User login
SGLT2 Inhibitors Reduce Portal Hypertension From Cirrhosis
SAN DIEGO — , new research shows.
“Our study found that SGLT2 inhibitors were associated with fewer portal hypertension complications and lower mortality, suggesting they may be a valuable addition to cirrhosis management,” first author Abhinav K. Rao, MD, of the Medical University of South Carolina, Charleston, South Carolina, told GI & Hepatology News.
The findings were presented at Digestive Disease Week (DDW) 2025.
Portal hypertension, a potentially life-threatening complication of cirrhosis, can be a key driver of additional complications including ascites and gastro-esophageal varices in cirrhosis.
Current treatments such as beta-blockers can prevent some complications, however, more effective therapies are needed.
SGLT2 inhibitors are often used in the treatment of cardiovascular disease as well as metabolic dysfunction–associated steatohepatitis (MASH)–mediated liver disease; research is lacking regarding their effects in portal hypertension in the broader population of people with cirrhosis.
“The therapeutic efficacy of SGLT2 inhibitors might be related to their ability to improve vascular function, making them attractive in portal hypertension,” Rao explained.
To investigate, Rao and colleagues evaluated data on 637,079 patients with cirrhosis in the TriNetX database, which includes patients in the United States from 66 healthcare organizations.
Patients were divided into three subgroups, including those with MASH, alcohol-associated, and other etiologies of cirrhosis.
Using robust 1:1 propensity score matching, patients in each subgroup were stratified as either having or not having been treated with SGLT2 inhibitors, limited to those who initiated the drugs within 1 year of their cirrhosis diagnosis to prevent immortal time bias. Patients were matched on other characteristics.
For the primary outcome of all-cause mortality, with an overall median follow-up of 2 years, patients prescribed SGLT2 inhibitors in the MASH cirrhosis (n = 47,385), alcohol-associated cirrhosis (n = 107,844), and other etiologies of cirrhosis (n = 59,499) groups all had a significantly lower risk for all-cause mortality than those not prescribed SGLT2 inhibitors (P < .05 for all).
SGLT2 Inhibitors in MASH Cirrhosis
Specifically looking at the MASH cirrhosis group, Rao described outcomes of the two groups of 3026 patients each who were and were not treated with SGLT2 inhibitors.
The patients had similar rates of esophageal varices (25% in the SGLT2 group and 22% in the no SGLT2 group), ascites (19% in each group), and a similar rate of 19% had hepatic encephalopathy (HE).
About 57% of patients in each treatment group used beta-blockers and 33% used glucagon-like peptide 1 (GLP-1) receptor agonists. Those with a history of liver transplantation, hemodialysis, or transjugular intrahepatic portosystemic shunt placement were excluded.
The secondary outcome results in those patients showed that treatment with SGLT2 inhibitors was associated with significantly reduced risks of developing portal hypertension complications including ascites, HE, spontaneous bacterial peritonitis (SBP), and hepatorenal syndrome (P < .05 for all).
Esophageal variceal bleeding was also reduced with SGLT-2 inhibitors; however the difference was not statistically significant.
Effects Diminished With Beta-Blocker Treatment
In a secondary analysis of patients in the MASH cirrhosis group treated with one type of a nonselective beta-blockers (n = 509) and another nonselective beta-blockers (n = 2561), the beneficial effects of SGLT2 inhibitors on portal hypertension, with the exception of HE and SBP, were found to be somewhat diminished, likely because patients were already benefitting from the beta-blockers, Rao noted.
Other Groups
In outcomes of the non–MASH-related cirrhosis groups, patients prescribed SGLT2 inhibitors also had a reduced risk for specific, as well as any portal hypertension complications (P < .05), Rao noted.
Overall, the findings add to previous studies on SGLT2 inhibitors in MASH and expand on the possible benefits, he said.
“Our findings validate these [previous] results and suggest potential benefits across for patients with other types of liver disease and raise the possibility of a beneficial effect in portal hypertension,” he said.
“Given the marked reduction in portal hypertension complications after SGLT2 inhibitor initiation, the associated survival benefit may not be surprising,” he noted.
“However, we were intrigued by the consistent reduction in portal hypertension complications across all cirrhosis types, especially since SGLT-2 inhibitors are most commonly used in patients with diabetes who have MASH-mediated liver disease.”
‘Real World Glimpse’ at SGLT2 Inhibitors; Limitations Need Noting
Commenting on the study, Rotonya M. Carr, MD, Division Head of Gastroenterology at the University of Washington, Seattle, said the study sheds important light on an issue previously addressed only in smaller cohorts.
“To date, there have only been a few small prospective, retrospective, and case series studies investigating SGTL2 inhibitors in patients with cirrhosis,” she told GI & Hepatology Newsv.
“This retrospective study is a real-world glimpse at how patients with cirrhosis may fare on these drugs — very exciting data.”
Carr cautioned, however, that, in addition to the retrospective study design, limitations included that the study doesn’t provide details on the duration of therapy, preventing an understanding of whether the results represent chronic, sustained use of SGLT2 inhibitors.
“[Therefore], we cannot interpret these results to mean that chronic, sustained use of SGTL2 inh is beneficial, or does not cause harm, in patients with cirrhosis.”
“While these data are provocative, more work needs to be done before we understand the full safety and efficacy of SGTL2 inhibitors for patients with cirrhosis,” Carr added.
“However, these data are very encouraging, and I am optimistic that we will indeed see both SGTL2 inhibitors and GLP-1s among the group of medications we use in the future for the primary management of patients with liver disease.”
The authors had no disclosures to report. Carr’s disclosures included relationships with Intercept and Novo Nordisk and research funding from Merck.
A version of this article appeared on Medscape.com.
SAN DIEGO — , new research shows.
“Our study found that SGLT2 inhibitors were associated with fewer portal hypertension complications and lower mortality, suggesting they may be a valuable addition to cirrhosis management,” first author Abhinav K. Rao, MD, of the Medical University of South Carolina, Charleston, South Carolina, told GI & Hepatology News.
The findings were presented at Digestive Disease Week (DDW) 2025.
Portal hypertension, a potentially life-threatening complication of cirrhosis, can be a key driver of additional complications including ascites and gastro-esophageal varices in cirrhosis.
Current treatments such as beta-blockers can prevent some complications, however, more effective therapies are needed.
SGLT2 inhibitors are often used in the treatment of cardiovascular disease as well as metabolic dysfunction–associated steatohepatitis (MASH)–mediated liver disease; research is lacking regarding their effects in portal hypertension in the broader population of people with cirrhosis.
“The therapeutic efficacy of SGLT2 inhibitors might be related to their ability to improve vascular function, making them attractive in portal hypertension,” Rao explained.
To investigate, Rao and colleagues evaluated data on 637,079 patients with cirrhosis in the TriNetX database, which includes patients in the United States from 66 healthcare organizations.
Patients were divided into three subgroups, including those with MASH, alcohol-associated, and other etiologies of cirrhosis.
Using robust 1:1 propensity score matching, patients in each subgroup were stratified as either having or not having been treated with SGLT2 inhibitors, limited to those who initiated the drugs within 1 year of their cirrhosis diagnosis to prevent immortal time bias. Patients were matched on other characteristics.
For the primary outcome of all-cause mortality, with an overall median follow-up of 2 years, patients prescribed SGLT2 inhibitors in the MASH cirrhosis (n = 47,385), alcohol-associated cirrhosis (n = 107,844), and other etiologies of cirrhosis (n = 59,499) groups all had a significantly lower risk for all-cause mortality than those not prescribed SGLT2 inhibitors (P < .05 for all).
SGLT2 Inhibitors in MASH Cirrhosis
Specifically looking at the MASH cirrhosis group, Rao described outcomes of the two groups of 3026 patients each who were and were not treated with SGLT2 inhibitors.
The patients had similar rates of esophageal varices (25% in the SGLT2 group and 22% in the no SGLT2 group), ascites (19% in each group), and a similar rate of 19% had hepatic encephalopathy (HE).
About 57% of patients in each treatment group used beta-blockers and 33% used glucagon-like peptide 1 (GLP-1) receptor agonists. Those with a history of liver transplantation, hemodialysis, or transjugular intrahepatic portosystemic shunt placement were excluded.
The secondary outcome results in those patients showed that treatment with SGLT2 inhibitors was associated with significantly reduced risks of developing portal hypertension complications including ascites, HE, spontaneous bacterial peritonitis (SBP), and hepatorenal syndrome (P < .05 for all).
Esophageal variceal bleeding was also reduced with SGLT-2 inhibitors; however the difference was not statistically significant.
Effects Diminished With Beta-Blocker Treatment
In a secondary analysis of patients in the MASH cirrhosis group treated with one type of a nonselective beta-blockers (n = 509) and another nonselective beta-blockers (n = 2561), the beneficial effects of SGLT2 inhibitors on portal hypertension, with the exception of HE and SBP, were found to be somewhat diminished, likely because patients were already benefitting from the beta-blockers, Rao noted.
Other Groups
In outcomes of the non–MASH-related cirrhosis groups, patients prescribed SGLT2 inhibitors also had a reduced risk for specific, as well as any portal hypertension complications (P < .05), Rao noted.
Overall, the findings add to previous studies on SGLT2 inhibitors in MASH and expand on the possible benefits, he said.
“Our findings validate these [previous] results and suggest potential benefits across for patients with other types of liver disease and raise the possibility of a beneficial effect in portal hypertension,” he said.
“Given the marked reduction in portal hypertension complications after SGLT2 inhibitor initiation, the associated survival benefit may not be surprising,” he noted.
“However, we were intrigued by the consistent reduction in portal hypertension complications across all cirrhosis types, especially since SGLT-2 inhibitors are most commonly used in patients with diabetes who have MASH-mediated liver disease.”
‘Real World Glimpse’ at SGLT2 Inhibitors; Limitations Need Noting
Commenting on the study, Rotonya M. Carr, MD, Division Head of Gastroenterology at the University of Washington, Seattle, said the study sheds important light on an issue previously addressed only in smaller cohorts.
“To date, there have only been a few small prospective, retrospective, and case series studies investigating SGTL2 inhibitors in patients with cirrhosis,” she told GI & Hepatology Newsv.
“This retrospective study is a real-world glimpse at how patients with cirrhosis may fare on these drugs — very exciting data.”
Carr cautioned, however, that, in addition to the retrospective study design, limitations included that the study doesn’t provide details on the duration of therapy, preventing an understanding of whether the results represent chronic, sustained use of SGLT2 inhibitors.
“[Therefore], we cannot interpret these results to mean that chronic, sustained use of SGTL2 inh is beneficial, or does not cause harm, in patients with cirrhosis.”
“While these data are provocative, more work needs to be done before we understand the full safety and efficacy of SGTL2 inhibitors for patients with cirrhosis,” Carr added.
“However, these data are very encouraging, and I am optimistic that we will indeed see both SGTL2 inhibitors and GLP-1s among the group of medications we use in the future for the primary management of patients with liver disease.”
The authors had no disclosures to report. Carr’s disclosures included relationships with Intercept and Novo Nordisk and research funding from Merck.
A version of this article appeared on Medscape.com.
SAN DIEGO — , new research shows.
“Our study found that SGLT2 inhibitors were associated with fewer portal hypertension complications and lower mortality, suggesting they may be a valuable addition to cirrhosis management,” first author Abhinav K. Rao, MD, of the Medical University of South Carolina, Charleston, South Carolina, told GI & Hepatology News.
The findings were presented at Digestive Disease Week (DDW) 2025.
Portal hypertension, a potentially life-threatening complication of cirrhosis, can be a key driver of additional complications including ascites and gastro-esophageal varices in cirrhosis.
Current treatments such as beta-blockers can prevent some complications, however, more effective therapies are needed.
SGLT2 inhibitors are often used in the treatment of cardiovascular disease as well as metabolic dysfunction–associated steatohepatitis (MASH)–mediated liver disease; research is lacking regarding their effects in portal hypertension in the broader population of people with cirrhosis.
“The therapeutic efficacy of SGLT2 inhibitors might be related to their ability to improve vascular function, making them attractive in portal hypertension,” Rao explained.
To investigate, Rao and colleagues evaluated data on 637,079 patients with cirrhosis in the TriNetX database, which includes patients in the United States from 66 healthcare organizations.
Patients were divided into three subgroups, including those with MASH, alcohol-associated, and other etiologies of cirrhosis.
Using robust 1:1 propensity score matching, patients in each subgroup were stratified as either having or not having been treated with SGLT2 inhibitors, limited to those who initiated the drugs within 1 year of their cirrhosis diagnosis to prevent immortal time bias. Patients were matched on other characteristics.
For the primary outcome of all-cause mortality, with an overall median follow-up of 2 years, patients prescribed SGLT2 inhibitors in the MASH cirrhosis (n = 47,385), alcohol-associated cirrhosis (n = 107,844), and other etiologies of cirrhosis (n = 59,499) groups all had a significantly lower risk for all-cause mortality than those not prescribed SGLT2 inhibitors (P < .05 for all).
SGLT2 Inhibitors in MASH Cirrhosis
Specifically looking at the MASH cirrhosis group, Rao described outcomes of the two groups of 3026 patients each who were and were not treated with SGLT2 inhibitors.
The patients had similar rates of esophageal varices (25% in the SGLT2 group and 22% in the no SGLT2 group), ascites (19% in each group), and a similar rate of 19% had hepatic encephalopathy (HE).
About 57% of patients in each treatment group used beta-blockers and 33% used glucagon-like peptide 1 (GLP-1) receptor agonists. Those with a history of liver transplantation, hemodialysis, or transjugular intrahepatic portosystemic shunt placement were excluded.
The secondary outcome results in those patients showed that treatment with SGLT2 inhibitors was associated with significantly reduced risks of developing portal hypertension complications including ascites, HE, spontaneous bacterial peritonitis (SBP), and hepatorenal syndrome (P < .05 for all).
Esophageal variceal bleeding was also reduced with SGLT-2 inhibitors; however the difference was not statistically significant.
Effects Diminished With Beta-Blocker Treatment
In a secondary analysis of patients in the MASH cirrhosis group treated with one type of a nonselective beta-blockers (n = 509) and another nonselective beta-blockers (n = 2561), the beneficial effects of SGLT2 inhibitors on portal hypertension, with the exception of HE and SBP, were found to be somewhat diminished, likely because patients were already benefitting from the beta-blockers, Rao noted.
Other Groups
In outcomes of the non–MASH-related cirrhosis groups, patients prescribed SGLT2 inhibitors also had a reduced risk for specific, as well as any portal hypertension complications (P < .05), Rao noted.
Overall, the findings add to previous studies on SGLT2 inhibitors in MASH and expand on the possible benefits, he said.
“Our findings validate these [previous] results and suggest potential benefits across for patients with other types of liver disease and raise the possibility of a beneficial effect in portal hypertension,” he said.
“Given the marked reduction in portal hypertension complications after SGLT2 inhibitor initiation, the associated survival benefit may not be surprising,” he noted.
“However, we were intrigued by the consistent reduction in portal hypertension complications across all cirrhosis types, especially since SGLT-2 inhibitors are most commonly used in patients with diabetes who have MASH-mediated liver disease.”
‘Real World Glimpse’ at SGLT2 Inhibitors; Limitations Need Noting
Commenting on the study, Rotonya M. Carr, MD, Division Head of Gastroenterology at the University of Washington, Seattle, said the study sheds important light on an issue previously addressed only in smaller cohorts.
“To date, there have only been a few small prospective, retrospective, and case series studies investigating SGTL2 inhibitors in patients with cirrhosis,” she told GI & Hepatology Newsv.
“This retrospective study is a real-world glimpse at how patients with cirrhosis may fare on these drugs — very exciting data.”
Carr cautioned, however, that, in addition to the retrospective study design, limitations included that the study doesn’t provide details on the duration of therapy, preventing an understanding of whether the results represent chronic, sustained use of SGLT2 inhibitors.
“[Therefore], we cannot interpret these results to mean that chronic, sustained use of SGTL2 inh is beneficial, or does not cause harm, in patients with cirrhosis.”
“While these data are provocative, more work needs to be done before we understand the full safety and efficacy of SGTL2 inhibitors for patients with cirrhosis,” Carr added.
“However, these data are very encouraging, and I am optimistic that we will indeed see both SGTL2 inhibitors and GLP-1s among the group of medications we use in the future for the primary management of patients with liver disease.”
The authors had no disclosures to report. Carr’s disclosures included relationships with Intercept and Novo Nordisk and research funding from Merck.
A version of this article appeared on Medscape.com.
FROM DDW 2025
AI-Enhanced Digital Collaborative Care Improves IBS Symptoms
SAN DIEGO — seen at Cleveland Clinic, Cleveland, Ohio, an observational study found.
Symptom tracking at 4-week intervals showed that “almost everybody got better” regardless of IBS subtype, with relief starting in the first 4 weeks, Stephen Lupe, PsyD, gastrointestinal psychologist and director of Behavioral Medicine, Department of Gastroenterology, Hepatology, and Nutrition at Cleveland Clinic, Cleveland, said in an interview with GI & Hepatology News.
The findings were presented at Digestive Disease Week (DDW) 2025.
Digital Boost to Collaborative Care Model
The combination of dietary interventions and brain-gut behavioral therapy has demonstrated excellent outcomes for patients with IBS, but patients struggle to access these needed services, Lupe noted. A medical home collaborative care model in which patients get care from a multidisciplinary team has been shown to be a good way to successfully deliver this combination of care.
“When you do collaborative in-person care, people get better quicker,” Lupe said.
However, scaling access to this model remains a challenge. For their study, Cleveland Clinic researchers added an AI-enhanced digital platform, Ayble Health, to the in-person collaborative care model to expand access to disease-management services and evaluated whether it improved clinical outcomes for study’s 171 participants, who were recruited via social media advertisements.
Here’s how the platform works. Once a patient enrolls in Ayble Health, a personalized care plan is recommended based on a virtual visit, screening questionnaire, and baseline survey.
The platform includes brain-gut programs, including guided audio content on mindfulness, hypnosis, meditation, cognitive behavioral therapy, and breathing techniques; personalized nutrition support to find and remove trigger foods, a food barcode scanner, and a comprehensive groceries database; and AI-powered wellness tools to help manage and track symptoms. Lupe worked with Ayble Health to develop the platform’s behavioral health content and care pathways.
Patients may choose to follow any combination of three care pathways: A care team overseen by gastro-psychologists, dietitians, and gastroenterologists; a holistic nutrition program including a personalized elimination diet; and a brain-gut behavioral therapy program with gut-directed hypnosis, cognitive behavioral therapy, and acceptance and commitment therapy. They go at their own pace, can connect with Ayble Health’s virtual care team to help with education and goal setting, and continue to consult their Cleveland Clinic providers as needed for evaluation and treatment.
“The care team is still there. We’ve just augmented it to make sure that as many people as possible get behavioral skills training and dietary support, with monitoring between visits — instead of the traditional, ‘I’ll see you in 6 months approach,” Lupe explained.
IBS Symptom Scores Improve
Of the study’s 171 patients, 20 had IBS-diarrhea, 23 had IBS-constipation, 32 had IBS-mixed, and 8 had IBS-unspecified. The remaining 88 patients reported IBS without indication of subtype.
At intake, all patients had active IBS symptoms, with scores ≥ 75 on the IBS symptom severity scale (IBS-SSS). Most patients enrolled in more than one care pathway, and 95% of participants completed at least 4 weeks on their chosen pathways.
Overall, patients saw an average 140-point decrease in IBS-SSS from intake through follow-up lasting up to 42 weeks. A drop in IBS-SSS score ≥ 50 points was considered a clinically meaningful change.
Symptom improvements occurred as early as week 4, were sustained and were uniform across IBS subtypes, suggesting that the AI-enhanced digital collaborative care model has wide utility in patients with IBS, Lupe said.
Patients with the most severe IBS symptoms showed the greatest improvement, but even 50% of those with mild symptoms had clinically meaningful changes in IBS-SSS.
Improvement in IBS symptoms was seen across all care pathways, but the combination of multiple pathways improved outcomes better than a single care pathway alone. The combination of nutrition and brain-gut behavioral therapy demonstrated the greatest reduction in IBS-SSS scores and proportion of patients achieving clinically meaningful results (95%).
The digital comprehensive car model for IBS is now “up and running” at Cleveland Clinic, and the team plans to proactively reach out to patients with gastrointestinal disorders recently seen at their center to alert them to the availability of this tool, Lupe said.
A randomized controlled trial is planned to further validate these observational findings, he added.
‘Wave of the Future’
The digital collaborative care model is “innovative, and I think is the wave of the future,” Kyle Staller, MD, MPH, gastroenterologist and director of the Gastrointestinal Motility Laboratory at Massachusetts General Hospital, Boston, who wasn’t involved in the study, told GI & Hepatology News.
“These digital platforms bundle nondrug options, such as cognitive-behavioral therapy, dietary therapy, hypnotherapy, so patients can choose what suits them, rather than the gastroenterologist hunting down each individual resource, which requires a lot of work,” Staller said.
The study “provides real-world evidence that a deliberative, digital, collaborative care model that houses various types of nondrug IBS treatment under one roof can provide meaningful benefit to patients,” Staller told GI & Hepatology News.
Importantly, he said, “patients chose which option they wanted. At the end of the day, the way that we should be thinking about IBS care is really making sure that we engage the patient with treatment choices,” Staller said.
This study had no specific funding. Three authors had relationships with Ayble Health. Lupe is a scientific advisor for Boomerang Health and paid lecturer for Takeda Pharmaceuticals. Staller disclosed having relationships with Mahana Therapeutics, Ardelyx Inc, Gemelli Biotech, Salix Pharmaceuticals, and Takeda Pharmaceuticals.
A version of this article appeared on Medscape.com.
SAN DIEGO — seen at Cleveland Clinic, Cleveland, Ohio, an observational study found.
Symptom tracking at 4-week intervals showed that “almost everybody got better” regardless of IBS subtype, with relief starting in the first 4 weeks, Stephen Lupe, PsyD, gastrointestinal psychologist and director of Behavioral Medicine, Department of Gastroenterology, Hepatology, and Nutrition at Cleveland Clinic, Cleveland, said in an interview with GI & Hepatology News.
The findings were presented at Digestive Disease Week (DDW) 2025.
Digital Boost to Collaborative Care Model
The combination of dietary interventions and brain-gut behavioral therapy has demonstrated excellent outcomes for patients with IBS, but patients struggle to access these needed services, Lupe noted. A medical home collaborative care model in which patients get care from a multidisciplinary team has been shown to be a good way to successfully deliver this combination of care.
“When you do collaborative in-person care, people get better quicker,” Lupe said.
However, scaling access to this model remains a challenge. For their study, Cleveland Clinic researchers added an AI-enhanced digital platform, Ayble Health, to the in-person collaborative care model to expand access to disease-management services and evaluated whether it improved clinical outcomes for study’s 171 participants, who were recruited via social media advertisements.
Here’s how the platform works. Once a patient enrolls in Ayble Health, a personalized care plan is recommended based on a virtual visit, screening questionnaire, and baseline survey.
The platform includes brain-gut programs, including guided audio content on mindfulness, hypnosis, meditation, cognitive behavioral therapy, and breathing techniques; personalized nutrition support to find and remove trigger foods, a food barcode scanner, and a comprehensive groceries database; and AI-powered wellness tools to help manage and track symptoms. Lupe worked with Ayble Health to develop the platform’s behavioral health content and care pathways.
Patients may choose to follow any combination of three care pathways: A care team overseen by gastro-psychologists, dietitians, and gastroenterologists; a holistic nutrition program including a personalized elimination diet; and a brain-gut behavioral therapy program with gut-directed hypnosis, cognitive behavioral therapy, and acceptance and commitment therapy. They go at their own pace, can connect with Ayble Health’s virtual care team to help with education and goal setting, and continue to consult their Cleveland Clinic providers as needed for evaluation and treatment.
“The care team is still there. We’ve just augmented it to make sure that as many people as possible get behavioral skills training and dietary support, with monitoring between visits — instead of the traditional, ‘I’ll see you in 6 months approach,” Lupe explained.
IBS Symptom Scores Improve
Of the study’s 171 patients, 20 had IBS-diarrhea, 23 had IBS-constipation, 32 had IBS-mixed, and 8 had IBS-unspecified. The remaining 88 patients reported IBS without indication of subtype.
At intake, all patients had active IBS symptoms, with scores ≥ 75 on the IBS symptom severity scale (IBS-SSS). Most patients enrolled in more than one care pathway, and 95% of participants completed at least 4 weeks on their chosen pathways.
Overall, patients saw an average 140-point decrease in IBS-SSS from intake through follow-up lasting up to 42 weeks. A drop in IBS-SSS score ≥ 50 points was considered a clinically meaningful change.
Symptom improvements occurred as early as week 4, were sustained and were uniform across IBS subtypes, suggesting that the AI-enhanced digital collaborative care model has wide utility in patients with IBS, Lupe said.
Patients with the most severe IBS symptoms showed the greatest improvement, but even 50% of those with mild symptoms had clinically meaningful changes in IBS-SSS.
Improvement in IBS symptoms was seen across all care pathways, but the combination of multiple pathways improved outcomes better than a single care pathway alone. The combination of nutrition and brain-gut behavioral therapy demonstrated the greatest reduction in IBS-SSS scores and proportion of patients achieving clinically meaningful results (95%).
The digital comprehensive car model for IBS is now “up and running” at Cleveland Clinic, and the team plans to proactively reach out to patients with gastrointestinal disorders recently seen at their center to alert them to the availability of this tool, Lupe said.
A randomized controlled trial is planned to further validate these observational findings, he added.
‘Wave of the Future’
The digital collaborative care model is “innovative, and I think is the wave of the future,” Kyle Staller, MD, MPH, gastroenterologist and director of the Gastrointestinal Motility Laboratory at Massachusetts General Hospital, Boston, who wasn’t involved in the study, told GI & Hepatology News.
“These digital platforms bundle nondrug options, such as cognitive-behavioral therapy, dietary therapy, hypnotherapy, so patients can choose what suits them, rather than the gastroenterologist hunting down each individual resource, which requires a lot of work,” Staller said.
The study “provides real-world evidence that a deliberative, digital, collaborative care model that houses various types of nondrug IBS treatment under one roof can provide meaningful benefit to patients,” Staller told GI & Hepatology News.
Importantly, he said, “patients chose which option they wanted. At the end of the day, the way that we should be thinking about IBS care is really making sure that we engage the patient with treatment choices,” Staller said.
This study had no specific funding. Three authors had relationships with Ayble Health. Lupe is a scientific advisor for Boomerang Health and paid lecturer for Takeda Pharmaceuticals. Staller disclosed having relationships with Mahana Therapeutics, Ardelyx Inc, Gemelli Biotech, Salix Pharmaceuticals, and Takeda Pharmaceuticals.
A version of this article appeared on Medscape.com.
SAN DIEGO — seen at Cleveland Clinic, Cleveland, Ohio, an observational study found.
Symptom tracking at 4-week intervals showed that “almost everybody got better” regardless of IBS subtype, with relief starting in the first 4 weeks, Stephen Lupe, PsyD, gastrointestinal psychologist and director of Behavioral Medicine, Department of Gastroenterology, Hepatology, and Nutrition at Cleveland Clinic, Cleveland, said in an interview with GI & Hepatology News.
The findings were presented at Digestive Disease Week (DDW) 2025.
Digital Boost to Collaborative Care Model
The combination of dietary interventions and brain-gut behavioral therapy has demonstrated excellent outcomes for patients with IBS, but patients struggle to access these needed services, Lupe noted. A medical home collaborative care model in which patients get care from a multidisciplinary team has been shown to be a good way to successfully deliver this combination of care.
“When you do collaborative in-person care, people get better quicker,” Lupe said.
However, scaling access to this model remains a challenge. For their study, Cleveland Clinic researchers added an AI-enhanced digital platform, Ayble Health, to the in-person collaborative care model to expand access to disease-management services and evaluated whether it improved clinical outcomes for study’s 171 participants, who were recruited via social media advertisements.
Here’s how the platform works. Once a patient enrolls in Ayble Health, a personalized care plan is recommended based on a virtual visit, screening questionnaire, and baseline survey.
The platform includes brain-gut programs, including guided audio content on mindfulness, hypnosis, meditation, cognitive behavioral therapy, and breathing techniques; personalized nutrition support to find and remove trigger foods, a food barcode scanner, and a comprehensive groceries database; and AI-powered wellness tools to help manage and track symptoms. Lupe worked with Ayble Health to develop the platform’s behavioral health content and care pathways.
Patients may choose to follow any combination of three care pathways: A care team overseen by gastro-psychologists, dietitians, and gastroenterologists; a holistic nutrition program including a personalized elimination diet; and a brain-gut behavioral therapy program with gut-directed hypnosis, cognitive behavioral therapy, and acceptance and commitment therapy. They go at their own pace, can connect with Ayble Health’s virtual care team to help with education and goal setting, and continue to consult their Cleveland Clinic providers as needed for evaluation and treatment.
“The care team is still there. We’ve just augmented it to make sure that as many people as possible get behavioral skills training and dietary support, with monitoring between visits — instead of the traditional, ‘I’ll see you in 6 months approach,” Lupe explained.
IBS Symptom Scores Improve
Of the study’s 171 patients, 20 had IBS-diarrhea, 23 had IBS-constipation, 32 had IBS-mixed, and 8 had IBS-unspecified. The remaining 88 patients reported IBS without indication of subtype.
At intake, all patients had active IBS symptoms, with scores ≥ 75 on the IBS symptom severity scale (IBS-SSS). Most patients enrolled in more than one care pathway, and 95% of participants completed at least 4 weeks on their chosen pathways.
Overall, patients saw an average 140-point decrease in IBS-SSS from intake through follow-up lasting up to 42 weeks. A drop in IBS-SSS score ≥ 50 points was considered a clinically meaningful change.
Symptom improvements occurred as early as week 4, were sustained and were uniform across IBS subtypes, suggesting that the AI-enhanced digital collaborative care model has wide utility in patients with IBS, Lupe said.
Patients with the most severe IBS symptoms showed the greatest improvement, but even 50% of those with mild symptoms had clinically meaningful changes in IBS-SSS.
Improvement in IBS symptoms was seen across all care pathways, but the combination of multiple pathways improved outcomes better than a single care pathway alone. The combination of nutrition and brain-gut behavioral therapy demonstrated the greatest reduction in IBS-SSS scores and proportion of patients achieving clinically meaningful results (95%).
The digital comprehensive car model for IBS is now “up and running” at Cleveland Clinic, and the team plans to proactively reach out to patients with gastrointestinal disorders recently seen at their center to alert them to the availability of this tool, Lupe said.
A randomized controlled trial is planned to further validate these observational findings, he added.
‘Wave of the Future’
The digital collaborative care model is “innovative, and I think is the wave of the future,” Kyle Staller, MD, MPH, gastroenterologist and director of the Gastrointestinal Motility Laboratory at Massachusetts General Hospital, Boston, who wasn’t involved in the study, told GI & Hepatology News.
“These digital platforms bundle nondrug options, such as cognitive-behavioral therapy, dietary therapy, hypnotherapy, so patients can choose what suits them, rather than the gastroenterologist hunting down each individual resource, which requires a lot of work,” Staller said.
The study “provides real-world evidence that a deliberative, digital, collaborative care model that houses various types of nondrug IBS treatment under one roof can provide meaningful benefit to patients,” Staller told GI & Hepatology News.
Importantly, he said, “patients chose which option they wanted. At the end of the day, the way that we should be thinking about IBS care is really making sure that we engage the patient with treatment choices,” Staller said.
This study had no specific funding. Three authors had relationships with Ayble Health. Lupe is a scientific advisor for Boomerang Health and paid lecturer for Takeda Pharmaceuticals. Staller disclosed having relationships with Mahana Therapeutics, Ardelyx Inc, Gemelli Biotech, Salix Pharmaceuticals, and Takeda Pharmaceuticals.
A version of this article appeared on Medscape.com.
FROM DDW 2025
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
William Kissick’s description of health care’s iron triangle in 1994 still resonates. Access, quality, and cost will always come at the expense of the others.1 In 2018, Congress passed the VA MISSION Act, allowing patients to pursue community care options for extended waits (> 28 days) or longer distance drive times of > 60 minutes for specialty care services, such as radiation oncology. According to Albanese et al, the VA MISSION Act sought to address gaps in care for veterans living in rural and underserved areas.2 The Veterans Health Administration (VHA) continues to increase community care spending, with a 13.8% increase in fiscal year 2024 and an expected cost of > $40 billion for 2025.3 One could argue this pays for access for remote patients and quality when services are unavailable, making it a direct application of the iron triangle.
The VA MISSION Act also bolstered the expansion of existing community care department staff to expediently facilitate and coordinate care and payments.2 Cost management and monitoring have become critical in predicting future staff requirements, maintaining functionality, and ensuring patients receive optimal care. The VHA purchases care through partner networks and defines these bundled health care services as standard episodes of care (SEOCs), which are “clinically related health care services for a specific unique illness or medical condition… over a defined period of time.”4 Medicare publishes its rates quarterly, and outpatient procedure pricing is readily available online.5 Along these same lines, the US Department of Veterans Affairs (VA) publishes a current list of available procedures and associated Current Procedure Technology (CPT) codes that are covered under its VA fee schedule for community care.
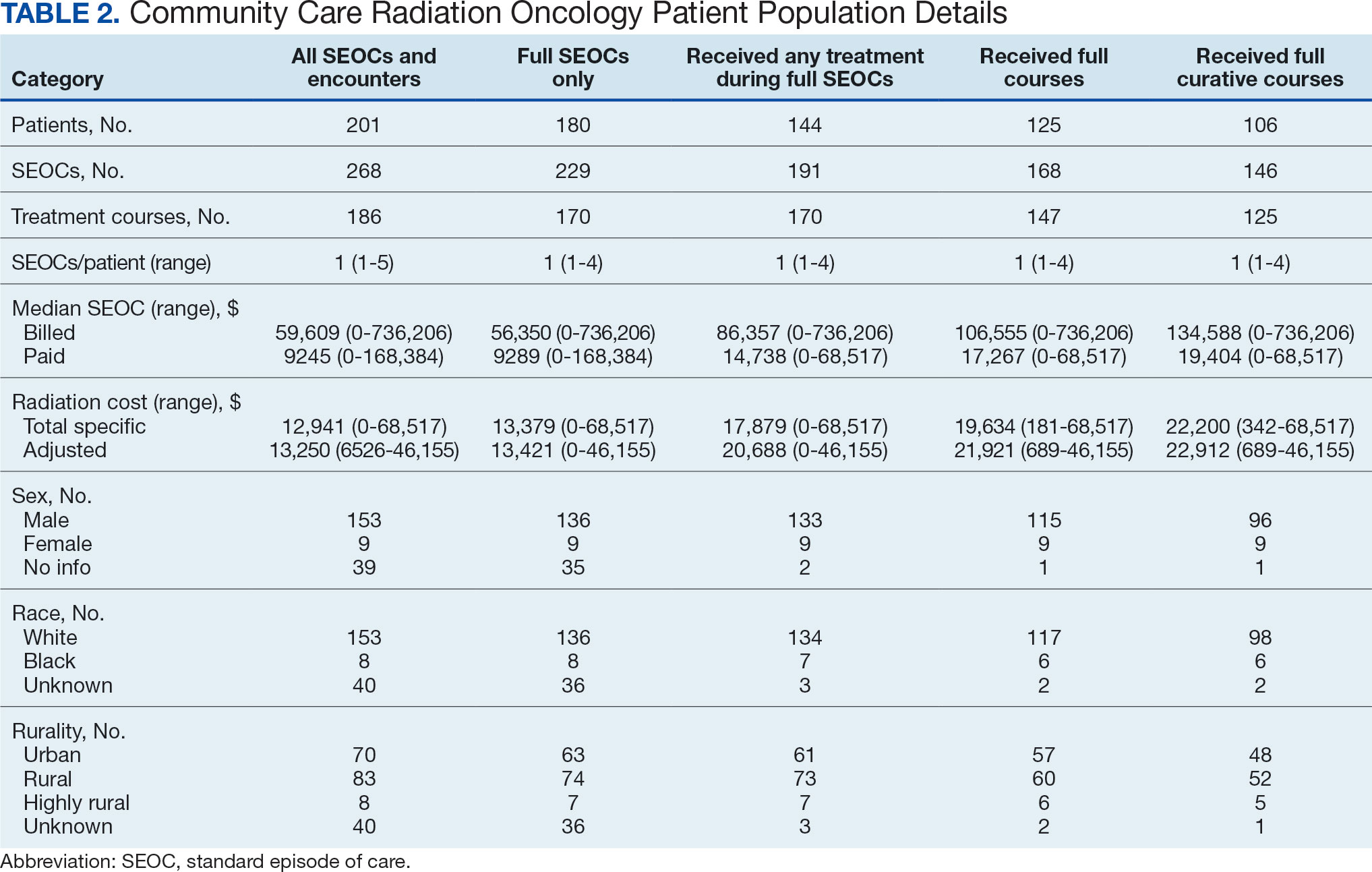
Unique challenges persist when using this system to accurately account for radiation oncology expenditures. This study was based on the current practices at the Richard L. Roudebush VA Medical Center (RLRVAMC), a large 1a hospital. A detailed analysis reveals the contemporaneous cost of radiation oncology cancer care from October 1, 2021, through February 1, 2024, highlights the challenges in SEOC definition and duration, communication issues between RLRVAMC and purchase partners, inconsistencies in billing, erroneous payments, and difficulty of cost categorization.
METHODS
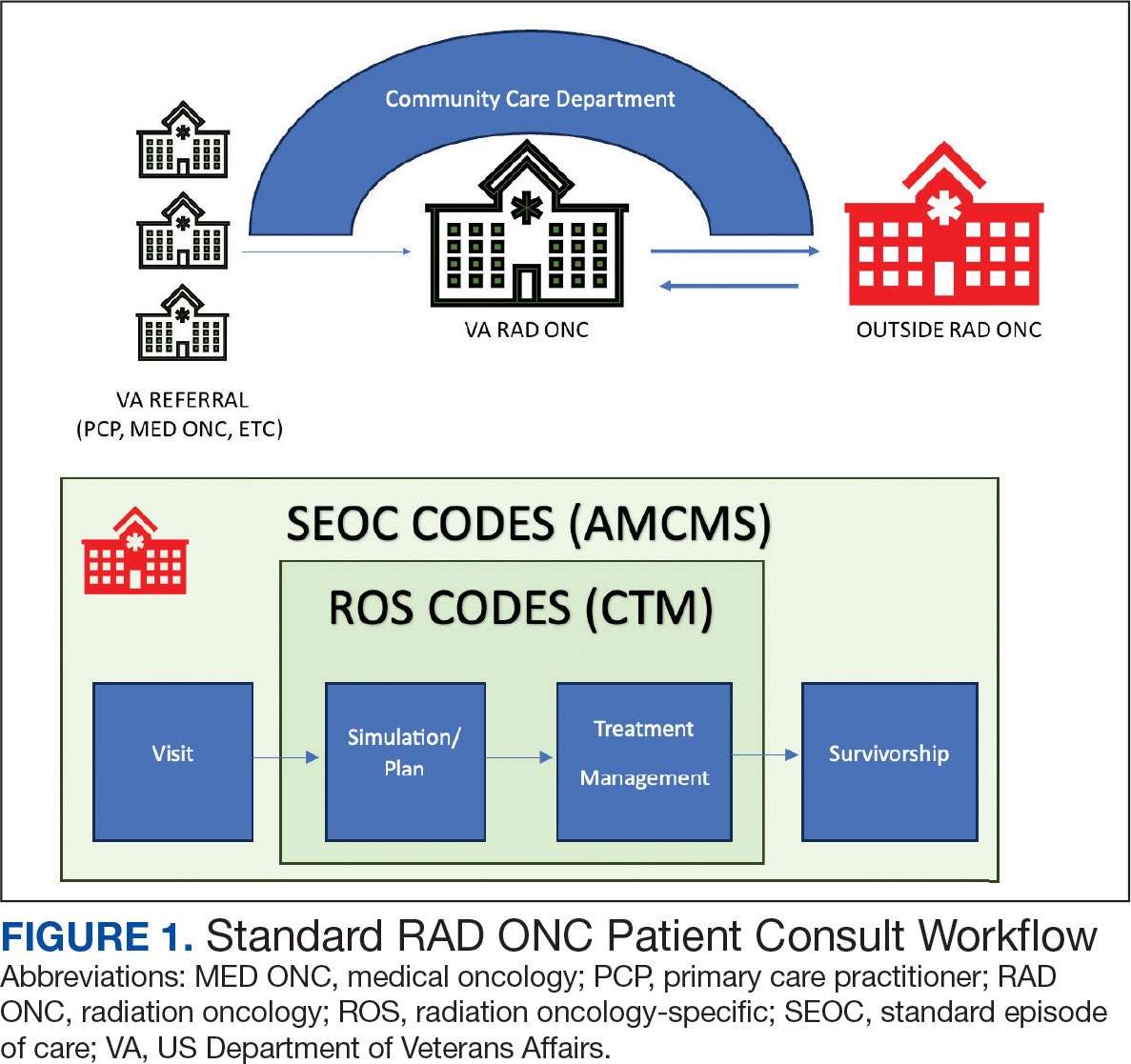
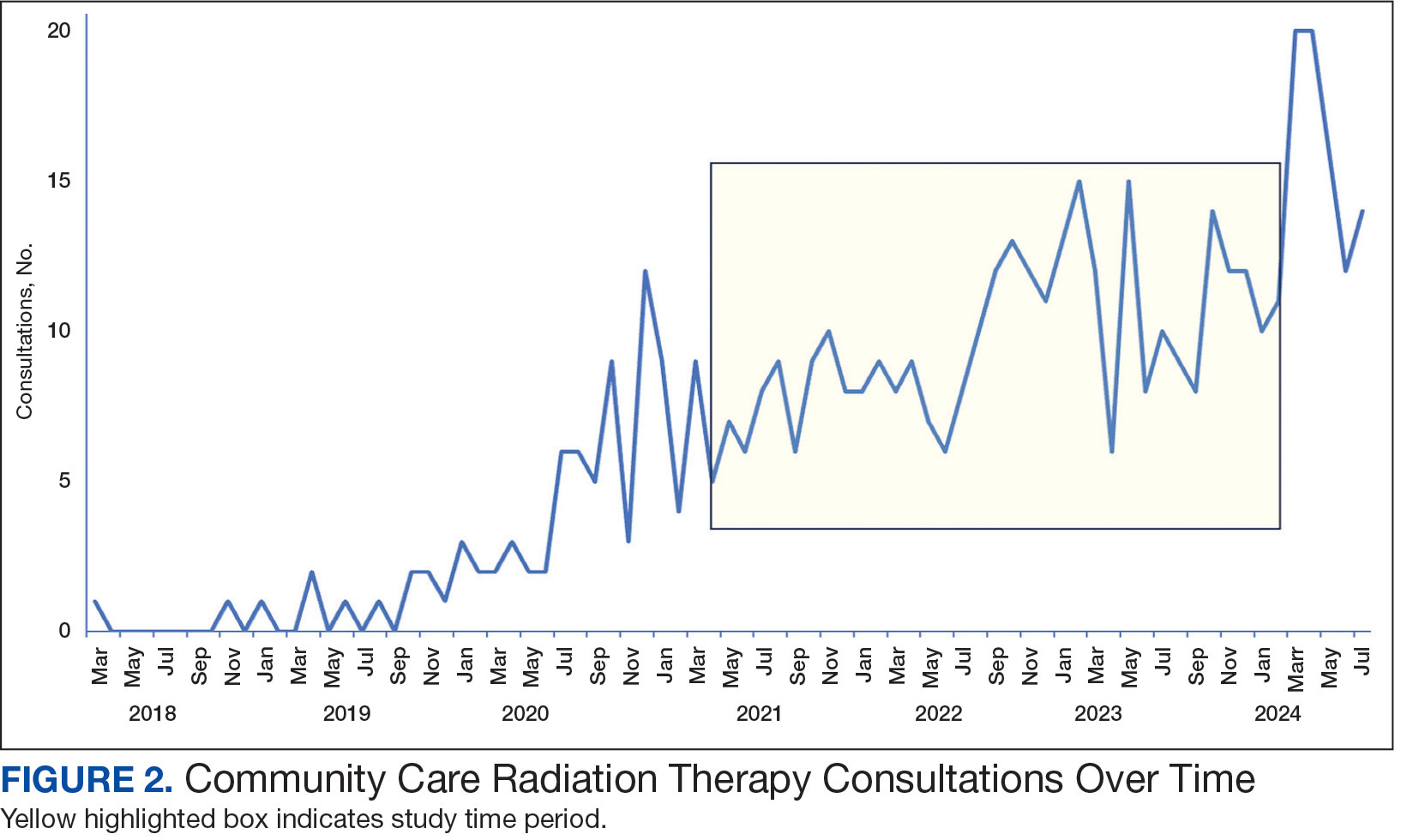
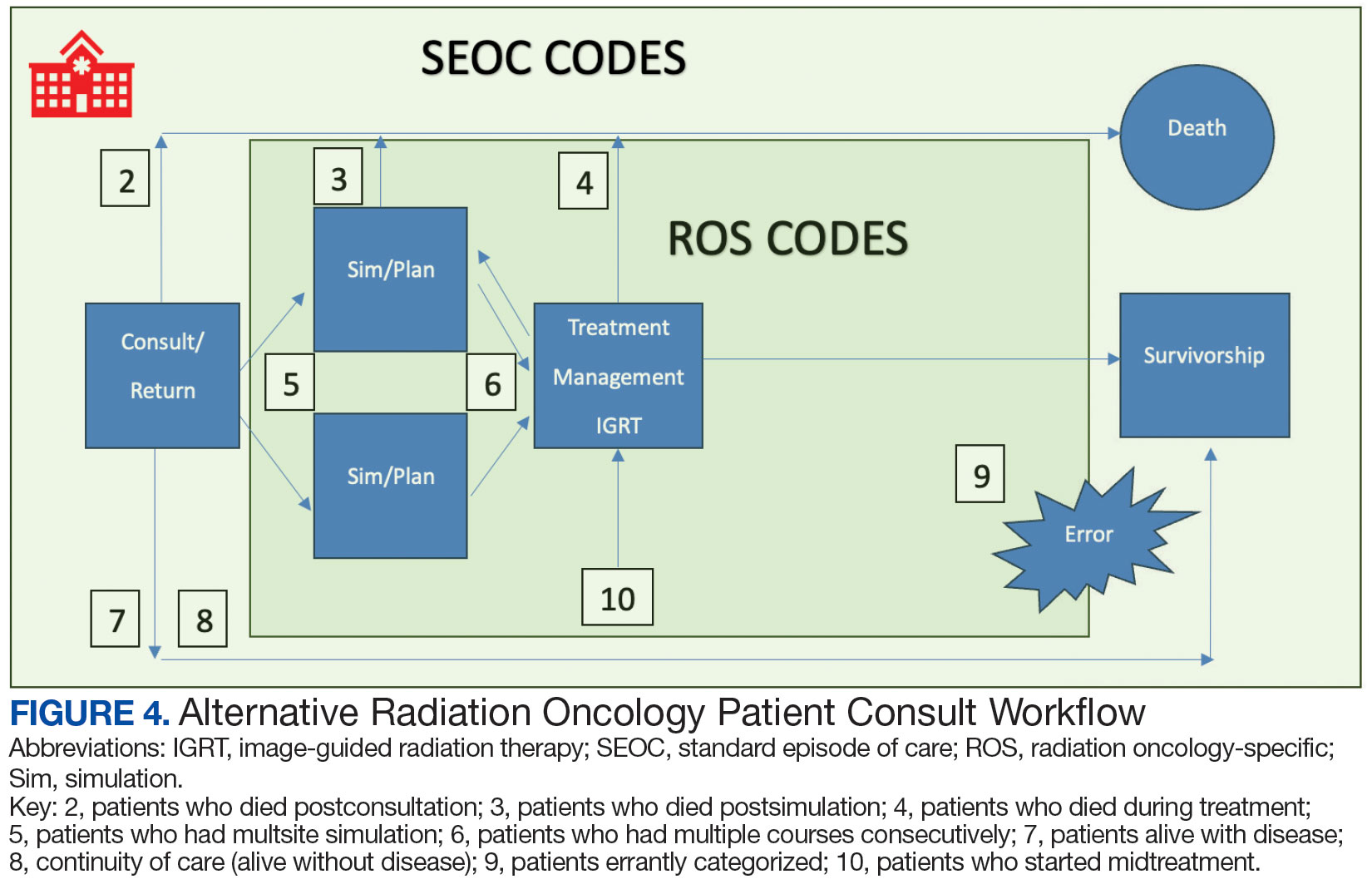
Community care radiation oncology-related costs were examined from October 1, 2021, to February 1, 2024 for RLRVAMC, 6 months prior to billing data extraction. Figure 1 shows a simple radiation oncology patient pathway with consultation or visit, simulation and planning, and treatment, with codes used to check billing. It illustrates the expected relationships between the VHA (radiation oncology, primary, and specialty care) and community care (clinicians and radiation oncology treatment sites).
VHA standard operating procedures for a patient requesting community-based radiation oncology care require a board-certified radiation oncologist at RLRVAMC to review and approve the outside care request. Community care radiation oncology consultation data were accessed from the VA Corporate Data Warehouse (CDW) using Pyramid Analytics (V25.2). Nurses, physicians, and community care staff can add comments, forward consultations to other services, and mark them as complete or discontinued, when appropriate. Consultations not completed within 91 days are automatically discontinued. All community care requests from 2018 through 2024 were extracted; analysis began April 1, 2021, 6 months prior to the cost evaluation date of October 1, 2021.
An approved consultation is reviewed for eligibility by a nurse in the community care department and assigned an authorization number (a VA prefix followed by 12 digits). Billing codes are approved and organized by the community care networks, and all procedure codes should be captured and labeled under this number. The VAMC Community Care department obtains initial correspondence from the treating clinicians. Subsequent records from the treating radiation oncologist are expected to be scanned into the electronic health record and made accessible via the VA Joint Legacy Viewer (JLV) and Computerized Patient Record System (CPRS).
Radiation Oncology SEOC
The start date of the radiation oncology SEOC is determined by the community care nurse based on guidance established by the VA. It can be manually backdated or delayed, but current practice is to start at first visit or procedure code entry after approval from the VAMC Radiation Oncology department. Approved CPT codes from SEOC versions between October 1, 2021, and February 1, 2024, are in eAppendix 1 (available at doi:10.12788/fp.0585). These generally include 10 types of encounters, about 115 different laboratory tests, 115 imaging studies, 25 simulation and planning procedures, and 115 radiation treatment codes. The radiation oncology SEOCs during the study period had an approval duration of 180 days. Advanced Medical Cost Management Solutions software (AMCMS) is the VHA data analytics platform for community care medical service costs. AMCMS includes all individual CPT codes billed by specific radiation oncology SEOC versions. Data are refreshed monthly, and all charges were extracted on September 12, 2024, > 6 months after the final evaluated service date to allow for complete billing returns.6
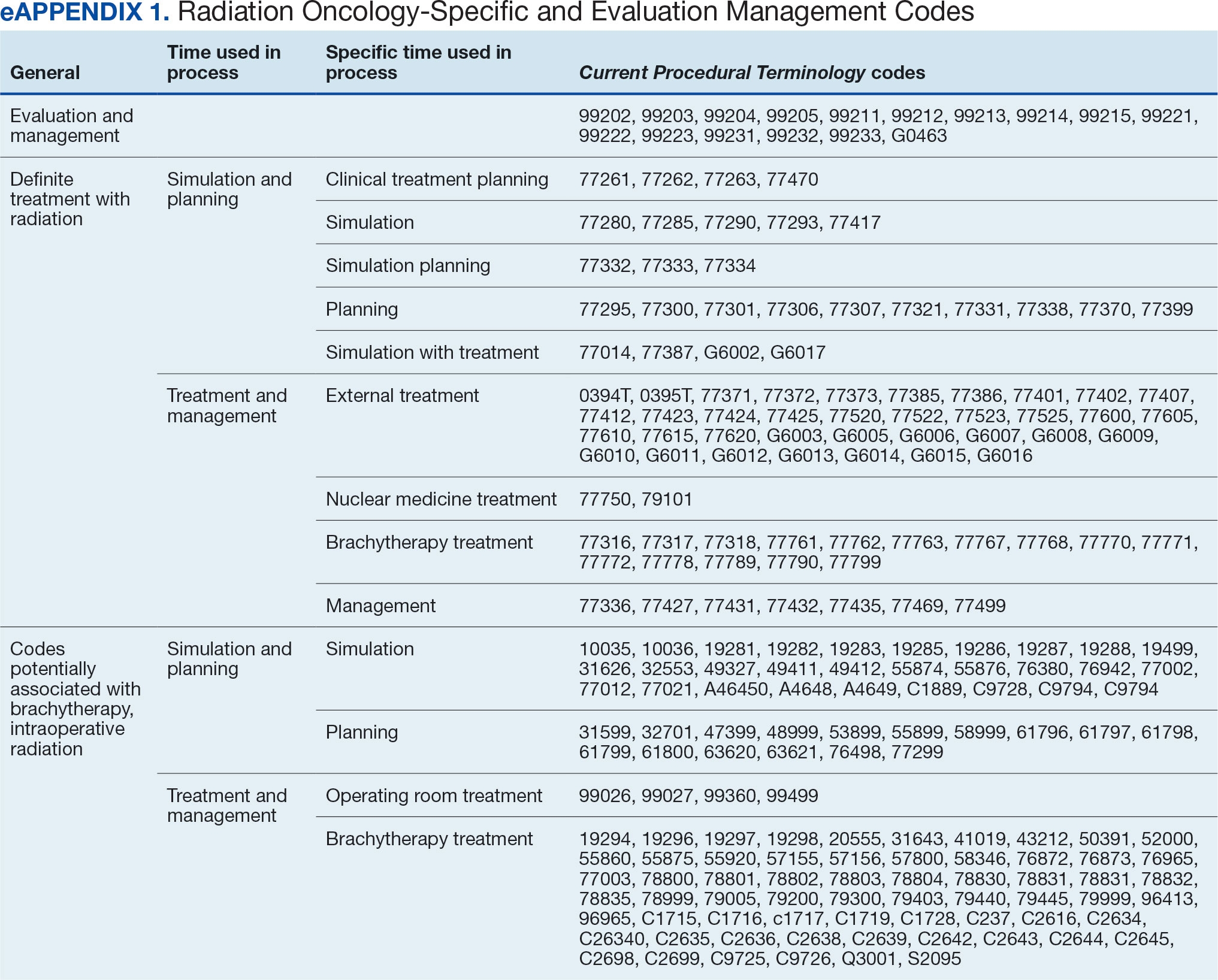
Radiation Oncology-Specific Costs
The VA Close to Me (CTM) program was used to find 84 specific radiation oncology CPT codes, nearly all within the 77.XXX or G6.XXX series, which included all radiation oncology-specific (ROS) codes (except visits accrued during consultation and return appointments). ROS costs are those that could not be performed by any other service and include procedures related to radiation oncology simulation, treatment planning, treatment delivery (with or without image guidance), and physician or physicist management. All ROS costs should be included in a patient’s radiation oncology SEOC. Other costs that may accompany operating room or brachytherapy administration did not follow a 77.XXX or G6.XXX pattern but were included in total radiation therapy operating costs.
Data obtained from AMCMS and CTM included patient name and identifier; CPT billed amount; CPT paid amount; dates of service; number of claims; International Classification of Diseases, Tenth Revision (ICD) diagnosis; and VA authorization numbers. Only CTM listed code modifiers. Only items categorized as paid were included in the analysis. Charges associated with discontinued consultations that had accrued costs also were included. Codes that were not directly related to ROS were separately characterized as other and further subcategorized.
Deep Dive Categorization
All scanned documents tagged to the community consultation were accessed and evaluated for completeness by a radiation oncologist (RS). The presence or absence of consultation notes and treatment summaries was evaluated based on necessity (ie, not needed for continuation of care or treatment was not given). In the absence of a specific completion summary or follow-up note detailing the treatment modality, number of fractions, and treatment sites, available documentation, including clinical notes and billing information, was used. Radical or curative therapies were identified as courses expected to eradicate disease, including stereotactic ablative radiotherapy to the brain, lung, liver, and other organs. Palliative therapies included whole-brain radiotherapy or other low-dose treatments. If the patient received the intended course, this was categorized as full. If incomplete, it was considered partial.
Billing Deviations
The complete document review allowed for close evaluation of paid therapy and identification of gaps in billing (eg, charges not found in extracted data that should have occurred) for external beam radiotherapy patients. Conversely, extra charges, such as an additional weekly treatment management charge (CPT code 77427), would be noted. Patients were expected to have the number of treatments specified in the summary, a clinical treatment planning code, and weekly treatment management notes from physicians and physicists every 5 fractions. Consultations and follow-up visits were expected to have 1 visit code; CPT codes 99205 and 99215, respectively, were used to estimate costs in their absence.
Costs were based on Medicare rates as of January 1 of the year in which they were accrued. 7-10 Duplicates were charges with the same code, date, billed quantity, and paid amounts for a given patient. These would always be considered erroneous. Medicare treatment costs for procedures such as intensity modulated radiotherapy (CPT code 77385 or 77386) are available on the Medicare website. When reviewing locality deviations for 77427, there was a maximum of 33% increase in Medicare rates. Therefore, for treatment codes, one would expect the range to be at least the Medicare rate and maximally 33% higher. These rates are negotiated with insurance companies, but this range was used for the purpose of reviewing and adjusting large data sets.
RESULTS
Since 2018, > 500 community care consults have been placed by radiation oncology for treatment in the community, with more following implementation of the VA MISSION Act. Use of radiation oncology community care services annually increased during the study period for this facility (Table 1, Figure 2). Of the 325 community care consults placed from October 1, 2021, to February 1, 2024, 248 radiation oncology SEOCs were recorded with charges for 181 patients (range, 1-5 SEOCs). Long drive time was the rationale for > 97% of patients directed to community care (Supplemental materials, available at doi:10.12788/fp.0585). Based on AMCMS data, $22.2 million was billed and $2.7 million was paid (20%) for 8747 CPT codes. Each community care interval cost the VA a median (range) of $5000 ($8-$168,000 (Figure 3).
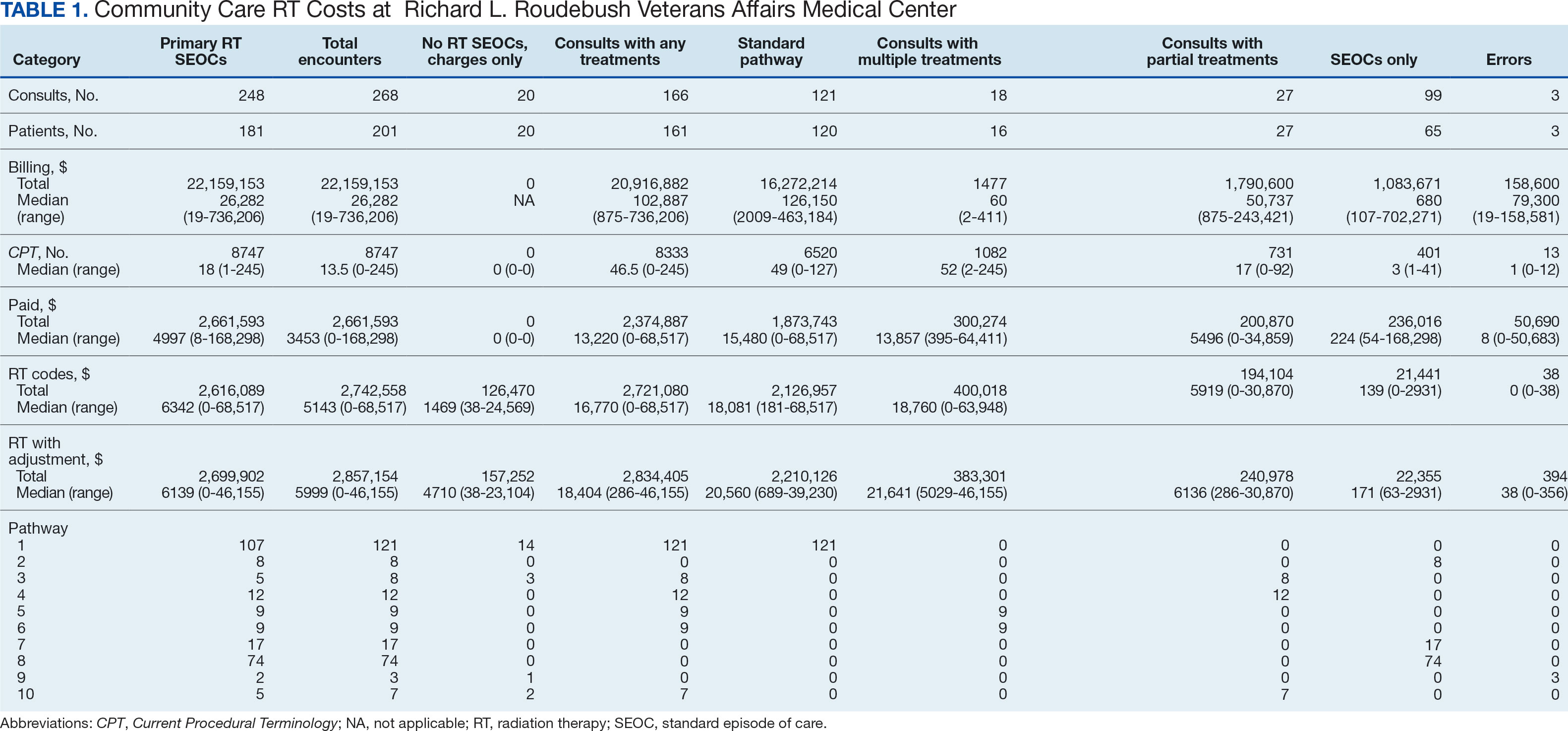
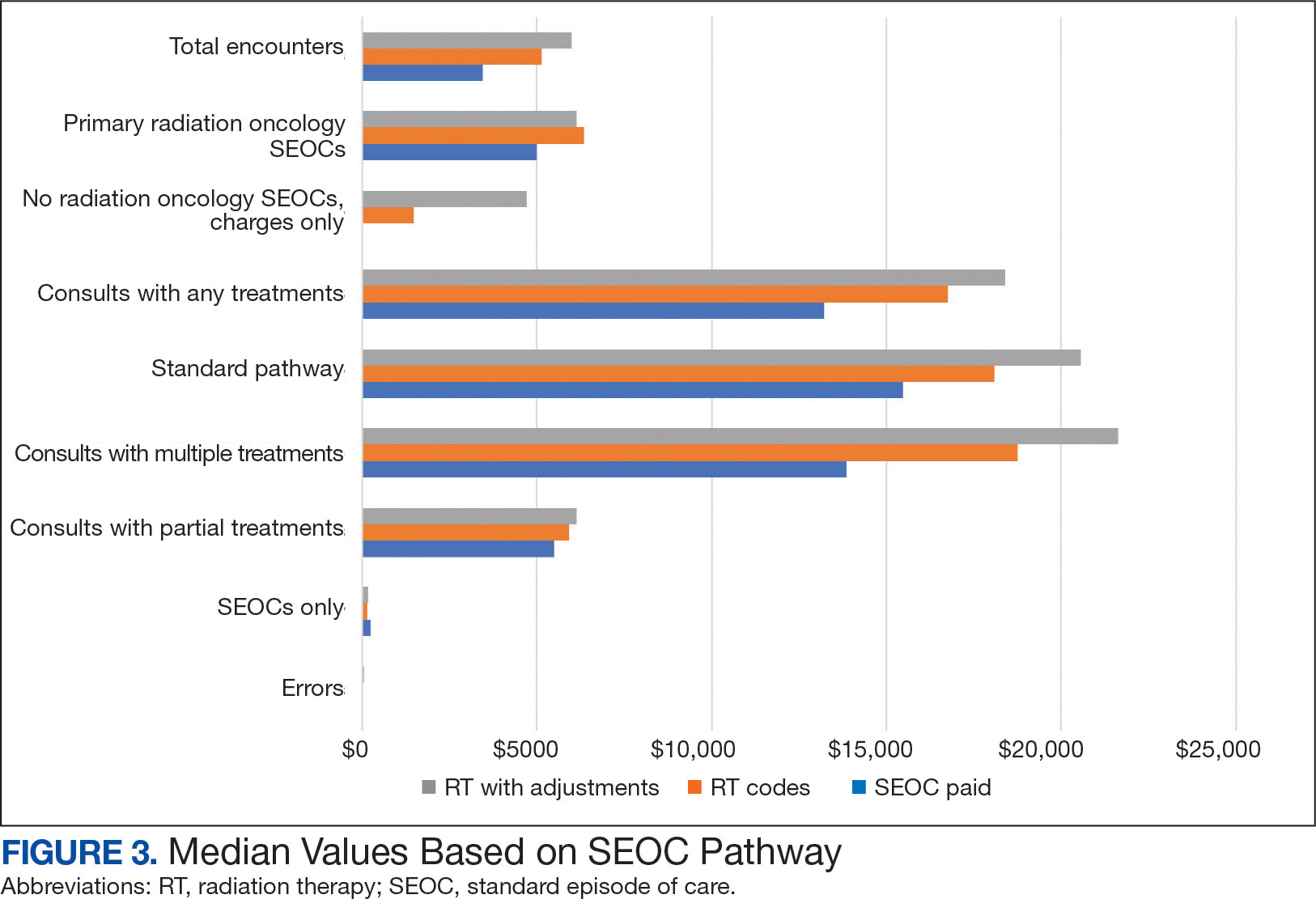
After reviewing ROS charges extracted from CTM, 20 additional patients had radiation oncology charges but did not have a radiation oncology SEOC for 268 episodes of care for 201 unique patients. In addition to the 20 patients who did not have a SEOC, 42 nonradiation oncology SEOCs contained 1148 radiation oncology codes, corresponding to almost $500,000 paid. Additional charges of about $416,000, which included biologic agents (eg, durvalumab, nivolumab), procedures (eg, mastectomies), and ambulance rides were inappropriately added to radiation oncology SEOCs.
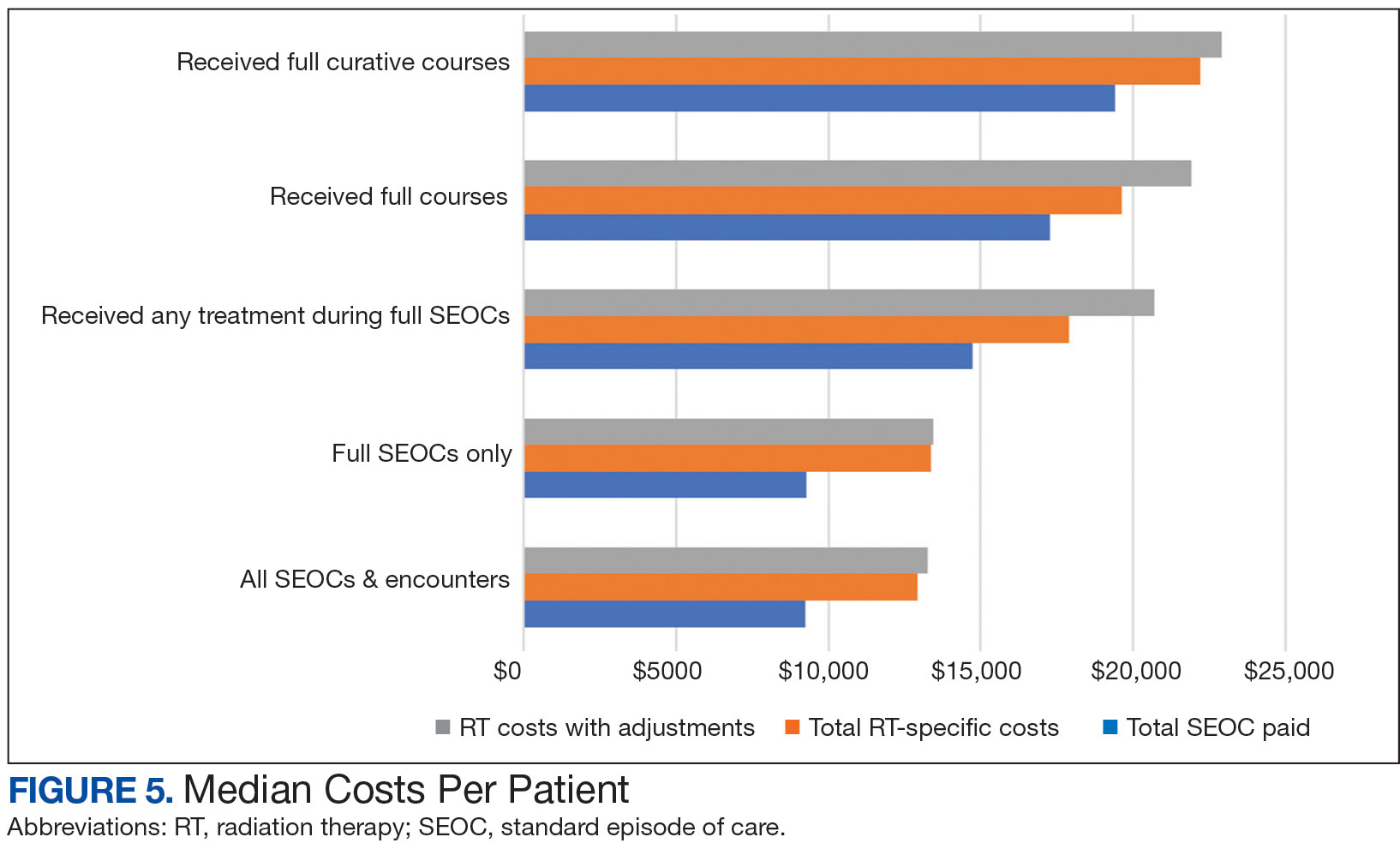
While 77% of consultations were scanned into CPRS and JLV, only 54% of completion summaries were available with an estimated $115,000 in additional costs. The total adjusted costs was about $2.9 million. Almost 37% of SEOCs were for visits only. For the 166 SEOCs where patients received any radiation treatment or planning, the median cost was $18,000. Differences in SEOC pathways are shown in Figure 4. One hundred twenty-one SEOCs (45%) followed the standard pathway, with median SEOC costs of $15,500; when corrected for radiation-specific costs, the median cost increased to $18,000. When adjusted for billing irregularities, the median cost was $20,600. Ninety-nine SEOCs (37%) were for consultation/ follow-up visits only, with a median cost of $220. When omitting shared scans and nonradiation therapy costs and correcting for billing gaps, the median cost decreased to $170. A median of $9200 was paid per patient, with $12,900 for radiation therapy-specific costs and $13,300 adjusted for billing deviations. Narrowing to the 106 patients who received full, radical courses, the median SEOC, ROS, and adjusted radiation therapy costs increased to $19,400, $22,200, and $22,900, respectively (Table 2, Figure 5). Seventy-one SEOCs (26%) had already seen a radiation oncologist before the VA radiation oncology department was aware, and 49 SEOCs (18%) had retroactive approvals (Supplemental materials available at doi:10.12788/fp.0585).
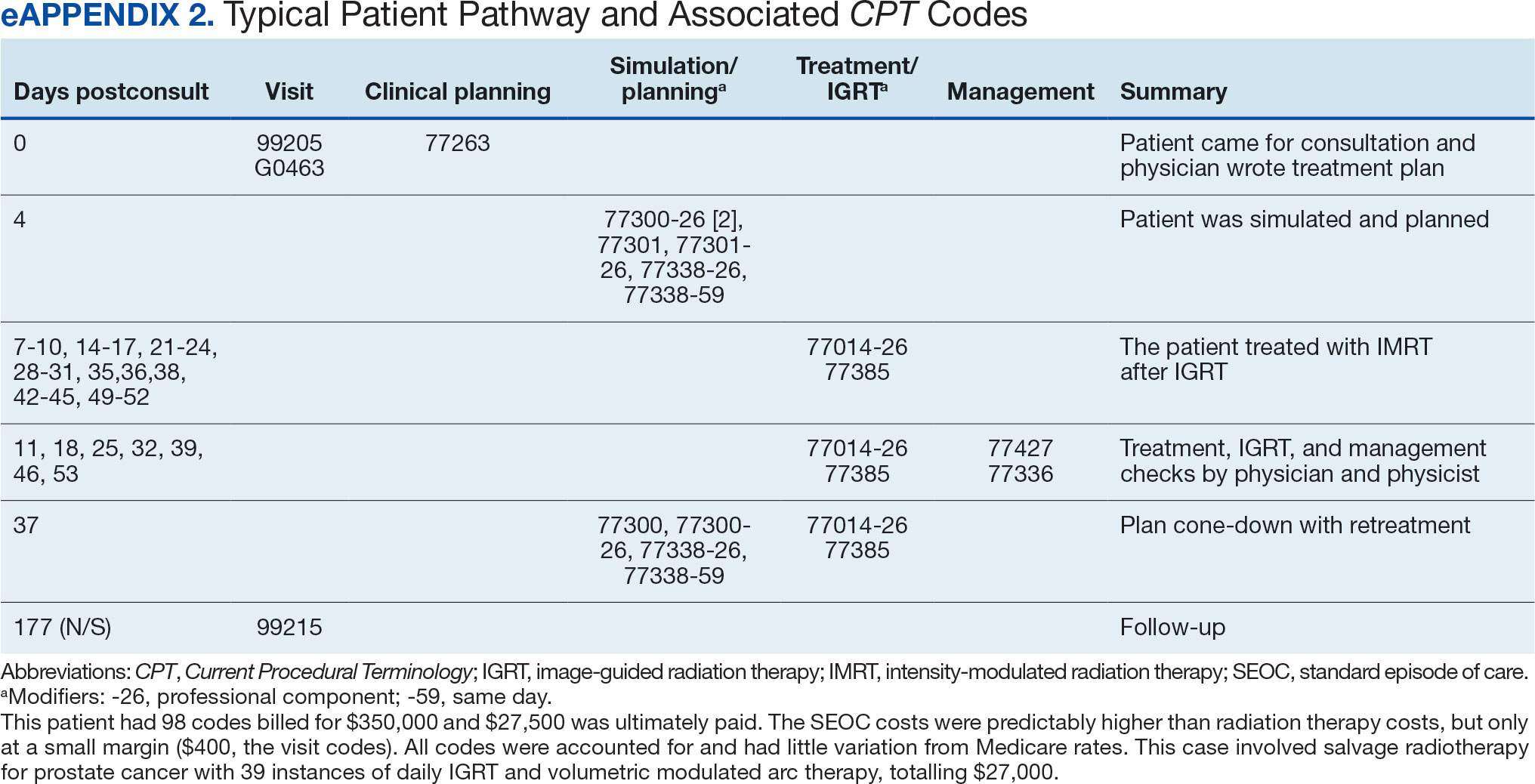
Every consultation charge was reviewed. A typical patient following the standard pathway (eAppendix 2, available at doi:10.12788/ fp.0585) exhibited a predictable pattern of consultation payment, simulation and planning, multiple radiation treatments interspersed with treatment management visits and a cone-down phase, and finishing with a follow-up visit. A less predictable case with excess CPT codes, gaps in charges, and an additional unexpected palliative course is shown in eAppendix 3 (available at doi:10.12788/fp.0585). Gaps occurred in 42% of SEOCs with missed bills costing as much as $12,000. For example, a patient with lung cancer had a treatment summary note for lung cancer after completion that showed the patient received 30 fractions of 2 Gy, a typical course. Only 10 treatment codes and 3 of 6 weekly treatment management codes were available. There was a gap of 20 volumetric modulated arc therapy treatments, 3 physics weekly status checks, 3 physician managements notes, and a computed tomography simulation charge.

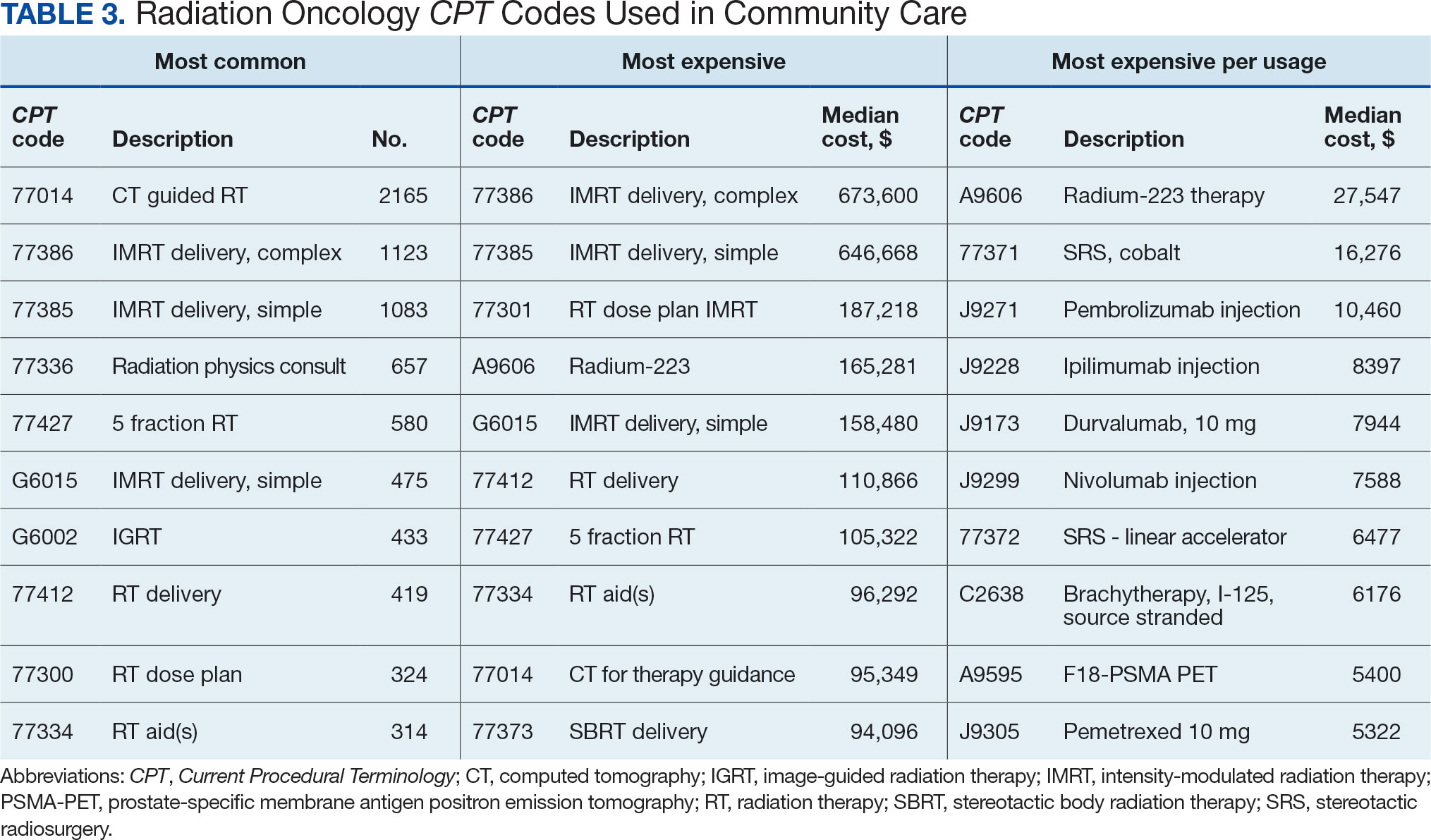
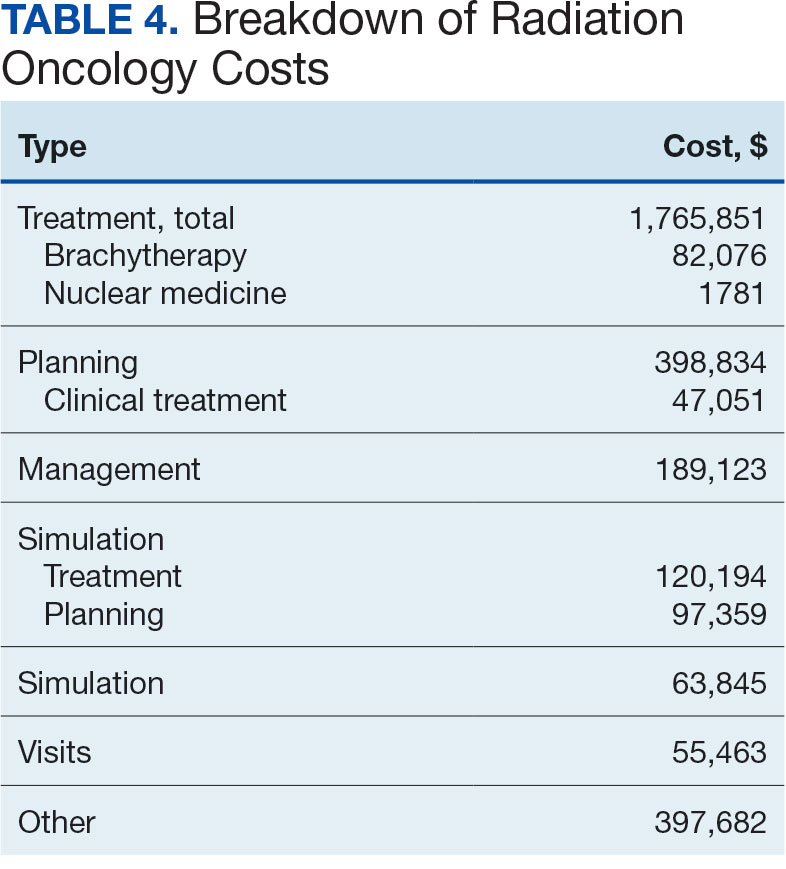
Between AMCMS and CTM, 10,005 CPT codes were evaluated; 1255 (12.5%) were unique to AMCMS (either related to the radiation oncology course, such as Evaluation and Management CPT codes or “other” unrelated codes) while 1158 (11.6%) were unique to CTM. Of the 7592 CPT codes shared between AMCMS and CTM, there was a discrepancy in 135 (1.8%); all were duplicates (CTM showed double payment while AMCMS showed $0 paid). The total CPT code costs came to $3.2 million with $560,000 unique to SEOCs and $500,000 unique to CTM. Treatment codes were the most common (33%) as shown in Table 3 and accounted for 55% of the cost ($1.8 million). About 700 CPT codes were considered “other,” typically for biologic therapeutic agents (Table 4 and eAppendix 4, available at doi:10.12788/fp.0585).

DISCUSSION
The current method of reporting radiation oncology costs used by VA is insufficient and misleading. Better data are needed to summarize purchased care costs to guide decisions about community care at the VA. Investigations into whether the extra costs for quality care (ie, expensive capital equipment, specialized staff, mandatory accreditations) are worthwhile if omitted at other facilities patients choose for their health care needs. No study has defined specialty care-specific costs by evaluating billing receipts from the CDW to answer the question. Kenamond et al highlight the need for radiation oncology for rural patients.11 Drive time was cited as the reason for community care referral for 97% of veterans, many of whom lived in rural locations. Of patients with rurality information who enrolled in community care, 57% came from rural or highly rural counties, and this ratio held for those who received full curative therapies. An executive administrator relying on AMCMS reports would see a median SEOC cost of $5000, but without ROS knowledge in coding, the administrator would miss many additional costs. For example, 2 patients who each had 5 SEOCs during the evaluated period, incurred a total cost of only $1800.
Additionally, an administrator could include miscategorized costs with significant ramifications. The 2 most expensive SEOCs were not typical radiation oncology treatments. A patient undergoing radium-223 dichloride therapy incurred charges exceeding $165,000, contributing disproportionately to the overall median cost analysis; this would normally be administered by the nuclear medicine department. Immunotherapy and chemotherapy are uniformly overseen by medical oncology services, but drug administration codes were still found in radiation oncology SEOCs. A patient (whose SEOC was discontinued but accrued charges) had an electrocardiogram interpretation for $8 as the SEOC cost; 3 other SEOCs continued to incur costs after being discontinued. There were 24 empty SEOCs for patients that had consults to the community, and 2 had notes stating treatment had been delivered yet there was no ROS costs or SEOC costs. Of the 268 encounters, 43% had some sort of billing irregularities (ie, missing treatment costs) that would be unlikely for a private practice to omit; it would be much more likely that the CDW miscategorized the payment despite confirmation of the 2 retrieval systems.
It would be inadvisable to make staffing decisions or forecast costs based on current SEOC reports without specialized curation. A simple yet effective improvement to the cost attribution process would be to restrict the analysis to encounters containing primary radiation treatment codes. This targeted approach allows more accurate identification of patients actively receiving radiation oncology treatment, while excluding those seen solely for consultations or follow-up visits. Implementing this refinement leads to a substantial increase in the median payment—from $5000 to $13,000—without requiring additional coding or data processing, thereby enhancing the accuracy of cost estimates with minimal effort.
Clarifying radiation oncology service costs requires addressing the time frame and services included, given laxity and interpretation of the SEOCs. VA community care departments have streamlined the reimbursement process at the expense of medical cost organization and accuracy; 86% of VA practitioners reported that ≥ 1 potential community health care partners had refused to work with the VA because of payment delays.12 Payments are contingent on correspondence from outside practices for community work. For radiation oncology, this includes the consultation but also critical radiation-related details of treatment, which were omitted nearly half the time. SEOC approval forms have many costly laboratory tests, imaging, and procedures that have little to do with radiation oncology cancer treatments but may be used in the workup and staging process; this creates noise when calculating radiation oncology fiscal cost.
The presumption that an episode of care equates to a completed radiation therapy course is incorrect; this occurs less than half of the time. An episode often refers to a return visit, or conversely, multiple treatment courses. As the patients’ medical homes are their VHA primary care practitioners, it would be particularly challenging to care for the patients without full treatment information, especially if adverse effects from therapy were to arise. As a tertiary specialty, radiation oncology does not seek out patients and are sent consultations from medical oncology, surgical, and medical oncologic specialties. Timesensitive processes such as workup, staging, and diagnosis often occur in parallel. This analysis revealed that patients see outside radiation oncologists prior to the VA. There are ≥ 100 patients who had radiation oncology codes without a radiation oncology SEOC or community care consultation, and in many cases, the consultation was placed after the patient was seen.
Given the lack of uniformity and standardization of patient traffic, the typical and expected pathways were insufficient to find the costs. Too many opportunities for errors and incorrect categorization of costs meant a different method would be necessary. Starting at the inception of the community care consult, only 1 diagnosis code can be entered. For patients with multiple diagnoses, one would not be able to tell what was treated without chart access. Radiation oncology consults come from primary and specialty care practitioners and nurses throughout the VA. Oftentimes, the referral would be solicited by the community radiation oncology clinic, diagnosing community specialty (ie, urology for a patient with prostate cancer), or indirectly from the patient through primary care. Many cases were retroactively approved as the veteran had already been consulted by the community care radiation oncologist. If the patient is drive-time eligible, it would be unlikely that they would leave and choose to return to the VA. There is no way for a facility VA service chief or administrator to mitigate VA community costs of care, especially as shown by the miscategorization of several codes. Database challenges exacerbate the issue: 1 patient changed her first and last name during this time frame, and 2 patients had the same name but different social security numbers. In order to strictly find costs between 2 discrete timepoints, 39 (15%) SEOCs were split and incomplete, and 6 SEOCs contained charges for 2 different patients. This was corrected, and all inadvertent charges were cancelled. Only 1 ICD code is allowed per community care consultation, so an investigation is required to find costs for patients with multiple sites of disease. Additionally, 5 of the patients marked for drive time were actually patients who received Gamma Knife and brachytherapy, services not available at the VA.
Hanks et al first attempted to calculate cost of radiation oncology services. External beam prostate cancer radiotherapy at 3 suburban California centers cost $6750 ($20,503 inflation adjusted) per patient before October 1984 and $5600 ($17,010 inflation adjusted) afterwards.13 According to the American Society for Radiation Oncology, Advocacy Radiation Oncology Case Rate Program Curative radiation courses should cost $20,000 to $30,000 and palliative courses should cost $10,000 to $15,000. These costs are consistent with totals demonstrated in this analysis and similar to the inflation-adjusted Hanks et al figures. Preliminary findings suggest that radiation treatment constituted more than half of the total expenditures, with a notable $4 million increase in adjusted cost compared to the Medicare rates, indicating significant variation. Direct comparisons with Medicaid or commercial payer rates remain unexplored.
Future Directions
During the study period, 201 patients received 186 courses of radiation therapy in the community, while 1014 patients were treated in-house for a total of 833 courses. A forthcoming analysis will directly compare the cost of in-house care with that of communitybased treatment, specifically breaking down expenditure differences by diagnosis. Future research should investigate strategies to align reimbursement with quality metrics, including the potential role of tertiary accreditation in incentivizing high-value care. Additional work is also warranted to assess patient out-ofpocket expenses across care settings and to benchmark VA reimbursement against Medicare, Medicaid, and private insurance rates. In any case, with the increasing possibility of fewer fractions for treatments such as stereotactic radiotherapy or palliative care therapy, there is a clear financial incentive to treat as frequently as allowed despite equal clinical outcomes.
CONCLUSIONS
Veterans increasingly choose to receive care closer to home if the option is available. In the VA iron triangle, cost comes at the expense of access but quantifying this has proved elusive in the cost accounting model currently used at the VA.1 The inclusion of all charges loosely associated with SEOCs significantly impairs the ability to conduct meaningful cost analyses. The current VA methodology not only introduces substantial noise into the data but also leads to a marked underestimation of the true cost of care delivered in community settings. Such misrepresentation risks driving policy decisions that could inappropriately reduce or eliminate in-house radiation oncology services. Categorizing costs effectively in the VA could assist in making managerial and administrative decisions and would prevent damaging service lines based on misleading or incorrect data. A system which differentiates between patients who have received any treatment codes vs those who have not would increase accuracy.
- Kissick W. Medicine’s Dilemmas: Infinite Needs Versus Finite Resources. 1st ed. Yale University Press; 1994.
- Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
- Office of Management and Budget (US). Budget of the United States Government, Fiscal Year 2025. Washington, DC: US Government Publishing Office; 2024. Available from: US Department of Veterans Affairs FY 2025 Budget Submission: Budget in Brief.
- US Department of Veterans Affairs. Veteran care claims. Accessed April 3, 2025. https://www.va.gov/COMMUNITYCARE/revenue-ops/Veteran-Care-Claims.asp
- US Centers for Medicare and Medicaid Services. Accessed April 3, 2025. Procedure price lookup https://www.medicare.gov/procedure-price-lookup
- US Department of Veterans Affairs. WellHive -Enterprise. Accessed April 3, 2025. https://department.va.gov/privacy/wp-content/uploads/sites/5/2023/05/FY23WellHiveEnterprisePIA.pdf
- US Centers for Medicare and Medicaid Services. RVU21a physician fee schedule, January 2021 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu21a
- US Centers for Medicare and Medicaid Services. RVU22a physician fee schedule, January 2022 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- US Centers for Medicare and Medicaid Services. RVU23a physician fee schedule, January 2023 release. Accessed April 3, 2025. https://www.cms.gov/medicare/medicare-fee-service-payment/physicianfeesched/pfs-relative-value-files/rvu23a
- US Centers for Medicare and Medicaid Services. RVU23a Medicare Physician Fee Schedule rates effective January 1, 2024, through March 8, 2024. Accessed on April 3, 2025. https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a
- Kenamond MC, Mourad WF, Randall ME, Kaushal A. No oncology patient left behind: challenges and solutions in rural radiation oncology. Lancet Reg Health Am. 2022;13:100289. doi:10.1016/j.lana.2022.100289
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Hanks GE, Dunlap K. A comparison of the cost of various treatment methods for early cancer of the prostate. Int J Radiat Oncol Biol Phys. 1986;12(10):1879-1881. doi:10.1016/0360-3016(86)90334-2
- American Society of Radiation Oncology. Radiation oncology case rate program (ROCR). Accessed April 3, 2025. https://www.astro.org/advocacy/key-issues-8f3e5a3b76643265ee93287d79c4fc40/rocr
William Kissick’s description of health care’s iron triangle in 1994 still resonates. Access, quality, and cost will always come at the expense of the others.1 In 2018, Congress passed the VA MISSION Act, allowing patients to pursue community care options for extended waits (> 28 days) or longer distance drive times of > 60 minutes for specialty care services, such as radiation oncology. According to Albanese et al, the VA MISSION Act sought to address gaps in care for veterans living in rural and underserved areas.2 The Veterans Health Administration (VHA) continues to increase community care spending, with a 13.8% increase in fiscal year 2024 and an expected cost of > $40 billion for 2025.3 One could argue this pays for access for remote patients and quality when services are unavailable, making it a direct application of the iron triangle.
The VA MISSION Act also bolstered the expansion of existing community care department staff to expediently facilitate and coordinate care and payments.2 Cost management and monitoring have become critical in predicting future staff requirements, maintaining functionality, and ensuring patients receive optimal care. The VHA purchases care through partner networks and defines these bundled health care services as standard episodes of care (SEOCs), which are “clinically related health care services for a specific unique illness or medical condition… over a defined period of time.”4 Medicare publishes its rates quarterly, and outpatient procedure pricing is readily available online.5 Along these same lines, the US Department of Veterans Affairs (VA) publishes a current list of available procedures and associated Current Procedure Technology (CPT) codes that are covered under its VA fee schedule for community care.
Unique challenges persist when using this system to accurately account for radiation oncology expenditures. This study was based on the current practices at the Richard L. Roudebush VA Medical Center (RLRVAMC), a large 1a hospital. A detailed analysis reveals the contemporaneous cost of radiation oncology cancer care from October 1, 2021, through February 1, 2024, highlights the challenges in SEOC definition and duration, communication issues between RLRVAMC and purchase partners, inconsistencies in billing, erroneous payments, and difficulty of cost categorization.
METHODS
Community care radiation oncology-related costs were examined from October 1, 2021, to February 1, 2024 for RLRVAMC, 6 months prior to billing data extraction. Figure 1 shows a simple radiation oncology patient pathway with consultation or visit, simulation and planning, and treatment, with codes used to check billing. It illustrates the expected relationships between the VHA (radiation oncology, primary, and specialty care) and community care (clinicians and radiation oncology treatment sites).

VHA standard operating procedures for a patient requesting community-based radiation oncology care require a board-certified radiation oncologist at RLRVAMC to review and approve the outside care request. Community care radiation oncology consultation data were accessed from the VA Corporate Data Warehouse (CDW) using Pyramid Analytics (V25.2). Nurses, physicians, and community care staff can add comments, forward consultations to other services, and mark them as complete or discontinued, when appropriate. Consultations not completed within 91 days are automatically discontinued. All community care requests from 2018 through 2024 were extracted; analysis began April 1, 2021, 6 months prior to the cost evaluation date of October 1, 2021.
An approved consultation is reviewed for eligibility by a nurse in the community care department and assigned an authorization number (a VA prefix followed by 12 digits). Billing codes are approved and organized by the community care networks, and all procedure codes should be captured and labeled under this number. The VAMC Community Care department obtains initial correspondence from the treating clinicians. Subsequent records from the treating radiation oncologist are expected to be scanned into the electronic health record and made accessible via the VA Joint Legacy Viewer (JLV) and Computerized Patient Record System (CPRS).
Radiation Oncology SEOC
The start date of the radiation oncology SEOC is determined by the community care nurse based on guidance established by the VA. It can be manually backdated or delayed, but current practice is to start at first visit or procedure code entry after approval from the VAMC Radiation Oncology department. Approved CPT codes from SEOC versions between October 1, 2021, and February 1, 2024, are in eAppendix 1 (available at doi:10.12788/fp.0585). These generally include 10 types of encounters, about 115 different laboratory tests, 115 imaging studies, 25 simulation and planning procedures, and 115 radiation treatment codes. The radiation oncology SEOCs during the study period had an approval duration of 180 days. Advanced Medical Cost Management Solutions software (AMCMS) is the VHA data analytics platform for community care medical service costs. AMCMS includes all individual CPT codes billed by specific radiation oncology SEOC versions. Data are refreshed monthly, and all charges were extracted on September 12, 2024, > 6 months after the final evaluated service date to allow for complete billing returns.6

Radiation Oncology-Specific Costs
The VA Close to Me (CTM) program was used to find 84 specific radiation oncology CPT codes, nearly all within the 77.XXX or G6.XXX series, which included all radiation oncology-specific (ROS) codes (except visits accrued during consultation and return appointments). ROS costs are those that could not be performed by any other service and include procedures related to radiation oncology simulation, treatment planning, treatment delivery (with or without image guidance), and physician or physicist management. All ROS costs should be included in a patient’s radiation oncology SEOC. Other costs that may accompany operating room or brachytherapy administration did not follow a 77.XXX or G6.XXX pattern but were included in total radiation therapy operating costs.
Data obtained from AMCMS and CTM included patient name and identifier; CPT billed amount; CPT paid amount; dates of service; number of claims; International Classification of Diseases, Tenth Revision (ICD) diagnosis; and VA authorization numbers. Only CTM listed code modifiers. Only items categorized as paid were included in the analysis. Charges associated with discontinued consultations that had accrued costs also were included. Codes that were not directly related to ROS were separately characterized as other and further subcategorized.
Deep Dive Categorization
All scanned documents tagged to the community consultation were accessed and evaluated for completeness by a radiation oncologist (RS). The presence or absence of consultation notes and treatment summaries was evaluated based on necessity (ie, not needed for continuation of care or treatment was not given). In the absence of a specific completion summary or follow-up note detailing the treatment modality, number of fractions, and treatment sites, available documentation, including clinical notes and billing information, was used. Radical or curative therapies were identified as courses expected to eradicate disease, including stereotactic ablative radiotherapy to the brain, lung, liver, and other organs. Palliative therapies included whole-brain radiotherapy or other low-dose treatments. If the patient received the intended course, this was categorized as full. If incomplete, it was considered partial.
Billing Deviations
The complete document review allowed for close evaluation of paid therapy and identification of gaps in billing (eg, charges not found in extracted data that should have occurred) for external beam radiotherapy patients. Conversely, extra charges, such as an additional weekly treatment management charge (CPT code 77427), would be noted. Patients were expected to have the number of treatments specified in the summary, a clinical treatment planning code, and weekly treatment management notes from physicians and physicists every 5 fractions. Consultations and follow-up visits were expected to have 1 visit code; CPT codes 99205 and 99215, respectively, were used to estimate costs in their absence.
Costs were based on Medicare rates as of January 1 of the year in which they were accrued. 7-10 Duplicates were charges with the same code, date, billed quantity, and paid amounts for a given patient. These would always be considered erroneous. Medicare treatment costs for procedures such as intensity modulated radiotherapy (CPT code 77385 or 77386) are available on the Medicare website. When reviewing locality deviations for 77427, there was a maximum of 33% increase in Medicare rates. Therefore, for treatment codes, one would expect the range to be at least the Medicare rate and maximally 33% higher. These rates are negotiated with insurance companies, but this range was used for the purpose of reviewing and adjusting large data sets.
RESULTS
Since 2018, > 500 community care consults have been placed by radiation oncology for treatment in the community, with more following implementation of the VA MISSION Act. Use of radiation oncology community care services annually increased during the study period for this facility (Table 1, Figure 2). Of the 325 community care consults placed from October 1, 2021, to February 1, 2024, 248 radiation oncology SEOCs were recorded with charges for 181 patients (range, 1-5 SEOCs). Long drive time was the rationale for > 97% of patients directed to community care (Supplemental materials, available at doi:10.12788/fp.0585). Based on AMCMS data, $22.2 million was billed and $2.7 million was paid (20%) for 8747 CPT codes. Each community care interval cost the VA a median (range) of $5000 ($8-$168,000 (Figure 3).



After reviewing ROS charges extracted from CTM, 20 additional patients had radiation oncology charges but did not have a radiation oncology SEOC for 268 episodes of care for 201 unique patients. In addition to the 20 patients who did not have a SEOC, 42 nonradiation oncology SEOCs contained 1148 radiation oncology codes, corresponding to almost $500,000 paid. Additional charges of about $416,000, which included biologic agents (eg, durvalumab, nivolumab), procedures (eg, mastectomies), and ambulance rides were inappropriately added to radiation oncology SEOCs.
While 77% of consultations were scanned into CPRS and JLV, only 54% of completion summaries were available with an estimated $115,000 in additional costs. The total adjusted costs was about $2.9 million. Almost 37% of SEOCs were for visits only. For the 166 SEOCs where patients received any radiation treatment or planning, the median cost was $18,000. Differences in SEOC pathways are shown in Figure 4. One hundred twenty-one SEOCs (45%) followed the standard pathway, with median SEOC costs of $15,500; when corrected for radiation-specific costs, the median cost increased to $18,000. When adjusted for billing irregularities, the median cost was $20,600. Ninety-nine SEOCs (37%) were for consultation/ follow-up visits only, with a median cost of $220. When omitting shared scans and nonradiation therapy costs and correcting for billing gaps, the median cost decreased to $170. A median of $9200 was paid per patient, with $12,900 for radiation therapy-specific costs and $13,300 adjusted for billing deviations. Narrowing to the 106 patients who received full, radical courses, the median SEOC, ROS, and adjusted radiation therapy costs increased to $19,400, $22,200, and $22,900, respectively (Table 2, Figure 5). Seventy-one SEOCs (26%) had already seen a radiation oncologist before the VA radiation oncology department was aware, and 49 SEOCs (18%) had retroactive approvals (Supplemental materials available at doi:10.12788/fp.0585).



Every consultation charge was reviewed. A typical patient following the standard pathway (eAppendix 2, available at doi:10.12788/ fp.0585) exhibited a predictable pattern of consultation payment, simulation and planning, multiple radiation treatments interspersed with treatment management visits and a cone-down phase, and finishing with a follow-up visit. A less predictable case with excess CPT codes, gaps in charges, and an additional unexpected palliative course is shown in eAppendix 3 (available at doi:10.12788/fp.0585). Gaps occurred in 42% of SEOCs with missed bills costing as much as $12,000. For example, a patient with lung cancer had a treatment summary note for lung cancer after completion that showed the patient received 30 fractions of 2 Gy, a typical course. Only 10 treatment codes and 3 of 6 weekly treatment management codes were available. There was a gap of 20 volumetric modulated arc therapy treatments, 3 physics weekly status checks, 3 physician managements notes, and a computed tomography simulation charge.


Between AMCMS and CTM, 10,005 CPT codes were evaluated; 1255 (12.5%) were unique to AMCMS (either related to the radiation oncology course, such as Evaluation and Management CPT codes or “other” unrelated codes) while 1158 (11.6%) were unique to CTM. Of the 7592 CPT codes shared between AMCMS and CTM, there was a discrepancy in 135 (1.8%); all were duplicates (CTM showed double payment while AMCMS showed $0 paid). The total CPT code costs came to $3.2 million with $560,000 unique to SEOCs and $500,000 unique to CTM. Treatment codes were the most common (33%) as shown in Table 3 and accounted for 55% of the cost ($1.8 million). About 700 CPT codes were considered “other,” typically for biologic therapeutic agents (Table 4 and eAppendix 4, available at doi:10.12788/fp.0585).



DISCUSSION
The current method of reporting radiation oncology costs used by VA is insufficient and misleading. Better data are needed to summarize purchased care costs to guide decisions about community care at the VA. Investigations into whether the extra costs for quality care (ie, expensive capital equipment, specialized staff, mandatory accreditations) are worthwhile if omitted at other facilities patients choose for their health care needs. No study has defined specialty care-specific costs by evaluating billing receipts from the CDW to answer the question. Kenamond et al highlight the need for radiation oncology for rural patients.11 Drive time was cited as the reason for community care referral for 97% of veterans, many of whom lived in rural locations. Of patients with rurality information who enrolled in community care, 57% came from rural or highly rural counties, and this ratio held for those who received full curative therapies. An executive administrator relying on AMCMS reports would see a median SEOC cost of $5000, but without ROS knowledge in coding, the administrator would miss many additional costs. For example, 2 patients who each had 5 SEOCs during the evaluated period, incurred a total cost of only $1800.
Additionally, an administrator could include miscategorized costs with significant ramifications. The 2 most expensive SEOCs were not typical radiation oncology treatments. A patient undergoing radium-223 dichloride therapy incurred charges exceeding $165,000, contributing disproportionately to the overall median cost analysis; this would normally be administered by the nuclear medicine department. Immunotherapy and chemotherapy are uniformly overseen by medical oncology services, but drug administration codes were still found in radiation oncology SEOCs. A patient (whose SEOC was discontinued but accrued charges) had an electrocardiogram interpretation for $8 as the SEOC cost; 3 other SEOCs continued to incur costs after being discontinued. There were 24 empty SEOCs for patients that had consults to the community, and 2 had notes stating treatment had been delivered yet there was no ROS costs or SEOC costs. Of the 268 encounters, 43% had some sort of billing irregularities (ie, missing treatment costs) that would be unlikely for a private practice to omit; it would be much more likely that the CDW miscategorized the payment despite confirmation of the 2 retrieval systems.
It would be inadvisable to make staffing decisions or forecast costs based on current SEOC reports without specialized curation. A simple yet effective improvement to the cost attribution process would be to restrict the analysis to encounters containing primary radiation treatment codes. This targeted approach allows more accurate identification of patients actively receiving radiation oncology treatment, while excluding those seen solely for consultations or follow-up visits. Implementing this refinement leads to a substantial increase in the median payment—from $5000 to $13,000—without requiring additional coding or data processing, thereby enhancing the accuracy of cost estimates with minimal effort.
Clarifying radiation oncology service costs requires addressing the time frame and services included, given laxity and interpretation of the SEOCs. VA community care departments have streamlined the reimbursement process at the expense of medical cost organization and accuracy; 86% of VA practitioners reported that ≥ 1 potential community health care partners had refused to work with the VA because of payment delays.12 Payments are contingent on correspondence from outside practices for community work. For radiation oncology, this includes the consultation but also critical radiation-related details of treatment, which were omitted nearly half the time. SEOC approval forms have many costly laboratory tests, imaging, and procedures that have little to do with radiation oncology cancer treatments but may be used in the workup and staging process; this creates noise when calculating radiation oncology fiscal cost.
The presumption that an episode of care equates to a completed radiation therapy course is incorrect; this occurs less than half of the time. An episode often refers to a return visit, or conversely, multiple treatment courses. As the patients’ medical homes are their VHA primary care practitioners, it would be particularly challenging to care for the patients without full treatment information, especially if adverse effects from therapy were to arise. As a tertiary specialty, radiation oncology does not seek out patients and are sent consultations from medical oncology, surgical, and medical oncologic specialties. Timesensitive processes such as workup, staging, and diagnosis often occur in parallel. This analysis revealed that patients see outside radiation oncologists prior to the VA. There are ≥ 100 patients who had radiation oncology codes without a radiation oncology SEOC or community care consultation, and in many cases, the consultation was placed after the patient was seen.
Given the lack of uniformity and standardization of patient traffic, the typical and expected pathways were insufficient to find the costs. Too many opportunities for errors and incorrect categorization of costs meant a different method would be necessary. Starting at the inception of the community care consult, only 1 diagnosis code can be entered. For patients with multiple diagnoses, one would not be able to tell what was treated without chart access. Radiation oncology consults come from primary and specialty care practitioners and nurses throughout the VA. Oftentimes, the referral would be solicited by the community radiation oncology clinic, diagnosing community specialty (ie, urology for a patient with prostate cancer), or indirectly from the patient through primary care. Many cases were retroactively approved as the veteran had already been consulted by the community care radiation oncologist. If the patient is drive-time eligible, it would be unlikely that they would leave and choose to return to the VA. There is no way for a facility VA service chief or administrator to mitigate VA community costs of care, especially as shown by the miscategorization of several codes. Database challenges exacerbate the issue: 1 patient changed her first and last name during this time frame, and 2 patients had the same name but different social security numbers. In order to strictly find costs between 2 discrete timepoints, 39 (15%) SEOCs were split and incomplete, and 6 SEOCs contained charges for 2 different patients. This was corrected, and all inadvertent charges were cancelled. Only 1 ICD code is allowed per community care consultation, so an investigation is required to find costs for patients with multiple sites of disease. Additionally, 5 of the patients marked for drive time were actually patients who received Gamma Knife and brachytherapy, services not available at the VA.
Hanks et al first attempted to calculate cost of radiation oncology services. External beam prostate cancer radiotherapy at 3 suburban California centers cost $6750 ($20,503 inflation adjusted) per patient before October 1984 and $5600 ($17,010 inflation adjusted) afterwards.13 According to the American Society for Radiation Oncology, Advocacy Radiation Oncology Case Rate Program Curative radiation courses should cost $20,000 to $30,000 and palliative courses should cost $10,000 to $15,000. These costs are consistent with totals demonstrated in this analysis and similar to the inflation-adjusted Hanks et al figures. Preliminary findings suggest that radiation treatment constituted more than half of the total expenditures, with a notable $4 million increase in adjusted cost compared to the Medicare rates, indicating significant variation. Direct comparisons with Medicaid or commercial payer rates remain unexplored.
Future Directions
During the study period, 201 patients received 186 courses of radiation therapy in the community, while 1014 patients were treated in-house for a total of 833 courses. A forthcoming analysis will directly compare the cost of in-house care with that of communitybased treatment, specifically breaking down expenditure differences by diagnosis. Future research should investigate strategies to align reimbursement with quality metrics, including the potential role of tertiary accreditation in incentivizing high-value care. Additional work is also warranted to assess patient out-ofpocket expenses across care settings and to benchmark VA reimbursement against Medicare, Medicaid, and private insurance rates. In any case, with the increasing possibility of fewer fractions for treatments such as stereotactic radiotherapy or palliative care therapy, there is a clear financial incentive to treat as frequently as allowed despite equal clinical outcomes.
CONCLUSIONS
Veterans increasingly choose to receive care closer to home if the option is available. In the VA iron triangle, cost comes at the expense of access but quantifying this has proved elusive in the cost accounting model currently used at the VA.1 The inclusion of all charges loosely associated with SEOCs significantly impairs the ability to conduct meaningful cost analyses. The current VA methodology not only introduces substantial noise into the data but also leads to a marked underestimation of the true cost of care delivered in community settings. Such misrepresentation risks driving policy decisions that could inappropriately reduce or eliminate in-house radiation oncology services. Categorizing costs effectively in the VA could assist in making managerial and administrative decisions and would prevent damaging service lines based on misleading or incorrect data. A system which differentiates between patients who have received any treatment codes vs those who have not would increase accuracy.
William Kissick’s description of health care’s iron triangle in 1994 still resonates. Access, quality, and cost will always come at the expense of the others.1 In 2018, Congress passed the VA MISSION Act, allowing patients to pursue community care options for extended waits (> 28 days) or longer distance drive times of > 60 minutes for specialty care services, such as radiation oncology. According to Albanese et al, the VA MISSION Act sought to address gaps in care for veterans living in rural and underserved areas.2 The Veterans Health Administration (VHA) continues to increase community care spending, with a 13.8% increase in fiscal year 2024 and an expected cost of > $40 billion for 2025.3 One could argue this pays for access for remote patients and quality when services are unavailable, making it a direct application of the iron triangle.
The VA MISSION Act also bolstered the expansion of existing community care department staff to expediently facilitate and coordinate care and payments.2 Cost management and monitoring have become critical in predicting future staff requirements, maintaining functionality, and ensuring patients receive optimal care. The VHA purchases care through partner networks and defines these bundled health care services as standard episodes of care (SEOCs), which are “clinically related health care services for a specific unique illness or medical condition… over a defined period of time.”4 Medicare publishes its rates quarterly, and outpatient procedure pricing is readily available online.5 Along these same lines, the US Department of Veterans Affairs (VA) publishes a current list of available procedures and associated Current Procedure Technology (CPT) codes that are covered under its VA fee schedule for community care.
Unique challenges persist when using this system to accurately account for radiation oncology expenditures. This study was based on the current practices at the Richard L. Roudebush VA Medical Center (RLRVAMC), a large 1a hospital. A detailed analysis reveals the contemporaneous cost of radiation oncology cancer care from October 1, 2021, through February 1, 2024, highlights the challenges in SEOC definition and duration, communication issues between RLRVAMC and purchase partners, inconsistencies in billing, erroneous payments, and difficulty of cost categorization.
METHODS
Community care radiation oncology-related costs were examined from October 1, 2021, to February 1, 2024 for RLRVAMC, 6 months prior to billing data extraction. Figure 1 shows a simple radiation oncology patient pathway with consultation or visit, simulation and planning, and treatment, with codes used to check billing. It illustrates the expected relationships between the VHA (radiation oncology, primary, and specialty care) and community care (clinicians and radiation oncology treatment sites).

VHA standard operating procedures for a patient requesting community-based radiation oncology care require a board-certified radiation oncologist at RLRVAMC to review and approve the outside care request. Community care radiation oncology consultation data were accessed from the VA Corporate Data Warehouse (CDW) using Pyramid Analytics (V25.2). Nurses, physicians, and community care staff can add comments, forward consultations to other services, and mark them as complete or discontinued, when appropriate. Consultations not completed within 91 days are automatically discontinued. All community care requests from 2018 through 2024 were extracted; analysis began April 1, 2021, 6 months prior to the cost evaluation date of October 1, 2021.
An approved consultation is reviewed for eligibility by a nurse in the community care department and assigned an authorization number (a VA prefix followed by 12 digits). Billing codes are approved and organized by the community care networks, and all procedure codes should be captured and labeled under this number. The VAMC Community Care department obtains initial correspondence from the treating clinicians. Subsequent records from the treating radiation oncologist are expected to be scanned into the electronic health record and made accessible via the VA Joint Legacy Viewer (JLV) and Computerized Patient Record System (CPRS).
Radiation Oncology SEOC
The start date of the radiation oncology SEOC is determined by the community care nurse based on guidance established by the VA. It can be manually backdated or delayed, but current practice is to start at first visit or procedure code entry after approval from the VAMC Radiation Oncology department. Approved CPT codes from SEOC versions between October 1, 2021, and February 1, 2024, are in eAppendix 1 (available at doi:10.12788/fp.0585). These generally include 10 types of encounters, about 115 different laboratory tests, 115 imaging studies, 25 simulation and planning procedures, and 115 radiation treatment codes. The radiation oncology SEOCs during the study period had an approval duration of 180 days. Advanced Medical Cost Management Solutions software (AMCMS) is the VHA data analytics platform for community care medical service costs. AMCMS includes all individual CPT codes billed by specific radiation oncology SEOC versions. Data are refreshed monthly, and all charges were extracted on September 12, 2024, > 6 months after the final evaluated service date to allow for complete billing returns.6

Radiation Oncology-Specific Costs
The VA Close to Me (CTM) program was used to find 84 specific radiation oncology CPT codes, nearly all within the 77.XXX or G6.XXX series, which included all radiation oncology-specific (ROS) codes (except visits accrued during consultation and return appointments). ROS costs are those that could not be performed by any other service and include procedures related to radiation oncology simulation, treatment planning, treatment delivery (with or without image guidance), and physician or physicist management. All ROS costs should be included in a patient’s radiation oncology SEOC. Other costs that may accompany operating room or brachytherapy administration did not follow a 77.XXX or G6.XXX pattern but were included in total radiation therapy operating costs.
Data obtained from AMCMS and CTM included patient name and identifier; CPT billed amount; CPT paid amount; dates of service; number of claims; International Classification of Diseases, Tenth Revision (ICD) diagnosis; and VA authorization numbers. Only CTM listed code modifiers. Only items categorized as paid were included in the analysis. Charges associated with discontinued consultations that had accrued costs also were included. Codes that were not directly related to ROS were separately characterized as other and further subcategorized.
Deep Dive Categorization
All scanned documents tagged to the community consultation were accessed and evaluated for completeness by a radiation oncologist (RS). The presence or absence of consultation notes and treatment summaries was evaluated based on necessity (ie, not needed for continuation of care or treatment was not given). In the absence of a specific completion summary or follow-up note detailing the treatment modality, number of fractions, and treatment sites, available documentation, including clinical notes and billing information, was used. Radical or curative therapies were identified as courses expected to eradicate disease, including stereotactic ablative radiotherapy to the brain, lung, liver, and other organs. Palliative therapies included whole-brain radiotherapy or other low-dose treatments. If the patient received the intended course, this was categorized as full. If incomplete, it was considered partial.
Billing Deviations
The complete document review allowed for close evaluation of paid therapy and identification of gaps in billing (eg, charges not found in extracted data that should have occurred) for external beam radiotherapy patients. Conversely, extra charges, such as an additional weekly treatment management charge (CPT code 77427), would be noted. Patients were expected to have the number of treatments specified in the summary, a clinical treatment planning code, and weekly treatment management notes from physicians and physicists every 5 fractions. Consultations and follow-up visits were expected to have 1 visit code; CPT codes 99205 and 99215, respectively, were used to estimate costs in their absence.
Costs were based on Medicare rates as of January 1 of the year in which they were accrued. 7-10 Duplicates were charges with the same code, date, billed quantity, and paid amounts for a given patient. These would always be considered erroneous. Medicare treatment costs for procedures such as intensity modulated radiotherapy (CPT code 77385 or 77386) are available on the Medicare website. When reviewing locality deviations for 77427, there was a maximum of 33% increase in Medicare rates. Therefore, for treatment codes, one would expect the range to be at least the Medicare rate and maximally 33% higher. These rates are negotiated with insurance companies, but this range was used for the purpose of reviewing and adjusting large data sets.
RESULTS
Since 2018, > 500 community care consults have been placed by radiation oncology for treatment in the community, with more following implementation of the VA MISSION Act. Use of radiation oncology community care services annually increased during the study period for this facility (Table 1, Figure 2). Of the 325 community care consults placed from October 1, 2021, to February 1, 2024, 248 radiation oncology SEOCs were recorded with charges for 181 patients (range, 1-5 SEOCs). Long drive time was the rationale for > 97% of patients directed to community care (Supplemental materials, available at doi:10.12788/fp.0585). Based on AMCMS data, $22.2 million was billed and $2.7 million was paid (20%) for 8747 CPT codes. Each community care interval cost the VA a median (range) of $5000 ($8-$168,000 (Figure 3).



After reviewing ROS charges extracted from CTM, 20 additional patients had radiation oncology charges but did not have a radiation oncology SEOC for 268 episodes of care for 201 unique patients. In addition to the 20 patients who did not have a SEOC, 42 nonradiation oncology SEOCs contained 1148 radiation oncology codes, corresponding to almost $500,000 paid. Additional charges of about $416,000, which included biologic agents (eg, durvalumab, nivolumab), procedures (eg, mastectomies), and ambulance rides were inappropriately added to radiation oncology SEOCs.
While 77% of consultations were scanned into CPRS and JLV, only 54% of completion summaries were available with an estimated $115,000 in additional costs. The total adjusted costs was about $2.9 million. Almost 37% of SEOCs were for visits only. For the 166 SEOCs where patients received any radiation treatment or planning, the median cost was $18,000. Differences in SEOC pathways are shown in Figure 4. One hundred twenty-one SEOCs (45%) followed the standard pathway, with median SEOC costs of $15,500; when corrected for radiation-specific costs, the median cost increased to $18,000. When adjusted for billing irregularities, the median cost was $20,600. Ninety-nine SEOCs (37%) were for consultation/ follow-up visits only, with a median cost of $220. When omitting shared scans and nonradiation therapy costs and correcting for billing gaps, the median cost decreased to $170. A median of $9200 was paid per patient, with $12,900 for radiation therapy-specific costs and $13,300 adjusted for billing deviations. Narrowing to the 106 patients who received full, radical courses, the median SEOC, ROS, and adjusted radiation therapy costs increased to $19,400, $22,200, and $22,900, respectively (Table 2, Figure 5). Seventy-one SEOCs (26%) had already seen a radiation oncologist before the VA radiation oncology department was aware, and 49 SEOCs (18%) had retroactive approvals (Supplemental materials available at doi:10.12788/fp.0585).



Every consultation charge was reviewed. A typical patient following the standard pathway (eAppendix 2, available at doi:10.12788/ fp.0585) exhibited a predictable pattern of consultation payment, simulation and planning, multiple radiation treatments interspersed with treatment management visits and a cone-down phase, and finishing with a follow-up visit. A less predictable case with excess CPT codes, gaps in charges, and an additional unexpected palliative course is shown in eAppendix 3 (available at doi:10.12788/fp.0585). Gaps occurred in 42% of SEOCs with missed bills costing as much as $12,000. For example, a patient with lung cancer had a treatment summary note for lung cancer after completion that showed the patient received 30 fractions of 2 Gy, a typical course. Only 10 treatment codes and 3 of 6 weekly treatment management codes were available. There was a gap of 20 volumetric modulated arc therapy treatments, 3 physics weekly status checks, 3 physician managements notes, and a computed tomography simulation charge.


Between AMCMS and CTM, 10,005 CPT codes were evaluated; 1255 (12.5%) were unique to AMCMS (either related to the radiation oncology course, such as Evaluation and Management CPT codes or “other” unrelated codes) while 1158 (11.6%) were unique to CTM. Of the 7592 CPT codes shared between AMCMS and CTM, there was a discrepancy in 135 (1.8%); all were duplicates (CTM showed double payment while AMCMS showed $0 paid). The total CPT code costs came to $3.2 million with $560,000 unique to SEOCs and $500,000 unique to CTM. Treatment codes were the most common (33%) as shown in Table 3 and accounted for 55% of the cost ($1.8 million). About 700 CPT codes were considered “other,” typically for biologic therapeutic agents (Table 4 and eAppendix 4, available at doi:10.12788/fp.0585).



DISCUSSION
The current method of reporting radiation oncology costs used by VA is insufficient and misleading. Better data are needed to summarize purchased care costs to guide decisions about community care at the VA. Investigations into whether the extra costs for quality care (ie, expensive capital equipment, specialized staff, mandatory accreditations) are worthwhile if omitted at other facilities patients choose for their health care needs. No study has defined specialty care-specific costs by evaluating billing receipts from the CDW to answer the question. Kenamond et al highlight the need for radiation oncology for rural patients.11 Drive time was cited as the reason for community care referral for 97% of veterans, many of whom lived in rural locations. Of patients with rurality information who enrolled in community care, 57% came from rural or highly rural counties, and this ratio held for those who received full curative therapies. An executive administrator relying on AMCMS reports would see a median SEOC cost of $5000, but without ROS knowledge in coding, the administrator would miss many additional costs. For example, 2 patients who each had 5 SEOCs during the evaluated period, incurred a total cost of only $1800.
Additionally, an administrator could include miscategorized costs with significant ramifications. The 2 most expensive SEOCs were not typical radiation oncology treatments. A patient undergoing radium-223 dichloride therapy incurred charges exceeding $165,000, contributing disproportionately to the overall median cost analysis; this would normally be administered by the nuclear medicine department. Immunotherapy and chemotherapy are uniformly overseen by medical oncology services, but drug administration codes were still found in radiation oncology SEOCs. A patient (whose SEOC was discontinued but accrued charges) had an electrocardiogram interpretation for $8 as the SEOC cost; 3 other SEOCs continued to incur costs after being discontinued. There were 24 empty SEOCs for patients that had consults to the community, and 2 had notes stating treatment had been delivered yet there was no ROS costs or SEOC costs. Of the 268 encounters, 43% had some sort of billing irregularities (ie, missing treatment costs) that would be unlikely for a private practice to omit; it would be much more likely that the CDW miscategorized the payment despite confirmation of the 2 retrieval systems.
It would be inadvisable to make staffing decisions or forecast costs based on current SEOC reports without specialized curation. A simple yet effective improvement to the cost attribution process would be to restrict the analysis to encounters containing primary radiation treatment codes. This targeted approach allows more accurate identification of patients actively receiving radiation oncology treatment, while excluding those seen solely for consultations or follow-up visits. Implementing this refinement leads to a substantial increase in the median payment—from $5000 to $13,000—without requiring additional coding or data processing, thereby enhancing the accuracy of cost estimates with minimal effort.
Clarifying radiation oncology service costs requires addressing the time frame and services included, given laxity and interpretation of the SEOCs. VA community care departments have streamlined the reimbursement process at the expense of medical cost organization and accuracy; 86% of VA practitioners reported that ≥ 1 potential community health care partners had refused to work with the VA because of payment delays.12 Payments are contingent on correspondence from outside practices for community work. For radiation oncology, this includes the consultation but also critical radiation-related details of treatment, which were omitted nearly half the time. SEOC approval forms have many costly laboratory tests, imaging, and procedures that have little to do with radiation oncology cancer treatments but may be used in the workup and staging process; this creates noise when calculating radiation oncology fiscal cost.
The presumption that an episode of care equates to a completed radiation therapy course is incorrect; this occurs less than half of the time. An episode often refers to a return visit, or conversely, multiple treatment courses. As the patients’ medical homes are their VHA primary care practitioners, it would be particularly challenging to care for the patients without full treatment information, especially if adverse effects from therapy were to arise. As a tertiary specialty, radiation oncology does not seek out patients and are sent consultations from medical oncology, surgical, and medical oncologic specialties. Timesensitive processes such as workup, staging, and diagnosis often occur in parallel. This analysis revealed that patients see outside radiation oncologists prior to the VA. There are ≥ 100 patients who had radiation oncology codes without a radiation oncology SEOC or community care consultation, and in many cases, the consultation was placed after the patient was seen.
Given the lack of uniformity and standardization of patient traffic, the typical and expected pathways were insufficient to find the costs. Too many opportunities for errors and incorrect categorization of costs meant a different method would be necessary. Starting at the inception of the community care consult, only 1 diagnosis code can be entered. For patients with multiple diagnoses, one would not be able to tell what was treated without chart access. Radiation oncology consults come from primary and specialty care practitioners and nurses throughout the VA. Oftentimes, the referral would be solicited by the community radiation oncology clinic, diagnosing community specialty (ie, urology for a patient with prostate cancer), or indirectly from the patient through primary care. Many cases were retroactively approved as the veteran had already been consulted by the community care radiation oncologist. If the patient is drive-time eligible, it would be unlikely that they would leave and choose to return to the VA. There is no way for a facility VA service chief or administrator to mitigate VA community costs of care, especially as shown by the miscategorization of several codes. Database challenges exacerbate the issue: 1 patient changed her first and last name during this time frame, and 2 patients had the same name but different social security numbers. In order to strictly find costs between 2 discrete timepoints, 39 (15%) SEOCs were split and incomplete, and 6 SEOCs contained charges for 2 different patients. This was corrected, and all inadvertent charges were cancelled. Only 1 ICD code is allowed per community care consultation, so an investigation is required to find costs for patients with multiple sites of disease. Additionally, 5 of the patients marked for drive time were actually patients who received Gamma Knife and brachytherapy, services not available at the VA.
Hanks et al first attempted to calculate cost of radiation oncology services. External beam prostate cancer radiotherapy at 3 suburban California centers cost $6750 ($20,503 inflation adjusted) per patient before October 1984 and $5600 ($17,010 inflation adjusted) afterwards.13 According to the American Society for Radiation Oncology, Advocacy Radiation Oncology Case Rate Program Curative radiation courses should cost $20,000 to $30,000 and palliative courses should cost $10,000 to $15,000. These costs are consistent with totals demonstrated in this analysis and similar to the inflation-adjusted Hanks et al figures. Preliminary findings suggest that radiation treatment constituted more than half of the total expenditures, with a notable $4 million increase in adjusted cost compared to the Medicare rates, indicating significant variation. Direct comparisons with Medicaid or commercial payer rates remain unexplored.
Future Directions
During the study period, 201 patients received 186 courses of radiation therapy in the community, while 1014 patients were treated in-house for a total of 833 courses. A forthcoming analysis will directly compare the cost of in-house care with that of communitybased treatment, specifically breaking down expenditure differences by diagnosis. Future research should investigate strategies to align reimbursement with quality metrics, including the potential role of tertiary accreditation in incentivizing high-value care. Additional work is also warranted to assess patient out-ofpocket expenses across care settings and to benchmark VA reimbursement against Medicare, Medicaid, and private insurance rates. In any case, with the increasing possibility of fewer fractions for treatments such as stereotactic radiotherapy or palliative care therapy, there is a clear financial incentive to treat as frequently as allowed despite equal clinical outcomes.
CONCLUSIONS
Veterans increasingly choose to receive care closer to home if the option is available. In the VA iron triangle, cost comes at the expense of access but quantifying this has proved elusive in the cost accounting model currently used at the VA.1 The inclusion of all charges loosely associated with SEOCs significantly impairs the ability to conduct meaningful cost analyses. The current VA methodology not only introduces substantial noise into the data but also leads to a marked underestimation of the true cost of care delivered in community settings. Such misrepresentation risks driving policy decisions that could inappropriately reduce or eliminate in-house radiation oncology services. Categorizing costs effectively in the VA could assist in making managerial and administrative decisions and would prevent damaging service lines based on misleading or incorrect data. A system which differentiates between patients who have received any treatment codes vs those who have not would increase accuracy.
- Kissick W. Medicine’s Dilemmas: Infinite Needs Versus Finite Resources. 1st ed. Yale University Press; 1994.
- Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
- Office of Management and Budget (US). Budget of the United States Government, Fiscal Year 2025. Washington, DC: US Government Publishing Office; 2024. Available from: US Department of Veterans Affairs FY 2025 Budget Submission: Budget in Brief.
- US Department of Veterans Affairs. Veteran care claims. Accessed April 3, 2025. https://www.va.gov/COMMUNITYCARE/revenue-ops/Veteran-Care-Claims.asp
- US Centers for Medicare and Medicaid Services. Accessed April 3, 2025. Procedure price lookup https://www.medicare.gov/procedure-price-lookup
- US Department of Veterans Affairs. WellHive -Enterprise. Accessed April 3, 2025. https://department.va.gov/privacy/wp-content/uploads/sites/5/2023/05/FY23WellHiveEnterprisePIA.pdf
- US Centers for Medicare and Medicaid Services. RVU21a physician fee schedule, January 2021 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu21a
- US Centers for Medicare and Medicaid Services. RVU22a physician fee schedule, January 2022 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- US Centers for Medicare and Medicaid Services. RVU23a physician fee schedule, January 2023 release. Accessed April 3, 2025. https://www.cms.gov/medicare/medicare-fee-service-payment/physicianfeesched/pfs-relative-value-files/rvu23a
- US Centers for Medicare and Medicaid Services. RVU23a Medicare Physician Fee Schedule rates effective January 1, 2024, through March 8, 2024. Accessed on April 3, 2025. https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a
- Kenamond MC, Mourad WF, Randall ME, Kaushal A. No oncology patient left behind: challenges and solutions in rural radiation oncology. Lancet Reg Health Am. 2022;13:100289. doi:10.1016/j.lana.2022.100289
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Hanks GE, Dunlap K. A comparison of the cost of various treatment methods for early cancer of the prostate. Int J Radiat Oncol Biol Phys. 1986;12(10):1879-1881. doi:10.1016/0360-3016(86)90334-2
- American Society of Radiation Oncology. Radiation oncology case rate program (ROCR). Accessed April 3, 2025. https://www.astro.org/advocacy/key-issues-8f3e5a3b76643265ee93287d79c4fc40/rocr
- Kissick W. Medicine’s Dilemmas: Infinite Needs Versus Finite Resources. 1st ed. Yale University Press; 1994.
- Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
- Office of Management and Budget (US). Budget of the United States Government, Fiscal Year 2025. Washington, DC: US Government Publishing Office; 2024. Available from: US Department of Veterans Affairs FY 2025 Budget Submission: Budget in Brief.
- US Department of Veterans Affairs. Veteran care claims. Accessed April 3, 2025. https://www.va.gov/COMMUNITYCARE/revenue-ops/Veteran-Care-Claims.asp
- US Centers for Medicare and Medicaid Services. Accessed April 3, 2025. Procedure price lookup https://www.medicare.gov/procedure-price-lookup
- US Department of Veterans Affairs. WellHive -Enterprise. Accessed April 3, 2025. https://department.va.gov/privacy/wp-content/uploads/sites/5/2023/05/FY23WellHiveEnterprisePIA.pdf
- US Centers for Medicare and Medicaid Services. RVU21a physician fee schedule, January 2021 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu21a
- US Centers for Medicare and Medicaid Services. RVU22a physician fee schedule, January 2022 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- US Centers for Medicare and Medicaid Services. RVU23a physician fee schedule, January 2023 release. Accessed April 3, 2025. https://www.cms.gov/medicare/medicare-fee-service-payment/physicianfeesched/pfs-relative-value-files/rvu23a
- US Centers for Medicare and Medicaid Services. RVU23a Medicare Physician Fee Schedule rates effective January 1, 2024, through March 8, 2024. Accessed on April 3, 2025. https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a
- Kenamond MC, Mourad WF, Randall ME, Kaushal A. No oncology patient left behind: challenges and solutions in rural radiation oncology. Lancet Reg Health Am. 2022;13:100289. doi:10.1016/j.lana.2022.100289
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Hanks GE, Dunlap K. A comparison of the cost of various treatment methods for early cancer of the prostate. Int J Radiat Oncol Biol Phys. 1986;12(10):1879-1881. doi:10.1016/0360-3016(86)90334-2
- American Society of Radiation Oncology. Radiation oncology case rate program (ROCR). Accessed April 3, 2025. https://www.astro.org/advocacy/key-issues-8f3e5a3b76643265ee93287d79c4fc40/rocr
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
Gastric Cancer Prevention: New AGA Update Reflects Latest High-Risk Screening and Surveillance Advice
Clinicians can help reduce gastric cancer incidence and mortality in high-risk groups through endoscopic screening and surveillance of precancerous conditions, such as gastric intestinal metaplasia (GIM), according to a new clinical practice update from AGA.
The update supports additional gastric guidance published so far in 2025, including a clinical guideline on the diagnosis and management of gastric premalignant conditions (GPMC) from the American College of Gastroenterology (ACG) and upper GI endoscopy quality indicators from ACG and the American Society for Gastrointestinal Endoscopy (ASGE).
“The synergy of these three publications coming out at the same time helps us to finally establish surveillance of high-risk gastric conditions in practice, as we do in the colon and esophagus,” said Douglas R. Morgan, MD, professor of medicine in gastroenterology and hepatology and director of Global Health programs in gastroenterology at the University of Alabama at Birmingham.
Morgan, who wasn’t involved with the AGA update, served as lead author for the ACG guideline and co-author of the ACG-ASGE quality indicators. He also co-authored the 2024 ACG clinical guideline on treating Helicobacter pylori infection, which has implications for gastric cancer.
“The AGA and ACG updates provide detail, while the QI document is an enforcer with medical, legal, and reimbursement implications,” he said. “We have an alignment of the stars with this overdue move toward concrete surveillance for high-risk lesions in the stomach.”
The clinical practice update was published in Gastroenterology.
Gastric Cancer Screening
, the authors wrote. The top ways to reduce mortality include primary prevention, particularly by eradicating H pylori, and secondary prevention through screening and surveillance.
High-risk groups in the United States should be considered for gastric cancer screening, including first-generation immigrants from high-incidence regions and potentially other non-White racial and ethnic groups, those with a family history of gastric cancer in a first-degree relative, and those with certain hereditary GI polyposis or hereditary cancer syndromes.
Endoscopy remains the best test for screening or surveillance of high-risk groups, the authors wrote, since it allows for direct visualization to endoscopically stage the mucosa, identify any concerning areas of neoplasia, and enable biopsies. Both endoscopic and histologic staging are key for risk stratification and surveillance decisions.
In particular, clinicians should use a high-definition white light endoscopy system with image enhancement, gastric mucosal cleansing, and insufflation to see the mucosa. As part of this, clinicians should allow for adequate visual inspection time, photodocumentation, and systematic biopsy protocol for mucosal staging, where appropriate.
As part of this, clinicians should consider H pylori eradication as an essential adjunct to endoscopic screening, the authors wrote. Opportunistic screening for H pylori should be considered in high-risk groups, and familial-based testing should be considered among adult household members of patients who test positive for H pylori.
Endoscopic Biopsy and Diagnosis
In patients with suspected gastric atrophy — with or without GIM — gastric biopsies should be obtained with a systematic approach, the authors wrote. Clinicians should take a minimum of five biopsies, sampling from the antrum/incisura and corpus.
Endoscopists should work with their pathologists on consistent documentation of histologic risk-stratification parameters when atrophic gastritis is diagnosed, the authors wrote. To inform clinical decision-making, this should include documentation of the presence or absence of H pylori infection, severity of atrophy or metaplasia, and histologic subtyping of GIM.
Although GIM and dysplasia are endoscopically detectable, these findings often go undiagnosed when endoscopists aren’t familiar with the characteristic visual features, the authors wrote. More training is needed, especially in the US, and although artificial intelligence tools appear promising for detecting early gastric neoplasia, data remain too preliminary to recommend routine use, the authors added.
Since indefinite and low-grade dysplasia can be difficult to identify by endoscopy and accurately diagnosis on histopathology, all dysplasia should be confirmed by an experienced gastrointestinal pathologist, the authors wrote. Clinicians should refer patients with visible or nonvisible dysplasia to an endoscopist or center with expertise in gastric neoplasia.
Endoscopic Management and Surveillance
If an index screening endoscopy doesn’t identify atrophy, GIM, or neoplasia, ongoing screening should be based on a patient’s risk factors and preferences. If the patient has a family history or multiple risk factors, ongoing screening should be considered. However, the optimal screening intervals in these scenarios aren’t well-defined.
Patients with confirmed gastric atrophy should undergo risk stratification, the authors wrote. Those with severe atrophic gastritis or multifocal/incomplete GIM would likely benefit from endoscopic surveillance, particularly if they have other risk factors such as family history. Surveillance should be considered every 3 years, though shorter intervals may be advisable for those with multiple risk factors such as severe GIM.
Patients with high-grade dysplasia or early gastric cancer should undergo endoscopic submucosal dissection (ESD), with the goal of en bloc, R0 resection to enable accurate pathologic staging and the intent to cure. Eradicating active H pylori infection is essential — but shouldn’t delay endoscopic intervention, the authors wrote.
In addition, patients with a history of successfully resected gastric dysplasia or cancer should undergo endoscopic surveillance. Although post-ESD surveillance intervals have been suggested in other recent AGA clinical practice updates, additional data are needed, particularly for US recommendations, the authors wrote.
Although type 1 gastric carcinoids in patients with atrophic gastritis are typically indolent, especially if less than 1 cm, endoscopists may consider resecting them and should resect lesions between 1and 2 cm. Patients with lesions over 2 cm should undergo cross-sectional imaging and be referred for surgical resection, given the risk for metastasis.
Patient-Centered Approach
The guideline authors suggested thinking about screening and surveillance on a patient-level basis. For instance, only those who are fit for endoscopic or potentially surgical treatment should be screened for gastric cancer and continued surveillance of GPMC, they wrote. If a person is no longer fit for endoscopic or surgical treatment, whether due to life expectancy or other comorbidities, then screening should be stopped.
In addition, to achieve health equity, clinicians should take a personalized approach to assess a patient’s risk for gastric cancer and determine whether to pursue screening and surveillance, the authors wrote. Modifiable risk factors — such as tobacco use, high-salt and processed food diets, and lack of health care — should also be addressed, since most of these risk factors disproportionately affect high-risk patients and represent healthcare disparities, they added.
“This update provides clinicians with a framework for understanding the natural history and epidemiology of gastric polyps, as well as guidance on best practices for the endoscopic detection and classification of gastric polyps, best practices for the endoscopic resection of gastric polyps, and best practices for endoscopic surveillance following resection,” said Hashem El-Serag, MD, professor and chair of medicine at the Baylor College of Medicine and director of the Texas Medical Center Digestive Diseases Center in Houston.
El-Serag, who wasn’t involved with the clinical practice update, has researched and published on consensus around the diagnosis and management of GIM.
“Stomach polyps are commonly found during routine endoscopic procedures. They are mostly asymptomatic and incidental, and therefore, clinicians may not be prepared ahead of time on how to deal with them,” he said. “The appropriate management requires proper identification and sampling of the polyp features and the uninvolved gastric mucosa, as well as a clear understanding of the risk factors and prognosis. Recent changes in the epidemiology and endoscopic management of gastric polyps makes this update timely and important.”
The update received no particular funding. The authors disclosed receiving grant support, having consultant relationships with, and serving in advisory roles for numerous pharmaceutical, biomedical, and biotechnology firms. Morgan and El-Serag reported having no relevant disclosures.
A version of this article appeared on Medscape.com.
Clinicians can help reduce gastric cancer incidence and mortality in high-risk groups through endoscopic screening and surveillance of precancerous conditions, such as gastric intestinal metaplasia (GIM), according to a new clinical practice update from AGA.
The update supports additional gastric guidance published so far in 2025, including a clinical guideline on the diagnosis and management of gastric premalignant conditions (GPMC) from the American College of Gastroenterology (ACG) and upper GI endoscopy quality indicators from ACG and the American Society for Gastrointestinal Endoscopy (ASGE).
“The synergy of these three publications coming out at the same time helps us to finally establish surveillance of high-risk gastric conditions in practice, as we do in the colon and esophagus,” said Douglas R. Morgan, MD, professor of medicine in gastroenterology and hepatology and director of Global Health programs in gastroenterology at the University of Alabama at Birmingham.
Morgan, who wasn’t involved with the AGA update, served as lead author for the ACG guideline and co-author of the ACG-ASGE quality indicators. He also co-authored the 2024 ACG clinical guideline on treating Helicobacter pylori infection, which has implications for gastric cancer.
“The AGA and ACG updates provide detail, while the QI document is an enforcer with medical, legal, and reimbursement implications,” he said. “We have an alignment of the stars with this overdue move toward concrete surveillance for high-risk lesions in the stomach.”
The clinical practice update was published in Gastroenterology.
Gastric Cancer Screening
, the authors wrote. The top ways to reduce mortality include primary prevention, particularly by eradicating H pylori, and secondary prevention through screening and surveillance.
High-risk groups in the United States should be considered for gastric cancer screening, including first-generation immigrants from high-incidence regions and potentially other non-White racial and ethnic groups, those with a family history of gastric cancer in a first-degree relative, and those with certain hereditary GI polyposis or hereditary cancer syndromes.
Endoscopy remains the best test for screening or surveillance of high-risk groups, the authors wrote, since it allows for direct visualization to endoscopically stage the mucosa, identify any concerning areas of neoplasia, and enable biopsies. Both endoscopic and histologic staging are key for risk stratification and surveillance decisions.
In particular, clinicians should use a high-definition white light endoscopy system with image enhancement, gastric mucosal cleansing, and insufflation to see the mucosa. As part of this, clinicians should allow for adequate visual inspection time, photodocumentation, and systematic biopsy protocol for mucosal staging, where appropriate.
As part of this, clinicians should consider H pylori eradication as an essential adjunct to endoscopic screening, the authors wrote. Opportunistic screening for H pylori should be considered in high-risk groups, and familial-based testing should be considered among adult household members of patients who test positive for H pylori.
Endoscopic Biopsy and Diagnosis
In patients with suspected gastric atrophy — with or without GIM — gastric biopsies should be obtained with a systematic approach, the authors wrote. Clinicians should take a minimum of five biopsies, sampling from the antrum/incisura and corpus.
Endoscopists should work with their pathologists on consistent documentation of histologic risk-stratification parameters when atrophic gastritis is diagnosed, the authors wrote. To inform clinical decision-making, this should include documentation of the presence or absence of H pylori infection, severity of atrophy or metaplasia, and histologic subtyping of GIM.
Although GIM and dysplasia are endoscopically detectable, these findings often go undiagnosed when endoscopists aren’t familiar with the characteristic visual features, the authors wrote. More training is needed, especially in the US, and although artificial intelligence tools appear promising for detecting early gastric neoplasia, data remain too preliminary to recommend routine use, the authors added.
Since indefinite and low-grade dysplasia can be difficult to identify by endoscopy and accurately diagnosis on histopathology, all dysplasia should be confirmed by an experienced gastrointestinal pathologist, the authors wrote. Clinicians should refer patients with visible or nonvisible dysplasia to an endoscopist or center with expertise in gastric neoplasia.
Endoscopic Management and Surveillance
If an index screening endoscopy doesn’t identify atrophy, GIM, or neoplasia, ongoing screening should be based on a patient’s risk factors and preferences. If the patient has a family history or multiple risk factors, ongoing screening should be considered. However, the optimal screening intervals in these scenarios aren’t well-defined.
Patients with confirmed gastric atrophy should undergo risk stratification, the authors wrote. Those with severe atrophic gastritis or multifocal/incomplete GIM would likely benefit from endoscopic surveillance, particularly if they have other risk factors such as family history. Surveillance should be considered every 3 years, though shorter intervals may be advisable for those with multiple risk factors such as severe GIM.
Patients with high-grade dysplasia or early gastric cancer should undergo endoscopic submucosal dissection (ESD), with the goal of en bloc, R0 resection to enable accurate pathologic staging and the intent to cure. Eradicating active H pylori infection is essential — but shouldn’t delay endoscopic intervention, the authors wrote.
In addition, patients with a history of successfully resected gastric dysplasia or cancer should undergo endoscopic surveillance. Although post-ESD surveillance intervals have been suggested in other recent AGA clinical practice updates, additional data are needed, particularly for US recommendations, the authors wrote.
Although type 1 gastric carcinoids in patients with atrophic gastritis are typically indolent, especially if less than 1 cm, endoscopists may consider resecting them and should resect lesions between 1and 2 cm. Patients with lesions over 2 cm should undergo cross-sectional imaging and be referred for surgical resection, given the risk for metastasis.
Patient-Centered Approach
The guideline authors suggested thinking about screening and surveillance on a patient-level basis. For instance, only those who are fit for endoscopic or potentially surgical treatment should be screened for gastric cancer and continued surveillance of GPMC, they wrote. If a person is no longer fit for endoscopic or surgical treatment, whether due to life expectancy or other comorbidities, then screening should be stopped.
In addition, to achieve health equity, clinicians should take a personalized approach to assess a patient’s risk for gastric cancer and determine whether to pursue screening and surveillance, the authors wrote. Modifiable risk factors — such as tobacco use, high-salt and processed food diets, and lack of health care — should also be addressed, since most of these risk factors disproportionately affect high-risk patients and represent healthcare disparities, they added.
“This update provides clinicians with a framework for understanding the natural history and epidemiology of gastric polyps, as well as guidance on best practices for the endoscopic detection and classification of gastric polyps, best practices for the endoscopic resection of gastric polyps, and best practices for endoscopic surveillance following resection,” said Hashem El-Serag, MD, professor and chair of medicine at the Baylor College of Medicine and director of the Texas Medical Center Digestive Diseases Center in Houston.
El-Serag, who wasn’t involved with the clinical practice update, has researched and published on consensus around the diagnosis and management of GIM.
“Stomach polyps are commonly found during routine endoscopic procedures. They are mostly asymptomatic and incidental, and therefore, clinicians may not be prepared ahead of time on how to deal with them,” he said. “The appropriate management requires proper identification and sampling of the polyp features and the uninvolved gastric mucosa, as well as a clear understanding of the risk factors and prognosis. Recent changes in the epidemiology and endoscopic management of gastric polyps makes this update timely and important.”
The update received no particular funding. The authors disclosed receiving grant support, having consultant relationships with, and serving in advisory roles for numerous pharmaceutical, biomedical, and biotechnology firms. Morgan and El-Serag reported having no relevant disclosures.
A version of this article appeared on Medscape.com.
Clinicians can help reduce gastric cancer incidence and mortality in high-risk groups through endoscopic screening and surveillance of precancerous conditions, such as gastric intestinal metaplasia (GIM), according to a new clinical practice update from AGA.
The update supports additional gastric guidance published so far in 2025, including a clinical guideline on the diagnosis and management of gastric premalignant conditions (GPMC) from the American College of Gastroenterology (ACG) and upper GI endoscopy quality indicators from ACG and the American Society for Gastrointestinal Endoscopy (ASGE).
“The synergy of these three publications coming out at the same time helps us to finally establish surveillance of high-risk gastric conditions in practice, as we do in the colon and esophagus,” said Douglas R. Morgan, MD, professor of medicine in gastroenterology and hepatology and director of Global Health programs in gastroenterology at the University of Alabama at Birmingham.
Morgan, who wasn’t involved with the AGA update, served as lead author for the ACG guideline and co-author of the ACG-ASGE quality indicators. He also co-authored the 2024 ACG clinical guideline on treating Helicobacter pylori infection, which has implications for gastric cancer.
“The AGA and ACG updates provide detail, while the QI document is an enforcer with medical, legal, and reimbursement implications,” he said. “We have an alignment of the stars with this overdue move toward concrete surveillance for high-risk lesions in the stomach.”
The clinical practice update was published in Gastroenterology.
Gastric Cancer Screening
, the authors wrote. The top ways to reduce mortality include primary prevention, particularly by eradicating H pylori, and secondary prevention through screening and surveillance.
High-risk groups in the United States should be considered for gastric cancer screening, including first-generation immigrants from high-incidence regions and potentially other non-White racial and ethnic groups, those with a family history of gastric cancer in a first-degree relative, and those with certain hereditary GI polyposis or hereditary cancer syndromes.
Endoscopy remains the best test for screening or surveillance of high-risk groups, the authors wrote, since it allows for direct visualization to endoscopically stage the mucosa, identify any concerning areas of neoplasia, and enable biopsies. Both endoscopic and histologic staging are key for risk stratification and surveillance decisions.
In particular, clinicians should use a high-definition white light endoscopy system with image enhancement, gastric mucosal cleansing, and insufflation to see the mucosa. As part of this, clinicians should allow for adequate visual inspection time, photodocumentation, and systematic biopsy protocol for mucosal staging, where appropriate.
As part of this, clinicians should consider H pylori eradication as an essential adjunct to endoscopic screening, the authors wrote. Opportunistic screening for H pylori should be considered in high-risk groups, and familial-based testing should be considered among adult household members of patients who test positive for H pylori.
Endoscopic Biopsy and Diagnosis
In patients with suspected gastric atrophy — with or without GIM — gastric biopsies should be obtained with a systematic approach, the authors wrote. Clinicians should take a minimum of five biopsies, sampling from the antrum/incisura and corpus.
Endoscopists should work with their pathologists on consistent documentation of histologic risk-stratification parameters when atrophic gastritis is diagnosed, the authors wrote. To inform clinical decision-making, this should include documentation of the presence or absence of H pylori infection, severity of atrophy or metaplasia, and histologic subtyping of GIM.
Although GIM and dysplasia are endoscopically detectable, these findings often go undiagnosed when endoscopists aren’t familiar with the characteristic visual features, the authors wrote. More training is needed, especially in the US, and although artificial intelligence tools appear promising for detecting early gastric neoplasia, data remain too preliminary to recommend routine use, the authors added.
Since indefinite and low-grade dysplasia can be difficult to identify by endoscopy and accurately diagnosis on histopathology, all dysplasia should be confirmed by an experienced gastrointestinal pathologist, the authors wrote. Clinicians should refer patients with visible or nonvisible dysplasia to an endoscopist or center with expertise in gastric neoplasia.
Endoscopic Management and Surveillance
If an index screening endoscopy doesn’t identify atrophy, GIM, or neoplasia, ongoing screening should be based on a patient’s risk factors and preferences. If the patient has a family history or multiple risk factors, ongoing screening should be considered. However, the optimal screening intervals in these scenarios aren’t well-defined.
Patients with confirmed gastric atrophy should undergo risk stratification, the authors wrote. Those with severe atrophic gastritis or multifocal/incomplete GIM would likely benefit from endoscopic surveillance, particularly if they have other risk factors such as family history. Surveillance should be considered every 3 years, though shorter intervals may be advisable for those with multiple risk factors such as severe GIM.
Patients with high-grade dysplasia or early gastric cancer should undergo endoscopic submucosal dissection (ESD), with the goal of en bloc, R0 resection to enable accurate pathologic staging and the intent to cure. Eradicating active H pylori infection is essential — but shouldn’t delay endoscopic intervention, the authors wrote.
In addition, patients with a history of successfully resected gastric dysplasia or cancer should undergo endoscopic surveillance. Although post-ESD surveillance intervals have been suggested in other recent AGA clinical practice updates, additional data are needed, particularly for US recommendations, the authors wrote.
Although type 1 gastric carcinoids in patients with atrophic gastritis are typically indolent, especially if less than 1 cm, endoscopists may consider resecting them and should resect lesions between 1and 2 cm. Patients with lesions over 2 cm should undergo cross-sectional imaging and be referred for surgical resection, given the risk for metastasis.
Patient-Centered Approach
The guideline authors suggested thinking about screening and surveillance on a patient-level basis. For instance, only those who are fit for endoscopic or potentially surgical treatment should be screened for gastric cancer and continued surveillance of GPMC, they wrote. If a person is no longer fit for endoscopic or surgical treatment, whether due to life expectancy or other comorbidities, then screening should be stopped.
In addition, to achieve health equity, clinicians should take a personalized approach to assess a patient’s risk for gastric cancer and determine whether to pursue screening and surveillance, the authors wrote. Modifiable risk factors — such as tobacco use, high-salt and processed food diets, and lack of health care — should also be addressed, since most of these risk factors disproportionately affect high-risk patients and represent healthcare disparities, they added.
“This update provides clinicians with a framework for understanding the natural history and epidemiology of gastric polyps, as well as guidance on best practices for the endoscopic detection and classification of gastric polyps, best practices for the endoscopic resection of gastric polyps, and best practices for endoscopic surveillance following resection,” said Hashem El-Serag, MD, professor and chair of medicine at the Baylor College of Medicine and director of the Texas Medical Center Digestive Diseases Center in Houston.
El-Serag, who wasn’t involved with the clinical practice update, has researched and published on consensus around the diagnosis and management of GIM.
“Stomach polyps are commonly found during routine endoscopic procedures. They are mostly asymptomatic and incidental, and therefore, clinicians may not be prepared ahead of time on how to deal with them,” he said. “The appropriate management requires proper identification and sampling of the polyp features and the uninvolved gastric mucosa, as well as a clear understanding of the risk factors and prognosis. Recent changes in the epidemiology and endoscopic management of gastric polyps makes this update timely and important.”
The update received no particular funding. The authors disclosed receiving grant support, having consultant relationships with, and serving in advisory roles for numerous pharmaceutical, biomedical, and biotechnology firms. Morgan and El-Serag reported having no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
New Fecal Product Expected to Enhance Microbiome Research
According to AGA’s Center for Gut Microbiome Research & Education, this critical resource will help advance the utility and reproducibility of microbiome-based diagnostics — “which still remain relatively meaningless clinically, although patients continue to buy direct-to-consumer tests, and a standard reference material will mean there’s a better way to ensure quality control and accuracy.”
Though not a therapeutic, Human Fecal Material RM is expected to speed up gastrointestinal (GI) therapeutics since many microbiome-based drugs are inspired by fecal transplants with human stool as the developmental starting point. A standardized reference material will be an important resource as industry develops and tests new drugs. It can be purchased online at the NIST Store (shop.nist.gov).
The product consists of eight frozen vials of exhaustively studied human feces suspended in aqueous solution. Available are more than 25 pages of data identifying the key microbes and biomolecules in the material. Scientists, including those working at biopharmaceutical and biotech companies, can use this material to further their research and develop new drugs that target the microbiome, including treatments that contain living bacteria.
Development
According to NIST, the stool material is “the most precisely measured, scientifically analyzed, and richly characterized human fecal standard ever produced.
“The project ran for about 6 years from start to finish, the last 2 for manufacturing, characterization, and writing,” said NIST molecular geneticist Scott A. Jackson, PhD, who helped develop the product. “We hope our reference material will lay the foundation for gut microbiome research to thrive and reach its full potential.”
As founder of NIST’s Complex Microbial Systems Group, Jackson is leading international efforts to improve microbiome and metagenomic measurements by organizing inter-lab studies and refining reference materials and methods.
The project collected stool from two cohorts of donors, ie, vegetarians and omnivores, with each cohort comprising four to six donors. Material from each cohort was pooled and homogenized before being aliquoted into 5000 vials per cohort. About 300 tubes from each cohort were picked, and aliquots then underwent multiomic analyses.
Offering his perspective on the new product, Sudhir K. Dutta, MBBS, associate professor in the Division of Gastroenterology and Hepatology at Johns Hopkins University School of Medicine, Baltimore, said, “This tool will be 100% useful for microbiome research.”
And according to Lori Holtz, MD, MSPH, professor of pediatric gastroenterology, hepatology, and nutrition at Washington University School of Medicine in St. Louis, Missouri, the material will aid microbiome research by allowing interpretability and repeatability across studies. “Microbiome research is a relatively new field, and protocols differ from group to group and lab to lab, so it’s been difficult to compare results across studies,” she told GI & Hepatology News. “A standard stool product will allow for greater comparability in preclinical studies and later clinical trials testing interventions to alter the microbiome.”
The NIST developers are looking forward to reaction from the GI research community. “Over the last several years, we’ve released smaller pilot batches of material to smaller groups of stakeholders,” said Jackson. “We’ve used the feedback on these earlier batches to inform the manufacturing and characterization of the final batch that was released in March, but we don’t yet have any feedback yet on the current material.”
Jackson, Dutta, and Holtz disclosed having no relevant competing interests.
A version of this article appeared on Medscape.com.
According to AGA’s Center for Gut Microbiome Research & Education, this critical resource will help advance the utility and reproducibility of microbiome-based diagnostics — “which still remain relatively meaningless clinically, although patients continue to buy direct-to-consumer tests, and a standard reference material will mean there’s a better way to ensure quality control and accuracy.”
Though not a therapeutic, Human Fecal Material RM is expected to speed up gastrointestinal (GI) therapeutics since many microbiome-based drugs are inspired by fecal transplants with human stool as the developmental starting point. A standardized reference material will be an important resource as industry develops and tests new drugs. It can be purchased online at the NIST Store (shop.nist.gov).
The product consists of eight frozen vials of exhaustively studied human feces suspended in aqueous solution. Available are more than 25 pages of data identifying the key microbes and biomolecules in the material. Scientists, including those working at biopharmaceutical and biotech companies, can use this material to further their research and develop new drugs that target the microbiome, including treatments that contain living bacteria.
Development
According to NIST, the stool material is “the most precisely measured, scientifically analyzed, and richly characterized human fecal standard ever produced.
“The project ran for about 6 years from start to finish, the last 2 for manufacturing, characterization, and writing,” said NIST molecular geneticist Scott A. Jackson, PhD, who helped develop the product. “We hope our reference material will lay the foundation for gut microbiome research to thrive and reach its full potential.”
As founder of NIST’s Complex Microbial Systems Group, Jackson is leading international efforts to improve microbiome and metagenomic measurements by organizing inter-lab studies and refining reference materials and methods.
The project collected stool from two cohorts of donors, ie, vegetarians and omnivores, with each cohort comprising four to six donors. Material from each cohort was pooled and homogenized before being aliquoted into 5000 vials per cohort. About 300 tubes from each cohort were picked, and aliquots then underwent multiomic analyses.
Offering his perspective on the new product, Sudhir K. Dutta, MBBS, associate professor in the Division of Gastroenterology and Hepatology at Johns Hopkins University School of Medicine, Baltimore, said, “This tool will be 100% useful for microbiome research.”
And according to Lori Holtz, MD, MSPH, professor of pediatric gastroenterology, hepatology, and nutrition at Washington University School of Medicine in St. Louis, Missouri, the material will aid microbiome research by allowing interpretability and repeatability across studies. “Microbiome research is a relatively new field, and protocols differ from group to group and lab to lab, so it’s been difficult to compare results across studies,” she told GI & Hepatology News. “A standard stool product will allow for greater comparability in preclinical studies and later clinical trials testing interventions to alter the microbiome.”
The NIST developers are looking forward to reaction from the GI research community. “Over the last several years, we’ve released smaller pilot batches of material to smaller groups of stakeholders,” said Jackson. “We’ve used the feedback on these earlier batches to inform the manufacturing and characterization of the final batch that was released in March, but we don’t yet have any feedback yet on the current material.”
Jackson, Dutta, and Holtz disclosed having no relevant competing interests.
A version of this article appeared on Medscape.com.
According to AGA’s Center for Gut Microbiome Research & Education, this critical resource will help advance the utility and reproducibility of microbiome-based diagnostics — “which still remain relatively meaningless clinically, although patients continue to buy direct-to-consumer tests, and a standard reference material will mean there’s a better way to ensure quality control and accuracy.”
Though not a therapeutic, Human Fecal Material RM is expected to speed up gastrointestinal (GI) therapeutics since many microbiome-based drugs are inspired by fecal transplants with human stool as the developmental starting point. A standardized reference material will be an important resource as industry develops and tests new drugs. It can be purchased online at the NIST Store (shop.nist.gov).
The product consists of eight frozen vials of exhaustively studied human feces suspended in aqueous solution. Available are more than 25 pages of data identifying the key microbes and biomolecules in the material. Scientists, including those working at biopharmaceutical and biotech companies, can use this material to further their research and develop new drugs that target the microbiome, including treatments that contain living bacteria.
Development
According to NIST, the stool material is “the most precisely measured, scientifically analyzed, and richly characterized human fecal standard ever produced.
“The project ran for about 6 years from start to finish, the last 2 for manufacturing, characterization, and writing,” said NIST molecular geneticist Scott A. Jackson, PhD, who helped develop the product. “We hope our reference material will lay the foundation for gut microbiome research to thrive and reach its full potential.”
As founder of NIST’s Complex Microbial Systems Group, Jackson is leading international efforts to improve microbiome and metagenomic measurements by organizing inter-lab studies and refining reference materials and methods.
The project collected stool from two cohorts of donors, ie, vegetarians and omnivores, with each cohort comprising four to six donors. Material from each cohort was pooled and homogenized before being aliquoted into 5000 vials per cohort. About 300 tubes from each cohort were picked, and aliquots then underwent multiomic analyses.
Offering his perspective on the new product, Sudhir K. Dutta, MBBS, associate professor in the Division of Gastroenterology and Hepatology at Johns Hopkins University School of Medicine, Baltimore, said, “This tool will be 100% useful for microbiome research.”
And according to Lori Holtz, MD, MSPH, professor of pediatric gastroenterology, hepatology, and nutrition at Washington University School of Medicine in St. Louis, Missouri, the material will aid microbiome research by allowing interpretability and repeatability across studies. “Microbiome research is a relatively new field, and protocols differ from group to group and lab to lab, so it’s been difficult to compare results across studies,” she told GI & Hepatology News. “A standard stool product will allow for greater comparability in preclinical studies and later clinical trials testing interventions to alter the microbiome.”
The NIST developers are looking forward to reaction from the GI research community. “Over the last several years, we’ve released smaller pilot batches of material to smaller groups of stakeholders,” said Jackson. “We’ve used the feedback on these earlier batches to inform the manufacturing and characterization of the final batch that was released in March, but we don’t yet have any feedback yet on the current material.”
Jackson, Dutta, and Holtz disclosed having no relevant competing interests.
A version of this article appeared on Medscape.com.
Treating Barrett’s Esophagus: Comparing EMR and ESD
Dear colleagues,
Many of us diagnose and treat patients with Barrett’s esophagus, estimated to affect up to 5.6% of the US adult population. There has been an expanding array of tools to help diagnose and effectively treat Barrett’s esophagus with dysplasia and malignancy. In particular, endoscopic submucosal dissection (ESD) has emerged as an important method for treating early cancer in the gastrointestinal tract.
But how do we incorporate ESD into our algorithm for management, especially with the popularity and effectiveness of endoscopic mucosal resection (EMR)? In this issue of Perspectives we aim to provide context for the use of ESD, as compared with EMR. Dr. Silvio de Melo discusses his preferred EMR technique and its many advantages in the management of BE, including for residual or refractory areas. In contrast, Dr. Mohamed Othman reviews the power of ESD and when we should consider this approach over EMR. We hope these discussions will facilitate your care for patients with Barrett’s esophagus.
We also welcome your thoughts on this topic — join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Endoscopic Mucosal Resection: The ‘Workhorse’ for Patient Care
BY SILVIO W. DE MELO JR, MD, AGAF
Barrett’s esophagus (BE) remains an important clinical problem, being one of the modifiable risk factors for esophageal adenocarcinoma. The care for BE is complex and requires several steps to correctly formulate a therapeutic plan. It starts with a proper endoscopic examination. It is recommended to spend at least 1 minute inspecting and evaluating every centimeter of the salmon-colored epithelium, looking for change in vascular pattern, erosions/ulcers, nodules, and/or masses. After the inspection, random biopsies every 1-2 cm plus targeted biopsies will guide you. It is still controversial if the addition of other sampling strategies, such as brushings or confocal endomicroscopy, is needed.
The introduction of radiofrequency ablation (RFA) was paramount in popularizing the treatment options for BE and sunsetting the previous dominant modality, photodynamic therapy (PDT). RFA proved to have a superior clinical efficacy in replacing the intestinal metaplasia/BE with neosquamous epithelium while boosting a much better safety profile, compared with PDT. However, RFA is most efficacious for “flat BE” and it is not an effective, nor recommended, method to treat nodular BE or early cancer, such as carcinoma in situ or nodular high-grade dysplasia. Endoscopic mucosal resection (EMR) is utilized to overcome those limitations.
There are several techniques utilized for EMR:
- The lift and snare technique.
- The snare-in-cap technique.
- The Band-snare technique.
The free-hand submucosal lift and snare is not frequently used in the esophagus. It is difficult to maintain visualization while being confident that one has the whole lesion inside the snare and that the distal (anal side) part of the lesion is free of any unwanted tissue (to minimize complications such as perforations or unwelcomed gastric resections). It is difficult after the first resection to lift an adjacent area, as the fluid easily leaks from the first resected spot, thus removing larger lesions in piece-meal fashion is challenging. This technique can be used in small (in my personal experience, less than 5 mm) lesions, but, given that there are better and safer alternatives, I almost never use this technique for my esophageal EMR cases. I prefer to use the band-snare technique even for lesions under 5 mm.
The snare-in-cap technique has been utilized in the esophagus. In this technique, a cap is attached to the distal end of the scope and the size of the resection is determined by the size of the cap, usually under 1.5 cm. Because of the risk of perforation without previous lifting, it is required that the lesion is lifted with a submucosal fluid, saline or any Food and Drug Administration–approved EMR solution. The lesion is then suctioned inside the cap where the snare had been previously opened inside the cap, the snare is closed, and the tissue is resected. The same limitations regarding the inability to remove larger lesions (greater than 1.5 cm) because of the challenge in lifting the adjacent area applies here. However, the perforation risk for this technique is higher than the traditional lift and the band and snare techniques. Thus, this technique has fallen out of favor for most endoscopists.
The third technique (band-snare EMR) is the one that most endoscopists use for endoscopic mucosal resection. It is a small variation of the already time-tested and very familiar procedure of esophageal variceal band ligation (EVL). There are multiple commercially available kits for esophageal EMR. The kit contains the chamber with the bands and a proprietary hexagonal snare used to resect the specimen.
The advantages of this technique are:
- It is widely commercially available.
- It builds on a familiar procedure, EVL, therefore the learning curve is short.
- The set-up is quick and the procedure can be completed safely and effectively.
- There is no need for injecting the submucosal with a lifting solution.
- Despite the band having a size limitation of 1 cm, one can remove larger lesions by repeating the band and resect process, using the rosette technique.
Band-snare EMR also has limitations:
- There are only six bands on each chamber. Depending on the size of the lesion, one may need to use multiple kits.
- It is not suitable for en bloc resection of lesions greater than 1 cm.
My experience with band EMR is that we can complete the procedure in under 1 hour. The dreaded complication of perforation occurs in under 1% of cases, most bleeding episodes can easily be controlled endoscopically, and the risk of post-EMR stricture is minimal. Therefore, band EMR is the most used technique for esophageal endoscopic resections.
Esophageal EMR is also effective for other indications in BE therapy, such as residual and recurrent BE. Band-snare EMR can be used for an en bloc resection or rosette technique for the areas resistant to ablation therapies with great success and safety.
From a financial standpoint, comparing EMR with endoscopic submucosal dissection (ESD), EMR is the superior strategy given that EMR is widely available, has a much shorter learning curve, has a greater safety profile, is applicable to a wider variety of indications, and has a more favorable return on investment. EMR should be the workhorse for the care of patients with BE, reserving ESD for specific indications.
In summary, there is no “one-size-fits-all” endoscopic therapy in the care of BE. Most Barrett’s patients can be successfully treated with a combination of ablation plus EMR, reserving ESD for select cases.
Dr. de Melo is section chief of gastroenterology at the Orlando VA Healthcare System, Orlando, Florida. He declares no conflicts of interest.
ESD Over EMR for Resecting Esophageal Lesions
BY MOHAMED O. OTHMAN, MD, AGAF
Although endoscopic submucosal dissection (ESD) is the preferred endoscopic resection method in the East, the adoption of this technique in the West, particularly in the United States, has faced many hurdles. Many endoscopists who routinely perform piecemeal endoscopic mucosal resection (EMR) question the utility of ESD, arguing that EMR is just as effective. While this may hold true in certain situations, the global trend in the endoscopic treatment of early esophageal squamous cell carcinoma, nodular Barrett’s esophagus (BE), and early esophageal adenocarcinoma (EAC) has clearly shifted toward ESD. In this perspective, I will summarize why ESD is preferred over EMR for these indications and explore why ESD has yet to gain widespread adoption in the United States.
The superiority of ESD over EMR has been well established in multiple publications from both Eastern and Western literature. Mejia-Perez et al, in a multicenter cohort study from eight centers in North America, compared outcomes of ESD vs EMR for BE with high-grade dysplasia (HGD) or T1a adenocarcinoma in 243 patients. ESD achieved significantly higher en bloc resection rates (89% vs 43%) and R0 resection rates (73% vs 56%), compared with EMR, along with a substantially lower recurrence/residual disease rate on follow-up (3.5% in the ESD group vs 31.4% in EMR group). Additionally, more patients required repeat endoscopic resection after EMR to treat residual or recurrent disease (EMR, 24.2% vs ESD, 3.5%; P < .001).
Han et al conducted a meta-analysis of 22 studies comparing ESD and EMR for early esophageal neoplasia, including both squamous cell carcinoma (SCC) and BE-associated lesions. ESD was associated with significantly higher curative resection rates than EMR (OR, 9.74; 95% CI, 4.83-19.62; P < .0001). Of note, lesion size was a critical factor in determining the advantage of ESD. For lesions ≤ 10 mm, curative resection rates were comparable between ESD and EMR. However, for lesions > 10 mm, ESD achieved significantly higher curative resection rates. This size-based recommendation has been adopted by the American Society of Gastrointestinal Endoscopy (ASGE) in their recent guidelines on ESD indications for esophageal lesions. ASGE guidelines favors ESD over EMR for SCC lesions > 15 mm and for nodular BE with dysplasia or early EAC > 20 mm.
ESD is particularly beneficial in patients who develop early adenocarcinoma after RFA or EMR. Mesureur et al evaluated the efficacy of salvage ESD for Barrett’s recurrence or residual BE following RFA. In their multicenter retrospective study of 56 patients, salvage ESD achieved an en bloc resection rate of 89.3%, despite significant fibrosis, with an R0 resection rate of 66%. At a median follow-up of 14 months, most patients remained in endoscopic remission without the need for esophagectomy.
Combining ESD with RFA has also been shown to accelerate the eradication of BE with dysplasia while reducing the number of required sessions. Our group demonstrated the high efficacy of ESD followed by RFA in 18 patients, most of whom had long-segment BE with HGD or EAC. On average, patients required only one to two RFA sessions after ESD to achieve complete eradication of intestinal metaplasia (CE-IM). Over a median follow-up of 42.5 months (IQR, 28-59.25), complete eradication of early esophageal cancer was achieved in 13 patients (100%), eradication of dysplasia in 15 patients (100%), and CE-IM in 14 patients (77.8%).
Despite the overwhelming evidence supporting ESD and the strong endorsement from professional societies, adoption in the West continues to lag. Several factors contribute to this gap. First, ESD has a steep learning curve. Our data showed that, on average, an untutored practitioner achieved competency after 150-250 procedures, a finding corroborated by other US groups.
Second, there is no specific CPT code for ESD in the United States. Physicians are forced to bill the procedure as EMR or use an unlisted code, resulting in reimbursement that does not reflect the time and complexity of the procedure. Our group showed that physician reimbursement for ESD is highly variable, ranging from $50 to $800 per case, depending on insurance type.
Third, the increasing emphasis on productivity and RVU generation in academic settings has hindered the growth of ESD training in many institutions. Still, the outlook for ESD in the United States remains encouraging. Multiple industry-sponsored training courses are held annually, and professional societies are investing heavily in expanding access to structured education in ESD. Industry is also innovating devices that improve procedural efficiency and safety. Adopting novel approaches, such as traction-assisted ESD, has made the technique more appealing to endoscopists concerned about long procedure times. For example, our group proposed a standardized esophageal ESD technique that incorporates specimen self-retraction. This method improves both safety and speed and has helped address several procedural challenges. We’ve demonstrated that consistency in technique can substantially expedite esophageal ESD.
Fast forward 5 years: We anticipate a dedicated CPT code for ESD, broader access to advanced resection tools, and an expanding number of fellowships offering structured ESD training. These developments are poised to eliminate many of the current barriers. In summary, with robust data supporting the efficacy of ESD in early esophageal cancer, the focus in the United States should shift toward mastering and integrating the technique, rather than dismissing it in favor of piecemeal EMR.
Dr. Othman is chief of the gastroenterology and hepatology section at Baylor College of Medicine and Medicine Subspecialities Service Line Chief at Baylor St Luke’s Medical Center, both in Houston. He declares no conflicts of interest.
Dear colleagues,
Many of us diagnose and treat patients with Barrett’s esophagus, estimated to affect up to 5.6% of the US adult population. There has been an expanding array of tools to help diagnose and effectively treat Barrett’s esophagus with dysplasia and malignancy. In particular, endoscopic submucosal dissection (ESD) has emerged as an important method for treating early cancer in the gastrointestinal tract.
But how do we incorporate ESD into our algorithm for management, especially with the popularity and effectiveness of endoscopic mucosal resection (EMR)? In this issue of Perspectives we aim to provide context for the use of ESD, as compared with EMR. Dr. Silvio de Melo discusses his preferred EMR technique and its many advantages in the management of BE, including for residual or refractory areas. In contrast, Dr. Mohamed Othman reviews the power of ESD and when we should consider this approach over EMR. We hope these discussions will facilitate your care for patients with Barrett’s esophagus.
We also welcome your thoughts on this topic — join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Endoscopic Mucosal Resection: The ‘Workhorse’ for Patient Care
BY SILVIO W. DE MELO JR, MD, AGAF
Barrett’s esophagus (BE) remains an important clinical problem, being one of the modifiable risk factors for esophageal adenocarcinoma. The care for BE is complex and requires several steps to correctly formulate a therapeutic plan. It starts with a proper endoscopic examination. It is recommended to spend at least 1 minute inspecting and evaluating every centimeter of the salmon-colored epithelium, looking for change in vascular pattern, erosions/ulcers, nodules, and/or masses. After the inspection, random biopsies every 1-2 cm plus targeted biopsies will guide you. It is still controversial if the addition of other sampling strategies, such as brushings or confocal endomicroscopy, is needed.
The introduction of radiofrequency ablation (RFA) was paramount in popularizing the treatment options for BE and sunsetting the previous dominant modality, photodynamic therapy (PDT). RFA proved to have a superior clinical efficacy in replacing the intestinal metaplasia/BE with neosquamous epithelium while boosting a much better safety profile, compared with PDT. However, RFA is most efficacious for “flat BE” and it is not an effective, nor recommended, method to treat nodular BE or early cancer, such as carcinoma in situ or nodular high-grade dysplasia. Endoscopic mucosal resection (EMR) is utilized to overcome those limitations.
There are several techniques utilized for EMR:
- The lift and snare technique.
- The snare-in-cap technique.
- The Band-snare technique.
The free-hand submucosal lift and snare is not frequently used in the esophagus. It is difficult to maintain visualization while being confident that one has the whole lesion inside the snare and that the distal (anal side) part of the lesion is free of any unwanted tissue (to minimize complications such as perforations or unwelcomed gastric resections). It is difficult after the first resection to lift an adjacent area, as the fluid easily leaks from the first resected spot, thus removing larger lesions in piece-meal fashion is challenging. This technique can be used in small (in my personal experience, less than 5 mm) lesions, but, given that there are better and safer alternatives, I almost never use this technique for my esophageal EMR cases. I prefer to use the band-snare technique even for lesions under 5 mm.
The snare-in-cap technique has been utilized in the esophagus. In this technique, a cap is attached to the distal end of the scope and the size of the resection is determined by the size of the cap, usually under 1.5 cm. Because of the risk of perforation without previous lifting, it is required that the lesion is lifted with a submucosal fluid, saline or any Food and Drug Administration–approved EMR solution. The lesion is then suctioned inside the cap where the snare had been previously opened inside the cap, the snare is closed, and the tissue is resected. The same limitations regarding the inability to remove larger lesions (greater than 1.5 cm) because of the challenge in lifting the adjacent area applies here. However, the perforation risk for this technique is higher than the traditional lift and the band and snare techniques. Thus, this technique has fallen out of favor for most endoscopists.
The third technique (band-snare EMR) is the one that most endoscopists use for endoscopic mucosal resection. It is a small variation of the already time-tested and very familiar procedure of esophageal variceal band ligation (EVL). There are multiple commercially available kits for esophageal EMR. The kit contains the chamber with the bands and a proprietary hexagonal snare used to resect the specimen.
The advantages of this technique are:
- It is widely commercially available.
- It builds on a familiar procedure, EVL, therefore the learning curve is short.
- The set-up is quick and the procedure can be completed safely and effectively.
- There is no need for injecting the submucosal with a lifting solution.
- Despite the band having a size limitation of 1 cm, one can remove larger lesions by repeating the band and resect process, using the rosette technique.
Band-snare EMR also has limitations:
- There are only six bands on each chamber. Depending on the size of the lesion, one may need to use multiple kits.
- It is not suitable for en bloc resection of lesions greater than 1 cm.
My experience with band EMR is that we can complete the procedure in under 1 hour. The dreaded complication of perforation occurs in under 1% of cases, most bleeding episodes can easily be controlled endoscopically, and the risk of post-EMR stricture is minimal. Therefore, band EMR is the most used technique for esophageal endoscopic resections.
Esophageal EMR is also effective for other indications in BE therapy, such as residual and recurrent BE. Band-snare EMR can be used for an en bloc resection or rosette technique for the areas resistant to ablation therapies with great success and safety.
From a financial standpoint, comparing EMR with endoscopic submucosal dissection (ESD), EMR is the superior strategy given that EMR is widely available, has a much shorter learning curve, has a greater safety profile, is applicable to a wider variety of indications, and has a more favorable return on investment. EMR should be the workhorse for the care of patients with BE, reserving ESD for specific indications.
In summary, there is no “one-size-fits-all” endoscopic therapy in the care of BE. Most Barrett’s patients can be successfully treated with a combination of ablation plus EMR, reserving ESD for select cases.
Dr. de Melo is section chief of gastroenterology at the Orlando VA Healthcare System, Orlando, Florida. He declares no conflicts of interest.
ESD Over EMR for Resecting Esophageal Lesions
BY MOHAMED O. OTHMAN, MD, AGAF
Although endoscopic submucosal dissection (ESD) is the preferred endoscopic resection method in the East, the adoption of this technique in the West, particularly in the United States, has faced many hurdles. Many endoscopists who routinely perform piecemeal endoscopic mucosal resection (EMR) question the utility of ESD, arguing that EMR is just as effective. While this may hold true in certain situations, the global trend in the endoscopic treatment of early esophageal squamous cell carcinoma, nodular Barrett’s esophagus (BE), and early esophageal adenocarcinoma (EAC) has clearly shifted toward ESD. In this perspective, I will summarize why ESD is preferred over EMR for these indications and explore why ESD has yet to gain widespread adoption in the United States.
The superiority of ESD over EMR has been well established in multiple publications from both Eastern and Western literature. Mejia-Perez et al, in a multicenter cohort study from eight centers in North America, compared outcomes of ESD vs EMR for BE with high-grade dysplasia (HGD) or T1a adenocarcinoma in 243 patients. ESD achieved significantly higher en bloc resection rates (89% vs 43%) and R0 resection rates (73% vs 56%), compared with EMR, along with a substantially lower recurrence/residual disease rate on follow-up (3.5% in the ESD group vs 31.4% in EMR group). Additionally, more patients required repeat endoscopic resection after EMR to treat residual or recurrent disease (EMR, 24.2% vs ESD, 3.5%; P < .001).
Han et al conducted a meta-analysis of 22 studies comparing ESD and EMR for early esophageal neoplasia, including both squamous cell carcinoma (SCC) and BE-associated lesions. ESD was associated with significantly higher curative resection rates than EMR (OR, 9.74; 95% CI, 4.83-19.62; P < .0001). Of note, lesion size was a critical factor in determining the advantage of ESD. For lesions ≤ 10 mm, curative resection rates were comparable between ESD and EMR. However, for lesions > 10 mm, ESD achieved significantly higher curative resection rates. This size-based recommendation has been adopted by the American Society of Gastrointestinal Endoscopy (ASGE) in their recent guidelines on ESD indications for esophageal lesions. ASGE guidelines favors ESD over EMR for SCC lesions > 15 mm and for nodular BE with dysplasia or early EAC > 20 mm.
ESD is particularly beneficial in patients who develop early adenocarcinoma after RFA or EMR. Mesureur et al evaluated the efficacy of salvage ESD for Barrett’s recurrence or residual BE following RFA. In their multicenter retrospective study of 56 patients, salvage ESD achieved an en bloc resection rate of 89.3%, despite significant fibrosis, with an R0 resection rate of 66%. At a median follow-up of 14 months, most patients remained in endoscopic remission without the need for esophagectomy.
Combining ESD with RFA has also been shown to accelerate the eradication of BE with dysplasia while reducing the number of required sessions. Our group demonstrated the high efficacy of ESD followed by RFA in 18 patients, most of whom had long-segment BE with HGD or EAC. On average, patients required only one to two RFA sessions after ESD to achieve complete eradication of intestinal metaplasia (CE-IM). Over a median follow-up of 42.5 months (IQR, 28-59.25), complete eradication of early esophageal cancer was achieved in 13 patients (100%), eradication of dysplasia in 15 patients (100%), and CE-IM in 14 patients (77.8%).
Despite the overwhelming evidence supporting ESD and the strong endorsement from professional societies, adoption in the West continues to lag. Several factors contribute to this gap. First, ESD has a steep learning curve. Our data showed that, on average, an untutored practitioner achieved competency after 150-250 procedures, a finding corroborated by other US groups.
Second, there is no specific CPT code for ESD in the United States. Physicians are forced to bill the procedure as EMR or use an unlisted code, resulting in reimbursement that does not reflect the time and complexity of the procedure. Our group showed that physician reimbursement for ESD is highly variable, ranging from $50 to $800 per case, depending on insurance type.
Third, the increasing emphasis on productivity and RVU generation in academic settings has hindered the growth of ESD training in many institutions. Still, the outlook for ESD in the United States remains encouraging. Multiple industry-sponsored training courses are held annually, and professional societies are investing heavily in expanding access to structured education in ESD. Industry is also innovating devices that improve procedural efficiency and safety. Adopting novel approaches, such as traction-assisted ESD, has made the technique more appealing to endoscopists concerned about long procedure times. For example, our group proposed a standardized esophageal ESD technique that incorporates specimen self-retraction. This method improves both safety and speed and has helped address several procedural challenges. We’ve demonstrated that consistency in technique can substantially expedite esophageal ESD.
Fast forward 5 years: We anticipate a dedicated CPT code for ESD, broader access to advanced resection tools, and an expanding number of fellowships offering structured ESD training. These developments are poised to eliminate many of the current barriers. In summary, with robust data supporting the efficacy of ESD in early esophageal cancer, the focus in the United States should shift toward mastering and integrating the technique, rather than dismissing it in favor of piecemeal EMR.
Dr. Othman is chief of the gastroenterology and hepatology section at Baylor College of Medicine and Medicine Subspecialities Service Line Chief at Baylor St Luke’s Medical Center, both in Houston. He declares no conflicts of interest.
Dear colleagues,
Many of us diagnose and treat patients with Barrett’s esophagus, estimated to affect up to 5.6% of the US adult population. There has been an expanding array of tools to help diagnose and effectively treat Barrett’s esophagus with dysplasia and malignancy. In particular, endoscopic submucosal dissection (ESD) has emerged as an important method for treating early cancer in the gastrointestinal tract.
But how do we incorporate ESD into our algorithm for management, especially with the popularity and effectiveness of endoscopic mucosal resection (EMR)? In this issue of Perspectives we aim to provide context for the use of ESD, as compared with EMR. Dr. Silvio de Melo discusses his preferred EMR technique and its many advantages in the management of BE, including for residual or refractory areas. In contrast, Dr. Mohamed Othman reviews the power of ESD and when we should consider this approach over EMR. We hope these discussions will facilitate your care for patients with Barrett’s esophagus.
We also welcome your thoughts on this topic — join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Endoscopic Mucosal Resection: The ‘Workhorse’ for Patient Care
BY SILVIO W. DE MELO JR, MD, AGAF
Barrett’s esophagus (BE) remains an important clinical problem, being one of the modifiable risk factors for esophageal adenocarcinoma. The care for BE is complex and requires several steps to correctly formulate a therapeutic plan. It starts with a proper endoscopic examination. It is recommended to spend at least 1 minute inspecting and evaluating every centimeter of the salmon-colored epithelium, looking for change in vascular pattern, erosions/ulcers, nodules, and/or masses. After the inspection, random biopsies every 1-2 cm plus targeted biopsies will guide you. It is still controversial if the addition of other sampling strategies, such as brushings or confocal endomicroscopy, is needed.
The introduction of radiofrequency ablation (RFA) was paramount in popularizing the treatment options for BE and sunsetting the previous dominant modality, photodynamic therapy (PDT). RFA proved to have a superior clinical efficacy in replacing the intestinal metaplasia/BE with neosquamous epithelium while boosting a much better safety profile, compared with PDT. However, RFA is most efficacious for “flat BE” and it is not an effective, nor recommended, method to treat nodular BE or early cancer, such as carcinoma in situ or nodular high-grade dysplasia. Endoscopic mucosal resection (EMR) is utilized to overcome those limitations.
There are several techniques utilized for EMR:
- The lift and snare technique.
- The snare-in-cap technique.
- The Band-snare technique.
The free-hand submucosal lift and snare is not frequently used in the esophagus. It is difficult to maintain visualization while being confident that one has the whole lesion inside the snare and that the distal (anal side) part of the lesion is free of any unwanted tissue (to minimize complications such as perforations or unwelcomed gastric resections). It is difficult after the first resection to lift an adjacent area, as the fluid easily leaks from the first resected spot, thus removing larger lesions in piece-meal fashion is challenging. This technique can be used in small (in my personal experience, less than 5 mm) lesions, but, given that there are better and safer alternatives, I almost never use this technique for my esophageal EMR cases. I prefer to use the band-snare technique even for lesions under 5 mm.
The snare-in-cap technique has been utilized in the esophagus. In this technique, a cap is attached to the distal end of the scope and the size of the resection is determined by the size of the cap, usually under 1.5 cm. Because of the risk of perforation without previous lifting, it is required that the lesion is lifted with a submucosal fluid, saline or any Food and Drug Administration–approved EMR solution. The lesion is then suctioned inside the cap where the snare had been previously opened inside the cap, the snare is closed, and the tissue is resected. The same limitations regarding the inability to remove larger lesions (greater than 1.5 cm) because of the challenge in lifting the adjacent area applies here. However, the perforation risk for this technique is higher than the traditional lift and the band and snare techniques. Thus, this technique has fallen out of favor for most endoscopists.
The third technique (band-snare EMR) is the one that most endoscopists use for endoscopic mucosal resection. It is a small variation of the already time-tested and very familiar procedure of esophageal variceal band ligation (EVL). There are multiple commercially available kits for esophageal EMR. The kit contains the chamber with the bands and a proprietary hexagonal snare used to resect the specimen.
The advantages of this technique are:
- It is widely commercially available.
- It builds on a familiar procedure, EVL, therefore the learning curve is short.
- The set-up is quick and the procedure can be completed safely and effectively.
- There is no need for injecting the submucosal with a lifting solution.
- Despite the band having a size limitation of 1 cm, one can remove larger lesions by repeating the band and resect process, using the rosette technique.
Band-snare EMR also has limitations:
- There are only six bands on each chamber. Depending on the size of the lesion, one may need to use multiple kits.
- It is not suitable for en bloc resection of lesions greater than 1 cm.
My experience with band EMR is that we can complete the procedure in under 1 hour. The dreaded complication of perforation occurs in under 1% of cases, most bleeding episodes can easily be controlled endoscopically, and the risk of post-EMR stricture is minimal. Therefore, band EMR is the most used technique for esophageal endoscopic resections.
Esophageal EMR is also effective for other indications in BE therapy, such as residual and recurrent BE. Band-snare EMR can be used for an en bloc resection or rosette technique for the areas resistant to ablation therapies with great success and safety.
From a financial standpoint, comparing EMR with endoscopic submucosal dissection (ESD), EMR is the superior strategy given that EMR is widely available, has a much shorter learning curve, has a greater safety profile, is applicable to a wider variety of indications, and has a more favorable return on investment. EMR should be the workhorse for the care of patients with BE, reserving ESD for specific indications.
In summary, there is no “one-size-fits-all” endoscopic therapy in the care of BE. Most Barrett’s patients can be successfully treated with a combination of ablation plus EMR, reserving ESD for select cases.
Dr. de Melo is section chief of gastroenterology at the Orlando VA Healthcare System, Orlando, Florida. He declares no conflicts of interest.
ESD Over EMR for Resecting Esophageal Lesions
BY MOHAMED O. OTHMAN, MD, AGAF
Although endoscopic submucosal dissection (ESD) is the preferred endoscopic resection method in the East, the adoption of this technique in the West, particularly in the United States, has faced many hurdles. Many endoscopists who routinely perform piecemeal endoscopic mucosal resection (EMR) question the utility of ESD, arguing that EMR is just as effective. While this may hold true in certain situations, the global trend in the endoscopic treatment of early esophageal squamous cell carcinoma, nodular Barrett’s esophagus (BE), and early esophageal adenocarcinoma (EAC) has clearly shifted toward ESD. In this perspective, I will summarize why ESD is preferred over EMR for these indications and explore why ESD has yet to gain widespread adoption in the United States.
The superiority of ESD over EMR has been well established in multiple publications from both Eastern and Western literature. Mejia-Perez et al, in a multicenter cohort study from eight centers in North America, compared outcomes of ESD vs EMR for BE with high-grade dysplasia (HGD) or T1a adenocarcinoma in 243 patients. ESD achieved significantly higher en bloc resection rates (89% vs 43%) and R0 resection rates (73% vs 56%), compared with EMR, along with a substantially lower recurrence/residual disease rate on follow-up (3.5% in the ESD group vs 31.4% in EMR group). Additionally, more patients required repeat endoscopic resection after EMR to treat residual or recurrent disease (EMR, 24.2% vs ESD, 3.5%; P < .001).
Han et al conducted a meta-analysis of 22 studies comparing ESD and EMR for early esophageal neoplasia, including both squamous cell carcinoma (SCC) and BE-associated lesions. ESD was associated with significantly higher curative resection rates than EMR (OR, 9.74; 95% CI, 4.83-19.62; P < .0001). Of note, lesion size was a critical factor in determining the advantage of ESD. For lesions ≤ 10 mm, curative resection rates were comparable between ESD and EMR. However, for lesions > 10 mm, ESD achieved significantly higher curative resection rates. This size-based recommendation has been adopted by the American Society of Gastrointestinal Endoscopy (ASGE) in their recent guidelines on ESD indications for esophageal lesions. ASGE guidelines favors ESD over EMR for SCC lesions > 15 mm and for nodular BE with dysplasia or early EAC > 20 mm.
ESD is particularly beneficial in patients who develop early adenocarcinoma after RFA or EMR. Mesureur et al evaluated the efficacy of salvage ESD for Barrett’s recurrence or residual BE following RFA. In their multicenter retrospective study of 56 patients, salvage ESD achieved an en bloc resection rate of 89.3%, despite significant fibrosis, with an R0 resection rate of 66%. At a median follow-up of 14 months, most patients remained in endoscopic remission without the need for esophagectomy.
Combining ESD with RFA has also been shown to accelerate the eradication of BE with dysplasia while reducing the number of required sessions. Our group demonstrated the high efficacy of ESD followed by RFA in 18 patients, most of whom had long-segment BE with HGD or EAC. On average, patients required only one to two RFA sessions after ESD to achieve complete eradication of intestinal metaplasia (CE-IM). Over a median follow-up of 42.5 months (IQR, 28-59.25), complete eradication of early esophageal cancer was achieved in 13 patients (100%), eradication of dysplasia in 15 patients (100%), and CE-IM in 14 patients (77.8%).
Despite the overwhelming evidence supporting ESD and the strong endorsement from professional societies, adoption in the West continues to lag. Several factors contribute to this gap. First, ESD has a steep learning curve. Our data showed that, on average, an untutored practitioner achieved competency after 150-250 procedures, a finding corroborated by other US groups.
Second, there is no specific CPT code for ESD in the United States. Physicians are forced to bill the procedure as EMR or use an unlisted code, resulting in reimbursement that does not reflect the time and complexity of the procedure. Our group showed that physician reimbursement for ESD is highly variable, ranging from $50 to $800 per case, depending on insurance type.
Third, the increasing emphasis on productivity and RVU generation in academic settings has hindered the growth of ESD training in many institutions. Still, the outlook for ESD in the United States remains encouraging. Multiple industry-sponsored training courses are held annually, and professional societies are investing heavily in expanding access to structured education in ESD. Industry is also innovating devices that improve procedural efficiency and safety. Adopting novel approaches, such as traction-assisted ESD, has made the technique more appealing to endoscopists concerned about long procedure times. For example, our group proposed a standardized esophageal ESD technique that incorporates specimen self-retraction. This method improves both safety and speed and has helped address several procedural challenges. We’ve demonstrated that consistency in technique can substantially expedite esophageal ESD.
Fast forward 5 years: We anticipate a dedicated CPT code for ESD, broader access to advanced resection tools, and an expanding number of fellowships offering structured ESD training. These developments are poised to eliminate many of the current barriers. In summary, with robust data supporting the efficacy of ESD in early esophageal cancer, the focus in the United States should shift toward mastering and integrating the technique, rather than dismissing it in favor of piecemeal EMR.
Dr. Othman is chief of the gastroenterology and hepatology section at Baylor College of Medicine and Medicine Subspecialities Service Line Chief at Baylor St Luke’s Medical Center, both in Houston. He declares no conflicts of interest.
Experts Recommend Medication for Pediatric MASLD Management
, according to a new joint perspective paper.
Pediatric MASLD is the number-one cause of chronic liver disease in children and the number-one reason for liver transplant listing in young adults aged 18-40 years, said corresponding author Jennifer A. Panganiban, MD, Children’s Hospital of Philadelphia, Philadelphia.
The paper, published in Obesity Pillars, represents “a call to action that has been long overdue,” Panganiban told GI & Hepatology News.
The goal of the authors was to bring global awareness to the recent changes in the pediatric MASLD landscape — especially in medication use — and to empower clinicians treating the disease, she explained.
The recommendations are based on a combination of the latest published evidence and clinical expertise from eight hepatologists/gastroenterologists and two physicians from the Obesity Medicine Association, Centennial, Colorado.
One of the major barriers to MASLD management in children is suboptimal screening resulting in underdiagnosis, said Panganiban. “Unfortunately, only up to 30% of children are being screened in their pediatrician’s office.”
The new guideline outlines the patient care process from screening, referral to a subspecialist, and workup; however, the primary focus is on treatment with medication options that were previously not available or underutilized, she said.
Successful and Sustainable Weight Loss
Adiposity and weight gain make MASLD worse, but weight reduction has been shown to improve the condition, the authors noted. Previous strategies for curbing MASLD in children with obesity have focused mainly on lifestyle changes, but with limited success.
Nevertheless, the authors recommend continuing physical activity and nutrition as treatments for MASLD in children, with a plan tailored specifically to the patient.
In addition, however, they suggest that anti-obesity medications started early in the disease may help reduce costs and improve future outcomes.
Although glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have not yet been studied specifically for pediatric MASLD, data from studies of pediatric obesity, diabetes, and other retrospective studies are encouraging, the authors wrote.
The GLP-1 RAs liraglutide and semaglutide are both approved by the US Food and Drug Administration (FDA) for managing obesity in children and adolescents aged 12 years or older, they noted. And a recent phase 3a randomized trial showed that liraglutide, not yet approved for children younger than 12 years, led to a mean change in body mass index of 5.8% from baseline to 56 weeks in children aged 6-11 years with obesity.
GLP-1 RAs not only are effective for weight management but also improve other metabolic dysfunction indicators including cholesterol and blood pressure, which makes these medications an even more beneficial option for individuals with obesity and MASLD, Panganiban and colleagues wrote.
For example, a recent single-center study of 111 children with MASLD (mean age, 15 years) showed a significant improvement in alanine aminotransferase levels with the use of GLP-1 RAs, although body mass index and weight were unchanged.
Regaining weight after discontinuing GLP-1 RAs is the main barrier to their use for MASLD, the authors noted. In addition, GLP-1 RAs are contraindicated in some situations, such as in those with a history of serious hypersensitivity, and in patients with a personal or family history of either medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 based on animal studies showing an association with the medications and thyroid C–cell tumors.
Other FDA-approved medication options for obesity in children include metformin, topiramate, and phentermine, as well as bupropion, lisdexamfetamine, and setmelanotide, the authors said.
Resmetirom, a thyroid hormone receptor-beta agonist, which is another significant breakthrough in MASLD for adults, has not yet been tested or approved for pediatric use.
In addition to medications, metabolic bariatric surgery has shown effectiveness in children with obesity and/or MASLD by reducing liver fat and reversing fibrosis, as shown in the Teen-LABS study, the authors wrote. However, long-term data on fibrosis reversal are limited, and cost and access remain barriers.
More Research Needed
The joint expert review is intended as an educational tool that may require updates and should not be interpreted as rules for individual patient care, the authors cautioned. And physical activity and nutrition remain the primary treatment of MASLD and should be continued in conjunction with other treatment modalities, they emphasized.
Looking ahead, research is needed to develop accurate and reliable noninvasive biomarkers to diagnose and assess obesity treatment efficacy, Panganiban told GI & Hepatology News.
Also needed are multicenter randomized control trials in children with obesity involving different medications that have been successful in the treatment of metabolic dysfunction–associated steatohepatitis/fibrosis in adults, such as GLP-1 RAs or resmetirom, she added.
Educating Clinicians on Early Identification
When obesity occurs in childhood, it starts a process of additional complications that arise in earlier ages in adults, said Saul J. Karpen, MD, chief scientific officer at the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia, in an interview.“Given the epidemic of obesity, altered diets, and reduced physical activities during younger ages, it is not easy to identify which children are at greater risk of MASLD,” said Karpen.
“It requires insight from the care providers and often imaging, a blood test, or a referral to a pediatric hepatologist, and not every region has easy access to such expertise,” Karpen said.
The new review is important because it highlights the fact that obesity and its consequences are not limited to adulthood, and that educated clinicians are in a position to get an early start on treatment in children, Karpen noted.
The guideline received no outside funding. Panganiban and Karpen had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, according to a new joint perspective paper.
Pediatric MASLD is the number-one cause of chronic liver disease in children and the number-one reason for liver transplant listing in young adults aged 18-40 years, said corresponding author Jennifer A. Panganiban, MD, Children’s Hospital of Philadelphia, Philadelphia.
The paper, published in Obesity Pillars, represents “a call to action that has been long overdue,” Panganiban told GI & Hepatology News.
The goal of the authors was to bring global awareness to the recent changes in the pediatric MASLD landscape — especially in medication use — and to empower clinicians treating the disease, she explained.
The recommendations are based on a combination of the latest published evidence and clinical expertise from eight hepatologists/gastroenterologists and two physicians from the Obesity Medicine Association, Centennial, Colorado.
One of the major barriers to MASLD management in children is suboptimal screening resulting in underdiagnosis, said Panganiban. “Unfortunately, only up to 30% of children are being screened in their pediatrician’s office.”
The new guideline outlines the patient care process from screening, referral to a subspecialist, and workup; however, the primary focus is on treatment with medication options that were previously not available or underutilized, she said.
Successful and Sustainable Weight Loss
Adiposity and weight gain make MASLD worse, but weight reduction has been shown to improve the condition, the authors noted. Previous strategies for curbing MASLD in children with obesity have focused mainly on lifestyle changes, but with limited success.
Nevertheless, the authors recommend continuing physical activity and nutrition as treatments for MASLD in children, with a plan tailored specifically to the patient.
In addition, however, they suggest that anti-obesity medications started early in the disease may help reduce costs and improve future outcomes.
Although glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have not yet been studied specifically for pediatric MASLD, data from studies of pediatric obesity, diabetes, and other retrospective studies are encouraging, the authors wrote.
The GLP-1 RAs liraglutide and semaglutide are both approved by the US Food and Drug Administration (FDA) for managing obesity in children and adolescents aged 12 years or older, they noted. And a recent phase 3a randomized trial showed that liraglutide, not yet approved for children younger than 12 years, led to a mean change in body mass index of 5.8% from baseline to 56 weeks in children aged 6-11 years with obesity.
GLP-1 RAs not only are effective for weight management but also improve other metabolic dysfunction indicators including cholesterol and blood pressure, which makes these medications an even more beneficial option for individuals with obesity and MASLD, Panganiban and colleagues wrote.
For example, a recent single-center study of 111 children with MASLD (mean age, 15 years) showed a significant improvement in alanine aminotransferase levels with the use of GLP-1 RAs, although body mass index and weight were unchanged.
Regaining weight after discontinuing GLP-1 RAs is the main barrier to their use for MASLD, the authors noted. In addition, GLP-1 RAs are contraindicated in some situations, such as in those with a history of serious hypersensitivity, and in patients with a personal or family history of either medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 based on animal studies showing an association with the medications and thyroid C–cell tumors.
Other FDA-approved medication options for obesity in children include metformin, topiramate, and phentermine, as well as bupropion, lisdexamfetamine, and setmelanotide, the authors said.
Resmetirom, a thyroid hormone receptor-beta agonist, which is another significant breakthrough in MASLD for adults, has not yet been tested or approved for pediatric use.
In addition to medications, metabolic bariatric surgery has shown effectiveness in children with obesity and/or MASLD by reducing liver fat and reversing fibrosis, as shown in the Teen-LABS study, the authors wrote. However, long-term data on fibrosis reversal are limited, and cost and access remain barriers.
More Research Needed
The joint expert review is intended as an educational tool that may require updates and should not be interpreted as rules for individual patient care, the authors cautioned. And physical activity and nutrition remain the primary treatment of MASLD and should be continued in conjunction with other treatment modalities, they emphasized.
Looking ahead, research is needed to develop accurate and reliable noninvasive biomarkers to diagnose and assess obesity treatment efficacy, Panganiban told GI & Hepatology News.
Also needed are multicenter randomized control trials in children with obesity involving different medications that have been successful in the treatment of metabolic dysfunction–associated steatohepatitis/fibrosis in adults, such as GLP-1 RAs or resmetirom, she added.
Educating Clinicians on Early Identification
When obesity occurs in childhood, it starts a process of additional complications that arise in earlier ages in adults, said Saul J. Karpen, MD, chief scientific officer at the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia, in an interview.“Given the epidemic of obesity, altered diets, and reduced physical activities during younger ages, it is not easy to identify which children are at greater risk of MASLD,” said Karpen.
“It requires insight from the care providers and often imaging, a blood test, or a referral to a pediatric hepatologist, and not every region has easy access to such expertise,” Karpen said.
The new review is important because it highlights the fact that obesity and its consequences are not limited to adulthood, and that educated clinicians are in a position to get an early start on treatment in children, Karpen noted.
The guideline received no outside funding. Panganiban and Karpen had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, according to a new joint perspective paper.
Pediatric MASLD is the number-one cause of chronic liver disease in children and the number-one reason for liver transplant listing in young adults aged 18-40 years, said corresponding author Jennifer A. Panganiban, MD, Children’s Hospital of Philadelphia, Philadelphia.
The paper, published in Obesity Pillars, represents “a call to action that has been long overdue,” Panganiban told GI & Hepatology News.
The goal of the authors was to bring global awareness to the recent changes in the pediatric MASLD landscape — especially in medication use — and to empower clinicians treating the disease, she explained.
The recommendations are based on a combination of the latest published evidence and clinical expertise from eight hepatologists/gastroenterologists and two physicians from the Obesity Medicine Association, Centennial, Colorado.
One of the major barriers to MASLD management in children is suboptimal screening resulting in underdiagnosis, said Panganiban. “Unfortunately, only up to 30% of children are being screened in their pediatrician’s office.”
The new guideline outlines the patient care process from screening, referral to a subspecialist, and workup; however, the primary focus is on treatment with medication options that were previously not available or underutilized, she said.
Successful and Sustainable Weight Loss
Adiposity and weight gain make MASLD worse, but weight reduction has been shown to improve the condition, the authors noted. Previous strategies for curbing MASLD in children with obesity have focused mainly on lifestyle changes, but with limited success.
Nevertheless, the authors recommend continuing physical activity and nutrition as treatments for MASLD in children, with a plan tailored specifically to the patient.
In addition, however, they suggest that anti-obesity medications started early in the disease may help reduce costs and improve future outcomes.
Although glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have not yet been studied specifically for pediatric MASLD, data from studies of pediatric obesity, diabetes, and other retrospective studies are encouraging, the authors wrote.
The GLP-1 RAs liraglutide and semaglutide are both approved by the US Food and Drug Administration (FDA) for managing obesity in children and adolescents aged 12 years or older, they noted. And a recent phase 3a randomized trial showed that liraglutide, not yet approved for children younger than 12 years, led to a mean change in body mass index of 5.8% from baseline to 56 weeks in children aged 6-11 years with obesity.
GLP-1 RAs not only are effective for weight management but also improve other metabolic dysfunction indicators including cholesterol and blood pressure, which makes these medications an even more beneficial option for individuals with obesity and MASLD, Panganiban and colleagues wrote.
For example, a recent single-center study of 111 children with MASLD (mean age, 15 years) showed a significant improvement in alanine aminotransferase levels with the use of GLP-1 RAs, although body mass index and weight were unchanged.
Regaining weight after discontinuing GLP-1 RAs is the main barrier to their use for MASLD, the authors noted. In addition, GLP-1 RAs are contraindicated in some situations, such as in those with a history of serious hypersensitivity, and in patients with a personal or family history of either medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 based on animal studies showing an association with the medications and thyroid C–cell tumors.
Other FDA-approved medication options for obesity in children include metformin, topiramate, and phentermine, as well as bupropion, lisdexamfetamine, and setmelanotide, the authors said.
Resmetirom, a thyroid hormone receptor-beta agonist, which is another significant breakthrough in MASLD for adults, has not yet been tested or approved for pediatric use.
In addition to medications, metabolic bariatric surgery has shown effectiveness in children with obesity and/or MASLD by reducing liver fat and reversing fibrosis, as shown in the Teen-LABS study, the authors wrote. However, long-term data on fibrosis reversal are limited, and cost and access remain barriers.
More Research Needed
The joint expert review is intended as an educational tool that may require updates and should not be interpreted as rules for individual patient care, the authors cautioned. And physical activity and nutrition remain the primary treatment of MASLD and should be continued in conjunction with other treatment modalities, they emphasized.
Looking ahead, research is needed to develop accurate and reliable noninvasive biomarkers to diagnose and assess obesity treatment efficacy, Panganiban told GI & Hepatology News.
Also needed are multicenter randomized control trials in children with obesity involving different medications that have been successful in the treatment of metabolic dysfunction–associated steatohepatitis/fibrosis in adults, such as GLP-1 RAs or resmetirom, she added.
Educating Clinicians on Early Identification
When obesity occurs in childhood, it starts a process of additional complications that arise in earlier ages in adults, said Saul J. Karpen, MD, chief scientific officer at the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia, in an interview.“Given the epidemic of obesity, altered diets, and reduced physical activities during younger ages, it is not easy to identify which children are at greater risk of MASLD,” said Karpen.
“It requires insight from the care providers and often imaging, a blood test, or a referral to a pediatric hepatologist, and not every region has easy access to such expertise,” Karpen said.
The new review is important because it highlights the fact that obesity and its consequences are not limited to adulthood, and that educated clinicians are in a position to get an early start on treatment in children, Karpen noted.
The guideline received no outside funding. Panganiban and Karpen had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.