during the Omicron wave with the Delta wave of COVID-19, the investigators also had data on the experience in 2020 with wild-type COVID-19 that preceded both variants. Overall, this showed a stepwise decline in skin symptoms overall, as well in as skin symptoms that occurred in the absence of systemic symptoms.
“The shift in the skin manifestations makes sense when you think about the change that is also being seen in the systemic symptoms,” said Dr. Freeman, referring to lower rates of cough and loss of smell but higher rates of sore throat and fatigue. “Omicron is achieving immune escape, which is why there is a shift in involved tissues,” she said in an interview.
Previous data collected during the wild-type COVID-19 stage of the pandemic by the same group of investigators showed that 17% of patients reported skin rash as the first symptom of COVID-19 infection, and 21% reported skin rash as the only clinical sign of infection.
In the Delta and Omicron waves, skin rash was an isolated initial symptom in only 0.8% and 0.5% of patients, respectively. (The authors noted that, in the United Kingdom, the first documented samples of the Delta variant were detected in October 2020, and the first documented samples of the Omicron variant were detected in November 2021.)
During the early stages of wild-type COVID, an acral rash was characteristic, occurring in 3.1% of patients, according to the U.K. data. In the Delta wave, acral rashes, at an incidence of 1.1% remained positively correlated with a diagnosis of COVID-19 infection. In the Omicron wave, acral rashes were observed in only 0.7% of patients and were no longer statistically correlated with a positive COVID diagnosis.
Characteristic cutaneous symptoms are evolving
Early in the course of the COVID-19 epidemic, more than 30 types of rashes were observed in patients with COVID-19 infection. Cutaneous symptoms continue to be diverse, but some, such as acral rash, are being seen less frequently. For example, the odds ratio of a positive COVID-19 diagnosis among those with an erythematopapular rash fell from 1.76 to 1.08 between the Delta and Omicron waves.
While specific cutaneous symptoms are less predictive of a diagnosis of COVID-19, clinicians should not discount cutaneous symptoms as a sign of disease, according to Veronique Bataille, MD, PhD, a consultant dermatologist at King’s College.
“You need to keep an open mind” regarding cutaneous signs and a diagnosis of COVID-19, Dr. Bataille, one of the coauthors of the U.K. report, said in an interview. In general, she considers a low threshold of suspicion appropriate. “If the patient has no past history of skin disease and no other triggers for a rash, then, in a high prevalence area, COVID must be suspected.”
In most cases, the rash resolves on its own, but Dr. Bataille emphasized the need for individualized care. Even as the risk of life-threatening COVID-19 infections appears to be diminishing with current variants, cutaneous manifestations can be severe.
“There are cases of long COVID affecting the skin, such as urticaria or a lichenoid erythematopapular rash, both of which can be very pruritic and difficult to control,” she said.
Dr. Freeman echoed the importance of an individualized approach. She agreed that most cutaneous symptoms are self-limited, but there are exceptions and treatments vary for the different types of skin involvement. “I think another point to consider when examining skin lesions is monkey pox. The fact that these are overlapping outbreaks should not be ignored. You need to be alert for both.”
Dr. Visconti, Dr. Freeman, and Dr. Bataille reported no potential conflicts of interest.
during the Omicron and Delta waves.
Among the key findings, the study shows that skin involvement during the Omicron wave was less frequent than during the Delta wave (11.4% vs. 17.6%), skin symptoms generally resolved more quickly, and that the risk for skin symptoms was similar whether patients had or had not been vaccinated, according to a team led by Alessia Visconti, PhD, a research fellow in the department of twin research and genetic epidemiology, King’s College, London.
These data are consistent with the experience of those dermatologists who have been following this area closely, according to Esther Freeman, MD, PhD, associate professor of dermatology at Harvard Medical School and director of MGH Global Health Dermatology at Massachusetts General Hospital, both in Boston.
“Anecdotally, we thought we were seeing fewer skin symptoms with Omicron versus Delta and the ancestral strains, and now this study shows it is true,” said Dr. Freeman, who is also principal investigator of the American Academy of Dermatology’s International Dermatology COVID-19 Registry.
The data also confirm that the skin is less likely to be involved than in past waves of COVID-19 infections.
“Up to this point, it was hard to know if we were seeing fewer referrals for COVID-related skin rashes or if clinicians had just become more comfortable with these rashes and were not referring them as often,” added Dr. Freeman, who was among the study coauthors.
Data captured from 348,691 patients
The data from the study was generated by 348,691 users in the United Kingdom of the ZOE COVID study app, a smartphone-based tool introduced relatively early in the pandemic. It asked users to provide demographic data, information on COVID-19 symptoms, including those involving the skin, and treatments. Of 33 COVID-related symptoms included in the app, five related to the skin (acral rash, burning rash, erythematopapular rash, urticarial rash, and unusual hair loss).
While the focus of this study was to compare skin manifestations during the Omicron wave with the Delta wave of COVID-19, the investigators also had data on the experience in 2020 with wild-type COVID-19 that preceded both variants. Overall, this showed a stepwise decline in skin symptoms overall, as well in as skin symptoms that occurred in the absence of systemic symptoms.
“The shift in the skin manifestations makes sense when you think about the change that is also being seen in the systemic symptoms,” said Dr. Freeman, referring to lower rates of cough and loss of smell but higher rates of sore throat and fatigue. “Omicron is achieving immune escape, which is why there is a shift in involved tissues,” she said in an interview.
Previous data collected during the wild-type COVID-19 stage of the pandemic by the same group of investigators showed that 17% of patients reported skin rash as the first symptom of COVID-19 infection, and 21% reported skin rash as the only clinical sign of infection.
In the Delta and Omicron waves, skin rash was an isolated initial symptom in only 0.8% and 0.5% of patients, respectively. (The authors noted that, in the United Kingdom, the first documented samples of the Delta variant were detected in October 2020, and the first documented samples of the Omicron variant were detected in November 2021.)
During the early stages of wild-type COVID, an acral rash was characteristic, occurring in 3.1% of patients, according to the U.K. data. In the Delta wave, acral rashes, at an incidence of 1.1% remained positively correlated with a diagnosis of COVID-19 infection. In the Omicron wave, acral rashes were observed in only 0.7% of patients and were no longer statistically correlated with a positive COVID diagnosis.
Characteristic cutaneous symptoms are evolving
Early in the course of the COVID-19 epidemic, more than 30 types of rashes were observed in patients with COVID-19 infection. Cutaneous symptoms continue to be diverse, but some, such as acral rash, are being seen less frequently. For example, the odds ratio of a positive COVID-19 diagnosis among those with an erythematopapular rash fell from 1.76 to 1.08 between the Delta and Omicron waves.
While specific cutaneous symptoms are less predictive of a diagnosis of COVID-19, clinicians should not discount cutaneous symptoms as a sign of disease, according to Veronique Bataille, MD, PhD, a consultant dermatologist at King’s College.
“You need to keep an open mind” regarding cutaneous signs and a diagnosis of COVID-19, Dr. Bataille, one of the coauthors of the U.K. report, said in an interview. In general, she considers a low threshold of suspicion appropriate. “If the patient has no past history of skin disease and no other triggers for a rash, then, in a high prevalence area, COVID must be suspected.”
In most cases, the rash resolves on its own, but Dr. Bataille emphasized the need for individualized care. Even as the risk of life-threatening COVID-19 infections appears to be diminishing with current variants, cutaneous manifestations can be severe.
“There are cases of long COVID affecting the skin, such as urticaria or a lichenoid erythematopapular rash, both of which can be very pruritic and difficult to control,” she said.
Dr. Freeman echoed the importance of an individualized approach. She agreed that most cutaneous symptoms are self-limited, but there are exceptions and treatments vary for the different types of skin involvement. “I think another point to consider when examining skin lesions is monkey pox. The fact that these are overlapping outbreaks should not be ignored. You need to be alert for both.”
Dr. Visconti, Dr. Freeman, and Dr. Bataille reported no potential conflicts of interest.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Sexual assault flagged as a possible psychosis trigger
A new study sheds light on some of the risk factors for the development of psychosis, including the potentially causative role of sexual assault.
Investigators conducted an exposome-wide association analysis on more than 155,000 individuals. Of more than 140 correlates of psychotic experiences that they identified, they narrowed it down to 36 variables, which they further explored using Mendelian randomization analysis.
On the other hand, having experienced a physical violent crime, cannabis use, and prolonged worry after embarrassment showed a pleiotropic association and appeared to be an aftereffect of psychotic experience.
“From a public health perspective, we need more investment in comprehensive strategies to prevent traumatic experiences at the population level to decrease the burden of psychosis,” senior author Sinan Gülöksüz, MD, PhD, associate professor in the department of psychiatry and neuropsychiatry, Maastricht University Medical Center, the Netherlands, said in an interview.
“From a clinical perspective, clinicians should be aware of the harmful influence of traumatic experiences on mental health and address this through interventions such as trauma-informed care,” he said.
The study was published online in JAMA Psychiatry.
‘Disentangling’ cause and effect
“Previous research has shown associations between psychosis and a few environmental factors, such as substance use, urbanicity, pregnancy complications, and traumatic experiences, but research has so far investigated only a few specific environmental factors by singling them out in individual studies,” Dr. Gülöksüz said.
“Yet, environment is a much more complex and interactive network that includes many factors shaping our health – where we live, what we eat, our lifestyle preferences and habits such as exercise and smoking, and our social surrounding,” he continued. “Rarely has it been possible to understand whether these environmental factors have causal roles in developing psychosis.”
To investigate the question, the researchers turned to the UK Biobank, one of the largest population-based datasets in the world. The current study focused on individuals with completed data on mental questionnaires that assessed psychotic experiences (n = 155,247; mean [SD] age, 55.94 [7.74] years; 57% female).
They began by conducting an exposome-wide association study, using logistic regression analyses with psychotic experiences as the outcome and adjusting all analyses for age and sex.
“Initially, we identified many associations between environmental factors and psychotic experiences in this large cohort,” Dr. Gülöksüz reported.
In the final multivariable model, variables associated with psychotic experiences were further analyzed using “genetically informed approaches to probe potential associations.”
The researchers utilized Mendelian randomization (MR) methodology “to disentangle cause and effect in this observational study,” Dr. Gülöksüz said. “This method reduces confounding and reverse causation in observational studies by using genetic variants that have been passed on from generation to generation randomly as instruments.”
MR analysis “has allowed us to assess whether these associations reflect potentially causal influences of environmental factors on psychotic experiences,” he added.
Well-studied and unexplored risk factors
The researchers identified 162 variables associated with psychotic experiences in the discovery dataset and were able to replicate 148. When these 148 variables were subjected to multivariable analyses, 36 were found to be statistically significantly associated with psychotic experiences. Of these variables, 28 had “significant genetic overlap” with psychotic experiences.
When the researchers conducted one-sample MR analyses, they found forward associations with three variables and reverse associations with three variables.
Forward associations were found with ever having experienced sexual assault (odds ratio [OR], 1.32; 95% confidence interval [CI], 1.14-1.52; P = 2.67), and forward associations (with pleiotropy) were found with ever having experienced a physically violent crime and risk-taking behavior (OR, 1.25, 95% CI, 1.11-1.41; P = 3.28 and OR, 1.21, 95% CI, 1.08-1.35; P = 1.34, respectively).
“The allele scores for these 3 variables explained 0.03% to 0.23% variance of the corresponding variable” and the F statistics “ranged from 21.53 to 181.84, indicating that the results did not suffer from a weak-instrument bias,” the authors reported.
The researchers calculated an instrument based on increasing psychotic experiences risk allele scores and found that these scores explained 0.14% variance of psychotic experiences (F statistic, 19.26).
Using that calculation, they found a reverse association with having experienced a physically violent crime (OR, 1.08; 95% CI, 1.04-1.13; P = 3.92 × 10-4), cannabis use (OR, 1.11; 95% CI, 1.06-1.15; P = 2.64 × 10-6), and worrying too long after embarrassment (OR, 1.06; 95% CI, 1.03-1.10; P = 3.96 × 10-4). They then validated these associations.
The presence of all five correlates was associated with tenfold increased odds of psychotic experiences (OR, 10.63; 95% CI, 8.27-13.65, P = 1.2 × 10-114).
“Associations with psychotic experiences were found with both well-studied and unexplored multiple correlated variables,” the authors stated.
Era of ‘big data’
In a comment, Chirag Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, who was not involved with the study, said he thought the study was “a nice example of a data-driven and comprehensive study of the environment coupled with attempts to triangulate evidence from genetics, made possible by biobank data.
“To guide public health policies and implementation of prevention strategies for psychosis, we need more systematic analyses and triangulate evidence with genetically informed methods to identify potentially modifiable risk factors in the era of ‘big data,’ ” he said.
“For instance, traumatic experiences contribute to poor mental and physical health, including psychosis,” Dr. Gülöksüz added.
The Kootstra Talent Fellowship, the Ophelia Research Project, and the Vidi Award from the Netherlands Scientific Organization provided funding to individual investigators. Dr. Gülöksüz and coauthors declared no relevant financial conflicts. Dr. Patel served as a reviewer on the study.
A version of this article first appeared on Medscape.com.
A new study sheds light on some of the risk factors for the development of psychosis, including the potentially causative role of sexual assault.
Investigators conducted an exposome-wide association analysis on more than 155,000 individuals. Of more than 140 correlates of psychotic experiences that they identified, they narrowed it down to 36 variables, which they further explored using Mendelian randomization analysis.
On the other hand, having experienced a physical violent crime, cannabis use, and prolonged worry after embarrassment showed a pleiotropic association and appeared to be an aftereffect of psychotic experience.
“From a public health perspective, we need more investment in comprehensive strategies to prevent traumatic experiences at the population level to decrease the burden of psychosis,” senior author Sinan Gülöksüz, MD, PhD, associate professor in the department of psychiatry and neuropsychiatry, Maastricht University Medical Center, the Netherlands, said in an interview.
“From a clinical perspective, clinicians should be aware of the harmful influence of traumatic experiences on mental health and address this through interventions such as trauma-informed care,” he said.
The study was published online in JAMA Psychiatry.
‘Disentangling’ cause and effect
“Previous research has shown associations between psychosis and a few environmental factors, such as substance use, urbanicity, pregnancy complications, and traumatic experiences, but research has so far investigated only a few specific environmental factors by singling them out in individual studies,” Dr. Gülöksüz said.
“Yet, environment is a much more complex and interactive network that includes many factors shaping our health – where we live, what we eat, our lifestyle preferences and habits such as exercise and smoking, and our social surrounding,” he continued. “Rarely has it been possible to understand whether these environmental factors have causal roles in developing psychosis.”
To investigate the question, the researchers turned to the UK Biobank, one of the largest population-based datasets in the world. The current study focused on individuals with completed data on mental questionnaires that assessed psychotic experiences (n = 155,247; mean [SD] age, 55.94 [7.74] years; 57% female).
They began by conducting an exposome-wide association study, using logistic regression analyses with psychotic experiences as the outcome and adjusting all analyses for age and sex.
“Initially, we identified many associations between environmental factors and psychotic experiences in this large cohort,” Dr. Gülöksüz reported.
In the final multivariable model, variables associated with psychotic experiences were further analyzed using “genetically informed approaches to probe potential associations.”
The researchers utilized Mendelian randomization (MR) methodology “to disentangle cause and effect in this observational study,” Dr. Gülöksüz said. “This method reduces confounding and reverse causation in observational studies by using genetic variants that have been passed on from generation to generation randomly as instruments.”
MR analysis “has allowed us to assess whether these associations reflect potentially causal influences of environmental factors on psychotic experiences,” he added.
Well-studied and unexplored risk factors
The researchers identified 162 variables associated with psychotic experiences in the discovery dataset and were able to replicate 148. When these 148 variables were subjected to multivariable analyses, 36 were found to be statistically significantly associated with psychotic experiences. Of these variables, 28 had “significant genetic overlap” with psychotic experiences.
When the researchers conducted one-sample MR analyses, they found forward associations with three variables and reverse associations with three variables.
Forward associations were found with ever having experienced sexual assault (odds ratio [OR], 1.32; 95% confidence interval [CI], 1.14-1.52; P = 2.67), and forward associations (with pleiotropy) were found with ever having experienced a physically violent crime and risk-taking behavior (OR, 1.25, 95% CI, 1.11-1.41; P = 3.28 and OR, 1.21, 95% CI, 1.08-1.35; P = 1.34, respectively).
“The allele scores for these 3 variables explained 0.03% to 0.23% variance of the corresponding variable” and the F statistics “ranged from 21.53 to 181.84, indicating that the results did not suffer from a weak-instrument bias,” the authors reported.
The researchers calculated an instrument based on increasing psychotic experiences risk allele scores and found that these scores explained 0.14% variance of psychotic experiences (F statistic, 19.26).
Using that calculation, they found a reverse association with having experienced a physically violent crime (OR, 1.08; 95% CI, 1.04-1.13; P = 3.92 × 10-4), cannabis use (OR, 1.11; 95% CI, 1.06-1.15; P = 2.64 × 10-6), and worrying too long after embarrassment (OR, 1.06; 95% CI, 1.03-1.10; P = 3.96 × 10-4). They then validated these associations.
The presence of all five correlates was associated with tenfold increased odds of psychotic experiences (OR, 10.63; 95% CI, 8.27-13.65, P = 1.2 × 10-114).
“Associations with psychotic experiences were found with both well-studied and unexplored multiple correlated variables,” the authors stated.
Era of ‘big data’
In a comment, Chirag Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, who was not involved with the study, said he thought the study was “a nice example of a data-driven and comprehensive study of the environment coupled with attempts to triangulate evidence from genetics, made possible by biobank data.
“To guide public health policies and implementation of prevention strategies for psychosis, we need more systematic analyses and triangulate evidence with genetically informed methods to identify potentially modifiable risk factors in the era of ‘big data,’ ” he said.
“For instance, traumatic experiences contribute to poor mental and physical health, including psychosis,” Dr. Gülöksüz added.
The Kootstra Talent Fellowship, the Ophelia Research Project, and the Vidi Award from the Netherlands Scientific Organization provided funding to individual investigators. Dr. Gülöksüz and coauthors declared no relevant financial conflicts. Dr. Patel served as a reviewer on the study.
A version of this article first appeared on Medscape.com.
A new study sheds light on some of the risk factors for the development of psychosis, including the potentially causative role of sexual assault.
Investigators conducted an exposome-wide association analysis on more than 155,000 individuals. Of more than 140 correlates of psychotic experiences that they identified, they narrowed it down to 36 variables, which they further explored using Mendelian randomization analysis.
On the other hand, having experienced a physical violent crime, cannabis use, and prolonged worry after embarrassment showed a pleiotropic association and appeared to be an aftereffect of psychotic experience.
“From a public health perspective, we need more investment in comprehensive strategies to prevent traumatic experiences at the population level to decrease the burden of psychosis,” senior author Sinan Gülöksüz, MD, PhD, associate professor in the department of psychiatry and neuropsychiatry, Maastricht University Medical Center, the Netherlands, said in an interview.
“From a clinical perspective, clinicians should be aware of the harmful influence of traumatic experiences on mental health and address this through interventions such as trauma-informed care,” he said.
The study was published online in JAMA Psychiatry.
‘Disentangling’ cause and effect
“Previous research has shown associations between psychosis and a few environmental factors, such as substance use, urbanicity, pregnancy complications, and traumatic experiences, but research has so far investigated only a few specific environmental factors by singling them out in individual studies,” Dr. Gülöksüz said.
“Yet, environment is a much more complex and interactive network that includes many factors shaping our health – where we live, what we eat, our lifestyle preferences and habits such as exercise and smoking, and our social surrounding,” he continued. “Rarely has it been possible to understand whether these environmental factors have causal roles in developing psychosis.”
To investigate the question, the researchers turned to the UK Biobank, one of the largest population-based datasets in the world. The current study focused on individuals with completed data on mental questionnaires that assessed psychotic experiences (n = 155,247; mean [SD] age, 55.94 [7.74] years; 57% female).
They began by conducting an exposome-wide association study, using logistic regression analyses with psychotic experiences as the outcome and adjusting all analyses for age and sex.
“Initially, we identified many associations between environmental factors and psychotic experiences in this large cohort,” Dr. Gülöksüz reported.
In the final multivariable model, variables associated with psychotic experiences were further analyzed using “genetically informed approaches to probe potential associations.”
The researchers utilized Mendelian randomization (MR) methodology “to disentangle cause and effect in this observational study,” Dr. Gülöksüz said. “This method reduces confounding and reverse causation in observational studies by using genetic variants that have been passed on from generation to generation randomly as instruments.”
MR analysis “has allowed us to assess whether these associations reflect potentially causal influences of environmental factors on psychotic experiences,” he added.
Well-studied and unexplored risk factors
The researchers identified 162 variables associated with psychotic experiences in the discovery dataset and were able to replicate 148. When these 148 variables were subjected to multivariable analyses, 36 were found to be statistically significantly associated with psychotic experiences. Of these variables, 28 had “significant genetic overlap” with psychotic experiences.
When the researchers conducted one-sample MR analyses, they found forward associations with three variables and reverse associations with three variables.
Forward associations were found with ever having experienced sexual assault (odds ratio [OR], 1.32; 95% confidence interval [CI], 1.14-1.52; P = 2.67), and forward associations (with pleiotropy) were found with ever having experienced a physically violent crime and risk-taking behavior (OR, 1.25, 95% CI, 1.11-1.41; P = 3.28 and OR, 1.21, 95% CI, 1.08-1.35; P = 1.34, respectively).
“The allele scores for these 3 variables explained 0.03% to 0.23% variance of the corresponding variable” and the F statistics “ranged from 21.53 to 181.84, indicating that the results did not suffer from a weak-instrument bias,” the authors reported.
The researchers calculated an instrument based on increasing psychotic experiences risk allele scores and found that these scores explained 0.14% variance of psychotic experiences (F statistic, 19.26).
Using that calculation, they found a reverse association with having experienced a physically violent crime (OR, 1.08; 95% CI, 1.04-1.13; P = 3.92 × 10-4), cannabis use (OR, 1.11; 95% CI, 1.06-1.15; P = 2.64 × 10-6), and worrying too long after embarrassment (OR, 1.06; 95% CI, 1.03-1.10; P = 3.96 × 10-4). They then validated these associations.
The presence of all five correlates was associated with tenfold increased odds of psychotic experiences (OR, 10.63; 95% CI, 8.27-13.65, P = 1.2 × 10-114).
“Associations with psychotic experiences were found with both well-studied and unexplored multiple correlated variables,” the authors stated.
Era of ‘big data’
In a comment, Chirag Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, who was not involved with the study, said he thought the study was “a nice example of a data-driven and comprehensive study of the environment coupled with attempts to triangulate evidence from genetics, made possible by biobank data.
“To guide public health policies and implementation of prevention strategies for psychosis, we need more systematic analyses and triangulate evidence with genetically informed methods to identify potentially modifiable risk factors in the era of ‘big data,’ ” he said.
“For instance, traumatic experiences contribute to poor mental and physical health, including psychosis,” Dr. Gülöksüz added.
The Kootstra Talent Fellowship, the Ophelia Research Project, and the Vidi Award from the Netherlands Scientific Organization provided funding to individual investigators. Dr. Gülöksüz and coauthors declared no relevant financial conflicts. Dr. Patel served as a reviewer on the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Evusheld for COVID-19: Lifesaving and free, but still few takers
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Malpractice lawyer gloats at win, then puts foot in mouth
During the closing arguments in a $10 million malpractice trial, attorney Robert McKenna III told jurors the claims against his client, a gastroenterologist, were baseless and equivalent to “extortion.” The patient’s family blamed the gastroenterologist for their father’s death, alleging the doctor perforated his colon during insertion of a feeding tube.
“I take pride in what I do, and I’ve got to tell you, in the 30 years I have been doing this, I have never seen a more insulting, factually devoid presentation in my entire career,” Mr. McKenna said, according to court transcripts. “On the strength of this evidence, they want you to award them $10 million. Welcome to America. Welcome to the personal injury machine, the personal injury industrial complex.”
After less than 30 minutes of deliberation, jurors returned a 12-0 verdict in favor of the physician.
However, Mr. McKenna, from Huntington Beach, Calif., described the case very differently to his staff in a celebration video, which he never expected to become public.
In the video, posted on Twitter and Instagram, Mr. McKenna bragged about how his legal team convinced jurors to doubt the patient’s official cause of death. He said the lawsuit involved a guy “that was probably negligently killed, but we kind of made it look like other people did it.”
“We actually had a death certificate that said he died the very way the plaintiff said he died, and we had to say, ‘No, you really shouldn’t believe what that death certificate says, or the coroner from the Orange County coroner’s office ... who says that it’s right,’” Mr. McKenna said in the video.
The 26-minute verdict was the fastest he’s ever received, Mr. McKenna says in the video, encouraging his partner to ring the firm’s victory bell.
“Overcoming all of those hurdles, we managed to sock three lawyers in the face,” Mr. McKenna said, referring to the plaintiffs’ lawyers.
The video of Mr. McKenna’s remarks is now in wide circulation after having been posted to online attorney forums, Instagram, where it’s been viewed more than 8,000 times, and Twitter, where views have reached over 3,000.
Jorge Ledezma, an Orange County, Calif., attorney who represented the patient’s family in the case, said the remarks make it appear as if Mr. McKenna tricked the jury.
“It was a drastic change from the comments he made to the jury during his closing arguments,” Mr. Ledezma said. “But the video is more important for what he doesn’t say. He doesn’t say his client did everything properly. He doesn’t say our case didn’t have any merit. He doesn’t say his client was a good doctor. Clearly, what he told the jury and what he believes are the exact opposite of each other.”
Mr. McKenna did not return multiple messages seeking comment for this story. In a statement to the LA Times, Mr. McKenna said his remarks were “intended purely as an internal briefing to our staff, using shorthand phrases which might understandably cause confusion for a lay audience unfamiliar with the case at hand, and the law in general.”
“I have expressed my apologies to my client, opposing counsel, and both the medical and legal communities,” Mr. McKenna said in the statement to the LA Times. “However, nothing about my remarks should call into question our very transparent trial strategy or the jury’s verdict in favor of my client.”
What happened to the patient?
Enrique Garcia Sanchez, 49, arrived at the critical care unit at South Coast Global Medical Center in Santa Ana, Calif., on Nov. 5, 2017, complaining of abdominal pain. He was diagnosed with acute pancreatitis, acute hypokalemia, and alcohol abuse, and transferred to the ICU, according to the family’s legal complaint.
Mr. Sanchez had a positive D-Dimer test, indicating a probable blood clot, and he appeared to be experiencing septic shock caused by pancreatitis, according to the complaint. By Nov. 17, Mr. Sanchez was suffering from respiratory failure and severe hypoxemia, and as a result, he was sedated. In addition, his abdomen was described as distended with decreased bowel sounds, according to court documents.
On. Nov. 18, a gastrointestinal specialist was consulted because of Mr. Sanchez’s prolonged intubation and oropharyngeal dysphagia, according to the lawsuit. On Nov. 21, air was leaking from Mr. Sanchez’s breathing tube with diffuse infiltration noted on the right side, and pneumonia.
Mr. Sanchez was eventually unable to swallow, and the gastroenterologist inserted a percutaneous endoscopic gastrostomy (PEG) tube, according to court records.
Mr. Sanchez’s condition worsened, and he developed respiratory distress, hypotension, and weakness during dialysis. On Dec. 9, 2017, physicians noted he had a bacterial infection, and he was later intubated on vent support because of progressive respiratory failure. Additionally, an internist reported that “fecal material” was observed per the PEG tube. Mr. Sanchez’s white blood cell count continued to rise, and his condition deteriorated. Mr. Sanchez died on Dec. 31, 2017.
A death certificate concluded that Mr. Sanchez died from complications of a PEG tube that perforated his colon, according to Mr. Ledezma. The plaintiffs’ legal team argued the gastroenterologist breached the standard of care by failing to ensure the tube was placed properly and failing to remedy the error after leakage was noted.
“Mr. Garcia died because of a misplaced PEG tube that perforated the colon, resulting in peritonitis and sepsis,” attorney Jose Robles said during his closing arguments. “Mr. Garcia had ascites, a contraindication for PEG tube placement. He had ileus, a contraindication for PEG tube placement. The standard of care requires that [the gastroenterologist] conduct a proper workup to confirm that a PEG tube placement can be done appropriately and safely.”
Mr. McKenna argued the gastroenterologist was not at fault for the patient’s death, and that complications from his pancreatitis ultimately killed him. During the trial, physicians who cared for Mr. Sanchez testified the patient had a less than 50% chance of survival.
“What he had was end-stage catastrophic [pancreatitis] that was affecting his organ system and aspiration pneumonia that made it impossible for him to try to breathe on his own,” Mr. McKenna said during closing arguments. “The man ... had a catastrophic injury that ate most of his pancreas. That is not a survivable event.”
Attorney faces backlash from legal community
Since his celebratory remarks were posted online, Mr. McKenna has faced much backlash, particularly from the legal community.
@mgvolada tweeted, “As an attorney I am revolted and I hope sanctions follow ... this is why people hate attorneys.”
@stevewieland, who identified himself as a trial lawyer, wrote he would not feel good about winning such a case.
“No wonder we get no love from the public,” he tweeted.
“Let’s see how the Court of Appeals thinks about your braggadocio and how this makes lawyers appear to the public,” tweeted @Stephen60134955, a self-identified attorney.
Mr. McKenna’s license remains active and in good standing with no disciplinary actions, according to the State Bar of California website.
Mr. Ledezma has filed a motion for a new trial, and a hearing on the motion is scheduled for Aug. 4, 2022. The motion was filed primarily because of issues during the trial, what Mr. Ledezma described as “inflammatory closing arguments,” and in small part, Mr. McKenna’s video remarks, he said.
If the motion is denied, the plaintiffs will move forward with an appeal, he said.
A version of this article first appeared on Medscape.com.
During the closing arguments in a $10 million malpractice trial, attorney Robert McKenna III told jurors the claims against his client, a gastroenterologist, were baseless and equivalent to “extortion.” The patient’s family blamed the gastroenterologist for their father’s death, alleging the doctor perforated his colon during insertion of a feeding tube.
“I take pride in what I do, and I’ve got to tell you, in the 30 years I have been doing this, I have never seen a more insulting, factually devoid presentation in my entire career,” Mr. McKenna said, according to court transcripts. “On the strength of this evidence, they want you to award them $10 million. Welcome to America. Welcome to the personal injury machine, the personal injury industrial complex.”
After less than 30 minutes of deliberation, jurors returned a 12-0 verdict in favor of the physician.
However, Mr. McKenna, from Huntington Beach, Calif., described the case very differently to his staff in a celebration video, which he never expected to become public.
In the video, posted on Twitter and Instagram, Mr. McKenna bragged about how his legal team convinced jurors to doubt the patient’s official cause of death. He said the lawsuit involved a guy “that was probably negligently killed, but we kind of made it look like other people did it.”
“We actually had a death certificate that said he died the very way the plaintiff said he died, and we had to say, ‘No, you really shouldn’t believe what that death certificate says, or the coroner from the Orange County coroner’s office ... who says that it’s right,’” Mr. McKenna said in the video.
The 26-minute verdict was the fastest he’s ever received, Mr. McKenna says in the video, encouraging his partner to ring the firm’s victory bell.
“Overcoming all of those hurdles, we managed to sock three lawyers in the face,” Mr. McKenna said, referring to the plaintiffs’ lawyers.
The video of Mr. McKenna’s remarks is now in wide circulation after having been posted to online attorney forums, Instagram, where it’s been viewed more than 8,000 times, and Twitter, where views have reached over 3,000.
Jorge Ledezma, an Orange County, Calif., attorney who represented the patient’s family in the case, said the remarks make it appear as if Mr. McKenna tricked the jury.
“It was a drastic change from the comments he made to the jury during his closing arguments,” Mr. Ledezma said. “But the video is more important for what he doesn’t say. He doesn’t say his client did everything properly. He doesn’t say our case didn’t have any merit. He doesn’t say his client was a good doctor. Clearly, what he told the jury and what he believes are the exact opposite of each other.”
Mr. McKenna did not return multiple messages seeking comment for this story. In a statement to the LA Times, Mr. McKenna said his remarks were “intended purely as an internal briefing to our staff, using shorthand phrases which might understandably cause confusion for a lay audience unfamiliar with the case at hand, and the law in general.”
“I have expressed my apologies to my client, opposing counsel, and both the medical and legal communities,” Mr. McKenna said in the statement to the LA Times. “However, nothing about my remarks should call into question our very transparent trial strategy or the jury’s verdict in favor of my client.”
What happened to the patient?
Enrique Garcia Sanchez, 49, arrived at the critical care unit at South Coast Global Medical Center in Santa Ana, Calif., on Nov. 5, 2017, complaining of abdominal pain. He was diagnosed with acute pancreatitis, acute hypokalemia, and alcohol abuse, and transferred to the ICU, according to the family’s legal complaint.
Mr. Sanchez had a positive D-Dimer test, indicating a probable blood clot, and he appeared to be experiencing septic shock caused by pancreatitis, according to the complaint. By Nov. 17, Mr. Sanchez was suffering from respiratory failure and severe hypoxemia, and as a result, he was sedated. In addition, his abdomen was described as distended with decreased bowel sounds, according to court documents.
On. Nov. 18, a gastrointestinal specialist was consulted because of Mr. Sanchez’s prolonged intubation and oropharyngeal dysphagia, according to the lawsuit. On Nov. 21, air was leaking from Mr. Sanchez’s breathing tube with diffuse infiltration noted on the right side, and pneumonia.
Mr. Sanchez was eventually unable to swallow, and the gastroenterologist inserted a percutaneous endoscopic gastrostomy (PEG) tube, according to court records.
Mr. Sanchez’s condition worsened, and he developed respiratory distress, hypotension, and weakness during dialysis. On Dec. 9, 2017, physicians noted he had a bacterial infection, and he was later intubated on vent support because of progressive respiratory failure. Additionally, an internist reported that “fecal material” was observed per the PEG tube. Mr. Sanchez’s white blood cell count continued to rise, and his condition deteriorated. Mr. Sanchez died on Dec. 31, 2017.
A death certificate concluded that Mr. Sanchez died from complications of a PEG tube that perforated his colon, according to Mr. Ledezma. The plaintiffs’ legal team argued the gastroenterologist breached the standard of care by failing to ensure the tube was placed properly and failing to remedy the error after leakage was noted.
“Mr. Garcia died because of a misplaced PEG tube that perforated the colon, resulting in peritonitis and sepsis,” attorney Jose Robles said during his closing arguments. “Mr. Garcia had ascites, a contraindication for PEG tube placement. He had ileus, a contraindication for PEG tube placement. The standard of care requires that [the gastroenterologist] conduct a proper workup to confirm that a PEG tube placement can be done appropriately and safely.”
Mr. McKenna argued the gastroenterologist was not at fault for the patient’s death, and that complications from his pancreatitis ultimately killed him. During the trial, physicians who cared for Mr. Sanchez testified the patient had a less than 50% chance of survival.
“What he had was end-stage catastrophic [pancreatitis] that was affecting his organ system and aspiration pneumonia that made it impossible for him to try to breathe on his own,” Mr. McKenna said during closing arguments. “The man ... had a catastrophic injury that ate most of his pancreas. That is not a survivable event.”
Attorney faces backlash from legal community
Since his celebratory remarks were posted online, Mr. McKenna has faced much backlash, particularly from the legal community.
@mgvolada tweeted, “As an attorney I am revolted and I hope sanctions follow ... this is why people hate attorneys.”
@stevewieland, who identified himself as a trial lawyer, wrote he would not feel good about winning such a case.
“No wonder we get no love from the public,” he tweeted.
“Let’s see how the Court of Appeals thinks about your braggadocio and how this makes lawyers appear to the public,” tweeted @Stephen60134955, a self-identified attorney.
Mr. McKenna’s license remains active and in good standing with no disciplinary actions, according to the State Bar of California website.
Mr. Ledezma has filed a motion for a new trial, and a hearing on the motion is scheduled for Aug. 4, 2022. The motion was filed primarily because of issues during the trial, what Mr. Ledezma described as “inflammatory closing arguments,” and in small part, Mr. McKenna’s video remarks, he said.
If the motion is denied, the plaintiffs will move forward with an appeal, he said.
A version of this article first appeared on Medscape.com.
During the closing arguments in a $10 million malpractice trial, attorney Robert McKenna III told jurors the claims against his client, a gastroenterologist, were baseless and equivalent to “extortion.” The patient’s family blamed the gastroenterologist for their father’s death, alleging the doctor perforated his colon during insertion of a feeding tube.
“I take pride in what I do, and I’ve got to tell you, in the 30 years I have been doing this, I have never seen a more insulting, factually devoid presentation in my entire career,” Mr. McKenna said, according to court transcripts. “On the strength of this evidence, they want you to award them $10 million. Welcome to America. Welcome to the personal injury machine, the personal injury industrial complex.”
After less than 30 minutes of deliberation, jurors returned a 12-0 verdict in favor of the physician.
However, Mr. McKenna, from Huntington Beach, Calif., described the case very differently to his staff in a celebration video, which he never expected to become public.
In the video, posted on Twitter and Instagram, Mr. McKenna bragged about how his legal team convinced jurors to doubt the patient’s official cause of death. He said the lawsuit involved a guy “that was probably negligently killed, but we kind of made it look like other people did it.”
“We actually had a death certificate that said he died the very way the plaintiff said he died, and we had to say, ‘No, you really shouldn’t believe what that death certificate says, or the coroner from the Orange County coroner’s office ... who says that it’s right,’” Mr. McKenna said in the video.
The 26-minute verdict was the fastest he’s ever received, Mr. McKenna says in the video, encouraging his partner to ring the firm’s victory bell.
“Overcoming all of those hurdles, we managed to sock three lawyers in the face,” Mr. McKenna said, referring to the plaintiffs’ lawyers.
The video of Mr. McKenna’s remarks is now in wide circulation after having been posted to online attorney forums, Instagram, where it’s been viewed more than 8,000 times, and Twitter, where views have reached over 3,000.
Jorge Ledezma, an Orange County, Calif., attorney who represented the patient’s family in the case, said the remarks make it appear as if Mr. McKenna tricked the jury.
“It was a drastic change from the comments he made to the jury during his closing arguments,” Mr. Ledezma said. “But the video is more important for what he doesn’t say. He doesn’t say his client did everything properly. He doesn’t say our case didn’t have any merit. He doesn’t say his client was a good doctor. Clearly, what he told the jury and what he believes are the exact opposite of each other.”
Mr. McKenna did not return multiple messages seeking comment for this story. In a statement to the LA Times, Mr. McKenna said his remarks were “intended purely as an internal briefing to our staff, using shorthand phrases which might understandably cause confusion for a lay audience unfamiliar with the case at hand, and the law in general.”
“I have expressed my apologies to my client, opposing counsel, and both the medical and legal communities,” Mr. McKenna said in the statement to the LA Times. “However, nothing about my remarks should call into question our very transparent trial strategy or the jury’s verdict in favor of my client.”
What happened to the patient?
Enrique Garcia Sanchez, 49, arrived at the critical care unit at South Coast Global Medical Center in Santa Ana, Calif., on Nov. 5, 2017, complaining of abdominal pain. He was diagnosed with acute pancreatitis, acute hypokalemia, and alcohol abuse, and transferred to the ICU, according to the family’s legal complaint.
Mr. Sanchez had a positive D-Dimer test, indicating a probable blood clot, and he appeared to be experiencing septic shock caused by pancreatitis, according to the complaint. By Nov. 17, Mr. Sanchez was suffering from respiratory failure and severe hypoxemia, and as a result, he was sedated. In addition, his abdomen was described as distended with decreased bowel sounds, according to court documents.
On. Nov. 18, a gastrointestinal specialist was consulted because of Mr. Sanchez’s prolonged intubation and oropharyngeal dysphagia, according to the lawsuit. On Nov. 21, air was leaking from Mr. Sanchez’s breathing tube with diffuse infiltration noted on the right side, and pneumonia.
Mr. Sanchez was eventually unable to swallow, and the gastroenterologist inserted a percutaneous endoscopic gastrostomy (PEG) tube, according to court records.
Mr. Sanchez’s condition worsened, and he developed respiratory distress, hypotension, and weakness during dialysis. On Dec. 9, 2017, physicians noted he had a bacterial infection, and he was later intubated on vent support because of progressive respiratory failure. Additionally, an internist reported that “fecal material” was observed per the PEG tube. Mr. Sanchez’s white blood cell count continued to rise, and his condition deteriorated. Mr. Sanchez died on Dec. 31, 2017.
A death certificate concluded that Mr. Sanchez died from complications of a PEG tube that perforated his colon, according to Mr. Ledezma. The plaintiffs’ legal team argued the gastroenterologist breached the standard of care by failing to ensure the tube was placed properly and failing to remedy the error after leakage was noted.
“Mr. Garcia died because of a misplaced PEG tube that perforated the colon, resulting in peritonitis and sepsis,” attorney Jose Robles said during his closing arguments. “Mr. Garcia had ascites, a contraindication for PEG tube placement. He had ileus, a contraindication for PEG tube placement. The standard of care requires that [the gastroenterologist] conduct a proper workup to confirm that a PEG tube placement can be done appropriately and safely.”
Mr. McKenna argued the gastroenterologist was not at fault for the patient’s death, and that complications from his pancreatitis ultimately killed him. During the trial, physicians who cared for Mr. Sanchez testified the patient had a less than 50% chance of survival.
“What he had was end-stage catastrophic [pancreatitis] that was affecting his organ system and aspiration pneumonia that made it impossible for him to try to breathe on his own,” Mr. McKenna said during closing arguments. “The man ... had a catastrophic injury that ate most of his pancreas. That is not a survivable event.”
Attorney faces backlash from legal community
Since his celebratory remarks were posted online, Mr. McKenna has faced much backlash, particularly from the legal community.
@mgvolada tweeted, “As an attorney I am revolted and I hope sanctions follow ... this is why people hate attorneys.”
@stevewieland, who identified himself as a trial lawyer, wrote he would not feel good about winning such a case.
“No wonder we get no love from the public,” he tweeted.
“Let’s see how the Court of Appeals thinks about your braggadocio and how this makes lawyers appear to the public,” tweeted @Stephen60134955, a self-identified attorney.
Mr. McKenna’s license remains active and in good standing with no disciplinary actions, according to the State Bar of California website.
Mr. Ledezma has filed a motion for a new trial, and a hearing on the motion is scheduled for Aug. 4, 2022. The motion was filed primarily because of issues during the trial, what Mr. Ledezma described as “inflammatory closing arguments,” and in small part, Mr. McKenna’s video remarks, he said.
If the motion is denied, the plaintiffs will move forward with an appeal, he said.
A version of this article first appeared on Medscape.com.
Do ICDs still ‘work’ in primary prevention given today’s recommended HF meds?
Contemporary guidelines highly recommend patients with heart failure with reduced ejection fraction (HFrEF) be on all four drug classes that together have shown clinical clout, including improved survival, in major randomized trials.
Although many such patients don’t receive all four drug classes, the more that are prescribed to those with primary-prevention implantable defibrillators (ICD), the better their odds of survival, a new analysis suggests.
The cohort study of almost 5,000 patients with HFrEF and such devices saw their all-cause mortality risk improve stepwise with each additional prescription they were given toward the full quadruple drug combo at the core of modern HFrEF guideline-directed medical therapy (GDMT). The four classes are sodium-glucose cotransporter 2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and renin-angiotensin system (RAS) inhibitors.
That inverse relation between risk and number of GDMT medications held whether patients had solo-ICD or defibrillating cardiac resynchronization therapy (CRT-D) implants, and were independent of device-implantation year and comorbidities, regardless of HFrEF etiology.
“If anybody had doubts about really pushing forward as much of these guideline-directed medical therapies as the patient tolerates, these data confirm that, by doing so, we definitely do better than with two medications or one medication,” Samir Saba, MD, University of Pittsburgh Medical Center, said in an interview.
The analysis begs an old and challenging question: Do primary-prevention ICDs confer clinically important survival gains over those provided by increasingly life-preserving recommended HFrEF medical therapy?
Given the study’s incremental survival bumps with each added GDMT med, “one ought to consider whether ICD therapy can still have an impact on overall survival in this population,” proposes a report published online in JACC Clinical Electrophysiology, with Dr. Saba as senior author.
In the adjusted analysis, the 2-year risk for death from any cause in HFrEF patients with primary-prevention devices fell 36% in those with ICDs and 30% in those with CRT-D devices for each added prescribed GDMT drug, from none up to either three or four such agents (P < .001 in both cases).
Only so much can be made of nonrandomized study results, Saba observed in an interview. But they are enough to justify asking whether primary-prevention ICDs are “still valuable” in HFrEF given current GDMT. One interpretation of the study, the published report noted, is that contemporary GDMT improves HFrEF survival so much that it eclipses any such benefit from a primary-prevention ICD.
Both defibrillators and the four core drug therapies boost survival in such cases, “so the fundamental question is, are they additive. Do we save more lives by having a defibrillator on top of the medications, or is it overlapping?” Dr. Saba asked. “We don’t know the answer.”
For now, at least, the findings could reassure clinicians as they consider whether to recommended a primary-prevention ICD when there might be reasons not to, as long there is full GDMT on board, “especially what we today define as quadruple guideline-directed medical therapy.”
Recently announced North American guidelines defining an HFrEF quadruple regimen prefer – beyond a beta-blocker, MRA, and SGLT2 inhibitor – that the selected RAS inhibitor be sacubitril/valsartan (Entresto, Novartis), with ACE inhibitors or angiotensin-receptor blockers (ARBs) as a substitute, if needed.
Nearly identical European guidelines on HFrEF quad therapy, unveiled in 2021, include but do not necessarily prefer sacubitril/valsartan over ACE inhibitors as the RAS inhibitor of choice.
GDMT a moving target
Primary-prevention defibrillators entered practice at a time when expected background GDMT consisted of beta-blockers and either ACE inhibitors or ARBs, the current report notes. In practice, many patients receive the devices without both drug classes optimally on board. Moreover, many who otherwise meet guidelines for such ICDs won’t tolerate the kind of maximally tolerated GDMT used in the major primary-prevention device trials.
Yet current guidelines give such devices a class I recommendation, based on the highest level of evidence, in HFrEF patients who remain symptomatic despite quad GDMT, observed Gregg C. Fonarow, MD, University of California Los Angeles Medical Center.
The current analysis “further reinforces the importance of providing all four foundational GDMTs” to all eligible HFrEF patients without contraindications who can tolerate them, he said in an interview. Such quad therapy “is associated with incremental 1-year survival advantages” in patients with primary-prevention devices. And in the major trials, “there were reductions in sudden deaths, as well as progressive heart failure deaths.”
But the current study also suggests that in practice “very few patients can actually get to all four drugs on GDMT,” Roderick Tung, MD, University of Arizona, Phoenix, said in an interview. Optimized GDMT in randomized trials probably represents the best-case scenario. “There is a difference between randomized data and real-world data, which is why we need both.”
And it asserts that “the more GDMT you’re on, the better you do,” he said. “But does that obviate the need for an ICD? I think that’s not clear,” in part because of potential confounding in the analysis. For example, patients who can take all four agents tend to be less sick than those who cannot.
“The ones who can get up to four are preselected, because they’re healthier,” Dr. Tung said. “There are real limitations – such as metabolic disturbances, acute kidney injury and cardiorenal syndrome, and hypotension – that actually make it difficult to initiate and titrate these medications.”
Indeed, the major primary-prevention ICD trials usually excluded the sickest patients with the most comorbidities, Dr. Saba observed, which raises issues about their relevance to clinical practice. But his group’s study controlled for many potential confounders by adjusting for, among other things, Elixhauser comorbidity score, ejection fraction, type of cardiomyopathy, and year of device implantation.
“We tried to level the playing field that way, to see if – despite all of this adjustment – the incremental number of heart failure medicines stills make a difference,” Dr. Saba said. “And our results suggest that yes, they still do.”
GDMT coverage in the real world
The analysis of patients with HFrEF involved 3,210 with ICD-only implants and 1,762 with CRT-D devices for primary prevention at a major medical center from 2010 to 2021. Of the total, 5% had not been prescribed any of the four GDMT agents, 20% had been prescribed only one, 52% were prescribed two, and 23% were prescribed three or four. Only 113 patients had been prescribed SGLT2 inhibitors, which have only recently been indicated for HFrEF.
Adjusted hazard ratios for death from any cause at 2 years for each added GDMT drug (P < .001 in each case), were 0.64 (95% confidence interval, 0.56-0.74) for ICD recipients, 0.70 (95% CI, 0.58-0.86) for those with a CRT-D device, 0.70 (95% CI, 0.60-0.81) for those with ischemic cardiomyopathy, and 0.61 (95% CI, 0.51-0.73) for patients with nonischemic disease.
The results “raise questions rather than answers,” Dr. Saba said. “At some point, someone will need to take patients who are optimized on their heart failure medications and then randomize them to defibrillator versus no defibrillator to see whether there is still an additive impact.”
Current best evidence suggests that primary-prevention ICDs in patients with guideline-based indications confer benefits that far outweigh any risks. But if the major primary-prevention ICD trials were to be repeated in patients on contemporary quad-therapy GDMT, Dr. Tung said, “would the benefit of ICD be attenuated? I think most of us believe it likely would.”
Still, he said, a background of modern GDMT could potentially “optimize” such trials by attenuating mortality from heart failure progression and thereby expanding the proportion of deaths that are arrhythmic, “which the defibrillator can prevent.”
Dr. Saba discloses receiving research support from Boston Scientific and Abbott; and serving on advisory boards for Medtronic and Boston Scientific. The other authors reported no relevant relationships. Dr. Tung has disclosed receiving speaker fees from Abbott and Boston Scientific. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Contemporary guidelines highly recommend patients with heart failure with reduced ejection fraction (HFrEF) be on all four drug classes that together have shown clinical clout, including improved survival, in major randomized trials.
Although many such patients don’t receive all four drug classes, the more that are prescribed to those with primary-prevention implantable defibrillators (ICD), the better their odds of survival, a new analysis suggests.
The cohort study of almost 5,000 patients with HFrEF and such devices saw their all-cause mortality risk improve stepwise with each additional prescription they were given toward the full quadruple drug combo at the core of modern HFrEF guideline-directed medical therapy (GDMT). The four classes are sodium-glucose cotransporter 2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and renin-angiotensin system (RAS) inhibitors.
That inverse relation between risk and number of GDMT medications held whether patients had solo-ICD or defibrillating cardiac resynchronization therapy (CRT-D) implants, and were independent of device-implantation year and comorbidities, regardless of HFrEF etiology.
“If anybody had doubts about really pushing forward as much of these guideline-directed medical therapies as the patient tolerates, these data confirm that, by doing so, we definitely do better than with two medications or one medication,” Samir Saba, MD, University of Pittsburgh Medical Center, said in an interview.
The analysis begs an old and challenging question: Do primary-prevention ICDs confer clinically important survival gains over those provided by increasingly life-preserving recommended HFrEF medical therapy?
Given the study’s incremental survival bumps with each added GDMT med, “one ought to consider whether ICD therapy can still have an impact on overall survival in this population,” proposes a report published online in JACC Clinical Electrophysiology, with Dr. Saba as senior author.
In the adjusted analysis, the 2-year risk for death from any cause in HFrEF patients with primary-prevention devices fell 36% in those with ICDs and 30% in those with CRT-D devices for each added prescribed GDMT drug, from none up to either three or four such agents (P < .001 in both cases).
Only so much can be made of nonrandomized study results, Saba observed in an interview. But they are enough to justify asking whether primary-prevention ICDs are “still valuable” in HFrEF given current GDMT. One interpretation of the study, the published report noted, is that contemporary GDMT improves HFrEF survival so much that it eclipses any such benefit from a primary-prevention ICD.
Both defibrillators and the four core drug therapies boost survival in such cases, “so the fundamental question is, are they additive. Do we save more lives by having a defibrillator on top of the medications, or is it overlapping?” Dr. Saba asked. “We don’t know the answer.”
For now, at least, the findings could reassure clinicians as they consider whether to recommended a primary-prevention ICD when there might be reasons not to, as long there is full GDMT on board, “especially what we today define as quadruple guideline-directed medical therapy.”
Recently announced North American guidelines defining an HFrEF quadruple regimen prefer – beyond a beta-blocker, MRA, and SGLT2 inhibitor – that the selected RAS inhibitor be sacubitril/valsartan (Entresto, Novartis), with ACE inhibitors or angiotensin-receptor blockers (ARBs) as a substitute, if needed.
Nearly identical European guidelines on HFrEF quad therapy, unveiled in 2021, include but do not necessarily prefer sacubitril/valsartan over ACE inhibitors as the RAS inhibitor of choice.
GDMT a moving target
Primary-prevention defibrillators entered practice at a time when expected background GDMT consisted of beta-blockers and either ACE inhibitors or ARBs, the current report notes. In practice, many patients receive the devices without both drug classes optimally on board. Moreover, many who otherwise meet guidelines for such ICDs won’t tolerate the kind of maximally tolerated GDMT used in the major primary-prevention device trials.
Yet current guidelines give such devices a class I recommendation, based on the highest level of evidence, in HFrEF patients who remain symptomatic despite quad GDMT, observed Gregg C. Fonarow, MD, University of California Los Angeles Medical Center.
The current analysis “further reinforces the importance of providing all four foundational GDMTs” to all eligible HFrEF patients without contraindications who can tolerate them, he said in an interview. Such quad therapy “is associated with incremental 1-year survival advantages” in patients with primary-prevention devices. And in the major trials, “there were reductions in sudden deaths, as well as progressive heart failure deaths.”
But the current study also suggests that in practice “very few patients can actually get to all four drugs on GDMT,” Roderick Tung, MD, University of Arizona, Phoenix, said in an interview. Optimized GDMT in randomized trials probably represents the best-case scenario. “There is a difference between randomized data and real-world data, which is why we need both.”
And it asserts that “the more GDMT you’re on, the better you do,” he said. “But does that obviate the need for an ICD? I think that’s not clear,” in part because of potential confounding in the analysis. For example, patients who can take all four agents tend to be less sick than those who cannot.
“The ones who can get up to four are preselected, because they’re healthier,” Dr. Tung said. “There are real limitations – such as metabolic disturbances, acute kidney injury and cardiorenal syndrome, and hypotension – that actually make it difficult to initiate and titrate these medications.”
Indeed, the major primary-prevention ICD trials usually excluded the sickest patients with the most comorbidities, Dr. Saba observed, which raises issues about their relevance to clinical practice. But his group’s study controlled for many potential confounders by adjusting for, among other things, Elixhauser comorbidity score, ejection fraction, type of cardiomyopathy, and year of device implantation.
“We tried to level the playing field that way, to see if – despite all of this adjustment – the incremental number of heart failure medicines stills make a difference,” Dr. Saba said. “And our results suggest that yes, they still do.”
GDMT coverage in the real world
The analysis of patients with HFrEF involved 3,210 with ICD-only implants and 1,762 with CRT-D devices for primary prevention at a major medical center from 2010 to 2021. Of the total, 5% had not been prescribed any of the four GDMT agents, 20% had been prescribed only one, 52% were prescribed two, and 23% were prescribed three or four. Only 113 patients had been prescribed SGLT2 inhibitors, which have only recently been indicated for HFrEF.
Adjusted hazard ratios for death from any cause at 2 years for each added GDMT drug (P < .001 in each case), were 0.64 (95% confidence interval, 0.56-0.74) for ICD recipients, 0.70 (95% CI, 0.58-0.86) for those with a CRT-D device, 0.70 (95% CI, 0.60-0.81) for those with ischemic cardiomyopathy, and 0.61 (95% CI, 0.51-0.73) for patients with nonischemic disease.
The results “raise questions rather than answers,” Dr. Saba said. “At some point, someone will need to take patients who are optimized on their heart failure medications and then randomize them to defibrillator versus no defibrillator to see whether there is still an additive impact.”
Current best evidence suggests that primary-prevention ICDs in patients with guideline-based indications confer benefits that far outweigh any risks. But if the major primary-prevention ICD trials were to be repeated in patients on contemporary quad-therapy GDMT, Dr. Tung said, “would the benefit of ICD be attenuated? I think most of us believe it likely would.”
Still, he said, a background of modern GDMT could potentially “optimize” such trials by attenuating mortality from heart failure progression and thereby expanding the proportion of deaths that are arrhythmic, “which the defibrillator can prevent.”
Dr. Saba discloses receiving research support from Boston Scientific and Abbott; and serving on advisory boards for Medtronic and Boston Scientific. The other authors reported no relevant relationships. Dr. Tung has disclosed receiving speaker fees from Abbott and Boston Scientific. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Contemporary guidelines highly recommend patients with heart failure with reduced ejection fraction (HFrEF) be on all four drug classes that together have shown clinical clout, including improved survival, in major randomized trials.
Although many such patients don’t receive all four drug classes, the more that are prescribed to those with primary-prevention implantable defibrillators (ICD), the better their odds of survival, a new analysis suggests.
The cohort study of almost 5,000 patients with HFrEF and such devices saw their all-cause mortality risk improve stepwise with each additional prescription they were given toward the full quadruple drug combo at the core of modern HFrEF guideline-directed medical therapy (GDMT). The four classes are sodium-glucose cotransporter 2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and renin-angiotensin system (RAS) inhibitors.
That inverse relation between risk and number of GDMT medications held whether patients had solo-ICD or defibrillating cardiac resynchronization therapy (CRT-D) implants, and were independent of device-implantation year and comorbidities, regardless of HFrEF etiology.
“If anybody had doubts about really pushing forward as much of these guideline-directed medical therapies as the patient tolerates, these data confirm that, by doing so, we definitely do better than with two medications or one medication,” Samir Saba, MD, University of Pittsburgh Medical Center, said in an interview.
The analysis begs an old and challenging question: Do primary-prevention ICDs confer clinically important survival gains over those provided by increasingly life-preserving recommended HFrEF medical therapy?
Given the study’s incremental survival bumps with each added GDMT med, “one ought to consider whether ICD therapy can still have an impact on overall survival in this population,” proposes a report published online in JACC Clinical Electrophysiology, with Dr. Saba as senior author.
In the adjusted analysis, the 2-year risk for death from any cause in HFrEF patients with primary-prevention devices fell 36% in those with ICDs and 30% in those with CRT-D devices for each added prescribed GDMT drug, from none up to either three or four such agents (P < .001 in both cases).
Only so much can be made of nonrandomized study results, Saba observed in an interview. But they are enough to justify asking whether primary-prevention ICDs are “still valuable” in HFrEF given current GDMT. One interpretation of the study, the published report noted, is that contemporary GDMT improves HFrEF survival so much that it eclipses any such benefit from a primary-prevention ICD.
Both defibrillators and the four core drug therapies boost survival in such cases, “so the fundamental question is, are they additive. Do we save more lives by having a defibrillator on top of the medications, or is it overlapping?” Dr. Saba asked. “We don’t know the answer.”
For now, at least, the findings could reassure clinicians as they consider whether to recommended a primary-prevention ICD when there might be reasons not to, as long there is full GDMT on board, “especially what we today define as quadruple guideline-directed medical therapy.”
Recently announced North American guidelines defining an HFrEF quadruple regimen prefer – beyond a beta-blocker, MRA, and SGLT2 inhibitor – that the selected RAS inhibitor be sacubitril/valsartan (Entresto, Novartis), with ACE inhibitors or angiotensin-receptor blockers (ARBs) as a substitute, if needed.
Nearly identical European guidelines on HFrEF quad therapy, unveiled in 2021, include but do not necessarily prefer sacubitril/valsartan over ACE inhibitors as the RAS inhibitor of choice.
GDMT a moving target
Primary-prevention defibrillators entered practice at a time when expected background GDMT consisted of beta-blockers and either ACE inhibitors or ARBs, the current report notes. In practice, many patients receive the devices without both drug classes optimally on board. Moreover, many who otherwise meet guidelines for such ICDs won’t tolerate the kind of maximally tolerated GDMT used in the major primary-prevention device trials.
Yet current guidelines give such devices a class I recommendation, based on the highest level of evidence, in HFrEF patients who remain symptomatic despite quad GDMT, observed Gregg C. Fonarow, MD, University of California Los Angeles Medical Center.
The current analysis “further reinforces the importance of providing all four foundational GDMTs” to all eligible HFrEF patients without contraindications who can tolerate them, he said in an interview. Such quad therapy “is associated with incremental 1-year survival advantages” in patients with primary-prevention devices. And in the major trials, “there were reductions in sudden deaths, as well as progressive heart failure deaths.”
But the current study also suggests that in practice “very few patients can actually get to all four drugs on GDMT,” Roderick Tung, MD, University of Arizona, Phoenix, said in an interview. Optimized GDMT in randomized trials probably represents the best-case scenario. “There is a difference between randomized data and real-world data, which is why we need both.”
And it asserts that “the more GDMT you’re on, the better you do,” he said. “But does that obviate the need for an ICD? I think that’s not clear,” in part because of potential confounding in the analysis. For example, patients who can take all four agents tend to be less sick than those who cannot.
“The ones who can get up to four are preselected, because they’re healthier,” Dr. Tung said. “There are real limitations – such as metabolic disturbances, acute kidney injury and cardiorenal syndrome, and hypotension – that actually make it difficult to initiate and titrate these medications.”
Indeed, the major primary-prevention ICD trials usually excluded the sickest patients with the most comorbidities, Dr. Saba observed, which raises issues about their relevance to clinical practice. But his group’s study controlled for many potential confounders by adjusting for, among other things, Elixhauser comorbidity score, ejection fraction, type of cardiomyopathy, and year of device implantation.
“We tried to level the playing field that way, to see if – despite all of this adjustment – the incremental number of heart failure medicines stills make a difference,” Dr. Saba said. “And our results suggest that yes, they still do.”
GDMT coverage in the real world
The analysis of patients with HFrEF involved 3,210 with ICD-only implants and 1,762 with CRT-D devices for primary prevention at a major medical center from 2010 to 2021. Of the total, 5% had not been prescribed any of the four GDMT agents, 20% had been prescribed only one, 52% were prescribed two, and 23% were prescribed three or four. Only 113 patients had been prescribed SGLT2 inhibitors, which have only recently been indicated for HFrEF.
Adjusted hazard ratios for death from any cause at 2 years for each added GDMT drug (P < .001 in each case), were 0.64 (95% confidence interval, 0.56-0.74) for ICD recipients, 0.70 (95% CI, 0.58-0.86) for those with a CRT-D device, 0.70 (95% CI, 0.60-0.81) for those with ischemic cardiomyopathy, and 0.61 (95% CI, 0.51-0.73) for patients with nonischemic disease.
The results “raise questions rather than answers,” Dr. Saba said. “At some point, someone will need to take patients who are optimized on their heart failure medications and then randomize them to defibrillator versus no defibrillator to see whether there is still an additive impact.”
Current best evidence suggests that primary-prevention ICDs in patients with guideline-based indications confer benefits that far outweigh any risks. But if the major primary-prevention ICD trials were to be repeated in patients on contemporary quad-therapy GDMT, Dr. Tung said, “would the benefit of ICD be attenuated? I think most of us believe it likely would.”
Still, he said, a background of modern GDMT could potentially “optimize” such trials by attenuating mortality from heart failure progression and thereby expanding the proportion of deaths that are arrhythmic, “which the defibrillator can prevent.”
Dr. Saba discloses receiving research support from Boston Scientific and Abbott; and serving on advisory boards for Medtronic and Boston Scientific. The other authors reported no relevant relationships. Dr. Tung has disclosed receiving speaker fees from Abbott and Boston Scientific. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
FROM JACC CLINICAL ELECTROPHYSIOLOGY
‘Striking’ disparities in CVD deaths persist across COVID waves
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MAYO CLINIC PROCEEDINGS
Potentially deadly bacteria detected in U.S. soil
, according to a new alert from the Centers for Disease Control and Prevention.
The bacteria, Burkholderia pseudomallei, was found along the Gulf Coast region in southern Mississippi. Typically, the bacteria are in tropical and subtropical climates, especially in parts of Southeast Asia, northern Australia, Central America, South America, Puerto Rico, and the U.S. Virgin Islands.
The bacteria can cause melioidosis, a rare and serious infectious disease that spreads to animals and humans through contact with contaminated soil and water via cuts, wounds, mucous membranes, breathing the bacteria in, or eating or drinking it. Worldwide, the disease is fatal in 10%-50% of those who become infected.
CDC and state officials are investigating the samples to find out how widespread the bacteria are within the United States. So far, modeling suggests that the environmental conditions on the Gulf Coast support the growth of B. pseudomallei.
“It is unclear how long the bacteria has been in the environment and where else it might be found in the U.S.,” according to the CDC statement. “CDC is alerting clinicians throughout the country of this discovery through a national health advisory, reminding them to be aware of the signs and symptoms of melioidosis and to consider melioidosis in patients that present with symptoms of the disease.”
Two unrelated people who live near the Gulf Coast region of Mississippi became sick with melioidosis recently – one in July 2020 and one in May 2022. Neither had traveled outside of the United States. The cases led the CDC and the Mississippi State Department of Health to collect environmental samples and test household products at the patients’ homes in June 2022. Three of the samples taken from soil and puddle water in the 2020 case tested positive for the bacteria.
Genomic sequencing revealed that both patients were infected with the same strain of the bacteria from the Western Hemisphere. They were hospitalized with sepsis due to pneumonia and had known risk factors for melioidosis. Both patients recovered after they were treated with antibiotics.
An average of 12 melioidosis cases are diagnosed in the United States each year, with most in people with recent travel to a country where the bacteria is endemic, or regularly found. Cases have also been linked to contaminated products imported from endemic countries. In late 2021, four cases in four states – Georgia, Kansas, Minnesota, and Texas – were linked to a contaminated aromatherapy spray that was imported, and Walmart issued a recall in November of that year, according to a CDC announcement. Two of the four people died.
Given the small number of cases found in the United States, the CDC believes the risk of melioidosis for the general population continues to be “very low,” and the risk of person-to-person spread is considered “extremely low.” But people who live on the Gulf Coast of Mississippi and who have health conditions that may put them at a higher risk, such as diabetes, chronic kidney disease, chronic lung disease, excessive alcohol use, and immunosuppressive conditions, should protect themselves.
The CDC recommends avoiding contact with soil or muddy water, particularly after heavy rains, and protecting open wounds with waterproof bandages. People should also wear waterproof boots when gardening, working in the yard, or doing agricultural work, which can prevent infection through the feet and lower legs, especially after flooding or storms. People should also wear gloves to protect their hands when working directly with soil.
Melioidosis has a wide range of symptoms, including fever, joint pain, headaches, coughing, chest pain, and belly pain. It can also cause conditions such as pneumonia, abscesses, and blood infections. The disease can infect any organ, including the brain. In most cases, symptoms appear within 1-21 days after exposure, with an average of 7 days after exposure.
The CDC’s health advisory for health professionals and public health officials shows that melioidosis is now considered to be locally endemic in areas of the Gulf Coast region in Mississippi.
“Once well-established in the soil, B. pseudomallei cannot feasibly be removed from the soil,” according to the advisory. “Public health efforts should focus primarily on improving identification of cases so that appropriate treatment can be administered.”
A version of this article first appeared on WebMD.com.
, according to a new alert from the Centers for Disease Control and Prevention.
The bacteria, Burkholderia pseudomallei, was found along the Gulf Coast region in southern Mississippi. Typically, the bacteria are in tropical and subtropical climates, especially in parts of Southeast Asia, northern Australia, Central America, South America, Puerto Rico, and the U.S. Virgin Islands.
The bacteria can cause melioidosis, a rare and serious infectious disease that spreads to animals and humans through contact with contaminated soil and water via cuts, wounds, mucous membranes, breathing the bacteria in, or eating or drinking it. Worldwide, the disease is fatal in 10%-50% of those who become infected.
CDC and state officials are investigating the samples to find out how widespread the bacteria are within the United States. So far, modeling suggests that the environmental conditions on the Gulf Coast support the growth of B. pseudomallei.
“It is unclear how long the bacteria has been in the environment and where else it might be found in the U.S.,” according to the CDC statement. “CDC is alerting clinicians throughout the country of this discovery through a national health advisory, reminding them to be aware of the signs and symptoms of melioidosis and to consider melioidosis in patients that present with symptoms of the disease.”
Two unrelated people who live near the Gulf Coast region of Mississippi became sick with melioidosis recently – one in July 2020 and one in May 2022. Neither had traveled outside of the United States. The cases led the CDC and the Mississippi State Department of Health to collect environmental samples and test household products at the patients’ homes in June 2022. Three of the samples taken from soil and puddle water in the 2020 case tested positive for the bacteria.
Genomic sequencing revealed that both patients were infected with the same strain of the bacteria from the Western Hemisphere. They were hospitalized with sepsis due to pneumonia and had known risk factors for melioidosis. Both patients recovered after they were treated with antibiotics.
An average of 12 melioidosis cases are diagnosed in the United States each year, with most in people with recent travel to a country where the bacteria is endemic, or regularly found. Cases have also been linked to contaminated products imported from endemic countries. In late 2021, four cases in four states – Georgia, Kansas, Minnesota, and Texas – were linked to a contaminated aromatherapy spray that was imported, and Walmart issued a recall in November of that year, according to a CDC announcement. Two of the four people died.
Given the small number of cases found in the United States, the CDC believes the risk of melioidosis for the general population continues to be “very low,” and the risk of person-to-person spread is considered “extremely low.” But people who live on the Gulf Coast of Mississippi and who have health conditions that may put them at a higher risk, such as diabetes, chronic kidney disease, chronic lung disease, excessive alcohol use, and immunosuppressive conditions, should protect themselves.
The CDC recommends avoiding contact with soil or muddy water, particularly after heavy rains, and protecting open wounds with waterproof bandages. People should also wear waterproof boots when gardening, working in the yard, or doing agricultural work, which can prevent infection through the feet and lower legs, especially after flooding or storms. People should also wear gloves to protect their hands when working directly with soil.
Melioidosis has a wide range of symptoms, including fever, joint pain, headaches, coughing, chest pain, and belly pain. It can also cause conditions such as pneumonia, abscesses, and blood infections. The disease can infect any organ, including the brain. In most cases, symptoms appear within 1-21 days after exposure, with an average of 7 days after exposure.
The CDC’s health advisory for health professionals and public health officials shows that melioidosis is now considered to be locally endemic in areas of the Gulf Coast region in Mississippi.
“Once well-established in the soil, B. pseudomallei cannot feasibly be removed from the soil,” according to the advisory. “Public health efforts should focus primarily on improving identification of cases so that appropriate treatment can be administered.”
A version of this article first appeared on WebMD.com.
, according to a new alert from the Centers for Disease Control and Prevention.
The bacteria, Burkholderia pseudomallei, was found along the Gulf Coast region in southern Mississippi. Typically, the bacteria are in tropical and subtropical climates, especially in parts of Southeast Asia, northern Australia, Central America, South America, Puerto Rico, and the U.S. Virgin Islands.
The bacteria can cause melioidosis, a rare and serious infectious disease that spreads to animals and humans through contact with contaminated soil and water via cuts, wounds, mucous membranes, breathing the bacteria in, or eating or drinking it. Worldwide, the disease is fatal in 10%-50% of those who become infected.
CDC and state officials are investigating the samples to find out how widespread the bacteria are within the United States. So far, modeling suggests that the environmental conditions on the Gulf Coast support the growth of B. pseudomallei.
“It is unclear how long the bacteria has been in the environment and where else it might be found in the U.S.,” according to the CDC statement. “CDC is alerting clinicians throughout the country of this discovery through a national health advisory, reminding them to be aware of the signs and symptoms of melioidosis and to consider melioidosis in patients that present with symptoms of the disease.”
Two unrelated people who live near the Gulf Coast region of Mississippi became sick with melioidosis recently – one in July 2020 and one in May 2022. Neither had traveled outside of the United States. The cases led the CDC and the Mississippi State Department of Health to collect environmental samples and test household products at the patients’ homes in June 2022. Three of the samples taken from soil and puddle water in the 2020 case tested positive for the bacteria.
Genomic sequencing revealed that both patients were infected with the same strain of the bacteria from the Western Hemisphere. They were hospitalized with sepsis due to pneumonia and had known risk factors for melioidosis. Both patients recovered after they were treated with antibiotics.
An average of 12 melioidosis cases are diagnosed in the United States each year, with most in people with recent travel to a country where the bacteria is endemic, or regularly found. Cases have also been linked to contaminated products imported from endemic countries. In late 2021, four cases in four states – Georgia, Kansas, Minnesota, and Texas – were linked to a contaminated aromatherapy spray that was imported, and Walmart issued a recall in November of that year, according to a CDC announcement. Two of the four people died.
Given the small number of cases found in the United States, the CDC believes the risk of melioidosis for the general population continues to be “very low,” and the risk of person-to-person spread is considered “extremely low.” But people who live on the Gulf Coast of Mississippi and who have health conditions that may put them at a higher risk, such as diabetes, chronic kidney disease, chronic lung disease, excessive alcohol use, and immunosuppressive conditions, should protect themselves.
The CDC recommends avoiding contact with soil or muddy water, particularly after heavy rains, and protecting open wounds with waterproof bandages. People should also wear waterproof boots when gardening, working in the yard, or doing agricultural work, which can prevent infection through the feet and lower legs, especially after flooding or storms. People should also wear gloves to protect their hands when working directly with soil.
Melioidosis has a wide range of symptoms, including fever, joint pain, headaches, coughing, chest pain, and belly pain. It can also cause conditions such as pneumonia, abscesses, and blood infections. The disease can infect any organ, including the brain. In most cases, symptoms appear within 1-21 days after exposure, with an average of 7 days after exposure.
The CDC’s health advisory for health professionals and public health officials shows that melioidosis is now considered to be locally endemic in areas of the Gulf Coast region in Mississippi.
“Once well-established in the soil, B. pseudomallei cannot feasibly be removed from the soil,” according to the advisory. “Public health efforts should focus primarily on improving identification of cases so that appropriate treatment can be administered.”
A version of this article first appeared on WebMD.com.
Acute pancreatitis: Procalcitonin algorithm safely reduces antibiotic overuse
A procalcitonin-based algorithm could safely reduce unnecessary usage of antibiotics in patients with acute pancreatitis, based on results of a randomized controlled trial.
Physicians should consider incorporating the decision-making process into their daily practice, suggested lead author Ajith K. Siriwardena, MD, of Manchester (England) University and colleagues, who also recommended that the algorithm be added to future guidelines.
“Overuse of antibiotics and the resultant emergence of multidrug resistant microorganisms is a potent threat to the welfare of humanity in the 21st century,” the investigators wrote in The Lancet Gastroenterology & Hepatology.
Antibiotic overuse is common in cases of acute pancreatitis, they noted, because clinical features are typically insufficient to distinguish between inflammation and infection. While measuring procalcitonin can help can detect infection, “indiscriminate measurement” of the biomarker is not cost effective, according to the investigators, leading previous reviews and analyses to conclude that further research is needed before widespread usage can be recommended.
Dr. Siriwardena and colleagues aimed to meet this need by conducting a randomized controlled trial involving 260 patients hospitalized for acute pancreatitis at Manchester Royal Infirmary. Patients were randomized in a near 1:1 ratio. Both the intervention group (n = 132) and the control group (n = 128) received guideline-based care; however, in addition to standard of care, procalcitonin was measured in the intervention group at days 0, 4, and 7 then weekly. Among these patients, antibiotics were stopped or not started when procalcitonin was below 1.0 ng/mL, but antibiotics were started or continued when procalcitonin was 1.0 ng/mL or more.
The primary outcome was presence or absence of antibiotic use during hospital stay. A range of secondary outcomes were also reported, included all-cause mortality, days of antibiotic use, rates of infection, and endoscopic, radiological, or surgical intervention.
Significantly fewer patients in the procalcitonin group received antibiotics during their stay, compared with the usual-care group (45% vs. 63%), which translated to an adjusted risk difference of –15.6% (P = .0071). Patients in the procalcitonin group who did receive antibiotics received about 1 day less of antibiotic treatment.
Despite the reduced antibiotic usage, length of hospital stay was similar between groups, as were rates of clinical infection, hospital-acquired infection, death, and adverse events, which suggests that the algorithm safely reduced antibiotic usage without negatively impacting clinical outcomes, according to investigators.
“Procalcitonin-based algorithms to guide antibiotic use should be considered in the care of this group of patients and be incorporated into future guidelines on the management of acute pancreatitis,” the investigators concluded.
Aaron Sasson, MD, director of the pancreatic cancer center and codirector of the gastrointestinal oncology team at Stony Brook (N.Y.) Medicine, said the study is noteworthy because it addresses an important topic with a large prospective randomized trial; however, he pointed out some limitations.
“There are several issues with this trial,” Dr. Sasson said in a written comment. “First, it included a large percentage of patients with mild acute pancreatitis, a group of patients for whom the use of antibiotics is not controversial. Secondly, the rate of infected pancreatic necrosis was 5% in both arms of the study, indicating the lack of severity of the cohort of patients.”
Dr. Sasson said that the algorithm “could be useful” to differentiate between inflammation and infection in patients with acute pancreatitis, “but only as an adjunct with other clinical parameters.”
He suggested that the algorithm would offer more utility if it could distinguish between pancreatic necrosis and infected pancreatic necrosis. “Unfortunately, this trial did not answer this question,” he said, noting that a similar trial involving “only patients with severe pancreatitis” would be needed.
The investigators and Dr. Sasson disclosed no competing interests.
A procalcitonin-based algorithm could safely reduce unnecessary usage of antibiotics in patients with acute pancreatitis, based on results of a randomized controlled trial.
Physicians should consider incorporating the decision-making process into their daily practice, suggested lead author Ajith K. Siriwardena, MD, of Manchester (England) University and colleagues, who also recommended that the algorithm be added to future guidelines.
“Overuse of antibiotics and the resultant emergence of multidrug resistant microorganisms is a potent threat to the welfare of humanity in the 21st century,” the investigators wrote in The Lancet Gastroenterology & Hepatology.
Antibiotic overuse is common in cases of acute pancreatitis, they noted, because clinical features are typically insufficient to distinguish between inflammation and infection. While measuring procalcitonin can help can detect infection, “indiscriminate measurement” of the biomarker is not cost effective, according to the investigators, leading previous reviews and analyses to conclude that further research is needed before widespread usage can be recommended.
Dr. Siriwardena and colleagues aimed to meet this need by conducting a randomized controlled trial involving 260 patients hospitalized for acute pancreatitis at Manchester Royal Infirmary. Patients were randomized in a near 1:1 ratio. Both the intervention group (n = 132) and the control group (n = 128) received guideline-based care; however, in addition to standard of care, procalcitonin was measured in the intervention group at days 0, 4, and 7 then weekly. Among these patients, antibiotics were stopped or not started when procalcitonin was below 1.0 ng/mL, but antibiotics were started or continued when procalcitonin was 1.0 ng/mL or more.
The primary outcome was presence or absence of antibiotic use during hospital stay. A range of secondary outcomes were also reported, included all-cause mortality, days of antibiotic use, rates of infection, and endoscopic, radiological, or surgical intervention.
Significantly fewer patients in the procalcitonin group received antibiotics during their stay, compared with the usual-care group (45% vs. 63%), which translated to an adjusted risk difference of –15.6% (P = .0071). Patients in the procalcitonin group who did receive antibiotics received about 1 day less of antibiotic treatment.
Despite the reduced antibiotic usage, length of hospital stay was similar between groups, as were rates of clinical infection, hospital-acquired infection, death, and adverse events, which suggests that the algorithm safely reduced antibiotic usage without negatively impacting clinical outcomes, according to investigators.
“Procalcitonin-based algorithms to guide antibiotic use should be considered in the care of this group of patients and be incorporated into future guidelines on the management of acute pancreatitis,” the investigators concluded.
Aaron Sasson, MD, director of the pancreatic cancer center and codirector of the gastrointestinal oncology team at Stony Brook (N.Y.) Medicine, said the study is noteworthy because it addresses an important topic with a large prospective randomized trial; however, he pointed out some limitations.
“There are several issues with this trial,” Dr. Sasson said in a written comment. “First, it included a large percentage of patients with mild acute pancreatitis, a group of patients for whom the use of antibiotics is not controversial. Secondly, the rate of infected pancreatic necrosis was 5% in both arms of the study, indicating the lack of severity of the cohort of patients.”
Dr. Sasson said that the algorithm “could be useful” to differentiate between inflammation and infection in patients with acute pancreatitis, “but only as an adjunct with other clinical parameters.”
He suggested that the algorithm would offer more utility if it could distinguish between pancreatic necrosis and infected pancreatic necrosis. “Unfortunately, this trial did not answer this question,” he said, noting that a similar trial involving “only patients with severe pancreatitis” would be needed.
The investigators and Dr. Sasson disclosed no competing interests.
A procalcitonin-based algorithm could safely reduce unnecessary usage of antibiotics in patients with acute pancreatitis, based on results of a randomized controlled trial.
Physicians should consider incorporating the decision-making process into their daily practice, suggested lead author Ajith K. Siriwardena, MD, of Manchester (England) University and colleagues, who also recommended that the algorithm be added to future guidelines.
“Overuse of antibiotics and the resultant emergence of multidrug resistant microorganisms is a potent threat to the welfare of humanity in the 21st century,” the investigators wrote in The Lancet Gastroenterology & Hepatology.
Antibiotic overuse is common in cases of acute pancreatitis, they noted, because clinical features are typically insufficient to distinguish between inflammation and infection. While measuring procalcitonin can help can detect infection, “indiscriminate measurement” of the biomarker is not cost effective, according to the investigators, leading previous reviews and analyses to conclude that further research is needed before widespread usage can be recommended.
Dr. Siriwardena and colleagues aimed to meet this need by conducting a randomized controlled trial involving 260 patients hospitalized for acute pancreatitis at Manchester Royal Infirmary. Patients were randomized in a near 1:1 ratio. Both the intervention group (n = 132) and the control group (n = 128) received guideline-based care; however, in addition to standard of care, procalcitonin was measured in the intervention group at days 0, 4, and 7 then weekly. Among these patients, antibiotics were stopped or not started when procalcitonin was below 1.0 ng/mL, but antibiotics were started or continued when procalcitonin was 1.0 ng/mL or more.
The primary outcome was presence or absence of antibiotic use during hospital stay. A range of secondary outcomes were also reported, included all-cause mortality, days of antibiotic use, rates of infection, and endoscopic, radiological, or surgical intervention.
Significantly fewer patients in the procalcitonin group received antibiotics during their stay, compared with the usual-care group (45% vs. 63%), which translated to an adjusted risk difference of –15.6% (P = .0071). Patients in the procalcitonin group who did receive antibiotics received about 1 day less of antibiotic treatment.
Despite the reduced antibiotic usage, length of hospital stay was similar between groups, as were rates of clinical infection, hospital-acquired infection, death, and adverse events, which suggests that the algorithm safely reduced antibiotic usage without negatively impacting clinical outcomes, according to investigators.
“Procalcitonin-based algorithms to guide antibiotic use should be considered in the care of this group of patients and be incorporated into future guidelines on the management of acute pancreatitis,” the investigators concluded.
Aaron Sasson, MD, director of the pancreatic cancer center and codirector of the gastrointestinal oncology team at Stony Brook (N.Y.) Medicine, said the study is noteworthy because it addresses an important topic with a large prospective randomized trial; however, he pointed out some limitations.
“There are several issues with this trial,” Dr. Sasson said in a written comment. “First, it included a large percentage of patients with mild acute pancreatitis, a group of patients for whom the use of antibiotics is not controversial. Secondly, the rate of infected pancreatic necrosis was 5% in both arms of the study, indicating the lack of severity of the cohort of patients.”
Dr. Sasson said that the algorithm “could be useful” to differentiate between inflammation and infection in patients with acute pancreatitis, “but only as an adjunct with other clinical parameters.”
He suggested that the algorithm would offer more utility if it could distinguish between pancreatic necrosis and infected pancreatic necrosis. “Unfortunately, this trial did not answer this question,” he said, noting that a similar trial involving “only patients with severe pancreatitis” would be needed.
The investigators and Dr. Sasson disclosed no competing interests.
FROM THE LANCET GASTROENTEROLOGY & HEPATOLOGY


