User login
Altha J. Stewart, MD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Altha J. Stewart, MD. Dr. Stewart is Senior Associate Dean for Community Health Engagement at the University of Tennessee Health Science Center (UTHSC)–Memphis. She also serves as Chief of the Division of Social and Community Psychiatry and Director, Center for Health in Justice Involved Youth at UTHSC, where she manages community-based programs serving children impacted by trauma and mental illness and their families. In 2018, she was elected President of the American Psychiatric Association, the first African American individual elected in the 175-year history of the organization.
Dr. Aftab: Structural racism in academic and organized psychiatry is an issue that is close to your heart. What is your perspective on the current state of structural racism in American psychiatry, and what do you think we can do about it?
Dr. Stewart: That’s a good question to start with because I think the conversations that we need to have in academia in general and in academic psychiatry specifically really do frame the current issues that we are facing, whether we’re talking about eliminating health disparities or achieving mental health equity. Historically, from the very beginning these discussions have been structured in a racist manner. The early days of American psychiatry were very clearly directed towards maintaining a system that excluded large segments of the population of the time, since a particularly violent form of chattel slavery was being practiced in this country.
The mental health care system was primarily designed for the landowning white men of some standing in society, and so there was never any intent to do much in the way of providing quality humane service to people who were not part of that group. What we have today is a system that was designed for a racist societal structure, that was intended to perpetuate certain behaviors, policies, and practices that had at their core a racist framework. We have to acknowledge and start from this beginning point. This is not to blame anyone currently alive. These are larger structural problems. Before we can begin setting up strategic plans and other actions, we have to go back and acknowledge how we got here. We have to accept the responsibility for being here, and then we have to allow the conversations that need to happen to happen in a safe way, without further alienating people, or maligning and demeaning people who are for the most part well-intentioned but perhaps operating on automatic pilot in a system that is structurally racist.
Dr. Aftab: Do you think that the conversations that need to happen are taking place?
Dr. Stewart: Yes, I think they are beginning to happen. I do a fair number of talks and grand rounds, and what I discover when I meet with different academic departments and different groups is that most places now have a diversity committee, or the residents and students have assigned themselves as diversity leaders. They are really pushing to have these conversations, to insert these conversations into the training and education curricula. The structures in power are so deeply entrenched that many people, particularly younger people, are easily frustrated by the lack of forward motion. One of the things that seasoned leaders in psychiatry have to do is to help everyone understand that the movement forward might be glacial in the beginning, but any movement forward is good when it comes to this. The psychiatrists of my generation talked about cultural competence in psychiatry, but generations of today talk about structural competence. These are similar concepts, except that cultural competency worked within the traditional model, while structural competency recognizes that the system itself needs to change. I find this development very encouraging.
Dr. Aftab: What do you see as some of the strengths of our profession?
Continue to: Dr. Stewart
Dr. Stewart: I am a hopeful optimist when it comes to psychiatry. I have dedicated my professional life to psychiatry and specifically to community psychiatry. Throughout the time that I have practiced psychiatry, I have been encouraged that what we do as a medical specialty really does improve the quality of life for the people we serve. Situationally right now, we’re in a unique position because the COVID pandemic has laid open and then laid bare the whole issue of how we deal with psychological distress, whether it’s diagnosed mental illness or a natural, normal response to a catastrophic event. We are the experts in this. This is our sweet spot, our wheelhouse, whatever analogy you prefer. This is the moment where we assert our expertise as the leaders—not as service add-ons, not as followers, not as adjuncts, but as the leaders.
I am so impressed with the next generation of psychiatrists. They have a wonderful blend of pride and privilege at what they have been able to accomplish to get to the point where they are doctors and psychiatrists, but they have aligned that with a strong core sense of social justice, and they are moved by their responsibility to the people in the society around them.
Another strength of our profession is what we consider to be the “art” of psychiatry. That is, the way we marry the relational aspects of psychiatry with the biological, technical, and digital aspects to arrive at a happy collaboration that benefits people. It is our great skill to engage people, to interact with them therapeutically, to recognize and acknowledge the nonverbal cues. This skill will be even more important in the age of online mental health services. I’m an “old-school” therapist. I like that face-to-face interaction. I think it’s important to preserve that aspect of our practice, even as we move towards online services.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Stewart: I don’t think we can afford to remain in status quo, because we need to constantly think and rethink, evaluate and re-evaluate, assess things in the light of new information. Particularly if we’re talking about people who rely on public funding to get even the bare minimum services, status quo doesn’t cut it. It’s not good enough. I had a teacher during my residency, a child psychiatrist, who used to say, “Good, better, best. Never let it rest, until your good is better and your better is best.” Something about that has stuck with me. As my career progressed, I heard variations of it, including one from former Surgeon General of the United States David Satcher, who was not a psychiatrist, but pulled together the group that published the first Surgeon General’s report on mental health, followed by the Surgeon General’s report on mental health, culture, race, and ethnicity. He had the penetrating insight that risk factors are not to be accepted as predictive factors due to protective factors. If I am at risk for mental illness or a chronic medical condition based on my race or ethnicity or socioeconomic status or employment status, this does not mean that I am destined to experience that illness. In fact, we are not doing our job if we accept these outcomes as inevitable and make no attempt to change them. So, for me, if we accept the status quo, we give up on the message of “Good, better, best. Never let it rest, until your good is better and your better is best.”
Continue to: Dr. Aftab
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Stewart: Well, this is going to sound harsh, and I do hope that the readers do not feel that I intend it to be harsh. We get in our own way. I work in the public sector, for example, and the reality is that there aren’t enough psychiatrists to provide all the necessary psychiatric services for the people who need them. So many mental health clinics and practices employ other mental health professionals, whether they are psychologists or nurse practitioners or physician assistants with special training in mental health to provide those services. To have a blanket concern about anyone who is not an MD practicing in what is considered “our area” just begs the question that if we can’t do it and we don’t have enough psychiatrists to do it, should people just not get mental health treatment? Is that the solution? I don’t think so. I don’t think that’s what people want, either, but because of the energy that gets aroused around these issues, we lose sight of that end goal. I think the answer is that we must take leadership for ensuring that our colleagues are well-trained, maybe not as well-trained as physicians, but well-trained enough to provide good care working under our supervision.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Stewart: I think we are moving naturally into the space of integrated or collaborative care. I think we’re going to have to acknowledge that going forward, the path to being a good psychiatrist means that we will also be consultants. Not just the consultation-liaison kind of consultant that we typically think of, but a consultant to the rest of medicine around shaping programs, addressing how we treat comorbid illness, looking at ways to minimize the morbidity and mortality associated with some of the chronic medical and mental diseases. We’re moving naturally in that direction. For some people, that must be frightening. All throughout medicine people are witnessing change, and we need to adapt. I would hope that the specialty that is designed to help others deal with change will figure out how to use those skills to help themselves deal with the changes that are coming!
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Altha J. Stewart, MD. Dr. Stewart is Senior Associate Dean for Community Health Engagement at the University of Tennessee Health Science Center (UTHSC)–Memphis. She also serves as Chief of the Division of Social and Community Psychiatry and Director, Center for Health in Justice Involved Youth at UTHSC, where she manages community-based programs serving children impacted by trauma and mental illness and their families. In 2018, she was elected President of the American Psychiatric Association, the first African American individual elected in the 175-year history of the organization.
Dr. Aftab: Structural racism in academic and organized psychiatry is an issue that is close to your heart. What is your perspective on the current state of structural racism in American psychiatry, and what do you think we can do about it?
Dr. Stewart: That’s a good question to start with because I think the conversations that we need to have in academia in general and in academic psychiatry specifically really do frame the current issues that we are facing, whether we’re talking about eliminating health disparities or achieving mental health equity. Historically, from the very beginning these discussions have been structured in a racist manner. The early days of American psychiatry were very clearly directed towards maintaining a system that excluded large segments of the population of the time, since a particularly violent form of chattel slavery was being practiced in this country.
The mental health care system was primarily designed for the landowning white men of some standing in society, and so there was never any intent to do much in the way of providing quality humane service to people who were not part of that group. What we have today is a system that was designed for a racist societal structure, that was intended to perpetuate certain behaviors, policies, and practices that had at their core a racist framework. We have to acknowledge and start from this beginning point. This is not to blame anyone currently alive. These are larger structural problems. Before we can begin setting up strategic plans and other actions, we have to go back and acknowledge how we got here. We have to accept the responsibility for being here, and then we have to allow the conversations that need to happen to happen in a safe way, without further alienating people, or maligning and demeaning people who are for the most part well-intentioned but perhaps operating on automatic pilot in a system that is structurally racist.
Dr. Aftab: Do you think that the conversations that need to happen are taking place?
Dr. Stewart: Yes, I think they are beginning to happen. I do a fair number of talks and grand rounds, and what I discover when I meet with different academic departments and different groups is that most places now have a diversity committee, or the residents and students have assigned themselves as diversity leaders. They are really pushing to have these conversations, to insert these conversations into the training and education curricula. The structures in power are so deeply entrenched that many people, particularly younger people, are easily frustrated by the lack of forward motion. One of the things that seasoned leaders in psychiatry have to do is to help everyone understand that the movement forward might be glacial in the beginning, but any movement forward is good when it comes to this. The psychiatrists of my generation talked about cultural competence in psychiatry, but generations of today talk about structural competence. These are similar concepts, except that cultural competency worked within the traditional model, while structural competency recognizes that the system itself needs to change. I find this development very encouraging.
Dr. Aftab: What do you see as some of the strengths of our profession?
Continue to: Dr. Stewart
Dr. Stewart: I am a hopeful optimist when it comes to psychiatry. I have dedicated my professional life to psychiatry and specifically to community psychiatry. Throughout the time that I have practiced psychiatry, I have been encouraged that what we do as a medical specialty really does improve the quality of life for the people we serve. Situationally right now, we’re in a unique position because the COVID pandemic has laid open and then laid bare the whole issue of how we deal with psychological distress, whether it’s diagnosed mental illness or a natural, normal response to a catastrophic event. We are the experts in this. This is our sweet spot, our wheelhouse, whatever analogy you prefer. This is the moment where we assert our expertise as the leaders—not as service add-ons, not as followers, not as adjuncts, but as the leaders.
I am so impressed with the next generation of psychiatrists. They have a wonderful blend of pride and privilege at what they have been able to accomplish to get to the point where they are doctors and psychiatrists, but they have aligned that with a strong core sense of social justice, and they are moved by their responsibility to the people in the society around them.
Another strength of our profession is what we consider to be the “art” of psychiatry. That is, the way we marry the relational aspects of psychiatry with the biological, technical, and digital aspects to arrive at a happy collaboration that benefits people. It is our great skill to engage people, to interact with them therapeutically, to recognize and acknowledge the nonverbal cues. This skill will be even more important in the age of online mental health services. I’m an “old-school” therapist. I like that face-to-face interaction. I think it’s important to preserve that aspect of our practice, even as we move towards online services.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Stewart: I don’t think we can afford to remain in status quo, because we need to constantly think and rethink, evaluate and re-evaluate, assess things in the light of new information. Particularly if we’re talking about people who rely on public funding to get even the bare minimum services, status quo doesn’t cut it. It’s not good enough. I had a teacher during my residency, a child psychiatrist, who used to say, “Good, better, best. Never let it rest, until your good is better and your better is best.” Something about that has stuck with me. As my career progressed, I heard variations of it, including one from former Surgeon General of the United States David Satcher, who was not a psychiatrist, but pulled together the group that published the first Surgeon General’s report on mental health, followed by the Surgeon General’s report on mental health, culture, race, and ethnicity. He had the penetrating insight that risk factors are not to be accepted as predictive factors due to protective factors. If I am at risk for mental illness or a chronic medical condition based on my race or ethnicity or socioeconomic status or employment status, this does not mean that I am destined to experience that illness. In fact, we are not doing our job if we accept these outcomes as inevitable and make no attempt to change them. So, for me, if we accept the status quo, we give up on the message of “Good, better, best. Never let it rest, until your good is better and your better is best.”
Continue to: Dr. Aftab
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Stewart: Well, this is going to sound harsh, and I do hope that the readers do not feel that I intend it to be harsh. We get in our own way. I work in the public sector, for example, and the reality is that there aren’t enough psychiatrists to provide all the necessary psychiatric services for the people who need them. So many mental health clinics and practices employ other mental health professionals, whether they are psychologists or nurse practitioners or physician assistants with special training in mental health to provide those services. To have a blanket concern about anyone who is not an MD practicing in what is considered “our area” just begs the question that if we can’t do it and we don’t have enough psychiatrists to do it, should people just not get mental health treatment? Is that the solution? I don’t think so. I don’t think that’s what people want, either, but because of the energy that gets aroused around these issues, we lose sight of that end goal. I think the answer is that we must take leadership for ensuring that our colleagues are well-trained, maybe not as well-trained as physicians, but well-trained enough to provide good care working under our supervision.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Stewart: I think we are moving naturally into the space of integrated or collaborative care. I think we’re going to have to acknowledge that going forward, the path to being a good psychiatrist means that we will also be consultants. Not just the consultation-liaison kind of consultant that we typically think of, but a consultant to the rest of medicine around shaping programs, addressing how we treat comorbid illness, looking at ways to minimize the morbidity and mortality associated with some of the chronic medical and mental diseases. We’re moving naturally in that direction. For some people, that must be frightening. All throughout medicine people are witnessing change, and we need to adapt. I would hope that the specialty that is designed to help others deal with change will figure out how to use those skills to help themselves deal with the changes that are coming!
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Altha J. Stewart, MD. Dr. Stewart is Senior Associate Dean for Community Health Engagement at the University of Tennessee Health Science Center (UTHSC)–Memphis. She also serves as Chief of the Division of Social and Community Psychiatry and Director, Center for Health in Justice Involved Youth at UTHSC, where she manages community-based programs serving children impacted by trauma and mental illness and their families. In 2018, she was elected President of the American Psychiatric Association, the first African American individual elected in the 175-year history of the organization.
Dr. Aftab: Structural racism in academic and organized psychiatry is an issue that is close to your heart. What is your perspective on the current state of structural racism in American psychiatry, and what do you think we can do about it?
Dr. Stewart: That’s a good question to start with because I think the conversations that we need to have in academia in general and in academic psychiatry specifically really do frame the current issues that we are facing, whether we’re talking about eliminating health disparities or achieving mental health equity. Historically, from the very beginning these discussions have been structured in a racist manner. The early days of American psychiatry were very clearly directed towards maintaining a system that excluded large segments of the population of the time, since a particularly violent form of chattel slavery was being practiced in this country.
The mental health care system was primarily designed for the landowning white men of some standing in society, and so there was never any intent to do much in the way of providing quality humane service to people who were not part of that group. What we have today is a system that was designed for a racist societal structure, that was intended to perpetuate certain behaviors, policies, and practices that had at their core a racist framework. We have to acknowledge and start from this beginning point. This is not to blame anyone currently alive. These are larger structural problems. Before we can begin setting up strategic plans and other actions, we have to go back and acknowledge how we got here. We have to accept the responsibility for being here, and then we have to allow the conversations that need to happen to happen in a safe way, without further alienating people, or maligning and demeaning people who are for the most part well-intentioned but perhaps operating on automatic pilot in a system that is structurally racist.
Dr. Aftab: Do you think that the conversations that need to happen are taking place?
Dr. Stewart: Yes, I think they are beginning to happen. I do a fair number of talks and grand rounds, and what I discover when I meet with different academic departments and different groups is that most places now have a diversity committee, or the residents and students have assigned themselves as diversity leaders. They are really pushing to have these conversations, to insert these conversations into the training and education curricula. The structures in power are so deeply entrenched that many people, particularly younger people, are easily frustrated by the lack of forward motion. One of the things that seasoned leaders in psychiatry have to do is to help everyone understand that the movement forward might be glacial in the beginning, but any movement forward is good when it comes to this. The psychiatrists of my generation talked about cultural competence in psychiatry, but generations of today talk about structural competence. These are similar concepts, except that cultural competency worked within the traditional model, while structural competency recognizes that the system itself needs to change. I find this development very encouraging.
Dr. Aftab: What do you see as some of the strengths of our profession?
Continue to: Dr. Stewart
Dr. Stewart: I am a hopeful optimist when it comes to psychiatry. I have dedicated my professional life to psychiatry and specifically to community psychiatry. Throughout the time that I have practiced psychiatry, I have been encouraged that what we do as a medical specialty really does improve the quality of life for the people we serve. Situationally right now, we’re in a unique position because the COVID pandemic has laid open and then laid bare the whole issue of how we deal with psychological distress, whether it’s diagnosed mental illness or a natural, normal response to a catastrophic event. We are the experts in this. This is our sweet spot, our wheelhouse, whatever analogy you prefer. This is the moment where we assert our expertise as the leaders—not as service add-ons, not as followers, not as adjuncts, but as the leaders.
I am so impressed with the next generation of psychiatrists. They have a wonderful blend of pride and privilege at what they have been able to accomplish to get to the point where they are doctors and psychiatrists, but they have aligned that with a strong core sense of social justice, and they are moved by their responsibility to the people in the society around them.
Another strength of our profession is what we consider to be the “art” of psychiatry. That is, the way we marry the relational aspects of psychiatry with the biological, technical, and digital aspects to arrive at a happy collaboration that benefits people. It is our great skill to engage people, to interact with them therapeutically, to recognize and acknowledge the nonverbal cues. This skill will be even more important in the age of online mental health services. I’m an “old-school” therapist. I like that face-to-face interaction. I think it’s important to preserve that aspect of our practice, even as we move towards online services.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Stewart: I don’t think we can afford to remain in status quo, because we need to constantly think and rethink, evaluate and re-evaluate, assess things in the light of new information. Particularly if we’re talking about people who rely on public funding to get even the bare minimum services, status quo doesn’t cut it. It’s not good enough. I had a teacher during my residency, a child psychiatrist, who used to say, “Good, better, best. Never let it rest, until your good is better and your better is best.” Something about that has stuck with me. As my career progressed, I heard variations of it, including one from former Surgeon General of the United States David Satcher, who was not a psychiatrist, but pulled together the group that published the first Surgeon General’s report on mental health, followed by the Surgeon General’s report on mental health, culture, race, and ethnicity. He had the penetrating insight that risk factors are not to be accepted as predictive factors due to protective factors. If I am at risk for mental illness or a chronic medical condition based on my race or ethnicity or socioeconomic status or employment status, this does not mean that I am destined to experience that illness. In fact, we are not doing our job if we accept these outcomes as inevitable and make no attempt to change them. So, for me, if we accept the status quo, we give up on the message of “Good, better, best. Never let it rest, until your good is better and your better is best.”
Continue to: Dr. Aftab
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Stewart: Well, this is going to sound harsh, and I do hope that the readers do not feel that I intend it to be harsh. We get in our own way. I work in the public sector, for example, and the reality is that there aren’t enough psychiatrists to provide all the necessary psychiatric services for the people who need them. So many mental health clinics and practices employ other mental health professionals, whether they are psychologists or nurse practitioners or physician assistants with special training in mental health to provide those services. To have a blanket concern about anyone who is not an MD practicing in what is considered “our area” just begs the question that if we can’t do it and we don’t have enough psychiatrists to do it, should people just not get mental health treatment? Is that the solution? I don’t think so. I don’t think that’s what people want, either, but because of the energy that gets aroused around these issues, we lose sight of that end goal. I think the answer is that we must take leadership for ensuring that our colleagues are well-trained, maybe not as well-trained as physicians, but well-trained enough to provide good care working under our supervision.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Stewart: I think we are moving naturally into the space of integrated or collaborative care. I think we’re going to have to acknowledge that going forward, the path to being a good psychiatrist means that we will also be consultants. Not just the consultation-liaison kind of consultant that we typically think of, but a consultant to the rest of medicine around shaping programs, addressing how we treat comorbid illness, looking at ways to minimize the morbidity and mortality associated with some of the chronic medical and mental diseases. We’re moving naturally in that direction. For some people, that must be frightening. All throughout medicine people are witnessing change, and we need to adapt. I would hope that the specialty that is designed to help others deal with change will figure out how to use those skills to help themselves deal with the changes that are coming!
Lithium and kidney disease: Understand the risks
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
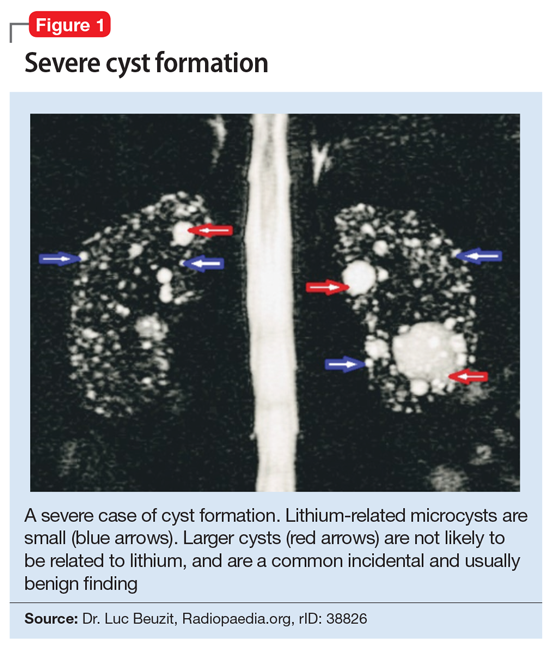
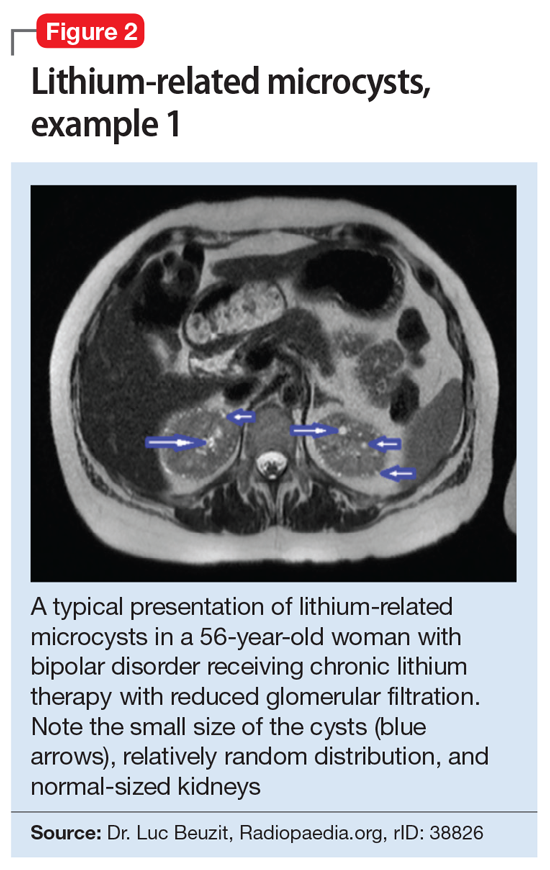
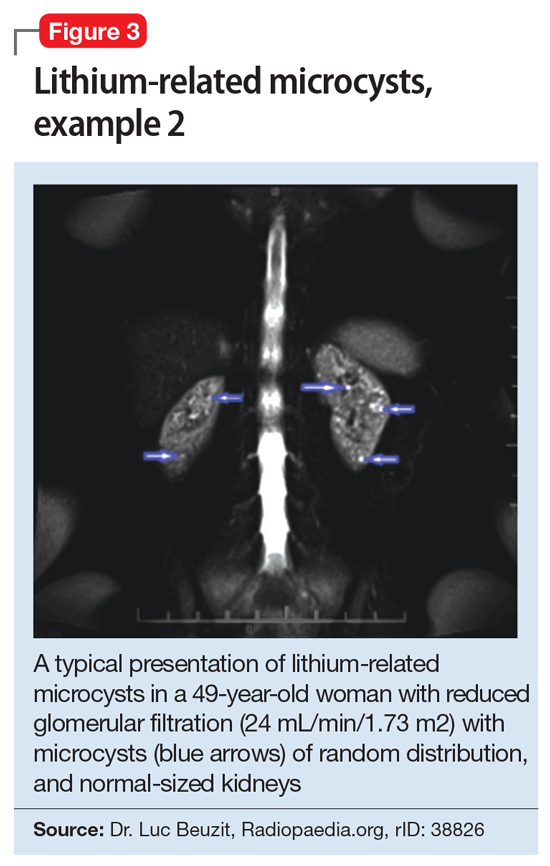
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
Managing deep infiltrating endometriosis
Deep endometriosis, including bowel endometriosis, happens in more than 10% of all women with endometriosis. Because it can be debilitating and difficult to diagnose, how is it identified?
Dr. Kho: Diagnosing deep infiltrating endometriosis (DIE) is one of the biggest challenges in the field. Currently, the gold standard is still histologic confirmation of endometriosis lesions, but I think the paradigm is shifting significantly. Advanced centers are now moving towards the use of imaging to visualize suspected endometriosis better. In skilled hands, ultrasonography and MRI can accurately detect DIE. The advantage of visualizing suspected deep endometriosis before surgery is that we can offer better counseling to patients and offer medical or surgical treatments or a combination of both. When the patient chooses surgery, we can be better prepared with a multidisciplinary team to remove the lesions completely.
The treatment of deep endometriosis can be challenging because it does not always respond to medical therapy, such as oral contraceptive pills or GnRH agonists. What treatment options have shown effectiveness?
Dr. Kho: It should be understood that we need to manage the symptoms and not the lesions. Just because there are endometriosis lesions identified does NOT mean that they need to be removed. The decision should be based on what the treatment goals are. A good discussion with the patient is important to determine what the treatment objectives are. Are we managing pain? Are we surgically treating infertility? Are we treating something else, such as bowel obstruction or blood in the urine? These questions and their answers should be first and foremost in the clinician’s mind.
Different medical treatment therapies are available and have been shown to be effective in managing endometriosis symptoms without having to immediately resort to surgery as with certain cases of suspected DIE. These medical management options include combined estrogen and progesterone contraceptive formulations, progesterone-only formulations, and GnRH agonists and antagonists. Patients should be counseled about the different options and their associated risks. Often, patients need to switch from one form to another when side effects encumber them.
If imaging or an exam reveals incidental findings of suspected deep endometriosis, it does not mean that treatment is required because some patients can be without symptoms or have no issues. But when the indication for pain or desire to achieve a pregnancy is clear, and the decision is to proceed with treatment, there are some guidelines:
- If the pain is severe and affects the patient’s daily quality of life, for example, with large, deep lesions, surgical excision appears to be effective in improving the patient’s quality of life.
- If infertility is the main indication, then other factors need to be considered. Has she had previous surgeries? What is her age? What is her ovarian reserve? What is the condition of the tubes? Is the use of artificial reproductive technology, or ART, an option for the couple? What is her cancer risk?
- Lastly, what is the extent of the lesion(s)? Both medical and surgical approaches for treatment carry risks. For surgery, complications can be severe with long-term consequences. Therefore, it is important to discuss all of this in detail with the patient.
When we understand what the main goal of the treatment is for endometriosis—such as either to treat pain or to achieve pregnancy, or both—we can tailor our surgical approach to achieve the goal(s). For example, in a patient who wants to achieve a pregnancy in the future, we may opt to do only what is necessary to remove endometriosis and relieve pain without impairing the patient’s fertility potential and overall quality of life.
Post-operatively, if the patient does not desire to become pregnant, medications to suppress the ovaries are an option to reduce the chance of disease recurrence.
What pearls can you provide when it comes to surgical techniques available and benefits for short- and long-term risks and limitations?
Dr. Kho: The surgeon should tailor his or her level of radicality to excise endometriosis to achieve the treatment goals that are mutually agreed on with the patient. For the surgeon bringing the patient into surgery, go in prepared. I strongly believe in preoperative imaging to provide the best mapping of the disease and in having other specialists or surgeons available—whether it be a urologist, a colorectal surgeon, a thoracic surgeon, a vascular surgeon, or an orthopedic surgeon (if needed)—depending on where the lesions are located.
Because we know that the best surgical outcomes come from surgeons with the highest volume, providers should be willing to refer patients with advanced disease to centers that specialize in the treatment of endometriosis. Many providers with this expertise undergo additional training to advance their skills. Advanced centers in endometriosis also provide patients with a multidisciplinary team to provide the best care.
What is the most optimal setup for a multidisciplinary team treating deep infiltrating endometriosis?
Dr. Kho: You will find that advanced centers for endometriosis will have in place a multidisciplinary team that includes a pain specialist, a surgical specialist, a pain psychologist, physical therapy, urologist, colorectal surgeon, and other sub-specialty surgeons to be able to look at the patients from all the different aspects. Often a reproductive endocrinologist or REI specialist would also be part of the group.
At our center, we have what we call a “benign tumor board,” where we discuss complex cases with representatives from different areas. This allows us to discuss the case, review what is known in the literature, and collectively develop a treatment plan.
Is there anything that was not covered in this discussion that you would like to mention?
Dr. Kho: Something that should be mentioned is that ovarian endometriosis is often associated with advanced endometriosis, often Stage 3 or 4. Clinicians should be aware that when imaging suggests the presence of ovarian endometriosis, studies show that the disease is isolated in the ovary in only 15% of cases—meaning that there is an 85% chance that endometriosis is present elsewhere in the pelvis. Therefore, the surgeon should be prepared for more extensive dissection when they bring the patient into surgery.
Deep endometriosis, including bowel endometriosis, happens in more than 10% of all women with endometriosis. Because it can be debilitating and difficult to diagnose, how is it identified?
Dr. Kho: Diagnosing deep infiltrating endometriosis (DIE) is one of the biggest challenges in the field. Currently, the gold standard is still histologic confirmation of endometriosis lesions, but I think the paradigm is shifting significantly. Advanced centers are now moving towards the use of imaging to visualize suspected endometriosis better. In skilled hands, ultrasonography and MRI can accurately detect DIE. The advantage of visualizing suspected deep endometriosis before surgery is that we can offer better counseling to patients and offer medical or surgical treatments or a combination of both. When the patient chooses surgery, we can be better prepared with a multidisciplinary team to remove the lesions completely.
The treatment of deep endometriosis can be challenging because it does not always respond to medical therapy, such as oral contraceptive pills or GnRH agonists. What treatment options have shown effectiveness?
Dr. Kho: It should be understood that we need to manage the symptoms and not the lesions. Just because there are endometriosis lesions identified does NOT mean that they need to be removed. The decision should be based on what the treatment goals are. A good discussion with the patient is important to determine what the treatment objectives are. Are we managing pain? Are we surgically treating infertility? Are we treating something else, such as bowel obstruction or blood in the urine? These questions and their answers should be first and foremost in the clinician’s mind.
Different medical treatment therapies are available and have been shown to be effective in managing endometriosis symptoms without having to immediately resort to surgery as with certain cases of suspected DIE. These medical management options include combined estrogen and progesterone contraceptive formulations, progesterone-only formulations, and GnRH agonists and antagonists. Patients should be counseled about the different options and their associated risks. Often, patients need to switch from one form to another when side effects encumber them.
If imaging or an exam reveals incidental findings of suspected deep endometriosis, it does not mean that treatment is required because some patients can be without symptoms or have no issues. But when the indication for pain or desire to achieve a pregnancy is clear, and the decision is to proceed with treatment, there are some guidelines:
- If the pain is severe and affects the patient’s daily quality of life, for example, with large, deep lesions, surgical excision appears to be effective in improving the patient’s quality of life.
- If infertility is the main indication, then other factors need to be considered. Has she had previous surgeries? What is her age? What is her ovarian reserve? What is the condition of the tubes? Is the use of artificial reproductive technology, or ART, an option for the couple? What is her cancer risk?
- Lastly, what is the extent of the lesion(s)? Both medical and surgical approaches for treatment carry risks. For surgery, complications can be severe with long-term consequences. Therefore, it is important to discuss all of this in detail with the patient.
When we understand what the main goal of the treatment is for endometriosis—such as either to treat pain or to achieve pregnancy, or both—we can tailor our surgical approach to achieve the goal(s). For example, in a patient who wants to achieve a pregnancy in the future, we may opt to do only what is necessary to remove endometriosis and relieve pain without impairing the patient’s fertility potential and overall quality of life.
Post-operatively, if the patient does not desire to become pregnant, medications to suppress the ovaries are an option to reduce the chance of disease recurrence.
What pearls can you provide when it comes to surgical techniques available and benefits for short- and long-term risks and limitations?
Dr. Kho: The surgeon should tailor his or her level of radicality to excise endometriosis to achieve the treatment goals that are mutually agreed on with the patient. For the surgeon bringing the patient into surgery, go in prepared. I strongly believe in preoperative imaging to provide the best mapping of the disease and in having other specialists or surgeons available—whether it be a urologist, a colorectal surgeon, a thoracic surgeon, a vascular surgeon, or an orthopedic surgeon (if needed)—depending on where the lesions are located.
Because we know that the best surgical outcomes come from surgeons with the highest volume, providers should be willing to refer patients with advanced disease to centers that specialize in the treatment of endometriosis. Many providers with this expertise undergo additional training to advance their skills. Advanced centers in endometriosis also provide patients with a multidisciplinary team to provide the best care.
What is the most optimal setup for a multidisciplinary team treating deep infiltrating endometriosis?
Dr. Kho: You will find that advanced centers for endometriosis will have in place a multidisciplinary team that includes a pain specialist, a surgical specialist, a pain psychologist, physical therapy, urologist, colorectal surgeon, and other sub-specialty surgeons to be able to look at the patients from all the different aspects. Often a reproductive endocrinologist or REI specialist would also be part of the group.
At our center, we have what we call a “benign tumor board,” where we discuss complex cases with representatives from different areas. This allows us to discuss the case, review what is known in the literature, and collectively develop a treatment plan.
Is there anything that was not covered in this discussion that you would like to mention?
Dr. Kho: Something that should be mentioned is that ovarian endometriosis is often associated with advanced endometriosis, often Stage 3 or 4. Clinicians should be aware that when imaging suggests the presence of ovarian endometriosis, studies show that the disease is isolated in the ovary in only 15% of cases—meaning that there is an 85% chance that endometriosis is present elsewhere in the pelvis. Therefore, the surgeon should be prepared for more extensive dissection when they bring the patient into surgery.
Deep endometriosis, including bowel endometriosis, happens in more than 10% of all women with endometriosis. Because it can be debilitating and difficult to diagnose, how is it identified?
Dr. Kho: Diagnosing deep infiltrating endometriosis (DIE) is one of the biggest challenges in the field. Currently, the gold standard is still histologic confirmation of endometriosis lesions, but I think the paradigm is shifting significantly. Advanced centers are now moving towards the use of imaging to visualize suspected endometriosis better. In skilled hands, ultrasonography and MRI can accurately detect DIE. The advantage of visualizing suspected deep endometriosis before surgery is that we can offer better counseling to patients and offer medical or surgical treatments or a combination of both. When the patient chooses surgery, we can be better prepared with a multidisciplinary team to remove the lesions completely.
The treatment of deep endometriosis can be challenging because it does not always respond to medical therapy, such as oral contraceptive pills or GnRH agonists. What treatment options have shown effectiveness?
Dr. Kho: It should be understood that we need to manage the symptoms and not the lesions. Just because there are endometriosis lesions identified does NOT mean that they need to be removed. The decision should be based on what the treatment goals are. A good discussion with the patient is important to determine what the treatment objectives are. Are we managing pain? Are we surgically treating infertility? Are we treating something else, such as bowel obstruction or blood in the urine? These questions and their answers should be first and foremost in the clinician’s mind.
Different medical treatment therapies are available and have been shown to be effective in managing endometriosis symptoms without having to immediately resort to surgery as with certain cases of suspected DIE. These medical management options include combined estrogen and progesterone contraceptive formulations, progesterone-only formulations, and GnRH agonists and antagonists. Patients should be counseled about the different options and their associated risks. Often, patients need to switch from one form to another when side effects encumber them.
If imaging or an exam reveals incidental findings of suspected deep endometriosis, it does not mean that treatment is required because some patients can be without symptoms or have no issues. But when the indication for pain or desire to achieve a pregnancy is clear, and the decision is to proceed with treatment, there are some guidelines:
- If the pain is severe and affects the patient’s daily quality of life, for example, with large, deep lesions, surgical excision appears to be effective in improving the patient’s quality of life.
- If infertility is the main indication, then other factors need to be considered. Has she had previous surgeries? What is her age? What is her ovarian reserve? What is the condition of the tubes? Is the use of artificial reproductive technology, or ART, an option for the couple? What is her cancer risk?
- Lastly, what is the extent of the lesion(s)? Both medical and surgical approaches for treatment carry risks. For surgery, complications can be severe with long-term consequences. Therefore, it is important to discuss all of this in detail with the patient.
When we understand what the main goal of the treatment is for endometriosis—such as either to treat pain or to achieve pregnancy, or both—we can tailor our surgical approach to achieve the goal(s). For example, in a patient who wants to achieve a pregnancy in the future, we may opt to do only what is necessary to remove endometriosis and relieve pain without impairing the patient’s fertility potential and overall quality of life.
Post-operatively, if the patient does not desire to become pregnant, medications to suppress the ovaries are an option to reduce the chance of disease recurrence.
What pearls can you provide when it comes to surgical techniques available and benefits for short- and long-term risks and limitations?
Dr. Kho: The surgeon should tailor his or her level of radicality to excise endometriosis to achieve the treatment goals that are mutually agreed on with the patient. For the surgeon bringing the patient into surgery, go in prepared. I strongly believe in preoperative imaging to provide the best mapping of the disease and in having other specialists or surgeons available—whether it be a urologist, a colorectal surgeon, a thoracic surgeon, a vascular surgeon, or an orthopedic surgeon (if needed)—depending on where the lesions are located.
Because we know that the best surgical outcomes come from surgeons with the highest volume, providers should be willing to refer patients with advanced disease to centers that specialize in the treatment of endometriosis. Many providers with this expertise undergo additional training to advance their skills. Advanced centers in endometriosis also provide patients with a multidisciplinary team to provide the best care.
What is the most optimal setup for a multidisciplinary team treating deep infiltrating endometriosis?
Dr. Kho: You will find that advanced centers for endometriosis will have in place a multidisciplinary team that includes a pain specialist, a surgical specialist, a pain psychologist, physical therapy, urologist, colorectal surgeon, and other sub-specialty surgeons to be able to look at the patients from all the different aspects. Often a reproductive endocrinologist or REI specialist would also be part of the group.
At our center, we have what we call a “benign tumor board,” where we discuss complex cases with representatives from different areas. This allows us to discuss the case, review what is known in the literature, and collectively develop a treatment plan.
Is there anything that was not covered in this discussion that you would like to mention?
Dr. Kho: Something that should be mentioned is that ovarian endometriosis is often associated with advanced endometriosis, often Stage 3 or 4. Clinicians should be aware that when imaging suggests the presence of ovarian endometriosis, studies show that the disease is isolated in the ovary in only 15% of cases—meaning that there is an 85% chance that endometriosis is present elsewhere in the pelvis. Therefore, the surgeon should be prepared for more extensive dissection when they bring the patient into surgery.
What’s lost, what’s saved
DDW is now history. While rejoicing that DDW happened (as opposed to when it couldn’t in 2020), the virtual format precluded all those hallway conversations, meetings with mentors and small group (after hour) discussions. This year, AGA saved substantial monies in travel costs. Of note, at Michigan Medicine, we track the miles patients did not have to travel because of our conversion to virtual care (currently about 30% of all ambulatory visits). To date, our “virtual first” protocol has saved over 24 million patient travel-miles since February 2020 (average of 62 miles per patient visit).
The pandemic forced rapid adoption of virtual care and alternative care delivery models. As patients adapted to telehealth, businesses saw opportunities. Health systems have begun to downsize their brick-and-mortar footprints for both clinical and office space. Hospital at Home models are developing as viable alternatives to inpatient care using a hybrid system of on-site nurses and remote physician supervision.
Digital health start-ups are developing rapidly, and equity funding for digital health companies has reached an all-time high of $26.5 billion in 2020. Multiple companies went public through traditional initial public offerings or special purpose acquisition companies. Sameer Berry, MD, recently collected an inventory of major GI digital health companies counted at least 16 with more appearing each month. These companies focus on management of a single condition (for example IBS or Celiac) or full-service virtual GI care that includes “at-risk” financial contracts
I am delighted to announce that Megan Adams, MD, JD, MSc, has been chosen to be the fourth editor in chief of GI & Hepatology News. She and her team will transition into editorial control during Fall 2021. I have known Megan since meeting her at an AGA young faculty function almost 10 years ago. She is extremely talented and knowledgeable about gastroenterology from a variety of viewpoints. She has recruited a strong and dedicated editorial board.
I have enjoyed the last 5 years leading the current board as we have brought breaking news to the GI community. I wish to publicly thank our editorial board and the Frontline staff who monthly publish AGA’s official newspaper.
John I. Allen, MD, MBA, AGAF
Editor in Chief
DDW is now history. While rejoicing that DDW happened (as opposed to when it couldn’t in 2020), the virtual format precluded all those hallway conversations, meetings with mentors and small group (after hour) discussions. This year, AGA saved substantial monies in travel costs. Of note, at Michigan Medicine, we track the miles patients did not have to travel because of our conversion to virtual care (currently about 30% of all ambulatory visits). To date, our “virtual first” protocol has saved over 24 million patient travel-miles since February 2020 (average of 62 miles per patient visit).
The pandemic forced rapid adoption of virtual care and alternative care delivery models. As patients adapted to telehealth, businesses saw opportunities. Health systems have begun to downsize their brick-and-mortar footprints for both clinical and office space. Hospital at Home models are developing as viable alternatives to inpatient care using a hybrid system of on-site nurses and remote physician supervision.
Digital health start-ups are developing rapidly, and equity funding for digital health companies has reached an all-time high of $26.5 billion in 2020. Multiple companies went public through traditional initial public offerings or special purpose acquisition companies. Sameer Berry, MD, recently collected an inventory of major GI digital health companies counted at least 16 with more appearing each month. These companies focus on management of a single condition (for example IBS or Celiac) or full-service virtual GI care that includes “at-risk” financial contracts
I am delighted to announce that Megan Adams, MD, JD, MSc, has been chosen to be the fourth editor in chief of GI & Hepatology News. She and her team will transition into editorial control during Fall 2021. I have known Megan since meeting her at an AGA young faculty function almost 10 years ago. She is extremely talented and knowledgeable about gastroenterology from a variety of viewpoints. She has recruited a strong and dedicated editorial board.
I have enjoyed the last 5 years leading the current board as we have brought breaking news to the GI community. I wish to publicly thank our editorial board and the Frontline staff who monthly publish AGA’s official newspaper.
John I. Allen, MD, MBA, AGAF
Editor in Chief
DDW is now history. While rejoicing that DDW happened (as opposed to when it couldn’t in 2020), the virtual format precluded all those hallway conversations, meetings with mentors and small group (after hour) discussions. This year, AGA saved substantial monies in travel costs. Of note, at Michigan Medicine, we track the miles patients did not have to travel because of our conversion to virtual care (currently about 30% of all ambulatory visits). To date, our “virtual first” protocol has saved over 24 million patient travel-miles since February 2020 (average of 62 miles per patient visit).
The pandemic forced rapid adoption of virtual care and alternative care delivery models. As patients adapted to telehealth, businesses saw opportunities. Health systems have begun to downsize their brick-and-mortar footprints for both clinical and office space. Hospital at Home models are developing as viable alternatives to inpatient care using a hybrid system of on-site nurses and remote physician supervision.
Digital health start-ups are developing rapidly, and equity funding for digital health companies has reached an all-time high of $26.5 billion in 2020. Multiple companies went public through traditional initial public offerings or special purpose acquisition companies. Sameer Berry, MD, recently collected an inventory of major GI digital health companies counted at least 16 with more appearing each month. These companies focus on management of a single condition (for example IBS or Celiac) or full-service virtual GI care that includes “at-risk” financial contracts
I am delighted to announce that Megan Adams, MD, JD, MSc, has been chosen to be the fourth editor in chief of GI & Hepatology News. She and her team will transition into editorial control during Fall 2021. I have known Megan since meeting her at an AGA young faculty function almost 10 years ago. She is extremely talented and knowledgeable about gastroenterology from a variety of viewpoints. She has recruited a strong and dedicated editorial board.
I have enjoyed the last 5 years leading the current board as we have brought breaking news to the GI community. I wish to publicly thank our editorial board and the Frontline staff who monthly publish AGA’s official newspaper.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Optimal Treatment for HR+/HER2- Breast Cancer Patients With Visceral Metastasis
In the past, chemotherapy was the go-to treatment for patients with HR+/HER2- breast cancer with visceral metastases, which most commonly involve lung and liver. Dr Adam M. Brufsky, of the University of Pittsburgh School of Medicine, reports that the advent of CDK4/6 inhibitors offers an opportunity for several different courses of action appropriate to subgroups within this population of patients.
Three CDK4/6 inhibitors are FDA approved for clinical practice. Dr Brufsky summarizes results from three studies of CDK4/6 inhibitors — abemaciclib, ribociclib, and palbociclib — where these agents were used as second-line therapy after progression on a nonsteroidal aromatase inhibitor. In combination with fulvestrant, all three offered significant benefits in progression-free survival (PFS) and two in overall survival (OS). Among those who derived survival benefit were patients who had visceral metatases, who constituted 56%, 27% and 60% of the respective studies.
For patients who show progression on CDK4/6 inhibitors, additional treatment can be determined by genomic sequencing. Combination therapy with alpelisib and fulvestrant has been shown to lengthen PFS in patients whose tumors have PIK3 mutations. Patients without PIK3 mutations can be treated with fulvestrant or a combination of fulvestrant and everolimus.
--
Adam M. Brufsky, MD, PhD, Professor, Department of Medicine, Division of Hematology-Oncology, University of Pittsburgh School of Medicine; Associate Chief, Department of Medicine, Division of Hematology-Oncology, UPMC-Hillman Cancer Center, Pittsburgh, Pennsylvania.
Adam M. Brufsky, MD, PhD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Seattle Genetics; Roche; AstraZeneca; Puma; Daiichi Sankyo.
In the past, chemotherapy was the go-to treatment for patients with HR+/HER2- breast cancer with visceral metastases, which most commonly involve lung and liver. Dr Adam M. Brufsky, of the University of Pittsburgh School of Medicine, reports that the advent of CDK4/6 inhibitors offers an opportunity for several different courses of action appropriate to subgroups within this population of patients.
Three CDK4/6 inhibitors are FDA approved for clinical practice. Dr Brufsky summarizes results from three studies of CDK4/6 inhibitors — abemaciclib, ribociclib, and palbociclib — where these agents were used as second-line therapy after progression on a nonsteroidal aromatase inhibitor. In combination with fulvestrant, all three offered significant benefits in progression-free survival (PFS) and two in overall survival (OS). Among those who derived survival benefit were patients who had visceral metatases, who constituted 56%, 27% and 60% of the respective studies.
For patients who show progression on CDK4/6 inhibitors, additional treatment can be determined by genomic sequencing. Combination therapy with alpelisib and fulvestrant has been shown to lengthen PFS in patients whose tumors have PIK3 mutations. Patients without PIK3 mutations can be treated with fulvestrant or a combination of fulvestrant and everolimus.
--
Adam M. Brufsky, MD, PhD, Professor, Department of Medicine, Division of Hematology-Oncology, University of Pittsburgh School of Medicine; Associate Chief, Department of Medicine, Division of Hematology-Oncology, UPMC-Hillman Cancer Center, Pittsburgh, Pennsylvania.
Adam M. Brufsky, MD, PhD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Seattle Genetics; Roche; AstraZeneca; Puma; Daiichi Sankyo.
In the past, chemotherapy was the go-to treatment for patients with HR+/HER2- breast cancer with visceral metastases, which most commonly involve lung and liver. Dr Adam M. Brufsky, of the University of Pittsburgh School of Medicine, reports that the advent of CDK4/6 inhibitors offers an opportunity for several different courses of action appropriate to subgroups within this population of patients.
Three CDK4/6 inhibitors are FDA approved for clinical practice. Dr Brufsky summarizes results from three studies of CDK4/6 inhibitors — abemaciclib, ribociclib, and palbociclib — where these agents were used as second-line therapy after progression on a nonsteroidal aromatase inhibitor. In combination with fulvestrant, all three offered significant benefits in progression-free survival (PFS) and two in overall survival (OS). Among those who derived survival benefit were patients who had visceral metatases, who constituted 56%, 27% and 60% of the respective studies.
For patients who show progression on CDK4/6 inhibitors, additional treatment can be determined by genomic sequencing. Combination therapy with alpelisib and fulvestrant has been shown to lengthen PFS in patients whose tumors have PIK3 mutations. Patients without PIK3 mutations can be treated with fulvestrant or a combination of fulvestrant and everolimus.
--
Adam M. Brufsky, MD, PhD, Professor, Department of Medicine, Division of Hematology-Oncology, University of Pittsburgh School of Medicine; Associate Chief, Department of Medicine, Division of Hematology-Oncology, UPMC-Hillman Cancer Center, Pittsburgh, Pennsylvania.
Adam M. Brufsky, MD, PhD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Seattle Genetics; Roche; AstraZeneca; Puma; Daiichi Sankyo.

Storing patients’ credit card information: Keep it safe
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
Credit cards have made it easier for psychiatrists who work in outpatient settings to collect payment for their services. Accepting credit cards saves time in sessions for clinical matters, leads to higher rates of collecting payments for patients who do not show up for appointments, and avoids having to manage bounced checks and collection agencies.1 No federal or state laws prohibit businesses from storing consumers’ credit card information. However, psychiatric practices are legally obligated to have safeguards in place to protect sensitive information and limit liability exposures.2 There are several steps to take when storing your patients’ credit card information.
Establish a payment policy. Create a policy that outlines your practice’s credit card procedures, including when credit cards will be charged and under what circumstances, how patients will be notified, and how credit card information will be stored.2 Give your patients a copy of this policy and review it with them at their first appointment and any time you change this policy.2 Get consent from your patients before using and storing their credit card information.2
Use secure methods to store this information. Most medical practices photocopy/write down their patients’ credit card information and store it in the patient’s electronic/paper medical record, or they use an online service to store it electronically.2 Online services usually provide a higher level of protection than the patient’s medical record.2 Ensure that electronic data that includes credit card numbers is robustly encrypted, or that paper records are locked in a secure place, such as in a safe or file drawer that requires a key/combination lock.3 Payment Card Industry (PCI) regulations prohibit storing a credit card’s security code (a three- or four-digit number on the front or back of the card).3 This code is used to allow merchants to verify whether a customer authorizing a transaction over the phone or via the internet physically possesses the card.3 PCI regulations also prohibit storing data contained in the card’s magnetic strip.3 This data contains information about the account that is not displayed on the card, assists with authorizing transactions, and ensures that credit cards cannot be easily counterfeited.3
Understand all federal and state laws and regulations. If your practice collects patient billing information, you are considered a “merchant” and are subject to federal and state laws and regulations that protect consumer credit card information.2 These laws and regulations include (but are not limited to)2:
- Health Insurance Portability and Accountability Act (HIPAA) and similar state privacy laws
- Federal Trade Commission Act (FTCA) and similar state business laws
- Payment Card Industry Data Security Standard (PCI DSS), which was not devised by federal or state government.
HIPAA and state privacy laws require psychiatrists to implement “reasonable” security measures to protect payment information, regardless of how that information is stored.2,4 Because HIPAA does not define “reasonable,” psychiatrists have latitude in determining which security measures to implement.2,4 Locking the information in a file cabinet and locking the room where the file cabinet is kept (for paper storage) or using HIPAA-compliant encrypted storage programs (for electronic storage) are examples of “reasonable” security measures.2
FTCA requires businesses to use “appropriate” and “reasonable” security measures to protect credit card information.2,5 Because FTCA does not specify these terms,2,5 psychiatrists have leeway in determining which security measures to implement. Federal law requires all businesses to delete a card’s expiration date and shorten the account information to include no more than the last 5 digits of the card number that is printed on all sales receipts.6 FTCA also requires businesses to get prior authorization from individuals before charging their credit card.2 For example, if a patient previously used a credit card to pay for a session, the psychiatrist cannot later use the credit card to charge for a missed appointment without notifying the patient and getting their authorization.2
PCI DSS applies to entities that store, process, and/or transmit cardholder data.7 Any business that accepts credit card payments must comply with PCI DSS, which includes 12 requirements.7 Examples of these requirements include using firewalls to protect cardholder data and restricting access to cardholder data to a “need-to-know” basis. Businesses that do not comply with PCI DSS can be subjected to fines and/or have their contracts terminated by the credit card companies.2 Fines can range from $5,000 to $100,000 per month for data breaches where you are found negligible.1
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
1. Braslow K. Benefits and costs of accepting credit cards in your practice. Current Psychiatry. 2017;16(5):17,29.
2. Wertheimer M. Keeping patient credit card and payment information on file. Psychiatric News. 2019;54(11):8.
3. Hephner L. 5 tips for proper handling of credit card information. Accessed April 22, 2020. https://paysimple.com/blog/5-tips-for-proper-handling-of-customer-credit-card-account-information/
4. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
5. Federal Trade Commission Act of 1914. 15 U.S.C. §§ 41-58, as amended (1914).
6. Federal Trade Commission. Slip showing? Federal law requires all businesses to truncate credit card information on receipts. Accessed April 22, 2020. https://www.ftc.gov/tips-advice/business-center/guidance/slip-showing-federal-law-requires-all-businesses-truncate
7. PCI Security Standards Council. Accessed April 22, 2020. https://www.pcisecuritystandards.org/
Harassment of health care workers: A survey
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
During the course of my residency training, I have experienced and witnessed patients and visitors harassing health care workers (HCWs) by cursing or directing racial slurs at them, making sexist comments, or threatening their lives. What should be the correct response to this harassment? To say nothing may avoid conflict, but the silence perpetuates such abuse. To speak up may provoke aggression or even a physical assault. Further, does our response change if it is not the patient but someone who is accompanying them who exhibits this behavior?
I conducted a survey of psychiatry HCWs at our institution to evaluate the prevalence of and factors associated with such harassment.
An all-too-common problem
In a December 2020 internal survey at the University of Missouri Department of Psychiatry, 59 of 158 HCWs responded, and 26 (44%) reported experiencing or witnessing on-the-job harassment or abuse. Factors that were statistically significantly associated with experiencing or witnessing on-the-job harassment or abuse included being non-White, working in a patient-facing position, and being a nonphysician patient-facing HCW (Table 1). Factors that were not significantly associated with experiencing or witnessing on-the-job harassment or abuse included clinical setting, HCW age, and HCW gender (Table 2).
In addition to comments from patients and visitors, respondents stated that the harassment or abuse also included:
- physically threatening behavior and assault
- reporting a HCW for HIPAA (Health Insurance Portability and Accountability Act) violations after the HCW declined to provide an early refill of a controlled substance
- being accused of being a bad person for declining to prescribe a specific medication
- insults about not being intelligent enough to be on the treatment team
- comments from colleagues.
At the most basic level of response, the emergency department (ED) remains under the Emergency Medical Treatment and Labor Act (EMTALA) obligation to see, screen, and stabilize any patient, and if psychiatry is consulted in the ED, we should similarly provide this standard of care. Beyond this, we can create behavioral plans for when a relevant diagnosis exists or does not exist, and patients and/or visitors can be terminated from their stay at the location/service/health care system. Whether or not a patient is receiving psychiatric care and/or treatment is irrelevant to the responses to harassment we might consider.
During the incident itself, we are empowered to remove ourselves from the patient encounter. Historically, HCWs have had strong opinions on the next steps, either deciding, “Yes, I am a professional and I will not be bullied,” or “No, I am a professional and I don’t need to deal with this.” Just as we prioritize our patients’ dignities, we should also respect our own and our colleagues’ dignities.
How harassment is handled at our facility
HCWs are commonly unsure whether to “call out” abusive comments during the encounter itself or afterwards. In our hospital, HCWs are encouraged to independently choose to immediately respond, immediately report to a supervisor or hospital security, or defer and report to leadership afterwards via the Patient Safety Network (PSN). The PSN is our hospital’s reporting system for medical errors, near misses, and abuse, neglect, and workplace violence. Relevant examples of abuse, neglect, and workplace violence include:
- Threats. Expression of intent to cause harm, including verbal or written threats and threatening body language
- Physical assault. Attacks ranging from slapping and beating to rape, the use of weapons, or homicide
- Sexual assault. Any type of sexual contact or behavior that occurs without the explicit consent of the recipient, such as forced sexual intercourse, forcible sodomy, child molestation, incest, fondling, and attempted rape.
Continue to: Once complete...
Once complete, the PSN report is sent to Risk Management and other relevant groups, such as a 5-person team of security investigators, who are trained in trauma-informed interviewing and re-directive techniques. This team can immediately speak to the patient face-to-face in the inpatient setting or follow-up via phone in the outpatient setting.
The PSN report may result in the creation of a behavior plan for the patient that outlines the behaviors of concern, staff interventions, and consequences for persistent violations. The behavior plan is saved in the patient’s medical chart, and an alert pops up every time the chart is opened. The behavior plan is reviewed once annually for revision or deletion, as appropriate.
Lessons from our facility’s policy
In our health care system, our primary response to HCW harassment is to create a patient behavior plan that lays out specific expectations, care parameters, and consequences (up to terminating a patient from the entire health care system, except for EMTALA-level care). Clinicians are encouraged to report harassment to hospital administration, and a team of security investigators discusses expectations with the patient and/or visitors to prevent further abuse. We believe that describing our policies may be helpful to other health care systems and HCWs who confront this widespread issue.
Garbage out: How much trash does a Mohs surgery practice produce?
left behind after surgical procedures. Their findings: Just two physicians – a surgeon and a surgical fellow – manage to produce nearly a ton of noncontaminated surgical waste annually even though they only see patients twice a week.
“While our emissions as Mohs surgeons are relatively small compared to other types of surgeries, we still emit a notable amount of greenhouse gases compared to nonmedical fields. Mohs surgeons tend to produce the most noncontaminated waste versus other categories, and that’s the category that could be most recyclable,” said Mohs surgeon Simon S. Yoo, MD, of Northwestern University, Chicago, who presented the results at the annual meeting of the American College of Mohs Surgery.
Dr. Yoo, who spoke in an interview, said the coronavirus pandemic spurred the waste analysis. “In the past year, there seemed to be many questions as to the environmental causes and impacts of the pandemic,” he said. “We decided to investigate the environmental impact of Mohs surgery.”
He and surgical fellow Alvin Li, MD, analyzed all waste produced by their clinic over a 3-week period when 106 procedures were performed. They discovered that the surgeries produced 25.8 kg of biohazardous waste (29%), 2.2 kg of packaging waste (3%), 56.4 kg of noncontaminated waste (63%), and 7.5 kg of sharps waste (8%).
“The majority of the waste we produced was noncontaminated and possibly recyclable,” Dr. Yoo said. “However, most of this waste and its packaging did not have clear recycling instructions and presented a significant barrier to recycling by our staff.”
The study authors extrapolated the waste amount to annual totals of 413.5 kg of biohazardous waste, 34.9 kg of packaging waste, 902.3 kg of noncontaminated waste, and 119.9 kg of sharps waste. That adds up to 1,471 kg. The total of noncontaminated waste is the equivalent of nearly 2,000 pounds – a ton.
Dr. Yoo and Dr. Li estimate that the waste produced annual emissions equal to 6.5 metric tons of carbon dioxide equivalent. They estimate that the amount of emissions produced by Mohs surgeons nationally each year is 7,592 metric tons of carbon dioxide equivalent, equal to emissions produced by 19 million miles of passenger automobile travel.
Still, Dr. Yoo said, Mohs surgeries appear to produce fewer emissions than some other operations. “We estimate that an individual Mohs procedure generates around 10 kg of carbon dioxide equivalent whereas a single hysterectomy generates about 380 kg; much of this is due to the use of volatile anesthetics.”
Environmental protection advocate Mary Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, urged colleagues to launch a similar waste-weighing project in their own clinics. “I challenge dermatologists to take a bag of your daily plastic waste and weigh it,” she said. “We’ll all be astounded by how much we throw away each day. Until you do that experiment yourself, you’ll have a hard time getting your arms around how much plastic we’re using.”
Dr. Maloney, a member of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues, urged colleagues to consider strategies to reduce plastic use specifically. “Look at everything you use and see if there’s a nonplastic equivalent,” she said. Even reducing the use of plastic writing pens can make a difference, she said, as can cutting back on syringes and revising procedures so gloves don’t have to be changed as often.
No study funding was reported. Dr. Yoo and Dr. Maloney report no disclosures.
left behind after surgical procedures. Their findings: Just two physicians – a surgeon and a surgical fellow – manage to produce nearly a ton of noncontaminated surgical waste annually even though they only see patients twice a week.
“While our emissions as Mohs surgeons are relatively small compared to other types of surgeries, we still emit a notable amount of greenhouse gases compared to nonmedical fields. Mohs surgeons tend to produce the most noncontaminated waste versus other categories, and that’s the category that could be most recyclable,” said Mohs surgeon Simon S. Yoo, MD, of Northwestern University, Chicago, who presented the results at the annual meeting of the American College of Mohs Surgery.
Dr. Yoo, who spoke in an interview, said the coronavirus pandemic spurred the waste analysis. “In the past year, there seemed to be many questions as to the environmental causes and impacts of the pandemic,” he said. “We decided to investigate the environmental impact of Mohs surgery.”
He and surgical fellow Alvin Li, MD, analyzed all waste produced by their clinic over a 3-week period when 106 procedures were performed. They discovered that the surgeries produced 25.8 kg of biohazardous waste (29%), 2.2 kg of packaging waste (3%), 56.4 kg of noncontaminated waste (63%), and 7.5 kg of sharps waste (8%).
“The majority of the waste we produced was noncontaminated and possibly recyclable,” Dr. Yoo said. “However, most of this waste and its packaging did not have clear recycling instructions and presented a significant barrier to recycling by our staff.”
The study authors extrapolated the waste amount to annual totals of 413.5 kg of biohazardous waste, 34.9 kg of packaging waste, 902.3 kg of noncontaminated waste, and 119.9 kg of sharps waste. That adds up to 1,471 kg. The total of noncontaminated waste is the equivalent of nearly 2,000 pounds – a ton.
Dr. Yoo and Dr. Li estimate that the waste produced annual emissions equal to 6.5 metric tons of carbon dioxide equivalent. They estimate that the amount of emissions produced by Mohs surgeons nationally each year is 7,592 metric tons of carbon dioxide equivalent, equal to emissions produced by 19 million miles of passenger automobile travel.
Still, Dr. Yoo said, Mohs surgeries appear to produce fewer emissions than some other operations. “We estimate that an individual Mohs procedure generates around 10 kg of carbon dioxide equivalent whereas a single hysterectomy generates about 380 kg; much of this is due to the use of volatile anesthetics.”
Environmental protection advocate Mary Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, urged colleagues to launch a similar waste-weighing project in their own clinics. “I challenge dermatologists to take a bag of your daily plastic waste and weigh it,” she said. “We’ll all be astounded by how much we throw away each day. Until you do that experiment yourself, you’ll have a hard time getting your arms around how much plastic we’re using.”
Dr. Maloney, a member of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues, urged colleagues to consider strategies to reduce plastic use specifically. “Look at everything you use and see if there’s a nonplastic equivalent,” she said. Even reducing the use of plastic writing pens can make a difference, she said, as can cutting back on syringes and revising procedures so gloves don’t have to be changed as often.
No study funding was reported. Dr. Yoo and Dr. Maloney report no disclosures.
left behind after surgical procedures. Their findings: Just two physicians – a surgeon and a surgical fellow – manage to produce nearly a ton of noncontaminated surgical waste annually even though they only see patients twice a week.
“While our emissions as Mohs surgeons are relatively small compared to other types of surgeries, we still emit a notable amount of greenhouse gases compared to nonmedical fields. Mohs surgeons tend to produce the most noncontaminated waste versus other categories, and that’s the category that could be most recyclable,” said Mohs surgeon Simon S. Yoo, MD, of Northwestern University, Chicago, who presented the results at the annual meeting of the American College of Mohs Surgery.
Dr. Yoo, who spoke in an interview, said the coronavirus pandemic spurred the waste analysis. “In the past year, there seemed to be many questions as to the environmental causes and impacts of the pandemic,” he said. “We decided to investigate the environmental impact of Mohs surgery.”
He and surgical fellow Alvin Li, MD, analyzed all waste produced by their clinic over a 3-week period when 106 procedures were performed. They discovered that the surgeries produced 25.8 kg of biohazardous waste (29%), 2.2 kg of packaging waste (3%), 56.4 kg of noncontaminated waste (63%), and 7.5 kg of sharps waste (8%).
“The majority of the waste we produced was noncontaminated and possibly recyclable,” Dr. Yoo said. “However, most of this waste and its packaging did not have clear recycling instructions and presented a significant barrier to recycling by our staff.”
The study authors extrapolated the waste amount to annual totals of 413.5 kg of biohazardous waste, 34.9 kg of packaging waste, 902.3 kg of noncontaminated waste, and 119.9 kg of sharps waste. That adds up to 1,471 kg. The total of noncontaminated waste is the equivalent of nearly 2,000 pounds – a ton.
Dr. Yoo and Dr. Li estimate that the waste produced annual emissions equal to 6.5 metric tons of carbon dioxide equivalent. They estimate that the amount of emissions produced by Mohs surgeons nationally each year is 7,592 metric tons of carbon dioxide equivalent, equal to emissions produced by 19 million miles of passenger automobile travel.
Still, Dr. Yoo said, Mohs surgeries appear to produce fewer emissions than some other operations. “We estimate that an individual Mohs procedure generates around 10 kg of carbon dioxide equivalent whereas a single hysterectomy generates about 380 kg; much of this is due to the use of volatile anesthetics.”
Environmental protection advocate Mary Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, urged colleagues to launch a similar waste-weighing project in their own clinics. “I challenge dermatologists to take a bag of your daily plastic waste and weigh it,” she said. “We’ll all be astounded by how much we throw away each day. Until you do that experiment yourself, you’ll have a hard time getting your arms around how much plastic we’re using.”
Dr. Maloney, a member of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues, urged colleagues to consider strategies to reduce plastic use specifically. “Look at everything you use and see if there’s a nonplastic equivalent,” she said. Even reducing the use of plastic writing pens can make a difference, she said, as can cutting back on syringes and revising procedures so gloves don’t have to be changed as often.
No study funding was reported. Dr. Yoo and Dr. Maloney report no disclosures.
FROM THE ACMS ANNUAL MEETING
How early can laser treatment for port wine stains in infants be initiated?
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
FROM ASLMS 2021
Private practice: The basics for psychiatry trainees
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
Many psychiatry trainees consider private practice as a career option or form of supplemental income. In my experience, however, residency training may provide limited introduction to the general steps involved in starting a practice. In this article, I briefly summarize what I learned while exploring the private practice option as a psychiatry resident.
A good specialty for private practice
Trainees in the earlier stages of their education should be aware that the first step toward private practice may actually occur during medical school, when they are considering which specialty to pursue. If a student is particularly interested in solo private practice, they may want to select a specialty with the potential for less overhead in an independent setting. Psychiatry typically has lower overhead costs than some other specialties. This gap widens even further with the increased popularity and acceptance of telepsychiatry.
Budgeting and finance
Once you decide to pursue private practice, you will want to consider whether you prefer solo practice or group practice, and part-time or full-time. If working for yourself, you will need to understand business planning and budgeting, including how to project revenue and expenses. When first starting in solo practice—especially if you are not taking over a previously established practice—it is useful to have secondary sources of income. This can be a part-time clinical position, working with on-demand health care companies, contracting, consulting, etc. Many new physicians begin with a full-time position and decide to initiate their private practice on a part-time basis. This approach provides a level of financial security that you otherwise would not have. However, a full-time position requires full-time energy, hours, and attention, and it can be challenging to balance full-time and part-time work. Whichever approach you decide to take, it can be most helpful to simply keep an open mind and always consider looking further into any new opportunity that interests you.
Insurance and licensing
You don’t have to wait to establish your own practice to purchase malpractice insurance. Shop around for the best rates and the coverage that most comprehensively fits your needs. If your training program allows “moonlighting,” you might need your own insurance to work at sites other than your training hospital. Many residents begin to apply for independent state licensure at the same time they begin pursuing moonlighting opportunities. It may be helpful not to wait until the last minute to do this, because the process has quite a few steps and can take a while. If your state requires letters of reference, think about which of your supervisors you can ask for one. If you plan to work in a state other than that of your training location, it may be helpful to simultaneously apply for your medical license in that state, because you will already be going through the process. Certain states offer reciprocity regarding medical licenses. The Interstate Medical Licensure Compact offers an expedited pathway to licensure for qualified physicians who want to practice in multiple states.1
Marketing your practice
Potential sources for building a panel of patients include referral networks, insurance panels, professional organizations, social media, networking, directories, and word of mouth. If you plan to accept health insurance, the directories provided by insurance panels will allow potential patients to find you when searching for practitioners who accept their plan. Professional organizations offer similar directories, and some private companies also provide directories, either for free or for a fee.
Use technology to your advantage
The exciting thing about starting a private practice today is that the technology available to support a small practice has drastically improved. Many software applications can help with scheduling and billing, which minimizes the need for office staff and enables you to be more productive. These programs typically are available via an online subscription that gives you access to an electronic medical record and other features for a monthly fee. Many of these programs provide add-ons such as a website for your practice and integrated telehealth services. While these programs typically perform many of the same functions, each has a different setup and varying workflows. An online search can facilitate a side-by-side comparison of the software programs that most closely meet your needs.
Seek out mentors and consultants
Finally, try to find a private practice mentor, and reach out to as many people as possible who have worked in any type of private practice setting. A mentor can alert you to factors you might not otherwise have considered. It also may be helpful to establish some form of supervision; such opportunities can be found through professional societies and other groups for private practice clinicians. In these groups, you also can ask other clinicians to recommend private practice and practice management consultants.
Stepping into the unknown can be an intimidating experience; however, you will not know what you are capable of until you try. Fortunately, psychiatry offers the flexibility to create a hybrid career that allows you to follow your passion and maintain your level of comfort. The American Psychiatric Association offers members additional information in the practice management resources section of its website.2
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook
1. Interstate Medical Licensure Compact. Information for physicians. 2020. Accessed March 8, 2021. https://www.imlcc.org/information-for-physicians
2. American Psychiatric Association. Online practice handbook. 2021. Accessed March 21, 2021. https://www.psychiatry.org/psychiatrists/practice/practice-management/starting-a-practice/online-practice-handbook