User login
Daratumumab approved for new indication in MM
The European Commission (EC) has approved a new indication for daratumumab (Darzalex®).
The drug is now authorized for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat adults with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant.
Daratumumab was previously approved by the EC for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, to treat adults with MM who have received at least one prior therapy.
In addition, daratumumab is EC-approved as monotherapy for adults with relapsed and refractory MM whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who have demonstrated disease progression on their last therapy.
The EC’s latest approval for daratumumab is based on results from the phase 3 ALCYONE (MMY3007) study.
Results from this study were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P<0.0001). Rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively. The 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and 0% of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation due to adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The European Commission (EC) has approved a new indication for daratumumab (Darzalex®).
The drug is now authorized for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat adults with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant.
Daratumumab was previously approved by the EC for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, to treat adults with MM who have received at least one prior therapy.
In addition, daratumumab is EC-approved as monotherapy for adults with relapsed and refractory MM whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who have demonstrated disease progression on their last therapy.
The EC’s latest approval for daratumumab is based on results from the phase 3 ALCYONE (MMY3007) study.
Results from this study were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P<0.0001). Rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively. The 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and 0% of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation due to adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The European Commission (EC) has approved a new indication for daratumumab (Darzalex®).
The drug is now authorized for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat adults with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant.
Daratumumab was previously approved by the EC for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, to treat adults with MM who have received at least one prior therapy.
In addition, daratumumab is EC-approved as monotherapy for adults with relapsed and refractory MM whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who have demonstrated disease progression on their last therapy.
The EC’s latest approval for daratumumab is based on results from the phase 3 ALCYONE (MMY3007) study.
Results from this study were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P<0.0001). Rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively. The 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and 0% of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation due to adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
Click for Credit: Alcohol use while breastfeeding; cigarette smoking in HCV; more
Here are 4 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Alcohol use during breastfeeding linked to cognitive harms in children
To take the posttest, go to: https://bit.ly/2vJyUDc
Expires July 30, 2019
2. Cigarette smoking epidemic among HCV-infected individuals
To take the posttest, go to: https://bit.ly/2B00JwX
Expires June 26, 2019
3. Pancreatic surveillance identified resectable cancers
To take the posttest, go to: https://bit.ly/2vuSKmj
Expires July 30, 2019
4. Autoimmune connective tissue disease predicted by interferon status, family history
To take the posttest, go to: https://bit.ly/2OkZHNS
Expires July 30, 2019
Here are 4 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Alcohol use during breastfeeding linked to cognitive harms in children
To take the posttest, go to: https://bit.ly/2vJyUDc
Expires July 30, 2019
2. Cigarette smoking epidemic among HCV-infected individuals
To take the posttest, go to: https://bit.ly/2B00JwX
Expires June 26, 2019
3. Pancreatic surveillance identified resectable cancers
To take the posttest, go to: https://bit.ly/2vuSKmj
Expires July 30, 2019
4. Autoimmune connective tissue disease predicted by interferon status, family history
To take the posttest, go to: https://bit.ly/2OkZHNS
Expires July 30, 2019
Here are 4 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Alcohol use during breastfeeding linked to cognitive harms in children
To take the posttest, go to: https://bit.ly/2vJyUDc
Expires July 30, 2019
2. Cigarette smoking epidemic among HCV-infected individuals
To take the posttest, go to: https://bit.ly/2B00JwX
Expires June 26, 2019
3. Pancreatic surveillance identified resectable cancers
To take the posttest, go to: https://bit.ly/2vuSKmj
Expires July 30, 2019
4. Autoimmune connective tissue disease predicted by interferon status, family history
To take the posttest, go to: https://bit.ly/2OkZHNS
Expires July 30, 2019
Summer is over, more health care changes are afoot
CMS has released its proposed rule (see related articles and a commentary) and included changes as substantial as I have seen in the last two decades. Additionally, the Affordable Care Act has been under continued attack despite its majority support from our citizenry. Loss of the individual mandate, allowance of “skinny” health plans, a rewrite of association plan rules, elimination of cost-sharing reductions and premium support – all have contributed to a shifting away from socialized medical costs and toward a system of individual responsibility for health. Depending on one’s political philosophy (and income), that may be bad or good.
Our article list this month will be interesting to many. The AGA produced a Clinical Practice Update about tumor seeding with endoscopic procedures. This should give us pause and make us reconsider our endoscopic practices. My wife (an endoscopy nurse in Minneapolis) has been asking for years whether pulling a PEG tube past an esophageal cancer might cause tumor seeding, and physicians have reassured her that there is no cause for worry. Turns out she was right (as usual). Deaths from liver disease in the U.S. have seen a dramatic increase since 1999, driven substantially by increasing alcohol use. Fecal transplants in irritable bowel syndrome? Possibly helpful, as reported in an article from Digestive Disease Week.®
As summer comes to an end, we head into a tumultuous fall that will be dominated by November elections.
John I. Allen, MD, MBA, AGAF
Editor in Chief
CMS has released its proposed rule (see related articles and a commentary) and included changes as substantial as I have seen in the last two decades. Additionally, the Affordable Care Act has been under continued attack despite its majority support from our citizenry. Loss of the individual mandate, allowance of “skinny” health plans, a rewrite of association plan rules, elimination of cost-sharing reductions and premium support – all have contributed to a shifting away from socialized medical costs and toward a system of individual responsibility for health. Depending on one’s political philosophy (and income), that may be bad or good.
Our article list this month will be interesting to many. The AGA produced a Clinical Practice Update about tumor seeding with endoscopic procedures. This should give us pause and make us reconsider our endoscopic practices. My wife (an endoscopy nurse in Minneapolis) has been asking for years whether pulling a PEG tube past an esophageal cancer might cause tumor seeding, and physicians have reassured her that there is no cause for worry. Turns out she was right (as usual). Deaths from liver disease in the U.S. have seen a dramatic increase since 1999, driven substantially by increasing alcohol use. Fecal transplants in irritable bowel syndrome? Possibly helpful, as reported in an article from Digestive Disease Week.®
As summer comes to an end, we head into a tumultuous fall that will be dominated by November elections.
John I. Allen, MD, MBA, AGAF
Editor in Chief
CMS has released its proposed rule (see related articles and a commentary) and included changes as substantial as I have seen in the last two decades. Additionally, the Affordable Care Act has been under continued attack despite its majority support from our citizenry. Loss of the individual mandate, allowance of “skinny” health plans, a rewrite of association plan rules, elimination of cost-sharing reductions and premium support – all have contributed to a shifting away from socialized medical costs and toward a system of individual responsibility for health. Depending on one’s political philosophy (and income), that may be bad or good.
Our article list this month will be interesting to many. The AGA produced a Clinical Practice Update about tumor seeding with endoscopic procedures. This should give us pause and make us reconsider our endoscopic practices. My wife (an endoscopy nurse in Minneapolis) has been asking for years whether pulling a PEG tube past an esophageal cancer might cause tumor seeding, and physicians have reassured her that there is no cause for worry. Turns out she was right (as usual). Deaths from liver disease in the U.S. have seen a dramatic increase since 1999, driven substantially by increasing alcohol use. Fecal transplants in irritable bowel syndrome? Possibly helpful, as reported in an article from Digestive Disease Week.®
As summer comes to an end, we head into a tumultuous fall that will be dominated by November elections.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Real-world challenges in managing ‘dual diagnosis’ patients
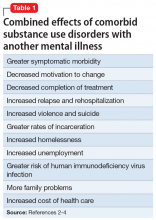
The term “dual diagnosis” describes the clinically challenging comorbidity of a substance use disorder (SUD) along with another major mental illness. Based on data from the Epidemiologic Catchment Area study, the lifetime prevalence of SUDs among patients with mental illness is approximately 30%, and is higher among patients with certain mental disorders, such as schizophrenia (47%), bipolar disorder (61%), and antisocial personality disorder (84%).1 These statistics highlight that addiction is often the rule rather than the exception among those with severe mental illness.1 Not surprisingly, the combined effects of having an SUD along with another mental illness are uniformly negative (Table 12-4).
Based on outcomes research, the core tenets of evidence-based dual-diagnosis treatment include the importance of integrated (rather than parallel) and simultaneous (rather than sequential) care, which means an ideal treatment program includes a unified, multidisciplinary team whose coordinated efforts focus on treating both disorders concurrently.2 Evidence-based psychotherapies for addiction, including motivational interviewing, cognitive-behavioral therapy, relapse prevention, contingency management, skills training, and/or case management, are a necessity,3,5 and must be balanced with rational and appropriate pharmacotherapy targeting both the SUD as well as the other disorder (Table 22,3,5-9).
3 ‘Real-world’ clinical challenges
Ideal vs real-world treatment
Treating patients with co-occurring disorders (CODs) within integrated dual-disorder treatment (IDDT) programs sounds straightforward. However, implementing evidence-based “best practice” treatment is a significant challenge in the real world for several reasons. First, individuals with CODs often struggle with poor insight, low motivation to change, and lack of access to health care. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), 52% of individuals with CODs in the U.S. received no treatment at all in 2016.10 For patients with dual disorders who do seek care, most are not given access to specialty SUD treatment10 and may instead find themselves treated by psychiatrists with limited SUD training who fail to provide evidence-based psychotherapies and underutilize pharmacotherapies for SUDs.11 In the setting of CODs, the “harm reduction model” can be conflated with therapeutic nihilism, resulting in the neglect of SUD issues, with clinicians expecting patients to seek SUD treatment on their own, through self-help groups such as Alcoholics Anonymous or in other community treatment programs staffed by nonprofessionals that often are not tailored to the unique needs of patients with dual disorders. Psychiatrists working with other mental health professionals who provide psychotherapy for SUDs often do so in parallel rather than in an evidence-based, integrated fashion.
IDDT programs are not widely available. One study found that fewer than 20% of addiction treatment programs and fewer than 10% of mental health programs in the U.S. met criteria for dual diagnosis–capable services.12 Getting treatment programs to become dual diagnosis–capable is possible, but it is a time-consuming and costly endeavor that, once achieved, requires continuous staff training and programmatic adaptations to interruptions in funding.13-16 With myriad barriers to the establishment and maintenance of IDDTs, many patients with dual disorders are left without access to the most effective and comprehensive care; as few as 4% of individuals with CODs are treated within integrated programs.17
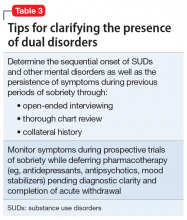
Diagnostic dilemmas
Establishing whether or not a patient with an active SUD has another serious mental illness (SMI) is a crucial first step for optimizing treatment, but diagnostic reliability can prove challenging and requires careful clinical assessment (Table 3). As always in psychiatry, accurate diagnosis is limited to careful clinical assessment18 and, in the case of possible dual disorders, is complicated by the fact that both SUDs as well as non-SUDs can result in the same psychiatric symptoms (eg, insomnia, anxiety, depression, manic behaviors, and psychosis). Clinicians must therefore distinguish between:
- Symptoms of substance intoxication or withdrawal vs independent symptoms of an underlying psychiatric disorder (that persist beyond a month after cessation of intoxication or withdrawal)
- Subclinical symptoms vs threshold mental illness, keeping in mind that some mood and anxiety states can be normal given social situations and stressors (eg, turmoil in relationships, employment difficulties, homelessness, etc.)
- Any mental illness (AMI) vs SMI. The latter is defined by SAMHSA as AMI that substantially interferes with or limits ≥1 major life activities.10
With these distinctions in mind, data from the 2016 National Survey on Drug Use and Health indicate that dual-diagnosis comorbidity was higher when the threshold for mental illness was lower—among the 19 million adults in the U.S. with SUDs, the past-year prevalence was 43% for AMI and 14% for SMI.10 Looking at substance-induced disorders vs “independent” disorders, the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions found that for individuals with SUDs, the past-year prevalence of an independent mood or anxiety disorder was 35% and 26%, respectively.19 Taken together, these findings illustrate the substantial rate of dual-diagnosis comorbidity, the diagnostic heterogeneity and range of severity of CODs,20 and the potential for both false negatives (eg, diagnosing a substance-induced syndrome when in fact a patient has an underlying disorder) and false positives (diagnosing a full-blown mental illness when symptoms are subclinical or substance-induced) when performing diagnostic assessments in the setting of known SUDs.
Continue to: False positives are more likely...
False positives are more likely when patients seeking treatment for non-SUDs don’t disclose active drug use, even when asked. Both patients and their treating clinicians may also be prone to underestimating the significant potential for morbidity associated with SUDs, such that substance-induced symptoms may be misattributed to a dual disorder. Diagnostic questioning and thorough chart review that includes careful assessment of whether psychiatric symptoms preceded the onset of substance use, and whether they persisted in the setting of extended sobriety, is therefore paramount for minimizing false positives when assessing for dual diagnoses.18,21 Likewise, random urine toxicology testing can be invaluable in verifying claims regarding sobriety.
Another factor that can complicate diagnosis is that there are often considerable secondary gains (eg, disability income, hospitalization, housing, access to prescription medications, and mitigation of the blame and stigma associated with addiction) associated with having a dual disorder as opposed to having “just” a SUD. As a result, for some patients, obtaining a non-SUD diagnosis can be highly incentivized.22,23 Clinicians must therefore be savvy about the high potential for malingering, embellishment, and mislabeling of symptoms when conducting diagnostic interviews. For example, in assessing for psychosis, the frequent endorsement of “hearing voices” in patients with SUDs often results in a diagnosis of schizophrenia or unspecified psychotic disorder,22 despite the fact that this symptom can occur during substance intoxication and withdrawal, is well documented among people without mental illness as well as those with non-psychotic disorders,24 and can resolve without medications or with non-antipsychotic pharmacotherapy.25
When assessing for dual disorders, diagnostic false positives and false negatives can both contribute to inappropriate treatment and unrealistic expectations for recovery, and therefore underscore the importance of careful diagnostic assessment. Even with diligent assessment, however, diagnostic clarity can prove elusive due to inadequate sobriety, inconsistent reporting, and poor memory.26 Therefore, for patients with known SUDs but diagnostic uncertainty about a dual disorder, the work-up should include a trial of prospective observation, with completion of appropriate detoxification, throughout a 1-month period of sobriety and in the absence of psychiatric medications, to determine if there are persistent symptoms that would justify a dual diagnosis. In research settings, such observations have revealed that most of depressive symptoms among alcoholics who present for substance abuse treatment resolve after a month of abstinence.27 A similar time course for resolution has been noted for anxiety, distress, fatigue, and depressive symptoms among individuals with cocaine dependence.28 These findings support the guideline established in DSM-IV that symptoms persisting beyond a month of sobriety “should be considered to be manifestations of an independent, non-substance-induced mental disorder,”29 while symptoms occurring within that month may well be substance-induced. Unfortunately, in real-world clinical practice, and particularly in outpatient settings, it can be quite difficult to achieve the requisite period of sobriety for reliable diagnosis, and patients are often prematurely prescribed medications (eg, an antidepressant, antipsychotic, or mood stabilizer) that can confound the cause of symptomatic resolution. Such prescriptions are driven by compelling pressures from patients to relieve their acute suffering, as well as the predilection of some clinicians to give patients “the benefit of doubt” in assessing for dual diagnoses. However, whether an inappropriate diagnosis or a prescription for an unnecessary medication represents a benefit is debatable at best.
Pharmacotherapy
A third real-world challenge in managing patients with dual disorders involves optimizing pharmacotherapy. Unfortunately, because patients with SUDs often are excluded from clinical trials, evidence-based guidance for patients with dual disorders is lacking. In addition, medications for both CODs often remain inaccessible to patients with dual disorders for 3 reasons:
- SUDs negatively impact medication adherence among patients with dual disorders, who sometimes point out that “it says right here on the bottle not to take this medication with drugs or alcohol!”
- Some self-help groups still espouse blanket opposition of any “psychotropic” medications, even when clearly indicated for patients with COD. Groups that recognize the importance of pharmacotherapy, such as Dual Diagnosis Anonymous (DDA), have emerged, but are not yet widely available.30
- Although there are increasing options for FDA-approved medications for SUDs, they are limited to the treatment of alcohol, opioid, and nicotine use disorders31; are often restricted due to hospital and health insurance formularies32; and remain underprescribed for patients with dual disorders.11
Continue to: Although underutilization of pharmacotherapy is...
Although underutilization of pharmacotherapy is a pitfall to be avoided in the treatment of patients with dual disorders, medication overutilization can be just as problematic. Patients with dual disorders are sometimes singularly focused on resolving acute anxiety, depression, or psychosis at the expense of working towards sobriety.33 Although the “self-medication hypothesis” is frequently invoked by patients and clinicians alike to suggest that substance use occurs in the service of “treating” underlying disorders,34 this theory has not been well supported in studies.35-37 Some patients may pledge dedication to abstinence, but still pressure physicians for a pharmacologic solution to their suffering. With expanding legalization of cannabis for both recreational and medical purposes, patients are increasingly seeking doctors’ recommendations for “medical marijuana” for a wide range of complaints, despite the fact that data supporting a therapeutic role for cannabis in the treatment of mental illness is sparse,38 whereas the potential harm in terms of either causing or worsening psychosis is well established.39,40 Clinicians must be knowledgeable about the abuse potential of prescribed medications, ranging from sleep aids, analgesics, and muscle relaxants to antidepressants and antipsychotics, while also being mindful of the psychological meaningfulness of seeking, prescribing, and not prescribing medications.41
Although the simultaneous treatment of patients with dual disorders that includes pharmacotherapy for both SUDs and CODs is vital for optimizing clinical outcomes, clinicians should strive for diagnostic accuracy and use medications judiciously. In addition, although pharmacotherapy often is necessary to deliver evidence-based treatment for patients with dual disorders, it is inadequate as standalone treatment and should be administered along with psychosocial interventions within an integrated, multidisciplinary treatment setting.
The keys to optimal outcomes
The treatment of patients with dual disorders can be challenging, to say the least. Ideal, evidence-based therapy in the form of an IDDT program can be difficult for clinicians to implement and for patients to access. Best efforts to perform meticulous clinical assessment to clarify diagnoses, use pharmacotherapy judiciously, work collaboratively in a multidisciplinary setting, and optimize treatment given available resources are keys to clinical success.
Bottom Line
Ideal treatment of patients with dual disorders consists of simultaneous, integrated interventions delivered by a multidisciplinary team. However, in the real world, limited resources, diagnostic challenges, and both over- and underutilization of pharmacotherapy often hamper optimal treatment.
Related Resources
- Substance Abuse and Mental Health Services Administration. Co-occurring disorders. https://www.samhsa.gov/disorders/co-occurring.
- National Alliance on Mental Illness. Dual diagnosis. https://www.nami.org/Learn-More/Mental-Health-Conditions/Related-Conditions/Dual-Diagnosis.
- Foundations Recovery Network. Dual diagnosis support groups. http://www.dualdiagnosis.org/resource/ddrn/self-helpsupport-groups/support-groups.
- Substance Abuse and Mental Health Services Administration. Integrated treatment for co-occurring disorders evidence-based practices (EBP) KIT. https://store.samhsa.gov/product/Integrated-Treatment-for-Co-Occurring-Disorders-Evidence-Based-Practices-EBP-KIT/SMA08-4367.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment for persons with co-occurring disorders. https://store.samhsa.gov/shin/content/SMA13-3992/SMA13-3992.pdf.
1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511-2518.
2. Drake RE, Mercer-McFadden C, Muesner KT, et al. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589-608.
3. Horsfall J, Cleary M, Hunt GE, et al. Psychosocial treatments for people with co-occurring severe mental illness and substance use disorders (dual diagnosis): a review of empiric evidence. Harv Rev Psychiatry. 2009;17(1):24-34.
4. Krawczyk N, Feder KA, Saloner B, et al. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 2017;175:157-163.
5. Brunette MF, Muesner KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(suppl 7):10-17.
6. Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31(4):513-536.
7. Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
8. Tsuang JT, Ho AP, Eckman TA, et al. Dual diagnosis treatment for patients with schizophrenia who are substance dependent. Psychatr Serv. 1997;48(7):887-889.
9. Rosen MI, Rosenheck RA, Shaner A, et al. Veterans who may need a payee to prevent misuse of funds for drugs. Psychiatr Serv. 2002;53(8):995-1000.
10. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health. HHS Publication No. SMA 17-5044, NSDUH Series H-52. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. Published September 2017. Accessed August 7, 2018.
11. Rubinsky AD, Chen C, Batki SL, et al. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res. 2015;69:150-157.
12. McGovern MP, Lambert-Harris C, McHugo GJ, et al. Improving the dual diagnosis capability of addiction and mental health treatment services: implementation factors associated with program level changes. J Dual Diag. 2010;6:237-250.
13. Reno R. Maintaining quality of care in a comprehensive dual diagnosis treatment program. Psychiatr Serv. 2001;52(5):673-675.
14. McGovern MP, Lambert-Harris, Gotham HJ, et al. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205-214.
15. Gotham HJ, Claus RE, Selig K, et al. Increasing program capabilities to provide treatment for co-occurring substance use and mental disorders: organizational characteristics. J Subs Abuse Treat. 2010;38(2):160-169.
16. Priester MA, Browne T, Iachini A, et al. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47-59.
17. Drake RE, Bond GR. Implementing integrated mental health and substance abuse services. J Dual Diagnosis. 2010;6(3-4):251-262.
18. Miele GM, Trautman KD, Hasin DS. Assessing comorbid mental and substance-use disorders: a guide for clinical practice. J Pract Psychiatry Behav Health. 1996;5:272-282.
19. Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2015;80(1):105-116.
20. Flynn PM, Brown BS. Co-occurring disorders in substance abuse treatment: Issues and prospects. J Subt Abuse Treat. 2008;34(1):36-47.
21. Grant BF, Stintson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807-816.
22. Pierre JM, Wirshing DA, Wirshing WC. “Iatrogenic malingering” in VA substance abuse treatment. Psych Services. 2003;54(2):253-254.
23. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry. 2004;161(9):1718.
24. Pierre JM. Hallucinations in non-psychotic disorders: Toward a differential diagnosis of “hearing voices.” Harv Rev Psychiatry. 2010;18(1):22-35.
25. Pierre JM. Nonantipsychotic therapy for monosymptomatic auditory hallucinations. Biol Psychiatry. 2010;68(7):e33-e34.
26. Shaner A, Roberts LJ, Eckman TA, et al. Sources of diagnostic uncertainty for chronically psychotic cocaine abusers. Psychiatr Serv. 1998;49(5):684-690.
27. Brown SA, Shuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49(5):412-417.
28. Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861-868.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994:210.
30. Roush S, Monica C, Carpenter-Song E, et al. First-person perspectives on Dual Diagnosis Anonymous (DDA): a qualitative study. J Dual Diagnosis. 2015;11(2):136-141.
31. Klein JW. Pharmacotherapy for substance abuse disorders. Med Clin N Am. 2016;100(4):891-910.
32. Horgan CM, Reif S, Hodgkin D, et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147-156.
33. Frances RJ. The wrath of grapes versus the self-medication hypothesis. Harvard Rev Psychiatry. 1997;4(5):287-289.
34. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231-244.
35. Hall DH, Queener JE. Self-medication hypothesis of substance use: testing Khantzian’s updated theory. J Psychoactive Drugs. 2007;39(2):151-158.
36. Henwood B, Padgett DK. Reevaluating the self-medication hypothesis among the dually diagnosed. Am J Addict. 2007;16(3):160-165.
37. Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alc Abuse. 2012;38(6):524-529.
38. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
39. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29.
40. Pierre JM. Risks of increasingly potent cannabis: the joint effects of potency and frequency. Current Psychiatry. 2017;16:14-20.
41. Zweben JE, Smith DE. Considerations in using psychotropic medication with dual diagnosis patients in recovery. J Psychoactive Drugs. 1989;21(2):221-228.
The term “dual diagnosis” describes the clinically challenging comorbidity of a substance use disorder (SUD) along with another major mental illness. Based on data from the Epidemiologic Catchment Area study, the lifetime prevalence of SUDs among patients with mental illness is approximately 30%, and is higher among patients with certain mental disorders, such as schizophrenia (47%), bipolar disorder (61%), and antisocial personality disorder (84%).1 These statistics highlight that addiction is often the rule rather than the exception among those with severe mental illness.1 Not surprisingly, the combined effects of having an SUD along with another mental illness are uniformly negative (Table 12-4).
Based on outcomes research, the core tenets of evidence-based dual-diagnosis treatment include the importance of integrated (rather than parallel) and simultaneous (rather than sequential) care, which means an ideal treatment program includes a unified, multidisciplinary team whose coordinated efforts focus on treating both disorders concurrently.2 Evidence-based psychotherapies for addiction, including motivational interviewing, cognitive-behavioral therapy, relapse prevention, contingency management, skills training, and/or case management, are a necessity,3,5 and must be balanced with rational and appropriate pharmacotherapy targeting both the SUD as well as the other disorder (Table 22,3,5-9).
3 ‘Real-world’ clinical challenges
Ideal vs real-world treatment
Treating patients with co-occurring disorders (CODs) within integrated dual-disorder treatment (IDDT) programs sounds straightforward. However, implementing evidence-based “best practice” treatment is a significant challenge in the real world for several reasons. First, individuals with CODs often struggle with poor insight, low motivation to change, and lack of access to health care. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), 52% of individuals with CODs in the U.S. received no treatment at all in 2016.10 For patients with dual disorders who do seek care, most are not given access to specialty SUD treatment10 and may instead find themselves treated by psychiatrists with limited SUD training who fail to provide evidence-based psychotherapies and underutilize pharmacotherapies for SUDs.11 In the setting of CODs, the “harm reduction model” can be conflated with therapeutic nihilism, resulting in the neglect of SUD issues, with clinicians expecting patients to seek SUD treatment on their own, through self-help groups such as Alcoholics Anonymous or in other community treatment programs staffed by nonprofessionals that often are not tailored to the unique needs of patients with dual disorders. Psychiatrists working with other mental health professionals who provide psychotherapy for SUDs often do so in parallel rather than in an evidence-based, integrated fashion.
IDDT programs are not widely available. One study found that fewer than 20% of addiction treatment programs and fewer than 10% of mental health programs in the U.S. met criteria for dual diagnosis–capable services.12 Getting treatment programs to become dual diagnosis–capable is possible, but it is a time-consuming and costly endeavor that, once achieved, requires continuous staff training and programmatic adaptations to interruptions in funding.13-16 With myriad barriers to the establishment and maintenance of IDDTs, many patients with dual disorders are left without access to the most effective and comprehensive care; as few as 4% of individuals with CODs are treated within integrated programs.17
Diagnostic dilemmas
Establishing whether or not a patient with an active SUD has another serious mental illness (SMI) is a crucial first step for optimizing treatment, but diagnostic reliability can prove challenging and requires careful clinical assessment (Table 3). As always in psychiatry, accurate diagnosis is limited to careful clinical assessment18 and, in the case of possible dual disorders, is complicated by the fact that both SUDs as well as non-SUDs can result in the same psychiatric symptoms (eg, insomnia, anxiety, depression, manic behaviors, and psychosis). Clinicians must therefore distinguish between:
- Symptoms of substance intoxication or withdrawal vs independent symptoms of an underlying psychiatric disorder (that persist beyond a month after cessation of intoxication or withdrawal)
- Subclinical symptoms vs threshold mental illness, keeping in mind that some mood and anxiety states can be normal given social situations and stressors (eg, turmoil in relationships, employment difficulties, homelessness, etc.)
- Any mental illness (AMI) vs SMI. The latter is defined by SAMHSA as AMI that substantially interferes with or limits ≥1 major life activities.10
With these distinctions in mind, data from the 2016 National Survey on Drug Use and Health indicate that dual-diagnosis comorbidity was higher when the threshold for mental illness was lower—among the 19 million adults in the U.S. with SUDs, the past-year prevalence was 43% for AMI and 14% for SMI.10 Looking at substance-induced disorders vs “independent” disorders, the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions found that for individuals with SUDs, the past-year prevalence of an independent mood or anxiety disorder was 35% and 26%, respectively.19 Taken together, these findings illustrate the substantial rate of dual-diagnosis comorbidity, the diagnostic heterogeneity and range of severity of CODs,20 and the potential for both false negatives (eg, diagnosing a substance-induced syndrome when in fact a patient has an underlying disorder) and false positives (diagnosing a full-blown mental illness when symptoms are subclinical or substance-induced) when performing diagnostic assessments in the setting of known SUDs.
Continue to: False positives are more likely...
False positives are more likely when patients seeking treatment for non-SUDs don’t disclose active drug use, even when asked. Both patients and their treating clinicians may also be prone to underestimating the significant potential for morbidity associated with SUDs, such that substance-induced symptoms may be misattributed to a dual disorder. Diagnostic questioning and thorough chart review that includes careful assessment of whether psychiatric symptoms preceded the onset of substance use, and whether they persisted in the setting of extended sobriety, is therefore paramount for minimizing false positives when assessing for dual diagnoses.18,21 Likewise, random urine toxicology testing can be invaluable in verifying claims regarding sobriety.
Another factor that can complicate diagnosis is that there are often considerable secondary gains (eg, disability income, hospitalization, housing, access to prescription medications, and mitigation of the blame and stigma associated with addiction) associated with having a dual disorder as opposed to having “just” a SUD. As a result, for some patients, obtaining a non-SUD diagnosis can be highly incentivized.22,23 Clinicians must therefore be savvy about the high potential for malingering, embellishment, and mislabeling of symptoms when conducting diagnostic interviews. For example, in assessing for psychosis, the frequent endorsement of “hearing voices” in patients with SUDs often results in a diagnosis of schizophrenia or unspecified psychotic disorder,22 despite the fact that this symptom can occur during substance intoxication and withdrawal, is well documented among people without mental illness as well as those with non-psychotic disorders,24 and can resolve without medications or with non-antipsychotic pharmacotherapy.25
When assessing for dual disorders, diagnostic false positives and false negatives can both contribute to inappropriate treatment and unrealistic expectations for recovery, and therefore underscore the importance of careful diagnostic assessment. Even with diligent assessment, however, diagnostic clarity can prove elusive due to inadequate sobriety, inconsistent reporting, and poor memory.26 Therefore, for patients with known SUDs but diagnostic uncertainty about a dual disorder, the work-up should include a trial of prospective observation, with completion of appropriate detoxification, throughout a 1-month period of sobriety and in the absence of psychiatric medications, to determine if there are persistent symptoms that would justify a dual diagnosis. In research settings, such observations have revealed that most of depressive symptoms among alcoholics who present for substance abuse treatment resolve after a month of abstinence.27 A similar time course for resolution has been noted for anxiety, distress, fatigue, and depressive symptoms among individuals with cocaine dependence.28 These findings support the guideline established in DSM-IV that symptoms persisting beyond a month of sobriety “should be considered to be manifestations of an independent, non-substance-induced mental disorder,”29 while symptoms occurring within that month may well be substance-induced. Unfortunately, in real-world clinical practice, and particularly in outpatient settings, it can be quite difficult to achieve the requisite period of sobriety for reliable diagnosis, and patients are often prematurely prescribed medications (eg, an antidepressant, antipsychotic, or mood stabilizer) that can confound the cause of symptomatic resolution. Such prescriptions are driven by compelling pressures from patients to relieve their acute suffering, as well as the predilection of some clinicians to give patients “the benefit of doubt” in assessing for dual diagnoses. However, whether an inappropriate diagnosis or a prescription for an unnecessary medication represents a benefit is debatable at best.
Pharmacotherapy
A third real-world challenge in managing patients with dual disorders involves optimizing pharmacotherapy. Unfortunately, because patients with SUDs often are excluded from clinical trials, evidence-based guidance for patients with dual disorders is lacking. In addition, medications for both CODs often remain inaccessible to patients with dual disorders for 3 reasons:
- SUDs negatively impact medication adherence among patients with dual disorders, who sometimes point out that “it says right here on the bottle not to take this medication with drugs or alcohol!”
- Some self-help groups still espouse blanket opposition of any “psychotropic” medications, even when clearly indicated for patients with COD. Groups that recognize the importance of pharmacotherapy, such as Dual Diagnosis Anonymous (DDA), have emerged, but are not yet widely available.30
- Although there are increasing options for FDA-approved medications for SUDs, they are limited to the treatment of alcohol, opioid, and nicotine use disorders31; are often restricted due to hospital and health insurance formularies32; and remain underprescribed for patients with dual disorders.11
Continue to: Although underutilization of pharmacotherapy is...
Although underutilization of pharmacotherapy is a pitfall to be avoided in the treatment of patients with dual disorders, medication overutilization can be just as problematic. Patients with dual disorders are sometimes singularly focused on resolving acute anxiety, depression, or psychosis at the expense of working towards sobriety.33 Although the “self-medication hypothesis” is frequently invoked by patients and clinicians alike to suggest that substance use occurs in the service of “treating” underlying disorders,34 this theory has not been well supported in studies.35-37 Some patients may pledge dedication to abstinence, but still pressure physicians for a pharmacologic solution to their suffering. With expanding legalization of cannabis for both recreational and medical purposes, patients are increasingly seeking doctors’ recommendations for “medical marijuana” for a wide range of complaints, despite the fact that data supporting a therapeutic role for cannabis in the treatment of mental illness is sparse,38 whereas the potential harm in terms of either causing or worsening psychosis is well established.39,40 Clinicians must be knowledgeable about the abuse potential of prescribed medications, ranging from sleep aids, analgesics, and muscle relaxants to antidepressants and antipsychotics, while also being mindful of the psychological meaningfulness of seeking, prescribing, and not prescribing medications.41
Although the simultaneous treatment of patients with dual disorders that includes pharmacotherapy for both SUDs and CODs is vital for optimizing clinical outcomes, clinicians should strive for diagnostic accuracy and use medications judiciously. In addition, although pharmacotherapy often is necessary to deliver evidence-based treatment for patients with dual disorders, it is inadequate as standalone treatment and should be administered along with psychosocial interventions within an integrated, multidisciplinary treatment setting.
The keys to optimal outcomes
The treatment of patients with dual disorders can be challenging, to say the least. Ideal, evidence-based therapy in the form of an IDDT program can be difficult for clinicians to implement and for patients to access. Best efforts to perform meticulous clinical assessment to clarify diagnoses, use pharmacotherapy judiciously, work collaboratively in a multidisciplinary setting, and optimize treatment given available resources are keys to clinical success.
Bottom Line
Ideal treatment of patients with dual disorders consists of simultaneous, integrated interventions delivered by a multidisciplinary team. However, in the real world, limited resources, diagnostic challenges, and both over- and underutilization of pharmacotherapy often hamper optimal treatment.
Related Resources
- Substance Abuse and Mental Health Services Administration. Co-occurring disorders. https://www.samhsa.gov/disorders/co-occurring.
- National Alliance on Mental Illness. Dual diagnosis. https://www.nami.org/Learn-More/Mental-Health-Conditions/Related-Conditions/Dual-Diagnosis.
- Foundations Recovery Network. Dual diagnosis support groups. http://www.dualdiagnosis.org/resource/ddrn/self-helpsupport-groups/support-groups.
- Substance Abuse and Mental Health Services Administration. Integrated treatment for co-occurring disorders evidence-based practices (EBP) KIT. https://store.samhsa.gov/product/Integrated-Treatment-for-Co-Occurring-Disorders-Evidence-Based-Practices-EBP-KIT/SMA08-4367.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment for persons with co-occurring disorders. https://store.samhsa.gov/shin/content/SMA13-3992/SMA13-3992.pdf.
The term “dual diagnosis” describes the clinically challenging comorbidity of a substance use disorder (SUD) along with another major mental illness. Based on data from the Epidemiologic Catchment Area study, the lifetime prevalence of SUDs among patients with mental illness is approximately 30%, and is higher among patients with certain mental disorders, such as schizophrenia (47%), bipolar disorder (61%), and antisocial personality disorder (84%).1 These statistics highlight that addiction is often the rule rather than the exception among those with severe mental illness.1 Not surprisingly, the combined effects of having an SUD along with another mental illness are uniformly negative (Table 12-4).
Based on outcomes research, the core tenets of evidence-based dual-diagnosis treatment include the importance of integrated (rather than parallel) and simultaneous (rather than sequential) care, which means an ideal treatment program includes a unified, multidisciplinary team whose coordinated efforts focus on treating both disorders concurrently.2 Evidence-based psychotherapies for addiction, including motivational interviewing, cognitive-behavioral therapy, relapse prevention, contingency management, skills training, and/or case management, are a necessity,3,5 and must be balanced with rational and appropriate pharmacotherapy targeting both the SUD as well as the other disorder (Table 22,3,5-9).
3 ‘Real-world’ clinical challenges
Ideal vs real-world treatment
Treating patients with co-occurring disorders (CODs) within integrated dual-disorder treatment (IDDT) programs sounds straightforward. However, implementing evidence-based “best practice” treatment is a significant challenge in the real world for several reasons. First, individuals with CODs often struggle with poor insight, low motivation to change, and lack of access to health care. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), 52% of individuals with CODs in the U.S. received no treatment at all in 2016.10 For patients with dual disorders who do seek care, most are not given access to specialty SUD treatment10 and may instead find themselves treated by psychiatrists with limited SUD training who fail to provide evidence-based psychotherapies and underutilize pharmacotherapies for SUDs.11 In the setting of CODs, the “harm reduction model” can be conflated with therapeutic nihilism, resulting in the neglect of SUD issues, with clinicians expecting patients to seek SUD treatment on their own, through self-help groups such as Alcoholics Anonymous or in other community treatment programs staffed by nonprofessionals that often are not tailored to the unique needs of patients with dual disorders. Psychiatrists working with other mental health professionals who provide psychotherapy for SUDs often do so in parallel rather than in an evidence-based, integrated fashion.
IDDT programs are not widely available. One study found that fewer than 20% of addiction treatment programs and fewer than 10% of mental health programs in the U.S. met criteria for dual diagnosis–capable services.12 Getting treatment programs to become dual diagnosis–capable is possible, but it is a time-consuming and costly endeavor that, once achieved, requires continuous staff training and programmatic adaptations to interruptions in funding.13-16 With myriad barriers to the establishment and maintenance of IDDTs, many patients with dual disorders are left without access to the most effective and comprehensive care; as few as 4% of individuals with CODs are treated within integrated programs.17
Diagnostic dilemmas
Establishing whether or not a patient with an active SUD has another serious mental illness (SMI) is a crucial first step for optimizing treatment, but diagnostic reliability can prove challenging and requires careful clinical assessment (Table 3). As always in psychiatry, accurate diagnosis is limited to careful clinical assessment18 and, in the case of possible dual disorders, is complicated by the fact that both SUDs as well as non-SUDs can result in the same psychiatric symptoms (eg, insomnia, anxiety, depression, manic behaviors, and psychosis). Clinicians must therefore distinguish between:
- Symptoms of substance intoxication or withdrawal vs independent symptoms of an underlying psychiatric disorder (that persist beyond a month after cessation of intoxication or withdrawal)
- Subclinical symptoms vs threshold mental illness, keeping in mind that some mood and anxiety states can be normal given social situations and stressors (eg, turmoil in relationships, employment difficulties, homelessness, etc.)
- Any mental illness (AMI) vs SMI. The latter is defined by SAMHSA as AMI that substantially interferes with or limits ≥1 major life activities.10
With these distinctions in mind, data from the 2016 National Survey on Drug Use and Health indicate that dual-diagnosis comorbidity was higher when the threshold for mental illness was lower—among the 19 million adults in the U.S. with SUDs, the past-year prevalence was 43% for AMI and 14% for SMI.10 Looking at substance-induced disorders vs “independent” disorders, the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions found that for individuals with SUDs, the past-year prevalence of an independent mood or anxiety disorder was 35% and 26%, respectively.19 Taken together, these findings illustrate the substantial rate of dual-diagnosis comorbidity, the diagnostic heterogeneity and range of severity of CODs,20 and the potential for both false negatives (eg, diagnosing a substance-induced syndrome when in fact a patient has an underlying disorder) and false positives (diagnosing a full-blown mental illness when symptoms are subclinical or substance-induced) when performing diagnostic assessments in the setting of known SUDs.
Continue to: False positives are more likely...
False positives are more likely when patients seeking treatment for non-SUDs don’t disclose active drug use, even when asked. Both patients and their treating clinicians may also be prone to underestimating the significant potential for morbidity associated with SUDs, such that substance-induced symptoms may be misattributed to a dual disorder. Diagnostic questioning and thorough chart review that includes careful assessment of whether psychiatric symptoms preceded the onset of substance use, and whether they persisted in the setting of extended sobriety, is therefore paramount for minimizing false positives when assessing for dual diagnoses.18,21 Likewise, random urine toxicology testing can be invaluable in verifying claims regarding sobriety.
Another factor that can complicate diagnosis is that there are often considerable secondary gains (eg, disability income, hospitalization, housing, access to prescription medications, and mitigation of the blame and stigma associated with addiction) associated with having a dual disorder as opposed to having “just” a SUD. As a result, for some patients, obtaining a non-SUD diagnosis can be highly incentivized.22,23 Clinicians must therefore be savvy about the high potential for malingering, embellishment, and mislabeling of symptoms when conducting diagnostic interviews. For example, in assessing for psychosis, the frequent endorsement of “hearing voices” in patients with SUDs often results in a diagnosis of schizophrenia or unspecified psychotic disorder,22 despite the fact that this symptom can occur during substance intoxication and withdrawal, is well documented among people without mental illness as well as those with non-psychotic disorders,24 and can resolve without medications or with non-antipsychotic pharmacotherapy.25
When assessing for dual disorders, diagnostic false positives and false negatives can both contribute to inappropriate treatment and unrealistic expectations for recovery, and therefore underscore the importance of careful diagnostic assessment. Even with diligent assessment, however, diagnostic clarity can prove elusive due to inadequate sobriety, inconsistent reporting, and poor memory.26 Therefore, for patients with known SUDs but diagnostic uncertainty about a dual disorder, the work-up should include a trial of prospective observation, with completion of appropriate detoxification, throughout a 1-month period of sobriety and in the absence of psychiatric medications, to determine if there are persistent symptoms that would justify a dual diagnosis. In research settings, such observations have revealed that most of depressive symptoms among alcoholics who present for substance abuse treatment resolve after a month of abstinence.27 A similar time course for resolution has been noted for anxiety, distress, fatigue, and depressive symptoms among individuals with cocaine dependence.28 These findings support the guideline established in DSM-IV that symptoms persisting beyond a month of sobriety “should be considered to be manifestations of an independent, non-substance-induced mental disorder,”29 while symptoms occurring within that month may well be substance-induced. Unfortunately, in real-world clinical practice, and particularly in outpatient settings, it can be quite difficult to achieve the requisite period of sobriety for reliable diagnosis, and patients are often prematurely prescribed medications (eg, an antidepressant, antipsychotic, or mood stabilizer) that can confound the cause of symptomatic resolution. Such prescriptions are driven by compelling pressures from patients to relieve their acute suffering, as well as the predilection of some clinicians to give patients “the benefit of doubt” in assessing for dual diagnoses. However, whether an inappropriate diagnosis or a prescription for an unnecessary medication represents a benefit is debatable at best.
Pharmacotherapy
A third real-world challenge in managing patients with dual disorders involves optimizing pharmacotherapy. Unfortunately, because patients with SUDs often are excluded from clinical trials, evidence-based guidance for patients with dual disorders is lacking. In addition, medications for both CODs often remain inaccessible to patients with dual disorders for 3 reasons:
- SUDs negatively impact medication adherence among patients with dual disorders, who sometimes point out that “it says right here on the bottle not to take this medication with drugs or alcohol!”
- Some self-help groups still espouse blanket opposition of any “psychotropic” medications, even when clearly indicated for patients with COD. Groups that recognize the importance of pharmacotherapy, such as Dual Diagnosis Anonymous (DDA), have emerged, but are not yet widely available.30
- Although there are increasing options for FDA-approved medications for SUDs, they are limited to the treatment of alcohol, opioid, and nicotine use disorders31; are often restricted due to hospital and health insurance formularies32; and remain underprescribed for patients with dual disorders.11
Continue to: Although underutilization of pharmacotherapy is...
Although underutilization of pharmacotherapy is a pitfall to be avoided in the treatment of patients with dual disorders, medication overutilization can be just as problematic. Patients with dual disorders are sometimes singularly focused on resolving acute anxiety, depression, or psychosis at the expense of working towards sobriety.33 Although the “self-medication hypothesis” is frequently invoked by patients and clinicians alike to suggest that substance use occurs in the service of “treating” underlying disorders,34 this theory has not been well supported in studies.35-37 Some patients may pledge dedication to abstinence, but still pressure physicians for a pharmacologic solution to their suffering. With expanding legalization of cannabis for both recreational and medical purposes, patients are increasingly seeking doctors’ recommendations for “medical marijuana” for a wide range of complaints, despite the fact that data supporting a therapeutic role for cannabis in the treatment of mental illness is sparse,38 whereas the potential harm in terms of either causing or worsening psychosis is well established.39,40 Clinicians must be knowledgeable about the abuse potential of prescribed medications, ranging from sleep aids, analgesics, and muscle relaxants to antidepressants and antipsychotics, while also being mindful of the psychological meaningfulness of seeking, prescribing, and not prescribing medications.41
Although the simultaneous treatment of patients with dual disorders that includes pharmacotherapy for both SUDs and CODs is vital for optimizing clinical outcomes, clinicians should strive for diagnostic accuracy and use medications judiciously. In addition, although pharmacotherapy often is necessary to deliver evidence-based treatment for patients with dual disorders, it is inadequate as standalone treatment and should be administered along with psychosocial interventions within an integrated, multidisciplinary treatment setting.
The keys to optimal outcomes
The treatment of patients with dual disorders can be challenging, to say the least. Ideal, evidence-based therapy in the form of an IDDT program can be difficult for clinicians to implement and for patients to access. Best efforts to perform meticulous clinical assessment to clarify diagnoses, use pharmacotherapy judiciously, work collaboratively in a multidisciplinary setting, and optimize treatment given available resources are keys to clinical success.
Bottom Line
Ideal treatment of patients with dual disorders consists of simultaneous, integrated interventions delivered by a multidisciplinary team. However, in the real world, limited resources, diagnostic challenges, and both over- and underutilization of pharmacotherapy often hamper optimal treatment.
Related Resources
- Substance Abuse and Mental Health Services Administration. Co-occurring disorders. https://www.samhsa.gov/disorders/co-occurring.
- National Alliance on Mental Illness. Dual diagnosis. https://www.nami.org/Learn-More/Mental-Health-Conditions/Related-Conditions/Dual-Diagnosis.
- Foundations Recovery Network. Dual diagnosis support groups. http://www.dualdiagnosis.org/resource/ddrn/self-helpsupport-groups/support-groups.
- Substance Abuse and Mental Health Services Administration. Integrated treatment for co-occurring disorders evidence-based practices (EBP) KIT. https://store.samhsa.gov/product/Integrated-Treatment-for-Co-Occurring-Disorders-Evidence-Based-Practices-EBP-KIT/SMA08-4367.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment for persons with co-occurring disorders. https://store.samhsa.gov/shin/content/SMA13-3992/SMA13-3992.pdf.
1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511-2518.
2. Drake RE, Mercer-McFadden C, Muesner KT, et al. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589-608.
3. Horsfall J, Cleary M, Hunt GE, et al. Psychosocial treatments for people with co-occurring severe mental illness and substance use disorders (dual diagnosis): a review of empiric evidence. Harv Rev Psychiatry. 2009;17(1):24-34.
4. Krawczyk N, Feder KA, Saloner B, et al. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 2017;175:157-163.
5. Brunette MF, Muesner KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(suppl 7):10-17.
6. Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31(4):513-536.
7. Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
8. Tsuang JT, Ho AP, Eckman TA, et al. Dual diagnosis treatment for patients with schizophrenia who are substance dependent. Psychatr Serv. 1997;48(7):887-889.
9. Rosen MI, Rosenheck RA, Shaner A, et al. Veterans who may need a payee to prevent misuse of funds for drugs. Psychiatr Serv. 2002;53(8):995-1000.
10. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health. HHS Publication No. SMA 17-5044, NSDUH Series H-52. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. Published September 2017. Accessed August 7, 2018.
11. Rubinsky AD, Chen C, Batki SL, et al. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res. 2015;69:150-157.
12. McGovern MP, Lambert-Harris C, McHugo GJ, et al. Improving the dual diagnosis capability of addiction and mental health treatment services: implementation factors associated with program level changes. J Dual Diag. 2010;6:237-250.
13. Reno R. Maintaining quality of care in a comprehensive dual diagnosis treatment program. Psychiatr Serv. 2001;52(5):673-675.
14. McGovern MP, Lambert-Harris, Gotham HJ, et al. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205-214.
15. Gotham HJ, Claus RE, Selig K, et al. Increasing program capabilities to provide treatment for co-occurring substance use and mental disorders: organizational characteristics. J Subs Abuse Treat. 2010;38(2):160-169.
16. Priester MA, Browne T, Iachini A, et al. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47-59.
17. Drake RE, Bond GR. Implementing integrated mental health and substance abuse services. J Dual Diagnosis. 2010;6(3-4):251-262.
18. Miele GM, Trautman KD, Hasin DS. Assessing comorbid mental and substance-use disorders: a guide for clinical practice. J Pract Psychiatry Behav Health. 1996;5:272-282.
19. Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2015;80(1):105-116.
20. Flynn PM, Brown BS. Co-occurring disorders in substance abuse treatment: Issues and prospects. J Subt Abuse Treat. 2008;34(1):36-47.
21. Grant BF, Stintson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807-816.
22. Pierre JM, Wirshing DA, Wirshing WC. “Iatrogenic malingering” in VA substance abuse treatment. Psych Services. 2003;54(2):253-254.
23. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry. 2004;161(9):1718.
24. Pierre JM. Hallucinations in non-psychotic disorders: Toward a differential diagnosis of “hearing voices.” Harv Rev Psychiatry. 2010;18(1):22-35.
25. Pierre JM. Nonantipsychotic therapy for monosymptomatic auditory hallucinations. Biol Psychiatry. 2010;68(7):e33-e34.
26. Shaner A, Roberts LJ, Eckman TA, et al. Sources of diagnostic uncertainty for chronically psychotic cocaine abusers. Psychiatr Serv. 1998;49(5):684-690.
27. Brown SA, Shuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49(5):412-417.
28. Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861-868.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994:210.
30. Roush S, Monica C, Carpenter-Song E, et al. First-person perspectives on Dual Diagnosis Anonymous (DDA): a qualitative study. J Dual Diagnosis. 2015;11(2):136-141.
31. Klein JW. Pharmacotherapy for substance abuse disorders. Med Clin N Am. 2016;100(4):891-910.
32. Horgan CM, Reif S, Hodgkin D, et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147-156.
33. Frances RJ. The wrath of grapes versus the self-medication hypothesis. Harvard Rev Psychiatry. 1997;4(5):287-289.
34. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231-244.
35. Hall DH, Queener JE. Self-medication hypothesis of substance use: testing Khantzian’s updated theory. J Psychoactive Drugs. 2007;39(2):151-158.
36. Henwood B, Padgett DK. Reevaluating the self-medication hypothesis among the dually diagnosed. Am J Addict. 2007;16(3):160-165.
37. Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alc Abuse. 2012;38(6):524-529.
38. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
39. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29.
40. Pierre JM. Risks of increasingly potent cannabis: the joint effects of potency and frequency. Current Psychiatry. 2017;16:14-20.
41. Zweben JE, Smith DE. Considerations in using psychotropic medication with dual diagnosis patients in recovery. J Psychoactive Drugs. 1989;21(2):221-228.
1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511-2518.
2. Drake RE, Mercer-McFadden C, Muesner KT, et al. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589-608.
3. Horsfall J, Cleary M, Hunt GE, et al. Psychosocial treatments for people with co-occurring severe mental illness and substance use disorders (dual diagnosis): a review of empiric evidence. Harv Rev Psychiatry. 2009;17(1):24-34.
4. Krawczyk N, Feder KA, Saloner B, et al. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 2017;175:157-163.
5. Brunette MF, Muesner KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(suppl 7):10-17.
6. Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31(4):513-536.
7. Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
8. Tsuang JT, Ho AP, Eckman TA, et al. Dual diagnosis treatment for patients with schizophrenia who are substance dependent. Psychatr Serv. 1997;48(7):887-889.
9. Rosen MI, Rosenheck RA, Shaner A, et al. Veterans who may need a payee to prevent misuse of funds for drugs. Psychiatr Serv. 2002;53(8):995-1000.
10. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health. HHS Publication No. SMA 17-5044, NSDUH Series H-52. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. Published September 2017. Accessed August 7, 2018.
11. Rubinsky AD, Chen C, Batki SL, et al. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res. 2015;69:150-157.
12. McGovern MP, Lambert-Harris C, McHugo GJ, et al. Improving the dual diagnosis capability of addiction and mental health treatment services: implementation factors associated with program level changes. J Dual Diag. 2010;6:237-250.
13. Reno R. Maintaining quality of care in a comprehensive dual diagnosis treatment program. Psychiatr Serv. 2001;52(5):673-675.
14. McGovern MP, Lambert-Harris, Gotham HJ, et al. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205-214.
15. Gotham HJ, Claus RE, Selig K, et al. Increasing program capabilities to provide treatment for co-occurring substance use and mental disorders: organizational characteristics. J Subs Abuse Treat. 2010;38(2):160-169.
16. Priester MA, Browne T, Iachini A, et al. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47-59.
17. Drake RE, Bond GR. Implementing integrated mental health and substance abuse services. J Dual Diagnosis. 2010;6(3-4):251-262.
18. Miele GM, Trautman KD, Hasin DS. Assessing comorbid mental and substance-use disorders: a guide for clinical practice. J Pract Psychiatry Behav Health. 1996;5:272-282.
19. Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2015;80(1):105-116.
20. Flynn PM, Brown BS. Co-occurring disorders in substance abuse treatment: Issues and prospects. J Subt Abuse Treat. 2008;34(1):36-47.
21. Grant BF, Stintson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807-816.
22. Pierre JM, Wirshing DA, Wirshing WC. “Iatrogenic malingering” in VA substance abuse treatment. Psych Services. 2003;54(2):253-254.
23. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry. 2004;161(9):1718.
24. Pierre JM. Hallucinations in non-psychotic disorders: Toward a differential diagnosis of “hearing voices.” Harv Rev Psychiatry. 2010;18(1):22-35.
25. Pierre JM. Nonantipsychotic therapy for monosymptomatic auditory hallucinations. Biol Psychiatry. 2010;68(7):e33-e34.
26. Shaner A, Roberts LJ, Eckman TA, et al. Sources of diagnostic uncertainty for chronically psychotic cocaine abusers. Psychiatr Serv. 1998;49(5):684-690.
27. Brown SA, Shuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49(5):412-417.
28. Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861-868.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994:210.
30. Roush S, Monica C, Carpenter-Song E, et al. First-person perspectives on Dual Diagnosis Anonymous (DDA): a qualitative study. J Dual Diagnosis. 2015;11(2):136-141.
31. Klein JW. Pharmacotherapy for substance abuse disorders. Med Clin N Am. 2016;100(4):891-910.
32. Horgan CM, Reif S, Hodgkin D, et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147-156.
33. Frances RJ. The wrath of grapes versus the self-medication hypothesis. Harvard Rev Psychiatry. 1997;4(5):287-289.
34. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231-244.
35. Hall DH, Queener JE. Self-medication hypothesis of substance use: testing Khantzian’s updated theory. J Psychoactive Drugs. 2007;39(2):151-158.
36. Henwood B, Padgett DK. Reevaluating the self-medication hypothesis among the dually diagnosed. Am J Addict. 2007;16(3):160-165.
37. Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alc Abuse. 2012;38(6):524-529.
38. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
39. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29.
40. Pierre JM. Risks of increasingly potent cannabis: the joint effects of potency and frequency. Current Psychiatry. 2017;16:14-20.
41. Zweben JE, Smith DE. Considerations in using psychotropic medication with dual diagnosis patients in recovery. J Psychoactive Drugs. 1989;21(2):221-228.
AGA Guideline: Treatment of opioid-induced constipation
For patients with suspected opioid-induced constipation, start by taking a careful history of defecation and dietary patterns, stool consistency, incomplete evacuation, and “alarm symptoms,” such as bloody stools or weight loss, state new guidelines from the American Gastroenterological Association in Gastroenterology.
Clinicians also should rule out other causes of constipation, such as pelvic outlet dysfunction, mechanical obstruction, metabolic abnormalities, and comorbidities or concurrent medications, wrote Seth D. Crockett, MD, MPH, of the University of North Carolina at Chapel Hill, together with his associates. The guideline was published online Sept. 1.
Opioid therapy can lead to a range of gastrointestinal symptoms, such as constipation, gastroesophageal reflux, nausea and vomiting, bloating, and abdominal pain. Among these, constipation is by far the most common and debilitating, the guideline notes. In past studies, 40%-80% of patients who received opioids developed opioid-induced constipation (OIC), a more severe presentation that involves a combination of reduced stool frequency in addition to other symptoms, such as harder stools, new or worsening straining during defecation, and a sense of incomplete rectal evacuation.
Treating OIC should start with lifestyle interventions, such as drinking more fluids, toileting as soon as possible when feeling the urge to defecate, and adding regular moderate exercise whenever tolerable, the guideline advises. For patients on oral or parenteral therapy, consider switching to an equianalgesic dose of a less-constipating opioid, such as transdermal fentanyl or oxycodone-naloxone combination therapy.
Many patients with OIC require interventions beyond lifestyle changes or opioid switching. For these patients, the guideline advises starting with conventional laxative therapies based on their safety, low cost, and “established efficacy” in the OIC setting. Options include stool softeners (docusate sodium), osmotic laxatives (polyethylene glycol, magnesium hydroxide, magnesium citrate, and lactulose), lubricants (mineral oil), and stimulant laxatives (bisacodyl, sodium picosulfate, and senna). “Of note, there is little evidence that routine use of stimulant laxatives is harmful to the colon, despite widespread concern to the contrary,” the guideline states. Although randomized, controlled trials have not evaluated particular laxative combinations or titrations for OIC, the best evidence supports stimulant and osmotic laxative therapy, the authors note.
Before deeming any case of OIC laxative refractory, ensure that a patient receives an adequate trial of at least two classes of laxatives administered on a regular schedule, not just “as needed,” the guideline specifies. For example, a patient might receive a 2-week trial of a daily osmotic laxative plus a stimulant laxative two to three times weekly. The guideline authors suggest restricting the use of enemas to rescue therapy. They also note that consuming more fiber tends not to help patients with OIC because fiber does not affect colonic motility.
For truly laxative-refractory OIC, the guidelines recommend escalating treatment to peripherally acting mu-opioid receptor antagonists (PAMORAs). These drugs restore the function of the enteric nervous system by blocking mu-opioid receptors in the gut. Among the PAMORAs, the guideline strongly recommends the use of naldemedine or naloxegol over no treatment, based on robust data from randomized, double-blind, placebo-controlled trials. In the phase 3 COMPOSE 1, 2, and 3 trials, about 52% of patients who received naldemedine achieved at least three spontaneous bowel movements per week, compared with 35% of patients who received placebo. Additionally, in a 52-week safety and efficacy study (COMPOSE 3), naldemedine was associated with one more spontaneous bowel movement per week versus placebo and with a low absolute increase in adverse events.
The guideline bases its strong recommendation for naloxegol on moderate-quality data from three studies, including two phase 3, double-blind, randomized, placebo-controlled trials. Although at least five randomized, controlled trials have evaluated methylnaltrexone, the evidence was low quality and therefore the guideline only conditionally recommends prescribing this PAMORA over no treatment.
The guideline also makes no recommendation on the use of the intestinal secretagogue lubiprostone or the 5HT agonist prucalopride. Studies of lubiprostone were limited by possible reporting bias and showed no clear treatment benefit, the authors state. They describe a similar evidence gap for prucalopride, noting that at least one trial ended early without publication of the findings. They recommend further studying lubiprostone as well as prucalopride and other highly selective 5-HT4 agonists for treating OIC. Head-to-head trials would help guide treatment choice for patients with laxative-refractory OIC, they add. “Cost-effectiveness studies are also lacking in this field, which could inform prescribing strategy, particularly for newer, more expensive agents.”
For patients with suspected opioid-induced constipation, start by taking a careful history of defecation and dietary patterns, stool consistency, incomplete evacuation, and “alarm symptoms,” such as bloody stools or weight loss, state new guidelines from the American Gastroenterological Association in Gastroenterology.
Clinicians also should rule out other causes of constipation, such as pelvic outlet dysfunction, mechanical obstruction, metabolic abnormalities, and comorbidities or concurrent medications, wrote Seth D. Crockett, MD, MPH, of the University of North Carolina at Chapel Hill, together with his associates. The guideline was published online Sept. 1.
Opioid therapy can lead to a range of gastrointestinal symptoms, such as constipation, gastroesophageal reflux, nausea and vomiting, bloating, and abdominal pain. Among these, constipation is by far the most common and debilitating, the guideline notes. In past studies, 40%-80% of patients who received opioids developed opioid-induced constipation (OIC), a more severe presentation that involves a combination of reduced stool frequency in addition to other symptoms, such as harder stools, new or worsening straining during defecation, and a sense of incomplete rectal evacuation.
Treating OIC should start with lifestyle interventions, such as drinking more fluids, toileting as soon as possible when feeling the urge to defecate, and adding regular moderate exercise whenever tolerable, the guideline advises. For patients on oral or parenteral therapy, consider switching to an equianalgesic dose of a less-constipating opioid, such as transdermal fentanyl or oxycodone-naloxone combination therapy.
Many patients with OIC require interventions beyond lifestyle changes or opioid switching. For these patients, the guideline advises starting with conventional laxative therapies based on their safety, low cost, and “established efficacy” in the OIC setting. Options include stool softeners (docusate sodium), osmotic laxatives (polyethylene glycol, magnesium hydroxide, magnesium citrate, and lactulose), lubricants (mineral oil), and stimulant laxatives (bisacodyl, sodium picosulfate, and senna). “Of note, there is little evidence that routine use of stimulant laxatives is harmful to the colon, despite widespread concern to the contrary,” the guideline states. Although randomized, controlled trials have not evaluated particular laxative combinations or titrations for OIC, the best evidence supports stimulant and osmotic laxative therapy, the authors note.
Before deeming any case of OIC laxative refractory, ensure that a patient receives an adequate trial of at least two classes of laxatives administered on a regular schedule, not just “as needed,” the guideline specifies. For example, a patient might receive a 2-week trial of a daily osmotic laxative plus a stimulant laxative two to three times weekly. The guideline authors suggest restricting the use of enemas to rescue therapy. They also note that consuming more fiber tends not to help patients with OIC because fiber does not affect colonic motility.
For truly laxative-refractory OIC, the guidelines recommend escalating treatment to peripherally acting mu-opioid receptor antagonists (PAMORAs). These drugs restore the function of the enteric nervous system by blocking mu-opioid receptors in the gut. Among the PAMORAs, the guideline strongly recommends the use of naldemedine or naloxegol over no treatment, based on robust data from randomized, double-blind, placebo-controlled trials. In the phase 3 COMPOSE 1, 2, and 3 trials, about 52% of patients who received naldemedine achieved at least three spontaneous bowel movements per week, compared with 35% of patients who received placebo. Additionally, in a 52-week safety and efficacy study (COMPOSE 3), naldemedine was associated with one more spontaneous bowel movement per week versus placebo and with a low absolute increase in adverse events.
The guideline bases its strong recommendation for naloxegol on moderate-quality data from three studies, including two phase 3, double-blind, randomized, placebo-controlled trials. Although at least five randomized, controlled trials have evaluated methylnaltrexone, the evidence was low quality and therefore the guideline only conditionally recommends prescribing this PAMORA over no treatment.
The guideline also makes no recommendation on the use of the intestinal secretagogue lubiprostone or the 5HT agonist prucalopride. Studies of lubiprostone were limited by possible reporting bias and showed no clear treatment benefit, the authors state. They describe a similar evidence gap for prucalopride, noting that at least one trial ended early without publication of the findings. They recommend further studying lubiprostone as well as prucalopride and other highly selective 5-HT4 agonists for treating OIC. Head-to-head trials would help guide treatment choice for patients with laxative-refractory OIC, they add. “Cost-effectiveness studies are also lacking in this field, which could inform prescribing strategy, particularly for newer, more expensive agents.”
For patients with suspected opioid-induced constipation, start by taking a careful history of defecation and dietary patterns, stool consistency, incomplete evacuation, and “alarm symptoms,” such as bloody stools or weight loss, state new guidelines from the American Gastroenterological Association in Gastroenterology.
Clinicians also should rule out other causes of constipation, such as pelvic outlet dysfunction, mechanical obstruction, metabolic abnormalities, and comorbidities or concurrent medications, wrote Seth D. Crockett, MD, MPH, of the University of North Carolina at Chapel Hill, together with his associates. The guideline was published online Sept. 1.
Opioid therapy can lead to a range of gastrointestinal symptoms, such as constipation, gastroesophageal reflux, nausea and vomiting, bloating, and abdominal pain. Among these, constipation is by far the most common and debilitating, the guideline notes. In past studies, 40%-80% of patients who received opioids developed opioid-induced constipation (OIC), a more severe presentation that involves a combination of reduced stool frequency in addition to other symptoms, such as harder stools, new or worsening straining during defecation, and a sense of incomplete rectal evacuation.
Treating OIC should start with lifestyle interventions, such as drinking more fluids, toileting as soon as possible when feeling the urge to defecate, and adding regular moderate exercise whenever tolerable, the guideline advises. For patients on oral or parenteral therapy, consider switching to an equianalgesic dose of a less-constipating opioid, such as transdermal fentanyl or oxycodone-naloxone combination therapy.
Many patients with OIC require interventions beyond lifestyle changes or opioid switching. For these patients, the guideline advises starting with conventional laxative therapies based on their safety, low cost, and “established efficacy” in the OIC setting. Options include stool softeners (docusate sodium), osmotic laxatives (polyethylene glycol, magnesium hydroxide, magnesium citrate, and lactulose), lubricants (mineral oil), and stimulant laxatives (bisacodyl, sodium picosulfate, and senna). “Of note, there is little evidence that routine use of stimulant laxatives is harmful to the colon, despite widespread concern to the contrary,” the guideline states. Although randomized, controlled trials have not evaluated particular laxative combinations or titrations for OIC, the best evidence supports stimulant and osmotic laxative therapy, the authors note.
Before deeming any case of OIC laxative refractory, ensure that a patient receives an adequate trial of at least two classes of laxatives administered on a regular schedule, not just “as needed,” the guideline specifies. For example, a patient might receive a 2-week trial of a daily osmotic laxative plus a stimulant laxative two to three times weekly. The guideline authors suggest restricting the use of enemas to rescue therapy. They also note that consuming more fiber tends not to help patients with OIC because fiber does not affect colonic motility.
For truly laxative-refractory OIC, the guidelines recommend escalating treatment to peripherally acting mu-opioid receptor antagonists (PAMORAs). These drugs restore the function of the enteric nervous system by blocking mu-opioid receptors in the gut. Among the PAMORAs, the guideline strongly recommends the use of naldemedine or naloxegol over no treatment, based on robust data from randomized, double-blind, placebo-controlled trials. In the phase 3 COMPOSE 1, 2, and 3 trials, about 52% of patients who received naldemedine achieved at least three spontaneous bowel movements per week, compared with 35% of patients who received placebo. Additionally, in a 52-week safety and efficacy study (COMPOSE 3), naldemedine was associated with one more spontaneous bowel movement per week versus placebo and with a low absolute increase in adverse events.
The guideline bases its strong recommendation for naloxegol on moderate-quality data from three studies, including two phase 3, double-blind, randomized, placebo-controlled trials. Although at least five randomized, controlled trials have evaluated methylnaltrexone, the evidence was low quality and therefore the guideline only conditionally recommends prescribing this PAMORA over no treatment.
The guideline also makes no recommendation on the use of the intestinal secretagogue lubiprostone or the 5HT agonist prucalopride. Studies of lubiprostone were limited by possible reporting bias and showed no clear treatment benefit, the authors state. They describe a similar evidence gap for prucalopride, noting that at least one trial ended early without publication of the findings. They recommend further studying lubiprostone as well as prucalopride and other highly selective 5-HT4 agonists for treating OIC. Head-to-head trials would help guide treatment choice for patients with laxative-refractory OIC, they add. “Cost-effectiveness studies are also lacking in this field, which could inform prescribing strategy, particularly for newer, more expensive agents.”
FROM GASTROENTEROLOGY
Nerve growth factor therapy speeds gastric ulcer healing
a recent study found.
Compared with young individuals, elderly people have significantly lower levels of NGF in gastric endothelial cells (GECs), a finding that is associated with impaired angiogenesis and delayed gastric ulcer healing.
“Our previous studies have shown that the gastric mucosa of aging individuals ... has increased susceptibility to injury and delayed healing owing to impaired angiogenesis, but the mechanisms are not fully elucidated,” wrote Amrita Ahluwalia, PhD, of Medical and Research Services at the Veterans Affairs Long Beach (Calif.) Healthcare System and her coauthors.
Mapping the drivers of angiogenesis in the gastric mucosa could lead to treatment options for elderly patients with injured or ulcerated gastric tissue. In prior trials (with rats), “treatment with VEGF [vascular endothelial growth factor] only partly reversed impaired angiogenesis in aging [GECs], indicating an essential role for other factor(s) in addition to VEGF,” the investigators wrote in the September issue of Cellular and Molecular Gastroenterology and Hepatology. They looked to NGF as another possible factor because recent studies had shown it could improve angiogenesis in the brain.
The present study measured NGF expression in rats and humans of varying ages, with NGF treatment and gene therapy performed in rats (in vitro and in vivo).
In vitro angiogenesis was 4.1-fold lower in GECs from aging rats (24 months of age) than it was in GECs from young rats (3 months of age; P less than .001). NGF protein and NGF mRNA levels were also significantly lower in aging GECs than they were with young GECs (NGF protein, 3.0-fold lower; NGF mRNA, 4.2-fold lower; P less than .001).
Treatment of aging rat GECs with exogenous NGF increased angiogenesis by 1.5-fold (P less than .001). Pretreatment with a PI3 kinase inhibitor or an mTOR inhibitor abolished this improvement, suggesting that the PI3 kinase/Akt and mTOR pathways are involved.
When NGF gene therapy was performed in aging GECs, NGF levels rose to the level of that in young GECs, with an accompanying restoration of angiogenesis (threefold increase; P less than .001). Proliferation of aging GECs also increased with gene therapy (P less than .001).
In vivo studies revealed that NGF expression and cell proliferation in aging rat gastric mucosa were lower than in younger rats. Of note, older rats treated with local NGF protein showed increased gastric mucosa angiogenesis and faster ulcer healing, compared with phosphate-buffered saline treatment.
Similar age-related NGF declines were found in humans. When gastric mucosa biopsies were collected from younger individuals (younger than 40 years old; n = 10) and compared with samples from an older population (at least 70 years old; n = 10), the investigators found that NGF expression was 5.5-fold lower in the older people (P less than .001).
“This clearly indicates human relevance of our experimental findings and also can explain impaired angiogenesis and delayed healing of injured gastric mucosa in aging individuals,” the investigators wrote.
“Aging gastropathy and its consequences are clinically critical issues,” the investigators noted, “especially because the aging U.S. population is growing rapidly and it is estimated that, by the year 2030, approximately 70 million Americans will be older than 65 years of age.” Gastric ulcers become more common with age, and individuals 70 years or older have an eightfold increased risk of associated complications, compared with people under 50 years.
The investigators noted that multiple growth factors likely play a role in stimulation of angiogenesis, including NGF, VEGF, epidermal growth factor, and basic fibroblast growth factor. “Further studies are necessary to investigate the role of other growth factors and cytokines in angiogenesis, [gastric ulcer] healing, and their impairment in aging,” the investigators concluded.
The study was funded by the Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The authors declared no conflicts of interest.
SOURCE: Ahluwalia A et al. CMGH. 2018 May 17. doi: 10.1016/j.jcmgh.2018.05.003
Although the incidence of gastric ulcers has been declining in the general population, hospitalization and mortality linked to gastric ulcers remains high in the elderly population. One of the major risk factors for gastric ulceration is the use of NSAIDs. It is estimated that 40% of individuals aged 65 years and older fill at least one prescription for an NSAID each year. Given that the elderly population (those aged 65 years and older) is anticipated to more than double by the year 2050, reaching 84 million, understanding the pathogenesis of gastric ulceration is increasingly relevant.
This study described a new role for nerve growth factor (NGF) in promoting angiogenesis during gastric ulcer repair. The authors observed that aged rats exhibited low NGF levels in gastric endothelial cells that corresponded with impaired ulcer healing of the gastric mucosa following injury. Local NGF treatment to aged rats significantly increased angiogenesis and gastric regeneration. Consistent with their in vivo rat model, analysis of human gastric biopsy specimens showed that individuals more than 70 years of age had decreased expression of NGF in gastric endothelial cells, compared with individuals younger than 40 years.
Ahluwalia and colleagues are the first to demonstrate the role of NGF in aging gastropathy, and their work highlights a key mechanism of angiogenesis during gastric repair that may inform future therapeutic strategies.
Amy Christine Engevik, PhD, is a postdoctoral fellow in the division of surgical sciences at Vanderbilt University Medical Center, Nashville, Tenn. She has no conflicts of interest.
Although the incidence of gastric ulcers has been declining in the general population, hospitalization and mortality linked to gastric ulcers remains high in the elderly population. One of the major risk factors for gastric ulceration is the use of NSAIDs. It is estimated that 40% of individuals aged 65 years and older fill at least one prescription for an NSAID each year. Given that the elderly population (those aged 65 years and older) is anticipated to more than double by the year 2050, reaching 84 million, understanding the pathogenesis of gastric ulceration is increasingly relevant.
This study described a new role for nerve growth factor (NGF) in promoting angiogenesis during gastric ulcer repair. The authors observed that aged rats exhibited low NGF levels in gastric endothelial cells that corresponded with impaired ulcer healing of the gastric mucosa following injury. Local NGF treatment to aged rats significantly increased angiogenesis and gastric regeneration. Consistent with their in vivo rat model, analysis of human gastric biopsy specimens showed that individuals more than 70 years of age had decreased expression of NGF in gastric endothelial cells, compared with individuals younger than 40 years.
Ahluwalia and colleagues are the first to demonstrate the role of NGF in aging gastropathy, and their work highlights a key mechanism of angiogenesis during gastric repair that may inform future therapeutic strategies.
Amy Christine Engevik, PhD, is a postdoctoral fellow in the division of surgical sciences at Vanderbilt University Medical Center, Nashville, Tenn. She has no conflicts of interest.
Although the incidence of gastric ulcers has been declining in the general population, hospitalization and mortality linked to gastric ulcers remains high in the elderly population. One of the major risk factors for gastric ulceration is the use of NSAIDs. It is estimated that 40% of individuals aged 65 years and older fill at least one prescription for an NSAID each year. Given that the elderly population (those aged 65 years and older) is anticipated to more than double by the year 2050, reaching 84 million, understanding the pathogenesis of gastric ulceration is increasingly relevant.
This study described a new role for nerve growth factor (NGF) in promoting angiogenesis during gastric ulcer repair. The authors observed that aged rats exhibited low NGF levels in gastric endothelial cells that corresponded with impaired ulcer healing of the gastric mucosa following injury. Local NGF treatment to aged rats significantly increased angiogenesis and gastric regeneration. Consistent with their in vivo rat model, analysis of human gastric biopsy specimens showed that individuals more than 70 years of age had decreased expression of NGF in gastric endothelial cells, compared with individuals younger than 40 years.
Ahluwalia and colleagues are the first to demonstrate the role of NGF in aging gastropathy, and their work highlights a key mechanism of angiogenesis during gastric repair that may inform future therapeutic strategies.
Amy Christine Engevik, PhD, is a postdoctoral fellow in the division of surgical sciences at Vanderbilt University Medical Center, Nashville, Tenn. She has no conflicts of interest.
a recent study found.
Compared with young individuals, elderly people have significantly lower levels of NGF in gastric endothelial cells (GECs), a finding that is associated with impaired angiogenesis and delayed gastric ulcer healing.
“Our previous studies have shown that the gastric mucosa of aging individuals ... has increased susceptibility to injury and delayed healing owing to impaired angiogenesis, but the mechanisms are not fully elucidated,” wrote Amrita Ahluwalia, PhD, of Medical and Research Services at the Veterans Affairs Long Beach (Calif.) Healthcare System and her coauthors.
Mapping the drivers of angiogenesis in the gastric mucosa could lead to treatment options for elderly patients with injured or ulcerated gastric tissue. In prior trials (with rats), “treatment with VEGF [vascular endothelial growth factor] only partly reversed impaired angiogenesis in aging [GECs], indicating an essential role for other factor(s) in addition to VEGF,” the investigators wrote in the September issue of Cellular and Molecular Gastroenterology and Hepatology. They looked to NGF as another possible factor because recent studies had shown it could improve angiogenesis in the brain.
The present study measured NGF expression in rats and humans of varying ages, with NGF treatment and gene therapy performed in rats (in vitro and in vivo).
In vitro angiogenesis was 4.1-fold lower in GECs from aging rats (24 months of age) than it was in GECs from young rats (3 months of age; P less than .001). NGF protein and NGF mRNA levels were also significantly lower in aging GECs than they were with young GECs (NGF protein, 3.0-fold lower; NGF mRNA, 4.2-fold lower; P less than .001).
Treatment of aging rat GECs with exogenous NGF increased angiogenesis by 1.5-fold (P less than .001). Pretreatment with a PI3 kinase inhibitor or an mTOR inhibitor abolished this improvement, suggesting that the PI3 kinase/Akt and mTOR pathways are involved.
When NGF gene therapy was performed in aging GECs, NGF levels rose to the level of that in young GECs, with an accompanying restoration of angiogenesis (threefold increase; P less than .001). Proliferation of aging GECs also increased with gene therapy (P less than .001).
In vivo studies revealed that NGF expression and cell proliferation in aging rat gastric mucosa were lower than in younger rats. Of note, older rats treated with local NGF protein showed increased gastric mucosa angiogenesis and faster ulcer healing, compared with phosphate-buffered saline treatment.
Similar age-related NGF declines were found in humans. When gastric mucosa biopsies were collected from younger individuals (younger than 40 years old; n = 10) and compared with samples from an older population (at least 70 years old; n = 10), the investigators found that NGF expression was 5.5-fold lower in the older people (P less than .001).
“This clearly indicates human relevance of our experimental findings and also can explain impaired angiogenesis and delayed healing of injured gastric mucosa in aging individuals,” the investigators wrote.
“Aging gastropathy and its consequences are clinically critical issues,” the investigators noted, “especially because the aging U.S. population is growing rapidly and it is estimated that, by the year 2030, approximately 70 million Americans will be older than 65 years of age.” Gastric ulcers become more common with age, and individuals 70 years or older have an eightfold increased risk of associated complications, compared with people under 50 years.
The investigators noted that multiple growth factors likely play a role in stimulation of angiogenesis, including NGF, VEGF, epidermal growth factor, and basic fibroblast growth factor. “Further studies are necessary to investigate the role of other growth factors and cytokines in angiogenesis, [gastric ulcer] healing, and their impairment in aging,” the investigators concluded.
The study was funded by the Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The authors declared no conflicts of interest.
SOURCE: Ahluwalia A et al. CMGH. 2018 May 17. doi: 10.1016/j.jcmgh.2018.05.003
a recent study found.
Compared with young individuals, elderly people have significantly lower levels of NGF in gastric endothelial cells (GECs), a finding that is associated with impaired angiogenesis and delayed gastric ulcer healing.
“Our previous studies have shown that the gastric mucosa of aging individuals ... has increased susceptibility to injury and delayed healing owing to impaired angiogenesis, but the mechanisms are not fully elucidated,” wrote Amrita Ahluwalia, PhD, of Medical and Research Services at the Veterans Affairs Long Beach (Calif.) Healthcare System and her coauthors.
Mapping the drivers of angiogenesis in the gastric mucosa could lead to treatment options for elderly patients with injured or ulcerated gastric tissue. In prior trials (with rats), “treatment with VEGF [vascular endothelial growth factor] only partly reversed impaired angiogenesis in aging [GECs], indicating an essential role for other factor(s) in addition to VEGF,” the investigators wrote in the September issue of Cellular and Molecular Gastroenterology and Hepatology. They looked to NGF as another possible factor because recent studies had shown it could improve angiogenesis in the brain.
The present study measured NGF expression in rats and humans of varying ages, with NGF treatment and gene therapy performed in rats (in vitro and in vivo).
In vitro angiogenesis was 4.1-fold lower in GECs from aging rats (24 months of age) than it was in GECs from young rats (3 months of age; P less than .001). NGF protein and NGF mRNA levels were also significantly lower in aging GECs than they were with young GECs (NGF protein, 3.0-fold lower; NGF mRNA, 4.2-fold lower; P less than .001).
Treatment of aging rat GECs with exogenous NGF increased angiogenesis by 1.5-fold (P less than .001). Pretreatment with a PI3 kinase inhibitor or an mTOR inhibitor abolished this improvement, suggesting that the PI3 kinase/Akt and mTOR pathways are involved.
When NGF gene therapy was performed in aging GECs, NGF levels rose to the level of that in young GECs, with an accompanying restoration of angiogenesis (threefold increase; P less than .001). Proliferation of aging GECs also increased with gene therapy (P less than .001).
In vivo studies revealed that NGF expression and cell proliferation in aging rat gastric mucosa were lower than in younger rats. Of note, older rats treated with local NGF protein showed increased gastric mucosa angiogenesis and faster ulcer healing, compared with phosphate-buffered saline treatment.
Similar age-related NGF declines were found in humans. When gastric mucosa biopsies were collected from younger individuals (younger than 40 years old; n = 10) and compared with samples from an older population (at least 70 years old; n = 10), the investigators found that NGF expression was 5.5-fold lower in the older people (P less than .001).
“This clearly indicates human relevance of our experimental findings and also can explain impaired angiogenesis and delayed healing of injured gastric mucosa in aging individuals,” the investigators wrote.
“Aging gastropathy and its consequences are clinically critical issues,” the investigators noted, “especially because the aging U.S. population is growing rapidly and it is estimated that, by the year 2030, approximately 70 million Americans will be older than 65 years of age.” Gastric ulcers become more common with age, and individuals 70 years or older have an eightfold increased risk of associated complications, compared with people under 50 years.
The investigators noted that multiple growth factors likely play a role in stimulation of angiogenesis, including NGF, VEGF, epidermal growth factor, and basic fibroblast growth factor. “Further studies are necessary to investigate the role of other growth factors and cytokines in angiogenesis, [gastric ulcer] healing, and their impairment in aging,” the investigators concluded.
The study was funded by the Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The authors declared no conflicts of interest.
SOURCE: Ahluwalia A et al. CMGH. 2018 May 17. doi: 10.1016/j.jcmgh.2018.05.003
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Nerve growth factor (NGF) therapy in aging rats improves angiogenesis and speeds gastric ulcer (GU) healing, suggesting possible applications in human medicine.
Major finding: Compared with young individuals, elderly people have 5.5-fold lower NGF expression in their gastric mucosa.
Study details: A prospective study involving rats and humans, with NGF therapy performed in rats.
Disclosures: The study was funded by the Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The authors declared no conflicts of interest.
Source: Ahluwalia et al. CMGH. 2018 May 17. doi: 10.1016/j.jcmgh.2018.05.003
Sunitinib for RCC: Side effects predictable, manageable, and reversible
A proactive side-effect management strategy enabled many patients with high-risk renal cell carcinoma (RCC) to stay on adjuvant sunitinib therapy in a recent clinical trial, researchers report.
With dose interruptions, dose reductions, and supportive medical therapy, adverse events on adjuvant sunitinib were predictable, manageable, and reversible, according to their report on the S-TRAC (Sunitinib as Adjuvant Treatment for High-Risk Renal Cell Carcinoma Following Nephrectomy) trial.
Patients did report reduced health-related quality of life, but with the exception of diarrhea and loss of appetite, those changes were generally not clinically significant, the investigators wrote. The report is in Annals of Oncology.
The safety profile for sunitinib was acceptable in adjuvant RCC treatment in S-TRAC, with no safety signals observed, said Michael Staehler, MD, PhD, department of urology, Ludwig Maximilian University of Munich, and his coauthors.
“It is likely proactive management contributed to preservation of global health status and quality of life, and alleviation of treatment-related symptoms, thereby enabling patients to remain on effective adjuvant therapy,” Dr. Staehler and his coauthors said.
Sunitinib is approved by the Food and Drug Administration for adjuvant treatment of patients at high risk of recurrent RCC after nephrectomy. That was based in part on previously reported results of the phase 3 S-TRAC study, which showed a 24% reduction in risk of a disease-free survival event versus placebo.
For the 306 patients randomized to sunitinib, 71% stayed on treatment for at least 8 months, reaching cycle 6 of therapy, while 56% finished the full year of treatment, Dr. Staehler and his coauthors said in this new report on S-TRAC focused on adverse events and patient-reported outcomes.
The most common adverse events in the sunitinib arm of S-TRAC included diarrhea in 56.9%, versus 21.4% in the placebo arm, palmar-plantar erythrodysesthesia (PPE) in 50.3% versus 10.2% for placebo, and hypertension in 36.9% versus 11.8% for placebo. The frequency of serious adverse events was similar between arms, according to the investigators, at 21.9% for sunitinib and 17.1% for placebo.
Adverse events were the most common reason for dose reduction, cited in 34.6% of cases, and for dose interruption, reported in 46.4%, they said.
Treatment discontinuations due to adverse events occurred in 28.1%, usually because of PPE.
The S-TRAC study investigators used the EORTC QLQ-C30 instrument to evaluate patient experience during treatment.
That evaluation included an analysis of global health status/quality of life score that favored placebo, with a mean difference in the overall means of –4.76. Although statistically significant (P greater than or equal to .0001), the point estimate of the difference was below the commonly accepted threshold that would indicate clinically meaningful deterioration, investigators said.
Similar patterns that were statistically significant but not clinically meaningful were seen for symptoms including fatigue and pain. However, patient-reported scores for diarrhea and loss of appetite did reach the level of a clinically meaningful difference.
But only one patient permanently discontinued sunitinib because of diarrhea, and none permanently discontinued because of loss of appetite, Dr. Staehler and his coauthors noted.
The work was sponsored by Pfizer. Dr. Staehler reported honoraria, consulting fees, and research grants from Pfizer, Bayer, GSK, Roche, BMS, Novartis, Exelixis, and AVEO. Coauthors reported disclosures related to Merck, Sanofi, Astellas, Celldex, Acerta, Janssen, and others.
SOURCE: Staehler M et al. Ann Oncol. 2018 Aug 23. doi: 10.1093/annonc/mdy329.
A proactive side-effect management strategy enabled many patients with high-risk renal cell carcinoma (RCC) to stay on adjuvant sunitinib therapy in a recent clinical trial, researchers report.
With dose interruptions, dose reductions, and supportive medical therapy, adverse events on adjuvant sunitinib were predictable, manageable, and reversible, according to their report on the S-TRAC (Sunitinib as Adjuvant Treatment for High-Risk Renal Cell Carcinoma Following Nephrectomy) trial.
Patients did report reduced health-related quality of life, but with the exception of diarrhea and loss of appetite, those changes were generally not clinically significant, the investigators wrote. The report is in Annals of Oncology.
The safety profile for sunitinib was acceptable in adjuvant RCC treatment in S-TRAC, with no safety signals observed, said Michael Staehler, MD, PhD, department of urology, Ludwig Maximilian University of Munich, and his coauthors.
“It is likely proactive management contributed to preservation of global health status and quality of life, and alleviation of treatment-related symptoms, thereby enabling patients to remain on effective adjuvant therapy,” Dr. Staehler and his coauthors said.
Sunitinib is approved by the Food and Drug Administration for adjuvant treatment of patients at high risk of recurrent RCC after nephrectomy. That was based in part on previously reported results of the phase 3 S-TRAC study, which showed a 24% reduction in risk of a disease-free survival event versus placebo.
For the 306 patients randomized to sunitinib, 71% stayed on treatment for at least 8 months, reaching cycle 6 of therapy, while 56% finished the full year of treatment, Dr. Staehler and his coauthors said in this new report on S-TRAC focused on adverse events and patient-reported outcomes.
The most common adverse events in the sunitinib arm of S-TRAC included diarrhea in 56.9%, versus 21.4% in the placebo arm, palmar-plantar erythrodysesthesia (PPE) in 50.3% versus 10.2% for placebo, and hypertension in 36.9% versus 11.8% for placebo. The frequency of serious adverse events was similar between arms, according to the investigators, at 21.9% for sunitinib and 17.1% for placebo.
Adverse events were the most common reason for dose reduction, cited in 34.6% of cases, and for dose interruption, reported in 46.4%, they said.
Treatment discontinuations due to adverse events occurred in 28.1%, usually because of PPE.
The S-TRAC study investigators used the EORTC QLQ-C30 instrument to evaluate patient experience during treatment.
That evaluation included an analysis of global health status/quality of life score that favored placebo, with a mean difference in the overall means of –4.76. Although statistically significant (P greater than or equal to .0001), the point estimate of the difference was below the commonly accepted threshold that would indicate clinically meaningful deterioration, investigators said.
Similar patterns that were statistically significant but not clinically meaningful were seen for symptoms including fatigue and pain. However, patient-reported scores for diarrhea and loss of appetite did reach the level of a clinically meaningful difference.
But only one patient permanently discontinued sunitinib because of diarrhea, and none permanently discontinued because of loss of appetite, Dr. Staehler and his coauthors noted.
The work was sponsored by Pfizer. Dr. Staehler reported honoraria, consulting fees, and research grants from Pfizer, Bayer, GSK, Roche, BMS, Novartis, Exelixis, and AVEO. Coauthors reported disclosures related to Merck, Sanofi, Astellas, Celldex, Acerta, Janssen, and others.
SOURCE: Staehler M et al. Ann Oncol. 2018 Aug 23. doi: 10.1093/annonc/mdy329.
A proactive side-effect management strategy enabled many patients with high-risk renal cell carcinoma (RCC) to stay on adjuvant sunitinib therapy in a recent clinical trial, researchers report.
With dose interruptions, dose reductions, and supportive medical therapy, adverse events on adjuvant sunitinib were predictable, manageable, and reversible, according to their report on the S-TRAC (Sunitinib as Adjuvant Treatment for High-Risk Renal Cell Carcinoma Following Nephrectomy) trial.
Patients did report reduced health-related quality of life, but with the exception of diarrhea and loss of appetite, those changes were generally not clinically significant, the investigators wrote. The report is in Annals of Oncology.
The safety profile for sunitinib was acceptable in adjuvant RCC treatment in S-TRAC, with no safety signals observed, said Michael Staehler, MD, PhD, department of urology, Ludwig Maximilian University of Munich, and his coauthors.
“It is likely proactive management contributed to preservation of global health status and quality of life, and alleviation of treatment-related symptoms, thereby enabling patients to remain on effective adjuvant therapy,” Dr. Staehler and his coauthors said.
Sunitinib is approved by the Food and Drug Administration for adjuvant treatment of patients at high risk of recurrent RCC after nephrectomy. That was based in part on previously reported results of the phase 3 S-TRAC study, which showed a 24% reduction in risk of a disease-free survival event versus placebo.
For the 306 patients randomized to sunitinib, 71% stayed on treatment for at least 8 months, reaching cycle 6 of therapy, while 56% finished the full year of treatment, Dr. Staehler and his coauthors said in this new report on S-TRAC focused on adverse events and patient-reported outcomes.
The most common adverse events in the sunitinib arm of S-TRAC included diarrhea in 56.9%, versus 21.4% in the placebo arm, palmar-plantar erythrodysesthesia (PPE) in 50.3% versus 10.2% for placebo, and hypertension in 36.9% versus 11.8% for placebo. The frequency of serious adverse events was similar between arms, according to the investigators, at 21.9% for sunitinib and 17.1% for placebo.
Adverse events were the most common reason for dose reduction, cited in 34.6% of cases, and for dose interruption, reported in 46.4%, they said.
Treatment discontinuations due to adverse events occurred in 28.1%, usually because of PPE.
The S-TRAC study investigators used the EORTC QLQ-C30 instrument to evaluate patient experience during treatment.
That evaluation included an analysis of global health status/quality of life score that favored placebo, with a mean difference in the overall means of –4.76. Although statistically significant (P greater than or equal to .0001), the point estimate of the difference was below the commonly accepted threshold that would indicate clinically meaningful deterioration, investigators said.
Similar patterns that were statistically significant but not clinically meaningful were seen for symptoms including fatigue and pain. However, patient-reported scores for diarrhea and loss of appetite did reach the level of a clinically meaningful difference.
But only one patient permanently discontinued sunitinib because of diarrhea, and none permanently discontinued because of loss of appetite, Dr. Staehler and his coauthors noted.
The work was sponsored by Pfizer. Dr. Staehler reported honoraria, consulting fees, and research grants from Pfizer, Bayer, GSK, Roche, BMS, Novartis, Exelixis, and AVEO. Coauthors reported disclosures related to Merck, Sanofi, Astellas, Celldex, Acerta, Janssen, and others.
SOURCE: Staehler M et al. Ann Oncol. 2018 Aug 23. doi: 10.1093/annonc/mdy329.
FROM ANNALS OF ONCOLOGY
Key clinical point: Adverse events on adjuvant sunitinib were predictable, manageable, and reversible, while decreases in health-related quality of life were not clinically meaningful except for those related to diarrhea and loss of appetite.
Major finding: The EORTC QLQ-C30 global health status/quality of life score favored placebo, with a mean difference in the overall means of –4.76 (P greater than or equal to .0001) that did not exceed the threshold that would indicate clinically meaningful deterioration.
Study details: Analysis of adverse events and patient-reported outcomes for 306 patients treated with sunitinib in the S-TRAC (Sunitinib as Adjuvant Treatment for High-Risk Renal Cell Carcinoma Following Nephrectomy) trial.
Disclosures: Pfizer sponsored the study. The authors reported disclosures related to Pfizer, Bayer, GSK, Roche, BMS, Novartis, Exelixis, AVEO, Merck, Sanofi, Astellas, Celldex, Acerta, Janssen, and others.
Source: Staehler M et al. Ann Oncol. 2018 Aug 23. doi: 10.1093/annonc/mdy329.
FDA approves two once-daily HIV drugs
Two once-daily oral HIV-1 medicines have been approved by the Food and Drug Administration, according to Merck: Delstrigo, a fixed-dose combination tablet of doravirine (100 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg), and Pifeltro (doravirine, 100 mg). Both drugs are indicated for treating HIV-1 infection in adult patients with no prior antiretroviral treatment.
Pifeltro is a nonnucleoside reverse transcriptase inhibitor to be used in combination with other antiretroviral medicines. Delstrigo contains a boxed warning regarding posttreatment acute exacerbation of hepatitis B infection.
Delstrigo and Pifeltro are not curative, according to the announcement by Merck, which manufactures both drugs.
The FDA approval of Delstrigo was based on findings from the DRIVE-AHEAD trial (NCT02403674), which randomized 728 participants with no history of antiretroviral treatment to receive once daily either Delstrigo or efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV 600 mg/FTC 200 mg/TDF 300 mg). Delstrigo was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with EFV/FTC/TDF (84% vs. 81%, respectively), according to Merck.
Pifeltro was approved based on the results of the DRIVE-FORWARD trial (NCT02275780), which randomized 766 participants with no history of antiretroviral treatment to receive either Pifeltro once daily or darunavir 800 mg plus ritonavir 100 mg (DRV+r) once daily, each in combination with either emtricitabine (FTC)/TDF or abacavir (ABC)/lamivudine (3TC), as selected by the investigator. Pifeltro was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with DRV+r, each in combination with FTC/TDF or ABC/3TC (84% vs. 80%, respectively), according to the Merck announcement.
Two once-daily oral HIV-1 medicines have been approved by the Food and Drug Administration, according to Merck: Delstrigo, a fixed-dose combination tablet of doravirine (100 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg), and Pifeltro (doravirine, 100 mg). Both drugs are indicated for treating HIV-1 infection in adult patients with no prior antiretroviral treatment.
Pifeltro is a nonnucleoside reverse transcriptase inhibitor to be used in combination with other antiretroviral medicines. Delstrigo contains a boxed warning regarding posttreatment acute exacerbation of hepatitis B infection.
Delstrigo and Pifeltro are not curative, according to the announcement by Merck, which manufactures both drugs.
The FDA approval of Delstrigo was based on findings from the DRIVE-AHEAD trial (NCT02403674), which randomized 728 participants with no history of antiretroviral treatment to receive once daily either Delstrigo or efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV 600 mg/FTC 200 mg/TDF 300 mg). Delstrigo was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with EFV/FTC/TDF (84% vs. 81%, respectively), according to Merck.
Pifeltro was approved based on the results of the DRIVE-FORWARD trial (NCT02275780), which randomized 766 participants with no history of antiretroviral treatment to receive either Pifeltro once daily or darunavir 800 mg plus ritonavir 100 mg (DRV+r) once daily, each in combination with either emtricitabine (FTC)/TDF or abacavir (ABC)/lamivudine (3TC), as selected by the investigator. Pifeltro was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with DRV+r, each in combination with FTC/TDF or ABC/3TC (84% vs. 80%, respectively), according to the Merck announcement.
Two once-daily oral HIV-1 medicines have been approved by the Food and Drug Administration, according to Merck: Delstrigo, a fixed-dose combination tablet of doravirine (100 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg), and Pifeltro (doravirine, 100 mg). Both drugs are indicated for treating HIV-1 infection in adult patients with no prior antiretroviral treatment.
Pifeltro is a nonnucleoside reverse transcriptase inhibitor to be used in combination with other antiretroviral medicines. Delstrigo contains a boxed warning regarding posttreatment acute exacerbation of hepatitis B infection.
Delstrigo and Pifeltro are not curative, according to the announcement by Merck, which manufactures both drugs.
The FDA approval of Delstrigo was based on findings from the DRIVE-AHEAD trial (NCT02403674), which randomized 728 participants with no history of antiretroviral treatment to receive once daily either Delstrigo or efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV 600 mg/FTC 200 mg/TDF 300 mg). Delstrigo was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with EFV/FTC/TDF (84% vs. 81%, respectively), according to Merck.
Pifeltro was approved based on the results of the DRIVE-FORWARD trial (NCT02275780), which randomized 766 participants with no history of antiretroviral treatment to receive either Pifeltro once daily or darunavir 800 mg plus ritonavir 100 mg (DRV+r) once daily, each in combination with either emtricitabine (FTC)/TDF or abacavir (ABC)/lamivudine (3TC), as selected by the investigator. Pifeltro was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with DRV+r, each in combination with FTC/TDF or ABC/3TC (84% vs. 80%, respectively), according to the Merck announcement.
Dose-dense MVAC credited with better bladder cancer survival
In patients with muscle-invasive bladder cancer, a dose-dense neoadjuvant chemotherapy regimen followed by cystectomy was associated with a higher rate of complete responses, compared with standard gemcitabine-platinum neoadjuvant chemotherapy and cystectomy, results of a retrospective analysis indicate.
Among 1,113 patients who underwent neoadjuvant chemotherapy (NAC) and cystectomy, a regimen of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) was associated with a nearly threefold greater likelihood that patients would have a complete response, compared with gemcitabine and cisplatin, reported Scott M. Gilbert, MD, of the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida, and colleagues.
“We also found that ddMVAC was associated with longer survival intervals and a lower risk of death than the other treatments examined, although those findings did not reach statistical significance, indicating that larger comparative studies are needed to definitively answer questions regarding survival,” they wrote in JAMA Oncology.
The investigators noted that, despite clear evidence of a survival benefit associated with neoadjuvant chemotherapy followed by cystectomy, compared with cystectomy alone in patients with muscle-invasive bladder cancer, “the rates of adoption and routine use of NAC have been modest.”
Gemcitabine and cisplatin have become the de facto standard of care because of favorable toxicity profile and response rates comparable to those seen with ddMVAC. Yet there are few studies comparing disease control and survival outcomes for different neoadjuvant chemotherapy regimens, they noted, which prompted the current study.
The investigators conducted a cross-sectional analysis of data on 1,113 patients with bladder cancer treated with cystectomy at their center from January 2007 through May 2017. They compared rates of downstaging, complete responses, and overall survival with ddMVAC, compared with gemcitabine combined with either cisplatin or carboplatin, other neoadjuvant combinations (including etoposide- fluorouracil- and paclitaxel-based regimens) or no neoadjuvant chemotherapy.
Of the 1,113 patients, 824 had disease stage T2 or greater, and of this group, 332 had received neoadjuvant chemotherapy.
They found that ddMVAC was associated with a 52.2% downstaging rate, compared with 41.3% for gemcitabine-cisplatin, and 27% for gemcitabine-carboplatin. Respective pathologic complete response rates were 41.3%, 24.5%, and 9.4% (P less than .001).
Downstaging rates for patients treated with other regimens and patients who did not receive neoadjuvant chemotherapy were 42% and 25.7%, respectively. Complete response rates (downstaging to pT0N0) were 24% and 10.7%.
In a multivariable logistic regression model controlling for age, comorbidities, sex, clinical stage, and chemotherapy regimen, ddMVAC was associated with a significantly higher likelihood of pathologic complete response, with an odds ratio of 2.67 (P less than .001). Similarly, a propensity-score model weighted for clinical and demographic characteristics showed an OR for complete response with ddMVAC of 1.52 (P = .05).
The 2-year Kaplan-Meier survival probability estimate for ddMVAC was 73.3%, compared with 62% for gemcitabine-cisplatin and 34.8% for gemcitabine-carboplatin (P = .002).
Regardless of chemotherapy type, a complete pathologic response was a significant predictor for overall survival (P less than .001).
Although ddMVAC showed a trend toward better overall survival in both logistic regression and propensity score models, neither reached statistical significance.
The authors did not report survival results for patients who did not receive neoadjuvant chemotherapy.
The investigators acknowledged that the study is limited by its nonrandomized design and the relatively small sample of patients treated with ddMVAC (46 patients).
The study was supported in part by a National Cancer Institute grant to the H. Lee Moffitt Cancer Center. The authors reported having no conflicts of interest.
SOURCE: Peyton CC et al. JAMA Oncology. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3542.
In patients with muscle-invasive bladder cancer, a dose-dense neoadjuvant chemotherapy regimen followed by cystectomy was associated with a higher rate of complete responses, compared with standard gemcitabine-platinum neoadjuvant chemotherapy and cystectomy, results of a retrospective analysis indicate.
Among 1,113 patients who underwent neoadjuvant chemotherapy (NAC) and cystectomy, a regimen of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) was associated with a nearly threefold greater likelihood that patients would have a complete response, compared with gemcitabine and cisplatin, reported Scott M. Gilbert, MD, of the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida, and colleagues.
“We also found that ddMVAC was associated with longer survival intervals and a lower risk of death than the other treatments examined, although those findings did not reach statistical significance, indicating that larger comparative studies are needed to definitively answer questions regarding survival,” they wrote in JAMA Oncology.
The investigators noted that, despite clear evidence of a survival benefit associated with neoadjuvant chemotherapy followed by cystectomy, compared with cystectomy alone in patients with muscle-invasive bladder cancer, “the rates of adoption and routine use of NAC have been modest.”
Gemcitabine and cisplatin have become the de facto standard of care because of favorable toxicity profile and response rates comparable to those seen with ddMVAC. Yet there are few studies comparing disease control and survival outcomes for different neoadjuvant chemotherapy regimens, they noted, which prompted the current study.
The investigators conducted a cross-sectional analysis of data on 1,113 patients with bladder cancer treated with cystectomy at their center from January 2007 through May 2017. They compared rates of downstaging, complete responses, and overall survival with ddMVAC, compared with gemcitabine combined with either cisplatin or carboplatin, other neoadjuvant combinations (including etoposide- fluorouracil- and paclitaxel-based regimens) or no neoadjuvant chemotherapy.
Of the 1,113 patients, 824 had disease stage T2 or greater, and of this group, 332 had received neoadjuvant chemotherapy.
They found that ddMVAC was associated with a 52.2% downstaging rate, compared with 41.3% for gemcitabine-cisplatin, and 27% for gemcitabine-carboplatin. Respective pathologic complete response rates were 41.3%, 24.5%, and 9.4% (P less than .001).
Downstaging rates for patients treated with other regimens and patients who did not receive neoadjuvant chemotherapy were 42% and 25.7%, respectively. Complete response rates (downstaging to pT0N0) were 24% and 10.7%.
In a multivariable logistic regression model controlling for age, comorbidities, sex, clinical stage, and chemotherapy regimen, ddMVAC was associated with a significantly higher likelihood of pathologic complete response, with an odds ratio of 2.67 (P less than .001). Similarly, a propensity-score model weighted for clinical and demographic characteristics showed an OR for complete response with ddMVAC of 1.52 (P = .05).
The 2-year Kaplan-Meier survival probability estimate for ddMVAC was 73.3%, compared with 62% for gemcitabine-cisplatin and 34.8% for gemcitabine-carboplatin (P = .002).
Regardless of chemotherapy type, a complete pathologic response was a significant predictor for overall survival (P less than .001).
Although ddMVAC showed a trend toward better overall survival in both logistic regression and propensity score models, neither reached statistical significance.
The authors did not report survival results for patients who did not receive neoadjuvant chemotherapy.
The investigators acknowledged that the study is limited by its nonrandomized design and the relatively small sample of patients treated with ddMVAC (46 patients).
The study was supported in part by a National Cancer Institute grant to the H. Lee Moffitt Cancer Center. The authors reported having no conflicts of interest.
SOURCE: Peyton CC et al. JAMA Oncology. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3542.
In patients with muscle-invasive bladder cancer, a dose-dense neoadjuvant chemotherapy regimen followed by cystectomy was associated with a higher rate of complete responses, compared with standard gemcitabine-platinum neoadjuvant chemotherapy and cystectomy, results of a retrospective analysis indicate.
Among 1,113 patients who underwent neoadjuvant chemotherapy (NAC) and cystectomy, a regimen of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) was associated with a nearly threefold greater likelihood that patients would have a complete response, compared with gemcitabine and cisplatin, reported Scott M. Gilbert, MD, of the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida, and colleagues.
“We also found that ddMVAC was associated with longer survival intervals and a lower risk of death than the other treatments examined, although those findings did not reach statistical significance, indicating that larger comparative studies are needed to definitively answer questions regarding survival,” they wrote in JAMA Oncology.
The investigators noted that, despite clear evidence of a survival benefit associated with neoadjuvant chemotherapy followed by cystectomy, compared with cystectomy alone in patients with muscle-invasive bladder cancer, “the rates of adoption and routine use of NAC have been modest.”
Gemcitabine and cisplatin have become the de facto standard of care because of favorable toxicity profile and response rates comparable to those seen with ddMVAC. Yet there are few studies comparing disease control and survival outcomes for different neoadjuvant chemotherapy regimens, they noted, which prompted the current study.
The investigators conducted a cross-sectional analysis of data on 1,113 patients with bladder cancer treated with cystectomy at their center from January 2007 through May 2017. They compared rates of downstaging, complete responses, and overall survival with ddMVAC, compared with gemcitabine combined with either cisplatin or carboplatin, other neoadjuvant combinations (including etoposide- fluorouracil- and paclitaxel-based regimens) or no neoadjuvant chemotherapy.
Of the 1,113 patients, 824 had disease stage T2 or greater, and of this group, 332 had received neoadjuvant chemotherapy.
They found that ddMVAC was associated with a 52.2% downstaging rate, compared with 41.3% for gemcitabine-cisplatin, and 27% for gemcitabine-carboplatin. Respective pathologic complete response rates were 41.3%, 24.5%, and 9.4% (P less than .001).
Downstaging rates for patients treated with other regimens and patients who did not receive neoadjuvant chemotherapy were 42% and 25.7%, respectively. Complete response rates (downstaging to pT0N0) were 24% and 10.7%.
In a multivariable logistic regression model controlling for age, comorbidities, sex, clinical stage, and chemotherapy regimen, ddMVAC was associated with a significantly higher likelihood of pathologic complete response, with an odds ratio of 2.67 (P less than .001). Similarly, a propensity-score model weighted for clinical and demographic characteristics showed an OR for complete response with ddMVAC of 1.52 (P = .05).
The 2-year Kaplan-Meier survival probability estimate for ddMVAC was 73.3%, compared with 62% for gemcitabine-cisplatin and 34.8% for gemcitabine-carboplatin (P = .002).
Regardless of chemotherapy type, a complete pathologic response was a significant predictor for overall survival (P less than .001).
Although ddMVAC showed a trend toward better overall survival in both logistic regression and propensity score models, neither reached statistical significance.
The authors did not report survival results for patients who did not receive neoadjuvant chemotherapy.
The investigators acknowledged that the study is limited by its nonrandomized design and the relatively small sample of patients treated with ddMVAC (46 patients).
The study was supported in part by a National Cancer Institute grant to the H. Lee Moffitt Cancer Center. The authors reported having no conflicts of interest.
SOURCE: Peyton CC et al. JAMA Oncology. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3542.
FROM JAMA ONCOLOGY
Key clinical point: Neoadjuvant chemotherapy with dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (ddMVAC) is associated with improved response and survival rates, compared with gemcitabine-based regimens.
Major finding: 2-year survival probability with ddMVAC was 73.3% vs. 62% for gemcitabine-cisplatin and 34.8% for gemcitabine-carboplatin (P = .002).
Study details: Retrospective cross-sectional analysis of data on 1,113 patients with muscle-invasive bladder cancer.
Disclosures: The study was supported in part by a National Cancer Institute grant to the H. Lee Moffitt Cancer Center. The authors reported having no conflicts of interest.
Source: Peyton CC et al. JAMA Oncology. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3542.
Sometimes talk is useless
“Alex, I understand that you are upset that you left your little bulldozer at home. Let’s try to think of something else you can play with here at the restaurant that is kind of like a bulldozer.”
Sounds like a reasonable strategy to calm an unruly preschooler, and it might have been had it not been the fifth attempt in a 45-minute dialogue between a mother and her overtired, misbehaving 3-year-old. There had been a lot of “I understand how you feel” and “use your words” woven into a gag-worthy and futile effort to forge a collaborative parent-child solution to the problem of an exhausted preschooler who is up past his bedtime in a public place.
My wife and I enjoy a night out with friends and prefer a quiet dining atmosphere. However, some evenings we eat earlier and choose a restaurant we know appeals to families with young children. At those meals, we anticipate being serenaded by a loud background buzz punctuated by the occasional shriek or short bout of crying. We expect a degree of childish behavior to come with the territory, and watching the dramas unfold brings back fond “been there, done that” memories. But, listening to those behaviors being horribly mismanaged can ruin even the most tolerant adult’s appetite in less time than it takes a parent to say, “I can see you’re unhappy, and we need to talk about why.”
In an op-ed piece, a psychotherapist asks the legitimate question, and provides the correct quick answer (“Which Is Better, Rewards or Punishments? Neither,” New York Times, Aug. 21, 2018). I couldn’t agree more. In my experience, rewards have a very short half-life and can become disastrously inflationary in the blink of an eye. On the other hand, punishments can be either too heavy-handed or so irrational that the child fails to make a logical connection between his misbehavior and his sentence.
Unfortunately, many child behavior advisers, including the op-ed author, offer alternatives to rewards and punishment that are unworkable in real-world circumstances, such as the restaurant scenario my wife and I endured.
While it sounds very democratic to ask a 3-year-old why he is misbehaving, more often than not it should be the parent who is asking what he or she could have done differently to avoid the situation. It is likely the child has been allowed to become overtired and/or the parent may be in denial about his/her child’s intolerance for stimulating environments.
Too often the parent takes too long to realize that the water is spilling over the dam and it is time to head for shore. Children who are overtired and having a tantrum can’t participate in a rational discussion about their feelings. If that dialogue needs to happen, and that is seldom, it should be the next day after the parent has time to consider his or her own mistakes.
When it comes to managing misbehaving children, I prefer well-tailored consequences and my favorite is a humanely crafted time-out. When presented and executed properly, a time-out can break the cycle of misbehavior and give both parent and child a chance to reconsider their positions.
But at 7 p.m. on a Friday evening in a busy restaurant, neither a time-out nor philosophizing with a 3-year-old is going to work. It’s time to ask for the check and head home to bed.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
“Alex, I understand that you are upset that you left your little bulldozer at home. Let’s try to think of something else you can play with here at the restaurant that is kind of like a bulldozer.”
Sounds like a reasonable strategy to calm an unruly preschooler, and it might have been had it not been the fifth attempt in a 45-minute dialogue between a mother and her overtired, misbehaving 3-year-old. There had been a lot of “I understand how you feel” and “use your words” woven into a gag-worthy and futile effort to forge a collaborative parent-child solution to the problem of an exhausted preschooler who is up past his bedtime in a public place.
My wife and I enjoy a night out with friends and prefer a quiet dining atmosphere. However, some evenings we eat earlier and choose a restaurant we know appeals to families with young children. At those meals, we anticipate being serenaded by a loud background buzz punctuated by the occasional shriek or short bout of crying. We expect a degree of childish behavior to come with the territory, and watching the dramas unfold brings back fond “been there, done that” memories. But, listening to those behaviors being horribly mismanaged can ruin even the most tolerant adult’s appetite in less time than it takes a parent to say, “I can see you’re unhappy, and we need to talk about why.”
In an op-ed piece, a psychotherapist asks the legitimate question, and provides the correct quick answer (“Which Is Better, Rewards or Punishments? Neither,” New York Times, Aug. 21, 2018). I couldn’t agree more. In my experience, rewards have a very short half-life and can become disastrously inflationary in the blink of an eye. On the other hand, punishments can be either too heavy-handed or so irrational that the child fails to make a logical connection between his misbehavior and his sentence.
Unfortunately, many child behavior advisers, including the op-ed author, offer alternatives to rewards and punishment that are unworkable in real-world circumstances, such as the restaurant scenario my wife and I endured.
While it sounds very democratic to ask a 3-year-old why he is misbehaving, more often than not it should be the parent who is asking what he or she could have done differently to avoid the situation. It is likely the child has been allowed to become overtired and/or the parent may be in denial about his/her child’s intolerance for stimulating environments.
Too often the parent takes too long to realize that the water is spilling over the dam and it is time to head for shore. Children who are overtired and having a tantrum can’t participate in a rational discussion about their feelings. If that dialogue needs to happen, and that is seldom, it should be the next day after the parent has time to consider his or her own mistakes.
When it comes to managing misbehaving children, I prefer well-tailored consequences and my favorite is a humanely crafted time-out. When presented and executed properly, a time-out can break the cycle of misbehavior and give both parent and child a chance to reconsider their positions.
But at 7 p.m. on a Friday evening in a busy restaurant, neither a time-out nor philosophizing with a 3-year-old is going to work. It’s time to ask for the check and head home to bed.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
“Alex, I understand that you are upset that you left your little bulldozer at home. Let’s try to think of something else you can play with here at the restaurant that is kind of like a bulldozer.”
Sounds like a reasonable strategy to calm an unruly preschooler, and it might have been had it not been the fifth attempt in a 45-minute dialogue between a mother and her overtired, misbehaving 3-year-old. There had been a lot of “I understand how you feel” and “use your words” woven into a gag-worthy and futile effort to forge a collaborative parent-child solution to the problem of an exhausted preschooler who is up past his bedtime in a public place.
My wife and I enjoy a night out with friends and prefer a quiet dining atmosphere. However, some evenings we eat earlier and choose a restaurant we know appeals to families with young children. At those meals, we anticipate being serenaded by a loud background buzz punctuated by the occasional shriek or short bout of crying. We expect a degree of childish behavior to come with the territory, and watching the dramas unfold brings back fond “been there, done that” memories. But, listening to those behaviors being horribly mismanaged can ruin even the most tolerant adult’s appetite in less time than it takes a parent to say, “I can see you’re unhappy, and we need to talk about why.”
In an op-ed piece, a psychotherapist asks the legitimate question, and provides the correct quick answer (“Which Is Better, Rewards or Punishments? Neither,” New York Times, Aug. 21, 2018). I couldn’t agree more. In my experience, rewards have a very short half-life and can become disastrously inflationary in the blink of an eye. On the other hand, punishments can be either too heavy-handed or so irrational that the child fails to make a logical connection between his misbehavior and his sentence.
Unfortunately, many child behavior advisers, including the op-ed author, offer alternatives to rewards and punishment that are unworkable in real-world circumstances, such as the restaurant scenario my wife and I endured.
While it sounds very democratic to ask a 3-year-old why he is misbehaving, more often than not it should be the parent who is asking what he or she could have done differently to avoid the situation. It is likely the child has been allowed to become overtired and/or the parent may be in denial about his/her child’s intolerance for stimulating environments.
Too often the parent takes too long to realize that the water is spilling over the dam and it is time to head for shore. Children who are overtired and having a tantrum can’t participate in a rational discussion about their feelings. If that dialogue needs to happen, and that is seldom, it should be the next day after the parent has time to consider his or her own mistakes.
When it comes to managing misbehaving children, I prefer well-tailored consequences and my favorite is a humanely crafted time-out. When presented and executed properly, a time-out can break the cycle of misbehavior and give both parent and child a chance to reconsider their positions.
But at 7 p.m. on a Friday evening in a busy restaurant, neither a time-out nor philosophizing with a 3-year-old is going to work. It’s time to ask for the check and head home to bed.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.