User login
NAFLD can regress with weight loss, activity
BOSTON – All phenotypes of nonalcoholic fatty liver disease (NAFLD) can progress or regress even without pharmacologic intervention, according to a prospective longitudinal study of 394 patients.
Weight loss and baseline NAFLD Activity Score predicted resolution of NAFLD, while weight gain and rising serum transaminases predicted progression to nonalcoholic steatohepatitis (NASH), Arun J. Sanyal, MD, said at the annual meeting of the American Association for the Study of Liver Diseases. Baseline and subsequent NAFLD Activity Score also was “a strong predictor of fibrosis progression or regression,” as was AST, portal inflammation, and baseline fibrosis stage, said Dr. Sanyal of Virginia Commonwealth University in Richmond, Va.
NAFLD comprises two main phenotypes, fatty liver and steatohepatitis. “The phenotype can change over time, and both phenotypes can be associated with fibrosis,” Dr. Sanyal noted. To better understand trends and clinical correlates for these phenotypes, he and his associates analyzed prospectively collected clinical and biopsy data from the NASH Clinical Research Network of the National Institutes of Diabetes and Digestive and Kidney Diseases. Each patient attended multiple clinic visits and underwent two liver biopsies at least 1 year and usually 4-5 years apart, which were interpreted by a masked central pathology review committee.
At baseline, 75 patients had fatty liver without steatohepatitis, of which only 13% resolved and 44% progressed to borderline or definite steatohepatitis. Similarly, among 74 patients with borderline steatohepatitis at baseline, only 22% regressed to fatty liver disease without steatohepatitis, while 43% progressed to definite steatohepatitis. The remaining 245 patients had definite steatohepatitis at baseline, of which 58% failed to regress at all, 20% regressed to borderline, 11% regressed to fatty liver disease without steatohepatitis, and 11% regressed to normal.
The investigators also performed a multivariable analysis of 197 patients with complete data. After the investigators controlled for serum insulin level, alkaline phosphatase level, NAS, and the presence of metabolic syndrome, each 10-U/L increase in ALT more than doubled the odds of progression from fatty liver without steatohepatitis to NASH (odds ratio, 2.2; 95% confidence interval, 1.1 to 4.1; P = .02). The association was even stronger for AST (OR, 3.5; 95% CI, 1.2 to 10.4; P = .03), and each 1-kg gain in body weight increased the odds of progression to NASH by 70% (OR, 1.7; 95% CI, 1.1 to 2.5; P = .01). In contrast, resolution of NAFLD was associated with weight loss (OR per 1 kg, 0.9; P less than .001) and lower baseline NAFLD Activity Score (OR, 0.7; P = .04).
About one in four patients had evidence of fibrosis at baseline, and 44% had at least stage 1 fibrosis at follow-up biopsy. Patients whose NAFLD progressed to a more severe phenotype were much more likely to have evidence of progressive fibrosis than were those whose NAFLD did not progress (OR, 7.2; 95% CI, 2.1 to 21.5; P less than .001), and there was no evidence that time between liver biopsies influenced this relationship. Among patients with definite NASH who had stage 0 fibrosis at baseline, 50% progressed to at least 1 fibrosis stage over the next 6.8 years, and 50% progressed to at least 2 stages over 9.6 years. Patients whose baseline NAFLD Activity Scores were between 1 and 4 were most likely to experience regression of fibrosis, while those with scores between 5 and 8 were more likely to have worsening fibrosis. Patients with severe baseline NAFLD Activity Score component scores for steatosis, lobular inflammation, and ballooning also were significantly more likely to have progressive fibrosis than were those with baseline NAFLD Activity Scores of 0 or 1. Furthermore, increasing NAFLD Activity Score over time predicted fibrosis progression.
Diabetes did not seem to affect fibrosis progression or regression, Dr. Sanyal noted. However, baseline portal inflammation predicted worsening fibrosis (P less than .01), as did baseline and subsequent elevations in AST (P less than .001), insulin (P = .03), and NAS (P less than 001), and baseline ballooning (P less than .01).
The investigators reported that the study had no sponsors. Dr. Sanyal disclosed ties to a wide number of drug companies.
BOSTON – All phenotypes of nonalcoholic fatty liver disease (NAFLD) can progress or regress even without pharmacologic intervention, according to a prospective longitudinal study of 394 patients.
Weight loss and baseline NAFLD Activity Score predicted resolution of NAFLD, while weight gain and rising serum transaminases predicted progression to nonalcoholic steatohepatitis (NASH), Arun J. Sanyal, MD, said at the annual meeting of the American Association for the Study of Liver Diseases. Baseline and subsequent NAFLD Activity Score also was “a strong predictor of fibrosis progression or regression,” as was AST, portal inflammation, and baseline fibrosis stage, said Dr. Sanyal of Virginia Commonwealth University in Richmond, Va.
NAFLD comprises two main phenotypes, fatty liver and steatohepatitis. “The phenotype can change over time, and both phenotypes can be associated with fibrosis,” Dr. Sanyal noted. To better understand trends and clinical correlates for these phenotypes, he and his associates analyzed prospectively collected clinical and biopsy data from the NASH Clinical Research Network of the National Institutes of Diabetes and Digestive and Kidney Diseases. Each patient attended multiple clinic visits and underwent two liver biopsies at least 1 year and usually 4-5 years apart, which were interpreted by a masked central pathology review committee.
At baseline, 75 patients had fatty liver without steatohepatitis, of which only 13% resolved and 44% progressed to borderline or definite steatohepatitis. Similarly, among 74 patients with borderline steatohepatitis at baseline, only 22% regressed to fatty liver disease without steatohepatitis, while 43% progressed to definite steatohepatitis. The remaining 245 patients had definite steatohepatitis at baseline, of which 58% failed to regress at all, 20% regressed to borderline, 11% regressed to fatty liver disease without steatohepatitis, and 11% regressed to normal.
The investigators also performed a multivariable analysis of 197 patients with complete data. After the investigators controlled for serum insulin level, alkaline phosphatase level, NAS, and the presence of metabolic syndrome, each 10-U/L increase in ALT more than doubled the odds of progression from fatty liver without steatohepatitis to NASH (odds ratio, 2.2; 95% confidence interval, 1.1 to 4.1; P = .02). The association was even stronger for AST (OR, 3.5; 95% CI, 1.2 to 10.4; P = .03), and each 1-kg gain in body weight increased the odds of progression to NASH by 70% (OR, 1.7; 95% CI, 1.1 to 2.5; P = .01). In contrast, resolution of NAFLD was associated with weight loss (OR per 1 kg, 0.9; P less than .001) and lower baseline NAFLD Activity Score (OR, 0.7; P = .04).
About one in four patients had evidence of fibrosis at baseline, and 44% had at least stage 1 fibrosis at follow-up biopsy. Patients whose NAFLD progressed to a more severe phenotype were much more likely to have evidence of progressive fibrosis than were those whose NAFLD did not progress (OR, 7.2; 95% CI, 2.1 to 21.5; P less than .001), and there was no evidence that time between liver biopsies influenced this relationship. Among patients with definite NASH who had stage 0 fibrosis at baseline, 50% progressed to at least 1 fibrosis stage over the next 6.8 years, and 50% progressed to at least 2 stages over 9.6 years. Patients whose baseline NAFLD Activity Scores were between 1 and 4 were most likely to experience regression of fibrosis, while those with scores between 5 and 8 were more likely to have worsening fibrosis. Patients with severe baseline NAFLD Activity Score component scores for steatosis, lobular inflammation, and ballooning also were significantly more likely to have progressive fibrosis than were those with baseline NAFLD Activity Scores of 0 or 1. Furthermore, increasing NAFLD Activity Score over time predicted fibrosis progression.
Diabetes did not seem to affect fibrosis progression or regression, Dr. Sanyal noted. However, baseline portal inflammation predicted worsening fibrosis (P less than .01), as did baseline and subsequent elevations in AST (P less than .001), insulin (P = .03), and NAS (P less than 001), and baseline ballooning (P less than .01).
The investigators reported that the study had no sponsors. Dr. Sanyal disclosed ties to a wide number of drug companies.
BOSTON – All phenotypes of nonalcoholic fatty liver disease (NAFLD) can progress or regress even without pharmacologic intervention, according to a prospective longitudinal study of 394 patients.
Weight loss and baseline NAFLD Activity Score predicted resolution of NAFLD, while weight gain and rising serum transaminases predicted progression to nonalcoholic steatohepatitis (NASH), Arun J. Sanyal, MD, said at the annual meeting of the American Association for the Study of Liver Diseases. Baseline and subsequent NAFLD Activity Score also was “a strong predictor of fibrosis progression or regression,” as was AST, portal inflammation, and baseline fibrosis stage, said Dr. Sanyal of Virginia Commonwealth University in Richmond, Va.
NAFLD comprises two main phenotypes, fatty liver and steatohepatitis. “The phenotype can change over time, and both phenotypes can be associated with fibrosis,” Dr. Sanyal noted. To better understand trends and clinical correlates for these phenotypes, he and his associates analyzed prospectively collected clinical and biopsy data from the NASH Clinical Research Network of the National Institutes of Diabetes and Digestive and Kidney Diseases. Each patient attended multiple clinic visits and underwent two liver biopsies at least 1 year and usually 4-5 years apart, which were interpreted by a masked central pathology review committee.
At baseline, 75 patients had fatty liver without steatohepatitis, of which only 13% resolved and 44% progressed to borderline or definite steatohepatitis. Similarly, among 74 patients with borderline steatohepatitis at baseline, only 22% regressed to fatty liver disease without steatohepatitis, while 43% progressed to definite steatohepatitis. The remaining 245 patients had definite steatohepatitis at baseline, of which 58% failed to regress at all, 20% regressed to borderline, 11% regressed to fatty liver disease without steatohepatitis, and 11% regressed to normal.
The investigators also performed a multivariable analysis of 197 patients with complete data. After the investigators controlled for serum insulin level, alkaline phosphatase level, NAS, and the presence of metabolic syndrome, each 10-U/L increase in ALT more than doubled the odds of progression from fatty liver without steatohepatitis to NASH (odds ratio, 2.2; 95% confidence interval, 1.1 to 4.1; P = .02). The association was even stronger for AST (OR, 3.5; 95% CI, 1.2 to 10.4; P = .03), and each 1-kg gain in body weight increased the odds of progression to NASH by 70% (OR, 1.7; 95% CI, 1.1 to 2.5; P = .01). In contrast, resolution of NAFLD was associated with weight loss (OR per 1 kg, 0.9; P less than .001) and lower baseline NAFLD Activity Score (OR, 0.7; P = .04).
About one in four patients had evidence of fibrosis at baseline, and 44% had at least stage 1 fibrosis at follow-up biopsy. Patients whose NAFLD progressed to a more severe phenotype were much more likely to have evidence of progressive fibrosis than were those whose NAFLD did not progress (OR, 7.2; 95% CI, 2.1 to 21.5; P less than .001), and there was no evidence that time between liver biopsies influenced this relationship. Among patients with definite NASH who had stage 0 fibrosis at baseline, 50% progressed to at least 1 fibrosis stage over the next 6.8 years, and 50% progressed to at least 2 stages over 9.6 years. Patients whose baseline NAFLD Activity Scores were between 1 and 4 were most likely to experience regression of fibrosis, while those with scores between 5 and 8 were more likely to have worsening fibrosis. Patients with severe baseline NAFLD Activity Score component scores for steatosis, lobular inflammation, and ballooning also were significantly more likely to have progressive fibrosis than were those with baseline NAFLD Activity Scores of 0 or 1. Furthermore, increasing NAFLD Activity Score over time predicted fibrosis progression.
Diabetes did not seem to affect fibrosis progression or regression, Dr. Sanyal noted. However, baseline portal inflammation predicted worsening fibrosis (P less than .01), as did baseline and subsequent elevations in AST (P less than .001), insulin (P = .03), and NAS (P less than 001), and baseline ballooning (P less than .01).
The investigators reported that the study had no sponsors. Dr. Sanyal disclosed ties to a wide number of drug companies.
AT THE LIVER MEETING 2016
Key clinical point: Phenotypes of nonalcoholic fatty liver disease can progress or regress over time.
Major finding: Resolution of NAFLD was associated with weight loss (OR per 1 kg, 0.9; P less than .001) and lower baseline NAFLD Activity Score (OR, 0.7; P = .04).
Data source: A prospective study of 394 patients with nonalcoholic fatty liver disease.
Disclosures: The investigators reported that the study had no sponsors. Dr. Sanyal disclosed ties to several drug companies.
Tumor boards linked to improved survival in hepatocellular carcinoma
BOSTON – Veterans were about 13% less likely to die within 5 years of hepatocellular carcinoma diagnosis when multidisciplinary tumor boards managed their care than if they did not, according to a large, multicenter observational study.
Seeing a hepatologist or surgeon within 30 days of diagnosis also significantly improved 5-year overall survival, even after controlling for age, race, Charlson-Deyo comorbidity index, Barcelona Clinic Liver Cancer (BCLC) stage, academic center and geographic region of care, and the distance patients lived from the nearest Veterans Affairs transplant center, Marina Serper, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases. “More studies are needed to understand how to best use multidisciplinary tumor boards to improve the care of patients with hepatocellular carcinoma,” she said.
Outcomes data for hepatocellular carcinoma mostly come from clinical trials; transplant centers; and Surveillance, Epidemiology, and End Results-Medicare analyses, noted Dr. Serper of the University of Pennsylvania in Philadelphia.
For a better look at veterans, she and her associates combined administrative, laboratory, and death data with medical chart reviews and information from the Organ Procurement and Transplantation Network’s Standard Transplant Analysis and Research file. The initial cohort included more than 6,800 veterans whose ICD-9CM diagnosis code indicated a malignant hepatic neoplasm. Excluding patients with neoplasms such as cholangiocarcinoma and those managed outside the VA left 3,989 VA patients with hepatocellular carcinoma.
In the multivariable analysis, use of multidisciplinary tumor boards was associated with a statistically significant 13% improvement in 5-year overall survival (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001). Improved survival also was linked with seeing certain specialists within 30 days of diagnosis, including hepatologists (HR, 0.77; P less than .001) and surgeons (HR, 0.72; P less than .001). Consulting with a hepatologist within 30 days of diagnosis, however, did not improve the chances of receiving curative therapy, such as liver transplantation, resection, local ablation, transarterial chemoembolization, or Y-90 radioembolization.
Care also varied substantially geographically and by academic affiliation, Dr. Serper noted. “Treatment of hepatocellular carcinoma is complex, as it depends as much on liver function as it does on tumor staging,” she emphasized. “Studies to improve multidisciplinary approaches for hepatocellular carcinoma in the community are needed to increase rates of curative therapy and improve clinical outcomes.”
Patients in this study averaged 62 years of age at diagnosis, 54% were white, 36% were within Milan criteria, and 45% had a Child-Turcotte-Pugh score of B or higher. Nearly 18% had macrovascular invasion at diagnosis, and 7% had metastatic disease. Nearly two-thirds of patients were BCLC stage A or B at diagnosis, and more than a third had underlying alcohol misuse and chronic hepatitis C virus infection.
The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
BOSTON – Veterans were about 13% less likely to die within 5 years of hepatocellular carcinoma diagnosis when multidisciplinary tumor boards managed their care than if they did not, according to a large, multicenter observational study.
Seeing a hepatologist or surgeon within 30 days of diagnosis also significantly improved 5-year overall survival, even after controlling for age, race, Charlson-Deyo comorbidity index, Barcelona Clinic Liver Cancer (BCLC) stage, academic center and geographic region of care, and the distance patients lived from the nearest Veterans Affairs transplant center, Marina Serper, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases. “More studies are needed to understand how to best use multidisciplinary tumor boards to improve the care of patients with hepatocellular carcinoma,” she said.
Outcomes data for hepatocellular carcinoma mostly come from clinical trials; transplant centers; and Surveillance, Epidemiology, and End Results-Medicare analyses, noted Dr. Serper of the University of Pennsylvania in Philadelphia.
For a better look at veterans, she and her associates combined administrative, laboratory, and death data with medical chart reviews and information from the Organ Procurement and Transplantation Network’s Standard Transplant Analysis and Research file. The initial cohort included more than 6,800 veterans whose ICD-9CM diagnosis code indicated a malignant hepatic neoplasm. Excluding patients with neoplasms such as cholangiocarcinoma and those managed outside the VA left 3,989 VA patients with hepatocellular carcinoma.
In the multivariable analysis, use of multidisciplinary tumor boards was associated with a statistically significant 13% improvement in 5-year overall survival (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001). Improved survival also was linked with seeing certain specialists within 30 days of diagnosis, including hepatologists (HR, 0.77; P less than .001) and surgeons (HR, 0.72; P less than .001). Consulting with a hepatologist within 30 days of diagnosis, however, did not improve the chances of receiving curative therapy, such as liver transplantation, resection, local ablation, transarterial chemoembolization, or Y-90 radioembolization.
Care also varied substantially geographically and by academic affiliation, Dr. Serper noted. “Treatment of hepatocellular carcinoma is complex, as it depends as much on liver function as it does on tumor staging,” she emphasized. “Studies to improve multidisciplinary approaches for hepatocellular carcinoma in the community are needed to increase rates of curative therapy and improve clinical outcomes.”
Patients in this study averaged 62 years of age at diagnosis, 54% were white, 36% were within Milan criteria, and 45% had a Child-Turcotte-Pugh score of B or higher. Nearly 18% had macrovascular invasion at diagnosis, and 7% had metastatic disease. Nearly two-thirds of patients were BCLC stage A or B at diagnosis, and more than a third had underlying alcohol misuse and chronic hepatitis C virus infection.
The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
BOSTON – Veterans were about 13% less likely to die within 5 years of hepatocellular carcinoma diagnosis when multidisciplinary tumor boards managed their care than if they did not, according to a large, multicenter observational study.
Seeing a hepatologist or surgeon within 30 days of diagnosis also significantly improved 5-year overall survival, even after controlling for age, race, Charlson-Deyo comorbidity index, Barcelona Clinic Liver Cancer (BCLC) stage, academic center and geographic region of care, and the distance patients lived from the nearest Veterans Affairs transplant center, Marina Serper, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases. “More studies are needed to understand how to best use multidisciplinary tumor boards to improve the care of patients with hepatocellular carcinoma,” she said.
Outcomes data for hepatocellular carcinoma mostly come from clinical trials; transplant centers; and Surveillance, Epidemiology, and End Results-Medicare analyses, noted Dr. Serper of the University of Pennsylvania in Philadelphia.
For a better look at veterans, she and her associates combined administrative, laboratory, and death data with medical chart reviews and information from the Organ Procurement and Transplantation Network’s Standard Transplant Analysis and Research file. The initial cohort included more than 6,800 veterans whose ICD-9CM diagnosis code indicated a malignant hepatic neoplasm. Excluding patients with neoplasms such as cholangiocarcinoma and those managed outside the VA left 3,989 VA patients with hepatocellular carcinoma.
In the multivariable analysis, use of multidisciplinary tumor boards was associated with a statistically significant 13% improvement in 5-year overall survival (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001). Improved survival also was linked with seeing certain specialists within 30 days of diagnosis, including hepatologists (HR, 0.77; P less than .001) and surgeons (HR, 0.72; P less than .001). Consulting with a hepatologist within 30 days of diagnosis, however, did not improve the chances of receiving curative therapy, such as liver transplantation, resection, local ablation, transarterial chemoembolization, or Y-90 radioembolization.
Care also varied substantially geographically and by academic affiliation, Dr. Serper noted. “Treatment of hepatocellular carcinoma is complex, as it depends as much on liver function as it does on tumor staging,” she emphasized. “Studies to improve multidisciplinary approaches for hepatocellular carcinoma in the community are needed to increase rates of curative therapy and improve clinical outcomes.”
Patients in this study averaged 62 years of age at diagnosis, 54% were white, 36% were within Milan criteria, and 45% had a Child-Turcotte-Pugh score of B or higher. Nearly 18% had macrovascular invasion at diagnosis, and 7% had metastatic disease. Nearly two-thirds of patients were BCLC stage A or B at diagnosis, and more than a third had underlying alcohol misuse and chronic hepatitis C virus infection.
The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
AT THE LIVER MEETING 2016
Key clinical point: The use of multidisciplinary tumor boards was associated with significantly improved overall survival in patients with hepatocellular carcinoma.
Major finding: The risk of death within 5 years dropped by about 13% (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001).
Data source: A retrospective study of 3,989 Veterans Affairs patients with hepatocellular carcinoma.
Disclosures: The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
Direct-acting antiretrovirals in genotype 1 HCV patients with mental health conditions safe, well tolerated
BOSTON – Direct-acting antiretroviral therapies are safe and well tolerated in hepatitis C virus patients with comorbid psychiatric conditions, a study showed.
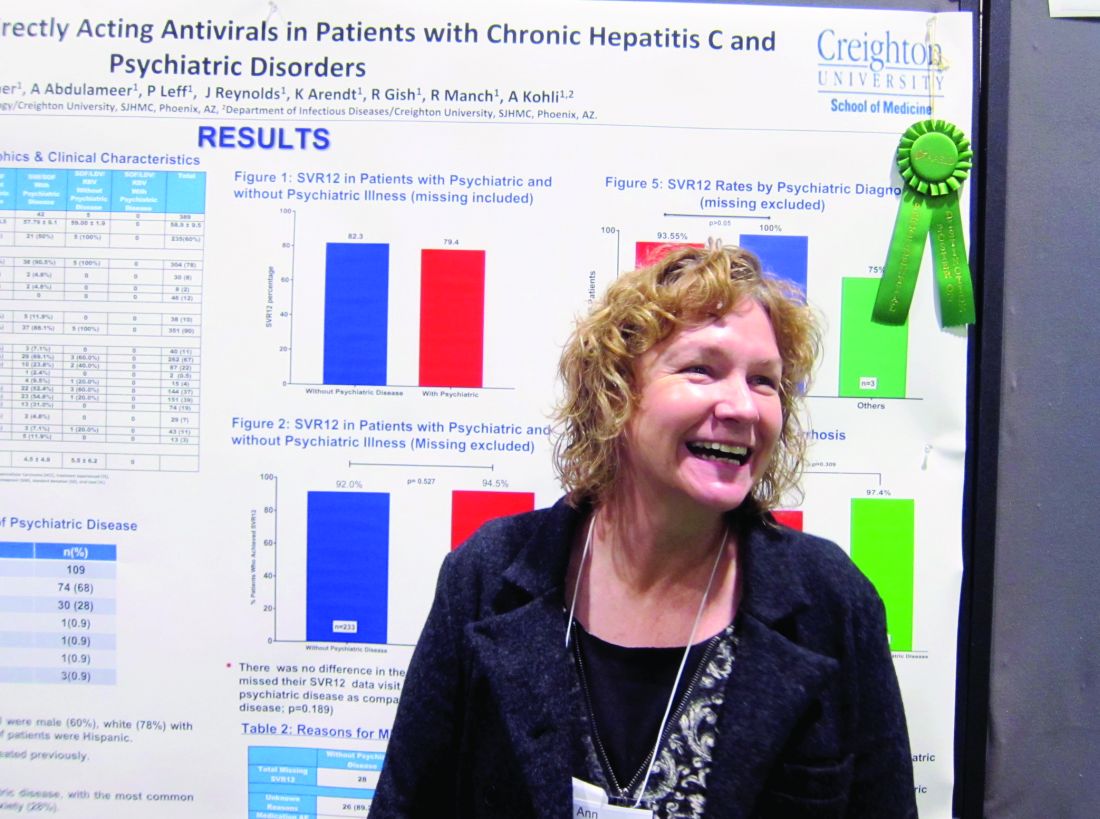
The finding that sustained virologic response (SVR) rates at 1 year were similar in genotype 1 HCV patients with and without psychiatric comorbidities could help end the stigma against treating this patient subgroup, stemming from when treatment with interferon risked psychiatric decompensation. That’s according to nurse practitioner Anne Moore, winner of this year’s Poster of Distinction Award at the American Association for the Study of Liver Diseases annual meeting.
She and her colleagues reviewed patient records for 588 adults diagnosed with genotype 1 HCV in Arizona between 2013 and 2016. They found 389 patients who’d been treated with either a combination of sofosbuvir and ledipasvir (with or without ribavirin) or sofosbuvir and simeprevir. Patients coinfected with HIV were included in this group; those who’d had a liver transplant were not. Just over three-quarters of the patients were white, 60% were male, 10% were Hispanic, and the average age was 59 years. A third of the patients had been diagnosed with cirrhosis.
Because medical and mental health records typically are not integrated, Ms. Moore and her colleagues instead used medical records to evaluate which medications had been prescribed, in order to determine likely psychiatric diagnoses. They determined that 27% of the 389 patients had a comorbid psychiatric diagnosis. Of these, 68% had depression and 28% had anxiety. Only one person was considered to have bipolar disorder; Ms. Moore said it was possible this group was underrepresented in the study, but that some persons considered to have depression might have had bipolar disorder instead.
Rates of SVR at 1 year were similar across the study – 82.3% in those without a comorbid psychiatric diagnosis vs. 79.4% of those who did have one – with no additional risk of patients with psychiatric diagnoses being lost to follow-up. DAA therapy was well tolerated in both groups.
Ms. Moore said that winning the award was a “huge surprise” but interpreted it to mean that liver specialists are struggling to find the best treatment algorithms for HCV, partly because of the number of restrictions often placed by payers on access to DAAs, but also because it is often difficult to know who will adhere to treatment.
“Gastroenterologists and hepatologists are not used to dealing with patients who have psychiatric conditions. They don’t understand them well, and so there is a reluctance to ‘go there.’ If that’s what has been holding you back, it doesn’t have to. You can treat these patients successfully,” Ms. Moore said.
She had no relevant financial disclosures.
BOSTON – Direct-acting antiretroviral therapies are safe and well tolerated in hepatitis C virus patients with comorbid psychiatric conditions, a study showed.
The finding that sustained virologic response (SVR) rates at 1 year were similar in genotype 1 HCV patients with and without psychiatric comorbidities could help end the stigma against treating this patient subgroup, stemming from when treatment with interferon risked psychiatric decompensation. That’s according to nurse practitioner Anne Moore, winner of this year’s Poster of Distinction Award at the American Association for the Study of Liver Diseases annual meeting.
She and her colleagues reviewed patient records for 588 adults diagnosed with genotype 1 HCV in Arizona between 2013 and 2016. They found 389 patients who’d been treated with either a combination of sofosbuvir and ledipasvir (with or without ribavirin) or sofosbuvir and simeprevir. Patients coinfected with HIV were included in this group; those who’d had a liver transplant were not. Just over three-quarters of the patients were white, 60% were male, 10% were Hispanic, and the average age was 59 years. A third of the patients had been diagnosed with cirrhosis.
Because medical and mental health records typically are not integrated, Ms. Moore and her colleagues instead used medical records to evaluate which medications had been prescribed, in order to determine likely psychiatric diagnoses. They determined that 27% of the 389 patients had a comorbid psychiatric diagnosis. Of these, 68% had depression and 28% had anxiety. Only one person was considered to have bipolar disorder; Ms. Moore said it was possible this group was underrepresented in the study, but that some persons considered to have depression might have had bipolar disorder instead.
Rates of SVR at 1 year were similar across the study – 82.3% in those without a comorbid psychiatric diagnosis vs. 79.4% of those who did have one – with no additional risk of patients with psychiatric diagnoses being lost to follow-up. DAA therapy was well tolerated in both groups.
Ms. Moore said that winning the award was a “huge surprise” but interpreted it to mean that liver specialists are struggling to find the best treatment algorithms for HCV, partly because of the number of restrictions often placed by payers on access to DAAs, but also because it is often difficult to know who will adhere to treatment.
“Gastroenterologists and hepatologists are not used to dealing with patients who have psychiatric conditions. They don’t understand them well, and so there is a reluctance to ‘go there.’ If that’s what has been holding you back, it doesn’t have to. You can treat these patients successfully,” Ms. Moore said.
She had no relevant financial disclosures.
BOSTON – Direct-acting antiretroviral therapies are safe and well tolerated in hepatitis C virus patients with comorbid psychiatric conditions, a study showed.
The finding that sustained virologic response (SVR) rates at 1 year were similar in genotype 1 HCV patients with and without psychiatric comorbidities could help end the stigma against treating this patient subgroup, stemming from when treatment with interferon risked psychiatric decompensation. That’s according to nurse practitioner Anne Moore, winner of this year’s Poster of Distinction Award at the American Association for the Study of Liver Diseases annual meeting.
She and her colleagues reviewed patient records for 588 adults diagnosed with genotype 1 HCV in Arizona between 2013 and 2016. They found 389 patients who’d been treated with either a combination of sofosbuvir and ledipasvir (with or without ribavirin) or sofosbuvir and simeprevir. Patients coinfected with HIV were included in this group; those who’d had a liver transplant were not. Just over three-quarters of the patients were white, 60% were male, 10% were Hispanic, and the average age was 59 years. A third of the patients had been diagnosed with cirrhosis.
Because medical and mental health records typically are not integrated, Ms. Moore and her colleagues instead used medical records to evaluate which medications had been prescribed, in order to determine likely psychiatric diagnoses. They determined that 27% of the 389 patients had a comorbid psychiatric diagnosis. Of these, 68% had depression and 28% had anxiety. Only one person was considered to have bipolar disorder; Ms. Moore said it was possible this group was underrepresented in the study, but that some persons considered to have depression might have had bipolar disorder instead.
Rates of SVR at 1 year were similar across the study – 82.3% in those without a comorbid psychiatric diagnosis vs. 79.4% of those who did have one – with no additional risk of patients with psychiatric diagnoses being lost to follow-up. DAA therapy was well tolerated in both groups.
Ms. Moore said that winning the award was a “huge surprise” but interpreted it to mean that liver specialists are struggling to find the best treatment algorithms for HCV, partly because of the number of restrictions often placed by payers on access to DAAs, but also because it is often difficult to know who will adhere to treatment.
“Gastroenterologists and hepatologists are not used to dealing with patients who have psychiatric conditions. They don’t understand them well, and so there is a reluctance to ‘go there.’ If that’s what has been holding you back, it doesn’t have to. You can treat these patients successfully,” Ms. Moore said.
She had no relevant financial disclosures.
AT THE LIVER MEETING 2016
Key clinical point:
Major finding: Sustained virologic response rates at 1 year were similar between genotype 1 HCV patients with and without a comorbid psychiatric diagnosis.
Data source: A retrospective analysis of medical records from 2013 to 2016 for 588 adults with genotype 1 hepatitis C virus.
Disclosures: Ms. Moore had no relevant financial disclosures.
VIDEO: PBC patients with compensated cirrhosis fare well on obeticholic acid
BOSTON – Patients with primary biliary cholangitis (PBC) who have compensated cirrhosis fared just as well on obeticholic acid (OCA) as did PBC patients without cirrhosis, according to an analysis of data from POISE, the pivotal clinical trial for approval of OCA for PBC.
The POISE trial included 36 individuals with PBC and compensated cirrhosis, since cirrhosis “is an endpoint for virtually all liver diseases,” John Vierling, MD, said in a video interview at the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To see how this group fared, Dr. Vierling and his coinvestigators performed a post hoc analysis of the POISE data to examine OCA’s safety and efficacy for patients with compensated cirrhosis. Patients with decompensated cirrhosis were not included in the trial.
Dr. Vierling, chief of hepatology at Baylor College of Medicine, Houston, noted that investigators worked hard to set the bar high for inclusion in the group with cirrhosis, to achieve very high specificity. “We did this by using very stringent criteria of liver biopsy, or transient elastography adjusted for a very high range of kilopascals required to diagnose cirrhosis in cholestatic patients,” he said. To be included, patients also had to have elevated total bilirubin levels and a baseline alkaline phosphatase level greater than five times the upper limit of normal.
Statistically, the patients were evenly distributed across the placebo arm and the two treatment arms, one of which dosed OCA at 10 mg/day; the other treatment arm had flexible dosing at 5-10 mg/day.
The POISE trial used a composite primary efficacy endpoint of achieving an alkaline phosphatase (ALP) less than 1.67 times the upper limit of normal, with total bilirubin within normal limits, and at least a 15% reduction in ALP.
“Significantly more OCA-treated patients with cirrhosis achieved the primary composite endpoint compared to placebo,” Dr. Vierling and his coauthors wrote in a poster presented at the annual meeting for the American Association for the Study of Liver Diseases. The difference was individually significant for all three values that made up the composite primary endpoint as well.
Secondary endpoints included gamma-glutamyltransferase, alanine aminotrasferase, and aspartate aminotransferase, all of which were significantly reduced among patients taking OCA. Patients on placebo saw these values rise over the time period of the study.
There were no new safety signals seen in the post hoc analysis of the group with cirrhosis that were not seen in the trial at large, said Dr. Vierling. Two individuals in the subgroup dropped out of the trial because of pruritis, a similar proportion to that seen in the full trial population.
The drug’s manufacturer, Intercept Pharmaceuticals, is working with the Food and Drug Administration to establish appropriate doses and intervals for obeticholic acid so it may be used safely in individuals with decompensated cirrhosis, said Dr. Vierling.
Obeticholic acid, a farnesoid-X receptor agonist, is an approved agent to use as add-on therapy to ursodeoxycholic acid (UDCA), or as monotherapy for patients who can’t tolerate UDCA.
Dr. Vierling disclosed financial relationships with Intercept Pharmaceuticals and with several other pharmaceutical companies. The study was funded by Intercept Pharmaceuticals.
koakes@frontlinemedcom.com
On Twitter @karioakes
BOSTON – Patients with primary biliary cholangitis (PBC) who have compensated cirrhosis fared just as well on obeticholic acid (OCA) as did PBC patients without cirrhosis, according to an analysis of data from POISE, the pivotal clinical trial for approval of OCA for PBC.
The POISE trial included 36 individuals with PBC and compensated cirrhosis, since cirrhosis “is an endpoint for virtually all liver diseases,” John Vierling, MD, said in a video interview at the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To see how this group fared, Dr. Vierling and his coinvestigators performed a post hoc analysis of the POISE data to examine OCA’s safety and efficacy for patients with compensated cirrhosis. Patients with decompensated cirrhosis were not included in the trial.
Dr. Vierling, chief of hepatology at Baylor College of Medicine, Houston, noted that investigators worked hard to set the bar high for inclusion in the group with cirrhosis, to achieve very high specificity. “We did this by using very stringent criteria of liver biopsy, or transient elastography adjusted for a very high range of kilopascals required to diagnose cirrhosis in cholestatic patients,” he said. To be included, patients also had to have elevated total bilirubin levels and a baseline alkaline phosphatase level greater than five times the upper limit of normal.
Statistically, the patients were evenly distributed across the placebo arm and the two treatment arms, one of which dosed OCA at 10 mg/day; the other treatment arm had flexible dosing at 5-10 mg/day.
The POISE trial used a composite primary efficacy endpoint of achieving an alkaline phosphatase (ALP) less than 1.67 times the upper limit of normal, with total bilirubin within normal limits, and at least a 15% reduction in ALP.
“Significantly more OCA-treated patients with cirrhosis achieved the primary composite endpoint compared to placebo,” Dr. Vierling and his coauthors wrote in a poster presented at the annual meeting for the American Association for the Study of Liver Diseases. The difference was individually significant for all three values that made up the composite primary endpoint as well.
Secondary endpoints included gamma-glutamyltransferase, alanine aminotrasferase, and aspartate aminotransferase, all of which were significantly reduced among patients taking OCA. Patients on placebo saw these values rise over the time period of the study.
There were no new safety signals seen in the post hoc analysis of the group with cirrhosis that were not seen in the trial at large, said Dr. Vierling. Two individuals in the subgroup dropped out of the trial because of pruritis, a similar proportion to that seen in the full trial population.
The drug’s manufacturer, Intercept Pharmaceuticals, is working with the Food and Drug Administration to establish appropriate doses and intervals for obeticholic acid so it may be used safely in individuals with decompensated cirrhosis, said Dr. Vierling.
Obeticholic acid, a farnesoid-X receptor agonist, is an approved agent to use as add-on therapy to ursodeoxycholic acid (UDCA), or as monotherapy for patients who can’t tolerate UDCA.
Dr. Vierling disclosed financial relationships with Intercept Pharmaceuticals and with several other pharmaceutical companies. The study was funded by Intercept Pharmaceuticals.
koakes@frontlinemedcom.com
On Twitter @karioakes
BOSTON – Patients with primary biliary cholangitis (PBC) who have compensated cirrhosis fared just as well on obeticholic acid (OCA) as did PBC patients without cirrhosis, according to an analysis of data from POISE, the pivotal clinical trial for approval of OCA for PBC.
The POISE trial included 36 individuals with PBC and compensated cirrhosis, since cirrhosis “is an endpoint for virtually all liver diseases,” John Vierling, MD, said in a video interview at the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To see how this group fared, Dr. Vierling and his coinvestigators performed a post hoc analysis of the POISE data to examine OCA’s safety and efficacy for patients with compensated cirrhosis. Patients with decompensated cirrhosis were not included in the trial.
Dr. Vierling, chief of hepatology at Baylor College of Medicine, Houston, noted that investigators worked hard to set the bar high for inclusion in the group with cirrhosis, to achieve very high specificity. “We did this by using very stringent criteria of liver biopsy, or transient elastography adjusted for a very high range of kilopascals required to diagnose cirrhosis in cholestatic patients,” he said. To be included, patients also had to have elevated total bilirubin levels and a baseline alkaline phosphatase level greater than five times the upper limit of normal.
Statistically, the patients were evenly distributed across the placebo arm and the two treatment arms, one of which dosed OCA at 10 mg/day; the other treatment arm had flexible dosing at 5-10 mg/day.
The POISE trial used a composite primary efficacy endpoint of achieving an alkaline phosphatase (ALP) less than 1.67 times the upper limit of normal, with total bilirubin within normal limits, and at least a 15% reduction in ALP.
“Significantly more OCA-treated patients with cirrhosis achieved the primary composite endpoint compared to placebo,” Dr. Vierling and his coauthors wrote in a poster presented at the annual meeting for the American Association for the Study of Liver Diseases. The difference was individually significant for all three values that made up the composite primary endpoint as well.
Secondary endpoints included gamma-glutamyltransferase, alanine aminotrasferase, and aspartate aminotransferase, all of which were significantly reduced among patients taking OCA. Patients on placebo saw these values rise over the time period of the study.
There were no new safety signals seen in the post hoc analysis of the group with cirrhosis that were not seen in the trial at large, said Dr. Vierling. Two individuals in the subgroup dropped out of the trial because of pruritis, a similar proportion to that seen in the full trial population.
The drug’s manufacturer, Intercept Pharmaceuticals, is working with the Food and Drug Administration to establish appropriate doses and intervals for obeticholic acid so it may be used safely in individuals with decompensated cirrhosis, said Dr. Vierling.
Obeticholic acid, a farnesoid-X receptor agonist, is an approved agent to use as add-on therapy to ursodeoxycholic acid (UDCA), or as monotherapy for patients who can’t tolerate UDCA.
Dr. Vierling disclosed financial relationships with Intercept Pharmaceuticals and with several other pharmaceutical companies. The study was funded by Intercept Pharmaceuticals.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM THE LIVER MEETING
SNP predicts liver cancer in hepatitis C patients regardless of SVR
BOSTON – rs4836493, a single nucleotide polymorphism of the gene encoding chondroitin sulfate synthase-3, significantly predicted hepatocellular carcinoma among patients with HCV even when they achieved sustained virologic response on pegylated interferon, according to a genome-wide association study.
The next step is to determine whether rs4836493 predicts liver cancer after SVR on the new direct-acting antiviral regimens, Basile Njei, MD, MPH, said during an oral presentation at the annual meeting of the American Association for the Study of Liver Diseases.
Cirrhotic patients face about a 2%-7% annual risk of hepatocellular carcinoma, noted Dr. Njei of Yale University, New Haven, Conn. “Recent studies show that people with HCV may still develop hepatocellular carcinoma even after achieving SVR,” he said.
To look for genetic predictors of this outcome, he and his associates genotyped 958 patients with HCV and advanced hepatic fibrosis from the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) study. This trial had evaluated long-term, low-dose pegylated interferon therapy (90 mcg per week for 3.5 years) as a means of keeping fibrosis from progressing among HCV patients who had failed peginterferon and ribavirin therapy.
A total of 63% of patients had cirrhosis, and 55 (5.7%) developed biopsy or imaging-confirmed hepatocellular carcinoma over a median of 80 months of follow-up, Dr. Njei said. After the researchers controlled for age, sex, Ishak fibrosis score, and SVR status, rs4836493 predicted hepatocellular carcinoma with a highly significant P value of .000004.
This SNP is located on the CHSY3 gene, which plays a role in the chondroitin polymerization, tissue development, and morphogenesis, according to Dr. Njei. Notably, the gene has been implicated in the biology of colorectal tumors, he added.
Dr. Njei and his associates genotyped patients by using the 610-Quad platform, which contains more than 600,000 SNPs. They double-checked results and conducted more genetic analyses using PLINK 1.9, a free, open-source software program for genome-wide association data. Three-quarters of patients in the study were white, 72% were male, and median age at enrollment was 50 years, he noted.
Linking a single SNP to liver cancer despite SVR is a striking finding, but it is also preliminary, Dr. Njei cautioned. “The SNP identified in our discovery genome-wide association study needs future replication and validation in patients who achieve SVR after receiving the new direct-acting antiviral therapies,” he said.
The National Institutes of Health provided partial funding. Dr. Njei and his coinvestigators had no relevant financial conflicts of interest.
BOSTON – rs4836493, a single nucleotide polymorphism of the gene encoding chondroitin sulfate synthase-3, significantly predicted hepatocellular carcinoma among patients with HCV even when they achieved sustained virologic response on pegylated interferon, according to a genome-wide association study.
The next step is to determine whether rs4836493 predicts liver cancer after SVR on the new direct-acting antiviral regimens, Basile Njei, MD, MPH, said during an oral presentation at the annual meeting of the American Association for the Study of Liver Diseases.
Cirrhotic patients face about a 2%-7% annual risk of hepatocellular carcinoma, noted Dr. Njei of Yale University, New Haven, Conn. “Recent studies show that people with HCV may still develop hepatocellular carcinoma even after achieving SVR,” he said.
To look for genetic predictors of this outcome, he and his associates genotyped 958 patients with HCV and advanced hepatic fibrosis from the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) study. This trial had evaluated long-term, low-dose pegylated interferon therapy (90 mcg per week for 3.5 years) as a means of keeping fibrosis from progressing among HCV patients who had failed peginterferon and ribavirin therapy.
A total of 63% of patients had cirrhosis, and 55 (5.7%) developed biopsy or imaging-confirmed hepatocellular carcinoma over a median of 80 months of follow-up, Dr. Njei said. After the researchers controlled for age, sex, Ishak fibrosis score, and SVR status, rs4836493 predicted hepatocellular carcinoma with a highly significant P value of .000004.
This SNP is located on the CHSY3 gene, which plays a role in the chondroitin polymerization, tissue development, and morphogenesis, according to Dr. Njei. Notably, the gene has been implicated in the biology of colorectal tumors, he added.
Dr. Njei and his associates genotyped patients by using the 610-Quad platform, which contains more than 600,000 SNPs. They double-checked results and conducted more genetic analyses using PLINK 1.9, a free, open-source software program for genome-wide association data. Three-quarters of patients in the study were white, 72% were male, and median age at enrollment was 50 years, he noted.
Linking a single SNP to liver cancer despite SVR is a striking finding, but it is also preliminary, Dr. Njei cautioned. “The SNP identified in our discovery genome-wide association study needs future replication and validation in patients who achieve SVR after receiving the new direct-acting antiviral therapies,” he said.
The National Institutes of Health provided partial funding. Dr. Njei and his coinvestigators had no relevant financial conflicts of interest.
BOSTON – rs4836493, a single nucleotide polymorphism of the gene encoding chondroitin sulfate synthase-3, significantly predicted hepatocellular carcinoma among patients with HCV even when they achieved sustained virologic response on pegylated interferon, according to a genome-wide association study.
The next step is to determine whether rs4836493 predicts liver cancer after SVR on the new direct-acting antiviral regimens, Basile Njei, MD, MPH, said during an oral presentation at the annual meeting of the American Association for the Study of Liver Diseases.
Cirrhotic patients face about a 2%-7% annual risk of hepatocellular carcinoma, noted Dr. Njei of Yale University, New Haven, Conn. “Recent studies show that people with HCV may still develop hepatocellular carcinoma even after achieving SVR,” he said.
To look for genetic predictors of this outcome, he and his associates genotyped 958 patients with HCV and advanced hepatic fibrosis from the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) study. This trial had evaluated long-term, low-dose pegylated interferon therapy (90 mcg per week for 3.5 years) as a means of keeping fibrosis from progressing among HCV patients who had failed peginterferon and ribavirin therapy.
A total of 63% of patients had cirrhosis, and 55 (5.7%) developed biopsy or imaging-confirmed hepatocellular carcinoma over a median of 80 months of follow-up, Dr. Njei said. After the researchers controlled for age, sex, Ishak fibrosis score, and SVR status, rs4836493 predicted hepatocellular carcinoma with a highly significant P value of .000004.
This SNP is located on the CHSY3 gene, which plays a role in the chondroitin polymerization, tissue development, and morphogenesis, according to Dr. Njei. Notably, the gene has been implicated in the biology of colorectal tumors, he added.
Dr. Njei and his associates genotyped patients by using the 610-Quad platform, which contains more than 600,000 SNPs. They double-checked results and conducted more genetic analyses using PLINK 1.9, a free, open-source software program for genome-wide association data. Three-quarters of patients in the study were white, 72% were male, and median age at enrollment was 50 years, he noted.
Linking a single SNP to liver cancer despite SVR is a striking finding, but it is also preliminary, Dr. Njei cautioned. “The SNP identified in our discovery genome-wide association study needs future replication and validation in patients who achieve SVR after receiving the new direct-acting antiviral therapies,” he said.
The National Institutes of Health provided partial funding. Dr. Njei and his coinvestigators had no relevant financial conflicts of interest.
AT THE LIVER MEETING 2016
Key clinical point: A single nucleotide polymorphism of the CHSY3 gene predicted liver cancer in patients who successfully completed treatment for chronic hepatitis C virus infection.
Major finding: After researchers controlled for sustained virologic response and other confounders, the rs4836493 variant predicted hepatocellular carcinoma with a P value of .000004.
Data source: A genome-wide association study of 958 HCV patients with advanced hepatic fibrosis from the HALT-C trial.
Disclosures: The National Institutes of Health provided partial funding. Dr. Njei and his coinvestigators had no relevant financial conflicts of interest.
Treatment withdrawal without prior liver biopsy found safe in well-controlled autoimmune hepatitis
BOSTON – Although current guidance calls for a liver biopsy prior to treatment withdrawal in autoimmune hepatitis (AIH), a retrospective observational analysis conducted by researchers from the Cleveland Clinic offers a different view.
“Maybe not everyone needs a liver biopsy before withdrawing treatment,” Yilien Alonso, MD, an internist at the Cleveland Clinic in Weston, Fla., said in an interview at the annual meeting of the American Association for the Study of Liver Diseases.
Both the European Association for Study of the Liver and the AASLD recommend liver biopsy prior to treatment withdrawal in AIH, but the expensive procedure is not without risk of morbidity and mortality. Dr. Alonso and her coinvestigators reviewed the records of 508 AIH patients seen at their institution between January 2001 and April 2015. After excluding the records of patients who’d had juvenile onset of AIH, or who were treated with agents other than corticosteroids and azathioprine, the researchers found 34 adults with similar pretreatment profiles who’d had treatment withdrawal after 2 years of excellent response to treatment, 10 of whom had a liver biopsy prior to treatment withdrawal.
The outcomes at 1 year post treatment withdrawal for all 34 were similar, with no difference in flare rates or reinitiation of treatment. In those who’d had the second liver biopsy, the fibrosis stage was noted at 1 year to have declined in three patients.
“If you have a stable patient population that you are tracking every 3-6 months, we don’t see why you can’t stop the treatment without having to have another invasive procedure,” Dr. Alonso said.
BOSTON – Although current guidance calls for a liver biopsy prior to treatment withdrawal in autoimmune hepatitis (AIH), a retrospective observational analysis conducted by researchers from the Cleveland Clinic offers a different view.
“Maybe not everyone needs a liver biopsy before withdrawing treatment,” Yilien Alonso, MD, an internist at the Cleveland Clinic in Weston, Fla., said in an interview at the annual meeting of the American Association for the Study of Liver Diseases.
Both the European Association for Study of the Liver and the AASLD recommend liver biopsy prior to treatment withdrawal in AIH, but the expensive procedure is not without risk of morbidity and mortality. Dr. Alonso and her coinvestigators reviewed the records of 508 AIH patients seen at their institution between January 2001 and April 2015. After excluding the records of patients who’d had juvenile onset of AIH, or who were treated with agents other than corticosteroids and azathioprine, the researchers found 34 adults with similar pretreatment profiles who’d had treatment withdrawal after 2 years of excellent response to treatment, 10 of whom had a liver biopsy prior to treatment withdrawal.
The outcomes at 1 year post treatment withdrawal for all 34 were similar, with no difference in flare rates or reinitiation of treatment. In those who’d had the second liver biopsy, the fibrosis stage was noted at 1 year to have declined in three patients.
“If you have a stable patient population that you are tracking every 3-6 months, we don’t see why you can’t stop the treatment without having to have another invasive procedure,” Dr. Alonso said.
BOSTON – Although current guidance calls for a liver biopsy prior to treatment withdrawal in autoimmune hepatitis (AIH), a retrospective observational analysis conducted by researchers from the Cleveland Clinic offers a different view.
“Maybe not everyone needs a liver biopsy before withdrawing treatment,” Yilien Alonso, MD, an internist at the Cleveland Clinic in Weston, Fla., said in an interview at the annual meeting of the American Association for the Study of Liver Diseases.
Both the European Association for Study of the Liver and the AASLD recommend liver biopsy prior to treatment withdrawal in AIH, but the expensive procedure is not without risk of morbidity and mortality. Dr. Alonso and her coinvestigators reviewed the records of 508 AIH patients seen at their institution between January 2001 and April 2015. After excluding the records of patients who’d had juvenile onset of AIH, or who were treated with agents other than corticosteroids and azathioprine, the researchers found 34 adults with similar pretreatment profiles who’d had treatment withdrawal after 2 years of excellent response to treatment, 10 of whom had a liver biopsy prior to treatment withdrawal.
The outcomes at 1 year post treatment withdrawal for all 34 were similar, with no difference in flare rates or reinitiation of treatment. In those who’d had the second liver biopsy, the fibrosis stage was noted at 1 year to have declined in three patients.
“If you have a stable patient population that you are tracking every 3-6 months, we don’t see why you can’t stop the treatment without having to have another invasive procedure,” Dr. Alonso said.
AT THE LIVER MEETING 2016
Key clinical point:
Major finding: Flare rates were similar post treatment withdrawal at 1 year in autoimmune hepatitis patients with and without a prior liver biopsy.
Data source: Retrospective observational analysis of 34 adults with well-controlled autoimmune hepatitis given treatment withdrawal with and without liver biopsy in large academic practice.
Disclosures: Dr. Alonso did not have any relevant disclosures.
Medicaid restrictions loosening on access to HCV therapies
BOSTON – State Medicaid programs have begun to loosen restrictions and improve transparency around access to direct-acting antiviral agents to treat hepatitis C virus infection, but inconsistencies are still the norm nationwide.
“Far too many states continue to restrict access in defiance to their obligations under the law,” said Robert Greenwald, clinical professor of law at Harvard University Center for Health Law and Policy Innovation (CHLPI), Boston.
Since 2014, the number of states that do not publish their access criteria for direct-acting antiviral agents (DAA) has dropped from 17 to 7, according to a report from CHLPI and the National Viral Hepatitis Roundtable (NVHR). Also during that period, 16 states relaxed or dropped fibrosis levels to qualify for access, and 7 decreased sobriety restrictions. The number of states that publish prescriber limitations has increased from 28 to 35. Because cost restrictions vary across insurance plans, some patients who need access get it, and others don’t, even if they have coverage, said Mr. Greenwald and Ryan Clary, NVHR executive director, who presented the report at the annual meeting of the American Association for the Study of Liver Diseases.
NVHR is a coalition of advocacy groups and local governmental agencies with interests in HCV, HIV, and infectious diseases. Its sponsors include AbbVie, Gilead Sciences, Merck, Bristol-Myers Squibb, Janssen, OraSure Technologies, Quest Diagnostics, and Walgreens. CHLPI is supported in part by Gilead, BMS, Johnson & Johnson, and ViiV Health Care.
In November 2015, the Centers for Medicaid & Medicare Services issued guidance to states, noting that while the cost of DAAs is prohibitive, states should use “sound clinical judgment” when determining access, and to “not unreasonably restrict coverage.” Varied interpretation of the guidance by state Medicaid directors means there is still a great deal of inconsistency in coverage.
Robert W. Zavoski, MD, Connecticut medical director of social services, noted that his state is making gains on balancing patient and taxpayer interests by emphasizing prevention, curbing reinfection rates, and using predictive modeling to determine the cost of HCV comorbidities.
Aligning incentives between institutions and payers that are based on long-term patient outcomes would mean not just lowered costs, but actual savings said Doug Dieterich, MD, professor of medicine at Mount Sinai Hospital in New York.
“It’s incredibly effective to treat hepatitis C virtually independent of the price of the drug. If patients remain in the same [health insurance] plan for 3-5 years after treatment, then the cost of treatment is very effective because the cost of health care drops precipitously – about 300% per patient – as soon as you cure hepatitis C,” Dr. Dieterich said.
Reducing the cost of treatments does not automatically result in better access, according John McHutchison, MD, executive vice president of clinical research at Gilead Sciences. Gilead’s DAAs (sofosbuvir and ledipasvir/sofosbuvir) were priced to the standard of care; however, miscalculations of demand drove up costs and “blew up” budgets, he said at the meeting.
Rebates to payers, discounts to patients, and the influx of new treatments to market are helping to drive down costs, Dr. McHutchison said, but while industry “wants to develop curative therapies, our system promotes chronic therapies from the financial perspective.”
The situation is compounded by the fact that state Medicaid programs negotiate individually with pharmaceutical companies and do not make their dealings public, said Brian Edlin, MD, chief medical officer for the CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. He called for more transparency regarding actual thresholds for profit and cure so that all restrictions could be dropped, and everyone could benefit. “How can much can we expect people to pay, what can we afford? All of that is out of the public domain,” he said.
Further dismantling of the barriers to care caused by high cost could be in the works if a recent proposal by FDA Commissioner Robert Califf, MD, gains traction. In an editorial published in JAMA, Dr. Califf called for collaboration between federal agencies to hasten and clarify public notification of the necessary criteria for a drug’s approval, coverage, and payment.
In the meantime, Mr. Greenwald said he and other advocates “are putting Medicaid directors on notice.”
BOSTON – State Medicaid programs have begun to loosen restrictions and improve transparency around access to direct-acting antiviral agents to treat hepatitis C virus infection, but inconsistencies are still the norm nationwide.
“Far too many states continue to restrict access in defiance to their obligations under the law,” said Robert Greenwald, clinical professor of law at Harvard University Center for Health Law and Policy Innovation (CHLPI), Boston.
Since 2014, the number of states that do not publish their access criteria for direct-acting antiviral agents (DAA) has dropped from 17 to 7, according to a report from CHLPI and the National Viral Hepatitis Roundtable (NVHR). Also during that period, 16 states relaxed or dropped fibrosis levels to qualify for access, and 7 decreased sobriety restrictions. The number of states that publish prescriber limitations has increased from 28 to 35. Because cost restrictions vary across insurance plans, some patients who need access get it, and others don’t, even if they have coverage, said Mr. Greenwald and Ryan Clary, NVHR executive director, who presented the report at the annual meeting of the American Association for the Study of Liver Diseases.
NVHR is a coalition of advocacy groups and local governmental agencies with interests in HCV, HIV, and infectious diseases. Its sponsors include AbbVie, Gilead Sciences, Merck, Bristol-Myers Squibb, Janssen, OraSure Technologies, Quest Diagnostics, and Walgreens. CHLPI is supported in part by Gilead, BMS, Johnson & Johnson, and ViiV Health Care.
In November 2015, the Centers for Medicaid & Medicare Services issued guidance to states, noting that while the cost of DAAs is prohibitive, states should use “sound clinical judgment” when determining access, and to “not unreasonably restrict coverage.” Varied interpretation of the guidance by state Medicaid directors means there is still a great deal of inconsistency in coverage.
Robert W. Zavoski, MD, Connecticut medical director of social services, noted that his state is making gains on balancing patient and taxpayer interests by emphasizing prevention, curbing reinfection rates, and using predictive modeling to determine the cost of HCV comorbidities.
Aligning incentives between institutions and payers that are based on long-term patient outcomes would mean not just lowered costs, but actual savings said Doug Dieterich, MD, professor of medicine at Mount Sinai Hospital in New York.
“It’s incredibly effective to treat hepatitis C virtually independent of the price of the drug. If patients remain in the same [health insurance] plan for 3-5 years after treatment, then the cost of treatment is very effective because the cost of health care drops precipitously – about 300% per patient – as soon as you cure hepatitis C,” Dr. Dieterich said.
Reducing the cost of treatments does not automatically result in better access, according John McHutchison, MD, executive vice president of clinical research at Gilead Sciences. Gilead’s DAAs (sofosbuvir and ledipasvir/sofosbuvir) were priced to the standard of care; however, miscalculations of demand drove up costs and “blew up” budgets, he said at the meeting.
Rebates to payers, discounts to patients, and the influx of new treatments to market are helping to drive down costs, Dr. McHutchison said, but while industry “wants to develop curative therapies, our system promotes chronic therapies from the financial perspective.”
The situation is compounded by the fact that state Medicaid programs negotiate individually with pharmaceutical companies and do not make their dealings public, said Brian Edlin, MD, chief medical officer for the CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. He called for more transparency regarding actual thresholds for profit and cure so that all restrictions could be dropped, and everyone could benefit. “How can much can we expect people to pay, what can we afford? All of that is out of the public domain,” he said.
Further dismantling of the barriers to care caused by high cost could be in the works if a recent proposal by FDA Commissioner Robert Califf, MD, gains traction. In an editorial published in JAMA, Dr. Califf called for collaboration between federal agencies to hasten and clarify public notification of the necessary criteria for a drug’s approval, coverage, and payment.
In the meantime, Mr. Greenwald said he and other advocates “are putting Medicaid directors on notice.”
BOSTON – State Medicaid programs have begun to loosen restrictions and improve transparency around access to direct-acting antiviral agents to treat hepatitis C virus infection, but inconsistencies are still the norm nationwide.
“Far too many states continue to restrict access in defiance to their obligations under the law,” said Robert Greenwald, clinical professor of law at Harvard University Center for Health Law and Policy Innovation (CHLPI), Boston.
Since 2014, the number of states that do not publish their access criteria for direct-acting antiviral agents (DAA) has dropped from 17 to 7, according to a report from CHLPI and the National Viral Hepatitis Roundtable (NVHR). Also during that period, 16 states relaxed or dropped fibrosis levels to qualify for access, and 7 decreased sobriety restrictions. The number of states that publish prescriber limitations has increased from 28 to 35. Because cost restrictions vary across insurance plans, some patients who need access get it, and others don’t, even if they have coverage, said Mr. Greenwald and Ryan Clary, NVHR executive director, who presented the report at the annual meeting of the American Association for the Study of Liver Diseases.
NVHR is a coalition of advocacy groups and local governmental agencies with interests in HCV, HIV, and infectious diseases. Its sponsors include AbbVie, Gilead Sciences, Merck, Bristol-Myers Squibb, Janssen, OraSure Technologies, Quest Diagnostics, and Walgreens. CHLPI is supported in part by Gilead, BMS, Johnson & Johnson, and ViiV Health Care.
In November 2015, the Centers for Medicaid & Medicare Services issued guidance to states, noting that while the cost of DAAs is prohibitive, states should use “sound clinical judgment” when determining access, and to “not unreasonably restrict coverage.” Varied interpretation of the guidance by state Medicaid directors means there is still a great deal of inconsistency in coverage.
Robert W. Zavoski, MD, Connecticut medical director of social services, noted that his state is making gains on balancing patient and taxpayer interests by emphasizing prevention, curbing reinfection rates, and using predictive modeling to determine the cost of HCV comorbidities.
Aligning incentives between institutions and payers that are based on long-term patient outcomes would mean not just lowered costs, but actual savings said Doug Dieterich, MD, professor of medicine at Mount Sinai Hospital in New York.
“It’s incredibly effective to treat hepatitis C virtually independent of the price of the drug. If patients remain in the same [health insurance] plan for 3-5 years after treatment, then the cost of treatment is very effective because the cost of health care drops precipitously – about 300% per patient – as soon as you cure hepatitis C,” Dr. Dieterich said.
Reducing the cost of treatments does not automatically result in better access, according John McHutchison, MD, executive vice president of clinical research at Gilead Sciences. Gilead’s DAAs (sofosbuvir and ledipasvir/sofosbuvir) were priced to the standard of care; however, miscalculations of demand drove up costs and “blew up” budgets, he said at the meeting.
Rebates to payers, discounts to patients, and the influx of new treatments to market are helping to drive down costs, Dr. McHutchison said, but while industry “wants to develop curative therapies, our system promotes chronic therapies from the financial perspective.”
The situation is compounded by the fact that state Medicaid programs negotiate individually with pharmaceutical companies and do not make their dealings public, said Brian Edlin, MD, chief medical officer for the CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. He called for more transparency regarding actual thresholds for profit and cure so that all restrictions could be dropped, and everyone could benefit. “How can much can we expect people to pay, what can we afford? All of that is out of the public domain,” he said.
Further dismantling of the barriers to care caused by high cost could be in the works if a recent proposal by FDA Commissioner Robert Califf, MD, gains traction. In an editorial published in JAMA, Dr. Califf called for collaboration between federal agencies to hasten and clarify public notification of the necessary criteria for a drug’s approval, coverage, and payment.
In the meantime, Mr. Greenwald said he and other advocates “are putting Medicaid directors on notice.”
VIDEO: Don’t be surprised by weight gain in men after HCV cure
BOSTON – In the new era of direct-acting antiviral (DAA) therapy, physicians will be seeing more and more patients who have achieved a cure of their hepatitis C virus (HCV). Once freed from the burden of a chronic illness, patients feel better and may eat better. Unexpected weight gain and potential associated health effects may be the next set of challenges patients and their physicians will face.
A single-center retrospective study of patients who had achieved sustained virologic response (SVR) after treatment for HCV found a small but significant weight gain in men, but not women. Additionally, according to noninvasive assessments, liver fat increased significantly in men, but not women, after SVR was achieved.
In a study of 63 patients (42 male, 67%) who received DAA treatment for HCV, mean weight gain for men after SVR was 2.8 pounds (range, –26 to +17; P = .0459), and body mass index (BMI) increased by a mean 0.50 kg/m2 (range, –3.6 to +3.33; P = .0176). No significant change was seen for women when pre- and posttreatment measures were compared.
Isaac Wasserman, a medical student at Mount Sinai Medical Center, New York, presented the results of the single-center retrospective study in a poster presentation at the annual meeting of the American Association for the Study of Liver Diseases.
To assess changes in liver fat, Mr. Wasserman and his coinvestigator used results of pre- and posttreatment transient elastography with controlled attenuation parameter (CAP). CAP measures the degree to which the ultrasound signal is attenuated by liver fat, he explained in a video interview.
For men, hepatic steatosis increased by this measure, with CAP measurements up by a mean 18 dB/m (range, –106 to +128, P = .0314). Mr. Wasserman and his colleagues wrote, “The change in liver fat was large enough to push 11% of the cohort (n = 7 of 63) into advanced steatosis (CAP greater than 300 dB/m).” Again, the women studied had no significant posttreatment change in liver fat.
Post-SVR weight gain appeared to be the culprit in the increased fat seen in the posttreatment livers. Mr. Wasserman and his colleagues in the abstract accompanying the presentation, “Changes in weight were positively correlated with changes in liver fat (P = .006).”
Mr. Wasserman said that he and his coinvestigators believe that social, and not biochemical or mechanistic, reasons underlie the weight gain and increased hepatic steatosis. They are planning further investigation of social and economic factors that may underlie the difference seen in this study, and hope to continue and expand data acquisition to validate their findings.
Mr. Wasserman reported no conflicts of interest or outside sources of funding for the study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
BOSTON – In the new era of direct-acting antiviral (DAA) therapy, physicians will be seeing more and more patients who have achieved a cure of their hepatitis C virus (HCV). Once freed from the burden of a chronic illness, patients feel better and may eat better. Unexpected weight gain and potential associated health effects may be the next set of challenges patients and their physicians will face.
A single-center retrospective study of patients who had achieved sustained virologic response (SVR) after treatment for HCV found a small but significant weight gain in men, but not women. Additionally, according to noninvasive assessments, liver fat increased significantly in men, but not women, after SVR was achieved.
In a study of 63 patients (42 male, 67%) who received DAA treatment for HCV, mean weight gain for men after SVR was 2.8 pounds (range, –26 to +17; P = .0459), and body mass index (BMI) increased by a mean 0.50 kg/m2 (range, –3.6 to +3.33; P = .0176). No significant change was seen for women when pre- and posttreatment measures were compared.
Isaac Wasserman, a medical student at Mount Sinai Medical Center, New York, presented the results of the single-center retrospective study in a poster presentation at the annual meeting of the American Association for the Study of Liver Diseases.
To assess changes in liver fat, Mr. Wasserman and his coinvestigator used results of pre- and posttreatment transient elastography with controlled attenuation parameter (CAP). CAP measures the degree to which the ultrasound signal is attenuated by liver fat, he explained in a video interview.
For men, hepatic steatosis increased by this measure, with CAP measurements up by a mean 18 dB/m (range, –106 to +128, P = .0314). Mr. Wasserman and his colleagues wrote, “The change in liver fat was large enough to push 11% of the cohort (n = 7 of 63) into advanced steatosis (CAP greater than 300 dB/m).” Again, the women studied had no significant posttreatment change in liver fat.
Post-SVR weight gain appeared to be the culprit in the increased fat seen in the posttreatment livers. Mr. Wasserman and his colleagues in the abstract accompanying the presentation, “Changes in weight were positively correlated with changes in liver fat (P = .006).”
Mr. Wasserman said that he and his coinvestigators believe that social, and not biochemical or mechanistic, reasons underlie the weight gain and increased hepatic steatosis. They are planning further investigation of social and economic factors that may underlie the difference seen in this study, and hope to continue and expand data acquisition to validate their findings.
Mr. Wasserman reported no conflicts of interest or outside sources of funding for the study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
BOSTON – In the new era of direct-acting antiviral (DAA) therapy, physicians will be seeing more and more patients who have achieved a cure of their hepatitis C virus (HCV). Once freed from the burden of a chronic illness, patients feel better and may eat better. Unexpected weight gain and potential associated health effects may be the next set of challenges patients and their physicians will face.
A single-center retrospective study of patients who had achieved sustained virologic response (SVR) after treatment for HCV found a small but significant weight gain in men, but not women. Additionally, according to noninvasive assessments, liver fat increased significantly in men, but not women, after SVR was achieved.
In a study of 63 patients (42 male, 67%) who received DAA treatment for HCV, mean weight gain for men after SVR was 2.8 pounds (range, –26 to +17; P = .0459), and body mass index (BMI) increased by a mean 0.50 kg/m2 (range, –3.6 to +3.33; P = .0176). No significant change was seen for women when pre- and posttreatment measures were compared.
Isaac Wasserman, a medical student at Mount Sinai Medical Center, New York, presented the results of the single-center retrospective study in a poster presentation at the annual meeting of the American Association for the Study of Liver Diseases.
To assess changes in liver fat, Mr. Wasserman and his coinvestigator used results of pre- and posttreatment transient elastography with controlled attenuation parameter (CAP). CAP measures the degree to which the ultrasound signal is attenuated by liver fat, he explained in a video interview.
For men, hepatic steatosis increased by this measure, with CAP measurements up by a mean 18 dB/m (range, –106 to +128, P = .0314). Mr. Wasserman and his colleagues wrote, “The change in liver fat was large enough to push 11% of the cohort (n = 7 of 63) into advanced steatosis (CAP greater than 300 dB/m).” Again, the women studied had no significant posttreatment change in liver fat.
Post-SVR weight gain appeared to be the culprit in the increased fat seen in the posttreatment livers. Mr. Wasserman and his colleagues in the abstract accompanying the presentation, “Changes in weight were positively correlated with changes in liver fat (P = .006).”
Mr. Wasserman said that he and his coinvestigators believe that social, and not biochemical or mechanistic, reasons underlie the weight gain and increased hepatic steatosis. They are planning further investigation of social and economic factors that may underlie the difference seen in this study, and hope to continue and expand data acquisition to validate their findings.
Mr. Wasserman reported no conflicts of interest or outside sources of funding for the study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
AT THE LIVER MEETING
Lack of health literacy and normal routine implicated in hepatitis B virus treatment nonadherence
BOSTON – Lack of health literacy, lack of routine, and being a nonnative speaker of English were predictors of treatment nonadherence in one-quarter of adults with hepatitis B virus in Australia, according to a study.
“Clinicians don’t know this is happening. We overlook it. Because it’s just one tablet a day, we think it’s quite easy, but when I took up this project, I completely underestimated the complexity of adherence and how many different factors can play into why a patient does or doesn’t adhere,” Suzanne Sheppard-Law, RN, MPH, PhD, a senior research fellow at the University of Technology Sydney, said in an interview about her prize-winning poster presentation at this year’s annual meeting of the American Association for the Study of Liver Diseases.
The findings grew from Dr. Sheppard-Law’s clinical practice where she noticed a trend in some patients whose treatment regimen would lose efficacy over time. When switched to other therapies, the pattern would be repeated. Dr. Sheppard-Law interviewed 29 of these patients in person to see if there were commonalities she could address.
“The deeper I dug, the more it all unfolded before me,” she said. It turned out that patients who’d been endorsing adherence were not. In some cases, patients were skipping their medications for days at a time.
This informal study lead to a more formal one focused on a patient’s level of health literacy. Dr. Sheppard-Law and her colleagues examined factors the World Health Organization says are implicated in adherence, including ones that are social and economic, and others that are related to clinical worker interactions, health systems, individual therapy and condition, and patient considerations. They conducted in-person interviews and worked with the patients as they completed the Newest Vital Sign health literacy survey online.
Just over a fifth of respondents said they followed a regular routine when taking their medication, such as taking it at a certain time every day; however, three-quarters of those surveyed said they didn’t think having a routine made any difference (P less than .001). Half of respondents were prescribed at least one additional daily medication to their antiretroviral pill. A third had no idea what type of medication was prescribed for their hepatitis B.
Whether the person was proficient in English, and the impact this had on perceived communication between the patient and clinician was another factor, as most of the patients in the study were immigrants to Australia who’d been living there, on average, about 19 years. Only 27% of the study group reported that they spoke English at home as their primary language.
“It has to be individually focused, person-centered care, is the conclusion I came to,” Dr. Sheppard-Law said. Although her findings do not indicate a need for more resources in the clinic, she did say that clinicians could help patients by asking them to repeat back to them what they have heard.
“I don’t believe it has to be more resource intense; you just need to be sure the patient understands at the beginning what they need to do. Then you have a better chance [they will adhere],” she said. Because patients with poor health literacy are unlikely to tell their clinician that they do not fully grasp what they are being told about their condition and their treatment, Dr. Sheppard-Law suggested asking patients at the end of their consultation to detail what their routine will be, what they will do if they lose their prescription, what they will do if they run out of medication, and asking if they understand that their medication must be taken daily. “They need to understand it’s not okay to skip a day,” she said. “It’s our responsibility to ensure they know that.”
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
BOSTON – Lack of health literacy, lack of routine, and being a nonnative speaker of English were predictors of treatment nonadherence in one-quarter of adults with hepatitis B virus in Australia, according to a study.
“Clinicians don’t know this is happening. We overlook it. Because it’s just one tablet a day, we think it’s quite easy, but when I took up this project, I completely underestimated the complexity of adherence and how many different factors can play into why a patient does or doesn’t adhere,” Suzanne Sheppard-Law, RN, MPH, PhD, a senior research fellow at the University of Technology Sydney, said in an interview about her prize-winning poster presentation at this year’s annual meeting of the American Association for the Study of Liver Diseases.
The findings grew from Dr. Sheppard-Law’s clinical practice where she noticed a trend in some patients whose treatment regimen would lose efficacy over time. When switched to other therapies, the pattern would be repeated. Dr. Sheppard-Law interviewed 29 of these patients in person to see if there were commonalities she could address.
“The deeper I dug, the more it all unfolded before me,” she said. It turned out that patients who’d been endorsing adherence were not. In some cases, patients were skipping their medications for days at a time.
This informal study lead to a more formal one focused on a patient’s level of health literacy. Dr. Sheppard-Law and her colleagues examined factors the World Health Organization says are implicated in adherence, including ones that are social and economic, and others that are related to clinical worker interactions, health systems, individual therapy and condition, and patient considerations. They conducted in-person interviews and worked with the patients as they completed the Newest Vital Sign health literacy survey online.
Just over a fifth of respondents said they followed a regular routine when taking their medication, such as taking it at a certain time every day; however, three-quarters of those surveyed said they didn’t think having a routine made any difference (P less than .001). Half of respondents were prescribed at least one additional daily medication to their antiretroviral pill. A third had no idea what type of medication was prescribed for their hepatitis B.
Whether the person was proficient in English, and the impact this had on perceived communication between the patient and clinician was another factor, as most of the patients in the study were immigrants to Australia who’d been living there, on average, about 19 years. Only 27% of the study group reported that they spoke English at home as their primary language.
“It has to be individually focused, person-centered care, is the conclusion I came to,” Dr. Sheppard-Law said. Although her findings do not indicate a need for more resources in the clinic, she did say that clinicians could help patients by asking them to repeat back to them what they have heard.
“I don’t believe it has to be more resource intense; you just need to be sure the patient understands at the beginning what they need to do. Then you have a better chance [they will adhere],” she said. Because patients with poor health literacy are unlikely to tell their clinician that they do not fully grasp what they are being told about their condition and their treatment, Dr. Sheppard-Law suggested asking patients at the end of their consultation to detail what their routine will be, what they will do if they lose their prescription, what they will do if they run out of medication, and asking if they understand that their medication must be taken daily. “They need to understand it’s not okay to skip a day,” she said. “It’s our responsibility to ensure they know that.”
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
BOSTON – Lack of health literacy, lack of routine, and being a nonnative speaker of English were predictors of treatment nonadherence in one-quarter of adults with hepatitis B virus in Australia, according to a study.
“Clinicians don’t know this is happening. We overlook it. Because it’s just one tablet a day, we think it’s quite easy, but when I took up this project, I completely underestimated the complexity of adherence and how many different factors can play into why a patient does or doesn’t adhere,” Suzanne Sheppard-Law, RN, MPH, PhD, a senior research fellow at the University of Technology Sydney, said in an interview about her prize-winning poster presentation at this year’s annual meeting of the American Association for the Study of Liver Diseases.
The findings grew from Dr. Sheppard-Law’s clinical practice where she noticed a trend in some patients whose treatment regimen would lose efficacy over time. When switched to other therapies, the pattern would be repeated. Dr. Sheppard-Law interviewed 29 of these patients in person to see if there were commonalities she could address.
“The deeper I dug, the more it all unfolded before me,” she said. It turned out that patients who’d been endorsing adherence were not. In some cases, patients were skipping their medications for days at a time.
This informal study lead to a more formal one focused on a patient’s level of health literacy. Dr. Sheppard-Law and her colleagues examined factors the World Health Organization says are implicated in adherence, including ones that are social and economic, and others that are related to clinical worker interactions, health systems, individual therapy and condition, and patient considerations. They conducted in-person interviews and worked with the patients as they completed the Newest Vital Sign health literacy survey online.
Just over a fifth of respondents said they followed a regular routine when taking their medication, such as taking it at a certain time every day; however, three-quarters of those surveyed said they didn’t think having a routine made any difference (P less than .001). Half of respondents were prescribed at least one additional daily medication to their antiretroviral pill. A third had no idea what type of medication was prescribed for their hepatitis B.
Whether the person was proficient in English, and the impact this had on perceived communication between the patient and clinician was another factor, as most of the patients in the study were immigrants to Australia who’d been living there, on average, about 19 years. Only 27% of the study group reported that they spoke English at home as their primary language.
“It has to be individually focused, person-centered care, is the conclusion I came to,” Dr. Sheppard-Law said. Although her findings do not indicate a need for more resources in the clinic, she did say that clinicians could help patients by asking them to repeat back to them what they have heard.
“I don’t believe it has to be more resource intense; you just need to be sure the patient understands at the beginning what they need to do. Then you have a better chance [they will adhere],” she said. Because patients with poor health literacy are unlikely to tell their clinician that they do not fully grasp what they are being told about their condition and their treatment, Dr. Sheppard-Law suggested asking patients at the end of their consultation to detail what their routine will be, what they will do if they lose their prescription, what they will do if they run out of medication, and asking if they understand that their medication must be taken daily. “They need to understand it’s not okay to skip a day,” she said. “It’s our responsibility to ensure they know that.”
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
AT THE LIVER MEETING 2016
Key clinical point:
Major finding: A quarter of adults with hepatitis B virus were treatment noncompliant in the past 30 days.
Data source: In-person and online survey of 277 adults with hepatitis B virus.
Disclosures: Dr. Sheppard-Law did not have any relevant disclosures.
Study eyed zinc for slowing progression of chronic liver disease
BOSTON – Oral zinc supplementation was associated with maintenance of liver function and suppression of hepatocellular carcinoma in a retrospective cohort study of 267 patients with chronic liver disease.
Additional analyses revealed stepwise inverse relationships between serum zinc levels and rates of de novo liver failure, hepatocellular carcinoma, and death, Atsushi Hosui, MD, PhD, said at the annual meeting of the American Association for the Study of Liver Diseases. No patients stopped zinc therapy because of adverse events, and there were no serious adverse events, although some patients experienced nausea, which can occur with zinc supplementation, noted Dr. Hosui of Osaka-Rosai Hospital, Japan.
To begin exploring hepatic correlates of zinc supplementation, Dr. Hosui and his associates retrospectively studied 267 patients in Japan with chronic liver diseases between 2006 and 2015. They had a median of 40 months of data for each patient. No patient had hepatocellular carcinoma at baseline. In all, 196 patients received zinc supplementation (average baseline zinc level, 51 mcg/dL), while 71 patients did not (62 mcg/dL). These two groups resembled each other in terms of etiologies of liver disease, but the zinc group was significantly older (73.2 vs. 66.4 years; P less than .0001), had a significantly higher average baseline bilirubin level (1.2 vs. 0.8 mg/dL; P less than .0001), and a significantly lower average platelet concentration and prothrombin time. (P less than .0001).
Despite having multiple indicators of worse liver disease, only 9.5% of zinc recipients developed hepatocellular carcinoma over 3 years, compared with 25% of patients in the control group (P = .005). Rates of liver failure and death were similar between the two groups, but sequential blood tests did indicate worsening liver disease among patients who did not receive zinc – their prothrombin times and branched-chain amino acid to tyrosine ratios steadily dropped over 3 years, while those in zinc recipients did not.
Next, the researchers stratified zinc recipients according to their serum zinc levels 6 months after starting supplementation. Notably, 3-year rates of mortality, liver failure, and death were significantly higher among patients whose zinc levels were lower than in patients who achieved higher serum zinc levels. For example, 3-year mortality rates were 28% among patients whose zinc level was 70-89 mcg/dL, versus 0% among patients whose zinc level was at least 90 mcg/dL (P = .02). Similarly, 3-year rates of liver failure were 3.6% among patients whose zinc level was 50-69 mcg/dL, versus 0% among patients whose serum zinc level was at least 70 mcg/dL (P = .03). Finally, over 3 years, hepatocellular carcinoma was diagnosed in 17% of patients whose zinc level was 50-69 mcg/dL, versus only 3.8% of patients whose zinc level was 70-89 mcg/dL.
“We suggest that oral zinc supplementation is effective for maintaining liver function and suppressing the development of hepatocellular carcinoma,” Dr. Hosui concluded. The data support a target serum zinc level of at least 70 mcg/dL to suppress liver-related events, including hepatocellular carcinoma, he added. The researchers are exploring clinical trials of zinc for these outcomes in Japan.
The investigators did not report funding for this study. They reported having no conflicts of interest.
BOSTON – Oral zinc supplementation was associated with maintenance of liver function and suppression of hepatocellular carcinoma in a retrospective cohort study of 267 patients with chronic liver disease.
Additional analyses revealed stepwise inverse relationships between serum zinc levels and rates of de novo liver failure, hepatocellular carcinoma, and death, Atsushi Hosui, MD, PhD, said at the annual meeting of the American Association for the Study of Liver Diseases. No patients stopped zinc therapy because of adverse events, and there were no serious adverse events, although some patients experienced nausea, which can occur with zinc supplementation, noted Dr. Hosui of Osaka-Rosai Hospital, Japan.
To begin exploring hepatic correlates of zinc supplementation, Dr. Hosui and his associates retrospectively studied 267 patients in Japan with chronic liver diseases between 2006 and 2015. They had a median of 40 months of data for each patient. No patient had hepatocellular carcinoma at baseline. In all, 196 patients received zinc supplementation (average baseline zinc level, 51 mcg/dL), while 71 patients did not (62 mcg/dL). These two groups resembled each other in terms of etiologies of liver disease, but the zinc group was significantly older (73.2 vs. 66.4 years; P less than .0001), had a significantly higher average baseline bilirubin level (1.2 vs. 0.8 mg/dL; P less than .0001), and a significantly lower average platelet concentration and prothrombin time. (P less than .0001).
Despite having multiple indicators of worse liver disease, only 9.5% of zinc recipients developed hepatocellular carcinoma over 3 years, compared with 25% of patients in the control group (P = .005). Rates of liver failure and death were similar between the two groups, but sequential blood tests did indicate worsening liver disease among patients who did not receive zinc – their prothrombin times and branched-chain amino acid to tyrosine ratios steadily dropped over 3 years, while those in zinc recipients did not.
Next, the researchers stratified zinc recipients according to their serum zinc levels 6 months after starting supplementation. Notably, 3-year rates of mortality, liver failure, and death were significantly higher among patients whose zinc levels were lower than in patients who achieved higher serum zinc levels. For example, 3-year mortality rates were 28% among patients whose zinc level was 70-89 mcg/dL, versus 0% among patients whose zinc level was at least 90 mcg/dL (P = .02). Similarly, 3-year rates of liver failure were 3.6% among patients whose zinc level was 50-69 mcg/dL, versus 0% among patients whose serum zinc level was at least 70 mcg/dL (P = .03). Finally, over 3 years, hepatocellular carcinoma was diagnosed in 17% of patients whose zinc level was 50-69 mcg/dL, versus only 3.8% of patients whose zinc level was 70-89 mcg/dL.
“We suggest that oral zinc supplementation is effective for maintaining liver function and suppressing the development of hepatocellular carcinoma,” Dr. Hosui concluded. The data support a target serum zinc level of at least 70 mcg/dL to suppress liver-related events, including hepatocellular carcinoma, he added. The researchers are exploring clinical trials of zinc for these outcomes in Japan.
The investigators did not report funding for this study. They reported having no conflicts of interest.
BOSTON – Oral zinc supplementation was associated with maintenance of liver function and suppression of hepatocellular carcinoma in a retrospective cohort study of 267 patients with chronic liver disease.
Additional analyses revealed stepwise inverse relationships between serum zinc levels and rates of de novo liver failure, hepatocellular carcinoma, and death, Atsushi Hosui, MD, PhD, said at the annual meeting of the American Association for the Study of Liver Diseases. No patients stopped zinc therapy because of adverse events, and there were no serious adverse events, although some patients experienced nausea, which can occur with zinc supplementation, noted Dr. Hosui of Osaka-Rosai Hospital, Japan.
To begin exploring hepatic correlates of zinc supplementation, Dr. Hosui and his associates retrospectively studied 267 patients in Japan with chronic liver diseases between 2006 and 2015. They had a median of 40 months of data for each patient. No patient had hepatocellular carcinoma at baseline. In all, 196 patients received zinc supplementation (average baseline zinc level, 51 mcg/dL), while 71 patients did not (62 mcg/dL). These two groups resembled each other in terms of etiologies of liver disease, but the zinc group was significantly older (73.2 vs. 66.4 years; P less than .0001), had a significantly higher average baseline bilirubin level (1.2 vs. 0.8 mg/dL; P less than .0001), and a significantly lower average platelet concentration and prothrombin time. (P less than .0001).
Despite having multiple indicators of worse liver disease, only 9.5% of zinc recipients developed hepatocellular carcinoma over 3 years, compared with 25% of patients in the control group (P = .005). Rates of liver failure and death were similar between the two groups, but sequential blood tests did indicate worsening liver disease among patients who did not receive zinc – their prothrombin times and branched-chain amino acid to tyrosine ratios steadily dropped over 3 years, while those in zinc recipients did not.
Next, the researchers stratified zinc recipients according to their serum zinc levels 6 months after starting supplementation. Notably, 3-year rates of mortality, liver failure, and death were significantly higher among patients whose zinc levels were lower than in patients who achieved higher serum zinc levels. For example, 3-year mortality rates were 28% among patients whose zinc level was 70-89 mcg/dL, versus 0% among patients whose zinc level was at least 90 mcg/dL (P = .02). Similarly, 3-year rates of liver failure were 3.6% among patients whose zinc level was 50-69 mcg/dL, versus 0% among patients whose serum zinc level was at least 70 mcg/dL (P = .03). Finally, over 3 years, hepatocellular carcinoma was diagnosed in 17% of patients whose zinc level was 50-69 mcg/dL, versus only 3.8% of patients whose zinc level was 70-89 mcg/dL.
“We suggest that oral zinc supplementation is effective for maintaining liver function and suppressing the development of hepatocellular carcinoma,” Dr. Hosui concluded. The data support a target serum zinc level of at least 70 mcg/dL to suppress liver-related events, including hepatocellular carcinoma, he added. The researchers are exploring clinical trials of zinc for these outcomes in Japan.
The investigators did not report funding for this study. They reported having no conflicts of interest.
AT THE LIVER MEETING 2016
Key clinical point: Oral zinc sulfate supplementation might help prevent the progression of chronic liver disease and associated hepatocellular carcinoma.
Major finding: Despite having multiple indicators of worse liver disease, only 9.5% of zinc recipients developed hepatocellular carcinoma over 3 years, compared with 25% of patients in the control group (P = .005).
Data source: A retrospective cohort study of 267 patients with chronic liver disease but no hepatocellular carcinoma at baseline.
Disclosures: The investigators did not report funding for this study. They reported having no conflicts of interest.