User login
Nocturnally pruritic rash
A 74-YEAR-OLD WOMAN presented with a 3-day history of an intensely pruritic rash that was localized to her upper arms, upper chest between her breasts, and upper back. The pruritus was much worse at night while the patient was in bed. Symptoms did not improve with over-the-counter topical corticosteroids.
The patient had a history of atrial fibrillation (for which she was receiving chronic anticoagulation therapy), hypertension, an implanted pacemaker, depression, and Parkinson disease. Her medications included carbidopa-levodopa, fluoxetine, hydrochlorothiazide, metoprolol tartrate, naproxen, and warfarin. She had no known allergies. She reported that she was a nonsmoker and drank 1 glass of wine per week.
There were no recent changes in soaps, detergents, lotions, or makeup, nor did the patient have any bug bites or plant exposure. She shared a home with her spouse and several pets: a dog, a cat, and a Bantam-breed chicken. The patient’s husband, who slept in a different bedroom, had no rash. Recently, the cat had been bringing its captured prey of rabbits into the home.
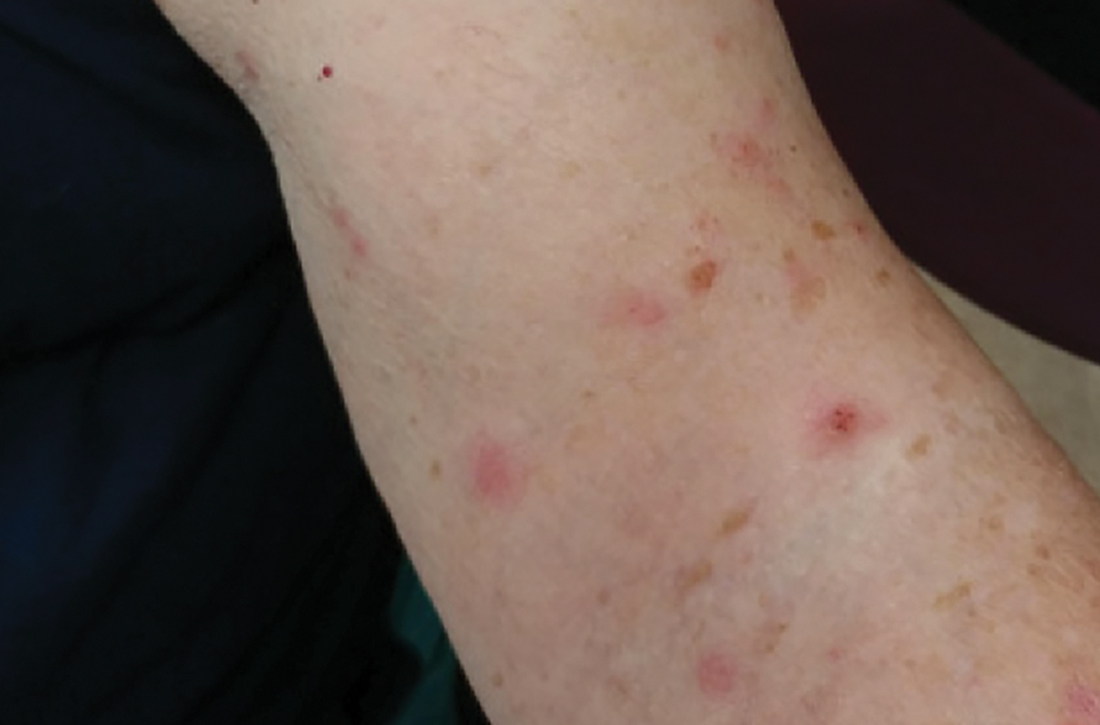
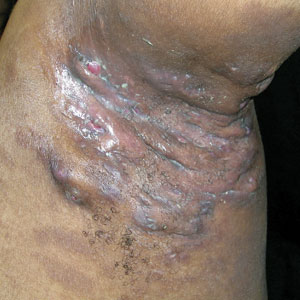
Review of systems was negative for fever, chills, shortness of breath, cough, throat swelling, and rhinorrhea. Physical examination revealed red/pink macules and papules scattered over the upper arms (FIGURE 1), chest, and upper back. Many lesions were excoriated but had no active bleeding or vesicles. Under dermatoscope, no burrowing was found; however, a small (< 1 mm) creature was seen moving rapidly across the skin surface. The physician (CTW) captured and isolated the creature using a sterile lab cup.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gamasoidosis
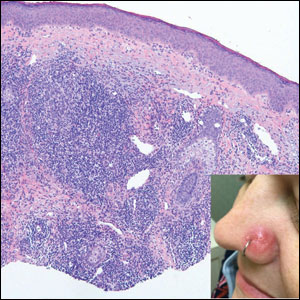
The collected sample (FIGURE 2) was examined and identified as an avian mite by a colleague who specializes in entomology, confirming the diagnosis of gamasoidosis.

Two genera of avian mites are responsible: Dermanyssus and Ornithonyssus. The most common culprits are the red poultry mite (D gallinae) and the northern fowl mite (O bursa). These small mites parasitize birds, such as poultry livestock, domesticated birds, and wild game birds. When unfed, the mite appears translucent brown and measures 0.3 to 0.7 mm in length, but after a blood meal, it appears red and increases in size to 1 mm. The mites tend to be active and feed at night and hide during the day.2 This explained the severe nighttime pruritus in this case.
Human infestation, although infrequent, can be a concern for those who work with poultry, or during the spring and summer seasons when young birds leave their nests and the mites migrate to find alternative hosts.3 The 1- to 2-mm erythematous maculopapules are often found with excoriations in covered areas.3,4 Unlike scabies, the genitalia and interdigital areas are spared.3,5
Differential for arthropod dermatoses
The differential diagnosis includes cimicosis, pulicosis, pediculosis corporis, and scabies.
Cimicosis is caused by bed bugs (from the insect Cimex genus). Bed bugs are oval and reddish brown, have 6 legs, and range in size from 1 to 7 mm. Most bed bugs hide in cracks or crevices of furniture and other surfaces (eg, bed frames, headboards, seams or holes of box springs or mattresses, or behind wallpaper, switch plates, and picture frames) by day and come out at night to feed on a sleeping host. Commonly, bed bugs will leave a series of bites grouped in rows (described as “breakfast, lunch, and dinner”). The bites can mimic urticaria, and bullous reactions may also occur.2
Continue to: Pulicosis
Pulicosis results from bites caused by a variety of flea species including, but not limited to, human, dog, oriental rat, sticktight, mouse, and chicken fleas. Fleas are small brown insects measuring about 2.5 mm in length, with flat sides and long hind legs. Their bites are most often arranged in a zigzag pattern around a host’s legs and waist. Hypersensitivity reactions may appear as papular urticaria, nodules, or bullae.2
Pediculosis corporis is caused by body lice. The adult louse is 2.5 to 3.5 mm in size, has 6 legs, and is a tan to greyish white color.6 Lice live in clothing, lay their eggs within the seams, and obtain blood meals from the host. Symptoms include generalized itching. The erythematous blue- and copper-colored macules, wheals, and lichenification can occur throughout the body, but spare the hands and feet. Secondary impetigo and furunculosis commonly occur.2
Scabies is caused by an oval mite that is ventrally flat, with dorsal spines. The mite is < 0.5 mm in size, appearing as a pinpoint of white. It burrows into its host’s skin, where it lives and lays eggs, causing pruritic papular lesions and ensuing excoriations. The mite burrows with a predilection for the finger web spaces, wrists, axillae, areolae, umbilicus, lower abdomen, genitals, and buttocks.2
Treatment involves a 3-step process
The mainstay of treatment is removal of the infested bird, decontamination of bedding and clothing, and use of oral antihistamines and topical corticosteroids.1,3,5 Bedding and clothing should be washed. Carpets, rugs, and curtains should be vacuumed and the vacuum bag placed in a sealed bag in the freezer for several hours before it can be thrown away. Eggs, larvae, nymphs, and adults are killed at 55 to 60 °F. Because humans are only incidental hosts and mites do not reproduce on them, the use of scabicidal agents, such as permethrin, is controversial.
Our patient was treated with permethrin cream before definitive identification of the mite. Once the mite was identified, the chicken was removed from the home and the patient’s bedding and clothing were decontaminated. The patient continued to apply over-the-counter topical steroids and take oral antihistamines for several more days after the chicken was removed from the home.
ACKNOWLEDGEMENT
The authors would like to acknowledge Patrick Liesch of the University of Wisconsin-Madison’s Department of Entomology, Insect Diagnostic Lab, for his help in identifying the avian mite.
1. Leib AE, Anderson BE. Pruritic dermatitis caused by bird mite infestation. Cutis. 2016;97:E6-E8.
2. Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377. doi: 10.1111/j.1365-2230.2012.04434.x
3. Baselga E, Drolet BA, Esterly NB. Avian mite dermatitis. Pediatrics. 1996;97:743-745.
4. James WD, Elston DM, Treat J, et al, eds. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatolog Treat. 2010;21:319-321. doi: 10.3109/09546630903287437
6. Centers for Disease Control and Prevention. Parasites. Reviewed September 12, 2019. Accessed August 4, 2022. www.cdc.gov/parasites/lice/body/biology.html
A 74-YEAR-OLD WOMAN presented with a 3-day history of an intensely pruritic rash that was localized to her upper arms, upper chest between her breasts, and upper back. The pruritus was much worse at night while the patient was in bed. Symptoms did not improve with over-the-counter topical corticosteroids.
The patient had a history of atrial fibrillation (for which she was receiving chronic anticoagulation therapy), hypertension, an implanted pacemaker, depression, and Parkinson disease. Her medications included carbidopa-levodopa, fluoxetine, hydrochlorothiazide, metoprolol tartrate, naproxen, and warfarin. She had no known allergies. She reported that she was a nonsmoker and drank 1 glass of wine per week.
There were no recent changes in soaps, detergents, lotions, or makeup, nor did the patient have any bug bites or plant exposure. She shared a home with her spouse and several pets: a dog, a cat, and a Bantam-breed chicken. The patient’s husband, who slept in a different bedroom, had no rash. Recently, the cat had been bringing its captured prey of rabbits into the home.
Review of systems was negative for fever, chills, shortness of breath, cough, throat swelling, and rhinorrhea. Physical examination revealed red/pink macules and papules scattered over the upper arms (FIGURE 1), chest, and upper back. Many lesions were excoriated but had no active bleeding or vesicles. Under dermatoscope, no burrowing was found; however, a small (< 1 mm) creature was seen moving rapidly across the skin surface. The physician (CTW) captured and isolated the creature using a sterile lab cup.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gamasoidosis
The collected sample (FIGURE 2) was examined and identified as an avian mite by a colleague who specializes in entomology, confirming the diagnosis of gamasoidosis.

Two genera of avian mites are responsible: Dermanyssus and Ornithonyssus. The most common culprits are the red poultry mite (D gallinae) and the northern fowl mite (O bursa). These small mites parasitize birds, such as poultry livestock, domesticated birds, and wild game birds. When unfed, the mite appears translucent brown and measures 0.3 to 0.7 mm in length, but after a blood meal, it appears red and increases in size to 1 mm. The mites tend to be active and feed at night and hide during the day.2 This explained the severe nighttime pruritus in this case.
Human infestation, although infrequent, can be a concern for those who work with poultry, or during the spring and summer seasons when young birds leave their nests and the mites migrate to find alternative hosts.3 The 1- to 2-mm erythematous maculopapules are often found with excoriations in covered areas.3,4 Unlike scabies, the genitalia and interdigital areas are spared.3,5
Differential for arthropod dermatoses
The differential diagnosis includes cimicosis, pulicosis, pediculosis corporis, and scabies.
Cimicosis is caused by bed bugs (from the insect Cimex genus). Bed bugs are oval and reddish brown, have 6 legs, and range in size from 1 to 7 mm. Most bed bugs hide in cracks or crevices of furniture and other surfaces (eg, bed frames, headboards, seams or holes of box springs or mattresses, or behind wallpaper, switch plates, and picture frames) by day and come out at night to feed on a sleeping host. Commonly, bed bugs will leave a series of bites grouped in rows (described as “breakfast, lunch, and dinner”). The bites can mimic urticaria, and bullous reactions may also occur.2
Continue to: Pulicosis
Pulicosis results from bites caused by a variety of flea species including, but not limited to, human, dog, oriental rat, sticktight, mouse, and chicken fleas. Fleas are small brown insects measuring about 2.5 mm in length, with flat sides and long hind legs. Their bites are most often arranged in a zigzag pattern around a host’s legs and waist. Hypersensitivity reactions may appear as papular urticaria, nodules, or bullae.2
Pediculosis corporis is caused by body lice. The adult louse is 2.5 to 3.5 mm in size, has 6 legs, and is a tan to greyish white color.6 Lice live in clothing, lay their eggs within the seams, and obtain blood meals from the host. Symptoms include generalized itching. The erythematous blue- and copper-colored macules, wheals, and lichenification can occur throughout the body, but spare the hands and feet. Secondary impetigo and furunculosis commonly occur.2
Scabies is caused by an oval mite that is ventrally flat, with dorsal spines. The mite is < 0.5 mm in size, appearing as a pinpoint of white. It burrows into its host’s skin, where it lives and lays eggs, causing pruritic papular lesions and ensuing excoriations. The mite burrows with a predilection for the finger web spaces, wrists, axillae, areolae, umbilicus, lower abdomen, genitals, and buttocks.2
Treatment involves a 3-step process
The mainstay of treatment is removal of the infested bird, decontamination of bedding and clothing, and use of oral antihistamines and topical corticosteroids.1,3,5 Bedding and clothing should be washed. Carpets, rugs, and curtains should be vacuumed and the vacuum bag placed in a sealed bag in the freezer for several hours before it can be thrown away. Eggs, larvae, nymphs, and adults are killed at 55 to 60 °F. Because humans are only incidental hosts and mites do not reproduce on them, the use of scabicidal agents, such as permethrin, is controversial.
Our patient was treated with permethrin cream before definitive identification of the mite. Once the mite was identified, the chicken was removed from the home and the patient’s bedding and clothing were decontaminated. The patient continued to apply over-the-counter topical steroids and take oral antihistamines for several more days after the chicken was removed from the home.
ACKNOWLEDGEMENT
The authors would like to acknowledge Patrick Liesch of the University of Wisconsin-Madison’s Department of Entomology, Insect Diagnostic Lab, for his help in identifying the avian mite.
A 74-YEAR-OLD WOMAN presented with a 3-day history of an intensely pruritic rash that was localized to her upper arms, upper chest between her breasts, and upper back. The pruritus was much worse at night while the patient was in bed. Symptoms did not improve with over-the-counter topical corticosteroids.
The patient had a history of atrial fibrillation (for which she was receiving chronic anticoagulation therapy), hypertension, an implanted pacemaker, depression, and Parkinson disease. Her medications included carbidopa-levodopa, fluoxetine, hydrochlorothiazide, metoprolol tartrate, naproxen, and warfarin. She had no known allergies. She reported that she was a nonsmoker and drank 1 glass of wine per week.
There were no recent changes in soaps, detergents, lotions, or makeup, nor did the patient have any bug bites or plant exposure. She shared a home with her spouse and several pets: a dog, a cat, and a Bantam-breed chicken. The patient’s husband, who slept in a different bedroom, had no rash. Recently, the cat had been bringing its captured prey of rabbits into the home.
Review of systems was negative for fever, chills, shortness of breath, cough, throat swelling, and rhinorrhea. Physical examination revealed red/pink macules and papules scattered over the upper arms (FIGURE 1), chest, and upper back. Many lesions were excoriated but had no active bleeding or vesicles. Under dermatoscope, no burrowing was found; however, a small (< 1 mm) creature was seen moving rapidly across the skin surface. The physician (CTW) captured and isolated the creature using a sterile lab cup.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Gamasoidosis
The collected sample (FIGURE 2) was examined and identified as an avian mite by a colleague who specializes in entomology, confirming the diagnosis of gamasoidosis.

Two genera of avian mites are responsible: Dermanyssus and Ornithonyssus. The most common culprits are the red poultry mite (D gallinae) and the northern fowl mite (O bursa). These small mites parasitize birds, such as poultry livestock, domesticated birds, and wild game birds. When unfed, the mite appears translucent brown and measures 0.3 to 0.7 mm in length, but after a blood meal, it appears red and increases in size to 1 mm. The mites tend to be active and feed at night and hide during the day.2 This explained the severe nighttime pruritus in this case.
Human infestation, although infrequent, can be a concern for those who work with poultry, or during the spring and summer seasons when young birds leave their nests and the mites migrate to find alternative hosts.3 The 1- to 2-mm erythematous maculopapules are often found with excoriations in covered areas.3,4 Unlike scabies, the genitalia and interdigital areas are spared.3,5
Differential for arthropod dermatoses
The differential diagnosis includes cimicosis, pulicosis, pediculosis corporis, and scabies.
Cimicosis is caused by bed bugs (from the insect Cimex genus). Bed bugs are oval and reddish brown, have 6 legs, and range in size from 1 to 7 mm. Most bed bugs hide in cracks or crevices of furniture and other surfaces (eg, bed frames, headboards, seams or holes of box springs or mattresses, or behind wallpaper, switch plates, and picture frames) by day and come out at night to feed on a sleeping host. Commonly, bed bugs will leave a series of bites grouped in rows (described as “breakfast, lunch, and dinner”). The bites can mimic urticaria, and bullous reactions may also occur.2
Continue to: Pulicosis
Pulicosis results from bites caused by a variety of flea species including, but not limited to, human, dog, oriental rat, sticktight, mouse, and chicken fleas. Fleas are small brown insects measuring about 2.5 mm in length, with flat sides and long hind legs. Their bites are most often arranged in a zigzag pattern around a host’s legs and waist. Hypersensitivity reactions may appear as papular urticaria, nodules, or bullae.2
Pediculosis corporis is caused by body lice. The adult louse is 2.5 to 3.5 mm in size, has 6 legs, and is a tan to greyish white color.6 Lice live in clothing, lay their eggs within the seams, and obtain blood meals from the host. Symptoms include generalized itching. The erythematous blue- and copper-colored macules, wheals, and lichenification can occur throughout the body, but spare the hands and feet. Secondary impetigo and furunculosis commonly occur.2
Scabies is caused by an oval mite that is ventrally flat, with dorsal spines. The mite is < 0.5 mm in size, appearing as a pinpoint of white. It burrows into its host’s skin, where it lives and lays eggs, causing pruritic papular lesions and ensuing excoriations. The mite burrows with a predilection for the finger web spaces, wrists, axillae, areolae, umbilicus, lower abdomen, genitals, and buttocks.2
Treatment involves a 3-step process
The mainstay of treatment is removal of the infested bird, decontamination of bedding and clothing, and use of oral antihistamines and topical corticosteroids.1,3,5 Bedding and clothing should be washed. Carpets, rugs, and curtains should be vacuumed and the vacuum bag placed in a sealed bag in the freezer for several hours before it can be thrown away. Eggs, larvae, nymphs, and adults are killed at 55 to 60 °F. Because humans are only incidental hosts and mites do not reproduce on them, the use of scabicidal agents, such as permethrin, is controversial.
Our patient was treated with permethrin cream before definitive identification of the mite. Once the mite was identified, the chicken was removed from the home and the patient’s bedding and clothing were decontaminated. The patient continued to apply over-the-counter topical steroids and take oral antihistamines for several more days after the chicken was removed from the home.
ACKNOWLEDGEMENT
The authors would like to acknowledge Patrick Liesch of the University of Wisconsin-Madison’s Department of Entomology, Insect Diagnostic Lab, for his help in identifying the avian mite.
1. Leib AE, Anderson BE. Pruritic dermatitis caused by bird mite infestation. Cutis. 2016;97:E6-E8.
2. Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377. doi: 10.1111/j.1365-2230.2012.04434.x
3. Baselga E, Drolet BA, Esterly NB. Avian mite dermatitis. Pediatrics. 1996;97:743-745.
4. James WD, Elston DM, Treat J, et al, eds. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatolog Treat. 2010;21:319-321. doi: 10.3109/09546630903287437
6. Centers for Disease Control and Prevention. Parasites. Reviewed September 12, 2019. Accessed August 4, 2022. www.cdc.gov/parasites/lice/body/biology.html
1. Leib AE, Anderson BE. Pruritic dermatitis caused by bird mite infestation. Cutis. 2016;97:E6-E8.
2. Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377. doi: 10.1111/j.1365-2230.2012.04434.x
3. Baselga E, Drolet BA, Esterly NB. Avian mite dermatitis. Pediatrics. 1996;97:743-745.
4. James WD, Elston DM, Treat J, et al, eds. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
Dermanyssus gallinae infestation: an unusual cause of scalp pruritus treated with permethrin shampoo. J Dermatolog Treat. 2010;21:319-321. doi: 10.3109/09546630903287437
6. Centers for Disease Control and Prevention. Parasites. Reviewed September 12, 2019. Accessed August 4, 2022. www.cdc.gov/parasites/lice/body/biology.html
56-year-old man • increased heart rate • weakness • intense sweating • horseradish consumption • Dx?
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; culehmann@gmail.com
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; culehmann@gmail.com
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; culehmann@gmail.com
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
Noncardiac inpatient has acute hypertension: Treat or not?
ILLUSTRATIVE CASE
A 48-year-old man is admitted to your family medicine service for cellulitis after failed outpatient therapy. He has presumed community-acquired methicillin-resistant Staphylococcus aureus infection of the left lower extremity and is receiving intravenous (IV) vancomycin. His BP this morning is 176/98 mm Hg, and the reading from the previous shift was 168/94 mm Hg. He is asymptomatic from this elevated BP. Based on protocol, his nurse is asking about treatment in response to the multiple elevated readings. How should you address the patient’s elevated BP, knowing that you will see him for a transition management appointment in 2 weeks?
Elevated BP is common in the adult inpatient setting. Prevalence estimates range from 25% to > 50%. Many factors can contribute to elevated BP in the acute illness setting, such as pain, anxiety, medication withdrawal, and volume status.2,3
Treatment of elevated BP in outpatients is well researched, with evidence-based guidelines for physicians. That is not the case for treatment of asymptomatic elevated BP in the inpatient setting. Most published guidance on inpatient management of acutely elevated BP recommends IV medications, such as hydralazine or labetalol, although there is limited evidence to support such recommendations. There is minimal evidence for outcomes-based benefit in treating acute elevations of inpatient BP, such as reduced myocardial injury or stroke; however, there is some evidence of adverse outcomes, such as hypotension and prolonged hospital stays.4-8
Although the possibility of intensifying antihypertensive therapy for those with known hypertension or those with presumed “new-onset” hypertension could theoretically lead to improved outcomes over the long term, there is little evidence to support this presumption. Rather, there is evidence that intensification of antihypertensive therapy at discharge is linked to short-term harms. This was demonstrated in a propensity-matched veteran cohort that included 4056 hospitalized older adults with hypertension (mean age, 77 years; 3961 men), equally split between those who received antihypertensive intensification at hospital discharge and those who did not. Within 30 days, patients receiving intensification had a higher risk of readmission (number needed to harm [NNH] = 27) and serious adverse events (NNH = 63).9
The current study aimed to put all these pieces together by quantifying the prevalence of hypertension in hospitalized patients, characterizing clinician response to patients’ acutely elevated BP, and comparing both short- and long-term outcomes in patients treated for acute BP elevations while hospitalized vs those who were not. The study also assessed the potential effects of antihypertensive intensification at discharge.
STUDY SUMMARY
Treatment of acute hypertension was associated with end-organ injury
This retrospective, propensity score–matched cohort study (N = 22,834) evaluated the electronic health records of all adult patients (age > 18 years) admitted to a medicine service with a noncardiovascular diagnosis over a 1-year period at 10 Cleveland Clinic hospitals, with 1 year of follow-up data.
Exclusion criteria included hospitalization for a cardiovascular diagnosis; admission for a cerebrovascular event or acute coronary syndrome within the previous 30 days; pregnancy; length of stay of less than 2 days or more than 14 days; and lack of outpatient medication data. Patients were propensity-score matched using BP, demographic features, comorbidities, hospital shift, and time since admission. Exposure was defined as administration of IV antihypertensive medication or a new class of oral antihypertensive medication.
Continue to: Outcomes were defined...
Outcomes were defined as a temporal association between acute hypertension treatment and subsequent end-organ damage, such as AKI (serum creatinine increase ≥ 0.3 mg/dL or 1.5 × initial value [Acute Kidney Injury Network definition]), myocardial injury (elevated troponin: > 0.029 ng/mL for troponin T; > 0.045 ng/mL for troponin I), and/or stroke (indicated by discharge diagnosis, with confirmation by chart review). Monitored outcomes included stroke and myocardial infarction (MI) within 30 days of discharge and BP control up to 1 year later.
The 22,834 patients had a mean (SD) age of 65.6 (17.9) years; 12,993 (56.9%) were women, and 15,963 (69.9%) were White. Of the 17,821 (78%) who had at least 1 inpatient hypertensive systolic BP (SBP) episode, defined as an SBP ≥ 140 mm Hg, 5904 (33.1%) received a new treatment. Of those receiving a new treatment, 4378 (74.2%) received only oral treatment, and 1516 (25.7%) received at least 1 dose of IV medication with or without oral dosing.
Using the propensity-matched sample (4520 treated for elevated BP matched to 4520 who were not treated), treated patients had higher rates of AKI (10.3% vs 7.9%; P < .001) and myocardial injury (1.2% vs 0.6%; P = .003). When assessed by SBP, nontreatment of BP was still superior up to an SBP of 199 mm Hg. At an SBP of ≥ 200 mm Hg, there was no difference in rates of AKI or MI between the treatment and nontreatment groups. There was no difference in stroke in either cohort, although the overall numbers were quite low.
Patients with and without antihypertensive intensification at discharge had similar rates of MI (0.1% vs 0.2%; P > .99) and stroke (0.5% vs 0.4%; P > .99) in a matched cohort at 30 days post discharge. At 1 year, BP control in the intensification vs no-intensification groups was nearly the same: maximum SBP was 157.2 mm Hg vs 157.8 mm Hg, respectively (P = .54) and maximum diastolic BP was 86.5 mm Hg vs 86.1 mm Hg, respectively (P = .49).
WHAT’S NEW
Previous research is confirmed in a more diverse population
Whereas previous research showed no benefit to intensification of treatment among hospitalized older male patients, this large, retrospective, propensity score–matched cohort study demonstrated the short- and long-term effects of treating acute, asymptomatic BP elevations in a younger, more generalizable population that included women. Regardless of treatment modality, there appeared to be more harm than good from treating these BP elevations.
In addition, the study appears to corroborate previous research showing that intensification of BP treatment at discharge did not lead to better outcomes.9 At the very least, the study makes a reasonable argument that treating acute BP elevations in noncardiac patients in the hospital setting is not beneficial.
CAVEATS
Impact of existing therapy could be underestimated
This study had several important limitations. First, 23% of treated participants were excluded from the propensity analysis without justification from the authors. Additionally, there was no reporting of missing data and how it was managed. The authors’ definition of treatment excluded dose intensification of existing antihypertensive therapy, which would undercount the number of treated patients. However, this could underestimate the actual harms of the acute antihypertensive therapy. The authors also included patients with atrial fibrillation and heart failure in the study population, even though they already may have been taking antihypertensive agents.
CHALLENGES TO IMPLEMENTATION
Potential delays in translating findings to patient care
Although several recent studies have shown the potential benefit of not treating asymptomatic acute BP elevations in inpatients, incorporating that information into electronic health record order sets or clinical decision support, and disseminating it to clinical end users, will take time. In the interim, despite these findings, patients may continue to receive IV or oral medications to treat acute, asymptomatic BP elevations while hospitalized for noncardiac diagnoses.
1. Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352. doi: 10.1001/jamainternmed.2020.7501
2. Jacobs ZG, Najafi N, Fang MC, et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019;14:144-150. doi: 10.12788/jhm.3087
3. Pasik SD, Chiu S, Yang J, et al. Assess before Rx: reducing the overtreatment of asymptomatic blood pressure elevation in the inpatient setting. J Hosp Med. 2019;14:151-156. doi: 10.12788/jhm.3190
4. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5:473-477. doi: 10.1016/j.jash.2011.07.002
5. Gauer R. Severe asymptomatic hypertension: evaluation and treatment. Am Fam Physician. 2017;95:492-500.
6. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11:193-198. doi: 10.1002/jhm.2510
7. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2018;12:7-15. doi: 10.1177/1753944717746613
8. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12:29-33. doi: 10.1111/j.1751-7176.2009.00196.x
9. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536. doi: 10.1001/jamainternmed.2019.3007
ILLUSTRATIVE CASE
A 48-year-old man is admitted to your family medicine service for cellulitis after failed outpatient therapy. He has presumed community-acquired methicillin-resistant Staphylococcus aureus infection of the left lower extremity and is receiving intravenous (IV) vancomycin. His BP this morning is 176/98 mm Hg, and the reading from the previous shift was 168/94 mm Hg. He is asymptomatic from this elevated BP. Based on protocol, his nurse is asking about treatment in response to the multiple elevated readings. How should you address the patient’s elevated BP, knowing that you will see him for a transition management appointment in 2 weeks?
Elevated BP is common in the adult inpatient setting. Prevalence estimates range from 25% to > 50%. Many factors can contribute to elevated BP in the acute illness setting, such as pain, anxiety, medication withdrawal, and volume status.2,3
Treatment of elevated BP in outpatients is well researched, with evidence-based guidelines for physicians. That is not the case for treatment of asymptomatic elevated BP in the inpatient setting. Most published guidance on inpatient management of acutely elevated BP recommends IV medications, such as hydralazine or labetalol, although there is limited evidence to support such recommendations. There is minimal evidence for outcomes-based benefit in treating acute elevations of inpatient BP, such as reduced myocardial injury or stroke; however, there is some evidence of adverse outcomes, such as hypotension and prolonged hospital stays.4-8
Although the possibility of intensifying antihypertensive therapy for those with known hypertension or those with presumed “new-onset” hypertension could theoretically lead to improved outcomes over the long term, there is little evidence to support this presumption. Rather, there is evidence that intensification of antihypertensive therapy at discharge is linked to short-term harms. This was demonstrated in a propensity-matched veteran cohort that included 4056 hospitalized older adults with hypertension (mean age, 77 years; 3961 men), equally split between those who received antihypertensive intensification at hospital discharge and those who did not. Within 30 days, patients receiving intensification had a higher risk of readmission (number needed to harm [NNH] = 27) and serious adverse events (NNH = 63).9
The current study aimed to put all these pieces together by quantifying the prevalence of hypertension in hospitalized patients, characterizing clinician response to patients’ acutely elevated BP, and comparing both short- and long-term outcomes in patients treated for acute BP elevations while hospitalized vs those who were not. The study also assessed the potential effects of antihypertensive intensification at discharge.
STUDY SUMMARY
Treatment of acute hypertension was associated with end-organ injury
This retrospective, propensity score–matched cohort study (N = 22,834) evaluated the electronic health records of all adult patients (age > 18 years) admitted to a medicine service with a noncardiovascular diagnosis over a 1-year period at 10 Cleveland Clinic hospitals, with 1 year of follow-up data.
Exclusion criteria included hospitalization for a cardiovascular diagnosis; admission for a cerebrovascular event or acute coronary syndrome within the previous 30 days; pregnancy; length of stay of less than 2 days or more than 14 days; and lack of outpatient medication data. Patients were propensity-score matched using BP, demographic features, comorbidities, hospital shift, and time since admission. Exposure was defined as administration of IV antihypertensive medication or a new class of oral antihypertensive medication.
Continue to: Outcomes were defined...
Outcomes were defined as a temporal association between acute hypertension treatment and subsequent end-organ damage, such as AKI (serum creatinine increase ≥ 0.3 mg/dL or 1.5 × initial value [Acute Kidney Injury Network definition]), myocardial injury (elevated troponin: > 0.029 ng/mL for troponin T; > 0.045 ng/mL for troponin I), and/or stroke (indicated by discharge diagnosis, with confirmation by chart review). Monitored outcomes included stroke and myocardial infarction (MI) within 30 days of discharge and BP control up to 1 year later.
The 22,834 patients had a mean (SD) age of 65.6 (17.9) years; 12,993 (56.9%) were women, and 15,963 (69.9%) were White. Of the 17,821 (78%) who had at least 1 inpatient hypertensive systolic BP (SBP) episode, defined as an SBP ≥ 140 mm Hg, 5904 (33.1%) received a new treatment. Of those receiving a new treatment, 4378 (74.2%) received only oral treatment, and 1516 (25.7%) received at least 1 dose of IV medication with or without oral dosing.
Using the propensity-matched sample (4520 treated for elevated BP matched to 4520 who were not treated), treated patients had higher rates of AKI (10.3% vs 7.9%; P < .001) and myocardial injury (1.2% vs 0.6%; P = .003). When assessed by SBP, nontreatment of BP was still superior up to an SBP of 199 mm Hg. At an SBP of ≥ 200 mm Hg, there was no difference in rates of AKI or MI between the treatment and nontreatment groups. There was no difference in stroke in either cohort, although the overall numbers were quite low.
Patients with and without antihypertensive intensification at discharge had similar rates of MI (0.1% vs 0.2%; P > .99) and stroke (0.5% vs 0.4%; P > .99) in a matched cohort at 30 days post discharge. At 1 year, BP control in the intensification vs no-intensification groups was nearly the same: maximum SBP was 157.2 mm Hg vs 157.8 mm Hg, respectively (P = .54) and maximum diastolic BP was 86.5 mm Hg vs 86.1 mm Hg, respectively (P = .49).
WHAT’S NEW
Previous research is confirmed in a more diverse population
Whereas previous research showed no benefit to intensification of treatment among hospitalized older male patients, this large, retrospective, propensity score–matched cohort study demonstrated the short- and long-term effects of treating acute, asymptomatic BP elevations in a younger, more generalizable population that included women. Regardless of treatment modality, there appeared to be more harm than good from treating these BP elevations.
In addition, the study appears to corroborate previous research showing that intensification of BP treatment at discharge did not lead to better outcomes.9 At the very least, the study makes a reasonable argument that treating acute BP elevations in noncardiac patients in the hospital setting is not beneficial.
CAVEATS
Impact of existing therapy could be underestimated
This study had several important limitations. First, 23% of treated participants were excluded from the propensity analysis without justification from the authors. Additionally, there was no reporting of missing data and how it was managed. The authors’ definition of treatment excluded dose intensification of existing antihypertensive therapy, which would undercount the number of treated patients. However, this could underestimate the actual harms of the acute antihypertensive therapy. The authors also included patients with atrial fibrillation and heart failure in the study population, even though they already may have been taking antihypertensive agents.
CHALLENGES TO IMPLEMENTATION
Potential delays in translating findings to patient care
Although several recent studies have shown the potential benefit of not treating asymptomatic acute BP elevations in inpatients, incorporating that information into electronic health record order sets or clinical decision support, and disseminating it to clinical end users, will take time. In the interim, despite these findings, patients may continue to receive IV or oral medications to treat acute, asymptomatic BP elevations while hospitalized for noncardiac diagnoses.
ILLUSTRATIVE CASE
A 48-year-old man is admitted to your family medicine service for cellulitis after failed outpatient therapy. He has presumed community-acquired methicillin-resistant Staphylococcus aureus infection of the left lower extremity and is receiving intravenous (IV) vancomycin. His BP this morning is 176/98 mm Hg, and the reading from the previous shift was 168/94 mm Hg. He is asymptomatic from this elevated BP. Based on protocol, his nurse is asking about treatment in response to the multiple elevated readings. How should you address the patient’s elevated BP, knowing that you will see him for a transition management appointment in 2 weeks?
Elevated BP is common in the adult inpatient setting. Prevalence estimates range from 25% to > 50%. Many factors can contribute to elevated BP in the acute illness setting, such as pain, anxiety, medication withdrawal, and volume status.2,3
Treatment of elevated BP in outpatients is well researched, with evidence-based guidelines for physicians. That is not the case for treatment of asymptomatic elevated BP in the inpatient setting. Most published guidance on inpatient management of acutely elevated BP recommends IV medications, such as hydralazine or labetalol, although there is limited evidence to support such recommendations. There is minimal evidence for outcomes-based benefit in treating acute elevations of inpatient BP, such as reduced myocardial injury or stroke; however, there is some evidence of adverse outcomes, such as hypotension and prolonged hospital stays.4-8
Although the possibility of intensifying antihypertensive therapy for those with known hypertension or those with presumed “new-onset” hypertension could theoretically lead to improved outcomes over the long term, there is little evidence to support this presumption. Rather, there is evidence that intensification of antihypertensive therapy at discharge is linked to short-term harms. This was demonstrated in a propensity-matched veteran cohort that included 4056 hospitalized older adults with hypertension (mean age, 77 years; 3961 men), equally split between those who received antihypertensive intensification at hospital discharge and those who did not. Within 30 days, patients receiving intensification had a higher risk of readmission (number needed to harm [NNH] = 27) and serious adverse events (NNH = 63).9
The current study aimed to put all these pieces together by quantifying the prevalence of hypertension in hospitalized patients, characterizing clinician response to patients’ acutely elevated BP, and comparing both short- and long-term outcomes in patients treated for acute BP elevations while hospitalized vs those who were not. The study also assessed the potential effects of antihypertensive intensification at discharge.
STUDY SUMMARY
Treatment of acute hypertension was associated with end-organ injury
This retrospective, propensity score–matched cohort study (N = 22,834) evaluated the electronic health records of all adult patients (age > 18 years) admitted to a medicine service with a noncardiovascular diagnosis over a 1-year period at 10 Cleveland Clinic hospitals, with 1 year of follow-up data.
Exclusion criteria included hospitalization for a cardiovascular diagnosis; admission for a cerebrovascular event or acute coronary syndrome within the previous 30 days; pregnancy; length of stay of less than 2 days or more than 14 days; and lack of outpatient medication data. Patients were propensity-score matched using BP, demographic features, comorbidities, hospital shift, and time since admission. Exposure was defined as administration of IV antihypertensive medication or a new class of oral antihypertensive medication.
Continue to: Outcomes were defined...
Outcomes were defined as a temporal association between acute hypertension treatment and subsequent end-organ damage, such as AKI (serum creatinine increase ≥ 0.3 mg/dL or 1.5 × initial value [Acute Kidney Injury Network definition]), myocardial injury (elevated troponin: > 0.029 ng/mL for troponin T; > 0.045 ng/mL for troponin I), and/or stroke (indicated by discharge diagnosis, with confirmation by chart review). Monitored outcomes included stroke and myocardial infarction (MI) within 30 days of discharge and BP control up to 1 year later.
The 22,834 patients had a mean (SD) age of 65.6 (17.9) years; 12,993 (56.9%) were women, and 15,963 (69.9%) were White. Of the 17,821 (78%) who had at least 1 inpatient hypertensive systolic BP (SBP) episode, defined as an SBP ≥ 140 mm Hg, 5904 (33.1%) received a new treatment. Of those receiving a new treatment, 4378 (74.2%) received only oral treatment, and 1516 (25.7%) received at least 1 dose of IV medication with or without oral dosing.
Using the propensity-matched sample (4520 treated for elevated BP matched to 4520 who were not treated), treated patients had higher rates of AKI (10.3% vs 7.9%; P < .001) and myocardial injury (1.2% vs 0.6%; P = .003). When assessed by SBP, nontreatment of BP was still superior up to an SBP of 199 mm Hg. At an SBP of ≥ 200 mm Hg, there was no difference in rates of AKI or MI between the treatment and nontreatment groups. There was no difference in stroke in either cohort, although the overall numbers were quite low.
Patients with and without antihypertensive intensification at discharge had similar rates of MI (0.1% vs 0.2%; P > .99) and stroke (0.5% vs 0.4%; P > .99) in a matched cohort at 30 days post discharge. At 1 year, BP control in the intensification vs no-intensification groups was nearly the same: maximum SBP was 157.2 mm Hg vs 157.8 mm Hg, respectively (P = .54) and maximum diastolic BP was 86.5 mm Hg vs 86.1 mm Hg, respectively (P = .49).
WHAT’S NEW
Previous research is confirmed in a more diverse population
Whereas previous research showed no benefit to intensification of treatment among hospitalized older male patients, this large, retrospective, propensity score–matched cohort study demonstrated the short- and long-term effects of treating acute, asymptomatic BP elevations in a younger, more generalizable population that included women. Regardless of treatment modality, there appeared to be more harm than good from treating these BP elevations.
In addition, the study appears to corroborate previous research showing that intensification of BP treatment at discharge did not lead to better outcomes.9 At the very least, the study makes a reasonable argument that treating acute BP elevations in noncardiac patients in the hospital setting is not beneficial.
CAVEATS
Impact of existing therapy could be underestimated
This study had several important limitations. First, 23% of treated participants were excluded from the propensity analysis without justification from the authors. Additionally, there was no reporting of missing data and how it was managed. The authors’ definition of treatment excluded dose intensification of existing antihypertensive therapy, which would undercount the number of treated patients. However, this could underestimate the actual harms of the acute antihypertensive therapy. The authors also included patients with atrial fibrillation and heart failure in the study population, even though they already may have been taking antihypertensive agents.
CHALLENGES TO IMPLEMENTATION
Potential delays in translating findings to patient care
Although several recent studies have shown the potential benefit of not treating asymptomatic acute BP elevations in inpatients, incorporating that information into electronic health record order sets or clinical decision support, and disseminating it to clinical end users, will take time. In the interim, despite these findings, patients may continue to receive IV or oral medications to treat acute, asymptomatic BP elevations while hospitalized for noncardiac diagnoses.
1. Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352. doi: 10.1001/jamainternmed.2020.7501
2. Jacobs ZG, Najafi N, Fang MC, et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019;14:144-150. doi: 10.12788/jhm.3087
3. Pasik SD, Chiu S, Yang J, et al. Assess before Rx: reducing the overtreatment of asymptomatic blood pressure elevation in the inpatient setting. J Hosp Med. 2019;14:151-156. doi: 10.12788/jhm.3190
4. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5:473-477. doi: 10.1016/j.jash.2011.07.002
5. Gauer R. Severe asymptomatic hypertension: evaluation and treatment. Am Fam Physician. 2017;95:492-500.
6. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11:193-198. doi: 10.1002/jhm.2510
7. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2018;12:7-15. doi: 10.1177/1753944717746613
8. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12:29-33. doi: 10.1111/j.1751-7176.2009.00196.x
9. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536. doi: 10.1001/jamainternmed.2019.3007
1. Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352. doi: 10.1001/jamainternmed.2020.7501
2. Jacobs ZG, Najafi N, Fang MC, et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019;14:144-150. doi: 10.12788/jhm.3087
3. Pasik SD, Chiu S, Yang J, et al. Assess before Rx: reducing the overtreatment of asymptomatic blood pressure elevation in the inpatient setting. J Hosp Med. 2019;14:151-156. doi: 10.12788/jhm.3190
4. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5:473-477. doi: 10.1016/j.jash.2011.07.002
5. Gauer R. Severe asymptomatic hypertension: evaluation and treatment. Am Fam Physician. 2017;95:492-500.
6. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11:193-198. doi: 10.1002/jhm.2510
7. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2018;12:7-15. doi: 10.1177/1753944717746613
8. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12:29-33. doi: 10.1111/j.1751-7176.2009.00196.x
9. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536. doi: 10.1001/jamainternmed.2019.3007
PRACTICE CHANGER
Manage blood pressure (BP) elevations conservatively in patients admitted for noncardiac diagnoses, as acute hypertension treatment may increase the risk for acute kidney injury (AKI) and myocardial injury.
STRENGTH OF RECOMMENDATION
C: Based on a single, large, retrospective cohort study.1
Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352.
When the public misplaces their trust
Not long ago, the grandmother of my son’s friend died of COVID-19 infection. She was elderly and unvaccinated. Her grandson had no regrets over her unvaccinated status. “Why would she inject poison into her body?” he said, and then expressed a strong opinion that she had died because the hospital physicians refused to give her ivermectin and hydroxychloroquine. My son, wisely, did not push the issue.
Soon thereafter, my personal family physician emailed a newsletter to his patients (me included) with 3 important messages: (1) COVID vaccines were available in the office; (2) He was not going to prescribe hydroxychloroquine, no matter how adamantly it was requested; and (3) He warned against threatening him or his staff with lawsuits or violence over refusal to prescribe any unproven medication.
How, as a country, have we come to this? A sizeable portion of the public trusts the advice of quacks, hacks, and political opportunists over that of the nation’s most expert scientists and physicians. The National Institutes of Health maintains a website with up-to-date recommendations on the use of treatments for COVID-19. They assess the existing evidence and make recommendations for or against a wide array of interventions. (They recommend against the use of both ivermectin and hydroxychloroquine.) The Centers for Disease Control and Prevention publishes extensively about the current knowledge on the safety and efficacy of vaccines. Neither agency is part of a “deep state” or conspiracy. They are comprised of some of the nation’s leading scientists, including physicians, trying to protect the public from disease and foster good health.
Sadly, some physicians have been a source of inaccurate vaccine information; some even prescribe ineffective treatments despite the evidence. These physicians are either letting their politics override their good sense or are improperly assessing the scientific literature, or both. Medical licensing agencies, and specialty certification boards, need to find ways to prevent this—ways that can survive judicial scrutiny and allow for legitimate scientific debate.
I have been tempted to just accept the current situation as the inevitable outcome of social media–fueled tribalism. But when we know that the COVID death rate among the unvaccinated is 9 times that of people who have received a booster dose,1 I can’t sit idly and watch the Internet pundits prevail. Instead, I continue to advise and teach my students to have confidence in trustworthy authorities and websites. Mistakes will be made; corrections will be issued. However, this is not evidence of malintent or incompetence, but rather, the scientific process in action.
I tell my students that one of the biggest challenges facing them and society is to figure out how to stop, or at least minimize the effects of, incorrect information, misleading statements, and outright lies in a society that values free speech. Physicians—young and old alike—must remain committed to communicating factual information to a not-always-receptive audience. And I wish my young colleagues luck; I hope that their passion for family medicine and their insights into social media may be just the combination that’s needed to redirect the public’s trust back to where it belongs during a health care crisis.
1. Fleming-Dutra KE. COVID-19 Epidemiology and Vaccination Rates in the United States. Presented to the Authorization Committee on Immunization Practices, July 19, 2022. Accessed August 9, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/02-COVID-Fleming-Dutra-508.pdf
Not long ago, the grandmother of my son’s friend died of COVID-19 infection. She was elderly and unvaccinated. Her grandson had no regrets over her unvaccinated status. “Why would she inject poison into her body?” he said, and then expressed a strong opinion that she had died because the hospital physicians refused to give her ivermectin and hydroxychloroquine. My son, wisely, did not push the issue.
Soon thereafter, my personal family physician emailed a newsletter to his patients (me included) with 3 important messages: (1) COVID vaccines were available in the office; (2) He was not going to prescribe hydroxychloroquine, no matter how adamantly it was requested; and (3) He warned against threatening him or his staff with lawsuits or violence over refusal to prescribe any unproven medication.
How, as a country, have we come to this? A sizeable portion of the public trusts the advice of quacks, hacks, and political opportunists over that of the nation’s most expert scientists and physicians. The National Institutes of Health maintains a website with up-to-date recommendations on the use of treatments for COVID-19. They assess the existing evidence and make recommendations for or against a wide array of interventions. (They recommend against the use of both ivermectin and hydroxychloroquine.) The Centers for Disease Control and Prevention publishes extensively about the current knowledge on the safety and efficacy of vaccines. Neither agency is part of a “deep state” or conspiracy. They are comprised of some of the nation’s leading scientists, including physicians, trying to protect the public from disease and foster good health.
Sadly, some physicians have been a source of inaccurate vaccine information; some even prescribe ineffective treatments despite the evidence. These physicians are either letting their politics override their good sense or are improperly assessing the scientific literature, or both. Medical licensing agencies, and specialty certification boards, need to find ways to prevent this—ways that can survive judicial scrutiny and allow for legitimate scientific debate.
I have been tempted to just accept the current situation as the inevitable outcome of social media–fueled tribalism. But when we know that the COVID death rate among the unvaccinated is 9 times that of people who have received a booster dose,1 I can’t sit idly and watch the Internet pundits prevail. Instead, I continue to advise and teach my students to have confidence in trustworthy authorities and websites. Mistakes will be made; corrections will be issued. However, this is not evidence of malintent or incompetence, but rather, the scientific process in action.
I tell my students that one of the biggest challenges facing them and society is to figure out how to stop, or at least minimize the effects of, incorrect information, misleading statements, and outright lies in a society that values free speech. Physicians—young and old alike—must remain committed to communicating factual information to a not-always-receptive audience. And I wish my young colleagues luck; I hope that their passion for family medicine and their insights into social media may be just the combination that’s needed to redirect the public’s trust back to where it belongs during a health care crisis.
Not long ago, the grandmother of my son’s friend died of COVID-19 infection. She was elderly and unvaccinated. Her grandson had no regrets over her unvaccinated status. “Why would she inject poison into her body?” he said, and then expressed a strong opinion that she had died because the hospital physicians refused to give her ivermectin and hydroxychloroquine. My son, wisely, did not push the issue.
Soon thereafter, my personal family physician emailed a newsletter to his patients (me included) with 3 important messages: (1) COVID vaccines were available in the office; (2) He was not going to prescribe hydroxychloroquine, no matter how adamantly it was requested; and (3) He warned against threatening him or his staff with lawsuits or violence over refusal to prescribe any unproven medication.
How, as a country, have we come to this? A sizeable portion of the public trusts the advice of quacks, hacks, and political opportunists over that of the nation’s most expert scientists and physicians. The National Institutes of Health maintains a website with up-to-date recommendations on the use of treatments for COVID-19. They assess the existing evidence and make recommendations for or against a wide array of interventions. (They recommend against the use of both ivermectin and hydroxychloroquine.) The Centers for Disease Control and Prevention publishes extensively about the current knowledge on the safety and efficacy of vaccines. Neither agency is part of a “deep state” or conspiracy. They are comprised of some of the nation’s leading scientists, including physicians, trying to protect the public from disease and foster good health.
Sadly, some physicians have been a source of inaccurate vaccine information; some even prescribe ineffective treatments despite the evidence. These physicians are either letting their politics override their good sense or are improperly assessing the scientific literature, or both. Medical licensing agencies, and specialty certification boards, need to find ways to prevent this—ways that can survive judicial scrutiny and allow for legitimate scientific debate.
I have been tempted to just accept the current situation as the inevitable outcome of social media–fueled tribalism. But when we know that the COVID death rate among the unvaccinated is 9 times that of people who have received a booster dose,1 I can’t sit idly and watch the Internet pundits prevail. Instead, I continue to advise and teach my students to have confidence in trustworthy authorities and websites. Mistakes will be made; corrections will be issued. However, this is not evidence of malintent or incompetence, but rather, the scientific process in action.
I tell my students that one of the biggest challenges facing them and society is to figure out how to stop, or at least minimize the effects of, incorrect information, misleading statements, and outright lies in a society that values free speech. Physicians—young and old alike—must remain committed to communicating factual information to a not-always-receptive audience. And I wish my young colleagues luck; I hope that their passion for family medicine and their insights into social media may be just the combination that’s needed to redirect the public’s trust back to where it belongs during a health care crisis.
1. Fleming-Dutra KE. COVID-19 Epidemiology and Vaccination Rates in the United States. Presented to the Authorization Committee on Immunization Practices, July 19, 2022. Accessed August 9, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/02-COVID-Fleming-Dutra-508.pdf
1. Fleming-Dutra KE. COVID-19 Epidemiology and Vaccination Rates in the United States. Presented to the Authorization Committee on Immunization Practices, July 19, 2022. Accessed August 9, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/02-COVID-Fleming-Dutra-508.pdf
COPD inhaler therapy: A path to success
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).
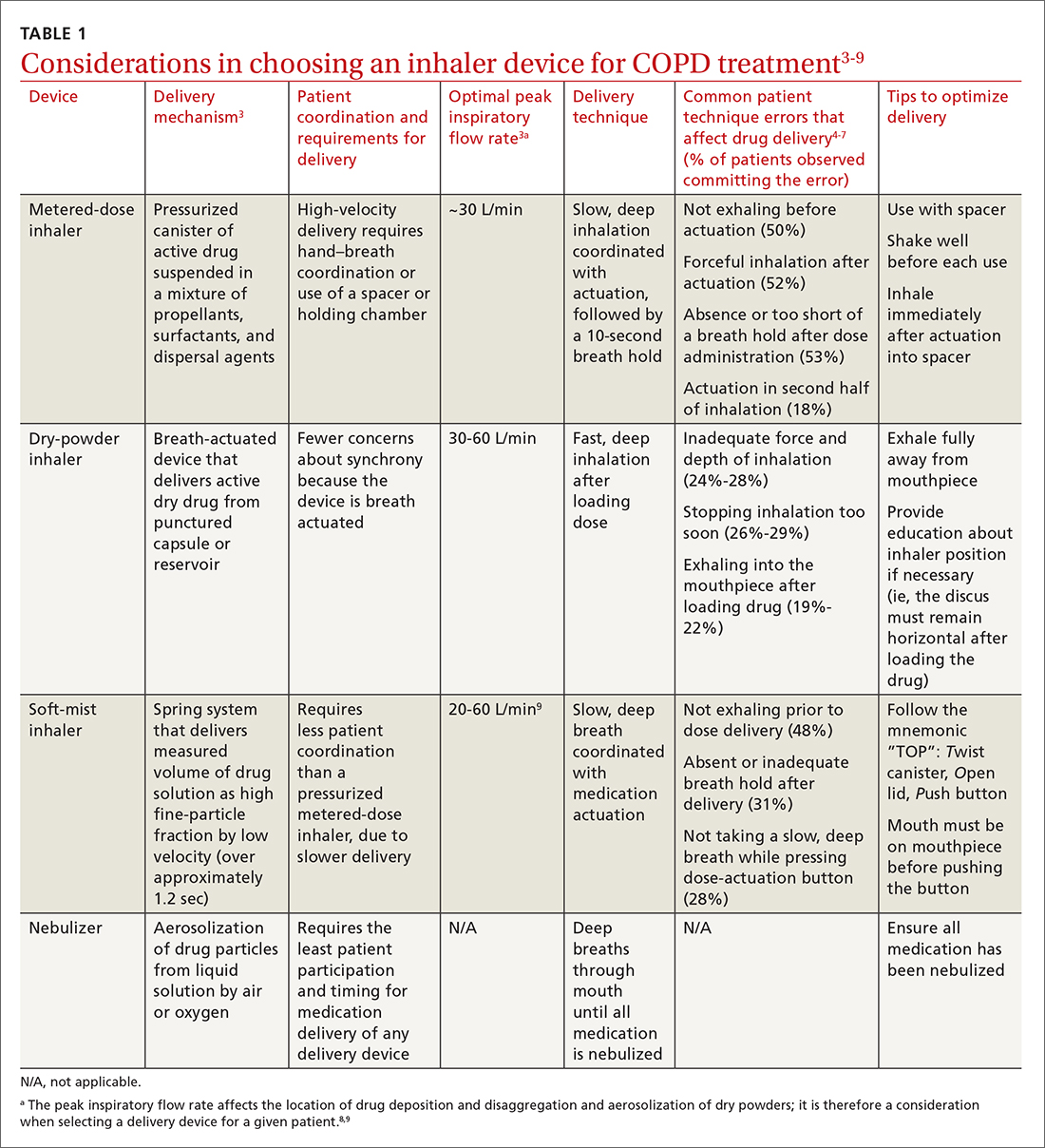
Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.
Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1
Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
awww.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
bwww.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; scalpelandyardstick@gmail.com
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).

Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.


Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1

Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
awww.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
bwww.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; scalpelandyardstick@gmail.com
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).

Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.


Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1

Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
awww.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
bwww.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; scalpelandyardstick@gmail.com
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
PRACTICE RECOMMENDATIONS
› Follow guideline advice that (1) in general, short-acting beta-agonists (SABAs) are not for daily use in stable chronic obstructive pulmonary disease (COPD) but (2) agents in this class of drugs might have a role in relieving occasional COPD-associated dyspnea. C
› Prescribe albuterol over levalbuterol when a SABA is indicated because of the lower cost of albuterol, its comparative efficacy, and its lower incidence of tachycardia and palpitations, even in patients with cardiovascular disease. B
› Avoid the use of an inhaled corticosteroid, or consider withdrawing inhaled corticosteroid therapy, in patients with COPD whose blood eosinophil count is < 100 cells/μL or who have repeated bouts of pneumonia or a history of mycobacterial infection. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The LIVE CHEST Challenge Championship is back!
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
What are we missing when it comes to obstructive sleep apnea and atrial fibrillation?
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Hidradenitis Suppurativa
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).
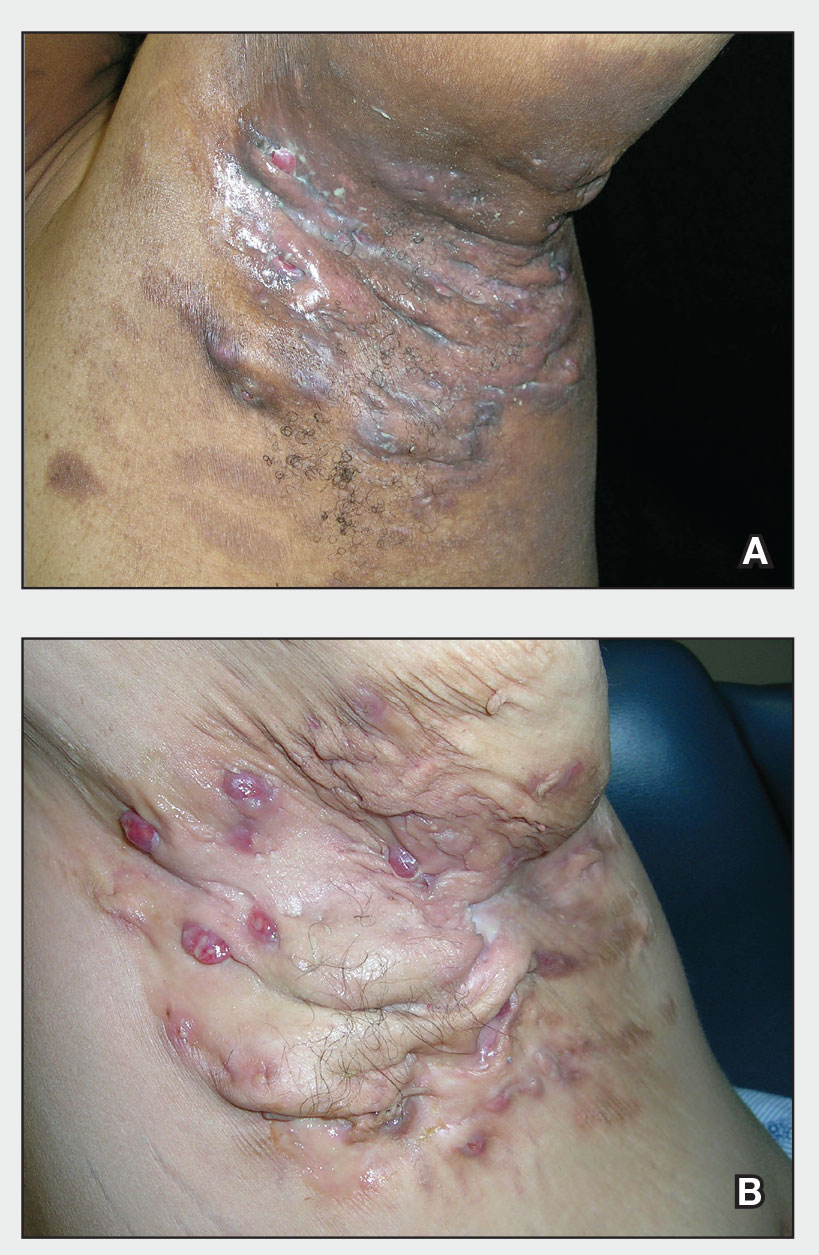
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
Erythematous Papule on the Nasal Ala
The Diagnosis: Cutaneous Lymphoid Hyperplasia
Cutaneous lymphoid hyperplasia (CLH)(also known as pseudolymphoma or lymphocytoma cutis) is a benign inflammatory condition that typically presents as a flesh-colored to erythematous or violaceous papule or nodule on the head or neck. Cutaneous lymphoid hyperplasia may arise in response to an antigenic stimulus, such as an insect bite, infectious agent (eg, Borrelia species), medication, or foreign body (eg, tattoos and piercings).1,2 Given the benign nature and potential for spontaneous resolution, treatment is conservative; however, high-potency topical steroids, cryosurgery, surgical excision, or local radiotherapy may lead to improvement.3 Our patient was started on clobetasol ointment 0.05% and topical tacrolimus 0.1%. After 3 months of use, she reported lesion improvement, but a new lesion appeared on the nose superior to the original. She was offered a steroid injection and liquid nitrogen freezing but was lost to follow-up.
The histopathologic features of CLH are variable and can resemble a cutaneous B- or T-cell lymphoma (quiz images). If there is B-cell predominance, histopathology typically shows a dense dermal infiltrate of lymphocytes admixed with sparse histiocytes, eosinophils, and plasma cells. Multiple germinal-center phenotype lymphoid follicles also may be seen.4 Histopathology of T-cell–predominant CLH commonly shows CD4+ T helper lymphocytes admixed with CD8+ T cells within the dermis with possible papillary dermal edema and red cell extravasation.5 Immunohistochemical stains for CD3, CD4, CD8, and CD20 usually are positive. Most lymphocytes are CD3+ T cells. Admixed clusters of CD20+ B cells may be present.
Angiolymphoid hyperplasia with eosinophilia is a vascular tumor of the skin composed of endothelial cells and inflammatory cells.6,7 The condition presents as single or multiple flesh-colored to purple papules most commonly on the face, scalp, and ears.8 Histologically, lesions appear as well-circumscribed collections of blood vessels composed of plump endothelial cells and an inflammatory infiltrate with lymphocytes and eosinophils (Figure 1A). Endothelial cells also may have an epithelioid appearance.7 Apparent fenestrations—holes within endothelial cells—may be present (Figure 1B). Surgical excision is the preferred treatment of angiolymphoid hyperplasia with eosinophilia. Success with laser and cryosurgery also has been reported.
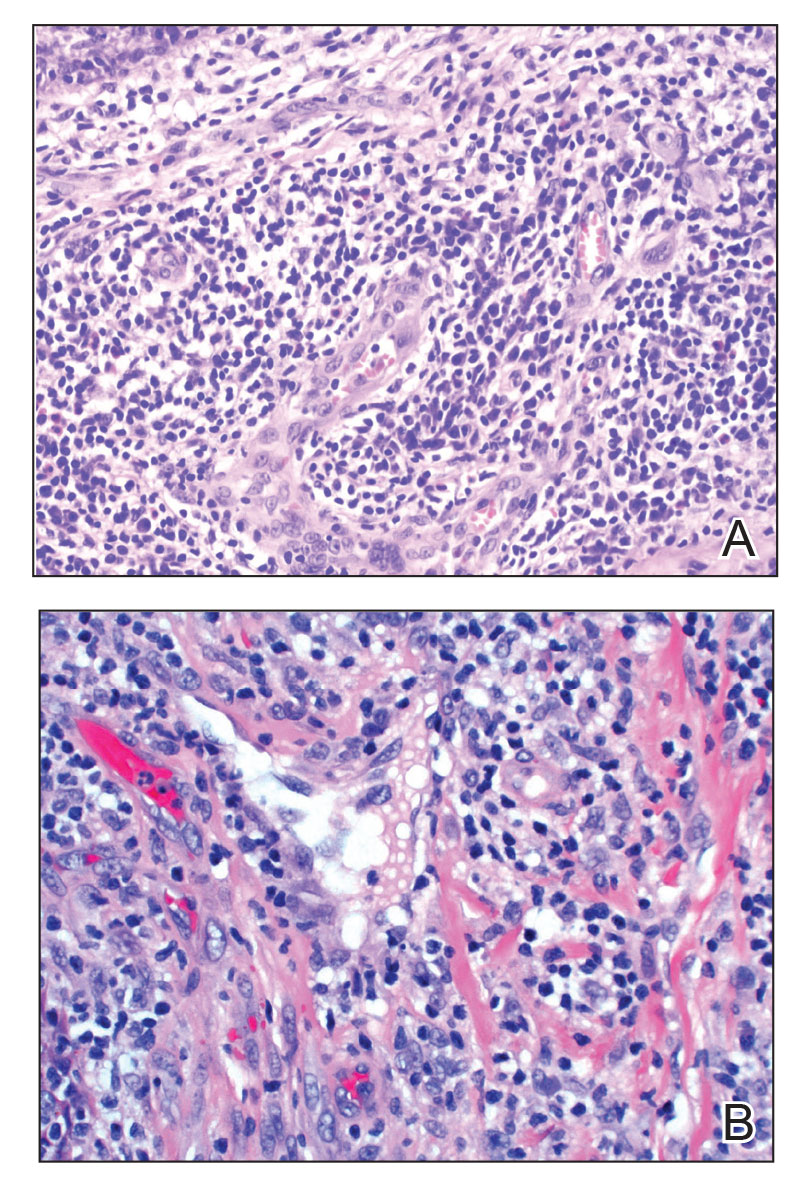
Granuloma faciale typically presents as a solitary redbrown papule or plaque on the face. Linear arborizing vessels and dilated follicular openings with brown globules frequently are seen on dermoscopy.9 Although it may resemble CLH clinically, the histopathology of granuloma faciale is characterized by a perivascular and interstitial dermal infiltrate of numerous eosinophils admixed with lymphocytes, plasma cells, and neutrophils underneath a grenz zone (Figure 2).10 Leukocytoclastic vasculitis may be seen in early lesions, and lesions can show variable angiocentric fibrosis.11 Treatment options include intralesional triamcinolone, topical steroids or calcineurin inhibitors, topical psoralen plus UVA, surgical excision, and laser therapy, but outcomes are variable.12
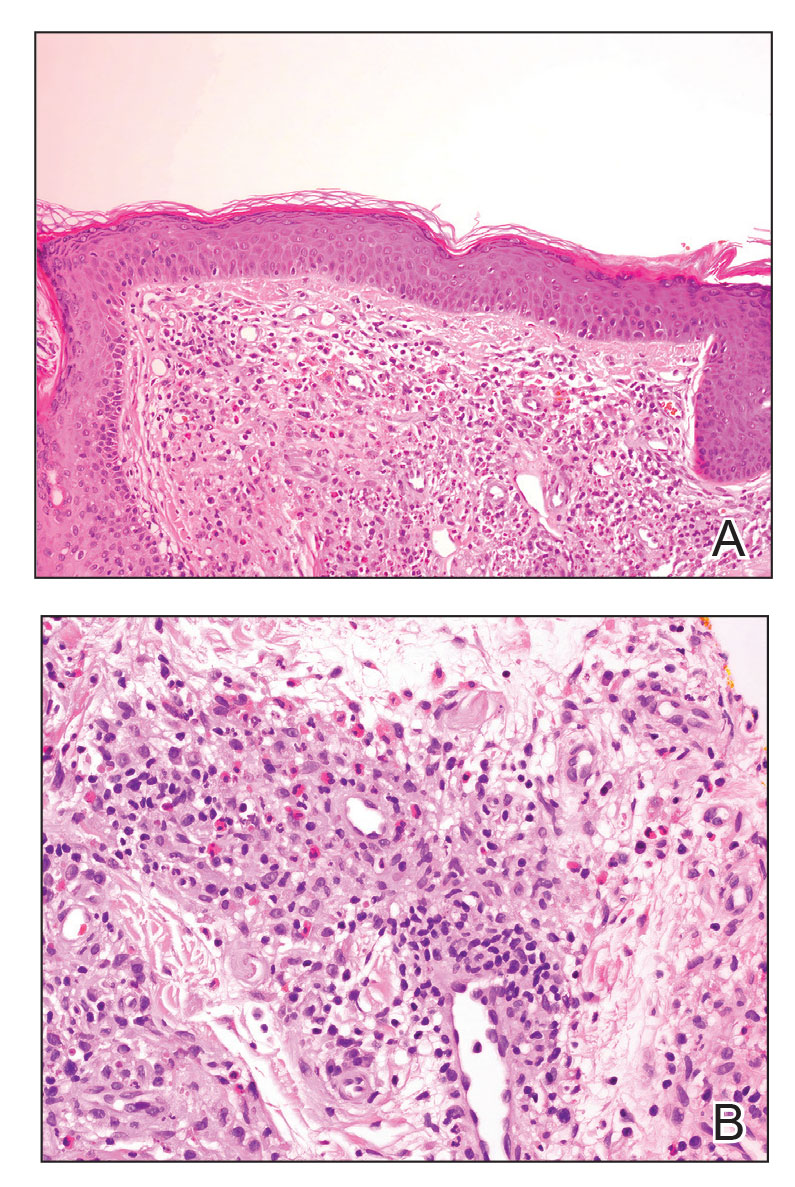
Leukemia cutis is a malignant hematopoietic skin infiltration that presents as multiple pink to red-brown, firm, hemorrhagic papules most frequently involving the head, neck, and trunk.13 Rarely, lesions of leukemia cutis may present as ulcers or bullae. Most lesions occur at presentation of systemic leukemia or in the setting of established leukemia. The cutaneous involvement portends a poor prognosis, strongly correlating with additional extramedullary leukemic involvement.14 Histologic features vary based on the specific type of leukemia (eg, acute myelogenous leukemia). Generally, neoplastic infiltration of the dermis and subcutaneous tissue in a nodular, diffuse, perivascular, or interstitial pattern is seen (Figure 3).15 Leukemia cutis typically resolves after successful treatment of the underlying leukemia.
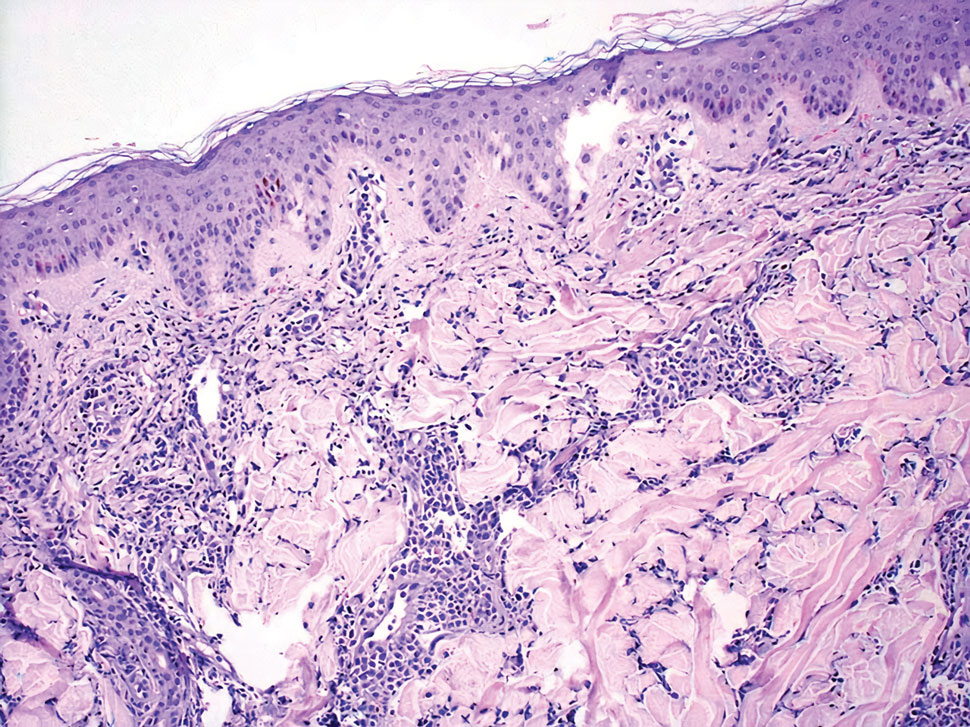
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma. In its early stages, MF presents as erythematous, brown, scaly patches and plaques. With progression to the tumor stage of disease, clonal expansion of CD4+ T cells leads to the development of purple papules and nodules.16 Microscopic findings of MF are dependent on the stage of disease. Early patch lesions show superficial or lichenoid lymphocytic infiltration of the epidermal basal layer.17 In the plaque stage, dermal infiltrates and epidermotropism become more pronounced, with increased atypical lymphocytes with cerebriform nuclei and interspersed inflammatory cells (Figure 4). In the tumor stage, lymphocytic infiltrates may involve the entirety of the dermis or extend into the subcutaneous tissue, and malignant cells become larger in size.17 Mycosis fungoides lesions typically stain positive for helper T-cell markers with a minority staining positive for CD8.
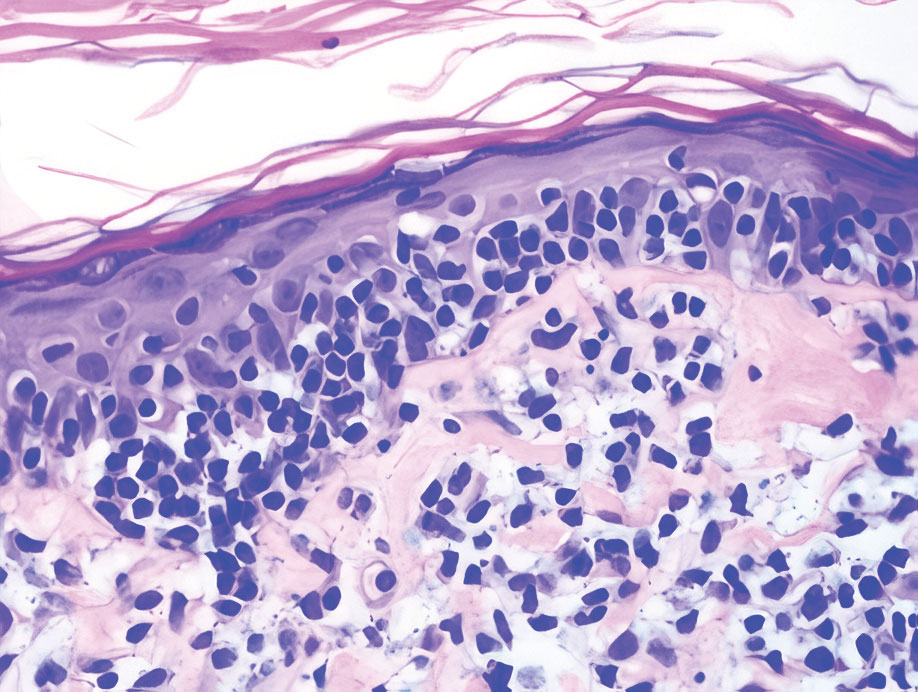
- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190:E398.
- Lackey JN, Xia Y, Cho S, et al. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis. 2007;79:445-448.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244, vii.
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol. 2005;36:505-511.
- Bergman R, Khamaysi Z, Sahar D, et al. Cutaneous lymphoid hyperplasia presenting as a solitary facial nodule: clinical, histopathological, immunophenotypical, and molecular studies. Arch Dermatol. 2006;142:1561-1566.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139:683-686.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781-796.
- Lallas A, Sidiropoulos T, Lefaki I, et al. Photo letter to the editor: dermoscopy of granuloma faciale. J Dermatol Case Rep. 2012;6:59-60.
- Oliveira CC, Ianhez PE, Marques SA, et al. Granuloma faciale: clinical, morphological and immunohistochemical aspects in a series of 10 patients. An Bras Dermatol. 2016;91:803-807.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Derm Venereol. 2018;98:14-18.
- Haidari W, Strowd LC. Clinical characterization of leukemia cutis presentation. Cutis. 2019;104:326-330; E3.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Desch JK, Smoller BR. The spectrum of cutaneous disease in leukemias. J Cutan Pathol. 1993;20:407-410.
- Yamashita T, Abbade LP, Marques ME, et al. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-828; quiz 829-830.
- Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
The Diagnosis: Cutaneous Lymphoid Hyperplasia
Cutaneous lymphoid hyperplasia (CLH)(also known as pseudolymphoma or lymphocytoma cutis) is a benign inflammatory condition that typically presents as a flesh-colored to erythematous or violaceous papule or nodule on the head or neck. Cutaneous lymphoid hyperplasia may arise in response to an antigenic stimulus, such as an insect bite, infectious agent (eg, Borrelia species), medication, or foreign body (eg, tattoos and piercings).1,2 Given the benign nature and potential for spontaneous resolution, treatment is conservative; however, high-potency topical steroids, cryosurgery, surgical excision, or local radiotherapy may lead to improvement.3 Our patient was started on clobetasol ointment 0.05% and topical tacrolimus 0.1%. After 3 months of use, she reported lesion improvement, but a new lesion appeared on the nose superior to the original. She was offered a steroid injection and liquid nitrogen freezing but was lost to follow-up.
The histopathologic features of CLH are variable and can resemble a cutaneous B- or T-cell lymphoma (quiz images). If there is B-cell predominance, histopathology typically shows a dense dermal infiltrate of lymphocytes admixed with sparse histiocytes, eosinophils, and plasma cells. Multiple germinal-center phenotype lymphoid follicles also may be seen.4 Histopathology of T-cell–predominant CLH commonly shows CD4+ T helper lymphocytes admixed with CD8+ T cells within the dermis with possible papillary dermal edema and red cell extravasation.5 Immunohistochemical stains for CD3, CD4, CD8, and CD20 usually are positive. Most lymphocytes are CD3+ T cells. Admixed clusters of CD20+ B cells may be present.
Angiolymphoid hyperplasia with eosinophilia is a vascular tumor of the skin composed of endothelial cells and inflammatory cells.6,7 The condition presents as single or multiple flesh-colored to purple papules most commonly on the face, scalp, and ears.8 Histologically, lesions appear as well-circumscribed collections of blood vessels composed of plump endothelial cells and an inflammatory infiltrate with lymphocytes and eosinophils (Figure 1A). Endothelial cells also may have an epithelioid appearance.7 Apparent fenestrations—holes within endothelial cells—may be present (Figure 1B). Surgical excision is the preferred treatment of angiolymphoid hyperplasia with eosinophilia. Success with laser and cryosurgery also has been reported.

Granuloma faciale typically presents as a solitary redbrown papule or plaque on the face. Linear arborizing vessels and dilated follicular openings with brown globules frequently are seen on dermoscopy.9 Although it may resemble CLH clinically, the histopathology of granuloma faciale is characterized by a perivascular and interstitial dermal infiltrate of numerous eosinophils admixed with lymphocytes, plasma cells, and neutrophils underneath a grenz zone (Figure 2).10 Leukocytoclastic vasculitis may be seen in early lesions, and lesions can show variable angiocentric fibrosis.11 Treatment options include intralesional triamcinolone, topical steroids or calcineurin inhibitors, topical psoralen plus UVA, surgical excision, and laser therapy, but outcomes are variable.12

Leukemia cutis is a malignant hematopoietic skin infiltration that presents as multiple pink to red-brown, firm, hemorrhagic papules most frequently involving the head, neck, and trunk.13 Rarely, lesions of leukemia cutis may present as ulcers or bullae. Most lesions occur at presentation of systemic leukemia or in the setting of established leukemia. The cutaneous involvement portends a poor prognosis, strongly correlating with additional extramedullary leukemic involvement.14 Histologic features vary based on the specific type of leukemia (eg, acute myelogenous leukemia). Generally, neoplastic infiltration of the dermis and subcutaneous tissue in a nodular, diffuse, perivascular, or interstitial pattern is seen (Figure 3).15 Leukemia cutis typically resolves after successful treatment of the underlying leukemia.

Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma. In its early stages, MF presents as erythematous, brown, scaly patches and plaques. With progression to the tumor stage of disease, clonal expansion of CD4+ T cells leads to the development of purple papules and nodules.16 Microscopic findings of MF are dependent on the stage of disease. Early patch lesions show superficial or lichenoid lymphocytic infiltration of the epidermal basal layer.17 In the plaque stage, dermal infiltrates and epidermotropism become more pronounced, with increased atypical lymphocytes with cerebriform nuclei and interspersed inflammatory cells (Figure 4). In the tumor stage, lymphocytic infiltrates may involve the entirety of the dermis or extend into the subcutaneous tissue, and malignant cells become larger in size.17 Mycosis fungoides lesions typically stain positive for helper T-cell markers with a minority staining positive for CD8.

The Diagnosis: Cutaneous Lymphoid Hyperplasia
Cutaneous lymphoid hyperplasia (CLH)(also known as pseudolymphoma or lymphocytoma cutis) is a benign inflammatory condition that typically presents as a flesh-colored to erythematous or violaceous papule or nodule on the head or neck. Cutaneous lymphoid hyperplasia may arise in response to an antigenic stimulus, such as an insect bite, infectious agent (eg, Borrelia species), medication, or foreign body (eg, tattoos and piercings).1,2 Given the benign nature and potential for spontaneous resolution, treatment is conservative; however, high-potency topical steroids, cryosurgery, surgical excision, or local radiotherapy may lead to improvement.3 Our patient was started on clobetasol ointment 0.05% and topical tacrolimus 0.1%. After 3 months of use, she reported lesion improvement, but a new lesion appeared on the nose superior to the original. She was offered a steroid injection and liquid nitrogen freezing but was lost to follow-up.
The histopathologic features of CLH are variable and can resemble a cutaneous B- or T-cell lymphoma (quiz images). If there is B-cell predominance, histopathology typically shows a dense dermal infiltrate of lymphocytes admixed with sparse histiocytes, eosinophils, and plasma cells. Multiple germinal-center phenotype lymphoid follicles also may be seen.4 Histopathology of T-cell–predominant CLH commonly shows CD4+ T helper lymphocytes admixed with CD8+ T cells within the dermis with possible papillary dermal edema and red cell extravasation.5 Immunohistochemical stains for CD3, CD4, CD8, and CD20 usually are positive. Most lymphocytes are CD3+ T cells. Admixed clusters of CD20+ B cells may be present.
Angiolymphoid hyperplasia with eosinophilia is a vascular tumor of the skin composed of endothelial cells and inflammatory cells.6,7 The condition presents as single or multiple flesh-colored to purple papules most commonly on the face, scalp, and ears.8 Histologically, lesions appear as well-circumscribed collections of blood vessels composed of plump endothelial cells and an inflammatory infiltrate with lymphocytes and eosinophils (Figure 1A). Endothelial cells also may have an epithelioid appearance.7 Apparent fenestrations—holes within endothelial cells—may be present (Figure 1B). Surgical excision is the preferred treatment of angiolymphoid hyperplasia with eosinophilia. Success with laser and cryosurgery also has been reported.

Granuloma faciale typically presents as a solitary redbrown papule or plaque on the face. Linear arborizing vessels and dilated follicular openings with brown globules frequently are seen on dermoscopy.9 Although it may resemble CLH clinically, the histopathology of granuloma faciale is characterized by a perivascular and interstitial dermal infiltrate of numerous eosinophils admixed with lymphocytes, plasma cells, and neutrophils underneath a grenz zone (Figure 2).10 Leukocytoclastic vasculitis may be seen in early lesions, and lesions can show variable angiocentric fibrosis.11 Treatment options include intralesional triamcinolone, topical steroids or calcineurin inhibitors, topical psoralen plus UVA, surgical excision, and laser therapy, but outcomes are variable.12

Leukemia cutis is a malignant hematopoietic skin infiltration that presents as multiple pink to red-brown, firm, hemorrhagic papules most frequently involving the head, neck, and trunk.13 Rarely, lesions of leukemia cutis may present as ulcers or bullae. Most lesions occur at presentation of systemic leukemia or in the setting of established leukemia. The cutaneous involvement portends a poor prognosis, strongly correlating with additional extramedullary leukemic involvement.14 Histologic features vary based on the specific type of leukemia (eg, acute myelogenous leukemia). Generally, neoplastic infiltration of the dermis and subcutaneous tissue in a nodular, diffuse, perivascular, or interstitial pattern is seen (Figure 3).15 Leukemia cutis typically resolves after successful treatment of the underlying leukemia.

Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma. In its early stages, MF presents as erythematous, brown, scaly patches and plaques. With progression to the tumor stage of disease, clonal expansion of CD4+ T cells leads to the development of purple papules and nodules.16 Microscopic findings of MF are dependent on the stage of disease. Early patch lesions show superficial or lichenoid lymphocytic infiltration of the epidermal basal layer.17 In the plaque stage, dermal infiltrates and epidermotropism become more pronounced, with increased atypical lymphocytes with cerebriform nuclei and interspersed inflammatory cells (Figure 4). In the tumor stage, lymphocytic infiltrates may involve the entirety of the dermis or extend into the subcutaneous tissue, and malignant cells become larger in size.17 Mycosis fungoides lesions typically stain positive for helper T-cell markers with a minority staining positive for CD8.

- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190:E398.
- Lackey JN, Xia Y, Cho S, et al. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis. 2007;79:445-448.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244, vii.
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol. 2005;36:505-511.
- Bergman R, Khamaysi Z, Sahar D, et al. Cutaneous lymphoid hyperplasia presenting as a solitary facial nodule: clinical, histopathological, immunophenotypical, and molecular studies. Arch Dermatol. 2006;142:1561-1566.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139:683-686.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781-796.
- Lallas A, Sidiropoulos T, Lefaki I, et al. Photo letter to the editor: dermoscopy of granuloma faciale. J Dermatol Case Rep. 2012;6:59-60.
- Oliveira CC, Ianhez PE, Marques SA, et al. Granuloma faciale: clinical, morphological and immunohistochemical aspects in a series of 10 patients. An Bras Dermatol. 2016;91:803-807.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Derm Venereol. 2018;98:14-18.
- Haidari W, Strowd LC. Clinical characterization of leukemia cutis presentation. Cutis. 2019;104:326-330; E3.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Desch JK, Smoller BR. The spectrum of cutaneous disease in leukemias. J Cutan Pathol. 1993;20:407-410.
- Yamashita T, Abbade LP, Marques ME, et al. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-828; quiz 829-830.
- Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190:E398.
- Lackey JN, Xia Y, Cho S, et al. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis. 2007;79:445-448.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244, vii.
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol. 2005;36:505-511.
- Bergman R, Khamaysi Z, Sahar D, et al. Cutaneous lymphoid hyperplasia presenting as a solitary facial nodule: clinical, histopathological, immunophenotypical, and molecular studies. Arch Dermatol. 2006;142:1561-1566.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139:683-686.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781-796.
- Lallas A, Sidiropoulos T, Lefaki I, et al. Photo letter to the editor: dermoscopy of granuloma faciale. J Dermatol Case Rep. 2012;6:59-60.
- Oliveira CC, Ianhez PE, Marques SA, et al. Granuloma faciale: clinical, morphological and immunohistochemical aspects in a series of 10 patients. An Bras Dermatol. 2016;91:803-807.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Derm Venereol. 2018;98:14-18.
- Haidari W, Strowd LC. Clinical characterization of leukemia cutis presentation. Cutis. 2019;104:326-330; E3.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Desch JK, Smoller BR. The spectrum of cutaneous disease in leukemias. J Cutan Pathol. 1993;20:407-410.
- Yamashita T, Abbade LP, Marques ME, et al. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-828; quiz 829-830.
- Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
A 35-year-old woman presented with a slowly growing, smooth, erythematous papule of 2 months’ duration on the left nasal ala surrounding a piercing (top, inset) that had been performed 4 years prior. A tangential biopsy was obtained for histopathologic evaluation.
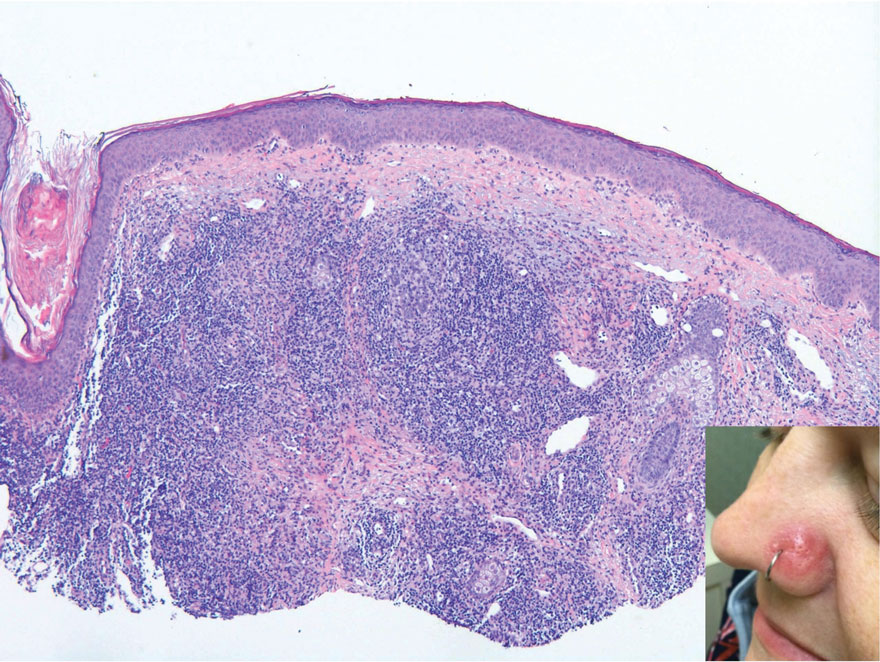
Novel study offers clues to sex bias in lupus
Systemic lupus erythematosus (SLE), or lupus, shows a marked sex bias, affecting about nine females for every one male, according to Susan Kovats, PhD, who studies sex differences in immunity at the Oklahoma Medical Research Foundation in Oklahoma City. This characteristic of lupus suggests that hormones are involved in the pathogenesis of the disease. It also suggests, Dr. Kovats said, that the X chromosome might play a role.
Though studies since the 1970s have indicated a significant role for hormones, the issue is still complex and not well understood, and relatively little research has been done on the molecular mechanisms that might be responsible. This may be because of difficulties with influencing the immune system in vitro, said George A. Robinson, PhD, of University College London’s Centre for Rheumatology.
But Dr. Robinson and his team found a unique way of investigating the role of sex chromosomes and hormones in the inflammatory profiles across subjects of different sex, gender, age, and disease status. In research published online in The Lancet Rheumatology, Dr. Robinson and his team looked at immune cells taken from both cisgender men and women and transgender men and women, and thus were able to “get a more physiological view of what sex hormones are doing to the immune system,” he said.
Dr. Kovats agreed that it was a useful approach. “The transgender people provided an opportunity to effectively separate sex hormone levels from chromosome content,” she said in an interview.
Methods and findings
Peripheral blood mononuclear cell (PBMC) samples were taken from cisgender individuals with and without juvenile-onset lupus and assessed for 28 immune-cell subsets, including different T-cell, B-cell, and monotype subsets. Subjects included 39 postpubertal cisgender men and women (17 men and 22 women) who did not have juvenile-onset lupus, and 35 postpubertal cisgender men and women (12 men and 23 women) who did have juvenile onset lupus. All were aged 16-25 years. The transgender group included five transgender men and five transgender women (aged 18-19) who were undergoing gender-affirming sex hormone treatment.
The analysis found that one of the key differences between young postpubertal cisgender men and age-matched cisgender women was that the men had significantly elevated frequencies of regulatory T cells (T-reg cells), and the T-reg cells from young cisgender men had greater suppressive capacity in vitro than did those from cisgender women. In addition, RNA sequencing data from isolated T-reg cells showed the transcriptomic signature of the cisgender men’s T-regs were significantly enriched for genes in the P13K-AKT signaling pathway. The frequency of T-reg cells was not influenced by sex hormones, but their transcriptomic profile was affected.
“These results are beginning to give us an indication of which genes might be differentially regulated by sex hormones and how these are associated with autoimmunity,” Dr. Robinson said. “We’ve also found that, depending on whether you’re a cisgender man or woman, you may have a different pathogenic process to developing lupus. It’s not necessarily that one mechanism drives the disease across both sexes.”
New approaches, better insights
Dr. Kovats was particularly impressed by the methods of this study. “It was a natural study, the kind of thing we can usually do only in mice,” she said.
“One problem with studies on the effects of hormones in disease is that historically researchers have not paid that much attention to the actual hormone levels in the humans they studied. They might look at 100 women and 100 men, roughly between the ages of 20 and 50. We’re starting to see more, but there aren’t a lot of studies correlating numbers of cells in blood with actual hormone levels in the person. And as we know, just because someone’s a certain age doesn’t mean that they have a textbook hormone level. Early menopause, birth-control pills, many things can affect those levels.”
The researchers hope that these findings will shed light on the mechanisms that create sexual bias in autoimmune diseases, particularly lupus, as well as help researchers to better understand the innate and adaptive immunological differences between men and women. It could also be useful in the clinical setting, Dr. Robinson said. Because of the extreme sex bias in lupus, doctors see far more women with the illness than men. When they do see men with lupus, they need to be able to consider how the patient’s sex affects the development and course of the disease. “I think that people need to start looking at patients as clinically different, depending on their sex and gender,” he said. Information like that analyzed in this study could help with that. This could be especially important because as Dr. Kovats pointed out, although men get lupus far less often than women, when they do have it, they tend to have more severe disease.
Help from machines
This study was groundbreaking in another area as well. The researchers used machine learning to analyze the data. “We’ve started working a lot more with these analysis methods to try to answer as much as we can with these smaller data sets,” Dr. Robinson said. “Rather than the conventional analysis that we would typically perform, we’re able to use machine learning and artificial intelligence to try and learn from the data and increase the numbers that we’re working with by using a training data set. This allows us to interrogate the data with a lot more precision.”
The authors declared no competing interests.
Systemic lupus erythematosus (SLE), or lupus, shows a marked sex bias, affecting about nine females for every one male, according to Susan Kovats, PhD, who studies sex differences in immunity at the Oklahoma Medical Research Foundation in Oklahoma City. This characteristic of lupus suggests that hormones are involved in the pathogenesis of the disease. It also suggests, Dr. Kovats said, that the X chromosome might play a role.
Though studies since the 1970s have indicated a significant role for hormones, the issue is still complex and not well understood, and relatively little research has been done on the molecular mechanisms that might be responsible. This may be because of difficulties with influencing the immune system in vitro, said George A. Robinson, PhD, of University College London’s Centre for Rheumatology.
But Dr. Robinson and his team found a unique way of investigating the role of sex chromosomes and hormones in the inflammatory profiles across subjects of different sex, gender, age, and disease status. In research published online in The Lancet Rheumatology, Dr. Robinson and his team looked at immune cells taken from both cisgender men and women and transgender men and women, and thus were able to “get a more physiological view of what sex hormones are doing to the immune system,” he said.
Dr. Kovats agreed that it was a useful approach. “The transgender people provided an opportunity to effectively separate sex hormone levels from chromosome content,” she said in an interview.
Methods and findings
Peripheral blood mononuclear cell (PBMC) samples were taken from cisgender individuals with and without juvenile-onset lupus and assessed for 28 immune-cell subsets, including different T-cell, B-cell, and monotype subsets. Subjects included 39 postpubertal cisgender men and women (17 men and 22 women) who did not have juvenile-onset lupus, and 35 postpubertal cisgender men and women (12 men and 23 women) who did have juvenile onset lupus. All were aged 16-25 years. The transgender group included five transgender men and five transgender women (aged 18-19) who were undergoing gender-affirming sex hormone treatment.
The analysis found that one of the key differences between young postpubertal cisgender men and age-matched cisgender women was that the men had significantly elevated frequencies of regulatory T cells (T-reg cells), and the T-reg cells from young cisgender men had greater suppressive capacity in vitro than did those from cisgender women. In addition, RNA sequencing data from isolated T-reg cells showed the transcriptomic signature of the cisgender men’s T-regs were significantly enriched for genes in the P13K-AKT signaling pathway. The frequency of T-reg cells was not influenced by sex hormones, but their transcriptomic profile was affected.
“These results are beginning to give us an indication of which genes might be differentially regulated by sex hormones and how these are associated with autoimmunity,” Dr. Robinson said. “We’ve also found that, depending on whether you’re a cisgender man or woman, you may have a different pathogenic process to developing lupus. It’s not necessarily that one mechanism drives the disease across both sexes.”
New approaches, better insights
Dr. Kovats was particularly impressed by the methods of this study. “It was a natural study, the kind of thing we can usually do only in mice,” she said.
“One problem with studies on the effects of hormones in disease is that historically researchers have not paid that much attention to the actual hormone levels in the humans they studied. They might look at 100 women and 100 men, roughly between the ages of 20 and 50. We’re starting to see more, but there aren’t a lot of studies correlating numbers of cells in blood with actual hormone levels in the person. And as we know, just because someone’s a certain age doesn’t mean that they have a textbook hormone level. Early menopause, birth-control pills, many things can affect those levels.”
The researchers hope that these findings will shed light on the mechanisms that create sexual bias in autoimmune diseases, particularly lupus, as well as help researchers to better understand the innate and adaptive immunological differences between men and women. It could also be useful in the clinical setting, Dr. Robinson said. Because of the extreme sex bias in lupus, doctors see far more women with the illness than men. When they do see men with lupus, they need to be able to consider how the patient’s sex affects the development and course of the disease. “I think that people need to start looking at patients as clinically different, depending on their sex and gender,” he said. Information like that analyzed in this study could help with that. This could be especially important because as Dr. Kovats pointed out, although men get lupus far less often than women, when they do have it, they tend to have more severe disease.
Help from machines
This study was groundbreaking in another area as well. The researchers used machine learning to analyze the data. “We’ve started working a lot more with these analysis methods to try to answer as much as we can with these smaller data sets,” Dr. Robinson said. “Rather than the conventional analysis that we would typically perform, we’re able to use machine learning and artificial intelligence to try and learn from the data and increase the numbers that we’re working with by using a training data set. This allows us to interrogate the data with a lot more precision.”
The authors declared no competing interests.
Systemic lupus erythematosus (SLE), or lupus, shows a marked sex bias, affecting about nine females for every one male, according to Susan Kovats, PhD, who studies sex differences in immunity at the Oklahoma Medical Research Foundation in Oklahoma City. This characteristic of lupus suggests that hormones are involved in the pathogenesis of the disease. It also suggests, Dr. Kovats said, that the X chromosome might play a role.
Though studies since the 1970s have indicated a significant role for hormones, the issue is still complex and not well understood, and relatively little research has been done on the molecular mechanisms that might be responsible. This may be because of difficulties with influencing the immune system in vitro, said George A. Robinson, PhD, of University College London’s Centre for Rheumatology.
But Dr. Robinson and his team found a unique way of investigating the role of sex chromosomes and hormones in the inflammatory profiles across subjects of different sex, gender, age, and disease status. In research published online in The Lancet Rheumatology, Dr. Robinson and his team looked at immune cells taken from both cisgender men and women and transgender men and women, and thus were able to “get a more physiological view of what sex hormones are doing to the immune system,” he said.
Dr. Kovats agreed that it was a useful approach. “The transgender people provided an opportunity to effectively separate sex hormone levels from chromosome content,” she said in an interview.
Methods and findings
Peripheral blood mononuclear cell (PBMC) samples were taken from cisgender individuals with and without juvenile-onset lupus and assessed for 28 immune-cell subsets, including different T-cell, B-cell, and monotype subsets. Subjects included 39 postpubertal cisgender men and women (17 men and 22 women) who did not have juvenile-onset lupus, and 35 postpubertal cisgender men and women (12 men and 23 women) who did have juvenile onset lupus. All were aged 16-25 years. The transgender group included five transgender men and five transgender women (aged 18-19) who were undergoing gender-affirming sex hormone treatment.
The analysis found that one of the key differences between young postpubertal cisgender men and age-matched cisgender women was that the men had significantly elevated frequencies of regulatory T cells (T-reg cells), and the T-reg cells from young cisgender men had greater suppressive capacity in vitro than did those from cisgender women. In addition, RNA sequencing data from isolated T-reg cells showed the transcriptomic signature of the cisgender men’s T-regs were significantly enriched for genes in the P13K-AKT signaling pathway. The frequency of T-reg cells was not influenced by sex hormones, but their transcriptomic profile was affected.
“These results are beginning to give us an indication of which genes might be differentially regulated by sex hormones and how these are associated with autoimmunity,” Dr. Robinson said. “We’ve also found that, depending on whether you’re a cisgender man or woman, you may have a different pathogenic process to developing lupus. It’s not necessarily that one mechanism drives the disease across both sexes.”
New approaches, better insights
Dr. Kovats was particularly impressed by the methods of this study. “It was a natural study, the kind of thing we can usually do only in mice,” she said.
“One problem with studies on the effects of hormones in disease is that historically researchers have not paid that much attention to the actual hormone levels in the humans they studied. They might look at 100 women and 100 men, roughly between the ages of 20 and 50. We’re starting to see more, but there aren’t a lot of studies correlating numbers of cells in blood with actual hormone levels in the person. And as we know, just because someone’s a certain age doesn’t mean that they have a textbook hormone level. Early menopause, birth-control pills, many things can affect those levels.”
The researchers hope that these findings will shed light on the mechanisms that create sexual bias in autoimmune diseases, particularly lupus, as well as help researchers to better understand the innate and adaptive immunological differences between men and women. It could also be useful in the clinical setting, Dr. Robinson said. Because of the extreme sex bias in lupus, doctors see far more women with the illness than men. When they do see men with lupus, they need to be able to consider how the patient’s sex affects the development and course of the disease. “I think that people need to start looking at patients as clinically different, depending on their sex and gender,” he said. Information like that analyzed in this study could help with that. This could be especially important because as Dr. Kovats pointed out, although men get lupus far less often than women, when they do have it, they tend to have more severe disease.
Help from machines
This study was groundbreaking in another area as well. The researchers used machine learning to analyze the data. “We’ve started working a lot more with these analysis methods to try to answer as much as we can with these smaller data sets,” Dr. Robinson said. “Rather than the conventional analysis that we would typically perform, we’re able to use machine learning and artificial intelligence to try and learn from the data and increase the numbers that we’re working with by using a training data set. This allows us to interrogate the data with a lot more precision.”
The authors declared no competing interests.
FROM THE LANCET RHEUMATOLOGY