User login
PVd improved survival in lenalidomide-exposed myeloma
CHICAGO – For patients with relapsed/refractory multiple myeloma previously exposed to lenalidomide, the combination of pomalidomide plus bortezomib and low‐dose dexamethasone (PVd) improved response and progression-free survival, results of the phase 3 OPTIMISMM trial showed.
Risk of disease progression or death was reduced by 39%, compared with bortezomib and low-dose dexamethasone alone (Vd), among patients in the trial, of whom approximately 70% were lenalidomide refractory, reported investigator Paul G. Richardson, MD, of the Dana-Farber Cancer Institute in Boston.
The improvements in efficacy seen with the PVd regimen were more pronounced in patients with only one prior line of therapy, and overall, the safety profile of the triplet regimen was consistent with known toxicities of each individual agent, Dr. Richardson reported.
Together, those results “would seem to support the use of this triplet [therapy] in first relapse in patients with relapsed/refractory myeloma and prior exposure to lenalidomide,” he said at the annual meeting of the American Society of Clinical Oncology.
“This study, importantly, evaluated a clinically relevant patient population and a growing patient population who receive upfront lenalidomide and maintenance in that setting and for whom lenalidomide is no longer a viable treatment option,” Dr. Richardson added.
Lenalidomide has become a mainstay of upfront myeloma treatment, and the Food and Drug Administration recently gave approval to lenalidomide as maintenance after autologous hematopoietic stem cell transplantation. Accordingly, it’s important to understand the benefits of triplet therapies in patients progressing on lenalidomide therapy and in whom lenalidomide is no longer a treatment option, Dr. Richardson said.
Pomalidomide, a potent oral immunomodulatory agent, is already approved for relapsed/refractory myeloma after two or more previous therapies that include lenalidomide and a proteasome inhibitor in patients who progress on or within 60 days of treatment.
In the OPTIMISMM trial, 559 patients who had received prior therapy, including at least two cycles of lenalidomide, were randomized to receive either PVd or Vd until disease progression or unacceptable toxicity.
Median progression-free survival was 11.20 months for PVd versus 7.10 months for Vd (hazard ratio, 0.61; 95% confidence interval, 0.49-0.77; P less than .0001).
Progression-free survival results were “even more encouraging” in the subset of patients with only one prior line of therapy, Dr. Richardson said, reporting a median of 20.73 months for PVd versus 11.63 for Vd (HR, 0.54; 95% CI, 0.36-0.82; P = .0027).
The overall response rate was 82.2% for PVd versus 50.0% for Vd (P less than .001). In patients with only one prior line of therapy, the overall response rate was 90.1% and 54.8% for PVd and Vd, respectively (P less than .001).
The progression-free survival advantage occurred regardless of whether patients were refractory to lenalidomide, Dr. Richardson added. Median progression-free survival for PVd versus Vd was 9.53 and 5.59 months, respectively, in the lenalidomide-refractory patients (P less than .001) and 22.01 versus 11.63 months in non–lenalidomide refractory patients (P less than .001).
The side effect profile of PVd was “very much as expected,” with more neutropenia seen with the PVd than with Vd, though rates of febrile neutropenia were low, Dr. Richardson said. Likewise, the rate of infection was higher in the triplet arm, but it was generally manageable, he added.
Deep vein thrombosis and pulmonary embolism rates were low in both arms, as were the rates of secondary primary malignancies. Analysis of minimal residual disease and quality of life are ongoing.
PVd could “arguably be now an important treatment platform for future directions in combination with other strategies,” Dr. Richardson said.
The study was supported by Celgene. Dr. Richardson reported advisory board work for Celgene, Novartis, Oncopeptides, Janssen, Amgen, and Takeda, and research funding from Bristol-Myers Squibb, Celgene, and Takeda.
SOURCE: Richardson PG et al. ASCO 2018, Abstract 8001.
CHICAGO – For patients with relapsed/refractory multiple myeloma previously exposed to lenalidomide, the combination of pomalidomide plus bortezomib and low‐dose dexamethasone (PVd) improved response and progression-free survival, results of the phase 3 OPTIMISMM trial showed.
Risk of disease progression or death was reduced by 39%, compared with bortezomib and low-dose dexamethasone alone (Vd), among patients in the trial, of whom approximately 70% were lenalidomide refractory, reported investigator Paul G. Richardson, MD, of the Dana-Farber Cancer Institute in Boston.
The improvements in efficacy seen with the PVd regimen were more pronounced in patients with only one prior line of therapy, and overall, the safety profile of the triplet regimen was consistent with known toxicities of each individual agent, Dr. Richardson reported.
Together, those results “would seem to support the use of this triplet [therapy] in first relapse in patients with relapsed/refractory myeloma and prior exposure to lenalidomide,” he said at the annual meeting of the American Society of Clinical Oncology.
“This study, importantly, evaluated a clinically relevant patient population and a growing patient population who receive upfront lenalidomide and maintenance in that setting and for whom lenalidomide is no longer a viable treatment option,” Dr. Richardson added.
Lenalidomide has become a mainstay of upfront myeloma treatment, and the Food and Drug Administration recently gave approval to lenalidomide as maintenance after autologous hematopoietic stem cell transplantation. Accordingly, it’s important to understand the benefits of triplet therapies in patients progressing on lenalidomide therapy and in whom lenalidomide is no longer a treatment option, Dr. Richardson said.
Pomalidomide, a potent oral immunomodulatory agent, is already approved for relapsed/refractory myeloma after two or more previous therapies that include lenalidomide and a proteasome inhibitor in patients who progress on or within 60 days of treatment.
In the OPTIMISMM trial, 559 patients who had received prior therapy, including at least two cycles of lenalidomide, were randomized to receive either PVd or Vd until disease progression or unacceptable toxicity.
Median progression-free survival was 11.20 months for PVd versus 7.10 months for Vd (hazard ratio, 0.61; 95% confidence interval, 0.49-0.77; P less than .0001).
Progression-free survival results were “even more encouraging” in the subset of patients with only one prior line of therapy, Dr. Richardson said, reporting a median of 20.73 months for PVd versus 11.63 for Vd (HR, 0.54; 95% CI, 0.36-0.82; P = .0027).
The overall response rate was 82.2% for PVd versus 50.0% for Vd (P less than .001). In patients with only one prior line of therapy, the overall response rate was 90.1% and 54.8% for PVd and Vd, respectively (P less than .001).
The progression-free survival advantage occurred regardless of whether patients were refractory to lenalidomide, Dr. Richardson added. Median progression-free survival for PVd versus Vd was 9.53 and 5.59 months, respectively, in the lenalidomide-refractory patients (P less than .001) and 22.01 versus 11.63 months in non–lenalidomide refractory patients (P less than .001).
The side effect profile of PVd was “very much as expected,” with more neutropenia seen with the PVd than with Vd, though rates of febrile neutropenia were low, Dr. Richardson said. Likewise, the rate of infection was higher in the triplet arm, but it was generally manageable, he added.
Deep vein thrombosis and pulmonary embolism rates were low in both arms, as were the rates of secondary primary malignancies. Analysis of minimal residual disease and quality of life are ongoing.
PVd could “arguably be now an important treatment platform for future directions in combination with other strategies,” Dr. Richardson said.
The study was supported by Celgene. Dr. Richardson reported advisory board work for Celgene, Novartis, Oncopeptides, Janssen, Amgen, and Takeda, and research funding from Bristol-Myers Squibb, Celgene, and Takeda.
SOURCE: Richardson PG et al. ASCO 2018, Abstract 8001.
CHICAGO – For patients with relapsed/refractory multiple myeloma previously exposed to lenalidomide, the combination of pomalidomide plus bortezomib and low‐dose dexamethasone (PVd) improved response and progression-free survival, results of the phase 3 OPTIMISMM trial showed.
Risk of disease progression or death was reduced by 39%, compared with bortezomib and low-dose dexamethasone alone (Vd), among patients in the trial, of whom approximately 70% were lenalidomide refractory, reported investigator Paul G. Richardson, MD, of the Dana-Farber Cancer Institute in Boston.
The improvements in efficacy seen with the PVd regimen were more pronounced in patients with only one prior line of therapy, and overall, the safety profile of the triplet regimen was consistent with known toxicities of each individual agent, Dr. Richardson reported.
Together, those results “would seem to support the use of this triplet [therapy] in first relapse in patients with relapsed/refractory myeloma and prior exposure to lenalidomide,” he said at the annual meeting of the American Society of Clinical Oncology.
“This study, importantly, evaluated a clinically relevant patient population and a growing patient population who receive upfront lenalidomide and maintenance in that setting and for whom lenalidomide is no longer a viable treatment option,” Dr. Richardson added.
Lenalidomide has become a mainstay of upfront myeloma treatment, and the Food and Drug Administration recently gave approval to lenalidomide as maintenance after autologous hematopoietic stem cell transplantation. Accordingly, it’s important to understand the benefits of triplet therapies in patients progressing on lenalidomide therapy and in whom lenalidomide is no longer a treatment option, Dr. Richardson said.
Pomalidomide, a potent oral immunomodulatory agent, is already approved for relapsed/refractory myeloma after two or more previous therapies that include lenalidomide and a proteasome inhibitor in patients who progress on or within 60 days of treatment.
In the OPTIMISMM trial, 559 patients who had received prior therapy, including at least two cycles of lenalidomide, were randomized to receive either PVd or Vd until disease progression or unacceptable toxicity.
Median progression-free survival was 11.20 months for PVd versus 7.10 months for Vd (hazard ratio, 0.61; 95% confidence interval, 0.49-0.77; P less than .0001).
Progression-free survival results were “even more encouraging” in the subset of patients with only one prior line of therapy, Dr. Richardson said, reporting a median of 20.73 months for PVd versus 11.63 for Vd (HR, 0.54; 95% CI, 0.36-0.82; P = .0027).
The overall response rate was 82.2% for PVd versus 50.0% for Vd (P less than .001). In patients with only one prior line of therapy, the overall response rate was 90.1% and 54.8% for PVd and Vd, respectively (P less than .001).
The progression-free survival advantage occurred regardless of whether patients were refractory to lenalidomide, Dr. Richardson added. Median progression-free survival for PVd versus Vd was 9.53 and 5.59 months, respectively, in the lenalidomide-refractory patients (P less than .001) and 22.01 versus 11.63 months in non–lenalidomide refractory patients (P less than .001).
The side effect profile of PVd was “very much as expected,” with more neutropenia seen with the PVd than with Vd, though rates of febrile neutropenia were low, Dr. Richardson said. Likewise, the rate of infection was higher in the triplet arm, but it was generally manageable, he added.
Deep vein thrombosis and pulmonary embolism rates were low in both arms, as were the rates of secondary primary malignancies. Analysis of minimal residual disease and quality of life are ongoing.
PVd could “arguably be now an important treatment platform for future directions in combination with other strategies,” Dr. Richardson said.
The study was supported by Celgene. Dr. Richardson reported advisory board work for Celgene, Novartis, Oncopeptides, Janssen, Amgen, and Takeda, and research funding from Bristol-Myers Squibb, Celgene, and Takeda.
SOURCE: Richardson PG et al. ASCO 2018, Abstract 8001.
REPORTING FROM ASCO 2018
Key clinical point:
Major finding: Risk of disease progression or death was reduced by 39% with pomalidomide plus bortezomib and low‐dose dexamethasone (PVd), compared with use of bortezomib and low-dose dexamethasone alone (Vd).
Study details: The phase 3 OPTIMISMM trial including 559 patients who had received prior therapy with at least two cycles of lenalidomide.
Disclosures: The study was supported by Celgene. Dr. Richardson reported advisory board work for Celgene, Novartis, Oncopeptides, Janssen, Amgen, and Takeda and research funding from Bristol-Myers Squibb, Celgene, and Takeda.
Source: Richardson PG et al. ASCO 2018, Abstract 8001.
Screen sooner and more often for those with family history of CRC
WASHINGTON – The number of first- and second-degree relatives with colorectal cancer can increase an individual’s risk for CRC, which could require screening to be done more frequently.
“There have been multiple guidelines reported as to what should be done for these individuals,” Harminder Singh, MD, of the University of Manitoba and his associates stated. “However, for the most part, they have not systematically analyzed the data.” He went on to say that, “more importantly, there’s been no recent AGA [American Gastroenterological Association] or Canadian Association of Gastroenterology statement, which led the development of this guideline.”
To address this issue, Dr. Singh and his colleagues conducted a systematic review of 10 literature searches to answer the following five questions concerning colorectal risk and screening practices: What is the effect of a family history of CRC on an individual’s risk of CRC? What is the effect of a family history of adenoma on an individual’s risk of CRC? At what age should CRC screening begin? Which screening tests are optimal? What are the optimal testing intervals for people with a family history of CRC or adenoma?
These questions were developed via an iterative online platform and then further developed and voted on by a team of specialists. GRADE (Grading of Recommendation Assessment, Development and Evaluation) was used to assess the quality of evidence to support these questions.
Similarly, individuals with two or more first-degree relatives with CRC had a two- to fourfold increased risk of developing CRC, compared with the general population. The review also found that, of the 20 recommendation statements from the review panel, there was consensus about 19 of them.
Colorectal cancer screening is recommended for all individuals with a family history of CRC or documented adenoma. Similarly, colonoscopy is recommended as the preferred test for individuals at the highest risk– those with one or more affected first-degree relatives. Fecal immunochemistry tests are considered a viable alternative except in patients with two or more first-degree relatives.
If a patient is considered to have an elevated risk of CRC because of family history, then screening should begin when they are aged 10 years younger than when that first-degree relative was diagnosed, and a 5-year screening interval should be followed after that.
Dr. Singh pointed out that the age of the affected first-degree relative should be considered when weighing an individual’s related risk of developing CRC. For example, having an first-degree relative who is diagnosed after the age of 75 is not likely to elevate an individual’s risk of developing CRC. Individuals with one or more second-degree relatives with CRC or nonadvanced adenoma do not appear to have an elevated risk of developing CRC and should be screened according to average-risk guidelines.
Dr. Singh reported receiving funding for from Merck Canada.
SOURCE: Leddin D. Gastroenterology. 2018 Jun. doi: 10.1016/S0016-5085(18)31083-7.
WASHINGTON – The number of first- and second-degree relatives with colorectal cancer can increase an individual’s risk for CRC, which could require screening to be done more frequently.
“There have been multiple guidelines reported as to what should be done for these individuals,” Harminder Singh, MD, of the University of Manitoba and his associates stated. “However, for the most part, they have not systematically analyzed the data.” He went on to say that, “more importantly, there’s been no recent AGA [American Gastroenterological Association] or Canadian Association of Gastroenterology statement, which led the development of this guideline.”
To address this issue, Dr. Singh and his colleagues conducted a systematic review of 10 literature searches to answer the following five questions concerning colorectal risk and screening practices: What is the effect of a family history of CRC on an individual’s risk of CRC? What is the effect of a family history of adenoma on an individual’s risk of CRC? At what age should CRC screening begin? Which screening tests are optimal? What are the optimal testing intervals for people with a family history of CRC or adenoma?
These questions were developed via an iterative online platform and then further developed and voted on by a team of specialists. GRADE (Grading of Recommendation Assessment, Development and Evaluation) was used to assess the quality of evidence to support these questions.
Similarly, individuals with two or more first-degree relatives with CRC had a two- to fourfold increased risk of developing CRC, compared with the general population. The review also found that, of the 20 recommendation statements from the review panel, there was consensus about 19 of them.
Colorectal cancer screening is recommended for all individuals with a family history of CRC or documented adenoma. Similarly, colonoscopy is recommended as the preferred test for individuals at the highest risk– those with one or more affected first-degree relatives. Fecal immunochemistry tests are considered a viable alternative except in patients with two or more first-degree relatives.
If a patient is considered to have an elevated risk of CRC because of family history, then screening should begin when they are aged 10 years younger than when that first-degree relative was diagnosed, and a 5-year screening interval should be followed after that.
Dr. Singh pointed out that the age of the affected first-degree relative should be considered when weighing an individual’s related risk of developing CRC. For example, having an first-degree relative who is diagnosed after the age of 75 is not likely to elevate an individual’s risk of developing CRC. Individuals with one or more second-degree relatives with CRC or nonadvanced adenoma do not appear to have an elevated risk of developing CRC and should be screened according to average-risk guidelines.
Dr. Singh reported receiving funding for from Merck Canada.
SOURCE: Leddin D. Gastroenterology. 2018 Jun. doi: 10.1016/S0016-5085(18)31083-7.
WASHINGTON – The number of first- and second-degree relatives with colorectal cancer can increase an individual’s risk for CRC, which could require screening to be done more frequently.
“There have been multiple guidelines reported as to what should be done for these individuals,” Harminder Singh, MD, of the University of Manitoba and his associates stated. “However, for the most part, they have not systematically analyzed the data.” He went on to say that, “more importantly, there’s been no recent AGA [American Gastroenterological Association] or Canadian Association of Gastroenterology statement, which led the development of this guideline.”
To address this issue, Dr. Singh and his colleagues conducted a systematic review of 10 literature searches to answer the following five questions concerning colorectal risk and screening practices: What is the effect of a family history of CRC on an individual’s risk of CRC? What is the effect of a family history of adenoma on an individual’s risk of CRC? At what age should CRC screening begin? Which screening tests are optimal? What are the optimal testing intervals for people with a family history of CRC or adenoma?
These questions were developed via an iterative online platform and then further developed and voted on by a team of specialists. GRADE (Grading of Recommendation Assessment, Development and Evaluation) was used to assess the quality of evidence to support these questions.
Similarly, individuals with two or more first-degree relatives with CRC had a two- to fourfold increased risk of developing CRC, compared with the general population. The review also found that, of the 20 recommendation statements from the review panel, there was consensus about 19 of them.
Colorectal cancer screening is recommended for all individuals with a family history of CRC or documented adenoma. Similarly, colonoscopy is recommended as the preferred test for individuals at the highest risk– those with one or more affected first-degree relatives. Fecal immunochemistry tests are considered a viable alternative except in patients with two or more first-degree relatives.
If a patient is considered to have an elevated risk of CRC because of family history, then screening should begin when they are aged 10 years younger than when that first-degree relative was diagnosed, and a 5-year screening interval should be followed after that.
Dr. Singh pointed out that the age of the affected first-degree relative should be considered when weighing an individual’s related risk of developing CRC. For example, having an first-degree relative who is diagnosed after the age of 75 is not likely to elevate an individual’s risk of developing CRC. Individuals with one or more second-degree relatives with CRC or nonadvanced adenoma do not appear to have an elevated risk of developing CRC and should be screened according to average-risk guidelines.
Dr. Singh reported receiving funding for from Merck Canada.
SOURCE: Leddin D. Gastroenterology. 2018 Jun. doi: 10.1016/S0016-5085(18)31083-7.
REPORTING FROM DDW 2018
Key clinical point: Patients with 1 or more first-degree relative with CRC should be screened more often.
Major finding: Patients with one or more first-degree relatives with CRC or adenoma had a twofold greater risk of developing CRC, compared with those without a family history of these diseases.
Study details: A systematic review of 10 literature searches assessing risk of CRC in those with a family history of CRC.
Disclosures: Dr. Singh has received funding from Merck Canada.
Source: Leddin D. Gastroenterology. 2018 Jun. doi: 10.1016/S0016-5085(18)31083-7.
EULAR recommendations on steroids: ‘As necessary, but as little as possible’
AMSTERDAM – Glucocorticosteroids remain an important therapeutic option for many patients with rheumatic and nonrheumatic disease, but careful assessment of their relative benefits and risks needs to be considered when prescribing, according to an expert summary of currently available European League Against Rheumatism (EULAR) recommendations.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
From rheumatoid arthritis (RA) and polymyalgia rheumatica (PMR) to vasculitis, myositis, and even gout, steroids are widely used in the rheumatic diseases, said Frank Buttgereit, MD, during a final plenary session at the European Congress of Rheumatology.
“These are strong-acting, rapidly acting, efficacious drugs,” observed Dr. Buttgereit, who is a professor in the department of rheumatology at Charité–Universitätsmedizin Berlin. While effective at reducing inflammation and providing immunosuppression, they are, of course, not without their well-known risks. Some of the well-documented risks he pointed out were the development of osteoporosis, myopathy, and edema; the disruption of lipid and carbohydrate metabolism; and the risk of developing glaucoma and cataracts.
“This leads to the question on how to optimize the use of these drugs,” Dr. Buttgereit said. “EULAR is constantly working to improve its guidelines,” and updating these in line with the available evidence, he added. “The bottom line is always give as much as necessary but as little as possible.”
Over the past few years, EULAR’s Glucocorticoid Task Force has been reviewing and updating recommendations on the use of these drugs and it has published several important documents clarifying their use in RA and in PMR. The task force has also published a viewpoint article on the long-term use of steroids, defining the conditions where an “acceptably low level of harm” might exist to enable their continued use. There have also been separate recommendations, published in 2010, on how to monitor these drugs (Ann Rheum Dis. 2010;69[11]:1913-9).
Clarifying the role of steroids in rheumatoid arthritis
The latest (2016) EULAR recommendations on the use of glucocorticosteroids were published last year (Ann Rheum Dis. 2017;76[6]:960-77) and included an important adjustment on when they should be initially used in RA, Dr. Buttgereit explained. Previous recommendations had said that steroids could be combined with disease-modifying antirheumatic drugs (DMARDs) but had suggested that they be used at a low dose. Now the wording has changed to focus on short-term use rather than dosing.
“Glucocorticoids can be given initially at different dosages, and using different routes of administration,” he said in a video interview at the EULAR Congress. The practice on what dose to give varies from country to country, he noted, so the recommendations are now being less prescriptive.
“We have made it clear that glucocorticoids should really be used only when initiating conventional synthetic DMARDs, but not necessarily if you switch to biologics or targeted synthetics because usually the onset of their actions is pretty fast,” Dr. Buttgereit said.
One thing that hasn’t changed is that steroid should be tapered down as “rapidly as clinically feasible” until, ideally, their full withdrawal. Although there are cases when that might not be possible, and their long-term use might be warranted. This is when you get into discussion about the benefit-to-risk ratio, he said.
Steroids for polymyalgia rheumatica
Steroids may be used as monotherapy in patients with PMR, Dr. Buttgereit observed, which is in contrast to other conditions such as RA. Although the evidence for use of steroids in PMR is limited, the EULAR Glucocorticoid Task Force and American College of Rheumatology recommended (Ann Rheum Dis. 2015;74[10]:1799-807) using a starting dose of a prednisolone-equivalent dose between 12.5 and 25 mg/day, and if there is an improvement in few weeks, the dose can start to be reduced. Tapering should be rapid at first to bring the dose down to 10 mg/day and followed by a more gradual dose-reduction phase.
“So, you can see we are giving more or less precise recommendations on how to start, how to taper,” Dr. Buttgereit said.
Balancing long-term benefit vs. harm
Balancing the long-term benefits and risks of steroids in rheumatic disease was the focus of a EULAR viewpoint article published 3 years ago in 2015 (Ann Rheum Dis. 2015;75[6]:952-7).
Three main messages can be drawn out of this work, Dr. Buttgereit said.
First, treatment with steroids for 3-6 months is associated with more benefits than risks if doses of 5 mg/day or less are used. There is one important exception to this, however, and that is the use of steroids in patients with comorbid cardiovascular disease.
Second, using doses of 10 mg/day for long periods tips the balance toward more risks than benefits, and “this means you should avoid this.”
Third, doses of 5-10 mg/day may be appropriate, but there are certain patient factors that will influence the benefit-to-harm ratio that need to be considered. These include older age, smoking, high alcohol consumption, and poor nutrition. There are also factors that may help protect the patients from risk, such as early diagnosis, low disease activity, low cumulative dose of steroids, and a shorter duration of treatment.
“It’s not only the dose, it’s also the absence or presence of risk factors and/or preventive measures,” that’s important, Dr. Buttgereit said.
Dr. Buttgereit has received consultancy fees, honoraria, and/or travel expenses from Amgen, Horizon Pharma, Mundipharma, Roche, and Pfizer and grant or study support from Amgen, Mundipharma, and Pfizer.
SOURCE: Buttgereit F. EULAR 2018 Congress, Abstract SP160.
AMSTERDAM – Glucocorticosteroids remain an important therapeutic option for many patients with rheumatic and nonrheumatic disease, but careful assessment of their relative benefits and risks needs to be considered when prescribing, according to an expert summary of currently available European League Against Rheumatism (EULAR) recommendations.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
From rheumatoid arthritis (RA) and polymyalgia rheumatica (PMR) to vasculitis, myositis, and even gout, steroids are widely used in the rheumatic diseases, said Frank Buttgereit, MD, during a final plenary session at the European Congress of Rheumatology.
“These are strong-acting, rapidly acting, efficacious drugs,” observed Dr. Buttgereit, who is a professor in the department of rheumatology at Charité–Universitätsmedizin Berlin. While effective at reducing inflammation and providing immunosuppression, they are, of course, not without their well-known risks. Some of the well-documented risks he pointed out were the development of osteoporosis, myopathy, and edema; the disruption of lipid and carbohydrate metabolism; and the risk of developing glaucoma and cataracts.
“This leads to the question on how to optimize the use of these drugs,” Dr. Buttgereit said. “EULAR is constantly working to improve its guidelines,” and updating these in line with the available evidence, he added. “The bottom line is always give as much as necessary but as little as possible.”
Over the past few years, EULAR’s Glucocorticoid Task Force has been reviewing and updating recommendations on the use of these drugs and it has published several important documents clarifying their use in RA and in PMR. The task force has also published a viewpoint article on the long-term use of steroids, defining the conditions where an “acceptably low level of harm” might exist to enable their continued use. There have also been separate recommendations, published in 2010, on how to monitor these drugs (Ann Rheum Dis. 2010;69[11]:1913-9).
Clarifying the role of steroids in rheumatoid arthritis
The latest (2016) EULAR recommendations on the use of glucocorticosteroids were published last year (Ann Rheum Dis. 2017;76[6]:960-77) and included an important adjustment on when they should be initially used in RA, Dr. Buttgereit explained. Previous recommendations had said that steroids could be combined with disease-modifying antirheumatic drugs (DMARDs) but had suggested that they be used at a low dose. Now the wording has changed to focus on short-term use rather than dosing.
“Glucocorticoids can be given initially at different dosages, and using different routes of administration,” he said in a video interview at the EULAR Congress. The practice on what dose to give varies from country to country, he noted, so the recommendations are now being less prescriptive.
“We have made it clear that glucocorticoids should really be used only when initiating conventional synthetic DMARDs, but not necessarily if you switch to biologics or targeted synthetics because usually the onset of their actions is pretty fast,” Dr. Buttgereit said.
One thing that hasn’t changed is that steroid should be tapered down as “rapidly as clinically feasible” until, ideally, their full withdrawal. Although there are cases when that might not be possible, and their long-term use might be warranted. This is when you get into discussion about the benefit-to-risk ratio, he said.
Steroids for polymyalgia rheumatica
Steroids may be used as monotherapy in patients with PMR, Dr. Buttgereit observed, which is in contrast to other conditions such as RA. Although the evidence for use of steroids in PMR is limited, the EULAR Glucocorticoid Task Force and American College of Rheumatology recommended (Ann Rheum Dis. 2015;74[10]:1799-807) using a starting dose of a prednisolone-equivalent dose between 12.5 and 25 mg/day, and if there is an improvement in few weeks, the dose can start to be reduced. Tapering should be rapid at first to bring the dose down to 10 mg/day and followed by a more gradual dose-reduction phase.
“So, you can see we are giving more or less precise recommendations on how to start, how to taper,” Dr. Buttgereit said.
Balancing long-term benefit vs. harm
Balancing the long-term benefits and risks of steroids in rheumatic disease was the focus of a EULAR viewpoint article published 3 years ago in 2015 (Ann Rheum Dis. 2015;75[6]:952-7).
Three main messages can be drawn out of this work, Dr. Buttgereit said.
First, treatment with steroids for 3-6 months is associated with more benefits than risks if doses of 5 mg/day or less are used. There is one important exception to this, however, and that is the use of steroids in patients with comorbid cardiovascular disease.
Second, using doses of 10 mg/day for long periods tips the balance toward more risks than benefits, and “this means you should avoid this.”
Third, doses of 5-10 mg/day may be appropriate, but there are certain patient factors that will influence the benefit-to-harm ratio that need to be considered. These include older age, smoking, high alcohol consumption, and poor nutrition. There are also factors that may help protect the patients from risk, such as early diagnosis, low disease activity, low cumulative dose of steroids, and a shorter duration of treatment.
“It’s not only the dose, it’s also the absence or presence of risk factors and/or preventive measures,” that’s important, Dr. Buttgereit said.
Dr. Buttgereit has received consultancy fees, honoraria, and/or travel expenses from Amgen, Horizon Pharma, Mundipharma, Roche, and Pfizer and grant or study support from Amgen, Mundipharma, and Pfizer.
SOURCE: Buttgereit F. EULAR 2018 Congress, Abstract SP160.
AMSTERDAM – Glucocorticosteroids remain an important therapeutic option for many patients with rheumatic and nonrheumatic disease, but careful assessment of their relative benefits and risks needs to be considered when prescribing, according to an expert summary of currently available European League Against Rheumatism (EULAR) recommendations.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
From rheumatoid arthritis (RA) and polymyalgia rheumatica (PMR) to vasculitis, myositis, and even gout, steroids are widely used in the rheumatic diseases, said Frank Buttgereit, MD, during a final plenary session at the European Congress of Rheumatology.
“These are strong-acting, rapidly acting, efficacious drugs,” observed Dr. Buttgereit, who is a professor in the department of rheumatology at Charité–Universitätsmedizin Berlin. While effective at reducing inflammation and providing immunosuppression, they are, of course, not without their well-known risks. Some of the well-documented risks he pointed out were the development of osteoporosis, myopathy, and edema; the disruption of lipid and carbohydrate metabolism; and the risk of developing glaucoma and cataracts.
“This leads to the question on how to optimize the use of these drugs,” Dr. Buttgereit said. “EULAR is constantly working to improve its guidelines,” and updating these in line with the available evidence, he added. “The bottom line is always give as much as necessary but as little as possible.”
Over the past few years, EULAR’s Glucocorticoid Task Force has been reviewing and updating recommendations on the use of these drugs and it has published several important documents clarifying their use in RA and in PMR. The task force has also published a viewpoint article on the long-term use of steroids, defining the conditions where an “acceptably low level of harm” might exist to enable their continued use. There have also been separate recommendations, published in 2010, on how to monitor these drugs (Ann Rheum Dis. 2010;69[11]:1913-9).
Clarifying the role of steroids in rheumatoid arthritis
The latest (2016) EULAR recommendations on the use of glucocorticosteroids were published last year (Ann Rheum Dis. 2017;76[6]:960-77) and included an important adjustment on when they should be initially used in RA, Dr. Buttgereit explained. Previous recommendations had said that steroids could be combined with disease-modifying antirheumatic drugs (DMARDs) but had suggested that they be used at a low dose. Now the wording has changed to focus on short-term use rather than dosing.
“Glucocorticoids can be given initially at different dosages, and using different routes of administration,” he said in a video interview at the EULAR Congress. The practice on what dose to give varies from country to country, he noted, so the recommendations are now being less prescriptive.
“We have made it clear that glucocorticoids should really be used only when initiating conventional synthetic DMARDs, but not necessarily if you switch to biologics or targeted synthetics because usually the onset of their actions is pretty fast,” Dr. Buttgereit said.
One thing that hasn’t changed is that steroid should be tapered down as “rapidly as clinically feasible” until, ideally, their full withdrawal. Although there are cases when that might not be possible, and their long-term use might be warranted. This is when you get into discussion about the benefit-to-risk ratio, he said.
Steroids for polymyalgia rheumatica
Steroids may be used as monotherapy in patients with PMR, Dr. Buttgereit observed, which is in contrast to other conditions such as RA. Although the evidence for use of steroids in PMR is limited, the EULAR Glucocorticoid Task Force and American College of Rheumatology recommended (Ann Rheum Dis. 2015;74[10]:1799-807) using a starting dose of a prednisolone-equivalent dose between 12.5 and 25 mg/day, and if there is an improvement in few weeks, the dose can start to be reduced. Tapering should be rapid at first to bring the dose down to 10 mg/day and followed by a more gradual dose-reduction phase.
“So, you can see we are giving more or less precise recommendations on how to start, how to taper,” Dr. Buttgereit said.
Balancing long-term benefit vs. harm
Balancing the long-term benefits and risks of steroids in rheumatic disease was the focus of a EULAR viewpoint article published 3 years ago in 2015 (Ann Rheum Dis. 2015;75[6]:952-7).
Three main messages can be drawn out of this work, Dr. Buttgereit said.
First, treatment with steroids for 3-6 months is associated with more benefits than risks if doses of 5 mg/day or less are used. There is one important exception to this, however, and that is the use of steroids in patients with comorbid cardiovascular disease.
Second, using doses of 10 mg/day for long periods tips the balance toward more risks than benefits, and “this means you should avoid this.”
Third, doses of 5-10 mg/day may be appropriate, but there are certain patient factors that will influence the benefit-to-harm ratio that need to be considered. These include older age, smoking, high alcohol consumption, and poor nutrition. There are also factors that may help protect the patients from risk, such as early diagnosis, low disease activity, low cumulative dose of steroids, and a shorter duration of treatment.
“It’s not only the dose, it’s also the absence or presence of risk factors and/or preventive measures,” that’s important, Dr. Buttgereit said.
Dr. Buttgereit has received consultancy fees, honoraria, and/or travel expenses from Amgen, Horizon Pharma, Mundipharma, Roche, and Pfizer and grant or study support from Amgen, Mundipharma, and Pfizer.
SOURCE: Buttgereit F. EULAR 2018 Congress, Abstract SP160.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: When dosing glucocorticoids, give as much as necessary but as little as possible.
Major finding:
Disclosures: Dr. Buttgereit has received consultancy fees, honoraria, and/or travel expenses from Amgen, Horizon Pharma, Mundipharma, Roche, and Pfizer and grant or study support from Amgen, Mundipharma, and Pfizer.
Source: Buttgereit F. EULAR 2018 Congress. Abstract SP160.
Anthrax vaccine recommendations updated in the event of a wide-area release
at their meeting.
The recommendations to the committee sought to optimize the use of Anthrax Vaccine Adsorbed (AVA) in post-exposure prophylaxis (PEP) in the event of a wide-area release of Bacillus anthracis spores. In this event, a mass vaccination effort would be undertaken, requiring expedited administration of AVA. ACIP now recommends that the intramuscular administration may be used over the traditional subcutaneous approach if there are any operational or logistical challenges that delay effective vaccination. Another recommendation from ACIP would allow two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. The committee also recommended that AbxPEP, an antimicrobial, be stopped 42 days after the first dose of AVA or 2 weeks after the last dose.
William A. Bower, MD, of the division of high-consequence pathogens and pathology at the Centers for Disease Control and Prevention, and the anthrax work group looked at three nonhuman primate studies and eight human immunogenicity and adverse event studies during the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE). All of the animal studies were used to predict human survival by vaccinating the nonhuman primates with AVA, then challenging them with B. anthracis. Using animal studies to predict human survival is common practice under the “animal rule.”
When Dr. Bower and the work group assessed the studies comparing intramuscular administration with subcutaneous administration of AVA, they rated the overall evidence as GRADE 2. However, they rated the adverse events data as GRADE 1.
Dr. Bower and his colleagues also reviewed dose-sparing studies to identify the feasibility of allowing two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. For these studies, the anthrax work group gave a GRADE score of 2.
The overall evidence score for microbial duration for PEP was judged to be GRADE 2.
“These forthcoming recommendations will be used by the CDC to inform state and local health departments to better prepare for an emergency response to a wide-area release of Bacillus anthracis spores,” said Dr. Bower.
The committee’s recommendations must be approved by the CDC’s director before they are considered official recommendations.
Dr. Bower did not report any relevant financial conflicts of interest.
at their meeting.
The recommendations to the committee sought to optimize the use of Anthrax Vaccine Adsorbed (AVA) in post-exposure prophylaxis (PEP) in the event of a wide-area release of Bacillus anthracis spores. In this event, a mass vaccination effort would be undertaken, requiring expedited administration of AVA. ACIP now recommends that the intramuscular administration may be used over the traditional subcutaneous approach if there are any operational or logistical challenges that delay effective vaccination. Another recommendation from ACIP would allow two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. The committee also recommended that AbxPEP, an antimicrobial, be stopped 42 days after the first dose of AVA or 2 weeks after the last dose.
William A. Bower, MD, of the division of high-consequence pathogens and pathology at the Centers for Disease Control and Prevention, and the anthrax work group looked at three nonhuman primate studies and eight human immunogenicity and adverse event studies during the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE). All of the animal studies were used to predict human survival by vaccinating the nonhuman primates with AVA, then challenging them with B. anthracis. Using animal studies to predict human survival is common practice under the “animal rule.”
When Dr. Bower and the work group assessed the studies comparing intramuscular administration with subcutaneous administration of AVA, they rated the overall evidence as GRADE 2. However, they rated the adverse events data as GRADE 1.
Dr. Bower and his colleagues also reviewed dose-sparing studies to identify the feasibility of allowing two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. For these studies, the anthrax work group gave a GRADE score of 2.
The overall evidence score for microbial duration for PEP was judged to be GRADE 2.
“These forthcoming recommendations will be used by the CDC to inform state and local health departments to better prepare for an emergency response to a wide-area release of Bacillus anthracis spores,” said Dr. Bower.
The committee’s recommendations must be approved by the CDC’s director before they are considered official recommendations.
Dr. Bower did not report any relevant financial conflicts of interest.
at their meeting.
The recommendations to the committee sought to optimize the use of Anthrax Vaccine Adsorbed (AVA) in post-exposure prophylaxis (PEP) in the event of a wide-area release of Bacillus anthracis spores. In this event, a mass vaccination effort would be undertaken, requiring expedited administration of AVA. ACIP now recommends that the intramuscular administration may be used over the traditional subcutaneous approach if there are any operational or logistical challenges that delay effective vaccination. Another recommendation from ACIP would allow two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. The committee also recommended that AbxPEP, an antimicrobial, be stopped 42 days after the first dose of AVA or 2 weeks after the last dose.
William A. Bower, MD, of the division of high-consequence pathogens and pathology at the Centers for Disease Control and Prevention, and the anthrax work group looked at three nonhuman primate studies and eight human immunogenicity and adverse event studies during the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE). All of the animal studies were used to predict human survival by vaccinating the nonhuman primates with AVA, then challenging them with B. anthracis. Using animal studies to predict human survival is common practice under the “animal rule.”
When Dr. Bower and the work group assessed the studies comparing intramuscular administration with subcutaneous administration of AVA, they rated the overall evidence as GRADE 2. However, they rated the adverse events data as GRADE 1.
Dr. Bower and his colleagues also reviewed dose-sparing studies to identify the feasibility of allowing two full doses or three half doses of AVA to be used to expand vaccine coverage for PEP in the event there is an inadequate vaccine supply. For these studies, the anthrax work group gave a GRADE score of 2.
The overall evidence score for microbial duration for PEP was judged to be GRADE 2.
“These forthcoming recommendations will be used by the CDC to inform state and local health departments to better prepare for an emergency response to a wide-area release of Bacillus anthracis spores,” said Dr. Bower.
The committee’s recommendations must be approved by the CDC’s director before they are considered official recommendations.
Dr. Bower did not report any relevant financial conflicts of interest.
REPORTING FROM AN ACIP MEETING
Voxelotor cut transfusions in compassionate use sickle cell cohort
WASHINGTON – An investigational drug for sickle cell disease (SCD) boosted hemoglobin levels while reducing hospitalizations and transfusion needs by approximately two-thirds in a small cohort of severely affected patients.
Lanetta Bronté, MD, who presented the findings at the annual meeting of the Foundation for Sickle Cell Disease Research, reviewed the FDA’s requirements for allowing expanded access to an investigational drug. The key point, she said, is that expanded access may be granted “for treatment of patients with serious or immediately life-threatening diseases or conditions that lack therapeutic alternatives.” And, she said, the potential for benefit should outweigh potential risk of taking the investigational drug.
Current treatments don’t really address serious disease-related complications for patients with advanced SCD, she said. Furthermore, these patients will often be excluded from clinical trials of new SCD therapies.
Voxelotor is a novel small molecule that stabilizes the sickle hemoglobin molecule as a monomer in its high oxygen state. Thus, polymerization of the hemoglobin molecules is inhibited, which decreases the amount of red blood cell damage. Other beneficial effects of voxelotor include improved rheology and reduced hemolysis, as well as a boost to the oxygen-carrying capacity of the sickle hemoglobin molecules, Dr. Bronté explained.
For the seven patients in Dr. Bronté’s clinic who were granted expanded access to voxelator, the disease burden of their end-stage SCD was heavy. All participants had iron overload, five were receiving frequent transfusions, two required chronic oxygen supplementation, and four had severe fatigue. One patient had end-stage renal disease, and another had experienced multiple organ failure and prolonged hospitalizations.
None of the patients qualified for participation in ongoing clinical trials of voxelotor, said Dr. Bronté, who is president of the Foundation for Sickle Cell Disease Research. She also maintains a private practice in Hollywood, Fla.
The four women and three men, aged 22-67 years, were treated with voxelotor for a range of 6-17 months under the FDA’s Expanded Access Program. All patients saw rapid increases in serum hemoglobin, with increases of at least 1 g/dL in five of the seven. Across the participants, increases ranged from 0.5-5.4 g/dL at 24 weeks, up from baseline values of 5.2-7.8 g/dL.
One marker of clinical efficacy that Dr. Bronté and her coauthors examined was the number of hospitalizations for pain from vaso-occlusive crises (VOCs). In the 24 weeks before beginning voxelotor, participants had a summed total of 28 hospitalizations. In the first 24 weeks of treatment, there were a total of nine VOC-related hospitalizations among the participants, a 67% decrease.
The total number of red blood cell transfusions required by the study population declined by a similar proportion, from 33 during the 24 weeks before voxelotor treatment to 13 during the first 24 weeks of treatment, a decrease of 60%.
Individual patients saw improvements related to some of their most troublesome SCD complications, Dr. Bronté reported. All four patients whose oxygen saturation levels had been below 95% on room air saw oxygen saturations improve to 98%-99% on voxelotor. The two patients who had moderate or moderately severe depression, as assessed by the Patient Health Questionnaire 9-item, had minimal or no depression on retest after 24 weeks of voxelotor treatment.
Voxelotor was generally well tolerated at a 900-mg once-daily oral dose. One patient developed grade 2 diarrhea after increasing the dose to 1,500 mg, but symptoms resolved after returning to the 900-mg dose. Another patient had transient mild diarrhea on 900 mg of voxelotor; the symptoms resolved without changing or stopping the drug, Dr. Bronté said. There were no serious treatment-related adverse events.
Among this seriously ill population, two patients died after beginning voxelotor treatment, but both deaths were judged to be unrelated to the treatment. Dr. Bronté reported that the two deceased patients, one of whom was on voxelotor for 16 months and the other for 7 months, did experience reduced transfusion needs and reduced VOC-related hospitalizations while on the drug, experiences that were similar to the surviving members of the cohort.
“Voxelotor administered via compassionate use demonstrated large improvements in anemia and hemolysis, including in patients with lower baseline hemoglobin than studied in clinical trials to date,” Dr. Bronté said.
Taken together with clinical improvements and improved patient-focused outcomes among a severely affected population, “these data … support ongoing investigation in controlled clinical trials to confirm the benefits of voxelotor in a broad range of patients with SCD,” she said.
The study was supported by Global Blood Therapeutics, the manufacturer of voxelotor. Dr. Bronté reported having no other conflicts of interest.
WASHINGTON – An investigational drug for sickle cell disease (SCD) boosted hemoglobin levels while reducing hospitalizations and transfusion needs by approximately two-thirds in a small cohort of severely affected patients.
Lanetta Bronté, MD, who presented the findings at the annual meeting of the Foundation for Sickle Cell Disease Research, reviewed the FDA’s requirements for allowing expanded access to an investigational drug. The key point, she said, is that expanded access may be granted “for treatment of patients with serious or immediately life-threatening diseases or conditions that lack therapeutic alternatives.” And, she said, the potential for benefit should outweigh potential risk of taking the investigational drug.
Current treatments don’t really address serious disease-related complications for patients with advanced SCD, she said. Furthermore, these patients will often be excluded from clinical trials of new SCD therapies.
Voxelotor is a novel small molecule that stabilizes the sickle hemoglobin molecule as a monomer in its high oxygen state. Thus, polymerization of the hemoglobin molecules is inhibited, which decreases the amount of red blood cell damage. Other beneficial effects of voxelotor include improved rheology and reduced hemolysis, as well as a boost to the oxygen-carrying capacity of the sickle hemoglobin molecules, Dr. Bronté explained.
For the seven patients in Dr. Bronté’s clinic who were granted expanded access to voxelator, the disease burden of their end-stage SCD was heavy. All participants had iron overload, five were receiving frequent transfusions, two required chronic oxygen supplementation, and four had severe fatigue. One patient had end-stage renal disease, and another had experienced multiple organ failure and prolonged hospitalizations.
None of the patients qualified for participation in ongoing clinical trials of voxelotor, said Dr. Bronté, who is president of the Foundation for Sickle Cell Disease Research. She also maintains a private practice in Hollywood, Fla.
The four women and three men, aged 22-67 years, were treated with voxelotor for a range of 6-17 months under the FDA’s Expanded Access Program. All patients saw rapid increases in serum hemoglobin, with increases of at least 1 g/dL in five of the seven. Across the participants, increases ranged from 0.5-5.4 g/dL at 24 weeks, up from baseline values of 5.2-7.8 g/dL.
One marker of clinical efficacy that Dr. Bronté and her coauthors examined was the number of hospitalizations for pain from vaso-occlusive crises (VOCs). In the 24 weeks before beginning voxelotor, participants had a summed total of 28 hospitalizations. In the first 24 weeks of treatment, there were a total of nine VOC-related hospitalizations among the participants, a 67% decrease.
The total number of red blood cell transfusions required by the study population declined by a similar proportion, from 33 during the 24 weeks before voxelotor treatment to 13 during the first 24 weeks of treatment, a decrease of 60%.
Individual patients saw improvements related to some of their most troublesome SCD complications, Dr. Bronté reported. All four patients whose oxygen saturation levels had been below 95% on room air saw oxygen saturations improve to 98%-99% on voxelotor. The two patients who had moderate or moderately severe depression, as assessed by the Patient Health Questionnaire 9-item, had minimal or no depression on retest after 24 weeks of voxelotor treatment.
Voxelotor was generally well tolerated at a 900-mg once-daily oral dose. One patient developed grade 2 diarrhea after increasing the dose to 1,500 mg, but symptoms resolved after returning to the 900-mg dose. Another patient had transient mild diarrhea on 900 mg of voxelotor; the symptoms resolved without changing or stopping the drug, Dr. Bronté said. There were no serious treatment-related adverse events.
Among this seriously ill population, two patients died after beginning voxelotor treatment, but both deaths were judged to be unrelated to the treatment. Dr. Bronté reported that the two deceased patients, one of whom was on voxelotor for 16 months and the other for 7 months, did experience reduced transfusion needs and reduced VOC-related hospitalizations while on the drug, experiences that were similar to the surviving members of the cohort.
“Voxelotor administered via compassionate use demonstrated large improvements in anemia and hemolysis, including in patients with lower baseline hemoglobin than studied in clinical trials to date,” Dr. Bronté said.
Taken together with clinical improvements and improved patient-focused outcomes among a severely affected population, “these data … support ongoing investigation in controlled clinical trials to confirm the benefits of voxelotor in a broad range of patients with SCD,” she said.
The study was supported by Global Blood Therapeutics, the manufacturer of voxelotor. Dr. Bronté reported having no other conflicts of interest.
WASHINGTON – An investigational drug for sickle cell disease (SCD) boosted hemoglobin levels while reducing hospitalizations and transfusion needs by approximately two-thirds in a small cohort of severely affected patients.
Lanetta Bronté, MD, who presented the findings at the annual meeting of the Foundation for Sickle Cell Disease Research, reviewed the FDA’s requirements for allowing expanded access to an investigational drug. The key point, she said, is that expanded access may be granted “for treatment of patients with serious or immediately life-threatening diseases or conditions that lack therapeutic alternatives.” And, she said, the potential for benefit should outweigh potential risk of taking the investigational drug.
Current treatments don’t really address serious disease-related complications for patients with advanced SCD, she said. Furthermore, these patients will often be excluded from clinical trials of new SCD therapies.
Voxelotor is a novel small molecule that stabilizes the sickle hemoglobin molecule as a monomer in its high oxygen state. Thus, polymerization of the hemoglobin molecules is inhibited, which decreases the amount of red blood cell damage. Other beneficial effects of voxelotor include improved rheology and reduced hemolysis, as well as a boost to the oxygen-carrying capacity of the sickle hemoglobin molecules, Dr. Bronté explained.
For the seven patients in Dr. Bronté’s clinic who were granted expanded access to voxelator, the disease burden of their end-stage SCD was heavy. All participants had iron overload, five were receiving frequent transfusions, two required chronic oxygen supplementation, and four had severe fatigue. One patient had end-stage renal disease, and another had experienced multiple organ failure and prolonged hospitalizations.
None of the patients qualified for participation in ongoing clinical trials of voxelotor, said Dr. Bronté, who is president of the Foundation for Sickle Cell Disease Research. She also maintains a private practice in Hollywood, Fla.
The four women and three men, aged 22-67 years, were treated with voxelotor for a range of 6-17 months under the FDA’s Expanded Access Program. All patients saw rapid increases in serum hemoglobin, with increases of at least 1 g/dL in five of the seven. Across the participants, increases ranged from 0.5-5.4 g/dL at 24 weeks, up from baseline values of 5.2-7.8 g/dL.
One marker of clinical efficacy that Dr. Bronté and her coauthors examined was the number of hospitalizations for pain from vaso-occlusive crises (VOCs). In the 24 weeks before beginning voxelotor, participants had a summed total of 28 hospitalizations. In the first 24 weeks of treatment, there were a total of nine VOC-related hospitalizations among the participants, a 67% decrease.
The total number of red blood cell transfusions required by the study population declined by a similar proportion, from 33 during the 24 weeks before voxelotor treatment to 13 during the first 24 weeks of treatment, a decrease of 60%.
Individual patients saw improvements related to some of their most troublesome SCD complications, Dr. Bronté reported. All four patients whose oxygen saturation levels had been below 95% on room air saw oxygen saturations improve to 98%-99% on voxelotor. The two patients who had moderate or moderately severe depression, as assessed by the Patient Health Questionnaire 9-item, had minimal or no depression on retest after 24 weeks of voxelotor treatment.
Voxelotor was generally well tolerated at a 900-mg once-daily oral dose. One patient developed grade 2 diarrhea after increasing the dose to 1,500 mg, but symptoms resolved after returning to the 900-mg dose. Another patient had transient mild diarrhea on 900 mg of voxelotor; the symptoms resolved without changing or stopping the drug, Dr. Bronté said. There were no serious treatment-related adverse events.
Among this seriously ill population, two patients died after beginning voxelotor treatment, but both deaths were judged to be unrelated to the treatment. Dr. Bronté reported that the two deceased patients, one of whom was on voxelotor for 16 months and the other for 7 months, did experience reduced transfusion needs and reduced VOC-related hospitalizations while on the drug, experiences that were similar to the surviving members of the cohort.
“Voxelotor administered via compassionate use demonstrated large improvements in anemia and hemolysis, including in patients with lower baseline hemoglobin than studied in clinical trials to date,” Dr. Bronté said.
Taken together with clinical improvements and improved patient-focused outcomes among a severely affected population, “these data … support ongoing investigation in controlled clinical trials to confirm the benefits of voxelotor in a broad range of patients with SCD,” she said.
The study was supported by Global Blood Therapeutics, the manufacturer of voxelotor. Dr. Bronté reported having no other conflicts of interest.
REPORTING FROM FSCDR 2018
Key clinical point:
Major finding: Transfusion requirements were cut by 60% in the first 24 weeks on voxelotor.
Study details: Open label case series of seven patients with end-stage SCD at a single center.
Disclosures: The study was funded by Global Blood Therapeutics, which manufactures voxelotor. Dr. Bronté reported having no other conflicts of interest.
In JIA, etanercept associated with lower uveitis risk compared with methotrexate
AMSTERDAM – Etanercept does not appear to increase the risk of uveitis relative to methotrexate in children with juvenile idiopathic arthritis (JIA), according to a large retrospective cohort study presented at the EULAR 2018 Congress.
“When we added in a fully adjusted hazard ratio, it showed a lower risk in the development of uveitis in patients on etanercept [compared with methotrexate],” reported Rebecca Davies, a research assistant at the University of Manchester (England).
These data were characterized as reassuring for JIA patients being considered for etanercept, but Ms. Davies was cautious about suggesting that etanercept has a protective effect. Even though hazard ratios were calculated with propensity-adjusted Cox regression analyses, Ms. Davies believes the best interpretation of these data is that etanercept therapy is not likely to contribute significantly to the risk of uveitis.
The substantial differences between the comparator groups provide one reason to refrain from speculating that etanercept is protective against uveitis. The risk of uveitis is greater both in younger patients and in the first year after diagnosis. Patients in the etanercept group were older than patients in the methotrexate group and they started etanercept a longer time after the JIA diagnosis.
“The initiation of etanercept later in the disease may mean that more of these patients had already passed through the window of greatest risk,” Ms. Davies explained.
The data for this study were drawn from a British Society for Pediatric and Adolescent Rheumatology cohort registry. Confined to patients first starting as opposed to restarting therapy, 1,009 patients initiating etanercept were compared to 508 patients initiating methotrexate.
In addition to an older age (11 vs. 9 years) and disease duration at start of therapy (3 vs. 1 years), a lower proportion of patients in the etanercept group had a persistent oligoarthritis subtype (5% vs. 17%). During follow-up, there were 15 cases (0.15%) of uveitis in the etanercept group and 18 (3.5%) in the methotrexate group.
The crude incidence of uveitis was 0.6 per 100 patient-years for etanercept versus 2.4 per 100 patient-years for methotrexate, according to Ms. Davies. After adjustment for a broad number of variables, including age, gender, disease scores, disease duration, baseline steroid use, and the presence of comorbidities, there was still a 70% lower risk of uveitis among those treated with etanercept (hazard ratio 0.30; 95% confidence interval, 0.1-0.9).
The low relative rate of uveitis after starting etanercept is discordant with several previous studies, according to Ms. Davies. In two retrospective studies conducted in the United States and one in Canada, etanercept treatment was associated with higher rates of uveitis than other tumor necrosis factor inhibitors. In a German study, etanercept was associated with a higher risk of uveitis than that of methotrexate.
Although a highly effective anti-inflammatory agent such as etanercept might be expected to have a protective effect against uveitis, at least relative to methotrexate, Ms. Davies suggested that the previous reports of potential causal association and the limitations of this retrospective analysis require a more cautious interpretation.
“It is possible that those considered to be at high risk of developing uveitis were kept away from etanercept,” said Ms. Davis, providing one of several explanations why skepticism is needed in regard to assuming uveitis protection from etanercept.
With up to 1 in 10 patients with JIA eventually developing sight-threatening uveitis, risk management is a priority, according to Ms. Davies. Current guidelines in the United Kingdom call for an ophthalmologist consult within 6 weeks of a diagnosis. Although several risk factors for uveitis have been published, the goal of this study was to determine whether exposure to etanercept is among these risks. According to Ms. Davies, these data suggest that this is not the case, but prospective studies comparing etanercept to other biologics would be particularly helpful in determining which therapy is most appropriate in order to reduce uveitis risk.
SOURCE: EULAR 2018 Congress. Abstract OP0351.
AMSTERDAM – Etanercept does not appear to increase the risk of uveitis relative to methotrexate in children with juvenile idiopathic arthritis (JIA), according to a large retrospective cohort study presented at the EULAR 2018 Congress.
“When we added in a fully adjusted hazard ratio, it showed a lower risk in the development of uveitis in patients on etanercept [compared with methotrexate],” reported Rebecca Davies, a research assistant at the University of Manchester (England).
These data were characterized as reassuring for JIA patients being considered for etanercept, but Ms. Davies was cautious about suggesting that etanercept has a protective effect. Even though hazard ratios were calculated with propensity-adjusted Cox regression analyses, Ms. Davies believes the best interpretation of these data is that etanercept therapy is not likely to contribute significantly to the risk of uveitis.
The substantial differences between the comparator groups provide one reason to refrain from speculating that etanercept is protective against uveitis. The risk of uveitis is greater both in younger patients and in the first year after diagnosis. Patients in the etanercept group were older than patients in the methotrexate group and they started etanercept a longer time after the JIA diagnosis.
“The initiation of etanercept later in the disease may mean that more of these patients had already passed through the window of greatest risk,” Ms. Davies explained.
The data for this study were drawn from a British Society for Pediatric and Adolescent Rheumatology cohort registry. Confined to patients first starting as opposed to restarting therapy, 1,009 patients initiating etanercept were compared to 508 patients initiating methotrexate.
In addition to an older age (11 vs. 9 years) and disease duration at start of therapy (3 vs. 1 years), a lower proportion of patients in the etanercept group had a persistent oligoarthritis subtype (5% vs. 17%). During follow-up, there were 15 cases (0.15%) of uveitis in the etanercept group and 18 (3.5%) in the methotrexate group.
The crude incidence of uveitis was 0.6 per 100 patient-years for etanercept versus 2.4 per 100 patient-years for methotrexate, according to Ms. Davies. After adjustment for a broad number of variables, including age, gender, disease scores, disease duration, baseline steroid use, and the presence of comorbidities, there was still a 70% lower risk of uveitis among those treated with etanercept (hazard ratio 0.30; 95% confidence interval, 0.1-0.9).
The low relative rate of uveitis after starting etanercept is discordant with several previous studies, according to Ms. Davies. In two retrospective studies conducted in the United States and one in Canada, etanercept treatment was associated with higher rates of uveitis than other tumor necrosis factor inhibitors. In a German study, etanercept was associated with a higher risk of uveitis than that of methotrexate.
Although a highly effective anti-inflammatory agent such as etanercept might be expected to have a protective effect against uveitis, at least relative to methotrexate, Ms. Davies suggested that the previous reports of potential causal association and the limitations of this retrospective analysis require a more cautious interpretation.
“It is possible that those considered to be at high risk of developing uveitis were kept away from etanercept,” said Ms. Davis, providing one of several explanations why skepticism is needed in regard to assuming uveitis protection from etanercept.
With up to 1 in 10 patients with JIA eventually developing sight-threatening uveitis, risk management is a priority, according to Ms. Davies. Current guidelines in the United Kingdom call for an ophthalmologist consult within 6 weeks of a diagnosis. Although several risk factors for uveitis have been published, the goal of this study was to determine whether exposure to etanercept is among these risks. According to Ms. Davies, these data suggest that this is not the case, but prospective studies comparing etanercept to other biologics would be particularly helpful in determining which therapy is most appropriate in order to reduce uveitis risk.
SOURCE: EULAR 2018 Congress. Abstract OP0351.
AMSTERDAM – Etanercept does not appear to increase the risk of uveitis relative to methotrexate in children with juvenile idiopathic arthritis (JIA), according to a large retrospective cohort study presented at the EULAR 2018 Congress.
“When we added in a fully adjusted hazard ratio, it showed a lower risk in the development of uveitis in patients on etanercept [compared with methotrexate],” reported Rebecca Davies, a research assistant at the University of Manchester (England).
These data were characterized as reassuring for JIA patients being considered for etanercept, but Ms. Davies was cautious about suggesting that etanercept has a protective effect. Even though hazard ratios were calculated with propensity-adjusted Cox regression analyses, Ms. Davies believes the best interpretation of these data is that etanercept therapy is not likely to contribute significantly to the risk of uveitis.
The substantial differences between the comparator groups provide one reason to refrain from speculating that etanercept is protective against uveitis. The risk of uveitis is greater both in younger patients and in the first year after diagnosis. Patients in the etanercept group were older than patients in the methotrexate group and they started etanercept a longer time after the JIA diagnosis.
“The initiation of etanercept later in the disease may mean that more of these patients had already passed through the window of greatest risk,” Ms. Davies explained.
The data for this study were drawn from a British Society for Pediatric and Adolescent Rheumatology cohort registry. Confined to patients first starting as opposed to restarting therapy, 1,009 patients initiating etanercept were compared to 508 patients initiating methotrexate.
In addition to an older age (11 vs. 9 years) and disease duration at start of therapy (3 vs. 1 years), a lower proportion of patients in the etanercept group had a persistent oligoarthritis subtype (5% vs. 17%). During follow-up, there were 15 cases (0.15%) of uveitis in the etanercept group and 18 (3.5%) in the methotrexate group.
The crude incidence of uveitis was 0.6 per 100 patient-years for etanercept versus 2.4 per 100 patient-years for methotrexate, according to Ms. Davies. After adjustment for a broad number of variables, including age, gender, disease scores, disease duration, baseline steroid use, and the presence of comorbidities, there was still a 70% lower risk of uveitis among those treated with etanercept (hazard ratio 0.30; 95% confidence interval, 0.1-0.9).
The low relative rate of uveitis after starting etanercept is discordant with several previous studies, according to Ms. Davies. In two retrospective studies conducted in the United States and one in Canada, etanercept treatment was associated with higher rates of uveitis than other tumor necrosis factor inhibitors. In a German study, etanercept was associated with a higher risk of uveitis than that of methotrexate.
Although a highly effective anti-inflammatory agent such as etanercept might be expected to have a protective effect against uveitis, at least relative to methotrexate, Ms. Davies suggested that the previous reports of potential causal association and the limitations of this retrospective analysis require a more cautious interpretation.
“It is possible that those considered to be at high risk of developing uveitis were kept away from etanercept,” said Ms. Davis, providing one of several explanations why skepticism is needed in regard to assuming uveitis protection from etanercept.
With up to 1 in 10 patients with JIA eventually developing sight-threatening uveitis, risk management is a priority, according to Ms. Davies. Current guidelines in the United Kingdom call for an ophthalmologist consult within 6 weeks of a diagnosis. Although several risk factors for uveitis have been published, the goal of this study was to determine whether exposure to etanercept is among these risks. According to Ms. Davies, these data suggest that this is not the case, but prospective studies comparing etanercept to other biologics would be particularly helpful in determining which therapy is most appropriate in order to reduce uveitis risk.
SOURCE: EULAR 2018 Congress. Abstract OP0351.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point:
Major finding: In children on new medication, the incidence of uveitis per 100 patients was 0.6 for etanercept and 2.4 for methotrexate.
Study details: Retrospective cohort registry.
Disclosures: The study was not funded by industry. Ms. Davies reports no potential conflicts of interest.
Source: EULAR 2018 Congress. Abstract OP0351.
Ob.gyn. workforce shortage looms
The average age of ob.gyns. is rising across the country, signaling physician shortages that are projected to worsen over the next several decades. The pinch may be felt especially in areas with high birth rates and few younger ob.gyns., according to a report from Doximity.
Doximity’s geographic projections paint a fine-grained picture in agreement with the American College of Obstetricians and Gynecologists’ projections of a national shortfall of up to 8,800 ob.gyns. by 2020, with the deficit of ob.gyns. potentially climbing to 22,000 by 2050.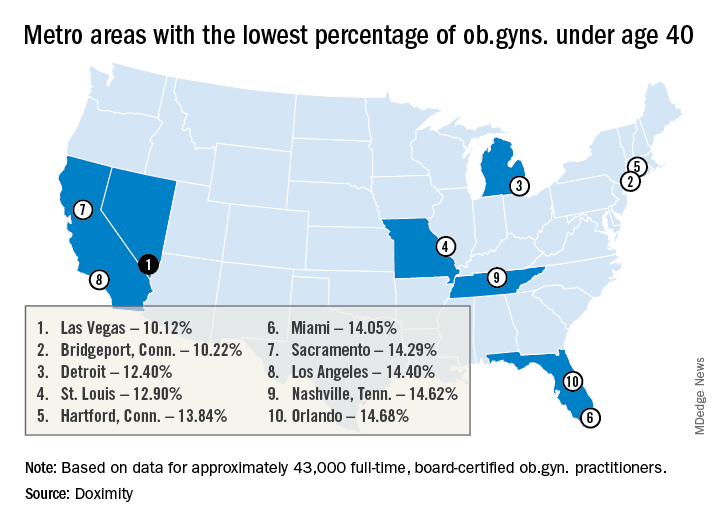
Pittsburgh and Bridgeport, Conn. top the list of metropolitan areas with the oldest average age of practicing ob.gyns., with average ages of 52.32 and 52.12 years, respectively. Las Vegas, Detroit, Miami, Los Angeles, New York, Boston, and Chicago all also made the top 15 cities in this list.
Because ob.gyns. start to leave the work force at age 59 years and retire at a median age of 64 years, this aging physician population could affect access to women’s health services within the decade, said Dr. Whaley. The fact that the burnout rate for ob.gyns. is only topped by emergency room physicians represents another area for concern, said Dr. Whaley of the University of California, Berkeley.
The report drew on data from the Centers for Medicare and Medicaid Services and board certification data. Doximity’s self-reported information from about 43,000 full-time board-certified ob.gyns. was also factored in. The top metropolitan areas were determined by 2010 census data and birth statistics came from federal databases.
With the data sliced another way, Pittsburgh also had the highest percentage of ob.gyns. aged 55 years or older, with 41.92% falling into this age group. Of the 50 metropolitan areas included in the Doximity report, 32 have an ob.gyn. workforce with at least one-third aged 55 years and up.
And there are many metropolitan areas where there’s a paucity of young ob.gyns., signaling future serious shortages, according to Doximity. In 12 of the metropolitan areas studied, less than 15% of the ob.gyn. workforce is aged 40 years or younger. Some of the cities with a potential “double hit” of older ob.gyns. and few younger physicians entering the profession include Las Vegas, Miami, Los Angeles, New York, and Chicago.
The Doximity methodology also took into account the average workload, in terms of the number of live births per physician per year, for ob.gyns. in the various metropolitan areas. There was a large variation in workload calculated this way: St. Louis has 247 live births per ob.gyn per year, while Louisville, Ky., has just 64, according to the report.
Factoring in workload information as well, the report gives a “shortage risk index” listing. Here, Las Vegas, Los Angeles, and Miami top the list. Cities considered at lowest risk for shortages according to this methodology include Baltimore, Denver, Portland, Ore., and Cleveland.
According to a 2017 ACOG workforce report, though women make up nearly half of those entering medical school, over four in five physicians entering ob.gyn. residencies (82.3% in 2016) are women. Further, women made up 58.7% of the ob.gyns. in active practice in 2017, outstripping all other surgical and medical specialties save pediatrics. Within 10 years, two-thirds of all ob.gyns. will be female, according to ACOG.
“While this study cannot determine causation for the variation in workloads, compensation, or shortages across metropolitan areas, we hope it will continue to serve as a baseline for the size of the challenge and prove helpful to health care employers, policymakers, patient advocates, and others interested in further study of this topic,” wrote Dr. Whaley and the report’s coauthors. “This information may also be helpful for ob.gyns. looking to live in areas with an increasing need for their expertise,” they said.
Dr. Whaley has received consulting fees from Castlight Health, Crossover Health, Doximity, Livongo, and Norwest Venture Partners. Doximity funded the production of the report.
The average age of ob.gyns. is rising across the country, signaling physician shortages that are projected to worsen over the next several decades. The pinch may be felt especially in areas with high birth rates and few younger ob.gyns., according to a report from Doximity.
Doximity’s geographic projections paint a fine-grained picture in agreement with the American College of Obstetricians and Gynecologists’ projections of a national shortfall of up to 8,800 ob.gyns. by 2020, with the deficit of ob.gyns. potentially climbing to 22,000 by 2050.
Pittsburgh and Bridgeport, Conn. top the list of metropolitan areas with the oldest average age of practicing ob.gyns., with average ages of 52.32 and 52.12 years, respectively. Las Vegas, Detroit, Miami, Los Angeles, New York, Boston, and Chicago all also made the top 15 cities in this list.
Because ob.gyns. start to leave the work force at age 59 years and retire at a median age of 64 years, this aging physician population could affect access to women’s health services within the decade, said Dr. Whaley. The fact that the burnout rate for ob.gyns. is only topped by emergency room physicians represents another area for concern, said Dr. Whaley of the University of California, Berkeley.
The report drew on data from the Centers for Medicare and Medicaid Services and board certification data. Doximity’s self-reported information from about 43,000 full-time board-certified ob.gyns. was also factored in. The top metropolitan areas were determined by 2010 census data and birth statistics came from federal databases.
With the data sliced another way, Pittsburgh also had the highest percentage of ob.gyns. aged 55 years or older, with 41.92% falling into this age group. Of the 50 metropolitan areas included in the Doximity report, 32 have an ob.gyn. workforce with at least one-third aged 55 years and up.
And there are many metropolitan areas where there’s a paucity of young ob.gyns., signaling future serious shortages, according to Doximity. In 12 of the metropolitan areas studied, less than 15% of the ob.gyn. workforce is aged 40 years or younger. Some of the cities with a potential “double hit” of older ob.gyns. and few younger physicians entering the profession include Las Vegas, Miami, Los Angeles, New York, and Chicago.
The Doximity methodology also took into account the average workload, in terms of the number of live births per physician per year, for ob.gyns. in the various metropolitan areas. There was a large variation in workload calculated this way: St. Louis has 247 live births per ob.gyn per year, while Louisville, Ky., has just 64, according to the report.
Factoring in workload information as well, the report gives a “shortage risk index” listing. Here, Las Vegas, Los Angeles, and Miami top the list. Cities considered at lowest risk for shortages according to this methodology include Baltimore, Denver, Portland, Ore., and Cleveland.
According to a 2017 ACOG workforce report, though women make up nearly half of those entering medical school, over four in five physicians entering ob.gyn. residencies (82.3% in 2016) are women. Further, women made up 58.7% of the ob.gyns. in active practice in 2017, outstripping all other surgical and medical specialties save pediatrics. Within 10 years, two-thirds of all ob.gyns. will be female, according to ACOG.
“While this study cannot determine causation for the variation in workloads, compensation, or shortages across metropolitan areas, we hope it will continue to serve as a baseline for the size of the challenge and prove helpful to health care employers, policymakers, patient advocates, and others interested in further study of this topic,” wrote Dr. Whaley and the report’s coauthors. “This information may also be helpful for ob.gyns. looking to live in areas with an increasing need for their expertise,” they said.
Dr. Whaley has received consulting fees from Castlight Health, Crossover Health, Doximity, Livongo, and Norwest Venture Partners. Doximity funded the production of the report.
The average age of ob.gyns. is rising across the country, signaling physician shortages that are projected to worsen over the next several decades. The pinch may be felt especially in areas with high birth rates and few younger ob.gyns., according to a report from Doximity.
Doximity’s geographic projections paint a fine-grained picture in agreement with the American College of Obstetricians and Gynecologists’ projections of a national shortfall of up to 8,800 ob.gyns. by 2020, with the deficit of ob.gyns. potentially climbing to 22,000 by 2050.
Pittsburgh and Bridgeport, Conn. top the list of metropolitan areas with the oldest average age of practicing ob.gyns., with average ages of 52.32 and 52.12 years, respectively. Las Vegas, Detroit, Miami, Los Angeles, New York, Boston, and Chicago all also made the top 15 cities in this list.
Because ob.gyns. start to leave the work force at age 59 years and retire at a median age of 64 years, this aging physician population could affect access to women’s health services within the decade, said Dr. Whaley. The fact that the burnout rate for ob.gyns. is only topped by emergency room physicians represents another area for concern, said Dr. Whaley of the University of California, Berkeley.
The report drew on data from the Centers for Medicare and Medicaid Services and board certification data. Doximity’s self-reported information from about 43,000 full-time board-certified ob.gyns. was also factored in. The top metropolitan areas were determined by 2010 census data and birth statistics came from federal databases.
With the data sliced another way, Pittsburgh also had the highest percentage of ob.gyns. aged 55 years or older, with 41.92% falling into this age group. Of the 50 metropolitan areas included in the Doximity report, 32 have an ob.gyn. workforce with at least one-third aged 55 years and up.
And there are many metropolitan areas where there’s a paucity of young ob.gyns., signaling future serious shortages, according to Doximity. In 12 of the metropolitan areas studied, less than 15% of the ob.gyn. workforce is aged 40 years or younger. Some of the cities with a potential “double hit” of older ob.gyns. and few younger physicians entering the profession include Las Vegas, Miami, Los Angeles, New York, and Chicago.
The Doximity methodology also took into account the average workload, in terms of the number of live births per physician per year, for ob.gyns. in the various metropolitan areas. There was a large variation in workload calculated this way: St. Louis has 247 live births per ob.gyn per year, while Louisville, Ky., has just 64, according to the report.
Factoring in workload information as well, the report gives a “shortage risk index” listing. Here, Las Vegas, Los Angeles, and Miami top the list. Cities considered at lowest risk for shortages according to this methodology include Baltimore, Denver, Portland, Ore., and Cleveland.
According to a 2017 ACOG workforce report, though women make up nearly half of those entering medical school, over four in five physicians entering ob.gyn. residencies (82.3% in 2016) are women. Further, women made up 58.7% of the ob.gyns. in active practice in 2017, outstripping all other surgical and medical specialties save pediatrics. Within 10 years, two-thirds of all ob.gyns. will be female, according to ACOG.
“While this study cannot determine causation for the variation in workloads, compensation, or shortages across metropolitan areas, we hope it will continue to serve as a baseline for the size of the challenge and prove helpful to health care employers, policymakers, patient advocates, and others interested in further study of this topic,” wrote Dr. Whaley and the report’s coauthors. “This information may also be helpful for ob.gyns. looking to live in areas with an increasing need for their expertise,” they said.
Dr. Whaley has received consulting fees from Castlight Health, Crossover Health, Doximity, Livongo, and Norwest Venture Partners. Doximity funded the production of the report.
Geriatric assessments could fine-tune cancer care for older adults
Fewer than 25% of older cancer patients currently get these assessments, which evaluate a person’s functioning (what he can and cannot do), psychological status, nutrition, cognition, social circumstances, and other, coexisting medical conditions, and which can predict the potential toxicity of chemotherapy.
The new guideline, ASCO’s first in the field of “geriatric oncology,” may have significant potential to change medical practice. “These recommendations will capture the attention of oncologists, I think, and that will be incredibly valuable,” said Corinne Leach, PhD, strategic director of cancer and aging research at the American Cancer Society.
They recognize a shifting demographic reality for cancer specialists, who are treating increasingly older patients as life spans lengthen across the globe. In the United States, 60% of patients newly diagnosed with cancer (an estimated 1.7 million people this year) are aged 65 years or older, as are more than 60% of cancer survivors.
Yet evidence about how best to treat older adults with cancer is weak because older adults are underrepresented in clinical trials. And most oncologists have received little training in how to manage older patients’ unique vulnerabilities.
When researchers asked 305 community oncologists about evaluating older patients, 89% acknowledged “the care of older adults with cancer needs to be improved,” according to a recently published study. Fewer than 25% said they were “very confident” they could identify dementia or accurately assess a patient’s functioning or risk of falling – factors associated with poorer outcomes for cancer treatment.
Still, resistance to change is evident. “We’re all inundated with trying to keep up with new standards of care, and I doubt there will be any broad acceptance of the rigor called for in this guideline,” said Frederick Schnell, MD, medical director of the Community Oncology Alliance.
The burden on physicians shouldn’t be significant, however: The streamlined assessments recommended in the ASCO guideline take only about 20 minutes to complete. Patients fill out surveys during most of that time; about five minutes is required for a nurse or physician assistant to administer several brief tests.
The assessment can identify people at increased risk of experiencing serious side effects from chemotherapy – infections, fatigue, diarrhea, dehydration and other problems that affect more than half of older patients. Physicians can then take steps to address these vulnerabilities, such as prescribing physical therapy for an older patient with muscle weakness or ordering a nutritional consultation for someone who has become malnourished. Also, they can alter chemotherapy regimens to minimize the potential for harm.
Currently, most oncologists decide whether older patients can benefit from chemotherapy by using the “eyeball test,” an assessment that relies primarily on their experience and judgment. “This isn’t enough to understand factors that put older adults at risk; it takes a deeper dive,” said Arti Hurria, MD, director of the Center for Cancer and Aging and professor of medical oncology and therapeutics research at City of Hope, a comprehensive cancer center in Duarte, Calif., and cochair of the panel that produced the new guidelines.
An oncologist walking into a room in a busy clinic might find an older patient already on the exam table, for instance, and miss the fact that he needed assistance getting out of a chair and getting into a gown – important signs of functional impairment that could be aggravated by chemotherapy, Dr. Hurria said. Or, “a very pleasant older patient might smile kindly at you and agree with everything you’re saying, and she might not have understood a thing you said” because of undetected cognitive impairment that could worsen and interfere with treatment, she explained.
William Dale, MD, a geriatrician and Arthur M. Coppola Family Chair in Supportive Care Medicine at City of Hope and another cochair of the guideline panel, tells of an 83-year-old woman whom he saw several years ago, with lung cancer metastasized to her brain. Her family requested a consultation because she’d become withdrawn and forgetful – a sign of accelerating cognitive impairment, they suspected.
Should she have chemotherapy and whole brain radiation, or would that worsen her memory lapses, the patient and family wondered?
One result stood out when Dale ordered a geriatric assessment: This older woman wasn’t cognitively impaired, she was psychologically distressed. “She wasn’t eating, she wasn’t interacting with other people, she appeared not to want treatment, but all this was due to depression,” Dr. Dale recalled. With counseling, the patient decided to undergo chemotherapy and radiation treatment, which he called “remarkably successful.”
Just as genetic tests are being used to personalize care for older cancer patients, geriatric assessments can be employed for this purpose – at considerably less expense, said Supriya Gupta Mohile, MD, editor in chief of the Journal of Geriatric Oncology and director of geriatric oncology at the James Wilmot Cancer Institute at the University of Rochester (N.Y.).
She tells of a 78-year-old man with invasive bladder cancer who came in for a consultation. From the medical chart, she learned the patient had hypertension, diabetes, and depression, all reasonably well controlled. From a geriatric assessment, she discovered that he lived alone, had cognitive impairment, relied on his daughter to deliver meals, and was at high risk of falling.
“The patient and his daughter were worried about his safety at home, his cognition getting worse, and fatigue [he was dealing with] and how that might affect his ability to function,” Dr. Mohile said. “His goal was to stay independent, at home, and not be hospitalized or go to rehabilitation.”
The standard of care for this condition was 3-4 months of chemotherapy before surgery, but Dr. Mohile recommended that the older patient skip chemotherapy and have surgery immediately, after reviewing the geriatric assessment with her patient and his family.
Every older patient considering chemotherapy should request an evaluation of this kind, even if your physician doesn’t offer it, said Heidi D. Klepin, MD, associate professor of hematology and oncology at Wake Forest University, Winston-Salem, N.C. “Ask for your doctor to consider your ability to do the things you most care about doing and for care to be individualized to your unique circumstances.”
KHN’s coverage of these topics is supported by John A. Hartford Foundation, Gordon and Betty Moore Foundation, and The SCAN Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Fewer than 25% of older cancer patients currently get these assessments, which evaluate a person’s functioning (what he can and cannot do), psychological status, nutrition, cognition, social circumstances, and other, coexisting medical conditions, and which can predict the potential toxicity of chemotherapy.
The new guideline, ASCO’s first in the field of “geriatric oncology,” may have significant potential to change medical practice. “These recommendations will capture the attention of oncologists, I think, and that will be incredibly valuable,” said Corinne Leach, PhD, strategic director of cancer and aging research at the American Cancer Society.
They recognize a shifting demographic reality for cancer specialists, who are treating increasingly older patients as life spans lengthen across the globe. In the United States, 60% of patients newly diagnosed with cancer (an estimated 1.7 million people this year) are aged 65 years or older, as are more than 60% of cancer survivors.
Yet evidence about how best to treat older adults with cancer is weak because older adults are underrepresented in clinical trials. And most oncologists have received little training in how to manage older patients’ unique vulnerabilities.
When researchers asked 305 community oncologists about evaluating older patients, 89% acknowledged “the care of older adults with cancer needs to be improved,” according to a recently published study. Fewer than 25% said they were “very confident” they could identify dementia or accurately assess a patient’s functioning or risk of falling – factors associated with poorer outcomes for cancer treatment.
Still, resistance to change is evident. “We’re all inundated with trying to keep up with new standards of care, and I doubt there will be any broad acceptance of the rigor called for in this guideline,” said Frederick Schnell, MD, medical director of the Community Oncology Alliance.
The burden on physicians shouldn’t be significant, however: The streamlined assessments recommended in the ASCO guideline take only about 20 minutes to complete. Patients fill out surveys during most of that time; about five minutes is required for a nurse or physician assistant to administer several brief tests.
The assessment can identify people at increased risk of experiencing serious side effects from chemotherapy – infections, fatigue, diarrhea, dehydration and other problems that affect more than half of older patients. Physicians can then take steps to address these vulnerabilities, such as prescribing physical therapy for an older patient with muscle weakness or ordering a nutritional consultation for someone who has become malnourished. Also, they can alter chemotherapy regimens to minimize the potential for harm.
Currently, most oncologists decide whether older patients can benefit from chemotherapy by using the “eyeball test,” an assessment that relies primarily on their experience and judgment. “This isn’t enough to understand factors that put older adults at risk; it takes a deeper dive,” said Arti Hurria, MD, director of the Center for Cancer and Aging and professor of medical oncology and therapeutics research at City of Hope, a comprehensive cancer center in Duarte, Calif., and cochair of the panel that produced the new guidelines.
An oncologist walking into a room in a busy clinic might find an older patient already on the exam table, for instance, and miss the fact that he needed assistance getting out of a chair and getting into a gown – important signs of functional impairment that could be aggravated by chemotherapy, Dr. Hurria said. Or, “a very pleasant older patient might smile kindly at you and agree with everything you’re saying, and she might not have understood a thing you said” because of undetected cognitive impairment that could worsen and interfere with treatment, she explained.
William Dale, MD, a geriatrician and Arthur M. Coppola Family Chair in Supportive Care Medicine at City of Hope and another cochair of the guideline panel, tells of an 83-year-old woman whom he saw several years ago, with lung cancer metastasized to her brain. Her family requested a consultation because she’d become withdrawn and forgetful – a sign of accelerating cognitive impairment, they suspected.
Should she have chemotherapy and whole brain radiation, or would that worsen her memory lapses, the patient and family wondered?
One result stood out when Dale ordered a geriatric assessment: This older woman wasn’t cognitively impaired, she was psychologically distressed. “She wasn’t eating, she wasn’t interacting with other people, she appeared not to want treatment, but all this was due to depression,” Dr. Dale recalled. With counseling, the patient decided to undergo chemotherapy and radiation treatment, which he called “remarkably successful.”
Just as genetic tests are being used to personalize care for older cancer patients, geriatric assessments can be employed for this purpose – at considerably less expense, said Supriya Gupta Mohile, MD, editor in chief of the Journal of Geriatric Oncology and director of geriatric oncology at the James Wilmot Cancer Institute at the University of Rochester (N.Y.).
She tells of a 78-year-old man with invasive bladder cancer who came in for a consultation. From the medical chart, she learned the patient had hypertension, diabetes, and depression, all reasonably well controlled. From a geriatric assessment, she discovered that he lived alone, had cognitive impairment, relied on his daughter to deliver meals, and was at high risk of falling.
“The patient and his daughter were worried about his safety at home, his cognition getting worse, and fatigue [he was dealing with] and how that might affect his ability to function,” Dr. Mohile said. “His goal was to stay independent, at home, and not be hospitalized or go to rehabilitation.”
The standard of care for this condition was 3-4 months of chemotherapy before surgery, but Dr. Mohile recommended that the older patient skip chemotherapy and have surgery immediately, after reviewing the geriatric assessment with her patient and his family.
Every older patient considering chemotherapy should request an evaluation of this kind, even if your physician doesn’t offer it, said Heidi D. Klepin, MD, associate professor of hematology and oncology at Wake Forest University, Winston-Salem, N.C. “Ask for your doctor to consider your ability to do the things you most care about doing and for care to be individualized to your unique circumstances.”
KHN’s coverage of these topics is supported by John A. Hartford Foundation, Gordon and Betty Moore Foundation, and The SCAN Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Fewer than 25% of older cancer patients currently get these assessments, which evaluate a person’s functioning (what he can and cannot do), psychological status, nutrition, cognition, social circumstances, and other, coexisting medical conditions, and which can predict the potential toxicity of chemotherapy.
The new guideline, ASCO’s first in the field of “geriatric oncology,” may have significant potential to change medical practice. “These recommendations will capture the attention of oncologists, I think, and that will be incredibly valuable,” said Corinne Leach, PhD, strategic director of cancer and aging research at the American Cancer Society.
They recognize a shifting demographic reality for cancer specialists, who are treating increasingly older patients as life spans lengthen across the globe. In the United States, 60% of patients newly diagnosed with cancer (an estimated 1.7 million people this year) are aged 65 years or older, as are more than 60% of cancer survivors.
Yet evidence about how best to treat older adults with cancer is weak because older adults are underrepresented in clinical trials. And most oncologists have received little training in how to manage older patients’ unique vulnerabilities.
When researchers asked 305 community oncologists about evaluating older patients, 89% acknowledged “the care of older adults with cancer needs to be improved,” according to a recently published study. Fewer than 25% said they were “very confident” they could identify dementia or accurately assess a patient’s functioning or risk of falling – factors associated with poorer outcomes for cancer treatment.
Still, resistance to change is evident. “We’re all inundated with trying to keep up with new standards of care, and I doubt there will be any broad acceptance of the rigor called for in this guideline,” said Frederick Schnell, MD, medical director of the Community Oncology Alliance.
The burden on physicians shouldn’t be significant, however: The streamlined assessments recommended in the ASCO guideline take only about 20 minutes to complete. Patients fill out surveys during most of that time; about five minutes is required for a nurse or physician assistant to administer several brief tests.
The assessment can identify people at increased risk of experiencing serious side effects from chemotherapy – infections, fatigue, diarrhea, dehydration and other problems that affect more than half of older patients. Physicians can then take steps to address these vulnerabilities, such as prescribing physical therapy for an older patient with muscle weakness or ordering a nutritional consultation for someone who has become malnourished. Also, they can alter chemotherapy regimens to minimize the potential for harm.
Currently, most oncologists decide whether older patients can benefit from chemotherapy by using the “eyeball test,” an assessment that relies primarily on their experience and judgment. “This isn’t enough to understand factors that put older adults at risk; it takes a deeper dive,” said Arti Hurria, MD, director of the Center for Cancer and Aging and professor of medical oncology and therapeutics research at City of Hope, a comprehensive cancer center in Duarte, Calif., and cochair of the panel that produced the new guidelines.
An oncologist walking into a room in a busy clinic might find an older patient already on the exam table, for instance, and miss the fact that he needed assistance getting out of a chair and getting into a gown – important signs of functional impairment that could be aggravated by chemotherapy, Dr. Hurria said. Or, “a very pleasant older patient might smile kindly at you and agree with everything you’re saying, and she might not have understood a thing you said” because of undetected cognitive impairment that could worsen and interfere with treatment, she explained.
William Dale, MD, a geriatrician and Arthur M. Coppola Family Chair in Supportive Care Medicine at City of Hope and another cochair of the guideline panel, tells of an 83-year-old woman whom he saw several years ago, with lung cancer metastasized to her brain. Her family requested a consultation because she’d become withdrawn and forgetful – a sign of accelerating cognitive impairment, they suspected.
Should she have chemotherapy and whole brain radiation, or would that worsen her memory lapses, the patient and family wondered?
One result stood out when Dale ordered a geriatric assessment: This older woman wasn’t cognitively impaired, she was psychologically distressed. “She wasn’t eating, she wasn’t interacting with other people, she appeared not to want treatment, but all this was due to depression,” Dr. Dale recalled. With counseling, the patient decided to undergo chemotherapy and radiation treatment, which he called “remarkably successful.”
Just as genetic tests are being used to personalize care for older cancer patients, geriatric assessments can be employed for this purpose – at considerably less expense, said Supriya Gupta Mohile, MD, editor in chief of the Journal of Geriatric Oncology and director of geriatric oncology at the James Wilmot Cancer Institute at the University of Rochester (N.Y.).
She tells of a 78-year-old man with invasive bladder cancer who came in for a consultation. From the medical chart, she learned the patient had hypertension, diabetes, and depression, all reasonably well controlled. From a geriatric assessment, she discovered that he lived alone, had cognitive impairment, relied on his daughter to deliver meals, and was at high risk of falling.
“The patient and his daughter were worried about his safety at home, his cognition getting worse, and fatigue [he was dealing with] and how that might affect his ability to function,” Dr. Mohile said. “His goal was to stay independent, at home, and not be hospitalized or go to rehabilitation.”
The standard of care for this condition was 3-4 months of chemotherapy before surgery, but Dr. Mohile recommended that the older patient skip chemotherapy and have surgery immediately, after reviewing the geriatric assessment with her patient and his family.
Every older patient considering chemotherapy should request an evaluation of this kind, even if your physician doesn’t offer it, said Heidi D. Klepin, MD, associate professor of hematology and oncology at Wake Forest University, Winston-Salem, N.C. “Ask for your doctor to consider your ability to do the things you most care about doing and for care to be individualized to your unique circumstances.”
KHN’s coverage of these topics is supported by John A. Hartford Foundation, Gordon and Betty Moore Foundation, and The SCAN Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Dupilumab reduces exacerbations, cuts glucocorticoid use in moderate to severe asthma
Among patients with moderate to severe asthma, dupilumab reduced exacerbations by almost 50%, while also allowing glucocorticoid-treated patients to cut their use of that medication by 70%, with no increased risk of exacerbation.
The pair of placebo-controlled studies – Liberty Asthma Quest and Liberty Asthma Venture – also showed treatment-associated stability in forced expiratory volume (FEV1) evidence of lung remodeling among those who took the antibody, Mario Castro, MD, of Washington University, St. Louis, and his colleagues reported in the New England Journal of Medicine.
By week 12, FEV1 it had already increased by 0.32 L, they said.
“An analysis of the postbronchodilator FEV1 slope showed a loss of lung function in patients who received placebo and no loss in those who received dupilumab, findings that suggest a potential effect of dupilumab on airway remodeling,” wrote Dr. Castro and his colleagues. “The slope analysis showed that patients who received placebo lost, on average, approximately 40 mL annually, which is consistent with data from other cohorts of patients with asthma.”
Dupilumab is an anti–interleukin-4 alpha antibody that blocks both IL-4 and IL-13. The Quest trial examined efficacy and safety of two doses (200 mg and 300 mg every 2 weeks), compared with placebo in patients with uncontrolled asthma. Venture examined efficacy and safety of 300 mg or placebo as add-on therapy for patients with severe asthma who were taking glucocorticoids.
Liberty Asthma Quest
This 52-week study randomized 1,902 patients with severe, uncontrolled asthma to placebo or dupilumab 200 mg or 300 mg every other week. The primary endpoints were annual rate of severe asthma exacerbations and the change in FEV1 by week 12. The study also looked at these endpoints in patients whose baseline eosinophil count was greater than 300 per cubic millimeter.
Patients were a mean of 48 years old with a mean baseline FEV1 of about 1.75 L (about 58% of the predicted normal value). They had a mean of two exacerbations per year and an average eosinophil count of about 350 per cubic millimeter.
Both doses outperformed placebo in all endpoints.
Among those taking 200 mg, the annual relapse rate was 0.46 versus 0.87 among those taking placebo – a significant 47.7% risk reduction. Among those taking 300 mg, the exacerbation rate was 0.52 versus 0.97; this translated to a significant 46% risk reduction.
The response rate was even greater among those with an eosinophil count greater than 300 per cubic millimeter: 0.37 for 200 mg and 0.40 for 300 mg versus the placebo rates of 1.08 and 1.24. This translated to risk reductions of 65.8% and 67.4%, respectively.
By week 12, FEV1 had significantly increased by 0.32 L in the 200-mg group and by 0.34 L in the 300-mg group, compared with nonsignificant increases among those taking placebo.
Again, patients with the high eosinophil counts experienced the greatest benefits, with FEV1 increasing by a mean of 0.43 L at 12 weeks in the 200-mg group and by 0.47 L in the 300-mg group, significantly better than either placebo comparator.
The benefit was already noticeable by the 2-week evaluation, the investigators noted.
Dupilumab appeared safe; injection-site reactions were the most common adverse event, occurring in 15% of the low-dose group and 18% of the high-dose group. However, 52 patients taking the drug experienced eosinophilia, compared with four of those taking placebo (4.1% vs. 0.6%).
Four of those taking the study drug experienced clinical symptoms associated with eosinophilia, including worsening eosinophilia and chronic eosinophilic pneumonia.
Liberty Asthma Venture
In this study, the effect of dupilumab on glucocorticoid use among 210 patients with severe asthma was examined. Patients were randomized to add-on dupilumab 300 mg every 2 weeks for 24 weeks. Glucocorticoids were tapered downward from weeks 4 to 20. The primary endpoints were percent reduction in glucocorticoid dose at week 24, and the percentage of patients who experienced a reduction of at least 50% in glucocorticoid dose.
These patients were a mean of 51 years old, with a mean of two severe asthma exacerbations in the past year. Their mean daily oral glucocorticoid dose at randomization was about 11 mg per day. Their mean prebronchodilator FEV1 was about 1.6 liters – about 52% of predicted value.
Oral glucocorticoid use decreased by a mean of 70.1% in the active group, compared with 41.9% in the placebo group, a statistically significant difference, Klaus F. Rabe, MD, of Christian Albrechts University, Kiel, Germany, and his coauthors wrote in the New England Journal of Medicine. The median change was even better: A 100% reduction in the active group and 50% reduction in the placebo group.
By week 24, 80% of those taking dupilumab had decreased their glucocorticoid intake by at least 50%, compared with 50% of the placebo group reaching this goal. The glucocorticoid dose was less than 5 mg/day in 69% of the dupilumab group, compared with 33% of the placebo group.
Like Quest, Venture showed a treatment advantage among patients with high baseline eosinophil count. “The magnitude of the effect was largest in patients with a higher eosinophil count at baseline,” the investigators wrote. “… The odds ratios [a 50% glucocorticoid reduction] for dupilumab versus placebo were 6.59 among patients with 300 or more cells per cubic millimeter at baseline and 2.91 among those with less than 300 cells per cubic millimeter at baseline.”
In a fully adjusted model at week 24, 48% of the patients in the dupilumab group were able to stop oral glucocorticoids entirely, compared with 25% of the placebo group.
Dupilumab was also associated with a significant 59% reduction in severe annual asthma exacerbations. FEV1 among the active group was 0.22 L better than that in the placebo group at week 24.
Again, patients with a higher baseline blood eosinophil count experienced greater treatment benefit; among these, the rate of severe asthma exacerbations was 71% lower than the rate in the placebo group, and FEV1 was 0.32 L higher.
The most frequent adverse events were viral infections (9% of the patients in the dupilumab group vs. 18% of those in the placebo group), bronchitis (7% vs. 6%), sinusitis (7% vs. 4%), influenza (3% vs. 6%), and eosinophilia (14% vs. 1%). Injection-site reactions occurred in 9% of those taking dupilumab and 4% of those taking placebo.
Antidrug antibodies developed in five patients in each group, without clinical effect.
Both trials were funded by Sanofi and Regeneron. Dr. Castro has received grant support from Sanofi. Dr. Rabe has received consulting and lecture fees from AstraZeneca, Boehringer Ingelheim, Novartis, Sanofi, and Teva Pharmaceutical Industries.
SOURCES: Castro M et al. N Engl J Med. 2018;378:2486-96; KF Rabe et al. N Engl J Med. 2018;378:2475-85.
Among patients with moderate to severe asthma, dupilumab reduced exacerbations by almost 50%, while also allowing glucocorticoid-treated patients to cut their use of that medication by 70%, with no increased risk of exacerbation.
The pair of placebo-controlled studies – Liberty Asthma Quest and Liberty Asthma Venture – also showed treatment-associated stability in forced expiratory volume (FEV1) evidence of lung remodeling among those who took the antibody, Mario Castro, MD, of Washington University, St. Louis, and his colleagues reported in the New England Journal of Medicine.
By week 12, FEV1 it had already increased by 0.32 L, they said.
“An analysis of the postbronchodilator FEV1 slope showed a loss of lung function in patients who received placebo and no loss in those who received dupilumab, findings that suggest a potential effect of dupilumab on airway remodeling,” wrote Dr. Castro and his colleagues. “The slope analysis showed that patients who received placebo lost, on average, approximately 40 mL annually, which is consistent with data from other cohorts of patients with asthma.”
Dupilumab is an anti–interleukin-4 alpha antibody that blocks both IL-4 and IL-13. The Quest trial examined efficacy and safety of two doses (200 mg and 300 mg every 2 weeks), compared with placebo in patients with uncontrolled asthma. Venture examined efficacy and safety of 300 mg or placebo as add-on therapy for patients with severe asthma who were taking glucocorticoids.
Liberty Asthma Quest
This 52-week study randomized 1,902 patients with severe, uncontrolled asthma to placebo or dupilumab 200 mg or 300 mg every other week. The primary endpoints were annual rate of severe asthma exacerbations and the change in FEV1 by week 12. The study also looked at these endpoints in patients whose baseline eosinophil count was greater than 300 per cubic millimeter.
Patients were a mean of 48 years old with a mean baseline FEV1 of about 1.75 L (about 58% of the predicted normal value). They had a mean of two exacerbations per year and an average eosinophil count of about 350 per cubic millimeter.
Both doses outperformed placebo in all endpoints.
Among those taking 200 mg, the annual relapse rate was 0.46 versus 0.87 among those taking placebo – a significant 47.7% risk reduction. Among those taking 300 mg, the exacerbation rate was 0.52 versus 0.97; this translated to a significant 46% risk reduction.
The response rate was even greater among those with an eosinophil count greater than 300 per cubic millimeter: 0.37 for 200 mg and 0.40 for 300 mg versus the placebo rates of 1.08 and 1.24. This translated to risk reductions of 65.8% and 67.4%, respectively.
By week 12, FEV1 had significantly increased by 0.32 L in the 200-mg group and by 0.34 L in the 300-mg group, compared with nonsignificant increases among those taking placebo.
Again, patients with the high eosinophil counts experienced the greatest benefits, with FEV1 increasing by a mean of 0.43 L at 12 weeks in the 200-mg group and by 0.47 L in the 300-mg group, significantly better than either placebo comparator.
The benefit was already noticeable by the 2-week evaluation, the investigators noted.
Dupilumab appeared safe; injection-site reactions were the most common adverse event, occurring in 15% of the low-dose group and 18% of the high-dose group. However, 52 patients taking the drug experienced eosinophilia, compared with four of those taking placebo (4.1% vs. 0.6%).
Four of those taking the study drug experienced clinical symptoms associated with eosinophilia, including worsening eosinophilia and chronic eosinophilic pneumonia.
Liberty Asthma Venture
In this study, the effect of dupilumab on glucocorticoid use among 210 patients with severe asthma was examined. Patients were randomized to add-on dupilumab 300 mg every 2 weeks for 24 weeks. Glucocorticoids were tapered downward from weeks 4 to 20. The primary endpoints were percent reduction in glucocorticoid dose at week 24, and the percentage of patients who experienced a reduction of at least 50% in glucocorticoid dose.
These patients were a mean of 51 years old, with a mean of two severe asthma exacerbations in the past year. Their mean daily oral glucocorticoid dose at randomization was about 11 mg per day. Their mean prebronchodilator FEV1 was about 1.6 liters – about 52% of predicted value.
Oral glucocorticoid use decreased by a mean of 70.1% in the active group, compared with 41.9% in the placebo group, a statistically significant difference, Klaus F. Rabe, MD, of Christian Albrechts University, Kiel, Germany, and his coauthors wrote in the New England Journal of Medicine. The median change was even better: A 100% reduction in the active group and 50% reduction in the placebo group.
By week 24, 80% of those taking dupilumab had decreased their glucocorticoid intake by at least 50%, compared with 50% of the placebo group reaching this goal. The glucocorticoid dose was less than 5 mg/day in 69% of the dupilumab group, compared with 33% of the placebo group.
Like Quest, Venture showed a treatment advantage among patients with high baseline eosinophil count. “The magnitude of the effect was largest in patients with a higher eosinophil count at baseline,” the investigators wrote. “… The odds ratios [a 50% glucocorticoid reduction] for dupilumab versus placebo were 6.59 among patients with 300 or more cells per cubic millimeter at baseline and 2.91 among those with less than 300 cells per cubic millimeter at baseline.”
In a fully adjusted model at week 24, 48% of the patients in the dupilumab group were able to stop oral glucocorticoids entirely, compared with 25% of the placebo group.
Dupilumab was also associated with a significant 59% reduction in severe annual asthma exacerbations. FEV1 among the active group was 0.22 L better than that in the placebo group at week 24.
Again, patients with a higher baseline blood eosinophil count experienced greater treatment benefit; among these, the rate of severe asthma exacerbations was 71% lower than the rate in the placebo group, and FEV1 was 0.32 L higher.
The most frequent adverse events were viral infections (9% of the patients in the dupilumab group vs. 18% of those in the placebo group), bronchitis (7% vs. 6%), sinusitis (7% vs. 4%), influenza (3% vs. 6%), and eosinophilia (14% vs. 1%). Injection-site reactions occurred in 9% of those taking dupilumab and 4% of those taking placebo.
Antidrug antibodies developed in five patients in each group, without clinical effect.
Both trials were funded by Sanofi and Regeneron. Dr. Castro has received grant support from Sanofi. Dr. Rabe has received consulting and lecture fees from AstraZeneca, Boehringer Ingelheim, Novartis, Sanofi, and Teva Pharmaceutical Industries.
SOURCES: Castro M et al. N Engl J Med. 2018;378:2486-96; KF Rabe et al. N Engl J Med. 2018;378:2475-85.
Among patients with moderate to severe asthma, dupilumab reduced exacerbations by almost 50%, while also allowing glucocorticoid-treated patients to cut their use of that medication by 70%, with no increased risk of exacerbation.
The pair of placebo-controlled studies – Liberty Asthma Quest and Liberty Asthma Venture – also showed treatment-associated stability in forced expiratory volume (FEV1) evidence of lung remodeling among those who took the antibody, Mario Castro, MD, of Washington University, St. Louis, and his colleagues reported in the New England Journal of Medicine.
By week 12, FEV1 it had already increased by 0.32 L, they said.
“An analysis of the postbronchodilator FEV1 slope showed a loss of lung function in patients who received placebo and no loss in those who received dupilumab, findings that suggest a potential effect of dupilumab on airway remodeling,” wrote Dr. Castro and his colleagues. “The slope analysis showed that patients who received placebo lost, on average, approximately 40 mL annually, which is consistent with data from other cohorts of patients with asthma.”
Dupilumab is an anti–interleukin-4 alpha antibody that blocks both IL-4 and IL-13. The Quest trial examined efficacy and safety of two doses (200 mg and 300 mg every 2 weeks), compared with placebo in patients with uncontrolled asthma. Venture examined efficacy and safety of 300 mg or placebo as add-on therapy for patients with severe asthma who were taking glucocorticoids.
Liberty Asthma Quest
This 52-week study randomized 1,902 patients with severe, uncontrolled asthma to placebo or dupilumab 200 mg or 300 mg every other week. The primary endpoints were annual rate of severe asthma exacerbations and the change in FEV1 by week 12. The study also looked at these endpoints in patients whose baseline eosinophil count was greater than 300 per cubic millimeter.
Patients were a mean of 48 years old with a mean baseline FEV1 of about 1.75 L (about 58% of the predicted normal value). They had a mean of two exacerbations per year and an average eosinophil count of about 350 per cubic millimeter.
Both doses outperformed placebo in all endpoints.
Among those taking 200 mg, the annual relapse rate was 0.46 versus 0.87 among those taking placebo – a significant 47.7% risk reduction. Among those taking 300 mg, the exacerbation rate was 0.52 versus 0.97; this translated to a significant 46% risk reduction.
The response rate was even greater among those with an eosinophil count greater than 300 per cubic millimeter: 0.37 for 200 mg and 0.40 for 300 mg versus the placebo rates of 1.08 and 1.24. This translated to risk reductions of 65.8% and 67.4%, respectively.
By week 12, FEV1 had significantly increased by 0.32 L in the 200-mg group and by 0.34 L in the 300-mg group, compared with nonsignificant increases among those taking placebo.
Again, patients with the high eosinophil counts experienced the greatest benefits, with FEV1 increasing by a mean of 0.43 L at 12 weeks in the 200-mg group and by 0.47 L in the 300-mg group, significantly better than either placebo comparator.
The benefit was already noticeable by the 2-week evaluation, the investigators noted.
Dupilumab appeared safe; injection-site reactions were the most common adverse event, occurring in 15% of the low-dose group and 18% of the high-dose group. However, 52 patients taking the drug experienced eosinophilia, compared with four of those taking placebo (4.1% vs. 0.6%).
Four of those taking the study drug experienced clinical symptoms associated with eosinophilia, including worsening eosinophilia and chronic eosinophilic pneumonia.
Liberty Asthma Venture
In this study, the effect of dupilumab on glucocorticoid use among 210 patients with severe asthma was examined. Patients were randomized to add-on dupilumab 300 mg every 2 weeks for 24 weeks. Glucocorticoids were tapered downward from weeks 4 to 20. The primary endpoints were percent reduction in glucocorticoid dose at week 24, and the percentage of patients who experienced a reduction of at least 50% in glucocorticoid dose.
These patients were a mean of 51 years old, with a mean of two severe asthma exacerbations in the past year. Their mean daily oral glucocorticoid dose at randomization was about 11 mg per day. Their mean prebronchodilator FEV1 was about 1.6 liters – about 52% of predicted value.
Oral glucocorticoid use decreased by a mean of 70.1% in the active group, compared with 41.9% in the placebo group, a statistically significant difference, Klaus F. Rabe, MD, of Christian Albrechts University, Kiel, Germany, and his coauthors wrote in the New England Journal of Medicine. The median change was even better: A 100% reduction in the active group and 50% reduction in the placebo group.
By week 24, 80% of those taking dupilumab had decreased their glucocorticoid intake by at least 50%, compared with 50% of the placebo group reaching this goal. The glucocorticoid dose was less than 5 mg/day in 69% of the dupilumab group, compared with 33% of the placebo group.
Like Quest, Venture showed a treatment advantage among patients with high baseline eosinophil count. “The magnitude of the effect was largest in patients with a higher eosinophil count at baseline,” the investigators wrote. “… The odds ratios [a 50% glucocorticoid reduction] for dupilumab versus placebo were 6.59 among patients with 300 or more cells per cubic millimeter at baseline and 2.91 among those with less than 300 cells per cubic millimeter at baseline.”
In a fully adjusted model at week 24, 48% of the patients in the dupilumab group were able to stop oral glucocorticoids entirely, compared with 25% of the placebo group.
Dupilumab was also associated with a significant 59% reduction in severe annual asthma exacerbations. FEV1 among the active group was 0.22 L better than that in the placebo group at week 24.
Again, patients with a higher baseline blood eosinophil count experienced greater treatment benefit; among these, the rate of severe asthma exacerbations was 71% lower than the rate in the placebo group, and FEV1 was 0.32 L higher.
The most frequent adverse events were viral infections (9% of the patients in the dupilumab group vs. 18% of those in the placebo group), bronchitis (7% vs. 6%), sinusitis (7% vs. 4%), influenza (3% vs. 6%), and eosinophilia (14% vs. 1%). Injection-site reactions occurred in 9% of those taking dupilumab and 4% of those taking placebo.
Antidrug antibodies developed in five patients in each group, without clinical effect.
Both trials were funded by Sanofi and Regeneron. Dr. Castro has received grant support from Sanofi. Dr. Rabe has received consulting and lecture fees from AstraZeneca, Boehringer Ingelheim, Novartis, Sanofi, and Teva Pharmaceutical Industries.
SOURCES: Castro M et al. N Engl J Med. 2018;378:2486-96; KF Rabe et al. N Engl J Med. 2018;378:2475-85.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Dupilumab allowed asthma patients to decrease glucocorticoids with no risk of asthma exacerbation.
Major finding: Dupilumab reduced exacerbations by almost 50%, while also allowing glucocorticoid-treated patients to cut their use of that medication by 70%.
Study details: Liberty Asthma Quest comprised 1,902 patients and Liberty Asthma Venture comprised 210. Both were randomized, placebo-controlled trials.
Disclosures: Both trials were funded by Sanofi and Regeneron. Dr. Castro has received grant support from Sanofi. Dr. Rabe has received consulting and lecture fees from AstraZeneca, Boehringer Ingelheim, Novartis, Sanofi, and Teva Pharmaceutical Industries.
Sources: Castro M et al. N Engl J Med. 2018;378:2486-96; Rabe KF et al. N Engl J Med. 2018;378:2475-85.
Intravascular Involvement of Cutaneous Squamous Cell Carcinoma
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinoma.1 With an estimated 700,000 cases reported annually in the United States, the incidence of cSCC continues to increase.2 Most patients with cSCC have an excellent prognosis after surgical clearance, with Mohs micrographic surgery (MMS) being the most successful treatment, followed by excision and electrodesiccation and curettage. A subset of patients with cSCC carry an increased risk of local recurrence, lymph node metastasis, and disease-specific death. A meta-analysis of 36 studies found that statistically significant risk factors for recurrence of cSCC included thickness greater than 2 mm (risk ratio [RR], 9.64; 95% CI, 1.30-1.52), invasion beyond the subcutaneous fat (RR, 7.61; 95% CI, 4.17-13.88), perineural invasion (RR, 4.30; 95% CI, 2.80-6.60), diameter greater than 20 mm (RR, 3.22; 95% CI, 1.91-5.45), location on temple (RR, 3.20; 95% CI, 1.12-9.15), and poor differentiation (RR, 2.66; 95% CI, 1.72-4.14).3 Additional risk factors for cSCC metastasis included location on the temple, ear, or lip, as well as a history of immunosuppression. Factors for disease-specific death were diameter greater than 20 mm, poor differentiation, location on the ear or lip, invasion beyond the subcutaneous fat, and perineural invasion.3 Perineural and/or lymphovascular invasion is considered high risk, but despite being linked to negative outcomes, there are no treatment guidelines based on lymphovascular (intravascular) invasion.4 We present a case of intravascular involvement found during MMS and treated with adjuvant radiotherapy after surgery. We share this case with the goal of discussing management in such cases and highlighting the need for improved definitive guidelines for high-risk cSCCs.
Case Report
A 72-year-old man presented with a rapidly growing lesion on the left side of the forehead of 1 year’s duration. His medical history was remarkable for B-cell lymphoma, which was currently in remission following chemotherapy 10 years prior. The lesion started as a small, red, dry patch that the patient initially thought was eczema. The site progressively enlarged to a red tumor measuring 2.4×2.0 cm (Figure 1), and the patient presented to the dermatology department for further evaluation. There was no clinical evidence of lymphadenopathy. A skin biopsy confirmed a moderately differentiated cSCC with a positive deep margin (Figure 2). Due to the tumor’s location, histology, size, and poorly defined borders, the patient was referred for treatment with MMS. The lesion was removed in a total of 2 stages and 4 sections. In addition to a proliferation of spindled tumor cells seen during surgery, which was consistent with cSCC, an intravascular component was noted despite clear margins after the surgery (Figure 3). The aggressive histology of intravascular involvement was subsequently confirmed by the academic dermatopathologist at our institution. With the evidence of an intravascular component of this patient’s cSCC, there was concern about further metastatic disease. After discussing the more aggressive histology type and size of the cSCC with the patient, he underwent subsequent computed tomography of the head, neck, and chest. Fortunately, this imaging did not show evidence of metastatic disease; thus, final staging of the cSCC was cT2N0M0. After interdisciplinary discussion and consultation with radiation oncology, the site of the cSCC was treated with adjuvant radiotherapy. The patient received a total of 6600 cGy delivered in 33 fractions of 200 cGy, each using an en face technique and 6 eV over a total treatment course of 48 days.
One year after undergoing MMS and adjuvant radiotherapy, the patient remains free of cSCC recurrence or metastases and still undergoes regular interdisciplinary monitoring. Without clear guidelines on the treatment of patients with intravascular involvement of cSCC, we relied on prior experience with similar cases.
Comment
This case highlights the challenge in managing patients with high-risk cSCC, as the current guidelines provided by the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) vary on the inclusion of intravascular involvement of cSCC as high risk and treatment is at the discretion of the provider in such circumstances.5-7 Both the AJCC and the NCCN have defined high-risk factors and staging for cSCC. The AJCC 8th edition (AJCC-8) revised guidelines include several high-risk factors of cSCC, including tumor diameter of 4 cm or larger leading to upstaging of a tumor from T2 to T3, invasion into or beyond the level of the subcutaneous tissue, depth of invasion greater than 6 mm, and large-caliber perineural invasion, and removed poorly differentiated histology from the AJCC-8 guidelines compared to the AJCC-7 guidelines. According to the AJCC-8 guidelines, location on the ear or lip, desmoplastic or spindle cell features, lymphovascular invasion, and immunosuppression do not affect tumor staging. The AJCC’s criteria for its TNM staging system strictly focus on features of the primary tumor and do not include clinical risk factors such as recurrence or immunosuppression. In contrast, the NCCN does include lymphovascular invasion as a high-risk factor of cSCC.
Intravascular invasion plays a considerable role in patient survival in certain cancers (eg, breast, gastric, prostate). In cutaneous malignancies, such as melanoma and SCC, metastasis more commonly occurs via lymphatic spread. When present, vascular invasion typically coexists with lymphatic involvement. The presence of microscopic lymphovascular invasion in cSCCs has not been definitively proven to increase the risk of metastases.8 However, multivariate analysis has shown that lymphovascular invasion independently predicts nodal metastasis and disease-specific death.9 As such, there are no guidelines on sentinel lymph node biopsy or adjuvant therapy in the setting of lymphovascular involvement of cSCCs. A survey-based study of 117 Mohs surgeons found a lack of consistency in their approaches to evaluation and management of high-risk SCCs. Most respondents noted perineural invasion and in-transit metastasis as the main findings that would lead to radiologic nodal staging, sentinel lymph node biopsy, or adjuvant radiotherapy, but they highlighted the lack of evidence-based treatment guidelines.4 High-risk cSCC can be treated via MMS or conventional surgery with safe excision margins. Adjuvant radiotherapy can reduce tumor recurrence and improve survival and therefore should be considered in cases of advanced or high-risk cSCCs, such as in our case.
The lack of consensus over the definition of high-risk cSCCs, a lack of high-quality therapeutic studies, and the absence of a prognostic model that integrates multiple risk factors all have made the prediction of outcomes and the formation of definitive management of cSCCs challenging. Multidisciplinary teams and vigilant monitoring are crucial in the successful management of high-risk cSCC, but further studies and reports are needed to develop definitive treatment algorithms.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Jambusaria-Pahlajani A, Hess SD, Katz KA, et al. Uncertainty in the perioperative management of high-risk cutaneous squamous cell carcinoma among Mohs surgeons. Arch Dermatol. 2010;146:1225-1231.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Amin MD, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed June 27, 2018.
- Lonie S, Niumsawatt V, Castley A. A prognostic dilemma of basal cell carcinoma with intravascular invasion. Plast Reconstr Surg Glob Open. 2016;4:e1046.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149:35-41.
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinoma.1 With an estimated 700,000 cases reported annually in the United States, the incidence of cSCC continues to increase.2 Most patients with cSCC have an excellent prognosis after surgical clearance, with Mohs micrographic surgery (MMS) being the most successful treatment, followed by excision and electrodesiccation and curettage. A subset of patients with cSCC carry an increased risk of local recurrence, lymph node metastasis, and disease-specific death. A meta-analysis of 36 studies found that statistically significant risk factors for recurrence of cSCC included thickness greater than 2 mm (risk ratio [RR], 9.64; 95% CI, 1.30-1.52), invasion beyond the subcutaneous fat (RR, 7.61; 95% CI, 4.17-13.88), perineural invasion (RR, 4.30; 95% CI, 2.80-6.60), diameter greater than 20 mm (RR, 3.22; 95% CI, 1.91-5.45), location on temple (RR, 3.20; 95% CI, 1.12-9.15), and poor differentiation (RR, 2.66; 95% CI, 1.72-4.14).3 Additional risk factors for cSCC metastasis included location on the temple, ear, or lip, as well as a history of immunosuppression. Factors for disease-specific death were diameter greater than 20 mm, poor differentiation, location on the ear or lip, invasion beyond the subcutaneous fat, and perineural invasion.3 Perineural and/or lymphovascular invasion is considered high risk, but despite being linked to negative outcomes, there are no treatment guidelines based on lymphovascular (intravascular) invasion.4 We present a case of intravascular involvement found during MMS and treated with adjuvant radiotherapy after surgery. We share this case with the goal of discussing management in such cases and highlighting the need for improved definitive guidelines for high-risk cSCCs.
Case Report
A 72-year-old man presented with a rapidly growing lesion on the left side of the forehead of 1 year’s duration. His medical history was remarkable for B-cell lymphoma, which was currently in remission following chemotherapy 10 years prior. The lesion started as a small, red, dry patch that the patient initially thought was eczema. The site progressively enlarged to a red tumor measuring 2.4×2.0 cm (Figure 1), and the patient presented to the dermatology department for further evaluation. There was no clinical evidence of lymphadenopathy. A skin biopsy confirmed a moderately differentiated cSCC with a positive deep margin (Figure 2). Due to the tumor’s location, histology, size, and poorly defined borders, the patient was referred for treatment with MMS. The lesion was removed in a total of 2 stages and 4 sections. In addition to a proliferation of spindled tumor cells seen during surgery, which was consistent with cSCC, an intravascular component was noted despite clear margins after the surgery (Figure 3). The aggressive histology of intravascular involvement was subsequently confirmed by the academic dermatopathologist at our institution. With the evidence of an intravascular component of this patient’s cSCC, there was concern about further metastatic disease. After discussing the more aggressive histology type and size of the cSCC with the patient, he underwent subsequent computed tomography of the head, neck, and chest. Fortunately, this imaging did not show evidence of metastatic disease; thus, final staging of the cSCC was cT2N0M0. After interdisciplinary discussion and consultation with radiation oncology, the site of the cSCC was treated with adjuvant radiotherapy. The patient received a total of 6600 cGy delivered in 33 fractions of 200 cGy, each using an en face technique and 6 eV over a total treatment course of 48 days.



One year after undergoing MMS and adjuvant radiotherapy, the patient remains free of cSCC recurrence or metastases and still undergoes regular interdisciplinary monitoring. Without clear guidelines on the treatment of patients with intravascular involvement of cSCC, we relied on prior experience with similar cases.
Comment
This case highlights the challenge in managing patients with high-risk cSCC, as the current guidelines provided by the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) vary on the inclusion of intravascular involvement of cSCC as high risk and treatment is at the discretion of the provider in such circumstances.5-7 Both the AJCC and the NCCN have defined high-risk factors and staging for cSCC. The AJCC 8th edition (AJCC-8) revised guidelines include several high-risk factors of cSCC, including tumor diameter of 4 cm or larger leading to upstaging of a tumor from T2 to T3, invasion into or beyond the level of the subcutaneous tissue, depth of invasion greater than 6 mm, and large-caliber perineural invasion, and removed poorly differentiated histology from the AJCC-8 guidelines compared to the AJCC-7 guidelines. According to the AJCC-8 guidelines, location on the ear or lip, desmoplastic or spindle cell features, lymphovascular invasion, and immunosuppression do not affect tumor staging. The AJCC’s criteria for its TNM staging system strictly focus on features of the primary tumor and do not include clinical risk factors such as recurrence or immunosuppression. In contrast, the NCCN does include lymphovascular invasion as a high-risk factor of cSCC.
Intravascular invasion plays a considerable role in patient survival in certain cancers (eg, breast, gastric, prostate). In cutaneous malignancies, such as melanoma and SCC, metastasis more commonly occurs via lymphatic spread. When present, vascular invasion typically coexists with lymphatic involvement. The presence of microscopic lymphovascular invasion in cSCCs has not been definitively proven to increase the risk of metastases.8 However, multivariate analysis has shown that lymphovascular invasion independently predicts nodal metastasis and disease-specific death.9 As such, there are no guidelines on sentinel lymph node biopsy or adjuvant therapy in the setting of lymphovascular involvement of cSCCs. A survey-based study of 117 Mohs surgeons found a lack of consistency in their approaches to evaluation and management of high-risk SCCs. Most respondents noted perineural invasion and in-transit metastasis as the main findings that would lead to radiologic nodal staging, sentinel lymph node biopsy, or adjuvant radiotherapy, but they highlighted the lack of evidence-based treatment guidelines.4 High-risk cSCC can be treated via MMS or conventional surgery with safe excision margins. Adjuvant radiotherapy can reduce tumor recurrence and improve survival and therefore should be considered in cases of advanced or high-risk cSCCs, such as in our case.
The lack of consensus over the definition of high-risk cSCCs, a lack of high-quality therapeutic studies, and the absence of a prognostic model that integrates multiple risk factors all have made the prediction of outcomes and the formation of definitive management of cSCCs challenging. Multidisciplinary teams and vigilant monitoring are crucial in the successful management of high-risk cSCC, but further studies and reports are needed to develop definitive treatment algorithms.
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer after basal cell carcinoma.1 With an estimated 700,000 cases reported annually in the United States, the incidence of cSCC continues to increase.2 Most patients with cSCC have an excellent prognosis after surgical clearance, with Mohs micrographic surgery (MMS) being the most successful treatment, followed by excision and electrodesiccation and curettage. A subset of patients with cSCC carry an increased risk of local recurrence, lymph node metastasis, and disease-specific death. A meta-analysis of 36 studies found that statistically significant risk factors for recurrence of cSCC included thickness greater than 2 mm (risk ratio [RR], 9.64; 95% CI, 1.30-1.52), invasion beyond the subcutaneous fat (RR, 7.61; 95% CI, 4.17-13.88), perineural invasion (RR, 4.30; 95% CI, 2.80-6.60), diameter greater than 20 mm (RR, 3.22; 95% CI, 1.91-5.45), location on temple (RR, 3.20; 95% CI, 1.12-9.15), and poor differentiation (RR, 2.66; 95% CI, 1.72-4.14).3 Additional risk factors for cSCC metastasis included location on the temple, ear, or lip, as well as a history of immunosuppression. Factors for disease-specific death were diameter greater than 20 mm, poor differentiation, location on the ear or lip, invasion beyond the subcutaneous fat, and perineural invasion.3 Perineural and/or lymphovascular invasion is considered high risk, but despite being linked to negative outcomes, there are no treatment guidelines based on lymphovascular (intravascular) invasion.4 We present a case of intravascular involvement found during MMS and treated with adjuvant radiotherapy after surgery. We share this case with the goal of discussing management in such cases and highlighting the need for improved definitive guidelines for high-risk cSCCs.
Case Report
A 72-year-old man presented with a rapidly growing lesion on the left side of the forehead of 1 year’s duration. His medical history was remarkable for B-cell lymphoma, which was currently in remission following chemotherapy 10 years prior. The lesion started as a small, red, dry patch that the patient initially thought was eczema. The site progressively enlarged to a red tumor measuring 2.4×2.0 cm (Figure 1), and the patient presented to the dermatology department for further evaluation. There was no clinical evidence of lymphadenopathy. A skin biopsy confirmed a moderately differentiated cSCC with a positive deep margin (Figure 2). Due to the tumor’s location, histology, size, and poorly defined borders, the patient was referred for treatment with MMS. The lesion was removed in a total of 2 stages and 4 sections. In addition to a proliferation of spindled tumor cells seen during surgery, which was consistent with cSCC, an intravascular component was noted despite clear margins after the surgery (Figure 3). The aggressive histology of intravascular involvement was subsequently confirmed by the academic dermatopathologist at our institution. With the evidence of an intravascular component of this patient’s cSCC, there was concern about further metastatic disease. After discussing the more aggressive histology type and size of the cSCC with the patient, he underwent subsequent computed tomography of the head, neck, and chest. Fortunately, this imaging did not show evidence of metastatic disease; thus, final staging of the cSCC was cT2N0M0. After interdisciplinary discussion and consultation with radiation oncology, the site of the cSCC was treated with adjuvant radiotherapy. The patient received a total of 6600 cGy delivered in 33 fractions of 200 cGy, each using an en face technique and 6 eV over a total treatment course of 48 days.



One year after undergoing MMS and adjuvant radiotherapy, the patient remains free of cSCC recurrence or metastases and still undergoes regular interdisciplinary monitoring. Without clear guidelines on the treatment of patients with intravascular involvement of cSCC, we relied on prior experience with similar cases.
Comment
This case highlights the challenge in managing patients with high-risk cSCC, as the current guidelines provided by the American Joint Committee on Cancer (AJCC) and the National Comprehensive Cancer Network (NCCN) vary on the inclusion of intravascular involvement of cSCC as high risk and treatment is at the discretion of the provider in such circumstances.5-7 Both the AJCC and the NCCN have defined high-risk factors and staging for cSCC. The AJCC 8th edition (AJCC-8) revised guidelines include several high-risk factors of cSCC, including tumor diameter of 4 cm or larger leading to upstaging of a tumor from T2 to T3, invasion into or beyond the level of the subcutaneous tissue, depth of invasion greater than 6 mm, and large-caliber perineural invasion, and removed poorly differentiated histology from the AJCC-8 guidelines compared to the AJCC-7 guidelines. According to the AJCC-8 guidelines, location on the ear or lip, desmoplastic or spindle cell features, lymphovascular invasion, and immunosuppression do not affect tumor staging. The AJCC’s criteria for its TNM staging system strictly focus on features of the primary tumor and do not include clinical risk factors such as recurrence or immunosuppression. In contrast, the NCCN does include lymphovascular invasion as a high-risk factor of cSCC.
Intravascular invasion plays a considerable role in patient survival in certain cancers (eg, breast, gastric, prostate). In cutaneous malignancies, such as melanoma and SCC, metastasis more commonly occurs via lymphatic spread. When present, vascular invasion typically coexists with lymphatic involvement. The presence of microscopic lymphovascular invasion in cSCCs has not been definitively proven to increase the risk of metastases.8 However, multivariate analysis has shown that lymphovascular invasion independently predicts nodal metastasis and disease-specific death.9 As such, there are no guidelines on sentinel lymph node biopsy or adjuvant therapy in the setting of lymphovascular involvement of cSCCs. A survey-based study of 117 Mohs surgeons found a lack of consistency in their approaches to evaluation and management of high-risk SCCs. Most respondents noted perineural invasion and in-transit metastasis as the main findings that would lead to radiologic nodal staging, sentinel lymph node biopsy, or adjuvant radiotherapy, but they highlighted the lack of evidence-based treatment guidelines.4 High-risk cSCC can be treated via MMS or conventional surgery with safe excision margins. Adjuvant radiotherapy can reduce tumor recurrence and improve survival and therefore should be considered in cases of advanced or high-risk cSCCs, such as in our case.
The lack of consensus over the definition of high-risk cSCCs, a lack of high-quality therapeutic studies, and the absence of a prognostic model that integrates multiple risk factors all have made the prediction of outcomes and the formation of definitive management of cSCCs challenging. Multidisciplinary teams and vigilant monitoring are crucial in the successful management of high-risk cSCC, but further studies and reports are needed to develop definitive treatment algorithms.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Jambusaria-Pahlajani A, Hess SD, Katz KA, et al. Uncertainty in the perioperative management of high-risk cutaneous squamous cell carcinoma among Mohs surgeons. Arch Dermatol. 2010;146:1225-1231.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Amin MD, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed June 27, 2018.
- Lonie S, Niumsawatt V, Castley A. A prognostic dilemma of basal cell carcinoma with intravascular invasion. Plast Reconstr Surg Glob Open. 2016;4:e1046.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149:35-41.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Jambusaria-Pahlajani A, Hess SD, Katz KA, et al. Uncertainty in the perioperative management of high-risk cutaneous squamous cell carcinoma among Mohs surgeons. Arch Dermatol. 2010;146:1225-1231.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24:171-194.
- Amin MD, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed June 27, 2018.
- Lonie S, Niumsawatt V, Castley A. A prognostic dilemma of basal cell carcinoma with intravascular invasion. Plast Reconstr Surg Glob Open. 2016;4:e1046.
- Carter JB, Johnson MM, Chua TL, et al. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149:35-41.
Resident Pearl
- Intravascular (also referred to as lymphovascular when involving vessels and/or lymphatics) invasion of cutaneous squamous cell carcinoma can be considered a high-risk factor that may warrant adjuvant therapy.