User login
DRESS Syndrome: Clinical Myths and Pearls
Drug rash with eosinophilia and systemic symptoms (DRESS syndrome), also known as drug-induced hypersensitivity syndrome, is an uncommon severe systemic hypersensitivity drug reaction. It is estimated to occur in 1 in every 1000 to 10,000 drug exposures.1 It can affect patients of all ages and typically presents 2 to 6 weeks after exposure to a culprit medication. Classically, DRESS syndrome presents with often widespread rash, facial edema, systemic symptoms such as fever, lymphadenopathy, and evidence of visceral organ involvement. Peripheral blood eosinophilia is frequently but not universally observed.1,2
Even with proper management, reported DRESS syndrome mortality rates worldwide are approximately 10%2 or higher depending on the degree and type of other organ involvement (eg, cardiac).3 Beyond the acute manifestations of DRESS syndrome, this condition is unique in that some patients develop late-onset sequelae such as myocarditis or autoimmune conditions even years after the initial cutaneous eruption.4 Therefore, longitudinal evaluation is a key component of management.
The clinical myths and pearls presented here highlight some of the commonly held assumptions regarding DRESS syndrome in an effort to illuminate subtleties of managing patients with this condition (Table).
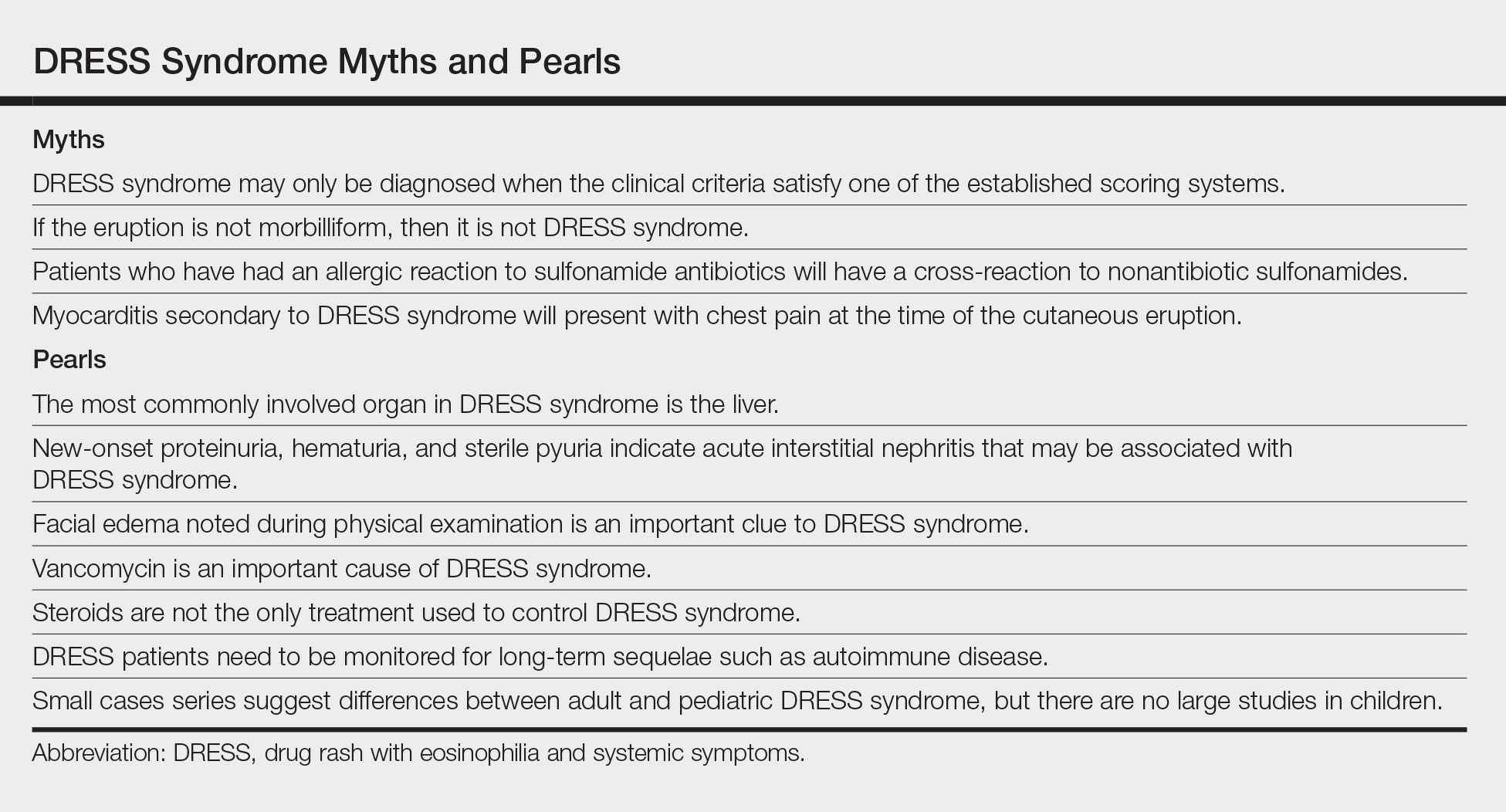
Myth: DRESS syndrome may only be diagnosed when the clinical criteria satisfy one of the established scoring systems.
Patients with DRESS syndrome can have heterogeneous manifestations. As a result, patients may develop a drug hypersensitivity with biological behavior and a natural history compatible with DRESS syndrome that does not fulfill published diagnostic criteria.5 The syndrome also may reveal its component manifestations gradually, thus delaying the diagnosis. The terms mini-DRESS and skirt syndrome have been employed to describe drug eruptions that clearly have systemic symptoms and more complex and pernicious biologic behavior than a simple drug exanthema but do not meet DRESS syndrome criteria. Ultimately, it is important to note that in clinical practice, DRESS syndrome exists on a spectrum of severity and the diagnosis remains a clinical one.
Pearl: The most commonly involved organ in DRESS syndrome is the liver.
Liver involvement is the most common visceral organ involved in DRESS syndrome and is estimated to occur in approximately 45.0% to 86.1% of cases.6,7 If a patient develops the characteristic rash, peripheral blood eosinophilia, and evidence of liver injury, DRESS syndrome must be included in the differential diagnosis.
Hepatitis presenting in DRESS syndrome can be hepatocellular, cholestatic, or mixed.6,7 Case series are varied in whether the transaminitis of DRESS syndrome tends to be more hepatocellular8 or cholestatic.7 Liver dysfunction in DRESS syndrome often lasts longer than in other severe cutaneous adverse drug reactions, and patients may improve anywhere from a few days in milder cases to months to achieve resolution of abnormalities.6,7 Severe hepatic involvement is thought to be the most notable cause of mortality.9
Pearl: New-onset proteinuria, hematuria, and sterile pyuria indicate acute interstitial nephritis that may be associated with DRESS syndrome.
Acute interstitial nephritis (AIN) is a drug-induced form of acute kidney injury that can co-occur with DRESS syndrome. Acute interstitial nephritis can present with some combination of acute kidney injury, morbilliform eruption, eosinophilia, fever, and sometimes eosinophiluria. Although AIN can be distinct from DRESS syndrome, there are cases of DRESS syndrome associated with AIN.10 In the correct clinical context, urinalysis may help by showing new-onset proteinuria, new-onset hematuria, and sterile pyuria. More common causes of acute kidney injury such as prerenal etiologies and acute tubular necrosis have a bland urinary sediment.
Myth: If the eruption is not morbilliform, then it is not DRESS syndrome.
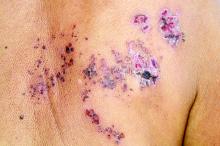
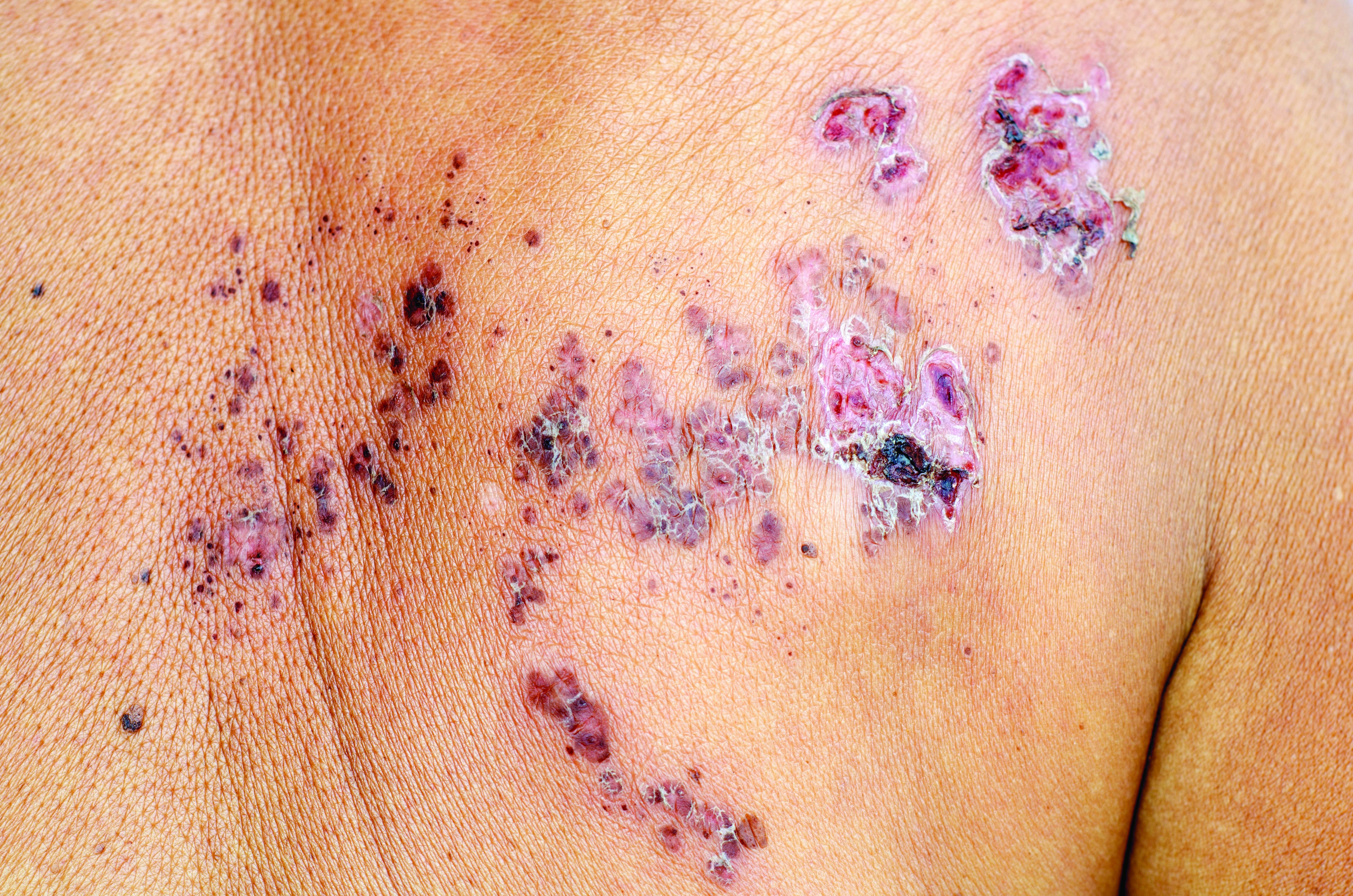
The most common morphology of DRESS syndrome is a morbilliform eruption (Figure 1), but urticarial and atypical targetoid (erythema multiforme–like) eruptions also have been described.9 Rarely, DRESS syndrome secondary to use of allopurinol or anticonvulsants may have a pustular morphology (Figure 2), which is distinguished from acute generalized exanthematous pustulosis by its delayed onset, more severe visceral involvement, and prolonged course.11
Another reported variant demonstrates overlapping features between Stevens-Johnson syndrome/toxic epidermal necrolysis and DRESS syndrome. It may present with mucositis, atypical targetoid lesions, and vesiculobullous lesions.12 It is unclear whether this reported variant is indeed a true subtype of DRESS syndrome, as Stevens-Johnson syndrome/toxic epidermal necrolysis may present with systemic symptoms, lymphadenopathy, hepatic, renal, and pulmonary complications, among other systemic disturbances.12
Pearl: Facial edema noted during physical examination is an important clue of DRESS syndrome.
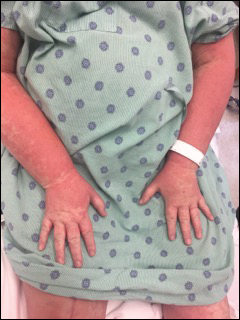
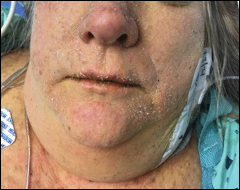
Perhaps the most helpful findings in the diagnosis of DRESS syndrome are facial edema and anasarca (Figure 3), as facial edema is not a usual finding in sepsis. Facial edema can be severe enough that the patient’s features are dramatically altered. It may be useful to ask family members if the patient’s face appears swollen or to compare the current appearance to the patient’s driver’s license photograph. An important complication to note is laryngeal edema, which may complicate airway management and may manifest as respiratory distress, stridor, and the need for emergent intubation.13
Myth: Patients who have had an allergic reaction to sulfonamide antibiotics will have a cross-reaction to nonantibiotic sulfonamides.
A common question is, if a patient has had a prior allergy to sulfonamide antibiotics, then are nonantibiotic sulfones such as a sulfonylurea, thiazide diuretic, or furosemide likely to cause a a cross-reaction? In one study (N=969), only 9.9% of patients with a prior sulfone antibiotic allergy developed hypersensitivity when exposed to a nonantibiotic sulfone, which is thought to be due to an overall increased propensity for hypersensitivity rather than a true cross-reaction. In fact, the risk for developing a hypersensitivity reaction to penicillin (14.0% [717/5115]) was higher than the risk for developing a reaction from a nonantibiotic sulfone among these patients.14 This study bolsters the argument that if there are other potential culprit medications and the time course for a patient’s nonantibiotic sulfone is not consistent with the timeline for DRESS syndrome, it may be beneficial to look for a different causative agent.
Pearl: Vancomycin is an important cause of DRESS syndrome.
Guidelines for treating endocarditis and osteomyelitis caused by methicillin-resistant Staphylococcus aureus infection recommend intravenous vancomycin for 4 to 6 weeks.15 This duration is within the relevant time frame of exposure for the development of DRESS syndrome de novo.
One case series noted that 37.5% (12/32) of DRESS syndrome cases in a 3-year period were caused by vancomycin, which notably was the most common medication associated with DRESS syndrome.16 There were caveats to this case series in that no standardized drug causality score was used and the sample size over the 3-year period was small; however, the increased use (and misuse) of antibiotics and perhaps increased recognition of rash in outpatient parenteral antibiotic therapy clinics may play a role if vancomycin-induced DRESS syndrome is indeed becoming more common.
Myth: Myocarditis secondary to DRESS syndrome will present with chest pain at the time of the cutaneous eruption.
Few patients with DRESS syndrome–associated myocarditis actually are symptomatic during their hospitalization.4 In asymptomatic patients, the primary team and consultants should be vigilant for the potential of subclinical myocarditis or the possibility of developing cardiac involvement after discharge, as myocarditis secondary to DRESS syndrome may present any time from rash onset up to 4 months later.4 Therefore, DRESS patients should be especially attentive to any new cardiac symptoms and notify their provider if any develop.
Although no standard cardiac screening guidelines exist for DRESS syndrome, some have recommended that baseline cardiac screening tests including electrocardiogram, troponin levels, and echocardiogram be considered at the time of diagnosis.5 If any testing is abnormal, DRESS syndrome–associated myocarditis should be suspected and an endomyocardial biopsy, which is the diagnostic gold standard, may be necessary.4 If the cardiac screening tests are normal, some investigators recommend serial outpatient echocardiograms for all DRESS patients, even those who remain asymptomatic.17 An alternative is an empiric approach in which a thorough review of systems is performed and testing is done if patients develop symptoms that are concerning for myocarditis.
Pearl: Steroids are not the only treatment used to control DRESS syndrome.
A prolonged taper of systemic steroids is the first-line treatment of DRESS syndrome. Steroids at the equivalent of 1 to 2 mg/kg daily (once or divided into 2 doses) of prednisone typically are used. For severe and/or recalcitrant DRESS syndrome, 2 mg/kg daily (once or divided into 2 doses) typically is used, and less than 1 mg/kg daily may be used for mini-DRESS syndrome.
Clinical improvement of DRESS syndrome has been demonstrated in several case reports with intravenous immunoglobulin, cyclosporine, cyclophosphamide, mycophenolate mofetil, and plasmapheresis.18-21 Each of these therapies typically were initiated as second-line therapeutic agents when initial treatment with steroids failed. It is important to note that large prospective studies regarding these treatments are lacking; however, there have been case reports of acute necrotizing eosinophilic myocarditis that did not respond to the combination of steroids and cyclosporine.4,22
Although there have been successful case reports using intravenous immunoglobulin, a 2012 prospective open-label clinical trial reported notable side effects in 5 of 6 (83.3%) patients with only 1 of 6 (16.6%) achieving the primary end point of control of fever/symptoms at day 7 and clinical remission without steroids on day 30.23
Pearl: DRESS patients need to be monitored for long-term sequelae such as autoimmune disease.
Several autoimmune conditions may develop as a delayed complication of DRESS syndrome, including autoimmune thyroiditis, systemic lupus erythematosus, type 1 diabetes mellitus, and autoimmune hemolytic anemia.24-26 Incidence rates of autoimmunity following DRESS syndrome range from 3% to 5% among small case series.24,25
Autoimmune thyroiditis, which may present as Graves disease, Hashimoto thyroiditis, or painless thyroiditis, is the most common autoimmune disorder to develop in DRESS patients and appears from several weeks to up to 3 years after DRESS.24 Therefore, all DRESS patients should be monitored longitudinally for several years for signs or symptoms suggestive of an autoimmune condition.5,24,26
Because no guidelines exist regarding serial monitoring for autoimmune sequelae, it may be reasonable to check thyroid function tests at the time of diagnosis and regularly for at least 2 years after diagnosis.5 Alternatively, clinicians may consider an empiric approach to laboratory testing that is guided by the development of clinical symptoms.
Pearl: Small cases series suggest differences between adult and pediatric DRESS syndrome, but there are no large studies in children.
Small case series have suggested there may be noteworthy differences between DRESS syndrome in adults and children. Although human herpesvirus 6 (HHV-6) positivity in DRESS syndrome in adults may be as high as 80%, 13% of pediatric patients in one cohort tested positive for HHV-6, though the study size was limited at 29 total patients.27 In children, DRESS syndrome secondary to antibiotics was associated with a shorter latency time as compared to cases secondary to nonantibiotics. In contrast to the typical 2- to 6-week timeline, Sasidharanpillai et al28 reported an average onset 5.8 days after drug administration in antibiotic-associated DRESS syndrome compared to 23.9 days for anticonvulsants, though this study only included 11 total patients. Other reports have suggested a similar trend.27
The role of HHV-6 positivity in pediatric DRESS syndrome and its influence on prognosis remains unclear. One study showed a worse prognosis for pediatric patients with positive HHV-6 antibodies.27 However, with such a small sample size—only 4 HHV-6–positive patients of 29 pediatric DRESS cases—larger studies are needed to better characterize the relationship between HHV-6 positivity and prognosis.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med, 2011;124:588-597.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
- Intarasupht J, Kanchanomai A, Leelasattakul W, et al. Prevalence, risk factors, and mortality outcome in the drug reaction with eosinophilia and systemic symptoms patients with cardiac involvement. Int J Dermatol. 2018;57:1187-1191.
- Bourgeois GP, Cafardi JA, Groysman V, et al. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66:E229-E236.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-693.e14; quiz 706-708.
- Lee T, Lee YS, Yoon SY, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69:407-415.
- Lin IC, Yang HC, Strong C, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-991.
- Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
- Walsh S, Diaz-Cano S, Higgins E, et al. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? a review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391-401.
- Raghavan R, Eknoyan G. Acute interstitial nephritis—a reappraisal and update. Clin Nephrol. 2014;82:149-162.
- Matsuda H, Saito K, Takayanagi Y, et al. Pustular-type drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms due to carbamazepine with systemic muscle involvement. J Dermatol. 2013;40:118-122.
- Wolf R, Davidovici B, Matz H, et al. Drug rash with eosinophilia and systemic symptoms versus Stevens-Johnson Syndrome—a case that indicates a stumbling block in the current classification. Int Arch Allergy Immunol. 2006;141:308-310.
- Kumar A, Goldfarb JW, Bittner EA. A case of drug rash with eosinophilia and systemic symptoms (DRESS) syndrome complicating airway management. Can J Anaesth. 2012;59:295-298.
- Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349:1628-1635.
- Berbari EF, Kanj SS, Kowalski TJ, et al; Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:E26-E46.
- Lam BD, Miller MM, Sutton AV, et al. Vancomycin and DRESS: a retrospective chart review of 32 cases in Los Angeles, California. J Am Acad Dermatol. 2017;77:973-975.
- Eppenberger M, Hack D, Ammann P, et al. Acute eosinophilic myocarditis with dramatic response to steroid therapy: the central role of echocardiography in diagnosis and follow-up. Tex Heart Inst J. 2013;40:326-330.
- Kirchhof MG, Wong A, Dutz JP. Cyclosporine treatment of drug-induced hypersensitivity syndrome. JAMA Dermatol. 2016;152:1254-1257.
- Singer EM, Wanat KA, Rosenbach MA. A case of recalcitrant DRESS syndrome with multiple autoimmune sequelae treated with intravenous immunoglobulins. JAMA Dermatol. 2013;149:494-495.
- Bommersbach TJ, Lapid MI, Leung JG, et al. Management of psychotropic drug-induced DRESS syndrome: a systematic review. Mayo Clin Proc. 2016;91:787-801.
- Alexander T, Iglesia E, Park Y, et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics. 2013;131:E945-E949.
- Daoulah A, Alqahtani AA, Ocheltree SR, et al. Acute myocardial infarction in a 56-year-old female patient treated with sulfasalazine. Am J Emerg Med. 2012;30:638.e1-638.e3.
- Joly P, Janela B, Tetart F, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol. 2012;148:543-544.
- Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Matta JM, Flores SM, Cherit JD. Drug reaction with eosinophilia and systemic symptoms (DRESS) and its relation with autoimmunity in a reference center in Mexico. An Bras Dermatol. 2017;92:30-33.
- Ahluwalia J, Abuabara K, Perman MJ, et al. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172:1090-1095.
- Sasidharanpillai S, Sabitha S, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms in children: a prospective study. Pediatr Dermatol. 2016;33:E162-E165.
Drug rash with eosinophilia and systemic symptoms (DRESS syndrome), also known as drug-induced hypersensitivity syndrome, is an uncommon severe systemic hypersensitivity drug reaction. It is estimated to occur in 1 in every 1000 to 10,000 drug exposures.1 It can affect patients of all ages and typically presents 2 to 6 weeks after exposure to a culprit medication. Classically, DRESS syndrome presents with often widespread rash, facial edema, systemic symptoms such as fever, lymphadenopathy, and evidence of visceral organ involvement. Peripheral blood eosinophilia is frequently but not universally observed.1,2
Even with proper management, reported DRESS syndrome mortality rates worldwide are approximately 10%2 or higher depending on the degree and type of other organ involvement (eg, cardiac).3 Beyond the acute manifestations of DRESS syndrome, this condition is unique in that some patients develop late-onset sequelae such as myocarditis or autoimmune conditions even years after the initial cutaneous eruption.4 Therefore, longitudinal evaluation is a key component of management.
The clinical myths and pearls presented here highlight some of the commonly held assumptions regarding DRESS syndrome in an effort to illuminate subtleties of managing patients with this condition (Table).
Myth: DRESS syndrome may only be diagnosed when the clinical criteria satisfy one of the established scoring systems.
Patients with DRESS syndrome can have heterogeneous manifestations. As a result, patients may develop a drug hypersensitivity with biological behavior and a natural history compatible with DRESS syndrome that does not fulfill published diagnostic criteria.5 The syndrome also may reveal its component manifestations gradually, thus delaying the diagnosis. The terms mini-DRESS and skirt syndrome have been employed to describe drug eruptions that clearly have systemic symptoms and more complex and pernicious biologic behavior than a simple drug exanthema but do not meet DRESS syndrome criteria. Ultimately, it is important to note that in clinical practice, DRESS syndrome exists on a spectrum of severity and the diagnosis remains a clinical one.
Pearl: The most commonly involved organ in DRESS syndrome is the liver.
Liver involvement is the most common visceral organ involved in DRESS syndrome and is estimated to occur in approximately 45.0% to 86.1% of cases.6,7 If a patient develops the characteristic rash, peripheral blood eosinophilia, and evidence of liver injury, DRESS syndrome must be included in the differential diagnosis.
Hepatitis presenting in DRESS syndrome can be hepatocellular, cholestatic, or mixed.6,7 Case series are varied in whether the transaminitis of DRESS syndrome tends to be more hepatocellular8 or cholestatic.7 Liver dysfunction in DRESS syndrome often lasts longer than in other severe cutaneous adverse drug reactions, and patients may improve anywhere from a few days in milder cases to months to achieve resolution of abnormalities.6,7 Severe hepatic involvement is thought to be the most notable cause of mortality.9
Pearl: New-onset proteinuria, hematuria, and sterile pyuria indicate acute interstitial nephritis that may be associated with DRESS syndrome.
Acute interstitial nephritis (AIN) is a drug-induced form of acute kidney injury that can co-occur with DRESS syndrome. Acute interstitial nephritis can present with some combination of acute kidney injury, morbilliform eruption, eosinophilia, fever, and sometimes eosinophiluria. Although AIN can be distinct from DRESS syndrome, there are cases of DRESS syndrome associated with AIN.10 In the correct clinical context, urinalysis may help by showing new-onset proteinuria, new-onset hematuria, and sterile pyuria. More common causes of acute kidney injury such as prerenal etiologies and acute tubular necrosis have a bland urinary sediment.
Myth: If the eruption is not morbilliform, then it is not DRESS syndrome.
The most common morphology of DRESS syndrome is a morbilliform eruption (Figure 1), but urticarial and atypical targetoid (erythema multiforme–like) eruptions also have been described.9 Rarely, DRESS syndrome secondary to use of allopurinol or anticonvulsants may have a pustular morphology (Figure 2), which is distinguished from acute generalized exanthematous pustulosis by its delayed onset, more severe visceral involvement, and prolonged course.11


Another reported variant demonstrates overlapping features between Stevens-Johnson syndrome/toxic epidermal necrolysis and DRESS syndrome. It may present with mucositis, atypical targetoid lesions, and vesiculobullous lesions.12 It is unclear whether this reported variant is indeed a true subtype of DRESS syndrome, as Stevens-Johnson syndrome/toxic epidermal necrolysis may present with systemic symptoms, lymphadenopathy, hepatic, renal, and pulmonary complications, among other systemic disturbances.12
Pearl: Facial edema noted during physical examination is an important clue of DRESS syndrome.
Perhaps the most helpful findings in the diagnosis of DRESS syndrome are facial edema and anasarca (Figure 3), as facial edema is not a usual finding in sepsis. Facial edema can be severe enough that the patient’s features are dramatically altered. It may be useful to ask family members if the patient’s face appears swollen or to compare the current appearance to the patient’s driver’s license photograph. An important complication to note is laryngeal edema, which may complicate airway management and may manifest as respiratory distress, stridor, and the need for emergent intubation.13

Myth: Patients who have had an allergic reaction to sulfonamide antibiotics will have a cross-reaction to nonantibiotic sulfonamides.
A common question is, if a patient has had a prior allergy to sulfonamide antibiotics, then are nonantibiotic sulfones such as a sulfonylurea, thiazide diuretic, or furosemide likely to cause a a cross-reaction? In one study (N=969), only 9.9% of patients with a prior sulfone antibiotic allergy developed hypersensitivity when exposed to a nonantibiotic sulfone, which is thought to be due to an overall increased propensity for hypersensitivity rather than a true cross-reaction. In fact, the risk for developing a hypersensitivity reaction to penicillin (14.0% [717/5115]) was higher than the risk for developing a reaction from a nonantibiotic sulfone among these patients.14 This study bolsters the argument that if there are other potential culprit medications and the time course for a patient’s nonantibiotic sulfone is not consistent with the timeline for DRESS syndrome, it may be beneficial to look for a different causative agent.
Pearl: Vancomycin is an important cause of DRESS syndrome.
Guidelines for treating endocarditis and osteomyelitis caused by methicillin-resistant Staphylococcus aureus infection recommend intravenous vancomycin for 4 to 6 weeks.15 This duration is within the relevant time frame of exposure for the development of DRESS syndrome de novo.
One case series noted that 37.5% (12/32) of DRESS syndrome cases in a 3-year period were caused by vancomycin, which notably was the most common medication associated with DRESS syndrome.16 There were caveats to this case series in that no standardized drug causality score was used and the sample size over the 3-year period was small; however, the increased use (and misuse) of antibiotics and perhaps increased recognition of rash in outpatient parenteral antibiotic therapy clinics may play a role if vancomycin-induced DRESS syndrome is indeed becoming more common.
Myth: Myocarditis secondary to DRESS syndrome will present with chest pain at the time of the cutaneous eruption.
Few patients with DRESS syndrome–associated myocarditis actually are symptomatic during their hospitalization.4 In asymptomatic patients, the primary team and consultants should be vigilant for the potential of subclinical myocarditis or the possibility of developing cardiac involvement after discharge, as myocarditis secondary to DRESS syndrome may present any time from rash onset up to 4 months later.4 Therefore, DRESS patients should be especially attentive to any new cardiac symptoms and notify their provider if any develop.
Although no standard cardiac screening guidelines exist for DRESS syndrome, some have recommended that baseline cardiac screening tests including electrocardiogram, troponin levels, and echocardiogram be considered at the time of diagnosis.5 If any testing is abnormal, DRESS syndrome–associated myocarditis should be suspected and an endomyocardial biopsy, which is the diagnostic gold standard, may be necessary.4 If the cardiac screening tests are normal, some investigators recommend serial outpatient echocardiograms for all DRESS patients, even those who remain asymptomatic.17 An alternative is an empiric approach in which a thorough review of systems is performed and testing is done if patients develop symptoms that are concerning for myocarditis.
Pearl: Steroids are not the only treatment used to control DRESS syndrome.
A prolonged taper of systemic steroids is the first-line treatment of DRESS syndrome. Steroids at the equivalent of 1 to 2 mg/kg daily (once or divided into 2 doses) of prednisone typically are used. For severe and/or recalcitrant DRESS syndrome, 2 mg/kg daily (once or divided into 2 doses) typically is used, and less than 1 mg/kg daily may be used for mini-DRESS syndrome.
Clinical improvement of DRESS syndrome has been demonstrated in several case reports with intravenous immunoglobulin, cyclosporine, cyclophosphamide, mycophenolate mofetil, and plasmapheresis.18-21 Each of these therapies typically were initiated as second-line therapeutic agents when initial treatment with steroids failed. It is important to note that large prospective studies regarding these treatments are lacking; however, there have been case reports of acute necrotizing eosinophilic myocarditis that did not respond to the combination of steroids and cyclosporine.4,22
Although there have been successful case reports using intravenous immunoglobulin, a 2012 prospective open-label clinical trial reported notable side effects in 5 of 6 (83.3%) patients with only 1 of 6 (16.6%) achieving the primary end point of control of fever/symptoms at day 7 and clinical remission without steroids on day 30.23
Pearl: DRESS patients need to be monitored for long-term sequelae such as autoimmune disease.
Several autoimmune conditions may develop as a delayed complication of DRESS syndrome, including autoimmune thyroiditis, systemic lupus erythematosus, type 1 diabetes mellitus, and autoimmune hemolytic anemia.24-26 Incidence rates of autoimmunity following DRESS syndrome range from 3% to 5% among small case series.24,25
Autoimmune thyroiditis, which may present as Graves disease, Hashimoto thyroiditis, or painless thyroiditis, is the most common autoimmune disorder to develop in DRESS patients and appears from several weeks to up to 3 years after DRESS.24 Therefore, all DRESS patients should be monitored longitudinally for several years for signs or symptoms suggestive of an autoimmune condition.5,24,26
Because no guidelines exist regarding serial monitoring for autoimmune sequelae, it may be reasonable to check thyroid function tests at the time of diagnosis and regularly for at least 2 years after diagnosis.5 Alternatively, clinicians may consider an empiric approach to laboratory testing that is guided by the development of clinical symptoms.
Pearl: Small cases series suggest differences between adult and pediatric DRESS syndrome, but there are no large studies in children.
Small case series have suggested there may be noteworthy differences between DRESS syndrome in adults and children. Although human herpesvirus 6 (HHV-6) positivity in DRESS syndrome in adults may be as high as 80%, 13% of pediatric patients in one cohort tested positive for HHV-6, though the study size was limited at 29 total patients.27 In children, DRESS syndrome secondary to antibiotics was associated with a shorter latency time as compared to cases secondary to nonantibiotics. In contrast to the typical 2- to 6-week timeline, Sasidharanpillai et al28 reported an average onset 5.8 days after drug administration in antibiotic-associated DRESS syndrome compared to 23.9 days for anticonvulsants, though this study only included 11 total patients. Other reports have suggested a similar trend.27
The role of HHV-6 positivity in pediatric DRESS syndrome and its influence on prognosis remains unclear. One study showed a worse prognosis for pediatric patients with positive HHV-6 antibodies.27 However, with such a small sample size—only 4 HHV-6–positive patients of 29 pediatric DRESS cases—larger studies are needed to better characterize the relationship between HHV-6 positivity and prognosis.
Drug rash with eosinophilia and systemic symptoms (DRESS syndrome), also known as drug-induced hypersensitivity syndrome, is an uncommon severe systemic hypersensitivity drug reaction. It is estimated to occur in 1 in every 1000 to 10,000 drug exposures.1 It can affect patients of all ages and typically presents 2 to 6 weeks after exposure to a culprit medication. Classically, DRESS syndrome presents with often widespread rash, facial edema, systemic symptoms such as fever, lymphadenopathy, and evidence of visceral organ involvement. Peripheral blood eosinophilia is frequently but not universally observed.1,2
Even with proper management, reported DRESS syndrome mortality rates worldwide are approximately 10%2 or higher depending on the degree and type of other organ involvement (eg, cardiac).3 Beyond the acute manifestations of DRESS syndrome, this condition is unique in that some patients develop late-onset sequelae such as myocarditis or autoimmune conditions even years after the initial cutaneous eruption.4 Therefore, longitudinal evaluation is a key component of management.
The clinical myths and pearls presented here highlight some of the commonly held assumptions regarding DRESS syndrome in an effort to illuminate subtleties of managing patients with this condition (Table).
Myth: DRESS syndrome may only be diagnosed when the clinical criteria satisfy one of the established scoring systems.
Patients with DRESS syndrome can have heterogeneous manifestations. As a result, patients may develop a drug hypersensitivity with biological behavior and a natural history compatible with DRESS syndrome that does not fulfill published diagnostic criteria.5 The syndrome also may reveal its component manifestations gradually, thus delaying the diagnosis. The terms mini-DRESS and skirt syndrome have been employed to describe drug eruptions that clearly have systemic symptoms and more complex and pernicious biologic behavior than a simple drug exanthema but do not meet DRESS syndrome criteria. Ultimately, it is important to note that in clinical practice, DRESS syndrome exists on a spectrum of severity and the diagnosis remains a clinical one.
Pearl: The most commonly involved organ in DRESS syndrome is the liver.
Liver involvement is the most common visceral organ involved in DRESS syndrome and is estimated to occur in approximately 45.0% to 86.1% of cases.6,7 If a patient develops the characteristic rash, peripheral blood eosinophilia, and evidence of liver injury, DRESS syndrome must be included in the differential diagnosis.
Hepatitis presenting in DRESS syndrome can be hepatocellular, cholestatic, or mixed.6,7 Case series are varied in whether the transaminitis of DRESS syndrome tends to be more hepatocellular8 or cholestatic.7 Liver dysfunction in DRESS syndrome often lasts longer than in other severe cutaneous adverse drug reactions, and patients may improve anywhere from a few days in milder cases to months to achieve resolution of abnormalities.6,7 Severe hepatic involvement is thought to be the most notable cause of mortality.9
Pearl: New-onset proteinuria, hematuria, and sterile pyuria indicate acute interstitial nephritis that may be associated with DRESS syndrome.
Acute interstitial nephritis (AIN) is a drug-induced form of acute kidney injury that can co-occur with DRESS syndrome. Acute interstitial nephritis can present with some combination of acute kidney injury, morbilliform eruption, eosinophilia, fever, and sometimes eosinophiluria. Although AIN can be distinct from DRESS syndrome, there are cases of DRESS syndrome associated with AIN.10 In the correct clinical context, urinalysis may help by showing new-onset proteinuria, new-onset hematuria, and sterile pyuria. More common causes of acute kidney injury such as prerenal etiologies and acute tubular necrosis have a bland urinary sediment.
Myth: If the eruption is not morbilliform, then it is not DRESS syndrome.
The most common morphology of DRESS syndrome is a morbilliform eruption (Figure 1), but urticarial and atypical targetoid (erythema multiforme–like) eruptions also have been described.9 Rarely, DRESS syndrome secondary to use of allopurinol or anticonvulsants may have a pustular morphology (Figure 2), which is distinguished from acute generalized exanthematous pustulosis by its delayed onset, more severe visceral involvement, and prolonged course.11


Another reported variant demonstrates overlapping features between Stevens-Johnson syndrome/toxic epidermal necrolysis and DRESS syndrome. It may present with mucositis, atypical targetoid lesions, and vesiculobullous lesions.12 It is unclear whether this reported variant is indeed a true subtype of DRESS syndrome, as Stevens-Johnson syndrome/toxic epidermal necrolysis may present with systemic symptoms, lymphadenopathy, hepatic, renal, and pulmonary complications, among other systemic disturbances.12
Pearl: Facial edema noted during physical examination is an important clue of DRESS syndrome.
Perhaps the most helpful findings in the diagnosis of DRESS syndrome are facial edema and anasarca (Figure 3), as facial edema is not a usual finding in sepsis. Facial edema can be severe enough that the patient’s features are dramatically altered. It may be useful to ask family members if the patient’s face appears swollen or to compare the current appearance to the patient’s driver’s license photograph. An important complication to note is laryngeal edema, which may complicate airway management and may manifest as respiratory distress, stridor, and the need for emergent intubation.13

Myth: Patients who have had an allergic reaction to sulfonamide antibiotics will have a cross-reaction to nonantibiotic sulfonamides.
A common question is, if a patient has had a prior allergy to sulfonamide antibiotics, then are nonantibiotic sulfones such as a sulfonylurea, thiazide diuretic, or furosemide likely to cause a a cross-reaction? In one study (N=969), only 9.9% of patients with a prior sulfone antibiotic allergy developed hypersensitivity when exposed to a nonantibiotic sulfone, which is thought to be due to an overall increased propensity for hypersensitivity rather than a true cross-reaction. In fact, the risk for developing a hypersensitivity reaction to penicillin (14.0% [717/5115]) was higher than the risk for developing a reaction from a nonantibiotic sulfone among these patients.14 This study bolsters the argument that if there are other potential culprit medications and the time course for a patient’s nonantibiotic sulfone is not consistent with the timeline for DRESS syndrome, it may be beneficial to look for a different causative agent.
Pearl: Vancomycin is an important cause of DRESS syndrome.
Guidelines for treating endocarditis and osteomyelitis caused by methicillin-resistant Staphylococcus aureus infection recommend intravenous vancomycin for 4 to 6 weeks.15 This duration is within the relevant time frame of exposure for the development of DRESS syndrome de novo.
One case series noted that 37.5% (12/32) of DRESS syndrome cases in a 3-year period were caused by vancomycin, which notably was the most common medication associated with DRESS syndrome.16 There were caveats to this case series in that no standardized drug causality score was used and the sample size over the 3-year period was small; however, the increased use (and misuse) of antibiotics and perhaps increased recognition of rash in outpatient parenteral antibiotic therapy clinics may play a role if vancomycin-induced DRESS syndrome is indeed becoming more common.
Myth: Myocarditis secondary to DRESS syndrome will present with chest pain at the time of the cutaneous eruption.
Few patients with DRESS syndrome–associated myocarditis actually are symptomatic during their hospitalization.4 In asymptomatic patients, the primary team and consultants should be vigilant for the potential of subclinical myocarditis or the possibility of developing cardiac involvement after discharge, as myocarditis secondary to DRESS syndrome may present any time from rash onset up to 4 months later.4 Therefore, DRESS patients should be especially attentive to any new cardiac symptoms and notify their provider if any develop.
Although no standard cardiac screening guidelines exist for DRESS syndrome, some have recommended that baseline cardiac screening tests including electrocardiogram, troponin levels, and echocardiogram be considered at the time of diagnosis.5 If any testing is abnormal, DRESS syndrome–associated myocarditis should be suspected and an endomyocardial biopsy, which is the diagnostic gold standard, may be necessary.4 If the cardiac screening tests are normal, some investigators recommend serial outpatient echocardiograms for all DRESS patients, even those who remain asymptomatic.17 An alternative is an empiric approach in which a thorough review of systems is performed and testing is done if patients develop symptoms that are concerning for myocarditis.
Pearl: Steroids are not the only treatment used to control DRESS syndrome.
A prolonged taper of systemic steroids is the first-line treatment of DRESS syndrome. Steroids at the equivalent of 1 to 2 mg/kg daily (once or divided into 2 doses) of prednisone typically are used. For severe and/or recalcitrant DRESS syndrome, 2 mg/kg daily (once or divided into 2 doses) typically is used, and less than 1 mg/kg daily may be used for mini-DRESS syndrome.
Clinical improvement of DRESS syndrome has been demonstrated in several case reports with intravenous immunoglobulin, cyclosporine, cyclophosphamide, mycophenolate mofetil, and plasmapheresis.18-21 Each of these therapies typically were initiated as second-line therapeutic agents when initial treatment with steroids failed. It is important to note that large prospective studies regarding these treatments are lacking; however, there have been case reports of acute necrotizing eosinophilic myocarditis that did not respond to the combination of steroids and cyclosporine.4,22
Although there have been successful case reports using intravenous immunoglobulin, a 2012 prospective open-label clinical trial reported notable side effects in 5 of 6 (83.3%) patients with only 1 of 6 (16.6%) achieving the primary end point of control of fever/symptoms at day 7 and clinical remission without steroids on day 30.23
Pearl: DRESS patients need to be monitored for long-term sequelae such as autoimmune disease.
Several autoimmune conditions may develop as a delayed complication of DRESS syndrome, including autoimmune thyroiditis, systemic lupus erythematosus, type 1 diabetes mellitus, and autoimmune hemolytic anemia.24-26 Incidence rates of autoimmunity following DRESS syndrome range from 3% to 5% among small case series.24,25
Autoimmune thyroiditis, which may present as Graves disease, Hashimoto thyroiditis, or painless thyroiditis, is the most common autoimmune disorder to develop in DRESS patients and appears from several weeks to up to 3 years after DRESS.24 Therefore, all DRESS patients should be monitored longitudinally for several years for signs or symptoms suggestive of an autoimmune condition.5,24,26
Because no guidelines exist regarding serial monitoring for autoimmune sequelae, it may be reasonable to check thyroid function tests at the time of diagnosis and regularly for at least 2 years after diagnosis.5 Alternatively, clinicians may consider an empiric approach to laboratory testing that is guided by the development of clinical symptoms.
Pearl: Small cases series suggest differences between adult and pediatric DRESS syndrome, but there are no large studies in children.
Small case series have suggested there may be noteworthy differences between DRESS syndrome in adults and children. Although human herpesvirus 6 (HHV-6) positivity in DRESS syndrome in adults may be as high as 80%, 13% of pediatric patients in one cohort tested positive for HHV-6, though the study size was limited at 29 total patients.27 In children, DRESS syndrome secondary to antibiotics was associated with a shorter latency time as compared to cases secondary to nonantibiotics. In contrast to the typical 2- to 6-week timeline, Sasidharanpillai et al28 reported an average onset 5.8 days after drug administration in antibiotic-associated DRESS syndrome compared to 23.9 days for anticonvulsants, though this study only included 11 total patients. Other reports have suggested a similar trend.27
The role of HHV-6 positivity in pediatric DRESS syndrome and its influence on prognosis remains unclear. One study showed a worse prognosis for pediatric patients with positive HHV-6 antibodies.27 However, with such a small sample size—only 4 HHV-6–positive patients of 29 pediatric DRESS cases—larger studies are needed to better characterize the relationship between HHV-6 positivity and prognosis.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med, 2011;124:588-597.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
- Intarasupht J, Kanchanomai A, Leelasattakul W, et al. Prevalence, risk factors, and mortality outcome in the drug reaction with eosinophilia and systemic symptoms patients with cardiac involvement. Int J Dermatol. 2018;57:1187-1191.
- Bourgeois GP, Cafardi JA, Groysman V, et al. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66:E229-E236.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-693.e14; quiz 706-708.
- Lee T, Lee YS, Yoon SY, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69:407-415.
- Lin IC, Yang HC, Strong C, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-991.
- Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
- Walsh S, Diaz-Cano S, Higgins E, et al. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? a review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391-401.
- Raghavan R, Eknoyan G. Acute interstitial nephritis—a reappraisal and update. Clin Nephrol. 2014;82:149-162.
- Matsuda H, Saito K, Takayanagi Y, et al. Pustular-type drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms due to carbamazepine with systemic muscle involvement. J Dermatol. 2013;40:118-122.
- Wolf R, Davidovici B, Matz H, et al. Drug rash with eosinophilia and systemic symptoms versus Stevens-Johnson Syndrome—a case that indicates a stumbling block in the current classification. Int Arch Allergy Immunol. 2006;141:308-310.
- Kumar A, Goldfarb JW, Bittner EA. A case of drug rash with eosinophilia and systemic symptoms (DRESS) syndrome complicating airway management. Can J Anaesth. 2012;59:295-298.
- Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349:1628-1635.
- Berbari EF, Kanj SS, Kowalski TJ, et al; Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:E26-E46.
- Lam BD, Miller MM, Sutton AV, et al. Vancomycin and DRESS: a retrospective chart review of 32 cases in Los Angeles, California. J Am Acad Dermatol. 2017;77:973-975.
- Eppenberger M, Hack D, Ammann P, et al. Acute eosinophilic myocarditis with dramatic response to steroid therapy: the central role of echocardiography in diagnosis and follow-up. Tex Heart Inst J. 2013;40:326-330.
- Kirchhof MG, Wong A, Dutz JP. Cyclosporine treatment of drug-induced hypersensitivity syndrome. JAMA Dermatol. 2016;152:1254-1257.
- Singer EM, Wanat KA, Rosenbach MA. A case of recalcitrant DRESS syndrome with multiple autoimmune sequelae treated with intravenous immunoglobulins. JAMA Dermatol. 2013;149:494-495.
- Bommersbach TJ, Lapid MI, Leung JG, et al. Management of psychotropic drug-induced DRESS syndrome: a systematic review. Mayo Clin Proc. 2016;91:787-801.
- Alexander T, Iglesia E, Park Y, et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics. 2013;131:E945-E949.
- Daoulah A, Alqahtani AA, Ocheltree SR, et al. Acute myocardial infarction in a 56-year-old female patient treated with sulfasalazine. Am J Emerg Med. 2012;30:638.e1-638.e3.
- Joly P, Janela B, Tetart F, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol. 2012;148:543-544.
- Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Matta JM, Flores SM, Cherit JD. Drug reaction with eosinophilia and systemic symptoms (DRESS) and its relation with autoimmunity in a reference center in Mexico. An Bras Dermatol. 2017;92:30-33.
- Ahluwalia J, Abuabara K, Perman MJ, et al. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172:1090-1095.
- Sasidharanpillai S, Sabitha S, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms in children: a prospective study. Pediatr Dermatol. 2016;33:E162-E165.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med, 2011;124:588-597.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
- Intarasupht J, Kanchanomai A, Leelasattakul W, et al. Prevalence, risk factors, and mortality outcome in the drug reaction with eosinophilia and systemic symptoms patients with cardiac involvement. Int J Dermatol. 2018;57:1187-1191.
- Bourgeois GP, Cafardi JA, Groysman V, et al. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66:E229-E236.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-693.e14; quiz 706-708.
- Lee T, Lee YS, Yoon SY, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69:407-415.
- Lin IC, Yang HC, Strong C, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-991.
- Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
- Walsh S, Diaz-Cano S, Higgins E, et al. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? a review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391-401.
- Raghavan R, Eknoyan G. Acute interstitial nephritis—a reappraisal and update. Clin Nephrol. 2014;82:149-162.
- Matsuda H, Saito K, Takayanagi Y, et al. Pustular-type drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms due to carbamazepine with systemic muscle involvement. J Dermatol. 2013;40:118-122.
- Wolf R, Davidovici B, Matz H, et al. Drug rash with eosinophilia and systemic symptoms versus Stevens-Johnson Syndrome—a case that indicates a stumbling block in the current classification. Int Arch Allergy Immunol. 2006;141:308-310.
- Kumar A, Goldfarb JW, Bittner EA. A case of drug rash with eosinophilia and systemic symptoms (DRESS) syndrome complicating airway management. Can J Anaesth. 2012;59:295-298.
- Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349:1628-1635.
- Berbari EF, Kanj SS, Kowalski TJ, et al; Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:E26-E46.
- Lam BD, Miller MM, Sutton AV, et al. Vancomycin and DRESS: a retrospective chart review of 32 cases in Los Angeles, California. J Am Acad Dermatol. 2017;77:973-975.
- Eppenberger M, Hack D, Ammann P, et al. Acute eosinophilic myocarditis with dramatic response to steroid therapy: the central role of echocardiography in diagnosis and follow-up. Tex Heart Inst J. 2013;40:326-330.
- Kirchhof MG, Wong A, Dutz JP. Cyclosporine treatment of drug-induced hypersensitivity syndrome. JAMA Dermatol. 2016;152:1254-1257.
- Singer EM, Wanat KA, Rosenbach MA. A case of recalcitrant DRESS syndrome with multiple autoimmune sequelae treated with intravenous immunoglobulins. JAMA Dermatol. 2013;149:494-495.
- Bommersbach TJ, Lapid MI, Leung JG, et al. Management of psychotropic drug-induced DRESS syndrome: a systematic review. Mayo Clin Proc. 2016;91:787-801.
- Alexander T, Iglesia E, Park Y, et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics. 2013;131:E945-E949.
- Daoulah A, Alqahtani AA, Ocheltree SR, et al. Acute myocardial infarction in a 56-year-old female patient treated with sulfasalazine. Am J Emerg Med. 2012;30:638.e1-638.e3.
- Joly P, Janela B, Tetart F, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol. 2012;148:543-544.
- Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Matta JM, Flores SM, Cherit JD. Drug reaction with eosinophilia and systemic symptoms (DRESS) and its relation with autoimmunity in a reference center in Mexico. An Bras Dermatol. 2017;92:30-33.
- Ahluwalia J, Abuabara K, Perman MJ, et al. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172:1090-1095.
- Sasidharanpillai S, Sabitha S, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms in children: a prospective study. Pediatr Dermatol. 2016;33:E162-E165.
Practice Points
- Drug rash with eosinophilia and systemic symptoms (DRESS syndrome) is a clinical diagnosis, and incomplete forms may not meet formal criteria-based diagnosis.
- Although DRESS syndrome typically has a morbilliform eruption, different rash morphologies may be observed.
- The myocarditis of DRESS syndrome may not present with chest pain; a high index of suspicion is warranted.
- Autoimmune sequelae are more frequent in patients who have had an episode of DRESS syndrome.
2018 Update on minimally invasive gynecologic surgery
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel
Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.
Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

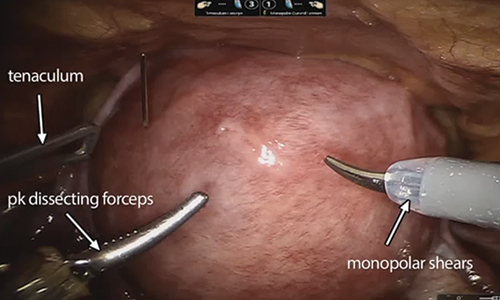
Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

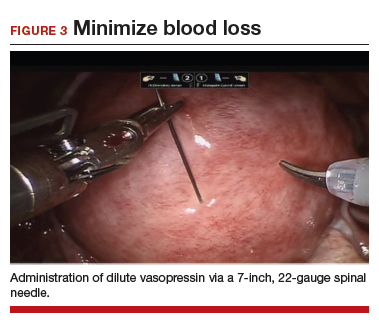
To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28
Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.
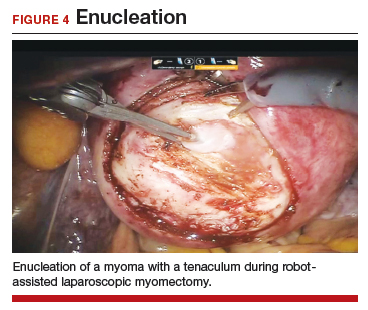
As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
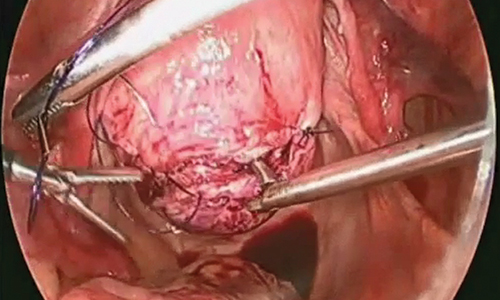
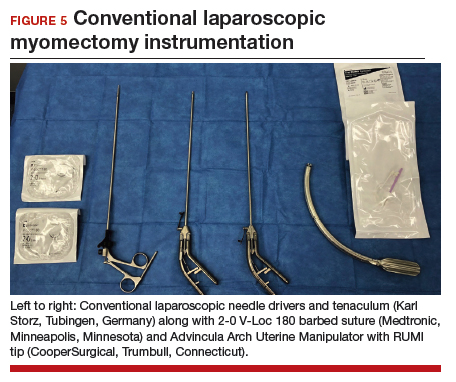
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.
Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Uterine fibroids are the most common solid pelvic tumor in women and a leading indication for hysterectomy in the United States.1 As a result, they represent significant morbidity for many women and are a major public health problem. By age 50, 70% of white women and 80% of black women have fibroids.2
Although fibroids are sometimes asymptomatic, the symptoms most commonly reported are abnormal uterine bleeding (AUB) with resultant anemia and bulk/pressure symptoms. Uterine fibroids also are associated with reproductive dysfunction, such as recurrent pregnancy loss, and even infertility.3
The clinical diagnosis of uterine fibroids is made based on a combination of physical examination and imaging studies, including pelvic ultrasonography, saline infusion sonography, and magnetic resonance imaging (MRI). When medical management, such as combination oral contraceptive pills, fails in patients with AUB and/or bulk predominant symptoms or patients present with compromised fertility, the only option for conservative surgical management is a myomectomy.4
The route of myomectomy—hysteroscopy, laparotomy, conventional laparoscopic myomectomy (LM), or robot-assisted laparoscopic myomectomy (RALM)—depends on the size, number, location, and consistency of the uterine fibroids and, to a certain extent, the indication for the myomectomy. In some cases, multiple routes must be used to achieve optimal results, and sometimes these procedures have to be staged. In this literature review and technical summary, we focus on conventional LM and RALM approaches.
Literature review: In the right hands, LM and RALM have clear benefits
In the past, laparotomy was the surgical route of choice for fibroid removal. This surgery was associated with a long hospital stay, a high rate of blood transfusions, postoperative pain, and a lengthy recovery period. As minimally invasive surgery gained popularity, conventional LM became more commonly performed and was accepted by many as the gold standard approach for myomectomy.5
LM has considerable advantages over laparotomy
Compared with the traditional, more invasive route, the conventional LM approach has many benefits. These include less blood loss, decreased postoperative pain, shorter recovery time, shorter hospitalization stay, and decreased perioperative complications.6 LM should be considered the first-line approach unless the size of an intramural myoma exceeds 10 to 12 cm or multiple myomas (consensus, approximately 4 or more) are present and necessitate several incisions according to their varying locations within the uterus.7,8 While this is a recommendation, reports have been published on the successful laparoscopic approach to myomas larger than 20 cm, demonstrating that a skilled, experienced surgeon can perform this procedure safely.9-11
Many studies comparing LM with the abdominal approach showed that LM is associated with decreased blood loss, less postoperative pain, shorter hospital stay, and quicker recovery.12-14 Unfortunately, myomectomy via conventional laparoscopy can be technically challenging, thereby limiting patient accessibility to this approach. Major challenges with conventional LM include enucleation of the fibroid along the correct plane and a multilayered hysterotomy closure.15 The obvious concern with the latter is the potential risk for uterine rupture when improperly performed as a result of deficient suturing skills. Accordingly, several cases of uterine rupture in the second and third trimester of pregnancy after LM led to recommendations for stricter selection criteria, which excluded patients with fibroids larger than 5 cm, multiple fibroids, and deep intramural fibroids.16
Continue to: The RALM approach
The RALM approach
RALM was developed as a surgical alternative and to help overcome conventional laparoscopy challenges, such as suturing, as well as to offer minimally invasive options to a broader patient pool. In 2004, Advincula and colleagues reported the first case series of 35 women who underwent RALM.17 Since that report was published, multiple retrospective studies have confirmed RALM’s safety, feasibility, and efficacy.
How RALM stacks up against laparotomy. Compared with traditional abdominal myomectomy (AM), RALM has been associated with less blood loss, shorter hospital stay, quicker recovery time, fewer complications, and higher costs.18 In a comparative analysis of surgical outcomes and costs of RALM versus AM, Nash and colleagues found that RALM patients required less intravenous narcotics, had shorter hospital stays, and had equivalent clinical outcomes compared with AM-treated patients.19 In addition, the authors observed a correlation between increased specimen size and decreased operative efficiency with RALM. Retrospective cohort studies by Mansour and colleagues and Sangha and colleagues echoed similar conclusions.20,21
RALM versus conventional LM. The comparisons between conventional LM and RALM are not as clear-cut, and although evidence strongly suggests a role for RALM, more comparative studies are needed.
In 2013, Pundir and colleagues completed a meta-analysis and systematic review comparing RALM with AM and LM.22 They reviewed 10 observational studies; 7 compared RALM with AM, 4 compared RALM with LM, and 1 study compared RALM with AM and LM (this was included in both groups). In the comparison between RALM and AM, estimated blood loss, blood transfusion, and length of hospital stay were significantly lower with RALM, risk of complication was similar, and operating time and costs were significantly higher. The cost findings were not too dissimilar to conclusions drawn by Advincula and colleagues in an earlier study.18
Further, when Pundir and colleagues compared RALM with LM, blood transfusion risk and costs were higher with RALM, but no significant differences were noted in estimated blood loss, operating time, length of hospital stay, and complications.22 In this analysis, RALM showed significant short-term benefits when compared with AM but no benefit when compared with LM.
Continue to: Benefits after RALM over time
Benefits after RALM over time
Long-term benefits from RALM, such as symptom recurrence rates and fertility outcomes, have been demonstrated. In 2015, Pitter and colleagues published the first paper on symptom recurrence after RALM.23 In this retrospective survey, 426 women underwent RALM for symptom relief or infertility across 3 practice sites; 62.9% reported being symptom free after 3 years. In addition, 80% of symptom-free women who had undergone RALM to improve fertility outcomes conceived after 3 years. The mean (SD) time to pregnancy was 7.9 (9.4) months. Overall, pregnancy rates improved and symptom recurrence increased with the interval of time since surgery.23
In another study, Pitter and colleagues reported on pregnancy outcomes in greater detail.24 They evaluated 872 women who underwent RALM between October 2005 and November 2010 at 3 centers. Of these women, 107 conceived, resulting in 127 pregnancies and 92 deliveries through 2011. The means (SD) for age at myomectomy, number of myomas removed, and myoma size were 34.8 (4.5) years, 3.9 (3.2), and 7.5 (3.0) cm (weight, 191.7 [144.8] g), respectively. Overall, the pregnancy outcomes in this study were comparable to those reported in the literature for conventional LM.
Cela and colleagues reported similar outcomes based on their review of 48 patients who underwent RALM between 2007 and 2011.25 Seven women became pregnant (8 pregnancies). There were no spontaneous abortions or uterine ruptures. Following suit, Kang and colleagues reported outcomes in 100 women who underwent RALM for deep intramural fibroids (FIGO type 2 to 5).26 The average (SD) number of fibroids was 3.8 (3.5) with a mean (SD) size of 7.5 (2.1) cm. All patients recovered without major complications, and 75% of those pursuing pregnancy conceived.
The importance of LM and RALM
After this brief review of the data on conventional LM and RALM, it is fair to conclude that both surgical options are a game changer for the minimally invasive management of uterine fibroids. Despite strong evidence that suggests laparoscopy is superior to laparotomy for myomectomy, the technical demands required for performing conventional LM may explain why it is underutilized and why the advantages of robotic surgery—with its 3-dimensional imaging and articulated instruments—make this approach an attractive alternative.
The myomectomy technique we prefer at our institution
At our medical center, we approach the majority of abdominal myomectomies via conventional LM or RALM. We carefully select candidates with the goal of ensuring a successful procedure and minimizing the risk of conversion. When selecting candidates, we consider these factors:
- size, number, location, and consistency of the fibroids
- patient’s body habitus, and
- relative size of the uterus to the length of the patient’s torso.
Additionally, any concerns raised during the preoperative workup regarding a suspected risk of occult leiomyosarcoma preclude a minimally invasive approach. Otherwise, deciding between
conventional LM and RALM is based on surgeon preference.
View these surgical techniques on the multimedia channel

Robot-assisted laparoscopic myomectomy
Arnold P. Advincula, MD, Victoria M. Fratto, MD, and Caroline Key
A systematic approach to surgery in a 39-year-old woman with heavy menstrual bleeding who desires future fertility. Features include robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair and demonstration of the ExCITE technique for tissue extraction.

Laparoscopic myomectomy technique
William H. Parker, MD
A step-by-step demonstration of the laparoscopic myomectomy technique used to resect a 7-cm posterior fibroid in a 44-year-old woman.
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, MPH
A surgical case of a 41-yearold woman with radiating lower abdominal pain and menorrhagia who desired removal of symptomatic myomas. Preoperative transvaginal ultrasonography revealed a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma.
Continue to: Preoperative MRI guides surgical approach
Preoperative MRI guides surgical approach
An MRI scan is a critical component of the patient’s preoperative evaluation. It helps to define the uterine architecture as it relates to fibroids and to rule out the presence of adenomyosis. In general, we do not offer RALM to patients who have more than 15 myomas, a single myoma that is larger than 12 to 15 cm, or when the uterus is more than 2 fingerbreadths above the umbilicus (unless the patient’s torso allows for an adequate insufflated workspace). We also try to avoid preoperative treatment with a gonadotropin–releasing hormone agonist to minimize softening of the myoma and blurring of the dissection planes.

Steps in the procedure

Once the patient is intubated, properly positioned, prepped, and draped, we turn our attention toward peritoneal entry. Factors that influence entry include the patient’s surgical history, radiologic imaging, physical examination (particularly the exam under anesthesia), and surgeon preference for optimizing access. Quite often we use a left upper quadrant entry via Palmer’s point, with subsequent port placement individualized to the patient’s pathology and abdominal topography. Three or more incisions are required to accommodate the camera and at least 2 to 3 operative instruments. Port sizes vary from 5 to 12 mm depending on the desired equipment and surgeon preference (conventional LM versus RALM [FIGURE 1]).
A uterine manipulator is a crucial tool used when performing LM.27 This instrument enables elevation of the uterus to allow for adequate visualization of the targeted myomas, traction-countertraction during enucleation, and strategic positioning during hysterotomy repair. We also use a bedside-mounted electric uterine positioning system that provides static orientation of the uterus by interfacing with the uterine manipulator, thereby obviating the need for a bedside assistant to provide that service (FIGURE 2).

To minimize blood loss during the course of the myomectomy, we inject a dilute concentration of vasopressin (20 U in 50 mL of saline) via a 7-inch, 22-gauge spinal needle into the myometrium surrounding the targeted myomas (FIGURE 3). Additional methods for mitigating blood loss include the use of vascular clamps, clips, or ties (both permanent and temporary) on the bilateral uterine arteries; intravaginal prostaglandins; intravenous tranexamic acid; gelatin-thrombin matrices; and cell salvage systems.28

Once we observe adequate myometrial blanching from the vasopressin administration, we make a strategic hysterotomy incision (preferably transverse) to allow the surgeon to more ergonomically close the defect. We then identify the pseudocapsule so that the surgeon can circumferentially enucleate the myoma and dissect it from its fibrous attachments to the surrounding myometrium.
Continue to: The energy devices used to perform the hysterotomy...
The energy devices used to perform the hysterotomy and enucleation are selected largely based on surgeon preference, but various instruments can be used to accomplish these steps, including an ultrasonically activated scalpel or such electrosurgical instruments as monopolar scissors or hooks.
A reliable tenaculum is critical to the success of any enucleation, whether the approach is conventional LM or RALM, in order to provide adequate traction on the myoma (FIGURE 4). We try to minimize the number of hysterotomy incisions not only to reduce further blood loss, as the majority of bleeding ensues from the surrounding myometrium, but also to minimize compromise of myometrial integrity. Additionally, we take care to avoid entry into the endometrial cavity.

As we enucleate a myoma, we place it in either the anterior or posterior cul de sac. Most important, if we enucleate multiple myomas, we keep careful track of their number. We string the myomas together with suture until we extract them to ensure this.
While hysterotomy closure can be performed with either barbed or nonbarbed sutures in a single- or a multi-layered fashion, we prefer to use a barbed suture.29,30 Just as enucleation requires appropriate instruments, suturing requires proper needle drivers (FIGURE 5). We advise judicious use of energy to minimize thermal effects and maintain the viability of the surrounding myometrium. Once we have sutured the myometrium closed, we place an adhesion barrier.

Although discussion of tissue extraction is beyond the scope of this Update, any surgeon embarking on either conventional LM or RALM must have a strategy for safe contained tissue extraction given the recent concerns over uncontained power morcellation.31,32
Surgical skill and careful patient selection are key to optimal outcomes
Patients seeking conservative surgical management of their uterine fibroids should be considered candidates for either a conventional LM or RALM. Both the scientific literature and technologic advances make these approaches viable options, especially when the surgeon’s skill is appropriate and the patient’s candidacy is adequately vetted. A well thought out surgical strategy from start to finish will ensure the chances for successful completion and optimized outcomes.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
- Matchar DB, Myers ER, Barber MW, et al. Management of uterine fibroids: summary. AHRQ Evidence Report Summaries. Rockville, MD; Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 01-E051.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Herrmann A, De Wilde RL. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014;3:31-38.
- Stoica RA, Bistriceanu I, Sima R, et al. Laparoscopic myomectomy. J Med Life. 2014;7:522-524.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Holub Z. Laparoscopic myomectomy: indications and limits. Ceska Gynekol. 2007;72:64-68.
- Sinha R, Hegde A, Mahajan C, et al. Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol. 2008;15:292-300.
- Aksoy H, Aydin T, Ozdamar O, et al. Successful use of laparoscopic myomectomy to remove a giant uterine myoma: a case report. J Med Case Rep. 2015;9:286.
- Damiani A, Melgrati L, Marziali M, et al. Laparoscopic myomectomy for very large myomas using an isobaric (gasless) technique. JSLS. 2005;9:434-438.
- Holzer A, Jirecek ST, Illievich UM, et al. Laparoscopic versus open myomectomy: a double-blind study to evaluate postoperative pain. Anesth Analg. 2006;102:1480-1484.
- Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21.
- Pluchino N, Litta P, Freschi L, et al. Comparison of the initial surgical experience with robotic and laparoscopic myomectomy. Int J Med Robot. 2014;10:208-212.
- Parker WH, Iacampo K, Long T. Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol. 2007;14:362-364.
- Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511-518.
- Advincula AP, Xu X, Goudeau S 4th, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14:698-705.
- Nash K, Feinglass J, Zei C, et al. Robotic-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparative analysis of surgical outcomes and costs. Arch Gynecol Obstet. 2012;285:435-440.
- Mansour FW, Kives S, Urbach DR, et al. Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Canada. 2012;34:353-358.
- Sangha R, Eisenstein D, George A, et al. Comparison of surgical outcomes for robotic assisted laparoscopic myomectomy compared to abdominal myomectomy (abstract 373). J Minim Invasive Gynecol. 2010;17(suppl):S90-S108.
- Pundir J, Pundir V, Walavalkar R, et al. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013; 20:335–345.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015. doi:10.1155/2015/967568.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013; 28:99-108.
- Cela V, Freschi L, Simi G, et al. Fertility and endocrine outcome after robot-assisted laparoscopic myomectomy (RALM). Gynecol Endocrinol. 2013;29:79-82.
- Kang SY, Jeung IC, Chung YJ, et al. Robot-assisted laparoscopic myomectomy for deep intramural myomas. Int J Med Robot. 2017;13. doi:10.1002/rcs.1742.
- van den Haak L, Alleblas C, Nieboer TE, et al. Efficacy and safety of uterine manipulators in laparoscopic surgery: a review. Arch Gynecol Obstet. 2015;292:1003-1011.
- Hickman LC, Kotlyar A, Shue S, et al. Hemostatic techniques for myomectomy: an evidence-based approach. J Minim Invasive Gynecol. 2016;23:497-504.
- Tulandi T, Einarsson JI. The use of barbed suture for laparoscopic hysterectomy and myomectomy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2014;21:210-216.
- Alessandri F, Remorgida V, Venturini PL, et al. Unidirectional barbed suture versus continuous suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. J Minim Invasive Gynecol. 2010;17:725-729.
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- Meurs EA, Brito LG, Ajao MO, et al. Comparison of morcellation techniques at the time of laparoscopic hysterectomy and myomectomy. J Minim Invasive Gynecol. 2017;24:843-849.
Canagliflozin approved for cardiovascular event risk reduction
The Food and Drug Administration has approved canagliflozin (Invokana) as a way to reduce the risk of major adverse cardiovascular events in patients with type 2 diabetes and cardiovascular disease, according to Janssen Pharmaceuticals.
The sodium–glucose cotransporter 2 inhibitor was first approved in 2013 to improve glycemic control in adults with type 2 diabetes.
FDA approval was based on results from the CANVAS (Canagliflozin Cardiovascular Assessment Study) trial, which included more than 10,000 adults with type 2 diabetes who either had cardiovascular disease or were at risk for cardiovascular disease. Overall, patients who received canagliflozin had a 14% lower risk of experiencing a major cardiovascular event over the control group, and patients with established cardiovascular disease had an 18% lower risk.
The most common adverse events associated with canagliflozin include female genital mycotic infections, urinary tract infection, and increased urination. Notably, canagliflozin also increases the risk of lower-extremity amputation, especially in those with a history of amputation.
“Americans living with type 2 diabetes are two to three times more likely to die from heart disease than adults without diabetes. With this approval, Invokana now plays an even more important role in the overall treatment mix with its demonstrated ability to reduce the risk of potentially devastating cardiovascular events,” Ralph A. DeFronzo, MD, professor and division chief of medicine and diabetes at the University of Texas, San Antonio, said in the press release.
The new indication applies to all formulations of canagliflozin.
Find the full press release on the Janssen website.
The Food and Drug Administration has approved canagliflozin (Invokana) as a way to reduce the risk of major adverse cardiovascular events in patients with type 2 diabetes and cardiovascular disease, according to Janssen Pharmaceuticals.
The sodium–glucose cotransporter 2 inhibitor was first approved in 2013 to improve glycemic control in adults with type 2 diabetes.
FDA approval was based on results from the CANVAS (Canagliflozin Cardiovascular Assessment Study) trial, which included more than 10,000 adults with type 2 diabetes who either had cardiovascular disease or were at risk for cardiovascular disease. Overall, patients who received canagliflozin had a 14% lower risk of experiencing a major cardiovascular event over the control group, and patients with established cardiovascular disease had an 18% lower risk.
The most common adverse events associated with canagliflozin include female genital mycotic infections, urinary tract infection, and increased urination. Notably, canagliflozin also increases the risk of lower-extremity amputation, especially in those with a history of amputation.
“Americans living with type 2 diabetes are two to three times more likely to die from heart disease than adults without diabetes. With this approval, Invokana now plays an even more important role in the overall treatment mix with its demonstrated ability to reduce the risk of potentially devastating cardiovascular events,” Ralph A. DeFronzo, MD, professor and division chief of medicine and diabetes at the University of Texas, San Antonio, said in the press release.
The new indication applies to all formulations of canagliflozin.
Find the full press release on the Janssen website.
The Food and Drug Administration has approved canagliflozin (Invokana) as a way to reduce the risk of major adverse cardiovascular events in patients with type 2 diabetes and cardiovascular disease, according to Janssen Pharmaceuticals.
The sodium–glucose cotransporter 2 inhibitor was first approved in 2013 to improve glycemic control in adults with type 2 diabetes.
FDA approval was based on results from the CANVAS (Canagliflozin Cardiovascular Assessment Study) trial, which included more than 10,000 adults with type 2 diabetes who either had cardiovascular disease or were at risk for cardiovascular disease. Overall, patients who received canagliflozin had a 14% lower risk of experiencing a major cardiovascular event over the control group, and patients with established cardiovascular disease had an 18% lower risk.
The most common adverse events associated with canagliflozin include female genital mycotic infections, urinary tract infection, and increased urination. Notably, canagliflozin also increases the risk of lower-extremity amputation, especially in those with a history of amputation.
“Americans living with type 2 diabetes are two to three times more likely to die from heart disease than adults without diabetes. With this approval, Invokana now plays an even more important role in the overall treatment mix with its demonstrated ability to reduce the risk of potentially devastating cardiovascular events,” Ralph A. DeFronzo, MD, professor and division chief of medicine and diabetes at the University of Texas, San Antonio, said in the press release.
The new indication applies to all formulations of canagliflozin.
Find the full press release on the Janssen website.
Nivo + ipi shows durable activity against metastatic melanoma
MUNICH – Four years on, the combination of the immune checkpoint inhibitors nivolumab and ipilimumab as well as nivolumab alone continue to show benefit as first-line therapies for patients with advanced malignant melanoma, compared with ipilimumab monotherapy, reported investigators in the CheckMate O67 trial.
Among 945 patients with previously untreated and unresectable stage III or IV malignant melanoma, median overall survival at a minimum of 48 months follow-up had not been reached for patients assigned to the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), compared with 36.9 months for patients assigned to nivolumab alone, and 19.9 months for patients assigned to ipilimumab alone, reported F. Stephen Hodi Jr., MD, of Dana-Farber Cancer Institute, Boston, and his colleagues.
“There’s a durable, sustained clinical benefit that can be achieved with first-line nivo plus ipi combination or nivo alone in patients with advanced melanoma,” he said at the European Society for Medical Oncology Congress. The study results were published online in The Lancet Oncology to coincide with the presentation.
The benefit of immunotherapy also was seen in patients whose tumors had BRAF mutations, and both the combination and nivolumab alone showed improved efficacy compared with ipilimumab alone regardless of tumor expression of the programmed death ligand-1 (PD-L1), the investigators reported.
As previously reported, investigators in CheckMate 067 randomly assigned 945 previously untreated patients with unresectable stage III or IV melanoma to nivolumab 3 mg/kg every 2 weeks or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses then nivolumab 3 mg/kg every 2 weeks, or ipilimumab 3 mg/kg every 3 weeks for four doses. Patients were stratified at baseline by PD-L1 expression, BRAF status, and American Joint Commission on Cancer M stage.
Earlier results from the trial, reported at the 2015 annual meeting of the American Society of Clinical Oncology, showed that after a minimum of 9 months follow-up, the risk of disease progression or death was reduced by 43% with nivolumab versus ipilimumab (hazard ratio, 0.57; P less than .001) and by 58% with nivolumab plus ipilimumab vs. ipilimumab (HR, 0.42; P less than .001).
At ESMO 2018, Dr. Hodi presented 4-year follow-up results from the trial, with the analysis conducted at a minimum of 4 years after randomization of the last patient to be enrolled.
Median follow-up was 46.9 months for the nivolumab-plus-ipilimumab arm, 36 months in the nivolumab arm, and 18.6 months in the ipilimumab arm.
Median overall survival in the intention-to-treat population, a coprimary endpoint with progression-free survival (PFS), was as noted before. The HR for death with the combination compared with ipilimumab was 0.54 (P less than .0001) and for nivolumab versus ipilimumab it was 0.65 (P less than .0001).
The 4-year OS rates were 53% in the combination arm, 46% in the nivolumab-alone arm, and 30% in the ipilimumab-alone arm.
Median PFS was 11.5 months with the checkpoint inhibitor combination, 6.9 months in the nivolumab-alone arm, and 2.9 months in the ipilimumab arm.
The HR for PFS with the combination compared with ipilimumab was 0.42 (P less than .0001), and for nivolumab versus ipilimumab it was 0.53 (P less than .0001).
The safety analysis, conducted in all patients who received at least one dose of study drugs, showed that 59% of patients treated with the nivolumab/ipilimumab combination had treatment-related grade 3 or 4 adverse events, compared with 22% for patients treated with nivolumab alone, and 28% of those who received ipilimumab alone.
The most common treatment-related grade 3 adverse events were diarrhea in the combination and nivolumab-alone arms, and colitis in the ipilimumab group. In all three study arms the most common grade 4 adverse event was increased lipase.
Over the 4 years of follow-up, four patients died from treatment-related causes: one patient from cardiomyopathy and one from liver necrosis in the combination group, one from neutropenia in the nivolumab group, and one from colon perforation in the ipilimumab group. All of the deaths occurred within the first 3 years of the follow-up.
The investigators did not report on serious adverse events in the current analysis.
Invited discussant Reinhard Dummer, MD, of University Hospital Zurich Skin Cancer Center in Switzerland, said that while the study shows improved response rates and duration of response and longer PFS and OS with the combination, it’s premature to state conclusively that the combination is superior, because the study was not powered to compare efficacy between the two nivolumab-containing arms.
“So unfortunately, we have results, but we are not really convinced that the combination is so much better,” he said.
He added that the 4-year overall survival results for each arm show a consistent difference in the curves between the nivolumab and ipilimumab-alone arms. He also pointed to encouraging data showing that among patients alive at 4 years, 71% in the combination group did not require subsequent therapy, compared with 50% in the nivolumab group, and 39% in the ipilimumab group.
Dr. Hodi has received grant/research support from, and is a nonpaid consultant to, Bristol-Myers Squibb, which supported Checkmate 067. Dr. Dummer reported advising/consulting roles with the company.
SOURCE: Hodi FS et al. Lancet Oncol. 2018 Oct 22. doi: 10.1016/S1470-2045(18)30700-9.
MUNICH – Four years on, the combination of the immune checkpoint inhibitors nivolumab and ipilimumab as well as nivolumab alone continue to show benefit as first-line therapies for patients with advanced malignant melanoma, compared with ipilimumab monotherapy, reported investigators in the CheckMate O67 trial.
Among 945 patients with previously untreated and unresectable stage III or IV malignant melanoma, median overall survival at a minimum of 48 months follow-up had not been reached for patients assigned to the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), compared with 36.9 months for patients assigned to nivolumab alone, and 19.9 months for patients assigned to ipilimumab alone, reported F. Stephen Hodi Jr., MD, of Dana-Farber Cancer Institute, Boston, and his colleagues.
“There’s a durable, sustained clinical benefit that can be achieved with first-line nivo plus ipi combination or nivo alone in patients with advanced melanoma,” he said at the European Society for Medical Oncology Congress. The study results were published online in The Lancet Oncology to coincide with the presentation.
The benefit of immunotherapy also was seen in patients whose tumors had BRAF mutations, and both the combination and nivolumab alone showed improved efficacy compared with ipilimumab alone regardless of tumor expression of the programmed death ligand-1 (PD-L1), the investigators reported.
As previously reported, investigators in CheckMate 067 randomly assigned 945 previously untreated patients with unresectable stage III or IV melanoma to nivolumab 3 mg/kg every 2 weeks or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses then nivolumab 3 mg/kg every 2 weeks, or ipilimumab 3 mg/kg every 3 weeks for four doses. Patients were stratified at baseline by PD-L1 expression, BRAF status, and American Joint Commission on Cancer M stage.
Earlier results from the trial, reported at the 2015 annual meeting of the American Society of Clinical Oncology, showed that after a minimum of 9 months follow-up, the risk of disease progression or death was reduced by 43% with nivolumab versus ipilimumab (hazard ratio, 0.57; P less than .001) and by 58% with nivolumab plus ipilimumab vs. ipilimumab (HR, 0.42; P less than .001).
At ESMO 2018, Dr. Hodi presented 4-year follow-up results from the trial, with the analysis conducted at a minimum of 4 years after randomization of the last patient to be enrolled.
Median follow-up was 46.9 months for the nivolumab-plus-ipilimumab arm, 36 months in the nivolumab arm, and 18.6 months in the ipilimumab arm.
Median overall survival in the intention-to-treat population, a coprimary endpoint with progression-free survival (PFS), was as noted before. The HR for death with the combination compared with ipilimumab was 0.54 (P less than .0001) and for nivolumab versus ipilimumab it was 0.65 (P less than .0001).
The 4-year OS rates were 53% in the combination arm, 46% in the nivolumab-alone arm, and 30% in the ipilimumab-alone arm.
Median PFS was 11.5 months with the checkpoint inhibitor combination, 6.9 months in the nivolumab-alone arm, and 2.9 months in the ipilimumab arm.
The HR for PFS with the combination compared with ipilimumab was 0.42 (P less than .0001), and for nivolumab versus ipilimumab it was 0.53 (P less than .0001).
The safety analysis, conducted in all patients who received at least one dose of study drugs, showed that 59% of patients treated with the nivolumab/ipilimumab combination had treatment-related grade 3 or 4 adverse events, compared with 22% for patients treated with nivolumab alone, and 28% of those who received ipilimumab alone.
The most common treatment-related grade 3 adverse events were diarrhea in the combination and nivolumab-alone arms, and colitis in the ipilimumab group. In all three study arms the most common grade 4 adverse event was increased lipase.
Over the 4 years of follow-up, four patients died from treatment-related causes: one patient from cardiomyopathy and one from liver necrosis in the combination group, one from neutropenia in the nivolumab group, and one from colon perforation in the ipilimumab group. All of the deaths occurred within the first 3 years of the follow-up.
The investigators did not report on serious adverse events in the current analysis.
Invited discussant Reinhard Dummer, MD, of University Hospital Zurich Skin Cancer Center in Switzerland, said that while the study shows improved response rates and duration of response and longer PFS and OS with the combination, it’s premature to state conclusively that the combination is superior, because the study was not powered to compare efficacy between the two nivolumab-containing arms.
“So unfortunately, we have results, but we are not really convinced that the combination is so much better,” he said.
He added that the 4-year overall survival results for each arm show a consistent difference in the curves between the nivolumab and ipilimumab-alone arms. He also pointed to encouraging data showing that among patients alive at 4 years, 71% in the combination group did not require subsequent therapy, compared with 50% in the nivolumab group, and 39% in the ipilimumab group.
Dr. Hodi has received grant/research support from, and is a nonpaid consultant to, Bristol-Myers Squibb, which supported Checkmate 067. Dr. Dummer reported advising/consulting roles with the company.
SOURCE: Hodi FS et al. Lancet Oncol. 2018 Oct 22. doi: 10.1016/S1470-2045(18)30700-9.
MUNICH – Four years on, the combination of the immune checkpoint inhibitors nivolumab and ipilimumab as well as nivolumab alone continue to show benefit as first-line therapies for patients with advanced malignant melanoma, compared with ipilimumab monotherapy, reported investigators in the CheckMate O67 trial.
Among 945 patients with previously untreated and unresectable stage III or IV malignant melanoma, median overall survival at a minimum of 48 months follow-up had not been reached for patients assigned to the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), compared with 36.9 months for patients assigned to nivolumab alone, and 19.9 months for patients assigned to ipilimumab alone, reported F. Stephen Hodi Jr., MD, of Dana-Farber Cancer Institute, Boston, and his colleagues.
“There’s a durable, sustained clinical benefit that can be achieved with first-line nivo plus ipi combination or nivo alone in patients with advanced melanoma,” he said at the European Society for Medical Oncology Congress. The study results were published online in The Lancet Oncology to coincide with the presentation.
The benefit of immunotherapy also was seen in patients whose tumors had BRAF mutations, and both the combination and nivolumab alone showed improved efficacy compared with ipilimumab alone regardless of tumor expression of the programmed death ligand-1 (PD-L1), the investigators reported.
As previously reported, investigators in CheckMate 067 randomly assigned 945 previously untreated patients with unresectable stage III or IV melanoma to nivolumab 3 mg/kg every 2 weeks or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses then nivolumab 3 mg/kg every 2 weeks, or ipilimumab 3 mg/kg every 3 weeks for four doses. Patients were stratified at baseline by PD-L1 expression, BRAF status, and American Joint Commission on Cancer M stage.
Earlier results from the trial, reported at the 2015 annual meeting of the American Society of Clinical Oncology, showed that after a minimum of 9 months follow-up, the risk of disease progression or death was reduced by 43% with nivolumab versus ipilimumab (hazard ratio, 0.57; P less than .001) and by 58% with nivolumab plus ipilimumab vs. ipilimumab (HR, 0.42; P less than .001).
At ESMO 2018, Dr. Hodi presented 4-year follow-up results from the trial, with the analysis conducted at a minimum of 4 years after randomization of the last patient to be enrolled.
Median follow-up was 46.9 months for the nivolumab-plus-ipilimumab arm, 36 months in the nivolumab arm, and 18.6 months in the ipilimumab arm.
Median overall survival in the intention-to-treat population, a coprimary endpoint with progression-free survival (PFS), was as noted before. The HR for death with the combination compared with ipilimumab was 0.54 (P less than .0001) and for nivolumab versus ipilimumab it was 0.65 (P less than .0001).
The 4-year OS rates were 53% in the combination arm, 46% in the nivolumab-alone arm, and 30% in the ipilimumab-alone arm.
Median PFS was 11.5 months with the checkpoint inhibitor combination, 6.9 months in the nivolumab-alone arm, and 2.9 months in the ipilimumab arm.
The HR for PFS with the combination compared with ipilimumab was 0.42 (P less than .0001), and for nivolumab versus ipilimumab it was 0.53 (P less than .0001).
The safety analysis, conducted in all patients who received at least one dose of study drugs, showed that 59% of patients treated with the nivolumab/ipilimumab combination had treatment-related grade 3 or 4 adverse events, compared with 22% for patients treated with nivolumab alone, and 28% of those who received ipilimumab alone.
The most common treatment-related grade 3 adverse events were diarrhea in the combination and nivolumab-alone arms, and colitis in the ipilimumab group. In all three study arms the most common grade 4 adverse event was increased lipase.
Over the 4 years of follow-up, four patients died from treatment-related causes: one patient from cardiomyopathy and one from liver necrosis in the combination group, one from neutropenia in the nivolumab group, and one from colon perforation in the ipilimumab group. All of the deaths occurred within the first 3 years of the follow-up.
The investigators did not report on serious adverse events in the current analysis.
Invited discussant Reinhard Dummer, MD, of University Hospital Zurich Skin Cancer Center in Switzerland, said that while the study shows improved response rates and duration of response and longer PFS and OS with the combination, it’s premature to state conclusively that the combination is superior, because the study was not powered to compare efficacy between the two nivolumab-containing arms.
“So unfortunately, we have results, but we are not really convinced that the combination is so much better,” he said.
He added that the 4-year overall survival results for each arm show a consistent difference in the curves between the nivolumab and ipilimumab-alone arms. He also pointed to encouraging data showing that among patients alive at 4 years, 71% in the combination group did not require subsequent therapy, compared with 50% in the nivolumab group, and 39% in the ipilimumab group.
Dr. Hodi has received grant/research support from, and is a nonpaid consultant to, Bristol-Myers Squibb, which supported Checkmate 067. Dr. Dummer reported advising/consulting roles with the company.
SOURCE: Hodi FS et al. Lancet Oncol. 2018 Oct 22. doi: 10.1016/S1470-2045(18)30700-9.
REPORTING FROM ESMO 2018
Key clinical point: Nivolumab and ipilimumab combined provide superior progression-free and overall survival compared with nivolumab or ipilimumab alone.
Major finding: At 4-year minimum follow-up the median overall survival with the combination had not be reached, vs. 36.9 months for nivolumab and 19.9 months for ipilimumab.
Study details: Randomized phase 3 trial of 945 patients with previously untreated stage III or IV malignant melanoma.
Disclosures: Dr. Hodi has received grant/research support from, and is a nonpaid consultant to, Bristol-Myers Squibb, which supported Checkmate 067. Dr. Dummer reported advising/consulting roles with the company.
Source: Hodi FS et al. Lancet Oncol. 2018 Oct 22. doi: 10.1016/S1470-2045(18)30700-9.
Death of a sales pitch
The EHR and our troubled health care system, Part 1
In 2000, the Institute of Medicine published “To Err Is Human,” a landmark study that warned that as many as 98,000 people die annually as a result of medical errors. One conclusion of the report stated, “When patients see multiple providers in different settings, none of whom has access to complete information, it becomes easier for things to go wrong.” Government and public reaction to the study resulted in the rushed integration of electronic health records into the U.S. medical system. EHR vendors promised solutions that included a dramatic reduction of preventable errors, a simplified system of physician communication, and the consolidation of a patient’s salient medical information into a single, transferable file. Now, almost 20 years later, these promises remain mostly unfilled. How did we get here?
Systems of medical records have been in place since 1600 B.C. For thousands of years, they consisted mainly of the patient’s diagnosis and the physician’s treatment. In 1968, the New England Journal of Medicine published the special article “Medical Records That Guide and Teach” by Lawrence L. Weed, MD. In the report, Dr. Weed advocated for the organization of medical records by problems rather than by a single diagnosis. This was the birth of our modern system. Medical records would now include lists of symptoms, findings, and problems that would organize the physician’s planning and allow third parties to confirm the initial diagnosis. Nearly concurrent with this publication, the next major innovation was developing in a very unusual location.
In 1999, Fortune magazine labeled Jack Welch “Manager of the Century” for his innovative work as CEO of General Electric. His techniques involved cutting waste and streamlining his workforce. While these methods were somewhat controversial, GE’s market value increased dramatically under his watch. The publishers at Fortune became interested in finding similar innovators in other fields. In this pursuit, they sent journalist Philip Longman to find the “Jack Welch” of health care.
Mr. Longman had recently lost his wife to breast cancer and was becoming obsessed with medical errors and health care quality integration. He set out to discover the best health care system in the United States. After months of research, Mr. Longman reached a startling conclusion. By nearly every metric, the Veterans Affairs system produced the highest quality of care. The key factor in upholding that quality appeared to be the EHR system VistA (Veterans Information Systems and Technology Architecture).
The development of VistA was a grassroots effort begun in the 1970s. Using Tandy computers and Wang processors, the VA “hardhats” sought to develop an electronic system for medical records and communication. This effort was initially opposed and driven underground by the central bureaucracy. Laptops were confiscated, people were fired. Still, development continued, and in 1978, the Decentralized Hospital Computer Program was launched at 20 VA sites. The national rollout occurred in 1994 under the name VistA.
VistA was developed by doctors, for doctors, and routinely enjoys the highest satisfaction rates among all available EHRs. VistA also is an open source model; its code is readily available on the VA website. After seeing the evidence of VistA’s efficacy, Representative Pete Stark (D-CA) introduced HR 6898 on Sept. 15, 2008. The bill would establish a large federal open source health IT system that private hospitals could leverage. The bill also mandated that only open source solutions would receive federal funding. As opposed to proprietary systems, open source models allow for rapid innovation, easy personal configuration, and incorporation of open source apps from unlimited numbers of contributors.
HR 6898 never passed, despite initial bipartisan support. By relying on lobbyists, marketing, and money, the proprietary EHR vendors killed the Stark bill. After a 4-month scramble, the Health Information Technology for Economic and Clinical Health Act (HITECH) passed, with EHR vendor support. HITECH established a certification system for EHRs. While the Stark bill envisioned a single, open source network, there were soon hundreds of certified EHR systems in the United States.
Before the HITECH Act, many EHRs existed, but several barriers blocked full implementation. Early systems were essentially electronic filing cabinets. Their developers had not anticipated the lack of standardization among physicians and hospital systems. The need for custom EHR bases frustrated the vendors. The question of marketing was omnipresent. Who was the actual customer? An economic model developed in which clinicians would bear the time and even financial costs as the benefits would be passed on to insurers, hospitals, and, presumably, the patients.
EHRs needed to become practical, affordable, and interoperable, but who was demanding this? Where was the financial motivation? In the beginning, vendors of EHRs had to convince doctors, the public, and the government of their worth. Now, essentially mandated by the HITECH Act, they only had to sell themselves to hospital administrators, who often had a different motive. Profits.
Many of today’s EHRs are simply modified billing platforms, and doctors are paying the price. The Meaningful Use standards were meant to provide financial incentives for EHR adoption. Stage 2 required EHRs to be able to transport clinical information from one system to another. Looking at our actual practices can provide a master class in the gap between “be able to” and “actually doing.” Again, who does the EHR vendor see as the customer? Certainly not the physician. My patients can list every type of inferior vena cava filter (or at least those with pending legal action), but most of them have never heard of an EHR. Just like “service lines,” EHRs can make it very difficult for patients to seek care outside of their primary system. Who would see this barrier in communication as a perk and not a deficiency? Hospital administrators. The free transfer of medical records is bad for business. Therefore, hospitals don’t prioritize it in their EHRs. The EHR vendors also benefit since an easy transfer of records would simplify a hospital’s transition from one EHR to another. So, as with most deficiencies in the EHR, physicians are left to find ways around these problems. Sometimes, we need to go to comical lengths.
Two months ago, a patient pointed to a large machine behind our check-in desk. “What is that,” he asked incredulously; it was a fax machine. While my competence with this apparatus is marginal (my office staff has taken to yelling “doctor faxing!” to alert one another that I am about to inadvertently copy or scan my documents into oblivion), faxes remain a mainstay of medical care. Abandoned by modern business practices as a relic of the 1980s, why are we constantly faxing medical information? Because we are not the customer.
Disruption is now a favorable term in business. Doctors are busy people. BUSY people. Most of us walk a tightrope, a razor-thin timeline. Will we see the next patient in time, the next surgery? Will we get the medical records done today? Will we get the dictations done before being suspended? Will we make the committee meeting, the conference call, the next clinic across town? Will we have dinner with our spouse or see our kids today? Will we make it to the parent-teacher conference inexplicably scheduled for 10:45 a.m. on a Tuesday??!! When deciding between work commitments and family, we side with work overwhelmingly (and depressingly). Explaining this to a layperson is an impossible feat. I have stopped trying, stopped making excuses. Only we know how catastrophic “disruption” can be. Disruption in a 40-patient clinic. Disruption in the trauma bay. I have seen physicians reduced to tears by this disruption. Some activities need disruption. Typing with your back to the patient. Onerous documentation to facilitate billing. Faxing medical records. Will these be disrupted? Who is the customer?
In 1999, the Institute of Medicine started this process, telling us, “To err is human.” I now respond with another Alexander Pope quote, “The same ambition can destroy or save.” The money and influence of EHR vendors destroyed the chance to nationalize the most successful EHR our country has ever seen. What happens now? EHRs are incontrovertibly associated with burnout. Burnout is incontrovertibly associated with outcomes ranging from early retirement to suicide. EHRs cause physician harm. Major vendors can follow the Big Tobacco play book and deny the obvious, but the burden of proof is shifting to them. With their billions of dollars in profits, what have they done to study this problem? To help?
Who is their customer?
Dr. Sheahan is the Claude C. Craighead Jr. Professor and Chair, division of vascular and endovascular surgery, Louisiana State University Health Sciences Center, New Orleans.
References
Institute of Medicine (US) Committee on Quality of Health Care in America. 2000. To Err Is Human: Building a Safer Health System. Washington: The National Academies Press.
Weed LL. Medical records that guide and teach. N Engl J Med. 1968 Mar 14;278(11):593-600.
Longman P. “Best Care Anywhere: Why VA Health Care Is Better Than Yours.” (Oakland: Berrett-Koehler Publishers).
The EHR and our troubled health care system, Part 1
The EHR and our troubled health care system, Part 1
In 2000, the Institute of Medicine published “To Err Is Human,” a landmark study that warned that as many as 98,000 people die annually as a result of medical errors. One conclusion of the report stated, “When patients see multiple providers in different settings, none of whom has access to complete information, it becomes easier for things to go wrong.” Government and public reaction to the study resulted in the rushed integration of electronic health records into the U.S. medical system. EHR vendors promised solutions that included a dramatic reduction of preventable errors, a simplified system of physician communication, and the consolidation of a patient’s salient medical information into a single, transferable file. Now, almost 20 years later, these promises remain mostly unfilled. How did we get here?
Systems of medical records have been in place since 1600 B.C. For thousands of years, they consisted mainly of the patient’s diagnosis and the physician’s treatment. In 1968, the New England Journal of Medicine published the special article “Medical Records That Guide and Teach” by Lawrence L. Weed, MD. In the report, Dr. Weed advocated for the organization of medical records by problems rather than by a single diagnosis. This was the birth of our modern system. Medical records would now include lists of symptoms, findings, and problems that would organize the physician’s planning and allow third parties to confirm the initial diagnosis. Nearly concurrent with this publication, the next major innovation was developing in a very unusual location.
In 1999, Fortune magazine labeled Jack Welch “Manager of the Century” for his innovative work as CEO of General Electric. His techniques involved cutting waste and streamlining his workforce. While these methods were somewhat controversial, GE’s market value increased dramatically under his watch. The publishers at Fortune became interested in finding similar innovators in other fields. In this pursuit, they sent journalist Philip Longman to find the “Jack Welch” of health care.
Mr. Longman had recently lost his wife to breast cancer and was becoming obsessed with medical errors and health care quality integration. He set out to discover the best health care system in the United States. After months of research, Mr. Longman reached a startling conclusion. By nearly every metric, the Veterans Affairs system produced the highest quality of care. The key factor in upholding that quality appeared to be the EHR system VistA (Veterans Information Systems and Technology Architecture).
The development of VistA was a grassroots effort begun in the 1970s. Using Tandy computers and Wang processors, the VA “hardhats” sought to develop an electronic system for medical records and communication. This effort was initially opposed and driven underground by the central bureaucracy. Laptops were confiscated, people were fired. Still, development continued, and in 1978, the Decentralized Hospital Computer Program was launched at 20 VA sites. The national rollout occurred in 1994 under the name VistA.
VistA was developed by doctors, for doctors, and routinely enjoys the highest satisfaction rates among all available EHRs. VistA also is an open source model; its code is readily available on the VA website. After seeing the evidence of VistA’s efficacy, Representative Pete Stark (D-CA) introduced HR 6898 on Sept. 15, 2008. The bill would establish a large federal open source health IT system that private hospitals could leverage. The bill also mandated that only open source solutions would receive federal funding. As opposed to proprietary systems, open source models allow for rapid innovation, easy personal configuration, and incorporation of open source apps from unlimited numbers of contributors.
HR 6898 never passed, despite initial bipartisan support. By relying on lobbyists, marketing, and money, the proprietary EHR vendors killed the Stark bill. After a 4-month scramble, the Health Information Technology for Economic and Clinical Health Act (HITECH) passed, with EHR vendor support. HITECH established a certification system for EHRs. While the Stark bill envisioned a single, open source network, there were soon hundreds of certified EHR systems in the United States.
Before the HITECH Act, many EHRs existed, but several barriers blocked full implementation. Early systems were essentially electronic filing cabinets. Their developers had not anticipated the lack of standardization among physicians and hospital systems. The need for custom EHR bases frustrated the vendors. The question of marketing was omnipresent. Who was the actual customer? An economic model developed in which clinicians would bear the time and even financial costs as the benefits would be passed on to insurers, hospitals, and, presumably, the patients.
EHRs needed to become practical, affordable, and interoperable, but who was demanding this? Where was the financial motivation? In the beginning, vendors of EHRs had to convince doctors, the public, and the government of their worth. Now, essentially mandated by the HITECH Act, they only had to sell themselves to hospital administrators, who often had a different motive. Profits.
Many of today’s EHRs are simply modified billing platforms, and doctors are paying the price. The Meaningful Use standards were meant to provide financial incentives for EHR adoption. Stage 2 required EHRs to be able to transport clinical information from one system to another. Looking at our actual practices can provide a master class in the gap between “be able to” and “actually doing.” Again, who does the EHR vendor see as the customer? Certainly not the physician. My patients can list every type of inferior vena cava filter (or at least those with pending legal action), but most of them have never heard of an EHR. Just like “service lines,” EHRs can make it very difficult for patients to seek care outside of their primary system. Who would see this barrier in communication as a perk and not a deficiency? Hospital administrators. The free transfer of medical records is bad for business. Therefore, hospitals don’t prioritize it in their EHRs. The EHR vendors also benefit since an easy transfer of records would simplify a hospital’s transition from one EHR to another. So, as with most deficiencies in the EHR, physicians are left to find ways around these problems. Sometimes, we need to go to comical lengths.
Two months ago, a patient pointed to a large machine behind our check-in desk. “What is that,” he asked incredulously; it was a fax machine. While my competence with this apparatus is marginal (my office staff has taken to yelling “doctor faxing!” to alert one another that I am about to inadvertently copy or scan my documents into oblivion), faxes remain a mainstay of medical care. Abandoned by modern business practices as a relic of the 1980s, why are we constantly faxing medical information? Because we are not the customer.
Disruption is now a favorable term in business. Doctors are busy people. BUSY people. Most of us walk a tightrope, a razor-thin timeline. Will we see the next patient in time, the next surgery? Will we get the medical records done today? Will we get the dictations done before being suspended? Will we make the committee meeting, the conference call, the next clinic across town? Will we have dinner with our spouse or see our kids today? Will we make it to the parent-teacher conference inexplicably scheduled for 10:45 a.m. on a Tuesday??!! When deciding between work commitments and family, we side with work overwhelmingly (and depressingly). Explaining this to a layperson is an impossible feat. I have stopped trying, stopped making excuses. Only we know how catastrophic “disruption” can be. Disruption in a 40-patient clinic. Disruption in the trauma bay. I have seen physicians reduced to tears by this disruption. Some activities need disruption. Typing with your back to the patient. Onerous documentation to facilitate billing. Faxing medical records. Will these be disrupted? Who is the customer?
In 1999, the Institute of Medicine started this process, telling us, “To err is human.” I now respond with another Alexander Pope quote, “The same ambition can destroy or save.” The money and influence of EHR vendors destroyed the chance to nationalize the most successful EHR our country has ever seen. What happens now? EHRs are incontrovertibly associated with burnout. Burnout is incontrovertibly associated with outcomes ranging from early retirement to suicide. EHRs cause physician harm. Major vendors can follow the Big Tobacco play book and deny the obvious, but the burden of proof is shifting to them. With their billions of dollars in profits, what have they done to study this problem? To help?
Who is their customer?
Dr. Sheahan is the Claude C. Craighead Jr. Professor and Chair, division of vascular and endovascular surgery, Louisiana State University Health Sciences Center, New Orleans.
References
Institute of Medicine (US) Committee on Quality of Health Care in America. 2000. To Err Is Human: Building a Safer Health System. Washington: The National Academies Press.
Weed LL. Medical records that guide and teach. N Engl J Med. 1968 Mar 14;278(11):593-600.
Longman P. “Best Care Anywhere: Why VA Health Care Is Better Than Yours.” (Oakland: Berrett-Koehler Publishers).
In 2000, the Institute of Medicine published “To Err Is Human,” a landmark study that warned that as many as 98,000 people die annually as a result of medical errors. One conclusion of the report stated, “When patients see multiple providers in different settings, none of whom has access to complete information, it becomes easier for things to go wrong.” Government and public reaction to the study resulted in the rushed integration of electronic health records into the U.S. medical system. EHR vendors promised solutions that included a dramatic reduction of preventable errors, a simplified system of physician communication, and the consolidation of a patient’s salient medical information into a single, transferable file. Now, almost 20 years later, these promises remain mostly unfilled. How did we get here?
Systems of medical records have been in place since 1600 B.C. For thousands of years, they consisted mainly of the patient’s diagnosis and the physician’s treatment. In 1968, the New England Journal of Medicine published the special article “Medical Records That Guide and Teach” by Lawrence L. Weed, MD. In the report, Dr. Weed advocated for the organization of medical records by problems rather than by a single diagnosis. This was the birth of our modern system. Medical records would now include lists of symptoms, findings, and problems that would organize the physician’s planning and allow third parties to confirm the initial diagnosis. Nearly concurrent with this publication, the next major innovation was developing in a very unusual location.
In 1999, Fortune magazine labeled Jack Welch “Manager of the Century” for his innovative work as CEO of General Electric. His techniques involved cutting waste and streamlining his workforce. While these methods were somewhat controversial, GE’s market value increased dramatically under his watch. The publishers at Fortune became interested in finding similar innovators in other fields. In this pursuit, they sent journalist Philip Longman to find the “Jack Welch” of health care.
Mr. Longman had recently lost his wife to breast cancer and was becoming obsessed with medical errors and health care quality integration. He set out to discover the best health care system in the United States. After months of research, Mr. Longman reached a startling conclusion. By nearly every metric, the Veterans Affairs system produced the highest quality of care. The key factor in upholding that quality appeared to be the EHR system VistA (Veterans Information Systems and Technology Architecture).
The development of VistA was a grassroots effort begun in the 1970s. Using Tandy computers and Wang processors, the VA “hardhats” sought to develop an electronic system for medical records and communication. This effort was initially opposed and driven underground by the central bureaucracy. Laptops were confiscated, people were fired. Still, development continued, and in 1978, the Decentralized Hospital Computer Program was launched at 20 VA sites. The national rollout occurred in 1994 under the name VistA.
VistA was developed by doctors, for doctors, and routinely enjoys the highest satisfaction rates among all available EHRs. VistA also is an open source model; its code is readily available on the VA website. After seeing the evidence of VistA’s efficacy, Representative Pete Stark (D-CA) introduced HR 6898 on Sept. 15, 2008. The bill would establish a large federal open source health IT system that private hospitals could leverage. The bill also mandated that only open source solutions would receive federal funding. As opposed to proprietary systems, open source models allow for rapid innovation, easy personal configuration, and incorporation of open source apps from unlimited numbers of contributors.
HR 6898 never passed, despite initial bipartisan support. By relying on lobbyists, marketing, and money, the proprietary EHR vendors killed the Stark bill. After a 4-month scramble, the Health Information Technology for Economic and Clinical Health Act (HITECH) passed, with EHR vendor support. HITECH established a certification system for EHRs. While the Stark bill envisioned a single, open source network, there were soon hundreds of certified EHR systems in the United States.
Before the HITECH Act, many EHRs existed, but several barriers blocked full implementation. Early systems were essentially electronic filing cabinets. Their developers had not anticipated the lack of standardization among physicians and hospital systems. The need for custom EHR bases frustrated the vendors. The question of marketing was omnipresent. Who was the actual customer? An economic model developed in which clinicians would bear the time and even financial costs as the benefits would be passed on to insurers, hospitals, and, presumably, the patients.
EHRs needed to become practical, affordable, and interoperable, but who was demanding this? Where was the financial motivation? In the beginning, vendors of EHRs had to convince doctors, the public, and the government of their worth. Now, essentially mandated by the HITECH Act, they only had to sell themselves to hospital administrators, who often had a different motive. Profits.
Many of today’s EHRs are simply modified billing platforms, and doctors are paying the price. The Meaningful Use standards were meant to provide financial incentives for EHR adoption. Stage 2 required EHRs to be able to transport clinical information from one system to another. Looking at our actual practices can provide a master class in the gap between “be able to” and “actually doing.” Again, who does the EHR vendor see as the customer? Certainly not the physician. My patients can list every type of inferior vena cava filter (or at least those with pending legal action), but most of them have never heard of an EHR. Just like “service lines,” EHRs can make it very difficult for patients to seek care outside of their primary system. Who would see this barrier in communication as a perk and not a deficiency? Hospital administrators. The free transfer of medical records is bad for business. Therefore, hospitals don’t prioritize it in their EHRs. The EHR vendors also benefit since an easy transfer of records would simplify a hospital’s transition from one EHR to another. So, as with most deficiencies in the EHR, physicians are left to find ways around these problems. Sometimes, we need to go to comical lengths.
Two months ago, a patient pointed to a large machine behind our check-in desk. “What is that,” he asked incredulously; it was a fax machine. While my competence with this apparatus is marginal (my office staff has taken to yelling “doctor faxing!” to alert one another that I am about to inadvertently copy or scan my documents into oblivion), faxes remain a mainstay of medical care. Abandoned by modern business practices as a relic of the 1980s, why are we constantly faxing medical information? Because we are not the customer.
Disruption is now a favorable term in business. Doctors are busy people. BUSY people. Most of us walk a tightrope, a razor-thin timeline. Will we see the next patient in time, the next surgery? Will we get the medical records done today? Will we get the dictations done before being suspended? Will we make the committee meeting, the conference call, the next clinic across town? Will we have dinner with our spouse or see our kids today? Will we make it to the parent-teacher conference inexplicably scheduled for 10:45 a.m. on a Tuesday??!! When deciding between work commitments and family, we side with work overwhelmingly (and depressingly). Explaining this to a layperson is an impossible feat. I have stopped trying, stopped making excuses. Only we know how catastrophic “disruption” can be. Disruption in a 40-patient clinic. Disruption in the trauma bay. I have seen physicians reduced to tears by this disruption. Some activities need disruption. Typing with your back to the patient. Onerous documentation to facilitate billing. Faxing medical records. Will these be disrupted? Who is the customer?
In 1999, the Institute of Medicine started this process, telling us, “To err is human.” I now respond with another Alexander Pope quote, “The same ambition can destroy or save.” The money and influence of EHR vendors destroyed the chance to nationalize the most successful EHR our country has ever seen. What happens now? EHRs are incontrovertibly associated with burnout. Burnout is incontrovertibly associated with outcomes ranging from early retirement to suicide. EHRs cause physician harm. Major vendors can follow the Big Tobacco play book and deny the obvious, but the burden of proof is shifting to them. With their billions of dollars in profits, what have they done to study this problem? To help?
Who is their customer?
Dr. Sheahan is the Claude C. Craighead Jr. Professor and Chair, division of vascular and endovascular surgery, Louisiana State University Health Sciences Center, New Orleans.
References
Institute of Medicine (US) Committee on Quality of Health Care in America. 2000. To Err Is Human: Building a Safer Health System. Washington: The National Academies Press.
Weed LL. Medical records that guide and teach. N Engl J Med. 1968 Mar 14;278(11):593-600.
Longman P. “Best Care Anywhere: Why VA Health Care Is Better Than Yours.” (Oakland: Berrett-Koehler Publishers).
Constipation because of deportation-related trauma
I recently saw Anaeli (not her real name), an 8-year-old Mexican American girl, in clinic for worsening constipation. Her mother brought her in because of a year’s worth of increasingly irregular bowel movements. Looking through her chart, it was easy to find the starting point of Anaeli’s constipation – it aligned with her father’s deportation. U.S. Immigration and Customs Enforcement had arrested him while he was dropping Anaeli off at school.
Family separation at the border has reignited awareness of the effects of adverse childhood events. As a young pediatrician training in San Diego, I see both the impact of immigration policies on children and the resulting need for trauma-informed care. We need coordinated efforts in homes, schools, and hospitals to effectively treat affected kids.
For the past year, Anaeli’s caregivers have struggled to do so. She has been acting out, frequently crying and throwing fits about going to school. Anaeli has missed about 30 days of school because of behavioral issues.
What does 30 fewer days of first grade look like? Anaeli’s language skills are at a standstill. She cannot follow complex directions like her peers. Because of her academic shortcomings, Anaeli earned an individualized education plan and a teacher’s aide to help her focus. This aide has adopted a “tough love” attitude. Anaeli’s mom reports that she is often disciplined by long time-outs in the classroom bathroom and worries that this discipline is causing Anaeli to withhold stool to a point of loosing control and soiling herself. Since working with the aide, Anaeli has been having daily “accidents,” stooling in her pants, despite being toilet trained for years.
After the appointment, I called the school three times and was finally able to get in touch with Anaeli’s aide. She expressed frustration over Anaeli’s “lack of trying” and “meltdown” reaction to discipline. She said Anaeli’s mom was not enforcing limits at home. She told me she had successfully used time-outs in the bathroom with her own children. When I reviewed the impact of childhood trauma and more appropriate approaches to discipline, the aide grew defensive and challenged me by asking if I have kids of my own.
While I disagreed with the aide’s methods, I understood her frustration. Anaeli is not easy to help. But she is just one of a generation of children affected by the deportation of a family member. Like them, Anaeli’s health is deeply affected by stress in a way that she many not be able to verbalize.
Trauma-informed care should be an essential lens for caregivers of children who have been separated from their family. Resolving Anaeli’s constipation will require a concerted effort by her mom, health providers, teachers, and aides to encourage good behavior, use measured disciplinary tactics, and consume a high-fiber diet. In doing so, we can provide children like her with the appropriate environment to build resilience.
Dr. Parekh is a pediatrician in San Diego. Email her at pdnews@mdedge.com.
I recently saw Anaeli (not her real name), an 8-year-old Mexican American girl, in clinic for worsening constipation. Her mother brought her in because of a year’s worth of increasingly irregular bowel movements. Looking through her chart, it was easy to find the starting point of Anaeli’s constipation – it aligned with her father’s deportation. U.S. Immigration and Customs Enforcement had arrested him while he was dropping Anaeli off at school.
Family separation at the border has reignited awareness of the effects of adverse childhood events. As a young pediatrician training in San Diego, I see both the impact of immigration policies on children and the resulting need for trauma-informed care. We need coordinated efforts in homes, schools, and hospitals to effectively treat affected kids.
For the past year, Anaeli’s caregivers have struggled to do so. She has been acting out, frequently crying and throwing fits about going to school. Anaeli has missed about 30 days of school because of behavioral issues.
What does 30 fewer days of first grade look like? Anaeli’s language skills are at a standstill. She cannot follow complex directions like her peers. Because of her academic shortcomings, Anaeli earned an individualized education plan and a teacher’s aide to help her focus. This aide has adopted a “tough love” attitude. Anaeli’s mom reports that she is often disciplined by long time-outs in the classroom bathroom and worries that this discipline is causing Anaeli to withhold stool to a point of loosing control and soiling herself. Since working with the aide, Anaeli has been having daily “accidents,” stooling in her pants, despite being toilet trained for years.
After the appointment, I called the school three times and was finally able to get in touch with Anaeli’s aide. She expressed frustration over Anaeli’s “lack of trying” and “meltdown” reaction to discipline. She said Anaeli’s mom was not enforcing limits at home. She told me she had successfully used time-outs in the bathroom with her own children. When I reviewed the impact of childhood trauma and more appropriate approaches to discipline, the aide grew defensive and challenged me by asking if I have kids of my own.
While I disagreed with the aide’s methods, I understood her frustration. Anaeli is not easy to help. But she is just one of a generation of children affected by the deportation of a family member. Like them, Anaeli’s health is deeply affected by stress in a way that she many not be able to verbalize.
Trauma-informed care should be an essential lens for caregivers of children who have been separated from their family. Resolving Anaeli’s constipation will require a concerted effort by her mom, health providers, teachers, and aides to encourage good behavior, use measured disciplinary tactics, and consume a high-fiber diet. In doing so, we can provide children like her with the appropriate environment to build resilience.
Dr. Parekh is a pediatrician in San Diego. Email her at pdnews@mdedge.com.
I recently saw Anaeli (not her real name), an 8-year-old Mexican American girl, in clinic for worsening constipation. Her mother brought her in because of a year’s worth of increasingly irregular bowel movements. Looking through her chart, it was easy to find the starting point of Anaeli’s constipation – it aligned with her father’s deportation. U.S. Immigration and Customs Enforcement had arrested him while he was dropping Anaeli off at school.
Family separation at the border has reignited awareness of the effects of adverse childhood events. As a young pediatrician training in San Diego, I see both the impact of immigration policies on children and the resulting need for trauma-informed care. We need coordinated efforts in homes, schools, and hospitals to effectively treat affected kids.
For the past year, Anaeli’s caregivers have struggled to do so. She has been acting out, frequently crying and throwing fits about going to school. Anaeli has missed about 30 days of school because of behavioral issues.
What does 30 fewer days of first grade look like? Anaeli’s language skills are at a standstill. She cannot follow complex directions like her peers. Because of her academic shortcomings, Anaeli earned an individualized education plan and a teacher’s aide to help her focus. This aide has adopted a “tough love” attitude. Anaeli’s mom reports that she is often disciplined by long time-outs in the classroom bathroom and worries that this discipline is causing Anaeli to withhold stool to a point of loosing control and soiling herself. Since working with the aide, Anaeli has been having daily “accidents,” stooling in her pants, despite being toilet trained for years.
After the appointment, I called the school three times and was finally able to get in touch with Anaeli’s aide. She expressed frustration over Anaeli’s “lack of trying” and “meltdown” reaction to discipline. She said Anaeli’s mom was not enforcing limits at home. She told me she had successfully used time-outs in the bathroom with her own children. When I reviewed the impact of childhood trauma and more appropriate approaches to discipline, the aide grew defensive and challenged me by asking if I have kids of my own.
While I disagreed with the aide’s methods, I understood her frustration. Anaeli is not easy to help. But she is just one of a generation of children affected by the deportation of a family member. Like them, Anaeli’s health is deeply affected by stress in a way that she many not be able to verbalize.
Trauma-informed care should be an essential lens for caregivers of children who have been separated from their family. Resolving Anaeli’s constipation will require a concerted effort by her mom, health providers, teachers, and aides to encourage good behavior, use measured disciplinary tactics, and consume a high-fiber diet. In doing so, we can provide children like her with the appropriate environment to build resilience.
Dr. Parekh is a pediatrician in San Diego. Email her at pdnews@mdedge.com.
Thiopurines linked to zoster in IBD patients
For patients with inflammatory bowel disease (IBD), thiopurine exposure was associated with a significantly increased risk of herpes zoster, compared with 5-aminosalicylic acid (5-ASA) monotherapy, according to the results of two large retrospective cohort studies.
In the multivariable analysis, thiopurine monotherapy was linked to about a 47% increase in the risk of herpes zoster, compared with 5-ASA monotherapy (adjusted hazard ratio, 1.47; 95% confidence interval, 1.31-1.65; P less than .001). Combination therapy with thiopurines and tumor necrosis factor antagonists conferred about a 65% increase in zoster risk (aHR, 1.65; 95% CI, 1.22-2.23; P = .001). However, tumor necrosis factor–antagonist monotherapy did not appear to significantly increase the risk of zoster when compared with 5-ASA monotherapy, reported Nabeel Khan, MD, of the University of Pennsylvania in Philadelphia, and his associates.
“Compared to [patients without] IBD, ulcerative colitis (UC) and Crohn’s disease (CD) each were associated with significantly increased risk of herpes zoster infection,” the researchers wrote online in Clinical Gastroenterology and Hepatology. “With the approval of a new and potentially safer vaccine for herpes zoster, the effects of immunization of patients with IBD should be investigated.”
Past studies have linked IBD with a 1.2- to 1.8-fold increase in the risk of zoster, but these studies date to the prebiologic era or excluded patients who were in their midsixties or older, the researchers wrote. “Additionally, these prior studies have not assessed the validity of the codes used to identify herpes zoster and also did not account for the impact of vaccination,” they added. “They also did not take into consideration the severity of the disease or degree of steroid exposure.”
Therefore, the researchers conducted two retrospective cohort studies of patients in the United States Department of Veterans Affairs between 2000 and 2016. The first cohort study compared the incidence of herpes zoster among patients with IBD who received 5-ASA alone with matched patients without IBD. The second cohort study measured the incidence of herpes zoster in patients with IBD who received various medications and combination regimen. “The VA has a predominantly older population, which makes it an ideal cohort to study herpes zoster incidence in a high-risk population,” the investigators noted. “Unlike insurance databases, the VA database can be validated internally and vaccination records are documented.”
After adjusting for age, race, sex, geographic region, disease flare, corticosteroid use, and baseline comorbidities, the estimated hazard of developing herpes zoster was 1.81 (95% confidence interval, 1.56-2.11) among patients with ulcerative colitis and 1.56 (95% CI, 1.28-1.91) among patients with Crohn’s disease, as compared with patients without IBD. Regardless of their age or the medications they were receiving, patients with IBD had a higher incidence of zoster than the oldest group of patients without IBD (older than 60 years), regardless of age or medication. “The highest risk of herpes zoster was observed in patients with IBD who were less than 60 years of age and on combination therapy,” the investigators wrote. “Patients with IBD younger than 50 years who were on combination therapy had higher risk of herpes zoster, compared with patients with IBD older than 60 years of age who were not on immunosuppressive therapy.” Based on the findings, they recommended studying the efficacy of widespread use of the new herpes zoster vaccine in patients with IBD.
Pfizer provided unrestricted research funding but was not otherwise involved in the study. One coinvestigator disclosed ties to Pfizer and several other pharmaceutical companies. The remaining investigators reported having no conflicts of interest.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Jan 5. doi: 10.1016/j.cgh.2017.12.052.
Patients with inflammatory bowel disease are thought to have altered immune regulation, which may increase the risk of systemic complications including infections like herpes zoster. Many of the prior studies assessing the risk of herpes zoster in IBD patients were done before the advent of biologics and excluded older patients, thereby limiting their utility. This study by Khan et al. aimed to better estimate the incidence and risk factors for development of herpes zoster and to determine the effect of immunosuppressant use on this risk. In two large, retrospective cohort studies they found that, compared with patients without IBD, patients with IBD had a significantly increased risk of developing herpes zoster. Furthermore, this risk was higher in those with recent or cumulative steroid use and in those treated with thiopurines (as monotherapy or in combination with anti-TNF agents). Interestingly, exposure to TNF antagonists alone was not associated with an increased risk of herpes zoster infection.
Richa Shukla, MD, assistant professor, section of gastroenterology and hepatology, Baylor College of Medicine, Houston.
Patients with inflammatory bowel disease are thought to have altered immune regulation, which may increase the risk of systemic complications including infections like herpes zoster. Many of the prior studies assessing the risk of herpes zoster in IBD patients were done before the advent of biologics and excluded older patients, thereby limiting their utility. This study by Khan et al. aimed to better estimate the incidence and risk factors for development of herpes zoster and to determine the effect of immunosuppressant use on this risk. In two large, retrospective cohort studies they found that, compared with patients without IBD, patients with IBD had a significantly increased risk of developing herpes zoster. Furthermore, this risk was higher in those with recent or cumulative steroid use and in those treated with thiopurines (as monotherapy or in combination with anti-TNF agents). Interestingly, exposure to TNF antagonists alone was not associated with an increased risk of herpes zoster infection.
Richa Shukla, MD, assistant professor, section of gastroenterology and hepatology, Baylor College of Medicine, Houston.
Patients with inflammatory bowel disease are thought to have altered immune regulation, which may increase the risk of systemic complications including infections like herpes zoster. Many of the prior studies assessing the risk of herpes zoster in IBD patients were done before the advent of biologics and excluded older patients, thereby limiting their utility. This study by Khan et al. aimed to better estimate the incidence and risk factors for development of herpes zoster and to determine the effect of immunosuppressant use on this risk. In two large, retrospective cohort studies they found that, compared with patients without IBD, patients with IBD had a significantly increased risk of developing herpes zoster. Furthermore, this risk was higher in those with recent or cumulative steroid use and in those treated with thiopurines (as monotherapy or in combination with anti-TNF agents). Interestingly, exposure to TNF antagonists alone was not associated with an increased risk of herpes zoster infection.
Richa Shukla, MD, assistant professor, section of gastroenterology and hepatology, Baylor College of Medicine, Houston.
For patients with inflammatory bowel disease (IBD), thiopurine exposure was associated with a significantly increased risk of herpes zoster, compared with 5-aminosalicylic acid (5-ASA) monotherapy, according to the results of two large retrospective cohort studies.
In the multivariable analysis, thiopurine monotherapy was linked to about a 47% increase in the risk of herpes zoster, compared with 5-ASA monotherapy (adjusted hazard ratio, 1.47; 95% confidence interval, 1.31-1.65; P less than .001). Combination therapy with thiopurines and tumor necrosis factor antagonists conferred about a 65% increase in zoster risk (aHR, 1.65; 95% CI, 1.22-2.23; P = .001). However, tumor necrosis factor–antagonist monotherapy did not appear to significantly increase the risk of zoster when compared with 5-ASA monotherapy, reported Nabeel Khan, MD, of the University of Pennsylvania in Philadelphia, and his associates.
“Compared to [patients without] IBD, ulcerative colitis (UC) and Crohn’s disease (CD) each were associated with significantly increased risk of herpes zoster infection,” the researchers wrote online in Clinical Gastroenterology and Hepatology. “With the approval of a new and potentially safer vaccine for herpes zoster, the effects of immunization of patients with IBD should be investigated.”
Past studies have linked IBD with a 1.2- to 1.8-fold increase in the risk of zoster, but these studies date to the prebiologic era or excluded patients who were in their midsixties or older, the researchers wrote. “Additionally, these prior studies have not assessed the validity of the codes used to identify herpes zoster and also did not account for the impact of vaccination,” they added. “They also did not take into consideration the severity of the disease or degree of steroid exposure.”
Therefore, the researchers conducted two retrospective cohort studies of patients in the United States Department of Veterans Affairs between 2000 and 2016. The first cohort study compared the incidence of herpes zoster among patients with IBD who received 5-ASA alone with matched patients without IBD. The second cohort study measured the incidence of herpes zoster in patients with IBD who received various medications and combination regimen. “The VA has a predominantly older population, which makes it an ideal cohort to study herpes zoster incidence in a high-risk population,” the investigators noted. “Unlike insurance databases, the VA database can be validated internally and vaccination records are documented.”
After adjusting for age, race, sex, geographic region, disease flare, corticosteroid use, and baseline comorbidities, the estimated hazard of developing herpes zoster was 1.81 (95% confidence interval, 1.56-2.11) among patients with ulcerative colitis and 1.56 (95% CI, 1.28-1.91) among patients with Crohn’s disease, as compared with patients without IBD. Regardless of their age or the medications they were receiving, patients with IBD had a higher incidence of zoster than the oldest group of patients without IBD (older than 60 years), regardless of age or medication. “The highest risk of herpes zoster was observed in patients with IBD who were less than 60 years of age and on combination therapy,” the investigators wrote. “Patients with IBD younger than 50 years who were on combination therapy had higher risk of herpes zoster, compared with patients with IBD older than 60 years of age who were not on immunosuppressive therapy.” Based on the findings, they recommended studying the efficacy of widespread use of the new herpes zoster vaccine in patients with IBD.
Pfizer provided unrestricted research funding but was not otherwise involved in the study. One coinvestigator disclosed ties to Pfizer and several other pharmaceutical companies. The remaining investigators reported having no conflicts of interest.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Jan 5. doi: 10.1016/j.cgh.2017.12.052.
For patients with inflammatory bowel disease (IBD), thiopurine exposure was associated with a significantly increased risk of herpes zoster, compared with 5-aminosalicylic acid (5-ASA) monotherapy, according to the results of two large retrospective cohort studies.
In the multivariable analysis, thiopurine monotherapy was linked to about a 47% increase in the risk of herpes zoster, compared with 5-ASA monotherapy (adjusted hazard ratio, 1.47; 95% confidence interval, 1.31-1.65; P less than .001). Combination therapy with thiopurines and tumor necrosis factor antagonists conferred about a 65% increase in zoster risk (aHR, 1.65; 95% CI, 1.22-2.23; P = .001). However, tumor necrosis factor–antagonist monotherapy did not appear to significantly increase the risk of zoster when compared with 5-ASA monotherapy, reported Nabeel Khan, MD, of the University of Pennsylvania in Philadelphia, and his associates.
“Compared to [patients without] IBD, ulcerative colitis (UC) and Crohn’s disease (CD) each were associated with significantly increased risk of herpes zoster infection,” the researchers wrote online in Clinical Gastroenterology and Hepatology. “With the approval of a new and potentially safer vaccine for herpes zoster, the effects of immunization of patients with IBD should be investigated.”
Past studies have linked IBD with a 1.2- to 1.8-fold increase in the risk of zoster, but these studies date to the prebiologic era or excluded patients who were in their midsixties or older, the researchers wrote. “Additionally, these prior studies have not assessed the validity of the codes used to identify herpes zoster and also did not account for the impact of vaccination,” they added. “They also did not take into consideration the severity of the disease or degree of steroid exposure.”
Therefore, the researchers conducted two retrospective cohort studies of patients in the United States Department of Veterans Affairs between 2000 and 2016. The first cohort study compared the incidence of herpes zoster among patients with IBD who received 5-ASA alone with matched patients without IBD. The second cohort study measured the incidence of herpes zoster in patients with IBD who received various medications and combination regimen. “The VA has a predominantly older population, which makes it an ideal cohort to study herpes zoster incidence in a high-risk population,” the investigators noted. “Unlike insurance databases, the VA database can be validated internally and vaccination records are documented.”
After adjusting for age, race, sex, geographic region, disease flare, corticosteroid use, and baseline comorbidities, the estimated hazard of developing herpes zoster was 1.81 (95% confidence interval, 1.56-2.11) among patients with ulcerative colitis and 1.56 (95% CI, 1.28-1.91) among patients with Crohn’s disease, as compared with patients without IBD. Regardless of their age or the medications they were receiving, patients with IBD had a higher incidence of zoster than the oldest group of patients without IBD (older than 60 years), regardless of age or medication. “The highest risk of herpes zoster was observed in patients with IBD who were less than 60 years of age and on combination therapy,” the investigators wrote. “Patients with IBD younger than 50 years who were on combination therapy had higher risk of herpes zoster, compared with patients with IBD older than 60 years of age who were not on immunosuppressive therapy.” Based on the findings, they recommended studying the efficacy of widespread use of the new herpes zoster vaccine in patients with IBD.
Pfizer provided unrestricted research funding but was not otherwise involved in the study. One coinvestigator disclosed ties to Pfizer and several other pharmaceutical companies. The remaining investigators reported having no conflicts of interest.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Jan 5. doi: 10.1016/j.cgh.2017.12.052.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: For patients with inflammatory bowel disease, thiopurine exposure was associated with a significantly increased risk of herpes zoster, compared with 5-aminosalicylic acid monotherapy.
Major finding: The adjusted hazard ratio was 1.47 (95% confidence interval, 1.31-1.65; P less than .001).
Study details: Two large retrospective cohort studies of veterans with and without inflammatory bowel disease.
Disclosures: Pfizer provided unrestricted research funding but was not otherwise involved in the study. One coinvestigator disclosed ties to Pfizer and several other pharmaceutical companies. The remaining investigators reported having no conflicts of interest.
Source: Khan N et al. Clin Gastroenterol Hepatol. 2018 Jan 5. doi: 10.1016/j.cgh.2017.12.052.
What Are the Clinical, Laboratory, and Electrodiagnostic Features of Zinc Deficiency-Induced Peripheral Neuropathy?
Reduced tendon reflexes and an abnormal Romberg test may be common in patients with this disorder.
WASHINGTON, DC—Patients with zinc deficiency-induced peripheral neuropathy may present with paresthesia, gait abnormalities, sensory deficits, reduced tendon reflexes, an abnormal Romberg test, and increased CSF protein, according to a study presented at the 2018 Annual Meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine.
Recognition of the features of zinc deficiency-induced peripheral neuropathy may help neurologists diagnose the disorder and manage patients, researchers said.
“Zinc, an essential trace element, plays a critical role in maintaining normal structural and functional conditions in the body,” said lead author Favio C. Bumanlag, Chief Technologist in the Department of Neurology at the Lewis Katz School of Medicine at Temple University in Philadelphia. “Peripheral nerves are susceptible to damage when zinc deficiency occurs.... Recognition of [zinc deficiency-induced peripheral neuropathy] will help physicians and technologists effectively manage patients.”
To study the clinical and electrophysiologic features of zinc deficiency-induced peripheral neuropathy, Mr. Bumanlag and Jin Luo, MD, PhD, Professor of Neurology and Pharmacology at Temple University, retrospectively reviewed charts in their neuromuscular clinic and EMG laboratory database to identify patients with peripheral neuropathy and zinc deficiency. They included charts from between January 1, 2015, and December 31, 2017, in their review. They excluded patients with abnormal copper levels.
Mr. Bumanlag and Dr. Luo obtained information about patients’ clinical presentations, past medical histories, BMI, neurologic examinations, and laboratory results. They also examined patients’ needle electromyograms and nerve conduction studies.
In all, they identified 12 patients with peripheral neuropathy and zinc deficiency. Patients had a mean age of 55.1. Six were female. Patients’ mean zinc level was 52.5 μg/dL, with a range of 37 μg/dL to 58 μg/dL (reference, 56–134 μg/dL). Mean copper level was 107.6 μg/dL, with a range of 84 μg/dL to 173 μg/dL (reference, 72–166 μg/dL). Eleven of the 12 patients had received an electrophysiologic evaluation.
Notable findings in presentation included paresthesia in 75 and gait abnormalities in 42%. One patient was obese (8%), and three patients had diarrhea (25%). Neurologic examination showed sensory deficits in 83%, reduced tendon reflexes in 67%, and an abnormal Romberg test in 67%. Four of five patients had increased CSF protein. Electrophysiologic evaluations showed features of demyelinating peripheral neuropathy (28%) and distally active denervation in the lower extremities.
“Zinc participates in more than 200 enzymatic reactions,” said the researchers. “Unfortunately, zinc deficiency-induced peripheral neuropathy is often misdiagnosed or delayed in diagnosis. Literature on zinc deficiency-induced peripheral neuropathy is sparse.”
Reduced tendon reflexes and an abnormal Romberg test may be common in patients with this disorder.
Reduced tendon reflexes and an abnormal Romberg test may be common in patients with this disorder.
WASHINGTON, DC—Patients with zinc deficiency-induced peripheral neuropathy may present with paresthesia, gait abnormalities, sensory deficits, reduced tendon reflexes, an abnormal Romberg test, and increased CSF protein, according to a study presented at the 2018 Annual Meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine.
Recognition of the features of zinc deficiency-induced peripheral neuropathy may help neurologists diagnose the disorder and manage patients, researchers said.
“Zinc, an essential trace element, plays a critical role in maintaining normal structural and functional conditions in the body,” said lead author Favio C. Bumanlag, Chief Technologist in the Department of Neurology at the Lewis Katz School of Medicine at Temple University in Philadelphia. “Peripheral nerves are susceptible to damage when zinc deficiency occurs.... Recognition of [zinc deficiency-induced peripheral neuropathy] will help physicians and technologists effectively manage patients.”
To study the clinical and electrophysiologic features of zinc deficiency-induced peripheral neuropathy, Mr. Bumanlag and Jin Luo, MD, PhD, Professor of Neurology and Pharmacology at Temple University, retrospectively reviewed charts in their neuromuscular clinic and EMG laboratory database to identify patients with peripheral neuropathy and zinc deficiency. They included charts from between January 1, 2015, and December 31, 2017, in their review. They excluded patients with abnormal copper levels.
Mr. Bumanlag and Dr. Luo obtained information about patients’ clinical presentations, past medical histories, BMI, neurologic examinations, and laboratory results. They also examined patients’ needle electromyograms and nerve conduction studies.
In all, they identified 12 patients with peripheral neuropathy and zinc deficiency. Patients had a mean age of 55.1. Six were female. Patients’ mean zinc level was 52.5 μg/dL, with a range of 37 μg/dL to 58 μg/dL (reference, 56–134 μg/dL). Mean copper level was 107.6 μg/dL, with a range of 84 μg/dL to 173 μg/dL (reference, 72–166 μg/dL). Eleven of the 12 patients had received an electrophysiologic evaluation.
Notable findings in presentation included paresthesia in 75 and gait abnormalities in 42%. One patient was obese (8%), and three patients had diarrhea (25%). Neurologic examination showed sensory deficits in 83%, reduced tendon reflexes in 67%, and an abnormal Romberg test in 67%. Four of five patients had increased CSF protein. Electrophysiologic evaluations showed features of demyelinating peripheral neuropathy (28%) and distally active denervation in the lower extremities.
“Zinc participates in more than 200 enzymatic reactions,” said the researchers. “Unfortunately, zinc deficiency-induced peripheral neuropathy is often misdiagnosed or delayed in diagnosis. Literature on zinc deficiency-induced peripheral neuropathy is sparse.”
WASHINGTON, DC—Patients with zinc deficiency-induced peripheral neuropathy may present with paresthesia, gait abnormalities, sensory deficits, reduced tendon reflexes, an abnormal Romberg test, and increased CSF protein, according to a study presented at the 2018 Annual Meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine.
Recognition of the features of zinc deficiency-induced peripheral neuropathy may help neurologists diagnose the disorder and manage patients, researchers said.
“Zinc, an essential trace element, plays a critical role in maintaining normal structural and functional conditions in the body,” said lead author Favio C. Bumanlag, Chief Technologist in the Department of Neurology at the Lewis Katz School of Medicine at Temple University in Philadelphia. “Peripheral nerves are susceptible to damage when zinc deficiency occurs.... Recognition of [zinc deficiency-induced peripheral neuropathy] will help physicians and technologists effectively manage patients.”
To study the clinical and electrophysiologic features of zinc deficiency-induced peripheral neuropathy, Mr. Bumanlag and Jin Luo, MD, PhD, Professor of Neurology and Pharmacology at Temple University, retrospectively reviewed charts in their neuromuscular clinic and EMG laboratory database to identify patients with peripheral neuropathy and zinc deficiency. They included charts from between January 1, 2015, and December 31, 2017, in their review. They excluded patients with abnormal copper levels.
Mr. Bumanlag and Dr. Luo obtained information about patients’ clinical presentations, past medical histories, BMI, neurologic examinations, and laboratory results. They also examined patients’ needle electromyograms and nerve conduction studies.
In all, they identified 12 patients with peripheral neuropathy and zinc deficiency. Patients had a mean age of 55.1. Six were female. Patients’ mean zinc level was 52.5 μg/dL, with a range of 37 μg/dL to 58 μg/dL (reference, 56–134 μg/dL). Mean copper level was 107.6 μg/dL, with a range of 84 μg/dL to 173 μg/dL (reference, 72–166 μg/dL). Eleven of the 12 patients had received an electrophysiologic evaluation.
Notable findings in presentation included paresthesia in 75 and gait abnormalities in 42%. One patient was obese (8%), and three patients had diarrhea (25%). Neurologic examination showed sensory deficits in 83%, reduced tendon reflexes in 67%, and an abnormal Romberg test in 67%. Four of five patients had increased CSF protein. Electrophysiologic evaluations showed features of demyelinating peripheral neuropathy (28%) and distally active denervation in the lower extremities.
“Zinc participates in more than 200 enzymatic reactions,” said the researchers. “Unfortunately, zinc deficiency-induced peripheral neuropathy is often misdiagnosed or delayed in diagnosis. Literature on zinc deficiency-induced peripheral neuropathy is sparse.”
Does Thymectomy Benefit Patients With Anti-MuSK Myasthenia Gravis?
Favorable clinical outcomes are not more likely in patients with anti-MuSK myasthenia gravis who undergo thymectomy versus patients who do not.
WASHINGTON, DC—Among patients with anti-muscle-specific kinase (MuSK) myasthenia gravis, thymectomy is not associated with greater likelihood of clinical improvement, according to an analysis of data from a multicenter cohort study. The results were presented at the 2018 Annual Meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM).
Although a randomized trial has demonstrated benefit from thymectomy in nonthymomatous antiacetylcholine receptor (AChR) antibody positive generalized myasthenia gravis, observational studies suggest that thymectomy may not be efficacious in anti-MuSK myasthenia gravis. Histologic studies have found that patients with anti-MuSK myasthenia gravis have less hyperplastic thymic tissue, compared with patients with anti-AChR myasthenia gravis.
To evaluate the therapeutic impact of thymectomy in patients with anti-MuSK myasthenia gravis, Katherine Clifford, a medical student at the University of Vermont Larner College of Medicine in Burlington, and colleagues analyzed data from a multicenter, retrospective, blinded review of rituximab treatment in patients with anti-MuSK myasthenia gravis. The primary outcome was favorable outcome on the Myasthenia Gravis Foundation of America (MGFA) Post-Intervention Status (PIS). The researchers defined a favorable outcome as an MGFA PIS score of minimal manifestations or better.
Secondary outcomes included prednisone dose; use of other immunosuppressant medications, IV immunoglobulin (IVIG), or plasma exchange (PLEX) treatment; and Myasthenia Gravis Status and Treatment Intensity (MGSTI).
Baseline characteristics were similar between patients with anti-MuSK myasthenia gravis who underwent thymectomy (n = 26) and those who did not (n = 29), including treatment with rituximab (42% vs 45%). Median follow-up was more than three years.
At last visit, 35% (nine of 26) of patients who underwent thymectomy had a favorable outcome, compared with 55% (16 of 29) of patients who did not undergo thymectomy. In addition, 69% of patients who underwent thymectomy were taking prednisone, compared with 41% of patients who did not undergo thymectomy (median dose, 10 mg/day vs 0 mg/day).
“After controlling for rituximab, baseline prednisone, and final IVIG/PLEX treatment, thymectomy was not associated with greater likelihood of favorable clinical outcome, but broad confidence intervals cannot exclude therapeutic effect (odds ratio, 0.43),” the investigators reported.
“The recent MGTX trial clearly demonstrated the benefit of thymectomy for patients with AChR antibody positive myasthenia gravis,” said A. Gordon Smith, MD, Cochair of the AANEM Annual Meeting Program Committee. “Ms. Clifford and her colleagues now provide compelling data suggesting thymectomy may not be effective in MuSK-positive myasthenia gravis.”
The study’s follow-up is long enough for the findings to be clinically “relevant to all physicians treating myasthenia gravis,” said Robert W. Irwin, MD, Cochair of the AANEM Annual Meeting Program Committee.
Favorable clinical outcomes are not more likely in patients with anti-MuSK myasthenia gravis who undergo thymectomy versus patients who do not.
Favorable clinical outcomes are not more likely in patients with anti-MuSK myasthenia gravis who undergo thymectomy versus patients who do not.
WASHINGTON, DC—Among patients with anti-muscle-specific kinase (MuSK) myasthenia gravis, thymectomy is not associated with greater likelihood of clinical improvement, according to an analysis of data from a multicenter cohort study. The results were presented at the 2018 Annual Meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM).
Although a randomized trial has demonstrated benefit from thymectomy in nonthymomatous antiacetylcholine receptor (AChR) antibody positive generalized myasthenia gravis, observational studies suggest that thymectomy may not be efficacious in anti-MuSK myasthenia gravis. Histologic studies have found that patients with anti-MuSK myasthenia gravis have less hyperplastic thymic tissue, compared with patients with anti-AChR myasthenia gravis.
To evaluate the therapeutic impact of thymectomy in patients with anti-MuSK myasthenia gravis, Katherine Clifford, a medical student at the University of Vermont Larner College of Medicine in Burlington, and colleagues analyzed data from a multicenter, retrospective, blinded review of rituximab treatment in patients with anti-MuSK myasthenia gravis. The primary outcome was favorable outcome on the Myasthenia Gravis Foundation of America (MGFA) Post-Intervention Status (PIS). The researchers defined a favorable outcome as an MGFA PIS score of minimal manifestations or better.
Secondary outcomes included prednisone dose; use of other immunosuppressant medications, IV immunoglobulin (IVIG), or plasma exchange (PLEX) treatment; and Myasthenia Gravis Status and Treatment Intensity (MGSTI).
Baseline characteristics were similar between patients with anti-MuSK myasthenia gravis who underwent thymectomy (n = 26) and those who did not (n = 29), including treatment with rituximab (42% vs 45%). Median follow-up was more than three years.
At last visit, 35% (nine of 26) of patients who underwent thymectomy had a favorable outcome, compared with 55% (16 of 29) of patients who did not undergo thymectomy. In addition, 69% of patients who underwent thymectomy were taking prednisone, compared with 41% of patients who did not undergo thymectomy (median dose, 10 mg/day vs 0 mg/day).
“After controlling for rituximab, baseline prednisone, and final IVIG/PLEX treatment, thymectomy was not associated with greater likelihood of favorable clinical outcome, but broad confidence intervals cannot exclude therapeutic effect (odds ratio, 0.43),” the investigators reported.
“The recent MGTX trial clearly demonstrated the benefit of thymectomy for patients with AChR antibody positive myasthenia gravis,” said A. Gordon Smith, MD, Cochair of the AANEM Annual Meeting Program Committee. “Ms. Clifford and her colleagues now provide compelling data suggesting thymectomy may not be effective in MuSK-positive myasthenia gravis.”
The study’s follow-up is long enough for the findings to be clinically “relevant to all physicians treating myasthenia gravis,” said Robert W. Irwin, MD, Cochair of the AANEM Annual Meeting Program Committee.
WASHINGTON, DC—Among patients with anti-muscle-specific kinase (MuSK) myasthenia gravis, thymectomy is not associated with greater likelihood of clinical improvement, according to an analysis of data from a multicenter cohort study. The results were presented at the 2018 Annual Meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM).
Although a randomized trial has demonstrated benefit from thymectomy in nonthymomatous antiacetylcholine receptor (AChR) antibody positive generalized myasthenia gravis, observational studies suggest that thymectomy may not be efficacious in anti-MuSK myasthenia gravis. Histologic studies have found that patients with anti-MuSK myasthenia gravis have less hyperplastic thymic tissue, compared with patients with anti-AChR myasthenia gravis.
To evaluate the therapeutic impact of thymectomy in patients with anti-MuSK myasthenia gravis, Katherine Clifford, a medical student at the University of Vermont Larner College of Medicine in Burlington, and colleagues analyzed data from a multicenter, retrospective, blinded review of rituximab treatment in patients with anti-MuSK myasthenia gravis. The primary outcome was favorable outcome on the Myasthenia Gravis Foundation of America (MGFA) Post-Intervention Status (PIS). The researchers defined a favorable outcome as an MGFA PIS score of minimal manifestations or better.
Secondary outcomes included prednisone dose; use of other immunosuppressant medications, IV immunoglobulin (IVIG), or plasma exchange (PLEX) treatment; and Myasthenia Gravis Status and Treatment Intensity (MGSTI).
Baseline characteristics were similar between patients with anti-MuSK myasthenia gravis who underwent thymectomy (n = 26) and those who did not (n = 29), including treatment with rituximab (42% vs 45%). Median follow-up was more than three years.
At last visit, 35% (nine of 26) of patients who underwent thymectomy had a favorable outcome, compared with 55% (16 of 29) of patients who did not undergo thymectomy. In addition, 69% of patients who underwent thymectomy were taking prednisone, compared with 41% of patients who did not undergo thymectomy (median dose, 10 mg/day vs 0 mg/day).
“After controlling for rituximab, baseline prednisone, and final IVIG/PLEX treatment, thymectomy was not associated with greater likelihood of favorable clinical outcome, but broad confidence intervals cannot exclude therapeutic effect (odds ratio, 0.43),” the investigators reported.
“The recent MGTX trial clearly demonstrated the benefit of thymectomy for patients with AChR antibody positive myasthenia gravis,” said A. Gordon Smith, MD, Cochair of the AANEM Annual Meeting Program Committee. “Ms. Clifford and her colleagues now provide compelling data suggesting thymectomy may not be effective in MuSK-positive myasthenia gravis.”
The study’s follow-up is long enough for the findings to be clinically “relevant to all physicians treating myasthenia gravis,” said Robert W. Irwin, MD, Cochair of the AANEM Annual Meeting Program Committee.
Medical calculator apps allow point of care, rapid decision-making
The most useful applications (apps) for health care professionals and students? Medical calculator apps (along with drug reference and disease diagnosis apps), according to surveys of clinicians and students.1,2 The utility of calculator apps to these groups is not surprising; calculator apps fall in the category of clinical decision-making apps, which also includes decision support systems, clinical treatment guidelines, disease diagnosis aids, differential diagnosis aids, laboratory test ordering, laboratory test interpretation, and medical exams.3 Calculator apps obviously save time as most health care providers have not memorized the many medical formulas and do not have computational speed. I have previously discussed other, more ObGyn-specific calculators, such as due date calculators.4,5 In this App Review column, however, I would like to highlight 3 general calculator apps: Calculate by QxMD, CliniCalc Medical Calculator, and Medscape. Researchers found all 3 apps 100% accurate and contained the most functions desired by internists.6 The apps are available at no cost and include many unique calculators. My colleagues and I actually used Calculate by QxMD to verify calculations in a previous study.7
A clinical example for how to apply calculators in practice is as follows: A multiparous patient at term has undergone an unscheduled cesarean delivery for arrest of dilation and intra-amniotic infection. You need to decide if the patient requires anti‑coagulants for deep venous thrombosis (DVT) prophylaxis and her necessary daily dose for gentamicin for postpartum infection prophylaxis. You can use Medscape’s body mass index (BMI) calculator to find out that this patient’s BMI is 45 kg/m2 and that DVT prophylaxis is in fact indicated. You also can use QxMD’s ideal body weight calculator to get the patient’s weight and determine the appropriate daily dose for gentamicin.
The TABLE provides more information on the apps, with its inclusions based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).7
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
1. Mosa AS, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
2. Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak. 2012;12:121.
3. Ventola CL. Mobile devices and apps for health care professionals: uses and benefits. P T. 2014;39:356-364.
4. Chen KT. Three good apps for calculating the date of delivery. OBG Manag. 2017;29:45-46.
5. Chen KT. ACOG app and applets: tools to augment your practice. OBG Manag. 2018;30:41-42.
6. Bierbrier R, Lo V, Wu RC. Evaluation of the accuracy of smartphone medical calculation apps. J Med Internet Res. 2014;16:e32.
7. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
The most useful applications (apps) for health care professionals and students? Medical calculator apps (along with drug reference and disease diagnosis apps), according to surveys of clinicians and students.1,2 The utility of calculator apps to these groups is not surprising; calculator apps fall in the category of clinical decision-making apps, which also includes decision support systems, clinical treatment guidelines, disease diagnosis aids, differential diagnosis aids, laboratory test ordering, laboratory test interpretation, and medical exams.3 Calculator apps obviously save time as most health care providers have not memorized the many medical formulas and do not have computational speed. I have previously discussed other, more ObGyn-specific calculators, such as due date calculators.4,5 In this App Review column, however, I would like to highlight 3 general calculator apps: Calculate by QxMD, CliniCalc Medical Calculator, and Medscape. Researchers found all 3 apps 100% accurate and contained the most functions desired by internists.6 The apps are available at no cost and include many unique calculators. My colleagues and I actually used Calculate by QxMD to verify calculations in a previous study.7
A clinical example for how to apply calculators in practice is as follows: A multiparous patient at term has undergone an unscheduled cesarean delivery for arrest of dilation and intra-amniotic infection. You need to decide if the patient requires anti‑coagulants for deep venous thrombosis (DVT) prophylaxis and her necessary daily dose for gentamicin for postpartum infection prophylaxis. You can use Medscape’s body mass index (BMI) calculator to find out that this patient’s BMI is 45 kg/m2 and that DVT prophylaxis is in fact indicated. You also can use QxMD’s ideal body weight calculator to get the patient’s weight and determine the appropriate daily dose for gentamicin.
The TABLE provides more information on the apps, with its inclusions based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).7

Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The most useful applications (apps) for health care professionals and students? Medical calculator apps (along with drug reference and disease diagnosis apps), according to surveys of clinicians and students.1,2 The utility of calculator apps to these groups is not surprising; calculator apps fall in the category of clinical decision-making apps, which also includes decision support systems, clinical treatment guidelines, disease diagnosis aids, differential diagnosis aids, laboratory test ordering, laboratory test interpretation, and medical exams.3 Calculator apps obviously save time as most health care providers have not memorized the many medical formulas and do not have computational speed. I have previously discussed other, more ObGyn-specific calculators, such as due date calculators.4,5 In this App Review column, however, I would like to highlight 3 general calculator apps: Calculate by QxMD, CliniCalc Medical Calculator, and Medscape. Researchers found all 3 apps 100% accurate and contained the most functions desired by internists.6 The apps are available at no cost and include many unique calculators. My colleagues and I actually used Calculate by QxMD to verify calculations in a previous study.7
A clinical example for how to apply calculators in practice is as follows: A multiparous patient at term has undergone an unscheduled cesarean delivery for arrest of dilation and intra-amniotic infection. You need to decide if the patient requires anti‑coagulants for deep venous thrombosis (DVT) prophylaxis and her necessary daily dose for gentamicin for postpartum infection prophylaxis. You can use Medscape’s body mass index (BMI) calculator to find out that this patient’s BMI is 45 kg/m2 and that DVT prophylaxis is in fact indicated. You also can use QxMD’s ideal body weight calculator to get the patient’s weight and determine the appropriate daily dose for gentamicin.
The TABLE provides more information on the apps, with its inclusions based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).7

Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
1. Mosa AS, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
2. Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak. 2012;12:121.
3. Ventola CL. Mobile devices and apps for health care professionals: uses and benefits. P T. 2014;39:356-364.
4. Chen KT. Three good apps for calculating the date of delivery. OBG Manag. 2017;29:45-46.
5. Chen KT. ACOG app and applets: tools to augment your practice. OBG Manag. 2018;30:41-42.
6. Bierbrier R, Lo V, Wu RC. Evaluation of the accuracy of smartphone medical calculation apps. J Med Internet Res. 2014;16:e32.
7. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
1. Mosa AS, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
2. Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak. 2012;12:121.
3. Ventola CL. Mobile devices and apps for health care professionals: uses and benefits. P T. 2014;39:356-364.
4. Chen KT. Three good apps for calculating the date of delivery. OBG Manag. 2017;29:45-46.
5. Chen KT. ACOG app and applets: tools to augment your practice. OBG Manag. 2018;30:41-42.
6. Bierbrier R, Lo V, Wu RC. Evaluation of the accuracy of smartphone medical calculation apps. J Med Internet Res. 2014;16:e32.
7. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.