User login
Collaboration is key to bridging the AYA cancer care divide
Survival gains among adolescents and young adults (AYAs) with cancer continue to lag behind outcomes for children and older adult patients. It’s a trend that spans decades, but clinicians and researchers are finally getting serious about trying to understand the underlying causes and are re-examining prevailing practices in an effort to address the discrepancies.
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist at Cleveland Clinic Children’s Hospital, Ohio, said in an interview. He’s specifically referring to the cancers that affect AYAs, who are broadly defined as patients aged 15 through 39 years. “A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults. The biology may be different in the adolescent and young adult patients, which may lead to different outcomes.”
In addition, the psychosocial needs in this age group differ vastly from those in other groups. “Many of these patients are in college or have just started their families, so we have to pay more attention to [issues related to] financial toxicity and fertility, for example,” said Dr Hanna, who is the director of pediatric bone marrow transplantation at the clinic. (The term “financial toxicity” describes the cumulative negative impact of the high cost of care, lost work time, and delays in reaching educational and career goals on patients with cancer and their families.)
Another factor that likely contributes to the outcome disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs.
A recent series of articles published in the journal Blood addressed these and other issues, among them, whether AYAs with acute lymphoblastic leukemia (ALL)1 or aggressive B-cell non-Hodgkin lymphomas (NHLs) 2 should be treated as children or adults; treatment strategies for those with acute myeloid leukemias (AMLs); 3 management of Hodgkin lymphoma;4 and psychosocial challenges and health-related quality of life (QoL) in AYAs with hematologic malignancies.5
In the introduction to the series, Jorge Cortes, MD, an assistant editor on the journal, wrote that hematologic malignancies in AYAs “represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population.”6
He noted, however, that “not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs often centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults,” noted Dr Cortes, professor and chair of the chronic myeloid leukemia section in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
Therapeutic options: pediatric or adult protocols?
In their article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15 through 20 years is supported by findings from numerous studies. In young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” because they have been shown to significantly improve outcomes, with long-term survival rates nearing 70%.1 Patients in these age groups require specific programs that factor in access to care and to trials, an increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
However, Kristen O’Dwyer, MD, and colleagues, argue in an article on AML treatment in AYAs that neither the pediatric nor adult approaches are ideally suited for AYAs because of the “distinguishing characteristics of AYAs with AML.” Rather, they conclude that AYA-specific approaches merit consideration.3
Similarly, Kieron Dunleavy, MD, and Thomas G Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that there is a “remarkable divide” in the treatment of patients younger than 18 years with lymphoma compared with their young adult counterparts, and that it underscores the need for collaboration in developing consensus regarding treatment of AYAs.2
Clinical setting: pediatric or adult?
Consideration is also being given to the clinical setting in which AYA patients receive their treatment. Lori Muffly, MD, MS, and colleagues have reported that survival was superior for AYA patients with ALL who were treated in pediatric cancer settings,7 and other researchers have reported similar findings.
However, those improved outcomes in the pediatric setting might be offset by a higher use of resources and therefore higher costs, based on recent findings in a Canadian study by Paul C Nathan, MD, and colleagues.8 Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the authors found that the cost of care was higher when treatment took place in a pediatric setting compared with in an adult institution, and that it was driven in part by higher hospitalization rates and longer hospital stays. These findings were true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
In an accompanying editorial, Helen M Parsons, PhD, and her co-authors wrote that adolescents who receive treatment in the pediatric setting “tended to seek more [emergency department (ED)] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship.9 They pointed out that the findings of higher inpatient days in the pediatric setting was not surprising given that induction therapies for pediatric ALL tend to be more complex and intensive than therapies commonly used in adults with ALL, and that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase,” they wrote, adding that further investigation was needed on this topic to better understand those trends. “The finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.” 9
The authors of the editorial emphasized that because of the differences in health care delivery and payment structures between the United States and Canada, where the Nathan study was done, it was important that similar studies are done in the United States to confirm these findings.
Disease and developmental biology
As Dr Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and that they may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr O'Dwyer and her colleagues noted,3 citing a recent European study of 5,564 patients with de novo AML that showed that the frequency of favorable cytogenetics was low in infants (13.7%), increased in children (25%) and young adults (44%), and decreased again in middle age and older patients.10
“Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr O'Dwyer and her colleagues wrote.3 It was also becoming more apparent that age influences the presence of AML-related molecular abnormalities, and recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr Boissel and Dr Baruchel also noted in their report that light was finally being shed on the “black hole” of understanding ALL biology in AYAs, and research has shown that there is a continuum between childhood and adult ALL.1 They concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, NC, said in an interview.
These patients have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, and in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added. “Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... these are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so.”
In a 2014 study, Dr Salsman and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life.11 The latter finding was surprising and highlights the importance of the social dimension in the lives of AYAs. “Patient after patient will say ‘I found out who my real friends are,’ ” he said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr Salsman and his colleagues are using those findings to develop interventions that can maximize self-care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves. For example, a randomized controlled pilot study that incorporates social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions is underway.
Another intervention targets emotional well-being through the use of web-based tools to increase positive affect. A proof-of-concept study showed that the approach was feasible and well received, and a larger-scale randomized controlled trial is being planned, he said.
Dr Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital. It was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr Cortes noted a paucity of clinical trials specifically designed for this population. “At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, describing AYA enrollment in clinical trials in cancer as “suboptimal at best.”6
Dr Salsman said these dismal enrolment numbers could in part be related to treatment setting. Data suggest that most AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers where the bulk of research is being done, he explained.
Dr Hanna agreed that more research involving AYAs was needed as is a better understanding of why enrollment is so much lower in this population. He pointed out that in 2017 the American Society of Clinical Oncology and Friends of Cancer Research released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.12 The organizations said that individuals aged 12 years and older should routinely be included in such trials because their drug metabolism is similar to adults, and inclusion of younger patients may also be appropriate if they are part of the population affected by the disease, depending on specific disease biology, action of the drug, and available safety information.
Officials at the Food and Drug Administration are considering that possibility, Dr Hanna said.
Dr Salsman added there has been an increase in recent years in the attention paid to disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups. For example, about 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population;13 the Institute of Medicine held a forum on the care of AYAs with cancer;14 and the National Cancer Institute held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology,15 he noted.
Dr Hanna added that “scientific groups such as Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now. One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically co-ordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.16
Next steps
Among the recommendations from authors in the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams and the involvement of multidisciplinary teams in care for this population.
Many centers are already working on models for collaborative care, Dr Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in helping clinical and supportive caregivers and their AYA patients “have a shared vision” as they work to maximize improvements in outcomes.
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr Hanna said. In the case of the community-powered advocacy organization Critical Mass, members have succeeded in getting lawmakers to introduce a bill in the US House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
1. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132:351-361.
2. Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375.
3. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132:362-368.
4. Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132:376-384.
5. Husson O, Huijgens PC, van der Graaf WTA. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood. 2018;132:385-392.
6. Cortes J. Introduction to a review series on adolescent and young adult malignant hematology. Blood. 2018;132:345-346.
7. Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
8. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. July 19, 2018. [Epub ahead of print.]
9. Parsons HM, Muffly L, Alvarez EM, Keegan THM. Does treatment setting matter? Evaluating resource utilization for adolescents treated in pediatric vs adult cancer institutions. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djy123/5056313?searchresult=1. Published July 19, 2018. Last accessed October 12, 2018.
10. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821-3830.
11. Salsman JM, Garcia SF, Yanez B, et al. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254.
12. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
13. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. Published online February 18, 2015. DOI 10.1007/s40124-015-0075-y.
14. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
15. Wilder Smith A, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999.
16. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016.
Survival gains among adolescents and young adults (AYAs) with cancer continue to lag behind outcomes for children and older adult patients. It’s a trend that spans decades, but clinicians and researchers are finally getting serious about trying to understand the underlying causes and are re-examining prevailing practices in an effort to address the discrepancies.
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist at Cleveland Clinic Children’s Hospital, Ohio, said in an interview. He’s specifically referring to the cancers that affect AYAs, who are broadly defined as patients aged 15 through 39 years. “A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults. The biology may be different in the adolescent and young adult patients, which may lead to different outcomes.”
In addition, the psychosocial needs in this age group differ vastly from those in other groups. “Many of these patients are in college or have just started their families, so we have to pay more attention to [issues related to] financial toxicity and fertility, for example,” said Dr Hanna, who is the director of pediatric bone marrow transplantation at the clinic. (The term “financial toxicity” describes the cumulative negative impact of the high cost of care, lost work time, and delays in reaching educational and career goals on patients with cancer and their families.)
Another factor that likely contributes to the outcome disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs.
A recent series of articles published in the journal Blood addressed these and other issues, among them, whether AYAs with acute lymphoblastic leukemia (ALL)1 or aggressive B-cell non-Hodgkin lymphomas (NHLs) 2 should be treated as children or adults; treatment strategies for those with acute myeloid leukemias (AMLs); 3 management of Hodgkin lymphoma;4 and psychosocial challenges and health-related quality of life (QoL) in AYAs with hematologic malignancies.5
In the introduction to the series, Jorge Cortes, MD, an assistant editor on the journal, wrote that hematologic malignancies in AYAs “represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population.”6
He noted, however, that “not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs often centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults,” noted Dr Cortes, professor and chair of the chronic myeloid leukemia section in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
Therapeutic options: pediatric or adult protocols?
In their article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15 through 20 years is supported by findings from numerous studies. In young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” because they have been shown to significantly improve outcomes, with long-term survival rates nearing 70%.1 Patients in these age groups require specific programs that factor in access to care and to trials, an increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
However, Kristen O’Dwyer, MD, and colleagues, argue in an article on AML treatment in AYAs that neither the pediatric nor adult approaches are ideally suited for AYAs because of the “distinguishing characteristics of AYAs with AML.” Rather, they conclude that AYA-specific approaches merit consideration.3
Similarly, Kieron Dunleavy, MD, and Thomas G Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that there is a “remarkable divide” in the treatment of patients younger than 18 years with lymphoma compared with their young adult counterparts, and that it underscores the need for collaboration in developing consensus regarding treatment of AYAs.2
Clinical setting: pediatric or adult?
Consideration is also being given to the clinical setting in which AYA patients receive their treatment. Lori Muffly, MD, MS, and colleagues have reported that survival was superior for AYA patients with ALL who were treated in pediatric cancer settings,7 and other researchers have reported similar findings.
However, those improved outcomes in the pediatric setting might be offset by a higher use of resources and therefore higher costs, based on recent findings in a Canadian study by Paul C Nathan, MD, and colleagues.8 Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the authors found that the cost of care was higher when treatment took place in a pediatric setting compared with in an adult institution, and that it was driven in part by higher hospitalization rates and longer hospital stays. These findings were true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
In an accompanying editorial, Helen M Parsons, PhD, and her co-authors wrote that adolescents who receive treatment in the pediatric setting “tended to seek more [emergency department (ED)] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship.9 They pointed out that the findings of higher inpatient days in the pediatric setting was not surprising given that induction therapies for pediatric ALL tend to be more complex and intensive than therapies commonly used in adults with ALL, and that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase,” they wrote, adding that further investigation was needed on this topic to better understand those trends. “The finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.” 9
The authors of the editorial emphasized that because of the differences in health care delivery and payment structures between the United States and Canada, where the Nathan study was done, it was important that similar studies are done in the United States to confirm these findings.
Disease and developmental biology
As Dr Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and that they may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr O'Dwyer and her colleagues noted,3 citing a recent European study of 5,564 patients with de novo AML that showed that the frequency of favorable cytogenetics was low in infants (13.7%), increased in children (25%) and young adults (44%), and decreased again in middle age and older patients.10
“Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr O'Dwyer and her colleagues wrote.3 It was also becoming more apparent that age influences the presence of AML-related molecular abnormalities, and recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr Boissel and Dr Baruchel also noted in their report that light was finally being shed on the “black hole” of understanding ALL biology in AYAs, and research has shown that there is a continuum between childhood and adult ALL.1 They concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, NC, said in an interview.
These patients have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, and in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added. “Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... these are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so.”
In a 2014 study, Dr Salsman and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life.11 The latter finding was surprising and highlights the importance of the social dimension in the lives of AYAs. “Patient after patient will say ‘I found out who my real friends are,’ ” he said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr Salsman and his colleagues are using those findings to develop interventions that can maximize self-care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves. For example, a randomized controlled pilot study that incorporates social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions is underway.
Another intervention targets emotional well-being through the use of web-based tools to increase positive affect. A proof-of-concept study showed that the approach was feasible and well received, and a larger-scale randomized controlled trial is being planned, he said.
Dr Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital. It was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr Cortes noted a paucity of clinical trials specifically designed for this population. “At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, describing AYA enrollment in clinical trials in cancer as “suboptimal at best.”6
Dr Salsman said these dismal enrolment numbers could in part be related to treatment setting. Data suggest that most AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers where the bulk of research is being done, he explained.
Dr Hanna agreed that more research involving AYAs was needed as is a better understanding of why enrollment is so much lower in this population. He pointed out that in 2017 the American Society of Clinical Oncology and Friends of Cancer Research released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.12 The organizations said that individuals aged 12 years and older should routinely be included in such trials because their drug metabolism is similar to adults, and inclusion of younger patients may also be appropriate if they are part of the population affected by the disease, depending on specific disease biology, action of the drug, and available safety information.
Officials at the Food and Drug Administration are considering that possibility, Dr Hanna said.
Dr Salsman added there has been an increase in recent years in the attention paid to disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups. For example, about 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population;13 the Institute of Medicine held a forum on the care of AYAs with cancer;14 and the National Cancer Institute held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology,15 he noted.
Dr Hanna added that “scientific groups such as Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now. One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically co-ordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.16
Next steps
Among the recommendations from authors in the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams and the involvement of multidisciplinary teams in care for this population.
Many centers are already working on models for collaborative care, Dr Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in helping clinical and supportive caregivers and their AYA patients “have a shared vision” as they work to maximize improvements in outcomes.
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr Hanna said. In the case of the community-powered advocacy organization Critical Mass, members have succeeded in getting lawmakers to introduce a bill in the US House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
Survival gains among adolescents and young adults (AYAs) with cancer continue to lag behind outcomes for children and older adult patients. It’s a trend that spans decades, but clinicians and researchers are finally getting serious about trying to understand the underlying causes and are re-examining prevailing practices in an effort to address the discrepancies.
“This is a very heterogeneous group of disorders,” Rabi Hanna, MD, a pediatric hematologist and oncologist at Cleveland Clinic Children’s Hospital, Ohio, said in an interview. He’s specifically referring to the cancers that affect AYAs, who are broadly defined as patients aged 15 through 39 years. “A few cancers, such as [acute lymphoblastic leukemia], are more common in children, and others, such as breast cancer, are more common in adults. The biology may be different in the adolescent and young adult patients, which may lead to different outcomes.”
In addition, the psychosocial needs in this age group differ vastly from those in other groups. “Many of these patients are in college or have just started their families, so we have to pay more attention to [issues related to] financial toxicity and fertility, for example,” said Dr Hanna, who is the director of pediatric bone marrow transplantation at the clinic. (The term “financial toxicity” describes the cumulative negative impact of the high cost of care, lost work time, and delays in reaching educational and career goals on patients with cancer and their families.)
Another factor that likely contributes to the outcome disparities between AYAs and other populations with cancer is the relative lack of clinical trial involvement among AYAs.
A recent series of articles published in the journal Blood addressed these and other issues, among them, whether AYAs with acute lymphoblastic leukemia (ALL)1 or aggressive B-cell non-Hodgkin lymphomas (NHLs) 2 should be treated as children or adults; treatment strategies for those with acute myeloid leukemias (AMLs); 3 management of Hodgkin lymphoma;4 and psychosocial challenges and health-related quality of life (QoL) in AYAs with hematologic malignancies.5
In the introduction to the series, Jorge Cortes, MD, an assistant editor on the journal, wrote that hematologic malignancies in AYAs “represent a unique challenge because of their special biological features and distinctive therapeutic requirements, as well as the unique medical, social, and psychological characteristics of this patient population.”6
He noted, however, that “not much has been done to explore unique molecular and biological features of AYA hematologic malignancies. The discussion on the management of AYAs often centers on whether these patients should be treated in a pediatric setting or an adult setting, or with regimens designed for children or for adults,” noted Dr Cortes, professor and chair of the chronic myeloid leukemia section in the department of leukemia at the University of Texas MD Anderson Cancer Center, Houston.
Therapeutic options: pediatric or adult protocols?
In their article on ALL in AYAs, Nicolas Boissel, MD, and André Baruchel, MD, note that the use of “fully pediatric protocols” in patients aged 15 through 20 years is supported by findings from numerous studies. In young adults, evidence increasingly supports “pediatric-inspired or even fully pediatric approaches” because they have been shown to significantly improve outcomes, with long-term survival rates nearing 70%.1 Patients in these age groups require specific programs that factor in access to care and to trials, an increased risk of acute toxicities, and treatment adherence, which can be particularly problematic in AYAs, they concluded.
However, Kristen O’Dwyer, MD, and colleagues, argue in an article on AML treatment in AYAs that neither the pediatric nor adult approaches are ideally suited for AYAs because of the “distinguishing characteristics of AYAs with AML.” Rather, they conclude that AYA-specific approaches merit consideration.3
Similarly, Kieron Dunleavy, MD, and Thomas G Gross, MD, note in an article on managing aggressive B-cell NHLs in AYAs that there is a “remarkable divide” in the treatment of patients younger than 18 years with lymphoma compared with their young adult counterparts, and that it underscores the need for collaboration in developing consensus regarding treatment of AYAs.2
Clinical setting: pediatric or adult?
Consideration is also being given to the clinical setting in which AYA patients receive their treatment. Lori Muffly, MD, MS, and colleagues have reported that survival was superior for AYA patients with ALL who were treated in pediatric cancer settings,7 and other researchers have reported similar findings.
However, those improved outcomes in the pediatric setting might be offset by a higher use of resources and therefore higher costs, based on recent findings in a Canadian study by Paul C Nathan, MD, and colleagues.8 Among 1,356 patients aged 15-17 years who were diagnosed with cancer between 1996 and 2010, the authors found that the cost of care was higher when treatment took place in a pediatric setting compared with in an adult institution, and that it was driven in part by higher hospitalization rates and longer hospital stays. These findings were true across different diagnoses, including leukemias, lymphomas, sarcomas, and germ cell tumors, but only during the initial treatment phase.
In an accompanying editorial, Helen M Parsons, PhD, and her co-authors wrote that adolescents who receive treatment in the pediatric setting “tended to seek more [emergency department (ED)] care immediately before diagnosis and during the initial treatment phase; these adolescents also used more home care services during initial treatment and survivorship.9 They pointed out that the findings of higher inpatient days in the pediatric setting was not surprising given that induction therapies for pediatric ALL tend to be more complex and intensive than therapies commonly used in adults with ALL, and that pediatric cancer hospitals tend to have a wider array of services, including psychosocial and family support services.
“What is less clear is why individuals seen in pediatric settings have higher rates of ED care directly before diagnosis and during the initial treatment phase,” they wrote, adding that further investigation was needed on this topic to better understand those trends. “The finding that adolescents treated in pediatric institutions had higher resource use across diagnostic groups demonstrates that resource utilization may be driven just as much by care setting as diagnosis.” 9
The authors of the editorial emphasized that because of the differences in health care delivery and payment structures between the United States and Canada, where the Nathan study was done, it was important that similar studies are done in the United States to confirm these findings.
Disease and developmental biology
As Dr Hanna noted, biological differences and changes over time suggest that different age groups need varying approaches to treatment and that they may have different outcomes with the same treatments.
For example, the biology of AML is known to change with age, Dr O'Dwyer and her colleagues noted,3 citing a recent European study of 5,564 patients with de novo AML that showed that the frequency of favorable cytogenetics was low in infants (13.7%), increased in children (25%) and young adults (44%), and decreased again in middle age and older patients.10
“Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYA,” Dr O'Dwyer and her colleagues wrote.3 It was also becoming more apparent that age influences the presence of AML-related molecular abnormalities, and recognition of age-related differences in disease biology “will provide the best opportunity to improve the clinical outcomes that have been static for decades.”
Dr Boissel and Dr Baruchel also noted in their report that light was finally being shed on the “black hole” of understanding ALL biology in AYAs, and research has shown that there is a continuum between childhood and adult ALL.1 They concluded that “risk stratification based on recent biology findings and sequential [minimum residual disease] evaluations should now be implemented, as well as new therapeutic options including immunotherapy and targeted therapies, at best within the setting of integrated pediatric and AYA protocols.”
Psychosocial factors
“Cancer is a non-normative event for AYAs. It is extremely disruptive to them physically, psychologically, and vocationally ... and this poses significant challenges,” John Salsman, PhD, director of clinical research in AYA oncology at Wake Forest University, Winston-Salem, NC, said in an interview.
These patients have 5-year survival rates that haven’t improved in tandem with those in pediatric and adult populations over the last 3 decades, and in addition to the financial toxicity and strain, they also have higher rates of depression and anxiety, including fear of recurrence, he added. “Quality of life is incredibly important, and these things need to be addressed because of the developmental changes AYAs are navigating; there are issues of positive body image, family and career decisions ... these are challenging for anyone, and when you throw a cancer diagnosis into the mix they become disproportionately so.”
In a 2014 study, Dr Salsman and his colleagues found that AYAs with cancer had poorer physical and emotional quality of life when compared with matched controls, but better social quality of life.11 The latter finding was surprising and highlights the importance of the social dimension in the lives of AYAs. “Patient after patient will say ‘I found out who my real friends are,’ ” he said. “There’s this refinement and deepening of the social network among some posttreatment survivors.”
Dr Salsman and his colleagues are using those findings to develop interventions that can maximize self-care in posttreatment survivorship – a time when AYAs may feel they have a new lease on life and may be more motivated to adhere to recommendations and take care of themselves. For example, a randomized controlled pilot study that incorporates social media apps and other technologies to build on the positive social components of their lives in promoting physical activity interventions is underway.
Another intervention targets emotional well-being through the use of web-based tools to increase positive affect. A proof-of-concept study showed that the approach was feasible and well received, and a larger-scale randomized controlled trial is being planned, he said.
Dr Salsman also praised the PRISM (Promoting Resilience in Stress Management) tool developed by researchers at Seattle Children’s Hospital. It was created to help AYAs with cancer and other illnesses learn coping skills to manage stress after their diagnosis and to boost quality of life beyond treatment. A digital app has also been developed to be used in conjunction with the program.
Trial enrollment
In his editorial introducing the Blood series on AYAs and cancer, Dr Cortes noted a paucity of clinical trials specifically designed for this population. “At the time of this writing, I could identify four therapeutic trials registered at www.clinicaltrials.gov that appeared to be somewhat specifically designed for AYAs (some included children also),” he wrote, describing AYA enrollment in clinical trials in cancer as “suboptimal at best.”6
Dr Salsman said these dismal enrolment numbers could in part be related to treatment setting. Data suggest that most AYAs with cancer are treated in community-based practices rather than comprehensive cancer centers where the bulk of research is being done, he explained.
Dr Hanna agreed that more research involving AYAs was needed as is a better understanding of why enrollment is so much lower in this population. He pointed out that in 2017 the American Society of Clinical Oncology and Friends of Cancer Research released a statement recommending that pediatric patients be considered for enrollment in later-phase trials for cancer types that span both adults and children.12 The organizations said that individuals aged 12 years and older should routinely be included in such trials because their drug metabolism is similar to adults, and inclusion of younger patients may also be appropriate if they are part of the population affected by the disease, depending on specific disease biology, action of the drug, and available safety information.
Officials at the Food and Drug Administration are considering that possibility, Dr Hanna said.
Dr Salsman added there has been an increase in recent years in the attention paid to disparities in survival improvements and trial involvement among AYAs with cancer, compared with other age groups. For example, about 5 years ago, the National Clinical Trials Network formed a working group that developed a number of specific objectives for incorporating more AYAs into cancer trials and finding better ways to study this population;13 the Institute of Medicine held a forum on the care of AYAs with cancer;14 and the National Cancer Institute held a state-of-the-science meeting that focused on identifying strategic priorities for AYA oncology,15 he noted.
Dr Hanna added that “scientific groups such as Southwest Oncology Group (SWOG) and Children’s Oncology Group (COG) also have AYA committees now. One of the success stories of working together between SWOG and COG was the intergroup study C10403 for patients with ALL. And now there are efforts for an intergroup AYA-AML task force to include representatives from each of the cooperative groups that historically co-ordinated myeloid disease clinical trials – COG, SWOG, Alliance, and ECOG-ACRIN,” he said.
In fact, all of the National Clinical Trials Network groups have some initiative in place to address AYA concerns, said Dr Salsman, who chairs the ECOG-ACRIN AYA oncology subcommittee.
Despite these efforts, and many others, long-term survival improvements among AYAs with cancer still fall short, compared with those of other age groups.16
Next steps
Among the recommendations from authors in the AYA series in Blood is a call for assessing AYA-specific therapy in future clinical trials, as well as improved collaboration between adult and pediatric teams and the involvement of multidisciplinary teams in care for this population.
Many centers are already working on models for collaborative care, Dr Salsman said, citing the Fort Worth AYA Oncology Coalition led by medical director Karen Albritton, MD, as an example of a program that has been successful in helping clinical and supportive caregivers and their AYA patients “have a shared vision” as they work to maximize improvements in outcomes.
Patients are also taking the lead in demanding better care and attention to their psychosocial needs, Dr Hanna said. In the case of the community-powered advocacy organization Critical Mass, members have succeeded in getting lawmakers to introduce a bill in the US House of Representatives that would allow college students to defer loan payments while undergoing cancer treatment.
1. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132:351-361.
2. Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375.
3. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132:362-368.
4. Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132:376-384.
5. Husson O, Huijgens PC, van der Graaf WTA. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood. 2018;132:385-392.
6. Cortes J. Introduction to a review series on adolescent and young adult malignant hematology. Blood. 2018;132:345-346.
7. Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
8. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. July 19, 2018. [Epub ahead of print.]
9. Parsons HM, Muffly L, Alvarez EM, Keegan THM. Does treatment setting matter? Evaluating resource utilization for adolescents treated in pediatric vs adult cancer institutions. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djy123/5056313?searchresult=1. Published July 19, 2018. Last accessed October 12, 2018.
10. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821-3830.
11. Salsman JM, Garcia SF, Yanez B, et al. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254.
12. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
13. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. Published online February 18, 2015. DOI 10.1007/s40124-015-0075-y.
14. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
15. Wilder Smith A, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999.
16. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016.
1. Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132:351-361.
2. Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375.
3. O’Dwyer K, Freyer DR, Horan JT. Treatment strategies for adolescent and young adult patients with acute myeloid leukemia. Blood. 2018;132:362-368.
4. Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132:376-384.
5. Husson O, Huijgens PC, van der Graaf WTA. Psychosocial challenges and health-related quality of life of adolescents and young adults with hematologic malignancies. Blood. 2018;132:385-392.
6. Cortes J. Introduction to a review series on adolescent and young adult malignant hematology. Blood. 2018;132:345-346.
7. Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895-903.
8. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. July 19, 2018. [Epub ahead of print.]
9. Parsons HM, Muffly L, Alvarez EM, Keegan THM. Does treatment setting matter? Evaluating resource utilization for adolescents treated in pediatric vs adult cancer institutions. https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djy123/5056313?searchresult=1. Published July 19, 2018. Last accessed October 12, 2018.
10. Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821-3830.
11. Salsman JM, Garcia SF, Yanez B, et al. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254.
12. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
13. Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. Published online February 18, 2015. DOI 10.1007/s40124-015-0075-y.
14. Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: summary of an Institute of Medicine workshop. Oncologist. 2015;20(2):186-195.
15. Wilder Smith A, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer. 2016;122(7):988-999.
16. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009-1016.
NIH Program Enhances Diversity Among Researchers
The NIH has chosen 13 researchers for the inaugural class of the Distinguished Scholars Program (DSP), launched earlier this year to build diversity within the NIH Intramural Research Program. “Nurturing diversity in the NIH Intramural Research Program is paramount to upholding our mission,” said NIH Director Francis Collins, MD, PhD. Research has shown that a “diversity of perspectives” is vital to the improved quality and number of discoveries, he adds.
The DSP aims to facilitate hiring and career progression of tenure-track investigators who have demonstrated commitment to promoting diversity and inclusion in the biomedical research workforce, according to the NIH. The DSP is unique in its focus on early-stage investigators, says Hannah A. Valantine, MD, NIH Chief Officer for Scientific Workforce Diversity. She says that is the “major point where we lose underrepresented groups from scientific careers.”
Dr. Collins says the DSP can serve as a model for universities to prevent the attrition of underrepresented groups, including women, blacks, Hispanics or Latinos, American Indians and Alaska Natives, Native Hawaiians and other Pacific Islanders, individuals with disabilities, and individuals from disadvantaged backgrounds.
The pilot program will fund 3 cohorts of up to 15 scholars each. Nominees are chosen for their scientific excellence and commitment to diversity and inclusion, shown through participation in activities, such as mentoring programs.
Scholars will receive 4 years of research support of up to $2.35 million from the DSP; their nominating institute or center will continue to fund their research throughout their tenure track. Each scholar also will be mentored by a highly experienced NIH senior investigator and receive professional leadership training, workshops on management skills, and networking opportunities with NIH leadership.
Source:
NIH selects first scholars in pioneering program to enhance diversity within inhouse research program [news release]. Bethesda, MD: National Institutes of Health; October 23, 2018. https://www.nih.gov/news-events/news-releases/nih-selects-first-scholars-pioneering-program-enhance-diversity-within-house-research-program . Accessed October 31, 2018.
The NIH has chosen 13 researchers for the inaugural class of the Distinguished Scholars Program (DSP), launched earlier this year to build diversity within the NIH Intramural Research Program. “Nurturing diversity in the NIH Intramural Research Program is paramount to upholding our mission,” said NIH Director Francis Collins, MD, PhD. Research has shown that a “diversity of perspectives” is vital to the improved quality and number of discoveries, he adds.
The DSP aims to facilitate hiring and career progression of tenure-track investigators who have demonstrated commitment to promoting diversity and inclusion in the biomedical research workforce, according to the NIH. The DSP is unique in its focus on early-stage investigators, says Hannah A. Valantine, MD, NIH Chief Officer for Scientific Workforce Diversity. She says that is the “major point where we lose underrepresented groups from scientific careers.”
Dr. Collins says the DSP can serve as a model for universities to prevent the attrition of underrepresented groups, including women, blacks, Hispanics or Latinos, American Indians and Alaska Natives, Native Hawaiians and other Pacific Islanders, individuals with disabilities, and individuals from disadvantaged backgrounds.
The pilot program will fund 3 cohorts of up to 15 scholars each. Nominees are chosen for their scientific excellence and commitment to diversity and inclusion, shown through participation in activities, such as mentoring programs.
Scholars will receive 4 years of research support of up to $2.35 million from the DSP; their nominating institute or center will continue to fund their research throughout their tenure track. Each scholar also will be mentored by a highly experienced NIH senior investigator and receive professional leadership training, workshops on management skills, and networking opportunities with NIH leadership.
Source:
NIH selects first scholars in pioneering program to enhance diversity within inhouse research program [news release]. Bethesda, MD: National Institutes of Health; October 23, 2018. https://www.nih.gov/news-events/news-releases/nih-selects-first-scholars-pioneering-program-enhance-diversity-within-house-research-program . Accessed October 31, 2018.
The NIH has chosen 13 researchers for the inaugural class of the Distinguished Scholars Program (DSP), launched earlier this year to build diversity within the NIH Intramural Research Program. “Nurturing diversity in the NIH Intramural Research Program is paramount to upholding our mission,” said NIH Director Francis Collins, MD, PhD. Research has shown that a “diversity of perspectives” is vital to the improved quality and number of discoveries, he adds.
The DSP aims to facilitate hiring and career progression of tenure-track investigators who have demonstrated commitment to promoting diversity and inclusion in the biomedical research workforce, according to the NIH. The DSP is unique in its focus on early-stage investigators, says Hannah A. Valantine, MD, NIH Chief Officer for Scientific Workforce Diversity. She says that is the “major point where we lose underrepresented groups from scientific careers.”
Dr. Collins says the DSP can serve as a model for universities to prevent the attrition of underrepresented groups, including women, blacks, Hispanics or Latinos, American Indians and Alaska Natives, Native Hawaiians and other Pacific Islanders, individuals with disabilities, and individuals from disadvantaged backgrounds.
The pilot program will fund 3 cohorts of up to 15 scholars each. Nominees are chosen for their scientific excellence and commitment to diversity and inclusion, shown through participation in activities, such as mentoring programs.
Scholars will receive 4 years of research support of up to $2.35 million from the DSP; their nominating institute or center will continue to fund their research throughout their tenure track. Each scholar also will be mentored by a highly experienced NIH senior investigator and receive professional leadership training, workshops on management skills, and networking opportunities with NIH leadership.
Source:
NIH selects first scholars in pioneering program to enhance diversity within inhouse research program [news release]. Bethesda, MD: National Institutes of Health; October 23, 2018. https://www.nih.gov/news-events/news-releases/nih-selects-first-scholars-pioneering-program-enhance-diversity-within-house-research-program . Accessed October 31, 2018.
How ovarian reserve testing can (and cannot) address your patients’ fertility concerns
CASE Your patient wants ovarian reserve testing. Is her request reasonable?
A 34-year-old woman, recently married, plans to delay attempting pregnancy for a few years. She requests ovarian reserve testing to inform this timeline.
This is not an unreasonable inquiry, given her age (<35 years), after which there is natural acceleration in the rate of decline in the quality of oocytes. Regardless of the results of testing, attempting pregnancy or pursuing fertility preservation as soon as possible (particularly in patients >35 years) is associated with better outcomes.
A woman is born with all the eggs she will ever have. Oocyte atresia occurs throughout a woman’s lifetime, from 1,000,000 eggs at birth to only 1,000 by the time of menopause.1 A woman’s ovarian reserve reflects the number of oocytes present in the ovaries and is the result of complex interactions of age, genetics, and environmental variables.
Ovarian reserve testing, however, only has been consistently shown to predict ovarian response to stimulation with gonadotropins; these tests might reflect in vitro fertilization (IVF) birth outcomes to a lesser degree, but have not been shown to predict natural fecundability.2,3 Essentially, ovarian reserve testing provides a partial view of reproductive potential.
Ovarian reserve testing also does not reflect an age-related decline in oocyte quality, particularly after age 35.4,5 As such, female age is the principal driver of fertility potential, regardless of oocyte number. A woman with abnormal ovarian reserve tests may benefit from referral to a fertility specialist for counseling that integrates her results, age, and medical history, with the caveat that abnormal results do not necessarily mean she needs assisted reproductive technology (ART) to conceive.
In this article, we review 6 common questions about the ovarian reserve, providing current data to support the answers.
Continue to: #1 What tests are part of an ovarian reserve assessment?
#1 What tests are part of an ovarian reserve assessment? What is their utility?
FSH and estradiol
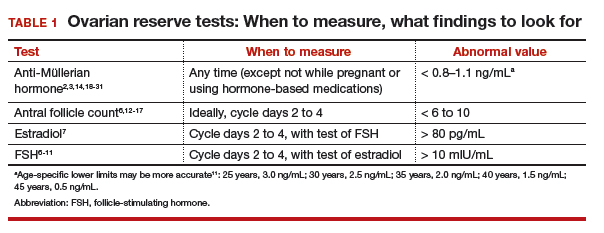
Follicle-stimulating hormone (FSH) and estradiol should be checked together in the early follicular phase (days 2 to 4 of the cycle). Elevated levels of one or both hormones suggest diminished ovarian reserve; an FSH level greater than 10 mIU/mL and/or an estradiol level greater than 80 pg/mL represent abnormal results6 (TABLE 1). Because FSH demonstrates significant intercycle variability, a single abnormal result should be confirmed in a subsequent cycle.7
Although the basal FSH level does not reflect egg quality or predict natural fecundity, an elevated FSH level predicts poor ovarian response (<3 or 4 eggs retrieved) to ovarian hyperstimulation, with good specificity.3,6,8,9 In patients younger than age 35 years undergoing IVF, basal FSH levels do not predict live birth or pregnancy loss.10 In older patients undergoing IVF, however, an elevated FSH level is associated with a reduced live birth rate (a 5% reduction in women <40 years to a 26% reduction in women >42 years) and a higher miscarriage rate, reflecting the positive correlation of oocyte aneuploidy and age.
In addition to high intercycle variability, an FSH level is reliable only in the setting of normal hypothalamic and pituitary function.7 Conditions such a prolactinoma (or other causes of hyperprolactinemia), other intracranial masses, prior central radiation, hormone-based medication use, and inadequate energy reserve (as the result of anorexia nervosa, resulting in hypothalamic suppression), might result in a low or inappropriately normal FSH level that does not reflect ovarian function.11
Antral follicle count
Antral follicle count (AFC) is defined as the total number of follicles measuring 2 to 10 mm, in both ovaries, in the early follicular phase (days 2 to 4 of the cycle). A count of fewer than 6 to 10 antral follicles in total is considered consistent with diminished ovarian reserve6,12,13 (TABLE 1). Antral follicle count is not predictive of natural fecundity but, rather, projects ovarian response during IVF. Antral follicle count has been shown to decrease by 5% a year with increasing age among women with or without infertility.14
Studies have highlighted concerns regarding interobserver and intraobserver variability in determining the AFC but, in experienced hands, the AFC is a reliable test of ovarian reserve.15,16 Visualization of antral follicles can be compromised in obese patients.11 Conversely, AFC sometimes also overestimates ovarian reserve, because atretic follicles might be included in the count.11,15 Last, AFC is reduced in patients who take a hormone-based medication but recovers with cessation of the medication.17 Ideally, a woman should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before AFC is measured.
Continue to: Anti-Müllerian hormone
Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
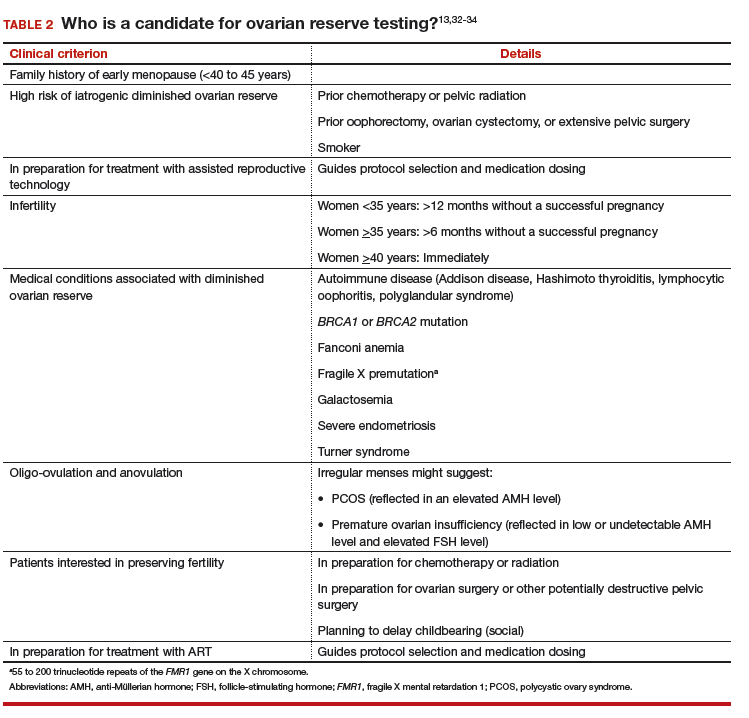
#2 Who should have ovarian reserve testing?
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.
Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?
#3 Can I reassure my patient about her reproductive potential using these tests?
Normal findings on ovarian reserve testing suggests that a woman might have a normal (that is, commensurate with age-matched peers) number of eggs in her ovaries. But normal test results do not mean she will have an easy time conceiving. Similarly, abnormal results do not mean that she will have difficulty conceiving.
Ovarian reserve testing reflects only the number of oocytes, not their quality, which is primarily determined by maternal age.35 Genetic testing of embryos during IVF shows that the percentage of embryos that are aneuploid (usually resulting from abnormal eggs) rises with advancing maternal age, beginning at 35 years.5 The increasing rate of oocyte aneuploidy is also reflected in the rising rate of loss of clinically recognized pregnancies with advancing maternal age: from 11% in women younger than age 34 to greater than 36% in women older than age 42.4
Furthermore, ovarian reserve testing does not reflect other potential genetic barriers to reproduction, such as a chromosomal translocation that can result in recurrent pregnancy loss. Fallopian tube obstruction and uterine issues, such as fibroids or septa, and male factors are also not reflected in ovarian reserve testing.
#4 My patient is trying to get pregnant and has abnormal ovarian reserve testing results. Will she need IVF?"
Not necessarily. Consultation with a fertility specialist to discuss the nuances of abnormal test results and management options is ideal but, essentially, as the American Society for Reproductive Medicine states, “evidence of [diminished ovarian reserve] does not necessarily equate with inability to conceive.” Furthermore, the Society states, “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of ART.”
Once counseled, patients might elect to pursue more aggressive treatment, but they might not necessarily need it. Age must figure significantly into treatment decisions, because oocyte quality—regardless of number—begins to decline at 35 years of age, with an associated increasing risk of infertility and miscarriage.
In a recently published study of 750 women attempting pregnancy, women with a low AMH level (<0.7 ng/mL) or high FSH level (>10 mIU/mL), or both, did not have a significantly lower likelihood of achieving spontaneous pregnancy within 1 year, compared with women with normal results of ovarian reserve testing.3
Continue to: #5 My patient is not ready to be pregnant
#5 My patient is not ready to be pregnant. If her results are abnormal, should she freeze eggs?
For patients who might be interested in seeking fertility preservation and ART, earlier referral to a reproductive specialist to discuss risks and benefits of oocyte or embryo cryopreservation is always preferable. The younger a woman is when she undergoes fertility preservation, the better. Among patients planning to delay conception, each one’s decision is driven by her personal calculations of the cost, risk, and benefit of egg or embryo freezing—a picture of which ovarian reserve testing is only one piece.
#6 Can these tests predict menopause?
Menopause is a clinical diagnosis, defined as 12 months without menses (without hormone use or other causes of amenorrhea). In such women, FSH levels are elevated, but biochemical tests are not part of the menopause diagnosis.36 In the years leading to menopause, FSH levels are highly variable and unreliable in predicting time to menopause.
AMH has been shown to correlate with time to menopause. (Once the AMH level becomes undetectable, menopause occurs in a mean of 6 years.37,38) Patients do not typically have serial AMH measurements, however, so it is not usually known when the hormone became undetectable. Therefore, AMH is not a useful test for predicting time to menopause.
Premature ovarian insufficiency (loss of ovarian function in women younger than age 40), should be considered in women with secondary amenorrhea of 4 months or longer. The diagnosis requires confirmatory laboratory assessment,36 and findings include an FSH level greater than 25 mIU/mL on 2 tests performed at least 1 month apart.39,40
Ovarian reserve tests: A partial view of reproductive potential
The answers we have provided highlight several key concepts and conclusions that should guide clinical practice and decisions made by patients:
- Ovarian reserve tests best serve to predict ovarian response during IVF; to a far lesser extent, they might predict birth outcomes from IVF. These tests have not, however, been shown to predict spontaneous pregnancy.
- Ovarian reserve tests should be administered purposefully, with counseling beforehand regarding their limitations.
- Abnormal ovarian reserve test results do not necessitate ART; however, they may prompt a patient to accelerate her reproductive timeline and consult with a reproductive endocrinologist to consider her age and health-related risks of infertility or pregnancy loss.
- Patients should be counseled that, regardless of the results of ovarian reserve testing, attempting conception or pursuing fertility preservation at a younger age (in particular, at <35 years of age) is associated with better outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1-21.
- Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012-1018.e1.
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367-1376.
- Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165(12):1380-1388.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 1,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17.
- Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Hum Reprod. 2004;19(3):590-595.
- Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2008;90(2):315-321.
- Roberts JE, Spandorfer S, Fasouliotis SJ, Kashyap S, Rosenwaks Z. Taking a basal follicle-stimulating hormone history is essential before initiating in vitro fertilization. Fertil Steril. 2005;83(1):37-41.
- Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Ferraretti AP, La Marca L, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-e50.
- Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A, et al. Infertile women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod. 2016;31(5):1034-1045.
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718.
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710.
- Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619.
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688-701.
- Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655.
- Hamdine O, Eijkemans MJ, Lentjes EW, Torrance HL, Macklon NS, Fauser BC, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30(1):170-178.
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855-864.
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159-163.
- Korsholm AS, Petersen KB, Bentzen JG, Hilsted LM, Andersen AN, Hvidman HW. Investigation of anti-Müllerian hormone concentrations in relation to natural conception rate and time to pregnancy. Reprod Biomed Online. 2018;36(5):568-575.
- Quinn MM, Kao CN, Ahmad AK, Haisenleder DJ, Santoro N, Eisenberg E, et al. Age-stratified thresholds of anti-Müllerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf).
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-129.
- Schiffner J, Roos J, Broomhead D, Helden JV, Godehardt E, Fehr D, et al. Relationship between anti-Müllerian hormone and antral follicle count across the menstrual cycle using the Beckman Coulter Access assay in comparison with Gen II manual assay. Clin Chem Lab Med. 2017;55(7):1025-1033.
- Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. 2018;35(5):777-783.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385.
- Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772.
- Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115.
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016 ;206:172-176.
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839-3847.
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633-634.
- National Collaborating Centre for Women’s and Children’s Health (UK). Menopause: Full Guideline. London: National Institute for Health and Care Excellence (UK); 2015 Nov 12. (NICE Guideline, No. 23). Premature ovarian insufficiency. Available from: www.ncbi.nlm.nih.gov/books/NBK343476/.
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680.
- van Rooij IA, den Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601-606.
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926-937.
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193-197.
CASE Your patient wants ovarian reserve testing. Is her request reasonable?
A 34-year-old woman, recently married, plans to delay attempting pregnancy for a few years. She requests ovarian reserve testing to inform this timeline.
This is not an unreasonable inquiry, given her age (<35 years), after which there is natural acceleration in the rate of decline in the quality of oocytes. Regardless of the results of testing, attempting pregnancy or pursuing fertility preservation as soon as possible (particularly in patients >35 years) is associated with better outcomes.
A woman is born with all the eggs she will ever have. Oocyte atresia occurs throughout a woman’s lifetime, from 1,000,000 eggs at birth to only 1,000 by the time of menopause.1 A woman’s ovarian reserve reflects the number of oocytes present in the ovaries and is the result of complex interactions of age, genetics, and environmental variables.
Ovarian reserve testing, however, only has been consistently shown to predict ovarian response to stimulation with gonadotropins; these tests might reflect in vitro fertilization (IVF) birth outcomes to a lesser degree, but have not been shown to predict natural fecundability.2,3 Essentially, ovarian reserve testing provides a partial view of reproductive potential.
Ovarian reserve testing also does not reflect an age-related decline in oocyte quality, particularly after age 35.4,5 As such, female age is the principal driver of fertility potential, regardless of oocyte number. A woman with abnormal ovarian reserve tests may benefit from referral to a fertility specialist for counseling that integrates her results, age, and medical history, with the caveat that abnormal results do not necessarily mean she needs assisted reproductive technology (ART) to conceive.
In this article, we review 6 common questions about the ovarian reserve, providing current data to support the answers.
Continue to: #1 What tests are part of an ovarian reserve assessment?
#1 What tests are part of an ovarian reserve assessment? What is their utility?
FSH and estradiol
Follicle-stimulating hormone (FSH) and estradiol should be checked together in the early follicular phase (days 2 to 4 of the cycle). Elevated levels of one or both hormones suggest diminished ovarian reserve; an FSH level greater than 10 mIU/mL and/or an estradiol level greater than 80 pg/mL represent abnormal results6 (TABLE 1). Because FSH demonstrates significant intercycle variability, a single abnormal result should be confirmed in a subsequent cycle.7
Although the basal FSH level does not reflect egg quality or predict natural fecundity, an elevated FSH level predicts poor ovarian response (<3 or 4 eggs retrieved) to ovarian hyperstimulation, with good specificity.3,6,8,9 In patients younger than age 35 years undergoing IVF, basal FSH levels do not predict live birth or pregnancy loss.10 In older patients undergoing IVF, however, an elevated FSH level is associated with a reduced live birth rate (a 5% reduction in women <40 years to a 26% reduction in women >42 years) and a higher miscarriage rate, reflecting the positive correlation of oocyte aneuploidy and age.
In addition to high intercycle variability, an FSH level is reliable only in the setting of normal hypothalamic and pituitary function.7 Conditions such a prolactinoma (or other causes of hyperprolactinemia), other intracranial masses, prior central radiation, hormone-based medication use, and inadequate energy reserve (as the result of anorexia nervosa, resulting in hypothalamic suppression), might result in a low or inappropriately normal FSH level that does not reflect ovarian function.11
Antral follicle count
Antral follicle count (AFC) is defined as the total number of follicles measuring 2 to 10 mm, in both ovaries, in the early follicular phase (days 2 to 4 of the cycle). A count of fewer than 6 to 10 antral follicles in total is considered consistent with diminished ovarian reserve6,12,13 (TABLE 1). Antral follicle count is not predictive of natural fecundity but, rather, projects ovarian response during IVF. Antral follicle count has been shown to decrease by 5% a year with increasing age among women with or without infertility.14
Studies have highlighted concerns regarding interobserver and intraobserver variability in determining the AFC but, in experienced hands, the AFC is a reliable test of ovarian reserve.15,16 Visualization of antral follicles can be compromised in obese patients.11 Conversely, AFC sometimes also overestimates ovarian reserve, because atretic follicles might be included in the count.11,15 Last, AFC is reduced in patients who take a hormone-based medication but recovers with cessation of the medication.17 Ideally, a woman should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before AFC is measured.
Continue to: Anti-Müllerian hormone
Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
#2 Who should have ovarian reserve testing?
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.
Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?
#3 Can I reassure my patient about her reproductive potential using these tests?
Normal findings on ovarian reserve testing suggests that a woman might have a normal (that is, commensurate with age-matched peers) number of eggs in her ovaries. But normal test results do not mean she will have an easy time conceiving. Similarly, abnormal results do not mean that she will have difficulty conceiving.
Ovarian reserve testing reflects only the number of oocytes, not their quality, which is primarily determined by maternal age.35 Genetic testing of embryos during IVF shows that the percentage of embryos that are aneuploid (usually resulting from abnormal eggs) rises with advancing maternal age, beginning at 35 years.5 The increasing rate of oocyte aneuploidy is also reflected in the rising rate of loss of clinically recognized pregnancies with advancing maternal age: from 11% in women younger than age 34 to greater than 36% in women older than age 42.4
Furthermore, ovarian reserve testing does not reflect other potential genetic barriers to reproduction, such as a chromosomal translocation that can result in recurrent pregnancy loss. Fallopian tube obstruction and uterine issues, such as fibroids or septa, and male factors are also not reflected in ovarian reserve testing.
#4 My patient is trying to get pregnant and has abnormal ovarian reserve testing results. Will she need IVF?"
Not necessarily. Consultation with a fertility specialist to discuss the nuances of abnormal test results and management options is ideal but, essentially, as the American Society for Reproductive Medicine states, “evidence of [diminished ovarian reserve] does not necessarily equate with inability to conceive.” Furthermore, the Society states, “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of ART.”
Once counseled, patients might elect to pursue more aggressive treatment, but they might not necessarily need it. Age must figure significantly into treatment decisions, because oocyte quality—regardless of number—begins to decline at 35 years of age, with an associated increasing risk of infertility and miscarriage.
In a recently published study of 750 women attempting pregnancy, women with a low AMH level (<0.7 ng/mL) or high FSH level (>10 mIU/mL), or both, did not have a significantly lower likelihood of achieving spontaneous pregnancy within 1 year, compared with women with normal results of ovarian reserve testing.3
Continue to: #5 My patient is not ready to be pregnant
#5 My patient is not ready to be pregnant. If her results are abnormal, should she freeze eggs?
For patients who might be interested in seeking fertility preservation and ART, earlier referral to a reproductive specialist to discuss risks and benefits of oocyte or embryo cryopreservation is always preferable. The younger a woman is when she undergoes fertility preservation, the better. Among patients planning to delay conception, each one’s decision is driven by her personal calculations of the cost, risk, and benefit of egg or embryo freezing—a picture of which ovarian reserve testing is only one piece.
#6 Can these tests predict menopause?
Menopause is a clinical diagnosis, defined as 12 months without menses (without hormone use or other causes of amenorrhea). In such women, FSH levels are elevated, but biochemical tests are not part of the menopause diagnosis.36 In the years leading to menopause, FSH levels are highly variable and unreliable in predicting time to menopause.
AMH has been shown to correlate with time to menopause. (Once the AMH level becomes undetectable, menopause occurs in a mean of 6 years.37,38) Patients do not typically have serial AMH measurements, however, so it is not usually known when the hormone became undetectable. Therefore, AMH is not a useful test for predicting time to menopause.
Premature ovarian insufficiency (loss of ovarian function in women younger than age 40), should be considered in women with secondary amenorrhea of 4 months or longer. The diagnosis requires confirmatory laboratory assessment,36 and findings include an FSH level greater than 25 mIU/mL on 2 tests performed at least 1 month apart.39,40
Ovarian reserve tests: A partial view of reproductive potential
The answers we have provided highlight several key concepts and conclusions that should guide clinical practice and decisions made by patients:
- Ovarian reserve tests best serve to predict ovarian response during IVF; to a far lesser extent, they might predict birth outcomes from IVF. These tests have not, however, been shown to predict spontaneous pregnancy.
- Ovarian reserve tests should be administered purposefully, with counseling beforehand regarding their limitations.
- Abnormal ovarian reserve test results do not necessitate ART; however, they may prompt a patient to accelerate her reproductive timeline and consult with a reproductive endocrinologist to consider her age and health-related risks of infertility or pregnancy loss.
- Patients should be counseled that, regardless of the results of ovarian reserve testing, attempting conception or pursuing fertility preservation at a younger age (in particular, at <35 years of age) is associated with better outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
CASE Your patient wants ovarian reserve testing. Is her request reasonable?
A 34-year-old woman, recently married, plans to delay attempting pregnancy for a few years. She requests ovarian reserve testing to inform this timeline.
This is not an unreasonable inquiry, given her age (<35 years), after which there is natural acceleration in the rate of decline in the quality of oocytes. Regardless of the results of testing, attempting pregnancy or pursuing fertility preservation as soon as possible (particularly in patients >35 years) is associated with better outcomes.
A woman is born with all the eggs she will ever have. Oocyte atresia occurs throughout a woman’s lifetime, from 1,000,000 eggs at birth to only 1,000 by the time of menopause.1 A woman’s ovarian reserve reflects the number of oocytes present in the ovaries and is the result of complex interactions of age, genetics, and environmental variables.
Ovarian reserve testing, however, only has been consistently shown to predict ovarian response to stimulation with gonadotropins; these tests might reflect in vitro fertilization (IVF) birth outcomes to a lesser degree, but have not been shown to predict natural fecundability.2,3 Essentially, ovarian reserve testing provides a partial view of reproductive potential.
Ovarian reserve testing also does not reflect an age-related decline in oocyte quality, particularly after age 35.4,5 As such, female age is the principal driver of fertility potential, regardless of oocyte number. A woman with abnormal ovarian reserve tests may benefit from referral to a fertility specialist for counseling that integrates her results, age, and medical history, with the caveat that abnormal results do not necessarily mean she needs assisted reproductive technology (ART) to conceive.
In this article, we review 6 common questions about the ovarian reserve, providing current data to support the answers.
Continue to: #1 What tests are part of an ovarian reserve assessment?
#1 What tests are part of an ovarian reserve assessment? What is their utility?
FSH and estradiol
Follicle-stimulating hormone (FSH) and estradiol should be checked together in the early follicular phase (days 2 to 4 of the cycle). Elevated levels of one or both hormones suggest diminished ovarian reserve; an FSH level greater than 10 mIU/mL and/or an estradiol level greater than 80 pg/mL represent abnormal results6 (TABLE 1). Because FSH demonstrates significant intercycle variability, a single abnormal result should be confirmed in a subsequent cycle.7
Although the basal FSH level does not reflect egg quality or predict natural fecundity, an elevated FSH level predicts poor ovarian response (<3 or 4 eggs retrieved) to ovarian hyperstimulation, with good specificity.3,6,8,9 In patients younger than age 35 years undergoing IVF, basal FSH levels do not predict live birth or pregnancy loss.10 In older patients undergoing IVF, however, an elevated FSH level is associated with a reduced live birth rate (a 5% reduction in women <40 years to a 26% reduction in women >42 years) and a higher miscarriage rate, reflecting the positive correlation of oocyte aneuploidy and age.
In addition to high intercycle variability, an FSH level is reliable only in the setting of normal hypothalamic and pituitary function.7 Conditions such a prolactinoma (or other causes of hyperprolactinemia), other intracranial masses, prior central radiation, hormone-based medication use, and inadequate energy reserve (as the result of anorexia nervosa, resulting in hypothalamic suppression), might result in a low or inappropriately normal FSH level that does not reflect ovarian function.11
Antral follicle count
Antral follicle count (AFC) is defined as the total number of follicles measuring 2 to 10 mm, in both ovaries, in the early follicular phase (days 2 to 4 of the cycle). A count of fewer than 6 to 10 antral follicles in total is considered consistent with diminished ovarian reserve6,12,13 (TABLE 1). Antral follicle count is not predictive of natural fecundity but, rather, projects ovarian response during IVF. Antral follicle count has been shown to decrease by 5% a year with increasing age among women with or without infertility.14
Studies have highlighted concerns regarding interobserver and intraobserver variability in determining the AFC but, in experienced hands, the AFC is a reliable test of ovarian reserve.15,16 Visualization of antral follicles can be compromised in obese patients.11 Conversely, AFC sometimes also overestimates ovarian reserve, because atretic follicles might be included in the count.11,15 Last, AFC is reduced in patients who take a hormone-based medication but recovers with cessation of the medication.17 Ideally, a woman should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before AFC is measured.
Continue to: Anti-Müllerian hormone
Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
#2 Who should have ovarian reserve testing?
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.
Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?
#3 Can I reassure my patient about her reproductive potential using these tests?
Normal findings on ovarian reserve testing suggests that a woman might have a normal (that is, commensurate with age-matched peers) number of eggs in her ovaries. But normal test results do not mean she will have an easy time conceiving. Similarly, abnormal results do not mean that she will have difficulty conceiving.
Ovarian reserve testing reflects only the number of oocytes, not their quality, which is primarily determined by maternal age.35 Genetic testing of embryos during IVF shows that the percentage of embryos that are aneuploid (usually resulting from abnormal eggs) rises with advancing maternal age, beginning at 35 years.5 The increasing rate of oocyte aneuploidy is also reflected in the rising rate of loss of clinically recognized pregnancies with advancing maternal age: from 11% in women younger than age 34 to greater than 36% in women older than age 42.4
Furthermore, ovarian reserve testing does not reflect other potential genetic barriers to reproduction, such as a chromosomal translocation that can result in recurrent pregnancy loss. Fallopian tube obstruction and uterine issues, such as fibroids or septa, and male factors are also not reflected in ovarian reserve testing.
#4 My patient is trying to get pregnant and has abnormal ovarian reserve testing results. Will she need IVF?"
Not necessarily. Consultation with a fertility specialist to discuss the nuances of abnormal test results and management options is ideal but, essentially, as the American Society for Reproductive Medicine states, “evidence of [diminished ovarian reserve] does not necessarily equate with inability to conceive.” Furthermore, the Society states, “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of ART.”
Once counseled, patients might elect to pursue more aggressive treatment, but they might not necessarily need it. Age must figure significantly into treatment decisions, because oocyte quality—regardless of number—begins to decline at 35 years of age, with an associated increasing risk of infertility and miscarriage.
In a recently published study of 750 women attempting pregnancy, women with a low AMH level (<0.7 ng/mL) or high FSH level (>10 mIU/mL), or both, did not have a significantly lower likelihood of achieving spontaneous pregnancy within 1 year, compared with women with normal results of ovarian reserve testing.3
Continue to: #5 My patient is not ready to be pregnant
#5 My patient is not ready to be pregnant. If her results are abnormal, should she freeze eggs?
For patients who might be interested in seeking fertility preservation and ART, earlier referral to a reproductive specialist to discuss risks and benefits of oocyte or embryo cryopreservation is always preferable. The younger a woman is when she undergoes fertility preservation, the better. Among patients planning to delay conception, each one’s decision is driven by her personal calculations of the cost, risk, and benefit of egg or embryo freezing—a picture of which ovarian reserve testing is only one piece.
#6 Can these tests predict menopause?
Menopause is a clinical diagnosis, defined as 12 months without menses (without hormone use or other causes of amenorrhea). In such women, FSH levels are elevated, but biochemical tests are not part of the menopause diagnosis.36 In the years leading to menopause, FSH levels are highly variable and unreliable in predicting time to menopause.
AMH has been shown to correlate with time to menopause. (Once the AMH level becomes undetectable, menopause occurs in a mean of 6 years.37,38) Patients do not typically have serial AMH measurements, however, so it is not usually known when the hormone became undetectable. Therefore, AMH is not a useful test for predicting time to menopause.
Premature ovarian insufficiency (loss of ovarian function in women younger than age 40), should be considered in women with secondary amenorrhea of 4 months or longer. The diagnosis requires confirmatory laboratory assessment,36 and findings include an FSH level greater than 25 mIU/mL on 2 tests performed at least 1 month apart.39,40
Ovarian reserve tests: A partial view of reproductive potential
The answers we have provided highlight several key concepts and conclusions that should guide clinical practice and decisions made by patients:
- Ovarian reserve tests best serve to predict ovarian response during IVF; to a far lesser extent, they might predict birth outcomes from IVF. These tests have not, however, been shown to predict spontaneous pregnancy.
- Ovarian reserve tests should be administered purposefully, with counseling beforehand regarding their limitations.
- Abnormal ovarian reserve test results do not necessitate ART; however, they may prompt a patient to accelerate her reproductive timeline and consult with a reproductive endocrinologist to consider her age and health-related risks of infertility or pregnancy loss.
- Patients should be counseled that, regardless of the results of ovarian reserve testing, attempting conception or pursuing fertility preservation at a younger age (in particular, at <35 years of age) is associated with better outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1-21.
- Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012-1018.e1.
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367-1376.
- Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165(12):1380-1388.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 1,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17.
- Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Hum Reprod. 2004;19(3):590-595.
- Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2008;90(2):315-321.
- Roberts JE, Spandorfer S, Fasouliotis SJ, Kashyap S, Rosenwaks Z. Taking a basal follicle-stimulating hormone history is essential before initiating in vitro fertilization. Fertil Steril. 2005;83(1):37-41.
- Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Ferraretti AP, La Marca L, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-e50.
- Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A, et al. Infertile women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod. 2016;31(5):1034-1045.
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718.
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710.
- Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619.
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688-701.
- Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655.
- Hamdine O, Eijkemans MJ, Lentjes EW, Torrance HL, Macklon NS, Fauser BC, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30(1):170-178.
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855-864.
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159-163.
- Korsholm AS, Petersen KB, Bentzen JG, Hilsted LM, Andersen AN, Hvidman HW. Investigation of anti-Müllerian hormone concentrations in relation to natural conception rate and time to pregnancy. Reprod Biomed Online. 2018;36(5):568-575.
- Quinn MM, Kao CN, Ahmad AK, Haisenleder DJ, Santoro N, Eisenberg E, et al. Age-stratified thresholds of anti-Müllerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf).
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-129.
- Schiffner J, Roos J, Broomhead D, Helden JV, Godehardt E, Fehr D, et al. Relationship between anti-Müllerian hormone and antral follicle count across the menstrual cycle using the Beckman Coulter Access assay in comparison with Gen II manual assay. Clin Chem Lab Med. 2017;55(7):1025-1033.
- Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. 2018;35(5):777-783.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385.
- Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772.
- Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115.
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016 ;206:172-176.
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839-3847.
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633-634.
- National Collaborating Centre for Women’s and Children’s Health (UK). Menopause: Full Guideline. London: National Institute for Health and Care Excellence (UK); 2015 Nov 12. (NICE Guideline, No. 23). Premature ovarian insufficiency. Available from: www.ncbi.nlm.nih.gov/books/NBK343476/.
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680.
- van Rooij IA, den Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601-606.
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926-937.
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193-197.
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1-21.
- Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;101(4):1012-1018.e1.
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367-1376.
- Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. 2007;165(12):1380-1388.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 1,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17.
- Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Hum Reprod. 2004;19(3):590-595.
- Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2008;90(2):315-321.
- Roberts JE, Spandorfer S, Fasouliotis SJ, Kashyap S, Rosenwaks Z. Taking a basal follicle-stimulating hormone history is essential before initiating in vitro fertilization. Fertil Steril. 2005;83(1):37-41.
- Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril. 2017;108(6):980-987.
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129-140.
- Ferraretti AP, La Marca L, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-e50.
- Hvidman HW, Bentzen JG, Thuesen LL, Lauritsen MP, Forman JL, Loft A, et al. Infertile women below the age of 40 have similar anti-Müllerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod. 2016;31(5):1034-1045.
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718.
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698-710.
- Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25(6):612-619.
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688-701.
- Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655.
- Hamdine O, Eijkemans MJ, Lentjes EW, Torrance HL, Macklon NS, Fauser BC, et al. Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Müllerian hormone. Hum Reprod. 2015;30(1):170-178.
- Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855-864.
- Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159-163.
- Korsholm AS, Petersen KB, Bentzen JG, Hilsted LM, Andersen AN, Hvidman HW. Investigation of anti-Müllerian hormone concentrations in relation to natural conception rate and time to pregnancy. Reprod Biomed Online. 2018;36(5):568-575.
- Quinn MM, Kao CN, Ahmad AK, Haisenleder DJ, Santoro N, Eisenberg E, et al. Age-stratified thresholds of anti-Müllerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf).
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-129.
- Schiffner J, Roos J, Broomhead D, Helden JV, Godehardt E, Fehr D, et al. Relationship between anti-Müllerian hormone and antral follicle count across the menstrual cycle using the Beckman Coulter Access assay in comparison with Gen II manual assay. Clin Chem Lab Med. 2017;55(7):1025-1033.
- Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. 2018;35(5):777-783.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385.
- Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764-1772.
- Dólleman M, Verschuren WM, Eijkemans MJ, Dollé ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Müllerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106-2115.
- Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305-1310.
- Kim CW, Shim HS, Jang H, Song YG. The effects of uterine artery embolization on ovarian reserve. Eur J Obstet Gynecol Reprod Biol. 2016 ;206:172-176.
- Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839-3847.
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606-614.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633-634.
- National Collaborating Centre for Women’s and Children’s Health (UK). Menopause: Full Guideline. London: National Institute for Health and Care Excellence (UK); 2015 Nov 12. (NICE Guideline, No. 23). Premature ovarian insufficiency. Available from: www.ncbi.nlm.nih.gov/books/NBK343476/.
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673-1680.
- van Rooij IA, den Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601-606.
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926-937.
- Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193-197.
Disability in Patients With Stiff Person Syndrome May Progress Faster Than Thought
A study emphasizes the importance of early treatment.
WASHINGTON, DC—Stiff person syndrome leads to disability if therapy is not initiated early in the disease course, according to a prospective study presented at the 2018 Annual Meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM). In addition, patients with stiff person syndrome may have “faster progression of disablement than originally reported and believed,” said lead study author Goran Rakocevic, MD. Dr. Rakocevic is Associate Professor of Neurology, Director of the Neuromuscular Electrodiagnostic Laboratory, Clinical Director of the Jefferson Weinberg ALS Center, and Director of the Neuromuscular Medicine Fellowship Program at Thomas Jefferson University in Philadelphia.
Stiff person syndrome is a disorder characterized by muscle rigidity and episodic spasms in axial and limb musculature, as well as heightened sensitivity to external stimuli. To describe the natural history of stiff person syndrome, the extent of accumulated disability, and associated clinical features, Dr. Rakocevic and his research colleagues conducted a prospective cohort study in patients followed for up to eight years in a single center.
The cohort included 57 patients with mean age at disease onset of 42 (range, 22 to 60). Of these, 32 patients were examined every six months for two years without receiving immune therapies. The investigators assessed disease progression using quantitative scales of stiffness and heightened sensitivity.
Patients’ most frequent initial symptoms were leg stiffness, paraspinal muscle rigidity, and painful spasms. Although no patients required assistance for ambulation during the first two years of the disease, 46 patients (80%) lost the ability to walk independently during follow-up, despite symptomatic medications. In the longitudinal cohort, the number of stiff areas increased, which was consistent with worsening functional status and quality of life. The researchers confirmed a strong association between stiff person syndrome and the HLA-DR and DQ haplotypes.
The study is the largest prospective study of patients with stiff person syndrome and the first to provide longitudinal data on the natural course of the disorder in a large patient subgroup using objective clinical measures, Dr. Rakocevic and colleagues said. “The study shows that stiff person syndrome is a progressive autoimmune disease that leads to disability if ... immunotherapy is not applied,” said the investigators.
“Early diagnosis and management of stiff person syndrome can be challenging,” said A. Gordon Smith, MD, Cochair of the AANEM Annual Meeting Program Committee. The study by Dr. Rakocevic’s team demonstrates “that stiff person syndrome causes progressive stiffness and functional decline, with 80% [of patients] becoming unable to walk independently,” he said. “Their research emphasizes the need to treat early and will help clinicians recognize stiff person syndrome earlier in its course.”
The study adds to neurologists’ understanding of the rare disorder, and its strengths include the length of follow-up and the number of patients, said Robert W. Irwin, MD, Cochair of the AANEM Annual Meeting Program Committee.
A study emphasizes the importance of early treatment.
A study emphasizes the importance of early treatment.
WASHINGTON, DC—Stiff person syndrome leads to disability if therapy is not initiated early in the disease course, according to a prospective study presented at the 2018 Annual Meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM). In addition, patients with stiff person syndrome may have “faster progression of disablement than originally reported and believed,” said lead study author Goran Rakocevic, MD. Dr. Rakocevic is Associate Professor of Neurology, Director of the Neuromuscular Electrodiagnostic Laboratory, Clinical Director of the Jefferson Weinberg ALS Center, and Director of the Neuromuscular Medicine Fellowship Program at Thomas Jefferson University in Philadelphia.
Stiff person syndrome is a disorder characterized by muscle rigidity and episodic spasms in axial and limb musculature, as well as heightened sensitivity to external stimuli. To describe the natural history of stiff person syndrome, the extent of accumulated disability, and associated clinical features, Dr. Rakocevic and his research colleagues conducted a prospective cohort study in patients followed for up to eight years in a single center.
The cohort included 57 patients with mean age at disease onset of 42 (range, 22 to 60). Of these, 32 patients were examined every six months for two years without receiving immune therapies. The investigators assessed disease progression using quantitative scales of stiffness and heightened sensitivity.
Patients’ most frequent initial symptoms were leg stiffness, paraspinal muscle rigidity, and painful spasms. Although no patients required assistance for ambulation during the first two years of the disease, 46 patients (80%) lost the ability to walk independently during follow-up, despite symptomatic medications. In the longitudinal cohort, the number of stiff areas increased, which was consistent with worsening functional status and quality of life. The researchers confirmed a strong association between stiff person syndrome and the HLA-DR and DQ haplotypes.
The study is the largest prospective study of patients with stiff person syndrome and the first to provide longitudinal data on the natural course of the disorder in a large patient subgroup using objective clinical measures, Dr. Rakocevic and colleagues said. “The study shows that stiff person syndrome is a progressive autoimmune disease that leads to disability if ... immunotherapy is not applied,” said the investigators.
“Early diagnosis and management of stiff person syndrome can be challenging,” said A. Gordon Smith, MD, Cochair of the AANEM Annual Meeting Program Committee. The study by Dr. Rakocevic’s team demonstrates “that stiff person syndrome causes progressive stiffness and functional decline, with 80% [of patients] becoming unable to walk independently,” he said. “Their research emphasizes the need to treat early and will help clinicians recognize stiff person syndrome earlier in its course.”
The study adds to neurologists’ understanding of the rare disorder, and its strengths include the length of follow-up and the number of patients, said Robert W. Irwin, MD, Cochair of the AANEM Annual Meeting Program Committee.
WASHINGTON, DC—Stiff person syndrome leads to disability if therapy is not initiated early in the disease course, according to a prospective study presented at the 2018 Annual Meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM). In addition, patients with stiff person syndrome may have “faster progression of disablement than originally reported and believed,” said lead study author Goran Rakocevic, MD. Dr. Rakocevic is Associate Professor of Neurology, Director of the Neuromuscular Electrodiagnostic Laboratory, Clinical Director of the Jefferson Weinberg ALS Center, and Director of the Neuromuscular Medicine Fellowship Program at Thomas Jefferson University in Philadelphia.
Stiff person syndrome is a disorder characterized by muscle rigidity and episodic spasms in axial and limb musculature, as well as heightened sensitivity to external stimuli. To describe the natural history of stiff person syndrome, the extent of accumulated disability, and associated clinical features, Dr. Rakocevic and his research colleagues conducted a prospective cohort study in patients followed for up to eight years in a single center.
The cohort included 57 patients with mean age at disease onset of 42 (range, 22 to 60). Of these, 32 patients were examined every six months for two years without receiving immune therapies. The investigators assessed disease progression using quantitative scales of stiffness and heightened sensitivity.
Patients’ most frequent initial symptoms were leg stiffness, paraspinal muscle rigidity, and painful spasms. Although no patients required assistance for ambulation during the first two years of the disease, 46 patients (80%) lost the ability to walk independently during follow-up, despite symptomatic medications. In the longitudinal cohort, the number of stiff areas increased, which was consistent with worsening functional status and quality of life. The researchers confirmed a strong association between stiff person syndrome and the HLA-DR and DQ haplotypes.
The study is the largest prospective study of patients with stiff person syndrome and the first to provide longitudinal data on the natural course of the disorder in a large patient subgroup using objective clinical measures, Dr. Rakocevic and colleagues said. “The study shows that stiff person syndrome is a progressive autoimmune disease that leads to disability if ... immunotherapy is not applied,” said the investigators.
“Early diagnosis and management of stiff person syndrome can be challenging,” said A. Gordon Smith, MD, Cochair of the AANEM Annual Meeting Program Committee. The study by Dr. Rakocevic’s team demonstrates “that stiff person syndrome causes progressive stiffness and functional decline, with 80% [of patients] becoming unable to walk independently,” he said. “Their research emphasizes the need to treat early and will help clinicians recognize stiff person syndrome earlier in its course.”
The study adds to neurologists’ understanding of the rare disorder, and its strengths include the length of follow-up and the number of patients, said Robert W. Irwin, MD, Cochair of the AANEM Annual Meeting Program Committee.
FDA expands approval of pembrolizumab in NSCLC
The Food and Drug Administration .
The drug is now approved for use in combination with carboplatin and either paclitaxel or nanoparticle albumin–bound (nab) paclitaxel for the first-line treatment of NSCLC, regardless of PD-L1 expression status.
This makes pembrolizumab the first anti-PD-1 therapy approved in the first-line setting both as monotherapy and in combination treatment for certain patients with metastatic NSCLC. All appropriate patients with metastatic squamous NSCLC or metastatic nonsquamous NSCLC and no EGFR or ALK mutations are now eligible to receive pembrolizumab-based treatment first-line.
The FDA’s approval is based on results from the phase 3 KEYNOTE-407 trial. This randomized, double-blind study enrolled patients with metastatic squamous NSCLC, regardless of tumor PD-L1 expression status, who had received no prior systemic treatment for metastatic disease.
Patients in the pembrolizumab arm (n = 278) received pembrolizumab and carboplatin every 3 weeks for four cycles, plus paclitaxel every 3 weeks for four cycles or nab-paclitaxel on days 1, 8, and 15 of every 3-week cycle for four cycles, followed by pembrolizumab every 3 weeks.
Patients in the control arm (n = 281) received the same regimen of carboplatin and paclitaxel/nab-paclitaxel, but placebo instead of pembrolizumab.
There was a significant improvement in overall response rate, progression-free survival, and overall survival in patients who received pembrolizumab.
The overall response rate was 58% in the pembrolizumab arm and 35% in the placebo arm (P = .0008). The median duration of response was 7.2 months and 4.9 months, respectively.
The median progression-free survival was 6.4 months in the pembrolizumab arm and 4.8 months in the placebo arm (P less than .0001). The median overall survival was 15.9 months and 11.3 months, respectively (P = .0017).
Safety data are available for the first 203 patients treated on the trial, 101 of them in the pembrolizumab arm.
Fifteen percent of patients discontinued pembrolizumab because of adverse events (AEs), and 43% of patients on pembrolizumab experienced AEs leading to dose interruption.
The most common AEs leading to dose interruption in the pembrolizumab arm were thrombocytopenia, neutropenia, anemia, asthenia, and diarrhea. The most frequent serious AEs in the pembrolizumab arm were febrile neutropenia, pneumonia, and urinary tract infection.
Additional details on this trial are available in the prescribing information, which can be found on the Keytruda website.
The Food and Drug Administration .
The drug is now approved for use in combination with carboplatin and either paclitaxel or nanoparticle albumin–bound (nab) paclitaxel for the first-line treatment of NSCLC, regardless of PD-L1 expression status.
This makes pembrolizumab the first anti-PD-1 therapy approved in the first-line setting both as monotherapy and in combination treatment for certain patients with metastatic NSCLC. All appropriate patients with metastatic squamous NSCLC or metastatic nonsquamous NSCLC and no EGFR or ALK mutations are now eligible to receive pembrolizumab-based treatment first-line.
The FDA’s approval is based on results from the phase 3 KEYNOTE-407 trial. This randomized, double-blind study enrolled patients with metastatic squamous NSCLC, regardless of tumor PD-L1 expression status, who had received no prior systemic treatment for metastatic disease.
Patients in the pembrolizumab arm (n = 278) received pembrolizumab and carboplatin every 3 weeks for four cycles, plus paclitaxel every 3 weeks for four cycles or nab-paclitaxel on days 1, 8, and 15 of every 3-week cycle for four cycles, followed by pembrolizumab every 3 weeks.
Patients in the control arm (n = 281) received the same regimen of carboplatin and paclitaxel/nab-paclitaxel, but placebo instead of pembrolizumab.
There was a significant improvement in overall response rate, progression-free survival, and overall survival in patients who received pembrolizumab.
The overall response rate was 58% in the pembrolizumab arm and 35% in the placebo arm (P = .0008). The median duration of response was 7.2 months and 4.9 months, respectively.
The median progression-free survival was 6.4 months in the pembrolizumab arm and 4.8 months in the placebo arm (P less than .0001). The median overall survival was 15.9 months and 11.3 months, respectively (P = .0017).
Safety data are available for the first 203 patients treated on the trial, 101 of them in the pembrolizumab arm.
Fifteen percent of patients discontinued pembrolizumab because of adverse events (AEs), and 43% of patients on pembrolizumab experienced AEs leading to dose interruption.
The most common AEs leading to dose interruption in the pembrolizumab arm were thrombocytopenia, neutropenia, anemia, asthenia, and diarrhea. The most frequent serious AEs in the pembrolizumab arm were febrile neutropenia, pneumonia, and urinary tract infection.
Additional details on this trial are available in the prescribing information, which can be found on the Keytruda website.
The Food and Drug Administration .
The drug is now approved for use in combination with carboplatin and either paclitaxel or nanoparticle albumin–bound (nab) paclitaxel for the first-line treatment of NSCLC, regardless of PD-L1 expression status.
This makes pembrolizumab the first anti-PD-1 therapy approved in the first-line setting both as monotherapy and in combination treatment for certain patients with metastatic NSCLC. All appropriate patients with metastatic squamous NSCLC or metastatic nonsquamous NSCLC and no EGFR or ALK mutations are now eligible to receive pembrolizumab-based treatment first-line.
The FDA’s approval is based on results from the phase 3 KEYNOTE-407 trial. This randomized, double-blind study enrolled patients with metastatic squamous NSCLC, regardless of tumor PD-L1 expression status, who had received no prior systemic treatment for metastatic disease.
Patients in the pembrolizumab arm (n = 278) received pembrolizumab and carboplatin every 3 weeks for four cycles, plus paclitaxel every 3 weeks for four cycles or nab-paclitaxel on days 1, 8, and 15 of every 3-week cycle for four cycles, followed by pembrolizumab every 3 weeks.
Patients in the control arm (n = 281) received the same regimen of carboplatin and paclitaxel/nab-paclitaxel, but placebo instead of pembrolizumab.
There was a significant improvement in overall response rate, progression-free survival, and overall survival in patients who received pembrolizumab.
The overall response rate was 58% in the pembrolizumab arm and 35% in the placebo arm (P = .0008). The median duration of response was 7.2 months and 4.9 months, respectively.
The median progression-free survival was 6.4 months in the pembrolizumab arm and 4.8 months in the placebo arm (P less than .0001). The median overall survival was 15.9 months and 11.3 months, respectively (P = .0017).
Safety data are available for the first 203 patients treated on the trial, 101 of them in the pembrolizumab arm.
Fifteen percent of patients discontinued pembrolizumab because of adverse events (AEs), and 43% of patients on pembrolizumab experienced AEs leading to dose interruption.
The most common AEs leading to dose interruption in the pembrolizumab arm were thrombocytopenia, neutropenia, anemia, asthenia, and diarrhea. The most frequent serious AEs in the pembrolizumab arm were febrile neutropenia, pneumonia, and urinary tract infection.
Additional details on this trial are available in the prescribing information, which can be found on the Keytruda website.
VA Reaches Milestone in Digitizing Claims Process
In a “significant modernization effort,” the VA has removed nearly 8 million paper files from 60 locations in under 22 months. The files are scanned into the electronic claims process system, which means faster claims decisions, the VA says.
According to the VA, it is a milestone in a years-long project to improve the veteran experience and streamline claims processes. The project began in 2013 when the VA began removing paper records from its regional offices to save space and money. It then expanded in 2016 when the VA launched the File Bank Extraction initiative, which removed more than 1.7 million paper claims files. In 2017, the agency began extracting 6.1 million paper records held in the Records Control Division (RCD) in St. Louis.
The records are temporarily stored in a secure facility certified by the National Archives and Records Administration, where they are inventoried, prioritized, and sent to VA vendors for scanning into the VA’s Veterans Benefits Management System.
The VA is negotiating to return the RCD’s leased warehouse space to the General Services Administration, estimating the move will save roughly $1.8 million per year.
Source:
VA achieves major milestone in effort to modernize claims processing [news release]. Washington, DC: U.S. Department of Veteran Affairs Office of Public Affairs and Media Relations; October 23,2018. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5131. Accessed October 31, 2018.
In a “significant modernization effort,” the VA has removed nearly 8 million paper files from 60 locations in under 22 months. The files are scanned into the electronic claims process system, which means faster claims decisions, the VA says.
According to the VA, it is a milestone in a years-long project to improve the veteran experience and streamline claims processes. The project began in 2013 when the VA began removing paper records from its regional offices to save space and money. It then expanded in 2016 when the VA launched the File Bank Extraction initiative, which removed more than 1.7 million paper claims files. In 2017, the agency began extracting 6.1 million paper records held in the Records Control Division (RCD) in St. Louis.
The records are temporarily stored in a secure facility certified by the National Archives and Records Administration, where they are inventoried, prioritized, and sent to VA vendors for scanning into the VA’s Veterans Benefits Management System.
The VA is negotiating to return the RCD’s leased warehouse space to the General Services Administration, estimating the move will save roughly $1.8 million per year.
Source:
VA achieves major milestone in effort to modernize claims processing [news release]. Washington, DC: U.S. Department of Veteran Affairs Office of Public Affairs and Media Relations; October 23,2018. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5131. Accessed October 31, 2018.
In a “significant modernization effort,” the VA has removed nearly 8 million paper files from 60 locations in under 22 months. The files are scanned into the electronic claims process system, which means faster claims decisions, the VA says.
According to the VA, it is a milestone in a years-long project to improve the veteran experience and streamline claims processes. The project began in 2013 when the VA began removing paper records from its regional offices to save space and money. It then expanded in 2016 when the VA launched the File Bank Extraction initiative, which removed more than 1.7 million paper claims files. In 2017, the agency began extracting 6.1 million paper records held in the Records Control Division (RCD) in St. Louis.
The records are temporarily stored in a secure facility certified by the National Archives and Records Administration, where they are inventoried, prioritized, and sent to VA vendors for scanning into the VA’s Veterans Benefits Management System.
The VA is negotiating to return the RCD’s leased warehouse space to the General Services Administration, estimating the move will save roughly $1.8 million per year.
Source:
VA achieves major milestone in effort to modernize claims processing [news release]. Washington, DC: U.S. Department of Veteran Affairs Office of Public Affairs and Media Relations; October 23,2018. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5131. Accessed October 31, 2018.
Progressive and Translucent Plaques on the Soles
The Diagnosis: Cutaneous Macroglobulinosis
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma that produces a circulating monoclonal IgM. Incidence in the United States is 1500 patients annually, most commonly men in their 70s.1 The disease process is largely indolent, with early symptoms consisting of generalized weakness, weight loss, and fatigue. Signs of lymphadenopathy, hepatosplenomegaly, and cytopenia may emerge as the disease progresses. Diagnostic criteria include bone marrow biopsy with plasmacytoid/plasmacellular infiltrate; IgM monoclonal gammopathy; and end-organ damage, which may include cutaneous manifestations.2
Cutaneous findings in Waldenström macroglobulinemia are nonspecific and secondary to the disease's hematologic manifestations, presenting as livedo reticularis, purpura, and mucosal bleeding.3 True cutaneous involvement of the disease is rare and was first described in 1978 by Tichenor.4 Specific cutaneous lesions have 2 separate clinical presentations: (1) a primary cutaneous infiltrate of lymphoplasmacytic cells, and (2) deposition of IgM in the dermis.5 Although the primary infiltrate of neoplastic cells appears as erythematous firm papules or plaques on the face and trunk, similar to other manifestations of leukemia cutis, deposition of IgM presents as translucent papules and plaques and is located more distally, particularly on the extensor extremities.6 These depositional plaques are not pruritic but may be tender if located over sites of pressure, as seen with the plantar presentation in our patient.
Histologically, cutaneous macroglobulinosis demonstrates IgM deposition in perieccrine, perivascular, or intravascular tissue that is periodic acid-Schiff (PAS) positive.7 Staining with Congo red and Alcian blue is negative. In our case, biopsy showed a nodular deposition of hypocellular globular material that stained brightly with PAS and PAS diastase. With Masson trichome stain, intensity of staining diminished, suggesting that the deposition was not composed of collagen; rather, this deposition appeared to consist of IgM storage papules on immunohistochemistry (Figure 1). Further workup revealed borderline pancytopenia and elevated globulins with a monoclonal peak on serum protein electrophoresis, confirming the diagnosis of cutaneous macroglobulinosis secondary to Waldenström macroglobulinemia.
A PubMed search of articles indexed for MEDLINE using the terms cutaneous, macroglobulinosis, macroglobulinemia, Waldenström's macroglobulinemia, Waldenström's macroglobulinaemia, and macroglobulinemia cutis revealed a total of 19 cases of cutaneous macroglobulinosis (including this case). The average age of presentation in these cases is 60 years (range, 29-83 years) with a predisposition for men (68% [13/19]). The development of cutaneous macroglobulinosis primarily has been noted following diagnosis of Waldenström macroglobulinemia (53% [10/19]), with some cases prior to diagnosis (37% [7/19]) or at the time of diagnosis (11% [2/19]). The presence of cutaneous lesions does not correlate with prognosis of the underlying malignancy.5,8,9
Systemic treatment of the underlying macroglobulinemia has been suggested for symptomatic cases of cutaneous macroglobulinosis.3 Prior therapy has consisted primarily of chlorambucil; however, treatment with rituximab, occasionally in conjunction with the proteasome inhibitor bortezomib, recently has been reported.10 Because of the symptomatic nature of our patient's lesions, she was referred to the oncology department and started on rituximab therapy. The lesions improved with therapy and have remained stable following treatment.
The differential diagnosis for tender pink papules and plaques on the arms and legs includes tophaceous gout, plantar fibromatosis, erythropoietic protoporphyria, and acral fibrokeratoma.
Gouty tophi commonly accumulate as painful, edematous, yellow to whitish nodules and tumors with erythema, often overlying joints or extensor surfaces. Histopathologic examination after formalin fixation shows needle-shaped clefts within feathery amorphous pink areas surrounded by granuloma (Figure 2).11 Yellow, needle-shaped, negatively birefringent crystals can be viewed under polarized microscopy in alcohol-fixed samples.
Plantar fibromatosis (Ledderhose disease) is a benign proliferation of the plantar aponeurosis linked to alcohol use; liver disease; and notably epilepsy,12 a component of our patient's medical history. Large nodules appear grossly on the plantar feet and may progress to contractures in more advanced lesions. Biopsy reveals bland hyperproliferation of fibroblasts in a background of fascial fibrous tissue (Figure 3).12 Clinically, this diagnosis is part of the differential diagnosis of plantar nodules but appears histologically different than cutaneous macroglobulinosis because there are no hyaline deposits in plantar fibromatosis.
Erythropoietic protoporphyria is a rare disorder that primarily arises due to a congenital deficiency in the ferrochelatase enzyme involved in heme biosynthesis. Erythropoietic protoporphyria is the most common porphyria among children and typically presents in infancy or early childhood as a painful photosensitivity with ensuing cutaneous manifestations and possible hepatobiliary disease. Edema and severe burning pain can be noted within minutes of sun exposure in a dose-response relationship.13 Histologic findings of erythropoietic protoporphyria differ based on acute or chronic skin changes. Acute lesions exhibit a predominantly neutrophilic interstitial dermal infiltrate with vacuoles and intercellular edema. Chronic changes include the accumulation of a PAS-positive, amorphous, hyalinelike substance, similar to the microscopic findings of cutaneous macroglobulinosis (Figure 4).13
An acral fibrokeratoma is a benign fibroepithelial tumor that clinically appears as a flesh-colored or slightly erythematous exophytic nodule that most commonly is found on the fingers or toes. Thought to arise from trauma to the affected area, it is histologically characterized by interwoven collagenous bundles with overlying epidermal hyperkeratosis, acanthosis, and deep thickened rete ridges14 (Figure 5). Although multiple acral fibrokeratomas have been reported (similar to presentations of prurigo nodularis),15 they more commonly appear as solitary lesions as opposed to the numerous translucent papules seen in our patient.
- Camp BJ, Magro CM. Cutaneous macroglobulinosis: a case series. J Cutan Pathol. 2012;39:962-970.
- Dimopoulos MA, Alexanian R. Waldenstrom's macroglobulinemia. Blood. 1994;83:1452-1459.
- D'Acunto C, Nigrisoli E, Liardo EV, et al. Painful plantar nodules: a specific manifestation of cutaneous macroglobulinosis. J Am Acad Dermatol. 2014;71:E251-E252.
- Tichenor RE. Macroglobulinemia cutis. Arch Dermatol. 1978;114:280-281.
- Gressier L, Hotz C, Lelièvre JD, et al. Cutaneous macroglobulinosis: a report of 2 cases. Arch Dermatol. 2010;146:165-169.
- Spicknall KE, Dubas LE, Mutasim DF. Cutaneous macroglobulinosis with monotypic plasma cells: a specific manifestation of Waldenström macroglobulinemia. J Cutan Pathol. 2013;40:442-444.
- Lüftl M, Sauter-Jenne B, Gramatzki M, et al. Cutaneous macroglobulinosis deposits in a patient with IgM paraproteinemia/incipient Waldenström macroglobulinemia. J Dtsch Dermatol Ges. 2010;8:1000-1003.
- Mascaro JM, Montserrat E, Estrach T, et al. Specific cutaneous manifestations of Waldenstrom macroglobulinaemia: a report of two cases. Br J Dermatol. 1982;106:217-222.
- Hanke CW, Steck WD, Bergfeld WF, et al. Cutaneous macroglobulinosis. Arch Dermatol. 1980;116:575-577.
- Oshio-Yoshii A, Fujimoto N, Shiba Y, et al. Cutaneous macroglobulinosis: successful treatment with rituximab. J Eur Acad Dermatol Venereol. 2017;31:E30-E31.
- Gupta A, Rai S, Sinha R, et al. Tophi as an initial manifestation of gout. J Cytol. 2009;26:165-166.
- Carroll P, Henshaw RM, Garwood C, et al. Plantar fibromatosis: pathophysiology, surgical and nonsurgical therapies: an evidence-based review. Foot Ankle Spec. 2018;11:168-176.
- Michaels BD, Del Rosso JQ, Mobini N, et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3:44-48.
- Boffeli TJ, Abben KW. Acral fibrokeratoma of the foot treated with excision and trap door flap closure: a case report. J Foot Ankle Surg. 2014;53:449-452.
- Reed RJ. Multiple acral fibrokeratomas (a variant of prurigo nodularis). discussion of classification of acral fibrous nodules and of histogenesis of acral fibrokeratomas. Arch Dermatol. 1971;103:287-297.
The Diagnosis: Cutaneous Macroglobulinosis
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma that produces a circulating monoclonal IgM. Incidence in the United States is 1500 patients annually, most commonly men in their 70s.1 The disease process is largely indolent, with early symptoms consisting of generalized weakness, weight loss, and fatigue. Signs of lymphadenopathy, hepatosplenomegaly, and cytopenia may emerge as the disease progresses. Diagnostic criteria include bone marrow biopsy with plasmacytoid/plasmacellular infiltrate; IgM monoclonal gammopathy; and end-organ damage, which may include cutaneous manifestations.2
Cutaneous findings in Waldenström macroglobulinemia are nonspecific and secondary to the disease's hematologic manifestations, presenting as livedo reticularis, purpura, and mucosal bleeding.3 True cutaneous involvement of the disease is rare and was first described in 1978 by Tichenor.4 Specific cutaneous lesions have 2 separate clinical presentations: (1) a primary cutaneous infiltrate of lymphoplasmacytic cells, and (2) deposition of IgM in the dermis.5 Although the primary infiltrate of neoplastic cells appears as erythematous firm papules or plaques on the face and trunk, similar to other manifestations of leukemia cutis, deposition of IgM presents as translucent papules and plaques and is located more distally, particularly on the extensor extremities.6 These depositional plaques are not pruritic but may be tender if located over sites of pressure, as seen with the plantar presentation in our patient.
Histologically, cutaneous macroglobulinosis demonstrates IgM deposition in perieccrine, perivascular, or intravascular tissue that is periodic acid-Schiff (PAS) positive.7 Staining with Congo red and Alcian blue is negative. In our case, biopsy showed a nodular deposition of hypocellular globular material that stained brightly with PAS and PAS diastase. With Masson trichome stain, intensity of staining diminished, suggesting that the deposition was not composed of collagen; rather, this deposition appeared to consist of IgM storage papules on immunohistochemistry (Figure 1). Further workup revealed borderline pancytopenia and elevated globulins with a monoclonal peak on serum protein electrophoresis, confirming the diagnosis of cutaneous macroglobulinosis secondary to Waldenström macroglobulinemia.

A PubMed search of articles indexed for MEDLINE using the terms cutaneous, macroglobulinosis, macroglobulinemia, Waldenström's macroglobulinemia, Waldenström's macroglobulinaemia, and macroglobulinemia cutis revealed a total of 19 cases of cutaneous macroglobulinosis (including this case). The average age of presentation in these cases is 60 years (range, 29-83 years) with a predisposition for men (68% [13/19]). The development of cutaneous macroglobulinosis primarily has been noted following diagnosis of Waldenström macroglobulinemia (53% [10/19]), with some cases prior to diagnosis (37% [7/19]) or at the time of diagnosis (11% [2/19]). The presence of cutaneous lesions does not correlate with prognosis of the underlying malignancy.5,8,9
Systemic treatment of the underlying macroglobulinemia has been suggested for symptomatic cases of cutaneous macroglobulinosis.3 Prior therapy has consisted primarily of chlorambucil; however, treatment with rituximab, occasionally in conjunction with the proteasome inhibitor bortezomib, recently has been reported.10 Because of the symptomatic nature of our patient's lesions, she was referred to the oncology department and started on rituximab therapy. The lesions improved with therapy and have remained stable following treatment.
The differential diagnosis for tender pink papules and plaques on the arms and legs includes tophaceous gout, plantar fibromatosis, erythropoietic protoporphyria, and acral fibrokeratoma.
Gouty tophi commonly accumulate as painful, edematous, yellow to whitish nodules and tumors with erythema, often overlying joints or extensor surfaces. Histopathologic examination after formalin fixation shows needle-shaped clefts within feathery amorphous pink areas surrounded by granuloma (Figure 2).11 Yellow, needle-shaped, negatively birefringent crystals can be viewed under polarized microscopy in alcohol-fixed samples.

Plantar fibromatosis (Ledderhose disease) is a benign proliferation of the plantar aponeurosis linked to alcohol use; liver disease; and notably epilepsy,12 a component of our patient's medical history. Large nodules appear grossly on the plantar feet and may progress to contractures in more advanced lesions. Biopsy reveals bland hyperproliferation of fibroblasts in a background of fascial fibrous tissue (Figure 3).12 Clinically, this diagnosis is part of the differential diagnosis of plantar nodules but appears histologically different than cutaneous macroglobulinosis because there are no hyaline deposits in plantar fibromatosis.

Erythropoietic protoporphyria is a rare disorder that primarily arises due to a congenital deficiency in the ferrochelatase enzyme involved in heme biosynthesis. Erythropoietic protoporphyria is the most common porphyria among children and typically presents in infancy or early childhood as a painful photosensitivity with ensuing cutaneous manifestations and possible hepatobiliary disease. Edema and severe burning pain can be noted within minutes of sun exposure in a dose-response relationship.13 Histologic findings of erythropoietic protoporphyria differ based on acute or chronic skin changes. Acute lesions exhibit a predominantly neutrophilic interstitial dermal infiltrate with vacuoles and intercellular edema. Chronic changes include the accumulation of a PAS-positive, amorphous, hyalinelike substance, similar to the microscopic findings of cutaneous macroglobulinosis (Figure 4).13

An acral fibrokeratoma is a benign fibroepithelial tumor that clinically appears as a flesh-colored or slightly erythematous exophytic nodule that most commonly is found on the fingers or toes. Thought to arise from trauma to the affected area, it is histologically characterized by interwoven collagenous bundles with overlying epidermal hyperkeratosis, acanthosis, and deep thickened rete ridges14 (Figure 5). Although multiple acral fibrokeratomas have been reported (similar to presentations of prurigo nodularis),15 they more commonly appear as solitary lesions as opposed to the numerous translucent papules seen in our patient.

The Diagnosis: Cutaneous Macroglobulinosis
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma that produces a circulating monoclonal IgM. Incidence in the United States is 1500 patients annually, most commonly men in their 70s.1 The disease process is largely indolent, with early symptoms consisting of generalized weakness, weight loss, and fatigue. Signs of lymphadenopathy, hepatosplenomegaly, and cytopenia may emerge as the disease progresses. Diagnostic criteria include bone marrow biopsy with plasmacytoid/plasmacellular infiltrate; IgM monoclonal gammopathy; and end-organ damage, which may include cutaneous manifestations.2
Cutaneous findings in Waldenström macroglobulinemia are nonspecific and secondary to the disease's hematologic manifestations, presenting as livedo reticularis, purpura, and mucosal bleeding.3 True cutaneous involvement of the disease is rare and was first described in 1978 by Tichenor.4 Specific cutaneous lesions have 2 separate clinical presentations: (1) a primary cutaneous infiltrate of lymphoplasmacytic cells, and (2) deposition of IgM in the dermis.5 Although the primary infiltrate of neoplastic cells appears as erythematous firm papules or plaques on the face and trunk, similar to other manifestations of leukemia cutis, deposition of IgM presents as translucent papules and plaques and is located more distally, particularly on the extensor extremities.6 These depositional plaques are not pruritic but may be tender if located over sites of pressure, as seen with the plantar presentation in our patient.
Histologically, cutaneous macroglobulinosis demonstrates IgM deposition in perieccrine, perivascular, or intravascular tissue that is periodic acid-Schiff (PAS) positive.7 Staining with Congo red and Alcian blue is negative. In our case, biopsy showed a nodular deposition of hypocellular globular material that stained brightly with PAS and PAS diastase. With Masson trichome stain, intensity of staining diminished, suggesting that the deposition was not composed of collagen; rather, this deposition appeared to consist of IgM storage papules on immunohistochemistry (Figure 1). Further workup revealed borderline pancytopenia and elevated globulins with a monoclonal peak on serum protein electrophoresis, confirming the diagnosis of cutaneous macroglobulinosis secondary to Waldenström macroglobulinemia.

A PubMed search of articles indexed for MEDLINE using the terms cutaneous, macroglobulinosis, macroglobulinemia, Waldenström's macroglobulinemia, Waldenström's macroglobulinaemia, and macroglobulinemia cutis revealed a total of 19 cases of cutaneous macroglobulinosis (including this case). The average age of presentation in these cases is 60 years (range, 29-83 years) with a predisposition for men (68% [13/19]). The development of cutaneous macroglobulinosis primarily has been noted following diagnosis of Waldenström macroglobulinemia (53% [10/19]), with some cases prior to diagnosis (37% [7/19]) or at the time of diagnosis (11% [2/19]). The presence of cutaneous lesions does not correlate with prognosis of the underlying malignancy.5,8,9
Systemic treatment of the underlying macroglobulinemia has been suggested for symptomatic cases of cutaneous macroglobulinosis.3 Prior therapy has consisted primarily of chlorambucil; however, treatment with rituximab, occasionally in conjunction with the proteasome inhibitor bortezomib, recently has been reported.10 Because of the symptomatic nature of our patient's lesions, she was referred to the oncology department and started on rituximab therapy. The lesions improved with therapy and have remained stable following treatment.
The differential diagnosis for tender pink papules and plaques on the arms and legs includes tophaceous gout, plantar fibromatosis, erythropoietic protoporphyria, and acral fibrokeratoma.
Gouty tophi commonly accumulate as painful, edematous, yellow to whitish nodules and tumors with erythema, often overlying joints or extensor surfaces. Histopathologic examination after formalin fixation shows needle-shaped clefts within feathery amorphous pink areas surrounded by granuloma (Figure 2).11 Yellow, needle-shaped, negatively birefringent crystals can be viewed under polarized microscopy in alcohol-fixed samples.

Plantar fibromatosis (Ledderhose disease) is a benign proliferation of the plantar aponeurosis linked to alcohol use; liver disease; and notably epilepsy,12 a component of our patient's medical history. Large nodules appear grossly on the plantar feet and may progress to contractures in more advanced lesions. Biopsy reveals bland hyperproliferation of fibroblasts in a background of fascial fibrous tissue (Figure 3).12 Clinically, this diagnosis is part of the differential diagnosis of plantar nodules but appears histologically different than cutaneous macroglobulinosis because there are no hyaline deposits in plantar fibromatosis.

Erythropoietic protoporphyria is a rare disorder that primarily arises due to a congenital deficiency in the ferrochelatase enzyme involved in heme biosynthesis. Erythropoietic protoporphyria is the most common porphyria among children and typically presents in infancy or early childhood as a painful photosensitivity with ensuing cutaneous manifestations and possible hepatobiliary disease. Edema and severe burning pain can be noted within minutes of sun exposure in a dose-response relationship.13 Histologic findings of erythropoietic protoporphyria differ based on acute or chronic skin changes. Acute lesions exhibit a predominantly neutrophilic interstitial dermal infiltrate with vacuoles and intercellular edema. Chronic changes include the accumulation of a PAS-positive, amorphous, hyalinelike substance, similar to the microscopic findings of cutaneous macroglobulinosis (Figure 4).13

An acral fibrokeratoma is a benign fibroepithelial tumor that clinically appears as a flesh-colored or slightly erythematous exophytic nodule that most commonly is found on the fingers or toes. Thought to arise from trauma to the affected area, it is histologically characterized by interwoven collagenous bundles with overlying epidermal hyperkeratosis, acanthosis, and deep thickened rete ridges14 (Figure 5). Although multiple acral fibrokeratomas have been reported (similar to presentations of prurigo nodularis),15 they more commonly appear as solitary lesions as opposed to the numerous translucent papules seen in our patient.

- Camp BJ, Magro CM. Cutaneous macroglobulinosis: a case series. J Cutan Pathol. 2012;39:962-970.
- Dimopoulos MA, Alexanian R. Waldenstrom's macroglobulinemia. Blood. 1994;83:1452-1459.
- D'Acunto C, Nigrisoli E, Liardo EV, et al. Painful plantar nodules: a specific manifestation of cutaneous macroglobulinosis. J Am Acad Dermatol. 2014;71:E251-E252.
- Tichenor RE. Macroglobulinemia cutis. Arch Dermatol. 1978;114:280-281.
- Gressier L, Hotz C, Lelièvre JD, et al. Cutaneous macroglobulinosis: a report of 2 cases. Arch Dermatol. 2010;146:165-169.
- Spicknall KE, Dubas LE, Mutasim DF. Cutaneous macroglobulinosis with monotypic plasma cells: a specific manifestation of Waldenström macroglobulinemia. J Cutan Pathol. 2013;40:442-444.
- Lüftl M, Sauter-Jenne B, Gramatzki M, et al. Cutaneous macroglobulinosis deposits in a patient with IgM paraproteinemia/incipient Waldenström macroglobulinemia. J Dtsch Dermatol Ges. 2010;8:1000-1003.
- Mascaro JM, Montserrat E, Estrach T, et al. Specific cutaneous manifestations of Waldenstrom macroglobulinaemia: a report of two cases. Br J Dermatol. 1982;106:217-222.
- Hanke CW, Steck WD, Bergfeld WF, et al. Cutaneous macroglobulinosis. Arch Dermatol. 1980;116:575-577.
- Oshio-Yoshii A, Fujimoto N, Shiba Y, et al. Cutaneous macroglobulinosis: successful treatment with rituximab. J Eur Acad Dermatol Venereol. 2017;31:E30-E31.
- Gupta A, Rai S, Sinha R, et al. Tophi as an initial manifestation of gout. J Cytol. 2009;26:165-166.
- Carroll P, Henshaw RM, Garwood C, et al. Plantar fibromatosis: pathophysiology, surgical and nonsurgical therapies: an evidence-based review. Foot Ankle Spec. 2018;11:168-176.
- Michaels BD, Del Rosso JQ, Mobini N, et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3:44-48.
- Boffeli TJ, Abben KW. Acral fibrokeratoma of the foot treated with excision and trap door flap closure: a case report. J Foot Ankle Surg. 2014;53:449-452.
- Reed RJ. Multiple acral fibrokeratomas (a variant of prurigo nodularis). discussion of classification of acral fibrous nodules and of histogenesis of acral fibrokeratomas. Arch Dermatol. 1971;103:287-297.
- Camp BJ, Magro CM. Cutaneous macroglobulinosis: a case series. J Cutan Pathol. 2012;39:962-970.
- Dimopoulos MA, Alexanian R. Waldenstrom's macroglobulinemia. Blood. 1994;83:1452-1459.
- D'Acunto C, Nigrisoli E, Liardo EV, et al. Painful plantar nodules: a specific manifestation of cutaneous macroglobulinosis. J Am Acad Dermatol. 2014;71:E251-E252.
- Tichenor RE. Macroglobulinemia cutis. Arch Dermatol. 1978;114:280-281.
- Gressier L, Hotz C, Lelièvre JD, et al. Cutaneous macroglobulinosis: a report of 2 cases. Arch Dermatol. 2010;146:165-169.
- Spicknall KE, Dubas LE, Mutasim DF. Cutaneous macroglobulinosis with monotypic plasma cells: a specific manifestation of Waldenström macroglobulinemia. J Cutan Pathol. 2013;40:442-444.
- Lüftl M, Sauter-Jenne B, Gramatzki M, et al. Cutaneous macroglobulinosis deposits in a patient with IgM paraproteinemia/incipient Waldenström macroglobulinemia. J Dtsch Dermatol Ges. 2010;8:1000-1003.
- Mascaro JM, Montserrat E, Estrach T, et al. Specific cutaneous manifestations of Waldenstrom macroglobulinaemia: a report of two cases. Br J Dermatol. 1982;106:217-222.
- Hanke CW, Steck WD, Bergfeld WF, et al. Cutaneous macroglobulinosis. Arch Dermatol. 1980;116:575-577.
- Oshio-Yoshii A, Fujimoto N, Shiba Y, et al. Cutaneous macroglobulinosis: successful treatment with rituximab. J Eur Acad Dermatol Venereol. 2017;31:E30-E31.
- Gupta A, Rai S, Sinha R, et al. Tophi as an initial manifestation of gout. J Cytol. 2009;26:165-166.
- Carroll P, Henshaw RM, Garwood C, et al. Plantar fibromatosis: pathophysiology, surgical and nonsurgical therapies: an evidence-based review. Foot Ankle Spec. 2018;11:168-176.
- Michaels BD, Del Rosso JQ, Mobini N, et al. Erythropoietic protoporphyria: a case report and literature review. J Clin Aesthet Dermatol. 2010;3:44-48.
- Boffeli TJ, Abben KW. Acral fibrokeratoma of the foot treated with excision and trap door flap closure: a case report. J Foot Ankle Surg. 2014;53:449-452.
- Reed RJ. Multiple acral fibrokeratomas (a variant of prurigo nodularis). discussion of classification of acral fibrous nodules and of histogenesis of acral fibrokeratomas. Arch Dermatol. 1971;103:287-297.
A 64-year-old woman with a medical history of Waldenström macroglobulinemia, multiple sclerosis, and epilepsy presented with slowly growing papules on the plantar feet of 21 months' duration. She was diagnosed with Waldenström macroglobulinemia incidentally on routine blood work 3 years prior and declined treatment because she was asymptomatic. Physical examination revealed a total of 20 firm, variably sized, light pink to purple, partially translucent and telangiectatic papules and plaques bilaterally on the plantar feet. A plaque from the right sole was biopsied.
Elagolix: A new treatment for pelvic pain caused by endometriosis
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3
Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
Pediatric Dermatology Workforce Shortage Explained
The Society for Pediatric Dermatology (SPD) was established in 1975, and the pediatric dermatology workforce shortage began shortly after. In 1986, Honig and Burke1 reported that opportunities in pediatric dermatology were limited and that pediatric dermatologists were predominantly located in larger teaching hospitals and selected private practice settings; furthermore, only approximately 20% had patient populations comprising more than 75% children.1 Positive changes have occurred since that time, with more practitioners dedicated to pediatric dermatology and increased opportunities within the specialty. The SPD has expanded to a thriving group of collegial pediatric dermatologists now topping 1200 members worldwide.
Although the SPD has strongly influenced practice development in pediatric dermatology, there are fewer than 300 board-certified pediatric dermatologists in the United States and approximately double that number of pediatric dermatology practitioners. The deficiency is glaring based on the national population alone. The US Census Bureau reported 325,719,178 individuals living in the United States (as of July 1, 2017).2 With approximately 75 million children in the United States and estimates that 22.8% of the population is younger than 18 years,3 there currently is 1 pediatric dermatologist for every 120,000 children or more.
As if the numbers alone were not adequate, a number of publications have addressed the benefits of pediatric dermatologists in both dermatology and pediatrics training and furthermore in pediatric care. A 2004 survey of dermatology program directors and chairpersons regarding the issue of the pediatric dermatology workforce shortage revealed that 45 of 94 (47.9%) programs employed a pediatric dermatologist and 24 (25.5%) had been looking to hire one for more than a year.4 Although more pediatric dermatologists have joined the workforce, it is not surprising that programs with no pediatric dermatologists want them. First, pediatric dermatologists dramatically improve the quality of training with regard to pediatric dermatology education and can increase dermatology residents’ comfort level with children. In a survey of a group of graduating third-year dermatology residents, dermatology residency program directors, and pediatric dermatology fellowship program directors by Nijhawan et al,5 residents who were trained in a program with one or more full-time pediatric dermatologists were more likely to feel competent treating children and to feel satisfied with their training program’s pediatric dermatology curriculum than residents without contact with a full-time pediatric dermatologist (50.0% vs 5.9% [P=.002] and 85.3% vs 52.9% [P<.001], respectively). The availability of a pediatric dermatology fellowship further enhanced satisfaction. Residents in programs with no full-time pediatric dermatologist on staff were more likely to be somewhat or extremely dissatisfied with their pediatric dermatology training. Residency program directors were more satisfied with their curriculums when there was one or more pediatric dermatologist on staff (P<.01).5
Programs with pediatric dermatologists also offer easy access to a mentor in the field. In a 2010 survey of pediatric dermatologists (published in 2014), Admani et al6 reported that 84% (91/109) of respondents (board-certified pediatric dermatologists) cited mentorship as the most important factor influencing their career choice. Exposure to the specialty was noted as a key motivating factor. In my opinion, the actual inclusion of a pediatric dermatology fellowship, whether the position is filled or not, appears to increase the chances of expansion and retention in the field.
Furthermore, due to the outpatient burden of skin disease in a pediatrics practice, providing pediatric trainees with contact with a pediatric dermatologist is needed.
As if there was not enough evidence that pediatric dermatologists are in high demand, SPD pediatric dermatology workforce surveys from the last 5 years, which will soon be updated, show similar indications.7,10 Fogel and Teng11 showed that 60% of surveyed pediatric dermatologists (N=226) were academic and 81% were salaried. Unlike previous data,1 the investigators showed that children constituted 79.5% of respondents’ patient populations.
For the medical student or resident seeking a career in pediatric dermatology, it appears that finding and working on projects with mentors likely is the key to stepping in the field. From my own experience, pediatric dermatologists are extremely friendly and open to supporting career development in earnest students. Reach out to potential mentors months before starting desired electives, as you are competing with other students and pediatrics, dermatology, and emergency medicine residents. Joining and attending meetings of the SPD is a great way to find direction in this friendly and collegial field. Additionally, pediatric dermatology sessions at the annual meetings of the American Academy of Dermatology are a wonderful way to experience the excitement of the field. As a pediatric dermatologist in practice for almost 2 decades, I can honestly say that the field is always intellectually stimulating and evolving rapidly through enhanced understanding of disease pathogenesis, genetics, and therapeutics. Helping children and their parents/guardians never gets boring.
The solution to improving the size and accessibility of the pediatric dermatology workforce is not simple and likely starts from the bottom up. More than 75% of pediatric dermatologists favor implementing systems to encourage medical students to pursue a career in pediatric dermatology.7 Increasing resident exposure to dedicated pediatric dermatology training time enhances satisfaction.5 Increased funding of fellowships can help these students and residents meet their goals. Current fellowship training programs now total 36, but not all approved institutions have been able to support a postgraduate year 5 (PGY-5) or higher fellow, and in my experience some institutions have avoided adding a fellow due to lack of funding internally. The average pediatric dermatologist earns $100,000 less than colleagues who treat adults, which is an impediment to the expansion of the field.10 This disparity may chase away practitioners, especially those with medical school debt. Debt forgiveness programs, enhanced practice development, and better base pay for pediatric dermatologists could positively impact growth in this specialty. Dermatology and pediatrics training programs need to dedicate more money and developmental support for pediatric dermatologists as a way to invest in the quality of pediatric dermatology education for their trainees. By recognizing the true value of the academic contributions of pediatric dermatologists, dermatology residency programs can invest in producing trainees with greater aplomb and acumen in pediatric dermatology.
- Honig PJ, Burke L. The subspecialty of pediatric dermatology. J Am Acad Dermatol. 1986;15:123-126.
- United States Census Bureau. QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed October 19, 2018.
- An aging nation: projected number of children and older adults. United States Census Bureau website. https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Published March 13, 2018. Accessed October 9, 2018.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Nijhawan RI, Mazza JM, Silverberg NB. Pediatric dermatology training survey of United States dermatology residency programs. Pediatr Dermatol. 2014;31:131-137.
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- 2014 Society for Pediatric Dermatology Peds Derm Training Survey. Society for Pediatric Dermatology website. https://pedsderm.net/site/assets/files/8639/06b-peds_training_survey_responses_final.pdf. Accessed October 9, 2018.
- ABD approved pediatric dermatology fellowship programs. Society for Pediatric Dermatology website. https://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Accessed October 9, 2018.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present and future [published online July 21, 2014]. Pediatr Dermatol. 2015;32:1-12.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
The Society for Pediatric Dermatology (SPD) was established in 1975, and the pediatric dermatology workforce shortage began shortly after. In 1986, Honig and Burke1 reported that opportunities in pediatric dermatology were limited and that pediatric dermatologists were predominantly located in larger teaching hospitals and selected private practice settings; furthermore, only approximately 20% had patient populations comprising more than 75% children.1 Positive changes have occurred since that time, with more practitioners dedicated to pediatric dermatology and increased opportunities within the specialty. The SPD has expanded to a thriving group of collegial pediatric dermatologists now topping 1200 members worldwide.
Although the SPD has strongly influenced practice development in pediatric dermatology, there are fewer than 300 board-certified pediatric dermatologists in the United States and approximately double that number of pediatric dermatology practitioners. The deficiency is glaring based on the national population alone. The US Census Bureau reported 325,719,178 individuals living in the United States (as of July 1, 2017).2 With approximately 75 million children in the United States and estimates that 22.8% of the population is younger than 18 years,3 there currently is 1 pediatric dermatologist for every 120,000 children or more.
As if the numbers alone were not adequate, a number of publications have addressed the benefits of pediatric dermatologists in both dermatology and pediatrics training and furthermore in pediatric care. A 2004 survey of dermatology program directors and chairpersons regarding the issue of the pediatric dermatology workforce shortage revealed that 45 of 94 (47.9%) programs employed a pediatric dermatologist and 24 (25.5%) had been looking to hire one for more than a year.4 Although more pediatric dermatologists have joined the workforce, it is not surprising that programs with no pediatric dermatologists want them. First, pediatric dermatologists dramatically improve the quality of training with regard to pediatric dermatology education and can increase dermatology residents’ comfort level with children. In a survey of a group of graduating third-year dermatology residents, dermatology residency program directors, and pediatric dermatology fellowship program directors by Nijhawan et al,5 residents who were trained in a program with one or more full-time pediatric dermatologists were more likely to feel competent treating children and to feel satisfied with their training program’s pediatric dermatology curriculum than residents without contact with a full-time pediatric dermatologist (50.0% vs 5.9% [P=.002] and 85.3% vs 52.9% [P<.001], respectively). The availability of a pediatric dermatology fellowship further enhanced satisfaction. Residents in programs with no full-time pediatric dermatologist on staff were more likely to be somewhat or extremely dissatisfied with their pediatric dermatology training. Residency program directors were more satisfied with their curriculums when there was one or more pediatric dermatologist on staff (P<.01).5
Programs with pediatric dermatologists also offer easy access to a mentor in the field. In a 2010 survey of pediatric dermatologists (published in 2014), Admani et al6 reported that 84% (91/109) of respondents (board-certified pediatric dermatologists) cited mentorship as the most important factor influencing their career choice. Exposure to the specialty was noted as a key motivating factor. In my opinion, the actual inclusion of a pediatric dermatology fellowship, whether the position is filled or not, appears to increase the chances of expansion and retention in the field.
Furthermore, due to the outpatient burden of skin disease in a pediatrics practice, providing pediatric trainees with contact with a pediatric dermatologist is needed.
As if there was not enough evidence that pediatric dermatologists are in high demand, SPD pediatric dermatology workforce surveys from the last 5 years, which will soon be updated, show similar indications.7,10 Fogel and Teng11 showed that 60% of surveyed pediatric dermatologists (N=226) were academic and 81% were salaried. Unlike previous data,1 the investigators showed that children constituted 79.5% of respondents’ patient populations.
For the medical student or resident seeking a career in pediatric dermatology, it appears that finding and working on projects with mentors likely is the key to stepping in the field. From my own experience, pediatric dermatologists are extremely friendly and open to supporting career development in earnest students. Reach out to potential mentors months before starting desired electives, as you are competing with other students and pediatrics, dermatology, and emergency medicine residents. Joining and attending meetings of the SPD is a great way to find direction in this friendly and collegial field. Additionally, pediatric dermatology sessions at the annual meetings of the American Academy of Dermatology are a wonderful way to experience the excitement of the field. As a pediatric dermatologist in practice for almost 2 decades, I can honestly say that the field is always intellectually stimulating and evolving rapidly through enhanced understanding of disease pathogenesis, genetics, and therapeutics. Helping children and their parents/guardians never gets boring.
The solution to improving the size and accessibility of the pediatric dermatology workforce is not simple and likely starts from the bottom up. More than 75% of pediatric dermatologists favor implementing systems to encourage medical students to pursue a career in pediatric dermatology.7 Increasing resident exposure to dedicated pediatric dermatology training time enhances satisfaction.5 Increased funding of fellowships can help these students and residents meet their goals. Current fellowship training programs now total 36, but not all approved institutions have been able to support a postgraduate year 5 (PGY-5) or higher fellow, and in my experience some institutions have avoided adding a fellow due to lack of funding internally. The average pediatric dermatologist earns $100,000 less than colleagues who treat adults, which is an impediment to the expansion of the field.10 This disparity may chase away practitioners, especially those with medical school debt. Debt forgiveness programs, enhanced practice development, and better base pay for pediatric dermatologists could positively impact growth in this specialty. Dermatology and pediatrics training programs need to dedicate more money and developmental support for pediatric dermatologists as a way to invest in the quality of pediatric dermatology education for their trainees. By recognizing the true value of the academic contributions of pediatric dermatologists, dermatology residency programs can invest in producing trainees with greater aplomb and acumen in pediatric dermatology.
The Society for Pediatric Dermatology (SPD) was established in 1975, and the pediatric dermatology workforce shortage began shortly after. In 1986, Honig and Burke1 reported that opportunities in pediatric dermatology were limited and that pediatric dermatologists were predominantly located in larger teaching hospitals and selected private practice settings; furthermore, only approximately 20% had patient populations comprising more than 75% children.1 Positive changes have occurred since that time, with more practitioners dedicated to pediatric dermatology and increased opportunities within the specialty. The SPD has expanded to a thriving group of collegial pediatric dermatologists now topping 1200 members worldwide.
Although the SPD has strongly influenced practice development in pediatric dermatology, there are fewer than 300 board-certified pediatric dermatologists in the United States and approximately double that number of pediatric dermatology practitioners. The deficiency is glaring based on the national population alone. The US Census Bureau reported 325,719,178 individuals living in the United States (as of July 1, 2017).2 With approximately 75 million children in the United States and estimates that 22.8% of the population is younger than 18 years,3 there currently is 1 pediatric dermatologist for every 120,000 children or more.
As if the numbers alone were not adequate, a number of publications have addressed the benefits of pediatric dermatologists in both dermatology and pediatrics training and furthermore in pediatric care. A 2004 survey of dermatology program directors and chairpersons regarding the issue of the pediatric dermatology workforce shortage revealed that 45 of 94 (47.9%) programs employed a pediatric dermatologist and 24 (25.5%) had been looking to hire one for more than a year.4 Although more pediatric dermatologists have joined the workforce, it is not surprising that programs with no pediatric dermatologists want them. First, pediatric dermatologists dramatically improve the quality of training with regard to pediatric dermatology education and can increase dermatology residents’ comfort level with children. In a survey of a group of graduating third-year dermatology residents, dermatology residency program directors, and pediatric dermatology fellowship program directors by Nijhawan et al,5 residents who were trained in a program with one or more full-time pediatric dermatologists were more likely to feel competent treating children and to feel satisfied with their training program’s pediatric dermatology curriculum than residents without contact with a full-time pediatric dermatologist (50.0% vs 5.9% [P=.002] and 85.3% vs 52.9% [P<.001], respectively). The availability of a pediatric dermatology fellowship further enhanced satisfaction. Residents in programs with no full-time pediatric dermatologist on staff were more likely to be somewhat or extremely dissatisfied with their pediatric dermatology training. Residency program directors were more satisfied with their curriculums when there was one or more pediatric dermatologist on staff (P<.01).5
Programs with pediatric dermatologists also offer easy access to a mentor in the field. In a 2010 survey of pediatric dermatologists (published in 2014), Admani et al6 reported that 84% (91/109) of respondents (board-certified pediatric dermatologists) cited mentorship as the most important factor influencing their career choice. Exposure to the specialty was noted as a key motivating factor. In my opinion, the actual inclusion of a pediatric dermatology fellowship, whether the position is filled or not, appears to increase the chances of expansion and retention in the field.
Furthermore, due to the outpatient burden of skin disease in a pediatrics practice, providing pediatric trainees with contact with a pediatric dermatologist is needed.
As if there was not enough evidence that pediatric dermatologists are in high demand, SPD pediatric dermatology workforce surveys from the last 5 years, which will soon be updated, show similar indications.7,10 Fogel and Teng11 showed that 60% of surveyed pediatric dermatologists (N=226) were academic and 81% were salaried. Unlike previous data,1 the investigators showed that children constituted 79.5% of respondents’ patient populations.
For the medical student or resident seeking a career in pediatric dermatology, it appears that finding and working on projects with mentors likely is the key to stepping in the field. From my own experience, pediatric dermatologists are extremely friendly and open to supporting career development in earnest students. Reach out to potential mentors months before starting desired electives, as you are competing with other students and pediatrics, dermatology, and emergency medicine residents. Joining and attending meetings of the SPD is a great way to find direction in this friendly and collegial field. Additionally, pediatric dermatology sessions at the annual meetings of the American Academy of Dermatology are a wonderful way to experience the excitement of the field. As a pediatric dermatologist in practice for almost 2 decades, I can honestly say that the field is always intellectually stimulating and evolving rapidly through enhanced understanding of disease pathogenesis, genetics, and therapeutics. Helping children and their parents/guardians never gets boring.
The solution to improving the size and accessibility of the pediatric dermatology workforce is not simple and likely starts from the bottom up. More than 75% of pediatric dermatologists favor implementing systems to encourage medical students to pursue a career in pediatric dermatology.7 Increasing resident exposure to dedicated pediatric dermatology training time enhances satisfaction.5 Increased funding of fellowships can help these students and residents meet their goals. Current fellowship training programs now total 36, but not all approved institutions have been able to support a postgraduate year 5 (PGY-5) or higher fellow, and in my experience some institutions have avoided adding a fellow due to lack of funding internally. The average pediatric dermatologist earns $100,000 less than colleagues who treat adults, which is an impediment to the expansion of the field.10 This disparity may chase away practitioners, especially those with medical school debt. Debt forgiveness programs, enhanced practice development, and better base pay for pediatric dermatologists could positively impact growth in this specialty. Dermatology and pediatrics training programs need to dedicate more money and developmental support for pediatric dermatologists as a way to invest in the quality of pediatric dermatology education for their trainees. By recognizing the true value of the academic contributions of pediatric dermatologists, dermatology residency programs can invest in producing trainees with greater aplomb and acumen in pediatric dermatology.
- Honig PJ, Burke L. The subspecialty of pediatric dermatology. J Am Acad Dermatol. 1986;15:123-126.
- United States Census Bureau. QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed October 19, 2018.
- An aging nation: projected number of children and older adults. United States Census Bureau website. https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Published March 13, 2018. Accessed October 9, 2018.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Nijhawan RI, Mazza JM, Silverberg NB. Pediatric dermatology training survey of United States dermatology residency programs. Pediatr Dermatol. 2014;31:131-137.
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- 2014 Society for Pediatric Dermatology Peds Derm Training Survey. Society for Pediatric Dermatology website. https://pedsderm.net/site/assets/files/8639/06b-peds_training_survey_responses_final.pdf. Accessed October 9, 2018.
- ABD approved pediatric dermatology fellowship programs. Society for Pediatric Dermatology website. https://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Accessed October 9, 2018.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present and future [published online July 21, 2014]. Pediatr Dermatol. 2015;32:1-12.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
- Honig PJ, Burke L. The subspecialty of pediatric dermatology. J Am Acad Dermatol. 1986;15:123-126.
- United States Census Bureau. QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed October 19, 2018.
- An aging nation: projected number of children and older adults. United States Census Bureau website. https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Published March 13, 2018. Accessed October 9, 2018.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Nijhawan RI, Mazza JM, Silverberg NB. Pediatric dermatology training survey of United States dermatology residency programs. Pediatr Dermatol. 2014;31:131-137.
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- 2014 Society for Pediatric Dermatology Peds Derm Training Survey. Society for Pediatric Dermatology website. https://pedsderm.net/site/assets/files/8639/06b-peds_training_survey_responses_final.pdf. Accessed October 9, 2018.
- ABD approved pediatric dermatology fellowship programs. Society for Pediatric Dermatology website. https://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Accessed October 9, 2018.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present and future [published online July 21, 2014]. Pediatr Dermatol. 2015;32:1-12.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
Delay in plasma exchange increases chance of poor outcomes in NMOSD
BERLIN – A delay in undertaking plasma exchange may predict a poorer outcome after a first attack of neuromyelitis optica spectrum disorder, while antibodies to myelin oligodendrocyte glycoprotein (MOG) appear to predict a more positive outcome.
“We saw that for each day of delay in plasma exchange, the Expanded Disability Status Scale [EDSS] at 6 months increased by about 0.028 points, indicating a worse prognosis,” Maxime Guillaume, MD, said at the annual congress of the European Committee for Treatment and Research in Multiples Sclerosis.
However, said Dr. Guillaume, a resident at Rouen University Hospital, France, steroids are still a reasonable first-line therapy as long as they are discontinued quickly if they don’t appear to be helping. Plasma exchange is most effective if administered less than 2 weeks after symptom onset.
His study examined 6-month outcomes among 214 attacks in 188 patients; some patients had several first attacks in different areas. Response was defined in two ways. First, patients were clinically classified as having a good response, a bad response, or no response to treatment. The second definition was based on the EDSS. Good response was an EDSS decrease of at least 2 points for an initial score of 3 or higher, or a decrease of 1 point if the initial score was less than 3. Poor response was an EDSS that decreased without reaching these thresholds.
The cohort was largely female, with a mean age of 38 years. Most (55%) were positive for antibodies against aquaporin-4. Anti-MOG antibodies were present in 30%. A total of 7.5% were negative for both antibodies, and the remainder had an undetermined serotype.
The clinical presentations varied. Most frequently, patients presented with myelitis only (44%). This was followed by optic neuritis only (34%), both myelitis and optic neuritis (8%), and myelitis plus brainstem involvement (5%). Other clinical manifestations were acute demyelinating encephalomyelitis, and encephalitis alone.
The most common treatment was methylprednisolone (73%), followed by plasma exchange (25%), which occurred at a median of 9 days after symptom onset.
Outcomes varied according to the definition of response. By clinical characteristics, there was a complete response in 41, a partial response in 122, and no response in 51. By change in EDSS, 136 had a good response and 27 a partial response; 51 were still considered nonresponders.
Dr. Guillaume conducted a multivariate analysis to determine predictive factors. In both definitions, anti-MOG antibodies nearly quadrupled the chance of a good treatment response, and delaying plasma exchange was associated with a significantly increased chance of a poor response. When judged by the clinical response definition, multiple lines of treatment also were associated with a poor response. This, he said, was another reflection of plasma exchange delay.
Dr. Guillaume had no financial disclosures.
BERLIN – A delay in undertaking plasma exchange may predict a poorer outcome after a first attack of neuromyelitis optica spectrum disorder, while antibodies to myelin oligodendrocyte glycoprotein (MOG) appear to predict a more positive outcome.
“We saw that for each day of delay in plasma exchange, the Expanded Disability Status Scale [EDSS] at 6 months increased by about 0.028 points, indicating a worse prognosis,” Maxime Guillaume, MD, said at the annual congress of the European Committee for Treatment and Research in Multiples Sclerosis.
However, said Dr. Guillaume, a resident at Rouen University Hospital, France, steroids are still a reasonable first-line therapy as long as they are discontinued quickly if they don’t appear to be helping. Plasma exchange is most effective if administered less than 2 weeks after symptom onset.
His study examined 6-month outcomes among 214 attacks in 188 patients; some patients had several first attacks in different areas. Response was defined in two ways. First, patients were clinically classified as having a good response, a bad response, or no response to treatment. The second definition was based on the EDSS. Good response was an EDSS decrease of at least 2 points for an initial score of 3 or higher, or a decrease of 1 point if the initial score was less than 3. Poor response was an EDSS that decreased without reaching these thresholds.
The cohort was largely female, with a mean age of 38 years. Most (55%) were positive for antibodies against aquaporin-4. Anti-MOG antibodies were present in 30%. A total of 7.5% were negative for both antibodies, and the remainder had an undetermined serotype.
The clinical presentations varied. Most frequently, patients presented with myelitis only (44%). This was followed by optic neuritis only (34%), both myelitis and optic neuritis (8%), and myelitis plus brainstem involvement (5%). Other clinical manifestations were acute demyelinating encephalomyelitis, and encephalitis alone.
The most common treatment was methylprednisolone (73%), followed by plasma exchange (25%), which occurred at a median of 9 days after symptom onset.
Outcomes varied according to the definition of response. By clinical characteristics, there was a complete response in 41, a partial response in 122, and no response in 51. By change in EDSS, 136 had a good response and 27 a partial response; 51 were still considered nonresponders.
Dr. Guillaume conducted a multivariate analysis to determine predictive factors. In both definitions, anti-MOG antibodies nearly quadrupled the chance of a good treatment response, and delaying plasma exchange was associated with a significantly increased chance of a poor response. When judged by the clinical response definition, multiple lines of treatment also were associated with a poor response. This, he said, was another reflection of plasma exchange delay.
Dr. Guillaume had no financial disclosures.
BERLIN – A delay in undertaking plasma exchange may predict a poorer outcome after a first attack of neuromyelitis optica spectrum disorder, while antibodies to myelin oligodendrocyte glycoprotein (MOG) appear to predict a more positive outcome.
“We saw that for each day of delay in plasma exchange, the Expanded Disability Status Scale [EDSS] at 6 months increased by about 0.028 points, indicating a worse prognosis,” Maxime Guillaume, MD, said at the annual congress of the European Committee for Treatment and Research in Multiples Sclerosis.
However, said Dr. Guillaume, a resident at Rouen University Hospital, France, steroids are still a reasonable first-line therapy as long as they are discontinued quickly if they don’t appear to be helping. Plasma exchange is most effective if administered less than 2 weeks after symptom onset.
His study examined 6-month outcomes among 214 attacks in 188 patients; some patients had several first attacks in different areas. Response was defined in two ways. First, patients were clinically classified as having a good response, a bad response, or no response to treatment. The second definition was based on the EDSS. Good response was an EDSS decrease of at least 2 points for an initial score of 3 or higher, or a decrease of 1 point if the initial score was less than 3. Poor response was an EDSS that decreased without reaching these thresholds.
The cohort was largely female, with a mean age of 38 years. Most (55%) were positive for antibodies against aquaporin-4. Anti-MOG antibodies were present in 30%. A total of 7.5% were negative for both antibodies, and the remainder had an undetermined serotype.
The clinical presentations varied. Most frequently, patients presented with myelitis only (44%). This was followed by optic neuritis only (34%), both myelitis and optic neuritis (8%), and myelitis plus brainstem involvement (5%). Other clinical manifestations were acute demyelinating encephalomyelitis, and encephalitis alone.
The most common treatment was methylprednisolone (73%), followed by plasma exchange (25%), which occurred at a median of 9 days after symptom onset.
Outcomes varied according to the definition of response. By clinical characteristics, there was a complete response in 41, a partial response in 122, and no response in 51. By change in EDSS, 136 had a good response and 27 a partial response; 51 were still considered nonresponders.
Dr. Guillaume conducted a multivariate analysis to determine predictive factors. In both definitions, anti-MOG antibodies nearly quadrupled the chance of a good treatment response, and delaying plasma exchange was associated with a significantly increased chance of a poor response. When judged by the clinical response definition, multiple lines of treatment also were associated with a poor response. This, he said, was another reflection of plasma exchange delay.
Dr. Guillaume had no financial disclosures.
REPORTING FROM ECTRIMS 2018
Key clinical point:
Major finding: Each day’s delay in receiving plasma exchange increased the mean 6-month EDSS by about 0.028 points.
Study details: The retrospective study comprised 214 attacks in 188 patients.
Disclosures: Dr. Guillaume had no financial disclosures.
Source: Guillaume M. et al. ECTRIMS 2018, Abstract 211.