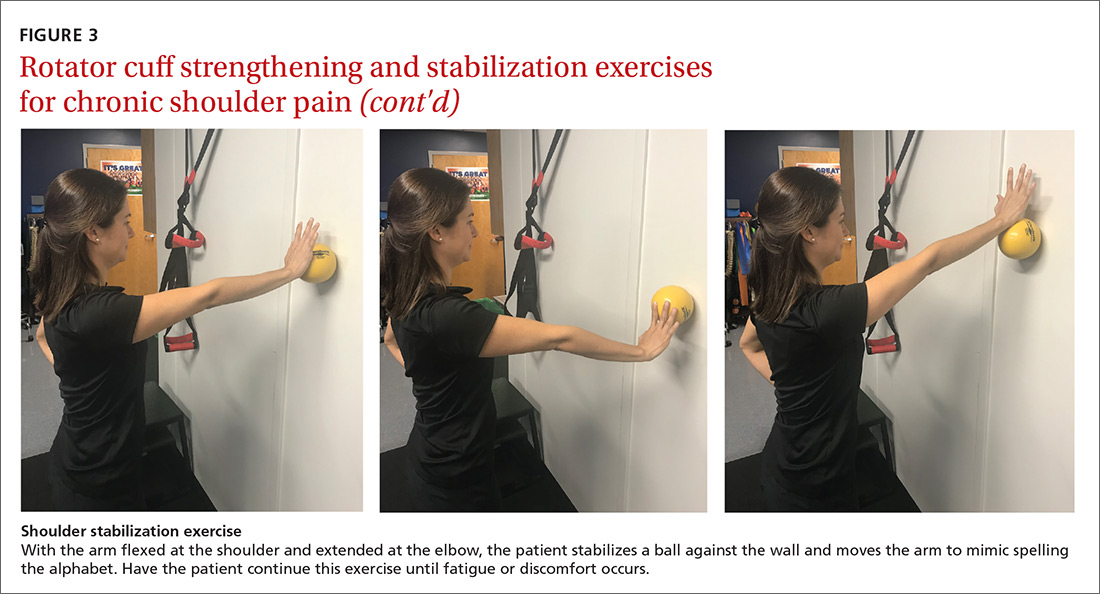
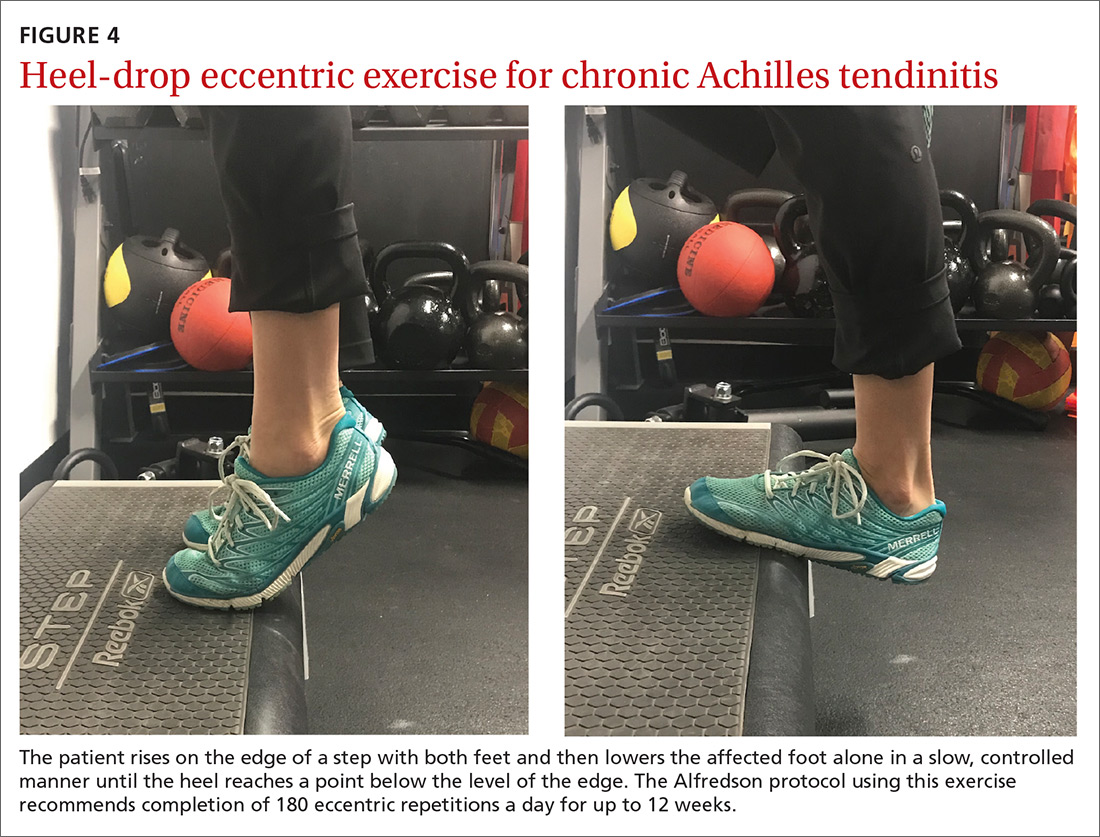
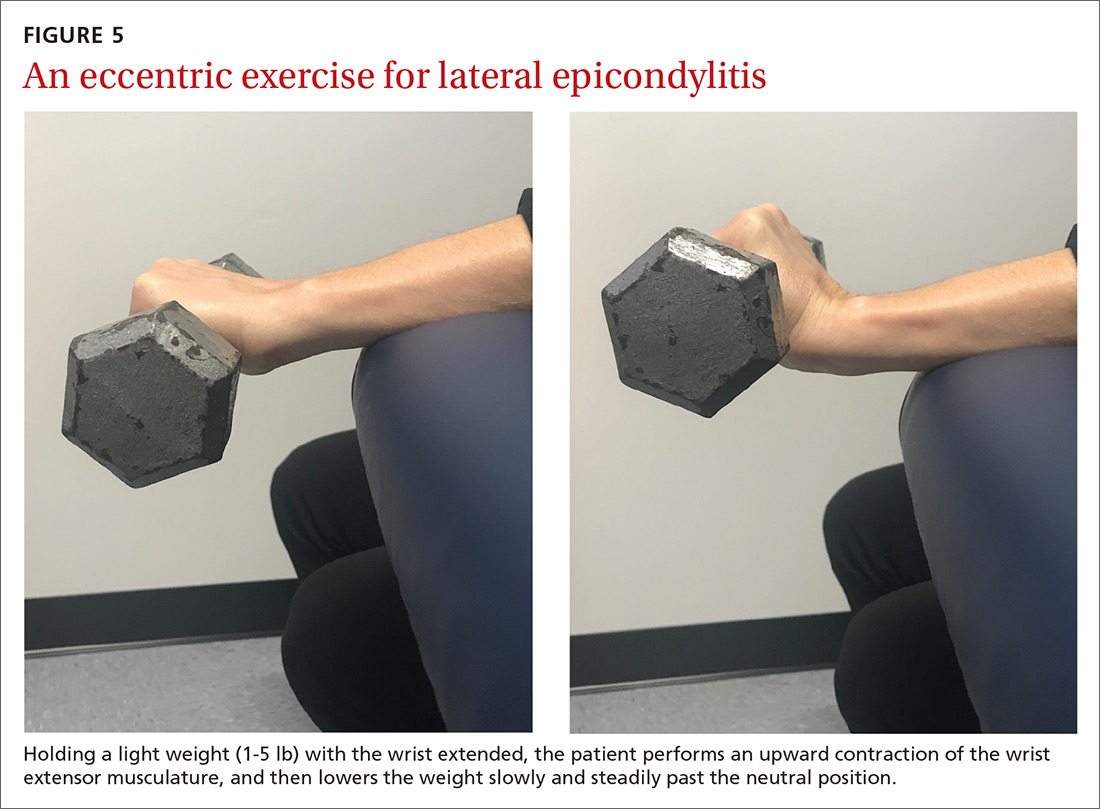
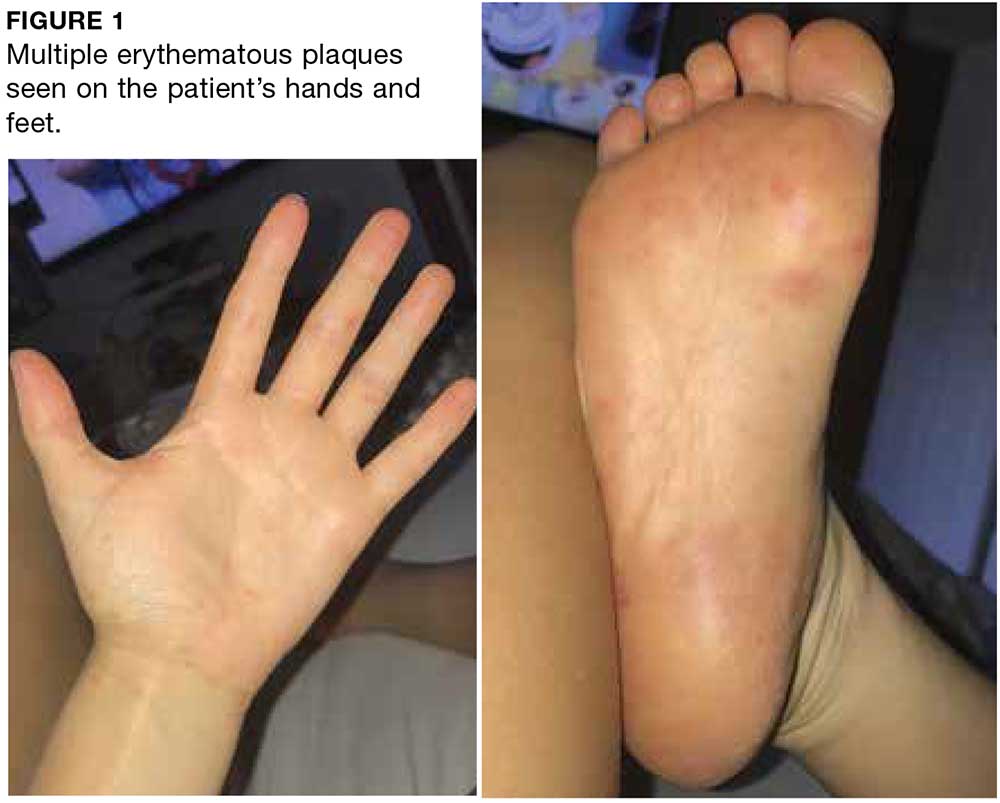
User login
How often does long-term PPI therapy cause clinically significant hypomagnesemia?
EVIDENCE SUMMARY
A systematic review and meta-analysis of observational studies examined the risk of hypomagnesemia, defined in various studies as serum magnesium levels of 1.6, 1.7, or 1.8 mg/dL.1 Two cohort studies, one case-control study, and 6 cross-sectional studies met inclusion criteria; 115,455 patients were enrolled. The studies were significantly heterogeneous (I2=89.1%), because of varying study designs, population sizes, and population characteristics.
PPI use increased the risk of hypomagnesemia (pooled odds ratio [OR]=1.5; 95% confidence interval [CI], 1.1-2.0) after adjustment for possible confounders such as use of diuretics.
Risk rises with long-term use, but severe hypomagnesemia is rare
Two more recent cohort studies produced conflicting results. Of 414 patients in a managed care cohort who received long-term PPIs, only 8 had mild hypomagnesemia (1.2-1.5 mg/dL) on nearly 14% of their combined 289 measurements. At final measurement, all patients had normal serum magnesium levels.2
A cross-sectional analysis of data from a retrospective cohort analysis of 9818 patients in the Netherlands found that any PPI use during the previous year was associated with an increased risk of hypomagnesemia (serum magnesium <1.73 mg/dL) compared with no use (adjusted OR=2; 95% CI, 1.4-2.9).3 The risk was greatest with use longer than 182 days (OR=3.0; 95% CI, 1.7-5.2). As with studies included in the meta-analysis, this study examined laboratory values exclusively. Only 3 of 724 PPI users had a serum magnesium level below 1.2 mg/dL, the point at which symptoms usually occur.
Case-control studies produce conflicting results
Two recent case-control studies also produced conflicting results. The first compared 154 outpatients who used PPIs for at least 6 months (mean, 27.5 months) with 84 nonusers.4 No association was found with hypomagnesemia (2.17 mg/dL vs 2.19 mg/dL), and none of the patients had a serum magnesium level below 1.7 mg/dL. The control group was poorly defined, however, and the study excluded patients taking diuretics.
Conversely, a study that compared 366 patients hospitalized with a primary or secondary diagnosis of hypomagnesemia (determined from an insurance claims database and defined as the presence of ICD-10 codes for hypomagnesemia or magnesium deficiency) with 1464 matched controls found that hospitalized patients with hypomagnesemia were more likely than controls to be current PPI users (adjusted OR=1.4; 95% CI, 1.1-1.9).5 Whether hypomagnesemia was the cause of the hospitalizations or an incidental finding wasn’t clear.
Concurrent use of diuretics and loop diuretics can increase risk
In a subgroup analysis of the second case-control study, PPI users who also used diuretics had an increased risk of hypomagnesemia (adjusted OR=1.7; 95% CI, 1.1-2.7) compared with patients who weren’t taking diuretics (adjusted OR=1.3; 95% CI, 0.8-1.9).5
Continue to: A comparison of the use of loop diuretics and...
A comparison of the use of loop diuretics and thiazides by patients taking PPIs found that concurrent use of loop diuretics increased serum magnesium reduction (−0.08 mg/dL; 95% CI, −0.14 to −0.02), but thiazides didn’t. Numbers were small: Of the 45 participants taking both a PPI and a loop diuretic, only 5 had hypomagnesemia (OR=7.2; 95% CI, 1.7-30.8).3
RECOMMENDATIONS
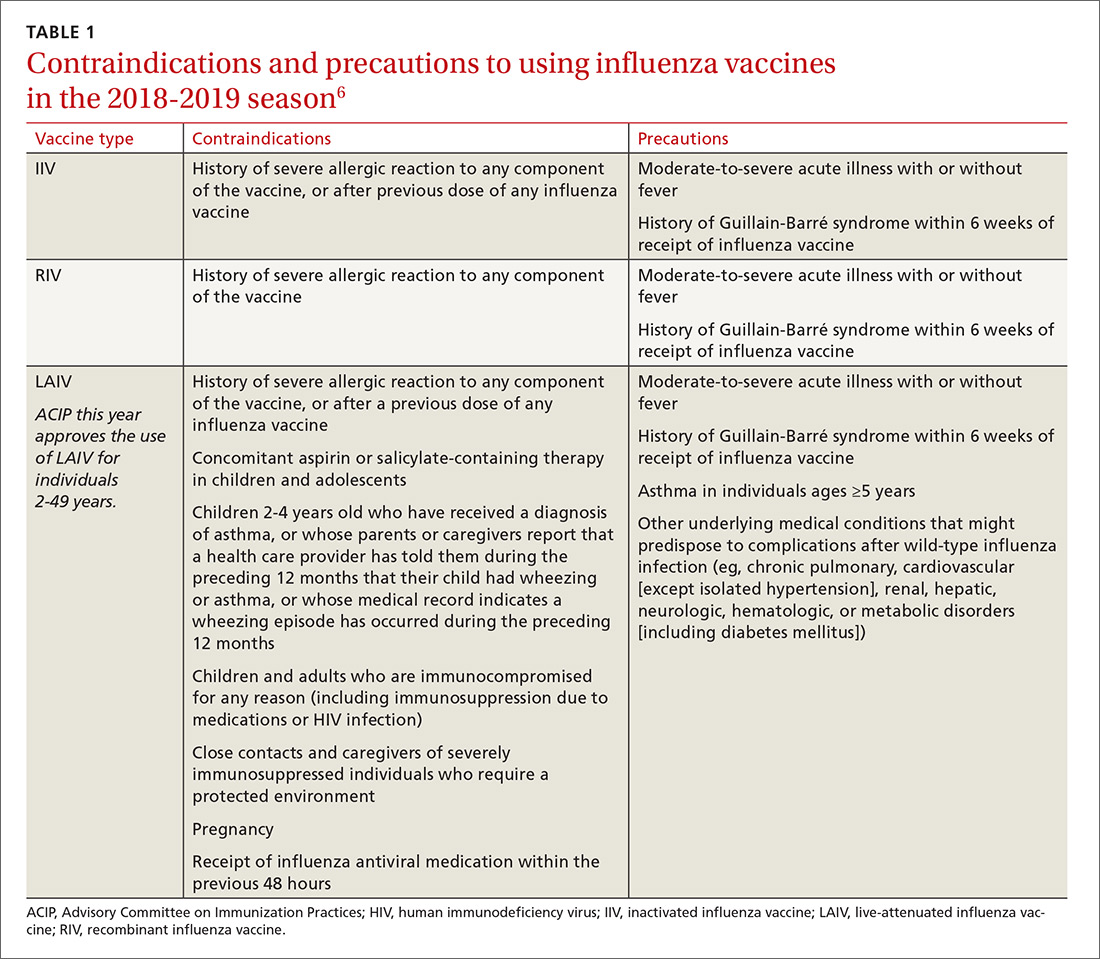
In 2011, the US Food and Drug Administration (FDA) warned of a possible increased risk of hypomagnesemia in patients taking PPIs long-term. The FDA advisory panel recommended evaluating serum magnesium before beginning long-term PPI therapy and in patients concurrently taking diuretics, digoxin, or other medications associated with hypomagnesemia.6
1. Park CH, Kim EH, Roh YH, et al. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558.
2. Sharara AI, Chalhoub JM, Hammoud N, et al. Low prevalence of hypomagnesemia in long-term recipients of proton pump inhibitors in a managed care cohort. Clin Gastroenterol Hepatol. 2016;14:317-321.
3. Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis. 2015;66:775-782.
4. Biyik M, Solak Y, Ucar R, et al. Hypomagnesemia among outpatient long-term proton pump inhibitor users. Am J Ther. 2014;24:e52-e55.
5. Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11:e1001736.
6. United States Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). 03/02/2011. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Accessed August 24, 2018.
EVIDENCE SUMMARY
A systematic review and meta-analysis of observational studies examined the risk of hypomagnesemia, defined in various studies as serum magnesium levels of 1.6, 1.7, or 1.8 mg/dL.1 Two cohort studies, one case-control study, and 6 cross-sectional studies met inclusion criteria; 115,455 patients were enrolled. The studies were significantly heterogeneous (I2=89.1%), because of varying study designs, population sizes, and population characteristics.
PPI use increased the risk of hypomagnesemia (pooled odds ratio [OR]=1.5; 95% confidence interval [CI], 1.1-2.0) after adjustment for possible confounders such as use of diuretics.
Risk rises with long-term use, but severe hypomagnesemia is rare
Two more recent cohort studies produced conflicting results. Of 414 patients in a managed care cohort who received long-term PPIs, only 8 had mild hypomagnesemia (1.2-1.5 mg/dL) on nearly 14% of their combined 289 measurements. At final measurement, all patients had normal serum magnesium levels.2
A cross-sectional analysis of data from a retrospective cohort analysis of 9818 patients in the Netherlands found that any PPI use during the previous year was associated with an increased risk of hypomagnesemia (serum magnesium <1.73 mg/dL) compared with no use (adjusted OR=2; 95% CI, 1.4-2.9).3 The risk was greatest with use longer than 182 days (OR=3.0; 95% CI, 1.7-5.2). As with studies included in the meta-analysis, this study examined laboratory values exclusively. Only 3 of 724 PPI users had a serum magnesium level below 1.2 mg/dL, the point at which symptoms usually occur.
Case-control studies produce conflicting results
Two recent case-control studies also produced conflicting results. The first compared 154 outpatients who used PPIs for at least 6 months (mean, 27.5 months) with 84 nonusers.4 No association was found with hypomagnesemia (2.17 mg/dL vs 2.19 mg/dL), and none of the patients had a serum magnesium level below 1.7 mg/dL. The control group was poorly defined, however, and the study excluded patients taking diuretics.
Conversely, a study that compared 366 patients hospitalized with a primary or secondary diagnosis of hypomagnesemia (determined from an insurance claims database and defined as the presence of ICD-10 codes for hypomagnesemia or magnesium deficiency) with 1464 matched controls found that hospitalized patients with hypomagnesemia were more likely than controls to be current PPI users (adjusted OR=1.4; 95% CI, 1.1-1.9).5 Whether hypomagnesemia was the cause of the hospitalizations or an incidental finding wasn’t clear.
Concurrent use of diuretics and loop diuretics can increase risk
In a subgroup analysis of the second case-control study, PPI users who also used diuretics had an increased risk of hypomagnesemia (adjusted OR=1.7; 95% CI, 1.1-2.7) compared with patients who weren’t taking diuretics (adjusted OR=1.3; 95% CI, 0.8-1.9).5
Continue to: A comparison of the use of loop diuretics and...
A comparison of the use of loop diuretics and thiazides by patients taking PPIs found that concurrent use of loop diuretics increased serum magnesium reduction (−0.08 mg/dL; 95% CI, −0.14 to −0.02), but thiazides didn’t. Numbers were small: Of the 45 participants taking both a PPI and a loop diuretic, only 5 had hypomagnesemia (OR=7.2; 95% CI, 1.7-30.8).3
RECOMMENDATIONS
In 2011, the US Food and Drug Administration (FDA) warned of a possible increased risk of hypomagnesemia in patients taking PPIs long-term. The FDA advisory panel recommended evaluating serum magnesium before beginning long-term PPI therapy and in patients concurrently taking diuretics, digoxin, or other medications associated with hypomagnesemia.6
EVIDENCE SUMMARY
A systematic review and meta-analysis of observational studies examined the risk of hypomagnesemia, defined in various studies as serum magnesium levels of 1.6, 1.7, or 1.8 mg/dL.1 Two cohort studies, one case-control study, and 6 cross-sectional studies met inclusion criteria; 115,455 patients were enrolled. The studies were significantly heterogeneous (I2=89.1%), because of varying study designs, population sizes, and population characteristics.
PPI use increased the risk of hypomagnesemia (pooled odds ratio [OR]=1.5; 95% confidence interval [CI], 1.1-2.0) after adjustment for possible confounders such as use of diuretics.
Risk rises with long-term use, but severe hypomagnesemia is rare
Two more recent cohort studies produced conflicting results. Of 414 patients in a managed care cohort who received long-term PPIs, only 8 had mild hypomagnesemia (1.2-1.5 mg/dL) on nearly 14% of their combined 289 measurements. At final measurement, all patients had normal serum magnesium levels.2
A cross-sectional analysis of data from a retrospective cohort analysis of 9818 patients in the Netherlands found that any PPI use during the previous year was associated with an increased risk of hypomagnesemia (serum magnesium <1.73 mg/dL) compared with no use (adjusted OR=2; 95% CI, 1.4-2.9).3 The risk was greatest with use longer than 182 days (OR=3.0; 95% CI, 1.7-5.2). As with studies included in the meta-analysis, this study examined laboratory values exclusively. Only 3 of 724 PPI users had a serum magnesium level below 1.2 mg/dL, the point at which symptoms usually occur.
Case-control studies produce conflicting results
Two recent case-control studies also produced conflicting results. The first compared 154 outpatients who used PPIs for at least 6 months (mean, 27.5 months) with 84 nonusers.4 No association was found with hypomagnesemia (2.17 mg/dL vs 2.19 mg/dL), and none of the patients had a serum magnesium level below 1.7 mg/dL. The control group was poorly defined, however, and the study excluded patients taking diuretics.
Conversely, a study that compared 366 patients hospitalized with a primary or secondary diagnosis of hypomagnesemia (determined from an insurance claims database and defined as the presence of ICD-10 codes for hypomagnesemia or magnesium deficiency) with 1464 matched controls found that hospitalized patients with hypomagnesemia were more likely than controls to be current PPI users (adjusted OR=1.4; 95% CI, 1.1-1.9).5 Whether hypomagnesemia was the cause of the hospitalizations or an incidental finding wasn’t clear.
Concurrent use of diuretics and loop diuretics can increase risk
In a subgroup analysis of the second case-control study, PPI users who also used diuretics had an increased risk of hypomagnesemia (adjusted OR=1.7; 95% CI, 1.1-2.7) compared with patients who weren’t taking diuretics (adjusted OR=1.3; 95% CI, 0.8-1.9).5
Continue to: A comparison of the use of loop diuretics and...
A comparison of the use of loop diuretics and thiazides by patients taking PPIs found that concurrent use of loop diuretics increased serum magnesium reduction (−0.08 mg/dL; 95% CI, −0.14 to −0.02), but thiazides didn’t. Numbers were small: Of the 45 participants taking both a PPI and a loop diuretic, only 5 had hypomagnesemia (OR=7.2; 95% CI, 1.7-30.8).3
RECOMMENDATIONS
In 2011, the US Food and Drug Administration (FDA) warned of a possible increased risk of hypomagnesemia in patients taking PPIs long-term. The FDA advisory panel recommended evaluating serum magnesium before beginning long-term PPI therapy and in patients concurrently taking diuretics, digoxin, or other medications associated with hypomagnesemia.6
1. Park CH, Kim EH, Roh YH, et al. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558.
2. Sharara AI, Chalhoub JM, Hammoud N, et al. Low prevalence of hypomagnesemia in long-term recipients of proton pump inhibitors in a managed care cohort. Clin Gastroenterol Hepatol. 2016;14:317-321.
3. Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis. 2015;66:775-782.
4. Biyik M, Solak Y, Ucar R, et al. Hypomagnesemia among outpatient long-term proton pump inhibitor users. Am J Ther. 2014;24:e52-e55.
5. Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11:e1001736.
6. United States Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). 03/02/2011. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Accessed August 24, 2018.
1. Park CH, Kim EH, Roh YH, et al. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558.
2. Sharara AI, Chalhoub JM, Hammoud N, et al. Low prevalence of hypomagnesemia in long-term recipients of proton pump inhibitors in a managed care cohort. Clin Gastroenterol Hepatol. 2016;14:317-321.
3. Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis. 2015;66:775-782.
4. Biyik M, Solak Y, Ucar R, et al. Hypomagnesemia among outpatient long-term proton pump inhibitor users. Am J Ther. 2014;24:e52-e55.
5. Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11:e1001736.
6. United States Food and Drug Administration. FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs). 03/02/2011. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Accessed August 24, 2018.
EVIDENCE-BASED ANSWER:
Rarely. Proton pump inhibitors (PPIs) may be associated with decreases in serum magnesium laboratory values to below 1.6 to 1.8 mg/dL, especially when used concurrently with diuretics and loop diuretics (strength of recommendation [SOR]: C, disease-oriented outcomes based on cohort, case-control, and cross-sectional studies). Clinically significant or symptomatic hypomagnesemia (below 1.2 mg/dL) appears to be quite rare, however.
4 pearls for treating musculoskeletal pain
Musculoskeletal complaints are one of the top reasons patients visit family physicians, with more than
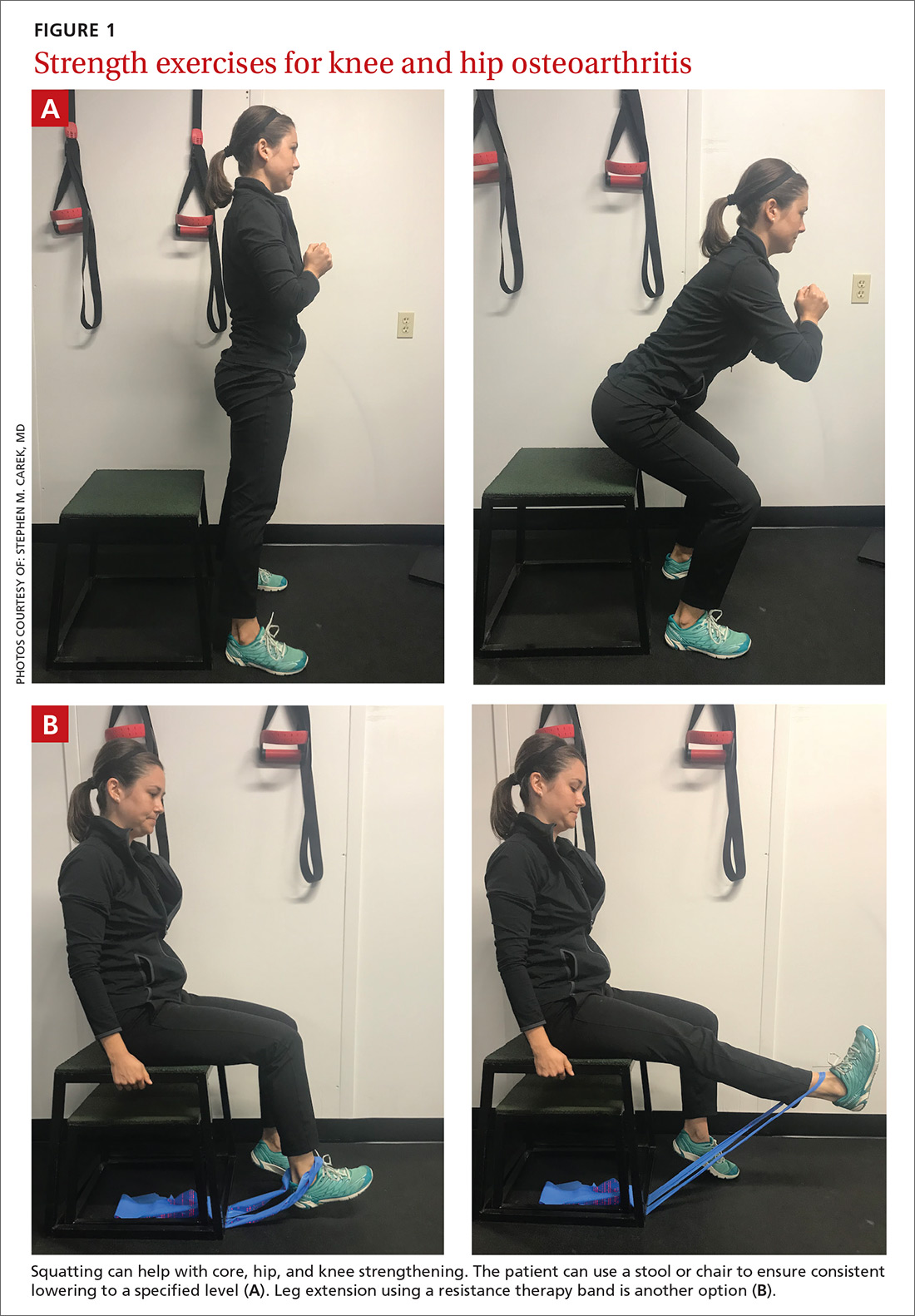
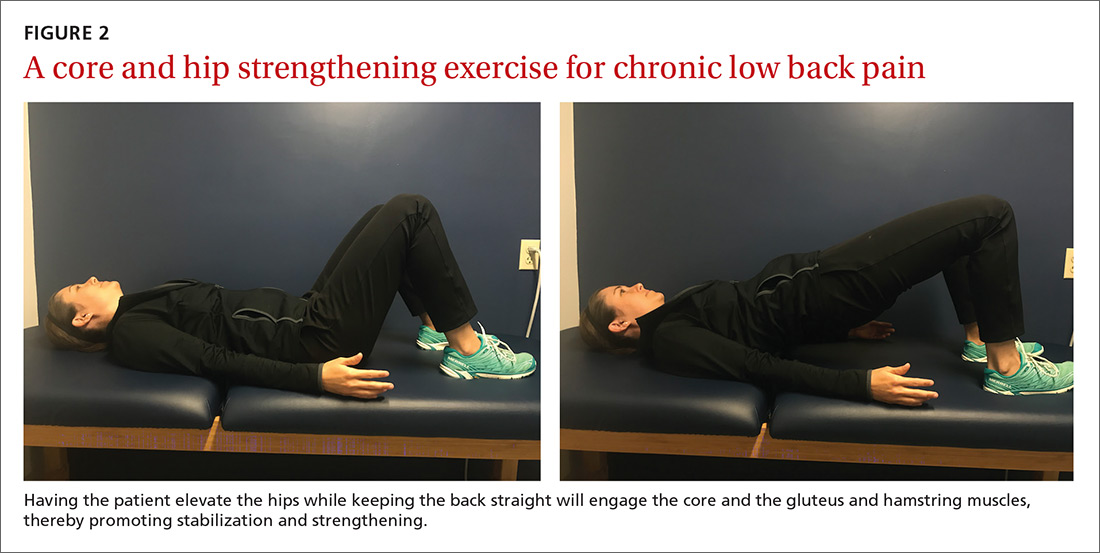
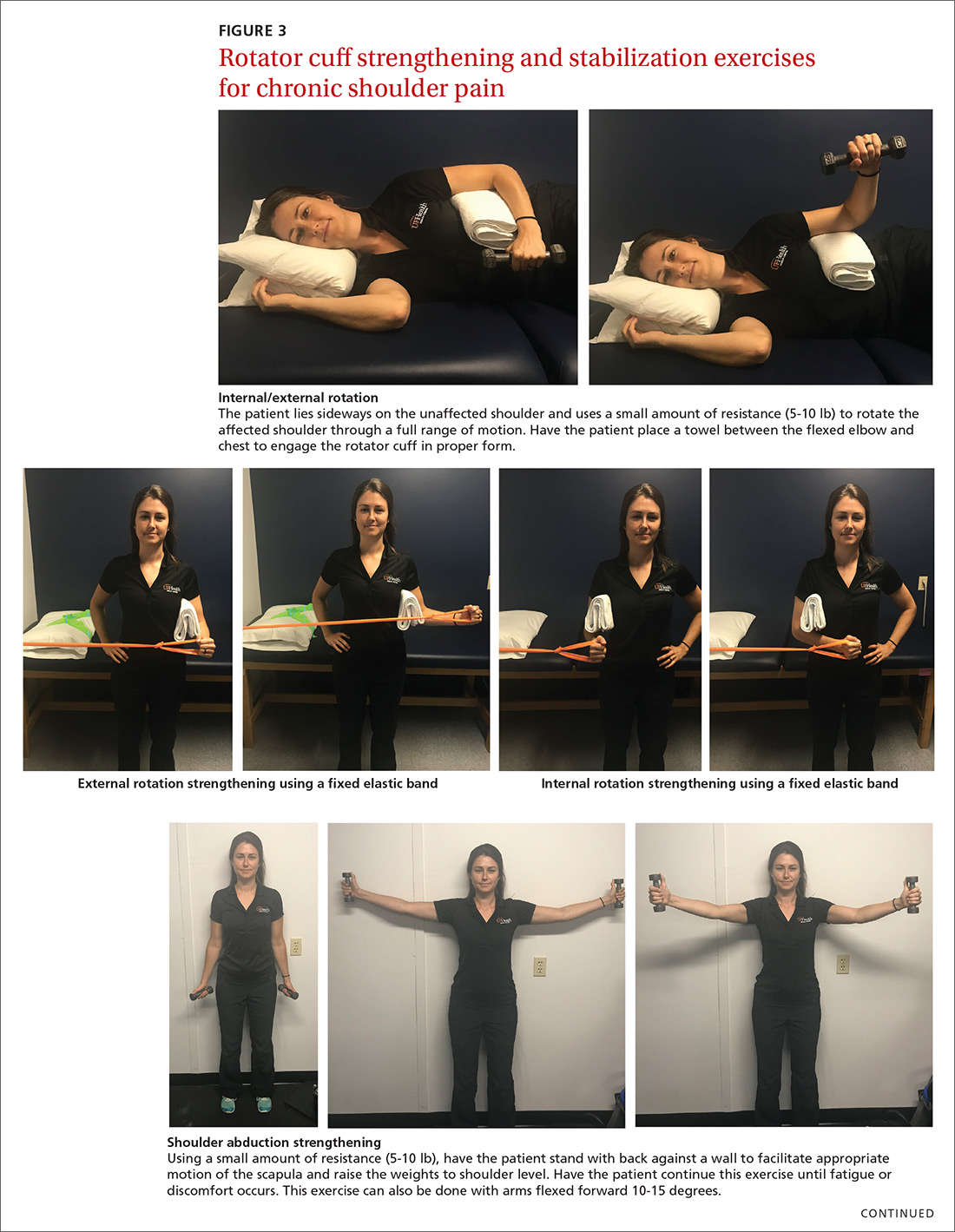
The article by Drs. Stephen and Peter Carek summarizes the value of specific exercises for hip and knee osteoarthritis (OA), chronic back pain, chronic shoulder pain, Achilles tendinitis, and lateral epicondylitis. This month’s PURL summarizes a negative randomized trial of treatment of knee OA with the popular over-the-counter combination of glucosamine and chondroitin. The findings? The group taking placebo actually had superior pain relief at 6 months!
What else works … and doesn’t? You may find that the following 4 “pearls,” taken from the literature, are also useful to know as you seek to manage patients’ musculoskeletal pain.
Pearl #1. Don’t use diazepam (valium) for acute low back pain. It doesn’t improve pain or function for this back pain. One hundred fourteen patients with acute low back pain were randomized to naproxen 500 mg bid as needed plus either placebo or diazepam 5 mg, 1 or 2 tablets, every 12 hours prn. At 7 days, 32% of the diazepam group reported moderate to severe pain and 22% of the placebo group did.2
Pearl #2. Use naproxen alone when treating acute low back pain. Three hundred twenty-three patients with acute low back pain were randomized to receive naproxen 500 mg bid plus placebo; naproxen plus oxycodone/acetaminophen; or naproxen plus cyclobenzaprine.3 At 7 days and 3 months, pain and function scores did not differ between groups.
Pearl #3. Don’t inject knees with corticosteroids. Enroll these patients in exercise and walking programs, which do provide benefit. One hundred forty patients with moderately severe knee OA were randomized to saline or triamcinolone 40 mg intra-articular injections every 3 months for 2 years.4 There was no difference in pain or function scores measured every 3 months and there was more cartilage degeneration in the triamcinolone group.
Continue to: Pearl #4
Pearl #4. Don’t dismiss the placebo effect. Eighty-three patients with chronic low back pain were randomized to either continue their current pain medications or to continue their current pain medication plus a placebo tablet twice daily for 3 weeks.5 They were told that placebos can have significant pain-relieving qualities. At 3 weeks, the patients taking placebo had less pain than those not taking placebo.
I’m not sure if we should start prescribing placebos, but this study is a strong reminder that we should harness the placebo effect, rather than dismiss it.
1. Peabody MR, O’Neill TR, Stelter KL, et al. Frequency and criticality of diagnoses in family medicine practices: from the National Ambulatory Medical Care Survey (NAMCS). J Am Board Fam Med. 2018;31:126-138.
2. Friedman BW, Irizarry E, Solorzano C, et al. Diazepam is no better than placebo when added to naproxen for acute low back pain. Ann Emerg Med. 2017;70:169-176.
3. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain. A randomized clinical trial. JAMA. 2015;314:1572-1580.
4. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
5. Carvalho C, Caetano JM, Cunha L, et al. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain. 2016;157:2766-2772.
Musculoskeletal complaints are one of the top reasons patients visit family physicians, with more than
The article by Drs. Stephen and Peter Carek summarizes the value of specific exercises for hip and knee osteoarthritis (OA), chronic back pain, chronic shoulder pain, Achilles tendinitis, and lateral epicondylitis. This month’s PURL summarizes a negative randomized trial of treatment of knee OA with the popular over-the-counter combination of glucosamine and chondroitin. The findings? The group taking placebo actually had superior pain relief at 6 months!
What else works … and doesn’t? You may find that the following 4 “pearls,” taken from the literature, are also useful to know as you seek to manage patients’ musculoskeletal pain.
Pearl #1. Don’t use diazepam (valium) for acute low back pain. It doesn’t improve pain or function for this back pain. One hundred fourteen patients with acute low back pain were randomized to naproxen 500 mg bid as needed plus either placebo or diazepam 5 mg, 1 or 2 tablets, every 12 hours prn. At 7 days, 32% of the diazepam group reported moderate to severe pain and 22% of the placebo group did.2
Pearl #2. Use naproxen alone when treating acute low back pain. Three hundred twenty-three patients with acute low back pain were randomized to receive naproxen 500 mg bid plus placebo; naproxen plus oxycodone/acetaminophen; or naproxen plus cyclobenzaprine.3 At 7 days and 3 months, pain and function scores did not differ between groups.
Pearl #3. Don’t inject knees with corticosteroids. Enroll these patients in exercise and walking programs, which do provide benefit. One hundred forty patients with moderately severe knee OA were randomized to saline or triamcinolone 40 mg intra-articular injections every 3 months for 2 years.4 There was no difference in pain or function scores measured every 3 months and there was more cartilage degeneration in the triamcinolone group.
Continue to: Pearl #4
Pearl #4. Don’t dismiss the placebo effect. Eighty-three patients with chronic low back pain were randomized to either continue their current pain medications or to continue their current pain medication plus a placebo tablet twice daily for 3 weeks.5 They were told that placebos can have significant pain-relieving qualities. At 3 weeks, the patients taking placebo had less pain than those not taking placebo.
I’m not sure if we should start prescribing placebos, but this study is a strong reminder that we should harness the placebo effect, rather than dismiss it.
Musculoskeletal complaints are one of the top reasons patients visit family physicians, with more than
The article by Drs. Stephen and Peter Carek summarizes the value of specific exercises for hip and knee osteoarthritis (OA), chronic back pain, chronic shoulder pain, Achilles tendinitis, and lateral epicondylitis. This month’s PURL summarizes a negative randomized trial of treatment of knee OA with the popular over-the-counter combination of glucosamine and chondroitin. The findings? The group taking placebo actually had superior pain relief at 6 months!
What else works … and doesn’t? You may find that the following 4 “pearls,” taken from the literature, are also useful to know as you seek to manage patients’ musculoskeletal pain.
Pearl #1. Don’t use diazepam (valium) for acute low back pain. It doesn’t improve pain or function for this back pain. One hundred fourteen patients with acute low back pain were randomized to naproxen 500 mg bid as needed plus either placebo or diazepam 5 mg, 1 or 2 tablets, every 12 hours prn. At 7 days, 32% of the diazepam group reported moderate to severe pain and 22% of the placebo group did.2
Pearl #2. Use naproxen alone when treating acute low back pain. Three hundred twenty-three patients with acute low back pain were randomized to receive naproxen 500 mg bid plus placebo; naproxen plus oxycodone/acetaminophen; or naproxen plus cyclobenzaprine.3 At 7 days and 3 months, pain and function scores did not differ between groups.
Pearl #3. Don’t inject knees with corticosteroids. Enroll these patients in exercise and walking programs, which do provide benefit. One hundred forty patients with moderately severe knee OA were randomized to saline or triamcinolone 40 mg intra-articular injections every 3 months for 2 years.4 There was no difference in pain or function scores measured every 3 months and there was more cartilage degeneration in the triamcinolone group.
Continue to: Pearl #4
Pearl #4. Don’t dismiss the placebo effect. Eighty-three patients with chronic low back pain were randomized to either continue their current pain medications or to continue their current pain medication plus a placebo tablet twice daily for 3 weeks.5 They were told that placebos can have significant pain-relieving qualities. At 3 weeks, the patients taking placebo had less pain than those not taking placebo.
I’m not sure if we should start prescribing placebos, but this study is a strong reminder that we should harness the placebo effect, rather than dismiss it.
1. Peabody MR, O’Neill TR, Stelter KL, et al. Frequency and criticality of diagnoses in family medicine practices: from the National Ambulatory Medical Care Survey (NAMCS). J Am Board Fam Med. 2018;31:126-138.
2. Friedman BW, Irizarry E, Solorzano C, et al. Diazepam is no better than placebo when added to naproxen for acute low back pain. Ann Emerg Med. 2017;70:169-176.
3. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain. A randomized clinical trial. JAMA. 2015;314:1572-1580.
4. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
5. Carvalho C, Caetano JM, Cunha L, et al. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain. 2016;157:2766-2772.
1. Peabody MR, O’Neill TR, Stelter KL, et al. Frequency and criticality of diagnoses in family medicine practices: from the National Ambulatory Medical Care Survey (NAMCS). J Am Board Fam Med. 2018;31:126-138.
2. Friedman BW, Irizarry E, Solorzano C, et al. Diazepam is no better than placebo when added to naproxen for acute low back pain. Ann Emerg Med. 2017;70:169-176.
3. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain. A randomized clinical trial. JAMA. 2015;314:1572-1580.
4. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
5. Carvalho C, Caetano JM, Cunha L, et al. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain. 2016;157:2766-2772.
Painful facial blisters, fever, and conjunctivitis
A 58-year-old woman with a history of hepatitis C, liver cirrhosis, hepatocellular carcinoma, hypothyroidism, and peripheral neuropathy presented to our clinic with left ear pain and blisters on her lips, nose, and mouth. On exam, the patient’s left tympanic membrane was opaque, and she had multiple 3- to 5-mm irregularly shaped ulcers on her right buccal mucosa, gingiva, and lips. She was given a diagnosis of acute otitis media and prescribed a course of amoxicillin. The physician, who was uncertain about the cause of her gingivostomatitis, took a “shotgun approach” and prescribed a nystatin/diphenhydramine/lidocaine mouthwash.
Three weeks later, the patient returned complaining of cloudy urine, dysuria, fever, vomiting, and “pink eye.” On exam, her right eye was mildly injected with no drainage. She had normal eye movements and no ophthalmoplegia. We diagnosed viral (vs allergic) conjunctivitis and pyelonephritis in this patient and advised her to use lubricant eyedrops and an oral antihistamine for the eye. We also started her on cefpodoxime (200 mg bid for 10 days) for pyelonephritis.
Three days later, the patient called our clinic and said that her right eye was not improving. We prescribed ofloxacin ophthalmic drops, 1 to 2 drops every 6 hours, for presumed bacterial conjunctivitis.
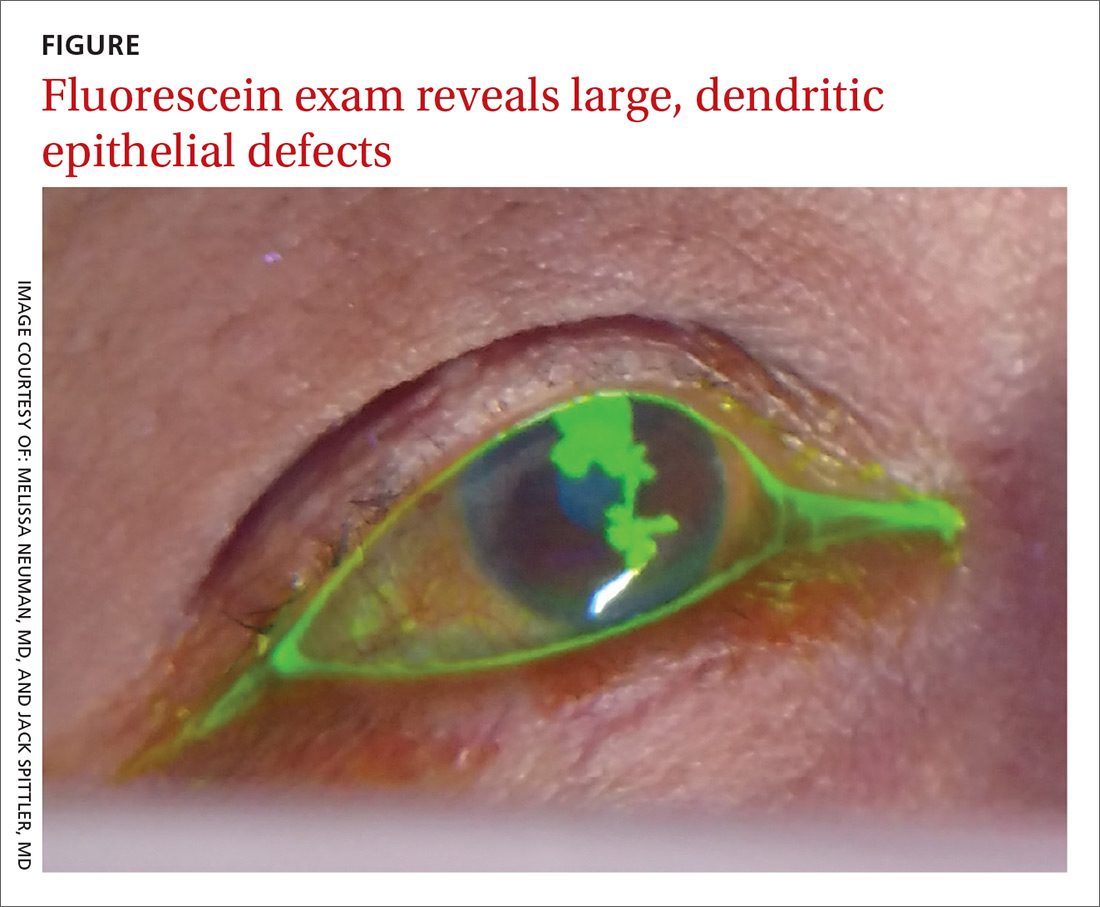
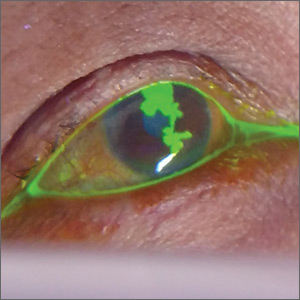
Four days later, she returned to our clinic; she had been using the ofloxacin drops and antihistamine but was experiencing worsening symptoms, including itching of her right eye, associated blurriness, and decreased vision. She had been using a warm compress on the eye and found that it was getting sticky and crusted. A gray corneal opacity was seen on physical exam, and a fluorescein exam was performed (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Herpes simplex virus keratitis
The patient was sent to the ophthalmology clinic, where a slit-lamp examination of the right eye showed 3+ injection, large dendritic epithelial defects spanning the majority of the cornea (with 10% haze), and trace nuclear sclerosis of the lens. These findings were consistent with a diagnosis of herpes simplex virus (HSV) keratitis, with a likely neurotrophic component (decreased sensation of the affected eye compared with that of the other eye). There was no evidence of secondary infection.
Discussion
The global incidence of HSV keratitis is approximately 1.5 million, including 40,000 new cases of monocular visual impairment or blindness each year.1 Primary infection with HSV-1 occurs following direct contact with infected mucosa or skin surfaces and inoculation. (Our patient likely transferred the infection by touching her eyes after touching her nose or mouth.) The virus remains in sensory ganglia for the lifetime of the host. Most ocular disease is thought to represent recurrent HSV (rather than a primary ocular infection).2 It has been proposed that HSV-1 latency may also occur in the cornea.
The symptoms of HSV keratitis include eye pain, redness, blurred vision, tearing, discharge, and sensitivity to light.
The 4 diagnostic categories
There are 4 categories of HSV keratitis, based on the location of the infection: epithelial, stromal, endotheliitis, and neurotrophic keratopathy.
Epithelial. The most common form, epithelial HSV manifests as dendritic or geographic lesions of the cornea.3 Geographic lesions occur when a dendrite widens and assumes an amoeboid shape.
Continue to: Stromal
Stromal. Stromal involvement accounts for 20% to 25% of presentations4 and may cause significant anterior chamber inflammation. Vision loss can result from permanent stromal scarring.5
Endotheliitis. Keratic precipitates (on top of stromal and epithelial edema) and a mild-to-moderate iritis are signs of endotheliitis.5
Neurotrophic keratopathy. This form of HSV keratitis is associated with corneal hypoesthesia or complete anesthesia secondary to damage of the corneal nerves, which can occur in any form of ocular HSV. Anesthesia may lead to nonhealing corneal epithelial defects.6 These defects, which are generally oval lesions, do not represent active viral disease and are made worse by antiviral drops. These lesions may cause stromal scarring, corneal perforation, or secondary bacterial infection.
Treatment consists of supportive care using artificial tears and prophylactic antibiotic eye drops, if appropriate; more advanced ophthalmologic treatments may be needed for advanced disease.7
Continue to: Other conditions, including conjunctivitis, have similar symptoms
Other conditions, including conjunctivitis, have similar symptoms
The differential for redness of the eye includes conditions such as conjunctivitis, glaucoma, and keratitis.
Conjunctivitis of any form—bacterial, viral, allergic, or toxic—involves injection of both the palpebral and bulbar conjunctiva.
Acute angle closure glaucoma can involve symptoms of headache, malaise, nausea, and vomiting. In addition, the pupil is fixed in mid-dilation, and the cornea becomes hazy.
Anterior uveitis/iritis causes sensitivity to light in both the affected and unaffected eyes, as well as ciliary flush (a red ring around the iris). Typically, there is no eye discharge.
Bacterial keratitis causes foreign body sensation and purulent discharge. This form of keratitis usually occurs due to improper wear of contact lenses.
Continue to: Viral keratitis...
Viral keratitis is characterized by photophobia, foreign body sensation, and watery discharge. A faint branching grey opacity may be seen on penlight exam, and dendrites may be seen with fluorescein.
Scleritis involves severe, boring pain of the eye in addition to photophobia and headache. It is usually associated with systemic inflammatory disorders.
Subconjunctival hemorrhage is asymptomatic and occurs following trauma.
Cellulitis manifests following trauma with a deep violet color and marked edema.
Continue to: Standard Tx
Standard Tx: Antiviral medications
Topical antiviral therapy is the standard treatment for epithelial HSV keratitis, although oral antiviral medications are equally effective. A randomized trial found that using an oral agent in addition to a topical antiviral did not improve outcomes.8 A 2015 systematic review found that topical antivirals acyclovir, ganciclovir, brivudine, trifluridine were equally effective in treatment outcome; 90% of patients healed within 2 weeks.9
Recurrent ocular HSV-1 infections are treated in the same way as the initial infection. Recurrent infection can be prevented with daily suppressive therapy. In one study, patients who took suppressive therapy (acyclovir 400 mg bid) for 1 year had 19% recurrence of ocular infection vs 32% in the placebo group.10
Prompt Tx is key. If the infection is superficial—involving only the outer layer of the cornea (epithelium)—the eye should heal without scarring with proper treatment. However, if the infection is not promptly treated or if deeper layers are involved, scarring of the cornea may occur. This can lead to vision loss or blindness.
Continue to: A missed opportunity for an earlier diagnosis
A missed opportunity for an earlier diagnosis
This case highlights the importance of conducting a thorough exam to identify findings that could shift the diagnosis from a simple allergic, viral, or bacterial conjunctivitis. It is always better to consider primary oral HSV infection than resort to a “shotgun approach” of treating candida and pain with an oral mixture. In this case, the ulcers and vesicles on the buccal mucosa, gingiva, and lips were a missed sign of primary HSV infection. Making this diagnosis might have prevented the ocular disease, as the treatment would have been an oral antiviral.
If conjunctivitis is refractory to usual management, the patient must be seen to rule out dangerous eye diagnoses such as HSV keratitis, preseptal or orbital cellulitis, or in the worst case, acute angle closure glaucoma. If there is uncertainty regarding diagnosis, a fluorescein exam is helpful. This simple in-office exam can facilitate a referral to Ophthalmology or the emergency department for a slit-lamp exam and appropriate therapy.
Our patient was started on valacyclovir 1 g bid, trifluridine eyedrops (5×/d), and erythromycin ophthalmic ointment (3×/d), with Ophthalmology follow-up in 1 week.
CORRESPONDENCE
John Spittler, MD, 3055 Roslyn St, Suite 100, Denver, CO 80238; John.Spittler@ucdenver.edu
1. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448-462.
2. Holland EJ, Mahanti RL, Belongia EA, et al. Ocular involvement in an outbreak of herpes gladiatorum. Am J Ophthalmol. 1992;114:680-684.
3. Cook SD. Herpes simplex virus in the eye. Br J Ophthalmol. 1992;76:365-366.
4. Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1-13.
5. Sekar Babu M, Balammal G, Sangeetha G, et al. A review on viral keratitis caused by herpes simplex virus. J Sci. 2011;1:1-10.
6. Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930-1936.
7. Bonini S, Rama P, Olzi D, et al. Neurotrophic keratitis. Eye. 2003;17:989-995.
8. Szentmáry N, Módis L, Imre L, et al. Diagnostics and treatment of infectious keratitis. Orv Hetil. 2017;158:1203-1212.
9. Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2015;(1):CD002898.
10. Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339:300-306.
A 58-year-old woman with a history of hepatitis C, liver cirrhosis, hepatocellular carcinoma, hypothyroidism, and peripheral neuropathy presented to our clinic with left ear pain and blisters on her lips, nose, and mouth. On exam, the patient’s left tympanic membrane was opaque, and she had multiple 3- to 5-mm irregularly shaped ulcers on her right buccal mucosa, gingiva, and lips. She was given a diagnosis of acute otitis media and prescribed a course of amoxicillin. The physician, who was uncertain about the cause of her gingivostomatitis, took a “shotgun approach” and prescribed a nystatin/diphenhydramine/lidocaine mouthwash.
Three weeks later, the patient returned complaining of cloudy urine, dysuria, fever, vomiting, and “pink eye.” On exam, her right eye was mildly injected with no drainage. She had normal eye movements and no ophthalmoplegia. We diagnosed viral (vs allergic) conjunctivitis and pyelonephritis in this patient and advised her to use lubricant eyedrops and an oral antihistamine for the eye. We also started her on cefpodoxime (200 mg bid for 10 days) for pyelonephritis.
Three days later, the patient called our clinic and said that her right eye was not improving. We prescribed ofloxacin ophthalmic drops, 1 to 2 drops every 6 hours, for presumed bacterial conjunctivitis.
Four days later, she returned to our clinic; she had been using the ofloxacin drops and antihistamine but was experiencing worsening symptoms, including itching of her right eye, associated blurriness, and decreased vision. She had been using a warm compress on the eye and found that it was getting sticky and crusted. A gray corneal opacity was seen on physical exam, and a fluorescein exam was performed (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Herpes simplex virus keratitis
The patient was sent to the ophthalmology clinic, where a slit-lamp examination of the right eye showed 3+ injection, large dendritic epithelial defects spanning the majority of the cornea (with 10% haze), and trace nuclear sclerosis of the lens. These findings were consistent with a diagnosis of herpes simplex virus (HSV) keratitis, with a likely neurotrophic component (decreased sensation of the affected eye compared with that of the other eye). There was no evidence of secondary infection.
Discussion
The global incidence of HSV keratitis is approximately 1.5 million, including 40,000 new cases of monocular visual impairment or blindness each year.1 Primary infection with HSV-1 occurs following direct contact with infected mucosa or skin surfaces and inoculation. (Our patient likely transferred the infection by touching her eyes after touching her nose or mouth.) The virus remains in sensory ganglia for the lifetime of the host. Most ocular disease is thought to represent recurrent HSV (rather than a primary ocular infection).2 It has been proposed that HSV-1 latency may also occur in the cornea.
The symptoms of HSV keratitis include eye pain, redness, blurred vision, tearing, discharge, and sensitivity to light.
The 4 diagnostic categories
There are 4 categories of HSV keratitis, based on the location of the infection: epithelial, stromal, endotheliitis, and neurotrophic keratopathy.
Epithelial. The most common form, epithelial HSV manifests as dendritic or geographic lesions of the cornea.3 Geographic lesions occur when a dendrite widens and assumes an amoeboid shape.
Continue to: Stromal
Stromal. Stromal involvement accounts for 20% to 25% of presentations4 and may cause significant anterior chamber inflammation. Vision loss can result from permanent stromal scarring.5
Endotheliitis. Keratic precipitates (on top of stromal and epithelial edema) and a mild-to-moderate iritis are signs of endotheliitis.5
Neurotrophic keratopathy. This form of HSV keratitis is associated with corneal hypoesthesia or complete anesthesia secondary to damage of the corneal nerves, which can occur in any form of ocular HSV. Anesthesia may lead to nonhealing corneal epithelial defects.6 These defects, which are generally oval lesions, do not represent active viral disease and are made worse by antiviral drops. These lesions may cause stromal scarring, corneal perforation, or secondary bacterial infection.
Treatment consists of supportive care using artificial tears and prophylactic antibiotic eye drops, if appropriate; more advanced ophthalmologic treatments may be needed for advanced disease.7
Continue to: Other conditions, including conjunctivitis, have similar symptoms
Other conditions, including conjunctivitis, have similar symptoms
The differential for redness of the eye includes conditions such as conjunctivitis, glaucoma, and keratitis.
Conjunctivitis of any form—bacterial, viral, allergic, or toxic—involves injection of both the palpebral and bulbar conjunctiva.
Acute angle closure glaucoma can involve symptoms of headache, malaise, nausea, and vomiting. In addition, the pupil is fixed in mid-dilation, and the cornea becomes hazy.
Anterior uveitis/iritis causes sensitivity to light in both the affected and unaffected eyes, as well as ciliary flush (a red ring around the iris). Typically, there is no eye discharge.
Bacterial keratitis causes foreign body sensation and purulent discharge. This form of keratitis usually occurs due to improper wear of contact lenses.
Continue to: Viral keratitis...
Viral keratitis is characterized by photophobia, foreign body sensation, and watery discharge. A faint branching grey opacity may be seen on penlight exam, and dendrites may be seen with fluorescein.
Scleritis involves severe, boring pain of the eye in addition to photophobia and headache. It is usually associated with systemic inflammatory disorders.
Subconjunctival hemorrhage is asymptomatic and occurs following trauma.
Cellulitis manifests following trauma with a deep violet color and marked edema.
Continue to: Standard Tx
Standard Tx: Antiviral medications
Topical antiviral therapy is the standard treatment for epithelial HSV keratitis, although oral antiviral medications are equally effective. A randomized trial found that using an oral agent in addition to a topical antiviral did not improve outcomes.8 A 2015 systematic review found that topical antivirals acyclovir, ganciclovir, brivudine, trifluridine were equally effective in treatment outcome; 90% of patients healed within 2 weeks.9
Recurrent ocular HSV-1 infections are treated in the same way as the initial infection. Recurrent infection can be prevented with daily suppressive therapy. In one study, patients who took suppressive therapy (acyclovir 400 mg bid) for 1 year had 19% recurrence of ocular infection vs 32% in the placebo group.10
Prompt Tx is key. If the infection is superficial—involving only the outer layer of the cornea (epithelium)—the eye should heal without scarring with proper treatment. However, if the infection is not promptly treated or if deeper layers are involved, scarring of the cornea may occur. This can lead to vision loss or blindness.
Continue to: A missed opportunity for an earlier diagnosis
A missed opportunity for an earlier diagnosis
This case highlights the importance of conducting a thorough exam to identify findings that could shift the diagnosis from a simple allergic, viral, or bacterial conjunctivitis. It is always better to consider primary oral HSV infection than resort to a “shotgun approach” of treating candida and pain with an oral mixture. In this case, the ulcers and vesicles on the buccal mucosa, gingiva, and lips were a missed sign of primary HSV infection. Making this diagnosis might have prevented the ocular disease, as the treatment would have been an oral antiviral.
If conjunctivitis is refractory to usual management, the patient must be seen to rule out dangerous eye diagnoses such as HSV keratitis, preseptal or orbital cellulitis, or in the worst case, acute angle closure glaucoma. If there is uncertainty regarding diagnosis, a fluorescein exam is helpful. This simple in-office exam can facilitate a referral to Ophthalmology or the emergency department for a slit-lamp exam and appropriate therapy.
Our patient was started on valacyclovir 1 g bid, trifluridine eyedrops (5×/d), and erythromycin ophthalmic ointment (3×/d), with Ophthalmology follow-up in 1 week.
CORRESPONDENCE
John Spittler, MD, 3055 Roslyn St, Suite 100, Denver, CO 80238; John.Spittler@ucdenver.edu
A 58-year-old woman with a history of hepatitis C, liver cirrhosis, hepatocellular carcinoma, hypothyroidism, and peripheral neuropathy presented to our clinic with left ear pain and blisters on her lips, nose, and mouth. On exam, the patient’s left tympanic membrane was opaque, and she had multiple 3- to 5-mm irregularly shaped ulcers on her right buccal mucosa, gingiva, and lips. She was given a diagnosis of acute otitis media and prescribed a course of amoxicillin. The physician, who was uncertain about the cause of her gingivostomatitis, took a “shotgun approach” and prescribed a nystatin/diphenhydramine/lidocaine mouthwash.
Three weeks later, the patient returned complaining of cloudy urine, dysuria, fever, vomiting, and “pink eye.” On exam, her right eye was mildly injected with no drainage. She had normal eye movements and no ophthalmoplegia. We diagnosed viral (vs allergic) conjunctivitis and pyelonephritis in this patient and advised her to use lubricant eyedrops and an oral antihistamine for the eye. We also started her on cefpodoxime (200 mg bid for 10 days) for pyelonephritis.
Three days later, the patient called our clinic and said that her right eye was not improving. We prescribed ofloxacin ophthalmic drops, 1 to 2 drops every 6 hours, for presumed bacterial conjunctivitis.
Four days later, she returned to our clinic; she had been using the ofloxacin drops and antihistamine but was experiencing worsening symptoms, including itching of her right eye, associated blurriness, and decreased vision. She had been using a warm compress on the eye and found that it was getting sticky and crusted. A gray corneal opacity was seen on physical exam, and a fluorescein exam was performed (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Herpes simplex virus keratitis
The patient was sent to the ophthalmology clinic, where a slit-lamp examination of the right eye showed 3+ injection, large dendritic epithelial defects spanning the majority of the cornea (with 10% haze), and trace nuclear sclerosis of the lens. These findings were consistent with a diagnosis of herpes simplex virus (HSV) keratitis, with a likely neurotrophic component (decreased sensation of the affected eye compared with that of the other eye). There was no evidence of secondary infection.
Discussion
The global incidence of HSV keratitis is approximately 1.5 million, including 40,000 new cases of monocular visual impairment or blindness each year.1 Primary infection with HSV-1 occurs following direct contact with infected mucosa or skin surfaces and inoculation. (Our patient likely transferred the infection by touching her eyes after touching her nose or mouth.) The virus remains in sensory ganglia for the lifetime of the host. Most ocular disease is thought to represent recurrent HSV (rather than a primary ocular infection).2 It has been proposed that HSV-1 latency may also occur in the cornea.
The symptoms of HSV keratitis include eye pain, redness, blurred vision, tearing, discharge, and sensitivity to light.
The 4 diagnostic categories
There are 4 categories of HSV keratitis, based on the location of the infection: epithelial, stromal, endotheliitis, and neurotrophic keratopathy.
Epithelial. The most common form, epithelial HSV manifests as dendritic or geographic lesions of the cornea.3 Geographic lesions occur when a dendrite widens and assumes an amoeboid shape.
Continue to: Stromal
Stromal. Stromal involvement accounts for 20% to 25% of presentations4 and may cause significant anterior chamber inflammation. Vision loss can result from permanent stromal scarring.5
Endotheliitis. Keratic precipitates (on top of stromal and epithelial edema) and a mild-to-moderate iritis are signs of endotheliitis.5
Neurotrophic keratopathy. This form of HSV keratitis is associated with corneal hypoesthesia or complete anesthesia secondary to damage of the corneal nerves, which can occur in any form of ocular HSV. Anesthesia may lead to nonhealing corneal epithelial defects.6 These defects, which are generally oval lesions, do not represent active viral disease and are made worse by antiviral drops. These lesions may cause stromal scarring, corneal perforation, or secondary bacterial infection.
Treatment consists of supportive care using artificial tears and prophylactic antibiotic eye drops, if appropriate; more advanced ophthalmologic treatments may be needed for advanced disease.7
Continue to: Other conditions, including conjunctivitis, have similar symptoms
Other conditions, including conjunctivitis, have similar symptoms
The differential for redness of the eye includes conditions such as conjunctivitis, glaucoma, and keratitis.
Conjunctivitis of any form—bacterial, viral, allergic, or toxic—involves injection of both the palpebral and bulbar conjunctiva.
Acute angle closure glaucoma can involve symptoms of headache, malaise, nausea, and vomiting. In addition, the pupil is fixed in mid-dilation, and the cornea becomes hazy.
Anterior uveitis/iritis causes sensitivity to light in both the affected and unaffected eyes, as well as ciliary flush (a red ring around the iris). Typically, there is no eye discharge.
Bacterial keratitis causes foreign body sensation and purulent discharge. This form of keratitis usually occurs due to improper wear of contact lenses.
Continue to: Viral keratitis...
Viral keratitis is characterized by photophobia, foreign body sensation, and watery discharge. A faint branching grey opacity may be seen on penlight exam, and dendrites may be seen with fluorescein.
Scleritis involves severe, boring pain of the eye in addition to photophobia and headache. It is usually associated with systemic inflammatory disorders.
Subconjunctival hemorrhage is asymptomatic and occurs following trauma.
Cellulitis manifests following trauma with a deep violet color and marked edema.
Continue to: Standard Tx
Standard Tx: Antiviral medications
Topical antiviral therapy is the standard treatment for epithelial HSV keratitis, although oral antiviral medications are equally effective. A randomized trial found that using an oral agent in addition to a topical antiviral did not improve outcomes.8 A 2015 systematic review found that topical antivirals acyclovir, ganciclovir, brivudine, trifluridine were equally effective in treatment outcome; 90% of patients healed within 2 weeks.9
Recurrent ocular HSV-1 infections are treated in the same way as the initial infection. Recurrent infection can be prevented with daily suppressive therapy. In one study, patients who took suppressive therapy (acyclovir 400 mg bid) for 1 year had 19% recurrence of ocular infection vs 32% in the placebo group.10
Prompt Tx is key. If the infection is superficial—involving only the outer layer of the cornea (epithelium)—the eye should heal without scarring with proper treatment. However, if the infection is not promptly treated or if deeper layers are involved, scarring of the cornea may occur. This can lead to vision loss or blindness.
Continue to: A missed opportunity for an earlier diagnosis
A missed opportunity for an earlier diagnosis
This case highlights the importance of conducting a thorough exam to identify findings that could shift the diagnosis from a simple allergic, viral, or bacterial conjunctivitis. It is always better to consider primary oral HSV infection than resort to a “shotgun approach” of treating candida and pain with an oral mixture. In this case, the ulcers and vesicles on the buccal mucosa, gingiva, and lips were a missed sign of primary HSV infection. Making this diagnosis might have prevented the ocular disease, as the treatment would have been an oral antiviral.
If conjunctivitis is refractory to usual management, the patient must be seen to rule out dangerous eye diagnoses such as HSV keratitis, preseptal or orbital cellulitis, or in the worst case, acute angle closure glaucoma. If there is uncertainty regarding diagnosis, a fluorescein exam is helpful. This simple in-office exam can facilitate a referral to Ophthalmology or the emergency department for a slit-lamp exam and appropriate therapy.
Our patient was started on valacyclovir 1 g bid, trifluridine eyedrops (5×/d), and erythromycin ophthalmic ointment (3×/d), with Ophthalmology follow-up in 1 week.
CORRESPONDENCE
John Spittler, MD, 3055 Roslyn St, Suite 100, Denver, CO 80238; John.Spittler@ucdenver.edu
1. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448-462.
2. Holland EJ, Mahanti RL, Belongia EA, et al. Ocular involvement in an outbreak of herpes gladiatorum. Am J Ophthalmol. 1992;114:680-684.
3. Cook SD. Herpes simplex virus in the eye. Br J Ophthalmol. 1992;76:365-366.
4. Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1-13.
5. Sekar Babu M, Balammal G, Sangeetha G, et al. A review on viral keratitis caused by herpes simplex virus. J Sci. 2011;1:1-10.
6. Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930-1936.
7. Bonini S, Rama P, Olzi D, et al. Neurotrophic keratitis. Eye. 2003;17:989-995.
8. Szentmáry N, Módis L, Imre L, et al. Diagnostics and treatment of infectious keratitis. Orv Hetil. 2017;158:1203-1212.
9. Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2015;(1):CD002898.
10. Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339:300-306.
1. Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448-462.
2. Holland EJ, Mahanti RL, Belongia EA, et al. Ocular involvement in an outbreak of herpes gladiatorum. Am J Ophthalmol. 1992;114:680-684.
3. Cook SD. Herpes simplex virus in the eye. Br J Ophthalmol. 1992;76:365-366.
4. Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1-13.
5. Sekar Babu M, Balammal G, Sangeetha G, et al. A review on viral keratitis caused by herpes simplex virus. J Sci. 2011;1:1-10.
6. Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930-1936.
7. Bonini S, Rama P, Olzi D, et al. Neurotrophic keratitis. Eye. 2003;17:989-995.
8. Szentmáry N, Módis L, Imre L, et al. Diagnostics and treatment of infectious keratitis. Orv Hetil. 2017;158:1203-1212.
9. Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2015;(1):CD002898.
10. Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339:300-306.
24-year-old with history of smoking tobacco and cannabis • dyspnea • chest tightness
THE CASE
A 24-year-old man with a history of smoking tobacco presented to the hospital with acute-onset chest tightness and dyspnea shortly after smoking cannabis. He was otherwise healthy and hemodynamically stable upon arrival to the emergency department. An electrocardiogram (EKG) was obtained.
THE DIAGNOSIS
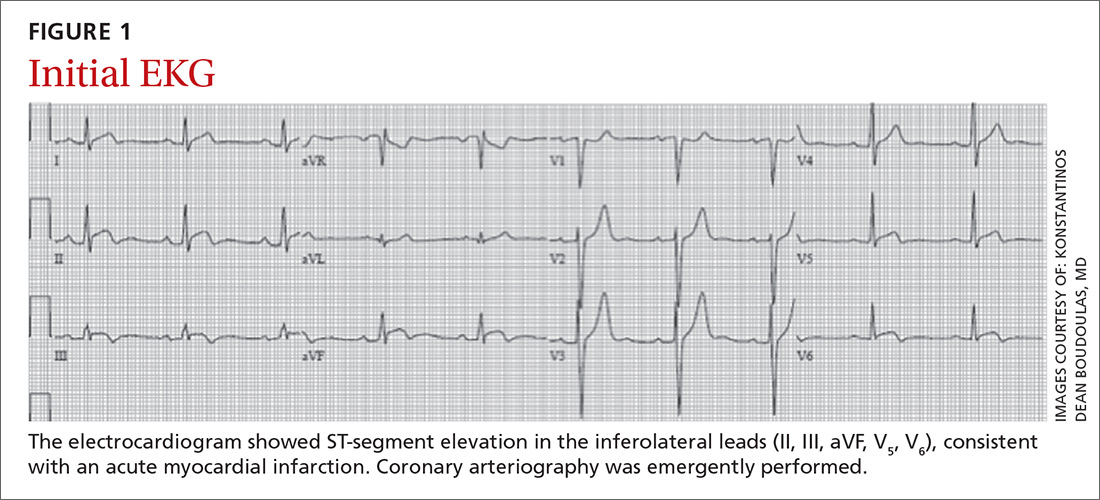
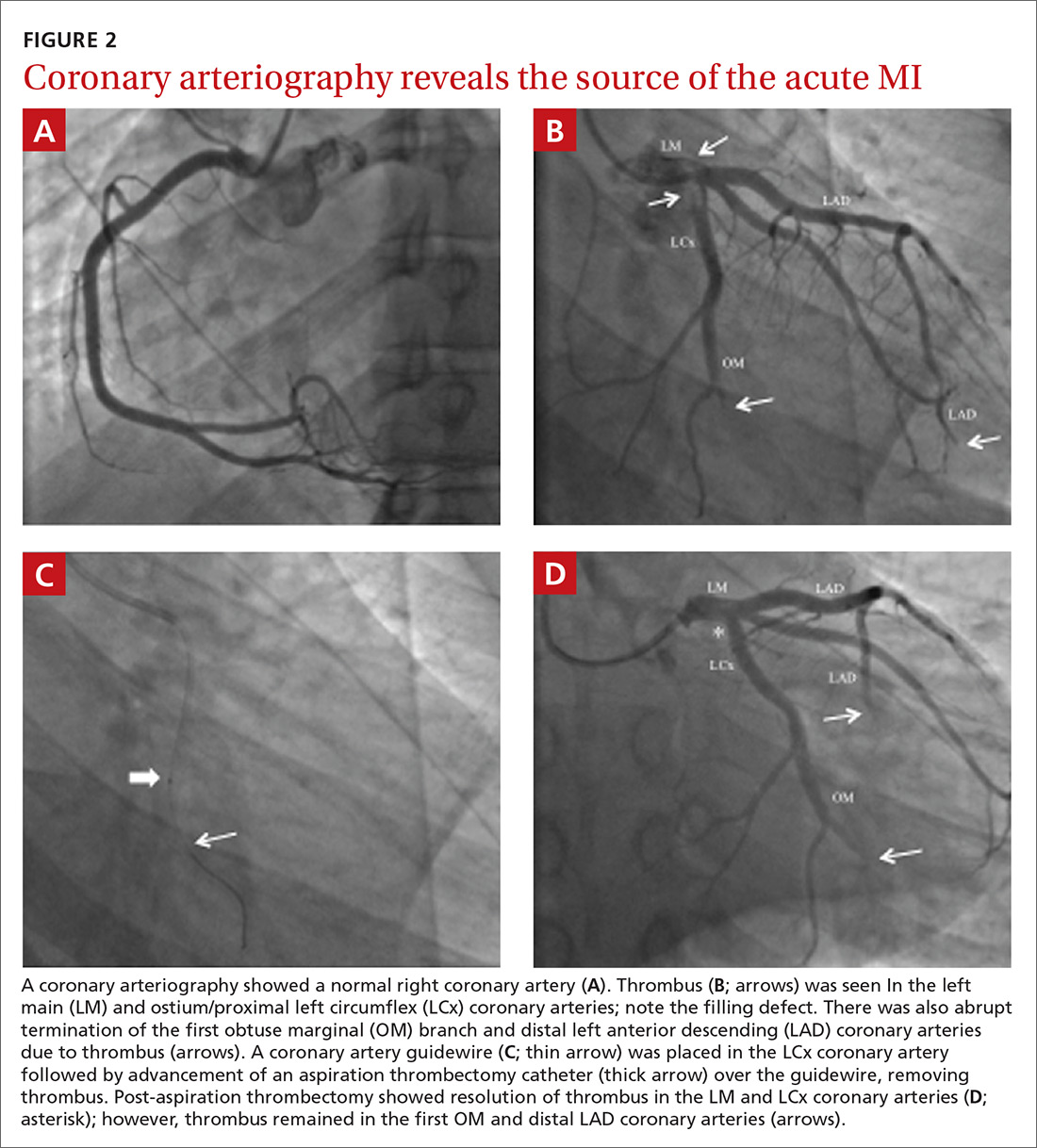
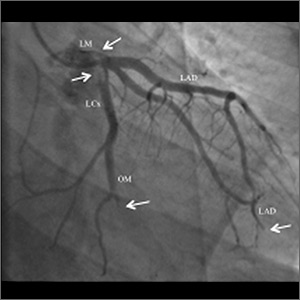
The EKG showed ST-segment elevation in the inferolateral leads, consistent with an acute myocardial infarction (AMI) (FIGURE 1). The patient was immediately transported to the cardiac catheterization laboratory, where coronary arteriography demonstrated a normal right coronary artery (FIGURE 2A). Diffuse thrombosis without atherosclerosis was seen throughout the left coronary arteries, including the left main artery, distal left anterior descending (LAD) artery, first diagonal branch of the LAD artery, ostial and proximal left circumflex (LCx) arteries, and first obtuse marginal (OM) branch of the LCx artery (FIGURE 2B).
DISCUSSION
The most common cause of AMI is underlying coronary atherosclerosis;1 however, AMI may occur due to in-situ thrombosis, thromboembolism, or coronary artery vasospasm, especially due to cocaine or other substance abuse. Occasionally, coronary arteries may be damaged due to viral myocarditis, autoimmune vasculitis, dissection of the ascending aorta, or dissection of a coronary artery, especially during pregnancy and postpartum.2,3
Cannabis and tobacco increase cardiovascular events
Smoking cannabis has been shown to increase adrenergic activity, resulting in an increased heart rate and elevated arterial pressure.4,5 These changes may increase myocardial oxygen demand and may result in a decrease in myocardial oxygen supply due to a decrease in the diastolic time.6 Smoking cannabis can also increase carboxyhemoglobin levels, which may compromise tissue oxygenation.
The risk for AMI has been shown to increase within the first hour of smoking cannabis.5 A few reports have documented cases of acute coronary syndrome following cannabis use; the majority of affected patients presented with chest pain within hours of smoking cannabis and were found to have a thrombus in a coronary artery, which was then treated medically or with percutaneous coronary intervention.7 Rare cases of cardiovascular death following cannabis use have also been reported.7,8
It has been suggested that coronary artery vasospasm may occur from cannabis use, which may precipitate thrombosis; however, this is not well defined.9 It is not clear if vasospasm was the inciting factor for thrombus formation in this case, as there was extensive and diffuse thrombus far greater than that expected solely from coronary artery vasospasm.
Continue to: AMI without atherosclerosis? Consider thrombosis
AMI without atherosclerosis? Consider thrombosis
In-situ coronary thrombosis should be considered in the differential diagnosis of a patient with an AMI without evidence of coronary atherosclerosis. Further, smoking cannabis immediately prior to symptom onset should heighten awareness for potential coronary thrombosis. Lifelong anticoagulation therapy may be indicated in these patients due to the catastrophic nature of the condition and limited data on this particular situation.
What’s recommended. Cessation of cannabis and tobacco smoking is recommended, as the use of these substances may contribute to the development of coronary thrombosis.3-7,9 A registry of patients with coronary thrombosis without coronary atherosclerosis, especially in states where cannabis is legal, would be useful to gauge the potential increase in such events. In addition, information related to the cardiovascular effects of using alternate routes of drug delivery, such as vaping devices, are limited; therefore, this practice should be closely monitored, as well.
Our patient’s outcome
Thrombus removal. In the cardiac catheterization laboratory, the majority of the thrombus was removed and coronary blood flow was improved using a thrombectomy catheter (FIGURE 2C). Residual thrombus remained in the very distal coronary arteries (FIGURE 2D), so heparin infusion was continued.
Imaging studies. Following the procedure, an echocardiogram demonstrated left ventricular (LV) regional wall motion abnormalities with moderately reduced LV systolic function and an ejection fraction (EF) of 35%. Troponin I levels peaked at 35 ng/mL. No LV apical thrombus or intracardiac defects (eg, patent foramen ovale, atrial septal defect, ventricular septal defect) that might have contributed to thromboembolism or paradoxical embolus were seen on echocardiogram or cardiac magnetic resonance imaging. In addition, ultrasound of the lower extremities did not demonstrate deep venous thrombosis.
Continue to: A toxicology screen
A toxicology screen was positive for tetrahydrocannabinol (THC) and negative for other substances. Hypercoagulable laboratory studies were normal, including anticardiolipin antibody IgG and IgM, factor V Leiden, prothrombin G20210A mutation, thrombin time, antithrombin III, and protein C and S activity. It was therefore believed that the AMI was due to in-situ coronary artery thrombus formation precipitated acutely by smoking cannabis—possibly with an underlying hypercoagulable state, even though no laboratory abnormalities were detected.
Our patient was discharged on lifelong aspirin 81 mg/d and oral rivaroxaban (maintenance dose of 20 mg/d). Metoprolol succinate 12.5 mg/d and lisinopril 2.5 mg/d were also initiated due
THE TAKEWAY
Coronary thrombosis can result in an AMI, even without underlying coronary atherosclerosis. Smoking cannabis may predispose individuals to in-situ coronary thrombosis and subsequent AMI. Although not often encountered in clinical practice, providers must be aware of this phenomenon in the differential diagnosis for AMI—particularly in young patients without traditional risk factors.
CORRESPONDENCE
Konstantinos Dean Boudoulas, MD, The Ohio State University Davis Lung and Heart Research Institute, 473 W. 12th Avenue, Suite 200, Columbus, Ohio 43210; kdboudoulas@osumc.edu
1. Davies MJ, Woolf N, Robertson WB. Pathology of acute myocardial infarction with particular reference to occlusive coronary thrombi. Br Heart J. 1976;38:659-664.
2. Pasupathy S, Air T, Dreyer RP, et al. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861-870.
3. Sharifi M, Frolich TG, Silverman IM. Myocardial infarction with angiographically normal coronary arteries. Chest. 1995;107:36-40.
4. Tatli E, Yilmaztepe M, Altun G, et al. Cannabis-induced coronary artery thrombosis and acute anterior myocardial infarction in a young man. Int J Cardiol. 2007;120:420-422.
5. Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805-2809.
6. Boudoulas KD, Borer JS, Boudoulas H. Heart rate, life expectancy and the cardiovascular system: therapeutic considerations. Cardiology. 2015;132:199-212.
7. Yurtdas M, Aydın MK. Acute myocardial infarction in a young man; fatal blow of the marijuana: a case report. Korean Circ J. 2012;42:641-645.
8. Jouanjus E, Lapeyre-Mestre M, Micallef J; French Association of the Regional Abuse and Dependence Monitoring Centres (CEIP-A) Working Group on Cannabis Complications. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc. 2014;3:e000638.
9. Hodcroft CJ, Rossiter MC, Buch AN. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J Emerg Med. 2014;47:277-281.
THE CASE
A 24-year-old man with a history of smoking tobacco presented to the hospital with acute-onset chest tightness and dyspnea shortly after smoking cannabis. He was otherwise healthy and hemodynamically stable upon arrival to the emergency department. An electrocardiogram (EKG) was obtained.
THE DIAGNOSIS
The EKG showed ST-segment elevation in the inferolateral leads, consistent with an acute myocardial infarction (AMI) (FIGURE 1). The patient was immediately transported to the cardiac catheterization laboratory, where coronary arteriography demonstrated a normal right coronary artery (FIGURE 2A). Diffuse thrombosis without atherosclerosis was seen throughout the left coronary arteries, including the left main artery, distal left anterior descending (LAD) artery, first diagonal branch of the LAD artery, ostial and proximal left circumflex (LCx) arteries, and first obtuse marginal (OM) branch of the LCx artery (FIGURE 2B).
DISCUSSION
The most common cause of AMI is underlying coronary atherosclerosis;1 however, AMI may occur due to in-situ thrombosis, thromboembolism, or coronary artery vasospasm, especially due to cocaine or other substance abuse. Occasionally, coronary arteries may be damaged due to viral myocarditis, autoimmune vasculitis, dissection of the ascending aorta, or dissection of a coronary artery, especially during pregnancy and postpartum.2,3
Cannabis and tobacco increase cardiovascular events
Smoking cannabis has been shown to increase adrenergic activity, resulting in an increased heart rate and elevated arterial pressure.4,5 These changes may increase myocardial oxygen demand and may result in a decrease in myocardial oxygen supply due to a decrease in the diastolic time.6 Smoking cannabis can also increase carboxyhemoglobin levels, which may compromise tissue oxygenation.
The risk for AMI has been shown to increase within the first hour of smoking cannabis.5 A few reports have documented cases of acute coronary syndrome following cannabis use; the majority of affected patients presented with chest pain within hours of smoking cannabis and were found to have a thrombus in a coronary artery, which was then treated medically or with percutaneous coronary intervention.7 Rare cases of cardiovascular death following cannabis use have also been reported.7,8
It has been suggested that coronary artery vasospasm may occur from cannabis use, which may precipitate thrombosis; however, this is not well defined.9 It is not clear if vasospasm was the inciting factor for thrombus formation in this case, as there was extensive and diffuse thrombus far greater than that expected solely from coronary artery vasospasm.
Continue to: AMI without atherosclerosis? Consider thrombosis
AMI without atherosclerosis? Consider thrombosis
In-situ coronary thrombosis should be considered in the differential diagnosis of a patient with an AMI without evidence of coronary atherosclerosis. Further, smoking cannabis immediately prior to symptom onset should heighten awareness for potential coronary thrombosis. Lifelong anticoagulation therapy may be indicated in these patients due to the catastrophic nature of the condition and limited data on this particular situation.
What’s recommended. Cessation of cannabis and tobacco smoking is recommended, as the use of these substances may contribute to the development of coronary thrombosis.3-7,9 A registry of patients with coronary thrombosis without coronary atherosclerosis, especially in states where cannabis is legal, would be useful to gauge the potential increase in such events. In addition, information related to the cardiovascular effects of using alternate routes of drug delivery, such as vaping devices, are limited; therefore, this practice should be closely monitored, as well.
Our patient’s outcome
Thrombus removal. In the cardiac catheterization laboratory, the majority of the thrombus was removed and coronary blood flow was improved using a thrombectomy catheter (FIGURE 2C). Residual thrombus remained in the very distal coronary arteries (FIGURE 2D), so heparin infusion was continued.
Imaging studies. Following the procedure, an echocardiogram demonstrated left ventricular (LV) regional wall motion abnormalities with moderately reduced LV systolic function and an ejection fraction (EF) of 35%. Troponin I levels peaked at 35 ng/mL. No LV apical thrombus or intracardiac defects (eg, patent foramen ovale, atrial septal defect, ventricular septal defect) that might have contributed to thromboembolism or paradoxical embolus were seen on echocardiogram or cardiac magnetic resonance imaging. In addition, ultrasound of the lower extremities did not demonstrate deep venous thrombosis.
Continue to: A toxicology screen
A toxicology screen was positive for tetrahydrocannabinol (THC) and negative for other substances. Hypercoagulable laboratory studies were normal, including anticardiolipin antibody IgG and IgM, factor V Leiden, prothrombin G20210A mutation, thrombin time, antithrombin III, and protein C and S activity. It was therefore believed that the AMI was due to in-situ coronary artery thrombus formation precipitated acutely by smoking cannabis—possibly with an underlying hypercoagulable state, even though no laboratory abnormalities were detected.
Our patient was discharged on lifelong aspirin 81 mg/d and oral rivaroxaban (maintenance dose of 20 mg/d). Metoprolol succinate 12.5 mg/d and lisinopril 2.5 mg/d were also initiated due
THE TAKEWAY
Coronary thrombosis can result in an AMI, even without underlying coronary atherosclerosis. Smoking cannabis may predispose individuals to in-situ coronary thrombosis and subsequent AMI. Although not often encountered in clinical practice, providers must be aware of this phenomenon in the differential diagnosis for AMI—particularly in young patients without traditional risk factors.
CORRESPONDENCE
Konstantinos Dean Boudoulas, MD, The Ohio State University Davis Lung and Heart Research Institute, 473 W. 12th Avenue, Suite 200, Columbus, Ohio 43210; kdboudoulas@osumc.edu
THE CASE
A 24-year-old man with a history of smoking tobacco presented to the hospital with acute-onset chest tightness and dyspnea shortly after smoking cannabis. He was otherwise healthy and hemodynamically stable upon arrival to the emergency department. An electrocardiogram (EKG) was obtained.
THE DIAGNOSIS
The EKG showed ST-segment elevation in the inferolateral leads, consistent with an acute myocardial infarction (AMI) (FIGURE 1). The patient was immediately transported to the cardiac catheterization laboratory, where coronary arteriography demonstrated a normal right coronary artery (FIGURE 2A). Diffuse thrombosis without atherosclerosis was seen throughout the left coronary arteries, including the left main artery, distal left anterior descending (LAD) artery, first diagonal branch of the LAD artery, ostial and proximal left circumflex (LCx) arteries, and first obtuse marginal (OM) branch of the LCx artery (FIGURE 2B).
DISCUSSION
The most common cause of AMI is underlying coronary atherosclerosis;1 however, AMI may occur due to in-situ thrombosis, thromboembolism, or coronary artery vasospasm, especially due to cocaine or other substance abuse. Occasionally, coronary arteries may be damaged due to viral myocarditis, autoimmune vasculitis, dissection of the ascending aorta, or dissection of a coronary artery, especially during pregnancy and postpartum.2,3
Cannabis and tobacco increase cardiovascular events
Smoking cannabis has been shown to increase adrenergic activity, resulting in an increased heart rate and elevated arterial pressure.4,5 These changes may increase myocardial oxygen demand and may result in a decrease in myocardial oxygen supply due to a decrease in the diastolic time.6 Smoking cannabis can also increase carboxyhemoglobin levels, which may compromise tissue oxygenation.
The risk for AMI has been shown to increase within the first hour of smoking cannabis.5 A few reports have documented cases of acute coronary syndrome following cannabis use; the majority of affected patients presented with chest pain within hours of smoking cannabis and were found to have a thrombus in a coronary artery, which was then treated medically or with percutaneous coronary intervention.7 Rare cases of cardiovascular death following cannabis use have also been reported.7,8
It has been suggested that coronary artery vasospasm may occur from cannabis use, which may precipitate thrombosis; however, this is not well defined.9 It is not clear if vasospasm was the inciting factor for thrombus formation in this case, as there was extensive and diffuse thrombus far greater than that expected solely from coronary artery vasospasm.
Continue to: AMI without atherosclerosis? Consider thrombosis
AMI without atherosclerosis? Consider thrombosis
In-situ coronary thrombosis should be considered in the differential diagnosis of a patient with an AMI without evidence of coronary atherosclerosis. Further, smoking cannabis immediately prior to symptom onset should heighten awareness for potential coronary thrombosis. Lifelong anticoagulation therapy may be indicated in these patients due to the catastrophic nature of the condition and limited data on this particular situation.
What’s recommended. Cessation of cannabis and tobacco smoking is recommended, as the use of these substances may contribute to the development of coronary thrombosis.3-7,9 A registry of patients with coronary thrombosis without coronary atherosclerosis, especially in states where cannabis is legal, would be useful to gauge the potential increase in such events. In addition, information related to the cardiovascular effects of using alternate routes of drug delivery, such as vaping devices, are limited; therefore, this practice should be closely monitored, as well.
Our patient’s outcome
Thrombus removal. In the cardiac catheterization laboratory, the majority of the thrombus was removed and coronary blood flow was improved using a thrombectomy catheter (FIGURE 2C). Residual thrombus remained in the very distal coronary arteries (FIGURE 2D), so heparin infusion was continued.
Imaging studies. Following the procedure, an echocardiogram demonstrated left ventricular (LV) regional wall motion abnormalities with moderately reduced LV systolic function and an ejection fraction (EF) of 35%. Troponin I levels peaked at 35 ng/mL. No LV apical thrombus or intracardiac defects (eg, patent foramen ovale, atrial septal defect, ventricular septal defect) that might have contributed to thromboembolism or paradoxical embolus were seen on echocardiogram or cardiac magnetic resonance imaging. In addition, ultrasound of the lower extremities did not demonstrate deep venous thrombosis.
Continue to: A toxicology screen
A toxicology screen was positive for tetrahydrocannabinol (THC) and negative for other substances. Hypercoagulable laboratory studies were normal, including anticardiolipin antibody IgG and IgM, factor V Leiden, prothrombin G20210A mutation, thrombin time, antithrombin III, and protein C and S activity. It was therefore believed that the AMI was due to in-situ coronary artery thrombus formation precipitated acutely by smoking cannabis—possibly with an underlying hypercoagulable state, even though no laboratory abnormalities were detected.
Our patient was discharged on lifelong aspirin 81 mg/d and oral rivaroxaban (maintenance dose of 20 mg/d). Metoprolol succinate 12.5 mg/d and lisinopril 2.5 mg/d were also initiated due
THE TAKEWAY
Coronary thrombosis can result in an AMI, even without underlying coronary atherosclerosis. Smoking cannabis may predispose individuals to in-situ coronary thrombosis and subsequent AMI. Although not often encountered in clinical practice, providers must be aware of this phenomenon in the differential diagnosis for AMI—particularly in young patients without traditional risk factors.
CORRESPONDENCE
Konstantinos Dean Boudoulas, MD, The Ohio State University Davis Lung and Heart Research Institute, 473 W. 12th Avenue, Suite 200, Columbus, Ohio 43210; kdboudoulas@osumc.edu
1. Davies MJ, Woolf N, Robertson WB. Pathology of acute myocardial infarction with particular reference to occlusive coronary thrombi. Br Heart J. 1976;38:659-664.
2. Pasupathy S, Air T, Dreyer RP, et al. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861-870.
3. Sharifi M, Frolich TG, Silverman IM. Myocardial infarction with angiographically normal coronary arteries. Chest. 1995;107:36-40.
4. Tatli E, Yilmaztepe M, Altun G, et al. Cannabis-induced coronary artery thrombosis and acute anterior myocardial infarction in a young man. Int J Cardiol. 2007;120:420-422.
5. Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805-2809.
6. Boudoulas KD, Borer JS, Boudoulas H. Heart rate, life expectancy and the cardiovascular system: therapeutic considerations. Cardiology. 2015;132:199-212.
7. Yurtdas M, Aydın MK. Acute myocardial infarction in a young man; fatal blow of the marijuana: a case report. Korean Circ J. 2012;42:641-645.
8. Jouanjus E, Lapeyre-Mestre M, Micallef J; French Association of the Regional Abuse and Dependence Monitoring Centres (CEIP-A) Working Group on Cannabis Complications. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc. 2014;3:e000638.
9. Hodcroft CJ, Rossiter MC, Buch AN. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J Emerg Med. 2014;47:277-281.
1. Davies MJ, Woolf N, Robertson WB. Pathology of acute myocardial infarction with particular reference to occlusive coronary thrombi. Br Heart J. 1976;38:659-664.
2. Pasupathy S, Air T, Dreyer RP, et al. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861-870.
3. Sharifi M, Frolich TG, Silverman IM. Myocardial infarction with angiographically normal coronary arteries. Chest. 1995;107:36-40.
4. Tatli E, Yilmaztepe M, Altun G, et al. Cannabis-induced coronary artery thrombosis and acute anterior myocardial infarction in a young man. Int J Cardiol. 2007;120:420-422.
5. Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805-2809.
6. Boudoulas KD, Borer JS, Boudoulas H. Heart rate, life expectancy and the cardiovascular system: therapeutic considerations. Cardiology. 2015;132:199-212.
7. Yurtdas M, Aydın MK. Acute myocardial infarction in a young man; fatal blow of the marijuana: a case report. Korean Circ J. 2012;42:641-645.
8. Jouanjus E, Lapeyre-Mestre M, Micallef J; French Association of the Regional Abuse and Dependence Monitoring Centres (CEIP-A) Working Group on Cannabis Complications. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc. 2014;3:e000638.
9. Hodcroft CJ, Rossiter MC, Buch AN. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J Emerg Med. 2014;47:277-281.
CDC recommendations for the 2018-2019 influenza season
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4
Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).
After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).
However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4
Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).
After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).
However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4
Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).
After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).
However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
Consider these exercises for chronic musculoskeletal conditions
Regular exercise confers several well-established benefits. In such conditions as coronary heart disease, stroke, heart failure, and diabetes, exercise has led to a reduction in mortality similar to that seen with pharmacotherapy.1 For patients with chronic musculoskeletal conditions, the benefits of exercise-based interventions are measurably reduced pain and improved daily function.2 However, prescribing of exercise is often neglected, with preference given to pharmacologic or surgical interventions.3 In part, the disregard of exercise as therapy results from unfamiliarity with appropriate exercise prescriptions,3 which include various forms of aerobic exercise, strength training, and stretching to increase flexibility (TABLE).
As is true of many therapeutic modalities, exercise must be tailored to the condition and to a patient’s preferences to optimize its benefits. In this review, we describe exercise regimens well suited for common musculoskeletal conditions, examine the effectiveness of exercise in each condition, and provide examples for use in treating patients.
Osteoarthritis of the hip and knee
Osteoarthritis (OA), one of the most common chronic joint diseases, erodes the articular cartilage and subchondral bone of a synovial joint, eventually leading to joint failure. Pain and diminished muscle strength restrict physical activity and can lead to decreased fitness and impaired muscle function. Exercise helps reduce pain and improve muscle function and quality of life in patients with hip or knee OA regardless of age, disease severity, or level of pain and dysfunction.2
Knee exercises. Activities suitable for patients with OA include muscle strengthening, aerobic conditioning, and range-of-motion (ROM) exercises.4-6 A 2015 Cochrane review of OA of the knee showed that exercise reduced pain and improved physical function and quality of life in patients who completed a treatment program, and that pain relief persisted up to 6 months after intervention.5
When designing an exercise prescription for patients with knee OA, consider quadriceps strengthening with an initial period of supervision, which may provide greater pain relief than nonspecific, unsupervised lower limb exercises.4 Enhanced strength of the lower limb may lessen force through the knee, thereby decreasing pain and improving overall physical function.7 Simple, teachable exercises include squats, step-ups, knee extension/flexion while sitting in a chair, and hip abduction/adduction while standing or lying down. Elastic bands, dumbbells, or cuff weights may be used to increase resistance.
Hip exercises. Exercise can significantly reduce pain and improve function for up to 6 months for patients with mild-to-moderate symptomatic hip OA.6 Types of exercise for hip OA include strength training of hip and core muscles, functional exercises that imitate movements in daily activities, and flexibility training. These exercises help reduce pain and increase ROM. Exercise should include resistance training and should not exceed the limit for acceptable pain.8
Aquatic therapy is also appropriate for exercise and strength training and can decrease pain and disability and improve quality of life.9 Supervised physical therapy, including strength training, manual therapy, and balance training, are important for reducing pain and improving function. Physical therapy can also enhance adherence to a prescribed exercise program.10
Continue to: Appropriate exercise prescriptions...
Appropriate exercise prescriptions for patients with knee or hip OA should focus on low-impact activities that can improve strength, flexibility, and function (FIGURE 1). A typical regimen would be 30 or more cumulative minutes daily of stationary cycling, water-based exercises, or strength training, 3 to 5 days per week. Individualize workout intensity for each patient, emphasizing that high-intensity, low-impact effort may yield greater strength gains and take less time to perform.11 A high-intensity exercise prescription focusing on quadriceps, hip, and core strengthening may consist of 3 sets of 8 repetitions with resistance set at 40% of the maximum resistance against which the patient can perform 1 repetition.7
Barriers to exercise in knee and hip OA include negative patient and provider perspectives on exercise and patients’ fear that increased activity may actually worsen OA.12 Depending on a patient’s personal preferences, ways to overcome these barriers and encourage adherence might be supervised exercises in an individual or group setting or audiotapes or videos of recommended exercises.10
Chronic low back pain
Chronic low back pain (LBP) is a large socioeconomic burden in the United States, with upward of $100 billion per year accounted for in health care costs and decreased worker productivity.13 The etiology of chronic LBP can be multifactorial and due to any of several conditions such as degenerative disc disease, spinal stenosis, spondylolisthesis, and facet arthropathy. Treatment is difficult, given that many common interventions—medications, massage, manipulation—have limited efficacy.14 However, for patients with nonspecific chronic LBP, exercise is an effective intervention for reducing pain and improving physical function.15
An effective approach is to design an exercise regimen for the individual by type, duration, and frequency of activity, administered under supervision to encourage adherence.16 Appropriate exercises emphasize resistance, strength training, and core stabilization, often focusing on whole body and trunk motion (FIGURE 2).17
Although yoga or Pilates classes may have a small effect on function, no high-quality evidence exists for their superiority to other forms of exercise.18,19 Back School, a therapeutic program that includes education on anatomy and biomechanics, optimal posture, ergonomics, and back exercises, has limited, low-quality evidence for treatment comparisons.20 Aerobic exercise, including treadmill, elliptical, or cycling exercises or walking outdoors can reduce pain and improve physical and psychologic functioning.21
Continue to: The most common reported adverse effect...
The most common reported adverse effect of exercise is a temporary exacerbation of back pain. However, having patients continue daily activities within the permitted limits of pain leads to more rapid recovery than rest or back-mobilizing exercises.15,22,23
Cautions. Exercise is contraindicated in patients with LBP arising from a serious medical condition, such as fracture, infection, cancer, or cauda equina syndrome.24 Importantly, exercise interventions recommended for acute LBP have not shown benefit for chronic LBP.
Chronic shoulder pain
With a prevalence ranging from 7% to 26% in the general population,25 chronic shoulder pain often interferes with essential activities of daily living. The etiology of chronic shoulder pain is broad and most commonly involves disorders of the rotator cuff, which functions in both motion and dynamic stabilization of the shoulder. The common term “rotator cuff pain syndrome” can cover such disorders as subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full thickness rotator cuff tears, calcific tendinitis, and subacromial bursitis. These pathologies may have overlapping presentations. Manual therapy and exercise, usually delivered as a component of structured physical therapy, focus on stretches and other exercises to increase ROM, stability, and strength of the rotator cuff musculature.26
A 2016 Cochrane review that evaluated manual therapy and exercise for chronic shoulder pain yielded limited high-quality evidence for effectiveness compared with placebo.27 Five trials found no important differences between manual therapy and exercise compared with glucocorticoid injection relative to overall pain, function, active shoulder abduction, and quality of life from 4 weeks up to 12 months.27 But compared with placebo, exercise has been more effective in reducing reported pain, especially in the context of strengthening regimens focused on flexion, extension, and internal and external rotation.28
For subacromial impingement syndrome, a 2017 meta-analysis found that a generalized exercise program relieves pain and improves function, ROM, and strength.29 A generalized shoulder-strengthening program includes exercises that focus on internal and external rotation, horizontal abduction, and shoulder stabilization (FIGURE 3). These exercises can be completed with 3 sets of 15 to 20 repetitions, which create a fatigue response that improves strength and targets local muscular endurance.30
Continue to: Achilles tendinopathy
Achilles tendinopathy
Achilles tendinopathy (also referred to as chronic Achilles tendinitis) is a degenerative condition of the Achilles tendon related to overuse that leads to pain, swelling, and impaired performance. It accounts for approximately 18% of injuries in runners and 4% of all patients presenting to sports medicine clinics.31 Eccentric muscle loading has become the dominant conservative intervention strategy for chronic Achilles tendinopathy.
For chronic tendinopathies, eccentric exercises subject greater force than concentric exercises through a controlled lengthening of a muscle-tendon unit, resulting in a greater remodeling stimulus of the tendon.32 Classically, the Alfredson protocol has been used to treat chronic Achilles tendinopathy. This program of eccentric heel-drop exercises recommends completion of 180 eccentric repetitions a day for up to 12 weeks (FIGURE 4).33 Exercises are performed slowly, and load can be increased when exercises are performed without pain or perhaps with mild nondisabling pain.
A variation of this protocol has allowed a gradual escalation of repetitions over a week up to the recommended 180 repetitions, and has shown improvements in pain reduction and function similar to that achieved with the primary protocol.34 Additionally, a 6-week “do as tolerated” program of eccentric exercises did not lead to lesser improvement for individuals with midportion Achilles tendinopathy.35
Several systematic reviews have supported the use of eccentric exercises for chronic Achilles tendinopathy,31,36,37 but no specific protocol or exercise regimen has demonstrated superiority. However, with the Alfredson protocol, improvement in pain and function in patients with chronic Achilles tendinopathy has persisted for up to 5 years.38
Lateral epicondylitis
Lateral epicondylitis (also called lateral epicondylosis or “tennis elbow”) is a disabling musculoskeletal condition that leads to pain and tenderness around the extensor mass of the lateral elbow. It is caused by microtrauma to the tendon, usually sustained through repetitive movement in a sporting activity, industrial work, or hobby. Affecting up to 3% of the US population, lateral epicondylitis is associated with pain and functional disability, as well as emotional and psychosocial consequences.39
Continue to: Proposed treatment and rehabilitation options...
Proposed treatment and rehabilitation options for patients with lateral epicondylitis have included massage, manipulation, taping, acupuncture, orthotic devices, ultrasound, activity modification, and rest. Exercise programs incorporating eccentric muscle activity are becoming increasingly popular for such conditions as Achilles and patellar tendinopathies, and they may translate well to other chronic tendinopathies, such as lateral epicondylitis.32
An eccentric exercise program for lateral epicondylitis, either in isolation or as an adjunct to other therapies, has decreased pain and improved function and grip strength from baseline measures.40 Compared with a standard exercise regimen without eccentric strength training, use of eccentric training improves such clinical measures as pain intensity and disability status, as it decreases tendon thickness and aids in recovering homogenous tendon structure.41
A sample exercise. The patient may sit in a chair and, with the forearm flexed and pronated over the edge of a table, grasp some form of resistance (bucket of water, training weight, resistance band) (FIGURE 5). The nonaffected hand can be used to help lift the affected wrist into full extension and then removed to allow lowering of the hand over several seconds into flexion. This activity can be performed in sets of 8 to 12 repetitions, 2 to 3 times a day, until the patient’s pain and function have improved.42
Overcoming barriers to exercise
A major concern across all studies assessing the therapeutic value of exercise is patient compliance and adherence to prescribed programs. Compliance and adherence are affected in part by psychosocial factors such as low literacy and poor social support. From a physician’s perspective, direct and indirect costs of treatment and rehabilitation of chronic musculoskeletal conditions may discourage the prescribing of supervised physical therapy.3
Steps to consider in overcoming these barriers would be advising an exercise regimen that requires only an initial period of supervision; educating patients about the benefits of an exercise program; exploring a patient’s expectations, beliefs, and fears; and developing strategies for long-term adherence.16 Supervision through physical therapy is often suggested. However, significant barriers may exist that impede a patient’s ability to attend or participate, in which case physician observation in the course of regularly scheduled clinical examinations could be considered.
Continue to: When prescribing exercises...
When prescribing exercises, be sure to address patient expectations regarding pain, duration, and limitations of exercise. It would be helpful for patients to know, for instance, that working through mild-to-moderate pain during exercise has been shown to shorten post-exercise recovery time and, in the short-term, improve relief from pain.43
Tailoring specific exercise prescriptions for a patient will make the regimen more satisfying for the individual and optimize adherence, which in turn will increase the potential for pain reduction and improved function. Secondary benefits would likely be weight loss and prevention (or regression) of cardiovascular disease. Continued evaluation by the physician or physical therapist should be part of ongoing management, as well as “refresher courses” to ensure understanding of, and adherence to, the exercise program. The potential benefits and limited risks of exercise, if done properly, make it a primary intervention for specific musculoskeletal conditions.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, PO Box 100237, Gainesville, FL 32610-0237; carek@ufl.edu.
1. Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
2. Babatunde OO, Jordan JL, van der Windt DA, et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12:e0178621.
3. Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner’s perspective - a qualitative study. BMC Fam Pract. 2013;14:128.
4. Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622-636.
5. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376.
6. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912.
7. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(suppl 5):S45-S52.
8. Fernandes L, Storheim K, Nordsletten L, et al. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90:592-601.
9. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
10. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956.
11. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
12. Brakke R, Singh J, Sullivan W. Physical therapy in persons with osteoarthritis. PM R. 2012;4:S53-S58.
13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24.
14. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
15. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335.
16. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
17. Searle A, Spink M, Ho A, et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155-1167.
18. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;(7):CD010265.
19. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(1):CD010671.
20. Parreira P, Heymans MW, van Tulder MW, et al. Back Schools for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(8):CD011674.
21. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil. 2015;94:358-365.
22. Malmivaara A, Häkkinen U, Aro T, et al. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351-355.
23. van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine (Phila Pa 1976). 2000;25:2784-2796.
24. Hoffmann TC, Maher CG, Briffa T, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016;188:510-518.
25. Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73-81.
26. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18:138-160.
27. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
28. van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216-1226.
29. Shire AR, Stæhr TAB, Overby JB, et al. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet Disord. 2017;18:158.
30. Ellenbecker TS, Cools A. Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 2010;44:319-327.
31. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
32. Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43:242-246.
33. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366.
34. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383.
35. Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44:59-67.
36. Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3-15.
37. Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286.
38. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214-218.
39. Alizadehkhaiyat O, Fisher AC, Kemp GJ, et al. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23:482-489.
40. Cullinane FL, Boocock MG, Trevelyan FC. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin Rehabil. 2014;28:3-19.
41. Croisier JL, Foidart-Dessalle M, Tinant F, et al. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269-275.
42. Söderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22:797-803.
43. Smith BE, Hendrick P, Smith TO, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51:1679-1687.
Regular exercise confers several well-established benefits. In such conditions as coronary heart disease, stroke, heart failure, and diabetes, exercise has led to a reduction in mortality similar to that seen with pharmacotherapy.1 For patients with chronic musculoskeletal conditions, the benefits of exercise-based interventions are measurably reduced pain and improved daily function.2 However, prescribing of exercise is often neglected, with preference given to pharmacologic or surgical interventions.3 In part, the disregard of exercise as therapy results from unfamiliarity with appropriate exercise prescriptions,3 which include various forms of aerobic exercise, strength training, and stretching to increase flexibility (TABLE).
As is true of many therapeutic modalities, exercise must be tailored to the condition and to a patient’s preferences to optimize its benefits. In this review, we describe exercise regimens well suited for common musculoskeletal conditions, examine the effectiveness of exercise in each condition, and provide examples for use in treating patients.
Osteoarthritis of the hip and knee
Osteoarthritis (OA), one of the most common chronic joint diseases, erodes the articular cartilage and subchondral bone of a synovial joint, eventually leading to joint failure. Pain and diminished muscle strength restrict physical activity and can lead to decreased fitness and impaired muscle function. Exercise helps reduce pain and improve muscle function and quality of life in patients with hip or knee OA regardless of age, disease severity, or level of pain and dysfunction.2
Knee exercises. Activities suitable for patients with OA include muscle strengthening, aerobic conditioning, and range-of-motion (ROM) exercises.4-6 A 2015 Cochrane review of OA of the knee showed that exercise reduced pain and improved physical function and quality of life in patients who completed a treatment program, and that pain relief persisted up to 6 months after intervention.5
When designing an exercise prescription for patients with knee OA, consider quadriceps strengthening with an initial period of supervision, which may provide greater pain relief than nonspecific, unsupervised lower limb exercises.4 Enhanced strength of the lower limb may lessen force through the knee, thereby decreasing pain and improving overall physical function.7 Simple, teachable exercises include squats, step-ups, knee extension/flexion while sitting in a chair, and hip abduction/adduction while standing or lying down. Elastic bands, dumbbells, or cuff weights may be used to increase resistance.
Hip exercises. Exercise can significantly reduce pain and improve function for up to 6 months for patients with mild-to-moderate symptomatic hip OA.6 Types of exercise for hip OA include strength training of hip and core muscles, functional exercises that imitate movements in daily activities, and flexibility training. These exercises help reduce pain and increase ROM. Exercise should include resistance training and should not exceed the limit for acceptable pain.8
Aquatic therapy is also appropriate for exercise and strength training and can decrease pain and disability and improve quality of life.9 Supervised physical therapy, including strength training, manual therapy, and balance training, are important for reducing pain and improving function. Physical therapy can also enhance adherence to a prescribed exercise program.10
Continue to: Appropriate exercise prescriptions...
Appropriate exercise prescriptions for patients with knee or hip OA should focus on low-impact activities that can improve strength, flexibility, and function (FIGURE 1). A typical regimen would be 30 or more cumulative minutes daily of stationary cycling, water-based exercises, or strength training, 3 to 5 days per week. Individualize workout intensity for each patient, emphasizing that high-intensity, low-impact effort may yield greater strength gains and take less time to perform.11 A high-intensity exercise prescription focusing on quadriceps, hip, and core strengthening may consist of 3 sets of 8 repetitions with resistance set at 40% of the maximum resistance against which the patient can perform 1 repetition.7
Barriers to exercise in knee and hip OA include negative patient and provider perspectives on exercise and patients’ fear that increased activity may actually worsen OA.12 Depending on a patient’s personal preferences, ways to overcome these barriers and encourage adherence might be supervised exercises in an individual or group setting or audiotapes or videos of recommended exercises.10
Chronic low back pain
Chronic low back pain (LBP) is a large socioeconomic burden in the United States, with upward of $100 billion per year accounted for in health care costs and decreased worker productivity.13 The etiology of chronic LBP can be multifactorial and due to any of several conditions such as degenerative disc disease, spinal stenosis, spondylolisthesis, and facet arthropathy. Treatment is difficult, given that many common interventions—medications, massage, manipulation—have limited efficacy.14 However, for patients with nonspecific chronic LBP, exercise is an effective intervention for reducing pain and improving physical function.15
An effective approach is to design an exercise regimen for the individual by type, duration, and frequency of activity, administered under supervision to encourage adherence.16 Appropriate exercises emphasize resistance, strength training, and core stabilization, often focusing on whole body and trunk motion (FIGURE 2).17
Although yoga or Pilates classes may have a small effect on function, no high-quality evidence exists for their superiority to other forms of exercise.18,19 Back School, a therapeutic program that includes education on anatomy and biomechanics, optimal posture, ergonomics, and back exercises, has limited, low-quality evidence for treatment comparisons.20 Aerobic exercise, including treadmill, elliptical, or cycling exercises or walking outdoors can reduce pain and improve physical and psychologic functioning.21
Continue to: The most common reported adverse effect...
The most common reported adverse effect of exercise is a temporary exacerbation of back pain. However, having patients continue daily activities within the permitted limits of pain leads to more rapid recovery than rest or back-mobilizing exercises.15,22,23
Cautions. Exercise is contraindicated in patients with LBP arising from a serious medical condition, such as fracture, infection, cancer, or cauda equina syndrome.24 Importantly, exercise interventions recommended for acute LBP have not shown benefit for chronic LBP.
Chronic shoulder pain
With a prevalence ranging from 7% to 26% in the general population,25 chronic shoulder pain often interferes with essential activities of daily living. The etiology of chronic shoulder pain is broad and most commonly involves disorders of the rotator cuff, which functions in both motion and dynamic stabilization of the shoulder. The common term “rotator cuff pain syndrome” can cover such disorders as subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full thickness rotator cuff tears, calcific tendinitis, and subacromial bursitis. These pathologies may have overlapping presentations. Manual therapy and exercise, usually delivered as a component of structured physical therapy, focus on stretches and other exercises to increase ROM, stability, and strength of the rotator cuff musculature.26
A 2016 Cochrane review that evaluated manual therapy and exercise for chronic shoulder pain yielded limited high-quality evidence for effectiveness compared with placebo.27 Five trials found no important differences between manual therapy and exercise compared with glucocorticoid injection relative to overall pain, function, active shoulder abduction, and quality of life from 4 weeks up to 12 months.27 But compared with placebo, exercise has been more effective in reducing reported pain, especially in the context of strengthening regimens focused on flexion, extension, and internal and external rotation.28
For subacromial impingement syndrome, a 2017 meta-analysis found that a generalized exercise program relieves pain and improves function, ROM, and strength.29 A generalized shoulder-strengthening program includes exercises that focus on internal and external rotation, horizontal abduction, and shoulder stabilization (FIGURE 3). These exercises can be completed with 3 sets of 15 to 20 repetitions, which create a fatigue response that improves strength and targets local muscular endurance.30
Continue to: Achilles tendinopathy
Achilles tendinopathy
Achilles tendinopathy (also referred to as chronic Achilles tendinitis) is a degenerative condition of the Achilles tendon related to overuse that leads to pain, swelling, and impaired performance. It accounts for approximately 18% of injuries in runners and 4% of all patients presenting to sports medicine clinics.31 Eccentric muscle loading has become the dominant conservative intervention strategy for chronic Achilles tendinopathy.
For chronic tendinopathies, eccentric exercises subject greater force than concentric exercises through a controlled lengthening of a muscle-tendon unit, resulting in a greater remodeling stimulus of the tendon.32 Classically, the Alfredson protocol has been used to treat chronic Achilles tendinopathy. This program of eccentric heel-drop exercises recommends completion of 180 eccentric repetitions a day for up to 12 weeks (FIGURE 4).33 Exercises are performed slowly, and load can be increased when exercises are performed without pain or perhaps with mild nondisabling pain.
A variation of this protocol has allowed a gradual escalation of repetitions over a week up to the recommended 180 repetitions, and has shown improvements in pain reduction and function similar to that achieved with the primary protocol.34 Additionally, a 6-week “do as tolerated” program of eccentric exercises did not lead to lesser improvement for individuals with midportion Achilles tendinopathy.35
Several systematic reviews have supported the use of eccentric exercises for chronic Achilles tendinopathy,31,36,37 but no specific protocol or exercise regimen has demonstrated superiority. However, with the Alfredson protocol, improvement in pain and function in patients with chronic Achilles tendinopathy has persisted for up to 5 years.38
Lateral epicondylitis
Lateral epicondylitis (also called lateral epicondylosis or “tennis elbow”) is a disabling musculoskeletal condition that leads to pain and tenderness around the extensor mass of the lateral elbow. It is caused by microtrauma to the tendon, usually sustained through repetitive movement in a sporting activity, industrial work, or hobby. Affecting up to 3% of the US population, lateral epicondylitis is associated with pain and functional disability, as well as emotional and psychosocial consequences.39
Continue to: Proposed treatment and rehabilitation options...
Proposed treatment and rehabilitation options for patients with lateral epicondylitis have included massage, manipulation, taping, acupuncture, orthotic devices, ultrasound, activity modification, and rest. Exercise programs incorporating eccentric muscle activity are becoming increasingly popular for such conditions as Achilles and patellar tendinopathies, and they may translate well to other chronic tendinopathies, such as lateral epicondylitis.32
An eccentric exercise program for lateral epicondylitis, either in isolation or as an adjunct to other therapies, has decreased pain and improved function and grip strength from baseline measures.40 Compared with a standard exercise regimen without eccentric strength training, use of eccentric training improves such clinical measures as pain intensity and disability status, as it decreases tendon thickness and aids in recovering homogenous tendon structure.41
A sample exercise. The patient may sit in a chair and, with the forearm flexed and pronated over the edge of a table, grasp some form of resistance (bucket of water, training weight, resistance band) (FIGURE 5). The nonaffected hand can be used to help lift the affected wrist into full extension and then removed to allow lowering of the hand over several seconds into flexion. This activity can be performed in sets of 8 to 12 repetitions, 2 to 3 times a day, until the patient’s pain and function have improved.42
Overcoming barriers to exercise
A major concern across all studies assessing the therapeutic value of exercise is patient compliance and adherence to prescribed programs. Compliance and adherence are affected in part by psychosocial factors such as low literacy and poor social support. From a physician’s perspective, direct and indirect costs of treatment and rehabilitation of chronic musculoskeletal conditions may discourage the prescribing of supervised physical therapy.3
Steps to consider in overcoming these barriers would be advising an exercise regimen that requires only an initial period of supervision; educating patients about the benefits of an exercise program; exploring a patient’s expectations, beliefs, and fears; and developing strategies for long-term adherence.16 Supervision through physical therapy is often suggested. However, significant barriers may exist that impede a patient’s ability to attend or participate, in which case physician observation in the course of regularly scheduled clinical examinations could be considered.
Continue to: When prescribing exercises...
When prescribing exercises, be sure to address patient expectations regarding pain, duration, and limitations of exercise. It would be helpful for patients to know, for instance, that working through mild-to-moderate pain during exercise has been shown to shorten post-exercise recovery time and, in the short-term, improve relief from pain.43
Tailoring specific exercise prescriptions for a patient will make the regimen more satisfying for the individual and optimize adherence, which in turn will increase the potential for pain reduction and improved function. Secondary benefits would likely be weight loss and prevention (or regression) of cardiovascular disease. Continued evaluation by the physician or physical therapist should be part of ongoing management, as well as “refresher courses” to ensure understanding of, and adherence to, the exercise program. The potential benefits and limited risks of exercise, if done properly, make it a primary intervention for specific musculoskeletal conditions.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, PO Box 100237, Gainesville, FL 32610-0237; carek@ufl.edu.
Regular exercise confers several well-established benefits. In such conditions as coronary heart disease, stroke, heart failure, and diabetes, exercise has led to a reduction in mortality similar to that seen with pharmacotherapy.1 For patients with chronic musculoskeletal conditions, the benefits of exercise-based interventions are measurably reduced pain and improved daily function.2 However, prescribing of exercise is often neglected, with preference given to pharmacologic or surgical interventions.3 In part, the disregard of exercise as therapy results from unfamiliarity with appropriate exercise prescriptions,3 which include various forms of aerobic exercise, strength training, and stretching to increase flexibility (TABLE).
As is true of many therapeutic modalities, exercise must be tailored to the condition and to a patient’s preferences to optimize its benefits. In this review, we describe exercise regimens well suited for common musculoskeletal conditions, examine the effectiveness of exercise in each condition, and provide examples for use in treating patients.
Osteoarthritis of the hip and knee
Osteoarthritis (OA), one of the most common chronic joint diseases, erodes the articular cartilage and subchondral bone of a synovial joint, eventually leading to joint failure. Pain and diminished muscle strength restrict physical activity and can lead to decreased fitness and impaired muscle function. Exercise helps reduce pain and improve muscle function and quality of life in patients with hip or knee OA regardless of age, disease severity, or level of pain and dysfunction.2
Knee exercises. Activities suitable for patients with OA include muscle strengthening, aerobic conditioning, and range-of-motion (ROM) exercises.4-6 A 2015 Cochrane review of OA of the knee showed that exercise reduced pain and improved physical function and quality of life in patients who completed a treatment program, and that pain relief persisted up to 6 months after intervention.5
When designing an exercise prescription for patients with knee OA, consider quadriceps strengthening with an initial period of supervision, which may provide greater pain relief than nonspecific, unsupervised lower limb exercises.4 Enhanced strength of the lower limb may lessen force through the knee, thereby decreasing pain and improving overall physical function.7 Simple, teachable exercises include squats, step-ups, knee extension/flexion while sitting in a chair, and hip abduction/adduction while standing or lying down. Elastic bands, dumbbells, or cuff weights may be used to increase resistance.
Hip exercises. Exercise can significantly reduce pain and improve function for up to 6 months for patients with mild-to-moderate symptomatic hip OA.6 Types of exercise for hip OA include strength training of hip and core muscles, functional exercises that imitate movements in daily activities, and flexibility training. These exercises help reduce pain and increase ROM. Exercise should include resistance training and should not exceed the limit for acceptable pain.8
Aquatic therapy is also appropriate for exercise and strength training and can decrease pain and disability and improve quality of life.9 Supervised physical therapy, including strength training, manual therapy, and balance training, are important for reducing pain and improving function. Physical therapy can also enhance adherence to a prescribed exercise program.10
Continue to: Appropriate exercise prescriptions...
Appropriate exercise prescriptions for patients with knee or hip OA should focus on low-impact activities that can improve strength, flexibility, and function (FIGURE 1). A typical regimen would be 30 or more cumulative minutes daily of stationary cycling, water-based exercises, or strength training, 3 to 5 days per week. Individualize workout intensity for each patient, emphasizing that high-intensity, low-impact effort may yield greater strength gains and take less time to perform.11 A high-intensity exercise prescription focusing on quadriceps, hip, and core strengthening may consist of 3 sets of 8 repetitions with resistance set at 40% of the maximum resistance against which the patient can perform 1 repetition.7
Barriers to exercise in knee and hip OA include negative patient and provider perspectives on exercise and patients’ fear that increased activity may actually worsen OA.12 Depending on a patient’s personal preferences, ways to overcome these barriers and encourage adherence might be supervised exercises in an individual or group setting or audiotapes or videos of recommended exercises.10
Chronic low back pain
Chronic low back pain (LBP) is a large socioeconomic burden in the United States, with upward of $100 billion per year accounted for in health care costs and decreased worker productivity.13 The etiology of chronic LBP can be multifactorial and due to any of several conditions such as degenerative disc disease, spinal stenosis, spondylolisthesis, and facet arthropathy. Treatment is difficult, given that many common interventions—medications, massage, manipulation—have limited efficacy.14 However, for patients with nonspecific chronic LBP, exercise is an effective intervention for reducing pain and improving physical function.15
An effective approach is to design an exercise regimen for the individual by type, duration, and frequency of activity, administered under supervision to encourage adherence.16 Appropriate exercises emphasize resistance, strength training, and core stabilization, often focusing on whole body and trunk motion (FIGURE 2).17
Although yoga or Pilates classes may have a small effect on function, no high-quality evidence exists for their superiority to other forms of exercise.18,19 Back School, a therapeutic program that includes education on anatomy and biomechanics, optimal posture, ergonomics, and back exercises, has limited, low-quality evidence for treatment comparisons.20 Aerobic exercise, including treadmill, elliptical, or cycling exercises or walking outdoors can reduce pain and improve physical and psychologic functioning.21
Continue to: The most common reported adverse effect...
The most common reported adverse effect of exercise is a temporary exacerbation of back pain. However, having patients continue daily activities within the permitted limits of pain leads to more rapid recovery than rest or back-mobilizing exercises.15,22,23
Cautions. Exercise is contraindicated in patients with LBP arising from a serious medical condition, such as fracture, infection, cancer, or cauda equina syndrome.24 Importantly, exercise interventions recommended for acute LBP have not shown benefit for chronic LBP.
Chronic shoulder pain
With a prevalence ranging from 7% to 26% in the general population,25 chronic shoulder pain often interferes with essential activities of daily living. The etiology of chronic shoulder pain is broad and most commonly involves disorders of the rotator cuff, which functions in both motion and dynamic stabilization of the shoulder. The common term “rotator cuff pain syndrome” can cover such disorders as subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full thickness rotator cuff tears, calcific tendinitis, and subacromial bursitis. These pathologies may have overlapping presentations. Manual therapy and exercise, usually delivered as a component of structured physical therapy, focus on stretches and other exercises to increase ROM, stability, and strength of the rotator cuff musculature.26
A 2016 Cochrane review that evaluated manual therapy and exercise for chronic shoulder pain yielded limited high-quality evidence for effectiveness compared with placebo.27 Five trials found no important differences between manual therapy and exercise compared with glucocorticoid injection relative to overall pain, function, active shoulder abduction, and quality of life from 4 weeks up to 12 months.27 But compared with placebo, exercise has been more effective in reducing reported pain, especially in the context of strengthening regimens focused on flexion, extension, and internal and external rotation.28
For subacromial impingement syndrome, a 2017 meta-analysis found that a generalized exercise program relieves pain and improves function, ROM, and strength.29 A generalized shoulder-strengthening program includes exercises that focus on internal and external rotation, horizontal abduction, and shoulder stabilization (FIGURE 3). These exercises can be completed with 3 sets of 15 to 20 repetitions, which create a fatigue response that improves strength and targets local muscular endurance.30
Continue to: Achilles tendinopathy
Achilles tendinopathy
Achilles tendinopathy (also referred to as chronic Achilles tendinitis) is a degenerative condition of the Achilles tendon related to overuse that leads to pain, swelling, and impaired performance. It accounts for approximately 18% of injuries in runners and 4% of all patients presenting to sports medicine clinics.31 Eccentric muscle loading has become the dominant conservative intervention strategy for chronic Achilles tendinopathy.
For chronic tendinopathies, eccentric exercises subject greater force than concentric exercises through a controlled lengthening of a muscle-tendon unit, resulting in a greater remodeling stimulus of the tendon.32 Classically, the Alfredson protocol has been used to treat chronic Achilles tendinopathy. This program of eccentric heel-drop exercises recommends completion of 180 eccentric repetitions a day for up to 12 weeks (FIGURE 4).33 Exercises are performed slowly, and load can be increased when exercises are performed without pain or perhaps with mild nondisabling pain.
A variation of this protocol has allowed a gradual escalation of repetitions over a week up to the recommended 180 repetitions, and has shown improvements in pain reduction and function similar to that achieved with the primary protocol.34 Additionally, a 6-week “do as tolerated” program of eccentric exercises did not lead to lesser improvement for individuals with midportion Achilles tendinopathy.35
Several systematic reviews have supported the use of eccentric exercises for chronic Achilles tendinopathy,31,36,37 but no specific protocol or exercise regimen has demonstrated superiority. However, with the Alfredson protocol, improvement in pain and function in patients with chronic Achilles tendinopathy has persisted for up to 5 years.38
Lateral epicondylitis
Lateral epicondylitis (also called lateral epicondylosis or “tennis elbow”) is a disabling musculoskeletal condition that leads to pain and tenderness around the extensor mass of the lateral elbow. It is caused by microtrauma to the tendon, usually sustained through repetitive movement in a sporting activity, industrial work, or hobby. Affecting up to 3% of the US population, lateral epicondylitis is associated with pain and functional disability, as well as emotional and psychosocial consequences.39
Continue to: Proposed treatment and rehabilitation options...
Proposed treatment and rehabilitation options for patients with lateral epicondylitis have included massage, manipulation, taping, acupuncture, orthotic devices, ultrasound, activity modification, and rest. Exercise programs incorporating eccentric muscle activity are becoming increasingly popular for such conditions as Achilles and patellar tendinopathies, and they may translate well to other chronic tendinopathies, such as lateral epicondylitis.32
An eccentric exercise program for lateral epicondylitis, either in isolation or as an adjunct to other therapies, has decreased pain and improved function and grip strength from baseline measures.40 Compared with a standard exercise regimen without eccentric strength training, use of eccentric training improves such clinical measures as pain intensity and disability status, as it decreases tendon thickness and aids in recovering homogenous tendon structure.41
A sample exercise. The patient may sit in a chair and, with the forearm flexed and pronated over the edge of a table, grasp some form of resistance (bucket of water, training weight, resistance band) (FIGURE 5). The nonaffected hand can be used to help lift the affected wrist into full extension and then removed to allow lowering of the hand over several seconds into flexion. This activity can be performed in sets of 8 to 12 repetitions, 2 to 3 times a day, until the patient’s pain and function have improved.42
Overcoming barriers to exercise
A major concern across all studies assessing the therapeutic value of exercise is patient compliance and adherence to prescribed programs. Compliance and adherence are affected in part by psychosocial factors such as low literacy and poor social support. From a physician’s perspective, direct and indirect costs of treatment and rehabilitation of chronic musculoskeletal conditions may discourage the prescribing of supervised physical therapy.3
Steps to consider in overcoming these barriers would be advising an exercise regimen that requires only an initial period of supervision; educating patients about the benefits of an exercise program; exploring a patient’s expectations, beliefs, and fears; and developing strategies for long-term adherence.16 Supervision through physical therapy is often suggested. However, significant barriers may exist that impede a patient’s ability to attend or participate, in which case physician observation in the course of regularly scheduled clinical examinations could be considered.
Continue to: When prescribing exercises...
When prescribing exercises, be sure to address patient expectations regarding pain, duration, and limitations of exercise. It would be helpful for patients to know, for instance, that working through mild-to-moderate pain during exercise has been shown to shorten post-exercise recovery time and, in the short-term, improve relief from pain.43
Tailoring specific exercise prescriptions for a patient will make the regimen more satisfying for the individual and optimize adherence, which in turn will increase the potential for pain reduction and improved function. Secondary benefits would likely be weight loss and prevention (or regression) of cardiovascular disease. Continued evaluation by the physician or physical therapist should be part of ongoing management, as well as “refresher courses” to ensure understanding of, and adherence to, the exercise program. The potential benefits and limited risks of exercise, if done properly, make it a primary intervention for specific musculoskeletal conditions.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, PO Box 100237, Gainesville, FL 32610-0237; carek@ufl.edu.
1. Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
2. Babatunde OO, Jordan JL, van der Windt DA, et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12:e0178621.
3. Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner’s perspective - a qualitative study. BMC Fam Pract. 2013;14:128.
4. Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622-636.
5. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376.
6. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912.
7. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(suppl 5):S45-S52.
8. Fernandes L, Storheim K, Nordsletten L, et al. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90:592-601.
9. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
10. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956.
11. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
12. Brakke R, Singh J, Sullivan W. Physical therapy in persons with osteoarthritis. PM R. 2012;4:S53-S58.
13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24.
14. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
15. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335.
16. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
17. Searle A, Spink M, Ho A, et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155-1167.
18. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;(7):CD010265.
19. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(1):CD010671.
20. Parreira P, Heymans MW, van Tulder MW, et al. Back Schools for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(8):CD011674.
21. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil. 2015;94:358-365.
22. Malmivaara A, Häkkinen U, Aro T, et al. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351-355.
23. van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine (Phila Pa 1976). 2000;25:2784-2796.
24. Hoffmann TC, Maher CG, Briffa T, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016;188:510-518.
25. Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73-81.
26. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18:138-160.
27. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
28. van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216-1226.
29. Shire AR, Stæhr TAB, Overby JB, et al. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet Disord. 2017;18:158.
30. Ellenbecker TS, Cools A. Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 2010;44:319-327.
31. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
32. Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43:242-246.
33. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366.
34. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383.
35. Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44:59-67.
36. Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3-15.
37. Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286.
38. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214-218.
39. Alizadehkhaiyat O, Fisher AC, Kemp GJ, et al. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23:482-489.
40. Cullinane FL, Boocock MG, Trevelyan FC. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin Rehabil. 2014;28:3-19.
41. Croisier JL, Foidart-Dessalle M, Tinant F, et al. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269-275.
42. Söderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22:797-803.
43. Smith BE, Hendrick P, Smith TO, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51:1679-1687.
1. Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
2. Babatunde OO, Jordan JL, van der Windt DA, et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12:e0178621.
3. Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner’s perspective - a qualitative study. BMC Fam Pract. 2013;14:128.
4. Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622-636.
5. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376.
6. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912.
7. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(suppl 5):S45-S52.
8. Fernandes L, Storheim K, Nordsletten L, et al. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90:592-601.
9. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
10. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956.
11. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
12. Brakke R, Singh J, Sullivan W. Physical therapy in persons with osteoarthritis. PM R. 2012;4:S53-S58.
13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24.
14. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
15. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335.
16. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
17. Searle A, Spink M, Ho A, et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155-1167.
18. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;(7):CD010265.
19. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(1):CD010671.
20. Parreira P, Heymans MW, van Tulder MW, et al. Back Schools for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(8):CD011674.
21. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil. 2015;94:358-365.
22. Malmivaara A, Häkkinen U, Aro T, et al. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351-355.
23. van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine (Phila Pa 1976). 2000;25:2784-2796.
24. Hoffmann TC, Maher CG, Briffa T, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016;188:510-518.
25. Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73-81.
26. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18:138-160.
27. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
28. van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216-1226.
29. Shire AR, Stæhr TAB, Overby JB, et al. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet Disord. 2017;18:158.
30. Ellenbecker TS, Cools A. Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 2010;44:319-327.
31. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
32. Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43:242-246.
33. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366.
34. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383.
35. Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44:59-67.
36. Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3-15.
37. Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286.
38. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214-218.
39. Alizadehkhaiyat O, Fisher AC, Kemp GJ, et al. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23:482-489.
40. Cullinane FL, Boocock MG, Trevelyan FC. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin Rehabil. 2014;28:3-19.
41. Croisier JL, Foidart-Dessalle M, Tinant F, et al. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269-275.
42. Söderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22:797-803.
43. Smith BE, Hendrick P, Smith TO, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51:1679-1687.
From The Journal of Family Practice | 2018;67(9):534-538,540-543.
PRACTICE RECOMMENDATIONS
› Consider quadriceps strengthening for knee osteoarthritis with an initial period of supervision, which can provide greater pain relief than nonspecific, unsupervised lower limb exercises. B
› Consider a generalized exercise program for subacromial impingement syndrome, to relieve shoulder pain and improve function, range of motion, and strength. A
› Bear in mind that the Alfredson protocol for Achilles tendinopathy has yielded improvement in pain and function for up to 5 years, although other exercise regimens have also proven initially effective. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
DTC test for BRCA mutations: What to tell patients who inquire
Resources
US Food & Drug Administration. FDA authorizes, with special controls, direct-to-consumer test that reports three mutations in the BRCA breast cancer genes. March 6, 2018. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm599560.htm. Accessed August 8, 2018.
US Preventive Services Task Force. Final recommendation statement: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. Available at:
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Accessed August 8, 2018.
Resources
US Food & Drug Administration. FDA authorizes, with special controls, direct-to-consumer test that reports three mutations in the BRCA breast cancer genes. March 6, 2018. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm599560.htm. Accessed August 8, 2018.
US Preventive Services Task Force. Final recommendation statement: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. Available at:
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Accessed August 8, 2018.
Resources
US Food & Drug Administration. FDA authorizes, with special controls, direct-to-consumer test that reports three mutations in the BRCA breast cancer genes. March 6, 2018. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm599560.htm. Accessed August 8, 2018.
US Preventive Services Task Force. Final recommendation statement: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. Available at:
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Accessed August 8, 2018.
Nonpharmacologic strategies for acute pain
Background: Pain is a very common cause for emergency room visits. Nonpharmacologic pain management is not well studied for adult patients visiting the ED.
Study design: Systematic review and meta-analysis.
Setting: Duke University, Durham, N.C.
Synopsis: Among the studies considered, 56 met inclusion criteria for summary analysis. Multiple interventions were used, mostly acupuncture and physical therapy. Of the 56 studies, 23 reported significantly reduced pain, compared with control; 24 showed no difference, compared with control; and 9 had no control group. Meta-analysis included 22 qualifying randomized, controlled trials and had a global standardized mean difference of –0.46 (95% confidence interval, –0.66 to –0.27) in favor of nonpharmacologic interventions for reducing pain.
Bottom line: Nonpharmacologic pain control approaches may be helpful for adult patients visiting the emergency room. Larger studies are needed to confirm efficacy.
Citation: JT Sakamoto et al. Are nonpharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department? A systematic review and meta‐analysis. Acad Emerg Med. 2018 Mar 15. doi: 10.1111/acem.13411.
Dr. Hasanein is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Pain is a very common cause for emergency room visits. Nonpharmacologic pain management is not well studied for adult patients visiting the ED.
Study design: Systematic review and meta-analysis.
Setting: Duke University, Durham, N.C.
Synopsis: Among the studies considered, 56 met inclusion criteria for summary analysis. Multiple interventions were used, mostly acupuncture and physical therapy. Of the 56 studies, 23 reported significantly reduced pain, compared with control; 24 showed no difference, compared with control; and 9 had no control group. Meta-analysis included 22 qualifying randomized, controlled trials and had a global standardized mean difference of –0.46 (95% confidence interval, –0.66 to –0.27) in favor of nonpharmacologic interventions for reducing pain.
Bottom line: Nonpharmacologic pain control approaches may be helpful for adult patients visiting the emergency room. Larger studies are needed to confirm efficacy.
Citation: JT Sakamoto et al. Are nonpharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department? A systematic review and meta‐analysis. Acad Emerg Med. 2018 Mar 15. doi: 10.1111/acem.13411.
Dr. Hasanein is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Pain is a very common cause for emergency room visits. Nonpharmacologic pain management is not well studied for adult patients visiting the ED.
Study design: Systematic review and meta-analysis.
Setting: Duke University, Durham, N.C.
Synopsis: Among the studies considered, 56 met inclusion criteria for summary analysis. Multiple interventions were used, mostly acupuncture and physical therapy. Of the 56 studies, 23 reported significantly reduced pain, compared with control; 24 showed no difference, compared with control; and 9 had no control group. Meta-analysis included 22 qualifying randomized, controlled trials and had a global standardized mean difference of –0.46 (95% confidence interval, –0.66 to –0.27) in favor of nonpharmacologic interventions for reducing pain.
Bottom line: Nonpharmacologic pain control approaches may be helpful for adult patients visiting the emergency room. Larger studies are needed to confirm efficacy.
Citation: JT Sakamoto et al. Are nonpharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department? A systematic review and meta‐analysis. Acad Emerg Med. 2018 Mar 15. doi: 10.1111/acem.13411.
Dr. Hasanein is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Obesity Extends Viral Shedding of Flu
Obesity not only makes flu more severe, but also lengthens the period of viral shedding for influenza A, according to a study by University of Michigan researchers, partly funded by the National Institute of Allergy and Infectious Diseases.
Over 3 flu seasons, the researchers monitored 1,783 people from 320 households in Managua, Nicaragua. During that time, 87 people became ill with influenza A and 58 with influenza B.
More than 40% of the adults aged > 18 years were obese, as defined by body mass. Obese adults with ≥ 2 symptoms of influenza A (n = 62) shed the virus 42% longer than did nonobese adults, or 5.2 days compared with 3.7 days, respectively. Obese adults with 1 or no symptoms of influenza A (n = 25) shed the virus 104% longer than nonobese adults—3.2 days compared with 1.6 days, respectively.
Obesity was not a risk factor for increased viral shedding duration in children aged 5 to 17 years or for adults with influenza B.
The researchers suggest that chronic inflammation caused by obesity may be responsible for the increased viral shedding. Reducing obesity rates could be an important target to limit the spread of viral infectious diseases, they suggest. The findings may have particular significance in the US, where in 2014 35% of adults were obese compared with 17.4% of adults in Nicaragua.
Obesity not only makes flu more severe, but also lengthens the period of viral shedding for influenza A, according to a study by University of Michigan researchers, partly funded by the National Institute of Allergy and Infectious Diseases.
Over 3 flu seasons, the researchers monitored 1,783 people from 320 households in Managua, Nicaragua. During that time, 87 people became ill with influenza A and 58 with influenza B.
More than 40% of the adults aged > 18 years were obese, as defined by body mass. Obese adults with ≥ 2 symptoms of influenza A (n = 62) shed the virus 42% longer than did nonobese adults, or 5.2 days compared with 3.7 days, respectively. Obese adults with 1 or no symptoms of influenza A (n = 25) shed the virus 104% longer than nonobese adults—3.2 days compared with 1.6 days, respectively.
Obesity was not a risk factor for increased viral shedding duration in children aged 5 to 17 years or for adults with influenza B.
The researchers suggest that chronic inflammation caused by obesity may be responsible for the increased viral shedding. Reducing obesity rates could be an important target to limit the spread of viral infectious diseases, they suggest. The findings may have particular significance in the US, where in 2014 35% of adults were obese compared with 17.4% of adults in Nicaragua.
Obesity not only makes flu more severe, but also lengthens the period of viral shedding for influenza A, according to a study by University of Michigan researchers, partly funded by the National Institute of Allergy and Infectious Diseases.
Over 3 flu seasons, the researchers monitored 1,783 people from 320 households in Managua, Nicaragua. During that time, 87 people became ill with influenza A and 58 with influenza B.
More than 40% of the adults aged > 18 years were obese, as defined by body mass. Obese adults with ≥ 2 symptoms of influenza A (n = 62) shed the virus 42% longer than did nonobese adults, or 5.2 days compared with 3.7 days, respectively. Obese adults with 1 or no symptoms of influenza A (n = 25) shed the virus 104% longer than nonobese adults—3.2 days compared with 1.6 days, respectively.
Obesity was not a risk factor for increased viral shedding duration in children aged 5 to 17 years or for adults with influenza B.
The researchers suggest that chronic inflammation caused by obesity may be responsible for the increased viral shedding. Reducing obesity rates could be an important target to limit the spread of viral infectious diseases, they suggest. The findings may have particular significance in the US, where in 2014 35% of adults were obese compared with 17.4% of adults in Nicaragua.
Woman, 18, With Sore Throat, Fever, and Painful Rash
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An 18-year-old woman presents to urgent care with a one-day history of sudden-onset sore throat, chills, malaise, and fever (102.5°F). On physical examination, the tonsils and pharynx are erythematous, and anterior cervical lymph nodes are tender on palpation. A rapid strep test is negative. The patient is instructed to use throat lozenges and take ibuprofen (400 mg every 8 h) as needed for pain.
On day 2, the patient’s fever resolves, but a painful rash develops on her palms and soles. Clinical examination reveals multiple erythematous plaques on the hands and feet (see Figure 1). The patient is diagnosed with hand-foot-and-mouth disease (HFMD).
DISCUSSION
HFMD is caused by enteroviruses, most commonly coxsackievirus A16 (CV-A16) or enterovirus 71 (EV71).1 However, a newly recognized strain, CV-A6, has caused worldwide outbreaks of HFMD in both children and adults. Although less common than CV-A16 or EV71, CV-A6 is associated with a more severe disease course.1 The CV-A6 strain was first identified in Finland in 2008 during a major outbreak of HFMD; it reached the United States in 2011.2,3 Accurate statistics on the prevalence of CV-A6 in the US are difficult to obtain because HFMD is not a reportable condition.
Clinical presentation
HFMD typically manifests with painful oral lesions, with or without a macular, maculopapular, or vesicular exanthema. If a fever is present, it is usually below 101°F. The oral lesions are typically benign and manifest as erythematous macules that progress to vesicles with an erythematous halo. These lesions tend to be painful and may interfere with eating or drinking. Signs and symptoms associated with the more virulent form, CV-A6, may include
- Higher fever
- Wider distribution of lesions
- More extensive skin involvement
- Longer duration
- Palmar and plantar desquamation
- Nail dystrophy.4,5
Laboratory diagnosis
Most symptomatic enterovirus infections are diagnosed based on clinical findings alone, reducing the need for laboratory testing. Laboratory confirmation may be warranted for more severe infections and during outbreaks. Molecular methods, such as reverse transcriptase polymerase chain reaction, are typically used for identifying enteroviruses, as they are rapid, sensitive, and widely available in hospital and commercial laboratories.6 Viral culture methods are labor-intensive, expensive, and reserved for typing the isolate. Serology is not useful in the diagnosis of acute infection.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis of HFMD includes conditions with oral lesions and maculopapular, vesicular lesions involving the palms and/or soles, as well as erythroderma.
Oral lesions. Aphthous ulcers are shallow, painful oral lesions not accompanied by skin rashes. Herpes gingivostomatitis, caused by herpes simplex virus (HSV), is often preceded by a prodrome of fever. The associated lesions manifest as vesicular clusters on a red base that evolve into large, painful ulcers. HSV mouth lesions can populate the gingivae, pharynx, hard palate, lips, and perioral skin. Skin lesions may occur unilaterally.
Rashes involving palms and soles. A number of conditions manifest with skin lesions similar to those of HFMD. An autoeczematization reaction consisting of a pruritic, papulovesicular eruption secondary to dermatophyte infection (eg, tinea pedis, tinea manuum, tinea cruris, tinea corporis, tinea capitis) should be ruled out. This type of reaction is thought to be a delayed hypersensitivity response to fungal antigens. Pruritus and the absence of mouth sores distinguishes this reaction from HFMD.7
Secondary syphilis can manifest with a short-lived macular rash involving the palm and soles, as well as oral mucous patches and generalized lymphadenopathy. Syphilis testing, including rapid plasma reagin or Venereal Disease Research Laboratory test with fluorescent treponemal antibody absorption, can rule out this diagnosis.
Erythema multiforme, which is more common in young adults, is characterized by target lesions on the palms and soles and erosions and/or bullae in the mouth and mucous membranes. It is usually preceded by a trigger, such as HSV infection.
Continue to: Erythroderma
Erythroderma. In addition to a rash, erythema multiforme can cause desquamation later in the disease course. Toxic shock syndrome (TSS), a life-threatening condition caused by Streptococcus or Staphylococcus, has an abrupt onset that is associated with high fever and hypotension. TSS causes a sunburn-like rash on the palms and soles that desquamates weeks after onset. Scarlet fever, caused by group A Streptococcus, can cause an erythematous rash with desquamation in children and adolescents. Scalded skin syndrome is a desquamative condition caused by Staphylococcus that occurs primarily in infants and young children.
Management
There is no specific antiviral treatment for HFMD, and thus management is mainly supportive. Fever and pain can be managed with ibuprofen or acetaminophen. Children who are unable to maintain oral hydration may require hospitalization for IV fluids.
Prevention of HFMD requires strict hand hygiene—washing with soap and water—as well as thoroughly cleaning and disinfecting surfaces that come in contact with infected oral secretions or feces.8
OUTCOME FOR THE CASE PATIENT
The patient was discharged and instructed to take ibuprofen as needed.
About three weeks later, the patient’s palms and soles began to peel. Clinical examination at follow-up revealed painful, diffuse, scaly desquamation of the hands and feet (see Figure 2). The patient also experienced loosening and shedding of the proximal nails (see Figure 3). She was diagnosed with postviral shedding.
Continue to: About eight weeks after the desquamatory rash manifested...
About eight weeks after the desquamatory rash manifested, complete resolution was seen. The patient experienced continued onychom
CONCLUSION
Clinicians should be mindful of the increasing incidence of HFMD in the adult population, since it may mimic other disease states. The extent and chronicity of this patient’s clinical manifestations were unusual and may have been caused by CV-A6.
1. Ben-Chetrit E, Wiener-Well Y, Shulman LM, et al. Coxsackievirus A6-related hand foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol. 2014;59(3):201-203.
2. Blomqvist S, Klemola P, Kajalainen S, et al. Co-circulation of coxsackie viruses A6 and A10 in hand, foot, mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49-54.
3. Downing C, Ramirez-Fort MK, Doan HQ, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60(4):381-386.
4. Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69(5):736-741.
5. Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14(1):83-86.
6. Pozo F, Casas I, Tenorio A, et al. Evaluation of a commercially available reverse transcription-PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol. 1998;36(6):1741-1745.
7. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128(2):e453-e457.
8. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898- e904.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An 18-year-old woman presents to urgent care with a one-day history of sudden-onset sore throat, chills, malaise, and fever (102.5°F). On physical examination, the tonsils and pharynx are erythematous, and anterior cervical lymph nodes are tender on palpation. A rapid strep test is negative. The patient is instructed to use throat lozenges and take ibuprofen (400 mg every 8 h) as needed for pain.
On day 2, the patient’s fever resolves, but a painful rash develops on her palms and soles. Clinical examination reveals multiple erythematous plaques on the hands and feet (see Figure 1). The patient is diagnosed with hand-foot-and-mouth disease (HFMD).
DISCUSSION
HFMD is caused by enteroviruses, most commonly coxsackievirus A16 (CV-A16) or enterovirus 71 (EV71).1 However, a newly recognized strain, CV-A6, has caused worldwide outbreaks of HFMD in both children and adults. Although less common than CV-A16 or EV71, CV-A6 is associated with a more severe disease course.1 The CV-A6 strain was first identified in Finland in 2008 during a major outbreak of HFMD; it reached the United States in 2011.2,3 Accurate statistics on the prevalence of CV-A6 in the US are difficult to obtain because HFMD is not a reportable condition.
Clinical presentation
HFMD typically manifests with painful oral lesions, with or without a macular, maculopapular, or vesicular exanthema. If a fever is present, it is usually below 101°F. The oral lesions are typically benign and manifest as erythematous macules that progress to vesicles with an erythematous halo. These lesions tend to be painful and may interfere with eating or drinking. Signs and symptoms associated with the more virulent form, CV-A6, may include
- Higher fever
- Wider distribution of lesions
- More extensive skin involvement
- Longer duration
- Palmar and plantar desquamation
- Nail dystrophy.4,5
Laboratory diagnosis
Most symptomatic enterovirus infections are diagnosed based on clinical findings alone, reducing the need for laboratory testing. Laboratory confirmation may be warranted for more severe infections and during outbreaks. Molecular methods, such as reverse transcriptase polymerase chain reaction, are typically used for identifying enteroviruses, as they are rapid, sensitive, and widely available in hospital and commercial laboratories.6 Viral culture methods are labor-intensive, expensive, and reserved for typing the isolate. Serology is not useful in the diagnosis of acute infection.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis of HFMD includes conditions with oral lesions and maculopapular, vesicular lesions involving the palms and/or soles, as well as erythroderma.
Oral lesions. Aphthous ulcers are shallow, painful oral lesions not accompanied by skin rashes. Herpes gingivostomatitis, caused by herpes simplex virus (HSV), is often preceded by a prodrome of fever. The associated lesions manifest as vesicular clusters on a red base that evolve into large, painful ulcers. HSV mouth lesions can populate the gingivae, pharynx, hard palate, lips, and perioral skin. Skin lesions may occur unilaterally.
Rashes involving palms and soles. A number of conditions manifest with skin lesions similar to those of HFMD. An autoeczematization reaction consisting of a pruritic, papulovesicular eruption secondary to dermatophyte infection (eg, tinea pedis, tinea manuum, tinea cruris, tinea corporis, tinea capitis) should be ruled out. This type of reaction is thought to be a delayed hypersensitivity response to fungal antigens. Pruritus and the absence of mouth sores distinguishes this reaction from HFMD.7
Secondary syphilis can manifest with a short-lived macular rash involving the palm and soles, as well as oral mucous patches and generalized lymphadenopathy. Syphilis testing, including rapid plasma reagin or Venereal Disease Research Laboratory test with fluorescent treponemal antibody absorption, can rule out this diagnosis.
Erythema multiforme, which is more common in young adults, is characterized by target lesions on the palms and soles and erosions and/or bullae in the mouth and mucous membranes. It is usually preceded by a trigger, such as HSV infection.
Continue to: Erythroderma
Erythroderma. In addition to a rash, erythema multiforme can cause desquamation later in the disease course. Toxic shock syndrome (TSS), a life-threatening condition caused by Streptococcus or Staphylococcus, has an abrupt onset that is associated with high fever and hypotension. TSS causes a sunburn-like rash on the palms and soles that desquamates weeks after onset. Scarlet fever, caused by group A Streptococcus, can cause an erythematous rash with desquamation in children and adolescents. Scalded skin syndrome is a desquamative condition caused by Staphylococcus that occurs primarily in infants and young children.
Management
There is no specific antiviral treatment for HFMD, and thus management is mainly supportive. Fever and pain can be managed with ibuprofen or acetaminophen. Children who are unable to maintain oral hydration may require hospitalization for IV fluids.
Prevention of HFMD requires strict hand hygiene—washing with soap and water—as well as thoroughly cleaning and disinfecting surfaces that come in contact with infected oral secretions or feces.8
OUTCOME FOR THE CASE PATIENT
The patient was discharged and instructed to take ibuprofen as needed.
About three weeks later, the patient’s palms and soles began to peel. Clinical examination at follow-up revealed painful, diffuse, scaly desquamation of the hands and feet (see Figure 2). The patient also experienced loosening and shedding of the proximal nails (see Figure 3). She was diagnosed with postviral shedding.
Continue to: About eight weeks after the desquamatory rash manifested...
About eight weeks after the desquamatory rash manifested, complete resolution was seen. The patient experienced continued onychom
CONCLUSION
Clinicians should be mindful of the increasing incidence of HFMD in the adult population, since it may mimic other disease states. The extent and chronicity of this patient’s clinical manifestations were unusual and may have been caused by CV-A6.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An 18-year-old woman presents to urgent care with a one-day history of sudden-onset sore throat, chills, malaise, and fever (102.5°F). On physical examination, the tonsils and pharynx are erythematous, and anterior cervical lymph nodes are tender on palpation. A rapid strep test is negative. The patient is instructed to use throat lozenges and take ibuprofen (400 mg every 8 h) as needed for pain.
On day 2, the patient’s fever resolves, but a painful rash develops on her palms and soles. Clinical examination reveals multiple erythematous plaques on the hands and feet (see Figure 1). The patient is diagnosed with hand-foot-and-mouth disease (HFMD).
DISCUSSION
HFMD is caused by enteroviruses, most commonly coxsackievirus A16 (CV-A16) or enterovirus 71 (EV71).1 However, a newly recognized strain, CV-A6, has caused worldwide outbreaks of HFMD in both children and adults. Although less common than CV-A16 or EV71, CV-A6 is associated with a more severe disease course.1 The CV-A6 strain was first identified in Finland in 2008 during a major outbreak of HFMD; it reached the United States in 2011.2,3 Accurate statistics on the prevalence of CV-A6 in the US are difficult to obtain because HFMD is not a reportable condition.
Clinical presentation
HFMD typically manifests with painful oral lesions, with or without a macular, maculopapular, or vesicular exanthema. If a fever is present, it is usually below 101°F. The oral lesions are typically benign and manifest as erythematous macules that progress to vesicles with an erythematous halo. These lesions tend to be painful and may interfere with eating or drinking. Signs and symptoms associated with the more virulent form, CV-A6, may include
- Higher fever
- Wider distribution of lesions
- More extensive skin involvement
- Longer duration
- Palmar and plantar desquamation
- Nail dystrophy.4,5
Laboratory diagnosis
Most symptomatic enterovirus infections are diagnosed based on clinical findings alone, reducing the need for laboratory testing. Laboratory confirmation may be warranted for more severe infections and during outbreaks. Molecular methods, such as reverse transcriptase polymerase chain reaction, are typically used for identifying enteroviruses, as they are rapid, sensitive, and widely available in hospital and commercial laboratories.6 Viral culture methods are labor-intensive, expensive, and reserved for typing the isolate. Serology is not useful in the diagnosis of acute infection.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis of HFMD includes conditions with oral lesions and maculopapular, vesicular lesions involving the palms and/or soles, as well as erythroderma.
Oral lesions. Aphthous ulcers are shallow, painful oral lesions not accompanied by skin rashes. Herpes gingivostomatitis, caused by herpes simplex virus (HSV), is often preceded by a prodrome of fever. The associated lesions manifest as vesicular clusters on a red base that evolve into large, painful ulcers. HSV mouth lesions can populate the gingivae, pharynx, hard palate, lips, and perioral skin. Skin lesions may occur unilaterally.
Rashes involving palms and soles. A number of conditions manifest with skin lesions similar to those of HFMD. An autoeczematization reaction consisting of a pruritic, papulovesicular eruption secondary to dermatophyte infection (eg, tinea pedis, tinea manuum, tinea cruris, tinea corporis, tinea capitis) should be ruled out. This type of reaction is thought to be a delayed hypersensitivity response to fungal antigens. Pruritus and the absence of mouth sores distinguishes this reaction from HFMD.7
Secondary syphilis can manifest with a short-lived macular rash involving the palm and soles, as well as oral mucous patches and generalized lymphadenopathy. Syphilis testing, including rapid plasma reagin or Venereal Disease Research Laboratory test with fluorescent treponemal antibody absorption, can rule out this diagnosis.
Erythema multiforme, which is more common in young adults, is characterized by target lesions on the palms and soles and erosions and/or bullae in the mouth and mucous membranes. It is usually preceded by a trigger, such as HSV infection.
Continue to: Erythroderma
Erythroderma. In addition to a rash, erythema multiforme can cause desquamation later in the disease course. Toxic shock syndrome (TSS), a life-threatening condition caused by Streptococcus or Staphylococcus, has an abrupt onset that is associated with high fever and hypotension. TSS causes a sunburn-like rash on the palms and soles that desquamates weeks after onset. Scarlet fever, caused by group A Streptococcus, can cause an erythematous rash with desquamation in children and adolescents. Scalded skin syndrome is a desquamative condition caused by Staphylococcus that occurs primarily in infants and young children.
Management
There is no specific antiviral treatment for HFMD, and thus management is mainly supportive. Fever and pain can be managed with ibuprofen or acetaminophen. Children who are unable to maintain oral hydration may require hospitalization for IV fluids.
Prevention of HFMD requires strict hand hygiene—washing with soap and water—as well as thoroughly cleaning and disinfecting surfaces that come in contact with infected oral secretions or feces.8
OUTCOME FOR THE CASE PATIENT
The patient was discharged and instructed to take ibuprofen as needed.
About three weeks later, the patient’s palms and soles began to peel. Clinical examination at follow-up revealed painful, diffuse, scaly desquamation of the hands and feet (see Figure 2). The patient also experienced loosening and shedding of the proximal nails (see Figure 3). She was diagnosed with postviral shedding.
Continue to: About eight weeks after the desquamatory rash manifested...
About eight weeks after the desquamatory rash manifested, complete resolution was seen. The patient experienced continued onychom
CONCLUSION
Clinicians should be mindful of the increasing incidence of HFMD in the adult population, since it may mimic other disease states. The extent and chronicity of this patient’s clinical manifestations were unusual and may have been caused by CV-A6.
1. Ben-Chetrit E, Wiener-Well Y, Shulman LM, et al. Coxsackievirus A6-related hand foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol. 2014;59(3):201-203.
2. Blomqvist S, Klemola P, Kajalainen S, et al. Co-circulation of coxsackie viruses A6 and A10 in hand, foot, mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49-54.
3. Downing C, Ramirez-Fort MK, Doan HQ, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60(4):381-386.
4. Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69(5):736-741.
5. Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14(1):83-86.
6. Pozo F, Casas I, Tenorio A, et al. Evaluation of a commercially available reverse transcription-PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol. 1998;36(6):1741-1745.
7. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128(2):e453-e457.
8. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898- e904.
1. Ben-Chetrit E, Wiener-Well Y, Shulman LM, et al. Coxsackievirus A6-related hand foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol. 2014;59(3):201-203.
2. Blomqvist S, Klemola P, Kajalainen S, et al. Co-circulation of coxsackie viruses A6 and A10 in hand, foot, mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49-54.
3. Downing C, Ramirez-Fort MK, Doan HQ, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60(4):381-386.
4. Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69(5):736-741.
5. Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14(1):83-86.
6. Pozo F, Casas I, Tenorio A, et al. Evaluation of a commercially available reverse transcription-PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol. 1998;36(6):1741-1745.
7. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128(2):e453-e457.
8. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898- e904.