User login
Endoscopic Procedure Shows Promise in Type 2 Diabetes Care
SAN DIEGO – .
In a new dose-finding study, the re-cellularization via electroporation therapy (ReCET, Endogenex) improved insulin sensitivity, beta-cell function, and other glycemic parameters at 12 and 48 weeks in 51 individuals with T2D. “The findings suggest that duodenal mucosal and submucosal recellularization are key therapeutic targets in type 2 diabetes management,” said Barham Abu Dayyeh, MD, director of Interventional Gastroenterology at Cedar-Sinai Hospital, Los Angeles, in a presentation at Digestive Disease Week (DDW) 2025.
The outpatient technique is based on pulsed electrical fields, or electroporation, which do not use heat. “It’s nonthermal regeneration, not just ablation. It’s regeneration of the duodenum as a treatment target that could potentially modify type 2 diabetes,” Abu Dayyeh told GI & Hepatology News.
Separately at DDW, Abu Dayyeh presented results from an artificial intelligence–based analysis of duodenal biopsies from 111 individuals with T2D and 120 control individuals without diabetes, demonstrating distinct mucosal features associated with metabolic disease, significant inflammation in the deep mucosa and submucosa with increased fibrosis, and gut-barrier dysfunction. The authors termed this set of abnormalities “diabetic duodenopathy.”
Abu Dayyeh likened the duodenum to a “conductor” of the “dysfunctional orchestra” of metabolic disease that includes T2D. “It’s tasked with integrating signals from the food that we eat and from our microbiome and communicates that metabolic response to downstream organs like the pancreas, liver, and adipose tissue.”
Currently, he said, “We use treatments that work downstream on components of this dysfunctional orchestra. So we work on the violinist and the flute player, but we do not go upstream to say maybe there’s an opportunity to put the orchestra conductor back in synch…We manage blood glycemia by lowering it, rather than looking at upstream disease-modifying targets that could reverse the course so you require less insulin and less medication.”
Abu Dayyeh envisions the ReCET procedure as an option for people struggling to control T2D with standard medications, or for early use to avoid or delay medications, particularly insulin. But it won’t replace medications. “On the contrary, I see it as enhancing and complementing medications,” he said.
Asked to comment, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, Cleveland, Ohio, told GI & Hepatology News, “Diabetes is a heterogeneous disease complex with numerous pathophysiological derangements. Although diabetic duodenopathy can be seen in some patients with diabetes, that wouldn’t explain the entire story behind diabetes pathogenesis in all people with diabetes. In a subgroup of people with duodenal involvement in their disease process, endoscopic procedures targeting the duodenum may play a role in the future.”
Glycemic Parameters Improve Following ReCET Procedure
The new study, called REGENT-1, was a multicenter, open-label, single-arm dose escalation of three levels of energy delivery in patients who had T2D for 10 years or less with A1c levels 7.5%-11% despite the use of one or more noninsulin glucose-lowering medications. Procedural success, defined as treatment of at least 6 cm of duodenum, was achieved in 100% of participants.
From a baseline A1c of 8.6%, there were dose-response drops at weeks 12 and 48 by energy delivery, with significant reductions at week 48 of 1.00 and 1.70 percentage points, respectively, among the 18 who received the middle dose and the 21 given the highest dose. Body weight also dropped in all three groups in a dose-response way, from 1.2% with the lowest to 6.2% with the highest energy delivery.
In mixed-meal tolerance testing, glucose area under the curve, homeostatic model assessment for insulin resistance, sensitivity index, beta-cell function, and disposition index (a measure of beta-cell response to insulin resistance) were all reduced from baseline at 48 weeks after ReCET, reaching statistical significance with the highest energy dose.
There were no device- or procedure-related serious adverse events.
Based on a literature search, Abu Dayyeh found that modern glucagon like peptide-1 receptor agonist medications have a stronger effect than ReCET or Roux-en-Y gastric bypass (RYGB) on beta-cell function (increases by 239% with semaglutide and 314% with tirzepatide vs 50% with ReCET and 74% for RYGB). However, ReCET procedure produced superior results for both insulin sensitivity (+487% for ReCET and +326% for bypass vs 30% and 62%, respectively for semaglutide and tirzepatide) and disposition index (+1032% for ReCET, +667% with tirzepatide, +642% for RYGB, and +367% for semaglutide).
Aminian commented, “The findings of this single arm clinical trial are promising. The next step is to incorporate a blinded control group who undergoes an endoscopy without any therapeutic intervention.”
In fact, such a study is underway. Results of “a multicenter, randomized, double-blind, sham-controlled study for assessing the safety and effectiveness of endoscopic intestinal re-cellularization therapy in individuals with type 2 diabetes (ReCET)” are expected in late 2026.
In the meantime, Amanian said about the current findings, “I’d argue that the observed improvement in diabetes parameters can be related to more intensive medical therapy during follow-up in this single arm study.”
In the trials, the procedure takes 30 minutes to an hour to perform. However, as the technology improves, “the vision of this is to be a 20-minute outpatient procedure eventually,” Abu Dayyeh said.
He envisions that eventually the procedure will become as accessible as colonoscopy is now, and that primary care physicians and endocrinologists would similarly refer patients to a gastroenterologist or surgeon to have it done. “They do the procedure and send your patient back, hopefully with a less complex management strategy, so you could manage them more efficiently without escalating care.”
Abu Dayyeh is a co-inventor of the ReCET procedure, with the technology licensed by the Mayo Clinic. He is a consultant for and/or reported receiving research support from Boston Scientific, Olympus, Medtronic, Metamodix, BFKW, Apollo Endosurgery, USGI, Endogastric Solutions, Spatz, and Cairn. Aminian had received grants and personal fees from Medtronic and Ethicon. He serves as a consultant for Medtronic, Ethicon, and Eli Lilly.
A version of this article appeared on Medscape.com.
SAN DIEGO – .
In a new dose-finding study, the re-cellularization via electroporation therapy (ReCET, Endogenex) improved insulin sensitivity, beta-cell function, and other glycemic parameters at 12 and 48 weeks in 51 individuals with T2D. “The findings suggest that duodenal mucosal and submucosal recellularization are key therapeutic targets in type 2 diabetes management,” said Barham Abu Dayyeh, MD, director of Interventional Gastroenterology at Cedar-Sinai Hospital, Los Angeles, in a presentation at Digestive Disease Week (DDW) 2025.
The outpatient technique is based on pulsed electrical fields, or electroporation, which do not use heat. “It’s nonthermal regeneration, not just ablation. It’s regeneration of the duodenum as a treatment target that could potentially modify type 2 diabetes,” Abu Dayyeh told GI & Hepatology News.
Separately at DDW, Abu Dayyeh presented results from an artificial intelligence–based analysis of duodenal biopsies from 111 individuals with T2D and 120 control individuals without diabetes, demonstrating distinct mucosal features associated with metabolic disease, significant inflammation in the deep mucosa and submucosa with increased fibrosis, and gut-barrier dysfunction. The authors termed this set of abnormalities “diabetic duodenopathy.”
Abu Dayyeh likened the duodenum to a “conductor” of the “dysfunctional orchestra” of metabolic disease that includes T2D. “It’s tasked with integrating signals from the food that we eat and from our microbiome and communicates that metabolic response to downstream organs like the pancreas, liver, and adipose tissue.”
Currently, he said, “We use treatments that work downstream on components of this dysfunctional orchestra. So we work on the violinist and the flute player, but we do not go upstream to say maybe there’s an opportunity to put the orchestra conductor back in synch…We manage blood glycemia by lowering it, rather than looking at upstream disease-modifying targets that could reverse the course so you require less insulin and less medication.”
Abu Dayyeh envisions the ReCET procedure as an option for people struggling to control T2D with standard medications, or for early use to avoid or delay medications, particularly insulin. But it won’t replace medications. “On the contrary, I see it as enhancing and complementing medications,” he said.
Asked to comment, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, Cleveland, Ohio, told GI & Hepatology News, “Diabetes is a heterogeneous disease complex with numerous pathophysiological derangements. Although diabetic duodenopathy can be seen in some patients with diabetes, that wouldn’t explain the entire story behind diabetes pathogenesis in all people with diabetes. In a subgroup of people with duodenal involvement in their disease process, endoscopic procedures targeting the duodenum may play a role in the future.”
Glycemic Parameters Improve Following ReCET Procedure
The new study, called REGENT-1, was a multicenter, open-label, single-arm dose escalation of three levels of energy delivery in patients who had T2D for 10 years or less with A1c levels 7.5%-11% despite the use of one or more noninsulin glucose-lowering medications. Procedural success, defined as treatment of at least 6 cm of duodenum, was achieved in 100% of participants.
From a baseline A1c of 8.6%, there were dose-response drops at weeks 12 and 48 by energy delivery, with significant reductions at week 48 of 1.00 and 1.70 percentage points, respectively, among the 18 who received the middle dose and the 21 given the highest dose. Body weight also dropped in all three groups in a dose-response way, from 1.2% with the lowest to 6.2% with the highest energy delivery.
In mixed-meal tolerance testing, glucose area under the curve, homeostatic model assessment for insulin resistance, sensitivity index, beta-cell function, and disposition index (a measure of beta-cell response to insulin resistance) were all reduced from baseline at 48 weeks after ReCET, reaching statistical significance with the highest energy dose.
There were no device- or procedure-related serious adverse events.
Based on a literature search, Abu Dayyeh found that modern glucagon like peptide-1 receptor agonist medications have a stronger effect than ReCET or Roux-en-Y gastric bypass (RYGB) on beta-cell function (increases by 239% with semaglutide and 314% with tirzepatide vs 50% with ReCET and 74% for RYGB). However, ReCET procedure produced superior results for both insulin sensitivity (+487% for ReCET and +326% for bypass vs 30% and 62%, respectively for semaglutide and tirzepatide) and disposition index (+1032% for ReCET, +667% with tirzepatide, +642% for RYGB, and +367% for semaglutide).
Aminian commented, “The findings of this single arm clinical trial are promising. The next step is to incorporate a blinded control group who undergoes an endoscopy without any therapeutic intervention.”
In fact, such a study is underway. Results of “a multicenter, randomized, double-blind, sham-controlled study for assessing the safety and effectiveness of endoscopic intestinal re-cellularization therapy in individuals with type 2 diabetes (ReCET)” are expected in late 2026.
In the meantime, Amanian said about the current findings, “I’d argue that the observed improvement in diabetes parameters can be related to more intensive medical therapy during follow-up in this single arm study.”
In the trials, the procedure takes 30 minutes to an hour to perform. However, as the technology improves, “the vision of this is to be a 20-minute outpatient procedure eventually,” Abu Dayyeh said.
He envisions that eventually the procedure will become as accessible as colonoscopy is now, and that primary care physicians and endocrinologists would similarly refer patients to a gastroenterologist or surgeon to have it done. “They do the procedure and send your patient back, hopefully with a less complex management strategy, so you could manage them more efficiently without escalating care.”
Abu Dayyeh is a co-inventor of the ReCET procedure, with the technology licensed by the Mayo Clinic. He is a consultant for and/or reported receiving research support from Boston Scientific, Olympus, Medtronic, Metamodix, BFKW, Apollo Endosurgery, USGI, Endogastric Solutions, Spatz, and Cairn. Aminian had received grants and personal fees from Medtronic and Ethicon. He serves as a consultant for Medtronic, Ethicon, and Eli Lilly.
A version of this article appeared on Medscape.com.
SAN DIEGO – .
In a new dose-finding study, the re-cellularization via electroporation therapy (ReCET, Endogenex) improved insulin sensitivity, beta-cell function, and other glycemic parameters at 12 and 48 weeks in 51 individuals with T2D. “The findings suggest that duodenal mucosal and submucosal recellularization are key therapeutic targets in type 2 diabetes management,” said Barham Abu Dayyeh, MD, director of Interventional Gastroenterology at Cedar-Sinai Hospital, Los Angeles, in a presentation at Digestive Disease Week (DDW) 2025.
The outpatient technique is based on pulsed electrical fields, or electroporation, which do not use heat. “It’s nonthermal regeneration, not just ablation. It’s regeneration of the duodenum as a treatment target that could potentially modify type 2 diabetes,” Abu Dayyeh told GI & Hepatology News.
Separately at DDW, Abu Dayyeh presented results from an artificial intelligence–based analysis of duodenal biopsies from 111 individuals with T2D and 120 control individuals without diabetes, demonstrating distinct mucosal features associated with metabolic disease, significant inflammation in the deep mucosa and submucosa with increased fibrosis, and gut-barrier dysfunction. The authors termed this set of abnormalities “diabetic duodenopathy.”
Abu Dayyeh likened the duodenum to a “conductor” of the “dysfunctional orchestra” of metabolic disease that includes T2D. “It’s tasked with integrating signals from the food that we eat and from our microbiome and communicates that metabolic response to downstream organs like the pancreas, liver, and adipose tissue.”
Currently, he said, “We use treatments that work downstream on components of this dysfunctional orchestra. So we work on the violinist and the flute player, but we do not go upstream to say maybe there’s an opportunity to put the orchestra conductor back in synch…We manage blood glycemia by lowering it, rather than looking at upstream disease-modifying targets that could reverse the course so you require less insulin and less medication.”
Abu Dayyeh envisions the ReCET procedure as an option for people struggling to control T2D with standard medications, or for early use to avoid or delay medications, particularly insulin. But it won’t replace medications. “On the contrary, I see it as enhancing and complementing medications,” he said.
Asked to comment, Ali Aminian, MD, professor of surgery and director of the Bariatric and Metabolic Institute at the Cleveland Clinic, Cleveland, Ohio, told GI & Hepatology News, “Diabetes is a heterogeneous disease complex with numerous pathophysiological derangements. Although diabetic duodenopathy can be seen in some patients with diabetes, that wouldn’t explain the entire story behind diabetes pathogenesis in all people with diabetes. In a subgroup of people with duodenal involvement in their disease process, endoscopic procedures targeting the duodenum may play a role in the future.”
Glycemic Parameters Improve Following ReCET Procedure
The new study, called REGENT-1, was a multicenter, open-label, single-arm dose escalation of three levels of energy delivery in patients who had T2D for 10 years or less with A1c levels 7.5%-11% despite the use of one or more noninsulin glucose-lowering medications. Procedural success, defined as treatment of at least 6 cm of duodenum, was achieved in 100% of participants.
From a baseline A1c of 8.6%, there were dose-response drops at weeks 12 and 48 by energy delivery, with significant reductions at week 48 of 1.00 and 1.70 percentage points, respectively, among the 18 who received the middle dose and the 21 given the highest dose. Body weight also dropped in all three groups in a dose-response way, from 1.2% with the lowest to 6.2% with the highest energy delivery.
In mixed-meal tolerance testing, glucose area under the curve, homeostatic model assessment for insulin resistance, sensitivity index, beta-cell function, and disposition index (a measure of beta-cell response to insulin resistance) were all reduced from baseline at 48 weeks after ReCET, reaching statistical significance with the highest energy dose.
There were no device- or procedure-related serious adverse events.
Based on a literature search, Abu Dayyeh found that modern glucagon like peptide-1 receptor agonist medications have a stronger effect than ReCET or Roux-en-Y gastric bypass (RYGB) on beta-cell function (increases by 239% with semaglutide and 314% with tirzepatide vs 50% with ReCET and 74% for RYGB). However, ReCET procedure produced superior results for both insulin sensitivity (+487% for ReCET and +326% for bypass vs 30% and 62%, respectively for semaglutide and tirzepatide) and disposition index (+1032% for ReCET, +667% with tirzepatide, +642% for RYGB, and +367% for semaglutide).
Aminian commented, “The findings of this single arm clinical trial are promising. The next step is to incorporate a blinded control group who undergoes an endoscopy without any therapeutic intervention.”
In fact, such a study is underway. Results of “a multicenter, randomized, double-blind, sham-controlled study for assessing the safety and effectiveness of endoscopic intestinal re-cellularization therapy in individuals with type 2 diabetes (ReCET)” are expected in late 2026.
In the meantime, Amanian said about the current findings, “I’d argue that the observed improvement in diabetes parameters can be related to more intensive medical therapy during follow-up in this single arm study.”
In the trials, the procedure takes 30 minutes to an hour to perform. However, as the technology improves, “the vision of this is to be a 20-minute outpatient procedure eventually,” Abu Dayyeh said.
He envisions that eventually the procedure will become as accessible as colonoscopy is now, and that primary care physicians and endocrinologists would similarly refer patients to a gastroenterologist or surgeon to have it done. “They do the procedure and send your patient back, hopefully with a less complex management strategy, so you could manage them more efficiently without escalating care.”
Abu Dayyeh is a co-inventor of the ReCET procedure, with the technology licensed by the Mayo Clinic. He is a consultant for and/or reported receiving research support from Boston Scientific, Olympus, Medtronic, Metamodix, BFKW, Apollo Endosurgery, USGI, Endogastric Solutions, Spatz, and Cairn. Aminian had received grants and personal fees from Medtronic and Ethicon. He serves as a consultant for Medtronic, Ethicon, and Eli Lilly.
A version of this article appeared on Medscape.com.
FROM DDW 2025
Semaglutide Therapy Improves Liver Histology in MASH
, an ongoing randomized placebo-controlled trial reported.
The glucagon-like peptide 1 receptor agonist (GLP-1 RA) is currently a candidate for treating MASH.
Preliminary results of the two-part phase 3, double-blind ESSENCE trial, conducted in at 253 clinical sites in 37 countries, were published in The New England Journal of Medicine.
A previous phase 2 study by Loomba et al suggested semaglutide was effective in reducing liver injury. “That study, however, did not show improvement in liver fibrosis, which this study has done,” study co-lead Philip Newsome, PhD, professor in the department of immunology and immunotherapy and Honorary Professor of Experimental Hepatology at the University of Birmingham in England, said in an interview.
“The results aligned with expectations in that the impact on liver fibrosis was anticipated — but with some uncertainty, so this study is important in that regard.”
Study Details
From May 2020 to April 2023, researchers led by Newsome and Arun J. Sanyal, MBBS, MD, of Stravitz-Sanyal Institute for Liver Disease and Metabolic Health at Virginia Commonwealth University School of Medicine, Richmond, randomized 1197 patients with a mean age of 56 years. Of these, 57% were women and 67.5% were White individuals. Mean body mass index was 34.6, and 55.9% had type 2 diabetes.
All had biopsy-defined MASH and fibrosis stage 2 or 3 according to the Nonalcoholic Steatohepatitis Clinical Research Network classification and a Nonalcoholic Fatty Liver Disease Activity Score ≥ 4.
Rates of fibrosis were 31.3% for stage 2 fibrosis and 68.8% for stage 3. Diverse geographic site locations included Asia (25.1%), Europe (25.3%), North America (35.0%), and South America (7.9%), and others (6.8%).
In a 2:1 ratio, they were assigned to receive once-weekly subcutaneous semaglutide at a dose of 2.4 mg or placebo for 240 weeks. A planned interim analysis of the first 800 patients was done at week 72, with primary endpoints being resolution of steatohepatitis without worsening of liver fibrosis and reduction in liver fibrosis without worsening of steatohepatitis.
Resolution of steatohepatitis without worsening of fibrosis occurred in 62.9% of the 534 patients in the semaglutide group and in 34.3% of the 266 patients in the placebo group (estimated difference, 28.7 percentage points; 95% CI, 21.1-36.2, P < .001).
A reduction in liver fibrosis without worsening of steatohepatitis was reported in 36.8% of semaglutide recipients and 22.4% of placebo recipients (estimated difference, 14.4 percentage points; 95% CI, 7.5-21.3, P < .001).
In secondary findings, the combined resolution of steatohepatitis and reduction in liver fibrosis was reported in 32.7% in the semaglutide group vs 16.1% in the placebo group (estimated difference, 16.5 percentage points; 95% CI, 10.2-22.8; P < .001).
The mean change in body weight was –10.5% with semaglutide and –2.0% with placebo (estimated difference, –8.5 percentage points; 95% CI, –9.6 to –7.4, P < .001). Mean changes in bodily pain scores did not differ significantly between arms.
The histologic benefits of semaglutide also emerged in improvements on all prespecified noninvasive tests — including aspartate transaminase and alanine transaminase levels and liver stiffness. Emerging evidence has suggested an association between reductions in liver stiffness and clinical benefit.
Gastrointestinal adverse events were more common in the semaglutide group.
Commenting on the study from a nonparticipant’s perspective, Naga P. Chalasani, MD, AGAF, professor of gastroenterology and hepatology at Indiana University School of Medicine, Indianapolis, said results from the ESSENCE trial were “long awaited and they certainly advance the field of MASH clinical trials substantially.”
Furthermore, he added, the results are well aligned with those of a phase 2b trial of semaglutide by Newsome and colleagues for what was then termed nonalcoholic steatohepatitis, and “they also align with what is known about the positive role of incretins, digestive hormones imitated by GLP-1s to improve liver health in patients with MASLD and MASH.”
“The results from this study certainly make a case for semaglutide to be the backbone therapy for diabetic or obese patients with MASH and fibrosis,” Chalasani said. “More than 80% of patients with MASH and fibrosis have either diabetes and/or obesity.”
He added that a better understanding is needed of how semaglutide works in patients with MASH cirrhosis since the previous small study was unsuccessful. “But this may need to be repeated as the published study was underpowered. Outcomes in the ESSENCE trial will help to clarify whether semaglutide will improve clinical outcomes beyond improving liver histology.”
According to Newsome, GLP-1s will become the backbone of therapy in MASH given their range of metabolic and liver benefit. But questions remain, he said. “Will there be further improvements with longer treatment with semaglutide? What noninvasive tests should we use to determine treatment success? Which patients will benefit from combination treatment?”
This study was supported by Novo Nordisk, the manufacturer of Wegovy. Sanyal reported having various financial relationships with multiple private-sector companies, including Novo Nordisk. Newsome reported consulting for Novo Nordisk and Boehringer Ingelheim. Several study coauthors reported having similar relationships with pharmaceutical companies or employment with Novo Nordisk. Chalasani declared being involved in several MASH clinical trials conducted by other pharmaceutical companies.
A version of this article appeared on Medscape.com.
, an ongoing randomized placebo-controlled trial reported.
The glucagon-like peptide 1 receptor agonist (GLP-1 RA) is currently a candidate for treating MASH.
Preliminary results of the two-part phase 3, double-blind ESSENCE trial, conducted in at 253 clinical sites in 37 countries, were published in The New England Journal of Medicine.
A previous phase 2 study by Loomba et al suggested semaglutide was effective in reducing liver injury. “That study, however, did not show improvement in liver fibrosis, which this study has done,” study co-lead Philip Newsome, PhD, professor in the department of immunology and immunotherapy and Honorary Professor of Experimental Hepatology at the University of Birmingham in England, said in an interview.
“The results aligned with expectations in that the impact on liver fibrosis was anticipated — but with some uncertainty, so this study is important in that regard.”
Study Details
From May 2020 to April 2023, researchers led by Newsome and Arun J. Sanyal, MBBS, MD, of Stravitz-Sanyal Institute for Liver Disease and Metabolic Health at Virginia Commonwealth University School of Medicine, Richmond, randomized 1197 patients with a mean age of 56 years. Of these, 57% were women and 67.5% were White individuals. Mean body mass index was 34.6, and 55.9% had type 2 diabetes.
All had biopsy-defined MASH and fibrosis stage 2 or 3 according to the Nonalcoholic Steatohepatitis Clinical Research Network classification and a Nonalcoholic Fatty Liver Disease Activity Score ≥ 4.
Rates of fibrosis were 31.3% for stage 2 fibrosis and 68.8% for stage 3. Diverse geographic site locations included Asia (25.1%), Europe (25.3%), North America (35.0%), and South America (7.9%), and others (6.8%).
In a 2:1 ratio, they were assigned to receive once-weekly subcutaneous semaglutide at a dose of 2.4 mg or placebo for 240 weeks. A planned interim analysis of the first 800 patients was done at week 72, with primary endpoints being resolution of steatohepatitis without worsening of liver fibrosis and reduction in liver fibrosis without worsening of steatohepatitis.
Resolution of steatohepatitis without worsening of fibrosis occurred in 62.9% of the 534 patients in the semaglutide group and in 34.3% of the 266 patients in the placebo group (estimated difference, 28.7 percentage points; 95% CI, 21.1-36.2, P < .001).
A reduction in liver fibrosis without worsening of steatohepatitis was reported in 36.8% of semaglutide recipients and 22.4% of placebo recipients (estimated difference, 14.4 percentage points; 95% CI, 7.5-21.3, P < .001).
In secondary findings, the combined resolution of steatohepatitis and reduction in liver fibrosis was reported in 32.7% in the semaglutide group vs 16.1% in the placebo group (estimated difference, 16.5 percentage points; 95% CI, 10.2-22.8; P < .001).
The mean change in body weight was –10.5% with semaglutide and –2.0% with placebo (estimated difference, –8.5 percentage points; 95% CI, –9.6 to –7.4, P < .001). Mean changes in bodily pain scores did not differ significantly between arms.
The histologic benefits of semaglutide also emerged in improvements on all prespecified noninvasive tests — including aspartate transaminase and alanine transaminase levels and liver stiffness. Emerging evidence has suggested an association between reductions in liver stiffness and clinical benefit.
Gastrointestinal adverse events were more common in the semaglutide group.
Commenting on the study from a nonparticipant’s perspective, Naga P. Chalasani, MD, AGAF, professor of gastroenterology and hepatology at Indiana University School of Medicine, Indianapolis, said results from the ESSENCE trial were “long awaited and they certainly advance the field of MASH clinical trials substantially.”
Furthermore, he added, the results are well aligned with those of a phase 2b trial of semaglutide by Newsome and colleagues for what was then termed nonalcoholic steatohepatitis, and “they also align with what is known about the positive role of incretins, digestive hormones imitated by GLP-1s to improve liver health in patients with MASLD and MASH.”
“The results from this study certainly make a case for semaglutide to be the backbone therapy for diabetic or obese patients with MASH and fibrosis,” Chalasani said. “More than 80% of patients with MASH and fibrosis have either diabetes and/or obesity.”
He added that a better understanding is needed of how semaglutide works in patients with MASH cirrhosis since the previous small study was unsuccessful. “But this may need to be repeated as the published study was underpowered. Outcomes in the ESSENCE trial will help to clarify whether semaglutide will improve clinical outcomes beyond improving liver histology.”
According to Newsome, GLP-1s will become the backbone of therapy in MASH given their range of metabolic and liver benefit. But questions remain, he said. “Will there be further improvements with longer treatment with semaglutide? What noninvasive tests should we use to determine treatment success? Which patients will benefit from combination treatment?”
This study was supported by Novo Nordisk, the manufacturer of Wegovy. Sanyal reported having various financial relationships with multiple private-sector companies, including Novo Nordisk. Newsome reported consulting for Novo Nordisk and Boehringer Ingelheim. Several study coauthors reported having similar relationships with pharmaceutical companies or employment with Novo Nordisk. Chalasani declared being involved in several MASH clinical trials conducted by other pharmaceutical companies.
A version of this article appeared on Medscape.com.
, an ongoing randomized placebo-controlled trial reported.
The glucagon-like peptide 1 receptor agonist (GLP-1 RA) is currently a candidate for treating MASH.
Preliminary results of the two-part phase 3, double-blind ESSENCE trial, conducted in at 253 clinical sites in 37 countries, were published in The New England Journal of Medicine.
A previous phase 2 study by Loomba et al suggested semaglutide was effective in reducing liver injury. “That study, however, did not show improvement in liver fibrosis, which this study has done,” study co-lead Philip Newsome, PhD, professor in the department of immunology and immunotherapy and Honorary Professor of Experimental Hepatology at the University of Birmingham in England, said in an interview.
“The results aligned with expectations in that the impact on liver fibrosis was anticipated — but with some uncertainty, so this study is important in that regard.”
Study Details
From May 2020 to April 2023, researchers led by Newsome and Arun J. Sanyal, MBBS, MD, of Stravitz-Sanyal Institute for Liver Disease and Metabolic Health at Virginia Commonwealth University School of Medicine, Richmond, randomized 1197 patients with a mean age of 56 years. Of these, 57% were women and 67.5% were White individuals. Mean body mass index was 34.6, and 55.9% had type 2 diabetes.
All had biopsy-defined MASH and fibrosis stage 2 or 3 according to the Nonalcoholic Steatohepatitis Clinical Research Network classification and a Nonalcoholic Fatty Liver Disease Activity Score ≥ 4.
Rates of fibrosis were 31.3% for stage 2 fibrosis and 68.8% for stage 3. Diverse geographic site locations included Asia (25.1%), Europe (25.3%), North America (35.0%), and South America (7.9%), and others (6.8%).
In a 2:1 ratio, they were assigned to receive once-weekly subcutaneous semaglutide at a dose of 2.4 mg or placebo for 240 weeks. A planned interim analysis of the first 800 patients was done at week 72, with primary endpoints being resolution of steatohepatitis without worsening of liver fibrosis and reduction in liver fibrosis without worsening of steatohepatitis.
Resolution of steatohepatitis without worsening of fibrosis occurred in 62.9% of the 534 patients in the semaglutide group and in 34.3% of the 266 patients in the placebo group (estimated difference, 28.7 percentage points; 95% CI, 21.1-36.2, P < .001).
A reduction in liver fibrosis without worsening of steatohepatitis was reported in 36.8% of semaglutide recipients and 22.4% of placebo recipients (estimated difference, 14.4 percentage points; 95% CI, 7.5-21.3, P < .001).
In secondary findings, the combined resolution of steatohepatitis and reduction in liver fibrosis was reported in 32.7% in the semaglutide group vs 16.1% in the placebo group (estimated difference, 16.5 percentage points; 95% CI, 10.2-22.8; P < .001).
The mean change in body weight was –10.5% with semaglutide and –2.0% with placebo (estimated difference, –8.5 percentage points; 95% CI, –9.6 to –7.4, P < .001). Mean changes in bodily pain scores did not differ significantly between arms.
The histologic benefits of semaglutide also emerged in improvements on all prespecified noninvasive tests — including aspartate transaminase and alanine transaminase levels and liver stiffness. Emerging evidence has suggested an association between reductions in liver stiffness and clinical benefit.
Gastrointestinal adverse events were more common in the semaglutide group.
Commenting on the study from a nonparticipant’s perspective, Naga P. Chalasani, MD, AGAF, professor of gastroenterology and hepatology at Indiana University School of Medicine, Indianapolis, said results from the ESSENCE trial were “long awaited and they certainly advance the field of MASH clinical trials substantially.”
Furthermore, he added, the results are well aligned with those of a phase 2b trial of semaglutide by Newsome and colleagues for what was then termed nonalcoholic steatohepatitis, and “they also align with what is known about the positive role of incretins, digestive hormones imitated by GLP-1s to improve liver health in patients with MASLD and MASH.”
“The results from this study certainly make a case for semaglutide to be the backbone therapy for diabetic or obese patients with MASH and fibrosis,” Chalasani said. “More than 80% of patients with MASH and fibrosis have either diabetes and/or obesity.”
He added that a better understanding is needed of how semaglutide works in patients with MASH cirrhosis since the previous small study was unsuccessful. “But this may need to be repeated as the published study was underpowered. Outcomes in the ESSENCE trial will help to clarify whether semaglutide will improve clinical outcomes beyond improving liver histology.”
According to Newsome, GLP-1s will become the backbone of therapy in MASH given their range of metabolic and liver benefit. But questions remain, he said. “Will there be further improvements with longer treatment with semaglutide? What noninvasive tests should we use to determine treatment success? Which patients will benefit from combination treatment?”
This study was supported by Novo Nordisk, the manufacturer of Wegovy. Sanyal reported having various financial relationships with multiple private-sector companies, including Novo Nordisk. Newsome reported consulting for Novo Nordisk and Boehringer Ingelheim. Several study coauthors reported having similar relationships with pharmaceutical companies or employment with Novo Nordisk. Chalasani declared being involved in several MASH clinical trials conducted by other pharmaceutical companies.
A version of this article appeared on Medscape.com.
Histamine Pathway a Target for Erythropoietic Protoporphyria?
An experimental study in zebrafish has suggested the decades-old, first-generation antihistamine chlorcyclizine and/or other antihistamines may be a strategy for treating erythropoietic protoporphyria (EPP)-associated liver disease by decreasing hepatic protoporphorin IX (PP-IX) accumulation.
Currently, liver transplantation is the primary treatment for this rare, painful, and life-threatening genetic disease, which is caused by excessive PP-IX accumulation and affects about 4000 people in the United States.
The findings could eventually lead to a simpler treatment that prevent shepatic damage at a much earlier stage, according to researchers led by M. Bishr Omary, MD, PhD, a professor in the Center for Advanced Biotechnology and Medicine and Robert Wood Johnson Medical School at Rutgers University in Piscataway, New Jersey.
Reporting in Cellular and Molecular Gastroenterology and Hepatology, the investigators found that chlorcyclizine reduced PP-IX levels. EPP is caused by mutations leading to deficiency of the enzyme ferrochelatase, which inserts iron into PP-IX to generate heme. The resulting condition is characterized by PP-IX accumulation, skin photosensitivity, cholestasis, and end-stage liver disease. “Despite available drugs that address photosensitivity, the treatment of EPP-related liver disease remains an unmet need,” Omary and colleagues wrote.
The Study
In order to trigger PP-IX overproduction and accumulation, the investigators administered delta-aminolevulinic acid and deferoxamine to zebrafish. These freshwater tropical fish share many physiological characteristics with humans and have been used to model human disease and develop drugs. Furthermore, these fish are transparent at the larval stage, allowing quantification and visualization of porphyrin, which is fluorescent.
The researchers had screened some 2500 approved and bioactive compounds and identified chlorcyclizine as a potent PP-IX–lowering agent.
High-throughput compound screening of ALA + DFO-treated zebrafish found that the HH-1 blocker reduced zebrafish liver PP-IX levels. The effect of chlorcyclizine was validated in porphyrin-loaded primary mouse hepatocytes, transgenic mice, and mice fed the porphyrinogenic compound 3,5-diethoxycarbonyl-1,4-dihydrocollidine.
Plasma and tissue PP-IX were measured by fluorescence; livers were analyzed by histology, immunoblotting, and quantitative polymerase chain reaction.
Chlorcyclizine-treated zebrafish larvae as well as the two types of mice all showed reduced hepatic PP-IX levels compared with controls. While the neurotransmitter played an important role in PP-IX accumulation in porphyrin-stressed hepatocytes, blockading notably decreased PP-IX levels.
Detailed analysis showed that chlorcyclizine appeared to work through multiple mechanisms, helping the liver clear toxic porphyrin buildup and reducing inflammation. It also decreased the presence of histamine-producing mast cells. The result was less liver injury, decreased porphyrin-triggered protein aggregation and oxidation, and increased clearance of s PP-I in stool.
Interestingly, in both mouse models, chlorcyclizine lowered PP-IX levels in female but not male mice in liver, erythrocytes, and bone marrow. This sex-specific effect appeared to be related to the greater speed at which male murines metabolize the drug, the authors explained in a news release. In rats, for example, the metabolism of chlorcyclizine is 8 times higher in male than in female livers.
The investigators plan to launch a clinical trial in EPP patients to evaluate the effectiveness of chlorcyclizine for both liver and skin involvement. And a phase 2 trial is already underway testing the antacid cimetidine for treating EPP skin manifestations. It is possible that the different antihistamines may act additively or synergistically.
This work was supported by National Institutes of Health (NIH) grants and the Henry and Mala Dorfman Family Professorship of Pediatric Hematology/Oncology.
Omary is a member of the NIH/National Institute of Diabetes and Digestive and Kidney Diseases Data and Safety Monitoring Board of the Porphyrias Consortium.
A provisional patent application has been submitted for the use of H1-receptor blockers with or without receptor blockers to treat protoporphyrias associated with PP-IX accumulation.
Mutations in the ferrochelatase (FECH) gene cause erythropoietic porphyria. EPP is characterized biochemically by liver and bone marrow accumulation of protoporphyrin-IX (PP-IX), and is characterized clinically by hepatic dysfunction with progression in 1-4% to advanced liver disease.
A recent study by Kuo and colleagues exemplifies a bench-to-bedside evolution comprising pharmacological screening, mechanistic dissection, and ultimately translation of this mechanism to human subjects to treat EPP. They utilized high-throughput compound screening in a zebrafish model to identify the anti-histamine, chlorcyclizine (CCZ), as a candidate EPP therapy. Chlorciclizine lowered hepatocyte PP-IX in multiple EPP models by blocking peripheral histamine production, and by inducing hepatocyte PP-IX efflux. The data represent advances in the realms of both clinical therapeutics and molecular pathophysiological discovery.
From a discovery standpoint, strategic compound screening that utilizes the LOPAC (library of pharmaceutically active compounds) and Prestwick libraries offers at least two key characteristics. First, these compounds have largely known targets. The known pharmacology of chlorcyclizine provided immediate clues to validate mechanism rapidly in hepatic HPP, a relatively poorly understood disease. Moreover, screening libraries comprising FDA-approved drugs can minimize lag time between discovery and translation to interventional trials in human subjects.
Beyond such strategic discovery considerations, perhaps more exciting is the therapeutic potential for anti-histaminergic therapy to mitigate hepatic manifestations in EPP. Specifically, other porphyrias with hepatic complications have FDA-approved treatments, such as anti-ALAS1 siRNAs to treat acute hepatic porphyria (AHP). No such treatment currently exists for liver dysfunction in EPP, yet CCZ and other histamine-1 receptor blockers hold such promise. Indeed, the H1 inhibitor, cimetidine, is currently in an active phase 2 trial to treat EPP (NCT05020184).
Given the already widespread use of antihistamines to symptomatically treat cutaneous EPP, we may not be too distant from pivoting and deploying readily available H1Bs like cimetidine to treat EPP liver manifestations as well. Given recent data by Kuo and colleagues, such an outcome should not be too far-FECHed.
Brian DeBosch, MD, PhD, is Center Director of the nutrition & molecular metabolism research program, in the Division of Gastroenterology, Hepatology & Nutrition at Indiana University School of Medicine, Indianapolis. He declares no conflicts of interest.
Mutations in the ferrochelatase (FECH) gene cause erythropoietic porphyria. EPP is characterized biochemically by liver and bone marrow accumulation of protoporphyrin-IX (PP-IX), and is characterized clinically by hepatic dysfunction with progression in 1-4% to advanced liver disease.
A recent study by Kuo and colleagues exemplifies a bench-to-bedside evolution comprising pharmacological screening, mechanistic dissection, and ultimately translation of this mechanism to human subjects to treat EPP. They utilized high-throughput compound screening in a zebrafish model to identify the anti-histamine, chlorcyclizine (CCZ), as a candidate EPP therapy. Chlorciclizine lowered hepatocyte PP-IX in multiple EPP models by blocking peripheral histamine production, and by inducing hepatocyte PP-IX efflux. The data represent advances in the realms of both clinical therapeutics and molecular pathophysiological discovery.
From a discovery standpoint, strategic compound screening that utilizes the LOPAC (library of pharmaceutically active compounds) and Prestwick libraries offers at least two key characteristics. First, these compounds have largely known targets. The known pharmacology of chlorcyclizine provided immediate clues to validate mechanism rapidly in hepatic HPP, a relatively poorly understood disease. Moreover, screening libraries comprising FDA-approved drugs can minimize lag time between discovery and translation to interventional trials in human subjects.
Beyond such strategic discovery considerations, perhaps more exciting is the therapeutic potential for anti-histaminergic therapy to mitigate hepatic manifestations in EPP. Specifically, other porphyrias with hepatic complications have FDA-approved treatments, such as anti-ALAS1 siRNAs to treat acute hepatic porphyria (AHP). No such treatment currently exists for liver dysfunction in EPP, yet CCZ and other histamine-1 receptor blockers hold such promise. Indeed, the H1 inhibitor, cimetidine, is currently in an active phase 2 trial to treat EPP (NCT05020184).
Given the already widespread use of antihistamines to symptomatically treat cutaneous EPP, we may not be too distant from pivoting and deploying readily available H1Bs like cimetidine to treat EPP liver manifestations as well. Given recent data by Kuo and colleagues, such an outcome should not be too far-FECHed.
Brian DeBosch, MD, PhD, is Center Director of the nutrition & molecular metabolism research program, in the Division of Gastroenterology, Hepatology & Nutrition at Indiana University School of Medicine, Indianapolis. He declares no conflicts of interest.
Mutations in the ferrochelatase (FECH) gene cause erythropoietic porphyria. EPP is characterized biochemically by liver and bone marrow accumulation of protoporphyrin-IX (PP-IX), and is characterized clinically by hepatic dysfunction with progression in 1-4% to advanced liver disease.
A recent study by Kuo and colleagues exemplifies a bench-to-bedside evolution comprising pharmacological screening, mechanistic dissection, and ultimately translation of this mechanism to human subjects to treat EPP. They utilized high-throughput compound screening in a zebrafish model to identify the anti-histamine, chlorcyclizine (CCZ), as a candidate EPP therapy. Chlorciclizine lowered hepatocyte PP-IX in multiple EPP models by blocking peripheral histamine production, and by inducing hepatocyte PP-IX efflux. The data represent advances in the realms of both clinical therapeutics and molecular pathophysiological discovery.
From a discovery standpoint, strategic compound screening that utilizes the LOPAC (library of pharmaceutically active compounds) and Prestwick libraries offers at least two key characteristics. First, these compounds have largely known targets. The known pharmacology of chlorcyclizine provided immediate clues to validate mechanism rapidly in hepatic HPP, a relatively poorly understood disease. Moreover, screening libraries comprising FDA-approved drugs can minimize lag time between discovery and translation to interventional trials in human subjects.
Beyond such strategic discovery considerations, perhaps more exciting is the therapeutic potential for anti-histaminergic therapy to mitigate hepatic manifestations in EPP. Specifically, other porphyrias with hepatic complications have FDA-approved treatments, such as anti-ALAS1 siRNAs to treat acute hepatic porphyria (AHP). No such treatment currently exists for liver dysfunction in EPP, yet CCZ and other histamine-1 receptor blockers hold such promise. Indeed, the H1 inhibitor, cimetidine, is currently in an active phase 2 trial to treat EPP (NCT05020184).
Given the already widespread use of antihistamines to symptomatically treat cutaneous EPP, we may not be too distant from pivoting and deploying readily available H1Bs like cimetidine to treat EPP liver manifestations as well. Given recent data by Kuo and colleagues, such an outcome should not be too far-FECHed.
Brian DeBosch, MD, PhD, is Center Director of the nutrition & molecular metabolism research program, in the Division of Gastroenterology, Hepatology & Nutrition at Indiana University School of Medicine, Indianapolis. He declares no conflicts of interest.
An experimental study in zebrafish has suggested the decades-old, first-generation antihistamine chlorcyclizine and/or other antihistamines may be a strategy for treating erythropoietic protoporphyria (EPP)-associated liver disease by decreasing hepatic protoporphorin IX (PP-IX) accumulation.
Currently, liver transplantation is the primary treatment for this rare, painful, and life-threatening genetic disease, which is caused by excessive PP-IX accumulation and affects about 4000 people in the United States.
The findings could eventually lead to a simpler treatment that prevent shepatic damage at a much earlier stage, according to researchers led by M. Bishr Omary, MD, PhD, a professor in the Center for Advanced Biotechnology and Medicine and Robert Wood Johnson Medical School at Rutgers University in Piscataway, New Jersey.
Reporting in Cellular and Molecular Gastroenterology and Hepatology, the investigators found that chlorcyclizine reduced PP-IX levels. EPP is caused by mutations leading to deficiency of the enzyme ferrochelatase, which inserts iron into PP-IX to generate heme. The resulting condition is characterized by PP-IX accumulation, skin photosensitivity, cholestasis, and end-stage liver disease. “Despite available drugs that address photosensitivity, the treatment of EPP-related liver disease remains an unmet need,” Omary and colleagues wrote.
The Study
In order to trigger PP-IX overproduction and accumulation, the investigators administered delta-aminolevulinic acid and deferoxamine to zebrafish. These freshwater tropical fish share many physiological characteristics with humans and have been used to model human disease and develop drugs. Furthermore, these fish are transparent at the larval stage, allowing quantification and visualization of porphyrin, which is fluorescent.
The researchers had screened some 2500 approved and bioactive compounds and identified chlorcyclizine as a potent PP-IX–lowering agent.
High-throughput compound screening of ALA + DFO-treated zebrafish found that the HH-1 blocker reduced zebrafish liver PP-IX levels. The effect of chlorcyclizine was validated in porphyrin-loaded primary mouse hepatocytes, transgenic mice, and mice fed the porphyrinogenic compound 3,5-diethoxycarbonyl-1,4-dihydrocollidine.
Plasma and tissue PP-IX were measured by fluorescence; livers were analyzed by histology, immunoblotting, and quantitative polymerase chain reaction.
Chlorcyclizine-treated zebrafish larvae as well as the two types of mice all showed reduced hepatic PP-IX levels compared with controls. While the neurotransmitter played an important role in PP-IX accumulation in porphyrin-stressed hepatocytes, blockading notably decreased PP-IX levels.
Detailed analysis showed that chlorcyclizine appeared to work through multiple mechanisms, helping the liver clear toxic porphyrin buildup and reducing inflammation. It also decreased the presence of histamine-producing mast cells. The result was less liver injury, decreased porphyrin-triggered protein aggregation and oxidation, and increased clearance of s PP-I in stool.
Interestingly, in both mouse models, chlorcyclizine lowered PP-IX levels in female but not male mice in liver, erythrocytes, and bone marrow. This sex-specific effect appeared to be related to the greater speed at which male murines metabolize the drug, the authors explained in a news release. In rats, for example, the metabolism of chlorcyclizine is 8 times higher in male than in female livers.
The investigators plan to launch a clinical trial in EPP patients to evaluate the effectiveness of chlorcyclizine for both liver and skin involvement. And a phase 2 trial is already underway testing the antacid cimetidine for treating EPP skin manifestations. It is possible that the different antihistamines may act additively or synergistically.
This work was supported by National Institutes of Health (NIH) grants and the Henry and Mala Dorfman Family Professorship of Pediatric Hematology/Oncology.
Omary is a member of the NIH/National Institute of Diabetes and Digestive and Kidney Diseases Data and Safety Monitoring Board of the Porphyrias Consortium.
A provisional patent application has been submitted for the use of H1-receptor blockers with or without receptor blockers to treat protoporphyrias associated with PP-IX accumulation.
An experimental study in zebrafish has suggested the decades-old, first-generation antihistamine chlorcyclizine and/or other antihistamines may be a strategy for treating erythropoietic protoporphyria (EPP)-associated liver disease by decreasing hepatic protoporphorin IX (PP-IX) accumulation.
Currently, liver transplantation is the primary treatment for this rare, painful, and life-threatening genetic disease, which is caused by excessive PP-IX accumulation and affects about 4000 people in the United States.
The findings could eventually lead to a simpler treatment that prevent shepatic damage at a much earlier stage, according to researchers led by M. Bishr Omary, MD, PhD, a professor in the Center for Advanced Biotechnology and Medicine and Robert Wood Johnson Medical School at Rutgers University in Piscataway, New Jersey.
Reporting in Cellular and Molecular Gastroenterology and Hepatology, the investigators found that chlorcyclizine reduced PP-IX levels. EPP is caused by mutations leading to deficiency of the enzyme ferrochelatase, which inserts iron into PP-IX to generate heme. The resulting condition is characterized by PP-IX accumulation, skin photosensitivity, cholestasis, and end-stage liver disease. “Despite available drugs that address photosensitivity, the treatment of EPP-related liver disease remains an unmet need,” Omary and colleagues wrote.
The Study
In order to trigger PP-IX overproduction and accumulation, the investigators administered delta-aminolevulinic acid and deferoxamine to zebrafish. These freshwater tropical fish share many physiological characteristics with humans and have been used to model human disease and develop drugs. Furthermore, these fish are transparent at the larval stage, allowing quantification and visualization of porphyrin, which is fluorescent.
The researchers had screened some 2500 approved and bioactive compounds and identified chlorcyclizine as a potent PP-IX–lowering agent.
High-throughput compound screening of ALA + DFO-treated zebrafish found that the HH-1 blocker reduced zebrafish liver PP-IX levels. The effect of chlorcyclizine was validated in porphyrin-loaded primary mouse hepatocytes, transgenic mice, and mice fed the porphyrinogenic compound 3,5-diethoxycarbonyl-1,4-dihydrocollidine.
Plasma and tissue PP-IX were measured by fluorescence; livers were analyzed by histology, immunoblotting, and quantitative polymerase chain reaction.
Chlorcyclizine-treated zebrafish larvae as well as the two types of mice all showed reduced hepatic PP-IX levels compared with controls. While the neurotransmitter played an important role in PP-IX accumulation in porphyrin-stressed hepatocytes, blockading notably decreased PP-IX levels.
Detailed analysis showed that chlorcyclizine appeared to work through multiple mechanisms, helping the liver clear toxic porphyrin buildup and reducing inflammation. It also decreased the presence of histamine-producing mast cells. The result was less liver injury, decreased porphyrin-triggered protein aggregation and oxidation, and increased clearance of s PP-I in stool.
Interestingly, in both mouse models, chlorcyclizine lowered PP-IX levels in female but not male mice in liver, erythrocytes, and bone marrow. This sex-specific effect appeared to be related to the greater speed at which male murines metabolize the drug, the authors explained in a news release. In rats, for example, the metabolism of chlorcyclizine is 8 times higher in male than in female livers.
The investigators plan to launch a clinical trial in EPP patients to evaluate the effectiveness of chlorcyclizine for both liver and skin involvement. And a phase 2 trial is already underway testing the antacid cimetidine for treating EPP skin manifestations. It is possible that the different antihistamines may act additively or synergistically.
This work was supported by National Institutes of Health (NIH) grants and the Henry and Mala Dorfman Family Professorship of Pediatric Hematology/Oncology.
Omary is a member of the NIH/National Institute of Diabetes and Digestive and Kidney Diseases Data and Safety Monitoring Board of the Porphyrias Consortium.
A provisional patent application has been submitted for the use of H1-receptor blockers with or without receptor blockers to treat protoporphyrias associated with PP-IX accumulation.
From Cellular and Molecular Gastroenterology and Hepatology
IgG-Guided Elimination Diet Beats Sham Diet for IBS Pain
An irritable bowel syndrome (IBS) elimination diet based on a novel IBS–specific, immunoglobulin G (IgG) was superior to a sham diet for abdominal pain, an 8-center, randomized double-blind controlled trial found.
While elimination diets can provide a personalized approach to dietary therapy, existing studies have had serious methodological issues, noted lead author Prashant Singh, MBBS, of the Division of Gastroenterology and Hepatology, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Mich., and colleagues in Gastroenterology.
For example, previous studies on IgG-based diets used assays developed without determining IBS trigger foods or establishing a 95% confidence interval–based cutoff using a healthy control comparison group.
Study Details
From June 2018 to December 2021, 238 IBS patients testing positive for at least one food on 18-food IgG ELISA (enzyme-linked immunosorbent assay) testing and an average daily abdominal pain intensity score of 3.0 to 7.5 on an 11.0-point scale during a 2-week run-in period were randomized for 8 weeks to an experimental antibody-guided diet or to a sham diet. The primary outcome was a 30% decrease in abdominal pain intensity (API) for 2 of the last 4 weeks of treatment.
The overall study population had a mean age of about 40 years, and more than three-quarters were female. The 3 IBS types – constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), and mixed bowel habits-predominant (IBS-M) – accounted for about a third each in both arms.
The experimental diet eliminated foods based on a positive ELISA result. Its sham counterpart had the same number of foods removed as the number of positive-testing food sensitivities, but the foods eliminated in the sham diet had tested negative on the IgG assay.
Participants reported daily abdominal pain intensity, bloating, and stool consistency, and frequency. They also reported dietary compliance and daily medication use.
Of the 238 randomized adults, 223 were included in the modified intention-to-treat analysis. A significantly greater proportion of subjects in the experimental group met the primary outcome than those in the sham group: 59.6% vs 42.1%, P = .02). “This highlights the potential effectiveness of a personalized elimination diet based on a novel IBS-specific IgG assay,” the authors wrote.
Symptom improvement between arms began to separate out at around 2 weeks, suggesting the effect of the experimental diet was relatively rapid in onset, and continued for at least 8 weeks. The durability of response, however, needs to be assessed in future studies “and it is unclear if there is a role for repeat IgG testing to monitor treatment response,” the authors wrote.
Subgroup analysis revealed that a higher proportion of those with IBS-C and IBS-M in the experimental diet group met the primary endpoint vs the sham group: 67.1% vs 35.8% and 66% vs 29.5%, respectively.
Interestingly, more patients in the experimental arm were noncompliant with their diet. “It is possible that subjects found the experimental diet more difficult to comply with compared with the sham diet or that because the experimental diet was more likely to improve symptoms, dietary indiscretion may have been more common in this group (a phenomenon seen with other elimination diets such as gluten-free diet in celiac disease),” the authors wrote.
Adverse events, deemed unrelated to either regimen, were 3 in the experimental arm vs 8 in the sham arm, which had 2 urinary tract infections.
The authors called for a larger, adequately powered study to assess the efficacy of an elimination diet based on this novel immunoglobulin G assay in patients with IBS-C and IBS-M. Future studies should perform detailed adherence assessments using food diaries.
“Mechanisms of how immunoglobulin G-antibody response to food antigen generates symptoms in irritable bowel syndrome are not well understood. Delineating this might provide new insights into food-related irritable bowel syndrome pathophysiology,” they concluded.
This study was funded by Biomerica Inc.
Symptoms in most people with irritable bowel syndrome (IBS) are perceived to be closely linked to diet. The low FODMAP diet has been pivotal for the treatment of IBS, and a range of other diet approaches are now on the research horizon.
Whilst IgE-mediated allergy is relatively rare, there has been research suggesting a role of IgG-mediated food sensitivity in causing symptoms in IBS, although the role of IgG testing and dietary elimination has been controversial. This study from Singh and colleagues suggests an IgG-based elimination diet could improve abdominal pain and global symptoms in two thirds of people with Rome IV IBS. Critically, the study is one of the largest so far and provides the most robust and detailed description of the trial diets to date.
The potential of a new diet approach is extremely appealing, especially as the low FODMAP diet is not universally effective. However, there is still some work to be done to transition the IgG-based elimination diet into guidelines and routine practice. Notably, some common foods restricted in IgG-based elimination diets are also high in FODMAPs leaving questions about the true driver of symptom benefit. Should convincing mechanistic studies and further additional RCT data validate these findings, this could present a major step forward for personalised nutrition in IBS.
Heidi Staudacher, PhD, is associate professor in the School of Translational Medicine, Monash University, Melbourne, Australia. She declared no conflicts of interest.
Symptoms in most people with irritable bowel syndrome (IBS) are perceived to be closely linked to diet. The low FODMAP diet has been pivotal for the treatment of IBS, and a range of other diet approaches are now on the research horizon.
Whilst IgE-mediated allergy is relatively rare, there has been research suggesting a role of IgG-mediated food sensitivity in causing symptoms in IBS, although the role of IgG testing and dietary elimination has been controversial. This study from Singh and colleagues suggests an IgG-based elimination diet could improve abdominal pain and global symptoms in two thirds of people with Rome IV IBS. Critically, the study is one of the largest so far and provides the most robust and detailed description of the trial diets to date.
The potential of a new diet approach is extremely appealing, especially as the low FODMAP diet is not universally effective. However, there is still some work to be done to transition the IgG-based elimination diet into guidelines and routine practice. Notably, some common foods restricted in IgG-based elimination diets are also high in FODMAPs leaving questions about the true driver of symptom benefit. Should convincing mechanistic studies and further additional RCT data validate these findings, this could present a major step forward for personalised nutrition in IBS.
Heidi Staudacher, PhD, is associate professor in the School of Translational Medicine, Monash University, Melbourne, Australia. She declared no conflicts of interest.
Symptoms in most people with irritable bowel syndrome (IBS) are perceived to be closely linked to diet. The low FODMAP diet has been pivotal for the treatment of IBS, and a range of other diet approaches are now on the research horizon.
Whilst IgE-mediated allergy is relatively rare, there has been research suggesting a role of IgG-mediated food sensitivity in causing symptoms in IBS, although the role of IgG testing and dietary elimination has been controversial. This study from Singh and colleagues suggests an IgG-based elimination diet could improve abdominal pain and global symptoms in two thirds of people with Rome IV IBS. Critically, the study is one of the largest so far and provides the most robust and detailed description of the trial diets to date.
The potential of a new diet approach is extremely appealing, especially as the low FODMAP diet is not universally effective. However, there is still some work to be done to transition the IgG-based elimination diet into guidelines and routine practice. Notably, some common foods restricted in IgG-based elimination diets are also high in FODMAPs leaving questions about the true driver of symptom benefit. Should convincing mechanistic studies and further additional RCT data validate these findings, this could present a major step forward for personalised nutrition in IBS.
Heidi Staudacher, PhD, is associate professor in the School of Translational Medicine, Monash University, Melbourne, Australia. She declared no conflicts of interest.
An irritable bowel syndrome (IBS) elimination diet based on a novel IBS–specific, immunoglobulin G (IgG) was superior to a sham diet for abdominal pain, an 8-center, randomized double-blind controlled trial found.
While elimination diets can provide a personalized approach to dietary therapy, existing studies have had serious methodological issues, noted lead author Prashant Singh, MBBS, of the Division of Gastroenterology and Hepatology, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Mich., and colleagues in Gastroenterology.
For example, previous studies on IgG-based diets used assays developed without determining IBS trigger foods or establishing a 95% confidence interval–based cutoff using a healthy control comparison group.
Study Details
From June 2018 to December 2021, 238 IBS patients testing positive for at least one food on 18-food IgG ELISA (enzyme-linked immunosorbent assay) testing and an average daily abdominal pain intensity score of 3.0 to 7.5 on an 11.0-point scale during a 2-week run-in period were randomized for 8 weeks to an experimental antibody-guided diet or to a sham diet. The primary outcome was a 30% decrease in abdominal pain intensity (API) for 2 of the last 4 weeks of treatment.
The overall study population had a mean age of about 40 years, and more than three-quarters were female. The 3 IBS types – constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), and mixed bowel habits-predominant (IBS-M) – accounted for about a third each in both arms.
The experimental diet eliminated foods based on a positive ELISA result. Its sham counterpart had the same number of foods removed as the number of positive-testing food sensitivities, but the foods eliminated in the sham diet had tested negative on the IgG assay.
Participants reported daily abdominal pain intensity, bloating, and stool consistency, and frequency. They also reported dietary compliance and daily medication use.
Of the 238 randomized adults, 223 were included in the modified intention-to-treat analysis. A significantly greater proportion of subjects in the experimental group met the primary outcome than those in the sham group: 59.6% vs 42.1%, P = .02). “This highlights the potential effectiveness of a personalized elimination diet based on a novel IBS-specific IgG assay,” the authors wrote.
Symptom improvement between arms began to separate out at around 2 weeks, suggesting the effect of the experimental diet was relatively rapid in onset, and continued for at least 8 weeks. The durability of response, however, needs to be assessed in future studies “and it is unclear if there is a role for repeat IgG testing to monitor treatment response,” the authors wrote.
Subgroup analysis revealed that a higher proportion of those with IBS-C and IBS-M in the experimental diet group met the primary endpoint vs the sham group: 67.1% vs 35.8% and 66% vs 29.5%, respectively.
Interestingly, more patients in the experimental arm were noncompliant with their diet. “It is possible that subjects found the experimental diet more difficult to comply with compared with the sham diet or that because the experimental diet was more likely to improve symptoms, dietary indiscretion may have been more common in this group (a phenomenon seen with other elimination diets such as gluten-free diet in celiac disease),” the authors wrote.
Adverse events, deemed unrelated to either regimen, were 3 in the experimental arm vs 8 in the sham arm, which had 2 urinary tract infections.
The authors called for a larger, adequately powered study to assess the efficacy of an elimination diet based on this novel immunoglobulin G assay in patients with IBS-C and IBS-M. Future studies should perform detailed adherence assessments using food diaries.
“Mechanisms of how immunoglobulin G-antibody response to food antigen generates symptoms in irritable bowel syndrome are not well understood. Delineating this might provide new insights into food-related irritable bowel syndrome pathophysiology,” they concluded.
This study was funded by Biomerica Inc.
An irritable bowel syndrome (IBS) elimination diet based on a novel IBS–specific, immunoglobulin G (IgG) was superior to a sham diet for abdominal pain, an 8-center, randomized double-blind controlled trial found.
While elimination diets can provide a personalized approach to dietary therapy, existing studies have had serious methodological issues, noted lead author Prashant Singh, MBBS, of the Division of Gastroenterology and Hepatology, Department of Internal Medicine, Michigan Medicine, Ann Arbor, Mich., and colleagues in Gastroenterology.
For example, previous studies on IgG-based diets used assays developed without determining IBS trigger foods or establishing a 95% confidence interval–based cutoff using a healthy control comparison group.
Study Details
From June 2018 to December 2021, 238 IBS patients testing positive for at least one food on 18-food IgG ELISA (enzyme-linked immunosorbent assay) testing and an average daily abdominal pain intensity score of 3.0 to 7.5 on an 11.0-point scale during a 2-week run-in period were randomized for 8 weeks to an experimental antibody-guided diet or to a sham diet. The primary outcome was a 30% decrease in abdominal pain intensity (API) for 2 of the last 4 weeks of treatment.
The overall study population had a mean age of about 40 years, and more than three-quarters were female. The 3 IBS types – constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), and mixed bowel habits-predominant (IBS-M) – accounted for about a third each in both arms.
The experimental diet eliminated foods based on a positive ELISA result. Its sham counterpart had the same number of foods removed as the number of positive-testing food sensitivities, but the foods eliminated in the sham diet had tested negative on the IgG assay.
Participants reported daily abdominal pain intensity, bloating, and stool consistency, and frequency. They also reported dietary compliance and daily medication use.
Of the 238 randomized adults, 223 were included in the modified intention-to-treat analysis. A significantly greater proportion of subjects in the experimental group met the primary outcome than those in the sham group: 59.6% vs 42.1%, P = .02). “This highlights the potential effectiveness of a personalized elimination diet based on a novel IBS-specific IgG assay,” the authors wrote.
Symptom improvement between arms began to separate out at around 2 weeks, suggesting the effect of the experimental diet was relatively rapid in onset, and continued for at least 8 weeks. The durability of response, however, needs to be assessed in future studies “and it is unclear if there is a role for repeat IgG testing to monitor treatment response,” the authors wrote.
Subgroup analysis revealed that a higher proportion of those with IBS-C and IBS-M in the experimental diet group met the primary endpoint vs the sham group: 67.1% vs 35.8% and 66% vs 29.5%, respectively.
Interestingly, more patients in the experimental arm were noncompliant with their diet. “It is possible that subjects found the experimental diet more difficult to comply with compared with the sham diet or that because the experimental diet was more likely to improve symptoms, dietary indiscretion may have been more common in this group (a phenomenon seen with other elimination diets such as gluten-free diet in celiac disease),” the authors wrote.
Adverse events, deemed unrelated to either regimen, were 3 in the experimental arm vs 8 in the sham arm, which had 2 urinary tract infections.
The authors called for a larger, adequately powered study to assess the efficacy of an elimination diet based on this novel immunoglobulin G assay in patients with IBS-C and IBS-M. Future studies should perform detailed adherence assessments using food diaries.
“Mechanisms of how immunoglobulin G-antibody response to food antigen generates symptoms in irritable bowel syndrome are not well understood. Delineating this might provide new insights into food-related irritable bowel syndrome pathophysiology,” they concluded.
This study was funded by Biomerica Inc.
FROM GASTROENTEROLOGY
Papilla Sphincterotomy Shows No Risk Reduction in Pancreas Divisum
SAN DIEGO — , suggesting that patients can be spared the intervention, which can carry risks of its own.
“This is a topic that has been debated for decades,” said first author Gregory A. Coté, MD, AGAF, Division Head, professor of medicine, Division of Gastroenterology & Hepatology, Oregon Health & Science University, in Portland, Oregon.
“Many doctors believe the procedure helps and offer it because we have limited options to help our patients, whereas others believe the procedure is harmful and doesn’t help,” he explained in a press briefing for the late-breaking study, presented at Digestive Disease Week (DDW) 2025.
The study’s findings supported the latter argument.
“Patients who underwent ERCP with sphincterotomy were just as likely as those who did not have this procedure to develop acute pancreatitis again,” Coté reported.
While clinical guidelines currently recommend ERCP as treatment for pancreas divisum, “these guidelines are likely to change based on this study,” he said.
Pancreas divisum, occurring in about 7%-10% of people, is an anatomic variation that can represent an obstructive risk factor for acute recurrent pancreatitis.
The common use of ERCP with minor papilla endoscopic sphincterotomy to treat the condition is based on prior retrospective studies showing that in patients who did develop acute pancreatitis, up to 70% with the treatment never developed acute pancreatitis again. However, there have been no studies comparing the use of the treatment with a control group.
Coté and colleagues conducted the multicenter SHARP trial, in which 148 patients with pancreas divisum were enrolled between September 2018 and August 2024 and randomized to receive either ERCP with minor papilla endoscopic sphincterotomy (n = 75) or a sham treatment (n = 73).
The patients, who had a median age of 51 years, had a median of 3 acute pancreatitis episodes prior to randomization.
With a median follow-up of 33.5 months (range, 6-48 months), 34.7% of patients in the ERCP arm experienced an acute pancreatitis incident compared with 43.8% in the sham arm, for a hazard ratio of 0.83 after adjusting for duct size and the number of episodes, which was not a statistically significant difference (P = .27).
A subgroup analysis further showed no indication of a treatment effect based on factors including age, diabetes status, sex, alcohol or tobacco use, or other factors.
“Compared with a sham ERCP group, we found that minor papillotomy did not reduce the risk of acute pancreatitis, incident chronic pancreatitis, endocrine pancreatic insufficiency or diabetes, or pancreas-related pain events,” Coté said.
The findings are particularly important because the treatment itself is associated with some risks, he added.
“Ironically, the problem with this procedure is that it can cause acute pancreatitis in 10%-20% of patients and may instigate other issues later,” such as the development of scarring of the pancreas related to incisions in the procedure.
“No one wants to offer an expensive procedure that has its own risks if it doesn’t help,” Coté said.
Based on the findings, “pancreas divisum anatomy should no longer be considered an indication for ERCP, even for idiopathic acute pancreatitis,” he concluded.
A version of this article appeared on Medscape.com.
SAN DIEGO — , suggesting that patients can be spared the intervention, which can carry risks of its own.
“This is a topic that has been debated for decades,” said first author Gregory A. Coté, MD, AGAF, Division Head, professor of medicine, Division of Gastroenterology & Hepatology, Oregon Health & Science University, in Portland, Oregon.
“Many doctors believe the procedure helps and offer it because we have limited options to help our patients, whereas others believe the procedure is harmful and doesn’t help,” he explained in a press briefing for the late-breaking study, presented at Digestive Disease Week (DDW) 2025.
The study’s findings supported the latter argument.
“Patients who underwent ERCP with sphincterotomy were just as likely as those who did not have this procedure to develop acute pancreatitis again,” Coté reported.
While clinical guidelines currently recommend ERCP as treatment for pancreas divisum, “these guidelines are likely to change based on this study,” he said.
Pancreas divisum, occurring in about 7%-10% of people, is an anatomic variation that can represent an obstructive risk factor for acute recurrent pancreatitis.
The common use of ERCP with minor papilla endoscopic sphincterotomy to treat the condition is based on prior retrospective studies showing that in patients who did develop acute pancreatitis, up to 70% with the treatment never developed acute pancreatitis again. However, there have been no studies comparing the use of the treatment with a control group.
Coté and colleagues conducted the multicenter SHARP trial, in which 148 patients with pancreas divisum were enrolled between September 2018 and August 2024 and randomized to receive either ERCP with minor papilla endoscopic sphincterotomy (n = 75) or a sham treatment (n = 73).
The patients, who had a median age of 51 years, had a median of 3 acute pancreatitis episodes prior to randomization.
With a median follow-up of 33.5 months (range, 6-48 months), 34.7% of patients in the ERCP arm experienced an acute pancreatitis incident compared with 43.8% in the sham arm, for a hazard ratio of 0.83 after adjusting for duct size and the number of episodes, which was not a statistically significant difference (P = .27).
A subgroup analysis further showed no indication of a treatment effect based on factors including age, diabetes status, sex, alcohol or tobacco use, or other factors.
“Compared with a sham ERCP group, we found that minor papillotomy did not reduce the risk of acute pancreatitis, incident chronic pancreatitis, endocrine pancreatic insufficiency or diabetes, or pancreas-related pain events,” Coté said.
The findings are particularly important because the treatment itself is associated with some risks, he added.
“Ironically, the problem with this procedure is that it can cause acute pancreatitis in 10%-20% of patients and may instigate other issues later,” such as the development of scarring of the pancreas related to incisions in the procedure.
“No one wants to offer an expensive procedure that has its own risks if it doesn’t help,” Coté said.
Based on the findings, “pancreas divisum anatomy should no longer be considered an indication for ERCP, even for idiopathic acute pancreatitis,” he concluded.
A version of this article appeared on Medscape.com.
SAN DIEGO — , suggesting that patients can be spared the intervention, which can carry risks of its own.
“This is a topic that has been debated for decades,” said first author Gregory A. Coté, MD, AGAF, Division Head, professor of medicine, Division of Gastroenterology & Hepatology, Oregon Health & Science University, in Portland, Oregon.
“Many doctors believe the procedure helps and offer it because we have limited options to help our patients, whereas others believe the procedure is harmful and doesn’t help,” he explained in a press briefing for the late-breaking study, presented at Digestive Disease Week (DDW) 2025.
The study’s findings supported the latter argument.
“Patients who underwent ERCP with sphincterotomy were just as likely as those who did not have this procedure to develop acute pancreatitis again,” Coté reported.
While clinical guidelines currently recommend ERCP as treatment for pancreas divisum, “these guidelines are likely to change based on this study,” he said.
Pancreas divisum, occurring in about 7%-10% of people, is an anatomic variation that can represent an obstructive risk factor for acute recurrent pancreatitis.
The common use of ERCP with minor papilla endoscopic sphincterotomy to treat the condition is based on prior retrospective studies showing that in patients who did develop acute pancreatitis, up to 70% with the treatment never developed acute pancreatitis again. However, there have been no studies comparing the use of the treatment with a control group.
Coté and colleagues conducted the multicenter SHARP trial, in which 148 patients with pancreas divisum were enrolled between September 2018 and August 2024 and randomized to receive either ERCP with minor papilla endoscopic sphincterotomy (n = 75) or a sham treatment (n = 73).
The patients, who had a median age of 51 years, had a median of 3 acute pancreatitis episodes prior to randomization.
With a median follow-up of 33.5 months (range, 6-48 months), 34.7% of patients in the ERCP arm experienced an acute pancreatitis incident compared with 43.8% in the sham arm, for a hazard ratio of 0.83 after adjusting for duct size and the number of episodes, which was not a statistically significant difference (P = .27).
A subgroup analysis further showed no indication of a treatment effect based on factors including age, diabetes status, sex, alcohol or tobacco use, or other factors.
“Compared with a sham ERCP group, we found that minor papillotomy did not reduce the risk of acute pancreatitis, incident chronic pancreatitis, endocrine pancreatic insufficiency or diabetes, or pancreas-related pain events,” Coté said.
The findings are particularly important because the treatment itself is associated with some risks, he added.
“Ironically, the problem with this procedure is that it can cause acute pancreatitis in 10%-20% of patients and may instigate other issues later,” such as the development of scarring of the pancreas related to incisions in the procedure.
“No one wants to offer an expensive procedure that has its own risks if it doesn’t help,” Coté said.
Based on the findings, “pancreas divisum anatomy should no longer be considered an indication for ERCP, even for idiopathic acute pancreatitis,” he concluded.
A version of this article appeared on Medscape.com.
FROM DDW 2025
Weekend Workout, Regular Exercise Are Equals at Lowering GI Disease Risk
SAN DIEGO — The session started with a question that many in the audience at Digestive Disease Week (DDW) 2025 seemed to relate to: “How many of you find yourself squeezing workouts into a weekend after a hectic work week?”
Although regular exercise three or more times a week is often viewed as preferable, Shiyi Yu, MD, a resident physician in the Department of Gastroenterology at Guangdong Provincial People’s Hospital in Guangzhou, China, had good news for weekend warriors.
Both patterns reduce digestive disease almost equally.
Her study compared weekend warriors with those she called “active regulars” and sedentary folks to see how activity patterns affect digestive disease risks.
Her bottom line: “Your gut does not care about your schedule.”
The researchers analyzed wrist-based accelerometer data from 89,595 participants in the UK Biobank. To categorize participants as active or inactive, they used the World Health Organization 2020 guidelines for physical activity, which recommend at least 150-300 minutes of moderate-intensity aerobic physical activity or at least 75-150 minutes of vigorous-intensity aerobic physical activity, or an equivalent combination throughout the week. Median age of participants was 63.3 years and 48.8% were men.
They divided participants into three groups:
- About 43% were weekend warriors who met or exceeded 150 minutes of moderate to vigorous physical activity (MVPA), with 50% or more of total MVPA achieved in 1-2 days.
- About 23% were active regulars who met or exceeded 150 minutes a week but spread over more days.
- About 34% were inactive participants who were active less than 150 minutes a week.
The researchers followed the participants for a median of 7.9 years, looking for the incidence of multiple digestive diseases, identified using the International Classification of Diseases, 10th Revision, codes. These included diverticulosis, constipation, metabolic dysfunction–associated steatotic liver disease, cholelithiasis, and gastroesophageal reflux disease.
,” Yu said. At the threshold ≥ 150 minutes, for instance, hazard ratios for any digestive disease were 0.83 for weekend warriors and 0.79 for active regulars, compared with sedentary participants.
The analysis was repeated using a median threshold ≥ 230.4 minutes of MVPA a week, and the researchers found the same results.
As a validation cohort, the researchers used more than 6,000 participants from the National Institutes of Health’s All of Us Research Program with over 6 months of wrist-based accelerometer data.
A recent meta-epidemiology study found that the weekend warrior pattern offers other health benefits, including reducing the risk for cardiovascular disease mortality, mental disorders, and metabolic syndrome.
A Pleasant Surprise
The digestive disease study’s findings were “a surprise and a pleasant one,” said Aasma Shaukat, MD, MPH, AGAF, professor of medicine and a gastroenterologist at NYU Grossman School of Medicine, New York City.
“We often think if we’re not able to exercise regularly, then there’s no hope for us,” said Shaukat, who moderated the session. “But this implies that even if we have time only during the weekend to engage in physical activity, it still confers benefits in reducing our risk of any GI health disorder, as well as cardiovascular or other health disorders, compared to people inactive at baseline.”
“It gives us flexibility in terms of how we structure our exercise. Obviously, people should try to get into the habit of doing regular activity; it’s more sustainable. But a good alternative, according to this research, is that packing all of that in over the weekend seems to confer benefit. So all is not lost.”
Will this change her conversation with patients moving forward? Absolutely, Shaukat said. She generally recommends physical activity for at least 30 minutes three times a week. Now Shaukat said she can tell patients: “If that’s not possible, take that time out during the weekend for your health”.
This study was funded by grants from the National Natural Science Foundation of China and its Regional Innovation and Development Joint Foundation. Yu and Shaukat reported no disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — The session started with a question that many in the audience at Digestive Disease Week (DDW) 2025 seemed to relate to: “How many of you find yourself squeezing workouts into a weekend after a hectic work week?”
Although regular exercise three or more times a week is often viewed as preferable, Shiyi Yu, MD, a resident physician in the Department of Gastroenterology at Guangdong Provincial People’s Hospital in Guangzhou, China, had good news for weekend warriors.
Both patterns reduce digestive disease almost equally.
Her study compared weekend warriors with those she called “active regulars” and sedentary folks to see how activity patterns affect digestive disease risks.
Her bottom line: “Your gut does not care about your schedule.”
The researchers analyzed wrist-based accelerometer data from 89,595 participants in the UK Biobank. To categorize participants as active or inactive, they used the World Health Organization 2020 guidelines for physical activity, which recommend at least 150-300 minutes of moderate-intensity aerobic physical activity or at least 75-150 minutes of vigorous-intensity aerobic physical activity, or an equivalent combination throughout the week. Median age of participants was 63.3 years and 48.8% were men.
They divided participants into three groups:
- About 43% were weekend warriors who met or exceeded 150 minutes of moderate to vigorous physical activity (MVPA), with 50% or more of total MVPA achieved in 1-2 days.
- About 23% were active regulars who met or exceeded 150 minutes a week but spread over more days.
- About 34% were inactive participants who were active less than 150 minutes a week.
The researchers followed the participants for a median of 7.9 years, looking for the incidence of multiple digestive diseases, identified using the International Classification of Diseases, 10th Revision, codes. These included diverticulosis, constipation, metabolic dysfunction–associated steatotic liver disease, cholelithiasis, and gastroesophageal reflux disease.
,” Yu said. At the threshold ≥ 150 minutes, for instance, hazard ratios for any digestive disease were 0.83 for weekend warriors and 0.79 for active regulars, compared with sedentary participants.
The analysis was repeated using a median threshold ≥ 230.4 minutes of MVPA a week, and the researchers found the same results.
As a validation cohort, the researchers used more than 6,000 participants from the National Institutes of Health’s All of Us Research Program with over 6 months of wrist-based accelerometer data.
A recent meta-epidemiology study found that the weekend warrior pattern offers other health benefits, including reducing the risk for cardiovascular disease mortality, mental disorders, and metabolic syndrome.
A Pleasant Surprise
The digestive disease study’s findings were “a surprise and a pleasant one,” said Aasma Shaukat, MD, MPH, AGAF, professor of medicine and a gastroenterologist at NYU Grossman School of Medicine, New York City.
“We often think if we’re not able to exercise regularly, then there’s no hope for us,” said Shaukat, who moderated the session. “But this implies that even if we have time only during the weekend to engage in physical activity, it still confers benefits in reducing our risk of any GI health disorder, as well as cardiovascular or other health disorders, compared to people inactive at baseline.”
“It gives us flexibility in terms of how we structure our exercise. Obviously, people should try to get into the habit of doing regular activity; it’s more sustainable. But a good alternative, according to this research, is that packing all of that in over the weekend seems to confer benefit. So all is not lost.”
Will this change her conversation with patients moving forward? Absolutely, Shaukat said. She generally recommends physical activity for at least 30 minutes three times a week. Now Shaukat said she can tell patients: “If that’s not possible, take that time out during the weekend for your health”.
This study was funded by grants from the National Natural Science Foundation of China and its Regional Innovation and Development Joint Foundation. Yu and Shaukat reported no disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — The session started with a question that many in the audience at Digestive Disease Week (DDW) 2025 seemed to relate to: “How many of you find yourself squeezing workouts into a weekend after a hectic work week?”
Although regular exercise three or more times a week is often viewed as preferable, Shiyi Yu, MD, a resident physician in the Department of Gastroenterology at Guangdong Provincial People’s Hospital in Guangzhou, China, had good news for weekend warriors.
Both patterns reduce digestive disease almost equally.
Her study compared weekend warriors with those she called “active regulars” and sedentary folks to see how activity patterns affect digestive disease risks.
Her bottom line: “Your gut does not care about your schedule.”
The researchers analyzed wrist-based accelerometer data from 89,595 participants in the UK Biobank. To categorize participants as active or inactive, they used the World Health Organization 2020 guidelines for physical activity, which recommend at least 150-300 minutes of moderate-intensity aerobic physical activity or at least 75-150 minutes of vigorous-intensity aerobic physical activity, or an equivalent combination throughout the week. Median age of participants was 63.3 years and 48.8% were men.
They divided participants into three groups:
- About 43% were weekend warriors who met or exceeded 150 minutes of moderate to vigorous physical activity (MVPA), with 50% or more of total MVPA achieved in 1-2 days.
- About 23% were active regulars who met or exceeded 150 minutes a week but spread over more days.
- About 34% were inactive participants who were active less than 150 minutes a week.
The researchers followed the participants for a median of 7.9 years, looking for the incidence of multiple digestive diseases, identified using the International Classification of Diseases, 10th Revision, codes. These included diverticulosis, constipation, metabolic dysfunction–associated steatotic liver disease, cholelithiasis, and gastroesophageal reflux disease.
,” Yu said. At the threshold ≥ 150 minutes, for instance, hazard ratios for any digestive disease were 0.83 for weekend warriors and 0.79 for active regulars, compared with sedentary participants.
The analysis was repeated using a median threshold ≥ 230.4 minutes of MVPA a week, and the researchers found the same results.
As a validation cohort, the researchers used more than 6,000 participants from the National Institutes of Health’s All of Us Research Program with over 6 months of wrist-based accelerometer data.
A recent meta-epidemiology study found that the weekend warrior pattern offers other health benefits, including reducing the risk for cardiovascular disease mortality, mental disorders, and metabolic syndrome.
A Pleasant Surprise
The digestive disease study’s findings were “a surprise and a pleasant one,” said Aasma Shaukat, MD, MPH, AGAF, professor of medicine and a gastroenterologist at NYU Grossman School of Medicine, New York City.
“We often think if we’re not able to exercise regularly, then there’s no hope for us,” said Shaukat, who moderated the session. “But this implies that even if we have time only during the weekend to engage in physical activity, it still confers benefits in reducing our risk of any GI health disorder, as well as cardiovascular or other health disorders, compared to people inactive at baseline.”
“It gives us flexibility in terms of how we structure our exercise. Obviously, people should try to get into the habit of doing regular activity; it’s more sustainable. But a good alternative, according to this research, is that packing all of that in over the weekend seems to confer benefit. So all is not lost.”
Will this change her conversation with patients moving forward? Absolutely, Shaukat said. She generally recommends physical activity for at least 30 minutes three times a week. Now Shaukat said she can tell patients: “If that’s not possible, take that time out during the weekend for your health”.
This study was funded by grants from the National Natural Science Foundation of China and its Regional Innovation and Development Joint Foundation. Yu and Shaukat reported no disclosures.
A version of this article appeared on Medscape.com.
FROM DDW 2025
Do GLP-1s Lower CRC Risk in Patients With Obesity and T2D?
SAN DIEGO — new research showed.
CRC risk was also lower for patients taking GLP-1s than the general population.
“Our findings show we might need to evaluate these therapies beyond their glycemic or weight loss [effects],” said first author Omar Al Ta’ani, MD, of the Allegheny Health Network, Pittsburgh.
This supports future prospective studies examining GLP-1s for CRC reduction, added Ta’ani, who presented the results at Digestive Disease Week (DDW) 2025.
Patients with type 2 diabetes and obesity are known to have a higher risk for CRC, stemming from metabolic risk factors. Whereas prior studies suggested that GLP-1s decrease the risk for CRC compared with other antidiabetic medications, studies looking at the risk for CRC associated with bariatric surgery have had more mixed results, Ta’ani said.
For the comparison, Ta’ani and colleagues conducted a retrospective analysis of the TriNetX database, identifying patients with type 2 diabetes and obesity (body mass index [BMI] > 30) enrolled in the database between 2005 and 2019.
Overall, the study included 94,098 GLP-1 users and 24,969 patients who underwent bariatric surgery. Those with a prior history of CRC were excluded.
Using propensity score matching, patients treated with GLP-1s were matched 1:1 with patients who had bariatric surgery based on wide-ranging factors including age, race, gender, demographics, diseases, medications, personal and family history, and hemoglobin A1c.
After the propensity matching, each group included 21,022 patients. About 64% in each group were women; their median age was 53 years and about 65% were White.
Overall, the results showed that patients on GLP-1s had a significantly lower CRC risk compared with those who had bariatric surgery (adjusted hazard ratio [aHR], 0.29; P < .0001). The lower risk was also observed among those with high obesity (defined as BMI > 35) compared with those who had surgery (aHR, 0.39; P < .0001).
The results were consistent across genders; however, the differences between GLP-1s and bariatric surgery were not observed in the 18- to 45-year-old age group (BMI > 30, P = .0809; BMI > 35, P = .2318).
Compared with the general population, patients on GLP-1s also had a reduced risk for CRC (aHR, 0.28; P < .0001); however, the difference was not observed between the bariatric surgery group and the general population (aHR, 1.11; P = .3).
Among patients with type 2 diabetes with CRC and a BMI > 30, the 5-year mortality rate was lower in the GLP-1 group vs the bariatric surgery group (aHR, 0.42; P < .001).
Speculating on the mechanisms of GLP-1s that could result in a greater reduction in CRC risk, Ta’ani explained that the key pathways linking type 2 diabetes, obesity, and CRC include hyperinsulinemia, chronic inflammation, and impaired immune surveillance.
Studies have shown that GLP-1s may be more effective in addressing the collective pathways, he said. They “may improve insulin resistance and lower systemic inflammation.”
Furthermore, GLP1s “inhibit tumor pathways like Wnt/beta-catenin and PI3K/Akt/mTOR signaling, which promote apoptosis and reduce tumor cell proliferation,” he added.
Bariatric Surgery Findings Questioned
Meanwhile, “bariatric surgery’s impact on CRC remains mixed,” said Ta’ani.
Commenting on the study, Vance L. Albaugh, MD, an assistant professor of metabolic surgery at the Metamor Institute, Pennington Biomedical Research Center, Baton Rouge, Louisiana, noted that prior studies, including a recent meta-analysis, suggest a potential benefit of bariatric surgery in cancer prevention.
“I think the [current study] is interesting, but it’s been pretty [well-reported] that bariatric surgery does decrease cancer incidence, so I find it questionable that this study shows the opposite of what’s in the literature,” Albaugh, an obesity medicine specialist and bariatric surgeon, said in an interview.
Ta’ani acknowledged the study’s important limitations, including that with a retrospective design, causality cannot be firmly established.
And, as noted by an audience member in the session’s Q&A, the study ended in 2019, which was before GLP-1s had taken off as anti-obesity drugs and before US Food and Drug Administration approvals for weight loss.
Participants were matched based on BMI, however, Ta’ani pointed out.
Albaugh agreed that the study ending in 2019 was a notable limitation. However, the relatively long study period — extending from 2005 to 2019 — was a strength.
“It’s nice to have a very long period to capture people who are diagnosed, because it takes a long time to develop CRC,” he said. “To evaluate effects [of more recent drug regimens], you would not be able to have the follow-up they had.”
Other study limitations included the need to adjust for ranges of obesity severity, said Albaugh. “The risk of colorectal cancer is probably much different for someone with a BMI of 60 vs a BMI of 30.”
Ultimately, a key question the study results raise is whether GLP-1 drugs have protective effects above and beyond that of weight loss, he said.
“I think that’s a very exciting question and that’s what I think the researchers’ next work should really focus on.”
Ta’ani had no disclosures to report. Albaugh reported that he had consulted for Novo Nordisk.
A version of this article appeared on Medscape.com.
SAN DIEGO — new research showed.
CRC risk was also lower for patients taking GLP-1s than the general population.
“Our findings show we might need to evaluate these therapies beyond their glycemic or weight loss [effects],” said first author Omar Al Ta’ani, MD, of the Allegheny Health Network, Pittsburgh.
This supports future prospective studies examining GLP-1s for CRC reduction, added Ta’ani, who presented the results at Digestive Disease Week (DDW) 2025.
Patients with type 2 diabetes and obesity are known to have a higher risk for CRC, stemming from metabolic risk factors. Whereas prior studies suggested that GLP-1s decrease the risk for CRC compared with other antidiabetic medications, studies looking at the risk for CRC associated with bariatric surgery have had more mixed results, Ta’ani said.
For the comparison, Ta’ani and colleagues conducted a retrospective analysis of the TriNetX database, identifying patients with type 2 diabetes and obesity (body mass index [BMI] > 30) enrolled in the database between 2005 and 2019.
Overall, the study included 94,098 GLP-1 users and 24,969 patients who underwent bariatric surgery. Those with a prior history of CRC were excluded.
Using propensity score matching, patients treated with GLP-1s were matched 1:1 with patients who had bariatric surgery based on wide-ranging factors including age, race, gender, demographics, diseases, medications, personal and family history, and hemoglobin A1c.
After the propensity matching, each group included 21,022 patients. About 64% in each group were women; their median age was 53 years and about 65% were White.
Overall, the results showed that patients on GLP-1s had a significantly lower CRC risk compared with those who had bariatric surgery (adjusted hazard ratio [aHR], 0.29; P < .0001). The lower risk was also observed among those with high obesity (defined as BMI > 35) compared with those who had surgery (aHR, 0.39; P < .0001).
The results were consistent across genders; however, the differences between GLP-1s and bariatric surgery were not observed in the 18- to 45-year-old age group (BMI > 30, P = .0809; BMI > 35, P = .2318).
Compared with the general population, patients on GLP-1s also had a reduced risk for CRC (aHR, 0.28; P < .0001); however, the difference was not observed between the bariatric surgery group and the general population (aHR, 1.11; P = .3).
Among patients with type 2 diabetes with CRC and a BMI > 30, the 5-year mortality rate was lower in the GLP-1 group vs the bariatric surgery group (aHR, 0.42; P < .001).
Speculating on the mechanisms of GLP-1s that could result in a greater reduction in CRC risk, Ta’ani explained that the key pathways linking type 2 diabetes, obesity, and CRC include hyperinsulinemia, chronic inflammation, and impaired immune surveillance.
Studies have shown that GLP-1s may be more effective in addressing the collective pathways, he said. They “may improve insulin resistance and lower systemic inflammation.”
Furthermore, GLP1s “inhibit tumor pathways like Wnt/beta-catenin and PI3K/Akt/mTOR signaling, which promote apoptosis and reduce tumor cell proliferation,” he added.
Bariatric Surgery Findings Questioned
Meanwhile, “bariatric surgery’s impact on CRC remains mixed,” said Ta’ani.
Commenting on the study, Vance L. Albaugh, MD, an assistant professor of metabolic surgery at the Metamor Institute, Pennington Biomedical Research Center, Baton Rouge, Louisiana, noted that prior studies, including a recent meta-analysis, suggest a potential benefit of bariatric surgery in cancer prevention.
“I think the [current study] is interesting, but it’s been pretty [well-reported] that bariatric surgery does decrease cancer incidence, so I find it questionable that this study shows the opposite of what’s in the literature,” Albaugh, an obesity medicine specialist and bariatric surgeon, said in an interview.
Ta’ani acknowledged the study’s important limitations, including that with a retrospective design, causality cannot be firmly established.
And, as noted by an audience member in the session’s Q&A, the study ended in 2019, which was before GLP-1s had taken off as anti-obesity drugs and before US Food and Drug Administration approvals for weight loss.
Participants were matched based on BMI, however, Ta’ani pointed out.
Albaugh agreed that the study ending in 2019 was a notable limitation. However, the relatively long study period — extending from 2005 to 2019 — was a strength.
“It’s nice to have a very long period to capture people who are diagnosed, because it takes a long time to develop CRC,” he said. “To evaluate effects [of more recent drug regimens], you would not be able to have the follow-up they had.”
Other study limitations included the need to adjust for ranges of obesity severity, said Albaugh. “The risk of colorectal cancer is probably much different for someone with a BMI of 60 vs a BMI of 30.”
Ultimately, a key question the study results raise is whether GLP-1 drugs have protective effects above and beyond that of weight loss, he said.
“I think that’s a very exciting question and that’s what I think the researchers’ next work should really focus on.”
Ta’ani had no disclosures to report. Albaugh reported that he had consulted for Novo Nordisk.
A version of this article appeared on Medscape.com.
SAN DIEGO — new research showed.
CRC risk was also lower for patients taking GLP-1s than the general population.
“Our findings show we might need to evaluate these therapies beyond their glycemic or weight loss [effects],” said first author Omar Al Ta’ani, MD, of the Allegheny Health Network, Pittsburgh.
This supports future prospective studies examining GLP-1s for CRC reduction, added Ta’ani, who presented the results at Digestive Disease Week (DDW) 2025.
Patients with type 2 diabetes and obesity are known to have a higher risk for CRC, stemming from metabolic risk factors. Whereas prior studies suggested that GLP-1s decrease the risk for CRC compared with other antidiabetic medications, studies looking at the risk for CRC associated with bariatric surgery have had more mixed results, Ta’ani said.
For the comparison, Ta’ani and colleagues conducted a retrospective analysis of the TriNetX database, identifying patients with type 2 diabetes and obesity (body mass index [BMI] > 30) enrolled in the database between 2005 and 2019.
Overall, the study included 94,098 GLP-1 users and 24,969 patients who underwent bariatric surgery. Those with a prior history of CRC were excluded.
Using propensity score matching, patients treated with GLP-1s were matched 1:1 with patients who had bariatric surgery based on wide-ranging factors including age, race, gender, demographics, diseases, medications, personal and family history, and hemoglobin A1c.
After the propensity matching, each group included 21,022 patients. About 64% in each group were women; their median age was 53 years and about 65% were White.
Overall, the results showed that patients on GLP-1s had a significantly lower CRC risk compared with those who had bariatric surgery (adjusted hazard ratio [aHR], 0.29; P < .0001). The lower risk was also observed among those with high obesity (defined as BMI > 35) compared with those who had surgery (aHR, 0.39; P < .0001).
The results were consistent across genders; however, the differences between GLP-1s and bariatric surgery were not observed in the 18- to 45-year-old age group (BMI > 30, P = .0809; BMI > 35, P = .2318).
Compared with the general population, patients on GLP-1s also had a reduced risk for CRC (aHR, 0.28; P < .0001); however, the difference was not observed between the bariatric surgery group and the general population (aHR, 1.11; P = .3).
Among patients with type 2 diabetes with CRC and a BMI > 30, the 5-year mortality rate was lower in the GLP-1 group vs the bariatric surgery group (aHR, 0.42; P < .001).
Speculating on the mechanisms of GLP-1s that could result in a greater reduction in CRC risk, Ta’ani explained that the key pathways linking type 2 diabetes, obesity, and CRC include hyperinsulinemia, chronic inflammation, and impaired immune surveillance.
Studies have shown that GLP-1s may be more effective in addressing the collective pathways, he said. They “may improve insulin resistance and lower systemic inflammation.”
Furthermore, GLP1s “inhibit tumor pathways like Wnt/beta-catenin and PI3K/Akt/mTOR signaling, which promote apoptosis and reduce tumor cell proliferation,” he added.
Bariatric Surgery Findings Questioned
Meanwhile, “bariatric surgery’s impact on CRC remains mixed,” said Ta’ani.
Commenting on the study, Vance L. Albaugh, MD, an assistant professor of metabolic surgery at the Metamor Institute, Pennington Biomedical Research Center, Baton Rouge, Louisiana, noted that prior studies, including a recent meta-analysis, suggest a potential benefit of bariatric surgery in cancer prevention.
“I think the [current study] is interesting, but it’s been pretty [well-reported] that bariatric surgery does decrease cancer incidence, so I find it questionable that this study shows the opposite of what’s in the literature,” Albaugh, an obesity medicine specialist and bariatric surgeon, said in an interview.
Ta’ani acknowledged the study’s important limitations, including that with a retrospective design, causality cannot be firmly established.
And, as noted by an audience member in the session’s Q&A, the study ended in 2019, which was before GLP-1s had taken off as anti-obesity drugs and before US Food and Drug Administration approvals for weight loss.
Participants were matched based on BMI, however, Ta’ani pointed out.
Albaugh agreed that the study ending in 2019 was a notable limitation. However, the relatively long study period — extending from 2005 to 2019 — was a strength.
“It’s nice to have a very long period to capture people who are diagnosed, because it takes a long time to develop CRC,” he said. “To evaluate effects [of more recent drug regimens], you would not be able to have the follow-up they had.”
Other study limitations included the need to adjust for ranges of obesity severity, said Albaugh. “The risk of colorectal cancer is probably much different for someone with a BMI of 60 vs a BMI of 30.”
Ultimately, a key question the study results raise is whether GLP-1 drugs have protective effects above and beyond that of weight loss, he said.
“I think that’s a very exciting question and that’s what I think the researchers’ next work should really focus on.”
Ta’ani had no disclosures to report. Albaugh reported that he had consulted for Novo Nordisk.
A version of this article appeared on Medscape.com.
FROM DDW 2025
ctDNA Positivity in Colorectal Cancer Links to Chemotherapy Response
SAN DIEGO — the results of the BESPOKE study showed.
“These findings highlight the value of utilizing ctDNA to select which patients should receive management chemotherapy and which patients can be potentially spared chemotherapy’s physical, emotional, and financial toxicities without compromising their long-term outcomes,” said first author Kim Magee of Natera, a clinical genetic testing company in Austin, Texas.
“ctDNA is emerging as the most powerful and prognostic biomarker in colorectal cancer,” said Magee, who presented the findings at Digestive Disease Week (DDW) 2025.
In stage II CRC, as many as 80% of patients are cured by surgery alone, while only about 5% benefit from chemotherapy. In stage III CRC, about half of patients are cured by surgery alone, while only 20% benefit from chemotherapy, and 30% recur despite chemotherapy, Magee explained.
The inability to pinpoint which patients will most benefit from chemotherapy means “we know we are needlessly treating [many] of these patients,” she said.
ctDNA Offers Insights Into Tumor’s Real-Time Status
Just as cells release fragments (cell-free DNA) into the blood as they regenerate, tumor cells also release fragments — ctDNA — which can represent a biomarker of a cancer’s current state, Magee explained.
Because the DNA fragments have a half-life of only about 2 hours, they represent a key snapshot in real time, “as opposed to imaging, which can take several weeks or months to show changes,” she said.
To determine the effects of ctDNA testing on treatment decisions and asymptomatic recurrence rates, Magee and colleagues analyzed data from the multicenter, prospective study, which used the Signatera (Natera) residual disease test.
The study included 1794 patients with resected stage II-III CRC who were treated with the standard of care between May 2020 and March 2023 who had complete clinical and laboratory data available.
ctDNA was collected 2-6 weeks post surgery and at surveillance months 2, 4, 6, and every 3 months through month 24.
Among the 1166 patients included in a final analysis, 694 (59.5%) patients received adjunctive chemotherapy, and 472 (40.5%) received no chemotherapy.
Among those with stage II CRC, a postoperative MRD positivity rate was 7.54%, while the rate in those with stage III disease was 28.35%.
Overall, 16.1% of patients had a recurrence by the trial end at 24 months.
The results showed that among patients who tested negative for ctDNA, the disease-free survival estimates were highly favorable, at 91.8% for stage II and 87.4% for stage III CRC.
Comparatively, for those who were ctDNA-positive, disease-free survival rates were just 45.9% and 35.5%, respectively, regardless of whether those patients received adjunctive chemotherapy.
At the study’s first ctDNA surveillance timepoint, patients who were ctDNA-positive with stage II and III CRC combined had substantially worse disease-free survival than patients who were ctDNA-negative (HR, 26.4; P < .0001).
Impact of Chemotherapy
Patients who were found to be MRD-positive on ctDNA testing and treated with chemotherapy had a 40.3% 2-year disease-free survival rate compared with just 24.7% among MRD-positive patients who did not receive chemotherapy.
Meanwhile, those who were MRD-negative and treated with chemotherapy had a substantially higher 2-year disease-free survival rate of 89.7% — nearly identical to the 89.5% observed in the no-chemotherapy group.
The findings underscored that “the adjuvant chemotherapy benefits were only observed among those who were ctDNA-positive,” Magee said.
“ctDNA can guide postsurgical treatment decisions by identifying which patients are most likely to benefit from chemotherapy, and in the surveillance setting, ctDNA can predict recurrence — usually ahead of scans,” she added. “This opens the opportunity to intervene and give those patients a second chance at cure.”
On the heels of major recent advances including CT, MRI, and PET-CT, “we believe that ctDNA represents the next major pivotal advancement in monitoring and eventually better understanding cancer diagnostics,” Magee said.
Commenting on the study, William M. Grady, MD, AGAF, medical director of the Fred Hutchinson Cancer Center Gastrointestinal Cancer Prevention Clinic, Seattle, said the BESPOKE trial represents a “well-done” study, adding to research underscoring that “MRD testing is a more accurate prognostic assay than the current standards of CT scan and CEA [carcinoembryonic antigen, a tumor marker] testing.”
However, “a limitation is that this is 2 years of follow-up, [while] 5-year follow-up data would be ideal,” he said in an interview, noting, importantly, that “a small number of patients who have no evidence of disease (NED) at 2 years develop recurrence by 5 years.”
Furthermore, more research demonstrating the outcomes of MRD detection is needed, Grady added.
“A caveat is that studies are still needed showing that if you change your care of patients based on the MRD result, that you improve outcomes,” he said. “These studies are being planned and initiated at this time, from my understanding.”
Oncologists treating patients with CRC are commonly performing MRD assessment with ctDNA assays; however, Grady noted that the practice is still not the standard of care.
Regarding the suggestion of ctDNA representing the next major, pivotal step in cancer monitoring, Grady responded that “I think this is aspirational, and further studies are needed to make this claim.”
However, “it does look like it has the promise to turn out to be true.”
Magee is an employee of Nater. Grady has been on the scientific advisory boards for Guardant Health and Freenome and has consulted for Karius.
A version of this article appeared on Medscape.com.
SAN DIEGO — the results of the BESPOKE study showed.
“These findings highlight the value of utilizing ctDNA to select which patients should receive management chemotherapy and which patients can be potentially spared chemotherapy’s physical, emotional, and financial toxicities without compromising their long-term outcomes,” said first author Kim Magee of Natera, a clinical genetic testing company in Austin, Texas.
“ctDNA is emerging as the most powerful and prognostic biomarker in colorectal cancer,” said Magee, who presented the findings at Digestive Disease Week (DDW) 2025.
In stage II CRC, as many as 80% of patients are cured by surgery alone, while only about 5% benefit from chemotherapy. In stage III CRC, about half of patients are cured by surgery alone, while only 20% benefit from chemotherapy, and 30% recur despite chemotherapy, Magee explained.
The inability to pinpoint which patients will most benefit from chemotherapy means “we know we are needlessly treating [many] of these patients,” she said.
ctDNA Offers Insights Into Tumor’s Real-Time Status
Just as cells release fragments (cell-free DNA) into the blood as they regenerate, tumor cells also release fragments — ctDNA — which can represent a biomarker of a cancer’s current state, Magee explained.
Because the DNA fragments have a half-life of only about 2 hours, they represent a key snapshot in real time, “as opposed to imaging, which can take several weeks or months to show changes,” she said.
To determine the effects of ctDNA testing on treatment decisions and asymptomatic recurrence rates, Magee and colleagues analyzed data from the multicenter, prospective study, which used the Signatera (Natera) residual disease test.
The study included 1794 patients with resected stage II-III CRC who were treated with the standard of care between May 2020 and March 2023 who had complete clinical and laboratory data available.
ctDNA was collected 2-6 weeks post surgery and at surveillance months 2, 4, 6, and every 3 months through month 24.
Among the 1166 patients included in a final analysis, 694 (59.5%) patients received adjunctive chemotherapy, and 472 (40.5%) received no chemotherapy.
Among those with stage II CRC, a postoperative MRD positivity rate was 7.54%, while the rate in those with stage III disease was 28.35%.
Overall, 16.1% of patients had a recurrence by the trial end at 24 months.
The results showed that among patients who tested negative for ctDNA, the disease-free survival estimates were highly favorable, at 91.8% for stage II and 87.4% for stage III CRC.
Comparatively, for those who were ctDNA-positive, disease-free survival rates were just 45.9% and 35.5%, respectively, regardless of whether those patients received adjunctive chemotherapy.
At the study’s first ctDNA surveillance timepoint, patients who were ctDNA-positive with stage II and III CRC combined had substantially worse disease-free survival than patients who were ctDNA-negative (HR, 26.4; P < .0001).
Impact of Chemotherapy
Patients who were found to be MRD-positive on ctDNA testing and treated with chemotherapy had a 40.3% 2-year disease-free survival rate compared with just 24.7% among MRD-positive patients who did not receive chemotherapy.
Meanwhile, those who were MRD-negative and treated with chemotherapy had a substantially higher 2-year disease-free survival rate of 89.7% — nearly identical to the 89.5% observed in the no-chemotherapy group.
The findings underscored that “the adjuvant chemotherapy benefits were only observed among those who were ctDNA-positive,” Magee said.
“ctDNA can guide postsurgical treatment decisions by identifying which patients are most likely to benefit from chemotherapy, and in the surveillance setting, ctDNA can predict recurrence — usually ahead of scans,” she added. “This opens the opportunity to intervene and give those patients a second chance at cure.”
On the heels of major recent advances including CT, MRI, and PET-CT, “we believe that ctDNA represents the next major pivotal advancement in monitoring and eventually better understanding cancer diagnostics,” Magee said.
Commenting on the study, William M. Grady, MD, AGAF, medical director of the Fred Hutchinson Cancer Center Gastrointestinal Cancer Prevention Clinic, Seattle, said the BESPOKE trial represents a “well-done” study, adding to research underscoring that “MRD testing is a more accurate prognostic assay than the current standards of CT scan and CEA [carcinoembryonic antigen, a tumor marker] testing.”
However, “a limitation is that this is 2 years of follow-up, [while] 5-year follow-up data would be ideal,” he said in an interview, noting, importantly, that “a small number of patients who have no evidence of disease (NED) at 2 years develop recurrence by 5 years.”
Furthermore, more research demonstrating the outcomes of MRD detection is needed, Grady added.
“A caveat is that studies are still needed showing that if you change your care of patients based on the MRD result, that you improve outcomes,” he said. “These studies are being planned and initiated at this time, from my understanding.”
Oncologists treating patients with CRC are commonly performing MRD assessment with ctDNA assays; however, Grady noted that the practice is still not the standard of care.
Regarding the suggestion of ctDNA representing the next major, pivotal step in cancer monitoring, Grady responded that “I think this is aspirational, and further studies are needed to make this claim.”
However, “it does look like it has the promise to turn out to be true.”
Magee is an employee of Nater. Grady has been on the scientific advisory boards for Guardant Health and Freenome and has consulted for Karius.
A version of this article appeared on Medscape.com.
SAN DIEGO — the results of the BESPOKE study showed.
“These findings highlight the value of utilizing ctDNA to select which patients should receive management chemotherapy and which patients can be potentially spared chemotherapy’s physical, emotional, and financial toxicities without compromising their long-term outcomes,” said first author Kim Magee of Natera, a clinical genetic testing company in Austin, Texas.
“ctDNA is emerging as the most powerful and prognostic biomarker in colorectal cancer,” said Magee, who presented the findings at Digestive Disease Week (DDW) 2025.
In stage II CRC, as many as 80% of patients are cured by surgery alone, while only about 5% benefit from chemotherapy. In stage III CRC, about half of patients are cured by surgery alone, while only 20% benefit from chemotherapy, and 30% recur despite chemotherapy, Magee explained.
The inability to pinpoint which patients will most benefit from chemotherapy means “we know we are needlessly treating [many] of these patients,” she said.
ctDNA Offers Insights Into Tumor’s Real-Time Status
Just as cells release fragments (cell-free DNA) into the blood as they regenerate, tumor cells also release fragments — ctDNA — which can represent a biomarker of a cancer’s current state, Magee explained.
Because the DNA fragments have a half-life of only about 2 hours, they represent a key snapshot in real time, “as opposed to imaging, which can take several weeks or months to show changes,” she said.
To determine the effects of ctDNA testing on treatment decisions and asymptomatic recurrence rates, Magee and colleagues analyzed data from the multicenter, prospective study, which used the Signatera (Natera) residual disease test.
The study included 1794 patients with resected stage II-III CRC who were treated with the standard of care between May 2020 and March 2023 who had complete clinical and laboratory data available.
ctDNA was collected 2-6 weeks post surgery and at surveillance months 2, 4, 6, and every 3 months through month 24.
Among the 1166 patients included in a final analysis, 694 (59.5%) patients received adjunctive chemotherapy, and 472 (40.5%) received no chemotherapy.
Among those with stage II CRC, a postoperative MRD positivity rate was 7.54%, while the rate in those with stage III disease was 28.35%.
Overall, 16.1% of patients had a recurrence by the trial end at 24 months.
The results showed that among patients who tested negative for ctDNA, the disease-free survival estimates were highly favorable, at 91.8% for stage II and 87.4% for stage III CRC.
Comparatively, for those who were ctDNA-positive, disease-free survival rates were just 45.9% and 35.5%, respectively, regardless of whether those patients received adjunctive chemotherapy.
At the study’s first ctDNA surveillance timepoint, patients who were ctDNA-positive with stage II and III CRC combined had substantially worse disease-free survival than patients who were ctDNA-negative (HR, 26.4; P < .0001).
Impact of Chemotherapy
Patients who were found to be MRD-positive on ctDNA testing and treated with chemotherapy had a 40.3% 2-year disease-free survival rate compared with just 24.7% among MRD-positive patients who did not receive chemotherapy.
Meanwhile, those who were MRD-negative and treated with chemotherapy had a substantially higher 2-year disease-free survival rate of 89.7% — nearly identical to the 89.5% observed in the no-chemotherapy group.
The findings underscored that “the adjuvant chemotherapy benefits were only observed among those who were ctDNA-positive,” Magee said.
“ctDNA can guide postsurgical treatment decisions by identifying which patients are most likely to benefit from chemotherapy, and in the surveillance setting, ctDNA can predict recurrence — usually ahead of scans,” she added. “This opens the opportunity to intervene and give those patients a second chance at cure.”
On the heels of major recent advances including CT, MRI, and PET-CT, “we believe that ctDNA represents the next major pivotal advancement in monitoring and eventually better understanding cancer diagnostics,” Magee said.
Commenting on the study, William M. Grady, MD, AGAF, medical director of the Fred Hutchinson Cancer Center Gastrointestinal Cancer Prevention Clinic, Seattle, said the BESPOKE trial represents a “well-done” study, adding to research underscoring that “MRD testing is a more accurate prognostic assay than the current standards of CT scan and CEA [carcinoembryonic antigen, a tumor marker] testing.”
However, “a limitation is that this is 2 years of follow-up, [while] 5-year follow-up data would be ideal,” he said in an interview, noting, importantly, that “a small number of patients who have no evidence of disease (NED) at 2 years develop recurrence by 5 years.”
Furthermore, more research demonstrating the outcomes of MRD detection is needed, Grady added.
“A caveat is that studies are still needed showing that if you change your care of patients based on the MRD result, that you improve outcomes,” he said. “These studies are being planned and initiated at this time, from my understanding.”
Oncologists treating patients with CRC are commonly performing MRD assessment with ctDNA assays; however, Grady noted that the practice is still not the standard of care.
Regarding the suggestion of ctDNA representing the next major, pivotal step in cancer monitoring, Grady responded that “I think this is aspirational, and further studies are needed to make this claim.”
However, “it does look like it has the promise to turn out to be true.”
Magee is an employee of Nater. Grady has been on the scientific advisory boards for Guardant Health and Freenome and has consulted for Karius.
A version of this article appeared on Medscape.com.
FROM DDW 2025
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Since 1999, annual deaths attributed to opioid overdose in the United States have increased from about 10,000 to about 50,000 in 2019.1 During the COVID-19 pandemic > 74,000 opioid overdose deaths occurred in the US from April 2020 to April 2021.2,3 Opioid-related overdoses now account for about 75% of all drug-related overdose deaths.1 In 2017, the cost of opioid overdose deaths and opioid use disorder (OUD) reached $1.02 trillion in the United States and $26 million in Indiana.4 The total deaths and costs would likely be higher if it were not for naloxone.
Naloxone hydrochloride was first patented in the 1960s and approved by the US Food and Drug Administration (FDA) in 1971 to treat opioid-related toxicity.1 It is the most frequently prescribed antidote for opioid toxicity due to its activity as a pure υ-opioid receptor competitive antagonist. Naloxone formulations include intramuscular, intravenous, subcutaneous, and intranasal delivery methods.5 According to the Centers for Disease Control and Prevention, clinicians should offer naloxone to patients at high risk for opioid-related adverse events. Risk factors include a history of overdose, opioid dosages of ≥ 50 morphine mg equivalents/day, and concurrent use of opioids with benzodiazepines.6
Intranasal naloxone 4 mg has become more accessible following the classification of opioid use as a public health emergency in 2017 and its over-the-counter availability since 2023. Intranasal naloxone 4 mg was approved by the FDA in 2015 for the prevention of opioid overdoses (accidental or intentional), which can be caused by heroin, fentanyl, carfentanil, hydrocodone, oxycodone, methadone, and other substances. 7 Fentanyl has most recently been associated with xylazine, a nonopioid tranquilizer linked to increased opioid overdose deaths.8 Recent data suggest that 34% of opioid overdose reversals involved ≥ 2 doses of intranasal naloxone 4 mg, which led to FDA approval of an intranasal naloxone 8 mg spray in April 2021.9-11
Veteran Health Indiana (VHI) has implemented several initiatives to promote naloxone prescribing. Established in 2020, the Opioid Overdose Education and Naloxone Distribution (OEND) program sought to prevent opioid-related deaths through education and product distribution. These criteria included an opioid prescription for ≥ 30 days. In 2021, the Stratification Tool for Opioid Risk Mitigation (STORM) was created to identify patients at high risk of opioid overdose and allowing pharmacists to prescribe naloxone for at-risk patients without restrictions, increasing accessibility.12
Recent cases of fentanyl-related overdoses involving stronger fentanyl analogues highlight the need for higher naloxone dosing to prevent overdose. A pharmacokinetic comparison of intranasal naloxone 8 mg vs 4 mg demonstrated maximum plasma concentrations of 10.3 ng/mL and 5.3 ng/mL, respectively. 13 Patients may be at an increased risk of precipitated opioid withdrawal when using intranasal naloxone 8 mg over 4 mg; however, some patients may benefit from achieving higher serum concentrations and therefore require larger doses of naloxone.
No clinical trials have demonstrated a difference in reversal rates between naloxone doses. No clinical practice guidelines support a specific naloxone formulation, and limited US Department of Veterans Affairs (VA)-specific guidance exists. VA Naloxone Rescue: Recommendations for Use states that selection of naloxone 8 mg should be based on shared decision-making between the patient and clinician and based on individual risk factors.12 The purpose of this study is to analyze data to determine if there is a difference in prescribing patterns of intranasal naloxone 4 mg and intranasal naloxone 8 mg.
METHODS
A retrospective chart reviews using the VA Computerized Patient Record System (CPRS) analyzed patients prescribed intranasal naloxone 4 mg or intranasal naloxone 8 mg at VHI. A patient list was generated based on active naloxone prescriptions between April 1, 2022, and April 1, 2023. Data were obtained exclusively through CPRS and patients were not contacted. This study was reviewed and deemed exempt by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
Patients were included if they were aged ≥ 18 years and had an active prescription for intranasal naloxone 4 mg or intranasal naloxone 8 mg during the trial period. Patients were excluded if their naloxone prescription was written by a non-VHI clinician, if the dose was not 4 mg or 8 mg, or if the dosage form was other than intranasal spray.
The primary endpoint was the comparison for prescribing patterns for intranasal naloxone 4 mg and intranasal naloxone 8 mg during the study period. Secondary endpoints included total naloxone prescriptions; monthly prescriptions; number of patients with repeated naloxone prescriptions; prescriber type by naloxone dose; clinic type by naloxone dose; and documented indication for naloxone use by dose.
Demographic data collected included baseline age, sex, race, comorbid mental health conditions, and active central nervous system depressant medications on patient profile (ie, opioids, gabapentinoids, benzodiazepines, antidepressants, antipsychotics). Opioid prescriptions that were active or discontinued within the last 3 months were also recorded. Comorbid mental health conditions were collected based on the most recent clinical note before initiating medication.
Prescription-related data included strength of medication prescribed (4 mg, 8 mg, or both), documented use of medication, prescriber name, prescriber discipline, prescription entered by, number of times naloxone was filled or refilled during the study period, indication, clinic location, and clinic name. If > 1 prescription was active during the study period, the number of refills, prescriber name and clinic location of the first prescription in the study period was recorded. Additionally, the indication of OUD was differentiated from substance use disorder (SUD) if the patient was only dependent on opioids, excluding tobacco or alcohol. Patients with SUDs may include opioid dependence in addition to other substance dependence (eg, cannabis, stimulants, gabapentinoids, or benzodiazepines).
Basic descriptive statistics, including mean, ranges, and percentages were used to characterize the study subjects. For nominal data, X2 tests were used. A 2-sided 5% significance level was used for all statistical tests.
RESULTS
A total of 1952 active naloxone prescriptions from 1739 patients met the inclusion criteria; none were eliminated based on the exclusion criteria and some were included multiple times because data were collected for each active prescription during the study period. One hundred one patients were randomized and included in the final analysis (Figure). Most patients identified as White (81%), male (90%), and had a mean (SD) age of 60.9 (14.2) years. Common mental health comorbidities included 59 patients with depression, 50 with tobacco use disorder, and 31 with anxiety. Eighty-four patients had opioid and 60 had antidepressants/antianxiety, and 40 had gabapentinoids prescriptions. Forty-three patients had ≥ 3 mental health comorbidities. Thirty-four patients had 2 active central nervous system depressant prescriptions, 30 had 3 active prescriptions, and 9 had ≥ 4 active prescriptions. Most patients (n = 83) had an active or recently discontinued opioid prescription (Table 1).
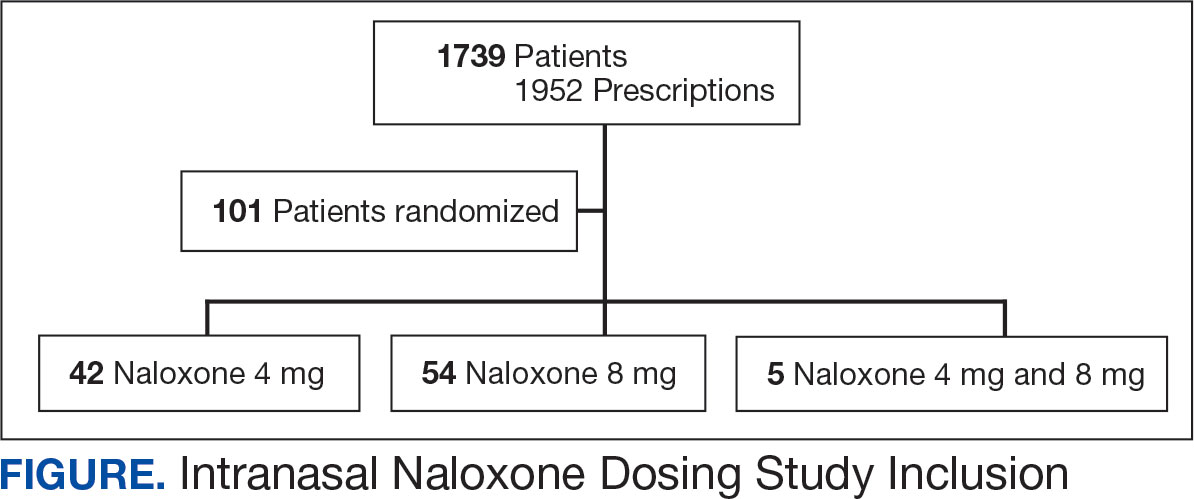
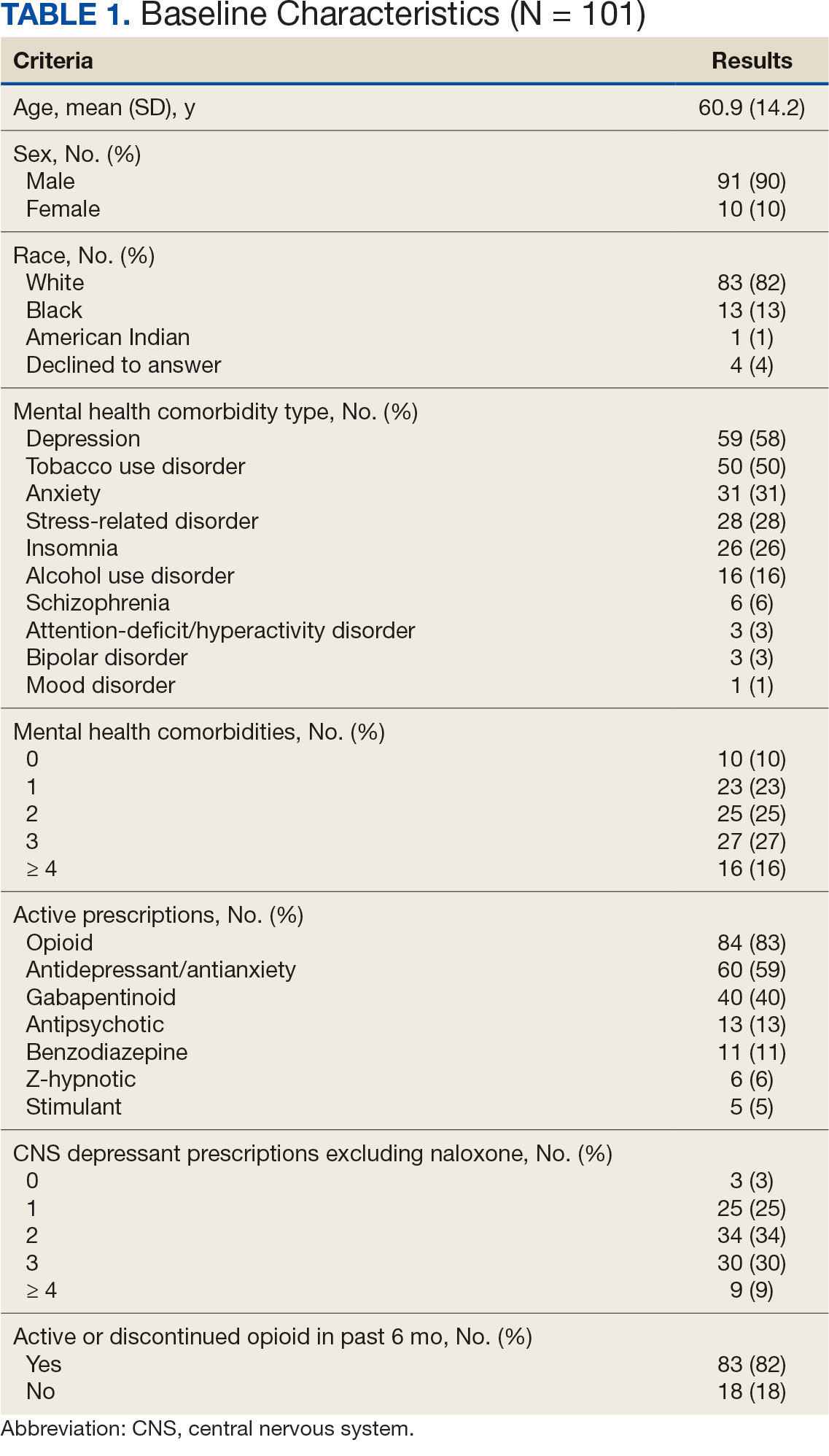
The 101 patients received 54 prescriptions for naloxone 8 mg and 47 for 4 mg (Table 2). Five patients received prescriptions for both the 4 mg and 8 mg intranasal naloxone formulations. Sixty-six patients had naloxone filled once (66%) during the study period. Intranasal naloxone 4 mg was prescribed to 30 patients by nurse practitioners, 17 patients by physicians, and not prescribed by pharmacists. Intranasal naloxone 8 mg was prescribed to 40 patients by pharmacists, 13 patients by physicians, and 6 patients by nurses. Patients who received prescriptions for both intranasal naloxone 4 mg and 8 mg were most routinely ordered by physicians (n = 3; 60%) in primary care (n = 2; 40%) for chronic opioid use (n = 2; 40%).
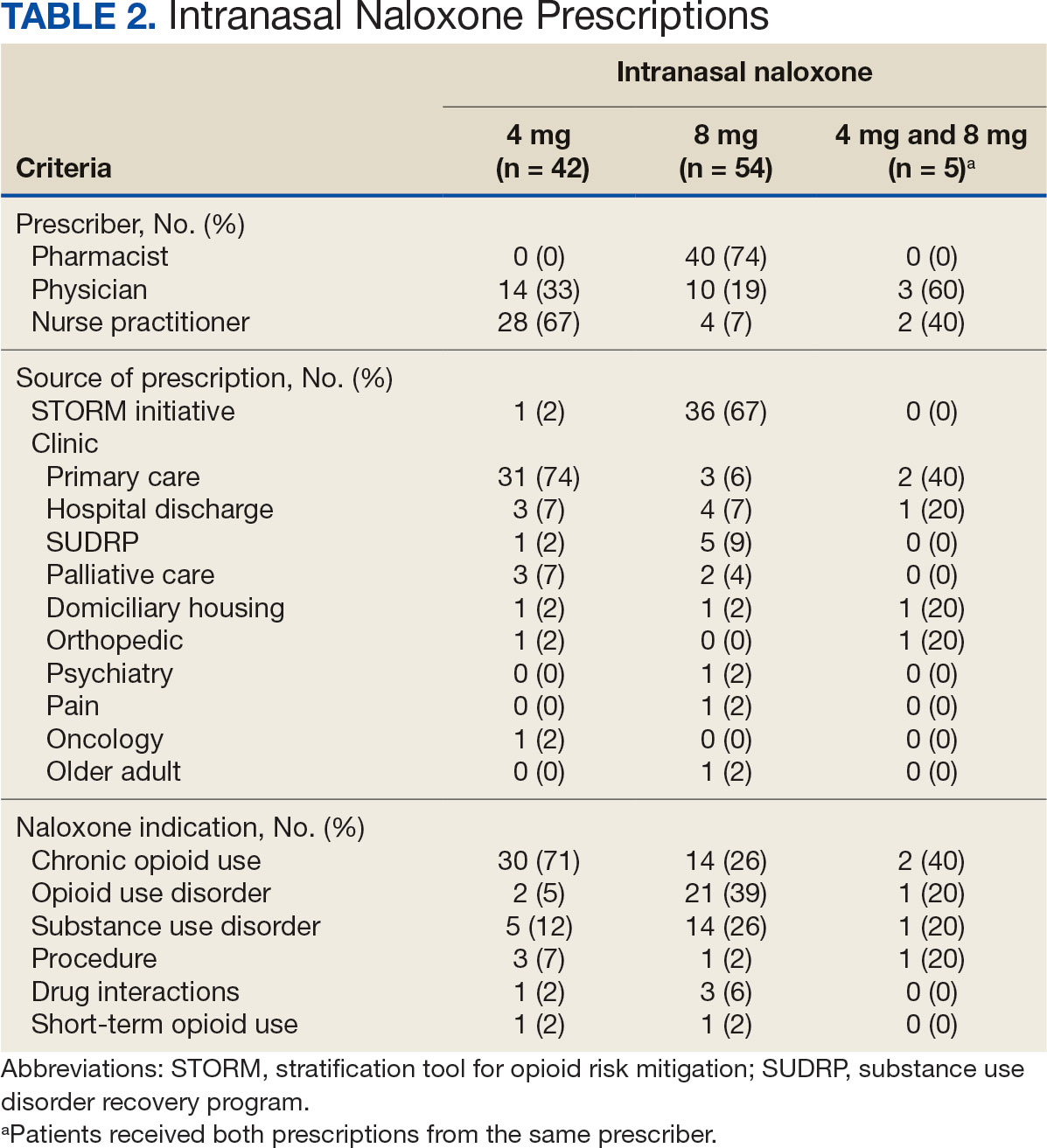
Patients access naloxone from many different VHI clinics. Primary care clinics prescribed the 4 mg formulation to 31 patients, 8 mg to 3 patients, and both to 2 patients. The STORM initiative was used for 37 of 106 prescriptions (35%): 4 mg intranasal naloxone was prescribed to 1 patient, 8 mg to 36 patients, and no patients received both formulations. Chronic opioid use was the most common indication (46%) with 30 patients prescribed intranasal naloxone 4 mg, 14 patients prescribed 8 mg, and 2 patients prescribed both. OUD was the indication for 24% of patients: 2 patients prescribed intranasal naloxone 4 mg, 21 patients prescribed 8 mg, and 1 patient prescribed both.
The 106 intranasal naloxone prescriptions were equally distributed across each month from April 1, 2022, to April 1, 2023. Of the 101 patients, 34 had multiple naloxone prescriptions filled during the study period. Pharmacists wrote 40 of 106 naloxone prescriptions (38%), all for the 8 mg formulation. Nurse practitioners prescribed naloxone 4 mg 30 times and 8 mg 6 times for 36 of 106 prescriptions (34%). Physicians prescribed 30 of 106 prescriptions (28%), including intranasal naloxone 4 mg 17 times and 8 mg 13 times.
Statistics were analyzed using a X2 test; however, it was determined that the expected frequencies made the tests inappropriate. Differences in prescribing patterns between naloxone doses, prescriber disciplines, source of the prescription, or indications were not statistically significant.
DISCUSSION
Many pharmacists possess a scope of practice under state law and/or institution policy to prescribe naloxone. In this study, pharmacists prescribed the most naloxone prescriptions compared to physicians and nurse practitioners. Initiatives such as OEND and STORM have given pharmacists at VHI an avenue to combat the growing opioid epidemic while expanding their scope of practice. A systematic review of 67 studies found that pharmacist-led OEND programs showed a statistically significant increase in naloxone orders. A statistical significance was likely met given the large sample sizes ranging from 10 to 217,000 individuals, whereas this study only assessed a small portion of patients.14 This study contributes to the overwhelming amount of data that highlights pharmacists’ impact on overall naloxone distribution.
The STORM initiative and primary care clinics were responsible for large portions of naloxone prescriptions in this study. STORM was used by pharmacists and contributed to more than half of the higher dose naloxone prescriptions. Following a discussion with members of the pain management team, pharmacists involved in STORM prescribing were revealed to exclusively prescribe intranasal naloxone 8 mg as opposed to 4 mg. At the risk of precipitating withdrawal from higher doses of naloxone, it was agreed that this risk was heavily outweighed by the benefit of successful opioid reversal. In this context, it is expected for this avenue of prescribing to influence naloxone prescribing patterns at VHI.
Prescribing in primary care clinics was shown to be equally as substantial. Primary care-based multidisciplinary transition clinics have been reported to be associated with increased access to OUD treatment.15 Primary care clinics at VHI, or patient aligned care teams (PACT), largely consist of multidisciplinary health care teams. PACT clinicians are heavily involved in transitions of care because one system provides patients with comprehensive acute and chronic care. Continuing to encourage naloxone distribution through primary care and using STORM affords various patient populations access to high-level care.
Notable differences were observed between indications for naloxone use and the corresponding dose. Patients with OUD or SUD were more likely to receive intranasal naloxone 8 mg as opposed to patients receiving intranasal naloxone for chronic opioid use, who were more likely to receive the 4 mg dose. This may be due to a rationale to provide a higher dose of naloxone to combat overdoses in the case of ingesting substances mixed with fentanyl or xylazine.12,13 Without standard of care guidelines, concerns remain for varying outcomes in opioid overdose prevention within vulnerable populations.
Limitations
Chart data were dependent on documentation, which may have omitted pertinent baseline characteristics and risk factors. Additional data collection could have further assessed a patient’s specific risk factors (eg, opioid dose in morphine equivalents) to draw conclusions to the dose of naloxone prescribed. The sample size was small, and the patient population was largely White and male, which minimized the generalizability of the results.
CONCLUSIONS
This study evaluated the differences in intranasal naloxone prescribing patterns within a veteran population at VHI over 12 months. Findings revealed that most prescriptions were written for intranasal naloxone 8 mg, by a pharmacist, in a primary care setting, and for chronic opioid use. The results revealed evidence of differing naloxone prescribing practices, which emphasize the need for clinical guidelines and better defined recommendations in relation to naloxone dosing.
The most evident gap in patient care could be addressed by urging the VA Pharmacy Benefits Management group to update naloxone recommendations for use to include more concrete dosing recommendations. Furthermore, it would be beneficial to re-educate clinicians on naloxone prescribing to increase awareness of different doses and the importance of equipping patients with the correct amount of naloxone in an emergency. Additional research assessing change in prescribing patterns is warranted as the use of higher dose naloxone becomes more routine.
- Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl). 2022;239(7):2063-2081. doi:10.1007/s00213-022-06125-5
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics, Centers for Disease Control and Prevention; 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740-1746. doi:10.15585/mmwr.mm7050e3
- Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541-546. doi:10.15585/mmwr.mm7015a1
- Lexicomp. Lexicomp Online. Accessed April 10, 2025. http://online.lexi.com
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi:10.15585/mmwr.rr7103a1
- Narcan (naloxone) FDA approval history. Drugs.com. Accessed April 10, 2025. https://www.drugs.com/history/narcan.html
- Centers for Disease Control and Prevention. What you should know about xylazine. May 16, 2024. Accessed April 10, 2025. https://www.cdc.gov/overdose-prevention/about/what-you-should-know-about-xylazine.html
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573-576. doi:10.1080/03007995.2017.1334637
- Kloxxado [package insert]. Hikma Pharmaceuticals USA Inc; 2021.
- FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. News release. FDA. April 30, 2021. Accessed April 10, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose
- US Department of Veterans Affairs, Pharmacy Benefits Management Services and National Formulary Committee in Collaboration with the VA National Harm Reduction Support & Development Workgroup. Naloxone Rescue: Recommendations for Use. June 2014. Updated March 2024. Accessed April 10, 2025. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Naloxone_Rescue_Guidance_March_2024.pdf
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243-1253. doi:10.1002/jcph.759
- Rawal S, Osae SP, Cobran EK, Albert A, Young HN. Pharmacists’ naloxone services beyond community pharmacy settings: a systematic review. Res Social Adm Pharm. 2023;19(2):243-265. doi:10.1016/j.sapharm.2022.09.002
- Incze MA, Sehgal SL, Hansen A, Garcia L, Stolebarger L. Evaluation of a primary care-based multidisciplinary transition clinic for patients newly initiated on buprenorphine in the emergency department. Subst Abus. 2023;44(3):220-225. doi:10.1177/08897077231188592
Since 1999, annual deaths attributed to opioid overdose in the United States have increased from about 10,000 to about 50,000 in 2019.1 During the COVID-19 pandemic > 74,000 opioid overdose deaths occurred in the US from April 2020 to April 2021.2,3 Opioid-related overdoses now account for about 75% of all drug-related overdose deaths.1 In 2017, the cost of opioid overdose deaths and opioid use disorder (OUD) reached $1.02 trillion in the United States and $26 million in Indiana.4 The total deaths and costs would likely be higher if it were not for naloxone.
Naloxone hydrochloride was first patented in the 1960s and approved by the US Food and Drug Administration (FDA) in 1971 to treat opioid-related toxicity.1 It is the most frequently prescribed antidote for opioid toxicity due to its activity as a pure υ-opioid receptor competitive antagonist. Naloxone formulations include intramuscular, intravenous, subcutaneous, and intranasal delivery methods.5 According to the Centers for Disease Control and Prevention, clinicians should offer naloxone to patients at high risk for opioid-related adverse events. Risk factors include a history of overdose, opioid dosages of ≥ 50 morphine mg equivalents/day, and concurrent use of opioids with benzodiazepines.6
Intranasal naloxone 4 mg has become more accessible following the classification of opioid use as a public health emergency in 2017 and its over-the-counter availability since 2023. Intranasal naloxone 4 mg was approved by the FDA in 2015 for the prevention of opioid overdoses (accidental or intentional), which can be caused by heroin, fentanyl, carfentanil, hydrocodone, oxycodone, methadone, and other substances. 7 Fentanyl has most recently been associated with xylazine, a nonopioid tranquilizer linked to increased opioid overdose deaths.8 Recent data suggest that 34% of opioid overdose reversals involved ≥ 2 doses of intranasal naloxone 4 mg, which led to FDA approval of an intranasal naloxone 8 mg spray in April 2021.9-11
Veteran Health Indiana (VHI) has implemented several initiatives to promote naloxone prescribing. Established in 2020, the Opioid Overdose Education and Naloxone Distribution (OEND) program sought to prevent opioid-related deaths through education and product distribution. These criteria included an opioid prescription for ≥ 30 days. In 2021, the Stratification Tool for Opioid Risk Mitigation (STORM) was created to identify patients at high risk of opioid overdose and allowing pharmacists to prescribe naloxone for at-risk patients without restrictions, increasing accessibility.12
Recent cases of fentanyl-related overdoses involving stronger fentanyl analogues highlight the need for higher naloxone dosing to prevent overdose. A pharmacokinetic comparison of intranasal naloxone 8 mg vs 4 mg demonstrated maximum plasma concentrations of 10.3 ng/mL and 5.3 ng/mL, respectively. 13 Patients may be at an increased risk of precipitated opioid withdrawal when using intranasal naloxone 8 mg over 4 mg; however, some patients may benefit from achieving higher serum concentrations and therefore require larger doses of naloxone.
No clinical trials have demonstrated a difference in reversal rates between naloxone doses. No clinical practice guidelines support a specific naloxone formulation, and limited US Department of Veterans Affairs (VA)-specific guidance exists. VA Naloxone Rescue: Recommendations for Use states that selection of naloxone 8 mg should be based on shared decision-making between the patient and clinician and based on individual risk factors.12 The purpose of this study is to analyze data to determine if there is a difference in prescribing patterns of intranasal naloxone 4 mg and intranasal naloxone 8 mg.
METHODS
A retrospective chart reviews using the VA Computerized Patient Record System (CPRS) analyzed patients prescribed intranasal naloxone 4 mg or intranasal naloxone 8 mg at VHI. A patient list was generated based on active naloxone prescriptions between April 1, 2022, and April 1, 2023. Data were obtained exclusively through CPRS and patients were not contacted. This study was reviewed and deemed exempt by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
Patients were included if they were aged ≥ 18 years and had an active prescription for intranasal naloxone 4 mg or intranasal naloxone 8 mg during the trial period. Patients were excluded if their naloxone prescription was written by a non-VHI clinician, if the dose was not 4 mg or 8 mg, or if the dosage form was other than intranasal spray.
The primary endpoint was the comparison for prescribing patterns for intranasal naloxone 4 mg and intranasal naloxone 8 mg during the study period. Secondary endpoints included total naloxone prescriptions; monthly prescriptions; number of patients with repeated naloxone prescriptions; prescriber type by naloxone dose; clinic type by naloxone dose; and documented indication for naloxone use by dose.
Demographic data collected included baseline age, sex, race, comorbid mental health conditions, and active central nervous system depressant medications on patient profile (ie, opioids, gabapentinoids, benzodiazepines, antidepressants, antipsychotics). Opioid prescriptions that were active or discontinued within the last 3 months were also recorded. Comorbid mental health conditions were collected based on the most recent clinical note before initiating medication.
Prescription-related data included strength of medication prescribed (4 mg, 8 mg, or both), documented use of medication, prescriber name, prescriber discipline, prescription entered by, number of times naloxone was filled or refilled during the study period, indication, clinic location, and clinic name. If > 1 prescription was active during the study period, the number of refills, prescriber name and clinic location of the first prescription in the study period was recorded. Additionally, the indication of OUD was differentiated from substance use disorder (SUD) if the patient was only dependent on opioids, excluding tobacco or alcohol. Patients with SUDs may include opioid dependence in addition to other substance dependence (eg, cannabis, stimulants, gabapentinoids, or benzodiazepines).
Basic descriptive statistics, including mean, ranges, and percentages were used to characterize the study subjects. For nominal data, X2 tests were used. A 2-sided 5% significance level was used for all statistical tests.
RESULTS
A total of 1952 active naloxone prescriptions from 1739 patients met the inclusion criteria; none were eliminated based on the exclusion criteria and some were included multiple times because data were collected for each active prescription during the study period. One hundred one patients were randomized and included in the final analysis (Figure). Most patients identified as White (81%), male (90%), and had a mean (SD) age of 60.9 (14.2) years. Common mental health comorbidities included 59 patients with depression, 50 with tobacco use disorder, and 31 with anxiety. Eighty-four patients had opioid and 60 had antidepressants/antianxiety, and 40 had gabapentinoids prescriptions. Forty-three patients had ≥ 3 mental health comorbidities. Thirty-four patients had 2 active central nervous system depressant prescriptions, 30 had 3 active prescriptions, and 9 had ≥ 4 active prescriptions. Most patients (n = 83) had an active or recently discontinued opioid prescription (Table 1).


The 101 patients received 54 prescriptions for naloxone 8 mg and 47 for 4 mg (Table 2). Five patients received prescriptions for both the 4 mg and 8 mg intranasal naloxone formulations. Sixty-six patients had naloxone filled once (66%) during the study period. Intranasal naloxone 4 mg was prescribed to 30 patients by nurse practitioners, 17 patients by physicians, and not prescribed by pharmacists. Intranasal naloxone 8 mg was prescribed to 40 patients by pharmacists, 13 patients by physicians, and 6 patients by nurses. Patients who received prescriptions for both intranasal naloxone 4 mg and 8 mg were most routinely ordered by physicians (n = 3; 60%) in primary care (n = 2; 40%) for chronic opioid use (n = 2; 40%).

Patients access naloxone from many different VHI clinics. Primary care clinics prescribed the 4 mg formulation to 31 patients, 8 mg to 3 patients, and both to 2 patients. The STORM initiative was used for 37 of 106 prescriptions (35%): 4 mg intranasal naloxone was prescribed to 1 patient, 8 mg to 36 patients, and no patients received both formulations. Chronic opioid use was the most common indication (46%) with 30 patients prescribed intranasal naloxone 4 mg, 14 patients prescribed 8 mg, and 2 patients prescribed both. OUD was the indication for 24% of patients: 2 patients prescribed intranasal naloxone 4 mg, 21 patients prescribed 8 mg, and 1 patient prescribed both.
The 106 intranasal naloxone prescriptions were equally distributed across each month from April 1, 2022, to April 1, 2023. Of the 101 patients, 34 had multiple naloxone prescriptions filled during the study period. Pharmacists wrote 40 of 106 naloxone prescriptions (38%), all for the 8 mg formulation. Nurse practitioners prescribed naloxone 4 mg 30 times and 8 mg 6 times for 36 of 106 prescriptions (34%). Physicians prescribed 30 of 106 prescriptions (28%), including intranasal naloxone 4 mg 17 times and 8 mg 13 times.
Statistics were analyzed using a X2 test; however, it was determined that the expected frequencies made the tests inappropriate. Differences in prescribing patterns between naloxone doses, prescriber disciplines, source of the prescription, or indications were not statistically significant.
DISCUSSION
Many pharmacists possess a scope of practice under state law and/or institution policy to prescribe naloxone. In this study, pharmacists prescribed the most naloxone prescriptions compared to physicians and nurse practitioners. Initiatives such as OEND and STORM have given pharmacists at VHI an avenue to combat the growing opioid epidemic while expanding their scope of practice. A systematic review of 67 studies found that pharmacist-led OEND programs showed a statistically significant increase in naloxone orders. A statistical significance was likely met given the large sample sizes ranging from 10 to 217,000 individuals, whereas this study only assessed a small portion of patients.14 This study contributes to the overwhelming amount of data that highlights pharmacists’ impact on overall naloxone distribution.
The STORM initiative and primary care clinics were responsible for large portions of naloxone prescriptions in this study. STORM was used by pharmacists and contributed to more than half of the higher dose naloxone prescriptions. Following a discussion with members of the pain management team, pharmacists involved in STORM prescribing were revealed to exclusively prescribe intranasal naloxone 8 mg as opposed to 4 mg. At the risk of precipitating withdrawal from higher doses of naloxone, it was agreed that this risk was heavily outweighed by the benefit of successful opioid reversal. In this context, it is expected for this avenue of prescribing to influence naloxone prescribing patterns at VHI.
Prescribing in primary care clinics was shown to be equally as substantial. Primary care-based multidisciplinary transition clinics have been reported to be associated with increased access to OUD treatment.15 Primary care clinics at VHI, or patient aligned care teams (PACT), largely consist of multidisciplinary health care teams. PACT clinicians are heavily involved in transitions of care because one system provides patients with comprehensive acute and chronic care. Continuing to encourage naloxone distribution through primary care and using STORM affords various patient populations access to high-level care.
Notable differences were observed between indications for naloxone use and the corresponding dose. Patients with OUD or SUD were more likely to receive intranasal naloxone 8 mg as opposed to patients receiving intranasal naloxone for chronic opioid use, who were more likely to receive the 4 mg dose. This may be due to a rationale to provide a higher dose of naloxone to combat overdoses in the case of ingesting substances mixed with fentanyl or xylazine.12,13 Without standard of care guidelines, concerns remain for varying outcomes in opioid overdose prevention within vulnerable populations.
Limitations
Chart data were dependent on documentation, which may have omitted pertinent baseline characteristics and risk factors. Additional data collection could have further assessed a patient’s specific risk factors (eg, opioid dose in morphine equivalents) to draw conclusions to the dose of naloxone prescribed. The sample size was small, and the patient population was largely White and male, which minimized the generalizability of the results.
CONCLUSIONS
This study evaluated the differences in intranasal naloxone prescribing patterns within a veteran population at VHI over 12 months. Findings revealed that most prescriptions were written for intranasal naloxone 8 mg, by a pharmacist, in a primary care setting, and for chronic opioid use. The results revealed evidence of differing naloxone prescribing practices, which emphasize the need for clinical guidelines and better defined recommendations in relation to naloxone dosing.
The most evident gap in patient care could be addressed by urging the VA Pharmacy Benefits Management group to update naloxone recommendations for use to include more concrete dosing recommendations. Furthermore, it would be beneficial to re-educate clinicians on naloxone prescribing to increase awareness of different doses and the importance of equipping patients with the correct amount of naloxone in an emergency. Additional research assessing change in prescribing patterns is warranted as the use of higher dose naloxone becomes more routine.
Since 1999, annual deaths attributed to opioid overdose in the United States have increased from about 10,000 to about 50,000 in 2019.1 During the COVID-19 pandemic > 74,000 opioid overdose deaths occurred in the US from April 2020 to April 2021.2,3 Opioid-related overdoses now account for about 75% of all drug-related overdose deaths.1 In 2017, the cost of opioid overdose deaths and opioid use disorder (OUD) reached $1.02 trillion in the United States and $26 million in Indiana.4 The total deaths and costs would likely be higher if it were not for naloxone.
Naloxone hydrochloride was first patented in the 1960s and approved by the US Food and Drug Administration (FDA) in 1971 to treat opioid-related toxicity.1 It is the most frequently prescribed antidote for opioid toxicity due to its activity as a pure υ-opioid receptor competitive antagonist. Naloxone formulations include intramuscular, intravenous, subcutaneous, and intranasal delivery methods.5 According to the Centers for Disease Control and Prevention, clinicians should offer naloxone to patients at high risk for opioid-related adverse events. Risk factors include a history of overdose, opioid dosages of ≥ 50 morphine mg equivalents/day, and concurrent use of opioids with benzodiazepines.6
Intranasal naloxone 4 mg has become more accessible following the classification of opioid use as a public health emergency in 2017 and its over-the-counter availability since 2023. Intranasal naloxone 4 mg was approved by the FDA in 2015 for the prevention of opioid overdoses (accidental or intentional), which can be caused by heroin, fentanyl, carfentanil, hydrocodone, oxycodone, methadone, and other substances. 7 Fentanyl has most recently been associated with xylazine, a nonopioid tranquilizer linked to increased opioid overdose deaths.8 Recent data suggest that 34% of opioid overdose reversals involved ≥ 2 doses of intranasal naloxone 4 mg, which led to FDA approval of an intranasal naloxone 8 mg spray in April 2021.9-11
Veteran Health Indiana (VHI) has implemented several initiatives to promote naloxone prescribing. Established in 2020, the Opioid Overdose Education and Naloxone Distribution (OEND) program sought to prevent opioid-related deaths through education and product distribution. These criteria included an opioid prescription for ≥ 30 days. In 2021, the Stratification Tool for Opioid Risk Mitigation (STORM) was created to identify patients at high risk of opioid overdose and allowing pharmacists to prescribe naloxone for at-risk patients without restrictions, increasing accessibility.12
Recent cases of fentanyl-related overdoses involving stronger fentanyl analogues highlight the need for higher naloxone dosing to prevent overdose. A pharmacokinetic comparison of intranasal naloxone 8 mg vs 4 mg demonstrated maximum plasma concentrations of 10.3 ng/mL and 5.3 ng/mL, respectively. 13 Patients may be at an increased risk of precipitated opioid withdrawal when using intranasal naloxone 8 mg over 4 mg; however, some patients may benefit from achieving higher serum concentrations and therefore require larger doses of naloxone.
No clinical trials have demonstrated a difference in reversal rates between naloxone doses. No clinical practice guidelines support a specific naloxone formulation, and limited US Department of Veterans Affairs (VA)-specific guidance exists. VA Naloxone Rescue: Recommendations for Use states that selection of naloxone 8 mg should be based on shared decision-making between the patient and clinician and based on individual risk factors.12 The purpose of this study is to analyze data to determine if there is a difference in prescribing patterns of intranasal naloxone 4 mg and intranasal naloxone 8 mg.
METHODS
A retrospective chart reviews using the VA Computerized Patient Record System (CPRS) analyzed patients prescribed intranasal naloxone 4 mg or intranasal naloxone 8 mg at VHI. A patient list was generated based on active naloxone prescriptions between April 1, 2022, and April 1, 2023. Data were obtained exclusively through CPRS and patients were not contacted. This study was reviewed and deemed exempt by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
Patients were included if they were aged ≥ 18 years and had an active prescription for intranasal naloxone 4 mg or intranasal naloxone 8 mg during the trial period. Patients were excluded if their naloxone prescription was written by a non-VHI clinician, if the dose was not 4 mg or 8 mg, or if the dosage form was other than intranasal spray.
The primary endpoint was the comparison for prescribing patterns for intranasal naloxone 4 mg and intranasal naloxone 8 mg during the study period. Secondary endpoints included total naloxone prescriptions; monthly prescriptions; number of patients with repeated naloxone prescriptions; prescriber type by naloxone dose; clinic type by naloxone dose; and documented indication for naloxone use by dose.
Demographic data collected included baseline age, sex, race, comorbid mental health conditions, and active central nervous system depressant medications on patient profile (ie, opioids, gabapentinoids, benzodiazepines, antidepressants, antipsychotics). Opioid prescriptions that were active or discontinued within the last 3 months were also recorded. Comorbid mental health conditions were collected based on the most recent clinical note before initiating medication.
Prescription-related data included strength of medication prescribed (4 mg, 8 mg, or both), documented use of medication, prescriber name, prescriber discipline, prescription entered by, number of times naloxone was filled or refilled during the study period, indication, clinic location, and clinic name. If > 1 prescription was active during the study period, the number of refills, prescriber name and clinic location of the first prescription in the study period was recorded. Additionally, the indication of OUD was differentiated from substance use disorder (SUD) if the patient was only dependent on opioids, excluding tobacco or alcohol. Patients with SUDs may include opioid dependence in addition to other substance dependence (eg, cannabis, stimulants, gabapentinoids, or benzodiazepines).
Basic descriptive statistics, including mean, ranges, and percentages were used to characterize the study subjects. For nominal data, X2 tests were used. A 2-sided 5% significance level was used for all statistical tests.
RESULTS
A total of 1952 active naloxone prescriptions from 1739 patients met the inclusion criteria; none were eliminated based on the exclusion criteria and some were included multiple times because data were collected for each active prescription during the study period. One hundred one patients were randomized and included in the final analysis (Figure). Most patients identified as White (81%), male (90%), and had a mean (SD) age of 60.9 (14.2) years. Common mental health comorbidities included 59 patients with depression, 50 with tobacco use disorder, and 31 with anxiety. Eighty-four patients had opioid and 60 had antidepressants/antianxiety, and 40 had gabapentinoids prescriptions. Forty-three patients had ≥ 3 mental health comorbidities. Thirty-four patients had 2 active central nervous system depressant prescriptions, 30 had 3 active prescriptions, and 9 had ≥ 4 active prescriptions. Most patients (n = 83) had an active or recently discontinued opioid prescription (Table 1).


The 101 patients received 54 prescriptions for naloxone 8 mg and 47 for 4 mg (Table 2). Five patients received prescriptions for both the 4 mg and 8 mg intranasal naloxone formulations. Sixty-six patients had naloxone filled once (66%) during the study period. Intranasal naloxone 4 mg was prescribed to 30 patients by nurse practitioners, 17 patients by physicians, and not prescribed by pharmacists. Intranasal naloxone 8 mg was prescribed to 40 patients by pharmacists, 13 patients by physicians, and 6 patients by nurses. Patients who received prescriptions for both intranasal naloxone 4 mg and 8 mg were most routinely ordered by physicians (n = 3; 60%) in primary care (n = 2; 40%) for chronic opioid use (n = 2; 40%).

Patients access naloxone from many different VHI clinics. Primary care clinics prescribed the 4 mg formulation to 31 patients, 8 mg to 3 patients, and both to 2 patients. The STORM initiative was used for 37 of 106 prescriptions (35%): 4 mg intranasal naloxone was prescribed to 1 patient, 8 mg to 36 patients, and no patients received both formulations. Chronic opioid use was the most common indication (46%) with 30 patients prescribed intranasal naloxone 4 mg, 14 patients prescribed 8 mg, and 2 patients prescribed both. OUD was the indication for 24% of patients: 2 patients prescribed intranasal naloxone 4 mg, 21 patients prescribed 8 mg, and 1 patient prescribed both.
The 106 intranasal naloxone prescriptions were equally distributed across each month from April 1, 2022, to April 1, 2023. Of the 101 patients, 34 had multiple naloxone prescriptions filled during the study period. Pharmacists wrote 40 of 106 naloxone prescriptions (38%), all for the 8 mg formulation. Nurse practitioners prescribed naloxone 4 mg 30 times and 8 mg 6 times for 36 of 106 prescriptions (34%). Physicians prescribed 30 of 106 prescriptions (28%), including intranasal naloxone 4 mg 17 times and 8 mg 13 times.
Statistics were analyzed using a X2 test; however, it was determined that the expected frequencies made the tests inappropriate. Differences in prescribing patterns between naloxone doses, prescriber disciplines, source of the prescription, or indications were not statistically significant.
DISCUSSION
Many pharmacists possess a scope of practice under state law and/or institution policy to prescribe naloxone. In this study, pharmacists prescribed the most naloxone prescriptions compared to physicians and nurse practitioners. Initiatives such as OEND and STORM have given pharmacists at VHI an avenue to combat the growing opioid epidemic while expanding their scope of practice. A systematic review of 67 studies found that pharmacist-led OEND programs showed a statistically significant increase in naloxone orders. A statistical significance was likely met given the large sample sizes ranging from 10 to 217,000 individuals, whereas this study only assessed a small portion of patients.14 This study contributes to the overwhelming amount of data that highlights pharmacists’ impact on overall naloxone distribution.
The STORM initiative and primary care clinics were responsible for large portions of naloxone prescriptions in this study. STORM was used by pharmacists and contributed to more than half of the higher dose naloxone prescriptions. Following a discussion with members of the pain management team, pharmacists involved in STORM prescribing were revealed to exclusively prescribe intranasal naloxone 8 mg as opposed to 4 mg. At the risk of precipitating withdrawal from higher doses of naloxone, it was agreed that this risk was heavily outweighed by the benefit of successful opioid reversal. In this context, it is expected for this avenue of prescribing to influence naloxone prescribing patterns at VHI.
Prescribing in primary care clinics was shown to be equally as substantial. Primary care-based multidisciplinary transition clinics have been reported to be associated with increased access to OUD treatment.15 Primary care clinics at VHI, or patient aligned care teams (PACT), largely consist of multidisciplinary health care teams. PACT clinicians are heavily involved in transitions of care because one system provides patients with comprehensive acute and chronic care. Continuing to encourage naloxone distribution through primary care and using STORM affords various patient populations access to high-level care.
Notable differences were observed between indications for naloxone use and the corresponding dose. Patients with OUD or SUD were more likely to receive intranasal naloxone 8 mg as opposed to patients receiving intranasal naloxone for chronic opioid use, who were more likely to receive the 4 mg dose. This may be due to a rationale to provide a higher dose of naloxone to combat overdoses in the case of ingesting substances mixed with fentanyl or xylazine.12,13 Without standard of care guidelines, concerns remain for varying outcomes in opioid overdose prevention within vulnerable populations.
Limitations
Chart data were dependent on documentation, which may have omitted pertinent baseline characteristics and risk factors. Additional data collection could have further assessed a patient’s specific risk factors (eg, opioid dose in morphine equivalents) to draw conclusions to the dose of naloxone prescribed. The sample size was small, and the patient population was largely White and male, which minimized the generalizability of the results.
CONCLUSIONS
This study evaluated the differences in intranasal naloxone prescribing patterns within a veteran population at VHI over 12 months. Findings revealed that most prescriptions were written for intranasal naloxone 8 mg, by a pharmacist, in a primary care setting, and for chronic opioid use. The results revealed evidence of differing naloxone prescribing practices, which emphasize the need for clinical guidelines and better defined recommendations in relation to naloxone dosing.
The most evident gap in patient care could be addressed by urging the VA Pharmacy Benefits Management group to update naloxone recommendations for use to include more concrete dosing recommendations. Furthermore, it would be beneficial to re-educate clinicians on naloxone prescribing to increase awareness of different doses and the importance of equipping patients with the correct amount of naloxone in an emergency. Additional research assessing change in prescribing patterns is warranted as the use of higher dose naloxone becomes more routine.
- Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl). 2022;239(7):2063-2081. doi:10.1007/s00213-022-06125-5
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics, Centers for Disease Control and Prevention; 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740-1746. doi:10.15585/mmwr.mm7050e3
- Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541-546. doi:10.15585/mmwr.mm7015a1
- Lexicomp. Lexicomp Online. Accessed April 10, 2025. http://online.lexi.com
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi:10.15585/mmwr.rr7103a1
- Narcan (naloxone) FDA approval history. Drugs.com. Accessed April 10, 2025. https://www.drugs.com/history/narcan.html
- Centers for Disease Control and Prevention. What you should know about xylazine. May 16, 2024. Accessed April 10, 2025. https://www.cdc.gov/overdose-prevention/about/what-you-should-know-about-xylazine.html
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573-576. doi:10.1080/03007995.2017.1334637
- Kloxxado [package insert]. Hikma Pharmaceuticals USA Inc; 2021.
- FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. News release. FDA. April 30, 2021. Accessed April 10, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose
- US Department of Veterans Affairs, Pharmacy Benefits Management Services and National Formulary Committee in Collaboration with the VA National Harm Reduction Support & Development Workgroup. Naloxone Rescue: Recommendations for Use. June 2014. Updated March 2024. Accessed April 10, 2025. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Naloxone_Rescue_Guidance_March_2024.pdf
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243-1253. doi:10.1002/jcph.759
- Rawal S, Osae SP, Cobran EK, Albert A, Young HN. Pharmacists’ naloxone services beyond community pharmacy settings: a systematic review. Res Social Adm Pharm. 2023;19(2):243-265. doi:10.1016/j.sapharm.2022.09.002
- Incze MA, Sehgal SL, Hansen A, Garcia L, Stolebarger L. Evaluation of a primary care-based multidisciplinary transition clinic for patients newly initiated on buprenorphine in the emergency department. Subst Abus. 2023;44(3):220-225. doi:10.1177/08897077231188592
- Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl). 2022;239(7):2063-2081. doi:10.1007/s00213-022-06125-5
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics, Centers for Disease Control and Prevention; 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740-1746. doi:10.15585/mmwr.mm7050e3
- Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541-546. doi:10.15585/mmwr.mm7015a1
- Lexicomp. Lexicomp Online. Accessed April 10, 2025. http://online.lexi.com
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi:10.15585/mmwr.rr7103a1
- Narcan (naloxone) FDA approval history. Drugs.com. Accessed April 10, 2025. https://www.drugs.com/history/narcan.html
- Centers for Disease Control and Prevention. What you should know about xylazine. May 16, 2024. Accessed April 10, 2025. https://www.cdc.gov/overdose-prevention/about/what-you-should-know-about-xylazine.html
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573-576. doi:10.1080/03007995.2017.1334637
- Kloxxado [package insert]. Hikma Pharmaceuticals USA Inc; 2021.
- FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. News release. FDA. April 30, 2021. Accessed April 10, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose
- US Department of Veterans Affairs, Pharmacy Benefits Management Services and National Formulary Committee in Collaboration with the VA National Harm Reduction Support & Development Workgroup. Naloxone Rescue: Recommendations for Use. June 2014. Updated March 2024. Accessed April 10, 2025. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Naloxone_Rescue_Guidance_March_2024.pdf
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243-1253. doi:10.1002/jcph.759
- Rawal S, Osae SP, Cobran EK, Albert A, Young HN. Pharmacists’ naloxone services beyond community pharmacy settings: a systematic review. Res Social Adm Pharm. 2023;19(2):243-265. doi:10.1016/j.sapharm.2022.09.002
- Incze MA, Sehgal SL, Hansen A, Garcia L, Stolebarger L. Evaluation of a primary care-based multidisciplinary transition clinic for patients newly initiated on buprenorphine in the emergency department. Subst Abus. 2023;44(3):220-225. doi:10.1177/08897077231188592
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas














