User login
Half of young adults with diabetes have diastolic dysfunction
Roughly half of adolescents and young adults with either type 1 or type 2 diabetes for about a decade had diastolic dysfunction, a direct precursor to heart failure, in a multicenter echocardiography survey of 479 American patients.
Using tissue Doppler echocardiography findings from 258 adolescents and young adults with type 1 diabetes, and 221 with type 2 diabetes, the study found at least one imaging marker of ventricular stiffness – diastolic dysfunction – in 58% of the patients with type 2 diabetes and in 47% of those with type 1 diabetes. The type 1 patients averaged 21 years of age with a median 12 years of diagnosed disease, while the type 2 patients had an average age of 25 years and a median 11 years disease duration.
The analysis also identified several measures that significantly linked with the presence of diastolic dysfunction: older age, female sex, nonwhite race, type 2 diabetes, higher heart rate, higher body mass index, higher systolic blood pressure, and higher hemoglobin A1c.
“Our data suggest targeting modifiable risk factors” in these patients in an effort to slow the process causing the diastolic dysfunction, Amy S. Shah, MD, said at the virtual annual scientific sessions of the American Diabetes Association. She particularly cited interventions aimed at reducing body mass index, lowering blood pressure, and improving glycemic control, as well as preventing type 2 diabetes in the first place.
Prevention of type 2 diabetes, as well as prevention of diastolic dysfunction development and progression, are key steps because of the substantial clinical consequences of diastolic dysfunction, triggered by stiffening of the left ventricle. Diastolic dysfunction leads to increased left ventricular diastolic pressure, left atrial dysfunction, and ultimately heart failure with preserved ejection fraction, a common diabetes complication that currently has no treatment with proven efficacy, said Dr. Shah, a pediatric endocrinologist and director of the Adolescent Type 2 Diabetes Program at Cincinnati Children’s Hospital Medical Center.
“It’s very concerning that diastolic dysfunction is so prevalent in this age group,” commented Robert A. Gabbay, MD, Chief Science & Medical Officer of the American Diabetes Association. “An important question is whether you can see an improvement by reversing risk factors.” He noted the importance of confirming the finding in additional cohorts as well as running prospective studies looking at the impact of risk factor modification.
Dr. Shah and her associates used data collected at four U.S. centers from patients enrolled in the SEARCH for Diabetes in Youth study who underwent a tissue Doppler examination during 2016-2019, and used three measures derived from the scans to identify diastolic dysfunction:
- The E/A ratio, which compares the early flow wave across the mitral valve (E) with the atrial flow wave (A) that occurs after atrial contraction. Lower values reflect worse pathology.
- The E/e’ ratio, which compares the early flow wave across the mitral valve (E) with the rate of cardiac wall relaxation in early diastole (e’). Higher values reflect worse pathology.
- The e’/a’ ratio, which compares the rate of cardiac wall relaxation in early diastole (e’) with the rate of cardiac wall relaxation in late diastole (a’). Lower values reflect worse pathology.
The most common abnormality involved the e’/a’ measure, which occurred in roughly 38% of the patients with type 2 diabetes and in about 23% of those with type 1 diabetes. Next most common was an abnormally high E/e’ ratio, and fewer than 10% of patients had an abnormally low E/A ratio. Both the E/A and E/e’ values were significantly worse among patients with type 2 diabetes compared with type 1 patients, while no statistically significant difference separated the two subgroups for prevalence of an e’/a’ abnormality after adjustment for body mass index, blood pressure, and HbA1c values.
Average body mass index among the 221 studied patients with type 2 diabetes was 38 kg/m2, 74% were girls or women, and 57% were non-Hispanic black and 24% non-Hispanic white. Mean blood pressure among the patients with type 2 diabetes was 123/80 mm Hg, while it was 110/72 mm Hg among the 258 patients with type 1 diabetes.
SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
SOURCE: Shah AS et al. ADA 2020 abstract 58-OR.
Roughly half of adolescents and young adults with either type 1 or type 2 diabetes for about a decade had diastolic dysfunction, a direct precursor to heart failure, in a multicenter echocardiography survey of 479 American patients.
Using tissue Doppler echocardiography findings from 258 adolescents and young adults with type 1 diabetes, and 221 with type 2 diabetes, the study found at least one imaging marker of ventricular stiffness – diastolic dysfunction – in 58% of the patients with type 2 diabetes and in 47% of those with type 1 diabetes. The type 1 patients averaged 21 years of age with a median 12 years of diagnosed disease, while the type 2 patients had an average age of 25 years and a median 11 years disease duration.
The analysis also identified several measures that significantly linked with the presence of diastolic dysfunction: older age, female sex, nonwhite race, type 2 diabetes, higher heart rate, higher body mass index, higher systolic blood pressure, and higher hemoglobin A1c.
“Our data suggest targeting modifiable risk factors” in these patients in an effort to slow the process causing the diastolic dysfunction, Amy S. Shah, MD, said at the virtual annual scientific sessions of the American Diabetes Association. She particularly cited interventions aimed at reducing body mass index, lowering blood pressure, and improving glycemic control, as well as preventing type 2 diabetes in the first place.
Prevention of type 2 diabetes, as well as prevention of diastolic dysfunction development and progression, are key steps because of the substantial clinical consequences of diastolic dysfunction, triggered by stiffening of the left ventricle. Diastolic dysfunction leads to increased left ventricular diastolic pressure, left atrial dysfunction, and ultimately heart failure with preserved ejection fraction, a common diabetes complication that currently has no treatment with proven efficacy, said Dr. Shah, a pediatric endocrinologist and director of the Adolescent Type 2 Diabetes Program at Cincinnati Children’s Hospital Medical Center.
“It’s very concerning that diastolic dysfunction is so prevalent in this age group,” commented Robert A. Gabbay, MD, Chief Science & Medical Officer of the American Diabetes Association. “An important question is whether you can see an improvement by reversing risk factors.” He noted the importance of confirming the finding in additional cohorts as well as running prospective studies looking at the impact of risk factor modification.
Dr. Shah and her associates used data collected at four U.S. centers from patients enrolled in the SEARCH for Diabetes in Youth study who underwent a tissue Doppler examination during 2016-2019, and used three measures derived from the scans to identify diastolic dysfunction:
- The E/A ratio, which compares the early flow wave across the mitral valve (E) with the atrial flow wave (A) that occurs after atrial contraction. Lower values reflect worse pathology.
- The E/e’ ratio, which compares the early flow wave across the mitral valve (E) with the rate of cardiac wall relaxation in early diastole (e’). Higher values reflect worse pathology.
- The e’/a’ ratio, which compares the rate of cardiac wall relaxation in early diastole (e’) with the rate of cardiac wall relaxation in late diastole (a’). Lower values reflect worse pathology.
The most common abnormality involved the e’/a’ measure, which occurred in roughly 38% of the patients with type 2 diabetes and in about 23% of those with type 1 diabetes. Next most common was an abnormally high E/e’ ratio, and fewer than 10% of patients had an abnormally low E/A ratio. Both the E/A and E/e’ values were significantly worse among patients with type 2 diabetes compared with type 1 patients, while no statistically significant difference separated the two subgroups for prevalence of an e’/a’ abnormality after adjustment for body mass index, blood pressure, and HbA1c values.
Average body mass index among the 221 studied patients with type 2 diabetes was 38 kg/m2, 74% were girls or women, and 57% were non-Hispanic black and 24% non-Hispanic white. Mean blood pressure among the patients with type 2 diabetes was 123/80 mm Hg, while it was 110/72 mm Hg among the 258 patients with type 1 diabetes.
SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
SOURCE: Shah AS et al. ADA 2020 abstract 58-OR.
Roughly half of adolescents and young adults with either type 1 or type 2 diabetes for about a decade had diastolic dysfunction, a direct precursor to heart failure, in a multicenter echocardiography survey of 479 American patients.
Using tissue Doppler echocardiography findings from 258 adolescents and young adults with type 1 diabetes, and 221 with type 2 diabetes, the study found at least one imaging marker of ventricular stiffness – diastolic dysfunction – in 58% of the patients with type 2 diabetes and in 47% of those with type 1 diabetes. The type 1 patients averaged 21 years of age with a median 12 years of diagnosed disease, while the type 2 patients had an average age of 25 years and a median 11 years disease duration.
The analysis also identified several measures that significantly linked with the presence of diastolic dysfunction: older age, female sex, nonwhite race, type 2 diabetes, higher heart rate, higher body mass index, higher systolic blood pressure, and higher hemoglobin A1c.
“Our data suggest targeting modifiable risk factors” in these patients in an effort to slow the process causing the diastolic dysfunction, Amy S. Shah, MD, said at the virtual annual scientific sessions of the American Diabetes Association. She particularly cited interventions aimed at reducing body mass index, lowering blood pressure, and improving glycemic control, as well as preventing type 2 diabetes in the first place.
Prevention of type 2 diabetes, as well as prevention of diastolic dysfunction development and progression, are key steps because of the substantial clinical consequences of diastolic dysfunction, triggered by stiffening of the left ventricle. Diastolic dysfunction leads to increased left ventricular diastolic pressure, left atrial dysfunction, and ultimately heart failure with preserved ejection fraction, a common diabetes complication that currently has no treatment with proven efficacy, said Dr. Shah, a pediatric endocrinologist and director of the Adolescent Type 2 Diabetes Program at Cincinnati Children’s Hospital Medical Center.
“It’s very concerning that diastolic dysfunction is so prevalent in this age group,” commented Robert A. Gabbay, MD, Chief Science & Medical Officer of the American Diabetes Association. “An important question is whether you can see an improvement by reversing risk factors.” He noted the importance of confirming the finding in additional cohorts as well as running prospective studies looking at the impact of risk factor modification.
Dr. Shah and her associates used data collected at four U.S. centers from patients enrolled in the SEARCH for Diabetes in Youth study who underwent a tissue Doppler examination during 2016-2019, and used three measures derived from the scans to identify diastolic dysfunction:
- The E/A ratio, which compares the early flow wave across the mitral valve (E) with the atrial flow wave (A) that occurs after atrial contraction. Lower values reflect worse pathology.
- The E/e’ ratio, which compares the early flow wave across the mitral valve (E) with the rate of cardiac wall relaxation in early diastole (e’). Higher values reflect worse pathology.
- The e’/a’ ratio, which compares the rate of cardiac wall relaxation in early diastole (e’) with the rate of cardiac wall relaxation in late diastole (a’). Lower values reflect worse pathology.
The most common abnormality involved the e’/a’ measure, which occurred in roughly 38% of the patients with type 2 diabetes and in about 23% of those with type 1 diabetes. Next most common was an abnormally high E/e’ ratio, and fewer than 10% of patients had an abnormally low E/A ratio. Both the E/A and E/e’ values were significantly worse among patients with type 2 diabetes compared with type 1 patients, while no statistically significant difference separated the two subgroups for prevalence of an e’/a’ abnormality after adjustment for body mass index, blood pressure, and HbA1c values.
Average body mass index among the 221 studied patients with type 2 diabetes was 38 kg/m2, 74% were girls or women, and 57% were non-Hispanic black and 24% non-Hispanic white. Mean blood pressure among the patients with type 2 diabetes was 123/80 mm Hg, while it was 110/72 mm Hg among the 258 patients with type 1 diabetes.
SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
SOURCE: Shah AS et al. ADA 2020 abstract 58-OR.
FROM ADA 2020
Key clinical point: .
Major finding: Tissue Doppler echocardiography detected diastolic dysfunction in 58% of patients with type 2 diabetes and 47% of type 1 patients.
Study details: SEARCH for Diabetes in Youth study, with 479 American adolescents and young adults with diabetes.
Disclosures: SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
Source: Shah AS et al. ADA 2020, Abstract 58-OR.
Starting new diabetes drugs less likely for racial minorities, Medicare Advantage beneficiaries
, according to researchers at the virtual annual scientific sessions of the American Diabetes Association.
Initiation of DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors was less likely among racial/ethnic minorities and Medicare Advantage beneficiaries in the retrospective analyses, leading the investigators to call for a better understanding of nonclinical factors that may be influencing treatment decisions.
Odds of new diabetes medication use were 55%-69% lower in patients in Medicare Advantage (MA) as compared to patients in commercial health plans, according to results of a separate study presented by Rozalina McCoy, MD, endocrinologist and researcher with Mayo Clinic, Rochester, Minn.
“The rates of use are increasing over time, but not enough in MA beneficiaries,” she said in her virtual presentation. “I think it really calls for more to be done to ensure access to and use of evidence based medications, by all people with type 2 diabetes.”
The likelihood of initiating a new diabetes drug was 29% lower among African Americans and 49% lower among Native Americans in a study of enrollees in the Look AHEAD (Action for Health in Diabetes) randomized trial, according to researcher Ahmed Elhussein, BMBCh, MPH, of the Johns Hopkins Bloomberg School of Public Health.
“This is particularly concerning, because they might have a greater need for these new diabetes medications, but reduced access,” Dr. Elhussein said in his presentation.
Disparities by race in diabetes drug use
The prevalence of type 2 diabetes in the United States is higher among racial and ethnic minorities, at about 12%-15%, versus about 7% in whites, according to Dr. Elhussein,
While the newer classes of diabetes medications have a lower risk of hyperglycemia and have cardiovascular and renal benefits, they also come at a higher cost, he added.
“This has created some concerns about access in particular for underserved groups,” he said in his presentation.
In their retrospective analysis, based on 4,892 patients enrolled in the Look AHEAD (Action for Health in Diabetes) randomized trial, Dr. Elhussein and coinvestigators identified 44% who had initiated a newer diabetes medication over a median follow-up of about 8 years.
They found black race was associated with significantly lower initiation of newer medications compared to whites, with a hazard ratio of 0.81 (95% confidence interval 0.80-0.94; P = 0.019), after adjustment for socioeconomic status.
New diabetes medication use was also significantly lower among American Indian/Alaskan Natives, with an HR of 0.51 and a confidence interval that did not include the null value of 1, according to the investigator.
No significant differences in new diabetes drug use were seen in Hispanics or those classified as other race/ethnicity, he added.
“We’d advocate for more study to try to understand what are the drivers of this disparity,” he said. “This would let us develop interventions that might help to increase access in these patient groups that might need them the most.”
Insurance type and diabetes drugs
Second-line medications, including GLP-1 receptor agonists and SGLT2 inhibitors, have “preferred” efficacy and side effect profiles, but are more costly than older, generic options such as sulfonylureas, which may affect the likelihood of their use, said Dr. McCoy, the Mayo Clinic researcher and lead author of the study on diabetes medication use by insurance type.
They analyzed 1.7 million individuals in a de-identified dataset (OptumLabs Data Warehouse) who were either privately insured or beneficiaries of Medicare Advantage, the private health plan alternative to fee-for-service Medicare.
After adjusting for race/ethnicity, baseline medications, age, gender, and other factors, odds of new medication use were significantly lower in the Medicare Advantage group, according to Dr. McCoy.
Odds ratios ranged from 0.61 (95% CI, 0.60-0.63) for DPP-4 inhibitors, to 0.45 (95% CI, 0.44-0.46) for GLP-1 receptor agonists, and to 0.31 (95% CI, 0.30-0.31) for SGLT2 inhibitors, she reported.
“This may be driven by affordability, because patients with Medicare Advantage plans are not able to access prescription savings cards (as compared to Medicare beneficiaries) and they also are more likely to have fixed incomes and not be able to afford the high costs of these drugs,” she said.
Dr. Elhussein reported no disclosures related to the research, while co-authors provided disclosures related to Abbott, Bigfoot Biomedical, Boehringer Ingelheim, Eli Lilly, MannKind, Medscape, Novo Nordisk, Sanofi US, and others. Dr McCoy likewise had no disclosures, while co-authors indicated disclosures related to Janssen Pharmaceuticals, the Centers for Medicare and Medicaid Services, and the U.S. Food and Drug Administration.
SOURCES: ADA 2020. Authors: McCoy R et al (38-OR), and Elhussein A, et al (37-OR).
, according to researchers at the virtual annual scientific sessions of the American Diabetes Association.
Initiation of DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors was less likely among racial/ethnic minorities and Medicare Advantage beneficiaries in the retrospective analyses, leading the investigators to call for a better understanding of nonclinical factors that may be influencing treatment decisions.
Odds of new diabetes medication use were 55%-69% lower in patients in Medicare Advantage (MA) as compared to patients in commercial health plans, according to results of a separate study presented by Rozalina McCoy, MD, endocrinologist and researcher with Mayo Clinic, Rochester, Minn.
“The rates of use are increasing over time, but not enough in MA beneficiaries,” she said in her virtual presentation. “I think it really calls for more to be done to ensure access to and use of evidence based medications, by all people with type 2 diabetes.”
The likelihood of initiating a new diabetes drug was 29% lower among African Americans and 49% lower among Native Americans in a study of enrollees in the Look AHEAD (Action for Health in Diabetes) randomized trial, according to researcher Ahmed Elhussein, BMBCh, MPH, of the Johns Hopkins Bloomberg School of Public Health.
“This is particularly concerning, because they might have a greater need for these new diabetes medications, but reduced access,” Dr. Elhussein said in his presentation.
Disparities by race in diabetes drug use
The prevalence of type 2 diabetes in the United States is higher among racial and ethnic minorities, at about 12%-15%, versus about 7% in whites, according to Dr. Elhussein,
While the newer classes of diabetes medications have a lower risk of hyperglycemia and have cardiovascular and renal benefits, they also come at a higher cost, he added.
“This has created some concerns about access in particular for underserved groups,” he said in his presentation.
In their retrospective analysis, based on 4,892 patients enrolled in the Look AHEAD (Action for Health in Diabetes) randomized trial, Dr. Elhussein and coinvestigators identified 44% who had initiated a newer diabetes medication over a median follow-up of about 8 years.
They found black race was associated with significantly lower initiation of newer medications compared to whites, with a hazard ratio of 0.81 (95% confidence interval 0.80-0.94; P = 0.019), after adjustment for socioeconomic status.
New diabetes medication use was also significantly lower among American Indian/Alaskan Natives, with an HR of 0.51 and a confidence interval that did not include the null value of 1, according to the investigator.
No significant differences in new diabetes drug use were seen in Hispanics or those classified as other race/ethnicity, he added.
“We’d advocate for more study to try to understand what are the drivers of this disparity,” he said. “This would let us develop interventions that might help to increase access in these patient groups that might need them the most.”
Insurance type and diabetes drugs
Second-line medications, including GLP-1 receptor agonists and SGLT2 inhibitors, have “preferred” efficacy and side effect profiles, but are more costly than older, generic options such as sulfonylureas, which may affect the likelihood of their use, said Dr. McCoy, the Mayo Clinic researcher and lead author of the study on diabetes medication use by insurance type.
They analyzed 1.7 million individuals in a de-identified dataset (OptumLabs Data Warehouse) who were either privately insured or beneficiaries of Medicare Advantage, the private health plan alternative to fee-for-service Medicare.
After adjusting for race/ethnicity, baseline medications, age, gender, and other factors, odds of new medication use were significantly lower in the Medicare Advantage group, according to Dr. McCoy.
Odds ratios ranged from 0.61 (95% CI, 0.60-0.63) for DPP-4 inhibitors, to 0.45 (95% CI, 0.44-0.46) for GLP-1 receptor agonists, and to 0.31 (95% CI, 0.30-0.31) for SGLT2 inhibitors, she reported.
“This may be driven by affordability, because patients with Medicare Advantage plans are not able to access prescription savings cards (as compared to Medicare beneficiaries) and they also are more likely to have fixed incomes and not be able to afford the high costs of these drugs,” she said.
Dr. Elhussein reported no disclosures related to the research, while co-authors provided disclosures related to Abbott, Bigfoot Biomedical, Boehringer Ingelheim, Eli Lilly, MannKind, Medscape, Novo Nordisk, Sanofi US, and others. Dr McCoy likewise had no disclosures, while co-authors indicated disclosures related to Janssen Pharmaceuticals, the Centers for Medicare and Medicaid Services, and the U.S. Food and Drug Administration.
SOURCES: ADA 2020. Authors: McCoy R et al (38-OR), and Elhussein A, et al (37-OR).
, according to researchers at the virtual annual scientific sessions of the American Diabetes Association.
Initiation of DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT2 inhibitors was less likely among racial/ethnic minorities and Medicare Advantage beneficiaries in the retrospective analyses, leading the investigators to call for a better understanding of nonclinical factors that may be influencing treatment decisions.
Odds of new diabetes medication use were 55%-69% lower in patients in Medicare Advantage (MA) as compared to patients in commercial health plans, according to results of a separate study presented by Rozalina McCoy, MD, endocrinologist and researcher with Mayo Clinic, Rochester, Minn.
“The rates of use are increasing over time, but not enough in MA beneficiaries,” she said in her virtual presentation. “I think it really calls for more to be done to ensure access to and use of evidence based medications, by all people with type 2 diabetes.”
The likelihood of initiating a new diabetes drug was 29% lower among African Americans and 49% lower among Native Americans in a study of enrollees in the Look AHEAD (Action for Health in Diabetes) randomized trial, according to researcher Ahmed Elhussein, BMBCh, MPH, of the Johns Hopkins Bloomberg School of Public Health.
“This is particularly concerning, because they might have a greater need for these new diabetes medications, but reduced access,” Dr. Elhussein said in his presentation.
Disparities by race in diabetes drug use
The prevalence of type 2 diabetes in the United States is higher among racial and ethnic minorities, at about 12%-15%, versus about 7% in whites, according to Dr. Elhussein,
While the newer classes of diabetes medications have a lower risk of hyperglycemia and have cardiovascular and renal benefits, they also come at a higher cost, he added.
“This has created some concerns about access in particular for underserved groups,” he said in his presentation.
In their retrospective analysis, based on 4,892 patients enrolled in the Look AHEAD (Action for Health in Diabetes) randomized trial, Dr. Elhussein and coinvestigators identified 44% who had initiated a newer diabetes medication over a median follow-up of about 8 years.
They found black race was associated with significantly lower initiation of newer medications compared to whites, with a hazard ratio of 0.81 (95% confidence interval 0.80-0.94; P = 0.019), after adjustment for socioeconomic status.
New diabetes medication use was also significantly lower among American Indian/Alaskan Natives, with an HR of 0.51 and a confidence interval that did not include the null value of 1, according to the investigator.
No significant differences in new diabetes drug use were seen in Hispanics or those classified as other race/ethnicity, he added.
“We’d advocate for more study to try to understand what are the drivers of this disparity,” he said. “This would let us develop interventions that might help to increase access in these patient groups that might need them the most.”
Insurance type and diabetes drugs
Second-line medications, including GLP-1 receptor agonists and SGLT2 inhibitors, have “preferred” efficacy and side effect profiles, but are more costly than older, generic options such as sulfonylureas, which may affect the likelihood of their use, said Dr. McCoy, the Mayo Clinic researcher and lead author of the study on diabetes medication use by insurance type.
They analyzed 1.7 million individuals in a de-identified dataset (OptumLabs Data Warehouse) who were either privately insured or beneficiaries of Medicare Advantage, the private health plan alternative to fee-for-service Medicare.
After adjusting for race/ethnicity, baseline medications, age, gender, and other factors, odds of new medication use were significantly lower in the Medicare Advantage group, according to Dr. McCoy.
Odds ratios ranged from 0.61 (95% CI, 0.60-0.63) for DPP-4 inhibitors, to 0.45 (95% CI, 0.44-0.46) for GLP-1 receptor agonists, and to 0.31 (95% CI, 0.30-0.31) for SGLT2 inhibitors, she reported.
“This may be driven by affordability, because patients with Medicare Advantage plans are not able to access prescription savings cards (as compared to Medicare beneficiaries) and they also are more likely to have fixed incomes and not be able to afford the high costs of these drugs,” she said.
Dr. Elhussein reported no disclosures related to the research, while co-authors provided disclosures related to Abbott, Bigfoot Biomedical, Boehringer Ingelheim, Eli Lilly, MannKind, Medscape, Novo Nordisk, Sanofi US, and others. Dr McCoy likewise had no disclosures, while co-authors indicated disclosures related to Janssen Pharmaceuticals, the Centers for Medicare and Medicaid Services, and the U.S. Food and Drug Administration.
SOURCES: ADA 2020. Authors: McCoy R et al (38-OR), and Elhussein A, et al (37-OR).
FROM ADA 2020
EMPA-REG OUTCOME: Empagliflozin cut insulin need in type 2 diabetes
Patients with type 2 diabetes treated with the SGLT2 inhibitor empagliflozin during the landmark EMPA-REG OUTCOME trial had a solidly reduced need to either start insulin treatment or intensify existing insulin treatment, compared with those given placebo, in a post-hoc analysis of the study’s findings.
“Empagliflozin markedly and durably delayed the need for insulin initiation, and reduced the need for large dose increases in patients already using insulin,” Muthiah Vaduganathan, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
The patients in the empagliflozin (Jardiance) arm of EMPA-REG OUTCOME had a 9% rate of initiating insulin treatment after 4 years in the study, compared with a 20% rate among patients who received placebo, a statistically significant 60% relative risk reduction. All patients in the trial continued on their background oral glucose-lowering medications.
Among the 48% of study patients who entered the study already using insulin as part of their usual regimen, 18% of those receiving empagliflozin required a significant increase in their insulin dosage (an increase of at least 20% from baseline) after 4 years. But among the control patients, 35% needed this level of insulin-dosage intensification, again a statistically significant difference that computed to a 58% relative reduction in the need for boosting the insulin dosage.
For both of these endpoints, the divergence between the empagliflozin and control arms became apparent within the first 6 months on treatment, and the between-group differences steadily increased during further follow-up. The analyses pooled the patients who received empagliflozin in the trial, which studied two different dosages of the drug.
Results add to the ‘risk and benefit conversation’
“This is one of the first studies to look at this question in a more granular fashion” in patients with type 2 diabetes receiving a drug from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class, said Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston. It provides “compelling” information to include when discussing oral diabetes-drug options with patients, he said in an interview.
Patients newly diagnosed with type 2 diabetes “often think about insulin” and their potential need to eventually start taking it, with the requirements it brings for training, monitoring, and drug delivery, along with the costs for insulin and glucose monitoring. “Patients are very attuned to potentially needing insulin and often ask about it. A reduced need for insulin will be an important part of the risk and benefit conversation” with patients about potential use of an SGLT2 inhibitor, he said.
Dr. Vaduganathan hypothesized that three factors could contribute to the impact of empagliflozin on insulin initiation and dosage level: a direct glycemic-control effect of the drug, the drug’s positive impact on overall well-being and function that could enhance patient movement, and the documented ability of treatment with empagliflozin and other drugs in its class to cut the rate of heart failure hospitalizations. This last feature is potentially relevant because insulin treatment often starts in patients with type 2 diabetes during a hospitalization, he noted.
Handelsman: Analysis shows no ‘spectacular effect’
The association of empagliflozin treatment with a reduced need for insulin seen in these data is consistent with expectations for patients with type 2 diabetes who receive an additional oral drug, commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of American in Tarzana, Calif. “In large part it has to do with patients on placebo having to get more insulin” because their additional oral-drug options were limited. Dr. Handelsman pointed out that during the period when the EMPA-REG OUTCOME trial ran, from 2010-2015, fewer oral drugs were available than today, and clinicians in the study were encouraged to treat patients to their goal glycemia level according to local guidelines. In addition to a modest but useful glycemic control effect from SGLT2 inhibitors that, on average, cut hemoglobin A1c levels by about 0.5%, they may also give a small boost to insulin sensitivity that can also defer the need to add or increase insulin. The level of insulin-treatment deference reported in the new analysis was “not a spectacular effect” he said in an interview.
The EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study followed 7,020 patients at 590 sites in 42 countries for a median of 3.1 years. The study’s primary endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction (excluding silent MI), or nonfatal stroke, and the results showed a statistically significant 14% relative risk reduction with empagliflozin treatment (N Engl J Med. 2015 Nov 26;373[22]:2117-28 ). The results also showed that 12 weeks into the study, before patients could receive any additional drugs, HbA1c levels averaged 0.54%-0.6% lower among the empagliflozin-treated patients than those in the placebo arm, with smaller between-group differences maintained through the balance of the study. At entry, more than half the enrolled patients were routinely treated with metformin, and close to half were receiving a sulfonyurea agent.
The EMPA-REG OUTCOME results were also notable as showing for the first time that treatment with an SGLT2 inhibitor drug produced a substantial decrease in heart failure hospitalizations, incident heart failure, and progression of renal dysfunction, effects subsequently confirmed and also found for other agents in this drug class.
EMPA-REG OUTCOME was funded in part by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin (Jardiance). Dr. Vaduganathan has been an advisor to Boehringer Ingelheim and to Amgen, AstraZeneca, Baxter, Bayer, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including Boehringer Ingelheim and Eli Lilly.
SOURCE: Vaduganathan M et al. ADA 2020, Abstract 30-OR.
Patients with type 2 diabetes treated with the SGLT2 inhibitor empagliflozin during the landmark EMPA-REG OUTCOME trial had a solidly reduced need to either start insulin treatment or intensify existing insulin treatment, compared with those given placebo, in a post-hoc analysis of the study’s findings.
“Empagliflozin markedly and durably delayed the need for insulin initiation, and reduced the need for large dose increases in patients already using insulin,” Muthiah Vaduganathan, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
The patients in the empagliflozin (Jardiance) arm of EMPA-REG OUTCOME had a 9% rate of initiating insulin treatment after 4 years in the study, compared with a 20% rate among patients who received placebo, a statistically significant 60% relative risk reduction. All patients in the trial continued on their background oral glucose-lowering medications.
Among the 48% of study patients who entered the study already using insulin as part of their usual regimen, 18% of those receiving empagliflozin required a significant increase in their insulin dosage (an increase of at least 20% from baseline) after 4 years. But among the control patients, 35% needed this level of insulin-dosage intensification, again a statistically significant difference that computed to a 58% relative reduction in the need for boosting the insulin dosage.
For both of these endpoints, the divergence between the empagliflozin and control arms became apparent within the first 6 months on treatment, and the between-group differences steadily increased during further follow-up. The analyses pooled the patients who received empagliflozin in the trial, which studied two different dosages of the drug.
Results add to the ‘risk and benefit conversation’
“This is one of the first studies to look at this question in a more granular fashion” in patients with type 2 diabetes receiving a drug from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class, said Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston. It provides “compelling” information to include when discussing oral diabetes-drug options with patients, he said in an interview.
Patients newly diagnosed with type 2 diabetes “often think about insulin” and their potential need to eventually start taking it, with the requirements it brings for training, monitoring, and drug delivery, along with the costs for insulin and glucose monitoring. “Patients are very attuned to potentially needing insulin and often ask about it. A reduced need for insulin will be an important part of the risk and benefit conversation” with patients about potential use of an SGLT2 inhibitor, he said.
Dr. Vaduganathan hypothesized that three factors could contribute to the impact of empagliflozin on insulin initiation and dosage level: a direct glycemic-control effect of the drug, the drug’s positive impact on overall well-being and function that could enhance patient movement, and the documented ability of treatment with empagliflozin and other drugs in its class to cut the rate of heart failure hospitalizations. This last feature is potentially relevant because insulin treatment often starts in patients with type 2 diabetes during a hospitalization, he noted.
Handelsman: Analysis shows no ‘spectacular effect’
The association of empagliflozin treatment with a reduced need for insulin seen in these data is consistent with expectations for patients with type 2 diabetes who receive an additional oral drug, commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of American in Tarzana, Calif. “In large part it has to do with patients on placebo having to get more insulin” because their additional oral-drug options were limited. Dr. Handelsman pointed out that during the period when the EMPA-REG OUTCOME trial ran, from 2010-2015, fewer oral drugs were available than today, and clinicians in the study were encouraged to treat patients to their goal glycemia level according to local guidelines. In addition to a modest but useful glycemic control effect from SGLT2 inhibitors that, on average, cut hemoglobin A1c levels by about 0.5%, they may also give a small boost to insulin sensitivity that can also defer the need to add or increase insulin. The level of insulin-treatment deference reported in the new analysis was “not a spectacular effect” he said in an interview.
The EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study followed 7,020 patients at 590 sites in 42 countries for a median of 3.1 years. The study’s primary endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction (excluding silent MI), or nonfatal stroke, and the results showed a statistically significant 14% relative risk reduction with empagliflozin treatment (N Engl J Med. 2015 Nov 26;373[22]:2117-28 ). The results also showed that 12 weeks into the study, before patients could receive any additional drugs, HbA1c levels averaged 0.54%-0.6% lower among the empagliflozin-treated patients than those in the placebo arm, with smaller between-group differences maintained through the balance of the study. At entry, more than half the enrolled patients were routinely treated with metformin, and close to half were receiving a sulfonyurea agent.
The EMPA-REG OUTCOME results were also notable as showing for the first time that treatment with an SGLT2 inhibitor drug produced a substantial decrease in heart failure hospitalizations, incident heart failure, and progression of renal dysfunction, effects subsequently confirmed and also found for other agents in this drug class.
EMPA-REG OUTCOME was funded in part by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin (Jardiance). Dr. Vaduganathan has been an advisor to Boehringer Ingelheim and to Amgen, AstraZeneca, Baxter, Bayer, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including Boehringer Ingelheim and Eli Lilly.
SOURCE: Vaduganathan M et al. ADA 2020, Abstract 30-OR.
Patients with type 2 diabetes treated with the SGLT2 inhibitor empagliflozin during the landmark EMPA-REG OUTCOME trial had a solidly reduced need to either start insulin treatment or intensify existing insulin treatment, compared with those given placebo, in a post-hoc analysis of the study’s findings.
“Empagliflozin markedly and durably delayed the need for insulin initiation, and reduced the need for large dose increases in patients already using insulin,” Muthiah Vaduganathan, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
The patients in the empagliflozin (Jardiance) arm of EMPA-REG OUTCOME had a 9% rate of initiating insulin treatment after 4 years in the study, compared with a 20% rate among patients who received placebo, a statistically significant 60% relative risk reduction. All patients in the trial continued on their background oral glucose-lowering medications.
Among the 48% of study patients who entered the study already using insulin as part of their usual regimen, 18% of those receiving empagliflozin required a significant increase in their insulin dosage (an increase of at least 20% from baseline) after 4 years. But among the control patients, 35% needed this level of insulin-dosage intensification, again a statistically significant difference that computed to a 58% relative reduction in the need for boosting the insulin dosage.
For both of these endpoints, the divergence between the empagliflozin and control arms became apparent within the first 6 months on treatment, and the between-group differences steadily increased during further follow-up. The analyses pooled the patients who received empagliflozin in the trial, which studied two different dosages of the drug.
Results add to the ‘risk and benefit conversation’
“This is one of the first studies to look at this question in a more granular fashion” in patients with type 2 diabetes receiving a drug from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class, said Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston. It provides “compelling” information to include when discussing oral diabetes-drug options with patients, he said in an interview.
Patients newly diagnosed with type 2 diabetes “often think about insulin” and their potential need to eventually start taking it, with the requirements it brings for training, monitoring, and drug delivery, along with the costs for insulin and glucose monitoring. “Patients are very attuned to potentially needing insulin and often ask about it. A reduced need for insulin will be an important part of the risk and benefit conversation” with patients about potential use of an SGLT2 inhibitor, he said.
Dr. Vaduganathan hypothesized that three factors could contribute to the impact of empagliflozin on insulin initiation and dosage level: a direct glycemic-control effect of the drug, the drug’s positive impact on overall well-being and function that could enhance patient movement, and the documented ability of treatment with empagliflozin and other drugs in its class to cut the rate of heart failure hospitalizations. This last feature is potentially relevant because insulin treatment often starts in patients with type 2 diabetes during a hospitalization, he noted.
Handelsman: Analysis shows no ‘spectacular effect’
The association of empagliflozin treatment with a reduced need for insulin seen in these data is consistent with expectations for patients with type 2 diabetes who receive an additional oral drug, commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of American in Tarzana, Calif. “In large part it has to do with patients on placebo having to get more insulin” because their additional oral-drug options were limited. Dr. Handelsman pointed out that during the period when the EMPA-REG OUTCOME trial ran, from 2010-2015, fewer oral drugs were available than today, and clinicians in the study were encouraged to treat patients to their goal glycemia level according to local guidelines. In addition to a modest but useful glycemic control effect from SGLT2 inhibitors that, on average, cut hemoglobin A1c levels by about 0.5%, they may also give a small boost to insulin sensitivity that can also defer the need to add or increase insulin. The level of insulin-treatment deference reported in the new analysis was “not a spectacular effect” he said in an interview.
The EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study followed 7,020 patients at 590 sites in 42 countries for a median of 3.1 years. The study’s primary endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction (excluding silent MI), or nonfatal stroke, and the results showed a statistically significant 14% relative risk reduction with empagliflozin treatment (N Engl J Med. 2015 Nov 26;373[22]:2117-28 ). The results also showed that 12 weeks into the study, before patients could receive any additional drugs, HbA1c levels averaged 0.54%-0.6% lower among the empagliflozin-treated patients than those in the placebo arm, with smaller between-group differences maintained through the balance of the study. At entry, more than half the enrolled patients were routinely treated with metformin, and close to half were receiving a sulfonyurea agent.
The EMPA-REG OUTCOME results were also notable as showing for the first time that treatment with an SGLT2 inhibitor drug produced a substantial decrease in heart failure hospitalizations, incident heart failure, and progression of renal dysfunction, effects subsequently confirmed and also found for other agents in this drug class.
EMPA-REG OUTCOME was funded in part by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin (Jardiance). Dr. Vaduganathan has been an advisor to Boehringer Ingelheim and to Amgen, AstraZeneca, Baxter, Bayer, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including Boehringer Ingelheim and Eli Lilly.
SOURCE: Vaduganathan M et al. ADA 2020, Abstract 30-OR.
FROM ADA 2020
CDC emphasizes pandemic not over, need to avoid large gatherings
Robert Redfield, MD, Director, CDC, and Jay C. Butler, MD, Deputy Director of Infectious Diseases and COVID-19 Response Incident Manager, CDC, discussed two new sets of CDC guidance on deciding to go out and attending group gatherings.
“We recognize that we’re all getting tired of staying at home; people long for the life that they had back in December, and as we head into the summer months, we know that Americans will be looking forward to reconnecting with family and friends and being able to attend events, and we want that to occur as safely as possible,” Butler said.
“Our recommendations evolved based on new information that becomes available, but it continues to be extremely important that we embrace the recommendations of social distancing, handwashing, and wearing a face covering when we’re in public as some of the key defenses that we have against this virus,” Redfield explained.
“The pandemic is not over and it’s important to recognize that. While COVID-19 is still making headlines everywhere, we know the pandemic hasn’t affected everyone everywhere in the same way,” Butler said.
He noted that it is important to prepare for next fall and winter, when we can expect influenza season to complicate matters. “If anything, we must be overly-prepared for what we might face later this year,” he continued, adding that it is important to get vaccinated against influenza. “[F]lu and COVID-19 could be circulating together as we move into the fall and winter months,” he concluded.
Americans Mostly Following Guidelines
The agency also presented data from an article published online June 12 in Morbidity and Mortality Weekly Report that “underscores the fact that American people have taken mitigation efforts seriously…and it demonstrates our collective spirit in responding to the pandemic,” Butler said.
In it, the researchers describe representative panel surveys conducted among 4042 adults aged 18 years or older in New York City and Los Angeles — the two most populous cities in the United States — and “broadly across the United States” during May 5 to May 12, 2020.
Most respondents supported stay-at-home orders and nonessential business closures (United States, 79.5%; New York City, 86.7%; Los Angeles, 81.5%) and always or often wore cloth face coverings in public (United States, 74.1%; New York City, 89.6%; Los Angeles, 89.8%). Respondents also agreed that nonessential workers should remain at home (United States, 67.3%; New York City, 76.6%; Los Angeles, 69.1%), report Mark É. Czeisler, from Monash University and Austin Health, both in Melbourne, Australia, and colleagues.
There was wide support with public health guidelines: more than 87% of individuals in each area agreed that individuals should keep six feet of distance between themselves and others, and more than 82% in each area said that people should limit gatherings to fewer than 10 individuals.
At the time the survey was conducted, most were against indoor dining at restaurants (United States, 66.6%; New York City, 81.5%; Los Angeles, 71.8%).
Adherence “Widespread,” Survey Finds
Most respondents said they were adhering to COVID-19 mitigation guidance, including self-isolating (United States, 77.3%; New York City, 84.6%; Los Angeles, 83.0%) and “always or often” kept at least six feet between themselves and others (New York City, 85.7%; Los Angeles, 82.6%).
More than 85% of respondents in each of the three cohorts said they always or often avoided groups of 10 or more individuals.
About 90% of respondents said they had been in a public area during the last week, with 74.1% of those saying they always or often covered their face in public; respondents in New York City (89.6%) and Los Angeles (89.8%) had higher percentages of this behavior compared with respondents from the United States overall.
Most respondents felt that restrictions in their state were balanced or too lax (United States, 84.3%; New York City, 89.7%; Los Angeles, 79.7%) and said they would feel unsafe if restrictions were eased nationwide at that time (United States, 74.3%; New York City, 81.5%; Los Angeles, 73.4%). However, some individuals who said they would feel unsafe still wanted community mitigation strategies eased and were willing to accept risks resulting from lifting restrictions (United States, 17.1%; New York City, 12.6%; Los Angeles, 12.7%).
“Reported prevalence of self-isolation and feeling safe if community mitigation strategies were lifted differed significantly by age, employment status, and essential worker status among adults in the U.S. survey cohort,” the authors write.
Reports of self-isolation were highest among persons aged 18 to 24 years (92.3%) and lowest among those aged 45 to 54 years (71.5%). Yet, young adults aged 18 to 24 years (43.1%) were more than twice as likely to say they would feel safe if community mitigation strategies were eased, compared with adults aged 65 years or older (19.2%).
Almost half (47.2%) of employed respondents in the US cohort were essential workers; essential workers were “significantly less likely” to report self-isolating when compared with nonessential workers (63.1% vs 80.6%). Some 37.7% of essential workers said they would feel safe if community mitigation strategies were eased, compared with 23.7% of nonessential workers.
“Respondents who were male, employed, or essential workers were significantly more likely to report having been in public areas in the past week. Among respondents who had been in public areas during the preceding week, significantly higher percentages of women, adults aged ≥ 65 years, retired persons, and those living in urban areas reported wearing cloth face coverings,” the authors explain.
The findings are subject to several limitations, including self-reporting and the fact that some respondents may have known someone who tested positive for COVID-19 or died from it, the authors note. Respondents were not representative of the US population and the findings may not be generalizable.
This article first appeared on Medscape.com.
Robert Redfield, MD, Director, CDC, and Jay C. Butler, MD, Deputy Director of Infectious Diseases and COVID-19 Response Incident Manager, CDC, discussed two new sets of CDC guidance on deciding to go out and attending group gatherings.
“We recognize that we’re all getting tired of staying at home; people long for the life that they had back in December, and as we head into the summer months, we know that Americans will be looking forward to reconnecting with family and friends and being able to attend events, and we want that to occur as safely as possible,” Butler said.
“Our recommendations evolved based on new information that becomes available, but it continues to be extremely important that we embrace the recommendations of social distancing, handwashing, and wearing a face covering when we’re in public as some of the key defenses that we have against this virus,” Redfield explained.
“The pandemic is not over and it’s important to recognize that. While COVID-19 is still making headlines everywhere, we know the pandemic hasn’t affected everyone everywhere in the same way,” Butler said.
He noted that it is important to prepare for next fall and winter, when we can expect influenza season to complicate matters. “If anything, we must be overly-prepared for what we might face later this year,” he continued, adding that it is important to get vaccinated against influenza. “[F]lu and COVID-19 could be circulating together as we move into the fall and winter months,” he concluded.
Americans Mostly Following Guidelines
The agency also presented data from an article published online June 12 in Morbidity and Mortality Weekly Report that “underscores the fact that American people have taken mitigation efforts seriously…and it demonstrates our collective spirit in responding to the pandemic,” Butler said.
In it, the researchers describe representative panel surveys conducted among 4042 adults aged 18 years or older in New York City and Los Angeles — the two most populous cities in the United States — and “broadly across the United States” during May 5 to May 12, 2020.
Most respondents supported stay-at-home orders and nonessential business closures (United States, 79.5%; New York City, 86.7%; Los Angeles, 81.5%) and always or often wore cloth face coverings in public (United States, 74.1%; New York City, 89.6%; Los Angeles, 89.8%). Respondents also agreed that nonessential workers should remain at home (United States, 67.3%; New York City, 76.6%; Los Angeles, 69.1%), report Mark É. Czeisler, from Monash University and Austin Health, both in Melbourne, Australia, and colleagues.
There was wide support with public health guidelines: more than 87% of individuals in each area agreed that individuals should keep six feet of distance between themselves and others, and more than 82% in each area said that people should limit gatherings to fewer than 10 individuals.
At the time the survey was conducted, most were against indoor dining at restaurants (United States, 66.6%; New York City, 81.5%; Los Angeles, 71.8%).
Adherence “Widespread,” Survey Finds
Most respondents said they were adhering to COVID-19 mitigation guidance, including self-isolating (United States, 77.3%; New York City, 84.6%; Los Angeles, 83.0%) and “always or often” kept at least six feet between themselves and others (New York City, 85.7%; Los Angeles, 82.6%).
More than 85% of respondents in each of the three cohorts said they always or often avoided groups of 10 or more individuals.
About 90% of respondents said they had been in a public area during the last week, with 74.1% of those saying they always or often covered their face in public; respondents in New York City (89.6%) and Los Angeles (89.8%) had higher percentages of this behavior compared with respondents from the United States overall.
Most respondents felt that restrictions in their state were balanced or too lax (United States, 84.3%; New York City, 89.7%; Los Angeles, 79.7%) and said they would feel unsafe if restrictions were eased nationwide at that time (United States, 74.3%; New York City, 81.5%; Los Angeles, 73.4%). However, some individuals who said they would feel unsafe still wanted community mitigation strategies eased and were willing to accept risks resulting from lifting restrictions (United States, 17.1%; New York City, 12.6%; Los Angeles, 12.7%).
“Reported prevalence of self-isolation and feeling safe if community mitigation strategies were lifted differed significantly by age, employment status, and essential worker status among adults in the U.S. survey cohort,” the authors write.
Reports of self-isolation were highest among persons aged 18 to 24 years (92.3%) and lowest among those aged 45 to 54 years (71.5%). Yet, young adults aged 18 to 24 years (43.1%) were more than twice as likely to say they would feel safe if community mitigation strategies were eased, compared with adults aged 65 years or older (19.2%).
Almost half (47.2%) of employed respondents in the US cohort were essential workers; essential workers were “significantly less likely” to report self-isolating when compared with nonessential workers (63.1% vs 80.6%). Some 37.7% of essential workers said they would feel safe if community mitigation strategies were eased, compared with 23.7% of nonessential workers.
“Respondents who were male, employed, or essential workers were significantly more likely to report having been in public areas in the past week. Among respondents who had been in public areas during the preceding week, significantly higher percentages of women, adults aged ≥ 65 years, retired persons, and those living in urban areas reported wearing cloth face coverings,” the authors explain.
The findings are subject to several limitations, including self-reporting and the fact that some respondents may have known someone who tested positive for COVID-19 or died from it, the authors note. Respondents were not representative of the US population and the findings may not be generalizable.
This article first appeared on Medscape.com.
Robert Redfield, MD, Director, CDC, and Jay C. Butler, MD, Deputy Director of Infectious Diseases and COVID-19 Response Incident Manager, CDC, discussed two new sets of CDC guidance on deciding to go out and attending group gatherings.
“We recognize that we’re all getting tired of staying at home; people long for the life that they had back in December, and as we head into the summer months, we know that Americans will be looking forward to reconnecting with family and friends and being able to attend events, and we want that to occur as safely as possible,” Butler said.
“Our recommendations evolved based on new information that becomes available, but it continues to be extremely important that we embrace the recommendations of social distancing, handwashing, and wearing a face covering when we’re in public as some of the key defenses that we have against this virus,” Redfield explained.
“The pandemic is not over and it’s important to recognize that. While COVID-19 is still making headlines everywhere, we know the pandemic hasn’t affected everyone everywhere in the same way,” Butler said.
He noted that it is important to prepare for next fall and winter, when we can expect influenza season to complicate matters. “If anything, we must be overly-prepared for what we might face later this year,” he continued, adding that it is important to get vaccinated against influenza. “[F]lu and COVID-19 could be circulating together as we move into the fall and winter months,” he concluded.
Americans Mostly Following Guidelines
The agency also presented data from an article published online June 12 in Morbidity and Mortality Weekly Report that “underscores the fact that American people have taken mitigation efforts seriously…and it demonstrates our collective spirit in responding to the pandemic,” Butler said.
In it, the researchers describe representative panel surveys conducted among 4042 adults aged 18 years or older in New York City and Los Angeles — the two most populous cities in the United States — and “broadly across the United States” during May 5 to May 12, 2020.
Most respondents supported stay-at-home orders and nonessential business closures (United States, 79.5%; New York City, 86.7%; Los Angeles, 81.5%) and always or often wore cloth face coverings in public (United States, 74.1%; New York City, 89.6%; Los Angeles, 89.8%). Respondents also agreed that nonessential workers should remain at home (United States, 67.3%; New York City, 76.6%; Los Angeles, 69.1%), report Mark É. Czeisler, from Monash University and Austin Health, both in Melbourne, Australia, and colleagues.
There was wide support with public health guidelines: more than 87% of individuals in each area agreed that individuals should keep six feet of distance between themselves and others, and more than 82% in each area said that people should limit gatherings to fewer than 10 individuals.
At the time the survey was conducted, most were against indoor dining at restaurants (United States, 66.6%; New York City, 81.5%; Los Angeles, 71.8%).
Adherence “Widespread,” Survey Finds
Most respondents said they were adhering to COVID-19 mitigation guidance, including self-isolating (United States, 77.3%; New York City, 84.6%; Los Angeles, 83.0%) and “always or often” kept at least six feet between themselves and others (New York City, 85.7%; Los Angeles, 82.6%).
More than 85% of respondents in each of the three cohorts said they always or often avoided groups of 10 or more individuals.
About 90% of respondents said they had been in a public area during the last week, with 74.1% of those saying they always or often covered their face in public; respondents in New York City (89.6%) and Los Angeles (89.8%) had higher percentages of this behavior compared with respondents from the United States overall.
Most respondents felt that restrictions in their state were balanced or too lax (United States, 84.3%; New York City, 89.7%; Los Angeles, 79.7%) and said they would feel unsafe if restrictions were eased nationwide at that time (United States, 74.3%; New York City, 81.5%; Los Angeles, 73.4%). However, some individuals who said they would feel unsafe still wanted community mitigation strategies eased and were willing to accept risks resulting from lifting restrictions (United States, 17.1%; New York City, 12.6%; Los Angeles, 12.7%).
“Reported prevalence of self-isolation and feeling safe if community mitigation strategies were lifted differed significantly by age, employment status, and essential worker status among adults in the U.S. survey cohort,” the authors write.
Reports of self-isolation were highest among persons aged 18 to 24 years (92.3%) and lowest among those aged 45 to 54 years (71.5%). Yet, young adults aged 18 to 24 years (43.1%) were more than twice as likely to say they would feel safe if community mitigation strategies were eased, compared with adults aged 65 years or older (19.2%).
Almost half (47.2%) of employed respondents in the US cohort were essential workers; essential workers were “significantly less likely” to report self-isolating when compared with nonessential workers (63.1% vs 80.6%). Some 37.7% of essential workers said they would feel safe if community mitigation strategies were eased, compared with 23.7% of nonessential workers.
“Respondents who were male, employed, or essential workers were significantly more likely to report having been in public areas in the past week. Among respondents who had been in public areas during the preceding week, significantly higher percentages of women, adults aged ≥ 65 years, retired persons, and those living in urban areas reported wearing cloth face coverings,” the authors explain.
The findings are subject to several limitations, including self-reporting and the fact that some respondents may have known someone who tested positive for COVID-19 or died from it, the authors note. Respondents were not representative of the US population and the findings may not be generalizable.
This article first appeared on Medscape.com.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available 670G hybrid closed-loop, particularly in young people with type 1 diabetes, new data suggest.
Automated insulin delivery systems are comprised of an insulin pump, continuous glucose monitor (CGM), and an automated insulin dosing algorithm.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented June 12 during the virtual American Diabetes Association (ADA) 80th Scientific Sessions. The AHCL is the algorithm used in Medtronic’s new MiniMed 780G system, which received a CE Mark on June 11 for the treatment of type 1 diabetes in people aged 7 to 80 years.
One trial, presented by Bruce W. Bode, MD, of Atlanta Diabetes Associates, Georgia, was the US pivotal safety study that will be submitted to the US Food and Drug Administration for approval of the Medtronic 780G.
Another trial, presented by Richard M. Bergenstal, MD, executive director of the International Diabetes Center at Park Nicollet, Minneapolis, Minnesota, was a separate comparison of the AHCL with the 670G. (The AHCL-based system used in the three trials was identical to the 780G except it didn’t include Bluetooth, which will be a feature of the final product.)
A third trial, presented by Martin de Bock, PhD, of the University of Otago, New Zealand, included the CE Mark dataset for the 780G.
In contrast to the 670G, the 780G adds automated correction boluses for high blood glucose levels (rather than simply adjusting the basal infusion) and allows for adjustment of target glucose levels down to 100 mg/dL rather than a minimum of 120 mg/dL.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction. And specifically compared to the 670G, the AHCL-based system reverts to open-loop far less often because it only exits closed-loop mode when the sensor stops working or during sensor changes, but not during hyperglycemia even above 300 mg/dL.
Asked to comment, session moderator Timothy S. Bailey, MD, president and CEO of the AMCR Institute, Escondido, California, told Medscape Medical News: “Automated insulin delivery systems are getting better and better.”
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They all take people from where they are now to better time-in-range, less time with hypoglycemia, and most important, they might make the quality of their lives better. That’s really underappreciated.”
One factor that has allowed for the improvements, Bailey said, is the recognition by regulatory bodies that the hybrid closed-loop devices are generally safer than current open-loop type 1 diabetes management so that fewer “safety” device features that interfere with tight glycemic control are necessary.
With first-generation closed-loop systems, “If a wide variety of conditions occur, users get kicked off [hybrid closed-loop mode]. Originally it was perceived by the regulatory agencies as a safety feature because they perceived the standard of care as safe. The new system was allowed to have fewer rules.”
Pivotal trial: Time-in-range improved, 96% say system easy to use
The goal of the AHCL system is to maximize the time-in-range of blood glucose between 70-180 mg/dL. Automated basal delivery of insulin is programmed to a set-point of 100 or 120 mg/dL, with dosing every 5 minutes.
The US pivotal trial was a single-arm, 16-center, in-home trial of 157 people with type 1 diabetes, including 39 adolescents aged 14-21 years and 118 adults aged 22-75 years. All had type 1 diabetes for at least 2 years, A1c levels below 10%, and had been using insulin pumps for at least 6 months, with or without CGMs.
After a 14-day run-in, they wore the systems with a 100 or 120 mg/dL set-point for 45 days, then switched to the other setpoint for another 45 days. Average A1c dropped from 7.5% to 7.0%, with the proportions having an A1c ≤ 7.0% increasing from 34% to 61%.
Overall time-in-range was 75% compared to 69% at baseline, with time below range (< 70 mg/dL) of 1.8%. Overnight time-in-range was 82%, with 1.5% below range. Time-in-range increased from 62% to 73% in the adolescents and from 71% to 75% in the adults.
There were no incidences of severe hypoglycemia or diabetic ketoacidosis, and no device-related serious adverse events.
Participants reported being in hybrid closed-loop, or auto-mode, 95% of the time, compared with 33% for those who had been previously using the 670G.
The number of AHCL exits was 1.3 per week, significantly less than with the 670G. Of those, 29% were user-initiated while the rest were implemented by the device, most often when the sensor wasn’t working.
In a study questionnaire, 96% reported that the system was easy to use.
AHCL vs 670G: Major improvements seen
Bergenstal presented data from the Fuzzy Logic Automated Insulin Regulation (FLAIR) study, funded by the National Institute of Diabetes and Digestive and Kidney Disease, comparing Medtronic’s AHCL-based system with the currently marketed 670G hybrid closed-loop, in 113 individuals with type 1 diabetes aged 14-29 years.
“This age group has traditionally been the most difficult group in which to optimize glucose management,” Bergenstal said.
FLAIR is believed to be the first-ever study comparing an investigational automated insulin delivery system with a commercially approved system, he noted. All participants used each automated insulin delivery system for 3 months in the randomized crossover trial.
The primary outcome, time spent above 180 mg/dL during the day combined with time below 54 mg/dL over 24 hours at baseline with the 670G and AHCL went from 42% to 37% to 34%, respectively, for the former and from 0.46% to 0.50% to 0.45%, respectively, for the latter.
The percentage time-in-range over 24 hours went from 57% at baseline to 67% with the AHCL versus 63% with the 670G. A1c levels dropped from 7.9% at baseline to 7.6% with the 670G and 7.4% with AHCL.
“Remember, these are the adolescents who are the toughest of the tough, yet there was a 10% increase in time-in-range ... this is very clinically significant,” Bergenstal said.
Even among 14 patients who had been using multiple daily injections without CGM prior to the study, a group often excluded from closed-loop studies, time-in-range improved from 45% at baseline to 63% with the 670G to 65% with AHCL.
“I’m making a plea not to exclude people just because they haven’t previously used technology,” Bergenstal said.
One patient who had dosed with extra insulin manually had a severe hypoglycemia event with AHCL. No patient had diabetic ketoacidosis.
The proportion of insulin given as auto-correction boluses was 36%, which is important as it means that the system was compensating for missed meal doses, a common phenomenon among teenagers, Bergenstal noted.
“There is still room for further improvement in glycemic control in this population of patients with type 1 diabetes, but AHCL represents a significant step forward,” he concluded.
New Zealand study: More data in youth show AHCL benefits
Unlike the US study populations of just teens aged 14 and older, and adults, the study data used for approval in the EU — from New Zealand — included a total of 60 patients with 20 children aged 7-15 years. It, too, was a 10-week randomized crossover clinical trial comparing the AHCL to a sensor-augmented pump system with an algorithm only for predictive low-glucose management (PLGM) and no adjustments for high blood glucose.
Time-in-range was 59% at baseline and 58% with PLGM, compared to 70.4% with AHCL, and most of the time-in-range improvement occurred at night. Time below 70 mg/dL dropped from 3.1% to 2.5% to 2.1%, respectively.
Similar to the US studies, participants spent 96% of the time in closed-loop mode with only 1.2 exits per week. On a questionnaire, 95% of patients agreed that the system was easy to use and 85% that the system improved their quality of life.
De Bock showed a slide with some quotes, including one from a parent saying, “We didn’t have to be fearful at night or have that thought when we opened her bedroom door in the morning that she might not be conscious,” and from a patient, “I forgot I had diabetes today.”
Bailey commented: “Of course these devices are not free. So, the challenge is how do we make them available, less expensive, and easy to use? We have our work cut out for us, but this is heartening data. Everything has gotten better but we’re not out of a job yet.”
Bailey has reported receiving research support from Abbott, Capillary Biomedical, Dexcom, Diasome, Eli Lilly, Kowa, Lexicon, Medtronic, Medtrum, Novo Nordisk, REMD, Sanofi, Senseonics, ViaCyte, vTv Therapeutics, Zealand Pharma, and consulting or speaking honoraria from Abbott, LifeScan, Novo Nordisk, Sanofi, and Medtronic. Bode has reported receiving consulting and speaker fees from Medtronic. Bergenstal has reported participating in clinical research, being an advisory board member, and/or serving as a consultant for Abbott Diabetes Care, Ascensia, CeQure, Dexcom, Eli Lilly, Hygieia, Senseonics, and United Healthcare. De Bock has reported receiving honoraria or expenses from Novo Nordisk, Sanofi, Pfizer, Medtronic, and Lilly, and research funds from Novo Nordisk and Medtronic.
This article first appeared on Medscape.com.
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available 670G hybrid closed-loop, particularly in young people with type 1 diabetes, new data suggest.
Automated insulin delivery systems are comprised of an insulin pump, continuous glucose monitor (CGM), and an automated insulin dosing algorithm.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented June 12 during the virtual American Diabetes Association (ADA) 80th Scientific Sessions. The AHCL is the algorithm used in Medtronic’s new MiniMed 780G system, which received a CE Mark on June 11 for the treatment of type 1 diabetes in people aged 7 to 80 years.
One trial, presented by Bruce W. Bode, MD, of Atlanta Diabetes Associates, Georgia, was the US pivotal safety study that will be submitted to the US Food and Drug Administration for approval of the Medtronic 780G.
Another trial, presented by Richard M. Bergenstal, MD, executive director of the International Diabetes Center at Park Nicollet, Minneapolis, Minnesota, was a separate comparison of the AHCL with the 670G. (The AHCL-based system used in the three trials was identical to the 780G except it didn’t include Bluetooth, which will be a feature of the final product.)
A third trial, presented by Martin de Bock, PhD, of the University of Otago, New Zealand, included the CE Mark dataset for the 780G.
In contrast to the 670G, the 780G adds automated correction boluses for high blood glucose levels (rather than simply adjusting the basal infusion) and allows for adjustment of target glucose levels down to 100 mg/dL rather than a minimum of 120 mg/dL.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction. And specifically compared to the 670G, the AHCL-based system reverts to open-loop far less often because it only exits closed-loop mode when the sensor stops working or during sensor changes, but not during hyperglycemia even above 300 mg/dL.
Asked to comment, session moderator Timothy S. Bailey, MD, president and CEO of the AMCR Institute, Escondido, California, told Medscape Medical News: “Automated insulin delivery systems are getting better and better.”
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They all take people from where they are now to better time-in-range, less time with hypoglycemia, and most important, they might make the quality of their lives better. That’s really underappreciated.”
One factor that has allowed for the improvements, Bailey said, is the recognition by regulatory bodies that the hybrid closed-loop devices are generally safer than current open-loop type 1 diabetes management so that fewer “safety” device features that interfere with tight glycemic control are necessary.
With first-generation closed-loop systems, “If a wide variety of conditions occur, users get kicked off [hybrid closed-loop mode]. Originally it was perceived by the regulatory agencies as a safety feature because they perceived the standard of care as safe. The new system was allowed to have fewer rules.”
Pivotal trial: Time-in-range improved, 96% say system easy to use
The goal of the AHCL system is to maximize the time-in-range of blood glucose between 70-180 mg/dL. Automated basal delivery of insulin is programmed to a set-point of 100 or 120 mg/dL, with dosing every 5 minutes.
The US pivotal trial was a single-arm, 16-center, in-home trial of 157 people with type 1 diabetes, including 39 adolescents aged 14-21 years and 118 adults aged 22-75 years. All had type 1 diabetes for at least 2 years, A1c levels below 10%, and had been using insulin pumps for at least 6 months, with or without CGMs.
After a 14-day run-in, they wore the systems with a 100 or 120 mg/dL set-point for 45 days, then switched to the other setpoint for another 45 days. Average A1c dropped from 7.5% to 7.0%, with the proportions having an A1c ≤ 7.0% increasing from 34% to 61%.
Overall time-in-range was 75% compared to 69% at baseline, with time below range (< 70 mg/dL) of 1.8%. Overnight time-in-range was 82%, with 1.5% below range. Time-in-range increased from 62% to 73% in the adolescents and from 71% to 75% in the adults.
There were no incidences of severe hypoglycemia or diabetic ketoacidosis, and no device-related serious adverse events.
Participants reported being in hybrid closed-loop, or auto-mode, 95% of the time, compared with 33% for those who had been previously using the 670G.
The number of AHCL exits was 1.3 per week, significantly less than with the 670G. Of those, 29% were user-initiated while the rest were implemented by the device, most often when the sensor wasn’t working.
In a study questionnaire, 96% reported that the system was easy to use.
AHCL vs 670G: Major improvements seen
Bergenstal presented data from the Fuzzy Logic Automated Insulin Regulation (FLAIR) study, funded by the National Institute of Diabetes and Digestive and Kidney Disease, comparing Medtronic’s AHCL-based system with the currently marketed 670G hybrid closed-loop, in 113 individuals with type 1 diabetes aged 14-29 years.
“This age group has traditionally been the most difficult group in which to optimize glucose management,” Bergenstal said.
FLAIR is believed to be the first-ever study comparing an investigational automated insulin delivery system with a commercially approved system, he noted. All participants used each automated insulin delivery system for 3 months in the randomized crossover trial.
The primary outcome, time spent above 180 mg/dL during the day combined with time below 54 mg/dL over 24 hours at baseline with the 670G and AHCL went from 42% to 37% to 34%, respectively, for the former and from 0.46% to 0.50% to 0.45%, respectively, for the latter.
The percentage time-in-range over 24 hours went from 57% at baseline to 67% with the AHCL versus 63% with the 670G. A1c levels dropped from 7.9% at baseline to 7.6% with the 670G and 7.4% with AHCL.
“Remember, these are the adolescents who are the toughest of the tough, yet there was a 10% increase in time-in-range ... this is very clinically significant,” Bergenstal said.
Even among 14 patients who had been using multiple daily injections without CGM prior to the study, a group often excluded from closed-loop studies, time-in-range improved from 45% at baseline to 63% with the 670G to 65% with AHCL.
“I’m making a plea not to exclude people just because they haven’t previously used technology,” Bergenstal said.
One patient who had dosed with extra insulin manually had a severe hypoglycemia event with AHCL. No patient had diabetic ketoacidosis.
The proportion of insulin given as auto-correction boluses was 36%, which is important as it means that the system was compensating for missed meal doses, a common phenomenon among teenagers, Bergenstal noted.
“There is still room for further improvement in glycemic control in this population of patients with type 1 diabetes, but AHCL represents a significant step forward,” he concluded.
New Zealand study: More data in youth show AHCL benefits
Unlike the US study populations of just teens aged 14 and older, and adults, the study data used for approval in the EU — from New Zealand — included a total of 60 patients with 20 children aged 7-15 years. It, too, was a 10-week randomized crossover clinical trial comparing the AHCL to a sensor-augmented pump system with an algorithm only for predictive low-glucose management (PLGM) and no adjustments for high blood glucose.
Time-in-range was 59% at baseline and 58% with PLGM, compared to 70.4% with AHCL, and most of the time-in-range improvement occurred at night. Time below 70 mg/dL dropped from 3.1% to 2.5% to 2.1%, respectively.
Similar to the US studies, participants spent 96% of the time in closed-loop mode with only 1.2 exits per week. On a questionnaire, 95% of patients agreed that the system was easy to use and 85% that the system improved their quality of life.
De Bock showed a slide with some quotes, including one from a parent saying, “We didn’t have to be fearful at night or have that thought when we opened her bedroom door in the morning that she might not be conscious,” and from a patient, “I forgot I had diabetes today.”
Bailey commented: “Of course these devices are not free. So, the challenge is how do we make them available, less expensive, and easy to use? We have our work cut out for us, but this is heartening data. Everything has gotten better but we’re not out of a job yet.”
Bailey has reported receiving research support from Abbott, Capillary Biomedical, Dexcom, Diasome, Eli Lilly, Kowa, Lexicon, Medtronic, Medtrum, Novo Nordisk, REMD, Sanofi, Senseonics, ViaCyte, vTv Therapeutics, Zealand Pharma, and consulting or speaking honoraria from Abbott, LifeScan, Novo Nordisk, Sanofi, and Medtronic. Bode has reported receiving consulting and speaker fees from Medtronic. Bergenstal has reported participating in clinical research, being an advisory board member, and/or serving as a consultant for Abbott Diabetes Care, Ascensia, CeQure, Dexcom, Eli Lilly, Hygieia, Senseonics, and United Healthcare. De Bock has reported receiving honoraria or expenses from Novo Nordisk, Sanofi, Pfizer, Medtronic, and Lilly, and research funds from Novo Nordisk and Medtronic.
This article first appeared on Medscape.com.
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available 670G hybrid closed-loop, particularly in young people with type 1 diabetes, new data suggest.
Automated insulin delivery systems are comprised of an insulin pump, continuous glucose monitor (CGM), and an automated insulin dosing algorithm.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented June 12 during the virtual American Diabetes Association (ADA) 80th Scientific Sessions. The AHCL is the algorithm used in Medtronic’s new MiniMed 780G system, which received a CE Mark on June 11 for the treatment of type 1 diabetes in people aged 7 to 80 years.
One trial, presented by Bruce W. Bode, MD, of Atlanta Diabetes Associates, Georgia, was the US pivotal safety study that will be submitted to the US Food and Drug Administration for approval of the Medtronic 780G.
Another trial, presented by Richard M. Bergenstal, MD, executive director of the International Diabetes Center at Park Nicollet, Minneapolis, Minnesota, was a separate comparison of the AHCL with the 670G. (The AHCL-based system used in the three trials was identical to the 780G except it didn’t include Bluetooth, which will be a feature of the final product.)
A third trial, presented by Martin de Bock, PhD, of the University of Otago, New Zealand, included the CE Mark dataset for the 780G.
In contrast to the 670G, the 780G adds automated correction boluses for high blood glucose levels (rather than simply adjusting the basal infusion) and allows for adjustment of target glucose levels down to 100 mg/dL rather than a minimum of 120 mg/dL.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction. And specifically compared to the 670G, the AHCL-based system reverts to open-loop far less often because it only exits closed-loop mode when the sensor stops working or during sensor changes, but not during hyperglycemia even above 300 mg/dL.
Asked to comment, session moderator Timothy S. Bailey, MD, president and CEO of the AMCR Institute, Escondido, California, told Medscape Medical News: “Automated insulin delivery systems are getting better and better.”
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They all take people from where they are now to better time-in-range, less time with hypoglycemia, and most important, they might make the quality of their lives better. That’s really underappreciated.”
One factor that has allowed for the improvements, Bailey said, is the recognition by regulatory bodies that the hybrid closed-loop devices are generally safer than current open-loop type 1 diabetes management so that fewer “safety” device features that interfere with tight glycemic control are necessary.
With first-generation closed-loop systems, “If a wide variety of conditions occur, users get kicked off [hybrid closed-loop mode]. Originally it was perceived by the regulatory agencies as a safety feature because they perceived the standard of care as safe. The new system was allowed to have fewer rules.”
Pivotal trial: Time-in-range improved, 96% say system easy to use
The goal of the AHCL system is to maximize the time-in-range of blood glucose between 70-180 mg/dL. Automated basal delivery of insulin is programmed to a set-point of 100 or 120 mg/dL, with dosing every 5 minutes.
The US pivotal trial was a single-arm, 16-center, in-home trial of 157 people with type 1 diabetes, including 39 adolescents aged 14-21 years and 118 adults aged 22-75 years. All had type 1 diabetes for at least 2 years, A1c levels below 10%, and had been using insulin pumps for at least 6 months, with or without CGMs.
After a 14-day run-in, they wore the systems with a 100 or 120 mg/dL set-point for 45 days, then switched to the other setpoint for another 45 days. Average A1c dropped from 7.5% to 7.0%, with the proportions having an A1c ≤ 7.0% increasing from 34% to 61%.
Overall time-in-range was 75% compared to 69% at baseline, with time below range (< 70 mg/dL) of 1.8%. Overnight time-in-range was 82%, with 1.5% below range. Time-in-range increased from 62% to 73% in the adolescents and from 71% to 75% in the adults.
There were no incidences of severe hypoglycemia or diabetic ketoacidosis, and no device-related serious adverse events.
Participants reported being in hybrid closed-loop, or auto-mode, 95% of the time, compared with 33% for those who had been previously using the 670G.
The number of AHCL exits was 1.3 per week, significantly less than with the 670G. Of those, 29% were user-initiated while the rest were implemented by the device, most often when the sensor wasn’t working.
In a study questionnaire, 96% reported that the system was easy to use.
AHCL vs 670G: Major improvements seen
Bergenstal presented data from the Fuzzy Logic Automated Insulin Regulation (FLAIR) study, funded by the National Institute of Diabetes and Digestive and Kidney Disease, comparing Medtronic’s AHCL-based system with the currently marketed 670G hybrid closed-loop, in 113 individuals with type 1 diabetes aged 14-29 years.
“This age group has traditionally been the most difficult group in which to optimize glucose management,” Bergenstal said.
FLAIR is believed to be the first-ever study comparing an investigational automated insulin delivery system with a commercially approved system, he noted. All participants used each automated insulin delivery system for 3 months in the randomized crossover trial.
The primary outcome, time spent above 180 mg/dL during the day combined with time below 54 mg/dL over 24 hours at baseline with the 670G and AHCL went from 42% to 37% to 34%, respectively, for the former and from 0.46% to 0.50% to 0.45%, respectively, for the latter.
The percentage time-in-range over 24 hours went from 57% at baseline to 67% with the AHCL versus 63% with the 670G. A1c levels dropped from 7.9% at baseline to 7.6% with the 670G and 7.4% with AHCL.
“Remember, these are the adolescents who are the toughest of the tough, yet there was a 10% increase in time-in-range ... this is very clinically significant,” Bergenstal said.
Even among 14 patients who had been using multiple daily injections without CGM prior to the study, a group often excluded from closed-loop studies, time-in-range improved from 45% at baseline to 63% with the 670G to 65% with AHCL.
“I’m making a plea not to exclude people just because they haven’t previously used technology,” Bergenstal said.
One patient who had dosed with extra insulin manually had a severe hypoglycemia event with AHCL. No patient had diabetic ketoacidosis.
The proportion of insulin given as auto-correction boluses was 36%, which is important as it means that the system was compensating for missed meal doses, a common phenomenon among teenagers, Bergenstal noted.
“There is still room for further improvement in glycemic control in this population of patients with type 1 diabetes, but AHCL represents a significant step forward,” he concluded.
New Zealand study: More data in youth show AHCL benefits
Unlike the US study populations of just teens aged 14 and older, and adults, the study data used for approval in the EU — from New Zealand — included a total of 60 patients with 20 children aged 7-15 years. It, too, was a 10-week randomized crossover clinical trial comparing the AHCL to a sensor-augmented pump system with an algorithm only for predictive low-glucose management (PLGM) and no adjustments for high blood glucose.
Time-in-range was 59% at baseline and 58% with PLGM, compared to 70.4% with AHCL, and most of the time-in-range improvement occurred at night. Time below 70 mg/dL dropped from 3.1% to 2.5% to 2.1%, respectively.
Similar to the US studies, participants spent 96% of the time in closed-loop mode with only 1.2 exits per week. On a questionnaire, 95% of patients agreed that the system was easy to use and 85% that the system improved their quality of life.
De Bock showed a slide with some quotes, including one from a parent saying, “We didn’t have to be fearful at night or have that thought when we opened her bedroom door in the morning that she might not be conscious,” and from a patient, “I forgot I had diabetes today.”
Bailey commented: “Of course these devices are not free. So, the challenge is how do we make them available, less expensive, and easy to use? We have our work cut out for us, but this is heartening data. Everything has gotten better but we’re not out of a job yet.”
Bailey has reported receiving research support from Abbott, Capillary Biomedical, Dexcom, Diasome, Eli Lilly, Kowa, Lexicon, Medtronic, Medtrum, Novo Nordisk, REMD, Sanofi, Senseonics, ViaCyte, vTv Therapeutics, Zealand Pharma, and consulting or speaking honoraria from Abbott, LifeScan, Novo Nordisk, Sanofi, and Medtronic. Bode has reported receiving consulting and speaker fees from Medtronic. Bergenstal has reported participating in clinical research, being an advisory board member, and/or serving as a consultant for Abbott Diabetes Care, Ascensia, CeQure, Dexcom, Eli Lilly, Hygieia, Senseonics, and United Healthcare. De Bock has reported receiving honoraria or expenses from Novo Nordisk, Sanofi, Pfizer, Medtronic, and Lilly, and research funds from Novo Nordisk and Medtronic.
This article first appeared on Medscape.com.
FROM ADA 2020
Ventricular tachycardia storm responds to magnetic stimulation
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.
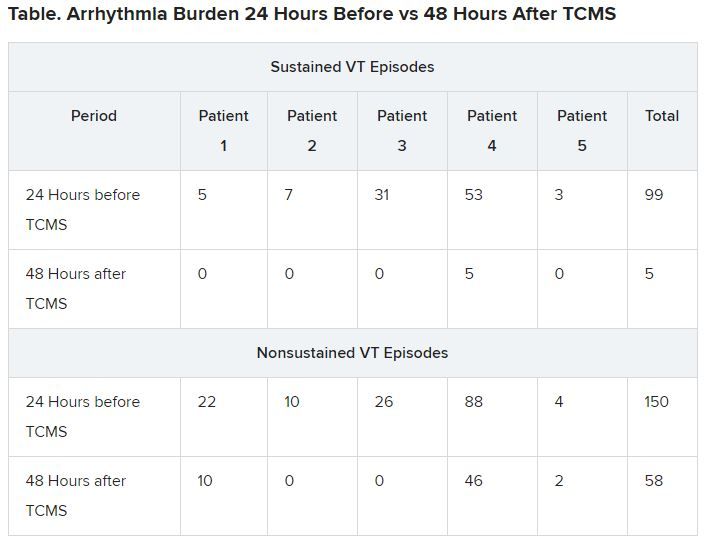
In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.

In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
In a pilot study of five patients with ventricular tachycardia (VT) storm that was refractory to antiarrhythmic drug therapy, treatment with noninvasive transcutaneous magnetic stimulation (TCMS) was associated with a lower arrhythmia burden.
The five patients were men aged 40 to 68 years with VT storm, defined as at least three episodes of sustained VT in the preceding 24 hours. The patients experienced a drop in both sustained and nonsustained VT with TCMS.
The study “aimed at developing a novel system for noninvasively and nondestructively interrupting the sympathetic tone,” corresponding author Timothy M. Markman, MD, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, told theheart.org | Medscape Cardiology. “We demonstrated that the technique was safe and that there was a strong signal of efficacy,” he added.
“We know that interrupting the sympathetic tone in these patients is beneficial,” said Markman, “but our strategies for doing so are mostly invasive and associated with a significant risk profile.”
The research letter was published online May 5 in the Journal of the American Medical Association. It was also presented during the virtual Heart Rhythm Society 2020 conference.
Growing body of evidence
Numerous studies have linked autonomic neuromodulation, including local blockade of the left stellate ganglion, with a reduction of cardiac sympathetic input in patients with VT storm, the authors write.
“This adds to a growing body of literature that autonomic neuromodulation is a valuable tool in the management of arrhythmias,” said Markman.
The use of magnetic stimulation to treat arrhythmias by targeting cardiac sympathetic innervation has been demonstrated in animal studies. The authors note that, to their knowledge, this is the first study involving humans.
Evidence suggests that TCMS may serve as a bridge for patients with difficult-to- treat VT to reduce VT and eliminate antiarrhythmic drug therapies and the associated risks, the authors say.
A lower VT burden
Five participants were included in the study. The patients were followed from March 2019 to June 2019. All had experienced at least three episodes of sustained VT (>30 sec) in the 24 hours preceding treatment. Patients with implantable cardiac devices were excluded.
The investigators used a figure 8 TCMS coil that was attached to a magnetic stimulation system positioned lateral to the C7 spinous process in approximation of the left stellate ganglion. TCMS was delivered at 80% of the left trapezius motor threshold at a frequency of 0.9 Hz for 60 minutes, the authors write. For one patient (patient no. 4), TCMS was shut off after 17 minutes, owing to the coil’s overheating. That resulted in the patient’s not being able to complete the protocol, they note.
Patients were monitored during and immediately after treatment for adverse events, including hemodynamic compromise, local discomfort, and skin irritation.
Results showed that compared to the 24-hour baseline period, sustained VT was reduced from 99 to five episodes, and nonsustained VT was reduced from 150 to 58 episodes in the 48 hours following TCMS.

In addition, 41 total external shocks were performed at the 24-hour baseline before TCMS. No external shocks were performed 48 hours after TCMS treatment.
Of the three patients who were not under sedation, none reported discomfort from TCMS.
Before TCMS treatment began, VT was refractory to a mean (SD) of 2.5 (2.1) antiarrhythmic drugs per patient. Within the 48-hour follow-up, patients received a mean of 1.2 (0.7) antiarrhythmic drugs. No additional antiarrhythmic drug was added, the authors note. Only patient no. 4, who did not complete the protocol, underwent ablation 36 hours post enrollment, they add.
The authors note some limitations, such as small case number. Markman told theheart.org | Medscape Cardiology that enrollment of patients in a randomized, sham-controlled trial to demonstrate efficacy is underway.
Physiology studies to evaluate the effects of this therapy while optimizing the technical aspects of the delivery of transcutaneous magnetic stimulation are also being conducted, he adds. Other limitations include the absence of control measures and exclusion of patients with implantable cardiac devices.
A potential addition to treatment
Gordon F. Tomaselli, MD, past president of the American Heart Association and current dean of the Albert Einstein College of Medicine, New York City, who was not involved in the research, told theheart.org | Medscape Cardiology that “the results are kind of interesting; it actually changes the function in the ganglion in the neck that actually innervates the heart, excites the heart, if you will.
“Clearly it wasn’t something that was just happening while this therapy was applied, but instead there’s some changes made when the sympathetic ganglion is targeted,” Tomaselli said. “They’re changing it functionally somehow, reducing the stimulating input to the heart, and in doing so, reducing the frequency of arrhythmias.”
Tomaselli suggested TCMS might be helpful in choosing among alternative treatments, such as sympathetic denervation. “It might also be a way to decide whether or not somebody might benefit, for example, from permanent dissection,” he said. “If you do this therapy, if it quiets things down but then it comes back after a while, you may consider denervation of that ganglion.”
Tomaselli adds that this treatment might be applied in different ways. “In some future iteration, it could even be implantable, could be patient activated or automatically activated ― for example, if a rapid heart rate is detected, that kind of thing.”
He noted that “there may be applications of this ultra-low frequency to other arrythmias, more common arrythmias, less life-threatening arrythmias, like atrial fibrillation; so there are a number of ways you might consider using this to treat cardiac rhythm disturbances by targeting the nervous system.”
Nazarian has consulted for Siemens, CardioSolv, and Circle Software and is a principle investigator for research funding to the University of Pennsylvania from Biosense-Webster, Siemens, ImriCor, and the National Institutes of Health. No other relevant financial relationships have been disclosed.
This story first appeared on Medscape.com.
Daily Recap: Stressed out primary care docs, ‘hospital at home’ for cancer patients
Here are the stories our MDedge editors across specialties think you need to know about today:
Racism, COVID-19 lead to sky-high stress levels
Primary care clinicians, already experiencing all-time high stress levels related to COVID-19, are now struggling to cope with the fallout from racism and the death of George Floyd, according to a survey conducted June 5-8.
When asked how the situation has affected their practices, 12% of the survey’s 586 respondents “drew clear connections between the current racial unrest and the health of their patients,” the Larry A. Green Center said in a recent statement. One-third of the clinicians also said that recent racism-related events have had a negative effect on their own well-being.
In a related survey of 1,111 patients conducted June 8 about 65% of patients said that racism affected emotional, psychological, and behavioral health.
“The fact that so many patients and clinicians agree that racism is a driver of health points to the incredible role primary care plays in creating safe spaces to process deep societal and personal issues,” said Christine Bechtel, cofounder of 3rd Conversation, a community of patients and providers. Read more.
Medical teams take to the streets
They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go. Many are medical professionals who see parallels between the front lines of COVID-19, where they confront stark racial imbalances among those stricken by the coronavirus, and what they see as racialized police brutality.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. “I am working full time and basically being at the protest after getting straight off of work,” said Ms. Butler, who is black. That’s tiring, she added, but so is being a black woman in America. Read more.
At-home management of type 1 diabetes, COVID-19
Although hyperglycemia and diabetic ketoacidosis are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
These new findings are still preliminary and were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
The published study includes data as of May 5 on 64 individuals from a total of 64 US sites. Since the paper was submitted, there are now 220 patients from 68 sites. There were two deaths in the preliminary report, Dr. Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Overall, 34.9% of patients were able to manage COVID-19 entirely at home. At the other extreme, 22.2% of patients overall were admitted to the intensive care unit. Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to the hospital. “Even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Dr. Ebekozien said. Read more.
‘Hospital at home’ for cancer patients
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations, 45% lower odds of ED visits, and 50% lower cumulative charges, as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Dr. Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000). Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Racism, COVID-19 lead to sky-high stress levels
Primary care clinicians, already experiencing all-time high stress levels related to COVID-19, are now struggling to cope with the fallout from racism and the death of George Floyd, according to a survey conducted June 5-8.
When asked how the situation has affected their practices, 12% of the survey’s 586 respondents “drew clear connections between the current racial unrest and the health of their patients,” the Larry A. Green Center said in a recent statement. One-third of the clinicians also said that recent racism-related events have had a negative effect on their own well-being.
In a related survey of 1,111 patients conducted June 8 about 65% of patients said that racism affected emotional, psychological, and behavioral health.
“The fact that so many patients and clinicians agree that racism is a driver of health points to the incredible role primary care plays in creating safe spaces to process deep societal and personal issues,” said Christine Bechtel, cofounder of 3rd Conversation, a community of patients and providers. Read more.
Medical teams take to the streets
They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go. Many are medical professionals who see parallels between the front lines of COVID-19, where they confront stark racial imbalances among those stricken by the coronavirus, and what they see as racialized police brutality.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. “I am working full time and basically being at the protest after getting straight off of work,” said Ms. Butler, who is black. That’s tiring, she added, but so is being a black woman in America. Read more.
At-home management of type 1 diabetes, COVID-19
Although hyperglycemia and diabetic ketoacidosis are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
These new findings are still preliminary and were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
The published study includes data as of May 5 on 64 individuals from a total of 64 US sites. Since the paper was submitted, there are now 220 patients from 68 sites. There were two deaths in the preliminary report, Dr. Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Overall, 34.9% of patients were able to manage COVID-19 entirely at home. At the other extreme, 22.2% of patients overall were admitted to the intensive care unit. Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to the hospital. “Even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Dr. Ebekozien said. Read more.
‘Hospital at home’ for cancer patients
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations, 45% lower odds of ED visits, and 50% lower cumulative charges, as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Dr. Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000). Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Racism, COVID-19 lead to sky-high stress levels
Primary care clinicians, already experiencing all-time high stress levels related to COVID-19, are now struggling to cope with the fallout from racism and the death of George Floyd, according to a survey conducted June 5-8.
When asked how the situation has affected their practices, 12% of the survey’s 586 respondents “drew clear connections between the current racial unrest and the health of their patients,” the Larry A. Green Center said in a recent statement. One-third of the clinicians also said that recent racism-related events have had a negative effect on their own well-being.
In a related survey of 1,111 patients conducted June 8 about 65% of patients said that racism affected emotional, psychological, and behavioral health.
“The fact that so many patients and clinicians agree that racism is a driver of health points to the incredible role primary care plays in creating safe spaces to process deep societal and personal issues,” said Christine Bechtel, cofounder of 3rd Conversation, a community of patients and providers. Read more.
Medical teams take to the streets
They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go. Many are medical professionals who see parallels between the front lines of COVID-19, where they confront stark racial imbalances among those stricken by the coronavirus, and what they see as racialized police brutality.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. “I am working full time and basically being at the protest after getting straight off of work,” said Ms. Butler, who is black. That’s tiring, she added, but so is being a black woman in America. Read more.
At-home management of type 1 diabetes, COVID-19
Although hyperglycemia and diabetic ketoacidosis are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
These new findings are still preliminary and were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
The published study includes data as of May 5 on 64 individuals from a total of 64 US sites. Since the paper was submitted, there are now 220 patients from 68 sites. There were two deaths in the preliminary report, Dr. Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Overall, 34.9% of patients were able to manage COVID-19 entirely at home. At the other extreme, 22.2% of patients overall were admitted to the intensive care unit. Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to the hospital. “Even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Dr. Ebekozien said. Read more.
‘Hospital at home’ for cancer patients
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations, 45% lower odds of ED visits, and 50% lower cumulative charges, as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Dr. Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000). Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Many COVID patients shed virus in feces, even without GI symptoms
Even without GI symptoms, many patients with COVID-19 shed viral RNA in feces, suggesting that stool testing and prevention of fecal-oral transmission may be needed to combat the ongoing pandemic, according to investigators.
A meta-analysis of 29 studies showed that 12% of patients with COVID-19 developed nausea, diarrhea, or vomiting, while 41% shed viral RNA in feces, reported lead author Sravanthi Parasa, MD, of Swedish Medical Center, Seattle.Writing in JAMA Network Open, Dr. Parasa and colleagues emphasized that respiratory symptoms remain the predominant form of disease; however, GI symptoms can occur.
“In fact, the first reported patient with COVID-19 in the U.S. reported GI symptoms of loose bowel movements and abdominal discomfort,” the investigators wrote, noting that the patient went on to test positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in both respiratory and stool specimens.
“This raises the question of inadvertent human-to-human transmission via the fecal route despite public health emphasis on droplet transmission and precautions for contact with respiratory secretions,” the investigators wrote.
To address this question, the investigators conducted a systematic review and meta-analysis involving 23 published and 6 preprint studies involving a total of 4,805 patients, all of whom tested positive for SARS-CoV-2 based on PCR results from nasopharyngeal swabs. Dr. Parasa and colleagues noted that most of the studies “scored between 8 and 10 on the MINORS quality assessment,” suggesting moderate quality.
Pooled data from these studies showed that 4.6% of patients reported nausea or vomiting, while 7.4% reported diarrhea. Such symptoms may serve as an early warning flag for clinicians, the investigators noted.
“[T]he presence of GI symptoms may portend a worse outcome for patients infected with SARS-CoV-2,” they wrote, citing a study by Pan and colleagues, which found that GI symptoms were associated with lower rates of recovery and hospital discharge.
Regardless of GI symptoms, 40.5% of patients in the meta-analysis tested positive for viral RNA in feces (95% confidence interval, 27.4%-55.1%). Duration of viral shedding in feces lasted up to 11 days after symptom onset, or in a single-patient case study, 18 days after hospitalization.
The investigators called these duration figures “particularly concerning,” especially in light of a study published by Xiao and colleagues, which showed that 23.3% of patients with negative respiratory tests were still shedding live virus in feces.
“[T]he fecal-oral route of transmission could be an additional potential source of infection spread,” wrote Dr. Parasa and colleagues. “Our results also suggest that testing of the virus in feces ... could be helpful in disease monitoring and surveillance.”
David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, said that the findings confirm what has been suspected for some time: GI disease is relatively common with COVID-19.
“The evidence is clear now that a sizable percentage of patients have GI symptoms,” Dr. Johnson said in an interview.
GI issues may precede respiratory signs, he added, so clinicians should be aware that nausea, vomiting, or diarrhea could be early indicators of COVID-19, and possibly, a worse outcome.
“The other highlight of this study is that stool shedding may be extended beyond respiratory shedding,” Dr. Johnson said.
He suggested that this finding could influence current CDC criteria, which define absence of infectious risk by two consecutive, negative nasopharyngeal swabs. Instead, fecal testing may be needed, he said, along with measures to prevent fecal-oral transmission.
Dr. Johnson expressed particular concern for risk of infection via toilet plume, in which toilet flushing aerosolizes viral particles.
“As much as people try to social distance by 6 feet – you can do that when you walk into a store, or a building, but you can’t necessarily do that when you walk into a public toilet, where the plume may have been expansive for a period of time,” he said. “That toilet may never really get cleaned to a high level of disinfection, and those droplets set up potential for fecal-oral spread.”
Dr. Sharma disclosed relationships with Medtronic, Fujifilm, Boston Scientific, and others. Dr. Johnson disclosed no relevant conflicts of interest.
SOURCE: Parasa S et al. JAMA Network Open. 2020 Jun 11. doi: 10.1001/jamanetworkopen.2020.11335.
Even without GI symptoms, many patients with COVID-19 shed viral RNA in feces, suggesting that stool testing and prevention of fecal-oral transmission may be needed to combat the ongoing pandemic, according to investigators.
A meta-analysis of 29 studies showed that 12% of patients with COVID-19 developed nausea, diarrhea, or vomiting, while 41% shed viral RNA in feces, reported lead author Sravanthi Parasa, MD, of Swedish Medical Center, Seattle.Writing in JAMA Network Open, Dr. Parasa and colleagues emphasized that respiratory symptoms remain the predominant form of disease; however, GI symptoms can occur.
“In fact, the first reported patient with COVID-19 in the U.S. reported GI symptoms of loose bowel movements and abdominal discomfort,” the investigators wrote, noting that the patient went on to test positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in both respiratory and stool specimens.
“This raises the question of inadvertent human-to-human transmission via the fecal route despite public health emphasis on droplet transmission and precautions for contact with respiratory secretions,” the investigators wrote.
To address this question, the investigators conducted a systematic review and meta-analysis involving 23 published and 6 preprint studies involving a total of 4,805 patients, all of whom tested positive for SARS-CoV-2 based on PCR results from nasopharyngeal swabs. Dr. Parasa and colleagues noted that most of the studies “scored between 8 and 10 on the MINORS quality assessment,” suggesting moderate quality.
Pooled data from these studies showed that 4.6% of patients reported nausea or vomiting, while 7.4% reported diarrhea. Such symptoms may serve as an early warning flag for clinicians, the investigators noted.
“[T]he presence of GI symptoms may portend a worse outcome for patients infected with SARS-CoV-2,” they wrote, citing a study by Pan and colleagues, which found that GI symptoms were associated with lower rates of recovery and hospital discharge.
Regardless of GI symptoms, 40.5% of patients in the meta-analysis tested positive for viral RNA in feces (95% confidence interval, 27.4%-55.1%). Duration of viral shedding in feces lasted up to 11 days after symptom onset, or in a single-patient case study, 18 days after hospitalization.
The investigators called these duration figures “particularly concerning,” especially in light of a study published by Xiao and colleagues, which showed that 23.3% of patients with negative respiratory tests were still shedding live virus in feces.
“[T]he fecal-oral route of transmission could be an additional potential source of infection spread,” wrote Dr. Parasa and colleagues. “Our results also suggest that testing of the virus in feces ... could be helpful in disease monitoring and surveillance.”
David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, said that the findings confirm what has been suspected for some time: GI disease is relatively common with COVID-19.
“The evidence is clear now that a sizable percentage of patients have GI symptoms,” Dr. Johnson said in an interview.
GI issues may precede respiratory signs, he added, so clinicians should be aware that nausea, vomiting, or diarrhea could be early indicators of COVID-19, and possibly, a worse outcome.
“The other highlight of this study is that stool shedding may be extended beyond respiratory shedding,” Dr. Johnson said.
He suggested that this finding could influence current CDC criteria, which define absence of infectious risk by two consecutive, negative nasopharyngeal swabs. Instead, fecal testing may be needed, he said, along with measures to prevent fecal-oral transmission.
Dr. Johnson expressed particular concern for risk of infection via toilet plume, in which toilet flushing aerosolizes viral particles.
“As much as people try to social distance by 6 feet – you can do that when you walk into a store, or a building, but you can’t necessarily do that when you walk into a public toilet, where the plume may have been expansive for a period of time,” he said. “That toilet may never really get cleaned to a high level of disinfection, and those droplets set up potential for fecal-oral spread.”
Dr. Sharma disclosed relationships with Medtronic, Fujifilm, Boston Scientific, and others. Dr. Johnson disclosed no relevant conflicts of interest.
SOURCE: Parasa S et al. JAMA Network Open. 2020 Jun 11. doi: 10.1001/jamanetworkopen.2020.11335.
Even without GI symptoms, many patients with COVID-19 shed viral RNA in feces, suggesting that stool testing and prevention of fecal-oral transmission may be needed to combat the ongoing pandemic, according to investigators.
A meta-analysis of 29 studies showed that 12% of patients with COVID-19 developed nausea, diarrhea, or vomiting, while 41% shed viral RNA in feces, reported lead author Sravanthi Parasa, MD, of Swedish Medical Center, Seattle.Writing in JAMA Network Open, Dr. Parasa and colleagues emphasized that respiratory symptoms remain the predominant form of disease; however, GI symptoms can occur.
“In fact, the first reported patient with COVID-19 in the U.S. reported GI symptoms of loose bowel movements and abdominal discomfort,” the investigators wrote, noting that the patient went on to test positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in both respiratory and stool specimens.
“This raises the question of inadvertent human-to-human transmission via the fecal route despite public health emphasis on droplet transmission and precautions for contact with respiratory secretions,” the investigators wrote.
To address this question, the investigators conducted a systematic review and meta-analysis involving 23 published and 6 preprint studies involving a total of 4,805 patients, all of whom tested positive for SARS-CoV-2 based on PCR results from nasopharyngeal swabs. Dr. Parasa and colleagues noted that most of the studies “scored between 8 and 10 on the MINORS quality assessment,” suggesting moderate quality.
Pooled data from these studies showed that 4.6% of patients reported nausea or vomiting, while 7.4% reported diarrhea. Such symptoms may serve as an early warning flag for clinicians, the investigators noted.
“[T]he presence of GI symptoms may portend a worse outcome for patients infected with SARS-CoV-2,” they wrote, citing a study by Pan and colleagues, which found that GI symptoms were associated with lower rates of recovery and hospital discharge.
Regardless of GI symptoms, 40.5% of patients in the meta-analysis tested positive for viral RNA in feces (95% confidence interval, 27.4%-55.1%). Duration of viral shedding in feces lasted up to 11 days after symptom onset, or in a single-patient case study, 18 days after hospitalization.
The investigators called these duration figures “particularly concerning,” especially in light of a study published by Xiao and colleagues, which showed that 23.3% of patients with negative respiratory tests were still shedding live virus in feces.
“[T]he fecal-oral route of transmission could be an additional potential source of infection spread,” wrote Dr. Parasa and colleagues. “Our results also suggest that testing of the virus in feces ... could be helpful in disease monitoring and surveillance.”
David A. Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, said that the findings confirm what has been suspected for some time: GI disease is relatively common with COVID-19.
“The evidence is clear now that a sizable percentage of patients have GI symptoms,” Dr. Johnson said in an interview.
GI issues may precede respiratory signs, he added, so clinicians should be aware that nausea, vomiting, or diarrhea could be early indicators of COVID-19, and possibly, a worse outcome.
“The other highlight of this study is that stool shedding may be extended beyond respiratory shedding,” Dr. Johnson said.
He suggested that this finding could influence current CDC criteria, which define absence of infectious risk by two consecutive, negative nasopharyngeal swabs. Instead, fecal testing may be needed, he said, along with measures to prevent fecal-oral transmission.
Dr. Johnson expressed particular concern for risk of infection via toilet plume, in which toilet flushing aerosolizes viral particles.
“As much as people try to social distance by 6 feet – you can do that when you walk into a store, or a building, but you can’t necessarily do that when you walk into a public toilet, where the plume may have been expansive for a period of time,” he said. “That toilet may never really get cleaned to a high level of disinfection, and those droplets set up potential for fecal-oral spread.”
Dr. Sharma disclosed relationships with Medtronic, Fujifilm, Boston Scientific, and others. Dr. Johnson disclosed no relevant conflicts of interest.
SOURCE: Parasa S et al. JAMA Network Open. 2020 Jun 11. doi: 10.1001/jamanetworkopen.2020.11335.
FROM JAMA NETWORK OPEN
Half of type 1 diabetes patients with COVID-19 manage at home
New preliminary data from the T1D Exchange suggest that, although hyperglycemia and diabetic ketoacidosis (DKA) are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
The new findings – the first US data on individuals with type 1 diabetes and COVID-19 – were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
Two UK studies are the only prior ones to previously examine the topic.
The newly published study includes data as of May 5 on 64 individuals from a total of 64 US sites, including 15 T1D Exchange member clinics and an additional 49 endocrinology clinics from around the country. Since the paper was submitted, there are now 220 patients from 68 sites. Another publication with a more detailed analysis of risk factors and adjustment for confounders is planned for later this year.
Some of the findings from the preliminary data have shifted, but many aspects remain consistent, Ebekozien told Medscape Medical News.
“One thing still very true, even with the unpublished findings, is the influence of A1c and glycemic management. ...With higher A1c levels, we’re seeing more COVID-19 hospitalizations and worse outcomes,” he said.
And as has been generally reported for COVID-19, high body mass index was a major risk factor in the preliminary dataset – and remains so.
There were two deaths in the preliminary report, both individuals with comorbidities in addition to type 1 diabetes, Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Interestingly, females predominate in both cohorts. That may be a reporting phenomenon, another factor that is being analyzed.
Hyperglycemia Remains a Major Risk Factor
The study is specifically being conducted by the T1D Exchange’s Quality Improvement Collaborative, which Ebekozien heads.
Data were obtained for 33 patients with type 1 diabetes who tested positive for COVID-19, and another 31 who were classified as “COVID-19–like” because they had symptoms consistent with COVID-19, as identified by the Centers for Disease Control and Prevention, but hadn’t been tested for the virus.
For all 64 patients, the mean age was 20.9 years and two thirds (65.6%) were aged 18 or younger. A higher proportion of the COVID-19–like patients were pediatric than the confirmed cases. The larger dataset includes more adult patients, Ebekozien told Medscape Medical News.
Overall, 60.9% of patients were female. Nearly half were white, a quarter Hispanic, and 18.8% black. More confirmed COVID-19 cases were black compared with suspected cases (30.3% vs 6.5%).
Median A1c for the overall group (including suspected COVID-19 cases) was 8.0%, but it was 8.5% among confirmed cases. Overall, six patients (9.8%) presented with new-onset type 1 diabetes after they developed COVID-19.
Hyperglycemia was present in half (32) of patients overall. DKA occurred in 19 people (30.2%): 15 of the confirmed COVID-19 cases (45.5%) versus just 4 (13.3%) of the COVID-19–like cases. Nausea was reported in 30.2% of patients overall.
Other symptoms were typical of COVID-19, including fever (41.3%), dry cough (38.1%), and shortness of breath (27.0%). Loss of taste and smell was less common, at just 9.5% overall.
Obesity was present in 39.7% of patients overall, with similar proportions in the confirmed and suspected COVID-19 groups. Hypertension and/or cardiovascular disease were present in 14.3% of patients overall, and the rate was similar between the two subgroups.
One of the two patients who died was a 79-year-old man who had hypertension and a prior stroke in addition to type 1 diabetes. The other was a 19-year-old woman with a history of asthma who developed a pulmonary embolism during the onset of COVID-19. Neither had DKA.
Even in Type 1 Diabetes, COVID-19 Can Be Managed at Home
Overall, 34.9% of patients were able to manage COVID-19 entirely at home, with 27.3% of the confirmed and 43.3% of the suspected cases able to do so.
At the other extreme, 22.2% of patients overall were admitted to the intensive care unit; 30.3% of the confirmed versus 13.3% of suspected cases.
Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to hospital.
“Interestingly, even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist. ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Ebekozien said.
“But, we’re seeing a good number of patients who are managed at home and the symptoms resolve in a week or two, and the illness runs its course, and they don’t have to even be seen,” he added.
The research team is also collecting data on barriers to remote care, including challenges with telemedicine and how frontline providers are navigating them.
“Those are all things that our future paper will be able to shed more light on,” he explained.
Endocrinologists around the country are invited to report cases of COVID-19 in patients with type 1 diabetes to the T1D Exchange by emailing QI@T1Dexchange.org.
And in fact, Ebekozien also requested that clinicians with a large type 1 diabetes population also report if they’ve had no COVID-19 cases.
“Even if they haven’t had a case, that’s very useful information for us to know. One of the things we want to calculate down the line is the incidence ratio. Not all participating sites have had a case.”
Endocrinologists from all the participating sites have formed a dedicated community that meets regularly via webinars to share information, he noted. “It’s been a very selfless effort to work collaboratively as a community to quickly answer critical questions.”
The Helmsley Charitable Trust funds the T1D Exchange Quality Improvement Collaborative. The T1D Exchange received financial support for this study from Abbott Diabetes, Dexcom, JDRF, Insulet Corporation, Lilly, Medtronic, and Tandem Diabetes Care. No other relevant financial relationships were reported.
This article first appeared on Medscape.com.
New preliminary data from the T1D Exchange suggest that, although hyperglycemia and diabetic ketoacidosis (DKA) are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
The new findings – the first US data on individuals with type 1 diabetes and COVID-19 – were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
Two UK studies are the only prior ones to previously examine the topic.
The newly published study includes data as of May 5 on 64 individuals from a total of 64 US sites, including 15 T1D Exchange member clinics and an additional 49 endocrinology clinics from around the country. Since the paper was submitted, there are now 220 patients from 68 sites. Another publication with a more detailed analysis of risk factors and adjustment for confounders is planned for later this year.
Some of the findings from the preliminary data have shifted, but many aspects remain consistent, Ebekozien told Medscape Medical News.
“One thing still very true, even with the unpublished findings, is the influence of A1c and glycemic management. ...With higher A1c levels, we’re seeing more COVID-19 hospitalizations and worse outcomes,” he said.
And as has been generally reported for COVID-19, high body mass index was a major risk factor in the preliminary dataset – and remains so.
There were two deaths in the preliminary report, both individuals with comorbidities in addition to type 1 diabetes, Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Interestingly, females predominate in both cohorts. That may be a reporting phenomenon, another factor that is being analyzed.
Hyperglycemia Remains a Major Risk Factor
The study is specifically being conducted by the T1D Exchange’s Quality Improvement Collaborative, which Ebekozien heads.
Data were obtained for 33 patients with type 1 diabetes who tested positive for COVID-19, and another 31 who were classified as “COVID-19–like” because they had symptoms consistent with COVID-19, as identified by the Centers for Disease Control and Prevention, but hadn’t been tested for the virus.
For all 64 patients, the mean age was 20.9 years and two thirds (65.6%) were aged 18 or younger. A higher proportion of the COVID-19–like patients were pediatric than the confirmed cases. The larger dataset includes more adult patients, Ebekozien told Medscape Medical News.
Overall, 60.9% of patients were female. Nearly half were white, a quarter Hispanic, and 18.8% black. More confirmed COVID-19 cases were black compared with suspected cases (30.3% vs 6.5%).
Median A1c for the overall group (including suspected COVID-19 cases) was 8.0%, but it was 8.5% among confirmed cases. Overall, six patients (9.8%) presented with new-onset type 1 diabetes after they developed COVID-19.
Hyperglycemia was present in half (32) of patients overall. DKA occurred in 19 people (30.2%): 15 of the confirmed COVID-19 cases (45.5%) versus just 4 (13.3%) of the COVID-19–like cases. Nausea was reported in 30.2% of patients overall.
Other symptoms were typical of COVID-19, including fever (41.3%), dry cough (38.1%), and shortness of breath (27.0%). Loss of taste and smell was less common, at just 9.5% overall.
Obesity was present in 39.7% of patients overall, with similar proportions in the confirmed and suspected COVID-19 groups. Hypertension and/or cardiovascular disease were present in 14.3% of patients overall, and the rate was similar between the two subgroups.
One of the two patients who died was a 79-year-old man who had hypertension and a prior stroke in addition to type 1 diabetes. The other was a 19-year-old woman with a history of asthma who developed a pulmonary embolism during the onset of COVID-19. Neither had DKA.
Even in Type 1 Diabetes, COVID-19 Can Be Managed at Home
Overall, 34.9% of patients were able to manage COVID-19 entirely at home, with 27.3% of the confirmed and 43.3% of the suspected cases able to do so.
At the other extreme, 22.2% of patients overall were admitted to the intensive care unit; 30.3% of the confirmed versus 13.3% of suspected cases.
Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to hospital.
“Interestingly, even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist. ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Ebekozien said.
“But, we’re seeing a good number of patients who are managed at home and the symptoms resolve in a week or two, and the illness runs its course, and they don’t have to even be seen,” he added.
The research team is also collecting data on barriers to remote care, including challenges with telemedicine and how frontline providers are navigating them.
“Those are all things that our future paper will be able to shed more light on,” he explained.
Endocrinologists around the country are invited to report cases of COVID-19 in patients with type 1 diabetes to the T1D Exchange by emailing QI@T1Dexchange.org.
And in fact, Ebekozien also requested that clinicians with a large type 1 diabetes population also report if they’ve had no COVID-19 cases.
“Even if they haven’t had a case, that’s very useful information for us to know. One of the things we want to calculate down the line is the incidence ratio. Not all participating sites have had a case.”
Endocrinologists from all the participating sites have formed a dedicated community that meets regularly via webinars to share information, he noted. “It’s been a very selfless effort to work collaboratively as a community to quickly answer critical questions.”
The Helmsley Charitable Trust funds the T1D Exchange Quality Improvement Collaborative. The T1D Exchange received financial support for this study from Abbott Diabetes, Dexcom, JDRF, Insulet Corporation, Lilly, Medtronic, and Tandem Diabetes Care. No other relevant financial relationships were reported.
This article first appeared on Medscape.com.
New preliminary data from the T1D Exchange suggest that, although hyperglycemia and diabetic ketoacidosis (DKA) are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
The new findings – the first US data on individuals with type 1 diabetes and COVID-19 – were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
Two UK studies are the only prior ones to previously examine the topic.
The newly published study includes data as of May 5 on 64 individuals from a total of 64 US sites, including 15 T1D Exchange member clinics and an additional 49 endocrinology clinics from around the country. Since the paper was submitted, there are now 220 patients from 68 sites. Another publication with a more detailed analysis of risk factors and adjustment for confounders is planned for later this year.
Some of the findings from the preliminary data have shifted, but many aspects remain consistent, Ebekozien told Medscape Medical News.
“One thing still very true, even with the unpublished findings, is the influence of A1c and glycemic management. ...With higher A1c levels, we’re seeing more COVID-19 hospitalizations and worse outcomes,” he said.
And as has been generally reported for COVID-19, high body mass index was a major risk factor in the preliminary dataset – and remains so.
There were two deaths in the preliminary report, both individuals with comorbidities in addition to type 1 diabetes, Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Interestingly, females predominate in both cohorts. That may be a reporting phenomenon, another factor that is being analyzed.
Hyperglycemia Remains a Major Risk Factor
The study is specifically being conducted by the T1D Exchange’s Quality Improvement Collaborative, which Ebekozien heads.
Data were obtained for 33 patients with type 1 diabetes who tested positive for COVID-19, and another 31 who were classified as “COVID-19–like” because they had symptoms consistent with COVID-19, as identified by the Centers for Disease Control and Prevention, but hadn’t been tested for the virus.
For all 64 patients, the mean age was 20.9 years and two thirds (65.6%) were aged 18 or younger. A higher proportion of the COVID-19–like patients were pediatric than the confirmed cases. The larger dataset includes more adult patients, Ebekozien told Medscape Medical News.
Overall, 60.9% of patients were female. Nearly half were white, a quarter Hispanic, and 18.8% black. More confirmed COVID-19 cases were black compared with suspected cases (30.3% vs 6.5%).
Median A1c for the overall group (including suspected COVID-19 cases) was 8.0%, but it was 8.5% among confirmed cases. Overall, six patients (9.8%) presented with new-onset type 1 diabetes after they developed COVID-19.
Hyperglycemia was present in half (32) of patients overall. DKA occurred in 19 people (30.2%): 15 of the confirmed COVID-19 cases (45.5%) versus just 4 (13.3%) of the COVID-19–like cases. Nausea was reported in 30.2% of patients overall.
Other symptoms were typical of COVID-19, including fever (41.3%), dry cough (38.1%), and shortness of breath (27.0%). Loss of taste and smell was less common, at just 9.5% overall.
Obesity was present in 39.7% of patients overall, with similar proportions in the confirmed and suspected COVID-19 groups. Hypertension and/or cardiovascular disease were present in 14.3% of patients overall, and the rate was similar between the two subgroups.
One of the two patients who died was a 79-year-old man who had hypertension and a prior stroke in addition to type 1 diabetes. The other was a 19-year-old woman with a history of asthma who developed a pulmonary embolism during the onset of COVID-19. Neither had DKA.
Even in Type 1 Diabetes, COVID-19 Can Be Managed at Home
Overall, 34.9% of patients were able to manage COVID-19 entirely at home, with 27.3% of the confirmed and 43.3% of the suspected cases able to do so.
At the other extreme, 22.2% of patients overall were admitted to the intensive care unit; 30.3% of the confirmed versus 13.3% of suspected cases.
Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to hospital.
“Interestingly, even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist. ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Ebekozien said.
“But, we’re seeing a good number of patients who are managed at home and the symptoms resolve in a week or two, and the illness runs its course, and they don’t have to even be seen,” he added.
The research team is also collecting data on barriers to remote care, including challenges with telemedicine and how frontline providers are navigating them.
“Those are all things that our future paper will be able to shed more light on,” he explained.
Endocrinologists around the country are invited to report cases of COVID-19 in patients with type 1 diabetes to the T1D Exchange by emailing QI@T1Dexchange.org.
And in fact, Ebekozien also requested that clinicians with a large type 1 diabetes population also report if they’ve had no COVID-19 cases.
“Even if they haven’t had a case, that’s very useful information for us to know. One of the things we want to calculate down the line is the incidence ratio. Not all participating sites have had a case.”
Endocrinologists from all the participating sites have formed a dedicated community that meets regularly via webinars to share information, he noted. “It’s been a very selfless effort to work collaboratively as a community to quickly answer critical questions.”
The Helmsley Charitable Trust funds the T1D Exchange Quality Improvement Collaborative. The T1D Exchange received financial support for this study from Abbott Diabetes, Dexcom, JDRF, Insulet Corporation, Lilly, Medtronic, and Tandem Diabetes Care. No other relevant financial relationships were reported.
This article first appeared on Medscape.com.
Electrosurgical choices lead to similar results
For endoscopists performing electrosurgical snare resection of large colorectal polyps, choosing between the blue foot pedal and the yellow foot pedal may be the least important step of the day, according to data from almost 1,000 patients.
Risks of severe adverse events and polyp recurrence were similar between cases in which blended current (yellow pedal) was used and those in which coagulation current (blue pedal) was used, reported lead author Heiko Pohl, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and colleagues.
“Although electrosurgical application is a fundamental aspect of polypectomy, various currents and settings are clinically used, and there are no accepted standards of practice,” the investigators wrote in Gastroenterology.
According to Dr. Pohl and colleagues, a 2004 study showed that the split between endoscopists using coagulation current and those using blended current was about 50-50 (46% vs. 46%), but no studies to date have tested the relative safety or efficacy of these approaches.
The investigators aimed to address this knowledge gap with a single-blinded study involving 928 patients who underwent endoscopic mucosal resection of nonpedunculated, large (20 mm or larger) colorectal polyps with an Erbe Vio® 300D electrosurgical unit (Erbe USA Inc., Marietta, Ga.) at 18 medical centers.
Patients were randomized in 2x2 factorial design involving clip closure versus no clip closure, and blended current (Endocut Q) versus pure coagulation current (Forced Coagulation). Although electrosurgical setting was initially a secondary intervention in the trial, post hoc analysis showed that interaction between the interventions was not significant (P = .957), allowing for the present, independent analysis of current type.
For this analysis, the primary outcome was severe adverse event rate, both during the procedure, and after the procedure for up to 30 days. Secondary outcomes included proportion of polyps completely excised and recurrence rate at time of first surveillance endoscopy.
Out of 928 patients randomized, 919 completed 30-day follow-up, and 675 underwent first surveillance colonoscopy. Baseline characteristics were similar between groups, apart from the proportion of individuals with more than one large polyp, which was significantly greater in the Endocut Q group (8.6% vs. 4.5%; P = .012), although the investigators noted that this imbalance did not affect main outcomes.
Rates of severe adverse events were similar between groups: 7.2% for the Endocut Q group and 7.9% for the Forced Coagulation group (P = .762). Groups also had similar rates of intra- and postprocedure adverse events, and types of adverse events.
Efficacy measures also revealed high similarity between cutting techniques. Endoscopists using Endocut achieved complete polyp removal 96% of the time, compared with 95% of the time when using Forced Coagulation (P = .267). Piecemeal resection rates were similar, at 90% and 87% for Endocut Q and Forced Coagulation, respectively (P = .270).
Although Endocut Q less frequently resulted in small residual tissue islands after initial snare resection (35% vs. 41%; P = .041), it more often caused intraprocedural bleeding that required treatment (17% vs. 11%; P = .006).
According to Dr. Pohl and colleagues, previous discussions have included concerns that such bleeding may impair visualization and therefore lead to higher rates of polyp recurrence; but surveillance colonoscopy, which was performed in 79% of patients, revealed a polyp recurrence rate of 17% for each group.
“Although we did not find a difference in recurrence between the two groups, our study cannot completely exclude this possibility,” the investigators added.
They also noted that six perforations occurred in the Endocut Q group, compared with three in the Forced Coagulation group, and suggested that this risk may be real, yet statistically unsupported by the present analysis because of sample size.
“Endoscopists using Endocut should therefore be aware of this potential risk and [ensure] that no muscularis propria is entrapped in the snare before electrosurgery is applied,” the investigators wrote.
Still, the investigators’ final conclusion supported the existing method of decision-making: personal choice.
“Overall, polyp resection with Endocut or Forced Coagulation did not differ with respect to severe adverse events, complete resection rate, or polyp recurrence,” they wrote. “This study therefore supports an individual approach based on endoscopist preference.”
The study was funded by Boston Scientific and the American College of Gastroenterology. The investigators disclosed additional relationships with Medtronic, Olympus, Cook Endoscopy, and others.
SOURCE: Pohl H et al. Gastroenterology. 2020 Mar 12. doi: 10.1053/j.gastro.2020.03.014.
There has long been a debate over which type of electrosurgical setting is best for colon polyp resection. Endoscopists can use either a blended current (yellow pedal) or a coagulation current (blue pedal). The choice is based on the endoscopists’ preference. However, few data have been available to support one setting versus the other. This study by Pohl et al. pursued the burning question of yellow or blue pedal? This single-blind randomized multicenter trial compared the two commonly used electrosurgical settings (Blended Current/Endocut Q vs. Forced Coagulation) for the resection of large colorectal polyps and found no difference in the risk of serious adverse events, complete resection rate, or polyp recurrence, thus supporting the current practice that electrosurgical settings can be selected based on endoscopist expertise and preference.
A few important highlights from this well designed study are worth mentioning. Although there was no significant difference in perforation, it should be noted that fewer patients had a perforation event in the Forced Coagulation group than in the Endocut Q group (3 vs. 6 patients; P = .320). In addition, the study demonstrated that the rate of polyp recurrence did not differ significantly between the two groups (17.4% vs. 16.5%; P = .762). while the rate of macroscopically visible recurrence was not statistically different, a histologic recurrence without visible polyp tissue was found slightly less frequent in the Forced Coagulation group than in the Endocut Q group (3.1% vs. 6.0%; P = .07). Finally, another important observation, intraprocedural bleeding requiring treatment, occurred less frequently during resection with Forced Coagulation than with Endocut Q (11% vs. 17%, P = .006); however, this difference did not affect overall safety and efficacy. This is an important finding since bleeding can affect the field of view during polypectomy, which that can potentially increase the risk of other serious adverse events such as perforation or increase the risk of recurrence because the endoscopist may not completely resect the polyp.
This study provides important insights into the potential risks associated with blended vs. coagulation currents. It further shows there is no difference in safety and efficacy of polypectomy using either a blended current or coagulation current, thus supporting current practice. However, the authors make it clear that a larger study will be needed to better answer such questions as polyp recurrence and perforation more definitively.
Frank G. Gress, MD, MBA, is senior faculty at the Icahn School of Medicine at Mount Sinai, New York; chief, division of gastroenterology and hepatology, and director of the Center for Interventional Endoscopy at Mount Sinai Hospital South Nassau. He has no conflicts.
There has long been a debate over which type of electrosurgical setting is best for colon polyp resection. Endoscopists can use either a blended current (yellow pedal) or a coagulation current (blue pedal). The choice is based on the endoscopists’ preference. However, few data have been available to support one setting versus the other. This study by Pohl et al. pursued the burning question of yellow or blue pedal? This single-blind randomized multicenter trial compared the two commonly used electrosurgical settings (Blended Current/Endocut Q vs. Forced Coagulation) for the resection of large colorectal polyps and found no difference in the risk of serious adverse events, complete resection rate, or polyp recurrence, thus supporting the current practice that electrosurgical settings can be selected based on endoscopist expertise and preference.
A few important highlights from this well designed study are worth mentioning. Although there was no significant difference in perforation, it should be noted that fewer patients had a perforation event in the Forced Coagulation group than in the Endocut Q group (3 vs. 6 patients; P = .320). In addition, the study demonstrated that the rate of polyp recurrence did not differ significantly between the two groups (17.4% vs. 16.5%; P = .762). while the rate of macroscopically visible recurrence was not statistically different, a histologic recurrence without visible polyp tissue was found slightly less frequent in the Forced Coagulation group than in the Endocut Q group (3.1% vs. 6.0%; P = .07). Finally, another important observation, intraprocedural bleeding requiring treatment, occurred less frequently during resection with Forced Coagulation than with Endocut Q (11% vs. 17%, P = .006); however, this difference did not affect overall safety and efficacy. This is an important finding since bleeding can affect the field of view during polypectomy, which that can potentially increase the risk of other serious adverse events such as perforation or increase the risk of recurrence because the endoscopist may not completely resect the polyp.
This study provides important insights into the potential risks associated with blended vs. coagulation currents. It further shows there is no difference in safety and efficacy of polypectomy using either a blended current or coagulation current, thus supporting current practice. However, the authors make it clear that a larger study will be needed to better answer such questions as polyp recurrence and perforation more definitively.
Frank G. Gress, MD, MBA, is senior faculty at the Icahn School of Medicine at Mount Sinai, New York; chief, division of gastroenterology and hepatology, and director of the Center for Interventional Endoscopy at Mount Sinai Hospital South Nassau. He has no conflicts.
There has long been a debate over which type of electrosurgical setting is best for colon polyp resection. Endoscopists can use either a blended current (yellow pedal) or a coagulation current (blue pedal). The choice is based on the endoscopists’ preference. However, few data have been available to support one setting versus the other. This study by Pohl et al. pursued the burning question of yellow or blue pedal? This single-blind randomized multicenter trial compared the two commonly used electrosurgical settings (Blended Current/Endocut Q vs. Forced Coagulation) for the resection of large colorectal polyps and found no difference in the risk of serious adverse events, complete resection rate, or polyp recurrence, thus supporting the current practice that electrosurgical settings can be selected based on endoscopist expertise and preference.
A few important highlights from this well designed study are worth mentioning. Although there was no significant difference in perforation, it should be noted that fewer patients had a perforation event in the Forced Coagulation group than in the Endocut Q group (3 vs. 6 patients; P = .320). In addition, the study demonstrated that the rate of polyp recurrence did not differ significantly between the two groups (17.4% vs. 16.5%; P = .762). while the rate of macroscopically visible recurrence was not statistically different, a histologic recurrence without visible polyp tissue was found slightly less frequent in the Forced Coagulation group than in the Endocut Q group (3.1% vs. 6.0%; P = .07). Finally, another important observation, intraprocedural bleeding requiring treatment, occurred less frequently during resection with Forced Coagulation than with Endocut Q (11% vs. 17%, P = .006); however, this difference did not affect overall safety and efficacy. This is an important finding since bleeding can affect the field of view during polypectomy, which that can potentially increase the risk of other serious adverse events such as perforation or increase the risk of recurrence because the endoscopist may not completely resect the polyp.
This study provides important insights into the potential risks associated with blended vs. coagulation currents. It further shows there is no difference in safety and efficacy of polypectomy using either a blended current or coagulation current, thus supporting current practice. However, the authors make it clear that a larger study will be needed to better answer such questions as polyp recurrence and perforation more definitively.
Frank G. Gress, MD, MBA, is senior faculty at the Icahn School of Medicine at Mount Sinai, New York; chief, division of gastroenterology and hepatology, and director of the Center for Interventional Endoscopy at Mount Sinai Hospital South Nassau. He has no conflicts.
For endoscopists performing electrosurgical snare resection of large colorectal polyps, choosing between the blue foot pedal and the yellow foot pedal may be the least important step of the day, according to data from almost 1,000 patients.
Risks of severe adverse events and polyp recurrence were similar between cases in which blended current (yellow pedal) was used and those in which coagulation current (blue pedal) was used, reported lead author Heiko Pohl, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and colleagues.
“Although electrosurgical application is a fundamental aspect of polypectomy, various currents and settings are clinically used, and there are no accepted standards of practice,” the investigators wrote in Gastroenterology.
According to Dr. Pohl and colleagues, a 2004 study showed that the split between endoscopists using coagulation current and those using blended current was about 50-50 (46% vs. 46%), but no studies to date have tested the relative safety or efficacy of these approaches.
The investigators aimed to address this knowledge gap with a single-blinded study involving 928 patients who underwent endoscopic mucosal resection of nonpedunculated, large (20 mm or larger) colorectal polyps with an Erbe Vio® 300D electrosurgical unit (Erbe USA Inc., Marietta, Ga.) at 18 medical centers.
Patients were randomized in 2x2 factorial design involving clip closure versus no clip closure, and blended current (Endocut Q) versus pure coagulation current (Forced Coagulation). Although electrosurgical setting was initially a secondary intervention in the trial, post hoc analysis showed that interaction between the interventions was not significant (P = .957), allowing for the present, independent analysis of current type.
For this analysis, the primary outcome was severe adverse event rate, both during the procedure, and after the procedure for up to 30 days. Secondary outcomes included proportion of polyps completely excised and recurrence rate at time of first surveillance endoscopy.
Out of 928 patients randomized, 919 completed 30-day follow-up, and 675 underwent first surveillance colonoscopy. Baseline characteristics were similar between groups, apart from the proportion of individuals with more than one large polyp, which was significantly greater in the Endocut Q group (8.6% vs. 4.5%; P = .012), although the investigators noted that this imbalance did not affect main outcomes.
Rates of severe adverse events were similar between groups: 7.2% for the Endocut Q group and 7.9% for the Forced Coagulation group (P = .762). Groups also had similar rates of intra- and postprocedure adverse events, and types of adverse events.
Efficacy measures also revealed high similarity between cutting techniques. Endoscopists using Endocut achieved complete polyp removal 96% of the time, compared with 95% of the time when using Forced Coagulation (P = .267). Piecemeal resection rates were similar, at 90% and 87% for Endocut Q and Forced Coagulation, respectively (P = .270).
Although Endocut Q less frequently resulted in small residual tissue islands after initial snare resection (35% vs. 41%; P = .041), it more often caused intraprocedural bleeding that required treatment (17% vs. 11%; P = .006).
According to Dr. Pohl and colleagues, previous discussions have included concerns that such bleeding may impair visualization and therefore lead to higher rates of polyp recurrence; but surveillance colonoscopy, which was performed in 79% of patients, revealed a polyp recurrence rate of 17% for each group.
“Although we did not find a difference in recurrence between the two groups, our study cannot completely exclude this possibility,” the investigators added.
They also noted that six perforations occurred in the Endocut Q group, compared with three in the Forced Coagulation group, and suggested that this risk may be real, yet statistically unsupported by the present analysis because of sample size.
“Endoscopists using Endocut should therefore be aware of this potential risk and [ensure] that no muscularis propria is entrapped in the snare before electrosurgery is applied,” the investigators wrote.
Still, the investigators’ final conclusion supported the existing method of decision-making: personal choice.
“Overall, polyp resection with Endocut or Forced Coagulation did not differ with respect to severe adverse events, complete resection rate, or polyp recurrence,” they wrote. “This study therefore supports an individual approach based on endoscopist preference.”
The study was funded by Boston Scientific and the American College of Gastroenterology. The investigators disclosed additional relationships with Medtronic, Olympus, Cook Endoscopy, and others.
SOURCE: Pohl H et al. Gastroenterology. 2020 Mar 12. doi: 10.1053/j.gastro.2020.03.014.
For endoscopists performing electrosurgical snare resection of large colorectal polyps, choosing between the blue foot pedal and the yellow foot pedal may be the least important step of the day, according to data from almost 1,000 patients.
Risks of severe adverse events and polyp recurrence were similar between cases in which blended current (yellow pedal) was used and those in which coagulation current (blue pedal) was used, reported lead author Heiko Pohl, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and colleagues.
“Although electrosurgical application is a fundamental aspect of polypectomy, various currents and settings are clinically used, and there are no accepted standards of practice,” the investigators wrote in Gastroenterology.
According to Dr. Pohl and colleagues, a 2004 study showed that the split between endoscopists using coagulation current and those using blended current was about 50-50 (46% vs. 46%), but no studies to date have tested the relative safety or efficacy of these approaches.
The investigators aimed to address this knowledge gap with a single-blinded study involving 928 patients who underwent endoscopic mucosal resection of nonpedunculated, large (20 mm or larger) colorectal polyps with an Erbe Vio® 300D electrosurgical unit (Erbe USA Inc., Marietta, Ga.) at 18 medical centers.
Patients were randomized in 2x2 factorial design involving clip closure versus no clip closure, and blended current (Endocut Q) versus pure coagulation current (Forced Coagulation). Although electrosurgical setting was initially a secondary intervention in the trial, post hoc analysis showed that interaction between the interventions was not significant (P = .957), allowing for the present, independent analysis of current type.
For this analysis, the primary outcome was severe adverse event rate, both during the procedure, and after the procedure for up to 30 days. Secondary outcomes included proportion of polyps completely excised and recurrence rate at time of first surveillance endoscopy.
Out of 928 patients randomized, 919 completed 30-day follow-up, and 675 underwent first surveillance colonoscopy. Baseline characteristics were similar between groups, apart from the proportion of individuals with more than one large polyp, which was significantly greater in the Endocut Q group (8.6% vs. 4.5%; P = .012), although the investigators noted that this imbalance did not affect main outcomes.
Rates of severe adverse events were similar between groups: 7.2% for the Endocut Q group and 7.9% for the Forced Coagulation group (P = .762). Groups also had similar rates of intra- and postprocedure adverse events, and types of adverse events.
Efficacy measures also revealed high similarity between cutting techniques. Endoscopists using Endocut achieved complete polyp removal 96% of the time, compared with 95% of the time when using Forced Coagulation (P = .267). Piecemeal resection rates were similar, at 90% and 87% for Endocut Q and Forced Coagulation, respectively (P = .270).
Although Endocut Q less frequently resulted in small residual tissue islands after initial snare resection (35% vs. 41%; P = .041), it more often caused intraprocedural bleeding that required treatment (17% vs. 11%; P = .006).
According to Dr. Pohl and colleagues, previous discussions have included concerns that such bleeding may impair visualization and therefore lead to higher rates of polyp recurrence; but surveillance colonoscopy, which was performed in 79% of patients, revealed a polyp recurrence rate of 17% for each group.
“Although we did not find a difference in recurrence between the two groups, our study cannot completely exclude this possibility,” the investigators added.
They also noted that six perforations occurred in the Endocut Q group, compared with three in the Forced Coagulation group, and suggested that this risk may be real, yet statistically unsupported by the present analysis because of sample size.
“Endoscopists using Endocut should therefore be aware of this potential risk and [ensure] that no muscularis propria is entrapped in the snare before electrosurgery is applied,” the investigators wrote.
Still, the investigators’ final conclusion supported the existing method of decision-making: personal choice.
“Overall, polyp resection with Endocut or Forced Coagulation did not differ with respect to severe adverse events, complete resection rate, or polyp recurrence,” they wrote. “This study therefore supports an individual approach based on endoscopist preference.”
The study was funded by Boston Scientific and the American College of Gastroenterology. The investigators disclosed additional relationships with Medtronic, Olympus, Cook Endoscopy, and others.
SOURCE: Pohl H et al. Gastroenterology. 2020 Mar 12. doi: 10.1053/j.gastro.2020.03.014.
FROM GASTROENTEROLOGY





