User login
Pembrolizumab improves event-free survival in early TNBC
The original trial data in more than 1,100 patients with early-stage TNBC indicated that adding pembrolizumab to chemotherapy prior to surgery and giving the drug for a year afterward improves event-free survival (EFS) over placebo by 37%.
Now, the researchers conducted a series of prespecified sensitivity and subgroup analyses, finding remarkably consistent EFS outcomes whether considering the addition of adjuvant chemotherapy, positive surgical margins, or disease characteristics such as nodal status and disease stage.
The analyses showed that the benefit with pembrolizumab over placebo was “robust,” said study presenter Peter Schmid, MD, PhD, Centre for Experimental Cancer Medicine, Barts Cancer Institute, Queen Mary University of London.
“These results further support pembrolizumab plus platinum-containing neoadjuvant chemotherapy followed by adjuvant pembrolizumab after surgery as a new standard of care treatment regimen for patients with high-risk, early-stage TNBC,” he said.
The research was presented at the San Antonio Breast Cancer Symposium on Dec. 7.
Hope S. Rugo, MD, who was invited to comment on the findings, noted that, while the sensitivity analyses showed the benefit with pembrolizumab was seen across the board, the numbers in each group of interest were “very small, making any impact unlikely.”
She continued that there also remain a number of unanswered questions, chief among them being: “Does everybody need a checkpoint inhibitor? Perhaps studies ... could help us understand which patients might do well with chemotherapy alone.”
Dr. Rugo, who is professor of medicine in the division of hematology and oncology at the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco, , added that “we need to understand the balance of risk and toxicity” asking whether there are patients whose risk of an immunotoxicity is “so high that we should not give them a checkpoint inhibitor.”
It is not clear what constitutes the optimal chemotherapy backbone. “Does everybody need carboplatin? Does everyone need a year of pembrolizumab, even with a pathologic complete response given the intriguing data from GeparNUEVO and previously the I-SPY trial?” she asked.
“Of course, we don’t know the answers to those questions,” she said, but it is nevertheless possible to draw a roadmap for the treatment of early TNBC, although the choice of adjuvant therapy following surgery is less clear.
Dr. Rugo conducted a Twitter poll to canvas opinion on what to give to patients following surgery, depending on whether or not they have a pathological complete response.
At 73%, most of almost 200 respondents said patients with a pathological complete response should continue pembrolizumab for 1 year, while 72% said that patients without a pathological complete response should receive combination therapy of pembrolizumab and either capecitabine or olaparib, depending on mutational status.
Dr. Schmid began his presentation by noting that KEYNOTE-522 was the first prospective, randomized, phase 3 trial of pembrolizumab in early TNBC in the neoadjuvant and adjuvant setting.
Previously presented results showed that adding neoadjuvant pembrolizumab to chemotherapy was associated with a clinically meaningful increase in pathological complete response, while continuing with adjuvant chemotherapy after surgery led to a clinically meaningful improvement in EFS.
Consequently, the Food and Drug Administration approved pembrolizumab in this setting for patients with high-risk early-stage TNBC.
He reminded the audience that the trial included 1,174 patients randomized 2:1 to pembrolizumab or placebo every 3 weeks alongside eight cycles of chemotherapy, followed by pembrolizumab over placebo alone for up to nine cycles after undergoing definitive surgery.
After a median follow-up of 39.1 months, 15.7% of patients treated with pembrolizumab experienced an event versus 23.9% of those in the placebo group, at a hazard ratio of 0.63 (P = .00031). At 36 months, the EFS rate was 84.5% with pembrolizumab and 76.8% in patients treated with placebo.
Dr. Schmid said that they then performed five prespecified sensitivity analyses, which revealed that the results were “consistent with the primary EFS in all five sensitivity analyses, showing the robustnesses of the event-free survival benefit in the pembrolizumab arm.”
The first analysis, he continued, is of “particular interest as it considered the impact of postsurgery new anticancer therapy. For example, the use of adjuvant capecitabine.”
Censoring 31 patients from the pembrolizumab arm who received the drug and 13 of those given placebo, the team found that the hazard ratio for EFS for pembrolizumab versus placebo was 0.64.
Removing “positive margin at last surgery” as part of the definition of EFS also did not change the results substantially, with the HR for EFS for pembrolizumab versus placebo at 0.65.
Subgroup analysis revealed “consistent EFS results,” Dr. Schmid said, irrespective of whether stratifying the patients by nodal status, overall disease stage, menopausal status, HER2 status, or lactate dehydrogenase levels.
While patients in both treatment arms who had nodal involvement had worse outcomes than those without, those in the pembrolizumab arm “still had improved outcomes, compared with placebo, suggesting that it provides benefit regardless of nodal status.”
“Similarly, the EFS benefit with pembrolizumab was irrespective of disease stage,” Dr. Schmid said. Although the EFS improvement was greater in patients with stage II rather than III disease, at a HR of 0.60 versus 0.68, it highlights “the importance of early intervention.”
He said that the “rate of adverse events with pembrolizumab was low, especially in the adjuvant setting.”
Following his presentation, Dr. Schmid was asked whether he would consider retrying immunotherapy in patients after progression on pembrolizumab.
He replied that this is currently a “data-free zone.”
However, he said: “If a patient responded immunotherapy initially, had a disease-free interval and then has recurrence, then I would consider, if the patient is PD-L1 [programmed death–ligand 1] positive, at that time to add immunotherapy. We can’t say whether those patients will derive the same benefit” as that seen in randomized controlled trials in later stage TNBC, he added, “but there is, in my opinion, little to lose, especially if we have already established the patient tolerates immunotherapy well in that setting.”
Dr. Schmid continued that he “personally found it reassuring” that, in the current study, even patients without a complete pathological response “still showed a substantially better event-free survival compared to patients without immunotherapy, so I personally would consider immunotherapy for those patients when they relapse but we can discuss what the optimal disease-free interval is.”
The study was funded by Merck Sharp and Dohme. Both Dr. Rugo and Dr. Schmid reported relationships numerous pharmaceutical companies.
.
The original trial data in more than 1,100 patients with early-stage TNBC indicated that adding pembrolizumab to chemotherapy prior to surgery and giving the drug for a year afterward improves event-free survival (EFS) over placebo by 37%.
Now, the researchers conducted a series of prespecified sensitivity and subgroup analyses, finding remarkably consistent EFS outcomes whether considering the addition of adjuvant chemotherapy, positive surgical margins, or disease characteristics such as nodal status and disease stage.
The analyses showed that the benefit with pembrolizumab over placebo was “robust,” said study presenter Peter Schmid, MD, PhD, Centre for Experimental Cancer Medicine, Barts Cancer Institute, Queen Mary University of London.
“These results further support pembrolizumab plus platinum-containing neoadjuvant chemotherapy followed by adjuvant pembrolizumab after surgery as a new standard of care treatment regimen for patients with high-risk, early-stage TNBC,” he said.
The research was presented at the San Antonio Breast Cancer Symposium on Dec. 7.
Hope S. Rugo, MD, who was invited to comment on the findings, noted that, while the sensitivity analyses showed the benefit with pembrolizumab was seen across the board, the numbers in each group of interest were “very small, making any impact unlikely.”
She continued that there also remain a number of unanswered questions, chief among them being: “Does everybody need a checkpoint inhibitor? Perhaps studies ... could help us understand which patients might do well with chemotherapy alone.”
Dr. Rugo, who is professor of medicine in the division of hematology and oncology at the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco, , added that “we need to understand the balance of risk and toxicity” asking whether there are patients whose risk of an immunotoxicity is “so high that we should not give them a checkpoint inhibitor.”
It is not clear what constitutes the optimal chemotherapy backbone. “Does everybody need carboplatin? Does everyone need a year of pembrolizumab, even with a pathologic complete response given the intriguing data from GeparNUEVO and previously the I-SPY trial?” she asked.
“Of course, we don’t know the answers to those questions,” she said, but it is nevertheless possible to draw a roadmap for the treatment of early TNBC, although the choice of adjuvant therapy following surgery is less clear.
Dr. Rugo conducted a Twitter poll to canvas opinion on what to give to patients following surgery, depending on whether or not they have a pathological complete response.
At 73%, most of almost 200 respondents said patients with a pathological complete response should continue pembrolizumab for 1 year, while 72% said that patients without a pathological complete response should receive combination therapy of pembrolizumab and either capecitabine or olaparib, depending on mutational status.
Dr. Schmid began his presentation by noting that KEYNOTE-522 was the first prospective, randomized, phase 3 trial of pembrolizumab in early TNBC in the neoadjuvant and adjuvant setting.
Previously presented results showed that adding neoadjuvant pembrolizumab to chemotherapy was associated with a clinically meaningful increase in pathological complete response, while continuing with adjuvant chemotherapy after surgery led to a clinically meaningful improvement in EFS.
Consequently, the Food and Drug Administration approved pembrolizumab in this setting for patients with high-risk early-stage TNBC.
He reminded the audience that the trial included 1,174 patients randomized 2:1 to pembrolizumab or placebo every 3 weeks alongside eight cycles of chemotherapy, followed by pembrolizumab over placebo alone for up to nine cycles after undergoing definitive surgery.
After a median follow-up of 39.1 months, 15.7% of patients treated with pembrolizumab experienced an event versus 23.9% of those in the placebo group, at a hazard ratio of 0.63 (P = .00031). At 36 months, the EFS rate was 84.5% with pembrolizumab and 76.8% in patients treated with placebo.
Dr. Schmid said that they then performed five prespecified sensitivity analyses, which revealed that the results were “consistent with the primary EFS in all five sensitivity analyses, showing the robustnesses of the event-free survival benefit in the pembrolizumab arm.”
The first analysis, he continued, is of “particular interest as it considered the impact of postsurgery new anticancer therapy. For example, the use of adjuvant capecitabine.”
Censoring 31 patients from the pembrolizumab arm who received the drug and 13 of those given placebo, the team found that the hazard ratio for EFS for pembrolizumab versus placebo was 0.64.
Removing “positive margin at last surgery” as part of the definition of EFS also did not change the results substantially, with the HR for EFS for pembrolizumab versus placebo at 0.65.
Subgroup analysis revealed “consistent EFS results,” Dr. Schmid said, irrespective of whether stratifying the patients by nodal status, overall disease stage, menopausal status, HER2 status, or lactate dehydrogenase levels.
While patients in both treatment arms who had nodal involvement had worse outcomes than those without, those in the pembrolizumab arm “still had improved outcomes, compared with placebo, suggesting that it provides benefit regardless of nodal status.”
“Similarly, the EFS benefit with pembrolizumab was irrespective of disease stage,” Dr. Schmid said. Although the EFS improvement was greater in patients with stage II rather than III disease, at a HR of 0.60 versus 0.68, it highlights “the importance of early intervention.”
He said that the “rate of adverse events with pembrolizumab was low, especially in the adjuvant setting.”
Following his presentation, Dr. Schmid was asked whether he would consider retrying immunotherapy in patients after progression on pembrolizumab.
He replied that this is currently a “data-free zone.”
However, he said: “If a patient responded immunotherapy initially, had a disease-free interval and then has recurrence, then I would consider, if the patient is PD-L1 [programmed death–ligand 1] positive, at that time to add immunotherapy. We can’t say whether those patients will derive the same benefit” as that seen in randomized controlled trials in later stage TNBC, he added, “but there is, in my opinion, little to lose, especially if we have already established the patient tolerates immunotherapy well in that setting.”
Dr. Schmid continued that he “personally found it reassuring” that, in the current study, even patients without a complete pathological response “still showed a substantially better event-free survival compared to patients without immunotherapy, so I personally would consider immunotherapy for those patients when they relapse but we can discuss what the optimal disease-free interval is.”
The study was funded by Merck Sharp and Dohme. Both Dr. Rugo and Dr. Schmid reported relationships numerous pharmaceutical companies.
.
The original trial data in more than 1,100 patients with early-stage TNBC indicated that adding pembrolizumab to chemotherapy prior to surgery and giving the drug for a year afterward improves event-free survival (EFS) over placebo by 37%.
Now, the researchers conducted a series of prespecified sensitivity and subgroup analyses, finding remarkably consistent EFS outcomes whether considering the addition of adjuvant chemotherapy, positive surgical margins, or disease characteristics such as nodal status and disease stage.
The analyses showed that the benefit with pembrolizumab over placebo was “robust,” said study presenter Peter Schmid, MD, PhD, Centre for Experimental Cancer Medicine, Barts Cancer Institute, Queen Mary University of London.
“These results further support pembrolizumab plus platinum-containing neoadjuvant chemotherapy followed by adjuvant pembrolizumab after surgery as a new standard of care treatment regimen for patients with high-risk, early-stage TNBC,” he said.
The research was presented at the San Antonio Breast Cancer Symposium on Dec. 7.
Hope S. Rugo, MD, who was invited to comment on the findings, noted that, while the sensitivity analyses showed the benefit with pembrolizumab was seen across the board, the numbers in each group of interest were “very small, making any impact unlikely.”
She continued that there also remain a number of unanswered questions, chief among them being: “Does everybody need a checkpoint inhibitor? Perhaps studies ... could help us understand which patients might do well with chemotherapy alone.”
Dr. Rugo, who is professor of medicine in the division of hematology and oncology at the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco, , added that “we need to understand the balance of risk and toxicity” asking whether there are patients whose risk of an immunotoxicity is “so high that we should not give them a checkpoint inhibitor.”
It is not clear what constitutes the optimal chemotherapy backbone. “Does everybody need carboplatin? Does everyone need a year of pembrolizumab, even with a pathologic complete response given the intriguing data from GeparNUEVO and previously the I-SPY trial?” she asked.
“Of course, we don’t know the answers to those questions,” she said, but it is nevertheless possible to draw a roadmap for the treatment of early TNBC, although the choice of adjuvant therapy following surgery is less clear.
Dr. Rugo conducted a Twitter poll to canvas opinion on what to give to patients following surgery, depending on whether or not they have a pathological complete response.
At 73%, most of almost 200 respondents said patients with a pathological complete response should continue pembrolizumab for 1 year, while 72% said that patients without a pathological complete response should receive combination therapy of pembrolizumab and either capecitabine or olaparib, depending on mutational status.
Dr. Schmid began his presentation by noting that KEYNOTE-522 was the first prospective, randomized, phase 3 trial of pembrolizumab in early TNBC in the neoadjuvant and adjuvant setting.
Previously presented results showed that adding neoadjuvant pembrolizumab to chemotherapy was associated with a clinically meaningful increase in pathological complete response, while continuing with adjuvant chemotherapy after surgery led to a clinically meaningful improvement in EFS.
Consequently, the Food and Drug Administration approved pembrolizumab in this setting for patients with high-risk early-stage TNBC.
He reminded the audience that the trial included 1,174 patients randomized 2:1 to pembrolizumab or placebo every 3 weeks alongside eight cycles of chemotherapy, followed by pembrolizumab over placebo alone for up to nine cycles after undergoing definitive surgery.
After a median follow-up of 39.1 months, 15.7% of patients treated with pembrolizumab experienced an event versus 23.9% of those in the placebo group, at a hazard ratio of 0.63 (P = .00031). At 36 months, the EFS rate was 84.5% with pembrolizumab and 76.8% in patients treated with placebo.
Dr. Schmid said that they then performed five prespecified sensitivity analyses, which revealed that the results were “consistent with the primary EFS in all five sensitivity analyses, showing the robustnesses of the event-free survival benefit in the pembrolizumab arm.”
The first analysis, he continued, is of “particular interest as it considered the impact of postsurgery new anticancer therapy. For example, the use of adjuvant capecitabine.”
Censoring 31 patients from the pembrolizumab arm who received the drug and 13 of those given placebo, the team found that the hazard ratio for EFS for pembrolizumab versus placebo was 0.64.
Removing “positive margin at last surgery” as part of the definition of EFS also did not change the results substantially, with the HR for EFS for pembrolizumab versus placebo at 0.65.
Subgroup analysis revealed “consistent EFS results,” Dr. Schmid said, irrespective of whether stratifying the patients by nodal status, overall disease stage, menopausal status, HER2 status, or lactate dehydrogenase levels.
While patients in both treatment arms who had nodal involvement had worse outcomes than those without, those in the pembrolizumab arm “still had improved outcomes, compared with placebo, suggesting that it provides benefit regardless of nodal status.”
“Similarly, the EFS benefit with pembrolizumab was irrespective of disease stage,” Dr. Schmid said. Although the EFS improvement was greater in patients with stage II rather than III disease, at a HR of 0.60 versus 0.68, it highlights “the importance of early intervention.”
He said that the “rate of adverse events with pembrolizumab was low, especially in the adjuvant setting.”
Following his presentation, Dr. Schmid was asked whether he would consider retrying immunotherapy in patients after progression on pembrolizumab.
He replied that this is currently a “data-free zone.”
However, he said: “If a patient responded immunotherapy initially, had a disease-free interval and then has recurrence, then I would consider, if the patient is PD-L1 [programmed death–ligand 1] positive, at that time to add immunotherapy. We can’t say whether those patients will derive the same benefit” as that seen in randomized controlled trials in later stage TNBC, he added, “but there is, in my opinion, little to lose, especially if we have already established the patient tolerates immunotherapy well in that setting.”
Dr. Schmid continued that he “personally found it reassuring” that, in the current study, even patients without a complete pathological response “still showed a substantially better event-free survival compared to patients without immunotherapy, so I personally would consider immunotherapy for those patients when they relapse but we can discuss what the optimal disease-free interval is.”
The study was funded by Merck Sharp and Dohme. Both Dr. Rugo and Dr. Schmid reported relationships numerous pharmaceutical companies.
.
FROM SABCS 2021
ASH studies look at racial disparities in ALL care, outcomes
Among almost 25,000 children and young adults up to 31 years old, all of whom participated in Children’s Oncology Group studies since 2004, 5-year event free survival (EFS) was 87.4% for White patients, 82.8% for Hispanic patients, and 81.9% for Black patients.
When socioeconomics and disease characteristics such as CNS involvement, white blood cell lineage, and induction status were taken into account, the risk of having a survival event fell from 37% to 11% higher for Hispanic patients versus White patients but from 45% to 32% for Black patients versus White patients.
However, there was no explicit adjustment in the study for acuity at presentation, body mass index, adherence to protocols, or Philadelphia chromosome (PH)-like disease, which is more common among Hispanic patients.
Even so, lead investigator Sumit Gupta, MD, a pediatric blood cancer specialist at the University of Toronto, said that even with the potential confounders, lingering differences in outcomes raise questions about equal access to care and other matters, and suggest that there are still “uncomfortable things to consider, things like ... structural racism” and a system that delivers “systemically different care to patients across racial” groups.
Another report presented at the meeting with 295 patients 18-40 years old found that Hispanic patients had 3-year overall survival comparable to that of White patients despite a higher prevalence of PH-like disease, perhaps because Hispanic patients had higher treatment adherence than did White patients at 76% versus 56%, said lead investigator Lori Muffly, MD, a bone and marrow transplant specialist at Stanford (Calif.) University.
However, Hispanic ALL patients were underrepresented in the study because the investigators didn’t recruit in Texas and Florida, states with higher percentages of young Hispanic ALL patients, and recruitment in California fell short of the prevalence of young Hispanic patients in that state. The work was a substudy of CALGB 10403, a trial of pediatric regimens in adolescents and young adults.
“It’s a relatively easy maneuver, going to where the patients are. When groups are thinking about multicenter trials, it has to be part of the dialogue from the beginning,” Dr. Muffly said.
Black patients in the review had fewer days in treatment and a higher prevalence of T-cell disease, and didn’t do as well as other groups.
Together, the studies “offer insight into the magnitude of racial and ethnic disparities in care among young people with” ALL, said Mikkael Sekeres, MD, chief of the division of hematology at the University of Miami, who moderated the presentations.
Dr. Gupta and his team found outcome differences only in relapsed B-cell ALL, not T-cell disease. B-cell disease has a more rigorous maintenance schedule, so it could be that there’s a difference in sticking to follow-up between various groups or less rigorous monitoring by pediatric oncologists in some groups, he said.
Dr. Gupta’s study was funded by the National Cancer Institute and others. Dr. Muffly didn’t report a funding source, but reported ties to Pfizer, Amgen, and other companies. Dr. Gupta is involved with Jazz Pharmaceuticals.
Among almost 25,000 children and young adults up to 31 years old, all of whom participated in Children’s Oncology Group studies since 2004, 5-year event free survival (EFS) was 87.4% for White patients, 82.8% for Hispanic patients, and 81.9% for Black patients.
When socioeconomics and disease characteristics such as CNS involvement, white blood cell lineage, and induction status were taken into account, the risk of having a survival event fell from 37% to 11% higher for Hispanic patients versus White patients but from 45% to 32% for Black patients versus White patients.
However, there was no explicit adjustment in the study for acuity at presentation, body mass index, adherence to protocols, or Philadelphia chromosome (PH)-like disease, which is more common among Hispanic patients.
Even so, lead investigator Sumit Gupta, MD, a pediatric blood cancer specialist at the University of Toronto, said that even with the potential confounders, lingering differences in outcomes raise questions about equal access to care and other matters, and suggest that there are still “uncomfortable things to consider, things like ... structural racism” and a system that delivers “systemically different care to patients across racial” groups.
Another report presented at the meeting with 295 patients 18-40 years old found that Hispanic patients had 3-year overall survival comparable to that of White patients despite a higher prevalence of PH-like disease, perhaps because Hispanic patients had higher treatment adherence than did White patients at 76% versus 56%, said lead investigator Lori Muffly, MD, a bone and marrow transplant specialist at Stanford (Calif.) University.
However, Hispanic ALL patients were underrepresented in the study because the investigators didn’t recruit in Texas and Florida, states with higher percentages of young Hispanic ALL patients, and recruitment in California fell short of the prevalence of young Hispanic patients in that state. The work was a substudy of CALGB 10403, a trial of pediatric regimens in adolescents and young adults.
“It’s a relatively easy maneuver, going to where the patients are. When groups are thinking about multicenter trials, it has to be part of the dialogue from the beginning,” Dr. Muffly said.
Black patients in the review had fewer days in treatment and a higher prevalence of T-cell disease, and didn’t do as well as other groups.
Together, the studies “offer insight into the magnitude of racial and ethnic disparities in care among young people with” ALL, said Mikkael Sekeres, MD, chief of the division of hematology at the University of Miami, who moderated the presentations.
Dr. Gupta and his team found outcome differences only in relapsed B-cell ALL, not T-cell disease. B-cell disease has a more rigorous maintenance schedule, so it could be that there’s a difference in sticking to follow-up between various groups or less rigorous monitoring by pediatric oncologists in some groups, he said.
Dr. Gupta’s study was funded by the National Cancer Institute and others. Dr. Muffly didn’t report a funding source, but reported ties to Pfizer, Amgen, and other companies. Dr. Gupta is involved with Jazz Pharmaceuticals.
Among almost 25,000 children and young adults up to 31 years old, all of whom participated in Children’s Oncology Group studies since 2004, 5-year event free survival (EFS) was 87.4% for White patients, 82.8% for Hispanic patients, and 81.9% for Black patients.
When socioeconomics and disease characteristics such as CNS involvement, white blood cell lineage, and induction status were taken into account, the risk of having a survival event fell from 37% to 11% higher for Hispanic patients versus White patients but from 45% to 32% for Black patients versus White patients.
However, there was no explicit adjustment in the study for acuity at presentation, body mass index, adherence to protocols, or Philadelphia chromosome (PH)-like disease, which is more common among Hispanic patients.
Even so, lead investigator Sumit Gupta, MD, a pediatric blood cancer specialist at the University of Toronto, said that even with the potential confounders, lingering differences in outcomes raise questions about equal access to care and other matters, and suggest that there are still “uncomfortable things to consider, things like ... structural racism” and a system that delivers “systemically different care to patients across racial” groups.
Another report presented at the meeting with 295 patients 18-40 years old found that Hispanic patients had 3-year overall survival comparable to that of White patients despite a higher prevalence of PH-like disease, perhaps because Hispanic patients had higher treatment adherence than did White patients at 76% versus 56%, said lead investigator Lori Muffly, MD, a bone and marrow transplant specialist at Stanford (Calif.) University.
However, Hispanic ALL patients were underrepresented in the study because the investigators didn’t recruit in Texas and Florida, states with higher percentages of young Hispanic ALL patients, and recruitment in California fell short of the prevalence of young Hispanic patients in that state. The work was a substudy of CALGB 10403, a trial of pediatric regimens in adolescents and young adults.
“It’s a relatively easy maneuver, going to where the patients are. When groups are thinking about multicenter trials, it has to be part of the dialogue from the beginning,” Dr. Muffly said.
Black patients in the review had fewer days in treatment and a higher prevalence of T-cell disease, and didn’t do as well as other groups.
Together, the studies “offer insight into the magnitude of racial and ethnic disparities in care among young people with” ALL, said Mikkael Sekeres, MD, chief of the division of hematology at the University of Miami, who moderated the presentations.
Dr. Gupta and his team found outcome differences only in relapsed B-cell ALL, not T-cell disease. B-cell disease has a more rigorous maintenance schedule, so it could be that there’s a difference in sticking to follow-up between various groups or less rigorous monitoring by pediatric oncologists in some groups, he said.
Dr. Gupta’s study was funded by the National Cancer Institute and others. Dr. Muffly didn’t report a funding source, but reported ties to Pfizer, Amgen, and other companies. Dr. Gupta is involved with Jazz Pharmaceuticals.
FROM ASH 2021
NHL: As a second-line treatment in phase 3 trial, tisa-cel disappoints
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
FROM ASH 2021
Are newer migraine therapies better? It depends
The findings, published in JAMA Network Open, “may imply that triptans will remain the current mainstay of specific acute migraine treatment,” suggested senior author Shuu-Jiun Wang, MD, from the National Yang Ming Chiao Tung University, and the Taipei Veterans General Hospital, both in Taipei, Taiwan, and his coauthors. However, lasmiditan (a 5-hydroxytryptamine1F receptor agonist) and rimegepant and ubrogepant (both calcitonin gene-related peptide [CGRP] antagonists) might still have unique advantages, since triptans are contraindicated for patients with cardiovascular risks, they said.
The systemic review and meta-analysis showed that, for the outcome of pain freedom and pain relief at 2 hours after the dose, the three newer agents worked better than placebo, but were inferior to most triptans. However, ubrogepant and rimegepant, which received U.S. Food and Drug Administration approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively, might be associated with fewer risks of adverse events (AEs), compared with triptans. “These new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction,” they wrote.
The meta-analysis included 64 randomized, controlled trials involving 46,442 participants (74%-87% female across studies; age range, 36-43 years). All studies examined clinically relevant outcomes in patients with International Headache Society criteria for migraine, and compared currently available migraine-specific acute treatments with each other or placebo. The drugs were examined at doses with widespread clinical use and included: ergotamine, dihydroergotamine, sumatriptan, zolmitriptan, naratriptan, rizatriptan, almotriptan, eletriptan, frovatriptan, lasmiditan, rimegepant, and ubrogepant.
The findings showed that all drug treatments were associated with a higher odds ratio for pain freedom, compared with placebo, except for sumatriptan, 10-mg nasal spray. The most effective drug was eletriptan 40 mg (OR, 5.59), and the least effective was lasmiditan 50 mg (OR, 1.65). Most triptans were associated with higher ORs for both pain freedom and pain relief at 2 hours, compared with lasmiditan, rimegepant, or ubrogepant, while comparisons between lasmiditan, rimegepant, and ubrogepant for these outcomes showed no statistically significant difference, they reported.
Lasmiditan was associated with the highest risk of any AEs, “however, the AEs were tolerable and were not considered serious. … Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan,” they wrote. Certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs, compared with the CGRP antagonists. “Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%).”
Finally, the authors noted that their observations of successful treatment with 5-hydroxytriptamine1F receptor agonists and CGRP antagonists “reveals that vasoconstriction is not essential for antimigraine therapy.” which could have implications for future pharmaceutical development.
Older and newer medications each have advantages
“Triptans will be around for a long time, but the newer medications are here to stay,” said Alan M. Rapoport, MD, in reaction to the study. “Before this publication, we knew that the 2-hour efficacy results of the newer medications were not quite as good as the faster-acting triptans; and after this network meta-analysis we are more sure of that,” said Dr. Rapoport, of the department of neurology at University of California, Los Angeles. “But the fact that the three newer medications do not constrict blood vessels and can easily be given even to patients with contraindications to triptans, or patients that simply are at greater risk due to obesity, smoking history, family history, diabetes, lack of exercise, or higher lipid levels, puts them into a desirable category.”
Calling it a “very carefully done” systematic review, Dr. Rapoport had a few caveats about the strength of the research. The trials that were included were not identically designed and were performed in different areas, by different investigators, on different patients, he noted. They were also not head-to-head trials “which ensures that the resultant data are more pure.” The studies also looked only at rapid results at 2 hours after dosing. “In my experience, patients are often satisfied with the response times from these newer agents; and doctors and patients both are happy that they are not vasoconstrictive,” he said. “The researchers also omitted studies looking at zolmitriptan nasal spray, which I have found to be rapid in onset and efficacious with few adverse events.”
Finally, Dr. Rapoport noted that one condition not examined in the review was medication overuse headache (MOH), which is “a major problem with patients that have high-frequency episodic migraine and chronic migraine. To our knowledge thus far, the two gepants (ubrogepant and rimegepant) do not appear to cause MOH when taken frequently, and these agents may end up being a treatment for this condition.”
Dr Wang reported receiving personal fees from Eli Lilly, Daiichi-Sankyo, Norvatis Taiwan, Biogen, Pfizer, and Bayer; and grants from AbbVie, Norvatis, Eli Lilly, Taiwan Ministry of Technology and Science, Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. No other disclosures were reported. Dr. Rapoport serves as an advisor for AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano; he is on the Speakers Bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries. He is Editor-in-Chief of Neurology Reviews.
The study was funded by the Ministry of Science and Technology, Taiwan, Ministry of Education, Taiwan, and the Brain Research Center, National Yang Ming Chiao Tung University.
The findings, published in JAMA Network Open, “may imply that triptans will remain the current mainstay of specific acute migraine treatment,” suggested senior author Shuu-Jiun Wang, MD, from the National Yang Ming Chiao Tung University, and the Taipei Veterans General Hospital, both in Taipei, Taiwan, and his coauthors. However, lasmiditan (a 5-hydroxytryptamine1F receptor agonist) and rimegepant and ubrogepant (both calcitonin gene-related peptide [CGRP] antagonists) might still have unique advantages, since triptans are contraindicated for patients with cardiovascular risks, they said.
The systemic review and meta-analysis showed that, for the outcome of pain freedom and pain relief at 2 hours after the dose, the three newer agents worked better than placebo, but were inferior to most triptans. However, ubrogepant and rimegepant, which received U.S. Food and Drug Administration approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively, might be associated with fewer risks of adverse events (AEs), compared with triptans. “These new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction,” they wrote.
The meta-analysis included 64 randomized, controlled trials involving 46,442 participants (74%-87% female across studies; age range, 36-43 years). All studies examined clinically relevant outcomes in patients with International Headache Society criteria for migraine, and compared currently available migraine-specific acute treatments with each other or placebo. The drugs were examined at doses with widespread clinical use and included: ergotamine, dihydroergotamine, sumatriptan, zolmitriptan, naratriptan, rizatriptan, almotriptan, eletriptan, frovatriptan, lasmiditan, rimegepant, and ubrogepant.
The findings showed that all drug treatments were associated with a higher odds ratio for pain freedom, compared with placebo, except for sumatriptan, 10-mg nasal spray. The most effective drug was eletriptan 40 mg (OR, 5.59), and the least effective was lasmiditan 50 mg (OR, 1.65). Most triptans were associated with higher ORs for both pain freedom and pain relief at 2 hours, compared with lasmiditan, rimegepant, or ubrogepant, while comparisons between lasmiditan, rimegepant, and ubrogepant for these outcomes showed no statistically significant difference, they reported.
Lasmiditan was associated with the highest risk of any AEs, “however, the AEs were tolerable and were not considered serious. … Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan,” they wrote. Certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs, compared with the CGRP antagonists. “Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%).”
Finally, the authors noted that their observations of successful treatment with 5-hydroxytriptamine1F receptor agonists and CGRP antagonists “reveals that vasoconstriction is not essential for antimigraine therapy.” which could have implications for future pharmaceutical development.
Older and newer medications each have advantages
“Triptans will be around for a long time, but the newer medications are here to stay,” said Alan M. Rapoport, MD, in reaction to the study. “Before this publication, we knew that the 2-hour efficacy results of the newer medications were not quite as good as the faster-acting triptans; and after this network meta-analysis we are more sure of that,” said Dr. Rapoport, of the department of neurology at University of California, Los Angeles. “But the fact that the three newer medications do not constrict blood vessels and can easily be given even to patients with contraindications to triptans, or patients that simply are at greater risk due to obesity, smoking history, family history, diabetes, lack of exercise, or higher lipid levels, puts them into a desirable category.”
Calling it a “very carefully done” systematic review, Dr. Rapoport had a few caveats about the strength of the research. The trials that were included were not identically designed and were performed in different areas, by different investigators, on different patients, he noted. They were also not head-to-head trials “which ensures that the resultant data are more pure.” The studies also looked only at rapid results at 2 hours after dosing. “In my experience, patients are often satisfied with the response times from these newer agents; and doctors and patients both are happy that they are not vasoconstrictive,” he said. “The researchers also omitted studies looking at zolmitriptan nasal spray, which I have found to be rapid in onset and efficacious with few adverse events.”
Finally, Dr. Rapoport noted that one condition not examined in the review was medication overuse headache (MOH), which is “a major problem with patients that have high-frequency episodic migraine and chronic migraine. To our knowledge thus far, the two gepants (ubrogepant and rimegepant) do not appear to cause MOH when taken frequently, and these agents may end up being a treatment for this condition.”
Dr Wang reported receiving personal fees from Eli Lilly, Daiichi-Sankyo, Norvatis Taiwan, Biogen, Pfizer, and Bayer; and grants from AbbVie, Norvatis, Eli Lilly, Taiwan Ministry of Technology and Science, Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. No other disclosures were reported. Dr. Rapoport serves as an advisor for AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano; he is on the Speakers Bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries. He is Editor-in-Chief of Neurology Reviews.
The study was funded by the Ministry of Science and Technology, Taiwan, Ministry of Education, Taiwan, and the Brain Research Center, National Yang Ming Chiao Tung University.
The findings, published in JAMA Network Open, “may imply that triptans will remain the current mainstay of specific acute migraine treatment,” suggested senior author Shuu-Jiun Wang, MD, from the National Yang Ming Chiao Tung University, and the Taipei Veterans General Hospital, both in Taipei, Taiwan, and his coauthors. However, lasmiditan (a 5-hydroxytryptamine1F receptor agonist) and rimegepant and ubrogepant (both calcitonin gene-related peptide [CGRP] antagonists) might still have unique advantages, since triptans are contraindicated for patients with cardiovascular risks, they said.
The systemic review and meta-analysis showed that, for the outcome of pain freedom and pain relief at 2 hours after the dose, the three newer agents worked better than placebo, but were inferior to most triptans. However, ubrogepant and rimegepant, which received U.S. Food and Drug Administration approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively, might be associated with fewer risks of adverse events (AEs), compared with triptans. “These new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction,” they wrote.
The meta-analysis included 64 randomized, controlled trials involving 46,442 participants (74%-87% female across studies; age range, 36-43 years). All studies examined clinically relevant outcomes in patients with International Headache Society criteria for migraine, and compared currently available migraine-specific acute treatments with each other or placebo. The drugs were examined at doses with widespread clinical use and included: ergotamine, dihydroergotamine, sumatriptan, zolmitriptan, naratriptan, rizatriptan, almotriptan, eletriptan, frovatriptan, lasmiditan, rimegepant, and ubrogepant.
The findings showed that all drug treatments were associated with a higher odds ratio for pain freedom, compared with placebo, except for sumatriptan, 10-mg nasal spray. The most effective drug was eletriptan 40 mg (OR, 5.59), and the least effective was lasmiditan 50 mg (OR, 1.65). Most triptans were associated with higher ORs for both pain freedom and pain relief at 2 hours, compared with lasmiditan, rimegepant, or ubrogepant, while comparisons between lasmiditan, rimegepant, and ubrogepant for these outcomes showed no statistically significant difference, they reported.
Lasmiditan was associated with the highest risk of any AEs, “however, the AEs were tolerable and were not considered serious. … Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan,” they wrote. Certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs, compared with the CGRP antagonists. “Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%).”
Finally, the authors noted that their observations of successful treatment with 5-hydroxytriptamine1F receptor agonists and CGRP antagonists “reveals that vasoconstriction is not essential for antimigraine therapy.” which could have implications for future pharmaceutical development.
Older and newer medications each have advantages
“Triptans will be around for a long time, but the newer medications are here to stay,” said Alan M. Rapoport, MD, in reaction to the study. “Before this publication, we knew that the 2-hour efficacy results of the newer medications were not quite as good as the faster-acting triptans; and after this network meta-analysis we are more sure of that,” said Dr. Rapoport, of the department of neurology at University of California, Los Angeles. “But the fact that the three newer medications do not constrict blood vessels and can easily be given even to patients with contraindications to triptans, or patients that simply are at greater risk due to obesity, smoking history, family history, diabetes, lack of exercise, or higher lipid levels, puts them into a desirable category.”
Calling it a “very carefully done” systematic review, Dr. Rapoport had a few caveats about the strength of the research. The trials that were included were not identically designed and were performed in different areas, by different investigators, on different patients, he noted. They were also not head-to-head trials “which ensures that the resultant data are more pure.” The studies also looked only at rapid results at 2 hours after dosing. “In my experience, patients are often satisfied with the response times from these newer agents; and doctors and patients both are happy that they are not vasoconstrictive,” he said. “The researchers also omitted studies looking at zolmitriptan nasal spray, which I have found to be rapid in onset and efficacious with few adverse events.”
Finally, Dr. Rapoport noted that one condition not examined in the review was medication overuse headache (MOH), which is “a major problem with patients that have high-frequency episodic migraine and chronic migraine. To our knowledge thus far, the two gepants (ubrogepant and rimegepant) do not appear to cause MOH when taken frequently, and these agents may end up being a treatment for this condition.”
Dr Wang reported receiving personal fees from Eli Lilly, Daiichi-Sankyo, Norvatis Taiwan, Biogen, Pfizer, and Bayer; and grants from AbbVie, Norvatis, Eli Lilly, Taiwan Ministry of Technology and Science, Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. No other disclosures were reported. Dr. Rapoport serves as an advisor for AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano; he is on the Speakers Bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries. He is Editor-in-Chief of Neurology Reviews.
The study was funded by the Ministry of Science and Technology, Taiwan, Ministry of Education, Taiwan, and the Brain Research Center, National Yang Ming Chiao Tung University.
FROM JAMA NETWORK OPEN
Delays in cancer referral, diagnosis linked with morbidities
These findings are based on a retrospective study of data from 11,716 cancer patients from the United Kingdom’s National Cancer Diagnosis Audit – an initiative that aimed to better understand the journey of cancer patients from primary care to diagnosis. Three-quarters of the study participants had at least one morbidity in their primary care record, according to the authors of the new research, which was published in Family Practice (2021 Nov 30. doi: 10.1093/fampra/cmab139).
In their analysis of all of the patient data, Minjoung M. Koo and colleagues found that the median time between first presenting to a primary care physician with cancer symptoms and being referred to a specialist was 5 days. For all patients studied, the median time to receiving a cancer diagnosis was 42 days, the investigators wrote.
Patients with multiple morbidities were 26% more likely to have their cancer diagnosed at least 60 days after the initial primary care consultation than were those without morbidities (95% confidence interval, 1.10-1.45). This was true after adjustment for confounders, including morbidity, sex, age, and cancer. Similarly, those with a Charlson score of 3 or above – signifying more severe comorbidities – had a 19% greater odds of being diagnosed more than 60 days after presenting to primary care (95% CI, 1.01-1.40)
Older adults ‘less likely to be screen-detected’
Dr. Fran Boyle, professor of medical oncology at the University of Sydney, Australia, said it wasn’t clear from the study whether people with multiple comorbidities may have symptoms that cloud the diagnostic process, or whether short primary care consultations may not allow for enough time to manage multiple issues.
“Older adults typically have more comorbidities, and they are less likely to be screen-detected; for example, breast cancer screening and bowel cancer screening typically stop after 75,” said Dr. Boyle, director of Patricia Ritchie Centre for Cancer Care and Research at Sydney’s Mater Hospital.
Dr. Boyle pointed to a recent systematic review in Australian rural oncology that suggested that patients with more comorbidities tend to be offered less intense treatment, and have higher operative mortality and morbidity, which can contribute to less effective therapy.
Referral delays seen in multiple patient groups
Ms. Koo, from the University College London and the National Disease Registration Service in the United Kingdom, and coauthors noted a nonsignificant trend toward increased intervals between primary care consultation and referral or diagnosis even in patients with one or more comorbidities.
A higher burden of comorbidities also meant patients were more likely to have more than one primary care consultation before being referred to a specialist. Those with three or more comorbidities were 21% more likely to have at least three consultations before referral, compared with patients with no comorbidities (95% CI, 1.05-1.40, P = .010).
Overall, 60% of the participants in the study experienced at least one investigation into whether they had cancer by a primary care clinician before being referred to a specialist.
Morbidities linked with emergency referral
The study also saw an association between morbidities and the likelihood of receiving an emergency referral. Those with three or more morbidities were 60% more likely to have an emergency referral than were those with no comorbidities. Those with a Charlson score of three or above were 61% more likely to be referred to an emergency department.
“The greater likelihood of clinical complexity or acute deterioration among individuals with multiple or severe chronic conditions means that an emergency referral may be clinically appropriate,” the authors wrote.
Commenting on the findings, Dr. Diane M. Harper, professor of family medicine at the University of Michigan, Ann Arbor, said primary care patients often have multiple chronic illnesses, and the relationship between the physician and patients determines how quickly symptoms of cancer are explored.
“What this work cannot explore is the quality of discussions between the physician and the patient, nor can it explore how the decision to go to the ED was made,” said Dr. Harper, president of the North American Primary Care Research Group. “Exploring these data would provide important information to the physician-patient dyad.”
Diagnostic difficulty might have been at play, according to authors
The investigators didn’t find any evidence of an interaction between cancer site, number of morbidities, and referral or diagnostic time, except in cases of colorectal cancer, where patients with multiple morbidities were more likely to experience a longer wait between primary care consultation and diagnosis.
The authors observed that diagnostic difficulty of the cancer might have been at play here, given that colorectal cancer can have a broad symptom signature.
“This was less often observed among patients diagnosed with a cancer that had a narrow symptom signature (“easy” diagnostic difficulty, e.g. breast cancer) or a broad symptom signature of mostly low PPVs (“hard” diagnostic difficulty, e.g. brain cancer),” they wrote.
The authors concluded that “it is reasonable to suggest that both improvement efforts and future research in this field should target patients with multiple or severe morbidity, and explore the reasons for prolonged diagnostic intervals in specialist care.”
The study was supported by Cancer Research UK. The authors and experts interviewed for this piece did not declare having any conflicts of interest.
These findings are based on a retrospective study of data from 11,716 cancer patients from the United Kingdom’s National Cancer Diagnosis Audit – an initiative that aimed to better understand the journey of cancer patients from primary care to diagnosis. Three-quarters of the study participants had at least one morbidity in their primary care record, according to the authors of the new research, which was published in Family Practice (2021 Nov 30. doi: 10.1093/fampra/cmab139).
In their analysis of all of the patient data, Minjoung M. Koo and colleagues found that the median time between first presenting to a primary care physician with cancer symptoms and being referred to a specialist was 5 days. For all patients studied, the median time to receiving a cancer diagnosis was 42 days, the investigators wrote.
Patients with multiple morbidities were 26% more likely to have their cancer diagnosed at least 60 days after the initial primary care consultation than were those without morbidities (95% confidence interval, 1.10-1.45). This was true after adjustment for confounders, including morbidity, sex, age, and cancer. Similarly, those with a Charlson score of 3 or above – signifying more severe comorbidities – had a 19% greater odds of being diagnosed more than 60 days after presenting to primary care (95% CI, 1.01-1.40)
Older adults ‘less likely to be screen-detected’
Dr. Fran Boyle, professor of medical oncology at the University of Sydney, Australia, said it wasn’t clear from the study whether people with multiple comorbidities may have symptoms that cloud the diagnostic process, or whether short primary care consultations may not allow for enough time to manage multiple issues.
“Older adults typically have more comorbidities, and they are less likely to be screen-detected; for example, breast cancer screening and bowel cancer screening typically stop after 75,” said Dr. Boyle, director of Patricia Ritchie Centre for Cancer Care and Research at Sydney’s Mater Hospital.
Dr. Boyle pointed to a recent systematic review in Australian rural oncology that suggested that patients with more comorbidities tend to be offered less intense treatment, and have higher operative mortality and morbidity, which can contribute to less effective therapy.
Referral delays seen in multiple patient groups
Ms. Koo, from the University College London and the National Disease Registration Service in the United Kingdom, and coauthors noted a nonsignificant trend toward increased intervals between primary care consultation and referral or diagnosis even in patients with one or more comorbidities.
A higher burden of comorbidities also meant patients were more likely to have more than one primary care consultation before being referred to a specialist. Those with three or more comorbidities were 21% more likely to have at least three consultations before referral, compared with patients with no comorbidities (95% CI, 1.05-1.40, P = .010).
Overall, 60% of the participants in the study experienced at least one investigation into whether they had cancer by a primary care clinician before being referred to a specialist.
Morbidities linked with emergency referral
The study also saw an association between morbidities and the likelihood of receiving an emergency referral. Those with three or more morbidities were 60% more likely to have an emergency referral than were those with no comorbidities. Those with a Charlson score of three or above were 61% more likely to be referred to an emergency department.
“The greater likelihood of clinical complexity or acute deterioration among individuals with multiple or severe chronic conditions means that an emergency referral may be clinically appropriate,” the authors wrote.
Commenting on the findings, Dr. Diane M. Harper, professor of family medicine at the University of Michigan, Ann Arbor, said primary care patients often have multiple chronic illnesses, and the relationship between the physician and patients determines how quickly symptoms of cancer are explored.
“What this work cannot explore is the quality of discussions between the physician and the patient, nor can it explore how the decision to go to the ED was made,” said Dr. Harper, president of the North American Primary Care Research Group. “Exploring these data would provide important information to the physician-patient dyad.”
Diagnostic difficulty might have been at play, according to authors
The investigators didn’t find any evidence of an interaction between cancer site, number of morbidities, and referral or diagnostic time, except in cases of colorectal cancer, where patients with multiple morbidities were more likely to experience a longer wait between primary care consultation and diagnosis.
The authors observed that diagnostic difficulty of the cancer might have been at play here, given that colorectal cancer can have a broad symptom signature.
“This was less often observed among patients diagnosed with a cancer that had a narrow symptom signature (“easy” diagnostic difficulty, e.g. breast cancer) or a broad symptom signature of mostly low PPVs (“hard” diagnostic difficulty, e.g. brain cancer),” they wrote.
The authors concluded that “it is reasonable to suggest that both improvement efforts and future research in this field should target patients with multiple or severe morbidity, and explore the reasons for prolonged diagnostic intervals in specialist care.”
The study was supported by Cancer Research UK. The authors and experts interviewed for this piece did not declare having any conflicts of interest.
These findings are based on a retrospective study of data from 11,716 cancer patients from the United Kingdom’s National Cancer Diagnosis Audit – an initiative that aimed to better understand the journey of cancer patients from primary care to diagnosis. Three-quarters of the study participants had at least one morbidity in their primary care record, according to the authors of the new research, which was published in Family Practice (2021 Nov 30. doi: 10.1093/fampra/cmab139).
In their analysis of all of the patient data, Minjoung M. Koo and colleagues found that the median time between first presenting to a primary care physician with cancer symptoms and being referred to a specialist was 5 days. For all patients studied, the median time to receiving a cancer diagnosis was 42 days, the investigators wrote.
Patients with multiple morbidities were 26% more likely to have their cancer diagnosed at least 60 days after the initial primary care consultation than were those without morbidities (95% confidence interval, 1.10-1.45). This was true after adjustment for confounders, including morbidity, sex, age, and cancer. Similarly, those with a Charlson score of 3 or above – signifying more severe comorbidities – had a 19% greater odds of being diagnosed more than 60 days after presenting to primary care (95% CI, 1.01-1.40)
Older adults ‘less likely to be screen-detected’
Dr. Fran Boyle, professor of medical oncology at the University of Sydney, Australia, said it wasn’t clear from the study whether people with multiple comorbidities may have symptoms that cloud the diagnostic process, or whether short primary care consultations may not allow for enough time to manage multiple issues.
“Older adults typically have more comorbidities, and they are less likely to be screen-detected; for example, breast cancer screening and bowel cancer screening typically stop after 75,” said Dr. Boyle, director of Patricia Ritchie Centre for Cancer Care and Research at Sydney’s Mater Hospital.
Dr. Boyle pointed to a recent systematic review in Australian rural oncology that suggested that patients with more comorbidities tend to be offered less intense treatment, and have higher operative mortality and morbidity, which can contribute to less effective therapy.
Referral delays seen in multiple patient groups
Ms. Koo, from the University College London and the National Disease Registration Service in the United Kingdom, and coauthors noted a nonsignificant trend toward increased intervals between primary care consultation and referral or diagnosis even in patients with one or more comorbidities.
A higher burden of comorbidities also meant patients were more likely to have more than one primary care consultation before being referred to a specialist. Those with three or more comorbidities were 21% more likely to have at least three consultations before referral, compared with patients with no comorbidities (95% CI, 1.05-1.40, P = .010).
Overall, 60% of the participants in the study experienced at least one investigation into whether they had cancer by a primary care clinician before being referred to a specialist.
Morbidities linked with emergency referral
The study also saw an association between morbidities and the likelihood of receiving an emergency referral. Those with three or more morbidities were 60% more likely to have an emergency referral than were those with no comorbidities. Those with a Charlson score of three or above were 61% more likely to be referred to an emergency department.
“The greater likelihood of clinical complexity or acute deterioration among individuals with multiple or severe chronic conditions means that an emergency referral may be clinically appropriate,” the authors wrote.
Commenting on the findings, Dr. Diane M. Harper, professor of family medicine at the University of Michigan, Ann Arbor, said primary care patients often have multiple chronic illnesses, and the relationship between the physician and patients determines how quickly symptoms of cancer are explored.
“What this work cannot explore is the quality of discussions between the physician and the patient, nor can it explore how the decision to go to the ED was made,” said Dr. Harper, president of the North American Primary Care Research Group. “Exploring these data would provide important information to the physician-patient dyad.”
Diagnostic difficulty might have been at play, according to authors
The investigators didn’t find any evidence of an interaction between cancer site, number of morbidities, and referral or diagnostic time, except in cases of colorectal cancer, where patients with multiple morbidities were more likely to experience a longer wait between primary care consultation and diagnosis.
The authors observed that diagnostic difficulty of the cancer might have been at play here, given that colorectal cancer can have a broad symptom signature.
“This was less often observed among patients diagnosed with a cancer that had a narrow symptom signature (“easy” diagnostic difficulty, e.g. breast cancer) or a broad symptom signature of mostly low PPVs (“hard” diagnostic difficulty, e.g. brain cancer),” they wrote.
The authors concluded that “it is reasonable to suggest that both improvement efforts and future research in this field should target patients with multiple or severe morbidity, and explore the reasons for prolonged diagnostic intervals in specialist care.”
The study was supported by Cancer Research UK. The authors and experts interviewed for this piece did not declare having any conflicts of interest.
FROM FAMILY PRACTICE
TKI/BiTE combo extends survival of older patients with Ph+ALL
ATLANTA – Older patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) are often not fit enough to withstand intensive chemotherapy and stem cell transplants, but remissions with alternative therapies are usually short lived.
Now, results from an ongoing study suggest that the combination of the
The new results were reported by investigators in the SWOG Cancer Research Network and come from a cohort of 25 patients with a median age of 73 years with newly diagnosed Ph+ALL or ALL with dasatinib-sensitive fusions of mutations (Ph-like ALL).
Nearly all (23 of 25 patients, 92%) had complete remissions, and 5 of 16 patients for whom minimal residual disease (MRD) data were available were MRD negative at day 28, said Anjali Advani, MD, from the Cleveland Clinic.
At a median follow-up of 1.7 years, the estimated 3-year disease-free survival rate was 80%, and the estimated overall survival rate was 85%, the investigators reported in a poster presentation at the annual meeting of the American Society of Hematology.
“I think the biggest question will be longer-term follow-up. We clearly see high remission rates in this population, but the issue is whether in these elderly patients who are not candidates for chemo we can prolong remission by the addition of other treatments, such as blinatumomab,” she said in an interview with this news organization.
“The follow-up is reasonable at this point, and as we get longer follow-up, if the current 3-year survival estimates hold up, that would be very encouraging,” she said.
Early promise
A leukemia specialist who was not involved in the study told this news organization that the results are promising, but added that it’s too early to make definitive judgments about the efficacy of the combination.
“People have used just a tyrosine kinase inhibitor and prednisone in these patients and gotten remissions, but they just don’t last,” said Peter Emanuel, MD, from CHI St. Vincent Infirmary in Little Rock, Ark.
“The promise with this approach is that you’re getting a longer-lasting remission – maybe not a cure, but a longer-lasting remission – without having to use intensive chemotherapy,” he said.
“It’s still a pretty small study, so I think this is going to require a bigger trial, looking at more patients, but it’s certainly very encouraging and very promising,” he added.
Hanno Hock, MD, PhD, a leukemia researcher at the Mass General Cancer Center in Boston, said in an interview that “the whole idea here is to add this newer agent, blinatumomab, to make those good initial responses more durable, and it looks like it is able to do that with very impressive initial data,” he said.
“The caveat is that this is still early, and one needs to wait and see how it all pans out, but it’s very well tolerated, and definitely the next logical step in trying to offer something to people who cannot tolerate more aggressive therapy such as transplant,” Dr. Hock added.
Study results
The new results come from a feasibility cohort of patients enrolled in the SWOG S1318 trial, which studied blinatumomab plus chemotherapy and prednisone in older patients with Ph-ALL, as well as blinatumomab, dasatinib, and prednisone in older adults with Ph+ ALL.
Patients 65 and older with newly diagnosed or relapsed/refractory Ph+ALL or Ph-like ALL and no central nervous system disease were eligible for the arm of the trial described here. All patients with data reported in this analysis had newly diagnosed ALL.
Patients first received a single induction cycle of dasatinib and prednisone and were then evaluated for response. Patients with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) would then undergo prednisone tapering while continuing dasatinib until day 84. Patients without a CR or CRi at day 28 who had remissions by day 56 then also continued dasatinib until day 84.
Those patients still in remission at day 84 went on to three cycles of blinatumomab and dasatinib, followed by dasatinib and prednisone maintenance until unacceptable toxicity or disease progression. Patients may remain on maintenance for up to 10 years after registration.
Patients who do not have a CR or CRi by day 84 can receive reinduction with up to two total cycles of blinatumomab, with those who get a remission moving on to the blinatumomab/ dasatinib combination and those who do not going off protocol.
Of the 25 patients, 23 had a CR following dasatinib/prednisone induction. As noted, 5 of 16 patients evaluable for MRD were MRD negative.
Four patients did not receive postremission therapy, two because of adverse events, one who went on to transplant, and one because of insurance issues.
In a safety review early in the study, 4 of 12 evaluable patients were found to have dose-limiting toxicities, including one case each of grade 3 dyspnea and gastrointestinal pain (in a single patient), hypertension, dyspnea, and hyperglycemia.
These adverse events were deemed acceptable by both U.S. Food and Drug Administration and National Cancer Institute reviewers, and this arm of the study was allowed to continue, Dr. Advani noted.
The study was funded by grants from the National Institutes of Health. Dr. Advani disclosed financial relationships with several companies. Dr. Emanuel and Dr. Hock have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Older patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) are often not fit enough to withstand intensive chemotherapy and stem cell transplants, but remissions with alternative therapies are usually short lived.
Now, results from an ongoing study suggest that the combination of the
The new results were reported by investigators in the SWOG Cancer Research Network and come from a cohort of 25 patients with a median age of 73 years with newly diagnosed Ph+ALL or ALL with dasatinib-sensitive fusions of mutations (Ph-like ALL).
Nearly all (23 of 25 patients, 92%) had complete remissions, and 5 of 16 patients for whom minimal residual disease (MRD) data were available were MRD negative at day 28, said Anjali Advani, MD, from the Cleveland Clinic.
At a median follow-up of 1.7 years, the estimated 3-year disease-free survival rate was 80%, and the estimated overall survival rate was 85%, the investigators reported in a poster presentation at the annual meeting of the American Society of Hematology.
“I think the biggest question will be longer-term follow-up. We clearly see high remission rates in this population, but the issue is whether in these elderly patients who are not candidates for chemo we can prolong remission by the addition of other treatments, such as blinatumomab,” she said in an interview with this news organization.
“The follow-up is reasonable at this point, and as we get longer follow-up, if the current 3-year survival estimates hold up, that would be very encouraging,” she said.
Early promise
A leukemia specialist who was not involved in the study told this news organization that the results are promising, but added that it’s too early to make definitive judgments about the efficacy of the combination.
“People have used just a tyrosine kinase inhibitor and prednisone in these patients and gotten remissions, but they just don’t last,” said Peter Emanuel, MD, from CHI St. Vincent Infirmary in Little Rock, Ark.
“The promise with this approach is that you’re getting a longer-lasting remission – maybe not a cure, but a longer-lasting remission – without having to use intensive chemotherapy,” he said.
“It’s still a pretty small study, so I think this is going to require a bigger trial, looking at more patients, but it’s certainly very encouraging and very promising,” he added.
Hanno Hock, MD, PhD, a leukemia researcher at the Mass General Cancer Center in Boston, said in an interview that “the whole idea here is to add this newer agent, blinatumomab, to make those good initial responses more durable, and it looks like it is able to do that with very impressive initial data,” he said.
“The caveat is that this is still early, and one needs to wait and see how it all pans out, but it’s very well tolerated, and definitely the next logical step in trying to offer something to people who cannot tolerate more aggressive therapy such as transplant,” Dr. Hock added.
Study results
The new results come from a feasibility cohort of patients enrolled in the SWOG S1318 trial, which studied blinatumomab plus chemotherapy and prednisone in older patients with Ph-ALL, as well as blinatumomab, dasatinib, and prednisone in older adults with Ph+ ALL.
Patients 65 and older with newly diagnosed or relapsed/refractory Ph+ALL or Ph-like ALL and no central nervous system disease were eligible for the arm of the trial described here. All patients with data reported in this analysis had newly diagnosed ALL.
Patients first received a single induction cycle of dasatinib and prednisone and were then evaluated for response. Patients with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) would then undergo prednisone tapering while continuing dasatinib until day 84. Patients without a CR or CRi at day 28 who had remissions by day 56 then also continued dasatinib until day 84.
Those patients still in remission at day 84 went on to three cycles of blinatumomab and dasatinib, followed by dasatinib and prednisone maintenance until unacceptable toxicity or disease progression. Patients may remain on maintenance for up to 10 years after registration.
Patients who do not have a CR or CRi by day 84 can receive reinduction with up to two total cycles of blinatumomab, with those who get a remission moving on to the blinatumomab/ dasatinib combination and those who do not going off protocol.
Of the 25 patients, 23 had a CR following dasatinib/prednisone induction. As noted, 5 of 16 patients evaluable for MRD were MRD negative.
Four patients did not receive postremission therapy, two because of adverse events, one who went on to transplant, and one because of insurance issues.
In a safety review early in the study, 4 of 12 evaluable patients were found to have dose-limiting toxicities, including one case each of grade 3 dyspnea and gastrointestinal pain (in a single patient), hypertension, dyspnea, and hyperglycemia.
These adverse events were deemed acceptable by both U.S. Food and Drug Administration and National Cancer Institute reviewers, and this arm of the study was allowed to continue, Dr. Advani noted.
The study was funded by grants from the National Institutes of Health. Dr. Advani disclosed financial relationships with several companies. Dr. Emanuel and Dr. Hock have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Older patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) are often not fit enough to withstand intensive chemotherapy and stem cell transplants, but remissions with alternative therapies are usually short lived.
Now, results from an ongoing study suggest that the combination of the
The new results were reported by investigators in the SWOG Cancer Research Network and come from a cohort of 25 patients with a median age of 73 years with newly diagnosed Ph+ALL or ALL with dasatinib-sensitive fusions of mutations (Ph-like ALL).
Nearly all (23 of 25 patients, 92%) had complete remissions, and 5 of 16 patients for whom minimal residual disease (MRD) data were available were MRD negative at day 28, said Anjali Advani, MD, from the Cleveland Clinic.
At a median follow-up of 1.7 years, the estimated 3-year disease-free survival rate was 80%, and the estimated overall survival rate was 85%, the investigators reported in a poster presentation at the annual meeting of the American Society of Hematology.
“I think the biggest question will be longer-term follow-up. We clearly see high remission rates in this population, but the issue is whether in these elderly patients who are not candidates for chemo we can prolong remission by the addition of other treatments, such as blinatumomab,” she said in an interview with this news organization.
“The follow-up is reasonable at this point, and as we get longer follow-up, if the current 3-year survival estimates hold up, that would be very encouraging,” she said.
Early promise
A leukemia specialist who was not involved in the study told this news organization that the results are promising, but added that it’s too early to make definitive judgments about the efficacy of the combination.
“People have used just a tyrosine kinase inhibitor and prednisone in these patients and gotten remissions, but they just don’t last,” said Peter Emanuel, MD, from CHI St. Vincent Infirmary in Little Rock, Ark.
“The promise with this approach is that you’re getting a longer-lasting remission – maybe not a cure, but a longer-lasting remission – without having to use intensive chemotherapy,” he said.
“It’s still a pretty small study, so I think this is going to require a bigger trial, looking at more patients, but it’s certainly very encouraging and very promising,” he added.
Hanno Hock, MD, PhD, a leukemia researcher at the Mass General Cancer Center in Boston, said in an interview that “the whole idea here is to add this newer agent, blinatumomab, to make those good initial responses more durable, and it looks like it is able to do that with very impressive initial data,” he said.
“The caveat is that this is still early, and one needs to wait and see how it all pans out, but it’s very well tolerated, and definitely the next logical step in trying to offer something to people who cannot tolerate more aggressive therapy such as transplant,” Dr. Hock added.
Study results
The new results come from a feasibility cohort of patients enrolled in the SWOG S1318 trial, which studied blinatumomab plus chemotherapy and prednisone in older patients with Ph-ALL, as well as blinatumomab, dasatinib, and prednisone in older adults with Ph+ ALL.
Patients 65 and older with newly diagnosed or relapsed/refractory Ph+ALL or Ph-like ALL and no central nervous system disease were eligible for the arm of the trial described here. All patients with data reported in this analysis had newly diagnosed ALL.
Patients first received a single induction cycle of dasatinib and prednisone and were then evaluated for response. Patients with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) would then undergo prednisone tapering while continuing dasatinib until day 84. Patients without a CR or CRi at day 28 who had remissions by day 56 then also continued dasatinib until day 84.
Those patients still in remission at day 84 went on to three cycles of blinatumomab and dasatinib, followed by dasatinib and prednisone maintenance until unacceptable toxicity or disease progression. Patients may remain on maintenance for up to 10 years after registration.
Patients who do not have a CR or CRi by day 84 can receive reinduction with up to two total cycles of blinatumomab, with those who get a remission moving on to the blinatumomab/ dasatinib combination and those who do not going off protocol.
Of the 25 patients, 23 had a CR following dasatinib/prednisone induction. As noted, 5 of 16 patients evaluable for MRD were MRD negative.
Four patients did not receive postremission therapy, two because of adverse events, one who went on to transplant, and one because of insurance issues.
In a safety review early in the study, 4 of 12 evaluable patients were found to have dose-limiting toxicities, including one case each of grade 3 dyspnea and gastrointestinal pain (in a single patient), hypertension, dyspnea, and hyperglycemia.
These adverse events were deemed acceptable by both U.S. Food and Drug Administration and National Cancer Institute reviewers, and this arm of the study was allowed to continue, Dr. Advani noted.
The study was funded by grants from the National Institutes of Health. Dr. Advani disclosed financial relationships with several companies. Dr. Emanuel and Dr. Hock have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASH 2021
Clinical Progress Note: Rhythm Control for Patients With Atrial Fibrillation
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3
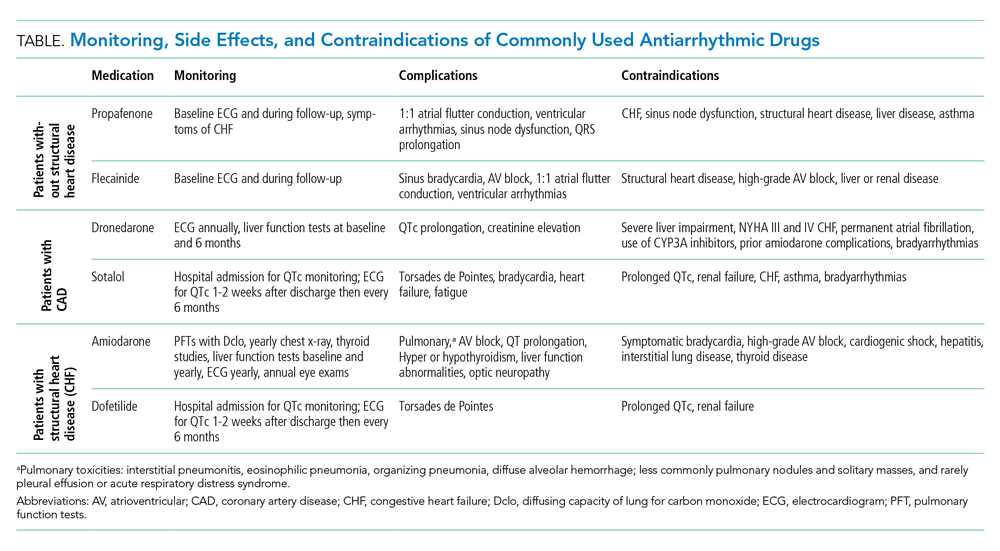
Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7
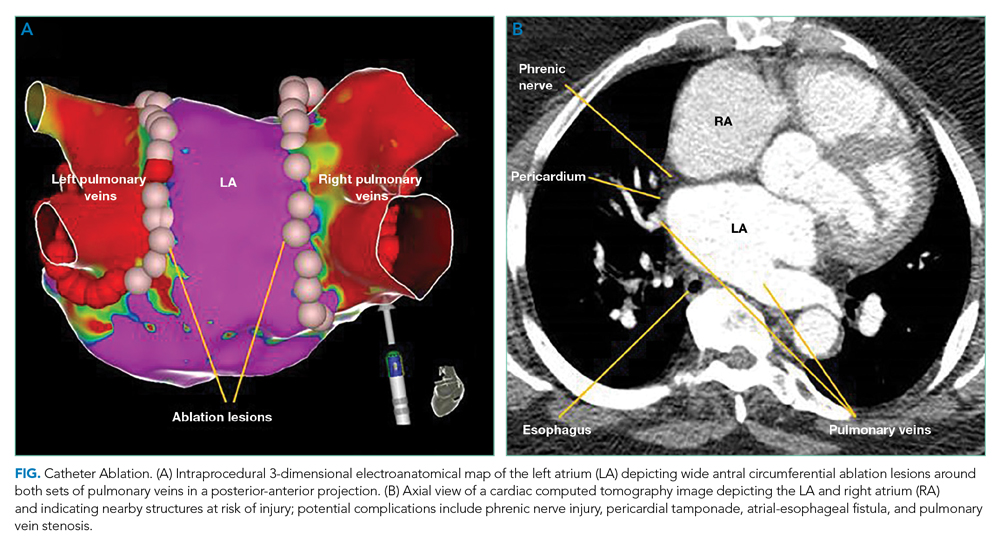
Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
© 2021 Society of Hospital Medicine
Beyond a Purple Journal: Improving Hospital-Based Addiction Care
Rosa* was one of my first patients as an intern rotating at the county hospital. Her marriage had disintegrated years earlier. To cope with depression, she hid a daily ritual of orange juice and vodka from her children. She worked as a cashier, until nausea and fatigue overwhelmed her.
The first time I met her she sat on the gurney: petite, tanned, and pregnant. Then I saw her yellow eyes and revised: temporal wasting, jaundiced, and swollen with ascites. Rosa didn’t know that alcohol could cause liver disease. Without insurance or access to primary care, her untreated alcohol use disorder (AUD) and depression had snowballed for years.
Midway through my intern year, I’d taken care of many people with AUD. However, I’d barely learned anything about it as a medical student, though we’d spent weeks studying esoteric diseases, that now––9 years after medical school––I still have not encountered.
Among the 28.3 million individuals in the United States with AUD, only 1% receive medication treatment.1 In the United States, unhealthy alcohol use accounts for more than 95,000 deaths each year.2 This number likely under-captures alcohol-related mortality and is higher now given recent reports of increasing alcohol-related deaths and prevalence of unhealthy alcohol use, especially among women, younger age groups, and marginalized populations.3-5
Rosa had alcohol-related hepatitis, which can cause severe inflammation and liver failure and quickly lead to death. As her liver failure progressed, I asked the gastroenterologists, “What other treatments can we offer? Is she a liver transplant candidate?” “Nothing” and “No” they answered.
Later, I emailed the hepatologist and transplant surgeon begging them to reevaluate her transplantation candidacy, but they told me there was no exception to the institution’s 6-month sobriety rule.
Maintaining a 6-month sobriety period is not an evidence-based criterion for transplantation. However, 50% of transplant centers do not perform transplantation prior to 6 months of alcohol abstinence for alcohol-related hepatitis due to concern for return to drinking after transplant.6 This practice may promote bias in patient selection for transplantation. A recent study found that individuals with alcohol-related liver disease transplanted before 6 months of abstinence had similar rates of survival and return to drinking compared to those who abstained from alcohol for 6 months and participated in AUD treatment before transplantation.7
There are other liver transplant practices that result in inequities for individuals with substance use disorders (SUD). Some liver transplant centers consider being on a medication for opioid use disorder a contraindication for transplantation—even if the individual is in recovery and abstaining from substances.8 Others mandate that individuals with alcohol-related liver disease attend Alcoholics Anonymous (AA) meetings prior to transplant. While mutual help groups, including AA, may benefit some individuals, different approaches work for different people.9 Other psychosocial interventions (eg, cognitive-behavioral therapy, contingency management, and residential treatment) and medications also help individuals reduce or stop drinking. Some meet their goals without any treatment. Addiction care works best when it respects autonomy and meets individuals where they are by allowing them to decide among options.
While organ allocations are a crystalized example of inequities in addiction care, they are also ethically complex. Many individuals—with and without SUD—die on waiting lists and must meet stringent transplantation criteria. However, we can at least remove the unnecessary biases that compound inequities in care people with SUD already face.
As Rosa’s liver succumbed, her kidneys failed too, and she required dialysis. She sensed what was coming. “I want everything…for now. I need to take care of my children.” I, too, wanted Rosa to live and see her youngest start kindergarten.
A few days before her discharge, I walked to the pharmacy and bought a purple journal. In a rare moment, I found Rosa alone in her room, without her ex-husband, sister, and mother, who rarely left her bedside. Together, we called AA and explored whether she could start participating in phone meetings from the hospital. I explained that one way to document a commitment to sobriety, as the transplant center’s rules dictated, was to attend and document AA meetings in this notebook. “In 5 months, you will be a liver transplant candidate,” I remember saying, wishing it to fruition.
I became Rosa’s primary care physician and saw her in clinic. Over the next few weeks, her skin took on an ashen tone. Sleep escaped her and her thoughts and speech blurred. Her walk slowed and she needed a wheelchair. The quiet fierceness that had defined her dissipated as encephalopathy took over. But until our last visit, she brought her purple journal, tracking the AA meetings she’d attended. Dialysis became intolerable, but not before Rosa made care arrangements for her girls. When that happened, she stopped dialysis and went to Mexico, where she died in her sleep after saying good-bye to her father.
Earlier access to healthcare and effective depression and AUD treatment could have saved Rosa’s life. While it was too late for her, as hospitalists we care for many others with substance-related complications and may miss opportunities to discuss and offer evidence-based addiction treatment. For example, we initiate the most up-to-date management for a patient’s gastrointestinal bleed but may leave the alcohol discussion for someone else. It is similar for other SUD: we treat cellulitis, epidural abscesses, bacteremia, chronic obstructive pulmonary disease, heart failure exacerbations, and other complications of SUD without addressing the root cause of the hospitalization—other than to prescribe abstinence from substance use or, at our worst, scold individuals for continuing to use.
But what can we offer? Most healthcare professionals still do not receive addiction education during training. Without tools, we enact temporizing measures, until patients return to the hospital or die.
In addition to increasing alcohol-related morbidity, there have also been increases in drug-related overdoses, fueled by COVID-19, synthetic opioids like fentanyl, and stimulants.10 In the 12-month period ending April 2021, more than 100,000 individuals died of drug-related overdoses, the highest number of deaths ever recorded in a year.11 Despite this, most healthcare systems remain unequipped to provide addiction services during hospitalization due to inadequate training, stigma, and lack of systems-based care.
Hospitalists and healthcare systems cannot be bystanders amid our worsening addiction crisis. We must empower clinicians with addiction education and ensure health systems offer evidence-based SUD services.
Educational efforts can close the knowledge gaps for both medical students and hospitalists. Medical schools should include foundational curricular content in screening, assessing, diagnosing, and treating SUD in alignment with standards set by the Liaison Committee on Medical Education, which accredits US medical schools. Residency programs can offer educational conferences, cased-based discussions, and addiction medicine rotations. Hospitalists can participate in educational didactics and review evidence-based addiction guidelines.12,13 While the focus here is on hospitalists, clinicians across practice settings and specialties will encounter patients with SUD, and all need to be well-versed in the diagnosis and treatment of addiction given the all-hands-on deck approach necessary amidst our worsening addiction crisis.
With one in nine hospitalizations involving individuals with SUD, and this number quickly rising, and with an annual cost to US hospitals of $13.2 billion, healthcare system leaders must invest in addiction care.14,15 Hospital-based addiction services could pay for themselves and save healthcare systems money while improving the patient and clinician experience.16One way to implement hospital-based addiction care is through an addiction consult team (ACT).17 While ACT compositions vary, most are interprofessional, offer evidence-based addiction treatment, and connect patients to community care.18 Our hospital’s ACT has nurses, patient navigators, and physicians who assess, diagnose, and treat SUD, and arrange follow-up addiction care.19 In addition to caring for individual patients, our ACT has led systems change. For example, we created order sets to guide clinicians, added medications to our hospital formulary to ensure access to evidence-based addiction treatment, and partnered with community stakeholders to streamline care transitions and access to psychosocial and medication treatment. Our team also worked with hospital leadership, nursing, and a syringe service program to integrate hospital harm reduction education and supply provision. Additionally, we are building capacity among staff, trainees, and clinicians through education and systems changes.
In hospitals without an ACT, leadership can finance SUD champions and integrate them into policy-level decision-making to implement best practices in addiction care and lead hospital-wide educational efforts. This will transform hospital culture and improve care as all clinicians develop essential addiction skills.
Addiction champions and ACTs could also advocate for equitable practices for patients with SUD to reduce the stigma that both prevents patients from seeking care and results in self-discharges.20 For example, with interprofessional support, we revised our in-hospital substance use policy. It previously entailed hospital security responding to substance use concerns, which unintentionally harmed patients and perpetuated stigma. Our revised policy ensures we offer medications for cravings and withdrawal, adequate pain management, and other services that address patients’ reasons for in-hospital substance use.
With the increasing prevalence of SUD among hospitalized patients, escalating substance-related deaths, rising healthcare costs, and the impact of addiction on health and well-being, addiction care, including ACTs and champions, must be adequately funded. However, sustainable financing remains a challenge.18
Caring for Rosa and others with SUD sparked my desire to learn about addiction, obtain addiction medicine board certification as a practicing hospitalist, and create an ACT that offers evidence-based addiction treatment. While much remains to be done, by collaborating with addiction champions and engaging hospital leadership, we have transformed our hospital’s approach to substance use care.
With the knowledge and resources I now have as an addiction medicine physician, I reimagine the possibilities for patients like Rosa.
Rosa died when living was possible.
*Name has been changed for patient privacy.
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. HHS Publication No. PEP21-07-01-003, NSDUH Series H-56. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Accessed December 1, 2021. www.samhsa.gov/data/
2. Centers for Disease Control and Prevention. Alcohol and public health: alcohol-related disease impact (ARDI) application, 2013. Average for United States 2006–2010 alcohol-attributable deaths due to excessive alcohol use. Accessed December 1, 2021. www.cdc.gov/ARDI
3. Spillane S, Shiels MS, Best AF, et al. Trends in alcohol-induced deaths in the United States, 2000-2016. JAMA Netw Open. 2020;3(2):e1921451. https://doi.org/ 10.1001/jamanetworkopen.2019.21451
4. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911-923. https://doi.org/10.1001/jamapsychiatry.2017.2161 https://doi.org/10.1001/jamapsychiatry.2017.2161
5. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the covid-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. https://doi.org/10.1001/jamanetworkopen.2020.22942
6. Bangaru S, Pedersen MR, Macconmara MP, Singal AG, Mufti AR. Survey of liver transplantation practices for severe acute alcoholic hepatitis. Liver Transpl. 2018;24(10):1357-1362. https://doi.org/10.1002/lt.25285
7. Herrick-Reynolds KM, Punchhi G, Greenberg RS, et al. Evaluation of early vs standard liver transplant for alcohol-associated liver disease. JAMA Surg. 2021;156(11):1026-1034. https://doi.org/10.1001/jamasurg.2021.3748
8. Fleming JN, Lai JC, Te HS, Said A, Spengler EK, Rogal SS. Opioid and opioid substitution therapy in liver transplant candidates: A survey of center policies and practices. Clin Transplant. 2017;31(12):e13119. https://doi.org/10.1111/ctr.13119
9. Klimas J, Fairgrieve C, Tobin H, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev. 2018;12(12):CD009269. https://doi.org/10.1002/14651858.CD009269.pub4
10. Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202–207. https://doi.org/10.15585/mmwr.mm7006a4
11. Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. Accessed November 18, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
12. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
13. California Bridge Program. Tools: Treat substance use disorders from the acute care setting. Accessed August 20, 2021. https://cabridge.org/tools
14. Peterson C, Li M, Xu L, Mikosz CA, Luo F. Assessment of annual cost of substance use disorder in US hospitals. JAMA Netw Open. 2021;4(3):e210242. https://doi.org/10.1001/jamanetworkopen.2021.0242
15. Suen LW, Makam AN, Snyder HR, et al. National prevalence of alcohol and other substance use disorders among emergency department visits and hospitalizations: NHAMCS 2014-2018. J Gen Intern Med. 2021;13:1-9. https://doi.org/10.1007/s11606-021-07069-w
16. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: Qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993
17. Priest KC, McCarty D. Making the business case for an addiction medicine consult service: a qualitative analysis. BMC Health Services Research. 2019;19(1):822. https://doi.org/10.1186/s12913-019-4670-4
18. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496
19. Martin M, Snyder HR, Coffa D, et al. Time to ACT: launching an Addiction Care Team (ACT) in an urban safety-net health system. BMJ Open Qual. 2021;10(1):e001111. https://doi.org/10.1136/bmjoq-2020-001111
20. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: A qualitative study. Subst Abus. 2020;41(4):519-525. https://doi.org/10.1080/08897077.2019.1671942
Rosa* was one of my first patients as an intern rotating at the county hospital. Her marriage had disintegrated years earlier. To cope with depression, she hid a daily ritual of orange juice and vodka from her children. She worked as a cashier, until nausea and fatigue overwhelmed her.
The first time I met her she sat on the gurney: petite, tanned, and pregnant. Then I saw her yellow eyes and revised: temporal wasting, jaundiced, and swollen with ascites. Rosa didn’t know that alcohol could cause liver disease. Without insurance or access to primary care, her untreated alcohol use disorder (AUD) and depression had snowballed for years.
Midway through my intern year, I’d taken care of many people with AUD. However, I’d barely learned anything about it as a medical student, though we’d spent weeks studying esoteric diseases, that now––9 years after medical school––I still have not encountered.
Among the 28.3 million individuals in the United States with AUD, only 1% receive medication treatment.1 In the United States, unhealthy alcohol use accounts for more than 95,000 deaths each year.2 This number likely under-captures alcohol-related mortality and is higher now given recent reports of increasing alcohol-related deaths and prevalence of unhealthy alcohol use, especially among women, younger age groups, and marginalized populations.3-5
Rosa had alcohol-related hepatitis, which can cause severe inflammation and liver failure and quickly lead to death. As her liver failure progressed, I asked the gastroenterologists, “What other treatments can we offer? Is she a liver transplant candidate?” “Nothing” and “No” they answered.
Later, I emailed the hepatologist and transplant surgeon begging them to reevaluate her transplantation candidacy, but they told me there was no exception to the institution’s 6-month sobriety rule.
Maintaining a 6-month sobriety period is not an evidence-based criterion for transplantation. However, 50% of transplant centers do not perform transplantation prior to 6 months of alcohol abstinence for alcohol-related hepatitis due to concern for return to drinking after transplant.6 This practice may promote bias in patient selection for transplantation. A recent study found that individuals with alcohol-related liver disease transplanted before 6 months of abstinence had similar rates of survival and return to drinking compared to those who abstained from alcohol for 6 months and participated in AUD treatment before transplantation.7
There are other liver transplant practices that result in inequities for individuals with substance use disorders (SUD). Some liver transplant centers consider being on a medication for opioid use disorder a contraindication for transplantation—even if the individual is in recovery and abstaining from substances.8 Others mandate that individuals with alcohol-related liver disease attend Alcoholics Anonymous (AA) meetings prior to transplant. While mutual help groups, including AA, may benefit some individuals, different approaches work for different people.9 Other psychosocial interventions (eg, cognitive-behavioral therapy, contingency management, and residential treatment) and medications also help individuals reduce or stop drinking. Some meet their goals without any treatment. Addiction care works best when it respects autonomy and meets individuals where they are by allowing them to decide among options.
While organ allocations are a crystalized example of inequities in addiction care, they are also ethically complex. Many individuals—with and without SUD—die on waiting lists and must meet stringent transplantation criteria. However, we can at least remove the unnecessary biases that compound inequities in care people with SUD already face.
As Rosa’s liver succumbed, her kidneys failed too, and she required dialysis. She sensed what was coming. “I want everything…for now. I need to take care of my children.” I, too, wanted Rosa to live and see her youngest start kindergarten.
A few days before her discharge, I walked to the pharmacy and bought a purple journal. In a rare moment, I found Rosa alone in her room, without her ex-husband, sister, and mother, who rarely left her bedside. Together, we called AA and explored whether she could start participating in phone meetings from the hospital. I explained that one way to document a commitment to sobriety, as the transplant center’s rules dictated, was to attend and document AA meetings in this notebook. “In 5 months, you will be a liver transplant candidate,” I remember saying, wishing it to fruition.
I became Rosa’s primary care physician and saw her in clinic. Over the next few weeks, her skin took on an ashen tone. Sleep escaped her and her thoughts and speech blurred. Her walk slowed and she needed a wheelchair. The quiet fierceness that had defined her dissipated as encephalopathy took over. But until our last visit, she brought her purple journal, tracking the AA meetings she’d attended. Dialysis became intolerable, but not before Rosa made care arrangements for her girls. When that happened, she stopped dialysis and went to Mexico, where she died in her sleep after saying good-bye to her father.
Earlier access to healthcare and effective depression and AUD treatment could have saved Rosa’s life. While it was too late for her, as hospitalists we care for many others with substance-related complications and may miss opportunities to discuss and offer evidence-based addiction treatment. For example, we initiate the most up-to-date management for a patient’s gastrointestinal bleed but may leave the alcohol discussion for someone else. It is similar for other SUD: we treat cellulitis, epidural abscesses, bacteremia, chronic obstructive pulmonary disease, heart failure exacerbations, and other complications of SUD without addressing the root cause of the hospitalization—other than to prescribe abstinence from substance use or, at our worst, scold individuals for continuing to use.
But what can we offer? Most healthcare professionals still do not receive addiction education during training. Without tools, we enact temporizing measures, until patients return to the hospital or die.
In addition to increasing alcohol-related morbidity, there have also been increases in drug-related overdoses, fueled by COVID-19, synthetic opioids like fentanyl, and stimulants.10 In the 12-month period ending April 2021, more than 100,000 individuals died of drug-related overdoses, the highest number of deaths ever recorded in a year.11 Despite this, most healthcare systems remain unequipped to provide addiction services during hospitalization due to inadequate training, stigma, and lack of systems-based care.
Hospitalists and healthcare systems cannot be bystanders amid our worsening addiction crisis. We must empower clinicians with addiction education and ensure health systems offer evidence-based SUD services.
Educational efforts can close the knowledge gaps for both medical students and hospitalists. Medical schools should include foundational curricular content in screening, assessing, diagnosing, and treating SUD in alignment with standards set by the Liaison Committee on Medical Education, which accredits US medical schools. Residency programs can offer educational conferences, cased-based discussions, and addiction medicine rotations. Hospitalists can participate in educational didactics and review evidence-based addiction guidelines.12,13 While the focus here is on hospitalists, clinicians across practice settings and specialties will encounter patients with SUD, and all need to be well-versed in the diagnosis and treatment of addiction given the all-hands-on deck approach necessary amidst our worsening addiction crisis.
With one in nine hospitalizations involving individuals with SUD, and this number quickly rising, and with an annual cost to US hospitals of $13.2 billion, healthcare system leaders must invest in addiction care.14,15 Hospital-based addiction services could pay for themselves and save healthcare systems money while improving the patient and clinician experience.16One way to implement hospital-based addiction care is through an addiction consult team (ACT).17 While ACT compositions vary, most are interprofessional, offer evidence-based addiction treatment, and connect patients to community care.18 Our hospital’s ACT has nurses, patient navigators, and physicians who assess, diagnose, and treat SUD, and arrange follow-up addiction care.19 In addition to caring for individual patients, our ACT has led systems change. For example, we created order sets to guide clinicians, added medications to our hospital formulary to ensure access to evidence-based addiction treatment, and partnered with community stakeholders to streamline care transitions and access to psychosocial and medication treatment. Our team also worked with hospital leadership, nursing, and a syringe service program to integrate hospital harm reduction education and supply provision. Additionally, we are building capacity among staff, trainees, and clinicians through education and systems changes.
In hospitals without an ACT, leadership can finance SUD champions and integrate them into policy-level decision-making to implement best practices in addiction care and lead hospital-wide educational efforts. This will transform hospital culture and improve care as all clinicians develop essential addiction skills.
Addiction champions and ACTs could also advocate for equitable practices for patients with SUD to reduce the stigma that both prevents patients from seeking care and results in self-discharges.20 For example, with interprofessional support, we revised our in-hospital substance use policy. It previously entailed hospital security responding to substance use concerns, which unintentionally harmed patients and perpetuated stigma. Our revised policy ensures we offer medications for cravings and withdrawal, adequate pain management, and other services that address patients’ reasons for in-hospital substance use.
With the increasing prevalence of SUD among hospitalized patients, escalating substance-related deaths, rising healthcare costs, and the impact of addiction on health and well-being, addiction care, including ACTs and champions, must be adequately funded. However, sustainable financing remains a challenge.18
Caring for Rosa and others with SUD sparked my desire to learn about addiction, obtain addiction medicine board certification as a practicing hospitalist, and create an ACT that offers evidence-based addiction treatment. While much remains to be done, by collaborating with addiction champions and engaging hospital leadership, we have transformed our hospital’s approach to substance use care.
With the knowledge and resources I now have as an addiction medicine physician, I reimagine the possibilities for patients like Rosa.
Rosa died when living was possible.
*Name has been changed for patient privacy.
Rosa* was one of my first patients as an intern rotating at the county hospital. Her marriage had disintegrated years earlier. To cope with depression, she hid a daily ritual of orange juice and vodka from her children. She worked as a cashier, until nausea and fatigue overwhelmed her.
The first time I met her she sat on the gurney: petite, tanned, and pregnant. Then I saw her yellow eyes and revised: temporal wasting, jaundiced, and swollen with ascites. Rosa didn’t know that alcohol could cause liver disease. Without insurance or access to primary care, her untreated alcohol use disorder (AUD) and depression had snowballed for years.
Midway through my intern year, I’d taken care of many people with AUD. However, I’d barely learned anything about it as a medical student, though we’d spent weeks studying esoteric diseases, that now––9 years after medical school––I still have not encountered.
Among the 28.3 million individuals in the United States with AUD, only 1% receive medication treatment.1 In the United States, unhealthy alcohol use accounts for more than 95,000 deaths each year.2 This number likely under-captures alcohol-related mortality and is higher now given recent reports of increasing alcohol-related deaths and prevalence of unhealthy alcohol use, especially among women, younger age groups, and marginalized populations.3-5
Rosa had alcohol-related hepatitis, which can cause severe inflammation and liver failure and quickly lead to death. As her liver failure progressed, I asked the gastroenterologists, “What other treatments can we offer? Is she a liver transplant candidate?” “Nothing” and “No” they answered.
Later, I emailed the hepatologist and transplant surgeon begging them to reevaluate her transplantation candidacy, but they told me there was no exception to the institution’s 6-month sobriety rule.
Maintaining a 6-month sobriety period is not an evidence-based criterion for transplantation. However, 50% of transplant centers do not perform transplantation prior to 6 months of alcohol abstinence for alcohol-related hepatitis due to concern for return to drinking after transplant.6 This practice may promote bias in patient selection for transplantation. A recent study found that individuals with alcohol-related liver disease transplanted before 6 months of abstinence had similar rates of survival and return to drinking compared to those who abstained from alcohol for 6 months and participated in AUD treatment before transplantation.7
There are other liver transplant practices that result in inequities for individuals with substance use disorders (SUD). Some liver transplant centers consider being on a medication for opioid use disorder a contraindication for transplantation—even if the individual is in recovery and abstaining from substances.8 Others mandate that individuals with alcohol-related liver disease attend Alcoholics Anonymous (AA) meetings prior to transplant. While mutual help groups, including AA, may benefit some individuals, different approaches work for different people.9 Other psychosocial interventions (eg, cognitive-behavioral therapy, contingency management, and residential treatment) and medications also help individuals reduce or stop drinking. Some meet their goals without any treatment. Addiction care works best when it respects autonomy and meets individuals where they are by allowing them to decide among options.
While organ allocations are a crystalized example of inequities in addiction care, they are also ethically complex. Many individuals—with and without SUD—die on waiting lists and must meet stringent transplantation criteria. However, we can at least remove the unnecessary biases that compound inequities in care people with SUD already face.
As Rosa’s liver succumbed, her kidneys failed too, and she required dialysis. She sensed what was coming. “I want everything…for now. I need to take care of my children.” I, too, wanted Rosa to live and see her youngest start kindergarten.
A few days before her discharge, I walked to the pharmacy and bought a purple journal. In a rare moment, I found Rosa alone in her room, without her ex-husband, sister, and mother, who rarely left her bedside. Together, we called AA and explored whether she could start participating in phone meetings from the hospital. I explained that one way to document a commitment to sobriety, as the transplant center’s rules dictated, was to attend and document AA meetings in this notebook. “In 5 months, you will be a liver transplant candidate,” I remember saying, wishing it to fruition.
I became Rosa’s primary care physician and saw her in clinic. Over the next few weeks, her skin took on an ashen tone. Sleep escaped her and her thoughts and speech blurred. Her walk slowed and she needed a wheelchair. The quiet fierceness that had defined her dissipated as encephalopathy took over. But until our last visit, she brought her purple journal, tracking the AA meetings she’d attended. Dialysis became intolerable, but not before Rosa made care arrangements for her girls. When that happened, she stopped dialysis and went to Mexico, where she died in her sleep after saying good-bye to her father.
Earlier access to healthcare and effective depression and AUD treatment could have saved Rosa’s life. While it was too late for her, as hospitalists we care for many others with substance-related complications and may miss opportunities to discuss and offer evidence-based addiction treatment. For example, we initiate the most up-to-date management for a patient’s gastrointestinal bleed but may leave the alcohol discussion for someone else. It is similar for other SUD: we treat cellulitis, epidural abscesses, bacteremia, chronic obstructive pulmonary disease, heart failure exacerbations, and other complications of SUD without addressing the root cause of the hospitalization—other than to prescribe abstinence from substance use or, at our worst, scold individuals for continuing to use.
But what can we offer? Most healthcare professionals still do not receive addiction education during training. Without tools, we enact temporizing measures, until patients return to the hospital or die.
In addition to increasing alcohol-related morbidity, there have also been increases in drug-related overdoses, fueled by COVID-19, synthetic opioids like fentanyl, and stimulants.10 In the 12-month period ending April 2021, more than 100,000 individuals died of drug-related overdoses, the highest number of deaths ever recorded in a year.11 Despite this, most healthcare systems remain unequipped to provide addiction services during hospitalization due to inadequate training, stigma, and lack of systems-based care.
Hospitalists and healthcare systems cannot be bystanders amid our worsening addiction crisis. We must empower clinicians with addiction education and ensure health systems offer evidence-based SUD services.
Educational efforts can close the knowledge gaps for both medical students and hospitalists. Medical schools should include foundational curricular content in screening, assessing, diagnosing, and treating SUD in alignment with standards set by the Liaison Committee on Medical Education, which accredits US medical schools. Residency programs can offer educational conferences, cased-based discussions, and addiction medicine rotations. Hospitalists can participate in educational didactics and review evidence-based addiction guidelines.12,13 While the focus here is on hospitalists, clinicians across practice settings and specialties will encounter patients with SUD, and all need to be well-versed in the diagnosis and treatment of addiction given the all-hands-on deck approach necessary amidst our worsening addiction crisis.
With one in nine hospitalizations involving individuals with SUD, and this number quickly rising, and with an annual cost to US hospitals of $13.2 billion, healthcare system leaders must invest in addiction care.14,15 Hospital-based addiction services could pay for themselves and save healthcare systems money while improving the patient and clinician experience.16One way to implement hospital-based addiction care is through an addiction consult team (ACT).17 While ACT compositions vary, most are interprofessional, offer evidence-based addiction treatment, and connect patients to community care.18 Our hospital’s ACT has nurses, patient navigators, and physicians who assess, diagnose, and treat SUD, and arrange follow-up addiction care.19 In addition to caring for individual patients, our ACT has led systems change. For example, we created order sets to guide clinicians, added medications to our hospital formulary to ensure access to evidence-based addiction treatment, and partnered with community stakeholders to streamline care transitions and access to psychosocial and medication treatment. Our team also worked with hospital leadership, nursing, and a syringe service program to integrate hospital harm reduction education and supply provision. Additionally, we are building capacity among staff, trainees, and clinicians through education and systems changes.
In hospitals without an ACT, leadership can finance SUD champions and integrate them into policy-level decision-making to implement best practices in addiction care and lead hospital-wide educational efforts. This will transform hospital culture and improve care as all clinicians develop essential addiction skills.
Addiction champions and ACTs could also advocate for equitable practices for patients with SUD to reduce the stigma that both prevents patients from seeking care and results in self-discharges.20 For example, with interprofessional support, we revised our in-hospital substance use policy. It previously entailed hospital security responding to substance use concerns, which unintentionally harmed patients and perpetuated stigma. Our revised policy ensures we offer medications for cravings and withdrawal, adequate pain management, and other services that address patients’ reasons for in-hospital substance use.
With the increasing prevalence of SUD among hospitalized patients, escalating substance-related deaths, rising healthcare costs, and the impact of addiction on health and well-being, addiction care, including ACTs and champions, must be adequately funded. However, sustainable financing remains a challenge.18
Caring for Rosa and others with SUD sparked my desire to learn about addiction, obtain addiction medicine board certification as a practicing hospitalist, and create an ACT that offers evidence-based addiction treatment. While much remains to be done, by collaborating with addiction champions and engaging hospital leadership, we have transformed our hospital’s approach to substance use care.
With the knowledge and resources I now have as an addiction medicine physician, I reimagine the possibilities for patients like Rosa.
Rosa died when living was possible.
*Name has been changed for patient privacy.
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. HHS Publication No. PEP21-07-01-003, NSDUH Series H-56. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Accessed December 1, 2021. www.samhsa.gov/data/
2. Centers for Disease Control and Prevention. Alcohol and public health: alcohol-related disease impact (ARDI) application, 2013. Average for United States 2006–2010 alcohol-attributable deaths due to excessive alcohol use. Accessed December 1, 2021. www.cdc.gov/ARDI
3. Spillane S, Shiels MS, Best AF, et al. Trends in alcohol-induced deaths in the United States, 2000-2016. JAMA Netw Open. 2020;3(2):e1921451. https://doi.org/ 10.1001/jamanetworkopen.2019.21451
4. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911-923. https://doi.org/10.1001/jamapsychiatry.2017.2161 https://doi.org/10.1001/jamapsychiatry.2017.2161
5. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the covid-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. https://doi.org/10.1001/jamanetworkopen.2020.22942
6. Bangaru S, Pedersen MR, Macconmara MP, Singal AG, Mufti AR. Survey of liver transplantation practices for severe acute alcoholic hepatitis. Liver Transpl. 2018;24(10):1357-1362. https://doi.org/10.1002/lt.25285
7. Herrick-Reynolds KM, Punchhi G, Greenberg RS, et al. Evaluation of early vs standard liver transplant for alcohol-associated liver disease. JAMA Surg. 2021;156(11):1026-1034. https://doi.org/10.1001/jamasurg.2021.3748
8. Fleming JN, Lai JC, Te HS, Said A, Spengler EK, Rogal SS. Opioid and opioid substitution therapy in liver transplant candidates: A survey of center policies and practices. Clin Transplant. 2017;31(12):e13119. https://doi.org/10.1111/ctr.13119
9. Klimas J, Fairgrieve C, Tobin H, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev. 2018;12(12):CD009269. https://doi.org/10.1002/14651858.CD009269.pub4
10. Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202–207. https://doi.org/10.15585/mmwr.mm7006a4
11. Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. Accessed November 18, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
12. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
13. California Bridge Program. Tools: Treat substance use disorders from the acute care setting. Accessed August 20, 2021. https://cabridge.org/tools
14. Peterson C, Li M, Xu L, Mikosz CA, Luo F. Assessment of annual cost of substance use disorder in US hospitals. JAMA Netw Open. 2021;4(3):e210242. https://doi.org/10.1001/jamanetworkopen.2021.0242
15. Suen LW, Makam AN, Snyder HR, et al. National prevalence of alcohol and other substance use disorders among emergency department visits and hospitalizations: NHAMCS 2014-2018. J Gen Intern Med. 2021;13:1-9. https://doi.org/10.1007/s11606-021-07069-w
16. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: Qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993
17. Priest KC, McCarty D. Making the business case for an addiction medicine consult service: a qualitative analysis. BMC Health Services Research. 2019;19(1):822. https://doi.org/10.1186/s12913-019-4670-4
18. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496
19. Martin M, Snyder HR, Coffa D, et al. Time to ACT: launching an Addiction Care Team (ACT) in an urban safety-net health system. BMJ Open Qual. 2021;10(1):e001111. https://doi.org/10.1136/bmjoq-2020-001111
20. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: A qualitative study. Subst Abus. 2020;41(4):519-525. https://doi.org/10.1080/08897077.2019.1671942
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. HHS Publication No. PEP21-07-01-003, NSDUH Series H-56. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Accessed December 1, 2021. www.samhsa.gov/data/
2. Centers for Disease Control and Prevention. Alcohol and public health: alcohol-related disease impact (ARDI) application, 2013. Average for United States 2006–2010 alcohol-attributable deaths due to excessive alcohol use. Accessed December 1, 2021. www.cdc.gov/ARDI
3. Spillane S, Shiels MS, Best AF, et al. Trends in alcohol-induced deaths in the United States, 2000-2016. JAMA Netw Open. 2020;3(2):e1921451. https://doi.org/ 10.1001/jamanetworkopen.2019.21451
4. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911-923. https://doi.org/10.1001/jamapsychiatry.2017.2161 https://doi.org/10.1001/jamapsychiatry.2017.2161
5. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the covid-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. https://doi.org/10.1001/jamanetworkopen.2020.22942
6. Bangaru S, Pedersen MR, Macconmara MP, Singal AG, Mufti AR. Survey of liver transplantation practices for severe acute alcoholic hepatitis. Liver Transpl. 2018;24(10):1357-1362. https://doi.org/10.1002/lt.25285
7. Herrick-Reynolds KM, Punchhi G, Greenberg RS, et al. Evaluation of early vs standard liver transplant for alcohol-associated liver disease. JAMA Surg. 2021;156(11):1026-1034. https://doi.org/10.1001/jamasurg.2021.3748
8. Fleming JN, Lai JC, Te HS, Said A, Spengler EK, Rogal SS. Opioid and opioid substitution therapy in liver transplant candidates: A survey of center policies and practices. Clin Transplant. 2017;31(12):e13119. https://doi.org/10.1111/ctr.13119
9. Klimas J, Fairgrieve C, Tobin H, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev. 2018;12(12):CD009269. https://doi.org/10.1002/14651858.CD009269.pub4
10. Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202–207. https://doi.org/10.15585/mmwr.mm7006a4
11. Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. Accessed November 18, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
12. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
13. California Bridge Program. Tools: Treat substance use disorders from the acute care setting. Accessed August 20, 2021. https://cabridge.org/tools
14. Peterson C, Li M, Xu L, Mikosz CA, Luo F. Assessment of annual cost of substance use disorder in US hospitals. JAMA Netw Open. 2021;4(3):e210242. https://doi.org/10.1001/jamanetworkopen.2021.0242
15. Suen LW, Makam AN, Snyder HR, et al. National prevalence of alcohol and other substance use disorders among emergency department visits and hospitalizations: NHAMCS 2014-2018. J Gen Intern Med. 2021;13:1-9. https://doi.org/10.1007/s11606-021-07069-w
16. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: Qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993
17. Priest KC, McCarty D. Making the business case for an addiction medicine consult service: a qualitative analysis. BMC Health Services Research. 2019;19(1):822. https://doi.org/10.1186/s12913-019-4670-4
18. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496
19. Martin M, Snyder HR, Coffa D, et al. Time to ACT: launching an Addiction Care Team (ACT) in an urban safety-net health system. BMJ Open Qual. 2021;10(1):e001111. https://doi.org/10.1136/bmjoq-2020-001111
20. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: A qualitative study. Subst Abus. 2020;41(4):519-525. https://doi.org/10.1080/08897077.2019.1671942
© 2021 Society of Hospital Medicine
The Kids Are Not Alright
“...but it all started to get worse during the pandemic.”
As the patient’s† door closed, I (JS) thought about what his father had shared: his 12-year-old son had experienced a slow decline in his mental health since March 2020. There had been a gradual loss of all the things his son needed for psychological well-being: school went virtual and extracurricular activities ceased, and with them went any sense of routine, normalcy, or authentic opportunities to socialize. His feelings of isolation and depression culminated in an attempt to end his own life. My mind shifted to other patients under our care: an 8-year-old with behavioral outbursts intensifying after school-based therapy ended, a 13-year-old who became suicidal from isolation and virtual bullying. These children’s families sought emergent care because they no longer had the resources to care for their children at home. My team left each of these rooms heartbroken, unsure of exactly what to say and aware of the limitations of our current healthcare system.
Before and during the COVID-19 pandemic, many pediatric providers have had similar experiences caring for countless patients who are “boarding”—awaiting transfer to a psychiatric facility for their primary acute psychiatric issue, initially in the emergency room, often for 5 days or more,1 then ultimately admitted to a general medical floor if an appropriate psychiatric bed is still not available.2 Unfortunately, just as parents have run out of resources to care for their children’s psychiatric needs, so too is our medical system lacking in resources to provide the acute care these children need in general hospitals.
This mental health crisis began before the COVID-19 pandemic3 but has only worsened in the wake of its resulting social isolation. During the pandemic, suicide hotlines had a 1000% increase in call volumes.4 COVID-19–induced bed closures simultaneously worsened an existing critical bed shortage5,6 and led to an increase in the average length of stay (LOS) for patients boarding in the emergency department (ED).7 In the state of Massachusetts, for example, psychiatric patients awaiting inpatient beds boarded for more than 10,000 hours in January 2021—more than ever before, and up approximately 4000 hours since January 2017.6 For pediatric patients, the average wait time is now 59 hours.6 In the first 6 months of the pandemic, 39% of children presenting to EDs for mental health complaints ended up boarding, which is an astounding figure and is unfortunately 7% higher than in 2019.8 Even these staggering numbers do not capture the full range of experiences, as many statistics do not account for time spent on inpatient units by patients who do not receive a bed placement after waiting hours to several days in the ED.
Shortages of space, as well as an underfunded and understaffed mental health workforce, lead to these prolonged, often traumatic boarding periods in hospitals designed to care for acute medical, rather than acute psychiatric, conditions. Patients awaiting psychiatric placement are waiting in settings that are chaotic, inconsistent, and lacking in privacy. A patient in the throes of psychosis or suicidality needs a therapeutic milieu, not one that interrupts their daily routine,2 disconnects them from their existing support networks, and is punctuated by the incessant clangs of bedside monitors and the hubbub of code teams. These environments are not therapeutic3 for young infants with fevers, let alone for teenagers battling suicidality and eating disorders. In fact, for these reasons, we suspect that many of our patients’ inpatient ”behavioral escalations” are in fact triggered by their hospital environment, which may contribute to the 300% increase in the number of pharmacological restraints used during mental health visits in the ED over the past 10 years.9
None of us imagined when we chose to pursue pediatrics a that significant—and at times predominant—portion of our training would encompass caring for patients with acute mental health concerns. And although we did not anticipate this crisis, we have now been tasked with managing it. Throughout the day, when we are called to see our patients with primarily psychiatric pathology, we are often at war with ourselves. We weigh forming deeply meaningful relationships with these patients against the potential of unintentionally retraumatizing them or forming bonds that will be abruptly severed when patients are transferred to a psychiatric facility, which often occurs with barely a few hours’ notice. Moreover, many healthcare workers have training ill-suited to meet the needs of these patients. Just as emergency physicians can diagnose appendicitis but rely on surgeons to provide timely surgical treatment, general pediatricians identify psychiatric crises but rely on psychiatrists for ideal treatment plans. And almost daily, we are called to an “escalating” patient and arrive minutes into a stressful situation that others expect us to extinguish expeditiously. Along with nursing colleagues and the behavioral response team, we enact the treatment plan laid out by our psychiatry colleagues and wonder whether there is a better way.
We propose the following changes to create a more ideal health system (Table). We acknowledge that each health system has unique resources, challenges, and patient populations. Thus, our recommendations are not comprehensive and are largely based on experiences within our own institutions and state, but they encompass many domains that impact and are affected by child and adolescent mental healthcare in the United States, ranging from program- and hospital-level innovation to community and legislative action.
UPSTREAM PREVENTION
Like all good health system designs, we recommend prioritizing prevention. This would entail funding programs and legislation such as H.R. 3180, the RISE from Trauma Act, and H.R. 8544, the STRONG Support for Children Act of 2020 (both currently under consideration in the US House of Representatives) that support early childhood development and prevent adverse childhood experiences and trauma, averting mental health diagnoses such as depression and attention-deficit/hyperactivity disorder before they begin.10
OUTPATIENT AND COMMUNITY RESOURCES
We recognize that schools and general pediatricians have far more exposure to children at risk for mental health crises than do subspecialists. Thus, we urge an equitable increase in access to mental healthcare in the community so that patients needing assistance are screened and diagnosed earlier in their illness, allowing for secondary prevention of worsening mental health disorders. We support increased funding for programs such as the Massachusetts Child Psychiatry Access Program, which allows primary care doctors to consult psychiatrists in real time, closing the gap between a primary care visit and specialty follow-up. Telehealth services will be key to improving access for patients themselves and to allow pediatricians to consult with mental health professionals to initiate care prior to specialist availability. We envision that strengthening school-based behavioral health resources will also help prevent ED visits. Behavioral healthcare should be integrated into schools and community centers while police presence is simultaneously reduced, as there is evidence of an increased likelihood of juvenile justice involvement for children with disabilities and mental health needs.11,12
WORKFORCE DEVELOPMENT AND TRAINING
Ensuring access necessitates increasing the capacity of our psychiatric workforce by encouraging graduates to pursue mental health occupations with concrete financial incentives such as loan repayment and training grants. We thus support legislation such as H.R. 6597, the Mental Health Professionals Workforce Shortage Loan Repayment Act of 2018 (currently under consideration in the US House of Representatives). This may also improve recruitment and retention of individuals who are underrepresented in medicine, one step in helping ensure children have access to linguistically appropriate and culturally sensitive care. Residency programs and hospital systems should expand their training and education to identify and stabilize patients in mental health in extremis through culturally sensitive curricula focused on behavioral de-escalation techniques, trauma-informed care, and psychopharmacology. Our own residency program created a 2-week mental health rotation13 that includes rotating with outpatient mental health providers and our hospital’s behavioral response team, a group of trauma-informed responders for behavioral emergencies. Similar training should be available for nursing and other allied health professionals, who are often the first responders to behavioral escalations.13
INSTITUTIONAL DEVELOPMENT AND CLINICAL PRACTICES
Ideally, patients requiring higher-intensity psychiatric care would be referred to specialized pediatric behavioral health urgent care centers so their conditions can be adequately evaluated and addressed by staff trained in psychiatric management and in therapeutic environments. We believe all providers caring for children with mental health needs should be trained in basic, but core, behavioral health and de-escalation competencies, including specialized training for children with comorbid medical and neurodevelopmental diagnoses, such as autism. These centers should have specific beds for young children and those with developmental or complex care needs, and services should be available in numerous languages and levels of health literacy to allow all families to participate in their child’s care. At the same time, even nonpsychiatric EDs and inpatient units should commit resources to developing a maximally therapeutic environment, including allowing adjunctive services such as child life services, group therapy, and pet and music therapy, and create environments that support, rather than disrupt, normal routines.
HEALTH SYSTEMS REFORM AND ADVOCACY
Underpinning all the above innovations are changes to our healthcare payment system and provider networks, including the need for insurance coverage and payment parity for behavioral health, to ensure care is not only accessible but affordable. Additionally, for durable change, we need more than just education—we need coalition building and advocacy. Many organizations, including the American Academy of Pediatrics and the Children’s Hospital Association, have begun this work, which we must all continue.14 Bringing in diverse partners, including health systems, providers, educators, hospital administrators, payors, elected officials, and communities, will prioritize children’s needs and create a more ideal pediatric behavioral healthcare system.15
The COVID-19 pandemic has highlighted the dire need for comprehensive mental healthcare in the United States, a need that existed before the pandemic and will persist in a more fragile state long after it ends. Our hope is that the pandemic serves as the catalyst necessary to promote the magnitude of investments and stakeholder buy-in necessary to improve pediatric mental health and engender a radical redesign of our behavioral healthcare system. Our patients are counting on us to act. Together, we can build a system that ensures that the kids will be alright.
†Patient details have been changed for patient privacy.
Acknowledgments
The authors thank Joanna Perdomo, MD, Amara Azubuike, JD, and Josh Greenberg, JD, for reading and providing feedback on earlier versions of this work.
1. “This is a crisis”: mom whose son has boarded 33 days for psych bed calls for state action. WBUR News. Updated March 2, 2021. Accessed August 4, 2021. www.wbur.org/news/2021/02/26/mental-health-boarding-hospitals
2. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813-824. https://doi.org/10.1016/S2215-0366(20)30307-2
3. Nash KA, Zima BT, Rothenberg C, et al. Prolonged emergency department length of stay for US pediatric mental health visits (2005-2015). Pediatrics. 2021;147(5):e2020030692. https://doi.org/10.1542/peds.2020-030692
4. Cloutier RL, Marshaall R. A dangerous pandemic pair: Covid19 and adolescent mental health emergencies. Am J Emerg Med. 2021;46:776-777. https://doi.org/10.1016/j.ajem.2020.09.008
5. Schoenberg S. Lack of mental health beds means long ER waits. CommonWealth Magazine. April 15, 2021. Accessed August 5, 2021. https://commonwealthmagazine.org/health-care/lack-of-mental-health-beds-means-long-er-waits/
6. Jolicoeur L, Mullins L. Mass. physicians call on state to address ER “boarding” of patients awaiting admission. WBUR News. Updated February 3, 2021. Accessed August 5, 2021. www.wbur.org/news/2021/02/02/emergency-department-er-inpatient-beds-boarding
7. Krass P, Dalton E, Doupnik SK, Esposito J. US pediatric emergency department visits for mental health conditions during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e218533. https://doi.org/10.1001/jamanetworkopen.2021.8533
8. Impact of COVID-19 on the Massachusetts Health Care System: Interim Report. Massachusetts Health Policy Commission. April 2021. Accessed September 25, 2021. www.mass.gov/doc/impact-of-covid-19-on-the-massachusetts-health-care-system-interim-report/download
9. Foster AA, Porter JJ, Monuteaux MC, Hoffmann JA, Hudgins JD. Pharmacologic restraint use during mental health visits in pediatric emergency departments. J Pediatr. 2021;236:276-283.e2. https://doi.org/10.1016/j.jpeds.2021.03.027
10. Brown NM, Brown SN, Briggs RD, Germán M, Belamarich PF, Oyeku SO. Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad Pediatr. 2017;17(4):349-355. https://doi.org/10.1016/j.acap.2016.08.013
11. Harper K, Ryberg R, Temkin D. Black students and students with disabilities remain more likely to receive out-of-school suspensions, despite overall declines. Child Trends. April 29, 2019. Accessed August 5, 2021. www.childtrends.org/publications/black-students-disabilities-out-of-school-suspensions
12. Whitaker A, Torres-Guillén S, Morton M, et al. Cops and no counselors: how the lack of school mental health staff is harming students. American Civil Liberties Union. Accessed August 6, 2021. www.aclu.org/report/cops-and-no-counselors
13. Education. Boston Combined Residence Program. Accessed August 5, 2021. https://msbcrp.wpengine.com/program/education/
14. American Academy of Pediatrics. Interim guidance on supporting the emotional and behavioral health needs of children, adolescents, and families during the COVID-19 pandemic. Updated July 28, 2021. Accessed August 5, 2021. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-on-supporting-the-emotional-and-behavioral-health-needs-of-children-adolescents-and-families-during-the-covid-19-pandemic/
15. Advocacy. Children’s Mental Health Campaign. Accessed August 4, 2021. https://childrensmentalhealthcampaign.org/advocacy
“...but it all started to get worse during the pandemic.”
As the patient’s† door closed, I (JS) thought about what his father had shared: his 12-year-old son had experienced a slow decline in his mental health since March 2020. There had been a gradual loss of all the things his son needed for psychological well-being: school went virtual and extracurricular activities ceased, and with them went any sense of routine, normalcy, or authentic opportunities to socialize. His feelings of isolation and depression culminated in an attempt to end his own life. My mind shifted to other patients under our care: an 8-year-old with behavioral outbursts intensifying after school-based therapy ended, a 13-year-old who became suicidal from isolation and virtual bullying. These children’s families sought emergent care because they no longer had the resources to care for their children at home. My team left each of these rooms heartbroken, unsure of exactly what to say and aware of the limitations of our current healthcare system.
Before and during the COVID-19 pandemic, many pediatric providers have had similar experiences caring for countless patients who are “boarding”—awaiting transfer to a psychiatric facility for their primary acute psychiatric issue, initially in the emergency room, often for 5 days or more,1 then ultimately admitted to a general medical floor if an appropriate psychiatric bed is still not available.2 Unfortunately, just as parents have run out of resources to care for their children’s psychiatric needs, so too is our medical system lacking in resources to provide the acute care these children need in general hospitals.
This mental health crisis began before the COVID-19 pandemic3 but has only worsened in the wake of its resulting social isolation. During the pandemic, suicide hotlines had a 1000% increase in call volumes.4 COVID-19–induced bed closures simultaneously worsened an existing critical bed shortage5,6 and led to an increase in the average length of stay (LOS) for patients boarding in the emergency department (ED).7 In the state of Massachusetts, for example, psychiatric patients awaiting inpatient beds boarded for more than 10,000 hours in January 2021—more than ever before, and up approximately 4000 hours since January 2017.6 For pediatric patients, the average wait time is now 59 hours.6 In the first 6 months of the pandemic, 39% of children presenting to EDs for mental health complaints ended up boarding, which is an astounding figure and is unfortunately 7% higher than in 2019.8 Even these staggering numbers do not capture the full range of experiences, as many statistics do not account for time spent on inpatient units by patients who do not receive a bed placement after waiting hours to several days in the ED.
Shortages of space, as well as an underfunded and understaffed mental health workforce, lead to these prolonged, often traumatic boarding periods in hospitals designed to care for acute medical, rather than acute psychiatric, conditions. Patients awaiting psychiatric placement are waiting in settings that are chaotic, inconsistent, and lacking in privacy. A patient in the throes of psychosis or suicidality needs a therapeutic milieu, not one that interrupts their daily routine,2 disconnects them from their existing support networks, and is punctuated by the incessant clangs of bedside monitors and the hubbub of code teams. These environments are not therapeutic3 for young infants with fevers, let alone for teenagers battling suicidality and eating disorders. In fact, for these reasons, we suspect that many of our patients’ inpatient ”behavioral escalations” are in fact triggered by their hospital environment, which may contribute to the 300% increase in the number of pharmacological restraints used during mental health visits in the ED over the past 10 years.9
None of us imagined when we chose to pursue pediatrics a that significant—and at times predominant—portion of our training would encompass caring for patients with acute mental health concerns. And although we did not anticipate this crisis, we have now been tasked with managing it. Throughout the day, when we are called to see our patients with primarily psychiatric pathology, we are often at war with ourselves. We weigh forming deeply meaningful relationships with these patients against the potential of unintentionally retraumatizing them or forming bonds that will be abruptly severed when patients are transferred to a psychiatric facility, which often occurs with barely a few hours’ notice. Moreover, many healthcare workers have training ill-suited to meet the needs of these patients. Just as emergency physicians can diagnose appendicitis but rely on surgeons to provide timely surgical treatment, general pediatricians identify psychiatric crises but rely on psychiatrists for ideal treatment plans. And almost daily, we are called to an “escalating” patient and arrive minutes into a stressful situation that others expect us to extinguish expeditiously. Along with nursing colleagues and the behavioral response team, we enact the treatment plan laid out by our psychiatry colleagues and wonder whether there is a better way.
We propose the following changes to create a more ideal health system (Table). We acknowledge that each health system has unique resources, challenges, and patient populations. Thus, our recommendations are not comprehensive and are largely based on experiences within our own institutions and state, but they encompass many domains that impact and are affected by child and adolescent mental healthcare in the United States, ranging from program- and hospital-level innovation to community and legislative action.
UPSTREAM PREVENTION
Like all good health system designs, we recommend prioritizing prevention. This would entail funding programs and legislation such as H.R. 3180, the RISE from Trauma Act, and H.R. 8544, the STRONG Support for Children Act of 2020 (both currently under consideration in the US House of Representatives) that support early childhood development and prevent adverse childhood experiences and trauma, averting mental health diagnoses such as depression and attention-deficit/hyperactivity disorder before they begin.10
OUTPATIENT AND COMMUNITY RESOURCES
We recognize that schools and general pediatricians have far more exposure to children at risk for mental health crises than do subspecialists. Thus, we urge an equitable increase in access to mental healthcare in the community so that patients needing assistance are screened and diagnosed earlier in their illness, allowing for secondary prevention of worsening mental health disorders. We support increased funding for programs such as the Massachusetts Child Psychiatry Access Program, which allows primary care doctors to consult psychiatrists in real time, closing the gap between a primary care visit and specialty follow-up. Telehealth services will be key to improving access for patients themselves and to allow pediatricians to consult with mental health professionals to initiate care prior to specialist availability. We envision that strengthening school-based behavioral health resources will also help prevent ED visits. Behavioral healthcare should be integrated into schools and community centers while police presence is simultaneously reduced, as there is evidence of an increased likelihood of juvenile justice involvement for children with disabilities and mental health needs.11,12
WORKFORCE DEVELOPMENT AND TRAINING
Ensuring access necessitates increasing the capacity of our psychiatric workforce by encouraging graduates to pursue mental health occupations with concrete financial incentives such as loan repayment and training grants. We thus support legislation such as H.R. 6597, the Mental Health Professionals Workforce Shortage Loan Repayment Act of 2018 (currently under consideration in the US House of Representatives). This may also improve recruitment and retention of individuals who are underrepresented in medicine, one step in helping ensure children have access to linguistically appropriate and culturally sensitive care. Residency programs and hospital systems should expand their training and education to identify and stabilize patients in mental health in extremis through culturally sensitive curricula focused on behavioral de-escalation techniques, trauma-informed care, and psychopharmacology. Our own residency program created a 2-week mental health rotation13 that includes rotating with outpatient mental health providers and our hospital’s behavioral response team, a group of trauma-informed responders for behavioral emergencies. Similar training should be available for nursing and other allied health professionals, who are often the first responders to behavioral escalations.13
INSTITUTIONAL DEVELOPMENT AND CLINICAL PRACTICES
Ideally, patients requiring higher-intensity psychiatric care would be referred to specialized pediatric behavioral health urgent care centers so their conditions can be adequately evaluated and addressed by staff trained in psychiatric management and in therapeutic environments. We believe all providers caring for children with mental health needs should be trained in basic, but core, behavioral health and de-escalation competencies, including specialized training for children with comorbid medical and neurodevelopmental diagnoses, such as autism. These centers should have specific beds for young children and those with developmental or complex care needs, and services should be available in numerous languages and levels of health literacy to allow all families to participate in their child’s care. At the same time, even nonpsychiatric EDs and inpatient units should commit resources to developing a maximally therapeutic environment, including allowing adjunctive services such as child life services, group therapy, and pet and music therapy, and create environments that support, rather than disrupt, normal routines.
HEALTH SYSTEMS REFORM AND ADVOCACY
Underpinning all the above innovations are changes to our healthcare payment system and provider networks, including the need for insurance coverage and payment parity for behavioral health, to ensure care is not only accessible but affordable. Additionally, for durable change, we need more than just education—we need coalition building and advocacy. Many organizations, including the American Academy of Pediatrics and the Children’s Hospital Association, have begun this work, which we must all continue.14 Bringing in diverse partners, including health systems, providers, educators, hospital administrators, payors, elected officials, and communities, will prioritize children’s needs and create a more ideal pediatric behavioral healthcare system.15
The COVID-19 pandemic has highlighted the dire need for comprehensive mental healthcare in the United States, a need that existed before the pandemic and will persist in a more fragile state long after it ends. Our hope is that the pandemic serves as the catalyst necessary to promote the magnitude of investments and stakeholder buy-in necessary to improve pediatric mental health and engender a radical redesign of our behavioral healthcare system. Our patients are counting on us to act. Together, we can build a system that ensures that the kids will be alright.
†Patient details have been changed for patient privacy.
Acknowledgments
The authors thank Joanna Perdomo, MD, Amara Azubuike, JD, and Josh Greenberg, JD, for reading and providing feedback on earlier versions of this work.
“...but it all started to get worse during the pandemic.”
As the patient’s† door closed, I (JS) thought about what his father had shared: his 12-year-old son had experienced a slow decline in his mental health since March 2020. There had been a gradual loss of all the things his son needed for psychological well-being: school went virtual and extracurricular activities ceased, and with them went any sense of routine, normalcy, or authentic opportunities to socialize. His feelings of isolation and depression culminated in an attempt to end his own life. My mind shifted to other patients under our care: an 8-year-old with behavioral outbursts intensifying after school-based therapy ended, a 13-year-old who became suicidal from isolation and virtual bullying. These children’s families sought emergent care because they no longer had the resources to care for their children at home. My team left each of these rooms heartbroken, unsure of exactly what to say and aware of the limitations of our current healthcare system.
Before and during the COVID-19 pandemic, many pediatric providers have had similar experiences caring for countless patients who are “boarding”—awaiting transfer to a psychiatric facility for their primary acute psychiatric issue, initially in the emergency room, often for 5 days or more,1 then ultimately admitted to a general medical floor if an appropriate psychiatric bed is still not available.2 Unfortunately, just as parents have run out of resources to care for their children’s psychiatric needs, so too is our medical system lacking in resources to provide the acute care these children need in general hospitals.
This mental health crisis began before the COVID-19 pandemic3 but has only worsened in the wake of its resulting social isolation. During the pandemic, suicide hotlines had a 1000% increase in call volumes.4 COVID-19–induced bed closures simultaneously worsened an existing critical bed shortage5,6 and led to an increase in the average length of stay (LOS) for patients boarding in the emergency department (ED).7 In the state of Massachusetts, for example, psychiatric patients awaiting inpatient beds boarded for more than 10,000 hours in January 2021—more than ever before, and up approximately 4000 hours since January 2017.6 For pediatric patients, the average wait time is now 59 hours.6 In the first 6 months of the pandemic, 39% of children presenting to EDs for mental health complaints ended up boarding, which is an astounding figure and is unfortunately 7% higher than in 2019.8 Even these staggering numbers do not capture the full range of experiences, as many statistics do not account for time spent on inpatient units by patients who do not receive a bed placement after waiting hours to several days in the ED.
Shortages of space, as well as an underfunded and understaffed mental health workforce, lead to these prolonged, often traumatic boarding periods in hospitals designed to care for acute medical, rather than acute psychiatric, conditions. Patients awaiting psychiatric placement are waiting in settings that are chaotic, inconsistent, and lacking in privacy. A patient in the throes of psychosis or suicidality needs a therapeutic milieu, not one that interrupts their daily routine,2 disconnects them from their existing support networks, and is punctuated by the incessant clangs of bedside monitors and the hubbub of code teams. These environments are not therapeutic3 for young infants with fevers, let alone for teenagers battling suicidality and eating disorders. In fact, for these reasons, we suspect that many of our patients’ inpatient ”behavioral escalations” are in fact triggered by their hospital environment, which may contribute to the 300% increase in the number of pharmacological restraints used during mental health visits in the ED over the past 10 years.9
None of us imagined when we chose to pursue pediatrics a that significant—and at times predominant—portion of our training would encompass caring for patients with acute mental health concerns. And although we did not anticipate this crisis, we have now been tasked with managing it. Throughout the day, when we are called to see our patients with primarily psychiatric pathology, we are often at war with ourselves. We weigh forming deeply meaningful relationships with these patients against the potential of unintentionally retraumatizing them or forming bonds that will be abruptly severed when patients are transferred to a psychiatric facility, which often occurs with barely a few hours’ notice. Moreover, many healthcare workers have training ill-suited to meet the needs of these patients. Just as emergency physicians can diagnose appendicitis but rely on surgeons to provide timely surgical treatment, general pediatricians identify psychiatric crises but rely on psychiatrists for ideal treatment plans. And almost daily, we are called to an “escalating” patient and arrive minutes into a stressful situation that others expect us to extinguish expeditiously. Along with nursing colleagues and the behavioral response team, we enact the treatment plan laid out by our psychiatry colleagues and wonder whether there is a better way.
We propose the following changes to create a more ideal health system (Table). We acknowledge that each health system has unique resources, challenges, and patient populations. Thus, our recommendations are not comprehensive and are largely based on experiences within our own institutions and state, but they encompass many domains that impact and are affected by child and adolescent mental healthcare in the United States, ranging from program- and hospital-level innovation to community and legislative action.
UPSTREAM PREVENTION
Like all good health system designs, we recommend prioritizing prevention. This would entail funding programs and legislation such as H.R. 3180, the RISE from Trauma Act, and H.R. 8544, the STRONG Support for Children Act of 2020 (both currently under consideration in the US House of Representatives) that support early childhood development and prevent adverse childhood experiences and trauma, averting mental health diagnoses such as depression and attention-deficit/hyperactivity disorder before they begin.10
OUTPATIENT AND COMMUNITY RESOURCES
We recognize that schools and general pediatricians have far more exposure to children at risk for mental health crises than do subspecialists. Thus, we urge an equitable increase in access to mental healthcare in the community so that patients needing assistance are screened and diagnosed earlier in their illness, allowing for secondary prevention of worsening mental health disorders. We support increased funding for programs such as the Massachusetts Child Psychiatry Access Program, which allows primary care doctors to consult psychiatrists in real time, closing the gap between a primary care visit and specialty follow-up. Telehealth services will be key to improving access for patients themselves and to allow pediatricians to consult with mental health professionals to initiate care prior to specialist availability. We envision that strengthening school-based behavioral health resources will also help prevent ED visits. Behavioral healthcare should be integrated into schools and community centers while police presence is simultaneously reduced, as there is evidence of an increased likelihood of juvenile justice involvement for children with disabilities and mental health needs.11,12
WORKFORCE DEVELOPMENT AND TRAINING
Ensuring access necessitates increasing the capacity of our psychiatric workforce by encouraging graduates to pursue mental health occupations with concrete financial incentives such as loan repayment and training grants. We thus support legislation such as H.R. 6597, the Mental Health Professionals Workforce Shortage Loan Repayment Act of 2018 (currently under consideration in the US House of Representatives). This may also improve recruitment and retention of individuals who are underrepresented in medicine, one step in helping ensure children have access to linguistically appropriate and culturally sensitive care. Residency programs and hospital systems should expand their training and education to identify and stabilize patients in mental health in extremis through culturally sensitive curricula focused on behavioral de-escalation techniques, trauma-informed care, and psychopharmacology. Our own residency program created a 2-week mental health rotation13 that includes rotating with outpatient mental health providers and our hospital’s behavioral response team, a group of trauma-informed responders for behavioral emergencies. Similar training should be available for nursing and other allied health professionals, who are often the first responders to behavioral escalations.13
INSTITUTIONAL DEVELOPMENT AND CLINICAL PRACTICES
Ideally, patients requiring higher-intensity psychiatric care would be referred to specialized pediatric behavioral health urgent care centers so their conditions can be adequately evaluated and addressed by staff trained in psychiatric management and in therapeutic environments. We believe all providers caring for children with mental health needs should be trained in basic, but core, behavioral health and de-escalation competencies, including specialized training for children with comorbid medical and neurodevelopmental diagnoses, such as autism. These centers should have specific beds for young children and those with developmental or complex care needs, and services should be available in numerous languages and levels of health literacy to allow all families to participate in their child’s care. At the same time, even nonpsychiatric EDs and inpatient units should commit resources to developing a maximally therapeutic environment, including allowing adjunctive services such as child life services, group therapy, and pet and music therapy, and create environments that support, rather than disrupt, normal routines.
HEALTH SYSTEMS REFORM AND ADVOCACY
Underpinning all the above innovations are changes to our healthcare payment system and provider networks, including the need for insurance coverage and payment parity for behavioral health, to ensure care is not only accessible but affordable. Additionally, for durable change, we need more than just education—we need coalition building and advocacy. Many organizations, including the American Academy of Pediatrics and the Children’s Hospital Association, have begun this work, which we must all continue.14 Bringing in diverse partners, including health systems, providers, educators, hospital administrators, payors, elected officials, and communities, will prioritize children’s needs and create a more ideal pediatric behavioral healthcare system.15
The COVID-19 pandemic has highlighted the dire need for comprehensive mental healthcare in the United States, a need that existed before the pandemic and will persist in a more fragile state long after it ends. Our hope is that the pandemic serves as the catalyst necessary to promote the magnitude of investments and stakeholder buy-in necessary to improve pediatric mental health and engender a radical redesign of our behavioral healthcare system. Our patients are counting on us to act. Together, we can build a system that ensures that the kids will be alright.
†Patient details have been changed for patient privacy.
Acknowledgments
The authors thank Joanna Perdomo, MD, Amara Azubuike, JD, and Josh Greenberg, JD, for reading and providing feedback on earlier versions of this work.
1. “This is a crisis”: mom whose son has boarded 33 days for psych bed calls for state action. WBUR News. Updated March 2, 2021. Accessed August 4, 2021. www.wbur.org/news/2021/02/26/mental-health-boarding-hospitals
2. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813-824. https://doi.org/10.1016/S2215-0366(20)30307-2
3. Nash KA, Zima BT, Rothenberg C, et al. Prolonged emergency department length of stay for US pediatric mental health visits (2005-2015). Pediatrics. 2021;147(5):e2020030692. https://doi.org/10.1542/peds.2020-030692
4. Cloutier RL, Marshaall R. A dangerous pandemic pair: Covid19 and adolescent mental health emergencies. Am J Emerg Med. 2021;46:776-777. https://doi.org/10.1016/j.ajem.2020.09.008
5. Schoenberg S. Lack of mental health beds means long ER waits. CommonWealth Magazine. April 15, 2021. Accessed August 5, 2021. https://commonwealthmagazine.org/health-care/lack-of-mental-health-beds-means-long-er-waits/
6. Jolicoeur L, Mullins L. Mass. physicians call on state to address ER “boarding” of patients awaiting admission. WBUR News. Updated February 3, 2021. Accessed August 5, 2021. www.wbur.org/news/2021/02/02/emergency-department-er-inpatient-beds-boarding
7. Krass P, Dalton E, Doupnik SK, Esposito J. US pediatric emergency department visits for mental health conditions during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e218533. https://doi.org/10.1001/jamanetworkopen.2021.8533
8. Impact of COVID-19 on the Massachusetts Health Care System: Interim Report. Massachusetts Health Policy Commission. April 2021. Accessed September 25, 2021. www.mass.gov/doc/impact-of-covid-19-on-the-massachusetts-health-care-system-interim-report/download
9. Foster AA, Porter JJ, Monuteaux MC, Hoffmann JA, Hudgins JD. Pharmacologic restraint use during mental health visits in pediatric emergency departments. J Pediatr. 2021;236:276-283.e2. https://doi.org/10.1016/j.jpeds.2021.03.027
10. Brown NM, Brown SN, Briggs RD, Germán M, Belamarich PF, Oyeku SO. Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad Pediatr. 2017;17(4):349-355. https://doi.org/10.1016/j.acap.2016.08.013
11. Harper K, Ryberg R, Temkin D. Black students and students with disabilities remain more likely to receive out-of-school suspensions, despite overall declines. Child Trends. April 29, 2019. Accessed August 5, 2021. www.childtrends.org/publications/black-students-disabilities-out-of-school-suspensions
12. Whitaker A, Torres-Guillén S, Morton M, et al. Cops and no counselors: how the lack of school mental health staff is harming students. American Civil Liberties Union. Accessed August 6, 2021. www.aclu.org/report/cops-and-no-counselors
13. Education. Boston Combined Residence Program. Accessed August 5, 2021. https://msbcrp.wpengine.com/program/education/
14. American Academy of Pediatrics. Interim guidance on supporting the emotional and behavioral health needs of children, adolescents, and families during the COVID-19 pandemic. Updated July 28, 2021. Accessed August 5, 2021. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-on-supporting-the-emotional-and-behavioral-health-needs-of-children-adolescents-and-families-during-the-covid-19-pandemic/
15. Advocacy. Children’s Mental Health Campaign. Accessed August 4, 2021. https://childrensmentalhealthcampaign.org/advocacy
1. “This is a crisis”: mom whose son has boarded 33 days for psych bed calls for state action. WBUR News. Updated March 2, 2021. Accessed August 4, 2021. www.wbur.org/news/2021/02/26/mental-health-boarding-hospitals
2. Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813-824. https://doi.org/10.1016/S2215-0366(20)30307-2
3. Nash KA, Zima BT, Rothenberg C, et al. Prolonged emergency department length of stay for US pediatric mental health visits (2005-2015). Pediatrics. 2021;147(5):e2020030692. https://doi.org/10.1542/peds.2020-030692
4. Cloutier RL, Marshaall R. A dangerous pandemic pair: Covid19 and adolescent mental health emergencies. Am J Emerg Med. 2021;46:776-777. https://doi.org/10.1016/j.ajem.2020.09.008
5. Schoenberg S. Lack of mental health beds means long ER waits. CommonWealth Magazine. April 15, 2021. Accessed August 5, 2021. https://commonwealthmagazine.org/health-care/lack-of-mental-health-beds-means-long-er-waits/
6. Jolicoeur L, Mullins L. Mass. physicians call on state to address ER “boarding” of patients awaiting admission. WBUR News. Updated February 3, 2021. Accessed August 5, 2021. www.wbur.org/news/2021/02/02/emergency-department-er-inpatient-beds-boarding
7. Krass P, Dalton E, Doupnik SK, Esposito J. US pediatric emergency department visits for mental health conditions during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4):e218533. https://doi.org/10.1001/jamanetworkopen.2021.8533
8. Impact of COVID-19 on the Massachusetts Health Care System: Interim Report. Massachusetts Health Policy Commission. April 2021. Accessed September 25, 2021. www.mass.gov/doc/impact-of-covid-19-on-the-massachusetts-health-care-system-interim-report/download
9. Foster AA, Porter JJ, Monuteaux MC, Hoffmann JA, Hudgins JD. Pharmacologic restraint use during mental health visits in pediatric emergency departments. J Pediatr. 2021;236:276-283.e2. https://doi.org/10.1016/j.jpeds.2021.03.027
10. Brown NM, Brown SN, Briggs RD, Germán M, Belamarich PF, Oyeku SO. Associations between adverse childhood experiences and ADHD diagnosis and severity. Acad Pediatr. 2017;17(4):349-355. https://doi.org/10.1016/j.acap.2016.08.013
11. Harper K, Ryberg R, Temkin D. Black students and students with disabilities remain more likely to receive out-of-school suspensions, despite overall declines. Child Trends. April 29, 2019. Accessed August 5, 2021. www.childtrends.org/publications/black-students-disabilities-out-of-school-suspensions
12. Whitaker A, Torres-Guillén S, Morton M, et al. Cops and no counselors: how the lack of school mental health staff is harming students. American Civil Liberties Union. Accessed August 6, 2021. www.aclu.org/report/cops-and-no-counselors
13. Education. Boston Combined Residence Program. Accessed August 5, 2021. https://msbcrp.wpengine.com/program/education/
14. American Academy of Pediatrics. Interim guidance on supporting the emotional and behavioral health needs of children, adolescents, and families during the COVID-19 pandemic. Updated July 28, 2021. Accessed August 5, 2021. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-on-supporting-the-emotional-and-behavioral-health-needs-of-children-adolescents-and-families-during-the-covid-19-pandemic/
15. Advocacy. Children’s Mental Health Campaign. Accessed August 4, 2021. https://childrensmentalhealthcampaign.org/advocacy
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Discontinuing Urate-Lowering Therapy on Admission
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™ " (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Clinical Scenario
An infected diabetic foot ulcer requiring intravenous antibiotics prompts admission for a 58-year-old man with hypertension, insulin-dependent diabetes mellitus, gout, stage 3 chronic kidney disease (CKD), and hyperlipidemia. On admission, the hospitalist discontinued the patient’s daily 300 mg of allopurinol, which had helped prevent a flare for more than 1 year. On day 3 of hospitalization, the patient developed right knee pain, swelling, and erythema. Due to concerns for septic arthritis, he underwent lab work, imaging, and joint aspiration, which confirmed the diagnosis of an acute gout flare. The prednisone he received for his gout flare caused hyperglycemia, requiring careful insulin titration during the remainder of his hospitalization.
Background
Gout, the most common form of inflammatory arthritis, affects 3.9% of the US population. Its incidence has doubled in the past 2 decades, partly due to an increase in risk factors for gout, including obesity, diabetes, hypertension, hyperlipidemia, and renal disease.1 Patients with gout incur high rates of hospitalization and costs related to the disease and its comorbidities.2 Volume depletion, diuretic use, fluid shifts, or discontinuation of gout medications put patients at high risk of developing acute flares during hospitalization.2-4
Acute inflammatory response to monosodium urate crystal deposition in joints causes gout flares. Over time, uncontrolled gout leads to chronic inflammatory damage, causing permanent deformities and disability. Patients with uncontrolled gout have decreased work productivity and higher healthcare utilization and costs than patients with controlled gout.5
Gout treatment has two components: acute flare management and long-term therapy to lower serum uric acid levels. Patients with frequent gout attacks (≥two annually), tophi, or radiographic damage require urate-lowering therapy (ULT) to prevent further damage. Additionally, ULT is conditionally recommended for patients with their first flare and concomitant CKD stage 3 or higher, serum uric acid >9 mg/dL, or urolithiasis. First-line ULT incorporates xanthine oxidase inhibitors, such as allopurinol, due to efficacy and low cost.6 Using a treat-to-target approach, allopurinol is titrated to achieve uric acid levels <6 mg/dL.6,7 Controlling gout can take many months and requires careful medication titration, lifestyle modifications, and clear communication with patients. Poor adherence to ULT treatment complicates overall gout control and partly results from patients’ and providers’ knowledge gaps about gout and gout medications.8,9 Prior studies demonstrated that poor adherence to ULT contributes to increased gout flares and resource utilization.6,9
Why You Might Think Stopping Urate-Lowering Therapy Is Helpful
In the authors’ experience, hospitalists discontinue ULT for three reasons. First, hospitalists hold ULT, particularly allopurinol, when a patient has either acute or chronic kidney injury, due to concern that decreased excretion of drug metabolites increases the risk of allopurinol hypersensitivity syndrome (AHS) and allopurinol toxicity.10 One small study reported a decrease or discontinuation of allopurinol in 21% of 73 admissions, citing concerns of using allopurinol in renal impairment.10 Oxipurinol, a renally excreted metabolite of allopurinol, accumulates at higher concentrations in individuals with kidney impairment. The belief that elevated concentrations increase the risk of adverse effects has guided past recommendations about safety and dosing of allopurinol in patients with CKD.11,12 Due to safety concerns, older guidelines and literature11 suggest not increasing allopurinol more than 300 mg daily in patients with CKD.
Second, clinicians may want to stop “nonessential” medications on admission in order to simplify a medication list. If a patient’s last gout flare occurred a long time ago, a clinician may think their gout no longer requires ULT.
Finally, ULT is discontinued during an acute gout flare because clinicians believe that continuing ULT will make flare symptoms worse. Allopurinol dissolves uric acid crystals, which can cause inflammation. The inflammation increases the risk of precipitating a gout flare when first starting allopurinol and during dose titration. Clinicians may feel that holding the medication during an acute flare avoids iatrogenesis that worsens the flare.
Why Stopping Urate-Lowering Therapy Is Not Helpful
While physicians cite concerns of using allopurinol in renal impairment,10 there are no absolute contraindications to allopurinol in kidney impairment. Clinicians can prescribe xanthine oxidase inhibitors to patients with moderate-to-severe CKD and can titrate allopurinol to doses greater than 300 mg daily safely in these same patients.6,7,12-14 Prior studies sparked concern that poor allopurinol metabolite excretion in CKD might contribute to AHS or toxicity. However, more recent studies show that patients with CKD can take allopurinol safely, but that they require slower up-titration to mitigate the risk of flares and AHS. Guidelines recommend a starting dose of ≤100 mg of allopurinol in patients with normal renal function, and even lower doses in patients with CKD.6 In studies showing safe dose titration in CKD, patients received an initial dose of allopurinol 50 mg daily, which increased by 50 mg every month.13,14 When hospitalists abruptly stop ULT during hospitalization in patients with CKD, those patients have to restart from the initial low dose and up-titrate slowly back to the lowest dose that achieves serum uric acid <6 mg/dL.6
Acute kidney injury (AKI) is not an absolute contraindication to allopurinol use, and the scant amount of published literature does not support discontinuation. In this acute situation, a patient may require a dose reduction in allopurinol to avoid toxicity depending on the severity of AKI. A discussion with inpatient pharmacy can help find a safe dose based on current creatinine clearance.
Physicians anecdotally recognize ULT discontinuation as a cause of inpatient gout flares. Clinicians and patients should view ULT as essential, even in patients who remain symptom-free for years. Between acute flares, a patient enters a potentially asymptomatic phase called “intercritical gout” that varies in duration. Urate deposition causing tophi and damage still occur during this phase, so patients must continue on ULT even if they have no recent flare history.
ULT that appears on any outpatient medication list needs verification of dose and compliance before ordering. If a patient is actually taking a lower dose than listed or not taking ULT at all, starting at a higher dose puts them at risk for flare and AHS, especially in patients with renal disease. Continuing ULT during hospitalization after verifying dose and compliance can potentially prevent gout flares and their downstream effects, including increased costs and potential side effects from additional pain medications.
Patients on chronic ULT should continue it during an acute gout flare.6,7 Literature and guidelines do not suggest that continuing ULT significantly worsens the intensity or duration of a flare. The initiation or up-titration of ULT, not the continuation of it, causes uric acid to dissolve, triggering an inflammatory response that increases the risk of gout flare. Therefore, guidelines recommend giving flare prophylaxis simultaneously for at least 3 to 6 months to prevent flares while starting and titrating ULT. Flare prophylaxis may continue longer depending on when a patient reaches a stable dose of ULT.6,7 While patients are receiving acute flare treatment, continuing ULT will help lower their serum uric acid levels over time.
To emphasize the importance of treating gout with ULT even further, the most recent American College of Rheumatology gout management guidelines conditionally recommend starting ULT during an acute flare for increased adherence. Small studies have shown that initiation of ULT does not precipitate attacks or significantly increase duration of flare. Input from patients influenced this recommendation, as they felt highly motivated to start ULT during acute flare due to symptoms.6
Additionally, due to comorbidities, inpatients often cannot tolerate standard flare therapies, such as nonsteroidal anti-inflammatory drugs, corticosteroids, or oral colchicine, to treat their acute symptoms. Moreover, patients often have other analgesics, such as opiates, prescribed for pain control. During an acute flare, hospitalists will likely need to add medications to treat the acute symptoms, but ULT should be considered an essential medication and continued as well.
When Stopping Urate-Lowering Therapy Might Be Helpful
Allopurinol can cause mild-to-severe cutaneous adverse reactions. AHS, a rare reaction that causes significant morbidity and mortality, presents with a rash, eosinophilia, fever, hepatitis, and progressive kidney failure. Risk factors for developing AHS include kidney impairment, higher starting doses, concurrent diuretic use, and presence of the genetic marker HLA B*5801.12 AHS usually occurs in the first 8 weeks of initiation of allopurinol, but can occur later in treatment, especially in those with risk factors—notably kidney impairment.12 When a patient on allopurinol develops a rash, the clinician should consider stopping allopurinol if concerned about AHS or, in milder cases, decrease the dose until the rash resolves.
What You Should Do Instead
When you see ULT on a patient’s medication list, verify the dose with the patient and continue it (even during an acute gout flare) unless a new rash has developed, or you are concerned about a drug-drug interaction. If a patient has a significant AKI, consider discussing dose modifications with your inpatient pharmacist.
Recommendations
- Consider ULT an essential medication and continue it during the hospitalization of a patient with a history of gout.
- Continue ULT while treating an acute gout flare.
- Continue ULT in patients with AKI and CKD, but discuss dose modifications with a pharmacist for AKI patients.
Conclusion
In the clinical scenario, the hospitalist did not treat ULT as an essential medication on admission, and the patient’s gout flared, leading to increased morbidity, resource utilization, and cost of hospitalization. Stopping ULT has downstream effects after discharge, including delays in achieving prior gout control. If ULT is discontinued, outpatient clinicians must restart it at lower doses and then up-titrate slowly, increasing the risk of flares and possibly contributing to nonadherence. During hospitalization, clinicians should continue ULT.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
1. Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol. 2018;45(4):574-579. https://doi.org/10.3899/jrheum.170806
2. Fisher MC, Pillinger MH, Keenan RT. Inpatient gout: a review. Curr Rheumatol Rep. 2014;16(11):458. https://doi.org/10.1007/s11926-014-0458-z
3. Zleik N, Elfishawi MM, Kvrgic Z, et al. Hospitalization increases the risk of acute arthritic flares in gout: a population-based study over 2 decades. J Rheumatol. 2018;45(8):1188-1191. https://doi.org/10.3899/jrheum.171320
4. Dubreuil M, Neogi T, Chen CA, et al. Increased risk of recurrent gout attacks with hospitalization. Am J Med. 2013;126(12):1138-1141.e1. https://doi.org/10.1016/j.amjmed.2013.06.026
5. Flores NM, Neuvo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. 2019;22(1):1-6. https://doi.org/10.1080/13696998.2018.1532904
6. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744-760. https://doi.org/10.1002/acr.24180
7. Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. https://doi.org/10.1002/acr.21773
8. Abhishek A, Doherty M. Education and non-pharmacological approaches for gout. Rheumatology (Oxford). 2018;57(suppl 1):i51-i58. https://doi.org/10.1093/rheumatology/kex421
9. Fields TR. The challenges of approaching and managing gout. Rheum Dis Clin North Am. 2019;45(1):145-157. https://doi.org/10.1016/j.rdc.2018.09.009
10. Huang IJ, Bays AM, Liew JW. Frequency of allopurinol dose reduction in hospitalized patients with gout flares. J Rheumatol. 2021;48(3):467-468. https://doi.org/10.3899/jrheum.201142
11. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56. https://doi.org/10.1016/0002-9343(84)90743-5
12. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016;12(4):235-242. https://doi.org/10.1038/nrrheum.2015.132
13. Stamp LK, Chapman PT, Barclay M, et al. The effect of kidney function on the urate lowering effect and safety of increasing allopurinol above doses based on creatinine clearance: a post hoc analysis of a randomized controlled trial. Arthritis Res Ther. 2017;19(1):283. https://doi.org/10.1186/s13075-017-1491-x
14. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421. https://doi.org/10.1002/art.30119
© 2021 Society of Hospital Medicine