User login
Using Intraindividual Variability to Evaluate Pediatric Epilepsy
The ability of a patient to focus over time is often impaired among children with epilepsy, and intraindividual variability may be an important way to measure such attentional problems according to a study published in Epilepsy & Behavior.
- Intraindividual variability, a measure of changes in an individual’s transient behavioral performance, has been identified in both pediatric and adult patients with epilepsy.
- Srnka et al evaluated intraindividual variability in 144 patients who had just been diagnosed with epilepsy, using the Connors Continuous Performance Task-II as a metric.
- The children with epilepsy were between the ages of 8 and 18 years and were compared to 82 healthy children.
- The researchers found a large difference in variability between the two groups, with an effect size difference of 0.68.
- They also discovered that intraindividual variability predicted intellectual functioning and academic achievement.
Srnka K, Seidenberg M, Hermann B, Jones J. Intraindividual variability in attentional vigilance in children with epilepsy. Epilepsy Behav. 2018;79:42-45.
The ability of a patient to focus over time is often impaired among children with epilepsy, and intraindividual variability may be an important way to measure such attentional problems according to a study published in Epilepsy & Behavior.
- Intraindividual variability, a measure of changes in an individual’s transient behavioral performance, has been identified in both pediatric and adult patients with epilepsy.
- Srnka et al evaluated intraindividual variability in 144 patients who had just been diagnosed with epilepsy, using the Connors Continuous Performance Task-II as a metric.
- The children with epilepsy were between the ages of 8 and 18 years and were compared to 82 healthy children.
- The researchers found a large difference in variability between the two groups, with an effect size difference of 0.68.
- They also discovered that intraindividual variability predicted intellectual functioning and academic achievement.
Srnka K, Seidenberg M, Hermann B, Jones J. Intraindividual variability in attentional vigilance in children with epilepsy. Epilepsy Behav. 2018;79:42-45.
The ability of a patient to focus over time is often impaired among children with epilepsy, and intraindividual variability may be an important way to measure such attentional problems according to a study published in Epilepsy & Behavior.
- Intraindividual variability, a measure of changes in an individual’s transient behavioral performance, has been identified in both pediatric and adult patients with epilepsy.
- Srnka et al evaluated intraindividual variability in 144 patients who had just been diagnosed with epilepsy, using the Connors Continuous Performance Task-II as a metric.
- The children with epilepsy were between the ages of 8 and 18 years and were compared to 82 healthy children.
- The researchers found a large difference in variability between the two groups, with an effect size difference of 0.68.
- They also discovered that intraindividual variability predicted intellectual functioning and academic achievement.
Srnka K, Seidenberg M, Hermann B, Jones J. Intraindividual variability in attentional vigilance in children with epilepsy. Epilepsy Behav. 2018;79:42-45.
Finding a Presurgical Role for Magnetoencephalography
Magnetoencephalography (MEG) may play an important role in the presurgical workup of patients with nonlesional refractory focal epilepsy suggests a recent observational study.
- Investigators observed 31 patients at an academic epilepsy center to determine if MEG would have had an impact on patient care; (they were unable to analyze the MEG early enough to influence the decision-making process).
- Had the test been integrated into the presurgical workup, 68% of patients would have received different management initially.
- MEG would have reduced the number of patients who received intracranial electrodes.
- MEG would also have led to the position of the electrodes being changed or provided adequate evidence to justify the use of an intracranial electrode.
- The results of the MEG studies would have let surgeons do direct surgery with no need for intracranial electrodes in 2 of 11 patients.
- 6 patients fared poorly after surgery, but MEG would have changed their outcomes in 3 of these patients by modifying the resection margin.
Mohamed IS, Bout hillier A, Bérubé A, et al. The clinical impact of integration of magnetoencephalography in the presurgical workup for refractory nonlesional epilepsy. Epilepsy Behav. 2018;79:34-41.
Magnetoencephalography (MEG) may play an important role in the presurgical workup of patients with nonlesional refractory focal epilepsy suggests a recent observational study.
- Investigators observed 31 patients at an academic epilepsy center to determine if MEG would have had an impact on patient care; (they were unable to analyze the MEG early enough to influence the decision-making process).
- Had the test been integrated into the presurgical workup, 68% of patients would have received different management initially.
- MEG would have reduced the number of patients who received intracranial electrodes.
- MEG would also have led to the position of the electrodes being changed or provided adequate evidence to justify the use of an intracranial electrode.
- The results of the MEG studies would have let surgeons do direct surgery with no need for intracranial electrodes in 2 of 11 patients.
- 6 patients fared poorly after surgery, but MEG would have changed their outcomes in 3 of these patients by modifying the resection margin.
Mohamed IS, Bout hillier A, Bérubé A, et al. The clinical impact of integration of magnetoencephalography in the presurgical workup for refractory nonlesional epilepsy. Epilepsy Behav. 2018;79:34-41.
Magnetoencephalography (MEG) may play an important role in the presurgical workup of patients with nonlesional refractory focal epilepsy suggests a recent observational study.
- Investigators observed 31 patients at an academic epilepsy center to determine if MEG would have had an impact on patient care; (they were unable to analyze the MEG early enough to influence the decision-making process).
- Had the test been integrated into the presurgical workup, 68% of patients would have received different management initially.
- MEG would have reduced the number of patients who received intracranial electrodes.
- MEG would also have led to the position of the electrodes being changed or provided adequate evidence to justify the use of an intracranial electrode.
- The results of the MEG studies would have let surgeons do direct surgery with no need for intracranial electrodes in 2 of 11 patients.
- 6 patients fared poorly after surgery, but MEG would have changed their outcomes in 3 of these patients by modifying the resection margin.
Mohamed IS, Bout hillier A, Bérubé A, et al. The clinical impact of integration of magnetoencephalography in the presurgical workup for refractory nonlesional epilepsy. Epilepsy Behav. 2018;79:34-41.
Comparing PreOp High-Gamma Modulation With Electrical Stimulation
Electrocorticographic (ECoG) high-γ modulation (HGM) can serve as a specific way to localize language preoperatively, when compared with electrical stimulation mapping (ESM), which is considered the gold standard, according to a recent meta-analysis. But the same analysis concluded that it was not sensitive enough when compared with ESM.
- The meta-analysis reviewed several metrics for diagnostic validity, including area under the summary receiver operating characteristic (SROC) curve, diagnostic odds ratio, and pooled estimates of sensitivity and specificity.
- To determine language mapping, the most common task used was overt picture naming.
- ECoG was analyzed at 50 to 400 Hz, with different studies using different bandwidths.
- Among the studies that looked at ESM, there were wide variations in pulse duration, train duration, and maximum current.
- The pooled diagnostic odds ratio was 6.44 and the AUC was 0.77, making HGM a fairly reliable way to ascertain electrodes overlying ESM cortical language sites.
Aryaa R, Horn PS, Crone NE, et al. ECoG high-gamma modulation versus electrical stimulation for presurgical language mapping. Epilepsy Behav. 2018;79:26-33.
Electrocorticographic (ECoG) high-γ modulation (HGM) can serve as a specific way to localize language preoperatively, when compared with electrical stimulation mapping (ESM), which is considered the gold standard, according to a recent meta-analysis. But the same analysis concluded that it was not sensitive enough when compared with ESM.
- The meta-analysis reviewed several metrics for diagnostic validity, including area under the summary receiver operating characteristic (SROC) curve, diagnostic odds ratio, and pooled estimates of sensitivity and specificity.
- To determine language mapping, the most common task used was overt picture naming.
- ECoG was analyzed at 50 to 400 Hz, with different studies using different bandwidths.
- Among the studies that looked at ESM, there were wide variations in pulse duration, train duration, and maximum current.
- The pooled diagnostic odds ratio was 6.44 and the AUC was 0.77, making HGM a fairly reliable way to ascertain electrodes overlying ESM cortical language sites.
Aryaa R, Horn PS, Crone NE, et al. ECoG high-gamma modulation versus electrical stimulation for presurgical language mapping. Epilepsy Behav. 2018;79:26-33.
Electrocorticographic (ECoG) high-γ modulation (HGM) can serve as a specific way to localize language preoperatively, when compared with electrical stimulation mapping (ESM), which is considered the gold standard, according to a recent meta-analysis. But the same analysis concluded that it was not sensitive enough when compared with ESM.
- The meta-analysis reviewed several metrics for diagnostic validity, including area under the summary receiver operating characteristic (SROC) curve, diagnostic odds ratio, and pooled estimates of sensitivity and specificity.
- To determine language mapping, the most common task used was overt picture naming.
- ECoG was analyzed at 50 to 400 Hz, with different studies using different bandwidths.
- Among the studies that looked at ESM, there were wide variations in pulse duration, train duration, and maximum current.
- The pooled diagnostic odds ratio was 6.44 and the AUC was 0.77, making HGM a fairly reliable way to ascertain electrodes overlying ESM cortical language sites.
Aryaa R, Horn PS, Crone NE, et al. ECoG high-gamma modulation versus electrical stimulation for presurgical language mapping. Epilepsy Behav. 2018;79:26-33.
Conference News Roundup—Society for Neuroscience
Transcranial Magnetic Stimulation Improves Memory in Older Adults
A painless and noninvasive brain stimulation technique may help improve some types of memory in older adults, investigators reported.
One possible explanation for age-related memory loss is degradation of the neural connections between the hippocampus and the cortex. Weakening of these connections may lead to difficulties in creating new memories of specific events and the locations of objects. Scientists hypothesized that strengthening the connections between the hippocampus and cortex through repetitive transcranial magnetic stimulation (TMS) may help the storage of new memories. TMS delivers painless magnetic pulses to a particular region of the brain, changing the activity of the neurons within the targeted area.
To determine whether TMS could improve memory, 15 healthy adults over the age of 64 received TMS to a part of the cortex that communicates with the hippocampus. Treatment lasted for five days. During a separate week, each participant received five days of sham treatment, in which the setup was the same, but the stimulation was too low to influence the neural connections. Before and after each five-day session, participants were asked to remember pictures of everyday objects and pictures of outdoor scenes associated with each one. The adults’ ability to recall the scenes associated with the objects improved after receiving TMS, but not after the sham treatment.
“Our study demonstrates that TMS could potentially be used as a way to improve memory for older adults experiencing age-related memory impairments,” said John A. Walker, PhD, postdoctorate fellow at Northwestern University in Evanston, Illinois. “TMS can be used to probe the relationship between brain networks and memory experimentally, opening new doors to understanding the network basis of cognitive decline in aging.”
Heading the Ball Hurts Women More Than Men
Intentionally hitting a soccer ball with the head, or “heading,” may have more adverse brain consequences for women than men, said researchers.
Heading does not typically result in a concussion, yet growing evidence links the move to CNS damage. Previous studies using diffusion tensor imaging (DTI) have revealed that heading damages the integrity of the axons. Women appear to be more vulnerable than men to problems associated with heading, as they report more symptoms that last longer, but the reason for these gender differences remains unknown.
To assess possible gender differences in the effects of heading, researchers used DTI to examine 49 male and 49 female amateur soccer players who were matched on age and frequency of heading. Higher levels of heading were associated with decreased axonal integrity in three brain regions for men and eight brain regions for women. In seven of the areas identified in women, the association between axonal integrity and heading was significantly stronger than it was in men.
“Given similar amounts of exposure to heading, women show a greater volume of abnormality that is significantly different from what is seen in men,” said lead author Todd G. Rubin, MD, a doctoral student at Albert Einstein College of Medicine in Bronx, New York. “Identifying and understanding the basis for differences in susceptibility to injury represent key steps in determining better treatments and guidelines for safer play.”
DBS Can Individualize Treatment for Parkinson’s Disease
A new approach to deep brain stimulation (DBS) adjusts itself to deliver the appropriate amount of stimulation in patients with Parkinson’s disease, according to new research. The approach could improve symptom management and reduce side effects.
DBS has been a valuable treatment for Parkinson’s disease by helping to quell the abnormal movements that are characteristic of the disease. Traditional DBS delivers a constant level of stimulation and cannot adapt if a patient’s symptoms vary over the course of a day. As a result, a patient may sometimes receive too little stimulation, which fails to control symptoms, or too much, which causes side effects such as dyskinesia.
To match stimulation to variations in patient symptoms throughout the day, researchers and engineers developed a novel implantable device that provides DBS and records activity from the surface of the brain. Similar to a cardiac pacemaker, this adaptive device can autoadjust its level of stimulation based on a physiologic signal—in this case, brain activity related to dyskinesia. A high dyskinesia signal indicated greater likelihood of unwanted side effects and caused the device to reduce the stimulation level. A low signal indicated a higher chance of symptoms returning and triggered an increase in stimulation.
The device was tested in two patients inside and outside of the laboratory. Neither patient reported discomfort, adverse events, or worsening symptoms. In addition, the battery used as much as 45% less energy than traditional DBS, which is an important advantage, since battery replacement requires surgery.
“Our study showed that totally implanted, adaptive DBS is feasible and can be used at home in patients,” said lead author Nicole C. Swann, PhD, Assistant Professor of Human Physiology at the University of Oregon in Eugene. “Adaptive stimulation represents one of the first major advances in DBS technology since this technique was first introduced for the treatment of Parkinson’s disease 25 years ago.”
Contact Sports May Impair Memory Temporarily
Sports-related head injuries may prevent the generation of new neurons in a brain region important for memory, said investigators.
Concussion can lead to cognitive impairments, and recent evidence indicates that subconcussive hits can cause damage. The hippocampus is particularly vulnerable. One way to test the effects of head impacts on the hippocampus is a memory assessment called the mnemonic similarity test (MST), which evaluates a person’s ability to distinguish between images that are novel, previously presented, or similar to images previously presented. Accumulating evidence suggests that MST scores are related to the hippocampus’s ability to generate new neurons.
To investigate changes in memory following sports-related head injuries, researchers assessed different types of athletes in two studies. In the first study, they compared athletes with concussion, uninjured athletes who played the same sport, same-sport athletes with musculoskeletal injuries, and healthy controls. Compared with the other three groups, concussed athletes performed worse on the MST when tested two to four weeks after their injury. The scores did not remain low, however. By the time the athletes were cleared to play, their scores had improved to normal levels.
In the second study, rugby players were given the MST before the season started, halfway through the season, and one month after their last game. Scores dropped midseason, compared with preseason scores, but recovered by the postseason assessment.
“Using a cognitive test believed to be sensitive to hippocampal neurogenesis, we found that athletes with concussion show impairments that resolve following recovery,” said lead author Melissa Danielle McCradden, PhD, a postdoctoral fellow at McMaster University in Toronto. “These findings represent, to the best of our knowledge, the first reported evidence in humans suggesting a brain change that might explain the cognitive and emotional symptoms associated with mild traumatic brain injury.”
Disrupted Brain Networks May Cause Gulf War Illness
The brains of veterans with Gulf War illness (GWI) show widespread communication abnormalities in networks that support various brain functions, researchers reported. The observed patterns of impairment provide objective neurophysiologic evidence to support the self-reported symptoms of veterans with GWI.
As many as 250,000 veterans who served in Iraq, Kuwait, and Saudi Arabia during the 1991 Gulf War may currently experience GWI. Symptoms include difficulty remembering things, trouble finding words while speaking, motor coordination, mood swings, fatigue, and chronic pain. GWI is thought to result from exposure to a mix of chemical and biological warfare agents and hazardous chemicals.
To better understand brain changes in GWI, researchers compared the brains of 22 veterans with GWI to the brains of 30 healthy veterans of similar age. Using resting state functional MRI, researchers analyzed patterns of communication among regions of the brain known to control different functions and behavior. They identified changes in functional networks related to many commonly reported GWI symptoms. Individuals with GWI showed clear deficits in neural communication in the sectors of the brain responsible for visual processing, mood regulation, motor coordination, sensory processing, and language command, but increased communication in networks related to pain perception during rest.
“The results from this study provide strong evidence of neuropathology in GWI patients from exposures to neurotoxic agents,” said lead author Kaundinya Gopinath, PhD, Assistant Professor of Radiology and Imaging Sciences at Emory University in Atlanta. Next, “the aim is to establish brain mechanisms underlying GWI, which in turn can lead to development of treatments.”
Prolonged Sedation May Cause Brain Abnormalities in Infants
Full-term infants who undergo repeated anesthesia and prolonged sedation are at risk for changes in brain development, according to investigators.
Developmental impacts of prenatal exposure to sedatives have been studied widely, but less is known about the immediate and long-term neurologic and developmental effects of prolonged sedation when administered to critically ill infants after birth. Prolonged administration of opioids and benzodiazepines, which commonly are used for infants undergoing surgery, is associated with a high incidence of drug tolerance and dependence. Although negative long-term outcomes have been associated with such drug exposures in infants, these studies could not exclude other possible causes, such as prematurity or heart problems.
To study neurologic effects of prolonged sedation, researchers conducted MRI scans on full-term infants who underwent life-saving surgery that required prolonged exposure to morphine and midazolam before one year of age. Brain imaging showed several brain MRI anomalies that were not present in healthy infants, including abnormalities in gray and white matter structures and the ventricles. The number of brain MRI abnormalities significantly correlated with the average daily dose of these sedative drugs. The higher the daily dose, the more MRI irregularities were seen. The patients also had more brain fluid and a smaller total brain volume, compared with healthy infants. This pattern has been associated with long-term neurodevelopmental outcomes such as autism spectrum disorder. Taken together, these preliminary findings indicate a potential negative impact of prolonged sedation on brain growth during the first year of life, the researchers said.
“We were surprised to find higher incidence of brain abnormalities in full-term infants who underwent life-saving surgery that required prolonged sedation,” said senior author Dusica Bajic, MD, PhD, Principal Investigator at Boston Children’s Hospital. “The constellation of MRI irregularities suggests prolonged sedation may potentially contribute to delayed brain growth.” Future investigations will explore the neural mechanisms of the observed developmental effects and whether early sedation exposure may lead to long-term neurobehavioral impacts.
The Brain Preferentially Reactivates Negative Memories During Sleep
The brain selectively reactivates negative memories during sleep, prioritizing the retention of these emotional memories, which may be of greater future relevance than neutral memories and thus more worth remembering, according to investigators.
Over the past two decades, neuroscientists have gained increased understanding of how sleep boosts and stabilizes memories in the human brain. In the current study, researchers presented 57 healthy volunteers with a series of neutral and negative images. While staring straight ahead, the volunteers saw all of the negative images on one side of their field of vision (left) and all of the neutral images on the other side (right). Because the brain processes visual information in the opposite hemisphere from where it is viewed, this method allowed researchers to “tag” one hemisphere with negative content and the other with neutral content, thus enabling them to track localized memories. Participants were then shown the previously seen images for memory tests, with some of the images shown immediately after the learning phase and the rest shown after a period of wakefulness or sleep. During all memory tests, volunteers viewed the images directly in front of them, rather than to either side, and researchers asked participants to state whether an image had originally appeared to the left or right.
Participants who stayed awake in between memory tests forgot some of the original image locations, but forgetting was similar for neutral and negative images. Participants who slept between tests, on the other hand, had a much better rate of recall for the negative images than for the neutral ones. EEG recordings made during the learning phase show that the brain has encoded the distinct types of memories in its two hemispheres, with the negative images strongly encoded in the hemisphere opposite to the side of presentation. Researchers are now analyzing data that they hypothesize will show that the waking EEG pattern corresponding to emotional memories is the same pattern that is reactivated most strongly during sleep.
“This [finding] would provide a long sought-after brain-based explanation of how sleep selectively stabilizes emotional memories,” said lead author Roy Cox, PhD, research fellow in psychiatry at Beth Israel Deaconess Medical Center in Boston. “Our research substantially advances the notion that sleep plays a fundamental and complex role in the offline reorganization of waking experiences.”
Transcranial Magnetic Stimulation Improves Memory in Older Adults
A painless and noninvasive brain stimulation technique may help improve some types of memory in older adults, investigators reported.
One possible explanation for age-related memory loss is degradation of the neural connections between the hippocampus and the cortex. Weakening of these connections may lead to difficulties in creating new memories of specific events and the locations of objects. Scientists hypothesized that strengthening the connections between the hippocampus and cortex through repetitive transcranial magnetic stimulation (TMS) may help the storage of new memories. TMS delivers painless magnetic pulses to a particular region of the brain, changing the activity of the neurons within the targeted area.
To determine whether TMS could improve memory, 15 healthy adults over the age of 64 received TMS to a part of the cortex that communicates with the hippocampus. Treatment lasted for five days. During a separate week, each participant received five days of sham treatment, in which the setup was the same, but the stimulation was too low to influence the neural connections. Before and after each five-day session, participants were asked to remember pictures of everyday objects and pictures of outdoor scenes associated with each one. The adults’ ability to recall the scenes associated with the objects improved after receiving TMS, but not after the sham treatment.
“Our study demonstrates that TMS could potentially be used as a way to improve memory for older adults experiencing age-related memory impairments,” said John A. Walker, PhD, postdoctorate fellow at Northwestern University in Evanston, Illinois. “TMS can be used to probe the relationship between brain networks and memory experimentally, opening new doors to understanding the network basis of cognitive decline in aging.”
Heading the Ball Hurts Women More Than Men
Intentionally hitting a soccer ball with the head, or “heading,” may have more adverse brain consequences for women than men, said researchers.
Heading does not typically result in a concussion, yet growing evidence links the move to CNS damage. Previous studies using diffusion tensor imaging (DTI) have revealed that heading damages the integrity of the axons. Women appear to be more vulnerable than men to problems associated with heading, as they report more symptoms that last longer, but the reason for these gender differences remains unknown.
To assess possible gender differences in the effects of heading, researchers used DTI to examine 49 male and 49 female amateur soccer players who were matched on age and frequency of heading. Higher levels of heading were associated with decreased axonal integrity in three brain regions for men and eight brain regions for women. In seven of the areas identified in women, the association between axonal integrity and heading was significantly stronger than it was in men.
“Given similar amounts of exposure to heading, women show a greater volume of abnormality that is significantly different from what is seen in men,” said lead author Todd G. Rubin, MD, a doctoral student at Albert Einstein College of Medicine in Bronx, New York. “Identifying and understanding the basis for differences in susceptibility to injury represent key steps in determining better treatments and guidelines for safer play.”
DBS Can Individualize Treatment for Parkinson’s Disease
A new approach to deep brain stimulation (DBS) adjusts itself to deliver the appropriate amount of stimulation in patients with Parkinson’s disease, according to new research. The approach could improve symptom management and reduce side effects.
DBS has been a valuable treatment for Parkinson’s disease by helping to quell the abnormal movements that are characteristic of the disease. Traditional DBS delivers a constant level of stimulation and cannot adapt if a patient’s symptoms vary over the course of a day. As a result, a patient may sometimes receive too little stimulation, which fails to control symptoms, or too much, which causes side effects such as dyskinesia.
To match stimulation to variations in patient symptoms throughout the day, researchers and engineers developed a novel implantable device that provides DBS and records activity from the surface of the brain. Similar to a cardiac pacemaker, this adaptive device can autoadjust its level of stimulation based on a physiologic signal—in this case, brain activity related to dyskinesia. A high dyskinesia signal indicated greater likelihood of unwanted side effects and caused the device to reduce the stimulation level. A low signal indicated a higher chance of symptoms returning and triggered an increase in stimulation.
The device was tested in two patients inside and outside of the laboratory. Neither patient reported discomfort, adverse events, or worsening symptoms. In addition, the battery used as much as 45% less energy than traditional DBS, which is an important advantage, since battery replacement requires surgery.
“Our study showed that totally implanted, adaptive DBS is feasible and can be used at home in patients,” said lead author Nicole C. Swann, PhD, Assistant Professor of Human Physiology at the University of Oregon in Eugene. “Adaptive stimulation represents one of the first major advances in DBS technology since this technique was first introduced for the treatment of Parkinson’s disease 25 years ago.”
Contact Sports May Impair Memory Temporarily
Sports-related head injuries may prevent the generation of new neurons in a brain region important for memory, said investigators.
Concussion can lead to cognitive impairments, and recent evidence indicates that subconcussive hits can cause damage. The hippocampus is particularly vulnerable. One way to test the effects of head impacts on the hippocampus is a memory assessment called the mnemonic similarity test (MST), which evaluates a person’s ability to distinguish between images that are novel, previously presented, or similar to images previously presented. Accumulating evidence suggests that MST scores are related to the hippocampus’s ability to generate new neurons.
To investigate changes in memory following sports-related head injuries, researchers assessed different types of athletes in two studies. In the first study, they compared athletes with concussion, uninjured athletes who played the same sport, same-sport athletes with musculoskeletal injuries, and healthy controls. Compared with the other three groups, concussed athletes performed worse on the MST when tested two to four weeks after their injury. The scores did not remain low, however. By the time the athletes were cleared to play, their scores had improved to normal levels.
In the second study, rugby players were given the MST before the season started, halfway through the season, and one month after their last game. Scores dropped midseason, compared with preseason scores, but recovered by the postseason assessment.
“Using a cognitive test believed to be sensitive to hippocampal neurogenesis, we found that athletes with concussion show impairments that resolve following recovery,” said lead author Melissa Danielle McCradden, PhD, a postdoctoral fellow at McMaster University in Toronto. “These findings represent, to the best of our knowledge, the first reported evidence in humans suggesting a brain change that might explain the cognitive and emotional symptoms associated with mild traumatic brain injury.”
Disrupted Brain Networks May Cause Gulf War Illness
The brains of veterans with Gulf War illness (GWI) show widespread communication abnormalities in networks that support various brain functions, researchers reported. The observed patterns of impairment provide objective neurophysiologic evidence to support the self-reported symptoms of veterans with GWI.
As many as 250,000 veterans who served in Iraq, Kuwait, and Saudi Arabia during the 1991 Gulf War may currently experience GWI. Symptoms include difficulty remembering things, trouble finding words while speaking, motor coordination, mood swings, fatigue, and chronic pain. GWI is thought to result from exposure to a mix of chemical and biological warfare agents and hazardous chemicals.
To better understand brain changes in GWI, researchers compared the brains of 22 veterans with GWI to the brains of 30 healthy veterans of similar age. Using resting state functional MRI, researchers analyzed patterns of communication among regions of the brain known to control different functions and behavior. They identified changes in functional networks related to many commonly reported GWI symptoms. Individuals with GWI showed clear deficits in neural communication in the sectors of the brain responsible for visual processing, mood regulation, motor coordination, sensory processing, and language command, but increased communication in networks related to pain perception during rest.
“The results from this study provide strong evidence of neuropathology in GWI patients from exposures to neurotoxic agents,” said lead author Kaundinya Gopinath, PhD, Assistant Professor of Radiology and Imaging Sciences at Emory University in Atlanta. Next, “the aim is to establish brain mechanisms underlying GWI, which in turn can lead to development of treatments.”
Prolonged Sedation May Cause Brain Abnormalities in Infants
Full-term infants who undergo repeated anesthesia and prolonged sedation are at risk for changes in brain development, according to investigators.
Developmental impacts of prenatal exposure to sedatives have been studied widely, but less is known about the immediate and long-term neurologic and developmental effects of prolonged sedation when administered to critically ill infants after birth. Prolonged administration of opioids and benzodiazepines, which commonly are used for infants undergoing surgery, is associated with a high incidence of drug tolerance and dependence. Although negative long-term outcomes have been associated with such drug exposures in infants, these studies could not exclude other possible causes, such as prematurity or heart problems.
To study neurologic effects of prolonged sedation, researchers conducted MRI scans on full-term infants who underwent life-saving surgery that required prolonged exposure to morphine and midazolam before one year of age. Brain imaging showed several brain MRI anomalies that were not present in healthy infants, including abnormalities in gray and white matter structures and the ventricles. The number of brain MRI abnormalities significantly correlated with the average daily dose of these sedative drugs. The higher the daily dose, the more MRI irregularities were seen. The patients also had more brain fluid and a smaller total brain volume, compared with healthy infants. This pattern has been associated with long-term neurodevelopmental outcomes such as autism spectrum disorder. Taken together, these preliminary findings indicate a potential negative impact of prolonged sedation on brain growth during the first year of life, the researchers said.
“We were surprised to find higher incidence of brain abnormalities in full-term infants who underwent life-saving surgery that required prolonged sedation,” said senior author Dusica Bajic, MD, PhD, Principal Investigator at Boston Children’s Hospital. “The constellation of MRI irregularities suggests prolonged sedation may potentially contribute to delayed brain growth.” Future investigations will explore the neural mechanisms of the observed developmental effects and whether early sedation exposure may lead to long-term neurobehavioral impacts.
The Brain Preferentially Reactivates Negative Memories During Sleep
The brain selectively reactivates negative memories during sleep, prioritizing the retention of these emotional memories, which may be of greater future relevance than neutral memories and thus more worth remembering, according to investigators.
Over the past two decades, neuroscientists have gained increased understanding of how sleep boosts and stabilizes memories in the human brain. In the current study, researchers presented 57 healthy volunteers with a series of neutral and negative images. While staring straight ahead, the volunteers saw all of the negative images on one side of their field of vision (left) and all of the neutral images on the other side (right). Because the brain processes visual information in the opposite hemisphere from where it is viewed, this method allowed researchers to “tag” one hemisphere with negative content and the other with neutral content, thus enabling them to track localized memories. Participants were then shown the previously seen images for memory tests, with some of the images shown immediately after the learning phase and the rest shown after a period of wakefulness or sleep. During all memory tests, volunteers viewed the images directly in front of them, rather than to either side, and researchers asked participants to state whether an image had originally appeared to the left or right.
Participants who stayed awake in between memory tests forgot some of the original image locations, but forgetting was similar for neutral and negative images. Participants who slept between tests, on the other hand, had a much better rate of recall for the negative images than for the neutral ones. EEG recordings made during the learning phase show that the brain has encoded the distinct types of memories in its two hemispheres, with the negative images strongly encoded in the hemisphere opposite to the side of presentation. Researchers are now analyzing data that they hypothesize will show that the waking EEG pattern corresponding to emotional memories is the same pattern that is reactivated most strongly during sleep.
“This [finding] would provide a long sought-after brain-based explanation of how sleep selectively stabilizes emotional memories,” said lead author Roy Cox, PhD, research fellow in psychiatry at Beth Israel Deaconess Medical Center in Boston. “Our research substantially advances the notion that sleep plays a fundamental and complex role in the offline reorganization of waking experiences.”
Transcranial Magnetic Stimulation Improves Memory in Older Adults
A painless and noninvasive brain stimulation technique may help improve some types of memory in older adults, investigators reported.
One possible explanation for age-related memory loss is degradation of the neural connections between the hippocampus and the cortex. Weakening of these connections may lead to difficulties in creating new memories of specific events and the locations of objects. Scientists hypothesized that strengthening the connections between the hippocampus and cortex through repetitive transcranial magnetic stimulation (TMS) may help the storage of new memories. TMS delivers painless magnetic pulses to a particular region of the brain, changing the activity of the neurons within the targeted area.
To determine whether TMS could improve memory, 15 healthy adults over the age of 64 received TMS to a part of the cortex that communicates with the hippocampus. Treatment lasted for five days. During a separate week, each participant received five days of sham treatment, in which the setup was the same, but the stimulation was too low to influence the neural connections. Before and after each five-day session, participants were asked to remember pictures of everyday objects and pictures of outdoor scenes associated with each one. The adults’ ability to recall the scenes associated with the objects improved after receiving TMS, but not after the sham treatment.
“Our study demonstrates that TMS could potentially be used as a way to improve memory for older adults experiencing age-related memory impairments,” said John A. Walker, PhD, postdoctorate fellow at Northwestern University in Evanston, Illinois. “TMS can be used to probe the relationship between brain networks and memory experimentally, opening new doors to understanding the network basis of cognitive decline in aging.”
Heading the Ball Hurts Women More Than Men
Intentionally hitting a soccer ball with the head, or “heading,” may have more adverse brain consequences for women than men, said researchers.
Heading does not typically result in a concussion, yet growing evidence links the move to CNS damage. Previous studies using diffusion tensor imaging (DTI) have revealed that heading damages the integrity of the axons. Women appear to be more vulnerable than men to problems associated with heading, as they report more symptoms that last longer, but the reason for these gender differences remains unknown.
To assess possible gender differences in the effects of heading, researchers used DTI to examine 49 male and 49 female amateur soccer players who were matched on age and frequency of heading. Higher levels of heading were associated with decreased axonal integrity in three brain regions for men and eight brain regions for women. In seven of the areas identified in women, the association between axonal integrity and heading was significantly stronger than it was in men.
“Given similar amounts of exposure to heading, women show a greater volume of abnormality that is significantly different from what is seen in men,” said lead author Todd G. Rubin, MD, a doctoral student at Albert Einstein College of Medicine in Bronx, New York. “Identifying and understanding the basis for differences in susceptibility to injury represent key steps in determining better treatments and guidelines for safer play.”
DBS Can Individualize Treatment for Parkinson’s Disease
A new approach to deep brain stimulation (DBS) adjusts itself to deliver the appropriate amount of stimulation in patients with Parkinson’s disease, according to new research. The approach could improve symptom management and reduce side effects.
DBS has been a valuable treatment for Parkinson’s disease by helping to quell the abnormal movements that are characteristic of the disease. Traditional DBS delivers a constant level of stimulation and cannot adapt if a patient’s symptoms vary over the course of a day. As a result, a patient may sometimes receive too little stimulation, which fails to control symptoms, or too much, which causes side effects such as dyskinesia.
To match stimulation to variations in patient symptoms throughout the day, researchers and engineers developed a novel implantable device that provides DBS and records activity from the surface of the brain. Similar to a cardiac pacemaker, this adaptive device can autoadjust its level of stimulation based on a physiologic signal—in this case, brain activity related to dyskinesia. A high dyskinesia signal indicated greater likelihood of unwanted side effects and caused the device to reduce the stimulation level. A low signal indicated a higher chance of symptoms returning and triggered an increase in stimulation.
The device was tested in two patients inside and outside of the laboratory. Neither patient reported discomfort, adverse events, or worsening symptoms. In addition, the battery used as much as 45% less energy than traditional DBS, which is an important advantage, since battery replacement requires surgery.
“Our study showed that totally implanted, adaptive DBS is feasible and can be used at home in patients,” said lead author Nicole C. Swann, PhD, Assistant Professor of Human Physiology at the University of Oregon in Eugene. “Adaptive stimulation represents one of the first major advances in DBS technology since this technique was first introduced for the treatment of Parkinson’s disease 25 years ago.”
Contact Sports May Impair Memory Temporarily
Sports-related head injuries may prevent the generation of new neurons in a brain region important for memory, said investigators.
Concussion can lead to cognitive impairments, and recent evidence indicates that subconcussive hits can cause damage. The hippocampus is particularly vulnerable. One way to test the effects of head impacts on the hippocampus is a memory assessment called the mnemonic similarity test (MST), which evaluates a person’s ability to distinguish between images that are novel, previously presented, or similar to images previously presented. Accumulating evidence suggests that MST scores are related to the hippocampus’s ability to generate new neurons.
To investigate changes in memory following sports-related head injuries, researchers assessed different types of athletes in two studies. In the first study, they compared athletes with concussion, uninjured athletes who played the same sport, same-sport athletes with musculoskeletal injuries, and healthy controls. Compared with the other three groups, concussed athletes performed worse on the MST when tested two to four weeks after their injury. The scores did not remain low, however. By the time the athletes were cleared to play, their scores had improved to normal levels.
In the second study, rugby players were given the MST before the season started, halfway through the season, and one month after their last game. Scores dropped midseason, compared with preseason scores, but recovered by the postseason assessment.
“Using a cognitive test believed to be sensitive to hippocampal neurogenesis, we found that athletes with concussion show impairments that resolve following recovery,” said lead author Melissa Danielle McCradden, PhD, a postdoctoral fellow at McMaster University in Toronto. “These findings represent, to the best of our knowledge, the first reported evidence in humans suggesting a brain change that might explain the cognitive and emotional symptoms associated with mild traumatic brain injury.”
Disrupted Brain Networks May Cause Gulf War Illness
The brains of veterans with Gulf War illness (GWI) show widespread communication abnormalities in networks that support various brain functions, researchers reported. The observed patterns of impairment provide objective neurophysiologic evidence to support the self-reported symptoms of veterans with GWI.
As many as 250,000 veterans who served in Iraq, Kuwait, and Saudi Arabia during the 1991 Gulf War may currently experience GWI. Symptoms include difficulty remembering things, trouble finding words while speaking, motor coordination, mood swings, fatigue, and chronic pain. GWI is thought to result from exposure to a mix of chemical and biological warfare agents and hazardous chemicals.
To better understand brain changes in GWI, researchers compared the brains of 22 veterans with GWI to the brains of 30 healthy veterans of similar age. Using resting state functional MRI, researchers analyzed patterns of communication among regions of the brain known to control different functions and behavior. They identified changes in functional networks related to many commonly reported GWI symptoms. Individuals with GWI showed clear deficits in neural communication in the sectors of the brain responsible for visual processing, mood regulation, motor coordination, sensory processing, and language command, but increased communication in networks related to pain perception during rest.
“The results from this study provide strong evidence of neuropathology in GWI patients from exposures to neurotoxic agents,” said lead author Kaundinya Gopinath, PhD, Assistant Professor of Radiology and Imaging Sciences at Emory University in Atlanta. Next, “the aim is to establish brain mechanisms underlying GWI, which in turn can lead to development of treatments.”
Prolonged Sedation May Cause Brain Abnormalities in Infants
Full-term infants who undergo repeated anesthesia and prolonged sedation are at risk for changes in brain development, according to investigators.
Developmental impacts of prenatal exposure to sedatives have been studied widely, but less is known about the immediate and long-term neurologic and developmental effects of prolonged sedation when administered to critically ill infants after birth. Prolonged administration of opioids and benzodiazepines, which commonly are used for infants undergoing surgery, is associated with a high incidence of drug tolerance and dependence. Although negative long-term outcomes have been associated with such drug exposures in infants, these studies could not exclude other possible causes, such as prematurity or heart problems.
To study neurologic effects of prolonged sedation, researchers conducted MRI scans on full-term infants who underwent life-saving surgery that required prolonged exposure to morphine and midazolam before one year of age. Brain imaging showed several brain MRI anomalies that were not present in healthy infants, including abnormalities in gray and white matter structures and the ventricles. The number of brain MRI abnormalities significantly correlated with the average daily dose of these sedative drugs. The higher the daily dose, the more MRI irregularities were seen. The patients also had more brain fluid and a smaller total brain volume, compared with healthy infants. This pattern has been associated with long-term neurodevelopmental outcomes such as autism spectrum disorder. Taken together, these preliminary findings indicate a potential negative impact of prolonged sedation on brain growth during the first year of life, the researchers said.
“We were surprised to find higher incidence of brain abnormalities in full-term infants who underwent life-saving surgery that required prolonged sedation,” said senior author Dusica Bajic, MD, PhD, Principal Investigator at Boston Children’s Hospital. “The constellation of MRI irregularities suggests prolonged sedation may potentially contribute to delayed brain growth.” Future investigations will explore the neural mechanisms of the observed developmental effects and whether early sedation exposure may lead to long-term neurobehavioral impacts.
The Brain Preferentially Reactivates Negative Memories During Sleep
The brain selectively reactivates negative memories during sleep, prioritizing the retention of these emotional memories, which may be of greater future relevance than neutral memories and thus more worth remembering, according to investigators.
Over the past two decades, neuroscientists have gained increased understanding of how sleep boosts and stabilizes memories in the human brain. In the current study, researchers presented 57 healthy volunteers with a series of neutral and negative images. While staring straight ahead, the volunteers saw all of the negative images on one side of their field of vision (left) and all of the neutral images on the other side (right). Because the brain processes visual information in the opposite hemisphere from where it is viewed, this method allowed researchers to “tag” one hemisphere with negative content and the other with neutral content, thus enabling them to track localized memories. Participants were then shown the previously seen images for memory tests, with some of the images shown immediately after the learning phase and the rest shown after a period of wakefulness or sleep. During all memory tests, volunteers viewed the images directly in front of them, rather than to either side, and researchers asked participants to state whether an image had originally appeared to the left or right.
Participants who stayed awake in between memory tests forgot some of the original image locations, but forgetting was similar for neutral and negative images. Participants who slept between tests, on the other hand, had a much better rate of recall for the negative images than for the neutral ones. EEG recordings made during the learning phase show that the brain has encoded the distinct types of memories in its two hemispheres, with the negative images strongly encoded in the hemisphere opposite to the side of presentation. Researchers are now analyzing data that they hypothesize will show that the waking EEG pattern corresponding to emotional memories is the same pattern that is reactivated most strongly during sleep.
“This [finding] would provide a long sought-after brain-based explanation of how sleep selectively stabilizes emotional memories,” said lead author Roy Cox, PhD, research fellow in psychiatry at Beth Israel Deaconess Medical Center in Boston. “Our research substantially advances the notion that sleep plays a fundamental and complex role in the offline reorganization of waking experiences.”
Know risk factors for ischemic colitis after AAA repair
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
CHICAGO – Postoperative ischemic colitis after abdominal aortic aneurysm (AAA) repair is a feared, potentially devastating complication with a mortality approaching 50%, but early diagnosis can mitigate that risk, Roy M. Fujitani, MD, said at a symposium on vascular surgery sponsored by Northwestern University in Chicago.
The most common etiology of ischemic colitis following AAA repair is hypoperfusion of the mesenteric vasculature leading to nonocclusive ischemia. Caught early – in the initial hyperactive phase of colonic ischemia – the complication is typically transient and can be managed medically without further sequelae. Improvement is generally noted within a day or 2, with complete resolution within 1-2 weeks.
The earliest indicator that a patient is in the hyperactive phase of ischemic colitis following completion of an AAA repair can be defecation while still on the operating table.
“When you’ve just completed an operation and the patient has a bowel movement right on the operating table, that always makes me very, very concerned because of the likelihood of an associated ischemic colitis,” the surgeon noted.
A conscious patient in the first phase of ischemic colitis will describe an urgent desire to defecate, along with crampy pain and loose bowel movements with or without blood in the stool.
In the second, paralytic phase of ischemic colitis, the pain diminishes in intensity but becomes more continuous and diffuse, usually in the lateral borders of the abdomen. The abdomen becomes distended and much more tender, and there are no bowel sounds.
In patients whose ischemic colitis has been misdiagnosed or undiagnosed, the shock phase comes next. This is marked by massive fluid, protein, and electrolyte loss through the gangrenous mucosa. The result is severe dehydration, metabolic acidosis, and hypovolemic shock.
Nonocclusive colonic ischemia most often affects the watershed areas of the colon, such as the Sudeck point at the rectosigmoid junction.
The two other etiologies of ischemic colitis occurring as a complication of AAA repair are acute arterial occlusion, typically caused by iatrogenic embolization from a proximal source, often during endovascular aneurysm repair (EVAR), or rarely, venous thrombosis.
Making the diagnosis
When a patient is suspected of having ischemic colitis, one of the easiest ways of advancing toward a diagnosis is to obtain an abdominal plain x-ray, which classically shows thumb printing indicative of submucosal edema. CT with IV contrast typically shows bowel wall thickening, pericolonic fat stranding, and – most significantly – there may be free air within the colonic wall, an indicator of more advanced ischemia that occurs shortly before transmural gangrenous changes.
Colonoscopy is, however, the mainstay of diagnosis. It should be performed in any patient where postoperative ischemic colitis is suspected.
Ischemic colitis risk factors and outcomes
Dr. Fujitani was senior author of the largest ever study of risk factors for and outcomes of postoperative ischemic colitis in patients undergoing contemporary methods of open and endovascular AAA repair. This retrospective analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database included 3,486 patients who underwent AAA repair in U.S. hospitals during 2011-2012. Twelve percent had an open repair, while the other 88% underwent EVAR.
The incidence of postoperative ischemic colitis was 2.2%. The median time of diagnosis was on postoperative day 2. The rate was nearly threefold higher in the open repair group: 5.2% versus 1.8%. However, the open-repair group had a higher rate of emergency admission, ruptured aneurysm before surgery, and other high-risk features. Upon multivariate analysis, the adjusted risk of postoperative ischemic colitis was no longer significantly different in the open-repair and EVAR groups.
The mean hospital length of stay in patients with postoperative ischemic colitis was 20 days, compared with 5 days in those without the complication. The unadjusted in-hospital mortality rate in patients with ischemic colitis was 39% versus 4% in those without ischemic colitis.
Of the 75 patients who developed postoperative ischemic colitis, 37 were managed medically, 38 surgically.
“What was quite surprising was that there was a 56.8% in-hospital mortality in the surgically treated patients. The point being that if you end up having ischemic colitis, there’s a 50% chance you’ll end up requiring an operation, and if you do undergo an operation you have more than a 50% chance of succumbing from the process,” Dr. Fujitani observed.
Dr. Fujitani and his coinvestigators scrutinized a plethora of potential risk factors for postoperative ischemic colitis. Six emerged as significant upon multivariate analysis: ruptured aneurysm before surgery, with an associated adjusted 4.1-fold increased risk; need for intra- or postoperative transfusion, with a 6-fold increased risk; renal failure requiring dialysis, with a 3.9-fold risk; proximal extension of the aneurysm, with a 2.2-fold elevation in risk; diabetes, with a 1.9-fold risk; and female sex, with an adjusted 1.75-fold increased risk (J Vasc Surg. 2016 Apr;63[4]:866-72).
Of note, these risk factors are largely unmodifiable, which underscores the importance of vigorous surveillance for possible signs of ischemic colitis during the first 4 days after AAA repair, especially in patients with multiple risk factors, Dr. Fujitani said.
Also, careful intraoperative assessment of the collateral mesenteric vascular anatomy is important in assessing a patient’s risk for postoperative ischemic colitis. This assessment should include the superior and inferior mesenteric arteries, as well as the celiac and internal iliac arteries. It’s worth bearing in mind that, even though collateral flow may appear adequate, it can be affected by hypovolemia, hypotension, or low cardiac output, the surgeon continued.
In the NSQIP data analysis, no patients who underwent reimplantation of the inferior mesenteric artery during open repair developed postoperative ischemic colitis. While this is an encouraging finding, the numbers were too small to draw definitive conclusions as to whether reimplantation of the artery is protective. It’s an important issue for further study, though, since so few of the recognized risk factors for the complication are modifiable, Dr. Fujitani noted.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
CHICAGO – Postoperative ischemic colitis after abdominal aortic aneurysm (AAA) repair is a feared, potentially devastating complication with a mortality approaching 50%, but early diagnosis can mitigate that risk, Roy M. Fujitani, MD, said at a symposium on vascular surgery sponsored by Northwestern University in Chicago.
The most common etiology of ischemic colitis following AAA repair is hypoperfusion of the mesenteric vasculature leading to nonocclusive ischemia. Caught early – in the initial hyperactive phase of colonic ischemia – the complication is typically transient and can be managed medically without further sequelae. Improvement is generally noted within a day or 2, with complete resolution within 1-2 weeks.
The earliest indicator that a patient is in the hyperactive phase of ischemic colitis following completion of an AAA repair can be defecation while still on the operating table.
“When you’ve just completed an operation and the patient has a bowel movement right on the operating table, that always makes me very, very concerned because of the likelihood of an associated ischemic colitis,” the surgeon noted.
A conscious patient in the first phase of ischemic colitis will describe an urgent desire to defecate, along with crampy pain and loose bowel movements with or without blood in the stool.
In the second, paralytic phase of ischemic colitis, the pain diminishes in intensity but becomes more continuous and diffuse, usually in the lateral borders of the abdomen. The abdomen becomes distended and much more tender, and there are no bowel sounds.
In patients whose ischemic colitis has been misdiagnosed or undiagnosed, the shock phase comes next. This is marked by massive fluid, protein, and electrolyte loss through the gangrenous mucosa. The result is severe dehydration, metabolic acidosis, and hypovolemic shock.
Nonocclusive colonic ischemia most often affects the watershed areas of the colon, such as the Sudeck point at the rectosigmoid junction.
The two other etiologies of ischemic colitis occurring as a complication of AAA repair are acute arterial occlusion, typically caused by iatrogenic embolization from a proximal source, often during endovascular aneurysm repair (EVAR), or rarely, venous thrombosis.
Making the diagnosis
When a patient is suspected of having ischemic colitis, one of the easiest ways of advancing toward a diagnosis is to obtain an abdominal plain x-ray, which classically shows thumb printing indicative of submucosal edema. CT with IV contrast typically shows bowel wall thickening, pericolonic fat stranding, and – most significantly – there may be free air within the colonic wall, an indicator of more advanced ischemia that occurs shortly before transmural gangrenous changes.
Colonoscopy is, however, the mainstay of diagnosis. It should be performed in any patient where postoperative ischemic colitis is suspected.
Ischemic colitis risk factors and outcomes
Dr. Fujitani was senior author of the largest ever study of risk factors for and outcomes of postoperative ischemic colitis in patients undergoing contemporary methods of open and endovascular AAA repair. This retrospective analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database included 3,486 patients who underwent AAA repair in U.S. hospitals during 2011-2012. Twelve percent had an open repair, while the other 88% underwent EVAR.
The incidence of postoperative ischemic colitis was 2.2%. The median time of diagnosis was on postoperative day 2. The rate was nearly threefold higher in the open repair group: 5.2% versus 1.8%. However, the open-repair group had a higher rate of emergency admission, ruptured aneurysm before surgery, and other high-risk features. Upon multivariate analysis, the adjusted risk of postoperative ischemic colitis was no longer significantly different in the open-repair and EVAR groups.
The mean hospital length of stay in patients with postoperative ischemic colitis was 20 days, compared with 5 days in those without the complication. The unadjusted in-hospital mortality rate in patients with ischemic colitis was 39% versus 4% in those without ischemic colitis.
Of the 75 patients who developed postoperative ischemic colitis, 37 were managed medically, 38 surgically.
“What was quite surprising was that there was a 56.8% in-hospital mortality in the surgically treated patients. The point being that if you end up having ischemic colitis, there’s a 50% chance you’ll end up requiring an operation, and if you do undergo an operation you have more than a 50% chance of succumbing from the process,” Dr. Fujitani observed.
Dr. Fujitani and his coinvestigators scrutinized a plethora of potential risk factors for postoperative ischemic colitis. Six emerged as significant upon multivariate analysis: ruptured aneurysm before surgery, with an associated adjusted 4.1-fold increased risk; need for intra- or postoperative transfusion, with a 6-fold increased risk; renal failure requiring dialysis, with a 3.9-fold risk; proximal extension of the aneurysm, with a 2.2-fold elevation in risk; diabetes, with a 1.9-fold risk; and female sex, with an adjusted 1.75-fold increased risk (J Vasc Surg. 2016 Apr;63[4]:866-72).
Of note, these risk factors are largely unmodifiable, which underscores the importance of vigorous surveillance for possible signs of ischemic colitis during the first 4 days after AAA repair, especially in patients with multiple risk factors, Dr. Fujitani said.
Also, careful intraoperative assessment of the collateral mesenteric vascular anatomy is important in assessing a patient’s risk for postoperative ischemic colitis. This assessment should include the superior and inferior mesenteric arteries, as well as the celiac and internal iliac arteries. It’s worth bearing in mind that, even though collateral flow may appear adequate, it can be affected by hypovolemia, hypotension, or low cardiac output, the surgeon continued.
In the NSQIP data analysis, no patients who underwent reimplantation of the inferior mesenteric artery during open repair developed postoperative ischemic colitis. While this is an encouraging finding, the numbers were too small to draw definitive conclusions as to whether reimplantation of the artery is protective. It’s an important issue for further study, though, since so few of the recognized risk factors for the complication are modifiable, Dr. Fujitani noted.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
CHICAGO – Postoperative ischemic colitis after abdominal aortic aneurysm (AAA) repair is a feared, potentially devastating complication with a mortality approaching 50%, but early diagnosis can mitigate that risk, Roy M. Fujitani, MD, said at a symposium on vascular surgery sponsored by Northwestern University in Chicago.
The most common etiology of ischemic colitis following AAA repair is hypoperfusion of the mesenteric vasculature leading to nonocclusive ischemia. Caught early – in the initial hyperactive phase of colonic ischemia – the complication is typically transient and can be managed medically without further sequelae. Improvement is generally noted within a day or 2, with complete resolution within 1-2 weeks.
The earliest indicator that a patient is in the hyperactive phase of ischemic colitis following completion of an AAA repair can be defecation while still on the operating table.
“When you’ve just completed an operation and the patient has a bowel movement right on the operating table, that always makes me very, very concerned because of the likelihood of an associated ischemic colitis,” the surgeon noted.
A conscious patient in the first phase of ischemic colitis will describe an urgent desire to defecate, along with crampy pain and loose bowel movements with or without blood in the stool.
In the second, paralytic phase of ischemic colitis, the pain diminishes in intensity but becomes more continuous and diffuse, usually in the lateral borders of the abdomen. The abdomen becomes distended and much more tender, and there are no bowel sounds.
In patients whose ischemic colitis has been misdiagnosed or undiagnosed, the shock phase comes next. This is marked by massive fluid, protein, and electrolyte loss through the gangrenous mucosa. The result is severe dehydration, metabolic acidosis, and hypovolemic shock.
Nonocclusive colonic ischemia most often affects the watershed areas of the colon, such as the Sudeck point at the rectosigmoid junction.
The two other etiologies of ischemic colitis occurring as a complication of AAA repair are acute arterial occlusion, typically caused by iatrogenic embolization from a proximal source, often during endovascular aneurysm repair (EVAR), or rarely, venous thrombosis.
Making the diagnosis
When a patient is suspected of having ischemic colitis, one of the easiest ways of advancing toward a diagnosis is to obtain an abdominal plain x-ray, which classically shows thumb printing indicative of submucosal edema. CT with IV contrast typically shows bowel wall thickening, pericolonic fat stranding, and – most significantly – there may be free air within the colonic wall, an indicator of more advanced ischemia that occurs shortly before transmural gangrenous changes.
Colonoscopy is, however, the mainstay of diagnosis. It should be performed in any patient where postoperative ischemic colitis is suspected.
Ischemic colitis risk factors and outcomes
Dr. Fujitani was senior author of the largest ever study of risk factors for and outcomes of postoperative ischemic colitis in patients undergoing contemporary methods of open and endovascular AAA repair. This retrospective analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database included 3,486 patients who underwent AAA repair in U.S. hospitals during 2011-2012. Twelve percent had an open repair, while the other 88% underwent EVAR.
The incidence of postoperative ischemic colitis was 2.2%. The median time of diagnosis was on postoperative day 2. The rate was nearly threefold higher in the open repair group: 5.2% versus 1.8%. However, the open-repair group had a higher rate of emergency admission, ruptured aneurysm before surgery, and other high-risk features. Upon multivariate analysis, the adjusted risk of postoperative ischemic colitis was no longer significantly different in the open-repair and EVAR groups.
The mean hospital length of stay in patients with postoperative ischemic colitis was 20 days, compared with 5 days in those without the complication. The unadjusted in-hospital mortality rate in patients with ischemic colitis was 39% versus 4% in those without ischemic colitis.
Of the 75 patients who developed postoperative ischemic colitis, 37 were managed medically, 38 surgically.
“What was quite surprising was that there was a 56.8% in-hospital mortality in the surgically treated patients. The point being that if you end up having ischemic colitis, there’s a 50% chance you’ll end up requiring an operation, and if you do undergo an operation you have more than a 50% chance of succumbing from the process,” Dr. Fujitani observed.
Dr. Fujitani and his coinvestigators scrutinized a plethora of potential risk factors for postoperative ischemic colitis. Six emerged as significant upon multivariate analysis: ruptured aneurysm before surgery, with an associated adjusted 4.1-fold increased risk; need for intra- or postoperative transfusion, with a 6-fold increased risk; renal failure requiring dialysis, with a 3.9-fold risk; proximal extension of the aneurysm, with a 2.2-fold elevation in risk; diabetes, with a 1.9-fold risk; and female sex, with an adjusted 1.75-fold increased risk (J Vasc Surg. 2016 Apr;63[4]:866-72).
Of note, these risk factors are largely unmodifiable, which underscores the importance of vigorous surveillance for possible signs of ischemic colitis during the first 4 days after AAA repair, especially in patients with multiple risk factors, Dr. Fujitani said.
Also, careful intraoperative assessment of the collateral mesenteric vascular anatomy is important in assessing a patient’s risk for postoperative ischemic colitis. This assessment should include the superior and inferior mesenteric arteries, as well as the celiac and internal iliac arteries. It’s worth bearing in mind that, even though collateral flow may appear adequate, it can be affected by hypovolemia, hypotension, or low cardiac output, the surgeon continued.
In the NSQIP data analysis, no patients who underwent reimplantation of the inferior mesenteric artery during open repair developed postoperative ischemic colitis. While this is an encouraging finding, the numbers were too small to draw definitive conclusions as to whether reimplantation of the artery is protective. It’s an important issue for further study, though, since so few of the recognized risk factors for the complication are modifiable, Dr. Fujitani noted.
He reported having no financial conflicts regarding his presentation.
Lipid variability predicts cardiovascular events, diabetes onset
ANAHEIM, CALIF. – , David D. Waters, MD, reported at the American Heart Association scientific sessions.
More specifically, above-average visit-to-visit variability in fasting triglycerides, LDL cholesterol, or HDL cholesterol in atorvastatin-treated patients with known coronary artery disease proved to be a strong and independent predictor of coronary and cardiovascular events in a post hoc analysis of the landmark Treating to New Targets (TNT) trial (N Engl J Med 2005;352:1425-35).
The TNT trial randomized more than 10,000 subjects with known coronary artery disease and a baseline LDL cholesterol level below 130 mg/dL to receive either 10 or 80 mg/day of atorvastatin, with fasting lipids measured in a central laboratory at 3 and 12 months, then annually. The trial demonstrated that high-intensity statin therapy was more effective at preventing cardiovascular events than moderate-intensity therapy, thereby ushering in major changes in clinical practice guidelines.
The TNT investigators had previously reported that higher visit-to-visit variability in LDL cholesterol was independently associated with an increased rate of cardiovascular events during the median 4.9 years of study follow-up. In a multivariate regression analysis, each 1 standard deviation increase in average successive variability – that is, the average absolute difference between successive LDL cholesterol values – was associated with a 16% increase in the risk of any coronary event, an 11% increase in risk of any cardiovascular event, a 10% increase in MI, an 17% increase in stroke, and a 23% higher all-cause mortality independent of assignment treatment, achieved LDL cholesterol, demographics, and baseline cardiovascular risk factors.
At the AHA meeting in Anaheim, Dr. Waters presented an expanded analysis of 9,572 TNT participants that incorporated visit-to-visit variability in HDL cholesterol and triglycerides (J Am Coll Cardiol. 2015 Apr 21;65[15]:1539-48). Patients with 1 standard deviation of average successive variability (ASV) in triglycerides – that is, more than 30 mg/dL of visit-to-visit variability – had a 9% increased risk of coronary events during follow-up in a multivariate analysis. Patients with more than 4 mg/dL of variability in HDL cholesterol had a 16% increased risk compared with those with lesser variability.
“For both coronary and cardiovascular events, most of the increased risk appears to reside in the uppermost quintile,” the cardiologist observed.
Indeed, when the investigators divided patients into quintiles of ASV, the top quintile in terms of triglyceride variability had a 34% greater risk of coronary events, a 31% increase in risk of cardiovascular events, a 63% increase in stroke, a 65% increase in nonfatal MI, and a 92% greater likelihood of new-onset diabetes compared with patients in the lowest quintile of ASV. In contrast, these risks were not significantly elevated in the second, third, and fourth quintiles.
Similarly, patients in the top quintile for HDL cholesterol ASV had a 50% greater rate of coronary events, a 56% increased risk of cardiovascular events, a 70% increase in stroke, and a 61% increase in nonfatal MI, compared with those in the lowest quintile. Again, risks weren’t significantly increased in the second through fourth quintiles. Unlike with triglycerides, greater variability in fasting HDL cholesterol over time wasn’t predictive of new-onset diabetes.
Observers noted that these findings could be clinically relevant for patients who remain at high residual risk for atherosclerotic cardiovascular events even after aggressive LDL cholesterol lowering.
Variability in levels of the three lipids was only weakly correlated.
Dr. Waters made a plea to his audience, “The mechanisms accounting for these associations are unknown. If you can suggest for me any possibility of what the causes are, I’d be very happy to hear it and go back to try to verify it.”
He reported serving as a consultant to Resverlogix, CSL Limited, the Medicines Company, Pfizer, and Sanofi-Aventis.
ANAHEIM, CALIF. – , David D. Waters, MD, reported at the American Heart Association scientific sessions.
More specifically, above-average visit-to-visit variability in fasting triglycerides, LDL cholesterol, or HDL cholesterol in atorvastatin-treated patients with known coronary artery disease proved to be a strong and independent predictor of coronary and cardiovascular events in a post hoc analysis of the landmark Treating to New Targets (TNT) trial (N Engl J Med 2005;352:1425-35).
The TNT trial randomized more than 10,000 subjects with known coronary artery disease and a baseline LDL cholesterol level below 130 mg/dL to receive either 10 or 80 mg/day of atorvastatin, with fasting lipids measured in a central laboratory at 3 and 12 months, then annually. The trial demonstrated that high-intensity statin therapy was more effective at preventing cardiovascular events than moderate-intensity therapy, thereby ushering in major changes in clinical practice guidelines.
The TNT investigators had previously reported that higher visit-to-visit variability in LDL cholesterol was independently associated with an increased rate of cardiovascular events during the median 4.9 years of study follow-up. In a multivariate regression analysis, each 1 standard deviation increase in average successive variability – that is, the average absolute difference between successive LDL cholesterol values – was associated with a 16% increase in the risk of any coronary event, an 11% increase in risk of any cardiovascular event, a 10% increase in MI, an 17% increase in stroke, and a 23% higher all-cause mortality independent of assignment treatment, achieved LDL cholesterol, demographics, and baseline cardiovascular risk factors.
At the AHA meeting in Anaheim, Dr. Waters presented an expanded analysis of 9,572 TNT participants that incorporated visit-to-visit variability in HDL cholesterol and triglycerides (J Am Coll Cardiol. 2015 Apr 21;65[15]:1539-48). Patients with 1 standard deviation of average successive variability (ASV) in triglycerides – that is, more than 30 mg/dL of visit-to-visit variability – had a 9% increased risk of coronary events during follow-up in a multivariate analysis. Patients with more than 4 mg/dL of variability in HDL cholesterol had a 16% increased risk compared with those with lesser variability.
“For both coronary and cardiovascular events, most of the increased risk appears to reside in the uppermost quintile,” the cardiologist observed.
Indeed, when the investigators divided patients into quintiles of ASV, the top quintile in terms of triglyceride variability had a 34% greater risk of coronary events, a 31% increase in risk of cardiovascular events, a 63% increase in stroke, a 65% increase in nonfatal MI, and a 92% greater likelihood of new-onset diabetes compared with patients in the lowest quintile of ASV. In contrast, these risks were not significantly elevated in the second, third, and fourth quintiles.
Similarly, patients in the top quintile for HDL cholesterol ASV had a 50% greater rate of coronary events, a 56% increased risk of cardiovascular events, a 70% increase in stroke, and a 61% increase in nonfatal MI, compared with those in the lowest quintile. Again, risks weren’t significantly increased in the second through fourth quintiles. Unlike with triglycerides, greater variability in fasting HDL cholesterol over time wasn’t predictive of new-onset diabetes.
Observers noted that these findings could be clinically relevant for patients who remain at high residual risk for atherosclerotic cardiovascular events even after aggressive LDL cholesterol lowering.
Variability in levels of the three lipids was only weakly correlated.
Dr. Waters made a plea to his audience, “The mechanisms accounting for these associations are unknown. If you can suggest for me any possibility of what the causes are, I’d be very happy to hear it and go back to try to verify it.”
He reported serving as a consultant to Resverlogix, CSL Limited, the Medicines Company, Pfizer, and Sanofi-Aventis.
ANAHEIM, CALIF. – , David D. Waters, MD, reported at the American Heart Association scientific sessions.
More specifically, above-average visit-to-visit variability in fasting triglycerides, LDL cholesterol, or HDL cholesterol in atorvastatin-treated patients with known coronary artery disease proved to be a strong and independent predictor of coronary and cardiovascular events in a post hoc analysis of the landmark Treating to New Targets (TNT) trial (N Engl J Med 2005;352:1425-35).
The TNT trial randomized more than 10,000 subjects with known coronary artery disease and a baseline LDL cholesterol level below 130 mg/dL to receive either 10 or 80 mg/day of atorvastatin, with fasting lipids measured in a central laboratory at 3 and 12 months, then annually. The trial demonstrated that high-intensity statin therapy was more effective at preventing cardiovascular events than moderate-intensity therapy, thereby ushering in major changes in clinical practice guidelines.
The TNT investigators had previously reported that higher visit-to-visit variability in LDL cholesterol was independently associated with an increased rate of cardiovascular events during the median 4.9 years of study follow-up. In a multivariate regression analysis, each 1 standard deviation increase in average successive variability – that is, the average absolute difference between successive LDL cholesterol values – was associated with a 16% increase in the risk of any coronary event, an 11% increase in risk of any cardiovascular event, a 10% increase in MI, an 17% increase in stroke, and a 23% higher all-cause mortality independent of assignment treatment, achieved LDL cholesterol, demographics, and baseline cardiovascular risk factors.
At the AHA meeting in Anaheim, Dr. Waters presented an expanded analysis of 9,572 TNT participants that incorporated visit-to-visit variability in HDL cholesterol and triglycerides (J Am Coll Cardiol. 2015 Apr 21;65[15]:1539-48). Patients with 1 standard deviation of average successive variability (ASV) in triglycerides – that is, more than 30 mg/dL of visit-to-visit variability – had a 9% increased risk of coronary events during follow-up in a multivariate analysis. Patients with more than 4 mg/dL of variability in HDL cholesterol had a 16% increased risk compared with those with lesser variability.
“For both coronary and cardiovascular events, most of the increased risk appears to reside in the uppermost quintile,” the cardiologist observed.
Indeed, when the investigators divided patients into quintiles of ASV, the top quintile in terms of triglyceride variability had a 34% greater risk of coronary events, a 31% increase in risk of cardiovascular events, a 63% increase in stroke, a 65% increase in nonfatal MI, and a 92% greater likelihood of new-onset diabetes compared with patients in the lowest quintile of ASV. In contrast, these risks were not significantly elevated in the second, third, and fourth quintiles.
Similarly, patients in the top quintile for HDL cholesterol ASV had a 50% greater rate of coronary events, a 56% increased risk of cardiovascular events, a 70% increase in stroke, and a 61% increase in nonfatal MI, compared with those in the lowest quintile. Again, risks weren’t significantly increased in the second through fourth quintiles. Unlike with triglycerides, greater variability in fasting HDL cholesterol over time wasn’t predictive of new-onset diabetes.
Observers noted that these findings could be clinically relevant for patients who remain at high residual risk for atherosclerotic cardiovascular events even after aggressive LDL cholesterol lowering.
Variability in levels of the three lipids was only weakly correlated.
Dr. Waters made a plea to his audience, “The mechanisms accounting for these associations are unknown. If you can suggest for me any possibility of what the causes are, I’d be very happy to hear it and go back to try to verify it.”
He reported serving as a consultant to Resverlogix, CSL Limited, the Medicines Company, Pfizer, and Sanofi-Aventis.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Variability in fasting lipids over time in statin-treated patients is of prognostic importance.
Major finding: More than 30 mg/dL of visit-to-visit variability in triglycerides was independently associated with a 34% increase in risk of coronary events and a 31% increase in cardiovascular events.
Study details: This was a post hoc analysis of the clinical impact of visit-to-visit variability in fasting lipids in 9,572 participants in the randomized, double-blind TNT trial, all of whom were on statin therapy.
Disclosures: The presenter reported serving as a consultant to Pfizer, which sponsored the TNT trial, as well as to several other companies.
FDA adds boxed warning to obeticholic acid label
The Food and Drug Administration is requiring a boxed warning on the label for obeticholic acid (Ocaliva) to highlight the correct weekly dosing regimen after incorrect daily dosing caused severe liver injury in patients with moderate to severe primary biliary cholangitis (PBC).
“FDA is adding a new Boxed Warning, FDA’s most prominent warning, to highlight this information in the prescribing information of the drug label,” FDA officials said in a statement Feb. 1. “To ensure correct dosing and reduce the risk of liver problems, FDA is clarifying the current recommendations for screening, dosing, monitoring, and managing PBC patients with moderate to severe liver disease taking Ocaliva.”
FDA recommends that “health care professionals should follow the Ocaliva dosing regimen in the drug label. … Dosing higher than recommended in the drug label can increase the risk for liver decompensation, liver failure, and sometimes death. Routinely monitor all patients for biochemical response, tolerability, and PBC progression, and reevaluate Child-Pugh classification to determine if dosage adjustment is needed.”
Manufacturer Intercept Pharmaceuticals was required to continue studying obeticholic acid in patients with advanced PBC as a condition of its FDA approval. Results from these studies are expected in 2023, FDA noted.
To report adverse medication events and side effects to the FDA, access the MedWatch program.
The Food and Drug Administration is requiring a boxed warning on the label for obeticholic acid (Ocaliva) to highlight the correct weekly dosing regimen after incorrect daily dosing caused severe liver injury in patients with moderate to severe primary biliary cholangitis (PBC).
“FDA is adding a new Boxed Warning, FDA’s most prominent warning, to highlight this information in the prescribing information of the drug label,” FDA officials said in a statement Feb. 1. “To ensure correct dosing and reduce the risk of liver problems, FDA is clarifying the current recommendations for screening, dosing, monitoring, and managing PBC patients with moderate to severe liver disease taking Ocaliva.”
FDA recommends that “health care professionals should follow the Ocaliva dosing regimen in the drug label. … Dosing higher than recommended in the drug label can increase the risk for liver decompensation, liver failure, and sometimes death. Routinely monitor all patients for biochemical response, tolerability, and PBC progression, and reevaluate Child-Pugh classification to determine if dosage adjustment is needed.”
Manufacturer Intercept Pharmaceuticals was required to continue studying obeticholic acid in patients with advanced PBC as a condition of its FDA approval. Results from these studies are expected in 2023, FDA noted.
To report adverse medication events and side effects to the FDA, access the MedWatch program.
The Food and Drug Administration is requiring a boxed warning on the label for obeticholic acid (Ocaliva) to highlight the correct weekly dosing regimen after incorrect daily dosing caused severe liver injury in patients with moderate to severe primary biliary cholangitis (PBC).
“FDA is adding a new Boxed Warning, FDA’s most prominent warning, to highlight this information in the prescribing information of the drug label,” FDA officials said in a statement Feb. 1. “To ensure correct dosing and reduce the risk of liver problems, FDA is clarifying the current recommendations for screening, dosing, monitoring, and managing PBC patients with moderate to severe liver disease taking Ocaliva.”
FDA recommends that “health care professionals should follow the Ocaliva dosing regimen in the drug label. … Dosing higher than recommended in the drug label can increase the risk for liver decompensation, liver failure, and sometimes death. Routinely monitor all patients for biochemical response, tolerability, and PBC progression, and reevaluate Child-Pugh classification to determine if dosage adjustment is needed.”
Manufacturer Intercept Pharmaceuticals was required to continue studying obeticholic acid in patients with advanced PBC as a condition of its FDA approval. Results from these studies are expected in 2023, FDA noted.
To report adverse medication events and side effects to the FDA, access the MedWatch program.
Total Shoulder Arthroplasty Using a Bone-Sparing, Precision Multiplanar Humeral Prosthesis
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).
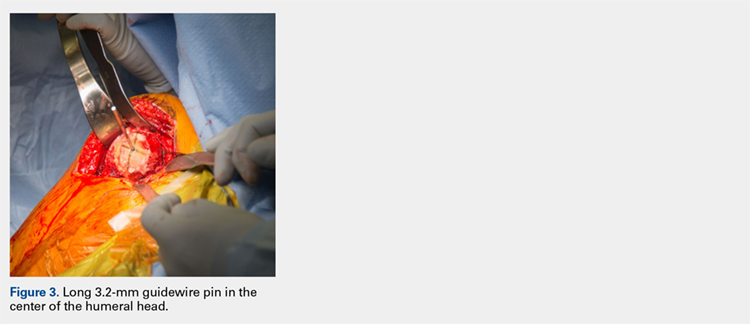
Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).
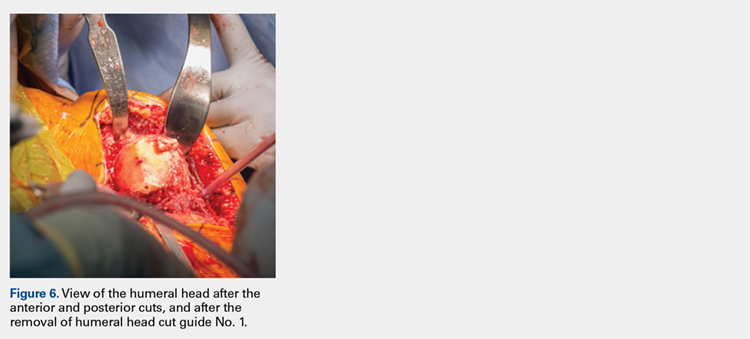
The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).
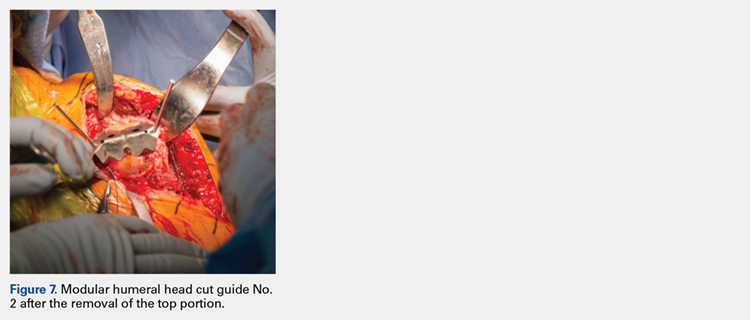
The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.
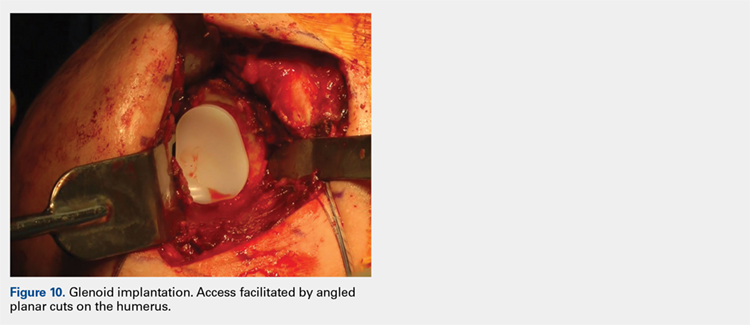
DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12
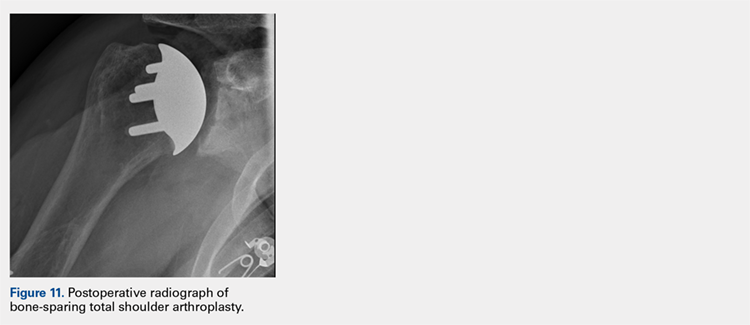
Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).

Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).

The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).

The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.

DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12

Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).

Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).

The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).

The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.

DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12

Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
TAKE-HOME POINTS
- Bone-preserving shoulder arthroplasty is now available and rapidly growing in the US.
- The calibrated, multiplanar instruments and prosthesis shown here allow surgeons to recreate the normal humerus shape with high precision.
- The elliptical, non-spherical design of the humerus prosthesis has shown improved shoulder kinematics compared to standard spherical prostheses.
- The implant rests on dense bone proximal to the anatomic neck where bone support is strong.
- Glenoid implant insertion is routinely performed using this technique and access is facilitated by the angled bone resections.
Special interest groups drive SHM engagement
As a professional society supporting an increasingly diverse membership base, SHM is perpetually challenged to create an environment that offers relevance and community to all. While the broad hospital medicine population and SHM are focused on the same goals – optimizing patient care and the system that delivers it – there are nuances within membership that require specific networks and platforms to build this environment of community.
SHM relies on both staff and volunteers to be an engine for leadership, innovation, and labor. Its volunteer corps is essential to delivering value to members and setting the strategic agenda for hospital medicine’s future. Over the last year, SHM has attempted to expand the infrastructure and opportunity for volunteer leadership by examining new approaches to allow pockets of membership to have their own voice. During the year to come, members will continue to help staff forge a new landscape for constituency engagement.
If you are a current volunteer leader, were interested in pursuing volunteer opportunities this past fall, or have simply been navigating SHM’s new website, you may be aware that the Committee structure has changed. There is also new publicity for things called “Special Interest Groups.” Many of our constituency-based Committees are in the process of transforming into Special Interest Groups, which will be officially launched during SHM’s Annual Conference in Orlando in April. They are adopting a more visible charge to create the most accessible and influence-able environment for the entire SHM community.
Committee-to-Special Interest Group transition is about both philosophy and mechanics. It aims to ensure that each constituency group can be readily shaped by the entire population it represents, and will work to create the infrastructure to facilitate that. SHM envisions Special Interest Groups being primary influencers over future content-development and policy objectives; their online communities serving as the principal means for socialization and dialogue around proposed ideas and initiatives. To that end, SHM invested in an entirely new platform for Hospital Medicine Exchange (HMX). If you have yet to explore the new HMX and opportunities for niche networking, visit www.hmxchange.org.
We have also developed a new governance model to encourage interactions between volunteer groups. While there is overlap within Committee and Special Interest Group constructs and likely many volunteers serving in both spheres, it is important to create parallel environments with discreet charges around function and membership engagement. As we continue to roll out the changes, we will rely on volunteers and members at large to help us best realize our intent.
During this transformation, existing volunteers are working with staff to determine the future. There will be some differences in the way Committees and Special Interest Groups function. The differences will be deliberate and designed to provide for fluid and thoughtful administration of business and membership engagement. There will also be consistent communication between Special Interest Groups and strategic and functional Committees with ongoing charges and oversight of existing SHM programs.
Special Interest Groups will have dedicated staff liaisons and volunteer leadership councils. Transforming Committees’ current volunteers will serve as inaugural council leaders with the process for future election being developed in concert by staff and volunteers over the next several months. Special Interest Group membership is open and free to all active SHM members. All current Special Interest Groups will facilitate live Special Interest Forums during SHM’s Annual Conference. If you attend Hospital Medicine 2018 in April, stop by these Special Interest Group Forums on the following topics to learn more:
• Advocacy and Public Policy
• Care for Vulnerable Populations
• Critical Care Medicine
• Health care Information Technology
• Hospitalists Trained in Family Medicine
• Med-Peds Hospitalists
• Multi-Site Hospital Leaders
• Nurse Practitioners and Physician Assistants
• Palliative Care
• Pediatric Hospitalists
• Perioperative Medicine
• Point of Care Ultrasound
• Practice Administrators/Practice Management
• Quality Improvement
• Medical Students and Residents
• Rural Hospitalists
Summaries of the live forums will be posted on corresponding HMX communities after the conference, complete with open comment periods for discussion. There will be an open application period during summer 2018 for new Special Interest Groups not defined above. The SHM Board will review applications in September 2018, and newly established groups will be convened in October to begin building HMX communities, confirming volunteer leader councils, and charting their course with a dedicated staff liaison.
The intent is simple – to provide an open-access mechanism for membership at large to collaborate amongst themselves, offer perspective, and validate or challenge SHM’s proposed initiatives and direction. Special Interest Groups and Committees alike are – and will be – an essential part of SHM’s future – as developers of content, voices of the populations we serve, and an apparatus for the implementation of our shared mission and vision.
SHM exists to serve its members and help them deliver exceptional patient care. We are always interested in your perspective and feedback. To offer your thoughts and ideas about Special Interest Groups or anything else related to membership, please email membership@hospitalmedicine.org.
Mr. Gray is vice president of membership at the Society of Hospital Medicine.
As a professional society supporting an increasingly diverse membership base, SHM is perpetually challenged to create an environment that offers relevance and community to all. While the broad hospital medicine population and SHM are focused on the same goals – optimizing patient care and the system that delivers it – there are nuances within membership that require specific networks and platforms to build this environment of community.
SHM relies on both staff and volunteers to be an engine for leadership, innovation, and labor. Its volunteer corps is essential to delivering value to members and setting the strategic agenda for hospital medicine’s future. Over the last year, SHM has attempted to expand the infrastructure and opportunity for volunteer leadership by examining new approaches to allow pockets of membership to have their own voice. During the year to come, members will continue to help staff forge a new landscape for constituency engagement.
If you are a current volunteer leader, were interested in pursuing volunteer opportunities this past fall, or have simply been navigating SHM’s new website, you may be aware that the Committee structure has changed. There is also new publicity for things called “Special Interest Groups.” Many of our constituency-based Committees are in the process of transforming into Special Interest Groups, which will be officially launched during SHM’s Annual Conference in Orlando in April. They are adopting a more visible charge to create the most accessible and influence-able environment for the entire SHM community.
Committee-to-Special Interest Group transition is about both philosophy and mechanics. It aims to ensure that each constituency group can be readily shaped by the entire population it represents, and will work to create the infrastructure to facilitate that. SHM envisions Special Interest Groups being primary influencers over future content-development and policy objectives; their online communities serving as the principal means for socialization and dialogue around proposed ideas and initiatives. To that end, SHM invested in an entirely new platform for Hospital Medicine Exchange (HMX). If you have yet to explore the new HMX and opportunities for niche networking, visit www.hmxchange.org.
We have also developed a new governance model to encourage interactions between volunteer groups. While there is overlap within Committee and Special Interest Group constructs and likely many volunteers serving in both spheres, it is important to create parallel environments with discreet charges around function and membership engagement. As we continue to roll out the changes, we will rely on volunteers and members at large to help us best realize our intent.
During this transformation, existing volunteers are working with staff to determine the future. There will be some differences in the way Committees and Special Interest Groups function. The differences will be deliberate and designed to provide for fluid and thoughtful administration of business and membership engagement. There will also be consistent communication between Special Interest Groups and strategic and functional Committees with ongoing charges and oversight of existing SHM programs.
Special Interest Groups will have dedicated staff liaisons and volunteer leadership councils. Transforming Committees’ current volunteers will serve as inaugural council leaders with the process for future election being developed in concert by staff and volunteers over the next several months. Special Interest Group membership is open and free to all active SHM members. All current Special Interest Groups will facilitate live Special Interest Forums during SHM’s Annual Conference. If you attend Hospital Medicine 2018 in April, stop by these Special Interest Group Forums on the following topics to learn more:
• Advocacy and Public Policy
• Care for Vulnerable Populations
• Critical Care Medicine
• Health care Information Technology
• Hospitalists Trained in Family Medicine
• Med-Peds Hospitalists
• Multi-Site Hospital Leaders
• Nurse Practitioners and Physician Assistants
• Palliative Care
• Pediatric Hospitalists
• Perioperative Medicine
• Point of Care Ultrasound
• Practice Administrators/Practice Management
• Quality Improvement
• Medical Students and Residents
• Rural Hospitalists
Summaries of the live forums will be posted on corresponding HMX communities after the conference, complete with open comment periods for discussion. There will be an open application period during summer 2018 for new Special Interest Groups not defined above. The SHM Board will review applications in September 2018, and newly established groups will be convened in October to begin building HMX communities, confirming volunteer leader councils, and charting their course with a dedicated staff liaison.
The intent is simple – to provide an open-access mechanism for membership at large to collaborate amongst themselves, offer perspective, and validate or challenge SHM’s proposed initiatives and direction. Special Interest Groups and Committees alike are – and will be – an essential part of SHM’s future – as developers of content, voices of the populations we serve, and an apparatus for the implementation of our shared mission and vision.
SHM exists to serve its members and help them deliver exceptional patient care. We are always interested in your perspective and feedback. To offer your thoughts and ideas about Special Interest Groups or anything else related to membership, please email membership@hospitalmedicine.org.
Mr. Gray is vice president of membership at the Society of Hospital Medicine.
As a professional society supporting an increasingly diverse membership base, SHM is perpetually challenged to create an environment that offers relevance and community to all. While the broad hospital medicine population and SHM are focused on the same goals – optimizing patient care and the system that delivers it – there are nuances within membership that require specific networks and platforms to build this environment of community.
SHM relies on both staff and volunteers to be an engine for leadership, innovation, and labor. Its volunteer corps is essential to delivering value to members and setting the strategic agenda for hospital medicine’s future. Over the last year, SHM has attempted to expand the infrastructure and opportunity for volunteer leadership by examining new approaches to allow pockets of membership to have their own voice. During the year to come, members will continue to help staff forge a new landscape for constituency engagement.
If you are a current volunteer leader, were interested in pursuing volunteer opportunities this past fall, or have simply been navigating SHM’s new website, you may be aware that the Committee structure has changed. There is also new publicity for things called “Special Interest Groups.” Many of our constituency-based Committees are in the process of transforming into Special Interest Groups, which will be officially launched during SHM’s Annual Conference in Orlando in April. They are adopting a more visible charge to create the most accessible and influence-able environment for the entire SHM community.
Committee-to-Special Interest Group transition is about both philosophy and mechanics. It aims to ensure that each constituency group can be readily shaped by the entire population it represents, and will work to create the infrastructure to facilitate that. SHM envisions Special Interest Groups being primary influencers over future content-development and policy objectives; their online communities serving as the principal means for socialization and dialogue around proposed ideas and initiatives. To that end, SHM invested in an entirely new platform for Hospital Medicine Exchange (HMX). If you have yet to explore the new HMX and opportunities for niche networking, visit www.hmxchange.org.
We have also developed a new governance model to encourage interactions between volunteer groups. While there is overlap within Committee and Special Interest Group constructs and likely many volunteers serving in both spheres, it is important to create parallel environments with discreet charges around function and membership engagement. As we continue to roll out the changes, we will rely on volunteers and members at large to help us best realize our intent.
During this transformation, existing volunteers are working with staff to determine the future. There will be some differences in the way Committees and Special Interest Groups function. The differences will be deliberate and designed to provide for fluid and thoughtful administration of business and membership engagement. There will also be consistent communication between Special Interest Groups and strategic and functional Committees with ongoing charges and oversight of existing SHM programs.
Special Interest Groups will have dedicated staff liaisons and volunteer leadership councils. Transforming Committees’ current volunteers will serve as inaugural council leaders with the process for future election being developed in concert by staff and volunteers over the next several months. Special Interest Group membership is open and free to all active SHM members. All current Special Interest Groups will facilitate live Special Interest Forums during SHM’s Annual Conference. If you attend Hospital Medicine 2018 in April, stop by these Special Interest Group Forums on the following topics to learn more:
• Advocacy and Public Policy
• Care for Vulnerable Populations
• Critical Care Medicine
• Health care Information Technology
• Hospitalists Trained in Family Medicine
• Med-Peds Hospitalists
• Multi-Site Hospital Leaders
• Nurse Practitioners and Physician Assistants
• Palliative Care
• Pediatric Hospitalists
• Perioperative Medicine
• Point of Care Ultrasound
• Practice Administrators/Practice Management
• Quality Improvement
• Medical Students and Residents
• Rural Hospitalists
Summaries of the live forums will be posted on corresponding HMX communities after the conference, complete with open comment periods for discussion. There will be an open application period during summer 2018 for new Special Interest Groups not defined above. The SHM Board will review applications in September 2018, and newly established groups will be convened in October to begin building HMX communities, confirming volunteer leader councils, and charting their course with a dedicated staff liaison.
The intent is simple – to provide an open-access mechanism for membership at large to collaborate amongst themselves, offer perspective, and validate or challenge SHM’s proposed initiatives and direction. Special Interest Groups and Committees alike are – and will be – an essential part of SHM’s future – as developers of content, voices of the populations we serve, and an apparatus for the implementation of our shared mission and vision.
SHM exists to serve its members and help them deliver exceptional patient care. We are always interested in your perspective and feedback. To offer your thoughts and ideas about Special Interest Groups or anything else related to membership, please email membership@hospitalmedicine.org.
Mr. Gray is vice president of membership at the Society of Hospital Medicine.
Impact of a Multicenter, Mentored Quality Collaborative on Hospital-Associated Venous Thromboembolism
Deep venous thrombosis and pulmonary embolism, collectively known as venous thromboembolism (VTE), affect up to 600,000 Americans a year.1 Most of these are hospital-associated venous thromboembolisms (HA-VTE).1,2 VTE poses a substantial risk of mortality and long-term morbidity, and its treatment poses a risk of major bleeding.1 As appropriate VTE prophylaxis (“prophylaxis”) can reduce the risk of VTE by 40% to 80% depending on the patient population,3 VTE risk assessment and prophylaxis is endorsed by multiple guidelines4-7 and supported by regulatory agencies.8-10
However, despite extensive study, consensus about the impact of prophylaxis4,11 and the optimal method of risk assessment4,5,7,12 is lacking. Meanwhile, implementation of prophylaxis in real-world settings is poor; only 40% to 60% of at-risk patients receive prophylaxis,13 and as few as <20% receive optimal prophylaxis.14 Both systematic reviews15,16 and experience with VTE prevention collaboratives17,18 found that multifaceted interventions and alerts may be most effective in improving prophylaxis rates, but without proof of improved VTE rates.15 There is limited experience with large-scale VTE prevention. Organizations like The Joint Commission (TJC)8 and the Surgical Care Improvement Project have promoted quality measures but without clear evidence of improvement.19 In addition, an analysis of over 20,000 medical patients at 35 hospitals found no difference in VTE rates between high- and low-performing hospitals,20 suggesting that aggressive prophylaxis efforts may not reduce VTE, at least among medical patients.21 However, a 5-hospital University of California collaborative was associated with improved VTE rates, chiefly among surgical patients.22
In 2011, Dignity Health targeted VTE for improvement after investigations of potentially preventable HA-VTE revealed variable patterns of prophylaxis. In addition, improvement seemed feasible because there is a proven framework for VTE quality improvement (QI) projects17,18 and a record of success with the following 3 specific strategies: quality mentorship,23 use of a simple VTE risk assessment method, and active surveillance (real-time monitoring targeting suboptimal prophylaxis with concurrent intervention). This active surveillance technique has been used successfully in prior improvement efforts, often termed measure-vention.17,18,22,24
METHODS
Setting and Participants
The QI collaborative was performed at 35 Dignity Health community hospitals in California, Arizona, and Nevada. Facilities ranged from 25 to 571 beds in size with a mixture of teaching and nonteaching hospitals. Prior to the initiative, prophylaxis improvement efforts were incomplete and inconsistent at study facilities. All adult acute care inpatients at all facilities were included except rehabilitation, behavioral health, skilled nursing, hospice, other nonacute care, and inpatient deliveries.
Design Overview
We performed a prospective, unblinded, open-intervention study of a QI collaborative in 35 community hospitals and studied the effect on prophylaxis and VTE rates with historical controls. The 35 hospitals were organized into 2 cohorts. In the “pilot” cohort, 9 hospitals (chosen to be representative of the various settings, size, and teaching status within the Dignity system) received funding from the Gordon and Betty Moore Foundation (GBMF) for intensive, individualized QI mentorship from experts as well as active surveillance (see “Interventions”). The pilot sites led the development of the VTE risk assessment and prophylaxis protocol (“VTE protocol”), measures, order sets, implementation tactics, and lessons learned, assisted by the mentor experts. Dissemination to the 26-hospital “spread” cohort was facilitated by the Dignity Health Hospital Engagement Network (HEN) infrastructure.
Timeline
Two of the pilot sites, acting as leads on the development of protocol and order set tools, formed improvement teams in March 2011, 6 to 12 months earlier than other Dignity sites. Planning and design work occurred from March 2011 to September 2012. Most implementation at the 35 hospitals occurred in a staggered fashion during calendar year (CY) 2012 and 2013 (see Figure 1). As few changes were made until mid-2012, we considered CY 2011 the baseline for comparison, CY 2012 to 2013 the implementation years, and CY 2014 the postimplementation period.
The project was reviewed by the Institutional Review Board (IRB) of Dignity Health and determined to be an IRB-exempt QI project.
Interventions
Collaborative Infrastructure
Multidisciplinary Teams
Improvement teams formed between March 2011 and September 2012. Members included a physician champion, frontline nurses and physicians, an administrative liaison, pharmacists, quality and data specialists, clinical informatics staff, and stakeholders from key clinical services. Teams met at least monthly at each site.
Physician Mentors
The 9 pilot sites received individualized mentorship provided by outside experts (IJ or GM) based on a model pioneered by the Society of Hospital Medicine’s (SHM) Mentored Implementation programs.23 Each pilot site completed a self-assessment survey17 (see supplementary Appendix A) about past efforts, team composition, current performance, aims, barriers, and opportunities. The mentors reviewed the completed questionnaire with each hospital and provided advice on the VTE protocol and order set design, measurement, and benchmarking during 3 webinar meetings scheduled at 0, 3, and 9 months, plus as-needed e-mail and phone correspondence. After each webinar, the mentors provided detailed improvement suggestions (see supplementary Appendix B). Several hospitals received mentor site visits, which focused on unit rounding, active surveillance, staff and provider education, and problem-solving sessions with senior leadership, physician leadership, and the improvement team.
VTE Protocol
After a literature review and consultation with the mentors, Dignity Health developed and implemented a VTE protocol, modified from a model used in previous improvement efforts.18,22-24 Its risk assessment method is often referred to as a “3 bucket” model because it assigns patients to high-, moderate-, or low-risk categories based on clinical factors (eg, major orthopedic surgery, prior VTE, and others), and the VTE protocol recommends interventions based on the risk category (see supplementary Appendix C). Dignity Health was transitioning to a single electronic health record (Cerner Corporation, North Kansas City, MO) during the study, and study hospitals were using multiple platforms, necessitating the development of both paper and electronic versions of the VTE protocol. The electronic version required completion of the VTE protocol for all inpatient admissions and transfers. The VTE protocol was completed in November 2011 and disseminated to other sites in a staggered fashion through November 2012. Completed protocols and improvement tips were shared by the project lead and by webinar sessions. Sites were also encouraged to implement a standardized practice that allowed nurses to apply sequential compression devices to at-risk patients without physician orders when indicated by protocol, when contraindications such as vascular disease or ulceration were absent.
Education
Staff were educated about the VTE protocol by local teams, starting between late 2011 and September 2012. The audience (physicians, nurses, pharmacists, etc.) and methods (conferences, fliers, etc.) were determined by local teams, following guidance by mentors and webinar content. Active surveillance provided opportunities for in-the-moment, patient-specific education and protocol reinforcement. Both mentors delivered educational presentations at pilot sites.
Active Surveillance
Sites were encouraged to perform daily review of prophylaxis adequacy for inpatients and correct lapses in real time (both under- and overprophylaxis). Inappropriate prophylaxis orders were addressed by contacting providers to change the order or document the rationale not to. Lapses in adherence to prophylaxis were addressed by nursing correction and education of involved staff. Active surveillance was funded for 10 hours a week at pilot sites. Spread sites received only minimal support from HEN monies. All sites used daily prophylaxis reports, enhanced to include contraindications like thrombocytopenia and coagulopathy, to facilitate efforts. Active surveillance began in May 2012 in the lead pilot hospitals and was implemented in other sites between October 2012 and February 2013.
Metrics
Prophylaxis Rates
Measurement of prophylaxis did not begin until 2012 to 2013; thus, the true baseline rate for prophylaxis was not captured. TJC metrics (VTE-1 and VTE-2)25 were consolidated into a composite TJC prophylaxis rate from January 2012 to December 2014 for both pilot and spread hospitals. These measures assess the percentage of adult inpatients who received VTE prophylaxis or have documentation of why no prophylaxis was given the day of or day after hospital admission (VTE-1) or the day of or day after ICU admission or transfer (VTE-2). These measures are met if any mechanical or pharmacologic prophylaxis was delivered.
In addition to the TJC metric, the 9 pilot hospitals monitored rates of protocol-compliant prophylaxis for 12 to 20 months. Each patient’s prophylaxis was considered protocol compliant if it was consistent with the prophylaxis protocol at the time of the audit or if contraindications were documented (eg, patients eligible for, but with contraindications to, pharmacologic prophylaxis had to have an order for mechanical prophylaxis or documented contraindication to both modalities). As this measure was initiated in a staggered fashion, the rate of protocol-compliant prophylaxis is summarized for consecutive months of measurement rather than consecutive calendar months.
HA-VTE Rates
VTE events were captured by review of electronic coding data for the International Classification of Diseases, 9th Revision (ICD-9) codes 415.11-415.19, 453.2, 453.40-453.42, and 453.8-453.89. HA-VTE was defined as either new VTE not present on admission (NPOA HA-VTE) or new VTE presenting in a readmitted patient within 30 days of discharge (Readmit HA-VTE). Cases were stratified based on whether the patient had undergone a major operation (surgery patients) or not (medical patients) as identified by Medicare Services diagnosis-related group codes.
Control Measures
Potential adverse events were captured by review of electronic coding data for ICD-9 codes 289.84 (heparin-induced thrombocytopenia [HIT]) and E934.2 (adverse effects because of anticoagulants).
Statistical Analysis
Statistical process control charts were used to depict changes in prophylaxis rates over the 3 years for which data was collected. For VTE and safety outcomes, Pearson χ2 value with relative risk (RR) calculations and 95% confidence intervals (CIs) were used to compare proportions between groups at baseline (CY 2011) versus postimplementation (CY 2014). Differences between the means of normally distributed data were calculated, and a 95% CI for the difference between the means was performed to assess statistical difference. Nonparametric characteristics were described by quartiles and interquartile range, and the 2-sided Mann-Whitney U test was performed to assess statistical difference between the CY 2011 and CY 2014 period.
Role of the Funding Source
The GBMF funded the collaborative and supported authorship of the manuscript but had no role in the design or conduct of the intervention, the collection or analysis of data, or the drafting of the manuscript.
RESULTS
Population Demographics
There were 1,155,069 adult inpatient admissions during the 4-year study period (264,280 in the 9 pilot sites, 890,789 in the 26 spread sites). There were no clinically relevant changes in gender distribution, mortality rate, median age, case mix index, or hospital length of stay in 2011 versus 2014. Men comprised 47.1% of the patient population in 2011 and 47.7% in 2014. The mortality rate was 2.7% in both years. Median age was 62 in 2011 and 63 in 2014. The mean case mix index (1.58 vs 1.65) and mean length of stay (4.29 vs 4.33 days) were similar in the 2 time periods.
Prophylaxis Rates
TJC Prophylaxis rates
Rates of Protocol-Compliant Prophylaxis
There were 34,071 active surveillance audits across the 20 months of reporting in the pilot cohort (mean, 1817 audits per month). The rate of protocol-compliant prophylaxis improved from 89% at month 1 of observation to 93% during month 2 and 97% by the last 3 months (Pearson χ2 P < .001 for both comparisons).
HA-VTE
HA-VTE characteristics
Improved HA-VTE over Time
Four hundred twenty-eight fewer HA-VTEs occurred in 2014 than in 2011 (RR 0.78; 95% CI, 0.73-0.85) (Table and Figure 3). Readmission HA-VTEs were reduced by 315 (RR 0.72; 95% CI, 0.65-0.80), while the reduction in NPOA HA-VTEs was less robust (RR 0.88; 95% CI, 0.79-0.99). Pilot sites enjoyed a more robust reduction in HA-VTEs than spread sites (26% vs 20%), largely because the pilot cohort enjoyed a 34% reduction in NPOA HA-VTEs and a 20% reduction in Readmit HA-VTEs, while the spread cohort only achieved reductions in Readmit HA-VTEs.
Safety
Rates of HIT and adverse effects because of anticoagulants were low (Table). The rate of HIT declined from 178 events in 2011 to 109 in 2014 (RR 0.66; 95% CI, 0.52-0.84), and the RR of anticoagulant adverse events remained stable (RR 1.01; 95% CI, 0.87-1.15).
DISCUSSION
Our QI project, based on a proven collaborative approach and mentorship,18,22,24 order set redesign, and active surveillance, was associated with 26% less VTEs in the pilot cohort and 20% less VTEs in the spread cohort. These gains, down to a final rate of approximately 4 HA-VTEs per 1000 admissions, occurred despite a low baseline HA-VTE rate. Dignity Health achieved these improvements in 35 hospitals with varied sizes, settings, ordering systems, and teaching statuses, achieving what is to our knowledge the largest VTE QI initiative yet reported.
Implementation experiences were not systematically recorded, and techniques were not compared with a control group. However, we believe that Dignity Health’s organizational commitment to improvement and centralized support were crucial for success. In addition, the pilot sites received grant support from the GBMF for intensive quality mentoring, a strategy with demonstrated value.23 Mentors and team members noted that system-wide revision to the computerized physician order entry system was easiest to implement, while active surveillance represented the most labor-intensive intervention. Other experiences echoed lessons from previous VTE mentorship efforts.17,18
The selection of a VTE protocol conducive to implementation and provider use was a key strategy. The ideal approach to VTE risk assessment is not known,12,26 but guidelines either offer no specific guidance7 or would require implementation of 3 different systems per hospital.4,5 Several of these are point scoring systems, which may have lower clinician acceptance or require programming to improve real-world use18,26,27; the Padua score was derived from a patient population that differs significantly from those in the United States.12 Our study provides more practical experience with a “3-bucket” model, which has previously shown high interobserver reliability, good clinician acceptance, and meaningful reductions of VTE, including in American patient populations.18,22,24
The value of VTE prophylaxis is still disputed in many inpatient groups. The overall rate of HA-VTE is low, so the per-patient benefit of prophylaxis is low, and many patients may be overprophylaxed.4,11,12 Recently, Flanders et al.20 reported that HA-VTE rates among 20,800 medical inpatients in Michigan were low (about 1%) and similar at hospitals in the top (mean prophylaxis rate 86%) or bottom (mean prophylaxis rate 56%) tertiles of performance. Possible explanations for the differences between their multicenter experience and ours include our sample size (55 times larger) and the possibility that targeting prophylaxis to patients at highest need (captured in our protocol-compliant prophylaxis rates) matters more than prophylaxing a percent of the population.
Further research is needed to develop simple, easy-to-implement methods to identify inpatients who do not, or no longer, require prophylaxis.12 Hospital systems also need methods to determine if prophylaxis improvement efforts can lower their HA-VTE rates and in which subpopulations. For example, a collaborative effort at the University of California lowered HA-VTE rates toward a common improved rate of 0.65% to 0.73%,22 while Dignity Health achieved improvement despite starting with an even lower baseline. In the University of California collaborative, benefits were limited chiefly to surgical patients, while Dignity Health achieved most improvement in medical patients, particularly in Readmit HA-VTE. If future research uncovers the reasons for these differences, it could help hospitals decide where to target improvement efforts.
Our study has several limitations. First, we used a nonrandomized time series design, so we cannot exclude other potential explanations for the change in VTE rates. However, there were no major changes in patient populations or concurrent projects likely to have influenced event rates. While we did not collect detailed demographic information on subjects, the broad inclusion criteria and multicenter design suggests a high degree of generalizability. Second, we followed inpatient VTE events and VTE-related readmissions, but not VTE treated in the outpatient setting. This did not change over the study, but the availability of all-oral therapy for VTE could have caused underdetection if clinic or emergency room doctors sent home more patients on oral therapy instead of readmitting them to the hospital. Third, implementation was enhanced by GBMF funds (at 9 sites, with the remainder benefitting from their experience), a shared electronic med
Strengths of our study include reductions in HA-VTE, both with and without access to GBMF funds, by using broadly available QI strategies.17 This real-world success and ease of dissemination are particularly important because the clinical trials of prophylaxis have been criticized for using highly selected patient populations,11 and prophylaxis QI studies show an inconsistent impact on VTE outcomes.15 In previous studies, two of the authors monitored orders for prophylaxis22,24; during this project, delivery for both pharmacologic and mechanical VTE prophylaxis was monitored, confirming that patient care actually changed.
CONCLUSION
Our multicenter VTE prophylaxis initiative, featuring a “3-bucket” VTE protocol, QI mentorship, and active surveillance as key interventions, was associated with improved prophylaxis rates and a reduction in HA-VTE by 22% with no increase in adverse events. This project provides a model for hospital systems seeking to optimize their prophylaxis efforts, and it supports the use of collaborative QI initiatives and SHM’s quality mentorship program as methods to drive improvement across health systems.
Disclosure
None of the authors have any conflicts of interest related to any topics or products discussed in the article. Dignity Health provided a stipend for writing the manuscript to GM and IJ, as noted in the article, but had no role in data analysis, writing, or decision to submit.
1. U.S. Department of Health and Human Services; National Heart, Lung, and Blood Institute. Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville: Office of the Surgeon General; 2008.
2. Heit JA, Melton LJ, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients versus community residents. Mayo Clin Proc. 2001;76(11):1102-1110. PubMed
3. Guyatt GH, Eikelboom JW, Gould MK. Approach to Outcome Measurement in the Prevention of Thrombosis in Surgical and Medical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e185S-e194S. doi:10.1378/chest.11-2289. PubMed
4. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in Nonsurgical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S. doi:10.1378/chest.11-2296. PubMed
5. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in Nonorthopedic Surgical Patients. Chest. 2012;141(2 suppl):e227S-e277S. PubMed
6. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in Orthopedic Surgery Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S. doi:10.1378/chest.11-2404. PubMed
7. Qaseem A, Chou R, Humphrey LL. Venous Thromboembolism Prophylaxis in Hospitalized Patients: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. PubMed
8. The Joint Commission. Performance Measurement Initiatives. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement. Accessed June 14, 2012.
9. National Quality Forum. National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures. http://www.qualityforum.org/Publications/2006/12/National_Voluntary_Consensus_Standards_for_Prevention_and_Care_of_Venous_Thromboembolism__Policy,_Preferred_Practices,_and_Initial_Performance_Measures.aspx. Accessed June 14, 2012.
10. Medicare Quality Improvement Committee. SCIP Project Information. Agency for Healthcare Research and Quality. http://www.qualitymeasures.ahrq.gov/content.aspx?id=35538&search=scip. Accessed March 2013.
11. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients and Those with Stroke: A Background Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. PubMed
12. Rothberg MB. Venous thromboembolism prophylaxis for medical patients: who needs it? JAMA Intern Med. 2014;174(10):1585-1586. PubMed
13. Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet. 2008;371(9610):387-394. PubMed
14. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4(8):E15-E21. PubMed
15. Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201. doi:10.1002/14651858.CD008201.pub2. PubMed
16. Lau BD, Haut ER. Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187-195. PubMed
17. Maynard G. Preventing hospital-associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. Rockville: Agency for Healthcare Research and Quality; 2015. https://www.ahrq.gov/sites/default/files/publications/files/vteguide.pdf. Accessed October 29, 2017.
18. Maynard G, Stein J. Designing and Implementing Effective VTE Prevention Protocols: Lessons from Collaboratives. J Thromb Thrombolysis. 2010;29(2):159-166. PubMed
19. Altom LK, Deierhoi RJ, Grams J, et al. Association between Surgical Care Improvement Program venous thromboembolism measures and postoperative events. Am J Surg. 2012;204(5):591-597. PubMed
20. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. PubMed
21. Finn KM, Greenwald JL. Update in Hospital Medicine: Evidence You Should Know. J Hosp Med. 2015;10(12):817-826. PubMed
22. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: Findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. PubMed
23. Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg Patient Safety and Quality Award. Mentored Implementation: Building Leaders and Achieving Results Through a Collaborative Improvement Model at the National Level. Jt Comm J Qual Patient Saf. 2012;38(7):301-310.
24. Maynard GA, Morris TA, Jenkins IH, et al. Optimizing prevention of hospital-acquired venous thromboembolism (VTE): Prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10-18. PubMed
25. The Joint Commission. Venous Thromboembolism Quality Measures. https://www.jointcommission.org/venous_thromboembolism/. Accessed October 13, 2017.
26. Maynard GA, Jenkins IH, Merli GJ. Venous thromboembolism prevention guidelines for medical inpatients: Mind the (implementation) Gap. J Hosp Med. 2013;8(10):582-588. PubMed
27. Elias P, Khanna R, Dudley A, et al. Automating Venous Thromboembolism Risk Calculation Using Electronic Health Record Data upon Hospital Admission: The Automated Padua Prediction Score. J Hosp Med. 2017;12(4):231-237. PubMed
Deep venous thrombosis and pulmonary embolism, collectively known as venous thromboembolism (VTE), affect up to 600,000 Americans a year.1 Most of these are hospital-associated venous thromboembolisms (HA-VTE).1,2 VTE poses a substantial risk of mortality and long-term morbidity, and its treatment poses a risk of major bleeding.1 As appropriate VTE prophylaxis (“prophylaxis”) can reduce the risk of VTE by 40% to 80% depending on the patient population,3 VTE risk assessment and prophylaxis is endorsed by multiple guidelines4-7 and supported by regulatory agencies.8-10
However, despite extensive study, consensus about the impact of prophylaxis4,11 and the optimal method of risk assessment4,5,7,12 is lacking. Meanwhile, implementation of prophylaxis in real-world settings is poor; only 40% to 60% of at-risk patients receive prophylaxis,13 and as few as <20% receive optimal prophylaxis.14 Both systematic reviews15,16 and experience with VTE prevention collaboratives17,18 found that multifaceted interventions and alerts may be most effective in improving prophylaxis rates, but without proof of improved VTE rates.15 There is limited experience with large-scale VTE prevention. Organizations like The Joint Commission (TJC)8 and the Surgical Care Improvement Project have promoted quality measures but without clear evidence of improvement.19 In addition, an analysis of over 20,000 medical patients at 35 hospitals found no difference in VTE rates between high- and low-performing hospitals,20 suggesting that aggressive prophylaxis efforts may not reduce VTE, at least among medical patients.21 However, a 5-hospital University of California collaborative was associated with improved VTE rates, chiefly among surgical patients.22
In 2011, Dignity Health targeted VTE for improvement after investigations of potentially preventable HA-VTE revealed variable patterns of prophylaxis. In addition, improvement seemed feasible because there is a proven framework for VTE quality improvement (QI) projects17,18 and a record of success with the following 3 specific strategies: quality mentorship,23 use of a simple VTE risk assessment method, and active surveillance (real-time monitoring targeting suboptimal prophylaxis with concurrent intervention). This active surveillance technique has been used successfully in prior improvement efforts, often termed measure-vention.17,18,22,24
METHODS
Setting and Participants
The QI collaborative was performed at 35 Dignity Health community hospitals in California, Arizona, and Nevada. Facilities ranged from 25 to 571 beds in size with a mixture of teaching and nonteaching hospitals. Prior to the initiative, prophylaxis improvement efforts were incomplete and inconsistent at study facilities. All adult acute care inpatients at all facilities were included except rehabilitation, behavioral health, skilled nursing, hospice, other nonacute care, and inpatient deliveries.
Design Overview
We performed a prospective, unblinded, open-intervention study of a QI collaborative in 35 community hospitals and studied the effect on prophylaxis and VTE rates with historical controls. The 35 hospitals were organized into 2 cohorts. In the “pilot” cohort, 9 hospitals (chosen to be representative of the various settings, size, and teaching status within the Dignity system) received funding from the Gordon and Betty Moore Foundation (GBMF) for intensive, individualized QI mentorship from experts as well as active surveillance (see “Interventions”). The pilot sites led the development of the VTE risk assessment and prophylaxis protocol (“VTE protocol”), measures, order sets, implementation tactics, and lessons learned, assisted by the mentor experts. Dissemination to the 26-hospital “spread” cohort was facilitated by the Dignity Health Hospital Engagement Network (HEN) infrastructure.
Timeline
Two of the pilot sites, acting as leads on the development of protocol and order set tools, formed improvement teams in March 2011, 6 to 12 months earlier than other Dignity sites. Planning and design work occurred from March 2011 to September 2012. Most implementation at the 35 hospitals occurred in a staggered fashion during calendar year (CY) 2012 and 2013 (see Figure 1). As few changes were made until mid-2012, we considered CY 2011 the baseline for comparison, CY 2012 to 2013 the implementation years, and CY 2014 the postimplementation period.
The project was reviewed by the Institutional Review Board (IRB) of Dignity Health and determined to be an IRB-exempt QI project.
Interventions
Collaborative Infrastructure
Multidisciplinary Teams
Improvement teams formed between March 2011 and September 2012. Members included a physician champion, frontline nurses and physicians, an administrative liaison, pharmacists, quality and data specialists, clinical informatics staff, and stakeholders from key clinical services. Teams met at least monthly at each site.
Physician Mentors
The 9 pilot sites received individualized mentorship provided by outside experts (IJ or GM) based on a model pioneered by the Society of Hospital Medicine’s (SHM) Mentored Implementation programs.23 Each pilot site completed a self-assessment survey17 (see supplementary Appendix A) about past efforts, team composition, current performance, aims, barriers, and opportunities. The mentors reviewed the completed questionnaire with each hospital and provided advice on the VTE protocol and order set design, measurement, and benchmarking during 3 webinar meetings scheduled at 0, 3, and 9 months, plus as-needed e-mail and phone correspondence. After each webinar, the mentors provided detailed improvement suggestions (see supplementary Appendix B). Several hospitals received mentor site visits, which focused on unit rounding, active surveillance, staff and provider education, and problem-solving sessions with senior leadership, physician leadership, and the improvement team.
VTE Protocol
After a literature review and consultation with the mentors, Dignity Health developed and implemented a VTE protocol, modified from a model used in previous improvement efforts.18,22-24 Its risk assessment method is often referred to as a “3 bucket” model because it assigns patients to high-, moderate-, or low-risk categories based on clinical factors (eg, major orthopedic surgery, prior VTE, and others), and the VTE protocol recommends interventions based on the risk category (see supplementary Appendix C). Dignity Health was transitioning to a single electronic health record (Cerner Corporation, North Kansas City, MO) during the study, and study hospitals were using multiple platforms, necessitating the development of both paper and electronic versions of the VTE protocol. The electronic version required completion of the VTE protocol for all inpatient admissions and transfers. The VTE protocol was completed in November 2011 and disseminated to other sites in a staggered fashion through November 2012. Completed protocols and improvement tips were shared by the project lead and by webinar sessions. Sites were also encouraged to implement a standardized practice that allowed nurses to apply sequential compression devices to at-risk patients without physician orders when indicated by protocol, when contraindications such as vascular disease or ulceration were absent.
Education
Staff were educated about the VTE protocol by local teams, starting between late 2011 and September 2012. The audience (physicians, nurses, pharmacists, etc.) and methods (conferences, fliers, etc.) were determined by local teams, following guidance by mentors and webinar content. Active surveillance provided opportunities for in-the-moment, patient-specific education and protocol reinforcement. Both mentors delivered educational presentations at pilot sites.
Active Surveillance
Sites were encouraged to perform daily review of prophylaxis adequacy for inpatients and correct lapses in real time (both under- and overprophylaxis). Inappropriate prophylaxis orders were addressed by contacting providers to change the order or document the rationale not to. Lapses in adherence to prophylaxis were addressed by nursing correction and education of involved staff. Active surveillance was funded for 10 hours a week at pilot sites. Spread sites received only minimal support from HEN monies. All sites used daily prophylaxis reports, enhanced to include contraindications like thrombocytopenia and coagulopathy, to facilitate efforts. Active surveillance began in May 2012 in the lead pilot hospitals and was implemented in other sites between October 2012 and February 2013.
Metrics
Prophylaxis Rates
Measurement of prophylaxis did not begin until 2012 to 2013; thus, the true baseline rate for prophylaxis was not captured. TJC metrics (VTE-1 and VTE-2)25 were consolidated into a composite TJC prophylaxis rate from January 2012 to December 2014 for both pilot and spread hospitals. These measures assess the percentage of adult inpatients who received VTE prophylaxis or have documentation of why no prophylaxis was given the day of or day after hospital admission (VTE-1) or the day of or day after ICU admission or transfer (VTE-2). These measures are met if any mechanical or pharmacologic prophylaxis was delivered.
In addition to the TJC metric, the 9 pilot hospitals monitored rates of protocol-compliant prophylaxis for 12 to 20 months. Each patient’s prophylaxis was considered protocol compliant if it was consistent with the prophylaxis protocol at the time of the audit or if contraindications were documented (eg, patients eligible for, but with contraindications to, pharmacologic prophylaxis had to have an order for mechanical prophylaxis or documented contraindication to both modalities). As this measure was initiated in a staggered fashion, the rate of protocol-compliant prophylaxis is summarized for consecutive months of measurement rather than consecutive calendar months.
HA-VTE Rates
VTE events were captured by review of electronic coding data for the International Classification of Diseases, 9th Revision (ICD-9) codes 415.11-415.19, 453.2, 453.40-453.42, and 453.8-453.89. HA-VTE was defined as either new VTE not present on admission (NPOA HA-VTE) or new VTE presenting in a readmitted patient within 30 days of discharge (Readmit HA-VTE). Cases were stratified based on whether the patient had undergone a major operation (surgery patients) or not (medical patients) as identified by Medicare Services diagnosis-related group codes.
Control Measures
Potential adverse events were captured by review of electronic coding data for ICD-9 codes 289.84 (heparin-induced thrombocytopenia [HIT]) and E934.2 (adverse effects because of anticoagulants).
Statistical Analysis
Statistical process control charts were used to depict changes in prophylaxis rates over the 3 years for which data was collected. For VTE and safety outcomes, Pearson χ2 value with relative risk (RR) calculations and 95% confidence intervals (CIs) were used to compare proportions between groups at baseline (CY 2011) versus postimplementation (CY 2014). Differences between the means of normally distributed data were calculated, and a 95% CI for the difference between the means was performed to assess statistical difference. Nonparametric characteristics were described by quartiles and interquartile range, and the 2-sided Mann-Whitney U test was performed to assess statistical difference between the CY 2011 and CY 2014 period.
Role of the Funding Source
The GBMF funded the collaborative and supported authorship of the manuscript but had no role in the design or conduct of the intervention, the collection or analysis of data, or the drafting of the manuscript.
RESULTS
Population Demographics
There were 1,155,069 adult inpatient admissions during the 4-year study period (264,280 in the 9 pilot sites, 890,789 in the 26 spread sites). There were no clinically relevant changes in gender distribution, mortality rate, median age, case mix index, or hospital length of stay in 2011 versus 2014. Men comprised 47.1% of the patient population in 2011 and 47.7% in 2014. The mortality rate was 2.7% in both years. Median age was 62 in 2011 and 63 in 2014. The mean case mix index (1.58 vs 1.65) and mean length of stay (4.29 vs 4.33 days) were similar in the 2 time periods.
Prophylaxis Rates
TJC Prophylaxis rates
Rates of Protocol-Compliant Prophylaxis
There were 34,071 active surveillance audits across the 20 months of reporting in the pilot cohort (mean, 1817 audits per month). The rate of protocol-compliant prophylaxis improved from 89% at month 1 of observation to 93% during month 2 and 97% by the last 3 months (Pearson χ2 P < .001 for both comparisons).
HA-VTE
HA-VTE characteristics
Improved HA-VTE over Time
Four hundred twenty-eight fewer HA-VTEs occurred in 2014 than in 2011 (RR 0.78; 95% CI, 0.73-0.85) (Table and Figure 3). Readmission HA-VTEs were reduced by 315 (RR 0.72; 95% CI, 0.65-0.80), while the reduction in NPOA HA-VTEs was less robust (RR 0.88; 95% CI, 0.79-0.99). Pilot sites enjoyed a more robust reduction in HA-VTEs than spread sites (26% vs 20%), largely because the pilot cohort enjoyed a 34% reduction in NPOA HA-VTEs and a 20% reduction in Readmit HA-VTEs, while the spread cohort only achieved reductions in Readmit HA-VTEs.
Safety
Rates of HIT and adverse effects because of anticoagulants were low (Table). The rate of HIT declined from 178 events in 2011 to 109 in 2014 (RR 0.66; 95% CI, 0.52-0.84), and the RR of anticoagulant adverse events remained stable (RR 1.01; 95% CI, 0.87-1.15).
DISCUSSION
Our QI project, based on a proven collaborative approach and mentorship,18,22,24 order set redesign, and active surveillance, was associated with 26% less VTEs in the pilot cohort and 20% less VTEs in the spread cohort. These gains, down to a final rate of approximately 4 HA-VTEs per 1000 admissions, occurred despite a low baseline HA-VTE rate. Dignity Health achieved these improvements in 35 hospitals with varied sizes, settings, ordering systems, and teaching statuses, achieving what is to our knowledge the largest VTE QI initiative yet reported.
Implementation experiences were not systematically recorded, and techniques were not compared with a control group. However, we believe that Dignity Health’s organizational commitment to improvement and centralized support were crucial for success. In addition, the pilot sites received grant support from the GBMF for intensive quality mentoring, a strategy with demonstrated value.23 Mentors and team members noted that system-wide revision to the computerized physician order entry system was easiest to implement, while active surveillance represented the most labor-intensive intervention. Other experiences echoed lessons from previous VTE mentorship efforts.17,18
The selection of a VTE protocol conducive to implementation and provider use was a key strategy. The ideal approach to VTE risk assessment is not known,12,26 but guidelines either offer no specific guidance7 or would require implementation of 3 different systems per hospital.4,5 Several of these are point scoring systems, which may have lower clinician acceptance or require programming to improve real-world use18,26,27; the Padua score was derived from a patient population that differs significantly from those in the United States.12 Our study provides more practical experience with a “3-bucket” model, which has previously shown high interobserver reliability, good clinician acceptance, and meaningful reductions of VTE, including in American patient populations.18,22,24
The value of VTE prophylaxis is still disputed in many inpatient groups. The overall rate of HA-VTE is low, so the per-patient benefit of prophylaxis is low, and many patients may be overprophylaxed.4,11,12 Recently, Flanders et al.20 reported that HA-VTE rates among 20,800 medical inpatients in Michigan were low (about 1%) and similar at hospitals in the top (mean prophylaxis rate 86%) or bottom (mean prophylaxis rate 56%) tertiles of performance. Possible explanations for the differences between their multicenter experience and ours include our sample size (55 times larger) and the possibility that targeting prophylaxis to patients at highest need (captured in our protocol-compliant prophylaxis rates) matters more than prophylaxing a percent of the population.
Further research is needed to develop simple, easy-to-implement methods to identify inpatients who do not, or no longer, require prophylaxis.12 Hospital systems also need methods to determine if prophylaxis improvement efforts can lower their HA-VTE rates and in which subpopulations. For example, a collaborative effort at the University of California lowered HA-VTE rates toward a common improved rate of 0.65% to 0.73%,22 while Dignity Health achieved improvement despite starting with an even lower baseline. In the University of California collaborative, benefits were limited chiefly to surgical patients, while Dignity Health achieved most improvement in medical patients, particularly in Readmit HA-VTE. If future research uncovers the reasons for these differences, it could help hospitals decide where to target improvement efforts.
Our study has several limitations. First, we used a nonrandomized time series design, so we cannot exclude other potential explanations for the change in VTE rates. However, there were no major changes in patient populations or concurrent projects likely to have influenced event rates. While we did not collect detailed demographic information on subjects, the broad inclusion criteria and multicenter design suggests a high degree of generalizability. Second, we followed inpatient VTE events and VTE-related readmissions, but not VTE treated in the outpatient setting. This did not change over the study, but the availability of all-oral therapy for VTE could have caused underdetection if clinic or emergency room doctors sent home more patients on oral therapy instead of readmitting them to the hospital. Third, implementation was enhanced by GBMF funds (at 9 sites, with the remainder benefitting from their experience), a shared electronic med
Strengths of our study include reductions in HA-VTE, both with and without access to GBMF funds, by using broadly available QI strategies.17 This real-world success and ease of dissemination are particularly important because the clinical trials of prophylaxis have been criticized for using highly selected patient populations,11 and prophylaxis QI studies show an inconsistent impact on VTE outcomes.15 In previous studies, two of the authors monitored orders for prophylaxis22,24; during this project, delivery for both pharmacologic and mechanical VTE prophylaxis was monitored, confirming that patient care actually changed.
CONCLUSION
Our multicenter VTE prophylaxis initiative, featuring a “3-bucket” VTE protocol, QI mentorship, and active surveillance as key interventions, was associated with improved prophylaxis rates and a reduction in HA-VTE by 22% with no increase in adverse events. This project provides a model for hospital systems seeking to optimize their prophylaxis efforts, and it supports the use of collaborative QI initiatives and SHM’s quality mentorship program as methods to drive improvement across health systems.
Disclosure
None of the authors have any conflicts of interest related to any topics or products discussed in the article. Dignity Health provided a stipend for writing the manuscript to GM and IJ, as noted in the article, but had no role in data analysis, writing, or decision to submit.
Deep venous thrombosis and pulmonary embolism, collectively known as venous thromboembolism (VTE), affect up to 600,000 Americans a year.1 Most of these are hospital-associated venous thromboembolisms (HA-VTE).1,2 VTE poses a substantial risk of mortality and long-term morbidity, and its treatment poses a risk of major bleeding.1 As appropriate VTE prophylaxis (“prophylaxis”) can reduce the risk of VTE by 40% to 80% depending on the patient population,3 VTE risk assessment and prophylaxis is endorsed by multiple guidelines4-7 and supported by regulatory agencies.8-10
However, despite extensive study, consensus about the impact of prophylaxis4,11 and the optimal method of risk assessment4,5,7,12 is lacking. Meanwhile, implementation of prophylaxis in real-world settings is poor; only 40% to 60% of at-risk patients receive prophylaxis,13 and as few as <20% receive optimal prophylaxis.14 Both systematic reviews15,16 and experience with VTE prevention collaboratives17,18 found that multifaceted interventions and alerts may be most effective in improving prophylaxis rates, but without proof of improved VTE rates.15 There is limited experience with large-scale VTE prevention. Organizations like The Joint Commission (TJC)8 and the Surgical Care Improvement Project have promoted quality measures but without clear evidence of improvement.19 In addition, an analysis of over 20,000 medical patients at 35 hospitals found no difference in VTE rates between high- and low-performing hospitals,20 suggesting that aggressive prophylaxis efforts may not reduce VTE, at least among medical patients.21 However, a 5-hospital University of California collaborative was associated with improved VTE rates, chiefly among surgical patients.22
In 2011, Dignity Health targeted VTE for improvement after investigations of potentially preventable HA-VTE revealed variable patterns of prophylaxis. In addition, improvement seemed feasible because there is a proven framework for VTE quality improvement (QI) projects17,18 and a record of success with the following 3 specific strategies: quality mentorship,23 use of a simple VTE risk assessment method, and active surveillance (real-time monitoring targeting suboptimal prophylaxis with concurrent intervention). This active surveillance technique has been used successfully in prior improvement efforts, often termed measure-vention.17,18,22,24
METHODS
Setting and Participants
The QI collaborative was performed at 35 Dignity Health community hospitals in California, Arizona, and Nevada. Facilities ranged from 25 to 571 beds in size with a mixture of teaching and nonteaching hospitals. Prior to the initiative, prophylaxis improvement efforts were incomplete and inconsistent at study facilities. All adult acute care inpatients at all facilities were included except rehabilitation, behavioral health, skilled nursing, hospice, other nonacute care, and inpatient deliveries.
Design Overview
We performed a prospective, unblinded, open-intervention study of a QI collaborative in 35 community hospitals and studied the effect on prophylaxis and VTE rates with historical controls. The 35 hospitals were organized into 2 cohorts. In the “pilot” cohort, 9 hospitals (chosen to be representative of the various settings, size, and teaching status within the Dignity system) received funding from the Gordon and Betty Moore Foundation (GBMF) for intensive, individualized QI mentorship from experts as well as active surveillance (see “Interventions”). The pilot sites led the development of the VTE risk assessment and prophylaxis protocol (“VTE protocol”), measures, order sets, implementation tactics, and lessons learned, assisted by the mentor experts. Dissemination to the 26-hospital “spread” cohort was facilitated by the Dignity Health Hospital Engagement Network (HEN) infrastructure.
Timeline
Two of the pilot sites, acting as leads on the development of protocol and order set tools, formed improvement teams in March 2011, 6 to 12 months earlier than other Dignity sites. Planning and design work occurred from March 2011 to September 2012. Most implementation at the 35 hospitals occurred in a staggered fashion during calendar year (CY) 2012 and 2013 (see Figure 1). As few changes were made until mid-2012, we considered CY 2011 the baseline for comparison, CY 2012 to 2013 the implementation years, and CY 2014 the postimplementation period.
The project was reviewed by the Institutional Review Board (IRB) of Dignity Health and determined to be an IRB-exempt QI project.
Interventions
Collaborative Infrastructure
Multidisciplinary Teams
Improvement teams formed between March 2011 and September 2012. Members included a physician champion, frontline nurses and physicians, an administrative liaison, pharmacists, quality and data specialists, clinical informatics staff, and stakeholders from key clinical services. Teams met at least monthly at each site.
Physician Mentors
The 9 pilot sites received individualized mentorship provided by outside experts (IJ or GM) based on a model pioneered by the Society of Hospital Medicine’s (SHM) Mentored Implementation programs.23 Each pilot site completed a self-assessment survey17 (see supplementary Appendix A) about past efforts, team composition, current performance, aims, barriers, and opportunities. The mentors reviewed the completed questionnaire with each hospital and provided advice on the VTE protocol and order set design, measurement, and benchmarking during 3 webinar meetings scheduled at 0, 3, and 9 months, plus as-needed e-mail and phone correspondence. After each webinar, the mentors provided detailed improvement suggestions (see supplementary Appendix B). Several hospitals received mentor site visits, which focused on unit rounding, active surveillance, staff and provider education, and problem-solving sessions with senior leadership, physician leadership, and the improvement team.
VTE Protocol
After a literature review and consultation with the mentors, Dignity Health developed and implemented a VTE protocol, modified from a model used in previous improvement efforts.18,22-24 Its risk assessment method is often referred to as a “3 bucket” model because it assigns patients to high-, moderate-, or low-risk categories based on clinical factors (eg, major orthopedic surgery, prior VTE, and others), and the VTE protocol recommends interventions based on the risk category (see supplementary Appendix C). Dignity Health was transitioning to a single electronic health record (Cerner Corporation, North Kansas City, MO) during the study, and study hospitals were using multiple platforms, necessitating the development of both paper and electronic versions of the VTE protocol. The electronic version required completion of the VTE protocol for all inpatient admissions and transfers. The VTE protocol was completed in November 2011 and disseminated to other sites in a staggered fashion through November 2012. Completed protocols and improvement tips were shared by the project lead and by webinar sessions. Sites were also encouraged to implement a standardized practice that allowed nurses to apply sequential compression devices to at-risk patients without physician orders when indicated by protocol, when contraindications such as vascular disease or ulceration were absent.
Education
Staff were educated about the VTE protocol by local teams, starting between late 2011 and September 2012. The audience (physicians, nurses, pharmacists, etc.) and methods (conferences, fliers, etc.) were determined by local teams, following guidance by mentors and webinar content. Active surveillance provided opportunities for in-the-moment, patient-specific education and protocol reinforcement. Both mentors delivered educational presentations at pilot sites.
Active Surveillance
Sites were encouraged to perform daily review of prophylaxis adequacy for inpatients and correct lapses in real time (both under- and overprophylaxis). Inappropriate prophylaxis orders were addressed by contacting providers to change the order or document the rationale not to. Lapses in adherence to prophylaxis were addressed by nursing correction and education of involved staff. Active surveillance was funded for 10 hours a week at pilot sites. Spread sites received only minimal support from HEN monies. All sites used daily prophylaxis reports, enhanced to include contraindications like thrombocytopenia and coagulopathy, to facilitate efforts. Active surveillance began in May 2012 in the lead pilot hospitals and was implemented in other sites between October 2012 and February 2013.
Metrics
Prophylaxis Rates
Measurement of prophylaxis did not begin until 2012 to 2013; thus, the true baseline rate for prophylaxis was not captured. TJC metrics (VTE-1 and VTE-2)25 were consolidated into a composite TJC prophylaxis rate from January 2012 to December 2014 for both pilot and spread hospitals. These measures assess the percentage of adult inpatients who received VTE prophylaxis or have documentation of why no prophylaxis was given the day of or day after hospital admission (VTE-1) or the day of or day after ICU admission or transfer (VTE-2). These measures are met if any mechanical or pharmacologic prophylaxis was delivered.
In addition to the TJC metric, the 9 pilot hospitals monitored rates of protocol-compliant prophylaxis for 12 to 20 months. Each patient’s prophylaxis was considered protocol compliant if it was consistent with the prophylaxis protocol at the time of the audit or if contraindications were documented (eg, patients eligible for, but with contraindications to, pharmacologic prophylaxis had to have an order for mechanical prophylaxis or documented contraindication to both modalities). As this measure was initiated in a staggered fashion, the rate of protocol-compliant prophylaxis is summarized for consecutive months of measurement rather than consecutive calendar months.
HA-VTE Rates
VTE events were captured by review of electronic coding data for the International Classification of Diseases, 9th Revision (ICD-9) codes 415.11-415.19, 453.2, 453.40-453.42, and 453.8-453.89. HA-VTE was defined as either new VTE not present on admission (NPOA HA-VTE) or new VTE presenting in a readmitted patient within 30 days of discharge (Readmit HA-VTE). Cases were stratified based on whether the patient had undergone a major operation (surgery patients) or not (medical patients) as identified by Medicare Services diagnosis-related group codes.
Control Measures
Potential adverse events were captured by review of electronic coding data for ICD-9 codes 289.84 (heparin-induced thrombocytopenia [HIT]) and E934.2 (adverse effects because of anticoagulants).
Statistical Analysis
Statistical process control charts were used to depict changes in prophylaxis rates over the 3 years for which data was collected. For VTE and safety outcomes, Pearson χ2 value with relative risk (RR) calculations and 95% confidence intervals (CIs) were used to compare proportions between groups at baseline (CY 2011) versus postimplementation (CY 2014). Differences between the means of normally distributed data were calculated, and a 95% CI for the difference between the means was performed to assess statistical difference. Nonparametric characteristics were described by quartiles and interquartile range, and the 2-sided Mann-Whitney U test was performed to assess statistical difference between the CY 2011 and CY 2014 period.
Role of the Funding Source
The GBMF funded the collaborative and supported authorship of the manuscript but had no role in the design or conduct of the intervention, the collection or analysis of data, or the drafting of the manuscript.
RESULTS
Population Demographics
There were 1,155,069 adult inpatient admissions during the 4-year study period (264,280 in the 9 pilot sites, 890,789 in the 26 spread sites). There were no clinically relevant changes in gender distribution, mortality rate, median age, case mix index, or hospital length of stay in 2011 versus 2014. Men comprised 47.1% of the patient population in 2011 and 47.7% in 2014. The mortality rate was 2.7% in both years. Median age was 62 in 2011 and 63 in 2014. The mean case mix index (1.58 vs 1.65) and mean length of stay (4.29 vs 4.33 days) were similar in the 2 time periods.
Prophylaxis Rates
TJC Prophylaxis rates
Rates of Protocol-Compliant Prophylaxis
There were 34,071 active surveillance audits across the 20 months of reporting in the pilot cohort (mean, 1817 audits per month). The rate of protocol-compliant prophylaxis improved from 89% at month 1 of observation to 93% during month 2 and 97% by the last 3 months (Pearson χ2 P < .001 for both comparisons).
HA-VTE
HA-VTE characteristics
Improved HA-VTE over Time
Four hundred twenty-eight fewer HA-VTEs occurred in 2014 than in 2011 (RR 0.78; 95% CI, 0.73-0.85) (Table and Figure 3). Readmission HA-VTEs were reduced by 315 (RR 0.72; 95% CI, 0.65-0.80), while the reduction in NPOA HA-VTEs was less robust (RR 0.88; 95% CI, 0.79-0.99). Pilot sites enjoyed a more robust reduction in HA-VTEs than spread sites (26% vs 20%), largely because the pilot cohort enjoyed a 34% reduction in NPOA HA-VTEs and a 20% reduction in Readmit HA-VTEs, while the spread cohort only achieved reductions in Readmit HA-VTEs.
Safety
Rates of HIT and adverse effects because of anticoagulants were low (Table). The rate of HIT declined from 178 events in 2011 to 109 in 2014 (RR 0.66; 95% CI, 0.52-0.84), and the RR of anticoagulant adverse events remained stable (RR 1.01; 95% CI, 0.87-1.15).
DISCUSSION
Our QI project, based on a proven collaborative approach and mentorship,18,22,24 order set redesign, and active surveillance, was associated with 26% less VTEs in the pilot cohort and 20% less VTEs in the spread cohort. These gains, down to a final rate of approximately 4 HA-VTEs per 1000 admissions, occurred despite a low baseline HA-VTE rate. Dignity Health achieved these improvements in 35 hospitals with varied sizes, settings, ordering systems, and teaching statuses, achieving what is to our knowledge the largest VTE QI initiative yet reported.
Implementation experiences were not systematically recorded, and techniques were not compared with a control group. However, we believe that Dignity Health’s organizational commitment to improvement and centralized support were crucial for success. In addition, the pilot sites received grant support from the GBMF for intensive quality mentoring, a strategy with demonstrated value.23 Mentors and team members noted that system-wide revision to the computerized physician order entry system was easiest to implement, while active surveillance represented the most labor-intensive intervention. Other experiences echoed lessons from previous VTE mentorship efforts.17,18
The selection of a VTE protocol conducive to implementation and provider use was a key strategy. The ideal approach to VTE risk assessment is not known,12,26 but guidelines either offer no specific guidance7 or would require implementation of 3 different systems per hospital.4,5 Several of these are point scoring systems, which may have lower clinician acceptance or require programming to improve real-world use18,26,27; the Padua score was derived from a patient population that differs significantly from those in the United States.12 Our study provides more practical experience with a “3-bucket” model, which has previously shown high interobserver reliability, good clinician acceptance, and meaningful reductions of VTE, including in American patient populations.18,22,24
The value of VTE prophylaxis is still disputed in many inpatient groups. The overall rate of HA-VTE is low, so the per-patient benefit of prophylaxis is low, and many patients may be overprophylaxed.4,11,12 Recently, Flanders et al.20 reported that HA-VTE rates among 20,800 medical inpatients in Michigan were low (about 1%) and similar at hospitals in the top (mean prophylaxis rate 86%) or bottom (mean prophylaxis rate 56%) tertiles of performance. Possible explanations for the differences between their multicenter experience and ours include our sample size (55 times larger) and the possibility that targeting prophylaxis to patients at highest need (captured in our protocol-compliant prophylaxis rates) matters more than prophylaxing a percent of the population.
Further research is needed to develop simple, easy-to-implement methods to identify inpatients who do not, or no longer, require prophylaxis.12 Hospital systems also need methods to determine if prophylaxis improvement efforts can lower their HA-VTE rates and in which subpopulations. For example, a collaborative effort at the University of California lowered HA-VTE rates toward a common improved rate of 0.65% to 0.73%,22 while Dignity Health achieved improvement despite starting with an even lower baseline. In the University of California collaborative, benefits were limited chiefly to surgical patients, while Dignity Health achieved most improvement in medical patients, particularly in Readmit HA-VTE. If future research uncovers the reasons for these differences, it could help hospitals decide where to target improvement efforts.
Our study has several limitations. First, we used a nonrandomized time series design, so we cannot exclude other potential explanations for the change in VTE rates. However, there were no major changes in patient populations or concurrent projects likely to have influenced event rates. While we did not collect detailed demographic information on subjects, the broad inclusion criteria and multicenter design suggests a high degree of generalizability. Second, we followed inpatient VTE events and VTE-related readmissions, but not VTE treated in the outpatient setting. This did not change over the study, but the availability of all-oral therapy for VTE could have caused underdetection if clinic or emergency room doctors sent home more patients on oral therapy instead of readmitting them to the hospital. Third, implementation was enhanced by GBMF funds (at 9 sites, with the remainder benefitting from their experience), a shared electronic med
Strengths of our study include reductions in HA-VTE, both with and without access to GBMF funds, by using broadly available QI strategies.17 This real-world success and ease of dissemination are particularly important because the clinical trials of prophylaxis have been criticized for using highly selected patient populations,11 and prophylaxis QI studies show an inconsistent impact on VTE outcomes.15 In previous studies, two of the authors monitored orders for prophylaxis22,24; during this project, delivery for both pharmacologic and mechanical VTE prophylaxis was monitored, confirming that patient care actually changed.
CONCLUSION
Our multicenter VTE prophylaxis initiative, featuring a “3-bucket” VTE protocol, QI mentorship, and active surveillance as key interventions, was associated with improved prophylaxis rates and a reduction in HA-VTE by 22% with no increase in adverse events. This project provides a model for hospital systems seeking to optimize their prophylaxis efforts, and it supports the use of collaborative QI initiatives and SHM’s quality mentorship program as methods to drive improvement across health systems.
Disclosure
None of the authors have any conflicts of interest related to any topics or products discussed in the article. Dignity Health provided a stipend for writing the manuscript to GM and IJ, as noted in the article, but had no role in data analysis, writing, or decision to submit.
1. U.S. Department of Health and Human Services; National Heart, Lung, and Blood Institute. Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville: Office of the Surgeon General; 2008.
2. Heit JA, Melton LJ, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients versus community residents. Mayo Clin Proc. 2001;76(11):1102-1110. PubMed
3. Guyatt GH, Eikelboom JW, Gould MK. Approach to Outcome Measurement in the Prevention of Thrombosis in Surgical and Medical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e185S-e194S. doi:10.1378/chest.11-2289. PubMed
4. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in Nonsurgical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S. doi:10.1378/chest.11-2296. PubMed
5. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in Nonorthopedic Surgical Patients. Chest. 2012;141(2 suppl):e227S-e277S. PubMed
6. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in Orthopedic Surgery Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S. doi:10.1378/chest.11-2404. PubMed
7. Qaseem A, Chou R, Humphrey LL. Venous Thromboembolism Prophylaxis in Hospitalized Patients: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. PubMed
8. The Joint Commission. Performance Measurement Initiatives. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement. Accessed June 14, 2012.
9. National Quality Forum. National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures. http://www.qualityforum.org/Publications/2006/12/National_Voluntary_Consensus_Standards_for_Prevention_and_Care_of_Venous_Thromboembolism__Policy,_Preferred_Practices,_and_Initial_Performance_Measures.aspx. Accessed June 14, 2012.
10. Medicare Quality Improvement Committee. SCIP Project Information. Agency for Healthcare Research and Quality. http://www.qualitymeasures.ahrq.gov/content.aspx?id=35538&search=scip. Accessed March 2013.
11. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients and Those with Stroke: A Background Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. PubMed
12. Rothberg MB. Venous thromboembolism prophylaxis for medical patients: who needs it? JAMA Intern Med. 2014;174(10):1585-1586. PubMed
13. Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet. 2008;371(9610):387-394. PubMed
14. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4(8):E15-E21. PubMed
15. Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201. doi:10.1002/14651858.CD008201.pub2. PubMed
16. Lau BD, Haut ER. Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187-195. PubMed
17. Maynard G. Preventing hospital-associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. Rockville: Agency for Healthcare Research and Quality; 2015. https://www.ahrq.gov/sites/default/files/publications/files/vteguide.pdf. Accessed October 29, 2017.
18. Maynard G, Stein J. Designing and Implementing Effective VTE Prevention Protocols: Lessons from Collaboratives. J Thromb Thrombolysis. 2010;29(2):159-166. PubMed
19. Altom LK, Deierhoi RJ, Grams J, et al. Association between Surgical Care Improvement Program venous thromboembolism measures and postoperative events. Am J Surg. 2012;204(5):591-597. PubMed
20. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. PubMed
21. Finn KM, Greenwald JL. Update in Hospital Medicine: Evidence You Should Know. J Hosp Med. 2015;10(12):817-826. PubMed
22. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: Findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. PubMed
23. Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg Patient Safety and Quality Award. Mentored Implementation: Building Leaders and Achieving Results Through a Collaborative Improvement Model at the National Level. Jt Comm J Qual Patient Saf. 2012;38(7):301-310.
24. Maynard GA, Morris TA, Jenkins IH, et al. Optimizing prevention of hospital-acquired venous thromboembolism (VTE): Prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10-18. PubMed
25. The Joint Commission. Venous Thromboembolism Quality Measures. https://www.jointcommission.org/venous_thromboembolism/. Accessed October 13, 2017.
26. Maynard GA, Jenkins IH, Merli GJ. Venous thromboembolism prevention guidelines for medical inpatients: Mind the (implementation) Gap. J Hosp Med. 2013;8(10):582-588. PubMed
27. Elias P, Khanna R, Dudley A, et al. Automating Venous Thromboembolism Risk Calculation Using Electronic Health Record Data upon Hospital Admission: The Automated Padua Prediction Score. J Hosp Med. 2017;12(4):231-237. PubMed
1. U.S. Department of Health and Human Services; National Heart, Lung, and Blood Institute. Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville: Office of the Surgeon General; 2008.
2. Heit JA, Melton LJ, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients versus community residents. Mayo Clin Proc. 2001;76(11):1102-1110. PubMed
3. Guyatt GH, Eikelboom JW, Gould MK. Approach to Outcome Measurement in the Prevention of Thrombosis in Surgical and Medical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e185S-e194S. doi:10.1378/chest.11-2289. PubMed
4. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in Nonsurgical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S. doi:10.1378/chest.11-2296. PubMed
5. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in Nonorthopedic Surgical Patients. Chest. 2012;141(2 suppl):e227S-e277S. PubMed
6. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in Orthopedic Surgery Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S. doi:10.1378/chest.11-2404. PubMed
7. Qaseem A, Chou R, Humphrey LL. Venous Thromboembolism Prophylaxis in Hospitalized Patients: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. PubMed
8. The Joint Commission. Performance Measurement Initiatives. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement. Accessed June 14, 2012.
9. National Quality Forum. National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures. http://www.qualityforum.org/Publications/2006/12/National_Voluntary_Consensus_Standards_for_Prevention_and_Care_of_Venous_Thromboembolism__Policy,_Preferred_Practices,_and_Initial_Performance_Measures.aspx. Accessed June 14, 2012.
10. Medicare Quality Improvement Committee. SCIP Project Information. Agency for Healthcare Research and Quality. http://www.qualitymeasures.ahrq.gov/content.aspx?id=35538&search=scip. Accessed March 2013.
11. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients and Those with Stroke: A Background Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. PubMed
12. Rothberg MB. Venous thromboembolism prophylaxis for medical patients: who needs it? JAMA Intern Med. 2014;174(10):1585-1586. PubMed
13. Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet. 2008;371(9610):387-394. PubMed
14. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4(8):E15-E21. PubMed
15. Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201. doi:10.1002/14651858.CD008201.pub2. PubMed
16. Lau BD, Haut ER. Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187-195. PubMed
17. Maynard G. Preventing hospital-associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. Rockville: Agency for Healthcare Research and Quality; 2015. https://www.ahrq.gov/sites/default/files/publications/files/vteguide.pdf. Accessed October 29, 2017.
18. Maynard G, Stein J. Designing and Implementing Effective VTE Prevention Protocols: Lessons from Collaboratives. J Thromb Thrombolysis. 2010;29(2):159-166. PubMed
19. Altom LK, Deierhoi RJ, Grams J, et al. Association between Surgical Care Improvement Program venous thromboembolism measures and postoperative events. Am J Surg. 2012;204(5):591-597. PubMed
20. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. PubMed
21. Finn KM, Greenwald JL. Update in Hospital Medicine: Evidence You Should Know. J Hosp Med. 2015;10(12):817-826. PubMed
22. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: Findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. PubMed
23. Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg Patient Safety and Quality Award. Mentored Implementation: Building Leaders and Achieving Results Through a Collaborative Improvement Model at the National Level. Jt Comm J Qual Patient Saf. 2012;38(7):301-310.
24. Maynard GA, Morris TA, Jenkins IH, et al. Optimizing prevention of hospital-acquired venous thromboembolism (VTE): Prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10-18. PubMed
25. The Joint Commission. Venous Thromboembolism Quality Measures. https://www.jointcommission.org/venous_thromboembolism/. Accessed October 13, 2017.
26. Maynard GA, Jenkins IH, Merli GJ. Venous thromboembolism prevention guidelines for medical inpatients: Mind the (implementation) Gap. J Hosp Med. 2013;8(10):582-588. PubMed
27. Elias P, Khanna R, Dudley A, et al. Automating Venous Thromboembolism Risk Calculation Using Electronic Health Record Data upon Hospital Admission: The Automated Padua Prediction Score. J Hosp Med. 2017;12(4):231-237. PubMed
© 2018 Society of Hospital Medicine