User login
Infectious disease pop quiz: Clinical challenges for the ObGyn
In this question-and-answer article (the second in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
9. For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin
500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
10. What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
11. In a pregnant woman with a history of recurrent herpes simplex virus infection, what is the best way to prevent an outbreak of lesions near term?
Obstetric patients with a history of recurrent herpes simplex infection should be treated with acyclovir 400 mg orally 3 times daily from 36 weeks until delivery. This
regimen significantly reduces the likelihood of a recurrent outbreak near the time of delivery, which if it occurred, would necessitate a cesarean delivery. In patients at increased risk for preterm delivery, the prophylactic regimen should be started earlier.
Valacyclovir, 500 mg orally twice daily, is an acceptable alternative but is significantly more expensive.
Continue to: 12. What are the best office-based tests for the diagnosis of bacterial vaginosis?...
12. What are the best office-based tests for the diagnosis of bacterial vaginosis?
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their
outer margin.
13. For a moderately ill pregnant woman, what is the most appropriate antibiotic combination for inpatient treatment of community-acquired pneumonia?
This patient should be treated with intravenous ceftriaxone (2 g every 24 hours) plus oral or intravenous azithromycin. The appropriate oral dose of azithromycin is 500 mg on day 1, then 250 mg daily for 4 doses. The appropriate intravenous dose of azithromycin is 500 mg every 24 hours. The goal is to provide appropriate coverage for the most likely pathogens: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and mycoplasmas. (Antibacterial drugs for community-acquired pneumonia. Med Lett Drugs Ther. 2021:63:10-14. Postma DF, van Werkoven CH, van Eldin LJ, et al; CAP-START Study Group. Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med. 2015;372: 1312-1323.)
14. What tests are best for the diagnosis of COVID-19 infection?
The 2 key diagnostic tests for COVID-19 infection are detecting antigen in nasopharyngeal washings or saliva by nucleic acid amplification tests and identifying groundglass opacities on computed tomography imaging of the chest. (Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460.)
15. What is the most appropriate treatment for a pregnant woman who is moderately to severely ill with COVID-19 infection?
Moderately to severely ill pregnant women with COVID-19 infection should be hospitalized and treated with supplementary oxygen, remdesivir, and dexamethasone. Other possible therapies include inhaled nitric oxide, baricitinib (a Janus kinase inhibitor), and tocilizumab (an anti-interleukin 6 receptor antibody). (RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693704. Kalil AC, Patterson TF, Mehta AK, et al; ACTT-2 Study Group. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. Berlin DA, Gulick RM, Martinez FJ, et al. Severe COVID19. N Engl J Med. 2020;383;2451-2460.)
16. What is the best test for the diagnosis of acute hepatitis A infection?
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
17. What are the best tests for identification of a patient with chronic hepatitis B infection?
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
18. What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
19. Should a postpartum patient with chronic hepatitis C infection be discouraged from breastfeeding her infant?
Hepatitis C is not a contraindication to breastfeeding. Although the virus has been identified in breast milk, the risk of transmission to the infant is exceedingly low.
20. What are the principal microorganisms that cause puerperal mastitis?
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used. ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
In this question-and-answer article (the second in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
9. For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin
500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
10. What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
11. In a pregnant woman with a history of recurrent herpes simplex virus infection, what is the best way to prevent an outbreak of lesions near term?
Obstetric patients with a history of recurrent herpes simplex infection should be treated with acyclovir 400 mg orally 3 times daily from 36 weeks until delivery. This
regimen significantly reduces the likelihood of a recurrent outbreak near the time of delivery, which if it occurred, would necessitate a cesarean delivery. In patients at increased risk for preterm delivery, the prophylactic regimen should be started earlier.
Valacyclovir, 500 mg orally twice daily, is an acceptable alternative but is significantly more expensive.
Continue to: 12. What are the best office-based tests for the diagnosis of bacterial vaginosis?...
12. What are the best office-based tests for the diagnosis of bacterial vaginosis?
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their
outer margin.
13. For a moderately ill pregnant woman, what is the most appropriate antibiotic combination for inpatient treatment of community-acquired pneumonia?
This patient should be treated with intravenous ceftriaxone (2 g every 24 hours) plus oral or intravenous azithromycin. The appropriate oral dose of azithromycin is 500 mg on day 1, then 250 mg daily for 4 doses. The appropriate intravenous dose of azithromycin is 500 mg every 24 hours. The goal is to provide appropriate coverage for the most likely pathogens: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and mycoplasmas. (Antibacterial drugs for community-acquired pneumonia. Med Lett Drugs Ther. 2021:63:10-14. Postma DF, van Werkoven CH, van Eldin LJ, et al; CAP-START Study Group. Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med. 2015;372: 1312-1323.)
14. What tests are best for the diagnosis of COVID-19 infection?
The 2 key diagnostic tests for COVID-19 infection are detecting antigen in nasopharyngeal washings or saliva by nucleic acid amplification tests and identifying groundglass opacities on computed tomography imaging of the chest. (Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460.)
15. What is the most appropriate treatment for a pregnant woman who is moderately to severely ill with COVID-19 infection?
Moderately to severely ill pregnant women with COVID-19 infection should be hospitalized and treated with supplementary oxygen, remdesivir, and dexamethasone. Other possible therapies include inhaled nitric oxide, baricitinib (a Janus kinase inhibitor), and tocilizumab (an anti-interleukin 6 receptor antibody). (RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693704. Kalil AC, Patterson TF, Mehta AK, et al; ACTT-2 Study Group. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. Berlin DA, Gulick RM, Martinez FJ, et al. Severe COVID19. N Engl J Med. 2020;383;2451-2460.)
16. What is the best test for the diagnosis of acute hepatitis A infection?
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
17. What are the best tests for identification of a patient with chronic hepatitis B infection?
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
18. What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
19. Should a postpartum patient with chronic hepatitis C infection be discouraged from breastfeeding her infant?
Hepatitis C is not a contraindication to breastfeeding. Although the virus has been identified in breast milk, the risk of transmission to the infant is exceedingly low.
20. What are the principal microorganisms that cause puerperal mastitis?
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used. ●
In this question-and-answer article (the second in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
9. For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin
500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
10. What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
11. In a pregnant woman with a history of recurrent herpes simplex virus infection, what is the best way to prevent an outbreak of lesions near term?
Obstetric patients with a history of recurrent herpes simplex infection should be treated with acyclovir 400 mg orally 3 times daily from 36 weeks until delivery. This
regimen significantly reduces the likelihood of a recurrent outbreak near the time of delivery, which if it occurred, would necessitate a cesarean delivery. In patients at increased risk for preterm delivery, the prophylactic regimen should be started earlier.
Valacyclovir, 500 mg orally twice daily, is an acceptable alternative but is significantly more expensive.
Continue to: 12. What are the best office-based tests for the diagnosis of bacterial vaginosis?...
12. What are the best office-based tests for the diagnosis of bacterial vaginosis?
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their
outer margin.
13. For a moderately ill pregnant woman, what is the most appropriate antibiotic combination for inpatient treatment of community-acquired pneumonia?
This patient should be treated with intravenous ceftriaxone (2 g every 24 hours) plus oral or intravenous azithromycin. The appropriate oral dose of azithromycin is 500 mg on day 1, then 250 mg daily for 4 doses. The appropriate intravenous dose of azithromycin is 500 mg every 24 hours. The goal is to provide appropriate coverage for the most likely pathogens: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and mycoplasmas. (Antibacterial drugs for community-acquired pneumonia. Med Lett Drugs Ther. 2021:63:10-14. Postma DF, van Werkoven CH, van Eldin LJ, et al; CAP-START Study Group. Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med. 2015;372: 1312-1323.)
14. What tests are best for the diagnosis of COVID-19 infection?
The 2 key diagnostic tests for COVID-19 infection are detecting antigen in nasopharyngeal washings or saliva by nucleic acid amplification tests and identifying groundglass opacities on computed tomography imaging of the chest. (Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460.)
15. What is the most appropriate treatment for a pregnant woman who is moderately to severely ill with COVID-19 infection?
Moderately to severely ill pregnant women with COVID-19 infection should be hospitalized and treated with supplementary oxygen, remdesivir, and dexamethasone. Other possible therapies include inhaled nitric oxide, baricitinib (a Janus kinase inhibitor), and tocilizumab (an anti-interleukin 6 receptor antibody). (RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693704. Kalil AC, Patterson TF, Mehta AK, et al; ACTT-2 Study Group. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. Berlin DA, Gulick RM, Martinez FJ, et al. Severe COVID19. N Engl J Med. 2020;383;2451-2460.)
16. What is the best test for the diagnosis of acute hepatitis A infection?
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
17. What are the best tests for identification of a patient with chronic hepatitis B infection?
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
18. What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
19. Should a postpartum patient with chronic hepatitis C infection be discouraged from breastfeeding her infant?
Hepatitis C is not a contraindication to breastfeeding. Although the virus has been identified in breast milk, the risk of transmission to the infant is exceedingly low.
20. What are the principal microorganisms that cause puerperal mastitis?
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used. ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Ginger for migraine: A new review
in patients who do not want to use or don’t have access to prescription medications, new data suggest.
Conducted by investigators at the National Institute of Mental Health and Neurosciences, Bangalore, India, the review showed ginger root can relieve migraine-related pain, nausea, and vomiting. However, the evidence does not support ginger’s use as a first-line therapy for acute migraine or for migraine prevention.
Study author Chittaranjan Andrade, MD, professor of clinical psychopharmacology and neurotoxicology at the institute, said in an interview that the evidence base is still “too small” to support formal clinical recommendations. However, he added, ginger can be considered as a viable “home-remedy option” for acute migraine.
The review was published online Dec. 2 in The Journal of Clinical Psychiatry.
Potential uses
Used for centuries in traditional medicine, much of the preclinical and clinical research has examined the potential of raw ginger, ginger extracts, and ginger constituents to prevent and treat a wide range of medical conditions. These include nausea and vomiting associated with pregnancy, chemotherapy, postoperative states, motion sickness, and other diseases and disorders, said Dr. Andrade.
Ginger has “long been recommended as an effective home remedy for the acute treatment of migraine, relieving both headache and the associated nausea,” Dr. Andrade noted.
One recommended recipe is stirring half a teaspoon of ground ginger into a glass of water and drinking the “ginger juice,” while another is to drink hot tea made from a teaspoon of freshly ground ginger.
“Patients with a number of common ailments, including migraine, are sometimes caught without medicines; or they may have poor access to medicines,” Dr. Andrade said. “I came across a reference to the use of ginger for migraine in a book on home remedies and I thought that if the research literature supports the use of ginger for migraine episodes, such patients could benefit.”
Large treatment gap
The review and meta-analysis included three randomized controlled trials with 227 patients looking at ginger versus placebo for the treatment.
One of the studies investigated the therapeutic efficacy of a specific proprietary formulation of ginger, combined with feverfew, while two trials were independent of industry.
Of these two, one examined the benefit of add-on dry ginger extract (400 mg; 5% active gingerols) in 50 patients who were also taking ketoprofen to treat migraine episodes, while the other examined the 3-month efficacy of daily dry ginger extract for migraine prophylaxis in 107 patients.
The two studies that examined the therapeutic efficacy of ginger versus placebo showed ginger reduced mean pain scores at 2 hours (mean difference, –1.27 [95% confidence interval, –1.46 to 1,07]) and also increased the proportion of patients who were pain free at 2 hours (RR, 1.79 [1.04 to 3.09]). In addition, compared to placebo, ginger halved the risk of migraine-related nausea and vomiting in all of the studies and was not associated with an increased risk of adverse events.
One RCT investigated prophylactic efficacy and found it to be more effective than placebo in bringing a ≥ 50% reduction in the frequency of monthly migraine episodes (in 42% versus 39% of patients, respectively), but the difference was not deemed statistically significant. In addition, there were no significant differences between the groups in days of pain, severe pain, days requiring use of analgesics, number of migraine episodes, and maximum duration of migraine episodes.
Dr. Andrade noted that ginger has many chemical constituents, including phenolic compounds, terpenes, polysaccharides, lipids, and organic acids of which 6-shogaol, 6-gingerol, and 10-dehydrogingerdione “may be important.”
It also has antioxidant and anti-inflammatory effects, lowering prostaglandins, and reducing several serum lipid and glycemic measures. Additionally, it has “putative” vasculoprotective effects, he added.
“Ginger has a large number of chemical constituents and we do not know which of these, separately or in combination, will help relieve migraine,” he said. “We won’t know the answer unless clinical trials are conducted with the individual constituents rather than with ginger extract.” He compared this to the study of omega-3 fatty acids rather than fish and nuts for various neuropsychiatric or cardiovascular indications.
Nevertheless, given the high global prevalence of migraine and the “large treatment gap [of migraine] in primary care,” it could be common for many affected patients to experience episodes of migraine headache “without recourse to recommended pharmacologic relief,” he noted. “In such cases, the availability of a simple home remedy, such as ginger, could be helpful.”
‘Good additional tool’
Commenting on the study for this news organization, Jessica Ailani, MD, director, MedStar Georgetown Headache Center and professor of clinical neurology, MedStar Georgetown University Hospital, Washington, said that for “people with migraine who are seeking treatment with minimal side effects that they can obtain without counsel of a health care provider, ginger is a good additional tool to have.”
Dr. Ailani, vice cochair of strategic planning in the MedStar department of neurology, who was not involved with the study, said that clinicians can “consider suggesting ginger to patients with migraine that have associated nausea who are interested in nonpharmacologic ways to treat symptoms.”
Since there are “many other effective ways to treat migraine,” she advises “conversing with the patient about speed of onset of efficacy, along with tolerability, and return of migraine symptoms as important factors to evaluate when choosing and staying with a treatment.”
Also commenting on the study for this news organization, Nada Hindiyeh, MD, clinical associate professor, department of neurology, Stanford (Calif.) University, called it a “nice summary of the objective research available for the use of ginger in acute and preventive treatment of migraine.”
Although there is insufficient literature evaluating ginger alone in migraine treatment, so “no definitive conclusions can be drawn,” since it appears to be safe and “somewhat helpful for migraine-associated nausea and vomiting and possibly in frequency of migraine reduction, it remains a considerable alternative for those seeking nonprescription options,” said Dr. Hindiyeh, who was not involved with the study.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made to charities. He has received payments for developing educational materials for scientific initiatives and programs. Dr. Ailani reports honoraria for independent consulting from various pharmaceutical companies and clinical trial grants to her institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly, Satsuma, and Zosano. Dr. Hindiyeh discloses no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in patients who do not want to use or don’t have access to prescription medications, new data suggest.
Conducted by investigators at the National Institute of Mental Health and Neurosciences, Bangalore, India, the review showed ginger root can relieve migraine-related pain, nausea, and vomiting. However, the evidence does not support ginger’s use as a first-line therapy for acute migraine or for migraine prevention.
Study author Chittaranjan Andrade, MD, professor of clinical psychopharmacology and neurotoxicology at the institute, said in an interview that the evidence base is still “too small” to support formal clinical recommendations. However, he added, ginger can be considered as a viable “home-remedy option” for acute migraine.
The review was published online Dec. 2 in The Journal of Clinical Psychiatry.
Potential uses
Used for centuries in traditional medicine, much of the preclinical and clinical research has examined the potential of raw ginger, ginger extracts, and ginger constituents to prevent and treat a wide range of medical conditions. These include nausea and vomiting associated with pregnancy, chemotherapy, postoperative states, motion sickness, and other diseases and disorders, said Dr. Andrade.
Ginger has “long been recommended as an effective home remedy for the acute treatment of migraine, relieving both headache and the associated nausea,” Dr. Andrade noted.
One recommended recipe is stirring half a teaspoon of ground ginger into a glass of water and drinking the “ginger juice,” while another is to drink hot tea made from a teaspoon of freshly ground ginger.
“Patients with a number of common ailments, including migraine, are sometimes caught without medicines; or they may have poor access to medicines,” Dr. Andrade said. “I came across a reference to the use of ginger for migraine in a book on home remedies and I thought that if the research literature supports the use of ginger for migraine episodes, such patients could benefit.”
Large treatment gap
The review and meta-analysis included three randomized controlled trials with 227 patients looking at ginger versus placebo for the treatment.
One of the studies investigated the therapeutic efficacy of a specific proprietary formulation of ginger, combined with feverfew, while two trials were independent of industry.
Of these two, one examined the benefit of add-on dry ginger extract (400 mg; 5% active gingerols) in 50 patients who were also taking ketoprofen to treat migraine episodes, while the other examined the 3-month efficacy of daily dry ginger extract for migraine prophylaxis in 107 patients.
The two studies that examined the therapeutic efficacy of ginger versus placebo showed ginger reduced mean pain scores at 2 hours (mean difference, –1.27 [95% confidence interval, –1.46 to 1,07]) and also increased the proportion of patients who were pain free at 2 hours (RR, 1.79 [1.04 to 3.09]). In addition, compared to placebo, ginger halved the risk of migraine-related nausea and vomiting in all of the studies and was not associated with an increased risk of adverse events.
One RCT investigated prophylactic efficacy and found it to be more effective than placebo in bringing a ≥ 50% reduction in the frequency of monthly migraine episodes (in 42% versus 39% of patients, respectively), but the difference was not deemed statistically significant. In addition, there were no significant differences between the groups in days of pain, severe pain, days requiring use of analgesics, number of migraine episodes, and maximum duration of migraine episodes.
Dr. Andrade noted that ginger has many chemical constituents, including phenolic compounds, terpenes, polysaccharides, lipids, and organic acids of which 6-shogaol, 6-gingerol, and 10-dehydrogingerdione “may be important.”
It also has antioxidant and anti-inflammatory effects, lowering prostaglandins, and reducing several serum lipid and glycemic measures. Additionally, it has “putative” vasculoprotective effects, he added.
“Ginger has a large number of chemical constituents and we do not know which of these, separately or in combination, will help relieve migraine,” he said. “We won’t know the answer unless clinical trials are conducted with the individual constituents rather than with ginger extract.” He compared this to the study of omega-3 fatty acids rather than fish and nuts for various neuropsychiatric or cardiovascular indications.
Nevertheless, given the high global prevalence of migraine and the “large treatment gap [of migraine] in primary care,” it could be common for many affected patients to experience episodes of migraine headache “without recourse to recommended pharmacologic relief,” he noted. “In such cases, the availability of a simple home remedy, such as ginger, could be helpful.”
‘Good additional tool’
Commenting on the study for this news organization, Jessica Ailani, MD, director, MedStar Georgetown Headache Center and professor of clinical neurology, MedStar Georgetown University Hospital, Washington, said that for “people with migraine who are seeking treatment with minimal side effects that they can obtain without counsel of a health care provider, ginger is a good additional tool to have.”
Dr. Ailani, vice cochair of strategic planning in the MedStar department of neurology, who was not involved with the study, said that clinicians can “consider suggesting ginger to patients with migraine that have associated nausea who are interested in nonpharmacologic ways to treat symptoms.”
Since there are “many other effective ways to treat migraine,” she advises “conversing with the patient about speed of onset of efficacy, along with tolerability, and return of migraine symptoms as important factors to evaluate when choosing and staying with a treatment.”
Also commenting on the study for this news organization, Nada Hindiyeh, MD, clinical associate professor, department of neurology, Stanford (Calif.) University, called it a “nice summary of the objective research available for the use of ginger in acute and preventive treatment of migraine.”
Although there is insufficient literature evaluating ginger alone in migraine treatment, so “no definitive conclusions can be drawn,” since it appears to be safe and “somewhat helpful for migraine-associated nausea and vomiting and possibly in frequency of migraine reduction, it remains a considerable alternative for those seeking nonprescription options,” said Dr. Hindiyeh, who was not involved with the study.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made to charities. He has received payments for developing educational materials for scientific initiatives and programs. Dr. Ailani reports honoraria for independent consulting from various pharmaceutical companies and clinical trial grants to her institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly, Satsuma, and Zosano. Dr. Hindiyeh discloses no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in patients who do not want to use or don’t have access to prescription medications, new data suggest.
Conducted by investigators at the National Institute of Mental Health and Neurosciences, Bangalore, India, the review showed ginger root can relieve migraine-related pain, nausea, and vomiting. However, the evidence does not support ginger’s use as a first-line therapy for acute migraine or for migraine prevention.
Study author Chittaranjan Andrade, MD, professor of clinical psychopharmacology and neurotoxicology at the institute, said in an interview that the evidence base is still “too small” to support formal clinical recommendations. However, he added, ginger can be considered as a viable “home-remedy option” for acute migraine.
The review was published online Dec. 2 in The Journal of Clinical Psychiatry.
Potential uses
Used for centuries in traditional medicine, much of the preclinical and clinical research has examined the potential of raw ginger, ginger extracts, and ginger constituents to prevent and treat a wide range of medical conditions. These include nausea and vomiting associated with pregnancy, chemotherapy, postoperative states, motion sickness, and other diseases and disorders, said Dr. Andrade.
Ginger has “long been recommended as an effective home remedy for the acute treatment of migraine, relieving both headache and the associated nausea,” Dr. Andrade noted.
One recommended recipe is stirring half a teaspoon of ground ginger into a glass of water and drinking the “ginger juice,” while another is to drink hot tea made from a teaspoon of freshly ground ginger.
“Patients with a number of common ailments, including migraine, are sometimes caught without medicines; or they may have poor access to medicines,” Dr. Andrade said. “I came across a reference to the use of ginger for migraine in a book on home remedies and I thought that if the research literature supports the use of ginger for migraine episodes, such patients could benefit.”
Large treatment gap
The review and meta-analysis included three randomized controlled trials with 227 patients looking at ginger versus placebo for the treatment.
One of the studies investigated the therapeutic efficacy of a specific proprietary formulation of ginger, combined with feverfew, while two trials were independent of industry.
Of these two, one examined the benefit of add-on dry ginger extract (400 mg; 5% active gingerols) in 50 patients who were also taking ketoprofen to treat migraine episodes, while the other examined the 3-month efficacy of daily dry ginger extract for migraine prophylaxis in 107 patients.
The two studies that examined the therapeutic efficacy of ginger versus placebo showed ginger reduced mean pain scores at 2 hours (mean difference, –1.27 [95% confidence interval, –1.46 to 1,07]) and also increased the proportion of patients who were pain free at 2 hours (RR, 1.79 [1.04 to 3.09]). In addition, compared to placebo, ginger halved the risk of migraine-related nausea and vomiting in all of the studies and was not associated with an increased risk of adverse events.
One RCT investigated prophylactic efficacy and found it to be more effective than placebo in bringing a ≥ 50% reduction in the frequency of monthly migraine episodes (in 42% versus 39% of patients, respectively), but the difference was not deemed statistically significant. In addition, there were no significant differences between the groups in days of pain, severe pain, days requiring use of analgesics, number of migraine episodes, and maximum duration of migraine episodes.
Dr. Andrade noted that ginger has many chemical constituents, including phenolic compounds, terpenes, polysaccharides, lipids, and organic acids of which 6-shogaol, 6-gingerol, and 10-dehydrogingerdione “may be important.”
It also has antioxidant and anti-inflammatory effects, lowering prostaglandins, and reducing several serum lipid and glycemic measures. Additionally, it has “putative” vasculoprotective effects, he added.
“Ginger has a large number of chemical constituents and we do not know which of these, separately or in combination, will help relieve migraine,” he said. “We won’t know the answer unless clinical trials are conducted with the individual constituents rather than with ginger extract.” He compared this to the study of omega-3 fatty acids rather than fish and nuts for various neuropsychiatric or cardiovascular indications.
Nevertheless, given the high global prevalence of migraine and the “large treatment gap [of migraine] in primary care,” it could be common for many affected patients to experience episodes of migraine headache “without recourse to recommended pharmacologic relief,” he noted. “In such cases, the availability of a simple home remedy, such as ginger, could be helpful.”
‘Good additional tool’
Commenting on the study for this news organization, Jessica Ailani, MD, director, MedStar Georgetown Headache Center and professor of clinical neurology, MedStar Georgetown University Hospital, Washington, said that for “people with migraine who are seeking treatment with minimal side effects that they can obtain without counsel of a health care provider, ginger is a good additional tool to have.”
Dr. Ailani, vice cochair of strategic planning in the MedStar department of neurology, who was not involved with the study, said that clinicians can “consider suggesting ginger to patients with migraine that have associated nausea who are interested in nonpharmacologic ways to treat symptoms.”
Since there are “many other effective ways to treat migraine,” she advises “conversing with the patient about speed of onset of efficacy, along with tolerability, and return of migraine symptoms as important factors to evaluate when choosing and staying with a treatment.”
Also commenting on the study for this news organization, Nada Hindiyeh, MD, clinical associate professor, department of neurology, Stanford (Calif.) University, called it a “nice summary of the objective research available for the use of ginger in acute and preventive treatment of migraine.”
Although there is insufficient literature evaluating ginger alone in migraine treatment, so “no definitive conclusions can be drawn,” since it appears to be safe and “somewhat helpful for migraine-associated nausea and vomiting and possibly in frequency of migraine reduction, it remains a considerable alternative for those seeking nonprescription options,” said Dr. Hindiyeh, who was not involved with the study.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made to charities. He has received payments for developing educational materials for scientific initiatives and programs. Dr. Ailani reports honoraria for independent consulting from various pharmaceutical companies and clinical trial grants to her institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly, Satsuma, and Zosano. Dr. Hindiyeh discloses no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
iPLEDGE rollout described as a failure, chaotic, and a disaster
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA updates risks, cautions for clotting-bleeding disorder on Janssen COVID-19 vaccine
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Cancer risk tied to some manufactured foods
SAN ANTONIO –
The findings were reported in three poster presentations (P1-09-01, P1-09-02 and P3-12-35) at the 2021 San Antonio Breast Cancer Symposium from the ongoing French NutriNet-Santé web-based study of 171,000 people that was launched in France in 2009 to investigate nutrition and health relationships. The authors of the analyses note that while evidence of deleterious health effects has been established for the dietary focus of their studies, and cancer risks have been suspected, strong evidence of a cancer association has been lacking.
Nitrates and nitrites are used in processed meats to increase shelf life and to avoid bacterial growth, said Eloi Chazelas, PhD, Nutritional Epidemiology Research Team (EREN) at Sorbonne Paris Nord University. Dr. Chazelas looked at consumption of nitrites and nitrates through repeated 24 hour dietary records, linked to a comprehensive food composition database. The study’s main outcome measure was adjusted associations between nitrite and nitrate exposures and the risk of cancer (overall and by main cancer sites).
During follow-up, 966 breast and 400 prostate cancers were diagnosed among 3,311 first incident cancer cases. Breast cancer risk was elevated (HR = 1.24 [1.03-1.48], P = 0.02) among higher consumers of nitrates from food additives, especially with potassium nitrate consumption (HR = 1.25 [1.04-1.50], P = 0.01). Elevated prostate cancer risk was associated with nitrites (HR = 1.58 [1.14-2.18], P = 0.008), specifically for sodium nitrite (HR = 1.62 [1.17-2.25], P = 0.004). Nitrates and nitrites from natural sources were not associated significantly with higher cancer risk, Dr. Chazelas said.
He and his team found that food additive nitrates were positively associated with breast cancer risk, and food additive nitrites were positively associated with prostate cancer risk. “While these results need confirmation in other large-scale prospective studies, they provide new insights in a context of lively debate around the ban of nitrite additives in the food industry,” said Dr. Chazelas, who is a doctoral candidate at Sorbonne Paris Nord University.
In “Breast and prostate cancer risk associated with nitrites and nitrates from food additives (P1-09-01),” the study included 102,046 adults from the French NutriNet-Santé prospective cohort (2009-2021). It examined associations between artificial sweetener intakes (total from all dietary sources, the most frequently consumed ones [aspartame e951, acesulfame-K e950 and sucralose e955]) and cancer risk (overall and by sites: breast, prostate and obesity-related cancers).
Overall cancer risk in people who consumed higher amounts of total sweeteners (i.e. above the median exposure in consumers) was elevated (n = 2,527 cases, hazard ratio = 1.12, 95 percent confidence interval = 1.00-1.25, P-trend=0.005), especially for aspartame (HR = 1.20 [1.05-1.38] P = 0.001) and acesulfame-K (HR = 1.18 [1.04-1.34] P = 0.003). Elevated breast cancer risks (among 723 cases) were observed for total sweeteners (HR = 1.25 [1.02-1.53] P = 0.01), for aspartame (HR = 1.33 [1.05-1.69] P = 0.007), and for acesulfame-K (HR = 1.39 [1.11-1.74] P = 0.003). Also, obesity-related cancers (1,509 cases) were increased for total sweeteners (HR = 1.16 [1.00-1.33] P = 0.02), for aspartame (HR = 1.22 [1.02-1.45] P = 0.01) and for acesulfame-K (HR = 1.23 [1.04-1.45] P = 0.01).
Artificial sweeteners are found in more than 10,000 foods and beverages, said Charlotte Debras, a doctoral candidate in nutritional epidemiology at Sorbonne Paris Nord University. “These findings provide important and novel insights for the ongoing re-evaluation of food additive sweeteners by the European Food Safety Authority and other health agencies globally,” she said.
Trans fatty acid intakes and cancer risk
Investigating associations between trans fatty acid intake (total ruminant [rTFAs], industrial [iTFAs], and corresponding specific isomers and cancer risk), the analysis of Gaëlle Wendeu-Foyet, PhD, Sorbonne Paris Nord University, found a total of 3,374 incident cancer cases (982 breast, 405 prostate) in an overall population of 104,909. Dietary intake of total TFAs was associated with higher prostate cancer risk (hazard ration for quartile 4 versus 1: 1.27, 1.11-1.77 P-trend = 0.005). Also, rTFAs were associated with increased overall cancer risk (1.16, 1.02-1.32 P-trend = 0.07), in particular the conjugated linoleic acid isomers (CLA) (1.19, 1.04-1.36 P-trend = 0.04). These associations were specifically observed for breast cancer (rTFAs: 1.35, 1.06-1.72 P-trend = 0.01; CLA: 1.29, 1.00-1.66 P-trend = 0.048), in particular before menopause (rTFAs: 1.68, 1.06-2.67 P-trend = 0.02; CLA: 2.013, 1.25-3.23 P-trend = 0.003). Several iTFAs were associated with overall (1.18, 1.06-1.31 P-trend = 0.02 for transdocosenoic acid), breast (isomer 18:2t: 1.30, 1.06-1.58 P-trend = 0.01; hexadecenoic acid: 1.28, 1.05-1.56 P-trend = 0.02) and prostate (transdocosenoic acid: 1.52, 1.09-2.12 P-trend = 0.07) cancer risks.
“These results support the WHO’s goal of achieving elimination from food supplies of industrially produced TFAs,” Dr. Foyet said. “The consumption of food products containing partially hydrogenated oils should be avoided.”
Nutrition, along with avoiding tobacco intake, is one of the main modifiable risk factors for chronic diseases. “There is a lot at stake in terms of prevention. This requires a combination of actions at the individual level to the public level by informing the public through food labeling,” Ms. Debras said.
It also requires influencing the context in which citizens evolve by encouraging manufacturers to improve their products (pricing policies, commitment charters for product reformulation, etc.), and limiting advertising and marketing for products of poor nutritional quality (especially among children),” she said.
SAN ANTONIO –
The findings were reported in three poster presentations (P1-09-01, P1-09-02 and P3-12-35) at the 2021 San Antonio Breast Cancer Symposium from the ongoing French NutriNet-Santé web-based study of 171,000 people that was launched in France in 2009 to investigate nutrition and health relationships. The authors of the analyses note that while evidence of deleterious health effects has been established for the dietary focus of their studies, and cancer risks have been suspected, strong evidence of a cancer association has been lacking.
Nitrates and nitrites are used in processed meats to increase shelf life and to avoid bacterial growth, said Eloi Chazelas, PhD, Nutritional Epidemiology Research Team (EREN) at Sorbonne Paris Nord University. Dr. Chazelas looked at consumption of nitrites and nitrates through repeated 24 hour dietary records, linked to a comprehensive food composition database. The study’s main outcome measure was adjusted associations between nitrite and nitrate exposures and the risk of cancer (overall and by main cancer sites).
During follow-up, 966 breast and 400 prostate cancers were diagnosed among 3,311 first incident cancer cases. Breast cancer risk was elevated (HR = 1.24 [1.03-1.48], P = 0.02) among higher consumers of nitrates from food additives, especially with potassium nitrate consumption (HR = 1.25 [1.04-1.50], P = 0.01). Elevated prostate cancer risk was associated with nitrites (HR = 1.58 [1.14-2.18], P = 0.008), specifically for sodium nitrite (HR = 1.62 [1.17-2.25], P = 0.004). Nitrates and nitrites from natural sources were not associated significantly with higher cancer risk, Dr. Chazelas said.
He and his team found that food additive nitrates were positively associated with breast cancer risk, and food additive nitrites were positively associated with prostate cancer risk. “While these results need confirmation in other large-scale prospective studies, they provide new insights in a context of lively debate around the ban of nitrite additives in the food industry,” said Dr. Chazelas, who is a doctoral candidate at Sorbonne Paris Nord University.
In “Breast and prostate cancer risk associated with nitrites and nitrates from food additives (P1-09-01),” the study included 102,046 adults from the French NutriNet-Santé prospective cohort (2009-2021). It examined associations between artificial sweetener intakes (total from all dietary sources, the most frequently consumed ones [aspartame e951, acesulfame-K e950 and sucralose e955]) and cancer risk (overall and by sites: breast, prostate and obesity-related cancers).
Overall cancer risk in people who consumed higher amounts of total sweeteners (i.e. above the median exposure in consumers) was elevated (n = 2,527 cases, hazard ratio = 1.12, 95 percent confidence interval = 1.00-1.25, P-trend=0.005), especially for aspartame (HR = 1.20 [1.05-1.38] P = 0.001) and acesulfame-K (HR = 1.18 [1.04-1.34] P = 0.003). Elevated breast cancer risks (among 723 cases) were observed for total sweeteners (HR = 1.25 [1.02-1.53] P = 0.01), for aspartame (HR = 1.33 [1.05-1.69] P = 0.007), and for acesulfame-K (HR = 1.39 [1.11-1.74] P = 0.003). Also, obesity-related cancers (1,509 cases) were increased for total sweeteners (HR = 1.16 [1.00-1.33] P = 0.02), for aspartame (HR = 1.22 [1.02-1.45] P = 0.01) and for acesulfame-K (HR = 1.23 [1.04-1.45] P = 0.01).
Artificial sweeteners are found in more than 10,000 foods and beverages, said Charlotte Debras, a doctoral candidate in nutritional epidemiology at Sorbonne Paris Nord University. “These findings provide important and novel insights for the ongoing re-evaluation of food additive sweeteners by the European Food Safety Authority and other health agencies globally,” she said.
Trans fatty acid intakes and cancer risk
Investigating associations between trans fatty acid intake (total ruminant [rTFAs], industrial [iTFAs], and corresponding specific isomers and cancer risk), the analysis of Gaëlle Wendeu-Foyet, PhD, Sorbonne Paris Nord University, found a total of 3,374 incident cancer cases (982 breast, 405 prostate) in an overall population of 104,909. Dietary intake of total TFAs was associated with higher prostate cancer risk (hazard ration for quartile 4 versus 1: 1.27, 1.11-1.77 P-trend = 0.005). Also, rTFAs were associated with increased overall cancer risk (1.16, 1.02-1.32 P-trend = 0.07), in particular the conjugated linoleic acid isomers (CLA) (1.19, 1.04-1.36 P-trend = 0.04). These associations were specifically observed for breast cancer (rTFAs: 1.35, 1.06-1.72 P-trend = 0.01; CLA: 1.29, 1.00-1.66 P-trend = 0.048), in particular before menopause (rTFAs: 1.68, 1.06-2.67 P-trend = 0.02; CLA: 2.013, 1.25-3.23 P-trend = 0.003). Several iTFAs were associated with overall (1.18, 1.06-1.31 P-trend = 0.02 for transdocosenoic acid), breast (isomer 18:2t: 1.30, 1.06-1.58 P-trend = 0.01; hexadecenoic acid: 1.28, 1.05-1.56 P-trend = 0.02) and prostate (transdocosenoic acid: 1.52, 1.09-2.12 P-trend = 0.07) cancer risks.
“These results support the WHO’s goal of achieving elimination from food supplies of industrially produced TFAs,” Dr. Foyet said. “The consumption of food products containing partially hydrogenated oils should be avoided.”
Nutrition, along with avoiding tobacco intake, is one of the main modifiable risk factors for chronic diseases. “There is a lot at stake in terms of prevention. This requires a combination of actions at the individual level to the public level by informing the public through food labeling,” Ms. Debras said.
It also requires influencing the context in which citizens evolve by encouraging manufacturers to improve their products (pricing policies, commitment charters for product reformulation, etc.), and limiting advertising and marketing for products of poor nutritional quality (especially among children),” she said.
SAN ANTONIO –
The findings were reported in three poster presentations (P1-09-01, P1-09-02 and P3-12-35) at the 2021 San Antonio Breast Cancer Symposium from the ongoing French NutriNet-Santé web-based study of 171,000 people that was launched in France in 2009 to investigate nutrition and health relationships. The authors of the analyses note that while evidence of deleterious health effects has been established for the dietary focus of their studies, and cancer risks have been suspected, strong evidence of a cancer association has been lacking.
Nitrates and nitrites are used in processed meats to increase shelf life and to avoid bacterial growth, said Eloi Chazelas, PhD, Nutritional Epidemiology Research Team (EREN) at Sorbonne Paris Nord University. Dr. Chazelas looked at consumption of nitrites and nitrates through repeated 24 hour dietary records, linked to a comprehensive food composition database. The study’s main outcome measure was adjusted associations between nitrite and nitrate exposures and the risk of cancer (overall and by main cancer sites).
During follow-up, 966 breast and 400 prostate cancers were diagnosed among 3,311 first incident cancer cases. Breast cancer risk was elevated (HR = 1.24 [1.03-1.48], P = 0.02) among higher consumers of nitrates from food additives, especially with potassium nitrate consumption (HR = 1.25 [1.04-1.50], P = 0.01). Elevated prostate cancer risk was associated with nitrites (HR = 1.58 [1.14-2.18], P = 0.008), specifically for sodium nitrite (HR = 1.62 [1.17-2.25], P = 0.004). Nitrates and nitrites from natural sources were not associated significantly with higher cancer risk, Dr. Chazelas said.
He and his team found that food additive nitrates were positively associated with breast cancer risk, and food additive nitrites were positively associated with prostate cancer risk. “While these results need confirmation in other large-scale prospective studies, they provide new insights in a context of lively debate around the ban of nitrite additives in the food industry,” said Dr. Chazelas, who is a doctoral candidate at Sorbonne Paris Nord University.
In “Breast and prostate cancer risk associated with nitrites and nitrates from food additives (P1-09-01),” the study included 102,046 adults from the French NutriNet-Santé prospective cohort (2009-2021). It examined associations between artificial sweetener intakes (total from all dietary sources, the most frequently consumed ones [aspartame e951, acesulfame-K e950 and sucralose e955]) and cancer risk (overall and by sites: breast, prostate and obesity-related cancers).
Overall cancer risk in people who consumed higher amounts of total sweeteners (i.e. above the median exposure in consumers) was elevated (n = 2,527 cases, hazard ratio = 1.12, 95 percent confidence interval = 1.00-1.25, P-trend=0.005), especially for aspartame (HR = 1.20 [1.05-1.38] P = 0.001) and acesulfame-K (HR = 1.18 [1.04-1.34] P = 0.003). Elevated breast cancer risks (among 723 cases) were observed for total sweeteners (HR = 1.25 [1.02-1.53] P = 0.01), for aspartame (HR = 1.33 [1.05-1.69] P = 0.007), and for acesulfame-K (HR = 1.39 [1.11-1.74] P = 0.003). Also, obesity-related cancers (1,509 cases) were increased for total sweeteners (HR = 1.16 [1.00-1.33] P = 0.02), for aspartame (HR = 1.22 [1.02-1.45] P = 0.01) and for acesulfame-K (HR = 1.23 [1.04-1.45] P = 0.01).
Artificial sweeteners are found in more than 10,000 foods and beverages, said Charlotte Debras, a doctoral candidate in nutritional epidemiology at Sorbonne Paris Nord University. “These findings provide important and novel insights for the ongoing re-evaluation of food additive sweeteners by the European Food Safety Authority and other health agencies globally,” she said.
Trans fatty acid intakes and cancer risk
Investigating associations between trans fatty acid intake (total ruminant [rTFAs], industrial [iTFAs], and corresponding specific isomers and cancer risk), the analysis of Gaëlle Wendeu-Foyet, PhD, Sorbonne Paris Nord University, found a total of 3,374 incident cancer cases (982 breast, 405 prostate) in an overall population of 104,909. Dietary intake of total TFAs was associated with higher prostate cancer risk (hazard ration for quartile 4 versus 1: 1.27, 1.11-1.77 P-trend = 0.005). Also, rTFAs were associated with increased overall cancer risk (1.16, 1.02-1.32 P-trend = 0.07), in particular the conjugated linoleic acid isomers (CLA) (1.19, 1.04-1.36 P-trend = 0.04). These associations were specifically observed for breast cancer (rTFAs: 1.35, 1.06-1.72 P-trend = 0.01; CLA: 1.29, 1.00-1.66 P-trend = 0.048), in particular before menopause (rTFAs: 1.68, 1.06-2.67 P-trend = 0.02; CLA: 2.013, 1.25-3.23 P-trend = 0.003). Several iTFAs were associated with overall (1.18, 1.06-1.31 P-trend = 0.02 for transdocosenoic acid), breast (isomer 18:2t: 1.30, 1.06-1.58 P-trend = 0.01; hexadecenoic acid: 1.28, 1.05-1.56 P-trend = 0.02) and prostate (transdocosenoic acid: 1.52, 1.09-2.12 P-trend = 0.07) cancer risks.
“These results support the WHO’s goal of achieving elimination from food supplies of industrially produced TFAs,” Dr. Foyet said. “The consumption of food products containing partially hydrogenated oils should be avoided.”
Nutrition, along with avoiding tobacco intake, is one of the main modifiable risk factors for chronic diseases. “There is a lot at stake in terms of prevention. This requires a combination of actions at the individual level to the public level by informing the public through food labeling,” Ms. Debras said.
It also requires influencing the context in which citizens evolve by encouraging manufacturers to improve their products (pricing policies, commitment charters for product reformulation, etc.), and limiting advertising and marketing for products of poor nutritional quality (especially among children),” she said.
FROM SABCS 2021
Breast cancer-related musculoskeletal pain alleviated with acupuncture
SAN ANTONIO –
Both techniques led to clinically meaningful and persistent reduction of pain, but electroacupuncture was more effective in reducing pain severity, according to study author Wanqing Iris Zhi, MD, PhD, of the Breast Medicine Service at Memorial Sloan Kettering Cancer Center in New York.
Among breast cancer survivors, Dr. Zhi said, chronic musculoskeletal pain is common and debilitating. In earlier results of the PEACE (Personalized Electroacupuncture versus Auricular Acupuncture Comparative Effectiveness) trial, both electroacupuncture and auricular acupuncture improved pain control better than usual care in cancer survivors. The comparative effectiveness between electroacupuncture and auricular acupuncture among breast cancer survivors, specifically for chronic musculoskeletal pain, remains unknown.
To evaluate potential differences between electroacupuncture and auricular acupuncture, Dr. Zhi et al. examined data from PEACE, a three-arm, parallel, single center randomized trial investigating electroacupuncture and auricular acupuncture for chronic musculoskeletal pain, compared with usual care. Among 360 cancer survivors in PEACE, mean age in 165 cancer survivors with a primary diagnosis of breast cancer was 60.3 years (35.8 percent non-White) with a mean of 5.4 years since their cancer diagnoses. Patients in both the electroacupuncture and auricular acupuncture groups received 10 weekly treatments. Change in mean Brief Pain Inventory (BPI) pain severity from baseline to week 12 was the primary endpoint, with BPI change to week 24 as a secondary endpoint. Usual care patients, after week 12, could receive 10 electroacupuncture treatments.
The most common locations of chronic musculoskeletal pain, Dr. Zhi observed, were lower back (24 percent), knee/leg (24 percent) and shoulder/elbow (14 percent). About 70 percent of patients were taking pain medication. Both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reductions among the evaluated breast cancer survivors. The change in BPI severity from baseline to week 12 was –0.29 (confidence interval, –0.08, 0.28) in the UC group. In the electroacupuncture group it was –2.65 (CI, –3.06, –2.25; P ≤0.001 from baseline) and –2.37 versus usual care (CI, –3.05, –1.68; P ≤0.001 versus UC). For the auricular acupuncture group, the change from baseline was –1.75 (CI, –2.15, –1.35; P ≤0.001 from baseline) and –1.46 versus usual care (CI, –2.14, –0.78; P ≤0.001 versus UC). The difference in BPI pain severity reduction from baseline between electroacupuncture and auricular acupuncture of –0.90 (CI, –1.45, –0.36) was statistically significant (P ≤0.001). Electroacupuncture also reduced pain severity significantly more than auricular acupuncture at week 24 (CI, –0.82, [–1.38, –0.27], P = 0.004).
Dr. Zhi concluded that among breast cancer survivors, although both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reduction, electroacupuncture was more effective at reducing pain severity.
She pointed out also that neither surgery type (mastectomy versus lumpectomy; P = 0.83) nor aromatase inhibitor versus tamoxifen versus neither (P = 0.59) was associated with BPI/severity response among electroacupuncture and auricular acupuncture patients.
“Both electroacupuncture and auricular acupuncture are significantly better than usual care, so it suggests that both acupuncture methods can be utilized for treating chronic muscle skeletal pain in breast cancer survivors, but electroacupuncture is preferred,” Dr. Zhi said.
“Auricular acupuncture can be more painful,” said PEACE principal investigator Jun Mao, MD, who is chair of integrative medicine at Memorial Sloan Kettering. “Ten percent of women could not tolerate the ear pain or discomfort. Electroacupuncture is generally well tolerated. People are more relaxed after treatment. If both are available, start with electroacupuncture,” he said.
SAN ANTONIO –
Both techniques led to clinically meaningful and persistent reduction of pain, but electroacupuncture was more effective in reducing pain severity, according to study author Wanqing Iris Zhi, MD, PhD, of the Breast Medicine Service at Memorial Sloan Kettering Cancer Center in New York.
Among breast cancer survivors, Dr. Zhi said, chronic musculoskeletal pain is common and debilitating. In earlier results of the PEACE (Personalized Electroacupuncture versus Auricular Acupuncture Comparative Effectiveness) trial, both electroacupuncture and auricular acupuncture improved pain control better than usual care in cancer survivors. The comparative effectiveness between electroacupuncture and auricular acupuncture among breast cancer survivors, specifically for chronic musculoskeletal pain, remains unknown.
To evaluate potential differences between electroacupuncture and auricular acupuncture, Dr. Zhi et al. examined data from PEACE, a three-arm, parallel, single center randomized trial investigating electroacupuncture and auricular acupuncture for chronic musculoskeletal pain, compared with usual care. Among 360 cancer survivors in PEACE, mean age in 165 cancer survivors with a primary diagnosis of breast cancer was 60.3 years (35.8 percent non-White) with a mean of 5.4 years since their cancer diagnoses. Patients in both the electroacupuncture and auricular acupuncture groups received 10 weekly treatments. Change in mean Brief Pain Inventory (BPI) pain severity from baseline to week 12 was the primary endpoint, with BPI change to week 24 as a secondary endpoint. Usual care patients, after week 12, could receive 10 electroacupuncture treatments.
The most common locations of chronic musculoskeletal pain, Dr. Zhi observed, were lower back (24 percent), knee/leg (24 percent) and shoulder/elbow (14 percent). About 70 percent of patients were taking pain medication. Both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reductions among the evaluated breast cancer survivors. The change in BPI severity from baseline to week 12 was –0.29 (confidence interval, –0.08, 0.28) in the UC group. In the electroacupuncture group it was –2.65 (CI, –3.06, –2.25; P ≤0.001 from baseline) and –2.37 versus usual care (CI, –3.05, –1.68; P ≤0.001 versus UC). For the auricular acupuncture group, the change from baseline was –1.75 (CI, –2.15, –1.35; P ≤0.001 from baseline) and –1.46 versus usual care (CI, –2.14, –0.78; P ≤0.001 versus UC). The difference in BPI pain severity reduction from baseline between electroacupuncture and auricular acupuncture of –0.90 (CI, –1.45, –0.36) was statistically significant (P ≤0.001). Electroacupuncture also reduced pain severity significantly more than auricular acupuncture at week 24 (CI, –0.82, [–1.38, –0.27], P = 0.004).
Dr. Zhi concluded that among breast cancer survivors, although both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reduction, electroacupuncture was more effective at reducing pain severity.
She pointed out also that neither surgery type (mastectomy versus lumpectomy; P = 0.83) nor aromatase inhibitor versus tamoxifen versus neither (P = 0.59) was associated with BPI/severity response among electroacupuncture and auricular acupuncture patients.
“Both electroacupuncture and auricular acupuncture are significantly better than usual care, so it suggests that both acupuncture methods can be utilized for treating chronic muscle skeletal pain in breast cancer survivors, but electroacupuncture is preferred,” Dr. Zhi said.
“Auricular acupuncture can be more painful,” said PEACE principal investigator Jun Mao, MD, who is chair of integrative medicine at Memorial Sloan Kettering. “Ten percent of women could not tolerate the ear pain or discomfort. Electroacupuncture is generally well tolerated. People are more relaxed after treatment. If both are available, start with electroacupuncture,” he said.
SAN ANTONIO –
Both techniques led to clinically meaningful and persistent reduction of pain, but electroacupuncture was more effective in reducing pain severity, according to study author Wanqing Iris Zhi, MD, PhD, of the Breast Medicine Service at Memorial Sloan Kettering Cancer Center in New York.
Among breast cancer survivors, Dr. Zhi said, chronic musculoskeletal pain is common and debilitating. In earlier results of the PEACE (Personalized Electroacupuncture versus Auricular Acupuncture Comparative Effectiveness) trial, both electroacupuncture and auricular acupuncture improved pain control better than usual care in cancer survivors. The comparative effectiveness between electroacupuncture and auricular acupuncture among breast cancer survivors, specifically for chronic musculoskeletal pain, remains unknown.
To evaluate potential differences between electroacupuncture and auricular acupuncture, Dr. Zhi et al. examined data from PEACE, a three-arm, parallel, single center randomized trial investigating electroacupuncture and auricular acupuncture for chronic musculoskeletal pain, compared with usual care. Among 360 cancer survivors in PEACE, mean age in 165 cancer survivors with a primary diagnosis of breast cancer was 60.3 years (35.8 percent non-White) with a mean of 5.4 years since their cancer diagnoses. Patients in both the electroacupuncture and auricular acupuncture groups received 10 weekly treatments. Change in mean Brief Pain Inventory (BPI) pain severity from baseline to week 12 was the primary endpoint, with BPI change to week 24 as a secondary endpoint. Usual care patients, after week 12, could receive 10 electroacupuncture treatments.
The most common locations of chronic musculoskeletal pain, Dr. Zhi observed, were lower back (24 percent), knee/leg (24 percent) and shoulder/elbow (14 percent). About 70 percent of patients were taking pain medication. Both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reductions among the evaluated breast cancer survivors. The change in BPI severity from baseline to week 12 was –0.29 (confidence interval, –0.08, 0.28) in the UC group. In the electroacupuncture group it was –2.65 (CI, –3.06, –2.25; P ≤0.001 from baseline) and –2.37 versus usual care (CI, –3.05, –1.68; P ≤0.001 versus UC). For the auricular acupuncture group, the change from baseline was –1.75 (CI, –2.15, –1.35; P ≤0.001 from baseline) and –1.46 versus usual care (CI, –2.14, –0.78; P ≤0.001 versus UC). The difference in BPI pain severity reduction from baseline between electroacupuncture and auricular acupuncture of –0.90 (CI, –1.45, –0.36) was statistically significant (P ≤0.001). Electroacupuncture also reduced pain severity significantly more than auricular acupuncture at week 24 (CI, –0.82, [–1.38, –0.27], P = 0.004).
Dr. Zhi concluded that among breast cancer survivors, although both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reduction, electroacupuncture was more effective at reducing pain severity.
She pointed out also that neither surgery type (mastectomy versus lumpectomy; P = 0.83) nor aromatase inhibitor versus tamoxifen versus neither (P = 0.59) was associated with BPI/severity response among electroacupuncture and auricular acupuncture patients.
“Both electroacupuncture and auricular acupuncture are significantly better than usual care, so it suggests that both acupuncture methods can be utilized for treating chronic muscle skeletal pain in breast cancer survivors, but electroacupuncture is preferred,” Dr. Zhi said.
“Auricular acupuncture can be more painful,” said PEACE principal investigator Jun Mao, MD, who is chair of integrative medicine at Memorial Sloan Kettering. “Ten percent of women could not tolerate the ear pain or discomfort. Electroacupuncture is generally well tolerated. People are more relaxed after treatment. If both are available, start with electroacupuncture,” he said.
FROM SABCS 2021
Doctors as trusted messengers
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
A pandemic silver lining? Dramatic drop in teen drug use
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.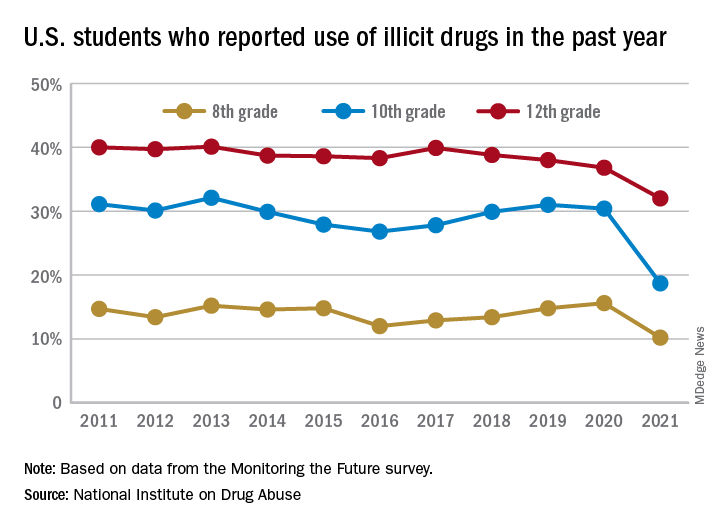
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Not All Pulmonary Nodules in Smokers are Lung Cancer
Identification of pulmonary nodules in older adults who smoke immediately brings concern for malignancy in the mind of clinicians. This is particularly the case in patients with significant smoking history. According to the National Cancer Institute in 2019, 12.9% of all new cancer cases were lung cancers.1 Screening for lung cancer, especially in patients with increased risk from smoking, is imperative to early detection and treatment. However, 20% of patients will be overdiagnosed by lung cancer-screening techniques.2 The rate of malignancy noted on a patient’s first screening computed tomography (CT) scan was between 3.7% and 5.5%.3
Rheumatoid arthritis (RA) is an autoimmune inflammatory condition that mainly affects the joints. Extraarticular manifestations can arise in various locations throughout the body, however. These manifestations are commonly observed in the skin, heart, and lungs.4 Prevalence of pulmonary rheumatoid nodules ranges from < 0.4% in radiologic studies to 32% in lung biopsies of patients with RA and nodules.5
Furthermore, there is a strong association between the risk of rheumatoid nodules in patients with positive serum rheumatoid factor (RF) and smoking history.6 Solitary pulmonary nodules in patients with RA can coexist with bronchogenic carcinoma, making their diagnosis more important.7
Case Presentation
A 54-year-old woman with a 30 pack-year smoking history and history of RA initially presented to the emergency department for cough and dyspnea for 5-day duration. Her initial diagnosis was bronchitis based on presenting symptom profile. A chest CT demonstrated 3 cavitary pulmonary nodules, 1 measuring 2.4 x 2.0 cm in the right middle lobe, and 2 additional nodules, measuring 1.8 x 1.4 and 1.5 x 1.4 in the left upper lobe (Figure). She had no improvement of symptoms after a 7-day course of doxycycline. The patient was taking methotrexate 15 mg weekly and golimumab 50 mg subcutaneously every 4 weeks as treatment for RA, prescribed by her rheumatologist.
Pulmonology was consulted and a positron emission tomography-CT (PET-CT) confirmed several cavitary pulmonary nodules involving both lungs with no suspicious fluorodeoxyglucose (FDG) uptake. The largest lesion was in the right middle lobe with FDG uptake of 1.9. Additional nodules were found in the left upper lobe, measuring 1.8 x 1.4 cm with FDG of 4.01, and in the left lung apex, measuring 1.5 x 1.4 cm with uptake of 3.53. CTguided percutaneous fine needle aspiration (PFNA) of the right middle lobe lung nodule demonstrated granuloma with central inflammatory debris. Grocott methenamine silver (GMS) stain was negative for fungal organism, acid-fast bacteria (AFB) stain was negative for acid-fast bacilli, and CD20 and CD3 immunostaining demonstrated mixed B- and T-cell populations. There was no evidence of atypia or malignancy. The biopsy demonstrated granuloma with central inflammatory debris on a background of densely fibrotic tissue and lympho-plasmatic inflammation. This finding confirmed the diagnosis of RA with pulmonary involvement.
Outpatient follow-up was established with a pulmonologist and rheumatologist. Methotrexate 15 mg weekly and golimumab subcutaneously 50 mg every 4 weeks were prescribed for the patient. The nodules are being monitored based on Fleischer guidelines with CT imaging 3 to 6 months following initial presentation. Further imaging will be considered at 18 to 24 months as well to further assess stability of the nodules and monitor for changes in size, shape, and necrosis. The patient also was encouraged to quit smoking. Her clinical course since the diagnosis has been stable.
Discussion
The differential diagnosis for new multiple pulmonary nodules on imaging studies is broad and includes infectious processes, such as tuberculosis, as well as other mycobacterial, fungal, and bacterial infections. Noninfectious causes of lung disease are an even broader category of consideration. Noninfectious pulmonary nodules differential includes sarcoidosis, granulomatous with polyangiitis, hypersensitivity pneumonitis, methotrexate drug reaction, pulmonary manifestations of systemic conditions, such as RA chronic granulomatous disease and malignancy.8 Bronchogenic carcinoma was suspected in this patient due to her smoking history. Squamous cell carcinoma was also considered as the lesion was cavitary. AFB and GMS stains were negative for fungi. Langerhans cell histiocytosis were considered but ruled out as these lesions contain larger numbers of eosinophils than described in the pathology report. Histoplasma and coccidiosis laboratory tests were obtained as the patient lived in a region endemic to both these fungi but were negative (Table). A diagnosis of rheumatoid nodule was made based on the clinical setting, typical radiographic, histopathology features, and negative cultures.
This case is unique due to the quality and location of the rheumatoid nodules within the lungs. Pulmonary manifestations of RA are usually subcutaneous or subpleural, solid, and peripherally located.9 This patient’s nodules were necrobiotic and located within the lung parenchyma. There was significant cavitation. These factors are atypical features of pulmonary RA.
Pulmonary RA can have many associated symptoms and remains an important factor in patient mortality. Estimates demonstrate that 10 to 20% of RA-related deaths are secondary to pulmonary manifestations.10 There are a wide array of symptoms and presentations to be aware of clinically. These symptoms are often nondescript, widely sensitive to many disease processes, and nonspecific to pulmonary RA. These symptoms include dyspnea, wheezing, and nonproductive cough.10 Bronchiectasis is a common symptom as well as small airway obstruction.10 Consolidated necrobiotic lesions are present in up to 20% of pulmonary RA cases.10 Generally these lesions are asymptomatic but can also be associated with pneumothorax, hemoptysis, and airway obstruction.10 Awareness of these symptoms is important for diagnosis and monitoring clinical improvement in patients.
Further workup is necessary to differentiate malignancy-related pulmonary nodules and other causes; if the index of suspicion is high for malignancy as in our case, the workup should be more aggressive. Biopsy is mandatory in such cases to rule out infections and malignancy, as it is highly sensitive and specific. The main problem hindering management is when a clinician fails to include this in their differential diagnosis. This further elucidates the importance of awareness of this diagnosis. Suspicious lesions in a proper clinical setting should be followed up by imaging studies and confirmatory histopathological diagnosis. Typical follow-up is 3 months after initial presentation to assess stability and possibly 18 to 24 months as well based on Fleischer guidelines.
Various treatment modalities have been tried as per literature, including tocilizumab and rituximab. 11,12 Our patient is currently being treated with golimumab based on outpatient rheumatologist recommendations.
Conclusions
This case demonstrates the importance of a careful workup to narrow a broad differential. Medical diagnosis of pulmonary nodules requires an in-depth workup, including clinical evaluation, laboratory and pulmonary functions tests, as well as various imaging studies.
1. Lung and Bronchus Cancer - Cancer Stat Facts. SEER. Accessed February 2, 2020. https://seer.cancer.gov /statfacts/html/lungb.html
2. Shaughnessy AF. One in Five Patients Overdiagnosed with Lung Cancer Screening. Am Fam Physician. 2014 Jul 15;90(2):112.
3. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369;910-919. doi:10.1056/NEJMoa1214726
4. Stamp LK, Cleland LG. Rheumatoid arthritis. In: Thompson LU, Ward WE, eds. Optimizing Women’s Health through Nutrition. CRC Press; 2008; 279-320.
5. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770-777. doi:10.1164/arrd.1985.131.5.770
6. Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, Turesson C; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601-606. doi:10.1136/ard.2005.039172
7. Shenberger KN, Schned AR, Taylor TH. Rheumatoid disease and bronchogenic carcinoma—case report and review of the literature. J Rheumatol. 1984;11:226–228.
8. Mukhopadhyay S, Wilcox BE, Myers JL, et al. Pulmonary necrotizing granulomas of unknown cause clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813-824. doi:10.1378/chest.12-2113
9. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. Published 2017 Aug 9. doi:10.1183/16000617.0012-2017
10. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448. doi:10.1513/pats.200703-045MS
11. Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72(2):166-171. doi:10.1007/s00393-012-1054-0
12. Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford). 2012;51(6):1132-1134. doi:10.1093/rheumatology/ker455
Identification of pulmonary nodules in older adults who smoke immediately brings concern for malignancy in the mind of clinicians. This is particularly the case in patients with significant smoking history. According to the National Cancer Institute in 2019, 12.9% of all new cancer cases were lung cancers.1 Screening for lung cancer, especially in patients with increased risk from smoking, is imperative to early detection and treatment. However, 20% of patients will be overdiagnosed by lung cancer-screening techniques.2 The rate of malignancy noted on a patient’s first screening computed tomography (CT) scan was between 3.7% and 5.5%.3
Rheumatoid arthritis (RA) is an autoimmune inflammatory condition that mainly affects the joints. Extraarticular manifestations can arise in various locations throughout the body, however. These manifestations are commonly observed in the skin, heart, and lungs.4 Prevalence of pulmonary rheumatoid nodules ranges from < 0.4% in radiologic studies to 32% in lung biopsies of patients with RA and nodules.5
Furthermore, there is a strong association between the risk of rheumatoid nodules in patients with positive serum rheumatoid factor (RF) and smoking history.6 Solitary pulmonary nodules in patients with RA can coexist with bronchogenic carcinoma, making their diagnosis more important.7
Case Presentation
A 54-year-old woman with a 30 pack-year smoking history and history of RA initially presented to the emergency department for cough and dyspnea for 5-day duration. Her initial diagnosis was bronchitis based on presenting symptom profile. A chest CT demonstrated 3 cavitary pulmonary nodules, 1 measuring 2.4 x 2.0 cm in the right middle lobe, and 2 additional nodules, measuring 1.8 x 1.4 and 1.5 x 1.4 in the left upper lobe (Figure). She had no improvement of symptoms after a 7-day course of doxycycline. The patient was taking methotrexate 15 mg weekly and golimumab 50 mg subcutaneously every 4 weeks as treatment for RA, prescribed by her rheumatologist.
Pulmonology was consulted and a positron emission tomography-CT (PET-CT) confirmed several cavitary pulmonary nodules involving both lungs with no suspicious fluorodeoxyglucose (FDG) uptake. The largest lesion was in the right middle lobe with FDG uptake of 1.9. Additional nodules were found in the left upper lobe, measuring 1.8 x 1.4 cm with FDG of 4.01, and in the left lung apex, measuring 1.5 x 1.4 cm with uptake of 3.53. CTguided percutaneous fine needle aspiration (PFNA) of the right middle lobe lung nodule demonstrated granuloma with central inflammatory debris. Grocott methenamine silver (GMS) stain was negative for fungal organism, acid-fast bacteria (AFB) stain was negative for acid-fast bacilli, and CD20 and CD3 immunostaining demonstrated mixed B- and T-cell populations. There was no evidence of atypia or malignancy. The biopsy demonstrated granuloma with central inflammatory debris on a background of densely fibrotic tissue and lympho-plasmatic inflammation. This finding confirmed the diagnosis of RA with pulmonary involvement.
Outpatient follow-up was established with a pulmonologist and rheumatologist. Methotrexate 15 mg weekly and golimumab subcutaneously 50 mg every 4 weeks were prescribed for the patient. The nodules are being monitored based on Fleischer guidelines with CT imaging 3 to 6 months following initial presentation. Further imaging will be considered at 18 to 24 months as well to further assess stability of the nodules and monitor for changes in size, shape, and necrosis. The patient also was encouraged to quit smoking. Her clinical course since the diagnosis has been stable.
Discussion
The differential diagnosis for new multiple pulmonary nodules on imaging studies is broad and includes infectious processes, such as tuberculosis, as well as other mycobacterial, fungal, and bacterial infections. Noninfectious causes of lung disease are an even broader category of consideration. Noninfectious pulmonary nodules differential includes sarcoidosis, granulomatous with polyangiitis, hypersensitivity pneumonitis, methotrexate drug reaction, pulmonary manifestations of systemic conditions, such as RA chronic granulomatous disease and malignancy.8 Bronchogenic carcinoma was suspected in this patient due to her smoking history. Squamous cell carcinoma was also considered as the lesion was cavitary. AFB and GMS stains were negative for fungi. Langerhans cell histiocytosis were considered but ruled out as these lesions contain larger numbers of eosinophils than described in the pathology report. Histoplasma and coccidiosis laboratory tests were obtained as the patient lived in a region endemic to both these fungi but were negative (Table). A diagnosis of rheumatoid nodule was made based on the clinical setting, typical radiographic, histopathology features, and negative cultures.
This case is unique due to the quality and location of the rheumatoid nodules within the lungs. Pulmonary manifestations of RA are usually subcutaneous or subpleural, solid, and peripherally located.9 This patient’s nodules were necrobiotic and located within the lung parenchyma. There was significant cavitation. These factors are atypical features of pulmonary RA.
Pulmonary RA can have many associated symptoms and remains an important factor in patient mortality. Estimates demonstrate that 10 to 20% of RA-related deaths are secondary to pulmonary manifestations.10 There are a wide array of symptoms and presentations to be aware of clinically. These symptoms are often nondescript, widely sensitive to many disease processes, and nonspecific to pulmonary RA. These symptoms include dyspnea, wheezing, and nonproductive cough.10 Bronchiectasis is a common symptom as well as small airway obstruction.10 Consolidated necrobiotic lesions are present in up to 20% of pulmonary RA cases.10 Generally these lesions are asymptomatic but can also be associated with pneumothorax, hemoptysis, and airway obstruction.10 Awareness of these symptoms is important for diagnosis and monitoring clinical improvement in patients.
Further workup is necessary to differentiate malignancy-related pulmonary nodules and other causes; if the index of suspicion is high for malignancy as in our case, the workup should be more aggressive. Biopsy is mandatory in such cases to rule out infections and malignancy, as it is highly sensitive and specific. The main problem hindering management is when a clinician fails to include this in their differential diagnosis. This further elucidates the importance of awareness of this diagnosis. Suspicious lesions in a proper clinical setting should be followed up by imaging studies and confirmatory histopathological diagnosis. Typical follow-up is 3 months after initial presentation to assess stability and possibly 18 to 24 months as well based on Fleischer guidelines.
Various treatment modalities have been tried as per literature, including tocilizumab and rituximab. 11,12 Our patient is currently being treated with golimumab based on outpatient rheumatologist recommendations.
Conclusions
This case demonstrates the importance of a careful workup to narrow a broad differential. Medical diagnosis of pulmonary nodules requires an in-depth workup, including clinical evaluation, laboratory and pulmonary functions tests, as well as various imaging studies.
Identification of pulmonary nodules in older adults who smoke immediately brings concern for malignancy in the mind of clinicians. This is particularly the case in patients with significant smoking history. According to the National Cancer Institute in 2019, 12.9% of all new cancer cases were lung cancers.1 Screening for lung cancer, especially in patients with increased risk from smoking, is imperative to early detection and treatment. However, 20% of patients will be overdiagnosed by lung cancer-screening techniques.2 The rate of malignancy noted on a patient’s first screening computed tomography (CT) scan was between 3.7% and 5.5%.3
Rheumatoid arthritis (RA) is an autoimmune inflammatory condition that mainly affects the joints. Extraarticular manifestations can arise in various locations throughout the body, however. These manifestations are commonly observed in the skin, heart, and lungs.4 Prevalence of pulmonary rheumatoid nodules ranges from < 0.4% in radiologic studies to 32% in lung biopsies of patients with RA and nodules.5
Furthermore, there is a strong association between the risk of rheumatoid nodules in patients with positive serum rheumatoid factor (RF) and smoking history.6 Solitary pulmonary nodules in patients with RA can coexist with bronchogenic carcinoma, making their diagnosis more important.7
Case Presentation
A 54-year-old woman with a 30 pack-year smoking history and history of RA initially presented to the emergency department for cough and dyspnea for 5-day duration. Her initial diagnosis was bronchitis based on presenting symptom profile. A chest CT demonstrated 3 cavitary pulmonary nodules, 1 measuring 2.4 x 2.0 cm in the right middle lobe, and 2 additional nodules, measuring 1.8 x 1.4 and 1.5 x 1.4 in the left upper lobe (Figure). She had no improvement of symptoms after a 7-day course of doxycycline. The patient was taking methotrexate 15 mg weekly and golimumab 50 mg subcutaneously every 4 weeks as treatment for RA, prescribed by her rheumatologist.
Pulmonology was consulted and a positron emission tomography-CT (PET-CT) confirmed several cavitary pulmonary nodules involving both lungs with no suspicious fluorodeoxyglucose (FDG) uptake. The largest lesion was in the right middle lobe with FDG uptake of 1.9. Additional nodules were found in the left upper lobe, measuring 1.8 x 1.4 cm with FDG of 4.01, and in the left lung apex, measuring 1.5 x 1.4 cm with uptake of 3.53. CTguided percutaneous fine needle aspiration (PFNA) of the right middle lobe lung nodule demonstrated granuloma with central inflammatory debris. Grocott methenamine silver (GMS) stain was negative for fungal organism, acid-fast bacteria (AFB) stain was negative for acid-fast bacilli, and CD20 and CD3 immunostaining demonstrated mixed B- and T-cell populations. There was no evidence of atypia or malignancy. The biopsy demonstrated granuloma with central inflammatory debris on a background of densely fibrotic tissue and lympho-plasmatic inflammation. This finding confirmed the diagnosis of RA with pulmonary involvement.
Outpatient follow-up was established with a pulmonologist and rheumatologist. Methotrexate 15 mg weekly and golimumab subcutaneously 50 mg every 4 weeks were prescribed for the patient. The nodules are being monitored based on Fleischer guidelines with CT imaging 3 to 6 months following initial presentation. Further imaging will be considered at 18 to 24 months as well to further assess stability of the nodules and monitor for changes in size, shape, and necrosis. The patient also was encouraged to quit smoking. Her clinical course since the diagnosis has been stable.
Discussion
The differential diagnosis for new multiple pulmonary nodules on imaging studies is broad and includes infectious processes, such as tuberculosis, as well as other mycobacterial, fungal, and bacterial infections. Noninfectious causes of lung disease are an even broader category of consideration. Noninfectious pulmonary nodules differential includes sarcoidosis, granulomatous with polyangiitis, hypersensitivity pneumonitis, methotrexate drug reaction, pulmonary manifestations of systemic conditions, such as RA chronic granulomatous disease and malignancy.8 Bronchogenic carcinoma was suspected in this patient due to her smoking history. Squamous cell carcinoma was also considered as the lesion was cavitary. AFB and GMS stains were negative for fungi. Langerhans cell histiocytosis were considered but ruled out as these lesions contain larger numbers of eosinophils than described in the pathology report. Histoplasma and coccidiosis laboratory tests were obtained as the patient lived in a region endemic to both these fungi but were negative (Table). A diagnosis of rheumatoid nodule was made based on the clinical setting, typical radiographic, histopathology features, and negative cultures.
This case is unique due to the quality and location of the rheumatoid nodules within the lungs. Pulmonary manifestations of RA are usually subcutaneous or subpleural, solid, and peripherally located.9 This patient’s nodules were necrobiotic and located within the lung parenchyma. There was significant cavitation. These factors are atypical features of pulmonary RA.
Pulmonary RA can have many associated symptoms and remains an important factor in patient mortality. Estimates demonstrate that 10 to 20% of RA-related deaths are secondary to pulmonary manifestations.10 There are a wide array of symptoms and presentations to be aware of clinically. These symptoms are often nondescript, widely sensitive to many disease processes, and nonspecific to pulmonary RA. These symptoms include dyspnea, wheezing, and nonproductive cough.10 Bronchiectasis is a common symptom as well as small airway obstruction.10 Consolidated necrobiotic lesions are present in up to 20% of pulmonary RA cases.10 Generally these lesions are asymptomatic but can also be associated with pneumothorax, hemoptysis, and airway obstruction.10 Awareness of these symptoms is important for diagnosis and monitoring clinical improvement in patients.
Further workup is necessary to differentiate malignancy-related pulmonary nodules and other causes; if the index of suspicion is high for malignancy as in our case, the workup should be more aggressive. Biopsy is mandatory in such cases to rule out infections and malignancy, as it is highly sensitive and specific. The main problem hindering management is when a clinician fails to include this in their differential diagnosis. This further elucidates the importance of awareness of this diagnosis. Suspicious lesions in a proper clinical setting should be followed up by imaging studies and confirmatory histopathological diagnosis. Typical follow-up is 3 months after initial presentation to assess stability and possibly 18 to 24 months as well based on Fleischer guidelines.
Various treatment modalities have been tried as per literature, including tocilizumab and rituximab. 11,12 Our patient is currently being treated with golimumab based on outpatient rheumatologist recommendations.
Conclusions
This case demonstrates the importance of a careful workup to narrow a broad differential. Medical diagnosis of pulmonary nodules requires an in-depth workup, including clinical evaluation, laboratory and pulmonary functions tests, as well as various imaging studies.
1. Lung and Bronchus Cancer - Cancer Stat Facts. SEER. Accessed February 2, 2020. https://seer.cancer.gov /statfacts/html/lungb.html
2. Shaughnessy AF. One in Five Patients Overdiagnosed with Lung Cancer Screening. Am Fam Physician. 2014 Jul 15;90(2):112.
3. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369;910-919. doi:10.1056/NEJMoa1214726
4. Stamp LK, Cleland LG. Rheumatoid arthritis. In: Thompson LU, Ward WE, eds. Optimizing Women’s Health through Nutrition. CRC Press; 2008; 279-320.
5. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770-777. doi:10.1164/arrd.1985.131.5.770
6. Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, Turesson C; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601-606. doi:10.1136/ard.2005.039172
7. Shenberger KN, Schned AR, Taylor TH. Rheumatoid disease and bronchogenic carcinoma—case report and review of the literature. J Rheumatol. 1984;11:226–228.
8. Mukhopadhyay S, Wilcox BE, Myers JL, et al. Pulmonary necrotizing granulomas of unknown cause clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813-824. doi:10.1378/chest.12-2113
9. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. Published 2017 Aug 9. doi:10.1183/16000617.0012-2017
10. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448. doi:10.1513/pats.200703-045MS
11. Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72(2):166-171. doi:10.1007/s00393-012-1054-0
12. Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford). 2012;51(6):1132-1134. doi:10.1093/rheumatology/ker455
1. Lung and Bronchus Cancer - Cancer Stat Facts. SEER. Accessed February 2, 2020. https://seer.cancer.gov /statfacts/html/lungb.html
2. Shaughnessy AF. One in Five Patients Overdiagnosed with Lung Cancer Screening. Am Fam Physician. 2014 Jul 15;90(2):112.
3. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369;910-919. doi:10.1056/NEJMoa1214726
4. Stamp LK, Cleland LG. Rheumatoid arthritis. In: Thompson LU, Ward WE, eds. Optimizing Women’s Health through Nutrition. CRC Press; 2008; 279-320.
5. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770-777. doi:10.1164/arrd.1985.131.5.770
6. Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, Turesson C; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601-606. doi:10.1136/ard.2005.039172
7. Shenberger KN, Schned AR, Taylor TH. Rheumatoid disease and bronchogenic carcinoma—case report and review of the literature. J Rheumatol. 1984;11:226–228.
8. Mukhopadhyay S, Wilcox BE, Myers JL, et al. Pulmonary necrotizing granulomas of unknown cause clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813-824. doi:10.1378/chest.12-2113
9. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. Published 2017 Aug 9. doi:10.1183/16000617.0012-2017
10. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448. doi:10.1513/pats.200703-045MS
11. Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72(2):166-171. doi:10.1007/s00393-012-1054-0
12. Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford). 2012;51(6):1132-1134. doi:10.1093/rheumatology/ker455
Medicare insulin negotiations seen saving $17 billion
Medicare could have saved more than $16.7 billion on three kinds of insulin products from 2011 to 2017 if it had secured the same discounts other federal health programs get through negotiations, House Democrats argue in a new report.
On Dec. 10, Democrats on the House Committee on Oversight and Reform released a final majority staff report, which they say is the culmination of an almost 3-year investigation into pharmaceutical pricing and business practices. The report draws from 1.5 million pages of internal company documents, the committee says.
Documents from insulin makers Eli Lilly, Novo Nordisk, and Sanofi indicate these firms “raised their prices in lockstep in order to maintain ‘pricing parity’,” with senior executives encouraging the practice, the committee staff writes in the report.
“In a discussion among Novo Nordisk employees about an Eli Lilly price increase for a different diabetes product on Dec. 24, 2015, a Novo Nordisk pricing analyst remarked, ‘[M]aybe Sanofi will wait until tomorrow morning to announce their price increase ... that’s all I want for Christmas,’” the report states.
House Democrats are seeking to use the report findings to aid their Senate colleagues’ attempt to pass the sweeping Build Back Better bill, which includes many provisions addressing drug costs.
It’s still unclear when the Senate will act on the measure. The House passed the Build Back Better bill, 220-213, in November. It includes a provision that would allow Medicare to negotiate the prices of certain drugs covered by Part D pharmacy plans.
That would mark a reversal of the stance taken when Congress created the pharmacy benefit in a 2003 law, which left negotiations to insurers that cover Part D plans.
Republicans have long argued insurers get the best deals on drugs for people on Medicare. Democrats say this approach sacrifices much of Medicare’s bargaining clout, scattering it among plans.
“This fight has been going on since the Medicare Part D legislation which gave away the store” to drugmakers, said Speaker Nancy Pelosi (D-CA) at a Dec. 10 press conference about the House Oversight report. “And they got used to having the store to themselves.”
The Endocrine Society is urging the Senate to protect the insulin affordability provisions included in the Build Back Better Act and move quickly to pass this crucial legislation.
“We implore all Senators to ensure these provisions are not scaled back. The Build Back Better Act represents the best opportunity to address the price of insulin. Millions of Americans cannot wait any longer for a solution,” it said in a statement issued Dec. 14.
Better deals for military, medicaid programs
Medicare is unusual among federal programs in that it doesn’t directly leverage its clout to lower drug costs.
Total Part D expenditures were approximately $105 billion last year, according to Medicare’s board of trustees. This spending is divided among the many insurers that run Part D plans, which then make a myriad of decisions about formularies and other factors that affect pricing.
For drugs administered by clinicians, and thus covered by Medicare Part B, the program pays a premium of the reported average sales price. Part B drug spending was $39 billion in 2019, an increase of about 11.6% from the previous year, according to the Medicare Payment Advisory Commission.
In contrast, federal law calls for steep reductions in drug prices for people on Medicaid.
The Department of Veterans Affairs (VA) and the Defense Department (DoD)’s Tricare program use several bargaining strategies to lower prices. To control costs, VA and DoD often use formularies of preferred drugs, steer patients to lower-cost drugs, and buy drugs in large volumes, “all of which increase their leverage with drug manufacturers,” the staff of the Congressional Budget Office (CBO) wrote in a Feb. 2021 report.
The CBO report examines how those different federal agencies’ approaches played out in terms of prices, net of applicable rebates, and discounts of 176 top-selling brand-name drugs in Medicare Part D.
The average price for this group of drugs was $118 in Medicaid. And for VA and DoD, the average prices were $190 and $184, respectively, for drugs dispensed at the agencies’ medical facilities or by mail.
But for Medicare Part D, the average price was $343, CBO said in the report, which was one of the sources consulted by House Oversight staff when developing their report released on Dec. 10.
Insulin still of interest, 100 years after its discovery
The House Oversight report runs to almost 270 pages. It addresses several issues with drug prices, including strategies pharmaceutical companies have used to thwart generic competition. On Monday, the trade group America’s Health Insurance Plans separately released its own report looking at patents and delays to the introduction of generic drugs.
Yet, much of the debate on drug prices has focused on one of the oldest widely produced prescription drugs, insulin.
Even with the allowance of generic competition for the essential medicine, branded versions of insulin have been some of the costliest products for Medicare in recent years. Eli Lilly, Novo Nordisk, and Sanofi dominate the insulin market.
Medicare Part D spent about $2.5 billion in 2019 on Sanofi’s Lantus Solostar insulin, or about $2,585 per person in the program using it. The program also paid about $1.1 billion for another form of Lantus, or about $2,746 per patient.
Medicare Part D also spent about $1.84 billion in 2019 on Novo Nordisk’s NovoLog FlexPen, or about $3,063 per person.
Medicare Part D’s drug spending dashboard also lists eight versions of Lilly’s Humalog, with combined 2019 spending of more than $2 billion. The cost per patient in Medicare Part D ranges from $5,619 to $1,462.
“Over the past 20 years, they have repeatedly and dramatically raised the list prices of their rapid-acting and long-acting insulins and reaped billions of dollars in revenues,” write the House Oversight staff in their report.
Republicans on the House Oversight and Reform Committee disagree with their Democratic colleagues on many points in the debate on drug prices, but they also looked at insulin as a cause for concern.
GOP members of the committee released a separate report on Dec. 10. They call for greater clarity into the role middlemen in the drug-supply chain – known as pharmaceutical benefit managers – may play in the rising costs of medicines. The GOP report notes that there are bills pending in the House that would seek to steer any discounts offered on insulin within the supply chain toward consumers (Insulin Price Reduction Act H.R. 4906, Insulin Cost Reduction Act H.R. 5623).
Democratic staff in the committee’s report seek to draw attention to how manufacturers priced their insulin products, including the comment by the Novo Nordisk employee about wishing for a price hike for a competitor’s product.
In a statement provided to this news organization, Novo Nordisk said the committee’s report reflects “a limited picture of the efforts put forth by our company and other companies to manage formulary access.”
“This glimpse into the complexity of pricing, formularies, and the health care system demonstrates why Novo Nordisk continues to advocate for comprehensive solutions,” Denmark’s Novo Nordisk said in the statement.
$35 a month for insulin?
Paris-based Sanofi said it makes insulin-pricing decisions independently from competitors. Sanofi said the net price of its insulins has declined by 53% since 2012, arguing the high prices charged to patients reflect decisions made elsewhere in the supply chain.
“Over the same period, the net price for commercial and Medicare Part D plans of Lantus has fallen 44.9%, while average out-of-pocket costs for patients with commercial insurance and Medicare Part D has risen approximately 82%,” Sanofi said.
“For all the focus on the growth of list prices, today, the average net price of Lantus is below 2006 levels. That is why we support policy reforms to require health plans to share negotiated savings with patients by requiring patient cost-sharing be tied to the net prices.”
Indianapolis-based Lilly offered a similar response in a statement to this news organization.
“Lilly, like other companies, monitors competitor list-price changes that are available through publicly available services,” the company said. “However, any changes we make to our list prices are independent decisions, and to the extent they consider competitors they are informed only through publicly available data.”
Despite rising insurance deductibles, the average monthly out-of-pocket cost for Lilly insulin has dropped 27% to $28.05 over the past 4 years, the company said in an interview. Lilly also noted that there are “several affordability options now available” allowing people to purchase their monthly prescription of its insulin for $35, “whether they are uninsured or use commercial insurance, Medicaid, or a participating Medicare Part D plan.”
In 2020, Lilly had announced that people with commercial insurance and those without insurance would be able to get monthly prescriptions of Lilly insulin for $35.
The Build Back Better Act would require insurers, including Medicare Part D plans and private group or individual health plans, to charge patient cost-sharing of no more than $35 per month for insulin products, said the staff of the nonprofit Kaiser Family Foundation (KFF) in a review of the bill.
“Private group or individual plans would not be required to cover all insulin products, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting), for no more than $35,” the KFF staff state in the report.
People enrolled in Medicare can already choose to enroll in a Part D plan participating in a federal test program that can secure certain insulin products for them at a monthly copayment of $35. In 2022, a total of 2,159 Part D plans will participate in this model, a 32% increase in participating plans since 2021, KFF said.
A version of this article first appeared on Medscape.com.
Medicare could have saved more than $16.7 billion on three kinds of insulin products from 2011 to 2017 if it had secured the same discounts other federal health programs get through negotiations, House Democrats argue in a new report.
On Dec. 10, Democrats on the House Committee on Oversight and Reform released a final majority staff report, which they say is the culmination of an almost 3-year investigation into pharmaceutical pricing and business practices. The report draws from 1.5 million pages of internal company documents, the committee says.
Documents from insulin makers Eli Lilly, Novo Nordisk, and Sanofi indicate these firms “raised their prices in lockstep in order to maintain ‘pricing parity’,” with senior executives encouraging the practice, the committee staff writes in the report.
“In a discussion among Novo Nordisk employees about an Eli Lilly price increase for a different diabetes product on Dec. 24, 2015, a Novo Nordisk pricing analyst remarked, ‘[M]aybe Sanofi will wait until tomorrow morning to announce their price increase ... that’s all I want for Christmas,’” the report states.
House Democrats are seeking to use the report findings to aid their Senate colleagues’ attempt to pass the sweeping Build Back Better bill, which includes many provisions addressing drug costs.
It’s still unclear when the Senate will act on the measure. The House passed the Build Back Better bill, 220-213, in November. It includes a provision that would allow Medicare to negotiate the prices of certain drugs covered by Part D pharmacy plans.
That would mark a reversal of the stance taken when Congress created the pharmacy benefit in a 2003 law, which left negotiations to insurers that cover Part D plans.
Republicans have long argued insurers get the best deals on drugs for people on Medicare. Democrats say this approach sacrifices much of Medicare’s bargaining clout, scattering it among plans.
“This fight has been going on since the Medicare Part D legislation which gave away the store” to drugmakers, said Speaker Nancy Pelosi (D-CA) at a Dec. 10 press conference about the House Oversight report. “And they got used to having the store to themselves.”
The Endocrine Society is urging the Senate to protect the insulin affordability provisions included in the Build Back Better Act and move quickly to pass this crucial legislation.
“We implore all Senators to ensure these provisions are not scaled back. The Build Back Better Act represents the best opportunity to address the price of insulin. Millions of Americans cannot wait any longer for a solution,” it said in a statement issued Dec. 14.
Better deals for military, medicaid programs
Medicare is unusual among federal programs in that it doesn’t directly leverage its clout to lower drug costs.
Total Part D expenditures were approximately $105 billion last year, according to Medicare’s board of trustees. This spending is divided among the many insurers that run Part D plans, which then make a myriad of decisions about formularies and other factors that affect pricing.
For drugs administered by clinicians, and thus covered by Medicare Part B, the program pays a premium of the reported average sales price. Part B drug spending was $39 billion in 2019, an increase of about 11.6% from the previous year, according to the Medicare Payment Advisory Commission.
In contrast, federal law calls for steep reductions in drug prices for people on Medicaid.
The Department of Veterans Affairs (VA) and the Defense Department (DoD)’s Tricare program use several bargaining strategies to lower prices. To control costs, VA and DoD often use formularies of preferred drugs, steer patients to lower-cost drugs, and buy drugs in large volumes, “all of which increase their leverage with drug manufacturers,” the staff of the Congressional Budget Office (CBO) wrote in a Feb. 2021 report.
The CBO report examines how those different federal agencies’ approaches played out in terms of prices, net of applicable rebates, and discounts of 176 top-selling brand-name drugs in Medicare Part D.
The average price for this group of drugs was $118 in Medicaid. And for VA and DoD, the average prices were $190 and $184, respectively, for drugs dispensed at the agencies’ medical facilities or by mail.
But for Medicare Part D, the average price was $343, CBO said in the report, which was one of the sources consulted by House Oversight staff when developing their report released on Dec. 10.
Insulin still of interest, 100 years after its discovery
The House Oversight report runs to almost 270 pages. It addresses several issues with drug prices, including strategies pharmaceutical companies have used to thwart generic competition. On Monday, the trade group America’s Health Insurance Plans separately released its own report looking at patents and delays to the introduction of generic drugs.
Yet, much of the debate on drug prices has focused on one of the oldest widely produced prescription drugs, insulin.
Even with the allowance of generic competition for the essential medicine, branded versions of insulin have been some of the costliest products for Medicare in recent years. Eli Lilly, Novo Nordisk, and Sanofi dominate the insulin market.
Medicare Part D spent about $2.5 billion in 2019 on Sanofi’s Lantus Solostar insulin, or about $2,585 per person in the program using it. The program also paid about $1.1 billion for another form of Lantus, or about $2,746 per patient.
Medicare Part D also spent about $1.84 billion in 2019 on Novo Nordisk’s NovoLog FlexPen, or about $3,063 per person.
Medicare Part D’s drug spending dashboard also lists eight versions of Lilly’s Humalog, with combined 2019 spending of more than $2 billion. The cost per patient in Medicare Part D ranges from $5,619 to $1,462.
“Over the past 20 years, they have repeatedly and dramatically raised the list prices of their rapid-acting and long-acting insulins and reaped billions of dollars in revenues,” write the House Oversight staff in their report.
Republicans on the House Oversight and Reform Committee disagree with their Democratic colleagues on many points in the debate on drug prices, but they also looked at insulin as a cause for concern.
GOP members of the committee released a separate report on Dec. 10. They call for greater clarity into the role middlemen in the drug-supply chain – known as pharmaceutical benefit managers – may play in the rising costs of medicines. The GOP report notes that there are bills pending in the House that would seek to steer any discounts offered on insulin within the supply chain toward consumers (Insulin Price Reduction Act H.R. 4906, Insulin Cost Reduction Act H.R. 5623).
Democratic staff in the committee’s report seek to draw attention to how manufacturers priced their insulin products, including the comment by the Novo Nordisk employee about wishing for a price hike for a competitor’s product.
In a statement provided to this news organization, Novo Nordisk said the committee’s report reflects “a limited picture of the efforts put forth by our company and other companies to manage formulary access.”
“This glimpse into the complexity of pricing, formularies, and the health care system demonstrates why Novo Nordisk continues to advocate for comprehensive solutions,” Denmark’s Novo Nordisk said in the statement.
$35 a month for insulin?
Paris-based Sanofi said it makes insulin-pricing decisions independently from competitors. Sanofi said the net price of its insulins has declined by 53% since 2012, arguing the high prices charged to patients reflect decisions made elsewhere in the supply chain.
“Over the same period, the net price for commercial and Medicare Part D plans of Lantus has fallen 44.9%, while average out-of-pocket costs for patients with commercial insurance and Medicare Part D has risen approximately 82%,” Sanofi said.
“For all the focus on the growth of list prices, today, the average net price of Lantus is below 2006 levels. That is why we support policy reforms to require health plans to share negotiated savings with patients by requiring patient cost-sharing be tied to the net prices.”
Indianapolis-based Lilly offered a similar response in a statement to this news organization.
“Lilly, like other companies, monitors competitor list-price changes that are available through publicly available services,” the company said. “However, any changes we make to our list prices are independent decisions, and to the extent they consider competitors they are informed only through publicly available data.”
Despite rising insurance deductibles, the average monthly out-of-pocket cost for Lilly insulin has dropped 27% to $28.05 over the past 4 years, the company said in an interview. Lilly also noted that there are “several affordability options now available” allowing people to purchase their monthly prescription of its insulin for $35, “whether they are uninsured or use commercial insurance, Medicaid, or a participating Medicare Part D plan.”
In 2020, Lilly had announced that people with commercial insurance and those without insurance would be able to get monthly prescriptions of Lilly insulin for $35.
The Build Back Better Act would require insurers, including Medicare Part D plans and private group or individual health plans, to charge patient cost-sharing of no more than $35 per month for insulin products, said the staff of the nonprofit Kaiser Family Foundation (KFF) in a review of the bill.
“Private group or individual plans would not be required to cover all insulin products, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting), for no more than $35,” the KFF staff state in the report.
People enrolled in Medicare can already choose to enroll in a Part D plan participating in a federal test program that can secure certain insulin products for them at a monthly copayment of $35. In 2022, a total of 2,159 Part D plans will participate in this model, a 32% increase in participating plans since 2021, KFF said.
A version of this article first appeared on Medscape.com.
Medicare could have saved more than $16.7 billion on three kinds of insulin products from 2011 to 2017 if it had secured the same discounts other federal health programs get through negotiations, House Democrats argue in a new report.
On Dec. 10, Democrats on the House Committee on Oversight and Reform released a final majority staff report, which they say is the culmination of an almost 3-year investigation into pharmaceutical pricing and business practices. The report draws from 1.5 million pages of internal company documents, the committee says.
Documents from insulin makers Eli Lilly, Novo Nordisk, and Sanofi indicate these firms “raised their prices in lockstep in order to maintain ‘pricing parity’,” with senior executives encouraging the practice, the committee staff writes in the report.
“In a discussion among Novo Nordisk employees about an Eli Lilly price increase for a different diabetes product on Dec. 24, 2015, a Novo Nordisk pricing analyst remarked, ‘[M]aybe Sanofi will wait until tomorrow morning to announce their price increase ... that’s all I want for Christmas,’” the report states.
House Democrats are seeking to use the report findings to aid their Senate colleagues’ attempt to pass the sweeping Build Back Better bill, which includes many provisions addressing drug costs.
It’s still unclear when the Senate will act on the measure. The House passed the Build Back Better bill, 220-213, in November. It includes a provision that would allow Medicare to negotiate the prices of certain drugs covered by Part D pharmacy plans.
That would mark a reversal of the stance taken when Congress created the pharmacy benefit in a 2003 law, which left negotiations to insurers that cover Part D plans.
Republicans have long argued insurers get the best deals on drugs for people on Medicare. Democrats say this approach sacrifices much of Medicare’s bargaining clout, scattering it among plans.
“This fight has been going on since the Medicare Part D legislation which gave away the store” to drugmakers, said Speaker Nancy Pelosi (D-CA) at a Dec. 10 press conference about the House Oversight report. “And they got used to having the store to themselves.”
The Endocrine Society is urging the Senate to protect the insulin affordability provisions included in the Build Back Better Act and move quickly to pass this crucial legislation.
“We implore all Senators to ensure these provisions are not scaled back. The Build Back Better Act represents the best opportunity to address the price of insulin. Millions of Americans cannot wait any longer for a solution,” it said in a statement issued Dec. 14.
Better deals for military, medicaid programs
Medicare is unusual among federal programs in that it doesn’t directly leverage its clout to lower drug costs.
Total Part D expenditures were approximately $105 billion last year, according to Medicare’s board of trustees. This spending is divided among the many insurers that run Part D plans, which then make a myriad of decisions about formularies and other factors that affect pricing.
For drugs administered by clinicians, and thus covered by Medicare Part B, the program pays a premium of the reported average sales price. Part B drug spending was $39 billion in 2019, an increase of about 11.6% from the previous year, according to the Medicare Payment Advisory Commission.
In contrast, federal law calls for steep reductions in drug prices for people on Medicaid.
The Department of Veterans Affairs (VA) and the Defense Department (DoD)’s Tricare program use several bargaining strategies to lower prices. To control costs, VA and DoD often use formularies of preferred drugs, steer patients to lower-cost drugs, and buy drugs in large volumes, “all of which increase their leverage with drug manufacturers,” the staff of the Congressional Budget Office (CBO) wrote in a Feb. 2021 report.
The CBO report examines how those different federal agencies’ approaches played out in terms of prices, net of applicable rebates, and discounts of 176 top-selling brand-name drugs in Medicare Part D.
The average price for this group of drugs was $118 in Medicaid. And for VA and DoD, the average prices were $190 and $184, respectively, for drugs dispensed at the agencies’ medical facilities or by mail.
But for Medicare Part D, the average price was $343, CBO said in the report, which was one of the sources consulted by House Oversight staff when developing their report released on Dec. 10.
Insulin still of interest, 100 years after its discovery
The House Oversight report runs to almost 270 pages. It addresses several issues with drug prices, including strategies pharmaceutical companies have used to thwart generic competition. On Monday, the trade group America’s Health Insurance Plans separately released its own report looking at patents and delays to the introduction of generic drugs.
Yet, much of the debate on drug prices has focused on one of the oldest widely produced prescription drugs, insulin.
Even with the allowance of generic competition for the essential medicine, branded versions of insulin have been some of the costliest products for Medicare in recent years. Eli Lilly, Novo Nordisk, and Sanofi dominate the insulin market.
Medicare Part D spent about $2.5 billion in 2019 on Sanofi’s Lantus Solostar insulin, or about $2,585 per person in the program using it. The program also paid about $1.1 billion for another form of Lantus, or about $2,746 per patient.
Medicare Part D also spent about $1.84 billion in 2019 on Novo Nordisk’s NovoLog FlexPen, or about $3,063 per person.
Medicare Part D’s drug spending dashboard also lists eight versions of Lilly’s Humalog, with combined 2019 spending of more than $2 billion. The cost per patient in Medicare Part D ranges from $5,619 to $1,462.
“Over the past 20 years, they have repeatedly and dramatically raised the list prices of their rapid-acting and long-acting insulins and reaped billions of dollars in revenues,” write the House Oversight staff in their report.
Republicans on the House Oversight and Reform Committee disagree with their Democratic colleagues on many points in the debate on drug prices, but they also looked at insulin as a cause for concern.
GOP members of the committee released a separate report on Dec. 10. They call for greater clarity into the role middlemen in the drug-supply chain – known as pharmaceutical benefit managers – may play in the rising costs of medicines. The GOP report notes that there are bills pending in the House that would seek to steer any discounts offered on insulin within the supply chain toward consumers (Insulin Price Reduction Act H.R. 4906, Insulin Cost Reduction Act H.R. 5623).
Democratic staff in the committee’s report seek to draw attention to how manufacturers priced their insulin products, including the comment by the Novo Nordisk employee about wishing for a price hike for a competitor’s product.
In a statement provided to this news organization, Novo Nordisk said the committee’s report reflects “a limited picture of the efforts put forth by our company and other companies to manage formulary access.”
“This glimpse into the complexity of pricing, formularies, and the health care system demonstrates why Novo Nordisk continues to advocate for comprehensive solutions,” Denmark’s Novo Nordisk said in the statement.
$35 a month for insulin?
Paris-based Sanofi said it makes insulin-pricing decisions independently from competitors. Sanofi said the net price of its insulins has declined by 53% since 2012, arguing the high prices charged to patients reflect decisions made elsewhere in the supply chain.
“Over the same period, the net price for commercial and Medicare Part D plans of Lantus has fallen 44.9%, while average out-of-pocket costs for patients with commercial insurance and Medicare Part D has risen approximately 82%,” Sanofi said.
“For all the focus on the growth of list prices, today, the average net price of Lantus is below 2006 levels. That is why we support policy reforms to require health plans to share negotiated savings with patients by requiring patient cost-sharing be tied to the net prices.”
Indianapolis-based Lilly offered a similar response in a statement to this news organization.
“Lilly, like other companies, monitors competitor list-price changes that are available through publicly available services,” the company said. “However, any changes we make to our list prices are independent decisions, and to the extent they consider competitors they are informed only through publicly available data.”
Despite rising insurance deductibles, the average monthly out-of-pocket cost for Lilly insulin has dropped 27% to $28.05 over the past 4 years, the company said in an interview. Lilly also noted that there are “several affordability options now available” allowing people to purchase their monthly prescription of its insulin for $35, “whether they are uninsured or use commercial insurance, Medicaid, or a participating Medicare Part D plan.”
In 2020, Lilly had announced that people with commercial insurance and those without insurance would be able to get monthly prescriptions of Lilly insulin for $35.
The Build Back Better Act would require insurers, including Medicare Part D plans and private group or individual health plans, to charge patient cost-sharing of no more than $35 per month for insulin products, said the staff of the nonprofit Kaiser Family Foundation (KFF) in a review of the bill.
“Private group or individual plans would not be required to cover all insulin products, just one of each dosage form (vial, pen) and insulin type (rapid-acting, short-acting, intermediate-acting, and long-acting), for no more than $35,” the KFF staff state in the report.
People enrolled in Medicare can already choose to enroll in a Part D plan participating in a federal test program that can secure certain insulin products for them at a monthly copayment of $35. In 2022, a total of 2,159 Part D plans will participate in this model, a 32% increase in participating plans since 2021, KFF said.
A version of this article first appeared on Medscape.com.