User login
FibroScan: M probe underestimates hepatic fat content
When performing transient elastography (FibroScan) to evaluate patients for hepatic steatosis, using an M probe instead of an XL probe may significantly underestimate hepatic fat content, according to investigators.
The findings, which were independent of body weight, suggest that probe-specific controlled attenuation parameter (CAP) thresholds are needed to accurately interpret FibroScan results, reported lead author Cyrielle Caussy, MD, PhD, of the University of California, San Diego, and colleagues.
“We have previously determined the optimal threshold of CAP using either [an] M or XL probe for the detection of ... nonalcoholic fatty liver disease (NAFLD),” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, head-to-head comparison of consecutive measurements of CAP with both the M and XL probes versus MRI-PDFF [proton density fat fraction] ... has not been reported yet.”
Dr. Caussy and colleagues set out to do just that. They enrolled 105 individuals with and without NAFLD who had a mean body mass index (BMI) of 30.6 kg/m2, as this represented a typical population screened for NAFLD. After evaluation for other causes of hepatic steatosis and liver disease, participants underwent MRI-PDFF, which served as a gold standard, followed by FibroScan using both M and XL probes on the same day.
The primary outcome was hepatic steatosis (MRI-PDFF of at least 5%), while the secondary outcome was MRI-PDFF–detected hepatic fat content of at least 10%, the latter of which has been “used in several therapeutic trials as inclusion criteria,” the investigators noted.
A total of 100 participants were included in the final analysis, of whom two-thirds (66%) underwent MRI and FibroScan on the same day, with a mean interval between test types of 11 days. Most participants (68%) had an MRI-PDFF of at least 5%, while almost half (48%) exceeded an MRI-PDFF of 10%.
The mean CAP measurement with the M probe was 310 dB/m, which was significantly lower than the mean value detected by the XL probe, which was 317 dB/m (P = .007). In participants with hepatic steatosis, when the M probe was used for those with a BMI of less than 30, and the XL probe was used for those with a BMI of 30 or more, the M probe still provided a significantly lower measure of hepatic fat content (312 vs. 345 dB/m; P = .0035).
“[T]hese results have direct application in routine clinical practice,” the investigators wrote, “as [they] will help clinicians interpreting CAP measurements depending on the type of probe used.”
Dr. Caussy and colleagues went on to offer a diagnostic algorithm involving optimal probe-specific thresholds for CAP based on hepatic fat content. Individuals screened with an M probe who have a CAP of 294 dB/m or more should be considered positive for NAFLD, while patients screened with an XL probe need to have a CAP of at least 307 dB/m to be NAFLD positive.
For the XL probe, but not the M probe, diagnostic accuracy depended upon an interquartile range of less than 30 dB/m. The investigators noted that this finding should alter the interpretation of a 2019 study by Eddowes and colleagues, which concluded that interquartile range was unrelated to diagnostic accuracy.
“As Eddowes et al. did not perform head-to-head comparison of CAP measurement with both the M and XL probes, this important difference could not have been observed,” the investigators wrote, noting that “an interquartile range of CAP below 30 dB/m should be considered as a quality indicator that significantly improves the diagnostic performance of CAP using the XL probe for the detection of hepatic steatosis in NAFLD.”
The investigators concluded by suggesting that their findings will drive research forward.
“The use of these new thresholds will help to further assess the clinical utility of CAP for the detection of hepatic steatosis and its cost-effectiveness, compared with other modalities, to develop optimal strategies for the screening of NAFLD,” they wrote.
The study was funded by Atlantic Philanthropies, the John A. Hartford Foundation, the American Gastroenterological Association, and others. The investigators disclosed no conflicts of interest.
SOURCE: Caussy C et al. Clin Gastro Hepatol. 2019 Dec 13. doi: 10.1016/j.cgh.2019.11.060.
When performing transient elastography (FibroScan) to evaluate patients for hepatic steatosis, using an M probe instead of an XL probe may significantly underestimate hepatic fat content, according to investigators.
The findings, which were independent of body weight, suggest that probe-specific controlled attenuation parameter (CAP) thresholds are needed to accurately interpret FibroScan results, reported lead author Cyrielle Caussy, MD, PhD, of the University of California, San Diego, and colleagues.
“We have previously determined the optimal threshold of CAP using either [an] M or XL probe for the detection of ... nonalcoholic fatty liver disease (NAFLD),” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, head-to-head comparison of consecutive measurements of CAP with both the M and XL probes versus MRI-PDFF [proton density fat fraction] ... has not been reported yet.”
Dr. Caussy and colleagues set out to do just that. They enrolled 105 individuals with and without NAFLD who had a mean body mass index (BMI) of 30.6 kg/m2, as this represented a typical population screened for NAFLD. After evaluation for other causes of hepatic steatosis and liver disease, participants underwent MRI-PDFF, which served as a gold standard, followed by FibroScan using both M and XL probes on the same day.
The primary outcome was hepatic steatosis (MRI-PDFF of at least 5%), while the secondary outcome was MRI-PDFF–detected hepatic fat content of at least 10%, the latter of which has been “used in several therapeutic trials as inclusion criteria,” the investigators noted.
A total of 100 participants were included in the final analysis, of whom two-thirds (66%) underwent MRI and FibroScan on the same day, with a mean interval between test types of 11 days. Most participants (68%) had an MRI-PDFF of at least 5%, while almost half (48%) exceeded an MRI-PDFF of 10%.
The mean CAP measurement with the M probe was 310 dB/m, which was significantly lower than the mean value detected by the XL probe, which was 317 dB/m (P = .007). In participants with hepatic steatosis, when the M probe was used for those with a BMI of less than 30, and the XL probe was used for those with a BMI of 30 or more, the M probe still provided a significantly lower measure of hepatic fat content (312 vs. 345 dB/m; P = .0035).
“[T]hese results have direct application in routine clinical practice,” the investigators wrote, “as [they] will help clinicians interpreting CAP measurements depending on the type of probe used.”
Dr. Caussy and colleagues went on to offer a diagnostic algorithm involving optimal probe-specific thresholds for CAP based on hepatic fat content. Individuals screened with an M probe who have a CAP of 294 dB/m or more should be considered positive for NAFLD, while patients screened with an XL probe need to have a CAP of at least 307 dB/m to be NAFLD positive.
For the XL probe, but not the M probe, diagnostic accuracy depended upon an interquartile range of less than 30 dB/m. The investigators noted that this finding should alter the interpretation of a 2019 study by Eddowes and colleagues, which concluded that interquartile range was unrelated to diagnostic accuracy.
“As Eddowes et al. did not perform head-to-head comparison of CAP measurement with both the M and XL probes, this important difference could not have been observed,” the investigators wrote, noting that “an interquartile range of CAP below 30 dB/m should be considered as a quality indicator that significantly improves the diagnostic performance of CAP using the XL probe for the detection of hepatic steatosis in NAFLD.”
The investigators concluded by suggesting that their findings will drive research forward.
“The use of these new thresholds will help to further assess the clinical utility of CAP for the detection of hepatic steatosis and its cost-effectiveness, compared with other modalities, to develop optimal strategies for the screening of NAFLD,” they wrote.
The study was funded by Atlantic Philanthropies, the John A. Hartford Foundation, the American Gastroenterological Association, and others. The investigators disclosed no conflicts of interest.
SOURCE: Caussy C et al. Clin Gastro Hepatol. 2019 Dec 13. doi: 10.1016/j.cgh.2019.11.060.
When performing transient elastography (FibroScan) to evaluate patients for hepatic steatosis, using an M probe instead of an XL probe may significantly underestimate hepatic fat content, according to investigators.
The findings, which were independent of body weight, suggest that probe-specific controlled attenuation parameter (CAP) thresholds are needed to accurately interpret FibroScan results, reported lead author Cyrielle Caussy, MD, PhD, of the University of California, San Diego, and colleagues.
“We have previously determined the optimal threshold of CAP using either [an] M or XL probe for the detection of ... nonalcoholic fatty liver disease (NAFLD),” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, head-to-head comparison of consecutive measurements of CAP with both the M and XL probes versus MRI-PDFF [proton density fat fraction] ... has not been reported yet.”
Dr. Caussy and colleagues set out to do just that. They enrolled 105 individuals with and without NAFLD who had a mean body mass index (BMI) of 30.6 kg/m2, as this represented a typical population screened for NAFLD. After evaluation for other causes of hepatic steatosis and liver disease, participants underwent MRI-PDFF, which served as a gold standard, followed by FibroScan using both M and XL probes on the same day.
The primary outcome was hepatic steatosis (MRI-PDFF of at least 5%), while the secondary outcome was MRI-PDFF–detected hepatic fat content of at least 10%, the latter of which has been “used in several therapeutic trials as inclusion criteria,” the investigators noted.
A total of 100 participants were included in the final analysis, of whom two-thirds (66%) underwent MRI and FibroScan on the same day, with a mean interval between test types of 11 days. Most participants (68%) had an MRI-PDFF of at least 5%, while almost half (48%) exceeded an MRI-PDFF of 10%.
The mean CAP measurement with the M probe was 310 dB/m, which was significantly lower than the mean value detected by the XL probe, which was 317 dB/m (P = .007). In participants with hepatic steatosis, when the M probe was used for those with a BMI of less than 30, and the XL probe was used for those with a BMI of 30 or more, the M probe still provided a significantly lower measure of hepatic fat content (312 vs. 345 dB/m; P = .0035).
“[T]hese results have direct application in routine clinical practice,” the investigators wrote, “as [they] will help clinicians interpreting CAP measurements depending on the type of probe used.”
Dr. Caussy and colleagues went on to offer a diagnostic algorithm involving optimal probe-specific thresholds for CAP based on hepatic fat content. Individuals screened with an M probe who have a CAP of 294 dB/m or more should be considered positive for NAFLD, while patients screened with an XL probe need to have a CAP of at least 307 dB/m to be NAFLD positive.
For the XL probe, but not the M probe, diagnostic accuracy depended upon an interquartile range of less than 30 dB/m. The investigators noted that this finding should alter the interpretation of a 2019 study by Eddowes and colleagues, which concluded that interquartile range was unrelated to diagnostic accuracy.
“As Eddowes et al. did not perform head-to-head comparison of CAP measurement with both the M and XL probes, this important difference could not have been observed,” the investigators wrote, noting that “an interquartile range of CAP below 30 dB/m should be considered as a quality indicator that significantly improves the diagnostic performance of CAP using the XL probe for the detection of hepatic steatosis in NAFLD.”
The investigators concluded by suggesting that their findings will drive research forward.
“The use of these new thresholds will help to further assess the clinical utility of CAP for the detection of hepatic steatosis and its cost-effectiveness, compared with other modalities, to develop optimal strategies for the screening of NAFLD,” they wrote.
The study was funded by Atlantic Philanthropies, the John A. Hartford Foundation, the American Gastroenterological Association, and others. The investigators disclosed no conflicts of interest.
SOURCE: Caussy C et al. Clin Gastro Hepatol. 2019 Dec 13. doi: 10.1016/j.cgh.2019.11.060.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
‘Hospital at home’ cuts ED visits and costs for cancer patients
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations (P = .001), 45% lower odds of ED visits (P = .037), and 50% lower cumulative charges (P = .001), as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, interim senior director of population sciences at the Huntsman Cancer Institute and distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000).
The hospital-at-home model of care provides hospital-level care in the comfort of the patient’s home and is a component of many healthcare systems worldwide. Although it was introduced in the United States more than 2 decades ago, it has not been widely adopted or studied specifically in oncology.
Most cancer treatment is provided on an outpatient basis, which means that patients experience significant adverse events, toxicities, and disease progression while they are at home. Thus, Mooney noted, patients tend to rely heavily on the ED and sometimes experience unplanned hospitalizations and 30-day readmissions.
“These care patterns are distressing to the patients and their families and tax healthcare resources,” she said. “They are even more concerning and salient as we endeavor to protect cancer patients and provide cancer care during a pandemic.”
Currently, strategies to evaluate and support cancer patients and caregivers at home are limited. In 2018, the Huntsman Cancer Institute implemented Huntsman at Home, a demonstration project to evaluate the utility of an oncology hospital-at-home model.
Significantly Fewer Unplanned Hospitalizations
Huntsman at Home is run by nurse practitioner and registered nurse teams who deliver acute-level care at home. Physicians provide backup support for both medical oncology and palliative care. Nurse practitioners also work directly with the patient’s oncology team to coordinate care needs, including services such as social work and physical therapy.
To evaluate the hospital-at-home model, Mooney and colleagues compared patients who were enrolled in the program with those who received usual care. The usual-care comparison group was drawn from patients who lived in the Salt Lake City area. These patients would have qualified for enrollment in the Huntsman at Home program, but they lived outside the 20-mile service area.
The cohort included 367 patients (169 Huntsman at Home patients and 198 usual-care patients). Of those patients, 77% had stage IV cancer. A range of cancer types was represented; the most common were colon, gynecologic, prostate, and lung cancers. As compared to the usual-care group, those in the home model were more likely to be women (61% vs 43%).
During the first 30 days after admission, Huntsman at Home patients had significantly fewer unplanned hospitalizations (19.5% vs 35.4%) and a shorter length of stay (1.4 vs 2.6 days). Their care was also less expensive. The estimated charges for the hospital-at-home patients was $10,238, compared with $21,363 for the usual-care patients. There was no real difference in stays in the intensive care unit between the two groups.
Mooney noted that since there have been few studies of the hospital-at-home model for oncology patients, the investigators’ initial focus was on patients at hospital discharge who needed continued acute-level care and those who had acute problems identified through their oncology care clinic. Therefore, patients were not admitted to the program directly from emergency services, and chemotherapy infusions were not provided, although these are “other areas to consider in an oncology hospital-at-home model.”
Other limitations of the study were that it was not a randomized trial, and the evaluation was from a single program located at one comprehensive cancer center.
“These findings provide the oncology community with an opportunity to rethink cancer care as solely hospital- and clinic-based and instead reimagine care delivery that moves with the patient with key components provided at home,” said Mooney. “We plan to continue the development and evaluation of Huntsman at Home and extend care to admission from the emergency department.”
She added that, together with Flatiron Health, they are validating a tool to prospectively predict, on the basis of the likelihood of ED use, which patients may benefit from Huntsman at Home support. They also plan to extend care to patients who live at a distance from the cancer center and in rural communities, and may include chemotherapy infusion services.
Palliative Care Patients Prefer Home-Based Treatment
In a discussion of the paper, Lynne Wagner, PhD, a professor in the Department of Social Sciences and Health Policy with the Wake Forest School of Medicine, Winston-Salem, North Carolina, and a member of the Wake Forest Baptist Comprehensive Cancer Center, explained that some “aspects of healthcare are more translatable to a virtual or alternative delivery model than others. An area of cancer care greatly in need of innovation and quality improvement pertains to the management of oncologic emergencies.”
She pointed out that optimal care for oncologic emergencies requires the “intersection of oncology and emergency medicine specialists,” but there are often no well-defined processes for care coordination in place.
“Emergency department utilization could be reduced through greater precision with regard to risk stratification and early intervention and improved outpatient management, including improved symptom management,” said Wagner.
Wagner suggested that research should incorporate patient-reported outcomes so as to measure patient-centered benefits of home-based care. “Patients who are receiving palliative care services prefer home-based care, and it’s reasonable to anticipate this finding would extrapolate to the investigator’s target population,” she said. “However, there may also be unanticipated consequences, potentially including increased anxiety or increased burden on caretakers.”
In addition, the tangible and intangible costs associated with traveling to receive healthcare services and time away from work can be reduced with home-based care, and this should also be quantified. “The costs associated with COVID infection should be estimated to realize the full economic value of this care model, given significant reductions in cohort exposure afforded by home-based visits,” Wagner added.
The Huntsman at Home program is funded by the Huntsman Cancer Institute. The evaluation was funded by the Cambia Health Foundation. Mooney has a consulting or advisory role with Cognitive Medical System, Inc, and has patents, royalties, and other intellectual property for the development of Symptom Care at Home, a remote symptom-monitoring platform developed through research grants funded by the National Cancer Institute. No royalties have been received to date. Wagner has relationships with Celgene, Eli Lilly, Gilead Sciences, and Johnson & Johnson.
This article first appeared on Medscape.com.
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations (P = .001), 45% lower odds of ED visits (P = .037), and 50% lower cumulative charges (P = .001), as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, interim senior director of population sciences at the Huntsman Cancer Institute and distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000).
The hospital-at-home model of care provides hospital-level care in the comfort of the patient’s home and is a component of many healthcare systems worldwide. Although it was introduced in the United States more than 2 decades ago, it has not been widely adopted or studied specifically in oncology.
Most cancer treatment is provided on an outpatient basis, which means that patients experience significant adverse events, toxicities, and disease progression while they are at home. Thus, Mooney noted, patients tend to rely heavily on the ED and sometimes experience unplanned hospitalizations and 30-day readmissions.
“These care patterns are distressing to the patients and their families and tax healthcare resources,” she said. “They are even more concerning and salient as we endeavor to protect cancer patients and provide cancer care during a pandemic.”
Currently, strategies to evaluate and support cancer patients and caregivers at home are limited. In 2018, the Huntsman Cancer Institute implemented Huntsman at Home, a demonstration project to evaluate the utility of an oncology hospital-at-home model.
Significantly Fewer Unplanned Hospitalizations
Huntsman at Home is run by nurse practitioner and registered nurse teams who deliver acute-level care at home. Physicians provide backup support for both medical oncology and palliative care. Nurse practitioners also work directly with the patient’s oncology team to coordinate care needs, including services such as social work and physical therapy.
To evaluate the hospital-at-home model, Mooney and colleagues compared patients who were enrolled in the program with those who received usual care. The usual-care comparison group was drawn from patients who lived in the Salt Lake City area. These patients would have qualified for enrollment in the Huntsman at Home program, but they lived outside the 20-mile service area.
The cohort included 367 patients (169 Huntsman at Home patients and 198 usual-care patients). Of those patients, 77% had stage IV cancer. A range of cancer types was represented; the most common were colon, gynecologic, prostate, and lung cancers. As compared to the usual-care group, those in the home model were more likely to be women (61% vs 43%).
During the first 30 days after admission, Huntsman at Home patients had significantly fewer unplanned hospitalizations (19.5% vs 35.4%) and a shorter length of stay (1.4 vs 2.6 days). Their care was also less expensive. The estimated charges for the hospital-at-home patients was $10,238, compared with $21,363 for the usual-care patients. There was no real difference in stays in the intensive care unit between the two groups.
Mooney noted that since there have been few studies of the hospital-at-home model for oncology patients, the investigators’ initial focus was on patients at hospital discharge who needed continued acute-level care and those who had acute problems identified through their oncology care clinic. Therefore, patients were not admitted to the program directly from emergency services, and chemotherapy infusions were not provided, although these are “other areas to consider in an oncology hospital-at-home model.”
Other limitations of the study were that it was not a randomized trial, and the evaluation was from a single program located at one comprehensive cancer center.
“These findings provide the oncology community with an opportunity to rethink cancer care as solely hospital- and clinic-based and instead reimagine care delivery that moves with the patient with key components provided at home,” said Mooney. “We plan to continue the development and evaluation of Huntsman at Home and extend care to admission from the emergency department.”
She added that, together with Flatiron Health, they are validating a tool to prospectively predict, on the basis of the likelihood of ED use, which patients may benefit from Huntsman at Home support. They also plan to extend care to patients who live at a distance from the cancer center and in rural communities, and may include chemotherapy infusion services.
Palliative Care Patients Prefer Home-Based Treatment
In a discussion of the paper, Lynne Wagner, PhD, a professor in the Department of Social Sciences and Health Policy with the Wake Forest School of Medicine, Winston-Salem, North Carolina, and a member of the Wake Forest Baptist Comprehensive Cancer Center, explained that some “aspects of healthcare are more translatable to a virtual or alternative delivery model than others. An area of cancer care greatly in need of innovation and quality improvement pertains to the management of oncologic emergencies.”
She pointed out that optimal care for oncologic emergencies requires the “intersection of oncology and emergency medicine specialists,” but there are often no well-defined processes for care coordination in place.
“Emergency department utilization could be reduced through greater precision with regard to risk stratification and early intervention and improved outpatient management, including improved symptom management,” said Wagner.
Wagner suggested that research should incorporate patient-reported outcomes so as to measure patient-centered benefits of home-based care. “Patients who are receiving palliative care services prefer home-based care, and it’s reasonable to anticipate this finding would extrapolate to the investigator’s target population,” she said. “However, there may also be unanticipated consequences, potentially including increased anxiety or increased burden on caretakers.”
In addition, the tangible and intangible costs associated with traveling to receive healthcare services and time away from work can be reduced with home-based care, and this should also be quantified. “The costs associated with COVID infection should be estimated to realize the full economic value of this care model, given significant reductions in cohort exposure afforded by home-based visits,” Wagner added.
The Huntsman at Home program is funded by the Huntsman Cancer Institute. The evaluation was funded by the Cambia Health Foundation. Mooney has a consulting or advisory role with Cognitive Medical System, Inc, and has patents, royalties, and other intellectual property for the development of Symptom Care at Home, a remote symptom-monitoring platform developed through research grants funded by the National Cancer Institute. No royalties have been received to date. Wagner has relationships with Celgene, Eli Lilly, Gilead Sciences, and Johnson & Johnson.
This article first appeared on Medscape.com.
Visits to the emergency department (ED) and hospitalizations are often frequent occurrences for cancer patients, but what if the “hospital” could be brought into the home instead?
A new American cohort study provides evidence that this can be a workable option for cancer patients. The authors report improved patient outcomes, with 56% lower odds of unplanned hospitalizations (P = .001), 45% lower odds of ED visits (P = .037), and 50% lower cumulative charges (P = .001), as compared with patients who received usual care.
“The oncology hospital-at-home model of care that extends acute-level care to the patient at home offers promise in addressing a long-term gap in cancer care service delivery,” said lead author Kathi Mooney, PhD, RN, interim senior director of population sciences at the Huntsman Cancer Institute and distinguished professor of nursing at the University of Utah, Salt Lake City. “In light of the current global pandemic, we are compelled to consider new ways to provide cancer care, and the oncology hospital-at-home model is on point to address critical elements of an improved cancer care delivery system.”
Mooney presented the findings during the virtual scientific program of the American Society of Clinical Oncology 2020 annual meeting (abstract 7000).
The hospital-at-home model of care provides hospital-level care in the comfort of the patient’s home and is a component of many healthcare systems worldwide. Although it was introduced in the United States more than 2 decades ago, it has not been widely adopted or studied specifically in oncology.
Most cancer treatment is provided on an outpatient basis, which means that patients experience significant adverse events, toxicities, and disease progression while they are at home. Thus, Mooney noted, patients tend to rely heavily on the ED and sometimes experience unplanned hospitalizations and 30-day readmissions.
“These care patterns are distressing to the patients and their families and tax healthcare resources,” she said. “They are even more concerning and salient as we endeavor to protect cancer patients and provide cancer care during a pandemic.”
Currently, strategies to evaluate and support cancer patients and caregivers at home are limited. In 2018, the Huntsman Cancer Institute implemented Huntsman at Home, a demonstration project to evaluate the utility of an oncology hospital-at-home model.
Significantly Fewer Unplanned Hospitalizations
Huntsman at Home is run by nurse practitioner and registered nurse teams who deliver acute-level care at home. Physicians provide backup support for both medical oncology and palliative care. Nurse practitioners also work directly with the patient’s oncology team to coordinate care needs, including services such as social work and physical therapy.
To evaluate the hospital-at-home model, Mooney and colleagues compared patients who were enrolled in the program with those who received usual care. The usual-care comparison group was drawn from patients who lived in the Salt Lake City area. These patients would have qualified for enrollment in the Huntsman at Home program, but they lived outside the 20-mile service area.
The cohort included 367 patients (169 Huntsman at Home patients and 198 usual-care patients). Of those patients, 77% had stage IV cancer. A range of cancer types was represented; the most common were colon, gynecologic, prostate, and lung cancers. As compared to the usual-care group, those in the home model were more likely to be women (61% vs 43%).
During the first 30 days after admission, Huntsman at Home patients had significantly fewer unplanned hospitalizations (19.5% vs 35.4%) and a shorter length of stay (1.4 vs 2.6 days). Their care was also less expensive. The estimated charges for the hospital-at-home patients was $10,238, compared with $21,363 for the usual-care patients. There was no real difference in stays in the intensive care unit between the two groups.
Mooney noted that since there have been few studies of the hospital-at-home model for oncology patients, the investigators’ initial focus was on patients at hospital discharge who needed continued acute-level care and those who had acute problems identified through their oncology care clinic. Therefore, patients were not admitted to the program directly from emergency services, and chemotherapy infusions were not provided, although these are “other areas to consider in an oncology hospital-at-home model.”
Other limitations of the study were that it was not a randomized trial, and the evaluation was from a single program located at one comprehensive cancer center.
“These findings provide the oncology community with an opportunity to rethink cancer care as solely hospital- and clinic-based and instead reimagine care delivery that moves with the patient with key components provided at home,” said Mooney. “We plan to continue the development and evaluation of Huntsman at Home and extend care to admission from the emergency department.”
She added that, together with Flatiron Health, they are validating a tool to prospectively predict, on the basis of the likelihood of ED use, which patients may benefit from Huntsman at Home support. They also plan to extend care to patients who live at a distance from the cancer center and in rural communities, and may include chemotherapy infusion services.
Palliative Care Patients Prefer Home-Based Treatment
In a discussion of the paper, Lynne Wagner, PhD, a professor in the Department of Social Sciences and Health Policy with the Wake Forest School of Medicine, Winston-Salem, North Carolina, and a member of the Wake Forest Baptist Comprehensive Cancer Center, explained that some “aspects of healthcare are more translatable to a virtual or alternative delivery model than others. An area of cancer care greatly in need of innovation and quality improvement pertains to the management of oncologic emergencies.”
She pointed out that optimal care for oncologic emergencies requires the “intersection of oncology and emergency medicine specialists,” but there are often no well-defined processes for care coordination in place.
“Emergency department utilization could be reduced through greater precision with regard to risk stratification and early intervention and improved outpatient management, including improved symptom management,” said Wagner.
Wagner suggested that research should incorporate patient-reported outcomes so as to measure patient-centered benefits of home-based care. “Patients who are receiving palliative care services prefer home-based care, and it’s reasonable to anticipate this finding would extrapolate to the investigator’s target population,” she said. “However, there may also be unanticipated consequences, potentially including increased anxiety or increased burden on caretakers.”
In addition, the tangible and intangible costs associated with traveling to receive healthcare services and time away from work can be reduced with home-based care, and this should also be quantified. “The costs associated with COVID infection should be estimated to realize the full economic value of this care model, given significant reductions in cohort exposure afforded by home-based visits,” Wagner added.
The Huntsman at Home program is funded by the Huntsman Cancer Institute. The evaluation was funded by the Cambia Health Foundation. Mooney has a consulting or advisory role with Cognitive Medical System, Inc, and has patents, royalties, and other intellectual property for the development of Symptom Care at Home, a remote symptom-monitoring platform developed through research grants funded by the National Cancer Institute. No royalties have been received to date. Wagner has relationships with Celgene, Eli Lilly, Gilead Sciences, and Johnson & Johnson.
This article first appeared on Medscape.com.
FROM ASCO 2020
Addressing suicide prevention among South Asian Americans
Multifaceted strategies are needed to address unique cultural factors
On first glance, the age-adjusted rate of suicide for Asian and Pacific Islander populations living in the United States looks comparatively low.
Over the past 2 decades in the United States, for example, the overall rate increased by 35%, from, 10.5 to 14.2 per 100,000 individuals. That compares with a rate of 7.0 per 100,000 among Asian and Pacific Islander communities.1
However, because of the aggregate nature (national suicide mortality data combine people of Asian, Native Hawaiian, and other Pacific Islander descent into a single group) in which these data are reported, a significant amount of salient information on subgroups of Asian Americans is lost.2 There is a growing body of research on the mental health of Asian Americans, but the dearth of information and research on suicide in South Asians is striking.3 In fact, a review of literature finds fewer than 10 articles on the topic that have been published in peer-reviewed journals in the last decade. to provide effective, culturally sensitive care.
Diverse group
There are 3.4 million individuals of South Asian descent in the United States. Geographically, South Asians may have familial and cultural/historical roots in Bangladesh, Bhutan, India, Maldives, Nepal, and Pakistan.4 They enjoy a rich diversity in terms of cultural and religious beliefs, language, socioeconomic status, modes of acculturation, and immigration patterns. Asian Indians are the largest group of South Asians in the United States. They are highly educated, with a larger proportion of them pursuing an undergraduate and/or graduate level education than the general population. The median household income of Asian Indians is also higher than the national average.5
In general, suicide, like all mental health issues, is a stigmatized and taboo topic in the South Asian community.6 Also, South Asian Americans are hesitant to seek mental health care because of a perceived inability of Western health care professionals to understand their cultural views. Extrapolation from data on South Asians in the United Kingdom, aggregate statistics for Asian Americans and Pacific Islanders, and studies on South Asians in the United States highlight two South Asian subgroups that are particularly vulnerable to suicide. These are young adults (aged 18-24 years) and women.7
Suicide is the second-leading cause of death for young Asian American men in the United States. Rates of lifetime suicidal ideation and attempts are higher among younger Asian Americans (aged 18-24 years) than among older Asian American adults. Young Asian American adults have been found to have higher levels of suicidal ideation than their white counterparts.8,9 Acculturation or assimilating into a different culture, familial violence as a child, hopelessness or a thought pattern with a pessimistic outlook, depression, and childhood sexual abuse have all been found to be positively correlated with suicidal ideation and attempted suicide in South Asian Americans. One study that conducted0 in-group analysis on undergraduate university students of South Asian descent living in New York found higher levels of hopelessness and depression in Asian Indians relative to Bangladeshi or Pakistani Americans.10
In addition, higher levels of suicidal ideation are reported in Asian Indians relative to Bangladeshi or Pakistani Americans. These results resemble findings from similar studies in the United Kingdom. A posited reason for these findings is a difference in religious beliefs. Pakistani and Bangladeshi Americans are predominantly Muslim, have stronger moral beliefs against suicide, and consider it a sin as defined by Islamic beliefs. Asian Indians, in contrast, are majority Hindu and believe in reincarnation – a context that might make suicide seem more permissible.11
South Asian women are particularly vulnerable to domestic violence, childhood sexual abuse, intimate partner violence, and/or familial violence. Cultural gender norms, traditional norms, and patriarchal ideology in the South Asian community make quantifying the level of childhood sexual abuse and familial violence a challenge. Furthermore, culturally, South Asian women are often considered subordinate relative to men, and discussion around family violence and childhood sexual abuse is avoided. Studies from the United Kingdom find a lack of knowledge around, disclosure of, and fear of reporting childhood sexual abuse in South Asian women. A study of a sample of representative South Asian American women found that 25.2% had experienced some form of childhood sexual abuse.12
Research also suggests that South Asians in the United States have some of the highest rates of intimate partner violence. Another study in the United States found that two out of five South Asian women have experienced physical and/or sexual intimate partner violence. This is much higher than the rate found in representative general U.S. population samples.
Literature suggests that exposure to these factors increases womens’ risk for suicidal ideation and attempted suicide. In the United Kingdom, research on South Asian women (aged 18-24 years) has found rates of attempted suicide to be three times higher than those of their white counterparts. Research from the United Kingdom and the United States suggests that younger married South Asian women are exposed to emotional and/or physical abuse from their spouse or in-laws, which is often a mediating factor in their increased risk for suicide.
Attempts to address suicide in the South Asian American community have to be multifaceted. An ideal approach would consist of educating, and connecting with, the community through ethnic media and trusted community sources, such as primary care doctors, caregivers, and social workers. In line with established American Psychological Association guidelines on caring for individuals of immigrant origin, health care professionals should document the patient’s number of generations in the country, number of years in the country, language fluency, family and community support, educational level, social status changes related to immigration, intimate relationships with people of different backgrounds, and stress related to acculturation. Special attention should be paid to South Asian women. Health care professionals should screen South Asian women for past and current intimate partner violence, provide culturally appropriate intimate partner violence resources, and be prepared to refer them to legal counseling services. Also, South Asian women should be screened for a history of exposure to familial violence and childhood sexual abuse.1
To adequately serve this population, there is a need to build capacity in the provision of culturally appropriate mental health services. Access to mental health care professionals through settings such as shelters for abused women, South Asian community–based organizations, youth centers, college counseling, and senior centers would encourage individuals to seek care without the threat of being stigmatized.
References
1. Hedegaard H et al. Suicide mortality in the United States, 1999–2017. NCHS Data Brief, No. 330. 2018 Nov.
2. Ahmad-Stout DJ and Nath SR. J College Stud Psychother. 2013 Jan 10;27(1):43-61.
3. Li H and Keshavan M. Asian J Psychiatry. 2011;4(1):1.
4. Nagaraj NC et al. J Immigr Minor Health. 2019 Oct;21(5):978-1003.
5. Nagaraj NC et al. J Comm Health. 2018;43(3):543-51.
6. Cao KO. Generations. 2014;30(4):82-5.
7. Hurwitz EJ et al. J Immigr Minor Health. 2006;8(3):251-61.
8. Polanco-Roman L et al. Cultur Divers Ethnic Minor Psychol. 2019 Dec 23. doi: 10.1037/cpd0000313.
9. Erausquin JT et al. J Youth Adolesc. 2019 Sep;48(9):1796-1805.
10. Lane R et al. Asian Am J Psychol. 2016;7(2):120-8.
11. Nath SR et al. Asian Am J Psychol. 2018;9(4):334-343.
12. Robertson HA et al. J Immigr Minor Health. 2016 Jul 31;18(4):921-7.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University (CMU) College of Medicine, Mt. Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in immigrant populations.
Multifaceted strategies are needed to address unique cultural factors
Multifaceted strategies are needed to address unique cultural factors
On first glance, the age-adjusted rate of suicide for Asian and Pacific Islander populations living in the United States looks comparatively low.
Over the past 2 decades in the United States, for example, the overall rate increased by 35%, from, 10.5 to 14.2 per 100,000 individuals. That compares with a rate of 7.0 per 100,000 among Asian and Pacific Islander communities.1
However, because of the aggregate nature (national suicide mortality data combine people of Asian, Native Hawaiian, and other Pacific Islander descent into a single group) in which these data are reported, a significant amount of salient information on subgroups of Asian Americans is lost.2 There is a growing body of research on the mental health of Asian Americans, but the dearth of information and research on suicide in South Asians is striking.3 In fact, a review of literature finds fewer than 10 articles on the topic that have been published in peer-reviewed journals in the last decade. to provide effective, culturally sensitive care.
Diverse group
There are 3.4 million individuals of South Asian descent in the United States. Geographically, South Asians may have familial and cultural/historical roots in Bangladesh, Bhutan, India, Maldives, Nepal, and Pakistan.4 They enjoy a rich diversity in terms of cultural and religious beliefs, language, socioeconomic status, modes of acculturation, and immigration patterns. Asian Indians are the largest group of South Asians in the United States. They are highly educated, with a larger proportion of them pursuing an undergraduate and/or graduate level education than the general population. The median household income of Asian Indians is also higher than the national average.5
In general, suicide, like all mental health issues, is a stigmatized and taboo topic in the South Asian community.6 Also, South Asian Americans are hesitant to seek mental health care because of a perceived inability of Western health care professionals to understand their cultural views. Extrapolation from data on South Asians in the United Kingdom, aggregate statistics for Asian Americans and Pacific Islanders, and studies on South Asians in the United States highlight two South Asian subgroups that are particularly vulnerable to suicide. These are young adults (aged 18-24 years) and women.7
Suicide is the second-leading cause of death for young Asian American men in the United States. Rates of lifetime suicidal ideation and attempts are higher among younger Asian Americans (aged 18-24 years) than among older Asian American adults. Young Asian American adults have been found to have higher levels of suicidal ideation than their white counterparts.8,9 Acculturation or assimilating into a different culture, familial violence as a child, hopelessness or a thought pattern with a pessimistic outlook, depression, and childhood sexual abuse have all been found to be positively correlated with suicidal ideation and attempted suicide in South Asian Americans. One study that conducted0 in-group analysis on undergraduate university students of South Asian descent living in New York found higher levels of hopelessness and depression in Asian Indians relative to Bangladeshi or Pakistani Americans.10
In addition, higher levels of suicidal ideation are reported in Asian Indians relative to Bangladeshi or Pakistani Americans. These results resemble findings from similar studies in the United Kingdom. A posited reason for these findings is a difference in religious beliefs. Pakistani and Bangladeshi Americans are predominantly Muslim, have stronger moral beliefs against suicide, and consider it a sin as defined by Islamic beliefs. Asian Indians, in contrast, are majority Hindu and believe in reincarnation – a context that might make suicide seem more permissible.11
South Asian women are particularly vulnerable to domestic violence, childhood sexual abuse, intimate partner violence, and/or familial violence. Cultural gender norms, traditional norms, and patriarchal ideology in the South Asian community make quantifying the level of childhood sexual abuse and familial violence a challenge. Furthermore, culturally, South Asian women are often considered subordinate relative to men, and discussion around family violence and childhood sexual abuse is avoided. Studies from the United Kingdom find a lack of knowledge around, disclosure of, and fear of reporting childhood sexual abuse in South Asian women. A study of a sample of representative South Asian American women found that 25.2% had experienced some form of childhood sexual abuse.12
Research also suggests that South Asians in the United States have some of the highest rates of intimate partner violence. Another study in the United States found that two out of five South Asian women have experienced physical and/or sexual intimate partner violence. This is much higher than the rate found in representative general U.S. population samples.
Literature suggests that exposure to these factors increases womens’ risk for suicidal ideation and attempted suicide. In the United Kingdom, research on South Asian women (aged 18-24 years) has found rates of attempted suicide to be three times higher than those of their white counterparts. Research from the United Kingdom and the United States suggests that younger married South Asian women are exposed to emotional and/or physical abuse from their spouse or in-laws, which is often a mediating factor in their increased risk for suicide.
Attempts to address suicide in the South Asian American community have to be multifaceted. An ideal approach would consist of educating, and connecting with, the community through ethnic media and trusted community sources, such as primary care doctors, caregivers, and social workers. In line with established American Psychological Association guidelines on caring for individuals of immigrant origin, health care professionals should document the patient’s number of generations in the country, number of years in the country, language fluency, family and community support, educational level, social status changes related to immigration, intimate relationships with people of different backgrounds, and stress related to acculturation. Special attention should be paid to South Asian women. Health care professionals should screen South Asian women for past and current intimate partner violence, provide culturally appropriate intimate partner violence resources, and be prepared to refer them to legal counseling services. Also, South Asian women should be screened for a history of exposure to familial violence and childhood sexual abuse.1
To adequately serve this population, there is a need to build capacity in the provision of culturally appropriate mental health services. Access to mental health care professionals through settings such as shelters for abused women, South Asian community–based organizations, youth centers, college counseling, and senior centers would encourage individuals to seek care without the threat of being stigmatized.
References
1. Hedegaard H et al. Suicide mortality in the United States, 1999–2017. NCHS Data Brief, No. 330. 2018 Nov.
2. Ahmad-Stout DJ and Nath SR. J College Stud Psychother. 2013 Jan 10;27(1):43-61.
3. Li H and Keshavan M. Asian J Psychiatry. 2011;4(1):1.
4. Nagaraj NC et al. J Immigr Minor Health. 2019 Oct;21(5):978-1003.
5. Nagaraj NC et al. J Comm Health. 2018;43(3):543-51.
6. Cao KO. Generations. 2014;30(4):82-5.
7. Hurwitz EJ et al. J Immigr Minor Health. 2006;8(3):251-61.
8. Polanco-Roman L et al. Cultur Divers Ethnic Minor Psychol. 2019 Dec 23. doi: 10.1037/cpd0000313.
9. Erausquin JT et al. J Youth Adolesc. 2019 Sep;48(9):1796-1805.
10. Lane R et al. Asian Am J Psychol. 2016;7(2):120-8.
11. Nath SR et al. Asian Am J Psychol. 2018;9(4):334-343.
12. Robertson HA et al. J Immigr Minor Health. 2016 Jul 31;18(4):921-7.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University (CMU) College of Medicine, Mt. Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in immigrant populations.
On first glance, the age-adjusted rate of suicide for Asian and Pacific Islander populations living in the United States looks comparatively low.
Over the past 2 decades in the United States, for example, the overall rate increased by 35%, from, 10.5 to 14.2 per 100,000 individuals. That compares with a rate of 7.0 per 100,000 among Asian and Pacific Islander communities.1
However, because of the aggregate nature (national suicide mortality data combine people of Asian, Native Hawaiian, and other Pacific Islander descent into a single group) in which these data are reported, a significant amount of salient information on subgroups of Asian Americans is lost.2 There is a growing body of research on the mental health of Asian Americans, but the dearth of information and research on suicide in South Asians is striking.3 In fact, a review of literature finds fewer than 10 articles on the topic that have been published in peer-reviewed journals in the last decade. to provide effective, culturally sensitive care.
Diverse group
There are 3.4 million individuals of South Asian descent in the United States. Geographically, South Asians may have familial and cultural/historical roots in Bangladesh, Bhutan, India, Maldives, Nepal, and Pakistan.4 They enjoy a rich diversity in terms of cultural and religious beliefs, language, socioeconomic status, modes of acculturation, and immigration patterns. Asian Indians are the largest group of South Asians in the United States. They are highly educated, with a larger proportion of them pursuing an undergraduate and/or graduate level education than the general population. The median household income of Asian Indians is also higher than the national average.5
In general, suicide, like all mental health issues, is a stigmatized and taboo topic in the South Asian community.6 Also, South Asian Americans are hesitant to seek mental health care because of a perceived inability of Western health care professionals to understand their cultural views. Extrapolation from data on South Asians in the United Kingdom, aggregate statistics for Asian Americans and Pacific Islanders, and studies on South Asians in the United States highlight two South Asian subgroups that are particularly vulnerable to suicide. These are young adults (aged 18-24 years) and women.7
Suicide is the second-leading cause of death for young Asian American men in the United States. Rates of lifetime suicidal ideation and attempts are higher among younger Asian Americans (aged 18-24 years) than among older Asian American adults. Young Asian American adults have been found to have higher levels of suicidal ideation than their white counterparts.8,9 Acculturation or assimilating into a different culture, familial violence as a child, hopelessness or a thought pattern with a pessimistic outlook, depression, and childhood sexual abuse have all been found to be positively correlated with suicidal ideation and attempted suicide in South Asian Americans. One study that conducted0 in-group analysis on undergraduate university students of South Asian descent living in New York found higher levels of hopelessness and depression in Asian Indians relative to Bangladeshi or Pakistani Americans.10
In addition, higher levels of suicidal ideation are reported in Asian Indians relative to Bangladeshi or Pakistani Americans. These results resemble findings from similar studies in the United Kingdom. A posited reason for these findings is a difference in religious beliefs. Pakistani and Bangladeshi Americans are predominantly Muslim, have stronger moral beliefs against suicide, and consider it a sin as defined by Islamic beliefs. Asian Indians, in contrast, are majority Hindu and believe in reincarnation – a context that might make suicide seem more permissible.11
South Asian women are particularly vulnerable to domestic violence, childhood sexual abuse, intimate partner violence, and/or familial violence. Cultural gender norms, traditional norms, and patriarchal ideology in the South Asian community make quantifying the level of childhood sexual abuse and familial violence a challenge. Furthermore, culturally, South Asian women are often considered subordinate relative to men, and discussion around family violence and childhood sexual abuse is avoided. Studies from the United Kingdom find a lack of knowledge around, disclosure of, and fear of reporting childhood sexual abuse in South Asian women. A study of a sample of representative South Asian American women found that 25.2% had experienced some form of childhood sexual abuse.12
Research also suggests that South Asians in the United States have some of the highest rates of intimate partner violence. Another study in the United States found that two out of five South Asian women have experienced physical and/or sexual intimate partner violence. This is much higher than the rate found in representative general U.S. population samples.
Literature suggests that exposure to these factors increases womens’ risk for suicidal ideation and attempted suicide. In the United Kingdom, research on South Asian women (aged 18-24 years) has found rates of attempted suicide to be three times higher than those of their white counterparts. Research from the United Kingdom and the United States suggests that younger married South Asian women are exposed to emotional and/or physical abuse from their spouse or in-laws, which is often a mediating factor in their increased risk for suicide.
Attempts to address suicide in the South Asian American community have to be multifaceted. An ideal approach would consist of educating, and connecting with, the community through ethnic media and trusted community sources, such as primary care doctors, caregivers, and social workers. In line with established American Psychological Association guidelines on caring for individuals of immigrant origin, health care professionals should document the patient’s number of generations in the country, number of years in the country, language fluency, family and community support, educational level, social status changes related to immigration, intimate relationships with people of different backgrounds, and stress related to acculturation. Special attention should be paid to South Asian women. Health care professionals should screen South Asian women for past and current intimate partner violence, provide culturally appropriate intimate partner violence resources, and be prepared to refer them to legal counseling services. Also, South Asian women should be screened for a history of exposure to familial violence and childhood sexual abuse.1
To adequately serve this population, there is a need to build capacity in the provision of culturally appropriate mental health services. Access to mental health care professionals through settings such as shelters for abused women, South Asian community–based organizations, youth centers, college counseling, and senior centers would encourage individuals to seek care without the threat of being stigmatized.
References
1. Hedegaard H et al. Suicide mortality in the United States, 1999–2017. NCHS Data Brief, No. 330. 2018 Nov.
2. Ahmad-Stout DJ and Nath SR. J College Stud Psychother. 2013 Jan 10;27(1):43-61.
3. Li H and Keshavan M. Asian J Psychiatry. 2011;4(1):1.
4. Nagaraj NC et al. J Immigr Minor Health. 2019 Oct;21(5):978-1003.
5. Nagaraj NC et al. J Comm Health. 2018;43(3):543-51.
6. Cao KO. Generations. 2014;30(4):82-5.
7. Hurwitz EJ et al. J Immigr Minor Health. 2006;8(3):251-61.
8. Polanco-Roman L et al. Cultur Divers Ethnic Minor Psychol. 2019 Dec 23. doi: 10.1037/cpd0000313.
9. Erausquin JT et al. J Youth Adolesc. 2019 Sep;48(9):1796-1805.
10. Lane R et al. Asian Am J Psychol. 2016;7(2):120-8.
11. Nath SR et al. Asian Am J Psychol. 2018;9(4):334-343.
12. Robertson HA et al. J Immigr Minor Health. 2016 Jul 31;18(4):921-7.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University (CMU) College of Medicine, Mt. Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in immigrant populations.
Do ObGyns agree that the practice of in-office IUD string checks should be halted?
In their Break This Practice Habit column, “The IUD string check: Benefit or burden?” (March 2020), Kathryn Fay, MD, and Lori Gawron, MD, MPH, argued that it is time to discontinue routine office visits and self-checks for IUD strings postinsertion as the practice is unsupported by data and costly. OBG Management polled readers: “Should the practice of counseling patients to present to the office for a string check after IUD insertion be halted?”
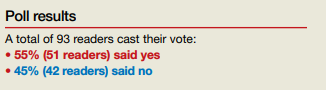
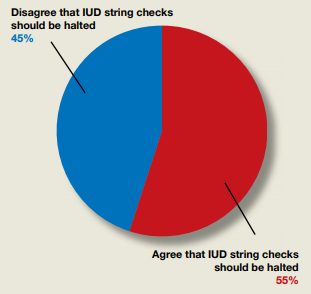
In their Break This Practice Habit column, “The IUD string check: Benefit or burden?” (March 2020), Kathryn Fay, MD, and Lori Gawron, MD, MPH, argued that it is time to discontinue routine office visits and self-checks for IUD strings postinsertion as the practice is unsupported by data and costly. OBG Management polled readers: “Should the practice of counseling patients to present to the office for a string check after IUD insertion be halted?”


In their Break This Practice Habit column, “The IUD string check: Benefit or burden?” (March 2020), Kathryn Fay, MD, and Lori Gawron, MD, MPH, argued that it is time to discontinue routine office visits and self-checks for IUD strings postinsertion as the practice is unsupported by data and costly. OBG Management polled readers: “Should the practice of counseling patients to present to the office for a string check after IUD insertion be halted?”


Fighting COVID and police brutality, medical teams take to streets to treat protesters
Amid clouds of choking tear gas, booming flash-bang grenades and other “riot control agents,” volunteer medics plunged into street protests over the past weeks to help the injured – sometimes rushing to the front lines as soon as their hospital shifts ended.
Known as “street medics,” these unorthodox teams of nursing students, veterinarians, doctors, trauma surgeons, security guards, ski patrollers, nurses, wilderness EMTs, and off-the-clock ambulance workers poured water – not milk – into the eyes of tear-gassed protesters. They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go.
So donning cloth masks to protect against the virus – plus helmets, makeshift shields and other gear to guard against rubber bullets, projectiles and tear gas – the volunteer medics organized themselves into a web of first responders to care for people on the streets. They showed up early, set up first-aid stations, established transportation networks and covered their arms, helmets and backpacks with crosses made of red duct tape, to signify that they were medics. Some stayed late into the night past curfews until every protester had left.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. She didn’t see it as a choice.
“I am working full time and basically being at the protest after getting straight off of work,” said Butler, who is black. That’s tiring, she added, but so is being a black woman in America.
After going out as a medic on her own, she soon met other volunteers. Together they used text-message chains to organize their efforts. One night, she responded to a man who had been shot with a rubber bullet in the chest; she said his torso had turned blue and purple from the impact. She also provided aid after a shooting near the protest left someone in critical condition.
“It’s hard, but bills need to be paid and justice needs to be served,” she said.
The street medic movement traces its roots, in part, to the 1960s protests, as well as the American Indian Movement and the Black Panther Party. Denver Action Medic Network offers a 20-hour training course that prepares them to treat patients in conflicts with police and large crowds; a four-hour session is offered to medical professionals as “bridge” training.
Since the coronavirus pandemic began, the Denver Action Medic Network has added new training guidelines: Don’t go to protests if sick or in contact with those who are infected; wear a mask; give people lots of space and use hand sanitizer. Jordan Garcia, a 39-year-old medic for over 20 years who works with the network of veteran street medics, said they also warn medics about the increased risk of transmission because of protesters coughing from tear gas, and urge them to get tested for the virus after the protests.
The number of volunteer medics swelled after George Floyd’s May 25 killing in Minneapolis. In Denver alone, at least 40 people reached out to the Denver Action Medic Network for training.
On June 3, Dr. Rupa Marya, an associate professor of medicine at the University of California,San Francisco, and the co-founder of the Do No Harm Coalition, which runs street medic training in the Bay Area, hosted a national webinar attended by over 3,000 medical professionals to provide the bridge training to be a street medic. In her online bio, Marya describes the coalition as “an organization of over 450 health workers committed to structural change” in addressing health problems.
“When we see suffering, that’s where we go,” Marya said. “And right now that suffering is happening on the streets.”
In the recent Denver protests, street medics responded to major head, face and eye injuries among protesters from what are sometimes described as “kinetic impact projectiles” or “less-than-lethal” bullets shot at protesters, along with tear-gas and flash-bang stun grenade canisters that either hit them or exploded in their faces.
Garcia, who by day works for an immigrant rights nonprofit, said that these weapons are not designed to be shot directly at people.
“We’re seeing police use these less-lethal weapons in lethal ways, and that is pretty upsetting,” Garcia said about the recent protests.
Denver police Chief Paul Pazen promised to make changes, including banning chokeholds and requiring SWAT teams to turn on their body cameras. Last week, a federal judge also issued a temporary injunction to stop Denver police from using tear gas and other less-than-lethal weapons in response to a class action lawsuit, in which a medic stated he was shot multiple times by police with pepper balls while treating patients. (Last week in North Carolina police were recorded destroying medic stations.)
Denver street medic Kevin Connell, a 30-year-old emergency room nurse, said he was hit with pepper balls in the back of his medic vest – which was clearly marked by red crosses – while treating a patient. He showed up to the Denver protests every night he did not have to work, he said, wearing a Kevlar medic vest, protective goggles and a homemade gas mask fashioned from a water bottle. As a member of the Denver Action Medic Network, Connell also served at the Standing Rock protests in North Dakota in a dispute over the building of the Dakota Access Pipeline.
“I mean, as bad as it sounds, it was only tear gas, pepper balls and rubber bullets that were being fired on us,” Connell said of his recent experience in Denver. “When I was at Standing Rock, they were using high-powered water hoses even when it was, like, freezing cold. … So I think the police here had a little bit more restraint.”
Still, first-time street medic Aj Mossman, a 31-year-old Denver emergency medical technician studying for nursing school, was shocked to be tear-gassed and struck in the back of the leg with a flash grenade while treating a protester on May 30. Mossman still has a large leg bruise.
The following night, Mossman, who uses the pronoun they, brought more protective gear, but said they are still having difficulty processing what felt like a war zone.
“I thought I understood what my black friends went through. I thought I understood what the black community went through,” said Mossman, who is white. “But I had absolutely no idea how violent the police were and how little they cared about who they hurt.”
For Butler, serving as a medic with others from various walks of life was inspiring. “They’re also out there to protect black and brown bodies. And that’s amazing,” she said. “That’s just a beautiful sight.”
This article originally appeared on Kaiser Health News, which is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Amid clouds of choking tear gas, booming flash-bang grenades and other “riot control agents,” volunteer medics plunged into street protests over the past weeks to help the injured – sometimes rushing to the front lines as soon as their hospital shifts ended.
Known as “street medics,” these unorthodox teams of nursing students, veterinarians, doctors, trauma surgeons, security guards, ski patrollers, nurses, wilderness EMTs, and off-the-clock ambulance workers poured water – not milk – into the eyes of tear-gassed protesters. They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go.
So donning cloth masks to protect against the virus – plus helmets, makeshift shields and other gear to guard against rubber bullets, projectiles and tear gas – the volunteer medics organized themselves into a web of first responders to care for people on the streets. They showed up early, set up first-aid stations, established transportation networks and covered their arms, helmets and backpacks with crosses made of red duct tape, to signify that they were medics. Some stayed late into the night past curfews until every protester had left.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. She didn’t see it as a choice.
“I am working full time and basically being at the protest after getting straight off of work,” said Butler, who is black. That’s tiring, she added, but so is being a black woman in America.
After going out as a medic on her own, she soon met other volunteers. Together they used text-message chains to organize their efforts. One night, she responded to a man who had been shot with a rubber bullet in the chest; she said his torso had turned blue and purple from the impact. She also provided aid after a shooting near the protest left someone in critical condition.
“It’s hard, but bills need to be paid and justice needs to be served,” she said.
The street medic movement traces its roots, in part, to the 1960s protests, as well as the American Indian Movement and the Black Panther Party. Denver Action Medic Network offers a 20-hour training course that prepares them to treat patients in conflicts with police and large crowds; a four-hour session is offered to medical professionals as “bridge” training.
Since the coronavirus pandemic began, the Denver Action Medic Network has added new training guidelines: Don’t go to protests if sick or in contact with those who are infected; wear a mask; give people lots of space and use hand sanitizer. Jordan Garcia, a 39-year-old medic for over 20 years who works with the network of veteran street medics, said they also warn medics about the increased risk of transmission because of protesters coughing from tear gas, and urge them to get tested for the virus after the protests.
The number of volunteer medics swelled after George Floyd’s May 25 killing in Minneapolis. In Denver alone, at least 40 people reached out to the Denver Action Medic Network for training.
On June 3, Dr. Rupa Marya, an associate professor of medicine at the University of California,San Francisco, and the co-founder of the Do No Harm Coalition, which runs street medic training in the Bay Area, hosted a national webinar attended by over 3,000 medical professionals to provide the bridge training to be a street medic. In her online bio, Marya describes the coalition as “an organization of over 450 health workers committed to structural change” in addressing health problems.
“When we see suffering, that’s where we go,” Marya said. “And right now that suffering is happening on the streets.”
In the recent Denver protests, street medics responded to major head, face and eye injuries among protesters from what are sometimes described as “kinetic impact projectiles” or “less-than-lethal” bullets shot at protesters, along with tear-gas and flash-bang stun grenade canisters that either hit them or exploded in their faces.
Garcia, who by day works for an immigrant rights nonprofit, said that these weapons are not designed to be shot directly at people.
“We’re seeing police use these less-lethal weapons in lethal ways, and that is pretty upsetting,” Garcia said about the recent protests.
Denver police Chief Paul Pazen promised to make changes, including banning chokeholds and requiring SWAT teams to turn on their body cameras. Last week, a federal judge also issued a temporary injunction to stop Denver police from using tear gas and other less-than-lethal weapons in response to a class action lawsuit, in which a medic stated he was shot multiple times by police with pepper balls while treating patients. (Last week in North Carolina police were recorded destroying medic stations.)
Denver street medic Kevin Connell, a 30-year-old emergency room nurse, said he was hit with pepper balls in the back of his medic vest – which was clearly marked by red crosses – while treating a patient. He showed up to the Denver protests every night he did not have to work, he said, wearing a Kevlar medic vest, protective goggles and a homemade gas mask fashioned from a water bottle. As a member of the Denver Action Medic Network, Connell also served at the Standing Rock protests in North Dakota in a dispute over the building of the Dakota Access Pipeline.
“I mean, as bad as it sounds, it was only tear gas, pepper balls and rubber bullets that were being fired on us,” Connell said of his recent experience in Denver. “When I was at Standing Rock, they were using high-powered water hoses even when it was, like, freezing cold. … So I think the police here had a little bit more restraint.”
Still, first-time street medic Aj Mossman, a 31-year-old Denver emergency medical technician studying for nursing school, was shocked to be tear-gassed and struck in the back of the leg with a flash grenade while treating a protester on May 30. Mossman still has a large leg bruise.
The following night, Mossman, who uses the pronoun they, brought more protective gear, but said they are still having difficulty processing what felt like a war zone.
“I thought I understood what my black friends went through. I thought I understood what the black community went through,” said Mossman, who is white. “But I had absolutely no idea how violent the police were and how little they cared about who they hurt.”
For Butler, serving as a medic with others from various walks of life was inspiring. “They’re also out there to protect black and brown bodies. And that’s amazing,” she said. “That’s just a beautiful sight.”
This article originally appeared on Kaiser Health News, which is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Amid clouds of choking tear gas, booming flash-bang grenades and other “riot control agents,” volunteer medics plunged into street protests over the past weeks to help the injured – sometimes rushing to the front lines as soon as their hospital shifts ended.
Known as “street medics,” these unorthodox teams of nursing students, veterinarians, doctors, trauma surgeons, security guards, ski patrollers, nurses, wilderness EMTs, and off-the-clock ambulance workers poured water – not milk – into the eyes of tear-gassed protesters. They stanched bleeding wounds and plucked disoriented teenagers from clouds of gas, entering dangerous corners where on-duty emergency health responders may fear to go.
So donning cloth masks to protect against the virus – plus helmets, makeshift shields and other gear to guard against rubber bullets, projectiles and tear gas – the volunteer medics organized themselves into a web of first responders to care for people on the streets. They showed up early, set up first-aid stations, established transportation networks and covered their arms, helmets and backpacks with crosses made of red duct tape, to signify that they were medics. Some stayed late into the night past curfews until every protester had left.
Iris Butler, a 21-year-old certified nursing assistant who works in a nursing home, decided to offer her skills after seeing a man injured by a rubber bullet on her first night at the Denver protests. She showed up as a medic every night thereafter. She didn’t see it as a choice.
“I am working full time and basically being at the protest after getting straight off of work,” said Butler, who is black. That’s tiring, she added, but so is being a black woman in America.
After going out as a medic on her own, she soon met other volunteers. Together they used text-message chains to organize their efforts. One night, she responded to a man who had been shot with a rubber bullet in the chest; she said his torso had turned blue and purple from the impact. She also provided aid after a shooting near the protest left someone in critical condition.
“It’s hard, but bills need to be paid and justice needs to be served,” she said.
The street medic movement traces its roots, in part, to the 1960s protests, as well as the American Indian Movement and the Black Panther Party. Denver Action Medic Network offers a 20-hour training course that prepares them to treat patients in conflicts with police and large crowds; a four-hour session is offered to medical professionals as “bridge” training.
Since the coronavirus pandemic began, the Denver Action Medic Network has added new training guidelines: Don’t go to protests if sick or in contact with those who are infected; wear a mask; give people lots of space and use hand sanitizer. Jordan Garcia, a 39-year-old medic for over 20 years who works with the network of veteran street medics, said they also warn medics about the increased risk of transmission because of protesters coughing from tear gas, and urge them to get tested for the virus after the protests.
The number of volunteer medics swelled after George Floyd’s May 25 killing in Minneapolis. In Denver alone, at least 40 people reached out to the Denver Action Medic Network for training.
On June 3, Dr. Rupa Marya, an associate professor of medicine at the University of California,San Francisco, and the co-founder of the Do No Harm Coalition, which runs street medic training in the Bay Area, hosted a national webinar attended by over 3,000 medical professionals to provide the bridge training to be a street medic. In her online bio, Marya describes the coalition as “an organization of over 450 health workers committed to structural change” in addressing health problems.
“When we see suffering, that’s where we go,” Marya said. “And right now that suffering is happening on the streets.”
In the recent Denver protests, street medics responded to major head, face and eye injuries among protesters from what are sometimes described as “kinetic impact projectiles” or “less-than-lethal” bullets shot at protesters, along with tear-gas and flash-bang stun grenade canisters that either hit them or exploded in their faces.
Garcia, who by day works for an immigrant rights nonprofit, said that these weapons are not designed to be shot directly at people.
“We’re seeing police use these less-lethal weapons in lethal ways, and that is pretty upsetting,” Garcia said about the recent protests.
Denver police Chief Paul Pazen promised to make changes, including banning chokeholds and requiring SWAT teams to turn on their body cameras. Last week, a federal judge also issued a temporary injunction to stop Denver police from using tear gas and other less-than-lethal weapons in response to a class action lawsuit, in which a medic stated he was shot multiple times by police with pepper balls while treating patients. (Last week in North Carolina police were recorded destroying medic stations.)
Denver street medic Kevin Connell, a 30-year-old emergency room nurse, said he was hit with pepper balls in the back of his medic vest – which was clearly marked by red crosses – while treating a patient. He showed up to the Denver protests every night he did not have to work, he said, wearing a Kevlar medic vest, protective goggles and a homemade gas mask fashioned from a water bottle. As a member of the Denver Action Medic Network, Connell also served at the Standing Rock protests in North Dakota in a dispute over the building of the Dakota Access Pipeline.
“I mean, as bad as it sounds, it was only tear gas, pepper balls and rubber bullets that were being fired on us,” Connell said of his recent experience in Denver. “When I was at Standing Rock, they were using high-powered water hoses even when it was, like, freezing cold. … So I think the police here had a little bit more restraint.”
Still, first-time street medic Aj Mossman, a 31-year-old Denver emergency medical technician studying for nursing school, was shocked to be tear-gassed and struck in the back of the leg with a flash grenade while treating a protester on May 30. Mossman still has a large leg bruise.
The following night, Mossman, who uses the pronoun they, brought more protective gear, but said they are still having difficulty processing what felt like a war zone.
“I thought I understood what my black friends went through. I thought I understood what the black community went through,” said Mossman, who is white. “But I had absolutely no idea how violent the police were and how little they cared about who they hurt.”
For Butler, serving as a medic with others from various walks of life was inspiring. “They’re also out there to protect black and brown bodies. And that’s amazing,” she said. “That’s just a beautiful sight.”
This article originally appeared on Kaiser Health News, which is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Adding low-dose ipi to pembro seems safer, still effective for advanced melanoma
The investigator, Daniel Olson, MD, of the University of Chicago, presented the study results as part of the American Society of Clinical Oncology virtual scientific program.
Pembrolizumab plus ipilimumab at 1 mg/kg generated a response rate of 27%, Dr. Olson reported. This is higher than the 15% response rate observed in historical controls who received ipilimumab alone after primary PD-1 failure (Lancet Oncol. 2019 Sep;20[9]:1239-1251), he noted.
“Treatment-related grade 3 to 4 toxicity occurred in 27% of patients” in the current trial, Dr. Olson added. He said this compares favorably to ipilimumab given at 3 mg/kg in combination with a PD-1 antibody first line, which produced a grade 3/4 adverse event rate of 59% in a prior trial (N Engl J Med 2017; 377:1345-1356).
Preserving efficacy while limiting toxicity
“The combination of PD-1 and CTLA-4 blockade is an incredibly potent combination, not only in melanoma, but across cancer types,” said Douglas Johnson, MD, an assistant professor at Vanderbilt University in Nashville, Tenn., and the discussant on Dr. Olson’s presentation.
Dr. Johnson noted, however, that the combination produces a high incidence of serious immune-related adverse events.
The goal of recent research has been finding a way to preserve the efficacy but limit the toxicity. The tack taken in the current study was to wait until primary PD-1 antibody failure to initiate the combination, then do so with an ipilimumab dose lower than the standard 3 mg/kg used in melanoma.
“The response rate was quite good,” Dr. Johnson said. “I think these are very favorable results.”
“It does seem like the sequential approach does decrease the total number of toxicities compared to using both agents in the front line,” he added. “Should we use 1 mg/kg or 3 mg/kg [ipilimumab] in this sort of sequential-type approach? I would say, at this point, they’re still both viable.”
However, for “patients who really need an upfront response ... we might favor giving combination upfront,” Dr. Johnson said.
Patients and treatment
The trial (NCT02743819) enrolled 70 patients with unresectable or metastatic melanoma that had progressed on a PD-1 antibody after a median treatment duration of 4.8 months. Patients had no prior exposure to a CTLA4 antibody.
Prior to entry, 86% of subjects had been treated with a PD-1 antibody alone, 14% with a PD-1 antibody in a non-CTLA4 antibody combination, and 7% with BRAF-directed therapy prior to PD-1 antibody treatment.
The patients’ median age was 64 years, and 67% were men. Overall, 89% of subjects had cutaneous melanoma, 10% acral melanoma, and 1% mucosal melanoma.
Half of patients had stage IV M1c or M1d disease. Ten percent had treated brain metastases at baseline, 24% had liver metastases, 28% had baseline lactate dehydrogenase (LDH) above the upper limit of normal, and 29% had BRAF mutations.
The patients were treated with ipilimumab at 1 mg/kg every 3 weeks for four doses. They received pembrolizumab at 200 mg every 3 weeks for up to 2 years.
Response details
There were 61 subjects evaluable for response, but all 70 patients were considered in the response rate. There were 5 complete responses and 14 partial responses, for a response rate of 27% (19/70). The median duration of response was 18.5 months.
“We did observe a substantially higher response rate among the PD-L1 negative subgroup, as compared to PD-L1-positive,” Dr. Olson said. “The responses observed in some of these higher-risk patients, and especially the responses we saw among many PD-L1-negative tumors, suggested that we might be capturing atypical responders with [pembrolizumab plus ipilimumab].”
“Most responses occurred in non-T-cell-inflamed or intermediate tumors,” Dr. Olson added. “Our trial enriched for non-T-cell inflamed tumor phenotypes, where we then observe[d] responses.”
“These patients responded across BRAF mutation status,” Dr. Johnson noted. “Patients who had elevated LDH, those who had liver metastases, brain metastases, also had comparable response rates to those lacking those more adverse prognostic features.”
Survival and safety
The median progression-free survival was 5 months, and the median overall survival was 24.7 months.
“The multiple durable responses we observed did translate into long-term survival for some patients,” Dr. Olson said.
Eighteen subjects (26%) had grade 3 adverse events at least possibly related to treatment. The most common were colitis/diarrhea in 9%, rash in 6%, and ALT/AST elevations in 6%. There was one grade 4 adverse event, a lipase elevation.
The median time to onset of high-grade adverse events was 55 days, which would fall between cycles 2 and 3 of ipilimumab “and is similar to the experience with [ipilimumab] in the front-line setting,” Dr. Olson said.
This study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company. Dr. Johnson is an advisor for Merck.
SOURCE: Olson D et al. ASCO 2020, Abstract 10004.
The investigator, Daniel Olson, MD, of the University of Chicago, presented the study results as part of the American Society of Clinical Oncology virtual scientific program.
Pembrolizumab plus ipilimumab at 1 mg/kg generated a response rate of 27%, Dr. Olson reported. This is higher than the 15% response rate observed in historical controls who received ipilimumab alone after primary PD-1 failure (Lancet Oncol. 2019 Sep;20[9]:1239-1251), he noted.
“Treatment-related grade 3 to 4 toxicity occurred in 27% of patients” in the current trial, Dr. Olson added. He said this compares favorably to ipilimumab given at 3 mg/kg in combination with a PD-1 antibody first line, which produced a grade 3/4 adverse event rate of 59% in a prior trial (N Engl J Med 2017; 377:1345-1356).
Preserving efficacy while limiting toxicity
“The combination of PD-1 and CTLA-4 blockade is an incredibly potent combination, not only in melanoma, but across cancer types,” said Douglas Johnson, MD, an assistant professor at Vanderbilt University in Nashville, Tenn., and the discussant on Dr. Olson’s presentation.
Dr. Johnson noted, however, that the combination produces a high incidence of serious immune-related adverse events.
The goal of recent research has been finding a way to preserve the efficacy but limit the toxicity. The tack taken in the current study was to wait until primary PD-1 antibody failure to initiate the combination, then do so with an ipilimumab dose lower than the standard 3 mg/kg used in melanoma.
“The response rate was quite good,” Dr. Johnson said. “I think these are very favorable results.”
“It does seem like the sequential approach does decrease the total number of toxicities compared to using both agents in the front line,” he added. “Should we use 1 mg/kg or 3 mg/kg [ipilimumab] in this sort of sequential-type approach? I would say, at this point, they’re still both viable.”
However, for “patients who really need an upfront response ... we might favor giving combination upfront,” Dr. Johnson said.
Patients and treatment
The trial (NCT02743819) enrolled 70 patients with unresectable or metastatic melanoma that had progressed on a PD-1 antibody after a median treatment duration of 4.8 months. Patients had no prior exposure to a CTLA4 antibody.
Prior to entry, 86% of subjects had been treated with a PD-1 antibody alone, 14% with a PD-1 antibody in a non-CTLA4 antibody combination, and 7% with BRAF-directed therapy prior to PD-1 antibody treatment.
The patients’ median age was 64 years, and 67% were men. Overall, 89% of subjects had cutaneous melanoma, 10% acral melanoma, and 1% mucosal melanoma.
Half of patients had stage IV M1c or M1d disease. Ten percent had treated brain metastases at baseline, 24% had liver metastases, 28% had baseline lactate dehydrogenase (LDH) above the upper limit of normal, and 29% had BRAF mutations.
The patients were treated with ipilimumab at 1 mg/kg every 3 weeks for four doses. They received pembrolizumab at 200 mg every 3 weeks for up to 2 years.
Response details
There were 61 subjects evaluable for response, but all 70 patients were considered in the response rate. There were 5 complete responses and 14 partial responses, for a response rate of 27% (19/70). The median duration of response was 18.5 months.
“We did observe a substantially higher response rate among the PD-L1 negative subgroup, as compared to PD-L1-positive,” Dr. Olson said. “The responses observed in some of these higher-risk patients, and especially the responses we saw among many PD-L1-negative tumors, suggested that we might be capturing atypical responders with [pembrolizumab plus ipilimumab].”
“Most responses occurred in non-T-cell-inflamed or intermediate tumors,” Dr. Olson added. “Our trial enriched for non-T-cell inflamed tumor phenotypes, where we then observe[d] responses.”
“These patients responded across BRAF mutation status,” Dr. Johnson noted. “Patients who had elevated LDH, those who had liver metastases, brain metastases, also had comparable response rates to those lacking those more adverse prognostic features.”
Survival and safety
The median progression-free survival was 5 months, and the median overall survival was 24.7 months.
“The multiple durable responses we observed did translate into long-term survival for some patients,” Dr. Olson said.
Eighteen subjects (26%) had grade 3 adverse events at least possibly related to treatment. The most common were colitis/diarrhea in 9%, rash in 6%, and ALT/AST elevations in 6%. There was one grade 4 adverse event, a lipase elevation.
The median time to onset of high-grade adverse events was 55 days, which would fall between cycles 2 and 3 of ipilimumab “and is similar to the experience with [ipilimumab] in the front-line setting,” Dr. Olson said.
This study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company. Dr. Johnson is an advisor for Merck.
SOURCE: Olson D et al. ASCO 2020, Abstract 10004.
The investigator, Daniel Olson, MD, of the University of Chicago, presented the study results as part of the American Society of Clinical Oncology virtual scientific program.
Pembrolizumab plus ipilimumab at 1 mg/kg generated a response rate of 27%, Dr. Olson reported. This is higher than the 15% response rate observed in historical controls who received ipilimumab alone after primary PD-1 failure (Lancet Oncol. 2019 Sep;20[9]:1239-1251), he noted.
“Treatment-related grade 3 to 4 toxicity occurred in 27% of patients” in the current trial, Dr. Olson added. He said this compares favorably to ipilimumab given at 3 mg/kg in combination with a PD-1 antibody first line, which produced a grade 3/4 adverse event rate of 59% in a prior trial (N Engl J Med 2017; 377:1345-1356).
Preserving efficacy while limiting toxicity
“The combination of PD-1 and CTLA-4 blockade is an incredibly potent combination, not only in melanoma, but across cancer types,” said Douglas Johnson, MD, an assistant professor at Vanderbilt University in Nashville, Tenn., and the discussant on Dr. Olson’s presentation.
Dr. Johnson noted, however, that the combination produces a high incidence of serious immune-related adverse events.
The goal of recent research has been finding a way to preserve the efficacy but limit the toxicity. The tack taken in the current study was to wait until primary PD-1 antibody failure to initiate the combination, then do so with an ipilimumab dose lower than the standard 3 mg/kg used in melanoma.
“The response rate was quite good,” Dr. Johnson said. “I think these are very favorable results.”
“It does seem like the sequential approach does decrease the total number of toxicities compared to using both agents in the front line,” he added. “Should we use 1 mg/kg or 3 mg/kg [ipilimumab] in this sort of sequential-type approach? I would say, at this point, they’re still both viable.”
However, for “patients who really need an upfront response ... we might favor giving combination upfront,” Dr. Johnson said.
Patients and treatment
The trial (NCT02743819) enrolled 70 patients with unresectable or metastatic melanoma that had progressed on a PD-1 antibody after a median treatment duration of 4.8 months. Patients had no prior exposure to a CTLA4 antibody.
Prior to entry, 86% of subjects had been treated with a PD-1 antibody alone, 14% with a PD-1 antibody in a non-CTLA4 antibody combination, and 7% with BRAF-directed therapy prior to PD-1 antibody treatment.
The patients’ median age was 64 years, and 67% were men. Overall, 89% of subjects had cutaneous melanoma, 10% acral melanoma, and 1% mucosal melanoma.
Half of patients had stage IV M1c or M1d disease. Ten percent had treated brain metastases at baseline, 24% had liver metastases, 28% had baseline lactate dehydrogenase (LDH) above the upper limit of normal, and 29% had BRAF mutations.
The patients were treated with ipilimumab at 1 mg/kg every 3 weeks for four doses. They received pembrolizumab at 200 mg every 3 weeks for up to 2 years.
Response details
There were 61 subjects evaluable for response, but all 70 patients were considered in the response rate. There were 5 complete responses and 14 partial responses, for a response rate of 27% (19/70). The median duration of response was 18.5 months.
“We did observe a substantially higher response rate among the PD-L1 negative subgroup, as compared to PD-L1-positive,” Dr. Olson said. “The responses observed in some of these higher-risk patients, and especially the responses we saw among many PD-L1-negative tumors, suggested that we might be capturing atypical responders with [pembrolizumab plus ipilimumab].”
“Most responses occurred in non-T-cell-inflamed or intermediate tumors,” Dr. Olson added. “Our trial enriched for non-T-cell inflamed tumor phenotypes, where we then observe[d] responses.”
“These patients responded across BRAF mutation status,” Dr. Johnson noted. “Patients who had elevated LDH, those who had liver metastases, brain metastases, also had comparable response rates to those lacking those more adverse prognostic features.”
Survival and safety
The median progression-free survival was 5 months, and the median overall survival was 24.7 months.
“The multiple durable responses we observed did translate into long-term survival for some patients,” Dr. Olson said.
Eighteen subjects (26%) had grade 3 adverse events at least possibly related to treatment. The most common were colitis/diarrhea in 9%, rash in 6%, and ALT/AST elevations in 6%. There was one grade 4 adverse event, a lipase elevation.
The median time to onset of high-grade adverse events was 55 days, which would fall between cycles 2 and 3 of ipilimumab “and is similar to the experience with [ipilimumab] in the front-line setting,” Dr. Olson said.
This study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company. Dr. Johnson is an advisor for Merck.
SOURCE: Olson D et al. ASCO 2020, Abstract 10004.
FROM ASCO 2020
Key clinical point: Low-dose ipilimumab (1 mg/kg) plus pembrolizumab given immediately after progression on a PD-1 antibody alone demonstrated antitumor activity and tolerability in patients with advanced melanoma, according to an investigator.
Major finding: There were 5 complete responses and 14 partial responses, for a response rate of 27%. The rate of grade 3/4 adverse events was 27%.
Study details: Phase 2 study of 70 patients, 61 of whom were evaluable for response.
Disclosures: The study was funded by an investigator-initiated grant from Merck. Dr. Olson had no disclosures. Some of his coinvestigators reported ties to the company.
Source: Olson D et al. ASCO 2020, Abstract 10004.
Upfront stereotactic radiosurgery an option for SCLC brain mets
Largest study of its kind
A new retrospective study provides some of the strongest support yet for considering first-line stereotactic radiosurgery (SRS) over whole-brain radiotherapy (WBRT) in carefully selected patients with brain metastases from small-cell lung cancer (SCLC), the researchers say.
As expected, WBRT was superior to focused SRS in lengthening the time to disease progression in the brain. However, this advantage did not appear to provide an improvement in overall survival (OS).
“This study suggests that the trade-offs inherent to first-line SRS without WBRT, including a shorter time to new brain metastases without an apparent difference in overall survival, may be similar to other settings where SRS alone is already well established,” lead author Chad Rusthoven, MD, told Medscape Medical News.
Upfront SRS may be “particularly attractive for SCLC patients with limited brain metastases and those at a higher risk of developing neurocognitive toxicity from WBRT, including older patients and those with a poor baseline performance status,” said Rusthoven, of the Department of Radiation Oncology, University of Colorado School of Medicine, Aurora.
Results of the FIRE-SCLC study – the largest analysis of first-line SRS for patients with SCLC brain metastases – were published online June 4 in JAMA Oncology.
The coauthors of an editorial in JAMA Oncology say the FIRE-SCLC study investigators should be “commended for conducting this important work and also for highlighting the inherent limitations of retrospective data.”
“Even after multivariable adjustment, OS may not be directly compared between the SRS and WBRT groups because selection bias is likely,” caution Cecile Le Pechoux, MD, and Antonin Levy, MD, PhD, from Institut Gustave-Roussy in Villejuif, France.
“Impressive” Outcomes
The researchers analyzed the outcomes of 710 patients (mean age, 68.5 years; 75% men; Karnofsky Performance Status score, ≥90) who underwent first-line SRS without prior treatment with WBRT or prophylactic cranial irradiation. They compared the SRS outcomes with outcomes of a cohort of 219 patients treated with first-line WBRT for SCLC brain metastases.
The SRS outcomes are “encouraging,” with a median OS of 8.5 months, median time to central nervous system (CNS) progression (TTCP) of 8.1 months, and median CNS progression-free survival (PFS) of 5.0 months, the study investigators say.
The outcomes are “particularly impressive” in patients with a single brain metastasis (median OS and TTCP, 11.0 months and 11.7 months, respectively), they note.
They found no significant differences in OS or TTCP after SRS in patients with two to four lesions and those with five to 10 lesions.
Median OS was 8.7 months with two to four lesions, 8.0 months with five to 10 lesions, and 5.5 months with 11 or more lesions. Corresponding median TTCP was 6.8, 6.1, and 4.7 months.
Local failures after SRS were rare. Most CNS progression occurred in the form of new lesions, which is in line with what’s been shown with SRS in other settings.
In propensity score–matched analyses that compared SRS with WBRT, median OS was higher with SRS (6.5 months vs 5.2 months with WBRT; P = .003). Median TTCP was improved with WBRT (SRS, 9.0 months vs WBRT, not reached; hazard ratio, 0.38; 95% confidence interval, 0.26 – 0.55; P < .001), with no significant difference in CNS PFS (median, 4.0 months for SRS vs 3.8 months for WBRT; P = .79).
The results were similar in multivariable analyses that compared SRS and WBRT, including subgroup analyses that controlled for extracranial metastases and extracranial disease control status.
Benchmark Data
“Although these retrospective data should not be used to conclude that OS is superior with SRS, the findings of this study suggest that the primary trade-offs associated with SRS without WBRT, including a shorter TTCP, are similar to other settings in which SRS alone is well established by multiple randomized clinical trials,” the researchers write.
These data, they say, provide a “benchmark for SRS outcomes and offer support to first-line SRS as a treatment option in carefully selected patients with small-cell lung cancer.”
In a news release, senior author Tyler Robin, MD, University of Colorado School of Medicine, notes that paradigms for the treatment of SCLC are “evolving,” with the integration of immunotherapy into SCLC management, less use of WBRT, and guideline updates advising routine brain MRI surveillance for all patients.
“These changes may be expected to increase the identification of small-cell lung cancer patients with limited brain metastases who may be candidates for first-line SRS,” said Robin.
SRS made mainstream headlines in 2015 when former President Jimmy Carter was successfully treated for melanoma brain metastases with it. At the time, SRS was relatively new. The approach is more targeted and less toxic than traditional WBRT. Carter was treated at Emory University in Atlanta, Georgia.
SRS is now widely available in the United States, but adoption has been slow, Rusthoven told Medscape Medical News.
“Delayed adoption of SRS for SCLC is related to a number of factors, including a concern for short-interval CNS progression with SCLC histology and the historical exclusion of SCLC patients from the landmark randomized trials that established SRS alone,” he said.
“We hope that this study will contribute to an increased interest in the role of SRS for carefully selected SCLC patients and that it will offer support to ongoing and developing prospective clinical trials evaluating first-line SRS alone for SCLC,” Rusthoven added.
Prospective Data “Eagerly” Needed
The French editorial writers say prospective data are “eagerly needed” for this patient population.
SRS, they conclude, “might be a promising treatment option” for patients with SCLC with brain metastases, but larger studies are needed, as prophylactic cranial irradiation or prophylactic-intent WBRT has been shown to improve survival. “Hopefully, the work of Rusthoven et al will be used for the development of further prospective trials in patients with SCLC with brain metastases,” they write.
The study was funded by a grant from the University of Colorado Cancer Center. Rusthoven has received research funding from Takeda outside the submitted work as well as honoraria for educational talks from Genentech and AstraZeneca outside this work. The original article contains a complete list of author disclosures. Le Pechoux has received institutional honoraria for participation in advisory boards from AstraZeneca, Nanobiotix, and Roche; institutional honoraria for participation to educational meetings from Amgen, AstraZeneca, Medscape, and Eli Lilly and Company; and personal honoraria from prIME Oncology for participation in educational meetings. Levy has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Largest study of its kind
Largest study of its kind
A new retrospective study provides some of the strongest support yet for considering first-line stereotactic radiosurgery (SRS) over whole-brain radiotherapy (WBRT) in carefully selected patients with brain metastases from small-cell lung cancer (SCLC), the researchers say.
As expected, WBRT was superior to focused SRS in lengthening the time to disease progression in the brain. However, this advantage did not appear to provide an improvement in overall survival (OS).
“This study suggests that the trade-offs inherent to first-line SRS without WBRT, including a shorter time to new brain metastases without an apparent difference in overall survival, may be similar to other settings where SRS alone is already well established,” lead author Chad Rusthoven, MD, told Medscape Medical News.
Upfront SRS may be “particularly attractive for SCLC patients with limited brain metastases and those at a higher risk of developing neurocognitive toxicity from WBRT, including older patients and those with a poor baseline performance status,” said Rusthoven, of the Department of Radiation Oncology, University of Colorado School of Medicine, Aurora.
Results of the FIRE-SCLC study – the largest analysis of first-line SRS for patients with SCLC brain metastases – were published online June 4 in JAMA Oncology.
The coauthors of an editorial in JAMA Oncology say the FIRE-SCLC study investigators should be “commended for conducting this important work and also for highlighting the inherent limitations of retrospective data.”
“Even after multivariable adjustment, OS may not be directly compared between the SRS and WBRT groups because selection bias is likely,” caution Cecile Le Pechoux, MD, and Antonin Levy, MD, PhD, from Institut Gustave-Roussy in Villejuif, France.
“Impressive” Outcomes
The researchers analyzed the outcomes of 710 patients (mean age, 68.5 years; 75% men; Karnofsky Performance Status score, ≥90) who underwent first-line SRS without prior treatment with WBRT or prophylactic cranial irradiation. They compared the SRS outcomes with outcomes of a cohort of 219 patients treated with first-line WBRT for SCLC brain metastases.
The SRS outcomes are “encouraging,” with a median OS of 8.5 months, median time to central nervous system (CNS) progression (TTCP) of 8.1 months, and median CNS progression-free survival (PFS) of 5.0 months, the study investigators say.
The outcomes are “particularly impressive” in patients with a single brain metastasis (median OS and TTCP, 11.0 months and 11.7 months, respectively), they note.
They found no significant differences in OS or TTCP after SRS in patients with two to four lesions and those with five to 10 lesions.
Median OS was 8.7 months with two to four lesions, 8.0 months with five to 10 lesions, and 5.5 months with 11 or more lesions. Corresponding median TTCP was 6.8, 6.1, and 4.7 months.
Local failures after SRS were rare. Most CNS progression occurred in the form of new lesions, which is in line with what’s been shown with SRS in other settings.
In propensity score–matched analyses that compared SRS with WBRT, median OS was higher with SRS (6.5 months vs 5.2 months with WBRT; P = .003). Median TTCP was improved with WBRT (SRS, 9.0 months vs WBRT, not reached; hazard ratio, 0.38; 95% confidence interval, 0.26 – 0.55; P < .001), with no significant difference in CNS PFS (median, 4.0 months for SRS vs 3.8 months for WBRT; P = .79).
The results were similar in multivariable analyses that compared SRS and WBRT, including subgroup analyses that controlled for extracranial metastases and extracranial disease control status.
Benchmark Data
“Although these retrospective data should not be used to conclude that OS is superior with SRS, the findings of this study suggest that the primary trade-offs associated with SRS without WBRT, including a shorter TTCP, are similar to other settings in which SRS alone is well established by multiple randomized clinical trials,” the researchers write.
These data, they say, provide a “benchmark for SRS outcomes and offer support to first-line SRS as a treatment option in carefully selected patients with small-cell lung cancer.”
In a news release, senior author Tyler Robin, MD, University of Colorado School of Medicine, notes that paradigms for the treatment of SCLC are “evolving,” with the integration of immunotherapy into SCLC management, less use of WBRT, and guideline updates advising routine brain MRI surveillance for all patients.
“These changes may be expected to increase the identification of small-cell lung cancer patients with limited brain metastases who may be candidates for first-line SRS,” said Robin.
SRS made mainstream headlines in 2015 when former President Jimmy Carter was successfully treated for melanoma brain metastases with it. At the time, SRS was relatively new. The approach is more targeted and less toxic than traditional WBRT. Carter was treated at Emory University in Atlanta, Georgia.
SRS is now widely available in the United States, but adoption has been slow, Rusthoven told Medscape Medical News.
“Delayed adoption of SRS for SCLC is related to a number of factors, including a concern for short-interval CNS progression with SCLC histology and the historical exclusion of SCLC patients from the landmark randomized trials that established SRS alone,” he said.
“We hope that this study will contribute to an increased interest in the role of SRS for carefully selected SCLC patients and that it will offer support to ongoing and developing prospective clinical trials evaluating first-line SRS alone for SCLC,” Rusthoven added.
Prospective Data “Eagerly” Needed
The French editorial writers say prospective data are “eagerly needed” for this patient population.
SRS, they conclude, “might be a promising treatment option” for patients with SCLC with brain metastases, but larger studies are needed, as prophylactic cranial irradiation or prophylactic-intent WBRT has been shown to improve survival. “Hopefully, the work of Rusthoven et al will be used for the development of further prospective trials in patients with SCLC with brain metastases,” they write.
The study was funded by a grant from the University of Colorado Cancer Center. Rusthoven has received research funding from Takeda outside the submitted work as well as honoraria for educational talks from Genentech and AstraZeneca outside this work. The original article contains a complete list of author disclosures. Le Pechoux has received institutional honoraria for participation in advisory boards from AstraZeneca, Nanobiotix, and Roche; institutional honoraria for participation to educational meetings from Amgen, AstraZeneca, Medscape, and Eli Lilly and Company; and personal honoraria from prIME Oncology for participation in educational meetings. Levy has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A new retrospective study provides some of the strongest support yet for considering first-line stereotactic radiosurgery (SRS) over whole-brain radiotherapy (WBRT) in carefully selected patients with brain metastases from small-cell lung cancer (SCLC), the researchers say.
As expected, WBRT was superior to focused SRS in lengthening the time to disease progression in the brain. However, this advantage did not appear to provide an improvement in overall survival (OS).
“This study suggests that the trade-offs inherent to first-line SRS without WBRT, including a shorter time to new brain metastases without an apparent difference in overall survival, may be similar to other settings where SRS alone is already well established,” lead author Chad Rusthoven, MD, told Medscape Medical News.
Upfront SRS may be “particularly attractive for SCLC patients with limited brain metastases and those at a higher risk of developing neurocognitive toxicity from WBRT, including older patients and those with a poor baseline performance status,” said Rusthoven, of the Department of Radiation Oncology, University of Colorado School of Medicine, Aurora.
Results of the FIRE-SCLC study – the largest analysis of first-line SRS for patients with SCLC brain metastases – were published online June 4 in JAMA Oncology.
The coauthors of an editorial in JAMA Oncology say the FIRE-SCLC study investigators should be “commended for conducting this important work and also for highlighting the inherent limitations of retrospective data.”
“Even after multivariable adjustment, OS may not be directly compared between the SRS and WBRT groups because selection bias is likely,” caution Cecile Le Pechoux, MD, and Antonin Levy, MD, PhD, from Institut Gustave-Roussy in Villejuif, France.
“Impressive” Outcomes
The researchers analyzed the outcomes of 710 patients (mean age, 68.5 years; 75% men; Karnofsky Performance Status score, ≥90) who underwent first-line SRS without prior treatment with WBRT or prophylactic cranial irradiation. They compared the SRS outcomes with outcomes of a cohort of 219 patients treated with first-line WBRT for SCLC brain metastases.
The SRS outcomes are “encouraging,” with a median OS of 8.5 months, median time to central nervous system (CNS) progression (TTCP) of 8.1 months, and median CNS progression-free survival (PFS) of 5.0 months, the study investigators say.
The outcomes are “particularly impressive” in patients with a single brain metastasis (median OS and TTCP, 11.0 months and 11.7 months, respectively), they note.
They found no significant differences in OS or TTCP after SRS in patients with two to four lesions and those with five to 10 lesions.
Median OS was 8.7 months with two to four lesions, 8.0 months with five to 10 lesions, and 5.5 months with 11 or more lesions. Corresponding median TTCP was 6.8, 6.1, and 4.7 months.
Local failures after SRS were rare. Most CNS progression occurred in the form of new lesions, which is in line with what’s been shown with SRS in other settings.
In propensity score–matched analyses that compared SRS with WBRT, median OS was higher with SRS (6.5 months vs 5.2 months with WBRT; P = .003). Median TTCP was improved with WBRT (SRS, 9.0 months vs WBRT, not reached; hazard ratio, 0.38; 95% confidence interval, 0.26 – 0.55; P < .001), with no significant difference in CNS PFS (median, 4.0 months for SRS vs 3.8 months for WBRT; P = .79).
The results were similar in multivariable analyses that compared SRS and WBRT, including subgroup analyses that controlled for extracranial metastases and extracranial disease control status.
Benchmark Data
“Although these retrospective data should not be used to conclude that OS is superior with SRS, the findings of this study suggest that the primary trade-offs associated with SRS without WBRT, including a shorter TTCP, are similar to other settings in which SRS alone is well established by multiple randomized clinical trials,” the researchers write.
These data, they say, provide a “benchmark for SRS outcomes and offer support to first-line SRS as a treatment option in carefully selected patients with small-cell lung cancer.”
In a news release, senior author Tyler Robin, MD, University of Colorado School of Medicine, notes that paradigms for the treatment of SCLC are “evolving,” with the integration of immunotherapy into SCLC management, less use of WBRT, and guideline updates advising routine brain MRI surveillance for all patients.
“These changes may be expected to increase the identification of small-cell lung cancer patients with limited brain metastases who may be candidates for first-line SRS,” said Robin.
SRS made mainstream headlines in 2015 when former President Jimmy Carter was successfully treated for melanoma brain metastases with it. At the time, SRS was relatively new. The approach is more targeted and less toxic than traditional WBRT. Carter was treated at Emory University in Atlanta, Georgia.
SRS is now widely available in the United States, but adoption has been slow, Rusthoven told Medscape Medical News.
“Delayed adoption of SRS for SCLC is related to a number of factors, including a concern for short-interval CNS progression with SCLC histology and the historical exclusion of SCLC patients from the landmark randomized trials that established SRS alone,” he said.
“We hope that this study will contribute to an increased interest in the role of SRS for carefully selected SCLC patients and that it will offer support to ongoing and developing prospective clinical trials evaluating first-line SRS alone for SCLC,” Rusthoven added.
Prospective Data “Eagerly” Needed
The French editorial writers say prospective data are “eagerly needed” for this patient population.
SRS, they conclude, “might be a promising treatment option” for patients with SCLC with brain metastases, but larger studies are needed, as prophylactic cranial irradiation or prophylactic-intent WBRT has been shown to improve survival. “Hopefully, the work of Rusthoven et al will be used for the development of further prospective trials in patients with SCLC with brain metastases,” they write.
The study was funded by a grant from the University of Colorado Cancer Center. Rusthoven has received research funding from Takeda outside the submitted work as well as honoraria for educational talks from Genentech and AstraZeneca outside this work. The original article contains a complete list of author disclosures. Le Pechoux has received institutional honoraria for participation in advisory boards from AstraZeneca, Nanobiotix, and Roche; institutional honoraria for participation to educational meetings from Amgen, AstraZeneca, Medscape, and Eli Lilly and Company; and personal honoraria from prIME Oncology for participation in educational meetings. Levy has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Ethical considerations in nutrition support because of provider bias
Case:
A 37-year-old woman presents with severe emaciation (body mass index, 9.4 kg/m2) because of chronic severe avoidant/restrictive food intake disorder. She had asked for parenteral nutrition (PN) for several years, whenever her providers pushed her to accept nutrition support, as she had experienced extreme distress because of presumed gastroparesis with enteral feeds or any time she tried to eat. All of her many physicians refused the request for PN on the basis that her intestine was believed to be functioning and her symptoms were functional, so they insisted on tube feeding. The medical team was angered by the request for PN, and very concerned that providing it would support her belief that she could not eat, which they likened to a delusion. They opined that refusal of appropriate therapy (enteral nutrition) did not constitute an indication for inappropriate therapy (PN). They also deemed her to have capacity, so her refusal of tube feeding was honored. She continued to deteriorate, and because of her inability to travel, along with financial and insurance-related issues, was unable to seek alternative care providers. The family provided access to highly credible external consultants, and begged that her providers initiate PN as a life-saving measure. Both were declined. She was taken by her family to the emergency department when she began to have difficulty ambulating and increasing confusion. In recognition of the severity of her starvation, she was to be admitted to the critical care unit. With minimal monitoring while awaiting transfer from the emergency department overnight, she developed severe hypoglycemia and sustained cardiac arrest. Although spontaneous circulation was resumed, she sustained anoxic brain injury, and died after removal of life-sustaining treatment.
Ethical considerations
This case illustrates how the practice of caring for certain patients may come with deep unconscious determinants and conflicts of expectation – the duty to treat can be unclear in cases of refractory eating disorders. Multiple clinical teams were angry at the patient and her family for requesting PN and refused external input.
Although other eating disorders have received more attention, there is little research specific to avoidant/restrictive food intake disorder. There is some consensus that someone at a very low weight because of anorexia nervosa cannot, by definition, have decisional capacity with regard to feeding. Certainly, reviews cite cognitive dysfunction as a common finding, far worse during starvation, in patients with anorexia nervosa,1,2 and nourishment over objection has been advised.3 Further, it is known that gastric dysfunction occurs with some frequency in the presence of starvation in patients with eating disorders.4 Moreover, the potential risks of PN should be contextualized and compared with the certainty of death in someone this starved. Finally, if the patient’s refusal to eat or be tube fed were a delusion, which is by definition “fixed,” refusing to provide PN, and allowing further starvation, would not be expected to have benefit in resolution of the delusion.
Issues related to nourishment can be highly emotive – from “starving to death” on the one hand and “force feeding” on the other. Delivery of adequate nutrition and hydration is considered a basic human right, and must be offered as part of basic care. At the same time, we have observed that the request for nutrition support creates severe moral distress and anger among clinicians treating patients with eating disorders or with fatal illness. Does a delusion preclude feeding, even if by less than ideal means? How should a physician react to feeding treatments they deem excessive or unnecessary? Does a treating team have a duty to consider input from specialists with expertise specific to the patient when such conflict occurs between the patient/family and the treating team? Speculation exists that onset of anorexia nervosa may be linked to a postinfectious condition – a post–viral disease brain reprogramming.5,6 Would an organic explanation change our attitude toward patients with eating disorders?
Medicine’s emotive harms
Clinicians hold more negative attitudes toward certain patients – our implicit bias. It has been suggested that nice patients may be preferred by clinicians and therefore receive more humanistic care.7 Clinicians hold more negative attitudes toward patients with eating disorders than toward other patients. Cases of starvation caused by eating disorders are often seen by clinicians as a form of deviance, which provokes a visceral reaction of anger and frustration. These reactions have been associated with patients’ lack of improvement and personality pathology and with clinicians’ stigmatizing beliefs and inexperience.8 One could argue that this type of unconscious partiality may be worse than intentional harm.
Families and patients often request a treatment as a way to exert their agency. We clinicians may experience ethical dissonance as a result, whether because of ego or because the desired treatment is less favorable (for example, parenteral vs. enteral nutrition). Should maintaining clinical obstinance overrule patient and family autonomy, particularly in the face of the availability of life-saving intervention, even if less desirable than other standard treatments?
Should the physicians have better considered the relative risk of PN? What is the true potential harm? Would it benefit the patient or family? While PN’s benefit is usually life prolongation, it is not without risk of infection, potential mucosal atrophy of the unused gut, hepatic dysfunction, high cost, and an increased complexity of care. However, the incidence of blood stream infections in hospitalized patients receiving PN is only 1 episode for every 100 patient-days of treatment.9 On the other hand, weight regain is a significant determinant of success for treating eating disorders.10 Does the small risk of line-related sepsis, unlikely to be fatal, outweigh the certainty of death from starvation? What is the source of providers’ anger toward such patients? Even when providers feel any hope of improved outcome to be unreasonable, does refusal to provide nourishment, even if less than ideally, improve the likelihood the family will “come to grips” with the situation? Is there an obligation to consider our contribution to the emotional harm to the family because of our refusal, especially if coupled with anger?
Duty of life-saving care
Treating a competent patient without consent is unlawful. Autonomy is the dominant ethical principle, and a mentally competent person has the right to refuse consent to medical treatment for any reason, even when that decision may lead to death. Authors urge that patient lives should not be intentionally shortened, including the withholding of life-prolonging medical treatments or interventions.11,12 Although starvation can compromise capacity, whether patients with severe starvation have truly lost their mental competence and right to self-determination is debated.13 Do physicians have a duty to provide nutrition support by whatever route a patient will accept as a life-saving measure or at least until nutritional stability and improved mental status can be attained?
Next steps
Despite potential concerns clinicians may have over the risks and disadvantages of PN, reeducation of clinician emotional responses toward providing it is needed. As illustrated by this case study, there are likely situations, not fitting the norm, when PN is warranted as a life-saving measure. An awareness of implicit bias we may experience is paramount in all situations. Case-by-case multidisciplinary evaluations are warranted based on guidelines from professional organizations,14 alongside core ethical principles, when considering nutrition support.
References
1. Guillaume S et al. Psychol Med. 2015 Dec;45(16):3377-91.
2. Katzman DK et al. Semin Clin Neuropsychiatry. 2001 Apr;6(2):146-52.
3. Elzakkers IF et al. Int J Eat Disord. 2014 Dec;47(8):845-52.
4. Robinson PH et al. Gut. 1988 Apr;29(4):458-64.
5. Breithaupt L et al. JAMA Psychiatry. 2019 Apr 24;76(8):800-9.
6. Sokol MS. J Child Adolesc Psychopharmacol. 2000;10(2):133-45.
7. Detsky AS, Baerlocher MO. JAMA. 2011 Jul;306(1):94-5.
8. Thompson-Brenner H et al. Psychiatr Serv. 2012 Jan;63(1):73-8.
9. Fonseca G et al. JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):171-5.
10. National Collaborating Centre for Mental Health. In: Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester, United Kingdom: British Psychological Society, 2004.
11. Keown J. Leg Stud. 2000 Mar;20(1):66-84.
12. Sayers GM et al. Postgrad Med J. 2006 Feb;82(964):79-83.
13. Miller I. BioSocieties. 2017;12:89-108.
14. A.S.P.E.N. Ethics Position Paper Task Force; Barrocas A et al. Nutr Clin Pract. 2010 Dec;25(6):672-9.
Dr. Anderson (@dochitect) is a clinical fellow in geriatric medicine at the University of California, San Francisco; Dr. Seres (@davidseres1) is an associate professor of medicine in the Institute of Human Nutrition, director of medical nutrition, and associate clinical ethicist at Columbia University Irving Medical Center, New York. They have no funding sources to declare and no conflicts of interest.
Case:
A 37-year-old woman presents with severe emaciation (body mass index, 9.4 kg/m2) because of chronic severe avoidant/restrictive food intake disorder. She had asked for parenteral nutrition (PN) for several years, whenever her providers pushed her to accept nutrition support, as she had experienced extreme distress because of presumed gastroparesis with enteral feeds or any time she tried to eat. All of her many physicians refused the request for PN on the basis that her intestine was believed to be functioning and her symptoms were functional, so they insisted on tube feeding. The medical team was angered by the request for PN, and very concerned that providing it would support her belief that she could not eat, which they likened to a delusion. They opined that refusal of appropriate therapy (enteral nutrition) did not constitute an indication for inappropriate therapy (PN). They also deemed her to have capacity, so her refusal of tube feeding was honored. She continued to deteriorate, and because of her inability to travel, along with financial and insurance-related issues, was unable to seek alternative care providers. The family provided access to highly credible external consultants, and begged that her providers initiate PN as a life-saving measure. Both were declined. She was taken by her family to the emergency department when she began to have difficulty ambulating and increasing confusion. In recognition of the severity of her starvation, she was to be admitted to the critical care unit. With minimal monitoring while awaiting transfer from the emergency department overnight, she developed severe hypoglycemia and sustained cardiac arrest. Although spontaneous circulation was resumed, she sustained anoxic brain injury, and died after removal of life-sustaining treatment.
Ethical considerations
This case illustrates how the practice of caring for certain patients may come with deep unconscious determinants and conflicts of expectation – the duty to treat can be unclear in cases of refractory eating disorders. Multiple clinical teams were angry at the patient and her family for requesting PN and refused external input.
Although other eating disorders have received more attention, there is little research specific to avoidant/restrictive food intake disorder. There is some consensus that someone at a very low weight because of anorexia nervosa cannot, by definition, have decisional capacity with regard to feeding. Certainly, reviews cite cognitive dysfunction as a common finding, far worse during starvation, in patients with anorexia nervosa,1,2 and nourishment over objection has been advised.3 Further, it is known that gastric dysfunction occurs with some frequency in the presence of starvation in patients with eating disorders.4 Moreover, the potential risks of PN should be contextualized and compared with the certainty of death in someone this starved. Finally, if the patient’s refusal to eat or be tube fed were a delusion, which is by definition “fixed,” refusing to provide PN, and allowing further starvation, would not be expected to have benefit in resolution of the delusion.
Issues related to nourishment can be highly emotive – from “starving to death” on the one hand and “force feeding” on the other. Delivery of adequate nutrition and hydration is considered a basic human right, and must be offered as part of basic care. At the same time, we have observed that the request for nutrition support creates severe moral distress and anger among clinicians treating patients with eating disorders or with fatal illness. Does a delusion preclude feeding, even if by less than ideal means? How should a physician react to feeding treatments they deem excessive or unnecessary? Does a treating team have a duty to consider input from specialists with expertise specific to the patient when such conflict occurs between the patient/family and the treating team? Speculation exists that onset of anorexia nervosa may be linked to a postinfectious condition – a post–viral disease brain reprogramming.5,6 Would an organic explanation change our attitude toward patients with eating disorders?
Medicine’s emotive harms
Clinicians hold more negative attitudes toward certain patients – our implicit bias. It has been suggested that nice patients may be preferred by clinicians and therefore receive more humanistic care.7 Clinicians hold more negative attitudes toward patients with eating disorders than toward other patients. Cases of starvation caused by eating disorders are often seen by clinicians as a form of deviance, which provokes a visceral reaction of anger and frustration. These reactions have been associated with patients’ lack of improvement and personality pathology and with clinicians’ stigmatizing beliefs and inexperience.8 One could argue that this type of unconscious partiality may be worse than intentional harm.
Families and patients often request a treatment as a way to exert their agency. We clinicians may experience ethical dissonance as a result, whether because of ego or because the desired treatment is less favorable (for example, parenteral vs. enteral nutrition). Should maintaining clinical obstinance overrule patient and family autonomy, particularly in the face of the availability of life-saving intervention, even if less desirable than other standard treatments?
Should the physicians have better considered the relative risk of PN? What is the true potential harm? Would it benefit the patient or family? While PN’s benefit is usually life prolongation, it is not without risk of infection, potential mucosal atrophy of the unused gut, hepatic dysfunction, high cost, and an increased complexity of care. However, the incidence of blood stream infections in hospitalized patients receiving PN is only 1 episode for every 100 patient-days of treatment.9 On the other hand, weight regain is a significant determinant of success for treating eating disorders.10 Does the small risk of line-related sepsis, unlikely to be fatal, outweigh the certainty of death from starvation? What is the source of providers’ anger toward such patients? Even when providers feel any hope of improved outcome to be unreasonable, does refusal to provide nourishment, even if less than ideally, improve the likelihood the family will “come to grips” with the situation? Is there an obligation to consider our contribution to the emotional harm to the family because of our refusal, especially if coupled with anger?
Duty of life-saving care
Treating a competent patient without consent is unlawful. Autonomy is the dominant ethical principle, and a mentally competent person has the right to refuse consent to medical treatment for any reason, even when that decision may lead to death. Authors urge that patient lives should not be intentionally shortened, including the withholding of life-prolonging medical treatments or interventions.11,12 Although starvation can compromise capacity, whether patients with severe starvation have truly lost their mental competence and right to self-determination is debated.13 Do physicians have a duty to provide nutrition support by whatever route a patient will accept as a life-saving measure or at least until nutritional stability and improved mental status can be attained?
Next steps
Despite potential concerns clinicians may have over the risks and disadvantages of PN, reeducation of clinician emotional responses toward providing it is needed. As illustrated by this case study, there are likely situations, not fitting the norm, when PN is warranted as a life-saving measure. An awareness of implicit bias we may experience is paramount in all situations. Case-by-case multidisciplinary evaluations are warranted based on guidelines from professional organizations,14 alongside core ethical principles, when considering nutrition support.
References
1. Guillaume S et al. Psychol Med. 2015 Dec;45(16):3377-91.
2. Katzman DK et al. Semin Clin Neuropsychiatry. 2001 Apr;6(2):146-52.
3. Elzakkers IF et al. Int J Eat Disord. 2014 Dec;47(8):845-52.
4. Robinson PH et al. Gut. 1988 Apr;29(4):458-64.
5. Breithaupt L et al. JAMA Psychiatry. 2019 Apr 24;76(8):800-9.
6. Sokol MS. J Child Adolesc Psychopharmacol. 2000;10(2):133-45.
7. Detsky AS, Baerlocher MO. JAMA. 2011 Jul;306(1):94-5.
8. Thompson-Brenner H et al. Psychiatr Serv. 2012 Jan;63(1):73-8.
9. Fonseca G et al. JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):171-5.
10. National Collaborating Centre for Mental Health. In: Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester, United Kingdom: British Psychological Society, 2004.
11. Keown J. Leg Stud. 2000 Mar;20(1):66-84.
12. Sayers GM et al. Postgrad Med J. 2006 Feb;82(964):79-83.
13. Miller I. BioSocieties. 2017;12:89-108.
14. A.S.P.E.N. Ethics Position Paper Task Force; Barrocas A et al. Nutr Clin Pract. 2010 Dec;25(6):672-9.
Dr. Anderson (@dochitect) is a clinical fellow in geriatric medicine at the University of California, San Francisco; Dr. Seres (@davidseres1) is an associate professor of medicine in the Institute of Human Nutrition, director of medical nutrition, and associate clinical ethicist at Columbia University Irving Medical Center, New York. They have no funding sources to declare and no conflicts of interest.
Case:
A 37-year-old woman presents with severe emaciation (body mass index, 9.4 kg/m2) because of chronic severe avoidant/restrictive food intake disorder. She had asked for parenteral nutrition (PN) for several years, whenever her providers pushed her to accept nutrition support, as she had experienced extreme distress because of presumed gastroparesis with enteral feeds or any time she tried to eat. All of her many physicians refused the request for PN on the basis that her intestine was believed to be functioning and her symptoms were functional, so they insisted on tube feeding. The medical team was angered by the request for PN, and very concerned that providing it would support her belief that she could not eat, which they likened to a delusion. They opined that refusal of appropriate therapy (enteral nutrition) did not constitute an indication for inappropriate therapy (PN). They also deemed her to have capacity, so her refusal of tube feeding was honored. She continued to deteriorate, and because of her inability to travel, along with financial and insurance-related issues, was unable to seek alternative care providers. The family provided access to highly credible external consultants, and begged that her providers initiate PN as a life-saving measure. Both were declined. She was taken by her family to the emergency department when she began to have difficulty ambulating and increasing confusion. In recognition of the severity of her starvation, she was to be admitted to the critical care unit. With minimal monitoring while awaiting transfer from the emergency department overnight, she developed severe hypoglycemia and sustained cardiac arrest. Although spontaneous circulation was resumed, she sustained anoxic brain injury, and died after removal of life-sustaining treatment.
Ethical considerations
This case illustrates how the practice of caring for certain patients may come with deep unconscious determinants and conflicts of expectation – the duty to treat can be unclear in cases of refractory eating disorders. Multiple clinical teams were angry at the patient and her family for requesting PN and refused external input.
Although other eating disorders have received more attention, there is little research specific to avoidant/restrictive food intake disorder. There is some consensus that someone at a very low weight because of anorexia nervosa cannot, by definition, have decisional capacity with regard to feeding. Certainly, reviews cite cognitive dysfunction as a common finding, far worse during starvation, in patients with anorexia nervosa,1,2 and nourishment over objection has been advised.3 Further, it is known that gastric dysfunction occurs with some frequency in the presence of starvation in patients with eating disorders.4 Moreover, the potential risks of PN should be contextualized and compared with the certainty of death in someone this starved. Finally, if the patient’s refusal to eat or be tube fed were a delusion, which is by definition “fixed,” refusing to provide PN, and allowing further starvation, would not be expected to have benefit in resolution of the delusion.
Issues related to nourishment can be highly emotive – from “starving to death” on the one hand and “force feeding” on the other. Delivery of adequate nutrition and hydration is considered a basic human right, and must be offered as part of basic care. At the same time, we have observed that the request for nutrition support creates severe moral distress and anger among clinicians treating patients with eating disorders or with fatal illness. Does a delusion preclude feeding, even if by less than ideal means? How should a physician react to feeding treatments they deem excessive or unnecessary? Does a treating team have a duty to consider input from specialists with expertise specific to the patient when such conflict occurs between the patient/family and the treating team? Speculation exists that onset of anorexia nervosa may be linked to a postinfectious condition – a post–viral disease brain reprogramming.5,6 Would an organic explanation change our attitude toward patients with eating disorders?
Medicine’s emotive harms
Clinicians hold more negative attitudes toward certain patients – our implicit bias. It has been suggested that nice patients may be preferred by clinicians and therefore receive more humanistic care.7 Clinicians hold more negative attitudes toward patients with eating disorders than toward other patients. Cases of starvation caused by eating disorders are often seen by clinicians as a form of deviance, which provokes a visceral reaction of anger and frustration. These reactions have been associated with patients’ lack of improvement and personality pathology and with clinicians’ stigmatizing beliefs and inexperience.8 One could argue that this type of unconscious partiality may be worse than intentional harm.
Families and patients often request a treatment as a way to exert their agency. We clinicians may experience ethical dissonance as a result, whether because of ego or because the desired treatment is less favorable (for example, parenteral vs. enteral nutrition). Should maintaining clinical obstinance overrule patient and family autonomy, particularly in the face of the availability of life-saving intervention, even if less desirable than other standard treatments?
Should the physicians have better considered the relative risk of PN? What is the true potential harm? Would it benefit the patient or family? While PN’s benefit is usually life prolongation, it is not without risk of infection, potential mucosal atrophy of the unused gut, hepatic dysfunction, high cost, and an increased complexity of care. However, the incidence of blood stream infections in hospitalized patients receiving PN is only 1 episode for every 100 patient-days of treatment.9 On the other hand, weight regain is a significant determinant of success for treating eating disorders.10 Does the small risk of line-related sepsis, unlikely to be fatal, outweigh the certainty of death from starvation? What is the source of providers’ anger toward such patients? Even when providers feel any hope of improved outcome to be unreasonable, does refusal to provide nourishment, even if less than ideally, improve the likelihood the family will “come to grips” with the situation? Is there an obligation to consider our contribution to the emotional harm to the family because of our refusal, especially if coupled with anger?
Duty of life-saving care
Treating a competent patient without consent is unlawful. Autonomy is the dominant ethical principle, and a mentally competent person has the right to refuse consent to medical treatment for any reason, even when that decision may lead to death. Authors urge that patient lives should not be intentionally shortened, including the withholding of life-prolonging medical treatments or interventions.11,12 Although starvation can compromise capacity, whether patients with severe starvation have truly lost their mental competence and right to self-determination is debated.13 Do physicians have a duty to provide nutrition support by whatever route a patient will accept as a life-saving measure or at least until nutritional stability and improved mental status can be attained?
Next steps
Despite potential concerns clinicians may have over the risks and disadvantages of PN, reeducation of clinician emotional responses toward providing it is needed. As illustrated by this case study, there are likely situations, not fitting the norm, when PN is warranted as a life-saving measure. An awareness of implicit bias we may experience is paramount in all situations. Case-by-case multidisciplinary evaluations are warranted based on guidelines from professional organizations,14 alongside core ethical principles, when considering nutrition support.
References
1. Guillaume S et al. Psychol Med. 2015 Dec;45(16):3377-91.
2. Katzman DK et al. Semin Clin Neuropsychiatry. 2001 Apr;6(2):146-52.
3. Elzakkers IF et al. Int J Eat Disord. 2014 Dec;47(8):845-52.
4. Robinson PH et al. Gut. 1988 Apr;29(4):458-64.
5. Breithaupt L et al. JAMA Psychiatry. 2019 Apr 24;76(8):800-9.
6. Sokol MS. J Child Adolesc Psychopharmacol. 2000;10(2):133-45.
7. Detsky AS, Baerlocher MO. JAMA. 2011 Jul;306(1):94-5.
8. Thompson-Brenner H et al. Psychiatr Serv. 2012 Jan;63(1):73-8.
9. Fonseca G et al. JPEN J Parenter Enteral Nutr. 2018 Jan;42(1):171-5.
10. National Collaborating Centre for Mental Health. In: Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. Leicester, United Kingdom: British Psychological Society, 2004.
11. Keown J. Leg Stud. 2000 Mar;20(1):66-84.
12. Sayers GM et al. Postgrad Med J. 2006 Feb;82(964):79-83.
13. Miller I. BioSocieties. 2017;12:89-108.
14. A.S.P.E.N. Ethics Position Paper Task Force; Barrocas A et al. Nutr Clin Pract. 2010 Dec;25(6):672-9.
Dr. Anderson (@dochitect) is a clinical fellow in geriatric medicine at the University of California, San Francisco; Dr. Seres (@davidseres1) is an associate professor of medicine in the Institute of Human Nutrition, director of medical nutrition, and associate clinical ethicist at Columbia University Irving Medical Center, New York. They have no funding sources to declare and no conflicts of interest.
Flat-topped papules on the neck, arms, and trunk
that typically occur on the trunk, extremities, or genitalia of children and young adults. However, it can affect people of all ages and all areas of skin. Lichen nitidus lesions may emerge in areas of trauma, often in a linear arrangement, called the Koebner phenomenon. It is not thought to be associated with any systemic disease. Nail involvement may be present. Oral lesions are not commonly seen. The diagnosis of LN is often a clinical one.
Histopathology for this patient showed a focally dense lymphohistiocytic infiltrate with multinucleate giant cells in the papillary dermis, associated with overlying epidermal atrophy and adjacent elongated rete ridges surrounding the infiltrate in a characteristic “ball and claw” pattern. These findings were consistent with a diagnosis of lichen nitidus.
The differential diagnosis includes lichen planus (LP). In LP, lesions tend to be larger and more violaceous. They tend to favor wrists, lower extremities, and genitalia. Oral and nail involvement are common. Histologically, a band-like lichenoid infiltrate in the dermis is present. Granulomatous inflammation and giant cells are absent. Direct immunofluorescence is positive for globular deposits of IgG, IgA, IgM and/or complement at the dermal-epidermal junction.
A hepatitis panel was drawn for this patient and was negative. Treatment for lichen nitidus is only needed if symptomatic because lesions will generally resolve spontaneously. Lesions may take months or years to resolve. For significant pruritus, topical corticosteroids or antihistamines may be used. Topical emollients are recommended. Topical tacrolimus has been reported to improve lesions. Oral steroids and light therapy have been reported to improve generalized lichen nitidus not responding to topical treatments.
The case and these photos were submitted byMs. Swartz of Nova Southeastern University, Ft. Lauderdale, Fla.; Dr. Chen and Dr. Walder of Bay Harbor Islands, Fla.; and Dr. Winslow of Pompano Beach, Fla. Donna Bilu Martin, MD, editor of this column, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
that typically occur on the trunk, extremities, or genitalia of children and young adults. However, it can affect people of all ages and all areas of skin. Lichen nitidus lesions may emerge in areas of trauma, often in a linear arrangement, called the Koebner phenomenon. It is not thought to be associated with any systemic disease. Nail involvement may be present. Oral lesions are not commonly seen. The diagnosis of LN is often a clinical one.
Histopathology for this patient showed a focally dense lymphohistiocytic infiltrate with multinucleate giant cells in the papillary dermis, associated with overlying epidermal atrophy and adjacent elongated rete ridges surrounding the infiltrate in a characteristic “ball and claw” pattern. These findings were consistent with a diagnosis of lichen nitidus.
The differential diagnosis includes lichen planus (LP). In LP, lesions tend to be larger and more violaceous. They tend to favor wrists, lower extremities, and genitalia. Oral and nail involvement are common. Histologically, a band-like lichenoid infiltrate in the dermis is present. Granulomatous inflammation and giant cells are absent. Direct immunofluorescence is positive for globular deposits of IgG, IgA, IgM and/or complement at the dermal-epidermal junction.
A hepatitis panel was drawn for this patient and was negative. Treatment for lichen nitidus is only needed if symptomatic because lesions will generally resolve spontaneously. Lesions may take months or years to resolve. For significant pruritus, topical corticosteroids or antihistamines may be used. Topical emollients are recommended. Topical tacrolimus has been reported to improve lesions. Oral steroids and light therapy have been reported to improve generalized lichen nitidus not responding to topical treatments.
The case and these photos were submitted byMs. Swartz of Nova Southeastern University, Ft. Lauderdale, Fla.; Dr. Chen and Dr. Walder of Bay Harbor Islands, Fla.; and Dr. Winslow of Pompano Beach, Fla. Donna Bilu Martin, MD, editor of this column, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
that typically occur on the trunk, extremities, or genitalia of children and young adults. However, it can affect people of all ages and all areas of skin. Lichen nitidus lesions may emerge in areas of trauma, often in a linear arrangement, called the Koebner phenomenon. It is not thought to be associated with any systemic disease. Nail involvement may be present. Oral lesions are not commonly seen. The diagnosis of LN is often a clinical one.
Histopathology for this patient showed a focally dense lymphohistiocytic infiltrate with multinucleate giant cells in the papillary dermis, associated with overlying epidermal atrophy and adjacent elongated rete ridges surrounding the infiltrate in a characteristic “ball and claw” pattern. These findings were consistent with a diagnosis of lichen nitidus.
The differential diagnosis includes lichen planus (LP). In LP, lesions tend to be larger and more violaceous. They tend to favor wrists, lower extremities, and genitalia. Oral and nail involvement are common. Histologically, a band-like lichenoid infiltrate in the dermis is present. Granulomatous inflammation and giant cells are absent. Direct immunofluorescence is positive for globular deposits of IgG, IgA, IgM and/or complement at the dermal-epidermal junction.
A hepatitis panel was drawn for this patient and was negative. Treatment for lichen nitidus is only needed if symptomatic because lesions will generally resolve spontaneously. Lesions may take months or years to resolve. For significant pruritus, topical corticosteroids or antihistamines may be used. Topical emollients are recommended. Topical tacrolimus has been reported to improve lesions. Oral steroids and light therapy have been reported to improve generalized lichen nitidus not responding to topical treatments.
The case and these photos were submitted byMs. Swartz of Nova Southeastern University, Ft. Lauderdale, Fla.; Dr. Chen and Dr. Walder of Bay Harbor Islands, Fla.; and Dr. Winslow of Pompano Beach, Fla. Donna Bilu Martin, MD, editor of this column, is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Meta-analysis: For large proximal polyps, clipping prevents bleeding
Prophylactically clipping large proximal colorectal lesions after resection may reduce risk of postprocedural bleeding, according to a meta-analysis involving nine randomized controlled trials.
Across all lesions, prophylactic clipping had no significant benefit, but when considering only large proximal lesions, clipping reduced bleeding risk by 63%, reported lead author Marco Spadaccini, MD, of Humanitas University, Rozzano, Italy, and colleagues.
According to the investigators, these findings emphasize the relevance of polyp size and location when assessing bleeding risk, which may influence future clinical guidance.
“Despite lack of high-quality evidence, prophylactic clipping has been advocated as a technique to reduce the risk of postprocedural bleeding,” the investigators wrote in Gastroenterology, referring to the European Society of Gastrointestinal Endoscopy recommendation that is based on patient risk factors.
Although previous meta-analyses reported that prophylactic clipping had no protective effect, these studies were “at high risk of bias” and predominantly evaluated lesions less than 20 mm in diameter, the investigators wrote.
Dr. Spadaccini and colleagues suggested that data from more recent, high-quality, randomized controlled trials could be used to identify subgroups that may benefit from clipping. This knowledge is particularly valuable considering the “costs and technical complexity” involved in the procedure, they noted.
The present meta-analysis comprised nine trials that included 7,197 colorectal lesions, of which 49.2% were proximally located and 22.5% were large (at least 20 mm in diameter).
Across all lesions, postprocedural bleeding occurred in 2.2% of clipped lesions and 3.3% of nonclipped lesions, a difference that was not statistically significant (P = .072). But for lesions 20 mm or larger, clipping was associated with a significantly lower rate of bleeding (4.3% vs. 7.6%; relative risk, 0.51; 95% CI, 0.33-0.78; P = .020). Similarly, clipping in the proximal location was independently associated with reduced bleeding risk (3.0% vs. 6.2%; RR, 0.53; 95% CI, 0.35-0.81; P less than .001). A multilevel meta-regression added further clarity by combining both size and location; it showed that clipping had a significant protective effect for large proximal lesions (RR, 0.37; 95% CI, 0.22-0.61; P = .021), but not for those that were small and proximal (RR, 0.88; 95% CI, 0.48-1.62; P = .581).
“According to our meta-analysis, routine practice of endoscopic clipping as a prophylactic intervention does not reduce the risk of postpolypectomy bleeding,” the investigators wrote. “However, clipping was effective in reducing the risk of postprocedural bleeding by nearly 50% for large lesions. If such lesions do not undergo endoscopic clipping, there was fourfold increase in the baseline risk of post-procedural bleeding as compared with those less than 20 mm.”
While the present analysis suggested that clipping was beneficial only for large lesions in the proximal colon, the investigators noted that the protective effect of clipping large lesions in the distal colon (RR, 0.70; 95% CI, 0.22-2.27) was “somewhat intermediate ... albeit not statistically significant” and driven by data from one trial.
“[T]his was not confirmed by other studies generating some uncertainty on the benefit of prophylactic clipping for large distal lesions,” the investigators wrote. “Thus, the decision for large and distal lesions should be tailored, especially taking into consideration other patient- and polyp-risk factors for postprocedural bleeding, such as the use of anti-thrombotic agents or intraprocedural bleeding.”
In contrast, the findings indicated that clipping is unnecessary for lesions less than 20 mm, the investigators wrote.
They went on to explain that clinical application of these findings could result in “significant cost savings” because one bleeding event would be prevented for every 23 large lesions clipped.
“Considering that clips are expensive and their placement might be technically demanding, prophylactic clipping tailored for a subgroup of higher-risk lesions/patients would decrease in parallel both adverse events and costs,” the investigators concluded.
The investigators reported no external funding or conflicts of interest.
ginews@gastro.org
SOURCE: Spadaccini M et al. Gastroenterology. 2020 Apr 1. doi: 10.1053/j.gastro.2020.03.051.
Prophylactically clipping large proximal colorectal lesions after resection may reduce risk of postprocedural bleeding, according to a meta-analysis involving nine randomized controlled trials.
Across all lesions, prophylactic clipping had no significant benefit, but when considering only large proximal lesions, clipping reduced bleeding risk by 63%, reported lead author Marco Spadaccini, MD, of Humanitas University, Rozzano, Italy, and colleagues.
According to the investigators, these findings emphasize the relevance of polyp size and location when assessing bleeding risk, which may influence future clinical guidance.
“Despite lack of high-quality evidence, prophylactic clipping has been advocated as a technique to reduce the risk of postprocedural bleeding,” the investigators wrote in Gastroenterology, referring to the European Society of Gastrointestinal Endoscopy recommendation that is based on patient risk factors.
Although previous meta-analyses reported that prophylactic clipping had no protective effect, these studies were “at high risk of bias” and predominantly evaluated lesions less than 20 mm in diameter, the investigators wrote.
Dr. Spadaccini and colleagues suggested that data from more recent, high-quality, randomized controlled trials could be used to identify subgroups that may benefit from clipping. This knowledge is particularly valuable considering the “costs and technical complexity” involved in the procedure, they noted.
The present meta-analysis comprised nine trials that included 7,197 colorectal lesions, of which 49.2% were proximally located and 22.5% were large (at least 20 mm in diameter).
Across all lesions, postprocedural bleeding occurred in 2.2% of clipped lesions and 3.3% of nonclipped lesions, a difference that was not statistically significant (P = .072). But for lesions 20 mm or larger, clipping was associated with a significantly lower rate of bleeding (4.3% vs. 7.6%; relative risk, 0.51; 95% CI, 0.33-0.78; P = .020). Similarly, clipping in the proximal location was independently associated with reduced bleeding risk (3.0% vs. 6.2%; RR, 0.53; 95% CI, 0.35-0.81; P less than .001). A multilevel meta-regression added further clarity by combining both size and location; it showed that clipping had a significant protective effect for large proximal lesions (RR, 0.37; 95% CI, 0.22-0.61; P = .021), but not for those that were small and proximal (RR, 0.88; 95% CI, 0.48-1.62; P = .581).
“According to our meta-analysis, routine practice of endoscopic clipping as a prophylactic intervention does not reduce the risk of postpolypectomy bleeding,” the investigators wrote. “However, clipping was effective in reducing the risk of postprocedural bleeding by nearly 50% for large lesions. If such lesions do not undergo endoscopic clipping, there was fourfold increase in the baseline risk of post-procedural bleeding as compared with those less than 20 mm.”
While the present analysis suggested that clipping was beneficial only for large lesions in the proximal colon, the investigators noted that the protective effect of clipping large lesions in the distal colon (RR, 0.70; 95% CI, 0.22-2.27) was “somewhat intermediate ... albeit not statistically significant” and driven by data from one trial.
“[T]his was not confirmed by other studies generating some uncertainty on the benefit of prophylactic clipping for large distal lesions,” the investigators wrote. “Thus, the decision for large and distal lesions should be tailored, especially taking into consideration other patient- and polyp-risk factors for postprocedural bleeding, such as the use of anti-thrombotic agents or intraprocedural bleeding.”
In contrast, the findings indicated that clipping is unnecessary for lesions less than 20 mm, the investigators wrote.
They went on to explain that clinical application of these findings could result in “significant cost savings” because one bleeding event would be prevented for every 23 large lesions clipped.
“Considering that clips are expensive and their placement might be technically demanding, prophylactic clipping tailored for a subgroup of higher-risk lesions/patients would decrease in parallel both adverse events and costs,” the investigators concluded.
The investigators reported no external funding or conflicts of interest.
ginews@gastro.org
SOURCE: Spadaccini M et al. Gastroenterology. 2020 Apr 1. doi: 10.1053/j.gastro.2020.03.051.
Prophylactically clipping large proximal colorectal lesions after resection may reduce risk of postprocedural bleeding, according to a meta-analysis involving nine randomized controlled trials.
Across all lesions, prophylactic clipping had no significant benefit, but when considering only large proximal lesions, clipping reduced bleeding risk by 63%, reported lead author Marco Spadaccini, MD, of Humanitas University, Rozzano, Italy, and colleagues.
According to the investigators, these findings emphasize the relevance of polyp size and location when assessing bleeding risk, which may influence future clinical guidance.
“Despite lack of high-quality evidence, prophylactic clipping has been advocated as a technique to reduce the risk of postprocedural bleeding,” the investigators wrote in Gastroenterology, referring to the European Society of Gastrointestinal Endoscopy recommendation that is based on patient risk factors.
Although previous meta-analyses reported that prophylactic clipping had no protective effect, these studies were “at high risk of bias” and predominantly evaluated lesions less than 20 mm in diameter, the investigators wrote.
Dr. Spadaccini and colleagues suggested that data from more recent, high-quality, randomized controlled trials could be used to identify subgroups that may benefit from clipping. This knowledge is particularly valuable considering the “costs and technical complexity” involved in the procedure, they noted.
The present meta-analysis comprised nine trials that included 7,197 colorectal lesions, of which 49.2% were proximally located and 22.5% were large (at least 20 mm in diameter).
Across all lesions, postprocedural bleeding occurred in 2.2% of clipped lesions and 3.3% of nonclipped lesions, a difference that was not statistically significant (P = .072). But for lesions 20 mm or larger, clipping was associated with a significantly lower rate of bleeding (4.3% vs. 7.6%; relative risk, 0.51; 95% CI, 0.33-0.78; P = .020). Similarly, clipping in the proximal location was independently associated with reduced bleeding risk (3.0% vs. 6.2%; RR, 0.53; 95% CI, 0.35-0.81; P less than .001). A multilevel meta-regression added further clarity by combining both size and location; it showed that clipping had a significant protective effect for large proximal lesions (RR, 0.37; 95% CI, 0.22-0.61; P = .021), but not for those that were small and proximal (RR, 0.88; 95% CI, 0.48-1.62; P = .581).
“According to our meta-analysis, routine practice of endoscopic clipping as a prophylactic intervention does not reduce the risk of postpolypectomy bleeding,” the investigators wrote. “However, clipping was effective in reducing the risk of postprocedural bleeding by nearly 50% for large lesions. If such lesions do not undergo endoscopic clipping, there was fourfold increase in the baseline risk of post-procedural bleeding as compared with those less than 20 mm.”
While the present analysis suggested that clipping was beneficial only for large lesions in the proximal colon, the investigators noted that the protective effect of clipping large lesions in the distal colon (RR, 0.70; 95% CI, 0.22-2.27) was “somewhat intermediate ... albeit not statistically significant” and driven by data from one trial.
“[T]his was not confirmed by other studies generating some uncertainty on the benefit of prophylactic clipping for large distal lesions,” the investigators wrote. “Thus, the decision for large and distal lesions should be tailored, especially taking into consideration other patient- and polyp-risk factors for postprocedural bleeding, such as the use of anti-thrombotic agents or intraprocedural bleeding.”
In contrast, the findings indicated that clipping is unnecessary for lesions less than 20 mm, the investigators wrote.
They went on to explain that clinical application of these findings could result in “significant cost savings” because one bleeding event would be prevented for every 23 large lesions clipped.
“Considering that clips are expensive and their placement might be technically demanding, prophylactic clipping tailored for a subgroup of higher-risk lesions/patients would decrease in parallel both adverse events and costs,” the investigators concluded.
The investigators reported no external funding or conflicts of interest.
ginews@gastro.org
SOURCE: Spadaccini M et al. Gastroenterology. 2020 Apr 1. doi: 10.1053/j.gastro.2020.03.051.
FROM GASTROENTEROLOGY