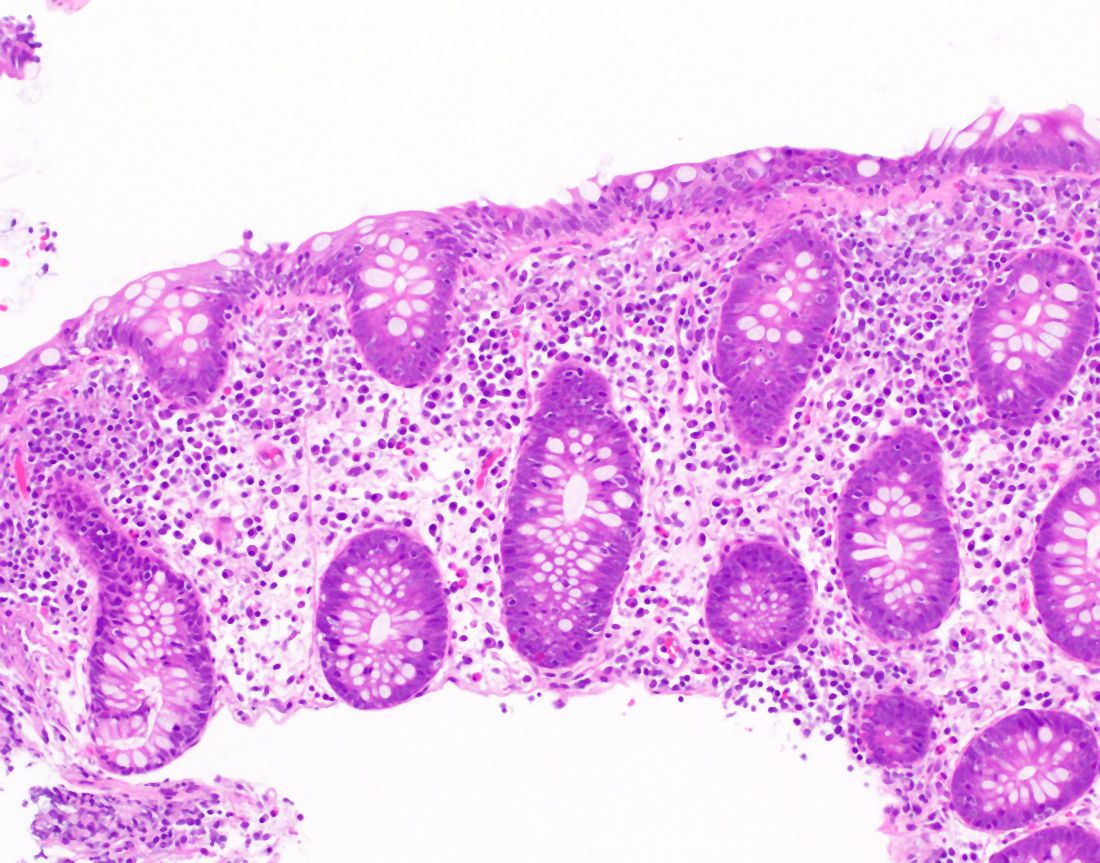
User login
Ocular Manifestations of Patients With Cutaneous Rosacea With and Without Demodex Infection
Acne rosacea is a chronic inflammatory disease that may affect the facial skin, eyes, and eyelids.1 It is characterized by transient or persistent flushing, facial erythema, and telangiectases, generally located on the central portion of the face, and may progress to papules and pustules.2,3 At the late stage of the disease, dermal edema or fibroplasia and sebaceous gland hypertrophy may cause phymatous alterations in the skin. In 2004, the National Rosacea Society Expert Committee developed a classification system for rosacea to standardize subtypes and variants that has since been widely accepted and continues to aid in research and epidemiologic studies.4 The committee defined 4 subtypes based on clinical characteristics: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea.2,3
Ocular rosacea may accompany mild, moderate, and severe dermatologic disease or may occur in the absence of diagnostic skin disease.5 Ocular signs include eyelid margin telangiectasia, spade-shaped infiltrates in the cornea, scleritis, and sclerokeratitis. Common symptoms include burning, stinging, light sensitivity, and foreign-body sensation. Ocular signs commonly seen in rosacea are meibomian gland dysfunction characterized by inspissation and inflammation of the meibomian glands (chalazia), conjunctivitis, honey crust and cylindrical collarette accumulation at the base of the eyelashes, irregularity of the eyelid margin architecture, and evaporative tear dysfunction.5,6
The physiopathology of rosacea is still unknown. Potential factors include genetic predisposition, abnormal inflammation, vascular dysfunction, and involvement of several microbial agents, such as commensal Demodex mites. The number of Demodex mites on normal skin flora is less than 5/cm2; however, the increased vascular dilation and capillary permeability associated with rosacea that result from sunlight and heat exposure increase the density of Demodex folliculorum.7 Elevated Demodex mite density has been observed in the lumens of the sebaceous follicles in patients with rosacea. However, because the severity of the clinical manifestations of the disease is not directly associated with the density of D folliculorum, it generally is accepted that D folliculorum is not a pathogenetic but rather an exacerbating factor.8 It has been reported that this species of mite is mostly found on the face and around the eyelashes and scalp of patients and that it can cause ocular surface inflammation.8
Most studies have researched ocular manifestations of rosacea but not ocular involvement in rosacea patients with and without Demodex mite infestation. In our study, we sought to compare the ocular surface, meibomian gland characteristics, and tear film abnormalities among patients with cutaneous rosacea with and without Demodex infestation.
Materials and Methods
We conducted a retrospective study of 60 patients with cutaneous rosacea. This study was approved by the ethics committee of the local hospital (2018/002-003), and all patients provided verbal and written informed consent before participating in the study. The study was carried out according to the guidelines of the Declaration of Helsinki.
Patient Selection and Evaluation
Patients diagnosed with rosacea by a dermatologist within 6 months were included in the study. Diagnosis of the disease was made after a detailed anamnesis and dermatologic examination. Rosacea was diagnosed if patients had an itching sensation, erythema and/or erythema attacks, and papules and pustules, and fulfilled the diagnostic criteria according to the National Rosacea Society. The skin disease was classified according to the subtypes as ETR, PPR, phymatous rosacea, or ocular rosacea.
The standard skin surface biopsy method was used in 60 patients for detecting Demodex density. When more than 5 mites were detected per square centimeter, the result was recorded as positive. Thirty consecutive, newly diagnosed patients with cutaneous acne rosacea with Demodex infestation and 30 consecutive, newly diagnosed sex- and age-matched patients with acne rosacea without Demodex infestation admitted to the dermatology outpatient clinic were included to this study. The patients who did not have any known dermatologic, systemic, or ocular diseases were included in the study. Patients who met any of the following criteria were excluded from the study: prior anti-inflammatory topical and/or systemic treatment for rosacea during the last 3 months, contact lens wear, eyelid surgery, or autoimmune disease requiring treatment.
Microscopic Demodex Examination
Demodex count was determined using a standardized skin surface biopsy, which is a noninvasive method. Every patient gave samples from the cheeks. This biopsy was repeated from the same site. A drop of cyanoacrylate was placed on a clean slide, pressed against a skin lesion, held in place for 1 minute, and removed. The obtained samples were evaluated under a light microscope (Nikon E200) with oil immersion. When more than 5 mites were detected per square centimeter, the result was recorded as positive.
Ophthalmologic Examination
A complete ophthalmologic examination including visual acuity assessment, standardized slit lamp examination, and fundus examination was done for all patients. Ocular rosacea was diagnosed on detection of 1 or more of the following: watery or bloodshot appearance, foreign-body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectases of the conjunctiva and eyelid margin, eyelid lid and periocular erythema, anterior blepharitis, meibomian gland dysfunction, or irregularity of eyelid margins. All patients were screened for the signs and symptoms of ocular rosacea and underwent other ophthalmologic examinations, including tear function tests. Tear functions were evaluated with Schirmer tests without anesthesia and fluorescein tear breakup time (TBUT). Tear film breakup time was assessed after instillation of 2% fluorescein staining under a cobalt blue filter. The time interval between the last complete blink and the appearance of the first dry spot was recorded. The mean of 3 consecutive measurements was obtained. The Schirmer test was performed without topical anesthesia using a standardized filter strip (Bio-Tech Vision Care). The amount of wetting was measured after 5 minutes. Meibomian gland expressibility was assessed by applying digital pressure to the eyelid margin.
Statistical Analysis
Statistical analysis of the study was performed with SPSS Statistics Version 22.0 (SPSS Inc). Continuous variables were reported as mean (SD), and categorical variables were reported as percentages and counts. Descriptive statistics for numerical variables were created. An independent sample t test was used for normally distributed continuous variables. The Kolmogorov-Smirnov test was used to determine normality. The Schirmer test without anesthesia and TBUT values among groups were compared using one-way analysis of variance. The differences were calculated using the multiple comparison Tukey test. P<.05 was considered statistically significant.
Results
Demographic Characteristics of Rosacea Patients
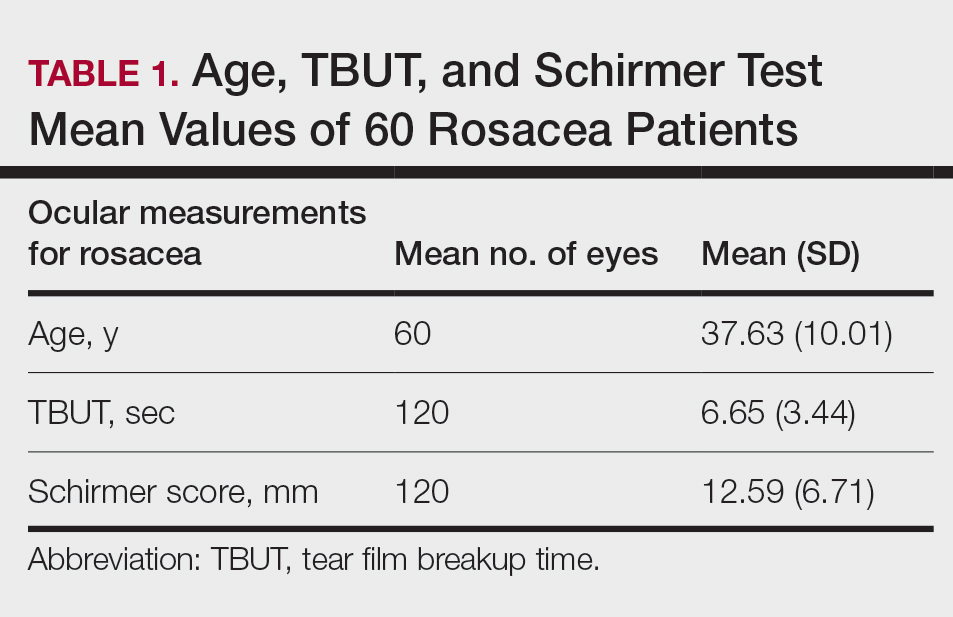
Sixty eyes of 30 newly diagnosed patients with acne rosacea with Demodex infestation and 60 eyes of 30 newly diagnosed patients with acne rosacea without Demodex infestation were enrolled in this study. The mean age (SD) of the 60 patients was 37.63 (10.01) years. The mean TBUT (SD) of the 120 eyes was 6.65 (3.44) seconds, and the mean Schirmer score (SD) was 12.59 (6.71) mm (Table 1).
Meibomian Gland Dysfunction vs Subgroup of Rosacea Patients
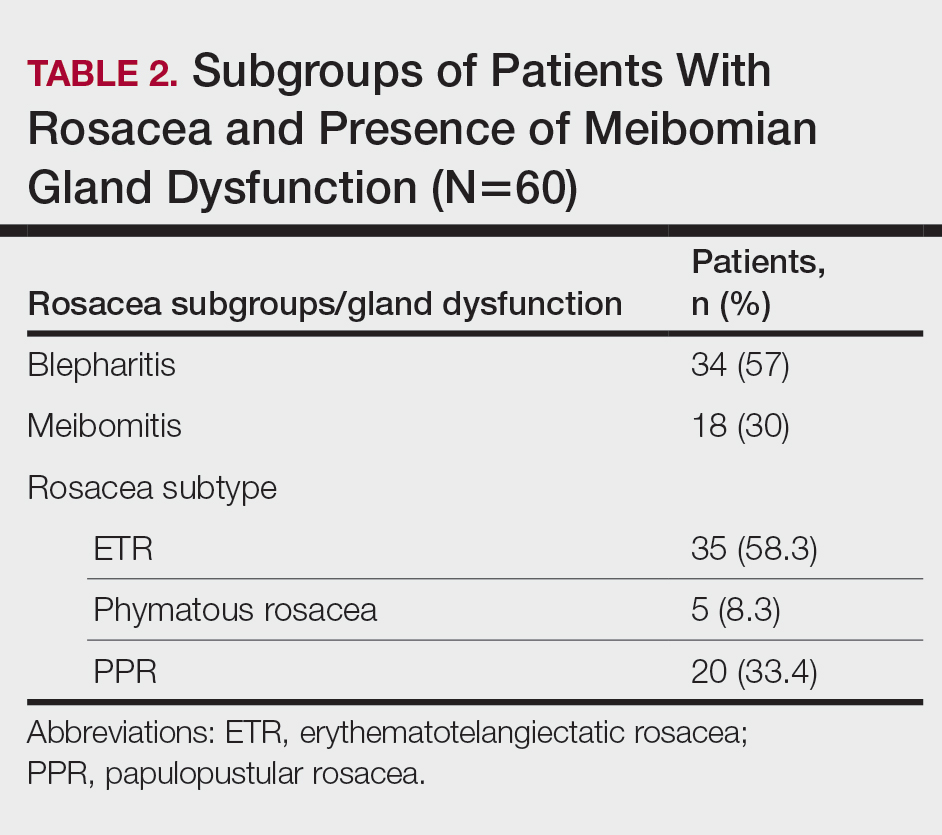
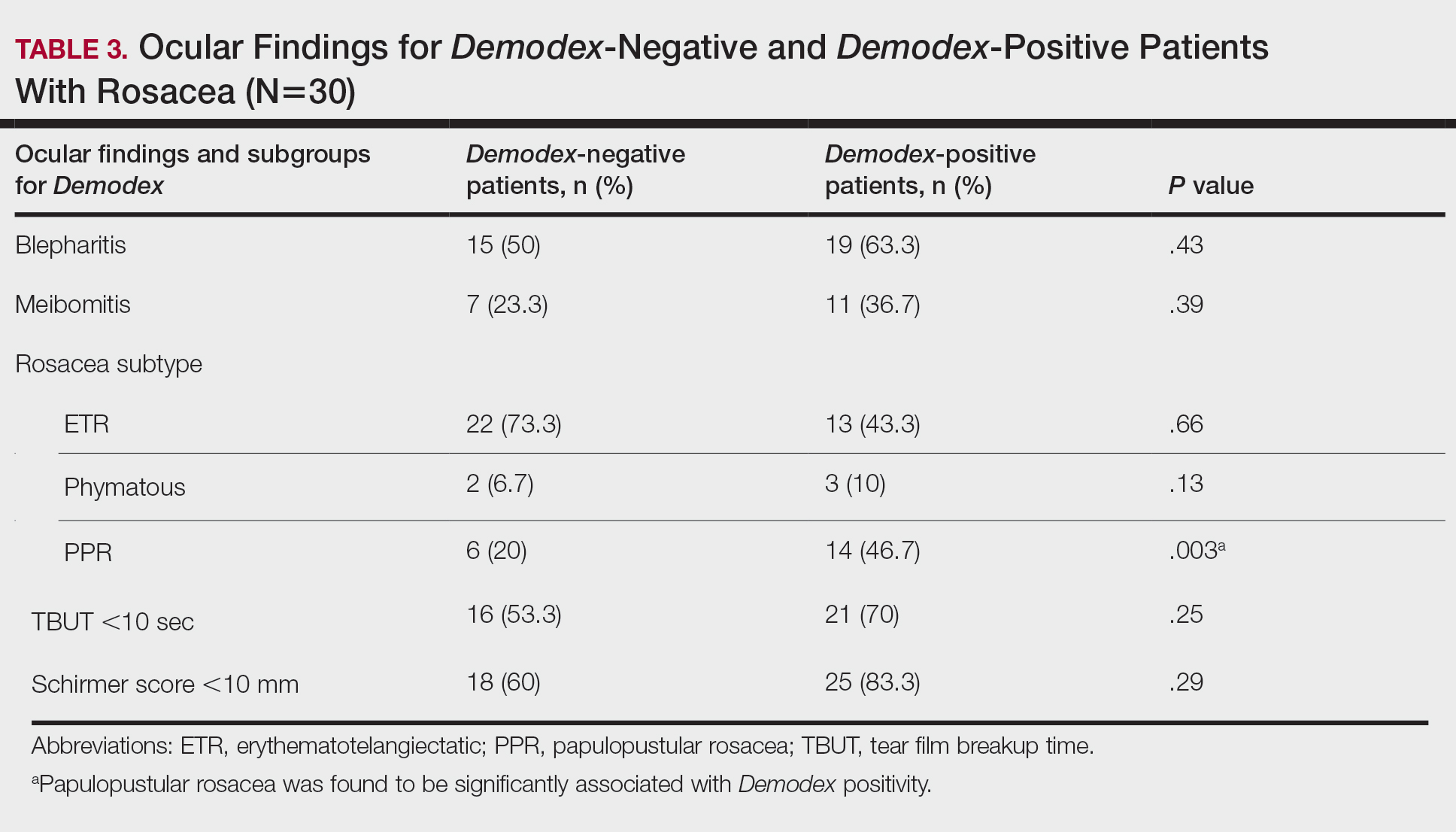
Thirty-four (57%) patients had blepharitis, and 18 (30%) patients had meibomitis. Thirty-five (58.3%) patients had ETR, 5 (8.3%) patients had phymatous rosacea, and 20 (33.4%) patients had PPR (Table 2). Of the Demodex-negative patients, 73.3% (22/30) had ETR, 20% (6/30) had PPR, and 6.7% (2/30) had phymatous rosacea. Of the Demodex-positive patients, 43.3% (13/30) had ETR, 46.7% (14/30) had PPR, and 10% (3/30) had phymatous rosacea (Table 3). Papulopustular rosacea was found to be significantly associated with Demodex positivity (P=.003); neither ETR nor phymatous rosacea was found to be significantly associated with Demodex infestation (P=.66 and P=.13, respectively)(Table 3).
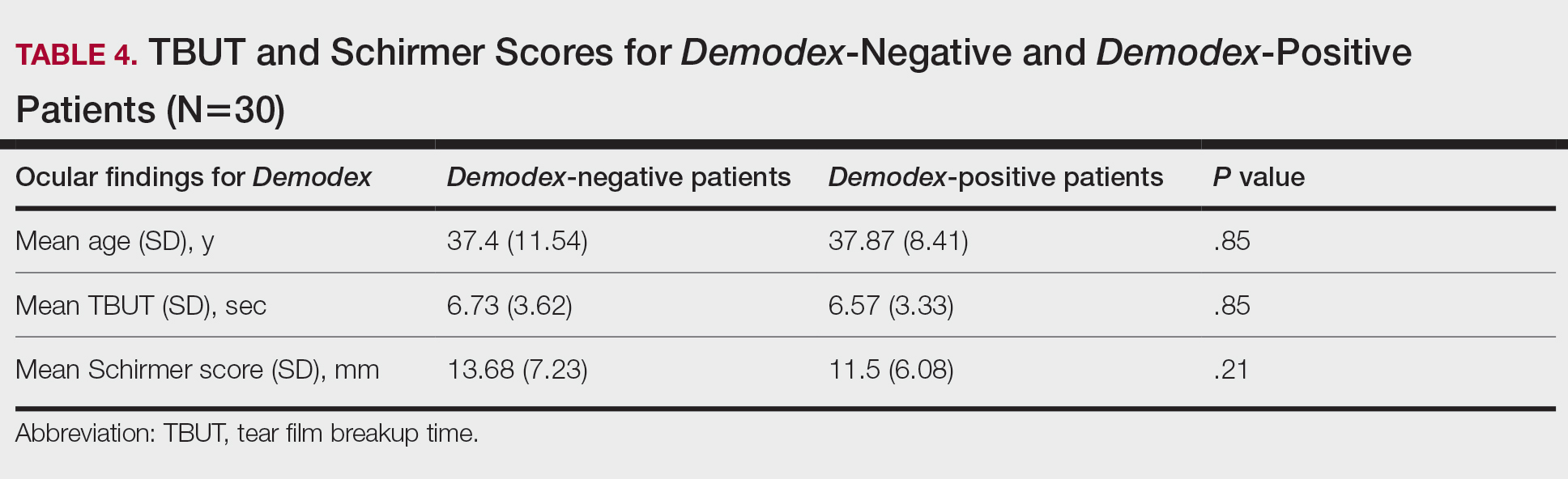
There was no statistically significant difference between the Demodex-negative and Demodex-positive groups for mean age (SD)(37.4 [11.54] years vs 37.87 [8.41] years; P=.85), mean TBUT (SD)(6.73 [3.62] seconds vs 6.57 [3.33] seconds; P=.85), and mean Schirmer score (SD)(13.68 [7.23] mm vs 11.5 [6.08] mm; P=.21)(Table 4).
Fifteen (50%) patients (30 eyes) in the Demodex-negative group and 19 (63.3%) patients (38 eyes) in the Demodex-positive group had blepharitis, with no statistically significant difference between the groups (P=.43). Seven (23.3%) patients (14 eyes) in the Demodex-negative group and 11 (36.7%) patients (22 eyes) in the Demodex-positive group had meibomitis, with no statistically significant difference between the groups (P=.39)(Table 3).
Sixteen (53.3%) patients (32 eyes) in the Demodex-negative group and 21 (70%) patients (42 eyes) in the Demodex-positive group had TBUT values less than 10 seconds. Eighteen (60%) patients (36 eyes) in the Demodex-negative group and 25 (83.3%) patients (50 eyes) in the Demodex-positive group had Schirmer scores less than 10 mm (Table 3). The 2 groups were not significantly different in dry eye findings (P=.25 and P=.29, respectively).
Comment
Inflammation in Rosacea
It is known that the density of nonfloral bacteria as well as D folliculorum and Demodex brevis increases in skin affected by rosacea compared to normal skin. Vascular dilation associated with rosacea that results from sunlight and heat causes increased capillary permeability and creates the ideal environment for the proliferation of D folliculorum. Demodex is thought to act as a vector for the activity of certain other microorganisms, particularly Bacillus oleronius, and thus initiates the inflammatory response associated with rosacea.9
One study reported that the inflammation associated with rosacea that was caused by Demodex and other environmental stimuli occurred through toll-like receptor 2 and various cytokines.10 It has been reported that the abnormal function of toll-like receptor 2 in the epidermis leads to the increased production of cathelicidin. Cathelicidin is an antimicrobial peptide with both vasoactive and proinflammatory activity and has been used as a basis to explain the pathogenesis of facial erythema, flushing, and telangiectasia in the context of rosacea.11,12 In addition, it has been reported that the increased secretion of proinflammatory cytokines such as IL-1 and gelatinase B in ocular rosacea leads to tearing film abnormalities that result from increased bacterial flora in the eyelids, which subsequently leads to decreased tear drainage and dry eyes.13 In addition, B oleronius isolated from a D folliculorum mite from patients with PPR produced proteins that induced an inflammatory immune response in 73% (16/22) of patients with rosacea.14
Ocular Findings in Rosacea Patients
In our study, PPR was found to be significantly associated with Demodex positivity compared to ETR and phymatous rosacea (P=.003). However, ocular inflammation findings such as blepharitis and meibomitis were not significantly different between Demodex-positive and Demodex-negative patients. Although the mean Schirmer score of Demodex-positive patients was lower than Demodex-negative patients, this difference was not statistically significant. We evaluated a TBUT of less than 10 seconds and a Schirmer score less than 10 mm as dry eye. Accordingly, the number of patients with dry eye was higher in the Demodex-positive group, but this difference was not statistically significant.
Chronic blepharitis, conjunctival inflammation, and meibomian gland dysfunction are among the most common findings of ocular rosacea.15,16 Patients with ocular rosacea commonly have dry eye and abnormal TBUT and Schirmer scores.17 In our study, we found that the fluorescein TBUT and Schirmer scores were more likely to be abnormal in the Demodex-positive group, but the difference between the 2 groups was not statistically significant.
It has been reported that proinflammatory cytokines due to a weakened immune system in rosacea patients were increased. The weakened immune system was further supported by the increased concentrations of proinflammatory cytokines such as IL-1 and matrix metalloproteinase 9 in these patients’ tears and the improvement of symptoms after the inhibition of these cytokines.11 Luo et al18 reported that Demodex inflammation causes dry eye, particularly with D brevis. Ayyildiz and Sezgin19 reported that Schirmer scores were significantly lower and that the Ocular Surface Disease Index had significantly increased in the Demodex-positive group compared to the Demodex-negative group (P=.001 for both). A Korean study reported that Demodex density was correlated with age, sex, and TBUT results, but there was no significant relationship between Demodex density and Schirmer scores.16
Sobolewska et al20 administered ivermectin cream 1% to 10 patients with cutaneous and ocular rosacea, but only to the forehead, chin, nose, cheeks, and regions close to the eyelids, and observed a significant improvement in blepharitis (P=.004). They stated that ivermectin, as applied only to the face, suppressed the proinflammatory cytokines associated with rosacea and showed anti-inflammatory effects by reducing Demodex mites.20Li et al21 demonstrated a strong correlation between ocular Demodex inflammation and serum reactivity to these bacterial proteins in patients with ocular rosacea, and they found that eyelid margin inflammation and facial rosacea correlated with reactivity to these proteins. These studies suggest a possible role for Demodex infestation and bacterial proteins in the etiology of rosacea.
Gonzalez-Hinojosa et al22 demonstrated that even though eyelash blepharitis was more common in PPR than ETR, there was no statistically significant association between rosacea and Demodex blepharitis. In our study, we found a significant correlation between PPR and Demodex positivity. Also, meibomian gland dysfunction was more common in the Demodex-positive group; however, this result was not statistically significant. One study compared patients with primary demodicosis and patients with rosacea with Demodex-induced blepharitis to healthy controls and found that patients with primary demodicosis and patients with rosacea did not have significantly different ocular findings.23 In contrast, Forton and De Maertelaer24 reported that patients with PPR had significantly more severe ocular manifestations compared with patients with demodicosis (P=.004).
Mizuno et al25 compared the normal (nonrosacea) population with and without Demodex-infested eyelashes and found that the 2 groups were not significantly different for meibomian gland dysfunction, fluorescein TBUT, or ocular surface discomfort.
Varying results have been reported regarding the association between Demodex and blepharitis or ocular surface discomfort with or without rosacea. In our study, we found that Demodex did not affect tear function tests or meibomian gland function in patients with rosacea. We believe this study is important because it demonstrates the effects of Demodex on ocular findings in patients with cutaneous rosacea.
Limitations
Our study has some limitations. The number of patients was relatively small, resulting in few significant differences between the comparison groups. A larger prospective research study is required to assess the prevalence of Demodex mites in the ocular rosacea population along with associated symptoms and findings.
Conclusion
Rosacea is a chronic disease associated with skin and ocular manifestations that range from mild to severe, that progresses in the form of attacks, and that requires long-term follow-up and treatment. Rosacea most often presents as a disease that causes ocular surface inflammation of varying degrees. Demodex infestation may increase cutaneous or ocular inflammation in rosacea. Therefore, every patient diagnosed with rosacea should be given a dermatologic examination to determine Demodex positivity and an ophthalmologic examination to determine ocular manifestations.
- O’Reilly N, Gallagher C, Reddy Katikireddy K, et al. Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci. 2012;53:3250-3259.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 suppl 1):S42-S43.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094.
- Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17:1-4.
- Erbagcı Z, Ozgoztası O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36:81‐86.
- Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97:242‐248.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Gold LM, Draelos ZD. New and emerging treatments for rosacea. Am J Clin Dermatol. 2015;16:457-461.
- Two AM, Del Rosso JQ. Kallikrein 5-mediated inflammation in rosacea: clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J Clin Aesthet Dermatol. 2014;7:20-25.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474-481.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74-87.
- Lee SH, Chun YS, Kim JH, et al. The relationship between Demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906-2911.
- Awais M, Anwar MI, Ilfikhar R, et al. Rosacea—the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34:161-166.
- Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9-S14.
- Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(suppl 1):S39-S41.
- Sobolewska B, Doycheva D, Deuter CM, et al. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea [published online April 7, 2020]. Ocul Immunol Inflamm. doi:10.1080/09273948.2020.1727531
- Li J, O‘Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. 2010;117:870-877.
- Gonzalez‐Hinojosa D, Jaime‐Villalonga A, Aguilar‐Montes G, et al. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66:36‐38.
- Sarac G, Cankaya C, Ozcan KN, et al. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J Cosmet Dermatol. 2020;19:1260-1265.
- Forton FMN, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99:47-52.
- Mizuno M, Kawashima M, Uchino M, et al. Demodex-mite infestation in cilia and its association with ocular surface parameters in Japanese volunteers. Eye Contact Lens. 2020;46:291-296.
Acne rosacea is a chronic inflammatory disease that may affect the facial skin, eyes, and eyelids.1 It is characterized by transient or persistent flushing, facial erythema, and telangiectases, generally located on the central portion of the face, and may progress to papules and pustules.2,3 At the late stage of the disease, dermal edema or fibroplasia and sebaceous gland hypertrophy may cause phymatous alterations in the skin. In 2004, the National Rosacea Society Expert Committee developed a classification system for rosacea to standardize subtypes and variants that has since been widely accepted and continues to aid in research and epidemiologic studies.4 The committee defined 4 subtypes based on clinical characteristics: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea.2,3
Ocular rosacea may accompany mild, moderate, and severe dermatologic disease or may occur in the absence of diagnostic skin disease.5 Ocular signs include eyelid margin telangiectasia, spade-shaped infiltrates in the cornea, scleritis, and sclerokeratitis. Common symptoms include burning, stinging, light sensitivity, and foreign-body sensation. Ocular signs commonly seen in rosacea are meibomian gland dysfunction characterized by inspissation and inflammation of the meibomian glands (chalazia), conjunctivitis, honey crust and cylindrical collarette accumulation at the base of the eyelashes, irregularity of the eyelid margin architecture, and evaporative tear dysfunction.5,6
The physiopathology of rosacea is still unknown. Potential factors include genetic predisposition, abnormal inflammation, vascular dysfunction, and involvement of several microbial agents, such as commensal Demodex mites. The number of Demodex mites on normal skin flora is less than 5/cm2; however, the increased vascular dilation and capillary permeability associated with rosacea that result from sunlight and heat exposure increase the density of Demodex folliculorum.7 Elevated Demodex mite density has been observed in the lumens of the sebaceous follicles in patients with rosacea. However, because the severity of the clinical manifestations of the disease is not directly associated with the density of D folliculorum, it generally is accepted that D folliculorum is not a pathogenetic but rather an exacerbating factor.8 It has been reported that this species of mite is mostly found on the face and around the eyelashes and scalp of patients and that it can cause ocular surface inflammation.8
Most studies have researched ocular manifestations of rosacea but not ocular involvement in rosacea patients with and without Demodex mite infestation. In our study, we sought to compare the ocular surface, meibomian gland characteristics, and tear film abnormalities among patients with cutaneous rosacea with and without Demodex infestation.
Materials and Methods
We conducted a retrospective study of 60 patients with cutaneous rosacea. This study was approved by the ethics committee of the local hospital (2018/002-003), and all patients provided verbal and written informed consent before participating in the study. The study was carried out according to the guidelines of the Declaration of Helsinki.
Patient Selection and Evaluation
Patients diagnosed with rosacea by a dermatologist within 6 months were included in the study. Diagnosis of the disease was made after a detailed anamnesis and dermatologic examination. Rosacea was diagnosed if patients had an itching sensation, erythema and/or erythema attacks, and papules and pustules, and fulfilled the diagnostic criteria according to the National Rosacea Society. The skin disease was classified according to the subtypes as ETR, PPR, phymatous rosacea, or ocular rosacea.
The standard skin surface biopsy method was used in 60 patients for detecting Demodex density. When more than 5 mites were detected per square centimeter, the result was recorded as positive. Thirty consecutive, newly diagnosed patients with cutaneous acne rosacea with Demodex infestation and 30 consecutive, newly diagnosed sex- and age-matched patients with acne rosacea without Demodex infestation admitted to the dermatology outpatient clinic were included to this study. The patients who did not have any known dermatologic, systemic, or ocular diseases were included in the study. Patients who met any of the following criteria were excluded from the study: prior anti-inflammatory topical and/or systemic treatment for rosacea during the last 3 months, contact lens wear, eyelid surgery, or autoimmune disease requiring treatment.
Microscopic Demodex Examination
Demodex count was determined using a standardized skin surface biopsy, which is a noninvasive method. Every patient gave samples from the cheeks. This biopsy was repeated from the same site. A drop of cyanoacrylate was placed on a clean slide, pressed against a skin lesion, held in place for 1 minute, and removed. The obtained samples were evaluated under a light microscope (Nikon E200) with oil immersion. When more than 5 mites were detected per square centimeter, the result was recorded as positive.
Ophthalmologic Examination
A complete ophthalmologic examination including visual acuity assessment, standardized slit lamp examination, and fundus examination was done for all patients. Ocular rosacea was diagnosed on detection of 1 or more of the following: watery or bloodshot appearance, foreign-body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectases of the conjunctiva and eyelid margin, eyelid lid and periocular erythema, anterior blepharitis, meibomian gland dysfunction, or irregularity of eyelid margins. All patients were screened for the signs and symptoms of ocular rosacea and underwent other ophthalmologic examinations, including tear function tests. Tear functions were evaluated with Schirmer tests without anesthesia and fluorescein tear breakup time (TBUT). Tear film breakup time was assessed after instillation of 2% fluorescein staining under a cobalt blue filter. The time interval between the last complete blink and the appearance of the first dry spot was recorded. The mean of 3 consecutive measurements was obtained. The Schirmer test was performed without topical anesthesia using a standardized filter strip (Bio-Tech Vision Care). The amount of wetting was measured after 5 minutes. Meibomian gland expressibility was assessed by applying digital pressure to the eyelid margin.
Statistical Analysis
Statistical analysis of the study was performed with SPSS Statistics Version 22.0 (SPSS Inc). Continuous variables were reported as mean (SD), and categorical variables were reported as percentages and counts. Descriptive statistics for numerical variables were created. An independent sample t test was used for normally distributed continuous variables. The Kolmogorov-Smirnov test was used to determine normality. The Schirmer test without anesthesia and TBUT values among groups were compared using one-way analysis of variance. The differences were calculated using the multiple comparison Tukey test. P<.05 was considered statistically significant.
Results
Demographic Characteristics of Rosacea Patients
Sixty eyes of 30 newly diagnosed patients with acne rosacea with Demodex infestation and 60 eyes of 30 newly diagnosed patients with acne rosacea without Demodex infestation were enrolled in this study. The mean age (SD) of the 60 patients was 37.63 (10.01) years. The mean TBUT (SD) of the 120 eyes was 6.65 (3.44) seconds, and the mean Schirmer score (SD) was 12.59 (6.71) mm (Table 1).
Meibomian Gland Dysfunction vs Subgroup of Rosacea Patients
Thirty-four (57%) patients had blepharitis, and 18 (30%) patients had meibomitis. Thirty-five (58.3%) patients had ETR, 5 (8.3%) patients had phymatous rosacea, and 20 (33.4%) patients had PPR (Table 2). Of the Demodex-negative patients, 73.3% (22/30) had ETR, 20% (6/30) had PPR, and 6.7% (2/30) had phymatous rosacea. Of the Demodex-positive patients, 43.3% (13/30) had ETR, 46.7% (14/30) had PPR, and 10% (3/30) had phymatous rosacea (Table 3). Papulopustular rosacea was found to be significantly associated with Demodex positivity (P=.003); neither ETR nor phymatous rosacea was found to be significantly associated with Demodex infestation (P=.66 and P=.13, respectively)(Table 3).
There was no statistically significant difference between the Demodex-negative and Demodex-positive groups for mean age (SD)(37.4 [11.54] years vs 37.87 [8.41] years; P=.85), mean TBUT (SD)(6.73 [3.62] seconds vs 6.57 [3.33] seconds; P=.85), and mean Schirmer score (SD)(13.68 [7.23] mm vs 11.5 [6.08] mm; P=.21)(Table 4).
Fifteen (50%) patients (30 eyes) in the Demodex-negative group and 19 (63.3%) patients (38 eyes) in the Demodex-positive group had blepharitis, with no statistically significant difference between the groups (P=.43). Seven (23.3%) patients (14 eyes) in the Demodex-negative group and 11 (36.7%) patients (22 eyes) in the Demodex-positive group had meibomitis, with no statistically significant difference between the groups (P=.39)(Table 3).
Sixteen (53.3%) patients (32 eyes) in the Demodex-negative group and 21 (70%) patients (42 eyes) in the Demodex-positive group had TBUT values less than 10 seconds. Eighteen (60%) patients (36 eyes) in the Demodex-negative group and 25 (83.3%) patients (50 eyes) in the Demodex-positive group had Schirmer scores less than 10 mm (Table 3). The 2 groups were not significantly different in dry eye findings (P=.25 and P=.29, respectively).
Comment
Inflammation in Rosacea
It is known that the density of nonfloral bacteria as well as D folliculorum and Demodex brevis increases in skin affected by rosacea compared to normal skin. Vascular dilation associated with rosacea that results from sunlight and heat causes increased capillary permeability and creates the ideal environment for the proliferation of D folliculorum. Demodex is thought to act as a vector for the activity of certain other microorganisms, particularly Bacillus oleronius, and thus initiates the inflammatory response associated with rosacea.9
One study reported that the inflammation associated with rosacea that was caused by Demodex and other environmental stimuli occurred through toll-like receptor 2 and various cytokines.10 It has been reported that the abnormal function of toll-like receptor 2 in the epidermis leads to the increased production of cathelicidin. Cathelicidin is an antimicrobial peptide with both vasoactive and proinflammatory activity and has been used as a basis to explain the pathogenesis of facial erythema, flushing, and telangiectasia in the context of rosacea.11,12 In addition, it has been reported that the increased secretion of proinflammatory cytokines such as IL-1 and gelatinase B in ocular rosacea leads to tearing film abnormalities that result from increased bacterial flora in the eyelids, which subsequently leads to decreased tear drainage and dry eyes.13 In addition, B oleronius isolated from a D folliculorum mite from patients with PPR produced proteins that induced an inflammatory immune response in 73% (16/22) of patients with rosacea.14
Ocular Findings in Rosacea Patients
In our study, PPR was found to be significantly associated with Demodex positivity compared to ETR and phymatous rosacea (P=.003). However, ocular inflammation findings such as blepharitis and meibomitis were not significantly different between Demodex-positive and Demodex-negative patients. Although the mean Schirmer score of Demodex-positive patients was lower than Demodex-negative patients, this difference was not statistically significant. We evaluated a TBUT of less than 10 seconds and a Schirmer score less than 10 mm as dry eye. Accordingly, the number of patients with dry eye was higher in the Demodex-positive group, but this difference was not statistically significant.
Chronic blepharitis, conjunctival inflammation, and meibomian gland dysfunction are among the most common findings of ocular rosacea.15,16 Patients with ocular rosacea commonly have dry eye and abnormal TBUT and Schirmer scores.17 In our study, we found that the fluorescein TBUT and Schirmer scores were more likely to be abnormal in the Demodex-positive group, but the difference between the 2 groups was not statistically significant.
It has been reported that proinflammatory cytokines due to a weakened immune system in rosacea patients were increased. The weakened immune system was further supported by the increased concentrations of proinflammatory cytokines such as IL-1 and matrix metalloproteinase 9 in these patients’ tears and the improvement of symptoms after the inhibition of these cytokines.11 Luo et al18 reported that Demodex inflammation causes dry eye, particularly with D brevis. Ayyildiz and Sezgin19 reported that Schirmer scores were significantly lower and that the Ocular Surface Disease Index had significantly increased in the Demodex-positive group compared to the Demodex-negative group (P=.001 for both). A Korean study reported that Demodex density was correlated with age, sex, and TBUT results, but there was no significant relationship between Demodex density and Schirmer scores.16
Sobolewska et al20 administered ivermectin cream 1% to 10 patients with cutaneous and ocular rosacea, but only to the forehead, chin, nose, cheeks, and regions close to the eyelids, and observed a significant improvement in blepharitis (P=.004). They stated that ivermectin, as applied only to the face, suppressed the proinflammatory cytokines associated with rosacea and showed anti-inflammatory effects by reducing Demodex mites.20Li et al21 demonstrated a strong correlation between ocular Demodex inflammation and serum reactivity to these bacterial proteins in patients with ocular rosacea, and they found that eyelid margin inflammation and facial rosacea correlated with reactivity to these proteins. These studies suggest a possible role for Demodex infestation and bacterial proteins in the etiology of rosacea.
Gonzalez-Hinojosa et al22 demonstrated that even though eyelash blepharitis was more common in PPR than ETR, there was no statistically significant association between rosacea and Demodex blepharitis. In our study, we found a significant correlation between PPR and Demodex positivity. Also, meibomian gland dysfunction was more common in the Demodex-positive group; however, this result was not statistically significant. One study compared patients with primary demodicosis and patients with rosacea with Demodex-induced blepharitis to healthy controls and found that patients with primary demodicosis and patients with rosacea did not have significantly different ocular findings.23 In contrast, Forton and De Maertelaer24 reported that patients with PPR had significantly more severe ocular manifestations compared with patients with demodicosis (P=.004).
Mizuno et al25 compared the normal (nonrosacea) population with and without Demodex-infested eyelashes and found that the 2 groups were not significantly different for meibomian gland dysfunction, fluorescein TBUT, or ocular surface discomfort.
Varying results have been reported regarding the association between Demodex and blepharitis or ocular surface discomfort with or without rosacea. In our study, we found that Demodex did not affect tear function tests or meibomian gland function in patients with rosacea. We believe this study is important because it demonstrates the effects of Demodex on ocular findings in patients with cutaneous rosacea.
Limitations
Our study has some limitations. The number of patients was relatively small, resulting in few significant differences between the comparison groups. A larger prospective research study is required to assess the prevalence of Demodex mites in the ocular rosacea population along with associated symptoms and findings.
Conclusion
Rosacea is a chronic disease associated with skin and ocular manifestations that range from mild to severe, that progresses in the form of attacks, and that requires long-term follow-up and treatment. Rosacea most often presents as a disease that causes ocular surface inflammation of varying degrees. Demodex infestation may increase cutaneous or ocular inflammation in rosacea. Therefore, every patient diagnosed with rosacea should be given a dermatologic examination to determine Demodex positivity and an ophthalmologic examination to determine ocular manifestations.
Acne rosacea is a chronic inflammatory disease that may affect the facial skin, eyes, and eyelids.1 It is characterized by transient or persistent flushing, facial erythema, and telangiectases, generally located on the central portion of the face, and may progress to papules and pustules.2,3 At the late stage of the disease, dermal edema or fibroplasia and sebaceous gland hypertrophy may cause phymatous alterations in the skin. In 2004, the National Rosacea Society Expert Committee developed a classification system for rosacea to standardize subtypes and variants that has since been widely accepted and continues to aid in research and epidemiologic studies.4 The committee defined 4 subtypes based on clinical characteristics: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea.2,3
Ocular rosacea may accompany mild, moderate, and severe dermatologic disease or may occur in the absence of diagnostic skin disease.5 Ocular signs include eyelid margin telangiectasia, spade-shaped infiltrates in the cornea, scleritis, and sclerokeratitis. Common symptoms include burning, stinging, light sensitivity, and foreign-body sensation. Ocular signs commonly seen in rosacea are meibomian gland dysfunction characterized by inspissation and inflammation of the meibomian glands (chalazia), conjunctivitis, honey crust and cylindrical collarette accumulation at the base of the eyelashes, irregularity of the eyelid margin architecture, and evaporative tear dysfunction.5,6
The physiopathology of rosacea is still unknown. Potential factors include genetic predisposition, abnormal inflammation, vascular dysfunction, and involvement of several microbial agents, such as commensal Demodex mites. The number of Demodex mites on normal skin flora is less than 5/cm2; however, the increased vascular dilation and capillary permeability associated with rosacea that result from sunlight and heat exposure increase the density of Demodex folliculorum.7 Elevated Demodex mite density has been observed in the lumens of the sebaceous follicles in patients with rosacea. However, because the severity of the clinical manifestations of the disease is not directly associated with the density of D folliculorum, it generally is accepted that D folliculorum is not a pathogenetic but rather an exacerbating factor.8 It has been reported that this species of mite is mostly found on the face and around the eyelashes and scalp of patients and that it can cause ocular surface inflammation.8
Most studies have researched ocular manifestations of rosacea but not ocular involvement in rosacea patients with and without Demodex mite infestation. In our study, we sought to compare the ocular surface, meibomian gland characteristics, and tear film abnormalities among patients with cutaneous rosacea with and without Demodex infestation.
Materials and Methods
We conducted a retrospective study of 60 patients with cutaneous rosacea. This study was approved by the ethics committee of the local hospital (2018/002-003), and all patients provided verbal and written informed consent before participating in the study. The study was carried out according to the guidelines of the Declaration of Helsinki.
Patient Selection and Evaluation
Patients diagnosed with rosacea by a dermatologist within 6 months were included in the study. Diagnosis of the disease was made after a detailed anamnesis and dermatologic examination. Rosacea was diagnosed if patients had an itching sensation, erythema and/or erythema attacks, and papules and pustules, and fulfilled the diagnostic criteria according to the National Rosacea Society. The skin disease was classified according to the subtypes as ETR, PPR, phymatous rosacea, or ocular rosacea.
The standard skin surface biopsy method was used in 60 patients for detecting Demodex density. When more than 5 mites were detected per square centimeter, the result was recorded as positive. Thirty consecutive, newly diagnosed patients with cutaneous acne rosacea with Demodex infestation and 30 consecutive, newly diagnosed sex- and age-matched patients with acne rosacea without Demodex infestation admitted to the dermatology outpatient clinic were included to this study. The patients who did not have any known dermatologic, systemic, or ocular diseases were included in the study. Patients who met any of the following criteria were excluded from the study: prior anti-inflammatory topical and/or systemic treatment for rosacea during the last 3 months, contact lens wear, eyelid surgery, or autoimmune disease requiring treatment.
Microscopic Demodex Examination
Demodex count was determined using a standardized skin surface biopsy, which is a noninvasive method. Every patient gave samples from the cheeks. This biopsy was repeated from the same site. A drop of cyanoacrylate was placed on a clean slide, pressed against a skin lesion, held in place for 1 minute, and removed. The obtained samples were evaluated under a light microscope (Nikon E200) with oil immersion. When more than 5 mites were detected per square centimeter, the result was recorded as positive.
Ophthalmologic Examination
A complete ophthalmologic examination including visual acuity assessment, standardized slit lamp examination, and fundus examination was done for all patients. Ocular rosacea was diagnosed on detection of 1 or more of the following: watery or bloodshot appearance, foreign-body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectases of the conjunctiva and eyelid margin, eyelid lid and periocular erythema, anterior blepharitis, meibomian gland dysfunction, or irregularity of eyelid margins. All patients were screened for the signs and symptoms of ocular rosacea and underwent other ophthalmologic examinations, including tear function tests. Tear functions were evaluated with Schirmer tests without anesthesia and fluorescein tear breakup time (TBUT). Tear film breakup time was assessed after instillation of 2% fluorescein staining under a cobalt blue filter. The time interval between the last complete blink and the appearance of the first dry spot was recorded. The mean of 3 consecutive measurements was obtained. The Schirmer test was performed without topical anesthesia using a standardized filter strip (Bio-Tech Vision Care). The amount of wetting was measured after 5 minutes. Meibomian gland expressibility was assessed by applying digital pressure to the eyelid margin.
Statistical Analysis
Statistical analysis of the study was performed with SPSS Statistics Version 22.0 (SPSS Inc). Continuous variables were reported as mean (SD), and categorical variables were reported as percentages and counts. Descriptive statistics for numerical variables were created. An independent sample t test was used for normally distributed continuous variables. The Kolmogorov-Smirnov test was used to determine normality. The Schirmer test without anesthesia and TBUT values among groups were compared using one-way analysis of variance. The differences were calculated using the multiple comparison Tukey test. P<.05 was considered statistically significant.
Results
Demographic Characteristics of Rosacea Patients
Sixty eyes of 30 newly diagnosed patients with acne rosacea with Demodex infestation and 60 eyes of 30 newly diagnosed patients with acne rosacea without Demodex infestation were enrolled in this study. The mean age (SD) of the 60 patients was 37.63 (10.01) years. The mean TBUT (SD) of the 120 eyes was 6.65 (3.44) seconds, and the mean Schirmer score (SD) was 12.59 (6.71) mm (Table 1).
Meibomian Gland Dysfunction vs Subgroup of Rosacea Patients
Thirty-four (57%) patients had blepharitis, and 18 (30%) patients had meibomitis. Thirty-five (58.3%) patients had ETR, 5 (8.3%) patients had phymatous rosacea, and 20 (33.4%) patients had PPR (Table 2). Of the Demodex-negative patients, 73.3% (22/30) had ETR, 20% (6/30) had PPR, and 6.7% (2/30) had phymatous rosacea. Of the Demodex-positive patients, 43.3% (13/30) had ETR, 46.7% (14/30) had PPR, and 10% (3/30) had phymatous rosacea (Table 3). Papulopustular rosacea was found to be significantly associated with Demodex positivity (P=.003); neither ETR nor phymatous rosacea was found to be significantly associated with Demodex infestation (P=.66 and P=.13, respectively)(Table 3).
There was no statistically significant difference between the Demodex-negative and Demodex-positive groups for mean age (SD)(37.4 [11.54] years vs 37.87 [8.41] years; P=.85), mean TBUT (SD)(6.73 [3.62] seconds vs 6.57 [3.33] seconds; P=.85), and mean Schirmer score (SD)(13.68 [7.23] mm vs 11.5 [6.08] mm; P=.21)(Table 4).
Fifteen (50%) patients (30 eyes) in the Demodex-negative group and 19 (63.3%) patients (38 eyes) in the Demodex-positive group had blepharitis, with no statistically significant difference between the groups (P=.43). Seven (23.3%) patients (14 eyes) in the Demodex-negative group and 11 (36.7%) patients (22 eyes) in the Demodex-positive group had meibomitis, with no statistically significant difference between the groups (P=.39)(Table 3).
Sixteen (53.3%) patients (32 eyes) in the Demodex-negative group and 21 (70%) patients (42 eyes) in the Demodex-positive group had TBUT values less than 10 seconds. Eighteen (60%) patients (36 eyes) in the Demodex-negative group and 25 (83.3%) patients (50 eyes) in the Demodex-positive group had Schirmer scores less than 10 mm (Table 3). The 2 groups were not significantly different in dry eye findings (P=.25 and P=.29, respectively).
Comment
Inflammation in Rosacea
It is known that the density of nonfloral bacteria as well as D folliculorum and Demodex brevis increases in skin affected by rosacea compared to normal skin. Vascular dilation associated with rosacea that results from sunlight and heat causes increased capillary permeability and creates the ideal environment for the proliferation of D folliculorum. Demodex is thought to act as a vector for the activity of certain other microorganisms, particularly Bacillus oleronius, and thus initiates the inflammatory response associated with rosacea.9
One study reported that the inflammation associated with rosacea that was caused by Demodex and other environmental stimuli occurred through toll-like receptor 2 and various cytokines.10 It has been reported that the abnormal function of toll-like receptor 2 in the epidermis leads to the increased production of cathelicidin. Cathelicidin is an antimicrobial peptide with both vasoactive and proinflammatory activity and has been used as a basis to explain the pathogenesis of facial erythema, flushing, and telangiectasia in the context of rosacea.11,12 In addition, it has been reported that the increased secretion of proinflammatory cytokines such as IL-1 and gelatinase B in ocular rosacea leads to tearing film abnormalities that result from increased bacterial flora in the eyelids, which subsequently leads to decreased tear drainage and dry eyes.13 In addition, B oleronius isolated from a D folliculorum mite from patients with PPR produced proteins that induced an inflammatory immune response in 73% (16/22) of patients with rosacea.14
Ocular Findings in Rosacea Patients
In our study, PPR was found to be significantly associated with Demodex positivity compared to ETR and phymatous rosacea (P=.003). However, ocular inflammation findings such as blepharitis and meibomitis were not significantly different between Demodex-positive and Demodex-negative patients. Although the mean Schirmer score of Demodex-positive patients was lower than Demodex-negative patients, this difference was not statistically significant. We evaluated a TBUT of less than 10 seconds and a Schirmer score less than 10 mm as dry eye. Accordingly, the number of patients with dry eye was higher in the Demodex-positive group, but this difference was not statistically significant.
Chronic blepharitis, conjunctival inflammation, and meibomian gland dysfunction are among the most common findings of ocular rosacea.15,16 Patients with ocular rosacea commonly have dry eye and abnormal TBUT and Schirmer scores.17 In our study, we found that the fluorescein TBUT and Schirmer scores were more likely to be abnormal in the Demodex-positive group, but the difference between the 2 groups was not statistically significant.
It has been reported that proinflammatory cytokines due to a weakened immune system in rosacea patients were increased. The weakened immune system was further supported by the increased concentrations of proinflammatory cytokines such as IL-1 and matrix metalloproteinase 9 in these patients’ tears and the improvement of symptoms after the inhibition of these cytokines.11 Luo et al18 reported that Demodex inflammation causes dry eye, particularly with D brevis. Ayyildiz and Sezgin19 reported that Schirmer scores were significantly lower and that the Ocular Surface Disease Index had significantly increased in the Demodex-positive group compared to the Demodex-negative group (P=.001 for both). A Korean study reported that Demodex density was correlated with age, sex, and TBUT results, but there was no significant relationship between Demodex density and Schirmer scores.16
Sobolewska et al20 administered ivermectin cream 1% to 10 patients with cutaneous and ocular rosacea, but only to the forehead, chin, nose, cheeks, and regions close to the eyelids, and observed a significant improvement in blepharitis (P=.004). They stated that ivermectin, as applied only to the face, suppressed the proinflammatory cytokines associated with rosacea and showed anti-inflammatory effects by reducing Demodex mites.20Li et al21 demonstrated a strong correlation between ocular Demodex inflammation and serum reactivity to these bacterial proteins in patients with ocular rosacea, and they found that eyelid margin inflammation and facial rosacea correlated with reactivity to these proteins. These studies suggest a possible role for Demodex infestation and bacterial proteins in the etiology of rosacea.
Gonzalez-Hinojosa et al22 demonstrated that even though eyelash blepharitis was more common in PPR than ETR, there was no statistically significant association between rosacea and Demodex blepharitis. In our study, we found a significant correlation between PPR and Demodex positivity. Also, meibomian gland dysfunction was more common in the Demodex-positive group; however, this result was not statistically significant. One study compared patients with primary demodicosis and patients with rosacea with Demodex-induced blepharitis to healthy controls and found that patients with primary demodicosis and patients with rosacea did not have significantly different ocular findings.23 In contrast, Forton and De Maertelaer24 reported that patients with PPR had significantly more severe ocular manifestations compared with patients with demodicosis (P=.004).
Mizuno et al25 compared the normal (nonrosacea) population with and without Demodex-infested eyelashes and found that the 2 groups were not significantly different for meibomian gland dysfunction, fluorescein TBUT, or ocular surface discomfort.
Varying results have been reported regarding the association between Demodex and blepharitis or ocular surface discomfort with or without rosacea. In our study, we found that Demodex did not affect tear function tests or meibomian gland function in patients with rosacea. We believe this study is important because it demonstrates the effects of Demodex on ocular findings in patients with cutaneous rosacea.
Limitations
Our study has some limitations. The number of patients was relatively small, resulting in few significant differences between the comparison groups. A larger prospective research study is required to assess the prevalence of Demodex mites in the ocular rosacea population along with associated symptoms and findings.
Conclusion
Rosacea is a chronic disease associated with skin and ocular manifestations that range from mild to severe, that progresses in the form of attacks, and that requires long-term follow-up and treatment. Rosacea most often presents as a disease that causes ocular surface inflammation of varying degrees. Demodex infestation may increase cutaneous or ocular inflammation in rosacea. Therefore, every patient diagnosed with rosacea should be given a dermatologic examination to determine Demodex positivity and an ophthalmologic examination to determine ocular manifestations.
- O’Reilly N, Gallagher C, Reddy Katikireddy K, et al. Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci. 2012;53:3250-3259.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 suppl 1):S42-S43.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094.
- Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17:1-4.
- Erbagcı Z, Ozgoztası O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36:81‐86.
- Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97:242‐248.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Gold LM, Draelos ZD. New and emerging treatments for rosacea. Am J Clin Dermatol. 2015;16:457-461.
- Two AM, Del Rosso JQ. Kallikrein 5-mediated inflammation in rosacea: clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J Clin Aesthet Dermatol. 2014;7:20-25.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474-481.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74-87.
- Lee SH, Chun YS, Kim JH, et al. The relationship between Demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906-2911.
- Awais M, Anwar MI, Ilfikhar R, et al. Rosacea—the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34:161-166.
- Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9-S14.
- Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(suppl 1):S39-S41.
- Sobolewska B, Doycheva D, Deuter CM, et al. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea [published online April 7, 2020]. Ocul Immunol Inflamm. doi:10.1080/09273948.2020.1727531
- Li J, O‘Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. 2010;117:870-877.
- Gonzalez‐Hinojosa D, Jaime‐Villalonga A, Aguilar‐Montes G, et al. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66:36‐38.
- Sarac G, Cankaya C, Ozcan KN, et al. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J Cosmet Dermatol. 2020;19:1260-1265.
- Forton FMN, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99:47-52.
- Mizuno M, Kawashima M, Uchino M, et al. Demodex-mite infestation in cilia and its association with ocular surface parameters in Japanese volunteers. Eye Contact Lens. 2020;46:291-296.
- O’Reilly N, Gallagher C, Reddy Katikireddy K, et al. Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci. 2012;53:3250-3259.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 suppl 1):S42-S43.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094.
- Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17:1-4.
- Erbagcı Z, Ozgoztası O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36:81‐86.
- Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97:242‐248.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Gold LM, Draelos ZD. New and emerging treatments for rosacea. Am J Clin Dermatol. 2015;16:457-461.
- Two AM, Del Rosso JQ. Kallikrein 5-mediated inflammation in rosacea: clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J Clin Aesthet Dermatol. 2014;7:20-25.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474-481.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74-87.
- Lee SH, Chun YS, Kim JH, et al. The relationship between Demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906-2911.
- Awais M, Anwar MI, Ilfikhar R, et al. Rosacea—the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34:161-166.
- Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9-S14.
- Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(suppl 1):S39-S41.
- Sobolewska B, Doycheva D, Deuter CM, et al. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea [published online April 7, 2020]. Ocul Immunol Inflamm. doi:10.1080/09273948.2020.1727531
- Li J, O‘Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. 2010;117:870-877.
- Gonzalez‐Hinojosa D, Jaime‐Villalonga A, Aguilar‐Montes G, et al. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66:36‐38.
- Sarac G, Cankaya C, Ozcan KN, et al. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J Cosmet Dermatol. 2020;19:1260-1265.
- Forton FMN, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99:47-52.
- Mizuno M, Kawashima M, Uchino M, et al. Demodex-mite infestation in cilia and its association with ocular surface parameters in Japanese volunteers. Eye Contact Lens. 2020;46:291-296.
Practice Points
- Rosacea is a common chronic inflammatory skin disease of the central facial skin and is of unknown origin. Patients with ocular rosacea may report dryness, itching, and photophobia.
- Demodex infestation may increase cutaneous or ocular inflammation in rosacea.
Results of Laboratory Monitoring in Patients Taking Isotretinoin for Acne
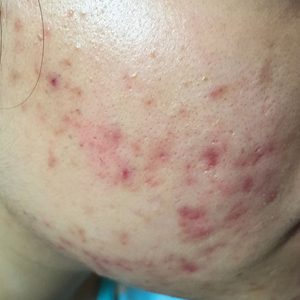
Introduced in 1982, isotretinoin is a retinoid derivative that has been widely used to treat various dermatologic conditions such as acne vulgaris, rosacea, hidradenitis suppurativa, and hair folliculitis. 1 It remains one of the most effective drugs for the treatment of all forms of acne vulgaris, especially the nodulocystic type, and exerts its effects via different mechanisms that affect the major domains involved in the pathogenesis of acne. 2 One month after treatment initiation, isotretinoin suppresses sebum production by decreasing the size and activity of sebaceous glands. In addition, it notably stabilizes keratinization of the skin and decreases the number of Propionibacterium acnes, which will minimize the inflammation associated with acne. 3,4 Despite its beneficial effects, isotretinoin therapy has been associated with several complications. The most commonly reported adverse effects include fissured lips, dry skin, eczema, epistaxis, dry eyes, gastrointestinal tract upset, angular stomatitis, and back pain. Less frequent systemic adverse effects have been reported and relate mainly to teratogenicity, pancreatitis, drug-induced hepatotoxicity, leukopenia, and thrombocytopenia. 5
Isotretinoin use has been associated with alterations in hepatic and lipid profiles; elevations of serum liver enzymes and triglycerides (TGs) following isotretinoin treatment have been reported.4 Consequently, different protocols for laboratory monitoring during isotretinoin therapy have been established and utilized by various health care institutes.6 Despite the time and economic investment involved, certain protocols recommend repetition of liver function tests and several other laboratory parameters following a baseline test.7 The aim of this study was to determine the prevalence of laboratory changes in alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, and TGs among patients with acne receiving isotretinoin therapy, as well as to link the initial and second laboratory readings of the aforementioned parameters following initiation of isotretinoin treatment.
Materials and Methods
This retrospective cohort design study obtained patient data, including laboratory test results, from the Electronic System for Integrated Health Information at King Khalid University Hospital (KKUH)(Riyadh, Saudi Arabia). All patients older than 16 years who presented with acne vulgaris to the dermatology department at KKUH; who received a course of isotretinoin for at least 4 weeks between 2011 and 2016; and who had available baseline readings of ALT, AST, cholesterol, and TGs, as well as 2 concurrent follow-up readings after isotretinoin treatment initiation, were included in this study. Patients with only 1 reading following treatment initiation and those receiving isotretinoin treatment for reasons other than acne were excluded. This study was approved by the institutional review board of the College of Medicine at King Saud University (Riyadh, Saudi Arabia)(E-18-3310).
Statistical Analysis
Data were entered into a Microsoft Excel document, and statistical analysis was performed using SPSS (version 22.0). Data were represented as numbers and percentages. Repeated measures analysis was performed using the Cochran Q test to compare proportions of abnormal laboratory values among 3 groups: baseline, first reading, and second reading. When test results were significant, a post hoc test was used to compare proportions between any 2 groups. Moreover, a Spearman rank correlation was performed to investigate the association between the daily isotretinoin dose and the laboratory parameters. Results with P<.05 were considered statistically significant.
Results
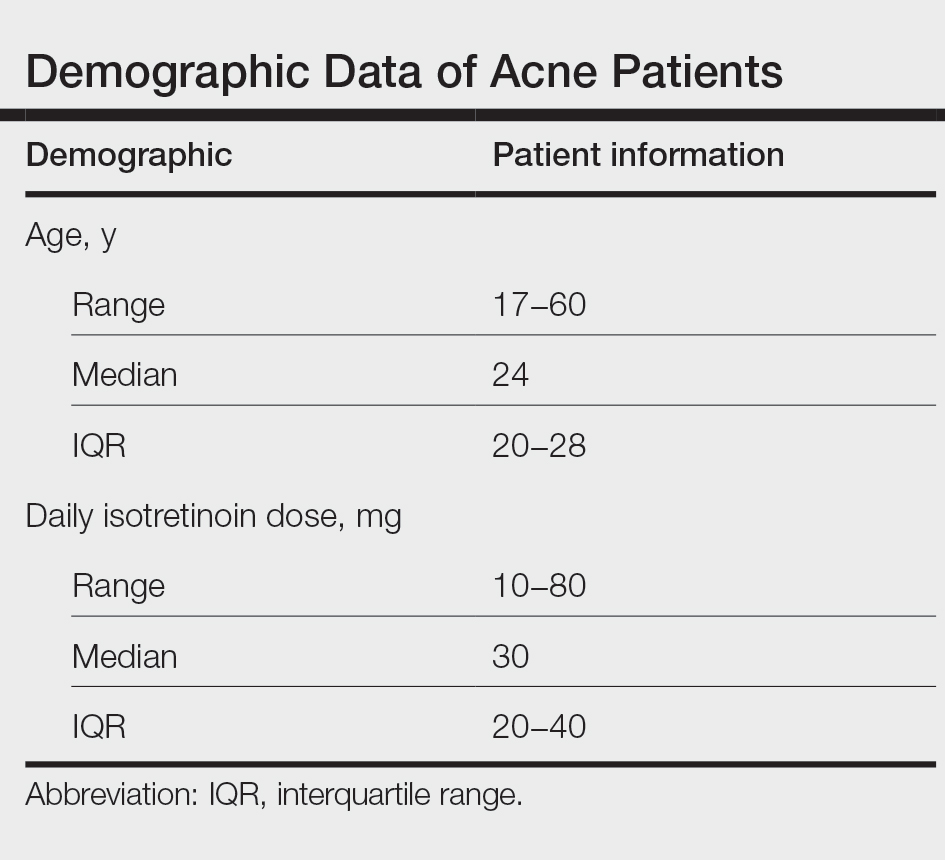
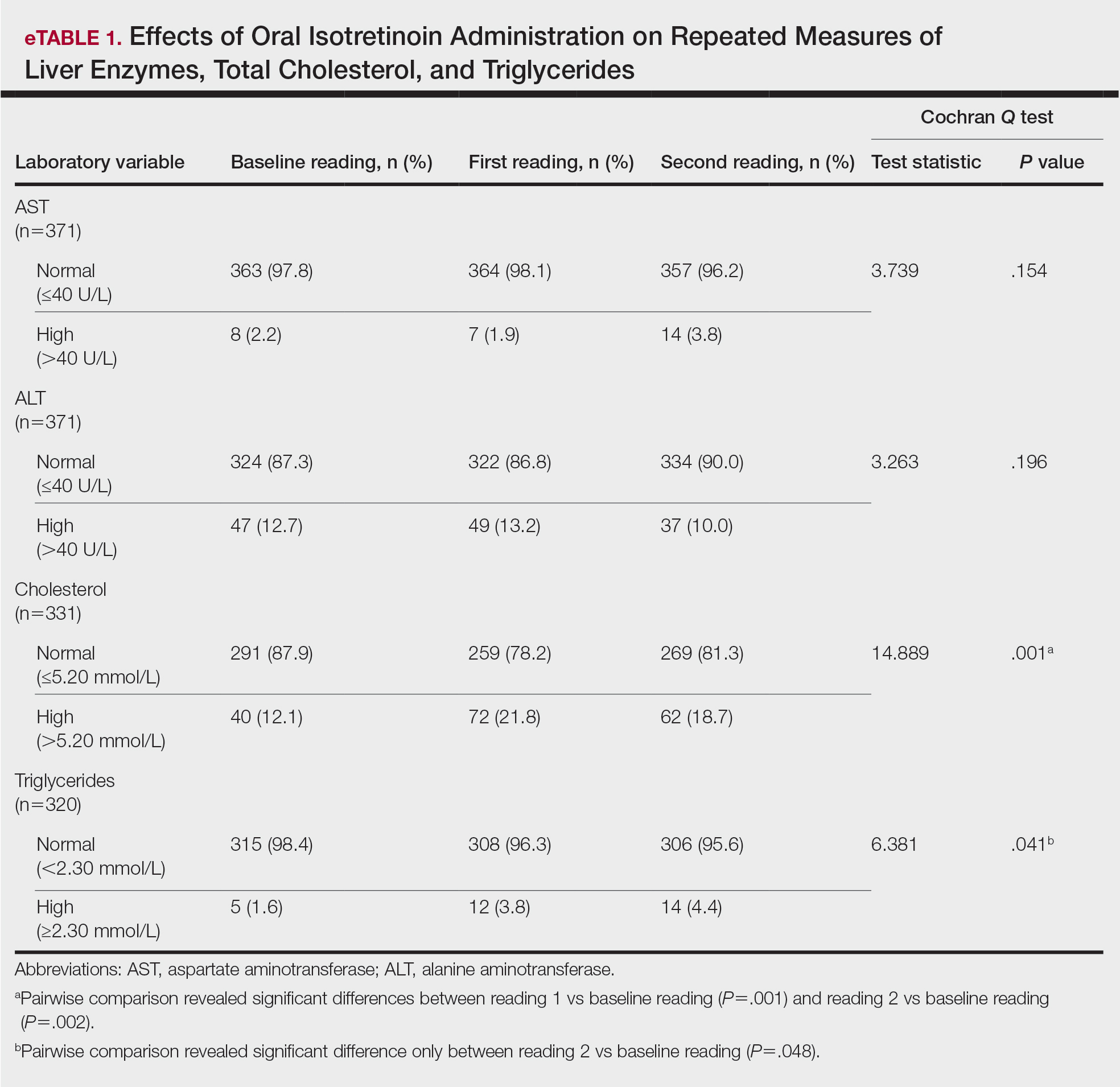
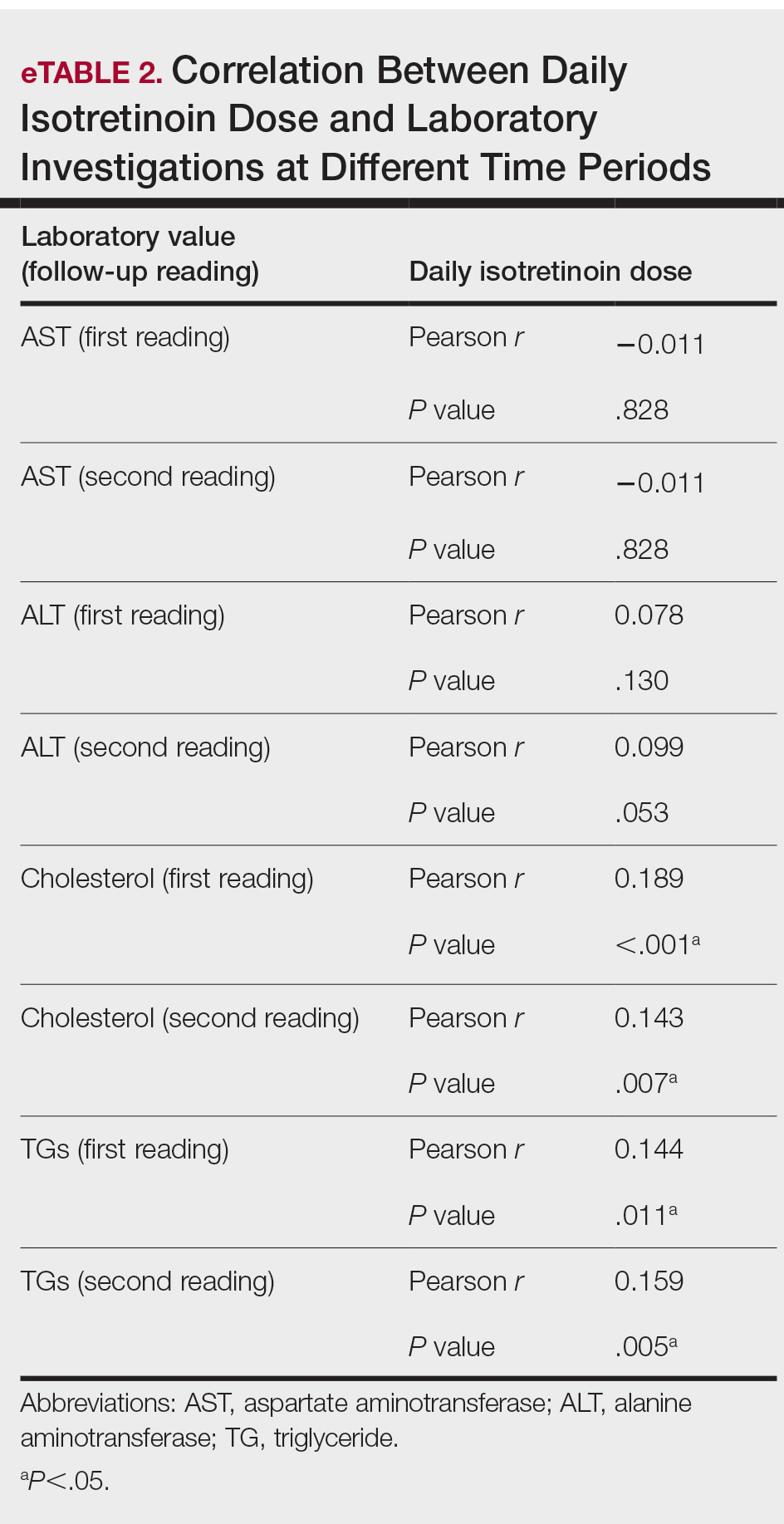
During the study period, treatment with oral isotretinoin was undertaken by 386 patients at KKUH. Several of these patients were excluded due to incomplete medical records. The age of the studied patients ranged from 17 to 60 years, with a median age of 24 years (interquartile range, 20−28 years). The daily administered dose ranged from 10 to 80 mg, with a median dose of 30 mg (interquartile range, 20−40 mg), as illustrated in the Table. Repeated-measures analysis of liver enzymes (AST and ALT), total cholesterol, and TGs is detailed in eTable 1. Eight (2.2%) of 371 patients showed abnormal baseline AST levels. The first follow-up measurements of AST revealed high levels in 7 (1.9%) patients. This figure doubled (14 [3.8%] patients) at the second follow-up, with no statistically significant differences (P>.05). Likewise, ALT showed abnormally high levels at baseline and at both the first and second follow-ups (47/371 [12.7%], 49/371 [13.2%], and 37/371 [10.0%], respectively) with no significant differences (P>.05). Furthermore, the proportions of high cholesterol levels at baseline and at both the first and second follow-ups (40/331 [12.1%], 72/331 [21.8%], and 62/331 [18.7%], respectively) showed a statistically significant difference (P=.001). The proportions of high cholesterol levels in both the first and second follow-ups were significantly higher than the baseline proportions (P=.001 and P=.002, respectively). However, the percentages of high cholesterol were reduced at the second reading relative to the first but with no significant differences. Regarding TGs, there was a statistically significant difference in the proportions of high levels over time (5/320 [1.6%], 12/320 [3.8%], and 14/320 [4.4%] at baseline and at the first and second readings, respectively). Moreover, pairwise comparison among the 3 readings revealed a significant difference between the second follow-up and the baseline levels (P=.048). eTable 2 demonstrates statistically significant positive weak associations between the daily administered isotretinoin dose and each of the cholesterol and TG levels, both at the first and second follow-up readings (P<.05).
Comment
Evaluation of the effects of isotretinoin on liver enzymes and lipids has suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT) and lipid profiles to various degrees.8 Furthermore, there are controversies regarding the routine laboratory monitoring of these patients. Some studies have reported severe alterations in serum liver transaminase and lipid levels, and they support the need for careful monitoring when treating patients with isotretinoin. However, other studies have reported that adverse effects are minimal, with no need for costly laboratory monitoring.9
Our study explored the profile of changes in liver aminotransferases (AST and ALT), cholesterol, and TGs in patients with acne who had been treated with oral isotretinoin. The cholesterol levels showed a nonprogressive increase, with a prevalence rate of 21.8% and 18.7% at the first and second follow-ups, respectively. Likewise, the frequency of high TG levels was 3.8% and 4.4%, respectively, with significant differences from the baseline levels (P=.041). However, liver enzymes were less affected by isotretinoin therapy than lipid profiles. Both AST and ALT showed nonsignificant minimal elevations during follow-up of the patients.
Similar to our findings, Zane et al6 at the University of California, San Francisco, studied 13,772 patients with acne who underwent oral isotretinoin therapy between 1995 and 2002. They reported a cumulative incidence of new abnormalities in patients with normal values at baseline at a frequency of 44% for TG levels, 31% for total cholesterol levels, and 11% for transaminase levels. Moreover, they suggested that these abnormalities generally were transient and reversible.6 Another retrospective study in Brazil included 130 patients who were treated with isotretinoin for 3 months and reported that TG levels had increased beyond the normal range in 11% of patients, whereas 8.6% had elevated AST levels and 7.3% had elevated ALT levels.8 Comparable to our findings, Kizilyel et al10 concluded that isotretinoin appeared to have a greater effect on lipids than on liver enzymes, and they recommended its use with careful monitoring.
The transient effects of isotretinoin therapy on lipid profiles were highlighted in an earlier study. It has been reported that the changes in low-density lipoprotein and TGs returned to baseline levels 2 months following termination of treatment.11 Although many studies have reported alterations in serum transaminase and lipid levels, other studies fail to report any such effects. Alcalay et al7 investigated 907 patients who completed a treatment course lasting 5 to 9 months. They reported that only 1.5% of patients had serum TG levels above 400 mg. Additionally, serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of treatment. They concluded that isotretinoin is a safe therapeutic drug and suggested that there is no need for routine laboratory follow-up in young healthy patients apart from a pregnancy test for females.7 In addition, Brito et al12 conducted a prospective clinical and laboratory evaluation of 150 patients being treated with oral isotretinoin prior to the start of therapy, 1 month after therapy initiation, and every 3 months thereafter until the completion of treatment. They found no statistically significant changes in liver transaminase, TG, or cholesterol levels.12 In another study of 30 participants, Baxter et al13 also reported no significant changes in TG or cholesterol levels measured at baseline or during treatment with isotretinoin. Furthermore, a systematic review and meta-analysis has estimated the laboratory changes that occur during isotretinoin therapy of acne vulgaris.14 The evidence revealed in this study does not support monthly laboratory testing for use of standard doses of oral isotretinoin for the typical patient with acne.
Conclusion
In our study, liver enzymes were less affected than lipids in patients who were treated with isotretinoin. Additionally, laboratory alterations in lipid profiles were nonprogressive and nonsevere. Consequently, isotretinoin may be administered with minimal concern for changes in serum transaminase and lipid profile. However, physicians should exercise caution when administering isotretinoin in patients with a history of abnormal findings.
- Kaymak Y, Ilter N. The results and side effects of systemic isotretinoin treatment in 100 patients with acne vulgaris. Dermatol Nurs. 2006;18:576-580.
- Al-Mutairi N, Manchanda Y, Nour-Eldin O, et al. Isotretinoin in acne vulgaris: a prospective analysis of 160 cases from Kuwait. J Drugs Dermatol. 2005;4:369-373.
- Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688-694.
- Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
- Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
- Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglycerides and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
- Bauer LB, Ornelas JN, Elston DM, et al. Isotretinoin: controversies, facts, and recommendations. Expert Rev Clin Pharmacol. 2016;9:1435-1442.
- Kizilyel O, Metin MS, Elmas ÖF, et al. Effects of oral isotretinoin on lipids and liver enzymes in acne patients. Cutis. 2014;94:234-238.
- Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
- Brito MDFDM, Sant’Anna IP, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin. An Bras Dermatol. 2010;85:331-337.
- Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2004;14:216-218.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
Introduced in 1982, isotretinoin is a retinoid derivative that has been widely used to treat various dermatologic conditions such as acne vulgaris, rosacea, hidradenitis suppurativa, and hair folliculitis. 1 It remains one of the most effective drugs for the treatment of all forms of acne vulgaris, especially the nodulocystic type, and exerts its effects via different mechanisms that affect the major domains involved in the pathogenesis of acne. 2 One month after treatment initiation, isotretinoin suppresses sebum production by decreasing the size and activity of sebaceous glands. In addition, it notably stabilizes keratinization of the skin and decreases the number of Propionibacterium acnes, which will minimize the inflammation associated with acne. 3,4 Despite its beneficial effects, isotretinoin therapy has been associated with several complications. The most commonly reported adverse effects include fissured lips, dry skin, eczema, epistaxis, dry eyes, gastrointestinal tract upset, angular stomatitis, and back pain. Less frequent systemic adverse effects have been reported and relate mainly to teratogenicity, pancreatitis, drug-induced hepatotoxicity, leukopenia, and thrombocytopenia. 5
Isotretinoin use has been associated with alterations in hepatic and lipid profiles; elevations of serum liver enzymes and triglycerides (TGs) following isotretinoin treatment have been reported.4 Consequently, different protocols for laboratory monitoring during isotretinoin therapy have been established and utilized by various health care institutes.6 Despite the time and economic investment involved, certain protocols recommend repetition of liver function tests and several other laboratory parameters following a baseline test.7 The aim of this study was to determine the prevalence of laboratory changes in alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, and TGs among patients with acne receiving isotretinoin therapy, as well as to link the initial and second laboratory readings of the aforementioned parameters following initiation of isotretinoin treatment.
Materials and Methods
This retrospective cohort design study obtained patient data, including laboratory test results, from the Electronic System for Integrated Health Information at King Khalid University Hospital (KKUH)(Riyadh, Saudi Arabia). All patients older than 16 years who presented with acne vulgaris to the dermatology department at KKUH; who received a course of isotretinoin for at least 4 weeks between 2011 and 2016; and who had available baseline readings of ALT, AST, cholesterol, and TGs, as well as 2 concurrent follow-up readings after isotretinoin treatment initiation, were included in this study. Patients with only 1 reading following treatment initiation and those receiving isotretinoin treatment for reasons other than acne were excluded. This study was approved by the institutional review board of the College of Medicine at King Saud University (Riyadh, Saudi Arabia)(E-18-3310).
Statistical Analysis
Data were entered into a Microsoft Excel document, and statistical analysis was performed using SPSS (version 22.0). Data were represented as numbers and percentages. Repeated measures analysis was performed using the Cochran Q test to compare proportions of abnormal laboratory values among 3 groups: baseline, first reading, and second reading. When test results were significant, a post hoc test was used to compare proportions between any 2 groups. Moreover, a Spearman rank correlation was performed to investigate the association between the daily isotretinoin dose and the laboratory parameters. Results with P<.05 were considered statistically significant.
Results
During the study period, treatment with oral isotretinoin was undertaken by 386 patients at KKUH. Several of these patients were excluded due to incomplete medical records. The age of the studied patients ranged from 17 to 60 years, with a median age of 24 years (interquartile range, 20−28 years). The daily administered dose ranged from 10 to 80 mg, with a median dose of 30 mg (interquartile range, 20−40 mg), as illustrated in the Table. Repeated-measures analysis of liver enzymes (AST and ALT), total cholesterol, and TGs is detailed in eTable 1. Eight (2.2%) of 371 patients showed abnormal baseline AST levels. The first follow-up measurements of AST revealed high levels in 7 (1.9%) patients. This figure doubled (14 [3.8%] patients) at the second follow-up, with no statistically significant differences (P>.05). Likewise, ALT showed abnormally high levels at baseline and at both the first and second follow-ups (47/371 [12.7%], 49/371 [13.2%], and 37/371 [10.0%], respectively) with no significant differences (P>.05). Furthermore, the proportions of high cholesterol levels at baseline and at both the first and second follow-ups (40/331 [12.1%], 72/331 [21.8%], and 62/331 [18.7%], respectively) showed a statistically significant difference (P=.001). The proportions of high cholesterol levels in both the first and second follow-ups were significantly higher than the baseline proportions (P=.001 and P=.002, respectively). However, the percentages of high cholesterol were reduced at the second reading relative to the first but with no significant differences. Regarding TGs, there was a statistically significant difference in the proportions of high levels over time (5/320 [1.6%], 12/320 [3.8%], and 14/320 [4.4%] at baseline and at the first and second readings, respectively). Moreover, pairwise comparison among the 3 readings revealed a significant difference between the second follow-up and the baseline levels (P=.048). eTable 2 demonstrates statistically significant positive weak associations between the daily administered isotretinoin dose and each of the cholesterol and TG levels, both at the first and second follow-up readings (P<.05).


Comment
Evaluation of the effects of isotretinoin on liver enzymes and lipids has suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT) and lipid profiles to various degrees.8 Furthermore, there are controversies regarding the routine laboratory monitoring of these patients. Some studies have reported severe alterations in serum liver transaminase and lipid levels, and they support the need for careful monitoring when treating patients with isotretinoin. However, other studies have reported that adverse effects are minimal, with no need for costly laboratory monitoring.9
Our study explored the profile of changes in liver aminotransferases (AST and ALT), cholesterol, and TGs in patients with acne who had been treated with oral isotretinoin. The cholesterol levels showed a nonprogressive increase, with a prevalence rate of 21.8% and 18.7% at the first and second follow-ups, respectively. Likewise, the frequency of high TG levels was 3.8% and 4.4%, respectively, with significant differences from the baseline levels (P=.041). However, liver enzymes were less affected by isotretinoin therapy than lipid profiles. Both AST and ALT showed nonsignificant minimal elevations during follow-up of the patients.
Similar to our findings, Zane et al6 at the University of California, San Francisco, studied 13,772 patients with acne who underwent oral isotretinoin therapy between 1995 and 2002. They reported a cumulative incidence of new abnormalities in patients with normal values at baseline at a frequency of 44% for TG levels, 31% for total cholesterol levels, and 11% for transaminase levels. Moreover, they suggested that these abnormalities generally were transient and reversible.6 Another retrospective study in Brazil included 130 patients who were treated with isotretinoin for 3 months and reported that TG levels had increased beyond the normal range in 11% of patients, whereas 8.6% had elevated AST levels and 7.3% had elevated ALT levels.8 Comparable to our findings, Kizilyel et al10 concluded that isotretinoin appeared to have a greater effect on lipids than on liver enzymes, and they recommended its use with careful monitoring.
The transient effects of isotretinoin therapy on lipid profiles were highlighted in an earlier study. It has been reported that the changes in low-density lipoprotein and TGs returned to baseline levels 2 months following termination of treatment.11 Although many studies have reported alterations in serum transaminase and lipid levels, other studies fail to report any such effects. Alcalay et al7 investigated 907 patients who completed a treatment course lasting 5 to 9 months. They reported that only 1.5% of patients had serum TG levels above 400 mg. Additionally, serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of treatment. They concluded that isotretinoin is a safe therapeutic drug and suggested that there is no need for routine laboratory follow-up in young healthy patients apart from a pregnancy test for females.7 In addition, Brito et al12 conducted a prospective clinical and laboratory evaluation of 150 patients being treated with oral isotretinoin prior to the start of therapy, 1 month after therapy initiation, and every 3 months thereafter until the completion of treatment. They found no statistically significant changes in liver transaminase, TG, or cholesterol levels.12 In another study of 30 participants, Baxter et al13 also reported no significant changes in TG or cholesterol levels measured at baseline or during treatment with isotretinoin. Furthermore, a systematic review and meta-analysis has estimated the laboratory changes that occur during isotretinoin therapy of acne vulgaris.14 The evidence revealed in this study does not support monthly laboratory testing for use of standard doses of oral isotretinoin for the typical patient with acne.
Conclusion
In our study, liver enzymes were less affected than lipids in patients who were treated with isotretinoin. Additionally, laboratory alterations in lipid profiles were nonprogressive and nonsevere. Consequently, isotretinoin may be administered with minimal concern for changes in serum transaminase and lipid profile. However, physicians should exercise caution when administering isotretinoin in patients with a history of abnormal findings.
Introduced in 1982, isotretinoin is a retinoid derivative that has been widely used to treat various dermatologic conditions such as acne vulgaris, rosacea, hidradenitis suppurativa, and hair folliculitis. 1 It remains one of the most effective drugs for the treatment of all forms of acne vulgaris, especially the nodulocystic type, and exerts its effects via different mechanisms that affect the major domains involved in the pathogenesis of acne. 2 One month after treatment initiation, isotretinoin suppresses sebum production by decreasing the size and activity of sebaceous glands. In addition, it notably stabilizes keratinization of the skin and decreases the number of Propionibacterium acnes, which will minimize the inflammation associated with acne. 3,4 Despite its beneficial effects, isotretinoin therapy has been associated with several complications. The most commonly reported adverse effects include fissured lips, dry skin, eczema, epistaxis, dry eyes, gastrointestinal tract upset, angular stomatitis, and back pain. Less frequent systemic adverse effects have been reported and relate mainly to teratogenicity, pancreatitis, drug-induced hepatotoxicity, leukopenia, and thrombocytopenia. 5
Isotretinoin use has been associated with alterations in hepatic and lipid profiles; elevations of serum liver enzymes and triglycerides (TGs) following isotretinoin treatment have been reported.4 Consequently, different protocols for laboratory monitoring during isotretinoin therapy have been established and utilized by various health care institutes.6 Despite the time and economic investment involved, certain protocols recommend repetition of liver function tests and several other laboratory parameters following a baseline test.7 The aim of this study was to determine the prevalence of laboratory changes in alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, and TGs among patients with acne receiving isotretinoin therapy, as well as to link the initial and second laboratory readings of the aforementioned parameters following initiation of isotretinoin treatment.
Materials and Methods
This retrospective cohort design study obtained patient data, including laboratory test results, from the Electronic System for Integrated Health Information at King Khalid University Hospital (KKUH)(Riyadh, Saudi Arabia). All patients older than 16 years who presented with acne vulgaris to the dermatology department at KKUH; who received a course of isotretinoin for at least 4 weeks between 2011 and 2016; and who had available baseline readings of ALT, AST, cholesterol, and TGs, as well as 2 concurrent follow-up readings after isotretinoin treatment initiation, were included in this study. Patients with only 1 reading following treatment initiation and those receiving isotretinoin treatment for reasons other than acne were excluded. This study was approved by the institutional review board of the College of Medicine at King Saud University (Riyadh, Saudi Arabia)(E-18-3310).
Statistical Analysis
Data were entered into a Microsoft Excel document, and statistical analysis was performed using SPSS (version 22.0). Data were represented as numbers and percentages. Repeated measures analysis was performed using the Cochran Q test to compare proportions of abnormal laboratory values among 3 groups: baseline, first reading, and second reading. When test results were significant, a post hoc test was used to compare proportions between any 2 groups. Moreover, a Spearman rank correlation was performed to investigate the association between the daily isotretinoin dose and the laboratory parameters. Results with P<.05 were considered statistically significant.
Results
During the study period, treatment with oral isotretinoin was undertaken by 386 patients at KKUH. Several of these patients were excluded due to incomplete medical records. The age of the studied patients ranged from 17 to 60 years, with a median age of 24 years (interquartile range, 20−28 years). The daily administered dose ranged from 10 to 80 mg, with a median dose of 30 mg (interquartile range, 20−40 mg), as illustrated in the Table. Repeated-measures analysis of liver enzymes (AST and ALT), total cholesterol, and TGs is detailed in eTable 1. Eight (2.2%) of 371 patients showed abnormal baseline AST levels. The first follow-up measurements of AST revealed high levels in 7 (1.9%) patients. This figure doubled (14 [3.8%] patients) at the second follow-up, with no statistically significant differences (P>.05). Likewise, ALT showed abnormally high levels at baseline and at both the first and second follow-ups (47/371 [12.7%], 49/371 [13.2%], and 37/371 [10.0%], respectively) with no significant differences (P>.05). Furthermore, the proportions of high cholesterol levels at baseline and at both the first and second follow-ups (40/331 [12.1%], 72/331 [21.8%], and 62/331 [18.7%], respectively) showed a statistically significant difference (P=.001). The proportions of high cholesterol levels in both the first and second follow-ups were significantly higher than the baseline proportions (P=.001 and P=.002, respectively). However, the percentages of high cholesterol were reduced at the second reading relative to the first but with no significant differences. Regarding TGs, there was a statistically significant difference in the proportions of high levels over time (5/320 [1.6%], 12/320 [3.8%], and 14/320 [4.4%] at baseline and at the first and second readings, respectively). Moreover, pairwise comparison among the 3 readings revealed a significant difference between the second follow-up and the baseline levels (P=.048). eTable 2 demonstrates statistically significant positive weak associations between the daily administered isotretinoin dose and each of the cholesterol and TG levels, both at the first and second follow-up readings (P<.05).


Comment
Evaluation of the effects of isotretinoin on liver enzymes and lipids has suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT) and lipid profiles to various degrees.8 Furthermore, there are controversies regarding the routine laboratory monitoring of these patients. Some studies have reported severe alterations in serum liver transaminase and lipid levels, and they support the need for careful monitoring when treating patients with isotretinoin. However, other studies have reported that adverse effects are minimal, with no need for costly laboratory monitoring.9
Our study explored the profile of changes in liver aminotransferases (AST and ALT), cholesterol, and TGs in patients with acne who had been treated with oral isotretinoin. The cholesterol levels showed a nonprogressive increase, with a prevalence rate of 21.8% and 18.7% at the first and second follow-ups, respectively. Likewise, the frequency of high TG levels was 3.8% and 4.4%, respectively, with significant differences from the baseline levels (P=.041). However, liver enzymes were less affected by isotretinoin therapy than lipid profiles. Both AST and ALT showed nonsignificant minimal elevations during follow-up of the patients.
Similar to our findings, Zane et al6 at the University of California, San Francisco, studied 13,772 patients with acne who underwent oral isotretinoin therapy between 1995 and 2002. They reported a cumulative incidence of new abnormalities in patients with normal values at baseline at a frequency of 44% for TG levels, 31% for total cholesterol levels, and 11% for transaminase levels. Moreover, they suggested that these abnormalities generally were transient and reversible.6 Another retrospective study in Brazil included 130 patients who were treated with isotretinoin for 3 months and reported that TG levels had increased beyond the normal range in 11% of patients, whereas 8.6% had elevated AST levels and 7.3% had elevated ALT levels.8 Comparable to our findings, Kizilyel et al10 concluded that isotretinoin appeared to have a greater effect on lipids than on liver enzymes, and they recommended its use with careful monitoring.
The transient effects of isotretinoin therapy on lipid profiles were highlighted in an earlier study. It has been reported that the changes in low-density lipoprotein and TGs returned to baseline levels 2 months following termination of treatment.11 Although many studies have reported alterations in serum transaminase and lipid levels, other studies fail to report any such effects. Alcalay et al7 investigated 907 patients who completed a treatment course lasting 5 to 9 months. They reported that only 1.5% of patients had serum TG levels above 400 mg. Additionally, serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of treatment. They concluded that isotretinoin is a safe therapeutic drug and suggested that there is no need for routine laboratory follow-up in young healthy patients apart from a pregnancy test for females.7 In addition, Brito et al12 conducted a prospective clinical and laboratory evaluation of 150 patients being treated with oral isotretinoin prior to the start of therapy, 1 month after therapy initiation, and every 3 months thereafter until the completion of treatment. They found no statistically significant changes in liver transaminase, TG, or cholesterol levels.12 In another study of 30 participants, Baxter et al13 also reported no significant changes in TG or cholesterol levels measured at baseline or during treatment with isotretinoin. Furthermore, a systematic review and meta-analysis has estimated the laboratory changes that occur during isotretinoin therapy of acne vulgaris.14 The evidence revealed in this study does not support monthly laboratory testing for use of standard doses of oral isotretinoin for the typical patient with acne.
Conclusion
In our study, liver enzymes were less affected than lipids in patients who were treated with isotretinoin. Additionally, laboratory alterations in lipid profiles were nonprogressive and nonsevere. Consequently, isotretinoin may be administered with minimal concern for changes in serum transaminase and lipid profile. However, physicians should exercise caution when administering isotretinoin in patients with a history of abnormal findings.
- Kaymak Y, Ilter N. The results and side effects of systemic isotretinoin treatment in 100 patients with acne vulgaris. Dermatol Nurs. 2006;18:576-580.
- Al-Mutairi N, Manchanda Y, Nour-Eldin O, et al. Isotretinoin in acne vulgaris: a prospective analysis of 160 cases from Kuwait. J Drugs Dermatol. 2005;4:369-373.
- Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688-694.
- Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
- Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
- Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglycerides and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
- Bauer LB, Ornelas JN, Elston DM, et al. Isotretinoin: controversies, facts, and recommendations. Expert Rev Clin Pharmacol. 2016;9:1435-1442.
- Kizilyel O, Metin MS, Elmas ÖF, et al. Effects of oral isotretinoin on lipids and liver enzymes in acne patients. Cutis. 2014;94:234-238.
- Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
- Brito MDFDM, Sant’Anna IP, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin. An Bras Dermatol. 2010;85:331-337.
- Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2004;14:216-218.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
- Kaymak Y, Ilter N. The results and side effects of systemic isotretinoin treatment in 100 patients with acne vulgaris. Dermatol Nurs. 2006;18:576-580.
- Al-Mutairi N, Manchanda Y, Nour-Eldin O, et al. Isotretinoin in acne vulgaris: a prospective analysis of 160 cases from Kuwait. J Drugs Dermatol. 2005;4:369-373.
- Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688-694.
- Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
- Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
- Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglycerides and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
- Bauer LB, Ornelas JN, Elston DM, et al. Isotretinoin: controversies, facts, and recommendations. Expert Rev Clin Pharmacol. 2016;9:1435-1442.
- Kizilyel O, Metin MS, Elmas ÖF, et al. Effects of oral isotretinoin on lipids and liver enzymes in acne patients. Cutis. 2014;94:234-238.
- Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
- Brito MDFDM, Sant’Anna IP, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin. An Bras Dermatol. 2010;85:331-337.
- Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2004;14:216-218.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44.
Practice Points
- Isotretinoin is the mainstay treatment for severe acne.
- Cost and convenience to patients should always be considered.
- Frequent monitoring for laboratory changes during isotretinoin treatment is not warranted.
Healthy weight gain in pregnancy: What the USPSTF recommends
REFERENCES
- US Preventive Services Task Force. Behavioral counseling interventions for healthy weight and weight gain in pregnancy: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:2087-2092. doi:10.1001/jama.2021.6949
- Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009. doi: 10.17226/12584
REFERENCES
- US Preventive Services Task Force. Behavioral counseling interventions for healthy weight and weight gain in pregnancy: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:2087-2092. doi:10.1001/jama.2021.6949
- Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009. doi: 10.17226/12584
REFERENCES
- US Preventive Services Task Force. Behavioral counseling interventions for healthy weight and weight gain in pregnancy: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:2087-2092. doi:10.1001/jama.2021.6949
- Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009. doi: 10.17226/12584
Married docs remove girl’s lethal facial tumor in ‘excruciatingly difficult’ procedure
In 2019, doctors in London saw a 5-year old girl from rural Ethiopia with an enormous tumor extending from her cheek to her lower jaw. Her name was Negalem and the tumor was a vascular malformation, a life-threatening web of tangled blood vessels.
Surgery to remove it was impossible, the doctors told the foundation advocating for the girl. The child would never make it off the operating table. After a closer examination, the London group still declined to do the procedure, but told the child’s parents and advocates that if anyone was going to attempt this, they’d need to get the little girl to New York.
In New York City, on 64th St. in Manhattan, is the Vascular Birthmark Institute, founded by Milton Waner, MD, who has exclusively treated hemangiomas and vascular malformations for the last 30 years. “I’m the only person in the [United] States whose practice is exclusively [treating] vascular anomalies,” Dr. Waner said in an interview.
Dr. Waner has assembled a multidisciplinary team of experts at the institute’s offices in Lenox Hill – including his wife Teresa O, MD, a facial plastic and reconstructive surgeon and neurospecialist. “People often ask how the hell do you spend so much time with your spouse?” Dr. Waner says. “We work extremely well together. We complement each other.”
Dr. O and Dr. Waner each manage half of the cases at VBI. And in January they received an email about Negalem. After corresponding with the child’s advocate and reviewing images,
The challenge with vascular malformations in children, Dr. Waner said, is that they have a fraction of the blood an adult has. Where adults have an average of 5 L of blood, a child this age has only 1 L. To lose 200 or 300 mL of blood, “that’s 20% or 30% of their blood volume,” Dr. Waner said. So the removal of such a mass, which requires a meticulous dissection around many blood vessels, carries a high risk of the child bleeding out.
There were some logistical hurdles, but the patient arrived in Manhattan in mid-June, at no cost to her family. The medical visa was organized by a volunteer who also work for USAID. Healing the Children Northeast paid for her travel and the Waner Kids Foundation paid for her hotel stay. Lenox Hill Hospital and Northwell Health covered all hospital costs and postsurgery care. And Dr. O and Dr. Waner did the planning, consult visits, and procedure pro bono.
The surgery was possible because of the generosity of several organizations, but the two surgeons still had a limited time to remove the mass. Under different circumstances, and with the luxury of more time, the patient would have undergone several rounds of sclerotherapy. This procedure, done by interventional radiologists, involves injecting a toxin into the blood vessels, which causes them to clot. Done prior to surgery it can help limit bleeding risk.
On June 23, the morning of the surgery, the patient underwent one round of sclerotherapy. However, it didn’t have the intended effect, Dr. Waner said, “because the lesion was just so massive.”
The team had planned several of their moves ahead of time. But this isn’t the sort of surgery you’d find in a textbook. Because it’s such a unique field, Dr. Waner and Dr. O have developed many of their own techniques along the way. This patient was much like the cases they treat every day, only “several orders of magnitudes greater,” Dr. Waner said. “On a scale of 1 to 10 she was a 12.”
The morning of the surgery, “I was very apprehensive,” Dr. Waner recalled. He vividly remembers the girl’s father repeatedly kissing her to say goodbye as she lay on the operating table, fully aware that this procedure was a life-threatening one. And from the beginning there were challenges, like getting her under anesthesia when the anatomy of her mouth, deformed by the tumor, didn’t allow the anesthesiologists to use their typical tubing. Then, once the skin was removed, it became clear how dilated and tangled the involved blood vessels were. There were many vital structures tangled in the anomaly. “The jugular vein was right there. The carotid artery was right there,” Dr. Waner said. It was extremely difficult to delineate and preserve them, he said.
“That’s why we really took our time. We just went very slowly and deliberately,” Dr. O said. The blood vessels were so dilated that their only option was to move painstakingly slow – otherwise a small nick could be devastating.
But even with the slow pace the surgery was “excruciatingly difficult,” Dr. Waner said. And early on in the dissection he wasn’t quite sure they’d make it out. The sclerotherapy hadn’t done much to prevent bleeding. “At one point every millimeter or 2 that we advanced we got into some bleeding,” Dr. Waner said. “Brisk bleeding.”
Once they got into the surgery they also realized that the growth had adhered to the jaw bone. “There were vessels traversing into the bone, which were hard to control,” Dr. O said.
But finally, both doctors realized they’d be able to remove it. With the lesion removed they began the work of reconstruction and reanimation.
The child’s jaw and cheek bone had grown beyond their normal size to support the growth. They had to shave them down to achieve facial symmetry. The tumor had also inhibited much of the child’s facial nerve control. With it gone, Dr. O began the work of finding all the facial nerve branches and assembling them to reanimate the child’s face.
Before medicine, Dr. O trained as an architect, which, according to Dr. Waner, has equipped her with very good spatial awareness – a valuable skill in the surgical reconstruction phase. After seeing a lecture by Dr. Waner, she immediately saw a fit for her unique interest and skill set. She did fellowship training with Dr. Waner in vascular anomalies, and then went on to specialize in facial nerve reanimation. The proof of Dr. O’s expertise is Negalem’s new, beautiful smile, Dr. Waner said.
The surgery drew out over 8 hours, as long as a day of surgeries for the two doctors. When Dr. O finally walked into the waiting room to inform the family of the success, the first words out of the father’s mouth were: “Is my daughter alive?”
A growth like Negalem had is not compatible with a normal life. Dr. Waner’s mantra is that every child has the right to look normal. But this case went beyond aesthetics. If the growth hadn’t been removed, the child was expected to live only 4-6 more years, Dr. Waner said. Without the surgery, she could have suffocated, starved without the ability to swallow, or suffered a fatal bleed.
Dr. O and Dr. Waner are uniquely equipped to do this kind of work, but both are adamant that treating vascular anomalies is a multidisciplinary, multimodal approach. Specialties in anesthesiology, radiology, lasers, facial nerves – they are all critical to these procedures. And often patients with these kinds of lesions require medical and radiologic interventions in addition to surgery. In this particular case, from logistics to post op, “it was a lot of teamwork,” Dr. O said, “a lot of international teams coming together.”
Though extremely difficult, “in the end the result was exactly what we wanted,” Dr. Waner said. Negalem can live a normal life. And as for the surgical duo, both feel very fortunate to do this work. Dr. O said, “I’m honored to have found this specialty and to be able to train with and work with Milton. I’m so happy to do what I do every day.”
A version of this article first appeared on Medscape.com.
In 2019, doctors in London saw a 5-year old girl from rural Ethiopia with an enormous tumor extending from her cheek to her lower jaw. Her name was Negalem and the tumor was a vascular malformation, a life-threatening web of tangled blood vessels.
Surgery to remove it was impossible, the doctors told the foundation advocating for the girl. The child would never make it off the operating table. After a closer examination, the London group still declined to do the procedure, but told the child’s parents and advocates that if anyone was going to attempt this, they’d need to get the little girl to New York.
In New York City, on 64th St. in Manhattan, is the Vascular Birthmark Institute, founded by Milton Waner, MD, who has exclusively treated hemangiomas and vascular malformations for the last 30 years. “I’m the only person in the [United] States whose practice is exclusively [treating] vascular anomalies,” Dr. Waner said in an interview.
Dr. Waner has assembled a multidisciplinary team of experts at the institute’s offices in Lenox Hill – including his wife Teresa O, MD, a facial plastic and reconstructive surgeon and neurospecialist. “People often ask how the hell do you spend so much time with your spouse?” Dr. Waner says. “We work extremely well together. We complement each other.”
Dr. O and Dr. Waner each manage half of the cases at VBI. And in January they received an email about Negalem. After corresponding with the child’s advocate and reviewing images,

The challenge with vascular malformations in children, Dr. Waner said, is that they have a fraction of the blood an adult has. Where adults have an average of 5 L of blood, a child this age has only 1 L. To lose 200 or 300 mL of blood, “that’s 20% or 30% of their blood volume,” Dr. Waner said. So the removal of such a mass, which requires a meticulous dissection around many blood vessels, carries a high risk of the child bleeding out.
There were some logistical hurdles, but the patient arrived in Manhattan in mid-June, at no cost to her family. The medical visa was organized by a volunteer who also work for USAID. Healing the Children Northeast paid for her travel and the Waner Kids Foundation paid for her hotel stay. Lenox Hill Hospital and Northwell Health covered all hospital costs and postsurgery care. And Dr. O and Dr. Waner did the planning, consult visits, and procedure pro bono.
The surgery was possible because of the generosity of several organizations, but the two surgeons still had a limited time to remove the mass. Under different circumstances, and with the luxury of more time, the patient would have undergone several rounds of sclerotherapy. This procedure, done by interventional radiologists, involves injecting a toxin into the blood vessels, which causes them to clot. Done prior to surgery it can help limit bleeding risk.
On June 23, the morning of the surgery, the patient underwent one round of sclerotherapy. However, it didn’t have the intended effect, Dr. Waner said, “because the lesion was just so massive.”
The team had planned several of their moves ahead of time. But this isn’t the sort of surgery you’d find in a textbook. Because it’s such a unique field, Dr. Waner and Dr. O have developed many of their own techniques along the way. This patient was much like the cases they treat every day, only “several orders of magnitudes greater,” Dr. Waner said. “On a scale of 1 to 10 she was a 12.”
The morning of the surgery, “I was very apprehensive,” Dr. Waner recalled. He vividly remembers the girl’s father repeatedly kissing her to say goodbye as she lay on the operating table, fully aware that this procedure was a life-threatening one. And from the beginning there were challenges, like getting her under anesthesia when the anatomy of her mouth, deformed by the tumor, didn’t allow the anesthesiologists to use their typical tubing. Then, once the skin was removed, it became clear how dilated and tangled the involved blood vessels were. There were many vital structures tangled in the anomaly. “The jugular vein was right there. The carotid artery was right there,” Dr. Waner said. It was extremely difficult to delineate and preserve them, he said.

“That’s why we really took our time. We just went very slowly and deliberately,” Dr. O said. The blood vessels were so dilated that their only option was to move painstakingly slow – otherwise a small nick could be devastating.
But even with the slow pace the surgery was “excruciatingly difficult,” Dr. Waner said. And early on in the dissection he wasn’t quite sure they’d make it out. The sclerotherapy hadn’t done much to prevent bleeding. “At one point every millimeter or 2 that we advanced we got into some bleeding,” Dr. Waner said. “Brisk bleeding.”
Once they got into the surgery they also realized that the growth had adhered to the jaw bone. “There were vessels traversing into the bone, which were hard to control,” Dr. O said.
But finally, both doctors realized they’d be able to remove it. With the lesion removed they began the work of reconstruction and reanimation.
The child’s jaw and cheek bone had grown beyond their normal size to support the growth. They had to shave them down to achieve facial symmetry. The tumor had also inhibited much of the child’s facial nerve control. With it gone, Dr. O began the work of finding all the facial nerve branches and assembling them to reanimate the child’s face.
Before medicine, Dr. O trained as an architect, which, according to Dr. Waner, has equipped her with very good spatial awareness – a valuable skill in the surgical reconstruction phase. After seeing a lecture by Dr. Waner, she immediately saw a fit for her unique interest and skill set. She did fellowship training with Dr. Waner in vascular anomalies, and then went on to specialize in facial nerve reanimation. The proof of Dr. O’s expertise is Negalem’s new, beautiful smile, Dr. Waner said.
The surgery drew out over 8 hours, as long as a day of surgeries for the two doctors. When Dr. O finally walked into the waiting room to inform the family of the success, the first words out of the father’s mouth were: “Is my daughter alive?”
A growth like Negalem had is not compatible with a normal life. Dr. Waner’s mantra is that every child has the right to look normal. But this case went beyond aesthetics. If the growth hadn’t been removed, the child was expected to live only 4-6 more years, Dr. Waner said. Without the surgery, she could have suffocated, starved without the ability to swallow, or suffered a fatal bleed.

Dr. O and Dr. Waner are uniquely equipped to do this kind of work, but both are adamant that treating vascular anomalies is a multidisciplinary, multimodal approach. Specialties in anesthesiology, radiology, lasers, facial nerves – they are all critical to these procedures. And often patients with these kinds of lesions require medical and radiologic interventions in addition to surgery. In this particular case, from logistics to post op, “it was a lot of teamwork,” Dr. O said, “a lot of international teams coming together.”
Though extremely difficult, “in the end the result was exactly what we wanted,” Dr. Waner said. Negalem can live a normal life. And as for the surgical duo, both feel very fortunate to do this work. Dr. O said, “I’m honored to have found this specialty and to be able to train with and work with Milton. I’m so happy to do what I do every day.”
A version of this article first appeared on Medscape.com.
In 2019, doctors in London saw a 5-year old girl from rural Ethiopia with an enormous tumor extending from her cheek to her lower jaw. Her name was Negalem and the tumor was a vascular malformation, a life-threatening web of tangled blood vessels.
Surgery to remove it was impossible, the doctors told the foundation advocating for the girl. The child would never make it off the operating table. After a closer examination, the London group still declined to do the procedure, but told the child’s parents and advocates that if anyone was going to attempt this, they’d need to get the little girl to New York.
In New York City, on 64th St. in Manhattan, is the Vascular Birthmark Institute, founded by Milton Waner, MD, who has exclusively treated hemangiomas and vascular malformations for the last 30 years. “I’m the only person in the [United] States whose practice is exclusively [treating] vascular anomalies,” Dr. Waner said in an interview.
Dr. Waner has assembled a multidisciplinary team of experts at the institute’s offices in Lenox Hill – including his wife Teresa O, MD, a facial plastic and reconstructive surgeon and neurospecialist. “People often ask how the hell do you spend so much time with your spouse?” Dr. Waner says. “We work extremely well together. We complement each other.”
Dr. O and Dr. Waner each manage half of the cases at VBI. And in January they received an email about Negalem. After corresponding with the child’s advocate and reviewing images,

The challenge with vascular malformations in children, Dr. Waner said, is that they have a fraction of the blood an adult has. Where adults have an average of 5 L of blood, a child this age has only 1 L. To lose 200 or 300 mL of blood, “that’s 20% or 30% of their blood volume,” Dr. Waner said. So the removal of such a mass, which requires a meticulous dissection around many blood vessels, carries a high risk of the child bleeding out.
There were some logistical hurdles, but the patient arrived in Manhattan in mid-June, at no cost to her family. The medical visa was organized by a volunteer who also work for USAID. Healing the Children Northeast paid for her travel and the Waner Kids Foundation paid for her hotel stay. Lenox Hill Hospital and Northwell Health covered all hospital costs and postsurgery care. And Dr. O and Dr. Waner did the planning, consult visits, and procedure pro bono.
The surgery was possible because of the generosity of several organizations, but the two surgeons still had a limited time to remove the mass. Under different circumstances, and with the luxury of more time, the patient would have undergone several rounds of sclerotherapy. This procedure, done by interventional radiologists, involves injecting a toxin into the blood vessels, which causes them to clot. Done prior to surgery it can help limit bleeding risk.
On June 23, the morning of the surgery, the patient underwent one round of sclerotherapy. However, it didn’t have the intended effect, Dr. Waner said, “because the lesion was just so massive.”
The team had planned several of their moves ahead of time. But this isn’t the sort of surgery you’d find in a textbook. Because it’s such a unique field, Dr. Waner and Dr. O have developed many of their own techniques along the way. This patient was much like the cases they treat every day, only “several orders of magnitudes greater,” Dr. Waner said. “On a scale of 1 to 10 she was a 12.”
The morning of the surgery, “I was very apprehensive,” Dr. Waner recalled. He vividly remembers the girl’s father repeatedly kissing her to say goodbye as she lay on the operating table, fully aware that this procedure was a life-threatening one. And from the beginning there were challenges, like getting her under anesthesia when the anatomy of her mouth, deformed by the tumor, didn’t allow the anesthesiologists to use their typical tubing. Then, once the skin was removed, it became clear how dilated and tangled the involved blood vessels were. There were many vital structures tangled in the anomaly. “The jugular vein was right there. The carotid artery was right there,” Dr. Waner said. It was extremely difficult to delineate and preserve them, he said.

“That’s why we really took our time. We just went very slowly and deliberately,” Dr. O said. The blood vessels were so dilated that their only option was to move painstakingly slow – otherwise a small nick could be devastating.
But even with the slow pace the surgery was “excruciatingly difficult,” Dr. Waner said. And early on in the dissection he wasn’t quite sure they’d make it out. The sclerotherapy hadn’t done much to prevent bleeding. “At one point every millimeter or 2 that we advanced we got into some bleeding,” Dr. Waner said. “Brisk bleeding.”
Once they got into the surgery they also realized that the growth had adhered to the jaw bone. “There were vessels traversing into the bone, which were hard to control,” Dr. O said.
But finally, both doctors realized they’d be able to remove it. With the lesion removed they began the work of reconstruction and reanimation.
The child’s jaw and cheek bone had grown beyond their normal size to support the growth. They had to shave them down to achieve facial symmetry. The tumor had also inhibited much of the child’s facial nerve control. With it gone, Dr. O began the work of finding all the facial nerve branches and assembling them to reanimate the child’s face.
Before medicine, Dr. O trained as an architect, which, according to Dr. Waner, has equipped her with very good spatial awareness – a valuable skill in the surgical reconstruction phase. After seeing a lecture by Dr. Waner, she immediately saw a fit for her unique interest and skill set. She did fellowship training with Dr. Waner in vascular anomalies, and then went on to specialize in facial nerve reanimation. The proof of Dr. O’s expertise is Negalem’s new, beautiful smile, Dr. Waner said.
The surgery drew out over 8 hours, as long as a day of surgeries for the two doctors. When Dr. O finally walked into the waiting room to inform the family of the success, the first words out of the father’s mouth were: “Is my daughter alive?”
A growth like Negalem had is not compatible with a normal life. Dr. Waner’s mantra is that every child has the right to look normal. But this case went beyond aesthetics. If the growth hadn’t been removed, the child was expected to live only 4-6 more years, Dr. Waner said. Without the surgery, she could have suffocated, starved without the ability to swallow, or suffered a fatal bleed.

Dr. O and Dr. Waner are uniquely equipped to do this kind of work, but both are adamant that treating vascular anomalies is a multidisciplinary, multimodal approach. Specialties in anesthesiology, radiology, lasers, facial nerves – they are all critical to these procedures. And often patients with these kinds of lesions require medical and radiologic interventions in addition to surgery. In this particular case, from logistics to post op, “it was a lot of teamwork,” Dr. O said, “a lot of international teams coming together.”
Though extremely difficult, “in the end the result was exactly what we wanted,” Dr. Waner said. Negalem can live a normal life. And as for the surgical duo, both feel very fortunate to do this work. Dr. O said, “I’m honored to have found this specialty and to be able to train with and work with Milton. I’m so happy to do what I do every day.”
A version of this article first appeared on Medscape.com.
Finding room for hope
Dear colleagues,
I’m thrilled to introduce the August edition of The New Gastroenterologist, which features an excellent line-up of articles! Summer has been in full swing, and gradually, we eased into aspects of our prepandemic routine. The fear, caution, and isolation that characterized the last year and a half was less pervasive, and the ability to reconnect in person felt both refreshing and liberating. While new threats of variants and rising infection rates have emerged, there is hope that, with the availability of vaccines, the worst of the pandemic may still be behind us.
One of the most difficult aspects of treating patients with inflammatory bowel disease is acute pain management. Dr. Jami Kinnucan and Dr. Mehwish Ahmed (University of Michigan) outline an expert approach on differentiating between visceral and somatic pain and how to manage each accordingly.
The diagnosis of microscopic colitis can be elusive because colonic mucosa typically appears endoscopically normal and the pathognomonic findings are histologic. Management can also be challenging given the frequently relapsing and remitting nature of its clinical course. The “In Focus” feature for August, written by Dr. June Tome, Dr. Amrit Kamboj, and Dr. Darrell Pardi (Mayo Clinic), is an absolute must-read as it provides a detailed review on the diagnosis, management, and therapeutic options for microscopic colitis.
As gastroenterologists, we are often asked to place percutaneous endoscopic gastrostomy (PEG) tubes. This can be a difficult situation to navigate especially when the indication or timing of placement seems questionable. In our ethics case for this quarter, Dr. David Seres and Dr. Jane Cowan (Columbia University) unpack the ethical considerations of PEG tube placement in order to facilitate discharge to subacute nursing facilities.
Months in quarantine have incited many to crave larger living spaces, lending to a chaotic housing market. Jon Solitro (FinancialMD) offers sound financial advice for physicians interested purchasing a home – including factors to consider when choosing a home, how much to spend, and whether or not to consider a doctor’s loan.
Success in research can be particularly difficult for fellows and early career gastroenterologists as they juggle the many responsibilities inherent to busy training programs or adjust to independent practice. Dr. Dionne Rebello and Dr. Michelle Long (Boston University) compile a list of incredibly helpful tips on how to optimize productivity. For those interested in ways to harness experiences in clinical medicine into health technology, Dr. Simon Matthews (Johns Hopkins) discusses his role as chief medical officer in a health tech start-up in our postfellowship pathways section.
Lastly, our DHPA Private Practice Perspectives article, written by Dr. George Dickstein (Greater Boston Gastroenterology), nicely summarizes lessons learned from the pandemic and how a practice can be adequately prepared for a post-pandemic surge of procedures.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu) or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m thrilled to introduce the August edition of The New Gastroenterologist, which features an excellent line-up of articles! Summer has been in full swing, and gradually, we eased into aspects of our prepandemic routine. The fear, caution, and isolation that characterized the last year and a half was less pervasive, and the ability to reconnect in person felt both refreshing and liberating. While new threats of variants and rising infection rates have emerged, there is hope that, with the availability of vaccines, the worst of the pandemic may still be behind us.
One of the most difficult aspects of treating patients with inflammatory bowel disease is acute pain management. Dr. Jami Kinnucan and Dr. Mehwish Ahmed (University of Michigan) outline an expert approach on differentiating between visceral and somatic pain and how to manage each accordingly.
The diagnosis of microscopic colitis can be elusive because colonic mucosa typically appears endoscopically normal and the pathognomonic findings are histologic. Management can also be challenging given the frequently relapsing and remitting nature of its clinical course. The “In Focus” feature for August, written by Dr. June Tome, Dr. Amrit Kamboj, and Dr. Darrell Pardi (Mayo Clinic), is an absolute must-read as it provides a detailed review on the diagnosis, management, and therapeutic options for microscopic colitis.
As gastroenterologists, we are often asked to place percutaneous endoscopic gastrostomy (PEG) tubes. This can be a difficult situation to navigate especially when the indication or timing of placement seems questionable. In our ethics case for this quarter, Dr. David Seres and Dr. Jane Cowan (Columbia University) unpack the ethical considerations of PEG tube placement in order to facilitate discharge to subacute nursing facilities.
Months in quarantine have incited many to crave larger living spaces, lending to a chaotic housing market. Jon Solitro (FinancialMD) offers sound financial advice for physicians interested purchasing a home – including factors to consider when choosing a home, how much to spend, and whether or not to consider a doctor’s loan.
Success in research can be particularly difficult for fellows and early career gastroenterologists as they juggle the many responsibilities inherent to busy training programs or adjust to independent practice. Dr. Dionne Rebello and Dr. Michelle Long (Boston University) compile a list of incredibly helpful tips on how to optimize productivity. For those interested in ways to harness experiences in clinical medicine into health technology, Dr. Simon Matthews (Johns Hopkins) discusses his role as chief medical officer in a health tech start-up in our postfellowship pathways section.
Lastly, our DHPA Private Practice Perspectives article, written by Dr. George Dickstein (Greater Boston Gastroenterology), nicely summarizes lessons learned from the pandemic and how a practice can be adequately prepared for a post-pandemic surge of procedures.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu) or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m thrilled to introduce the August edition of The New Gastroenterologist, which features an excellent line-up of articles! Summer has been in full swing, and gradually, we eased into aspects of our prepandemic routine. The fear, caution, and isolation that characterized the last year and a half was less pervasive, and the ability to reconnect in person felt both refreshing and liberating. While new threats of variants and rising infection rates have emerged, there is hope that, with the availability of vaccines, the worst of the pandemic may still be behind us.
One of the most difficult aspects of treating patients with inflammatory bowel disease is acute pain management. Dr. Jami Kinnucan and Dr. Mehwish Ahmed (University of Michigan) outline an expert approach on differentiating between visceral and somatic pain and how to manage each accordingly.
The diagnosis of microscopic colitis can be elusive because colonic mucosa typically appears endoscopically normal and the pathognomonic findings are histologic. Management can also be challenging given the frequently relapsing and remitting nature of its clinical course. The “In Focus” feature for August, written by Dr. June Tome, Dr. Amrit Kamboj, and Dr. Darrell Pardi (Mayo Clinic), is an absolute must-read as it provides a detailed review on the diagnosis, management, and therapeutic options for microscopic colitis.
As gastroenterologists, we are often asked to place percutaneous endoscopic gastrostomy (PEG) tubes. This can be a difficult situation to navigate especially when the indication or timing of placement seems questionable. In our ethics case for this quarter, Dr. David Seres and Dr. Jane Cowan (Columbia University) unpack the ethical considerations of PEG tube placement in order to facilitate discharge to subacute nursing facilities.
Months in quarantine have incited many to crave larger living spaces, lending to a chaotic housing market. Jon Solitro (FinancialMD) offers sound financial advice for physicians interested purchasing a home – including factors to consider when choosing a home, how much to spend, and whether or not to consider a doctor’s loan.
Success in research can be particularly difficult for fellows and early career gastroenterologists as they juggle the many responsibilities inherent to busy training programs or adjust to independent practice. Dr. Dionne Rebello and Dr. Michelle Long (Boston University) compile a list of incredibly helpful tips on how to optimize productivity. For those interested in ways to harness experiences in clinical medicine into health technology, Dr. Simon Matthews (Johns Hopkins) discusses his role as chief medical officer in a health tech start-up in our postfellowship pathways section.
Lastly, our DHPA Private Practice Perspectives article, written by Dr. George Dickstein (Greater Boston Gastroenterology), nicely summarizes lessons learned from the pandemic and how a practice can be adequately prepared for a post-pandemic surge of procedures.
If you have interest in contributing or have ideas for future TNG topics, please contact me (vijayarao@medicine.bsd.uchicago.edu) or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Microscopic colitis: A common, yet often overlooked, cause of chronic diarrhea
Microscopic colitis is an inflammatory disease of the colon and a frequent cause of chronic or recurrent watery diarrhea, particularly in older persons. MC consists of two subtypes, collagenous colitis (CC) and lymphocytic colitis (LC). While the primary symptom is diarrhea, other signs and symptoms such as abdominal pain, weight loss, and dehydration or electrolyte abnormalities may also be present depending on disease severity.1 In MC, the colonic mucosa usually appears normal on colonoscopy, and the diagnosis is made by histologic findings of intraepithelial lymphocytosis with (CC) or without (LC) a prominent subepithelial collagen band. The management approaches to CC and LC are similar and should be directed based on the severity of symptoms.2 We review the epidemiology, risk factors, pathophysiology, diagnosis, and clinical management for this condition, as well as novel therapeutic approaches.
Epidemiology
Although the incidence of MC increased in the late twentieth century, more recently, it has stabilized with an estimated incidence varying from 1 to 25 per 100,000 person-years.3-5 A recent meta-analysis revealed a pooled incidence of 4.85 per 100,000 persons for LC and 4.14 per 100,000 persons for CC.6 Proposed explanations for the rising incidence in the late twentieth century include improved clinical awareness of the disease, possible increased use of drugs associated with MC, and increased performance of diagnostic colonoscopies for chronic diarrhea. Since MC is now well-recognized, the recent plateau in incidence rates may reflect decreased detection bias.
The prevalence of MC ranges from 10%-20% in patients undergoing colonoscopy for chronic watery diarrhea.6,7 The prevalence of LC is approximately 63.1 cases per 100,000 person-years and, for CC, is 49.2 cases per 100,000 person-years.6-8 Recent studies have demonstrated increasing prevalence of MC likely resulting from an aging population.9,10
Risk stratification
Female gender, increasing age, concomitant autoimmune disease, and the use of certain drugs, including NSAIDs, proton pump inhibitors (PPIs), statins, and selective serotonin reuptake inhibitors (SSRIs), have been associated with an increased risk of MC.11,12 Autoimmune disorders, including celiac disease (CD), rheumatoid arthritis, hypothyroidism, and hyperthyroidism, are more common in patients with MC. The association with CD, in particular, is clinically important, as CD is associated with a 50-70 times greater risk of MC, and 2%-9% of patients with MC have CD.13,14
Several medications have been associated with MC. In a British multicenter prospective study, MC was associated with the use of NSAIDs, PPIs, and SSRIs;15 however, recent studies have questioned the association of MC with some of these medications, which might worsen diarrhea but not actually cause MC.16
An additional risk factor for MC is smoking. A recent meta-analysis demonstrated that current and former smokers had an increased risk of MC (odds ratio, 2.99; 95% confidence interval, 2.15-4.15 and OR, 1.63; 95% CI, 1.37-1.94, respectively), compared with nonsmokers.17 Smokers develop MC at a younger age, and smoking is associated with increased disease severity and decreased likelihood of attaining remission.18,19
Pathogenesis
The pathogenesis of MC remains largely unknown, although there are several hypotheses. The leading proposed mechanisms include reaction to luminal antigens, dysregulated collagen metabolism, genetic predisposition, autoimmunity, and bile acid malabsorption.
MC may be caused by abnormal epithelial barrier function, leading to increased permeability and reaction to luminal antigens, including dietary antigens, certain drugs, and bacterial products, 20,21 which themselves lead to the immune dysregulation and intestinal inflammation seen in MC. This mechanism may explain the association of several drugs with MC. Histological changes resembling LC are reported in patients with CD who consume gluten; however, large population-based studies have not found specific dietary associations with the development of MC.22
Another potential mechanism of MC is dysregulated collagen deposition. Collagen accumulation in the subepithelial layer in CC may result from increased levels of fibroblast growth factor, transforming growth factor–beta and vascular endothelial growth factor.23 Nonetheless, studies have not found an association between the severity of diarrhea in patients with CC and the thickness of the subepithelial collagen band.
Thirdly, autoimmunity and genetic predisposition have been postulated in the pathogenesis of MC. As previously discussed, MC is associated with several autoimmune diseases and predominantly occurs in women, a distinctive feature of autoimmune disorders. Several studies have demonstrated an association between MC and HLA-DQ2 and -DQ3 haplotypes,24 as well as potential polymorphisms in the serotonin transporter gene promoter.25 It is important to note, however, that only a few familial cases of MC have been reported to date.26
Lastly, bile acid malabsorption may play a role in the etiology of MC. Histologic findings of inflammation, along with villous atrophy and collagen deposition, have been reported in the ileum of patients with MC;27,28 however, because patients with MC without bile acid malabsorption may also respond to bile acid binders such as cholestyramine, these findings unlikely to be the sole mechanism explaining the development of the disease.
Despite the different proposed mechanisms for the pathogenesis of MC, no definite conclusions can be drawn because of the limited size of these studies and their often conflicting results.

Clinical features
Clinicians should suspect MC in patients with chronic or recurrent watery diarrhea, particularly in older persons. Other risk factors include female gender, use of certain culprit medications, smoking, and presence of other autoimmune diseases. The clinical manifestations of MC subtypes LC and CC are similar with no significant clinical differences.1,2 In addition to diarrhea, patients with MC may have abdominal pain, fatigue, and dehydration or electrolyte abnormalities depending on disease severity. Patients may also present with fecal urgency, incontinence, and nocturnal stools. Quality of life is often reduced in these patients, predominantly in those with severe or refractory symptoms.29,30 The natural course of MC is highly variable, with some patients achieving spontaneous resolution after one episode and others developing chronic symptoms.
Diagnosis
The differential diagnosis of chronic watery diarrhea is broad and includes malabsorption/maldigestion, inflammatory bowel disease (IBD), irritable bowel syndrome, and medication side effects. In addition, although gastrointestinal infections typically cause acute or subacute diarrhea, some can present with chronic diarrhea. Malabsorption/maldigestion may occur because of CD, lactose intolerance, and pancreatic insufficiency, among other conditions. A thorough history, regarding recent antibiotic and medication use, travel, and immunosuppression, should be obtained in patients with chronic diarrhea. Additionally, laboratory and endoscopic evaluation with random biopsies of the colon can further help differentiate these diseases from MC. A few studies suggest fecal calprotectin may be used to differentiate MC from other noninflammatory conditions such as irritable bowel syndrome, as well as to monitor disease activity. This test is not expected to distinguish MC from other inflammatory causes of diarrhea, such as IBD, and therefore, its role in clinical practice is uncertain.31
The diagnosis of MC is made by biopsy of the colonic mucosa demonstrating characteristic pathologic features.32 Unlike in diseases such as Crohn’s disease or ulcerative colitis, the colon usually appears normal in MC, although mild nonspecific changes, such as erythema or edema, may be visualized. There is no consensus on the ideal location to obtain biopsies for MC or whether biopsies from both the left and the right colon are required.2,33 The procedure of choice for the diagnosis of MC is colonoscopy with random biopsies taken throughout the colon. More limited evaluation by flexible sigmoidoscopy with biopsies may miss cases of MC as inflammation and collagen thickening are not necessarily uniform throughout the colon; however, in a patient that has undergone a recent colonoscopy for colon cancer screening without colon biopsies, a flexible sigmoidoscopy may be a reasonable next test for evaluation of MC, provided biopsies are obtained above the rectosigmoid colon.34
The MC subtypes are differentiated based on histology. The hallmark of LC is less than 20 intraepithelial lymphocytes per 100 surface epithelial cells (normal, less than 5) (Figure 1A). CC is characterized by a thickened subepithelial collagen band greater than 7-10 micrometers (normal, less than 5) (Figure 1B). For a subgroup of patients with milder abnormalities that do not meet these histological criteria, the terms “microscopic colitis, not otherwise specified” or “microscopic colitis, incomplete” may be used.35 These patients often respond to standard treatments for MC. There is an additional subset of patients with biopsy demonstrating features of both CC and LC simultaneously, as well as patients transitioning from one MC subtype to another over time.32,35
Management approach
The first step in management of patients with MC includes stopping culprit medications if there is a temporal relationship between the initiation of the medication and the onset of diarrhea, as well as encouraging smoking cessation. These steps alone, however, are unlikely to achieve clinical remission in most patients. A stepwise pharmacological approach is used in the management of MC based on disease severity (Figure 2). For patients with mild symptoms, antidiarrheal medications, such as loperamide, may be helpful.36 Long-term use of loperamide at therapeutic doses no greater than 16 mg daily appears to be safe if required to maintain symptom response. For those with persistent symptoms despite antidiarrheal medications, bismuth subsalicylate at three 262 mg tablets three times daily for 6-8 weeks can be considered. Long-term use of bismuth subsalicylate is not advised, especially at this dose, because of possible neurotoxicity.37
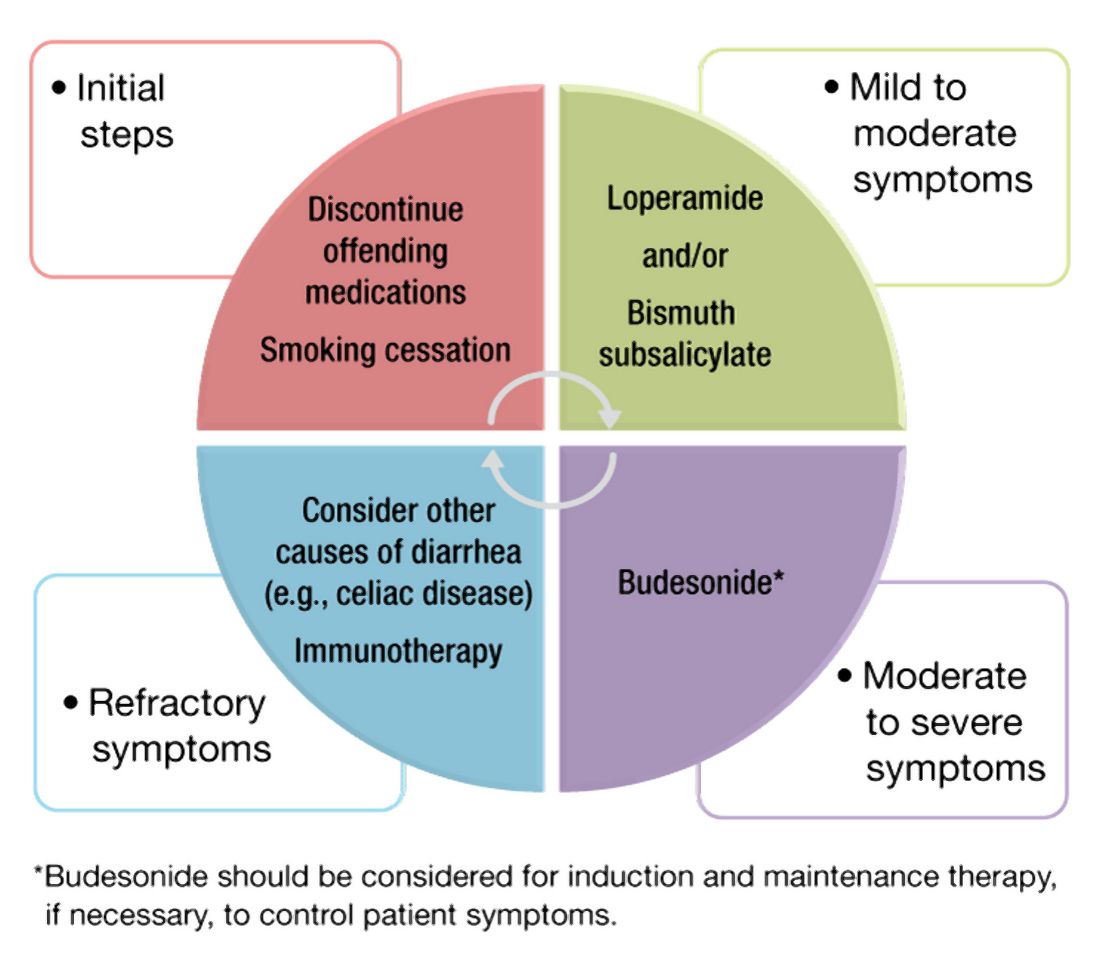
For patients refractory to the above treatments or those with moderate-to-severe symptoms, an 8-week course of budesonide at 9 mg daily is the first-line treatment.38 The dose was tapered before discontinuation in some studies but not in others. Both strategies appear effective. A recent meta-analysis of nine randomized trials demonstrated pooled ORs of 7.34 (95% CI, 4.08-13.19) and 8.35 (95% CI, 4.14-16.85) for response to budesonide induction and maintenance, respectively.39
Cholestyramine is another medication considered in the management of MC and warrants further investigation. To date, no randomized clinical trials have been conducted to evaluate bile acid sequestrants in MC, but they should be considered before placing patients on immunosuppressive medications. Some providers use mesalamine in this setting, although mesalamine is inferior to budesonide in the induction of clinical remission in MC.40
Despite high rates of response to budesonide, relapse after discontinuation is frequent (60%-80%), and time to relapse is variable41,42 The American Gastroenterological Association recommends budesonide for maintenance of remission in patients with recurrence following discontinuation of induction therapy. The lowest effective dose that maintains resolution of symptoms should be prescribed, ideally at 6 mg daily or lower.38 Although budesonide has a greater first-pass metabolism, compared with other glucocorticoids, patients should be monitored for possible side effects including hypertension, diabetes, and osteoporosis, as well as ophthalmologic disease, including cataracts and glaucoma.
For those who are intolerant to budesonide or have refractory symptoms, concomitant disorders such as CD that may be contributing to symptoms must be excluded. Immunosuppressive medications – such as thiopurines and biologic agents, including tumor necrosis factor–alpha inhibitors or vedolizumab – may be considered in refractory cases.43,44 Of note, there are limited studies evaluating the use of these medications for MC. Lastly, surgeries including ileostomy with or without colectomy have been performed in the most severe cases for resistant disease that has failed numerous pharmacological therapies.45
Patients should be counseled that, while symptoms from MC can be quite bothersome and disabling, there appears to be a normal life expectancy and no association between MC and colon cancer, unlike with other inflammatory conditions of the colon such as IBD.46,47
Conclusion and future outlook
As a common cause of chronic watery diarrhea, MC will be commonly encountered in primary care and gastroenterology practices. The diagnosis should be suspected in patients presenting with chronic or recurrent watery diarrhea, especially with female gender, autoimmune disease, and increasing age. The management of MC requires an algorithmic approach directed by symptom severity, with a subgroup of patients requiring maintenance therapy for relapsing symptoms. The care of patients with MC will continue to evolve in the future. Further work is needed to explore long-term safety outcomes with budesonide and the role of immunomodulators and newer biologic agents for patients with complex, refractory disease.
Dr. Tome is with the department of internal medicine at the Mayo Clinic, Rochester, Minn. Dr. Kamboj, and Dr. Pardi are with the division of gastroenterology and hepatology at the Mayo Clinic. Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta and Otsuka. The other authors have no conflicts of interest to report.
References
1. Nyhlin N et al. Aliment Pharmacol Ther. 2014;39:963-72.
2. Miehlke S et al. United European Gastroenterol J. 2020;20-8.
3. Pardi DS et al. Gut. 2007;56:504-8.
4. Fernández-Bañares F et al. J Crohn’s Colitis.2016;10(7):805-11.
5. Gentile NM et al. Clin Gastroenterol Hepatol. 2014;12(5):838-42.
6. Tong J et al. Am J Gastroenterol. 2015;110:265-76.
7. Olesen M et al. Gut. 2004;53(3):346-50.
8. Bergman D et al. Aliment Pharmacol Ther. 2019;49(11):1395-400.
9. Guagnozzi D et al. Dig Liver Dis. 2012;44(5):384-8.
10. Münch A et al. J Crohns Colitis. 2012;6(9):932-45.
11. Macaigne G et al. Am J Gastroenterol. 2014; 09(9):1461-70.
12. Verhaegh BP et al. Aliment Pharmacol Ther. 2016;43(9):1004-13.
13. Stewart M et al. Aliment Pharmacol Ther. 2011;33(12):1340-9.
14. Green PHR et al. Clin Gastroenterol Hepatol. 2009;7(11):1210-6.
15. Masclee GM et al. Am J Gastroenterol. 2015;110:749-59.
16. Zylberberg H et al. Ailment Pharmacol Ther. 2021 Jun;53(11)1209-15.
17. Jaruvongvanich V et al. Inflamm Bowel Dis. 2019;25(4):672-8.
18. Fernández-Bañares F et al. Inflamm Bowel Dis. 2013; 19(7):1470-6.
19. Yen EF et al. Inflamm Bowel Dis. 2012;18(10):1835-41.
20. Barmeyer C et al. J Gastroenterol. 2017;52(10):1090-100.
21. Morgan DM et al. Clin Gastroenterol Hepatol. 2020;18(4):984-6.
22. Larsson JK et al. Eur J Clin Nutr. 2016;70:1309-17.
23. Madisch A et al.. Inflamm Bowel Dis. 2011;17(11):2295-8.
24. Stahl E et al. Gastroenterology. 2020;159(2):549-61.
25. Sikander A et al. Dig Dis Sci. 2015; 60:887-94.
26. Abdo AA et al. Can J Gastroenterol. 2001;15(5):341-3.
27. Fernandez-Bañares F et al. Dig Dis Sci.2001;46(10):2231-8.
28. Lyutakov I et al. Eur J Gastroenterol Hepatol. 2021;1;33(3):380-7.
29. Hjortswang H et al. Dig Liver Dis. 2011 Feb;43(2):102-9.
30. Cotter TG= et al. Gut. 2018;67(3):441-6.
31. Von Arnim U et al. Clin Exp Gastroenterol. 2016;9:97-103.
32. Langner C et al. Histopathology. 2015;66:613-26.
33. ASGE Standards of Practice Committee and Sharaf RN et al. Gastrointest Endosc. 2013;78:216-24.
34. Macaigne G et al. Clin Res Hepatol Gastroenterol. 2017;41(3):333-40.
35. Bjørnbak C et al. Aliment Pharmacol Ther. 2011;34(10):1225-34.
36. Pardi DS et al. Gastroenterology. 2016;150(1):247-74.
37. Masannat Y and Nazer E. West Virginia Med J. 2013;109(3):32-4.
38. Nguyen GC et al. Gastroenterology. 2016; 150(1):242-6.
39. Sebastian S et al. Eur J Gastroenterol Hepatol. 2019 Aug;31(8):919-27.
40. Miehlke S et al. Gastroenterology. 2014;146(5):1222-30.
41. Gentile NM et al. Am J Gastroenterol. 2013;108:256-9.
42. Münch A et al. Gut. 2016; 65(1):47-56.
43. Cotter TG et al. Aliment Pharmacol Ther. 2017; 46(2):169-74.
44. Esteve M et al. J Crohns Colitis. 2011;5(6):612-8.
45. Cottreau J et al. Clin J Gastroenterol. 2016;9:140-4.
46. Kamboj AK et al. Program No. P1876. ACG 2018 Annual Scientific Meeting Abstracts. Philadelphia, Pennsylvania: American College of Gastroenterology.
47. Yen EF et al. Dig Dis Sci. 2012;57:161-9.
Microscopic colitis is an inflammatory disease of the colon and a frequent cause of chronic or recurrent watery diarrhea, particularly in older persons. MC consists of two subtypes, collagenous colitis (CC) and lymphocytic colitis (LC). While the primary symptom is diarrhea, other signs and symptoms such as abdominal pain, weight loss, and dehydration or electrolyte abnormalities may also be present depending on disease severity.1 In MC, the colonic mucosa usually appears normal on colonoscopy, and the diagnosis is made by histologic findings of intraepithelial lymphocytosis with (CC) or without (LC) a prominent subepithelial collagen band. The management approaches to CC and LC are similar and should be directed based on the severity of symptoms.2 We review the epidemiology, risk factors, pathophysiology, diagnosis, and clinical management for this condition, as well as novel therapeutic approaches.
Epidemiology
Although the incidence of MC increased in the late twentieth century, more recently, it has stabilized with an estimated incidence varying from 1 to 25 per 100,000 person-years.3-5 A recent meta-analysis revealed a pooled incidence of 4.85 per 100,000 persons for LC and 4.14 per 100,000 persons for CC.6 Proposed explanations for the rising incidence in the late twentieth century include improved clinical awareness of the disease, possible increased use of drugs associated with MC, and increased performance of diagnostic colonoscopies for chronic diarrhea. Since MC is now well-recognized, the recent plateau in incidence rates may reflect decreased detection bias.
The prevalence of MC ranges from 10%-20% in patients undergoing colonoscopy for chronic watery diarrhea.6,7 The prevalence of LC is approximately 63.1 cases per 100,000 person-years and, for CC, is 49.2 cases per 100,000 person-years.6-8 Recent studies have demonstrated increasing prevalence of MC likely resulting from an aging population.9,10
Risk stratification
Female gender, increasing age, concomitant autoimmune disease, and the use of certain drugs, including NSAIDs, proton pump inhibitors (PPIs), statins, and selective serotonin reuptake inhibitors (SSRIs), have been associated with an increased risk of MC.11,12 Autoimmune disorders, including celiac disease (CD), rheumatoid arthritis, hypothyroidism, and hyperthyroidism, are more common in patients with MC. The association with CD, in particular, is clinically important, as CD is associated with a 50-70 times greater risk of MC, and 2%-9% of patients with MC have CD.13,14
Several medications have been associated with MC. In a British multicenter prospective study, MC was associated with the use of NSAIDs, PPIs, and SSRIs;15 however, recent studies have questioned the association of MC with some of these medications, which might worsen diarrhea but not actually cause MC.16
An additional risk factor for MC is smoking. A recent meta-analysis demonstrated that current and former smokers had an increased risk of MC (odds ratio, 2.99; 95% confidence interval, 2.15-4.15 and OR, 1.63; 95% CI, 1.37-1.94, respectively), compared with nonsmokers.17 Smokers develop MC at a younger age, and smoking is associated with increased disease severity and decreased likelihood of attaining remission.18,19
Pathogenesis
The pathogenesis of MC remains largely unknown, although there are several hypotheses. The leading proposed mechanisms include reaction to luminal antigens, dysregulated collagen metabolism, genetic predisposition, autoimmunity, and bile acid malabsorption.
MC may be caused by abnormal epithelial barrier function, leading to increased permeability and reaction to luminal antigens, including dietary antigens, certain drugs, and bacterial products, 20,21 which themselves lead to the immune dysregulation and intestinal inflammation seen in MC. This mechanism may explain the association of several drugs with MC. Histological changes resembling LC are reported in patients with CD who consume gluten; however, large population-based studies have not found specific dietary associations with the development of MC.22
Another potential mechanism of MC is dysregulated collagen deposition. Collagen accumulation in the subepithelial layer in CC may result from increased levels of fibroblast growth factor, transforming growth factor–beta and vascular endothelial growth factor.23 Nonetheless, studies have not found an association between the severity of diarrhea in patients with CC and the thickness of the subepithelial collagen band.
Thirdly, autoimmunity and genetic predisposition have been postulated in the pathogenesis of MC. As previously discussed, MC is associated with several autoimmune diseases and predominantly occurs in women, a distinctive feature of autoimmune disorders. Several studies have demonstrated an association between MC and HLA-DQ2 and -DQ3 haplotypes,24 as well as potential polymorphisms in the serotonin transporter gene promoter.25 It is important to note, however, that only a few familial cases of MC have been reported to date.26
Lastly, bile acid malabsorption may play a role in the etiology of MC. Histologic findings of inflammation, along with villous atrophy and collagen deposition, have been reported in the ileum of patients with MC;27,28 however, because patients with MC without bile acid malabsorption may also respond to bile acid binders such as cholestyramine, these findings unlikely to be the sole mechanism explaining the development of the disease.
Despite the different proposed mechanisms for the pathogenesis of MC, no definite conclusions can be drawn because of the limited size of these studies and their often conflicting results.

Clinical features
Clinicians should suspect MC in patients with chronic or recurrent watery diarrhea, particularly in older persons. Other risk factors include female gender, use of certain culprit medications, smoking, and presence of other autoimmune diseases. The clinical manifestations of MC subtypes LC and CC are similar with no significant clinical differences.1,2 In addition to diarrhea, patients with MC may have abdominal pain, fatigue, and dehydration or electrolyte abnormalities depending on disease severity. Patients may also present with fecal urgency, incontinence, and nocturnal stools. Quality of life is often reduced in these patients, predominantly in those with severe or refractory symptoms.29,30 The natural course of MC is highly variable, with some patients achieving spontaneous resolution after one episode and others developing chronic symptoms.
Diagnosis
The differential diagnosis of chronic watery diarrhea is broad and includes malabsorption/maldigestion, inflammatory bowel disease (IBD), irritable bowel syndrome, and medication side effects. In addition, although gastrointestinal infections typically cause acute or subacute diarrhea, some can present with chronic diarrhea. Malabsorption/maldigestion may occur because of CD, lactose intolerance, and pancreatic insufficiency, among other conditions. A thorough history, regarding recent antibiotic and medication use, travel, and immunosuppression, should be obtained in patients with chronic diarrhea. Additionally, laboratory and endoscopic evaluation with random biopsies of the colon can further help differentiate these diseases from MC. A few studies suggest fecal calprotectin may be used to differentiate MC from other noninflammatory conditions such as irritable bowel syndrome, as well as to monitor disease activity. This test is not expected to distinguish MC from other inflammatory causes of diarrhea, such as IBD, and therefore, its role in clinical practice is uncertain.31
The diagnosis of MC is made by biopsy of the colonic mucosa demonstrating characteristic pathologic features.32 Unlike in diseases such as Crohn’s disease or ulcerative colitis, the colon usually appears normal in MC, although mild nonspecific changes, such as erythema or edema, may be visualized. There is no consensus on the ideal location to obtain biopsies for MC or whether biopsies from both the left and the right colon are required.2,33 The procedure of choice for the diagnosis of MC is colonoscopy with random biopsies taken throughout the colon. More limited evaluation by flexible sigmoidoscopy with biopsies may miss cases of MC as inflammation and collagen thickening are not necessarily uniform throughout the colon; however, in a patient that has undergone a recent colonoscopy for colon cancer screening without colon biopsies, a flexible sigmoidoscopy may be a reasonable next test for evaluation of MC, provided biopsies are obtained above the rectosigmoid colon.34
The MC subtypes are differentiated based on histology. The hallmark of LC is less than 20 intraepithelial lymphocytes per 100 surface epithelial cells (normal, less than 5) (Figure 1A). CC is characterized by a thickened subepithelial collagen band greater than 7-10 micrometers (normal, less than 5) (Figure 1B). For a subgroup of patients with milder abnormalities that do not meet these histological criteria, the terms “microscopic colitis, not otherwise specified” or “microscopic colitis, incomplete” may be used.35 These patients often respond to standard treatments for MC. There is an additional subset of patients with biopsy demonstrating features of both CC and LC simultaneously, as well as patients transitioning from one MC subtype to another over time.32,35
Management approach
The first step in management of patients with MC includes stopping culprit medications if there is a temporal relationship between the initiation of the medication and the onset of diarrhea, as well as encouraging smoking cessation. These steps alone, however, are unlikely to achieve clinical remission in most patients. A stepwise pharmacological approach is used in the management of MC based on disease severity (Figure 2). For patients with mild symptoms, antidiarrheal medications, such as loperamide, may be helpful.36 Long-term use of loperamide at therapeutic doses no greater than 16 mg daily appears to be safe if required to maintain symptom response. For those with persistent symptoms despite antidiarrheal medications, bismuth subsalicylate at three 262 mg tablets three times daily for 6-8 weeks can be considered. Long-term use of bismuth subsalicylate is not advised, especially at this dose, because of possible neurotoxicity.37

For patients refractory to the above treatments or those with moderate-to-severe symptoms, an 8-week course of budesonide at 9 mg daily is the first-line treatment.38 The dose was tapered before discontinuation in some studies but not in others. Both strategies appear effective. A recent meta-analysis of nine randomized trials demonstrated pooled ORs of 7.34 (95% CI, 4.08-13.19) and 8.35 (95% CI, 4.14-16.85) for response to budesonide induction and maintenance, respectively.39
Cholestyramine is another medication considered in the management of MC and warrants further investigation. To date, no randomized clinical trials have been conducted to evaluate bile acid sequestrants in MC, but they should be considered before placing patients on immunosuppressive medications. Some providers use mesalamine in this setting, although mesalamine is inferior to budesonide in the induction of clinical remission in MC.40
Despite high rates of response to budesonide, relapse after discontinuation is frequent (60%-80%), and time to relapse is variable41,42 The American Gastroenterological Association recommends budesonide for maintenance of remission in patients with recurrence following discontinuation of induction therapy. The lowest effective dose that maintains resolution of symptoms should be prescribed, ideally at 6 mg daily or lower.38 Although budesonide has a greater first-pass metabolism, compared with other glucocorticoids, patients should be monitored for possible side effects including hypertension, diabetes, and osteoporosis, as well as ophthalmologic disease, including cataracts and glaucoma.
For those who are intolerant to budesonide or have refractory symptoms, concomitant disorders such as CD that may be contributing to symptoms must be excluded. Immunosuppressive medications – such as thiopurines and biologic agents, including tumor necrosis factor–alpha inhibitors or vedolizumab – may be considered in refractory cases.43,44 Of note, there are limited studies evaluating the use of these medications for MC. Lastly, surgeries including ileostomy with or without colectomy have been performed in the most severe cases for resistant disease that has failed numerous pharmacological therapies.45
Patients should be counseled that, while symptoms from MC can be quite bothersome and disabling, there appears to be a normal life expectancy and no association between MC and colon cancer, unlike with other inflammatory conditions of the colon such as IBD.46,47
Conclusion and future outlook
As a common cause of chronic watery diarrhea, MC will be commonly encountered in primary care and gastroenterology practices. The diagnosis should be suspected in patients presenting with chronic or recurrent watery diarrhea, especially with female gender, autoimmune disease, and increasing age. The management of MC requires an algorithmic approach directed by symptom severity, with a subgroup of patients requiring maintenance therapy for relapsing symptoms. The care of patients with MC will continue to evolve in the future. Further work is needed to explore long-term safety outcomes with budesonide and the role of immunomodulators and newer biologic agents for patients with complex, refractory disease.
Dr. Tome is with the department of internal medicine at the Mayo Clinic, Rochester, Minn. Dr. Kamboj, and Dr. Pardi are with the division of gastroenterology and hepatology at the Mayo Clinic. Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta and Otsuka. The other authors have no conflicts of interest to report.
References
1. Nyhlin N et al. Aliment Pharmacol Ther. 2014;39:963-72.
2. Miehlke S et al. United European Gastroenterol J. 2020;20-8.
3. Pardi DS et al. Gut. 2007;56:504-8.
4. Fernández-Bañares F et al. J Crohn’s Colitis.2016;10(7):805-11.
5. Gentile NM et al. Clin Gastroenterol Hepatol. 2014;12(5):838-42.
6. Tong J et al. Am J Gastroenterol. 2015;110:265-76.
7. Olesen M et al. Gut. 2004;53(3):346-50.
8. Bergman D et al. Aliment Pharmacol Ther. 2019;49(11):1395-400.
9. Guagnozzi D et al. Dig Liver Dis. 2012;44(5):384-8.
10. Münch A et al. J Crohns Colitis. 2012;6(9):932-45.
11. Macaigne G et al. Am J Gastroenterol. 2014; 09(9):1461-70.
12. Verhaegh BP et al. Aliment Pharmacol Ther. 2016;43(9):1004-13.
13. Stewart M et al. Aliment Pharmacol Ther. 2011;33(12):1340-9.
14. Green PHR et al. Clin Gastroenterol Hepatol. 2009;7(11):1210-6.
15. Masclee GM et al. Am J Gastroenterol. 2015;110:749-59.
16. Zylberberg H et al. Ailment Pharmacol Ther. 2021 Jun;53(11)1209-15.
17. Jaruvongvanich V et al. Inflamm Bowel Dis. 2019;25(4):672-8.
18. Fernández-Bañares F et al. Inflamm Bowel Dis. 2013; 19(7):1470-6.
19. Yen EF et al. Inflamm Bowel Dis. 2012;18(10):1835-41.
20. Barmeyer C et al. J Gastroenterol. 2017;52(10):1090-100.
21. Morgan DM et al. Clin Gastroenterol Hepatol. 2020;18(4):984-6.
22. Larsson JK et al. Eur J Clin Nutr. 2016;70:1309-17.
23. Madisch A et al.. Inflamm Bowel Dis. 2011;17(11):2295-8.
24. Stahl E et al. Gastroenterology. 2020;159(2):549-61.
25. Sikander A et al. Dig Dis Sci. 2015; 60:887-94.
26. Abdo AA et al. Can J Gastroenterol. 2001;15(5):341-3.
27. Fernandez-Bañares F et al. Dig Dis Sci.2001;46(10):2231-8.
28. Lyutakov I et al. Eur J Gastroenterol Hepatol. 2021;1;33(3):380-7.
29. Hjortswang H et al. Dig Liver Dis. 2011 Feb;43(2):102-9.
30. Cotter TG= et al. Gut. 2018;67(3):441-6.
31. Von Arnim U et al. Clin Exp Gastroenterol. 2016;9:97-103.
32. Langner C et al. Histopathology. 2015;66:613-26.
33. ASGE Standards of Practice Committee and Sharaf RN et al. Gastrointest Endosc. 2013;78:216-24.
34. Macaigne G et al. Clin Res Hepatol Gastroenterol. 2017;41(3):333-40.
35. Bjørnbak C et al. Aliment Pharmacol Ther. 2011;34(10):1225-34.
36. Pardi DS et al. Gastroenterology. 2016;150(1):247-74.
37. Masannat Y and Nazer E. West Virginia Med J. 2013;109(3):32-4.
38. Nguyen GC et al. Gastroenterology. 2016; 150(1):242-6.
39. Sebastian S et al. Eur J Gastroenterol Hepatol. 2019 Aug;31(8):919-27.
40. Miehlke S et al. Gastroenterology. 2014;146(5):1222-30.
41. Gentile NM et al. Am J Gastroenterol. 2013;108:256-9.
42. Münch A et al. Gut. 2016; 65(1):47-56.
43. Cotter TG et al. Aliment Pharmacol Ther. 2017; 46(2):169-74.
44. Esteve M et al. J Crohns Colitis. 2011;5(6):612-8.
45. Cottreau J et al. Clin J Gastroenterol. 2016;9:140-4.
46. Kamboj AK et al. Program No. P1876. ACG 2018 Annual Scientific Meeting Abstracts. Philadelphia, Pennsylvania: American College of Gastroenterology.
47. Yen EF et al. Dig Dis Sci. 2012;57:161-9.
Microscopic colitis is an inflammatory disease of the colon and a frequent cause of chronic or recurrent watery diarrhea, particularly in older persons. MC consists of two subtypes, collagenous colitis (CC) and lymphocytic colitis (LC). While the primary symptom is diarrhea, other signs and symptoms such as abdominal pain, weight loss, and dehydration or electrolyte abnormalities may also be present depending on disease severity.1 In MC, the colonic mucosa usually appears normal on colonoscopy, and the diagnosis is made by histologic findings of intraepithelial lymphocytosis with (CC) or without (LC) a prominent subepithelial collagen band. The management approaches to CC and LC are similar and should be directed based on the severity of symptoms.2 We review the epidemiology, risk factors, pathophysiology, diagnosis, and clinical management for this condition, as well as novel therapeutic approaches.
Epidemiology
Although the incidence of MC increased in the late twentieth century, more recently, it has stabilized with an estimated incidence varying from 1 to 25 per 100,000 person-years.3-5 A recent meta-analysis revealed a pooled incidence of 4.85 per 100,000 persons for LC and 4.14 per 100,000 persons for CC.6 Proposed explanations for the rising incidence in the late twentieth century include improved clinical awareness of the disease, possible increased use of drugs associated with MC, and increased performance of diagnostic colonoscopies for chronic diarrhea. Since MC is now well-recognized, the recent plateau in incidence rates may reflect decreased detection bias.
The prevalence of MC ranges from 10%-20% in patients undergoing colonoscopy for chronic watery diarrhea.6,7 The prevalence of LC is approximately 63.1 cases per 100,000 person-years and, for CC, is 49.2 cases per 100,000 person-years.6-8 Recent studies have demonstrated increasing prevalence of MC likely resulting from an aging population.9,10
Risk stratification
Female gender, increasing age, concomitant autoimmune disease, and the use of certain drugs, including NSAIDs, proton pump inhibitors (PPIs), statins, and selective serotonin reuptake inhibitors (SSRIs), have been associated with an increased risk of MC.11,12 Autoimmune disorders, including celiac disease (CD), rheumatoid arthritis, hypothyroidism, and hyperthyroidism, are more common in patients with MC. The association with CD, in particular, is clinically important, as CD is associated with a 50-70 times greater risk of MC, and 2%-9% of patients with MC have CD.13,14
Several medications have been associated with MC. In a British multicenter prospective study, MC was associated with the use of NSAIDs, PPIs, and SSRIs;15 however, recent studies have questioned the association of MC with some of these medications, which might worsen diarrhea but not actually cause MC.16
An additional risk factor for MC is smoking. A recent meta-analysis demonstrated that current and former smokers had an increased risk of MC (odds ratio, 2.99; 95% confidence interval, 2.15-4.15 and OR, 1.63; 95% CI, 1.37-1.94, respectively), compared with nonsmokers.17 Smokers develop MC at a younger age, and smoking is associated with increased disease severity and decreased likelihood of attaining remission.18,19
Pathogenesis
The pathogenesis of MC remains largely unknown, although there are several hypotheses. The leading proposed mechanisms include reaction to luminal antigens, dysregulated collagen metabolism, genetic predisposition, autoimmunity, and bile acid malabsorption.
MC may be caused by abnormal epithelial barrier function, leading to increased permeability and reaction to luminal antigens, including dietary antigens, certain drugs, and bacterial products, 20,21 which themselves lead to the immune dysregulation and intestinal inflammation seen in MC. This mechanism may explain the association of several drugs with MC. Histological changes resembling LC are reported in patients with CD who consume gluten; however, large population-based studies have not found specific dietary associations with the development of MC.22
Another potential mechanism of MC is dysregulated collagen deposition. Collagen accumulation in the subepithelial layer in CC may result from increased levels of fibroblast growth factor, transforming growth factor–beta and vascular endothelial growth factor.23 Nonetheless, studies have not found an association between the severity of diarrhea in patients with CC and the thickness of the subepithelial collagen band.
Thirdly, autoimmunity and genetic predisposition have been postulated in the pathogenesis of MC. As previously discussed, MC is associated with several autoimmune diseases and predominantly occurs in women, a distinctive feature of autoimmune disorders. Several studies have demonstrated an association between MC and HLA-DQ2 and -DQ3 haplotypes,24 as well as potential polymorphisms in the serotonin transporter gene promoter.25 It is important to note, however, that only a few familial cases of MC have been reported to date.26
Lastly, bile acid malabsorption may play a role in the etiology of MC. Histologic findings of inflammation, along with villous atrophy and collagen deposition, have been reported in the ileum of patients with MC;27,28 however, because patients with MC without bile acid malabsorption may also respond to bile acid binders such as cholestyramine, these findings unlikely to be the sole mechanism explaining the development of the disease.
Despite the different proposed mechanisms for the pathogenesis of MC, no definite conclusions can be drawn because of the limited size of these studies and their often conflicting results.

Clinical features
Clinicians should suspect MC in patients with chronic or recurrent watery diarrhea, particularly in older persons. Other risk factors include female gender, use of certain culprit medications, smoking, and presence of other autoimmune diseases. The clinical manifestations of MC subtypes LC and CC are similar with no significant clinical differences.1,2 In addition to diarrhea, patients with MC may have abdominal pain, fatigue, and dehydration or electrolyte abnormalities depending on disease severity. Patients may also present with fecal urgency, incontinence, and nocturnal stools. Quality of life is often reduced in these patients, predominantly in those with severe or refractory symptoms.29,30 The natural course of MC is highly variable, with some patients achieving spontaneous resolution after one episode and others developing chronic symptoms.
Diagnosis
The differential diagnosis of chronic watery diarrhea is broad and includes malabsorption/maldigestion, inflammatory bowel disease (IBD), irritable bowel syndrome, and medication side effects. In addition, although gastrointestinal infections typically cause acute or subacute diarrhea, some can present with chronic diarrhea. Malabsorption/maldigestion may occur because of CD, lactose intolerance, and pancreatic insufficiency, among other conditions. A thorough history, regarding recent antibiotic and medication use, travel, and immunosuppression, should be obtained in patients with chronic diarrhea. Additionally, laboratory and endoscopic evaluation with random biopsies of the colon can further help differentiate these diseases from MC. A few studies suggest fecal calprotectin may be used to differentiate MC from other noninflammatory conditions such as irritable bowel syndrome, as well as to monitor disease activity. This test is not expected to distinguish MC from other inflammatory causes of diarrhea, such as IBD, and therefore, its role in clinical practice is uncertain.31
The diagnosis of MC is made by biopsy of the colonic mucosa demonstrating characteristic pathologic features.32 Unlike in diseases such as Crohn’s disease or ulcerative colitis, the colon usually appears normal in MC, although mild nonspecific changes, such as erythema or edema, may be visualized. There is no consensus on the ideal location to obtain biopsies for MC or whether biopsies from both the left and the right colon are required.2,33 The procedure of choice for the diagnosis of MC is colonoscopy with random biopsies taken throughout the colon. More limited evaluation by flexible sigmoidoscopy with biopsies may miss cases of MC as inflammation and collagen thickening are not necessarily uniform throughout the colon; however, in a patient that has undergone a recent colonoscopy for colon cancer screening without colon biopsies, a flexible sigmoidoscopy may be a reasonable next test for evaluation of MC, provided biopsies are obtained above the rectosigmoid colon.34
The MC subtypes are differentiated based on histology. The hallmark of LC is less than 20 intraepithelial lymphocytes per 100 surface epithelial cells (normal, less than 5) (Figure 1A). CC is characterized by a thickened subepithelial collagen band greater than 7-10 micrometers (normal, less than 5) (Figure 1B). For a subgroup of patients with milder abnormalities that do not meet these histological criteria, the terms “microscopic colitis, not otherwise specified” or “microscopic colitis, incomplete” may be used.35 These patients often respond to standard treatments for MC. There is an additional subset of patients with biopsy demonstrating features of both CC and LC simultaneously, as well as patients transitioning from one MC subtype to another over time.32,35
Management approach
The first step in management of patients with MC includes stopping culprit medications if there is a temporal relationship between the initiation of the medication and the onset of diarrhea, as well as encouraging smoking cessation. These steps alone, however, are unlikely to achieve clinical remission in most patients. A stepwise pharmacological approach is used in the management of MC based on disease severity (Figure 2). For patients with mild symptoms, antidiarrheal medications, such as loperamide, may be helpful.36 Long-term use of loperamide at therapeutic doses no greater than 16 mg daily appears to be safe if required to maintain symptom response. For those with persistent symptoms despite antidiarrheal medications, bismuth subsalicylate at three 262 mg tablets three times daily for 6-8 weeks can be considered. Long-term use of bismuth subsalicylate is not advised, especially at this dose, because of possible neurotoxicity.37

For patients refractory to the above treatments or those with moderate-to-severe symptoms, an 8-week course of budesonide at 9 mg daily is the first-line treatment.38 The dose was tapered before discontinuation in some studies but not in others. Both strategies appear effective. A recent meta-analysis of nine randomized trials demonstrated pooled ORs of 7.34 (95% CI, 4.08-13.19) and 8.35 (95% CI, 4.14-16.85) for response to budesonide induction and maintenance, respectively.39
Cholestyramine is another medication considered in the management of MC and warrants further investigation. To date, no randomized clinical trials have been conducted to evaluate bile acid sequestrants in MC, but they should be considered before placing patients on immunosuppressive medications. Some providers use mesalamine in this setting, although mesalamine is inferior to budesonide in the induction of clinical remission in MC.40
Despite high rates of response to budesonide, relapse after discontinuation is frequent (60%-80%), and time to relapse is variable41,42 The American Gastroenterological Association recommends budesonide for maintenance of remission in patients with recurrence following discontinuation of induction therapy. The lowest effective dose that maintains resolution of symptoms should be prescribed, ideally at 6 mg daily or lower.38 Although budesonide has a greater first-pass metabolism, compared with other glucocorticoids, patients should be monitored for possible side effects including hypertension, diabetes, and osteoporosis, as well as ophthalmologic disease, including cataracts and glaucoma.
For those who are intolerant to budesonide or have refractory symptoms, concomitant disorders such as CD that may be contributing to symptoms must be excluded. Immunosuppressive medications – such as thiopurines and biologic agents, including tumor necrosis factor–alpha inhibitors or vedolizumab – may be considered in refractory cases.43,44 Of note, there are limited studies evaluating the use of these medications for MC. Lastly, surgeries including ileostomy with or without colectomy have been performed in the most severe cases for resistant disease that has failed numerous pharmacological therapies.45
Patients should be counseled that, while symptoms from MC can be quite bothersome and disabling, there appears to be a normal life expectancy and no association between MC and colon cancer, unlike with other inflammatory conditions of the colon such as IBD.46,47
Conclusion and future outlook
As a common cause of chronic watery diarrhea, MC will be commonly encountered in primary care and gastroenterology practices. The diagnosis should be suspected in patients presenting with chronic or recurrent watery diarrhea, especially with female gender, autoimmune disease, and increasing age. The management of MC requires an algorithmic approach directed by symptom severity, with a subgroup of patients requiring maintenance therapy for relapsing symptoms. The care of patients with MC will continue to evolve in the future. Further work is needed to explore long-term safety outcomes with budesonide and the role of immunomodulators and newer biologic agents for patients with complex, refractory disease.
Dr. Tome is with the department of internal medicine at the Mayo Clinic, Rochester, Minn. Dr. Kamboj, and Dr. Pardi are with the division of gastroenterology and hepatology at the Mayo Clinic. Dr. Pardi has grant funding from Pfizer, Vedanta, Seres, Finch, Applied Molecular Transport, and Takeda and has consulted for Vedanta and Otsuka. The other authors have no conflicts of interest to report.
References
1. Nyhlin N et al. Aliment Pharmacol Ther. 2014;39:963-72.
2. Miehlke S et al. United European Gastroenterol J. 2020;20-8.
3. Pardi DS et al. Gut. 2007;56:504-8.
4. Fernández-Bañares F et al. J Crohn’s Colitis.2016;10(7):805-11.
5. Gentile NM et al. Clin Gastroenterol Hepatol. 2014;12(5):838-42.
6. Tong J et al. Am J Gastroenterol. 2015;110:265-76.
7. Olesen M et al. Gut. 2004;53(3):346-50.
8. Bergman D et al. Aliment Pharmacol Ther. 2019;49(11):1395-400.
9. Guagnozzi D et al. Dig Liver Dis. 2012;44(5):384-8.
10. Münch A et al. J Crohns Colitis. 2012;6(9):932-45.
11. Macaigne G et al. Am J Gastroenterol. 2014; 09(9):1461-70.
12. Verhaegh BP et al. Aliment Pharmacol Ther. 2016;43(9):1004-13.
13. Stewart M et al. Aliment Pharmacol Ther. 2011;33(12):1340-9.
14. Green PHR et al. Clin Gastroenterol Hepatol. 2009;7(11):1210-6.
15. Masclee GM et al. Am J Gastroenterol. 2015;110:749-59.
16. Zylberberg H et al. Ailment Pharmacol Ther. 2021 Jun;53(11)1209-15.
17. Jaruvongvanich V et al. Inflamm Bowel Dis. 2019;25(4):672-8.
18. Fernández-Bañares F et al. Inflamm Bowel Dis. 2013; 19(7):1470-6.
19. Yen EF et al. Inflamm Bowel Dis. 2012;18(10):1835-41.
20. Barmeyer C et al. J Gastroenterol. 2017;52(10):1090-100.
21. Morgan DM et al. Clin Gastroenterol Hepatol. 2020;18(4):984-6.
22. Larsson JK et al. Eur J Clin Nutr. 2016;70:1309-17.
23. Madisch A et al.. Inflamm Bowel Dis. 2011;17(11):2295-8.
24. Stahl E et al. Gastroenterology. 2020;159(2):549-61.
25. Sikander A et al. Dig Dis Sci. 2015; 60:887-94.
26. Abdo AA et al. Can J Gastroenterol. 2001;15(5):341-3.
27. Fernandez-Bañares F et al. Dig Dis Sci.2001;46(10):2231-8.
28. Lyutakov I et al. Eur J Gastroenterol Hepatol. 2021;1;33(3):380-7.
29. Hjortswang H et al. Dig Liver Dis. 2011 Feb;43(2):102-9.
30. Cotter TG= et al. Gut. 2018;67(3):441-6.
31. Von Arnim U et al. Clin Exp Gastroenterol. 2016;9:97-103.
32. Langner C et al. Histopathology. 2015;66:613-26.
33. ASGE Standards of Practice Committee and Sharaf RN et al. Gastrointest Endosc. 2013;78:216-24.
34. Macaigne G et al. Clin Res Hepatol Gastroenterol. 2017;41(3):333-40.
35. Bjørnbak C et al. Aliment Pharmacol Ther. 2011;34(10):1225-34.
36. Pardi DS et al. Gastroenterology. 2016;150(1):247-74.
37. Masannat Y and Nazer E. West Virginia Med J. 2013;109(3):32-4.
38. Nguyen GC et al. Gastroenterology. 2016; 150(1):242-6.
39. Sebastian S et al. Eur J Gastroenterol Hepatol. 2019 Aug;31(8):919-27.
40. Miehlke S et al. Gastroenterology. 2014;146(5):1222-30.
41. Gentile NM et al. Am J Gastroenterol. 2013;108:256-9.
42. Münch A et al. Gut. 2016; 65(1):47-56.
43. Cotter TG et al. Aliment Pharmacol Ther. 2017; 46(2):169-74.
44. Esteve M et al. J Crohns Colitis. 2011;5(6):612-8.
45. Cottreau J et al. Clin J Gastroenterol. 2016;9:140-4.
46. Kamboj AK et al. Program No. P1876. ACG 2018 Annual Scientific Meeting Abstracts. Philadelphia, Pennsylvania: American College of Gastroenterology.
47. Yen EF et al. Dig Dis Sci. 2012;57:161-9.
Study eyes impact of isotretinoin on triglycerides, other lab measures
.
“Isotretinoin is a very effective treatment for severe acne,” Varsha Parthasarathy said at the annual meeting of the Society for Pediatric Dermatology. “However, initiating this medication requires a complex process of laboratory testing,” which includes human chorionic gonadotropin pregnancy testing, because isotretinoin is a teratogen, as well as lipid labs and liver function tests, she noted. “Importantly, triglycerides are measured due to an association in adults between isotretinoin and hypertriglyceridemia-associated pancreatitis. However, these findings in children are limited to case reports, as are findings of retinoid-induced hepatotoxicity.”
To identify the role of isotretinoin on changes in lipids, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and to determine the impact on treatment course, Ms. Parthasarathy, a 4-year medical student at George Washington University, Washington, and colleagues retrospectively reviewed the charts of 130 patients aged 12-21 years who were cared for at Children’s National Hospital between January 2012 and October 2020. Nearly two-thirds (65%) were male, their average age was 16 years, and the mean time to obtain follow-up labs after starting isotretinoin was 3.25 months.
Between baseline and follow-up, the researchers observed increases in total cholesterol, triglycerides, and LDL (P less than .001 for all associations) and a decrease in HDL (P = .001), but there were no significant changes in AST or ALT levels. These findings were consistent with prior studies in adults examining the utility of these laboratory tests, most notably a 2016 study by Timothy J. Hansen, MD, and colleagues.
Among the 13 patients with elevated triglycerides at baseline, 9 (69%) were overweight or obese. Of the 20 patients with elevated triglycerides at follow-up, 11 patients (55%) were obese. At follow-up, 11 patients had levels of 200-500 mg/dL (grade I elevation), and 2 patients had levels of 501-1,000 mg/dL (grade II elevation). Isotretinoin was stopped in the latter two patients, who also had obesity as a risk factor for their hypertriglyceridemia.
“None of these patients had clinical sequelae from their hypertriglyceridemia, such as pancreatitis at baseline or follow-up,” Ms. Parthasarathy said. “However, since pancreatitis would be expected to be exceedingly rare, the sample size may be limited in identifying this adverse effect.”
She noted that while isotretinoin might cause a significant increase in lipid levels, the mean levels remained within normal limits at both baseline and follow-up. “Of the patients with elevated triglycerides at baseline and follow-up, obesity may have been a potential risk factor,” she said. “This could suggest a possible strategy for reduced testing in nonobese isotretinoin patients, which can be further explored in larger study populations.”
In addition, “there was a lack of significant change in AST and ALT in this study and adult studies, as well as minimal evidence for pediatric retinoid-induced hepatotoxicity, which raises the question of the necessity of baseline and follow-up comprehensive metabolic panel testing,” Ms. Parthasarathy added. “Clinicians must weigh the laboratory values with the costs of laboratory testing, including opportunity costs such as time, monetary costs, and the discomfort of testing for pediatric patients.”
The study’s senior author was A. Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital, Washington. The researchers reported having no relevant financial disclosures.
.
“Isotretinoin is a very effective treatment for severe acne,” Varsha Parthasarathy said at the annual meeting of the Society for Pediatric Dermatology. “However, initiating this medication requires a complex process of laboratory testing,” which includes human chorionic gonadotropin pregnancy testing, because isotretinoin is a teratogen, as well as lipid labs and liver function tests, she noted. “Importantly, triglycerides are measured due to an association in adults between isotretinoin and hypertriglyceridemia-associated pancreatitis. However, these findings in children are limited to case reports, as are findings of retinoid-induced hepatotoxicity.”
To identify the role of isotretinoin on changes in lipids, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and to determine the impact on treatment course, Ms. Parthasarathy, a 4-year medical student at George Washington University, Washington, and colleagues retrospectively reviewed the charts of 130 patients aged 12-21 years who were cared for at Children’s National Hospital between January 2012 and October 2020. Nearly two-thirds (65%) were male, their average age was 16 years, and the mean time to obtain follow-up labs after starting isotretinoin was 3.25 months.
Between baseline and follow-up, the researchers observed increases in total cholesterol, triglycerides, and LDL (P less than .001 for all associations) and a decrease in HDL (P = .001), but there were no significant changes in AST or ALT levels. These findings were consistent with prior studies in adults examining the utility of these laboratory tests, most notably a 2016 study by Timothy J. Hansen, MD, and colleagues.
Among the 13 patients with elevated triglycerides at baseline, 9 (69%) were overweight or obese. Of the 20 patients with elevated triglycerides at follow-up, 11 patients (55%) were obese. At follow-up, 11 patients had levels of 200-500 mg/dL (grade I elevation), and 2 patients had levels of 501-1,000 mg/dL (grade II elevation). Isotretinoin was stopped in the latter two patients, who also had obesity as a risk factor for their hypertriglyceridemia.
“None of these patients had clinical sequelae from their hypertriglyceridemia, such as pancreatitis at baseline or follow-up,” Ms. Parthasarathy said. “However, since pancreatitis would be expected to be exceedingly rare, the sample size may be limited in identifying this adverse effect.”
She noted that while isotretinoin might cause a significant increase in lipid levels, the mean levels remained within normal limits at both baseline and follow-up. “Of the patients with elevated triglycerides at baseline and follow-up, obesity may have been a potential risk factor,” she said. “This could suggest a possible strategy for reduced testing in nonobese isotretinoin patients, which can be further explored in larger study populations.”
In addition, “there was a lack of significant change in AST and ALT in this study and adult studies, as well as minimal evidence for pediatric retinoid-induced hepatotoxicity, which raises the question of the necessity of baseline and follow-up comprehensive metabolic panel testing,” Ms. Parthasarathy added. “Clinicians must weigh the laboratory values with the costs of laboratory testing, including opportunity costs such as time, monetary costs, and the discomfort of testing for pediatric patients.”
The study’s senior author was A. Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital, Washington. The researchers reported having no relevant financial disclosures.
.
“Isotretinoin is a very effective treatment for severe acne,” Varsha Parthasarathy said at the annual meeting of the Society for Pediatric Dermatology. “However, initiating this medication requires a complex process of laboratory testing,” which includes human chorionic gonadotropin pregnancy testing, because isotretinoin is a teratogen, as well as lipid labs and liver function tests, she noted. “Importantly, triglycerides are measured due to an association in adults between isotretinoin and hypertriglyceridemia-associated pancreatitis. However, these findings in children are limited to case reports, as are findings of retinoid-induced hepatotoxicity.”
To identify the role of isotretinoin on changes in lipids, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and to determine the impact on treatment course, Ms. Parthasarathy, a 4-year medical student at George Washington University, Washington, and colleagues retrospectively reviewed the charts of 130 patients aged 12-21 years who were cared for at Children’s National Hospital between January 2012 and October 2020. Nearly two-thirds (65%) were male, their average age was 16 years, and the mean time to obtain follow-up labs after starting isotretinoin was 3.25 months.
Between baseline and follow-up, the researchers observed increases in total cholesterol, triglycerides, and LDL (P less than .001 for all associations) and a decrease in HDL (P = .001), but there were no significant changes in AST or ALT levels. These findings were consistent with prior studies in adults examining the utility of these laboratory tests, most notably a 2016 study by Timothy J. Hansen, MD, and colleagues.
Among the 13 patients with elevated triglycerides at baseline, 9 (69%) were overweight or obese. Of the 20 patients with elevated triglycerides at follow-up, 11 patients (55%) were obese. At follow-up, 11 patients had levels of 200-500 mg/dL (grade I elevation), and 2 patients had levels of 501-1,000 mg/dL (grade II elevation). Isotretinoin was stopped in the latter two patients, who also had obesity as a risk factor for their hypertriglyceridemia.
“None of these patients had clinical sequelae from their hypertriglyceridemia, such as pancreatitis at baseline or follow-up,” Ms. Parthasarathy said. “However, since pancreatitis would be expected to be exceedingly rare, the sample size may be limited in identifying this adverse effect.”
She noted that while isotretinoin might cause a significant increase in lipid levels, the mean levels remained within normal limits at both baseline and follow-up. “Of the patients with elevated triglycerides at baseline and follow-up, obesity may have been a potential risk factor,” she said. “This could suggest a possible strategy for reduced testing in nonobese isotretinoin patients, which can be further explored in larger study populations.”
In addition, “there was a lack of significant change in AST and ALT in this study and adult studies, as well as minimal evidence for pediatric retinoid-induced hepatotoxicity, which raises the question of the necessity of baseline and follow-up comprehensive metabolic panel testing,” Ms. Parthasarathy added. “Clinicians must weigh the laboratory values with the costs of laboratory testing, including opportunity costs such as time, monetary costs, and the discomfort of testing for pediatric patients.”
The study’s senior author was A. Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital, Washington. The researchers reported having no relevant financial disclosures.
FROM SPD 2021
AGA Clinical Practice Update: Early complications after bariatric/metabolic surgery
The American Gastroenterological Association recently published a clinical practice update concerning endoscopic evaluation and management of early complications after bariatric/metabolic surgery.
The seven best practice advice statements, based on available evidence and expert opinion, range from a general call for high familiarity with available interventions to specific approaches for managing postoperative leaks.
According to lead author Vivek Kumbhari, MD, PhD, director of advanced endoscopy, department of gastroenterology and hepatology, Mayo Clinic, Jacksonville, Fla., and colleagues, the update was written in consideration of increasing rates of bariatric/metabolic surgery.
“Bariatric/metabolic surgery is unmatched with respect to its weight loss and metabolic benefits,” the investigators wrote in Clinical Gastroenterology and Hepatology. “The selection criteria will continue to broaden, likely resulting in increasing numbers of less robust patients undergoing surgery (e.g., children, elderly, and those with significant cardiorespiratory comorbidities).”
Although the 90-day overall complication rate across all patients undergoing bariatric/metabolic surgery is only 4%, Dr. Kumbhari and colleagues noted that this rate is considerably higher, at 20.1%, among patients aged older than 65 years.
“As utilization escalates, so will the number of patients who suffer early complications,” they wrote.
The first three items of best practice advice describe who should be managing complications after bariatric/metabolic surgery, and how.
Foremost, Dr. Kumbhari and colleagues called for a multidisciplinary approach; they suggested that endoscopists should work closely with related specialists, such as bariatric/metabolic surgeons and interventional radiologists.
“Timely communication between the endoscopist, radiologist, surgeon, nutritionists, and inpatient medical team or primary care physician will result in efficient, effective care with prompt escalation and deescalation,” they wrote. “Daily communication is advised.”
The next two best practice advice statements encourage high familiarity with endoscopic treatments, postsurgical anatomy, interventional radiology, and surgical interventions, including risks and benefits of each approach.
“The endoscopist should ... have expertise in interventional endoscopy techniques, including but not limited to using concomitant fluoroscopy, stent deployment and retrieval, pneumatic balloon dilation, incisional therapies, endoscopic suturing, and managing percutaneous drains,” the investigators wrote. “Having the ability to perform a wide array of therapies will enhance the likelihood that the optimal endoscopic strategy will be employed, as opposed to simply performing a technique with which the endoscopist has experience.”
Following these best practices, Dr. Kumbhari and colleagues advised screening patients with postoperative complications for comorbidities, both medical in nature (such as infection) and psychological.
“Patients often have higher depression and anxiety scores, as well as a lower physical quality of life, and medical teams sometimes neglect the patient’s psychological state,” they wrote. “It is imperative that the multidisciplinary team recognize and acknowledge the patient’s psychological comorbidities and engage expertise to manage them.”
Next, the investigators advised that endoscopic intervention should be considered regardless of time interval since surgery, including the immediate postoperative period.
“Endoscopy is often indicated as the initial therapeutic modality, and it can safely be performed,” Dr. Kumbhari and colleagues wrote. “When endoscopy is performed, it is advised to use carbon dioxide for insufflation. Caution should be used when advancing the endoscope into the small bowel, as it is best to minimize pressure along the fresh staple lines. In cases in which the patient is critically ill or the interventional endoscopist does not have extensive experience with such a scenario, the endoscopy should be performed in the operating room with a surgeon present (preferably the surgeon who performed the operation).”
Dr. Kumbhari and colleagues discussed functional stenosis, which can precipitate and propagate leaks. They noted that “downstream stenosis is frequently seen at the level of the incisura angularis or in the proximal stomach when a sleeve gastrectomy is performed in a patient with a prior laparoscopic adjustable gastric band.”
To address stenosis, the update calls for “aggressive dilation” using a large pneumatic balloon, preferably with fluoroscopy to make sure the distal end of the balloon does not cross the pylorus. The investigators noted that endoscopic suturing may be needed if a tear involving the muscularis propria is encountered.
Lastly, the clinical practice update offers comprehensive guidance for managing staple-line leaks, which “most commonly occur along the staple line of the proximal stomach.”
As leaks are thought to stem from ischemia, “most leaks are not present upon completion of the operation, and they develop over the subsequent weeks, often in the setting of downstream stenosis,” the investigators wrote.
To guide management of staple-line leaks, the investigators presented a treatment algorithm that incorporates defect size, time since surgery, and presence or absence of stenosis.
For example, a defect smaller than 10 mm occurring within 6 weeks of surgery and lacking stenosis may be managed with a percutaneous drain and diversion. In contrast, a defect of similar size, also without stenosis, but occurring later than 6 weeks after the initial procedure, should be managed with endoscopic internal drainage or vacuum therapy.
“Clinicians should recognize that the goal for endoscopic management of staple-line leaks is often not necessarily initial closure of the leak site, but rather techniques to promote drainage of material from the perigastric collection into the gastric lumen such that the leak site closes by secondary intention,” wrote Dr. Kumbhari and colleagues.
The clinical practice update was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Boston Scientific, Medtronic, Apollo Endosurgery, and others.
The American Gastroenterological Association recently published a clinical practice update concerning endoscopic evaluation and management of early complications after bariatric/metabolic surgery.
The seven best practice advice statements, based on available evidence and expert opinion, range from a general call for high familiarity with available interventions to specific approaches for managing postoperative leaks.
According to lead author Vivek Kumbhari, MD, PhD, director of advanced endoscopy, department of gastroenterology and hepatology, Mayo Clinic, Jacksonville, Fla., and colleagues, the update was written in consideration of increasing rates of bariatric/metabolic surgery.
“Bariatric/metabolic surgery is unmatched with respect to its weight loss and metabolic benefits,” the investigators wrote in Clinical Gastroenterology and Hepatology. “The selection criteria will continue to broaden, likely resulting in increasing numbers of less robust patients undergoing surgery (e.g., children, elderly, and those with significant cardiorespiratory comorbidities).”
Although the 90-day overall complication rate across all patients undergoing bariatric/metabolic surgery is only 4%, Dr. Kumbhari and colleagues noted that this rate is considerably higher, at 20.1%, among patients aged older than 65 years.
“As utilization escalates, so will the number of patients who suffer early complications,” they wrote.
The first three items of best practice advice describe who should be managing complications after bariatric/metabolic surgery, and how.
Foremost, Dr. Kumbhari and colleagues called for a multidisciplinary approach; they suggested that endoscopists should work closely with related specialists, such as bariatric/metabolic surgeons and interventional radiologists.
“Timely communication between the endoscopist, radiologist, surgeon, nutritionists, and inpatient medical team or primary care physician will result in efficient, effective care with prompt escalation and deescalation,” they wrote. “Daily communication is advised.”
The next two best practice advice statements encourage high familiarity with endoscopic treatments, postsurgical anatomy, interventional radiology, and surgical interventions, including risks and benefits of each approach.
“The endoscopist should ... have expertise in interventional endoscopy techniques, including but not limited to using concomitant fluoroscopy, stent deployment and retrieval, pneumatic balloon dilation, incisional therapies, endoscopic suturing, and managing percutaneous drains,” the investigators wrote. “Having the ability to perform a wide array of therapies will enhance the likelihood that the optimal endoscopic strategy will be employed, as opposed to simply performing a technique with which the endoscopist has experience.”
Following these best practices, Dr. Kumbhari and colleagues advised screening patients with postoperative complications for comorbidities, both medical in nature (such as infection) and psychological.
“Patients often have higher depression and anxiety scores, as well as a lower physical quality of life, and medical teams sometimes neglect the patient’s psychological state,” they wrote. “It is imperative that the multidisciplinary team recognize and acknowledge the patient’s psychological comorbidities and engage expertise to manage them.”
Next, the investigators advised that endoscopic intervention should be considered regardless of time interval since surgery, including the immediate postoperative period.
“Endoscopy is often indicated as the initial therapeutic modality, and it can safely be performed,” Dr. Kumbhari and colleagues wrote. “When endoscopy is performed, it is advised to use carbon dioxide for insufflation. Caution should be used when advancing the endoscope into the small bowel, as it is best to minimize pressure along the fresh staple lines. In cases in which the patient is critically ill or the interventional endoscopist does not have extensive experience with such a scenario, the endoscopy should be performed in the operating room with a surgeon present (preferably the surgeon who performed the operation).”
Dr. Kumbhari and colleagues discussed functional stenosis, which can precipitate and propagate leaks. They noted that “downstream stenosis is frequently seen at the level of the incisura angularis or in the proximal stomach when a sleeve gastrectomy is performed in a patient with a prior laparoscopic adjustable gastric band.”
To address stenosis, the update calls for “aggressive dilation” using a large pneumatic balloon, preferably with fluoroscopy to make sure the distal end of the balloon does not cross the pylorus. The investigators noted that endoscopic suturing may be needed if a tear involving the muscularis propria is encountered.
Lastly, the clinical practice update offers comprehensive guidance for managing staple-line leaks, which “most commonly occur along the staple line of the proximal stomach.”
As leaks are thought to stem from ischemia, “most leaks are not present upon completion of the operation, and they develop over the subsequent weeks, often in the setting of downstream stenosis,” the investigators wrote.
To guide management of staple-line leaks, the investigators presented a treatment algorithm that incorporates defect size, time since surgery, and presence or absence of stenosis.
For example, a defect smaller than 10 mm occurring within 6 weeks of surgery and lacking stenosis may be managed with a percutaneous drain and diversion. In contrast, a defect of similar size, also without stenosis, but occurring later than 6 weeks after the initial procedure, should be managed with endoscopic internal drainage or vacuum therapy.
“Clinicians should recognize that the goal for endoscopic management of staple-line leaks is often not necessarily initial closure of the leak site, but rather techniques to promote drainage of material from the perigastric collection into the gastric lumen such that the leak site closes by secondary intention,” wrote Dr. Kumbhari and colleagues.
The clinical practice update was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Boston Scientific, Medtronic, Apollo Endosurgery, and others.
The American Gastroenterological Association recently published a clinical practice update concerning endoscopic evaluation and management of early complications after bariatric/metabolic surgery.
The seven best practice advice statements, based on available evidence and expert opinion, range from a general call for high familiarity with available interventions to specific approaches for managing postoperative leaks.
According to lead author Vivek Kumbhari, MD, PhD, director of advanced endoscopy, department of gastroenterology and hepatology, Mayo Clinic, Jacksonville, Fla., and colleagues, the update was written in consideration of increasing rates of bariatric/metabolic surgery.
“Bariatric/metabolic surgery is unmatched with respect to its weight loss and metabolic benefits,” the investigators wrote in Clinical Gastroenterology and Hepatology. “The selection criteria will continue to broaden, likely resulting in increasing numbers of less robust patients undergoing surgery (e.g., children, elderly, and those with significant cardiorespiratory comorbidities).”
Although the 90-day overall complication rate across all patients undergoing bariatric/metabolic surgery is only 4%, Dr. Kumbhari and colleagues noted that this rate is considerably higher, at 20.1%, among patients aged older than 65 years.
“As utilization escalates, so will the number of patients who suffer early complications,” they wrote.
The first three items of best practice advice describe who should be managing complications after bariatric/metabolic surgery, and how.
Foremost, Dr. Kumbhari and colleagues called for a multidisciplinary approach; they suggested that endoscopists should work closely with related specialists, such as bariatric/metabolic surgeons and interventional radiologists.
“Timely communication between the endoscopist, radiologist, surgeon, nutritionists, and inpatient medical team or primary care physician will result in efficient, effective care with prompt escalation and deescalation,” they wrote. “Daily communication is advised.”
The next two best practice advice statements encourage high familiarity with endoscopic treatments, postsurgical anatomy, interventional radiology, and surgical interventions, including risks and benefits of each approach.
“The endoscopist should ... have expertise in interventional endoscopy techniques, including but not limited to using concomitant fluoroscopy, stent deployment and retrieval, pneumatic balloon dilation, incisional therapies, endoscopic suturing, and managing percutaneous drains,” the investigators wrote. “Having the ability to perform a wide array of therapies will enhance the likelihood that the optimal endoscopic strategy will be employed, as opposed to simply performing a technique with which the endoscopist has experience.”
Following these best practices, Dr. Kumbhari and colleagues advised screening patients with postoperative complications for comorbidities, both medical in nature (such as infection) and psychological.
“Patients often have higher depression and anxiety scores, as well as a lower physical quality of life, and medical teams sometimes neglect the patient’s psychological state,” they wrote. “It is imperative that the multidisciplinary team recognize and acknowledge the patient’s psychological comorbidities and engage expertise to manage them.”
Next, the investigators advised that endoscopic intervention should be considered regardless of time interval since surgery, including the immediate postoperative period.
“Endoscopy is often indicated as the initial therapeutic modality, and it can safely be performed,” Dr. Kumbhari and colleagues wrote. “When endoscopy is performed, it is advised to use carbon dioxide for insufflation. Caution should be used when advancing the endoscope into the small bowel, as it is best to minimize pressure along the fresh staple lines. In cases in which the patient is critically ill or the interventional endoscopist does not have extensive experience with such a scenario, the endoscopy should be performed in the operating room with a surgeon present (preferably the surgeon who performed the operation).”
Dr. Kumbhari and colleagues discussed functional stenosis, which can precipitate and propagate leaks. They noted that “downstream stenosis is frequently seen at the level of the incisura angularis or in the proximal stomach when a sleeve gastrectomy is performed in a patient with a prior laparoscopic adjustable gastric band.”
To address stenosis, the update calls for “aggressive dilation” using a large pneumatic balloon, preferably with fluoroscopy to make sure the distal end of the balloon does not cross the pylorus. The investigators noted that endoscopic suturing may be needed if a tear involving the muscularis propria is encountered.
Lastly, the clinical practice update offers comprehensive guidance for managing staple-line leaks, which “most commonly occur along the staple line of the proximal stomach.”
As leaks are thought to stem from ischemia, “most leaks are not present upon completion of the operation, and they develop over the subsequent weeks, often in the setting of downstream stenosis,” the investigators wrote.
To guide management of staple-line leaks, the investigators presented a treatment algorithm that incorporates defect size, time since surgery, and presence or absence of stenosis.
For example, a defect smaller than 10 mm occurring within 6 weeks of surgery and lacking stenosis may be managed with a percutaneous drain and diversion. In contrast, a defect of similar size, also without stenosis, but occurring later than 6 weeks after the initial procedure, should be managed with endoscopic internal drainage or vacuum therapy.
“Clinicians should recognize that the goal for endoscopic management of staple-line leaks is often not necessarily initial closure of the leak site, but rather techniques to promote drainage of material from the perigastric collection into the gastric lumen such that the leak site closes by secondary intention,” wrote Dr. Kumbhari and colleagues.
The clinical practice update was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Boston Scientific, Medtronic, Apollo Endosurgery, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Mutational signature may reveal underlying link between red meat and CRC
A mechanistic link between red meat consumption and colorectal cancer (CRC) has been identified in the form of an alkylating mutational signature, according to investigators.
This is the first time a colorectal mutational signature has been associated with a component of diet, which demonstrates the value of large-scale molecular epidemiologic studies and suggests potential for early, precision dietary intervention, reported lead author Carino Gurjao, MSc, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, and colleagues.
“Red meat consumption has been consistently linked to the incidence of colorectal cancer,” the investigators wrote in Cancer Discovery. “The suggested mechanism is mutagenesis through alkylating damage induced by N-nitroso-compounds (NOCs), which are metabolic products of blood heme iron or meat nitrites/nitrates. Nevertheless, this mutational damage is yet to be observed directly in patients’ tumors.”
To this end, the investigators turned to three long-term, large-scale, prospective cohort studies: the Nurses’ Health Studies I and II, and the Health Professionals Follow-up Study. These databases include nearly 300,000 individuals with follow-up dating back as far as 1976. The investigators identified 900 cases of primary, untreated CRC with adequate tissue for analysis, then, for each case, performed whole exome sequencing on both tumor tissue and normal colorectal tissue.
This revealed an alkylating mutational signature previously undescribed in CRC that was significantly associated with consumption of red meat prior to diagnosis, but not other dietary or lifestyle factors. The signature occurred most frequently in tumors and normal crypts in the distal colon and rectum.
According to the investigators, the presence of the alkylating signature in normal colorectal crypts “suggests that mutational changes due to such damage may start to occur early in the path of colorectal carcinogenesis.”
Further analysis showed that tumors harboring common KRAS and PIK3CA driver mutations had the highest levels of alkylating damage, with higher levels predicting worse survival.
“These results ... further implicate the role of red meat in CRC initiation and progression,” the investigators concluded.
Early findings, important implications
Cosenior author Kana Wu, MD, PhD, principal research scientist in the department of nutrition at Harvard School of Public Health, Boston, noted that these are early findings, although they may pave the way toward new dietary recommendations and methods of food production.
“While more detailed analysis needs to be conducted, and our results need to be confirmed in other studies, this study is a promising first step to better understand the biological mechanisms underlying the role of red and processed meats in colorectal cancers,” Dr. Wu said in an interview. “It is important to gain more insight into the biological mechanisms so we can improve dietary guidelines for cancer prevention and guide food reformulation efforts to lower cancer risk.”
For now, Dr. Wu predicted that standing dietary recommendations will remain unchanged.
“This study will not alter current diet recommendations to limit intake of red and processed meats,” Dr. Wu said, referring to similar recommendations across several organizations, including the American Heart Association, the World Cancer Research Fund/American Institute for Cancer Research, and the American Cancer Society.
“For example,” Dr. Wu said, “the WCRF/AICR recommends limiting consumption of red and processed meat to ‘no more than moderate amounts [12-18 ounces per week] of red meat, such as beef, pork, and lamb, and [to] eat little, if any, processed meat.’”
Possible biomarker?
According to Patricia Thompson-Carino, PhD, deputy director of the Stony Brook (N.Y.) Cancer Center, the study provides convincing evidence linking red meat consumption with development of CRC.
“Higher frequency of the signature in the distal colon is compelling for its consistency with epidemiologic evidence,” Dr. Thompson-Carino said in an interview. “Combined with the observed worse survival in patients harboring the signature and association with oncogenic KRAS and PIK3CA driver mutations, this study significantly elevates the biological plausibility that red meat is a modifiable source of NOC mutagenicity and carcinogenesis in humans.”
The signature could be used as a biomarker to detect exposure to NOCs, and susceptibility to CRC, she added.
Still, Dr. Thompson-Carino suggested that more work is needed to fully elucidate underlying mechanisms of action, which are needed to accurately shape dietary guidance.
“Key to advancing red meat dietary recommendations will be understanding the relationships between the new mutation signature and the NOCs derived from red meat and their source, whether endogenous [for example, intestinal N-nitrosation] or exogenous [for example, chemical preservation or charring],” she said. The study was supported by the National Institutes of Health, the Stand Up To Cancer Colorectal Cancer Dream Team Translational Research Grant (coadministered by the American Association for Cancer Research), the Project P Fund, and others. The investigators, Dr. Wu, and Dr. Thompson-Carino reported no conflicts of interest related to this study.
A mechanistic link between red meat consumption and colorectal cancer (CRC) has been identified in the form of an alkylating mutational signature, according to investigators.
This is the first time a colorectal mutational signature has been associated with a component of diet, which demonstrates the value of large-scale molecular epidemiologic studies and suggests potential for early, precision dietary intervention, reported lead author Carino Gurjao, MSc, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, and colleagues.
“Red meat consumption has been consistently linked to the incidence of colorectal cancer,” the investigators wrote in Cancer Discovery. “The suggested mechanism is mutagenesis through alkylating damage induced by N-nitroso-compounds (NOCs), which are metabolic products of blood heme iron or meat nitrites/nitrates. Nevertheless, this mutational damage is yet to be observed directly in patients’ tumors.”
To this end, the investigators turned to three long-term, large-scale, prospective cohort studies: the Nurses’ Health Studies I and II, and the Health Professionals Follow-up Study. These databases include nearly 300,000 individuals with follow-up dating back as far as 1976. The investigators identified 900 cases of primary, untreated CRC with adequate tissue for analysis, then, for each case, performed whole exome sequencing on both tumor tissue and normal colorectal tissue.
This revealed an alkylating mutational signature previously undescribed in CRC that was significantly associated with consumption of red meat prior to diagnosis, but not other dietary or lifestyle factors. The signature occurred most frequently in tumors and normal crypts in the distal colon and rectum.
According to the investigators, the presence of the alkylating signature in normal colorectal crypts “suggests that mutational changes due to such damage may start to occur early in the path of colorectal carcinogenesis.”
Further analysis showed that tumors harboring common KRAS and PIK3CA driver mutations had the highest levels of alkylating damage, with higher levels predicting worse survival.
“These results ... further implicate the role of red meat in CRC initiation and progression,” the investigators concluded.
Early findings, important implications
Cosenior author Kana Wu, MD, PhD, principal research scientist in the department of nutrition at Harvard School of Public Health, Boston, noted that these are early findings, although they may pave the way toward new dietary recommendations and methods of food production.
“While more detailed analysis needs to be conducted, and our results need to be confirmed in other studies, this study is a promising first step to better understand the biological mechanisms underlying the role of red and processed meats in colorectal cancers,” Dr. Wu said in an interview. “It is important to gain more insight into the biological mechanisms so we can improve dietary guidelines for cancer prevention and guide food reformulation efforts to lower cancer risk.”
For now, Dr. Wu predicted that standing dietary recommendations will remain unchanged.
“This study will not alter current diet recommendations to limit intake of red and processed meats,” Dr. Wu said, referring to similar recommendations across several organizations, including the American Heart Association, the World Cancer Research Fund/American Institute for Cancer Research, and the American Cancer Society.
“For example,” Dr. Wu said, “the WCRF/AICR recommends limiting consumption of red and processed meat to ‘no more than moderate amounts [12-18 ounces per week] of red meat, such as beef, pork, and lamb, and [to] eat little, if any, processed meat.’”
Possible biomarker?
According to Patricia Thompson-Carino, PhD, deputy director of the Stony Brook (N.Y.) Cancer Center, the study provides convincing evidence linking red meat consumption with development of CRC.
“Higher frequency of the signature in the distal colon is compelling for its consistency with epidemiologic evidence,” Dr. Thompson-Carino said in an interview. “Combined with the observed worse survival in patients harboring the signature and association with oncogenic KRAS and PIK3CA driver mutations, this study significantly elevates the biological plausibility that red meat is a modifiable source of NOC mutagenicity and carcinogenesis in humans.”
The signature could be used as a biomarker to detect exposure to NOCs, and susceptibility to CRC, she added.
Still, Dr. Thompson-Carino suggested that more work is needed to fully elucidate underlying mechanisms of action, which are needed to accurately shape dietary guidance.
“Key to advancing red meat dietary recommendations will be understanding the relationships between the new mutation signature and the NOCs derived from red meat and their source, whether endogenous [for example, intestinal N-nitrosation] or exogenous [for example, chemical preservation or charring],” she said. The study was supported by the National Institutes of Health, the Stand Up To Cancer Colorectal Cancer Dream Team Translational Research Grant (coadministered by the American Association for Cancer Research), the Project P Fund, and others. The investigators, Dr. Wu, and Dr. Thompson-Carino reported no conflicts of interest related to this study.
A mechanistic link between red meat consumption and colorectal cancer (CRC) has been identified in the form of an alkylating mutational signature, according to investigators.
This is the first time a colorectal mutational signature has been associated with a component of diet, which demonstrates the value of large-scale molecular epidemiologic studies and suggests potential for early, precision dietary intervention, reported lead author Carino Gurjao, MSc, of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, and colleagues.
“Red meat consumption has been consistently linked to the incidence of colorectal cancer,” the investigators wrote in Cancer Discovery. “The suggested mechanism is mutagenesis through alkylating damage induced by N-nitroso-compounds (NOCs), which are metabolic products of blood heme iron or meat nitrites/nitrates. Nevertheless, this mutational damage is yet to be observed directly in patients’ tumors.”
To this end, the investigators turned to three long-term, large-scale, prospective cohort studies: the Nurses’ Health Studies I and II, and the Health Professionals Follow-up Study. These databases include nearly 300,000 individuals with follow-up dating back as far as 1976. The investigators identified 900 cases of primary, untreated CRC with adequate tissue for analysis, then, for each case, performed whole exome sequencing on both tumor tissue and normal colorectal tissue.
This revealed an alkylating mutational signature previously undescribed in CRC that was significantly associated with consumption of red meat prior to diagnosis, but not other dietary or lifestyle factors. The signature occurred most frequently in tumors and normal crypts in the distal colon and rectum.
According to the investigators, the presence of the alkylating signature in normal colorectal crypts “suggests that mutational changes due to such damage may start to occur early in the path of colorectal carcinogenesis.”
Further analysis showed that tumors harboring common KRAS and PIK3CA driver mutations had the highest levels of alkylating damage, with higher levels predicting worse survival.
“These results ... further implicate the role of red meat in CRC initiation and progression,” the investigators concluded.
Early findings, important implications
Cosenior author Kana Wu, MD, PhD, principal research scientist in the department of nutrition at Harvard School of Public Health, Boston, noted that these are early findings, although they may pave the way toward new dietary recommendations and methods of food production.
“While more detailed analysis needs to be conducted, and our results need to be confirmed in other studies, this study is a promising first step to better understand the biological mechanisms underlying the role of red and processed meats in colorectal cancers,” Dr. Wu said in an interview. “It is important to gain more insight into the biological mechanisms so we can improve dietary guidelines for cancer prevention and guide food reformulation efforts to lower cancer risk.”
For now, Dr. Wu predicted that standing dietary recommendations will remain unchanged.
“This study will not alter current diet recommendations to limit intake of red and processed meats,” Dr. Wu said, referring to similar recommendations across several organizations, including the American Heart Association, the World Cancer Research Fund/American Institute for Cancer Research, and the American Cancer Society.
“For example,” Dr. Wu said, “the WCRF/AICR recommends limiting consumption of red and processed meat to ‘no more than moderate amounts [12-18 ounces per week] of red meat, such as beef, pork, and lamb, and [to] eat little, if any, processed meat.’”
Possible biomarker?
According to Patricia Thompson-Carino, PhD, deputy director of the Stony Brook (N.Y.) Cancer Center, the study provides convincing evidence linking red meat consumption with development of CRC.
“Higher frequency of the signature in the distal colon is compelling for its consistency with epidemiologic evidence,” Dr. Thompson-Carino said in an interview. “Combined with the observed worse survival in patients harboring the signature and association with oncogenic KRAS and PIK3CA driver mutations, this study significantly elevates the biological plausibility that red meat is a modifiable source of NOC mutagenicity and carcinogenesis in humans.”
The signature could be used as a biomarker to detect exposure to NOCs, and susceptibility to CRC, she added.
Still, Dr. Thompson-Carino suggested that more work is needed to fully elucidate underlying mechanisms of action, which are needed to accurately shape dietary guidance.
“Key to advancing red meat dietary recommendations will be understanding the relationships between the new mutation signature and the NOCs derived from red meat and their source, whether endogenous [for example, intestinal N-nitrosation] or exogenous [for example, chemical preservation or charring],” she said. The study was supported by the National Institutes of Health, the Stand Up To Cancer Colorectal Cancer Dream Team Translational Research Grant (coadministered by the American Association for Cancer Research), the Project P Fund, and others. The investigators, Dr. Wu, and Dr. Thompson-Carino reported no conflicts of interest related to this study.
FROM CANCER DISCOVERY
California to pay victims of forced, coerced sterilizations
SACRAMENTO (AP) – California is poised to approve reparations of up to $25,000 to some of the thousands of people – some as young as 13 – who were sterilized decades ago because the government deemed them unfit to have children.
The payments will make California at least the third state – following Virginia and North Carolina – to compensate victims of the so-called eugenics movement that peaked in the 1930s. Supporters of the movement believed sterilizing people with mental illnesses, physical disabilities, and other traits they deemed undesirable would improve the human race.
While California sterilized more than 20,000 people before its law was repealed in 1979, only a few hundred are still alive. The state has set aside $7.5 million for the reparations program, part of its $262.6 billion operating budget that is awaiting Gov. Gavin Newsom’s signature.
California’s proposal is unique because it also would pay women the state coerced to get sterilized while they were in prison, some as recently as 2010. First exposed by the Center for Investigative Reporting in 2013, a subsequent audit found California sterilized 144 women between 2005 and 2013 with little or no evidence that officials counseled them or offered alternative treatment.
While all of the women signed consent forms, officials in 39 cases did not do everything that was legally required to obtain their permission.
“We must address and face our horrific history,” said Lorena Garcia Zermeño, policy and communications coordinator for the advocacy group California Latinas for Reproductive Justice. “This isn’t something that just happened in the past.”
California’s forced sterilization program started in 1909, following similar laws in Indiana and Washington. It was by far the largest program, accounting for about a third of everyone sterilized in the United States under those laws.
California’s law was so prominent that it inspired similar practices in Nazi Germany, according to Paul Lombardo, a law professor at Georgia State University, Atlanta, and an expert on the eugenics movement.
“The promise of eugenics at the very earliest is: ‘We could do away with all the state institutions – prisons, hospitals, asylums, orphanages,’” Mr. Lombardo said. “People who were in them just wouldn’t be born after awhile if you sterilized all of their parents.”
In California, victims include Mary Franco, who was sterilized in 1934 when she was just 13. Paperwork described her as “feeble minded” because of “sexual deviance,” according to her niece, Stacy Cordova, who has researched her case.
Ms. Cordova said Franco actually was molested by a neighbor. She said her family put Ms. Franco in an institution to protect the family’s reputation.
Ms. Cordova said her late aunt loved children and wanted to have a family. She married briefly when she was about 17, but Ms. Cordova said the marriage was annulled when the man discovered Ms. Franco couldn’t have children. She lived a lonely life in a Mexican culture that revered big families, Ms. Cordova said.
“I don’t know if it is justice. Money doesn’t pay for what happened to them. But it’s great to know that this is being recognized,” said Ms. Cordova, who has advocated for the state to pay survivors. “For me, this is not about the money. This is about the memory.”
Relatives like Ms. Cordova aren’t eligible for the payments, only direct victims are.
Sterilizations in California prisons appear to date to 1999, when the state changed its policy for unknown reasons to include a sterilization procedure known as “tubal ligation” as part of inmates’ medical care. Over the next decade, women reported they were coerced into this procedure, with some not fully understanding the ramifications.
A state law passed in 2014 bans sterilizations for the purpose of birth control at state prisons and local jails. The law permits sterilizations that are “medically necessary,” such as removing cancer, and requires facilities to report each year how many people were sterilized and for what reason.
Questionable sterilizations also occurred in facilities run by local governments. In 2018, the Los Angeles County Board of Supervisors apologized after more than 200 women were sterilized at the Los Angeles–University of Southern California Medical Center between 1968 and 1974.
Those people are not eligible for reparations under California’s program. But advocates say they hope to include them in the future.
“It’s only the beginning,” said state Assemblywoman Wendy Carrillo, a Democrat from Los Angeles who has been advocating for reparations. “I can’t imagine the trauma, the depression, the stress of being incarcerated, being rehabilitated and trying to start your life again in society, wanting to start a family, only to find out that that choice was taken away from you.”
Of the people California sterilized under its old eugenics law, just a few hundred are still alive, according to research conducted by the Sterilization and Social Justice Lab. Including the inmates who were sterilized most recently, advocates estimate more than 600 people would be eligible for reparations.
But finding them will be difficult, with advocates predicting only about 25% of eligible people will ultimately apply for reparations and be paid.
California’s Victim Compensation Board will run the program, with $2 million used to find victims by advertising and poring through state records. The state also set aside $1 million for plaques to honor the victims, leaving $4.5 million for reparations.
A version of this article appeared on Medscape.com.
Associated Press © 2021
SACRAMENTO (AP) – California is poised to approve reparations of up to $25,000 to some of the thousands of people – some as young as 13 – who were sterilized decades ago because the government deemed them unfit to have children.
The payments will make California at least the third state – following Virginia and North Carolina – to compensate victims of the so-called eugenics movement that peaked in the 1930s. Supporters of the movement believed sterilizing people with mental illnesses, physical disabilities, and other traits they deemed undesirable would improve the human race.
While California sterilized more than 20,000 people before its law was repealed in 1979, only a few hundred are still alive. The state has set aside $7.5 million for the reparations program, part of its $262.6 billion operating budget that is awaiting Gov. Gavin Newsom’s signature.
California’s proposal is unique because it also would pay women the state coerced to get sterilized while they were in prison, some as recently as 2010. First exposed by the Center for Investigative Reporting in 2013, a subsequent audit found California sterilized 144 women between 2005 and 2013 with little or no evidence that officials counseled them or offered alternative treatment.
While all of the women signed consent forms, officials in 39 cases did not do everything that was legally required to obtain their permission.
“We must address and face our horrific history,” said Lorena Garcia Zermeño, policy and communications coordinator for the advocacy group California Latinas for Reproductive Justice. “This isn’t something that just happened in the past.”
California’s forced sterilization program started in 1909, following similar laws in Indiana and Washington. It was by far the largest program, accounting for about a third of everyone sterilized in the United States under those laws.
California’s law was so prominent that it inspired similar practices in Nazi Germany, according to Paul Lombardo, a law professor at Georgia State University, Atlanta, and an expert on the eugenics movement.
“The promise of eugenics at the very earliest is: ‘We could do away with all the state institutions – prisons, hospitals, asylums, orphanages,’” Mr. Lombardo said. “People who were in them just wouldn’t be born after awhile if you sterilized all of their parents.”
In California, victims include Mary Franco, who was sterilized in 1934 when she was just 13. Paperwork described her as “feeble minded” because of “sexual deviance,” according to her niece, Stacy Cordova, who has researched her case.
Ms. Cordova said Franco actually was molested by a neighbor. She said her family put Ms. Franco in an institution to protect the family’s reputation.
Ms. Cordova said her late aunt loved children and wanted to have a family. She married briefly when she was about 17, but Ms. Cordova said the marriage was annulled when the man discovered Ms. Franco couldn’t have children. She lived a lonely life in a Mexican culture that revered big families, Ms. Cordova said.
“I don’t know if it is justice. Money doesn’t pay for what happened to them. But it’s great to know that this is being recognized,” said Ms. Cordova, who has advocated for the state to pay survivors. “For me, this is not about the money. This is about the memory.”
Relatives like Ms. Cordova aren’t eligible for the payments, only direct victims are.
Sterilizations in California prisons appear to date to 1999, when the state changed its policy for unknown reasons to include a sterilization procedure known as “tubal ligation” as part of inmates’ medical care. Over the next decade, women reported they were coerced into this procedure, with some not fully understanding the ramifications.
A state law passed in 2014 bans sterilizations for the purpose of birth control at state prisons and local jails. The law permits sterilizations that are “medically necessary,” such as removing cancer, and requires facilities to report each year how many people were sterilized and for what reason.
Questionable sterilizations also occurred in facilities run by local governments. In 2018, the Los Angeles County Board of Supervisors apologized after more than 200 women were sterilized at the Los Angeles–University of Southern California Medical Center between 1968 and 1974.
Those people are not eligible for reparations under California’s program. But advocates say they hope to include them in the future.
“It’s only the beginning,” said state Assemblywoman Wendy Carrillo, a Democrat from Los Angeles who has been advocating for reparations. “I can’t imagine the trauma, the depression, the stress of being incarcerated, being rehabilitated and trying to start your life again in society, wanting to start a family, only to find out that that choice was taken away from you.”
Of the people California sterilized under its old eugenics law, just a few hundred are still alive, according to research conducted by the Sterilization and Social Justice Lab. Including the inmates who were sterilized most recently, advocates estimate more than 600 people would be eligible for reparations.
But finding them will be difficult, with advocates predicting only about 25% of eligible people will ultimately apply for reparations and be paid.
California’s Victim Compensation Board will run the program, with $2 million used to find victims by advertising and poring through state records. The state also set aside $1 million for plaques to honor the victims, leaving $4.5 million for reparations.
A version of this article appeared on Medscape.com.
Associated Press © 2021
SACRAMENTO (AP) – California is poised to approve reparations of up to $25,000 to some of the thousands of people – some as young as 13 – who were sterilized decades ago because the government deemed them unfit to have children.
The payments will make California at least the third state – following Virginia and North Carolina – to compensate victims of the so-called eugenics movement that peaked in the 1930s. Supporters of the movement believed sterilizing people with mental illnesses, physical disabilities, and other traits they deemed undesirable would improve the human race.
While California sterilized more than 20,000 people before its law was repealed in 1979, only a few hundred are still alive. The state has set aside $7.5 million for the reparations program, part of its $262.6 billion operating budget that is awaiting Gov. Gavin Newsom’s signature.
California’s proposal is unique because it also would pay women the state coerced to get sterilized while they were in prison, some as recently as 2010. First exposed by the Center for Investigative Reporting in 2013, a subsequent audit found California sterilized 144 women between 2005 and 2013 with little or no evidence that officials counseled them or offered alternative treatment.
While all of the women signed consent forms, officials in 39 cases did not do everything that was legally required to obtain their permission.
“We must address and face our horrific history,” said Lorena Garcia Zermeño, policy and communications coordinator for the advocacy group California Latinas for Reproductive Justice. “This isn’t something that just happened in the past.”
California’s forced sterilization program started in 1909, following similar laws in Indiana and Washington. It was by far the largest program, accounting for about a third of everyone sterilized in the United States under those laws.
California’s law was so prominent that it inspired similar practices in Nazi Germany, according to Paul Lombardo, a law professor at Georgia State University, Atlanta, and an expert on the eugenics movement.
“The promise of eugenics at the very earliest is: ‘We could do away with all the state institutions – prisons, hospitals, asylums, orphanages,’” Mr. Lombardo said. “People who were in them just wouldn’t be born after awhile if you sterilized all of their parents.”
In California, victims include Mary Franco, who was sterilized in 1934 when she was just 13. Paperwork described her as “feeble minded” because of “sexual deviance,” according to her niece, Stacy Cordova, who has researched her case.
Ms. Cordova said Franco actually was molested by a neighbor. She said her family put Ms. Franco in an institution to protect the family’s reputation.
Ms. Cordova said her late aunt loved children and wanted to have a family. She married briefly when she was about 17, but Ms. Cordova said the marriage was annulled when the man discovered Ms. Franco couldn’t have children. She lived a lonely life in a Mexican culture that revered big families, Ms. Cordova said.
“I don’t know if it is justice. Money doesn’t pay for what happened to them. But it’s great to know that this is being recognized,” said Ms. Cordova, who has advocated for the state to pay survivors. “For me, this is not about the money. This is about the memory.”
Relatives like Ms. Cordova aren’t eligible for the payments, only direct victims are.
Sterilizations in California prisons appear to date to 1999, when the state changed its policy for unknown reasons to include a sterilization procedure known as “tubal ligation” as part of inmates’ medical care. Over the next decade, women reported they were coerced into this procedure, with some not fully understanding the ramifications.
A state law passed in 2014 bans sterilizations for the purpose of birth control at state prisons and local jails. The law permits sterilizations that are “medically necessary,” such as removing cancer, and requires facilities to report each year how many people were sterilized and for what reason.
Questionable sterilizations also occurred in facilities run by local governments. In 2018, the Los Angeles County Board of Supervisors apologized after more than 200 women were sterilized at the Los Angeles–University of Southern California Medical Center between 1968 and 1974.
Those people are not eligible for reparations under California’s program. But advocates say they hope to include them in the future.
“It’s only the beginning,” said state Assemblywoman Wendy Carrillo, a Democrat from Los Angeles who has been advocating for reparations. “I can’t imagine the trauma, the depression, the stress of being incarcerated, being rehabilitated and trying to start your life again in society, wanting to start a family, only to find out that that choice was taken away from you.”
Of the people California sterilized under its old eugenics law, just a few hundred are still alive, according to research conducted by the Sterilization and Social Justice Lab. Including the inmates who were sterilized most recently, advocates estimate more than 600 people would be eligible for reparations.
But finding them will be difficult, with advocates predicting only about 25% of eligible people will ultimately apply for reparations and be paid.
California’s Victim Compensation Board will run the program, with $2 million used to find victims by advertising and poring through state records. The state also set aside $1 million for plaques to honor the victims, leaving $4.5 million for reparations.
A version of this article appeared on Medscape.com.
Associated Press © 2021